Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Adaptimmune Therapeutics PLC | a17-14214_48k.htm |
Exhibit 99.1
NY-ESO SPEAR T-cells in Synovial Sarcoma June 6, 2017 ASCO Update

Disclaimer This presentation contains “forward-looking statements,” as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as “believe,” “may”, “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect” and other words of similar meaning. These forward-looking statements involve certain risks and uncertainties. Such risks and uncertainties could cause our actual results to differ materially from those indicated by such forward-looking statements, and include, without limitation: the success, cost and timing of our product development activities and clinical trials; our ability to submit an IND and successfully advance our technology platform to improve the safety and effectiveness of our existing TCR therapeutic candidates; the rate and degree of market acceptance of T-cell therapy generally and of our TCR therapeutic candidates; government regulation and approval, including, but not limited to, the expected regulatory approval timelines for TCR therapeutic candidates; and our ability to protect our proprietary technology and enforce our intellectual property rights; amongst others. For a further description of the risks and uncertainties that could cause our actual results to differ materially from those expressed in these forward-looking statements, as well as risks relating to our business in general, we refer you to our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on May 10, 2017 and our other SEC filings. We urge you to consider these factors carefully in evaluating the forward-looking statements herein and you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. The forward-looking statements contained in this presentation speak only as of the date the statements were made and we do not undertake any obligation to update such forward-looking statements to reflect subsequent events or circumstances. We intend that all forward-looking statements be subject to the safe-harbor provisions of the PSLRA. 2
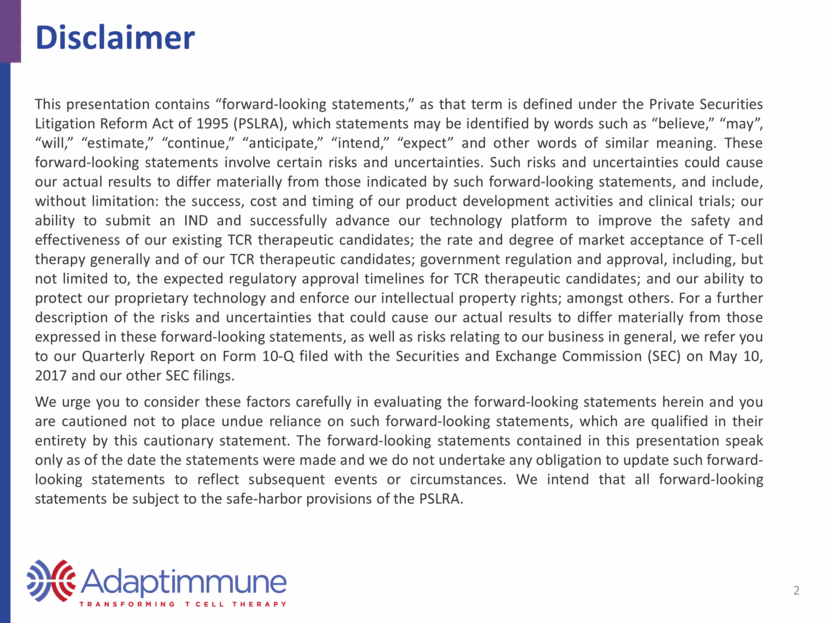
Synovial Sarcoma Rare cancer; ~20% of all soft tissue sarcomas a Peak incidence among people in their 30s Tumors primarily in extremities Near joints and tendons Treatment includes radiotherapy, chemotherapy, and surgery Many patients undergo “radical” treatments including amputations Patients have poor prognoses b, c 5-yr overall survival ~52% Unresectable, recurrent, and/or metastatic disease almost universally fatal NY-ESO expression observed >70% of cases d Overview 3 a Herzog CE. J Pediatr Hematol Oncol 2005 b Corey RM, et al. Cancer Med 2014 c Minchom A, et al. Sarcoma 2010 d Lai JP, et al. OncoImmunol 2012 e Zhao Y, et al. J Immunol 2012 NY-ESO-1 expression by IHC in Synovial Sarcoma
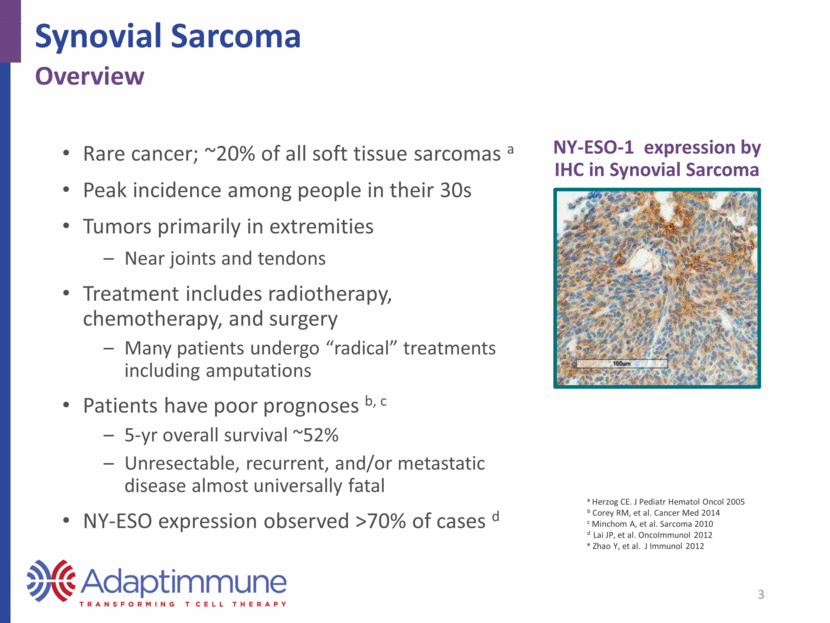
