Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Adaptimmune Therapeutics PLC | a17-10157_1ex99d2.htm |
| 8-K - 8-K - Adaptimmune Therapeutics PLC | a17-10157_18k.htm |
Exhibit 99.1
March 2017 Corporate Presentation
Disclaimer This presentation contains “forward-looking statements,” as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as “believe,” “may”, “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect” and other words of similar meaning. These forward-looking statements involve certain risks and uncertainties. Such risks and uncertainties could cause our actual results to differ materially from those indicated by such forward-looking statements, and include, without limitation: the success, cost and timing of our product development activities and clinical trials; our ability to submit an IND and successfully advance our technology platform to improve the safety and effectiveness of our existing TCR therapeutic candidates; the rate and degree of market acceptance of T-cell therapy generally and of our TCR therapeutic candidates; government regulation and approval, including, but not limited to, the expected regulatory approval timelines for TCR therapeutic candidates; and our ability to protect our proprietary technology and enforce our intellectual property rights; amongst others. For a further description of the risks and uncertainties that could cause our actual results to differ materially from those expressed in these forward-looking statements, as well as risks relating to our business in general, we refer you to our Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC) on March 13, 2017 and our other SEC filings. We urge you to consider these factors carefully in evaluating the forward-looking statements herein and you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. The forward-looking statements contained in this presentation speak only as of the date the statements were made and we do not undertake any obligation to update such forward-looking statements to reflect subsequent events or circumstances. We intend that all forward-looking statements be subject to the safe-harbor provisions of the PSLRA. 2
