Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Blueprint Medicines Corp | bpmc_8kpresentation.htm |
Exhibit 99.1
|
|
A Blueprint for a Healthier Tomorrow April 5, 2017 |
|
|
Forward-looking statements 2 This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. In this presentation, forward-looking statements include, without limitation, statements about plans and timelines for the clinical development of BLU-285, BLU-554 and BLU-667 and the ability of Blueprint Medicines Corporation (the “Company”) to implement those clinical development plans; the potential benefits of the Company’s current and future drug candidates in treating patients; plans and timelines for regulatory submissions, filings or discussions; plans and timelines for the development and commercialization of companion diagnostics for the Company’s current or future drug candidates; plans and timelines for current or future discovery programs; plans and timelines for future collaborations, if any, with strategic partners; the future financial performance of the Company; expectations regarding potential milestones in 2017; expectations regarding the Company’s existing cash, cash equivalents and investments; and the Company’s strategy, business plans and focus. The Company has based these forward-looking statements on management’s current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks, uncertainties and other important factors, many of which are beyond the Company’s control and may cause actual results, performance or achievements to differ materially from those expressed or implied by any forward-looking statements. These risks and uncertainties include, without limitation, risks and uncertainties related to the delay of any current or future clinical trials or the development of the Company’s drug candidates, including BLU-285, BLU-554 and BLU-667; the Company's advancement of multiple early-stage efforts; the Company’s ability to successfully demonstrate the efficacy and safety of its drug candidates; the preclinical and clinical results for the Company’s drug candidates, which may not support further development of such drug candidates; actions or decisions of regulatory agencies or authorities, which may affect the initiation, timing and progress of current or future clinical trials; the Company’s ability to obtain, maintain and enforce patent and other intellectual property protection for any drug candidates it is developing; the Company’s ability to develop and commercialize companion diagnostics for its current and future drug