Attached files
| file | filename |
|---|---|
| EX-3.5 - EXHIBIT 3.5 - Protea Biosciences Group, Inc. | v463651_ex3-5.htm |
| EX-3.4 - EXHIBIT 3.4 - Protea Biosciences Group, Inc. | v463651_ex3-4.htm |
| EX-3.3 - EXHIBIT 3.3 - Protea Biosciences Group, Inc. | v463651_ex3-3.htm |
| EX-3.2 - EXHIBIT 3.2 - Protea Biosciences Group, Inc. | v463651_ex3-2.htm |
| 8-K - FORM 8-K - Protea Biosciences Group, Inc. | v463651_8k.htm |
Exhibit 3.1
AMENDED AND RESTATED
CONFIDENTIAL PRIVATE PLACEMENT MEMORANDUM

Protea Biosciences Group, Inc.
Up to $5,000,000
500 Units of Securities Consisting of
Shares of Common Stock and Warrants to Purchase Common Stock
This Amended and Restated Confidential Private Placement Memorandum together with all exhibits and documents incorporated by reference (the “Memorandum”) relates to an offering (“Offering”) by Protea Biosciences Group, Inc., a Delaware corporation (“”Protea,” the “Company,” “we,” “us” and “our”) of up to $5,000,000 of units of our securities (the “Units”), consisting of a maximum of (a) 66,666,667 shares of common stock, par value $0.0001 per share (the “Common Stock”), (b) 18 month warrants to purchase up to 66,666,667 shares of Common Stock at an exercise price of $0.09 per share (the “Class A Warrants”), and (c) five year warrants purchase up to 66,666,667 shares of Common Stock at an exercise price of $0.1125 per share (the “Class B Warrants” and together with the Class A Warrants the “Warrants”). The Common Stock and the Warrants are sometimes referred to herein collectively as the “Securities.”
A minimum of 50 Units for aggregate gross proceeds of $500,000 (the “Minimum Offering”) and a maximum of 500 Units for aggregate gross proceeds of $5,000,000 (the “Maximum Offering”) are being offered by the Company in this Offering. Each Unit consists of (a) 133,333.33shares of Common Stock, (b) Class A Warrants to purchase 133,333.33 shares of Common Stock at an exercise price of $0.09 per share, and (c) Class B Warrants to purchase 133,333.33 shares of Common Stock at an exercise price of $0.1125 per share. The minimum subscription from qualified investors shall be one full Unit for a minimum purchase price of $10,000. However, the Company and the Placement Agent described below may accept subscriptions from qualified investors for a lesser amount at their sole discretion. The Company and the Placement Agent reserve the right to reject any proposed subscription.
On October 24, 2016, we amended our Certificate of Incorporation which now currently permits us to issue up to 500,000,000 shares of Common Stock. Our 134,226,310 outstanding shares of Common Stock, plus all additional shares of Common Stock that are issuable upon conversion of currently outstanding notes and exercise of currently outstanding options and warrants (the “Common Stock Equivalents”) totals 243,957,380 shares of Common Stock. In addition, we intend to consummate a 1-for-25 reverse stock split of our outstanding Common Stock following the Termination Date of this Offering (the “Reverse Stock Split”), which reverse split has previously been authorized by our stockholders, thereby reducing the outstanding number of shares of Common Stock and Common Stock Equivalents to 19,742,923 shares of Common Stock after giving effect to the sale of the Maximum offering offered hereby. Consummation of the Reverse Stock Split will require (a) the filing of an amendment to our Certificate of Incorporation with the Delaware Secretary of State, and (b) obtaining the approval of the Financial Industry Regulatory Authority (“FINRA”).
The number of shares of Common Stock included in the Units and the number of shares of Common Stock issuable upon the exercise of the Warrants (the “Warrant Shares”) are each subject to equitable pro-rata adjustment based on the 1:25 Reverse Stock Split or any other subsequent forward or reverse stock split.
Upon acceptance by the Company after the date hereof of subscriptions from qualified investors for the Minimum Offering, the Placement Agent (as defined herein) and the Company shall have the right at any time and prior to the Termination Date (as defined below), to effect periodic closings (each a “Closing”) for subscriptions for Securities from investors until the earlier of (i) the date upon which subscriptions for the Maximum Offering offered hereunder have been accepted, (ii) December 31, 2016 (subject to the right of the Company and the Placement Agent to extend the Offering until March 31, 2017 without further notice to investors), or (iii) the date upon which the Company elects to terminate the Offering (the “Termination Date”). The Securities are being offered by the Company on an “all-or-none” basis for the Minimum Offering and on a “reasonable best efforts” basis for the Maximum Offering. No assurance can be given that all or any portion of the Securities offered hereby will be sold. The Company may terminate the Offering at any time even if Units having an aggregate purchase price equal to the Maximum Offering has not been sold. The subscription amount for the Units of Securities will be held in escrow for the benefit of subscribers by Signature Bank (the “Escrow Agent”) until completion of the Minimum Offering and satisfaction of all the conditions to each Closing.
Neither the Common Stock nor the Warrants included in the Units will be listed on any securities exchange. The Company’s Common Stock is currently traded on the OTC Markets under the symbol “PRGB.” On October 28, 2016, the last reported sale price of our Common Stock on the OTC Markets was $0.14 per share.
This Memorandum amends and restates in its entirety, the Company’s private placement memorandum dated September 29, 2016.
THE SECURITIES OFFERED HEREBY HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), OR THE SECURITIES LAWS OF ANY STATE AND MAY NOT BE OFFERED OR SOLD IN THE UNITED STATES OR TO UNITED STATES PERSONS UNLESS THE SECURITIES ARE REGISTERED UNDER THE SECURITIES ACT OR AN EXEMPTION FROM THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT IS AVAILABLE. THESE SECURITIES ARE BEING OFFERED AND SOLD IN RELIANCE ON EXEMPTIONS FROM THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND SUCH LAWS. NEITHER THE SECURITIES AND EXCHANGE COMMISSION (THE “COMMISSION”) NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES, NOR HAVE ANY OF THE FOREGOING PASSED UPON OR ENDORSED THE MERITS OF THE OFFERING OR THE ACCURACY OR ADEQUACY OF THIS MEMORANDUM. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE. THESE ARE SPECULATIVE SECURITIES WHICH INVOLVE A HIGH DEGREE OF RISK. ONLY THOSE INVESTORS WHO CAN BEAR THE LOSS OF THEIR ENTIRE INVESTMENT SHOULD INVEST IN THESE SECURITIES. PLEASE SEE THE SECTION ENTITLED “RISK FACTORS” BEGINNING ON PAGE 21 OF THIS PRIVATE PLACEMENT MEMORANDUM (THE “MEMORANDUM”).
|
Aggregate Offering Price (1) |
Placement Agent Commissions (2) |
Proceeds to the Company (3) (4) | |
| Minimum Offering | $500,000 | $60,000 | $440,000 |
| Maximum Offering | $5,000,000 | $600,000 | $4,400,000 |
The Units of Securities offered hereby are speculative securities and involve a high degree of risk. See “Risk Factors.”
(see footnotes on following page)
LAIDLAW & COMPANY (UK) LTD.
Placement Agent
the date of this Memorandum is October 31, 2016
| 2 |
| (1) | The price of the securities was determined by negotiation and may not bear any relationship to the price of our Common Stock, assets, book value or results of operations or any other generally accepted criteria of value. |
We have agreed to pay Laidlaw & Company (UK) Ltd. (the “Placement Agent” or “Laidlaw”) (i) a cash commission in the amount often percent (10%) of the gross proceeds of the Offering received from investors other than certain investors previously identified to the Placement Agent by the Company (“Protea Investors”), (ii) two percent (2%) of the gross proceeds of the Offering received from Protea Investors, and (iii) an activation fee of $25,000. In addition, the Placement Agent will be entitled to receive three (3) year warrants to purchase such number of shares of Common Stock of the Company equal to (x) ten percent (10%), in the case of investors other than Protea Investors, of the aggregate number of securities sold in the Offering at an exercise price equal to the lowest price per share of the securities sold in the Offering. The Placement Agent shall also be entitled to a non-allocable expense reimbursement in the amount of two percent (2%) of the gross proceeds of the Offering, including amounts received from Protea Investors. After the Termination Date, assuming the closing on at least $1,000,000 in gross proceeds for the Units, the Company will pay the Placement Agent a non-refundable financial advisory fee of $150,000, to be paid monthly, at the rate of $25,000 per month for a term of six months beginning on the Termination Date. For additional information regarding the Placement Agent, see the section entitled “Offering Period and Subscription Procedures.” We shall also issue legal counsel to the Placement Agent one Unit of the Securities at the closing of the Minimum Offering.
| (2) | The amount of total proceeds set forth in the above table does not include deductions for expenses related to this Offering, including filing, printing, current and accrued legal fees, accounting fees, transfer agent and other miscellaneous expenses, estimated to be approximately $400,000. |
| (3) | No assurance can be given that all or any portion of the Securities offered hereby will be sold. The Placement Agent and Company have established an escrow account (the “Escrow Account”) with Signature Bank as escrow agent. The subscription amount for the Securities will be paid to the Escrow Account by wire and held in escrow until satisfaction of all the conditions to a closing. |
The Securities offered hereby are speculative, involve a high degree of risk and should not be purchased by anyone who cannot afford the loss of their entire investment. Prospective investors should consider carefully the information under “Risk Factors” before purchasing such SECURITIES.
NONE OF THE SECURITIES HAVE been registered with or approved by the Commission or any state securities commission, nor has any commission or state authority passed on the accuracy or adequacy of this memorandum. Any representation to the contrary is a criminal offense. This memorandum does not constitute an offer in any jurisdiction in which an offer is not authorized.
SUBSCRIPTIONS WILL BE ACCEPTED ONLY FROM “ACCREDITED INVESTORS” AS DEFINED IN RULE 501 OF REGULATION D (SEE “INVESTOR SUITABILITY STANDARDS”).
This Memorandum contains forward-looking statements that involve risks and uncertainties. Our actual results may differ significantly from the results discussed in such forward-looking statements. Factors that might cause differences include, but are not limited to, those discussed in “Risk Factors.”
The Securities has not been registered under the Securities Act of 1933, as amended (the “Securities Act”) or any state securities laws. These securities are being offered and sold in reliance upon an exemption provided by section 4(A)(2) and Rule 506 of Regulation D of the securities act and similar available exemptions under the securities laws of the states relating to a transaction not involving a public offering. The Securities are subject to restrictions on transferability and resale and may not be transferred or resold except as permitted under the Securities Act pursuant to registration or exemption therefrom. The Securities has not been approved or disapproved by the Securities and Exchange Commission or any other federal or state regulatory authorities, nor have any federal or state authorities passed upon or endorsed the merits of this Offering, the Securities or the accuracy or adequacy of this Memorandum. Any representation to the contrary is a criminal offense.
Investment in the Securities offered by the Company involves a high degree of risk. In making an investment decision you must rely on your own examination of the issuer and the terms of this Offering, including the merits and risks involved. You should retain your own professional advisors to review and evaluate the financial, economic, tax and other consequences of this investment. You should not invest any funds in this Offering unless you can afford to lose your entire investment.
| 3 |
The Securities will be “restricted securities” for the purposes of federal and state securities laws. There is no public market for the Securities and it is expected that there will not be a market for the resale of the Securities in the near future. The Securities may not be offered, sold, or pledged except pursuant to an effective registration statement under the Securities Act, or an exemption from registration under the Securities Act. We may require an opinion of counsel that an exemption is available, and such counsel and opinion must be reasonably satisfactory to us. Therefore, you should only acquire the Securities for investment, solely for your own account, and without any view toward resale or distribution. You should be aware that you will be required to bear the financial risks of this investment for an indefinite period of time.
All documents referred to in this Memorandum are provided herewith, available upon request from the Company, or will be provided prior to the signing of the Subscription Agreement and should be reviewed by you and your advisors for a complete understanding of their provisions. The statements in this Memorandum with respect to any documents are necessarily summaries of the document’s terms and are qualified in their entirety by reference to the underlying document.
In making an investment decision, you must rely on your own examination of the Company and the terms of the Subscription Agreement, including the merits and risks involved. We will endeavor to make available to every investor, during the course of their investigation and prior to any purchase of Securities, the opportunity to ask questions of and receive answers concerning the terms and conditions of this Offering and to provide any appropriate additional information necessary to verify the accuracy of the information contained in this Memorandum. You and your advisors are encouraged to ask questions concerning this Offering and such other matters as you deem pertinent in connection with understanding the information in the Memorandum. We will make every effort to respond fully to such questions and supply all available information that you or your advisors reasonably request. You agree to advise us in writing if you are relying on any such information.
Any requests for additional information should be made to Mr. Stephen Turner, CEO of the Company at 1311 Pineview Drive, Suite 501, Morgantown, West Virginia 26505, telephone (304) 292-2226; Stephen.turner@proteabio.com.
The Securities are subject to prior sale, cancellation, withdrawal or modification of the Offering without notice. We may accept or reject subscriptions received in this Offering without notice, in whole or in part, for any reason. We are not required to accept subscriptions in the order in which they are received. Except as required by certain state’s securities laws, subscriptions that have we have accepted may not be withdrawn by any subscriber.
CONFIDENTIALITY AND LIMITATIONS OF THIS PRIVATE PLACEMENT MEMORANDUM
This Memorandum contains information that is confidential and proprietary to the Company. It discusses trade and business secrets of the Company and is intended for use only by the party to whom it is transmitted by our employees or agents, and only for the purposes of permitting such persons to decide whether to purchase the Securities described herein. This Memorandum may not be reproduced in whole or in part, or used for any other purpose; nor may any of its contents be disclosed without our prior written consent. The recipient agrees to return this Memorandum to us upon request. Acceptance of this Memorandum constitutes agreement to the above conditions.
The information contained in this Memorandum may at times represent our best estimate of our expected business performance, based upon assumptions believed to be reasonable. No representation or warranty is made, however, as to the accuracy or completeness of such assumptions, and nothing contained herein should be relied upon as a promise or representation as to any future performance or event. In addition, investors should take note that the information contained herein is only offered to be accurate as of the date of this Memorandum. Neither the delivery of this Memorandum, nor any sale made hereunder, shall, under any circumstances, create the implication that there has been no change in the affairs of our business, or that the information contained herein is correct as of any date other than the date of its creation referenced on the cover page.
The distribution of this Memorandum and this Offering of the securities described herein may be restricted by law in certain jurisdictions.
This Memorandum does not constitute, and may not be used for or in connection with, an offer or solicitation by anyone in any jurisdiction in which such offering or solicitation is not authorized, or to any person to whom it is unlawful to make such offer or solicitation. We have not taken any action that would permit an offering of the securities or the circulation or distribution of this Memorandum or any offering materials in relation to our business or the Securities in any country or jurisdiction where action for that purpose is required by law.
Prospective investors are not to construe the contents of this Memorandum as legal, investment or tax advice. Prospective investors should consult their advisors as to legal, investment, tax and related matters concerning an investment by such prospective investors in the company.
| 4 |
information provided outside this Memorandum
No person has been authorized to give any information outside of this Memorandum or to make any representation in connection with this Offering. If any such information is given or if any such representations are made, such information or representation must not be relied upon as having been authorized by us.
Any sale of Securities shall be made solely by and in accordance with the terms of the Subscription Agreement, which may be accepted or rejected, in whole or in part, by us in our sole discretion for any reason.
SPECIAL NOTE ON FORWARD-LOOKING STATEMENTS
This Memorandum contains “forward-looking statements” as defined in Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Such forward-looking statements involve risks and uncertainties and include, but are not limited to, statements regarding future events and our plans and expectations. The words “believes,” “anticipates,” “expects,” “intends,” “projects,” “plans,” “forecasts,” “estimates,” “will” and other similar expressions identify forward-looking statements pertaining to, among other things, financial projections regarding our operations, and our allocation and use of the funds raised in this Offering. Such statements reflect only current views with respect to future events and financial performance or operations and speak only as of the date the statements are made.
Our actual results may differ materially from our forward looking statements. Factors that may cause or contribute to such differences include, but are not limited to, those discussed in the section “Risk Factors” as well as those discussed elsewhere in this Memorandum and the documents referenced herein.
Although we believe that the assumptions underlying our forward-looking statements are reasonable, any of the assumptions could prove inaccurate. There can be no assurance that the results contemplated in such forward-looking statements will be realized and our actual results are likely to differ from those anticipated in our forward-looking statements. In addition, as disclosed under “Risk Factors,” the business and operations of the Company are subject to substantial risks that increase the uncertainties inherent in the forward-looking statements included in this Memorandum.
The inclusion of forward-looking information should not be regarded as a representation by us or any other person that the future events, plans or expectations contemplated will be achieved. We disclaim any obligation to revise forward-looking statements to reflect subsequent events or circumstances or the occurrence of unanticipated events.
BY ACCEPTING DELIVERY OF THIS MEMORANDUM, OR ANY OTHER MATERIAL IN CONNECTION WITH THIS OFFERING, THE OFFEREE AGREES TO KEEP STRICTLY CONFIDENTIAL THE CONTENTS OF THIS MEMORANDUM AND SUCH OTHER MATERIAL, AND TO NOT DISCLOSE SUCH CONTENTS TO ANY THIRD PARTY OR OTHERWISE USE THE CONTENTS FOR ANY PURPOSE OTHER THAN EVALUATION BY SUCH OFFEREE OF AN INVESTMENT IN THE SHARES. INVESTORS SHALL NOT COPY ALL OR ANY PORTION OF THIS MEMORANDUM OR ANY SUCH OTHER MATERIAL AND RETURN THIS MEMORANDUM AND ALL SUCH OTHER MATERIAL TO THE COMPANY IF THE OFFEREE DOES NOT SUBSCRIBE TO PURCHASE ANY SHARES, OR IF THE OFFEREE’S SUBSCRIPTION IS NOT ACCEPTED, OR IF THIS OFFERING IS TERMINATED OR WITHDRAWN.
EXCLUSIVE NATURE OF PRIVATE PLACEMENT MEMORANUDM
EXCEPT FOR SUCH INFORMATION THAT IS CONTAINED IN THIS MEMORANDUM OR IS OTHERWISE PROVIDED BY THE COMPANY IN RESPONSE TO REQUESTS FROM PROSPECTIVE INVESTORS OR THEIR ADVISORS, NO PERSON OR ENTITY HAS BEEN AUTHORIZED TO GIVE ANY INFORMATION OR TO MAKE ANY REPRESENTATIONS CONCERNING THE COMPANY OR THE SECURITIES REFERENCED HEREIN, AND IF GIVEN OR MADE, SUCH INFORMATION OR REPRESENTATION(S) SHOULD NOT BE RELIED UPON AS HAVING BEEN AUTHORIZED BY THE COMPANY.
THE COMPANY DISCLAIMS ANY AND ALL LIABILITIES FOR REPRESENTATIONS OR WARRANTIES EXPRESSED OR IMPLIED, CONTAINED IN, OR OMISSIONS FROM, THIS MEMORANDUM, OR ANY OTHER WRITTEN OR ORAL COMMUNICATION TRANSMITTED OR MADE AVAILABLE TO THE RECIPIENT.
THIS MEMORANDUM DOES NOT PURPORT TO BE ALL-INCLUSIVE OR TO CONTAIN ALL OF THE INFORMATION THAT A PROSPECTIVE INVESTOR MAY DESIRE IN EVALUATING AN INVESTMENT IN THE COMPANY.
STATEMENTS MADE IN THIS MEMORANDUM ARE MADE AS OF THE DATE HEREOF UNLESS OTHERWISE STATED AND ARE SUBJECT TO CHANGE, COMPLETION OR AMENDMENT WITHOUT NOTICE. NEITHER THE DELIVERY OF THIS MEMORANDUM, AT ANY TIME, NOR THE SALE OF THE SECURITIES HEREUNDER, SHALL UNDER ANY CIRCUMSTANCES, CREATE AN IMPLICATION THAT THE INFORMATION HEREIN IS CORRECT AS OF ANY TIME SUBSEQUENT TO THE DATE HEREOF.
| 5 |
CERTAIN PROVISIONS OF VARIOUS AGREEMENTS ARE SUMMARIZED IN THIS MEMORANDUM, BUT PROSPECTIVE INVESTORS SHOULD NOT ASSUME THAT THE SUMMARIES ARE COMPLETE.
THIS MEMORANDUM DOES NOT CONSTITUTE AN OFFER TO SELL OR SOLICITATION OF AN OFFER TO BUY ANY UNIT OR SECURITY OTHER THAN THOSE OFFERED HEREBY, NOR DOES IT CONSTITUTE AN OFFER TO SELL OR A SOLICITATION OF AN OFFER TO BUY FROM ANY PERSON IN ANY STATE OR OTHER JURISDICTION IN WHIH SUCH AN OFFER OR SOLICITATION WOULD BE UNLAWFUL, OR IN WHICH THE PERSON OR ENTITY MAKING SUCH OFFER OR SOLICITATION IS NOT QUALIFIED TO DO SO, OR TO A PERSON OR ENTITY TO WHOM IT IS UNLAWFUL TO MAKE SUCH AN OFFER OR SOLICITATION.
THE COMPANY RESERVES THE RIGHT AT ITS SOLE DISCRETION AND FOR ANY REASON WHATSOEVER TO MODIFY, AMEND AND/OR WITHDRAW ALL OR A PORTION OF THE OFFERING, AND/OR ACCEPT OR REJECT IN WHOLE OR IN PART ANY PROSPECTIVE INVESTMENT IN THE SHARES, OR TO ALLOT TO ANY PROSPECTIVE INVESTOR LESS THAN THE AMOUNT OF SHARES SUCH INVESTOR DESIRES TO PURCHASE. THE COMPANY SHALL HAVE NO LIABILITY WHATSOEVER TO ANY OFFEREE AND/OR INVESTOR IN THE EVENT THAT ANY OF THE FOREGOING SHALL OCCUR.
SPECULATIVE; HIGH RISK; DUE DILIGENCE
THE SECURITIES REFERENCED HEREIN ARE HIGHLY SPECULATIVE AND ANY INVESTMENT INVOLVES A HIGH DEGREE OF RISK. INVESTORS SHOULD BE PREPARED TO BEAR THE ECONOMIC RISK OF THEIR INVESTMENT FOR AN INDEFINITE PERIOD OF TIME AND BE AWARE THAT THEY MAY SUSTAIN A LOSS OF THEIR ENTIRE INVESTMENT.
EACH INVESTOR WILL BE REQUIRED TO REPRESENT THAT THEY ARE FAMILIAR WITH AND UNDERSTAND THE TERMS, RISKS, AND MERITS OF THE OFFERING DESCRIBED HEREIN AND ALL ATTACHMENTS TO THIS MEMORANDUM.
THIS MEMORANDUM HAS BEEN PREPARED FOR INFORMATIONAL PURPOSES ONLY IN ORDER TO ASSIST PROSPECTIVE INVESTORS IN EVALUATING AN INVESTMENT IN THE COMPANY. PROSPECTIVE INVESTORS ARE EXPECTED TO CONDUCT THEIR OWN INVESTIGATIONS WITH REGARD TO THE COMPANY AND ITS PROSPECTS. IN MAKING AN INVESTMENT DECISION, INVESTORS MUST CONDUCT AND RELY ON THEIR OWN EXAMINATIONS OF THE COMPANY AND THE TERMS OF THE OFFERING, INCLUDING THE MERITS AND RISKS INVOLVED IN MAKING AN INVESTMENT DECISION WITH RESPECT TO THIS MEMORANDUM.
SECURITIES LAWS; REGISTRATION
THE OFFER AND SALE OF THE SECURITIES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR ANY STATE SECURITIES LAWS IN RELIANCE UPON EXEMPTIONS FROM REGISTRATION PROVIDED BY SECTION 4(2) OF THE SECURITIES ACT OF 1933, AS AMENDED, AND REGULATION D PROMULGATED THEREUNDER, AND SIMILAR EXEMPTIONS FROM REGISTRATION PROVIDED B Y CERTAIN STATE SECURITIES LAWS.
THE SECURITIES HAVE NOT BEEN REGISTERED WITH OR APPROVED OR DISAPPROVED BY THE SECURITIES AND EXCHANGE COMMISSION OR BY ANY STATE SECURITIES COMMISSION, NOR HAS THE SECURITIES AND EXCHANGE COMMISSION OR ANY SUCH STATE SECURITIES COMMISSION PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PRIVATE PLACEMENT MEMORANDUM.
RESTRICTIONS; TRANSFERABILITY
THESE SECURITIES ARE SUBJECT TO RESTRICTIONS ON TRANSFERABILITY AND RESALE. THE SECURITIES MAY NOT BE TRANSFERRED OR RESOLD EXCEPT AS PERMITTED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, APPLICABLE STATE SECURITIES LAWS PURSUANT TO REGISTRATION OR EXEMPTION THEREFROM, AND/OR THE COMPANY’S OPERATING AGREEMENT.
REGULATION D PROMULGATED UNDER THE SECURITIES ACT; INVESTOR SUITABILITY; DISCLOSURES; BACKGROUND CHECKS
| 6 |
THE SECURITIES HEREIN ARE BEING OFFERED IN RELIANCE UPON CERTAIN EXEMPTION(S) FROM THE REGISTRATION REQUIREMENTS OF THE ACT. IN PARTICULAR, THE SECURITIES ARE BEING OFFERED IN RELIANCE UPON RULE 506(BB), PROMULGATED UNDER REGULATION D OF THE ACT. ACCORDINGLY, NEITHER THE ISSUER OF SUCH SECURITY OR THE SECURITIES THEMSELVES ARE SUBJECT TO COMPLIANCE WITH THE SPECIFIC DISCLOSURE REQUIREMENTS APPLICABLE TO SECURITIES WHICH ARE REGISTERED UNDER THE ACT.
THE SECURITIES HEREIN ARE BEING OFFERED EXCLUSIVELY TO THOSE CERTAIN INVESTORS WHO MAY QUALIFY AS “ACCREDITED INVESTORS” AND POSSESS THE QUALIFICATIONS NECESSARY TO PERMIT THE SECURITIES TO BE OFFERED AND SOLD IN RELIANCE UPON CERTAIN EXEMPTIONS AND/OR AS DEFINED IN REGULATION D UNDER THE SECURITIES ACT OF 1933.
INVESTORS ARE ADVISED THAT THEY MAY NEED TO MEET CERTAIN MINIMUM ANNUAL INCOME OR NET WORTH THRESHOLDS PROVIDED IN THE INVESTOR SUITABILITY QUESTIONNAIRE AS WELL AS OTHER ADDITIONAL SUITABILITY REQUIREMENTS AS MAY BE DETERMINED BY THE COMPANY. INVESTORS ARE FURTHER ADVISED THAT THEY MAY IN CONNECTION WITH SUCH SUITABILITY REQUIREMENTS, BE REQUIRED TO SUBMIT FINANCIAL STATEMENTS,W-2 FORMS, 1099 FORMS, SCHEDULE K-1 OF 1065 FORMS, 1040 FORMS, TAX RETURNS, A SUITABILITY QUESTIONNAIRE, AND/OR OTHER DOCUMENTATION WHICH MAY INCLUDE BANK STATEMENTS, BROKERAGE STATEMENTS, CERTIFICATES OF DEPOSITS, TAX ASSESSMENTS AND CREDIT REPORTS.
THE COMPANY WILL TAKE REASONABLE STEPS TO VERIFY AN INVESTORS “ACCREDITED INVESTOR” STATUS AND THE COMPANY MAY, IN ITS SOLE DISCRETION, DECLINE TO ADMIT ANY PROSPECTIVE INVESTOR OF THE INTEREST DESCRIBED HEREIN.
NOTICE TO NON-UNITED STATES RESIDENTS
IT IS THE RESPONSIBILITY OF ANY ENTITY WISHING TO PURCHASE THE COMMON STOCK HEREIN TO SATISFY THEMSELVES AS TO FULL OBSERVANCE OF THE LAWS OF ANY RELEVANT TERRITORY OUTSIDE THE UNITED STATES IN CONNECTION WITH ANY SUCH PURCHASE, INCLUDING OBTAINING ANY REQUIRED GOVERNMENTAL OR OTHER CONSENTS OR OBSERVING ANY OTHER APPLICABLE FORMALITIES.
