Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - SOPHIRIS BIO INC. | ex32-2.htm |
| EX-32.1 - EXHIBIT 32.1 - SOPHIRIS BIO INC. | ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - SOPHIRIS BIO INC. | ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - SOPHIRIS BIO INC. | ex31-1.htm |
| EX-23.1 - EXHIBIT 23.1 - SOPHIRIS BIO INC. | ex23-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2016
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO
Commission file number: 001-36054
Sophiris Bio Inc.
(Exact name of registrant as specified in its charter)
|
British Columbia |
98-1008712 |
|
(State or Other Jurisdiction of |
(I.R.S. Employer |
|
Incorporation or Organization) |
Identification No.) |
|
|
|
|
1258 Prospect Street, La Jolla, California |
92037 |
|
(Address of Principal Executive Offices) |
(Zip Code) |
858-777-1760
(Registrant’s Telephone Number, Including Area Code)
Indicate by check mark if the registrant is a well-known season issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filer pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
|
|
|
|
|
Non-accelerated filer |
☐ (Do not check if a smaller reporting company) |
Smaller reporting company |
☒ |
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
As of June 30, 2016, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the registrant’s common shares held by non-affiliates of the registrant was approximately $46.0 million, based on the closing price of the registrant’s common shares on The NASDAQ Capital Market on June 30, 2016 of $2.15.
As of March 13, 2017, the registrant had 30,111,153 common shares (no par value) outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement for the 2017 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission by April 30, 2017 are incorporated by reference into Part III of this report.
SOPHIRIS BIO INC.
TABLE OF CONTENTS
|
|
|
Page |
|
PART I. | ||
|
Item 1. |
Business |
1 |
|
Item 1A. |
Risk Factors |
27 |
|
Item 1B. |
Unresolved Staff Comments |
53 |
|
Item 2. |
Properties |
53 |
|
Item 3. |
Legal Proceedings |
53 |
|
Item 4. |
Mine Safety Disclosures |
53 |
|
|
| |
|
PART II. | ||
|
Item 5. |
Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities |
54 |
|
Item 6. |
Selected Financial Data |
55 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
56 |
|
Item 7A. |
Quantitative and Qualitative Disclosures About Market Risk |
70 |
|
Item 8. |
Financial Statements and Supplementary Data |
71 |
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
97 |
|
Item 9A. |
Controls and Procedures |
97 |
|
Item 9B. |
Other Information |
97 |
|
|
| |
|
PART III. | ||
|
Item 10. |
Directors, Executive Officers and Corporate Governance |
98 |
|
Item 11. |
Executive Compensation |
98 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters |
98 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
98 |
|
Item 14. |
Principal Accounting Fees and Services |
98 |
|
|
| |
|
PART IV. | ||
|
Item 15. |
Exhibits, Financial Statement Schedules |
99 |
|
Signatures |
103 | |
PART I.
Forward-Looking Statements
This Annual Report on Form 10-K and the information incorporated herein by reference contain forward-looking statements that involve a number of risks and uncertainties. Although our forward-looking statements reflect the good faith judgment of our management, these statements can only be based on facts and factors currently known by us. Consequently, these forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from results and outcomes discussed in the forward-looking statements.
Forward-looking statements include statements about our strategies, objectives, discoveries, clinical trials, development programs, financial forecasts and other statements that are not historical facts, including statements which may be preceded by the words “intend,” “will,” “plan,” “expect,” “anticipate,” “estimate,” “aim,” “seek,” “suggest,” “may,” “believe,” “hope” or similar words. Similarly, statements that describe our future plans, strategies, intentions, expectations, objectives, goals or prospects and other statements that are not historical facts are also forward-looking statements. These statements include but are not limited to statements under the captions “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as other sections in this Annual Report on Form 10-K. You should be aware that the occurrence of any of the events discussed under the heading “Item 1A. Risk Factors” and elsewhere in this report could substantially harm our business, results of operations and financial condition and that if any of these events occurs, the trading price of our common shares could decline and you could lose all or a part of the value of your common shares. The cautionary statements made in this report are intended to be applicable to all related forward-looking statements wherever they may appear in this Annual Report on Form 10-K. We urge you not to place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report on Form 10-K. Except as required by law, we assume no obligation to update our forward-looking statements, even if new information becomes available in the future.
All dollar amounts are expressed in U.S. dollars unless otherwise noted. All amounts are expressed on an as-converted from Canadian dollar to U.S. dollar basis are calculated using the conversion rate as of December 31, 2016 unless otherwise noted.
Item 1. Business
Overview
We are a clinical-stage biopharmaceutical company focused on developing innovative products for the treatment of urological diseases. We are headquartered in San Diego, California and our common shares currently trade on The NASDAQ Capital Market. We are currently developing topsalysin (PRX302) as a treatment for clinically significant localized prostate cancer and as a treatment for the lower urinary tract symptoms of benign prostatic hyperplasia, or BPH, commonly referred to as an enlarged prostate. In 2004, we licensed exclusive rights to topsalysin from UVIC Industry Partnerships Inc., or UVIC, and The Johns Hopkins University, or Johns Hopkins, for the treatment of prostate cancer and in 2009, we licensed exclusive rights to topsalysin from UVIC and Johns Hopkins for the treatment of the symptoms of BPH. In April 2010, we entered into an exclusive license agreement with Kissei Pharmaceuticals Co., Ltd., or Kissei, pursuant to which we granted Kissei the right to develop and commercialize topsalysin in Japan for the treatment of the symptoms of BPH, prostate cancer, prostatitis or other diseases of the prostate.
Topsalysin, a genetically modified recombinant protein, is delivered via ultrasound-guided injection directly into the prostate. This membrane-disrupting protein is selectively activated by enzymatically active prostate specific antigen, or PSA, which is only present in the prostate, leading to localized cell death and tissue disruption without damage to neighboring tissue and nerves. This method of administration limits the circulation of the drug in the body, and we believe that this limited systemic exposure to the drug, together with how the drug is activated in the prostate, greatly diminishes the risk of side effects.
In May 2015, we initiated a single-center, open-label Phase 2a proof of concept clinical trial of topsalysin for the treatment of localized low to intermediate risk prostate cancer. We believe that the highly targeted mechanism by which topsalysin selectively destroys prostate tissue in BPH makes topsalysin a potential targeted focal treatment for localized prostate cancer. The clinical trial utilized previously obtained magnetic resonance imaging, or MRI, images of each patient’s prostate mapped to real time 3D ultrasound to target the delivery of topsalysin directly into and around a pre-identified clinically significant tumor. A clinically significant tumor was defined in our study as, either a Gleason score 6 (pattern 3+3) and >3mm Maximum Cancer Core Length, or MCCL, or Gleason score 7 (pattern 3+4 or 4+3) < 10 mm MCCL, which is thought to have the potential to progress and would therefore warrant treatment. (A Gleason pattern is a grading system utilized to describe how aggressive a prostate tumor is and how likely it is to spread. Generally, there are five recognized Gleason histological patterns and a higher Gleason pattern indicates a more aggressive tumor.) Patients received a transperineal administration of topsalysin under general anesthesia at a dose higher than used in our completed Phase 3 BPH PLUS-1 trial but less than the highest dose used in our previous prostate cancer trial. The primary objective of the trial was to assess the safety and tolerability of topsalysin when used to selectively target and focally ablate a clinically significant tumor. The potential efficacy was evidenced by histological changes, indicating tumor ablation at six months following treatment. The clinical trial was conducted at a single center, the University College London, which is well known for the focal treatment of prostate cancer in the United Kingdom.
A total of 18 patients with localized low to intermediate risk prostate cancer were enrolled in the Phase 2a proof of concept clinical trial. On June 9, 2016, we announced the biopsy results from all 18 patients enrolled in the Phase 2a proof of concept study of topsalysin for the treatment of localized prostate cancer. The one-time administration of topsalysin was well tolerated with no serious adverse events and no new safety signals being reported. Topsalysin demonstrated an ability to ablate tumor cells in 50 percent of patients (9/18 patients) six months after treatment in a patient population with pre-identified, clinically significant prostate cancer. In preparation for the presentation of the Phase 2a proof of concept clinical trial data for an upcoming medical conference, we recently determined that 2 patients who were initially reported as having no response to treatment should have been reported as having a partial response to treatment. Taking into account the updated results, topsalysin demonstrated an ability to ablate tumor cells in more than 60 percent of patients (11/18 patients) six months after treatment in a patient population with pre-identified, low to intermediate risk prostate cancer.
All 18 patients enrolled completed the study. Biopsy data at six months following treatment showed that:
|
|
● |
Two patients experienced complete ablation of their targeted tumor with no evidence of any tumor remaining at six months; |
|
|
● |
Nine patients experienced a partial response, defined as either a reduction in the maximum cancer core length or a reduction in Gleason pattern; and |
|
|
● |
Seven patients had no response to treatment. |
Detailed results from this study will be presented at a future medical conference.
In March 2017, we initiated a second Phase 2 clinical trial to confirm the dose and optimize the delivery of topsalysin for the treatment of clinically significant localized prostate cancer. This study will also utilize previously obtained MRI images of each patient’s prostate mapped to real time 3D ultrasound to target the delivery of topsalysin directly into and around a pre-identified clinically significant tumor. The primary objective of the study is safety and tolerability of an injection of topsalysin and the key efficacy variable is focal ablation of a clinically significant lesion on biopsy after six months. Approximately 40 patients will be enrolled across clinical sites in the UK and US. Patient screening into the study has begun.
We expect to receive biopsy data for all patients for biopsies conducted six months after the initial dose in late 2017 or early 2018. Based upon the results of the 6-month biopsy, the study includes an option to potentially re-treat the targeted lesion area with a second dose of topsalysin, with a targeted biopsy to occur six months following the second dose. In order to be eligible for a second dose, the patient cannot have experienced a significant adverse event attributable to topsalysin or the dosing procedure from the first dose and the patient will need to have had a clinical response from the first dose but still have the presence of a clinically significant lesion area. We expect to have final biopsy data on all patients who receive a second dose by the third quarter of 2018.
We have also completed the first of two Phase 3 clinical trials that we believe would be required to obtain marketing approval for topsalysin for the treatment of the symptoms of BPH. In October 2013, we initiated our first Phase 3 clinical trial, which we refer to as the “PLUS-1” trial, of topsalysin for the treatment of the lower urinary tract symptoms of BPH. The Phase 3 “PLUS-1” trial was an international, multicenter, randomized, double-blind, and vehicle-controlled trial to assess the efficacy and safety of a single intraprostatic administration of topsalysin (0.6 µg/g prostate) for the treatment of the lower urinary symptoms of BPH. Patients were randomized on a 1:1 ratio to either topsalysin or vehicle-only injection, and then monitored for one year. A total of 479 patients with moderate to severe BPH were enrolled and randomized by September 2014. On November 10, 2015, we announced final results from this trial. Topsalysin demonstrated a statistically significant improvement in International Prostate Symptom Score, or IPSS, total score from baseline over 12 months compared to the vehicle-only control group (7.60 vs. 6.58 point overall improvement; p = 0.043), the primary endpoint of the trial. (IPSS is a patient recorded, composite assessment that takes into account factors such as ability to empty the bladder, frequency of urination, intermittency of urination, urgency of urination, weak strength of urine stream, straining while urinating, and having to urinate at night after going to bed.) Topsalysin continues to demonstrate a favorable safety profile, with no evidence of any treatment related sexual or cardiovascular side effects.
We are currently not planning on pursuing a second Phase 3 trial in BPH, unless we secure a development partner to fund such new clinical trial or obtain other financing. There can be no assurance that such funding or a development partner will be available on acceptable terms or at all. For that reason, we cannot currently estimate when the clinical development required to seek the regulatory approvals needed to commercialize topsalysin for the treatment of the symptoms of BPH will be completed.
Topsalysin - Mechanism of Action
Topsalysin is a genetically altered form of the naturally occurring protein proaerolysin. In nature, proaerolysin is produced by Aeromonas bacteria, which are commonly found as a contaminant in fresh water and fresh water fish. We have altered the sequence encoding the bacterial protein so that topsalysin is only activated by active PSA (as shown in the figure below), an enzyme that is produced in large quantities in the prostate of men with prostate cancer and BPH.
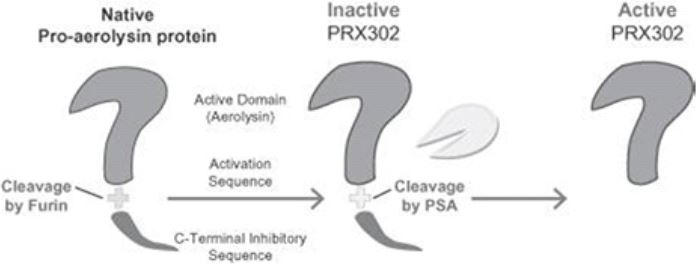 |
Topsalysin binds to the GPI-anchored receptors on the cell surface of prostate cells. Once activated by PSA, topsalysin combines with other activated topsalysin molecules, forming stable transmembrane pores that induce cell death. Topsalysin has not been detected in plasma following injection into the prostate. The prostate specific activation of topsalysin by enzymatically active PSA thus limits exposure of non-prostate tissues to the drug’s activity, contributing to the safety of the therapy.
The mechanism of action is shown in the figure below.
Topsalysin Mechanism of Action
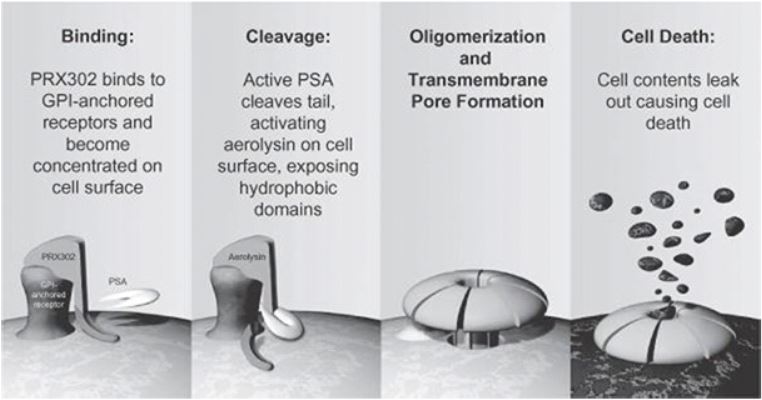 |
Background on Clinically Significant Localized Prostate Cancer
Prostate cancer is the fourth leading cause of death due to cancer in the United States. As a result of an increase in life expectancy along with the current practice of formal and informal screening using prostate-specific antigen, PSA, blood tests, disease treatment has shifted towards early detection of localized disease.
According to the National Cancer Institute, there were approximately 181,000 new cases of prostate cancer in the United States identified in 2016 with approximately 80% of patients diagnosed with localized disease (disease that has not progressed beyond the confines of the prostate). In the United States, approximately 27,000 were expected to die from prostate cancer in 2016 and it is currently the second leading cause of death in men from cancer.
Prostate cancer grows very slowly and research has shown that, in many cases, patients with early localized disease have a low likelihood of the cancer spreading beyond the confines of the prostate. These patients may elect to undergo active surveillance, which does not offer any therapeutic benefit but means that their doctor will continue to monitor the patient (typically PSA levels, digital rectal exams and periodic or as indicated biopsies) for any progression of disease. The information collected by the doctor during active surveillance is used to determine if a patient can remain in active surveillance or should undergo treatment. The complex psychological impact that results from a cancer diagnosis is demonstrated by a significant proportion of men (about 10% in most studies) electing to undergo treatment, even though they have had no evidence of biochemical or histopathological progression of their disease during active surveillance.
Current Therapies for Localized Prostate Cancer
Patients with localized prostate cancer who elect to treat their prostate cancer have traditionally been offered radical treatments in the form of surgery to remove the entire prostate and/or whole gland radiation. Potential side effects and toxicities from radical treatments can be significant and permanent. Men who have undergone radical treatments have experienced the following side effects and toxicity rates: erectile dysfunction 30% - 90%, incontinence 5% - 20% and rectal toxicity (which could include proctitis (inflammation of the rectum) with bleeding and bowel problems such as diarrhea) 5% - 20%.
The increasing use of multi-parametric magnetic resonance imaging (mpMRI) of the prostate and advances in software to co-register previously obtained mpMRI images with live 3D ultrasound images enables physicians to more accurately target their prostate biopsies. Consequently, it is increasingly possible to more confidently identify men with clinically significant lesions. This enables physicians and patients to make a more informed decision about the clinical significance of their disease and whether their disease requires radical treatment or whether they may be a candidate for targeted focal therapy. The objective of targeted focal therapy is to remove the significant disease while preserving as much of the prostate as possible thereby potentially avoiding many of the complications and side effects associated with the radical whole gland treatments. There are several focal targeted therapies currently being offered to patients such as targeted laser ablation, high-intensity focused ultrasound, cryoablation, radiofrequency ablation and photodynamic therapy each with the aim of reducing the treatment impact to the surrounding anatomical structures, potentially leading to lower rates of side effects while retaining the cancer control benefits that the whole gland radical treatments offer. This focal targeted approach to the treatment of prostate cancer is consistent with the management of almost all other solid organ cancers (breast, kidney, liver and pancreas) in which organ preservation is fundamental to functional preservation.
Topsalysin for the Targeted Focal Treatment of Clinically Significant Localized Prostate Cancer
The intraprostatic injection of topsalysin represents a highly targeted approach for potentially treating clinically significant localized prostate cancer that is confined within the encapsulated prostate gland for two reasons:
|
|
● |
a targeted focal delivery of an intraprostatic injection of topsalysin directly into and around a pre-identified tumor(s) within the prostate is now possible; and |
|
|
● |
topsalysin has a highly targeted mechanism of action, activated specifically only within the prostate. |
Using advancements in MRI and 3D ultrasound imaging, physicians are able to deliver the injection of topsalysin directly into the tumors located within the prostate. The increased use of multi-parametric magnetic resonance imaging (mpMRI) and advances in software to co-register the mpMRI images with live 3D ultrasound images also means that physicians are now able to locate tumors within the prostate and take more accurate biopsies from a patient, enabling the diagnosis of clinically significant lesions. These technical advances are enabling physicians and patients to make a more informed decision about the clinical significance of their disease and whether their disease requires radical treatment or they would be candidates for active surveillance. In addition, these advances make it possible to identify patients with clinically significant lesions that could be candidates for targeted ablation with a focal therapy. The targeted focal treatment of localized prostate cancer is consistent with the treatment approach frequently used for other solid tumors such as breast and liver cancer, where the objective is to remove the tumor and preserve as much of the organ as possible.
The mechanism of action of topsalysin allows for a highly targeted therapeutic activity in localized disease. Topsalysin is only activated in the presence of enzymatically active PSA which is found surrounding prostate cancer lesions. Therefore, we believe topsalysin has the potential to provide a focal targeted therapy for the ablation of localized prostate cancer while potentially avoiding many of the complications and side effects associated with radical treatments.
Background on BPH
BPH is a non-cancerous enlargement of the prostate gland that commonly affects men who are age 50 and older. BPH causes a restriction in urine flow from the urethra resulting in lower urinary tract symptoms, or LUTS. BPH, and its associated clinical manifestations of LUTS, is one of the most common medical conditions of aging men in the United States, with approximately 70% men aged 60-69 years and 80% of men older than the age of 70 being affected by BPH. The number of men with symptoms of BPH is expected to increase as the male population ages. Our market research suggests that as many as 36 million men in the United States are affected by BPH with approximately five million of these men suffering from bothersome symptoms. Symptomatic BPH greatly diminishes a patient’s quality of life. It causes a significant array of LUTS, including increased urinary frequency, urgency to urinate, frequent night-time urination, weak urine stream, and incomplete emptying of the bladder. In addition, men with BPH symptoms are predisposed to a higher risk of urinary tract infections, urinary stone formation, bladder damage, and in very late stage and/or unattended cases, renal damage.
Current Therapies for BPH
Physicians and patients choose treatments for the symptoms of BPH primarily based on the severity of symptoms, the patient’s quality of life and the presence of other medical conditions. Treatment options include watchful waiting, lifestyle changes, oral medications, minimally invasive surgical therapies or more aggressive surgical therapies, such as transurethral resection of the prostate, or TURP, or open prostatectomy. Our market research indicates that approximately three million men in the United States are taking oral drug therapy and there were approximately 200,000 surgical procedures for the treatment of the symptoms of BPH conducted in 2011.
The effectiveness of treatments for the symptoms of BPH is measured by IPSS and improvement in peak urine flow rate, or Qmax. IPSS is a patient recorded, composite assessment that takes into account factors such as ability to empty the bladder, frequency of urination, intermittency of urination, urgency of urination, weak strength of urine stream, straining while urinating, and having to urinate at night after going to bed. This index is measured on a 0 to 35 scale with 0 being defined as having no problems and 35 defined as the high end of severe symptoms. Patients are typically considered to have mild symptoms with IPSS of 1 to 7, moderate symptoms with scores of 8 to 19 and severe symptoms with scores of 20 to 35. An improvement of 3 points in IPSS is generally considered clinically meaningful by urologists. IPSS is a validated primary clinical endpoint used to assess the treatment benefit in BPH clinical trials and has served as the primary efficacy endpoint for the approval of many products for the treatment of the symptoms of BPH. An approximate 2 point difference in IPSS improvement between active and control has historically been utilized by the FDA to approve oral therapies, although the FDA has not provided guidance that a 2 point difference is required for approval of treatment for the symptoms of BPH.
Oral Drug Therapy
The most common form of therapy for men experiencing mild to moderate LUTS associated with BPH is oral drug therapy. Current classes of oral medications available for treatment of the symptoms of BPH include alpha-blockers, 5-alpha-reductase inhibitors, or 5-ARIs, a combination of an alpha-blocker and 5-ARI, and a phosphodiesterase Type 5 inhibitor, or PDE5. An alpha-blocker provides rapid relief of BPH symptoms, but does not prevent continued growth of the prostate. Examples of alpha-blockers include terazosin, doxazosin, tamsulosin, alfuzosin, and silodosin. Frequently reported side effects of alpha-blockers include hypotension, or low blood pressure, dizziness and feeling of weakness. 5-ARIs, such as finasteride and dutasteride, reduce the size of the prostate and thus provide symptom relief. It may take up to six months from starting treatment with 5-ARI for the prostate to reduce in size and for patients to experience the benefit of treatment. Side effects include sexual dysfunction. In addition, tadalafil (marketed by Eli Lilly as Cialis®), a PDE5 inhibitor (a class of drugs typically prescribed for erectile dysfunction), was shown to improve IPSS after four weeks of dosing and has been approved for treatment of the symptoms of BPH. Headache and dyspepsia, or indigestion, are the most commonly observed side effects of Cialis®, which is not recommended for use in combination with an alpha-blocker because of the risk of hypotension.
Many men will discontinue oral drug therapy due to inadequate response and/or the side effects mentioned above. Another drawback of the currently available oral therapies is the necessity of taking one or more pills daily. Published patient survey data (N=2,166) suggests that as many as 57% of patients taking oral drug therapy discontinue use within the first three years.
In previously completed clinical trials, each of these classes of oral medications has typically produced approximately 3 to 6 point reductions in IPSS, but the actual magnitude of treatment benefit observed compared to placebo is generally two to three points.
Minimally Invasive Surgical Therapies
Minimally invasive surgical therapies used to treat the symptoms of BPH include transurethral microwave thermotherapy, or TUMT, transurethral needle ablation, or TUNA, Urolift®, a system which lifts and holds the enlarged prostate tissue away from the urethra, and green laser treatment, which delivers high energy to ablate the prostatic tissue as an alternative to TURP. These treatments, frequently referred to as MIST, are generally less effective than surgical procedures in reducing the size of the prostate gland and often require retreatment within three years. However, these treatments may require catheterization and are still associated with pain and the potential for complications such as bleeding and long-lasting side effects such as urinary incontinence and sexual dysfunction, including erectile dysfunction and retrograde ejaculation (semen flowing backward into the bladder). A new TUNA, known as the Rezum System, has been recently approved. Rezum delivers radiofrequency generated thermal therapy in the form of water vapor via a transurethral needle. Studies of MIST procedures have shown varying improvements in IPSS, with TUNA and TUMT showing improvement in IPSS of approximately 10 to 13 points.
Other Surgical Options
Surgical procedures such as TURP typically reduce the size of the prostate gland and relieve the pressure on the urethra by ablating the prostate tissue that blocks the flow of urine. Studies of surgical procedures have generally shown reductions in IPSS of approximately 16 points. TURP is performed under spinal or general anesthesia, which carries the risk of side effects. TURP may result in nerve damage, bleeding (sometimes requiring transfusion), and long-lasting side effects, such as urinary incontinence and sexual dysfunction, including erectile dysfunction and retrograde ejaculation.
Topsalysin for the Treatment of the Symptoms of BPH
In our completed Phase 3 clinical trial, topsalysin significantly improved symptoms of BPH through 12 months of follow-up after a single treatment. Topsalysin is designed to be a safe, simple and convenient treatment that provides rapid and sustained relief of BPH symptoms. It is delivered through a targeted injection into the prostate, precisely ablating the prostate tissue without damaging neighboring tissue and nerves. This method of administration limits the circulation of the drug in the body and we believe that this limited systemic exposure to the drug, together with how the drug is activated in the body, greatly diminishes the risk of side effects.
The injection of topsalysin is individualized to each patient based on the size of the prostate and the drug is delivered in a procedure that can be performed in an urologist’s office. The entire process can be completed during a short office visit, and the actual injection of the drug into each of the two lobes of the prostate takes approximately four minutes. A physician administering topsalysin may elect to administer a local anesthetic before injection. Most urologists are familiar with the transrectal route of administration, as it is the same method urologists use to take biopsies of the prostate.
Topsalysin Transrectal Administration Schematic
 |
Market research we conducted with 100 urologists in 2012 has shown that topsalysin compares favorably to both oral therapies and procedures on a number of key attributes related to effectiveness, safety, tolerability, and burden placed on the patient. Specifically, when shown results from our Phase 2b clinical trial, the physicians viewed topsalysin as being more effective and having a better side effect profile than currently available oral drugs. Administration of topsalysin was also perceived as more effective, safer, and easier to perform than MIST procedures, TUNA and TUMT. When compared to TURP surgery, topsalysin was also perceived as safer and easier to administer. In this market research, physicians indicated a willingness to consider topsalysin as an alternative to both oral therapies and surgical procedures and also viewed topsalysin as a potential new choice for men who have discontinued oral therapy and are not willing to undergo a surgical procedure.
Clinical Overview
To date, we have completed eight clinical trials of topsalysin and we have an on-going Phase 2b clinical trial for the treatment of men with histologically proven, clinically significant localized prostate cancer. Five of our completed clinical trials were for the treatment of the symptoms of BPH and three were for the treatment of prostate cancer. In the eight completed clinical trials a total of 365 patients with moderate to severe BPH and 48 patients with prostate cancer have been treated with topsalysin for an estimated combined topsalysin exposure of 413 patients.
We have completed a clinical trial of topsalysin for the treatment of localized low to intermediate risk prostate cancer. The one-time administration of topsalysin was well tolerated with no serious adverse events and no new safety signals being reported. Patients received a transperineal administration of topsalysin under general anesthesia at a dose higher than used in our completed Phase 3 BPH PLUS-1 trial but less than the highest dose used in our previous prostate cancer trial. Topsalysin demonstrated an ability to ablate tumor cells in 60 percent of patients (11/18 patients) six months after treatment in a patient population with pre-identified, clinically significant prostate cancer.
We have completed two clinical trials of topsalysin for the treatment of locally recurrent prostate cancer. The patients in these two small open-label studies were patients who had previously undergone radiation for the treatment of their prostate cancer and showed signs of disease progression evidenced by rising levels of PSA. The results from these clinical trials demonstrated that topsalysin was well-tolerated and showed early signs of therapeutic activity following a single intraprostatic treatment.
In each of the clinical trials for BPH topsalysin was administered as a single intraprostatic treatment with 12 months of follow up. To date, no patients have been administered more than one exposure to topsalysin. The first five trials for topsalysin (two prostate cancer trials and three BPH trials) used the transperineal route of administration for the intraprostatic injection and the two most recent clinical trials in BPH, including our completed PLUS-1 trial, used the transrectal route. The transrectal route appears to be as well tolerated as the transperineal route and is more familiar to urologists.
All of the completed clinical trials of topsalysin for the treatment of the symptoms of BPH have shown clinically meaningful, sustained efficacy with regard to improvement in LUTS, as measured by IPSS and Qmax, the standard measures of the treatment of symptoms for BPH. Topsalysin has been well-tolerated in all completed clinical trials to date. Adverse events in our completed clinical trials were typically mild and transient in nature, limited to local discomfort and irritative urinary symptoms that generally occurred on the same day as topsalysin injection. There have been no drug-related sexual or cardiovascular side effects reported.
Clinical Development of Topsalysin
Our clinical program for topsalysin is summarized below.
Completed Clinical Development in Prostate Cancer
|
CLINICAL TRIAL |
|
STATUS |
|
TRIAL DESIGN |
|
PRX302-2-07 Phase 2a |
|
Completed |
|
Phase 2a 6 month proof of concept trial with topsalysin in patients who had histologically proven, localized low to intermediate risk prostate cancer.
18 patients |
|
Dosing: Varied based upon prostate volume and the size of the lesion to be injected but the dose did not exceed 5µg/gm of prostate and when normalized for lesion size up to 1000 ug/gram of tumor lesion | ||||
|
PRX302-1-02 Phase 2a |
|
Completed |
|
Open-label, safety, 12 month dose escalation & volume escalation of a single transperineal intraprostatic treatment of topsalysin in patients who had previously undergone radiation treatment of their prostate cancer
6 patients Dosing: 6.0µg/g, 12.0µg/g Volume: 20% to 40% of prostate volume |
|
Phase 1 |
|
Completed |
|
Open-label, safety, 12 month dose-escalation of a single transperineal intraprostatic treatment of topsalysin in patients who had previously undergone radiation treatment of their prostate cancer
24 patients Dosing: 0.03µg/g, 0.09µg/g, 0.3µg/g, 0.6µg/g, 1.2µg/g, 2.1µg/g, 3.0µg/g Volume: Fixed at 10% of prostate volume |
On-going Development in Localized Prostate Cancer
|
CLINICAL TRIAL |
|
STATUS |
|
TRIAL DESIGN |
|
PRX302-2-08 Phase 2b Dose Confirmation and Delivery Optimization Trial |
|
On-going |
|
Phase 2b 6 to 12 month trial with topsalysin in patients who have histologically proven, clinically significant localized prostate cancer to confirm the dose and optimize the delivery of a single and potentially a second transperineal intraprostatic treatment of topsalysin
Number of patients: 40 Dosing: Varied based upon prostate volume and size of the lesion up to 12 ug/gram prostate and 1,000 ug/gram of tumor lesion |
Completed Clinical Development in BPH
|
CLINICAL TRIAL |
|
STATUS |
|
TRIAL DESIGN |
|
PLUS-1 Phase 3 Trial #1 for the treatment of the symptoms of BPH |
|
Completed |
|
Prospective, randomized, double-blind, placebo-controlled clinical trial of a single transrectal intraprostatic treatment of topsalysin, which will utilize the IPSS outcome measure evaluated at 12 months as the primary endpoint
479 patients, 239 on topsalysin; 240 on vehicle Dosing: 0.6µg/g Volume: 20% of prostate volume |
|
|
|
|
|
|
|
PRX302-2-03 TRIUMPH Phase 2b |
|
Completed |
|
Randomized, double-blinded, placebo-controlled trial of a single transperineal intraprostatic treatment of topsalysin
92 patients; 61 on topsalysin; 31 on vehicle Dosing: 0.6 µg/g Volume: 20% of prostate volume |
| CLINICAL TRIAL | STATUS | TRIAL DESIGN | ||
|
PRX302-2-06 Transrectal Trial Phase 1/2 |
|
Completed |
|
Randomized dose-escalation, multicenter trial of a single transrectal intraprostatic treatment of topsalysin
40 patients; 32 on topsalysin in 4 dosing cohorts; 8 on placebo Dosing: 0.15µg/g, 0.30µg/g, 0.60µg/g, 1.2µg/g Volume: 20% of prostate volume |
|
PRX302-2-02 Phase 2a |
|
Completed |
|
Open-label, safety, volume escalation clinical trial of a single transperineal intraprostatic treatment of topsalysin
18 patients Dosing: 0.3µg/g, 0.6µg/g, 0.9µg/g Volume: 10 to 30% of prostate volume |
|
|
|
|
|
|
|
PRX302-2-01 Phase 1 |
|
Completed |
|
Open-label, safety, dose-escalation clinical trial of a single transperineal intraprostatic treatment of topsalysin
15 patients Dosing: 0.025µg/g, 0.072µg/g, 0.25µg/g, 0.35µg/g Volume: 1.5 to 2.0 mL |
Planned Clinical Development in BPH
|
CLINICAL TRIAL |
|
STATUS |
|
TRIAL DESIGN |
|
Phase 3 Trial #2 for the treatment of the symptoms of BPH |
|
Planned but initiation dependent upon receipt of funding to run the study |
|
Prospective, randomized, double-blind, placebo-controlled clinical trial of a single transrectal intraprostatic treatment of topsalysin
Dosing: 0.6µg/g Volume: 20% of prostate volume |
|
|
|
|
|
|
|
Open-Label Safety Study Phase 3 for the treatment of the symptoms of BPH |
|
Planned but initiation dependent upon receipt of funding to run the study |
|
Safety of repeat dose and long-term safety of transrectal intraprostatic treatment of topsalysin
Approximately 100 patients Dosing: 0.6µg/g Volume: 20% of prostate volume |
Clinical Development in Prostate Cancer
On-going Clinical Development in Localized Prostate Cancer
A Phase 2b open-label clinical trial has been initiated, to confirm the dose and optimize the delivery of topsalysin for the treatment of clinically significant localized prostate cancer. The primary objective of the study is safety and tolerability of an injection of topsalysin and the key efficacy variable is focal ablation of a clinically significant lesion on biopsy after six months.
This study will utilize previously obtained MRI images of each patient’s prostate mapped to real time 3D ultrasound images to target the delivery of topsalysin directly into and around a pre-identified clinically significant legion area.
Six months following-treatment with topsalysin, a targeted biopsy of the treated area will be conducted. We expect to receive biopsy data for all patients conducted six months after the initial dose in late 2017 or early 2018. Based upon the results of the 6-month biopsy, the study includes an option to potentially re-treat the targeted lesion area with a second dose of topsalysin, with a targeted biopsy to occur six months following the second dose. In order to be eligible for a second dose, the patient cannot have experienced a significant adverse event attributable to topsalysin or the dosing procedure from the first dose and the patient will need to have had a clinical response from the first dose but still have the presence of a clinically significant lesion area. We expect to have final biopsy data on all patients who receive a second dose by the third quarter of 2018.
Completed Clinical Development
Phase 2a Proof of Concept Trial in Localized Prostate Cancer
In May 2015, we initiated a single-center, open-label Phase 2a proof of concept clinical trial of topsalysin for the treatment of localized low to intermediate risk prostate cancer. We believe that the highly targeted mechanism by which topsalysin selectively destroys prostate tissue in BPH makes topsalysin a potential targeted focal treatment for localized prostate cancer. The clinical trial utilized previously obtained magnetic resonance imaging, or MRI, images of each patient’s prostate mapped to real time 3D ultrasound to target the delivery of topsalysin directly into and around a pre-identified clinically significant tumor. A clinically significant tumor was defined in our study as, either a Gleason score 6 (pattern 3+3) and >3mm Maximum Cancer Core Length, or MCCL, or Gleason score 7 (pattern 3+4 or 4+3) < 10 mm MCCL, which is thought to have the potential to progress and would therefore warrant treatment. (A Gleason pattern is a grading system utilized to describe how aggressive a prostate tumor is and how likely it is to spread. Generally, there are five recognized Gleason histological patterns and a higher Gleason pattern indicates a more aggressive tumor.) Patients received a transperineal administration of topsalysin under general anesthesia at a dose higher than used in our completed Phase 3 BPH PLUS-1 trial but less than the highest dose used in our previous prostate cancer trial. The primary objective of the trial was to assess the safety and tolerability of topsalysin when used to selectively target and focally ablate a clinically significant tumor. The potential efficacy was evidenced by histological changes, indicating tumor ablation at six months following treatment. The clinical trial was conducted at a single center, the University College London, which is well known for the focal treatment of prostate cancer in the United Kingdom.
On June 9, 2016, we announced the biopsy results from all 18 patients enrolled in the Phase 2a proof of concept study of topsalysin for the treatment of localized prostate cancer. The one-time administration of topsalysin was well tolerated with no serious adverse events and no new safety signals being reported. Topsalysin demonstrated an ability to ablate tumor cells in 50 percent of patients (9/18 patients) six months after treatment in a patient population with pre-identified, clinically significant prostate cancer. In preparation for the presentation of the Phase 2a proof of concept clinical trial data for an upcoming medical conference, we recently determined that 2 patients who were initially reported as having no response to treatment should have been reported as having a partial response to treatment. Taking into account the updated results, topsalysin demonstrated an ability to ablate tumor cells in more than 60 percent of patients (11/18 patients) six months after treatment in a patient population with pre-identified, low to intermediate risk prostate cancer.
All 18 patients enrolled completed the study. Biopsy data at six months following treatment showed that:
|
|
● |
Two patients experienced complete ablation of their targeted tumor with no evidence of any tumor remaining at six months; |
|
|
● |
Nine patients experienced a partial response, defined as either a reduction in the maximum cancer core length or a reduction in Gleason pattern; and |
|
|
● |
Seven patients had no response to treatment. |
Detailed results from this study will be presented at a future medical conference.
Phase 2 Open-Label Clinical Trial in Prostate Cancer
In September 2009, we completed a Phase 2 clinical trial of topsalysin in six patients with biopsy-proven, locally-recurrent prostate cancer that, following radiation therapy, showed signs of disease progression evidenced by rising levels of PSA. Therapeutic activity in the form of overall decreases in PSA levels and in the number of adenocarcinoma-positive biopsy cores following topsalysin treatment was observed in two of six patients.
Phase 1 Open-Label Clinical Trial in Prostate Cancer
In May 2008, we completed a multicenter, open-label, dose-escalation Phase 1 clinical trial of topsalysin in 24 patients in the United States with biopsy-proven, locally-recurrent prostate cancer that, following radiation therapy, showed signs of disease progression evidenced by rising levels of PSA. Elevated and rising levels of PSA can be a sign of the presence or progression of prostate cancer. The primary clinical endpoint of this clinical trial was to examine the safety and tolerability of topsalysin with therapeutic activity as the secondary clinical endpoint. Clinical trial results demonstrated that topsalysin was well-tolerated and showed early signs of therapeutic activity following a single intraprostatic treatment.