Adaptimmune Positioned for Delivery in 2017/18 Three wholly-owned INDs open (MAGE-A10, -A4 and AFP) in 8 tumor types Momentum in patient screening and recruitment Initial data likely 2H 2017 and 1H 2018 Significant progress with NY-ESO* program Plan to initiate registration study around end 2017, subject to regulatory process Initial data from MRCLS, NSCLC and ovarian studies likely 2H 2017 and 1H 2018 Initiation of combination study with Keytruda® 3 * Under option to GSK
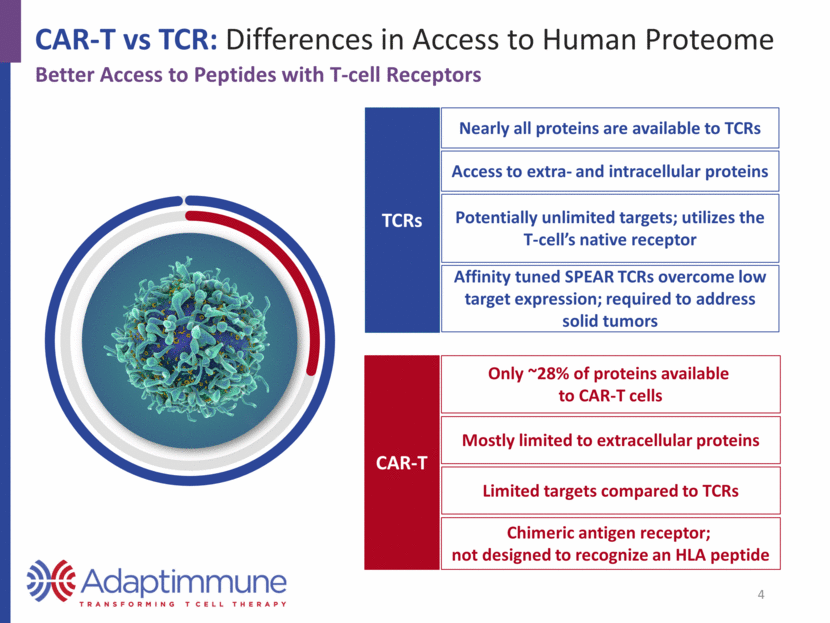
Nearly all proteins are available to TCRs Potentially unlimited targets; utilizes the T-cell’s native receptor Affinity tuned SPEAR TCRs overcome low target expression; required to address solid tumors Access to extra- and intracellular proteins CAR-T vs TCR: Differences in Access to Human Proteome TCRs CAR-T Better Access to Peptides with T-cell Receptors Only ~28% of proteins available to CAR-T cells Chimeric antigen receptor; not designed to recognize an HLA peptide Mostly limited to extracellular proteins Limited targets compared to TCRs 4
T-cells Play Critical Role in Cell-Mediated Immunity Cells display status by presenting peptides on their surface using HLA molecules Peptides in peptide-HLA complexes change if cell is damaged, cancerous or infected T-cells generally only recognize different “non-self” cells due to thymic selection T-cell recognition based on affinity of the TCR to the target peptide-HLA complex Multiple TCRs bind target peptide-HLA, which cluster to form an immune synapse T-cells release cytolytic granules, inducing target cell death However, T-cells have trouble recognizing cancer cells as targets Cancer peptides are derived from normal, “self” proteins Thymic selection deleted T-cells with high affinity to “self” proteins including cancer peptides Cancer cells express a lower number of targets 5 TCRs Eliminate Damaged/Diseased/Foreign Cells, but Cancer Evades Detection