You should rely only on the information contained in this Memorandum or any Memorandum supplement or amendment. We have not authorized any other person to provide you with information that is different from, or adds to, that contained in this Memorandum. If anyone provides you with different or inconsistent information, you should not rely on it. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. You should assume that the information contained in this Memorandum is accurate only as of the date of this Memorandum, regardless of the time of delivery of this Memorandum or of any sale of our Securities. Our business, financial condition, results of operations and prospects may have changed since that date. We are not making an offer of any securities in any jurisdiction.
| 7 |
TABLE OF CONTENTS
| ABOUT THIS MEMORANDUM | 9 |
| MARKET DATA | 9 |
| SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS | 9 |
| COMPANY SUMMARY | 10 |
SUMMARY OF THE OFFERING |
17 |
| RISK FACTORS | 21 |
| USE OF PROCEEDS | 35 |
| DIVIDEND POLICY | 35 |
| CAPITALIZATION | 36 |
| MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS | 37 |
| BUSINESS | 38 |
| MANAGEMENT | 54 |
| PRINCIPAL STOCKHOLDERS | 56 |
| CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS | 58 |
| DESCRIPTION OF CAPITAL STOCK | 59 |
| RESTRICTIONS ON TRANSFER OF SECURITIES | 62 |
| INVESTOR SUITABILITY STANDARDS | 62 |
| OFFERING PERIOD AND SUBSCRIPTION PROCEDURES | 63 |
| REPORTS AND AVAILABLE INFORMATION | 65 |
| ADDITIONAL INFORMATION | 65 |
No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this Memorandum. You must not rely on any unauthorized information or representations. This Memorandum is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this Memorandum is current only as of its date.
LIST OF EXHIBITS
| Exhibit A | Subscription Agreement |
| Exhibit B: | Registration Rights Agreement |
| Exhibit C: | Form of Class A Warrant |
| Exhibit D: | Form of Class B Warrant |
| Exhibit E: | Annual Report on Form 10-K for the fiscal year ended December 31, 2015 |
| Exhibit F: | Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2016 |
We encourage all potential Investors to review carefully the attached exhibits and this Memorandum
| 8 |
ABOUT THIS MEMORANDUM
Throughout this Memorandum, unless otherwise designated or the context suggests otherwise,
| · | all references to the “Company,” the “registrant,” “Protea,” “we,” “our,” or “us” in this Memorandum mean Protea Biosciences Group, Inc. and its consolidated subsidiaries; |
| · | “year” or “fiscal year” mean the year ending December 31; |
| · | all dollar or $ references when used in this Memorandum refer to United States dollars. |
MARKET DATA
Market data and certain industry data and forecasts used throughout this Memorandum were obtained from internal company surveys, market research, consultant surveys, publicly available information, reports of governmental agencies and industry publications and surveys. Industry surveys, publications, consultant surveys and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but the accuracy and completeness of such information is not guaranteed. We have not independently verified any of the data from third party sources, nor have we ascertained the underlying economic assumptions relied upon therein. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based on our management’s knowledge of the industry, have not been independently verified. Forecasts are particularly likely to be inaccurate, especially over long periods of time. Statements as to our market position are based on the most currently available data. While we are not aware of any misstatements regarding the industry data presented in this Memorandum, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” in this Memorandum.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Memorandum contains “forward-looking statements.” Forward-looking statements reflect the current view about future events. When used in this Memorandum, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward-looking statements. Such statements, include, but are not limited to, statements contained in this Memorandum relating to our business strategy, our future operating results and liquidity and capital resources outlook. Forward-looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions. Because forward–looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward-looking statements. They are neither statements of historical fact nor guarantees of assurance of future performance. We caution you therefore against relying on any of these forward-looking statements. Important factors that could cause actual results to differ materially from those in the forward-looking statements include, without limitation:
| · | the results of clinical trials and the regulatory approval process; |
| · | our ability to raise capital to fund continuing operations; |
| · | market acceptance of any products that may be approved for commercialization; |
| · | our ability to protect our intellectual property rights; |
| · | the impact of any infringement actions or other litigation brought against us; |
| · | competition from other providers and products; our ability to develop and commercialize new and improved products and services; |
| · | changes in government regulation; |
| · | our ability to complete capital raising transactions; |
| · | and other factors (including the risks contained in the section of this Memorandum entitled “Risk Factors”) relating to our industry, our operations and results of operations. |
Should one or more of these risks or uncertainties materialize, or should the underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned.
Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
| 9 |
COMPANY SUMMARY
This summary provides a brief overview of the key aspects of our business and our securities. The reader should read the entire Memorandum carefully, especially the risks of investing in our Common Stock discussed under “Risk Factors.” Some of the statements contained in this Memorandum, including statements under “Summary” and “Risk Factors” as well as those noted in the documents incorporated herein by reference, are forward-looking statements and may involve a number of risks and uncertainties. Our actual results and future events may differ significantly based upon a number of factors. The reader should not put undue reliance on the forward-looking statements in this document, which speak only as of the date on the cover of this Memorandum.
About Our Business
Protea is an emerging growth, molecular information company that has developed a revolutionary platform technology, which enables the direct analysis, mapping and display of biologically active molecules in living cells and tissue samples. The technology platform offers new, unprecedented capabilities useful to the pharmaceutical, diagnostic, clinical research, agricultural and life science industries.
“Molecular information” refers to the generation and bioinformatic processing of very large data sets, known as “big data,” obtained by applying the Company’s technology to identify and characterize the proteins, metabolites, lipids and other molecules which are the biologically active molecular products of all living cells and life forms.
Our technology is used to improve pharmaceutical development and life science research productivity and outcomes, and to extend and add value to other technologies that are used in research and development (“R&D”), such as three-dimensional tissue models, biomarker discovery, synthetic biologicals and mass spectrometry. In particular, the Company believes that its ability to rapidly provide comprehensive molecular image-based datasets addresses a universal need of the pharmaceutical, diagnostic and clinical research and life science industries.
Known as LAESI ® (Laser Ablation Electrospray Ionization), our technology platform is exclusively licensed from The George Washington University (“GWU”). We have subsequently completed the development of the first fully automated LAESI instruments and requisite proprietary software suites, used for LAESI data analysis and for molecular imaging – the direct analysis and visualization of molecules in cells and tissue. LAESI technology is currently the subject of eleven issued patents and over 40 peer-reviewed publications.
LAESI is intended to meet the broad need of the biologist for the direct, unbiased identification and characterization of molecules in biological samples. By virtue of LAESI’s improved speed and the comprehensive datasets it generates, the Company is pursuing its vision of what it believes will be a new era of human molecular information, where the molecular networks of human disease will be clearly elucidated, with data more rapidly available, thereby accelerating pharmaceutical development and improving healthcare.
Our Business Strategy and Products and Services
Protea has applied its core technology to establish commercial leadership in the emerging field of mass spectrometry imaging (“MSI”) services. MSI is a revolutionary capability that for the first time enables the identification of all classes of biologically active molecules produced by cells and combines this with the ability to instantly spatially-display the molecules in tissue histology sections. MSI can be performed with no sample preparation and no labeling or antibody techniques, thereby integrating direct molecular identification with tissue pathology.
The Company intends to achieve its business objectives by leveraging its core MSI technology and associated bioanalytical mass spectrometry platforms to improve the availability, comprehensiveness and usefulness of molecular information to address the needs of pharmaceutical research, biomarker discovery, agriculture and other life science research markets.
The Company’s commercial development is centered in three business lines:
| · | Molecular Information Services – the Company believes that it is the commercial leader in providing multimodal MSI, combining LAESI, MALDI, and optical microscopy. Our proprietary services enable the identification and quantitation of both small molecules (e.g., lipids and metabolites) and large molecules (e.g. proteins) and our services portfolio, inclusive of MSI, proteomics, metabolomics, lipidomics and bioinformatics is unique in the industry. Our clients include major pharmaceutical, chemical and biotechnology companies; |
| 10 |
| · | LAESI Instruments, Software and Consumables – we offer our proprietary LAESI DP-1000 instrument, software and bioanalytical consumables to corporate and academic research laboratories; and |
| · | Molecular Diagnostics and Clinical Research – we apply our multimodal MSI technologies and workflows in partnership with top-tier medical research institutions to co-develop new, molecular diagnostic tests that the company believes can be used to improve the diagnosis and prognosis of cancer and provide requisite information useful in predicting the outcome of cancer treatment. Our current test development programs target the differential diagnosis and prognosis of malignant melanoma and the molecular profiling of subpopulations of cells in lung adenocarcinoma. |
Molecular Information Services
We believe we are the commercial leader in the offering of MSI services. Our clients send their tissue and biofluid samples directly to the Company’s laboratory, where samples are analyzed by the Company’s state of the art mass spectrometry instrumentation, scientific staff and bioinformatics capabilities. We combine our proprietary LAESI platform with matrix-assisted laser desorption ionization (“MALDI”), and liquid chromatography mass spectrometry (LCMS) workflows. Our clients include major pharmaceutical, biotechnology, chemical and medical device and consumer products companies, and both academic and government institutions. The services unit is operated within a Quality by Design environment, which is necessary to meet the internal research and development standards of pharmaceutical and clinical research clients.
The Company’s MSI services represent a revolutionary capability that for the first time enables the identification of all classes of biologically-active molecules produced by cells, and combines this with the ability to instantly spatially-display the molecules (both two- and three-dimensional) in tissue histology sections. MSI can be performed without sample preparation, labeling or antibody techniques, thereby integrating direct molecular identification with tissue pathology for the first time. Since the sample is not touched, data is unbiased and more rapidly available. Providing molecular information that rapidly answers questions critical to the drug development process, such as, “is my drug actually getting inside the target cells,” “is my immune modulating agent changing the molecular output of the tumor cell,” or “how long did it take for the drug to enter the target cells” as well as questions of drug concentration and duration.
We have, in collaboration with industry leaders, developed advanced bioanalytical workflows for the characterization of protein biotherapeutics, such as monoclonal antibodies, to address regulatory requirements for safety, efficacy, and bioactivity in manufacturing and storage of these products. To this end, we have applied proteomics and metabolomics technologies along with LAESI direct analysis to aid in optimizing the expression systems used to produce monoclonal antibodies for drug discovery purposes. Key collaborations and partnerships have been formed to address the bioinformatics analysis required including statistical and pathway analysis.
In January 2014, the Company, as a subcontractor to GWU, was awarded a multi-year project with the Defense Advanced Research Projects Agency (“DARPA”). In addition to Protea, The Stanford Research Institute International and GE Global Research also collaborate on the project entitled, “New Tools for Comparative Systems Biology of Threat Agent Action Mechanisms.” A $15 million five year project, the goal of DARPA’s Rapid Threat Assessment (“RTA”) program is to develop new tools and methods to elucidate the mechanism of action of a threat agent, drug, biologic or chemical on living cells within 30 days from exposure. Uncovering the mechanism of action of such agents in 30 days, compared to the years currently required, could be key to the development of effective countermeasures. The molecular networks within living cells are vast and complex. Conventional approaches fail to capture the system-wide response of a living cell to a threat agent. The Company believes this technology will be applicable to preclinical drug research as well.
LAESI instruments, software and consumables
LAESI technology was invented in the laboratory of Professor Akos Vertes, Ph.D., Dept. of Chemistry, GWU, in 2007 and was exclusively-licensed to Protea in 2008. The LAESI DP-1000 instrument, the Company’s prototype embodiment of its proprietary LAESI technology, integrates with laboratory instruments known as mass spectrometers. LAESI employs a proprietary (patented) method that utilizes the water content in a sample (native or applied) to transition the sample into a gas state, where it can be analyzed by a mass spectrometer. LAESI accomplishes this without requiring the sample to be touched. By eliminating sample preparation, the biological sample can be analyzed without the possible contamination, bias or sample loss that occurs with current techniques, which require the introduction of chemicals or the destruction of the sample itself in order to enable analysis by mass spectrometry.
This technology enables the direct identification of proteins, lipids and metabolites in tissues, cells and biofluids such as serum and urine. Data is available in seconds to minutes, allowing rapid time to results and the capacity to analyze thousands of samples in a single work period. As an example, a researcher testing a new drug’s effects on living cells can analyze changes in the cells’ metabolism across a specific time course, thereby almost immediately obtaining data as to the activity of the new drug. The Company believes that LAESI technology has the potential to significantly improve the availability of molecular information in pharmaceutical research as well as many other fields including agriculture, pathology, biomarker discovery, biodefense and forensics.
| 11 |
LAESI instruments employ proprietary software developed by the Company that creates “molecular maps” - the ability to instantly display the distribution of molecules throughout tissue sample in both two- and three-dimensional imaging. The Company currently offers the LAESI Desktop Software and ProteaPlot software that facilitates operating the instrument and the storage and display of datasets in a user friendly, intuitive software environment. The ProteaPlot software includes tools for post-processing of LAESI-MS data, generation of molecular maps/images and mass spectral comparison for regions of interest. LAESI software displays the data obtained by mass spectrometry analysis combined with actual images of the tissue and cell samples. Thus, mass spectrometry data can be integrated with a sample’s tissue architecture.
We have developed and brought to market related, proprietary consumable products for use in molecular analysis including REDIchips TM (Resonance-Enhanced Desorption Ionization - sample target plates with nanopost array surfaces for matrix-free laser desorption ionization of small molecules) and Progenta TM (acid labile surfactants use to solubilize proteins for mass spectrometry analysis).
Molecular Diagnostics and Clinical Research
The Company is employing both its proprietary LAESI platform and MALDI methodologies to create comprehensive, tissue-based molecular profiles to improve the differential diagnosis of cancer. Our bioinformatics capability allows the integration of the Company’s MSI data files with related pathology, gene expression and demographic datasets, with the purpose of improving human disease state detection, assessment and management. The Company has entered into collaborative research partnerships with top tier academic centers to develop and validate new, proprietary, MSI–based cancer diagnostic tests.
We have established a collaborative research partnership with the Memorial Sloan-Kettering Cancer Center and the Dana-Farber Cancer Institute. The first target of the collaboration is early stage lung adenocarcinoma. The objectives of the collaboration are to demonstrate that different cancer cell sub-groups within a lung cancer will have different molecular profiles and will behave differently. The goal is to define these molecular differences and to identify the sub-group of cancer cells with the worst prognosis that are most likely to recur thereby enabling earlier treatment intervention, and to use these findings to achieve lung adenocarcinoma tumor cell “molecular profiling,” leading to more precise treatment selection and higher survivor rates.
We established a collaborative research initiative with The Yale University School of Medicine that employs our MSI technology to differentiate benign melanocytic nevi from malignant melanoma. We believe that our core MSI technology can identify unique protein expression profiles that can discriminate between benign melanocytic nevi and malignant melanoma.
In April 2016 we entered into an exclusive license agreement for technology with Yale University related to the differential diagnosis of melanoma, specifically designated as a "Method of Differentiating Benign Melanocytic Nevi from Malignant Melanoma." The technology was co-invented by Dr. Rossitza Lazova, of the Department of Dermatology at Yale School of Medicine, and Dr. Erin Seeley our Clinical Imaging Principal Investigator. Under the terms of the license agreement, we have been granted the exclusive worldwide rights to commercialize the technology. We are obligated to pay expenses for the preparation, filing and prosecution of future related patent applications governed by the license agreement and related license fees. Unless earlier terminated in accordance with its terms, the Yale license agreement expires upon the later of 20 years from the effective date or the end of the term of the last underlying patent to expire.
To support our MSI–based diagnostic development, we developed new software known as “Histology Guided Mass Spec Imaging (HG-MSI)” that enables pathologists to combine traditional microscopy and histology with high resolution mass spectrometry molecular imaging. Clinicians can share, annotate and direct the analysis of specific tissue morphologies and cell subpopulations by MSI. Molecular profiling data are collected from discrete locations within a tissue section using a histology stained section as a guide. The digital tissue scans are visually analyzed by pathologists by logging onto a secure website portal and they then annotate specific cellular areas for further analysis.
We intend to expand our collaborations with major medical research centers to develop additional molecular profiles for the improved differential diagnosis and prognosis of cancer.
Recent Developments
We entered into a Memorandum of Understanding (the “MOU”) with Agilent Technologies, Inc. (NYSE: A) (“Agilent”), to develop new bioanalytical workflows in order to meet the emerging needs of the growing biopharmaceutical industry. Under the terms of the MOU, Protea, using Agilent instrumentation combined with its expertise, will develop workflows to improve the characterization of protein therapeutics including monoclonal antibodies and new methods for the field of metabolomics. We believe, along with Agilent, that the field of biotherapeutics is advancing rapidly and is in need of new, innovative solutions that identify changes in the metabolic profiles of cells due to disease processes and drug interactions.
We have announced new workflows to support the bioanalytical needs of immuno-oncology, an emerging frontier of cancer treatment that utilizes the body’s own immune system to fight diseases. Our new workflows help provide both visual and analytical certainty of therapeutic efficacy and apply the Company’s core MSI technology to the analysis of drug target tissues and tumor microenvironments.
| 12 |
We have commercialized a consumable product out of the DARPA research grant that is currently being sold to research laboratories performing small molecule analysis. This product called REDIchip (Resonance-Enhanced Desorption Ionization - sample target plates with nanopost array surfaces for matrix-free laser desorption ionization of small molecules) enables researchers to rapidly profile small molecules and metabolites utilizing a MALDI platform. This product does not require the addition of a traditional chemical matrix, but is able to detect and quantitate small molecules due to the highly structured nanopost array. These products are being used with researchers investigating metabolites and small molecule drugs, and they have potential applications within clinical mass spectrometry pain panel management operations.
In April 2016, we entered into an exclusive license agreement for technology with Yale University related to the differential diagnosis of melanoma, specifically designated as a "Method of Differentiating Benign Melanocytic Nevi from Malignant Melanoma." The technology was co-invented by Dr. Rossitza Lazova, of the Department of Dermatology at Yale School of Medicine, and Dr. Erin Seeley our Clinical Imaging Principal Investigator. Under the terms of the license agreement, we have been granted the exclusive worldwide rights to commercialize the technology. We are obligated to pay expenses for the preparation, filing and prosecution of future related patent applications governed by the license agreement and related license fees. Unless earlier terminated in accordance with its terms, the Yale license agreement expires upon the later of 20 years from the effective date or the end of the term of the last underlying patent to expire.
Risk Factors
Our business is subject to a number of risks. You should be aware of these risks before making an investment decision. These risks are discussed more fully in the section of this Memorandum titled “Risk Factors,” which begins on page 21 of this Memorandum and includes:
| · | We have a history of losses; | |
| · | We will be required to raise additional financing; | |
| · | We have a significant amount of indebtedness; | |
| · | We may be unable to protect our intellectual property; | |
| · | Market acceptance of our products is still uncertain; | |
| · | We face significant competition; and | |
| · | Investors in this offering will incur substantial dilution. |
The Senior Notes
In March 2016, the Company sold $655,000 principal amount of original issue discount unsecured note due September 4, 2016 and providing an estimated 23% yield to maturity (the “CV Note”) to one accredited investor, pursuant to which the Company received an aggregate gross proceeds of $500,000. The CV Note does not accrue additional interest and included legal fees of $5,000, which was added to the principal amount. The Company also issued to the investor an aggregate of 108,675 shares of our common stock and five year warrants to purchase up to 1,637,500 additional shares of common stock at an exercise price of $0.75, subject to adjustment in certain events as provided therein.
In connection with our sale of the 2016 CV Note, the Company paid to Laidlaw & Company (UK) Ltd., who acted as the placement agent, an aggregate of approximately $60,000 in cash compensation, representing fees and an expense allowance. In addition, we issued to the placement agent (or its designees) a five-year warrant to purchase an aggregate of approximately 19,650 shares of our common stock at an exercise price of $6.25 per share.
On September 8, 2016, the Company paid off the CV Note with the proceeds of a 10% original issue discount secured promissory note of the Company purchased for $650,000 in cash by another private lender (the “Secured Note”). The Secured Notes is in the principal face amount of $720,000, and is due and payable on October 15, 2016. Our obligation to repay the Secured Note is secured by a continuing first priority lien and security interest in the accounts receivable and inventory of the Company and its subsidiary, Protea Biosciences Group, Inc.
In the event that we are unable to retire the Secured Note by its October 15, 2016 maturity date, the holder may commence suit against the Company and seek to foreclose on the accounts receivable and inventory of our operating subsidiary. Such actions would have a material adverse effect on our financial condition and business prospects. See “Risk Factors.”
| 13 |
The Bridge Note Offerings.
The Prior Bridge Note Offerings.
In May, June, July and September 2016, the Company received an aggregate of $1,736,000 in gross cash proceeds from 37 accredited investors in connection with the sale of a total principal amount of $2,170,000 of 20% OID unsecured convertible debentures (the “Bridge Notes”) due November 20, 2016, November 30, 2016, December 10, 2016 December 30, 2016, January 29, 2017 and March 26, 2017, being six months following the dates of issuance of each of the Bridge Notes. Such Bridge Notes (a) were issued with (b) bear interest at a rate of 10% per annum, and (c) are convertible at any time by the holders into our common stock at a conversion price of $0.25. In addition, we issued to the note holders three-year warrants to purchase up to 6,510,001 shares of common stock of the Company at an exercise price of $0.325 per share (the “Bridge Note Warrants”).
In connection with our sale of the Bridge Notes, we paid to Laidlaw, who acted as the placement agent, an aggregate of approximately $206,400.00 in cash compensation, representing fees and an expense allowance. In addition, we issued to the placement agent (or its designees) a five-year warrant to purchase an aggregate of approximately 1,519,000 shares of our common stock at an exercise price of $0.25 per share.
The Bridge Notes and Warrants issued in connection with sales made in May, June, July and September 2016 contained weighted average anti-dilution adjustment provisions, whereas, the Bridge Notes and Warrants sold in the September 2016 contain full-ratchet anti-dilution provisions which, in substance entitles the holder to reduce his or its conversion or exercise price then in effect to any lower price in which the Company sells Common Stock or other convertible securities or warrants (with limited exceptions). In addition, the issuance of the CV Note and the Secured Note violated certain contractual covenants contained in the Bridge Notes subscription agreements issued in connection with the May, June and July 2016 sales of Bridge Notes and Warrants Accordingly, we offered to the holders of the May, June, July and September 2016 Bridge Notes and Warrants (i) an opportunity to exchange such securities for the Bridge Notes and Warrants containing full-ratchet anti-dilution protection, and (ii) requested that such holders waive the default resulting from our sale of the CV Note and Secured Note. As at the date hereof, all holders of such Bridge Notes and Warrants agreed to the exchange and waived the default.
Our Capitalization
We are currently authorized by our Certificate of Incorporation to issue up to 500,000,000 shares of Common Stock. As of September 30, 2016, there were (i) issued and outstanding, an aggregate of 134,226,310 shares of our Common Stock, (ii) 82,946,270 additional shares of Common Stock issuable upon exercise of outstanding warrants, (iii) 10,134,800 additional shares of Common Stock issuable upon conversion of outstanding convertible notes, and (iv) 10,800,086 additional shares of Common Stock issuable upon exercise of stock options. Accordingly, as of September 30, 2016, our fully-diluted Common Stock was approximately 243,957,380 shares of Common Stock. After giving effect to the sale of all 500 Units in this Offering (assuming none are sold to Protea Investors), our fully-diluted Common Stock would be up to approximately 443,956,880 shares of Common Stock (including the warrants to be issued to the Placement Agents and shares of Common Stock we will be required to issue as a result of existing anti-dilution adjustment provisions).
The Increase in Authorized Capital Stock and Reverse Stock Split
The closing market price of our Common Stock on the OTCQB at October 28, 2016 was $0.14 per share, and during the six months ended September 30, 2016 the closing market prices for our Common Stock on the OTCQB ranged between $0.27 and $0.095 per share. On December 1, 2015, we obtained shareholder approval to seek discretionary authority to consummate a reverse stock split of our issued and outstanding shares of Common Stock within a range of between one-for-15 to one-for-25, as and when determined by our Board to be in our best interests without further approval from our stockholders. Such stockholder approval granted our Board discretionary authority to determine the exact ratio of the reverse stock split based upon the market price of our Common Stock on the date of such determination and with such reverse stock split to be effective at such time and date, if at all, as determined by the Board in its sole discretion.
We filed with the SEC a definitive consent solicitation statement on Schedule Rule 14A (the “Proxy Statement”) on September 23, 2016, obtained written consents from the holders of record on August 22, 2016 of a majority of our 134,226,310 outstanding shares of Common Stock for an amendment to our certificate of incorporation increasing our authorized Common Stock to 500,000,000 shares (the “Charter Amendment”). We filed the Charter Amended in Delaware on October 24, 2016.
We intend to consummate a 1-for-25 reverse stock split of our outstanding Common Stock which has previously been authorized by our stockholders following to the Termination Date of this Offering described below (the “Reverse Stock Split”), thereby reducing the outstanding number of shares of Common Stock and Common Stock Equivalents to prior to this Offering to 5,326,820 shares of Common Stock and 10,007,520 shares of Common Stock, assuming the sale of all 500 Units of Securities offered hereby. Consummation of the Reverse Stock Split will require (a) the filing with the Delaware Secretary of State of a further amendment to our Certificate of Incorporation, and (b) obtaining the approval of the Financial Industry Regulatory Authority (“FINRA”).
| 14 |
No fractional shares of Common Stock will be issued in connection with the Reverse Stock Split, and all such fractional interests will be rounded down to the nearest whole number. Issued and outstanding stock options, convertible notes and warrants will be split on the same basis and exercise prices will be adjusted accordingly.
There can be no assurance that we will receive FINRA approval to consummate the Reverse Stock Split.
Additional information regarding our issued and outstanding securities may be found in the section of this Memorandum titled “Description of Securities.”
Organizational History
We were incorporated as SRKP 5, Inc., in Delaware on May 24, 2005. Prior to the Reverse Merger (as defined below) and split-off (as described below), our business was to provide software solutions to deliver geo-location targeted coupon advertising to mobile internet devices.
On September 2, 2011, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Protea Biosciences, Inc., a Delaware corporation (“PBI”), and our wholly-owned subsidiary formed to complete the merger. Under the terms of the Merger Agreement, our subsidiary merged with and into PBI, as a result of which PBI became our wholly owned subsidiary (the “Reverse Merger”). In the Reverse Merger, each share of PBI Common Stock was automatically converted into one share of our Common Stock, all shares of PBI Common Stock in treasury were canceled and we assumed all of PBI’s rights and obligations for outstanding convertible securities and warrants. Upon the Reverse Merger, we discontinued our prior business, and our business became the business of PBI and its subsidiaries.
Corporate Information
Our principal executive office is located 1311 Pineview Drive, Suite 501, Morgantown, West Virginia 26505. Our telephone number is 1-304-292-2226. Our web address is http://proteabio.com. Information included on our website is not part of this Memorandum.
Implications of Being an Emerging Growth Company
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an emerging growth company until the earlier of (i) December 31, 2019, the last day of the fiscal year following the fifth anniversary of the date of the first sale of our Common Stock pursuant to an effective registration statement under the Securities Act; (ii) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under applicable SEC rules. We expect that we will remain an emerging growth company for the foreseeable future, but cannot retain our emerging growth company status indefinitely and will no longer qualify as an emerging growth company on or before December 31, 2019. We refer to the Jumpstart Our Business Startups Act of 2012 herein as the "JOBS Act," and references herein to "emerging growth company" have the meaning associated with it in the JOBS Act. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from specified disclosure requirements that are applicable to other public companies that are not emerging growth companies.
These exemptions include:
| · | being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced "Management's Discussion and Analysis of Financial Condition and Results of Operations" disclosure; |
| · | not being required to comply with the requirement of auditor attestation of our internal controls over financial reporting; |
| · | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements; |
| · | reduced disclosure obligations regarding executive compensation; and |
| · | not being required to hold a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
| 15 |
For as long as we continue to be an emerging growth company, we expect that we will take advantage of the reduced disclosure obligations available to us as a result of that classification. We have taken advantage of certain of those reduced reporting burdens in this Memorandum. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
An emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected to avail ourselves of this extended transition period and, as a result, we will not be required to adopt new or revised accounting standards on the dates on which adoption of such standards is required for other public reporting companies.