candidates, including a companion diagnostic for BLU-554 with Vantaa Medical Systems, Inc. and a companion diagnostic for BLU-285 with QIAGEN Manchester Limited; and the success of the Company’s rare genetic disease collaboration with Alexion Pharma Holding and its cancer immunotherapy collaboration with F. Hoffmann-La Roche Ltd and Hoffmann-La Roche Inc. These and other risks and uncertainties are described in greater detail under “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2016, as filed with the Securities and Exchange Commission (“SEC”) on March 9, 2017, and any other filings the Company may make with the SEC in the future. The Company cannot guarantee future results, outcomes, levels of activity, performance, developments, or achievements, and there can be no assurance that the Company’s expectations, intentions, anticipations, beliefs, or projections will result or be achieved or accomplished. The forward-looking statements in this presentation are made only as of the date hereof, and except as required by law, the Company undertakes no obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or otherwise. This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company’s industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of the Company’s future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk. |
|
|
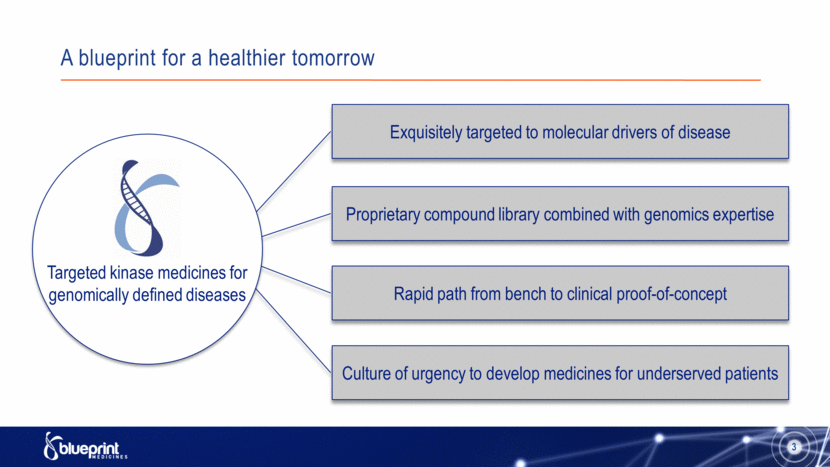
A blueprint for a healthier tomorrow 3 Proprietary compound library combined with genomics expertise Exquisitely targeted to molecular drivers of disease Rapid path from bench to clinical proof-of-concept Culture of urgency to develop medicines for underserved patients Targeted kinase medicines for genomically defined diseases |
|
|
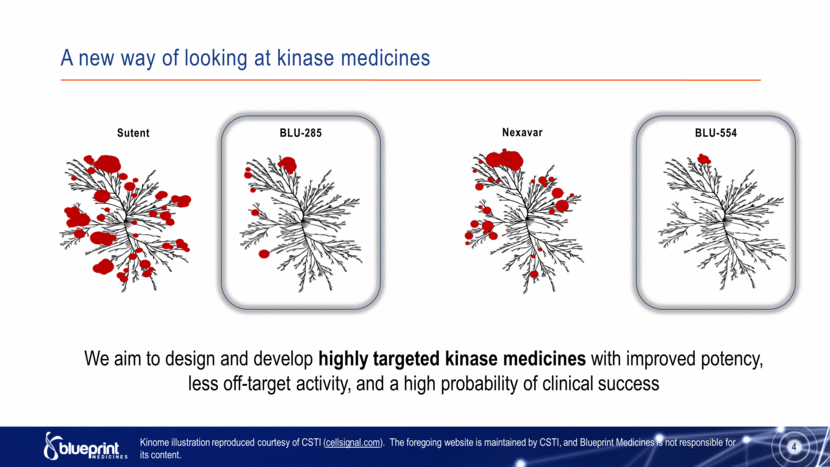
A new way of looking at kinase medicines 4 BLU-554 Nexavar Sutent BLU-285 We aim to design and develop highly targeted kinase medicines with improved potency, less off-target activity, and a high probability of clinical success Kinome illustration reproduced courtesy of CSTI (cellsignal.com). The foregoing website is maintained by CSTI, and Blueprint Medicines is not responsible for its content. |
|
|
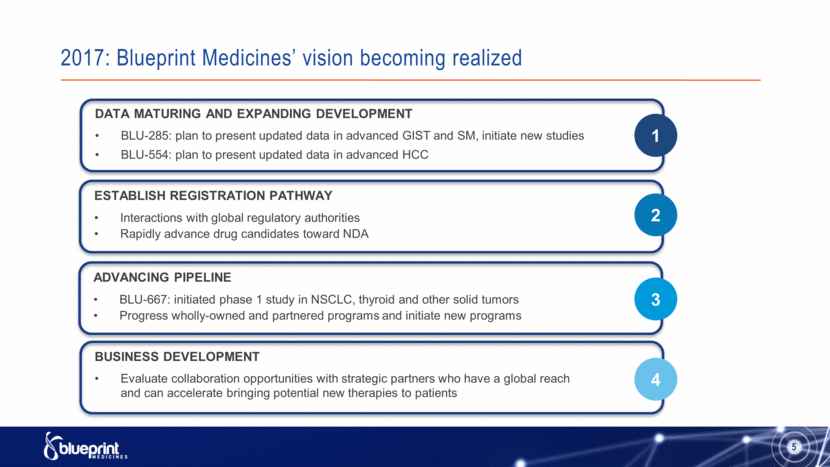