Key Study Design Elements 4 NY-ESO in synovial sarcoma Design Element Overview Objectives Primary – response rate by RECIST v1.1 Secondary – overall survival, safety, duration of response, progression-free survival Exploratory – persistence, phenotype, and function of SPEAR T-cells; mechanisms of resistance and sensitivity Procedures HLA and antigen screen, apheresis, manufacture, lymphodepletion, SPEAR T-cell infusion Follow-up - disease assessed at weeks 4, 8, 12; then every 3 months until progression) Long-term follow-up every 6 months for 5 years, then annually through year 15 Eligibility Age > 4; ECOG 0-1 or Lansky >60 (for children age <10) Pathologically confirmed synovial sarcoma Measurable disease per RECIST v1.1 NY-ESO-1 expression by IHC (high: 2+ or 3+ in > 50% cells; low: > 1+ in > 1% to < 50% cells) HLA-A*02:01, HLA-A*02:05, HLA-A*02:06 Cohorts Cohort 1: High NY-ESO / Flu 30 mg/m2/day x 4 + Cy 1800 mg/m2/day x 2 Cohort 2: Low NY-ESO / NY-ESO / Flu 30 mg/m2/day x 4 + Cy 1800 mg/m2/day x 2 Cohort 3: High NY-ESO / Cy 1800 mg/m2/day x 2 Cohort 4: High NY-ESO / Flu 30 mg/m2/day x 3 + Cy 600 mg/m2/day x 3
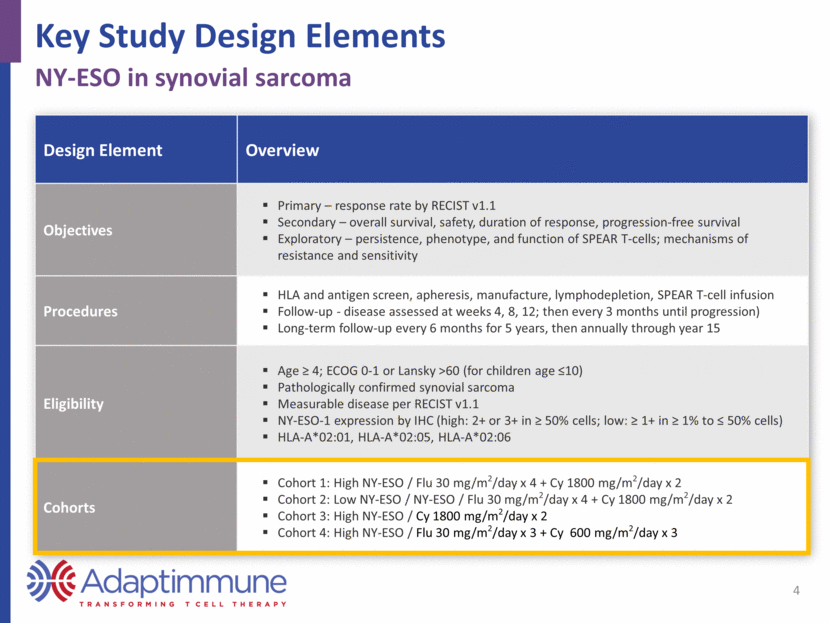
Response Summary 5 Responses observed in every cohort Measure Cohort 1 (closed) N=12 Cohort 2 (ongoing) N=5 Cohort 3 (closed) N=5 Cohort 4 (ongoing) N=6 Best overall response: N (%) CR PR SD PD Not assessed 1 (8) 5 (42) 6 (50) 0 (0) 0 (0) 0 (0) 2 (40) 1 (20) 1 (20) 1 (20) 0 (0) 1 (20) 4 (80) 0 (0) 0 (0) 0 (0) 3 (50) 3 (50) 0 (0) 0 (0) ORR: Confirmed, CR+PR: N (%) 6 (50) a 2 (40) 1 (20) 3 (50) Median PFS: weeks (range) 15 (8, 38) 12 (0.3, 14) 12 (8, 38) NE Median response duration: weeks (range) 30.9 (13, 72) 7.5 (6, 