No topsalysin treatment-related serious adverse events were reported and the treatment-related adverse effects that were reported were mild and were primarily associated with the injection procedure.
Clinical Development in BPH
Completed Clinical Development in BPH
PLUS-1 Randomized, Double-Blind, Placebo-Controlled Transrectal Route of Injection Clinical Trial
In October 2013 we initiated our first Phase 3 clinical trial, which we refer to as the “PLUS-1” trial, of topsalysin for the treatment of the lower urinary tract symptoms of BPH. The Phase 3 “PLUS-1” trial is an international, multicenter, randomized, double-blind, and vehicle-controlled trial to assess the efficacy and safety of a single intraprostatic administration of topsalysin (0.6 µg/g prostate) for the treatment of the symptoms of BPH. Patients were randomized in a 1:1 ratio to either topsalysin or vehicle-only injection, and then monitored for one year. A total of 479 patients with moderate to severe BPH were enrolled and randomized by September 2014. The 52-week completion rate was 91.9%, with a similar number of premature withdrawals from study for the topsalysin group (8.8%) vs. the vehicle group (7.5%). On average, the injection itself was completed in less than four minutes. This Phase 3 clinical trial used the IPSS total score change from baseline over 52 weeks using the repeated measures linear mixed model as the primary endpoint. Secondary endpoints included Qmax (maximum urine flow) change from baseline over 52 weeks.
Treatment groups were well balanced at baseline, including average IPSS total score (21.2 points both groups), Qmax (maximum urine flow) (9.5 mL/sec both groups), total prostate volume (49.8 mL for topsalysin vs. 48.1 mL vehicle), prior BPH treatment (55.2% topsalysin vs. 55.1% vehicle), and quality of life (4.5 points both groups, “mostly dissatisfied” to “unhappy” with current urinary condition).
The results of this trial were:
|
|
• |
Topsalysin demonstrated statistical significance over vehicle – The primary efficacy endpoint of the IPSS total score change from baseline over 52 weeks was analyzed, per guidance from the FDA, using the repeated measures linear mixed model applied to the modified intent-to-treat population of every patient randomized and dosed with study drug. Topsalysin demonstrated a statistically significant improvement in IPSS total score from baseline over 12 months compared to the vehicle-only control group (7.60 vs. 6.58 point overall improvement; p = 0.043), the primary endpoint of the study. |
|
|
• |
Improvement was clinically meaningful, rapid and sustained – In a secondary efficacy analysis of IPSS total score using an ANCOVA model and last observation carried forward, or LOCF, to impute missing post-baseline data, the improvement in IPSS for topsalysin was well sustained over the 52 weeks following the single administration. The maximal effect of 8.31 points improvement in IPSS vs vehicle 6.89 points (p = 0.012) was achieved at Week 18 with 8.04 points of improvement for topsalysin still remaining at Week 52 vs 6.64 points for patients treated with vehicle only (p = 0.022) representing an end-of-study preservation of 97% of the peak benefit. |
|
|
• |
Improvement in Qmax – Secondary efficacy endpoints included analysis of Qmax (maximum urine flow) change from baseline over 52 weeks by the repeated measures linear mixed model, which showed overall improvement of 1.77 mL/sec for topsalysin, representing a statistical trend that narrowly missed statistical significance (p = 0.055) compared to the vehicle group. |
|
|
• |
Improvement in Quality of Life was clinically meaningful – An additional efficacy endpoint was the patient self-assessment of disease-specific Quality of Life. On the 0 to 6 point Quality of Life (QOL) from the IPSS questionnaire, the topsalysin average change from the 4.5 point baseline was a sustained 1.6 to 1.7 points improvement from Weeks 18 through 52, which was statistically significantly superior to vehicle for every post-baseline visit beginning at Week 18 (reaching p = 0.004). |
|
|
• |
Topsalysin was well-tolerated – Topsalysin treatment was generally well-tolerated, and no patient was withdrawn from the trial or had their study drug injection altered because of an adverse event, or AE. The safety profile was consistent with that reported in the TRIUMPH Phase 2 trial. Adverse events occurring in >5% of patients treated with topsalysin regardless of assessed relatedness to study treatment are set forth in the table below. These AEs are not unexpected manifestations of the intraprostatic cellular destruction and resultant inflammation integral to the topsalysin mechanism of action. The median duration for each of these adverse events was typically less than one day. In general, these adverse events were mild or moderate, transient, began within the first few days after treatment (primarily on the same day as the study drug injection) and were resolved without consequences. |
Adverse Events Occurring in >5% of Subjects treated with topsalysin
|
Vehicle (N=240) |
Topsalysin (N=239) |
|||||||||||||||
|
Adverse Event(1) |
n (%) |
n (%) |
||||||||||||||
|
Dysuria (e.g., burning, pain, or discomfort on urination) |
20 | (8.3 |
) |
48 | (20.1 |
) | ||||||||||
|
Haematuria (microscopic or visible red blood cells in urine) |
36 | (15.0 |
) |
45 | (18.8 |
) | ||||||||||
|
Pollakiuria (frequent urination) |
14 | (5.8 |
) |
23 | (9.6 |
) | ||||||||||
|
Pyrexia (fever) |
10 | (4.2 |
) |
21 | (8.8 |
) | ||||||||||
|
Perineal Pain |
13 | (5.4 |
) |
21 | (8.8 |
) | ||||||||||
(1) (MedDRA Dictionary Preferred Terms)
The incidence of serious adverse events, or SAEs was similar in both treatment groups. There were two SAEs assessed by the investigator as at least possibly related to treatment for topsalysin and one such SAE for vehicle. The topsalysin-related SAEs were moderate events of “acute non-infectious prostatitis” and “fever following prostate procedure” not unexpected manifestations of the intraprostatic cellular destruction and resultant inflammation integral to the topsalysin mechanism of action. The vehicle-related SAE was a mild event of “urinary tract infection.” There were no treatment related sexual dysfunction or cardiovascular side effects reported in this clinical trial.
In order to seek regulatory approval for topsalysin for the treatment of the symptoms of BPH, we would be required to conduct a second Phase 3 clinical trial and we do not expect to commence any additional Phase 3 clinical trials unless we raise the additional funds required to conduct such trial.
TRIUMPH Phase 2b Randomized, Double-Blind, Placebo-Controlled Clinical Trial
In 2010, we completed TRIUMPH, a multicenter, randomized, double-blinded, placebo-controlled Phase 2b clinical trial of topsalysin in 92 patients with moderate to severe BPH symptoms who were randomized to topsalysin or vehicle on a 2:1 ratio. The primary objective of this clinical trial was to evaluate the effect on symptoms of BPH of topsalysin versus placebo. Patients randomized to placebo, which is referred to as the vehicle, were administered by injection an equivalent volume of phosphate-buffered saline that did not include active drug product. The patient population that we used to evaluate efficacy in this clinical trial, as defined by the clinical trial protocol, was the efficacy evaluable, or EE, population of patients, which was defined as those 73 patients who (1) received the full treatment, (2) completed three month assessments, and (3) had no major protocol violation, as determined by a blinded, independent review panel of urology experts. The intent-to-treat, or ITT, and safety patient populations consisted of all 92 patients who received any study drug. Our efficacy analyses in this clinical trial used the LOCF method to impute missing post-baseline data.
The results of this clinical trial were:
|
|
• |
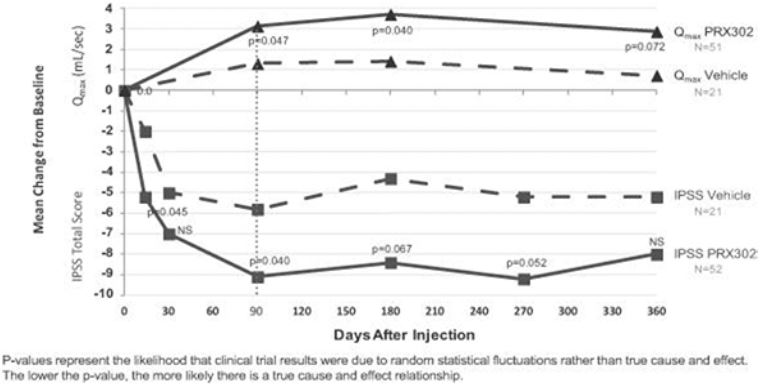
Topsalysin improved LUTS due to BPH – We achieved the primary endpoint of this clinical trial, which was a statistically significant improvement in IPSS at three months following injection for patients treated with topsalysin versus patients who received vehicle. Topsalysin treatment resulted in a 9.1 average reduction of IPSS, as compared to a 5.8 average reduction in patients who received vehicle (p=0.040). |
|
|
• |
Improvement was clinically meaningful, rapid and sustained – Improvement in IPSS was observed as early as 14 days following injection and was sustained through the twelfth month of observation. This improvement in IPSS was clinically meaningful, and superior to vehicle. |
|
|
• |
Improvement in Qmax – Topsalysin treatment resulted in an approximately 3.1 mL/sec average increase in Qmax at three months, as compared to 1.3 mL/sec for vehicle (p=0.047). The improvement in Qmax for topsalysin was apparent from the first post-baseline assessment and sustained through the twelfth month of observation. |
|
|
• |
Topsalysin was well-tolerated – The topsalysin injection was well-tolerated by patients in this clinical trial. The most common adverse events that were potentially attributable to topsalysin are set forth in the table below. These adverse events generally are not unexpected manifestations of the intraprostatic cellular destruction and inflammation integral to the topsalysin mechanism of action. The median duration for each of these adverse events was typically less than two days. In general, these adverse events were mild and transient, began within the first few days after treatment (primarily on the same day as the study drug injection) and were resolved without further complications. |
There were no drug-related erectile dysfunction or cardiovascular side effects reported in this clinical trial. In addition, 16.1% of patients in the vehicle group dropped out of the study due to lack of efficacy and the need for alternative therapy as compared to 3.3% of patients in the active group.
Adverse Events Occurring in >5% of Subjects treated with topsalysin (ITT Population)
|
Adverse Event(1) |
Vehicle (N=31) |
Topsalysin (N=61) |
||||||
|
Hematuria, or presence of red blood cells in urine |
11 | (35.5) | 18 | (29.5) | ||||
|
Dysuria, or painful urination |
2 | (6.5) | 17 | (27.9) | ||||
|
Pollakiuria, or increased frequency of urination |
5 | (16.1) | 14 | (23.0) | ||||
|
Micturition Urgency, or urgency of urination |
3 | (9.7) | 13 | (21.3) | ||||
|
Perineal Pain |
0 | (0.0) | 7 | (11.5) | ||||
|
Vertigo |
2 | (6.5) | 4 | (6.6) | ||||
|
Malaise |
0 | (0.0) | 4 | (6.6) | ||||
|
(1) |
MedDRA Dictionary-coded preferred terms. |
In summary, these results demonstrate that topsalysin is able to maintain a treatment benefit based on both measures of efficacy, IPSS and Qmax, which is clinically meaningful and sustained for the 12 months of monitoring in this clinical trial.
IPSS and Qmax in the Phase 2b BPH TRIUMPH Clinical Trial
N=73 Efficacy-Evaluable Patients using LOCF; 52 topsalysin and 21 Vehicle
 |
In our studies and other intraprostatic injection studies, vehicle response rates of 5 to 7 point improvements in IPSS have been observed. We believe that the vehicle response is due in part to the fluid injection potentially ablating prostate cells.
Although the clinical trial protocol did not specify an ITT population analysis, an improvement of 8.2 points in IPSS was observed in the active group of the ITT population. This was not statistically significant when compared to an improvement in the vehicle group of 7.2 points. Thirteen percent of the active group and 23% of the vehicle group were included in the ITT population but not included in the EE population because they were deemed major protocol violators based on confounding factors. Examples of confounding factors were taking prohibited medications, including other medications to treat the symptoms of BPH, or undergoing prohibited procedures during the clinical trial.
Transrectal Phase 1/2, Randomized, Double-Blind, Placebo-Controlled Clinical Trial in BPH
In March 2012, we completed dosing in a multicenter, randomized, double-blinded, vehicle-controlled Phase 1/2 clinical trial of topsalysin using the transrectal route of administration for the intraprostatic injection of topsalysin. Each of the previous clinical trials used transrectal ultrasound to guide the intraprostatic injection, but this clinical trial was the first to use the rectum as the route of administration rather than passing the needle through the perineum. The transrectal route has the advantage of being very similar to the routine prostate biopsy procedure, and therefore requires little extra training for the practicing urologist. The primary endpoint of this clinical trial was to evaluate the three-month safety and tolerability of escalating doses of topsalysin. The safety data from this new route of administration of topsalysin were needed for a comparison with the safety profile obtained from our previously-conducted Phase 1 and 2 clinical trials, which utilized a transperineal route of administration.
We enrolled 40 patients with moderate to severe BPH symptoms in this clinical trial who were randomized to topsalysin or placebo in a 4:1 ratio within each of the four escalating dose cohorts. All patients in this clinical trial received a single, transrectal, intraprostatic treatment of study drug or vehicle at 20% of the patient’s prostate volume, in four sequential cohorts according to escalating topsalysin dose: 0.15, 0.30, 0.60, and 1.20 µg/g prostate. Dose escalation decisions were guided by an independent data monitoring committee for each new cohort after all patients in the previous cohort had been followed for at least 15 days after study drug administration.
The results of this clinical trial showed that topsalysin was generally well-tolerated. The side effect profile in this transrectal clinical trial was consistent with the side effects reported in the previous, transperineal topsalysin clinical trials, indicating that topsalysin injection by the transrectal route was tolerated at least as well as the transperineal route. There was one serious adverse event that was deemed by the investigator to be related to injection of topsalysin in this clinical trial. This serious adverse event of urinary retention required an indwelling catheter followed by TUNA. There were no reports of sepsis in this clinical trial. With the switch to a transrectal route of administration, there is a potential risk of sepsis as currently the rate of sepsis with prostate biopsies in the United States is approximately 3-5%. However, prostate biopsies involve as many as 20 punctures and a large needle, whereas topsalysin administration requires only two punctures with a smaller needle. There were no drug-related erectile dysfunction or cardiovascular side effects reported in this clinical trial.
The small sample size of only eight patients on topsalysin and two patients on vehicle in each cohort was insufficient to show statistically significant improvements in BPH symptoms compared to vehicle. Although improvement in IPSS was noted on average for all dose cohorts through 12 months, there is no meaningful difference between topsalysin and vehicle-treated patients. We do not believe that any conclusions about efficacy can be drawn from this study due to the small sample size.
In our TRIUMPH clinical trial, we observed post-injection transient elevations of two markers: PSA, a marker of prostate tissue disruption, and serum C-reactive protein, or CRP, a non-specific marker of associated inflammation. Post-injection transient elevations in PSA and CRP were also observed in the transrectal study, suggesting that the targeted delivery of topsalysin to the prostrate is successfully achieved with either the transperineal or the transrectal route of administration.
Phase 2a Open-Label Clinical Trial in BPH (PRX302-2-02)
In 2009, we completed an open-label, multicenter, Phase 2a clinical trial in BPH to evaluate the safety and tolerability of topsalysin. We enrolled 18 patients with moderate to severe BPH symptoms who were either unresponsive to, intolerant to or unwilling to use oral medications for treatment of the symptoms of BPH. In this clinical trial, three cohorts of six patients each received a single treatment of topsalysin administered via transperineal injection. We measured therapeutic activity through changes in IPSS, Qmax, and quality of life scores compared to baseline scores at screening. In addition, we monitored changes in prostate volume. In this clinical trial, topsalysin was well-tolerated and patients attained meaningful symptomatic relief through follow up of 12 months following a single treatment. Based on the results of this clinical trial, we identified 20% of total prostate volume as our volume dose for our Phase 2b clinical trial.
Phase 1 Open-Label Clinical Trial in BPH (PRX302-2-01)
In 2008, we completed an open-label, multicenter, Phase 1 clinical trial in BPH to evaluate the dose of topsalysin needed to demonstrate therapeutic activity following a single treatment, as well as to evaluate safety and tolerability. We enrolled 15 patients with moderate to severe BPH symptoms who were either unresponsive to, intolerant to or unwilling to use oral medications for treatment of the symptoms of BPH. We administered topsalysin to five cohorts of three patients each at escalating doses of topsalysin. Topsalysin was well-tolerated.
Plans for Future Clinical Development in BPH
In order to seek regulatory approval for topsalysin for the treatment of the symptoms of BPH, we will be required to successfully conduct a second Phase 3 clinical trial.
We are currently not planning on pursuing a second Phase 3 trial in BPH, unless we secure a development partner to fund such new clinical trial or obtain other financing. There can be no assurance that such funding or a development partner will be available on acceptable terms or at all. For that reason, we cannot currently estimate when the clinical development required to seek the regulatory approvals needed to commercialize topsalysin for the treatment of the symptoms of BPH will be completed.
To date, no patients have been administered more than one treatment of topsalysin. Assuming sufficient capital resources, we are planning to initiate an open label repeat dose clinical trial in which patients from our transrectal clinical trial, as well as patients from our first Phase 3 clinical trial, will be eligible to receive a repeat dose of topsalysin, at least 12 months after their first dose. We believe this repeat dose Phase 3 clinical trial is supported by results from our pre-clinical study of repeat dosing in monkeys. In this pre-clinical study, two treatments of topsalysin were given to monkeys 56 days apart. Data from this study indicated that topsalysin resulted in ablation of cells after both the first and the second dose, even in the presence of circulating antibodies, and did not result in hypersensitivity.
Our Strategy
Our business strategy is to develop and commercialize innovative products for the treatment of urological diseases. The elements of our strategy include the following:
|
|
• |
Complete a Phase 2b of topsalysin for the treatment of clinically significant localized prostate cancer. In March 2017 we initiated a Phase 2b clinical trial of topsalysin for the treatment of clinically significant localized prostate cancer. The trial is being conducted at multiple centers and is expected to enroll approximately 40 patients. The primary objective will be to assess the safety and tolerability of topsalysin and the key efficacy variable is the focal ablation of a clinically significant lesion on biopsy after six months. |
|
|
• |
Evaluate options to continue the clinical development of topsalysin for the treatment of the symptoms of BPH. Topsalysin previously achieved its primary efficacy endpoint in a completed Phase 3 clinical trial in patients with moderate to severe BPH symptoms. In order to seek marketing approval in this indication we would be required to conduct a second Phase 3 clinical trial. We are currently evaluating options to further advance the clinical development of topsalysin for the treatment of BPH. We will require significant additional funding to advance topsalysin in clinical development for the treatment of BPH. We could use dilutive funding options such as an equity financing and non-dilutive funding options such as a partnering arrangement or royalty agreement to fund future clinical development of topsalysin. |
Competition
In the treatment of clinically significant localized prostate cancer we expect that topsalysin will compete with radical treatments such as prostatectomy and radiation as well as a number of other targeted focal therapies which are gaining traction such as brachytherapy, high-intensity focused ultrasound, cryotherapy, laser ablation, cyber, radiofrequency ablation and photodynamic therapy.
The increasing use of multi-parametric magnetic resonance imaging (mpMRI) of the prostate and advances in software to co-register previously obtained mpMRI images with live 3D ultrasound images enables physicians to more accurately target their prostate biopsies. Consequently, it is increasingly possible to more confidently identify men with clinically significant lesions. Thereby, enabling physcians and patients to make a more informed decision about the clinical significance of their disease and whether their disease requires a radical treatment approach with the potential for significant morbidity or whether they maybe a candidate for targeted focal therapy where the objective is to remove the significant disease while preserving the as much of the prostate as possible and potentially avoiding many of the complications and side effects associated with the radical whole gland treatments.
In 2016 Nymox Pharmaceuticals announced the clinical trial results from 18 months with the intraprostatic administration of their investigational therapy NX-1207 (fexapotide triflutate) in patients with low grade localized (T1c) prostate cancer. In January 2016, Steba Biotechnology submitted a Marketing Authorization Application to the European Medicine Agency for the focal treatment of patients with low risk localized prostate cancer, with their vascular–targeted photodynamic therapy TOOKAD.
We expect that topsalysin will compete with the current treatment options for the symptoms of BPH, which include oral drug therapy and surgery. Oral drug therapies include alpha-blockers, such as tamsulosin (marketed under various trade names by numerous companies, including as Flomax® by Astellas Pharma), alfuzosin (marketed in the United States by Sanofi as Uroxatral®), doxazosin (marketed by Pfizer as Cardura® and CarduraXL®) and silodosin (marketed by Watson Pharmaceuticals as Rapaflo® in the United States), (b) 5-alpha reductase inhibitors, such as dutasteride (marketed by GlaxoSmithKline plc as Avodart®) and finasteride (marketed by Merck & Co., Inc. as Proscar®), and (c) combinations of a-blockers and 5-alpha reductase inhibitors such as tamsulosin and dutasteride (marketed by GSK as Jalyn®). In addition, Eli Lilly and Company’s oral drug tadalafil (marketed as Cialis®), a PDE5 inhibitor, obtained FDA approval for the treatment of the symptoms of BPH in October 2011. Several MIST procedures are available, including transurethral microwave thermotherapy, or TUMT, TUNA, photo-selective vaporization of prostate, holmium laser enucleation of the prostate, transurethral electro vaporization of the prostate, Urolift, which is designed to open the urethra directly without the need to resect or ablate prostate tissue and interstitial laser coagulation. A new TUNA, Rezum by NxThera which delivers radiofrequency generated thermal therapy in the form of water vapor via a transurethral needle, received approval in September 2015 and became available on the US markets late 2016. Currently, the most commonly used MIST procedures are laser ablations of the prostate, TUMT, and TUNA. Surgery for BPH treatment is usually considered in patients who fail drug therapy as a result of side effects or inadequate relief of symptoms, have refractory urinary retention, or have recurrent urinary tract infections. Alternatively, surgery may be the initial treatment in patients with severe urinary symptoms. Surgical procedures for BPH include TURP, as well as other procedures such as transurethral incision of the prostate and transurethral vaporization of the prostate.
In addition, there are other treatments that are currently in clinical development for the treatment of the symptoms of BPH. In late 2016, Procept BioRobotics announced the completion of enrollment with 184 patients in a global Phase 3 clinical trial to evaluate the AquaBeam System, a waterjet ablation therapy for endoscopic resection of prostate tissue. Light Sciences Oncology Inc.’s AptocineTM is currently in Phase 2 clinical trials. In 2014, Nymox Pharmaceuticals announced that the injectable NX-1207 for the treatment of the symptoms of BPH did not meet its clinical endpoints in two completed Phase 3 clinical trials. In 2015, Nymox Pharmaceuticals announced that NX-1207 for the treatment of the symptoms of BPH met its primary endpoint in its pivotal Phase 3 extension trial.
Sales and Marketing
We do not currently have a sales, marketing or distribution organization. We intend to commercialize topsalysin alone by establishing, either internally or through a contract sales force, a urology sales force to sell topsalysin, if approved, in the United States, or through partnership. We plan to partner with third parties to commercialize topsalysin outside the United States.
Specifically, we intend to:
|
|
• |
establish a sales force in the United States of experienced urology and other specialty-care sales representatives; |
|
|
• |
build a marketing organization; |
|
|
• |
establish commercialization alliances with larger or more specialized pharmaceutical and sales organizations; and |
|
|
• |
generate and use pharmacoeconomic data to support the cost savings and therapeutic benefits of topsalysin. |
Manufacturing
We neither currently possess nor do we plan to develop our own manufacturing capabilities. All of our manufacturing is, and will be, outsourced to third parties with oversight by our internal managers. In 2012, we entered into a manufacturing and supply agreement with Boehringer Ingelheim RCV GmbH & Co KG, or BI, to manufacture topsalysin. The manufacture of topsalysin drug substance starts with a vial of the working cell bank of Aeromonas salmonicida bacteria which is then processed through four consecutive stages involving: batch fermentation and harvest, purification using immobilized metal affinity chromatography, purification using an ionic exchange chromatography and bulk formulation of topsalysin drug substance. The entire manufacturing process takes approximately two weeks.
There has been a successful scale-up up to the commercial batch size for drug substance. The finalization of the commercial fill finish process, for the production of drug product is still underway but we expect to reformulate our drug product prior to completing the commercial fill finish process and completing any further Phase 3 clinical trials. Although topsalysin is manufactured from readily available materials using standard pharmaceutical methods and equipment, any replacement of BI as our manufacturer may lead to significant delays and increase our costs. Further, BI currently procures an ingredient used in the formulation of topsalysin from a multinational industrial biotech company which is a single source supplier. We currently have adequate supply of clinical trial product for our planned Phase 2 clinical trial in prostate cancer but reformulation of the drug product could result in future delays in the completion of Phase 3 clinical trials if not completed when expected.
Supply Agreement with Boehringer Ingelheim RCV GmbH & Co KG
In June 2012, we entered into a technology transfer and supply agreement with BI, for the provision of technology transfer services and for the establishment of certain manufacturing processes for, and the manufacture of, purified topsalysin, the diluting agent for use in topsalysin drug products and placebos, and a placebo to be used in clinical trials. We will be required to make payments based upon the provision and completion of certain tasks specified in the agreement. Starting in 2013, the prices of BI’s services have been adjusted annually based on the average of the Austrian trade index and the average Standard Wages Index, both as of July of the previous year, subject to certain restrictions. BI will be required to manufacture the products in line with certain project timelines. If we postpone the performance of any services, we may be required to pay certain postponement fees. Additionally, if we cancel any services we will be required to pay the entire cost for such services and the entire cost of any materials that cannot be returned by BI to the appropriate vendor or otherwise used by BI. If we are required to have any product manufactured outside our expected manufacturing cycles due to an unforeseen loss of product, we will have to work with BI to arrange an available manufacturing slot and our receipt of drug product may be delayed. BI must provide all services under the agreement, including the manufacture, packaging, storing and delivery of topsalysin drug products, in accordance with cGMP (as defined below), as specified by the FDA. The agreement has an initial term of six years and will automatically renew for a single five-year period unless either party objects to such renewal at least two-years prior to the expiration of the agreement. Either party may terminate the agreement early for cause, including for any uncured material breach of the agreement, the other party’s insolvency or the assignment of the other party’s rights or obligations to a direct competitor of the non-assigning party. Additionally, we have the right to terminate the agreement immediately upon the rejection or non-approval of a regulatory filing due to medical, safety or regulatory concerns or in the event that we abandon our clinical program for topsalysin due to any clinical failure, subject in each case to payment of specified termination costs to BI.
Intellectual Property
We hold commercial rights to topsalysin in major markets, including, Canada, the United States, Europe and Asia (except Japan where we have licensed the rights to Kissei). We in-licensed topsalysin from UVIC and Johns Hopkins. Our success will depend in large part on our ability to obtain, maintain, defend and enforce patents and other proprietary technology rights. We file and prosecute patent applications to protect our proprietary discoveries. In addition to patent protection, we also seek to rely on trade secret protection, trademark protection and know-how to expand our proprietary position around our technology, discoveries and inventions that we consider important to our business. We also seek to protect our intellectual property in part by entering into confidentiality agreements and/or invention assignment agreements with our employees, consultants, scientific advisors, and certain consultants and investigators that grant us ownership of any discoveries or inventions made by them. Further, we seek trademark protection in Canada, the United States and certain other countries where available and when we deem appropriate. We have registered the Sophiris trademark, which we use in connection with our pharmaceutical research and development services as well as our clinical-stage product candidates in Europe, Canada, Japan and the United States.
Patents and patent applications covering topsalysin which we license or own are covered by issued patents and patent applications under the following four patent families:
|
● |
Proaerolysin Containing Protease Activation Sequences and Methods of Use for Treatment of Prostate Cancer (exclusively licensed); |
|
● |
Method of Treating the Symptoms of Benign Prostatic Hyperplasia (BPH) Using Modified Pore-Forming Proteins (exclusively licensed); |
|
● |
Method for Treating Prostatitis Utilizing Modified Pore-Forming Protein Proaerolysin (exclusively licensed). |
|
● |
Formulations and Methods of Administration (owned by Sophiris); and |
We own or have exclusively licensed six issued United States patents related to our prostate program: US 7,838,266 (prostate cancer) expiring in 2022, US 7,282,476 (prostate cancer) expiring in 2023, US 7,745,395 (prostate cancer) expiring in 2023, US 8,278,279 (prostatitis) expiring in 2029, US 8,901,070 (prostatitis) expiring in 2029 and US 8,916,161 (BPH) expiring in 2026, as well as eight issued patents in countries including Australia, China, the European Patent Office (including 16 validation states), India, Japan, Hong Kong, and South Africa expiring in 2022, eight patents in the European Patent Office (including 13 validation states), Canada, Japan, Korea, China, Australia, New Zealand, Israel, Singapore, and South Africa expiring in 2026, and additional pending U.S. and/or foreign patent applications in Australia, Canada, the European Patent Office, and India variously set to expire in 2022, 2026, 2029, or 2031. This portfolio includes issued U.S. patents that cover the composition of topsalysin or methods of using topsalysin to treat prostatitis or prostate cancer, and methods of using topsalysin to treat the symptoms of BPH. This portfolio includes two issued Chinese patents. To date, we have not sought to enforce any issued patents in China. We cannot give any assurances that we will be able to enforce our patents in China to the same degree that we could in the United States.
Technology Licenses
Exclusive License Agreement with UVIC Industry Partnerships Inc. and The Johns Hopkins University for Prostate Cancer
In September 2004, we entered into an exclusive license agreement with UVIC and Johns Hopkins, with respect to the use of topsalysin for the development of therapeutics for prostate cancer. This agreement was amended on December 8, 2004 and July 1, 2010. Such amendments did not change the material terms of the agreement. For the term of this agreement, we have an exclusive right of first option to obtain a license for future improvements to the patent rights covered by the agreement. In addition, we have the right to grant sublicenses to third parties under the agreement provided that such sublicenses meet certain criteria.
In order to secure the license, we paid an initial license fee of CND$75,000, or $62,000, applying the conversion rate as of the date of payment, and a reimbursement fee of CND$28,000, or $24,000, applying the conversion rate as of the date of payment, to cover expenses associated with the filing and maintenance fees of patents covered by the agreement. In addition, we are required to pay an annual license maintenance fee and are obligated to pay a percentage of gross sales for licensed products sold by us, our affiliates or our sublicensees during the term of the agreement. Such percentage is in the low single-digits and is subject to adjustment in certain circumstances. We are also required to make payments based upon the achievement of specific development and regulatory milestones totaling up to approximately CND$3.6 million, or $2.6 million, as converted. During the year ended December 31, 2016, we accrued a $0.1 million milestone payment as a result of the completion of Phase 1 clinical activities associated with topsalysin for the treatment of prostate cancer. We expect to pay this amount in the first quarter of 2017.
In the event we receive consideration for granting a sublicense, we are obligated to pay UVIC and Johns Hopkins a percentage of such consideration, which percentage is in the 20-29% range, including any future consideration we may receive under our exclusive license agreement with Kissei relating to development of therapeutics for the treatment of prostate cancer. Furthermore, we issued 3,420 common shares to Johns Hopkins and 1,710 common shares to UVIC in partial consideration for the rights granted to us under the agreement.
Under the terms of the agreement, we are required to use reasonable commercial efforts to develop and commercialize the technology covered by the agreement, and in this regard, have agreed to put a business plan in place. Our failure to commercialize the technology covered by the agreement may result in termination of the agreement.
The term of the agreement will, on a country-by-country basis, continue until expiration of the last to expire issued patent or, if no patent has issued in such country, then 20 years after the effective date of the agreement.
UVIC and Johns Hopkins have a unilateral right to terminate the agreement upon notice if we become insolvent, cease to carry out our business, subject the licensed technology to any security interest or breach any of our obligations under this agreement if such breach has remained uncured for 60 days following written notice thereof. In addition, the agreement may automatically terminate in the event we undergo bankruptcy proceedings.
Exclusive License Agreement with UVIC Industry Partnerships Inc. and The Johns Hopkins University for BPH
In October 2009, we entered into an exclusive license agreement with UVIC and Johns Hopkins with respect to the use of topsalysin for the development of therapeutics for the symptoms of BPH and other non-cancer diseases and conditions of the prostate. The agreement was amended in July 2010. Such amendment did not change the material terms of the agreement. We have the right to grant sublicenses to third parties under the agreement provided that such sublicenses meet certain criteria.
In order to secure the license, we paid an initial license fee of CND$45,000, or $39,000, applying the conversion rate as of the date of payment. In addition, we are required to pay an annual license maintenance fee and are obligated to pay a percentage of gross sales for licensed products sold by us, our affiliates or our sublicensees during the term of the agreement. Such percentage is in the low single-digits. Furthermore, we are required to make payments based upon the achievement of specific development and regulatory milestones separated among the indications of BPH and two additional therapeutic indications selected by us, totaling up to approximately CND$1.3 million, or $0.9 million, as converted. In the event we receive consideration for granting a sublicense, we are obligated to pay UVIC and Johns Hopkins a percentage of such consideration, which percentage is in the 10-19% range, depending upon the rights granted under the sublicense agreement. To the extent we receive any milestone payments relating to the development of therapeutics for the treatment of the symptoms of BPH under our exclusive license agreement with Kissei Pharmaceutical Co., Ltd., or Kissei, we are obligated to pay a percentage of such consideration, which percentage is in the 10-19% range, to UVIC and Johns Hopkins; however, pursuant to a separate agreement which we entered into in 2003 with Dr. J. Thomas Buckley, one of our founders, the aggregate amount of such consideration payable by us to UVIC and Johns Hopkins is reduced by 25%.
Under the terms of the agreement, we are required to use reasonable commercial efforts to develop and commercialize the technology covered by the agreement, and in this regard, we have agreed to put a business plan covering the marketing and commercialization of such technology in place. Our failure to commercialize the technology covered by the agreement may result in termination of the agreement.
The term of the agreement will, on a country-by-country basis, continue until expiration of the last to expire issued patent or, if no patent has issued in such country, then 20 years after the effective date of the agreement. UVIC and Johns Hopkins have a unilateral right to terminate the agreement upon notice if we become insolvent, cease to carry out our business, subject the licensed technology to any third-party security interest or breach any of our obligations under this agreement if such breach has remained uncured for 60 days following written notice thereof. In addition, the agreement may automatically terminate in the event we undergo bankruptcy proceedings.
Strategic Relationship with Kissei Pharmaceutical Co., Ltd.
In April 2010, we entered into an exclusive license agreement with Kissei, for the development and commercialization of topsalysin (and other products covered by the licensed patents) in Japan for the treatment of the symptoms of BPH, prostate cancer, prostatitis or other diseases of the prostate. Under the terms of the license, Kissei is permitted to sublicense its rights if certain conditions are met.
In order to secure the license, Kissei paid us an up-front payment of $3.0 million. During the year ended December 31, 2013, we recorded as revenue a $5.0 million non-refundable milestone payment due from Kissei upon the achievement of certain development activities. In addition, we remain eligible to receive up to approximately $67.0 million in additional payments contingent upon achievement of specified development, regulatory and commercial milestones, some of which are in Kissei’s sole discretion to achieve, separated among the indications of BPH, prostate cancer, and prostatitis or other diseases of the prostate, as well as the achievement of overall accumulated gross sales levels for such indications. The additional $67.0 million of non-refundable milestone payments is comprised as follows: aggregate milestone payments of $12.0 million are related to the BPH indication, of which $7.0 million relates to the completion of regulatory approvals and $5.0 million relates to the achievement of certain product sale goals; a total of $21.0 million is related to the prostate cancer indication, of which $7.0 million relates to the completion of development activities, $7.0 million relates to the completion of regulatory approvals and $7.0 million relates to the achievement of certain product sale goals; and a total of $21.0 million is related to prostatitis or other diseases of the prostate, of which $7.0 million relates to the completion of development activities, $7.0 million relates to the completion of regulatory approvals and $7.0 million relates to the achievement of certain product sale goals. An additional $13.0 million of aggregate milestone payments are not indication specific, of which $5.0 million relates to the completion of regulatory approvals and $8.0 million relates to the achievement of certain product sale goals. In addition, we may receive a drug supply fee and royalty payments in the 20-29% range as a percentage of future net sales of licensed products sold under the agreement. The royalties payable by Kissei are subject to reductions or offsets in certain circumstances. Kissei’s royalty obligations continue until the later of expiration of the last valid claim in the licensed patents covering the applicable licensed product, or 10 years after first commercial sale of such licensed product in Japan. Kissei is responsible for all costs associated with the development, regulatory approval, commercialization and marketing of topsalysin in Japan.
Kissei may unilaterally terminate the agreement, provided that if such termination occurs after commercial launch of a product under the agreement, Kissei must provide us with six months prior written notice. Absent early termination, the exclusive license agreement will remain in effect until Kissei or its sublicensees or affiliates discontinue the sale of products under the agreement.
Regulatory Overview
Our business and operations are subject to a variety of U.S. federal, state and local, and foreign supranational, national, provincial and municipal laws, regulations and trade practices. The FDA and comparable regulatory authorities in state and local jurisdictions and in other countries impose substantial and burdensome requirements upon companies involved in the clinical development, manufacture, marketing and distribution of drugs and biologics. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, recordkeeping, approval, advertising and promotion, and export and import of our product candidate.
U.S. Government Regulation
U.S. Drug Development Process
In the United States, the FDA regulates drugs and biologic products under the Federal Food, Drug and Cosmetic Act, or FDCA, its implementing regulations, and other laws, including, in the case of biologics, the Public Health Service Act. Our product candidate, topsalysin, is subject to regulation by the FDA as a biologic. Biologics require the submission of a BLA to the FDA and approval of the BLA by the FDA before marketing in the United States. The process of obtaining regulatory approvals for commercial sale and distribution and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial civil or criminal sanctions. These sanctions could include the FDA’s refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, imposition of a clinical hold on clinical trials, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil and/or criminal penalties. The process required by the FDA before a biologic may be marketed in the United States generally involves the following:
|
|
• |
completion of preclinical laboratory tests, animal studies and formulation studies performed in accordance with the FDA’s current Good Laboratory Practices, or GLP, regulations; |
|
|
• |
submission to the FDA of an investigational new drug application, or IND, which must become effective before human clinical trials in the United States may begin; |
|
|
• |
performance of adequate and well-controlled human clinical trials in accordance with the FDA’s current good clinical practices, or GCP, regulations to establish the safety and efficacy of the product candidate for its intended use; |
|
|
• |
submission to the FDA of a BLA; |
|
|
• |
satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the product is produced to assess compliance with the FDA’s current good manufacturing practice standards, or cGMP, regulations to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; |
|
|
• |
potential audits by the FDA of the nonclinical and clinical trial sites that generated the data in support of the BLA; |
|
|
• |
possible review of the BLA by an external Advisory Committee to the FDA, whose recommendations are not binding on the FDA; and |
|
|
• |
FDA review and approval of the BLA prior to any commercial marketing or sale. |
Before testing any compounds with potential therapeutic value in humans, the drug candidate enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, stability and formulation, as well as animal studies to assess the potential toxicity and activity of the product candidate. The conduct of the preclinical tests must comply with federal regulations and requirements including GLPs. The sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. The FDA may also impose clinical holds on a product candidate at any time before or during clinical trials due to safety concerns or non-compliance, or for other reasons.
