Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ALPINE IMMUNE SCIENCES, INC. | a17-11802_18k.htm |
Exhibit 99.1
Alpine Immune Sciences and Nivalis Therapeutics Combination COPYRIGHT © 2017 ALPHINE IMMUNE SCIENCES, INC. AND NIVALIS THERAPEUTICS, INC. ALL RIGHTS RESERVED.
Forward Looking Statements This presentation includes forward-looking statements regarding Nivalis, Alpine (including Alpine’s platform technology, potential therapies, clinical and regulatory objectives and milestone and royalty payment potential), the proposed merger transaction between Nivalis and Alpine, the cash available to the combined company post-merger, and other matters. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “plan,” “intend,” and other similar expressions among others. Actual results could differ materially and these statements are subject to important risks, including that the conditions to the closing of the transaction are not satisfied, that available cash is lower than estimated, that Alpine’s discovery-stage and pre-clinical programs do not advance into the clinic or result in approved products on a timely or cost effective basis or at all. Risks are or will be detailed in Nivalis’ filings with the SEC, including the most recent Annual Report on Form 10-K, and Current Reports on Form 8-K filed with the SEC. In connection with the proposed transaction between Nivalis and Alpine, Nivalis intends to file relevant materials with the SEC, including a registration statement that will contain a proxy statement, prospectus, and risk factors. Nivalis and Alpine undertake no obligation to update the forward –looking statements as a result of new information or otherwise.

Participants and Additional Information No Offer or Solicitation This communication is not intended to and does not constitute an offer to sell or the solicitation of an offer to subscribe for or buy or an invitation to purchase or subscribe for any securities or the solicitation of any vote in any jurisdiction pursuant to the proposed transaction or otherwise, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the United States Securities Act of 1933, as amended. Subject to certain exceptions to be approved by the relevant regulators or certain facts to be ascertained, the public offer will not be made directly or indirectly, in or into any jurisdiction where to do so would constitute a violation of the laws of such jurisdiction, or by use of the mails or by any means or instrumentality (including without limitation, facsimile transmission, telephone and the internet) of interstate or foreign commerce, or any facility of a national securities exchange, of any such jurisdiction. Important Additional Information Will be Filed with the SEC In connection with the proposed transaction between Nivalis and Alpine, Nivalis intends to file relevant materials with the SEC, including a registration statement that will contain a proxy statement and prospectus. NIVALIS URGES INVESTORS AND STOCKHOLDERS TO READ THESE MATERIALS CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT NIVALIS, THE PROPOSED TRANSACTION AND RELATED MATTERS. Potential investors and existing stockholders will be able to obtain free copies of the proxy statement, prospectus and other documents filed by Nivalis with the SEC (when they become available) through the website maintained by the SEC at www.sec.gov. In addition, potential investors and existing stockholders be able to obtain free copies of the proxy statement, prospectus and other documents filed by Nivalis with the SEC by contacting Investor Relations by mail at Attn: Investor Relations, 3122 Sterling Circle, Boulder, Colorado, 80301. Potential investors and existing stockholders are urged to read the proxy statement, prospectus and the other relevant materials when they become available before making any voting or investment decision with respect to the proposed transaction. Participants in the Solicitation Nivalis and Alpine, and each of their respective directors and executive officers and certain of their other members of management and employees, may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about Nivalis’ directors and executive officers is included in Nivalis’ Annual Report on Form 10-K for the year ended December 31, 2016, filed with the SEC on February 13, 2017, and the proxy statement for Nivalis’ 2017 annual meeting of stockholders, filed with the SEC on April 6, 2017. Additional information regarding these persons and their interests in the transaction will be included in the proxy statement relating to the transaction when filed with the SEC. These documents can be obtained free of charge from sources indicated above.