Affinity Optimization is Critical to Address Majority of Antigens Adaptimmune is the Only Company with this Proprietary Technology T-cells bind to targets on cancer cells Cancer downregulates targets to avoid detection Most naturally occurring anti-tumor T-cells are low affinity (require more targets) SPEAR T-cells are affinity enhanced to overcome this problem Proprietary preclinical engineering ensures tumor-specific response Optimal specificity and affinity for antitumor activity Demonstrated efficacy in solid tumors 6
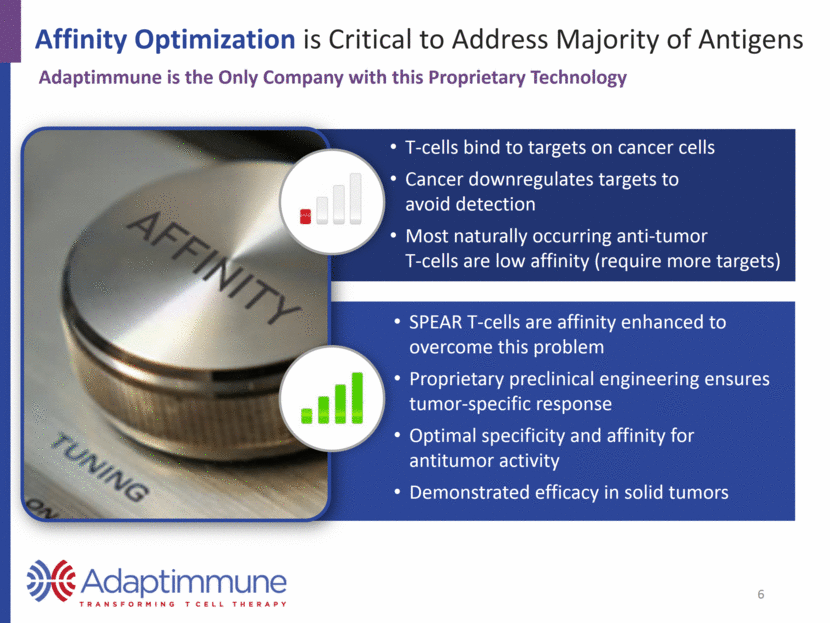
Developing Novel TCR Therapies Utilizing Proprietary Technology Platform to Develop Multiple Approaches Cancer Testis Antigens Largely exclusive to tumor tissue; shown to be good targets Developing a franchise with overlapping expression profiles Examples: NY-ESO, MAGE-A10 & -A4 Non-CTA Targets Includes oncofetal proteins and differentiation markers Closely associated with single tumor types Example: AFP Multiple HLAs Expanding research efforts to target multiple HLAs Looking beyond foundational data in HLA-A2 Next generation SPEAR T-cells Data on dnTGF-receptor construct at SITC 2016 Also evaluating combination approaches 7
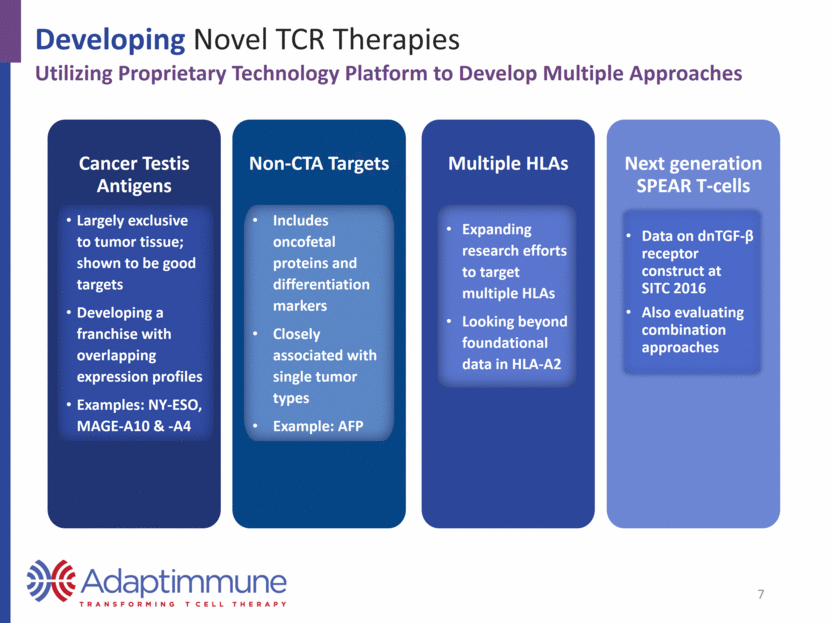
Adaptimmune Pipeline Overview Multiple Targets with Near-Term Clinical Milestones Clinical data in synovial sarcoma and multiple myeloma Active trials in synovial sarcoma, MRCLS, ovarian and non-small cell lung cancer (NSCLC) Planned registration studies in synovial sarcoma NY-ESO IND open Studies enrolling in head & neck, melanoma, urothelial (bladder), and NSCLC IND open Study in hepatocellular cancer in 2017 IND open (announced January 2017) Multi-tumor study in 2017 MAGE-A10 AFP MAGE-A4 GSK option Wholly-owned 8
Adaptimmune Pipeline 9
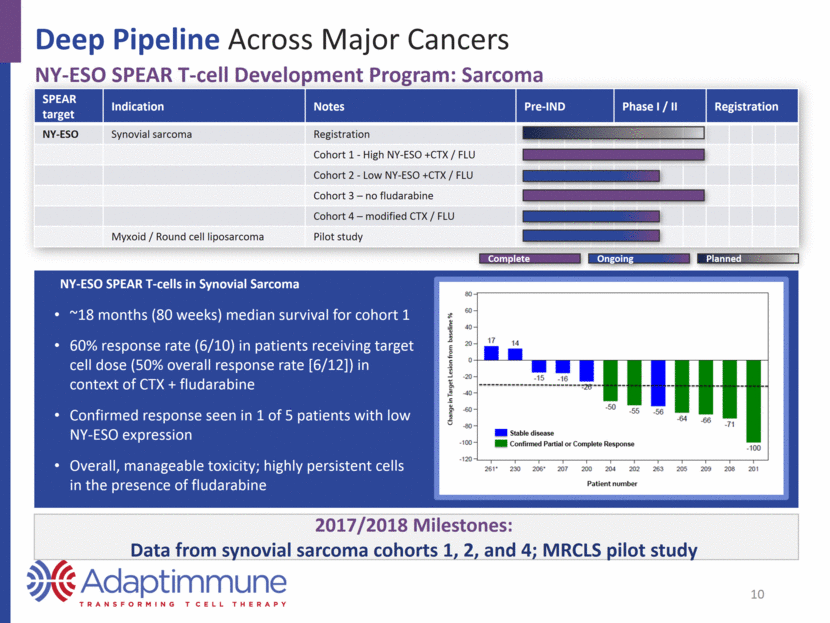
Deep Pipeline Across Major Cancers NY-ESO SPEAR T-cell Development Program: Sarcoma SPEAR target Indication Notes Pre-IND Phase I / II Registration NY-ESO Synovial sarcoma Registration Cohort 1 - High NY-ESO +CTX / FLU Cohort 2 - Low NY-ESO +CTX / FLU Cohort 3 – no fludarabine Cohort 4 – modified CTX / FLU Myxoid / Round cell liposarcoma Pilot study NY-ESO