We are also a “smaller reporting company” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and have elected to take advantage of certain of the scaled disclosure available for smaller reporting companies.
| 16 |
SUMMARY OF THE OFFERING
| The Offering: |
The Company proposes to offer, only to “accredited investors,” as that term is defined in Rule 501 of Regulation D, as promulgated under the Securities Act of 1933, as amended (the “Securities Act”), up to of up to $5,000,000 of units of our securities (the “Units”). The Units consist of a maximum of:
· 66,666,667 shares of our common stock, par value $0.0001 per share (the “Common Stock”),
· 18 month warrants to purchase up to 66,666,667 shares of common stock, par value $0.0001 per share (the “Common Stock”), at an exercise price of $0.09 per share (the “Class A Warrants”), and
· five year warrants purchase up to 66,666,667 shares of Common Stock at an exercise price of $0.1125 per share (the “Class B Warrants” and together with the Class A Warrants the “Warrants”).
The Common Stock and the Warrants are sometimes referred to herein collectively as the “Securities.”
A minimum of 50 Units for aggregate gross proceeds of $500,000 (the “Minimum Offering”) and a maximum of 500 Units for aggregate gross proceeds of $5,000,000 (the “Maximum Units”) are being offered by the Company in this Offering. Each Unit consists of:
· 133,333.33 shares of Common Stock,
· Class A Warrants to purchase 133,333.33 shares of Common Stock at an exercise price of $0.09 per share, and
· Class B Warrants to purchase 133,333.33 shares of Common Stock at an exercise price of $0.1125 per share.
The minimum subscription from qualified investors shall be one full Unit for a minimum purchase price of $10,000. However, the Company and the Placement Agent described below may accept subscriptions from qualified investors for aa lesser amount in their sole discretion...
The Company and the Placement Agent reserve the right to reject any proposed subscription.
The Offering will be made as a private placement to such accredited investors (the “Purchasers”) pursuant to Section 4(a)(2) of the Securities Act and/or Rule 506 promulgated thereunder.
|
| Terms of the Warrants |
The Class A Warrants will have a term of 18 months and will entitle the holders to purchase up to 66,666,667 shares of Common Stock at an exercise price of $0.09 per share, if all 500 Units are sold in this Offering. Upon consummation of the Reverse Stock Split, the maximum number of shares of Common Stock issuable upon full exercise of the Class A Warrants will be 2,666,660 shares of Common Stock (the “Class A Warrant Shares”).
The Class B Warrants will have a term of five years and will entitle the holders to purchase up to 66,666,667 shares of Common Stock at an exercise price of $0.1125 per share, if all 500 Units are sold in this Offering. Upon consummation of the Reverse Stock Split, the maximum number of shares of Common Stock issuable upon full exercise of the Class B Warrants will be 2,666,660 shares (the “Class B Warrant Shares” and together with the Class A Warrant Shares, the “Warrant Shares”).
Upon consummation of the 1:25 Reverse Stock Split, the 133,333,000 Warrant Shares shall be reduced to 5,333,320 Warrant Shares, and the exercise prices of the Class A Warrants and Class B Warrants shall be increased to $2.25 and $2.8125, respectively.
The exercises prices of the Class A Warrants and Class B Warrants as well as the number of shares issuable upon exercise of the Class A and Class B Warrants shall both be adjusted if the Company sells Common Stock or securities convertible into or issuable for common stock at a price per share that is lower than their respective exercise prices (as adjusted by the Reverse Stock Split); in which event, the exercise prices would be adjusted to the lower price.
|
| 17 |
| Registration Rights |
The Company will be required to file within 45 days of the Termination Date (the “Filing Deadline”) a registration statement (the “Registration Statement”) registering for resale all shares of Common Stock and all shares of shares of Common Stock issuable upon exercise of the Warrants (collectively, the “Registrable Shares”). The Company has agreed to use its reasonable best efforts to have the Registration Statement declared effective within 30 days of being notified by the SEC that the Registration Statement will not be reviewed by the SEC (and in such case of no SEC review, not later than 60 days after the Filing Deadline) or within 180 days after the Filing Deadline in the event the SEC provides comments to the Registration Statement (the “Effectiveness Deadline”). If the Registration Statement is not filed on or before the Filing Deadline declared effective on or before the Effectiveness Deadline or after the effective date of a Registration Statement, such Registration ceases for any reason to remain continuously effective as to all Registrable Securities included in such registration statement, or the holders are otherwise not permitted to utilize the prospectus therein to resell such Registrable Securities, for more than ten (10) consecutive calendar days or more than an aggregate of fifteen (15) calendar days (which need not be consecutive calendar days) during any 12-month period (the “Continuing Effectiveness”), then the Company shall pay to each holder of Registrable Shares an amount in cash equal to one-percent (1.0%) of such holder’s investment in the Offering on every thirty (30) day anniversary of such Filing Deadline failure, Effectiveness Deadline failure, or Continuing Effectiveness failure until such failure is cured. The payment amount shall be prorated for partial thirty (30) day periods. The maximum aggregate amount of payments to be made by the Company as the result of such failures, whether by reason of a Filing Deadline failure, Effectiveness Deadline failure Continuing Effectiveness failure, or any combination thereof, shall be an amount equal to 6% of each holder’s investment amount. Notwithstanding the foregoing, no payments shall be owed with respect to any period during which all of the holder’s Registrable Shares may be sold by such holder under Rule 144 without volume or manner-of-sale restrictions pursuant to Rule 144. Moreover, no such payments shall be due and payable with respect to any Registrable Shares if the Company is unable to register due to limits imposed by the SEC’s interpretation of Rule 415 under the Securities Act. The Company shall maintain the Registration Statement until all Registrable Securities covered by such Registration Statement have been sold, thereunder or pursuant to Rule 144 or until Rule 144 of the Securities Act is available to investors without volume or manner-of-sale restrictions pursuant to Rule 144, whichever is earlier.
The registration rights agreement provides that the Company and the managing underwriter in connection with any public offering shall, in the exercise of their joint good faith judgment, determine that immediate resales by holders of Registrable Securities could have a material adverse effect on the Company’s ability to complete the any public offering, the Company and such managing underwriter or placement agent may restrict resales of such Registrable Securities (a “Lock-Up”) for a period of up to ninety (90) days following such public offering. It is anticipated therefore, that Investors’ Registrable Securities may not be publicly sold by them for a period of 90 days after the date the Commission declares any registration statement related to such public offering is declared effective.
|
| SEC Filings |
The Company will be responsible for timely filing of all required documents including Form D, and will pay for all legal opinions of Company counsel associated with all future Rule 144 sales of the Common Stock and Warrant Shares.
|
| Investors: | All investors must be “accredited investors” as defined under Rule 501 of Regulation D and amended by the Dodd-Frank Wall Street Reform and Consumer Protection Act, and meet all other suitability requirements set forth herein under the caption “Investor Suitability Requirements,” and as contained in the subscription documents attached as Exhibits to this Memorandum. |
| Escrow: |
It is anticipated that all funds from this Offering shall be held in a non-interest bearing trust escrow/trust account with Signature Bank, or another nationally recognized escrow agent, as escrow agent, and shall comply with all applicable FINRA and Commission rules, and state and federal laws. The subscription amount for the Units will be paid to the escrow account established by the escrow agent, by either check or wire, and held in escrow until satisfaction of all the conditions to the Closing.
|
| Subscription Procedures: |
Accredited investors interested in subscribing for Units in this Offering must do the following:
· Deliver a completed and executed Subscription Agreement, which is attached to this Memorandum as Exhibit A, to the Placement Agent at the address provided in the Subscription Agreement.
· Deliver the purchase price in the amount of $10,000 per Unit to the Escrow Agent by check or by wire transfer using the wire transfer instructions provided in the Subscription Agreement.
Funds and executed documents will be held in escrow until a Closing of this Offering at which time escrowed funds and documents will be released by the Escrow Agent. Promptly following a Closing, certificates for the Common Stock and Warrants purchased in this Offering will be issued to the subscribers. If this Offering is not completed for any reason, all proceeds deposited into escrow will be returned to the subscribers without interest or deduction.
|
| 18 |
| Restrictions on Transfer: |
The Securities offered will be restricted as to transferability under state and federal laws regulating securities. The issuance of the Securities has not been registered under the Securities Act, or any other similar state statutes, in reliance upon exemptions from the registration requirements contained therein. Accordingly, the securities will be “restricted securities” as defined in Rule 144 of the Securities Act. As “restricted securities,” an investor must hold them indefinitely and may not dispose of or otherwise sell them without registration under the Securities Act and any applicable state securities laws unless exemptions from registrations are available. Moreover, in the event an investor desires to sell or otherwise dispose of any of the Securities, the investor will be required to furnish the Company with an opinion of counsel acceptable to the Company that the transfer would not violate the registration requirements of the Securities Act or applicable state securities laws.
|
| Placement Agent: |
Laidlaw & Company (UK) Ltd. (the “Placement Agent” or “Laidlaw”), a New York, New York-based, registered broker-dealer member FINRA/SIPC, is acting as the placement agent for the Offering. The Offering will be made on a “reasonable efforts, all or none” basis with respect to the Minimum Offering and thereafter on a “reasonable efforts” basis for up to the Maximum Offering, as described in this Memorandum. The Placement Agent’s principal business address is 546 Fifth Avenue, New York, New York 10036.
We have agreed to pay the Placement Agent: (i) a cash commission in the amount often percent (10%) of the gross proceeds of the Offering received from investors other than certain investors previously identified to the Placement Agent by the Company (“Protea Investors”), (ii) two percent (2%) of the gross proceeds of the Offering received from Protea Investors, and (iii) an activation fee of $25,000. In addition, the Placement Agent will be entitled to receive three (3) year warrants to purchase such number of shares of Common Stock of the Company equal to (x) ten percent (10%), in the case of investors other than Protea Investors, of the aggregate number of securities sold in the Offering at an exercise price equal to the lowest price per share of the securities sold in the Offering. The Placement Agent shall also be entitled to a non-allocable expense reimbursement in the amount of two percent (2%) of the gross proceeds of the Offering, including amounts received from Protea Investors. After the Termination Date, assuming the closing on at least $1,000,000 in gross proceeds for the Units, the Company will pay the Placement Agent a non-refundable financial advisory fee of $150,000, to be paid monthly, at the rate of $25,000 per month for a term of six months beginning on the Termination Date. For additional information regarding the Placement Agent, see the section entitled “Offering Period and Subscription Procedure.”
|
| Risk Factors: | See “Risk Factors” and the other information in this Memorandum for a discussion of the factors that you should carefully consider before deciding to invest in the Securities. |
| 19 |
The following table sets forth the number of shares of Common Stock of the Company and number of shares of fully-diluted Common Stock of the Company outstanding at September 30, 2016 and to be outstanding after giving effect to the Maximum offering of all 500 Units of Securities offered hereby, both before and after giving effect to the contemplated 1:25 Reverse Stock Split:
|
Before 1:25 Reverse Stock Split |
After 1:25 Reverse Stock Split | ||
|
Common stock outstanding prior to the Offering |
134,226,310 shares | 5,369,052 shares | |
| Fully-Diluted Common Stock outstanding before the Offering | 243,957,380 shares (1) | 9,758,295 shares (1) | |
| Common Stock outstanding upon issuance of Common Stock in the Units |
200,892,810 shares (2)
|
8,035,712 shares (2)
| |
| Fully-Diluted Common Stock outstanding upon issuance of all Common Stock and exercise of all Warrants in the Units |
443,956,880 shares (1)
|
17,758,275 shares (1)
| |
| Use of proceeds | Assuming we sell 50 Units of Securities in this Offering, we estimate that the net proceeds to us from this Offering will be approximately $360,000, after estimated offering expenses payable by us, and including all commissions and fees payable to the Placement Agent. Assuming we sell all 500 Units of Securities in this Offering, we estimate that the net proceeds to us from this Offering will be approximately $4,000,000, after estimated offering expenses payable by us, and including all commissions and fees payable to the Placement Agent. We intend to use the net proceeds of this Offering to fund research and development of new products, to increase our sales and marketing efforts, to build infrastructure, including hiring of additional personnel, and for working capital and other general corporate purposes. For additional information, please refer to the section entitled “Use of Proceeds” on page 35 of this Memorandum. | ||
| Trading symbol: | Our Common Stock is currently quoted on the OTCQB under the symbol “PRGB.” | ||
| Risk Factors: | You should carefully consider the information set forth in this Memorandum and, in particular, the specific factors set forth in the “Risk Factors” section beginning on page 21 of this Memorandum before deciding whether or not to invest in shares of our Common Stock. | ||
(1) Fully-Diluted Common Stock means all issued shares of Common Stock, plus all shares of Common Stock issuable upon conversion of all outstanding convertible notes (including Bridge Notes), the Securities included in the Units and exercise of all other issued and outstanding warrants and stock options. Fully-diluted Common Stock:
| · | Includes (i) 1,020,800 shares of Common Stock issuable upon conversion of $400,000 of convertible notes, and (ii) the conversion of all Bridge Notes and exercise of all Bridge Note Warrants at a conversion price and exercise price of $0.075 per share, into a maximum of 64,993,581 additional shares of Common Stock; |
| · | Includes 83,266,937 shares of Common Stock issuable upon the exercise of outstanding warrants at a weighted average exercise price of $0.567 per share, including (i) 2,751,250 warrants issued to Laidlaw, in their capacity as placement agent in two private placements consummated in the fourth quarter of 2015, the first quarter of 2016, and May, June, July and September 2016, respectively, and (ii) 3,000,000 shares issuable at $0.40 upon exercise of warrants issued to certain of our directors. |
| · | Does not include one Unit to be issued to the Placement Agent’s legal counsel. |
(2) Includes outstanding shares plus a maximum of 66,666,667 of Common Stock included in the Units. Does not include 10,000 shares of Common Stock to be issued to the Placement Agent’s legal counsel.
| 20 |
RISK FACTORS
Our business is subject to many risks and uncertainties, which may affect our future financial performance. If any of the events or circumstances described below occur, our business and financial performance could be adversely affected, our actual results could differ materially from our expectations, and the price of our stock could decline. The risks and uncertainties discussed below are not the only ones we face. There may be additional risks and uncertainties not currently known to us or that we currently do not believe are material that may adversely affect our business and financial performance. You should carefully consider the risks described below, together with all other information included in this Memorandum including our financial statements and related notes, before making an investment decision. The statements contained in this Memorandum that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our Common Stock could decline, and investors in our securities may lose all or part of their investment.
Risks Related to Our Business
We are an emerging growth company with a limited operating history and limited sales to date.
The Company is subject to all of the risks inherent in the establishment of an emerging growth company including the absence of an operating history and the risk that we may be unable to successfully develop, manufacture and sell our products. There can be no assurance that the Company will be able to execute its business plan, including without limitation the Company’s plans to develop, then manufacture, market and sell its technologies, products and services. The Company has engaged in limited manufacturing operations to date and although the Company believes that its plans to conduct manufacturing of its products internally will work, there is no assurance that this will be the case. The Company began to sell products and services in the fourth quarter of 2007 and sales to date are limited. There can be no assurance that the Company’s sales projections and marketing plans will be achieved as anticipated and planned. It is likely that losses will be incurred during the early stages of operations. The Company believes that its future success will depend on its ability to develop and introduce its instruments and services for mass spec molecular imaging, to meet a wide range of customer needs and achieve market acceptance. The Company cannot assure prospective investors that it will be able to successfully develop and market its products or that it will recover the initial investment that must be made to develop and market such products.
We have incurred net losses since inception.
We incurred a net loss of $1,807,598 for the quarter ended June 30, 2016, $9,574,434 for the year ended December 31, 2015 and $11,474,770 for the year ended December 31, 2014. As of June 30, 2016, the Company has an accumulated deficit of $82,490,749 since inception. Our independent registered public accountants issued an opinion on our audited financial statements as of and for the year ended December 31, 2015 that contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. We can provide no assurance as to when, or if, we will be profitable in the future. Even if the Company achieves profitability, it may not be able to sustain such profitability
We must raise additional working capital.
At the present time, our ability to continue as a going concern is dependent upon raising capital from financing transactions. To stay in business, we will need to raise additional working capital through public or private sales of our equity securities, debt financing or short-term bank loans, or a combination of the foregoing. We are currently in the process of seeking to obtain additional working capital through the private placement of additional bridge notes and warrants. Based on our current spending levels, management estimates that the Company will need to raise approximately $6,000,000 in additional working capital to maintain current operations through the next twelve calendar months.
There can be no assurance that we will be able to raise sufficient additional working capital financing either from this Offering or from the sale of additional Bridge Notes in the concurrent Bridge Note Offering to sustain our operations on acceptable terms, or at all. If such financing is not available on satisfactory terms or is not available at all, we may be required to delay, scale back or eliminate the development of business opportunities and our operations and our financial condition may be materially adversely affected. Debt financing, if obtained, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt and could increase our expenses and require that our assets be provided as a security for such debt. Debt financing would also be required to be repaid regardless of our operating results. Equity financing, if obtained, could result in dilution to our then existing stockholders.
| 21 |
There is no assurance that we will be able to obtain FINRA approval for our proposed Reverse Stock Split.
Consummation of the Reverse Stock Split will require (a) the filing with the Delaware Secretary of State of a further amendment to our Certificate of Incorporation, and (b) obtaining the approval of the FINRA. There can be no assurance that we will obtain FINRA approval to consummate the Reverse Stock Split.
Issuance of Common Stock to fund our operations or upon the conversion of convertible debt or equity securities or exercise of outstanding warrants and options may dilute your investment.
We have been operating at a loss since inception and our working capital requirements continue to be significant. We have been supporting our business through the sale of debt and equity since inception. We will need additional funding for developing products and services, increasing our sales and marketing capabilities, technologies and assets, as well as for working capital requirements and other operating and general corporate purposes. Our working capital requirements depend and will continue to depend on numerous factors including the timing of revenues, the expense involved in development of our products, and capital improvements. If we are unable to generate sufficient revenue and cash flow from operations, we will need to seek additional financing through the sale of additional shares of Common Stock, warrants and/or convertible debt securities to provide the capital required to maintain or expand our operations, which may have the effect of diluting our existing stockholders or restricting our ability to run our business.
After completion of this Offering, in the event we are required to sell additional shares of Common Stock or securities convertible into or exercisable for Common Stock, the percentage ownership of our fully diluted Common Stock held by purchasers of the Units of Securities in this Offering could be subject to dilution and reduction.
We have significant short-term indebtedness.
As of the date of this Memorandum, we have total indebtedness of $11,106,982, which includes $6,313,967 that is due within twelve months. Of this short-term component, $2,695,056 is owed to certain members of the Company’s Board of Directors or their affiliates, $995,626 is represented by notes owed primarily to three governmental agencies of the State of West Virginia, $2,135,536 is represented by convertible notes issued in 2016, $487,705 is related to capitalized leases. We are currently in discussions with existing directors holding $2,695,056 of related party indebtedness to defer payment of an estimated $2,021,292 or 75% of the total amount owed to them. There can be no assurance that the maturity dates of the balance of our obligations owed to the existing directors and shareholders, and the governmental agencies of the State of West Virginia can or will be extended, if required, nor do we know the final terms and conditions of any extensions, if any. If such obligations are not extended, we will be required to apply additional net proceeds of this offering to further reduce our debt obligations. See “Use of Proceeds” and “Certain Relationships and Related Transactions” elsewhere in this Memorandum.
We depend on the pharmaceutical and biotechnology industries.
Over the past several years, some areas of our businesses have grown significantly as a result of an increase in the sales of our bioanalytical instrument platform known as “LAESI®” and the increase in pharmaceutical, academic and clinical research laboratory outsourcing of their clinical drug research support activities. We believe that due to the significant investment in facilities and personnel required to support drug development, pharmaceutical, academic and clinical research laboratories look to purchase our bioanalytical instrument platforms and solutions technology to meet and administer their drug research requirements. Our revenues depend greatly on the expenditures made by these pharmaceutical and academic/clinical research laboratory companies in research and development. In some instances, companies in these industries are reliant on their ability to raise capital in order to fund their research and development projects. Accordingly, economic factors and industry trends that affect our clients in these industries also affect our business. If companies in these industries were to reduce the number of research and development projects they conduct or outsource, our business could be materially adversely affected.
Changes in government regulation or in practices relating to the pharmaceutical industry could change the need for the services we provide.
Governmental agencies throughout the world, but particularly in the United States, strictly regulate the drug development process. Changes in regulation, such as regulatory submissions to meet the internal research and development standards of pharmaceutical research, a relaxation in existing regulatory requirements, the introduction of simplified drug approval procedures or an increase in regulatory requirements that we may have difficulty satisfying or that make our services less competitive, could substantially change the demand for our services. Also, if the government increases efforts to contain drug costs and pharmaceutical companies profits from new drugs, our customers may spend less, or reduce their growth in spending on research and development.
| 22 |
We may be affected by health care reform.
In March 2010, the United States Congress enacted the Patient Protection and Affordable Care Act (“PPACA”) which is intended over time to expand health insurance coverage and impose health industry cost containment measures. PPACA legislation and the accompanying regulations may significantly impact the pharmaceutical and biotechnology industries as it is implemented over the next several years. In addition, the U.S. Congress, various state legislatures and European and Asian governments may consider various types of health care reform in order to control growing health care costs. We are unable to predict what legislative proposals will be adopted in the future, if any.
Implementation of health care reform legislation may have certain benefits but also may contain costs that could limit the profits that can be made from the development of new drugs. This could adversely affect research and development expenditures by pharmaceutical and biotechnology companies, which could in turn decrease the business opportunities available to us both in the United States and abroad. In addition, new laws or regulations may create a risk of liability, increase our costs or limit our service offerings.
A reduction in research and development budgets at pharmaceutical companies and clinical research institutions may adversely affect our business.
Our customers include researchers at pharmaceutical companies and academic/clinical research laboratory institutions. Our ability to continue to grow and win new business is dependent in large part upon the ability and willingness of the pharmaceutical and biotechnology industries to continue to spend on research and development and to outsource their product equipment and service needs. Fluctuations in the research and development budgets of these researchers and their organizations could have a significant effect on the demand for our products and services. Research and development budgets fluctuate due to changes in available resources, mergers of pharmaceutical companies and spending priorities and institutional budgetary policies of academic/clinical research organizations. Our business could be adversely affected by any significant decrease in life sciences research and development expenditures by pharmaceutical and academic/clinical research companies. Similarly, economic factors and industry trends that affect our clients in these industries also affect our business.
We rely on a limited number of key customers, the importance of which may vary dramatically from year to year, and a loss of one or more of these key customers may adversely affect our operating results.
A small number of customers have accounted for a substantial portion of our revenues in 2015. Five customers accounted for approximately 52% of our gross revenue in fiscal 2015 and five customers accounted for approximately 42% of our gross revenues in fiscal 2014. One large pharmaceutical company accounted for 20% of our gross revenue in 2015, and this customer will continue to be a significant contributor to revenue in 2016. The loss of a significant amount of business from one of our major customers would materially and adversely affect our results of operations until such time, if ever, as we are able to replace the lost business. Significant clients or projects in any one period may not continue to be significant clients or projects in other periods. In any given year, there is a possibility that a single pharmaceutical or academic/ clinical research laboratory company may account for 5% or more of our gross revenue or that our business may be dependent on one or more large projects. To the extent that we are dependent on any single customer, we are subject to the risks faced by that customer to the extent that such risks impede the customer's ability to stay in business and make timely payments to us.
We may bear financial risk if we underprice our contracts or overrun cost estimates.
Since some of our contracts are structured as fixed price or fee-for-service, we bear the financial risk if we initially underprice our contracts or otherwise overrun our cost estimates. Such underpricing or significant cost overruns could have a material adverse effect on our business, results of operations, financial condition, and cash flows.
A default in our credit facility could materially and adversely affect our operating results and our financial condition.
The Company has an outstanding line of credit with United Bank. This credit facility requires us to adhere to certain contractual covenants. If there were an event of default under our credit facility that was not cured or waived, the lenders of the defaulted debt could cause all amounts outstanding with respect to that debt to be due and payable immediately. Although the outstanding balance of this line of credit is not payable until July 2018, the amount due and the status of the payment terms have been unchanged since the first quarter of 2014. We cannot assure that our assets or cash flow would be sufficient to fully repay borrowings under the credit facility, either upon the 2018 maturity date or earlier if an event of default should occur, or that we would be able to refinance or restructure the payments becoming due on the credit facility. Please see Note 3 to the Consolidated Financial Statements, “Bank Line of Credit,” for additional detail regarding our credit facility.
| 23 |
We might incur expenses to develop products that are never successfully commercialized.
We have incurred and expect to continue to incur research and development and other expenses in connection with our products business. The potential products to which we devote resources might never be successfully developed or commercialized by us for numerous reasons including:
| · | our inability to develop products that address our customers’ needs; | |
| · | competitive products with superior performance; | |
| · | patent conflicts or unenforceable intellectual property rights; | |
| · | demand for the particular product; | |
| · | other factors that could make the product uneconomical; and | |
| · | termination of pre-existing license agreements. |
Incurring expenses for a potential product that is not successfully developed and/or commercialized could have a material adverse effect on our business, financial condition, prospects and stock price.
Our business uses biological and hazardous materials, which could injure people or violate laws, resulting in liability that could adversely impact our financial condition and business.
Our activities involve the controlled use of potentially harmful biological materials as well as hazardous materials and chemicals. We cannot completely eliminate the risk of accidental contamination or injury from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for damages that result and any liability could exceed our insurance coverage and ability to pay. Any contamination or injury could also damage our reputation, which is critical to obtaining new business. In addition, we are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations is significant and if changes are made to impose additional requirements, these costs could increase and have an adverse impact on our financial condition and results of operations.
Hardware or software failures, delays in the operations of our computer and communications systems or the failure to implement system enhancements could harm our business.
Our success depends on the efficient and uninterrupted operation of our computer and communications systems. A failure of our network or data gathering procedures could impede the processing of data, delivery of databases and services, client orders and day-to-day management of our business and could result in the corruption or loss of data. While all of our operations have disaster recovery plans in place, they might not adequately protect us. Despite any precautions we take, damage from fire, floods, hurricanes, power loss, telecommunications failures, computer viruses, break-ins and similar events at our computer facilities could result in interruptions in the flow of data to our servers and from our servers to our clients. In addition, any failure by our computer environment to provide our required data communications capacity could result in interruptions in our service. In the event of a delay in the delivery of data, we could be required to transfer our data collection operations to an alternative provider of server hosting services. Such a transfer could result in delays in our ability to deliver our products and services to our clients. Additionally, significant delays in the planned delivery of system enhancements, improvements and inadequate performance of the systems once they are completed could damage our reputation and harm our business. Finally, long-term disruptions in the infrastructure caused by events such as natural disasters, the outbreak of war, the escalation of hostilities and acts of terrorism, particularly involving cities in which we have offices, could adversely affect our businesses. Although we carry property and business interruption insurance, our coverage might not be adequate to compensate us for all losses that may occur.
We rely on third parties for important services.
We depend on third parties to provide us with services critical to our business. The failure of any of these third parties to adequately provide the needed services including, without limitation, licensed intellectual property rights, could have a material adverse effect on our business.
We license a significant portion of our intellectual property from third parties; if the Company fails to remain in compliance with these agreements the Company’s business may be adversely affected.
