Attached files
| file | filename |
|---|---|
| EX-31.4 - EX-31.4 - Jazz Pharmaceuticals plc | d364332dex314.htm |
| EX-31.3 - EX-31.3 - Jazz Pharmaceuticals plc | d364332dex313.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K/A
(Amendment No. 1)
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2016
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-33500
JAZZ PHARMACEUTICALS PUBLIC LIMITED COMPANY
(Exact name of registrant as specified in its charter)
| Ireland | 98-1032470 | |||
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
Fifth Floor, Waterloo Exchange
Waterloo Road, Dublin 4, Ireland
011-353-1-634-7800
(Address, including zip code, and telephone number, including area code, of
registrant’s principal executive offices)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |||
| Ordinary shares, nominal value $0.0001 per share |
The NASDAQ Stock Market LLC | |||
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☒ | Accelerated filer ☐ | |||||
|
Non-accelerated filer ☐ (Do not check if a smaller reporting company) |
Smaller reporting company ☐ | |||||
|
Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, as of June 30, 2016, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $5,545,347,783 based upon the last sale price reported for the registrant’s ordinary shares on such date on the NASDAQ Global Select Market. The calculation of the aggregate market value of voting and non-voting common equity excludes 21,264,016 ordinary shares of the registrant held by executive officers, directors, and shareholders that the registrant concluded were affiliates of the registrant on that date. Exclusion of such shares should not be construed to indicate that any such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant or that such person is controlled by or under common control with the registrant.
As of April 18, 2017, a total of 60,016,634 ordinary shares, nominal value $0.0001 per share, of the registrant were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
Table of Contents
EXPLANATORY NOTE
The registrant is filing this Amendment No. 1 to Annual Report on Form 10-K/A, or this Amendment (also referred to herein as this report), to amend the Annual Report on Form 10-K for the fiscal year ended December 31, 2016 (Commission File Number 001-33500), or the 2016 Annual Report on Form 10-K, as filed by the registrant with the Securities and Exchange Commission, or the SEC, on February 28, 2017. The principal purpose of this Amendment is to include in Part III the information that was to be incorporated by reference from the proxy statement for the registrant’s 2017 Annual General Meeting of Shareholders, as well as to update certain of the information included on the cover page of the 2016 Annual Report on Form 10-K and in the list of exhibits included in Item 15 and the Exhibit Index of this report. This Amendment hereby amends the cover page, Part III, Items 10 through 14, and Part IV, Item 15 of the 2016 Annual Report on Form 10-K. In addition, as required by Rule 12b-15 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, new certifications by the registrant’s principal executive officer and principal financial officer are filed as exhibits to this Amendment.
No attempt has been made in this Amendment to modify or update the other disclosures presented in the 2016 Annual Report on Form 10-K. This Amendment does not reflect events occurring after the filing of the original report (i.e., those events occurring after February 28, 2017) or modify or update those disclosures that may be affected by subsequent events. Accordingly, this Amendment should be read in conjunction with the 2016 Annual Report on Form 10-K and the registrant’s other filings with the SEC.
Table of Contents
JAZZ PHARMACEUTICALS PLC
2016 ANNUAL REPORT ON FORM 10-K
Amendment No. 1
| Page | ||||
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are subject to the “safe harbor” created by those sections. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “predict,” “propose,” “intend,” “continue,” “potential,” “possible,” “foreseeable,” “likely,” “unforeseen” and similar expressions intended to identify forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance, time frames or achievements to be materially different from any future results, performance, time frames or achievements expressed or implied by the forward-looking statements. We discuss many of these risks, uncertainties and other factors in greater detail under the heading “Risk Factors” in Part I, Item 1A of our 2016 Annual Report on Form 10-K, as filed with the SEC on February 28, 2017. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this filing. You should read this report completely and with the understanding that our actual future results may be materially different from what we expect. We hereby qualify our forward-looking statements by our cautionary statements. Except as required by law, we assume no obligation to update our forward-looking statements publicly, or to update the reasons that actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
BASIS OF PRESENTATION
In this report, unless otherwise indicated or the context otherwise requires, all references to “Jazz Pharmaceuticals,” “the registrant,” “the company,” “we,” “us,” and “our” refer to Jazz Pharmaceuticals plc and its consolidated subsidiaries, except when the context makes clear that the time period being referenced is prior to January 18, 2012, in which case such terms are references to Jazz Pharmaceuticals, Inc. and its consolidated subsidiaries. On January 18, 2012, the businesses of Jazz Pharmaceuticals, Inc. and Azur Pharma Public Limited Company, or Azur Pharma, were combined in a merger transaction, or the Azur Merger, in connection with which Azur Pharma was re-named Jazz Pharmaceuticals plc, and we became the parent company of and successor to Jazz Pharmaceuticals, Inc., with Jazz Pharmaceuticals, Inc. becoming our wholly owned subsidiary. Jazz Pharmaceuticals, Inc. was treated as the acquiring company in the Azur Merger for accounting purposes, and as a result, the historical consolidated financial statements of Jazz Pharmaceuticals, Inc. became our consolidated financial statements. In addition, on June 12, 2012, we completed our acquisition of EUSA Pharma Inc., or the EUSA Acquisition. In January 2014, we completed our acquisition of a controlling interest in Gentium S.r.l., or Gentium, and in July 2016, we completed our acquisition of Celator Pharmaceuticals, Inc., or Celator, which we refer to as the Celator Acquisition.
1
Table of Contents
Item 10. Directors, Executive Officers and Corporate Governance
DIRECTORS AND EXECUTIVE OFFICERS
Our Board of Directors
Our board of directors is divided into three classes, designated Class I, Class II and Class III. The term of the Class I directors will expire on the date of our 2018 annual general meeting of shareholders; the term of the Class II directors will expire on the date of our 2019 annual general meeting of shareholders; and the term of the Class III directors will expire on the date of our 2017 annual general meeting of shareholders. At each annual general meeting of shareholders, successors to the directors whose terms expire at that annual general meeting are put forward for election for a three-year term.
The following is a brief biography of each member of our board of directors, including their respective ages as of April 25, 2017, with each biography including information regarding the specific experience, qualifications, attributes or skills that led the nominating and corporate governance committee and our board of directors to determine that each member of our board of directors should serve as a director.
Class I Directors Continuing in Office Until the 2018 Annual General Meeting
Peter Gray, age 62, has served as a member of our board of directors since May 2013 and was appointed as chairperson of our audit committee in April 2014. Mr. Gray currently serves as Chairman of the board of directors of UDG Healthcare plc, an international provider of healthcare services. He is also a Chairman of two privately-held companies providing outsourced services to the biopharma industry and chairs a non-profit educational establishment. In September 2011, Mr. Gray retired from his position as Chief Executive Officer of ICON plc, a global provider of outsourced development services to the pharmaceutical, biotechnology and medical device industries, which he held since November 2002. At ICON plc, Mr. Gray previously served as Group Chief Operating Officer from June 2001 to November 2002 and Chief Financial Officer from June 1997 to June 2001. From November 1983 to November 1989, Mr. Gray served as senior financial officer at Elan Corporation plc, a pharmaceutical company. Mr. Gray holds a degree in law from Trinity College Dublin and qualified as a chartered accountant in 1981. Based on his experience as Chief Executive Officer and Chief Financial Officer of ICON plc, Mr. Gray brings to our board of directors and audit committee over 20 years of experience in financial and operational management within the pharmaceutical industry.
Kenneth W. O’Keefe, age 50, has served as a member of our board of directors since the closing of the Azur Merger in January 2012 and was a director of Jazz Pharmaceuticals, Inc. from 2004 until the closing of the Azur Merger. Since November 2015, he has been Chief Executive Officer of Beecken Petty O’Keefe & Company, a private equity firm, which he co-founded. From January 2011 to November 2015, he was Managing Partner, and from 1997 to January 2011, he was Managing Director, of Beecken Petty O’Keefe & Company. He serves on the boards of several privately-held healthcare companies. He received a B.A. from Northwestern University and an M.B.A. from the University of Chicago. As a member of Beecken Petty O’Keefe & Company, Mr. O’Keefe brings to our board of directors significant expertise in accounting and financial matters and in analyzing and evaluating financial statements, as well as substantial experience managing private equity investments. He serves or has served on the audit committee of several companies in the healthcare industry. As the former chairperson of our audit committee, Mr. O’Keefe brings to our board of directors detailed knowledge of our financial position and financial statements.
Elmar Schnee, age 57, has served as a member of our board of directors since August 2014 and previously served as a director of Gentium (now a subsidiary of Jazz Pharmaceuticals plc) from May 2012 until April 2014. Mr. Schnee has served as Chief Operating Officer of MindMaze SA, a neuro-technology company, since June 2016. From November 2013 to August 2015, Mr. Schnee served as a non-executive director of Cardiorentis Ltd., a biopharmaceutical company, where he served as Chairman and Chief Executive Officer from October 2011 until
2
Table of Contents
November 2013. From 2003 to 2011, Mr. Schnee held various positions at Merck KGaA, a global pharmaceutical and chemical group. He joined Merck KGaA in 2003 as Managing Director of Merck Santé S.A.S. In January 2004, Mr. Schnee assumed responsibility for global operations of the ethical pharmaceuticals division of Merck KGaA, and in November 2005, Mr. Schnee was appointed as Deputy Member of the Executive Board responsible for the pharmaceuticals business. In 2006, he was appointed as a member of the Executive Board and General Partner of Merck KGaA, with responsibility for global pharmaceutical activities, and served in this position until 2011. Prior to Merck KGaA, Mr. Schnee held senior positions in strategy, business development and marketing at UCB SA, Sanofi-Synthélabo SA, Migliara/Kaplan Associates, Inc. and Fisons Pharmaceuticals PLC. In addition, Mr. Schnee currently serves on the boards of directors of two pharmaceutical companies, Stallergenes-Greer and Santhera Pharmaceuticals Holding AG, and four privately-held life sciences companies. Mr. Schnee holds both a bachelor’s degree in marketing and a master’s degree in marketing and general management from the Swiss Institute of Business Administration in Zurich. With his experience as Chairman and Chief Executive Officer of Cardiorentis Ltd., his operational experience at Merck KGaA and other companies and his experience serving on the boards of directors of life sciences companies, including Gentium, Mr. Schnee brings to our board of directors significant management expertise and industry knowledge.
Catherine A. Sohn, Pharm. D., age 64, has served as a member of our board of directors since July 2012 and was appointed as chairperson of our nominating and corporate governance committee in August 2013. Since January 2014, Dr. Sohn has served as an independent director on the board of directors of Neuralstem, Inc., a publicly-traded life sciences company, where she is currently serving as Chairperson of the nominating and corporate governance committee. Since November 2012, Dr. Sohn has also served as an independent director on the board of directors of Landec Corporation, a publicly-traded life sciences company, where she is currently serving as Chairperson of the compensation committee. From 1998 to 2010, she was Senior Vice President, Worldwide Business Development and Strategic Alliances at GlaxoSmithKline Consumer Healthcare. From 1994 to 1998, she was Vice President, Worldwide Strategic Product Development at SmithKline Beecham Pharmaceuticals plc in the pharmaceutical division. From 1982 to 1994, she held a series of positions in Medical Affairs, Pharmaceutical Business Development and U.S. Product Marketing at SmithKline Beecham Pharmaceuticals plc and its predecessor, Smith, Kline & French. Dr. Sohn holds the positions of Adjunct Professor at the University of California, San Francisco and Dean’s Professor at the University of the Sciences in Philadelphia. She received a Doctor of Pharmacy from the University of California, San Francisco, School of Pharmacy and a Certificate of Professional Development from the Wharton School at the University of Pennsylvania. Dr. Sohn was named Woman of the Year by the Healthcare Businesswomen’s Association (2003) and Distinguished Alumnus of the Year by the University of California, San Francisco (2000) and is a Certified Licensing Professional and a National Association of Corporate Directors (NACD) Board Leadership Fellow. Dr. Sohn brings to our board of directors three decades of product development, strategic marketing and business development transaction experience in the pharmaceutical industry and a global perspective that is directly relevant to our company.
Class II Directors Continuing in Office Until the 2019 Annual General Meeting
Paul L. Berns, age 50, has served as a member of our board of directors since the closing of the Azur Merger in January 2012 and was a director of Jazz Pharmaceuticals, Inc. from 2010 until the closing of the Azur Merger. Mr. Berns is a consultant to the pharmaceutical industry. From March 2014 to June 2016, he served as the Chief Executive Officer and President of Anacor Pharmaceuticals, Inc., a biopharmaceutical company, which was acquired by Pfizer Inc. in June 2016. He also served as a member of the board of directors of Anacor Pharmaceuticals, Inc. from 2012 until 2016, including as Chairman of its board of directors from 2013 until 2016. From September 2012 to March 2014, he was a self-employed consultant to the pharmaceutical industry. From March 2006 to September 2012, he served as President and Chief Executive Officer, and as a member of the board of directors, of Allos Therapeutics, Inc., a pharmaceutical company acquired by Spectrum Pharmaceuticals, Inc. From July 2005 to March 2006, Mr. Berns was a self-employed consultant to the pharmaceutical industry. From June 2002 to July 2005, Mr. Berns was President, Chief Executive Officer and a director of Bone Care International, Inc., a specialty pharmaceutical company that was acquired by Genzyme Corporation in 2005. From 2001 to 2002, Mr. Berns served as Vice President and General Manager of the Immunology, Oncology and Pain Therapeutics
3
Table of Contents
business unit of Abbott Laboratories, a pharmaceutical company. From 2000 to 2001, he served as Vice President, Marketing of BASF Pharmaceuticals/Knoll, a pharmaceutical company, and from 1990 to 2000, Mr. Berns held various positions, including senior management roles, at Bristol-Myers Squibb Company, a pharmaceutical company. Mr. Berns previously served on the boards of directors of Cellectar Biosciences, Inc. (formerly Novelos Therapeutics, Inc.) from November 2013 to June 2016 and XenoPort, Inc. from 2005 to May 2016. Mr. Berns received a B.S. in Economics from the University of Wisconsin. With his experience as Chief Executive Officer of Allos Therapeutics, Inc., Anacor Pharmaceuticals, Inc. and Bone Care International Inc., and his experience serving on the boards of directors of public companies, Mr. Berns provides significant management expertise and industry knowledge to our board of directors.
Patrick G. Enright, age 55, has served as a member of our board of directors since the closing of the Azur Merger in January 2012 and was a director of Jazz Pharmaceuticals, Inc. from 2009 until the closing of the Azur Merger. Since 2006, Mr. Enright has served as a Managing Director of Longitude Capital, a venture capital firm, of which he is a founder. From 2002 through 2006, Mr. Enright was a Managing Director of Pequot Ventures, a venture capital investment firm, where he co-led the life sciences investment practice. He currently serves on the boards of directors of Aimmune Therapeutics, Inc., a biopharmaceutical company, Corcept Therapeutics Incorporated, a pharmaceutical company, and several privately-held companies. Previously, Mr. Enright served on the board of directors of Esperion Therapeutics, Inc., a pharmaceutical company, from April 2013 to June 2016. Mr. Enright received a B.S. from Stanford University and an M.B.A. from the Wharton School at the University of Pennsylvania. Based on his experience as a venture capital investor focused on life sciences companies and his past work in the pharmaceutical industry, Mr. Enright brings to our board of directors over 25 years of operating experience and financial expertise in the life sciences industry.
Seamus Mulligan, age 56, has served as a member of our board of directors since the closing of the Azur Merger in January 2012 and was a founder and principal investor of Azur Pharma. Since 2014, Mr. Mulligan has served as Chairman and Chief Executive Officer of Adapt Pharma Ltd., a specialty pharmaceutical company, and since 2006, Mr. Mulligan has also served as Executive Chairman of Circ Pharma Limited and its subsidiaries, a pharmaceutical development stage group. Mr. Mulligan served as our Chief Business Officer, International Business Development from the closing of the Azur Merger until February 2013. Mr. Mulligan served as Azur Pharma’s Chairman and Chief Executive Officer and as a member of its board of directors from 2005 until the closing of the Azur Merger. From 1984 until 2004, he held various positions with Elan Corporation, plc, a pharmaceutical company, most recently as Executive Vice President, Business and Corporate Development, and prior to that position, held the roles of President of Elan Pharmaceutical Technologies, the drug delivery division of Elan Corporation, plc, Executive Vice President, Pharmaceutical Operations, Vice President, U.S. Operations and Vice President, Product Development. He served as a member of the board of directors of the U.S. National Pharmaceutical Council until 2004. Mr. Mulligan received a B.Sc. (Pharm) and M.Sc. from Trinity College Dublin. As a founder of Azur Pharma and a pharmaceutical industry executive, Mr. Mulligan brings to our board of directors an expertise in business development and over 30 years of experience in the pharmaceutical industry.
Norbert G. Riedel, Ph.D., age 59, has served as a member of our board of directors since May 2013 and was appointed chairperson of our compensation committee in August 2013. Since September 2015, Dr. Riedel has served as Chief Executive Officer and President of Aptinyx, Inc., a biopharmaceutical company spun out of its predecessor company, Naurex, Inc., where Dr. Riedel served as Chief Executive Officer and President from January 2014 to September 2015. From 2001 to January 2013, he served as Corporate Vice President and Chief Scientific Officer of Baxter International Inc., a diversified healthcare company, where from 1998 to 2001, he also served as President and General Manager of the recombinant therapeutic proteins business unit and Vice President of Research and Development of the bioscience business unit. From 1996 to 1998, Dr. Riedel served as head of worldwide biotechnology and worldwide core research functions at Hoechst-Marion Roussel, now Sanofi, a global pharmaceutical company. Dr. Riedel served on the board of directors of Ariad Pharmaceuticals, Inc., an oncology company, from May 2011 until the company was acquired in February 2017. Dr. Riedel serves on the board of directors of the Illinois Biotechnology Industry Organization. Dr. Riedel is also a member of the Austrian Academy of Sciences. Dr. Riedel is an Adjunct Professor at Boston University School of Medicine and an Adjunct Professor
4
Table of Contents
of Medicine at Northwestern University’s Feinberg School of Medicine. Dr. Riedel holds a Diploma in biochemistry and a Ph.D. in biochemistry from the University of Frankfurt. Dr. Riedel brings significant scientific, drug discovery and development, and commercial expertise to our board of directors with over 20 years of experience in the biotechnology and pharmaceutical industries.
Class III Directors Continuing in Office Until the 2017 Annual General Meeting
Bruce C. Cozadd, age 53, has served as our Chairman and Chief Executive Officer since the closing of the Azur Merger in January 2012. He co-founded Jazz Pharmaceuticals, Inc. and has served (and continues to serve) as Chairman and Chief Executive Officer of Jazz Pharmaceuticals, Inc. since April 2009. From 2003 until 2009, he served as Jazz Pharmaceuticals, Inc.’s Executive Chairman and as a member of its board of directors. From 1991 until 2001, he held various positions with ALZA Corporation, a pharmaceutical company acquired by Johnson & Johnson, most recently as Executive Vice President and Chief Operating Officer, with responsibility for research and development, manufacturing and sales and marketing. Previously at ALZA Corporation, he held the roles of Chief Financial Officer and Vice President, Corporate Planning and Analysis. He serves on the boards of directors and compensation committees of Cerus Corporation, a biomedical products company, and Threshold Pharmaceuticals, Inc., a clinical stage biopharmaceutical company, and on the boards of The Nueva School, a non-profit organization, and SFJAZZ, a non-profit organization. He received a B.S. from Yale University and an M.B.A. from the Stanford Graduate School of Business. As our Chief Executive Officer, he brings to our board of directors a detailed knowledge of our business.
Heather Ann McSharry, age 55, has served as a member of our board of directors since May 2013. Ms. McSharry currently serves as a non-executive director on the boards of directors of several public and private companies, including CRH plc, an international building materials group, and Greencore Group plc, an international manufacturer of convenience foods, where she also serves as Chair of its remuneration committee. From 2006 to 2009, Ms. McSharry was Managing Director Ireland of Reckitt Benckiser, a multinational health, home and hygiene consumer products company. From 1989 to 2006, she held various positions at Boots Healthcare, a leading global consumer healthcare company, most recently as Managing Director of Boots Healthcare Ireland Limited. From 2007 to 2011, Ms. McSharry served on the board of directors of the Bank of Ireland, where she was a member of its audit committee from 2009 to 2011. Ms. McSharry served on the board of the Industrial Development Agency in Ireland from 2010 to 2014, where she was Chair of the audit and finance committee. Ms. McSharry holds a Bachelor of Commerce and a Master of Business Studies degree from University College Dublin. Ms. McSharry brings to our board of directors almost 30 years of experience in multiple international industries, including healthcare, consumer goods and financial services.
Rick E Winningham, age 57, has served as a member of our board of directors since the closing of the Azur Merger in January 2012 and was a director of Jazz Pharmaceuticals, Inc. from 2010 until the closing of the Azur Merger. In May 2014, Mr. Winningham was appointed as Lead Independent Director of our board of directors. Mr. Winningham has served as Chairman of the board of directors of Theravance Biopharma, Inc., a biopharmaceutical company, since July 2013. He has served as Chief Executive Officer of Theravance Biopharma, Inc. since its spin-off from Innoviva, Inc. in June 2014. From October 2001 to August 2014, Mr. Winningham served as Chief Executive Officer of Innoviva, Inc., where he also served as Chairman of the Board of Directors from April 2010 to October 2014. From 1997 to 2001, he served as President of Bristol-Myers Squibb Oncology/Immunology/Oncology Therapeutics Network and, from 2000 to 2001, as President of Global Marketing. Mr. Winningham is a member of Biotechnology Industry Organization’s board of directors and serves on the Health Section Governing Board Standing Committee on Reimbursement. He served as a member of the board of directors of the California Healthcare Institute, or CHI, from November 2011 to March 2015 and served as its Chairman from January 2014 until CHI merged with Bay Area Bioscience Association to become the California Life Sciences Association, or CLSA, in March 2015. Mr. Winningham was Chairman of CLSA from March 2015 until November 2015. Mr. Winningham is also a member of the board of directors of OncoMed Pharmaceuticals, Inc., a clinical stage biotechnology company. Mr. Winningham holds an M.B.A. from Texas Christian University and a B.S. from Southern Illinois University. Mr. Winningham’s experience in senior management positions in the
5
Table of Contents
pharmaceutical industry provides significant industry knowledge and operational and management expertise to our board of directors.
Committee Membership
The following table provides membership information for 2016 for each of the audit committee, compensation committee and nominating and corporate governance committee of our board of directors:
| Name | Audit | Compensation |
Nominating and Corporate Governance | |||
| Paul L. Berns |
M | |||||
| Patrick G. Enright |
M | |||||
| Peter Gray |
C | |||||
| Heather Ann McSharry |
M | M | ||||
| Kenneth W. O’Keefe |
M | |||||
| Norbert G. Riedel, Ph.D. |
C | |||||
| Elmar Schnee |
M | |||||
| Catherine A. Sohn, Pharm. D. |
C | |||||
| Rick E Winningham |
M |
C = committee chairperson; M = committee member
Our Executive Officers
The following table provides information regarding our executive officers as of April 25, 2017.
| Name | Age | Position | ||||||
| Bruce C. Cozadd |
53 | Chairman and Chief Executive Officer | ||||||
| Russell J. Cox |
53 | Executive Vice President and Chief Operating Officer | ||||||
| Suzanne Sawochka Hooper |
51 | Executive Vice President and General Counsel | ||||||
| Matthew P. Young |
47 | Executive Vice President and Chief Financial Officer | ||||||
| Iain McGill |
44 | Senior Vice President, Jazz Pharmaceuticals Europe and Rest of World | ||||||
| Michael P. Miller |
60 | Senior Vice President, U.S. Commercial | ||||||
| Karen Smith, M.D., Ph.D. |
49 | Global Head of Research & Development and Chief Medical Officer | ||||||
| Paul Treacy |
56 | Senior Vice President, Technical Operations | ||||||
| Karen J. Wilson |
53 | Senior Vice President, Finance and Principal Accounting Officer | ||||||
Bruce C. Cozadd. Biographical information regarding Mr. Cozadd is set forth above under “Our Board of Directors.”
Russell J. Cox was appointed our Executive Vice President and Chief Operating Officer as of May 2014 and served as our Executive Vice President and Chief Commercial Officer from March 2012 until May 2014 and our Senior Vice President, Sales and Marketing from the closing of the Azur Merger in January 2012 until March 2012. Prior to the closing of the Azur Merger, he served in a variety of senior management roles since joining Jazz Pharmaceuticals, Inc. in 2010. From January 2009 to January 2010, he was Senior Vice President and Chief Commercial Officer of Ipsen Group, a pharmaceutical company, and from 2007 until December 2008, he was Vice President of Marketing at Tercica, Inc. (acquired by Ipsen Group), a biotechnology company. From 2003 to 2007, he was with Scios Inc. (acquired by Johnson & Johnson in 2003), where he also held the role of Vice President, Marketing. Prior to 2003, Mr. Cox was with Genentech, Inc. for 12 years, where he was a Product Team Leader responsible for the Growth Hormone franchise and led numerous product launches as a Group Product Manager. In 2015, Mr. Cox joined the board of directors of Aeglea BioTherapeutics, Inc., a biotechnology company. Mr. Cox received a B.S. in Biomedical Science from Texas A&M University.
6
Table of Contents
Suzanne Sawochka Hooper was appointed our Executive Vice President and General Counsel as of March 2012. From 1999 through early 2012, she was a partner in the law firm Cooley LLP. Ms. Hooper served for several years as a member of Cooley’s Management Committee and as Vice Chair of the firm’s Business Department. While at Cooley, Ms. Hooper practiced corporate and securities law, primarily with companies and investors in the life sciences industry. Ms. Hooper received a J.D. from the University of California, Berkeley, Boalt Hall School of Law and a B.A. in Political Science from the University of California, Santa Barbara. Ms. Hooper is a member of the State Bar of California.
Matthew P. Young was appointed our Executive Vice President and Chief Financial Officer as of February 2015 and previously served as our Senior Vice President and Chief Financial Officer from March 2014 to February 2015 and as our Senior Vice President, Corporate Development from April 2013 to March 2014. Prior to joining us, Mr. Young worked in investment banking for approximately 20 years. From February 2009 to April 2013, Mr. Young served as a managing director in global healthcare of Barclays Capital Inc., an investment banking firm, where his role included acting as the co-head of life sciences at Barclays Capital. From 2007 to 2008, Mr. Young served as a managing director of Citigroup Global Markets Inc., an investment banking firm, and from 2003 to 2007, as a managing director of Lehman Brothers Inc., an investment banking firm. From 1992 to 2003, Mr. Young served in various capacities at other investment banking firms. In 2015, he joined the board of directors of PRA Health Sciences, Inc., a contract research company, where he currently serves on the compensation and audit committees. He is also a member of the board of directors and Chairman of the audit committee of CytomX Therapeutics, Inc., a biopharmaceutical company. Mr. Young received a B.S. in Economics and an M.B.A. from the Wharton School of the University of Pennsylvania.
Iain McGill was appointed our Senior Vice President, Jazz Pharmaceuticals Europe and Rest of World as of March 2015. He served as Head of EUSA International and Senior Vice President, Jazz Pharmaceuticals from March 2014 to March 2015 and our Chief Commercial Officer, EUSA Pharma, from June 2012, when he joined Jazz Pharmaceuticals in connection with the EUSA Acquisition. From October 2011 until he joined Jazz Pharmaceuticals, Mr. McGill served as Chief Commercial Officer at EUSA Pharma (Europe) Ltd., where he previously served from August 2010 to September 2011 as President Europe, International & Global Marketing and from January 2010 to July 2010 as President of Europe. From 2006 to 2009, Mr. McGill served as Vice President and Global Business Manager at Wyeth, a pharmaceutical company acquired by Pfizer Inc. In 2016, he joined the board of directors and the audit committee of Otonomy Inc., a biopharmaceutical company. Mr. McGill began his pharmaceutical career in sales and over 20 years, held various positions in sales management, market research, marketing, business development and general management at Syntex Corporation (acquired by Roche Holding Ltd.), Roche Holding Ltd. and Novartis AG. Mr. McGill received a B.Sc in Biochemistry from the University of London.
Michael P. Miller was appointed our Senior Vice President, U.S. Commercial as of April 2014. From April 2010 to January 2014, Mr. Miller was Senior Vice President and Chief Commercial Officer of Vivus, Inc., a biopharmaceutical company. From February 2006 to April 2010, Mr. Miller served as Vice President, Sales and Marketing, leading the HER Family Oncology Franchise, of Genentech, Inc., a biotechnology company and wholly owned subsidiary of Roche Holding Ltd. From January 2003 to December 2005, Mr. Miller served as the Senior Vice President, Chief Commercial Officer of Connetics Corporation, a specialty pharmaceutical company acquired by Stiefel Laboratories, Inc. Previously, from 1997 to 2001, he served as Vice President of the Urology Business Unit of ALZA Corporation, a pharmaceutical company acquired by Johnson & Johnson. Prior to 1997, Mr. Miller served 13 years in various sales and marketing positions at Syntex Corporation, a pharmaceutical company acquired by Roche Holding Ltd. Mr. Miller received a B.S. in Business Administration and Finance from the University of San Francisco and an M.B.A. in Information and Computer Systems from San Francisco State University.