Clinical trials involve the administration of the product candidate to human subjects under the supervision of qualified investigators, generally physicians not employed by or under the clinical trial sponsor’s control. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety and effectiveness. Each protocol must be submitted to the FDA as part of the IND. Clinical trials must be conducted in accordance with GCPs. Further, each clinical trial must be reviewed and approved by an IRB at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of clinical trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the form and content of the informed consent that must be signed by each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
|
|
• |
Phase 1. The product candidate is initially introduced into a limited population of healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for some diseases, or when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients with the disease or condition for which the product candidate is intended to gain an early indication of its effectiveness. |
|
|
• |
Phase 2. The product candidate is evaluated in a limited patient population (but larger than in Phase 1) to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted indications and to assess dosage tolerance, optimal dosage and dosing schedule. |
|
|
• |
Phase 3. Clinical trials are undertaken to further evaluate dosage, and provide substantial evidence of clinical efficacy and safety in an expanded patient population (such as several hundred to several thousand) at geographically dispersed clinical trial sites. Phase 3 clinical trials are typically conducted when Phase 2 clinical trials demonstrate that a dose range of the product candidate is effective and has an acceptable safety profile. These trials typically have at least two groups of patients who, in a blinded fashion, receive either the product or a placebo. Phase 3 clinical trials are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. Generally, two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of a BLA. |
Post-approval studies, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication to further assess the biologic’s safety and effectiveness after BLA approval. Phase 4 studies can be initiated by the drug sponsor or as a condition of BLA approval by the FDA.
Annual progress reports detailing the results of the clinical trials must be submitted to the FDA and written IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events or any finding from tests in laboratory animals that suggests a significant risk for human subjects.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the biologic and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final biologic product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests, proposed labeling and other relevant information are submitted to the FDA in the form of a BLA requesting approval to market the product for one or more specified indications. The submission of a BLA is subject to the payment of substantial user fees.
Once the FDA receives a BLA, it has 60 days to review the BLA to determine if it is substantially complete and the data is readable, before it accepts the BLA for filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the BLA. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act, or PDUFA, the FDA has 12 months from submission in which to complete its initial review of a standard BLA and make a decision on the application, and eight months from submission for a priority BLA, and such goal is referred to as the PDUFA date. The FDA does not always meet its PDUFA dates for either standard or priority BLAs. The review process and the PDUFA date may be extended by three months if the FDA requests or the BLA sponsor otherwise provides additional information or clarification regarding information already provided in the submission within the last three months before the PDUFA date.
After the BLA submission is accepted for filing, the FDA reviews the BLA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug or biological products or drug or biological products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. During the approval process, the FDA also will determine whether a risk evaluation and mitigation strategy, or REMS, is necessary to assure the safe use of the product. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS; the FDA will not approve the BLA without an approved REMS, if required. Development of a REMS can substantially increase the costs of obtaining approval.
Before approving a BLA, the FDA will typically inspect the facilities at which the product is manufactured. The FDA will not approve the BLA unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a BLA, the FDA will typically inspect one or more clinical sites to assure that the clinical studies were conducted in compliance with GCP requirements. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information before a BLA can be approved.
The FDA will issue a complete response letter if the agency decides not to approve the BLA. The complete response letter describes all of the specific deficiencies in the BLA identified by the FDA. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical trials. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or withdraw the application.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may require post marketing studies, sometimes referred to as Phase 4 testing, which involves clinical trials designed to further assess drug safety and effectiveness and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized. After approval, certain changes to the approved biologic, such as adding new indications, manufacturing changes or additional labeling claims, are subject to further FDA review and approval. Depending on the nature of the change proposed, a BLA supplement must be filed and approved before the change may be implemented. For many proposed post-approval changes to a BLA, the FDA has up to 180 days to review the application. As with new BLAs, the review process is often significantly extended by the FDA requests for additional information or clarification.
Post-Approval Requirements
Any biologic products for which we or our collaborators receive FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements, which include, among others, restrictions on direct-to-consumer advertising, promoting biologics for uses or in patient populations that are not described in the product’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities, and promotional activities involving the internet. The FDA closely regulates the post-approval marketing and promotion of biologics, and although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such off-label uses. Failure to comply with these or other FDA requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, suspension of manufacturing, seizure of product, injunctive action, mandated corrective advertising or communications with healthcare professionals, possible civil or criminal penalties, or other negative consequences, including adverse publicity.
We will rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products. Our collaborators may also utilize third parties for some or all of a product we are developing with such collaborator. Manufacturers are required to comply with applicable FDA manufacturing requirements contained in the FDA’s cGMP regulations. cGMP regulations require among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation. Drug manufacturers and other entities involved in the manufacture and distribution of approved biologics are required to register their establishments with the FDA and certain state agencies, and are subject to periodic inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
U.S. Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of the FDA approval of our biologic product candidate, some of our United States patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may intend to apply for restoration of patent term for one of our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant BLA.
Market exclusivity provisions under the FDCA can also delay the submission or the approval of certain applications of other companies seeking to reference another company’s BLA. We believe that if topsalysin is approved as a biological product under a BLA, it should qualify for a 12-year period of exclusivity currently permitted by the Biologics Price Competition and Innovation Act of 2009, or BPCIA. Under the BPCIA, an application for a biosimilar product cannot be approved by the FDA until 12 years after the original branded product was approved under a BLA. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement to one of the patents listed with the FDA by the innovator BLA holder. The BPCIA is complex and is only beginning to be interpreted and implemented by the FDA.
U.S. Foreign Corrupt Practices Act
The U.S. Foreign Corrupt Practices Act, to which we are subject, prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity.
U.S. Federal and State Health Regulation Laws
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the biopharmaceutical industry in recent years. These laws include anti-kickback statutes, false claims statutes patient data privacy and security laws, and physician sunshine laws and regulations, many of which may become more applicable if our product candidates are approved and we begin commercialization.
The federal health care program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce or in return for purchasing, leasing, ordering, or arranging for the purchase, lease, or order of any health care item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not satisfy the requirements of an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
Federal false claims laws and civil monetary penalties laws prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor, including commercial payors.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created additional federal criminal statutes that prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters. HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and its implementing regulations, also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information. We are also subject to state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
The Physician Payments Sunshine Act, and its implementing regulations, require certain manufacturers of drugs, devices, biological and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually information related to certain payments or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members. Additional state laws require pharmaceutical companies to implement a comprehensive compliance program and/or limit expenditure for, or payments to, individual medical or health professionals.
Because of the breadth of these laws and the narrowness of the applicable exceptions and safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to penalties, including potentially significant criminal and civil and/or administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
In the United States and foreign jurisdictions, there have been and continue to be a number of initiatives that seek to promote changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. For example, in March 2010 the Patient Protection and Affordable Health Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the PPACA, was enacted, which includes measures to significantly change the way health care is financed by both governmental and private insurers. Among the provisions of the PPACA of greatest importance to the pharmaceutical and biotechnology industry are the following:
|
|
• |
an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; |
|
|
• |
new requirements on certain manufacturers of drugs, devices, biological products and medical supplies to report annually certain financial arrangements, including reporting any “transfer of value” made or distributed to physicians and teaching hospitals and reporting annually certain ownership and investment interests held by physicians and their immediate family members; |
|
|
• |
a new requirement to annually report drug samples that certain manufacturers and authorized distributors provide to physicians; |
|
|
• |
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
|
|
• |
a licensure framework for follow-on biological products; |
|
|
• |
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program, an extension of manufacturers’ Medicaid rebate liability, an expansion of the eligibility criteria for people to participate in the Medicaid program, and the creation of a new Medicare Part D coverage gap discount program; |
|
|
• |
creation of the Independent Payment Advisory Board which will have authority to recommend certain changes to the Medicare program that could result in reduced payments for prescription drugs and those recommendations could have the effect of law even if Congress does not act on the recommendations; and |
|
|
• |
establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. |
Since its enactment there have been judicial and Congressional challenges to other aspects of the PPACA, and we expect there will be additional challenges and amendments to the PPACA in the future.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. For example, in August 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This included reductions to Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and, following passage of the Bipartisan Budget Act of 2015, will stay in effect through 2025 unless additional Congressional action is taken. Additionally, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals and imaging centers. Further, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products. For example, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
We expect that additional federal and state healthcare reform measures will be adopted in the future, any of which could result in reduced demand for our products or other adverse effects.
Europe / Rest of World Government Regulation
In addition to regulations in the United States, we, and our collaborators, will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products.
Whether or not we, or our collaborators, obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country.
If we, or our collaborators, fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidate for which we obtain regulatory approval. In the United States and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payors including government health administrative authorities, managed care providers, private health insurers and other organizations. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a drug product does not assure that other payors will also provide coverage for the drug product.
Patients who are prescribed medicine for the treatment of their conditions generally rely on third-party payors to reimburse all or part of the associated costs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Therefore, successful commercialization of our product will depend in part on the availability of third-party payor reimbursement for the cost of our products and/or payment to the physician for administering our product.
Employees
As of December 31, 2016, we had five full-time employees, two of whom have Ph.D. degrees. None of our employees are covered by collective bargaining agreements and we consider relations with our employees to be good.
Research and Development Expenses
Research and development expenses consist primarily of costs associated with the clinical development of topsalysin. Research and development expenses are the primary source of our expenses and totaled $3.5 million, $9.9 million and $24.7 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Corporate Information
We file annual, quarterly, current reports, proxy statements and other information with the Securities and Exchange Commission (SEC). Our primary website can be found at http://www.sophiris.com. We make available free of charge at this website (under the “Investors — Financial Information” caption) all of our reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, including our Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K and amendments to those reports. These reports are made available on the website as soon as reasonably practicable after their filing with, or furnishing to, the SEC. The SEC maintains an internet site that contains our public filings with the SEC and other information regarding the Company, at www.sec.gov. These reports and other information concerning the Company may also be accessed at the SEC’s Public Reference Room at 100 F Street, NE, Washington DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Furthermore, we also make available on our website free of charge, and in print to any shareholder who requests it, the Committee Charters for our Audit, Compensation, and Governance and Nominating Committees, as well as the Code of Business Conduct and Ethics that applies to all directors, officers and employees of the Company. Amendments to these documents or waivers related to the Code of Business Conduct and Ethics will be made available on our website as soon as reasonably practicable after their execution. The contents of the websites referred to in this paragraph are not incorporated into this Annual Report. Further, our references to the URLs for these websites are intended to be inactive textual reference only.
Our predecessor, Protox Pharmaceuticals Inc., was incorporated in January 2002. We were formed in May 2003 under the predecessor to the British Columbia Business Corporations Act, or the BCBCA, by the amalgamation of Stratos Biotechnologies Inc., Nucleus BioScience Inc. and Brightwave Ventures Inc. under the name SNB Capital Corp. In July 2004, we acquired all the shares of Protox Pharmaceuticals Inc. in a plan of arrangement under the BCBCA and changed its name to Protox Therapeutics Inc. In 2012, we changed our name to Sophiris Bio Inc. We are governed by the Business Corporations Act of British Columbia. Our operations were initially located in Vancouver, British Columbia. In April 2011, we relocated our core activities and headquarters from Vancouver, British Columbia to San Diego, California.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of our initial public offering in August 2013, (b) in which we have total annual gross revenue of at least $1.0 billion, or (c) in which we are deemed to be a large accelerated filer, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. We refer to the Jumpstart Our Business Startups Act of 2012 herein as the “JOBS Act,” and references herein to “emerging growth company” shall have the meaning associated with it in the JOBS Act.”
Item 1A. Risk Factors
Risks Related to Our Business and Industry
We will require significant funding to complete the development and commercialization of topsalysin and we may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our development program or commercialization efforts or cease operations.
Our operations have consumed substantial amounts of cash since inception. Since inception, we have raised approximately $146 million from the sale of equity securities in private placements and public offerings, $21 million from the issuance of debt securities, and $11 million from the exercise of common share purchase warrants. We will need to continue to spend substantial amounts to continue clinical development of topsalysin. We are currently evaluating options to further advance the clinical development of topsalysin. We have initiated a second Phase 2 clinical trial to confirm the dose and optimize the delivery of topsalysin for the treatment of localized prostate cancer. We will require significant additional funding to advance topsalysin in clinical development outside of this second Phase 2 clinical trial. We could use dilutive funding options such as an equity financing and non-dilutive funding options such as a partnering arrangement or royalty agreement to fund future clinical development of topsalysin. Other than our second Phase 2 clinical trial for the treatment of localized prostate cancer, at this point in time we do not plan on pursuing new clinical trials, including a second Phase 3 trial in BPH, unless we obtain additional financing or secure a development partner to fund such new clinical trials. There can be no assurance that such funding or a development partner will be available on acceptable terms or at all.
We expect that our existing cash, cash equivalents and securities available-for-sale, together with interest thereon, will only be sufficient to fund our operations to the end of 2018. However, changing circumstances may cause us to consume capital significantly faster than we currently anticipate, and we may need to spend more money than currently expected because of circumstances beyond our control. Any clinical development efforts, including our second Phase 2 clinical trial and our ongoing operations will require significant funding.
We expect to finance future cash needs through public or private equity offerings, debt financings or strategic partnerships and alliances and licensing arrangements. In addition, as part of our offering of common shares in August 2016, we agreed not to sell any equity securities for 90 days from the closing. We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us we may need to significantly delay, scale back or discontinue the development or commercialization of topsalysin. We also could be required to:
|
|
• |
seek collaborators for one or more of our current or future product candidates on terms that are less favorable than might otherwise be available; |
|
|
• |
relinquish or license on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves; or |
|
|
• |
seek a third party to acquire us or our assets. |
Any of the above events could significantly harm our business, prospects, financial condition and results of operations and cause the price of our common shares to decline.
We are an early stage company with no approved products and no revenue from commercialization of our product candidate.
We have not completed the development of any product candidates and, accordingly, have not begun to commercialize, or generate any product revenues from any product candidate. We are at an early stage of development of our product candidate, topsalysin, for the treatment of the lower urinary tract symptoms of benign prostatic hyperplasia, or BPH and for the treatment of clinically significant localized prostate cancer. Topsalysin requires significant additional clinical testing and investment prior to seeking marketing approval for either the treatment of the symptoms of BPH or the treatment of prostate cancer. On November 10, 2015, we announced final results from our Phase 3 "PLUS-1" study of topsalysin as a treatment for lower urinary tract symptoms of BPH. However, in order to seek regulatory approval for the treatment of the symptoms of BPH, we would be required to conduct a second Phase 3 clinical trial. At this point in time we do not plan on pursuing a second Phase 3 trial in BPH unless we obtain additional financing or secure a development partner to fund such new clinical trial. There can be no assurance that such funding or a development partner will be available on acceptable terms or at all. In May 2015, we initiated a Phase 2a proof of concept clinical trial of topsalysin for the treatment of localized low to intermediate risk prostate cancer and on June 9, 2016, we announced the biopsy data of all 18 patients. We have initiated a second Phase 2 clinical trial to confirm the dose and optimize the delivery of topsalysin for the treatment of localized prostate cancer, which will be conducted at two or more clinical trial sites. While we believe that we may be able to seek regulatory approval for topsalysin for the treatment of localized prostate cancer with one Phase 3 clinical trial, we have not discussed late-stage clinical development in this indication with the Food and Drug Administration, or FDA, or foreign regulatory authorities and these authorities may disagree with our assessment and require additional clinical trials or other studies before we can submit for regulatory approval. We will continue to refine our development plans for topsalysin for the treatment of localized prostate cancer based on the results of our second Phase 2 clinical trial and discussions with regulatory agencies and may change our assessment of required clinical trials and our development plan. A commitment of substantial resources by us and potential partners will be required to conduct additional clinical trials for topsalysin to meet applicable regulatory standards, obtain required regulatory approvals, and to successfully commercialize this product candidate for the treatment in either indication. Topsalysin is not expected to be commercially available for either indication for several years, if at all, and any projected timelines for commercialization are subject to a number of factors that are outside our control. There is no assurance that we will be able to commercialize topsalysin within the time periods we expect or that our clinical trials will support the regulatory approvals needed to commercialize topsalysin at all.
We are highly dependent on the success of our sole product candidate, topsalysin and we may not be able to successfully obtain regulatory or marketing approval for, or successfully commercialize, this product candidate.
To date, we have expended significant time, resources and effort on the development of topsalysin for the lower urinary tract symptoms of BPH and for the treatment of clinically significant localized prostate cancer, including conducting preclinical and clinical trials. We have no product candidates in our clinical development pipeline other than topsalysin, which we are developing for those two potential indications. Our ability to generate product revenues and to achieve commercial success in the near term will initially depend almost entirely on our ability to successfully raise capital to fund our development programs and to develop, obtain regulatory approval for and then successfully commercialize topsalysin for either of these indications in the United States and the European Economic Area, or EEA. Before we can market and sell topsalysin in the United States or foreign jurisdictions for any indication, we will need to commence and complete additional clinical trials, manage clinical, preclinical, and manufacturing activities, obtain necessary regulatory approvals from the Food and Drug Administration, or FDA, in the United States and from similar foreign regulatory agencies in other jurisdictions, obtain manufacturing supply, build a commercial organization or enter into a marketing collaboration with a third party, and in some jurisdictions, obtain reimbursement authorization, among other things. We cannot assure you that we will be able to successfully complete the necessary preclinical studies and clinical trials and/or obtain regulatory approvals and sufficient commercial manufacturing supply for topsalysin in either indication. If we do not receive regulatory approvals, our business, prospects, financial condition and results of operations will be adversely affected. Even if we obtain the regulatory approvals to market and sell topsalysin, we may never generate significant revenues from any commercial sales of topsalysin for several reasons, including because the market for topsalysin may be smaller than we anticipate, topsalysin may not be adopted by physicians and payors or because topsalysin may not be as efficacious or safe as other treatment options. If we fail to successfully commercialize topsalysin, we may be unable to generate sufficient revenues to sustain and grow our business and our business, prospects, financial condition and results of operations will be adversely affected.
The clinical trial protocol and design for our completed and any additional future Phase 3 clinical trials of topsalysin may not be sufficient to allow us to submit a BLA to the FDA in the indication of lower urinary tract symptoms of BPH or demonstrate safety or efficacy at the level required by the FDA for product approval.
Our initial Phase 3 clinical trial in the treatment of lower urinary tract symptoms of BPH and any additional Phase 3 clinical trial of topsalysin in this indication use the International Prostate Symptom Score, or IPSS, outcome measure evaluated at total change from baseline over 52 weeks as the primary endpoint. Secondary endpoints include Qmax (maximum urine flow) change from baseline (maximum urine flow) over 52 weeks. The IPSS outcome measure, which is a validated primary efficacy endpoint used to assess the treatment benefit in BPH clinical trials, is a patient recorded, composite assessment that takes into account factors such as ability to empty the bladder, frequency of urination, intermittency of urination and the urgency of urination. The IPSS outcome measure is subjective in nature and requires patients in the trial to accurately and retroactively assess numerous symptoms. The subjective nature of the IPSS outcome measure may make efficacy more difficult to demonstrate than for clinical trials for therapies that can show objective measures of efficacy.
We have not requested a special protocol assessment, or SPA, which drug development companies sometimes use to obtain an agreement with the FDA concerning the design and size of a clinical trial intended to form the primary basis of an effectiveness claim. Without the concurrence of the FDA on an SPA or otherwise, we cannot be certain that the design, conduct and data analysis approach for our initial Phase 3 clinical trial and any future Phase 3 clinical trials has or will generate data sufficient to establish the effectiveness of topsalysin for treatment of BPH symptoms to the FDA’s satisfaction, and therefore allow us to submit or receive approval of a Biologics License Application, or BLA for topsalysin. If the FDA requires us, or we otherwise determine, to amend our protocols, change our clinical trial designs, increase enrollment targets or conduct additional clinical trials, our ability to obtain regulatory approval on the timeline we have projected would be jeopardized and we could be required to make significant additional expenditures related to clinical development.
Further, even if we achieve positive results on the endpoints for a clinical trial, the FDA may disagree with our interpretation of the data and deem the results insufficient to demonstrate efficacy at the level required by the FDA for product approval. It is possible that we may make modifications to the clinical trial protocols or designs of our future clinical trials that delay enrollment or completion of such clinical trials and could delay regulatory approval of topsalysin for the treatment of symptoms of BPH. Any failure to obtain approval for topsalysin on the timeline that we currently anticipate, or at all, would have a material and adverse impact on our business, prospects, financial condition and results of operations.
Our clinical trials may fail to adequately demonstrate safety and efficacy of topsalysin for either indication being pursued. Failure to meet the safety or efficacy standards for the trial would prevent or delay regulatory approval and commercialization.
Clinical development is expensive, takes many years to complete and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process and topsalysin is subject to the risks of failure inherent in drug development. Success in early clinical trials does not mean that later clinical trials will be successful because product candidates in later-stage clinical trials may fail to demonstrate sufficient safety or efficacy despite having progressed through initial clinical testing, even at statistically significant levels. We will be required to demonstrate through well-controlled clinical trials of topsalysin that our product candidate is safe and effective for use in its target indication before we can obtain regulatory approvals for its commercial sale. Companies frequently suffer significant setbacks in late-stage clinical trials, even after earlier clinical trials have shown promising results. Any future clinical trials of topsalysin may not be successful for a variety of reasons, including faults in the clinical trial designs, the failure to enroll a sufficient number of patients, undesirable side effects and other safety concerns and the inability to demonstrate sufficient efficacy. If topsalysin fails to demonstrate sufficient safety or efficacy, we would experience potentially significant delays in, or be required to abandon our development of, topsalysin, which would have a material and adverse impact on our business, prospects, financial condition and results of operations.
On November 10, 2015, we announced the final results from our initial Phase 3 clinical trial of topsalysin for the treatment of lower urinary tract symptoms of BPH and we are currently considering an additional Phase 3 clinical trial for topsalysin to examine whether topsalysin will effectively relieve BPH symptoms as measured at 52 weeks following treatment, which second trial will be required by the FDA before we can seek marketing approval of topsalysin in this indication. The results of the initial Phase 3 clinical trial may not be predictive of the second required Phase 3 clinical trial in the same indication. Further, even if we meet the primary and secondary endpoints in both trials, if topsalysin is slow to achieve effectiveness, this may limit its commercial potential relative to therapies that demonstrate more immediate effect on the symptoms of BPH. The FDA has not agreed upon the amount of IPSS treatment effect that must be demonstrated in the required Phase 3 clinical trials in order for marketing approval to be granted; however, historically the oral medications approved for the treatment of BPH have shown approximately a 2 point improvement in IPSS between active and control. There is no assurance that the FDA will not require that we demonstrate a 2 point improvement, which was not seen in the PLUS-1 clinical trial.
On June 9, 2016, we announced the biopsy data at six months on all 18 patients enrolled in our Phase 2a proof of concept clinical trial of topsalysin for the treatment of localized low to intermediate risk prostate cancer. The results of the Phase 2a proof of concept clinical trial may not be predictive of the results of our next Phase 2 study to confirm dosing and optimize delivery.
If any of the clinical trials of topsalysin fail to demonstrate sufficient safety and efficacy, we would experience potentially significant delays in, or be required to abandon our development program, which would have a material and adverse impact on our business, prospects, financial condition and results of operations.
We may seek a partner for the continued development and commercialization of topsalysin for the treatment of the symptoms of BPH. If we seek a partner and are unable to find a partner or such partnership is unsuccessful, we may be unable to commercialize topsalysin for this indication.
We may seek a third-party partner for financial and scientific resources for the further clinical development and commercialization of topsalysin for the treatment of the symptoms of BPH, including the required second Phase 3 clinical trial. There is no assurance that we will be able to find such a partner and, if we do, we may have to relinquish a significant portion of the future economic value of topsalysin to such partner. Also, a partner will likely significantly limit our control over the course of clinical development of topsalysin. Our ability to recognize revenue from a successful partnering arrangement of the sort we are contemplating may be impaired by several factors, including:
|
|
● |
a partner may shift its priorities and resources away from topsalysin due to many reasons, including a change in business strategy, a merger, acquisition, sale or downsizing of its company or business unit; |
|
|
● |
successfully identifying a new partner and negotiating an agreement could be more difficult or the terms less advantageous because we have already established a partnership for Japan; |
|
|
● |
a partner may have the ability to unilaterally cease development of topsalysin; |
|
|
● |
a partner may change the success criteria for topsalysin as a treatment for the symptoms of BPH thereby delaying or ceasing clinical development of topsalysin; |
|
|
● |
a partner could develop a product that competes, either directly or indirectly, with topsalysin; |
|
|
● |
a partner with commercialization obligations may not commit sufficient financial or human resources to the marketing, distribution or sale of topsalysin; |
|
|
● |
a partner could terminate our agreement; |
|
|
● |
a dispute could arise between us and a partner concerning the research, development or commercialization of topsalysin which could delay or terminate development and, possibly, result in costly litigation or arbitration which may divert management attention and resources; and |
|
|
● |
a partner may use our proprietary information or intellectual property in such a way as to invite litigation from a third party or fail to maintain or prosecute intellectual property rights such that our rights are jeopardized. |
In addition, any adverse developments that occur during any clinical trials conducted by or under the supervision of a partner may affect our ability to obtain regulatory approval or commercialize topsalysin for the treatment of prostate cancer.
Further, if a partnership terminates or is otherwise unsuccessful, we may need to seek out and establish an alternative partnership. This may not be possible, or we may not be able to do so on terms which are acceptable to us, in which case, it may be necessary for us to cease the development of topsalysin for the treatment of symptoms of BPH or conduct the remaining clinical development on our own and with our own funds.
Any of these events would have a material adverse effect on our results of operations and financial condition.
Topsalysin is subject to extensive regulation, and we may not obtain regulatory approvals for topsalysin.
The clinical development, manufacturing, labeling, packaging, storage, tracking, recordkeeping, advertising, promotion, export, import, marketing and distribution and other possible activities relating to our product candidate are, and for any other biologic or drug candidate that we may develop will be, subject to extensive regulation by the FDA in the United States and other regulatory agencies in foreign jurisdictions. Topsalysin is subject to regulation in the United States as a biologic. Biologics require the submission of a BLA, and we are not permitted to market topsalysin in the United States until we obtain approval from the FDA of a BLA. To market topsalysin in the EEA, which includes the 27 member states of the European Union plus Norway, Liechtenstein and Iceland, we must submit a Marketing Authorization Application, or MAA, to the European Medicines Agency, or EMA, for approval under the EMA’s centralized procedure, which if the marketing authorization is granted, will enable us to market the product throughout the entire territory of the EEA. A BLA or MAA must be supported by extensive clinical and preclinical data, as well as extensive information regarding chemistry, manufacturing and controls, or CMC, sufficient to demonstrate the safety and effectiveness of the applicable product candidate to the satisfaction of FDA and EMA, respectively.
Regulatory approval of a BLA or an MAA is not guaranteed, and the approval process is expensive and will take several years. The FDA and foreign regulatory entities also have substantial discretion in the approval process. The number and types of preclinical studies and clinical trials that will be required for BLA or MAA approval varies depending on the product candidate, the disease or the condition that the product candidate is designed to target and the regulations applicable to any particular product candidate. Despite the time and expense associated with preclinical studies and clinical trials, failure can occur at any stage, and we could encounter problems that cause us to repeat or perform additional preclinical studies or clinical trials or generate additional CMC data. The FDA, EMA and similar foreign authorities could delay, limit or deny approval of a product candidate for many reasons, including because they:
|
|
• |
may not deem our product candidate to be adequately safe and effective; |
|
|
• |
may not find the data from our preclinical studies and clinical trials or CMC data to be sufficient to support a claim of safety and efficacy; |
|
|
• |
may not approve the manufacturing processes or facilities associated with our product candidate; |
|
|
• |
may conclude that we have not sufficiently demonstrated long-term stability of the formulation of the drug product for which we are seeking marketing approval; |
|
|
• |
may change approval policies (including with respect to our product candidate’s class of biologics) or adopt new regulations; or |
|
|
• |
may not accept a submission due to, among other reasons, the content or formatting of the submission. |
Obtaining approval of a BLA is a lengthy, expensive and uncertain process. As part of the U.S. Prescription Drug User Fee Act, the FDA has a goal to review and act on a percentage of all submissions in a given time frame. The general review goal for a BLA is 12 months from the submission date for a standard application and eight months from the submission date for a priority review application. The FDA’s review goals are subject to change, and it is unknown whether the review of a BLA for topsalysin will be completed within the FDA’s target timelines or will be delayed. Moreover, the duration of the FDA’s review may depend on the number and types of other BLAs that are submitted to the FDA around the same time period or are pending. Generally, public concern regarding the safety of drug products could delay or limit our ability to obtain regulatory approval, result in the inclusion of unfavorable information in our labeling, or require us to undertake other activities that may entail additional costs.
We have not submitted an application for approval or obtained FDA approval for any product. This lack of experience may impede our ability to obtain FDA approval in a timely manner, if at all, for topsalysin. In addition, failure to comply with FDA and other applicable U.S. and foreign regulatory requirements, either before or after product approval, may subject us to administrative or judicially imposed sanctions, including:
|
|
• |
warning letters; |
|
|
• |
civil and criminal penalties; |
|
|
• |
injunctions; |
|
|
• |
withdrawal of approved products; |
|
|
• |
product seizure or detention; |
|
|
• |
product recalls; |
|
|
• |
total or partial suspension of production; and |
|
|
• |
refusal to approve pending BLAs or supplements to approved BLAs. |
Even if we believe that data collected from our preclinical studies and clinical trials of our product candidate are promising, our data may not be sufficient to support marketing approval by the FDA or any foreign regulatory authority, or regulatory interpretation of these data and procedures may be unfavorable. In addition, the FDA’s regulatory review of BLAs for product candidates intended for widespread use by a large proportion of the general population is becoming increasingly focused on safety, which may lead to increased scrutiny of the safety data we submit in any BLA for topsalysin. Even if approved, a product candidate may not be approved for all indications requested and such approval may be subject to limitations on the indicated uses for which the biologic may be marketed, restricted distribution methods or other limitations. Our business and reputation may be harmed by any failure or significant delay in obtaining regulatory approval for the sale of our product candidate. We cannot predict when or whether regulatory approval will be obtained for any product candidate we develop.
To market any biologics outside of the United States, we and current or future collaborators must comply with numerous and varying regulatory and compliance related requirements of other countries. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods, including obtaining reimbursement and pricing approval in select markets. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks associated with FDA approval as well as additional, presently unanticipated, risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others, including the risk that our product candidates may not be approved for all indications requested and that such approval may be subject to limitations on the indicated uses for which the drug may be marketed.
Topsalysin may cause undesirable side effects or have other properties that may delay or prevent its regulatory approval or commercialization or limit its commercial potential.
Undesirable side effects caused by topsalysin could cause us or regulatory authorities to interrupt, delay, suspend or terminate clinical trials and could result in a more restrictive label or the delay or denial of marketing approval by the FDA or other regulatory authorities. This, in turn, could limit or prevent us from commercializing topsalysin and generating revenues from its sale. The most common adverse events observed in patients who received topsalysin in our initial Phase 3 clinical trial for the treatment of lower urinary tract symptoms of BPH that were potentially attributable to topsalysin included painful urination, the presence of red blood cells in urine, frequent urination and urinary urgency, fever, and perineal pain. Each of the foregoing adverse events occurred in greater than 5% of the topsalysin population. Further, the incidence of serious AEs, or SAEs, was similar in patients treated with topsalysin and vehicle. There were two SAEs assessed by the investigator as at least possibly related to treatment for topsalysin and one such SAE for vehicle. The topsalysin-related SAEs were moderate events of “acute non-infectious prostatitis” and “fever following prostate procedure” not unexpected manifestations of the intraprostatic cellular destruction and resultant inflammation integral to the topsalysin mechanism of action. The vehicle-related SAE was a mild event of “urinary tract infection.” The adverse events which occurred in our Phase 2a localized prostate cancer trial were similar in nature to the adverse events noted in our BPH program and no SAEs were reported. Although the SAEs were moderate and not unexpected, they may not be fully indicative of the adverse events that would be encountered in commercial use or in larger trials. Results from our future clinical trials could reveal a high and unacceptable severity and prevalence of these or other side effects. In such an event, our trials could be suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of topsalysin for its targeted indication. Further, such side effects could affect patient recruitment or the ability of enrolled patients to complete a trial or result in potential product liability claims. Any of these occurrences may have a material and adverse impact on our business, prospects, financial condition and results of operations.
In addition, if topsalysin receives marketing approval for the treatment of the symptoms of BPH or prostate cancer, or both, and we or others later identify undesirable side effects caused by topsalysin, a number of significant negative consequences could result, including:
|
|
• |
regulatory authorities may withdraw their approval of topsalysin; |
|
|
• |
regulatory authorities may require that we demonstrate a larger clinical benefit by conducting additional clinical trials for approval to offset the risk; |
|
|
• |
regulatory authorities may require the addition of labeling statements or warnings that could diminish the usage of the product or otherwise limit the commercial success of topsalysin; |
|
|
• |
we may be required to change the way topsalysin is administered; |
|
|
• |
we may choose to recall, withdraw or discontinue sale of topsalysin; |
|
|
• |
we could be sued and held liable for harm caused to patients; |
|
|
• |
we may not be able to enter into collaboration agreements on acceptable terms and execute on our business model; and |
|
|
• |
our reputation may suffer. |
Any one or a combination of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase the costs and expenses of commercializing topsalysin, which in turn could delay or prevent us from generating any revenues from the sale of the product, which could significantly harm our business, prospects, financial condition and results of operations.
We may experience delays in the commencement or completion of our clinical trials, which could result in increased costs to us and delay our ability to pursue regulatory approval and generate product revenues.
Delays in the commencement or completion of clinical testing could significantly impact our product development costs and could result in the need for additional financing. Although we have completed the first of two required Phase 3 clinical trials of topsalysin for the treatment of the symptoms of BPH and completed a Phase 2a proof of concept clinical trial for the treatment of localized low to intermediate risk prostate cancer, and have commenced our next planned Phase 2 trial for the treatment of clinically significant localized prostate cancer in the first quarter of 2017 for which we have funds, we do not know whether or when we will be able to fund any additional clinical trials for either the treatment of clinically significant localized prostate cancer or the treatment of the symptoms of BPH, or if any future trials will be completed on time, or at all.
Further, the commencement or completion of clinical trials can be delayed for a variety of reasons, including delays in or related to:
|
|
• |
raising sufficient capital or securing a development partner to fund the clinical trial; |
|
|
• |
obtaining regulatory approval, or feedback on trial design necessary, to commence a clinical trial; |
|
|
• |
identifying, recruiting and training suitable clinical investigators; |
|
|
• |
identifying, recruiting and enrolling suitable patients to participate in a clinical trial; |
|
|
• |
catastrophic loss of drug product due to shipping delays or delays in customs in connection with delivery of drug product to foreign countries for use in clinical trials; |
|
|
• |
reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites; |
|
|
• |
obtaining sufficient quantities of topsalysin and the diluent used with topsalysin for use in clinical trials and completing reformulation of topsalysin for commercial fill and finish for use in any future Phase 3 clinical trials; |
|
|
• |
having patients complete a trial or return for post-treatment follow-up; |
|
|
• |
adding new clinical trial sites; |
|
|
• |
failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
|
|
• |
failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions; |
|
|
• |
unforeseen safety issues or any determination that a clinical trial presents unacceptable health risks; |
|
|
• |
obtaining institutional review board, or IRB, approval to conduct a clinical trial at a prospective site; and |
|
|
• |
retaining patients who have initiated a clinical trial but may withdraw due to adverse side effects from the therapy, insufficient efficacy, fatigue with the clinical trial process or personal issues. |
Any delays in the commencement or completion of our clinical trials will delay our timeline to obtain regulatory approval for our product candidate. In addition, many of the factors that cause, or lead to, a delay in the commencement of clinical trials may also ultimately lead to the denial of regulatory approval for a product candidate. We do not expect to commence enrollment of our second required Phase 3 clinical trial in this indication until we have raised the additional capital required to fund such second Phase 3 clinical trial.
We may face competition to enroll prostate cancer and BPH patients in our future clinical trials from other clinical trials for other sponsors including potential competitors. Patient enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating. Delays in enrollment in any future clinical trials of topsalysin would result in delays in our ability to pursue regulatory approval of topsalysin.
Changes in regulatory requirements and guidance also may occur and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to IRBs for re-examination, which may impact the costs, timing and successful completion of a clinical trial. If we experience delays in the completion of, or if we must terminate, any clinical trial of topsalysin, our ability to obtain regulatory approval for that product candidate will be delayed and the commercial prospects, if any, for the product candidate may be harmed. If we ultimately commercialize topsalysin, other therapies for the same indications may have been introduced to the market during the period we have been delayed and such therapies may have established a competitive advantage over our product candidates.
We rely on third parties to manufacture topsalysin and an ingredient used in the diluent used to administer topsalysin, and we intend to rely on third parties to manufacture commercial supplies of topsalysin, if and when it is approved. The development and commercialization of topsalysin could be stopped or delayed if any such third party fails to provide us with sufficient quantities of the product or the diluent or fails to do so at acceptable quality levels or prices or fails to maintain or achieve satisfactory regulatory compliance.
We do not currently have nor do we plan to acquire the infrastructure or capability internally to manufacture our clinical drug supplies for use in the conduct of our clinical trials, and we lack the resources and the capability to manufacture topsalysin on a clinical or commercial scale. Instead, we currently rely on our third-party manufacturing partner, Boehringer Ingelheim RCV GmbH & Co KG, or BI, located in Austria for the production of topsalysin and located in Germany for fill and testing services, pursuant to an agreement which we entered into in 2011. Although we have entered into an agreement for the manufacture of clinical supplies and initial commercial supplies of topsalysin, BI may not perform as agreed, may be unable to comply with these cGMP requirements and with FDA, state and foreign regulatory requirements or may terminate its agreement with us.
We have completed scale-up up to the commercial batch size for topsalysin drug substance, but the finalization of the commercial fill finish process for the production of drug product is still underway. In addition, we have decided to pursue the reformulation of topsalysin, including the diluent. Reformulation could result in significant delays in the commencement of future clinical trials. Moreover, we have not entered into a commercial supply agreement with BI and BI has not demonstrated that it will be capable of manufacturing the filled and finished topsalysin on a large commercial scale. If BI is unable or unwilling to manufacture the filled and finished topsalysin on a large commercial scale, we may be required to identify a new manufacturer which could cause significant delays in finalizing the current commercial fill finish process and could cause delays to future planned clinical trials.
