Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - Ontrak, Inc. | ex23-1.htm |
As filed with the Securities and Exchange Commission on April 20, 2017
Registration No. 333-216007
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 3
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CATASYS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
8090 |
|
88-0464853 |
|
(State of Incorporation) |
|
(Primary Standard Industrial Classification |
|
(IRS Employer Identification No.) |
11601 Wilshire Boulevard, Suite 1100
Los Angeles, California 90025
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Terren S. Peizer
Chief Executive Officer
c/o Catasys, Inc.
11601 Wilshire Boulevard, Suite 1100
Los Angeles, California 90025
(310) 444-4300
(Name, address, including zip code, and telephone number, including, area code, of agent for service)
Copies to:
|
Kenneth R. Koch, Esq. Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. The Chrysler Center 666 Third Avenue New York, NY 10017 (212) 935-3000 (telephone number) (212) 983-3115 (facsimile number) |
Mitchell S. Nussbaum, Esq. Lili Taheri, Esq. |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
Non-accelerated filer |
|
☐ (Do not check if a smaller reporting company) |
|
Smaller reporting company |
|
☒ |
| Emerging growth company | ☐ |
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act . ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
PRELIMINARY PROSPECTUS |
|
SUBJECT TO COMPLETION |
|
DATED APRIL 20, 2017 |
|
$15,000,000 Shares of Common Stock
Catasys, Inc. |
|
 |
We are offering up to of shares of our common stock, $0.0001 par value per share.
Our common stock is quoted on the OTCQB Marketplace under the symbol “CATS”. On March 30, 2017, the last reported sale price for our common stock as reported on the OTCQB Marketplace was $9.66 per share, as adjusted to reflect the 1:6 reverse stock split of the Company’s common stock that will be effected in connection with this offering. We have applied to list our common stock on The NASDAQ Capital Market under the symbol “CATS”. No assurance can be given that our application will be approved.
Investing in our common stock involves a high degree of risk. Please read “Risk Factors ” beginning on page 13 of this prospectus for a discussion of factors you should consider before buying shares of our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
Per Share |
Total |
|||||||
|
Public offering price |
$ | $ | ||||||
|
Underwriting discounts and commissions(1) |
$ | $ | ||||||
|
Proceeds, before expenses, to us |
$ | $ | ||||||
|
(1) |
Please refer to “Underwriting” beginning on page 65 of this prospectus for additional information regarding underwriting compensation. |
We have granted a 45-day option to the representative of the underwriters to purchase up to additional shares of our common stock on the same terms and conditions set forth above, solely to cover over-allotments, if any.
The underwriters expect to deliver the shares to the purchasers in the offering on or about , 2017, subject to customary closing conditions.
Joseph Gunnar & Co.
The date of this prospectus is , 2017
|
|
TABLE OF CONTENTS
|
|
Page |
|
Prospectus Summary |
1 |
|
Risk Factors |
8 |
|
Special Note Regarding Forward-Looking Statements |
23 |
|
Use of Proceeds |
24 |
|
Price Range of Our Common Stock |
25 |
|
Dividend Policy |
25 |
|
Capitalization |
26 |
|
Dilution |
28 |
|
Selected Consolidated Financial Data |
29 |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
31 |
|
Business |
39 |
|
Management and Certain Corporate Governance Matters |
47 |
|
Executive Compensation |
52 |
|
Certain Relationships and Related Person Transactions |
57 |
|
Security Ownership of Certain Beneficial Owners and Management |
59 |
|
Description of Capital Stock |
61 |
|
Shares Eligible for Future Sale |
63 |
|
Underwriting |
65 |
|
Legal Matters |
73 |
|
Experts |
73 |
|
Where You Can Find Additional Information |
73 |
|
Index to Consolidated Financial Statements |
F-1 |
About This Prospectus
You should rely only on information contained in this prospectus. We have not, and the underwriters have not, authorized anyone to provide you with additional information or information different from that contained in this prospectus. We are not making an offer of these securities in any state or other jurisdiction where the offer is not permitted. The information in this prospectus may only be accurate as of the date on the front of this prospectus regardless of time of delivery of this prospectus or any sale of our securities.
No person is authorized in connection with this prospectus to give any information or to make any representations about us, the common stock hereby or any matter discussed in this prospectus, other than the information and representations contained in this prospectus. If any other information or representation is given or made, such information or representation may not be relied upon as having been authorized by us. This prospectus does not constitute an offer to sell, or a solicitation of an offer to buy our common stock in any circumstance under which the offer or solicitation is unlawful. Neither the delivery of this prospectus nor any distribution of our common stock in accordance with this prospectus shall, under any circumstances, imply that there has been no change in our affairs since the date of this prospectus.
Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. You are required to inform yourself about, and to observe any restrictions relating to, this offering and the distribution of this prospectus.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, or will be filed as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find Additional Information.”
As used in this prospectus, unless the context indicates or otherwise requires, “the Company,” “our Company,” “we,” “us,” and “our” refer to Catasys, Inc., a Delaware corporation, and its consolidated subsidiaries.
PROSPECTUS SUMMARY
This summary does not contain all of the information that should be considered before investing in our common stock. Investors should carefully read this prospectus, and the registration statement of which this prospectus is a part, in their entirety before investing in our common stock, including the information discussed under “Risk Factors” in this prospectus. As used in this prospectus, unless the context indicates or otherwise requires, “the Company,” “our Company,” “we,” “us,” and “our” refer to Catasys, Inc., a Delaware corporation, and its consolidated subsidiaries.
A 1:6 reverse stock split of our common stock will be effected prior to the closing of this offering. All share amounts in this prospectus have been retroactively adjusted to give effect to this reverse stock split, except for the financial statements and notes thereto.
Our Company
Overview
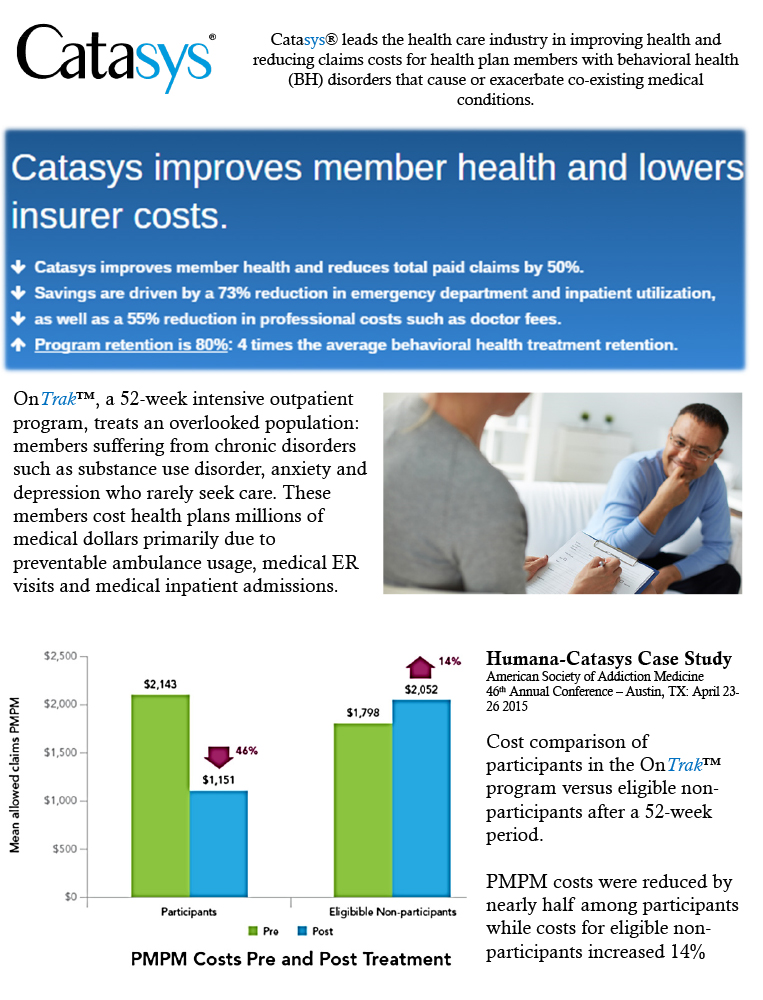
We provide data analytics based specialized behavioral health management and integrated treatment services to health plans through our OnTrak solution. Our OnTrak solution is designed to improve member health and at the same time lower costs to the insurer for underserved populations where behavioral health conditions are causing or exacerbating co-existing medical conditions. The program utilizes proprietary analytics, member engagement and patient centric treatment that integrates evidence-based medical and psychosocial interventions along with care coaching in a 52-week outpatient program. Our initial focus was members with substance use disorders, but we have expanded our solution to assist members with anxiety and depression. We currently operate our OnTrak solutions in Florida, Georgia, Illinois, Kansas, Kentucky, Louisiana, Massachusetts, Missouri, New Jersey, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, West Virginia and Wisconsin. We provide services to commercial (employer funded), managed Medicare Advantage, and managed Medicaid and duel eligible (Medicare and Medicaid) populations.
Our Strategy
Our business strategy is to provide a quality integrated medical and behavioral program to help health plans and other organizations treat and manage health plan members whose behavioral health conditions are exacerbating co-existing medical conditions resulting in increased in-patient medical costs. Our initial focus was members with substance use disorder, and we have expanded our solution into anxiety disorders and depression.
Key elements of our business strategy include:
|
|
● |
Demonstrating the potential for improved clinical outcomes and reduced cost associated with using our OnTrak solution with key managed care and other third-party payors; |
|
|
● |
Educating third-party payors on the disproportionately high cost of their substance dependent population; |
|
|
● |
Providing our OnTrak solution to third-party payors for reimbursement on a case rate, fee for service, or monthly fee basis; and |
|
|
● |
Generating outcomes data from our OnTrak solution to demonstrate cost reductions and utilization of this outcomes data to facilitate broader adoption. |
As an early entrant into offering integrated medical and behavioral programs for substance dependence, we believe we will be well positioned to address increasing market demand. We believe our OnTrak solution will help fill the gap that exists today: a lack of programs that focus on smaller populations with disproportionately higher costs driven by behavioral health conditions that improve patient care while controlling overall treatment costs.
Recent Developments
Amendments to Outstanding Warrants and Extension of Existing Debentures
In March 2017, we entered into amendments with the holders of certain outstanding warrants issued on April 17, 2015 and July 30, 2015 to eliminate certain anti-dilution provisions in such warrants, which caused us to reflect an associated liability of $5.3 million on our balance sheet as of December 31, 2016. Such amendments are contingent upon and will not take effect until the closing of this offering, which must occur on or before April 30, 2017. For each warrant share underlying the warrants so amended, the holder received the right to purchase an additional .2 shares of common stock. Two of the holders of such warrants, which owners hold warrants to purchase an aggregate of 11,049 shares of common stock, did not agree to the amendment. The warrant holders agreeing to the amendment include Acuitas Group Holdings, LLC (“Acuitas”), one hundred percent (100%) of which is owned by Terren S. Peizer, Chairman and Chief Executive Officer of the Company, and another accredited investor, who will own warrants to purchase 187,002 and 79,545 shares of the Company’s common stock, respectively, if and when the amendments take effect.
Additionally, in March 2017, we and the holders of an aggregate of approximately $10 million of our existing convertible debentures agreed to extend the maturity dates of such debentures until the earlier of the closing of this offering or April 30, 2017.
Financing Activities
In January 2017, we entered into a Subscription Agreement (the “Subscription Agreement”) with Acuitas, pursuant to which we will receive aggregate gross proceeds of $1,300,000 (the “Loan Amount”) in consideration of the issuance of (i) an 8% Series B Convertible Debenture due March 31, 2017 (the “January 2017 Convertible Debenture”) at a conversion price of $0.85 and (ii) five-year warrants to purchase shares of our common stock in an amount equal to one hundred percent (100%) of the initial number of shares of common stock issuable upon the conversion of the January 2017 Convertible Debenture, at an exercise price of $5.10 per share (the “January 2017 Warrants”). The Loan Amount is payable in tranches through March 2017. In addition, any warrants issued in conjunction with the December 2016 Convertible Debenture currently outstanding with Acuitas have been increased by an additional 25% warrant coverage, exercisable for an aggregate of 127,883 shares of our common stock.
The January 2017 Warrants include, among other things, price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the January 2017 Warrants will be adjusted if we issue shares of our common stock at a price that is less than the then exercise price of the January 2017 Warrants. Such price protection provisions will remain in effect until the earliest of (i) the termination date of the January 2017 Warrants, (ii) such time as the January 2017 Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange. The holder of the January 2017 Convertible Debenture has agreed to extend the maturity of the January 2017 Convertible Debenture from March 31, 2017 to the earlier of the closing date of this offering or April 30, 2017.
In connection with the Subscription Agreement described above, the number of warrants to purchase shares of our common stock previously issued in December 2016 to Shamus, LLC, a company owned by David E. Smith, a member of the Company’s board of directors (the “Shamus Warrants”), were increased from 75% to 100% warrant coverage, exercisable for an aggregate of 58,824 shares of our common stock.
2017 Stock Incentive Plan
On February 27, 2017, our Board of Directors and our stockholders approved the adoption of the 2017 Stock Incentive Plan (the “2017 Plan”). The 2017 Plan allows the Company, under the direction of the Board of Directors or a committee thereof, to make grants of stock options, restricted and unrestricted stock and other stock-based awards to employees, including our executive officers, consultants and directors. The 2017 Plan allows for the issuance of up to 2,333,334 additional shares of Common Stock pursuant to new awards granted under the 2017 Plan and up to approximately 250,000 shares of Common Stock that are represented by options outstanding under the 2010 Plan (defined below) that are forfeited, expire or are cancelled without delivery of shares of Common Stock or which result in the forfeiture of shares of Common Stock back to the Company. This description is qualified in its entirety by reference to the actual terms of the 2017 Plan, a copy of which is attached as Exhibit B to the Company’s preliminary Information Statement on Schedule 14C, filed with the Securities and Exchange Commission on February 28, 2017.
New Directors
On March 11, 2017, our Board of Directors appointed Marc Cummins and Richard J. Berman to serve on our Board of Directors and our Audit Committee, effective immediately. There are no arrangements or understandings between Messrs. Cummins or Berman and any other person pursuant to which they were appointed as our directors. There are no transactions to which we are a party and in which Messrs. Cummins or Berman have a material interest that are required to be disclosed under Item 404(a) of Regulation S-K. Mr. Cummins previously served on our Board of Directors until his resignation on December 15, 2010, and Mr. Berman has not previously held any position at the Company. Neither individual has family relations with any of our directors or executive officers.
Risks Associated with Our Business
Our business and ability to execute our business strategy are subject to a number of risks of which you should be aware before you decide to buy our common stock. In particular, you should consider the following risks, which are discussed more fully in the section entitled “Risk Factors” in this prospectus, as well as the other risks described in “Risk Factors.”
|
|
• |
We expect to continue to incur substantial operating losses and may be unable to obtain additional financing, causing our independent registered public accounting firm to express substantial doubt about our ability to continue as a going concern. |
|
|
• |
We will need additional funding, and we cannot guarantee that we will find adequate sources of capital in the future. |
|
|
• |
We depend on key personnel, the loss of which could impact the ability to manage our business. |
|
|
• |
Our OnTrak solutions may not become widely accepted, which could limit our growth. |
|
|
• |
We may be subject to future litigation, which could result in substantial liabilities that may exceed our insurance coverage. |
|
|
• |
If third-party payors fail to provide coverage and adequate payment rates for our OnTrak solution, our revenue and prospects for profitability will be harmed. |
|
|
• |
We may not be able to achieve promised savings for our OnTrak contracts, which could result in loss of customers and pricing levels insufficient to cover our costs or ensure profitability. |
|
|
• |
Confidentiality agreements with employees, treating physicians and others may not adequately prevent disclosure of trade secrets and other proprietary information. |
|
|
• |
We or our healthcare professionals may be subject to regulatory, enforcement and investigative proceedings, which could adversely affect our financial condition or operations. |
|
|
• |
We may not fully comply with complex and increasing regulation by state and federal authorities, which could negatively impact our business operations. |
|
|
• |
Our share price is volatile and may be influenced by numerous factors, some of which are beyond our control. |
|
• |
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval. |
Annual Meetings
We are required by Section 2.1 of our by-laws and Sections 211(b) and (c) of the Delaware General Corporation Law to hold regular annual meetings. In light of our historical liquidity constraints, we have not held regular annual meetings, electing instead to handle matters by written consent of the Company’s stockholders to save on the financial and administrative resources required to prepare for and hold such meetings. While we believe that no material adverse consequences have resulted from the Company’s failure to hold regular annual meetings, we intend to hold such meetings in the future, beginning in 2018.
Corporate Information
We were incorporated in the State of Delaware on September 29, 2003. Our principal executive offices are located at 11601 Wilshire Blvd, Suite 1100, Los Angeles, California 90025, and our telephone number is (310) 444-4300.
We are a “smaller reporting company” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and have elected to take advantage of certain of the scaled disclosure available for smaller reporting companies.
Our corporate website address is www.catasys.com. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to reports filed pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended, are available free of charge on our website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission. The Securities and Exchange Commission maintains an internet site that contains our public filings with the Securities and Exchange Commission and other information regarding our company, at www.sec.gov. These reports and other information concerning our company may also be accessed at the Securities and Exchange Commission’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the Securities and Exchange Commission at 1-800-SEC-0330. The contents of these websites are not incorporated into this prospectus. Further, our references to the URLs for these websites are intended to be inactive textual reference only.
The Offering
|
Common stock offered by us |
shares |
|
Common stock to be outstanding after this offering |
shares (1) |
|
Common stock outstanding before this offering |
shares (1) |
|
Option to purchase additional shares |
We have granted the representative of the underwriters a 45-day option to purchase up to additional shares of our common stock from us at the public offering price, less underwriting discounts and commissions, to cover over-allotments, if any. |
|
Use of proceeds |
We estimate that the net proceeds from this offering will be approximately $ million, or approximately $ million if the underwriters exercise their option to purchase additional shares in full, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds of this offering to repay certain of our outstanding convertible debentures and for working capital and general corporate purposes. See “Use of Proceeds” for a more complete description of the intended use of proceeds from this offering. |
|
Risk factors |
Investing in our securities involves a high degree of risk and purchasers may lose their entire investment. You should read the “Risk Factors” section of this prospectus beginning on page 14 for a discussion of certain factors to consider carefully before deciding to purchase any shares of our common stock. |
|
OTCQB Marketplace symbol |
“CATS”. |
|
Proposed NASDAQ Capital Market Symbol |
“CATS”. No assurance can be given that our shares of common stock will be approved for listing on NASDAQ. |
|
Lock-up |
We, our directors, officers and any other 5% or greater holder of outstanding shares of our common stock have agreed with the underwriters not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our common stock or securities convertible into common stock for a period of ninety (90) days after the date of this prospectus, without the prior written consent of the representative of the underwriters. See “Underwriting” section on page 65. |
(1) The number of shares of our common stock to be outstanding after this offering is based on 9,383,740 shares of common stock outstanding as of March 30, 2017, and assumes the effectiveness of a 1:6 reverse stock split of our common stock that will be effected prior to the closing of the offering and the sale of shares of common stock at $ per share, and excludes:
|
|
• |
243,853 shares of common stock issuable upon the exercise of outstanding stock options as of March 30, 2017, at a weighted average exercise price of $38.40 per share, as of March 30, 2017; |
|
|
• |
1,684,578 shares of common stock issuable upon the exercise of outstanding warrants as of March 30, 2017, at a weighted average exercise price of $4.88 per share, as of March 30, 2017; |
|
|
• |
50,774 shares of common stock reserved for future issuance under the 2010 Stock Incentive Plan, as of March 30, 2017; and |
|
|
• |
3,350,732 shares of common stock reserved for future issuance under our Convertible Debentures with conversion prices of $1.80 (2,363,055 shares), $5.10 (280,282 shares) and $6.60 (707,395 shares), as of March 30, 2017; and |
|
|
• |
_________ shares of common stock underlying the warrants to be issued to the representative of the underwriters in connection with this offering. |
Unless otherwise indicated, all information contained in this prospectus, and the number of shares of common stock outstanding, assumes no exercise by the underwriters of their option to purchase up to an additional of shares of our common stock to cover over-allotments, if any.
Summary Consolidated Financial Data
The following summary consolidated financial data should be read together with our audited consolidated financial statements and accompanying notes and “Management Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this prospectus. Our summary statements of operations data for the years ended December 31, 2016 and 2015 and the selected balance sheets data as of December 31, 2016 are derived from our audited consolidated financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of results to be expected for any future period. The summary financial data in this section are not intended to replace our audited consolidated financial statements and the related notes:
|
Twelve Months Ended |
||||||||
|
(In thousands, except per share amounts) |
December 31, |
|||||||
|
2016 |
2015 |
|||||||
|
Revenues |
||||||||
|
Healthcare services revenues |
$ | 7,075 | $ | 2,705 | ||||
|
Operating expenses |
||||||||
|
Cost of healthcare services |
4,670 | 2,433 | ||||||
| General and administrative | 8,838 | 9,049 | ||||||
|
Depreciation and amortization |
141 | 122 | ||||||
|
Total operating expenses |
13,649 | 11,604 | ||||||
|
Loss from operations |
(6,574 | ) | (8,899 | ) | ||||
|
Interest and other income |
106 | 64 | ||||||
|
Interest expense |
(5,354 | ) | (2,590 | ) | ||||
|
Loss on impairment of intangible assets |
- | (88 | ) | |||||
|
Loss on exchange of warrants |
- | (4,410 | ) | |||||
| Loss on debt extinguishment | (2,424 | ) | (195 | ) | ||||
|
Change in fair value of warrant liability |
2,093 | 11,665 | ||||||
|
Change in fair value of derivative liability |
(5,774 | ) | (2,761 | ) | ||||
|
Loss from operations before provision for income taxes |
(17,927 | ) | (7,214 | ) | ||||
|
Provision for income taxes |
9 | 9 | ||||||
|
Net loss |
$ | (17,936 | ) | $ | (7,223 | ) | ||
|
Basic and diluted net loss per share: |
$ | (1.95 | ) | $ | (1.07 | ) | ||
|
Basic weighted number of shares outstanding |
9,179 | 6,729 | ||||||
| As of December 31, 2016 | ||||||||||||
|
Actual |
|
Pro Forma As Adjusted |
||||||||||
|
(in thousands) |
(unaudited) |
(unaudited) |
||||||||||
|
Consolidated Balance Sheet Data: |
||||||||||||
|
Cash and cash equivalents |
$ | 851 | $ | 851 | $ | 11,528 | ||||||
|
Total assets |
3,104 | 3,104 | 13,781 | |||||||||
|
Total liabilities |
28,432 | 7,018 | 4,195 | |||||||||
|
Accumulated deficit |
(279,719 | ) | (274,506 | ) | (274,506 | ) | ||||||
|
Total stockholders’ equity (deficit) |
$ | (25,328 | ) | $ | (3,914 | ) | $ | 9,586 | ||||
The preceding table presents a summary of our audited balance sheet data as of December 31, 2016:
|
● |
on an actual basis; | |
|
● |
the unaudited pro forma balance sheet data as of December 31, 2016 gives effect to (i) the conversion of the 12% Original Issue Discount Convertible Debenture in the amount of $4,130, (ii) the conversion of the 8% Series B Convertible Debenture in the amount of $3,060, (iii) the partial elimination of the warrant liability by removing the anti-dilution provision in certain of the warrant agreements, and (iv) the payment of Terren Peizer’s deferred salary of $1,060 with common stock, each of which is expected to become effective as of the close of this offering; and | |
|
● |
on pro-forma as adjusted basis to give effect to the receipt of the estimated net proceeds from the sale of shares of common stock in this offering at the public offering price of $ per share, after deducting the underwriting discounts and commissions and estimated expenses payable by us and the use of $2,823 of the proceeds therefrom to pay Acuitas’ 8% Series B Convertible Debenture at closing. |
RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with the other information contained in this prospectus, including our consolidated financial statements and the related notes, before making any decision to invest in shares of our common stock. This prospectus contains forward-looking statements. If any of the events discussed in the risk factors below occurs, our business, prospects, results of operations, financial condition and cash flows could be materially harmed. If that were to happen, the trading price of our common stock could decline, and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks not currently known to us or other factors not perceived by us to present significant risks to our business at this time also may impair our business operations.
Risks related to our business
We have a limited operating history, expect to continue to incur substantial operating losses and may be unable to obtain additional financing, causing our independent registered public accounting firm to express substantial doubt about our ability to continue as a going concern.
We have been unprofitable since our inception in 2003 and expect to incur substantial additional operating losses and negative cash flow from operations for at least the next twelve months. As of December 31, 2016, these conditions raised substantial doubt as to our ability to continue as a going concern. At December 31, 2016, cash and cash equivalents was approximately $851,000 and accumulated deficit was approximately $280 million. During the year ended December 31, 2016, our cash and cash equivalents used by operating activities was $5.7 million. Although we have taken actions to increase our revenue and we are seeking to obtain additional financing, there can be no assurance that we will be successful in our efforts. We may not be successful in raising necessary funds on acceptable terms or at all, and we may not be able to offset our operating losses by sufficient reductions in expenses and increases in revenue. If this occurs, we may be unable to meet our cash obligations as they become due and we may be required to further delay or reduce operating expenses and curtail our operations, which would have a material adverse effect on us.
We may fail to successfully manage and grow our business, which could adversely affect our results of operations, financial condition and business.
Continued expansion could put significant strain on our management, operational and financial resources. The need to comply with the rules and regulations of the SEC will continue to place significant demands on our financial and accounting staff, financial, accounting and information systems, and our internal controls and procedures, any of which may not be adequate to support our anticipated growth. The need to comply with the state and federal healthcare, security and privacy regulation will continue to place significant demands on our staff and our policies and procedures, any of which may not be adequate to support our anticipated growth. We may not be able to effectively hire, train, retain, motivate and manage required personnel. Our failure to manage growth effectively could limit our ability to satisfy our reporting obligations, or achieve our marketing, commercialization and financial goals.
We will need additional funding, and we cannot guarantee that we will find adequate sources of capital in the future.
We have incurred negative cash flows from operations since inception and have expended, and expect to continue to expend, substantial funds to grow our business. As of March 30, 2017, we estimate that our existing cash and cash equivalents will be sufficient to fund our operating expenses and capital requirements through April 30, 2017. Actual cash fees collected and expenses incurred may significantly impact this estimate. We will require additional funds before we achieve positive cash flows and we may never become cash flow positive.
If we raise additional funds by issuing equity securities, such financing will result in further dilution to our stockholders. Any equity securities issued also may provide for rights, preferences or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, these debt securities would have rights, preferences and privileges senior to those of holders of our common stock, and the terms of the debt securities issued could impose significant restrictions on our operations.
We do not know whether additional financing will be available on commercially acceptable terms, or at all. If adequate funds are not available or are not available on commercially acceptable terms, we may need to continue to downsize, curtail program development efforts or halt our operations altogether.
Our programs may not be as effective as we believe them to be, which could limit our potential revenue growth.
Our belief in the efficacy of our OnTrak solution is based on a limited experience with a relatively small number of patients. Such results may not be statistically significant, have not been subjected to close scientific scrutiny, and may not be indicative of the long-term future performance of treatment with our programs. If the initially indicated results cannot be successfully replicated or maintained over time, utilization of our programs could decline substantially. There are no standardized methods for measuring efficacy of programs such as ours. Even if we believe our solutions are effective, our customers could determine they are not utilizing different outcomes measures. In addition, even if our customers determine our solutions are effective they may discontinue them because they determine that the aggregate cost savings are not sufficient or that our programs do not have a high enough return on investment. Our success is dependent on our ability to enroll third-party payor members in our OnTrak solutions. Large scale outreach and enrollment efforts have not been conducted and then only for limited time periods, and we may not be able to achieve the anticipated enrollment rates.
Our OnTrak solution may not become widely accepted, which could limit our growth.
Our ability to achieve further marketplace acceptance for our OnTrak solution may be dependent on our ability to contract with a sufficient number of third party payors and to demonstrate financial and clinical outcomes from those agreements. If we are unable to secure sufficient contracts to achieve recognition or acceptance of our OnTrak solution or if our program does not demonstrate the expected level of clinical improvement and cost savings, it is unlikely that we will be able to achieve widespread market acceptance.
Disappointing results for our Solutions or failure to attain our publicly disclosed milestones could adversely affect market acceptance and have a material adverse effect on our stock price.
Disappointing results, later-than-expected press release announcements or termination of evaluations, pilot programs or commercial OnTrak solutions could have a material adverse effect on the commercial acceptance of our solutions, our stock price and on our results of operations. In addition, announcements regarding results, or anticipation of results, may increase volatility in our stock price. In addition to numerous upcoming milestones, from time to time we provide financial guidance and other forecasts to the market. While we believe that the assumptions underlying projections and forecasts we make publicly available are reasonable, projections and forecasts are inherently subject to numerous risks and uncertainties. Any failure to achieve milestones, or to do so in a timely manner, or to achieve publicly announced guidance and forecasts, could have a material adverse effect on our results of operations and the price of our common stock.
Our industry is highly competitive, and we may not be able to compete successfully.
The healthcare business in general, and the behavioral health treatment business in particular, are highly competitive. While we believe our products and services are unique, we operate in highly competitive markets. We compete with other healthcare management service organizations, care management and disease management companies, including Managed Behavioral Healthcare Organizations (MBHOs), other specialty healthcare and managed care companies, and healthcare technology companies that are offering treatment and support of behavioral health on-line and on mobile devices. Most of our competitors are significantly larger and have greater financial, marketing and other resources than us. We believe that our ability to offer customers a comprehensive and integrated behavioral health solution, including the utilization of our analytical models and innovative member engagement methodologies, will enable us to compete effectively. However, there can be no assurance that we will not encounter more effective competition in the future, that we will have financial resources to continue to improve our offerings or that we will be successful improving them, which would limit our ability to maintain or increase our business.
Our competitors may develop and introduce new processes and products that are equal or superior to our programs in treating behavioral health conditions. Accordingly, we may be adversely affected by any new processes and products developed by our competitors.
We depend on key personnel, the loss of which could impact the ability to manage our business.
We are highly dependent on our senior management and key operating and technical personnel. The loss of the services of any member of our senior management and key operating and technical personnel could have a material adverse effect on our business, operating results and financial condition. We also rely on consultants and advisors to assist us in formulating our strategy. All of our consultants and advisors are either self-employed or employed by other organizations, and they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, that may affect their ability to contribute to us.
We will need to hire additional employees in order to achieve our objectives. There is currently intense competition for skilled executives and employees with relevant expertise, and this competition is likely to continue. The inability to attract and retain sufficient personnel could adversely affect our business, operating results and financial condition.
We may be subject to future litigation, which could result in substantial liabilities that may exceed our insurance coverage.
All significant medical treatments and procedures, including treatment utilizing our programs, involve the risk of serious injury or death. While we have not been the subject of any such claims, our business entails an inherent risk of claims for personal injuries and substantial damage awards. We cannot control whether individual physicians and therapists will apply the appropriate standard of care in determining how to treat their patients. While our agreements typically require physicians to indemnify us for their negligence, there can be no assurance they will be willing and financially able to do so if claims are made. In addition, our license agreements require us to indemnify physicians, hospitals or their affiliates for losses resulting from our negligence.
We currently have insurance coverage for personal injury claims, directors’ and officers’ liability insurance coverage, and errors and omissions insurance. We may not be able to maintain adequate liability insurance at acceptable costs or on favorable terms. We expect that liability insurance will be more difficult to obtain and that premiums will increase over time and as the volume of patients treated with our programs increases. In the event of litigation, we may sustain significant damages or settlement expense (regardless of a claim's merit), litigation expense and significant harm to our reputation.
If third-party payors fail to provide coverage and adequate payment rates for our solutions, our revenue and prospects for profitability will be harmed.
Our future revenue growth will depend in part upon our ability to contract with health plans and other insurance payors for our OnTrak solutions. To date, we have not received a significant amount of revenue from our OnTrak solutions from health plans and other insurance payors, and acceptance of our OnTrak solutions is critical to the future prospects of our business. In addition, insurance payors are increasingly attempting to contain healthcare costs, and may not cover or provide adequate payment for our programs. Adequate insurance reimbursement might not be available to enable us to realize an appropriate return on investment in research and product development, and the lack of such reimbursement could have a material adverse effect on our operations and could adversely affect our revenues and earnings.
We may not be able to achieve promised savings for our OnTrak contracts, which could result in pricing levels insufficient to cover our costs or ensure profitability.
We anticipate that many of our OnTrak contracts will be based upon anticipated or guaranteed levels of savings for our customers and achieving other operational metrics resulting in incentive fees based on savings. If we are unable to meet or exceed promised savings, achieve agreed upon operational metrics, or favorably resolve contract billing and interpretation issues with our customers, we may be required to refund from the amount of fees paid to us any difference between savings that were guaranteed and the savings, if any, which were actually achieved; or we may fail to earn incentive fees based on savings. Accordingly, during or at the end of the contract terms, we may be required to refund some or all of the fees paid for our services. This exposes us to significant risk that contracts negotiated and entered into may ultimately be unprofitable. In addition, managed care operations are at risk for costs incurred to provide agreed upon services under our solution. Therefore, failure to anticipate or control costs could have a materially adverse effect on our business.
Our ability to utilize net operating loss carryforwards may be limited.
As of December 31, 2016, we had net operating loss carryforwards (NOLs) of approximately $222 million for federal income tax purposes that will begin to expire in 2024. These NOLs may be used to offset future taxable income, to the extent we generate any taxable income, and thereby reduce or eliminate our future federal income taxes otherwise payable. Section 382 of the Internal Revenue Code imposes limitations on a corporation's ability to utilize NOLs if it experiences an ownership change as defined in Section 382. In general terms, an ownership change may result from transactions increasing the ownership of certain stockholders in the stock of a corporation by more than 50% over a three-year period. In the event that an ownership change has occurred, or were to occur, utilization of our NOLs would be subject to an annual limitation under Section 382 determined by multiplying the value of our stock at the time of the ownership change by the applicable long-term tax-exempt rate as defined in the Internal Revenue Code. Any unused annual limitation may be carried over to later years. We may be found to have experienced an ownership change under Section 382 as a result of events in the past or the issuance of shares of common stock, or a combination thereof. If so, the use of our NOLs, or a portion thereof, against our future taxable income may be subject to an annual limitation under Section 382, which may result in expiration of a portion of our NOLs before utilization.
We have not held regular annual meetings in the past, and if we are required by the Delaware Court of Chancery to hold an annual meeting pursuant to Section 211(c) of the Delaware General Corporation Law (the “DGCL”), it could result in the unanticipated expenditure of funds, time and other Company resources.
