Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Zyla Life Sciences | a17-12765_1ex99d1.htm |
| 8-K - 8-K - Zyla Life Sciences | a17-12765_18k.htm |
Exhibit 99.2
Specialty Pharmaceutical Company Focused on Pain Egalet Investor Day 1

2 Agenda Welcome Managing Pain in Today’s Environment Commercial Market Access Clinical and Regulatory Update Q&A Business Development Update First Quarter Financial Results Q&A Closing Remarks Bob Radie, President and Chief Executive Officer Dr. Cole Brown with Drs. Fudin, Goldstein and Rauck Patrick Shea, Chief Commercial Officer Sean Phillips, Vice President, Market Access Jeffrey Dayno, M.D., Chief Medical Officer Mark Strobeck, Ph.D., Chief Operating Officer Stan Musial, Chief Financial Officer Bob Radie

3 Forward Looking Statements Statements included in this presentation that are not historical in nature are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management's current expectations, and are subject to known and unknown uncertainties, risks and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward-looking statements by terminology such as “may,” “could,” “plans,” “future,” “expects,” “goal,” “intends,” “assess,” “continue to,” “potential,” “anticipates,” “believes,” “estimates,” or “predicts,” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to, (i) statements regarding the potential market size for our products, (ii) the timing or likelihood of regulatory filings and approvals for our products and product candidates; (iii) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (iv) the impact of our existing commercial presence on the commercialization of our new products; (v) the impact of the addition of our new products on our market presence; (vi) statements regarding the expansion of new prescribers and prescriptions for our products; (vii) the timing of the expansion of dosage levels for our products; (viii) the implications for the success of our products based on our current demand experience; (ix) our expectations regarding our path to sustainability and growth, including our business development plans; (x) the strategic imperatives with regard to our products, including our goals with regard to market access; (xi) the eventual outcome of the FDA’s actions relating to abuse-deterrent products; and (xii) our expectations regarding our finances, including our expenses, and our funding sources, our use of funds and potential payments under our notes and our royalty rights agreements. In addition, we, through our senior management, from time to time make forward-looking public statements concerning our expected future operations and performance and other developments. Actual results could differ materially from those discussed due to a number of factors, including, but not limited to: our ability to obtain regulatory approval of our product candidates; our ability to successfully commercialize SPRIX®, ARYMO® ER and OXAYDO®; our ability to execute on our sales and marketing strategy, including developing relationships with customers, physicians, payors and other constituencies; the accuracy of our estimates of the size and characteristics of the potential markets for our product candidates and our ability to serve those markets; unexpected safety or efficacy data; competitive factors; changes in the regulatory environment for our products; any further FDA action relating to abuse-deterrent products; general market conditions; our need for future capital; our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; and other risk factors described in our filings with the United States Securities and Exchange Commission. Egalet assumes no obligation to update or revise any forward-looking statements contained in this presentation whether as a result of new information or future events, except as may be required by law. See Sprix.com, Oxaydo.com and Arymoer.com for full prescribing information including boxed warning and medication guide.

4 Pain is a Huge Problem Annual costs associated with chronic pain, including healthcare cost estimates and value of lost productivity. to 100 Million Americans Live with Chronic Pain1 > Pain affects more Americans than Heart Disease + Cancer + Diabetes2 BILLION 1 of the most common reasons Americans access the health care system3 Institute of Medicine Report from the Committee on Advancing Pain Research, Care, and Education: Relieving Pain in America, A Blueprint for Transforming Prevention, Care, Education and Research. The National Academies Press, 2011. http://books.nap.edu/openbook.php?record_id=13172&page=1. NIH Research Portfolio reporting Tools (RePORT), March 2013. https://report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=57 Institute of Medicine Report from the Committee on Advancing Pain Research, Care, and Education: Relieving Pain in America, A Blueprint for Transforming Prevention, Care, Education and Research. The National Academies Press, 2011. http://books.nap.edu/openbook.php?record_id=13172&page=1.
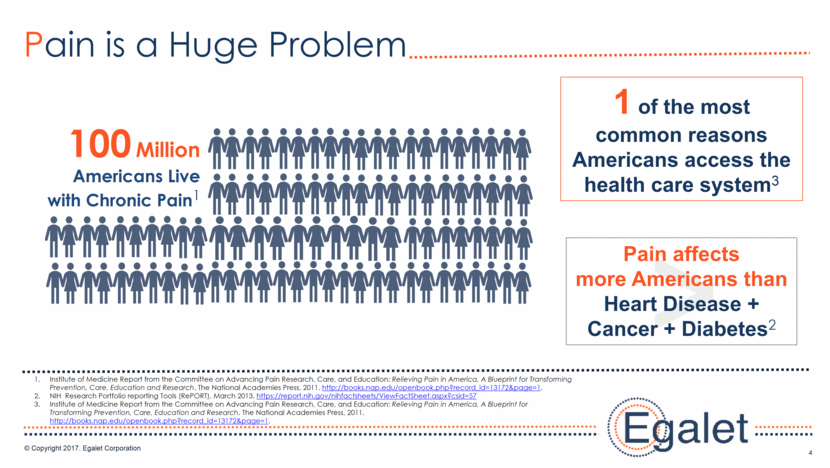
5 Prescription Opioid Abuse is Growing 2013, the US Centers for Disease Control and Prevention (CDC) http://www.pharmacytimes.com/contributor/jeffrey-fudin/2015/01/abuse-deterrent-opioid-formulations-purpose-practicality-and-paradigms. Understanding the Epidemic: Centers for Disease Control https://www.cdc.gov/drugoverdose/epidemic/index.html 75% Opioids caused of overdoses in 20131 91 Americans die every day from an opioid overdose2
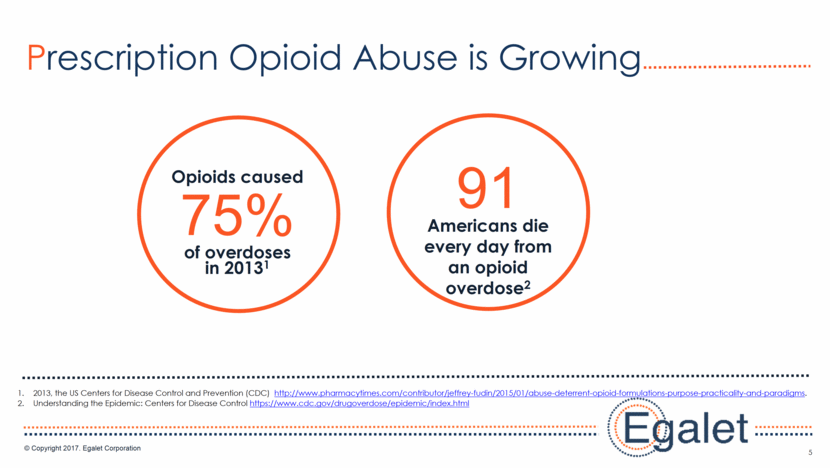
6 Pain Management Opioid Addiction Treatment Overdose Reversal Interventions to reduce mortality and link to treatment Future: Safe, effective less addictive options Solutions to the Opioid Crisis New and innovative medications and technologies Today: ADFs and NSAIDs
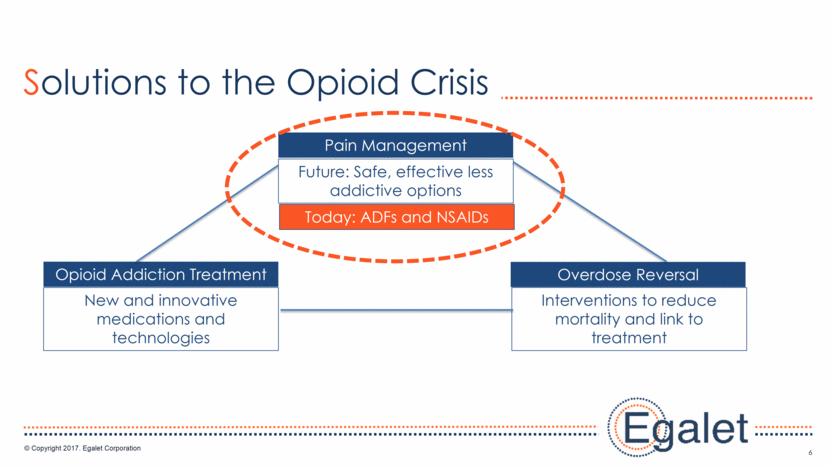
7 Build R&D Foundation Establish Ensure Sustainability Fuel Growth Established technology Filed patents Secured capital Completed IPO Progressed pipeline Licensed/Acquired products Established commercial Generated revenue ARYMO® ER approval Drive revenue growth Commercial focus LCM/acquisitions Specialty focus: Today: Pain Tomorrow: CNS, orphan Aggressive M&A Add late-stage or commercial products Invest in key business areas Establish competitive advantage Commercial Presence 2013 2014 - 2016 2017 2019 Egalet: Accomplishments and Path Forward
8 Approved Late-Stage Early-Stage SPRIX® (ketorolac tromethamine) Nasal Spray Short-term pain Egalet-002, AD, ER oxycodone Chronic pain Egalet-004, AD, ER Hydrocodone Chronic pain OXAYDO® (oxycodone HCl, USP) Tablets for oral use only – CII Acute and chronic pain Egalet-003, AD Stimulant Ph 1 Ph 2 NDA Filed Marketed Guardian Technology Commercial Products SPRIX® Nasal Spray for outside U.S. Short-term pain Preclinical Partnered Products ARYMO® ER (morphine sulfate) extended-release tablets – CII Chronic pain Pivotal Commercial Focus
9 Driving Growth ® ®
Treating Those Living with Pain in Today’s Environment 10 Dr. Cole Brown with Drs. Fudin, Goldstein and Rauck