2017: Blueprint Medicines’ vision becoming realized DATA MATURING AND EXPANDING DEVELOPMENT BLU-285: plan to present updated data in advanced GIST and SM, initiate new studies BLU-554: plan to present updated data in advanced HCC ESTABLISH REGISTRATION PATHWAY Interactions with global regulatory authorities Rapidly advance drug candidates toward NDA ADVANCING PIPELINE BLU-667: initiated phase 1 study in NSCLC, thyroid and other solid tumors Progress wholly-owned and partnered programs and initiate new programs BUSINESS DEVELOPMENT Evaluate collaboration opportunities with strategic partners who have a global reach and can accelerate bringing potential new therapies to patients 1 2 3 4 5 |
|
|
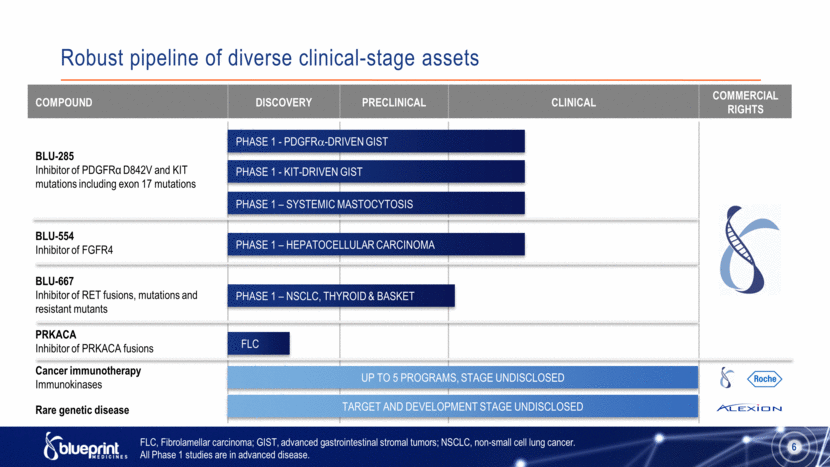
Robust pipeline of diverse clinical-stage assets FLC, Fibrolamellar carcinoma; GIST, advanced gastrointestinal stromal tumors; NSCLC, non-small cell lung cancer. All Phase 1 studies are in advanced disease. 6 compound Discovery Preclinical Clinical Commercial rights BLU-285 Inhibitor of PDGFRα D842V and KIT mutations including exon 17 mutations BLU-554 Inhibitor of FGFR4 BLU-667 Inhibitor of RET fusions, mutations and resistant mutants PRKACA Inhibitor of PRKACA fusions Cancer immunotherapy Immunokinases Rare genetic disease PHASE 1 - PDGFR-DRIVEN GIST PHASE 1 - KIT-DRIVEN GIST PHASE 1 – SYSTEMIC MASTOCYTOSIS PHASE 1 – HEPATOCELLULAR CARCINOMA PHASE 1 – NSCLC, THYROID & BASKET FLC Up to 5 programs, STAGE UNDISCLOSED Target and development stage undisclosed |
|
|
BLU-285 Phase 1 Study in Advanced Gastrointestinal Stromal Tumors (GIST) |
|
|
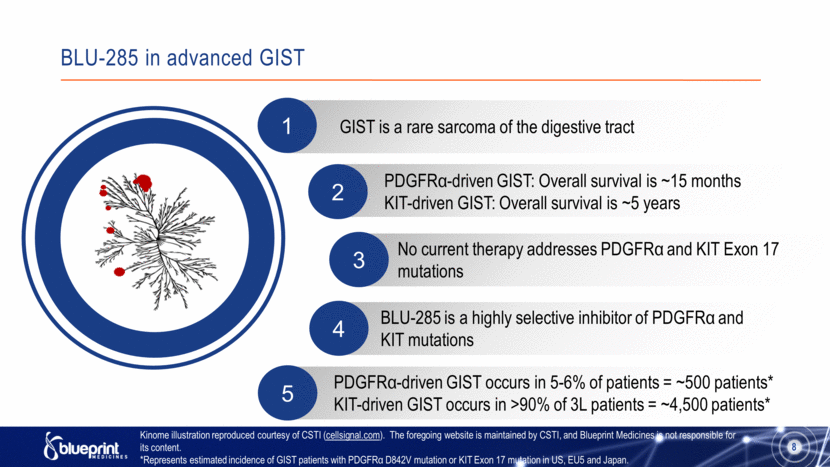
2 GIST is a rare sarcoma of the digestive tract 1 PDGFRα-driven GIST: Overall survival is ~15 months KIT-driven GIST: Overall survival is ~5 years No current therapy addresses PDGFRα and KIT Exon 17 mutations BLU-285 is a highly selective inhibitor of PDGFRα and KIT mutations 3 8 4 PDGFRα-driven GIST occurs in 5-6% of patients = ~500 patients* KIT-driven GIST occurs in >90% of 3L patients = ~4,500 patients* 5 Kinome illustration reproduced courtesy of CSTI (cellsignal.com). The foregoing website is maintained by CSTI, and Blueprint Medicines is not responsible for its content. *Represents estimated incidence of GIST patients with PDGFRα D842V mutation or KIT Exon 17 mutation in US, EU5 and Japan. BLU-285 in advanced GIST |
|
|
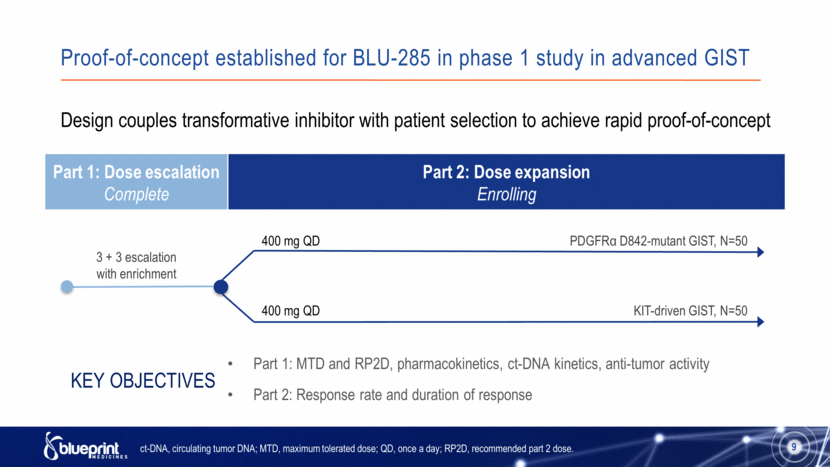
Proof-of-concept established for BLU-285 in phase 1 study in advanced GIST Part 1: Dose escalation Complete Part 2: Dose expansion Enrolling 9 PDGFRα D842-mutant GIST, N=50 KIT-driven GIST, N=50 3 + 3 escalation with enrichment 400 mg QD Design couples transformative inhibitor with patient selection to achieve rapid proof-of-concept Part 1: MTD and RP2D, pharmacokinetics, ct-DNA kinetics, anti-tumor activity Part 2: Response rate and duration of response Key Objectives ct-DNA, circulating tumor DNA; MTD, maximum tolerated dose; QD, once a day; RP2D, recommended part 2 dose. 400 mg QD 9 |