Section 2.1 of the Company’s By-Laws provides that an annual meeting shall be held each year on a date and at a time designated by the Company’s Board of Directors, and Section 211(b) of the DGCL provides for an annual meeting of stockholders to be held for the election of directors. Section 211(c) of the DGCL provides that if there is a failure to hold the annual meeting for a period of 13 months after the latest to occur of the organization of the corporation, its last annual meeting or last action by written consent to elect directors in lieu of an annual meeting, the Delaware Court of Chancery may order a meeting to be held upon the application of any stockholder or director. Section 211(c) also provides that the failure to hold an annual meeting shall not affect otherwise valid corporate acts or result in a forfeiture or dissolution of the corporation.
We have not held regular annual meetings in the past because a substantial majority of our stock is owned by a small number of stockholders, making it easy to obtain written consent in lieu of a meeting when necessary. In light of our historical liquidity constraints, handling matters by written consent has allowed the Company to save on the financial and administrative resources required to prepare for and hold such annual meetings. Additionally, we have applied to list our common stock on The Nasdaq Capital Market and expect that our common stock will be listed on The Nasdaq Capital Market prior to the completion of this offering. Pursuant to Nasdaq’s corporate governance requirements, the Company will be obligated to hold regular annual meetings in the future, and it is currently contemplated that we will hold such meetings beginning in 2018.
To our knowledge, no stockholder or director has requested the Company’s management to hold such an annual meeting and no stockholder or director has applied to the Delaware Court of Chancery seeking an order directing the Company to hold a meeting. However, if one or more stockholders or directors were to apply to the Delaware Court of Chancery seeking such an order, and if the Delaware Court of Chancery were to order an annual meeting before the Company was prepared to hold one, the preparation for the annual meeting and the meeting itself could result in the unanticipated expenditure of funds, time, and other Company resources.
Risks related to our intellectual property
Confidentiality agreements with employees, treating physicians and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary technology and processes, we rely in part on confidentiality provisions in our agreements with employees, treating physicians, and others. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
We may be subject to claims that we infringe the intellectual property rights of others, and unfavorable outcomes could harm our business.
Our future operations may be subject to claims, and potential litigation, arising from our alleged infringement of patents, trade secrets, trademarks or copyrights owned by other third parties. Within the healthcare, drug and bio-technology industry, many companies actively pursue infringement claims and litigation, which makes the entry of competitive products more difficult. We may experience claims or litigation initiated by existing, better-funded competitors and by other third parties. Court-ordered injunctions may prevent us from continuing to market existing products or from bringing new products to market and the outcome of litigation and any resulting loss of revenues and expenses of litigation may substantially affect our ability to meet our expenses and continue operations.
Risks related to our industry
Recent changes in insurance and health care laws have created uncertainty in the health care industry.
The Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act, each enacted in March 2010, generally known as the Health Care Reform Law, significantly expanded health insurance coverage to uninsured Americans and changed the way health care is financed by both governmental and private payers. The 2016 federal elections, which resulted in the election of the Republican presidential nominee and Republican majorities in both houses of Congress, is likely to prompt renewed legislative efforts to significantly modify or repeal the Health Care Reform Law, is likely to impact how the executive branch implements the law, and may impact how the federal government responds to lawsuits challenging the Health Care Reform Law. We cannot predict what further reform proposals, if any, will be adopted, when they may be adopted, or what impact they may have on our business. There may also be other risks and uncertainties associated with the Health Care Reform Law. If we fail to comply or are unable to effectively manage such risks and uncertainties, our financial condition and results of operations could be adversely affected.
Our policies and procedures may not fully comply with complex and increasing regulation by state and federal authorities, which could negatively impact our business operations.
The healthcare industry is highly regulated and continues to undergo significant changes as third-party payors, such as Medicare and Medicaid, traditional indemnity insurers, managed care organizations and other private payors, increase efforts to control cost, utilization and delivery of healthcare services. Healthcare companies are subject to extensive and complex federal, state and local laws, regulations and judicial decisions. Our failure or the failure of our treating physicians, to comply with applicable healthcare laws and regulations may result in the imposition of civil or criminal sanctions that we cannot afford, or require redesign or withdrawal of our programs from the market.
We or our healthcare professionals may be subject to regulatory, enforcement and investigative proceedings, which could adversely affect our financial condition or operations.
We or one or more of our healthcare professionals could become the subject of regulatory, enforcement, or other investigations or proceedings, and our relationships, business structure, and interpretations of applicable laws and regulations may be challenged. The defense of any such challenge could result in substantial cost and a diversion of management’s time and attention. In addition, any such challenge could require significant changes to how we conduct our business and could have a material adverse effect on our business, regardless of whether the challenge ultimately is successful. If determination is made that we or one or more of our healthcare professionals has failed to comply with any applicable laws or regulations, our business, financial condition and results of operations could be adversely affected.
Our business practices may be found to constitute illegal fee-splitting or corporate practice of medicine, which may lead to penalties and adversely affect our business.
Many states, including California where our principal executive offices are located, have laws that prohibit business corporations, such as us, from practicing medicine, exercising control over medical judgments or decisions of physicians or other health care professionals (such as nurses or nurse practitioners), or engaging in certain business arrangements with physicians or other health care professionals, such as employment of physicians and other health care professionals or fee-splitting. The state laws and regulations and administrative and judicial decisions that enumerate the specific corporate practice and fee-splitting rules vary considerably from state to state and are enforced by both the courts and government agencies, each with broad discretion. Courts, government agencies or other parties, including physicians, may assert that we are engaged in the unlawful corporate practice of medicine, fee-splitting, or payment for referrals by providing administrative and other services in connection with our treatment programs. As a result of such allegations, we could be subject to civil and criminal penalties, our contracts could be found invalid and unenforceable, in whole or in part, or we could be required to restructure our contractual arrangements. If so, we may be unable to restructure our contractual arrangements on favorable terms, which would adversely affect our business and operations.
Our business practices may be found to violate anti-kickback, physician self-referral or false claims laws, which may lead to penalties and adversely affect our business.
The healthcare industry is subject to extensive federal and state regulation with respect to kickbacks, physician self-referral arrangements, false claims and other fraud and abuse issues.
The federal anti-kickback law (the “Anti-Kickback Law”) prohibits, among other things, knowingly and willfully offering, paying, soliciting, receiving, or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for, or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program. “Remuneration” is broadly defined to include anything of value, such as, for example, cash payments, gifts or gift certificates, discounts, or the furnishing of services, supplies, or equipment. The Anti-Kickback Law is broad, and it prohibits many arrangements and practices that are lawful in businesses outside of the health care industry.
Recognizing the breadth of the Anti-Kickback Law and the fact that it may technically prohibit many innocuous or beneficial arrangements within the health care industry, the Office of Inspector General (“OIG”) has issued a series of regulations, known as the “safe harbors.” Compliance with all requirements of a safe harbor immunizes the parties to the business arrangement from prosecution under the Anti-Kickback Law. The failure of a business arrangement to fit within a safe harbor does not necessarily mean that the arrangement is illegal or that the OIG will pursue prosecution. Still, in the absence of an applicable safe harbor, a violation of the Anti-Kickback Law may occur even if only one purpose of an arrangement is to induce referrals. The penalties for violating the Anti-Kickback Law can be severe. These sanctions include criminal and civil penalties, imprisonment, and possible exclusion from the federal health care programs. Many states have adopted laws similar to the Anti-Kickback Law, and some apply to items and services reimbursable by any payor, including private insurers.
In addition, the federal ban on physician self-referrals, commonly known as the Stark Law, prohibits, subject to certain exceptions, physician referrals of Medicare patients to an entity providing certain “designated health services” if the physician or an immediate family member of the physician has any financial relationship with the entity. A “financial relationship” is created by an investment interest or a compensation arrangement. Penalties for violating the Stark Law include the return of funds received for all prohibited referrals, fines, civil monetary penalties, and possible exclusion from the federal health care programs. In addition to the Stark Law, many states have their own self-referral bans, which may extend to all self-referrals, regardless of the payor.
The federal False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government. Under the False Claims Act, a person acts knowingly if he has actual knowledge of the information or acts in deliberate ignorance or in reckless disregard of the truth or falsity of the information. Specific intent to defraud is not required. Violations of other laws, such as the Anti-Kickback Law or the FDA prohibitions against promotion of off-label uses of drugs, can lead to liability under the federal False Claims Act. The qui tam provisions of the False Claims Act allow a private individual to bring an action on behalf of the federal government and to share in any amounts paid by the defendant to the government in connection with the action. The number of filings of qui tam actions has increased significantly in recent years. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 and $11,000 for each false claim. Conduct that violates the False Claims Act may also lead to exclusion from the federal health care programs. Given the number of claims likely to be at issue, potential damages under the False Claims Act for even a single inappropriate billing arrangement could be significant. In addition, various states have enacted similar laws modeled after the False Claims Act that apply to items and services reimbursed under Medicaid and other state health care programs, and, in several states, such laws apply to claims submitted to all payors.
On May 20, 2009, the Federal Enforcement and Recovery Act of 2009, or FERA, became law, and it significantly amended the federal False Claims Act. Among other things, FERA eliminated the requirement that a claim must be presented to the federal government. As a result, False Claims Act liability extends to any false or fraudulent claim for government money, regardless of whether the claim is submitted to the government directly, or whether the government has physical custody of the money. FERA also specifically imposed False Claims Act liability if an entity “knowingly and improperly avoids or decreases an obligation to pay or transmit money or property to the Government.” As a result, the knowing and improper failure to return an overpayment can serve as the basis for a False Claims Act action. In March 2010, Congress passed the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, collectively the ACA, which also made sweeping changes to the federal False Claims Act. The ACA also established that Medicare and Medicaid overpayments must be reported and returned within 60 days of identification or when any corresponding cost report is due.
Finally, the Health Insurance Portability and Accountability Act of 1996 and its implementing regulations created the crimes of health care fraud and false statements relating to health care matters. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including a private insurer. The false statements statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the delivery of or payment for health care benefits, items, or services. A violation of this statute is a felony and may result in fines, imprisonment, or exclusion from the federal health care programs.
Federal or state authorities may claim that our fee arrangements, our agreements and relationships with contractors, hospitals and physicians, or other activities violate fraud and abuse laws and regulations. If our business practices are found to violate any of these laws or regulations, we may be unable to continue with our relationships or implement our business plans, which would have an adverse effect on our business and results of operations. Further, defending our business practices could be time consuming and expensive, and an adverse finding could result in substantial penalties or require us to restructure our operations, which we may not be able to do successfully.
Our business practices may be subject to state regulatory and licensure requirements.
Our business practices may be regulated by state regulatory agencies that generally have discretion to issue regulations and interpret and enforce laws and rules. These regulations can vary significantly from jurisdiction to jurisdiction, and the interpretation of existing laws and rules also may change periodically. Some of our business and related activities may be subject to state health care-related regulations and requirements, including managed health care, utilization review (UR) or third-party administrator-related regulations and licensure requirements. These regulations differ from state to state, and may contain network, contracting, and financial and reporting requirements, as well as specific standards for delivery of services, payment of claims, and adequacy of health care professional networks. If a determination is made that we have failed to comply with any applicable state laws or regulations, our business, financial condition and results of operations could be adversely affected.
We may be subject to healthcare anti-fraud initiatives, which may lead to penalties and adversely affect our business.
State and federal government agencies are devoting increased attention and resources to anti-fraud initiatives against healthcare providers and the entities and individuals with whom they do business, and such agencies may define fraud expansively to include our business practices, including the receipt of fees in connection with a healthcare business that is found to violate any of the complex regulations described above. While to our knowledge we have not been the subject of any anti-fraud investigations, if such a claim were made, defending our business practices could be time consuming and expensive and an adverse finding could result in substantial penalties or require us to restructure our operations, which we may not be able to do successfully.
Our use and disclosure of patient information is subject to privacy and security regulations, which may result in increased costs.
In providing administrative services to healthcare providers and operating our treatment programs, we may collect, use, disclose, maintain and transmit patient information in ways that will be subject to many of the numerous state, federal and international laws and regulations governing the collection, use, disclosure, storage, privacy and security of patient-identifiable health information, including the administrative simplification requirements of the Health Insurance Portability and Accountability Act of 1996 and its implementing regulations (HIPAA) and the Health Information Technology for Economic and Clinical Health Act of 2009 (HITECH). The HIPAA Privacy Rule restricts the use and disclosure of patient information (“Protected Health Information” or “PHI”), and requires safeguarding that information. The HIPAA Security Rule and HITECH establish elaborate requirements for safeguarding PHI transmitted or stored electronically. HIPAA applies to covered entities, which may include healthcare facilities and also includes health plans that will contract for the use of our programs and our services. HIPAA and HITECH require covered entities to bind contractors that use or disclose protected health information (or “Business Associates”) to compliance with certain aspects of the HIPAA Privacy Rule and all of the HIPAA Security Rule. In addition to contractual liability, Business Associates are also directly subject to regulation by the federal government. Direct liability means that we are subject to audit, investigation and enforcement by federal authorities. HITECH imposes new breach notification obligations requiring us to report breaches of “Unsecured Protected Health Information” or PHI that has not been encrypted or destroyed in accordance with federal standards. Business Associates must report such breaches so that their covered entity customers may in turn notify all affected patients, the federal government, and in some cases, local or national media outlets. We may be required to indemnify our covered entity customers for costs associated with breach notification and the mitigation of harm resulting from breaches that we cause. If we are providing management services that include electronic billing on behalf of a physician practice or facility that is a covered entity, we may be required to conduct those electronic transactions in accordance with the HIPAA regulations governing the form and format of those transactions. Services provided under our OnTrak solution not only require us to comply with HIPAA and HITECH but also Title 42 Part 2 of the Code of Federal Regulations (“Part 2”). Part 2 is a federal, criminal law that severely restricts our ability to use and disclose drug and alcohol treatment information obtained from federally-supported treatment facilities. Our operations must be carefully structured to avoid liability under this law. Our OnTrak solution qualifies as a federally funded treatment facility which requires us to disclose information on members only in compliance with Title 42. In addition to the federal privacy regulations, there are a number of state laws governing the privacy and security of health and personal information. The penalties for violation of these laws vary widely and the area is rapidly evolving. We believe that we have taken the steps required of us to comply with health information privacy and security laws and regulations in all jurisdictions, both state and federal. However, we may not be able to maintain compliance in all jurisdictions where we do business. Failure to maintain compliance, or changes in state or federal privacy and security laws could result in civil and/or criminal penalties and could have a material adverse effect on our business, including significant reputational damage associated with a breach. If regulations change or it is determined that we are not in compliance with privacy regulations we may be required to modify aspects of our program which may adversely affect program results and our business or profitability. Under HITECH, we are subject to prosecution or administrative enforcement and increased civil and criminal penalties for non-compliance, including a new, four-tiered system of monetary penalties. We are also subject to enforcement by state attorneys general who were given authority to enforce HIPAA under HITECH.
Certain of our professional healthcare employees, such as nurses, must comply with individual licensing requirements.
All of our healthcare professionals who are subject to licensing requirements, such as our care coaches, are licensed in the state in which they provide professional services in person. While we believe our nurses provide coaching and not professional services, one or more states may require our healthcare professionals to obtain licensure if providing services telephonically across state lines to the state’s residents. Healthcare professionals who fail to comply with these licensure requirements could face fines or other penalties for practicing without a license, and we could be required to pay those fines on behalf of our healthcare professionals. If we are required to obtain licenses for our nurses in states where they provide telephonic coaching, it would significantly increase the cost of providing our product. In addition, new and evolving agency interpretations, federal or state legislation or regulations, or judicial decisions could lead to the implementation of out-of-state licensure requirements in additional states, and such changes would increase the cost of services and could have a material adverse effect on our business.
Security breaches, loss of data and other disruptions could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we collect and store sensitive data, including legally protected patient health information, personally identifiable information about our employees, intellectual property, and proprietary business information. We manage and maintain our applications and data utilizing an off-site co-location facility. These applications and data encompass a wide variety of business critical information including research and development information, commercial information and business and financial information.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers, viruses, breaches or interruptions due to employee error or malfeasance, terrorist attacks, earthquakes, fire, flood, other natural disasters, power loss, computer systems failure, data network failure, Internet failure or lapses in compliance with privacy and security mandates. Any such virus, breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, lost or stolen. We have measures in place that are designed to detect and respond to such security incidents and breaches of privacy and security mandates. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as HIPAA, government enforcement actions and regulatory penalties. We may also be required to indemnify our customers for costs associated with having their data on our system breached. Unauthorized access, loss or dissemination could also interrupt our operations, including our ability to bill our customers, provide customer support services, conduct research and development activities, process and prepare company financial information, manage various general and administrative aspects of our business and damage our reputation, or we may lose one or more of our customers, especially if they felt their data may be breached, any of which could adversely affect our business.
Risks related to our common stock
Our common stock has limited trading volume, and it is therefore susceptible to high price volatility.
Our common stock is quoted on the OTCQB under the symbol “CATS” and has limited trading volume. As such, our common stock is more susceptible to significant and sudden price changes than stocks that are widely followed by the investment community and actively traded on an exchange. The liquidity of our common stock depends upon the presence in the marketplace of willing buyers and sellers. We cannot assure you that you will be able to find a buyer for your shares. If we successfully list the common stock on a securities exchange or obtain trading authorization, we will not be able to assure you that an organized public market for our securities will develop or that there will be any private demand for the common stock. We could also subsequently fail to satisfy the standards for continued national securities exchange trading, such as standards having to do with a minimum share price, the minimum number of public shareholders or the aggregate market value of publicly held shares. Any holder of our securities should regard them as a long-term investment and should be prepared to bear the economic risk of an investment in our securities for an indefinite period.
Our common stock is considered a “penny stock” and may be difficult to sell.
Our common stock is subject to certain rules and regulations relating to “penny stock.” Penny stocks are generally equity securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system). The penny stock rules require a broker−dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker−dealer must also provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker−dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. In addition, the penny stock rules generally require that prior to a transaction in a penny stock, the broker−dealer make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for a stock that becomes subject to the penny stock rules. Since the Company’s securities are subject to the penny stock rules, investors in the Company may find it more difficult to sell their securities.
Failure to maintain effective internal controls could adversely affect our operating results and the market for our common stock.
Section 404 of the Sarbanes-Oxley Act of 2002 requires that we maintain internal control over financial reporting that meets applicable standards. As with many smaller companies with small staff, material weaknesses in our financial controls and procedures may be discovered. If we are unable, or are perceived as unable, to produce reliable financial reports due to internal control deficiencies, investors could lose confidence in our reported financial information and operating results, which could result in a negative market reaction and adversely affect our ability to raise capital.
Approximately 78% of our outstanding common stock is beneficially owned by our chairman and chief executive officer, who has the ability to substantially influence the election of directors and other matters submitted to stockholders.
10,619,760 shares are beneficially held of record by Acuitas Group Holdings, LLC (“Acuitas”), whose sole managing member is our Chairman and Chief Executive Officer, which represents current beneficial ownership of approximately 78% of our outstanding shares of common stock. As a result, he has and is expected to continue to have the ability to significantly influence the election of our Board of Directors and the outcome of all other matters submitted to our stockholders. His interest may not always coincide with our interests or the interests of other stockholders, and he may act in a manner that advances his best interests and not necessarily those of other stockholders. One consequence to this substantial influence or control is that it may be difficult for investors to remove management of our Company. It could also deter unsolicited takeovers, including transactions in which stockholders might otherwise receive a premium for their shares over then current market prices.
Our stock price may be subject to substantial volatility, and the value of our stockholders' investment may decline.
The price at which our common stock will trade may fluctuate as a result of a number of factors, including the number of shares available for sale in the market, quarterly variations in our operating results and actual or anticipated announcements of our OnTrak solution, announcements regarding new or discontinued OnTrak solution contracts, new products or services by us or competitors, regulatory investigations or determinations, acquisitions or strategic alliances by us or our competitors, recruitment or departures of key personnel, the gain or loss of significant customers, changes in the estimates of our operating performance, actual or threatened litigation, market conditions in our industry and the economy as a whole.
Numerous factors, including many over which we have no control, may have a significant impact on the market price of our common stock, including:
|
|
● |
announcements of new products or services by us or our competitors; |
|
|
● |
current events affecting the political, economic and social situation in the United States; |
|
|
● |
trends in our industry and the markets in which we operate; |
|
|
● |
changes in financial estimates and recommendations by securities analysts; |
|
|
● |
acquisitions and financings by us or our competitors; |
|
|
● |
the gain or loss of a significant customer; |
|
|
● |
quarterly variations in operating results; |
|
|
● |
the operating and stock price performance of other companies that investors may consider to be comparable; |
|
|
● |
purchases or sales of blocks of our securities; and |
|
|
● |
issuances of stock. |
Furthermore, stockholders may initiate securities class action lawsuits if the market price of our stock drops significantly, which may cause us to incur substantial costs and could divert the time and attention of our management.
Future sales of common stock by existing stockholders, or the perception that such sales may occur, could depress our stock price.
The market price of our common stock could decline as a result of sales by, or the perceived possibility of sales by, our existing stockholders. We have completed a number of private placements of our common stock and other securities over the last several years, and we have effective resale registration statements pursuant to which the purchasers can freely resell their shares into the market. In addition, most of our outstanding shares are eligible for public resale pursuant to Rule 144 under the Securities Act of 1933, as amended. As of March 30, 2017, approximately 8.3 million shares of our common stock are held by our affiliates and may be sold pursuant to an effective registration statement or in accordance with the volume and other limitations of Rule 144 or pursuant to other exempt transactions. Future sales of common stock by significant stockholders, including those who acquired their shares in private placements or who are affiliates, or the perception that such sales may occur, could depress the price of our common stock.
Future issuances of common stock and hedging activities may depress the trading price of our common stock.
Any future issuance of equity securities, including the issuance of shares upon direct registration, the conversion of our 12% Original Issue Discount Convertible Debenture (the “Convertible Debenture”), our 8% Senior Convertible Debenture (the “December 2016 Convertible Debenture”), or our January 2017 Convertible Debenture upon satisfaction of our obligations, compensation of vendors, exercise of outstanding warrants, or effectuation of a reverse stock split, could dilute the interests of our existing stockholders, and could substantially decrease the trading price of our common stock. As of March 30, 2017, we have outstanding options to purchase approximately 243,853 shares of our common stock and warrants to purchase approximately 1,684,578 shares of our common stock at prices ranging from $1.80 to $6,360.00 per share. We may issue equity securities in the future for a number of reasons, including to finance our operations and business strategy, in connection with acquisitions, to adjust our ratio of debt to equity, to satisfy our obligations upon the exercise of outstanding warrants or options or for other reasons.
There may be future sales or other dilution of our equity, which may adversely affect the market price of our common stock.
In the future, we may need to raise additional funds through public or private financing, which might include sales of equity securities. The issuance of any additional shares of common stock or securities convertible into, exchangeable for, or that represent the right to receive common stock or the exercise of such securities could be substantially dilutive to holders of shares of our common stock. Holders of shares of our common stock have no preemptive rights that entitle holders to purchase their pro rata share of any offering of shares of any class or series. The market price of our common stock could decline as a result of sales of shares of our common stock made after this offering or the perception that such sales could occur. Because our decision to issue securities in any future offering will depend on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of our future offerings. Thus, our stockholders bear the risk of our future offerings reducing the market price of our common stock and diluting their interests in our Company.
Provisions in our certificate of incorporation and Delaware law could discourage a change in control, or an acquisition of us by a third party, even if the acquisition would be favorable to you.
Our certificate of incorporation and the Delaware General Corporation Law contain provisions that may have the effect of making more difficult or delaying attempts by others to obtain control of our Company, even when these attempts may be in the best interests of stockholders. For example, our certificate of incorporation authorizes our Board of Directors, without stockholder approval, to issue one or more series of preferred stock, which could have voting and conversion rights that adversely affect or dilute the voting power of the holders of common stock. Delaware law also imposes conditions on certain business combination transactions with “interested stockholders.” These provisions and others that could be adopted in the future could deter unsolicited takeovers or delay or prevent changes in our control or management, including transactions in which stockholders might otherwise receive a premium for their shares over then current market prices. These provisions may also limit the ability of stockholders to approve transactions that they may deem to be in their best interests.
We do not expect to pay dividends in the foreseeable future.
We have paid no cash dividends on our common stock to date, and we intend to retain our future earnings, if any, to fund the continued development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Further, any payment of cash dividends will also depend on our financial condition, results of operations, capital requirements and other factors, including contractual restrictions to which we may be subject, and will be at the discretion of our Board of Directors.
A number of our outstanding warrants contain anti-dilution provisions that, if triggered, could cause substantial dilution to our then-existing stockholders and adversely affect our stock price.
A number of our outstanding warrants contain anti-dilution provisions. As a result, if we, in the future, issue or grant any rights to purchase any of our common stock or other securities convertible into our common stock for a per share price less than the exercise price of our warrants, the exercise price, or in the case of some of our warrants the exercise price and number of shares of common stock, will be reduced. If our available funds and cash generated from operations are insufficient to satisfy our liquidity requirements in the future, then we may need to raise substantial additional funds in the future to support our working capital requirements and for other purposes. If shares of our common stock or securities exercisable for our common stock are issued in consideration of such funds at an effective per share price lower than our existing warrants, then the anti-dilution provisions would be triggered, thus possibly causing substantial dilution to our then-existing shareholders if such warrants are exercised. Such anti-dilution provisions may also make it more difficult for us to obtain financing.
The exercise of our outstanding warrants may result in a dilution of our current stockholders' voting power and an increase in the number of shares eligible for future resale in the public market, which may negatively impact the market price of our stock.
The exercise of some or all of our outstanding warrants could significantly dilute the ownership interests of our existing stockholders. As of March 30, 2017, we had outstanding warrants to purchase an aggregate of 1,684,578 shares of common stock at exercise prices ranging from $1.80 to $18.00 per share. To the extent warrants are exercised, additional shares of common stock will be issued, and such issuance may dilute existing stockholders and increase the number of shares eligible for resale in the public market.
In addition to the dilutive effects described above, the exercise of those warrants would lead to a potential increase in the number of shares eligible for resale in the public market. Sales of substantial numbers of such shares in the public market could adversely affect the market price of our shares.
Risks related to this offering
There is not now, and there may never be, an active, liquid and orderly trading market for our common stock, which may make it difficult for you to sell your shares of our common stock.
There is not now, nor has there been since our inception, any significant volume of trading activity in our common stock or an active market for shares of our common stock, and an active trading market for our shares may never develop or be sustained after this offering. As a result, investors in our common stock must bear the economic risk of holding those shares for an indefinite period of time. Although our common stock is quoted on the OTCQB Marketplace, or OTCQB, over-the-counter quotation system, trading of our common stock on such system has only recently commenced and continues to be extremely limited and sporadic and at very low volumes. Although we have applied to list our common stock on the NASDAQ Capital Market and expect that our common stock will be listed on the NASDAQ Capital Market prior to the completion of this offering, an active trading market for our common stock may never develop or be sustained. If an active market for our common stock does not develop, it may be difficult for you to sell the shares you purchase in this offering without depressing the market price for the shares or at all. Further, an unestablished trading market for our common stock may also impair our ability to raise capital by selling additional equity in the future, and may impair our ability to enter into strategic partnerships or acquire companies or products by using shares of our common stock as consideration.
Our common stock has limited trading volume, and it is therefore susceptible to high price volatility.
Our common stock is quoted on the OTCQB, under the symbol “CATS” and has limited trading volume. As such, our common stock is more susceptible to significant and sudden price changes than stocks that are widely followed by the investment community and actively traded on a securities exchange. The liquidity of our common stock depends upon the presence in the marketplace of willing buyers and sellers. We cannot assure you that you will be able to find a buyer for your shares. If we successfully list the common stock on a securities exchange or obtain trading authorization, we will not be able to assure you that an organized public market for our securities will develop or that there will be any private demand for the common stock. We could also subsequently fail to satisfy the standards for continued national securities exchange trading, such as standards having to do with a minimum share price, the minimum number of public shareholders or the aggregate market value of publicly held shares. Any holder of our securities should regard them as a long-term investment and should be prepared to bear the economic risk of an investment in our securities for an indefinite period.
If at any time our common stock is subject to the Securities and Exchange Commission’s “penny stock” rules, broker-dealers may experience difficulty in completing customer transactions and trading activity in our securities may be adversely affected.
If at any time our common stock is not listed on a national securities exchange, including the NASDAQ Capital Market, or we have net tangible assets of $5,000,000 or less and our common stock has a market price per share of less than $5.00, as is currently the case, transactions in our common stock will be subject to the Securities and Exchange Commission’s “penny stock” rules. If our common stock is subject to the “penny stock” rules promulgated under the Exchange Act, broker-dealers may find it difficult to effectuate customer transactions and trading activity in our securities may be adversely affected. For any transaction involving a penny stock, unless exempt, the rules require:
|
|
● |
that a broker or dealer approve a person’s account for transactions in penny stocks; and |
|
● |
the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. |
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must:
|
● |
obtain financial information and investment experience objectives of the person; and |
|
● |
make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. |
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the Securities and Exchange Commission relating to the penny stock market, which, in highlight form:
|
● |
sets forth the basis on which the broker or dealer made the suitability determination; and |
|
● |
that the broker or dealer received a signed, written agreement from the investor prior to the transaction.. |
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our common stock and cause a decline in the market value of our stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
We will incur increased costs associated with, and our management will need to devote substantial time and effort to, compliance with public company reporting and other requirements.
We have applied to list our common stock on the NASDAQ Capital Market and, although no assurance can be given that our application will be approved, we expect that our common stock will be listed on the NASDAQ Capital Market prior to the completion of this offering. As a public company listed on the NASDAQ Capital Market, and particularly if and after we cease to be a “smaller reporting company,” we will incur significant legal, accounting and other expenses that we did not incur prior to the listing of our common stock on the NASDAQ Capital Market. In addition, the rules and regulations of the Securities and Exchange Commission and the NASDAQ Capital Market impose numerous requirements on public companies, including requirements relating to our corporate governance practices, with which we will need to comply. Our management and other personnel will need to devote substantial time to gaining expertise regarding operations as a public company on NASDAQ and compliance with applicable laws and regulations, and our efforts and initiatives to comply with those requirements could be expensive.
Failure to maintain effective internal controls could adversely affect our operating results and the market for our common stock.
Section 404 of the Sarbanes-Oxley Act of 2002 requires that we maintain internal control over financial reporting that meets applicable standards. As with many smaller companies with small staff, material weaknesses in our financial controls and procedures may be discovered. If we are unable, or are perceived as unable, to produce reliable financial reports due to internal control deficiencies, investors could lose confidence in our reported financial information and operating results, which could result in a negative market reaction and adversely affect our ability to raise capital.
Our stock price may be subject to substantial volatility, and the value of our stockholders' investment may decline.
The price at which our common stock will trade may fluctuate as a result of a number of factors, including the number of shares available for sale in the market, quarterly variations in our operating results and actual or anticipated announcements of our OnTrak Solution, announcements regarding new or discontinued OnTrak Solution contracts, new products or services by us or competitors, regulatory investigations or determinations, acquisitions or strategic alliances by us or our competitors, recruitment or departures of key personnel, the gain or loss of significant customers, changes in the estimates of our operating performance, actual or threatened litigation, market conditions in our industry and the economy as a whole.
Numerous factors, including many over which we have no control, may have a significant impact on the market price of our common stock, including:
|
|
● |
announcements of new products or services by us or our competitors; |
|
|
● |
current events affecting the political, economic and social situation in the United States and other countries where we operate; |
|
|
● |
trends in our industry and the markets in which we operate; |
|
|
● |
adoption of new laws, rules and regulations affecting the health care industry; |
|
|
● |
changes in financial estimates and recommendations by securities analysts; |
|
|
● |
acquisitions and financings by us or our competitors; |
|
|
● |
the gain or loss of a significant customer; |
|
|
● |
quarterly variations in operating results; |
|
|
● |
the operating and stock price performance of other companies that investors may consider to be comparable; |
|
|
● |
purchases or sales of blocks of our securities; and |
|
|
● |
issuances of stock. |
Furthermore, stockholders may initiate securities class action lawsuits if the market price of our stock drops significantly, which may cause us to incur substantial costs and could divert the time and attention of our management.
If you purchase shares of our common stock in this offering, you will suffer immediate dilution of your investment.
We expect the public offering price of our common stock to be substantially higher than the pro forma net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our pro forma as adjusted net tangible book value per share after this offering. Based on the public offering price of $ per share, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, you will experience immediate dilution of $ per share, representing the difference between our as adjusted pro forma net tangible book value per share after this offering and the assumed public offering price.
In addition, as of March 30, 2017, we had outstanding stock options to purchase 243,853 shares of common stock and an outstanding warrants to purchase 1,684,578 shares of our common stock. To the extent these outstanding options or warrants are exercised, there may be further dilution to investors in this offering.
Future sales of common stock by existing stockholders, or the perception that such sales may occur, could depress our stock price.
The market price of our common stock could decline as a result of sales by, or the perceived possibility of sales by, our existing stockholders. We have completed a number of private placements of our common stock and other securities over the last several years and most of our outstanding shares are eligible for public resale pursuant to Rule 144 under the Securities Act of 1933, as amended. As of March 30, 2017, approximately 8.3 million shares of our common stock are held by our affiliates and may be sold pursuant to an effective registration statement or in accordance with the volume and other limitations of Rule 144 or pursuant to other exempt transactions. In addition, all of our directors and officers are subject to lock-up agreements with the underwriters of this offering that restrict the stockholders’ ability to transfer shares of our common stock for at least six months from the date of this prospectus. The lock-up agreements limit the number of shares of common stock that may be sold immediately following the public offering. Future sales of common stock by significant stockholders, including those who acquired their shares in private placements or who are affiliates, or the perception that such sales may occur, could depress the price of our common stock.
Future issuances of common stock and hedging activities may depress the trading price of our common stock.