Rationale for Nivalis and Alpine Combination Unique opportunity for Nivalis to combine with a growing immunotherapy company with multiple product opportunities in immuno-oncology and inflammatory diseases Well-capitalized private company with experienced healthcare investors OrbiMed Advisors, Frazier Healthcare Partners, and Alpine BioVentures will invest a combined $17 million just prior to close Combined company is expected to have ~$90 million in cash and cash equivalents at close expected to support three future INDs Expect multiple scientific presentations throughout next 18 months Up to $530 million in additional potential milestones from Kite collaboration validates transformative nature of platform

Alpine – Nivalis Combination Specifics Nivalis conducted an extensive review of strategic alternatives All-stock transaction is intended to qualify as a “reorganization” within the meaning of Section 368(a) of the tax code Nivalis stockholders will own about 26% and Alpine stockholders about 74% of the combined company upon completion of the proposed transaction Transaction approved by boards of directors of both companies Expected to close in Q3-2017, subject to the approval of Nivalis stockholders and other closing conditions

Alpine – Nivalis Combination Specifics Company will retain the Alpine Immune Sciences name Combined entity is expected to have ~$90 million at closing Nivalis contributes $44 million cash Alpine management team has successfully brought immunotherapies to market Dr. Mitchell Gold will be Chairman and CEO Headquarters in Seattle, WA Board of Directors: Two Nivalis designees, four Alpine designees, & one mutually-appointed independent designee
Introduction to Alpine Immune Sciences April 28, 2017