|
|
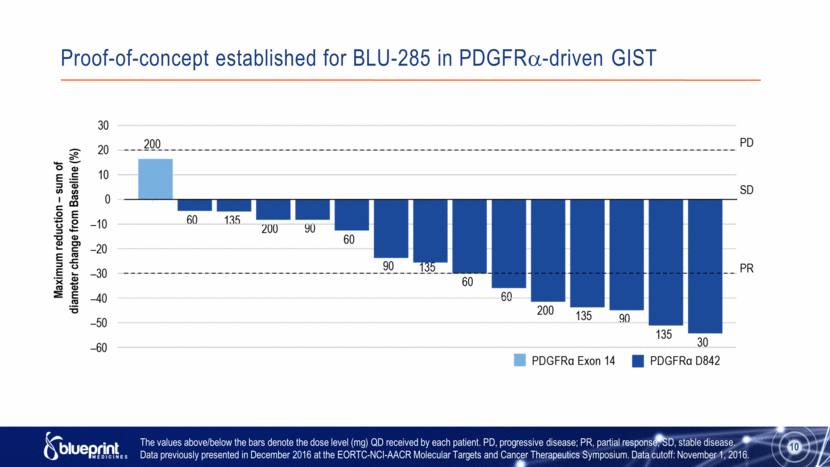
Proof-of-concept established for BLU-285 in PDGFR-driven GIST The values above/below the bars denote the dose level (mg) QD received by each patient. PD, progressive disease; PR, partial response; SD, stable disease. Data previously presented in December 2016 at the EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium. Data cutoff: November 1, 2016. 10 |
|
|
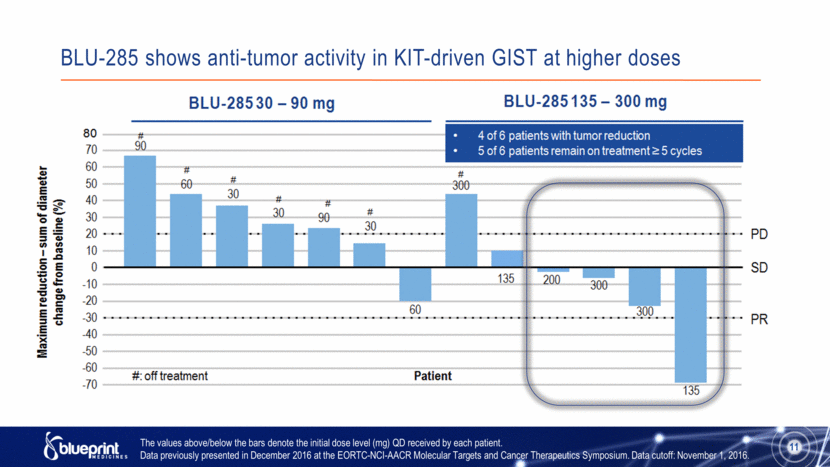
BLU-285 shows anti-tumor activity in KIT-driven GIST at higher doses The values above/below the bars denote the initial dose level (mg) QD received by each patient. Data previously presented in December 2016 at the EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium. Data cutoff: November 1, 2016. 11 |
|
|
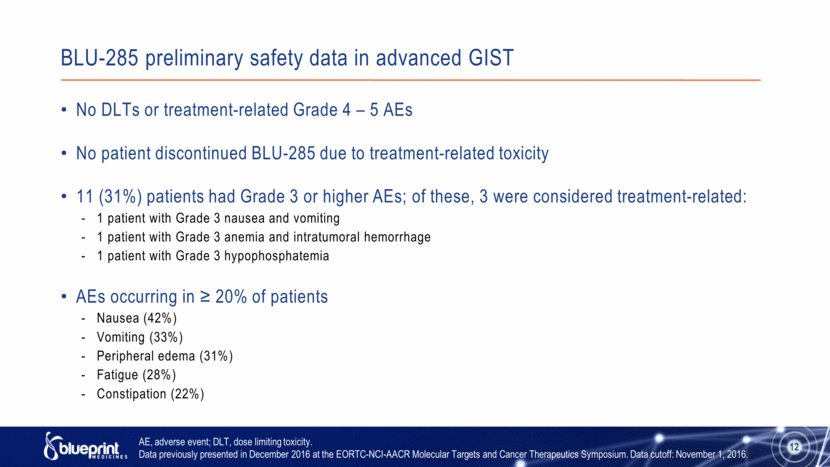
BLU-285 preliminary safety data in advanced GIST No DLTs or treatment-related Grade 4 – 5 AEs No patient discontinued BLU-285 due to treatment-related toxicity 11 (31%) patients had Grade 3 or higher AEs; of these, 3 were considered treatment-related: 1 patient with Grade 3 nausea and vomiting 1 patient with Grade 3 anemia and intratumoral hemorrhage 1 patient with Grade 3 hypophosphatemia AEs occurring in ≥ 20% of patients Nausea (42%) Vomiting (33%) Peripheral edema (31%) Fatigue (28%) Constipation (22%) AE, adverse event; DLT, dose limiting toxicity. Data previously presented in December 2016 at the EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium. Data cutoff: November 1, 2016. 12 |
|
|
BLU-285 Phase 1 study in Advanced Systemic Mastocytosis (SM) |
|
|
2 1 Advanced SM is a rare and severe disease that shortens life expectancy Prognosis is poor with ~3-5 years overall survival with current treatments focused on symptomatic relief KIT D816V mutation is a key driver in ~90-95% of patients1 3 14 4 Advanced SM (including smoldering) = ~4,400 patients2 Indolent SM = ~16,100 patients2 5 Skin urticaria pigmentosa Image from: Hartmann K et al, 2015.1Garcia-Montero AC et al, 2006. 2SM patient estimates represent prevalence in US, EU5 and Japan. BLU-285 in advanced systemic mastocytosis BLU-285 is a highly selective inhibitor of KIT D816V |
|
|
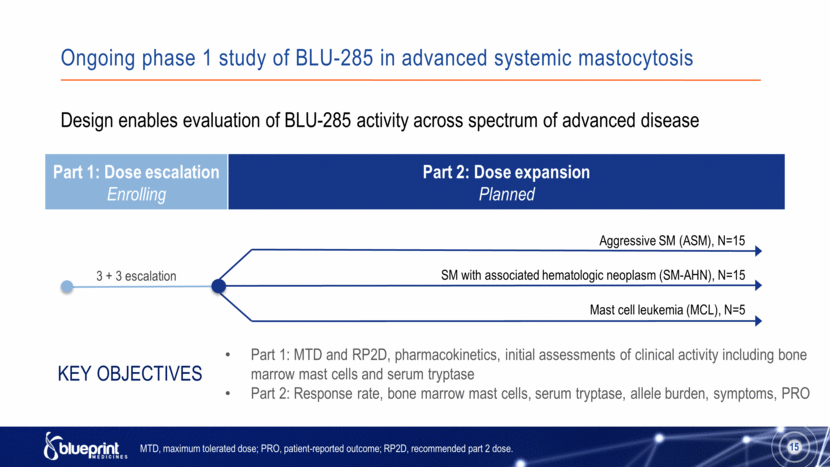