Jeffrey Fudin, Pharm.D. Registered Pharmacist and Credentialed Pain Management Specialist Adjunct Associate Professor, Western New England University College of Pharmacy and University of Connecticut School of Pharmacy Director PGY2 Pain Residency Stratton VA Medical Center in Albany, NY Founder & Chair, PROMPT (Professionals for Rational Opioid Monitoring & Pharmacotherapy) Director, Scientific and Clinical Affairs, REMITIGATE, LLC in Delmar NY Owner & Managing Editor, Pain Dr.com Section Editor, Pain Medicine 11
Jeffrey Goldstein, D.O. Trained in both Orthopedic Surgery and Emergency Medicine Board certified in Emergency Medicine Founder and Director of Fountain Medical Group Member of the New York State Pain Society and the American Academy of Pain Medicine 12
Richard Rauck, M.D. Board certified in Pain Medicine and Anesthesiology Founder of the Carolinas Pain Institute Founder and Medical Director for The Center for Clinical Research President and founding member of Sceptor Pain Foundation President of World Institute of Pain Founder and board member of the Pain Society of the Carolinas Board member of the North American Neuromodulation Society 13

Commercial Opportunity 14 Patrick Shea Chief Commercial Officer

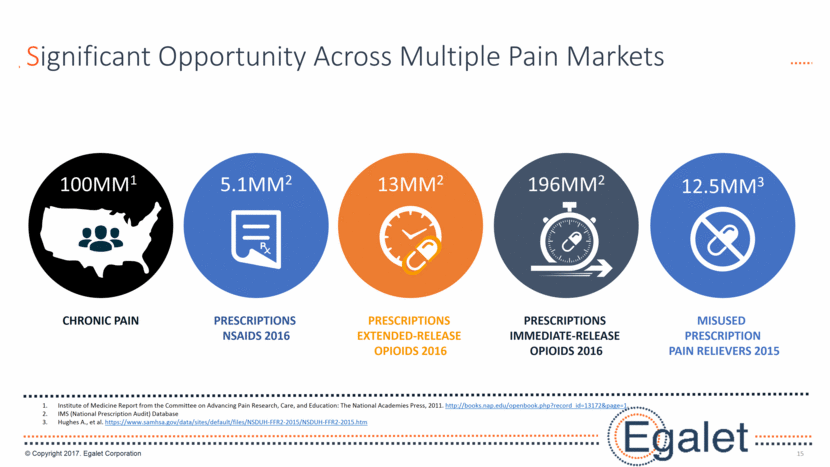
15 Significant Opportunity Across Multiple Pain Markets 100MM1 CHRONIC PAIN 13MM2 PRESCRIPTIONS EXTENDED-RELEASE OPIOIDS 2016 PRESCRIPTIONS IMMEDIATE-RELEASE OPIOIDS 2016 196MM2 12.5MM3 MISUSED PRESCRIPTION PAIN RELIEVERS 2015 5.1MM2 PRESCRIPTIONS NSAIDS 2016 Institute of Medicine Report from the Committee on Advancing Pain Research, Care, and Education: The National Academies Press, 2011. http://books.nap.edu/openbook.php?record_id=13172&page=1. IMS (National Prescription Audit) Database Hughes A., et al. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR2-2015/NSDUH-FFR2-2015.htm
16 Establishing a Solid Foundation

17 Experienced Integrated Commercial Team Driving Execution Sales Marketing Market Access (Account Management) Commercial Operations Analytics
SPRIX® Nasal Spray 2017 Brand Direction 18

19 Average Quarterly TRx Growth of 20%* *Excludes quarter launched Number of Unique HCPs Written 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 0 200 400 600 800 1,000 1,200 1,400 1,600 1,800 2,000 2015 - 1 2015 - 2 2015 - 3 2015 - 4 2016 - 1 2016 - 2 2016 - 3 2016 - 4 2017 - 1 Total Number of HCP Writers SPRIX # of Prescribers and Average RX per HCP Average per HCP 3,313 3,675 4,455 5,428 6,311 3,000 3,500 4,000 4,500 5,000 5,500 6,000 6,500 2016 - 1 2016 - 2 2016 - 3 2016 - 4 2017 - 1 SPRIX ® TRx Volume

20 SPRIX® Nasal Spray, Effective Pain Management Option in Dynamic Environment Indicated in adult patients for the short-term (up to 5 days) management of moderate to moderately severe pain that requires analgesia at the opioid level.
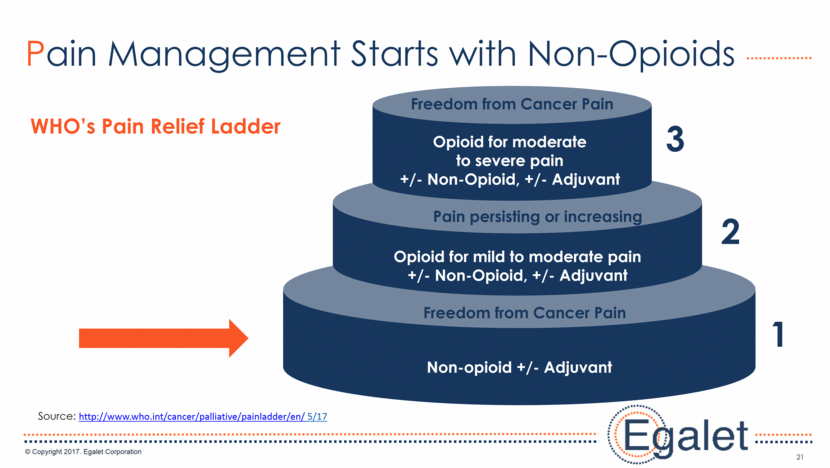
21 Pain Management Starts with Non-Opioids WHO’s Pain Relief Ladder 1 Non-opioid +/- Adjuvant Freedom from Cancer Pain Opioid for mild to moderate pain +/- Non-Opioid, +/- Adjuvant Opioid for moderate to severe pain +/- Non-Opioid, +/- Adjuvant Pain persisting or increasing Freedom from Cancer Pain 2 3 Source: http://www.who.int/cancer/palliative/painladder/en/ 5/17
22 SPRIX® 2017 Strategic Imperatives Sales Force Execution Operationalize Total Office Call Increase Depth and Breadth of Prescribing Effectively Communicate Value Proposition Increase Brand Touch Points