Company Overview Company developing novel potential therapies focused on targeting IgSF proteins in the immune synapse Seed financing from Alpine BioVentures and a $48 million Series A from OrbiMed, Frazier Healthcare, and Alpine BioVentures Collaboration with Kite Pharma for use of two TIPs™ with Kite’s CAR-T and TCR platforms Headcount currently ~35 employees Expect vIgD™ molecule in clinic in 2H-2018 for autoimmune/inflammation
Snapshot of Alpine Immune Sciences Our novel variant Immunoglobulin Domain (vIgD™) platform is based on directed evolution of an IgSF structure to multiple binding partners in the immune synapse First-in-class, multi-valent molecules with dual functionality and potential significant activity Dual ICOS/CD28 agonists/antagonists (first in class) Multiple additional discovery stage and pre-clinical programs underway (single checkpoint antagonists, multi-checkpoint antagonists, and costimulatory agonists/antagonists) vIgD™ platform also potentially capable of TREG depletion, TREG activation and T cell inhibitory receptor antagonists vIgD™ program in inflammatory disease demonstrated in vivo proof of concept
APC/T cell immune synapse (yellow) Image: Brian Cobb, Case Western Immune system activation involves multiple interactions of cell surface molecules in the immune synapse Three-dimensional structure of the synapse is critical to the final outcome of immune cell effector functions vIgD™ molecules are evolved to efficiently mediate complex multi-protein interactions Alpine Immune Sciences’ vIgDs™ are Functional Constituents of the Immune Synapse

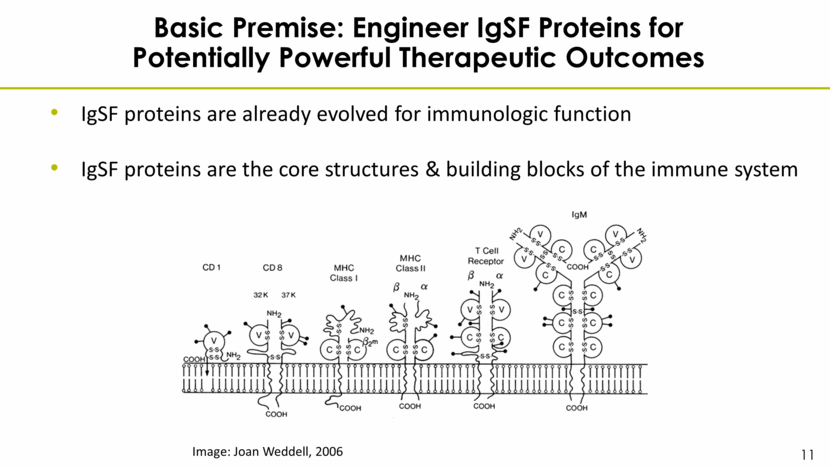
Basic Premise: Engineer IgSF Proteins for Potentially Powerful Therapeutic Outcomes IgSF proteins are already evolved for immunologic function IgSF proteins are the core structures & building blocks of the immune system Image: Joan Weddell, 2006
