Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - IMMUNOMEDICS INC | a17-12537_1ex99d2.htm |
| EX-3.1 - EX-3.1 - IMMUNOMEDICS INC | a17-12537_1ex3d1.htm |
| 8-K - 8-K - IMMUNOMEDICS INC | a17-12537_18k.htm |
Exhibit 99.1
[LOGO]
This presentation, in addition to historical information, may contain certain forward-looking statements made pursuant to the Private Securities Litigation Reform Act of 1995. Such statements, including statements regarding clinical trials (including the funding therefor, anticipated patient enrollment, trial outcomes, timing or associated costs), regulatory applications and related timelines, out-licensing arrangements, forecasts of future operating results, potential collaborations, and capital raising activities, involve significant risks and uncertainties and actual results could differ materially from those expressed or implied herein. Factors that could cause such differences include, but are not limited to, the Company’s dependence on business collaborations or availability of required financing from capital markets, or other sources on acceptable terms, if at all, in order to further develop our products and finance our operations, new product development (including clinical trials outcome and regulatory requirements/actions), the risk that we or any of our collaborators may be unable to secure regulatory approval of and market our drug candidates, risks associated with the outcome of pending litigation (including applicable court approval of pending settlements) and competitive risks to marketed products, and the Company’s ability to repay its outstanding indebtedness, if and when required, as well as the risks discussed in the Company’s filings with the Securities and Exchange Commission. The Company is not under any obligation, and the Company expressly disclaims any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. Forward-Looking Statements 2

Business Update and Situation Overview 3

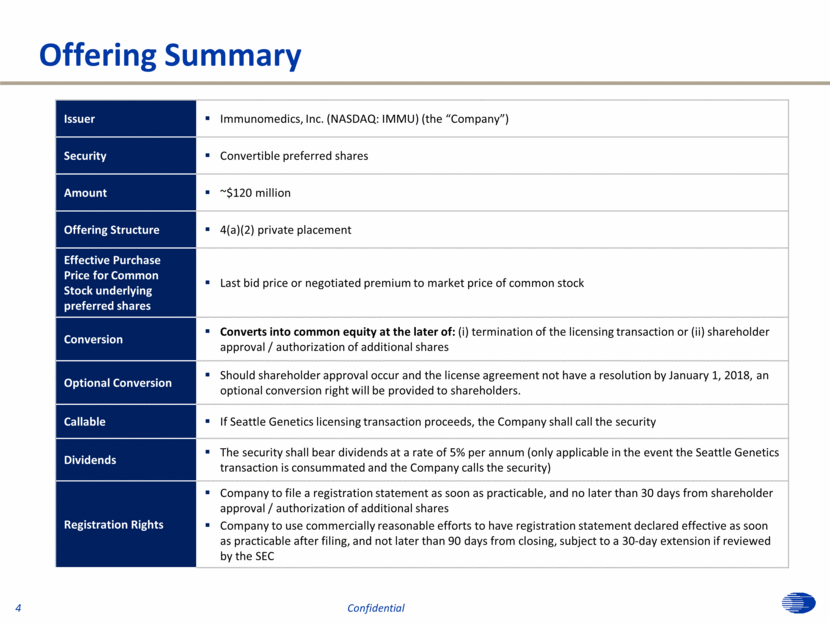
Offering Summary 4 Confidential Issuer Immunomedics, Inc. (NASDAQ: IMMU) (the “Company”) Security Convertible preferred shares Amount ~$120 million Offering Structure 4(a)(2) private placement Effective Purchase Price for Common Stock underlying preferred shares Last bid price or negotiated premium to market price of common stock Conversion Converts into common equity at the later of: (i) termination of the licensing transaction or (ii) shareholder approval / authorization of additional shares Optional Conversion Should shareholder approval occur and the license agreement not have a resolution by January 1, 2018, an optional conversion right will be provided to shareholders. Callable If Seattle Genetics licensing transaction proceeds, the Company shall call the security