Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Recro Pharma, Inc. | d382822dex991.htm |
| 8-K - 8-K - Recro Pharma, Inc. | d382822d8k.htm |
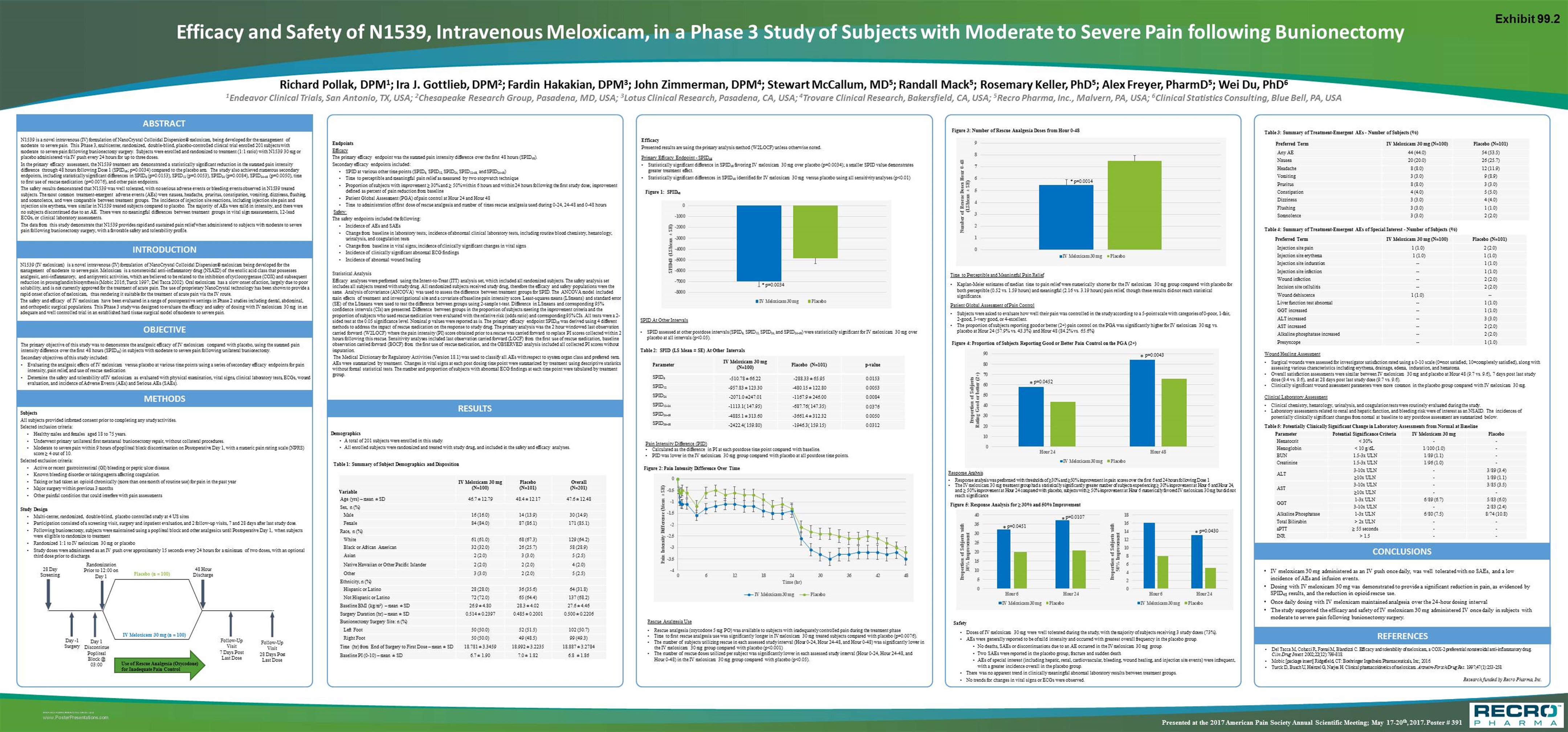
N1539 is a novel intravenous (IV) formulation of NanoCrystal Colloidal Dispersion® meloxicam, being developed for the management of moderate to severe pain. This Phase 3, multicenter, randomized, double-blind, placebo-controlled clinical trial enrolled 201 subjects with moderate to severe pain following bunionectomy surgery. Subjects were enrolled and randomized to treatment (1:1 ratio) with N1539 30 mg or placebo administered via IV push every 24 hours for up to three doses. In the primary efficacy assessment, the N1539 treatment arm demonstrated a statistically significant reduction in the summed pain intensity difference through 48 hours following Dose 1 (SPID48; p=0.0034) compared to the placebo arm. The study also achieved numerous secondary endpoints, including statistically significant differences in SPID6 (p=0.0153), SPID12 (p=0.0053), SPID24 (p=0.0084), SPID24-48 (p=0.0050), time to first use of rescue medication (p=0.0076), and other pain endpoints. The safety results demonstrated that N1539 was well tolerated, with no serious adverse events or bleeding events observed in N1539 treated subjects. The most common treatment-emergent adverse events (AEs) were nausea, headache, pruritus, constipation, vomiting, dizziness, flushing, and somnolence, and were comparable between treatment groups. The incidence of injection site reactions, including injection site pain and injection site erythema, were similar in N1539 treated subjects compared to placebo. The majority of AEs were mild in intensity, and there were no subjects discontinued due to an AE. There were no meaningful differences between treatment groups in vital sign measurements, 12-lead ECGs, or clinical laboratory assessments. The data from this study demonstrate that N1539 provides rapid and sustained pain relief when administered to subjects with moderate to severe pain following bunionectomy surgery, with a favorable safety and tolerability profile. ABSTRACT Richard Pollak, DPM1; Ira J. Gottlieb, DPM2; Fardin Hakakian, DPM3; John Zimmerman, DPM4; Stewart McCallum, MD5; Randall Mack5; Rosemary Keller, PhD5; Alex Freyer, PharmD5; Wei Du, PhD6 1Endeavor Clinical Trials, San Antonio, TX, USA; 2Chesapeake Research Group, Pasadena, MD, USA; 3Lotus Clinical Research, Pasadena, CA, USA; 4Trovare Clinical Research, Bakersfield, CA, USA; 5Recro Pharma, Inc., Malvern, PA, USA; 6Clinical Statistics Consulting, Blue Bell, PA, USA Efficacy and Safety of N1539, Intravenous Meloxicam, in a Phase 3 Study of Subjects with Moderate to Severe Pain following Bunionectomy The primary objective of this study was to demonstrate the analgesic efficacy of IV meloxicam compared with placebo, using the summed pain intensity difference over the first 48 hours (SPID48) in subjects with moderate to severe pain following unilateral bunionectomy. Secondary objectives of this study included: Evaluating the analgesic effects of IV meloxicam versus placebo at various time points