Ongoing phase 1 study of BLU-285 in advanced systemic mastocytosis Part 1: Dose escalation Enrolling Part 2: Dose expansion Planned 15 3 + 3 escalation Design enables evaluation of BLU-285 activity across spectrum of advanced disease Part 1: MTD and RP2D, pharmacokinetics, initial assessments of clinical activity including bone marrow mast cells and serum tryptase Part 2: Response rate, bone marrow mast cells, serum tryptase, allele burden, symptoms, PRO Key Objectives MTD, maximum tolerated dose; PRO, patient-reported outcome; RP2D, recommended part 2 dose. Aggressive SM (ASM), N=15 SM with associated hematologic neoplasm (SM-AHN), N=15 Mast cell leukemia (MCL), N=5 15 |
|
|
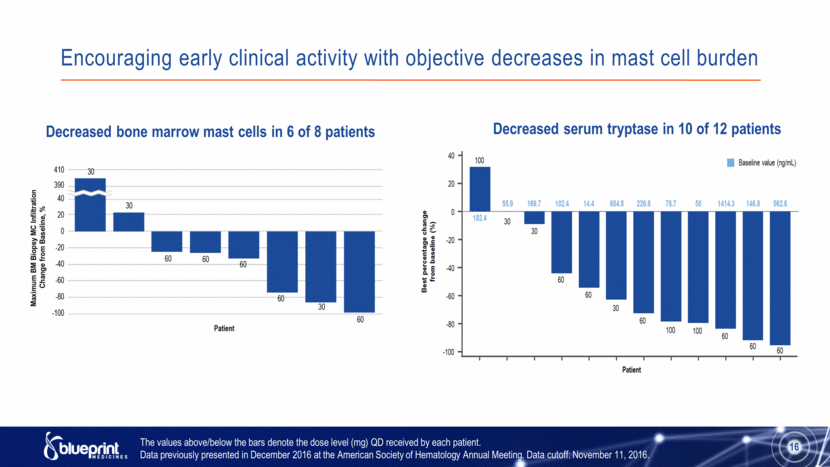
Encouraging early clinical activity with objective decreases in mast cell burden 16 Decreased bone marrow mast cells in 6 of 8 patients Decreased serum tryptase in 10 of 12 patients The values above/below the bars denote the dose level (mg) QD received by each patient. Data previously presented in December 2016 at the American Society of Hematology Annual Meeting. Data cutoff: November 11, 2016. |
|
|
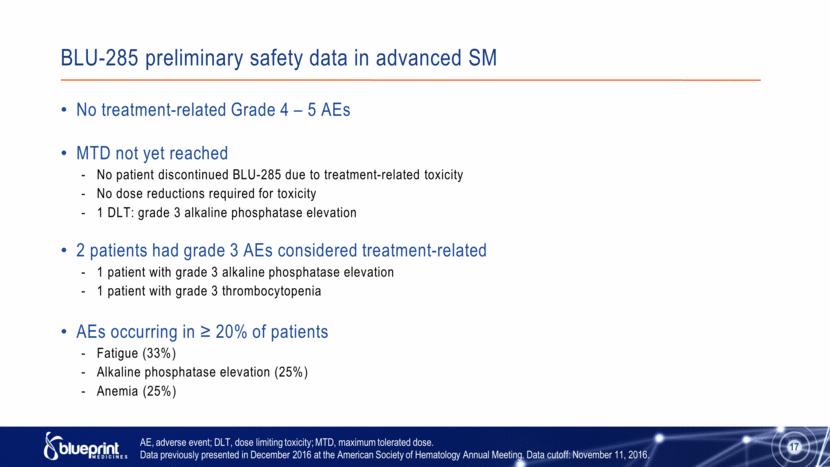
BLU-285 preliminary safety data in advanced SM No treatment-related Grade 4 – 5 AEs MTD not yet reached No patient discontinued BLU-285 due to treatment-related toxicity No dose reductions required for toxicity 1 DLT: grade 3 alkaline phosphatase elevation 2 patients had grade 3 AEs considered treatment-related 1 patient with grade 3 alkaline phosphatase elevation 1 patient with grade 3 thrombocytopenia AEs occurring in ≥ 20% of patients Fatigue (33%) Alkaline phosphatase elevation (25%) Anemia (25%) 17 AE, adverse event; DLT, dose limiting toxicity; MTD, maximum tolerated dose. Data previously presented in December 2016 at the American Society of Hematology Annual Meeting. Data cutoff: November 11, 2016. |
|
|
BLU-554 Phase 1 Study in Advanced Hepatocellular Carcinoma (HCC) |
|
|
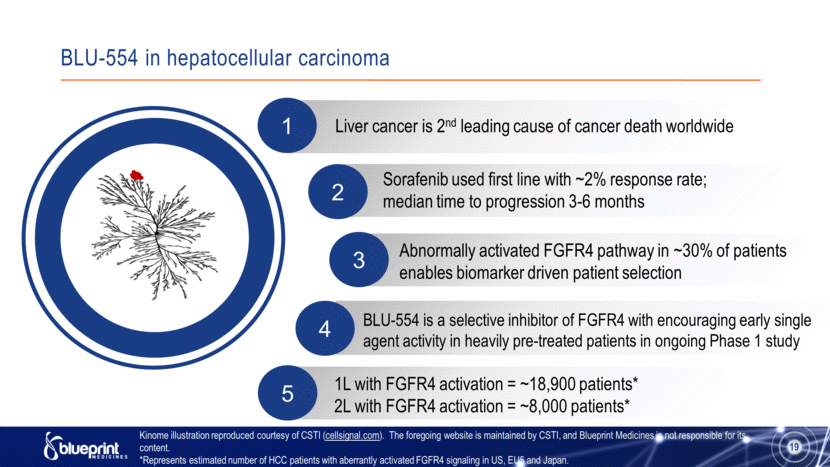
2 1 Abnormally activated FGFR4 pathway in ~30% of patients enables biomarker driven patient selection 3 BLU-554 in hepatocellular carcinoma 19 4 BLU-554 is a selective inhibitor of FGFR4 with encouraging early single agent activity in heavily pre-treated patients in ongoing Phase 1 study 5 1L with FGFR4 activation = ~18,900 patients* 2L with FGFR4 activation = ~8,000 patients* Sorafenib used first line with ~2% response rate; median time to progression 3-6 months Liver cancer is 2nd leading cause of cancer death worldwide Kinome illustration reproduced courtesy of CSTI (cellsignal.com). The foregoing website is maintained by CSTI, and Blueprint Medicines is not responsible for its content. *Represents estimated number of HCC patients with aberrantly activated FGFR4 signaling in US, EU5 and Japan. |