The Company has entered into a number of technology license agreements with various universities for the exclusive use of a significant portion of the patent-based intellectual property that the Company uses. Generally, the license agreements imposes, and we expect that future license agreements will impose, various diligence, milestone payment, royalty and other obligations on us. If we fail to comply with our obligations under these agreements, or if we file for bankruptcy, we may be required to make certain payments to the licensor, we may lose the exclusivity of our license, or the licensor may have the right to terminate the license, in which event we would not be able to develop or market products covered by the license. Additionally, the milestone and other payments associated with these licenses could materially and adversely affect our business, financial condition and results of operations. While the Company is currently in compliance with the respective terms of these agreements, if there are one or more breaches thereunder, such as the failure to pay the applicable royalties, and one or more of these agreements are terminated, the Company will not be able to use such technology and the Company’s business may be adversely affected.
| 24 |
In some cases, patent prosecution of our licensed technology may be controlled solely by the licensor. If our licensors fail to obtain and maintain patent or other protection for the proprietary intellectual property we in-license from them, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, and our competitors could market competing products using the intellectual property. In certain cases, we may control the prosecution of patents resulting from licensed technology. In the event we breach any of our obligations related to such prosecution, we may incur significant liability to our licensing partners. Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues. Disputes may arise regarding intellectual property subject to a licensing agreement, including, but not limited to:
| · | the scope of rights granted under the license agreement and other interpretation-related issues; | |
| · | the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; | |
| · | the sublicensing of patent and other rights; | |
| · | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; | |
| · | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our collaborators; and | |
| · | the priority of invention of patented technology. |
If disputes over intellectual property and other rights that we have in-licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected drug candidates. If we fail to comply with any such obligations to our licensor, such licensor may terminate their licenses to us, in which case we would not be able to market products covered by these licenses. The loss of any of our current licensing arrangements and potentially other licenses that we enter into in the future, would have a material adverse effect on our business.
We may be unable to obtain or maintain patent or other intellectual property protection for any products or processes that we may develop.
The Company faces risks and uncertainties related to intellectual property rights. The Company may be unable to obtain or maintain its patents or other intellectual property protection for any products or processes that it may develop; third parties may obtain patents covering the manufacture, use or sale of these products or processes which may prevent the Company from commercializing its technology; or any patents that the Company may obtain may not prevent other companies from competing with it by designing their products or conducting their activities so as to avoid the coverage of the Company’s patents.
Since patent applications in the U.S. are maintained in secrecy for at least portions of their pendency periods (published on U.S. patent issuance or, if earlier, 18 months from the earliest filing date for most applications) and since other publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we are the first to make the inventions to be covered by our patent applications. The patent position of biopharmaceutical firms generally is highly uncertain and involves complex legal and factual questions. The U.S. Patent and Trademark Office has not established a consistent policy regarding the breadth of claims that it will allow in biotechnology patents.
Proceedings to obtain, enforce or defend patents and to defend against charges of infringement are time consuming and expensive activities, and it is possible that the Company could become involved in such proceedings. Unfavorable outcomes in these proceedings could limit the Company’s activities and any patent rights that the Company may obtain, which could adversely affect its business or financial condition. Even if such proceedings ultimately are determined to be without merit, they can be expensive and distracting for the Company’s operations and personnel.
In addition, the Company’s success will depend in part on the ability of the Company to preserve its trade secrets. The Company cannot ensure investors that the obligations to maintain the confidentiality of trade secrets or proprietary information will not wrongfully be breached by employees, consultants, advisors or others or that the Company’s trade secrets or proprietary know how will not otherwise become known or be independently developed by competitors in such a manner that the Company has no legal recourse.
| 25 |
We may infringe the intellectual property rights of others, which may prevent or delay our development efforts and prevent us from commercializing or increase the costs of commercializing our products.
Our commercial success depends significantly on our ability to operate without infringing the patents and other intellectual property rights of third parties. For example, there could be issued patents of which we are not aware that our current or potential future product candidates infringe. There also could be patents that we believe we do not infringe, but that we may ultimately be found to infringe.
Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our drug candidates or potential products infringe. For example, pending applications may exist that claim or can be amended to claim subject matter that our product candidates or potential products infringe. Competitors may file continuing patent applications claiming priority to already issued patents in the form of continuation, divisional, or continuation-in-part applications, in order to maintain the pendency of a patent family and attempt to cover our product candidates.
Third parties may assert that we are employing their proprietary technology without authorization and may sue us for patent or other intellectual property infringement. These lawsuits are costly and could adversely affect our business, financial condition and results of operations and divert the attention of managerial and scientific personnel. If we are sued for patent infringement, we would need to demonstrate that our drug candidates, potential products or methods either do not infringe the claims of the relevant patent or that the patent claims are invalid, and we may not be able to do this. Proving invalidity is difficult. For example, in the U.S., proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could have a material adverse effect on us. In addition, we may not have sufficient resources to bring these actions to a successful conclusion. If a court holds that any third-party patents are valid, enforceable and cover our products or their use, the holders of any of these patents may be able to block our ability to commercialize our products unless we acquire or obtain a license under the applicable patents or until the patents expire.
We may not be able to enter into licensing arrangements or make other arrangements at a reasonable cost or on reasonable terms. Any inability to secure licenses or alternative technology could result in delays in the introduction of our products or lead to prohibition of the manufacture or sale of products by us. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, in any such proceeding or litigation, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our drug candidates or force us to cease some of our business operations, which could materially and adversely affect our business, financial condition and results of operations. Any claims by third parties that we have misappropriated their confidential information or trade secrets could have a similar material and adverse effect on our business, financial condition and results of operations. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
Any claims or lawsuits relating to infringement of intellectual property rights brought by or against us will be costly and time consuming and may adversely affect our business, financial condition and results of operations.
We may be required to initiate litigation to enforce or defend our licensed and owned intellectual property. These lawsuits can be very time consuming and costly. There is a substantial amount of litigation involving patent and other intellectual property rights in the biopharmaceutical industry generally. Such litigation or proceedings could substantially increase our operating expenses and reduce the resources available for development activities or any future sales, marketing or distribution activities.
In any infringement litigation, any award of monetary damages we receive may not be commercially valuable. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during litigation. Moreover, there can be no assurance that we will have sufficient financial or other resources to file and pursue such infringement claims, which typically last for years before they are resolved. Further, any claims we assert against a perceived infringer could provoke these parties to assert counterclaims against us alleging that we have infringed their patents. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
In addition, our licensed patents and patent applications, and patents and patent applications that we may own or license in the future, could face other challenges, such as interference proceedings, opposition proceedings, re-examination proceedings and other forms of post-grant review. Any of these challenges, if successful, could result in the invalidation of, or in a narrowing of the scope of, any of our licensed patents and patent applications and patents and patent applications that we may own or license in the future subject to challenge. Any of these challenges, regardless of their success, would likely be time consuming and expensive to defend and resolve and would divert our management and scientific personnel’s time and attention.
| 26 |
In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the market price of our Common Stock.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on drug candidates throughout the world would be prohibitively expensive. Competitors may use our licensed and owned technologies in jurisdictions where we have not licensed or obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we may obtain or license patent protection, but where patent enforcement is not as strong as that in the U.S. These products may compete with our products in jurisdictions where we do not have any issued or licensed patents and any future patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our licensed patents and future patents we may own, or marketing of competing products in violation of our proprietary rights generally. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the U.S. As a result, we may encounter significant problems in protecting and defending our licensed and owned intellectual property both in the U.S. and abroad. Proceedings to enforce our future patent rights, if any, in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
We are in a highly competitive market.
The Company is engaged in the highly competitive field of biotechnology. Competition from numerous existing biotechnology companies and others entering the proteomics field is intense and expected to increase. Many of these companies are larger, more established and recognized in the marketplace, and/or have substantially greater financial and business resources than the Company. Moreover, competitors who are able to develop and to commence commercial sales of their products before the Company may enjoy a significant competitive advantage. Likewise, innovations by competitors could cause the Company’s products or services to become obsolete or less attractive in the marketplace, adversely affecting sales and/or sales projections. The Company cannot assure investors that its technology will enable it to compete successfully in the future.
We may expand our business through acquisitions.
We occasionally review acquisition candidates. Factors which may affect our ability to grow successfully through acquisitions include:
| · | inability to obtain financing; |
| · | difficulties and expenses in connection with integrating the acquired companies and achieving the expected benefits; |
| · | diversion of management’s attention from current operations; |
| · | the possibility that we may be adversely affected by risk factors facing the acquired companies; |
| · | acquisitions could be dilutive to earnings, or in the event of acquisitions made through the issuance of our Common Stock to the shareholders of the acquired company, dilutive to the percentage of ownership of our existing stockholders; |
| · | potential losses resulting from undiscovered liabilities of acquired companies not covered by the indemnification we may obtain from the seller; and |
| · | loss of key employees of the acquired companies. |
We are dependent on certain key personnel.
The success of the Company is dependent to a significant degree upon the skill and experience of its founders and other key personnel including Stephen Turner, Matthew Powell and others. The loss of the services of any of these individuals would adversely affect the Company’s business. Although the Company has obtained key man life insurance policies on Mr. Turner, its President and CEO, there is no assurance that policy proceeds would cover all potential costs or operational challenges that would result from the loss of services from Mr. Turner and in any event, such policy would not cover the lives or loss of these other individuals. The Company cannot assure prospective investors that it would be able to find adequate replacements for these key individuals. In addition, the Company believes that its future success will depend in large part upon its continued ability to attract and retain highly skilled employees, who are in great demand.
| 27 |
We do not currently have a full-time Chief Financial Officer.
At the present time, the function of chief financial officer is being performed in an interim basis by Stephen Turner, our President. We have engaged the services of a consultant on a part-time basis to assist Mr. Turner in connection with financial and accounting matters, pending our obtaining the services of a full-time senior executive to fill the position of our chief financial and accounting officer. Although we intend to diligently undertake a search for another qualified person, there can be no assurance that we will be able to find an acceptable qualified person to serve as our permanent chief financial and accounting officer.
We are developing products in a rapidly evolving field and there are no assurances that the results of our research and development efforts will not be rendered obsolete by the research efforts and technological activities of others.
The bioanalytics field in which the Company is developing products is rapidly evolving. The Company cannot assure prospective investors that any results of the Company’s research and development efforts will not be rendered obsolete by the research efforts and technological activities of others, including the efforts and activities of governments, major research facilities and large multinational corporations. While the Company believes that its initial efforts to develop its bioanalytics technology platform have been successful thus far, there can be no assurance that the Company will be able to successfully expand its operations in the future, to commercialize, market and sell products and services at projected levels, or to fully develop the technology in a timely and successful manner.
There is no assurance that the Company’s manufacturing plans will be successful.
The Company employs internal and contract manufacturing. There is no assurance that the Company’s manufacturing plans will be successful. While the Company has a quality assurance program for its products, there nonetheless is inherent in any manufacturing process the risk of product defects or manufacturing problems that could result in potential liability for product liability risks.
Sale of European Subsidiary
As described in greater detail in the “Business” and “Certain Relationships and Related Transactions” sections below, on December 12, 2014 the Company completed the sale of 100% of the issued and outstanding capital stock of ProteaBio Europe SAS (“Protea Europe”), a wholly-owned subsidiary of Protea Biosciences, Inc., the Company’s wholly owned subsidiary, to AzurRx BioPharma, Inc. (“AzurRx”) pursuant to the terms and conditions of a stock purchase and sale agreement, dated as of May 21, 2014. Effective upon the closing of the Protea Europe sale, Thijs Spoor, a former director of the Company, was appointed to serve as a director and the Executive Chairman of AzurRx. While the Board did not establish a special committee to evaluate the fairness of this related party transaction or obtain a fairness opinion, our Board nevertheless believes that the terms of the transaction are no less favorable to the Company and its shareholders than such terms that would have been obtained from an unaffiliated third party.
In connection with the consummation of the Protea Europe sales, we received (i) $300,000, inclusive of the forgiveness of a $100,000 Company note, (ii) 100 shares of Common Stock of AzurRx that is convertible into 33% of the issued and outstanding Common Stock of AzurRx and the right to receive certain other contingent consideration to be paid upon the satisfaction of certain events. While the Protea Europe sale agreement contemplates certain contingent payments to us, there can be no assurance that we will ever recoup our $4.0 million investment in Protea Europe.
In November 2015, we converted 29 shares of Common Stock of AzurRx into 707,416 shares of Common Stock of AzurRx. Subsequently, we sold such shares of Common Stock of AzurRx and received gross proceeds of $910,000 as of December 31, 2015.
In the first quarter of 2016, the Company notified AzurRx of its intent to convert 71 of its Series A convertible Preferred Stock, $0.0001 par value per share, into shares of AzurRx Common Stock, $0.0001 par value per share, pursuant to the terms and conditions of the Certificate of Designations. The conversion rate was 24,393 shares of Common Stock per share of Common Stock. The Company received 1,731,949 shares of AzurRx Common Stock upon conversion. The Company sold 1,016,941 shares of AzurRx Common Stock during the first quarter of 2016 receiving net proceeds of $637,100. The Company sold an additional 550,000 shares during the second quarter of 2016 receiving net proceeds of $675,000.
As of June 30, 2016, the Company’s ownership interest in AzurRx included 265,757 shares of AzurRx Common Stock, which represented an ownership stake in AzurRx of 3.6% (on a fully-diluted basis). The Company previously accounted for its investment in AzurRx using the “equity method.” However, considering the reduction in the Company’s interest in AzurRx during the three months ended March 31, 2016, the Company discontinued the use of the “equity method” of accounting for this investment and is now accounting for the investment under the cost method.
| 28 |
In July 2016, the Company sold an additional 140,000 shares of AzurRx Common Stock generating net proceeds of $190,000. After this sale, the Company held 125,757 shares AzurRx Common Stock, which represented an ownership stake in AzurRx of 1.7% (on a fully-diluted basis).
Unfavorable general economic conditions may materially adversely affect our business.
While it is difficult for us to predict the impact of general economic conditions on our business, these conditions could reduce customer demand for some of our products or services which could cause our revenue to decline. Also, our customers that are especially reliant on the credit and capital markets may not be able to obtain adequate access to credit or equity funding, which could affect their ability to make timely payments to us. Moreover, we rely on obtaining additional capital and/or additional funding to provide working capital to support our operations. We regularly evaluate alternative financing sources. Further changes in the commercial capital markets or in the financial stability of our investors and creditors may impact the ability of our investors and creditors to provide additional financing. In addition, the financial condition of our credit facility providers, which is beyond our control, may adversely change. Any decrease in our access to borrowings under our credit facility, tightening of lending standards and other changes to our sources of liquidity could adversely impact our ability to obtain the financing we need to continue operating the business in our current manner. For these reasons, among others, if the economic conditions stagnate or decline, our operating results and financial condition could be adversely affected.
Risks Relating to this Offering and Ownership of Our Securities
There will be restrictions on resale of the Securities and there is no assurance that they will be registered for resale.
None of the shares of Common Stock and Warrants included in the Units or the Common Stock issuable upon exercise of the Warrants may be sold unless, at the time of such intended sale, there is a current registration statement covering the resale of the Common Stock and the shares of Common Stock issuable upon exercise of the Warrants, or there exists an exemption from registration under the Securities Act, and such Securities or underlying Common Stock has been registered, qualified, or deemed to be exempt under applicable state “blue sky” laws in the state of residence of the seller or in the state where sales are being effected. In the first quarter of 2017, but not later than March 31, 2017, the Company intends to file a registration statement covering the resale of the shares of Common Stock and the shares of Common Stock issuable upon the exercise of the Warrants included in the Units (the “Registrable Securities”). If such registration statement is not filed and declared effective covering the resale of any of the Registrable Securities, investors will be precluded from disposing of such Common Stock unless such Registrable Securities become eligible to be disposed of under the exemptions provided by Rule 144 under the Securities Act without restriction. If the Registrable Securities are not registered for resale under the Securities Act, or exempt therefrom, and registered or qualified under applicable securities or “blue sky” laws, or deemed exempt therefrom, the value of such Registrable Securities will be greatly reduced.
The Company was at one time a “shell company” as defined in Rule 12b-2 under the Exchange Act. Pursuant to Rule 144(i), securities issued by a current or former shell company that otherwise meet the holding period and other requirements of Rule 144 nevertheless cannot be sold in reliance on Rule 144 unless at the time of a proposed sale pursuant to Rule 144, the Company is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and has filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months (or for such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports. As a result, the restrictive legends on certificates for the Registrable Shares cannot be removed except in connection with an actual sale meeting the foregoing requirements or pursuant to an effective registration statement.
We have significant discretion over the use of certain of the net proceeds.
A significant portion of the net proceeds of this Offering will be applied to working capital and other general corporate purposes. Accordingly, our management will have broad discretion as to the application of such proceeds. There can be no assurance that management’s use of proceeds generated through this Offering will prove optimal or translate into revenue or profitability for the Company. Investors are urged to consult with their attorneys, accountants and personal investment advisors prior to making any decision to invest in the Company.
The offering price for the Securities has been arbitrarily determined by us.
The offering price of the Common Stock was arbitrarily determined by us after consultation with our Placement Agent. The price of the Units does not necessarily bear any relationship to established valuation criteria such as earnings, book value or assets. Rather, the price of the Units may be derived as a result of our negotiations with the investors based upon various factors including prevailing market conditions, our future prospects and our capital structure. These prices do not necessarily accurately reflect the actual value of the Common Stock or Warrants the price that may be realized upon disposition of the Common Stock issuable under the Common Stock and Warrants.
An investment in our Securities is speculative and there can be no assurance of any return on any such investment.
An investment in the Common Stock and Warrants included in the Units is highly speculative and there is no assurance that investors will obtain any return on their investment. Investors will be subject to substantial risks involved in an investment in the Company, including the risk of losing their entire investment.
| 29 |
Your ownership interest is subject to dilution.
If you purchase Units of Securities in this Offering, you will experience immediate dilution in the value of your Common Stock received upon exercise. In addition, each investor’s proportionate ownership interest may be diluted when we issue additional shares of our Common Stock. We may raise additional capital in the future through additional sales of shares of our Common Stock or other securities convertible or exercisable for Common Stock, and your percentage interest in our outstanding Common Stock would be diluted if you do not participate in such additional sales.
There is no active public trading market for our Common Stock and we cannot assure you that an active trading market will develop in the near future.
Our Common Stock is quoted under the symbol “PRGB” in the over-the-counter markets, including the OTCQB tier of the OTC Markets Group, Inc.; however, it is not listed on any stock exchange and there is currently very limited trading in our securities. We cannot assure you that an active trading market for our Common Stock will develop in the future due to a number of factors, including the fact that we are a small company that is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. There may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales. We cannot give you any assurance that an active public trading market for our Common Stock will develop or be sustained. You may not be able to liquidate your shares quickly or at the market price if trading in our Common Stock is not active.
Our share price could be volatile and our trading volume may fluctuate substantially.
The market price of our Common Stock may experience volatility. Many factors could have a significant impact on the future price of our Common Stock including:
| · | our failure to successfully implement our business objectives; |
| · | compliance with ongoing regulatory requirements; |
| · | market acceptance of our products; |
| · | technological innovations, new commercial products or drug discovery efforts and clinical activities by us or our competitors; |
| · | changes in government regulations; |
| · | general economic conditions and other external factors; |
| · | actual or anticipated fluctuations in our quarterly financial and operating results; |
| · | the degree of trading liquidity in our Common Stock; and |
| · | our ability to meet the minimum standards required for remaining listed on the OTC Markets. |
These factors also include ones beyond our control such as market conditions within our industry and changes in the pharmaceutical and biotechnology industries. In addition, in recent years, the stock market has experienced significant price and volume fluctuations. The stock market, and in particular the market for pharmaceutical and biotechnology company stocks, has also experienced significant decreases in value in the past. This volatility and valuation decline has affected the market prices of securities issued by many companies, often for reasons unrelated to their operating performance, and might adversely affect the price of our Common Stock.
We have established Preferred Stock which can be designated by the Company’s Board of Directors without shareholder approval.
The Company has authorized 10,000,000 shares of Preferred Stock, of which none are issued and outstanding. The shares of series of Preferred Stock of the Company may be issued from time to time in one or more series, each of which shall have a distinctive designation or title as shall be determined by the Board of Directors of the Company prior to the issuance of any shares thereof. The Preferred Stock shall have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof as adopted by the Board of Directors. In each such case, we will not need any further action or vote by our stockholders. One of the effects of undesignated Preferred Stock may be to enable the Board of Directors to render more difficult or to discourage an attempt to obtain control of us by means of a tender offer, proxy contest, merger or otherwise, and thereby to protect the continuity of our management. The issuance of shares of Preferred Stock pursuant to the Board of Director's authority described above may adversely affect the rights of holders of Common Stock. For example, Preferred Stock issued by us may rank senior to the Common Stock as to dividend rights, liquidation preference or both, may have full or limited voting rights and may be convertible into shares of Common Stock. Accordingly, the issuance of shares of Preferred Stock may discourage bids for the Common Stock at a premium or may otherwise adversely affect the market price of the Common Stock.
| 30 |
Stockholders holding aggregate of 26,984,986 shares of our Common Stock, including our directors, officers and certain key employees, are subject to a market standoff agreement which provides that the purchaser will not sell, assign or otherwise transfer or dispose of any Common Stock, warrants or other securities of the Company if so requested by an underwriter for a period not to exceed 180 days. If our securities are locked up at the request of any underwriter, the price of our Common Stock could decline if there are substantial sales of our Common Stock following the “lock-up” period, particularly sales by our directors, executive officers and significant stockholders, or if there is a large number of shares of our Common Stock available for sale.
Our Certificate of Incorporation provides our directors with limited liability.
Our Certificate of Incorporation states that our directors shall not be personally liable to us or any stockholder for monetary damages for breach of fiduciary duty as a director, except for any matter in respect of which such director shall be liable under Section 174 of the Delaware General Corporation Law (the “DGCL”) or shall be liable because the director (1) shall have breached his duty of loyalty to us or our stockholders, (2) shall have acted not in good faith or in a manner involving intentional misconduct or a knowing violation of law or, in failing to act, shall have acted not in good faith or in a manner involving intentional misconduct or a knowing violation of law, or (3) shall have derived an improper personal benefit. Article Seven further states that the liability of our directors shall be eliminated or limited to the fullest extent permitted by the DGCL, as it may be amended. These provisions may discourage stockholders from bringing suit against a director for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by stockholders on our behalf against a director.
Certain provisions of our Certificate of Incorporation and Delaware law make it more difficult for a third party to acquire us and make a takeover more difficult to complete, even if such a transaction were in the stockholders’ interest.
Our Certificate of Incorporation and the DGCL contain provisions that may have the effect of making it more difficult or delaying attempts by others to obtain control of the Company, even when these attempts may be in the best interests of our stockholders.
We also are subject to the anti-takeover provisions of the DGCL, which prohibit us from engaging in a “business combination” with an “interested stockholder” unless the business combination is approved in a prescribed manner and prohibit the voting of shares held by persons acquiring certain numbers of shares without obtaining requisite approval. This statute has the effect of making it more difficult to effect a change in control of a Delaware company.
Our financial controls and procedures may not be sufficient to ensure timely and reliable reporting of financial information, which, as a public company, could materially harm our stock price.
As a public reporting company, we require significant financial resources to maintain our public reporting status. We cannot assure you we will be able to maintain adequate resources to ensure that we will not have any future material weakness in our system of internal controls. The effectiveness of our controls and procedures may in the future be limited by a variety of factors including:
| · | faulty human judgment and simple errors, omissions or mistakes; | |
| · | fraudulent action of an individual or collusion of two or more people; | |
| · | inappropriate management override of procedures; and | |
| · | the possibility that any enhancements to controls and procedures may still not be adequate to assure timely and accurate financial information. |
Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
| 31 |
Despite these controls, because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance of achieving their control objectives. Furthermore, smaller reporting companies like us face additional limitations. Smaller reporting companies employ fewer individuals and can find it difficult to employ resources for complicated transactions and effective risk management. Additionally, smaller reporting companies tend to utilize general accounting software packages that lack a rigorous set of software controls.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015 based on the criteria established in “Internal Control - Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management concluded as a result of material weaknesses in our internal control over financial reporting, our disclosure controls and procedures were not effective as of December 31, 2015. The ineffectiveness of our disclosure controls and procedures was due to the following material weaknesses our internal control over financial reporting, which are common to many small companies: (1) lack of sufficient personnel commensurate with the Company’s reporting requirements; (2) the Company did not consistently establish appropriate authorities and responsibilities in pursuit of the Company’s financial reporting objectives; and (3) insufficient written documentation or training of internal control policies and procedures which provide staff with guidance or framework for accounting and disclosing financial transactions.
If we fail to have effective controls and procedures for financial reporting in place, we could be unable to provide timely and accurate financial information and be subject to investigation by the Securities and Exchange Commission (the “SEC”) and civil or criminal sanctions.
We must implement additional and expensive procedures and controls in order to grow our business and organization and to satisfy new reporting requirements, which will increase our costs and require additional management resources.
As a public reporting company, we are required to comply with the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”) and the related rules and regulations of the SEC, including the requirements that we maintain disclosure controls and procedures and adequate internal control over financial reporting. In the future, if our securities are listed on a national exchange, we may also be required to comply with marketplace rules and heightened corporate governance standards. Compliance with the Sarbanes-Oxley Act and other SEC and national exchange requirements will increase our costs and require additional management resources. We recently have begun upgrading our procedures and controls and will need to continue to implement additional procedures and controls as we grow our business and organization and to satisfy new reporting requirements. If we are unable to complete the required assessment as to the adequacy of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act or if we fail to maintain internal control over financial reporting, our ability to produce timely, accurate and reliable periodic financial statements could be impaired.
If we do not maintain adequate internal control over financial reporting, investors could lose confidence in the accuracy of our periodic reports filed under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Additionally, our ability to obtain additional financing could be impaired or a lack of investor confidence in the reliability and accuracy of our public reporting could cause our stock price to decline.
Our directors, officers and principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
Our officers, directors and principal stockholders collectively beneficially own approximately 20% of our outstanding Common Stock. As a result, these stockholders, if they act together, will be able to control the management and affairs of our company and most matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of our Common Stock. This concentration of ownership may not be in the best interests of our other stockholders.
We have not paid dividends in the past and do not expect to pay dividends in the future, and any return on investment may be limited to the value of our stock.
We have never paid cash dividends on our Common Stock and do not anticipate paying cash dividends on our Common Stock in the foreseeable future. We currently intend to retain any future earnings to support the development of our business and do not anticipate paying cash dividends in the foreseeable future. Our payment of any future dividends will be at the discretion of our Board after taking into account various factors, including, but not limited to, our financial condition, operating results, cash needs, growth plans and the terms of any credit agreements that we may be a party to at the time. In addition, our ability to pay dividends on our Common Stock may be limited by Delaware state law. Accordingly, investors must rely on sales of their Common Stock after price appreciation, which may never occur, as the only way to realize a return on their investment. Investors seeking cash dividends should not purchase our Common Stock.
| 32 |
Risks Related to Our Reverse Stock Split
The reverse stock split may decrease the liquidity of the shares of our Common Stock.
The liquidity of the shares of our Common Stock may be affected adversely by the reverse stock split given the reduced number of shares that will be outstanding following the reverse stock split, especially if the market price of our Common Stock does not increase as a result of the reverse stock split. In addition, the reverse stock split may increase the number of stockholders who own odd lots (less than 100 shares) of our Common Stock, creating the potential for such stockholders to experience an increase in the cost of selling their shares and greater difficulty effecting such sales.
Following the Reverse Stock Split, the resulting market price of our Common Stock may decline and may not attract new investors, including institutional investors. Consequently, the trading liquidity of our Common Stock may not improve.
Although we believe that a higher market price of our Common Stock may help generate greater or broader investor interest, there can be no assurance that the reverse stock split will result in the share price resulting from such Reverse Stock Split declining below $1.875, or that such share price that will attract new investors, including institutional investors. In addition, there can be no assurance that the market price of our Common Stock will satisfy the investing requirements of those investors. As a result, the trading liquidity of our Common Stock may not necessarily improve.
If securities or industry analysts do not publish research or reports about our business, or publish negative reports about our business, our share price and trading volume could decline.
The trading market for our Common Stock will, to some extent, depend on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. If one or more of the analysts who cover us downgrade our shares or change their opinion of our shares, our share price would likely decline. If one or more of these analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Risks Relating to this Offering
We have significant discretion over the use of the gross proceeds.
Substantially all of the net proceeds of this Offering will be used for working capital and general corporate purposes. The proceeds shall be used to carry out our business plan, pay salaries to our employees and satisfy our expenses, foreseeable and unforeseeable. As is the case with any business, it should be expected that certain expenses unforeseeable to management at this juncture will arise in the future. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flow. Investors are urged to consult with their attorneys, accountants and personal investment advisors prior to making any decision to invest in the Company.