Karen Smith, M.D., Ph.D., was appointed our Global Head of Research and Development and Chief Medical Officer as of April 2015. From January 2011 to March 2015, she was Senior Vice President, Global Medical Affairs and Global Therapeutic Area Head (Dermatology) for Allergan, Inc., a multi-specialty health care
7
Table of Contents
company. From October 2007 to December 2010, Dr. Smith served initially as Vice President, External Medical Relations and then Vice President, Global Development at AstraZeneca LP, a global innovation-driven biopharmaceutical company. From 2002 to 2007, Dr. Smith held a variety of management and medical roles with Bristol-Myers Squibb Company, a global biopharmaceutical company, in Australia, Canada, and the United States, most recently as the Head of U.S. Clinical Operations. In 2001, Dr. Smith was the Chief Executive Officer of Boron Molecular, a specialist fine chemicals manufacturing company. Dr. Smith is also a member of the board of directors of Forward Pharma A/S, a biotechnology company, and serves on the Women’s Advisory Board for Ironman Corporation. Dr. Smith holds a B.A.Sc. and a B.Sc. from the Curtin University of Technology, an M.D. from the University of Warwick, a Ph.D. in oncology molecular genetics from the University of Western Australia, an M.B.A. from the University of New England (Australia) and an L.L.M. in medical law from the University of Salford.
Paul Treacy was appointed our Senior Vice President, Technical Operations in July 2014. From April 2010 to May 2013, he was Head of CMC, Supply Chain and Manufacturing at Janssen Alzheimer Immunotherapy Research & Development, LLC, a biotechnology company and a subsidiary of Johnson & Johnson. From August 2005 to April 2010, he served as General Manager of Janssen Biologics Ireland, a biopharmaceutical company and a subsidiary of Johnson & Johnson. From August 2002 to August 2005, Mr. Treacy was Vice President, Manufacturing Operations at Centocor Inc., a subsidiary of Johnson & Johnson, and from February 1999 to August 2002, he served as Executive Director, Operations, at Centocor BV. Mr. Treacy received a B.S. and an M.S. in Microbiology and a Higher Diploma in Computer Science from University College Cork and a Higher Diploma in Pharmaceutical Manufacturing Technology from Trinity College Dublin.
Karen J. Wilson was appointed our Senior Vice President, Finance and Principal Accounting Officer as of February 2013 and served as our Vice President, Finance and Principal Accounting Officer from the closing of the Azur Merger in January 2012 until February 2013. Prior to the Azur Merger, she served as Jazz Pharmaceuticals, Inc.’s Vice President, Finance beginning in February 2011 and was appointed Principal Accounting Officer in March 2011. From 2009 to January 2011, Ms. Wilson served as Vice President of Finance and Principal Accounting Officer at PDL BioPharma, Inc., a biotechnology company. From 2005 to 2009, she served as a principal at the consulting firm Wilson Crisler LLC. Prior to that, from 2001 to 2004, she was Chief Financial Officer of ViroLogic, Inc., a biosciences company. Prior to joining ViroLogic, Ms. Wilson served as Chief Financial Officer and Vice President of Operations for Novare Surgical Systems, Inc. from 1999 to 2001. Prior to 1999, Ms. Wilson worked for Deloitte & Touche LLP for ten years, serving clients in both the medical and technology fields. Ms. Wilson is a Certified Public Accountant in the State of California and received a B.S. in Business from the University of California, Berkeley.
8
Table of Contents
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Exchange Act requires our directors and executive officers, and persons who own more than ten percent of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of our ordinary shares and other equity securities. Such persons are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us and written representations that no other reports were required, during the fiscal year ended December 31, 2016, we believe that all Section 16(a) filing requirements applicable to our executive officers, directors and greater than ten percent beneficial owners were complied with, except for one Form 4 of our executive officer Mr. Miller, which was inadvertently filed one day late on January 21, 2016, reporting the sale of shares of the company’s ordinary shares on January 15, 2016 pursuant to a Rule 10b5-1 trading plan adopted by Mr. Miller.
9
Table of Contents
CERTAIN CORPORATE GOVERNANCE MATTERS
Audit Committee
We have a standing audit committee that is currently composed of three directors (Mr. Gray, Ms. McSharry and Mr. O’Keefe). Our board of directors has determined that each of Mr. Gray, Ms. McSharry and Mr. O’Keefe meets the independence requirements of Rule 10A-3 of the Exchange Act and the listing standards of the NASDAQ Stock Market LLC, or the NASDAQ, with respect to audit committee members. Our board of directors has also determined that each of Mr. Gray, Ms. McSharry and Mr. O’Keefe qualifies as an “audit committee financial expert” within the meaning of SEC regulations. In making this determination, our board of directors considered the overall knowledge, experience and familiarity of each with accounting matters, analyzing and evaluating financial statements, and, in the case of Mr. O’Keefe, managing private equity investments. Mr. Gray serves as chairperson of the audit committee.
Code of Conduct
Our code of conduct applies to all of our employees, directors and officers, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, and those of our subsidiaries. The code of conduct is available on our website at www.jazzpharmaceuticals.com under the section entitled “About” under “Corporate Ethics.” We intend to satisfy the disclosure requirements under Item 5.05 of Form 8-K regarding an amendment to, or waiver from, a provision of our code of conduct by posting such information on our website at the website address and location specified above.
Director Nominations
No material changes have been made to the procedures by which shareholders may recommend nominees to our board of directors.
10
Table of Contents
Item 11. Executive Compensation
EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
The following Compensation Discussion and Analysis describes the material elements of compensation for the following individuals who served as our principal executive officer, principal financial officer and three other most highly compensated executive officers as of December 31, 2016. These individuals are our named executive officers, or NEOs, for 2016.
| Bruce C. Cozadd | Chairman and Chief Executive Officer (CEO) | |
| Matthew P. Young | Executive Vice President and Chief Financial Officer (CFO) | |
| Russell J. Cox | Executive Vice President and Chief Operating Officer (COO) | |
| Suzanne Sawochka Hooper | Executive Vice President and General Counsel (GC) | |
| Karen Smith, M.D., Ph.D. | Global Head of Research and Development and Chief Medical Officer (CMO) |
Table of Contents to Compensation Discussion and Analysis
| 12 | ||||
| 12 | ||||
| 13 | ||||
| 14 | ||||
| 14 | ||||
| 14 | ||||
| 14 | ||||
| 15 | ||||
| Competitive Assessment of Compensation – Peer Companies and Market Data |
16 | |||
| 18 | ||||
| 2016 Advisory Vote on Executive Compensation and Shareholder Engagement |
19 | |||
| Key Components and Design of the Executive Compensation Program |
20 | |||
| 20 | ||||
| 21 | ||||
| 23 | ||||
| 23 | ||||
| 26 | ||||
| 2016 Compensation Decisions for Our Named Executive Officers |
27 | |||
| 27 | ||||
| 27 | ||||
| 28 | ||||
| 34 | ||||
| 34 | ||||
| 34 | ||||
| 35 | ||||
| 36 | ||||
| Risk Assessment Concerning Compensation Practices and Policies |
36 | |||
11
Table of Contents
Our compensation policies and elements are intended to provide the necessary incentives to properly align our executive officers’ performance with the interests of our shareholders while maintaining equitable and competitive executive compensation practices that enable us to attract and retain the highest caliber of executive officers.
In 2016, we delivered solid growth for two of our key products, Xyrem and Defitelio, while completing multiple corporate development transactions and advancing and expanding our product development pipeline. We continued to invest in research and development activities, which included clinical development of new product candidates, activities related to line extensions and new indications for existing products and the generation of additional clinical data for existing products, all in our sleep and hematology/oncology therapeutic areas.
|
|
|
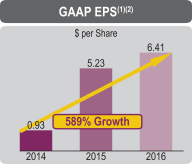
| (1) | GAAP net income and GAAP net income per diluted share (GAAP EPS) for the 2014 and 2015 periods are attributable to Jazz Pharmaceuticals plc. |
| (2) | For 2014, GAAP net income included, and GAAP EPS reflects, acquired in-process research and development costs of $202.6 million, primarily for the acquisition of rights to JZP-110 and rights to defibrotide in the Americas. |
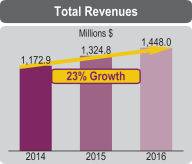
| Financial | • | 2016 total revenues of $1,488.0 million increased approximately 12% over 2015 | ||
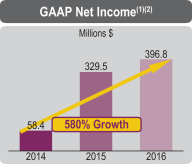
| • | 2016 GAAP net income of $396.8 million compared to $329.5 million in 2015 | |||
| Xyrem | • | 2016 net sales of Xyrem of $1,107.6 million increased approximately 16% over 2015 | ||
| • | U.S. Food and Drug Administration, or FDA, approval of our manufacturing facility in Athlone, Ireland, and Xyrem shipments to the U.S. | |||
| Defitelio/defibrotide | • | 2016 net sales of Defitelio of $109.0 million increased approximately 54% over 2015 | ||
| • | FDA approval of Defitelio new drug application, or NDA, followed by U.S. launch | |||
| Clinical/Regulatory | • | Patient enrollment completed in three Phase 3 studies evaluating JZP-110, a late-stage investigational compound being developed for potential treatment of excessive sleepiness in patients with obstructive sleep apnea, or OSA, and patients with narcolepsy (with the announcement of positive efficacy results and preliminary safety findings in the two JZP-110 Phase 3 studies in patients with OSA occurring in the first quarter of 2017) | ||
| • | Rolling NDA submission initiated for Vyxeos (CPX-351) for acute myeloid leukemia (which submission was completed in the first quarter of 2017) | |||
| • | Patient enrollment completed in Phase 3 study of Xyrem in pediatric narcolepsy patients with cataplexy (with the announcement of positive top-line efficacy results in the second quarter of 2017) | |||
| • | Clinical sites activated in Phase 3 study of defibrotide for prevention of hepatic veno-occlusive disease, or VOD, in high-risk patients following hematopoietic stem cell transplantation (with the first patient enrolled in the first quarter of 2017) | |||
| • | Announced development of two late-stage oxybate product candidates, JZP-507 and JZP-258, which have the potential to offer clinically meaningful benefits to patients compared to Xyrem (with the first patient enrolled in the JZP-258 Phase 3 study in the first quarter of 2017) | |||
| Corporate Development |
• | In July 2016, completed the Celator Acquisition, which broadened our hematology/oncology portfolio with the acquisition of worldwide development and commercialization rights to Vyxeos and the CombiPlex technology platform | ||
| • | Investment in Arrivo Bioventures LLC, or Arrivo, for the opportunity to access potential innovative products and product candidates | |||
| • | Agreement with Pfenex, Inc., or Pfenex, for worldwide rights to develop and commercialize multiple early-stage hematology product candidates | |||
12
Table of Contents
Key Features of Our Executive Compensation Program
| What We Do | What We Don’t Do | |||||
| ☑ | Design executive compensation to align pay with performance
|
☒ | No excessive change in control or severance payments
| |||
| ☑ | Balance short-term and long-term incentive compensation, with the majority of executive compensation being “at-risk”
|
☒ | No “single-trigger” cash or equity change in control benefits | |||
| ☑ | Use same performance bonus plan for all non-sales employees, including executives; base CEO’s bonus 100% on pre-established corporate performance goals
|
☒ | No repricing of underwater stock options without prior shareholder approval | |||
| ☑ | Establish maximum payout amount under performance bonus plan and require threshold level of achievement for payout with respect to financial metrics
|
☒ | No excessive perquisites | |||
| ☑ | Maintain share ownership guidelines
|
☒ | No tax gross ups on severance or change in control benefits
| |||
| ☑ | Provide “double-trigger” change in control benefits
|
☒ | No post-termination retirement or pension benefits that are not available to employees generally
| |||
| ☑ | Prohibit hedging and pledging by executive officers and directors
|
☒ | No guaranteed bonuses or base salary increases
| |||
| ☑ | Have 100% independent directors on the compensation committee
|
|||||
| ☑ | Hire independent compensation consultant who reports directly to the compensation committee |
13
Table of Contents
2016 Pay-for-Performance Overview
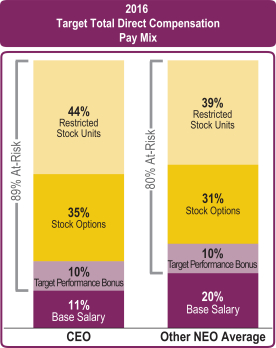
A significant portion of target total direct compensation for our CEO and other NEOs is structured in the form of “at-risk” compensation, consisting of annual performance bonus and equity incentive awards, with the performance bonus payouts and equity award values dependent upon our company performance. This aligns our executives’ interests with those of our shareholders for near- and long-term performance. Target total direct compensation for 2016, as shown below, reflects annual base salary paid, annual target performance bonus and the grant date fair value of equity awards granted during the year as reported in the Summary Compensation Table.
Compensation Philosophy and Objectives
Our executive compensation program is designed with the following objectives and philosophy:
| • | Attract, incentivize, reward and retain talented individuals with relevant experience in the life sciences industry through a competitive pay structure. We reward individuals fairly over time and seek to retain those individuals who continue to meet our high expectations. |
| • | Deliver balanced total compensation package to accomplish our business objectives and mission. Our executive compensation program focuses on total compensation, combining short- and long-term components, cash and equity, and fixed and contingent payments, in the proportions that we believe are the most appropriate to incentivize and reward our executive officers for achieving our corporate goals while minimizing incentives for excessive risk taking or unethical conduct. |
| • | Align pay with our performance. Our annual bonus awards are not earned unless pre-determined levels of performance are achieved against annual corporate objectives approved by our board of directors at the beginning of the year. Likewise, our stock option awards will not provide realizable value and our restricted stock unit, or RSU, awards will not provide increased value unless there is an increase in the value of our shares, which benefits all shareholders. We also have executive share ownership guidelines to further support our ownership culture and align the interests of executive officers and shareholders. |
How We Determine Executive Compensation
Role of Our Compensation Committee and Executive Officers
The compensation committee is (and was at all times during 2016) composed entirely of independent directors, as defined by Rule 5605(a)(2) of the NASDAQ listing standards. Our compensation committee meets as often as it determines necessary to carry out its duties and responsibilities through regularly scheduled meetings and, if necessary, special meetings. Our compensation committee also has the authority to take certain actions by written consent of all members. The agenda for each compensation committee meeting is usually developed by members of
14
Table of Contents
our human resources department and our CEO, with input from members of our legal department, and is reviewed with the chairperson of the compensation committee. In 2016, the compensation committee met five times and did not act by unanimous written consent. As of the date of this report, in 2017, the compensation committee has met twice and has not acted by unanimous written consent.
The compensation committee reviews and oversees our compensation policies, plans and programs and reviews and generally determines the compensation to be paid to the executive officers, including the NEOs. Either the compensation committee or the independent members of our board of directors, upon recommendation from the compensation committee, approve certain compensation of our CEO, and references in this Compensation Discussion and Analysis to our board of directors approving our CEO’s compensation refer to the independent members of our board of directors. The compensation committee does not delegate any of its functions to others in determining executive compensation.
In making executive compensation determinations, the compensation committee considers recommendations from our CEO. In making his recommendations, our CEO receives input from our human resources department and from the individuals who manage or report directly to the other executive officers, and he reviews various third party compensation surveys and compensation data provided by the independent compensation consultant to the compensation committee, as described below. While our CEO discusses his recommendations for the other executive officers with the compensation committee, he does not participate in the deliberations and recommendations to our board of directors concerning, or our board of directors’ determination of, his own compensation. Members of our human resources and legal departments also attend compensation committee meetings.
Below are the highlights of the annual cycle our compensation committee follows in reviewing and making decisions with respect to our executive compensation program.

Role of the Independent Compensation Consultant
The compensation committee engages an independent compensation consultant each year to provide a competitive compensation assessment with respect to the executive officers to assist the compensation committee in making annual compensation decisions. Since 2010, Radford, an Aon Hewitt Company and a subsidiary of Aon plc, has been engaged by the compensation committee each year to provide peer company and industry compensation data and provide the compensation committee with advice regarding executive officers’ compensation, including base salaries, performance-based bonuses and long-term equity compensation, and similar advice regarding non-executive directors’ compensation. The compensation committee has also consulted with Radford to update the peer company and industry compensation data on an annual basis and as needed with respect to specific questions that arise and on an advisory basis with respect to addressing other responsibilities arising under the compensation committee charter, including trends and best practices regarding executive compensation and compensation committees, in order to help inform the compensation committee’s decisions. Radford reports directly to the compensation committee, which maintains the authority to direct Radford’s work and engagement, and advises the compensation committee and our human resources department on projects from time to time. Radford interacts with management to gain access to company information that is required to perform services and to understand the culture and policies of the organization. Radford attends compensation committee meetings, and the compensation committee and Radford meet in executive session with no members of management present, as needed, to address various compensation matters, including deliberations regarding our CEO’s compensation.
15
Table of Contents
In assessing Radford’s independence from management in providing executive compensation services to the compensation committee, the compensation committee considered that Radford is only engaged by, takes direction from, and reports to, the compensation committee for such services and, accordingly, only the compensation committee has the right to terminate or replace Radford as its compensation consultant at any time. The compensation committee also analyzed whether the work of Radford as a compensation consultant with respect to executive and director compensation raised any conflict of interest, taking into consideration the following factors:
| ✓ | the provision of other services to our company by Radford and its affiliates; | ✓ | any business or personal relationship of the individual compensation advisors with any compensation committee member; | |||||
|
✓ |
the amount of fees we paid to Radford and its affiliates as a percentage of Radford’s total revenue; |
✓ |
Radford’s policies and procedures that are designed to prevent conflicts of interest; and | |||||
|
✓ |
any business or personal relationship of Radford or the individual compensation advisors employed by it with any executive officer of our company; |
✓ |
any ordinary shares of our company owned by Radford or the individual compensation advisors employed by it. |
The compensation committee has determined, based on its analysis of the above factors, that the work of Radford and the individual compensation advisors employed by Radford as compensation consultants to our company has not created any conflict of interest.
Competitive Assessment of Compensation – Peer Companies and Market Data
Because we aim to attract and retain the most highly qualified executive officers in an extremely competitive market, the compensation committee believes that it is important when making its compensation decisions to be informed as to the current practices of comparable public companies with which we compete for top talent. To this end, the compensation committee reviews market data for each executive officer’s position, compiled by Radford as described below, including information relating to the mix and levels of compensation for executive officers in the life sciences industry, with a focus on target total direct compensation in line with the compensation committee’s holistic approach to executive compensation.
2016 Peer Group. When developing a proposed list of our peer group companies to be used in connection with making compensation decisions for 2016, Radford reexamined our compensation philosophy and peer group criteria and companies to recommend changes to our 2015 peer group company list to reflect our growth, the increase in our revenues and market capitalization and the consolidation in our industry. Radford recommended companies:
| • | in the life sciences industry (specifically biotechnology and specialty bio/pharma companies) with non-generic commercial products on the market; |
| • | with revenue of approximately one-fourth (0.25x) to three times (3x) our then-projected revenue (resulting in a range of generally $300 million to $4 billion in revenue); and |
| • | with market values of approximately one-fourth (0.25x) to four times (4x) our market capitalization at the time (resulting in a range of between $2.5 billion to $40 billion in market capitalization). |
Based on these criteria, in October 2015, to form our 2016 peer group, Radford recommended, and our compensation committee approved, eliminating from our peer group Cubist Pharmaceuticals, Inc., Pharmacyclics, Inc. and Salix Pharmaceuticals, Ltd. (which were acquired since the 2015 peer group company list was approved) and adding Anacor Pharmaceuticals, Inc., Horizon Pharma plc, Ionis Pharmaceuticals, Inc., Shire plc and The Medicines Company.
16
Table of Contents
2017 Peer Group. When developing a proposed list of our peer group companies to be used in connection with making compensation decisions for 2017, Radford recommended companies based on the same criteria used for the 2016 peer group, adjusted for then-current revenue and market values. Based on these criteria, in July 2016, Radford recommended and our compensation committee approved eliminating Anacor Pharmaceuticals, Inc. (which was acquired in June 2016) from our peers to form our 2017 peer group.
| Name | Peer Group Inclusion | |||||
| 2015 | 2016 | 2017 | ||||
| Actelion Ltd. | ✓ | ✓ | ✓ | |||
| Alexion Pharmaceuticals, Inc. | ✓ | ✓ | ✓ | |||
| Alkermes plc | ✓ | ✓ | ✓ | |||
| Anacor Pharmaceuticals, Inc. | ✓ | |||||
| BioMarin Pharmaceutical Inc. | ✓ | ✓ | ✓ | |||
| Cubist Pharmaceuticals, Inc. | ✓ | |||||
| Endo International plc | ✓ | ✓ | ✓ | |||
| Horizon Pharma plc | ✓ | ✓ | ||||
| Incyte Corporation | ✓ | ✓ | ✓ | |||
| Ionis Pharmaceuticals, Inc. | ✓ | ✓ | ||||
| Mallinckrodt plc | ✓ | ✓ | ✓ | |||
| Medivation, Inc. | ✓ | ✓ | ✓ | |||
| Pharmacyclics, Inc. | ✓ | |||||
| Regeneron Pharmaceuticals, Inc. | ✓ | ✓ | ✓ | |||
| Salix Pharmaceuticals, Ltd. | ✓ | |||||
| Seattle Genetics Inc. | ✓ | ✓ | ✓ | |||
| Shire plc | ✓ | ✓ | ||||
| The Medicines Company | ✓ | ✓ | ||||
| United Therapeutics Corporation | ✓ | ✓ | ✓ | |||
| Vertex Pharmaceuticals Incorporated | ✓ | ✓ | ✓ | |||
| Peer Group Metrics ($ in millions) | ||||||
| Peer Revenue – 50th Percentile | 1,121 | 780 | 1,144 | |||
| Jazz Revenue | 1,006 | 1,278 | 1,352 | |||
| Jazz Revenue Percentile Rank | 47th | 62nd | 55th | |||
| Peer Market Cap – 50th Percentile | 9,062 | 9,812 | 8,229 | |||
| Jazz Market Cap | 9,834 | 9,301 | 8,683 | |||
| Jazz Market Cap Percentile Rank | 56th | 47th | 51st | |||
The Jazz percentile ranks shown above reflect trailing 12 months’ revenue and 30-day average market capitalization for our company and the median of each peer group, measured as of the time Radford prepared its final recommendations regarding each peer group for the compensation committee.
2016 Market Data. In early 2016, Radford completed an assessment of executive compensation based on our 2016 peer group to inform the compensation committee’s determinations of executive compensation for 2016. This assessment used market data that was compiled from multiple sources, including: (i) data from the Radford Global Life Sciences Survey with respect to the 2016 peer group companies listed above, or the peer survey data; (ii) the 2016 peer group companies’ publicly disclosed information, or public peer data; and (iii) data from public biotechnology and pharmaceutical companies in the Radford Global Life Sciences Survey that had revenue from $300 million to $4 billion, or the general survey data, which included survey data with respect to our selected 2016 peer group companies. The components of the market data were based on the availability of sufficient comparative data for an executive officer’s position. Generally, peer survey data and public peer data are used in establishing
17
Table of Contents
market data reference points, and the general survey data is used when there is a lack of peer survey data and public peer data for an executive officer’s position. The peer survey data, the general survey data, and the public peer data, collectively referred to in this report as market data, were reviewed by the compensation committee, with the assistance of Radford, and used as one reference point, in addition to other factors, in setting our NEOs’ compensation.
Use of 2016 Market Data. The compensation committee reviews target total direct compensation, comprising both target total cash compensation and equity compensation, against the market data described above primarily to ensure that our executive compensation program, as a whole, is positioned competitively to attract and retain the highest caliber of executive officers and that the total direct compensation opportunity for the executive officer group is aligned with our corporate objectives and strategic needs. The compensation committee does not have a specific target compensation level for the NEOs and does not otherwise use a formulaic approach to setting pay at a particular positioning within the market data; rather, the compensation committee reviews a range of market data reference points (generally at the 25th, 50th, 60th and 75th percentiles of the market data) with respect to target total direct compensation, target total cash compensation (including both base salary and the target annual performance bonus) and equity compensation (valued based on an approximation of grant date fair value) as one factor before making compensation determinations. The compensation committee believes that over-reliance on benchmarking can result in compensation that is unrelated to the value delivered by our executive officers because compensation benchmarking does not take into account company to company variations among actual roles with similar titles or the specific performance of the executive officers.
Factors Used in Determining Executive Compensation
Our compensation committee sets the compensation of our executive officers at levels that the compensation committee determines to be competitive and appropriate for each NEO, using the compensation committee’s professional experience and judgment. The compensation committee’s pay decisions are not driven by a particular target level of compensation to market data, and the compensation committee does not otherwise use a formulaic approach to setting executive pay. Instead, the compensation committee believes that executive pay decisions require consideration of multiple relevant factors, which may vary from year to year. The figure below reflects the factors the compensation committee considers in determining and approving the amount, form and mix of pay for our NEOs.
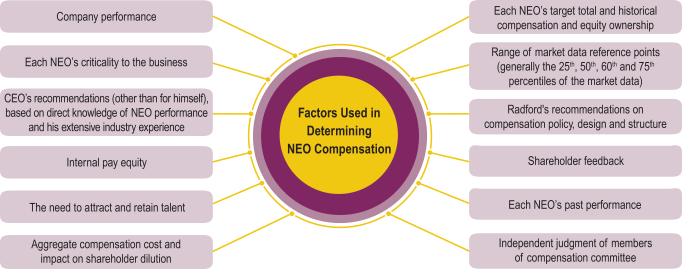
18
Table of Contents
2016 Advisory Vote on Executive Compensation and Shareholder Engagement
At our 2016 Annual General Meeting, the shareholders approved, on an advisory basis, the compensation of the NEOs, as disclosed in the proxy statement for that meeting pursuant to the compensation disclosure rules of the SEC. The compensation committee reviewed the final vote results for the proposal, and, given the significant level of shareholder support (approximately 93% of total votes cast with respect to the advisory proposal), concluded that our compensation program continues to provide a competitive pay-for-performance package that effectively incentivizes the NEOs and encourages long-term retention. Accordingly, the compensation committee and, with respect to our CEO’s compensation, our board of directors, determined not to make any significant changes to our executive compensation policies or decisions as a result of the vote. Our compensation committee and, with respect to our CEO’s compensation, our board of directors, will continue to consider the outcome of our say-on-pay votes and our shareholders’ views when making future compensation decisions for the NEOs.
We also intend to continue to engage with our shareholders on topics of particular concern to shareholders, including executive compensation matters. Shareholder feedback, including through direct discussions and prior shareholder votes, is reported to our compensation committee throughout the year. The graphic below describes our shareholder outreach and engagement and our related discussions and actions with respect to our 2016 Annual General Meeting.
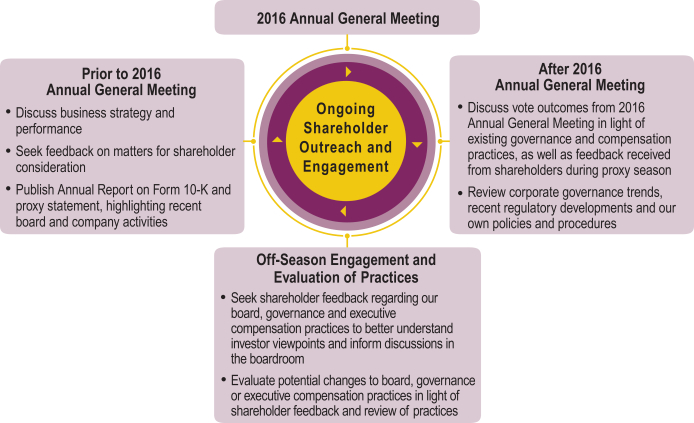
19
Table of Contents
Key Components and Design of the Executive Compensation Program
Our compensation program focuses on target total direct compensation, which consists of base salary, target bonus opportunity (which, together with base salary, we refer to as target total cash compensation), and long-term equity awards (valued based on an approximation of grant date fair value). We also offer our executive officers severance benefits upon certain types of involuntary terminations in connection with a change in control. The table below captioned “Components of Total Direct Compensation” provides an explanation of key features of each of the primary components of our executive compensation program and why we provide the particular compensation component.
The compensation committee takes a holistic approach to compensation and seeks to ensure that the aggregate level of pay across all of the pay elements is meeting the company’s desired objectives for each executive officer. The compensation committee does not have any formal policies for allocating compensation among salary, performance bonus opportunity and equity grants. Instead, the compensation committee uses its subjective judgment to establish a total compensation program for each NEO that is a mix of current, short-term and long-term incentive compensation, and cash and non-cash compensation, which it believes appropriate to achieve the goals of our executive compensation program and our corporate goals.
Because we believe it is important to our success to pursue long-term corporate objectives, to avoid excessive risk taking, and to preserve our cash resources, a significant portion of the NEOs’ total direct compensation is comprised of “at-risk” compensation, consisting of performance-based bonus opportunities and long-term equity awards, which align the executive officers’ incentives with the interests of our shareholders. This allocation between “at-risk” and fixed compensation is consistent with our pay-for-performance philosophy.