SPEAR T-cells in Synovial Sarcoma ~18 months (80 weeks) median survival for cohort 1 60% response rate (6/10) in patients receiving target cell dose (50% overall response rate [6/12]) in context of CTX + fludarabine Confirmed response seen in 1 of 5 patients with low NY-ESO expression Overall, manageable toxicity; highly persistent cells in the presence of fludarabine 2017/2018 Milestones: Data from synovial sarcoma cohorts 1, 2, and 4; MRCLS pilot study 10 Planned Complete Ongoing
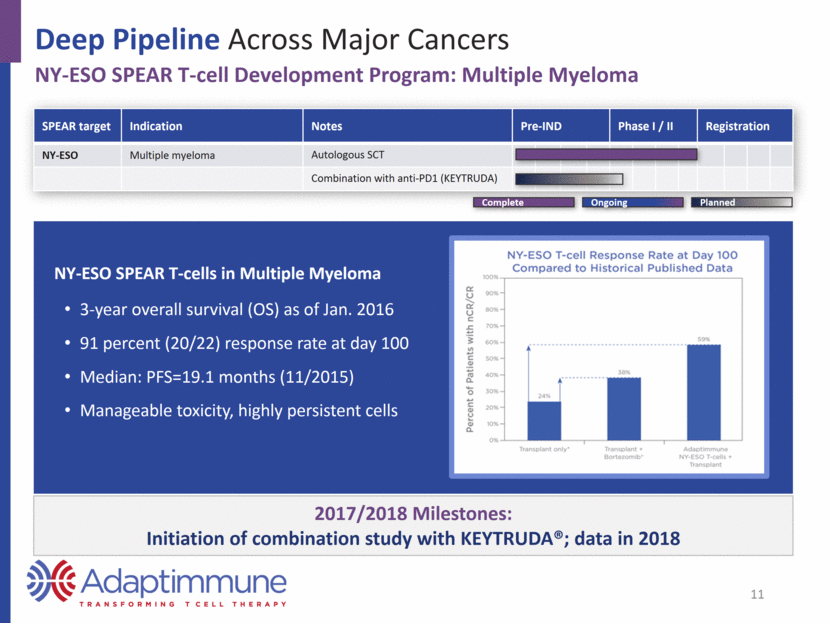
2017/2018 Milestones: Initiation of combination study with KEYTRUDA®; data in 2018 Deep Pipeline Across Major Cancers NY-ESO SPEAR T-cell Development Program: Multiple Myeloma SPEAR target Indication Notes Pre-IND Phase I / II Registration NY-ESO Multiple myeloma Autologous SCT Combination with anti-PD1 (KEYTRUDA) NY-ESO SPEAR T-cells in Multiple Myeloma 3-year overall survival (OS) as of Jan. 2016 91 percent (20/22) response rate at day 100 Median: PFS=19.1 months (11/2015) Manageable toxicity, highly persistent cells 11 Planned Complete Ongoing
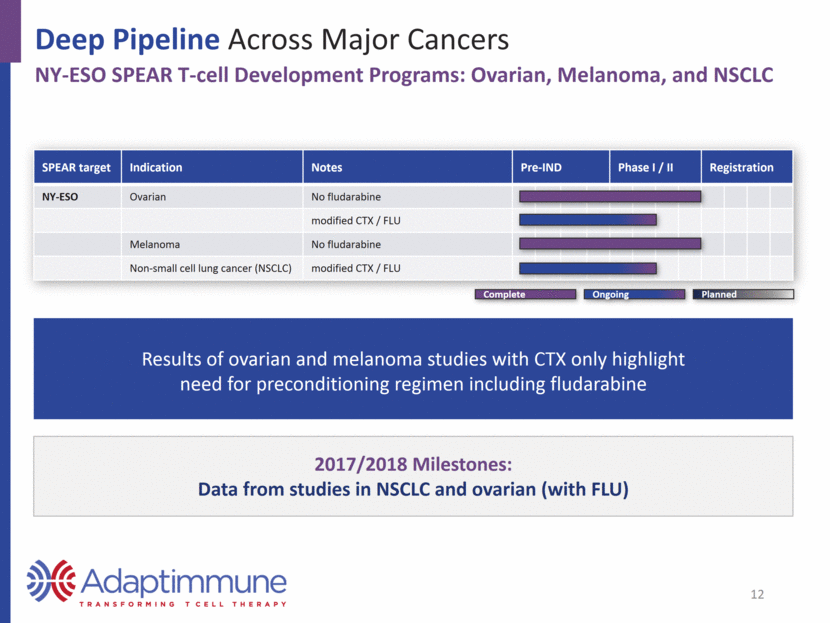
2017/2018 Milestones: Data from studies in NSCLC and ovarian (with FLU) Deep Pipeline Across Major Cancers NY-ESO SPEAR T-cell Development Programs: Ovarian, Melanoma, and NSCLC Results of ovarian and melanoma studies with CTX only highlight need for preconditioning regimen including fludarabine SPEAR target Indication Notes Pre-IND Phase I / II Registration NY-ESO Ovarian No fludarabine modified CTX / FLU Melanoma No fludarabine Non-small cell lung cancer (NSCLC) modified CTX / FLU 12 Planned Complete Ongoing
Frequency of Grade 3+ CRS: NY-ESO SPEAR-T vs CAR-Ts Adaptimmune NY-ESO SPEAR T-cell therapy 8.6% No Grade 5 CRS All cases resolved 18% CD19 CAR-T in DLBCL/PMBCL 27% 23% 39% JCAR 017 CAR-T in Pediatric ALL JCAR 014 CAR-T in Adult ALL JCAR 015 CAR-T in ALL 8% 10% JCAR 014 CAR-T in CLL JCAR 014 CAR-T in NHL 13
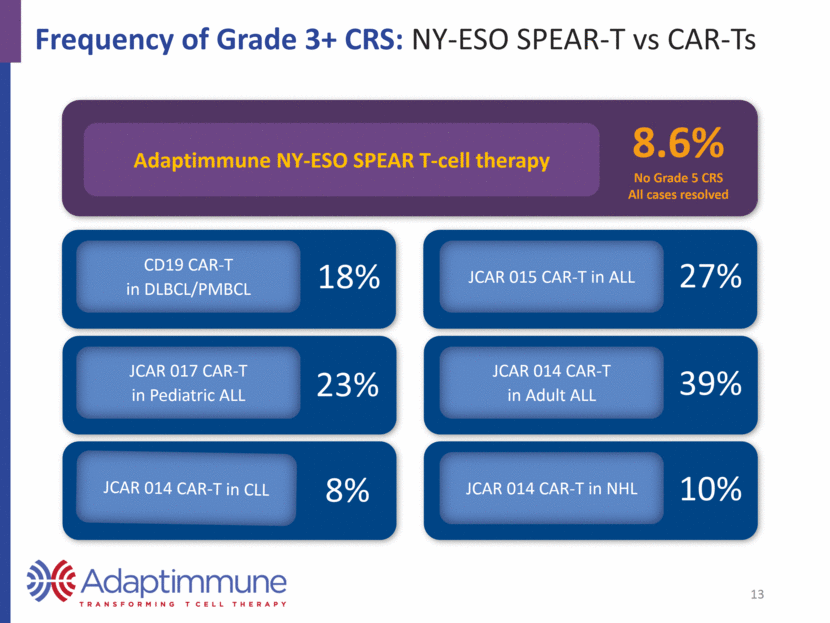