23 Sales Force Focused on SPRIX® Loyalists & IM Ketorolac Prescribers Current Writers (2,250 HCPs) 50% of Call Targets Cover 79% of 2016 Writers Cover 83% of 2016 TRx New Targets (2,250 HCPs) 50% of Call Targets IM Ketorolac (SPRIX naïve) NEW OPPORTUNITY – Know and Use ketorolac Specialties Primary Care Nurse Practitioners Physician Assistants Pain, PM&R Neurology, Urology, OBGYN
In-depth interviews with IM, GP/FP, Sports Medicine, General Surgeons, Orthopedic Surgeons and Pain Specialists who prescribe IM ketorolac Responded favorably to novel formulation of familiar, trusted molecule Positive reaction to SPRIX®, most compelling aspects include: Opioid-level analgesia in an NSAID “It’s extremely important that SPRIX is not an opioid because clinicians are looking for alternatives all the time” (Primary Care Physician) Rapid onset of effect (perceived to be comparable to IM formulation) “If the onset is really as quick as IM, SPRIX is a very interesting product”(Orthopedic Surgeon) Reduction in the amount of post-operative opioid analgesia required “Data showing one-quarter to one-third less post-op morphine use with SPRIX tells me that it’s working, and that’s very compelling” (General Surgeon) 24 Positive Feedback from IM Ketorolac Prescribers Source: February 2017 Core Visual Aid Qualitative Market Research
25 Patient Support—SPRIX® Direct Program Cardinal Health Specialty Pharmacy Quick home delivery of SPRIX with instructions for use Registered pharmacist available for 24-hour support Generous co-pay program for commercially insured patients, $0

OXAYDO® 2017 Brand Direction 26

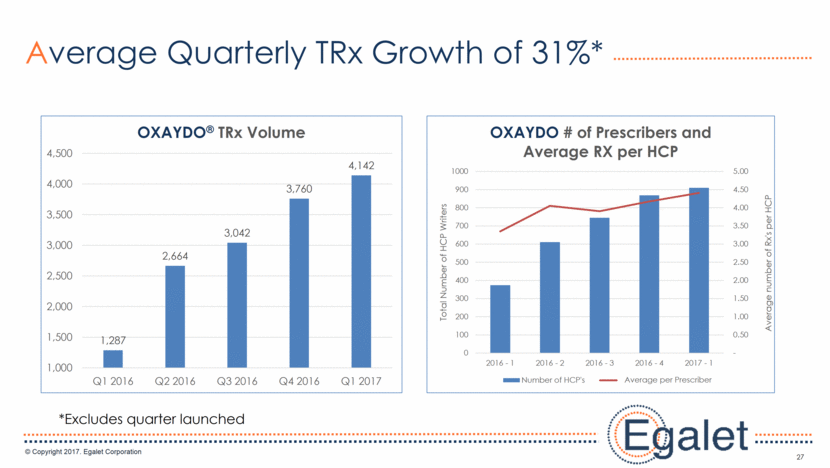
27 Average Quarterly TRx Growth of 31%* *Excludes quarter launched 1,287 2,664 3,042 3,760 4,142 1,000 1,500 2,000 2,500 3,000 3,500 4,000 4,500 Q1 2016 Q2 2016 Q3 2016 Q4 2016 Q1 2017 OXAYDO ® TRx Volume - 0.50 1.00 1.50 2.00 2.50 3.00 3.50 4.00 4.50 5.00 0 100 200 300 400 500 600 700 800 900 1000 2016 - 1 2016 - 2 2016 - 3 2016 - 4 2017 - 1 Average number of Rx's per HCP Total Number of HCP Writers OXAYDO # of Prescribers and Average RX per HCP Number of HCP's Average per prescriber Average per Prescriber
28 OXAYDO®, IR Opioid Designed to Discourage Abuse Indicated for the management of acute and chronic moderate to severe pain where the use of an opioid analgesic is appropriate.
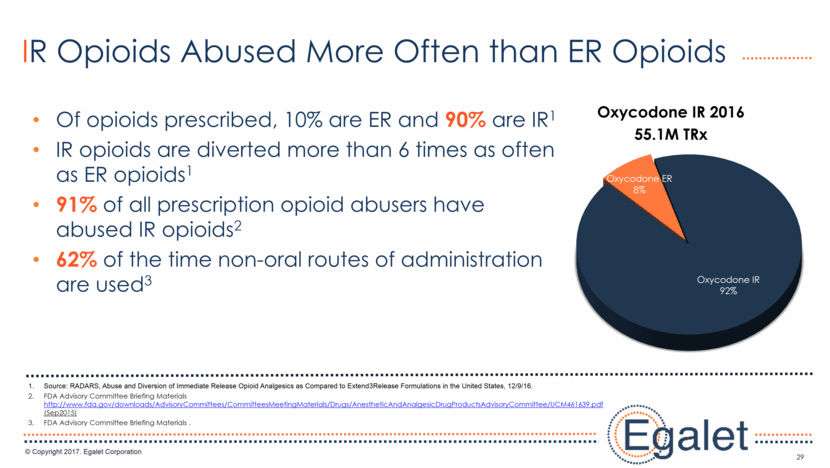
29 IR Opioids Abused More Often than ER Opioids Of opioids prescribed, 10% are ER and 90% are IR1 IR opioids are diverted more than 6 times as often as ER opioids1 91% of all prescription opioid abusers have abused IR opioids2 62% of the time non-oral routes of administration are used3 Source: RADARS, Abuse and Diversion of Immediate Release Opioid Analgesics as Compared to Extend3Release Formulations in the United States, 12/9/16. FDA Advisory Committee Briefing Materials http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndAnalgesicDrugProductsAdvisoryCommittee/UCM461639.pdf (Sep2015) FDA Advisory Committee Briefing Materials . Oxycodone IR 92% Oxycodone ER 8% Oxycodone IR 2016 55.1M TRx
30 Educating on Opioid Epidemic

In-depth interviews with target Pain HCPs, IMs, PCPs, & PM&R HCPs are aware of opioid issues & vast majority believe that “the OXAYDOs of the world” are a step in the right direction Majority of HCPs saw visual aid cover as relevant and clearly depicting the issue of diversion and opioid abuse “The headline says it all. This clearly shows the issues we have when opioids find their way into the wrong hands.” -- Pain Specialist “This is a great image! I see a city in shambles due to the amount of opioids on the street!” -- Pain Specialist Showing the abuse statistics provides a solid foundation to support the diversion story “This is really shocking I was not aware [how much] originated with friends and families I guess that makes sense.” -- Pain Specialist “I love the idea of discouraging abuse. I’d use it for chronic patients instead of Percocet; anyone you’d otherwise give 5 mg Percocet.” -- Nurse Practitioner --Pain Specialist. 31 Positive Physician Feedback Source: Sept 2016 Core Visual Aid Qualitative Market Research
32 2017 OXAYDO® Strategic Imperatives Sales Force Targeting and Execution Enable Patient Access (Affordable and Available) Augment Value Proposition Increase Productivity with Current Prescribers & Drive Trial with Non-Writers Communicate Brand Positioning