BI currently procures an ingredient used in the current formulation of topsalysin from a multinational industrial biotech company which is a single source supplier, on a purchase order basis. If our single source provider is unable to or decides to no longer supply BI or us with an ingredient for the diluent, we could experience delays in obtaining product for clinical trials until we procured another source or until we reformulate the product and we may be required to contract with another source in order to assure adequate commercial supply. Reformulation could result in significant further delays as we would be required to conduct additional clinical trials.
If our third-party manufacturer cannot successfully manufacture material that conforms to our specifications and the applicable regulatory authorities’ strict regulatory requirements, or pass regulatory inspection, they will not be able to secure or maintain regulatory approval for the manufacturing facilities. In addition, we have no control over the ability of any third-party manufacturer to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or any other applicable regulatory authorities do not approve these facilities for the manufacture of our products or if they withdraw any such approval in the future, or if our suppliers or third-party manufacturer decide they no longer want to supply our biologic or manufacture our products, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our products. We might be unable to identify manufacturers for long-term commercial supply on acceptable terms or at all. Manufacturers are subject to ongoing periodic unannounced inspection by the FDA and other governmental authorities to ensure strict compliance with government regulations. Currently, our contract manufacturer is located outside the United States and the FDA has recently increased the number of foreign drug manufacturers which it inspects. As a result, our third-party manufacturer may be subject to increased scrutiny.
The facilities used by our third-party manufacturer to manufacture topsalysin and any other potential product candidates that we may develop in the future must be approved by the applicable regulatory authorities, including the FDA, pursuant to inspections that will be conducted after we submit our BLA to the FDA. We do not control the manufacturing processes of BI and are currently completely dependent on BI for the production of topsalysin in accordance with cGMPs, which include, among other things, quality control, quality assurance and the maintenance of records and documentation.
If we were to experience an unexpected loss of topsalysin supply, we could experience delays in our future clinical trials as BI would need to manufacture additional topsalysin and would need sufficient lead time to schedule a manufacturing slot. This is due to the fact that, given its nature, topsalysin cannot be manufactured in the BI facility at the same time as other biologics.
Topsalysin is manufactured by starting with cells which are stored in a cell bank. We have one master cell bank and multiple working cell banks and believe we would have adequate backup should any cell bank be lost in a catastrophic event. However, it is possible that we could lose multiple cell banks and have our manufacturing severely impacted by the need to replace the cell banks.
The manufacture of biopharmaceutical products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. We and our contract manufacturers must comply with cGMP regulations and guidelines. Manufacturers of biopharmaceutical products often encounter difficulties in production, particularly in scaling up and validating initial production and contamination. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if microbial, viral or other contaminations are discovered in our products or in the manufacturing facilities in which our products are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. We cannot assure you that any stability or other issues relating to the manufacture of any of our products will not occur in the future. Additionally, our manufacturer may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If our manufacturer were to encounter any of these difficulties, or otherwise fail to comply with their contractual obligations, our ability to provide any product candidates to patients in clinical trials would be jeopardized. Any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at additional expense or terminate clinical trials completely.
Any adverse developments affecting clinical or commercial manufacturing of our products may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls, the need to reformulate our product or other interruptions in the supply of our products. We may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives. Accordingly, failures or difficulties faced at any level of our supply chain could materially adversely affect our business and delay or impede the development and commercialization of any of our products or product candidates and could have a material adverse effect on our business, prospects, financial condition and results of operations.
We have relied upon and expect to rely upon multiple CROs to conduct and oversee our completed and any future clinical trials for topsalysin. If any of our CROs does not meet our deadlines or otherwise conduct the trials as required or if any CRO experiences regulatory compliance issues we may not be able to obtain regulatory approval for or commercialize our product candidate when expected or at all.
We have used multiple CROs for our clinical trials of topsalysin and expect to rely upon CROs for any future clinical trials. We also rely upon medical institutions, clinical investigators and contract laboratories to conduct our trials in accordance with our clinical protocols and in accordance with applicable legal and regulatory requirements. These third parties play a significant role in the conduct of these trials and the subsequent collection and analysis of data from the clinical trials. There is no guarantee that any such third party will devote adequate time and resources to our clinical trial. If any of our CROs or any other third parties upon which we rely for administration and conduct of our clinical trials do not successfully carry out their contractual duties or obligations or fail to meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or if they otherwise perform in a substandard manner, our clinical trials may be extended, delayed, suspended or terminated, and we may not be able to complete development of and ultimately obtain approval for and successfully commercialize topsalysin. We will rely heavily on these third parties for the execution of our future clinical trials and will control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on CROs does not relieve us of our regulatory responsibilities.
We and our CROs are required to comply with current Good Clinical Practice, or GCP, which are regulations and guidelines enforced by the FDA, the competent authorities of the Member States of the EEA and comparable foreign regulatory authorities for products in clinical development. Regulatory authorities enforce these GCP regulations through periodic inspections of clinical trial sponsors, principal investigators and clinical trial sites. If we or any of our CROs fail to comply with applicable GCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and our submission of marketing applications may be delayed or the FDA may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, the FDA will determine that any of our clinical trials comply or complied with applicable GCP regulations. In addition, our clinical trials must be conducted with product produced under the current Good Manufacturing Practice, or cGMP, regulations enforced by the FDA, and our clinical trials require a large number of test subjects. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process. Moreover, our business may be implicated if any of our CROs violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
Switching or adding CROs can involve substantial cost and require extensive management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays may occur, which can materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationship with our CROs, there can be no assurance that we will not encounter such challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, prospects, financial condition or results of operations.
Any adverse developments that occur during any clinical trials conducted by Kissei may affect our ability to obtain regulatory approval or commercialize topsalysin.
Kissei Pharmaceutical Co., Ltd., or Kissei, retains the rights to develop and commercialize topsalysin in Japan for the treatment of the symptoms of BPH, prostate cancer, prostatitis or other diseases of the prostate. If serious adverse events occur during any other clinical trials Kissei decides to conduct with respect to topsalysin, the FDA and other regulatory authorities may delay, limit or deny approval of topsalysin or require us to conduct additional clinical trials as a condition to marketing approval, which would increase our costs. If we receive FDA approval for topsalysin and a new and serious safety issue is identified in connection with clinical trials conducted by Kissei, the FDA and other regulatory authorities may withdraw their approval of the product or otherwise restrict our ability to market and sell our product. In addition, treating physicians may be less willing to administer our product due to concerns over such adverse events, which would limit our ability to commercialize topsalysin.
We face significant competition from other pharmaceutical and biotechnology companies and from minimally invasive surgical therapies and surgical alternatives, and our operating results will suffer if we fail to compete effectively.
The biotechnology and pharmaceutical industries are intensely competitive. We have competitors both in the United States and international markets, including major multinational pharmaceutical companies, biotechnology companies and universities and other research institutions. Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff, experienced marketing and manufacturing organizations and well-established sales forces. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis, products that are more effective, easier to administer and/or less costly than topsalysin.
We expect that topsalysin will compete with the current treatment options for the treatment of clinically significant localized prostate cancer, which include surgical options such as laparoscopic and radical prostatectomy or radiation. In addition, there are other focal targeted therapies which are gaining traction that are currently in clinical development or have been recently approved which include: brachytherapy, cryotherapy, high focused ultrasound, cyber knife, radio frequency ablation and laser ablation. In addition, in 2016, Nymox Pharmaceuticals announced the clinical trial results from 18 months with the intraprostatic administration of their investigational therapy NX-1207 (fexapotide triflutate) in patients with low grade localized (T1c) prostate cancer, and, in January 2016, Steba Biotechnology submitted a Marketing Authorization Application to the European Medicine Agency for the focal treatment of patients with low risk localized prostate cancer, with their vascular –targeted photodynamic therapy TOOKAD.
We expect that topsalysin will compete with the current treatment options for the symptoms of BPH, which include oral drug therapy and surgery. Oral drug therapies include (a) alpha-blockers, such as tamsulosin (marketed under various trade names by numerous companies, including as Flomax® by Astellas Pharma), alfuzosin (marketed in the United States by Sanofi as Uroxatral®), doxazosin (marketed by Pfizer as Cardura® and Cardura® XL) and silodosin (marketed by Watson Pharmaceuticals as Rapaflo® in the United States), (b) 5-alpha reductase inhibitors, such as dutasteride (marketed by GlaxoSmithKline plc as Avodart®) and finasteride (marketed by Merck & Co., Inc. as Proscar®), (c) combinations of a-blockers and 5-alpha reductase inhibitors such as tamsulosin and dutasteride (marketed by GSK as Jalyn®) and (d) tadalafil (marketed as Cialis® by Eli Lilly), a PDE5 inhibitor which obtained FDA approval for the treatment of the symptoms of BPH in October 2011. Several minimally invasive surgical therapies, or MIST, are available, including transurethral microwave thermotherapy, or TUMT, transurethral needle ablation, or TUNA, photo-selective vaporization of prostate, holmium laser enucleation of the prostate, transurethral electrovaporization of the prostate, interstitial laser coagulation, and the UroLift® system (marketed by NeoTract, Inc.), which is an implant delivered into the body via a small needle and designed to hold prostate tissue out of the way of the blocked urethra. Currently, the most commonly used MIST procedures are laser ablations of the prostate, TUMT, and TUNA. Surgery for BPH treatment is usually considered in patients who fail drug therapy as a result of side effects or inadequate relief of symptoms, have refractory urinary retention, or have recurrent urinary tract infections. Alternatively, surgery may be the initial treatment in patients with severe urinary symptoms. Surgical procedures for BPH include transurethral resection of the prostate, as well as other procedures such as transurethral incision of the prostate and transurethral vaporization of the prostate. In addition, there are other treatments that are currently in clinical development for the treatment of the symptoms of BPH. Light Sciences Oncology Inc.’s AptocineTM is currently in Phase 2 clinical trials; in 2015, Nymox Pharmaceuticals announced that the injectable NX-1207 for the treatment of the symptoms of BPH met its primary endpoint in its pivotal Phase 3 extension trial; and in late 2015, Procept BioRobotics announced the first patients had been treated in a Phase 3 clinical trial to evaluate the AquaBeam System, a waterjet ablation therapy for endoscopic resection of prostate tissue.
The availability and price of our competitors’ products and procedures could limit the demand, and the price we are able to charge, for topsalysin. We will not successfully execute on our business objectives if the market acceptance of topsalysin is inhibited by price competition, if physicians are reluctant to switch from existing products or procedures to topsalysin or if physicians switch to other new products or surgeries or choose to reserve topsalysin for use in limited patient populations. In addition, established pharmaceutical companies may invest heavily to accelerate discovery and development of novel compounds or to in-license and develop novel compounds that could make topsalysin obsolete.
Any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to be approved and overcome price competition and to be commercially successful. Accordingly, our competitors may succeed in obtaining patent protection, obtaining FDA approval or discovering, developing and commercializing products before we do, which would have a material adverse impact on our business. The inability to compete with existing products or subsequently introduced products would have a material adverse impact on our business, prospects, financial condition and results of operations.
Even if we obtain and maintain approval for topsalysin from the FDA in either indication, we may never obtain approval for topsalysin outside of the United States, which would limit our market opportunities and adversely affect our business.
Sales of topsalysin outside of the United States will be subject to foreign regulatory requirements governing clinical trials and marketing approval. Even if the FDA grants marketing approval for a product candidate, comparable regulatory authorities of foreign countries must also approve the manufacturing and marketing of the product candidates in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials. In many countries outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that country. In some cases, the price that we intend to charge for our products is also subject to approval. We may decide to submit an MAA to the EMA for approval in the EEA. As with the FDA, obtaining approval of an MAA from the EMA is a similarly lengthy and expensive process and the EMA has its own procedures for approval of product candidates. Even if a product is approved, the FDA or the EMA, as the case may be, may limit the indications for which the product may be marketed, require extensive warnings on the product labeling or require expensive and time-consuming clinical trials or reporting as conditions of approval. Regulatory authorities in countries outside of the United States and the EEA also have requirements for approval of drug candidates with which we must comply prior to marketing in those countries. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. Further, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries and regulatory approval in one country does not ensure approval in any other country, while a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory approval process in others. Also, regulatory approval for any of our product candidates may be withdrawn. If we fail to comply with the regulatory requirements in international markets and/or receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of topsalysin will be harmed and our business will be adversely affected.
We will be, with respect to any product candidate for which we obtain FDA approval, subject to ongoing FDA obligations and continued regulatory review, which may result in significant additional expense.
Any regulatory approvals that we obtain for our product candidate may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including post-marketing studies and clinical trials and surveillance to monitor the safety and efficacy of the product candidate. In addition, if the FDA or a comparable foreign regulatory authority, like the EMA, approves a product candidate, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export, tracking and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMPs for marketed drugs and drugs used in clinical trials and GCPs for any clinical trials that we conduct post-approval. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
|
|
• |
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
|
|
• |
fines, warning letters or holds on clinical trials; |
|
|
• |
refusal by the FDA to approve pending applications or supplements to approved applications filed by us or our strategic partners, or suspension or revocation of product license approvals; |
|
|
• |
product seizure or detention, or refusal to permit the import or export of products; and |
|
|
• |
injunctions, the imposition of civil or criminal penalties, or exclusions. |
The FDA’s policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would have a material adverse effect on our business, prospects, financial condition and results of operations.
Moreover, the federal Drug Supply Chain Security Act, imposes obligations on manufacturers of pharmaceutical products, among others, related to product tracking and tracing. Among the requirements of this new federal legislation, manufacturers will be required to provide certain information regarding the drug product to individuals and entities to which product ownership is transferred, label drug product with a product identifier, and keep certain records regarding the drug product. Further, manufacturers have drug product investigation, quarantine, disposition, and notification responsibilities related to counterfeit, diverted, stolen, and intentionally adulterated products, as well as products that are the subject of fraudulent transactions or which are otherwise unfit for distribution such that they would be reasonably likely to result in serious health consequences or death.
If we fail to comply with health care laws, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
Even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, certain federal and state healthcare laws and regulations, including those pertaining to fraud and abuse and patients’ rights, are and will be applicable to our business. We could be subject to healthcare regulation by both the federal government and the states in which we conduct our business. The health care laws and regulations that may affect our ability to operate include, without limitation: anti-kickback statutes, false claims statutes patient data privacy and security laws, and physician sunshine laws and regulations, many of which may become more applicable if our product candidates are approved and we begin commercialization. If our operations are found to be in violation of any of these laws or regulations, we may be subject to penalties, including administrative, civil and criminal penalties, damages, fines, disgorgement, imprisonment, and exclusion from participation in federal healthcare programs, as well as contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations. Any such penalties could adversely affect our ability to operate our business and our financial results. Any action against us for violation of these laws and regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with these laws and regulations may prove costly.
We will need to increase the size of our organization and the scope of our outside vendor relationships, and we may experience difficulties in managing growth.
As of December 31, 2016, we had five full-time employees. In May 2016, we had a reduction in force of five employees to preserve our cash resources while we pursue strategic alternatives. If we obtain additional capital we may have to rehire these employees or identify and hire replacements. In addition, we have engaged part-time individual consultants to assist us with managing vendors and CROs, project management, regulatory compliance and business development. We will need to expand our managerial, operational, financial and other resources in order to manage our operations and clinical trials, continue our research and development activities, and commercialize our product candidate. Our management and scientific personnel, systems and facilities currently in place may not be adequate to support our future growth. Our need to effectively manage our operations, growth and various projects requires that we:
|
|
• |
manage our clinical trials effectively; |
|
|
• |
manage our internal development efforts effectively while carrying out our contractual obligations to licensors, contractors and other third parties; |
|
|
• |
continue to improve our operational, financial and management controls and reporting systems and procedures; |
|
|
• |
attract and retain sufficient numbers of talented employees; and |
|
|
• |
manage our regulatory compliance oversight and infrastructure. |
To date, we have utilized the services of third-party vendors to perform tasks including clinical trial management, statistics and analysis, regulatory affairs, formulation development and other drug development functions. Our growth strategy may also entail expanding our group of contractors or consultants to implement these tasks going forward. Because we rely on numerous consultants, effectively outsourcing many key functions of our business, we will need to be able to effectively manage these consultants to ensure that they successfully carry out their contractual obligations and meet expected deadlines. However, if we are unable to effectively manage our outsourced activities or if the quality or accuracy of the services provided by consultants is compromised for any reason, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for our product candidate or otherwise advance our business. There can be no assurance that we will be able to manage our existing consultants or find other competent outside contractors and consultants on economically reasonable terms, or at all. If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, we may be unable to successfully implement the tasks necessary to further develop and commercialize our product candidate and, accordingly, may not achieve our research, development and commercialization goals.
Our limited operating history makes evaluating our business and future prospects difficult.
Our predecessor, Protox Pharmaceuticals Inc., was incorporated in January 2002. We were formed in May 2003 under the predecessor to the British Columbia Business Corporations Act, or the BCBCA, by the amalgamation of Stratos Biotechnologies Inc., Nucleus BioScience Inc. and Brightwave Ventures Inc. under the name SNB Capital Corp. In July 2004, we acquired all the shares of Protox Pharmaceuticals Inc. in a plan of arrangement under the BCBCA and changed its name to Protox Therapeutics Inc. In 2011, we formed a wholly-owned U.S. subsidiary incorporated in Delaware, Protox Therapeutics Corp. In 2012, we changed our name to Sophiris Bio Inc. and changed the name of our subsidiary to Sophiris Bio Corp. In 2012, Sophiris Bio Corp. formed a wholly-owned subsidiary incorporated in Delaware, Sophiris Bio Holding Corp. We face considerable risks and difficulties as a company with limited operating history, particularly as a consolidated entity with an operating subsidiary that also has a limited operating history. If we do not successfully address these risks, our business, prospects, operating results and financial condition will be materially and adversely harmed. Our limited operating history makes it particularly difficult for us to predict our future operating results and appropriately budget for our expenses. In the event that actual results differ from our estimates or we adjust our estimates in future periods, our operating results and financial position could be materially affected. We have limited experience as a consolidated operating entity, and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the pharmaceutical or biotechnology areas.
Our ability to generate revenues from topsalysin will be subject to attaining significant market acceptance among physicians, patients and healthcare payors.
Topsalysin, if approved in either indication for which we are currently pursuing development or any other indication, may not attain market acceptance among physicians, patients, healthcare payors or the medical community. We believe that the degree of market acceptance and our ability to generate revenues from topsalysin will depend on a number of factors, including:
|
|
• |
timing of market introduction of our products as well as competitive drugs; |
|
|
• |
efficacy and safety of topsalysin; |
|
|
• |
the clinical indication(s) for which topsalysin is approved; |
|
|
• |
continued projected growth of the urological disease markets, including incidence of BPH and prostate cancer; |
|
|
• |
acceptance by patients, primary care specialists and key specialists, including urologists for BPH and urologists and oncologists for prostate cancer; |
|
|
• |
potential or perceived advantages or disadvantages of topsalysin over alternative treatments, for BPH including cost of treatment and relative convenience and ease of administration and length of sustained benefits from treatment; |
|
|
• |
potential or perceived advantages or disadvantages of topsalysin over alternative treatments, for BPH including cost of treatment and relative convenience and ease of administration and length of sustained benefits from treatment; |
|
|
• |
strength of sales, marketing and distribution support; |
|
|
• |
the price of topsalysin, both in absolute terms and relative to alternative treatments; |
|
|
• |
the effect of current and future healthcare laws; |
|
|
• |
availability of coverage and adequate coverage, reimbursement and pricing from government and other third-party payors; and |
|
|
• |
product labeling or product insert requirements of the FDA or other regulatory authorities. |
If topsalysin is approved in either or both indications but fails to attain market acceptance by physicians, patients, health care payors, or the medical community, we may not be able to generate significant revenue to achieve or sustain profitability, which would have a material adverse effect on our business, prospects, financial condition and results of operations.
Coverage and reimbursement may not be available, or may be available at only limited levels, for topsalysin, which could make it difficult for us to sell topsalysin profitably.
Market acceptance and sales of topsalysin will depend in large part on global reimbursement policies and may be affected by future healthcare reform measures, both in the United States and other key international markets. Patients who are prescribed medicine for the treatment of their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their prescription drugs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Therefore, successful commercialization of our product will depend in part on the availability of governmental and third-party payor reimbursement for the cost of topsalysin and/or payment to the physician for administering topsalysin. In the United States, no uniform policy of coverage and reimbursement for drug products exists among third-party payors. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained. One third-party payor’s decision to cover a particular medical product or service does not assure that other payors will also provide coverage for the medical product or service, or to provide coverage at an adequate reimbursement rate. As a result, the coverage determination process will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that adequate coverage and reimbursement will be obtained. Further, a third-party payor’s decision to provide coverage for a medical product or service does not imply that an adequate reimbursement rate will be approved. The market for our product candidates will depend significantly on access to third-party payors’ formularies, or lists of treatments for which third-party payors provide coverage and reimbursement.
Third-party payors establish coverage and reimbursement policies for new products, including product candidates like topsalysin. In particular, in the United States, private health insurers and other third-party payors often provide reimbursement for treatments based on the level at which the government (through the Medicare or Medicaid programs) provides reimbursement for such treatments. In the United States, the EEA and other significant or potentially significant markets for our product candidate, government authorities and third-party payors are increasingly attempting to limit or regulate the price of medical products and services, particularly for new and innovative products and therapies, which has resulted in lower average selling prices. Further, the increased emphasis on managed healthcare in the United States and on country and regional pricing and reimbursement controls in Canada and the EEA will put additional pressure on product pricing, coverage, reimbursement and utilization, which may adversely affect our product sales and results of operations. These pressures can arise from policies and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and healthcare reform, coverage and reimbursement policies and pricing in general. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the PPACA, became law in the United States. PPACA substantially changes the way healthcare is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. Among the provisions of the PPACA of greatest importance to the pharmaceutical industry are the following: (i) an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; (ii) an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively; (iii) a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts to negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; (iv) extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; (v) expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals with income at or below 133% of the Federal Poverty Level, thereby potentially increasing manufacturers’ Medicaid rebate liability; (vi) expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; (vii) expansion of health care fraud and abuse laws, including the federal civil False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance; and (viii) a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. Since its enactment there have been judicial and Congressional challenges to other aspects of the PPACA, and we expect there will be additional challenges and amendments to the PPACA in the future. Other legislative changes have been proposed and adopted in the United States since the PPACA. For example, through the process created by the Budget Control Act of 2011, there are automatic reductions of Medicare payments to providers up to 2% per fiscal year, which went into effect in April 2013 and, following passage of the Bipartisan Budget Act of 2015, will remain in effect through 2025 unless additional Congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers. Further, recently there has been heightened governmental scrutiny over the manner in which drug manufacturers set prices for their marketed products. We expect that additional federal and state healthcare reform measures will be adopted in the future, any of which could result in reduced demand for our products or other adverse effects on our business.
In the EEA, the success of topsalysin, if approved, will depend largely on obtaining and maintaining government reimbursement, because in many European countries patients are unlikely to use therapies that are not reimbursed by the government. Negotiating prices with governmental authorities can delay commercialization by 12 months or more. Reimbursement policies may adversely affect our ability to sell our products on a profitable basis. In many international markets, governments control the prices of prescription pharmaceuticals, including through the implementation of reference pricing, price cuts, rebates, revenue-related taxes and profit control, and expect prices of prescription pharmaceuticals to decline over the life of the product or as volumes increase. Recently, many countries in the EEA have increased the amount of discounts required on pharmaceutical products and other therapies, and we expect these discounts to continue as countries attempt to manage healthcare expenditures, especially in light of current economic conditions. As a result of these pricing practices, it may become difficult to achieve profitability or expected rates of growth in revenue or results of operations. Any shortfalls in revenue could adversely affect our business, prospects, financial condition and results of operations.
Certain countries have a very difficult reimbursement environment and we may not obtain reimbursement or pricing approval, if required, in all countries where we expect to market a product, or we may obtain reimbursement approval at a level that would make marketing a product in certain countries not viable.
We expect to experience pricing pressures in connection with the sale of topsalysin, if approved, and any other products that we may develop, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative proposals. If we fail to successfully secure and maintain adequate coverage and reimbursement for our products or are significantly delayed in doing so, we will have difficulty achieving market acceptance of our products and expected revenue and profitability which would have a material adverse effect on our business, prospects, financial condition and results of operations.
Our business and operations would suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our current and any future CROs and other contractors and consultants and collaborators are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we have not experienced any such material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture topsalysin and conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidate could be delayed.
Business interruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, systems failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions. The occurrence of any of these business interruptions could seriously harm our business and financial condition and increase our costs and expenses. A majority of our management operates in our principal executive offices located in San Diego, California. If our San Diego offices were affected by a natural or man-made disaster, particularly those that are characteristic of the region, such as wildfires and earthquakes, or other business interruption, our ability to manage our domestic and foreign operations could be impaired, which could materially and adversely affect our results of operations and financial condition. We currently rely, and intend to rely in the future, on our third-party manufacturer, BI, which is located in Austria and Germany, to produce our supply of topsalysin. Our ability to obtain supplies topsalysin could be disrupted, and our results of operations and financial condition could be materially and adversely affected if the operations of BI were affected by a man-made or natural disaster or other business interruption. The ultimate impact of such events on us, our significant suppliers and our general infrastructure is unknown.
Our business involves the use of hazardous materials, and we and our third-party manufacturer must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our third-party manufacturer’s activities involve the controlled storage, use and disposal of hazardous materials owned by us, including the components of topsalysin and other hazardous compounds. Specifically, the cleavage of the PSA-sensitive activation sequence of topsalysin in the manufacturing process could potentially lead to the release of the C-terminal inhibitory peptide resulting in the formation of active aerolysin, a pore-forming hemolytic toxin. We and our manufacturer are subject to federal, state and local as well as foreign laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. Although we believe that the safety procedures utilized by our third-party manufacturer for handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. BI, our third-party manufacturer, does not manufacture topsalysin in its facility at the same time as it manufactures other biologics due to the toxic nature of aerolysin. In the event of an accident, state, federal or foreign authorities may curtail the use of these materials and interrupt our business operations. We do not currently maintain hazardous materials insurance coverage. If we are subject to any liability as a result of our third-party manufacturer’s activities involving hazardous materials, our business and financial condition may be adversely affected. In the future we may seek to establish longer term third-party manufacturing arrangements, pursuant to which we would seek to obtain contractual indemnification protection from such third-party manufacturers potentially limiting this liability exposure.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products.
We face an inherent risk of product liability as a result of the clinical testing and, if approved, the commercialization of topsalysin. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state or foreign consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidate. Even a successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
|
|
• |
decreased demand for our product or product candidates that we may develop; |
|
|
• |
injury to our reputation; |
|
|
• |
withdrawal of clinical trial participants; |
|
|
• |
initiation of investigations by regulators; |
|
|
• |
costs to defend the related litigation; |
|
|
• |
a diversion of management’s time and our resources; |
|
|
• |
substantial monetary awards to clinical trial participants or patients; |
|
|
• |
product recalls, withdrawals or labeling, marketing or promotional restrictions; |
|
|
• |
loss of revenue; |
|
|
• |
exhaustion of any available insurance and our capital resources; |
|
|
• |
the inability to commercialize our products or product candidates; and |
|
|
• |
a decline in our share price. |
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. We currently carry product liability insurance covering our clinical studies and commercial product sales in the amount of $10 million in the aggregate.
Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. If we determine that it is prudent to increase our product liability coverage due to the commercial launch of any product, we may be unable to obtain such increased coverage on acceptable terms or at all. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
If we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive biotechnology and pharmaceuticals industries depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. We are highly dependent on our management and scientific and medical personnel, including our Chief Executive Officer and President, Randall E. Woods and our Chief Operating Officer and Head of Research and Development, Allison Hulme Ph.D. In order to retain valuable employees at our company, in addition to salary and cash incentives, we provide incentive stock options that vest over time. The value to employees of stock options that vest over time will be significantly affected by movements in our share price that are beyond our control, and may at any time be insufficient to counteract more lucrative offers from other companies.
Our scientific team in particular has expertise in many different aspects of drug development, and may be difficult to retain or replace. We conduct our operations at our facilities in San Diego, California and this region is headquarters to many other biopharmaceutical companies and many academic and research institutions and therefore we face increased competition for personnel in this location. Competition for skilled personnel in our market is very intense and competition for experienced scientists may limit our ability to hire and retain highly qualified personnel on acceptable terms.
In addition, we have scientific and clinical advisors who assist us in formulating our product development and clinical strategies. These advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us, or may have arrangements with other companies to assist in the development of products that may compete with ours.
Despite our efforts to retain valuable employees, members of our management and scientific and development teams may terminate their employment with us on short notice. Although we have written employment arrangements with all of our employees, these employment arrangements provide for at-will employment, which means that our employees can leave our employment at any time, with or without notice. In addition, we recently completed a reduction in workforce in May 2016 through which five of our ten employees were terminated. The loss of the services of any of our executive officers or other key employees and our inability to find suitable replacements could potentially harm our business, financial condition and prospects. We do not maintain “key man” insurance policies on the lives of these individuals or the lives of any of our other employees.
Our employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other illegal activity by our employees, independent contractors, consultants, commercial partners and vendors. Misconduct by these parties could include intentional, reckless and/or negligent conduct that fails to: comply with the laws of the FDA and other similar regulatory bodies; provide true, complete and accurate information to the FDA and other similar regulatory bodies; comply with manufacturing standards we have established; comply with federal and state healthcare fraud and abuse and health regulatory laws and other similar foreign fraudulent misconduct laws; or report financial information or data accurately or disclose unauthorized activities to us. These laws may impact, among other things, our activities with principal investigators and research subjects, as well as our sales, marketing and education programs. In particular, the promotion, sales, and marketing of health care items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Misconduct could also involve the improper use or disclosure of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. Additionally, we are subject to state and foreign equivalents of each of the healthcare laws described above, some of which may be broader in scope and may apply regardless of the payor.
We have adopted a Code of Business Conduct and Ethics, but it is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent inappropriate conduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws. Efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs. It is possible that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgement, individual imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations. Defending against any such actions can be costly, time-consuming and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell any products we may develop, we may not be able to effectively market and sell our products and generate product revenue.
We are developing topsalysin for large patient populations served by urologists and oncologists as well as general practice physicians, which number in the tens of thousands in the United States. Traditional pharmaceutical companies employ groups of sales representatives numbering in the thousands to call on this large of a number of physicians. We do not currently have an organization for the sale, marketing or distribution of topsalysin and we must build this organization or make arrangements with third parties to perform these functions in order to commercialize topsalysin and any future products. We intend to establish (either internally or through a contract sales force) a sales force to sell topsalysin, if approved, in the United States, although any partnership that we establish for the development of topsalysin for the treatment of the symptoms of BPH will likely provide U.S. commercialization rights or co-commercialization rights to the partner for this indication. We plan to partner with third parties to commercialize topsalysin outside the United States. The establishment and development of our own sales force or the establishment of a contract sales force to market any products we may develop in the United States will be expensive and time consuming and could delay any product launch, and we cannot be certain that we would be able to successfully develop this capacity. If we are unable to establish our sales and marketing capability or any other non-technical capabilities necessary to commercialize any products we may develop, we will need to contract with third parties to market and sell such products in the United States. We currently possess limited resources and may not be successful in establishing our own internal sales force or in establishing arrangements with third parties on acceptable terms, if at all.
We have incurred significant operating losses since our inception and anticipate that we will continue to incur losses for the foreseeable future.
We have a limited operating history and we have financed our operations primarily through equity and debt financings and have incurred significant operating losses since our inception. We had a net loss of $11.2 million, $14.2 million, and $30.7 million during the years ended December 31, 2016, 2015 and 2014, respectively. As of December 31, 2016, we had an accumulated deficit of $140.9 million. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our shareholders’ equity and working capital. Our losses have resulted principally from costs incurred in our research activities for topsalysin. We anticipate that our operating losses will substantially increase over the next several years as we continue development of topsalysin, including the conduct of any future clinical trials for the treatment of the symptoms of BPH and our completed proof of concept clinical trial and future clinical trials for the treatment of clinically significant localized prostate cancer and the conduct of any future clinical trials for the treatment of symptoms of BPH. In addition, if we obtain regulatory approval of topsalysin, we may incur significant sales and marketing expenses and outsourced manufacturing expenses, as well as continued development expenses. Because of the numerous risks and uncertainties associated with developing pharmaceutical products, we are unable to predict the extent of any future losses or whether or when we will become profitable.
We have not generated any product revenue and may never become profitable.
Our ability to become profitable depends upon our ability to develop and commercialize topsalysin. To date, other than the upfront payment we received from Kissei and the $5.0 million milestone payment we received in April 2013 from Kissei for the achievement of development milestones, we have not generated any revenue from topsalysin and we do not know when, or if, we will generate any future revenue. Our ability to generate future revenue depends on a number of factors, including:
|
|
• |
successfully completing the clinical development topsalysin in one or both indications; |
|
|
• |
obtaining U.S. and/or foreign regulatory approvals for topsalysin in one or both indications; |
|
|
• |
manufacturing commercial quantities of topsalysin at acceptable costs levels if regulatory approvals are received; |
|
|
• |
achieving broad market acceptance of topsalysin in the medical community and with third-party payors and patients; and |
|
|
• |
creating an internal commercial infrastructure or identifying and entering into one or more strategic collaborations to effectively market and sell topsalysin. |
We may never be able to successfully develop or commercialize topsalysin in either indication. Even if we do obtain regulatory approval to commercialize topsalysin, which we do not expect to occur for several years, we may never generate product sales and may never achieve or sustain profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress the market price of our common shares and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish intellectual property rights to our product candidates.
We may seek additional capital through a combination of public and private equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our shareholders. Debt financings may be coupled with an equity component, such as warrants to purchase shares, which could also result in dilution of our existing shareholders’ ownership. The incurrence of indebtedness would result in increased fixed payment obligations and could also result in certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and share price.
As widely reported, global credit and financial markets have experienced extreme disruptions in the past several years, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates, and uncertainty about economic stability. There can be no assurance that further deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, volatile business environment and continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate further, or do not improve, it may make any necessary debt or equity financing more difficult to complete, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and share price and could require us to delay or abandon development or commercialization plans. In addition, there is a risk that one or more of our current service providers, manufacturers and other partners may not survive these difficult economic times, which could directly affect our ability to attain our operating goals on schedule and on budget.
At December 31, 2016, we had $29.0 million of cash, cash equivalents securities available-for-sale. While we are not aware of any downgrades, material losses, or other significant deterioration in the fair value of our cash equivalents since December 31, 2016, no assurance can be given that further deterioration in conditions of the global credit and financial markets would not negatively impact our current portfolio of cash equivalents or our ability to meet our financing objectives. Further dislocations in the credit market may adversely impact the value and/or liquidity of cash equivalents owned by us.
Fluctuations in foreign currency exchange rates could result in changes in our reported revenues and earnings.
We currently incur expenses denominated in foreign currencies, specifically in connection with our manufacturing and supply agreement with Boehringer Ingelheim RCV GmbH & Co KG for the manufacture of topsalysin, for which payments are denominated in euro. In addition, we are utilizing several clinical vendors which are located in various countries outside of the United States. These clinical vendors invoice us in the local currency of the vendor. We do not engage in foreign currency hedging arrangements for our accounts payable, and, consequently, foreign currency fluctuations may adversely affect our earnings. During the years ended December 31, 2016 and 2015, 6.5% and 12.8% respectively, of our operating expenses were denominated in currencies other than the U.S. dollar. Going forward we anticipate that our sales and expenses, if any, will be denominated in the local currency of the country in which they occur. We may decide to manage this risk by hedging our foreign currency exposure, principally through derivative contracts. Even if we decide to enter into such hedging transactions, we cannot be sure that such hedges will be effective or that the costs of such hedges will not exceed their benefits. Fluctuations in the rate of exchange between the U.S. dollar and foreign currencies, primarily the euro, could result in material amounts of cash being required to settle the hedge transactions or could adversely affect our financial results.
Risks Related to our Intellectual Property
If we are unable to obtain or protect intellectual property rights related to our product candidates, we may not be able to compete effectively in our market.
We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our product candidates. The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we own or in-license may fail to result in issued patents with claims that cover the products in Canada, the United States or in other foreign countries. If this were to occur, early generic competition could be expected against product candidates in development. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing based on a pending patent application. Even if patents do successfully issue, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed or invalidated.
Composition-of-matter patents on the biological or chemical active pharmaceutical ingredient are generally considered to be the strongest form of intellectual property protection for pharmaceutical products, as such patents provide protection without regard to any method of use. We cannot be certain that the claims in our patent applications covering composition-of-matter of topsalysin will be considered patentable by the U.S. Patent and Trademark Office, or U.S. PTO, and courts in the United States or by the patent offices and courts in foreign countries. Method-of-use patents protect the use of a product for the specified method. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. Moreover, even if competitors do not actively promote their product for our targeted indications, physicians may prescribe these products off-label. Although off-label prescriptions may infringe or contribute to the infringement of method-of-use patents, the practice is common and such infringement is difficult to prevent or prosecute. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims. If the patent applications we hold with respect to topsalysin fail to issue or if their breadth or strength of protection is threatened, it could dissuade companies from collaborating with us to develop them, and threaten our ability to commercialize, our products. We cannot offer any assurances about which, if any, patents will issue or whether any issued patents will be found not invalid and not unenforceable or will go unthreatened by third parties. Further, if we encounter delays in regulatory approvals, the period of time during which we could market topsalysin under patent protection could be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we were the first to file any patent application related to topsalysin. Furthermore, if third parties have filed such patent applications, an interference proceeding in the United States can be provoked by a third party or instituted by us to determine who was the first to invent any of the subject matter covered by the patent claims of our applications.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our drug discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. Although we expect all of our employees to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques.
The Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law in September 2011 and includes a number of significant changes to U.S. patent law. These include changes in the way patent applications will be prosecuted and may also affect patent litigation. The U.S. PTO is currently developing regulations and procedures to administer the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act will not become effective until one year or 18 months after its enactment. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the cost of prosecuting our patent applications, our ability to obtain patents based on our patent applications and our ability to enforce or defend our issued patents. An inability to obtain, enforce and defend patents covering our proprietary technologies would materially and adversely affect our business prospects and financial condition. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States and Canada. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have any such enforceable trade secret protection, we may not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition.
Third party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and inter party reexamination proceedings before the U.S. PTO. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we, and our collaborators, are developing product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of topsalysin. Because patent applications can take many years to issue, there may be currently pending patent applications, which may later result in issued patents that our product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. We are aware of at least one third-party patent that may be relevant to our product candidates. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our product candidates, any molecules formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license under the applicable patents, or until such patents expire. Similarly, if any third-party patent were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy, the holders of any such patent may be able to block our ability to develop and commercialize the applicable product candidate unless we obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all. Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates, and we have done so from time to time. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize one or more of our product candidates, which could harm our business significantly. We cannot provide any assurances that third-party patents do not exist which might be enforced against our products, resulting in either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation to third parties.