An investment in the Company’s is speculative and there can be no assurance of any return on any such investment.
An investment in the Company is speculative and there is no assurance that investors will obtain any return on their investment. Investors will be subject to substantial risks involved in an investment in the Company, including the risk of losing their entire investment.
Investor funds will not accrue interest while in escrow prior to Closing.
All funds delivered in connection with subscriptions for the Securities will be held in a non-interest bearing escrow account with the Escrow Agent until the Closing of the Offering, if any. If we are unable to sell and receive payments for the Minimum Offering prior to the Termination Date, investor subscriptions will be returned without interest or deduction. Investors in the Securities offered hereby may not have the use of such funds or receive interest thereon pending the completion of the Offering.
The Securities will be offered by us on a “reasonable efforts” basis, and we may not raise the Minimum or Maximum Offering.
We are offering the Securities in the Minimum Offering on a “reasonable efforts, all or none” basis and the Securities in the Maximum Offering on a “reasonable efforts” basis. In a reasonable efforts offering such as the one described in this Memorandum, there is no assurance that we will sell the Minimum or Maximum Offering amounts. Accordingly, we may close upon amounts less than the Maximum Offering but not less than the Minimum Offering. This could delay, or curtail, certain aspects of our planned activities. Even if the Maximum Offering amount is sold, we expect to need to raise additional funds, which may include equity securities, in order to finance our operations. Increasing the amount of additional equity we raise will further dilute investors participating in this Offering.
| 33 |
There will be restrictions on resale of the Securities and the shares and there is no assurance of the registration of the Securities.
None of the Securities may be resold unless at the time of such intended sale there is a current registration statement covering the resale of the Securities or there exists an exemption from registration under the Securities Act, and such Securities have been registered, qualified, or deemed to be exempt under applicable securities or “blue sky” laws in the state of residence of the seller or in the state where sales are being effected. If no registration statement is filed with the Securities and Exchange Commission and declared effective covering the resale of any of the Securities sold pursuant to this Offering, investors will be precluded from disposing of such securities unless such securities may become eligible to be disposed of under the exemptions provided by Rule 144 under the Securities Act without restriction. If the Securities are not registered for resale under the Securities Act, or exempt therefrom, and registered or qualified under applicable securities or “blue sky” laws, or deemed exempt therefrom, the value of the such securities will be greatly reduced.
The offering price for the Units has been arbitrarily determined.
The offering price of the Units was arbitrarily determined through negotiations with the Placement Agent. The price of the Units and the terms of the Securities do not necessarily bear any relationship to established valuation criteria such as earnings, book value or assets. Rather, the price of the Units was derived as a result of our negotiations with the Placement Agent based upon various factors including prevailing market conditions, our future prospects and our capital structure. This price does not necessarily accurately reflect the actual value of the Units or the price that may be realized upon disposition of the Securities (including any securities issuable upon exercise of the Warrants).
IN ADDITION TO THE ABOVE RISKS, BUSINESSES ARE OFTEN SUBJECT TO RISKS NOT FORESEEN OR FULLY APPRECIATED BY MANAGEMENT. IN REVIEWING THIS MEMORANDUM, POTENTIAL INVESTORS SHOULD KEEP IN MIND THAT OTHER POSSIBLE RISKS MAY ADVERSELY IMPACT THE COMPANY’S BUSINESS OPERATIONS AND THE VALUE OF THE COMPANY’S SECURITIES.
| 34 |
USE OF PROCEEDS
Assuming the sale of all $5,000,000 of our 500 Units of Common Stock and Warrants at $10,000 per Unit, after deducting commissions and estimated offering expenses payable by us, will be approximately $4,000,000. Such net proceeds will be applied, approximately, as follows:
| · | approximately $720,000 will be used to retire a notes issued in September 2016 (1); |
| · | approximately, $1,000,000 will be used to increase our sales and marketing efforts; |
| · | approximately $250,000 will be used for research and development of new products; |
| · | approximately $1,000,000 to build infrastructure, including hiring of additional personnel; and |
| · | the balance of for working capital and other general corporate purposes. |
(1) Does not include (i) approximately $2,170,000 face amount of six month convertible Bridge Notes issued in May, June, July and September 2016 that, unless converted into our Common Stock, mature on various dates between December 31, 2016 and January 29, 2017, and (ii) an maximum $3,000,000 face amount of six month additional Bridge Notes to be issued between the date of this Memorandum and December 31, 2016 in the concurrent Bridge Note Offering. These Bridge Notes are and will be convertible at any time by the holders at a price of $0.25 per share. To the extent that the holders of these Bridge Notes do not elect to convert them prior to their maturity dates, we will be required to reduce net proceeds otherwise dedicated to our sales and marketing efforts, research and development and building infrastructure in order to pay such Bridge Notes.
Our expected use of the net proceeds from this Offering represents our current intentions based upon our present plans and business condition. As of the date of this Memorandum, we cannot predict with complete certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the actual amounts that we will spend on the uses set forth above. The costs and timing of development activities are highly uncertain, subject to substantial risks and can often change. Due to the many variables inherent to the development of our drug candidates, we cannot currently predict the stage of development we expect the net proceeds of this offering to achieve for our clinical trials, preclinical studies and drug candidates.
Our management will have broad discretion in the application of the net proceeds in the category of other working capital and general corporate purposes. For example, if we identify opportunities that we believe are in the best interests of our stockholders, we may use a portion of the net proceeds from this Offering to acquire, invest in or license complementary products, technologies or businesses, although we have no current understandings, agreements or commitments to do so. In addition, the amounts and timing of our actual expenditures will depend upon numerous factors, including the results of our research and development efforts, the timing and success of preclinical studies, our ongoing clinical trials or clinical trials we may commence in the future and the timing of regulatory submissions. Depending on the outcome of these activities and other unforeseen events, our plans and priorities may change and we may apply the net proceeds of this Offering toward different uses and in different proportions than we currently anticipate.
Pending use of the proceeds from this Offering as described above, we intend to invest the net proceeds of this Offering in short-term, interest-bearing, investment-grade securities or certificates of deposit.
DIVIDEND POLICY
We have never declared or paid any cash dividends on our Common Stock and we currently do not anticipate paying any cash dividends for the foreseeable future. Instead, we anticipate that all of our earnings on our Common Stock will be used to provide working capital, to support our operations, and to finance the growth and development of our business, or investment in, businesses, technologies or products that complement our existing business. Any future determination relating to dividend policy will be made at the discretion of our Board and will depend on a number of factors, including, but not limited to, our future earnings, capital requirements, financial condition, future prospects, applicable Delaware law, which provides that dividends are only payable out of surplus or current net profits and other factors our Board might deem relevant.
| 35 |
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2016, on:
| · | an actual basis; and |
| · | on a pro forma as adjusted basis giving effect to the foregoing and assumes the sale of Maximum Offering amount by us in this Offering. |
| June 30, 2016 | ||||||||
| Actual | As Adjusted | |||||||
| Cash and cash equivalents* | $ | 174,005 | $ | 4,174,005 | ||||
| Indebtedness due within one year (1)(2) | $ | 6,114,015 | 5,114,015 | |||||
| Total long term debt - net of current portion (A)(B) | $ | 4,918,508 | 4,918,508 | |||||
| Stockholders’ equity: | ||||||||
| Common stock, $0.0001 par value, 250,000,000 shares authorized, 134,226,310 actual shares and as adjusted shares outstanding (3) | 13,402 | 13,402 | ||||||
| Preferred stock, $0.0001 par value, 10,000,000 shares authorized; 0 shares outstanding, as adjusted | -0- | 500 | ||||||
| Additional paid-in capital | 71,618,843 | 76,218,343 | ||||||
| Accumulated deficit | (82,490,749 | ) | (82,490,749 | ) | ||||
| Accumulated other comprehensive (loss) income | (85 | ) | (85 | ) | ||||
| Total stockholders' equity (deficit) | (10,858,589 | ) | (6,258,589 | ) | ||||
| Total capitalization | $ | 173,934 | 3,773,934 | |||||
(1) On July 13, 2016, the Company and United Bank entered into a Letter Agreement that extended to July 2018 the maturity date of a $3,000,000 line of credit facility between the Company and the bank that was previously payable on demand. As at June 30, 2016, all $3,000,000 had been drawn under the line of credit. In addition, in May, June, July and September 2016 the Company issued convertible Prior Bridge Notes that had a total outstanding balance of $2,170,000 as of September 26, 2016.
(2) As adjusted, cash increased based on (a) the maximum estimated $4,250,000 of net proceeds of this offering, and (b) the estimated $1,929,320 net proceeds from the sale of all of the Bridge Notes in the concurrent Bridge Note Offering, before payment of related party debt of $673,764 and a third party note of $655,000. Indebtedness also decreased by $1,321,625 as a result of such payments.
*Excludes a bank overdraft of $276,900 that is presented as a current liability on the Company’s consolidated balance sheet.
(3) The number of shares of our Common Stock prior to and to be outstanding immediately after this Offering is based on 134,226,310 shares of our Common Stock outstanding as of September 26, 2016. If all 500 Units of Securities are sold in this Offering and all shares of Common Stock and all Warrants were converted and exercised for Common Stock, our pro-forma outstanding Common Stock would be 243,957,380 share of Common Stock, not including one Unit of Securities issued to the Placement Agent’s legal counsel.
The actual and as adjusted number of shares of our Common Stock outstanding, above excludes:
| · | 8,780,006 shares of Common Stock issuable upon exercise of options granted under the 2002 Equity incentive plan and the 2013 Equity Incentive Plan of which 5,439,005 shares have vested to date, and 7,869,994 additional shares we reserved for issuance under such plans. |
| · | 80,515,687 shares of Common Stock issuable upon exercise of outstanding warrants with an exercise prices ranging from $0.25 to $2.25 per share. |
| · | 2,751,250 shares of Common Stock issuable upon exercise of warrants issued to Laidlaw & Co. (UK) Ltd. or its assigns in connection with acting as placement agent in private placements of our securities consummated in 2015, the first quarter of 2016 and in May, June, July and September 2016. |
| · | 1,020,800 shares of Common Stock issuable upon conversion of $400,000 of convertible notes as at June, 2016 and 9,114,000 shares of Common Stock issuable upon conversion of $2,170,000 of convertible notes issued in May, June, July and September 2016. |
| 36 |
MARKET FOR OUR COMMON STOCK
Our Common Stock is currently quoted on OTCQB under the symbol “PRGB,” however it is not listed on any stock exchange, and there is currently limited trading in our securities. Subject to meeting qualification requirements for listing of our Common Stock on the Nasdaq Capital Market, by consummating the contemplated Public Offering and effecting the reverse stock split, in a range between 1-for-15 and 1-for-50, with the exact reverse split ratio to be determined by our Board of Directors, in its sole discretion, we intend to list our Common Stock on The Nasdaq Stock Market under the symbol “PRGB.”
The quotation of our Common Stock began on or about April 15, 2014. On October 28, 2016, the last reported sales price of our Common Stock was $0.14 per share and we had 134,226,310 shares of our Common Stock issued and outstanding held by approximately 517 stockholders of record.
As of October 28, 2016 we also have outstanding:
| · | warrants to purchase up to 83,266,937 shares of our Common Stock at exercise prices ranging between $0.25 and $2.25 per share, subject to adjustment in certain circumstances as provided therein; and |
| · | options to purchase up to 8,780,086 shares of our Common Stock at a weighted average exercise price of $0.69 per share, subject to adjustment in certain circumstances as provided therein. |
The following table sets forth the high and low closing bid prices for our Common Stock for the fiscal quarter indicated as reported on the OTCQB. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions. Our Common Stock is very thinly traded and, thus, pricing of our Common Stock on the OTC Markets does not necessarily represent its fair market value.
| Period | High | Low | ||||||
| Quarter ending June 30, 2014 (from April 11, 2014) | $ | 2.10 | $ | 0.70 | ||||
| Quarter ending September 30, 2014 | 0.88 | 0.51 | ||||||
| Quarter ending December 31, 2014 | 0.55 | 0.20 | ||||||
| Quarter ending June 30, 2015 | 0.60 | 0.12 | ||||||
| Quarter ending June 30, 2015 | 0.539 | 0.20 | ||||||
| Quarter ending September 30, 2015 | 0.47 | 0.16 | ||||||
| Quarter ending December 31, 2015 | 0.25 | 0.10 | ||||||
| Quarter Ending June 30, 2016 | 0.35 | 0.11 | ||||||
| Quarter Ending September 30, 2016 | 0.20 | 0.095 | ||||||
| Quarter Ending December 31, 2016 (through October 28, 2016) | 0.10 | 0.14 | ||||||
| 37 |
BUSINESS
Company Overview
Protea is an emerging growth, molecular information company that has developed a revolutionary platform technology, which enables the direct analysis, mapping and display of biologically active molecules in living cells and tissue samples. The technology platform offers new, unprecedented capabilities useful to the pharmaceutical, diagnostic, clinical research, agricultural and life science industries.
“Molecular information” refers to the generation and bio-informatic processing of very large data sets, known as “big data,” obtained by applying the Company’s technology to identify and characterize the proteins, metabolites, lipids and other molecules which are the biologically active molecular products of all living cells and life forms.
Our technology is used to improve pharmaceutical development and life science research productivity and outcomes and to extend and add value to other technologies that are used in research and development (“R&D”), such as three-dimensional tissue models, biomarker discovery, synthetic biologicals and mass spectrometry. In particular, the Company believes that its ability to rapidly provide comprehensive molecular image-based datasets addresses a universal need of the pharmaceutical, diagnostic and clinical research and life science industries. To date, we have established a number of collaborative research programs with major medical facilities. See “Strategic Relationships” below.
Known as LAESI ® (Laser Ablation Electrospray Ionization), our technology platform is exclusively licensed from The George Washington University (“GWU”). We have subsequently completed the development of the first fully automated LAESI instruments and requisite proprietary software suites, used for LAESI data analysis and for molecular imaging – the direct analysis and visualization of molecules in cells and tissue. LAESI technology is currently the subject of eleven issued patents and over 40 peer-reviewed publications.
LAESI is intended to meet the broad need of the biologist for the direct, unbiased identification and characterization of molecules in biological samples. By virtue of LAESI’s improved speed and the comprehensive datasets it generates, the Company is pursuing its vision of what it believes will be a new era of human molecular information, where the molecular networks of human disease will be clearly elucidated, with data more rapidly available, thereby accelerating pharmaceutical development and improving healthcare.
Our Business Strategy and Products and Services
Protea has applied its core technology to establish commercial leadership in the emerging field of mass spectrometry imaging (“MSI”) services. MSI is a revolutionary capability that for the first time enables the identification of all classes of biologically-active molecules produced by cells, and combines this with the ability to instantly spatially-display the molecules in tissue histology sections. MSI can be performed with no sample preparation, and no labeling or antibody techniques, thereby integrating direct molecular identification with tissue pathology.
The Company intends to achieve its business objectives by leveraging its core MSI technology and associated bioanalytical mass spectrometry platforms to improve the availability, comprehensiveness and usefulness of molecular information to address the needs of pharmaceutical research, biomarker discovery, agriculture and other life science research markets.
The Company’s commercial development is centered in three business lines:
| · | Molecular Information Services – the Company believes that we are the commercial leader in providing multimodal MSI services, combining LAESI, MALDI, and optical microscopy. Our proprietary services enable the identification and quantitation of both small molecules (e.g. lipids and metabolites) and large molecules (e.g. proteins) and our services portfolio, inclusive of MSI, proteomics, metabolomics, lipidomics and bioinformatics is unique in the industry. Our clients include major pharmaceutical, chemical and biotechnology companies; |
| · | LAESI Instruments, Software and Consumables – we offer our proprietary LAESI DP-1000 instrument, software and bioanalytical consumables to corporate and academic research laboratories; and |
| · | Molecular Diagnostics and Clinical Research – we apply our multimodal MSI technologies and workflows in partnership with top-tier medical research institutions to co-develop new, molecular diagnostic tests that the Company believes can be used to improve the diagnosis and prognosis of cancer and provide requisite information useful in predicting the outcome of cancer treatment. Our current test development programs target the differential diagnosis and prognosis of malignant melanoma and the molecular profiling of subpopulations of cells in lung adenocarcinoma. |
| 38 |
Industry and Market Overview
We believe there is a large and broad-based market opportunity for molecular information. The market opportunity can be viewed in terms of the primary markets for, and the platform technologies that generate, molecular information.
Molecular Information Markets: The Preclinical Pharmaceutical Research and Biomarker Discovery Markets
Preclinical pharmaceutical research refers to the discovery and testing of new therapeutic compound candidates prior to their use in human clinical trials. For every 5,000-10,000 preclinical drug candidates, only five enter human clinical trials. There is a substantial failure rate, estimated that, even after completing all preclinical toxicology and efficacy testing, 90% of drug candidates fail after entering human testing (Nature Reviews 2004).
There is a great need to obtain higher quality datasets more rapidly to better identify the most promising drug candidates. The Pharmaceutical Research and Manufacturers of America (“PhRMA”), an industry group comprised of 45 United States-based biopharmaceutical companies had a total of $49.58 billion in research and development expenditures in 2012. Of this, $11.82 billion was spent on pre-human/preclinical functions, which represented 23.8% of all R&D expenditures. This figure, which is only comprised of R&D by PhRMA member companies, does not include R&D expenditures by government agencies such as the National Institute of Health (NIH) and National Institute of Cancer (NIC) or domestic and international biopharmaceutical companies that are not members of PhRMA.
Biomarker discovery is the process of finding specific molecules, or panels of molecules, that have been found to be regulated in conjunction with specific disease states or drug treatments relevant to human health and disease. The global market for biomarkers in 2011 was $13.8 billion and expected to reach $37.68 billion in 2022. The global market for biomarkers includes biomarker development and technologies, biomarker diagnostics and biomarker services. Biomarker development and technologies accounted for 53.1% of the global market in 2011 and is expected to account for 43.3% of the 2022 market. In 2011, biomarker diagnostics and biomarker services accounted for 40.2% and 6.7% of the market and are expected to account for 49.8% and 6.9% of the market in 2022, respectively (Visiongain 2012. Biomarkers: Technological and Commercial Outlook 2012 - 2022).
Biomarker discovery is the essential starting point for the development of new prognostic and diagnostic tests, as well as for the emerging field of predictive medicine, where biomarkers can identify those patients who will respond well to specific therapies. The human disease biomarker sector seeks to identify and validate biomolecules that are associated with the onset and progression of a specific disease, and thus can become new diagnostics, or biomarkers, to be used for personalized medicine, as well as companion diagnostics to guide new pharmaceutical development for specific patient subgroups.
Biomarker discovery can be classified into four major sub segments: genomics, proteomics, bioinformatics, metabolomics and other, which is based on the technology used and class of molecule that is of interest. Genomics is focused on the analysis of DNA through the use of polymerase chain reaction or DNA sequencing to extract genetic information from biological samples. The Human Genome Project, which was completed in 2003, marked the sequencing of an entire human genome that led to the discovery that there are 20,000 - 25,000 human genes. Proteomics is the study of proteins, which are synthesized by DNA and are biologically active. It is estimated that there are up to 400,000 distinct proteins in the human body. Bioinformatics is comprised of tools and technologies such as software programs that are used to analyze large sets of data.
Additional molecular information markets addressed by Protea include agriculture, microbiology, consumer products and medical devices.
Molecular Information Platform Technologies
The two primary technologies for identifying and characterizing the biologically active molecules that are produced by cells are mass spectrometry and indirect molecular imaging.
Mass Spectrometry
Mass spectrometry is a 50 year old sector and represents the backbone of the bioanalytics industry. Mass spectrometers are used in a wide range of research applications in academic, pharmaceutical and chemical/industrial based laboratories worldwide, including their use to identify molecules in biological samples based on their “mass to charge” ratios.
According to TechNavio’s 2011 - 2015 Global Mass Spectrometry Market report, the global mass spectrometry market was valued at U.S. $3.9 billion in 2013 and is expected to reach US $5.9 billion by 2018, growing at a compound annual growth rate (“CAGR”) of 8.7% from 2013 to 2018. In 2013, it was estimated that there was a total installed base of 6,200 mass spectrometers in the large pharmaceutical companies sub-segment alone. The CAGR for the installed base for this sub-segment is expected to be 3.7% during the period 2011-2015. In 2013, a total of 715 mass spectrometers were expected to be installed, which includes both new mass spectrometer systems and replaced mass spectrometer systems in this sub-segment. Mass spectrometers are used extensively in the biopharmaceutical, industrial, chemical, materials and forensics industries as well as in academic research institutions.
| 39 |
According to the SDI Report, the mass spectrometry instrument market can be segmented into three major sub segments: Biopharmaceuticals, Academia and Industrial which represented 40%, 29% and 31% of the market, respectively, as cited in Strategic Directions International, Inc. and Global Assessment Report 12 th Edition, The Laboratory Analytical & Life Science Instrumentation Industry Market Forecast 2012 - 2016, October 2012 .
Protea estimates that the current LAESI instruments are compatible with approximately 6,000 mass spectrometers worldwide that are produced by Waters Corp. (NYSE:WAT) and Thermo Fisher Scientific (NYSE:TMO). Protea’s LAESI platform development roadmap includes enabling LAESI instrument compatibility with mass spectrometers from all major manufacturers.
The global mass spectrometry market is dominated by five key players: Agilent Technologies Inc., Bruker Corp., Danaher Corp., Thermo Fisher Scientific Inc. and Waters Corp.
| Company | Market Cap USD ($B) | Revenue 2015 USD ($B) | ||||||
| Agilent (NYSE:A) | 15.15 | B | 4.04 | M | ||||
| Bruker (Nasdaq:BRKR) | 3.65 | B | 1.62 | M | ||||
| Danaher (NYSE:DHR) | 53.61 | B | 20.56 | M | ||||
| Thermo Fisher (NYSE:TMO) | 62.64 | B | 16.97 | M | ||||
| Waters Corp. (NYSE:WAT) | 12.85 | B | 2.04 | M | ||||
Source: Capital IQ as of October 28, 2016.
Quantitative Whole Body Autoradiography (“QWBA”)
Autoradiography is defined as an image recorded on a photographic film or plate produced by the radiation emitted from a specimen, such as a section of tissue that has been treated or injected with a radioactively labeled isotope.
Traditional bioanalytical measurements determine concentrations of drug and metabolites in plasma; however, most drugs exert their effects in defined target tissues. As there is no clear relation between concentrations in plasma and those in tissue, alternative methods must be employed to study the absorption, distribution, metabolism and excretion properties of new therapeutic agents. The FDA requires distribution data in most new NDA applications prior to Phase II study initiation. Quantitative whole-body autoradiography is currently used in the drug development process to determine the distribution and concentrations of radiolabeled test compounds in laboratory animals. Quantitative whole-body autoradiography can provide information on tissue PKs, penetration, accumulation and retention. Although the technique is considered the industry standard for performing preclinical tissue distribution studies, It's disadvantages include the high cost and the radiolabeling of the molecule of interest and radiolabeling can take 90 days longer depending on the complexity (synthesis process and label required). Another shortcoming of the technique is that QWBA can take weeks to perform and only indicates the drug associated radioactivity. Additional studies then need to be performed to obtain the drug profile in tissues.
Mass spec imaging eliminates the need for a radiolabel, takes days instead of weeks, looks at the molecule and its metabolites directly eliminating the need for additional profiling studies, and is significantly less expensive than QWBA.
The Total Global indirect molecular imaging market is estimated at $7.3 billion according to a 2009 report by Scienta Advisors /Kolorama Information. The overall pre-clinical imaging (in vivo) market was valued at $790 million in 2012, and is estimated to grow at an annual growth rate of 14.5% over the next five years per a January 2013 Pre-Clinical Molecular Imaging Market report by marketsandmarkets.com. The global market for molecular imaging devices was valued at $2 billion in 2012. This market is expected to reach $2.2 billion in 2013 and nearly $3 billion by 2018, with a CAGR of 6.2% for the period of 2013 to 2018 according to a November 2013 report by BCC-Global Markets for Molecular Imaging Technology and Devices.
Limitations of Existing Technologies
There are a number of limitations to the existing platforms, as described below:
Mass Spectrometry
| · | Sample Preparation is Required: Mass spectrometry requires sample preparation that is tedious, time consuming and results in operating complexity that requires experienced and highly skilled users. |
| 40 |
| · | Loss of Spatial Distribution Information: Traditional mass spectrometry requires that a sample be homogenized, sometimes purified, or have additional chemicals introduced to it in order to be analyzed by a mass spectrometer. These processes destroy the tissue structure, which eliminates the ability to show where a specific molecule is located within a given tissue region. Understanding where a specific compound is localized could provide insight into a given disease or treatment action. |
| · | Limited Type and Number of Compatible Samples: Current mass spectrometry analysis occurs under vacuum conditions and therefore cannot analyze living cell samples such as bacterial colonies or cell cultures because the sample will be immediately destroyed as a result of the vacuum conditions. These are sample types used extensively in preclinical pharmaceutical research and biomarker discovery. |
| · | Limited Imaging Capability: Mass spectra can be very complex data sets that are difficult to interpret and can have limited relevance to biology-centric applications. Additionally, traditional mass spectrometry analyses do not provide direct, spatial relationships with the sample. Normally, bulk sample is processed and analyzed, which causes loss of the spatial association and value. |
Quantitative Whole Body Autoradiography:
| · | require radioactive labels that must be incorporated or synthesized in the drug to generate the images. As a result, they have half-lives, which can be as short as a few minutes in duration. The synthesis of these compounds takes time and requires highly trained technicians. |
| · | are only able to analyze a few molecules in a single analysis. Typically, only the labeled molecules can be visualized and if the label does not become a part of any metabolites, then they remain invisible to the process. |
| · | have resolutions that only reach the millimeter-scale level. While millimeter resolution enables the visualization of large anatomical components such as bones and organs, it is not adequate for tissue or cell-specific molecular analysis. |
| · | can only detect the drug associated radioactivity and not the distribution of the molecule and its metabolites. Further investigation and follow on studies are required to identify the actual distribution profile. |
| · | require special licensing for handling radioactive samples. |
Advantages of LAESI Technology Platform
We believe that our proprietary LAESI TM platform is disruptive technology that provides us with certain competitive advances over existing technologies and which we believe will become a new industry standard.
The LAESI platform eliminates two of the major drawbacks of traditional mass spectrometry - sample preparation and loss of spatial information. LAESI can analyze a wide range of sample types with no sample preparation required. In addition, samples such as a tissue section or cells can be analyzed “as is,” even live cells and bacterial colonies. LAESI technology generates big data molecular profiles of tissue sections, biofluids (blood, urine and serum) and many other sample types, including horticulture specimens, cell lines & pellets, bacterial colonies, hair fibers, contact lenses and hydrogels.
Enables Mass Spectrometry Imaging: Protea’s LAESI technology directly analyzes biological samples without the need to apply chemicals or introduce tags or tracers. Proprietary software developed at the company enables two- and three-dimensional direct molecular imaging, displaying the distribution of molecules in biological samples.
Numerous Molecules per Analysis: It is not unusual to detect over 1,000 individual molecules in a single experiment. Larger databases aid the “molecular eyesight” of a researcher, improving their prospects to find new biomarkers or molecular data that will provide new research insight.
No Sample Preparation Required: Protea’s LAESI technology analyzes samples directly without the need for sample preparation other than standard tissue sectioning. Virtually all sample types can be inserted directly into the LAESI instrument where they are ablated (put into gas phase), ionized (charged) and sent into the mass spectrometer, with this process taking a fraction of a second. LAESI instrumentation employs user-friendly software that eliminates the operation complexity of traditional mass spectrometry. Molecular information is acquired on samples in their native state; such data can be more useful, particularly to biologists.
| 41 |
Minimal Sample Destruction: Because of the small spot size, the sample is only locally destroyed by the laser ablation pulse. With the use of positional stages, LAESI allows, the spatial localization of biomolecules within a sample, thereby enabling two- and three-dimensional direct molecular imaging.