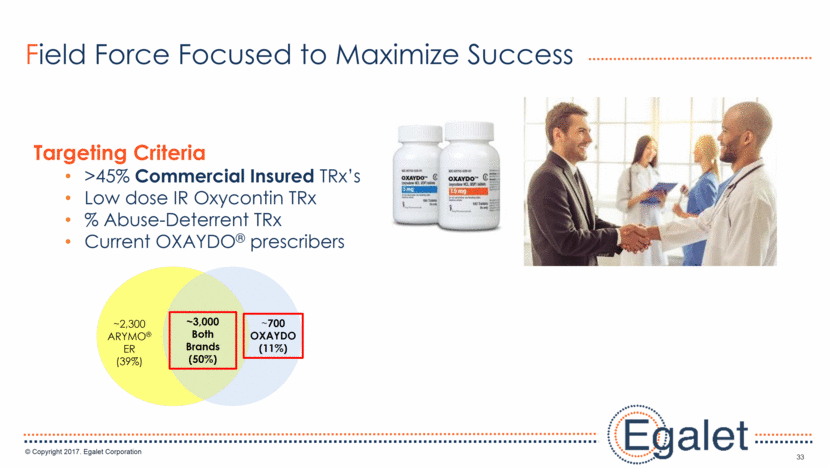
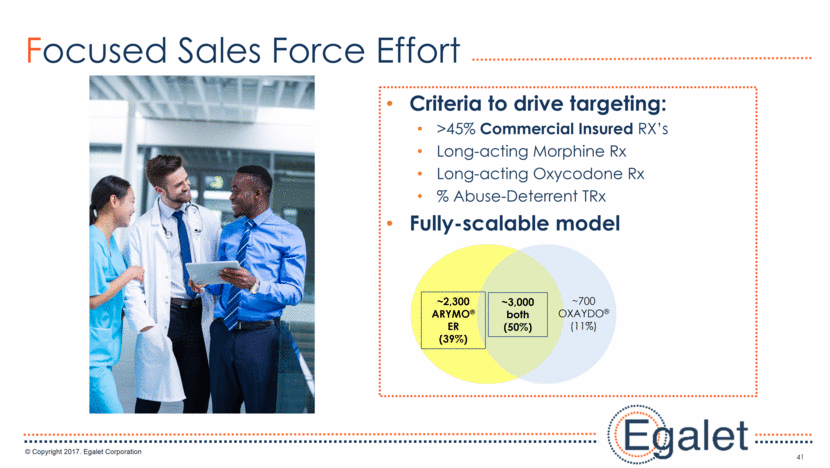
33 Field Force Focused to Maximize Success Targeting Criteria >45% Commercial Insured TRx’s Low dose IR Oxycontin TRx % Abuse-Deterrent TRx Current OXAYDO® prescribers ~3,000 Both Brands (50%) ~2,300 ARYMO® ER (39%) ~700 OXAYDO(11%)
34 Patient Support Program Co-pay program for commercially insured patients pay no more than $15 Pharmacy locater will help connect patients with a pharmacy that stocks or will carry OXAYDO®

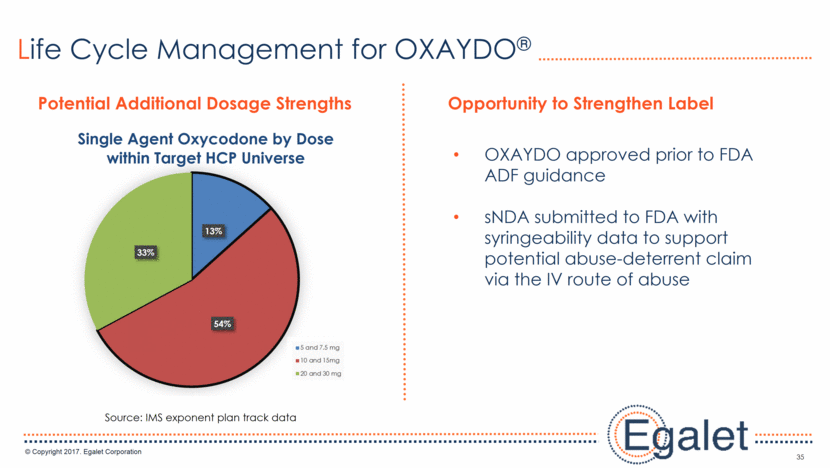
35 Life Cycle Management for OXAYDO® OXAYDO approved prior to FDA ADF guidance sNDA submitted to FDA with syringeability data to support potential abuse-deterrent claim via the IV route of abuse Potential Additional Dosage Strengths Opportunity to Strengthen Label Source: IMS exponent plan track data 13% 54% 33% Single Agent Oxycodone by Dose within Target HCP Universe 5 and 7.5 mg 10 and 15mg
ARYMO® ER 2017 Brand Direction 36

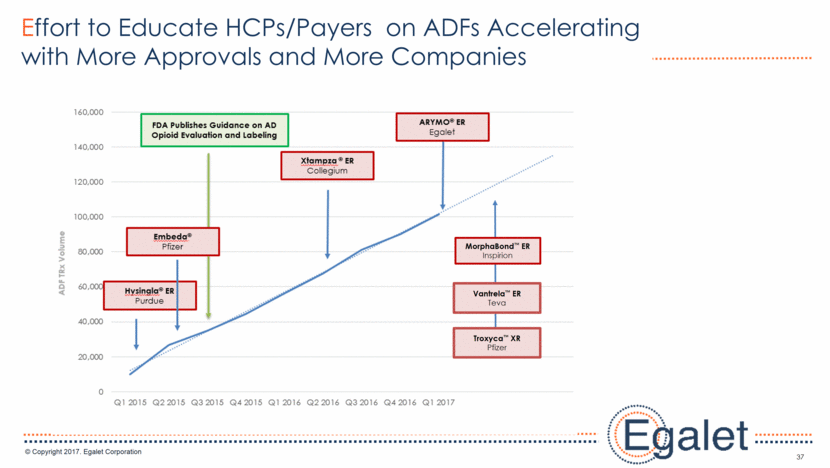
37 Effort to Educate HCPs/Payers on ADFs Accelerating with More Approvals and More Companies MorphaBond™ ER Inspirion Vantrela™ ER Teva Troxyca™ XR Pfizer
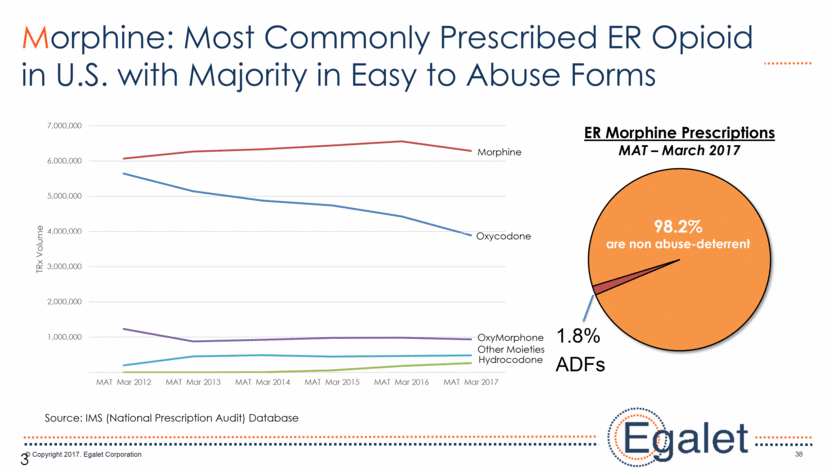
38 38 Morphine: Most Commonly Prescribed ER Opioid in U.S. with Majority in Easy to Abuse Forms 98.2% are non abuse-deterrent 1.8% ADFs ER Morphine Prescriptions MAT – March 2017 Source: IMS (National Prescription Audit) Database Other Moieties Hydrocodone OxyMorphone Oxycodone Morphine 1,000,000 2,000,000 3,000,000 4,000,000 5,000,000 6,000,000 7,000,000 MAT Mar 2012 MAT Mar 2013 MAT Mar 2014 MAT Mar 2015 MAT Mar 2016 MAT Mar 2017 TRx Volume
39 ARYMO® ER Strategic Imperatives Accelerate Product Adoption: Sales Force Execution Secure Formulary Access in Prioritized Segments Overcome Pharmacy Stocking Barriers Ensure Successful New Patient Starts and Adherence Activate Advocacy Networks and Policymakers to Support Preferred Access for ADFs