Any future issuance of equity securities could dilute the interests of our existing stockholders, and could substantially decrease the trading price of our common stock. As of March 30, 2017, we have outstanding options to purchase approximately 243,853 shares of our common stock and warrants to purchase approximately 1,684,578 shares of our common stock at exercise prices ranging from $1.80 to $6,360.00 per share. We may issue equity securities in the future for a number of reasons, including to finance our operations and business strategy, in connection with acquisitions, to adjust our ratio of debt to equity, to satisfy our obligations upon the exercise of outstanding warrants or options or for other reasons.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements in this prospectus that are not descriptions of historical facts, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research and development costs, timing and likelihood of success, plans and objectives of management for future operations, and future results of anticipated products, are forward-looking statements that are based on management’s current expectations and assumptions and are subject to risks and uncertainties. If such risks or uncertainties materialize or such assumptions prove incorrect, our business, operating results, financial condition and stock price could be materially negatively affected. In some cases, you can identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would” or the negative of these terms or other comparable terminology. Factors that could cause actual results to differ materially from those currently anticipated include those set forth in the section titled “Risk Factors” including, without limitation, risks relating to:
|
|
• |
our dependence on a widespread acceptance of our OnTrak Solution for our continued growth; |
|
|
• |
our need for additional funds in order to pursue our business plan and the uncertainty of whether we will be able to obtain the funding we need; |
|
|
• |
our ability to enroll members in our OnTrak Solutions and achieve promised savings for our OnTrak contracts; |
|
|
• |
competition in our industry; |
|
|
• |
our dependence on the retention of key personnel; |
|
|
• |
our ability to protect our intellectual property rights; |
|
|
• |
our dependence on third-party payors to provide coverage and adequate payment rates for our programs; |
|
|
• |
our ability to comply with complex and increasing regulation by state and federal authorities; |
|
|
• |
the impact of healthcare reform legislation; |
|
|
• |
regulatory developments in the United States and foreign countries; |
|
|
• |
our history of operating losses since our inception; |
|
|
• |
our ability to continue to operate as a going concern; and |
|
|
• |
our liquidity. |
We operate in a very competitive and rapidly-changing environment and new risks emerge from time to time. As a result, it is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. The forward-looking statements included in this prospectus speak only as of the date hereof, and except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations. For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
USE OF PROCEEDS
We estimate that we will receive net proceeds of approximately $ million (or approximately $ million if the underwriters’ option to purchase additional shares is exercised in full) from the sale of the shares of common stock offered by us in this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The holders of our December 2016 Convertible Debentures and January 2017 Convertible Debenture have the right to require us to repay $ of the 2016 Convertible Debentures and January 2017 Convertible Debenture out of the proceeds of this offering, although the holders of both series of debentures have agreed to extend the maturity date to the earlier of the closing date of this offering or April 30, 2017. We intend to use $4.5 million of the net proceeds to pay debentures held by Acuitas and Mark Cummins, and the remaining $1.5 million of debentures are expected to convert into common stock as of the closing of this offering. We intend to use the remaining net proceeds of this offering for working capital and general corporate purposes.
We believe that our existing cash and cash equivalents, together with the net proceeds from this offering, will be sufficient to fund our operating expenses and capital expenditure requirements for at least the next 18 months. The amount and timing of our actual expenditures will depend upon numerous factors, including the status of our expansion strategy, and other factors described under “Risk Factors” in this prospectus, as well as the amount of cash used in our operations. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the use of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our stock. Pending their use, we plan to invest the net proceeds from this offering in money market funds short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
PRICE RANGE OF OUR COMMON STOCK
Our common stock is quoted on the OTCQB under the symbol “CATS.” The last reported sale price for our common stock on the OTCQB on March 30, 2017 was $9.66 per share.
The table below sets forth the high and low sale prices for our common stock as reported on the OTCQB during the periods indicated. The quotations below reflect inter-dealer prices and do not include retail markup, markdown or commissions. In addition, these quotations may not necessarily represent actual transactions.
|
2017 |
High |
Low |
||||||
|
1st Quarter (through March 30, 2017) |
$ | 12.18 | $ | 4.98 | ||||
|
2016 |
High |
Low |
||||||
|
4th Quarter |
$ | 7.68 | $ | 4.74 | ||||
|
3rd Quarter |
9.30 | 3.60 | ||||||
|
2nd Quarter |
5.28 | 1.98 | ||||||
|
1st Quarter |
3.48 | 1.50 | ||||||
|
2015 |
High |
Low |
||||||
|
4th Quarter |
$ | 4.74 | $ | 1.74 | ||||
|
3rd Quarter |
10.14 | 3.66 | ||||||
|
2nd Quarter |
12.60 | 5.70 | ||||||
|
1st Quarter |
14.70 | 10.32 | ||||||
|
2014 |
High |
Low |
||||||
|
4th Quarter |
$ | 13.80 | $ | 9.36 | ||||
|
3rd Quarter |
13.74 | 9.06 | ||||||
|
2nd Quarter |
12.30 | 4.68 | ||||||
|
1st Quarter |
9.60 | 4.62 | ||||||
Stockholders
As of March 30, 2017, there were approximately 68 stockholders of record of our 9,383,740 outstanding shares of common stock. On March 30, 2017 the last reported sale price for our common stock as reported on the OTCQB Marketplace was $9.66 per share.
DIVIDEND POLICY
We have never declared or paid dividends on our common stock and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. Payment of cash dividends, if any, in the future will be at the discretion of our board of directors and will depend on applicable law and then-existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business.
CAPITALIZATION
The following table sets forth our cash and cash equivalents as well as capitalization as of December 31, 2016:
|
|
• |
on an actual basis; |
|
|
• |
on a pro forma basis giving effect to (i) the conversion of the 12% Original Issue Discount Convertible Debenture in the amount of $4,130, (ii) the conversion of the 8% Series B Convertible Debenture in the amount of $3,060 (iii) the partial elimination of the warrant liability by removing the anti-dilution provision in certain of the warrant agreements, and (iv) the payment of Terren Peizer’s deferred salary of $1,060 with common stock; and |
|
|
• |
on a pro forma as adjusted basis to give further effect to the issuance and sale by us of shares of common stock in this offering at the public offering price of $ per share, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us and the use of $2,823 of the proceeds from this offering to repay Acuitas’ 8% Series B Convertible Debenture in full. |
You should read this table together with “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” as well as our consolidated financial statements and related notes appearing elsewhere in this prospectus.
|
(In thousands, except for number of shares) |
As of December 31, 2016 |
|||||||||||
|
Actual |
Pro Forma (unaudited) |
Pro Forma As Adjusted (unaudited) |
||||||||||
|
Cash and cash equivalents |
$ | 851 | $ | 851 | $ | 11,528 | ||||||
|
Preferred stock, $0.0001 par value; 50,000,000 shares authorized; no shares issued and outstanding |
— | — | — | |||||||||
|
Common stock, $0.0001 par value; 500,000,000 shares authorized; and 9,214,743, [ __ ], and [ __ ] shares issued and outstanding, actual, pro forma, and pro forma as adjusted, respectively |
6 | 8 | 9 | |||||||||
|
Short term debt |
9,796 | 2,823 | — | |||||||||
|
Short term derivative liability |
8,122 | — | — | |||||||||
|
Warrant liabilities |
5,307 | 48 | 48 | |||||||||
|
Additional paid-in capital |
254,385 | 270,584 | 284,082 | |||||||||
|
Accumulated deficit |
(279,719 | ) | (274,584 | ) | (274,506 | ) | ||||||
|
Total Stockholders' equity (deficit) |
(25,328 | ) | (3,914 | ) | 9,586 | |||||||
|
Total capitalization |
$ | 254,385 | $ | 270,584 | $ | 284,082 | ||||||
The number of shares of common stock to be outstanding after this offering is based on 9,214,743 shares of common stock outstanding as of December 31, 2016, which does not include:
|
• |
244,046 shares of common stock issuable upon the exercise of outstanding stock options as of December 31, 2016, at a weighted average exercise price of $39.06 per share; |
|
• |
1,313,360 shares of common stock issuable upon the exercise of outstanding warrants as of December 31, 2016, at a weighted average exercise price of $4.82 per share; |
|
• |
50,581 shares of common stock reserved for future issuance under our equity incentive plan as of December 31, 2016; |
|
• |
3,198,881 shares of common stock reserved for future issuance under our Convertible Debenture as of December 31, 2016; and | |
| • | _______ shares of common stock underlying warrants to be issued to the representative of the underwriters in connection with the offering. |
DILUTION
Investors purchasing shares of our common stock in this offering will experience immediate and substantial dilution in the as adjusted net tangible book value of their shares of common stock. Dilution in pro forma as adjusted net tangible book value represents the difference between the public offering price per share and the pro forma as adjusted net tangible book value per share of our common stock immediately after the offering.
The historical net tangible book value of our common stock as of December 31, 2016 was $(25,328,000), or $(0.46) per share. Historical net tangible book value per share of our common stock represents our total tangible assets (total assets less intangible assets) less total liabilities divided by the number of shares of common stock outstanding as of that date.
On a pro forma basis, after giving effect to (i) the conversion of the 12% Original Issue Discount Convertible Debenture in the amount of $4,130,000 (ii) the conversion of the 8% Series B Convertible Debenture in the amount of $3,060,000 (iii) the partial elimination of the warrant liability by removing the anti-dilution provision in certain of the warrant agreements, and (iv) the payment of Terren Peizer's deferred salary of $1,060 with common stock, each of which will become effective as of the close of this offering, our pro forma net tangible book value as of December 31, 2016 would have been approximately $3,914,000, or approximately $0.42 per share of our common stock.
After giving effect to the issuance and sale of shares of common stock in this offering, at the public offering price of $ per share, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, and the use of $2,823,000 to repay Acuitas’ 8% Series B Convertible Debenture in full, our pro forma as adjusted net tangible book value as of December 31, 2016 would have been approximately $ million, or $ per share of common stock. This amount represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to existing stockholders and an immediate dilution of $ per share to new investors purchasing our common stock in this offering.
The following table illustrates this dilution on a per share basis to new investors:
|
Assumed Public offering price per share |
$ | |||||||
|
Pro forma net tangible book value per share as of December 31, 2016 |
$ | (0.42 | ) | |||||
|
Increase in pro forma net tangible book value per share attributable to new investors purchasing shares in this offering |
||||||||
|
Pro forma as adjusted net tangible book value per share after giving effect to this offering |
||||||||
|
Dilution in pro forma as adjusted net tangible book value per share to new investors participating in this offering |
$ |
If the underwriters exercise their option in full to purchase an additional shares of common stock in this offering, the pro forma as adjusted net tangible book value per share after the offering would be $ per share, the increase in the pro forma net tangible book value per share to existing stockholders would be $ per share and the dilution to new investors purchasing our common stock in this offering would be $ per share.
The number of shares of common stock to be outstanding after this offering is based on 9,214,743 shares of common stock outstanding as of December 31, 2016, which does not include:
|
• |
244,046 shares of common stock issuable upon the exercise of outstanding stock options as of December 31, 2016, at a weighted average exercise price of $39.06 per share; |
|
• |
1,313,360 shares of common stock issuable upon the exercise of outstanding warrants as of December 31, 2016, 2016, at a weighted average exercise price of $4.82 per share; |
|
• |
50,581 shares of common stock reserved for future issuance under our equity incentive plan as of December 31, 2016; |
|
• |
3,198,881 shares of common stock reserved for future issuance under our Convertible Debenture as of December 31, 2016; and | |
| • | shares of common stock underlying the warrants to be issued to the representative of the underwriters in connection with the offering. |
To the extent that outstanding exercisable options or warrants are exercised, you may experience further dilution. If all outstanding exercisable options and warrants with exercise prices below $ per share were exercised, our pro forma as adjusted net tangible book value as of December 31, 2016 (calculated on the basis of the assumptions set forth above) would have been approximately $ million, or approximately $ per share, causing immediate dilution of $ per share to new investors purchasing shares in this offering.
In addition, we may choose to raise additional capital due to market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital by issuing equity securities or convertible debt, your ownership will be further diluted.
SELECTED CONSOLIDATED FINANCIAL DATA
The following table summarizes our selected consolidated financial data for the periods and as of the dates indicated. Our selected statements of operations data for each of the years ended December 31, 2016 and 2015, and our selected balance sheet data as of December 31, 2016, have been derived from our audited consolidated financial statements and related notes included elsewhere in this prospectus. Our selected financial data should be read together with the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and with our financial statements and their related notes, which are included elsewhere in this prospectus. Our historical results are not indicative of the results that may be expected in the future.
|
Twelve Months Ended |
||||||||
|
(In thousands, except per share amounts) |
December 31, |
|||||||
|
2016 |
2015 |
|||||||
|
Revenues |
||||||||
|
Healthcare services revenues |
$ | 7,075 | $ | 2,705 | ||||
|
Operating expenses |
||||||||
|
Cost of healthcare services |
4,670 | 2,433 | ||||||
| General and administrative | 8,838 | 9,049 | ||||||
|
Depreciation and amortization |
141 | 122 | ||||||
|
Total operating expenses |
13,649 | 11,604 | ||||||
|
Loss from operations |
(6,574 | ) | (8,899 | ) | ||||
|
Interest and other income |
106 | 64 | ||||||
|
Interest expense |
(5,354 | ) | (2,590 | ) | ||||
|
Loss on impairment of intangible assets |
- | (88 | ) | |||||
|
Loss on exchange of warrants |
- | (4,410 | ) | |||||
| Loss on debt extinguishment | (2,424 | ) | (195 | ) | ||||
|
Change in fair value of warrant liability |
2,093 | 11,665 | ||||||
|
Change in fair value of derivative liability |
(5,774 | ) | (2,761 | ) | ||||
|
Loss from operations before provision for income taxes |
(17,927 | ) | (7,214 | ) | ||||
|
Provision for income taxes |
9 | 9 | ||||||
|
Net loss |
$ | (17,936 | ) | $ | (7,223 | ) | ||
|
Basic and diluted net loss per share: |
$ | (1.95 | ) | $ | (1.07 | ) | ||
|
Basic weighted number of shares outstanding |
9,179 | 6,729 | ||||||
|
As of December 31, |
||||||||
|
(In thousands) |
2016 |
2015 |
||||||
|
Consolidated Balance Sheet Data: |
||||||||
|
Cash and cash equivalents |
$ | 851 | $ | 916 | ||||
|
Total assets |
3,104 | 2,880 | ||||||
|
Total liabilities |
28,432 | 11,604 | ||||||
|
Accumulated deficit |
(279,719 | ) | (261,783 | ) | ||||
|
Total stockholders’ deficit |
(25,328 | ) | (8,724 | ) | ||||
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with “Selected Consolidated Financial Data” and our consolidated financial statements and related notes appearing elsewhere in this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties as described under the heading “Special Note Regarding Forward-Looking Statements” elsewhere in this prospectus. You should review the disclosure under the heading “Risk Factors” in this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
OVERVIEW
General
We provide data analytics based specialized behavioral health management and integrated treatment services to health plans through our OnTrak solution. Our OnTrak solution is designed to improve member health and at the same time lower costs to the insurer for underserved populations where behavioral health conditions are causing or exacerbating co-existing medical conditions. The program utilizes proprietary analytics, member engagement and patient centric treatment that integrates evidence-based medical and psychosocial interventions along with care coaching in a 52-week outpatient program. Our initial focus was members with substance use disorders, but we have expanded our solution to assist members with anxiety and depression. We currently operate our OnTrak solutions in Florida, Georgia, Illinois, Kansas, Kentucky, Louisiana, Massachusetts, Missouri, New Jersey, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, West Virginia and Wisconsin. We provide services to commercial (employer funded), managed Medicare Advantage, and managed Medicaid and duel eligible (Medicare and Medicaid) populations.
Recent Developments
Amendments to Outstanding Warrants and Extension of Existing Debentures
In March 2017, we entered into amendments with the holders of certain outstanding warrants issued on April 17, 2015 and July 30, 2015 to eliminate certain anti-dilution provisions in such warrants, which caused us to reflect an associated liability of $5.3 million on our balance sheet as of December 31, 2016. Such amendments are contingent upon and will not take effect until the closing of this offering, which must occur on or before April 30, 2017. For each warrant share underlying the warrants so amended, the holder received the right to purchase an additional .2 shares of common stock. Two of the holders of such warrants, which owners hold warrants to purchase an aggregate of 11,049 shares of common stock, did not agree to the amendment. The warrant holders agreeing to the amendment include Acuitas Group Holdings, LLC (“Acuitas”), one hundred percent (100%) of which is owned by Terren S. Peizer, Chairman and Chief Executive Officer of the Company, and another accredited investor, who will own warrants to purchase 187,002 and 79,545 shares of the Company’s common stock, respectively, if and when the amendments take effect.
Additionally, in March 2017, we and the holders of an aggregate of approximately $10 million of our existing convertible debentures agreed to extend the maturity dates of such debentures until the earlier of the closing of this offering or April 30, 2017.
Financing Activity
In January 2017, we entered into a Subscription Agreement (the “Subscription Agreement”) with Acuitas, pursuant to which the Company will receive aggregate gross proceeds of $1,300,000 (the “Loan Amount”) in consideration of the issuance of (i) an 8% Series B Convertible Debenture due March 31, 2017 (the “January 2017 Convertible Debenture”) and (ii) five-year warrants to purchase shares of the Company’s common stock in an amount equal to one hundred percent (100%) of the initial number of shares of common stock issuable upon the conversion of the January 2017 Convertible Debenture, at an exercise price of $5.10 per share (the “January 2017 Warrants”). The Loan Amount is payable in tranches through March 2017. In addition, any warrants issued in conjunction with the December 2016 Convertible Debenture currently outstanding with Acuitas have been increased by an additional 25% warrant coverage, exercisable for an aggregate of 137,883 shares of the Company’s common stock. The holder of the January 2017 Convertible Debenture has agreed to extend the maturity of the January 2017 Convertible Debenture from March 31, 2017 to the earlier of the closing date of this offering or April 30, 2017.
The January 2017 Warrants include, among other things, price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the January 2017 Warrants will be adjusted if the Company issues shares of common stock at a price that is less than the then exercise price of the January 2017 Warrants. Such price protection provisions will remain in effect until the earliest of (i) the expiration date of the January 2017 Warrants, (ii) such time as the January 2017 Warrants are exercised or (iii) contemporaneously with the listing of the Company’s shares of common stock on a registered national securities exchange.
In connection with the Subscription Agreement described above, the number of Shamus Warrants were increased from 75% to 100% warrant coverage, exercisable for an aggregate of 58,824 shares of the Company’s common stock.
2017 Stock Incentive Plan
On February 27, 2017, the Board of Directors and our stockholders approved the adoption of the 2017 Stock Incentive Plan (the “2017 Plan”), subject to complying with the notification requirements of Regulation 14C under the Exchange Act (“Regulation 14C”). The 2017 Plan allows the Company, under the direction of the Board of Directors or a committee thereof, to make grants of stock options, restricted and unrestricted stock and other stock-based awards to employees, including the Company’s executive officers, consultants and directors. The 2017 Plan allows for the issuance of up to 2,333,334 additional shares of Common Stock pursuant to new awards granted under the 2017 Plan and up to approximately 250,000 shares of Common Stock that are represented by options outstanding under the 2010 Plan (defined below) that are forfeited, expire or are cancelled without delivery of shares of Common Stock or which result in the forfeiture of shares of Common Stock back to the Company. This description is qualified in its entirety by reference to the actual terms of the 2017 Plan, a copy of which is attached as Exhibit B to the Company’s preliminary Information Statement on Schedule 14C, filed with the Securities and Exchange Commission on February 28, 2017.
New Directors
On March 11, 2017, our Board of Directors appointed Marc Cummins and Richard J. Berman. to serve on the Board of Directors and our Audit Committee, effective immediately. There are no arrangements or understandings between Messrs. Cummins or Berman and any other person pursuant to which they were appointed as our directors. There are no transactions to which we are a party and in which Messrs. Cummins or Berman have a material interest that are required to be disclosed under Item 404(a) of Regulation S-K. Mr. Cummins previously served on the Board of Directors until his resignation on December 15, 2010, and Mr. Berman has not previously held any position at the Company. Neither individual has family relations with any of our directors or executive officers.
Annual Meetings
We are required by Section 2.1 of our by-laws and Sections 211(b) and (c) of the Delaware General Corporation Law to hold regular annual meetings. In light of our historical liquidity constraints, we have not held regular annual meetings, electing instead to handle matters by written consent of the Company’s stockholders to save on the financial and administrative resources required to prepare for and hold such meetings. While we believe that no material adverse consequences have resulted from the Company’s failure to hold regular annual meetings, we intend to hold such meetings in the future, beginning in 2018.
Operations
We currently operate our OnTrak solutions in eighteen states. We provide services to commercial (employer funded), managed Medicare Advantage, and managed Medicaid and duel eligible (Medicare and Medicaid) populations. We have generated fees from our launched programs and expect to increase enrollment and fees throughout 2017. However, there can be no assurance that we will generate such fees or that new programs will launch as we expect.
RESULTS OF OPERATIONS
The table below and the discussion that follows summarize our results of operations and certain selected operating statistics for the last two fiscal years ended December 31, 2016 and 2015:
|
Twelve Months Ended |
||||||||
|
(In thousands, except per share amounts) |
December 31, |
|||||||
|
2016 |
2015 |
|||||||
|
Revenues |
||||||||
|
Healthcare services revenues |
$ | 7,075 | $ | 2,705 | ||||
|
Operating expenses |
||||||||
|
Cost of healthcare services |
4,670 | 2,433 | ||||||
| General and administrative | 8,838 | 9,049 | ||||||
|
Depreciation and amortization |
141 | 122 | ||||||
|
Total operating expenses |
13,649 | 11,604 | ||||||
|
Loss from operations |
(6,574 | ) | (8,899 | ) | ||||
|
Interest and other income |
106 | 64 | ||||||
|
Interest expense |
(5,354 | ) | (2,590 | ) | ||||
|
Loss on impairment of intangible assets |
- | (88 | ) | |||||
|
Loss on exchange of warrants |
- | (4,410 | ) | |||||
| Loss on debt extinguishment | (2,424 | ) | (195 | ) | ||||
|
Change in fair value of warrant liability |
2,093 | 11,665 | ||||||
|
Change in fair value of derivative liability |
(5,774 | ) | (2,761 | ) | ||||
|
Loss from operations before provision for income taxes |
(17,927 | ) | (7,214 | ) | ||||
|
Provision for income taxes |
9 | 9 | ||||||
|
Net loss |
$ | (17,936 | ) | $ | (7,223 | ) | ||
|
Basic and diluted net loss per share: |
$ | (1.95 | ) | $ | (1.07 | ) | ||
|
Basic weighted number of shares outstanding |
9,179 | 6,729 | ||||||
Year ended December 31, 2016 compared with year ended December 31, 2015
Summary of Consolidated Operating Results
Loss from operations before provision for income taxes for the year ended December 31, 2016 was $17.9 million compared with $7.2 million for the year ended December 31, 2015. The increase in loss from operations was primarily due to the decrease in the change in fair value of warrants of $9.6 million, the increase in the change in fair value of derivative liability of $3.0 million, an increase in interest expense of $2.8 million, the increase in the loss on debt extinguishment of $2.2 million, offset by the decrease in the loss from operations of $2.3 million, and a decrease in the loss on the exchange of warrants of $4.4 million.
Revenues
During the year ended December 31, 2016, we have launched OnTrak in several new populations, which has resulted in a significant increase in the number of patients enrolled in our solutions compared with the same period in 2015. For the twelve months ended December 31, 2016, enrollment increased by more than 57% over the same period in 2015. Recognized revenue increased by $4.4 million, or 162%, for the year ended December 31, 2016, compared with the same period in 2015. We reserve a portion, and in some cases all, of the fees we receive related to enrolled members, as the fees are subject to performance guarantees or are received as case rates in advance at the time of enrollment. Fees deferred for performance guarantees are recognized when those guarantees are satisfied and fees received in advance are recognized ratably over the period of enrollment. Deferred revenue decreased by $158,000 since December 31, 2015.
Operating Expenses
Cost of Healthcare Services
Cost of healthcare services consists primarily of salaries related to our care coaches, healthcare provider claims payments to our network of physicians and psychologists, and fees charged by our third party administrators for processing these claims. The increase of $2.2 million in cost of healthcare services for the year ended December 31, 2016 compared with the same period in 2015, relates primarily to the increase in members being treated, the addition of care coaches, outreach staff, community care coordinators and other staff to manage the increasing number of enrolled members. In addition, we hire staff in preparation for anticipated future customer contracts and corresponding increases in members eligible for OnTrak. The costs for such staff are included in Cost of Healthcare Services during training and ramp-up periods.
General and Administrative Expenses
Total general and administrative expense decreased by $211,000 for the year ended December 31, 2016, compared with the same period in 2015. The decrease was primarily due to decreases in share-based compensation expense related to stock options issued to our board of directors during the first quarter of 2015, investor relations, and costs for legal services.
Depreciation and Amortization
Depreciation and amortization was immaterial for the years ended December 31, 2016 and 2015.
Interest Expense
Interest expense for the year ended December 31, 2016 increased by $2.8 million compared with the same period in 2015. The expense is directly related to the multiple financings that were done during 2016 and 2015.
Loss on Exchange of Warrant
The loss of $4.4 million on the exchange of warrants related to the exchange of 3,546,204 warrants for 3,546,204 shares of common stock during 2015. There was no such warrant exchange during 2016.
Loss on Debt Extinguishment
The loss of $2.4 million on the debt extinguishment related to exchange of the August 2016 promissory notes for the December 2016 8% senior convertible debentures. There was no such debt extinguishment during 2015.
Change in Fair Value of Warrant Liabilities
We have issued warrants to purchase common stock in February 2012, April 2015, July 2015, August 2016, and December 2016. The warrants are being accounted for as liabilities in accordance with FASB accounting rules, due to anti-dilution provisions in some warrants that protect the holders from declines in our stock price, which is considered outside our control. The warrants are marked-to-market each reporting period, using the Black-Scholes pricing model, until they are completely settled or expire.
The decrease in the change in fair value of warrants of $9.6 million for the twelve months ended December 31, 2016 primarily related to the exchange of warrants during 2015.
As a result of the warrant amendments we will no longer be required to mark to market the warrants after the closing of the offering.
Change in fair value of derivative liability
The increase in the change in fair value of derivative liabilities was $3.0 million for the twelve months ended December 31, 2016 compared with the same period in 2015. The derivative liability was the result of the issuance of the July 2015 convertible debenture.
As a result of the debenture conversions, we will no longer be required to mark to market the derivative liability after the closing of the offering.
LIQUIDITY AND CAPITAL RESOURCES
Liquidity and Going Concern
As of March 30, 2017, we had a balance of approximately $311,000 cash on hand. We had working capital deficit of approximately $20.7 million as of December 31, 2016. We have incurred significant net losses and negative operating cash flows since our inception. We expect to continue to incur negative cash flows and net losses for the next twelve months. Our current cash burn rate is approximately $450,000 per month. We expect our current cash resources and contracted bridge loans to cover our operations through April 30, 2017; however, delays in cash collections, revenue, or unforeseen expenditures could impact this estimate. We are in need of additional capital, however, there is no assurance that additional capital can be timely raised in an amount which is sufficient for us or on terms favorable to us and our stockholders, if at all. If we do not obtain additional capital, there is significant doubt as to whether we can continue to operate as a going concern and whether we will need to curtail or cease operations or seek bankruptcy relief. If we discontinue operations, we may not have sufficient funds to pay any amounts to our stockholders.
Our ability to fund our ongoing operations and continue as a going concern is dependent on increasing the number of members that are eligible for our solutions by signing new contracts, identifying more eligible members in existing contracts, and generating fees from existing and new contracts and the success of management’s plan to increase revenue and continue to control expenses. We currently operate our OnTrak solutions in eighteen states. We provide services to commercial (employer funded), managed Medicare Advantage, and managed Medicaid and duel eligible (Medicare and Medicaid) populations. We have generated fees from our launched programs and expect to increase enrollment and fees throughout 2017. However, there can be no assurance that we will generate such fees or that new programs will launch as we expect. We are in need of additional capital, however, there is no assurance that additional capital can be timely raised in an amount which is sufficient for us or on terms favorable to us and our stockholders, if at all. If we do not obtain additional capital, there is a significant doubt as to whether we can continue to operate as a going concern and we will need to curtail or cease operations or seek bankruptcy relief. If we discontinue operations, we may not have sufficient funds to pay any amounts to our stockholders.
Cash Flows
We used $5.7 million of cash from operating activities during the year ended December 31, 2016 compared with $5.2 million during the same period in 2015. The increase in cash used in operating activities reflects the increase in the number of enrolled members and the addition of staff in preparation for anticipated future increases in members eligible for OnTrak. Significant non-cash adjustments to operating activities for the year ended December 31, 2016 included an amortization of debt discount and issuance costs of $4.7 million, a loss on debt extinguishment of $2.4 million, share-based compensation of $697,000, and a $5.8 million fair value adjustment on derivative liability, offset by a fair value adjustment on warrant liability of $2.1 million.
Capital expenditures for the year ended December 31, 2016 were not material. We anticipate that capital expenditures will increase in the future as we replace our computer systems that are reaching their useful lives, upgrade equipment to support our increased number of enrolled members, and enhance the reliability and security of our systems. These future capital expenditure requirements will depend upon many factors, including obsolescence or failure of our systems, progress with expanding the adoption of our solutions, our marketing efforts, the necessity of, and time and costs involved in obtaining, regulatory approvals, competing technological and market developments, and our ability to establish collaborative arrangements, effective commercialization, marketing activities and other arrangements.
Our net cash provided by financing activities was $5.8 million for the year ended December 31, 2016, compared with net cash provided by financing activities of $5.5 million for the year ended December 31, 2015. Cash provided by financing activities for the year ended December 31, 2016 primarily consisted of the net proceeds from the issuance of the 8% senior convertible debenture.
As discussed above, we currently expend cash at a rate of approximately $450,000 per month. We also anticipate cash inflow to increase during 2017 as we continue to service our executed contracts and sign new contracts. We expect our current cash resources to cover our operations through April 30, 2017; however, delays in cash collections, revenue, or unforeseen expenditures could impact this estimate. We are in need of additional capital; however, there is no assurance that additional capital can be timely raised in an amount which is sufficient for us or on terms favorable to us and our stockholders, if at all. If we do not obtain additional capital, there is a significant doubt as to whether we can continue to operate as a going concern, and we will need to curtail or cease operations or seek bankruptcy relief. If we discontinue operations, we may not have sufficient funds to pay any amounts to our stockholders.
OFF BALANCE SHEET ARRANGEMENTS
As of December 31, 2016, we had no off-balance sheet arrangements.
CRITICAL ACCOUNTING ESTIMATES
The discussion and analysis of our financial condition and results of operations is based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). U.S. GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and the disclosure of contingent assets and liabilities. We base our estimates on experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that may not be readily apparent from other sources. On an on-going basis, we evaluate the appropriateness of our estimates and we maintain a thorough process to review the application of our accounting policies. Our actual results may differ from these estimates.
We consider our critical accounting estimates to be those that (1) involve significant judgments and uncertainties, (2) require estimates that are more difficult for management to determine, and (3) may produce materially different results when using different assumptions. We have discussed these critical accounting estimates, the basis for their underlying assumptions and estimates, and the nature of our related disclosures herein with the audit committee of our Board of Directors. We believe our accounting policies related to share-based compensation expense, the estimation of the fair value of warrant liabilities, and the estimation of the fair value of our derivative liabilities involve our most significant judgments and estimates that are material to our consolidated financial statements. They are discussed further below.
Share-based compensation expense
We account for the issuance of stock, stock options and warrants for services from non-employees based on an estimate of the fair value of options and warrants issued using the Black-Scholes pricing model. This model’s calculations include the exercise price, the market price of shares on grant date, weighted average assumptions for risk-free interest rates, expected life of the option or warrant, expected volatility of our stock and expected dividend yield.
The amounts recorded in the financial statements for share-based compensation expense could vary significantly if we were to use different assumptions. For example, the assumptions we have made for the expected volatility of our stock price have been based on the historical volatility of our stock, measured over a period generally commensurate with the expected term. If we were to use a different volatility than the actual volatility of our stock price, there may be a significant variance in the amounts of share-based expense from the amounts reported. The weighted average expected option term for the years ended December 31, 2016 and 2015 reflects the application of the simplified method set out in SEC Staff Accounting Bulletin No. 107, which defines the life as the average of the contractual term of the options and the weighted average vesting period for all option tranches.
From time to time, we retain terminated employees as part-time consultants upon their resignation from the Company. Because the employees continue to provide services to us, their options continue to vest in accordance with the terms set forth under their original grants. Due to the change in classification of the option awards, the options are considered modified at the date of termination. The modifications are treated as exchanges of the original awards in return for the issuance of new awards. At the date of termination, the unvested options are no longer accounted for as employee awards and are accounted for as new non-employee awards. The accounting for the portion of the total grants that have already vested and have been previously expensed as equity awards is not changed. There were no employees moved to consulting status for the year ended December 31, 2016. There was one employee moved to consulting status for the year ended December 31, 2015. The employee was 100% vested at the date of termination so no entry was recorded, and the employee is no longer a consultant as of December 31, 2016.
Warrant Liabilities
We have issued warrants to purchase common stock in February 2012, April 2015, July 2015, August 2016, and December 2016. The warrants are being accounted for as liabilities in accordance with FASB accounting rules, due to anti-dilution provisions in some warrants that protect the holders from declines in our stock price, which is considered outside our control. The warrants are marked-to-market each reporting period, using the Black-Scholes pricing model, until they are completely settled or expire.
The warrant liabilities were calculated using the Black-Scholes model based upon the following assumptions:
|
December 31, 2016 |
December 31, 2015 |
|||||||
|
Expected volatility |
104.31 |
% |
133.19 | % | ||||
|
Risk-free interest rate |
1.20%-1.93 |
% |
0.65-1.76 | % | ||||
|
Weighted average expected lives in years |
2.25-4.99 | 0.99-4.29 | ||||||
|
Expected dividend |
0 |
% |
0 | % | ||||
For the year ended December 31, 2016, we recorded a net gain of $2.1 million, compared with a net gain of $11.7 million for the same period in 2015, related to the revaluation of our warrant liabilities.
We will continue to mark the warrants to market value each reporting period, using the Black-Scholes pricing model until they are completely settled or expire.
Derivative Liabilities
In July 2015, we entered into a $3.55 million 12% Original Issue Discount Convertible Debenture due January 18, 2016 with Acuitas (the “July 2015 Convertible Debenture”). The conversion price of the July 2015 Convertible Debenture was $11.40 per share, subject to adjustments, including for issuances of common stock and common stock equivalents below the then current conversion price. In October 2015, we entered into an amendment of the July 2015 Convertible Debenture which extended the maturity date of the July 2015 Convertible Debenture from January 18, 2016 to January 18, 2017. In addition, the conversion price of the July 2015 Convertible Debenture was subsequently adjusted to $1.80 per share. The July 2015 Convertible Debenture is unsecured, bears interest at a rate of 12% per annum payable in cash or shares of common stock, subject to certain conditions, at our option, and is subject to mandatory prepayment upon the consummation of certain future financings. The derivative liability associated with the July 2015 Convertible Debenture was calculated using the Black-Scholes model based upon the following assumptions:
|
December 31, 2016 |
December 31, 2015 |
|||||||
|
Expected volatility |
104.31 |
% |
133.19 | % | ||||
|
Risk-free interest rate |
0.44 |
% |
0.23 | % | ||||
|
Weighted average expected lives in years |
0.05 | 1.05 | ||||||
|
Expected dividend |
0 |
% |
0 | % | ||||
The expected volatility assumption for the year ended December 31, 2016 was based on the historical volatility of our stock, measured over a period generally commensurate with the expected term. The weighted average expected lives in years for 2016 reflect the application of the simplified method set out in Security and Exchange Commission (SEC) Staff Accounting Bulletin (SAB) 107 (and as amended by SAB 110), which defines the life as the average of the contractual term of the options and the weighted average vesting period for all option tranches. We use historical data to estimate the rate of forfeitures assumption for awards granted to employees.
For the years ended December 31, 2016 and 2015, we recognized a loss of $5.8 million and $2.8 million, respectively, related to the revaluation of our derivative liability.