Dividends The security shall bear dividends at a rate of 5% per annum (only applicable in the event the Seattle Genetics transaction is consummated and the Company calls the security) Registration Rights Company to file a registration statement as soon as practicable, and no later than 30 days from shareholder approval / authorization of additional shares Company to use commercially reasonable efforts to have registration statement declared effective as soon as practicable after filing, and not later than 90 days from closing, subject to a 30-day extension if reviewed by the SEC
Start confirmatory Phase 3 clinical trial in mTNBC (Q3 2017) Execute CRO Agreement Site selection / trial initiation / patient enrollment (US & EU) Continue CMC preparations for commercial launch Begin transfer of manufacturing to large-scale CMO Pre-approval inspection activities continue Phase 2 and Phase 3 clinical trial materials manufactured Commercial drug manufacturing continues Submit BLA for Accelerated Approval in mTNBC Late 2017 / early 2018 (pending FDA input on process validation) Explore potential strategic partnerships to maximize the value of the pipeline, including regional partnerships for IMMU-132 Key Business Objectives for 2017 5 Confidential

Key Updates Termination of Development and License Agreement with Seattle Genetics The mutually agreed upon termination of the licensing agreement between Immunomedics and Seattle Genetics is subject to court approval Immunomedics will retain all rights to IMMU-132 No payments or expense reimbursements will be made by either party Management Update Cynthia Sullivan has agreed to resign from all director, officer and other positions of the Company and any of its affiliates, upon execution of the contemplated Settlement Agreement, effective as of the date of the Settlement Agreement, which is to be entered into and is subject to court approval. Michael Garone was appointed interim CEO, effective upon the execution and effectiveness of the Settlement Agreement. The Board plans to retain an executive search firm to identify a permanent CEO for the Company Board to also evaluate additional senior management appointments Dr. David Goldenberg will remain a director of the Company, but has agreed to resign from all officer positions with the Company, including as chief scientific officer and chief patent officer, effective upon the execution of the settlement agreement. 6 Confidential

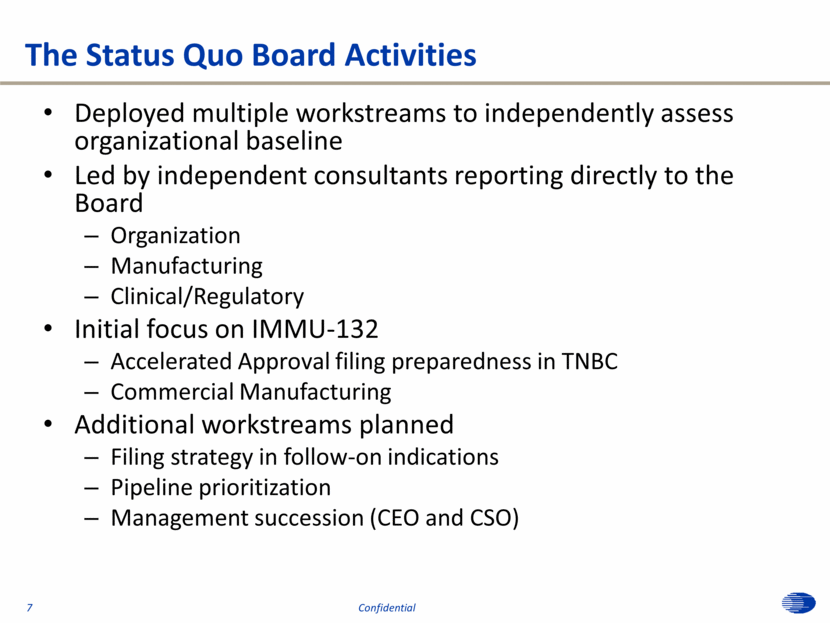
The Status Quo Board Activities Deployed multiple workstreams to independently assess organizational baseline Led by independent consultants reporting directly to the Board Organization Manufacturing Clinical/Regulatory Initial focus on IMMU-132 Accelerated Approval filing preparedness in TNBC Commercial Manufacturing Additional workstreams planned Filing strategy in follow-on indications Pipeline prioritization Management succession (CEO and CSO) 7 Confidential