using a series of secondary efficacy endpoints for pain intensity, pain relief, and use of rescue medication Determine the safety and tolerability of IV meloxicam as evaluated with physical examination, vital signs, clinical laboratory tests, ECGs, wound evaluation, and incidence of Adverse Events (AEs) and Serious AEs (SAEs). OBJECTIVE Subjects All subjects provided informed consent prior to completing any study activities. Selected inclusion criteria: Healthy males and females aged 18 to 75 years. Underwent primary unilateral first metatarsal bunionectomy repair, without collateral procedures. Moderate to severe pain within 9 hours of popliteal block discontinuation on Postoperative Day 1, with a numeric pain rating scale (NPRS) score ≥ 4 out of 10. Selected exclusion criteria: Active or recent gastrointestinal (GI) bleeding or peptic ulcer disease. Known bleeding disorder or taking agents affecting coagulation. Taking or had taken an opioid chronically (more than one month of routine use) for pain in the past year Major surgery within previous 3 months Other painful condition that could interfere with pain assessments Study Design Multi-center, randomized, double-blind, placebo controlled study at 4 US sites Participation consisted of a screening visit, surgery and inpatient evaluation, and 2 follow-up visits, 7 and 28 days after last study dose. Following bunionectomy, subjects were maintained using a popliteal block and other analgesics until Postoperative Day 1, when subjects were eligible to randomize to treatment Randomized 1:1 to IV meloxicam 30 mg or placebo Study doses were administered as an IV push over approximately 15 seconds every 24 hours for a minimum of two doses, with an optional third dose prior to discharge. METHODS RESULTS Demographics A total of 201 subjects were enrolled in this study. All enrolled subjects were randomized and treated with study drug, and included in the safety and efficacy analyses. Endpoints Efficacy The primary efficacy endpoint was the summed pain intensity difference over the first 48 hours (SPID48). Secondary efficacy endpoints included: SPID at various other time points (SPID6, SPID12, SPID24, SPID12-48, and SPID24-48) Time to perceptible and meaningful pain relief as measured by two stopwatch technique Proportion of subjects with improvement ≥ 30% and ≥ 50% within 6 hours and within 24 hours following the first study dose; improvement defined as percent of pain reduction from baseline Patient Global Assessment (PGA) of pain control at Hour 24 and Hour 48 Time to administration of first dose of rescue analgesia and number of times rescue analgesia used during 0-24, 24-48 and 0-48 hours Safety: The safety endpoints included the following: Incidence of AEs and SAEs Change from baseline in laboratory tests; incidence of abnormal clinical laboratory tests, including routine blood chemistry, hematology, urinalysis, and coagulation tests Change from baseline in vital signs; incidence of clinically significant changes in vital signs Incidence of clinically significant abnormal ECG findings Incidence of abnormal wound healing Statistical Analysis Efficacy