20
Table of Contents
Components of Total Direct Compensation
| Component | Key Features | Purpose | ||
|
Base Salary |
• Fixed cash compensation |
• Provides fixed level of compensation that is competitive within our industry and geographic areas | ||
|
• No amount is guaranteed |
||||
|
• Amounts are reviewed and determined annually, and are generally effective by March 1 each year
|
||||
|
Performance Bonus Award |
• Cash compensation under the performance bonus plan, which is “at-risk” because the realized value is dependent upon achievement of performance objectives |
• Provides financial incentives for our executives officers to achieve key corporate objectives that drive our business
• Rewards our executive officers for attaining corporate objectives and, for executive officers other than our CEO, their individual contributions toward such achievements | ||
|
• Target bonuses are reviewed and determined annually and expressed as a percentage of base salary earned |
||||
|
• Bonus opportunity is directly dependent on achievement of specific corporate objectives derived from our annual corporate goals |
||||
|
• Actual bonuses paid shortly after the end of each year, based on the extent corporate goals are attained as determined by the compensation committee, and for executive officers other than our CEO, their individual contributions toward such achievements
|
||||
|
Long-Term Incentive Compensation |
• Equity compensation generally in the form of stock options and RSUs granted under the 2011 Equity Incentive Plan, which is “at-risk” because the realized value is dependent upon our share price
• Awards are discretionary and reviewed and generally granted annually, early in the year, at time of hire or promotion or in other rare circumstances such as recognition of outstanding performance
• Awards to executive officers are granted shortly after annual or quarterly financial results released to public
• Stock options and RSUs generally vest over a 4-year period subject to executive officer’s continued service with us; stock option exercise price is set equal to fair market value on date of grant (i.e., closing price on NASDAQ Global Select Market)
• We have executive share ownership guidelines to further support our ownership culture and align the interests of executive officers and shareholders
|
• Fosters ownership culture
• Links compensation to long-term success
• Stock options are a key aspect of our pay-for-performance culture, by providing a return to our executive officers only if the market price of our ordinary shares appreciates over the stock option term
• RSU awards cover fewer shares than the stock options that deliver a similar value to an executive officer, and as a result, RSU awards enable the company to minimize dilution to shareholders while reinforcing the importance of shareholder value creation
• RSU awards provide a return based on the market price of our ordinary shares; if our share price declines, RSU awards correspondingly decline in value but still maintain value, and therefore, a mix of RSU awards and stock options aligns executive officers’ interests with those of shareholders by minimizing incentive for short-term risk taking at the expense of realizing long-term value
|
21
Table of Contents
Other Benefits. Executive officers based in the U.S. are eligible to participate in all of our benefit plans, such as the 401(k) Plan (see the section below “Description of Compensation Arrangements–401(k) Plan”), our medical, dental, vision, short-term disability, long-term disability and group life insurance plans and our Employee Stock Purchase Plan, or ESPP, in each case generally on the same basis as other employees. We also have a section 125 flexible benefits healthcare plan and a flexible benefits childcare plan under which employees can set aside pre-tax funds to pay for qualified healthcare expenses and qualified childcare expenses not reimbursed by insurance. We do not currently offer pension or other retirement benefits in the U.S., but do offer pension or other retirement benefits in certain other countries.
Severance Benefits upon Change in Control. Executive officers based in the U.S. are also eligible to participate in our Amended and Restated Executive Change in Control and Severance Benefit Plan, or the change in control plan, which is described below under the headings “Additional Compensation Information—Change in Control Plan” and “Potential Payments upon Termination or Change in Control—Amended and Restated Executive Change in Control and Severance Benefit Plan.” The change in control plan provides certain severance benefits to participants, in connection with specified involuntary termination events, including termination without cause and constructive termination, following a change in control. Certain executive officers who are not employed by our U.S. affiliates receive comparable change in control benefits pursuant to their employment agreements. The compensation committee believes these severance benefits are important from a retention perspective to provide some level of protection to our executives who might be terminated following a change in control and that the amounts are reasonable and maintain the competitiveness of our executive compensation and retention program. The compensation committee believes this structure serves to mitigate the distraction and loss of key executive officers that may occur in connection with rumored or actual fundamental corporate changes. Such payments protect the interests of our shareholders by enhancing executive focus during rumored or actual change in control activity, retaining executives despite the uncertainty that generally exists while a transaction is under consideration and encouraging the executives responsible for negotiating potential transactions to do so with independence and objectivity. We do not provide any tax gross up payments on severance benefits.
Clawback Requirement. As a public company, if we are required to restate our financial results due to our material noncompliance with any financial reporting requirements under the federal securities laws as a result of misconduct, our CEO and CFO may be legally required to reimburse our company for any bonus or other incentive-based or equity-based compensation they receive in accordance with the provisions of section 304 of the Sarbanes-Oxley Act of 2002.
22
Table of Contents
2016 Performance Bonus Program
The corporate objectives and relative weightings established by the board of directors for the 2016 performance bonus program that were communicated to the NEOs in early 2016 are described in the chart below. Product development objectives were given higher weight than in prior years, reflecting our increased focus on advancing our product candidate pipeline. The total revenue objective described below included stretch goals with the opportunity to earn up to an additional 15% bonus program funding.
Following the end of the year, after adding together the resulting bonus pool funding percentages for the quantitative and qualitative objectives based on their relative weightings of 75% and 25%, respectively, the compensation committee approved an overall bonus pool funding percentage of 99.7% of the target bonus pool for the 2016 plan year, as further described below.
The compensation committee did not set specific objectives for individual executive officers. Each executive officer is responsible for contributing to the corporate objectives, individually and as part of the leadership team, with each objective deemed to be important in determining the level of the company’s performance during the year. In approving individual bonus awards, the compensation committee considers the individual contribution towards the company’s achievement of the corporate objectives by each executive officer (other than our CEO). The actual bonus payments approved for each of the NEOs for 2016 are described below under “2016 Compensation Decisions for Our Named Executive Officers.”
Each of the three main quantitative objectives for 2016, with a total relative overall weighting of 75%, is described in the table and accompanying footnotes below, including each objective’s weighting, actual results and performance multipliers, as well as the bonus pool funding percentage resulting from the level of achievement of the quantitative objectives.
The compensation committee defined an algorithm with respect to each main quantitative objective (as well as the total revenue stretch goals discussed below) for calculating the bonus pool funding attributable to the extent of achievement for each such objective. With respect to the total revenue objective, the compensation committee approved three related additional, or stretch, goals, each with its own individual weighting. The compensation committee set specific threshold and maximum levels of achievement for the total revenue objective and the related stretch goals, as well as for the adjusted net income objective, which are described in the footnotes to the table below. For the main quantitative product development objective, the compensation committee did not set a threshold performance level; rather, an overall achievement of between 0% and 200%, measured against certain unweighted criteria as described in more detail below, was determined by the compensation committee and used to calculate the applicable bonus pool funding percentage attributable to such objective.
23
Table of Contents
| Quantitative Objectives |
Weighting | Actual Results | Multiplier | Bonus Pool Funding(3) | ||||||
| 1. | Total Revenue Objective: Achieve total revenue in 2016 of $1,522 million (at budgeted foreign currency exchange rates)(1) | 30% | Below target: Total revenue of $1,488 million, as reported | 89%(2) | 26.8% | |||||
| • Stretch goal: Achieve certain Xyrem year-over-year bottle volume growth(4) |
7% | Below threshold | N/A | 0% | ||||||
| • Stretch goal: Exceed budgeted Defitelio U.S. sales volume(5) |
4% | Below threshold | N/A | 0% | ||||||
| • Stretch goal: Exceed budgeted ex-U.S. Erwinaze and Defitelio sales(6) |
4% | Below threshold | N/A | 0% | ||||||
| 2. | Product Development Objective: Strong execution of late-stage development programs and ensure ongoing development of our drug portfolio(7) | 25% | Achieved at 100% level(7) | 100% | 25.0% | |||||
| 3. | Adjusted Net Income Objective: Achieve non-GAAP adjusted net income* in 2016 of $702 million(1) | 20% | Below target: non-GAAP adjusted net income* of $627.2 million, as reported | 82%(8) | 16.4% | |||||
|
| ||||||||||
| Total | 68.2% | |||||||||
|
| ||||||||||
|
| ||||||||||
| (1) | If the specified threshold annual performance level was met (90% of target for the total revenue objective and the adjusted net income objective), then a pre-established scaled performance multiplier (ranging from 50% to 150% for the total revenue objective and 50% to 200% for the adjusted net income objective) would be used to calculate the applicable bonus pool funding percentage attributable to such quantitative objective. The performance multiplier would be zero if performance was below the threshold level, 50% if performance was at the threshold level, and then scaled for performance between 51% and the applicable maximum level. The performance multiplier was capped for performance above the specified maximum performance level (110% of target for the total revenue objective and 120% of target for the adjusted net income objective). |
| (2) | To calculate the performance multiplier, the reported revenue of $1,488 million was increased by <$1 million to adjust for the impact of foreign currency exchange rates that were less favorable than the budgeted rates. |
| (3) | The percentages in this column represent, for each quantitative corporate objective, the weight of the quantitative objective multiplied by the performance multiplier that corresponds to the actual achievement of such quantitative objective. |
| (4) | With respect to the Xyrem bottle growth stretch goal, 9% annual bottle volume growth would have resulted in 3.5% added to the total bonus pool funding percentage, and 10% annual bottle volume growth would have resulted in another 3.5% added to the total bonus pool funding percentage. Actual achievement for 2016 was below the threshold level of achievement at 5.7% bottle volume growth. |
| (5) | With respect to the Defitelio U.S. sales volume stretch goal, the performance levels were set at achievement above the budgeted U.S. sales volume. Exceeding the sales volume budget by 10% would have resulted in 2% added to the total bonus pool funding percentage, and exceeding the sales volume budget by 20% would have resulted in a another 2% added to the total bonus pool funding percentage. Actual Defitelio U.S. sales volume for 2016 met budget but did not exceed budget by 10% or more. |
| * | Non-GAAP adjusted net income is a non-GAAP financial measure that both (i) excludes certain items from our GAAP reported net income and (ii) includes certain tax-related adjustments. For 2016, the items excluded from our GAAP reported net income to arrive at non-GAAP adjusted net income consisted of intangible asset amortization, share-based compensation expense, upfront and milestone payments, transaction and integration related costs, expenses related to certain legal proceedings and restructuring, non-cash interest expense, and loss on extinguishment and modification of debt. In addition, as set forth in footnote (8) to this table, solely for purposes of calculating the performance multiplier for 2016, the impact of the Celator Acquisition was excluded completely and non-GAAP adjusted net income was determined using the same calculation as was used to establish the performance objective, which did not give effect to the modification of the company’s calculation of its non-GAAP income tax provision for purposes of reporting its non-GAAP adjusted net income in 2016. |
24
Table of Contents
| (6) | With respect to the ex-U.S. Erwinaze and Defitelio sales stretch goal, the performance levels were set at achievement above budgeted sales. Achieving $130 million in ex-U.S. Erwinaze and Defitelio net sales would have resulted in 2% added to the total bonus pool funding percentage, and achieving $136 million in ex-U.S. Erwinaze and Defitelio net sales would have resulted in another 2% added to the total bonus pool funding percentage, in each case, based on budgeted foreign currency exchange rates. Actual achievement for 2016 ex-U.S. net Erwinaze and Defitelio sales of $111 million was below the threshold level of achievement. To calculate the level of achievement, actual sales of $111 million were increased by <$1 million to adjust for the impact of foreign currency exchange rates that were less favorable than the budgeted rates. |
| (7) | With respect to the product development objective, the compensation committee determined that the actual achievement by the company was 100%, resulting in a performance multiplier of 100%, and therefore, a 25% bonus pool funding percentage, based on achievement with respect to the unweighted performance criteria as described below: |
| Performance Category | Unweighted Performance Criteria and Results | |||||
| JZP-110 Clinical Activities |
The performance criteria consisted of the completion of enrollment and producing top-line results from three Phase 3 clinical studies for JZP-110 by the end of 2016. Enrollment for all Phase 3 studies of JZP-110 was completed on schedule; however, top-line results were not available until the first half of 2017 and therefore, the performance criteria were not fully met. | |||||
| Research and Development Activities for Our Existing Products | This performance criteria in this category required us to advance certain research and development activities related to line extensions and/or new indications for our sleep and hematology/oncology products, with the specified performance criteria consisting of: (i) progress in our JZP-507 development program; (ii) initiation of a Phase 3 study for a low-sodium oxybate formulation; (iii) progress in our oxybate once-nightly formulation program; (iv) completion of specified enrollment in our Phase 3 clinical trial of Xyrem to assess the safety and efficacy of Xyrem in pediatric narcolepsy patients with cataplexy; (v) selection of candidates in the hematology/oncology area for investigational new drug (IND)-enabling studies; and (vi) achievement of asset investment management approval for a potential new indication for defibrotide, in each case, by or before the end of 2016. We achieved each of these performance criteria. | |||||
| Approval of the Defibrotide NDA and Initiation of VOD study | The performance criteria consisted of obtaining approval of our defibrotide NDA by FDA in the first quarter of 2016, and enrolling the first patient in our study of defibrotide for the prevention of VOD, in the third quarter of 2016. The defibrotide NDA was approved on schedule in March 2016. With respect to the Phase 3 clinical study for the prevention of VOD, we activated clinical sites but were not able to enroll our first patient until the first quarter of 2017. | |||||
| In determining that the actual achievement by the company was 100% for the product development objective, the compensation committee employed a holistic analysis that took into account the compensation committee’s assessment of the degree to which the product development objective criteria were met as a whole against the backdrop of competing development priorities. In this regard, the compensation committee took into account the fact that the addition of Vyxeos through the Celator Acquisition in the third quarter of 2016 and the initiation of the rolling NDA submission for Vyxeos in the third quarter of 2016 required dedication of significant development resources that made achieving the established performance criteria more difficult. In addition, certain of the 2016 development criteria were aggressive and set at challenging levels, including the approval of our defibrotide NDA by the FDA in the first quarter of 2016 and producing top-line results from our three Phase 3 clinical studies for JZP-110 by the end of 2016 (particularly in light of the enrollment rates in those studies at the time the performance criteria were established). After considering the extent to which the performance criteria had been met as a whole against the backdrop of competing priorities, and after factoring in the difficulty of achievement of the performance criteria that were met and that were not met, the |
25
Table of Contents
| compensation committee determined that on balance, the achievement by the company was at the 100% level. |
| (8) | For purposes of calculating the performance multiplier, non-GAAP adjusted net income was determined using the same calculation as was used to establish the performance objective, which did not give effect to the modification of the company’s calculation of its non-GAAP income tax provision for purposes of reporting its non-GAAP adjusted net income, which modification commenced in the second quarter of 2016. In addition, for purposes of calculating the performance multiplier, the impact of the Celator Acquisition was excluded completely, as the Celator Acquisition was completed in July 2016, after the time that the performance objective was established. |
The qualitative corporate objectives approved by the board of directors fell into two categories: (1) progress on corporate development activities, with a relative weighting of 15%, and (2) a demonstrated commitment to and progress on certain operational, efficiency and organizational goals, with a relative weighting of 10%. Achievement of the qualitative objectives is inherently less objectively measurable than with respect to the quantitative objectives.
Corporate Development Objective. The objective relating to progress on corporate development activities consisted of executing on our corporate strategy with an emphasis on deal readiness and a view to one or more corporate development transactions in 2016 that would meaningfully diversify our business and grow revenues over time. The multiplier applied to the corporate development objective ranged from 0% to 200%, based on the compensation committee’s determination of the extent to which the corporate development objective was achieved during the year. In considering the company’s corporate development accomplishments in 2016, the compensation committee noted that that we had completed several transactions in 2016 that we believe will meaningfully diversity our business and grow revenues over time. In this regard, the compensation committee weighed heavily our critical and strategic acquisition of Celator, which broadened our hematology/oncology portfolio with the acquisition of worldwide development and commercialization rights to Vyxeos and the CombiPlex technology platform, and also considered our success in executing our agreements with Arrivo and Pfenex, our overall deal readiness and our robust and thoughtful corporate development process that led to the evaluation of several other opportunities during the year. In large part due to the criticality of the Celator Acquisition as well as our success in executing on transactions such as our agreements with Arrivo and Pfenex that we believe have the potential to diversify and add future revenue-generating products to our portfolio, the compensation committee determined that as whole, our overall achievement resulted in a multiplier of 150% and, therefore, a 22.5% bonus pool funding percentage for the 2016 corporate development objective.
Operational, Efficiency and Organizational Objective. With respect to the operational, efficiency and organizational objective, the compensation committee established three sub goals. Because the sub goals are not objectively measurable, they were not assigned individual weightings. The multiplier applied to the organizational corporate objective ranged from 0% to 200%, based on the compensation committee’s determination of the extent to which the aggregate organizational corporate objective, including sub goals, were achieved, as whole, during the year. The organizational corporate objective sub goals were:
| • | strengthening operations to ensure product continuity and optimize patient access and safety across all products; |
| • | creating and improving on meaningful operating efficiencies across the company; and |
| • | continuing progress towards strong performance management and accountability with a company commitment to employee development. |
In evaluating the organizational corporate objective, the compensation committee determined the following accomplishments to be relevant: (i) successful and timely physician and patient enrollment in the Xyrem risk evaluation and mitigation strategy program; (ii) completion of construction (on time and according to budget), and receipt of regulatory approval, of our Athlone manufacturing facility; (iii) demonstrated, measurable progress toward
26
Table of Contents
employee development and organizational and operational improvements; and (iv) significant efforts to mitigate product supply and other operational risks. The compensation committee also balanced these accomplishments against certain challenges experienced in 2016, including supply interruptions of Erwinaze in the U.S. and other countries in the third and fourth quarters of 2016. After taking into consideration both our accomplishments and challenges with respect to these sub goals, the compensation committee determined that as whole, our overall achievement resulted in a multiplier of 90% and therefore, a 9% bonus pool funding percentage for the 2016 operational, efficiency and organizational objective.
2016 Compensation Decisions for Our Named Executive Officers
In making compensation decisions for 2016, the compensation committee considered the factors discussed in “Factors Used in Determining Executive Compensation” above and the compensation committee’s specific compensation objectives for 2016. Our compensation committee did not use a formula or assign a particular weight to any one factor in determining each NEO’s target total direct compensation. Rather, our compensation committee’s determination of the target total direct compensation, mix of cash and equity and fixed and “at-risk” pay opportunities was a subjective, individualized decision for each NEO. The compensation committee reviewed and considered each element of pay in the context of the overall target total direct compensation for each NEO. When the compensation committee made changes to one element of pay, those changes were made in the context of the levels of the other elements of pay, and the resulting target total direct compensation for each NEO. As a result, the 2016 pay decisions for each NEO are presented holistically in this section.
The compensation committee also reviewed the target total cash compensation (base salary plus target performance bonus), target equity award grants and target total direct compensation for similarly-situated executives of our peer companies. However, as described above, the compensation committee believes that over-reliance on benchmarking can result in compensation that is unrelated to the value delivered by our executive officers because compensation benchmarking does not take into account company by company variations among actual roles with similar titles or the specific performance of the executive officers.
Summary of 2016 Compensation Decisions
Target Total Cash Compensation. The compensation committee increased each NEO’s base salary for 2016, and the new base salary rates were effective February 20, 2016. There were no changes to the 2016 target performance bonuses (expressed as a percentage of base salary) for any of the NEOs because the compensation committee determined the current percentages remained at appropriate levels and were consistent with our philosophy that the target percentages should generally vary based on each NEO’s job level in order to promote internal equity for positions of similar scope and impact and to reinforce teamwork across the executive group. Mr. Cozadd’s annual target performance bonus was set at a higher percentage than the percentages for other NEOs to reflect that he has ultimate responsibility for our company’s performance. Mr. Cozadd’s target bonus percentage has remained the same since 2012.
Target Equity Compensation and Impact on Target Total Direct Compensation. In determining the appropriate size of 2016 equity award grants, the compensation committee (and the board of directors, with respect to Mr. Cozadd) initially aimed to deliver equity awards to each executive officer of a similar grant date value to those delivered to each executive officer in 2015 (with the exception of Dr. Smith, whose 2015 award was made in connection with her commencement of employment and therefore included an inducement premium). The compensation committee and board of directors understood the resulting grants would generally fall at a lower positioning within the market data for each NEO than in 2015 (with the exception of Dr. Smith because her prior grants were in connection with commencement of employment), but the compensation committee and board of directors were mindful that our share price had fallen over the previous year and believed those amounts would be appropriate to serve the retention and incentive purposes of the awards. As a result of the company’s 90-day average
27
Table of Contents
share price used to calculate the size of the awards, each NEO’s resulting equity award grant date value and target total direct compensation for 2016 were lower than in 2015, as shown in the tables below.
Form of Equity Awards. Approximately 50% of the potential value of each NEO’s equity award was delivered in the form of stock options, and 50% of the potential value was delivered in the form of RSUs, in each case based on an approximation of grant date fair value, using an approximately 2.5 to 1 ratio of stock option grants to RSUs, in order to mitigate dilution and to reflect the increased value of receiving shares at full value without the payment of an exercise price. The 50/50 value split was consistent with our historical practices and took into consideration peer practices and market data. The actual share amounts granted to each executive officer were determined by applying the company’s 90-day average share price (as of December 31, 2015) to the grant date fair value of the award, which the compensation committee and, in the case of Mr. Cozadd, the board of directors, intended to deliver (dividing such value by the average share price, in the case of RSUs and applying a Black-Scholes option pricing model calculation using the average share price, in the case of stock options). A 90-day average share price was used, rather than a single day share price, in order to provide a more stabilized share value less susceptible to possible swings in the market. The exercise price of each stock option is equal to our closing share price on NASDAQ Global Select Market on the date of grant. The shares subject to the option awards vest over four years, with 25% vesting on the one-year anniversary of the grant date and the remainder vesting in equal monthly installments thereafter over the remaining 36 months. The RSUs vest over four years in equal annual installments.
On an annual basis, the compensation committee reviews market trends, including market peer use of performance-based vesting for equity awards, which are often favored by proxy advisory firms and certain institutional investors. For 2016, the compensation committee determined that equity awards vesting over time continued to be the most appropriate incentive structure for our executive officers. Our time-based vesting schedules deliver retention incentives for the company over the long-term and, unlike awards that vest based on pre-determined operational or market goals, do not create incentives for inappropriate short-term risk taking at the expense of realizing long-term value or the potential incentive for unethical conduct. In addition, we deliver a meaningful portion of compensation in the form of annual incentive compensation that is directly tied to, and incentivizes our executives to work towards, achievement of our key corporate goals. The key purposes served by time-vesting options and RSUs for 2016 are further discussed above in the chart captioned “Components of Total Direct Compensation.”
Individual NEO Compensation Decisions
Below are summaries, for each NEO individually, of the compensation committee’s decisions about 2016 target total direct compensation and the changes from each NEO’s 2015 target total direct compensation. As described above, when making the 2016 compensation decisions, the compensation committee focused primarily on the target total direct compensation for each NEO while considering the factors set forth in the section titled “Factors Used in Determining Executive Compensation” and the compensation committee’s specific compensation objectives for 2016. The footnotes to the tables also include the actual performance bonus paid to each of the NEOs for 2016 and how that actual bonus compared to each NEO’s target bonus.