9) 21 b NE CR=complete response; PR=partial response; SD=stable disease; PD=progressive disease; ORR=objective response rate; PFS=progression-free survival; NE=not evaluable a ORR among 10 patients who received target dose: 6/10 (60%) b One response ongoing at 21 weeks Data cutoff 30 Mar 2017
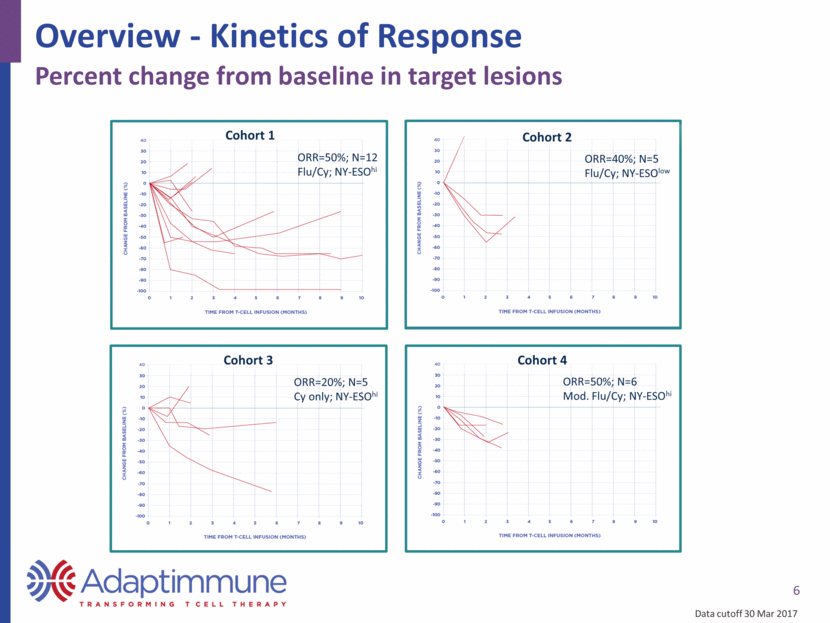
6 Overview - Kinetics of Response Percent change from baseline in target lesions Cohort 1 Cohort 2 Cohort 3 Cohort 4 ORR=50%; N=12 Flu/Cy; NY-ESOhi ORR=40%; N=5 Flu/Cy; NY-ESOlow ORR=20%; N=5 Cy only; NY-ESOhi ORR=50%; N=6 Mod. Flu/Cy; NY-ESOhi Data cutoff 30 Mar 2017
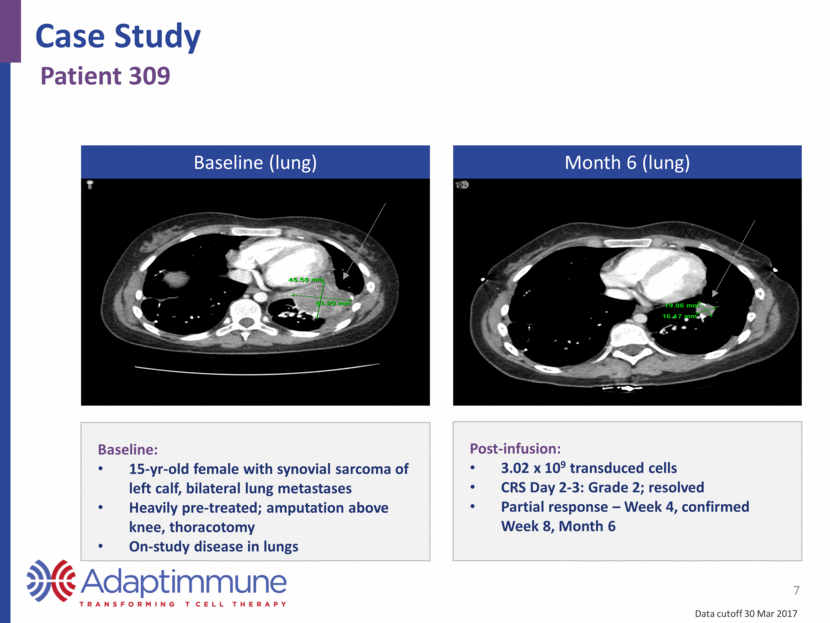
7 Baseline (lung) Month 6 (lung) Baseline: 15-yr-old female with synovial sarcoma of left calf, bilateral lung metastases Heavily pre-treated; amputation above knee, thoracotomy On-study disease in lungs Post-infusion: 3.02 x 109 transduced cells CRS Day 2-3: Grade 2; resolved Partial response – Week 4, confirmed Week 8, Month 6 Case Study Patient 309 Data cutoff 30 Mar 2017
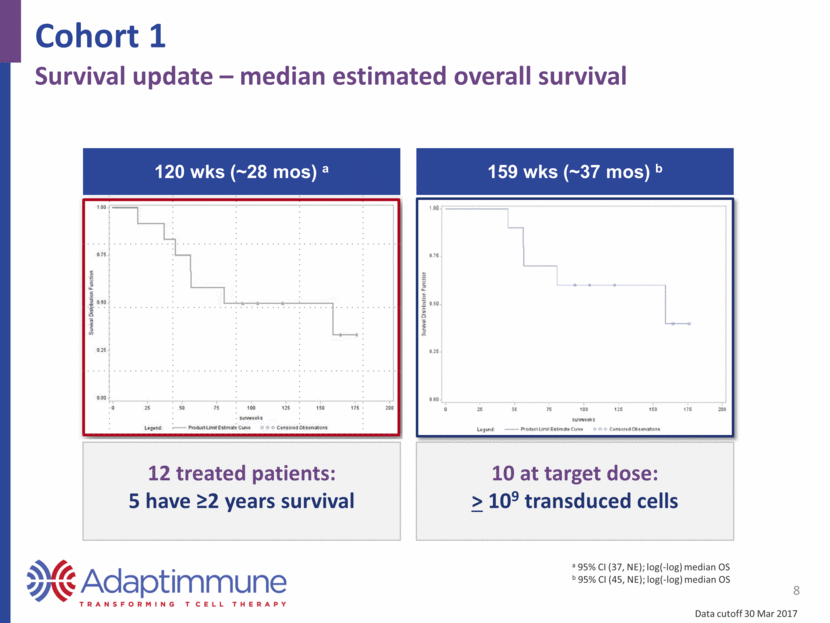
Cohort 1 8 Survival update – median estimated overall survival 120 wks (~28 mos) a 12 treated patients: 5 have >2 years survival 159 wks (~37 mos) b a 95% CI (37, NE); log(-log) median OS b 95% CI (45, NE); log(-log) median OS 10 at target dose: > 109 transduced cells Data cutoff 30 Mar 2017