If we fail to comply with our obligations in the agreements under which we license rights to technology from third parties, we could lose license rights that are important to our business.
We are a party to a number of technology license agreements that are essential to our business and expect to enter into additional license agreements in the future. For example, we have exclusive licenses to topsalysin from UVIC Industry Partnerships Inc. and The Johns Hopkins University. The agreements governing these exclusive licenses include provisions that permit the licensors to terminate the license agreements in a number of situations, including if we grant a security interest on the licensed technology. These licensors might claim that filings made by Oxford with the U.S. PTO or foreign jurisdictions in 2011 imposed a security interest on the applicable technology. However, no claims from these licensors have been made to date regarding violations of these license agreements as a result of these filings. Furthermore, if any such claims are made in the future, we believe that such claims would not have merit and we would vigorously defend and reject such claims. If we fail to comply with our obligations under our license agreements, or we are insolvent or subject to a bankruptcy proceeding, the applicable licensor may have the right to terminate such license agreement, in which event we would not be able to market products covered by such license agreement, including topsalysin. We may also be subjected to litigation or other potential disputes under our license agreements if we fail to comply with our obligations under those agreements. The loss of our rights to technology that we have licensed under certain agreements would have a material adverse effect on our business.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Interference proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our collaborators or licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common shares.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the U.S. PTO and foreign patent agencies in several stages over the lifetime of the patent. The U.S. PTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we or our licensors fail to maintain the patents and patent applications covering our product candidates, our competitors might be able to enter the market, which would have a material adverse effect on our business.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
We employ individuals who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of our employees’ former employers or other third parties. We may also be subject to claims that former employers or other third parties have an ownership interest in our patents. Litigation may be necessary to defend against these claims. There is no guarantee of success in defending these claims, and if we are successful, litigation could result in substantial cost and be a distraction to our management and other employees.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries, including China, do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our product candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Ownership of Our Common Shares
U.S. holders of our shares may suffer adverse tax consequences if we are characterized as a passive foreign investment company after 2012.
Generally, if for any taxable year 75% or more of our gross income is passive income, or at least 50% of the average quarterly value of our assets (which may be determined in part by the market value of our ordinary shares, which is subject to change) are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company, or PFIC, for United States federal income tax purposes. Based on the composition of our gross income and gross assets and the nature of our business, we expect that we were a PFIC for the taxable years ending December 31, 2012, 2013, 2014 and 2015 and that we will likely be a PFIC for the taxable year ending December 31, 2016. In 2017 and for future years, our status as a passive foreign investment company will also depend on whether we are a “controlled foreign corporation” for U.S. federal income tax purposes, how quickly we utilize the cash proceeds from our IPO in our business and other factors. If we are a PFIC for 2016 or any subsequent year, U.S. holders of our shares may suffer adverse tax consequences. Gains realized by non-corporate U.S. holders on the sale of our ordinary shares would be taxed as ordinary income, rather than as capital gain, and the preferential tax rate applicable to dividends received on our ordinary shares would be lost. Interest charges would also be added to taxes on gains and dividends realized by all U.S. holders.
A U.S. holder may avoid these adverse tax consequences by timely making a qualified electing fund election. For each year that we would meet the PFIC gross income or asset test, an electing U.S. holder would be required to include in gross income its pro rata share of our net ordinary income and net capital gains, if any. A U.S. holder may make a qualified electing fund election only if we commit to provide U.S. holders with their pro rata share of our net ordinary income and net capital gains. Because we intend to provide this information, a U.S. holder should be eligible to make a qualified electing fund election.
A U.S. holder may also mitigate the adverse tax consequences of being a PFIC by timely making a mark-to-market election. Generally, for each year that we would meet the PFIC gross income or asset test, an electing U.S. holder would include in gross income the increase in the value of its shares during each of its taxable years and deduct from gross income the decrease in the value of such shares during each of its taxable years. A mark-to-market election may be made and maintained only if our shares are regularly traded on a qualified exchange. While we anticipate that these requirements will be satisfied following our IPO, whether our shares are regularly traded on a qualified exchange is an annual determination based on facts that, in part, are beyond our control. Accordingly, we can provide no assurances that a U.S. holder will be eligible to make a mark-to-market election. You should consult your own tax advisor as to the specific tax consequences to you in the event we are characterized as a PFIC for the taxable year ending December 31, 2016 or any subsequent year.
The financial reporting obligations of being a public company require significant company resources and management attention.
We are subject to the public company reporting obligations under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the rules and regulations regarding corporate governance practices, including those under the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, and the listing requirements of The NASDAQ Capital Market. As a result, we have incurred, and will continue to incur, significant legal, accounting and other expenses that we did not incur as a private company, particularly after we are no longer an “emerging growth company” as defined in the JOBS Act. Further, the need to establish the corporate infrastructure demanded of a public company may divert management’s attention from implementing our growth strategy. We have made, and will continue to make, changes to our corporate governance standards, disclosure controls and financial reporting and accounting systems to meet our reporting obligations. Any changes that we make to comply with these obligations may not be sufficient to allow us to satisfy our obligations as a public company on a timely basis, or at all, which could subject us to delisting of our common shares, fines, sanctions and other regulatory action and potentially civil litigation. In addition, we incur significant legal, accounting, reporting and other expenses in order to maintain a listing on The NASDAQ Capital Market. These expenses relate to, among other things, the obligation to present financial information according to U.S. GAAP in the United States. We are also required to comply with certain disclosure and filing requirements under applicable securities laws in Canada as a reporting issuer in certain provinces.
The price of our common shares is likely to be highly volatile, and you could lose all or part of your investment.
Prior to our IPO in 2013, there was no public market for our common shares in the United States. The trading price of our common shares has been volatile and is likely to continue to be volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including limited trading volume. In addition to the other risk factors discussed in this section, these factors include:
|
|
• |
the outcome of our pursuit of strategic alternatives, including whether we raise any additional capital to fund our ongoing operations; |
|
|
• |
the results of our completed and future clinical trials of topsalysin or changes in the development status of topsalysin; |
|
|
• |
any adverse development or perceived adverse development with respect to our submission of a BLA to the FDA for topsalysin; |
|
|
• |
unanticipated serious safety concerns related to the use of topsalysin; |
|
|
• |
adverse regulatory decisions, including failure to receive regulatory approval for topsalysin; |
|
|
• |
our decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial; |
|
|
• |
our ability to obtain resources for us and our clinical trial programs on our desired schedule; |
|
|
• |
inability to obtain adequate commercial supply for any approved product or inability to do so at acceptable prices; |
|
|
• |
developments concerning our commercial partners, including but not limited to, those with manufacturers; |
|
|
• |
competition from existing technologies and products or new technologies and products that may emerge; |
|
|
• |
announcements of significant acquisitions, strategic partnerships, joint ventures, new products, capital commitments or other events by us or our competitors; |
|
|
• |
the inability to establish collaborations or termination of a collaboration; |
|
|
• |
actual or anticipated variations in our quarterly operating results; |
|
|
• |
failure to meet the estimates and projections of the investment community or that we may otherwise provide to the public; |
|
|
• |
our cash position; |
|
|
• |
announcement or expectation of additional financing efforts; |
|
|
• |
issuances of debt or equity securities; |
|
|
• |
our inability to successfully enter new markets or develop additional product candidates; |
|
|
• |
actual or anticipated fluctuations in our competitors’ operating results or changes in their growth rate; |
|
|
• |
sales of our common shares by us, or our shareholders in the future; |
|
|
• |
trading volume of our common shares on The NASDAQ Capital Market and price; |
|
|
• |
market conditions in our industry; |
|
|
• |
overall performance of the equity markets and general political and economic conditions; |
|
|
• |
introduction of new products or services by us or our competitors; |
|
|
• |
additions or departures of key management, scientific or other personnel; |
|
|
• |
publication of research reports about us or our industry or positive or negative recommendations or withdrawal of research coverage by securities or industry analysts; |
|
|
• |
changes in the market valuation of similar companies; |
|
|
• |
disputes or other developments related to intellectual property and other proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies and product candidates; |
|
|
• |
changes in laws or regulations and policies applicable to product candidates, including but not limited to clinical trial requirements for approvals; |
|
|
• |
changes in accounting practices; |
|
|
• |
significant lawsuits, including patent or shareholder litigation; and |
|
|
• |
other events or factors, many of which are beyond our control. |
Furthermore, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political and market conditions such as recessions, interest rate changes or international currency fluctuations, may negatively impact the market price of our common shares.
Sales of a substantial number of our common shares in the public market by our existing shareholders could cause our share price to fall.
Sales of a substantial number of our common shares in the public market or the perception that these sales might occur, could depress the market price of our common shares and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common shares.
Future sales and issuances of our common shares or rights to purchase common shares by us, including pursuant to our equity incentive plan, could result in additional dilution of the percentage ownership of our shareholders and could cause our share price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations, including commercialization efforts, expanded research and development activities and costs associated with operating as a public company. To the extent we raise additional capital by issuing equity or convertible securities, our shareholders may experience substantial dilution. We may sell common shares, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common shares, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing shareholders, and new investors could gain rights superior to our existing shareholders.
Pursuant to our equity incentive plan, our management is authorized to grant options to our employees, directors and consultants. The number of shares available for future grant under our plan is equal to 10% of all shares of our issued and outstanding common shares at any time. Currently, the number of shares available for issuance under our equity incentive plan each year automatically increases when we issue additional common shares. If our board of directors elects to grant additional options each year our shareholders may experience additional dilution, which could cause our share price to fall.
We are at risk of securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and biochemical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
We do not intend to pay dividends on our common shares so any returns will be limited to the value of our shares.
We have never declared or paid any cash dividend on our common shares. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to shareholders will therefore be limited to the increase, if any, of our share price.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth company until December 31, 2018, although circumstances could cause us to lose that status earlier, including if the market value of our common shares held by non-affiliates exceeds $700 million as of any December 31 before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31, or if we issue more than $1.0 billion in non-convertible debt during any three year period before that time, in which case we would no longer be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” which would allow us to take advantage of many of the same exemptions from disclosure requirements including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our share price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Our charter documents, certain related party contracts and certain Canadian legislation could delay or deter a change of control, limit attempts by our shareholders to replace or remove our current management and limit the market price of our common shares.
Our authorized preferred shares are available for issuance from time to time at the discretion of our board of directors, without shareholder approval. Our articles grant our board of directors the authority, subject to the BCBCA, to determine the special rights and restrictions granted to or imposed on any unissued series of preferred shares, and those rights may be superior to those of our common shares.
In addition, provisions in the BCBCA and in our articles, may have the effect of delaying or preventing changes in our management, including provisions that:
|
|
• |
prohibit cumulative voting in the election of directors; and |
|
|
• |
require the approval of our board of directors or the holders of a supermajority of our outstanding share capital to amend our articles and our notice of articles. |
These provisions may frustrate or prevent any attempts by our shareholders to replace or remove our current management by making it more difficult for shareholders to replace members of our board of directors, which is responsible for appointing the members of our management. Any of the foregoing could prevent or delay a change of control and may deprive or limit strategic opportunities to our shareholders to sell their shares.
Risks Related To Being A Canadian Entity
We are governed by the corporate laws in British Columbia, Canada which in some cases have a different effect on shareholders than the corporate laws in Delaware, United States.
The material differences between the BCBCA as compared to the Delaware General Corporation Law, or the DGCL, which may be of most interest to shareholders include the following: (i) for material corporate transactions (such as mergers and amalgamations, other extraordinary corporate transactions, amendments to our articles) the BCBCA generally requires two-thirds majority vote by shareholders, whereas DGCL generally only requires a majority vote of shareholders for similar material corporate transactions; (ii) the quorum for shareholders meetings is not prescribed under the BCBCA and is only two persons representing 5% of the issued shares under our articles, whereas under DGCL, quorum requires a minimum of one-third of the shares entitled to vote to be present and companies’ certificates of incorporation frequently require a higher percentage to be present; (iii) under the BCBCA a holder of 5% or more of our common shares can requisition a special meeting at which any matters that can be voted on at our annual meeting can be considered, whereas the DGCL does not give this right; (iv) our articles require two-thirds majority vote by shareholders to pass a resolution for one or more directors to be removed, whereas DGCL only requires the affirmative vote of a majority of the shareholders; however, many public company charters limit removal of directors to a removal for cause; and (v) our articles may be amended by resolution of our directors to alter our authorized share structure, including to (a) consolidate or subdivide any of our shares and (b) create additional classes or series of shares, whereas under DGCL, a majority vote by shareholders is generally required to amend a corporation’s certificate of incorporation and a separate class vote may be required to authorize alterations to a corporation’s authorized share structure. We cannot predict if investors will find our common shares less attractive because of these material differences. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our share price may be more volatile.
|
Item 1B. |
Unresolved Staff Comments |
None.
|
Item 2. |
Properties |
Our corporate headquarters are located in San Diego, California. The facility we lease encompasses approximately 2,002 square feet of office space. The lease for this facility expires in May 2018. We believe that our facility is sufficient to meet our needs and that suitable additional space will be available as and when needed.
|
Item 3. |
Legal Proceedings |
We are not currently party to any material legal proceedings.
|
Item 4. |
Mine Safety Disclosures |
None.
Part II.
|
Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Our common shares are currently traded on The NASDAQ Capital Market under the symbol “SPHS.”
The following table sets forth the high and low sales prices for our common shares for the period January 1, 2015 through December 31, 2016.
|
2015 |
High |
Low |
||||||
|
First Quarter |
$ | 1.04 | $ | 0.42 | ||||
|
Second Quarter |
1.64 | 0.54 | ||||||
|
Third Quarter |
1.13 | 0.71 | ||||||
|
Fourth Quarter |
3.85 | 0.67 | ||||||
|
2016 |
||||||||
|
First Quarter |
$ | 2.41 | $ | 1.35 | ||||
|
Second Quarter |
2.33 | 0.80 | ||||||
|
Third Quarter |
8.55 | 2.05 | ||||||
|
Fourth Quarter |
3.30 | 2.20 | ||||||
Holders of Record
As of March 13, 2017, there were approximately 10 shareholders of record of our common shares, which included Cede & Co., a nominee for Depository Trust Company, or DTC, and CDS & Co., a nominee for The Canadian Depository for Securities Ltd., or CDS. Common shares that are held by financial institutions as nominees for beneficial owners are deposited into participant accounts at either DTC or CDS, and are considered to be held of record by Cede & Co. or CDS & Co. as one shareholder.
Dividends
We have not paid any cash dividends on our common shares since inception and do not anticipate paying cash dividends in the foreseeable future. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business.
Securities Authorized for Issuance under Equity Compensation Plans
Information about our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K.
Repurchases of Equity Securities
There were no repurchases of equity securities during the fourth quarter of 2016.
|
Item 6. |
Selected Financial Data |
The following data has been derived from our audited financial statements, including the consolidated balance sheets at December 31, 2016 and 2015 and the related consolidated statements of operations and comprehensive loss for the three years ended December 31, 2016 and related notes appearing elsewhere in this Annual Report on Form 10-K. The statement of operations data for the years ended December 31, 2012 and 2013 and the balance sheet data as of December 31, 2014, 2013 and 2012 are derived from our audited consolidated financial statements that are not included in this Annual Report on Form 10-K. You should read the selected financial data set forth below in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this Annual Report on Form 10-K.
|
For the Years Ended December 31, |
||||||||||||||||||||
|
2016 |
2015 |
2014 |
2013 |
2012 |
||||||||||||||||
|
(In thousands, except per share data) |
||||||||||||||||||||
|
Consolidated Statement of Operations Data: |
||||||||||||||||||||
|
Revenues |
$ | — | $ | — | $ | — | $ | 5,000 | $ | — | ||||||||||
|
Operating expenses: |
||||||||||||||||||||
|
Research and development |
3,538 | 9,862 | 24,708 | 10,279 | 13,523 | |||||||||||||||
|
General and administrative |
6,768 | 3,626 | 5,332 | 4,511 | 5,685 | |||||||||||||||
|
Total operating expenses |
10,306 | 13,488 | 30,040 | 14,790 | 19,208 | |||||||||||||||
|
Loss from operations |
(10,306 |
) |
(13,488 |
) |
(30,040 |
) |
(9,790 |
) |
(19,208 |
) | ||||||||||
|
Other income (expense): |
||||||||||||||||||||
|
Interest expense |
(373 |
) |
(690 |
) |
(726 |
) |
(1,308 |
) |
(1,988 |
) | ||||||||||
|
Interest income |
37 | 22 | 51 | — | 108 | |||||||||||||||
|
Gain (loss) on revaluation of warrant liability |
(330 |
) |
— | 49 | 689 | — | ||||||||||||||
|
Loss on extinguishment of debt |
(180 |
) |
— | — | — | — | ||||||||||||||
|
Other expense, net |
(12 |
) |
(41 |
) |
(46 |
) |
(240 |
) |
(106 |
) | ||||||||||
|
Total other expense |
(858 |
) |
(709 |
) |
(672 |
) |
(859 |
) |
(1,986 |
) | ||||||||||
|
Net loss before income tax expense |
(11,164 |
) |
(14,197 |
) |
(30,712 |
) |
(10,649 |
) |
(21,194 |
) | ||||||||||
|
Income tax expense |
— | — | — | (500 |
) |
— | ||||||||||||||
|
Net loss |
$ | (11,164 |
) |
$ | (14,197 |
) |
$ | (30,712 |
) |
$ | (11,149 |
) |
$ | (21,194 |
) | |||||
|
Basic and diluted net loss per common share(1) (2) |
$ | (0.49 |
) |
$ | (0.84 |
) |
$ | (1.85 |
) |
$ | (1.39 |
) |
$ | (6.94 |
) | |||||
|
Weighted average shares used to calculate net loss per common share (1) (2) |
23,002 | 16,881 | 16,586 | 8,029 | 3,054 | |||||||||||||||
|
(1) |
See Note 4 of our Notes to the Consolidated Financial Statements for an explanation of the method used to calculate the basic and diluted net loss per common share and the number of shares used in the computation of the per share amounts. |
|
(2) |
Reflects the 52-for-1 share consolidation of our common shares for the years ending December 31, 2013 and 2012. |
|
As of December 31, |
||||||||||||||||||||
|
2016 |
2015 |
2014 |
2013 |
2012 |
||||||||||||||||
|
(In thousands) |
||||||||||||||||||||
| Consolidated Balance Sheet Data: | ||||||||||||||||||||
|
Cash, cash equivalents and securities available-for-sale |
$ | 29,001 | $ | 8,381 | $ | 22,695 | $ | 48,149 | $ | 9,721 | ||||||||||
|
Working capital |
27,754 | 5,610 | 19,998 | 41,267 | 815 | |||||||||||||||
|
Total assets |
29,998 | 8,892 | 25,591 | 51,892 | 11,529 | |||||||||||||||
|
Promissory notes, including current portion |
— | 5,343 | 5,941 | 6,877 | 12,021 | |||||||||||||||
|
Warrant liability |
13,396 | — | — | 883 | — | |||||||||||||||
|
Stock-based compensation liability |
57 | 168 | 22 | 202 | — | |||||||||||||||
|
Accumulated deficit |
(140,920 |
) |
(129,756 |
) |
(115,559 |
) |
(84,847 |
) |
(73,698 |
) | ||||||||||
|
Total shareholders’ equity (deficit) |
14,324 | 1,906 | 14,688 | 40,279 | (5,105 |
) | ||||||||||||||
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
You should read the following discussion and analysis in conjunction with “Item 8. Financial Statements and Supplementary Data” included below in this Annual Report on Form 10-K. Operating results are not necessarily indicative of results that may occur in future periods.
This discussion and analysis contains forward-looking statements that involve a number of risks, uncertainties and assumptions. Actual events or results may differ materially from our expectations. Important factors that could cause actual results to differ materially from those stated or implied by our forward-looking statements include, but are not limited to, those set forth in “Item 1A. Risk Factors” in this Annual Report on Form 10-K. All forward-looking statements included in this Annual Report on Form 10-K are based on information available to us as of the time we file this Annual Report on Form 10-K and, except as required by law, we undertake no obligation to update publicly or revise any forward-looking statements.
All dollar amounts are expressed in U.S. dollars unless otherwise noted. All amounts converted from Canadian dollars to U.S. dollars are calculated using the conversion rate as of December 31, 2016 unless otherwise noted.
Overview
Background
We are a clinical-stage biopharmaceutical company focused on developing innovative products for the treatment of urological diseases. We are headquartered in San Diego, California and our common shares currently trade on The NASDAQ Capital Market. We are currently developing topsalysin (PRX302) as a treatment for the lower urinary tract symptoms of benign prostatic hyperplasia, or BPH, commonly referred to as an enlarged prostate and as a treatment for clinically significant localized prostate cancer. In 2004, we licensed exclusive rights to topsalysin from UVIC Industry Partnerships Inc., or UVIC, and The Johns Hopkins University, or Johns Hopkins, for the treatment of prostate cancer and in 2009, we licensed exclusive rights to topsalysin from UVIC and Johns Hopkins for the treatment of the symptoms of BPH. In April 2010, we entered into an exclusive license agreement with Kissei Pharmaceuticals Co., Ltd., or Kissei, pursuant to which we granted Kissei the right to develop and commercialize topsalysin in Japan for the treatment of the symptoms of BPH, prostate cancer, prostatitis or other diseases of the prostate.
Topsalysin, a genetically modified recombinant protein, is delivered via ultrasound-guided injection directly into the prostate. This membrane-disrupting protein is selectively activated by enzymatically active prostate specific antigen, or PSA, which is only present in the prostate, leading to localized cell death and tissue disruption without damage to neighboring tissue and nerves. This method of administration limits the circulation of the drug in the body, and we believe that this limited systemic exposure to the drug, together with how the drug is activated in the prostate, greatly diminishes the risk of side effects.
In May 2015, we initiated a single-center, open-label Phase 2a proof of concept clinical trial of topsalysin for the treatment of localized low to intermediate risk prostate cancer. We believe that the highly targeted mechanism by which topsalysin selectively destroys prostate tissue in BPH makes topsalysin a potential targeted focal treatment for localized prostate cancer. The clinical trial utilized previously obtained magnetic resonance imaging, or MRI, images of each patient’s prostate mapped to real time 3D ultrasound to target the delivery of topsalysin directly into and around a pre-identified clinically significant tumor. A clinically significant tumor was defined in our study as, either a Gleason score 6 (pattern 3+3) and >3mm Maximum Cancer Core Length, or MCCL, or Gleason score 7 (pattern 3+4 or 4+3) < 10 mm MCCL, which is thought to have the potential to progress and would therefore warrant treatment. (A Gleason pattern is a grading system utilized to describe how aggressive a prostate tumor is and how likely it is to spread. Generally, there are five recognized Gleason histological patterns and a higher Gleason pattern indicates a more aggressive tumor.) Patients received a transperineal administration of topsalysin under general anesthesia at a dose higher than used in our completed Phase 3 BPH PLUS-1 trial but less than the highest dose used in our previous prostate cancer trial. The primary objective of the trial was to assess the safety and tolerability of topsalysin when used to selectively target and focally ablate a clinically significant tumor. The potential efficacy was evidenced by histological changes, indicating tumor ablation at six months following treatment. The clinical trial was conducted at a single center, the University College London, which is well known for the focal treatment of prostate cancer in the United Kingdom.
A total of 18 patients with localized low to intermediate risk prostate cancer were enrolled in the Phase 2a proof of concept clinical trial. On June 9, 2016, we announced the biopsy results from all 18 patients enrolled in the Phase 2a proof-of-concept study of topsalysin for the treatment of localized prostate cancer. The one-time administration of topsalysin was well tolerated with no serious adverse events and no new safety signals being reported. Topsalysin demonstrated an ability to ablate tumor cells in 50 percent of patients (9/18 patients) six months after treatment in a patient population with pre-identified, clinically significant prostate cancer. In preparation for the presentation of the Phase 2a proof of concept clinical trial data for an upcoming medical conference, we recently determined that 2 patients who were initially reported as having no response to treatment should have been reported as having a partial response to treatment. Taking into account the updated results, topsalysin demonstrated an ability to ablate tumor cells in more than 60 percent of patients (11/18 patients) six months after treatment in a patient population with pre-identified, low to intermediate risk prostate cancer.
All 18 patients enrolled completed the study. Biopsy data at six months following treatment showed that:
|
|
● |
Two patients experienced complete ablation of their targeted tumor with no evidence of any tumor remaining at six months; |
|
|
● |
Nine patients experienced a partial response, defined as either a reduction in the maximum cancer core length or a reduction in Gleason pattern; and |
|
|
● |
Seven patients had no response to treatment. |
Detailed results from this study will be presented at a future medical conference.
In March 2017, we initiated a second Phase 2 clinical trial to confirm the dose and optimize the delivery of topsalysin for the treatment of clinically significant localized prostate cancer. This study will also utilize previously obtained MRI images of each patient’s prostate mapped to real time 3D ultrasound to target the delivery of topsalysin directly into and around a pre-identified clinically significant tumor. The primary objective of the study is safety and tolerability of an injection of topsalysin and the key efficacy variable is focal ablation of a clinically significant lesion on biopsy after six months. Approximately 40 patients will be enrolled across clinical sites in the UK and US. Patient screening into the study.
We expect to receive biopsy data for all patients conducted six months after the initial dose in late 2017 or early 2018. Based upon the results of the 6-month biopsy, the study includes an option to potentially re-treat the targeted lesion area with a second dose of topsalysin, with a targeted biopsy to occur six months following the second dose. We expect to have final biopsy data on all patients who receive a second dose by the third quarter of 2018.
We have also completed the first of two Phase 3 clinical trials that we believe would be required to obtain marketing approval for topsalysin for the treatment of the symptoms of BPH. In October 2013 we initiated our first Phase 3 clinical trial, which we refer to as the “PLUS-1” trial, of topsalysin for the treatment of the lower urinary tract symptoms of BPH. The Phase 3 “PLUS-1” trial was an international, multicenter, randomized, double-blind, and vehicle-controlled trial to assess the efficacy and safety of a single intraprostatic administration of topsalysin (0.6 µg/g prostate) for the treatment of the lower urinary symptoms of BPH. Patients were randomized on a 1:1 ratio to either topsalysin or vehicle-only injection, and then monitored for one year. A total of 479 patients with moderate to severe BPH were enrolled and randomized by September 2014. On November 10, 2015, we announced final results from this trial. Topsalysin demonstrated a statistically significant improvement in International Prostate Symptom Score, IPSS, total score from baseline over 12 months compared to the vehicle-only control group (7.60 vs. 6.58 point overall improvement; p = 0.043), the primary endpoint of the trial. (IPSS is a patient recorded, composite assessment that takes into account factors such as ability to empty the bladder, frequency of urination, intermittency of urination, urgency of urination, weak strength of urine stream, straining while urinating, and having to urinate at night after going to bed.) Topsalysin continues to demonstrate a favorable safety profile, with no evidence of any treatment related sexual or cardiovascular side effects.
We are currently not planning on pursuing a second Phase 3 trial in BPH, unless we secure a development partner to fund such new clinical trial or obtain other financing. There can be no assurance that such funding or a development partner will be available on acceptable terms or at all. For that reason, we cannot currently estimate when the clinical development required to seek the regulatory approvals needed to commercialize topsalysin for the treatment of the symptoms of BPH will be completed.
We completed a reduction in workforce in May 2016 through which five of our ten employees were terminated. We incurred a charge of approximately $81,000 during the year ended December 31, 2016, which is included in operating expenses, related to cash severance and continuation of benefits in connection with the workforce reduction. No additional cash payments are expected to be made related to this reduction in workforce. In addition, we incurred a non-cash stock-based compensation charge of approximately $76,000 associated with the modification of stock options for individuals included in the reduction in workforce.
On May 11, 2016, we completed an offering in which we raised net proceeds of approximately $4.6 million by selling 3,571,428 common shares at a price of $1.40 per share. Additionally, for each common share purchased, the investors received a warrant to purchase one-half of a common share at an exercise price of $1.40 per full share, exercisable for a five-year period. During the year ended December 31, 2016, 1,775,714 of these warrants were exercised which generated proceeds of $2.5 million. We will use the proceeds of this offering for working capital and general corporate purposes.
On May 12, 2016, we announced that we had engaged Oppenheimer & Co. Inc. as our financial advisor to assist with the evaluation of various strategic alternatives.
On August 26, 2016, we completed a public offering in which we issued 7,475,000 common shares at a price of $4.00 per share. We received $27.4 million, net of underwriters’ discounts, commissions and offering costs. Additionally, for each common share purchased, the investors received a warrant to purchase 0.75 of a common share at an exercise price of $4.00 per full share, exercisable during a five-year period. We intend to use the net proceeds of this offering to fund our second Phase 2 clinical trial for the treatment of localized prostate cancer. In addition, we used approximately $4.2 million of the proceeds to retire debt and will use the remaining proceeds of this offering for working capital and general corporate purposes, which may include research and development expenses, general and administrative expenses and manufacturing expenses.
Kissei Pharmaceuticals License Agreement
In April 2010, the Company entered into an exclusive license agreement for the development and commercialization of topsalysin (and other products covered by the licensed patent). The agreement with Kissei Pharmaceuticals Co., Ltd., a Japanese pharmaceutical company, or Kissei covers the development and commercialization of topsalysin in Japan for the treatment of the symptoms of BPH, prostate cancer, prostatitis or other diseases of the prostate. Pursuant to the agreement in 2010, the Company received an upfront license payment of $3.0 million. The Company has determined that the deliverables under this agreement included the license, the transfer of relevant technical information and participation in a periodic development meeting. The Company recognized the entire upfront license payment upon receipt as the license was deemed to have stand-alone value and no significant undelivered performance obligations were identified in connection with the license.
During the year ended December 31, 2013, the Company recorded as revenue a $5.0 million non-refundable substantive milestone payment due from Kissei upon the achievement of certain development activities during this period. In accordance with the Company’s revenue recognition policy, the Company recognizes the receipt of milestone payments in accordance with the milestone method in the period in which the underlying triggering event occurs.
Topsalysin License Agreement for Prostate Cancer
In 2004, we licensed exclusive rights to topsalysin for the treatment of prostate cancer under an agreement with UVIC and Johns Hopkins. We have agreed to make cumulative milestone payments over the lifecycle of topsalysin of up to CND$3.6 million, or $2.6 million, as converted, on the achievement of certain clinical and regulatory milestones and to pay royalties on commercial sales of resulting products. From the inception of the agreement, we have paid milestone payments of CND$0.1 million, or $0.1 million, applying the historical conversion rate at each payment date and at December 31, 2016, CND$0.1 million is included in accrued expenses. We have to date completed three clinical trials in patients with prostate cancer.
Topsalysin License Agreement for BPH
In 2009, we licensed exclusive rights to topsalysin under an agreement with UVIC and Johns Hopkins with respect to the use of topsalysin for the treatment of the symptoms of BPH and other non-cancer diseases and conditions of the prostate, with the exception of prostate cancer. The license agreement requires us to make payments of CND$1.3 million, or $0.9 million, as converted, on the achievement of certain clinical and regulatory milestones and to pay royalties on commercial sales of resulting products. During the year ended December 31, 2013, we expensed a $0.1 million milestone payment due under the license agreement upon the completion of our last Phase 2b clinical trial prior to commencing a Phase 3 clinical trial. This amount was recorded as research and development expense. In addition, in the second quarter of 2013 we paid UVIC and Johns Hopkins a sub-license royalty of $0.4 million payable under the license agreement associated with our $5.0 million milestone payment from Kissei. This amount was recorded as a component of research and development expense. From the inception of the agreement, we have incurred sub-license fees of $0.6 million and milestone payments of $0.1 million under this agreement.
Financial Operations Overview
Revenues
Our revenues to date consist of a $3.0 million up-front payment received from Kissei in 2010 and a $5.0 million non-refundable milestone payment for our achievement of certain development activities in 2013. We have no products approved for sale, and we have not generated any revenues from product sales.
Other than potential development milestones from Kissei, we do not expect to receive any revenues from topsalysin until we obtain regulatory approval and commercialize such product or until we potentially enter into additional collaborative agreements with third parties for the development and commercialization of topsalysin, which additional agreements will not likely occur until we complete the development of topsalysin. If our development efforts for topsalysin, or the efforts of Kissei or any future collaborator, result in clinical success and regulatory approval or collaboration agreements with other third parties, we may generate revenues from topsalysin. However, we may never generate revenues from topsalysin as we or any collaborator may never succeed in obtaining regulatory approval or commercializing this product.
Research and Development Expenses
Research and development expenses can be driven by a number of factors including: (a) the scope of clinical development and research programs pursued; (b) the type and size of clinical trials undertaken; (c) the number of clinical trials that are active during a particular period of time; (d) the rate of patient enrollment; (e) regulatory activities to support the clinical programs; and (f) Chemistry, Manufacturing and Controls, or CMC, activities associated with process development, scale-up and manufacture of drugs used in clinical trials; and such expenses are ultimately a function of decisions made to continue the development and testing of a product candidate based on supporting safety and efficacy results from clinical trial.
The majority of our operating expenses to date have been incurred in research and development activities related to topsalysin. Research and development expenses include:
|
|
• |
external research and development expenses incurred under agreements with clinical research organizations, or CROs, and investigative sites and clinical trial costs, as well as payments to consultants; |
|
|
• |
employee related expenses, including salaries, benefits, travel and stock-based compensation expense; |
|
|
• |
third-party manufacturing expenses; and |
|
|
• |
facilities, depreciation and other allocated expenses. |
We expense research and development costs as incurred. We account for nonrefundable advance payments for goods and services that will be used in future research and development activities as expenses when the service has been performed or when the goods have been consumed. Since our inception in January 2002 through December 31, 2016, we have incurred a total of $98.0 million in research and development expenses.
At this time, due to the risks inherent in the clinical trial process and given the stage of our product development program, we are unable to estimate with any certainty the costs we will incur in the continued development of topsalysin for potential approval and commercialization in two indications. Clinical development timelines, the probability of success and development costs can differ materially from expectations. However, we do expect our research and development expenses to continue for the foreseeable future as we advance topsalysin through clinical development. The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. Any failure by us or delay in completing clinical trials, or in obtaining regulatory approvals, could lead to increased research and development expense and, in turn, have a material adverse effect on our results of operations.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related benefits including stock-based compensation. Other general and administrative expenses include allocated facility-related costs not otherwise included in research and development expenses, travel expenses, market research expenses and professional fees for auditing, tax, investor relations and legal services. We expect general and administrative expenses to remain fairly consistent over the next year but we do expect that general and administrative expenses will increase as we move towards commercialization of our drug candidates in future periods.
Interest Expense
Interest expense primarily represents interest payable to Oxford Finance, LLC, or Oxford, amortization of our debt discount and issuance costs associated with Oxford related financings. On September 2, 2016, we repaid the outstanding balance of the Oxford Loan and Security Agreement in full.
Interest Income
We earn interest income from interest-bearing cash and investment accounts.
Gain (Loss) on Revaluation of Warrant Liability
In connection with the offering completed on May 11, 2016, we issued 1,785,714 warrants to purchase our common shares. These warrants may require us to pay the warrant holder cash under certain provisions of the warrant and therefore we account for these warrants as a liability. As a result of these warrants being classified as a liability, we are required to calculate their fair value at each reporting date. The fair value of these warrants is calculated utilizing a Black-Scholes pricing model. We calculated the initial fair value of these warrants on May 11, 2016, the date the warrants were issued. On various dates from May 11, 2016 through December 31, 2016, the warrant holders exercised 1,775,714 warrants and as a result we revalued the fair value of the underlying warrants on each exercise date. The fair value of the exercised warrants was reclassified from the warrant liability to contributed surplus upon exercise. As of December 31, 2016, only 10,000 warrants remain outstanding from the May 11, 2016 offering for which the fair value was remeasured as of December 31, 2016.
In connection with the offering completed on August 26, 2016, we issued 5,606,250 warrants to purchase our common shares. These warrants may require us to pay the warrant holder cash under certain provisions of the warrant and therefore we account for these warrants as a liability. As a result of these warrants being classified as a liability, we are required to calculate the fair value of these warrants at each reporting date. The fair value of these warrants is calculated utilizing a Black-Scholes pricing model. We calculated the initial fair value of these warrants on August 26, 2016, the date the warrants were issued. As of December 31, 2016, the fair value was remeasured.
Loss on the Early Extinguishment of Debt
On September 2, 2016, we repaid the outstanding balance of the Oxford Loan and Security Agreement in full. We made a total payoff payment of $4.2 million to Oxford, which included the final payment of $300,000, a prepayment fee of $39,000, accrued interest of $2,000 and legal fees of $4,000. We had $159,000 of unamortized debt premium as of the date of the payoff. The debt repayment was accounted for as an extinguishment as per ASC 470-50, “Debt: Modification and Extinguishments”, and a loss on early extinguishment of the debt totaling $180,000 was recorded for the ended December 31, 2016, consisting of the final payment and the prepayment fee which was offset by the unamortized debt premium.
Other Expense, Net
Other expense consists primarily of foreign exchange gains and losses and on occasion income or expense of an unusual nature. Foreign exchange gains and losses result from the settlement of foreign currency transactions and from the remeasurement of monetary assets and liabilities denominated in currencies other than our functional currency.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in conformity with generally accepted accounting principles in the United States. The preparation of our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the revenues and expenses incurred during the reported periods. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in the notes to our consolidated financial statements appearing at the end of this Annual Report on Form 10-K, we believe that the following accounting policies are critical to understanding and evaluating our reported financial results.
Revenue Recognition
We may enter into product development agreements with collaborative partners for the research and development of products for the treatment of urological diseases. The terms of the agreements may include nonrefundable signing and licensing fees, milestone payments and royalties on any product sales derived from collaborations. These multiple element arrangements are analyzed to determine whether the deliverables can be separated or whether they must be accounted for as a single unit of accounting. License fees are recognized as revenue when persuasive evidence of an arrangement exists, the fee is fixed or determinable, delivery or performance has substantially completed and collection is reasonably assured.
We recognize up front license payments as revenue upon delivery of the license only if the license has stand-alone value to the customer and if the agreement includes a general right of return, the delivery or performance of undelivered items is considered probable and within our control. The payment is generally allocated to the separate units of accounting based on their relative selling prices. The selling price of each deliverable is determined using vendor specific objective evidence of selling prices, if it exists; otherwise, third-party evidence of selling prices. If neither vendor specific objective evidence nor third-party evidence exists, we use our best estimate of the selling price for each deliverable. The payment allocated is limited to the amount that is not contingent on the delivery of additional items or fulfillment of other performance conditions.