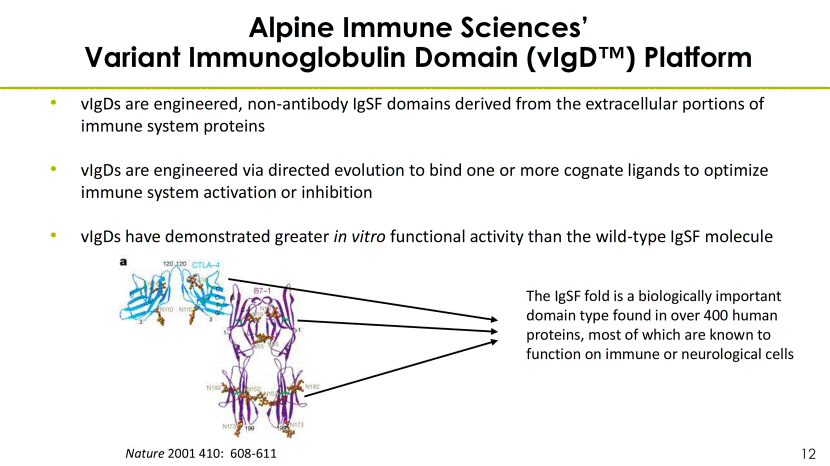
Alpine Immune Sciences’ Variant Immunoglobulin Domain (vIgD™) Platform vIgDs are engineered, non-antibody IgSF domains derived from the extracellular portions of immune system proteins vIgDs are engineered via directed evolution to bind one or more cognate ligands to optimize immune system activation or inhibition vIgDs have demonstrated greater in vitro functional activity than the wild-type IgSF molecule Nature 2001 410: 608-611 The IgSF fold is a biologically important domain type found in over 400 human proteins, most of which are known to function on immune or neurological cells
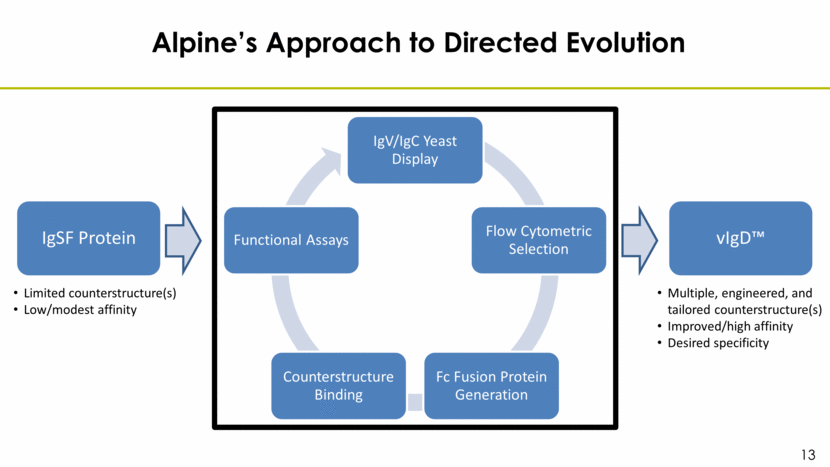
Alpine’s Approach to Directed Evolution IgSF Protein Limited counterstructure(s) Low/modest affinity vIgD™ Multiple, engineered, and tailored counterstructure(s) Improved/high affinity Desired specificity IgV/IgC Yeast Display Flow Cytometric Selection Fc Fusion Protein Generation Counterstructure Binding Functional Assays
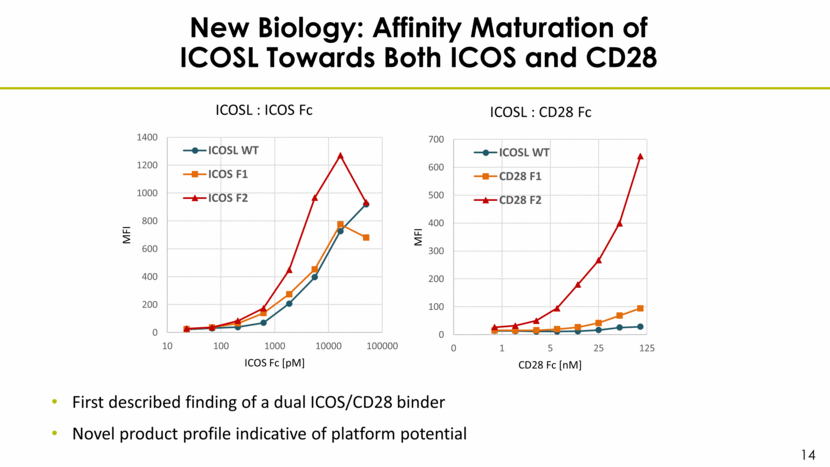
New Biology: Affinity Maturation of ICOSL Towards Both ICOS and CD28 First described finding of a dual ICOS/CD28 binder Novel product profile indicative of platform potential 0 200 400 600 800 1000 1200 1400 10 100 1000 10000 100000 MFI ICOS Fc [pM] ICOSL : ICOS Fc ICOSL WT ICOS F1 ICOS F2 0 100 200 300 400 500 600 700 0 1 5 25 125 MFI CD28 Fc [nM] ICOSL : CD28 Fc ICOSL WT CD28 F1 CD28 F2
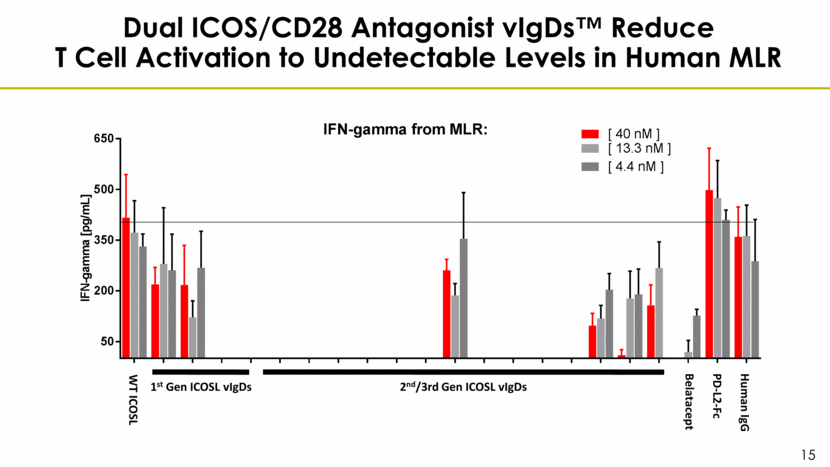
Dual ICOS/CD28 Antagonist vIgDs™ Reduce T Cell Activation to Undetectable Levels in Human MLR WT ICOSL 1st Gen ICOSL vIgDs 2nd/3rd Gen ICOSL vIgDs Belatacept PD-L2-Fc Human IgG