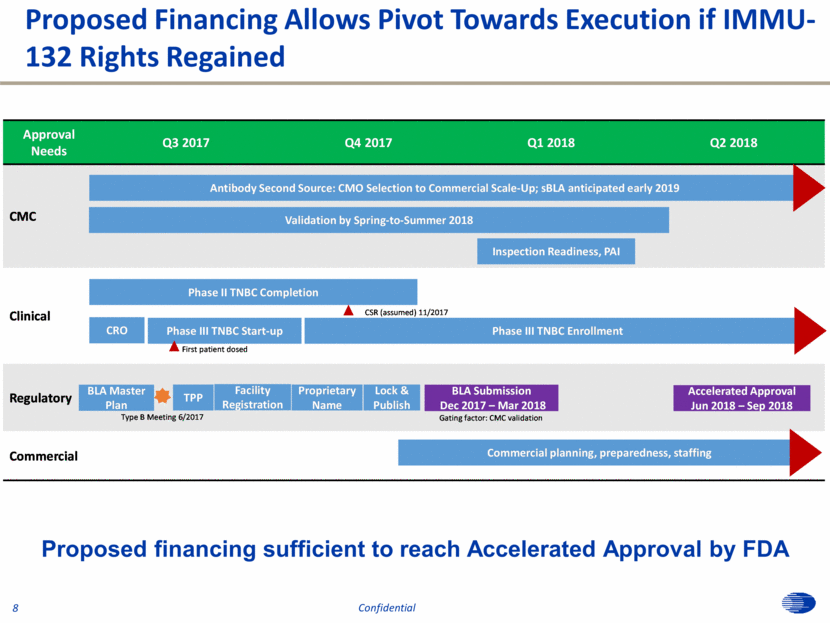
Proposed Financing Allows Pivot Towards Execution if IMMU-132 Rights Regained 8 Proposed financing sufficient to reach Accelerated Approval by FDA Confidential Approval Needs Q3 2017 Q4 2017 Q1 2018 Q2 2018 CMC Clinical Regulatory Commercial CRO Phase III TNBC Start - up First patient dosed Phase III TNBC Enrollment Phase II TNBC Completion CSR ( assumed) 11/2017 BLA Master Plan TPP Type B Meeting 6/2017 Lock & Publish Antibody Second Source: CMO Selection to Commercial Scale - Up; sBLA anticipated early 2019 Validation by Spring - to - Summer 2018 Commercial planning, preparedness, staffing Inspection Readiness, PAI Facility Registration Proprietary Name BLA Submission Dec 2017 – Mar 2018 Accelerated Approval Jun 2018 – Sep 2018 Gating factor: CMC validation
IMMU ADC Technology 9

Unique approach to ADC therapeutics for cancer Highly cancer-specific antibodies based on 30 years of experience Utilize antibodies with dual activity Moderately potent payloads increased therapeutic index Proprietary linker designed for SN-38 High drug-to-antibody ratio (~7.6:1) Rapid payload release at or inside tumor SN-38 payload Active metabolite more potent than parent compound, irinotecan (a commonly used chemotherapeutic) ADCs’ unique chemistry avoids low solubility and selectively delivers SN-38 to the tumor What Makes IMMU’s ADCs Different? 10 Confidential
IMMU-132 11

Overview of IMMU-132 (Sacituzumab Govitecan) 12 Confidential Single-arm Phase 2 study Enrolled 100 assessable TNBC patients with at least 2 prior therapies required for BLA submission Patient follow-up ongoing Third-party “blinded” confirmation of response assessment ongoing Phase 3 confirmatory trial Enrollment planned to commence in mid-2017 Selected a CRO to run trial in U.S. and Europe BLA submission for accelerated approval Expected late 2017 / early 2018 Key Highlights Key Updates on Clinical Development for TNBC Breakthrough Therapy Designation granted in metastatic triple-negative breast cancer (mTNBC) Validated mechanism of action Binds Trop-2 Delivers enhanced SN-38 concentrations at or in the tumor Exploring treatment for multiple solid cancers Pursuing TNBC, UC, NSCLC and SCLC and additional solid cancer indications Strong results in Phase 2 study for TNBC 29% ORR in 85 patients treated Promising durable activity – achieved median PFS / OS of 6.0 / 18.8 months, respectively Acceptable safety profile in heavily pretreated patients Note: TNBC = triple-negative breast cancer, UC = urothelial cancer, NSCLC = non-small-cell lung cancer, SCLC = small-cell lung cancer.