Whenever we determine that an arrangement should be accounted for as a single unit of accounting, it must determine the period over which the performance obligations will be performed and revenue recognized. If we cannot reasonably estimate the timing and the level of effort to complete its performance obligations under the arrangement, then revenue under the arrangement is recognized on a straight-line basis over the period we expect to complete the performance obligations.
We evaluate milestone payments on an individual basis and recognizes revenue from non-refundable milestone payments when the earnings process is complete and the payment is reasonably assured. Non-refundable milestone payments related to arrangements under which we have continuing performance obligations are recognized as revenue upon achievement of the associated milestone, provided that (i) the milestone event is substantive and its achievability was not reasonably assured at the inception of the agreement and (ii) the amount of the milestone payment is reasonable in relation to the effort expended or the risk associated with the milestone event. Any amounts received under agreements in advance of performance, if deemed substantive, are recorded as deferred revenue and recognized as revenue as we complete our performance obligations. A milestone event is considered substantive if (i) the milestone is commensurate with either (a) our performance to achieve the milestone or (b) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from our performance to achieve the milestone; (ii) it relates solely to past performance and (iii) it is reasonable relative to all of the deliverables and payment terms (including other potential milestone consideration) within the arrangement. If any portion of the milestone payment does not relate to our performance, does not relate solely to past performance or is refundable or adjustable based on future performance, the milestone is not considered to be substantive. Milestone payments are not bifurcated into substantive and non-substantive components. Payments related to the achievement of non-substantive milestones is deferred and recognized over our remaining performance period.
Royalty revenue will be recognized upon the sale of the related products provided that we have no remaining performance obligations under the arrangement.
Accrued Research and Development Expenses
Clinical trial costs are recorded as a component of research and development expenses. We accrue and expense clinical trial activities performed by third parties based upon estimates of the percentage of work completed of the total work over the life of the individual study in accordance with agreements established with clinical research organizations and clinical trial sites. We determine the estimates through discussions with internal clinical personnel and external service providers as to the progress or stage of completion of trials or services and the agreed-upon fee to be paid for such services based on facts and circumstances known by us as of each balance sheet date. However, actual costs and timing of clinical trials are highly uncertain, subject to risks and may change depending upon a number of factors, including our clinical development plan.
If the actual timing of the performance of services or the level of effort varies from the estimate, we will adjust the accrual accordingly. Adjustments to prior period estimates have not been material.
Examples of estimated accrued research and development expenses include:
|
|
• |
fees to clinical research organizations in connection with clinical studies; |
|
|
• |
fees to investigative sites in connection with clinical studies; |
|
|
• |
fees to vendors in connection with preclinical development activities; |
|
|
• |
fees to vendors associated with the development of companion diagnostics; and |
|
|
• |
fees to vendors related to product manufacturing, development and distribution of clinical supplies. |
Nonrefundable advance payments for goods and services that will be used or rendered in future research and development activities are recorded as a prepaid expense and recognized as expense in the period that the related goods are consumed or services are performed.
Essentially all of our research and development expenses related to topsalysin during the years ended December 31, 2016, 2015 and 2014. We recognized research and development expenses as follows (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Clinical research and development |
$ | 2,974 | $ | 8,866 | $ | 20,604 | ||||||
|
Pre-clinical research and development |
— | 1 | 2 | |||||||||
|
Manufacturing |
421 | 738 | 3,452 | |||||||||
|
Stock-based compensation expense |
143 | 257 | 650 | |||||||||
| $ | 3,538 | $ | 9,862 | $ | 24,708 | |||||||
Warrant Liability
In connection with the offering we completed on May 11, 2016, we issued 1,785,714 warrants to purchase common shares. These warrants may require us to pay the warrant holder cash under certain provisions of the warrant and therefore we are accounting for these warrants as a liability. As a result of these warrants being classified as a liability, we are required to calculate the fair value of these warrants at each reporting date. The fair value of these warrants is calculated utilizing a Black-Scholes pricing model. We calculated the initial fair value of these warrants on May 11, 2016, the date the warrants were issued. On various dates, warrant holders exercised 1,775,714 warrants and as a result we were required to revalue the fair value of the underlying warrants on the various exercise dates. The calculated fair value for the exercised warrants was reclassified from the warrant liability to contributed surplus. Fair value of warrants was remeasured on December 31, 2016. The following inputs were utilized in the Black-Scholes pricing model during the year ended December 31, 2016:
|
Initial Fair Value May 11, 2016 |
Weighted Average at Various Exercise Dates |
December 31, 2016 |
||||||||||
|
Stock price |
$ | 1.12 | $ | 3.50 | $ | 2.80 | ||||||
|
Exercise price |
$ | 1.40 | $ | 1.40 | $ | 1.40 | ||||||
|
Risk-free interest rate |
1.20 |
% |
1.05 |
% |
1.78 |
% | ||||||
|
Volatility |
130.64 |
% |
132.05 |
% |
144.25 |
% | ||||||
|
Dividend yield |
0.00 |
% |
0.00 |
% |
0.00 |
% | ||||||
|
Expected life in years |
5.00 | 4.85 | 4.36 | |||||||||
|
Calculated fair value per warrant |
$ | 0.95 | $ | 3.20 | $ | 2.55 | ||||||
In connection with the offering we completed on August 26, 2016, we issued 5,606,250 warrants to purchase common shares. These warrants may require us to pay the warrant holder cash under certain provisions of the warrant and therefore we are accounting for these warrants as a liability. As a result of these warrants being classified as liabilities, we are required to calculate the fair value of these warrants at each reporting date. The fair value of these warrants are calculated utilizing a Black-Scholes pricing model. We calculated the initial fair value of these warrants on August 26, 2016, the date the warrants were issued. Fair value of the warrants was remeasured on December 31, 2016. The following inputs were utilized in the Black-Scholes pricing model during the year ended December 31, 2016:
|
August 26, 2016 |
December 31, 2016 |
|||||||
|
Stock price |
$ | 3.52 | $ | 2.80 | ||||
|
Exercise price |
$ | 4.00 | $ | 4.00 | ||||
|
Risk-free interest rate |
1.23 |
% |
1.85 |
% | ||||
|
Volatility |
135.04 |
% |
140.47 |
% | ||||
|
Dividend yield |
0.00 |
% |
0.00 |
% | ||||
|
Expected life in years |
5.00 | 4.65 | ||||||
|
Calculated fair value per warrant |
$ | 3.04 | $ | 2.38 | ||||
Certain inputs utilized in our Black-Scholes fair value calculation may fluctuate in future periods based upon factors which are outside of the Company’s control. A significant change in one or more of these inputs used in the calculation of the fair value may cause a significant change to the fair value of our warrant liability which could also result in material non-cash gain or loss being reported in our consolidated statement of operations and comprehensive loss. A 10% change in our closing stock price on December 31, 2016 would result in a $1.4 million change to the fair value of our warrant liability at December 31, 2016. A 10% change in our stock price volatility at December 31, 2016 would result in a change of $0.8 million to our warrant liability at December 31, 2016. A 10% change in the risk-free interest rate at December 31, 2016 would not have a material effect on the fair value of our warrant liability at December 31, 2016.
Stock-based Compensation
We expense the fair value of employee stock options over the vesting period. Stock-based compensation expense is measured using the fair value of the award at the grant date, net of estimated forfeitures, and is adjusted annually to reflect actual forfeitures. The fair value of each stock-based award is estimated using the Black-Scholes pricing model and is expensed using graded vesting over the vesting period.
We recognized stock-based compensation expense as follows (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Research and development |
$ | 143 | $ | 257 | $ | 650 | ||||||
|
General and administrative |
282 | 519 | 1,441 | |||||||||
|
Total |
$ | 425 | $ | 776 | $ | 2,091 | ||||||
As of December 31, 2016 there were $2.1 million of unrecognized compensation costs related to non-vested stock options. As of December 31, 2016 we expect to recognize those costs over weighted average period of 1.6 years.
The fair value of options granted during the year ended December 31, 2016, 2015 and 2014 were estimated at the date of grant using the following assumptions:
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Expected life of the option term (years) |
3.9 | 3.5 | 3.7 | |||||||||
|
Risk-free interest rate |
1.5 |
% |
1.0 |
% |
1.2 |
% | ||||||
|
Dividend yield |
0.0 |
% |
0.0 |
% |
0.0 |
% | ||||||
|
Volatility |
144.0 |
% |
128.4 |
% |
76.2 |
% | ||||||
Expected Life of the Option Term – This is the period of time that the options granted are expected to remain unexercised. Options granted during the year have a contractual term of five to ten years. We estimate the expected life of the option term based on actual past behavior for similar options.
Risk-Free Interest Rate – This is the Canadian or United States Treasury rate, as applicable, for the week of each option grant during the year having a term that most closely resembles the expected life of the option.
Dividend Yield – We have never declared or paid dividends on common shares and have no plans to do so in the foreseeable future.
Volatility – Volatility is a measure of the amount by which a financial variable such as a share price has fluctuated or is expected to fluctuate during a period. We considered the historical volatility from our Canadian initial public offering through the dates of grants.
Prior to the Company’s IPO, the Company had issued its stock options with a Canadian dollar denominated exercise price. Subsequent to the Company’s IPO, the Company issues its stock options with a U.S. dollar denominated exercise price.
Effective November 13, 2013, the Company voluntarily delisted from the Toronto Stock Exchange, or TSX. As a result of the delisting from the TSX and the change in the Company’s functional currency to the U.S. dollar, the stock options granted with exercise prices denominated in Canadian dollars are considered dual indexed as defined in ASC 718, “Compensation, Stock Compensation”. As a result, the Company is required to account for these stock options as a liability. Historically these options had been accounted for as equity. The estimated fair value is determined using the Black-Scholes pricing model based on the estimated value of the underlying common shares at the valuation measurement date, the remaining service period of the stock options, risk-free interest rates, expected dividends and expected volatility of the price of the underlying common shares. As of November 13, 2013, the Company calculated the initial fair value of the vested awards of $163,000. This fair value was initially recorded as a deduction from Contributed Surplus. At each reporting period subsequent to November 13, 2013, the Company adjusts the fair value of the stock-based compensation liability and any corresponding increase or decrease to the stock-based compensation liability is recorded as an adjustment to Contributed Surplus and/or compensation expense on the consolidated statement of operations and comprehensive loss but in no case will the amount of stock-based compensation expense be less than the original grant date fair value of the stock options.
The fair value of the stock-based compensation liability was $57,000 and $168,000 at December 31, 2016 and 2015, respectively. As the calculated fair value of the stock options at December 31, 2016 was less than the original grant date fair value no additional compensation expense was recorded in the consolidated statement of operations and comprehensive loss. The change in the fair value of the stock-based compensation liability of $111,000 for the year ended December 31, 2016 was recorded as an adjustment to Contributed Surplus.
The following inputs were utilized in the Black-Scholes pricing model for the options granted with exercise prices denominated in Canadian dollars at December 31, 2016 and 2015:
|
December 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Stock price at the end of each reporting period |
$ | 2.80 | $ | 1.78 | ||||
|
Weighted average exercise price |
$ | 11.06 | $ | 13.12 | ||||
|
Risk-free interest rate |
0.85 |
% |
0.91 |
% | ||||
|
Volatility |
120.81 |
% |
182.74 |
% | ||||
|
Dividend yield |
0.00 |
% |
0.00 |
% | ||||
|
Expected life in years |
0.85 | 1.53 | ||||||
|
Calculated fair value per stock option |
$ | 0.33 | $ | 0.74 | ||||
Fair value of financial instruments
The Company measures certain financial assets and liabilities at fair value based on the exchange price that would be received for an asset or paid for to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. The carrying amounts of the Company’s financial instruments, including cash and cash equivalents and accounts payable and accrued expenses, approximate fair value due to their short maturities.
The Company follows ASC 820-10, “Fair Value Measurements and Disclosures,” which among other things, defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, a three-tier fair value hierarchy has been established, which prioritizes the inputs used in measuring fair value as follows:
Level 1 – Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date.
Level 2 – Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument’s anticipated life.
Level 3 – Inputs reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board, or FASB, issued new guidance related to revenue recognition (ASU No. 2014-09 Revenue from Contracts with Customers (Topic 606)). Subsequently the FASB has issued additional guidance (ASU Nos. 2015-14; 2016-08; 2016-10; 2016-12; 2016-20 Revenue from Contracts with Customers (Topic 606)). The guidance establishes principles for reporting information about the nature, amount, timing, and uncertainty of revenue and cash flows arising from an entity’s contracts with customers. The guidance is effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period. Early adoption is permitted only as of annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. Two methods of adoption are permitted: (a) full retrospective adoption, meaning the standard is applied to all periods presented or (b) modified retrospective adoption, meaning the cumulative effect of applying the new guidance is recognized as an adjustment to the opening retained earnings balance. We did not recognize any revenue from contracts with customers in the years ended December 31, 2016, 2015 and 2014. Although we are still evaluating the impact of the new standard, we anticipate that the impact will not be material to the consolidated financial statements as we do not currently generate revenues from contracts with customers.
In February 2016, the FASB issued ASU, No. 2016-02, “Leases (Topic 842)”. This guidance requires lessees to recognize a lease liability and a right-of-use asset with the exception of short-term leases. In addition, lessees are required to classify leases as either operating or finance based on current criteria of whether or not the lease is effectively a financed purchase by the lessee. The new standard is effective for the annual reporting period beginning after December 15, 2018 and early adoption is permitted. Although we are in the process of evaluating the impact of this guidance on its consolidated financial statements and related disclosures, we expect that our operating lease will be subject to the new standard and recognized as operating lease liabilities and right-of-use assets upon adoption.
In March 2016, the FASB issued ASU No. 2016-09, “Compensation – Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting”. This guidance is intended to simply the accounting for stock compensation. Several aspects of the accounting for share-based payment award transactions are simplified, including: (a) income tax consequences, (b) classification of awards as either equity or liabilities; and (c) classification on the statement of cash flows. The new standard is effective for annual periods beginning after December 15, 2016, and interim periods therein. This guidance may be applied either prospectively, retrospectively or using a modified retrospective transition method, depending on the area covered in this update. We will adopt ASU 2016-09 in 2017, and we do not anticipate this guidance to have a significant impact on our financial statements at the time of adoption.
In August 2016, the FASB issued ASU 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments”, addressing eight specific cash flow issues in an effort to reduce diversity in practice. The amended guidance is effective for fiscal years beginning after December 31, 2017, and for interim periods within those years. Early adoption is permitted. We are in the process of evaluating the impact of this guidance on our consolidated financial statements.
Net Operating Loss Carryforwards
As of December 31, 2016 we had Canadian and U.S. federal tax net operating loss carryforwards of $121.8 million and $1.4 million, respectively, which begin to expire in 2026 and 2034, respectively. As of December 31, 2016, we also had Canadian investment tax credits and U.S. federal research and development tax credits of $2.8 million and $1.6 million, respectively. The Canadian investment tax credits and U.S. federal research and development tax credits will begin to expire in 2017 and 2031, respectively.
JOBS Act
In April 2012, the JumpStart Our Business Startups Act of 2012, or the JOBS Act, was signed into law. The JOBS Act contains provisions that, among other things, reduce certain reporting requirements for an “emerging growth company.” As an “emerging growth company,” we are electing not to take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision not to take advantage of the extended transition period is irrevocable. In addition, we are in the process of evaluating the benefits of relying on the other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, if as an “emerging growth company” we choose to rely on such exemptions, we may not be required to, among other things, (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis), and (iv) disclose certain executive compensation-related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive Officer’s compensation to median employee compensation. These exemptions will apply for a period of five years following the completion of our initial public offering or until we no longer meet the requirements of being an “emerging growth company,” whichever is earlier.
Results of Operations
Comparison of the Years Ended December 31, 2016 and 2015
The following table summarizes the results of our operations for the year ended December 31, 2016 and 2015, together with the changes in those items in dollars (in thousands):
|
For the Years Ended December 31, |
Change 2016 vs. |
|||||||||||
|
2016 |
2015 |
2015 |
||||||||||
|
Research and development expenses |
$ | 3,538 | $ | 9,862 | (6,324 |
) | ||||||
|
General and administrative expenses |
6,768 | 3,626 | 3,142 | |||||||||
|
Interest expense |
(373 |
) |
(690 |
) |
317 | |||||||
|
Interest income |
37 | 22 | 15 | |||||||||
|
Loss on revaluation of warrant liability |
(330 |
) |
— | (330 |
) | |||||||
|
Loss on extinguishment of debt |
(180 |
) |
— | (180 |
) | |||||||
|
Other expense, net |
(12 |
) |
(41 |
) |
29 | |||||||
Research and development expenses. Research and development expenses were $3.5 million in the year ended December 31, 2016 compared to $9.9 million in the year ended December 31, 2015. The decrease in research and development costs is attributable to the following:
|
|
• |
a $5.9 million decrease in costs associated with our Phase 3 PLUS-1 clinical trial of topsalysin as the trial is completed, specifically a $3.9 million decrease in cost associated with our clinical research organizations, $1.3 million decrease for patient visits to our clinical site investigators as sites and a $0.7 million decrease primarily in costs associated with data tracking, consulting, clinical supplies, site inspections and travel of the Phase 3 PLUS-1 clinical trial; |
|
• |
a $0.6 million decrease in the costs associated with our completed Phase 2a proof of concept clinical trial for the treatment of localized low to intermediate risk prostate cancer; and |
|
|
• |
a $0.4 million decrease in the costs associated with the manufacturing activities for topsalysin. |
These decreases are partially offset by an increase of $0.3 million for costs associated with our Phase 2b for the treatment of localized prostate cancer, an increase of $0.1 million in personnel related costs and an increase of $0.1 million milestone payment due to UVIC and Johns Hopkins for the completion of Phase 1 clinical activities associated topsalysin for prostate cancer.
General and administrative expenses. General and administrative expenses were $6.8 million in the year ended December 31, 2016 compared to $3.6 million for the year ended December 31, 2015. The increase is primarily due to the inclusion of $1.6 million in closing costs which were allocated to the warrants issued in our registered direct transaction and public offering, both completed in the year ending December 31, 2016, a $0.8 million increase in personnel related costs and a $0.7 million in increases for legal, accounting, consulting and professional services.
Interest expense. Interest expense was $0.4 million in the years ended December 31, 2016 compared to $0.7 million for the year ended December 31, 2015. Interest expense is related to the Company’s promissory notes with Oxford. The Company repaid the outstanding principal balance in September 2016.
Interest income. Interest income was $37,000 for the year ended December 31, 2016 compared to $22,000 for the year ended December 31, 2015. The increase is due to the increase in the average balances of the interest-bearing cash and investment accounts period over period.
Loss on revaluation of warrant liability. Loss on revaluation of the warrant liability was $0.3 million for the year ended December 31, 2016. The non-cash loss is associated with the change in the fair value of our warrant liability.
Loss on early extinguishment of debt. Loss on early extinguishment of debt was $0.2 million for the year ended December 31, 2016. This consists of the final payment and prepayment fee offset by our unamortized debt premium resulting from the payoff of our loan with Oxford.
Other expense, net. Other expense, net was $12,000 for the year ended December 31, 2016 compared to $41,000 for the year ended December 31, 2015. This change was primarily due to a decrease in foreign exchange losses associated with foreign currency transactions.
Comparison of the Years Ended December 31, 2015 and 2014
The following table summarizes the results of our operations for the year ended December 31, 2015 and 2014, together with the changes in those items in dollars (in thousands):
|
For the Years Ended December 31, |
Change 2015 vs. |
|||||||||||
|
2015 |
2014 |
2014 |
||||||||||
|
Research and development expenses |
$ | 9,862 | $ | 24,708 | (14,846 |
) | ||||||
|
General and administrative expenses |
3,626 | 5,332 | (1,706 |
) | ||||||||
|
Interest expense |
(690 |
) |
(726 |
) |
36 | |||||||
|
Interest income |
22 | 51 | (29 |
) | ||||||||
|
Gain on revaluation of warrant liability |
— | 49 | (49 |
) | ||||||||
|
Other expense, net |
(41 |
) |
(46 |
) |
5 | |||||||
Research and development expenses. Research and development expenses were $9.9 million in the year ended December 31, 2015 compared to $24.7 million in the year ended December 31, 2014. The decrease in research and development costs is attributable to the following:
|
|
• |
a $12.2 million decrease in costs associated with our Phase 3 PLUS-1 clinical trial of topsalysin as the trial is completed, specifically a $2.0 million decrease for patient recruitment as enrollment was completed in September 2014, a $4.7 million decrease for patient visits to our clinical site investigators as sites have completed the final patient visits, a $1.4 million decrease in cost associated with our clinical research organizations, a $0.3 million decrease in clinical supply management and a $3.8 million decrease primarily in costs associated with the startup, consulting, training and travel of the Phase 3 PLUS-1 clinical trial; |
|
|
• |
a $2.7 million decrease in the costs associated with the manufacturing activities for topsalysin, due the completion of a manufacturing campaign in the year ended December 31, 2014; |
|
|
• |
a $0.4 million decrease in stock-based compensation expense. Research and development expenses included stock-based compensation expense of $0.2 million for the year ended December 31, 2015 as compared to $0.6 million for the year ended December 31, 2014. The decrease in the non-cash stock-based compensation expense is primarily associated with stock options granted to employees and directors in October 2013. As we utilize the graded amortization method to expense the fair value of our stock options, the expense recorded for the stock options decrease over the vesting period and therefore the expense for our October 2013 grants decreased from the year ended December 31, 2014 to the year ended December 31, 2015; and |
|
|
• |
a $0.3 million decrease in the personnel related costs. |
These decreases are partially offset by an increase of $0.9 million for costs associated with our Phase 2a proof of concept clinical trial for localized low to intermediate risk prostate cancer.
General and administrative expenses. General and administrative expenses were $3.6 million in the year ended December 31, 2015 compared to $5.3 million for the year ended December 31, 2014. The decrease is primarily due to a decrease in non-cash stock-based compensation expense of $0.9 million. The decrease in the non-cash stock-based compensation expense is primarily associated with significant stock options granted to employees and directors in October 2013. As we utilize the graded amortization method to expense the fair value of our stock options, the expense recorded for the stock options decreases over the vesting period and therefore the expense for our October 2013 grants decreased from the year ended December 31, 2014 to the year ended December 31, 2015. In addition, there was a $0.3 million decrease in personnel related cost and $0.5 million primarily associated with decreases in legal, travel, professional and consulting expenses.
Interest expense. Interest expense was $0.7 million in the years ended December 31, 2015 and 2014. Interest expense related to the Company’s promissory notes with Oxford is expected to decline in future periods as the total principal outstanding on the loan is paid down.
Interest income. Interest income was $22,000 for the year ended December 31, 2015 compared to $51,000 for the year ended December 31, 2014. The decrease is due to the decrease in the average balances of the interest-bearing cash and investment accounts period over period.
Gain on revaluation of warrant liability. Gain on revaluation of the warrant liability was $49,000 for the year ended December 31, 2014. The non-cash gain is associated with the change in the fair value of our outstanding warrants with exercise prices denominated in Canadian dollars. All of our outstanding warrants with exercise prices denominated in Canadian dollars expired in March 2015.
Other expense, net. Other expense, net was $41,000 for the year ended December 31, 2015 compared to $46,000 for the year ended December 31, 2014. This change was primarily due to a decrease in foreign exchange losses associated with foreign currency transactions.
Liquidity and Capital Resources
Overview
Since our inception, our operations have been primarily financed through public and private equity sales, debt financings and payments from Kissei. Since inception, we have devoted our resources to funding and conducting research and development programs, including discovery research, preclinical studies and clinical trial activities.
At December 31, 2016, we had cash, cash equivalents and securities available-for-sale of $29.0 million, representing an increase of $20.6 million from December 31, 2015. We had working capital of $27.8 million at December 31, 2016, an increase of $22.1 million from December 31, 2015. The increase in working capital from December 31, 2015 to December 31, 2016 was the result of a completed public offering in August 2016, in which we issued 7,475,000 common shares at a price of $4.00 per share, and warrants to purchase 5,606,250 common shares and received proceeds of $27.4 million, net of underwriters’ discounts, commissions and offering costs and a completed public offering in May 2016, in which we issued 3,571,428 common shares at a price of $1.40 per share and warrants to purchase common shares, and received net proceeds of approximately $4.6 million. During the year ended December 31, 2016, 1,775,714 of our common share purchase warrants were exercised which generated proceeds of $2.5 million. We expect that our cash, cash equivalents and securities available-for-sale will be sufficient to fund our operations to the end of 2018. At this point in time we do not plan on pursuing a second Phase 3 trial in BPH unless we obtain additional financing. We could use dilutive funding options to fund a second Phase 3 trial in BPH such as an equity financing and non-dilutive funding options such as a partnering arrangement or royalty agreement. There can be no assurance that such funding will be available on acceptable terms or at all.
Future Operations
We have devoted substantial resources to developing topsalysin, protecting and enhancing our intellectual property and providing general and administrative support for these activities. We have not generated any revenue from product sales and, to date, have funded our operations primarily through public and private equity security sales, debt financings and payments from Kissei.
We will require significant additional capital to fund our operations and complete development of topsalysin and there is no assurance that we will obtain additional capital.
Our future capital requirements will depend on, and could increase significantly as a result of many factors, including:
|
|
• |
progress in, and the costs of, our clinical trials, including our second Phase 2 clinical trial for localized prostate cancer and an additional Phase 3 clinical trial for BPH, preclinical studies and other research and development activities for topsalysin; |
|
|
• |
the costs and timing of regulatory approvals; |
|
|
• |
our ability to maintain our strategic license with Kissei and its ability to achieve applicable milestones and establish and maintain additional strategic collaborations, including licensing and other arrangements; |
|
|
• |
the costs involved in filing, prosecuting, enforcing and defending patent claims and other intellectual property rights; |
|
|
• |
the costs of obtaining and securing manufacturing supply for clinical or commercial production of product candidates; and |
|
|
• |
the costs of establishing, or contracting for, sales and marketing capabilities if we obtain regulatory approvals to market topsalysin. |
Until we can generate significant continuing revenues, we expect to satisfy our future cash needs through private and public sales of our securities, debt financings, by establishing additional strategic collaborations for topsalysin or from exercise of outstanding common share purchase warrants and stock options.
Cash Flows
The following table shows a summary of our cash flows for the years ended December 31, 2016, 2015 and 2014 (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Net cash provided by (used in): |
||||||||||||
|
Operating activities |
$ | (10,329 |
) |
$ | (14,357 |
) |
$ | (26,230 |
) | |||
|
Investing activities |
(13,721 |
) |
16,066 | 14,691 | ||||||||
|
Financing activities |
30,971 | 50 | 839 | |||||||||
|
Effect of exchange rate changes on cash and cash equivalents |
(2 |
) |
(1 |
) |
(16 |
) | ||||||
|
Net increase (decrease) in cash and cash equivalents |
$ | 6,919 | $ | 1,758 | $ | (10,716 |
) | |||||
Operating Activities
Net cash used in operating activities decreased to $10.3 million for the year ended December 31, 2016 compared to $14.4 million for the year ended December 31, 2015. The decrease in net cash used in operating activities of $4.0 million was primarily due to the net cash outflow impact of the decrease in our net loss from period to period and a decrease in funds used for the payment of accounts payable and accrued expenses for the year ended December 31, 2016. The decrease in our net loss from December 31, 2015 to December 31, 2016 is primarily a result of the decrease in our research and development expenses associated with our completed Phase 3 clinical trial for the treatment of the symptoms of BPH and a decrease in costs associated with manufacturing activities for topsalysin, offset by the non-cash loss recorded for the revaluation of our common share purchase warrants.
Net cash used in operating activities decreased to $14.4 million for the year ended December 31, 2015 compared to $26.2 million for the year ended December 31, 2014. The decrease in net cash used in operating activities of $11.9 million was primarily due to the net cash outflow impact of the decrease in our net loss from period to period offset by the increase in cash payments made to our clinical research organizations primarily for investigator sites in the period ended December 31, 2015. The decrease in our net loss from December 31, 2014 to December 31, 2015 is primarily a result of the decrease in our research and development expenses associated with our Phase 3 clinical trial for the treatment of the symptoms of BPH as the trial is completed and a decrease in costs associated with manufacturing activities for topsalysin.
Investing Activities
Net cash used in investing activities was $13.7 million for the year ended December 31, 2016, compared to net cash provided by investing activities of $16.1 million for the year ended December 31, 2015. The increase in net cash used in investing activities from December 31, 2015 to December 31, 2016 represents the usage of our cash and cash equivalents to purchase securities classified as available-for-sale.
Net cash provided by investing activities was $16.1 million for the year ended December 31, 2015, compared to $14.7 million for the year ended December 31, 2014. The increase in net cash provided by investing activities from December 31, 2014 to December 31, 2015 represents the usage of the proceeds from the maturity of securities classified as available-for-sale to fund our operations and to a lesser extent to purchase securities with maturities less than 90 days which are classified as cash and cash equivalents.
Financing Activities
Net cash provided by financing activities was $31.0 million for the year ended December 31, 2016, as compared to $50,000 for the year ended December 31, 2015. The increase in cash provided by financing activities for the year ended December 31, 2016 is primarily related to the receipt of the proceeds from our completed common share offerings of $33.5 million, net of issuance costs. We also received proceeds of $2.6 million from the exercise of warrants and stock options during the year ended December 31, 2016. These cash inflows were offset by $5.1 million of principal payments on our loan with Oxford.
Net cash provided by financing activities was $50,000 for the year ended December 31, 2015, as compared to $0.8 million for the year ended December 31, 2014. The cash provided by financing activities for the year ended December 31, 2015 reflects the proceeds of $0.8 million from the common share purchases from Aspire Capital offset by $0.7 in principal payments on our Oxford loan. The cash provided in financing activities during the year ended December 31, 2014 reflects the proceeds of $1.9 million, net from the common share purchase from Aspire Capital and cash received from Oxford of $2.4 million from our loan. These funds are offset by the settlement of our outstanding principal of $3.4 million due under the original loan.
Contractual Obligations and Commitments
The following is a summary of our contractual obligations as of December 31, 2016 (in thousands):
|
Payments due by period |
||||||||||||||||||||
|
Total |
Less than |
1-3 |
3-5 |
More than |
||||||||||||||||
|
Operating lease obligation relating to facility(1) |
$ | 173 | $ | 122 | $ | 51 | $ | — | $ | — | ||||||||||
|
Total |
$ | 173 | $ | 122 | $ | 51 | $ | — | $ | — | ||||||||||
|
(1) |
We currently lease an office facility comprising our headquarters in San Diego, California under a non-cancelable lease. The lease, as amended, expires in May 2018 and the minimum rent is $8,946 per month, subject to annual cost of living increases, plus our pro rata share of certain operating costs and other expenses. |
Off-balance Sheet Arrangements
We do not have any off-balance sheet arrangements (as defined by applicable SEC regulations) that are reasonably likely to have a current or future material effect on our financial condition, results of operations, liquidity, capital expenditures or capital resources.
|
Item 7A. |
Qualitative and Quantitative Disclosures About Market Risk |
Under SEC rules and regulations, as a smaller reporting company, we are not required to provide the information required by this item.
|
Item 8. |
Financial Statements and Supplementary Data |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Sophiris Bio Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations and comprehensive loss, of shareholders’ equity and of cash flows present fairly, in all material respects, the financial position of Sophiris Bio Inc. and its subsidiaries as of December 31, 2016 and 2015, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016 in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
San Diego, California
March 27, 2017
Sophiris Bio Inc.
Consolidated Balance Sheets
(In thousands, except share amounts)
|
December 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Assets: |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 12,800 | $ | 5,881 | ||||
|
Securities available-for-sale |
16,201 | 2,500 | ||||||
|
Other receivables |
128 | 8 | ||||||
|
Prepaid expenses |
846 | 467 | ||||||
|
Total current assets |
29,975 | 8,856 | ||||||
|
Property and equipment, net |
4 | 17 | ||||||
|
Other long-term assets |
19 | 19 | ||||||
|
Total assets |
$ | 29,998 | $ | 8,892 | ||||
|
Liabilities and shareholders’ equity: |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 459 | $ | 909 | ||||
|
Accrued expenses |
1,762 | 566 | ||||||
|
Current portion of promissory notes |
— | 1,771 | ||||||
|
Total current liabilities |
2,221 | 3,246 | ||||||
|
Long-term promissory notes |
— | 3,572 | ||||||
|
Warrant liability |
13,396 | — | ||||||
|
Stock-based compensation liability |
57 | 168 | ||||||
|
Total liabilities |
15,674 | 6,986 | ||||||
|
Commitments and contingencies (Note 16) |
||||||||
|
Shareholders’ equity: |
||||||||
|
Common shares, unlimited authorized shares, no par value; 30,107,644 and 17,244,736 shares issued and outstanding at December 31, 2016 and 2015, respectively |
131,245 | 113,880 | ||||||
|
Contributed surplus |
23,900 | 17,683 | ||||||
|
Accumulated other comprehensive gain |
99 | 99 | ||||||
|
Accumulated deficit |
(140,920 |
) |
(129,756 |
) | ||||
|
Total shareholders’ equity |
14,324 | 1,906 | ||||||
|
Total liabilities and shareholders’ equity |
$ | 29,998 | $ | 8,892 | ||||
The accompanying notes are an integral part of these audited consolidated financial statements.
Sophiris Bio Inc.
Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except per share amounts)
|
|
|
For the Years Ended December 31, |
| |||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
| |||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
3,538 |
|
|
$ |
9,862 |
|
|
$ |
24,708 |
|
|
General and administrative |
|
|
6,768 |
|
|
|
3,626 |
|
|
|
5,332 |
|
|
Total operating expenses |
|
|
10,306 |
|
|
|
13,488 |
|
|
|
30,040 |
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense |
|
|
(373 |
) |
|
|
(690 |
) |
|
|
(726 |
) |
|
Interest income |
|
|
37 |
|
|
|
22 |
|
|
|
51 |
|
|
Gain (loss) on revaluation of warrant liability |
|
|
(330 |
) |
|
|
— |
|
|
|
49 |
|
|
Loss on extinguishment of debt |
(180 |
) |
— |
— |
||||||||
|
Other expense, net |
|
|
(12 |
) |
|
|
(41 |
) |
|
|
(46 |
) |
|
Total other expense |
|
|
(858 |
) |
|
|
(709 |
) |
|
|
(672 |
) |
|
Net loss |
|
$ |
(11,164 |
) |
|
$ |
(14,197 |
) |
|
$ |
(30,712 |
) |
|
Basic and diluted loss per share |
|
$ |
(0.49 |
) |
|
$ |
(0.84 |
) |
|
$ |
(1.85 |
) |
|
Weighted average number of outstanding shares – basic and diluted |
|
|
23,002 |
|
|
|
16,881 |
|
|
|
16,586 |
|
|
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized gain on securities available-for-sale |
|
|
— |
|
|
|
— |
|
|
|
1 |
|
|
Total other comprehensive loss |
|
$ |
(11,164 |
) |
|
$ |
(14,197 |
) |
|
$ |
(30,711 |
) |
The accompanying notes are an integral part of these audited consolidated financial statements.
Sophiris Bio Inc.
Consolidated Statements of Shareholders’ Equity
(In thousands, except share amounts)
|
Common |
Contributed |
|
Accumulated |
|
||||||||||||||||||||
|
Shares |
Amount |
Surplus |
Deficit |
Income |
Equity |
|||||||||||||||||||
|
Balance at January 1, 2014 |
16,149,871 | $ | 111,204 | $ | 13,824 | $ | (84,847 |
) |
$ | 98 | $ | 40,279 | ||||||||||||
|
Issuance of common shares, net of issuance costs of $109 |
694,865 | 1,891 | — | — | — | 1,891 | ||||||||||||||||||
|
Reclassification of historic fair value of warrants |
— | — | 834 | — | — | 834 | ||||||||||||||||||
|
Change in the fair value of stock-based compensation liability recorded to contributed surplus |
— | — | 180 | — | — | 180 | ||||||||||||||||||
|
Issuance of warrants with secured promissory note |
— | — | 124 | — | — | 124 | ||||||||||||||||||
|
Stock-based compensation expense |
— | — | 2,091 | — | — | 2,091 | ||||||||||||||||||
|
Net loss |
— | — | — | (30,712 |
) |
— | (30,712 |
) | ||||||||||||||||
|
Other comprehensive income |
— | — | — | — | 1 | 1 | ||||||||||||||||||
|
Balance at December 31, 2014 |
16,844,736 | $ | 113,095 | $ | 17,053 | $ | (115,559 |
) |
$ | 99 | $ | 14,688 | ||||||||||||
|
Issuance of common shares |
400,000 | 785 | — | — | — | 785 | ||||||||||||||||||
|
Reclassification of historic fair value of warrants |
— | — | — | — | — | — | ||||||||||||||||||
|
Change in the fair value of stock-based compensation liability recorded to contributed surplus |
— | — | (146 |
) |
— | — | (146 |
) | ||||||||||||||||
|
Stock-based compensation expense |
— | — | 776 | — | — | 776 | ||||||||||||||||||
|
Net loss |
— | — | — | (14,197 |
) |
— | (14,197 |
) | ||||||||||||||||
|
Balance at December 31, 2015 |
17,244,736 | $ | 113,880 | $ | 17,683 | $ | (129,756 |
) |
$ | 99 | $ | 1,906 | ||||||||||||
|
Issuance of common shares and warrants, net of issuance cost of $1,366 |
11,046,428 | 33,534 | — | — | — | 33,534 | ||||||||||||||||||
|
Exercise of warrants |
1,775,714 | 2,486 | — | — | — | 2,486 | ||||||||||||||||||
|
Exercise of stock options |
40,766 | 92 | — | — | — | 92 | ||||||||||||||||||
|
Initial valuation of warrant liability upon issuance of warrants |
— | (18,747 |
) |
— | — | — | (18,747 |
) | ||||||||||||||||
|
Valuation of exercised warrants reclassified from warrant liability to contributed surplus |
— | — | 5,681 | — | — | 5,681 | ||||||||||||||||||
|
Change in the fair value of stock-based compensation liability recorded to contributed surplus |
— | — | 111 | — | — | 111 | ||||||||||||||||||
|
Stock-based compensation expense |
— | — | 425 | — | — | 425 | ||||||||||||||||||
|
Net loss |
— | — | — | (11,164 |
) |
— | (11,164 |
) | ||||||||||||||||
|
Balance at December 31, 2016 |
30,107,644 | $ | 131,245 | $ | 23,900 | $ | (140,920 |
) |
$ | 99 | $ | 14,324 | ||||||||||||
The accompanying notes are an integral part of these audited consolidated financial statements.
Sophiris Bio Inc.
