Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Artemis Therapeutics, Inc. | zk1719983.htm |
Exhibit 99.1

Corporate PresentationMay 2017

Forward Looking Statements This presentation contains express or implied forward-looking statements within the Private Securities Litigation Reform Act of 1995 and other U.S. Federal securities laws. These forward-looking statements and their implications are based on the current expectations of our management only. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: we may encounter delays or obstacles in launching and/or successfully completing our clinical trials; our products may not be approved by regulatory agencies, our technology and our methods may not be accepted by the scientific community; unforeseen scientific difficulties may develop with our process; results in the laboratory may not translate to equally good results in real clinical settings; results of preclinical studies may not correlate with the results of human clinical trials; our patents may not be sufficient; our products may harm recipients; inability to timely develop and introduce new technologies, products and applications, which could cause our actual results or performance to differ materially from those contemplated in such forward-looking statements. Except as otherwise required by law, we undertake no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. For a more detailed description of the risks and uncertainties affecting us, reference is made to our reports filed from time to time with the Securities and Exchange Commission. 2

LEAD ASSET ATMS-101 – proprietary anti-parasitic/anti-viral VALUE PROPOSITION One asset, two lead programs, both orphan indications in US & EULow risk : High return - treatment of p. falciparum malariaHigh Risk : High Return - treatment of cytomegalovirus (CMV) infection in transplant patients Potential addition of congenital CMV Potential applicability to other viruses and parasites POSITIVE DATA Clinical efficacy signal in p. falciparum malaria with good safety and tolerability profile In vitro potency against CMV similar to ganciclovir (standard of care) IP &EXCLUSIVITY Extensive patent portfolio, further applications in process Orphan Drug Designation, filing anticipated 2Q2017 LEADERSHIP Team and advisors with proven track record in life sciences Proven success developing compounds from the clinic to the market Artemis TherapeuticsDeveloping novel treatments for life-threatening infections 3

Leadership 4 Chief Scientific Officer: Dana Wolf, MD, PhDTed & Frances Chanock Professor of Virology &Head of Clinical Virology,Hadassah Hebrew University Medical CenterPost-doc research in virology, UCSDVisiting Scientist, Stanford Univ. School of Medicine Chief Executive Officer: Peter Payne, B. Sc. (HONS)Founder & President Gennaker Bio, LLCSVP, Bus Dev & Corporate Strategy ChimerixChief Executive Officer eXcelerate ResearchChief Business Officer TcLand ExpressionVP NovaQuest Capital ManagementVP, Corporate Development Quintiles Chief Financial Officer: Chanan MorrisVP Finance & Operations Infinity GroupCFO Galmed PharmaceuticalsVP Finance Mango DSPVP Finance Elam EL Industries Ltd

Programs with Different Risk:Return ProfilesProof of Concept already demonstrated for chemical class 5 Artesunate (ATS)Anti-malarialDecades of use Artesunate (ATS)Efficacy vs. CMV in vitro Used in 6 clinical cases ATMS-101Enhanced thermal stability3 x more active vs. malariaImproved safety & tolerabilityEvaluated in Phase I & II studies* ATMS-10112 x greater potency than ATS vs. CMV in vitroSimilar potency to ganciclovir* Next Generation artemisinin development MalariaLow Risk:High ReturnAtypical in Biotech CMVHigh Risk:High ReturnTypical in Biotech * Data on file – Artemis Therapeutics

Multiple Potential Indications 6 Treatment of CMV in Hematopoietic Cell Transplant Pre-Clinical Phase I Phase II Phase III Treatment of Malaria Treatment of CMV in Solid Organ Transplant Treatment of Congenital CMV Other potential anti-parasitic and anti-viral indications being explored in pre-clinical testing2 additional programs to be named 1H2018

MalariaOpportunity Overview Endemic in 91 countries worldwide229 million cases and 429,000 deaths in 2015 (WHO Report of 12/16)Largely impacts sub-saharan Africa (90% of cases, 92% of deaths)S-E Asia, Latin America and Middle East also at risk But Treatment needs to be cheap Artemisinins work in malariaRapid clinical pathNon-dilutive funding opportunitiesNo commercial risk to be takenValue Proposition includes Orphan Drug Designation & Tropical Disease Priority Review Voucher So, why is Malaria low risk:high return? 7

CMV in TransplantationOpportunity Overview CDC estimates 50 – 80 % of population infected by age 40Can cause severe and life-threatening illness in patients with compromised immune systems. Patients undergoing Hematopoietic Cell Transplant or Solid Organ Transplant at high risk of acquiring infection or reactivation of existing infection. Recent Phase III success with letermovir (Merck) in HCT Current treatments limited in use by toxicity and resistanceHistory of clinical failures, eg, maribavir (Shire), brincidofovir (Chimerix)Novel & unique MOA of ATMS-101 allows for niche in marketGlobal pat. pop’n. > 190K cases per yearUS market potential > $1 B Value Proposition includes Orphan Drug Designation, Premium Pricing So, why is CMV high risk:high return? 8 But