28
Table of Contents
Bruce C. Cozadd, Chairman and CEO
| 2015 Pay ($) | 2016 Pay ($) | Change (%) | 2016 Pay Relative to Market Data (percentile) (1) | |||||
| Target Total Cash Compensation | 1,744,616 | 1,842,308 | 5.6 | <50th | ||||
| Base Salary (2) |
875,000 | 925,000 | ||||||
| Target Performance Bonus (3) |
869,616 | 917,308 | ||||||
| Target Equity Compensation (4) | 9,052,724 | 6,933,442 | (23.4) | 60th | ||||
| Options |
4,182,445 | 3,109,285 | ||||||
| RSUs |
4,870,279 | 3,824,157 | ||||||
|
Target Total Direct Compensation (5) |
10,797,340 | 8,775,750 | (18.7) | 60th |
| (1) | Reflects where the 2016 target compensation fell within the market data at the time reviewed and approved by the compensation committee. For equity compensation, the percentile corresponds to the target equity compensation value approved by the compensation committee; the actual target equity compensation delivered (as presented in the chart) reflects the fair value of the awards as of the grant date, in accordance with FASB Accounting Standards Codification Topic 718, Compensation—Stock Compensation, or ASC 718, which was slightly lower than the target equity compensation value approved by the compensation committee as a result of the compensation committee’s methodology of using a 90-day share price average to calculate the shares to be delivered, as described above. |
| (2) | Represents annual base salary rate for the applicable year. |
| (3) | Target amounts are as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively, and reflect the target percentage of base salary earned for each year. The 2016 amount reflects a target performance bonus of 100% of base salary earned, unchanged from the target performance bonus percentage for 2015. The actual 2016 performance bonus paid was $914,600, reflecting 99.7% of the target performance bonus, based entirely on the overall 2016 bonus pool funding percentage of 99.7%. The compensation committee (with approval from the board of directors) determined that the overall 2016 bonus pool funding percentage of 99.7% was applicable to Mr. Cozadd, because, as CEO, Mr. Cozadd is responsible for the company meeting all of its objectives. |
| (4) | Target equity compensation dollar amounts represent the grant date fair value of each stock option and RSU award, as applicable, and have been calculated in accordance with ASC 718 as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively. See the Grants of Plan-Based Awards Table for the number of shares subject to each award. |
| (5) | The compensation committee and board of directors designed Mr. Cozadd’s target total direct compensation to be competitive compared to the market data, appropriate from an internal equity perspective and more heavily weighted towards equity compensation, in line with our pay-for-performance philosophy. Consistent with the approach for 2016 equity award grants described above, the compensation committee and board of directors generally aimed to deliver equity awards to Mr. Cozadd of a similar grant date value to those delivered to him in 2015. Even though the compensation committee and board of directors understood the resulting grants would generally fall at a lower positioning within the market data than in 2015, the compensation committee and board of directors were mindful that our share price had fallen over the previous year and believed that a similar aggregate equity amount, taken together with Mr. Cozadd’s target total cash compensation (resulting in his target total direct compensation approximating the 60th percentile of the market data) was appropriate. As described above, Mr. Cozadd’s target bonus percentage remained the same as in 2015, and while the increase in his base salary resulted in a higher target performance bonus opportunity, Mr. Cozadd’s target total cash compensation for 2016 was still below the median of the market data. |
29
Table of Contents
Matthew P. Young, Executive Vice President and CFO
| 2015 Pay ($) | 2016 Pay ($) | Change (%) | 2016 Pay Relative to Market Data (percentile) (1) | |||||
| Target Total Cash Compensation | 731,173 | 802,192 | 9.7 | <60th | ||||
| Base Salary (2) |
475,000 | 520,000 | ||||||
| Target Performance Bonus (3) |
256,173 | 282,192 | ||||||
| Target Equity Compensation (4) | 2,498,360 | 2,012,935 | (19.4) | <75th | ||||
| Options |
1,153,778 | 902,696 | ||||||
| RSUs |
1,344,582 | 1,110,239 | ||||||
|
Target Total Direct Compensation (5) |
3,229,533 | 2,815,127 | (12.8) | <75th |
| (1) | Reflects where the 2016 target compensation fell within the market data at the time reviewed and approved by the compensation committee. For equity compensation, the percentile corresponds to the target equity compensation value approved by the compensation committee; the actual target equity compensation delivered (as presented in the chart) reflects the fair value of the awards as of the grant date, in accordance with ASC 718, which was slightly lower than the target equity compensation value approved by the compensation committee as a result of the compensation committee’s methodology of using a 90-day share price average to calculate the shares to be delivered. |
| (2) | Represents annual base salary rate for the applicable year. |
| (3) | Target amounts are as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively, and reflect the target percentage of base salary earned for each year. The 2016 amount reflects a target performance bonus of 55% of base salary earned, unchanged from the target performance bonus percentage for 2015. The actual 2016 performance bonus paid was $300,000, reflecting 106.3% of target performance bonus, based on the overall 2016 bonus pool funding percentage of 99.7% and Mr. Young’s significant individual contributions to such achievement. Specifically, the compensation committee considered Mr. Young’s overall leadership of our finance organization and his performance with respect to execution of corporate development priorities in 2016, particularly with respect to the Celator Acquisition. |
| (4) | Target equity compensation dollar amounts represent the grant date fair value of each stock option and RSU award, as applicable, and have been calculated in accordance with ASC 718 as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively. See the Grants of Plan-Based Awards Table for the number of shares subject to each award. |
| (5) | The compensation committee designed Mr. Young’s target total direct compensation to be competitive compared to the market data, appropriate from an internal equity perspective and more heavily weighted towards equity compensation, in line with our pay-for-performance philosophy. For 2016, the compensation committee decided that all of our executive vice presidents should receive the same size equity awards as each other to encourage the leadership team to work together to drive the business. Consistent with the approach for 2016 equity award grants described above, the compensation committee generally aimed to deliver equity awards to the executive vice presidents of a similar grant date value to those delivered to executive vice presidents in 2015. Even though the compensation committee understood the resulting grants would generally fall at a lower positioning within the market data than in 2015, the compensation committee was mindful that our share price had fallen over the previous year and believed that a similar aggregate equity amount, taken together with Mr. Young’s target total cash compensation (resulting in his target total direct compensation falling below the 75th percentile of the market data) was appropriate because of Mr. Young’s significant individual contributions, growth and increased experience in his role. The compensation committee determined it was appropriate to increase Mr. Young’s base salary from an internal pay equity perspective, in an amount necessary to bring his base salary closer to those of other NEOs who contribute similarly, given the criticality of Mr. Young’s role as our CFO. As described above, Mr. Young’s target bonus percentage remained the same as in 2015, and the increase in his base salary resulted in the higher target performance bonus opportunity shown above. |
30
Table of Contents
Russell J. Cox, Executive Vice President and COO
| 2015 Pay ($) | 2016 Pay ($) | Change (%) | 2016 Pay Relative to Market Data (percentile) (1) | |||||
| Target Total Cash Compensation |
850,385 | 889,135 | 4.6 | <50th | ||||
| Base Salary (2) |
550,000 | 575,000 | ||||||
| Target Performance Bonus (3) |
300,385 | 314,135 | ||||||
| Target Equity Compensation (4) |
2,498,360 | 2,012,935 | (19.4) | <60th | ||||
| Options |
1,153,778 | 902,696 | ||||||
| RSUs |
1,344,582 | 1,110,239 | ||||||
|
Target Total Direct Compensation (5) |
3,348,745 | 2,902,070 | (13.3) | <60th |
| (1) | Reflects where the 2016 target compensation fell within the market data at the time reviewed and approved by the compensation committee. For equity compensation, the percentile corresponds to the target equity compensation value approved by the compensation committee; the actual target equity compensation delivered (as presented in the chart) reflects the fair value of the awards as of the grant date, in accordance with ASC 718, which was slightly lower than the target equity compensation value approved by the compensation committee as a result of the compensation committee’s methodology of using a 90-day share price average to calculate the shares to be delivered. |
| (2) | Represents annual base salary rate for the applicable year. |
| (3) | Target amounts are as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively, and reflect the target percentage of base salary earned for each year. The 2016 amount reflects a target performance bonus of 55% of base salary earned, unchanged from the target performance bonus percentage for 2015. The actual 2016 performance bonus paid was $313,200, reflecting 99.7% of target performance bonus, based on the overall 2016 bonus pool funding percentage of 99.7%. For 2016, the compensation committee determined that applying the overall 2016 bonus pool funding percentage of 99.7% was appropriate for Mr. Cox, because, as COO, Mr. Cox is responsible for the company meeting all of its operational objectives. |
| (4) | Target equity compensation dollar amounts represent the grant date fair value of each stock option and RSU award, as applicable, and have been calculated in accordance with ASC 718 as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively. See the Grants of Plan-Based Awards Table for the number of shares subject to each award. |
| (5) | The compensation committee designed Mr. Cox’s target total direct compensation to be competitive compared to the market data, appropriate from an internal equity perspective and more heavily weighted towards equity compensation, in line with our pay-for-performance philosophy. For 2016, the compensation committee decided that all of our executive vice presidents should receive the same size equity awards as each other to encourage the leadership team to work together to drive the business. In line with the approach for 2016 equity award grants described above, the compensation committee generally aimed to deliver equity awards to the executive vice presidents of a similar grant date value to those delivered to executive vice presidents in 2015. Even though the compensation committee understood the resulting grants would generally fall at a lower positioning within the market data than in 2015, the compensation committee was mindful that our share price had fallen over the previous year and believed that a similar aggregate equity amount, taken together with Mr. Cox’s target total cash compensation (resulting in his target total direct compensation falling below the 60th percentile of the market data) was appropriate because of Mr. Cox’s scope of responsibility and the criticality of his role. The compensation committee determined it was appropriate to increase Mr. Cox’s base salary in an amount necessary to reflect his increased scope of responsibility and oversight of significant functions within the organization, as well as to maintain competitive positioning relative to the market data and the other NEOs. As described above, Mr. Cox’s target bonus percentage remained the same as in 2015, and the increase in his base salary resulted in the higher target performance bonus opportunity shown above. |
31
Table of Contents
Suzanne Sawochka Hooper, Executive Vice President and GC
| 2015 Pay ($) | 2016 Pay ($) | Change (%) | 2016 Pay Relative to Market Data (percentile)(1) | |||||
| Target Total Cash Compensation |
773,731 | 811,635 | 4.9 | 75th | ||||
| Base Salary (2) |
500,000 | 525,000 | ||||||
| Target Performance Bonus (3) |
273,731 | 286,635 | ||||||
| Target Equity Compensation (4) |
2,498,360 | 2,012,935 | (19.4) | <75th | ||||
| Options |
1,153,778 | 902,696 | ||||||
| RSUs |
1,344,582 | 1,110,239 | ||||||
|
Target Total Direct Compensation (5) |
3,272,091 | 2,824,570 | (13.7) | <75th |
| (1) | Reflects where the 2016 target compensation fell within the market data at the time reviewed and approved by the compensation committee. For equity compensation, the percentile corresponds to the target equity compensation value approved by the compensation committee; the actual target equity compensation delivered (as presented in the chart) reflects the fair value of the awards as of the grant date, in accordance with ASC 718, which was slightly lower than the target equity compensation value approved by the compensation committee as a result of the compensation committee’s methodology of using a 90-day share price average to calculate the shares to be delivered. |
| (2) | Represents annual base salary rate for the applicable year. |
| (3) | Target amounts are as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively, and reflect the target percentage of base salary earned for each year. The 2016 amount reflects a target performance bonus of 55% of base salary earned, unchanged from the target performance bonus percentage for 2015. The actual 2016 performance bonus paid was $300,000, reflecting 104.7% of target performance bonus, based on the overall 2016 bonus pool funding percentage of 99.7% and Ms. Hooper’s significant individual contributions to such achievement. Specifically, the compensation committee considered Ms. Hooper’s overall leadership, her oversight of complex strategic matters relating to Xyrem and our other products and her performance with respect to execution of corporate development priorities in 2016. |
| (4) | Target equity compensation dollar amounts represent the grant date fair value of each stock option and RSU award, as applicable, and have been calculated in accordance with ASC 718 as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively. See the Grants of Plan-Based Awards Table for the number of shares subject to each award. |
| (5) | The compensation committee designed Ms. Hooper’s target total direct compensation to be competitive compared to the market data, appropriate from an internal equity perspective and more heavily weighted towards equity compensation, in line with our pay-for-performance philosophy. For 2016, the compensation committee decided that all of our executive vice presidents should receive the same size equity awards as each other to encourage the leadership team to work together to drive the business. Consistent with the approach for 2016 equity award grants described above, the compensation committee generally aimed to deliver equity awards to the executive vice presidents of a similar grant date value to those delivered to executive vice presidents in 2015. Even though the compensation committee understood the resulting grants would generally fall at a lower positioning within the market data than in 2015, the compensation committee was mindful that our share price had fallen over the previous year and believed that a similar aggregate equity amount, taken together with Ms. Hooper’s target total cash compensation (resulting in her target total direct compensation falling below the 75th percentile of the market data) was appropriate because of Ms. Hooper’s significant experience and her sustained contribution and overall criticality to our business. The compensation committee determined it was appropriate to increase Ms. Hooper’s base salary even though her target total direct compensation would remain at the high end of the range of market data for her position given her salary at the time of joining the company and because of her contributions to achieving strategic initiatives in line with corporate objectives. As described above, Ms. Hooper’s target bonus percentage remained the same as in 2015, and the increase in her base salary resulted in the higher target performance bonus opportunity shown above. |
32
Table of Contents
Karen Smith, M.D., Ph.D., Global Head of Research and Development and CMO
| 2015 Pay ($) | 2016 Pay ($) | Change (%) | 2016 Pay Relative to Market Data (percentile) (1) | |||||
| Target Total Cash Compensation | 627,091 | 723,269 | 15.3 | <50th | ||||
| Base Salary (2) |
475,000 | 500,000 | ||||||
| Target Performance Bonus (3) |
152,091 | 223,269 | ||||||
| Target Equity Compensation (4) | 2,288,453 | 1,341,956 | (41.4) | <50th | ||||
| Options |
1,034,350 | 601,797 | ||||||
| RSUs |
1,254,103 | 740,159 | ||||||
|
Target Total Direct Compensation (5) |
2,915,544 | 2,065,225 | (29.2) | <50th |
| (1) | Reflects where the 2016 target compensation fell within the market data at the time reviewed and approved by the compensation committee. For equity compensation, the percentile corresponds to the target equity compensation value approved by the compensation committee; the actual target equity compensation delivered (as presented in the chart) reflects the fair value of the awards as of the grant date, in accordance with ASC 718, which was slightly lower than the target equity compensation value approved by the compensation committee as a result of the compensation committee’s methodology of using a 90-day share price average to calculate the shares to be delivered. |
| (2) | Represents annual base salary rate for the applicable year. |
| (3) | Target amounts are as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively, and reflect the target percentage of base salary earned for each year. The 2016 amount reflects a target performance bonus of 45% of base salary earned, unchanged from the target performance bonus percentage for 2015. The actual 2016 performance bonus paid was $245,000, reflecting 109.7% of target performance bonus, based on the overall 2016 bonus pool funding percentage of 99.7% and Dr. Smith’s significant individual contributions to such achievement. Specifically, the compensation committee considered that Dr. Smith led a large and complex portion of our organization to execute the quantitative and qualitative objectives described above. The compensation committee also particularly recognized her performance with respect to execution of product development priorities in 2016. |
| (4) | Target equity compensation dollar amounts represent the grant date fair value of each stock option and RSU award, as applicable, and have been calculated in accordance with ASC 718 as reported in the Grants of Plan-Based Awards Table for 2015 and 2016, respectively. See the Grants of Plan-Based Awards Table for the number of shares subject to each award. |
| (5) | The compensation committee designed Dr. Smith’s target total direct compensation to be competitive compared to the market data, appropriate from an internal equity perspective and more heavily weighted towards equity compensation, in line with our pay-for-performance philosophy. For 2016, the compensation committee decided that all of our senior vice presidents on the executive committee should generally receive the same size equity awards as each other to encourage the leadership team to work together to drive the business; however, the compensation committee determined it was appropriate to increase the value of Dr. Smith’s equity awards to reflect a value that was closer to the value received by executive vice presidents given the scope of Dr. Smith’s responsibility and role on the leadership team. Consistent with the approach for 2016 equity award grants described above, the compensation committee generally aimed to deliver equity awards to the Dr. Smith of a similar grant date value to those delivered to her in 2015 (not including the one-time inducement premium she received in connection with her commencement of employment in 2015). Even though the compensation committee understood the resulting grants would generally fall at a lower positioning within the market data than in 2015, the compensation committee was mindful that our share price had fallen over the previous year and believed that a similar aggregate equity amount, taken together with Dr. Smith’s |
33
Table of Contents
| target total cash compensation (resulting in her target total direct compensation falling below the 50th percentile of the market data) was appropriate given Dr. Smith’s tenure with the company. The compensation committee determined it was appropriate to increase Dr. Smith’s base salary from an internal pay equity perspective, in an amount necessary to bring her base salary closer to those of other NEOs who contribute similarly, given the increasing scope of Dr. Smith’s role as our CMO and her increasing experience. As described above, Dr. Smith’s target bonus percentage remained the same as in 2015, and the increase in her base salary resulted in the higher target performance bonus opportunity shown above. |
Additional Compensation Information
Ownership Guidelines for Executive Officers
In February 2013, we adopted share ownership guidelines for our CEO and certain other employees who serve on our executive committee, including our NEOs. Under the guidelines, these individuals are expected to own a number of the company’s ordinary shares with a value equal to three times base salary for the company’s chief executive officer and one times base salary for each other member of the company’s executive committee. The guidelines provide that the individuals subject to the guidelines are expected to establish the minimum ownership levels within five years of the company’s adoption of the guidelines (or within five years of the date an officer first becomes subject to the guidelines).
Ownership Guidelines and Compliance
| Name | Ownership Requirement |
Actual Ownership(1) |
In Compliance | Compliance Deadline | ||||
| Bruce C. Cozadd |
3.0x | 35.0x | Yes | 2018 | ||||
| Matthew P. Young |
1.0x | 3.7x | Yes | 2019 | ||||
| Russell J. Cox |
1.0x | 6.4x | Yes | 2018 | ||||
| Suzanne Sawochka Hooper |
1.0x | 5.0x | Yes | 2018 | ||||
| Karen Smith, M.D., Ph.D. |
1.0x | 1.1x | Yes | 2020 |
| (1) | Actual ownership calculated based on (a) value of shares owned as of March 31, 2017, using the closing price of the company’s ordinary shares on March 31, 2017 of $145.13, divided by (b) 2017 base salary. |
The value of the company’s ordinary shares for purposes of determining the number of shares subject to these guidelines in a given year is determined as the product of (i) the number of ordinary shares credited as held by the individual and (ii) the greater of (a) the closing price of the company’s ordinary shares on the applicable date, or (b) the purchase or exercise price paid for such shares. Shares that count toward satisfaction of these guidelines include: shares owned outright by the individual (including RSUs that have vested but not yet settled, net of taxes); shares retained after an option exercise or issuance under another type of equity award granted under the company’s equity incentive plans; shares retained after purchase under the ESPP; and shares held in trust for the benefit of the individual. The compensation committee has discretion to develop an alternative individual guideline or an alternative method of complying with the applicable individual guideline for an individual covered by the guidelines if compliance would place a significant hardship on such individual.
Our compensation committee periodically reviews the terms of our change in control plan against market data to ensure that the benefits we offer remain appropriate. In 2016, the compensation committee reviewed the benefits offered under the change in control plan and determined that the general “double-trigger” structure and benefits remained appropriate. While the levels and amounts of benefits remain the same, the compensation committee approved the following modifications and clarifications to the plan:
34
Table of Contents
| • | an executive’s termination due to death or disability will be considered an involuntary termination without cause entitling the executive to receive severance benefits if such termination occurs within the twelve months following a change in control transaction; |
| • | cash severance payments are calculated based on an executive’s salary and bonus at the time of termination or immediately prior to the change in control, if higher; |
| • | certain modifications to the definitions of cause, change in control and good reason (specifically, “cause” was revised to clarify and broaden the actions taken by the executive which could constitute “cause” for termination; “change in control” was revised to lower the threshold regarding an accumulation of ownership by a single shareholder or group that would constitute a change in control from 50% to 30%, to reflect Irish takeover methods and add a change in the majority of the incumbent board of directors as a change in control; “good reason” was modified to provide that the reduction in base salary by more than 10% would constitute good reason even if the reduction was part of a company-wide or executive-wide salary reduction, that such reduction could take place in a series of one or more smaller reductions that totaled 10%, and to include the baseline rate for comparison for measuring such reduction; and clarifications were made to ensure that, after a change in control, an executive who retains the same position but with substantially reduced authorities, duties or responsibility will have grounds to resign for good reason); |
| • | certain other clarifications, including regarding eligibility for employees of our U.S. affiliates and timing for equity acceleration; and |
| • | updates in accordance with U.S. and Irish law. |
The modifications to the change in control plan were intended as refinements aimed to provide greater clarity, reflect market practice and improvements for both the executives and our company and updates in applicable law since the plan was originally adopted in 2007. Only our executive officers who are employees of our U.S. affiliates are eligible to participate in the change in control plan, which includes all of our NEOs. Certain executive officers who are not employed by our U.S. affiliates receive comparable change in control benefits pursuant to their employment agreements. The compensation committee believes that the change in control benefits we provide are representative of market practice, both in terms of design and cost, and are sufficient to retain our current executive team and to recruit talented executive officers in the future. The terms of the change in control plan are described below under the heading “Potential Payments upon Termination or Change in Control—Amended and Restated Executive Change in Control and Severance Benefit Plan.”
Equity Grant Timing and Equity Plan Information
Our equity incentive grant policy, which was initially approved by our board of directors after the Azur Merger and amended and restated most recently in April 2015, provides that all equity grants that are approved for executive officers will be granted on the second trading day following the filing date of our next quarterly or annual report filed under the Exchange Act that occurs after the date on which such grants are approved by our board of directors or compensation committee, as applicable. Accordingly, our equity incentive grant policy requires that grants to our executive officers, if any, be made shortly after we have released information about our financial performance to the public for the applicable annual or quarterly period, so that the market will have an opportunity to absorb the financial and other information included in our annual and periodic reports before such grants are awarded. As a result, the timing of equity awards is not coordinated in a manner that intentionally benefits our executive officers; rather, the policy is designed with the objective that the market price of our ordinary shares at the time of grant can generally be expected to reflect our then-current results and prospects.
We currently grant equity awards to the NEOs, including stock options and RSUs, under the 2011 Equity Incentive Plan, or the 2011 Plan. The 2011 Plan was adopted by Jazz Pharmaceuticals, Inc.’s board of directors and approved by Jazz Pharmaceuticals, Inc.’s stockholders in connection with their approval of the Azur Merger in December 2011 and was assumed by us upon the completion of the Azur Merger. Before the 2011 Plan was adopted, we granted stock options under our 2007 Equity Incentive Plan, or the 2007 Plan, which was adopted by Jazz Pharmaceuticals, Inc.’s board of directors and approved by Jazz Pharmaceuticals, Inc.’s stockholders in
35
Table of Contents
connection with Jazz Pharmaceuticals, Inc.’s initial public offering. Awards granted under the 2007 Plan continue to be governed by the terms of the 2007 Plan, but subsequent equity awards have been, and continue to be, awarded under the 2011 Plan. The 2011 Plan affords the compensation committee the flexibility to utilize a broad array of equity incentives and performance cash incentives in order to secure and retain the services of employees of our company and its subsidiaries and to provide long-term incentives that align the interests of employees with the interests of our shareholders.
Additional long-term equity incentives are provided through the ESPP, which we assumed upon the completion of the Azur Merger. Pursuant to the ESPP, all eligible employees, including the NEOs, may allocate up to 15% of their base salary to purchase our stock at a 15% discount to the market price, subject to specified limits.
Accounting and Tax Considerations
Under Financial Accounting Standard Board ASC Topic 718, or ASC 718, the company is required to estimate and record an expense for each award of equity compensation (including stock options and RSUs) over the vesting period of the award. We record share-based compensation expense on an ongoing basis according to ASC 718. The compensation committee has considered, and may in the future consider, the grant of performance-based or other types of stock awards to executive officers in lieu of or in addition to stock option and time-based RSU grants in light of the accounting impact of ASC 718 and other considerations.
Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, limits companies to a deduction for federal income tax purposes of not more than $1 million of compensation paid to certain executive officers in a calendar year. Compensation above $1 million may be deducted if it is “performance-based compensation,” as defined in the Code and accompanying regulations. To maintain flexibility in compensating executive officers in a manner designed to promote the company’s goals, the compensation committee has considered and determined not to establish a policy at this time for determining which forms of incentive compensation awarded to executive officers shall be designed to qualify as “performance-based compensation” for purposes of section 162(m) or requiring all compensation to be deductible. The compensation committee intends to continue to evaluate the effects of the compensation limits of section 162(m) on any compensation it proposes to grant, and the compensation committee intends to continue to provide future compensation in a manner consistent with the best interests of the company and its shareholders.
Risk Assessment Concerning Compensation Practices and Policies
The compensation committee annually reviews the company’s compensation policies and practices to assess whether they encourage employees to take inappropriate risks. After reviewing each of the company’s compensation plans, and the checks and balances built into, and oversight of, each plan, in February 2017, the compensation committee determined that any risks arising from our compensation policies and practices for our employees are not reasonably likely to have a material adverse effect on our company as a whole. In addition, the compensation committee believes that the mix and design of the elements of executive compensation do not encourage management to assume excessive risks, and significant compensation decisions, as well as decisions concerning the compensation of the company’s executive officers, include subjective considerations by the compensation committee or the board of directors, which restrain the influence of formulae or objective factors on excessive risk taking. Finally, the mix of short-term compensation (in the form of salary and annual bonus, if any), and long-term compensation (in the form of stock options and RSUs) also prevents undue focus on short-term results and helps align the interests of the company’s executive officers with the interests of our shareholders.
36
Table of Contents
SUMMARY COMPENSATION TABLE
Summary of Compensation
The following table sets forth certain summary information for the years indicated with respect to the compensation earned by the NEOs during fiscal years 2016, 2015 and 2014, as applicable.
| Name and Principal Position | Year | Salary ($)(1) |
Bonus ($)(2) |
Stock Awards ($)(3) |
Option Awards ($)(4) |
Non-Equity Incentive Plan Compensation ($)(5) |
All Other Compensation ($)(6) |
Total ($) | ||||||||
| Bruce C. Cozadd |
2016 | 917,308 | — | 3,824,157 | 3,109,285 | 914,600 | 5,622 | 8,770,972 | ||||||||
| Chairman and CEO |
2015 | 869,616 | — | 4,870,279 | 4,182,445 | 750,500 | 4,622 | 10,677,462 | ||||||||
| 2014 | 830,000 | — | 5,498,457 | 4,194,452 | 1,020,900 | 11,692 | 11,555,501 | |||||||||
| Matthew P. Young |
2016 | 513,077 | — | 1,110,239 | 902,696 | 300,000 | 4,710 | 2,830,722 | ||||||||
| Executive Vice President and CFO |
2015 | 465,769 | — | 1,344,582 | 1,153,778 | 225,000 | 3,710 | 3,192,839 | ||||||||
| 2014 | 402,904 | 1,563,726 | 1,185,144 | 250,000 | 3,620 | 3,405,394 | ||||||||||
| Russell J. Cox |
2016 | 571,154 | — | 1,110,239 | 902,696 | 313,200 | 5,622 | 2,902,911 | ||||||||
| Executive Vice President and COO |
2015 | 546,154 | — | 1,344,582 | 1,153,778 | 255,000 | 4,622 | 3,304,136 | ||||||||
| 2014 | 493,462 | — | 2,681,998 | 1,991,469 | 320,000 | 11,759 | 5,498,688 | |||||||||
| Suzanne Sawochka Hooper |
2016 | 521,154 | — | 1,110,239 | 902,696 | 300,000 | 5,622 | 2,839,711 | ||||||||
| Executive Vice President and GC |
2015 | 497,692 | — | 1,344,582 | 1,153,778 | 240,000 | 4,622 | 3,240,674 | ||||||||
| 2014 | 483,462 | 1,666,199 | 1,271,046 | 320,000 | 3,710 | 3,744,417 | ||||||||||
| Karen Smith, M.D., Ph.D. (7) |
2016 | 496,154 | — | 740,159 | 601,797 | 245,000 | 4,695 | 2,087,805 | ||||||||
| Global Head of Research and Development and CMO |
2015 | 337,981 | 290,000 | 1,254,103 | 1,034,350 | 130,000 | 67,492 | 3,113,926 | ||||||||
| (1) | The dollar amounts in this column represent base salary earned during the indicated fiscal year. 2016 base salary rates were effective February 20, 2016. For more information on salaries in 2016, see “Compensation Discussion and Analysis—2016 Compensation Decisions for Our Named Executive Officers—Individual NEO Compensation Decisions” above. |
| (2) | The dollar amounts in this column represent a cash signing bonus of $100,000 and a relocation bonus of $190,000 paid to Dr. Smith in 2015. See “Description of Compensation Arrangements—Executive Employment Agreements” below. |
| (3) | The dollar amounts in this column reflect the aggregate grant date fair value of all RSU awards granted during the indicated fiscal year computed in accordance with ASC 718. The grant date fair value of each RSU award is measured based on the closing price of our ordinary shares on the date of grant. These amounts do not necessarily correspond to the actual value recognized or that may be recognized by the NEOs. |
| (4) | The dollar amounts in this column reflect the aggregate grant date fair value of all stock option awards granted during the indicated fiscal year. These amounts have been calculated in accordance with ASC 718, using the Black-Scholes option-pricing model and excluding the effect of estimated forfeitures. Assumptions used in the calculation of these amounts are included in the notes to our audited consolidated financial statements included in the company’s 2016 Annual Report on Form 10-K. These amounts do not necessarily correspond to the actual value recognized or that may be recognized by the NEOs. |
| (5) | The dollar amounts in this column represent the cash bonus awarded under the performance bonus plan for the indicated fiscal year. For more information on the cash bonus awards for 2016, see “Compensation Discussion and Analysis—2016 Performance Bonus Program” and “Compensation Discussion and Analysis—2016 Compensation Decisions for Our Named Executive Officers” above. |
| (6) | The dollar amounts in this column for 2016 include group term life insurance premiums paid and matching contributions under the 401(k) Plan of up to $3,000. |
| (7) | Dr. Smith joined us as our Global Head of Research and Development and CMO in April 2015. |
37
Table of Contents
Grants of Plan-Based Awards
The following table shows, for the fiscal year ended December 31, 2016, certain information regarding grants of plan-based awards to the NEOs.
GRANTS OF PLAN-BASED AWARDS IN FISCAL 2016
| Name | Award Type | Grant Date | Approval Date |
Estimated Possible Payouts Under Non- Equity Incentive Plan Awards Target ($)(1) |
All Other Stock Awards: Number of Shares of Stock or Units (#)(2) |
All Other Option Awards: Number of Securities Underlying Options (#)(2) |
Exercise or Base Option Awards ($/Sh)(3) |
Grant Date Fair Value of Stock and Option Awards ($)(4) | ||||||||
| Bruce C. Cozadd |
Annual Cash | — | — | 917,308 | — | — | — | — | ||||||||
| Annual Option | 2/25/2016 | 2/11/2016 | — | — | 77,500 | 123.36 | 3,109,285 | |||||||||
| Annual RSU | 2/25/2016 | 2/11/2016 | — | 31,000 | — | — | 3,824,157 | |||||||||
| Matthew P. Young |
Annual Cash | — | — | 282,192 | — | — | — | — | ||||||||
| Annual Option | 2/25/2016 | 2/10/2016 | — | — | 22,500 | 123.36 | 902,696 | |||||||||
| Annual RSU | 2/25/2016 | 2/10/2016 | — | 9,000 | — | — | 1,110,239 | |||||||||
| Russell J. Cox |
Annual Cash | — | — | 314,135 | — | — | — | — | ||||||||
| Annual Option | 2/25/2016 | 2/10/2016 | — | — | 22,500 | 123.36 | 902,696 | |||||||||
| Annual RSU | 2/25/2016 | 2/10/2016 | — | 9,000 | — | — | 1,110,239 | |||||||||
| Suzanne Sawochka Hooper |
Annual Cash | — | — | 286,635 | — | — | — | — | ||||||||
| Annual Option | 2/25/2016 | 2/10/2016 | — | — | 22,500 | 123.36 | 902,696 | |||||||||
| Annual RSU | 2/25/2016 | 2/10/2016 | — | 9,000 | — | — | 1,110,239 | |||||||||
| Karen Smith, M.D., Ph.D. |
Annual Cash | — | — | 223,269 | — | — | — | — | ||||||||
| Annual Option | 2/25/2016 | 2/10/2016 | — | — | 15,000 | 123.36 | 601,797 | |||||||||
| Annual RSU | 2/25/2016 | 2/10/2016 | — | 6,000 | — | — | 740,159 | |||||||||
| (1) | This column sets forth the target bonus amount for each NEO for the year ended December 31, 2016 under the performance bonus plan. There are no thresholds or maximum bonus amounts for each individual officer established under the performance bonus plan. Target bonuses were set as a percentage of each NEO’s base salary earned for the fiscal year ended December 31, 2016 and were 100% for Mr. Cozadd, 55% for each of Messrs. Young and Cox and Ms. Hooper and 45% for Dr. Smith. The dollar value of the actual bonus award earned for the year ended December 31, 2016 for each NEO is set forth in the Summary Compensation Table above. As such, the amounts set forth in this column do not represent either additional or actual compensation earned by the NEOs for the year ended December 31, 2016. For a description of the performance bonus plan, see “Compensation Discussion and Analysis—2016 Performance Bonus Program” above. |
| (2) | Annual and initial stock options and RSU awards were granted under the 2011 Plan. Each of the annual stock option awards listed in the table above vested as to 25% of the ordinary shares underlying the stock options upon the one year anniversary of the grant date and vest as to the remainder of the shares in 36 equal monthly installments thereafter. Each of the annual RSU awards vest in four equal annual installments on the anniversary of the grant date. As a general matter, the vested portion of stock options granted to the NEOs will expire three months after each NEO’s last day of service, subject to extension upon certain termination situations, such as death or disability, and RSUs will cease vesting upon each NEO’s last day of service. Stock option and RSU awards are subject to potential vesting acceleration as described below under the headings “Description of Compensation Arrangements—Equity Compensation Arrangements—2011 Equity Incentive Plan” and “Potential Payments upon Termination or Change in Control—Amended and Restated Executive Change in Control Plan and Severance Benefit Plan” below. See also “Description of Compensation Arrangements—Equity Compensation Arrangements—2011 Equity Incentive Plan” below for a general description of the material terms of the 2011 Plan. |
38
Table of Contents
| (3) | Stock options were granted with an exercise price equal to 100% of the fair market value on the date of grant which was $123.36 per share for the February 25, 2016 annual grants. |
| (4) | The dollar amounts in this column represent the grant date fair value of each stock option and RSU award, as applicable, granted to the NEOs in 2016. These amounts have been calculated in accordance with ASC 718. The grant date fair value of each stock option is calculated using the Black-Scholes option-pricing model and excluding the effect of estimated forfeitures. Assumptions used in the calculation of these amounts are included in the notes to our audited consolidated financial statements included in the company’s 2016 Annual Report on Form 10-K. The grant date fair value of each RSU award is measured based on the closing price of our ordinary shares on the date of grant. |
Description of Compensation Arrangements
Executive Employment Agreements
We do not have employment agreements currently in effect with any of our NEOs. Like other employees, executive officers are eligible for annual salary increases, participation in the performance bonus plan and discretionary equity grants. We have employment agreements in effect with certain employees based outside of the United States.
From time to time, we have provided an offer letter in connection with the commencement of employment of an executive officer based in the United States, which describes such executive officer’s initial terms of employment. For example, in March 2015, we provided an offer letter to Dr. Smith, which was amended and restated in July 2015, that included her initial base salary, a hiring bonus of $100,000 payable in connection with commencement of employment and a relocation bonus of $190,000. The employment of Dr. Smith, as is the case for all of our employees based in the United States, is at-will and not governed by the terms of their respective offer letters.
Amended and Restated Executive Change in Control and Severance Benefit Plan
Each of the NEOs is a participant in the change in control plan, a description of which is included below under the heading “Potential Payments upon Termination or Change in Control—Amended and Restated Executive Change in Control and Severance Benefit Plan.”
Equity Compensation Arrangements
Since the Azur Merger, we have granted stock options and RSU awards to employees, including the NEOs, under the 2011 Plan. From the initial public offering of Jazz Pharmaceuticals, Inc. until the Azur Merger, we granted stock options to our employees, including some of the NEOs, under the 2007 Plan. For more information on our current equity compensation program and decisions regarding the grants of equity awards in 2016 for our NEOs, see “Compensation Discussion and Analysis—2016 Compensation Decisions for Our Named Executive Officers.” The following is a brief summary of the material terms of each of our equity compensation plans.