High Throughput Sample Profiling: The LAESI platform also functions as a high throughput processing system for well plate and bacterial analysis. With the ability to analyze samples in seconds, researchers are able to quickly screen libraries of compounds, strains, and other assays very rapidly.
Improvements in Resolution: Protea's LAESI technology has the ability to produce images that have micron-scale resolution, enabling imaging at the cellular level. This gives Protea the ability to image a select number of cells of interest in a given biological sample.
Improvement in Analysis Speed: Due to the elimination of sample preparation and the rapid speed of LAESI, data can be made available in seconds to minutes. This factor makes LAESI attractive to users that have high-throughput molecular information applications (i.e., a large number of samples must be analyzed a limited number of times).
A LAESI Mass Spec Imaging Data File
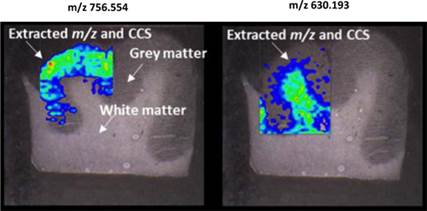
LAESI image of human brain tissue showing marked localization in the gray matter (left) for the lipid at 756 m/z, and white matter (right) of a lipid (m/z 630). This dataset shows the power of LAESI to provide distinct spatial localization visualized for these two lipids, which would be lost with a traditional approach. (Anal Chem. 2015 Jan 20; 87(2): 1137–1144. Source: The Company.)
Our Technology
LAESI Mass Spectrometry
Protea exclusively licensed the technology known as Laser Ablation Electrospray Ionization (“LAESI”) from GWU in 2008, invented in the laboratory of Professor Akos Vertes, Ph.D. Protea has completed the development of a novel bioanalytical instrument platform known as the LAESI DP-1000 Direct Ionization System. This technology enables the direct identification of proteins, lipids and metabolites in tissue, cells and biofluids, such as serum and urine, without any sample preparation prior to analysis. By eliminating sample preparation, the biological sample can be analyzed without the possible contamination, bias or sample loss that occurs with the current techniques, which require the introduction of chemicals, or the destruction of the sample itself, in order to enable analysis by mass spectrometry. The ProteaPlot software then can display the data obtained by mass spectrometry analysis combined with actual images of the tissue and cell samples. Thus, mass spectrometry data can be evaluated in the context of the biology of the sample; this allows the integration of mass spectrometry data with current pathology and microscopic imaging techniques. Data is available in seconds to minutes, allowing rapid time to results and the capacity to analyze thousands of samples in a single work period. As an example, a researcher testing a new drug’s effects on living cells can analyze changes in the cells’ metabolism across a specific time course, thereby almost immediately obtaining data as to the activity of the new drug. The Company believes that LAESI technology has the potential to significantly improve the availability of molecular information in pharmaceutical research, as well as many other fields, including agriculture, pathology, biomarker discovery, biodefense and forensics.
| 42 |
LAESI Illustration
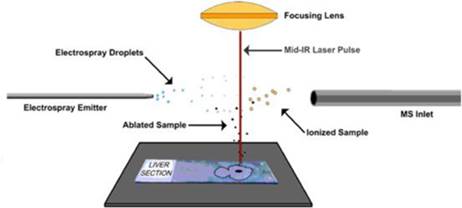
As depicted above, LAESI utilizes a mid-range infrared laser pulse that rapidly (microseconds) boils the water in a given sample creating an ablation event that results in the biomolecules from that particular area being ejected vertically where they meet a stream of electrospray droplets and become charged biomolecules or ions. The ionized sample then moves through an inlet tube and into the mass spectrometer where the mass analyzer determines the mass-to-charge ratio of the representative biomolecules and their relative abundance is determined by the detector.
Key LAESI Components
| 43 |
ProteaPlot TM Direct Molecular Mass Spec Imaging Software
Using its own internal software development team, Protea developed the software platform ProteaPlot to address two other limitations of traditional mass spectrometry: the inability to determine the location of a molecule or ion in a sample and the difficulty a non-analytical chemist has in interpreting mass spectrometry data. ProteaPlot is designed to create images known as ion maps or “molecular maps,” from traditional mass spectrometry data (mass spectrum). These molecular maps depict the distribution of a specific molecule in a given sample and the relative intensity (abundance) of that molecule.
The image of a rat brain below is a screen shot from the ProteaPlot software program. A traditional mass spectrum can be seen on the right side of the image. The image to the left is an ion map of clozapine, an anti-psychotic drug prescribed for the treatment of schizophrenia, with a mass-to-charge ratio (m/z) of 270 Da. The colorimetric scale represents the relative intensity of this ion with blue representing the regions with the lowest relative intensity, the areas in red representing the regions with the highest relative intensity and the areas in black representing regions where that molecule is not present at all.
ProteaPlot Software Screen showing molecular (ion) distribution image.
Molecular (ion) Map of a dosed pharmaceutical, clozapine, using ProteaPlot Software. The histologically stained section is highlighted below to show the correlation of the dosed drug to its target in the cerebral cortex and other regions in the brain.
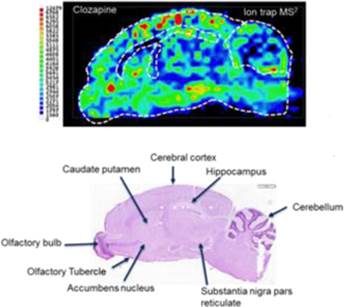
The ability to display molecular information obtained from LAESI as direct molecular images is of great advantage to pathologists who can analyze samples of choice with no sample preparation, and can rapidly obtain molecular information along with traditional microscopic analysis. Also, the remaining sample is not destroyed so ion location information can be obtained and the ion maps can be visualized over the sample of origin for integration with more traditional biological analyses such as histology.
| 44 |
Proprietary MSI Software
In January 2015, the Company announced it had completed the in-house development of a novel workflow and software suite. Known as “Histology Guided Mass Spec Imaging (HG-MSI),” the workflow and software enables pathologists to combine traditional microscopy and histology with high resolution mass spectrometry molecular imaging, allowing researchers to share, annotate and direct the analysis of specific tissue morphologies and cell subpopulations by mass spec imaging. With HG-MSI, molecular profiling data are collected from discrete locations within a tissue section using a histology stained section as a guide. The digital tissue scans are visually analyzed by pathologists, who then annotate specific areas for further analysis. The annotated areas are then targeted by mass spectrometry to acquire a chemical fingerprint of the representative area. This chemical information can then be used to identify specific molecules of interest and map the biomolecules present in a visualized morphology. This workflow is applicable to all classes of biomolecules (e.g., proteins, peptides, lipids, metabolites) and can be carried out on both fresh frozen and formalin fixed, paraffin embedded (FFPE) tissue specimens.
This web interface can be accessed via any device (e.g., laptop, tablet, cell phone, etc.) allowing the end user to visualize, annotate and download microscopy images from anywhere in the world. The development of the ProteaScope portal and HG-MSI workflow and service enables researchers to obtain molecular information from specific regions of tissues, combining optical morphology with spatial biomolecular information Clinicians can share, annotate and direct the analysis of specific tissue morphologies and cell subpopulations by MSI. Understanding the molecular differences between select cell sub-populations supports the identification and characterization of biomarkers within these regions that improves the differential diagnosis of cancer.
Our Business Strategy
Protea intends to achieve its business objectives by leveraging its knowhow and technology to improve the availability, comprehensiveness and usefulness of molecular information to address the needs of the preclinical pharmaceutical research, biomarker discovery and other life science markets. Key strategic elements are the following:
| · | Maintain and advance our commercial leadership in developing and providing mass spectrometry imaging services, combined with existing bioanalytical workflows, to improve the availability, comprehensiveness, and usefulness of molecular information to address the needs of our pharmaceutical research, biomarker discovery, agriculture and other life science research clients. |
| · | Collaborate with platform extending companies including three-dimensional tissue suppliers, biomarker discovery and other commercial technology platforms where the Company’s technology can advance and add value to their capabilities. |
| · | Advance the Company’s market by developing and validating an expanding portfolio of LAESI capabilities and continuously expand the commercial applications for the technology. Our customers continuously provide guidance for new applications and workflows which we can address with the LAESI platform. |
| · | Continuously advance and upgrade our software offering, and introduce new software-driven data bioinformatics capabilities. Protea is focused on creating software and analytical tools that enhance the value of the data, including bioinformatics datamining tools. In January 2015, we announced our development of new software, known as “Histology Guided Mass Spec Imaging (HG-MSI).” The software enables pathologists to combine traditional microscopy and histology with high resolution mass spectrometry molecular imaging, allowing researchers to share, annotate, and direct the analysis of specific tissue morphologies and cell subpopulations by mass spec imaging. |
| · | Partner with first tier medical research laboratories to apply our core MSI technology and workflows to create new, molecular diagnostic tests that can be used to improve the diagnosis and prognosis of cancer. Our current programs target the differential diagnosis and prognosis of malignant melanoma and the characterization of subpopulations of cells in lung adenocarcinoma. |
Our Commercial Offering
Molecular Information Services
We believe we are the commercial leader in the offering of MSI services. Our clients send their tissue and biofluid samples directly to the Company’s laboratory, where samples are analyzed utilizing the Company’s state of the art mass spectrometry instrumentation, scientific expertise and bioinformatics capabilities. We combine our proprietary LAESI platform with MALDI workflows. Our clients include major pharmaceutical, biotechnology, chemical and medical device and consumer products companies. The services unit is operated within a “Quality by Design” environment, which is necessary to meet the internal research and development standards of pharmaceutical and clinical research clients.
| 45 |
The Company’s MSI services represent a revolutionary capability that for the first time enables the identification of all classes of biologically-active molecules produced by cells, and combines this with the ability to instantly spatially-display the molecules (both two- and three-dimensional) in tissue histology sections. MSI can be performed without sample preparation, labeling or antibody techniques, thereby for the first time integrating direct molecular identification with tissue pathology. Since the sample is not touched, data is unbiased and more rapidly available, providing molecular information that rapidly answers questions critical to the drug development process, such as, “is my drug actually getting inside the target cells,” “is my immune modulating agent changing the molecular output of the tumor cell,” or “how long did it take for the drug to enter the target cells” as well as questions of drug concentration and duration.
We have developed workflows specifically for the characterization of therapeutic antibodies, to provide both visual and analytical certainty of therapeutic efficacy, and validation of therapeutic design. Once target tissues are imaged, data analysis can include quantitation of the dosed therapeutic, distribution analysis, and other bioinformatics workflows, including statistical and pathway analysis.
In January 2014, the Company, as a subcontractor to GWU, was awarded a multi-year project with the Defense Advanced Research Projects Agency (DARPA). In addition to Protea, The Stanford Research Institute International and GE Global Research also collaborate on the project entitled, “New Tools for Comparative Systems Biology of Threat Agent Action Mechanisms.” A $14.6 million five year project, the goal of DARPA’s Rapid Threat Assessment (“RTA”) program is to develop new tools and methods to elucidate the mechanism of action of a threat agent, drug, biologic or chemical on living cells within 30 days from exposure. Uncovering the mechanism of action of such agents in 30 days, compared to the years currently required, could be key to the development of effective countermeasures. The molecular networks within living cells are vast and complex. Conventional approaches fail to capture the system-wide response of a living cell to a threat agent.
LAESI Instrument, Software and Consumables
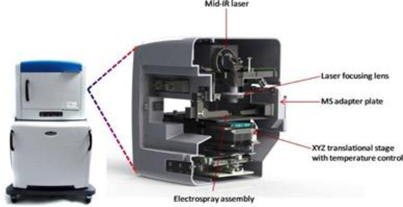
LAESI technology was invented in the laboratory of Professor Akos Vertes, Ph.D., Dept. of Chemistry, GWU, in 2007 and was exclusively-licensed to Protea in 2008. The LAESI DP-1000 instrument, the Company’s prototype embodiment of its proprietary LAESI technology, integrates with laboratory instruments known as mass spectrometers.
This technology enables the direct identification of proteins, lipids and metabolites in tissues, cells and biofluids, such as serum and urine. Data is available in seconds to minutes, allowing rapid time to results and the capacity to analyze thousands of samples in a single work period. As an example, a researcher testing a new drug’s effects on living cells can analyze changes in the cells’ metabolism across a specific time course, thereby almost immediately obtaining data as to the activity of the new drug. The Company believes that LAESI technology has the potential to significantly improve the availability of molecular information in pharmaceutical research, as well as many other fields, including agriculture, pathology, biomarker discovery, biodefense and forensics.
LAESI instruments employ proprietary software developed by the Company that creates “molecular maps” - the ability to instantly display the distribution of molecules throughout tissue sample in both two- and three-dimensional imaging. The Company currently offers the LAESI Desktop Software and ProteaPlot software that facilitates operating the instrument, and the storage and display of datasets in a user friendly, intuitive software environment. The LAESI and ProteaPlot software includes tools for post-processing of LAESI-MS data, generation of molecular maps/images, and mass spectral comparison for regions of interest. LAESI software display the data obtained by mass spectrometry analysis combined with actual images of the tissue and cell samples. Thus, mass spectrometry data can be integrated with a sample’s tissue architecture.
We have developed and brought to market related, proprietary consumable products for use in molecular analysis, including REDIchips TM (Resonance-Enhanced Desorption Ionization - sample target plates with nanopost array surfaces for matrix-free laser desorption ionization of small molecules); and, Progenta TM a(acid labile surfactants use to solubilize proteins for mass spectrometry analysis).
| 46 |
LAESI Applications
Direct Molecular Imaging of Tissues and Cell samples
The LAESI is capable of rapidly analyzing and mapping the spatial distribution of biomolecules in tissue. The LAESI DP-1000 currently has a resolution of 200 (micron) that allows it to analyze a small number of cells per ablation, which occurs in a matter of seconds. The LAESI DP-1000 can analyze and image both human and animal tissue. It is not unusual for over a thousand individual molecular entities to be identified in a single experiment. Below is a LAESI mass spec image of two molecules shown simultaneously in a mouse pup section.
LAESI 2 dimensional map image of four molecules shown simultaneously in a mouse pup brain section
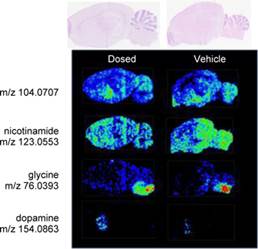
Rapid Profiling of Biofluids and Assays
LAESI is capable of rapidly analyzing micro titer well plates filled with biofluids such as blood, urine or serum. The LAESI Desktop Software makes the automation of this type of analysis very easy for the user. A grid pattern can be quickly drawn for 96, 384 or 1536 sample well plates and the number of ablations per well can be set. Once the user’s settings have been entered the analysis will assess each well systematically and takes less than ten seconds to complete per well. This profiling speed is incredibly useful for researchers with a large number of samples interested in a rapid “snapshot” of the molecular content in a well. This screening application can aid in assay monitoring and the detection of specific compound presence or production.
In Vivo Direct Molecular Imaging
Because LAESI operates at ambient pressure (non-vacuum) it is the only laser based mass spectrometry imaging technology that can analyze vacuum incompatible samples such as living cell cultures and bacterial colonies. Due to this unique capability, LAESI can be used to analyze a bacterial colony and then that colony can be re-incubated and analyzed at a later time, enabling biodynamics studies on living samples for the first time. Below is a LAESI mass spec image of a bacterial colony. This application can be used to rapidly screen and discover potential new pharmaceuticals produced from bacteria and fungi. In addition, LAESI can be used to monitor the production of a specific compound being overexpressed for increased production and yield in bioprocessing workflows.
| 47 |
LAESI 3 dimensional ion molecular map image of a live bacterial colony showing the spatial distribution of an antibiotic molecule (green) and a biomolecule produced by the bacterial cells (orange)
Three-Dimensional LAESI Mass Spectrometry Imaging
LAESI is the only laser based mass spectrometry imaging technology that performs a volumetric sampling. All other mass spectrometry imaging technologies perform surface analysis or desorption whereby they are only extracting small amounts of molecular information. This is a key, unique advantage, as the LAESI DP-1000 mid-IR laser enables it to penetrate samples in the Z-plane, which enables 3 dimensional analysis and imaging. The ProteaPlot software then is able to recompile the molecular data into planes representing each layer of sample analyzed by the LAESI system. Below is a three-dimensional LAESI mass spectrometry image that assessed the distribution of a molecule in a contact lens.
LAESI 2 Dimensional (left) and 3 Dimensional (right) Ion Molecular Map Image of a Contact Lens, displaying the presence, location and intensity of a molecule
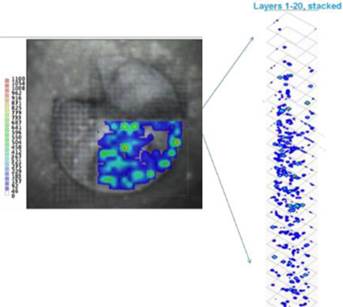
Source: The Company
Molecular Diagnostics and Clinical Research
The Company is employing both its proprietary LAESI platform and MALDI methodologies to create comprehensive, tissue-based molecular profiles to improve the differential diagnosis of cancer. Our bioinformatics capability allows the integration of the Company’s MSI data files with related pathology, gene expression and demographic datasets, with the purpose of improving human disease state detection, assessment and management. The Company has entered into collaborative research partnerships with top tier academic centers to develop and validate new, proprietary, mass spec imaging - based cancer diagnostic tests.
We have established a collaborative research partnership with the Memorial Sloan-Kettering Cancer Center and the Dana-Farber Cancer Institute. The first target of the collaboration is early stage lung adenocarcinoma. The objectives of the collaboration are to demonstrate that different cancer cell sub-groups within a lung cancer will have different molecular profiles and will behave differently. The goal is to define these molecular differences, to identify the sub-group of cancer cells with the worst prognosis that are most likely to recur, thereby enabling earlier treatment intervention, and to use these findings to achieve lung adenocarcinoma tumor cell “molecular profiling,” leading to more precise treatment selection and higher survivor rates.
| 48 |
The market for analysis of indeterminate or borderline analysis is $720 million annually, based upon current pricing. Moreover, 41% of metastatic melanomas were missed by experts in a recent study of borderline melanocytic lesions (need reference). About 500,000 skin biopsies are labeled as indeterminate or borderline every year.
We are advancing our MSI melanoma test with further clinical studies.
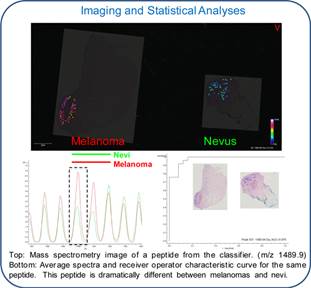
Source: “Mass Spectrometry Imaging - an objective and reliable method to differentiate between benign melanocytic nevi and malignant melanomas”, Rossitza Lazova, MD 1 and Erin H. Seeley, PhD 2 ; 1 Department of Dermatology, Yale University School of Medicine, New Haven, CT. 2 Protea Biosciences, Inc., Morgantown, WV ; October, 2015 Annual meeting of the American Society of Dermatopathology, San Francisco, CA.
Intellectual Property
Protea currently owns eight patents (with additional pending applications) and has an exclusive license to fourteen additional patents and other pending applications owned by GWU. The subjects of the patent applications include: 1) Laser Ablation Electrospray Ionization (“LAESI”) for high throughput and imaging mass spectrometry (two- and three-dimensional biomolecular imaging); 2) nanopost arrays (“NAPA”) for high sensitivity and matrix-free analysis of biological samples in MALDI mass spectrometers; 3) novel acid-cleavable chemical surfactants; and 4) protein microscope.
Research and Development
The Company’s primary research focus is advancing the capabilities of its proprietary LAESI technology platform, related technologies and software. New advances and applications for the LAESI technology platform and software include increasing spatial resolution, improving sensitivity, expanding applications for LAESI direct molecular imaging services, developing new software database structures and next generation instrumentation.
In addition, through the development of diagnostics, the Company is developing intellectual property and knowledge relating to the presence or absence of specific molecules in normal and disease-specific tissues, to be used to validate a new tissue analytics-based class of tests for the differential diagnosis of cancer.
The Company devotes most of its R&D resources to advancing its core LAESI technology platform. New advances and applications for the LAESI technology platform are in development, including the integration of the LAESI-DP 1000 system with expanded mass spectrometer vendor coverage and advancing the spatial resolution of the LAESI technology.
In 2014, we teamed with GWU, Stanford Research International (SRI) and GE Global Research and were awarded up to $14.6 million for a cooperative agreement from DARPA’s Rapid Threat Assessment program.
| 49 |
The goal of DARPA’s Rapid Threat Assessment program is to develop new tools and methods to rapidly define the mechanism of action of a threat agent, drug, biologic or chemical on living cells - within 30 days from the time of exposure. Uncovering the mechanism of action of such agents in 30 days, compared to the years currently required, will aid the development of threat mitigations and countermeasures. Such a breakthrough capability could also reduce the strategic advantage associated with rapidly emerging chemical or biological threats in a national security context. Current methods fail to capture the system-wide response of living cells to a threat agent. To accomplish this objective, Protea has developed a novel silicon wafer known as a “REDIchip” (resonance enhanced desorption ionization), exclusively licensed to Protea by GWU that will help to achieve the goal of real time threat identification on the battlefield.
The company has successfully commercialized the first generation REDIchip product in late 2015. REDIchip can be used on commercially available MALDI mass spectrometers and has numerous applications outside of rapid threat assessment such as biomarker discover, clinical research, polymer science, food safety and others. The Company believes that REDIchip has advantages over existing MALDI mass spectrometry due to the ability to analyze samples without the addition of a chemical matrix, which enables a more sensitive, rapid, and quantitative workflow. The REDIchip is easily integrated into existing MALDI-MS instrumentation and can improve throughput, reduces background signal, and enables reproducible small molecule characterization and quantitation results.
Sales and Marketing
The Company markets its products and services worldwide, utilizing a combination of field sales, distributors, in-house sales support and web-based marketing. The Company attends exhibitions in the U.S. and Europe to present its products, and in the last 12 months has exhibited at over eight major industry exhibitions and over ten regional conferences. Protea's sales and marketing staff consists of three field sales professionals, two inside sales representatives and two marketing professionals. In addition, Protea established a global co-marketing agreement with Waters Corp. (NYSE:WAT), a major mass spectrometry focused company headquartered in Milford, Massachusetts in 2012.
In 2015, Protea announced a joint collaboration with Agilent Technologies, Inc. (NYSE: A) (“Agilent”), to develop new bioanalytical workflows in order to meet the emerging needs of the growing biopharmaceutical industry. Under the terms of the Memorandum of Understanding, Protea, using Agilent instrumentation combined with its technology, will develop workflows that focus on developing new methods for the field of biotherapeutics.
In March 2016, the Company entered into an agreement with an advertising firm for certain services to be rendered to the Company over an estimated ninety (90) day period
Protea also engages distributor partners globally including VWR International and Fisher Scientific.
Competition
The Company believes that its technology differs substantially from what is currently on the market. Bioanalytics is a major sector of the biotechnology industry, and competition is expected to be broad-based. However, the Company also believes that the mass spec industry affords opportunities for commercial partnerships and that industry participants can also be potential partners. The following list of competitors is not intended to be exhaustive, and there are other existing competitors and there likely will be new potential competitors in the future. Many competitors, including most of the companies identified below, are much larger than Protea in terms of capital, employees and other measures of size and have been in business longer than Protea and thus may have greater market acceptance or brand recognition:
| · | Imabiotech (Loos, France). Imabiotech develops software that enables quantitative MALDI mass spectrometry imaging and offers only MALDI based mass spectrometry services to pharmaceutical and biotechnology companies. |
| · | Kinemed (Emeryville, CA). Kinemed works with major pharmaceutical and biotechnology companies to making predictions of efficacy and toxicity to accelerate drug development and reduce the overall cost. |
| · | Prosolia (Indianapolis, IN). Prosolia develops and markets DESI, a mass spectrometry imaging technology and provides DESI based services to pharmaceutical and biotechnology companies. |
| · | Molecular Imaging, Inc. (Ann Arbor, MI). Molecular Imaging is a CRO that provides in vivo pharmacology and small animal in vivo imaging services to pharmaceutical and biotechnology companies. |
| 50 |
Strategic Relationships
Over the past eight years we have established collaborative research initiatives with certain major universities and teaching medical facilities. These include:
Agreement with The George Washington University (“GWU”)
In December 2008, the Company entered into an Exclusive License Agreement, as amended on February 22, 2010 and from time to time thereafter with GWU (Washington, D.C.) for the LAESI technology developed in the laboratory of Akos Vertes Ph.D., Professor of Chemistry, Professor of Biochemistry & Molecular Biology, Founder and Co-Director of the W.M. Keck Institute for Proteomics Technology and Applications, the Department of Chemistry, who is a science advisor to the Company. Under the terms of the license agreement, the Company has the exclusive, worldwide rights to commercialize the technology. The Company is obligated to pay expenses for the preparation, filing and prosecution of future related patent applications governed by the license agreement and related license fees, annual royalties equal to 5% of the net sales of products and processes sold by the Company, or an affiliate that utilizes the subject technology, after taking into account the annual minimum royalty fees described below, and 50% of payments received by the Company in connection with any sublicense of the technology under the agreement. On the first anniversary of either the date on which the Company first sells a product or service utilizing the technology underlying the agreement or the date on which the Company enters into its first sublicense agreement, whichever occurs first (the “First Sale Date”), the Company is required to pay GWU a non-refundable minimum royalty payment equal to $5,000. The university is also entitled to the following non-refundable minimum royalty payments on each subsequent anniversary of the First Sale Date: second anniversary: $10,000; third anniversary: $15,000; fourth anniversary and continuing annually through the expiration or termination of the agreement: $20,000.
Unless earlier terminated in accordance with its terms, the agreement expires upon the later of 20 years from the effective date or the end of the term of the last underlying patent to expire. Currently, the LAESI patent (US 7,964,843) is the underlying patent and will expire on May 21, 2026. The Company has made all the payments required under the agreement, and is otherwise in full compliance with the terms of the agreement.
In November 2012, the Company entered into a Patent License Agreement with GWU for Laser Desorption/Ionization and Peptide Sequencing on Laser-Induced Silicon Microcolumn Arrays (Matrix) and Nanophotonic Production, Modulation and Switching of Ions by Silicon Microcolumn Arrays technology developed in the laboratory of Akos Vertes Ph.D. Under the terms of the license agreement, the Company has an exclusive, worldwide license to make, have made, use, import, offer for sale and sell the licensed products. The Company paid a license initiation fee of $25,000 and a license diligence resource fee of $12,500 in 2013. In addition, the Company is required to pay $20,000 each year thereafter to develop and commercialize the products, $30,000 milestone payment upon the first sale of a licensed product, and royalties will be 7% of the net sales of licensed products and 5% of the net sales of combination products for combined cumulative net sales up to $50 million. Thereafter, royalties reduce to 6% and 4%, respectively, after taking into account quarterly minimum royalties of $1,500 for the first four quarters after the first sale of a licensed product, $2,500 for the next four quarters, $3,500 for the next four quarters and $6,000 for each succeeding quarter. The Company has made all the payments required under the Patent License Agreement, and the Company is otherwise in full compliance with the terms of the Patent License Agreement.
Agreement with West Virginia University (“WVU”)
On December 21, 2005, the Company entered into an Exclusive License Agreement (the “WVU Agreement”) with the West Virginia University Research Corporation, a nonprofit West Virginia corporation (“WVURC”) acting for and on behalf of West Virginia University. Under the terms of the WVU Agreement, the Company is required to pay (i) a license fee equal to $25,000 due within 90 days of the date on which the first notice of allowance is issued by the United States Patent and Trademark Office or a similar foreign country with respect to the technology underlying the WVU Agreement; (ii) annual royalties equal to 4% of the gross sales of products and services that utilize the subject technology which are payable semi-annually within 30 days of June 30 and December 31 of each calendar year covering gross sales received during the preceding year; and (iii) expenses for the preparation, filing and prosecution of related patent applications. In the event the Company is required to license any intellectual property from third parties in order to practice or commercialize the technology underlying the WVU Agreement, the royalty payments will be reduced by the lesser of (a) 50% or (b) the royalty and licensing fees actually incurred by the Company to license the intellectual property rights from such third party. If such a reduction is applicable, WVURC is entitled to earned royalties of at least 2% on gross sales of products and services that utilize the WVU subject technology. For any sublicense granted to sub-licensees, WVURC is entitled to 10% of any license fee and other payments or fees received from the sublicensee, which is due and payable within ten days of receipt by the Company from the sublicensee. At present, the Company sponsors collaborative research in the WVU School of Medicine Department of Pathology and the Mary Babb Randolph Cancer Center (MBRCC).
| 51 |
Unless earlier terminated in accordance with its terms, the WVU Agreement automatically terminates upon the later of: (i) the expiration of the last patent to expire issued in respect of the licensed technology, or (ii) 20 years from the first commercial sale by the Company of the last licensed product included in the subject technology by amendment to the WVU Agreement. At present, there are no patent applications being pursued by WVU in respect of the technology licensed to the Company under the WVU Agreement. The Company has made all the payments required under the WVU Agreement, and the Company is otherwise in full compliance with the terms of the WVU Agreement.