EFFECTS OF INFLATION
Our most liquid assets are cash and cash equivalents. Because of their liquidity, these assets are not directly affected by inflation. Because we intend to retain and continue to use our equipment, furniture and fixtures and leasehold improvements, we believe that the incremental inflation related to replacement costs of such items will not materially affect our operations. However, the rate of inflation affects our expenses, such as those for employee compensation and contract services, which could increase our level of expenses and the rate at which we use our resources.
BUSINESS
Overview
We provide data analytics based specialized behavioral health management and integrated treatment services to health plans through our OnTrak solution. Our OnTrak solution is designed to improve member health and at the same time lower costs to the insurer for underserved populations where behavioral health conditions are causing or exacerbating co-existing medical conditions. The program utilizes proprietary analytics, member engagement and patient centric treatment that integrates evidence-based medical and psychosocial interventions along with care coaching in a 52-week outpatient program. Our initial focus was members with substance use disorders, but we have expanded our solution to assist members with anxiety and depression. We currently operate our OnTrak solutions in Florida, Georgia, Illinois, Kansas, Kentucky, Louisiana, Massachusetts, Missouri, New Jersey, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, West Virginia and Wisconsin. We provide services to commercial (employer funded), managed Medicare Advantage, and managed Medicaid and duel eligible (Medicare and Medicaid) populations.
We have not been profitable since being incorporated in 2003 and may continue to incur operating losses for at least the next twelve months. As of December 31, 2016, these conditions raised substantial doubt as to our ability to continue as a going concern.
We believe that our business and operations as outlined above are in substantial compliance with applicable laws and regulations. However, the healthcare industry is highly regulated, and the criteria are often vague and subject to change and interpretation by various federal and state legislatures, courts, enforcement and regulatory authorities. Our future prospects are subject to the legal, regulatory, commercial and scientific risks outlined below and in Item 1.A “Risk Factors.”
Substance Dependence
Scientific research indicates that not only can drugs interfere with normal brain functioning, but they can also have long-lasting effects that persist even after the drug is no longer being used. Data indicates that at some point, changes may occur in the brain that can turn drug and alcohol abuse into substance dependence—a chronic, relapsing, and sometimes fatal disease. Those dependent on drugs may suffer from compulsive drug craving and usage and be unable to stop drug use or remain drug abstinent without effective treatment. Professional medical treatment may be necessary to end this physiologically-based compulsive behavior.
Substance dependence is a worldwide problem with prevalence rates continuing to rise despite the efforts by national and local health authorities to curtail its growth. Substance dependence disorders affect many people and have wide-ranging social consequences. In 2015, an estimated 20.8 million adults in the United States (U.S.) met the criteria for substance dependence, according to the National Survey of Drug Use and Health.
We believe the best results in treating substance dependence can be achieved in programs such as our OnTrak solution that integrate psychosocial and medical treatment modalities and provide longer term support on an out-patient basis.
Anxiety Disorders
According to the National Institute of Mental Health, anxiety disorders are the most common mental illness in the U.S., affecting an estimated 18% of adults, or approximately 43 million people age 18 years or older. People with anxiety disorders are:
|
● |
Three to five times more likely to go to the doctor; and |
|
● |
Six times more likely to be hospitalized for psychiatric disorders. |
Mood Disorders
In 2015, an estimated 16.1 million U.S. adults aged 18 or older, or approximately 6.7% of all U.S. adults, had at least one major depressive episode in the past year, according to the National Institute of Mental Health. Patients with substance dependence and mood disorders were ranked four out of the top 10 reasons leading to readmission rates for Medicaid patients.
Our Market
The true impact of behavioral health is often under-identified by organizations that provide healthcare benefits. The reality is that individuals with behavioral health conditions:
|
● |
are prevalent in any organization; |
|
● |
cost health plans and employers a disproportionate amount of money; |
|
● |
have higher rates of absenteeism and lower rates of productivity; and |
|
● |
have co-morbid medical conditions which incur increased costs for the treatment of these conditions compared to a non-substance dependent population. |
When considering behavioral health-related costs, many organizations have historically only looked at direct treatment costs–usually behavioral claims. The reality is that individuals with behavioral health conditions generally have overall poorer health and lower compliance, which leads to more expensive treatment for related, and even seemingly unrelated, co-occurring medical conditions. In fact, for the members we seek to engage our solutions, costs associated with behavioral health treatment are a small portion of their overall healthcare claims.
According to the U.S. Census Bureau in 2014, there were over 283 million lives in the U.S. covered by various private managed care programs, including Preferred Provider Organizations (PPOs), Health Maintenance Organizations (HMOs), self-insured employers and managed Medicare/Medicaid programs. Each year, based on our analysis, approximately 1.9% of commercial plan members will have a substance dependence diagnosis, and that figure may be lesser or greater for specific payors depending on the health plan demographics and location. A smaller, high-cost subset of this population drives the majority of the claims costs for the overall substance dependent population. For commercial members with substance dependence and a total annual claims cost of at least $7,500, the average annual per member claims cost is $30,000, compared with an average of $3,250 for a commercial non-substance dependent member, according to our research.
Our Customers
Our customers provide health insurance to individuals or groups (Contracted Membership). We contract with our customers to provide our OnTrak solution to the customers’ Contracted Membership generally in specific lines of business (e.g., commercial, Medicare, Medicaid, etc.) and/or specific states or other geographical areas and for specific indications, such as substance use disorders and, more recently, anxiety and depression. We refer to the Contracted Membership to whom we are providing the OnTrak solution as Covered Lives. Generally, we receive data relating to the Covered Lives on a regular basis from our customers. We use that data to identify members who meet our contractual eligibility requirements (Eligible Members) and we attempt to engage and enroll those members in our OnTrak solution. Our Eligible Members can fluctuate significantly from month to month due to fluctuations in our customers’ Contracted Membership and changes in eligibility due to changes in claims or eligibility data provided to us by our customers. Based on our analysis of the data provided to us by our customers, approximately 0.045% of the adult Contracted Membership in a commercial line of business is anticipated to be eligible for our OnTrak solution. Based on our analysis, Medicare and Medicaid lines of business average approximately 2.5 times the number of Eligible Members for our OnTrak solution as the same number of Covered Lives in a commercial line of business. Further, our preliminary data analysis shows that adding anxiety and depression indications to our Covered Lives is anticipated to increase our pool of Eligible Members substantially. Based on the latest data provided by one of our customers that has contracted for us to provide OnTrak for anxiety, adding the anxiety and depression indications are anticipated to increase the number of Eligible Members by approximately four times over substance use disorders alone. There are fluctuations in the number of Eligible Members across customers and geographies. Our analysis to date is based on limited data, and in some cases like anxiety and depression, very limited data. There can be no assurance that the data we have analyzed to date will be predictive of the future or that the portion of Covered Lives that are eligible for our programs will not change in the future. In addition, the percentage of Eligible Members in any lines of Covered Lives may fluctuate substantially from period to period.
Our Solution: OnTrak
OnTrak™
Our OnTrak solution combines evidence-based medical and psychosocial treatments with elements of population health management and ongoing member support to help health plans treat members with substance dependence, anxiety and depression and to improve member health and lower the overall health plan costs of these members. We believe the benefits of our OnTrak solution include improved clinical outcomes and decreased costs for the payor, and improved quality of life and productivity for the member.
Although the healthcare services industry is competitive, we believe OnTrak is the only solution of its kind. The OnTrak solution was developed by behavioral health experts with years of clinical experience. This experience has helped to form key areas of expertise that we believe sets our solution apart from other solutions, including member engagement, working directly with the member treatment team and a more fully integrated treatment offering.
Our OnTrak solution includes the following components: identification of impactable members, member engagement, enrollment/referral, provider network, outpatient medical treatment, outpatient psychosocial treatment, care coaching, monitoring and reporting, and our proprietary web-based clinical information platform (eOnTrak).
We assist health plans to identify those members who incur significant costs and may be appropriate for enrollment into OnTrak. We then engage and enroll targeted members into our program through direct mailings and telephonic outreach, and referral through health plan sources. After enrollment, our contracted network of providers provide treatments utilizing integrated medical and psychosocial treatment modalities, including our proprietary OnTrak therapy modules for anxiety, depression and substance use disorders to help members develop improved coping skills and a recovery support network. Throughout the treatment process, our care coaches work directly with members to keep them engaged in treatment by proactively supporting members to enhance motivation, minimize lapses and enable lifestyle modifications consistent with the recovery goals. Periodically, we will provide outcomes reporting on clinical and financial metrics to our customers to demonstrate the extent of the program’s value.
Clinical and financial outcomes from the OnTrak solution have been promising with OnTrak enrolled members achieving an average gross cost reduction of more than 50% for the year after enrollment compared to the 12 months prior to enrollment. In addition, to date, approximately 80% of members who have remained eligible have been retained in the program.
OnTrak
Our proprietary OnTrak solution is designed to improve treatment outcomes and lower the utilization of medical and behavioral health plan services by high utilization and high risk enrollees. Our OnTrak solution includes medical and psychosocial interventions; a proprietary web based clinical information platform and database, psychosocial programs and integrated care coaching services.
Another important aspect of the Catasys solution is that the solution is flexible and can be altered in a modular way to enable us to partner with payors to meet their needs. As a service delivery model, the OnTrak solution can be modified to cover particular populations and provide for varying levels of service. In this way, OnTrak can work with payors to identify, engage and treat a broader spectrum of patients in a way that is consistent with payors’ business needs.
Our value proposition to our customers includes that the OnTrak solution is designed for the following benefits:
|
|
● |
A specific program aimed at addressing high-cost conditions by improving patient health and thereby reducing overall healthcare costs can benefit health plans; |
|
● |
Increased worker productivity by reducing workplace absenteeism, compensation claims and job related injuries; |
|
● |
Decreased emergency room and inpatient utilization; |
|
● |
Decreased readmission rates; and |
|
● |
Healthcare cost savings (including medical, behavioral and pharmaceutical). |
Our Strategy
Our business strategy is to provide a quality integrated medical and behavioral program to help health plans and other organizations treat and manage health plan members whose behavioral health conditions are exacerbating co-existing medical conditions resulting in increased in-patient medical costs. Our initial focus was members with substance use disorder, and we have expanded our solution into anxiety disorders and depression.
Key elements of our business strategy include:
|
● |
Demonstrating the potential for improved clinical outcomes and reduced cost associated with using our OnTrak solution with key managed care and other third-party payors; |
|
● |
Educating third-party payors on the disproportionately high cost of their substance dependent population; |
|
● |
Providing our OnTrak solution to third-party payors for reimbursement on a case rate, fee for service, or monthly fee basis; and |
|
● |
Generating outcomes data from our OnTrak solution to demonstrate cost reductions and utilization of this outcomes data to facilitate broader adoption. |
As an early entrant into offering integrated medical and behavioral programs for substance dependence, we believe we will be well positioned to address increasing market demand. We believe our OnTrak solution will help fill the gap that exists today: a lack of programs that focus on smaller populations with disproportionately higher costs driven by behavioral health conditions that improve patient care while controlling overall treatment costs.
Our Operations
Healthcare Services
Our OnTrak solution combines innovative medical and psychosocial treatments with elements of traditional disease management, case management, and ongoing member support to help organizations treat and manage populations struggling with substance dependence, depression, and anxiety to improve their health and thereby decrease their overall health care costs.
As of March 30, 2017, we have contracts with seven health plans, two of which have merged and are in the process of integrating operations. We are enrolling patients under five of these contracts.
We are currently marketing our OnTrak solution to managed care health plans on a case rate, monthly fee, or fee for service basis, which involves educating third party payors on the disproportionately high cost of their substance dependent population and demonstrating the potential for improved clinical outcomes and reduced cost associated with using our program.
Competition
Healthcare Services
Our OnTrak solution to date has focused primarily on substance dependence, anxiety and depression, and is marketed to health plans and other insurance payers. While we believe our products and services are unique, we operate in highly competitive markets. We compete with other healthcare management service organizations, care management and disease management companies, including managed behavioral health organizations (MBHOs) that manage behavioral health benefits, perform utilization reviews, provide case management and patient coaching, and pay their network of providers for behavioral health services delivered. Most of our competitors are significantly larger and have greater financial, marketing and other resources than us. We compete with companies such as Hummingbird and Health Integrated that offer coaching, social media, and in the case of Health Integrated, more comprehensive products to address the costs of members with substance dependence and other behavioral health conditions. There are several companies that seek to utilize digital approaches to treat behavioral health conditions or use data analytics and digital technologies to identify health plan members with behavior health conditions and try to match them to treatment providers. Some of these companies also utilize care coaches or on-line therapists to enhance their digital models. A small number of these have secured reimbursement for their products or services from health plans. We believe these providers are serving a different segment of the market and do not provide an end-to-end integrated solution with financial outcomes. We believe our product is the most comprehensive one to focus exclusively on engaging and treating high cost members whose anxiety, depression, or substance use disorders are exacerbating co-existing medical conditions through our network of providers using specially designed treatment regimens.
In addition, managed care companies may seek to provide similar specialty healthcare services directly to their members, rather than by contracting with us for such services. Behavioral health conditions, including substance dependence, are typically managed for insurance companies by internal divisions or third-parties (MBHOs) frequently under capitated arrangements. Under such arrangements, MBHOs are paid a fixed monthly fee and must pay providers for provided services, which gives such entities an incentive to decrease cost and utilization of services by members. We compete to differentiate our integrated program for high utilizing substance dependence members, which seeks to increase treatment and impact the overall health care costs of the members, from the population utilization management programs that MBHOs offer to manage a health benefit.
We believe that our ability to offer customers an outcomes based comprehensive and integrated solution, including the utilization of innovative medical and psychosocial treatments and engagement methodologies, and our network of treatment providers, will enable us to compete effectively. However, there can be no assurance that we will not encounter more effective competition in the future, which would limit our ability to maintain or increase our business.
Once we contract with a third-party payor, we implement our program in conjunction with the third party payor and then commence outreach to eligible members to enroll them in our OnTrak solution. In this enrollment process, we compete against numerous other providers of behavioral health treatment programs, facilities and providers for those members that elect to receive treatment for their behavioral health conditions (see Treatment Programs below). We believe we provide members with a lower cost and more comprehensive solutions, but members may choose to receive care from other providers. To the extent a member selects a different provider that is part of a health plan network of providers, the cost of such treatment may be paid in whole or in part by our health plan customer.
Treatment Programs
There are over 14,000 facilities reporting to the SAMHSA that provide substance dependence treatment. Well-known examples of residential treatment programs include the Betty Ford Center®, Caron Foundation®, Hazelden® and Sierra Tucson®. In addition, individual physicians may provide substance dependence treatment in the course of their practices. Many of these traditional treatment programs have established name recognition.
Trademarks
We rely on a combination of trademark, trade secret and copyright laws and contractual restrictions to protect the proprietary aspects of our technology. Our branded trade names on which we rely include the following:
|
|
● |
OnTrak™; and |
|
|
● |
eOnTrak™. |
We require that, as a condition of their employment, employees assign to us their interests in inventions, original works of authorship, copyrights and similar intellectual property rights conceived or developed by them during their employment with us.
Financial Information about Segments
We manage and report our operations through one business segment: Healthcare Services. This segment includes the OnTrak solution marketed to health plans and other third party payors.
Government Regulation
Overview
We believe that our business and operations as outlined above are in substantial compliance with applicable laws and regulations. Only a treating physician can determine if our OnTrak solution is appropriate for any individual patient. Our future prospects are subject to the legal, regulatory, commercial and scientific risks outlined below and under the caption “Risk Factors.”
The healthcare industry is highly regulated and continues to undergo significant changes as third-party payors, such as Medicare and Medicaid, traditional indemnity insurers, managed care organizations and other private payors, increase efforts to control cost, utilization and delivery of healthcare services. Healthcare companies are subject to extensive and complex federal, state and local laws, regulations and judicial decisions.
Health Care Reform
The Affordable Care Act or the ACA was enacted into law in 2010. The provisions of the ACA are comprehensive and varied and are generally directed at implementing health insurance reforms to increase health insurance coverage and reduce the number of uninsured and reshaping the health care delivery system to increase quality and efficiency and reduce cost. Certain provisions of the ACA took effect immediately or within a few months, while others will be phased in over time, ranging from one year to ten years. Because of the complexity of health care reform generally, additional legislation is likely to be considered and enacted over time. The ACA, and any subsequent health care reform legislation, will require the promulgation of substantial regulations with significant effect on the health care industry. Thus, the health care industry will be subjected to significant new statutory and regulatory requirements, and consequently to structural and operational changes and challenges, for a substantial period of time.
Reimbursement
Reimbursement from federal health care programs such as Medicare and Medicaid are subject to complex statutory and regulatory requirements, administrative rulings, interpretations of policy, determinations by fiscal intermediaries and government funding restrictions, all of which may materially increase or decrease reimbursement to our company.
Fraud and Abuse
Health care fraud and abuse laws have been enacted at the federal and state levels to regulate both the provision of services to government program beneficiaries and the methods and requirements for submitting claims for services rendered to such beneficiaries. Under these laws, individuals and organizations can be penalized for various activities, including submitting claims for services that are not provided, are billed in a manner other than as actually provided, are not medically necessary, are provided by an improper person, are accompanied by an illegal inducement to utilize or refrain from utilizing a service or product, or are billed in a manner that does not comply with applicable government requirements. Both individuals and organizations are subject to prosecution under the criminal and civil fraud and abuse statutes relating to health care providers.
The federal anti-kickback law (the “Anti-Kickback Law”) prohibits, among other things, knowingly and willfully offering or receiving remuneration to induce the referral of items or services that are reimbursable by a federal health care program. The Office of Inspector General has issued a series of regulations, known as the “safe harbors” which immunizes the parties to the business arrangement from prosecution under the Anti-Kickback Law. The failure of a business arrangement to fit within a safe harbor does not necessarily mean that the arrangement is illegal. Many states have adopted laws similar to the Anti-Kickback Law, and some apply to items and services reimbursable by any payor, including private insurers.
The so-called Stark Law prohibits physician referrals of Medicare patients to an entity providing certain “designated health services” if the physician or an immediate family member of the physician has any financial relationship with the entity and the financial relationship does not fall within one of the enumerated exceptions to the Stark Law. In addition to the Stark Law, many states have their own self-referral bans, which may extend to all self-referrals, regardless of the payor.
The federal False Claims Act imposes liability for the submission of false or fraudulent claims for payment to the federal government. The knowing and improper failure to return an overpayment can serve as the basis for a False Claims Act action and Medicare and Medicaid overpayments must be reported and returned within 60 days of identification. The qui tam provisions of the False Claims Act allow a private individual to bring an action on behalf of the federal government and to share in any amounts paid by the defendant to the government in connection with the action. Various states have enacted similar laws modeled after the False Claims Act that apply to items and services reimbursed under Medicaid and other state health care programs, and, in several states, such laws apply to claims submitted to all payors.
The Health Insurance Portability and Accountability Act of 1996 prohibits the knowing and willful execution of a scheme to defraud any health care benefit program, including a private insurer. It also prohibits falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the delivery of or payment for health care benefits, items, or services.
State and Federal Privacy and Data Security Laws
The Health Insurance Portability and Accountability Act of 1996 and its implementing regulations (HIPAA) and the Health Information Technology for Economic and Clinical Health Act of 2009 and its implementing regulations (HITECH) govern the collection, use, disclosure, maintenance and transmission of identifiable patient information (“Protected Health Information” or “PHI”). HIPAA and HITECH apply to covered entities, which may include health plans as well as to those entities that contract with covered entities (“Business Associates”). HITECH imposes breach notification obligations that require the reporting of breaches of “Unsecured Protected Health Information” or PHI that has not been encrypted or destroyed in accordance with federal standards.
Federal Regulations (Title 42 Part 2 of the Code of Federal Regulations) set forth very specific rules governing the use and disclosure of drug and alcohol treatment information obtained from federally-supported treatment facilities.
In addition to the federal privacy and security laws and regulations, most states have enacted data security laws governing other types of personal data such as employee and customer information.
State Managed Care Laws
State insurance and managed cared laws and regulations regulate the contractual relationships with managed care organizations, utilization review programs and third-party administrator activities. These regulations differ from state to state, and may contain network, contracting, and financial and reporting requirements, as well as specific standards for delivery of services, payment of claims, and adequacy of health care professional networks.
Corporate practice of medicine and fee-splitting and laws
Many states prohibit business corporations from practicing medicine, exercising control over medical judgments or decisions of physicians or other health care professionals (such as nurses or nurse practitioners), or engaging in certain business arrangements with physicians or other health care professionals, such as employment of physicians and other health care professionals or fee-splitting. The state laws and regulations and administrative and judicial decisions that enumerate the specific corporate practice and fee-splitting rules vary considerably from state to state and are enforced by both the courts and government agencies, each with broad discretion.
State Laws Governing Licensure of Healthcare Professionals
State professional licensing boards contain requirements for the licensure of health care professionals and typically require a healthcare professional who is providing professional services in that state to be licensed. Some state licensing boards specifically address the licensure of professionals who are providing services via telephone or other electronic means.
Employees
As of March 30, 2017, we employed 90 full-time employees. We are not a party to any labor agreements and none of our employees are represented by a labor union.
Facilities
Information concerning our principal facilities, all of which were leased at March 30, 2017, is set forth below:
|
Location |
Use |
Approximate |
|
| ||
|
11601 Wilshire Blvd. |
Principal executive and administrative offices |
9,120 |
| |||
Our principal executive and administrative offices are located in Los Angeles, California, and consists of leased office space totaling approximately 9,120 square feet, which will expire in April 2019. Our base rent is approximately $31,000 per month, subject to annual adjustments, with aggregate minimum lease commitments at March 30, 2017, totaling approximately $769,000.
We believe that the current office space is adequate to meet our needs.
Legal Proceedings
Neither we nor our subsidiary is currently a party to, nor is our property the subject of, any material legal proceedings.
MANAGEMENT AND CERTAIN CORPORATE GOVERNANCE MATTERS
Directors, Executive Officers and Certain Other Non-Executive Officers
The following table lists our executive officers and directors serving at March 30, 2017. Our executive officers are elected annually by our board of directors and serve at the discretion of the board of directors. Each current director is serving a term that will expire at our next annual meeting. There are no family relationships among any of our directors or executive officers.
|
Name |
|
Age |
|
Position |
|
Officer/ Director Since |
| ||
|
Terren S. Peizer |
|
|
57 |
|
Director, Chairman of the Board and Chief Executive Officer |
|
|
2003 |
|
|
|
|
|
|
|
|
|
|
| |
|
Richard A. Anderson |
|
|
47 |
|
Director, President and Chief Operating Officer |
|
|
2003 |
|
|
|
|
|
|
|
|
|
|
| |
|
Susan Etzel |
|
|
43 |
|
Chief Financial Officer |
|
|
2011 |
|
|
|
|
|
|
|
|
|
|
| |
|
Richard A. Berman (1) (2) |
|
|
72 |
|
Director, Chairman of the Audit Committee, and Member of the Compensation Committee |
|
|
2014 |
|
|
|
|
|
|
|
|
|
| ||
|
David E. Smith (3) |
|
|
70 |
|
Director, and Member of the Nominations and Corporate Governance Committee |
|
|
2014 |
|
|
|
|
|
|
|
|
|
|
| |
|
Marvin Igelman (1), (2), (3) |
|
|
54 |
|
Director, Chairman of the Nominations and Corporate Governance Committee, Member of the Compensation Committee, and Member of the Audit Committee |
|
|
2014 |
|
|
|
|
|
|
|
|
|
|
| |
|
Steve Gorlin(3) |
|
|
79 |
|
Director, and Member of the Nominations and Corporate Governance Committee |
|
|
2014 |
|
|
Richard J. Berman(1) |
74 |
Director, and Member of the Audit Committee |
2017 |
||||||
|
Marc Cummins(1) |
57 |
Director, and Member of the Audit Committee |
2017 |
||||||
|
(1) |
Member of the Audit Committee |
|
(2) |
Member of the Compensation Committee |
|
(3) |
Member of the Nominations and Governance Committee |
Business Experience
The following is a brief account of the education and business experience of our current directors and executive officers:
Terren S. Peizer is the founder of our Company and an entrepreneur, investor, and financier with a particular interest in healthcare, having founded and successfully commercialized several healthcare companies. He has served as its Chief Executive Officer and Chairman of the Board of Directors since the Company’s inception in 2004. Mr. Peizer is also the founder, Chairman and CEO NeurMedix, Inc., a biotechnology company with a focus on inflammatory, neurological and neuro-degenerative diseases. In addition to his roles with Catasys and NeurMedix, Mr. Peizer is Chairman of Acuitas Group Holdings, LLC, his personal investment vehicle, and holding company that is the owner of all of his portfolio company interests. Through Acuitas, Mr. Peizer owns Crede Capital Group, LLC an industry leader in investing in micro and small capitalization equities, having invested over $1.2 billion directly into portfolio companies. Mr. Peizer has been the largest beneficial shareholder of, and has held various senior executive positions with, several other publicly-traded growth companies, including having served as Chairman of Cray, Inc. a supercomputer company. Mr. Peizer has a background in venture capital, investing, mergers and acquisitions, corporate finance, and previously held senior executive positions with the investment banking firms Goldman Sachs, First Boston, and Drexel Burnham Lambert. He received his B.S.E. in Finance from The Wharton School of Finance and Commerce.
We believe Mr. Peizers’s qualifications to serve on our board of directors include his role as an investor and executive positions in several private and public companies, including numerous companies in the healthcare field. He has extensive knowledge and experience in the financial and healthcare industries, and provides extensive insight and experience with capital markets and publicly traded companies at all stages of development.
Richard A. Anderson has served as a director since July 2003 and as a member of our management team since April 2005. He has been our President and Chief Operating Officer since July 2008; in this role he has been primarily responsible for the creation and leadership of our OnTrak solution. He has more than twenty-five years of experience in business development, strategic planning, operations, finance and management, with more than 15 years of that in the healthcare field. Prior to joining the Company, he held senior level financial and operational positions in healthcare and financial companies, and served as a director in PriceWaterhouseCoopers LLP’s business assurance and transaction support practices. He received a B.A. in Business Economics from University of California, Santa Barbara.
We believe Mr. Anderson’s qualifications to serve on the board of directors include his business and healthcare experience, including a diversified background as an executive and in operational roles in both public and private companies. His leadership of our product creation gives him a breadth of knowledge and valuable understanding of our business, operations and customers.
Susan E. Etzel has served as the Company’s Chief Financial Officer since July 2011 and prior to that was the Company’s Corporate Controller since February 2011. Prior to joining the Company, she acted as the Controller of Clearant, Inc., a developer of a universal pathogen inactivation technology, from July 2005 until February 2011. Prior to joining the Clearant she held a senior level auditor position at Arthur Anderson LLP. She received a Bachelor of Business Economics with an emphasis in Accounting from the University of California, Santa Barbara.
Richard A. Berman is the interim dean of the USF Patel College of Global Sustainability, visiting social entrepreneurship professor in the Muma College of Business, and a professor in the institute of innovation and discovery. As a recognized global leader, Mr. Berman has held positions in health care, education, politics and management. He has worked with several foreign governments, the United Nations, the U.S. Department of Health and Welfare, the FDA, and as a cabinet level official for the state of New York. He has also worked with McKinsey & Co, NYU Medical Center, Westchester Medical, Korn-Ferry International, Howe-Lewis International and numerous startup companies. In 1995, Mr. Berman was selected by Manhattanville College to serve as its tenth President. Mr. Berman is credited with the turnaround of the College, where he served until 2009. Mr. Berman serves on the board of several organizations and is an elected member of the National Academy of Medicine of the National Academy of Sciences (Formerly known as the Institute of Medicine). Mr. Berman received his BBA, MBA, and MPH from the University of Michigan and holds honorary doctorates from Manhattanville College and New York Medical College.
We believe Mr. Berman’s qualifications to serve on our board of directors include his extensive experience as an executive in several healthcare firms. In addition, as a board member of a health plan we believe he has an understanding of our customer base and current developments and strategies in the health insurance industry.
David E. Smith is the President, Chief Executive Officer and Chief Investment Officer of the Trading Advisor. Mr. Smith was the founder and Chief Executive Officer of Coast Asset Management. Mr. Smith has worked in various capacities in the securities industry, including as Vice President of Security Pacific Bank , and Oppenheimer and Company as a bond arbitrageur, and he is also a successful investor in small cap growth companies. Mr. Smith has an MBA from the University of California at Berkeley.
We believe Mr. Smith’s qualifications to serve on our board of directors include his extensive background in the banking and securities industries, as well as his experience in corporate governance and management.
Marvin Igelman is the Chief Executive Officer of Breaking Data Corporation, formally known as Sprylogics International Inc. (TSX: BKD), a leader in the semantic search technology sector. Previously, he was Chief Executive Officer of Unomobi, Inc. a mobile advertising and messaging platform that was acquired in February 2010 by Poynt Corporation and was previously on the Board of Directors of Jamba Juice (NASDAQ: JMBA). Mr. Igelman was also founder, President and Chief Executive Officer of Brandera Inc., which operated Portfolios.com, a leading online business-to-business site for the Graphic Arts and creative community, and has served as a business development consultant for numerous technology companies, and established a number of other successful ventures. Mr. Igelman has a Bachelor of Laws from Osgoode Hall Law School.
We believe Mr. Igelman’s qualifications to serve on our board of directors include his extensive business development experience, and his current and past executive experience in numerous private and publicly traded companies.
Steve Gorlin is an entrepreneur who has founded numerous successful biotechnology and pharmaceutical companies over the last 40 years, including Hycor Biomedical, Inc. (acquired by Agilent), Theragenics Corporation (NYSE: TGX), CytRx Corporation (NASDAQ: CYTR), Medicis Pharmaceutical Corporation (sold to Valeant for approximately $2.6 billion), Entremed, Inc. (NASDAQ: ENMD), MRI Interventions (MRIC), DARA BioSciences, Inc. (NASDAQ: DARA), MiMedx (NASDAQ: MDXG), and Medivation, Inc. (NASDAQ: MDVN). Mr. Gorlin served many years on the Business Advisory Council to the Johns Hopkins School of Medicine and on theJohns Hopkins BioMedical Engineering Advisory Board and as well as on the Board of Andrews Institute. He is presently a member of the Research Institute Advisory Committee (RIAC) of Massachusetts General Hospital. Mr. Gorlin founded a number of non-medical related companies, including Perma-Fix, Inc., Pretty Good Privacy, Inc. (sold to Network Associates), Judicial Correction Services, Inc. (solt to Correctional Healthcare), and NTC China. He started the Touch Foundation, a nonprofit organization for the blind and was a principal financial contributor to the founding of Camp Kudzu for diabetic children. He presently serves as Vice Chairman of NantKwest (NASDAQ: NK), CEO of NantibodyFc, Executive Chairman of ViCapsys and Aperisys and serves on the Boards of Medovex, Inc. (MDVX), Catasys, Inc. and NTC China, Inc.
We believe Mr. Gorlin’s qualifications to serve on our board of directors include his experience in the healthcare industry, his extensive business development experience, and his current and past executive experience in numerous private and publicly traded companies.
Richard J. Berman was Chairman of National Investment Managers, a company with $12 billion pension administration assets from 2006-2011. Mr. Berman is a director of four public healthcare companies: Advaxis, Inc., Caladrius Biosciences, Inc. MetaStat Inc (Chairman) and Cryoport Inc. From 1998-2000, he was employed by Internet Commerce Corporation (now Easylink Services) as Chairman and CEO, and was a director from 1998-2012. Previously, Mr. Berman was Senior Vice President of Bankers Trust Company, where he started the M&A and Leveraged Buyout Departments; created the largest battery company in the world in the 1980’s by merging Prestolite, General Battery and Exide and advised on over $4 billion of M&A transactions (completed over 300 deals). He is a past Director of the Stern School of Business of NYU where he obtained his BS and MBA. He also has US and foreign law degrees from Boston College and The Hague Academy of International Law, respectively.
We believe Mr. Berman’s qualifications to serve on our board of directors include his experience in the healthcare industry, and his current and past experience in numerous private and publicly traded companies.
Marc Cummins is a partner of Trispan LLP, a global multi-strategy asset manager. Previously, Mr. Cummins was the Founder and Managing Partner of Prime Capital, an investment firm focused on investing in public and private companies in the consumer sector. Prior to founding Prime, Mr. Cummins spent seven years as Managing Partner of Catterton Partners, the largest private equity firm in the U.S. focused exclusively on the consumer sector. Prior to Catterton, Mr. Cummins spent fourteen years at Donaldson, Lufkin & Jenrette (“DLJ”) as Managing Director of DLJ’s Consumer Products and Specialty Distribution Group.
Mr. Cummins has acted as a principal investor and advisor to leading consumer related companies and has sat on the boards of numerous public and private businesses.
Mr. Cummins received a B.A. in Economics, magna cum laude, from Middlebury College, where he was honored as a Middlebury College Scholar and is a member of Phi Beta Kappa. Mr. Cummins also received an M.B.A. in Finance with honors from The Wharton School at the University of Pennsylvania.
We believe that Mr. Cummins qualifications to serve on our board of directors include his experience in the banking and securities industry, and his current and past executive experience in numerous private and publicly traded companies.
Code of Ethics
Our Board of Directors has adopted a code of ethics applicable to our chief executive officer, chief financial officer and persons performing similar functions. Our code of ethics is listed hereto as Exhibit 14.1 and is accessible on our website at http://www.catasys.com. Disclosure regarding any amendments to, or waivers from, provisions of the code of ethics will be included in a Current Report on Form 8-K within four business days following the date of the amendment or waiver.
Independence of the Board of Directors
Our common stock is traded on the OTCQB. Our Board of Directors has determined that five of the members of our Board of Directors qualify as “independent,” as defined by the listing standards of the NASDAQ. Consistent with these considerations, after review of all relevant transactions and relationships between each director, or any of his family members, and the Company, its senior management and its independent auditors, the Board has determined further that Messrs. Richard A. Berman, Richard J. Berman, Igelman, Gorlin and Cummins are independent under the listing standards of NASDAQ. In making this determination, our Board of Directors considered that there were no new transactions or relationships between its current independent directors and the Company, its senior management and its independent auditors since last making this determination.
Committees of the Board of Directors
Audit committee
During 2016, the audit committee consisted of two directors, Messrs. Berman and Igelman. Our Board of Directors has determined that each of the members of the audit committee were independent as defined by the NASDAQ rules, meet the applicable requirements for audit committee members, including Rule 10A-3(b) under the Exchange Act, and that Mr. Berman qualifies as an “audit committee financial expert” as defined by Item 401(h)(2) of Regulation S-K. The duties and responsibilities of the audit committee include (i) selecting, evaluating and, if appropriate, replacing our independent registered accounting firm, (ii) reviewing the plan and scope of audits, (iii) reviewing our significant accounting policies, any significant deficiencies in the design or operation of internal controls or material weakness therein and any significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of their evaluation and (iv) overseeing related auditing matters.