Consolidated Statements of Cash Flows
(In thousands)
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Cash flows used in operating activities |
||||||||||||
|
Net loss for the period |
$ | (11,164 |
) |
$ | (14,197 |
) |
$ | (30,712 |
) | |||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
|
Stock-based compensation |
425 | 776 | 2,091 | |||||||||
|
Amortization of debt discount |
81 | 137 | 188 | |||||||||
|
Depreciation of property and equipment |
12 | 20 | 47 | |||||||||
|
Amortization of promissory note issuance costs |
— | — | 38 | |||||||||
|
Amortization of discount on available-for-sale securities |
20 | 5 | 39 | |||||||||
|
Change in fair value warrant liability |
330 | — | (49 |
) | ||||||||
|
Noncash portion of loss on early extinguishment of debt |
(159 |
) |
— | — | ||||||||
|
Payment of original issue discount |
(124 |
) |
— | — | ||||||||
|
Foreign exchange transaction loss |
— | 6 | 1 | |||||||||
|
Other |
— | — | 3 | |||||||||
|
Changes in operating assets and liabilities: |
||||||||||||
|
Other receivables |
(120 |
) |
8 | 33 | ||||||||
|
Prepaid expenses |
(379 |
) |
2,358 | 734 | ||||||||
|
Accounts payable |
(446 |
) |
(1,728 |
) |
1,171 | |||||||
|
Accrued expenses |
1,195 | (1,742 |
) |
186 | ||||||||
|
Net cash used in operating activities |
(10,329 |
) |
(14,357 |
) |
(26,230 |
) | ||||||
|
Cash flows (used in) provided by investing activities |
||||||||||||
|
Purchases of property and equipment |
— | — | (9 |
) | ||||||||
|
Maturity of securities available-for-sale |
2,750 | 26,169 | 43,201 | |||||||||
|
Purchases of securities available-for-sale |
(16,471 |
) |
(10,103 |
) |
(28,501 |
) | ||||||
|
Net cash (used in) provided by investing activities |
(13,721 |
) |
16,066 | 14,691 | ||||||||
|
Cash flows provided by financing activities |
||||||||||||
|
Proceeds from the issuance of common shares and warrants, net of paid issuance costs |
33,534 | 785 | 1,891 | |||||||||
|
Proceeds from the exercise of warrants |
2,486 | — | — | |||||||||
|
Proceeds from exercise of stock options |
92 | — | — | |||||||||
|
Payment of issuance costs in connection with public offering |
— | — | (53 |
) | ||||||||
|
Cash received from the issuance of promissory notes |
— | — | 2,362 | |||||||||
|
Principal payments on notes payable |
(5,141 |
) |
(735 |
) |
(3,361 |
) | ||||||
|
Net cash provided by financing activities |
30,971 | 50 | 839 | |||||||||
|
Effect of exchange rate changes on cash and cash equivalents |
(2 |
) |
(1 |
) |
(16 |
) | ||||||
|
Net increase (decrease) in cash and cash equivalents |
6,919 | 1,758 | (10,716 |
) | ||||||||
|
Cash and cash equivalents at beginning of period |
5,881 | 4,123 | 14,839 | |||||||||
|
Cash and cash equivalents at end of period |
$ | 12,800 | $ | 5,881 | $ | 4,123 | ||||||
|
Supplemental disclosures of cash flow information: |
||||||||||||
|
Cash paid for interest |
$ | 334 | $ | 559 | $ | 495 | ||||||
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Supplemental disclosures of non-cash investing and financing activities: |
||||||||||||
|
Valuation of warrant liability upon issuance of warrants |
$ | 18,747 | $ | — | $ | — | ||||||
|
Reclassification of fair value of warrant liability to equity as a result of the amendment of the underlying common share purchase warrants |
$ | — | $ | — | $ | 834 | ||||||
|
Value of warrants issued in connection with promissory notes |
$ | — | $ | — | $ | 124 | ||||||
|
Valuation of exercised warrants reclassified from warrant liability to contributed surplus |
$ | 5,681 | $ | — | $ | — | ||||||
|
Change in the fair value of stock-based compensation liability recorded to contributed surplus |
$ | (111 |
) |
$ | 146 | $ | (180 |
) | ||||
|
Unrealized gain on securities available-for sale |
$ | — | $ | — | $ | (1 |
) | |||||
The accompanying notes are an integral part of these audited consolidated financial statements.
Sophiris Bio Inc.
Notes to the Consolidated Financial Statements
|
|
|
1. |
Nature of the business |
Company
Sophiris Bio Inc., or the Company, or Sophiris, is a clinical-stage biopharmaceutical company currently developing topsalysin for treatment of the symptoms of for the treatment of clinically significant localized prostate cancer and benign prostatic hyperplasia, or BPH, commonly referred to as an enlarged prostate. The Company is governed by the British Columbia Business Corporations Act. The Company’s operations were initially located in Vancouver, British Columbia until April 2011, when its core activities and headquarters relocated from Vancouver, British Columbia to San Diego, California.
The consolidated financial statements include the accounts of Sophiris Bio Inc. and its wholly-owned subsidiaries, Sophiris Bio Corp. and Sophiris Bio Holding Corp., both of which are incorporated in the State of Delaware.
|
2. |
Summary of significant accounting policies |
Significant accounting policies followed by the Company in the preparation of its consolidated financial statements are as follows:
Basis of consolidation
The consolidated financial statements include the accounts of the Company, Sophiris Bio Corp. and Sophiris Bio Holding Corp. All intercompany balances and transactions have been eliminated for purposes of consolidation.
Basis of presentation and use of estimates
The accompanying consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States or GAAP.
GAAP requires the Company’s management to make estimates and judgments that may affect the reported amounts of assets, liabilities, revenue, expenses and related disclosures. The Company bases estimates and judgments on historical experience and on various other factors that it believes to be reasonable under the circumstances. The significant estimates in these consolidated financial statements include stock-based compensation expense, fair value of the warrant liability and accrued research and development expenses, including accruals related to the Company’s clinical trial(s). The Company’s actual results may differ from these estimates. The Company evaluates its estimates on an ongoing basis. Changes in estimates are reflected in reported results in the period in which they become known by the Company’s management.
Foreign currency
Historically gains and losses resulting from foreign currency translation were recorded in accumulated other comprehensive gain (loss), which is a separate component of shareholders’ equity. Foreign currency transaction gains and losses are recognized as a component of other expense.
Cash and cash equivalents
Cash equivalents are short-term, highly liquid investments with an original maturity of three months or less at the date of purchase.
Securities Available-for-Sale
Investments with an original maturity of more than three months when purchased have been classified by management as securities available-for-sale. Such investments are carried at fair value, with unrealized gains and losses included as a component of accumulated other comprehensive gain (loss) in shareholders’ equity. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities are included in interest income. No other-than-temporary impairments were identified for the investment securities held by the Company as of December 31, 2016 and 2015. The cost of investment securities classified as available-for-sale is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion are included in interest income. The cost of securities sold is based on the specific-identification method. The Company has classified all of its investment securities as available-for-sale, including any of those with maturities beyond one year, as current assets on the consolidated balance sheets based on the highly liquid nature of the investment securities and because these investment securities are considered available for use in current operations.
Concentration of credit risk
Financial instruments, which potentially subject the Company to concentration of credit risk, consist primarily of cash and cash equivalents and investment securities classified as available-for-sale. The Company maintains deposits in federally insured financial institutions in excess of federally insured limits. Management believes that the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. Additionally, the Company has adopted an investment policy that includes guidelines relative to credit quality, diversification of maturities and liquidity.
Property and equipment
Property and equipment are recorded at cost and depreciated using the straight-line method, based on their estimated useful lives as follows:
|
Asset classification |
Estimated useful life (in years) |
|||||
|
Equipment |
3 | - | 5 | |||
|
Computer hardware |
3 | |||||
|
Software |
3 | - | 5 | |||
|
Leasehold improvements |
Lesser of useful life or lease term | |||||
|
Furniture and fixtures |
5 | |||||
Repairs and maintenance costs are expensed as incurred.
The Company reviews its long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate. Each impairment test is based on a comparison of the undiscounted cash flow to the recorded value of the asset. If impairment is indicated, the asset will be written down to its estimated fair value on a discounted cash flow basis. The Company has not recognized any impairment losses through December 31, 2016.
Promissory notes
Promissory notes are recognized initially at fair value. Promissory notes are subsequently carried at amortized cost; any difference between the initial fair market value and the redemption value is recognized in the statement of operations and comprehensive loss over the period of the notes payable using the effective interest method.
The fair value of the promissory notes when issued with equity is recognized initially at the fair value of similar promissory notes issued on a standalone basis. The equity that is issued with borrowings is valued at fair value using the Black-Scholes valuation model.
Revenue recognition
The Company may enter into product development agreements with collaborative partners for the research and development of products for the treatment of urological diseases. The terms of the agreements may include nonrefundable signing and licensing fees, milestone payments and royalties on any product sales derived from collaborations. These multiple element arrangements are analyzed to determine whether the deliverables can be separated or whether they must be accounted for as a single unit of accounting. License fees are recognized as revenue when persuasive evidence of an arrangement exists, the fee is fixed or determinable, delivery or performance has substantially completed and collection is reasonably assured.
The Company recognizes up front license payments as revenue upon delivery of the license only if the license has stand-alone value to the customer and if the agreement includes a general right of return, the delivery or performance of undelivered items is considered probable and within the control of the Company. The payment is generally allocated to the separate units of accounting based on their relative selling prices. The selling price of each deliverable is determined using vendor specific objective evidence of selling prices, if it exists; otherwise, third-party evidence of selling prices. If neither vendor specific objective evidence nor third-party evidence exists, the Company uses its’ best estimate of the selling price for each deliverable. The payment allocated is limited to the amount that is not contingent on the delivery of additional items or fulfillment of other performance conditions.
Whenever the Company determines that an arrangement should be accounted for as a single unit of accounting, it must determine the period over which the performance obligations will be performed and revenue recognized. If the Company cannot reasonably estimate the timing and the level of effort to complete its performance obligations under the arrangement, then revenue under the arrangement is recognized on a straight-line basis over the period the Company is expected to complete its performance obligations.
The Company evaluates milestone payments on an individual basis and recognizes revenue from non-refundable milestone payments when the earnings process is complete and the payment is reasonably assured. Non-refundable milestone payments related to arrangements under which the Company has continuing performance obligations are recognized as revenue upon achievement of the associated milestone, provided that (i) the milestone event is substantive and its achievability was not reasonably assured at the inception of the agreement and (ii) the amount of the milestone payment is reasonable in relation to the effort expended or the risk associated with the milestone event. Any amounts received under agreements in advance of performance, if deemed substantive, are recorded as deferred revenue and recognized as revenue as the Company completes its performance obligations. A milestone event is considered substantive if (i) the milestone is commensurate with either (a) the Company’s performance to achieve the milestone or (b) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the Company’s performance to achieve the milestone; (ii) it relates solely to past performance and (iii) it is reasonable relative to all of the deliverables and payment terms (including other potential milestone consideration) within the arrangement. If any portion of the milestone payment does not relate to the Company’s performance, does not relate solely to past performance or is refundable or adjustable based on future performance, the milestone is not considered to be substantive. Milestone payments are not bifurcated into substantive and non-substantive components. Payments related to the achievement of non-substantive milestones is deferred and recognized over the Company’s remaining performance period.
Royalty revenue will be recognized upon the sale of the related products provided the Company has no remaining performance obligations under the arrangement.
Research and development expenses
Research and development costs are charged to expense as incurred. Research and development expenses comprise costs incurred in performing research and development activities, including personnel-related costs, stock-based compensation, facilities, research-related overhead, clinical trial costs, contracted services, manufacturing, license fees and other external costs. The Company accounts for nonrefundable advance payments for goods and services that will be used in future research and development activities as expenses when the service has been performed or when the goods have been consumed rather than when the payment is made.
Accrued research and development expenses
Clinical trial costs are recorded as a component of research and development expenses. The Company accrues and expenses clinical trial activities performed by third parties based upon estimates of the percentage of work completed of the total work over the life of the individual study in accordance with agreements established with clinical research organizations and clinical trial sites. The Company determines the estimates through discussions with internal clinical personnel and external service providers as to the progress or stage of completion of trials or services and the agreed-upon fee to be paid for such services based on facts and circumstances known to the Company as of each balance sheet date. However, actual costs and timing of clinical trials are highly uncertain, subject to risks and may change depending upon a number of factors, including the Company’s clinical development plan.
If the actual timing of the performance of services or the level of effort varies from the estimate, the Company will adjust the accrual accordingly. Adjustments to prior period estimates have not been material.
Examples of estimated accrued research and development expenses include:
|
|
• |
fees to clinical research organizations in connection with clinical studies; |
|
|
• |
fees to investigative sites in connection with clinical studies; |
|
|
• |
fees to vendors in connection with preclinical development activities; |
|
|
• |
fees to vendors associated with the development of companion diagnostics; and |
|
|
• |
fees to vendors related to product manufacturing, development and distribution of clinical supplies. |
Nonrefundable advance payments for goods and services that will be used or rendered in future research and development activities, are recorded as a prepaid expense and recognized as expense in the period that the related goods are consumed or services are performed.
Dividend Policy
The Company has never declared or paid any cash dividends on its capital shares. The Company intends to retain all available funds and any future earnings to support its operations and finance the growth and development of its business. The Company does not intend to pay cash dividends on its common shares for the foreseeable future. Any future determination related to the Company’s dividend policy will be made at the discretion of its board of directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors of the Company’s board of directors may deem relevant.
Stock-based compensation
The Company expenses the fair value of employee stock options over the vesting period. Compensation expense is measured using the fair value of the award at the grant date, net of estimated forfeitures, and is adjusted annually to reflect actual forfeitures. The fair value of each stock-based award is estimated using the Black-Scholes pricing model and is expensed using graded amortization over the vesting period.
The Company accounts for stock options granted to non-employees, which primarily consist of consultants of the Company, using the fair value approach. Stock options granted to non-employees are subject to revaluation each reporting period over their vesting terms.
Prior to the Company’s initial public offering, or IPO, the Company had issued its stock options with a Canadian dollar denominated exercise price. Subsequent to the Company’s IPO, the Company issues its stock options with a U.S. dollar denominated exercise price.
Effective November 13, 2013, the Company voluntarily delisted from the Toronto Stock Exchange, or TSX. As a result of the delisting from the TSX and the change in the Company’s functional currency to the U.S. dollar, the stock options granted with exercise prices denominated in Canadian dollars are considered dual indexed as defined in Accounting Standards Codification, or ASC, topic 718, “Compensation, Stock Compensation”. As a result, the Company is required to account for these stock options as a liability. Historically these options had been accounted for as equity. The estimated fair value is determined using the Black-Scholes pricing model based on the estimated value of the underlying common shares at the valuation measurement date, the remaining service period of the stock options, risk-free interest rates, expected dividends and expected volatility of the price of the underlying common shares. The fair value of the stock-based compensation liability was $57,000 at December 31, 2016. As the calculated fair value of the stock options at December 31, 2016 was less than the original grant date fair value no additional compensation expense was recorded in the consolidated statement of operations and comprehensive loss. The change in the fair value of the stock-based compensation liability was recorded as an adjustment to Contributed Surplus of ($111,000) and $146,000 for the years ended December 31, 2016 and 2015, respectively.
Warrant Liability
In connection with the offerings we completed in the year ended December 31, 2016, we issued warrants to purchase common shares. These warrants may require us to pay the warrant holder cash under certain provisions of the warrant and therefore we are accounting for these warrants as a liability in accordance with ASC 480 “Distinguishing Liabilities from Equity”. As a result of these warrants being classified as liabilities, we are required to calculate the fair value of these warrants at each reporting date. The fair value of these warrants are calculated utilizing a Black-Scholes pricing model. We calculated the initial fair value of these warrants at the date the warrants were issued. At each reporting date we are required to remeasure the fair value of the warrant liability and any corresponding increase or decrease to the warrant liability is recorded as a component of other income or expense in our consolidated statement of operations and comprehensive loss. In addition, if a warrant holder exercises warrants we are required to revalue the fair value of the underlying warrants on the date of exercise and reclassify the change in the fair value from the warrant liability to contributed surplus.
Certain inputs utilized in our Black-Scholes fair value calculation may fluctuate in future periods based upon factors which are outside of the Company’s control. A significant change in one or more of these inputs used in the calculation of the fair value may cause a significant change to the fair value of our warrant liability which could also result in material non-cash gain or loss being reported in our consolidated statement of operations and comprehensive loss. A 10% change in our closing stock price on December 31, 2016 would result in a $1.4 million change to the fair value of our warrant liability at December 31, 2016. A 10% change in our stock price volatility at December 31, 2016 would result in a change of $0.8 million to our warrant liability at December 31, 2016. A 10% change in the risk-free interest rate at December 31, 2016 would not have a material effect on the fair value of our warrant liability at December 31, 2016.
Income taxes
The Company accounts for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted rates in effect for the year in which these temporary differences are expected to be recovered or settled. Valuation allowances are provided if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company provides reserves for potential payments of tax to various tax authorities related to uncertain tax positions and other issues. Reserves are based on a determination of whether and how much of a tax benefit taken by the Company in its tax filing is more likely than not to be realized following resolution of any potential contingencies present related to the tax benefit. Potential interest and penalties associated with such uncertain tax positions are recorded as components of income tax expense.
Segment reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker, or CODM. The Company’s Chief Executive Officer serves as its CODM. The Company views its operations and manages its business as one segment operating primarily in the United States. As of December 31, 2016, all of the Company’s assets were located in the United States of America with the exception of $36,000 of cash and cash equivalents located in Canada. All of the Company’s property and equipment was located within the United States as of December 31, 2016.
Fair value of financial instruments
The Company measures certain financial assets and liabilities at fair value based on the exchange price that would be received for an asset or paid for to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. The carrying amounts of the Company’s financial instruments, including cash equivalents, accounts payable and accrued expenses, approximate fair value due to their short maturities.
The Company follows ASC 820-10, “Fair Value Measurements and Disclosures,” which among other things, defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, a three-tier fair value hierarchy has been established, which prioritizes the inputs used in measuring fair value as follows:
Level 1 – Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date.
Level 2 – Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument’s anticipated life.
Level 3 – Inputs reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model.
Recent accounting pronouncements
In May 2014, the Financial Accounting Standards Board, or FASB, issued new guidance related to revenue recognition (Accounting Standards Update, or ASU, No. 2014-09 Revenue from Contracts with Customers (Topic 606)). Subsequently the FASB has issued additional guidance (ASU Nos. 2015-14; 2016-08; 2016-10; 2016-12; 2016-20 Revenue from Contracts with Customers (Topic 606)). The guidance establishes principles for reporting information about the nature, amount, timing, and uncertainty of revenue and cash flows arising from an entity’s contracts with customers. The guidance is effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period. Early adoption is permitted only as of annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. Two methods of adoption are permitted: (a) full retrospective adoption, meaning the standard is applied to all periods presented or (b) modified retrospective adoption, meaning the cumulative effect of applying the new guidance is recognized as an adjustment to the opening retained earnings balance. The Company does not intend to adopt the new guidance early and is in the process of determining the adoption method. The Company did not recognize any revenue from contracts with customers in the years ended December 31, 2016, 2015 and 2014. Although the Company is still evaluating the impact of the new standard, we anticipate that the impact will not be material to the consolidated financial statements as we do not currently generate revenues from contracts with customers.
.
In February 2016, the FASB issued ASU No. 2016-02, “Leases (Topic 842)”. This guidance requires lessees to recognize a lease liability and a right-of-use asset with the exception of short-term leases. In addition, lessees are required to classify leases as either operating or finance based on current criteria of whether or not the lease is effectively a financed purchase by the lessee. The new standard is effective for the annual reporting period beginning after December 15, 2018 and early adoption is permitted. Although the Company is in the process of evaluating the impact of this guidance on its consolidated financial statements and related disclosures, the Company expects that its operating lease will be subject to the new standard and recognized as operating lease liabilities and right-of-use assets upon adoption.
In March 2016, the FASB issued ASU No. 2016-09, “Compensation – Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting”. This guidance is intended to simply the accounting for stock compensation. Several aspects of the accounting for share-based payment award transactions are simplified, including: (a) income tax consequences, (b) classification of awards as either equity or liabilities; and (c) classification on the statement of cash flows. The new standard is effective for annual periods beginning after December 15, 2016, and interim periods therein. This guidance may be applied either prospectively, retrospectively or using a modified retrospective transition method, depending on the area covered in this update. We will adopt ASU 2016-09 in 2017, and we do not anticipate this guidance to have a significant impact on our financial statements at the time of adoption.
In August 2016, the FASB issued ASU 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments”, addressing eight specific cash flow issues in an effort to reduce diversity in practice. The amended guidance is effective for fiscal years beginning after December 31, 2017, and for interim periods within those years. Early adoption is permitted. The Company is in the process of evaluating the impact of this guidance on its consolidated financial statements.
|
3. |
Reduction in workforce |
The Company completed a reduction in workforce in May 2016 through which five of its ten employees were terminated. The Company incurred a charge of approximately $81,000 during May 2016, which is included in operating expenses, related to cash severance and continuation of benefits in connection with the workforce reduction. No additional cash payments are expected to be made related to this reduction in workforce. In addition, the Company incurred a non-cash stock-based compensation charge of approximately $76,000 during May 2016 associated with the modification of stock options for individuals included in the reduction in workforce. See additional discussion regarding the modification of stock options for terminated employees at Note 13.
|
|
|
4. |
Net loss per common share |
Basic net loss per share is calculated by dividing the net loss attributable to common shareholders by the weighted-average number of common shares outstanding during the period, without consideration for potentially dilutive securities. Diluted net loss per share is computed by dividing the net loss by the weighted-average number of potentially dilutive securities outstanding for the period determined using the treasury-stock method. For purposes of this calculation, stock options and warrants are considered to be potentially dilutive securities and are only included in the calculation of diluted net loss per share when their effect is dilutive.
The following table presents the computation of basic and diluted net loss per share (in thousands, except per share amounts):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Net loss per share: |
||||||||||||
|
Net loss |
$ | (11,164 |
) |
$ | (14,197 |
) |
$ | (30,712 |
) | |||
|
Weighted-average common shares – basic and diluted |
23,002 | 16,881 | 16,586 | |||||||||
|
Net loss per share – basic and diluted per share |
$ | (0.49 |
) |
$ | (0.84 |
) |
$ | (1.85 |
) | |||
The following dilutive securities have been excluded from the computation of diluted weighted-average shares outstanding as of the year ended December 31, 2016, 2015 and 2014 as the Company recorded a net loss in all periods and, therefore, they would be anti-dilutive (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Options to purchase common shares |
2,868 | 1,677 | 1,378 | |||||||||
|
Common share purchase warrants |
5,965 | 589 | 1,001 | |||||||||
|
5. |
Securities Available-for-Sale |
Securities available-for-sale consisted of the following as of December 31, 2016 (in thousands):
|
December 31, 2016 |
||||||||||||||||
|
Amortized |
Unrealized |
Estimated |
||||||||||||||
|
Cost |
Gain |
Loss |
Fair Value |
|||||||||||||
|
Commercial paper |
$ | 3,890 | $ | — | $ | — | $ | 3,890 | ||||||||
|
U.S. government sponsored enterprise securities |
12,311 | — | — | 12,311 | ||||||||||||
| $ | 16,201 | $ | — | $ | — | $ | 16,201 | |||||||||
The amortized cost and estimated fair value of the Company securities available-for-sale by contractual maturity as of December 31, 2016 are shown below (in thousands):
|
December 31, 2016 |
||||||||||||||||
|
Amortized |
Unrealized |
Estimated |
||||||||||||||
|
Cost |
Gain |
Loss |
Fair Value |
|||||||||||||
|
Within one year |
$ | 16,201 | $ | — | $ | — | $ | 16,201 | ||||||||
|
After one year |
— | — | — | — | ||||||||||||
| $ | 16,201 | $ | — | $ | — | $ | 16,201 | |||||||||
Securities available-for-sale consisted of the following as of December 31, 2015 (in thousands):
|
December 31, 2015 |
||||||||||||||||
|
Amortized |
Unrealized |
Estimated |
||||||||||||||
|
Cost |
Gain |
Loss |
Fair Value |
|||||||||||||
|
Commercial paper |
$ | 750 | $ | — | $ | — | $ | 750 | ||||||||
|
U.S. government sponsored enterprise securities |
1,750 | — | — | 1,750 | ||||||||||||
| $ | 2,500 | $ | — | $ | — | $ | 2,500 | |||||||||
The amortized cost and estimated fair value of the Company securities available-for-sale by contractual maturity as of December 31, 2015 are shown below (in thousands):
|
December 31, 2015 |
||||||||||||||||
|
Amortized |
Unrealized |
Estimated |
||||||||||||||
|
Cost |
Gain |
Loss |
Fair Value |
|||||||||||||
|
Within one year |
$ | 2,500 | $ | — | $ | — | $ | 2,500 | ||||||||
|
After one year |
— | — | — | — | ||||||||||||
| $ | 2,500 | $ | — | $ | — | $ | 2,500 | |||||||||
|
|
|
6. |
Fair value measurement and financial instruments |
As of December 31, 2016 the Company has $28.5 million of securities consisting of money market funds, commercial paper, and U.S. government sponsored enterprise securities with maturities that range from three days to eleven months with an overall average time to maturity of approximately five months. The Company has the ability to liquidate these investments without restriction. The Company determines fair value for securities with Level 1 inputs through quoted market prices. The Company determines fair value for securities with Level 2 inputs through broker or dealer quotations or alternative pricing sources with reasonable levels of price transparency. The Company’s Level 2 securities have been initially valued at the transaction price and subsequently valued, at the end of each reporting period, typically utilizing third party pricing services or other observable market data. The pricing services utilize industry standard valuation models, including both income and market based approaches and observable market inputs to determine value. These observable market inputs include reportable trades, benchmark yields, credit spreads, broker/dealer quotes, bids, offers, and other industry and economic events. The Company’s Level 3 inputs are unobservable inputs based on the Company’s assessment that market participants would use in pricing the instruments.
The following table presents the Company’s assets and liabilities that are measured at fair value on a recurring basis for the periods presented (in thousands):
|
December 31, 2016 |
Level 1 |
Level 2 |
Level 3 |
|||||||||||||
|
Assets: |
||||||||||||||||
|
Money market funds |
$ | 57 | $ | 57 | $ | — | $ | — | ||||||||
|
Commercial paper |
16,085 | — | 16,085 | — | ||||||||||||
|
U.S. government sponsored enterprise securities |
12,311 | — | 12,311 | — | ||||||||||||
|
Total assets |
$ | 28,453 | $ | 57 | $ | 28,396 | $ | — | ||||||||
|
Liabilities: |
||||||||||||||||
|
Warrant liability |
$ | 13,396 | $ | — | $ | — | $ | 13,396 | ||||||||
|
Stock-based compensation liability |
57 | — | — | 57 | ||||||||||||
|
Total liabilities |
$ | 13,453 | $ | — | $ | — | $ | 13,453 | ||||||||
|
December 31, 2015 |
Level 1 |
Level 2 |
Level 3 |
|||||||||||||
|
Assets: |
||||||||||||||||
|
Money market funds |
$ | 87 | $ | 87 | $ | — | $ | — | ||||||||
|
Commercial paper |
1,850 | — | 1,850 | — | ||||||||||||
|
U.S. government sponsored enterprise securities |
5,549 | — | 5,549 | — | ||||||||||||
|
Total assets |
$ | 7,486 | $ | 87 | $ | 7,399 | $ | — | ||||||||
|
Liabilities: |
||||||||||||||||
|
Stock-based compensation liability |
$ | 168 | $ | — | $ | — | $ | 168 | ||||||||
|
Total liabilities |
$ | 168 | $ | — | $ | — | $ | 168 | ||||||||
Warrant liability
In connection with the offering completed on May 11, 2016, the Company issued 1,785,714 warrants to purchase its common shares. These warrants may require the Company to pay the warrant holder cash under certain provisions of the warrant and therefore the Company is accounting for these warrants as a liability. As a result of these warrants being classified as a liability, the Company is required to calculate their fair value at each reporting date. The fair value of these warrants is calculated utilizing a Black-Scholes pricing model. The Company calculated the initial fair value of these warrants on May 11, 2016, the date the warrants were issued. On various dates from May 11, 2016 through December 31, 2016, the warrant holders exercised 1,775,714 warrants and as a result the Company revalued the fair value of the underlying warrants on each exercise date. The fair value of the exercised warrants was reclassified from the warrant liability to contributed surplus upon exercise. As of December 31, 2016, only 10,000 warrants remain outstanding from the May 11, 2016 offering for which the fair value was remeasured as of December 31, 2016. The following inputs were utilized in the Black-Scholes pricing model during the year ended December 31, 2016:
|
Initial Fair Value Assessment May 11, 2016 |
Weighted Average Values Utilized on the Various Exercise Dates |
December 31, 2016 |
||||||||||
|
Stock price |
$ | 1.12 | $ | 3.50 | $ | 2.80 | ||||||
|
Exercise price |
$ | 1.40 | $ | 1.40 | $ | 1.40 | ||||||
|
Risk-free interest rate |
1.20 |
% |
1.05 |
% |
1.78 |
% | ||||||
|
Volatility |
130.64 |
% |
132.05 |
% |
144.25 |
% | ||||||
|
Dividend yield |
0.00 |
% |
0.00 |
% |
0.00 |
% | ||||||
|
Expected life in years |
5.00 | 4.85 | 4.36 | |||||||||
|
Calculated fair value per warrant |
$ | 0.95 | $ | 3.20 | $ | 2.55 | ||||||
In connection with the offering completed on August 26, 2016, the Company issued 5,606,250 warrants to purchase its common shares. These warrants may require the Company to pay the warrant holder cash under certain provisions of the warrant and therefore the Company is accounting for these warrants as a liability. As a result of these warrants being classified as a liability, the Company is required to calculate the fair value of these warrants at each reporting date. The fair value of these warrants is calculated utilizing a Black-Scholes pricing model. The Company calculated the initial fair value of these warrants on August 26, 2016, the date the warrants were issued. As of December 31, 2016, the fair value was remeasured. The following inputs were utilized in the Black-Scholes pricing model during the year ended December 31, 2016:
|
Initial Fair Value Assessment |
||||||||
|
August 26, 2016 |
December 31, 2016 |
|||||||
|
Stock price |
$ | 3.52 | $ | 2.80 | ||||
|
Exercise price |
$ | 4.00 | $ | 4.00 | ||||
|
Risk-free interest rate |
1.23 |
% |
1.85 |
% | ||||
|
Volatility |
135.04 |
% |
140.47 |
% | ||||
|
Dividend yield |
0.00 |
% |
0.00 |
% | ||||
|
Expected life in years |
5.00 | 4.65 | ||||||
|
Calculated fair value per warrant |
$ | 3.04 | $ | 2.38 | ||||
The following table presents a reconciliation of the warrant liability measured at fair value using unobservable inputs (Level 3) (in thousands):
|
Year Ended December 31, 2016 |
||||
|
Liabilities: |
||||
|
Balance at beginning of period |
$ | — | ||
|
Calculated fair value of warrants on May 11, 2016, date of issuance |
1,687 | |||
|
Calculated fair value of warrants on August 26, 2016, date of issuance |
17,060 | |||
|
Fair value of warrants exercised and recorded as an adjustment to contributed capital |
(5,681 |
) | ||
|
Increase in the fair value of warrant liability |
330 | |||
|
Balance at end of period |
$ | 13,396 | ||
Stock-based compensation liability
The Company calculates the fair value of the stock-based compensation liability for those stock options with exercise prices denominated in Canadian Dollars (level 3) at each reporting period utilizing a Black-Scholes pricing model. The following inputs were utilized in the Black-Scholes pricing model:
|
December 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Stock price at the end of each reporting period |
$ | 2.80 | $ | 1.78 | ||||
|
Weighted average exercise price |
$ | 11.06 | $ | 13.12 | ||||
|
Risk-free interest rate |
0.85 |
% |
0.91 |
% | ||||
|
Volatility |
120.81 |
% |
182.74 |
% | ||||
|
Dividend yield |
0.00 |
% |
0.00 |
% | ||||
|
Expected life in years |
0.85 | 1.53 | ||||||
|
Calculated fair value per stock option |
$ | 0.33 | $ | 0.74 | ||||
The following table presents a reconciliation of the stock-based compensation liability measured at fair value using unobservable inputs (Level 3) (in thousands):
|
For the Years Ended December 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Liabilities: |
||||||||
|
Balance at beginning of period |
$ | 168 | $ | 22 | ||||
|
Change in fair value of stock-based compensation liability recorded as an adjustment to contributed surplus |
(111 |
) |
146 | |||||
|
Balance at end of period |
$ | 57 | $ | 168 | ||||
The Company recognizes transfers into and out of levels within the fair value hierarchy at the end of the reporting period in which the actual event or change in circumstances that caused the transfer occurs. There were no transfers of assets or liabilities between the fair value measurement classifications.
|
|
|
7. |
Prepaid expenses |
Prepaid expenses as of December 31, 2016 and 2015 consisted of the following (in thousands):
|
December 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Prepaid insurance |
$ | 273 | $ | 261 | ||||
|
Prepaid research and development expenses |
546 | 176 | ||||||
|
Other prepaid expenses |
27 | 30 | ||||||
|
Prepaid Expenses |
$ | 846 | $ | 467 | ||||
As of December 31, 2016 and 2015, prepaid research and development expenses includes $0.5 million and $0.2 million, respectively for upfront fees paid to the Company’s clinical research organizations assisting with the Company’s clinical trials. The upfront fees will be relieved in future periods based upon work completed.
|
8. |
Property and equipment |
Property and equipment consisted of the following (in thousands):
|
December 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Equipment |
$ | 5 | $ | 5 | ||||
|
Computer hardware and software |
23 | 43 | ||||||
|
Leasehold improvements |
155 | 155 | ||||||
|
Furniture and fixtures |
72 | 72 | ||||||
| 255 | 275 | |||||||
|
Less: accumulated depreciation |
(251 |
) |
(258 |
) | ||||
|
Property and equipment, net |
$ | 4 | $ | 17 | ||||
Depreciation expense was $12,000, $20,000 and $47,000 for the years ended December 31, 2016, 2015 and 2014, respectively.
|
9. |
Accrued expenses |
Accrued expenses as of December 31, 2016 and 2015 consisted of the following (in thousands):
|
December 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Accrued personnel related costs |
$ | 1,491 | $ | 224 | ||||
|
Accrued interest |
— | 42 | ||||||
|
Accrued research and development expenses |
87 | 78 | ||||||
|
Accrued audit and tax services |
129 | 182 | ||||||
|
Other accrued expenses |
55 | 40 | ||||||
|
Accrued expenses |
$ | 1,762 | $ | 566 | ||||
|
10. |
Promissory notes |
On June 30, 2014, the Company entered into a $6.0 million Loan and Security Agreement with Oxford Finance LLC, or Oxford. The principal borrowed under the loan included fixed interest of 9.504% per annum. The Company had the option to prepay the outstanding balance of the loan in full, subject to a prepayment fee of 1% to 3% depending upon when the prepayment occurred. Upon the final repayment of the loan on the maturity date, by prepayment, or upon acceleration, the Company was required to pay Oxford an additional fee of $300,000. This additional fee was recorded as a debt discount and was recognized as interest expense over the life of the loan utilizing the effective interest method.
On September 2, 2016, the Company repaid the outstanding principal balance of the Oxford loan. The total payoff was approximately $4.2 million, which included the final payment of $300,000, a prepayment fee of $39,000, accrued interest of $2,000 and legal fees of $4,000. The Company had $159,000 of unamortized debt premium as of the date of the payoff. The debt repayment was accounted for as an extinguishment as per ASC 470-50, “Debt: Modification and Extinguishments”, and a loss on early extinguishment of the debt totaling $180,000 was recorded for the year ended December 31, 2016, consisting of the final payment and prepayment fee which was offset by the unamortized debt premium.
The following table summarizes interest expense (in thousands) for the periods presented:
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Stated interest |
$ | 292 | $ | 553 | $ | 500 | ||||||
|
Amortization of debt discount |
81 | 137 | 188 | |||||||||
|
Amortization of promissory notes issuance costs |
— | — | 38 | |||||||||
|
Interest expense |
$ | 373 | $ | 690 | $ | 726 | ||||||
|
11. |
Shareholders’ equity |
Shares issued in public offering
On August 26, 2016, the Company completed a public offering whereby it issued 7,475,000 common shares at a price of $4.00 per share. The Company received $27.4 million, net of underwriters’ discounts, commissions and offering cost. Additionally, for each common share purchased, the investors received a warrant to purchase 0.75 of a common share of the Company at an exercise price of $4.00 per full share for a period of five years from August 26, 2016.
In connection with this offering, the Company entered into a Purchase Agreement with Piper Jaffray & Co., as representative of the several underwriters named therein, or the August Purchase Agreement.
For a period of two years after August 26, 2016, the August Purchase Agreement prohibits the Company from entering into a variable rate transaction and prohibits the Company from issuing any securities which have a price that will be determined at a future date.
The common share warrants are recorded as a liability and then marked to market each period through earnings in other income (expense) each period as the warrants included in this transaction contain a “fundamental change” provision, which may in certain circumstances allow the common share warrants to be redeemed for cash at an amount equal to the Black-Scholes Value, as defined by the warrant agreements. In addition, the warrants include a “failure to timely deliver shares” provision, which may require the Company to pay cash to the warrant holder in certain circumstances as defined by the warrant agreements. See a discussion on the calculation of the fair value associated with these warrants at Note 6.
In connection with this offering the Company incurred offering costs of approximately $2.5 million. The Company allocated these offering costs between the estimated fair value of the common shares and the fair value of the warrants on the date of their issuance. The Company allocated approximately $1.1 million to the common shares which was recorded as a reduction to equity. The remaining $1.4 million was allocated to the warrants. The amount allocated to the warrants was expensed and included as a component of general and administrative expenses for the year ended December 31, 2016 as the warrants are classified as liabilities.
Shares issued in registered direct transaction
On May 11, 2016, the Company completed an offering in which net proceeds of approximately $4.6 million was raised by selling 3,571,428 common shares at a price of $1.40 per share. Additionally, for each common share purchased, the investors received a warrant to purchase one-half of a common share of the Company at an exercise price of $1.40 per full share for a period of five years from May 11, 2016. During the year ended December 31, 2016, 1,775,714 of these warrants were exercised which generated proceeds of $2.5 million.
The common share warrants are recorded as a liability and then marked to market each period through earnings in other income (expense) each period as the warrants included in this transaction contain a “fundamental change” provision, which may in certain circumstances allow the common share warrants to be redeemed for cash at an amount equal to the Black-Scholes Value, as defined by the warrant agreements. In addition, the warrants include a “failure to timely deliver shares” provision, which may require the Company to pay cash to the warrant holder in certain circumstances as defined by the warrant agreements. See a discussion on the calculation of the fair value associated with these warrants at Note 6.