Neurotoxicity: NY-ESO SPEAR-T vs CAR-Ts NY-ESO SPEAR T-cells: Not associated with the type and severity of neurotoxicity events seen with CAR-T 34% Grade 3 or 4 3% Grade 5 CD19 CAR-T in DLBCL/PMBCL 23% Severe JCAR 017 CAR-T in Pediatric ALL JCAR 014 CAR-T in Adult ALL JCAR 015 CAR-T in ALL JCAR 014 CAR-T in CLL JCAR 014 CAR-T in NHL 25% Grade 3-5 29% Grade 3+ 39% Grade 3+ 10% Severe 14
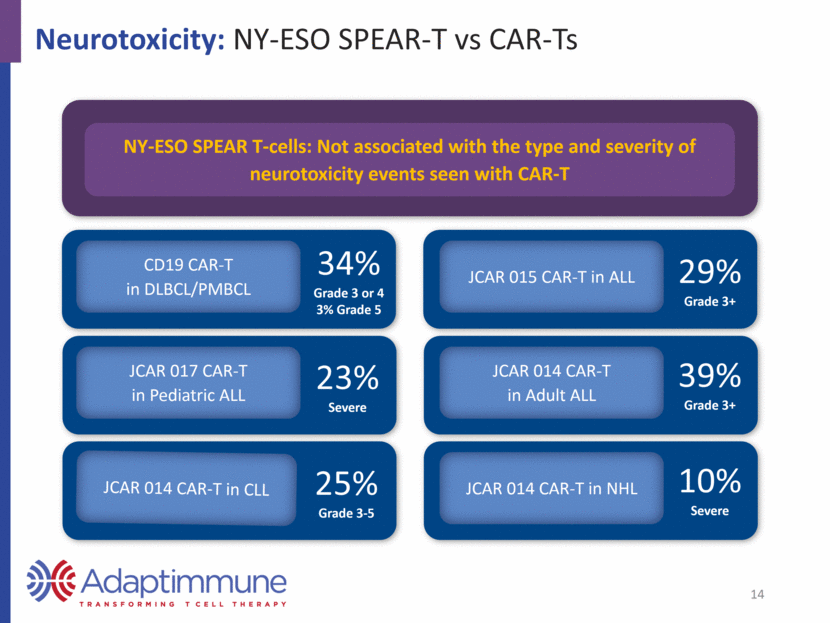
Deep Pipeline Across Major Cancers MAGE-A10 and -A4: Expressed Across a Wide Range of Tumors MAGE-A10 Expression* MAGE-A4 Expression* US1 Europe2 Urothelial 16,390 52,374 Head and neck 9,570 43,704 Ovarian 14,240 42,716 Melanoma 10,130 22,199 Lung 158,080 353,580 Esophageal 15,690 39,504 Gastric 10,730 107,313 Source: seer.cancer.gov; http://www.cancer.org/; 2016 data Source: eco.iarc.fr/eucan; 2012 data * Antigen expression in table is not exhaustive Estimated Annual Deaths 15 Source: TCGA Research Network: http://cancergenome.nih.gov, March 2017. 0 20 40 60 80 100 Positive tumors (%) 0 20 40 60 80 100 Positive tumors (%)
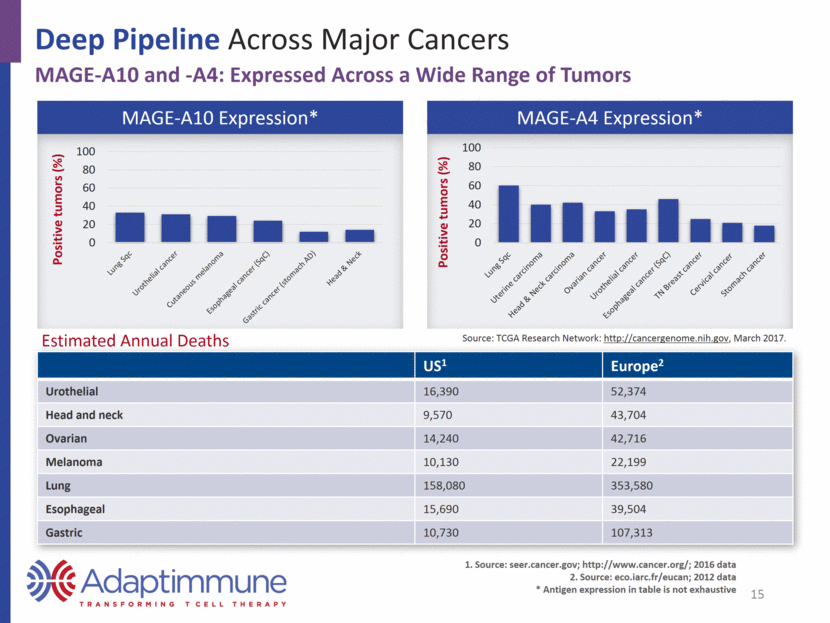
Deep Pipeline Across Major Cancers MAGE-A10 and -A4 SPEAR T-cell Development Programs: Multiple Cancers SPEAR target Indication Notes Pre-IND Phase I / II Registration MAGE-A10 Non-small cell lung cancer (NSCLC) modified CTX / FLU Urothelial (bladder), melanoma, H&N modified CTX / FLU MAGE-A4 Urothelial, melanoma, H&N, ovarian, NSCLC, esophageal, gastric 2017/2018 Milestones: Data from NSCLC and triple tumor studies of MAGE-A10 SPEAR T-cells 2017/2018 Milestones: Data from multi-tumor study of MAGE-A4 SPEAR T-cells 16 Planned Complete Ongoing
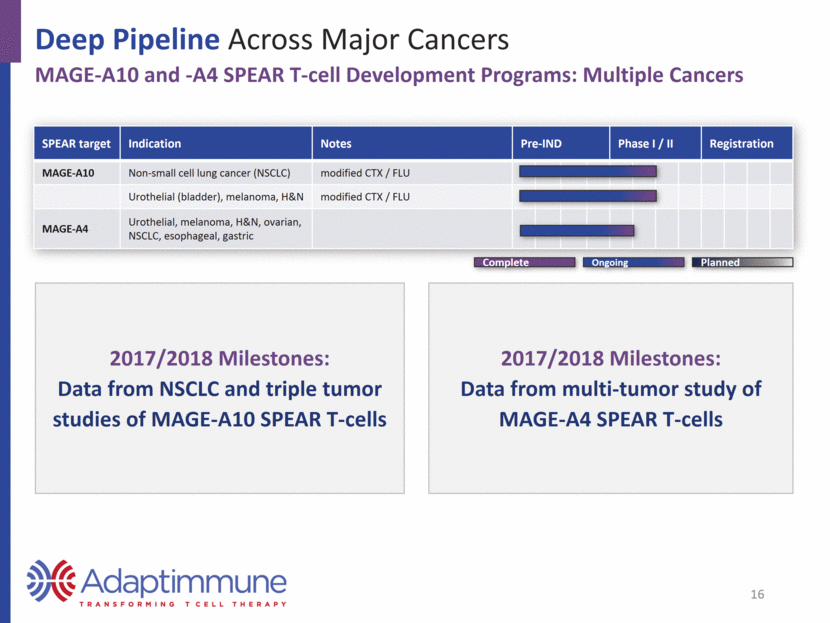
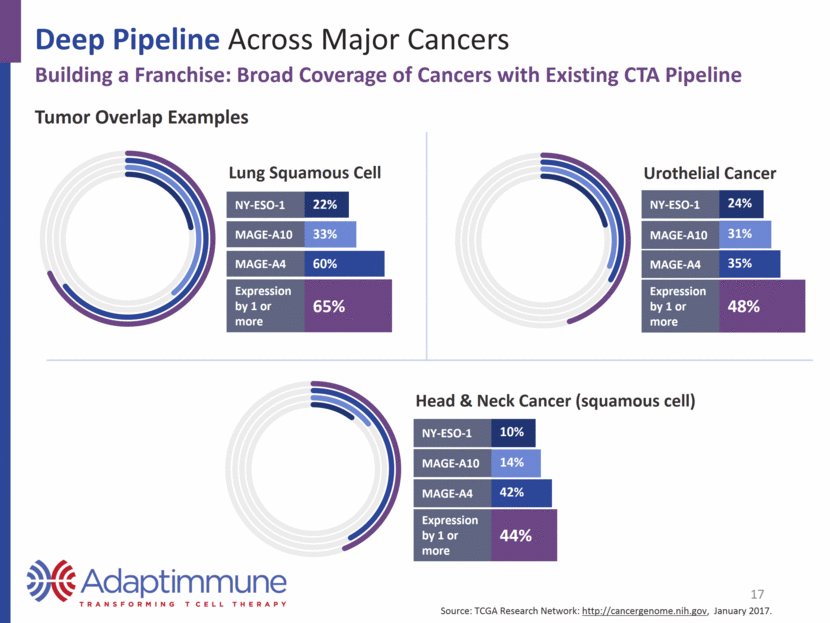
Deep Pipeline Across Major Cancers Building a Franchise: Broad Coverage of Cancers with Existing CTA Pipeline Lung Squamous Cell NY-ESO-1 22% MAGE-A10 33% MAGE-A4 60% Expression by 1 or more 65% Head & Neck Cancer (squamous cell) NY-ESO-1 10% MAGE-A10 14% MAGE-A4 42% Expression by 1 or more 44% Tumor Overlap Examples 17 Urothelial Cancer NY-ESO-1 24% MAGE-A10 31% MAGE-A4 35% Expression by 1 or more 48% Source: TCGA Research Network: http://cancergenome.nih.gov, January 2017.