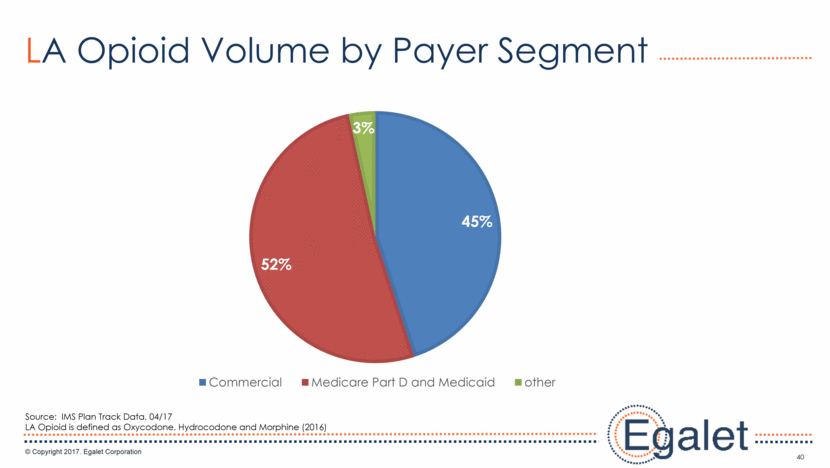
40 LA Opioid Volume by Payer Segment Source: IMS Plan Track Data, 04/17 LA Opioid is defined as Oxycodone, Hydrocodone and Morphine (2016) 45% 52% 3% Commercial Medicare Part D and Medicaid other
41 Focused Sales Force Effort Criteria to drive targeting: >45% Commercial Insured RX’s Long-acting Morphine Rx Long-acting Oxycodone Rx % Abuse-Deterrent TRx Fully-scalable model ~3,000 both (50%) ~2,300 ARYMO® ER (39%) ~700 OXAYDO®(11%)
42 ARYMO® ER: Value Proposition Centered on Proprietary Guardian™ Technology An opioid agonist indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.

43 Educating on Opioid Epidemic

44 Patient Support Program Commercially insured patients pay $0 for first prescription and as little as $10 for subsequent prescriptions Pharmacy Locater will help connect patients with a pharmacy that stocks or will carry ARYMO® ER

In depth interviews with HCPs who prescribe extended-release opioids and treat chronic pain (Pain Specialists, PCPs and NPs/PAs) HCPs find the ARYMO® ER “story” compelling and straight-forward The key “take-away” is that Guardian™ Technology produces a hard ER morphine sulfate tablet that resists multiple forms of physical manipulation and chemical extraction, with a focus on decreasing the risk of abuse via injection “If you can’t crush it enough to dissolve it for injection, you probably can’t snort it, either.”--Pain Specialist The graphic image of the hydrogel and syringe is broadly viewed as the most compelling and impactful element of the visual aid “You would have to be an idiot to try to inject that goop.” -- PCP Post Launch Feedback HCPs happy to have a “true” ADF ER morphine and learn more about ARYMO ER HCPs not satisfied with Embeda: having issues getting Embeda and glad to have new option HCPs are curious about Guardian Technology 45 Positive Physician Feedback Source: Feb 2017 Core Visual Aid Market Research
Strategic Imperative: Market Access 46 Sean Phillips Vice President, Market Access

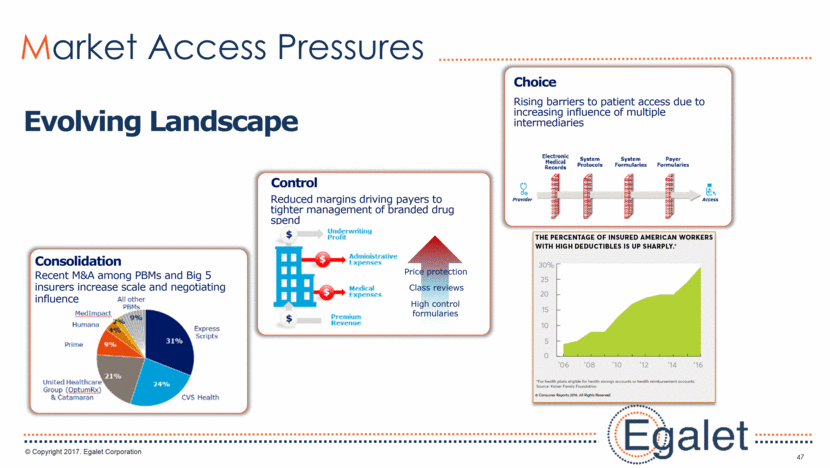
47 Market Access Pressures Evolving Landscape Recent M&A among PBMs and Big 5 insurers increase scale and negotiating influence Consolidation Reduced margins driving payers to tighter management of branded drug spend Price protection Class reviews High control formularies Control Rising barriers to patient access due to increasing influence of multiple intermediaries Choice
48 Coverage Goals for Market Access Vary By Payer Segment Focus on ease of prescribing for physicians Commercial Coverage That Eliminates NDCB / PA Restrictions FFS Medicaid Coverage That Eliminates ST / PA Restrictions Preferred Brand Tier Managed Medicaid Medicare Part D
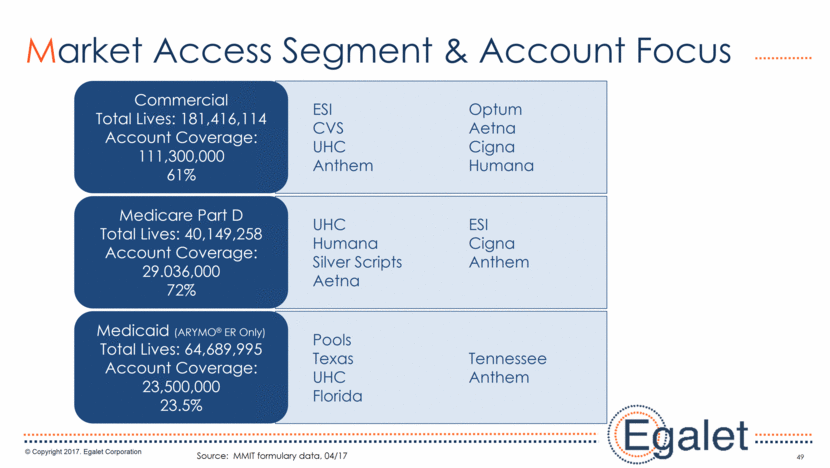
Pools Texas Tennessee UHC Anthem Florida UHC ESI Humana Cigna Silver Scripts Anthem Aetna ESI Optum CVS Aetna UHC Cigna Anthem Humana 49 Market Access Segment & Account Focus Source: MMIT formulary data, 04/17 Commercial Total Lives: 181,416,114 Account Coverage: 111,300,000 61% Medicare Part D Total Lives: 40,149,258 Account Coverage: 29.036,000 72% Medicaid (ARYMO® ER Only) Total Lives: 64,689,995 Account Coverage: 23,500,000 23.5%
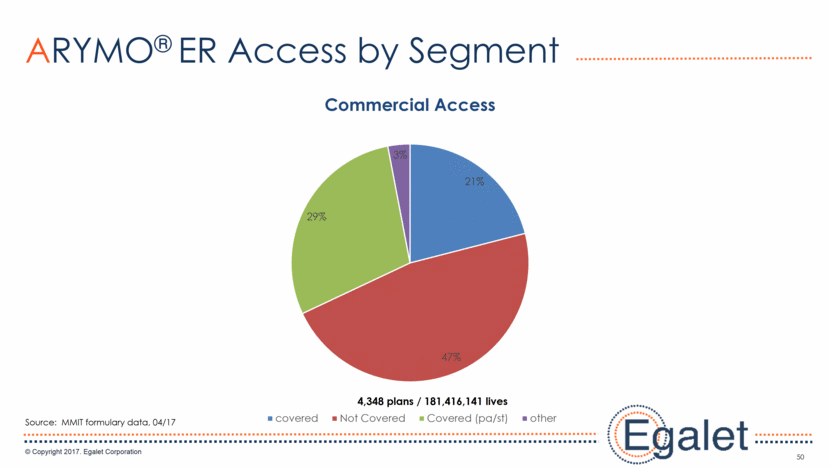
50 ARYMO® ER Access by Segment Source: MMIT formulary data, 04/17 4,348 plans / 181,416,141 lives 21% 47% 29% 3% Commercial Access covered Not Covered Covered (pa/st) other
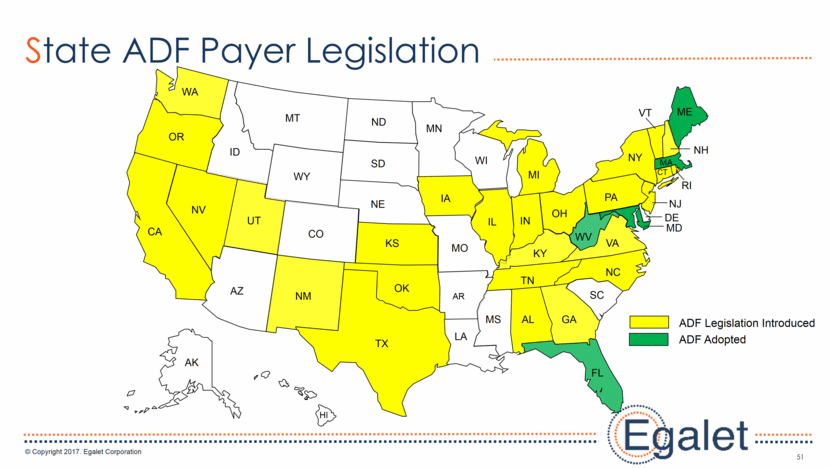
51 State ADF Payer Legislation AK HI WA OR CA NV ID MT WY UT AZ NM CO ND SD NE KS MN IA MO WI IL IN MI OH PA NY TX OK AR MS LA AL GA FL SC NC TN KY WV VA RI MD DE NJ MA CT ME VT NH ADF Legislation Introduced ADF Adopted
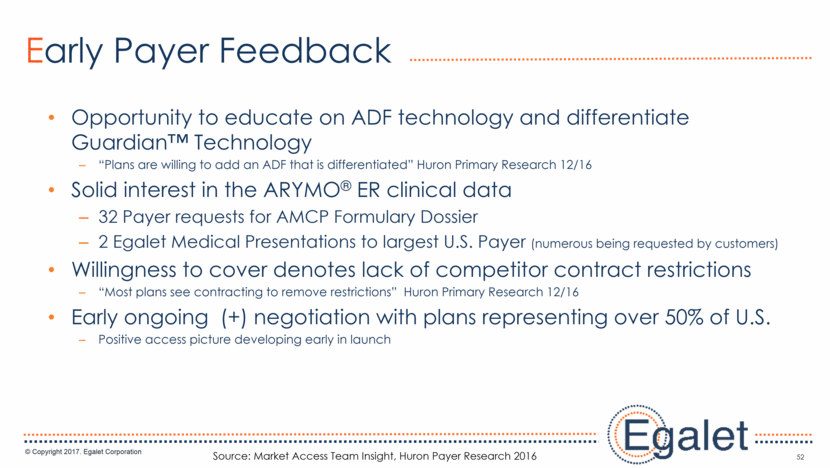
Opportunity to educate on ADF technology and differentiate Guardian™ Technology “Plans are willing to add an ADF that is differentiated” Huron Primary Research 12/16 Solid interest in the ARYMO® ER clinical data 32 Payer requests for AMCP Formulary Dossier 2 Egalet Medical Presentations to largest U.S. Payer (numerous being requested by customers) Willingness to cover denotes lack of competitor contract restrictions “Most plans see contracting to remove restrictions” Huron Primary Research 12/16 Early ongoing (+) negotiation with plans representing over 50% of U.S. Positive access picture developing early in launch 52 Early Payer Feedback Source: Market Access Team Insight, Huron Payer Research 2016
53 Portfolio is Poised for Continued Growth Market opportunities: SPRIX®: Large and responsive IM ketorolac universe OXAYDO®: Additional higher IR doses and label expansion ARYMO® ER: ADF tailwinds and majority of morphine market in easy to abuse forms Experienced and fully-integrated commercial team Track record of TRx growth Market Access success is high priority