A copy of the audit committee’s written charter is publicly available through the “Investors-Governance” section of our website at www.catasys.com.
Nominations and governance committee
Our nominations and governance committee consists of one member who is independent as defined by the NASDAQ rules. During 2016, the committee consisted of three directors, Messrs. Smith, Igelman, and Gorlin, and did not hold any meetings. The committee nominates new directors and periodically oversees corporate governance matters.
The charter of the nominations and governance committee provides that the committee will consider Board candidates recommended for consideration by our stockholders, provided the stockholders provide information regarding candidates as required by the charter or reasonably requested by us within the timeframe proscribed in Rule 14a-8 of Regulation 14A under the Exchange Act, and other applicable rules and regulations. Recommendation materials are required to be sent to the nominations and governance committee c/o Catasys, Inc., 11601 Wilshire Boulevard, Suite 1100, Los Angeles, California 90025. There are no specific minimum qualifications required to be met by a director nominee recommended for a position on our Board of Directors, nor are there any specific qualities or skills that are necessary for one or more of our Board of Directors to possess, other than as are necessary to meet any requirements under the rules and regulations applicable to us. The nominations and governance committee considers a potential candidate's experience, areas of expertise, and other factors relative to the overall composition of our Board of Directors.
The nominations and governance committee considers director candidates that are suggested by members of our Board of Directors, as well as management and stockholders. Although it has not previously utilized, the committee may also retain a third-party executive search firm to identify candidates. The process for identifying and evaluating nominees for director, including nominees recommended by stockholders, involves reviewing potentially eligible candidates, conducting background and reference checks, interviews with the candidate and others (as schedules permit), meeting to consider and approve the candidate and, as appropriate, preparing and presenting to the full Board of Directors an analysis with respect to particular recommended candidates. The nominations and governance committee endeavors to identify director nominees who have the highest personal and professional integrity, have demonstrated exceptional ability and judgment, and, together with other director nominees and members, are expected to serve the long term interest of our stockholders and contribute to our overall corporate goals.
A copy of the nominations and governance committee’s written charter is publicly available through the “Investors-Governance” section of our website at www.catasys.com.
Compensation committee
The compensation committee consists of up to three directors who are independent as defined by the NASDAQ rules. During 2016, the committee consisted of two directors, Messrs. Berman and Igelman, and did not hold any meetings. The compensation committee reviews and recommends to the board of directors for approval the compensation of our executive officers.
A copy of our compensation committee written charter is publicly available through the “Investors-Governance” section of our website at www.catasys.com.
EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth the total compensation paid during the last two fiscal years ended December 31, 2016 and 2015 to (1) our Chief Executive Officer, and (2) our two next most highly compensated executive officers who earned more than $100,000 during the fiscal year ended December 31, 2016 and were serving as executive officers as of such date.
|
Name and Principal Position |
Year |
Salary ($) |
Option Award |
All |
Total ($) |
|||||||||||||
|
Terren S. Peizer, |
2016 |
450,000 | (1) | - | 14,627 | 464,627 | ||||||||||||
|
Chairman & |
2015 |
450,000 | (1) | - | 18,899 | 468,899 | ||||||||||||
|
Chief Executive Officer |
||||||||||||||||||
|
Richard A. Anderson, |
2016 |
386,548 | - | 28,473 | 415,021 | |||||||||||||
|
President and |
2015 |
379,077 | - | 28,231 | 407,308 | |||||||||||||
|
Chief Operating Officer |
||||||||||||||||||
|
Susan Etzel, |
2016 |
170,000 | - | - | 170,000 | |||||||||||||
|
Chief Financial Officer |
2015 |
170,000 | - | - | 170,000 | |||||||||||||
|
(1) |
Mr. Peizer deferred part of his salary for the 2016 and 2015 years. |
|
(2) |
Includes group life insurance premiums and medical benefits. |
Narrative Disclosures to Summary Compensation Table
Chief Executive Officer
We entered into a five-year employment agreement with our Chairman and Chief Executive Officer, Terren S. Peizer, effective as of September 29, 2003, which automatically renews after each five-year term. Mr. Peizer received an annual base salary of $450,000 in each of 2016 and 2015, part of which was deferred. Mr. Peizer is also eligible for an annual bonus targeted at 100% of his base salary based on goals and milestones established and reevaluated on an annual basis by mutual agreement between Mr. Peizer and the Board of Directors. Mr. Peizer did not receive any annual bonus during the fiscal years ended December 31, 2016 and 2015. His base salary and bonus target will be adjusted each year to not be less than the median compensation of similarly positioned CEO’s of similarly situated companies. Mr. Peizer receives executive benefits including group medical and dental insurance, term life insurance equal to 150% of his salary, accidental death and long-term disability insurance, grossed up for taxes. There were no equity awards granted to Mr. Peizer during 2016 or 2015. All unvested options vest immediately in the event of a change in control, termination without good cause or resignation with good reason. In the event that Mr. Peizer is terminated without good cause or resigns with good reason prior to the end of the term, he will receive a lump sum payment equal to the remainder of his base salary and targeted bonus for the year of termination, plus three years of additional salary, bonuses and benefits. If any of the provisions above result in an excise tax, we will make an additional “gross up” payment to eliminate the impact of the tax on Mr. Peizer.
President and Chief Operating Officer
We entered into a four-year employment agreement with our President and Chief Operating Officer, Richard A. Anderson, effective April 19, 2005, as amended on July 16, 2008. After the initial four-year term, the employment agreement automatically renews for additional three-year terms unless otherwise terminated. Mr. Anderson’s agreement renewed for an additional three-year term in April 2015. Mr. Anderson received an annual base salary of $386,548 in 2016 and $379,077 in 2015. Mr. Anderson is eligible for an annual bonus targeted at 50% of his base salary based on achieving certain milestones. Mr. Anderson did not receive any annual bonus during the fiscal years ended December 31, 2016 and 2015. Mr. Anderson’s compensation will be adjusted each year by an amount not less than the Consumer Price Index. Mr. Anderson received executive benefits, including group medical and dental insurance, term life insurance, accidental death and long-term disability insurance. There were no equity awards granted to Mr. Anderson in 2016 or 2015. All unvested options will vest immediately in the event of a change in control, termination without cause or resignation with good reason. In the event of termination without good cause or resignation with good reason prior to the end of the term, upon execution of a mutual general release, Mr. Anderson will receive a lump sum payment equal to one year of salary and bonus, and will receive continued medical benefits for one year unless he becomes eligible for coverage under another employer's plan. If he is terminated without cause or resigns with good reason within twelve months following a change in control, upon execution of a general release he will receive a lump sum payment equal to eighteen months salary, 150% of the targeted bonus, and will receive continued medical benefits for 18 months unless he becomes eligible for coverage under another employer's plan.
Chief Financial Officer
We entered into a two-year employment agreement with Ms. Etzel effective January 1, 2013. Beginning January 1, 2015, Ms. Etzel is employed on an at-will basis. Ms. Etzel received an annual base salary of $170,000 in 2016 and $170,000 in 2015, and she may be eligible to an annual bonus, to be determined solely by the Company, contingent on achieving certain individual goals and milestones and the overall performance and profitability of the Company. Ms. Etzel did not receive any annual bonus during the years ended December 31, 2016 and 2015.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
The following table sets forth all outstanding equity awards held by our named executive officers as of December 31, 2016.
|
Name |
Number of |
Number of Securities Underlying Unexercised Options (#) Unexer- cisable |
Option |
Option Expiration Date | |||||||||
|
Terren S. Peizer |
192 | - | 739.20 |
02/07/18 | |||||||||
| 225 | - | 739.20 |
06/20/18 | ||||||||||
| 400 | - | 1,160.60 |
10/27/19 | ||||||||||
| 24,750 | - | 105.60 |
12/06/20 | ||||||||||
| 25,567 | - | ||||||||||||
|
Richard A. Anderson |
123 | - | 672.00 |
02/07/18 | |||||||||
| 144 | - | 672.00 |
06/20/18 | ||||||||||
| 208 | - | 1,056.00 |
10/27/19 | ||||||||||
| 24,750 | - | 96.00 |
12/06/20 | ||||||||||
| 25,225 | - | ||||||||||||
|
Susan Etzel |
271 | - | 48.00 |
05/24/21 | |||||||||
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE-IN-CONTROL
Potential payments upon termination
The following summarizes the payments that the named executive officers would have received if their employment had terminated on December 31, 2016.
If Mr. Peizer's employment had terminated due to disability, he would have received insurance and other fringe benefits for a period of one year thereafter, with a value equal to $10,000. If Mr. Peizer had been terminated without good cause or resigned for good reason, he would have received a lump sum payment of $2,731,000, based upon: (i) three years of additional salary at $450,000 per year; (ii) three years of additional bonus of $450,000 per year; and (iii) three years of fringe benefits, with a value equal to $31,000.
If Mr. Anderson had been or is terminated without good cause or resigned for good reason, he would have received a lump sum payment of $580,000 based upon one year's salary plus the full targeted bonus of 50% of base salary. In addition, medical benefits would continue for up to one year, with a value equal to $28,000.
Potential payments upon change in control
Upon a change in control, the unvested stock options of each of our named executive officers would have vested, with the values set forth above.
If Mr. Peizer had been terminated without good cause or resigned for good reason within twelve months following a change in control, he would have received a lump sum payment of $2,731,000, as described above, plus a tax gross up of $683,000.
If Mr. Anderson had been terminated without good cause or resigned for good reason within twelve months following a change in control, he would have received a lump sum payment of $870,000, based upon one-and-a-half year's salary plus one-and-a-half the full targeted bonus of 50% of base salary. In addition, medical benefits would continue for up to one-and-a-half years, with a value equal to $42,000.
DIRECTOR COMPENSATION
The following table provides information regarding compensation that was earned or paid to the individuals who served as non-employee directors during the year ended December 31, 2016. Except as set forth in the table, during 2016, directors did not earn nor receive cash compensation or compensation in the form of stock awards, option awards or any other form.
|
Name |
Option awards ($) (1) |
Total |
||||||
|
Richard Berman |
167,500 | 167,500 | ||||||
|
David Smith |
134,000 | 134,000 | ||||||
|
Marvin Igelman |
134,000 | 134,000 | ||||||
|
Steve Gorlin |
134,000 | 134,000 | ||||||
Notes to director compensation table:
|
(1) |
Amounts reflect the compensation expense recognized in the Company's financial statements in 2016 for non-employee director stock options granted in 2015, in accordance with FASB ASC Topic 718. As such, these amounts do not correspond to the compensation actually realized by each director for the period. See notes to consolidated financial statements included elsewhere in this prospectus for further information on the assumptions used to value stock options granted to non-employee directors. |
Outstanding equity awards for non-employee directors, as of December 31, 2016, were as follows:
|
Options outstanding |
Aggregate grant date fair market value options outstanding |
|||||||
|
Richard Berman |
41,667 | $ | 502,500 | |||||
|
David Smith |
33,334 | 402,000 | ||||||
|
Marvin Igelman |
33,334 | 402,000 | ||||||
|
Steve Gorlin |
33,334 | 402,000 | ||||||
|
Total |
141,669 | $ | 1,708,500 | |||||
There were a total of 141,669 stock options outstanding as of December 31, 2016, with an aggregate grant date fair value of $1,708,500, the last of which vest in February 2017. There were no options granted to non-employee directors during 2016.
EQUITY COMPENSATION PLAN INFORMATION
The following table provides certain aggregate information with respect to all of the Company’s equity compensation plans in effect as of December 31, 2016.
|
Plan Category |
(a) Number of securities to be issued upon exercise of outstanding options, warrants and right |
(b) Weighted-average exercise price of outstanding options, warrants and rights |
(c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
|
Equity compensation plans approved by security holders (1) |
244,046 | $ | 39.06 | 50,581 | ||||||||
|
Equity compensation plans not approved by security holders |
- | - | - | |||||||||
|
Total |
244,046 | $ | 39.06 | 50,581 | ||||||||
|
1) |
We adopted our 2010 Stock Incentive Plan (the “2010 Plan”) in 2011. Under the 2010 Plan, we could grant incentive stock options, non-qualified stock option, restricted and unrestricted stock awards and other stock-based awards. As of December 31, 2016, 50,581 equity awards remained reserved for future issuance under the 2010 Plan. |
2010 Stock Incentive Plan
We adopted our 2010 Stock Incentive Plan, or the 2010 Plan, in 2011. Under the 2010 Plan, we may grant incentive stock options, non-qualified stock options, restricted and unrestricted stock awards and other stock-based awards. The 2010 Plan will expire on December 9, 2020. As of December 31, 2016, we have 244,046 stock options issued and 50,581 are reserved for issuance of future awards under the 2010 Plan.
The 2010 Plan provides that no participant may receive awards for more than 31,250 shares of common stock in any fiscal year.
In accordance with the terms of the 2010 Plan, our board of directors has authorized our Compensation Committee to administer the 2010 Plan. The Compensation Committee may delegate part of its authority and powers under the 2010 Plan to one or more of our directors and/or officers, but only the Compensation Committee can make awards to participants who are our directors or executive officers. In accordance with the provisions of the 2010 Plan, our Compensation Committee determines the terms of awards, including:
|
• |
which employees, directors and consultants shall be granted options and other awards; |
|
• |
the number of shares of our common stock subject to options and other awards; |
|
• |
the exercise price of each option, which generally shall not be less than fair market value on the date of grant; |
|
• |
the schedule upon which options become exercisable; |
|
• |
the termination or cancellation provisions applicable to options; |
|
• |
the terms and conditions of other awards, including conditions for repurchase, termination or cancellation, issue price and repurchase price; and |
|
• |
all other terms and conditions upon which each award may be granted in accordance with the 2010 Plan. |
Upon a merger, consolidation or sale of all or substantially all of our assets, the administrator of the 2010 Plan, or the board of directors of any corporation assuming our obligations, may, in its sole discretion, take any one or more of the following actions pursuant to our plan, as to some or all outstanding awards:
|
• |
provide that outstanding options will be substituted for shares of the successor corporation or consideration payable with respect to our outstanding stock in connection with the corporate transaction; |
|
• |
provide that the outstanding options must be exercised within a certain number of days, either to the extent the options are then exercisable, or at our board of directors’ discretion, any such options being made partially or fully exercisable; |
|
• |
terminate outstanding options in exchange for payment of an amount equal to the difference between (a) the consideration payable upon consummation of the corporate transaction to a holder of the number of shares into which such option would have been exercisable to the extent then exercisable (or, in our Board of Directors’ discretion, any such options being made partially or fully exercisable) and (b) the aggregate exercise price of those options; |
|
• |
provide that outstanding stock grants will be substituted for shares of the successor corporation or consideration payable with respect to our outstanding stock in connection with the corporate transaction; |
|
• |
the terms and conditions of other awards, including conditions for repurchase, termination or cancellation, issue price and repurchase price; and |
|
• |
terminate outstanding stock grants in exchange for payment of any amount equal to the consideration payable upon consummation of the corporate transaction to a holder of the same number of shares comprising the stock grant, to the extent the stock grant is no longer subject to any forfeiture or repurchase rights (or, at our Board of Directors’ discretion, all forfeiture and repurchase rights being waived upon the corporate transaction). |
2017 Stock Incentive Plan
On February 27, 2017, the Board of Directors and our stockholders approved the adoption of the 2017 Stock Incentive Plan (the “2017 Plan”), subject to complying with the notification requirements of Regulation 14C. The 2017 Plan allows the Company, under the direction of the Board of Directors or a committee thereof, to make grants of stock options, restricted and unrestricted stock and other stock-based awards to employees, including the Company’s executive officers, consultants and directors. The 2017 Plan allows for the issuance of up to 2,333,334 additional shares of Common Stock pursuant to new awards granted under the 2017 Plan and up to approximately 250,000 shares of Common Stock that are represented by options outstanding under the 2010 Plan (defined below) that are forfeited, expire or are cancelled without delivery of shares of Common Stock or which result in the forfeiture of shares of Common Stock back to the Company. This description is qualified in its entirety by reference to the actual terms of the 2017 Plan, a copy of which is attached as Exhibit B to the Company’s preliminary Information Statement on Schedule 14C, filed with the Securities and Exchange Commission on February 28, 2017.
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
The following is a description of transactions, since January 1, 2015, to which we have been a party and the amount involved exceeded $120,000, and in which any of our executive officers, directors or holders of more than 5% of any class of our voting securities, or an affiliate or immediate family member thereof, had a direct or indirect material interest, other than compensation, termination and change of control arrangements, which are described under “Executive and Director Compensation.”
Review and Approval of Transactions with Related Persons
Either the audit committee or the Board of Directors approves all related party transactions. The procedure for the review, approval or ratification for related party transactions involves discussing the transaction with management, discussing the transaction with our independent registered public accounting firm, reviewing financial statements and related disclosures and reviewing the details of major deals and transactions to ensure that they do not involve related transactions. Members of management have been informed and understand that they are to bring related party transactions to the audit committee or the Board of Directors for pre-approval. These policies and procedures are evidenced in the audit committee charter and our code of ethics.
Certain Transactions
In July 2015, we entered into a $3.55 million, 12% Original Issue Discount Convertible Debenture due January 18, 2016 (the “July 2015 Convertible Debenture”) with Acuitas Group Holdings, LLC (“Acuitas”), one hundred percent (100%) of which is owned by Terren S. Peizer, Chairman and Chief Executive Officer of the Company, and five-year warrants to purchase 155,835 shares of our common stock, at an exercise price of $11.40 per share, subject to adjustment, including for issuances of common stock and common stock equivalents below the then current conversion or exercise price, as the case may be (the “July 2015 Warrants”).
The conversion price of the July 2015 Convertible Debenture is $11.40 per share, subject to adjustments, including for issuances of common stock and common stock equivalents below the then current conversion or exercise price, as the case may be. The July 2015 Convertible Debenture is unsecured, bears interest at a rate of 12% per annum payable in cash or shares of common stock, is subject to certain conditions, at our option, and is subject to mandatory prepayment upon the consummation of certain future financings.
The conversion price of the July 2015 Convertible Debenture and the July 2015 Warrants were subsequently adjusted to $1.80 per share based upon the September Offering.
In September 2015, we entered into a Stock Purchase Agreement with Acuitas, relating to the sale and issuance of approximately 250,000 shares of common stock for gross proceeds of $463,000 (the “September Offering”).
In October 2015, we entered into Stock Purchase Agreements with each of Acuitas, Shamus, LLC (“Shamus”), a Company owned by David E. Smith, a member of our board of directors, and Steve Gorlin, a member of our board of directors, pursuant to which we received gross proceeds of $2.0 million from the sale of approximately 1.1 million shares of the Company’s common stock, at a purchase price of $1.80 per share (the “October Offering”).
In August 2016, Acuitas loaned us $225,000. No terms were discussed nor were any agreements executed in connection with such loan, but the $225,000 was paid back out of the August 2016 Notes.
In August 2016, we entered into subscription agreements with three accredited investors, (collectively, the “Investors”), including Shamus, pursuant to which we issued to the Investors short-term senior promissory notes in the aggregate principal amount of $2.8 million (the “August 2016 Notes”) and five-year warrants to purchase up to an aggregate of 145,834 shares of our common stock, at an exercise price of $6.60 per share (the “August 2016 Warrants”).
The August 2016 Warrants include price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the August 2016 Warrants will be adjusted in the event we issue shares of our common stock for consideration per share less than the then exercise price of the August 2016 Warrants, to the lowest consideration per share for the shares issued or sold in such transaction. The price protection will be in effect until the earliest of (i) the termination date of the August 2016 Warrants, (ii) such time as the Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange.
In addition, in August 2016, Acuitas, agreed to exchange its existing promissory note for short-term senior promissory notes, in the aggregate principal amount of $2.8 million plus accrued interest, in the form substantially identical to the form of the August 2016 Notes. Acuitas also agreed to exchange certain of its outstanding warrants to purchase an aggregate of 338,005 shares of our common stock at an exercise price of $1.98 per share, for warrants to purchase an aggregate of 498,927 shares of our common stock at an exercise price of $6.60 per share, in the form substantially identical to the form of the August 2016 Warrants.
In December 2016, we exchanged the August 2016 Notes issued to the Investors, which had an aggregate outstanding principal amount of $5.6 million, for (i) 8% Convertible Debentures in the same principal amount due on March 15, 2017 (the “Debentures”) and (ii) five-year warrants to purchase shares of the Company’s common stock in amount equal to forty percent (40%) of the initial number of shares of common stock issuable upon conversion of each Investor’s Debentures, at an exercise price of $6.60 per share (the “December 2016 Warrants”). The holders of the Debentures have agreed to extend the maturity of the Debentures from March 15, 2017 to the earlier of the closing date of this offering or April 30, 2017, and it is expected that $2.8 million of the Debentures will convert the full principal amount into common stock as of the closing date.
The December 2016 Warrants include a price protection provision pursuant to which, subject to certain exempt issuances, the then exercise price of the December 2016 Warrants will be adjusted if the Company issues shares of common stock at a price that is less than the then exercise price of the December 2016 Warrants. Such price protection provisions will remain in effect until the earliest of (i) the termination date of the December 2016 Warrants, (ii) such time as the December 2016 Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange.
In December 2016, we entered into an agreement with Shamus pursuant to which the Company received gross proceeds of $300,000 for the sale of (i) an 8% Series B Convertible Debenture due March 31, 2017 (the “December 2016 Convertible Debenture”) and (ii) five-year warrants to purchase shares of the Company’s common stock in an amount equal to seventy-five percent (75%) of the initial number of shares of common stock issuable upon the conversion of the December 2016 Convertible Debenture, at an exercise price of $5.10 per share (the “Shamus Warrants”). The holder of the December 2016 Convertible Debenture has agreed to extend the maturity of the Debentures from March 31, 2017 to the earlier of the closing date of this offering, and it is expected that the December 2016 Convertible Debenture will convert the full principal amount into common stock as of the closing date.
The Shamus Warrants include price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the Shamus Warrants will be adjusted if the Company issues shares of common stock at a price that is less than the then exercise price of the Shamus Warrants. Such mechanism will remain in effect until the earliest of (i) the termination date of the Shamus Warrants, (ii) such time as the Shamus Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange.
In January 2017, we entered into a Subscription Agreement (the “Subscription Agreement”) with Acuitas, pursuant to which the Company will receive aggregate gross proceeds of $1,300,000 (the “Loan Amount”) in consideration of the issuance of (i) an 8% Series B Convertible Debenture due March 31, 2017 (the “January 2017 Convertible Debenture”) and (ii) five-year warrants to purchase shares of the Company’s common stock in an amount equal to one hundred percent (100%) of the initial number of shares of common stock issuable upon the conversion of the January 2017 Convertible Debenture, at an exercise price of $5.10 per share (the “January 2017 Warrants”). The Loan Amount is payable in tranches through March 2017. In addition, any warrants issued in conjunction with the December 2016 Convertible Debenture currently outstanding with Acuitas have been increased by an additional 25% warrant coverage, exercisable for an aggregate of 137,883 shares of the Company’s common stock. The holders of the January 2017 Convertible Debentures have agreed to extend the maturity of the Debentures from March 31, 2017 to the earlier of the closing date of this offering or April 30, 2017 and to convert the full principal amount into common stock as of the closing date. The holder of the January 2017 Convertible Debenture has agreed to extend the maturity of the January 2017 Convertible Debenture from March 31, 2017 to the earlier of the closing date of this offering or April 30, 2017.
The January 2017 Warrants include, among other things, price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the January 2017 Warrants will be adjusted if the Company issues shares of common stock at a price that is less than the then exercise price of the January 2017 Warrants. Such price protection provisions will remain in effect until the earliest of (i) the expiration date of the January 2017 Warrants, (ii) such time as the January 2017 Warrants are exercised or (iii) contemporaneously with the listing of the Company’s shares of common stock on a registered national securities exchange.
In connection with the Subscription Agreement described above, the number of Shamus Warrants were increased from 75% to 100% warrant coverage, exercisable for an aggregate of 58,824 shares of the Company’s common stock.
In March 2017, we entered into amendments with the holders of certain outstanding warrants issued on April 17, 2015 and July 30, 2015 to eliminate certain anti-dilution provisions in such warrants, which caused us to reflect an associated liability of $5.3 million on our balance sheet as of December 31, 2016. Such amendments are contingent upon and will not take effect until the closing of this offering, which must occur on or before April 30, 2017. For each warrant share underlying the warrants so amended, the holder received the right to purchase an additional .2 shares of common stock. Two of the holders of such warrants, which owners hold warrants to purchase an aggregate of 11,049 shares of common stock, did not agree to the amendment. The warrant holders agreeing to the amendment include Acuitas Group Holdings, LLC (“Acuitas”), one hundred percent (100%) of which is owned by Terren S. Peizer, Chairman and Chief Executive Officer of the Company, and another accredited investor, who will own warrants to purchase 31,167 and 13,258 shares of the Company’s common stock, respectively, if and when the amendments take effect.
Additionally, in March 2017, we and the holders of an aggregate of approximately $10 million of our existing convertible debentures agreed to extend the maturity dates of such debentures until the earlier of the closing of this offering or April 30, 2017.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of March 30, 2017 for (a) each stockholder known by us to own beneficially more than 5% of our common stock (b) our named executive officers, (c) each of our directors, and (d) all of our current directors and executive officers as a group. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. We deem shares of common stock that may be acquired by an individual or group within 60 days of March 30, 2017 pursuant to the exercise of options or warrants to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them based on information provided to us by these stockholders. Percentage of ownership is based on 9,383,740 shares of common stock outstanding on March 30, 2017.
|
Total |
||||||||||||||||
|
Shares |
common |
|||||||||||||||
|
Common |
beneficially |
stock |
Percent |
|||||||||||||
|
stock |
owned |
beneficially |
of |
|||||||||||||
|
Name of beneficial owner (1) |
owned (2) |
(3) |
owned |
class (3) |
||||||||||||
|
Directors and Named Executive Officers: |
||||||||||||||||
|
Terren S. Peizer (4) |
6,393,042 | 4,128,718 | 10,619,760 | 78.0 |
% | |||||||||||
|
Richard A. Anderson (5) |
- | 25,225 | 25,225 | * | ||||||||||||
|
Susan E. Etzel (6) |
- | 271 | 271 | * | ||||||||||||
|
Richard A. Berman (7) |
- | 41,667 | 41,667 | * | ||||||||||||
|
David E. Smith (8) |
1,832,253 | 265,803 | 2,098,056 | 21.7 |
% | |||||||||||
|
Marvin Igelman (9) |
- | 41,667 | 41,667 | * | ||||||||||||
|
Steve Gorlin (10) |
56,250 | 33,334 | 89,584 | * | ||||||||||||
|
Richard J. Berman (11) |
13,333 | - | 13,333 | * | ||||||||||||
|
Marc Cummins (12) |
30,318 | 67,168 | 97,485 | * | ||||||||||||
|
All directors and named executive officers as a group (9 persons) |
8,325,196 | 4,701,853 | 13,027,049 | 92.5 |
% | |||||||||||
* Less than 1%
|
(1) |
Except as set forth below, the mailing address of all individuals listed is c/o Catasys, Inc., 11601 Wilshire Boulevard, Suite 1100, Los Angeles, California 90025. |
|
(2) |
The number of shares beneficially owned includes shares of common stock in which a person has sole or shared voting power and/or sole or shared investment power. Except as noted below, each person named reportedly has sole voting and investment powers with respect to the common stock beneficially owned by that person, subject to applicable community property and similar laws. |
|
(3) |
On March 30, 2017, there were 9,383,740 shares of common stock outstanding. Common stock not outstanding but which underlies options and rights (including warrants) vested as of or vesting within 60 days after March 30, 2017, is deemed to be outstanding for the purpose of computing the percentage of the common stock beneficially owned by each named person (and the directors and executive officers as a group), but is not deemed to be outstanding for any other purpose. |
|
(4) |
Consists of warrants to purchase 1,181,747 shares of common stock, options to purchase 25,567 shares of common stock, and convertible debentures to purchase 3,019,408 shares of common stock. 6,393,042 shares of common stock are held of record by Acuitas Group Holdings, LLC, a limited liability company 100% owned by Terren S. Peizer, and as such, Mr. Peizer may be deemed to beneficially own or control. Mr. Peizer disclaims beneficial ownership of any such securities. |
|
(5) |
Includes options to purchase 25,225 shares of common stock, which are exercisable within the next 60 days. |
|
(6) |
Includes options to purchase 271 shares of common stock, which are exercisable within the next 60 days. |
|
(7) |
Incudes options to purchase 41,667 shares of common stock, which are exercisable within the next 60 days. |
|
(8) |
Consists of 1,830,683 shares of common stock held by Shamus, LLC ("Shamus"). As the sole member of Shamus, The Coast Fund L.P. ("Coast Fund") may be deemed to beneficially own all common stock beneficially owned by Shamus. Similarly, as the managing general partner of the Coast Fund, Coast Offshore Management (Cayman), Ltd. ("Coast Offshore Management") may be deemed to beneficially own all common stock beneficially owned by the Coast Fund. Except to the extent it is deemed to beneficially own any common stock beneficially owned by Shamus, neither the Coast Fund nor Coast Offshore Management beneficially owns any common stock. As the president of Coast Offshore Management, Mr. Smith may be deemed to beneficially own all common stock beneficially owned by Coast Offshore Management, Coast Fund and Shamus. In addition, Mr. Smith directly owns (i) 1,571 shares of common stock and (ii) 33,334 shares of common stock issuable upon the exercise of options granted to Mr. Smith for his service on our board of directors that are either currently exercisable or will become exercisable within the next 60 days and (iii) warrants to purchase 172,450 shares of common stock. As a result, Mr. Smith may be deemed to beneficially own, in the aggregate, 2,098,056 shares of our common stock. |
|
(9) |
Includes options to purchase 41,667 shares of common stock, which are exercisable within the next 60 days. |
|
(10) |
Consists of 56,250 shares of common stock and options to purchase 33,334 shares of common stock, which are exercisable within the next 60 days. |
|
(11) |
Consists of 13,333 shares of common stock. |
|
(12) |
Consists of 30,318 shares of common stock, warrants to purchase 28,409 shares of common stock, and convertible debentures to purchase 34,759 shares of common stock. |
DESCRIPTION OF CAPITAL STOCK
The following describes the material terms of our capital stock. The following description does not purport to be complete and is subject to, and qualified in its entirety by reference to, our certificate of incorporation and by-laws, each as amended, which are attached as exhibits to the registration statement of which this prospectus forms a part. All of our stockholders are urged to read our certificate of incorporation and by-laws, each as amended, carefully and in their entirety.
General
Our certificate of incorporation authorizes us to issue up to 500,000,000 shares of common stock, par value $0.0001 per share, and 50,000,000 shares of preferred stock, par value $0.0001 per share, none of which is currently outstanding.
As of March 30, 2017, there were 9,383,740 shares of our common stock issued and outstanding, held by approximately 68 record holders. In addition, as of March 30, 2017, there were warrants and options outstanding to purchase approximately 1,928,431 shares of our common stock.
The following description of our capital stock is not complete and is subject to and qualified in its entirety by our certificate of incorporation and by-laws, which are filed as exhibits to the registration statement of which this prospectus is a part, and by the relevant provisions of the Delaware General Corporation Law.
Common Stock
The holders of common stock are entitled to one vote per share on all matters to be voted upon by stockholders. Subject to preferences that may be applicable to any outstanding preferred stock, holders of common stock are entitled to receive ratably dividends as may be declared by our Board of Directors out of funds legally available for that purpose. In the event of our liquidation, dissolution, or winding up, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preferences of any outstanding preferred stock. The common stock has no preemptive or conversion rights, other subscription rights, or redemption or sinking fund provisions. All issued and outstanding shares of common stock are fully paid and non-assessable.
Anti-Takeover Provisions of Delaware Law and Charter Provisions
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a publicly-held Delaware corporation from engaging in a “business combination,” except under certain circumstances, with an “interested stockholder” for a period of three years following the date such person became an “interested stockholder” unless:
|
● |
before such person became an interested stockholder, the board of directors of the corporation approved either the business combination or the transaction that resulted in the interested stockholder becoming an interested stockholder; |
|
● |
upon the consummation of the transaction that resulted in the interested stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding shares held by directors who also are officers of the corporation and shares held by employee stock plans; or |
|
● |
at or following the time such person became an interested stockholder, the business combination is approved by the board of directors of the corporation and authorized at a meeting of stockholders by the affirmative vote of the holders of 66 2/3% of the outstanding voting stock of the corporation which is not owned by the interested stockholder. |
The term “interested stockholder” generally is defined as a person who, together with affiliates and associates, owns, or, within the three years prior to the determination of interested stockholder status, owned, 15% or more of a corporation’s outstanding voting stock. The term “business combination” includes mergers, asset or stock sales and other similar transactions resulting in a financial benefit to an interested stockholder. Section 203 makes it more difficult for an “interested stockholder” to effect various business combinations with a corporation for a three-year period. The existence of this provision would be expected to have an anti-takeover effect with respect to transactions not approved in advance by our Board of Directors, including discouraging attempts that might result in a premium over the market price for the shares of common stock held by stockholders.
The ability of our Board of Directors to issue shares of preferred stock and to set the voting rights, preferences and other terms thereof, without further stockholder action, may be deemed to have an anti-takeover effect and may discourage takeover attempts not first approved by our Board of Directors, including takeovers which stockholders may deem to be in their best interests. If takeover attempts are discouraged, temporary fluctuations in the market price of our common stock, which may result from actual or rumored takeover attempts, may be inhibited. These provisions, together with the ability of our Board of Directors to issue preferred stock without further stockholder action could also delay or frustrate the removal of incumbent directors or the assumption of control by stockholders, even if the removal or assumption would be beneficial to our stockholders. These provisions could also discourage or inhibit a merger, tender offer or proxy contests, even if favorable to the interests of stockholders, and could depress the market price of our common stock. In addition, our bylaws may be amended by action of the board of directors, which could have further anti-takeover effects, and which could limit the price investors would be willing to pay in the future for shares of our common stock.
Warrants
As of March 30, 2017, we had warrants outstanding for the number of shares of our common stock at the exercise prices and expiration dates set forth below. Warrants entitle the holder to purchase shares of our common stock at the specified exercise price at any time prior to the expiration date.
|
Expiration Date |
Number of |
Weighted-Average |
||||||
|
February 2019 |
4,167 | $ | 18.00 | |||||
|
April 2019 |
50,000 | $ | 12.00 | |||||
|
April 2020 |
77,337 | $ | 1.80 | |||||
|
July 2020 |
155,835 | $ | 1.80 | |||||
|
August 2021 |
644,762 | $ | 5.10 | |||||
|
December 2021 |
381,259 | $ | 5.10 | |||||
|
January 2022 |
211,413 | $ | 5.10 | |||||
|
February 2022 |
61,765 | $ | 5.10 | |||||
|
March 2022 |
98,040 | $ | 5.10 | |||||
|
Total: |
1,684,578 | $ | 4.88 | |||||
Transfer Agent
The transfer agent for our securities is American Stock Transfer & Trust Company, LLC whose address is 6201 15th Avenue, Brooklyn, NY 11219, and whose telephone number is 1-800-937-5449.