|
|
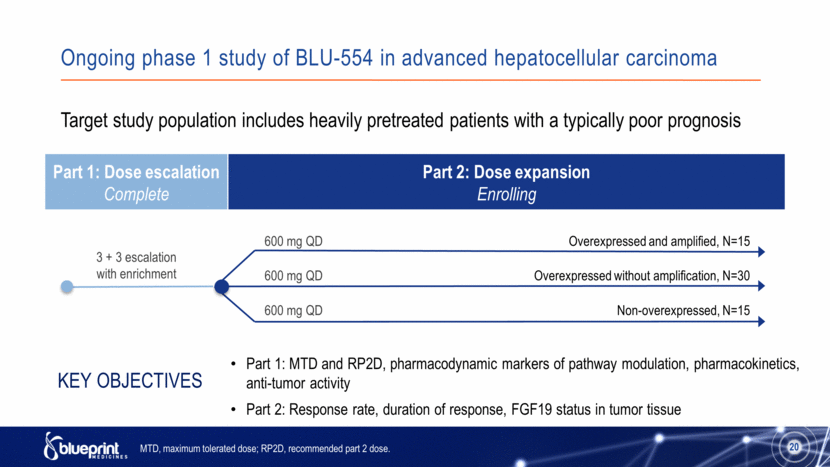
Ongoing phase 1 study of BLU-554 in advanced hepatocellular carcinoma Part 1: Dose escalation Complete Part 2: Dose expansion Enrolling 20 3 + 3 escalation with enrichment Target study population includes heavily pretreated patients with a typically poor prognosis Part 1: MTD and RP2D, pharmacodynamic markers of pathway modulation, pharmacokinetics, anti-tumor activity Part 2: Response rate, duration of response, FGF19 status in tumor tissue Key Objectives MTD, maximum tolerated dose; RP2D, recommended part 2 dose. Overexpressed and amplified, N=15 Overexpressed without amplification, N=30 Non-overexpressed, N=15 600 mg QD 600 mg QD 600 mg QD 20 |
|
|
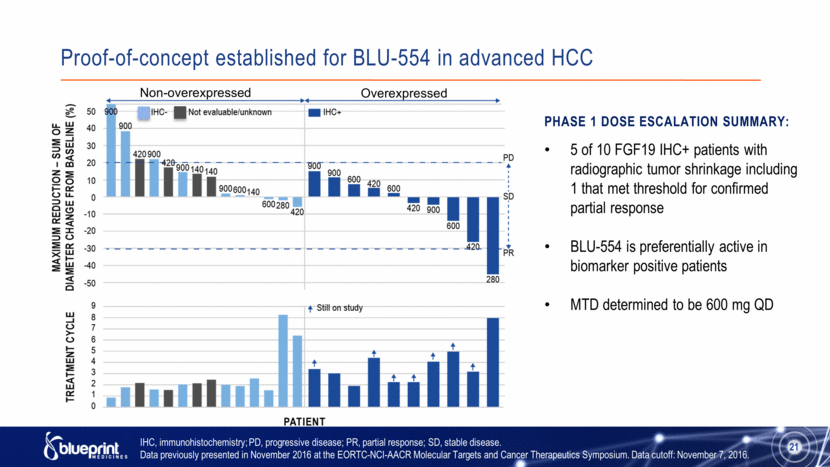
Proof-of-concept established for BLU-554 in advanced HCC 21 IHC, immunohistochemistry; PD, progressive disease; PR, partial response; SD, stable disease. Data previously presented in November 2016 at the EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium. Data cutoff: November 7, 2016. Non-overexpressed Overexpressed Phase 1 Dose Escalation Summary: 5 of 10 FGF19 IHC+ patients with radiographic tumor shrinkage including 1 that met threshold for confirmed partial response BLU-554 is preferentially active in biomarker positive patients MTD determined to be 600 mg QD |
|
|
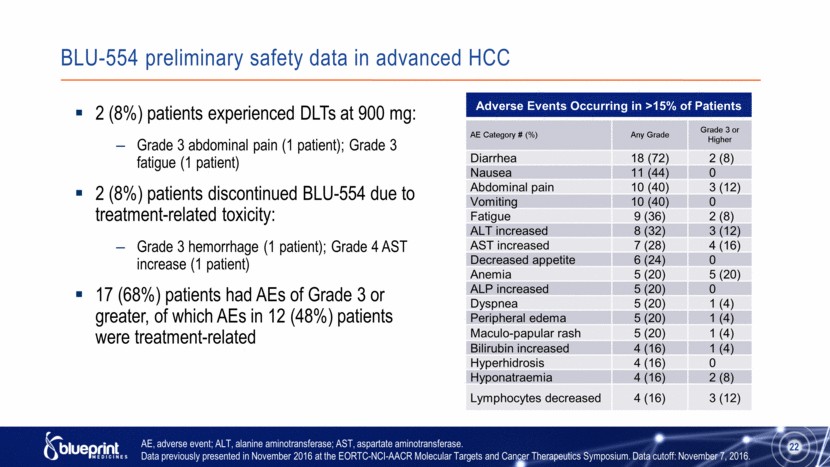
BLU-554 preliminary safety data in advanced HCC Adverse Events Occurring in >15% of Patients AE Category # (%) Any Grade Grade 3 or Higher Diarrhea 18 (72) 2 (8) Nausea 11 (44) 0 Abdominal pain 10 (40) 3 (12) Vomiting 10 (40) 0 Fatigue 9 (36) 2 (8) ALT increased 8 (32) 3 (12) AST increased 7 (28) 4 (16) Decreased appetite 6 (24) 0 Anemia 5 (20) 5 (20) ALP increased 5 (20) 0 Dyspnea 5 (20) 1 (4) Peripheral edema 5 (20) 1 (4) Maculo-papular rash 5 (20) 1 (4) Bilirubin increased 4 (16) 1 (4) Hyperhidrosis 4 (16) 0 Hyponatraemia 4 (16) 2 (8) Lymphocytes decreased 4 (16) 3 (12) 2 (8%) patients experienced DLTs at 900 mg: Grade 3 abdominal pain (1 patient); Grade 3 fatigue (1 patient) 2 (8%) patients discontinued BLU-554 due to treatment-related toxicity: Grade 3 hemorrhage (1 patient); Grade 4 AST increase (1 patient) 17 (68%) patients had AEs of Grade 3 or greater, of which AEs in 12 (48%) patients were treatment-related AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase. Data previously presented in November 2016 at the EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium. Data cutoff: November 7, 2016. 22 |
|
|
BLU-667 RET Inhibitor |
|
|