2011 Equity Incentive Plan
In connection with the Azur Merger, Jazz Pharmaceuticals, Inc.’s board of directors adopted the 2011 Plan in October 2011, and its stockholders approved the 2011 Plan at the special meeting of the stockholders held in December 2011. The 2011 Plan became effective immediately before the consummation of the Azur Merger and was assumed and adopted by us upon the consummation of the Azur Merger. The following is a brief summary of the material terms of the 2011 Plan.
Administration. The board of directors has delegated its authority to administer the 2011 Plan to the compensation committee. Subject to the terms of the 2011 Plan, the board of directors or a committee authorized by the board determines recipients, dates of grant, the numbers and types of stock awards to be granted, and the terms and conditions of the stock awards, including the period of their exercisability and vesting. The compensation committee has the authority to delegate its administrative powers under the 2011 Plan to a subcommittee consisting of members of the compensation committee and may, at any time, revest in itself some or all of the power previously
39
Table of Contents
delegated to the subcommittee. Our board of directors may also delegate to one or more of our officers the authority to designate employees who are not officers to be recipients of certain stock awards and the number of shares subject to such stock awards, provided that our board of directors must specify the total number of shares that may be subject to the stock awards granted by such officer(s) and such officer(s) may not grant a stock award to himself or herself.
Types of Awards. The 2011 Plan provides for the grant of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock awards, RSU awards, other stock awards, and performance awards that may be settled in cash, shares, or other property, which may be granted to employees, including officers.
Corporate Transactions. In the event of certain significant corporate transactions (as defined in the 2011 Plan and described below), our board of directors will have the discretion to take one or more of the following actions with respect to outstanding stock awards (contingent upon the closing or completion of such corporate transaction), unless otherwise provided in the stock award agreement or other written agreement with the participant or unless otherwise provided by our board of directors at the time of grant:
| • | arrange for assumption, continuation, or substitution of a stock award by a surviving or acquiring corporation (or its parent company); |
| • | arrange for the assignment of any reacquisition or repurchase rights applicable to any shares issued pursuant to a stock award to the surviving or acquiring corporation (or its parent company); |
| • | accelerate the vesting, in whole or in part, and exercisability of a stock award and provide for its termination if it is not exercised at or prior to the corporate transaction; |
| • | arrange for the lapse of any reacquisition or repurchase rights applicable to any shares issued pursuant to a stock award; |
| • | cancel or arrange for the cancellation of a stock award, to the extent not vested or exercised prior to the effective time of the corporate transaction, in exchange for such cash consideration, if any, as the board of directors may consider appropriate; or |
| • | make a payment equal to the excess, if any, of (a) the value of the property that the participant would have received upon the exercise of the stock award over (b) any exercise price payable in connection with such exercise. |
Our board of directors need not take the same action for each stock award or with regard to all participants.
For purposes of the 2011 Plan, a “corporate transaction” generally means (i) a sale or disposition of all or substantially all our assets or a sale or disposition of at least 90% of our outstanding securities; (ii) a merger, consolidation or similar transaction after which we are not the surviving corporation; or (iii) a merger, consolidation or similar transaction after which we are the surviving corporation but our ordinary shares are converted or exchanged into other property.
Change in Control. The board of directors has the discretion to provide additional acceleration of vesting and exercisability upon or after a change in control (as defined in the 2011 Plan and described below) as may be provided in a stock award agreement or any other written agreement between us or any of our affiliates and a participant. The forms of stock option agreement and RSU award agreement adopted by the board of directors under the 2011 Plan provide that in the event a participant’s service relationship with us or a successor entity is terminated due to an involuntary termination without cause (as defined in the stock award agreement and as described below) within 12 months following, or one month prior to, the effective date of a change in control, the vesting (and in the case of stock options, exercisability) of the stock award will accelerate in full.
For purposes of the 2011 Plan and the forms of stock option agreement and RSU award agreement issued thereunder, a “change in control” generally means (i) a person or group acquires ownership of more than 50% (reduced to 30%, effective as of November 2016) of the combined voting power of our outstanding securities (other than directly from our company); (ii) a merger transaction involving us (revised as of November 2016 to mean certain compromises or arrangements sanctioned by the Irish courts, certain schemes, contracts or offers that have become
40
Table of Contents
binding on all of our shareholders, certain takeover bids, certain offers or reverse takeover transactions or a reorganization, merger, statutory share exchange, consolidation or similar transaction involving us), after which our shareholders do not own more than 50% of the combined voting power of the surviving entity or its parent in substantially the same proportion as their ownership of our outstanding voting securities immediately before the transaction (or, as of November 2016, a person or group acquires ownership of more than 30% of the combined voting power of the surviving entity or its parent, or at least a majority of the members of the board of directors of the parent (or the surviving entity, if there is no parent) following such transaction are not incumbent board members (as defined in (v) below) at the time our board of directors approves the transaction); (iii) our shareholders or our board of directors approves a complete dissolution or liquidation of our company, or a complete dissolution or liquidation of our company otherwise occurs (except for a liquidation into a parent company); (iv) a sale, lease, license or other disposition of all or substantially all of our assets; or (v) individuals who were members of our board of directors on the date of adoption of the 2011 Plan (or members of our board of directors approved or recommended by a majority vote of such members still in office), referred to as “incumbent board members,” cease to constitute at least a majority of our board of directors.
An “involuntary termination without cause” generally means that a participant’s service relationship with us is terminated for any reason other than for the following reasons (and not upon a participant’s death or disability): (i) participant’s commission of any felony or crime involving fraud, dishonesty or moral turpitude under the laws of the United States or any state thereof (with respect to Irish participants, the participant’s conviction for any criminal offense (other than an offense under any road traffic legislation in Ireland, the United Kingdom or elsewhere for which a fine or non-custodial penalty is imposed) or any offense under any regulation or legislation relating to insider dealing, fraud or dishonesty); (ii) participant’s attempted commission of or participation in a fraud or act of dishonesty against us; (iii) participant’s intentional, material violation of any contract or agreement with us or of any statutory duty owed to us; (iv) participant’s unauthorized use or disclosure of our confidential information or trade secrets; or (v) participant’s gross misconduct.
2007 Equity Incentive Plan
The 2007 Plan, which was initially adopted by the Jazz Pharmaceuticals, Inc. board of directors and approved by the Jazz Pharmaceuticals, Inc. stockholders in connection with its initial public offering, was continued and assumed by us upon consummation of the Azur Merger. The following is a brief summary of the material terms of the 2007 Plan.
Administration. The board of directors has delegated its authority to administer the 2007 Plan to the compensation committee. Subject to the terms of the 2007 Plan, the board of directors or a committee authorized by the board determines recipients, dates of grant, the numbers and types of stock awards to be granted, and the terms and conditions of the stock awards, including the period of their exercisability and vesting.
Types of Awards. The 2007 Plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock awards, RSU awards, stock appreciation rights, performance stock awards and other forms of equity compensation, which may be granted to employees, including officers, non-employee directors, and consultants. Incentive stock options may be granted only to employees, including executive officers.
Corporate Transactions. Pursuant to the 2007 Plan, in the event of a corporate transaction (as defined in the 2007 Plan and described below), the board of directors will have the discretion to take one or more of the following actions with respect to outstanding stock awards (contingent upon the closing or completion of such corporate transaction), unless otherwise provided in the stock award agreement or other written agreement with the participant or unless otherwise provided by our board of directors at the time of grant:
| • | arrange for assumption, continuation, or substitution of a stock award by a surviving or acquiring corporation (or its parent company); |
| • | arrange for the assignment of any reacquisition or repurchase rights applicable to any shares issued pursuant to a stock award to the surviving or acquiring corporation (or its parent company); |
41
Table of Contents
| • | accelerate the vesting and exercisability of a stock award and provide for its termination if it is not exercised at or prior to the corporate transaction; |
| • | arrange for the lapse of any reacquisition or repurchase rights applicable to any shares issued pursuant to a stock award; |
| • | cancel or arrange for the cancellation of a stock award, to the extent not vested or exercised prior to the effective time of the corporate transaction, in exchange for such cash consideration as the board of directors may consider appropriate; or |
| • | make a payment equal to the excess, if any, of (a) the value of the property that the participant would have received upon the exercise of the stock award over (b) any exercise price payable in connection with such exercise. |
The board of directors need not take the same action for each stock award. For purposes of the 2007 Plan, a “corporate transaction” generally means (i) a sale or disposition of all or substantially all our assets or a sale or disposition of at least 90% of our outstanding securities; (ii) a merger, consolidation or similar transaction after which we are not the surviving corporation; or (iii) a merger, consolidation or similar transaction after which we are the surviving corporation but our ordinary shares are converted or exchanged into other property.
Change in Control. The board of directors has the discretion to provide additional acceleration of vesting and exercisability upon or after a change in control (as defined in the 2007 Plan and described below) as may be provided in a stock award agreement or any other written agreement between us or any of our affiliates and a participant. The forms of stock option agreement and RSU award agreement adopted by the board of directors under the 2007 Plan provide that in the event a participant’s service relationship with us or a successor entity is terminated due to an involuntary termination without cause (as defined in the stock award agreement and as described below) within 12 months following, or one month prior to, the effective date of a change in control, the vesting (and in the case of stock options, exercisability) of the stock award will accelerate in full. For purposes of the 2007 Plan and the forms of stock option agreement and RSU award agreement issued thereunder, a “change in control” generally means (i) a person or group acquires ownership of more than 50% of the combined voting power of our outstanding securities (other than in connection with a financing or a repurchase program); (ii) a merger, consolidation or similar transaction involving us, after which our shareholders do not own more than 50% of the combined voting power of the surviving entity or its parent in substantially the same proportion as their ownership of our outstanding voting securities immediately before the transaction; (iii) our shareholders or our board of directors approves a complete dissolution or liquidation of our company, or a complete dissolution or liquidation of our company otherwise occurs (except for a liquidation into a parent company); (iv) a sale, lease, license or other disposition of all or substantially all of our assets; or (v) individuals who are members of our board of directors on the date of adoption of the 2007 Plan (or members of our board of directors approved or recommended by a majority vote of such members still in office) cease to constitute at least a majority of our board of directors.
The term “involuntary termination without cause” has a similar meaning as under the 2011 Plan, as described above.
2007 Employee Stock Purchase Plan
Additional long-term equity incentives are provided through the ESPP, which was amended and restated by Jazz Pharmaceuticals, Inc.’s board of directors in October 2011 and approved by its stockholders in December 2011, to be effective immediately prior to the Azur Merger, and, in October 2012, amended and restated by our compensation committee. The ESPP was assumed by us upon the consummation of the Azur Merger. The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of section 423 of the Code. Under the ESPP, all of our regular employees and employees of any of our parent or subsidiary companies designated by the board of directors as eligible to participate may participate and may contribute, normally through payroll deductions, up to 15% of their earnings up to a total of $15,000 per purchase period for the purchase of our ordinary shares under the ESPP. The ESPP is currently offered to our regular employees in Ireland and in the United States, including the NEOs. The ESPP is implemented through a series of offerings of purchase rights to eligible employees. Under the ESPP, we
42
Table of Contents
may specify offerings with a duration of not more than 27 months, and may specify shorter purchase periods within each offering. Each offering will have one or more purchase dates on which our ordinary shares will be purchased for employees participating in the offering. Unless otherwise determined by the board of directors, ordinary shares are purchased for accounts of employees participating in the ESPP at a price per share equal to the lower of (a) 85% of the fair market value of an ordinary share on the first date of an offering or (b) 85% of the fair market value of an ordinary share on the date of purchase.
Performance Bonus Plan
We maintain a performance bonus plan to reward executive officers and other employees for successful achievement of company-wide and individual performance objectives on an annual basis. More information regarding the performance bonus plan is provided above under the headings “Compensation Discussion and Analysis—2016 Performance Bonus Program” and “Compensation Discussion and Analysis—2016 Compensation Decisions for Our Named Executive Officers.”
401(k) Plan
Our employees based in the United States are eligible to participate in the 401(k) Plan. The 401(k) Plan is intended to qualify as a tax-qualified plan under section 401 of the Code. Employee contributions are held and invested by the 401(k) Plan’s trustee. The 401(k) Plan provides that each participant may contribute a portion of his or her pre-tax compensation, up to a statutory annual limit, which was $18,000 for employees under age 50, and $24,000 for employees age 50 and over in 2016. The 401(k) Plan also permits us to make discretionary contributions and matching contributions, subject to established limits and a vesting schedule. In 2013, we began making discretionary matching contributions, which for 2016 was subject to an annual limit of $3,000 per employee.
Additional Benefits
The NEOs are eligible to participate in our benefit plans generally available to all employees, as described in “Compensation Discussion and Analysis—Key Components and Design of the Executive Compensation Program.”
Pension Benefits
Other than with respect to tax-qualified defined contribution plans such as the 401(k) Plan, the NEOs do not participate in any plan that provides for retirement payments and benefits, or payments and benefits that will be provided primarily following retirement.
Nonqualified Deferred Compensation
During the year ended December 31, 2016, the NEOs did not contribute to, or earn any amounts with respect to, any defined contribution or other plan sponsored by us that provides for the deferral of compensation on a basis that is not tax-qualified.
43
Table of Contents
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth, for the fiscal year ended December 31, 2016, certain information regarding outstanding equity awards at fiscal year-end for the NEOs.
OUTSTANDING EQUITY AWARDS AT 2016 FISCAL YEAR-END TABLE
| Option Awards | Stock Awards | |||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#)(1) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested (#)(2) |
Market Value of Not Vested ($)(3) | ||||||
|
|
| |||||||||||
| Bruce C. Cozadd |
— | 77,500 (5) | 123.36 | 2/24/2026 | 31,000 (13) | 3,379,930 | ||||||
| 33,229 | 39,271 (6) | 175.19 | 2/25/2025 | 20,850 (14) | 2,273,276 | |||||||
| 32,022 | 16,762 (4)(7) | 166.62 | 2/26/2024 | 14,372 (4)(15) | 1,566,979 | |||||||
| 68,009 | 5,952 (4)(8) | 59.13 | 3/4/2023 | 9,582 (4)(16) | 1,044,725 | |||||||
| 109,284 | — (4) | 46.83 | 8/8/2022 | — | — | |||||||
| 6,895 | — (4) | 11.48 | 3/7/2020 | — | — | |||||||
| Matthew P. Young |
— | 22,500 (5) | 123.36 | 2/24/2026 | 9,000 (13) | 981,270 | ||||||
| 9,166 | 10,834 (6) | 175.19 | 2/25/2025 | 5,756 (14) | 627,577 | |||||||
| 8,072 | 4,428 (9) | 130.23 | 5/11/2024 | 3,124 (17) | 340,610 | |||||||
| 6,375 | 2,625 (7) | 166.62 | 2/26/2024 | 2,250 (15) | 245,318 | |||||||
| 22,000 | 2,000 (10) | 58.72 | 5/2/2023 | 3,000 (18) | 327,090 | |||||||
| Russell J. Cox |
— | 22,500 (5) | 123.36 | 2/24/2026 | 9,000 (13) | 981,270 | ||||||
| 9,166 | 10,834 (6) | 175.19 | 2/25/2025 | 5,756 (14) | 627,577 | |||||||
| 8,750 | 6,250 (11) | 135.44 | 8/6/2024 | 3,750 (19) | 408,863 | |||||||
| 14,166 | 5,834 (7) | 166.62 | 2/26/2024 | 5,000 (15) | 545,150 | |||||||
| 25,781 | 1,719 (8) | 59.13 | 3/4/2023 | 3,437 (16) | 374,736 | |||||||
| 70,000 | — | 46.83 | 8/8/2022 | — | — | |||||||
| 44,200 | — | 8.23 | 8/24/2020 | — | — | |||||||
| Suzanne Sawochka Hooper |
— | 22,500 (5) | 123.36 | 2/24/2026 | 9,000 (13) | 981,270 | ||||||
| 9,166 | 10,834 (6) | 175.19 | 2/25/2025 | 5,756 (14) | 627,577 | |||||||
| 14,166 | 5,834 (7) | 166.62 | 2/26/2024 | 5,000 (15) | 545,150 | |||||||
| 30,000 | 2,000 (8) | 59.13 | 3/4/2023 | 4,000 (16) | 436,120 | |||||||
| 47,172 | — | 46.83 | 8/8/2022 | — | — | |||||||
| Karen Smith, M.D., Ph.D. |
— | 15,000 (5) | 123.36 | 2/24/2026 | 6,000 (13) | 654,180 | ||||||
| 7,408 | 10,372 (12) | 176.51 | 5/10/2025 | 5,122 (20) | 558,452 | |||||||
| (1) | In addition to the specific vesting schedule for each stock award, each unvested stock award is subject to the general terms of the 2011 Plan or 2007 Plan, as applicable, including the potential for future vesting acceleration described above under the heading “Description of Compensation Arrangements—Equity Compensation Arrangements” as well as the potential vesting acceleration under the terms of the change in control plan described below under the heading “Potential Payments upon Termination or Change in Control—Amended and Restated Executive Change in Control and Severance Benefit Plan.” |
| (2) | Each RSU award vests in four equal annual installments on the anniversary of the grant date. |
| (3) | The market values of the RSU awards that have not vested are calculated by multiplying the number of shares underlying the RSU awards shown in the table by $109.03, the closing price of our ordinary shares on December 30, 2016. |
44
Table of Contents
| (4) | The number of shares reported reflects the transfer of beneficial ownership of a portion of the indicated stock option and RSU awards in 2015 to Mr. Cozadd’s former spouse pursuant to a domestic relations order. |
| (5) | The unexercisable shares subject to this stock option award as of December 31, 2016 vested with respect to 25% of the shares underlying the stock option on February 25, 2017, and the remainder vests monthly from March 25, 2017 to February 25, 2020. |
| (6) | The unexercisable shares subject to this stock option award as of December 31, 2016 vest monthly from January 26, 2017 to February 26, 2019. |
| (7) | The unexercisable shares subject to this stock option award as of December 31, 2016 vest monthly from January 27, 2017 to February 27, 2018. |
| (8) | The unexercisable shares subject to this stock option award as of December 31, 2016 vest monthly from January 5, 2017 to March 5, 2017. |
| (9) | The unexercisable shares subject to this stock option award as of December 31, 2016 vest monthly from January 12, 2017 to May 12, 2018. |
| (10) | The unexercisable shares subject to this stock option award as of December 31, 2016 vest monthly from January 22, 2017 to April 22, 2017. |
| (11) | The unexercisable shares subject to this stock option award as of December 31, 2016 vest monthly from January 7, 2017 to August 7, 2018. |
| (12) | The unexercisable shares subject to this stock option award as of December 31, 2016 vest monthly from January 13, 2017 to April 13, 2019. |
| (13) | RSUs awarded on February 25, 2016. |
| (14) | RSUs awarded on February 26, 2015. |
| (15) | RSUs awarded on February 27, 2014. |
| (16) | RSUs awarded on March 5, 2013. |
| (17) | RSUs awarded on May 12, 2014. |
| (18) | RSUs awarded on May 3, 2013. |
| (19) | RSUs awarded on August 7, 2014. |
| (20) | RSUs awarded on May 11, 2015. |
45
Table of Contents
Option Exercises and Stock Vested
The following table provides information on RSUs vested and stock options exercised, including the number of shares acquired upon exercise and the value realized, determined as described below, for the NEOs in the year ended December 31, 2016.
| Option Awards | Stock Awards | |||||||
| Name | Number of Acquired on |
Value Realized on Exercise ($)(1) |
Number of (#) |
Value Realized on ($)(2) | ||||
| Bruce C. Cozadd |
— | — | 39,371 | 5,358,656 | ||||
| Matthew P. Young |
— | — | 7,607 | 1,061,056 | ||||
| Russell J. Cox |
— | — | 18,481 | 2,575,209 | ||||
| Suzanne Sawochka Hooper |
— | — | 17,169 | 2,367,771 | ||||
| Karen Smith, M.D., Ph.D. |
— | — | 1,983 | 294,600 | ||||
| (1) | The value realized on exercise is based on the difference between the closing price of our ordinary shares on the date of exercise and the applicable exercise price of those options and does not represent actual amounts received by the NEOs as a result of the option exercises. |
| (2) | The value realized on vesting is based on the number of shares underlying the RSUs that vested and the closing price of our ordinary shares on the vesting date. |
Potential Payments upon Termination or Change in Control
Amended and Restated Executive Change in Control and Severance Benefit Plan
The change in control plan provides that, in the event that an executive’s employment terminates due to an involuntary termination without cause or a constructive termination, in each case upon or within 12 months following a change in control (as such terms are defined in the change in control plan and described generally below), and assuming all of the other conditions of the change in control plan are met, each executive who is a participant in the change in control plan would be entitled to the following benefits under the change in control plan:
| • | A single, lump sum cash severance payment equal to the sum of: (i) the applicable base salary described below, multiplied by the applicable percentage set forth below; plus (ii) the product of (A) the applicable base salary, (B) the applicable bonus percentage described below and (C) the applicable percentage set forth below; plus (iii) the product of (A) the applicable base salary, (B) the applicable bonus percentage and (C) the quotient obtained by dividing the number of full months that an executive is employed in the year of the termination by 12. |
| ¡ | The “applicable base salary” is the higher of the executive’s base salary in effect (i) on the date of termination (without giving effect to any reduction in base salary that would constitute grounds for a constructive termination) or (ii) immediately prior to the change in control, without giving effect to any voluntary pay reduction taken by the executive during the 12 months preceding the date of termination or the change in control. |
| ¡ | The “applicable percentage” as of February 2016 was 200% for our CEO, executive chairman or president (currently only Mr. Cozadd), 150% for senior vice presidents and above (which includes our NEOs other than Mr. Cozadd) and 100% for vice presidents. |
| ¡ | The “applicable bonus percentage” is the greater of (i) the highest amount of any annual bonus paid to the executive for either of the last two calendar years prior to (A) the date of termination or (B) the change in control, in each case expressed as a percentage of the executive’s base salary for the applicable year, and (ii) the higher of the executive’s target bonus for the calendar year in which (A) the termination occurs or (B) the change in control occurs, in each case expressed as a percentage of the executive’s base salary for such year. |
46
Table of Contents
| • | Full payment of all of the applicable COBRA premiums for any health, dental or vision plan sponsored by us for a period of up to (i) 24 months for our CEO, executive chairman or president, (ii) 18 months for executive vice presidents and senior vice presidents (which includes our NEOs other than Mr. Cozadd), and (iii) 12 months for vice presidents, provided that the executive timely elects continued coverage. |
| • | Acceleration in full of the vesting and exercisability, as applicable, of outstanding stock options and other equity awards held by the executive. |
The following key terms are defined in the change in control plan:
| • | A “change in control” generally means: (i) a person or group acquires ownership of more than 30% of the combined voting power of our outstanding securities (other than directly from our company); (ii) certain compromises or arrangements sanctioned by the Irish courts, certain schemes, contracts or offers that have become binding on all of our shareholders, certain takeover bids, certain offers or reverse takeover transactions, or a reorganization, merger, statutory share exchange, consolidation or similar transaction involving us, after which our shareholders do not own more than 50% of the combined voting power of the surviving entity or its parent in substantially the same proportion as their ownership of our outstanding voting securities immediately before the transaction, or a person or group acquires ownership of more than 30% of the combined voting power of the surviving entity or its parent, or at least a majority of the members of the board of directors of the parent (or the surviving entity, if there is no parent) following such transaction are not incumbent board members (as defined in (v) below) at the time our board of directors approves the transaction; (iii) our shareholders or our board of directors approves a complete dissolution or liquidation of our company, or a complete dissolution or liquidation of our company otherwise occurs (except for a liquidation into a parent company); (iv) a sale, lease, license or other disposition of all or substantially all of our assets; or (v) individuals who were members of our board of directors as of February 10, 2016 (or members of our board of directors approved or recommended by a majority vote of such members still in office), referred to as “incumbent board members,” cease to constitute at least a majority of the board of directors. |
| • | An “involuntary termination without cause” generally means an executive’s employment is terminated for any reason other than for the following reasons: (i) the executive’s unauthorized use or disclosure of confidential information or trade secrets which causes material harm to us; (ii) the executive’s material breach of any agreement with us (or the executive’s material violation of any statutory duty owed to us) after an opportunity to cure; (iii) the executive’s material failure to comply with our written policies or rules after an opportunity to cure; (iv) the executive’s conviction or plea of guilty or no contest to any crime involving fraud, dishonesty or moral turpitude; (v) the executive’s gross misconduct; (vi) the executive’s continued failure to perform his or her assigned duties after notification; or (vii) the executive’s failure to reasonably cooperate in good faith with any governmental or internal investigation of us or our directors, officers or employees. An “involuntary termination without cause” also includes an executive’s termination of employment due to death or disability. |
| • | A “constructive termination” generally means an executive resigns employment after any of the following actions are taken or events occur without the executive’s written consent: (i) one or more reductions in the executive’s base salary that results in a total reduction in the executive’s base salary, as in effect immediately prior to the change in control or any higher base salary in effect following the change in control, by more than 10%; (ii) a relocation of the executive’s principal place of employment that increases the executive’s one-way commute by more than 35 miles; (iii) a substantial reduction in the executive’s authority, duties or responsibilities that are in effect immediately prior to the change in control, provided that if the executive holds the same position but the size of the executive’s employing entity or business unit has decreased significantly or our company or the executive’s employing entity ceases to be a publicly-traded corporation, the executive’s authority, duties and responsibilities will be considered to be substantially reduced; (iv) a reduction in the executive’s title; or (v) a substantial increase in executive’s required business travel as compared with the executive’s required business travel prior to the change in control. |
47
Table of Contents
We benefit by requiring the executive to execute an effective general waiver and release of claims in order to be eligible to receive benefits under the change in control plan. All other benefits (such as life insurance, disability coverage and 401(k) Plan eligibility) will terminate as of the executive’s termination date.
The change in control plan does not provide for the gross up of any excise taxes imposed by section 4999 of the Code. If any of the severance benefits payable under the change in control plan would constitute a “parachute payment” within the meaning of section 280G of the Code, subject to the excise tax imposed by section 4999 of the Code, the change in control plan provides for a best after-tax analysis with respect to such payments, under which the executive will receive whichever of the following two alternative forms of payment would result in executive’s receipt, on an after-tax basis, of the greater amount of the transaction payment notwithstanding that all or some portion of the transaction payment may be subject to the excise tax: (i) payment in full of the entire amount of the transaction payment, or (ii) payment of only a part of the transaction payment so that the executive receives the largest payment possible without the imposition of the excise tax.
The executive would not receive benefits under the change in control plan in certain circumstances, including if (i) the executive voluntarily terminates employment with us to accept employment with another entity that is controlled, directly or indirectly, by us or is otherwise affiliated with us; (ii) the executive does not confirm in writing that he or she is subject to agreements with us relating to proprietary and confidential information and our code of conduct; or (iii) the executive does not return all company property. In addition, benefits would be terminated under the change in control plan if the executive willfully breaches his or her agreements with us relating to proprietary and confidential information or our code of conduct or engages in certain solicitation or business interference activities.
The structure and amount of benefits provided under the change in control plan are intended to balance our goals of attracting and retaining highly qualified individuals, providing the appropriate incentive for such individuals to perform in the best interests of our shareholders and maintaining responsible pay practices. Our compensation committee periodically reviews market data to gain a general understanding of the change in control benefits offered by our competitors and reviews the benefits offered under the change in control plan against such market data to ensure that the benefits under the change in control plan remain appropriate.
Equity Compensation Plans
The 2011 Plan and 2007 Plan and award agreements thereunder provide for potential vesting acceleration upon an executive’s termination in connection with a change in control and, at the discretion of the board of directors, upon certain change in control events, as further described above under the heading “Description of Compensation Arrangements—Equity Compensation Arrangements.”
48
Table of Contents
Potential Payments upon Termination or Change in Control Table
The following table estimates the potential severance payments and benefits under the change in control plan to which the NEOs would be entitled in connection with specified termination events, calculated as if each NEO’s employment had terminated as of December 31, 2016. In addition, the table sets forth the amounts to which the NEOs would be entitled under the 2011 Plan and 2007 Plan if, upon a corporate transaction or change in control transaction, the board of directors exercised its discretion to accelerate the vesting and exercisability of stock options and the vesting of RSU awards, and such event occurred on December 31, 2016.
There are no other agreements, arrangements or plans that entitle any NEOs to severance, perquisites or other benefits upon termination of employment or a change in control. For purposes of the table below, we have assumed that none of the potential severance benefits payable under the change in control plan would be subject to the excise tax imposed by section 4999 of the Code and therefore would not be reduced in accordance with the terms of the change in control plan.