analyses were performed using the Intent-to-Treat (ITT) analysis set, which included all randomized subjects. The safety analysis set includes all subjects treated with study drug. All randomized subjects received study drug, therefore the efficacy and safety populations were the same. Analysis of covariance (ANCOVA) was used to assess the difference between treatment groups for SPID. The ANCOVA model included main effects of treatment and investigational site and a covariate of baseline pain intensity score. Least-squares means (LSmeans) and standard error (SE) of the LSmeans were used to test the difference between groups using 2-sample t-test. Difference in LSmeans and corresponding 95% confidence intervals (CIs) are presented. Difference between groups in the proportion of subjects meeting the improvement criteria and the proportion of subjects who used rescue medication were evaluated with the relative risk (odds ratio) and corresponding 95% CIs. All tests were a 2-sided test at the 0.05 significance level. Nominal p values were reported as is. The primary efficacy endpoint SPID48 was derived using 4 different methods to address the impact of rescue medication on the response to study drug. The primary analysis was the 2 hour windowed last observation carried forward (W2LOCF) where the pain intensity (PI) score obtained prior to a rescue was carried forward to replace PI scores collected within 2 hours following this rescue. Sensitivity analyses included last observation carried forward (LOCF) from the first use of rescue medication, baseline observation carried forward (BOCF) from the first use of rescue medication, and the OBSERVED analysis included all collected PI scores without imputation. The Medical Dictionary for Regulatory Activities (Version 18.1) was used to classify all AEs with respect to system organ class and preferred term. AEs were summarized by treatment. Changes in vital signs at each post dosing time point were summarized by treatment using descriptive statistics without formal statistical tests. The number and proportion of subjects with abnormal ECG findings at each time point were tabulated by treatment group. Safety Doses of IV meloxicam 30 mg were well tolerated during the study, with the majority of subjects receiving 3 study doses (73%). AEs were generally reported to be of mild intensity and occurred with greatest overall frequency in the placebo group. No deaths, SAEs or discontinuations due to an AE occurred in the IV meloxicam 30 mg group. Two SAEs were reported in the placebo group; fracture and sudden death AEs of special interest (including hepatic, renal, cardiovascular, bleeding, wound healing, and injection site events) were infrequent, with a greater incidence overall in the placebo group. There was no apparent trend in clinically meaningful abnormal laboratory results between treatment groups. No trends for changes in vital signs or ECGs were observed. IV meloxicam 30 mg administered as an IV push once daily, was well tolerated with no SAEs, and a low incidence of AEs and infusion events. Dosing with IV meloxicam 30 mg was demonstrated to provide a