AEs > Grade 3 in >10% of Patients 9 NY-ESO SPEAR T-cells continue to be well-tolerated Adverse Event a Cohort 1 N=12 (%) Cohort 2 N=5 (%) Cohort 3 N=5 (%) Cohort 4 N=6 (%) Total N=28 (%) White blood cell count decreased 11 (92) 4 (80) 5 (100) 3 (50) 23 (82) Lymphocyte count decreased 12 (100) 4 (80) 3 (60) 1 (17) 20 (71) Neutrophil count decreased 10 (83) 4 (80) 4 (80) 2 (33) 20 (71) Anemia 10 (83) 2 (40) 3 (60) 2 (33) 17 (61) Platelet count decreased 8 (67) 2 (40) 4 (80) 2 (33) 16 (57) Dyspnea 1 (8) 2 (40) 3 (60) 0 (0) 6 (21) Febrile neutropenia 3 (25) 1 (20) 0 (0) 1 (17) 5 (18) Cytokine Release Syndrome 2 (17) 1 (20) 1 (20) 0 (0) 4 (14) Pyrexia 1 (8) 1 (20) 2 (40) 0 (0) 4 (14) Fatigue 1 (8) 1 (20) 0 (0) 1 (17) 3 (11) Hypoxia 0 (0) 2 (40) 1 (20) 0 (0) 3 (11) a Excludes laboratory abnormalities except for hematologic terms Data cutoff 30 Mar 2017 One fatal related SAE due to bone marrow failure (reported ASCO 2016)

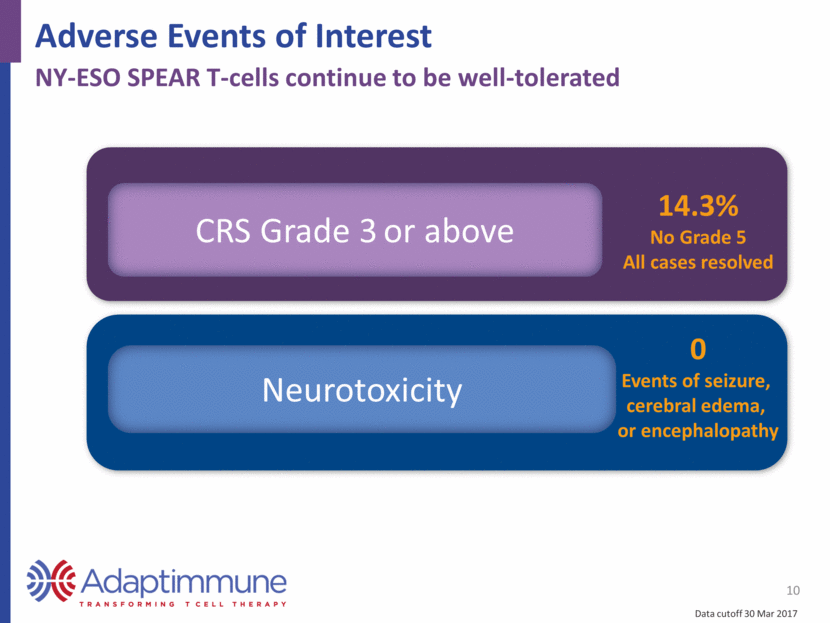
Adverse Events of Interest 10 NY-ESO SPEAR T-cells continue to be well-tolerated CRS Grade 3 or above 14.3% No Grade 5 All cases resolved Neurotoxicity 0 Events of seizure, cerebral edema, or encephalopathy Data cutoff 30 Mar 2017
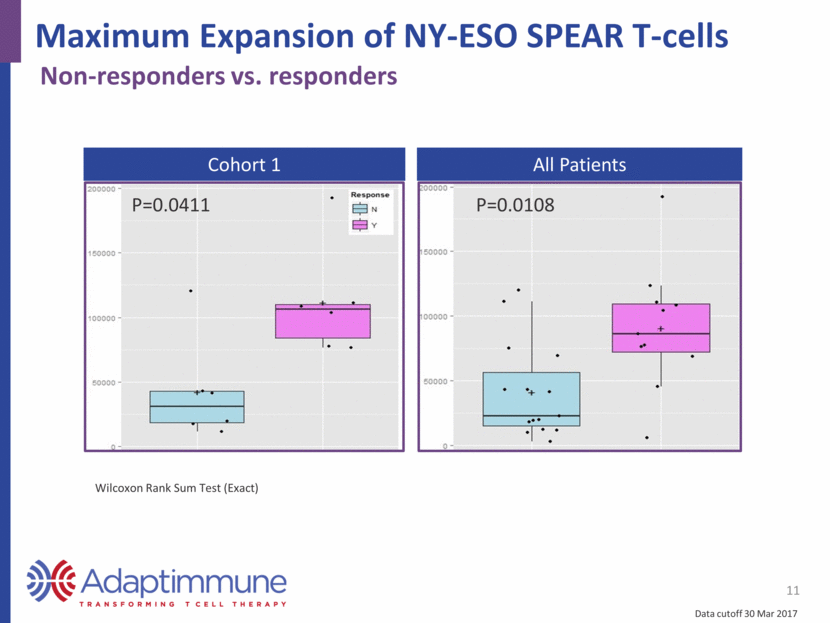
Maximum Expansion of NY-ESO SPEAR T-cells 11 Non-responders vs. responders Cohort 1 All Patients P=0.0411 P=0.0108 Data cutoff 30 Mar 2017 Wilcoxon Rank Sum Test (Exact)
Conclusions 12 Data update: NY-ESO in synovial sarcoma Initial efficacy results encouraging Cohort 1 survival data is promising NY-ESO SPEAR T-cells continue to be well-tolerated Maximal expansion appears to correlate with response
NY-ESO SPEAR T-cells in Synovial Sarcoma June 6, 2017 ASCO Update