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
AS OF DECEMBER 31, 2016
| Name | Benefit | Involuntary Termination Without Cause or Constructive Termination in Connection with a Change of Control($)(1) |
2011 Plan and 2007 Plan—Certain Corporate Transactions($)(2) | |||
| Bruce C. Cozadd |
Lump Sum Cash Severance Payment | 5,263,250 | — | |||
| COBRA Payments | 44,739 | — | ||||
| Vesting Acceleration (3) | 8,561,915 | 8,561,915 | ||||
|
|
| |||||
| Benefit Total | 13,869,904 | 8,561,915 | ||||
|
|
| |||||
| Matthew P. Young |
Lump Sum Cash Severance Payment | 1,586,644 | — | |||
| COBRA Payments | 49,940 | — | ||||
| Vesting Acceleration (3) | 2,622,484 | 2,622,484 | ||||
|
|
| |||||
| Benefit Total | 4,259,068 | 2,622,484 | ||||
|
|
| |||||
| Russell J. Cox |
Lump Sum Cash Severance Payment | 1,794,689 | — | |||
| COBRA Payments | 53,169 | — | ||||
| Vesting Acceleration (3) | 3,023,373 | 3,023,373 | ||||
|
|
| |||||
| Benefit Total | 4,871,231 | 3,023,373 | ||||
|
|
| |||||
| Suzanne Sawochka Hooper |
Lump Sum Cash Severance Payment | 1,656,234 | — | |||
| COBRA Payments | 36,206 | — | ||||
| Vesting Acceleration (3) | 2,689,917 | 2,689,917 | ||||
|
|
| |||||
| Benefit Total | 4,382,357 | 2,689,917 | ||||
|
|
| |||||
| Karen Smith, M.D., Ph.D. |
Lump Sum Cash Severance Payment | 1,312,500 | — | |||
| COBRA Payments | 53,383 | — | ||||
| Vesting Acceleration (3) | 1,212,632 | 1,212,632 | ||||
|
|
| |||||
| Benefit Total | 2,578,515 | 1,212,632 | ||||
|
|
| |||||
|
|
|
| (1) | These benefits would be payable under the change in control plan if the involuntary termination without cause or constructive termination occurred upon or within 12 months following a change in control and assuming such termination took place on December 31, 2016. The forms of stock option and RSU agreements under the 2011 Plan and the 2007 Plan provide for the same vesting acceleration benefit as shown here under the change in control plan, therefore no separate vesting acceleration benefit is listed. Pursuant to the change in control plan, an involuntary termination without cause also includes an individual’s death or disability. |
| (2) | These benefits would be payable under the 2011 Plan and the 2007 Plan if, upon a corporate transaction event, the board of directors exercised its discretion to accelerate the vesting and exercisability of outstanding stock options and RSU awards, assuming the vesting acceleration took place on December 31, 2016. For a description of the potential vesting acceleration provisions in the 2011 Plan and the 2007 Plan, see “Description of Compensation Arrangements—Equity Compensation Arrangements” above. |
| (3) | The value of stock option and RSU award vesting acceleration is based on the closing price of $109.03 per ordinary share as of December 30, 2016, minus, in the case of stock options, the exercise price of the unvested stock option shares subject to acceleration. |
49
Table of Contents
DIRECTOR COMPENSATION
Non-Employee Director Compensation Policy
Pursuant to our non-employee director compensation policy, or director compensation policy, each non-employee director was entitled to receive the following cash compensation for board services, as applicable, for 2016:
| • | a $55,000 (increased to $60,000 effective May 2016) annual retainer for service as a member of our board of directors (paid quarterly); |
| • | a supplemental $25,000 (increased to $50,000 effective May 2016) annual retainer for service as the Lead Independent Director (paid quarterly); |
| • | a supplemental annual retainer for the chairs of the following board committees in the following amounts: $25,000 for the chairperson of the audit committee, $22,500 for the chairperson of the compensation committee, $20,000 for the chairperson of the nominating and corporate governance committee and $22,500 for the chairperson of the transaction committee (each paid quarterly); and |
| • | a supplemental annual retainer for each member of the following board committees other than the chairs, in the following amounts: $15,000 for service as a member of the audit committee, $12,500 for service as a member of the compensation committee, $10,000 for service as a member of the nominating and corporate governance committee and $12,500 for service as a member of the transaction committee (each paid quarterly). |
Our director compensation policy was originally approved by our board of directors in May 2013 and has been amended as follows: in August 2013 to, among other things, provide for cash retainers for the chairperson and members of the transaction committee; in May 2014 to provide for compensation to our Lead Independent Director and revise the number of initial and continuing equity grants; in October 2014 to provide for a tax equalization payment on any Irish tax that may be paid on company reimbursement of reasonable travel, lodging and meal expenses related to service on the board of directors; in April 2015 to revise the number of initial and continuing equity grants; and in May 2016 to increase the annual retainer for service as a member of our board of directors, increase the annual retainer for service as our Lead Independent Director and revise the number of initial and continuing RSU awards, as discussed below.
The director compensation policy currently provides for the automatic grant of equity awards to our non-employee directors over the period of their service on our board of directors. Any individual who first becomes a non-employee director is automatically granted the following: (a) an initial option to purchase 5,695 ordinary shares that vests with respect to one-third of the shares on the first anniversary of the date of such individual’s election or appointment to the board of directors, and, with respect to the balance, in a series of 24 successive equal monthly installments thereafter and (b) an initial RSU award covering 2,280 ordinary shares that vests in equal annual installments over three years from the date of such individual’s election or appointment to the board of directors, subject in each case to the non-employee director’s continuous service through such dates. If a non-employee director does not stand for reelection at an annual general meeting of our shareholders in the year in which his or her term expires or otherwise resigns effective at an annual general meeting of our shareholders and, in either case, the non-employee director’s continuous service terminates at such meeting, then effective as of the date of such meeting, any unvested portion of the initial option award will become vested and exercisable, and any
50
Table of Contents
unvested portion of the initial RSU award will become vested, in each case with respect to the portion of the award that would have vested through the anniversary of the award’s vesting commencement date in the year of that meeting. From April 2015 until May 2016, the number of ordinary shares subject to the initial RSU award was 2,185 ordinary shares.
Under the current director compensation policy, each continuing non-employee director will automatically be granted the following continuing grants in connection with each annual general meeting: (a) a continuing option to purchase 3,415 ordinary shares that vests in a series of 12 successive equal monthly installments measured from the date of the annual general meeting of our shareholders with respect to which the option is granted and (b) a continuing RSU award covering 1,365 ordinary shares that vests in full on the first anniversary of the date of the annual general meeting of our shareholders with respect to which the RSU award is granted, subject in each case to the non-employee director’s continuous service through such dates. If a director is elected or appointed as a director for the first time other than at an annual general meeting, in order to receive automatic continuing grants, the director must have first joined the board at least four calendar months before the date of the applicable annual general meeting. If a director is elected or appointed as a director for the first time at an annual general meeting, the director will not receive automatic continuing grants for such meeting. If a non-employee director does not stand for reelection at an annual general meeting of our shareholders in the year in which his or her term expires or otherwise resigns effective at an annual general meeting of our shareholders and, in either case, the non-employee director’s continuous service terminates at such meeting, then effective as of the date of such meeting, any unvested portion of the continuing option award will become vested and exercisable in full and any unvested portion of a continuing RSU award will become vested in full. From April 2015 until May 2016, the number of ordinary shares subject to each continuing RSU award was 1,310 ordinary shares.
The automatic initial and continuing options and RSU awards are granted under the Amended and Restated 2007 Non-Employee Directors Stock Award Plan, or 2007 Directors Plan, unless the board determines such options or RSU awards will be granted under the 2007 Plan.
The grant date of these equity awards is the second trading day following the filing date of our next quarterly or annual report filed under the Exchange Act that occurs after the date the director first joined our board of directors (with respect to the automatic initial option and RSU awards) or the date of our annual general meeting (with respect to the automatic continuing option and RSU awards). The other terms and conditions applicable to equity awards made to our non-employee directors are included below under the heading “Equity Compensation Plans.”
In addition, our non-employee directors are reimbursed for travel and other reasonable expenses incurred in attending board or committee meetings, as are our employees who serve as directors. If any reimbursement payment is subject to tax imposed by the Irish Revenue Commissioners, each non-employee director is also entitled to a tax equalization payment in order to allow them to retain the full reimbursement payment.
Directors Continuing Education
In furtherance of our ongoing commitment to the continuing education of our directors, our nominating and corporate governance committee adopted a policy for the reimbursement of director continuing education in February 2013, as amended in February 2014. Under this policy, we will pay or reimburse each director for enrollment fees and reasonable expenses incurred in connection with attending and participating each year in one director continuing education program and in one healthcare industry continuing education program, each sponsored by an outside provider.
51
Table of Contents
Directors Deferred Compensation Plan
In May 2007, the Jazz Pharmaceuticals, Inc. board of directors adopted the Directors Deferred Compensation Plan, which was amended and restated in August 2010. The Directors Deferred Compensation Plan, as amended and restated, is referred to in this report as the Directors Deferred Plan. We continued and assumed the Directors Deferred Plan in connection with the Azur Merger. The Directors Deferred Plan allows each non-employee director to elect to defer receipt of all or a portion of his or her annual retainer fees to a future date or dates. Amounts deferred under the Directors Deferred Plan are credited as our ordinary shares to a phantom stock account, and the number of shares credited is based on the amount of the retainer fees deferred divided by the market value of our ordinary shares on the first trading day of the first open window period following the date the retainer fees were deemed earned. On the tenth business day following the day of separation from the board of directors or the occurrence of a change in control, or as soon thereafter as practical once the non-employee director has provided the necessary information for electronic deposit of the deferred shares, each non-employee director will receive (or commence receiving, depending upon whether the director has elected to receive distributions from his phantom stock account in a lump sum or in installments over time) a distribution from his phantom stock account in our ordinary shares. The Directors Deferred Plan may be amended or terminated at any time by the board of directors. The Directors Deferred Plan in form and operation is intended to be compliant with section 409A of the Code.
Although we continue to maintain the Directors Deferred Plan, since the closing of the Azur Merger we have not permitted our non-employee directors to defer any annual retainer fees under the Directors Deferred Plan.
Ownership Guidelines for Directors
In February 2013, our board of directors adopted share ownership guidelines for the company’s non-employee directors. Under the guidelines, each non-employee director is expected to own a number of the company’s ordinary shares with a value equal to three times his or her annual cash retainer. The guidelines provide that the individuals subject to the guidelines are expected to establish the minimum ownership levels within five years of the company’s adoption of the guidelines (or within five years of the date a director first becomes subject to them). As of March 31, 2017, each non-employee director was in compliance with his or her share ownership requirement under the guidelines.
Equity Compensation Plans
The 2007 Directors Plan, which was initially adopted by the Jazz Pharmaceuticals, Inc. board of directors and approved by the Jazz Pharmaceuticals, Inc. stockholders in connection with its initial public offering, was continued and assumed by us upon the consummation of the Azur Merger. The automatic initial option awards and continuing option awards under our director compensation policy described above are granted under the 2007 Directors Plan unless otherwise determined by our board of directors. Although the automatic initial RSU awards and continuing RSU awards under the director compensation policy described above have been granted under the 2007 Plan, in August 2016, our shareholders approved an amendment and restatement of the 2007 Directors Plan to expand the types of stock awards that may be granted under that plan, allowing our board of directors to grant RSU awards under the 2007 Directors Plan.
With respect to options granted under the 2007 Directors Plan and 2007 Plan, if a non-employee director’s service relationship with us or any of our affiliates, whether as a non-employee director or subsequently as our employee, director or consultant or that of any of our affiliates, ceases for any reason other than disability or death, or, with respect to options granted under the 2007 Directors Plan only, after any 12-month period following a change in control, the optionee may exercise any vested options for a period of three months following the cessation of service. If such optionee’s service relationship with us, or any of our affiliates, ceases due to disability or death (or an optionee dies within a certain period following cessation of service), the optionee or a beneficiary may exercise the option for a period of 12 months in the event of disability, and 18 months in the event of death. With respect to options granted under the 2007 Directors Plan, if such optionee’s service terminates within 12 months following a specified change in control transaction, the optionee may exercise any vested portion of the option for a period of
52
Table of Contents
12 months following the effective date of such a transaction. The option term may be extended in the event that exercise of the option following termination of service is prohibited by applicable securities laws. In no event, however, may an option be exercised beyond the expiration of its term.
With respect to RSU awards granted under the 2007 Directors Plan and 2007 Plan, if a non-employee director’s service relationship with us or any of our affiliates, whether as a non-employee director or subsequently as our employee, director or consultant or that of any of our affiliates, ceases for any reason, any RSU awards that were unvested as of the date of such termination will be forfeited.
In the event of certain significant corporate transactions (which generally have a meaning similar to “corporate transaction” under the 2011 Plan), all outstanding awards under the 2007 Directors Plan may be assumed, continued or substituted for by any surviving or acquiring entity (or its parent company). If the surviving or acquiring entity (or its parent company) elects not to assume, continue or substitute for such awards, then (a) with respect to any such awards that are held by participants then performing services for us or our affiliates, the vesting and exercisability of such awards will be accelerated in full and such awards will be terminated if not exercised (if applicable) prior to the effective date of the corporate transaction and (b) all other outstanding awards will terminate if not exercised prior to the effective date of the corporate transaction. The board of directors may also provide that the holder of an outstanding award not assumed in the corporate transaction will surrender such award in exchange for a payment equal to the excess of (i) the value of the property that the holder would have received upon exercise of the award, over (ii) the exercise price otherwise payable in connection with the exercise. In addition, the vesting and exercisability of awards under the 2007 Directors Plan held by non-employee directors who are either required to resign their position as a condition of a specified change in control transaction (which generally has a similar meaning as a “change in control” under the 2011 Plan) or are removed from their position in connection with such a change in control will be accelerated in full.
The treatment of outstanding options and RSU awards under the 2007 Plan in the event of certain significant corporate transactions or a specified change in control transaction is described above under the heading “Executive Compensation—Description of Compensation Arrangements—Equity Compensation Arrangements—2007 Equity Incentive Plan.”
2016 Equity Grants
In accordance with our non-employee director compensation policy described above, we made automatic continuing grants to each of our non-employee directors as a result of their continuing on the board of directors through our annual general meeting in August 2016, which continuing grants were comprised of an option to purchase 3,415 ordinary shares and an RSU award covering 1,365 ordinary shares. All options granted to non-employee directors during 2016 were granted under the 2007 Directors Plan and all RSU awards granted during 2016 were granted under the 2007 Plan.
53
Table of Contents
Director Compensation Table
The following table sets forth certain information with respect to the compensation of all of our non-employee directors for the fiscal year ended December 31, 2016.
Mr. Cozadd, our Chairman and CEO, is not listed in the following table because he is our employee. Mr. Cozadd’s compensation is described under “Executive Compensation.” Mr. Cozadd received no additional compensation for serving on our board of directors in 2016.
DIRECTOR COMPENSATION FOR FISCAL 2016
| Name | Fees Earned ($)(1) |
Stock Awards ($)(2)(4) |
Option Awards ($)(3)(4) |
All Other Compensation ($)(5) |
Total ($) | |||||
| Paul L. Berns |
70,815 | 188,479 | 146,440 | — | 405,734 | |||||
| Patrick G. Enright |
70,815 | 188,479 | 146,440 | — | 405,734 | |||||
| Peter Gray |
95,815 | 188,479 | 146,440 | — | 430,734 | |||||
| Heather Ann McSharry |
95,815 | 188,479 | 146,440 | — | 430,734 | |||||
| Seamus Mulligan |
80,815 | 188,479 | 146,440 | — | 415,734 | |||||
| Kenneth W. O’Keefe |
73,315 | 188,479 | 146,440 | — | 408,234 | |||||
| Norbert G. Riedel, Ph.D. |
93,315 | 188,479 | 146,440 | — | 428,234 | |||||
| Elmar Schnee |
68,315 | 188,479 | 146,440 | 7,450 | 410,684 | |||||
| Catherine A. Sohn, Pharm. D. |
90,815 | 188,479 | 146,440 | 3,950 | 429,684 | |||||
| Rick E Winningham |
109,890 | 188,479 | 146,440 | — | 444,809 |
| (1) | The dollar amounts in this column represent each non-employee director’s actual annual cash retainer earned for board services in 2016, which is equal to the aggregate of his or her annual retainer of $55,000 (increased to $60,000 effective May 2016) plus his or her annual retainers for service on one or more board committees, and for Mr. Winningham, for service as Lead Independent Director. Each non-employee director’s total fees were earned and payable in four quarterly installments subject to the non-employee director’s continuous service at the end of each quarter. Fees paid to each of Ms. McSharry and Messrs. Gray, Mulligan and Schnee were paid in Euro. The conversion to U.S. dollars was calculated based on the average exchange rate for each quarter as reported by the OANDA Corporation. Following the Azur Merger, the board of directors did not permit cash retainer fees to be deferred by our non-employee directors pursuant to the Directors Deferred Plan. The total number of shares previously credited to each individual non-employee director’s phantom stock account under the Directors Deferred Plan as of December 31, 2016 were as follows: 4,691 shares for Mr. Berns; 9,929 shares for Mr. Enright; 22,249 shares for Mr. O’Keefe; and no shares for the other non-employee directors. |
| (2) | The dollar amounts in this column reflect the aggregate grant date fair value of RSU awards computed in accordance with ASC 718. The grant date fair value of each RSU award is measured based on the closing price of our ordinary shares on the date of grant. These amounts do not necessarily correspond to the actual value recognized or that may be recognized by the non-employee directors. |
| (3) | The dollar amounts in this column represent the aggregate grant date fair value of each stock option award granted to our non-employee directors in 2016. These amounts have been calculated in accordance with ASC 718, using the Black-Scholes option-pricing model and excluding the effect of estimated forfeitures. Assumptions used in the calculation of these amounts are included in the notes to our audited consolidated financial statements included in the company’s 2016 Annual Report on Form 10-K. These amounts do not necessarily correspond to the actual value recognized or that may be recognized by the non-employee directors. |
54
Table of Contents
| (4) | The aggregate number of shares subject to outstanding stock options and RSU awards held by the non-employee directors listed in the table above as of December 31, 2016 was as follows: 19,130 shares subject to outstanding stock options and 1,365 shares subject to outstanding RSUs for each of Messrs. Berns, Enright, Mulligan and Winningham; 22,630 shares subject to outstanding stock options and 1,365 shares subject to outstanding RSUs for Dr. Sohn; 14,630 shares subject to outstanding stock options and 1,365 shares subject to outstanding RSUs for Mr. O’Keefe; 18,130 shares subject to outstanding stock options and 1,365 shares subject to outstanding RSUs for each of Ms. McSharry, Mr. Gray and Dr. Riedel; and 11,830 shares subject to outstanding stock options and 2,198 shares subject to outstanding RSUs for Mr. Schnee. |
| (5) | The dollar amount in this column for Mr. Schnee and Dr. Sohn represents reimbursed continuing director education fees. |
Compensation Committee Report(1)
The compensation committee has reviewed and discussed with management the Compensation Discussion and Analysis contained herein. Based on this review and discussion, the compensation committee has recommended to the board of directors that the Compensation Discussion and Analysis be included in our proxy statement for the 2017 annual general meeting of shareholders and be included in the company’s Annual Report on Form 10-K we filed with the SEC for the fiscal year ended December 31, 2016.
Respectfully submitted,
The Compensation Committee of the Board of Directors
Dr. Norbert G. Riedel, Ph.D. (Chair)
Mr. Paul L. Berns
Mr. Patrick G. Enright
| (1) | The material in this report is not “soliciting material,” is not deemed “filed” with the Commission and is not to be incorporated by reference in any filing of the registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. |
55
Table of Contents
Compensation Committee Interlocks and Insider Participation
During 2016 our compensation committee was composed of three directors: Messrs. Berns and Enright, and Dr. Riedel. None of the members of our compensation committee during 2016 has at any time been our officer or employee. None of our executive officers serve, or in the past fiscal year has served, as a member of the board of directors or the compensation committee of any entity that has one or more of its executive officers serving on our board of directors or compensation committee.
Compensation Consultant Fees
The compensation committee engages an independent compensation consultant each year to provide a competitive compensation assessment with respect to the executive officers to assist the compensation committee in making annual compensation decisions. Since 2010, Radford has been engaged by the compensation committee each year to provide peer company and industry compensation data and provide the compensation committee with advice regarding executive officers’ compensation, including base salaries, performance-based bonuses and long-term equity compensation, and similar advice regarding directors’ compensation. In 2016, the cost of Radford’s executive compensation and director compensation consulting services provided to the compensation committee was approximately $104,000. In addition, in 2016 management also engaged Radford to provide survey data relating to non-executive employee compensation and other affiliates of Aon to provide director and officer liability insurance-related services, pension-related services, other insurance brokerage services and risk services. The aggregate cost of such other consulting services provided in 2016 by Radford and other affiliates of Aon (not related to Radford’s executive compensation and director compensation consulting services provided to the compensation committee) was approximately $138,000, of which approximately $126,000 related to various insurance-related and benefits consulting services and approximately $12,000 related to general survey data. Although the compensation committee was aware of the nature of the services performed by affiliates of Aon and the non-executive employee compensation survey data provided by Radford, the compensation committee did not review and approve such services and surveys, as those were reviewed and approved by management in the ordinary course of business.
56
Table of Contents
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
EQUITY COMPENSATION PLAN INFORMATION
The following table provides certain information as of December 31, 2016 with respect to all of our equity compensation plans in effect on that date.
| Plan Category (1) |
Number of securities to warrants and rights (a) |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for (c) |
|||||||||
| Equity compensation plans approved by security holders: |
||||||||||||
| Amended and Restated 2011 Equity Incentive Plan |
4,912,156 | $96.84 (2) | 8,672,451 (3) | |||||||||
| 2007 Equity Incentive Plan |
439,425 | $18.60 (4) | 882,400 (5) | |||||||||
| 2007 Employee Stock Purchase Plan |
N/A | N/A | 442,510 (6) | |||||||||
| Amended and Restated 2007 Non-Employee Directors Stock Award Plan |
158,500 | $122.55 | 304,497 (7) | |||||||||
| Equity compensation plans not approved by security holders: | ||||||||||||
| Amended and Restated Directors Deferred Compensation Plan |
36,869 (8) | N/A | 163,816 (9) | |||||||||
|
|
|
|
|
|||||||||
| Total |
5,546,950 | 10,465,674 | ||||||||||
| (1) | Each of the equity compensation plans set forth in this table was originally adopted by Jazz Pharmaceuticals, Inc. and assumed and adopted by us in connection with the Azur Merger. In addition, each option that was outstanding under Jazz Pharmaceuticals, Inc.’s equity compensation plans was converted into an option to acquire, on substantially the same terms and conditions as were applicable under such option before the Azur Merger, the number of our ordinary shares equal to the number of shares of Jazz Pharmaceuticals, Inc.’s common stock subject to such option immediately prior to the Azur Merger, at an exercise price per ordinary share equal to the exercise price per share of Jazz Pharmaceuticals, Inc.’s common stock otherwise purchasable pursuant to such option, and each other equity award that was outstanding under Jazz Pharmaceuticals, Inc.’s equity compensation plans was converted into a right to receive, on substantially the same terms and conditions as were applicable under such equity award before the Azur Merger, the number of our ordinary shares equal to the number of shares of Jazz Pharmaceuticals, Inc.’s common stock subject to such equity award immediately prior to the Azur Merger. Other than with respect to the Directors Deferred Plan, each of the equity compensation plans set forth in this table was approved by Jazz Pharmaceuticals, Inc.’s stockholders. |
| (2) | The weighted-average exercise price takes into account 982,763 ordinary shares under the 2011 Plan issuable upon vesting of outstanding RSUs, which have no exercise price. The weighted-average exercise price, excluding such outstanding RSUs, is $121.06. |
| (3) | As of December 31, 2016, an aggregate of up to 19,036,985 of our ordinary shares were authorized for issuance under the 2011 Plan, of which 8,672,451 shares remained available for future issuance. The number of ordinary shares reserved for issuance under the 2011 Plan includes up to 3,335,255 ordinary shares subject to stock awards that were originally granted under the 2007 Plan and the 2003 Equity Incentive Plan that may become available for issuance under the 2011 Plan pursuant to the terms of the 2011 Plan and the 2007 Plan. In addition, the number of shares reserved for issuance under the 2011 Plan automatically increases on January 1 of each year for a period of ten years, starting on January 1, 2013 and |
57
Table of Contents
| continuing through January 1, 2022, by the least of (a) 4.5% of the total number of ordinary shares outstanding on December 31 of the preceding calendar year, (b) 5,000,000 ordinary shares, or (c) such lesser number of ordinary shares as determined by our board of directors. On January 1, 2017, the number of shares authorized for issuance under the 2011 Plan increased by 2,692,247 shares pursuant to this automatic share increase provision. |
| (4) | The weighted-average exercise price takes into account 14,483 ordinary shares under the 2007 Plan issuable upon vesting of outstanding RSUs, which have no exercise price. The weighted-average exercise price, excluding such outstanding RSUs, is $19.24. |
| (5) | As of December 31, 2016, an aggregate of 7,495,137 ordinary shares were authorized for issuance under the 2007 Plan, of which 882,400 shares remained available for future issuance. |
| (6) | As of December 31, 2016, an aggregate of 2,660,000 ordinary shares were authorized for issuance under the ESPP, of which 442,510 shares remained available for future issuance, and up to a maximum of 175,000 ordinary shares may be purchased in the current purchase period. The number of shares reserved for issuance under the ESPP automatically increases on January 1 of each year for a period of ten years, starting on January 1, 2013 and continuing through January 1, 2022, by the least of (a) 1.5% of the total number of our ordinary shares outstanding on December 31 of the preceding calendar year, (b) 1,000,000 ordinary shares, or (c) such lesser amount as may be approved by our board of directors. On January 1, 2017, no additional shares were reserved for issuance under the ESPP pursuant to this automatic share increase provision. |
| (7) | As of December 31, 2016, an aggregate of 903,938 ordinary shares were authorized for issuance under the 2007 Directors Plan, of which 304,497 shares remained available for future issuance. The number of shares remaining available for issuance under the 2007 Directors Plan as shown in the table above has been reduced by the number of shares credited to our non-employee directors’ stock accounts under the Directors Deferred Plan prior to August 15, 2010. The number of shares reserved for issuance under the 2007 Directors Plan automatically increased on January 1 of each year starting on January 1, 2008 and continuing through January 1, 2016, by the sum of (a) the excess of (i) the number of shares subject to options granted during the preceding calendar year under the 2007 Directors Plan, over (ii) the number of shares added back to the share reserve under the 2007 Directors Plan during the preceding calendar year and (b) for the automatic annual increases that occurred on or prior to January 1, 2010 only, the aggregate number of shares credited to our non-employee directors’ stock accounts under the Directors Deferred Plan during the preceding calendar year. |
| (8) | Represents shares credited to individual non-employee director stock accounts in lieu of director fees as of December 31, 2016 under the Directors Deferred Plan. There is no exercise price for these shares. Distributions under the Directors Deferred Plan are funded (i) with shares reserved under the 2007 Directors Plan for amounts credited to our non-employee directors’ stock accounts prior to August 15, 2010 and (ii) with shares reserved under the Directors Deferred Plan for amounts credited to our non-employee directors’ stock accounts on or after August 15, 2010. |
| (9) | Amounts credited to our non-employee directors’ stock accounts prior to August 15, 2010 pursuant to the Directors Deferred Plan are funded with shares reserved under the 2007 Directors Plan. In August 2010, a separate reserve of 200,000 shares was created under the Directors Deferred Plan which funds all distributions of amounts credited to our non-employee directors’ stock accounts on or after August 15, 2010 pursuant to the Directors Deferred Plan. Since the Azur Merger, non-employee directors have not been permitted to defer director fees pursuant to the Directors Deferred Plan. A description of the Directors Deferred Plan is provided under “Executive Compensation—Director Compensation—Directors Deferred Compensation Plan.” |
58
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding the ownership of our ordinary shares as of March 31, 2017 (except as noted) by: (i) each director; (ii) each of our NEOs identified in Item 11 of this report; (iii) all of our executive officers and directors as a group; and (iv) all those known by us to be beneficial owners of more than five percent of our ordinary shares.