Memorial Sloan-Kettering Cancer Center and the Dana-Farber Cancer Institute
We have established a collaborative research partnership with the Memorial Sloan-Kettering Cancer Center and the Dana-Farber Cancer Institute. The first target of the collaboration is early stage lung adenocarcinoma. The objectives of the collaboration are to demonstrate that different cancer cell sub-groups within a lung cancer will have different molecular profiles and will behave differently. The goal is to define these molecular differences and to identify the sub-group of cancer cells with the worst prognosis that are most likely to recur thereby enabling earlier treatment intervention, and to use these findings to achieve lung adenocarcinoma tumor cell “molecular profiling,” leading to more precise treatment selection and higher survivor rates.
Yale License Agreement
We established a collaborative research initiative with The Yale University School of Medicine that employs the Company’s MSI technology to differentiate melanoma from benign spitz nevi cells. In a recent publication, we showed that MSI can differentiate between benign melanocytic nevi and malignant melanomas in formalin-fixed, paraffin-embedded tissue sections based on proteomic differences, that MSI is an objective and reliable method that may be helpful in difficult cases, in which rendering a firm diagnosis of either benign nevus or malignant melanoma may be very difficult.
In April 2016 we entered into an exclusive license agreement for technology with Yale University related to the differential diagnosis of melanoma, specifically designated as a "Method of Differentiating Benign Melanocytic Nevi from Malignant Melanoma." The technology was co-invented by Dr. Rossitza Lazova, of the Department of Dermatology at Yale School of Medicine, and Dr. Erin Seeley our Clinical Imaging Principal Investigator. Under the terms of the license agreement, we have been granted the exclusive worldwide rights to commercialize the technology. We are obligated to pay expenses for the preparation, filing and prosecution of future related patent applications governed by the license agreement and related license fees. We are obligated to pay a non-refundable royalty of $5,000 payable to Yale University in May 2016, and an annual license maintenance royalty of $2,500 in April 2017 and an annual license maintenance royalty of $5,000 in each anniversary year thereafter. We also pay a non-refundable royalty of $5,000 upon our making our first sale of a licensed product, $7,500 when we file for an approval for commercial sale with a government regulatory agency and $10,000 upon our “first sale” of a licensed product that is approved by such governmental agency. In addition, we will pay Yale an earned royalty of 2.5% on worldwide cumulative net sales of licensed products by our company or under any sub-license or affiliate arrangements, to accrue within thirty (30) days from the end of each calendar quarter, subject to the payment of minimum annual royalties of $10,000, payable on the first day of January in the year following our first sale of licensed products (the “Minimum Royalty Effective Date”), and increasing to $15,000 on the first anniversary of the Minimum Royalty Effective Date, $20,000 on the second anniversary of the Minimum Royalty Effective Date, $30,000 on the third anniversary of the Minimum Royalty Effective Date, $40,000 on the fourth anniversary of the Minimum Royalty Effective Date and $50,000 on the fifth anniversary of the Minimum Royalty Effective Date and each subsequent anniversary year thereafter.
Unless earlier terminated in accordance with its terms, the Yale license agreement expires upon the later of 20 years from the effective date or the end of the term of the last underlying patent to expire.
Sale of European Subsidiary
In December 2014, Protea Biosciences, Inc., our wholly-owned subsidiary, completed the sale of 100% of the issued and outstanding capital stock of ProteaBio Europe SAS, to AzurRx BioPharma, Inc. (“AzurRx”). Pursuant to the terms of the Stock Purchase and Sale Agreement (the “SPA”), we received the following consideration in exchange for the subsidiary:
| (i) | an aggregate amount of $300,000 including $200,000 in cash and $100,000 from the forgiveness of outstanding indebtedness of the Company owed to AzurRx; |
| (ii) | 100 shares of Common Stock of AzurRx (the “Preferred Stock”) that is convertible into 33% of the issued and outstanding Common Stock of AzurRx; and |
| (iii) | the right to receive certain other contingent consideration to be paid upon the satisfaction of certain events, including (a) a one-time milestone payment of $2,000,000 due within (10) days of receipt of the first approval by the FDA of a New Drug Application or Biologics License Application for a Business Product (as such term is defined in the SPA); (b) royalty payments equal to 2.5% of net sales of Business Product up to $100,000,000 and 1.5% of net sales of Business Product in excess of $100,000,000 and (c) ten percent (10%) of the Transaction Value (as defined in the SPA) received in connection with a sale or transfer of the pharmaceutical development business of Protea Europe. |
| 52 |
Effective upon the closing of the Sale, Thijs Spoor, a former director of the Company, was appointed as the sole director and Executive Chairman of AzurRx. While Mr. Spoor was a director of the Company, he did not receive any compensation for his services rendered as an executive officer or director of AzurRx. Pursuant to the SPA, so long as the Company owns such number of shares of Preferred Stock that are convertible into twenty percent (20%) or more of the issued and outstanding Common Stock of AzurRx, the Company will have the right to designate at least one member of AzurRx's board of directors.
In November 2015, the Company converted 29 shares of Common Stock of AzurRx into 707,416 shares of Common Stock of AzurRx. Subsequently, the Company entered into various stock purchase agreements for the sale of such shares of Common Stock of AzurRx and received gross proceeds of $910,000 as of December 31, 2015.
In the first quarter of 2016, the Company notified AzurRx of its intent to convert 71 of its Series A convertible Preferred Stock, $0.0001 par value per share, into shares of AzurRx Common Stock, $0.0001 par value per share, pursuant to the terms and conditions of the Certificate of Designations. The conversion rate was 24,393 shares of Common Stock per share of Common Stock. The Company received 1,731,949 shares of AzurRx Common Stock upon conversion. The Company sold 1,016,941 shares of AzurRx Common Stock during the first quarter of 2016 receiving net proceeds of $637,100. The Company sold an additional 550,000 shares during the second quarter of 2016 receiving net proceeds of $675,000.
As of June 30, 2016, the Company’s ownership interest in AzurRx included 265,757 shares of AzurRx Common Stock, which represented an ownership stake in AzurRx of 3.6% (on a fully-diluted basis). The Company previously accounted for its investment in AzurRx using the “equity method.” However, considering the reduction in the Company’s interest in AzurRx during the three months ended March 31, 2016, the Company discontinued the use of the “equity method” of accounting for this investment and is now accounting for the investment under the cost method.
In July 2016, the Company sold an additional 140,000 shares of AzurRx Common Stock generating net proceeds of $190,000. After this sale, the Company held 125,757 shares AzurRx Common Stock, which represented an ownership stake in AzurRx of 1.7% (on a fully-diluted basis).
Government Regulation
The Company’s products and services are sold for research use only and are not subject to U.S. Food and Drug Administration or other government agency approval.
Sources and Availability of Raw Materials
The Company does not believe that it has any critical issues of availability of raw materials or vendors where there are not multiple sources for the raw materials or vendor support that its business requires.
Environmental Matters
We are subject to various laws and governmental regulations concerning environmental matters and employee safety and health in the United States and other countries. U.S. federal environmental legislation that affects us includes the Toxic Substances Control Act, the Resource Conservation and Recovery Act, the Clean Air Act, the Clean Water Act, the Safe Drinking Water Act and the Comprehensive Environmental Response Compensation and Liability Act (CERCLA). We are also subject to regulation by the Occupational Safety and Health Administration (OSHA) concerning employee safety and health matters. The United States Environmental Protection Agency (EPA), OSHA, and other federal agencies have the authority to promulgate regulations that have an effect on Protea’s operations. As of the date of this Memorandum, we did not have any accrued liabilities related to environmental matters.
Employees
As of the date of this Memorandum, the Company currently has 24 full-time and 1 part-time employee, consisting of 10 technicians and scientists (4 PhD level), 8 management/administrative (2 PhD level), and 7 sales and marketing (1 PhD level). None of our employees are represented by a union.
| 53 |
Facilities
The Company leases laboratory and office space of approximately 10,412 square feet located at White Birch Towers II, 1311 Pineview Drive, Suite 501, Morgantown, WV 26505. The lease has an initial five-year term beginning on April 1, 2012, (the “Initial Term”). The rent during the Initial Term is equal to $16.10 per square foot per year or $13,969 per month. The Company has the exclusive option to renew the term of the lease for an additional five years following the expiration of the Initial Term, (the “Renewal Option”). The Renewal Option must be exercised at least 120 days prior to the end of the Initial Term. If the Renewal Option is exercised, the rent payable during the Renewal Period shall be equal to $17.75 per square foot per year. We have the option to terminate the lease after reaching the thirty-seventh month of the then existing term upon 90 days advance written notice to the lessor and the payment of an amount equal to two months’ rent.
Legal Proceedings
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
There are currently no pending legal proceedings to which the Company or any of its subsidiaries is a party or of which any of their property is the subject that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results. As far as we are aware, no governmental authority is contemplating any such proceeding.
MANAGEMENT
The names, positions and ages of our directors and executive officers as of the date of this Memorandum, are as follows:
| Name | Age | Position | Officer/Director Since | ||||
| Stephen Turner | 70 | Chief Executive Officer, Interim Chief Financial Officer and Chairman of the Board | 2001 | * | |||
| David Halverson (1) | 50 | Vice President and Chief Operating Officer | 2016 | ||||
| Matthew Powell | 40 | Vice President, Research & Development | 2006 | * | |||
| Stanley Hostler | 87 | Vice President, Secretary and Director | 2006 | * | |||
| Steven Antoline | 59 | Director | 2010 | * | |||
| Leonard Harris | 79 | Director | 2003 | * | |||
| Ed Roberson | 70 | Director | 2009 | * | |||
| Scott Segal | 60 | Director | 2008 | * | |||
| Josiah T. Austin | 68 | Director | 2013 | * | |||
| Patrick Gallagher | 51 | Director | 2015 | ||||
| Maged Shenouda | 51 | Director | 2015 |
| * | Represents the date on which such person was appointed to the referenced office of Protea Biosciences, Inc. |
| (1) | Greg Kilby, our former Vice President and Chief Operating Officer, resigned on or about September 15, 2016. Mr. Kilby’s resignation was for personal reasons, but he has agreed to continue to provide us with his part time consulting services following his resignation. We are in the process of discussing the terms of such consulting arrangement which we expect to complete within the next month or two. |
There are no family relationships among any of our executive officers and directors. None of our directors has, during the past ten years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K.
Business Experience
The following is a brief summary of the background of each of our directors and executive officers.
Stephen Turner is Chief Executive Officer and Chairman of the Board, positions he has held since founding the Company in July, 2001. From 1999 to 2001 he served as President and CEO of Quorum Sciences, Inc. From 1984 to 1997 he was President and CEO of Oncor, Inc. He founded Bethesda Research Laboratories, Inc. in 1975 and served as its Chairman and CEO from 1975 to 1983, at which time BRL became the molecular biology division of Life Technologies, Inc. Prior to commencing his career in biotechnology, Mr. Turner held the position of Director of Marketing for the Clinical Microbiology Division of Becton, Dickinson & Co. He received his B.A. from Stanford University in 1967. In 1994 he received the Ernst & Young Entrepreneur of the Year Award in Life Sciences for the Washington D.C. region. Mr. Turner was appointed to serve as a director of the Company because he is the founder of Protea and his deep knowledge of our products and market opportunity led the Board to determine that he should serve as a director.
| 54 |
David Halverson was appointed as our Vice President and Chief Operating Officer in September 2016. Mr. Halverson has been with Protea since 2013. From 2006 to 2011 he served in several positions with Huntingdon Life Sciences including Head of European and US sales. From 2001 to 2006 he served as a Director of Sales for PPD Discovery and Quintiles Preclinical Groups. From 1993 to 2001 he served as a Staff Scientist in the Drug Metabolism Group at Covance Laboratories. He graduated from Kemper Military Academy in 1987 and received a commission as 2LT in the US Army and served on active duty, Wisconsin National Guard, and Army Reserve through 2000. Mr. Halverson was appointed to serve as the Chief Operating Officer because of his in-depth knowledge of Protea’s product lines and wealth of experience negotiating collaborations and contracts within analytical service based companies.
Matthew Powell, Ph.D. is Vice President, Research & Development and Chief Scientific Officer since 2006. He received his Ph.D. in Analytical Chemistry from West Virginia University in 2005. Dr. Powell is considered by the Company to be an expert in the field of biological mass spectrometry and is an inventor of several proprietary bioanalytical technologies in development at the Company.
Stanley Hostler is Vice President, Secretary and Director. He has been a Director of the Company since January 2006 and Vice President and Secretary since June 2006. Mr. Hostler is an attorney with a career practice in the field of labor and employment law. From 2000 to 2010 he served as Special Assistant to the Governor of the State of West Virginia. From 2002 to 2004 he served as Counsel to the Prim Law Firm. From 2000 to 2010 he served on the West Virginia University Foundation Board of Directors, and from 1995 to 2010 on the Advisory Committee of the WVU School of Medicine. He is a graduate of the West Virginia University School of Law (1965). Mr. Hostler’s legal experience and business contacts and relationships with West Virginia University and the State of West Virginia have been an asset to the Company and led the Board to determine that he should serve as a director of the Company.
Steven Antoline joined the Board of Directors in April 2010. He is a successful owner, developer and manager of coal and natural resource properties and inventor of new equipment for coal mining. From 1996 to 2006, he was President and owner of Superior Highwall Mining, Inc., which was sold to a partnership comprised of Lehman Bros. (60%) and Tennessee Valley Ventures (40%). He received his Bachelor of Arts from West Virginia University. Mr. Antoline was appointed to serve as a director of the Company because of his prior experience in the development and sale of companies, and in working with investment bankers.
Leonard Harris joined the Board of Directors in April 2003. Since 1977, he is the founder and Chief Executive Officer of Southern Computer Consultants, Inc. located in Frederick, Maryland, a company which provides products and services to the United States government and Fortune 500 corporations. Mr. Harris’ extensive experience in technology-based corporate development, which provides support and guidance to the Company’s LAESI instrument platform, led the Board to determine that he should serve as a director of the Company.
Ed Roberson joined the Board of Directors in September 2009. From July 2006 to June 2010 he served as Chairman of the Board of the Methodist Healthcare System. He received his MBA in accounting in 1972 from the University of Georgia. From 2006-2011 he was President of Beacon Financial in Memphis, Tennessee, and from 2006-2007 President of Conwood LLC. He was a director of the Paragon National Bank from 2004 to 2016. He is currently a director of Consol Energy. From 1972 to 1992, Mr. Roberson was employed by KPMG, most recently as partner. Mr. Roberson’s experience, both as a Partner with KPMG and subsequently as a CEO, led the Board to determine that he should serve as a director of the Company.
Scott Segal joined the Board of Directors in February 2008. He is a practicing attorney, specializing in the fields of personal injury, product liability and related matters, and is the President of the Segal Law Firm, Charleston, West Virginia. He received his JD from the West Virginia University School of Law in 1981, and has been a member of the American Bar Association from 1981 to present. Mr. Segal has extensive relationships within the State of West Virginia and is considered by the Company to be an expert in several areas which may have use for the Company’s technology, including forensics and occupational health, which led the Board to determine that he should serve as a director of the Company.
Josiah T. Austin joined the Board of Directors in January 2013. Mr. Austin serves as the managing member of El Coronado Holdings, L.L.C., a privately owned investment holding company which invests in public and private companies. He and his family own and operate agricultural properties in the states of Arizona, Montana, and northern Sonora, Mexico through El Coronado Ranch & Cattle Company, L.L.C. and other entities. Mr. Austin previously served on the Board of Directors of Monterey Bay Bancorp of Watsonville, California, and is a prior board member of New York Bancorp, Inc., and North Fork Bancorporation. He has served as a director of Goodrich Petroleum, Inc. since April 2002 and was named to the Board of Directors of Novogen Limited in September 2010. Mr. Austin also serves as a trustee of the Cuenca Los Ojos Foundation Trust, a non-profit organization working to preserve and restore the biodiversity of the borderland region between the United States and Mexico through land protection, habitat restoration and wildlife reintroduction. Mr. Austin graduated from the University of Denver with a Bachelor of Science in Finance in 1971.
| 55 |
Patrick Gallagher joined the Board of Directors in April 2015. Mr. Gallagher is a CFA and an accomplished Capital Markets executive, advisor and investor with a distinguished record of success in both the public and private markets with a focus on healthcare, agriculture and industrials. He has over two decades of experience on Wall Street and extensive experience in alternative investments, research and marketing. He is a Managing Director and Head of Institutional Sales for Laidlaw & Company (UK) Ltd., a healthcare focused investment bank. Mr. Gallagher co-founded Black Diamond Research, LLC (“BDR”), an independent sell-side research firm specializing in healthcare and industrial investing, financing and operations, serving the institutional investing community at large. As CEO of BDR, Mr. Gallagher oversaw institutional research and sales. Prior, he held various sales positions at Kidder Peabody, PaineWebber, New Vernon Associates and Concept Capital. Mr. Gallagher served as VP of Business Development and Investor Relations as well as a strategic consultant for Kinex Pharmaceuticals, a biotechnology firm focused on next-generation therapies in oncology and immunology. He also serves as an advisor to CHD Biosciences, a novel antimicrobial company. Mr. Gallagher sits on the board of directors for BioSig Technologies, Inc., a medical technology company that is developing a proprietary platform in the electrophysiology space. Mr. Gallagher received his B.S. from the University of Vermont, his M.B.A. from Pennsylvania State University and is a CFA charter holder.
Maged Shenouda joined the Board of Directors in April 2015. Mr. Shenouda has over 25 years of experience in the pharmaceutical and securities industries. Most recently, Mr. Shenouda was the Head of Business Development and Licensing at Retrophin, Inc. Prior to that, he served as the Head of East Coast Operations at Blueprint Life Science Group, a strategic investor relations consultancy. Mr. Shenouda spent the bulk of his career as an equity analyst with senior level positions at Stifel Nicolaus, UBS and JP Morgan, covering a broad range of small and large cap biotechnology companies. Mr. Shenouda started his sell-side career with Citigroup and Bear Stearns where his coverage universe focused on U.S. and European pharmaceutical companies. Before entering Wall Street, Mr. Shenouda was a management consultant with PricewaterhouseCoopers’ Pharmaceutical Consulting practice and also spent time in pharmaceutical sales, having worked as a hospital representative and managed care specialist for Abbott Laboratories’ Pharmaceutical Products Division. Mr. Shenouda earned a B.S. in pharmacy from St. John's University and is a registered pharmacist in New Jersey and California. He also received an M.B.A. from Rutgers University Graduate School of Management.
PRINCIPAL STOCKHOLDERS
The following table sets forth certain information, as of October 28, 2016, with respect to the holdings of (1) each person who is the beneficial owner of more than 5% of our Common Stock, (2) each of our directors, (3) each executive officer, and (4) all of our current directors and executive officers as a group.
Beneficial ownership before the date of this Memorandum is determined in accordance with the rules of the Securities and Exchange Commission and includes any shares of Common Stock over which a person exercises sole or shared voting or investment power, or of which a person has a right to acquire ownership at any time within 60 days of the date of this Memorandum. Except as otherwise indicated, we believe that the persons named in this table have sole voting and investment power with respect to all shares of Common Stock held by them. Applicable percentage ownership in the following table is based on 134,226,310 shares of Common Stock plus, for each individual, any securities that individual has the right to acquire within 60 days of October 28, 2016.
To the best of our knowledge, except as otherwise indicated, each of the persons named in the table has sole voting and investment power with respect to the shares of our Common Stock beneficially owned by such person, except to the extent such power may be shared with a spouse. To our knowledge, none of the shares listed below are held under a voting trust or similar agreement, except as noted. To our knowledge, there is no arrangement, including any pledge by any person of securities of the Company, the operation of which may at a subsequent date result in a change in control of the Company.
| Name and Address of Beneficial Owner |
Title | Beneficially Owned* |
Percent of Class** |
|||||||
| Officers and Directors | ||||||||||
| Stephen Turner | Chief Executive Officer and Chairman of the Board | 2,615,525 | (1) | 1.95 | % | |||||
| Stanley Hostler | Secretary and Director | 9,139,222 | (2) | 6.67 | % | |||||
| David Halverson | Vice President and Chief Operating Officer | 142,188 | (3) | - | * | |||||
| Matthew Powell | Vice President, Research & Development | 185,000 | (4) | - | * | |||||
| Steve Antoline | Director | 16,912,678 | (5) | 11.82 | % | |||||
| Leonard Harris | Director | 4,039,958 | (6) | 2.99 | %* | |||||
| Ed Roberson | Director | 645,717 | (7) | - | * | |||||
| Scott Segal | Director | 3,391,483 | (8) | 2.51 | %* | |||||
| Josiah T. Austin | Director | 9,614,381 | (9) | 6.93 | %* | |||||
| Patrick Gallagher | Director | 130,001 | (10) | - | * | |||||
| Maged Shenouda | Director | 150,001 | (11) | - | * | |||||
| Officers and Directors as a Group (total of 13 persons) | 46,966,154 | 30.50 | % | |||||||
| 5% Stockholders | ||||||||||
| El Coronado Holdings, LLC | 14,460,162 | (12) | 10.48 | % | ||||||
| Summit Resources, Inc. | - | 15,348,629 | (13) | 10.73 | % | |||||
| Andreas Wawrla | 18,105,550 | (14) | 12.79 | % | ||||||
| 56 |
| * | Represents ownership under 1%. |
| (1) | Includes 2,197,375 shares of Common Stock, 168,153 shares of Common Stock to be acquired upon the exercise of warrants and 250,000 shares of Common Stock to be acquired upon the exercise of stock options. |
| (2) | Includes 3,702,702 shares of Common Stock, 1,324,204 shares of Common Stock to be acquired upon the exercise of warrants and 716,001 shares of Common Stock to be acquired upon the exercise of stock options. Also includes 2,381,659 shares of Common Stock held by Mr. Hostler’s wife, Virginia Child and 903,422 shares of Common Stock to be acquired upon the exercise of warrants held by Mr. Hostler’s wife, Virginia Child. Also includes 111,234 shares of Common Stock to be acquired upon the exercise of warrants jointly held by Stanley Hostler and Virginia Child. |
| (3) | Includes 142,188 shares of Common Stock to be acquired upon the exercise of stock options. |
| (4) | Includes 185,000 shares of Common Stock to be acquired upon the exercise of stock options. |
| (5) | Includes 1,514,048 shares of Common Stock owned of record by Antoline Trust. Also includes 6,319,426 shares of Common Stock and 9,029,203 shares of Common Stock to be acquired upon the exercise of warrants owned of record by Summit Resources, Inc. As the trustee of the Steve A. Antoline 2006 Irrevocable Trust (the “Antoline Trust”) and president of Summit Resources, Inc., Mr. Antoline has voting and dispositive control over any securities owned of record by the Antoline Trust and Summit Resources, Inc. Therefore, he may be deemed to beneficially own the shares of Common Stock and the shares of Common Stock to be acquired upon the exercise of warrants held of record by the Antoline Trust and Summit Resources, Inc. Includes 50505050,001 shares of Common Stock to be acquired upon the exercise of stock options. |
| (6) | Includes 3,026,735 shares of Common Stock, 713,222 shares of Common Stock to be acquired upon the exercise of warrants and 300,001 shares of Common Stock to be acquired upon the exercise of stock options. |
| (7) | Includes 355,456 shares of Common Stock, 57,260 shares of Common Stock to be acquired upon the exercise of warrants and 233,001 shares of Common Stock to be acquired upon the exercise of stock options. |
| (8) | Includes 2,466,177 shares of Common Stock, 525,305 shares of Common Stock to be acquired upon the exercise of warrants and 400,001 shares of Common Stock to be acquired upon the exercise of stock options. |
| (9) | Includes 4,841,411 shares of Common Stock, 200,001 shares of Common Stock to be acquired upon the exercise of stock options, 3,966,908 shares to be acquired upon the exercise of warrants, and 606,061 shares of Common Stock upon the conversion of a convertible debenture. |
| (10) | Includes 80,000 shares of Common Stock and 50,001 shares of Common Stock to be acquired upon the exercise of stock options. |
| (11) | Includes 100,000 shares of Common Stock and 50,001 shares of Common Stock to be acquired upon the exercise of stock options. |
| (12) | Includes 10,493,250 shares of Common Stock and 3,966,908 shares of Common Stock to be acquired upon the exercise of warrants. |
| (13) | Includes 6,319,426 shares of Common Stock and 9,029,203 shares of Common Stock to be acquired upon the exercise of warrants. As president of Summit Resources, Inc., Mr. Antoline has voting and dispositive control over any securities owned of record by Summit Resources, Inc. Therefore, he may be deemed to beneficially own the shares of Common Stock and the shares of Common Stock to be acquired upon the exercise of warrants held of record by Summit Resources, Inc. |
| (14) | Includes 10,605,556 shares of Common Stock and 7,500,000 shares of Common Stock to be acquired upon the exercise of warrants. |
| 57 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Described below are transactions or series of transactions that occurred from January 1, 2015 through the date of this Memorandum (the “Period Reported”) between us and our executive officers, directors or the beneficial owners of 5% or more of our Common Stock, and certain persons affiliated with or related to these persons, including family members, in which they had or will have a direct or indirect material interest in an amount that exceeds the lesser of $120,000 or 1% of the average of our total assets as of year-end for the last two completed fiscal years, other than compensation arrangements that are otherwise described under “Executive Compensation,” above, which information is incorporated herein by reference. See “Principal Stockholders,” above for information relating to outstanding options and warrants to purchase our Common Stock held by such persons, which information is incorporated herein by reference.
During 2014, the Company received advances equal to an aggregate of $265,000 from Stanley Hostler, a director of the Company. The Company has repaid $125,000 to Stanley Hostler. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this Memorandum.
On May 22, 2014 and October 10, 2014, the Company received advances equal to $30,000 from Stephen and Nancy Turner, our Chief Executive Officer and director of the Company and his spouse. The Company has repaid $20,000 to Mr. Turner. No terms of repayment have been specified on the remaining aforementioned advance as of the date of this Memorandum.
During 2014, the Company received advances equal to an aggregate of $1,415,000 from Summit. In exchange for a portion of the advances received, the Company entered into Note and Warrant Purchase Agreements and issued (a) one-year promissory notes bearing simple interest at the rate of 10% per annum to Summit in an aggregate principal amount of $1,415,000 and (b) five-year warrants to purchase up to 1,415,000 shares of Common Stock at an exercise price of $0.80 per share. On June 3, 2014, the Board approved an increase in the total offering amount of the promissory notes issuable to $1,500,000 from $900,000. The fair value of these warrants was estimated to be $101,177, which was recorded as a discount to the promissory note, and will be accreted based on the repayment of the obligation. The Company repaid $250,000 as of December 31, 2014. Accretion expense of $10,950 was recognized during the year ended December 31, 2014. The outstanding balance, net of discount, was $1,074,773 as of December 31, 2014. On March 20, 2015, the Company converted the $165,000 of outstanding principal and unpaid accrued interest of $105,078, and issued Summit 1,080,312 shares of Common Stock at a conversion price of $0.25 per share and as an inducement to convert, warrants in aggregate of 540,156 to purchase shares of Common Stock at an exercise price of $0.50 per share. The Company recognized accretion expense of $7,227 during the three months ended June 30, 2015. In addition, the Company recognized debt conversion inducement cost related to the fair value of the warrants issued to Summit of $31,455 during the three months ended June 30, 2015. As of December 31, 2015, the outstanding balance was $1,000,000 or $917,000, net of discount.