vIgDs™ Can Be Formatted Specifically for Various Therapeutic Applications Oncology CAR-T (TIP™) mAb Costimulatory Agonist Costimulatory V-mAb Fc Target 1 Target 2 vIgD™ Multi-Checkpoint Antagonist Costimulatory Agonist Fc Localizer Localized vIgD™ ICOSL ECD Fc vIgD™ ICOSL Autoimmune

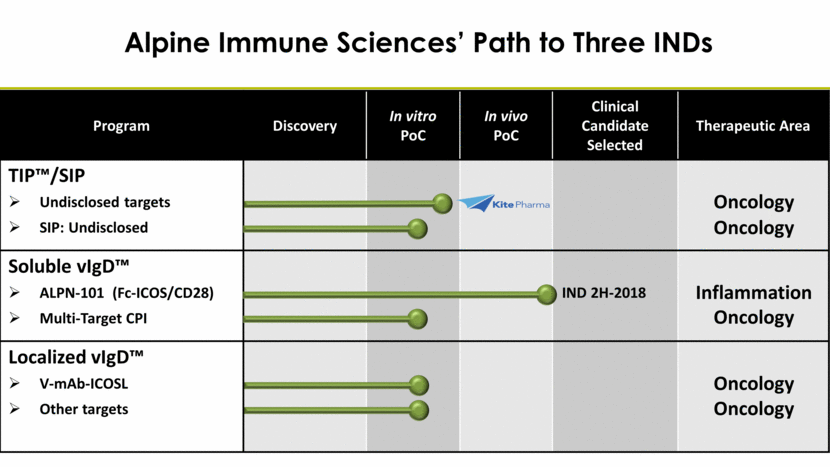
Alpine Immune Sciences’ Path to Three INDs Program Discovery In vitro PoC In vivo PoC Clinical Candidate Selected Therapeutic Area TIP™/SIP Undisclosed targets SIP: Undisclosed Soluble vIgD™ ALPN-101 (Fc-ICOS/CD28) Multi-Target CPI Localized vIgD™ V-mAb-ICOSL Other targets Oncology Inflammation Oncology Oncology Oncology Oncology IND 2H-2018
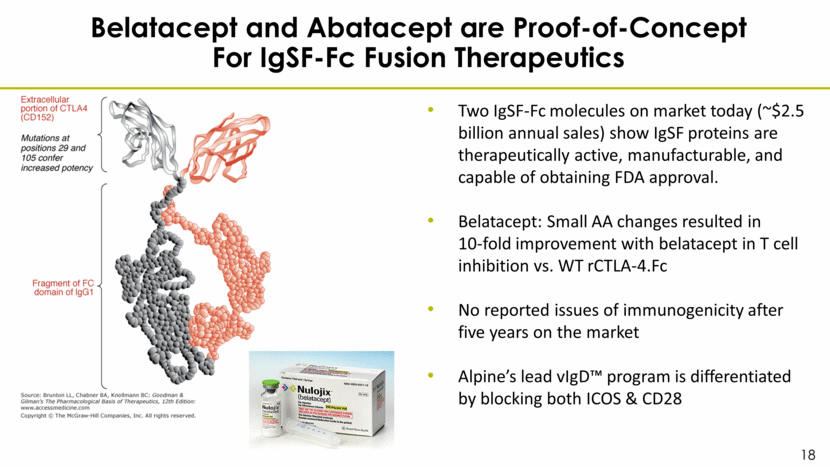
Belatacept and Abatacept are Proof-of-Concept For IgSF-Fc Fusion Therapeutics Two IgSF-Fc molecules on market today (~$2.5 billion annual sales) show IgSF proteins are therapeutically active, manufacturable, and capable of obtaining FDA approval. Belatacept: Small AA changes resulted in 10-fold improvement with belatacept in T cell inhibition vs. WT rCTLA-4.Fc No reported issues of immunogenicity after five years on the market Alpine’s lead vIgD™ program is differentiated by blocking both ICOS & CD28
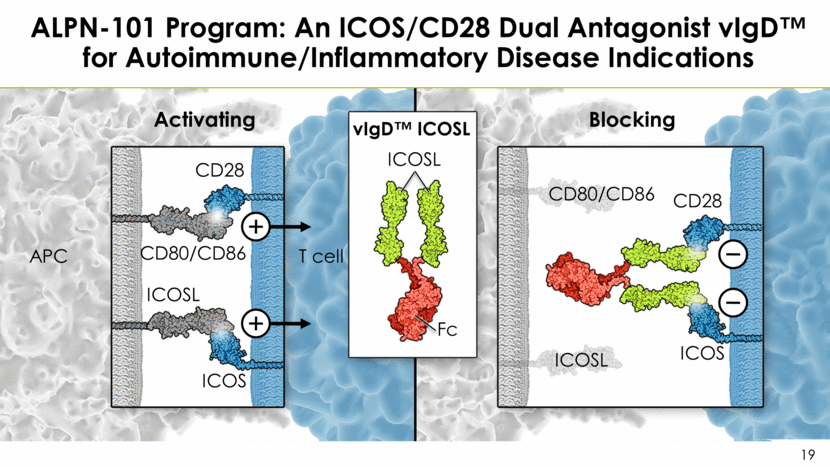
ALPN-101 Program: An ICOS/CD28 Dual Antagonist vIgD™ for Autoimmune/Inflammatory Disease Indications APC T cell Fc vIgD™ ICOSL CD80/CD86 CD28 ICOS ICOSL CD80/CD86 CD28 ICOS ICOSL Activating Blocking ICOSL
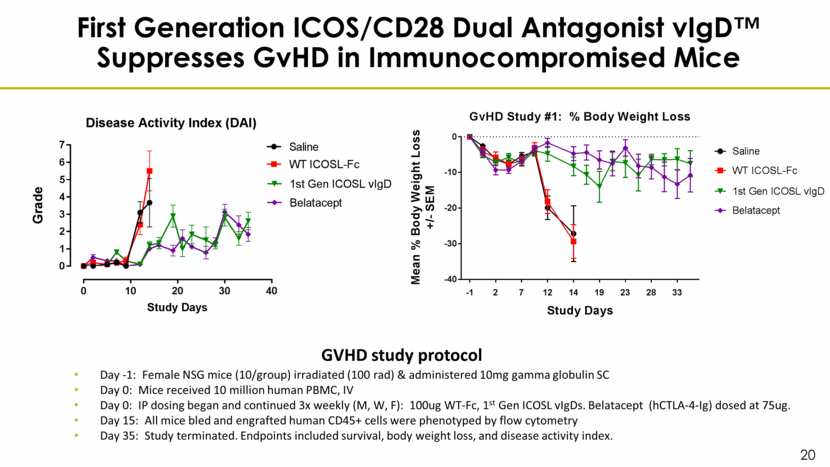