Current Trading Symbol and Exchange Listing
Our common stock is quoted for trading on the OTCQB under the symbol “CATS.” We have applied to list our common stock on The NASDAQ Capital Market under the symbol “CATS.” No assurance can be given that our application will be approved.
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of shares of our common stock in the public market, or the availability of such shares for sale in the public market, could adversely affect the market price of our common stock prevailing from time to time. As described below, only a limited number of shares are currently available for sale due to contractual and legal restrictions on resale. Nonetheless, sales of our common stock, or the perception that these sales could occur, could adversely affect prevailing market prices for our common stock and could impair our future ability to raise equity capital in the future.
Based on the number of shares outstanding as of March , 2017, upon the closing of this offering, shares of common stock will be outstanding, assuming no exercise of outstanding options or warrants and no exercise of the underwriters’ option to purchase additional shares. Of the outstanding shares, all of the shares of common stock sold in this offering (including pursuant to the underwriters’ exercise of their option to purchase additional shares) will be freely tradable, except that any shares held by our affiliates, as that term is defined in Rule 144 under the Securities Act, may only be sold in compliance with the limitations described below.
The remaining shares of our common stock outstanding after this offering are restricted securities, as that term is defined in Rule 144 under the Securities Act, or are subject to lock-up agreements with us as described below. Following the expiration of the lock-up period, restricted securities may be sold in the public market only if registered or if their resale qualifies for exemption from registration described below under Rule 144 promulgated under the Securities Act.
Rule 144.
In general, persons who have beneficially owned restricted shares of our common stock for at least six months, and any affiliate of ours who owns either restricted or unrestricted shares of our common stock, are entitled to sell their securities without registration with the Securities and Exchange Commission under an exemption from registration provided by Rule 144 under the Securities Act.
In general, a person who has beneficially owned restricted shares of our common stock for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, a sale and (ii) we have been subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Persons who have beneficially owned restricted shares of our common stock for at least six months, but who are our affiliates at the time of, or any time during the 90 days preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of either of the following:
|
● |
1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after the closing of this offering based on the number of shares of our common stock outstanding as of , 2017 and assuming no exercise of the underwriters’ option to purchase additional shares of our common stock; or |
|
● |
the average weekly trading volume of our common stock on the NASDAQ Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale; |
provided, in each case, that we have been subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Such sales both by affiliates and by non-affiliates must also comply with the manner of sale, current public information and notice provisions of Rule 144.
Lock-Up Agreements
All of our directors, executive officers and any other 5% or greater holder of our outstanding common stock are subject to lock-up agreements or market standoff provisions that, subject to certain exceptions, prohibit them from offering for sale, selling, contracting to sell, granting any option for the sale of, transferring or otherwise disposing of any shares of our common stock, options or warrants to acquire shares of our common stock or any security or instrument related to our common stock, or entering into any swap, hedge or other arrangement that transfers any of the economic consequences of ownership of our common stock, for a period of three (3) months following the date of this prospectus without the prior written consent of the representative of the underwriters. See the section of this prospectus titled “Underwriting.”
Equity Incentive Plans
We have filed a Form S-8 registration statement under the Securities Act of 1933, as amended, to register shares of our common stock issued or reserved for issuance under our equity compensation plans and agreements. Accordingly, the shares covered by this registration statement are eligible for sale in the public markets, subject to vesting restrictions, the lock-up agreements described above and Rule 144 limitations applicable to affiliates.
UNDERWRITING
Joseph Gunnar & Co., LLC is acting as representative of the underwriters (the “Representative”). Subject to the terms and conditions of an underwriting agreement between us and the Representative, we have agreed to sell to each underwriter named below, and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of shares of common stock listed next to its name in the following table:
|
Name of Underwriter |
Number of |
|||
|
Joseph Gunnar & Co., LLC |
||||
|
Total |
||||
The underwriters are committed to purchase all the shares of common stock offered by us if they purchase any shares of common stock. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated. The underwriters are not obligated to purchase the shares of common stock covered by the underwriters’ over-allotment option described below. The underwriters are offering the shares of common stock, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Discounts and Commissions
The underwriters propose initially to offer the shares of common stock to the public at the public offering price set forth on the cover page of this prospectus and to dealers at those prices less a concession not in excess of $ per share of common stock. If all of the shares of common stock offered by us are not sold at the public offering price, the underwriters may change the offering price and other selling terms by means of a supplement to this prospectus.
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information assumes either no exercise or full exercise of the over-allotment option we granted to the Representatives of the underwriters.
|
Total |
||||||||||||
| Per Share |
Without |
With |
||||||||||
|
Public offering price |
$ | $ | $ | |||||||||
|
Underwriting discount (7%) |
$ | $ | $ | |||||||||
|
Proceeds, before expenses, to us |
$ | $ | $ | |||||||||
We have agreed to pay a non-accountable expense allowance to the Representative of the underwriters equal to 1% of the gross proceeds received at the closing of the offering. The non-accountable expense allowance of 1% is not payable with respect to the shares sold upon exercise of the underwriters’ over-allotment option. We have paid an expense deposit of $50,000 to the Representative, which will be applied against the out-of-pocket accountable expenses that will be paid by us to the underwriters in connection with this offering, and will be reimbursed to us to the extent not actually incurred in compliance with FINRA Rule 5110(f)(2)(C).
We have also agreed to pay the Representative’s expenses relating to the offering, including (a) all actual filing fees incurred in connection with the review of this offering by the Financial Industry Regulatory Authority, or FINRA; (b) all fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed $15,000 in the aggregate; (c) the costs associated with bound volumes of the public offering materials as well as commemorative mementos and lucite tombstones not to exceed $2,000; (d) the fees and expenses of the Representative’s legal counsel not to exceed $75,000; (e) $29,500 for the underwriters’ use of Ipreo’s book-building, prospectus tracking and compliance software for this offering; and (f) up to $20,000 of the Representative’s actual accountable road show expenses for the offering.
The total estimated expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts, commissions and expenses, are approximately $ and are payable by us.
Over-Allotment Option
We have granted a 45-day option to the Representative of the underwriters to purchase up to additional shares of our common stock at a public offering price of $ per share, solely to cover over-allotments, if any. The underwriters may exercise this option for 45 days from the date of this prospectus solely to cover sales of shares of common stock by the underwriters in excess of the total number of shares of common stock set forth in the table above. If any of these additional shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered. We will pay the expenses associated with the exercise of the over-allotment option.
Representative’s Warrants
We have agreed to issue to the Representative the Representative’s warrants to purchase up to shares of common stock (5% of the shares of common stock sold in this offering). We are registering hereby the issuance of the Representative’s warrants and the shares of common stock issuable upon exercise of the warrants. The Representative’s warrants will be exercisable at any time, and from time to time, in whole or in part, during the four-year period commencing one year from the effective date of the registration statement at a per share exercise price equal to 125% of the public offering price per share of common stock in the offering. The Representative’s warrants and the shares of common stock underlying the warrants have been deemed compensation by FINRA and are, therefore, subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The Representatives (or permitted assignees under the Rule) will not sell, transfer, assign, pledge or hypothecate these warrants or the securities underlying these warrants, nor will it engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of these warrants or the underlying securities for a period of 180 days after the effective date. The exercise price and number of shares of common stock issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend, extraordinary cash dividend or our recapitalization, reorganization, merger or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
Lock-Up Agreements
Pursuant to “lock-up” agreements, we, our executive officers and directors, and any other 5% or greater holder of our outstanding common stock, have agreed, without the prior written consent of the Representative not to directly or indirectly, offer to sell, sell, pledge or otherwise transfer or dispose of any of shares of (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of) our common stock, enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of our common stock, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable for common stock or any other securities of ours or publicly disclose the intention to do any of the foregoing, subject to customary exceptions, for a period of three (3) months after the date of this prospectus.
Right of First Refusal
Until twelve (12) months from the effective date of this registration statement, the Representative shall have an irrevocable right of first refusal to act as sole investment banker, sole book-runner, and/or sole placement agent, at the Representative’s sole discretion, for each and every future underwritten registered public offering on terms customary to the Representative. The Representative shall have the sole right to determine whether or not any other broker-dealer shall have the right to participate in any such offering and the economic terms of any such participation. The Representative will not have more than one opportunity to waive or terminate the right of first refusal in consideration of any payment or fee.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities that may be incurred in connection with this offering, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
OTCQB and NASDAQ
Our shares of common are quoted on the OTCQB under the symbol “CATS.” We have applied to list our common stock on The Nasdaq Capital Market under the symbol “CATS”, prior to the completion of this offering. No assurance can be given that such listing will be approved; however, it is a condition of the underwriters’ obligation that our shares of common stock have been approved for listing on The Nasdaq Capital Market.
Price Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of our securities, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our securities. In connection with the offering, the underwriters may purchase and sell our securities in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares of securities than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of securities in the offering. The underwriters may close out any covered short position by either exercising the over-allotment option or purchasing shares of securities in the open market. In determining the source of shares of securities to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. “Naked” short sales are sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our securities in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of securities made by the underwriters in the open market before the completion of the offering.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our securities or preventing or retarding a decline in the market price of our securities. As result, the price of our securities may be higher than the price that might otherwise exist in the open market.
The underwriters have advised us that, pursuant to Regulation M under the Exchange Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of our securities, including the imposition of penalty bids. This means that if the Representative of the underwriters purchases securities in the open market in stabilizing transactions or to cover short sales, the Representative can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them. The underwriters make no representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our securities. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares of securities to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the Representative to underwriters and selling group members that may make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ websites and any information contained in any other website maintained by the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part.
Other Relationships
From time to time, certain of the underwriters and their affiliates have provided, and may provide in the future, various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions. However, except as disclosed in this prospectus, we have no present arrangements with any of the underwriters for any further services.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws. Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area — Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
|
|
(a) |
to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
|
|
(b) |
to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements); |
|
|
(c) |
to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of ours or any underwriter for any such offer; or |
|
|
(d) |
in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the Prospectus Directive. |
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France. Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D. 744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the ISA), nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Societ—$$—Aga e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
|
|
● |
to Italian qualified investors, as defined in Article 100 of Decree no.58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
|
|
● |
in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
|
|
● |
made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
|
|
● |
in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissão do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor have we received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by us.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to us.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
LEGAL MATTERS
The validity of the shares of the common stock offered by this prospectus will be passed upon for us by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., New York, New York. The underwriters are being represented by Loeb & Loeb LLP, New York, New York.
EXPERTS
The consolidated balance sheets of Catasys, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations, stockholders’ equity and cash flows for each of the years in the two-year period ended December 31, 2016, have been audited by Rose, Snyder & Jacobs LLP, independent registered public accounting firm, as stated in its report which is incorporated herein by reference. Such financial statements have been incorporated herein by reference in reliance on the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the Securities and Exchange Commission, or the SEC, a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock being offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC's website at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street NE, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities. You may also request a copy of these filings, at no cost, by writing us at 11095 11601 Wilshire Blvd, Suite 1100, Los Angeles, California 90025 or telephoning us at (310) 444-4300.
We are subject to the information and periodic reporting requirements of the Exchange Act, and we file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information are available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at http:// www.catasys.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this prospectus.
INDEX TO FINANCIAL STATEMENTS
|
Audited Financial Statements |
|
|
Report of Independent Registered Public Accounting Firm |
F-2 |
|
Consolidated Balance Sheets as of December 31, 2016 and 2015 |
F-3 |
|
Consolidated Statements of Operations for the Years Ended December 31, 2016 and 2015 |
F-4 |
|
Consolidated Statements of Stockholders’ Equity (Deficit) for Years Ended December 31, 2016 and 2015 |
F-5 |
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2016 and 2015 |
F-6 |
|
Notes to Consolidated Financial Statements |
F-7 |
|
PART I - FINANCIAL INFORMATION |
Item 1. Financial Statements
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Catasys, Inc.
We have audited the accompanying consolidated balance sheets of Catasys, Inc. and Subsidiaries (the “Company”) as of December 31, 2016 and 2015, and the related consolidated statements of operations, stockholders’ equity (deficit) and cash flows for each of the years then ended 2016 and 2015. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Catasys, Inc. and Subsidiaries as of December 31, 2016 and 2015, and the consolidated results of their operations and their cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has continued to incur significant operating losses and negative cash flows from operations during the year ended December 31, 2016 and continues to have negative working capital at December 31, 2016. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans regarding those matters also are described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to that matter.
/s/ Rose, Snyder & Jacobs LLP
Encino, California
February 27, 2017
CATASYS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
(In thousands, except for number of shares) |
December 31, |
December 31, |
||||||
|
2016 |
2015 |
|||||||
|
ASSETS |
||||||||
|
Current assets |
||||||||
|
Cash and cash equivalents |
$ | 851 | $ | 916 | ||||
|
Receivables, net of allowance for doubtful accounts of $0 and $0, respectively |
1,052 | 590 | ||||||
|
Prepaids and other current assets |
420 | 575 | ||||||
|
Total current assets |
2,323 | 2,081 | ||||||
|
Long-term assets |
||||||||
|
Property and equipment, net of accumulated depreciation of $1,620 and $1,491, respectively |
410 | 412 | ||||||
|
Deposits and other assets |
371 | 387 | ||||||
|
Total Assets |
$ | 3,104 | $ | 2,880 | ||||
|
LIABILITIES AND STOCKHOLDERS' DEFICIT |
||||||||
|
Current liabilities |
||||||||
|
Accounts payable |
$ | 870 | $ | 753 | ||||
|
Accrued compensation and benefits |
2,089 | 1,703 | ||||||
|
Deferred revenue |
1,525 | 1,683 | ||||||
|
Other accrued liabilities |
575 | 682 | ||||||
|
Short term debt, related party, net of discount of $216 and $0, respectively |
9,796 | - | ||||||
|
Short term derivative liability |
8,122 | - | ||||||
|
Total current liabilities |
22,977 | 4,821 | ||||||
|
Long-term liabilities |
||||||||
|
Deferred rent and other long-term liabilities |
117 | 198 | ||||||
|
Long term convertible debt, related party, net of discount $0 and $0, respectively |
- | 3,662 | ||||||
|
Capital leases |
31 | 66 | ||||||
|
Long term derivative liability |
- | 2,348 | ||||||
|
Warrant liabilities |
5,307 | 509 | ||||||
|
Total Liabilities |
28,432 | 11,604 | ||||||
|
Commitments and contingencies (note 8) |
||||||||
|
Stockholders' deficit |
||||||||
|
Preferred stock, $0.0001 par value; 50,000,000 shares authorized; no shares issued and outstanding |
- | - | ||||||
|
Common stock, $0.0001 par value; 500,000,000 shares authorized; 55,288,458 and 55,007,761 shares issued and outstanding at December 31, 2016 and December 31, 2015, respectively |
6 | 6 | ||||||
|
Additional paid-in-capital |
254,385 | 253,053 | ||||||
|
Accumulated deficit |
(279,719 | ) | (261,783 | ) | ||||
|
Total Stockholders' deficit |
(25,328 | ) | (8,724 | ) | ||||
|
Total Liabilities and Stockholders' Deficit |
$ | 3,104 | $ | 2,880 | ||||
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
CATASYS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
|
Twelve Months Ended |
||||||||
|
(In thousands, except per share amounts) |
December 31, |
|||||||
|
2016 |
2015 |
|||||||
|
Revenues |
||||||||
|
Healthcare services revenues |
$ | 7,075 | $ | 2,705 | ||||
|
Operating expenses |
||||||||
|
Cost of healthcare services |
4,670 | 2,433 | ||||||
|
General and administrative |
8,838 | 9,049 | ||||||
|
Depreciation and amortization |
141 | 122 | ||||||
|
Total operating expenses |
13,649 | 11,604 | ||||||
|
Loss from operations |
(6,574 | ) | (8,899 | ) | ||||
|
Interest and other income |
106 | 64 | ||||||
|
Interest expense |
(5,354 | ) | (2,590 | ) | ||||
|
Loss on impairment of intangible assets |
- | (88 | ) | |||||
|
Loss on exchange of warrants |
- | (4,410 | ) | |||||
|
Loss on debt extinguishment |
(2,424 | ) | (195 | ) | ||||
|
Change in fair value of warrant liability |
2,093 | 11,665 | ||||||
|
Change in fair value of derivative liability |
(5,774 | ) | (2,761 | ) | ||||
|
Loss from operations before provision for income taxes |
(17,927 | ) | (7,214 | ) | ||||
|
Provision for income taxes |
9 | 9 | ||||||
|
Net loss |
$ | (17,936 | ) | $ | (7,223 | ) | ||
|
Basic and diluted net loss per share: |
$ | (0.33 | ) | $ | (0.18 | ) | ||
|
Basic weighted number of shares outstanding |
55,074 | 40,372 | ||||||
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
CATASYS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (DEFICIT)
|
(Amounts in thousands, except for number of shares) |
Common Stock |
Additional Paid-In |
Other Comprehensive |
Accumulated |
||||||||||||||||||||
|
Shares |
Amount |
Capital |
Income |
Deficit |
Total |
|||||||||||||||||||
|
Balance at December 31, 2014 |
25,244,485 | $ | 3 | $ | 213,333 | $ | - | $ | (254,560 | ) | $ | (41,224 | ) | |||||||||||
|
Warrant Exchange |
21,277,220 | 2 | 35,531 | - | - | 35,533 | ||||||||||||||||||
|
Common stock issued for outside services |
76,055 | - | 172 | 172 | ||||||||||||||||||||
|
Common stock issued in private placement, net of expenses |
8,410,001 | 1 | 2,620 | - | - | 2,621 | ||||||||||||||||||
|
Share-based Compensation Expense |
- | - | 1,397 | - | - | 1,397 | ||||||||||||||||||
|
Net loss |
- | - | - | - | (7,223 | ) | (7,223 | ) | ||||||||||||||||
|
Balance at December 31, 2015 |
55,007,761 | $ | 6 | $ | 253,053 | $ | - | $ | (261,783 | ) | $ | (8,724 | ) | |||||||||||
|
Warrants Exercised |
45,697 | - | 46 | 46 | ||||||||||||||||||||
|
Common stock issued for outside services |
235,000 | - | 235 | 235 | ||||||||||||||||||||
|
Extinguishment of Debt |
354 | 354 | ||||||||||||||||||||||
|
Share-based Compensation Expense |
697 | 697 | ||||||||||||||||||||||
|
Net loss |
(17,936 | ) | (17,936 | ) | ||||||||||||||||||||
|
Balance at December 31, 2016 |
55,288,458 | $ | 6 | $ | 254,385 | $ | - | $ | (279,719 | ) | $ | (25,328 | ) | |||||||||||
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
CATASYS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
Twelve Months Ended |
||||||||
|
(In thousands) |
December 31, |
|||||||
|
2016 |
2015 |
|||||||
|
Operating activities: |
||||||||
|
Net loss |
$ | (17,936 | ) | $ | (7,223 | ) | ||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
|
Depreciation and amortization |
141 | 122 | ||||||
|
Loss on disposal of intangible assets |
- | 88 | ||||||
|
Amortization of debt discount and issuance costs included in interest expense |
4,651 | 2,324 | ||||||
|
Loss on debt extinguishment |
2,424 | 195 | ||||||
|
Warrants issued for services |
- | 168 | ||||||
|
Provision for doubtful accounts |
47 | 10 | ||||||
|
Deferred rent |
(70 | ) | (44 | ) | ||||
|
Share-based compensation expense |
697 | 1,397 | ||||||
|
Common stock issued for consulting services |
235 | 172 | ||||||
|
Fair value adjustment on warrant liability |
(2,093 | ) | (11,665 | ) | ||||
|
Loss on exchange of warrants |
- | 4,410 | ||||||
|
Fair value adjustment on derivative liability |
5,774 | 2,761 | ||||||
|
Changes in current assets and liabilities: |
||||||||
|
Receivables |
(509 | ) | (111 | ) | ||||
|
Prepaids and other current assets |
155 | 17 | ||||||
|
Deferred revenue |
(158 | ) | 1,329 | |||||
|
Accounts payable and other accrued liabilities |
916 | 882 | ||||||
|
Net cash used in operating activities of continuing operations |
$ | (5,726 | ) | $ | (5,168 | ) | ||
|
Investing activities: |
||||||||
|
Purchases of property and equipment |
$ | (106 | ) | $ | (107 | ) | ||
|
Deposits and other assets |
16 | - | ||||||
|
Net cash used in investing activities |
$ | (90 | ) | $ | (107 | ) | ||
|
Financing activities: |
||||||||
|
Proceeds from the issuance of common stock and warrants |
$ | - | $ | 2,463 | ||||
|
Proceeds from the issuance of convertible debt, related party |
300 | 5,910 | ||||||
|
Payments on convertible debenture |
- | (2,681 | ) | |||||
|
Proceeds from the issuance of senior promissory note, related party |
5,505 | - | ||||||
|
Transactions costs |
- | (185 | ) | |||||
|
Capital lease obligations |
(54 | ) | (24 | ) | ||||
|
Net cash provided by financing activities |
$ | 5,751 | $ | 5,483 | ||||
|
Net increase (decrease) in cash and cash equivalents |
$ | (65 | ) | $ | 208 | |||
|
Cash and cash equivalents at beginning of period |
916 | 708 | ||||||
|
Cash and cash equivalents at end of period |
$ | 851 | $ | 916 | ||||
|
Supplemental disclosure of cash paid |
||||||||
|
Interest |
$ | 149 | $ | 271 | ||||
|
Income taxes |
$ | 48 | $ | 41 | ||||
|
Supplemental disclosure of non-cash activity |
||||||||
|
Common stock issued for exercise of warrants |
$ | 46 | $ | - | ||||
|
Property and equipment acquired through capital leases and other financing |
$ | 34 | $ | 54 | ||||
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
CATASYS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
Note 1. Summary of Significant Accounting Policies
Description of Business
We provide data analytics based specialized behavioral health management and integrated treatment services to health plans through our OnTrak solution. Our OnTrak solution is designed to improve member health and at the same time lower costs to the insurer for underserved populations where behavioral health conditions are causing or exacerbating co-existing medical conditions. The program utilizes proprietary analytics, member engagement and patient centric treatment that integrates evidence-based medical and psychosocial interventions along with care coaching in a 52-week outpatient program. Our initial focus was members with substance use disorders, but we have expanded our solution to assist members with anxiety and depression. We currently operate our OnTrak solutions in Florida, Georgia, Illinois, Kansas, Kentucky, Louisiana, Massachusetts, Missouri, New Jersey, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, West Virginia and Wisconsin. We provide services to commercial (employer funded), managed Medicare Advantage, and managed Medicaid and duel eligible (Medicare and Medicaid) populations.
Basis of Consolidation and Presentation and Going Concern
Our financial statements have been prepared on the basis that we will continue as a going concern. At December 31, 2016, cash and cash equivalents was $851,000 and we had a working capital deficit of approximately $20.7 million. We have incurred significant operating losses and negative cash flows from operations since our inception. During the twelve months December 31, 2016, our cash used in operating activities from continuing operations was $5.7 million. We anticipate that we could continue to incur negative cash flows and net losses for the next twelve months. The financial statements do not include any adjustments relating to the recoverability of the carrying amount of the recorded assets or the amount of liabilities that might result from the outcome of this uncertainty. As of December 31, 2016, these conditions raised substantial doubt as to our ability to continue as a going concern. We expect our current cash resources to cover expenses through March 31, 2017; however delays in cash collections, revenue, or unforeseen expenditures could negatively impact our estimate. We are in need of additional capital, however, there is no assurance that additional capital can be timely raised in an amount which is sufficient for us or on terms favorable to us and our stockholders, if at all. If we do not obtain additional capital, there is a significant doubt as to whether we can continue to operate as a going concern and we will need to curtail or cease operations or seek bankruptcy relief. If we discontinue operations, we may not have sufficient funds to pay any amounts to stockholders.
Our ability to fund our ongoing operations and continue as a going concern is dependent on increasing the number of members that are eligible for our programs by signing new contracts and generating fees from existing and new contracts and the success of management’s plan to increase revenue and continue to control expenses. We currently operate our OnTrak solutions in Florida, Georgia, Illinois, Kansas, Kentucky, Louisiana, Massachusetts, Missouri, New Jersey, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia, West Virginia and Wisconsin. We provide services to commercial (employer funded), managed Medicare Advantage, and managed Medicaid and duel eligible (Medicare and Medicaid) populations. We have generated fees from our launched solutions and expect to increase enrollment and fees throughout 2017. However, there can be no assurance that we will generate such fees or that new solutions will launch as we expect.
All inter-company transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America (“U.S. GAAP”) requires management to make estimates and assumptions that affect the reported amounts in the financial statements and disclosed in the accompanying notes. Significant areas requiring the use of management estimates include expense accruals, accounts receivable allowances, accrued claims payable, the useful life of depreciable and amortizable assets, the evaluation of asset impairment, the valuation of warrant liabilities, the valuation of derivative liabilites, and shared-based compensation. Actual results could differ from those estimates.
Revenue Recognition
Our Catasys contracts are generally designed to provide cash fees to us on a monthly basis based on enrolled members. To the extent our contracts may include a minimum performance guarantee; we reserve a portion of the monthly fees that may be at risk until the performance measurement period is completed. To the extent we receive case rates or other fees in advance that are not subject to performance guarantees, we recognize the case rate ratably over the twelve months of our program.
Cost of Services
Cost of healthcare services consists primarily of salaries related to our care coaches, healthcare provider claims payments, and fees charged by our third party administrators for processing these claims. Salaries and fees charged by our third party administrators for processing claims are expensed when incurred and healthcare provider claims payments are recognized in the period in which an eligible member receives services. We contract with doctors and licensed behavioral healthcare professionals, on a fee-for-service basis. We determine that a member has received services when we receive a claim or in the absence of a claim, by utilizing member data recorded in the eOnTrakTM database within the contracted timeframe, with all required billing elements correctly completed by the service provider.
Share-Based Compensation
Our 2010 Stock Incentive Plan, as amended (“the Plan”), provides for the issuance of up to 1,825,000 shares of our common stock. Incentive stock options (ISOs) under Section 422A of the Internal Revenue Code and non-qualified options (NSOs) are authorized under the Plan. We have granted stock options to executive officers, employees, members of our board of directors, and certain outside consultants. The terms and conditions upon which options become exercisable vary among grants, but option rights expire no later than ten years from the date of grant and employee and board of director awards generally vest over three to five years. At December 31, 2016, we had an aggregate of 1,464,089 vested and unvested shares outstanding and 303,674 shares available for future awards.
Total share-based compensation expense attributable to operations were $697,000 and $1.4 million for the years ended December 31, 2016 and 2015, respectively.
Stock Options – Employees and Directors
We measure and recognize compensation expense for all share-based payment awards made to employees and directors based on estimated fair values on the date of grant. We estimate the fair value of share-based payment awards using the Black-Scholes option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in the consolidated statements of operations.
There were no options granted for the year ended December 31, 2016 and 1.3 million options granted for the year ended December 31, 2015.
Stock Options and Warrants – Non-employees
We account for the issuance of stock options and warrants for services from non-employees by estimating the fair value of stock options and warrants issued using the Black-Scholes pricing model. This model’s calculations incorporate the exercise price, the market price of shares on grant date, the weighted average risk-free interest rate, expected life of the option or warrant, expected volatility of our stock and expected dividends.
For options and warrants issued as compensation to non-employees for services that are fully vested and non-forfeitable at the time of issuance, the estimated value is recorded in equity and expensed when the services are performed and benefit is received. For unvested shares, the change in fair value during the period is recognized in expense using the graded vesting method.
From time to time, we have retained terminated employees as part-time consultants upon their departure from the company. Because the employees continue to provide services to us, their options continue to vest in accordance with the original terms. Due to the change in classification of the option awards, the options are considered modified at the date of termination. The modifications are treated as exchanges of the original awards in return for the issuance of new awards. At the date of termination, the unvested options are no longer accounted for as employee awards under FASB’s accounting rules for share-based expense but are instead accounted for as new non-employee awards. The accounting for the portion of the total grants that have already vested and have been previously expensed as equity awards is not changed. There were no employees moved to consulting status for the twelve months ended December 31, 2016. There was one employee moved to consulting status for the twelve months ended December 31, 2015. The employee was 100% vested at the date of termination so no entry was recorded, and the employee is no longer a consultant as of December 31, 2016.
Income Taxes
We account for income taxes using the liability method in accordance with Accounting Standards Committee (“ASC”) 740 “Income Taxes”. To date, no current income tax liability has been recorded due to our accumulated net losses. Deferred tax assets and liabilities are recognized for temporary differences between the financial statement carrying amounts of assets and liabilities and the amounts that are reported in the tax returns. Deferred tax assets and liabilities are recorded on a net basis; however, our net deferred tax assets have been fully reserved by a valuation allowance due to the uncertainty of our ability to realize future taxable income and to recover our net deferred tax assets.
Basic and Diluted Income (Loss) per Share
Basic income (loss) per share is computed by dividing the net income (loss) to common stockholders for the period by the weighted average number of shares of common stock outstanding during the period. Diluted income (loss) per share is computed by dividing the net income (loss) for the period by the weighted average number of shares of common stock and dilutive common equivalent shares outstanding during the period.
Common equivalent shares, consisting of approximately 9,344,214 and 3,277,744 of shares as of December 31, 2016 and 2015, respectively, issuable upon the exercise of stock options and warrants, have been excluded from the diluted earnings per share calculation because their effect is anti-dilutive.
Cash and Cash Equivalents
We consider all highly liquid investments with an original maturity of three months or less to be cash equivalents. Financial instruments that potentially subject us to a concentration of credit risk consist of cash and cash equivalents. Cash is deposited with what we believe are highly credited, quality financial institutions. The deposited cash may exceed Federal Deposit Insurance Corporation (“FDIC”) insured limits. At December 31, 2016, cash and cash equivalents exceeding federally insured limits totaled $676,000.
Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Assets and liabilities recorded at fair value in the consolidated balance sheets are categorized based upon the level of judgment associated with the inputs used to measure fair value. The fair value hierarchy distinguishes between (1) market participant assumptions developed based on market data obtained from independent sources (observable inputs) and (2) an entity’s own assumptions about market participant assumptions developed based on the best information available in the circumstances (unobservable inputs). The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level I) and the lowest priority to unobservable inputs (Level III). The three levels of the fair value hierarchy are described below:
|
Level Input: |
Input Definition: | |
|
Level I |
Inputs are unadjusted, quoted prices for identical assets or liabilities in active markets at the measurement date. | |
|
Level II |
Inputs, other than quoted prices included in Level I, that are observable for the asset or liability through corroboration with market data at the measurement date. | |
|
Level III |
Unobservable inputs that reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. |
The following tables summarize fair value measurements by level at December 31, 2016 and 2015, respectively, for assets and liabilities measured at fair value on a recurring basis:
| Balance at December 31, 2015 | ||||||||||||||||
|
(Amounts in thousands) |
Level I |
Level II |
Level III |
Total |
||||||||||||
|
Certificates of deposit |
122 | - | - | 122 | ||||||||||||
|
Total assets |
122 | - | - | 122 | ||||||||||||
|
Warrant liabilities |
- | - | 509 | 509 | ||||||||||||
|
Derivative Liability |
- | - | 2,348 | 2,348 | ||||||||||||
|
Total liabilities |
- | - | 2,857 | 2,857 | ||||||||||||
| Balance at December 31, 2016 | ||||||||||||||||
|
(Amounts in thousands) |
Level I |
Level II |
Level III |
Total |
||||||||||||
|
Certificates of deposit |
106 | - | - | 106 | ||||||||||||
|
Total assets |
106 | - | - | 106 | ||||||||||||
|
Warrant liabilities |
- | - | 5,307 | 5,307 | ||||||||||||
|
Derivative Liability |
8,122 | 8,122 | ||||||||||||||
|
Total liabilities |
- | - | 13,429 | 13,429 | ||||||||||||
Financial instruments classified as Level III in the fair value hierarchy as of December 31, 2016, represent our liabilities measured at market value on a recurring basis which include warrant liabilities and derivative liabilities resulting from recent debt and equity financings. In accordance with current accounting rules, the warrant liabilities and derivative liabilities are being marked-to-market each quarter-end until they are completely settled or expire. The warrants and derivative liabilities are valued using the Black-Scholes option-pricing model, using both observable and unobservable inputs and assumptions consistent with those used in our estimate of fair value of employee stock options. See Warrant Liabilities below.
The following table summarizes our fair value measurements using significant Level III inputs, and changes therein, for the years ended December 31, 2016 and 2015:
|
Level III |
Level III |
||||||||
|
Warrant |
Derivative |
||||||||
|
(Dollars in thousands) |
Liabilities |
(Dollars in thousands) |
Liabilities |
||||||
|
Balance as of December 31, 2014 |
$ | 40,585 |
Balance as of December 31, 2014 |
$ | - | ||||
|
Issuance (exercise) of warrants, net |
2,712 | Issuance (exercise) of derivatives, net | 1,019 | ||||||
|
Change in fair value |
(11,665 | ) | Change in fair value | 2,761 | |||||
|
Exchange of warrants |
(31,123 | ) | Debt Modification | (1,432 | ) | ||||
|
Balance as of December 31, 2015 |
$ | 509 |
Balance as of December 31, 2015 |
$ | 2,348 | ||||
|
Issuance (exercise) of warrants, net |
4,821 | Issuance (exercise) of derivatives, net | - | ||||||
|
Change in fair value |
(2,093 | ) | Change in fair value | 5,774 | |||||
|
Warrant Exchanged |
2,070 | Debt Modification | - | ||||||
|
Balance as of December 31, 2016 |
$ | 5,307 |
Balance as of December 31, 2016 |
$ | 8,122 | ||||
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. Additions and improvements to property and equipment are capitalized at cost. Expenditures for maintenance and repairs are charged to expense as incurred. Depreciation is computed using the straight-line method over the estimated useful lives of the related assets, which range from two to seven years for furniture and equipment. Leasehold improvements are amortized over the lesser of the estimated useful lives of the assets or the related lease term, which is typically five to seven years.
Capital Leases
Assets held under capital leases include computer equipment, and are recorded at the lower of the net present value of the minimum lease payments or the fair value of the leased asset at the inception of the lease. Depreciation expense is computed using the straight-line method over the estimated useful lives of the assets. All lease agreements meet at least one of the four requirements of a capital lease in accordance with ASC 840 of the codification.
Warrant Liabilities
In conjunction with the Securities Purchase Agreements entered into in April 2015 (the “April Offering”), the Company issued five year warrants to purchase an aggregate of 530,303 shares of our common stock, at an exercise price of $2.00 per share, subject to adjustment, including for issuances of common stock and common stock equivalents below the then current conversion or exercise price, as the case may be (the “April 2015 Warrants”). The exercise price of the April 2015 Warrants was subsequently adjusted to $0.30 per share based upon the September Offering. In July 2016, 66,288 of the April 2015 Warrants were exercised via a cashless exercise, and 464,015 of the April 2015 Warrants remain outstanding as of December 31, 2016.