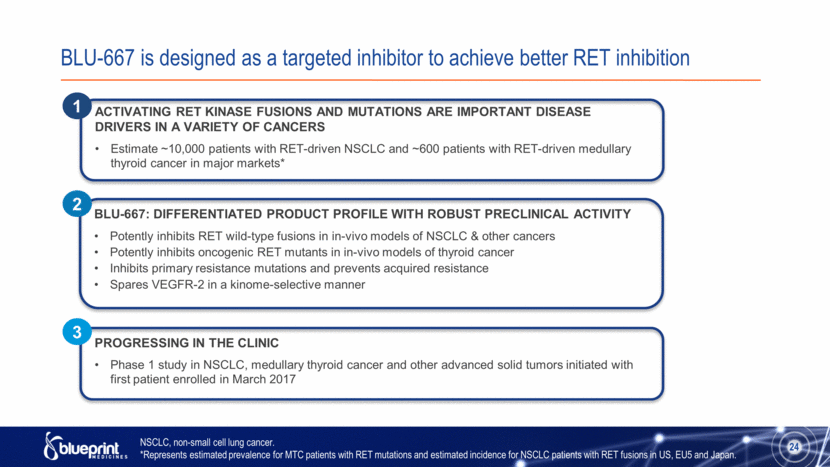
BLU-667 is designed as a targeted inhibitor to achieve better RET inhibition ACTIVATING RET KINASE FUSIONS AND MUTATIONS ARE IMPORTANT DISEASE DRIVERS IN A VARIETY OF CANCERS Estimate ~10,000 patients with RET-driven NSCLC and ~600 patients with RET-driven medullary thyroid cancer in major markets* BLU-667: DIFFERENTIATED PRODUCT PROFILE WITH ROBUST PRECLINICAL ACTIVITY Potently inhibits RET wild-type fusions in in-vivo models of NSCLC & other cancers Potently inhibits oncogenic RET mutants in in-vivo models of thyroid cancer Inhibits primary resistance mutations and prevents acquired resistance Spares VEGFR-2 in a kinome-selective manner PROGRESSING IN THE CLINIC Phase 1 study in NSCLC, medullary thyroid cancer and other advanced solid tumors initiated with first patient enrolled in March 2017 1 2 3 24 NSCLC, non-small cell lung cancer. *Represents estimated prevalence for MTC patients with RET mutations and estimated incidence for NSCLC patients with RET fusions in US, EU5 and Japan. |
|
|
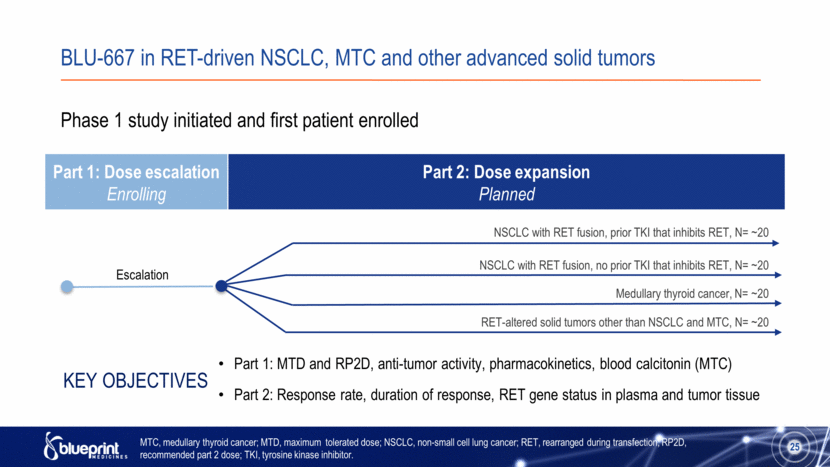
BLU-667 in RET-driven NSCLC, MTC and other advanced solid tumors Part 1: Dose escalation Enrolling Part 2: Dose expansion Planned 25 Escalation Phase 1 study initiated and first patient enrolled Part 1: MTD and RP2D, anti-tumor activity, pharmacokinetics, blood calcitonin (MTC) Part 2: Response rate, duration of response, RET gene status in plasma and tumor tissue Key Objectives NSCLC with RET fusion, prior TKI that inhibits RET, N= ~20 NSCLC with RET fusion, no prior TKI that inhibits RET, N= ~20 Medullary thyroid cancer, N= ~20 RET-altered solid tumors other than NSCLC and MTC, N= ~20 MTC, medullary thyroid cancer; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; RET, rearranged during transfection; RP2D, recommended part 2 dose; TKI, tyrosine kinase inhibitor. 25 |
|
|
PRKACA |
|
|
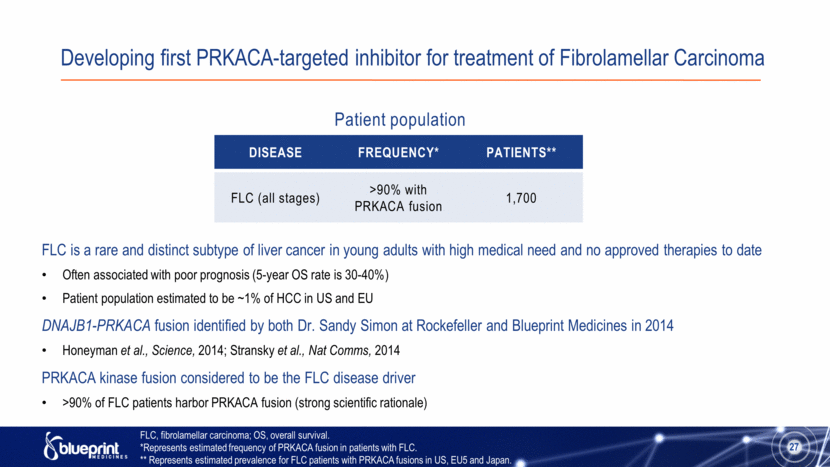
Developing first PRKACA-targeted inhibitor for treatment of Fibrolamellar Carcinoma FLC is a rare and distinct subtype of liver cancer in young adults with high medical need and no approved therapies to date Often associated with poor prognosis (5-year OS rate is 30-40%) Patient population estimated to be ~1% of HCC in US and EU DNAJB1-PRKACA fusion identified by both Dr. Sandy Simon at Rockefeller and Blueprint Medicines in 2014 Honeyman et al., Science, 2014; Stransky et al., Nat Comms, 2014 PRKACA kinase fusion considered to be the FLC disease driver >90% of FLC patients harbor PRKACA fusion (strong scientific rationale) 27 Disease Frequency* Patients** FLC (all stages) >90% with PRKACA fusion 1,700 Patient population FLC, fibrolamellar carcinoma; OS, overall survival. *Represents estimated frequency of PRKACA fusion in patients with FLC. ** Represents estimated prevalence for FLC patients with PRKACA fusions in US, EU5 and Japan. |