First Generation ICOS/CD28 Dual Antagonist vIgD™ Suppresses GvHD in Immunocompromised Mice Day -1: Female NSG mice (10/group) irradiated (100 rad) & administered 10mg gamma globulin SC Day 0: Mice received 10 million human PBMC, IV Day 0: IP dosing began and continued 3x weekly (M, W, F): 100ug WT-Fc, 1st Gen ICOSL vIgDs. Belatacept (hCTLA-4-Ig) dosed at 75ug. Day 15: All mice bled and engrafted human CD45+ cells were phenotyped by flow cytometry Day 35: Study terminated. Endpoints included survival, body weight loss, and disease activity index. GVHD study protocol -1 2 7 12 14 19 23 28 33 -40 -30 -20 -10 0 GvHD Study #1: % Body Weight Loss Study Days M e a n % B o d y W e i g h t L o s s + / - S E M Saline WT ICOSL-Fc 1st Gen ICOSL vIgD Belatacept 0 10 20 30 40 0 1 2 3 4 5 6 7 Disease Activity Index (DAI) Study Days G r a d e Saline WT ICOSL-Fc 1st Gen ICOSL vIgD Belatacept
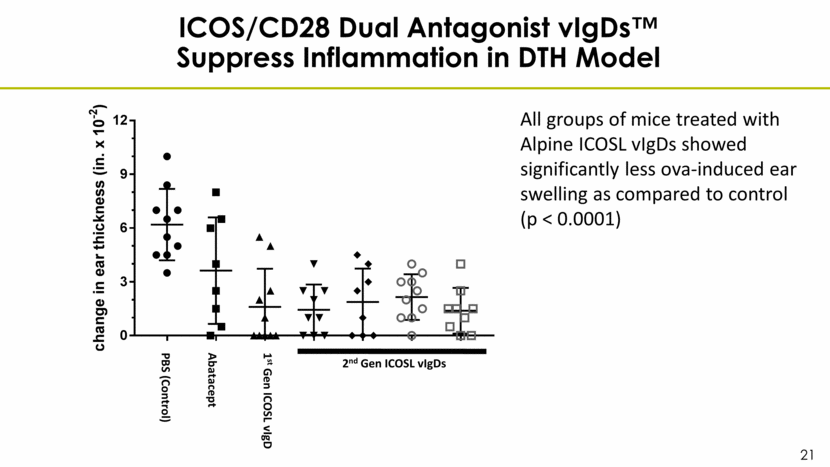
ICOS/CD28 Dual Antagonist vIgDs™ Suppress Inflammation in DTH Model All groups of mice treated with Alpine ICOSL vIgDs showed significantly less ova-induced ear swelling as compared to control (p < 0.0001) PBS (Control) Abatacept 1st Gen ICOSL vIgD 2nd Gen ICOSL vIgDs
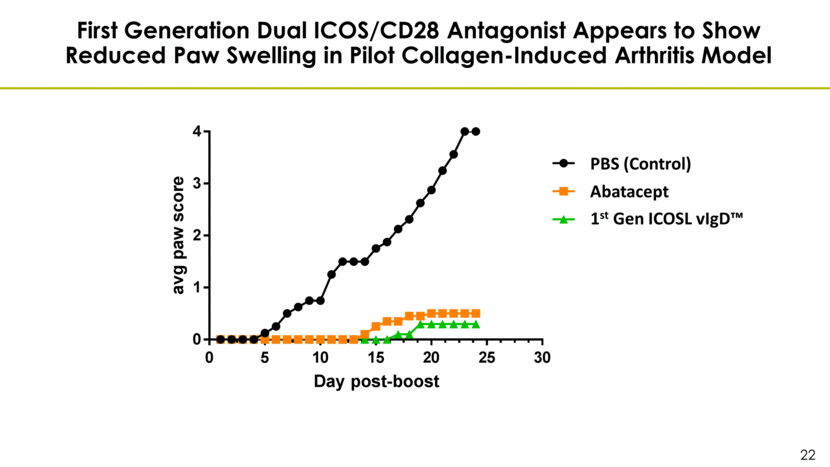
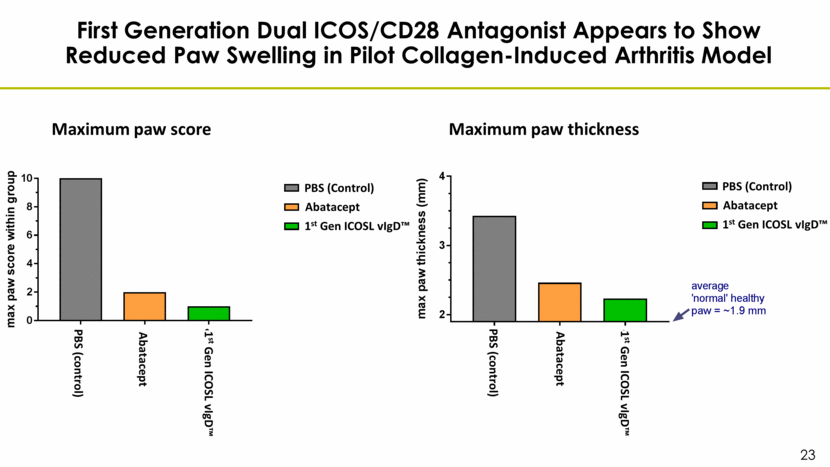
First Generation Dual ICOS/CD28 Antagonist Appears to Show Reduced Paw Swelling in Pilot Collagen-Induced Arthritis Model Abatacept 1st Gen ICOSL vIgD™ PBS (Control) 0 5 10 15 20 25 30 0 1 2 3 4 Mean CIA 1701 clinical scores Day post-boost a v g p a w s c o r e PBS Orencia AIS83
First Generation Dual ICOS/CD28 Antagonist Appears to Show Reduced Paw Swelling in Pilot Collagen-Induced Arthritis Model Maximum paw score Abatacept 1st Gen ICOSL vIgD™ PBS (Control) Maximum paw thickness Abatacept 1st Gen ICOSL vIgD™ PBS (Control) 1st Gen ICOSL vIgD™ Abatacept PBS (control) 1st Gen ICOSL vIgD™ Abatacept PBS (control)
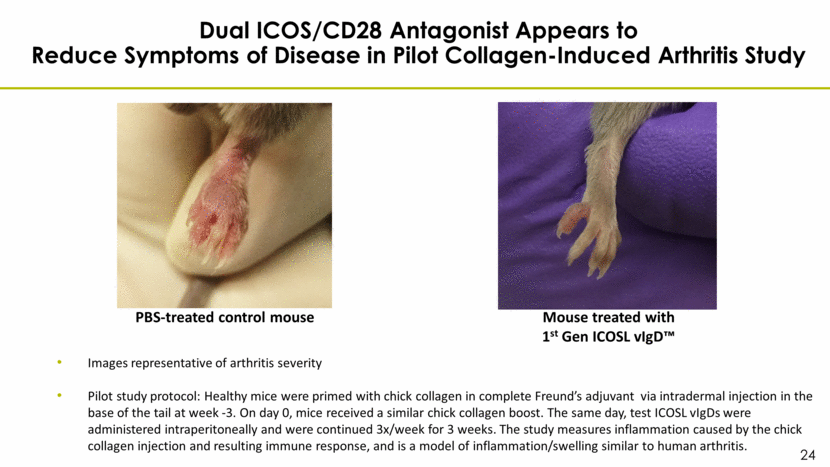