Regulatory and Pipeline Update 54 Jeffrey Dayno, M.D. Chief Medical Officer

ADFs: Evolving Regulatory Landscape Dynamic regulatory landscape around ADFs but momentum is building Currently 9 approved ER/LA opioids with abuse-deterrent labeling First IR opioid with AD labeling approved last month Exclusivity decisions based on ‘route of abuse’ approach “CDER's Woodcock suggests exclusivity rules outdated for abuse-deterrent technology development.” Pink Sheet Increasing post-marketing data in support of effectiveness of ADFs in real world 55 “Opioid abuse, misuse, and addiction constitute one of the most urgent and immediate public health threats facing our nation. It is also the biggest public health crisis facing the FDA. . . . I will make sure the FDA is aggressive, forward leaning, and fully engaged in combating this epidemic.” – Scott Gottlieb, M.D.
FDA’s Current Position FDA working to address opioid epidemic as a part of the larger HHS response; one of the FDA’s highest priorities FDA Opioid Action Plan: Provides framework for FDA response to challenge of opioid epidemic Balancing access to effective pain medications for appropriate patients while limiting the risk of opioid misuse/abuse Supporting development and use of progressively better AD opioids FDA looks forward to the day, not far in the future, when the majority of opioids on the market will be abuse-deterrent 56 FDA Perspective on Abuse-Deterrent Opioid Development Douglas C. Throckmorton, MD Deputy Director for Regulatory Programs CDER, FDA CBI Abuse-Deterrent Formulations Summit March 7-8, 2017
Leadership in AD Opioid Development Leadership roles: Spoke on behalf of Branded Industry Working Group at FDA Public Meeting on draft Guidance for Generic ADFs (Oct. 31, 2016) Co-hosted Category 1 Focus Group Industry meeting (2015 & 2016) Innovating through new tools: ALERRT® Scale (Assessing Level of Effort and Resources Required for Tampering; PinneyAssociates) Physical/chemical barrier provides a deterrent layer on its own Recognized across the field for both clinical and regulatory expertise: Speaker at many of the main industry meetings on AD opioid development 57
Multiple Publications of AD Clinical Data 58 Clinical Relevance of Pharmacokinetic Profile of ARYMO ER Journal of Opioid Management April 2017 Oral Human Abuse Potential Study Pain Medicine September 2016 Intranasal Human Abuse Potential Study Pain Medicine September 2016 Review Manuscript on AD Opioid Development Postgraduate Medicine January 2017

Strengthen the label Category 1 (IV syringeability data) labeling supplement for OXAYDO 5/7.5 mg Submitted 30 Sept 2016 Goal date 30 March 2017 Expand the label Addition of 10/15 mg dosage strengths Based on demonstration of BE for OXAYDO 15 mg compared to reference agent (Roxicodone) Interaction with food seen with 15 mg dose; similar profile as seen in prior study PAS submitted 17 Feb 2107 Goal date 17 June 2017 59 OXAYDO® LCM Development Plan