|
|
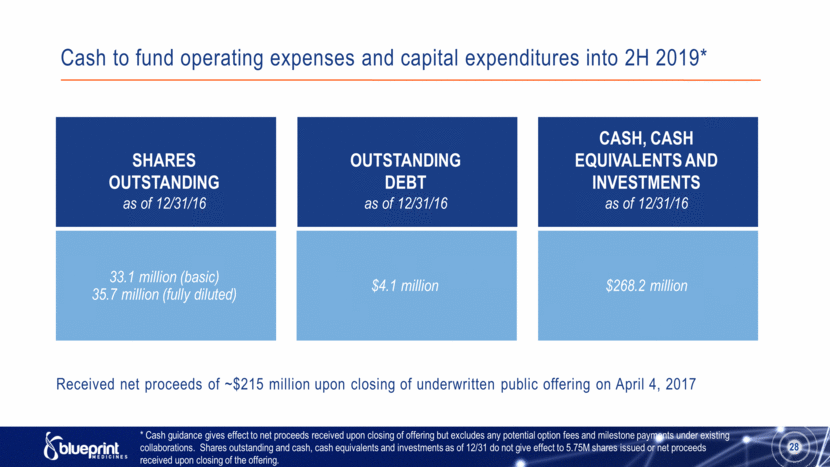
Cash to fund operating expenses and capital expenditures into 2H 2019* 28 SHARES OUTSTANDING as of 12/31/16 33.1 million (basic) 35.7 million (fully diluted) OUTSTANDING DEBT as of 12/31/16 $4.1 million CASH, CASH EQUIVALENTS AND INVESTMENTS as of 12/31/16 $268.2 million * Cash guidance gives effect to net proceeds received upon closing of offering but excludes any potential option fees and milestone payments under existing collaborations. Shares outstanding and cash, cash equivalents and investments as of 12/31 do not give effect to 5.75M shares issued or net proceeds received upon closing of the offering. Received net proceeds of ~$215 million upon closing of underwritten public offering on April 4, 2017 |
|
|
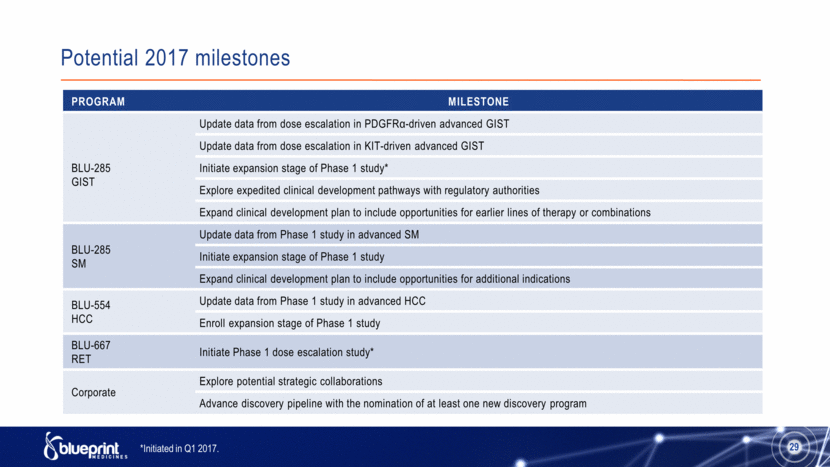
Potential 2017 milestones 29 Program Milestone BLU-285 GIST Update data from dose escalation in PDGFRα-driven advanced GIST Update data from dose escalation in KIT-driven advanced GIST Initiate expansion stage of Phase 1 study* Explore expedited clinical development pathways with regulatory authorities Expand clinical development plan to include opportunities for earlier lines of therapy or combinations BLU-285 SM Update data from Phase 1 study in advanced SM Initiate expansion stage of Phase 1 study Expand clinical development plan to include opportunities for additional indications BLU-554 HCC Update data from Phase 1 study in advanced HCC Enroll expansion stage of Phase 1 study BLU-667 RET Initiate Phase 1 dose escalation study* Corporate Explore potential strategic collaborations Advance discovery pipeline with the nomination of at least one new discovery program *Initiated in Q1 2017. |
|
|
A blueprint for a healthier tomorrow Proprietary discovery platform for highly selective and potent kinase drug candidates Strategy focused on genomically defined cancers and rare diseases Proof-of-concept demonstrated in 4 patient populations in 3 diseases with 2 candidates 3 wholly owned candidates in clinic with opportunities for rapid development Advancing early-stage pipeline; collaborations with Roche and Alexion Strong executive team and company culture focused on patient outcomes and urgency 30 |
|
|
Thank you 31 |