Dual ICOS/CD28 Antagonist Appears to Reduce Symptoms of Disease in Pilot Collagen-Induced Arthritis Study Images representative of arthritis severity Pilot study protocol: Healthy mice were primed with chick collagen in complete Freund’s adjuvant via intradermal injection in the base of the tail at week -3. On day 0, mice received a similar chick collagen boost. The same day, test ICOSL vIgDs were administered intraperitoneally and were continued 3x/week for 3 weeks. The study measures inflammation caused by the chick collagen injection and resulting immune response, and is a model of inflammation/swelling similar to human arthritis. PBS-treated control mouse Mouse treated with 1st Gen ICOSL vIgD™
Summary of Dual ICOS/CD28 Antagonist vIgD™ Program Individual ICOSL domains identified with increased affinity for ICOS & CD28 Minimal mutations (2-4) confer large increases in functional activity A single affinity maturation program results in lead candidates for multiple applications (oncology & autoimmune/inflammation) In vivo proof of concept data in GVHD, DTH, and CIA models Alpine plans to file IND 2H-2018 for autoimmune/inflammation
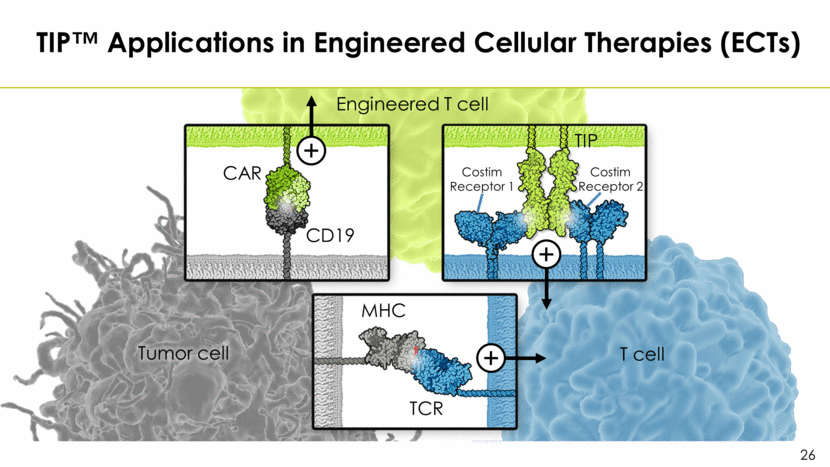
TIP™ Applications in Engineered Cellular Therapies (ECTs) Engineered T cell Tumor cell T cell CAR CD19 TIP Costim Receptor 1 TCR MHC Costim Receptor 2
Kite Pharma Collaboration Summary Kite Pharma licensed two TIPs™ for use in CAR-T, TCR, and TIL therapies $5.5 million up front cash plus research support Up to $530 million in additional research, clinical, and regulatory milestones Low single-digit royalties on sales Alpine retains rights to all vIgDs™ and vIgD applications to specified targets as well as all non-Kite targets for TIP™ programs Alpine to complete in vitro work with agreed-upon endpoints Kite to perform in vivo studies and is responsible for manufacturing and clinical trials