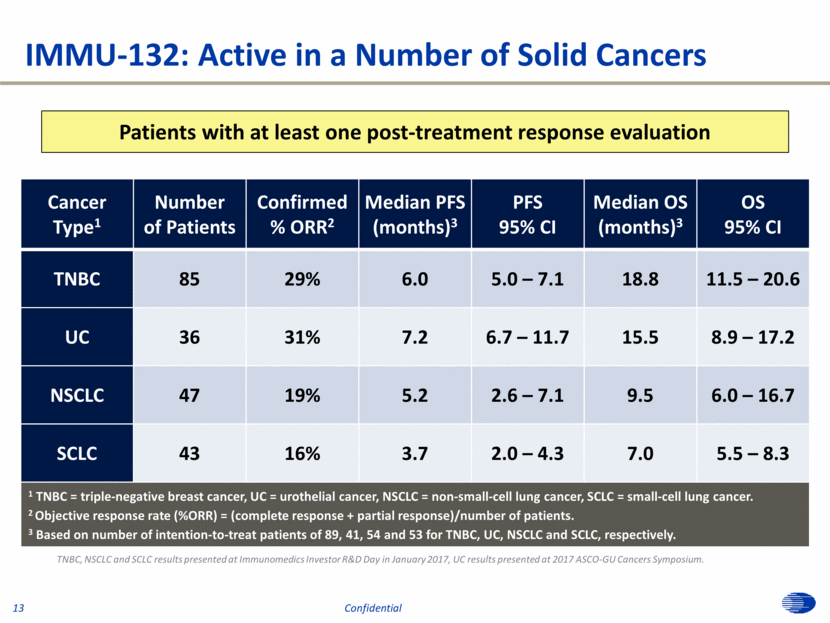
Cancer Type1 Number of Patients Confirmed % ORR2 Median PFS (months)3 PFS 95% CI Median OS (months)3 OS 95% CI TNBC 85 29% 6.0 5.0 – 7.1 18.8 11.5 – 20.6 UC 36 31% 7.2 6.7 – 11.7 15.5 8.9 – 17.2 NSCLC 47 19% 5.2 2.6 – 7.1 9.5 6.0 – 16.7 SCLC 43 16% 3.7 2.0 – 4.3 7.0 5.5 – 8.3 1 TNBC = triple-negative breast cancer, UC = urothelial cancer, NSCLC = non-small-cell lung cancer, SCLC = small-cell lung cancer. 2 Objective response rate (%ORR) = (complete response + partial response)/number of patients. 3 Based on number of intention-to-treat patients of 89, 41, 54 and 53 for TNBC, UC, NSCLC and SCLC, respectively. Patients with at least one post-treatment response evaluation IMMU-132: Active in a Number of Solid Cancers TNBC, NSCLC and SCLC results presented at Immunomedics Investor R&D Day in January 2017, UC results presented at 2017 ASCO-GU Cancers Symposium. 13 Confidential
IMMU-132: Best Response from mTNBC Patients (N=85) Confirmed ORR (RECIST 1.1) = 29% Median # prior therapies = 5 (range, 2 – 12) Presented at Immunomedics Investor R&D Day in January 2017. 14 Confidential -100 -80 -60 -40 -20 0 20 40 60 80 B e s t R e s p o n s e B e s t % c h a n g e i n T L f r o m b a s e l i n e Complete response (confirmed) Partial response (confirmed) Partial response (pending) Partial response (unconfirmed) Stable disease Progression 79/85 response assessable pts who completed 1 treatment cycle are represented 3 progressed, but TL measurement unavailable 3 did not have a CT response assessment Overall response assessment descriptor
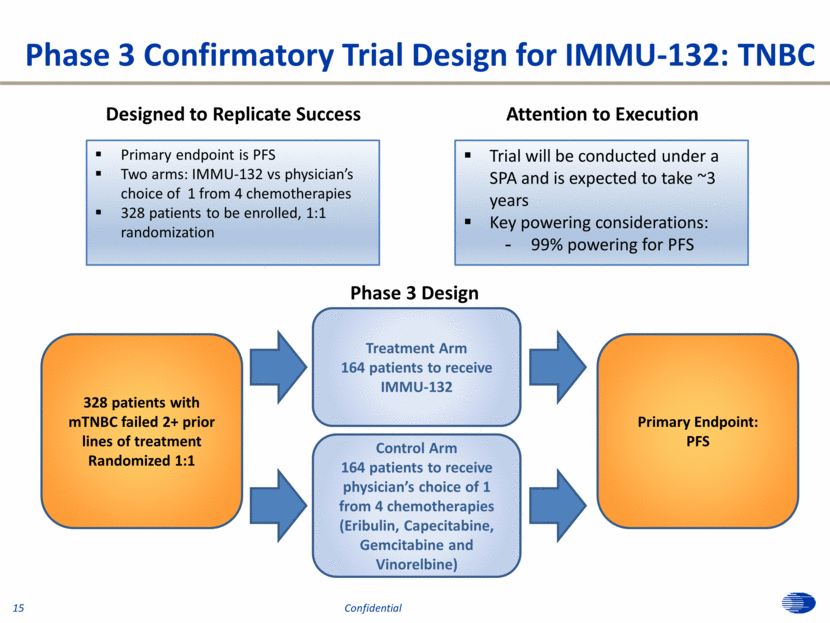
Phase 3 Confirmatory Trial Design for IMMU-132: TNBC Designed to Replicate Success Primary endpoint is PFS Two arms: IMMU-132 vs physician’s choice of 1 from 4 chemotherapies 328 patients to be enrolled, 1:1 randomization Attention to Execution Trial will be conducted under a SPA and is expected to take ~3 years Key powering considerations: 99% powering for PFS Phase 3 Design Control Arm 164 patients to receive physician’s choice of 1 from 4 chemotherapies (Eribulin, Capecitabine, Gemcitabine and Vinorelbine) Primary Endpoint: PFS Treatment Arm 164 patients to receive IMMU-132 328 patients with mTNBC failed 2+ prior lines of treatment Randomized 1:1 15 Confidential
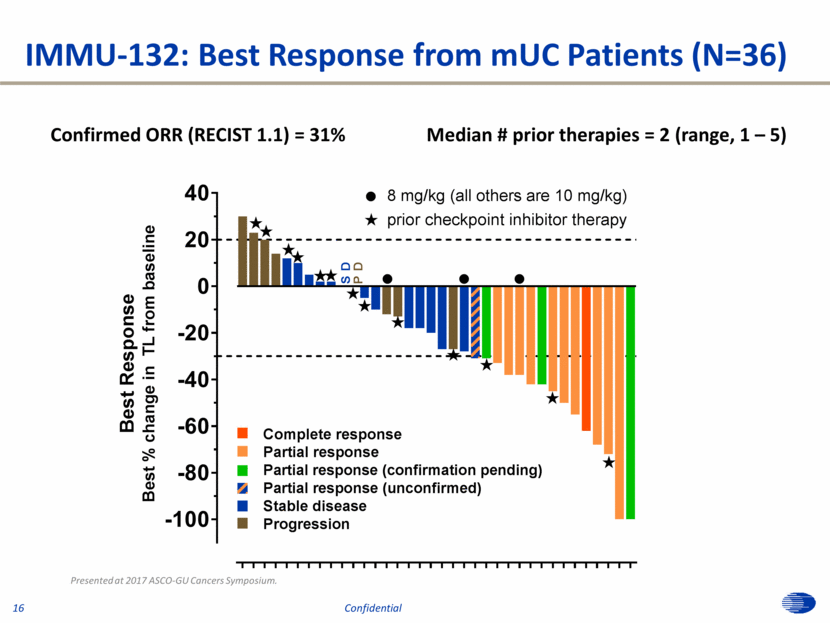
IMMU-132: Best Response from mUC Patients (N=36) Presented at 2017 ASCO-GU Cancers Symposium. Confirmed ORR (RECIST 1.1) = 31% Median # prior therapies = 2 (range, 1 – 5) 16 Confidential -100 -80 -60 -40 -20 0 20 40 B e s t R e s p o n s e B e s t % c h a n g e i n T L f r o m b a s e l i n e P D S D Complete response Partial response Partial response (confirmation pending) Partial response (unconfirmed) Stable disease Progression l « l l « « « « « l 8 mg/kg (all others are 10 mg/kg) prior checkpoint inhibitor therapy « « « « « « « «