significant reduction in pain, as evidenced by SPID48 results, and the reduction in opioid rescue use. Once daily dosing with IV meloxicam maintained analgesia over the 24-hour dosing interval The study supported the efficacy and safety of IV meloxicam 30 mg administered IV once daily in subjects with moderate to severe pain following bunionectomy surgery. CONCLUSIONS Del Tacca M, Colucci R, Fornai M, Blandizzi C. Efficacy and tolerability of meloxicam, a COX‑2 preferential nonsteroidal anti-inflammatory drug. Clin Drug Invest. 2002;22(12):799-818. Mobic [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2016. Turck D, Busch U, Heinzel G, Narjes H. Clinical pharmacokinetics of meloxicam. Arzneim-Forsch/Drug Res. 1997;47(1):253-258. REFERENCES Research funded by Recro Pharma, Inc. Presented at the 2017 American Pain Society Annual Scientific Meeting; May 17-20th, 2017. Poster # 391 INTRODUCTION N1539 (IV meloxicam) is a novel intravenous (IV) formulation of NanoCrystal Colloidal Dispersion® meloxicam being developed for the management of moderate to severe pain. Meloxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the enolic acid class that possesses analgesic, anti-inflammatory, and antipyretic activities, which are believed to be related to the inhibition of cyclooxygenase (COX) and subsequent reduction in prostaglandin biosynthesis (Mobic 2016; Turck 1997; Del Tacca 2002). Oral meloxicam has a slow onset of action, largely due to poor solubility, and is not currently approved for the treatment of acute pain. The use of proprietary NanoCrystal technology has been shown to provide a rapid onset of action of meloxicam, thus rendering it suitable for the treatment of acute pain via the IV route. The safety and efficacy of IV meloxicam have been evaluated in a range of postoperative settings in Phase 2 studies including dental, abdominal, and orthopedic surgical populations. This Phase 3 study was designed to evaluate the efficacy and safety of dosing with IV meloxicam 30 mg in an adequate and well controlled trial in an established hard tissue surgical model of moderate to severe pain. Table 1: Summary of Subject Demographics and Disposition Efficacy Presented results are using the primary analysis method (W2LOCF) unless otherwise noted. Primary Efficacy Endpoint - SPID48 Statistically significant difference in SPID48 favoring IV meloxicam 30 mg over placebo (p=0.0034); a smaller SPID value demonstrates greater treatment effect. Statistically significant differences in SPID48 identified for IV meloxicam 30 mg versus placebo using all sensitivity analyses (p<0.01) Figure 1: SPID48 Rescue Analgesia Use Rescue analgesia (oxycodone 5 mg PO) was available to subjects with inadequately controlled pain during the treatment phase Time to first rescue analgesia use was significantly longer in IV meloxicam 30 mg treated subjects compared with placebo (p=0.0076). The number of subjects utilizing rescue in each assessed study interval (Hour 0-24, Hour 24-48, and Hour 0-48) was significantly lower in the IV meloxicam 30 mg group compared with placebo (p<0.001) The number of rescue doses utilized per