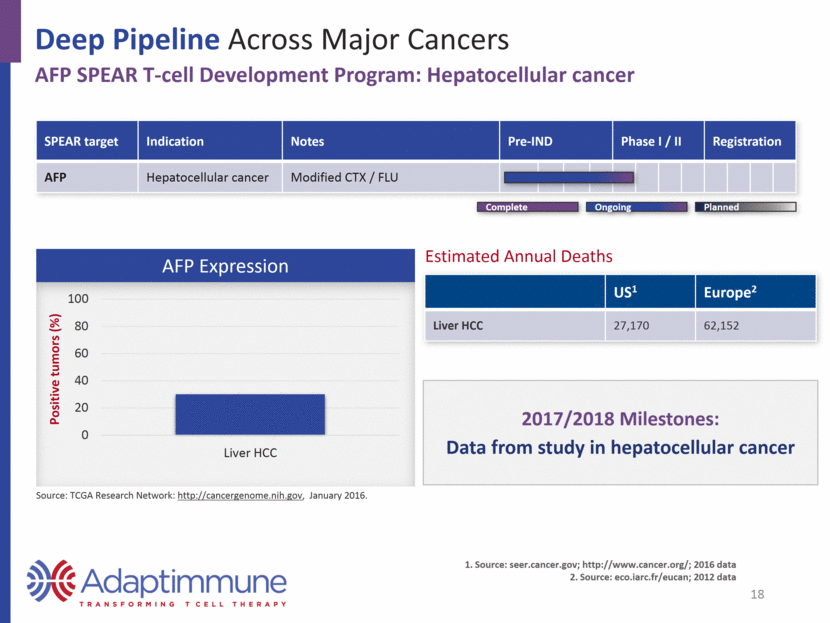
Deep Pipeline Across Major Cancers AFP SPEAR T-cell Development Program: Hepatocellular cancer SPEAR target Indication Notes Pre-IND Phase I / II Registration AFP Hepatocellular cancer Modified CTX / FLU AFP Expression US1 Europe2 Liver HCC 27,170 62,152 2017/2018 Milestones: Data from study in hepatocellular cancer Estimated Annual Deaths 18 Planned Complete Ongoing Source: TCGA Research Network: http://cancergenome.nih.gov, January 2016. Source: seer.cancer.gov; http://www.cancer.org/; 2016 data Source: eco.iarc.fr/eucan; 2012 data 0 20 40 60 80 100 Liver HCC Positive tumors (%)
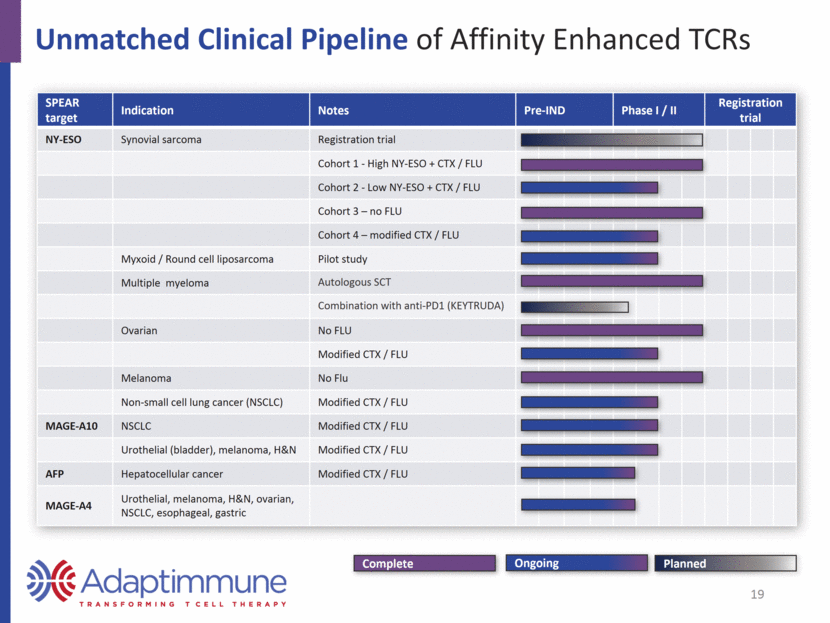
Unmatched Clinical Pipeline of Affinity Enhanced TCRs SPEAR target Indication Notes Pre-IND Phase I / II Registration trial NY-ESO Synovial sarcoma Registration trial Cohort 1 - High NY-ESO + CTX / FLU Cohort 2 - Low NY-ESO + CTX / FLU Cohort 3 – no FLU Cohort 4 – modified CTX / FLU Myxoid / Round cell liposarcoma Pilot study Multiple myeloma Autologous SCT Combination with anti-PD1 (KEYTRUDA) Ovarian No FLU Modified CTX / FLU Melanoma No Flu Non-small cell lung cancer (NSCLC) Modified CTX / FLU MAGE-A10 NSCLC Modified CTX / FLU Urothelial (bladder), melanoma, H&N Modified CTX / FLU AFP Hepatocellular cancer Modified CTX / FLU MAGE-A4 Urothelial, melanoma, H&N, ovarian, NSCLC, esophageal, gastric Planned Complete Ongoing 19
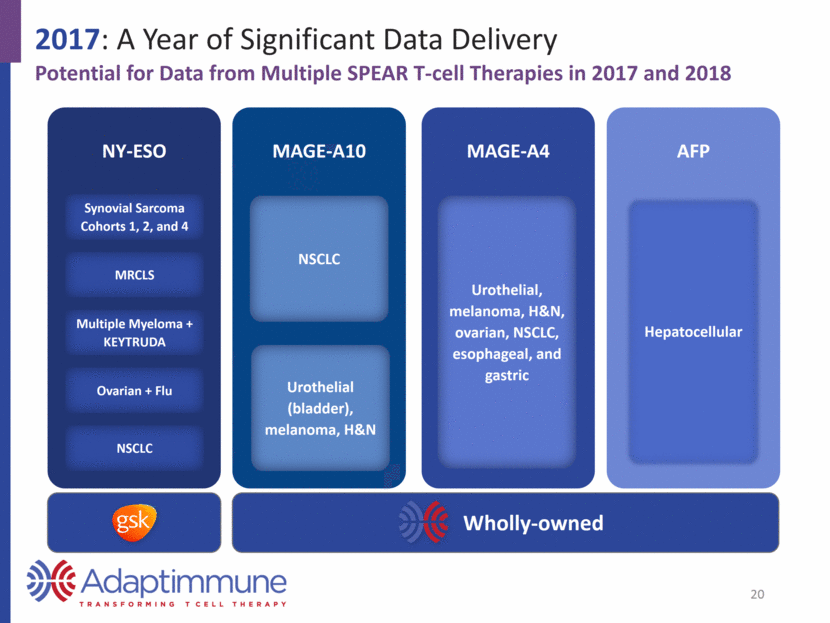
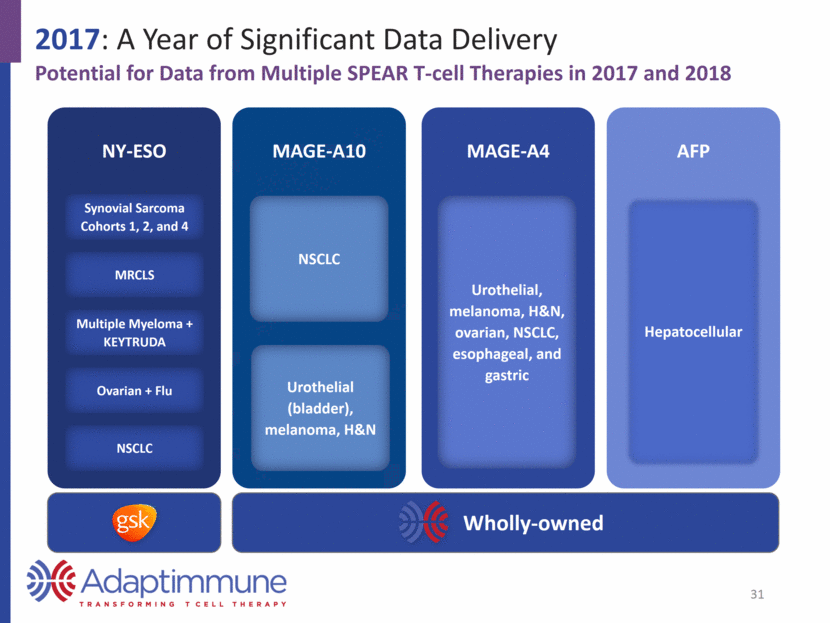
2017: A Year of Significant Data Delivery Potential for Data from Multiple SPEAR T-cell Therapies in 2017 and 2018 NY-ESO Synovial Sarcoma Cohorts 1, 2, and 4 MRCLS Multiple Myeloma + KEYTRUDA Ovarian + Flu NSCLC MAGE-A10 NSCLC Urothelial (bladder), melanoma, H&N MAGE-A4 Urothelial, melanoma, H&N, ovarian, NSCLC, esophageal, and gastric AFP Hepatocellular 20 Wholly-owned