IMMU-132: Best Response from mNSCLC Patients (N=47) Presented at Immunomedics Investor R&D Day in January 2017. Confirmed ORR (RECIST 1.1) = 19% Median # prior therapies = 3 (range, 1 – 7) 17 Confidential -80 -60 -40 -20 0 20 40 B e s t % c h a n g e i n t a r g e t l e s i o n s f r o m b a s e l i n e Partial response (confirmed) (PR) Unconfirmed PR (PRu) (stable disease) Stable disease Progression « Ä « « « « « « « « « « Ä Ä Ä Ä Ä « Squamous cell histology 8 mg/kg starting dose Prior checkpoint inhibitor Tx Ä Ä + + early CT assessment after 2 doses

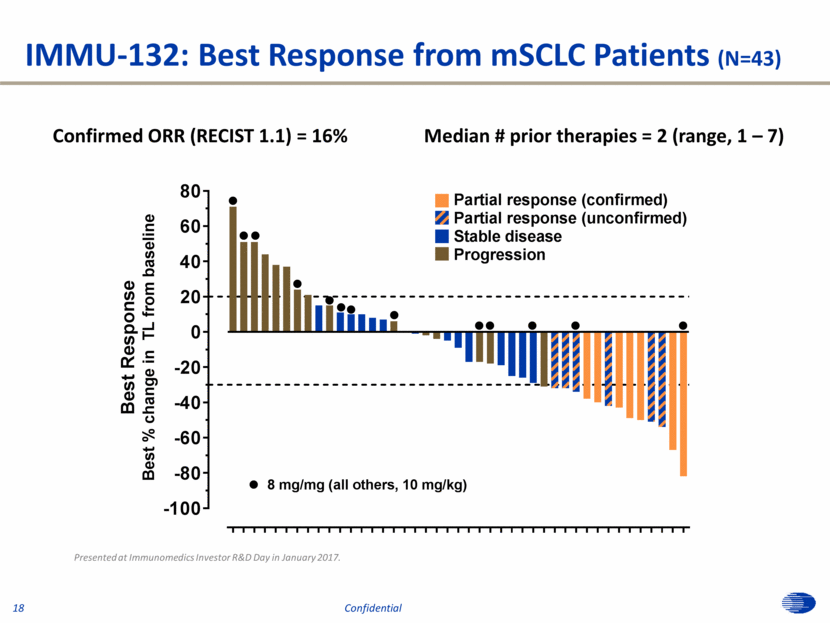
IMMU-132: Best Response from mSCLC Patients (N=43) Confirmed ORR (RECIST 1.1) = 16% Median # prior therapies = 2 (range, 1 – 7) Presented at Immunomedics Investor R&D Day in January 2017. 18 Confidential -100 -80 -60 -40 -20 0 20 40 60 80 B e s t R e s p o n s e B e s t % c h a n g e i n T L f r o m b a s e l i n e l l l l l l l l l l l l l Partial response (confirmed) Partial response (unconfirmed) Stable disease Progression l 8 mg/mg (all others, 10 mg/kg)
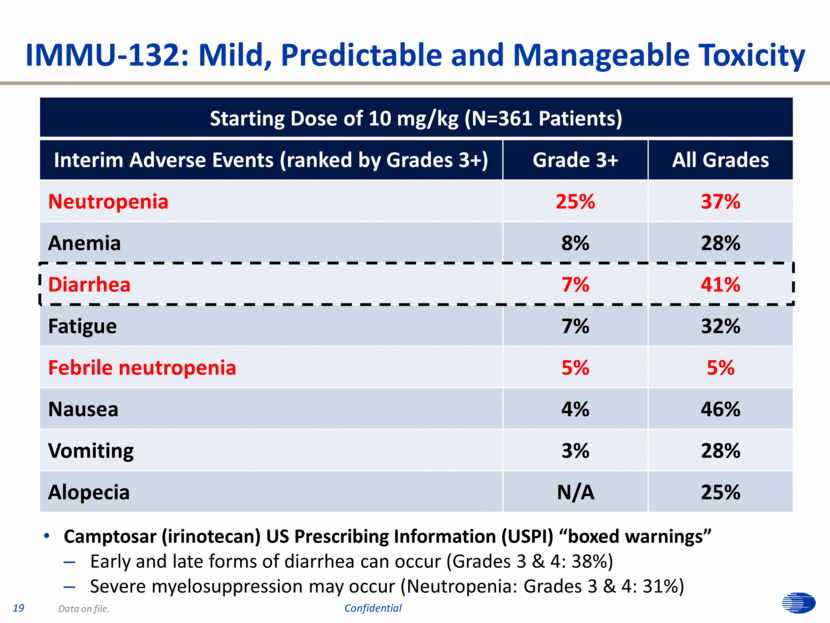