On March 20, 2015, the Company issued Common Stock in connection with the conversion of $550,262 outstanding principal and accrued unpaid interest of certain convertible promissory notes (the “Notes”) to Summit Resources, Inc. The Notes were converted into 2,201,046 shares of Common Stock determined by dividing the Notes by $0.25 per share. In connection with the conversion of the Notes, the Summit Resources, Inc. also received a 5 year warrant to purchase 1,100,523 shares of the Company’s Common Stock, exercisable at $0.50 per share.
On July 1, 2015, the Company received aggregate gross proceeds of $200,000 from one director and issued a 10% Convertible Promissory Note due on December 31, 2015. The note is convertible into Common Stock at a conversion price of $0.33 per share. The Company also issued 90,910 shares of Common Stock as commitment fee to the director with a price per share of $0.33.
In 2015, the Company received advances equal to an aggregate of $193,000 from Leo Harris a director of the Company. No terms of repayment have been specified on the aforementioned advances as of the date of this Memorandum.
In 2015, the Company received advances equal to an aggregate of $725,000 from Stanley Hostler a director of the Company. The Company repaid $25,000 to Stanley Hostler. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this Memorandum.
On January 30, 2015, the Company received advances equal to an aggregate of $57,500 from Steve and Nancy Turner, our Chief Executive Officer and director of the Company and his spouse. No terms of repayment have been specified on the aforementioned advances as of the date of this Memorandum.
In 2015, the Company received advances equal to an aggregate of $255,000 from Steve Antoline, a director of the Company. The Company repaid $90,000 to Steve Antoline. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this Memorandum.
| 58 |
In 2015, the Company received advances equal to an aggregate of $72,000 from Josiah Austin, a director of the Company. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this Memorandum.
In 2015, the Company received an advance equal to $25,000 from Scott Segal, a director of the Company. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this Memorandum.
In 2015, the Company received advances equal to an aggregate of $30,000 from Ed Roberson, a director of the Company. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this Memorandum.
Patrick Gallagher, one of our Directors, is a Managing Director and Head of Institutional Sales of Laidlaw & Co., an affiliate of the entity that acted as placement agent for the Company’s winter 2013, winter 2014 and fall 2015 private placements, for which the placement agent received cash fees aggregating $1,689,078 and warrants to purchase an aggregate of 7,147,975 shares of Common Stock at exercise prices between $0.25 and $0.50, and was reimbursed for approximately $187,500 expenses. The placement agent and its affiliates beneficially own shares of our Common Stock. See “Security Ownership of Certain Beneficial Owners and Management” and “Description of Securities—Warrants.” The placement agent and its affiliates may provide investment banking and other services to us in the future.
For the four months ended April 30, 2016, the Company’s Board of Directors authorized the conversion of an aggregate of $33,333 of accrued interest on promissory notes issued by the Company to Summit Resources, Inc. (“Summit”), an affiliate of Steven Antoline, who is a director of the Company, and $8,060 of accounts payable by the Company to Summit, into 165,573 shares of Common Stock at the rate of $0.25.
For the four months ended April 30, 2016, the Company issued warrants to purchase 450,000 shares of Common Stock to Summit as consideration for advances. The warrants are exercisable at an exercise price of $0.40 per share for a five year term.
For the four months ended April 30, 2016, the Company received advances equal to an aggregate of $176,375 from certain current directors and related parties. Repayment of $41,875 has been made on the aforementioned advances and repayment of $51,500 on prior advances. No terms of repayment have been specified on the balance of the aforementioned advances.
In August 2016, the Company received advances equal to an aggregate of $44,000 from Stanley and Carl Hostler. No terms of repayment have been specified on this specific advance.
Our $3,000,000 line of credit with United Bank is unconditionally guaranteed by three of our directors and the estate of a former board member, to the maximum extent of $750,000 by each of such individuals. In February 2016, and in consideration for their agreement to extend their personal guarantees of our $3,000,000 line of credit, we agreed to issue, on a pro-rata basis, to such directors and the estate of our former board member, three year warrants to purchase 3,000,000 shares of our Common Stock at an exercise price $0.40 per share.
As of the date of this Memorandum, we are indebted to certain members of our board of directors and the estate of a former director for loans and advances made to our Company over the past five years in the aggregate amount of $2,695,100.
DESCRIPTION OF CAPITAL STOCK
The following description of our securities is only a summary, and is qualified in its entirety by reference to the actual terms and provisions of the capital stock contained in our articles of incorporation and our bylaws.
Common Stock
Our Certificate of Incorporation currently permits us to issue up to 500,000,000 shares of Common Stock. Our 134,226,310 outstanding shares of Common Stock, plus all additional shares of Common Stock that are issuable upon conversion of outstanding notes and exercise of outstanding options and warrants (the “Outstanding Common Stock Equivalents”) totals 242,837,881 shares of Common Stock and Common Stock Equivalents.
We are currently authorized by our Certificate of Incorporation to issue up to 500,000,000 shares of Common Stock. As of September 26, 2016, there were (i) issued and outstanding, an aggregate of 134,226,310 shares of our Common Stock, (ii) 83,266,937 additional shares of Common Stock issuable upon exercise of outstanding warrants, (iii) 10,003,550 additional shares of Common Stock issuable upon conversion of outstanding convertible notes, and 8,780,086 additional shares of Common Stock issuable upon exercise of stock options. Accordingly, as at September 26, 2016, our fully-diluted Common Stock was approximately 243,957,380 shares of Common Stock. After giving effect to the sale of all 500 Units in this Offering, our fully-diluted Common Stock would be approximately 443,956,880 shares of Common Stock.
| 59 |
Based on our current and anticipated fully-diluted Common Stock and additional shares that are potentially issuable upon conversion of Bridge Notes, exercise of Bridge Note Warrants, as well as the exercise of the Class A Warrants and Class B Warrants that we propose to issue and sell in this Offering, we do not have enough authorized shares of Common Stock reserved for issuance. Accordingly, as set in this Memorandum we intend to seek stockholder approval to increase the total number of shares of Common Stock we are authorized to issue under our Certificate of Incorporation to up to 500,000,000 shares and consummate a 1:25 reverse stock split.
On October 24, 2016, we amended our Certificate of Incorporation which now currently permits us to issue up to 500,000,000 shares of Common Stock. We intend to consummate the Reverse Stock Split on or prior to the Termination Date of this Offering. Consummation of the Reverse Stock Split will require (a) the filing with the Delaware Secretary of State of a further amendment to our Certificate of Incorporation, and (b) obtaining the approval of the Financial Industry Regulatory Authority.
No fractional shares of Common Stock will be issued in connection with the Reverse Stock Split, and all such fractional interests will be rounded down to the nearest whole number. Issued and outstanding stock options, convertible notes and warrants will be split on the same basis and exercise prices will be adjusted accordingly.
The holders of our Common Stock are entitled to the following rights:
Voting Rights
Each share of our Common Stock entitles its holder to one vote per share on all matters to be voted or consented upon by the stockholders. Holders of our Common Stock are not entitled to cumulative voting rights with respect to the election of directors.
Dividend Rights
Subject to limitations under Delaware law and preferences that may apply to any shares of preferred stock that we may decide to issue in the future, holders of our Common Stock are entitled to receive ratably such dividends or other distributions, if any, as may be declared by our Board out of funds legally available therefor
Liquidation Rights
In the event of the liquidation, dissolution or winding up of our business, the holders of our Common Stock are entitled to share ratably in the assets available for distribution after the payment of all of our debts and other liabilities, subject to the prior rights of the holders of our preferred stock.
Other Matters
The holders of our Common Stock have no subscription, redemption or conversion privileges. Our Common Stock does not entitle its holders to preemptive rights. All of the outstanding shares of our Common Stock are fully paid and non-assessable. The rights, preferences and privileges of the holders of our Common Stock are subject to the rights of the holders of shares of any series of preferred stock which we may issue in the future.
Preferred Stock
Our authorized preferred stock consists of 10,000,000 shares of preferred stock, par value $0.0001 per share. As of the date of this Memorandum, no shares of preferred stock are outstanding. Our Board has the authority to issue preferred stock in one or more classes or series and to fix the designations, powers, preferences, and rights, and the qualifications, limitations or restrictions thereof including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption, redemption prices, liquidation preferences and the number of shares constituting any class or series, without further vote or action by the stockholders.
Warrants
The Class A Warrants will have a term of 18 months and will entitle the holders to purchase up to 66,666,667 shares of Common Stock at an exercise price of $0.09 per share, if all 500 Units are sold in this Offering. Upon consummation of the Reverse Stock Split, the maximum number of shares of Common Stock issuable upon full exercise of the Class A Warrants will be 2,666,660 shares of Common Stock (the “Class A Warrant Shares”).
The Class B Warrants will have a term of five years and will entitle the holders to purchase up to 66,666,667 shares of Common Stock at an exercise price of $0.1125 per share, if all 500 Units are sold in this Offering. Upon consummation of the Reverse Stock Split, the maximum number of shares of Common Stock issuable upon full exercise of the Class B Warrants will be 2,666,660 shares of Common Stock (the “Class B Warrant Shares” and together with the Class A Warrant Shares, the “Warrant Shares”).
| 60 |
Upon consummation of the 1:25 Reverse Stock Split, the 133,333,000 Warrant Shares shall be reduced to 5,333,320 Warrant Shares, and the exercise prices of the Class A Warrants and Class B Warrants shall be increased to $2.25 and $2.8125, respectively.
The exercises prices of the Class A Warrants and Class B Warrants shall both be adjusted if the Company sells Common Stock or securities convertible into or issuable for common stock at a price per share that is lower than their respective exercise prices (as adjusted by the Reverse Stock Split); in which event, the exercise prices would be adjusted to the lower price.
The form of Class A Warrant is annexed as Exhibit C to this Memorandum and the form of Class B warrant is annexed as Exhibit D to this Memorandum.
Anti-Dilution Adjustments.
An aggregate of 21,214,642 shares of the Company’s Common Stock issued in connection with warrants and convertible notes are subject to full ratchet anti-dilution rights until the earlier of (1) five years from the closing date on which the purchaser received the securities or (2) the date on which any purchaser no longer owns shares of Common Stock, if the Company issues or sells any shares of Common Stock or any Common Stock Equivalent pursuant to which shares may be acquired at a price less than $0.25 per share (the “Base Price”) (subject to appropriate adjustments for any stock dividend, stock split, stock combination, reclassification or similar transaction). In addition, 42,394,317 shares of the Company Common Stock issuable under other warrants and convertible notes are subject to weighted average anti-dilution adjustments if Common Stock or Common Stock Equivalents are issued at exercise or conversion prices lower than those contained in such warrants or convertible notes; which adjustments requires the Company to issue additional shares of Common Stock to each purchaser, for no additional consideration, in an amount sufficient such that the pro rata portion of the purchase price paid by such purchaser for the shares of Common Stock then held, when divided by the total number of shares of Common Stock then held by such purchaser plus those shares of Common Stock issued as a result of the dilutive issuance will equal the applicable conversion price or exercise price.
As of the date of this Memorandum, there are other warrants to purchase a total of 82,688,812 shares of Common Stock outstanding at the exercise prices ranging between $0.25 per share and $2.25 per share. However, as a result of the sale of Common Stock convertible into our Common Stock at a Conversion Price of $0.075 per share, the exercise price of the 42,394,317 warrants that are each exercisable at $0.25 per share shall be reduced to $0.075 per share ($1.875 after the Reverse Stock Split) based on the “full ratchet” anti-dilution provisions set forth therein, and the exercise prices other warrants shall be subject to reduction in their exercise prices based on the weighted average anti-dilution adjustments set forth therein.
Governing Documents that May Have an Antitakeover Effect
Certain provisions of our Certificate of Incorporation, as amended, and our Second Amended and Restated Bylaws (the “Bylaws”), which are discussed below could discourage or make it more difficult to accomplish a proxy contest, change in our management or the acquisition of control by a holder of a substantial amount of our voting stock.
Our Certificate of Incorporation provides that our Board has the authority to issue preferred stock in one or more classes or series and fix such designations, powers, preferences and rights and the qualifications thereof without further vote by our stockholders. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control of the Company without further action by the stockholders and may adversely affect the voting and other rights of the holders of our Common Stock.
Our Bylaws limit the ability to call special meetings of the stockholders to the Chairman of the Board, or the Chief Executive Officer, or, if there is no Chairman or Chief Executive Officer, then by the President. The stockholders have no right to request or call a special meeting; however, any action of stockholders required to be taken at any annual or special meeting, may be taken without a meeting, and without prior notice, provide that the written consent is signed by the holders of majority of the total voting power of outstanding shares of stock of the Company entitled to vote.
Our Bylaws provide that the removal of a director from the Board, with cause, must be by affirmative vote of not less than a majority of the voting power of our issued and outstanding stock entitled to vote generally in the election of directors (voting as a single class), excluding stock entitled to vote only upon the happening of a fact or event unless such fact or event shall have occurred, is required to remove a director from the Board with or without cause.
Transfer Agent and Registrar
Our transfer agent and registrar is Island Stock Transfer, located at 15500 Roosevelt Blvd, Clearwater, FL 33760. Its telephone number is 1-727-289-0010.
| 61 |
RESTRICTIONS ON TRANSFER OF SECURITIES
The Securities (including any shares issuable upon exercise of the Warrants) are subject to restrictions on transfer and have not been registered under the Securities Act. Such Securities must be held indefinitely unless:
| · | there is in effect a registration statement under the Securities Act covering the proposed disposition or transfer and such disposition or transfer is made in accordance with such registration statement; |
| · | you notify us of the proposed disposition or transfer and obtain a legal opinion from our counsel or from outside counsel, at your cost and reasonably satisfactory to us, that such disposition or transfer will not require registration under the Securities Act; |
| · | the Securities are sold pursuant to an exemption from the registration requirements of the Securities Act afforded by Rule 144 of the Securities Act or similar rule then in effect, (or Section 4(1) under the Securities Act and our counsel, or an outside counsel reasonably satisfactory to us, provides a legal opinion, at your cost, that such disposition is exempt from registration under the Securities Act; or |
| · | the restrictive legend may be removed, without volume or manner of sale requirements, pursuant to Rule 144 under the Securities Act, if applicable, or Section 4(1) under the Securities Act, and we have, or our counsel has, at our cost, instructed our transfer agent as to such legend removal. |
The Securities issued in this Offering (including the shares issuable upon exercise of the Warrants), will bear a legend setting forth these restrictions on transfer and any legends required by state securities laws.
Investor Suitability Standards
PURCHASE OF THE SECURITIES INVOLVES SIGNIFICANT RISKS AND IS A SUITABLE INVESTMENT ONLY FOR CERTAIN TYPES OF POTENTIAL INVESTORS. SEE “RISK FACTORS.”
The purchase of the Securities is suitable only for investors who have no need for liquidity in their investments and who have adequate means of providing for their current needs and contingencies even if the investment in the Securities results in a total loss. The Securities will be sold only to prospective investors that qualify as “accredited investors” under Regulation D promulgated under the Securities Act and as amended by the Dodd-Frank Wall Street Reform and Consumer Protection Act. “Accredited Investors” are those investors which make certain written representations that evidence that the investor comes within one of the following categories:
| (1) | any bank as defined in Section 3(a)(2) of the Securities Act or any savings and loan association or other institution as defined in Section 3(a)(5)(A) of the Securities Act whether acting in its individual or fiduciary capacity; any broker dealer registered pursuant to Section 15 of the Exchange Act; any insurance company as defined in Section 2(13) of the Securities Act; any investment company registered under the Investment Company Act of 1940 or a business development company as defined in Section 2(a)(48) of that act; any Small Business Investment Company licensed by the U.S. Small Business Administration under Section 301(c) or (d) of the Small Business Investment Act of 1958; any plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions, for the benefit of its employees, if such plan has total assets in excess of $5,000,000; any employee benefit plan within the meaning of the Employee Retirement Income Security Act of 1974, if the investment decision is made by a plan fiduciary, as defined in Section 3(21) of such act, which is either a bank, savings and loan association, insurance company, or registered investment adviser, or if the employee benefit plan has total assets in excess of $5,000,000, or, if a self-directed plan, with investment decisions made solely by persons that are accredited investors; |
| (2) | any private business development company as defined in Section 202(a)(22) of the Investment Advisers Act of 1940; |
| (3) | any organization described in Section 501(c)(3) of the Internal Revenue Code, corporation, Massachusetts or similar business trust, or partnership, not formed for the specific purpose of acquiring the Securities offered, with total assets in excess of $5,000,000; |
| (4) | any director or executive officer of the Company; |
| (5) | any natural person whose individual net worth, or joint net worth with that person's spouse, presently exceeds $1,000,000. For purposes of calculating net worth under this paragraph, (i) the primary residence shall not be included as an asset, (ii) to the extent that the indebtedness that is secured by the primary residence is in excess of the fair market value of the primary residence, the excess amount shall be included as a liability, and (iii) if the amount of outstanding indebtedness that is secured by the primary residence exceeds the amount outstanding 60 days prior to investing in this Offering, other than as a result of the acquisition of the primary residence, the amount of such excess shall be included as a liability. |
| 62 |
| (6) | any natural person who had an individual income in excess of $200,000 in each of the two most recent years or joint income with that person's spouse in excess of $300,000 in each of those years and has a reasonable expectation of reaching that same income level in the current year; |
| (7) | any trust with total assets in excess of $5,000,000, not formed for the specific purpose of acquiring the Securities offered, whose purchase is directed by a sophisticated person as described in Rule 506(b)(2)(ii) of Regulation D; and |
| (8) | any entity in which all of the equity owners are accredited investors. |
Prospective investors will be required to represent in writing that they meet the suitability standards set forth above, which represent minimum suitability requirements for prospective investors. Satisfaction of such standards by a prospective investor does not mean that the Securities are a suitable investment for such investor. In addition, certain states may impose additional or different suitability standards which may be more restrictive.
As used in this Memorandum, the term “net worth” means the excess of total assets over total liabilities. In determining income, an investor should add to his or her adjusted gross income any amounts attributable to tax-exempt income received, losses claimed as a limited partner in any limited partnership, deductions claimed for depreciation, contributions to an IRA or Keogh retirement plan, alimony payments and any amount by which from long-term capital gains has been reduced in arriving at adjusted gross income. For purposes of calculating net worth under this paragraph, (i) the primary residence shall not be included as an asset, (ii) to the extent that the indebtedness that is secured by the primary residence is in excess of the fair market value of the primary residence, the excess amount shall be included as a liability, and (iii) if the amount of outstanding indebtedness that is secured by the primary residence exceeds the amount outstanding 60 days prior to the execution of this Subscription Agreement, other than as a result of the acquisition of the primary residence, the amount of such excess shall be included as a liability.
We may make or cause to be made such further inquiry and obtain such additional information as we deem appropriate with regard to the suitability of prospective investors. We may reject subscriptions in whole or in part if, in our discretion, we deem such action to be in our best interests. If the Offering is oversubscribed, we will determine at our option, whether over-subscriptions will be accepted and if so, which subscriptions will be accepted.
If any information furnished or representations made by a prospective investor or others acting on its behalf mislead us as to the suitability or other circumstances of such investor, or if, because of any error or misunderstanding as to such circumstances, a copy of this Memorandum is delivered to any such prospective investor, the delivery of this Memorandum to such prospective investor shall not be deemed to be an offer and this Memorandum must be returned to us immediately.
OFFERING PERIOD AND SUBSCRIPTION PROCEDURES
The Company is offering up to $5,000,000 of units of our securities (the “Units”), consisting of a maximum of (a) 66,666,667 shares of our common stock, par value $0.0001 per share (the “Common Stock”), (b) 18 month warrants to purchase up to 66,666,667 shares of common stock, par value $0.0001 per share (the “Common Stock”), at an exercise price of $0.09 per share (the “Class A Warrants”), and (c) five year warrants purchase up to 66,666,667 shares of Common Stock at an exercise price of $0.1125 per share (the “Class B Warrants” and together with the Class A Warrants the “Warrants”). The Common Stock included in the Units is being offered at a price of $0.075 per share. The Common Stock and the Warrants are sometimes referred to herein collectively as the “Securities.”
A minimum of 50 Units for aggregate gross proceeds of $500,000 (the “Minimum Offering”) and a maximum of 500 Units for aggregate gross proceeds of $5,000,000 (the “Maximum Offering”) are being offered by the Company in this Offering. Each Unit consists of (a) 133,333.33 shares of Common Stock, (b) Class A Warrants to purchase 133,333.33 shares of Common Stock at an exercise price of $0.09 per share, and (c) Class B Warrants to purchase 133,333.33 shares of Common Stock at an exercise price of $0.1125 per share. The minimum subscription from qualified investors shall be one full Unit for a minimum purchase price of $10,000. However, the Company and the Placement Agent described below may accept subscriptions from qualified investors for a lesser amount at their sole discretion. The Company and the Placement Agent reserve the right to reject any proposed subscription.
Upon acceptance by the Company after the date hereof of subscriptions from qualified investors for the Minimum Offering, the Placement Agent (as defined herein) and the Company shall have the right at any time and prior to the Termination Date (as defined below), to effect periodic closings (each a “Closing”) for subscriptions for Securities from investors until the earlier of (i) the date upon which subscriptions for the Maximum Offering offered hereunder have been accepted, (ii) December 31, 2016 (subject to the right of the Company and the Placement Agent to extend the Offering until as late as March 31, 2017 without further notice to investors), or (iii) the date upon which the Company elects to terminate the Offering (the “Termination Date”). The Offering will be made on an “all or none” basis for the Minimum Offering and on a “reasonable efforts” basis for up to the Maximum Offering. The Company may terminate the Offering at any time even if Securities having an aggregate purchase price equal to the Maximum Offering has not been sold. The subscription amount for the Securities will be held in escrow for the benefit of subscribers by Signature Bank (the “Escrow Agent”) until satisfaction of all the conditions to each Closing.
| 63 |
Affiliates, and the officers and directors, of the Company, may purchase Securities in the Offering for their own accounts on the same terms as set forth herein.
We shall be responsible for paying the legal fees and expenses incurred in connection with the registration of the Securities being sold in this Offering under the state blue sky laws of the various states in which the Securities are sold.
If after careful review of this Memorandum, completion of your investigation of the Company, consideration of the risks involved in an investment in the Securities, satisfaction of all questions or concerns related to such an investment decision, and your determination that you meet the suitability requirements provided herein and in the subscription documents, you wish to subscribe for the Securities, then review, complete and deliver the subscription documents and the purchase price as directed herein prior to the date the Offering terminates.
By signing and returning the Subscription Agreement to us, you will:
Commit to purchase the number of Securities that you enter on the signature page, at the price specified on that page, if we accept your subscription;
Make various representations and warranties to us, including that you:
| · | Recognize that an investment in our Securities is speculative and involves a high degree of risk, |
| · | Are a knowledgeable and experienced investor, and an accredited investor within the meaning of Regulation D under the Securities Act and as amended by the Dodd-Frank Wall Street Reform and Consumer Protection Act, |
| · | Are purchasing the Securities for your own account, for investment, not with a view to the resale or distribution of the Securities until a registration statement is declared effective by the Commission or you are permitted to sell under Rule 144 and that the Securities (including the Warrant Shares) will contain a restrictive legend to that effect, |
| · | Must bear the economic risk of your investment in the Securities unless and until a registration statement is declared effective by the Commission or you are permitted to sell under Rule 144, which rule does not become available for at least six months and contains specified limitations and requirements, and |
| · | Were given access to any information about us that you requested, including the opportunity to ask questions of our management. |
To subscribe for the Securities offered herein:
| · | Review, complete execute and deliver to the Placement Agent (at the address provided in the Subscription Agreement) prior to the Termination Date the Subscription Agreement attached to this Memorandum as Exhibit A; and |
| · | Deliver to the Escrow Agent, prior to the Termination Date, the full purchase price for the Securities you wish to purchase by check or by wire transfer using the wire transfer instructions provided in the Subscription Agreement. Wires should include the account number and the Escrow Agent’s routing number (as indicated in Exhibit A attached hereto - the Subscription Agreement). |
The subscription documents and the funds representing the purchase price will be held by the Escrow Agent until acceptance of the subscription and satisfaction of all closing conditions to this Offering. You may not withdraw funds deposited into escrow. The Company may accept any subscription in whole or in part, or reject any subscription, in its sole discretion for any reason whatsoever and terminate this Offering at any time prior to its acceptance of subscriptions. In the event that your subscription is rejected or this Offering is otherwise terminated or withdrawn, funds delivered by you to the Escrow Agent will be returned to you without interest or deduction. If this Offering is oversubscribed, the Company may determine, in its sole discretion, to reject subscriptions in whole or in part or to allocate to any prospective investor less than the number of Securities to which the investor subscribed, subject to the Company’s obligation to return to any prospective investor funds transmitted by such investor in respect of a rejected subscription, in whole or in part.
| 64 |
We have agreed to pay Laidlaw & Company (UK) Ltd. (the “Placement Agent” or “Laidlaw”) (i) a cash commission in the amount often percent (10%) of the gross proceeds of the Offering received from investors other than certain investors previously identified to the Placement Agent by the Company (“Protea Investors”), (ii) two percent (2%) of the gross proceeds of the Offering received from Protea Investors, and (iii) an activation fee of $25,000. In addition, the Placement Agent will be entitled to receive three (3) year warrants to purchase such number of shares of Common Stock of the Company equal to (x) ten percent (10%), in the case of investors other than Protea Investors, of the aggregate number of securities sold in the Offering at an exercise price equal to the lowest price per share of the securities sold in the Offering. The Placement Agent shall also be entitled to a non-allocable expense reimbursement in the amount of two percent (2%) of the gross proceeds of the Offering, including amounts received from Protea Investors. After the Termination Date, assuming the closing on at least $1,000,000 in gross proceeds for the Units, the Company will pay the Placement Agent a non-refundable financial advisory fee of $150,000, to be paid monthly, at the rate of $25,000 per month for a term of six months beginning on the Termination Date.
No assurance can be given that all or any portion of Securities offered hereby will be sold. The Placement Agent and the Company have established an escrow account at Signature Bank, the Escrow Agent.
On each Closing date of the Offering, the Escrow Agent will release the funds and the subscription documents pursuant to the terms and conditions of the escrow agreement. Promptly following each such Closing date, the Company will issue to the investors the certificates representing the Common Stock and Warrants purchased in this Offering.
REPORTS AND AVAILABLE INFORMATION
A copy of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2015 is annexed to this Memorandum as Exhibit F and a copy of the Company’s Quarterly Report on Form 10-Q for the fiscal period ended June 30, 2016 is annexed to this Memorandum as Exhibit G. Prospective Investors are strongly urged to review in detail such Exhibits, in addition to this Memorandum.
The Company files reports and other information with the Commission. Such reports, statements and other information filed by the Company with the Commission can be inspected and copied at the public reference facilities maintained by the Commission at 100 F Street, N.E, Washington, D.C. 20549. You can request copies of these documents, upon payment of a duplicating fee, by writing to the Commission. Please call the Commission at 1-800-SEC-0330 for further information on the operation of the public reference room. The Company’s filings are also available to the public through the Commission’s website at www.sec.gov.
ADDITIONAL INFORMATION
We will make available to each prospective investor the opportunity to ask questions of, and receive answers from, us or a person acting on our behalf concerning the terms and conditions of this Offering, the Company or any other relevant matters. The Company will respond with any additional information necessary and not of a proprietary nature to verify the accuracy of the information set forth in this Memorandum, to the extent that we possess such information or can acquire it without unreasonable effort or expense.
Any investor may ask questions and receive answers concerning the terms and conditions of the Offering or request additional information to verify the information contained herein by contacting a representative of the Placement Agent.
Any additional information furnished by the Company may be proprietary and confidential.
| 65 |

Up to $5,000,000
Common Stock and Warrants
CONFIDENTIAL PRIVATE PLACEMENT MEMORANDUM
October 31, 2016