subject was significantly lower in each assessed study interval (Hour 0-24, Hour 24-48, and Hour 0-48) in the IV meloxicam 30 mg group compared with placebo (p<0.05). Time to Perceptible and Meaningful Pain Relief Kaplan-Meier estimates of median time to pain relief were numerically shorter for the IV meloxicam 30 mg group compared with placebo for both perceptible (0.52 vs. 1.59 hours) and meaningful (2.16 vs. 3.19 hours) pain relief, though these results did not reach statistical significance. Patient Global Assessment of Pain Control Subjects were asked to evaluate how well their pain was controlled in the study according to a 5-point scale with categories of 0-poor, 1-fair, 2-good, 3-very good, or 4-excellent. The proportion of subjects reporting good or better (2+) pain control on the PGA was significantly higher for IV meloxicam 30 mg vs. placebo at Hour 24 (57.9% vs. 43.3%) and Hour 48 (84.2% vs. 65.6%) Figure 2: Pain Intensity Difference Over Time Response Analysis Response analysis was performed with thresholds of ≥30% and ≥50% improvement in pain scores over the first 6 and 24 hours following Dose 1 The IV meloxicam 30 mg treatment group had a statistically significantly greater number of subjects experiencing ≥ 30% improvement at Hour 6 and Hour 24, and ≥ 50% improvement at Hour 24 compared with placebo; subjects with ≥ 50% improvement at Hour 6 numerically favored IV meloxicam 30 mg but did not reach significance Variable IV Meloxicam 30 mg (N=100) Placebo (N=101) Overall (N=201) Age (yrs) – mean ± SD 46.7 ± 12.79 48.4 ± 12.17 47.6 ± 12.48 Sex, n (%) Male 16 (16.0) 14 (13.9) 30 (14.9) Female 84 (84.0) 87 (86.1) 171 (85.1) Race, n (%) White 61 (61.0) 68 (67.3) 129 (64.2) Black or African American 32 (32.0) 26 (25.7) 58 (28.9) Asian 2 (2.0) 3 (3.0) 5 (2.5) Native Hawaiian or Other Pacific Islander 2 (2.0) 2 (2.0) 4 (2.0) Other 3 (3.0) 2 (2.0) 5 (2.5) Ethnicity, n (%) Hispanic or Latino 28 (28.0) 36 (35.6) 64 (31.8) Not Hispanic or Latino 72 (72.0) 65 (64.4) 137 (68.2) Baseline BMI (kg/m2) – mean ± SD 26.9 ± 4.80 28.3 ± 4.02 27.6 ± 4.46 Surgery Duration (hr) – mean ± SD 0.514 ± 0.2397 0.485 ± 0.2001 0.500 ± 0.2206 Bunionectomy Surgery Site: n (%) Left Foot 50 (50.0) 52 (51.5) 102 (50.7) Right Foot 50 (50.0) 49 (48.5) 99 (49.3) Time (hr) from End of Surgery to First Dose – mean ± SD 18.781 ± 3.3459 18.992 ± 3.2235 18.887 ± 3.2784 Baseline PI (0-10) – mean ± SD 6.7 ± 1.90 7.0 ± 1.82 6.8 ± 1.86 * p=0.0034 SPID At Other Intervals SPID assessed at other postdose intervals (SPID6, SPID12, SPID24, and SPID24-48) were statistically significant for IV meloxicam 30 mg over placebo at all intervals (p<0.05). Parameter IV Meloxicam 30 mg (N=100) Placebo (N=101) p-value SPID6 -510.78 ± 66.22 -288.33 ± 65.95 0.0153 SPID12 -957.83 ± 123.30 -480.15 ± 122.80 0.0053 SPID24 -2071.0 ±247.01 -1167.9 ± 246.00 0.0084 SPID12-24 -1113.1( 147.95) -687.76( 147.35) 0.0376 SPID24-48 -4885.1 ± 313.60 -3661.4 ± 312.32 0.0050 SPID36-48 -2422.4( 159.80) -1946.3( 159.15) 0.0312 Table 2: SPID (LS Mean ± SE) At Other Intervals Pain Intensity Difference (PID) Calculated as the difference in PI at each postdose time point