Starting Dose of 10 mg/kg (N=361 Patients) Interim Adverse Events (ranked by Grades 3+) Grade 3+ All Grades Neutropenia 25% 37% Anemia 8% 28% Diarrhea 7% 41% Fatigue 7% 32% Febrile neutropenia 5% 5% Nausea 4% 46% Vomiting 3% 28% Alopecia N/A 25% IMMU-132: Mild, Predictable and Manageable Toxicity Data on file. Camptosar (irinotecan) US Prescribing Information (USPI) “boxed warnings” Early and late forms of diarrhea can occur (Grades 3 & 4: 38%) Severe myelosuppression may occur (Neutropenia: Grades 3 & 4: 31%) 19 Confidential
IMMU-132 Patent Portfolio 32 issued U.S. and 16 foreign patents covering composition of matter, synthesis and uses of IMMU-132 Company’s main composition of matter patents expire in 2023 in the U.S., and in 2029 in Europe In addition, IMMU-132 has patent coverage through 2033 for uses in various cancers at different dose schedules, and other proprietary features of the antibody-drug conjugate Patent applications are being prosecuted in all major countries, with patents issued in Australia, Canada, China, Europe, Israel, Japan and South Korea IMMU-132 has regulatory exclusivity of 12 years in the US and 10 years in Europe Moreover, the product has been granted Orphan Drug status in the US and EU for certain secondary indications 20 Confidential
IMMU Pipeline 21
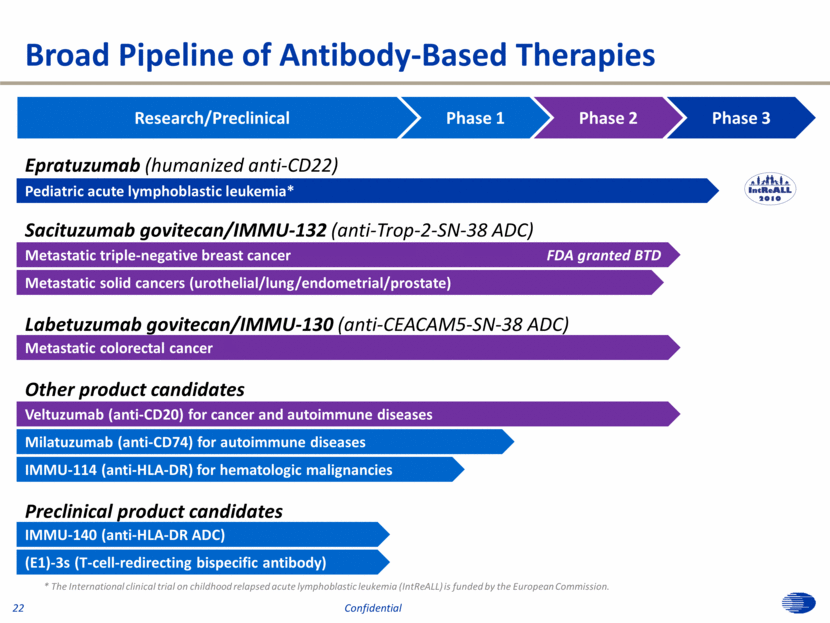