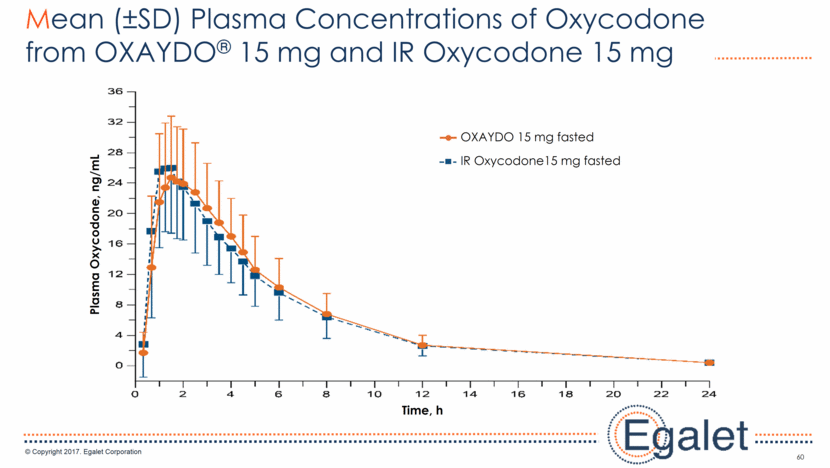
60 Mean (±SD) Plasma Concentrations of Oxycodone from OXAYDO® 15 mg and IR Oxycodone 15 mg IR Oxycodone15 mg fasted OXAYDO 15 mg fasted Time, h Plasma Oxycodone, ng/mL
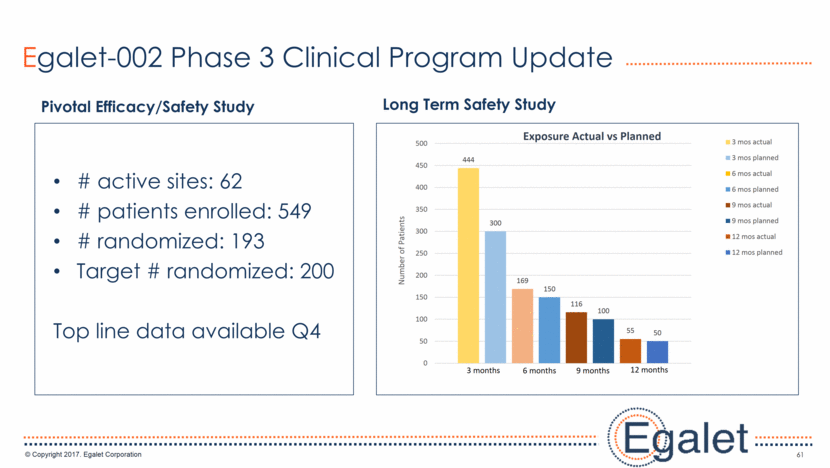
Egalet-002 Phase 3 Clinical Program Update 61 Pivotal Efficacy/Safety Study Long Term Safety Study # active sites: 62 # patients enrolled: 549 # randomized: 193 Target # randomized: 200 Top line data available Q4 444 300 169 150 116 100 55 50 0 50 100 150 200 250 300 350 400 450 500 Number of Patients Exposure Actual vs Planned 3 mos actual 3 mos planned 6 mos actual 6 mos planned 9 mos actual 9 mos planned 12 mos actual 12 mos planned 3 months 6 months 12 months 9 months
Commercial, Market Access and Clinical Q&A 62

Mark Strobeck, Ph.D. Chief Operating Officer Business Development 63

64 Build R&D Foundation Establish Ensure Sustainability Fuel Growth Established technology Filed patents Secured capital Completed IPO Progressed pipeline Licensed/Acquired products Established commercial Generated revenue ARYMO® ER approval Drive revenue growth Commercial focus LCM/acquisitions Specialty focus: Today: Pain Tomorrow: CNS, orphan Aggressive M&A Add late-stage or commercial products Invest in key business areas Establish competitive advantage Commercial Presence 2013 2014 - 2016 2017 2019 Egalet’s Path to Sustainability
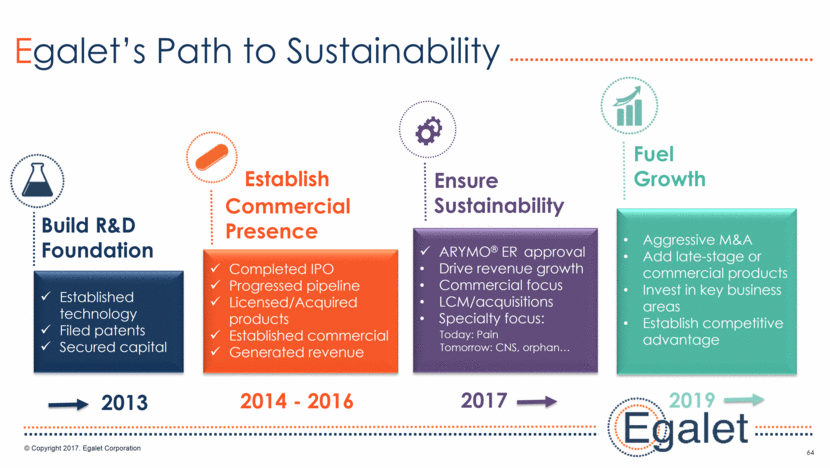
BD Strategy: Add New Revenue Streams 65 Out-license Marketed Products ex-US Out-license Marketed Products in US outside of target universe (e.g. surgery) In-license commercial/late-stage products in pain or CNS Out-license early stage pipeline candidates Establish Research Collaborations outside of core therapeutic areas Grow Value of Marketed Products Add New Products Monetize Established Technology Acquire companies with Ph III / commercial products
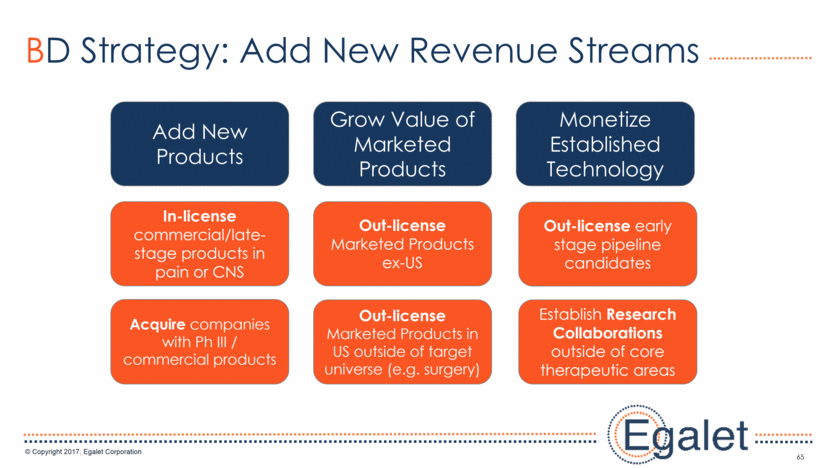
Prioritization of Potential Product Acquisitions Stage of Development Deal Likelihood Marketed Clinical Pain Other Specialty LOE >2 yr Generics Available High Med Low Sprix Opioid Other 66
SPRIX® Life Cycle Management Enhance commercial footprint - Seek partnerships with organizations targeting physician groups not being called on by Egalet Signed deal with Septodont Looking to add one new co-promote partner in 2017 Seek EX-U.S. opportunities for SPRIX Teva relationship in place for Israel, West Bank and the Gaza Strip Seek to enhance our IP protection on SPRIX to extend patent runway 67