| Beneficial Ownership (2) | ||||||||
| Name and Address of Beneficial Owner (1) | Number of Shares | Percentage of Total |
||||||
| 5% Shareholders: |
||||||||
| Putnam Investments, LLC (3) |
7,734,707 | 12.9% | ||||||
| One Post Office Square |
||||||||
| Boston, MA 02109 |
||||||||
| FMR LLC (4) |
7,590,328 | 12.7% | ||||||
| 245 Summer Street |
||||||||
| Boston, MA 02210 |
||||||||
| The Vanguard Group (5) |
4,371,250 | 7.3% | ||||||
| 100 Vanguard Blvd. |
||||||||
| Malvern, PA 19355 |
||||||||
| BlackRock, Inc. (6) |
3,290,129 | 5.5% | ||||||
| 55 East 52nd Street |
||||||||
| New York, NY 10055 |
||||||||
| Named Executive Officers and Directors: |
||||||||
| Bruce C. Cozadd (7) |
523,450 | * | ||||||
| Matthew P. Young (8) |
77,605 | * | ||||||
| Russell J. Cox (9) |
212,499 | * | ||||||
| Suzanne Sawochka Hooper (10) |
132,763 | * | ||||||
| Karen Smith, M.D., Ph.D. (11) |
19,700 | * | ||||||
| Paul L. Berns (12) |
26,836 | * | ||||||
| Patrick G. Enright (13) |
248,205 | * | ||||||
| Peter Gray (14) |
22,665 | * | ||||||
| Heather Ann McSharry (15) |
21,987 | * | ||||||
| Seamus Mulligan (16) |
1,064,919 | 1.8% | ||||||
| Kenneth W. O’Keefe (17) |
48,767 | * | ||||||
| Norbert G. Riedel, Ph.D. (18) |
20,864 | * | ||||||
| Elmar Schnee (19) |
13,536 | * | ||||||
| Catherine A. Sohn, Pharm.D. (20) |
26,527 | * | ||||||
| Rick E Winningham (21) |
32,302 | * | ||||||
| All directors and executive officers as a group (19 persons) (22) |
2,633,087 | 4.3% | ||||||
| * | Less than 1%. |
| (1) | Unless otherwise provided in the table above or in the notes below, the address for each of the beneficial owners listed is c/o Fifth Floor, Waterloo Exchange, Waterloo Road, Dublin 4, Ireland. |
| (2) | This table is based upon information supplied by officers and directors as well as Schedules 13G or 13D filed with the SEC by beneficial owners of more than five percent of our ordinary shares. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, we believe that each of the shareholders named in this table has sole voting and investment power with respect to the |
59
Table of Contents
| ordinary shares indicated as beneficially owned. Applicable percentages are based on 59,988,162 ordinary shares outstanding on March 31, 2017, adjusted as required by rules promulgated by the SEC. The number of shares beneficially owned includes ordinary shares issuable pursuant to the exercise of stock options that are exercisable and RSUs that will vest within 60 days of March 31, 2017, and shares credited to individual non-employee director phantom stock accounts under our Directors Deferred Plan as of March 31, 2017. Amounts credited to individual non-employee director phantom stock accounts under our Directors Deferred Plan are payable solely in our ordinary shares, but such shares do not have current voting or investment power. Shares issuable pursuant to the exercise of stock options that are exercisable and RSUs that will vest within 60 days of March 31, 2017 and shares issuable pursuant to our Directors Deferred Plan are deemed to be outstanding and beneficially owned by the person to whom such shares are issuable for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. |
| (3) | This information is based on a Schedule 13G/A filed with the SEC on February 14, 2017 by Putnam Investments, LLC d/b/a Putnam Investments, or Putnam, on behalf of itself and on behalf of Putnam Investment Management, LLC, or PIM, The Putnam Advisory Company, LLC, or PAC, and Putnam Capital Spectrum Fund, or PCSF. According to the Schedule 13G/A, as of December 31, 2016, Putnam has sole power to vote or direct the vote of 77,786 ordinary shares and sole power to dispose or the direct the disposition of 7,734,707 ordinary shares. Of these shares, PIM, a wholly-owned subsidiary of Putnam, is the beneficial owner of 7,652,569 ordinary shares, with sole power to vote or direct the vote of 1,380 ordinary shares and sole power to dispose or direct the disposition of 7,652,569 ordinary shares in its capacity as investment adviser to the Putnam family of mutual funds; and PAC, a wholly owned subsidiary of Putnam, is the beneficial owner of 82,138 ordinary shares, with sole power to vote or direct the vote of 76,406 ordinary shares and sole power to dispose or direct the disposition of 82,138 ordinary shares in its capacity as investment adviser to Putnam’s institutional clients. As part of the Putnam family of funds, and the 7,652,569 shares beneficially owned by PIM, PCSF is the beneficial owner of, and has sole power to vote or direct the vote of, and sole power to dispose or direct the disposition of, 5,418,684 shares. The Schedule 13G/A provides information only as of December 31, 2016 and, consequently, the beneficial ownership of the above-mentioned entities may have changed between December 31, 2016 and March 31, 2017. |
| (4) | This information is based on a Schedule 13G/A filed with the SEC on February 14, 2017 by FMR LLC, or FMR, and Abigail P. Johnson. According to the Schedule 13G/A, as of December 31, 2016, FMR has sole power to vote or direct the vote of 359,219 ordinary shares and the sole power to dispose or direct the disposition of 7,590,328 ordinary shares, and Ms. Johnson has the sole power to dispose or direct the disposition of 7,590,328 ordinary shares. The Schedule 13G/A indicates that FMR is acting as a parent holding company or control person for a number of its relevant entities that beneficially owned the ordinary shares being reported, including FMR Co., Inc., an investment adviser reported as beneficially owning 5% or greater of our ordinary shares. In addition, Ms. Johnson is a Director, the Chairman, and the Chief Executive Officer of FMR. Ms. Johnson and members of her family are the predominant owners, directly or through trusts, of Series B voting common shares of FMR, representing 49% of the voting power of FMR. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, or Investment Company Act, to form a controlling group with respect to FMR. Neither FMR nor Ms. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies registered under the Investment Company Act, or the Fidelity Funds, advised by Fidelity Management & Research Company, or FMRC, a wholly owned subsidiary of FMR, which power resides with the Fidelity Funds’ Boards of Trustees. FMRC carries out the voting of the shares under written guidelines established by the Fidelity Funds’ Boards of Trustees. The Schedule 13G/A provides information only as of December 31, 2016 and, consequently, the beneficial ownership of the |
60
Table of Contents
| above-mentioned persons and entities may have changed between December 31, 2016 and March 31, 2017. |
| (5) | This information is based on a Schedule 13G/A filed with the SEC on February 10, 2017 by The Vanguard Group, or Vanguard. According to the Schedule 13G/A, as of December 31, 2016, Vanguard has sole power to vote or direct the vote of 35,436 ordinary shares, shared power to vote or direct the vote of 10,669 ordinary shares, sole power to dispose or direct the disposition of 4,323,445 ordinary shares, and shared power to dispose or direct the disposition of 47,805 shares. The Schedule 13G/A also indicates that Vanguard Fiduciary Trust Company, a wholly-owned subsidiary of Vanguard, is the beneficial owner of 19,836 ordinary shares as a result of its serving as investment manager of collective trust accounts, and Vanguard Investments Australia, Ltd., a wholly-owned subsidiary of Vanguard, is the beneficial owner of 43,569 ordinary shares as a result of its serving as investment manager of Australian investment offerings. The Schedule 13G/A provides information only as of December 31, 2016 and, consequently, the beneficial ownership of the above-mentioned entities may have changed between December 31, 2016 and March 31, 2017. |
| (6) | This information is based on a Schedule 13G/A filed with the SEC on January 25, 2017 by BlackRock, Inc., or BlackRock. According to the Schedule 13G/A, as of December 31, 2016, BlackRock has sole power to vote or direct the vote of 3,059,254 ordinary shares and sole power to dispose or direct the disposition of 3,290,129 ordinary shares. The Schedule 13G/A also identifies certain subsidiaries that acquired the ordinary shares being reported by BlackRock as parent holding company or control person, none of which individually are reported as beneficially owning 5% or greater of our ordinary shares. The Schedule 13G/A provides information only as of December 31, 2016 and, consequently, the beneficial ownership of the above-mentioned entities may have changed between December 31, 2016 and March 31, 2017. In this regard, we received a report on Form TR-1 (Voting Rights Attached to Shares–Article 12(1) of Directive 2004/109/EC, Financial Instruments–Article 11(3) of the Commission Directive 2007/14/EC), or the Form TR-1, from BlackRock indicating a change in interest in our ordinary shares, as required by the laws of the United Kingdom. The Form TR-1 discloses that, as of March 20, 2017, BlackRock holds less than 5% of voting rights attached to our ordinary shares. Because the “voting rights attached to shares” disclosed pursuant to the Form TR-1 under the laws of the United Kingdom is not necessarily the same as “beneficial ownership” as defined under the rules of the SEC, the table above does not reflect the change in ownership disclosed in the Form TR-1. |
| (7) | Includes 293,047 ordinary shares Mr. Cozadd has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (8) | Includes 58,966 ordinary shares Mr. Young has the right to acquire pursuant to options exercisable and 4,562 shares Mr. Young is expected to receive pursuant to RSUs scheduled to vest, in each case within 60 days of March 31, 2017. |
| (9) | Includes 186,541 ordinary shares Mr. Cox has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (10) | Includes 113,701 ordinary shares Ms. Hooper has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (11) | Includes 13,946 ordinary shares Dr. Smith has the right to acquire pursuant to options exercisable and 1,708 shares Dr. Smith is expected to receive pursuant to RSUs scheduled to vest, in each case within 60 days of March 31, 2017. |
| (12) | Includes 4,691 ordinary shares issuable to Mr. Berns pursuant to our Directors Deferred Plan as of March 31, 2017 and 18,276 ordinary shares Mr. Berns has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (13) | Includes 9,929 ordinary shares issuable to Mr. Enright pursuant to our Directors Deferred Plan as of March 31, 2017 and 18,276 ordinary shares Mr. Enright has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. Also includes 215,677 ordinary shares held by Longitude Venture Partners, L.P. and 4,323 ordinary shares held by Longitude Capital Associates, L.P. The funds named in this |
61
Table of Contents
| footnote (13) are referred to in this footnote as the Longitude Funds. Each of Mr. Enright and Juliet Tammenoms Bakker is a managing member of Longitude Capital Partners, LLC, which is the general partner of each of the Longitude Funds, and may be deemed to have shared voting and dispositive power with respect to the ordinary shares held by or issuable to the Longitude Funds. Each of Mr. Enright and Ms. Bakker disclaims beneficial ownership of all such ordinary shares except to the extent of such person’s proportionate pecuniary interest therein. |
| (14) | Includes 17,276 ordinary shares Mr. Gray has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (15) | Includes 17,276 ordinary shares Ms. McSharry has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (16) | Includes 18,276 ordinary shares Mr. Mulligan has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (17) | Includes 2,000 ordinary shares held by The Kenneth W. O’Keefe Trust U/A/D 2/12/1997, of which Mr. O’Keefe is the sole trustee and sole beneficiary, 22,249 ordinary shares issuable to Mr. O’Keefe pursuant to our Directors Deferred Plan as of March 31, 2017 and 13,776 ordinary shares Mr. O’Keefe has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (18) | Includes 17,276 ordinary shares Dr. Riedel has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (19) | Includes 10,559 ordinary shares Mr. Schnee has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (20) | Includes 21,776 ordinary shares Dr. Sohn has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (21) | Includes 18,276 ordinary shares Mr. Winningham has the right to acquire pursuant to options exercisable within 60 days of March 31, 2017. |
| (22) | Includes 220,000 ordinary shares held by entities affiliated with certain of our non-employee directors, 943,941 ordinary shares that our executive officers and non-employee directors have the right to acquire pursuant to options exercisable within 60 days of March 31, 2017, 8,770 ordinary shares that our executive officers and non-employee directors are expected to receive pursuant to RSUs scheduled to vest within 60 days of March 31, 2017, and 36,869 ordinary shares issuable to non-employee directors pursuant to our Directors Deferred Plan as of March 31, 2017. See footnotes (7) through (21) above. |
62
Table of Contents
Item 13. Certain Relationships and Related Transactions, and Director Independence
Policy and Procedures for Review of Related Party Transactions
We have adopted a Related Party Transaction Policy that sets forth our procedures for the identification, review, consideration and approval or ratification of “related-person transactions.” For purposes of our policy, a “related-person transaction” is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related person” are, were or will be participants and in which the amount involved exceeds $120,000. Transactions involving compensation for services provided to us as an employee or director are not covered by this policy. A “related person” is any executive officer, director or beneficial owner of more than 5% of any class of our voting securities, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, if a transaction has been identified as a related-person transaction (including any transaction that was not a related-person transaction when originally consummated or any transaction that was not initially identified as a related-person transaction prior to consummation), our management must present information regarding the related-person transaction to our audit committee (or, if audit committee approval would be inappropriate, to another independent body of our board of directors) for review, consideration and approval or ratification. The presentation must include a description of, among other things, the material facts, the interests, direct and indirect, of the related person(s), the benefits to us of the transaction and whether the transaction is on terms that are comparable to the terms available to or from, as the case may be, an unrelated third party or to or from employees generally. Under the policy, we will, on an annual basis, collect information that our GC reasonably necessary from each director, executive officer and (to the extent feasible) significant shareholder to enable us to identify any existing or potential related-person transactions and to effectuate the terms of the policy. In addition, under our code of conduct, our employees and directors have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest to our GC, or, if the employee is an executive officer, to our board of directors. In considering related-person transactions, our audit committee (or other independent body of our board of directors) will take into account the relevant available facts and circumstances including, but not limited to, the risks, costs and benefits to us, the terms of the transaction, the availability of other sources for comparable services or products and, if applicable, the impact on a director’s independence in the event that the related person is a director, immediate family member of a director or an entity with which a director is affiliated.
The policy requires that, in determining whether to approve, ratify or reject a related-person transaction, our audit committee (or other independent body of our board of directors) must consider, in light of known circumstances, whether the transaction is in, or is not inconsistent with, our best interests and those of our shareholders, as our audit committee (or other independent body of our board of directors) determines in the good faith exercise of its discretion.
Transactions with Related Persons; Indemnification
Transactions with Related Persons. Since January 1, 2016, we have not engaged in any transactions, nor are any such transactions currently proposed, in which we were a participant and the amount involved exceeded $120,000, and in which any related person had or will have a direct or indirect material interest.
Indemnification. We have entered into indemnification agreements with our directors, executive officers and certain other of our officers and employees. These indemnification agreements require us, under the circumstances and to the extent provided for therein, to indemnify such persons to the fullest extent permitted by applicable law against certain expenses and other amounts incurred by any such person as a result of such person being made a party to certain actions, suits, proceedings and other actions by reason of the fact that such person is or was a director, officer, employee, consultant, agent or fiduciary of our company or any of our subsidiaries or other affiliated enterprises. The rights of each person who is a party to an indemnification agreement are in addition to any other rights such person may have under our Amended and Restated Memorandum and Articles of Association,
63
Table of Contents
the Irish Companies Act 2014, any other agreement, a vote of the shareholders of our company, a resolution of directors of our company or otherwise. We believe that these agreements are necessary to attract and retain qualified persons as our officers and directors. We also maintain directors’ and officers’ liability insurance.
Director Independence
As required under the NASDAQ listing standards, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by the board of directors. Our board of directors consults with counsel to ensure that the board’s determinations are consistent with relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in the applicable NASDAQ listing standards, as in effect from time to time. Consistent with these considerations, after review of all relevant transactions or relationships between each director, or any of his or her family members, and our company, our senior management and our independent registered public accounting firm, the board of directors affirmatively determined that all of our current directors are independent directors within the meaning of the applicable NASDAQ listing standards, except that Mr. Cozadd, our Chairman and CEO, is not independent by virtue of his employment with our company. In addition, our board of directors has determined that each member of the audit committee, compensation committee and nominating and corporate governance committee meets the applicable NASDAQ and SEC rules and regulations regarding “independence” and that each member is free of any relationship that would impair his or her individual exercise of independent judgment with regard to the company. In determining that Seamus Mulligan is independent within the meaning of the applicable NASDAQ listing standards, the Board considered Mr. Mulligan’s former employment relationship with us, which ended more than three years ago.
64
Table of Contents
Item 14. Principal Accounting Fees and Services
Independent Registered Public Accounting Firm Fees and Services
In connection with the audit of our 2016 financial statements, we entered into an engagement agreement with KPMG, Dublin, or KPMG, which sets forth the terms under which KPMG performed audit and tax services for the company.
The following table represents aggregate fees billed to us for the years ended December 31, 2016 and 2015 by KPMG, our independent registered public accounting firm (in thousands):
| Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Audit Fees |
$ | 1,578 | $ | 1,381 | ||||
| Audit-Related Fees |
71 | 73 | ||||||
| Tax Fees |
1,568 | 901 | ||||||
| Tax compliance services |
1,192 | 491 | ||||||
| Tax advisory services |
376 | 410 | ||||||
| All Other Fees |
3 | 3 | ||||||
|
|
|
|||||||
| Total Fees |
$ | 3,220 | $ | 2,358 | ||||
Audit Fees: Consists of fees and expenses for professional services in respect of the audit of the company’s consolidated financial statements and of our internal control over financial reporting, the review of quarterly consolidated financial statements and statutory audits.
Audit-Related Fees: Consists of fees for assurance and related services related to audit and other attestation services performed by KPMG as required by statute, regulation or contract and which are not reported under “Audit Fees.”
Tax Fees: Consists of fees and expenses for professional services for tax compliance, tax advice and tax planning. Tax compliance services consist of professional services related to domestic and international tax compliance, and assistance with domestic and international tax return preparation. Tax advisory service fees relate to tax advice and planning services provided to us in connection with significant transactions undertaken by the company in 2016 and 2015. During the year ended December 31, 2016, fees and expenses of approximately $1,192,000 were billed in connection with tax compliance services, and fees and expenses of approximately $376,000 were billed in connection with tax advice and planning services. During the year ended December 31, 2015, fees and expenses of approximately $491,000 were billed in connection with tax compliance services, and fees and expenses of approximately $410,000 were billed in connection with tax advice and planning services.
All Other Fees: Consists of fees for products and services other than the services described above. For the years ended December 31, 2016 and 2015, these fees were paid in connection with access to the online accounting and tax research tool of KPMG.
All of the services and fees described above were approved by our audit committee.
As shown in the table above, less than 12% of the total fees that KPMG billed us for in 2016 were for services other than audit, audit-related and tax compliance services.
Pre-Approval Policies and Procedures
Our audit committee has a policy and procedures for the pre-approval of audit and non-audit services rendered by our independent registered public accounting firm. Our policy generally requires the pre-approval of specified services in the defined categories of audit services, audit-related services, and tax services up to specified
65
Table of Contents
amounts. Pre-approval may also be given as part of the audit committee’s approval of the scope of the engagement of the independent auditor or on an individual explicit case-by-case basis before the independent auditor is engaged to provide each service. The pre-approval of services may be delegated to one or more of the audit committee’s members, but the decision must be reported to the full audit committee at its next scheduled meeting.
Our audit committee determined that the rendering of the services other than audit services by our independent registered public accounting firm is compatible with maintaining the principal accountant’s independence.
66
Table of Contents
Item 15. Exhibits and Financial Statement Schedules
(a) The following documents are filed as part of the registrant’s Annual Report on Form 10-K filed with the SEC on February 28, 2017:
1. Index to Financial Statements:
See Index to Consolidated Financial Statements in Item 8 of the Annual Report on Form 10-K.
2. Index to Financial Statement Schedules:
The following financial statement schedule of Jazz Pharmaceuticals plc was filed as part of the Annual Report on Form 10-K on page F-47 thereof and should be read in conjunction with the consolidated financial statements of Jazz Pharmaceuticals plc.
Schedule II: Valuation and Qualifying Accounts
All other schedules were omitted because they are not applicable, not required under the instructions, or the requested information is shown in the consolidated financial statements or related notes thereto.
(b) Exhibits—The following exhibits are included herein or incorporated herein by reference.
| Exhibit Number |
Description of Document | |
| 2.1 |
Agreement and Plan of Merger and Reorganization, dated as of September 19, 2011, by and among Azur Pharma Limited (now Jazz Pharmaceuticals plc), Jaguar Merger Sub Inc., Jazz Pharmaceuticals, Inc. and Seamus Mulligan, solely in his capacity as the Indemnitors’ Representative (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals, Inc.’s Current Report on Form 8-K (File No. 001-33500) filed with the SEC on September 19, 2011). | |
| 2.2 |
Letter Agreement, dated as of January 17, 2012, by and among Jazz Pharmaceuticals plc, Jaguar Merger Sub Inc., Jazz Pharmaceuticals, Inc. and Seamus Mulligan, solely in his capacity as the Indemnitors’ Representative (incorporated by reference to Exhibit 2.2 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on January 18, 2012). | |
| 2.3 |
Agreement and Plan of Merger, dated as of April 26, 2012, by and among Jazz Pharmaceuticals plc, Jewel Merger Sub Inc., EUSA Pharma Inc., and Essex Woodlands Health Ventures, Inc., Mayflower L.P., and Bryan Morton, in their capacity as the representatives of the equity holders of EUSA Pharma Inc. (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on April 27, 2012). | |
| 2.4 |
Assignment, dated as of June 11, 2012, by and among Jazz Pharmaceuticals plc and Jazz Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 2.1B in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on June 12, 2012). | |
| 2.5 |
Tender Offer Agreement, dated December 19, 2013, by and among Jazz Pharmaceuticals Public Limited Company, Jazz Pharmaceuticals Italy S.r.l. and Gentium S.p.A. (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K/A (File No. 001-33500), as filed with the SEC on December 20, 2013). | |
| 2.6† |
Asset Purchase Agreement, dated January 13, 2014, by and among Jazz Pharmaceuticals International III Limited, Aerial BioPharma, LLC and Jazz Pharmaceuticals plc (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on January 13, 2014). | |
67
Table of Contents
| 2.7† |
Assignment Agreement, dated July 1, 2014, by and among Jazz Pharmaceuticals International II Limited, Sigma-Tau Pharmaceuticals, Inc., Jazz Pharmaceuticals plc and Gentium S.p.A. (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on August 5, 2014). | |
| 2.8 |
Amended and Restated Agreement for the Acquisition of the Topaz Portfolio Business of Jazz Pharmaceuticals plc, dated March 20, 2015, between Jazz Pharmaceuticals plc and Essex Bidco Limited (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on March 23, 2015). | |
| 2.9 |
Agreement and Plan of Merger, dated as of May 27, 2016, by and among Jazz Pharmaceuticals plc, Plex Merger Sub, Inc., and Celator Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on May 31, 2016). | |
| 3.1 |
Amended and Restated Memorandum and Articles of Association of Jazz Pharmaceuticals plc, as amended on August 4, 2016 (incorporated herein by reference to Exhibit 3.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 4.1 |
Reference is made to Exhibit 3.1. | |
| 4.2A |
Investor Rights Agreement, dated July 7, 2009 by and between Jazz Pharmaceuticals, Inc. and the other parties named therein (incorporated herein by reference to Exhibit 10.88 in Jazz Pharmaceuticals, Inc.’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on July 7, 2009). | |
| 4.2B |
Assignment, Assumption and Amendment Agreement, dated as of January 18, 2012, by and among Jazz Pharmaceuticals, Inc., Jazz Pharmaceuticals plc and the other parties named therein (incorporated herein by reference to Exhibit 4.7B in the Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2011, as filed by Jazz Pharmaceuticals plc on behalf of and as successor to Jazz Pharmaceuticals, Inc. with the SEC on February 28, 2012). | |
| 4.2C |
Indenture, dated as of August 13, 2014, by and among Jazz Pharmaceuticals plc, Jazz Investments I Limited and U.S. Bank National Association (incorporated herein by reference to Exhibit 4.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on August 13, 2014). | |
| 4.2D |
Form of 1.875% Exchangeable Senior Note due 2021 (incorporated herein by reference to Exhibit 4.2 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on August 13, 2014). | |
| 10.1† |
Supply Agreement, dated as of April 1, 2010, by and between Jazz Pharmaceuticals, Inc. and Siegfried (USA) Inc. (incorporated herein by reference to Exhibit 10.54 in Jazz Pharmaceuticals, Inc.’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2010, as filed with the SEC on May 6, 2010). | |
| 10.2† |
Master Services Agreement, dated April 15, 2011, by and between Jazz Pharmaceuticals, Inc., CuraScript, Inc. and Express Scripts Specialty Distribution Services, Inc. (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals, Inc.’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2011, as filed with the SEC on May 9, 2011). | |
| 10.3† |
Royalty Bearing Licence Agreement and Supply Agreement Re Erwinia-Derived Asparaginase, dated July 22, 2005, between Public Health England (formerly Health Protection Agency) and EUSA Pharma SAS (formerly OPi, S.A.), as amended on each of December 22, 2009, March 23, 2012 and August 8, 2012 (incorporated herein by reference to Exhibit 10.11 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q/A (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 9, 2012). | |
| 10.4 |
Novation Agreement relating to Royalty Bearing Licence Agreement and Supply Agreement re Erwinia-Derived Asparaginase, dated as of May 13, 2015, by and among EUSA Pharma SAS, the Secretary of State for Health acting through Public Health England and Porton Biopharma Limited (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2015, as filed with the SEC on August 5, 2015). | |
68
Table of Contents
| 10.5† |
Master Manufacturing Services Agreement, dated as of October 1, 2015, by and between Jazz Pharmaceuticals Ireland Limited and Patheon Pharmaceuticals Inc. (incorporated herein by reference to Exhibit 10.5 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2015, as filed with the SEC on February 23, 2016). | |
| 10.6A |
Credit Agreement, dated as of June 18, 2015, among Jazz Pharmaceuticals plc, Jazz Securities Limited, Jazz Pharmaceuticals, Inc., Jazz Financing I Limited, Jazz Pharmaceuticals Ireland Limited, the lenders party thereto and Bank of America, N.A., as Collateral Agent, Administrative Agent, Swing Line Lender and L/C Issuer (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on June 18, 2015). | |
| 10.6B |
Amendment No. 1, dated as of July 12, 2016, to Credit Agreement, dated as of June 18, 2015, among Jazz Pharmaceuticals plc, Jazz Securities Limited, Jazz Pharmaceuticals, Inc., Jazz Financing I Limited, Jazz Pharmaceuticals Ireland Limited, the lenders party thereto and Bank of America, N.A., as Collateral Agent, Administrative Agent, Swing Line Lender and L/C Issuer (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.7A |
Commercial Lease, dated as of June 2, 2004, by and between Jazz Pharmaceuticals, Inc. and The Board of Trustees of the Leland Stanford Junior University (incorporated herein by reference to Exhibit 10.52 in Jazz Pharmaceuticals, Inc.’s registration statement on Form S-1, as amended (File No. 333-141164), as filed with the SEC on March 27, 2007). | |
| 10.7B |
First Amendment of Lease, dated June 1, 2009, by and between Jazz Pharmaceuticals, Inc. and Wheatley-Fields, LLC, successor in interest to The Board of Trustees of the Leland Stanford Junior University (incorporated herein by reference to Exhibit 10.86 in Jazz Pharmaceuticals, Inc.’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on June 4, 2009). | |
| 10.7C |
Second Amendment of Lease, dated February 28, 2012, by and between Jazz Pharmaceuticals, Inc. and Wheatley-Fields, LLC, successor in interest to The Board of Trustees of the Leland Stanford Junior University (incorporated herein by reference to Exhibit 10.31 in the Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2011, as filed by Jazz Pharmaceuticals plc on behalf of and as successor to Jazz Pharmaceuticals, Inc. with the SEC on February 28, 2012). | |
| 10.8 |
Lease, dated May 8, 2012, by and between John Ronan and Castle Cove Property Developments Limited and Jazz Pharmaceuticals plc (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.9 |
Commercial Lease, dated as of January 7, 2015, by and between The Board of Trustees of the Leland Stanford Junior University and Jazz Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 10.10 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2014, as filed with the SEC on February 24, 2015). | |
| 10.10+ |
Form of Indemnification Agreement between Jazz Pharmaceuticals plc and its officers and directors (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on January 18, 2012). | |
| 10.11+ |
Offer Letter from Jazz Pharmaceuticals, Inc. to Suzanne Sawochka Hooper (incorporated herein by reference to Exhibit 10.19 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2012, as filed with the SEC on May 8, 2012). | |
| 10.12+ |
Offer Letter from Jazz Pharmaceuticals, Inc. to Matthew Young (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2014, as filed with the SEC on May 8, 2014). | |
| 10.13A+ |
Employment Agreement by and between EUSA Pharma Inc. and Iain McGill (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2014, as filed with the SEC on November 4, 2014). | |
69
Table of Contents
| 10.13B+ |
Amendment to Employment Agreement by and between Iain McGill and EUSA Pharma (Europe) Limited (incorporated herein by reference to Exhibit 10.15B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2014, as filed with the SEC on February 24, 2015). | |
| 10.13C+ |
Amended and Restated Schedule 3 to Employment Agreement by and between Jazz Pharmaceuticals UK Ltd and Iain McGill (incorporated herein by reference to Exhibit 10.5 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.13D+ |
Change in Control Stock Award Acceleration Agreement by and between Jazz Pharmaceuticals plc and Iain McGill (incorporated herein by reference to Exhibit 10.6 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.14+ |
Offer Letter from Jazz Pharmaceuticals, Inc. to Michael Miller (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2014, as filed with the SEC on November 4, 2014). | |
| 10.15A+ |
Employment Agreement by and between Jazz Pharmaceuticals Ireland Limited and Paul Treacy (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2014, as filed with the SEC on November 4, 2014). | |
| 10.15B+ |
Amendment to Employment Agreement by and between Jazz Pharmaceuticals Ireland Limited and Paul Treacy (incorporated herein by reference to Exhibit 10.17B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2014, as filed with the SEC on February 24, 2015). | |
| 10.15C+ |
Amended and Restated Schedule 3 to Employment Agreement by and between Jazz Pharmaceuticals Ireland Ltd. and Paul Treacy (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.15D+ |
Change in Control Stock Award Acceleration Agreement by and between Jazz Pharmaceuticals plc and Paul Treacy (incorporated herein by reference to Exhibit 10.4 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.16+ |
Amended and Restated Offer Letter, dated as of July 29, 2015, from Jazz Pharmaceuticals, Inc. to Karen Smith, M.D., Ph.D. (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2015, as filed with the SEC on November 9, 2015). | |
| 10.17A+ |
Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 99.3 in Jazz Pharmaceuticals plc’s registration statement on Form S-8 (File No. 333-179075), as filed with the SEC on January 18, 2012). | |
| 10.17B+ |
Jazz Pharmaceuticals plc 2007 Equity Incentive Plan Sub-Plan Governing Awards to Participants in the Republic of Ireland (incorporated herein by reference to Exhibit 10.3B in the Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2011, as filed by Jazz Pharmaceuticals plc on behalf of and as successor to Jazz Pharmaceuticals Inc. with the SEC on February 28, 2012). | |
| 10.17C+ |
Form of Notice of Grant of Stock Options and Form of Option Agreement (U.S.) under the Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.27C in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.17D+ |
Form of Notice of Grant of Stock Options and Form of Option Agreement (Irish) under Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.27D in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
70
Table of Contents
| 10.17E+ |
Form of Restricted Stock Unit Grant Notice and Form of Restricted Stock Unit Award Agreement (U.S.) under the Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.27E in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.17F+ |
Form of Restricted Stock Unit Grant Notice and Form of Restricted Stock Unit Award Agreement (Irish) under the Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.27F in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.17G+ |
Jazz Pharmaceuticals plc 2007 Equity Incentive Plan - Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.17H+ |
Jazz Pharmaceuticals plc 2007 Equity Incentive Plan - Form of Non-U.S. Restricted Stock Unit Award Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18A+ |
Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 99.1 in Jazz Pharmaceuticals plc’s registration statement on Form S-8 (File No. 333-179075), as filed with the SEC on January 18, 2012). | |
| 10.18B+ |
Jazz Pharmaceuticals plc 2011 Equity Incentive Plan Sub-Plan Governing Awards to Participants in the Republic of Ireland (incorporated herein by reference to Exhibit 10.39B in the Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2011, as filed by Jazz Pharmaceuticals plc on behalf of and as successor to Jazz Pharmaceuticals Inc. with the SEC on February 28, 2012). | |
| 10.18C+ |
Form of Option Grant Notice and Form of Stock Option Agreement (U.S.) under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.7 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.18D+ |
Form of Stock Option Grant Notice and Form of Option Agreement (Irish) under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.8 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.18E+ |
Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.28E in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.18F+ |
Form of Restricted Stock Unit Grant Notice and Form of Restricted Stock Unit Award Agreement (U.S.) under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.9 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.18G+ |
Form of Restricted Stock Unit Grant Notice and Form of Restricted Stock Unit Award Agreement (Irish) under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.10 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.18H+ |
Form of Non-U.S. Restricted Stock Unit Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.28H in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
71
Table of Contents
| 10.18I+ |
Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of U.S. Option Grant Notice and Form of U.S. Option Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18J+ |
Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of U.S. Restricted Stock Unit Award Grant Notice and Form of U.S. Restricted Stock Unit Award Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.4 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18K+ |
Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.5 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18L+ |
Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of Non-U.S. Restricted Stock Unit Award Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.6 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18M+ |
Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2016, as filed with the SEC on May 10, 2016). | |
| 10.18N+ |
Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of Non-U.S. Restricted Stock Unit Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2016, as filed with the SEC on May 10, 2016). | |
| 10.18O+ |
Amended and Restated 2011 Equity Incentive Plan (approved August 4, 2016) (incorporated herein by reference to Exhibit 10.8 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.18P+ |
Amended and Restated 2011 Equity Incentive Plan (approved November 3, 2016) (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.18Q+ |
Form of U.S. Restricted Stock Unit Award Grant Notice and Form of U.S. Restricted Stock Unit Award Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.6 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.18R+ |
Form of U.S. Option Grant Notice and Form of U.S. Option Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.7 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.18S+ |
Form of Non-U.S. Restricted Stock Unit Award Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.8 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.19+ |
Jazz Pharmaceuticals plc Amended and Restated Directors Deferred Compensation Plan (incorporated herein by reference to Exhibit 99.6 in Jazz Pharmaceuticals plc’s registration statement on Form S-8 (File No. 333-179075), as filed with the SEC on January 18, 2012). | |
72
Table of Contents
| 10.20A+ | Jazz Pharmaceuticals plc Amended and Restated 2007 Non-Employee Directors Stock Option Plan (incorporated herein by reference to Exhibit 99.4 in Jazz Pharmaceuticals plc’s registration statement on Form S-8 (File No. 333-179075), as filed with the SEC on January 18, 2012). | |
| 10.20B+ | Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2007 Non-Employee Directors Stock Option Plan (incorporated herein by reference to Exhibit 10.30B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.20C+ | Jazz Pharmaceuticals plc Amended and Restated 2007 Non-Employee Directors Stock Option Plan - Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement (approved August 1, 2013) (incorporated herein by reference to Exhibit 10.7 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.20D+ | Amended and Restated 2007 Non-Employee Directors Stock Award Plan (approved August 4, 2016) (incorporated herein by reference to Exhibit 10.9 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.20E+ | Amended and Restated 2007 Non-Employee Directors Stock Award Plan (approved November 3, 2016) (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.20F+ | Form of Non-U.S. Restricted Stock Unit Award Grant Notice and Form of Restricted Stock Unit Award Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2007 Non-Employee Directors Stock Award Plan (incorporated herein by reference to Exhibit 10.4 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.20G+ | Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement under the Jazz Pharmaceuticals plc Amended and Restated Non-Employee Directors 2007 Stock Award Plan (incorporated herein by reference to Exhibit 10.5 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.21A+ | Jazz Pharmaceuticals plc 2007 Employee Stock Purchase Plan, as amended and restated (incorporated herein by reference to Exhibit 10.31A in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.21B+ | Jazz Pharmaceuticals plc 2007 Employee Stock Purchase Plan Sub-Plan Governing Purchase Rights to Participants in the Republic of Ireland (incorporated by reference herein to Exhibit 10.14C in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2012, as filed with the SEC on May 8, 2012 ). | |
| 10.22A+ | Jazz Pharmaceuticals plc Cash Bonus Plan for U.S. Affiliates (approved November 4, 2015) (incorporated herein by reference to Exhibit 10.22B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2015, as filed with the SEC on February 23, 2016). | |
| 10.22B+ | Jazz Pharmaceuticals plc Cash Bonus Plan for U.S. Affiliates (approved November 3, 2016) (incorporated herein by reference to Exhibit 10.22B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 10.22C+ | Jazz Pharmaceuticals Cash Bonus Plan for International Affiliates (2014) (incorporated herein by reference to Exhibit 10.24D in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2013, as filed with the SEC on February 25, 2014). | |
73
Table of Contents
| 10.22D+ | Jazz Pharmaceuticals Cash Bonus Plan (Ireland and Other Specified Affiliates) (Calendar Year 2016) (incorporated herein by reference to Exhibit 10.22D in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2015, as filed with the SEC on February 23, 2016). | |
| 10.22E+ | Jazz Pharmaceuticals Cash Bonus Plan (Ireland and Other Specified Affiliates) (Calendar Year 2017) (incorporated herein by reference to Exhibit 10.22E in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 10.23+ | Jazz Pharmaceuticals plc Amended and Restated Executive Change in Control and Severance Benefit Plan (approved February 10, 2016) (incorporated herein by reference to Exhibit 10.23 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2015, as filed with the SEC on February 23, 2016). | |
| 10.24+ | Jazz Pharmaceuticals plc 2015 Executive Officer Compensation Arrangements (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2015, as filed with the SEC on May 7, 2015). | |
| 10.25A+ | Jazz Pharmaceuticals plc Non-Employee Director Compensation Policy (approved April 30, 2015) (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2015, as filed with the SEC on August 5, 2015). | |
| 10.25B+ | Amended and Restated Non-Employee Director Compensation Policy (approved May 5, 2016) (incorporated herein by reference to Exhibit 10.7 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.27 | Tender and Support Agreement, dated as of May 27, 2016, by and among Jazz Pharmaceuticals plc, Plex Merger Sub, Inc. and each of the persons set forth on Schedule A attached thereto (incorporated herein by reference to Exhibit 99.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on May 31, 2016). | |
| 21.1 | Subsidiaries of Jazz Pharmaceuticals plc (incorporated herein by reference to Exhibit 21.1 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 23.1 | Consent of KPMG, Independent Registered Public Accounting Firm (incorporated herein by reference to Exhibit 23.1 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 24.1 | Power of Attorney (included on the signature page to Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 31.1 | Certification of Chief Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended (incorporated herein by reference to Exhibit 31.1 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 31.2 | Certification of Chief Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended (incorporated herein by reference to Exhibit 31.2 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 31.3 | Certification of Chief Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended. | |
| 31.4 | Certification of Chief Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended. | |
74
Table of Contents
| 32.1* | Certifications of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (incorporated herein by reference to Exhibit 32.1 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017).
