Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AERIE PHARMACEUTICALS INC | d305020dex991.htm |
| 8-K - 8-K - AERIE PHARMACEUTICALS INC | d305020d8k.htm |
 Rhopressa TM (netarsudil ophthalmic solution) 0.02% Rocket 4 Phase 3 6-Month Topline Results 1 Exhibit 99.2 |
 Important
Information Any discussion of the potential use or expected success of our
product candidates is subject to our product candidates being approved by
regulatory authorities. The information in this presentation is current
only as of its date and may have changed or may change in the future. We
undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in
this presentation is accurate or complete.
Certain statements in this presentation are “forward-looking statements”
within the meaning of the federal securities laws. Words such as
“may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,”
“potential” or similar expressions are intended to identify
these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could
cause actual results to differ materially from those contemplated by the statements. In
evaluating these statements, you should specifically consider various
factors that may cause our actual results to differ materially from any
forward-looking statements. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk
Factors” and “Management’s Discussion and Analysis of Financial
Condition and Results of Operations.” In particular, the topline
Rocket 4 data presented herein is preliminary and based solely on
information available to us as of the date of this presentation and additional information about the results may be disclosed at any time. Such forward-looking statements only speak as of the date they
are made. We undertake no obligation to publicly update or revise any
forward-looking statements, whether because of new information,
future events or otherwise, except as otherwise required by law.
2 |
 Rhopressa TM Achieves Positive 6-Month Safety and Efficacy Results • Safety data over the 6 months were consistent with observations in previous Rhopressa TM 3-month and 12-month Phase 3 clinical trials • There were no drug-related serious adverse events and no evidence of treatment-related systemic effects • The main adverse event for Rhopressa™ was conjunctival hyperemia, which was reported in ~48% of patients, scored as mild for ~75% of the patients and sporadic • Rhopressa TM achieved primary efficacy endpoint at month 3 and performance at months 4, 5 and 6 remained within the non-inferiority range compared to timolol at baseline IOPs < 25 mmHg, and also < 27 mmHg • Rhopressa demonstrated stable and consistent efficacy across all baseline IOPs in the trial from Week 2 to Month 6 across multiple statistical analyses (PP/ITT/LOCF) 3 TM |
 Rocket 4
Trial Design Patients randomized
1:1 Primary endpoints: • Efficacy: Mean IOP at Weeks 2 and 6 and Month 3 for subjects with
baseline IOP > 20 mmHg and <25 mmHg
(N= 423 subjects per protocol)
• Safety: Ocular and systemic safety during a 6-month treatment period Patients with open angle glaucoma (OAG) or ocular hypertension (OHT) with IOP >20 mmHg and < 30 mmHg at 8am, N=708 subjects randomized at 52 US sites Rhopressa™ (AR-13324) 0.02% QD (PM) Timolol 0.5% BID (AM and PM) 4 ClinicalTrials.gov Identifier: NCT02558374 |
 Patient
Disposition (Topline 6-Month) Rhopressa
QD N = 351 Timolol BID N = 357 Completed Month 6 243 (69.2%) 314 (88.0%) Discontinued Prior to Month 6 108 (30.8%) 43 (12.0%) Discontinued Adverse Event Withdrawal of Consent Non-Compliant Lost to Follow-up Lack of Efficacy Disallowed Concurrent Medication Investigator Decision Protocol Violation Other 68 (19.4%) 12 (3.4%) 1 (0.3%) 1 (0.3%) 12 (3.4%) 1 (0.3%) 2 (0.6%) 5 (1.4%) 6 (1.7%) 8 (2.2%) 16 (4.5%) 2 (0.6%) 3 (0.8%) 1 (0.3%) 3 (0.8%) 4 (1.1%) 4 (1.1%) 2 (0.6%) 5 ++ Data on File Based on Rocket 4 Topline 6-month safety TM |
 Patient
Disposition (Topline 6-Month) (For Baseline IOP <25mmHg)
Rhopressa QD N = 214 Timolol BID N = 209 Completed Month 6 160 (74.8%) 188 (90.0%) Discontinued Prior to Month 6 54 (25.2%) 21 (10.0%) Discontinued Adverse Event Withdrawal of Consent Non-Compliant Lost to Follow-up Lack of Efficacy Disallowed Concurrent Medication Investigator Decision Protocol Violation Other 34 (15.9%) 7 (3.3%) 1 (0.5%) 0 3 (1.4%) 1 (0.5%) 2 (0.9%) 3 (1.4%) 3 (1.4%) 5 (2.4%) 7 (3.3%) 2 (1.0%) 1 (0.5%) 0 2 (1.0%) 1 (0.5%) 2 (1.0%) 1 (0.5%) 6 ++ Data on File Based on Rocket 4 Topline 3-month and 6-month safety, Rocket 1 3-month, Rocket 2 3-month