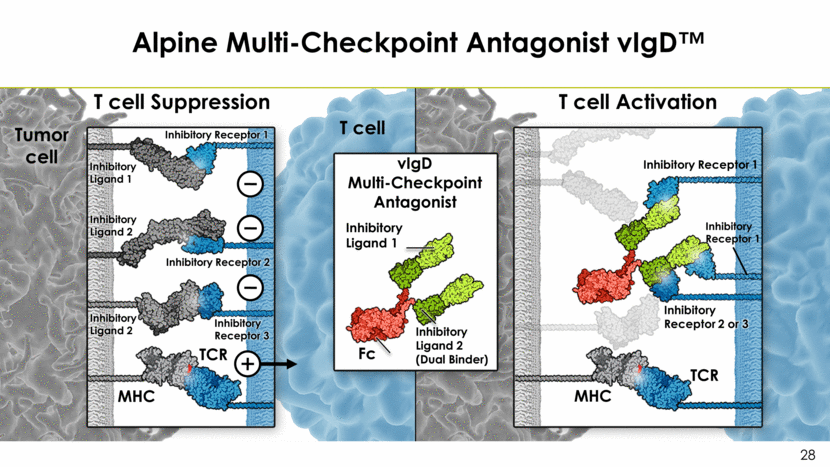
Alpine Multi-Checkpoint Antagonist vIgD™ T cell Suppression T cell Activation MHC Inhibitory Receptor 1 Inhibitory Ligand 1 TCR Inhibitory Ligand 2 Inhibitory Ligand 1 Fc vIgD Multi-Checkpoint Antagonist Inhibitory Receptor 2 or 3 MHC Inhibitory Receptor 1 TCR Tumor cell T cell Inhibitory Ligand 2 Inhibitory Receptor 2 Inhibitory Receptor 3 Inhibitory Receptor 1 Inhibitory Ligand 2 (Dual Binder)
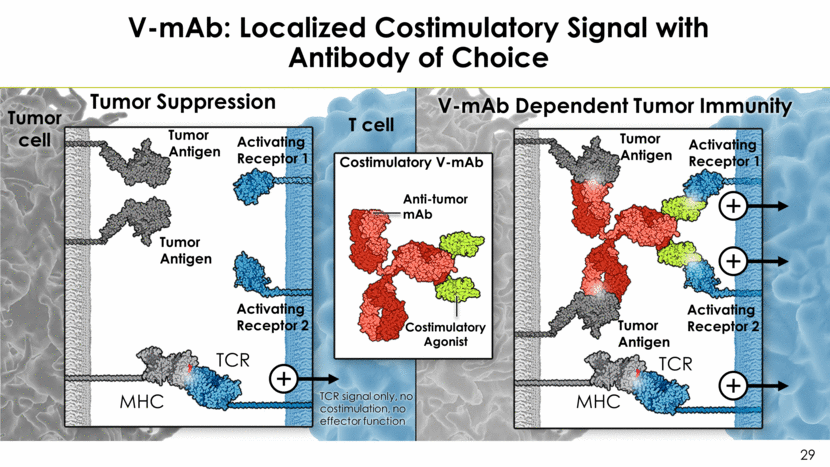
V-mAb: Localized Costimulatory Signal with Antibody of Choice Anti-tumor mAb Tumor Suppression V-mAb Dependent Tumor Immunity Costimulatory Agonist Costimulatory V-mAb Tumor cell T cell TCR MHC Activating Receptor 1 Tumor Antigen TCR MHC Tumor Antigen TCR signal only, no costimulation, no effector function Activating Receptor 2 Activating Receptor 1 Activating Receptor 2 Tumor Antigen Tumor Antigen
Summary of Alpine Immune Sciences Alpine Immune Sciences vIgD™ platform focused on affinity-enhanced IgSF immune targets Initial preclinical validation in immuno-oncology and inflammation Novel platform potentially capable of TREG depletion, TREG activation, and T cell inhibitory receptor agonists Broad range of targets creates potential partnering opportunities Kite Pharma collaboration supports TIP™ platform, with potential additional research, clinical, and regulatory milestone payments. Expect to have ~$90 million in cash & cash equivalents at closing of merger Balance sheet supports bringing three products into the clinic, with the first IND filing expected 2H-2018 with our dual ICOS/CD28 antagonist
ALPHINE IMMUNE SCIENCES COPYRIGHT © 2017 ALPHINE IMMUNE SCIENCES, INC. AND NIVALIS THERAPEUTICS, INC. ALL RIGHTS RESERVED.