Beyond the Clinical Pipeline 21
Leading Innovation in Engineered T-cell Therapy Addressing Depth and Durability in Solid Tumors Combination studies starting in 2017 Enhancing resistance to tumor microenvironment: 5 programs and growing Enhancing T-cell potency and function: 11 programs and growing Enhancement of Class-I restricted CD4 T-cell function Enhancement of cytotoxic function Enhancement of epitope spreading Other internal programs in development Partnership with Bellicum 22 Block effects of immunosuppression (e.g., TGF-) Overcoming metabolic restrictions of tumor environment Other internal programs in development
Leading Innovation in Engineered T-cell Therapy Innovative Partnership with Bellicum Staged collaboration to evaluate Bellicum’s “GoTCR” switch technology Technology could complement our next generation efforts Provides potential on/off switch to T-cell May further enhance SPEAR T-cell proliferation, activation and persistence Preclinical POC will be completed in 2017 Potential to proceed into co-development / co-commercialization phase in 2017/2018 23
Leading Innovation in Engineered T-cell Therapy Allogeneic Approach to TCR T-cell Therapy Partnered with Universal Cells Benefits of allogeneic approach include Progenitor cell line evaluated; T-cell differentiation ongoing Pre-IND meeting in planning Allows one manufacturing batch to treat numerous patients Enhanced control and standardization of manufactured product Eliminates risk of rejection by host and GvHD Decreases manufacturing costs Scalable for unlimited commercial manufacture 24
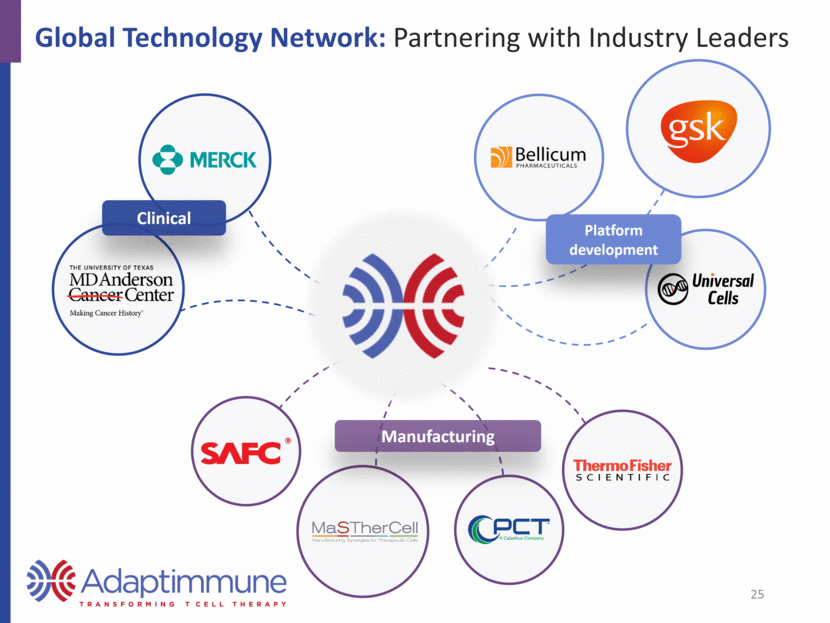
Global Technology Network: Partnering with Industry Leaders Manufacturing Platform development Clinical 25
Optimizing T-cell Product Manufacturing 26

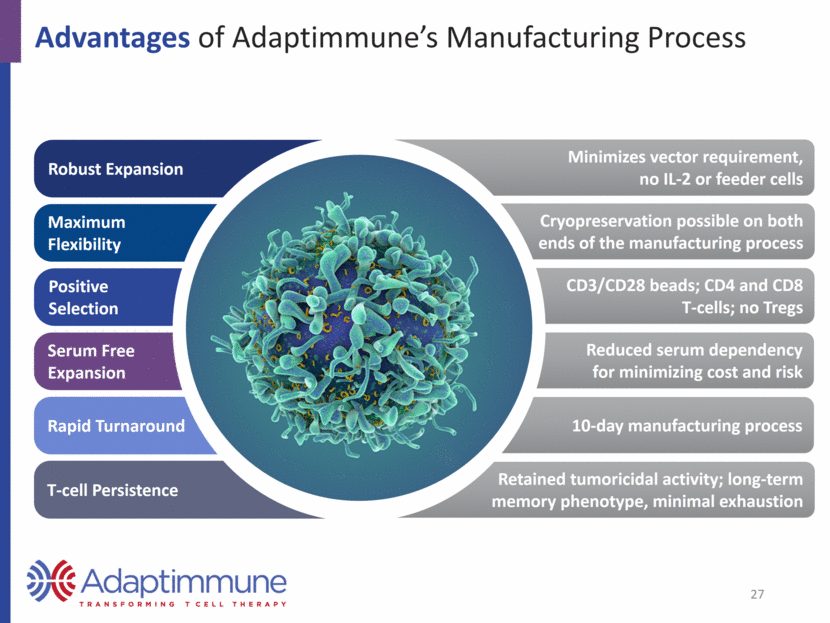