In May 2015, we entered into the Exchange Agreements whereby 21,277,220 warrants, at an exercise price of $0.58 per shares, issued by the Company between December 2011 and May 2014, were exchanged for 21,277,220 shares of common stock (the “Warrant Exchange”). We recognized a $0 and $4.4 million loss related to the Warrant Exchange for the years ended December 31, 2016 and 2015, respectively.
In July 2015, we entered into a $3.55 million 12% Original Issue Discount Convertible Debenture due January 18, 2016 (the “July 2015 Convertible Debenture”) with Acuitas Group Holdings, LLC (“Acuitas”), 100% owned by Terren S. Peizer, our Chairman and Chief Executive Officer, and five-year warrants to purchase 935,008 shares of our common stock, at an exercise price of $1.90 per share, subject to adjustment, including for issuances of common stock and common stock equivalents below the then current conversion or exercise price, as the case may be (the “July 2015 Warrants”).
The conversion price of the July 2015 Convertible Debenture is $1.90 per share, subject to adjustments, including for issuances of common stock and common stock equivalents below the then current conversion or exercise price, as the case may be. The July 2015 Convertible Debenture is unsecured, bears interest at a rate of 12% per annum payable in cash or shares of common stock, subject to certain conditions, at our option, and is subject to mandatory prepayment upon the consummation of certain future financings.
In September 2015, the conversion price of the July 2015 Convertible Debenture and the July 2015 Warrants were subsequently adjusted to $0.30 per share, based upon the Stock Purchase Agreement with Acuitas, relating to the sale and issuance of approximately 1.5 million shares of Common Stock for gross proceeds of $463,000 (the “September Offering”).
In March 2016, we entered into a promissory note with Acuitas Group Holdings, LLC (“Acuitas”), pursuant to which we received aggregate gross proceeds of $900,000 for the issuance of the note with a principal amount of $900,000 (the “March 2016 Promissory Note”). The March 2016 Promissory Note is due within 30 days of demand by Acuitas (the “Maturity Date”), and carries an interest rate on any unpaid principal amount of 8% per annum until the Maturity Date, after which the interest will increase to 12% per annum. In addition, we issued Acuitas five-year warrants to purchase an aggregate of 450,000 shares of our common stock, at an exercise price of $0.47 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “March 2016 Warrants”). The number of warrants were subsequently increased to 640,909 and the exercise price of the March 2016 Warrants was subsequently reduced to $0.33 per share based upon the May 2016 Promissory Note.
In April 2016, we amended and restated the March 2016 Promissory Note to increase the principal amount by $400,000, for a total of $1.3 million (the “April Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 200,000 shares of our common stock, at an exercise price of $0.47 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “April 2016 Warrants”). The number of warrants were subsequently increased to 284,848 and the exercise price of the April 2016 Warrants was subsequently reduced to $0.33 per share based upon the May 2016 Promissory Note.
In May 2016, we amended and restated the April 2016 Promissory Note to increase the principal amount by $405,000, for a total of $1.7 million (the “May Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 306,818 shares of our common stock, at an exercise price of $0.33 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “May 2016 Warrants”).
In June 2016, we amended and restated the May 2016 Promissory Note to increase the principal amount by $480,000, for a total of $2.2 million (the “June 2016 Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 363,636 shares of our common stock, at an exercise price of $0.33 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “June 2016 Warrants”).
In July 2016, we amended and restated the June 2016 Promissory Note to increase the principal amount by $570,000, for a total of $2.8 million (the “July 2016 Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 431,818 shares of our common stock at an exercise price of $0.33 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “July 2016 Warrants”)
In August 2016, we entered into subscription agreements with three accredited investors, including Shamus (collectively, the “Investors”), pursuant to which we issued to the Investors short-term senior promissory notes in the aggregate principal amount of $2.8 million (the “August 2016 Notes”) and five-year warrants to purchase up to an aggregate of 875,000 shares of our common stock, at an exercise price of $1.10 per share (the “August 2016 Warrants”).
The August 2016 Warrants include price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the August 2016 Warrants will be adjusted, in the event we issue shares of our common stock for consideration per share less than the then exercise price of the August 2016 Warrants, to the lowest consideration per share for the shares issued or sold in such transaction. The price protection will be in effect until the earliest of (i) the termination date of the August 2016 Warrants, (ii) such time as the Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange.
In addition, in August 2016, Acuitas agreed to exchange its July 2016 Promissory Note for a short-term senior promissory note, in the aggregate principal amount of $2.8 million plus accrued interest, in the form substantially identical to the form of the August 2016 Notes. Acuitas also agreed to exchange certain of its outstanding warrants to purchase an aggregate of 2,028,029 shares of our common stock at an exercise price of $0.33 per share for warrants to purchase an aggregate of 2,993,561 shares of common stock at an exercise price of $1.10 per share, in the form substantially identical to the form of the August 2016 Warrants.
In December 2016, we exchanged the August 2016 Notes issued to the Investors, which had an aggregate outstanding principal amount of $5.6 million, for (i) 8% Convertible Debentures in the same principal amount due on March 15, 2017 (the “Debentures”) and (ii) five-year warrants to purchase shares of the Company’s common stock in amount equal to forty percent (40%) of the initial number of shares of common stock, or 2,022,835 warrants, issuable upon conversion of each Investor’s Debentures, at an exercise price of $1.10 per share (the “December 2016 Warrants”).
The December 2016 Warrants include a price protection provision pursuant to which, subject to certain exempt issuances, the then exercise price of the December 2016 Warrants will be adjusted if the Company issues shares of common stock at a price that is less than the then exercise price of the December 2016 Warrants. Such price protection provisions will remain in effect until the earliest of (i) the termination date of the December 2016 Warrants, (ii) such time as the December 2016 Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange.
In December 2016, we entered into an agreement with Shamus pursuant to which the Company received gross proceeds of $300,000 for the sale of (i) an 8% Series B Convertible Debenture due March 31, 2017 (the “December 2016 Convertible Debenture”) and (ii) five-year warrants to purchase shares of the Company’s common stock in an amount equal to seventy-five percent (75%) of the initial number of shares of common stock, or 264,706 warrants, issuable upon the conversion of the December 2016 Convertible Debenture, at an exercise price of $0.85 per share (the “Shamus Warrants”).
The Shamus Warrants include price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the Shamus Warrants will be adjusted if the Company issues shares of common stock at a price that is less than the then exercise price of the Shamus Warrants. Such mechanism will remain in effect until the earliest of (i) the termination date of the Shamus Warrants, (ii) such time as the Shamus Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange.
The warrant liabilities were calculated using the Black-Scholes model based on upon the following assumptions:
|
December 31, 2016 |
December 31, 2015 |
|||||||||
|
Expected volatility |
104.31% |
|
133.19% | |||||||
|
Risk-free interest rate |
1.20% | - | 1.93% |
|
0.65 | - | 1.76% | |||
|
Weighted average expected lives in years |
2.25 | - | 4.99 | 0.99 | - | 4.29 | ||||
|
Expected dividend |
0% |
|
0% | |||||||
For the years ended December 31, 2016 and 2015, we recognized a gain of $2.1 million and a gain of $11.7 million, respectively, related to the revaluation of our warrant liabilities.
Concentration of Credit Risk
Financial instruments, which potentially subject us to a concentration of risk, include cash and accounts receivable. All of our customers are based in the United States at this time and we are not subject to exchange risk for accounts receivable.
The Company maintains its cash in domestic financial institutions subject to insurance coverage issued by the Federal Deposit Insurance Corporation (FDIC). Under FDIC rules, the company is entitled to aggregate coverage as defined by the Federal regulation per account type per separate legal entity per financial institution. The Company has incurred no losses as a result of any credit risk exposures.
For the year ended December 31, 2016, two customers accounted for approximately 78% of revenues and two customers accounted for approximately 81% of accounts receivable.
For the year ended December 31, 2015, three customers accounted for approximately 82% of revenues and three customers accounted for approximately 90% of accounts receivable.
Derivative Liability
In July 2015, we entered into a $3.55 million 12% Original Issue Discount Convertible Debenture due January 18, 2016 with Acuitas (the “July 2015 Convertible Debenture”). The conversion price of the July 2015 Convertible Debenture is $1.90 per share, subject to adjustments, including for issuances of common stock and common stock equivalents below the then current conversion or exercise price, as the case may be. In October 2015, we entered into an amendment of the July 2015 Convertible Debenture which extended the maturity date of the July 2015 Convertible Debenture from January 18, 2016 to January 18, 2017. In addition, the conversion price of the July 2015 Convertible Debenture was subsequently adjusted to $0.30 per share. The July 2015 Convertible Debenture is unsecured, bears interest at a rate of 12% per annum payable in cash or shares of common stock, subject to certain conditions, at our option, and is subject to mandatory prepayment upon the consummation of certain future financings. The derivative liability associated with the July 2015 Convertible Debenture was calculated using the Black-Scholes model based upon the following assumptions:
|
December 31, 2016 |
December 31, 2015 |
|||||||
|
Expected volatility |
104.31 |
% |
133.19 | % | ||||
|
Risk-free interest rate |
0.44 |
% |
0.23 | % | ||||
|
Weighted average expected lives in years |
0.05 | 1.05 | ||||||
|
Expected dividend |
0 |
% |
0 | % | ||||
The expected volatility assumption for the twelve months ended December 31, 2016 was based on the historical volatility of our stock, measured over a period generally commensurate with the expected term. The weighted average expected lives in years for 2016 reflect the application of the simplified method set out in Security and Exchange Commission (SEC) Staff Accounting Bulletin (SAB) 107 (and as amended by SAB 110), which defines the life as the average of the contractual term of the options and the weighted average vesting period for all option tranches. We use historical data to estimate the rate of forfeitures assumption for awards granted to employees.
For the twelve months ended December 31, 2016 and 2015, we recognized a loss of $5.8 million and $2.8 million related to the revaluation of our derivative liability, respectively.
Recently Issued or Newly Adopted Accounting Pronouncements
In April 2016, the FASB issued Accounting Standards Update (“ASU”) 2016-10, Revenue from Contracts with Customers (Topic 606), which amends certain aspects of the Board’s new revenue standard, ASU 2014-09, Revenue from Contracts with Customers. The standard should be adopted concurrently with adoption of ASU 2014-09, which is effective for annual and interim periods beginning after December 15, 2017. Early adoption is permitted. The Company has not yet selected a transition method nor has it determined the effect of the standard on its ongoing financial reporting.
In March 2016, the FASB issued ASU 2016-09, Compensation — Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting (“ASU 2016-09”), which outlines new provisions intended to simplify various aspects related to accounting for share-based payments and their presentation in the financial statements. The standard is effective for the Company for fiscal years beginning after December 15, 2016, and interim periods within those annual periods. Early adoption is permitted. The adoption of ASU 2016-09 did not have a material effect on our consolidated financial positon or results of operations.
In February 2015, the FASB issued ASU, Consolidation (Topic 810): Amendments to the Consolidation Analysis (“ASU 2015-02”). ASU 2015-02 modifies existing consolidation guidance for reporting organizations that are required to evaluate whether they should consolidate certain legal entities. ASU 2015-02 is effective for fiscal years and interim periods within those years beginning after December 15, 2015, and requires either a retrospective or a modified retrospective approach to adoptions. Early adoption is permitted. The adoption of ASU 2015-02 did not have a material effect on our consolidated financial position or results of operations.
In August 2014, the FASB issued FASB ASU 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (“ASU 2014-15”). ASU 2014-15 changes the disclosure of uncertainties about an entity’s ability to continue as a going concern. Under U.S. GAAP, continuation of a reporting entity as a going concern is presumed as the basis for preparing financial statements unless and until the entity’s liquidation becomes imminent. Even if an entity’s liquidation is not imminent, there may be conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern. Because there is no guidance in U.S. GAAP about management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern or to provide related note disclosures, there is diversity in practice whether, when, and how an entity discloses the relevant conditions and events in its financial statements. As a result, these changes require an entity’s management to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that financial statements are issued. Substantial doubt is defined as an indication that it is probable that an entity will be unable to meet its obligations as they become due within one year after the date that financial statements are issued. If management has concluded that substantial doubt exists, then the following disclosures should be made in the financial statements: (i) principal conditions or events that raised the substantial doubt, (ii) management’s evaluation of the significance of those conditions or events in relation to the entity’s ability to meet its obligations, (iii) management’s plans that alleviated the initial substantial doubt or, if substantial doubt was not alleviated, management’s plans that are intended to at least mitigate the conditions or events that raise substantial doubt, and (iv) if the latter in (iii) is disclosed, an explicit statement that there is substantial doubt about the entity’s ability to continue as a going concern. ASU 2014-15 is effective for periods beginning after December 15, 2016. The adoption of ASU 2014-15 did not have a material effect on our consolidated financial position or results of operations.
Note 2. Accounts Receivable
Accounts receivables consisted of the following as of December 31, 2016 and 2015:
|
December 31, |
||||||||
|
(in thousands) |
2016 |
2015 |
||||||
|
Healthcare fees |
$ | 1,050 | $ | 587 | ||||
|
Other |
2 | 3 | ||||||
|
Total receivables |
$ | 1,052 | $ | 590 | ||||
|
Less allowance for doubtful accounts |
- | - | ||||||
|
Total receivables, net |
$ | 1,052 | $ | 590 | ||||
We use the specific identification method for recording the provision for doubtful accounts, which was $0 as of December 31, 2016 and 2015.
Note 3. Property and Equipment
Property and equipment consisted of the following as of December 31, 2016 and 2015:
|
(in thousands) |
2016 |
2015 |
||||||
|
Furniture and equipment |
$ | 1,712 | $ | 1,585 | ||||
|
Leasehold improvements |
318 | 318 | ||||||
|
Total property and equipment |
2,030 | 1,903 | ||||||
|
Less accumulated depreciation and amortization |
(1,620 | ) | (1,491 | ) | ||||
|
Total property and equipment, net |
$ | 410 | $ | 412 | ||||
Depreciation expense was $141,000 and $110,000 for the years ended December 31, 2016 and 2015, respectively.
Note 4. Capital Lease Obligations
We lease certain computer equipment under agreements entered into during 2016 that are classified as capital leases. The computer equipment under capital leases is included in furniture and equipment on our consolidated balance sheets and was $103,000 and $110,000 at December 31, 2016 and 2015, respectively. Accumulated depreciation of the leased equipment at December 31, 2016 and 2015 was approximately $47,000 and $43,000, respectively.
The future minimum lease payments required under the capital leases and the present values of the net minimum lease payments as of December 31, 2016, is as follows:
|
(in thousands) |
Amount |
|||
|
Year ending December 31, |
||||
|
2017 |
$ | 46 | ||
|
2018 |
34 | |||
|
2019 |
2 | |||
|
Total minimum lease payments |
82 | |||
|
Less amounts representing interest |
(11 | ) | ||
|
Capital lease obligations, net of interest |
71 | |||
|
Less current maturities of capital lease obligations |
(40 | ) | ||
|
Long-term capital lease obligations |
$ | 31 | ||
Note 5. Income Taxes
As of December 31, 2016, the Company had net federal operating loss carry forwards and state operating loss carry forwards of approximately $222 million and $170 million, respectively. The net federal operating loss carry forwards begin to expire in 2024, and net state operating loss carry forwards begin to expire in 2016. The majority of the foreign net operating loss carry forwards expire over the next seven years.
The primary components of temporary differences which give rise to our net deferred tax assets are as follows:
|
2016 |
2015 |
|||||||
|
(in thousands) |
||||||||
|
Federal, state and foreign net operating losses |
$ | 78,466 | $ | 78,474 | ||||
|
Stock based compensation |
7,429 | 7,879 | ||||||
|
Accrued liabilities |
664 | 585 | ||||||
|
Other temporary differences |
4,894 | 3,045 | ||||||
|
Valuation allowance |
(91,453 | ) | (89,983 | ) | ||||
| $ | - | $ | - | |||||
The Company has provided a valuation allowance in full on its net deferred tax assets in accordance with ASC 740 Income Taxes. Because of the Company's continued losses, management assessed the realizability of its net deferred tax assets as being less than the more-likely-than-not criteria set forth by ASC 740. Furthermore, certain portions of the Company's net operating loss carryforwards were acquired, and therefore subject to further limitation set forth under the federal tax code, which could further limit the Company's ability to realize its deferred tax assets.
A reconciliation between the statutory federal income tax rate and the effective income tax rate for the years ended December 31, is as follows
|
2016 |
2015 |
|||||||
|
Federal statutory rate |
-34.0 | % | -34.0 | % | ||||
|
State taxes, net of federal benefit |
26.2 | % | 16.8 | % | ||||
|
Non-deductible goodwill |
0.0 | % | 0.0 | % | ||||
|
ISO / ESPP |
0.2 | % | 2.1 | % | ||||
|
Other |
-0.5 | % | -27.0 | % | ||||
|
Change in valuation allowance |
8.0 | % | 42.1 | % | ||||
|
Tax provision |
-0.1 | % | 0.0 | % | ||||
Current accounting rules require that companies recognize in the consolidated financial statements the impact of a tax position, if that position is more likely than not of being sustained on audit, based on the technical merits of the position. We file income tax returns in the U.S. federal jurisdiction and various state and foreign jurisdictions. Tax years that remain subject to examinations by tax authorities are 2011 through 2015. The federal and material foreign jurisdictions statutes of limitations began to expire in 2011. There are no current income tax audits in any jurisdictions for open tax years and, as of December 31, 2016, there have been no material changes to our tax positions.
The Company has adopted guidance issued by the FASB that clarifies the accounting for uncertainty in income taxes recognized in an enterprise's financial statements and prescribes a recognition threshold of more likely than not and a measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. In making this assessment, a company must determine whether it is more likely than not that a tax position will be sustained upon examination, based solely on the technical merits of the position and must assume that the tax position will be examined by taxing authorities. Our policy is to include interest and penalties related to unrecognized tax benefits in income tax expense. There were no interest and penalties for the years ended December 31, 2016 and 2015, respectively. The Company files income tax returns with the Internal Revenue Service (“IRS”) and the state of California. For jurisdictions in which tax filings are prepared, the Company is no longer subject to income tax examinations by state tax authorities for tax years through 2010, and by the IRS for tax years through 2011. The Company’s net operating loss carryforwards are subject to IRS examination until they are fully utilized and such tax years are closed.
Note 6. Equity Financings
In October 2015, we entered into Stock Purchase Agreements (the “Purchase Agreements”) with each of Acuitas, Shamus, and Steve Gorlin, pursuant to which the Company received gross proceeds of $2.0 million for the sale of approximately 6.7 million shares of the Company’s common stock, at a purchase price of $0.30 per share.
In September 2015, we entered into a Stock Purchase Agreement with Acuitas, relating to the sale and issuance of approximately 1.5 million shares of common stock for gross proceeds of $463,000 (the “September Offering”). In May 2015, we entered into the Exchange Agreements whereby 21,277,220 warrants, at an exercise price of $0.58 per shares, issued by the Company between December 2011 and May 2014, were exchanged for 21,277,220 shares of common stock to eliminate the liability associated with these warrants.
Note 7. Share-based Compensation
The Plan provides for the issuance of up to 1,825,000 shares of our common stock. Incentive stock options, under Section 422A of the Internal Revenue Code, non-qualified options, stock appreciation rights, limited stock appreciation rights and restricted stock grants are authorized under the Plan. We grant all such share-based compensation awards at no less than the fair market value of our stock on the date of grant, and have granted stock and stock options to executive officers, employees, members of our Board of Directors and certain outside consultants. The terms and conditions upon which options become exercisable vary among grants; however, option rights expire no later than ten years from the date of grant and employee and Board of Director awards generally vest over three to five years on a straight-line basis. At December 31, 2016, we had 1,464,089 vested and unvested stock options outstanding and 303,674 shares reserved for future awards. Total share-based compensation expense amounted to $697,000 million and $1.4 million for the years ended December 31, 2016 and 2015, respectively.
Stock Options – Employees and Directors
There were no options issued to employees or directors during 2016.
There were 250,000 options issued to employees during 2015.
For the twelve months ended December 31, 2015, we granted 1,050,000 to our non-employee directors.
Stock option activity for employee and director grants is summarized as follows:
|
Weighted Avg. |
||||||||
|
Shares |
Exercise Price |
|||||||
|
Balance, December 31, 2014 |
378,000 | $ | 19.59 | |||||
|
2015 |
||||||||
|
Granted |
1,300,000 | 2.20 | ||||||
|
Cancelled/Expired |
(207,000 | ) | 3.29 | |||||
|
Balance, December 31, 2015 |
1,471,000 | $ | 6.51 | |||||
|
2016 |
||||||||
|
Granted |
- | - | ||||||
|
Cancelled/Expired |
(7,000 | ) | 11.31 | |||||
|
Balance, December 31, 2016 |
1,464,000 | $ | 6.49 | |||||
The weighted average remaining contractual life and weighted average exercise price of options outstanding as of December 31, 2016 were as follows:
|
Options Outstanding |
Options Exercisable |
||||||||||||||||||||||
|
Range of Exercise Prices |
Shares |
Weighted Average Remaining Life (yrs) |
Weighted Average Price |
Shares |
Weighted Average Price |
||||||||||||||||||
| $0.00 | to | $20.00 | 1,455,000 | 7.07 | $ | 5.48 | 1,293,000 | $ | 5.89 | ||||||||||||||
| $20.01 | to | $1,000.00 | 9,000 | 2.03 | 158.98 | 9,000 | 158.98 | ||||||||||||||||
| 1,464,000 | 7.04 | $ | 6.46 | 1,302,000 | $ | 6.99 | |||||||||||||||||
Share-based compensation expense relating to stock options granted to employees and directors was $697,000 and $1.4 million for the years ended December 31, 2016 and 2015, respectively.
As of December 31, 2016, there was $318,000 of unrecognized compensation costs related to non-vested share-based compensation arrangements granted to employees and directors under the Plan. These costs are expected to be recognized over a weighted-average period of 1.3 years.
Stock Options and Warrants – Non-employees
In addition to stock options granted under the Plan, we have also granted options and warrants to purchase our common stock to certain non-employees that have been approved by our Board of Directors. There were no options granted during 2016 and 2015, respectively.
Warrants granted to non-employees outstanding as of December 31, 2016 and 2015, respectively, are summarized as follows:
|
December 31, 2016 |
||||||||
|
Description |
Shares |
Weighted Average Exercise Price |
||||||
|
Warrants issued in connection with equity offering |
- | $ | - | |||||
|
Warrants issued in connection with debt agreement |
6,156,102 | 0.85 | ||||||
|
Warrants issued for services |
- | - | ||||||
| 6,156,102 | $ | 0.85 | ||||||
|
December 31, 2015 |
||||||||
|
Description |
Shares |
Weighted Average Exercise Price |
||||||
|
Warrants issued in connection with equity offering |
- | $ | - | |||||
|
Warrants issued in connection with debt agreement |
1,465,311 | 0.30 | ||||||
|
Warrants issued for services |
341,251 | 2.16 | ||||||
| 1,806,562 | $ | 0.65 | ||||||
There were no warrants to purchase common stock issued for consulting services for the twelve months ended December 31, 2016. There were 300,000 warrants to purchase common stock issued for investor relations services for the twelve months ended December 31, 2015.
Share-based compensation expense relating to stock options and warrants granted to non-employees amounted to $0 and $3,000 for the years ended December 31, 2016 and 2015, respectively.
Common Stock
In October 2015, we issued 200,000 common shares in connection with the April Offering.
In May 2015, we entered into the Exchange Agreements whereby 21,277,220 warrants, at an exercise price of $0.58 per shares, issued by the Company between December 2011 and May 2014, were exchanged for 21,277,220 shares of common stock to eliminate the liability associated with these warrants.
During 2016 and 2015, we issued 235,000 and 76,000 shares of common stock, respectively, for consulting services valued at $235,000 and $172,000, respectively. Generally, the costs associated with shares issued for services are being amortized to the related expense on a straight-line basis over the related service periods.
Employee Stock Purchase Plan
Our qualified employee stock purchase plan (ESPP), approved by our Board of Directors and shareholders and adopted in June 2006, provides that eligible employees (employed at least 90 days) have the option to purchase shares of our common stock at a price equal to 85% of the lesser of the fair market value as of the first day or the last day of each offering period. Purchase options are granted semi-annually and are limited to the number of whole shares that can be purchased by an amount equal to up to 10% of a participant’s annual base salary. As of December 31, 2016, there were no shares of our common stock issued pursuant to the ESPP. There was no share-based compensation expense relating to the ESPP for the years ended December 31, 2016 and 2015, respectively.
Note 8. Commitments and Contingencies
Operating Lease Commitments
We incurred rent expense of approximately $296,000 and $294,000 for the years ended December 31, 2016 and 2015, respectively.
Our principal executive and administrative offices are located in Los Angeles, California and consist of leased office space totaling approximately 9,120 square feet. The initial term of the lease expires in April 2019. Our base rent is currently approximately $31,000 per month, subject to annual adjustments.
Rent expense is calculated using the straight-line method based on the total minimum lease payments over the initial term of the lease. Landlord tenant improvement allowances and rent expense exceeding actual rent payments are accounted for as deferred rent liability in the balance sheet and amortized on a straight-line basis over the initial term of the respective leases.
Future minimum payments, by year and in the aggregate, under non-cancelable operating leases with initial or remaining terms of one year or more, consist of the following at December 31, 2016:
|
(In thousands) |
||||
|
Year |
Amount |
|||
|
2017 |
$ | 376 | ||
|
2018 |
$ | 387 | ||
|
2019 |
$ | 99 | ||
Clinical Research Commitments
None.
Legal Proceedings
From time to time, we may be involved in certain legal actions and claims arising in the ordinary course of business. The Company was not a party to any specific legal actions or claims at December 31, 2016.
Note 9. Related Party Disclosure
Mr. Gorlin, an affiliate of the company, entered into securities purchase agreements during the fiscal year ended December 31, 2015, and received approximately 300,000 shares of common stock in exchange for gross proceeds of approximately $90,000.
Terren Peizer, Chairman and Chief Executive Officer, transferred his securities ownership in Catasys to Acuitas from Crede Capital Group, LLC during 2015. Mr. Peizer owns 100% of both entities.
In August 2016, Acuitas loaned us $225,000. No terms were discussed nor were any agreements executed in connection with such loan, but the $225,000 was paid back out of the August 2016 Notes.
In August 2016, Acuitas, agreed to exchange its existing promissory note for short-term senior promissory notes, in the aggregate principal amount of $2.8 million plus accrued interest, in the form substantially identical to the form of the August 2016 Notes. Acuitas also agreed to exchange certain of its outstanding warrants to purchase an aggregate of 2,028,029 shares of our common stock at an exercise price of $0.33 per share, for warrants to purchase an aggregate of 2,993,561 shares of our common stock at an exercise price of $1.10 per share, in the form substantially identical to the form of the August 2016 Warrants.
In December 2016, we exchanged the August 2016 Notes issued to the Acuitas, which had an aggregate outstanding principal amount of $2.8 million, for (i) 8% Convertible Debentures in the same principal amount due on March 15, 2017 (the “Debentures”) and (ii) five-year warrants to purchase shares of the Company’s common stock in amount equal to forty percent (40%) of the initial number of shares of common stock issuable upon conversion of each Investor’s Debentures, at an exercise price of $1.10 per share (the “December 2016 Warrants”).
Acuitas entered into securities purchase agreements as of December 31, 2015 and received approximately 6,953,334 shares of common stock in exchange for gross proceeds of approximately $2.1 million. In addition, Acuitas received warrants to purchase an aggregate 935,008 shares of common stock, at a price of $0.30 per share, as of December 31, 2015.
In addition, we have a $3.7 million Convertible Debenture outstanding with Acuitas, which includes $577,000 in interest as of December 31, 2016. We also have accounts payable outstanding with Mr. Peizer for travel and expenses of $194,000 and $130,000 as of December 31, 2016 and 2015, respectively.
In August 2016, we entered into subscription agreements with Shamus, pursuant to which we issued short-term senior promissory notes in the aggregate principal amount of $1.0 million and five-year warrants to purchase up to an aggregate of 318,182 shares of our common stock, at an exercise price of $1.10 per share. 3
In December 2016, we exchanged the subscription agreement with Shamus, which had an aggregate outstanding principal amount of $1.0 million, for (i) 8% Convertible Debentures in the same principal amount due on March 15, 2017 (the “Debentures”) and (ii) five-year warrants to purchase shares of the Company’s common stock in amount equal to forty percent (40%) of the initial number of shares of common stock issuable, or 363,636 warrants, upon conversion of each, at an exercise price of $1.10 per share.
In addition, in December 2016, we entered into an agreement with Shamus pursuant to which we received gross proceeds of $300,000 for the sale of (i) an 8% Series B Convertible Debenture due March 31, 2017 (the “December 2016 Convertible Debenture”) and (ii) five-year warrants to purchase shares of the Company’s common stock in an amount equal to seventy-five percent (75%) of the initial number of shares of common stock issuable, or 264,706 warrants, upon the conversion of the December 2016 Convertible Debenture, at an exercise price of $0.85 per share (the “Shamus Warrants”).
Shamus entered into securities purchase agreements during the fiscal years ended December 31, 2015 and received approximately 956,667 shares of common stock in exchange for gross proceeds of approximately $287,000.
Note 10. Short-term Debt
In April 2015, we entered into a Securities Purchase Agreement with several institutional accredited investors, pursuant to which we received aggregate gross proceeds of $2.0 million from the investors for the sale of approximately $2.12 million principal amount of 12% Original Issue Discount Convertible Debentures due January 18, 2016 (the “April 2015 Bridge Notes”) and the April 2015 Warrants. The closing of the April 2015 Bridge Notes transaction occurred on April 17, 2015. We received aggregate net proceeds of $1,815,000. We used $560,000 of the net proceeds to repay our outstanding indebtedness due to Acuitas incurred by way of short term, interest free loans in the first and second quarters of 2015.
The conversion price of the April 2015 Bridge Notes and the exercise price of the April 2015 Warrants was $2.00 per share, subject to adjustment, including for issuances of common stock and common stock equivalents below the then current conversion or exercise price, as the case may be. The April 2015 Bridge Notes were unsecured, bear interest at a rate of 12% per annum payable quarterly in cash or shares of common stock, subject to certain conditions, at our option, and were subject to mandatory prepayment upon the consummation of certain future financings. The exercise price of the April 2015 Warrants was subsequently adjusted to $0.30 per share based upon the September Offering.
In July 2015, we entered into a promissory note with Acuitas, pursuant to which we received gross proceeds of $3.35 million for the sale of $3.35 million in principal amount (the “Promissory Note”). The Promissory Note was due on August 21, 2015, and carried an interest rate on any unpaid principal amount of 8% per annum until the maturity date, after which the interest rate would increase to 12% per annum. We used approximately $2.2 million of the net proceeds of this transaction to redeem the April 2015 Bridge Notes. Following the redemption, all of the April 2015 Bridge Notes were extinguished.
In July 2015, we issued the July 2015 Convertible Debenture and the July 2015 Warrants for the Promissory Note.
The conversion price of the July 2015 Convertible Debenture was $1.90 per share, subject to adjustments, including for issuances of common stock and common stock equivalents below the then current conversion or exercise price, as the case may be. The July 2015 Convertible Debenture is unsecured, bears interest at a rate of 12% per annum payable in cash or shares of common stock, subject to certain conditions, at our option, and is subject to mandatory prepayment upon the consummation of certain future financings.
The conversion price of the July 2015 Convertible Debenture and the exercise price of the July 2015 Warrants were subsequently adjusted to $0.30 per share based upon the September Offering.
In October 2015, we amended the July 2015 Convertible Debenture which extended the maturity date of the July 2015 Convertible Debenture from January 18, 2016 to January 18, 2017 and extended the date we must consummate a public offering from December 31, 2015 to June 30, 2016. In accordance with ASC 470-50, Debt Modifications and Extinguishments, we recognized a $195,000 loss on extinguishment of debt in connection with the loan modification.
In March 2016, we entered into a promissory note with Acuitas Group Holdings, LLC (“Acuitas”), pursuant to which we received aggregate gross proceeds of $900,000 for the issuance of the note with a principal amount of $900,000 (the “March 2016 Promissory Note”). The March 2016 Promissory Note is due within 30 days of demand by Acuitas (the “Maturity Date”), and carries an interest rate on any unpaid principal amount of 8% per annum until the Maturity Date, after which the interest will increase to 12% per annum. In addition, we issued Acuitas five-year warrants to purchase an aggregate of 450,000 shares of our common stock, at an exercise price of $0.47 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “March 2016 Warrants”). The number of warrants were subsequently increased to 640,909 and the exercise price of the March 2016 Warrants was subsequently reduced to $0.33 per share based upon the May 2016 Promissory Note.
In April 2016, we amended and restated the March 2016 Promissory Note to increase the principal amount by $400,000, for a total of $1.3 million (the “April Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 200,000 shares of our common stock, at an exercise price of $0.47 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “April 2016 Warrants”). The number of warrants were subsequently increased to 284,848 and the exercise price of the April 2016 Warrants was subsequently reduced to $0.33 per share based upon the May 2016 Promissory Note.
In May 2016, we amended and restated the April 2016 Promissory Note to increase the principal amount by $405,000, for a total of $1.7 million (the “May Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 306,818 shares of our common stock, at an exercise price of $0.33 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “May 2016 Warrants”).
In June 2016, we amended and restated the May 2016 Promissory Note to increase the principal amount by $480,000, for a total of $2.2 million (the “June 2016 Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 363,636 shares of our common stock, at an exercise price of $0.33 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “June 2016 Warrants”).
In July 2016, we amended and restated the June 2016 Promissory Note to increase the principal amount by $570,000, for a total of $2.8 million (the “July 2016 Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 431,818 shares of our common stock at an exercise price of $0.33 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “July 2016 Warrants”).
In August 2016, Acuitas loaned us $225,000. No terms were discussed nor were any agreements executed in connection with such loan, but the $225,000 was paid back out of the August 2016 Notes.
In August 2016, we entered into subscription agreements (each, the “Subscription Agreement”) with three accredited investors, including Shamus, (collectively, the “Investors”), pursuant to which we issued to the Investors short-term senior promissory notes in the aggregate principal amount of $2.8 million (the “August 2016 Notes”) and five-year warrants to purchase up to an aggregate of 875,000 shares of our common stock, at an exercise price of $1.10 per share (the “August 2016 Warrants”).
The August 2016 Warrants include price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the August 2016 Warrants will be adjusted, in the event we issue shares of our common stock for consideration per share less than the then exercise price of the August 2016 Warrants, to the lowest consideration per share for the shares issued or sold in such transaction. The price protection will be in effect until the earliest of (i) the termination date of the August 2016 Warrants, (ii) such time as the Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange.