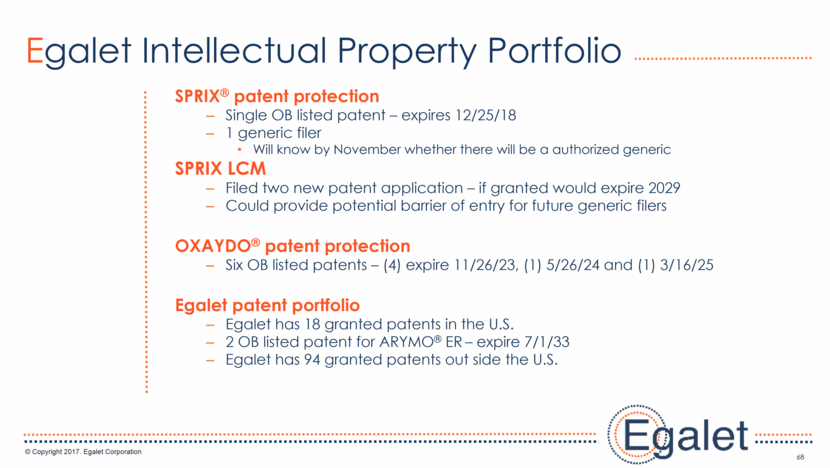
Egalet Intellectual Property Portfolio SPRIX® patent protection Single OB listed patent – expires 12/25/18 1 generic filer Will know by November whether there will be a authorized generic SPRIX LCM Filed two new patent application – if granted would expire 2029 Could provide potential barrier of entry for future generic filers OXAYDO® patent protection Six OB listed patents – (4) expire 11/26/23, (1) 5/26/24 and (1) 3/16/25 Egalet patent portfolio Egalet has 18 granted patents in the U.S. 2 OB listed patent for ARYMO® ER – expire 7/1/33 Egalet has 94 granted patents out side the U.S. 68
Monetize Established Technology Will seek to find appropriate partners with the resources to develop these projects Find partners interested in using the Guardian™ Technology to solve an issue with delivery of their product or manufacturing Improve PK characteristics Combination product development Interested in continuous manufacturing 69

Stan Musial, Chief Financial Officer First Quarter Financial Results 70

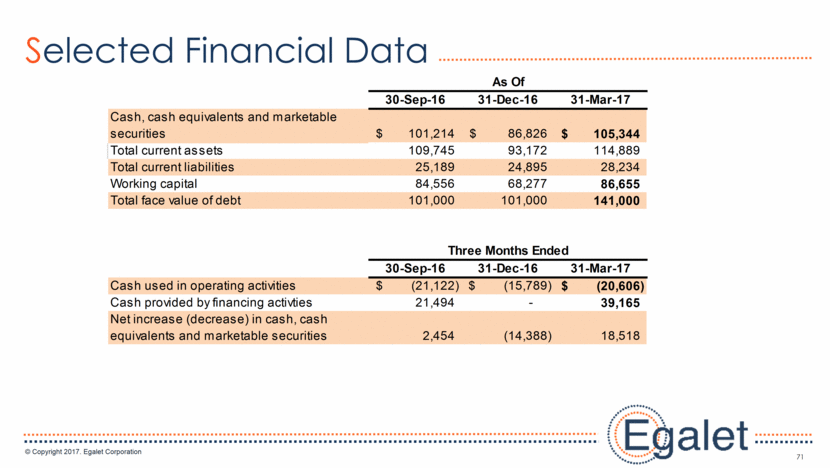
71 Selected Financial Data 30-Sep-16 31-Dec-16 31-Mar-17 Cash, cash equivalents and marketable securities 101,214 $ 86,826 $ 105,344 $ Total current assets 109,745 93,172 114,889 Total current liabilities 25,189 24,895 28,234 Working capital 84,556 68,277 86,655 Total face value of debt 101,000 101,000 141,000 30-Sep-16 31-Dec-16 31-Mar-17 Cash used in operating activities (21,122) $ (15,789) $ (20,606) $ Cash provided by financing activties 21,494 - 39,165 Net increase (decrease) in cash, cash equivalents and marketable securities 2,454 (14,388) 18,518 As Of Three Months Ended
72 First Quarter 2017 Financial Results Three Months Ended 3/31/2016 3/31/2017 Change Revenues Net product sales $2,563 $5,427 $2,864 Collaboration revenues 100 — (100) Total revenue 2,663 5,427 2,764 Cost and Expenses Cost of sales 882 1,325 443 Amortization of product rights 501 503 2 General and administrative 5,998 8,491 2,493 Sales and marketing 6,202 9,258 3,056 Research and development 6,119 6,520 401 Total costs and expenses 19,702 26,097 6,395 Loss from operations (17,039) (20,670) (3,631) Other (income) expense: Change in fair value of derivative liability (610) (12) 598 Interest expense, net 2,309 4,534 2,225 Other gain (3) 181 184 Loss on foreign currency exchange (2) — 2 1,694 4,703 3,009 Loss before provision for income taxes (18,733) (25,373) (6,640) Provision (benefit) for income taxes (185) — 185 Net loss $(18,548) $(25,373) $(6,825)
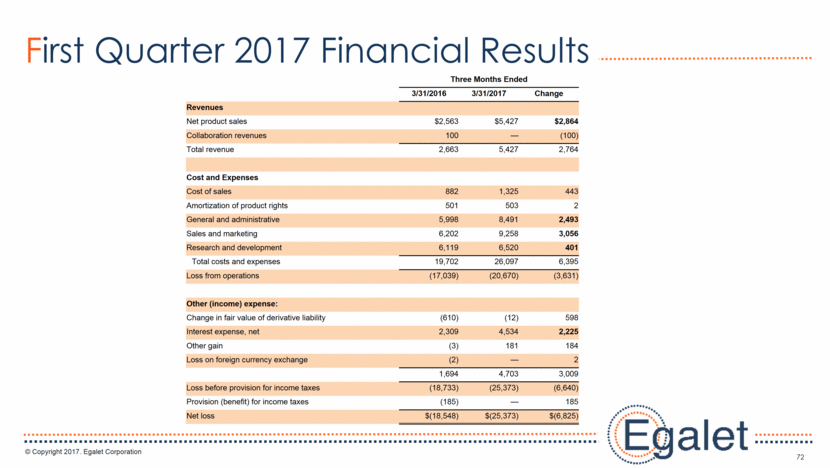
73 Financial Expectations for 2017 Continue growth of SPRIX® and OXAYDO® revenue Building demand for ARYMO® ER Total operating expenses in 2017 to be approximately the same as 2016 Lower R&D due to winding down of clinical trials Higher S&M due to sales force expansion and ARYMO ER launch Growing G&A to support larger commercial organization and impact of OPC costs OPC costs will be approximately $6-8 million during 2017, which represent the requirements for the class-wide extended-release/long-acting opioid analgesics as part of the Opioid Post-Marketing Requirement Consortium or OPC Interest expense is a combination of cash interest on 5.5% Convertible Notes, 13.0% Senior Secured Notes and non-cash amortization of debt discount

Business Development and Financial Q&A 74

75 Commercial Focus in 2017

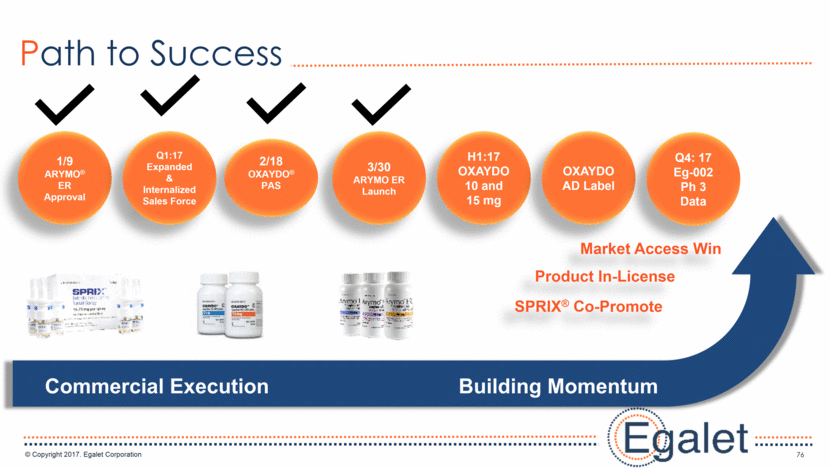
76 Path to Success H1:17 OXAYDO 10 and 15 mg SPRIX® Co-Promote 1/9 ARYMO® ER Approval OXAYDO AD Label 3/30 ARYMO ER Launch 2/18 OXAYDO® PAS Q1:17 Expanded & Internalized Sales Force Commercial Execution Building Momentum Product In-License Market Access Win Q4: 17 Eg-002 Ph 3 Data
Thank You 77 @EgaletCorp Egalet.com