compared with baseline. PID was lower in the IV meloxicam 30 mg group compared with placebo at all postdose time points. Figure 3: Number of Rescue Analgesia Doses from Hour 0-48 * p=0.0014 Figure 4: Proportion of Subjects Reporting Good or Better Pain Control on the PGA (2+) Figure 5: Response Analysis for ≥ 30% and 50% Improvement * * * Preferred Term IV Meloxicam 30 mg (N=100) Placebo (N=101) Any AE 44 (44.0) 54 (53.5) Nausea 20 (20.0) 26 (25.7) Headache 8 (8.0) 12 (11.9) Vomiting 3 (3.0) 9 (8.9) Pruritus 8 (8.0) 3 (3.0) Constipation 4 (4.0) 5 (5.0) Dizziness 3 (3.0) 4 (4.0) Flushing 3 (3.0) 1 (1.0) Somnolence 3 (3.0) 2 (2.0) Table 3: Summary of Treatment-Emergent AEs - Number of Subjects (%) Wound Healing Assessment Surgical wounds were assessed for investigator satisfaction rated using a 0-10 scale (0=not satisfied; 10=completely satisfied), along with assessing various characteristics including erythema, drainage, edema, induration, and hematoma. Overall satisfaction assessments were similar between IV meloxicam 30 mg and placebo at Hour 48 (9.7 vs. 9.6), 7 days post last study dose (9.4 vs. 9.6), and at 28 days post last study dose (9.7 vs. 9.6). Clinically significant wound assessment parameters were more common in the placebo group compared with IV meloxicam 30 mg. 28 Day Screening Follow-Up Visit 28 Days Post Last Dose Day -1 Surgery Follow-Up Visit 7 Days Post Last Dose Placebo (n = 100) Use of Rescue Analgesia (Oxycodone) for Inadequate Pain Control IV Meloxicam 30 mg (n = 100) Randomization Prior to 12:00 on Day 1 48 Hour Discharge Day 1 Discontinue Popliteal Block @ 03:00 Preferred Term IV Meloxicam 30 mg (N=100) Placebo (N=101) Injection site pain 1 (1.0) 2 (2.0) Injection site erythema 1 (1.0) 1 (1.0) Injection site induration -- 1 (1.0) Injection site infection -- 1 (1.0) Wound infection -- 2 (2.0) Incision site cellulitis -- 2 (2.0) Wound dehiscence 1 (1.0) -- Liver function test abnormal -- 1 (1.0) GGT increased -- 1 (1.0) ALT increased -- 3 (3.0) AST increased -- 2 (2.0) Alkaline phosphatase increased -- 2 (2.0) Presyncope -- 1 (1.0) Parameter Potential Significance Criteria IV Meloxicam 30 mg Placebo Hematocrit < 30% - - Hemoglobin < 10 g/dL 1/100 (1.0) - BUN 1.5-3x ULN 1/89 (1.1) - Creatinine 1.5-3x ULN 1/96 (1.0) - ALT 3-10x ULN - 3/89 (3.4) ≥10x ULN - 1/89 (1.1) AST 3-10x ULN - 3/85 (3.5) ≥10x ULN - - GGT 1-3x ULN 6/89 (6.7) 5/83 (6.0) 3-10x ULN - 2/83 (2.4) Alkaline Phosphatase 1-3x ULN 6/80 (7.5) 8/74 (10.8) Total Bilirubin > 2x ULN - - aPTT ≥ 55 seconds - - INR > 1.5 - - Table 4: Summary of Treatment-Emergent AEs of Special Interest - Number of Subjects (%) Table 5: Potentially Clinically Significant Change in Laboratory Assessments from Normal at Baseline Clinical Laboratory Assessment Clinical chemistry, hematology, urinalysis, and coagulation tests were routinely evaluated during the study. Laboratory assessments related to renal and hepatic function, and bleeding risk were of interest as an NSAID. The incidences of potentially clinically significant changes from normal at baseline to any postdose assessment are summarized below. p=0.0451 p=0.0107 p=0.0430 Exhibit 99.2