Advantages of Adaptimmune’s Manufacturing Process Robust Expansion Maximum Flexibility Positive Selection Serum Free Expansion Rapid Turnaround T-cell Persistence Minimizes vector requirement, no IL-2 or feeder cells Cryopreservation possible on both ends of the manufacturing process CD3/CD28 beads; CD4 and CD8 T-cells; no Tregs Reduced serum dependency for minimizing cost and risk 10-day manufacturing process Retained tumoricidal activity; long-term memory phenotype, minimal exhaustion 27
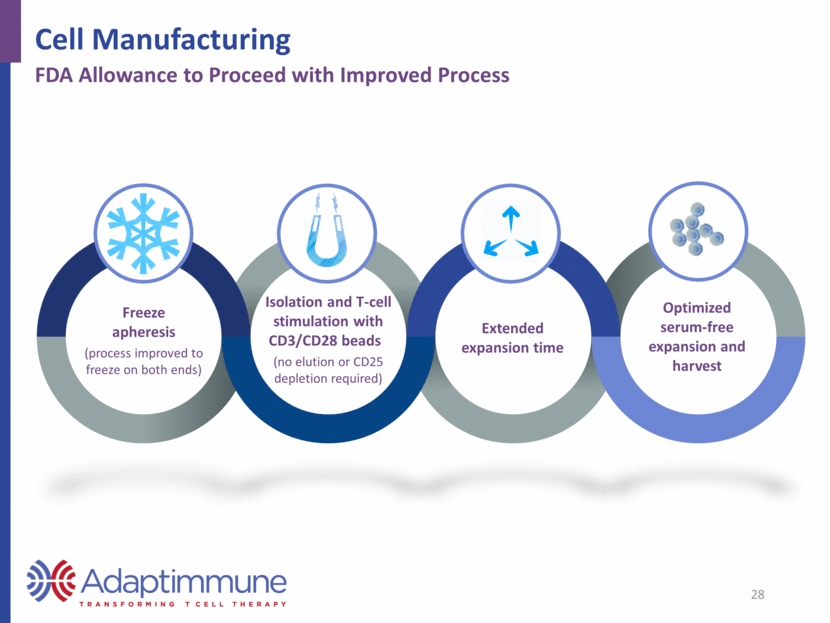
Freeze apheresis (process improved to freeze on both ends) Isolation and T-cell stimulation with CD3/CD28 beads (no elution or CD25 depletion required) Extended expansion time Optimized serum-free expansion and harvest 28 Cell Manufacturing FDA Allowance to Proceed with Improved Process
Financial Update 29

Financial Update * Guidance excludes any new business development and is based on current company assumptions ** Total liquidity position is a non GAAP financial measure, which is explained and reconciled to the most directly comparable financial measures prepared in accordance with GAAP Financial position as of December 31, 2016 $158.8 million of cash and cash equivalents $22.7 million of short-term deposits Combined represents a total liquidity position of $181.5 million** March 2017 public offering (15.7M ADS, $4.20 per ADS) ~$61.8 million net proceeds, including impact of underwriters option 30 Funds Operations through Mid-2019*

2017: A Year of Significant Data Delivery Potential for Data from Multiple SPEAR T-cell Therapies in 2017 and 2018 NY-ESO Synovial Sarcoma Cohorts 1, 2, and 4 MRCLS Multiple Myeloma + KEYTRUDA Ovarian + Flu NSCLC MAGE-A10 NSCLC Urothelial (bladder), melanoma, H&N MAGE-A4 Urothelial, melanoma, H&N, ovarian, NSCLC, esophageal, and gastric AFP Hepatocellular 31 Wholly-owned
March 2017 Corporate Presentation