Discontinuation prior to Month 3 rate ~ 15% (in ROCKET 1, 2 and 4)
TM |
 Safety/Tolerability Overview of Rhopressa
TM (Topline 6-Month) • There were no drug-related serious adverse events (SAEs) • There was no evidence of treatment-related systemic effects (e.g., clinical laboratory or hematology values, heart rate or blood pressure) • The most common adverse event was conjunctival hyperemia with ~48% incidence and was scored as mild for ~75% of the patients – ~ 20% of all patients had hyperemia at baseline – Only ~10% of patients had hyperemia on each study visit day from week 2 to month 6 • Other ocular AEs – AEs occurring in ~5-25% of subjects receiving Rhopressa included: cornea verticillata, conjunctival hemorrhage, lacrimation increased, erythema of eyelid and vision blurred 7 ++Data on File Based on Rocket 4 Topline 6-month safety TM |
 Rhopressa TM Phase 3 Safety Profile (Topline 6-Month) Adverse Events
( 5% in any group) Rhopressa QD N = 351 Timolol BID N = 357 Eye Disorders Conjunctival Hyperemia 168 (47.9%) 33 (9.2%) Cornea Verticillata 86 (24.5%) 0 (0.0%) Conjunctival Hemorrhage 56 (16.0%) 11 (3.1%) Lacrimation Increased 26 (7.4%) 5 (1.4%) Erythema of Eyelid 26 (7.4%) 2 (0.4%) Vision Blurred 22 ( 6.3%) 4 (1.1%) Administration Site Conditions Instillation Site Pain 83 (23.6%) 92 (25.8%) Instillation Site Erythema 36 (10.3%) 4 (1.1%) Patients with known contraindications or hypersensitivity to timolol were excluded 8 ++Data on File Based on Rocket 4 Topline 6-month safety TM |
 9 |
 When
Present, ~75% of Rhopressa TM
Hyperemia Graded as Mild For illustrative purposes only Grade Image Description 0 None/Normal 1 Mild 2 Moderate 3 Severe 10 ++ Data on File Based on Rocket 4 Topline 6-month safety |
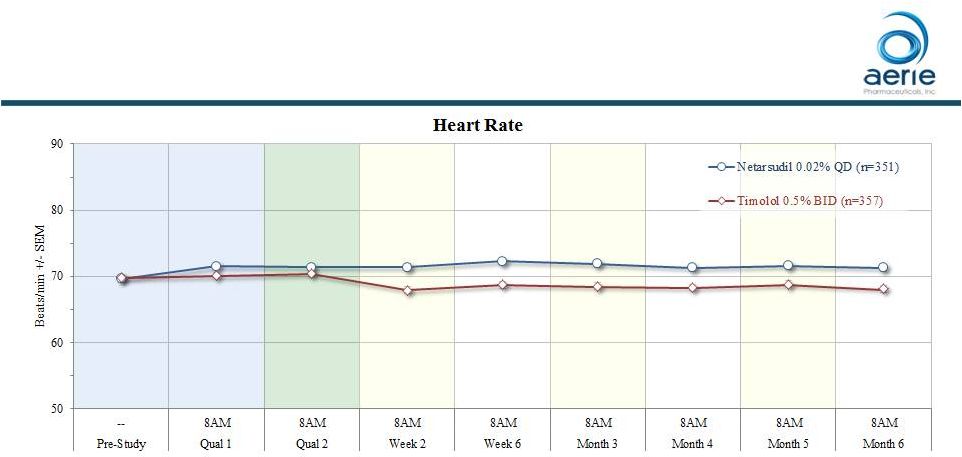 Timolol
Caused Statistically Significant Reduction in Heart Rate (Rocket 4)
• Timolol – reduced mean heart rate by 2 - 3 beats per minute (average across all patients; p < 0.0001) – maximum change from baseline ~40 beats per minute • Despite all measures to exclude patients with possible negative sensitivity to beta-blockers 11 ++ Data on File Based on Rocket 4 Topline 6-month safety |
 Rhopressa Achieved Non-Inferiority in the Primary Efficacy Analysis for Baseline IOP < 25 mmHg and Maintained Stable Efficacy Mean IOP at Each Time Point (PP) – Topline 6-Month 12 • Rhopressa™ performance remained within the non-inferiority range ++ Data on File Based on Rocket 4 Topline 6-month safety TM |
 Mean IOP
at Each Time Point (PP) – Topline 6-Month
13 ++ Data on File Based on Rocket 4 Topline 6-month safety Rhopressa TM Achieved Non-Inferiority for Baseline IOP < 27 mmHg and Maintained Stable Efficacy • Rhopressa™ performance remained within the non-inferiority range |
 Rhopressa TM Efficacy Stable through 6 Months 8am IOP Baseline < 27 mmHg BL W2 W6 M3 M4 M5 M6 ++ Data on File Based on Rocket 4 Topline 6-month 8am IOP 14 BL W2 W6 M3 M4 M5 M6 Baseline < 25 mmHg Rhopressa™ (n=186) Rhopressa™ (n=240) |
 Rhopressa TM Once-Daily Performance Summary To Date Well researched in over 2,000 clinical patients Well researched in over 2,000 clinical patients 200+ Ophthalmologists’ and Optometrists’ experience with the new drug class 200+ Ophthalmologists’ and Optometrists’ experience with the new drug class Once-daily consistent efficacy demonstrated in 4 Phase 3 trials (Rocket 1, 2, 4 and Mercury 1) Once-daily consistent efficacy demonstrated in 4 Phase 3 trials (Rocket 1, 2, 4 and Mercury 1) Stable efficacy through 12 months Stable efficacy through 12 months Well tolerated with no evidence of treatment-related serious or systemic effects Well tolerated with no evidence of treatment-related serious or systemic effects Continue to explore additional differentiating attributes e.g., 24-hour IOP control, trabecular outflow and anti-fibrotic effects Continue to explore additional differentiating attributes e.g., 24-hour IOP control, trabecular outflow and anti-fibrotic effects 15 |