| |
| 101.INS | XBRL Instance Document (incorporated herein by reference to Exhibit 101.INS in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.SCH | XBRL Taxonomy Extension Schema Document (incorporated herein by reference to Exhibit 101.SCH in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document (incorporated herein by reference to Exhibit 101.CAL in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document (incorporated herein by reference to Exhibit 101.DEF in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.LAB | XBRL Taxonomy Extension Labels Linkbase Document (incorporated herein by reference to Exhibit 101.LAB in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document (incorporated herein by reference to Exhibit 101.PRE in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| + | Indicates management contract or compensatory plan. |
| † | Confidential treatment has been granted for portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
| * | The certifications attached as Exhibit 32.1 accompany the Annual Report on Form 10-K pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, and shall not be deemed “filed” by the Registrant for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. |
75
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Amendment No. 1 to its Annual Report on Form 10-K/A to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: April 25, 2017 | Jazz Pharmaceuticals public limited company | |||
| (Registrant) | ||||
| /S/ BRUCE C. COZADD | ||||
|
Bruce C. Cozadd Chairman and Chief Executive Officer and Director (Principal Executive Officer) | ||||
76
Table of Contents
EXHIBIT INDEX
| Exhibit Number |
Description of Document | |
|
2.1 |
Agreement and Plan of Merger and Reorganization, dated as of September 19, 2011, by and among Azur Pharma Limited (now Jazz Pharmaceuticals plc), Jaguar Merger Sub Inc., Jazz Pharmaceuticals, Inc. and Seamus Mulligan, solely in his capacity as the Indemnitors’ Representative (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals, Inc.’s Current Report on Form 8-K (File No. 001-33500) filed with the SEC on September 19, 2011). | |
| 2.2 | Letter Agreement, dated as of January 17, 2012, by and among Jazz Pharmaceuticals plc, Jaguar Merger Sub Inc., Jazz Pharmaceuticals, Inc. and Seamus Mulligan, solely in his capacity as the Indemnitors’ Representative (incorporated by reference to Exhibit 2.2 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on January 18, 2012). | |
| 2.3 | Agreement and Plan of Merger, dated as of April 26, 2012, by and among Jazz Pharmaceuticals plc, Jewel Merger Sub Inc., EUSA Pharma Inc., and Essex Woodlands Health Ventures, Inc., Mayflower L.P., and Bryan Morton, in their capacity as the representatives of the equity holders of EUSA Pharma Inc. (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on April 27, 2012). | |
| 2.4 | Assignment, dated as of June 11, 2012, by and among Jazz Pharmaceuticals plc and Jazz Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 2.1B in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on June 12, 2012). | |
| 2.5 | Tender Offer Agreement, dated December 19, 2013, by and among Jazz Pharmaceuticals Public Limited Company, Jazz Pharmaceuticals Italy S.r.l. and Gentium S.p.A. (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K/A (File No. 001-33500), as filed with the SEC on December 20, 2013). | |
| 2.6† | Asset Purchase Agreement, dated January 13, 2014, by and among Jazz Pharmaceuticals International III Limited, Aerial BioPharma, LLC and Jazz Pharmaceuticals plc (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on January 13, 2014). | |
| 2.7† | Assignment Agreement, dated July 1, 2014, by and among Jazz Pharmaceuticals International II Limited, Sigma-Tau Pharmaceuticals, Inc., Jazz Pharmaceuticals plc and Gentium S.p.A. (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on August 5, 2014). | |
| 2.8 | Amended and Restated Agreement for the Acquisition of the Topaz Portfolio Business of Jazz Pharmaceuticals plc, dated March 20, 2015, between Jazz Pharmaceuticals plc and Essex Bidco Limited (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on March 23, 2015). | |
| 2.9 | Agreement and Plan of Merger, dated as of May 27, 2016, by and among Jazz Pharmaceuticals plc, Plex Merger Sub, Inc., and Celator Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 2.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on May 31, 2016). | |
| 3.1 | Amended and Restated Memorandum and Articles of Association of Jazz Pharmaceuticals plc, as amended on August 4, 2016 (incorporated herein by reference to Exhibit 3.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 4.1 | Reference is made to Exhibit 3.1. | |
| 4.2A | Investor Rights Agreement, dated July 7, 2009 by and between Jazz Pharmaceuticals, Inc. and the other parties named therein (incorporated herein by reference to Exhibit 10.88 in Jazz Pharmaceuticals, Inc.’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on July 7, 2009). | |
77
Table of Contents
| 4.2B | Assignment, Assumption and Amendment Agreement, dated as of January 18, 2012, by and among Jazz Pharmaceuticals, Inc., Jazz Pharmaceuticals plc and the other parties named therein (incorporated herein by reference to Exhibit 4.7B in the Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2011, as filed by Jazz Pharmaceuticals plc on behalf of and as successor to Jazz Pharmaceuticals, Inc. with the SEC on February 28, 2012). | |
| 4.2C | Indenture, dated as of August 13, 2014, by and among Jazz Pharmaceuticals plc, Jazz Investments I Limited and U.S. Bank National Association (incorporated herein by reference to Exhibit 4.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on August 13, 2014). | |
| 4.2D | Form of 1.875% Exchangeable Senior Note due 2021 (incorporated herein by reference to Exhibit 4.2 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on August 13, 2014). | |
| 10.1† | Supply Agreement, dated as of April 1, 2010, by and between Jazz Pharmaceuticals, Inc. and Siegfried (USA) Inc. (incorporated herein by reference to Exhibit 10.54 in Jazz Pharmaceuticals, Inc.’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2010, as filed with the SEC on May 6, 2010). | |
| 10.2† | Master Services Agreement, dated April 15, 2011, by and between Jazz Pharmaceuticals, Inc., CuraScript, Inc. and Express Scripts Specialty Distribution Services, Inc. (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals, Inc.’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2011, as filed with the SEC on May 9, 2011). | |
| 10.3† | Royalty Bearing Licence Agreement and Supply Agreement Re Erwinia-Derived Asparaginase, dated July 22, 2005, between Public Health England (formerly Health Protection Agency) and EUSA Pharma SAS (formerly OPi, S.A.), as amended on each of December 22, 2009, March 23, 2012 and August 8, 2012 (incorporated herein by reference to Exhibit 10.11 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q/A (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 9, 2012). | |
| 10.4 | Novation Agreement relating to Royalty Bearing Licence Agreement and Supply Agreement re Erwinia-Derived Asparaginase, dated as of May 13, 2015, by and among EUSA Pharma SAS, the Secretary of State for Health acting through Public Health England and Porton Biopharma Limited (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2015, as filed with the SEC on August 5, 2015).
| |
| 10.5† | Master Manufacturing Services Agreement, dated as of October 1, 2015, by and between Jazz Pharmaceuticals Ireland Limited and Patheon Pharmaceuticals Inc. (incorporated herein by reference to Exhibit 10.5 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2015, as filed with the SEC on February 23, 2016). | |
| 10.6A | Credit Agreement, dated as of June 18, 2015, among Jazz Pharmaceuticals plc, Jazz Securities Limited, Jazz Pharmaceuticals, Inc., Jazz Financing I Limited, Jazz Pharmaceuticals Ireland Limited, the lenders party thereto and Bank of America, N.A., as Collateral Agent, Administrative Agent, Swing Line Lender and L/C Issuer (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on June 18, 2015). | |
| 10.6B | Amendment No. 1, dated as of July 12, 2016, to Credit Agreement, dated as of June 18, 2015, among Jazz Pharmaceuticals plc, Jazz Securities Limited, Jazz Pharmaceuticals, Inc., Jazz Financing I Limited, Jazz Pharmaceuticals Ireland Limited, the lenders party thereto and Bank of America, N.A., as Collateral Agent, Administrative Agent, Swing Line Lender and L/C Issuer (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.7A | Commercial Lease, dated as of June 2, 2004, by and between Jazz Pharmaceuticals, Inc. and The Board of Trustees of the Leland Stanford Junior University (incorporated herein by reference to Exhibit 10.52 in Jazz Pharmaceuticals, Inc.’s registration statement on Form S-1, as amended (File No. 333-141164), as filed with the SEC on March 27, 2007). | |
78
Table of Contents
| 10.7B | First Amendment of Lease, dated June 1, 2009, by and between Jazz Pharmaceuticals, Inc. and Wheatley-Fields, LLC, successor in interest to The Board of Trustees of the Leland Stanford Junior University (incorporated herein by reference to Exhibit 10.86 in Jazz Pharmaceuticals, Inc.’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on June 4, 2009). | |
| 10.7C | Second Amendment of Lease, dated February 28, 2012, by and between Jazz Pharmaceuticals, Inc. and Wheatley-Fields, LLC, successor in interest to The Board of Trustees of the Leland Stanford Junior University (incorporated herein by reference to Exhibit 10.31 in the Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2011, as filed by Jazz Pharmaceuticals plc on behalf of and as successor to Jazz Pharmaceuticals, Inc. with the SEC on February 28, 2012). | |
| 10.8 | Lease, dated May 8, 2012, by and between John Ronan and Castle Cove Property Developments Limited and Jazz Pharmaceuticals plc (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.9 | Commercial Lease, dated as of January 7, 2015, by and between The Board of Trustees of the Leland Stanford Junior University and Jazz Pharmaceuticals, Inc. (incorporated herein by reference to Exhibit 10.10 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2014, as filed with the SEC on February 24, 2015). | |
| 10.10+ | Form of Indemnification Agreement between Jazz Pharmaceuticals plc and its officers and directors (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on January 18, 2012). | |
| 10.11+ | Offer Letter from Jazz Pharmaceuticals, Inc. to Suzanne Sawochka Hooper (incorporated herein by reference to Exhibit 10.19 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2012, as filed with the SEC on May 8, 2012). | |
| 10.12+ | Offer Letter from Jazz Pharmaceuticals, Inc. to Matthew Young (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2014, as filed with the SEC on May 8, 2014). | |
| 10.13A+ | Employment Agreement by and between EUSA Pharma Inc. and Iain McGill (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2014, as filed with the SEC on November 4, 2014). | |
| 10.13B+ | Amendment to Employment Agreement by and between Iain McGill and EUSA Pharma (Europe) Limited (incorporated herein by reference to Exhibit 10.15B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2014, as filed with the SEC on February 24, 2015). | |
| 10.13C+ | Amended and Restated Schedule 3 to Employment Agreement by and between Jazz Pharmaceuticals UK Ltd and Iain McGill (incorporated herein by reference to Exhibit 10.5 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016).
| |
| 10.13D+ | Change in Control Stock Award Acceleration Agreement by and between Jazz Pharmaceuticals plc and Iain McGill (incorporated herein by reference to Exhibit 10.6 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016).
| |
| 10.14+ | Offer Letter from Jazz Pharmaceuticals, Inc. to Michael Miller (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2014, as filed with the SEC on November 4, 2014). | |
| 10.15A+ | Employment Agreement by and between Jazz Pharmaceuticals Ireland Limited and Paul Treacy (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2014, as filed with the SEC on November 4, 2014). | |
79
Table of Contents
| 10.15B+ | Amendment to Employment Agreement by and between Jazz Pharmaceuticals Ireland Limited and Paul Treacy (incorporated herein by reference to Exhibit 10.17B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2014, as filed with the SEC on February 24, 2015). | |
| 10.15C+ | Amended and Restated Schedule 3 to Employment Agreement by and between Jazz Pharmaceuticals Ireland Ltd. and Paul Treacy (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.15D+ | Change in Control Stock Award Acceleration Agreement by and between Jazz Pharmaceuticals plc and Paul Treacy (incorporated herein by reference to Exhibit 10.4 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.16+ | Amended and Restated Offer Letter, dated as of July 29, 2015, from Jazz Pharmaceuticals, Inc. to Karen Smith, M.D., Ph.D. (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2015, as filed with the SEC on November 9, 2015). | |
| 10.17A+ | Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 99.3 in Jazz Pharmaceuticals plc’s registration statement on Form S-8 (File No. 333-179075), as filed with the SEC on January 18, 2012). | |
| 10.17B+ | Jazz Pharmaceuticals plc 2007 Equity Incentive Plan Sub-Plan Governing Awards to Participants in the Republic of Ireland (incorporated herein by reference to Exhibit 10.3B in the Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2011, as filed by Jazz Pharmaceuticals plc on behalf of and as successor to Jazz Pharmaceuticals Inc. with the SEC on February 28, 2012). | |
| 10.17C+ | Form of Notice of Grant of Stock Options and Form of Option Agreement (U.S.) under the Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.27C in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.17D+ | Form of Notice of Grant of Stock Options and Form of Option Agreement (Irish) under Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.27D in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.17E+ | Form of Restricted Stock Unit Grant Notice and Form of Restricted Stock Unit Award Agreement (U.S.) under the Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.27E in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.17F+ | Form of Restricted Stock Unit Grant Notice and Form of Restricted Stock Unit Award Agreement (Irish) under the Jazz Pharmaceuticals plc 2007 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.27F in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.17G+ | Jazz Pharmaceuticals plc 2007 Equity Incentive Plan - Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.17H+ | Jazz Pharmaceuticals plc 2007 Equity Incentive Plan - Form of Non-U.S. Restricted Stock Unit Award Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18A+ | Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 99.1 in Jazz Pharmaceuticals plc’s registration statement on Form S-8 (File No. 333-179075), as filed with the SEC on January 18, 2012). | |
80
Table of Contents
| 10.18B+ | Jazz Pharmaceuticals plc 2011 Equity Incentive Plan Sub-Plan Governing Awards to Participants in the Republic of Ireland (incorporated herein by reference to Exhibit 10.39B in the Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2011, as filed by Jazz Pharmaceuticals plc on behalf of and as successor to Jazz Pharmaceuticals Inc. with the SEC on February 28, 2012). | |
| 10.18C+ | Form of Option Grant Notice and Form of Stock Option Agreement (U.S.) under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.7 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.18D+ | Form of Stock Option Grant Notice and Form of Option Agreement (Irish) under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.8 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.18E+ | Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.28E in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.18F+ | Form of Restricted Stock Unit Grant Notice and Form of Restricted Stock Unit Award Agreement (U.S.) under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.9 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.18G+ | Form of Restricted Stock Unit Grant Notice and Form of Restricted Stock Unit Award Agreement (Irish) under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.10 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2012, as filed with the SEC on August 7, 2012). | |
| 10.18H+ | Form of Non-U.S. Restricted Stock Unit Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement under the Jazz Pharmaceuticals plc 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.28H in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.18I+ | Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of U.S. Option Grant Notice and Form of U.S. Option Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18J+ | Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of U.S. Restricted Stock Unit Award Grant Notice and Form of U.S. Restricted Stock Unit Award Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.4 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18K+ | Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.5 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18L+ | Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of Non-U.S. Restricted Stock Unit Award Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement (approved July 31, 2013) (incorporated herein by reference to Exhibit 10.6 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.18M+ | Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement (incorporated herein by reference to Exhibit 10.1 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2016, as filed with the SEC on May 10, 2016). | |
81
Table of Contents
| 10.18N+ | Jazz Pharmaceuticals plc 2011 Equity Incentive Plan - Form of Non-U.S. Restricted Stock Unit Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2016, as filed with the SEC on May 10, 2016). | |
| 10.18O+ | Amended and Restated 2011 Equity Incentive Plan (approved August 4, 2016) (incorporated herein by reference to Exhibit 10.8 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.18P+ | Amended and Restated 2011 Equity Incentive Plan (approved November 3, 2016) (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.18Q+ | Form of U.S. Restricted Stock Unit Award Grant Notice and Form of U.S. Restricted Stock Unit Award Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.6 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.18R+ | Form of U.S. Option Grant Notice and Form of U.S. Option Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.7 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.18S+ | Form of Non-U.S. Restricted Stock Unit Award Grant Notice and Form of Non-U.S. Restricted Stock Unit Award Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2011 Equity Incentive Plan (incorporated herein by reference to Exhibit 10.8 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.19+ | Jazz Pharmaceuticals plc Amended and Restated Directors Deferred Compensation Plan (incorporated herein by reference to Exhibit 99.6 in Jazz Pharmaceuticals plc’s registration statement on Form S-8 (File No. 333-179075), as filed with the SEC on January 18, 2012). | |
| 10.20A+ | Jazz Pharmaceuticals plc Amended and Restated 2007 Non-Employee Directors Stock Option Plan (incorporated herein by reference to Exhibit 99.4 in Jazz Pharmaceuticals plc’s registration statement on Form S-8 (File No. 333-179075), as filed with the SEC on January 18, 2012). | |
| 10.20B+ | Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2007 Non-Employee Directors Stock Option Plan (incorporated herein by reference to Exhibit 10.30B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.20C+ | Jazz Pharmaceuticals plc Amended and Restated 2007 Non-Employee Directors Stock Option Plan - Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement (approved August 1, 2013) (incorporated herein by reference to Exhibit 10.7 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2013, as filed with the SEC on November 5, 2013). | |
| 10.20D+ | Amended and Restated 2007 Non-Employee Directors Stock Award Plan (approved August 4, 2016) (incorporated herein by reference to Exhibit 10.9 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
| 10.20E+ | Amended and Restated 2007 Non-Employee Directors Stock Award Plan (approved November 3, 2016) (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
82
Table of Contents
| 10.20F+ | Form of Non-U.S. Restricted Stock Unit Award Grant Notice and Form of Restricted Stock Unit Award Agreement under the Jazz Pharmaceuticals plc Amended and Restated 2007 Non-Employee Directors Stock Award Plan (incorporated herein by reference to Exhibit 10.4 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.20G+ | Form of Non-U.S. Option Grant Notice and Form of Non-U.S. Option Agreement under the Jazz Pharmaceuticals plc Amended and Restated Non-Employee Directors 2007 Stock Award Plan (incorporated herein by reference to Exhibit 10.5 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended September 30, 2016, as filed with the SEC on November 8, 2016). | |
| 10.21A+ | Jazz Pharmaceuticals plc 2007 Employee Stock Purchase Plan, as amended and restated (incorporated herein by reference to Exhibit 10.31A in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2012, as filed with the SEC on February 26, 2013). | |
| 10.21B+ | Jazz Pharmaceuticals plc 2007 Employee Stock Purchase Plan Sub-Plan Governing Purchase Rights to Participants in the Republic of Ireland (incorporated by reference herein to Exhibit 10.14C in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2012, as filed with the SEC on May 8, 2012 ). | |
| 10.22A+ | Jazz Pharmaceuticals plc Cash Bonus Plan for U.S. Affiliates (approved November 4, 2015) (incorporated herein by reference to Exhibit 10.22B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2015, as filed with the SEC on February 23, 2016). | |
| 10.22B+ | Jazz Pharmaceuticals plc Cash Bonus Plan for U.S. Affiliates (approved November 3, 2016) (incorporated herein by reference to Exhibit 10.22B in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 10.22C+ | Jazz Pharmaceuticals Cash Bonus Plan for International Affiliates (2014) (incorporated herein by reference to Exhibit 10.24D in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2013, as filed with the SEC on February 25, 2014). | |
| 10.22D+ | Jazz Pharmaceuticals Cash Bonus Plan (Ireland and Other Specified Affiliates) (Calendar Year 2016) (incorporated herein by reference to Exhibit 10.22D in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2015, as filed with the SEC on February 23, 2016). | |
| 10.22E+ | Jazz Pharmaceuticals Cash Bonus Plan (Ireland and Other Specified Affiliates) (Calendar Year 2017) (incorporated herein by reference to Exhibit 10.22E in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 10.23+ | Jazz Pharmaceuticals plc Amended and Restated Executive Change in Control and Severance Benefit Plan (approved February 10, 2016) (incorporated herein by reference to Exhibit 10.23 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2015, as filed with the SEC on February 23, 2016). | |
| 10.24+ | Jazz Pharmaceuticals plc 2015 Executive Officer Compensation Arrangements (incorporated herein by reference to Exhibit 10.3 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended March 31, 2015, as filed with the SEC on May 7, 2015). | |
| 10.25A+ | Jazz Pharmaceuticals plc Non-Employee Director Compensation Policy (approved April 30, 2015) (incorporated herein by reference to Exhibit 10.2 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2015, as filed with the SEC on August 5, 2015). | |
| 10.25B+ | Amended and Restated Non-Employee Director Compensation Policy (approved May 5, 2016) (incorporated herein by reference to Exhibit 10.7 in Jazz Pharmaceuticals plc’s Quarterly Report on Form 10-Q (File No. 001-33500) for the period ended June 30, 2016, as filed with the SEC on August 9, 2016). | |
83
Table of Contents
| 10.27 | Tender and Support Agreement, dated as of May 27, 2016, by and among Jazz Pharmaceuticals plc, Plex Merger Sub, Inc. and each of the persons set forth on Schedule A attached thereto (incorporated herein by reference to Exhibit 99.1 in Jazz Pharmaceuticals plc’s Current Report on Form 8-K (File No. 001-33500), as filed with the SEC on May 31, 2016). | |
| 21.1 | Subsidiaries of Jazz Pharmaceuticals plc (incorporated herein by reference to Exhibit 21.1 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 23.1 | Consent of KPMG, Independent Registered Public Accounting Firm (incorporated herein by reference to Exhibit 23.1 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 24.1 | Power of Attorney (included on the signature page to Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 31.1 | Certification of Chief Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended (incorporated herein by reference to Exhibit 31.1 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 31.2 | Certification of Chief Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended (incorporated herein by reference to Exhibit 31.2 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 31.3 | Certification of Chief Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended. | |
| 31.4 | Certification of Chief Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended. | |
| 32.1* | Certifications of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (incorporated herein by reference to Exhibit 32.1 in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017). | |
| 101.INS | XBRL Instance Document (incorporated herein by reference to Exhibit 101.INS in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.SCH | XBRL Taxonomy Extension Schema Document (incorporated herein by reference to Exhibit 101.SCH in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document (incorporated herein by reference to Exhibit 101.CAL in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document (incorporated herein by reference to Exhibit 101.DEF in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.LAB | XBRL Taxonomy Extension Labels Linkbase Document (incorporated herein by reference to Exhibit 101.LAB in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document (incorporated herein by reference to Exhibit 101.PRE in Jazz Pharmaceuticals plc’s Annual Report on Form 10-K (File No. 001-33500) for the period ended December 31, 2016, as filed with the SEC on February 28, 2017) | |
| + | Indicates management contract or compensatory plan. |
84
Table of Contents
| † | Confidential treatment has been granted for portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
| * | The certifications attached as Exhibit 32.1 accompany the Annual Report on Form 10-K pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, and shall not be deemed “filed” by the Registrant for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. |
85