In addition, in August 2016, Acuitas agreed to exchange its July 2016 Promissory Note for a short-term senior promissory note in the aggregate principal amount of $2.8 million including accrued interest, in the form substantially identical to the form of the August 2016 Notes. Acuitas also agreed to exchange certain of its outstanding warrants to purchase an aggregate 2,028,029 shares of our common stock at an exercise price of $0.33 per share for warrants to purchase an aggregate 2,993,561 shares of common stock at an exercise price of $1.10 per share.
In December 2016, we exchanged the August 2016 Notes issued to the Investors, which had an aggregate outstanding principal amount of $5.6 million, for (i) 8% Convertible Debentures in the same principal amount due on March 15, 2017 (the “Debentures”) and (ii) five-year warrants to purchase shares of the Company’s common stock in amount equal to forty percent (40%) of the initial number of shares of common stock, or 2,022,835 warrants, issuable upon conversion of each Investor’s Debentures, at an exercise price of $1.10 per share (the “December 2016 Warrants”).
The December 2016 Warrants include a price protection provision pursuant to which, subject to certain exempt issuances, the then exercise price of the December 2016 Warrants will be adjusted if the Company issues shares of common stock at a price that is less than the then exercise price of the December 2016 Warrants. Such price protection provisions will remain in effect until the earliest of (i) the termination date of the December 2016 Warrants, (ii) such time as the December 2016 Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange.
In December 2016, we entered into an agreement with Shamus pursuant to which the Company received gross proceeds of $300,000 for the sale of (i) an 8% Series B Convertible Debenture due March 31, 2017 (the “December 2016 Convertible Debenture”) and (ii) five-year warrants to purchase shares of the Company’s common stock in an amount equal to seventy-five percent (75%) of the initial number of shares of common stock, or 264,706 warrants, issuable upon the conversion of the December 2016 Convertible Debenture, at an exercise price of $0.85 per share (the “Shamus Warrants”).
The Shamus Warrants include price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the Shamus Warrants will be adjusted if the Company issues shares of common stock at a price that is less than the then exercise price of the Shamus Warrants. Such mechanism will remain in effect until the earliest of (i) the termination date of the Shamus Warrants, (ii) such time as the Shamus Warrants are exercised or (iii) contemporaneously with the listing of our shares of common stock on a registered national securities exchange.
Note 11. Subsequent Events
Amendments to Outstanding Warrants and Extension of Existing Debentures
In March 2017, we entered into amendments with the holders of certain outstanding warrants issued on April 17, 2015 and July 30, 2015 to eliminate certain anti-dilution provisions in such warrants, which caused us to reflect an associated liability of $5.3 million on our balance sheet as of December 31, 2016. Such amendments are contingent upon and will not take effect until the closing of this offering, which must occur on or before April 30, 2017. For each warrant share underlying the warrants so amended, the holder received the right to purchase an additional .2 shares of common stock. Two of the holders of such warrants, which owners hold warrants to purchase an aggregate of 11,049 shares of common stock, did not agree to the amendment. The warrant holders agreeing to the amendment include Acuitas Group Holdings, LLC (“Acuitas”), one hundred percent (100%) of which is owned by Terren S. Peizer, Chairman and Chief Executive Officer of the Company, and another accredited investor, who will own warrants to purchase 187,002 and 79,545 shares of the Company’s common stock, respectively, if and when the amendments take effect.
Additionally, in March 2017, we and the holders of an aggregate of approximately $10 million of our existing convertible debentures agreed to extend the maturity dates of such debentures until the earlier of the closing of this offering or April 30, 2017.
Financing Activities
In January 2017, we entered into a Subscription Agreement (the “Subscription Agreement”) with Acuitas, pursuant to which the Company will receive aggregate gross proceeds of $1,300,000 (the “Loan Amount”) in consideration of the issuance of (i) an 8% Series B Convertible Debenture due March 31, 2017 (the “January 2017 Convertible Debenture”) and (ii) five-year warrants to purchase shares of the Company’s common stock in an amount equal to one hundred percent (100%) of the initial number of shares of common stock issuable upon the conversion of the January 2017 Convertible Debenture, at an exercise price of $0.85 per share (the “January 2017 Warrants”). The Loan Amount is payable in tranches through March 2017. In addition, any warrants issued in conjunction with the December 2016 Convertible Debenture currently outstanding with Acuitas have been increased by an additional 25% warrant coverage, exercisable for an aggregate of 827,293 shares of the Company’s common stock.
The January 2017 Warrants include, among other things, price protection provisions pursuant to which, subject to certain exempt issuances, the then exercise price of the January 2017 Warrants will be adjusted if the Company issues shares of common stock at a price that is less than the then exercise price of the January 2017 Warrants. Such price protection provisions will remain in effect until the earliest of (i) the termination date of the January 2017 Warrants, (ii) such time as the January 2017 Warrants are exercised or (iii) contemporaneously with the listing of the Company’s shares of common stock on a registered national securities exchange.
In connection with the Subscription Agreement described above, the number of Shamus Warrants were increased from 75% to 100% warrant coverage, exercisable for an aggregate of 352,941 shares of the Company’s common stock.
2017 Stock Incentive Plan
On February 27, 2017, the Board of Directors and our stockholders approved the adoption of the 2017 Stock Incentive Plan (the “2017 Plan”). The 2017 Plan allows the Company, under the direction of the Board of Directors or a committee thereof, to make grants of stock options, restricted and unrestricted stock and other stock-based awards to employees, including the Company’s executive officers, consultants and directors. The 2017 Plan allows for the issuance of up to 14,000,000 additional shares of Common Stock pursuant to new awards granted under the 2017 Plan and up to approximately 1,500,000 shares of Common Stock that are represented by options outstanding under the 2010 Plan (defined below) that are forfeited, expire or are cancelled without delivery of shares of Common Stock or which result in the forfeiture of shares of Common Stock back to the Company. This description is qualified in its entirety by reference to the actual terms of the 2017 Plan, a copy of which is attached as Exhibit B to the Company’s preliminary Information Statement on Schedule 14C, filed with the Securities and Exchange Commission on February 28, 2017.
New Directors
On March 11, 2017, our Board of Directors appointed Marc Cummins and Richard J. Berman. to serve on the Board of Directors and its Audit Committee, effective immediately. There are no arrangements or understandings between Messrs. Cummins or Berman and any other person pursuant to which they were appointed as directors of the Company. There are no transactions to which the Company is a party and in which Messrs. Cummins or Berman have a material interest that are required to be disclosed under Item 404(a) of Regulation S-K. Mr. Cummins previously served on the Board of Directors until his resignation on December 15, 2010, and Mr. Berman has not previously held any position at the Company. Neither individual has family relations with any directors or executive officers of the Company.
Reverse Stock Split
A 1:6 reverse stock split of the Company’s common stock and a corresponding decrease in the number of shares of the Company’s common stock that the Company is authorized to issue will be effected in connection with the Company’s public offering of its common stock that is described in the Prospectus of which these financial statements form a part. The reverse split will combine each 6 shares of the Company’s issued and outstanding common stock into 1 share of common stock. No fractional shares will be issued in connection with the reverse split, and any fractional shares resulting from the reverse split will paid in cash. The reverse split will be effective upon the filing of a certificate of amendment to the Company’s Certificate of Incorporation with the Delaware Secretary of State. The Company has not reflected the effect of the proposed 1:6 reverse split of its common stock in these financial statements.
|
|
[___] Shares of
Common Stock
 |
PROSPECTUS
Joseph Gunnar & Co.
___, 2017
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table indicates the expenses to be incurred in connection with the offering described in this registration statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimated except the Securities and Exchange Commission registration fee, the Financial Industry Regulatory Authority, Inc., or FINRA, filing fee and the NASDAQ Capital Market listing fee.
|
Amount to be paid |
||||
|
SEC registration fee |
$ | 2,000.00 | ||
|
FINRA filing fee |
3,087.50 | |||
|
NASDAQ Capital Market listing fee |
* | |||
|
Blue sky qualification fees and expenses |
* | |||
|
Printing and engraving expenses |
* | |||
|
Legal fees and expenses |
* | |||
|
Accounting fees and expenses |
* | |||
|
Transfer agent and registrar fees and expenses |
* | |||
|
Miscellaneous expenses |
* | |||
|
Total |
$ | * | ||
* To be filed by amendment.
Item 14. Indemnification of Directors and Officers.
The certificate of incorporation and the by-laws of our company, each as amended to date, provide that our company will indemnify, to the fullest extent permitted by the General Corporation Law of the State of Delaware, each person who is or was a director, officer, employee or agent of our company, or who serves or served any other enterprise or organization at the request of our company. Pursuant to Delaware law, this includes elimination of liability for monetary damages for breach of the directors’ fiduciary duty of care to our company and its stockholders. These provisions do not eliminate the directors’ duty of care and, in appropriate circumstances, equitable remedies such as injunctive or other forms of non-monetary relief will remain available under Delaware law. In addition, each director will continue to be subject to liability for breach of the director’s duty of loyalty to our company, for acts or omissions not in good faith or involving intentional misconduct, for knowing violations of law, for any transaction from which the director derived an improper personal benefit, and for payment of dividends or approval of stock repurchases or redemptions that are unlawful under Delaware law. The provision also does not affect a director’s responsibilities under any other laws, such as the federal securities laws or state or federal environmental laws.
Section 145 of the Delaware General Corporation Law permits a corporation to indemnify any director or officer of a corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with any action, suit or proceeding brought by reason of the fact that such person is or was a director or officer of the corporation, if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, if he or she had no reasonable cause to believe his or her conduct was unlawful. In a derivative action (i.e., one brought by or on behalf of the corporation), indemnification may be provided only for expenses actually and reasonably incurred by any director or officer in connection with the defense or settlement of such an action or suit if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, except that no indemnification shall be provided if such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the Delaware Chancery Court or the court in which the action or suit was brought shall determine that such person is fairly and reasonably entitled to indemnity for such expenses despite such adjudication of liability.
Pursuant to Section 102(b)(7) of the Delaware General Corporation Law, Article VI of our certificate of incorporation eliminates the liability of a director to us or our stockholders for monetary damages for such a breach of fiduciary duty as a director, except for liabilities arising:
|
|
• |
from any breach of the director’s duty of loyalty to us or our stockholders; |
|
• |
from acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
|
• |
under Section 174 of the Delaware General Corporation Law; and |
|
• |
from any transaction from which the director derived an improper personal benefit. |
We have entered into agreements with our directors and executive officers that require us to indemnify these persons against expenses, judgments, fines, settlements and other amounts actually and reasonably incurred (including expenses of a derivative action) in connection with any proceeding, whether actual or threatened, to which any such person may be made a party by reason of the fact that the person is or was a director or officer of our company or any of our affiliated enterprises, provided the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to our company’s best interests and, with respect to any criminal proceeding, had no reasonable cause to believe that his or her conduct was unlawful. The indemnification agreements will also establish procedures that will apply if a claim for indemnification arises under the agreements.
We maintain a policy of directors’ and officers’ liability insurance that insures its directors and officers against the cost of defense, settlement or payment of a judgment under some circumstances.
The foregoing discussion of our certificate of incorporation, by-laws, indemnification agreements and Delaware law is not intended to be exhaustive and is qualified in its entirety by such certificate of incorporation, by-laws, indemnification agreements or law.
Reference is made to our undertakings in Item 17 with respect to liabilities arising under the Securities Act.
Reference is also made to the form of underwriting agreement filed as Exhibit 1.1 to this registration statement for the indemnification agreements between us and the underwriters.
Item 15. Recent Sales of Unregistered Securities.
The following summarizes all sales of unregistered securities by us within the past three years:
In January 2014, we entered into securities purchase agreements with several investors, including Crede III, relating to the sale and issuance of an aggregate of 287,357 shares of common stock, and warrants (the “January Warrants”) to purchase an aggregate of 287,357 shares of common stock at an exercise price of $3.48 per share for aggregate gross proceeds of approximately $1.0 million (the “January Offering”). The January Warrants expire in January 2019, and contain anti-dilution provisions. As a result, if we, in the future, issue or grant any rights to purchase any of our common stock, or other security convertible into our common stock, for a per share price less than the exercise price of the January Warrants, the exercise price of the January Warrants will be reduced to such lower price, subject to customary exceptions. The January Offering provides that in the event that we effectuate a Reverse Split and the VWAP period declines from the closing price on the trading date immediately prior to the effective date of the Reverse Split, that we shall issue the Adjustment Shares, as described above. The common stock and the warrants were issued pursuant to the exemption afforded by Rule 506 of Regulation D promulgated under the Securities Act.
In May 2014, we entered into securities purchase agreements with several investors, including Crede III and Shamus, relating to the sale and issuance of an aggregate of 431,035 shares of common stock and warrants (the “May Warrants”) to purchase an aggregate of 431,035 shares of common stock at an exercise price of $3.48 per share for aggregate gross proceeds of approximately $1.5 million (the “May Offering”). The May Warrants expire in May 2019, and contain anti-dilution provisions. As a result, if we, in the future, issue or grant any rights to purchase any of our common stock, or other security convertible into our common stock, for a per share price less than the exercise price of the May Warrants, the exercise price of the May Warrants will be reduced to such lower price, subject to customary exceptions. The May Offering provide that in the event that we effectuate a Reverse Split and the VWAP period declines from the closing price on the trading date immediately prior to the effective date of the Reverse Split, that we shall issue the Adjustment Shares to the investors, as described above. Chardan Capital Markets, LLC acted as the placement agent for this Offering, in consideration for which it received 33,333 of our unregistered shares of common stock. The common stock and the warrants were issued pursuant to the exemption afforded by Rule 506 of Regulation D promulgated under the Securities Act.
In September 2014, we entered into the securities purchase agreements with several investors, relating to the sale and issuance of an aggregate of 125,000 shares of common at an exercise price of $12.00 per share for aggregate gross proceeds of approximately $1.5 million. Chardan Capital Markets, LLC acted as the sole placement agent for this offering, in consideration for which it received 9,166 of our unregistered shares of common stock. The shares of common stock were issued pursuant to the exemption afforded by Rule 506 of Regulation D promulgated under the Securities Act.
In December 2014, we entered into the securities purchase agreements with several investors, including Crede III and Steve Gorlin, a member of our board of directors, relating to the sale and issuance of an aggregate of 75,000 shares of common at an exercise price of $12.00 per share for aggregate gross proceeds of approximately $1.1 million. The shares of common stock were issued pursuant to the exemption afforded by Rule 506 of Regulation D promulgated under the Securities Act.
In April 2015, we entered into a Securities Purchase Agreement with several institutional accredited investors, pursuant to which we received aggregate gross proceeds of $2.0 million from the investors for the sale of approximately $2.12 million principal amount of 12% Original Issue Discount Convertible Debentures due January 18, 2016, or the Bridge Notes, and five-year warrants to purchase an aggregate of 88,384 shares of our shares of common stock, par value $0.0001 per share, or our common stock, at an exercise price of $12.00 per share, or the April 2015 Warrants. We received aggregate net proceeds of $1,815,000. The conversion price of the Bridge Notes and the exercise price of the April 2015 Warrants is $12.00 per share, subject to adjustment, including for issuances of common stock and common stock equivalents below the then current conversion or exercise price, as the case may be. The Bridge Notes are unsecured, bear interest at a rate of 12% per annum payable quarterly in cash or shares of common stock, subject to certain conditions, at our option, and are subject to mandatory prepayment upon the consummation of certain future financings. We are obligated to offer to repay the Bridge Notes, and any interest payable thereon, out of the proceeds of the offering contemplated by this prospectus. The investors will receive 33,334 additional shares of common stock if we have not consummated a public offering of at least $5.0 million in gross proceeds by September 30, 2015. The offering contemplated by this prospectus would fulfill that requirement if consummated prior to September 30, 2015. The investors are also entitled, until April 17, 2016, to participate in certain of our future financings. These securities were issued pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended, and Rule 506 of Regulation D promulgated thereunder.
On May 18, 2015, we entered into Warrant Exchange Agreements, or the exchange agreements, with 15 warrant holders that held warrants for the purchase of up to an aggregate of 3,546,200 shares of our common stock, at an exercise price of $3.48 per share, that were originally issued by us in private placements consummated on various dates between December 2011 and May 2014. Pursuant to the exchange agreements, the warrant holders collectively agreed to surrender for cancellation their warrants in exchange for an aggregate of 3,546,200 of the our shares of common stock. This transaction hereinafter the “Warrant Exchange”. The issuances of our shares of common stock to the warrant holders pursuant to the exchange agreements were made pursuant to the exemption from the registration requirements of the Securities Act of 1933, as amended, provided by Section 3(a)(9) thereof.
In March 2016, we entered into a promissory note with Acuitas, pursuant to which we received aggregate gross proceeds of $900,000 for the sale of $900,000 in principal amount (the "March 2016 Promissory Note"). The March 2016 Promissory Note is due within 30 business days of demand by Acuitas (the "Maturity Date"), and carries an interest rate on any unpaid principal amount of 8% per annum until the Maturity Date, after which the interest will increase to 12% per annum. In addition, we issued Acuitas five-year warrants to purchase an aggregate 75,000 shares of our common stock, at an exercise price of $2.82 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “March 2016 Warrants”). The number of warrants were subsequently increased to 106,818 and the exercise price of the March 2016 Warrants were subsequently reduced to $1.98 per share based upon the May 2016 Promissory Note. The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
In April 2016, we amended and restated the March 2016 Promissory Note to increase the amount by $400,000, for a total of $1.3 million (the “April 2016 Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 33,334 shares of our common stock, at an exercise price of $2.82 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “April 2016 Warrants”). The number of warrants were subsequently increased to 47,475 and the exercise price of the April 2016 Warrants were subsequently reduced to $1.98 per share based upon the May 2016 Promissory Note. The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
In May 2016, we amended and restated the April 2016 Promissory Note to increase the amount by $405,000, for a total of $1.7 million (the “May 2016 Promissory Note”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 51,136 shares of our common stock, at an exercise price of $1.98 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “May 2016 Warrants”). The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
In June 2016, we amended and restated the May 2016 Promissory Note to increase the amount by $480,000, for a total of $2.2 million (the “June 2016 Promissory Notes”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 60,606 shares of our common stock, at an exercise price of $1.98 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “June 2016 Warrants”). The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
In July 2016, we amended and restated the June 2016 Promissory notes to increase the amount by $570,000, for a total of $2.8 million (the “July 2016 Promissory Notes”). In connection with the amendment, we issued Acuitas five-year warrants to purchase an additional 71,970 shares of our common stock, at an exercise price of $1.98 per share, which warrants include, subject to certain exceptions, a full-ratchet anti-dilution protection (the “July 2016 Warrants”). The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
In August 2016, we entered into subscription agreements (each, the Subscription Agreement”) with three accredited investors, including Shamus, LLC, a company owned by David E. Smith, a member of our board of directors (collectively, the “Investors”), pursuant to which we issued to the Investors short-term senior promissory notes in the aggregate principal amount of $2,750,000 (the “Notes”) and five-year warrants to purchase aggregate of up to 145,834 shares of our common stock, at an exercise price of $6.60 per share (the “Warrants”). The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
In August 2016, we exchanged our $2.8 million July 2016 Promissory Notes for a senior promissory note in the aggregate principal amount of $2.8 million, in the form substantially identical to the Notes. Acuitas also agreed to exchange the July 2016 warrants for five-year warrants to purchase aggregate of up to 498,927 shares of our common stock, at an exercise price of $6.60 per share, in the form substantially identical to the Warrants. The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
In December 2016, we exchanged our senior promissory notes in the aggregate principal amount of $5.6 million for an 8% Series B Convertible Debenture and received five-year warrants to purchase an aggregate of up to 337,139 shares of our common stock, at an exercise price of $5.10 per share. The securities were issued without registration pursuant to exemption afforded by Section 3(a)(9) of the Securities Act of 1933, as amended.
In December 2016, we entered into a transaction with Shamus to which we received gross proceeds of $300,000 for the sale of an 8% Series B Convertible Denture and five-year warrants to purchase an aggregate of up to 44,118 shares of our common stock, at an exercise price of $5.10 per share. The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
In January 2017, we entered into a subscription agreement with Acuitas for which we will receive gross proceeds of $1.3 million for the sale of an 8% Series B Convertible Debentures and five-year warrants to purchase an aggregate of up to 254,902 shares of common stock, at an exercise price of $5.10 per share. The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
In connection with the January 2017 offering, Shamus received an additional five-year warrant to purchase an aggregate of up to 14,706 shares of common stock, at an exercise price of $5.10 per share. The warrants were issued without registration pursuant to exemption afforded by Section 4(a)(2) of the Securities Act of 1933, as amended.
Item 16. Exhibits and Financial Statements Schedules.
(a) Exhibits.
See the Exhibit Index immediately following the signature page hereto, which is incorporated into this Item 16(a) by reference.
(b) Financial Statements Schedules.
No financial statement schedules are provided because the information called for is not applicable or not required or is shown in the financial statements or the notes thereto.
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Los Angeles, California, on April 20, 2017.
|
|
|
CATASYS, INC. | ||
|
|
By: |
|
/s/ Terren S. Peizer | |
|
|
|
Terren S. Peizer | ||
|
Chairman of the Board of Directors and Chief Executive Officer (Principal Executive Officer) | ||||
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature |
|
Title(s) |
|
Date |
|
|
|
|
|
|
|
/s/ TERREN S. PEIZER |
|
Chairman of the Board of Directors |
|
April 20, 2017 |
|
Terren S. Peizer |
|
and Chief Executive Officer |
|
|
|
|
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ SUSAN ETZEL |
|
Chief Financial Officer |
|
April 20, 2017 |
|
Susan Etzel |
|
(Principal Financial and |
|
|
|
|
|
Accounting Officer) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ RICHARD A. ANDERSON |
|
President, Chief Operating Officer |
|
April 20, 2017 |
|
Richard A. Anderson |
|
and Director |
|
|
|
/s/ RICHARD BERMAN |
|
Director |
|
April 20, 2017 |
|
Richard Berman |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ DAVID E. SMITH |
|
Director |
|
April 20, 2017 |
|
David Smith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ MARVIN IGELMAN |
|
Director |
|
April 20, 2017 |
|
Marvin Igelman |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/ STEVE GORLIN |
|
Director |
|
April 20, 2017 |
|
Steve Gorlin |
|
|
|
|
|
/s/ RICHARD J. BERMAN |
|
Director |
April 20, 2017 | |
|
Richard J. Berman |
|
|||
|
/s/ MARC CUMMINS |
Director |
|
April 20, 2017 | |
|
Marc Cummins |
EXHIBIT INDEX UPDATE
|
No. |
Description | ||
|
1.1** |
Form of Underwriting Agreement. | ||
|
3.1 |
Certificate of Incorporation of Catasys, Inc., filed with the Secretary of State of the State of Delaware on September 29, 2003, incorporated by reference to exhibit of the same number of Catasys Inc.’s Form 8-K filed with the Securities and Exchange Commission on September 30, 2003. | ||
|
3.2 |
|
Certificate of Amendment to Certificate of Incorporation of Catasys, Inc., incorporated by reference to exhibit of the same number to Catasys, Inc.’s annual report on Form 10-K filed with the Securities and Exchange Commission for the year ended December 31, 2010. | |
|
3.3 |
|
Certificate of Amendment, as corrected by the Certificate of Correction, to Certificate of Incorporation of Catasys, Inc., incorporated by reference to exhibit of the same number to Catasys, Inc.’s Registration Statement on Form S-1/A filed with Securities and Exchange Commission on September 9, 2011. | |
|
3.4 |
|
Certificate of Amendment of the Certificate of Incorporation of Catasys, Inc., incorporated by reference to exhibit 3.1 of Catasys, Inc.’s current report on Form 8-K filed with the Securities and Exchange Commission on August 10, 2012. | |
|
3.5 |
|
Certificate of Amendment of the Certificate of Incorporation of Catasys, Inc., incorporated by reference to exhibit 3.1 of Catasys, Inc.’s current report on Form 8-K filed with the Securities and Exchange Commission on May 7, 2013. | |
|
3.6 |
|
By-Laws of Catasys, Inc., incorporated by reference to exhibit of the same number of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on September 30, 2003. | |
|
4.1 |
|
Specimen Common Stock Certificate, incorporated by reference to exhibit of the same number to Catasys Inc.’s annual report on Form 10-K filed with the Securities and Exchange Commission for the year ended December 31, 2005. | |
|
4.2 |
|
Form of Common Stock Purchase Warrant incorporated by reference to exhibit 4.2 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on July 31, 2015. | |
|
4.3 |
|
Form of 12% Original Issue Discount Convertible Debenture Due January 18, 2016 incorporated by reference to Exhibit 4.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on July 31, 2015. | |
|
4.4 |
|
Form of 8% Promissory Note, dated July 22, 2015, incorporated by reference to Exhibit 4.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on July 24, 2015. | |
|
4.5 |
|
Form on Common Stock Purchase Warrant incorporated by reference to Exhibit 4.2of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on April 21, 2015. | |
|
4.6 |
|
Form of 12% Original Issue Discount Convertible Debenture Due January 18, 2016 incorporated by reference to Exhibit 4.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on April 21, 2015. | |
|
4.7 |
|
Form of Warrant incorporated by reference to Exhibit 4.1 of Catasys, Inc.’s current report on Form 8-K filed with the Securities and Exchange Commission on February 3, 2014. | |
|
4.8 |
|
Form of Warrant incorporated by reference to Exhibit 4.1 of Catasys, Inc.’s current report on Form 8-K filed with the Securities and Exchange Commission on May 30, 2014. | |
|
4.9 |
|
Form of 8% Promissory Note, dated March 30, 2016, incorporated by reference to Exhibit 4.9 of Catasys, Inc.’s current report on form 10-K filed with the Securities and Exchange Commission on March 30, 2016. | |
|
4.10 |
Form of First Amendment and Restated 8% Promissory Note, dated April 27, 2016, incorporated by reference to Exhibit 4.1 of Catasys, Inc.’s current report on Form 10-Q filed with the Securities and Exchange Commission on May 13, 2016. | ||
|
4.11 |
Form of Common Stock Purchase Warrant, dated April 27, 2016, incorporated by reference to Exhibit 4.2 of Catasys, Inc.’s current report on Form 10-Q filed with the Securities and Exchange Commission on May 13, 2016. | ||
|
4.13 |
Form of 8% Promissory Note, dated March 30, 2016, incorporated by reference to Exhibit 4.3 of Catasys, Inc.’s Form 10-Q filed with the Securities and Exchange Commission on May 13, 2016. | ||
|
4.14 |
Form of Common Stock Purchase Warrant, dated March 30, 2016, incorporated by reference to Exhibit 4.4 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on May 13, 2016. | ||
|
4.15 |
Form of Second Amended and Restated Promissory Note, dated May 24, 2016, incorporated by reference to Exhibit 4.1 of Catasys Inc’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.16 |
Form of Common Stock Purchase Warrant, dated May 24, 2016, incorporated by reference to Exhibit 4.2 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.17 |
Form of Third Amended and Restated Promissory Note, dated June 2, 2016, incorporated by reference to Exhibit 4.3 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.18 |
Form on Common Stock Purchase Warrant, dated June 2, 2016, incorporated by reference to Exhibit 4.4 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.19 |
Form of Fourth Amended and Restated Promissory Note, dated June 22, 2016, incorporated by reference to Exhibit 4.5 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.20 |
Form of Common Stock Purchase Warrant, dated June 22, 2016, incorporated by reference to Exhibit 4.6 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.21 |
Form of Fifth Amended and Restated Promissory Note, dated July 5, 2016, incorporated by reference to Exhibit 4.7 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.22 |
Form of Common Stock Purchase Warrant, dated July 5, 2016, incorporated by reference to Exhibit 4.8 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.23 |
Form of Sixth Amended and Restated Promissory Note, dated July 21, 2016, incorporated by reference to Exhibit 4.9 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.24 |
Form of Common Stock Purchase Warrant, dated July 21, 2016, incorporated by reference to Exhibit 4.10 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.25 |
Form of Senior Promissory Note, dated August 15, 2016, incorporated by reference to Exhibit 4.13 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.26 |
Form of Common Stock Purchase Warrant, dated August 15, 2016, incorporated by reference to Exhibit 4.14 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||
|
4.27 |
Form of 8% Senior Convertible Debenture due March 15, 2017, incorporated by reference to Exhibit 4.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on December 23, 2016. | ||
|
4.28 |
Form of Common Stock Purchase Warrant, incorporated by reference to Exhibit 4.2 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on December 23, 2016. | ||
|
4.29 |
8% Series B Convertible Debenture, dated December 29, 2016, incorporated by reference to Exhibit 4.1 of Catasys, Inc’s Form 8-K filed with the Securities and Exchange Commission on December 30, 2016. | ||
|
4.30 |
Common Stock Purchase Warrant, dated December 29, 2016, incorporated by reference to Exhibit 4.2 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on December 30, 2016. | ||
|
4.31 |
8% Series B Convertible Debenture, dated January 31, 2017, incorporated by reference to Exhibit 4.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on February 1, 2017. | ||
|
4.32 |
Common Stock Purchase Warrant, dated January 31, 2017, incorporated by reference to Exhibit 4.2 filed with the Securities and Exchange Commission on February 1, 2017. | ||
| 4.33 | Form of Amendment to Warrant dated March 29, 2017, incorporated by reference to Exhibit 4.33 filed with the Securities and Exchange Commission on March 31, 2017. | ||
|
4.34** |
Form of Representative’s Warrant (included in Exhibit 1.1) | ||
|
5.1** |
Opinion of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. | ||
|
10.1# |
|
Employment Agreement between Catasys, Inc. and Terren S. Peizer, dated September 29, 2003, incorporated by reference to exhibit of the same number to Catasys Inc.’s annual report on Form 10-K filed with the Securities and Exchange Commission for the year ended December 31, 2005. | |
|
10.2# |
|
Employment Agreement between Catasys, Inc. and Richard A. Anderson, dated April 19, 2005, incorporated by reference to exhibit of the same number to Catasys Inc.’s annual report on Form 10-K filed with the Securities and Exchange Commission for the year ended December 31, 2005. | |
| 10.3# | Amendment to Employment Agreement of Richard A. Anderson, dated July 16, 2008, incorporated by reference to Exhibit 10.1 of Catasys Inc.’s current report on Form 8-K filed with the Securities and Exchange Commission on July 18, 2008. | ||||
|
10.4# |
Form of Stock Option Grant Notice, incorporated by reference to exhibit 10.4 of Catasys, Inc.'s Form 10-K filed with the Securities and Exchange Commission on March 31, 2015. | ||||
|
10.5# |
2010 Stock Incentive Plan incorporated by reference to exhibit C of Catasys, Inc.’s Information Statement on Schedule 14C filed with the Securities and Exchange Commission on June 4, 2012. | ||||
|
10.6 |
Amendment to 12% Original Issue Discount Convertible Debenture incorporated by reference to exhibit 10.2 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on October 16, 2015. | ||||
|
10.7 |
Securities Purchase Agreement, dated October 16, 2015, incorporated by reference to Exhibit 10.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on October 16, 2015. | ||||
|
10.8 |
Stock Purchase Agreement, dated September 17, 2015, incorporated by reference to Exhibit 10.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on September 18, 2015. | ||||
|
10.9 |
Securities Purchase Agreement between Catasys, Inc. and accredited investors dated July 30, 2015 incorporated by reference to Exhibit 10.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on July 31, 2015. | ||||
|
10.10 |
Form of Lock-Up Agreement incorporated by reference to Exhibit 10.2 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on May 20, 2015. | ||||
|
10.11 |
Form of Warrant Exchange Agreement incorporated by reference to Exhibit 10.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on May 20, 2015. | ||||
|
10.12 |
Securities Purchase Agreement between Catasys, Inc. and accredited investors dated April 16, 2015 incorporated by reference to Exhibit 10.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on April 21, 2015. | ||||
|
10.13 |
Office Lease between Catasys, Inc. and Trizec Wilshire Center, LLC dated November 6, 2013, incorporated by reference to Exhibit 10.1 of Catasys, Inc.’s quarterly report on Form 10-Q filed with the Securities and Exchange Commission on November 13, 2013. | ||||
|
10.14 |
First Amendment to the Office Lease between Catasys, Inc. and Trizec Wilshire Center, LLC dated March 6, 2015, incorporated by reference to exhibit 10.27 of Catasys, Inc.’s Form 10-K filed with the securities and Exchange Commission on March 31, 2015. | ||||
|
10.15 |
Form of Subscription Agreement, dated August 15, 2016, between Catasys, Inc. and accredited investors incorporated by reference to Exhibit 10.1 of Catasys Inc.’s Form 10-Q filed with the Securities and Exchange Commission on August 15, 2016. | ||||
|
10.16 |
Form of Securities and Exchange Agreement, between Catasys, Inc. and Acuitas Group Holdings, LLC, incorporated by reference to Exhibit 10.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on December 23, 2016. | ||||
|
10.17 |
Subscription Agreement, between Catasys, Inc. and Acuitas Group Holdings, LLC, incorporated by reference to Exhibit 10.1 of Catasys, Inc.’s Form 8-K filed with the Securities and Exchange Commission on February 1, 2017. | ||||
|
10.18 |
Form of Exchange Agreement, dated August 15, 2016, by and between Catasys, Inc. and Acuitas Group Holdings, LLC, incorporated by reference to Exhibit 4.6 of Catasys, Inc.’s Form 10-Q filed with the Securities and Exchange Commission on November 14, 2016. | ||||
|
10.19# |
2017 Stock Incentive Plan, incorporated by reference to Exhibit B of Catasys, Inc.’s Preliminary Information Statement on Schedule 14C filed with the Securities and Exchange Commission on February 28, 2017. | ||||
|
14.1 |
Code of Conduct and Ethics, incorporated by reference to exhibit of the same number of Catasys Inc.’s annual report on Form 10-K filed with the Securities and Exchange Commission for the year ended December 31, 2003. | ||||
|
21.1 |
Subsidiaries of the Company, incorporated by reference to Exhibit 21.1 of Catasys Inc.’s annual report on Form 10-K filed with the Securities and Exchange Commission on February 28, 2017. | ||||
|
23.1* |
Consent of Independent Registered Public Accounting Firm – Rose, Snyder & Jacobs LLP. | ||||
|
23.2** |
Consent of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. (contained in Exhibit 5.1). | ||||
|
24.1 |
Power of Attorney (see signature page to original filing of this Registration Statement on Form S-1). | ||||
|
101.INS* |
XBRL Instance Document | ||||
|
101.SCH* |
XBRL Taxonomy Extension Schema Document | ||||
|
101.CAL* |
XBRL Taxonomy Extension Calculation Linkbase Document | ||||
|
101.DEF* |
XBRL Taxonomy Extension Definition Linkbase Document | ||||
|
101.LAB* |
XBRL Taxonomy Extension Label Linkbase Document | ||||
|
101.PRE* |
XBRL Taxonomy Extension Presentation Linkbase Document | ||||
* Filed herewith.
** To be filed by amendment.
# Management contract or compensatory plan or arrangement.