Congenital CMVOpportunity Overview Major unmet medical need, biggest cause of non-hereditary sensorineural hearing loss in childrenTransmitted from pregnant mother to unborn childLive births/year – US 3,978,497* Congenital Infection (1/150) 26,523 Congenital Zika US (cum. 2016-17) 1,762** Everyone has heard of Zika, very few have heard of congenital CMVValue proposition includes Orphan Drug Designation, Rare Pediatric Disease Priority Review Voucher and value-based premium pricing 9 * CDC 2015 data** CDC April 2017 data
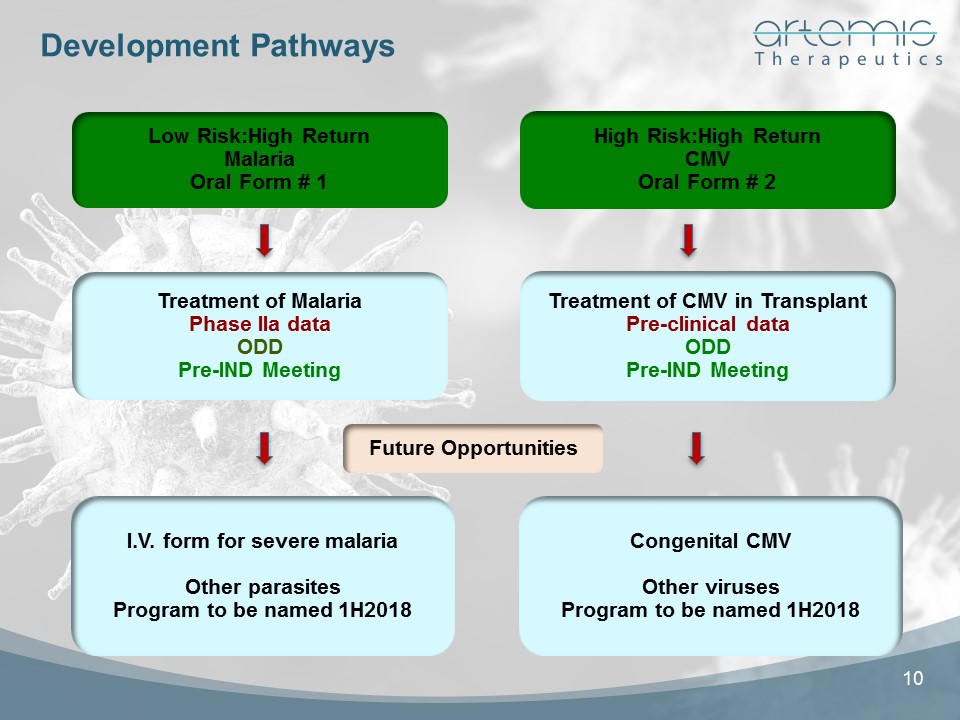
High Risk:High ReturnCMVOral Form # 2 I.V. form for severe malariaOther parasitesProgram to be named 1H2018 Treatment of CMV in TransplantPre-clinical dataODDPre-IND Meeting Low Risk:High ReturnMalariaOral Form # 1 Congenital CMVOther virusesProgram to be named 1H2018 Development Pathways Treatment of MalariaPhase IIa dataODDPre-IND Meeting 10 Future Opportunities

Company ComparisonsAnti-virals – As of April 25, 2017 11 Chimerix: CMRXClinical Stage: II (ADV)Mkt Cap (Mio): $ 282.6 CoCrystal: COCPClinical Stage: I (HCV)Mkt Cap (Mio): $ 196.2 Contravir: CTRVClinical Stage: II (HBV)Mkt Cap (Mio): $ 52.3 Anadys: ANDSClinical Stage: II (HCV)Acquired (Mio): $ 230.0 BioCryst: BCRXClinical Stage: ApprovedMkt Cap (Mio): $ 521.7 Regulus Therapeutics: RGLSClinical Stage: II (HCV)Mkt Cap (Mio): $ 76.8

Artemis TherapeuticsAttractive Investment Opportunity ATMS-101 has strong clinical and commercial potential addressing areas of high unmet medical need in significant markets.Clinical efficacy and safety demonstrated in one indicationStrong pre-clinical efficacy signal in second indicationPotential additional indications Large unmet medical need & estimated >$1 BN US market for CMV in transplant patientsSignificant unmet need in congenital CMV Value proposition includes Orphan Drug designation and one of Tropical Disease Priority Review Voucher or Rare Pediatric Disease Priority Review VoucherExperienced team & advisors 12