Research/Preclinical Phase 1 Phase 2 Phase 3 Sacituzumab govitecan/IMMU-132 (anti-Trop-2-SN-38 ADC) Metastatic triple-negative breast cancer FDA granted BTD Epratuzumab (humanized anti-CD22) Labetuzumab govitecan/IMMU-130 (anti-CEACAM5-SN-38 ADC) Metastatic solid cancers (urothelial/lung/endometrial/prostate) Metastatic colorectal cancer Milatuzumab (anti-CD74) for autoimmune diseases IMMU-140 (anti-HLA-DR ADC) Veltuzumab (anti-CD20) for cancer and autoimmune diseases Other product candidates Broad Pipeline of Antibody-Based Therapies Pediatric acute lymphoblastic leukemia* * The International clinical trial on childhood relapsed acute lymphoblastic leukemia (IntReALL) is funded by the European Commission. Preclinical product candidates (E1)-3s (T-cell-redirecting bispecific antibody) IMMU-114 (anti-HLA-DR) for hematologic malignancies 22 Confidential
Financial Highlights 23

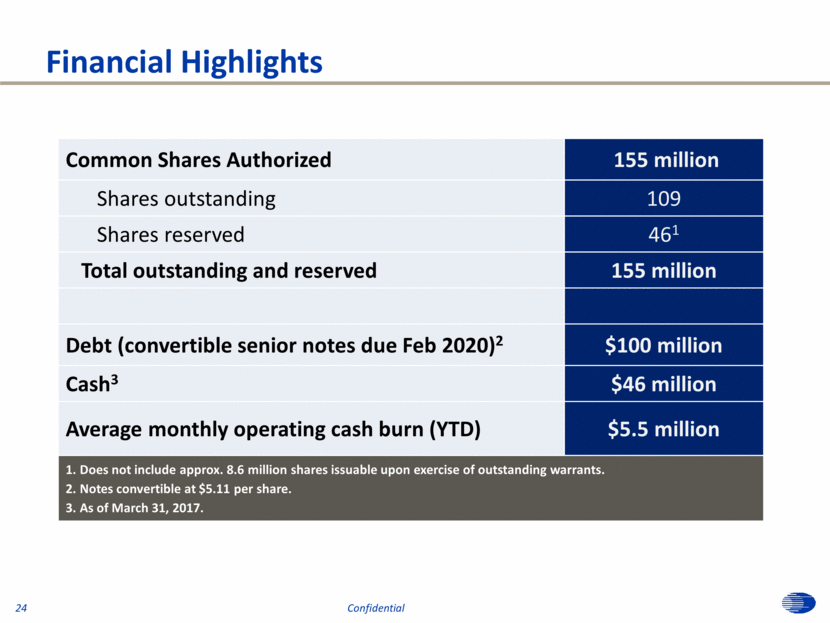
Financial Highlights Common Shares Authorized 155 million Shares outstanding 109 Shares reserved 461 Total outstanding and reserved 155 million Debt (convertible senior notes due Feb 2020)2 $100 million Cash3 $46 million Average monthly operating cash burn (YTD) $5.5 million 1. Does not include approx. 8.6 million shares issuable upon exercise of outstanding warrants. 2. Notes convertible at $5.11 per share. 3. As of March 31, 2017. 24 Confidential
May 2017
[LOGO]