In connection with this offering the Company incurred offering costs of approximately $0.4 million. The Company allocated these offering costs between the estimated fair value of the common shares and the fair value of the warrants on the date of their issuance. The Company allocated approximately $0.3 million to the common shares which was recorded as a reduction to equity. The remaining $0.1 million was allocated to the warrants. This amount was expensed and included as a component of general and administrative expenses for the year ended December 31, 2016 as the warrants are classified as liabilities.
Common stock purchase agreement with Aspire Capital
On May 16, 2014, the Company entered into a common stock purchase agreement, or the Purchase Agreement, with Aspire Capital Fund, LLC, or Aspire Capital, which provided that Aspire Capital was committed to purchase up to an aggregate of $15.0 million of the Company’s common shares over the approximately 30-month term of the Purchase Agreement.
In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, the Company issued to Aspire Capital 90,635 of the Company’s common shares. Upon the execution of the Purchase Agreement, the Company sold to Aspire Capital 604,230 common shares at $3.31 per share for net proceeds of $1.9 million which was recorded as in increase to common shares on the balance sheet. The Company incurred offering costs of $0.1 million associated with this transaction.
During the fourth quarter of 2015, Aspire Capital purchased 400,000 common shares under the Purchase Agreement, resulting in net proceeds to the Company of $0.8 million.
The Company did not issue any securities under the Aspire Purchase Agreement during the year ended December 31, 2016. This agreement expired on December 23, 2016.
Authorized
As of December 31, 2016 and 2015, the Company had unlimited shares of no par common shares authorized. There were 30.1 million and 17.2 million common shares issued and outstanding as of December 31, 2016 and 2015, respectively.
Shares reserved for future issuance
The shares reserved for future issuance as of December 31, 2016, 2015 and 2014 consisted of the following (in thousands):
|
December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Common share purchase warrants |
5,965 | 589 | 1,001 | |||||||||
|
Stock options |
||||||||||||
|
Granted and outstanding |
2,868 | 1,677 | 1,378 | |||||||||
|
Reserved for future grants |
143 | 8 | 306 | |||||||||
| 8,976 | 2,274 | 2,685 | ||||||||||
|
12. |
Common share purchase warrants |
At December 31, 2016 and 2015 there were 5,965,000 and 589,000 common share purchase warrants outstanding at a weighted average exercise price of $4.97 and $22.56, respectively. During the year ended December 31, 2016, 7,392,000 common share purchase warrants were issued, 1,775,714 warrants were exercised and 240,000 warrants expired unexercised.
The following table summarizes the expiration dates for the Company’s outstanding common share purchase warrants as of December 31, 2016 (in thousands):
|
Number of warrants outstanding |
Exercise Price |
Expiration date | ||||
| 240 | $ | 26.06 |
March 28, 2017 | |||
| 27 | $ | 28.17 |
July 15, 2018 | |||
| 10 | $ | 1.40 |
May 11, 2021 | |||
| 82 | $ | 2.19 |
June 30, 2021 | |||
| 5,606 | $ | 4.00 |
August 26, 2021 | |||
| 5,965 | ||||||
|
13. |
Stock-based compensation plan |
The Company’s Amended and Restated 2011 Stock Option plan, or the Plan, provides for the granting of options for the purchase of common shares of the Company at the fair value of the Company’s common shares on the date of the option grant. Options are granted to employees, directors and non-employees. The board of directors or a committee appointed by the board of directors administers the Plan and has discretion as to the number, vesting period and expiry date of each option award. Historically the Company granted options with an exercise price denominated in Canadian dollars prior to the Company’s U.S. IPO. Following the Company’s U.S. IPO the Company has granted options with an exercise price denominated in U.S. dollars.
The Plan is based on a cumulative percentage of options issuable up to 10% of the Company’s outstanding common shares. As of December 31, 2016, 2015 and 2014, there were 142,566, 7,639 and 306,137 shares, respectively, registered and available to be issued under the Plan.
During the year ended December 31, 2016, the Company issued options to purchase 1,289,801 common shares to its directors and employees. These options vest over a three year period for employees and over a one year period for directors. The contractual period for the granted options is five to ten years.
The Company received $92,000 for the exercise of options to purchase common shares during the year ended December 31, 2016, none of which are subject to repurchase. The aggregate intrinsic value of options exercised is calculated as the difference between the exercise price of the option and the closing market price of the Company’s common stock on the date of the exercise. The aggregate intrinsic value of options exercised during the year ended December 31, 2016 was $0.1 million.
The Company recognized stock-based compensation expense as follows (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Research and development |
$ | 143 | $ | 257 | $ | 650 | ||||||
|
General and administrative |
282 | 519 | 1,441 | |||||||||
|
Total |
$ | 425 | $ | 776 | $ | 2,091 | ||||||
In connection with the Company’s May 2016 completed reduction in workforce, the Company’s Board of Directors approved certain modifications to the outstanding stock options of terminated employees. All outstanding vested stock options were modified so that the expiration date of the vested options at the time of each employee’s termination would be extended to the remaining life of the stock options. As a result of this modification, the Company recorded non-cash stock-based compensation expense of approximately $76,000 during the year ended December 31, 2016. In addition, all outstanding unvested stock options were modified so that the expiration date of the unvested options on the date of each employee’s termination would be extended for one year from the termination date and the unvested options would automatically vest if a change of control occurred prior to the modified option expiration date. An additional stock-based compensation charge associated with the modification of the unvested options will be recorded if a change in control occurs prior to the expiration of the unvested options.
As of December 31, 2016 there were $2.1 million of unrecognized compensation costs related to unvested stock options. As of December 31, 2016 the Company expects to recognize those costs over weighted average period of 1.6 years.
The fair values of options granted during the year ended December 31, 2016, 2015 and 2014 were estimated at the date of grant using the following weighted-average assumptions:
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Expected life of the option term (years) |
3.9 | 3.5 | 3.7 | |||||||||
|
Risk-free interest rate |
1.5 |
% |
1.0 |
% |
1.2 |
% | ||||||
|
Dividend rate |
0.0 |
% |
0.0 |
% |
0.0 |
% | ||||||
|
Volatility |
144.0 |
% |
128.4 |
% |
76.2 |
% | ||||||
Expected Life of the Option Term – This is the period of time that the options granted are expected to remain unexercised. Options granted have a contractual term of five to ten years. The Company estimates the expected life of the option term based on actual past behavior for similar options.
Risk-Free Interest Rate – This is the United States Treasury rates, as applicable, for the week of each option grant during the year having a term that most closely resembles the expected life of the option.
Dividend Rate – The Company has never declared or paid dividends on common shares and has no plans to do so in the foreseeable future.
Volatility – Volatility is a measure of the amount by which a financial variable such as a share price has fluctuated or is expected to fluctuate during a period. The Company considered the historical volatility from its Canadian initial public offering through the dates of grants.
The following table summarizes stock option activity, including options issued to employees, directors and non-employees (in thousands, except per share and contractual term data):
|
Options |
Weighted |
Weighted Average |
Aggregate |
|||||||||||||
|
Outstanding at January 1, 2014 |
1,362 | $ | 7.10 | 4.5 | $ | — | ||||||||||
|
Options granted |
87 | 2.57 | ||||||||||||||
|
Options expired |
(5 |
) |
24.91 | |||||||||||||
|
Options forfeited |
(66 |
) |
8.77 | |||||||||||||
|
Outstanding at December 31, 2014 |
1,378 | $ | 6.65 | 3.6 | $ | — | ||||||||||
|
Options granted |
302 | 0.54 | ||||||||||||||
|
Options expired |
(3 |
) |
29.16 | |||||||||||||
|
Outstanding at December 31, 2015 |
1,677 | $ | 5.52 | 2.9 | $ | 374 | ||||||||||
|
Options granted |
1,290 | 2.21 | ||||||||||||||
|
Options exercised |
(41 |
) |
2.26 | |||||||||||||
|
Options expired |
(56 |
) |
28.94 | |||||||||||||
|
Options forfeited |
(2 |
) |
4.41 | |||||||||||||
|
Outstanding at December 31, 2016 |
2,868 | $ | 3.63 | 4.8 | $ | 1,431 | ||||||||||
|
Vested or expected to vest at December 31, 2016 |
2,799 | $ | 3.42 | 4.7 | $ | 1,390 | ||||||||||
|
Exercisable at December 31, 2016 |
1,454 | $ | 4.62 | 1.9 | $ | 442 | ||||||||||
The total amounts for options outstanding, vested or expected to vest, and exercisable at December 31, 2016 include options with exercise prices denominated in Canadian dollars and U.S. Dollars. The Canadian dollar amounts have been converted to U.S. dollars for purposes of the calculation.
The weighted average fair value of options granted during the years ended December 31, 2016, 2015 and 2014 was approximately $1.88, $0.42, and $1.41, respectively.
The aggregate intrinsic value was calculated as the difference between the exercise price of the stock options converted to U.S. dollars and the fair value of the Company’s common stock as of the respective balance sheet date. The Company settles employee stock option exercises with newly issued common shares.
|
|
|
14. |
License agreements |
Kissei Agreement
In April 2010, the Company entered into an exclusive license agreement for the development and commercialization of topsalysin (and other products covered by the licensed patent). The agreement with Kissei Pharmaceuticals Co., Ltd., a Japanese pharmaceutical company, or Kissei, covers the development and commercialization of topsalysin in Japan for the treatment of the symptoms of BPH, prostate cancer, prostatitis or other diseases of the prostate. Pursuant to the agreement in 2010, the Company received an upfront license payment of $3.0 million. The Company has determined that the deliverables under this agreement included the license, the transfer of relevant technical information and participation in a periodic development meeting. The Company recognized the entire upfront license payment upon receipt as the license was deemed to have stand-alone value and no significant undelivered performance obligations were identified in connection with the license.
The agreement also notes that the Company shall supply Kissei with bulk material under a separate supply agreement for use in future clinical studies and, if approved, for commercial sales. The license agreement also notes that if the Company is unwilling or unable to supply Kissei with the necessary bulk material that Kissei will have the option to manufacture the bulk material themselves or they can outsource the manufacturing to a third party. To date the Company and Kissei have not signed a supply agreement.
The agreement also provides that the Company shall have full responsibility, including financial responsibility, for filing, prosecuting and maintaining all of the patents in Japan during the term of the agreement. The filing of patents is an administrative and perfunctory deliverable. The associated costs are immaterial. The prosecution and maintenance of patents is not considered an undelivered performance obligation.
During the year ended December 31, 2013, the Company recorded as revenue a $5.0 million non-refundable substantive milestone payment due from Kissei upon the achievement of certain development activities during the year ended December 31, 2013, as such milestone had been achieved during this period. In accordance with the Company’s revenue recognition policy, the Company recognizes the receipt of milestone payments in accordance with the milestone method in the period in which the underlying triggering event occurs. The Company received payment for the milestone in April 2013.
In addition to the upfront license payment and the $5.0 million milestone payment recognized as revenue during the year ended December 31, 2013, the Company is entitled to receive up to $67.0 million of non-refundable milestone payments as follows: a total of $12.0 million for the BPH indication, of which $7.0 million relates to the completion of regulatory approvals and $5.0 million relates to the achievement of certain product sale goals; a total of $21.0 million for the prostate cancer indication, of which $7.0 million relates to the completion of certain development activities, $7.0 million relates to the completion of regulatory approvals and $7.0 million relates to the achievement of certain product sale goals; and a total of $21.0 million for prostatitis or other diseases of the prostate, of which $7.0 million relates to the completion of certain development activities, $7.0 million relates to the completion of regulatory approvals and $7.0 million relates to the achievement of certain product sale goals. An additional $13.0 million of aggregate milestone payments are not indication specific, of which $5.0 million relates to the completion of regulatory approvals and $8.0 million relates to the achievement of certain product sale goals.
Management evaluated the nature of the events triggering these additional milestone payments, and concluded that these events fall into two categories: (a) events which involve the performance of the Company’s obligations under the Kissei license agreement, and (b) events which do not involve the performance of the Company’s obligations under the Kissei license agreement.
Milestone payments which involve the performance of the Company’s obligations include activities related to the completion of development activities and regulatory approvals in the United States. Management concluded that each of these payments constitutes a substantive milestone. This conclusion was based primarily on the facts that (i) each triggering event represents a specific outcome that can be achieved only through successful performance by the Company of one or more of its deliverables, (ii) achievement of each triggering event was subject to inherent risk and was not reasonably assured at the inception of the agreement, (iii) each of these milestones is non-refundable, (iv) substantial effort is required to complete each milestone, (v) the amount of each milestone payment is reasonable in relation to the value created in achieving the milestone, (vi) a substantial amount of time is expected to pass between the up-front payment and the potential milestone payments, and (vii) the milestone payments relate solely to past performance. Based on the foregoing, the Company recognizes any revenue from these milestone payments under the milestone method in the period in which the underlying triggering event occurs.
Milestone payments which do not involve the performance of the Company’s obligations include the completion of development activities, regulatory approvals and certain product sale goals in Japan, all of which are areas in which the Company has no pertinent contractual responsibilities under the agreement. Management concluded that these milestones are not substantive and will be recognized in accordance with the Company’s accounting policy for revenue recognition. The following table breaks down the remaining unpaid milestone payments by indication or, in the case of milestones not associated with a specific indication, by triggering events and by involvement of the Company:
|
Milestone Payments Performance (in millions) |
Milestone Payments Not Performance (in millions) |
|||||||
|
Milestones by Indication |
||||||||
|
BPH |
— | $ | 12 | |||||
|
Prostate cancer |
— | $ | 21 | |||||
|
Prostatitis and other diseases of the prostate |
— | $ | 21 | |||||
|
Milestones Not Associated with an Indication |
||||||||
|
Gross sale targets |
— | $ | 8 | |||||
|
Regulatory approvals |
$ | 5 | — | |||||
The Company may also receive a drug supply fee, assuming the Company supplies material to Kissei, and royalty payments in the 20-29% range as a percentage of future net sales of licensed products sold under the agreement.
Kissei is not currently studying topsalysin for the treatment of prostate cancer, prostatitis or other diseases of the prostate. In addition, Kissei has the option to sublicense the development and commercialization for topsalysin in their territory.
Topsalysin license agreement for Benign Prostate Hyperplasia
In 2009, the Company signed an exclusive license agreement with UVIC Industry Partnerships Inc., or UVIC, and The Johns Hopkins University, or Johns Hopkins, with respect to the use of topsalysin for the treatment of the symptoms of benign prostate hyperplasia and other non-cancer diseases and conditions of the prostate. The license agreement requires the Company to make payments of CND$1.3 million in the aggregate on the achievement of certain clinical and regulatory milestones and to pay royalties on commercial sales of resulting products. To the extent the Company receives any milestone payments relating to the development of therapeutics for the treatment of the symptoms of BPH under its exclusive license agreement with Kissei, the Company is obligated to pay a percentage of such consideration, which percentage is in the 10-19% range, to UVIC and Johns Hopkins; however, pursuant to a separate agreement which the Company entered into in 2003 with Dr. J. Thomas Buckley, one of the Company’s founders, the aggregate amount of such consideration payable by the Company to UVIC and Johns Hopkins is reduced by 25% .
From the inception of the agreement, the Company has incurred sub-license fees of $0.6 million and milestone payments of $0.1 million under this agreement.
Topsalysin License Agreement for Prostate Cancer
In 2004, the Company licensed exclusive rights to topsalysin for the treatment of prostate cancer under an agreement with UVIC and Johns Hopkins. The Company has agreed to make cumulative milestone payments over the lifecycle of topsalysin of up to CND$3.6 million on the achievement of certain clinical and regulatory milestones and to pay royalties on commercial sales of resulting products. From the inception of the agreement the Company has paid milestone payments of CND$0.1 million and at December 31, 2016, CND$0.1 million is included in accrued expenses. To date, the Company has completed three clinical trials in patients with prostate cancer.
|
|
|
15. |
Income taxes |
The component of the loss before provision for income taxes were as follows (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
United States |
$ | (521 |
) |
$ | (1,389 |
) |
$ | (2,147 |
) | |||
|
Canada |
(10,643 |
) |
(12,808 |
) |
(28,565 |
) | ||||||
|
Loss before provision for income taxes |
$ | (11,164 |
) |
$ | (14,197 |
) |
$ | (30,712 |
) | |||
The components of the provision for income taxes from continuing operations is as follows (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Current Tax: |
||||||||||||
|
Canada |
$ | — | $ | — | $ | — | ||||||
|
US |
— | — | — | |||||||||
|
State |
— | — | — | |||||||||
| $ | — | $ | — | $ | — | |||||||
|
Deferred Tax: |
||||||||||||
|
Canada |
$ | — | $ | — | $ | — | ||||||
|
US |
— | — | — | |||||||||
|
State |
— | — | — | |||||||||
| — | — | — | ||||||||||
| $ | — | $ | — | $ | — | |||||||
A reconciliation of income taxes to the amount computed by applying the statutory federal income tax rate to the net loss is as follows (in thousands, except income tax rates):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Combined federal and provincial income tax rates |
26.00 |
% |
26.00 |
% |
26.00 |
% | ||||||
|
Income tax benefit at statutory rates |
$ | (2,902 |
) |
$ | (3,691 |
) |
$ | (7,982 |
) | |||
|
State income tax, net of federal benefit |
1 | (63 |
) |
(88 |
) | |||||||
|
Permanent items |
(17 |
) |
36 | 34 | ||||||||
|
Tax credits |
— | (105 |
) |
(1,007 |
) | |||||||
|
Non-deductible stock-based compensation |
81 | 155 | 253 | |||||||||
|
Foreign accrual property income |
69 | 48 | 67 | |||||||||
|
Expired NOLs |
79 | 887 | 299 | |||||||||
|
Return to provision true up |
60 | (320 |
) |
83 | ||||||||
|
Uncertain tax positions |
68 | 296 | — | |||||||||
|
Rate differential |
60 | (175 |
) |
(229 |
) | |||||||
|
Rate change |
183 | — | — | |||||||||
|
Other |
(112 |
) |
(57 |
) |
152 | |||||||
|
Revaluation of warrant liability |
86 | — | (13 |
) | ||||||||
|
CTA |
(312 |
) |
(1,702 |
) |
2,028 | |||||||
|
Change in valuation allowance |
2,656 | 4,691 | 6,403 | |||||||||
|
Income tax expense |
$ | — | $ | — | $ | — | ||||||
Significant components of the Company’s deferred tax assets as of December 31, 2016 and 2015 are shown below (in thousands):
|
December 31, |
||||||||
|
2016 |
2015 |
|||||||
|
Deferred tax assets: |
||||||||
|
Net operating loss carryforwards (non-capital losses) |
$ | 32,140 | $ | 29,730 | ||||
|
Scientific research and development |
2,509 | 2,509 | ||||||
|
Tax credits |
4,066 | 4,145 | ||||||
|
Stock based compensation |
1,116 | 1,287 | ||||||
|
Other, net |
529 | 91 | ||||||
|
Share issue costs |
1,042 | 668 | ||||||
|
Total deferred tax assets, net, before valuation allowance |
41,402 | 38,430 | ||||||
|
Valuation allowance |
(41,402 |
) |
(38,430 |
) | ||||
|
Net deferred tax assets |
$ | — | $ | — | ||||
Under current GAAP, in a classified statement of financial position, deferred tax assets and liabilities are separated into a current amount and a non-current amount on the basis of the classification of the related asset or liability for financial reporting. Deferred tax assets and liabilities that are not related to an asset or liability for financial reporting are classified according to the expected reversal date of the temporary difference.
On November 20, 2015 the FASB issued ASU 2015-17, Income Taxes (Topic 740) Balance Sheet classification of Deferred Taxes, which requires non-current classification of all deferred tax assets and liabilities for all public entities for annual periods beginning after December 15, 2016. The Company elected to early adopt ASU 2015-17 on a retrospective basis and presented all of its deferred tax assets and liabilities as non-current as of December 31, 2016 and 2015.
Due to the operating losses since inception, a valuation allowance has been recognized to offset net deferred assets as realization of such deferred tax assets is not more likely than not. During the years ended December 31, 2016 and 2015, the valuation allowance on the deferred tax assets increased by $3.0 million and $4.7 million, respectively.
At December 31, 2016, the Company has net operating loss carryforwards for income tax purposes in Canada and in the United States which may be used to reduce taxable income. The income tax benefit, if any, of these losses has not been recorded due to the uncertainty of its recovery. Based upon statute, losses are expected to expire as follows (in thousands):
|
Expiration date |
Canada |
U.S. Federal |
Total |
|||||||||
|
2026 |
$ | 3,389 | $ | — | $ | 3,389 | ||||||
|
2027 |
5,006 | — | 5,006 | |||||||||
|
2028 |
5,696 | — | 5,696 | |||||||||
|
2029 |
4,539 | — | 4,539 | |||||||||
|
2030 |
4,176 | — | 4,176 | |||||||||
|
2031 |
12,230 | — | 12,230 | |||||||||
|
2032 |
18,380 | — | 18,380 | |||||||||
|
2033 |
13,699 | — | 13,699 | |||||||||
|
2034 |
29,608 | 208 | 29,816 | |||||||||
|
2035 |
14,288 | 1,148 | 15,436 | |||||||||
|
2036 |
10,792 | — | 10,792 | |||||||||
| $ | 121,803 | $ | 1,356 | $ | 123,159 | |||||||
In addition, the Company has $1.3 million of U.S. state net operating loss carryforwards which begin to expire in 2034.
At December 31, 2016, the Company had investment tax credits in Canada and research and development tax credits in the United States that expire as follows (in thousands):
|
Expiration date |
Canada |
U.S. Federal |
Total |
|||||||||
|
2017 |
$ | 140 | — | $ | 140 | |||||||
|
2018 |
200 | — | 200 | |||||||||
|
2019 |
194 | — | 194 | |||||||||
|
2020 |
41 | — | 41 | |||||||||
|
2021 |
9 | — | 9 | |||||||||
|
2023 |
33 | — | 33 | |||||||||
|
2024 |
112 | — | 112 | |||||||||
|
2025 |
236 | — | 236 | |||||||||
|
2026 |
229 | — | 229 | |||||||||
|
2027 |
356 | — | 356 | |||||||||
|
2028 |
447 | — | 447 | |||||||||
|
2029 |
565 | — | 565 | |||||||||
|
2030 |
176 | — | 176 | |||||||||
|
2031 |
26 | 56 | 82 | |||||||||
|
2032 |
— | 335 | 335 | |||||||||
|
2033 |
— | 249 | 249 | |||||||||
|
2034 |
— | 908 | 908 | |||||||||
|
2035 |
— | 42 | 42 | |||||||||
| $ | 2,764 | $ | 1,590 | $ | 4,354 | |||||||
In addition, the Company has $0.6 million of California research and development tax credits which carry forward indefinitely as well as foreign tax credits in Canada of $0.2 million that begin to expire in 2023.
The Company’s Canadian tax years are subject to inspection from 2011 forward. The Company’s United States federal and California 2011 tax returns are subject to examination by taxing authorities.
The future utilization of the Company’s research and development credit carry forwards and net operating loss carry forwards to offset future taxable income may be subject to an annual limitation as a result of ownership changes that may have occurred previously or may occur in the future. The Tax Reform Act of 1986, or the Act, limits a company’s ability to utilize certain tax credit carry forwards and net operating loss carry forwards in the event of a cumulative change in ownerships in excess of 50% as defined in the Act.
In 2011, the Company adopted the recognition and measurement principals under ASC740, “Income Taxes” (ASC740) regarding the recognition of tax benefits. In accordance with ASC740, tax benefits are only recognized when a position is more likely than not of being sustained. Tax benefits are then measured using a cumulative benefit approach whereby the largest amount of tax benefit that is more likely than not of being sustained is recognized.
The following table summarizes the activity related to the Company’s unrecognized tax benefits (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2016 |
2015 |
2014 |
||||||||||
|
Beginning balance |
$ | 325 | $ | — | $ | — | ||||||
|
Increase related to prior year tax positions |
103 | 304 | — | |||||||||
|
Increase related to current year tax positions |
— | 21 | — | |||||||||
|
Ending balance |
$ | 428 | $ | 325 | $ | — | ||||||
The amount of unrecognized tax benefit that, if recognized and realized, would affect the effective tax rate is zero as of December 31, 2016. To the extent unrecognized tax benefits are recognized at a time such valuation allowance no longer exists, the addition amount that would affect the effective tax rate is approximately $0.4 million. The Company does not anticipate any significate decreases in its unrecognized tax benefits over the next 12 months. The Company recognizes interest and/or penalties related to income tax matters in income tax expense. For the years ended December 31, 2016 and 2015, the Company has not recognized any interest or penalties related to income taxes.
On December 18, 2015, the Protecting Americans from Tax Hikes Act was signed into law reinstating the federal research and development credit for 2015. Accordingly, the impact related to the 2015 federal research and development credit was treated as a discrete tax item during the fourth quarter of 2015.
|
16. |
Commitments and contingencies |
Operating leases
The Company leases a facility, comprising the Company’s headquarters, located in San Diego, California under a non-cancelable lease. During September 2016, the Company exercised a one-year lease extension on its headquarters in San Diego, California. As a result of this extension, the expiration date for the Company’s headquarters was extended from May 2017 to May 2018. The rent on the Company’s headquarters is currently $8,946 per month.
Total rent expense under operating leases was $0.1 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Future minimum lease payments under non-cancelable operating leases at December 31, 2016 are as follows (in thousands):
|
Future rent payments |
||||
|
2017 |
$ | 122 | ||
|
2018 |
51 | |||
|
Total |
$ | 173 | ||
License agreements
The Company has license agreements with third parties that require the Company to make annual license maintenance payments and contingent future payments upon the success of licensed products that include milestone and/or royalties. Minimum future payments over the next five years are not material.
|
17. |
401(k) Plan |
Effective July 2012, the Company established a deferred compensation plan, or the 401(k) Plan, pursuant to Section 401(k) of the Internal Revenue Code of 1986 where by all employees, subject to certain age requirement can contribute pretax earnings to the plan. The Company makes safe harbor contributions to the 401(k) Plan up to 4% of eligible compensation, subject to limitations under the Code. The Company’s total contributions to the 401(k) Plan were $0.1 million for each of the years ended December 31, 2016, 2015 and 2014.
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosures |
None.
|
Item 9A. |
Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our periodic and current reports that we file with the SEC is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable and not absolute assurance of achieving the desired control objectives. In reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. In addition, the design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, control may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
As of December 31, 2016, we carried out an evaluation, under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act. Based on this evaluation, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2016.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Exchange Act Rule 13a-15(f). Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our principal executive and financial officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
As of December 31, 2016, our management assessed the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013 Framework). Based on this assessment, our management concluded that, as of December 31, 2016, our internal control over financial reporting was effective based on those criteria. We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the quarter ended December 31, 2016 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
|
Item 9B. |
Other Information |
Not applicable.
Part III.
Certain information required by Part III of this Annual Report on Form 10-K is omitted from this report because the registrant will file a definitive Proxy Statement within 120 days after the end of its fiscal year pursuant to Regulation 14A for its 2017 Annual Meeting of Stockholders to be held within 180 days of December 31, 2016, referred to as the Proxy Statement, and the information included therein is incorporated herein by reference.
|
Item 10. |
Directors, Executive Officers and Corporate Governance |
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Election of Directors” “Executive Officers” and “Section 16(a) Beneficial Ownership Reporting Compliance.”
We have adopted a code of ethics for directors, officers (including our principal executive officer, principal financial officer and principal accounting officer) and employees, known as the Code of Business Conduct and Ethics. The Code of Business Conduct and Ethics is available on our website at http://www.sophirisbio.com under the Corporate Governance section of our Investor Relations page. We will promptly disclose on our website (i) the nature of any amendment to the policy that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and (ii) the nature of any waiver, including an implicit waiver, from a provision of the policy that is granted to one of these specified individuals that is required to be disclosed pursuant to SEC rules and regulations, the name of such person who is granted the waiver and the date of the waiver.
|
Item 11. |
Executive Compensation |
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Executive and Director Compensation” and “Compensation Committee Interlocks and Insider Participation.”
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Principal Shareholders” and “Equity Compensation Plan Information.”
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Election of Directors” and “Certain Relationships and Related Party Transactions.”
|
Item 14. |
Principal Accounting Fees and Services |
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the section entitled “Principal Accountant Fees and Services” and “Pre-Approval Policies and Procedures.”
Part IV.
|
Item 15. |
Exhibits, Financial Statements and Schedules |
(a) Documents filed as part of this report.
1. Financial Statements. We have filed the following documents as part of this Annual Report:
|
|
Page |
|
Report of Independent Registered Public Accounting Firm |
71 |
|
Balance Sheets |
72 |
|
Statements of Operations and Comprehensive Loss |
73 |
|
Statements of Shareholders’ Equity |
74 |
|
Statements of Cash Flows |
75 |
|
Notes to Financial Statements |
77 |
2. Financial Statement Schedules.
All schedules are omitted because they are not applicable or the required information is shown in the Financial Statements or notes thereto.
(b) Exhibits
The following exhibits are filed as part of, or incorporated by reference into, this report:
|
Exhibit |
|
Description of Exhibit |
|
Incorporated by Reference or Attached Hereto | |
|
|
|
|
|
|
|
|
3.1 |
|
|
Certificate of Amalgamation of the Company, dated January 1, 2005 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
3.2 |
|
|
Notice of Articles of the Company |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
3.3 |
|
|
Articles of the Company |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
4.1 |
|
|
Form of Common Share Certificate |
|
Incorporated by reference to the Amendment No. 4 to the Registrant’s Form S-1/A (SEC File No. 333-186724) filed on July 15, 2013. |
|
|
|
|
|
|
|
|
4.2 |
|
|
Form of Common Share Purchase Warrant Issued in connection with the subsequent closings pursuant to our Investment Agreement by and between the Company, Warburg Pincus Private Equity X, L.P. and Warburg Pincus X Partners, L.P., dated September 28, 2010. |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
4.3 |
|
|
Common Share Purchase Warrant Issued to Oxford Finance LLC |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
4.4 |
|
|
Common Share Purchase Warrant Issued to Oxford Finance LLC |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
4.5 |
|
|
Omnibus Amendment to Warrants to Purchase Common Shares dated January 31, 2014, 2014 by and between the Company and Warburg Pincus Private Equity X, L.P. and Warburg Pincus X Partners, L.P |
|
Incorporated by reference to the Current Report on Form 8-K February 6, 2014. |
|
|
|
|
|
|
|
|
4.6 |
|
|
Omnibus Amendment to Warrants to Purchase Common Shares dated February 14, 2014 by and between the Company and Oxford Finance LLC |
|
Incorporated by reference to the Current Report on Form 8-K filed on February 18, 2014. |
|
|
|
|
|
|
|
|
4.7 |
|
|
Common Share Purchase Warrant Issued to Oxford Finance LLC dated June 30, 2014 |
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed on August 7, 2014. |
|
|
|
|
|
|
|
|
4.8 |
|
|
Common Share Purchase Warrant Issued to Oxford Finance LLC dated June 30, 2014 |
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed on August 7, 2014. |
|
|
|
|
|
|
|
|
4.9 |
|
|
Registration Rights Agreement by and between the Company and Aspire Capital Fund, LLC dated May 16, 2014. |
|
Incorporated by reference to the Current Report on Form 8-K filed on May 19, 2014. |
|
4.10 |
Form of Common Share Purchase Warrant Issued in connection with the Company’s May 2016 Financing |
Incorporated by reference to the Current Report on Form 8-K filed on May 11, 2016. | |||
|
4.11 |
Form of Common Share Purchase Warrant Issued in connection with the Company’s August 2016 Financing |
Incorporated by reference to the Current Report on Form 8-K filed on August 23, 2016. | |||
|
|
|
|
|
|
|
|
10.1 |
|
|
Amended and Restated 2011 Stock Option Plan |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.2 |
+ |
|
Form of Option Certificate |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.3 |
+ |
|
Form of Indemnification Agreement by and between the Company and each of its directors |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.4 |
+ |
|
Employment Agreement by and between Sophiris Bio Corp. and Allison Hulme, Ph.D., dated March 31, 2011 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.5 |
+ |
|
Employment Agreement between Sophiris Bio Corp. and Randall E. Woods, dated August 16, 2012 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.6 |
+ |
|
Employment Agreement between Sophiris Bio Corp. and Peter Slover, dated March 19, 2012 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
10.7 |
* |
|
Exclusive License Agreement effective September 30, 2004 by and among UVIC Industry Partnerships Inc., The Johns Hopkins University and the Company |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.8 |
|
|
Amendment to Exclusive License Agreement by and among UVIC Industry Partnerships Inc., The Johns Hopkins University and the Company, dated January 10, 2005 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.9 |
* |
|
Exclusive License Agreement effective October 16, 2009 by and among UVIC Industry Partnerships Inc., The Johns Hopkins University and the Company |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
10.10 |
* |
|
Exclusive License Agreement by and between the Company and Kissei Pharmaceuticals Co., Ltd., dated April 28, 2010 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.11 |
|
|
Exclusive License Amending Agreement by and among UVIC Industry Partnerships Inc., The Johns Hopkins University and the Company, dated July 1, 2010, with respect to the Exclusive License Agreement effective September 30, 2004 by and among UVIC Industry Partnerships Inc., The Johns Hopkins University and the Company |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.12 |
|
|
Exclusive License Amending Agreement by and among UVIC Industry Partnerships Inc., The Johns Hopkins University and the Company, dated July 1, 2010, with respect to the Exclusive License Agreement effective October 16, 2009 by and among UVIC Industry Partnerships Inc., The Johns Hopkins University and the Company |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.13 |
|
|
Standard Lease by and between Allison-Zongker, L.P. and the Company, dated April 15, 2011 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.14 |
|
|
First Amendment to that Certain Lease Agreement dated April 15, 2011 by and between Allison-Zongker, L.P. and the Company, effective April 2, 2012 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.15 |
|
|
Indemnification Letter Agreement by and between the Company, Warburg Pincus Private Equity X, L.P. and Warburg Pincus X Partners, L.P., dated November 19, 2010 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.16 |
* |
|
Technology Transfer and Supply Agreement by and between Boehringer Ingelheim RCV GmbH & Co KG and the Company, dated June 29, 2012 |
|
Incorporated by reference to the Registrant’s Form S-1 (SEC File No. 333-186724) filed on February 15, 2013. |
|
|
|
|
|
|
|
|
10.17 |
|
|
Non-employee Director Compensation Program |
|
Incorporated by reference to the Current Report on Form 8-K filed on October 31, 2013. |
|
10.18 |
Agreement Respecting Intellectual Property by and between the Company and Dr. J. Thomas Buckley, dated February 12, 2003, as amended by the Amendment Agreement dated May 5, 2004 |
Incorporated by reference to the Amendment No. 4 to the Registrant’s Form S-1/A (SEC File No. 333-186724) filed on July 15, 2013. | |||
|
10.19 |
+ |
Officer Change in Control Severance Benefit Agreement by and between Randall E. Woods and the Company |
Incorporated by reference to the Quarterly Report on Form 10-Q filed on November 12, 2014. | ||
|
10.20 |
+ |
|
Officer Change in Control Severance Benefit Agreement by and between Allison Hulme and the Company |
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed on November 12, 2014. |
|
10.21 |
+ |
|
Officer Change in Control Severance Benefit Agreement by and between Peter T. Slover and the Company |
|
Incorporated by reference to the Quarterly Report on Form 10-Q filed on November 12, 2014. |
|
10.22 |
Letter Agreement, dated May 6, 2016, by and between the Company and Roth Capital Partners, LLC |
Incorporated by reference to the Current Report on Form 8-K filed on May 11, 2016. |
|
10.23 |
Form of Securities Purchase Agreement, dated May 6, 2016, by and between the Company and the Purchasers thereto |
Incorporated by reference to the Current Report on Form 8-K filed on May 11, 2016. | |||
|
23.1 |
|
|
Consent of Independent Registered Public Accounting Firm |
|
Attached hereto |
|
24.1 |
|
|
Power of Attorney (included on signature page) |
|
Attached hereto |
|
31.1 |
|
|
Certification of Chief Executive Officer pursuant to Rules 13a-14 and 15d-14 promulgated pursuant to the Securities Exchange Act of 1934, as amended |
|
Attached hereto |
|
31.2 |
|
|
Certification of Chief Financial Officer pursuant to Rules 13a-14 and 15d-14 promulgated pursuant to the Securities Exchange Act of 1934, as amended |
|
Attached hereto |
|
|
|
|
|
|
|
|
32.1 |
|
|
Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
|
Attached hereto |
|
|
|
|
|
|
|
|
32.2 |
|
|
Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
|
Attached hereto |
| 101.INS | ** | XBRL Instance Document | Attached hereto | ||
| 101.SCH | ** | XBRL Taxonomy Extension Schema Document | Attached hereto | ||
| 101.CAL | ** | XBRL Taxonomy Extension Calculation Linkbase Document | Attached hereto | ||
| 101.DEF | ** | XBRL Taxonomy Extension Definition Linkbase Document | Attached hereto | ||
| 101.LAB | ** | XBRL Taxonomy Extension Label Linkbase Document | Attached hereto | ||
| 101.PRE | ** | XBRL Taxonomy Extension Presentation Linkbase Document | Attached hereto |
|
+ |
Indicates management contract or compensatory plan. |
|
* |
Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
|
** |
In accordance with Rule 406T of Regulation S-T, the XBRL related information in Exhibit 101 to this Annual Report on Form 10-K is deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act, is deemed not filed for purposes of Section 18 of the Exchange Act, and otherwise is not subject to liability under these sections. |
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on the 27th day of March, 2017.
|
|
SOPHIRIS BIO INC. | |
|
|
|
|
|
|
By: |
/s/ Randall E. Woods |
|
|
|
Randall E. Woods |
|
|
|
Chief Executive Officer and President |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Randall E. Woods and Peter T. Slover, and each of them, his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or their or his substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Randall E. Woods |
|
Chief Executive Officer, President and Director |
|
March 27, 2017 |
|
Randall E. Woods |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/s/ Peter T. Slover |
|
Chief Financial Officer |
|
March 27, 2017 |
|
Peter T. Slover |
|
(Principal Financial Officer and Principal Accounting Officer) |
|
|
|
|
|
|
|
|
|
/s/ Lars Ekman, M.D., Ph.D. |
|
Executive Chairman and Director |
|
March 27, 2017 |
|
Lars Ekman, M.D., Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Allison Hulme, Ph.D. |
|
Chief Operating Officer and Director |
|
March 27, 2017 |
|
Allison Hulme, Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ John Geltosky, Ph.D. |
|
Director |
|
March 27, 2017 |
|
John Geltosky, Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Jim Heppell |
|
Director |
|
March 27, 2017 |
|
Jim Heppell |
|
|
|
|
|
|
|
|
|
|
|
/s/ Gerald Proehl |
|
Director |
|
March 27, 2017 |
|
Gerald Proehl |
|
|
|
|
103
