Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - Great Basin Scientific, Inc. | gbsn-ex322_275.htm |
| EX-32.1 - EX-32.1 - Great Basin Scientific, Inc. | gbsn-ex321_278.htm |
| EX-31.2 - EX-31.2 - Great Basin Scientific, Inc. | gbsn-ex312_276.htm |
| EX-31.1 - EX-31.1 - Great Basin Scientific, Inc. | gbsn-ex311_277.htm |
| EX-23.2 - EX-23.2 - Great Basin Scientific, Inc. | gbsn-ex232_1091.htm |
| EX-23.1 - EX-23.1 - Great Basin Scientific, Inc. | gbsn-ex231_1352.htm |
| EX-3.1 - EX-3.1 - Great Basin Scientific, Inc. | gbsn-ex31_1092.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-36662
GREAT BASIN SCIENTIFIC, INC.
(Exact name of Registrant as specified in its Charter)
|
Delaware |
|
83-0361454 |
|
(State or other jurisdiction of |
|
(I.R.S. Employer |
|
|
|
|
|
420 East South Temple, Suite 520 |
|
84111 |
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (801) 990-1055
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common stock, par value $0.0001 per share
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☐ NO ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). YES ☒ NO ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definition of “large accelerated filer”, “accelerated filer”, and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
Non-accelerated filer |
☐ (Do not check if a small reporting company) |
Small reporting company |
☒ |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the shares of common stock on The NASDAQ Stock Market on June 30, 2016 was $12.6 million.
The number of shares of Registrant’s Common Stock outstanding as of March 17, 2017 was 1,485,904,493.
|
|
|
Page |
|
|
|
|
|
Item 1. |
3 |
|
|
Item 1A. |
21 |
|
|
Item 1B. |
50 |
|
|
Item 2. |
50 |
|
|
Item 3. |
50 |
|
|
Item 4. |
51 |
|
|
|
|
|
|
|
|
|
|
Item 5. |
52 |
|
|
Item 6. |
53 |
|
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
54 |
|
Item 7A. |
66 |
|
|
Item 8. |
66 |
|
|
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
66 |
|
Item 9A. |
67 |
|
|
|
|
|
|
|
|
|
|
Item 10. |
69 |
|
|
Item 11. |
72 |
|
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
76 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
83 |
|
Item 14. |
87 |
|
|
|
|
|
|
|
|
|
|
Item 15. |
89 |
|
|
Item 16. |
94 |
|
|
|
95 |
|
|
|
F-1 |
i
CAUTIONARY NOTE CONCERNING FORWARD-LOOKING STATEMENTS
This annual report on Form 10-K contains forward-looking statements that involve risks and uncertainties. You should not place undue reliance on these forward-looking statements. Our actual results could differ materially from those anticipated in the forward-looking statements for many reasons, including the reasons described in this report at sections “Item 1A. Risk Factors,” “Item 7. Management Discussion and Analysis of Financial Condition and Result of Operations,” and “Item 1. Business”. In some cases, you can identify these forward- looking statements by terms such as “anticipate,” “believe,” “continue,” “could,” “depends,” “estimate,” “expects,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms or other similar expressions, although not all forward-looking statements contain those words.
We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of known and unknown risks, uncertainties and assumptions, including risks described in the section titled “Risk Factors” and elsewhere in this 10-K. Factors that could cause actual results to differ materially from those discussed in the forward-looking statements include, but are not limited to:
|
|
• |
our expectation that for the foreseeable future, substantially all of our revenue will be derived from sales of our C. diff, Group B Strep, Staph ID/R and STEC diagnostic tests; |
|
|
• |
our ability to expand our sales and marketing capabilities to increase demand for C. diff, Group B Strep, Staph ID/R and STEC and any other diagnostic tests we may develop and gain approval for; |
|
|
• |
our ability to develop additional revenue opportunities, including new diagnostic tests; |
|
|
• |
the timing of regulatory submissions; |
|
|
• |
our ability to maintain regulatory approval of our C. diff, Group B Strep, Staph ID/R and STEC diagnostic tests and to obtain and maintain regulatory approval for any other diagnostic test we may develop; |
|
|
• |
approvals for clinical trials may be delayed or withheld by regulatory agencies; |
|
|
• |
pre-clinical and clinical studies may not be successful or confirm earlier results or may not meet expectations, regulatory requirements or performance thresholds for commercial success; |
|
|
• |
risks relating to the timing and costs of clinical trials and other expenses; |
|
|
• |
management and employee operations and execution risks; |
|
|
• |
loss of key personnel; |
|
|
• |
competition in the markets we serve; |
|
|
• |
our ability to manufacture C. diff, Group B Strep, Staph ID/R and STEC and any other diagnostic tests we may develop at sufficient volumes to meet customer needs; |
|
|
• |
our ability to reduce the cost to manufacture our C. diff, Group B Strep, Staph ID/R and STEC and any other diagnostic tests; |
|
|
• |
risks related to market acceptance of diagnostic tests; |
|
|
• |
intellectual property risks; |
|
|
• |
assumptions regarding the size of the available market, benefits of our diagnostic tests, product pricing and timing of product launches; |
|
|
• |
our ability to fund our working capital requirements; |
|
|
• |
risks associated with the uncertainty of future financial results; |
|
|
• |
risks related to our proposed March 2017 Reverse Stock Split; |
|
|
• |
risks related to our outstanding convertible notes, warrants and convertible preferred stock; |
1
|
|
• |
risks associated with our reliance on third party suppliers and other organizations that provide goods and services to us. |
These risks are not exhaustive. Other sections of this annual report on Form 10-K may include additional factors that could adversely impact our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. You should read this annual report on Form 10-K with the understanding that our actual future results, levels of activity, performance and achievements may be materially different from what we expect. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this annual report on Form 10-K or to conform these statements to actual results or to changes in our expectations.
We qualify all of our forward-looking statements by these cautionary statements.
Unless the context requires otherwise references to “Great Basin Scientific,” “Great Basin”, our “Company,” “we,” “us” or “our” refer to Great Basin Scientific, Inc., a Delaware corporation, doing business as Great Basin Corporation.
2
Overview
We are a molecular diagnostic testing company focused on the development and commercialization of our patented, molecular diagnostic platform designed to test for infectious disease, especially hospital-acquired infections. We believe that laboratories in small to medium sized hospitals, those under 400 beds, are in need of simpler and more affordable molecular diagnostic testing methods. We market a system that combines both affordability and ease-of-use, when compared to other commercially available molecular testing methods, which we believe will accelerate the adoption of molecular testing in small to medium sized hospitals. Our system includes an analyzer, which we provide for our customers’ use without charge in the United States, and a diagnostic cartridge, which we sell to our customers. For purposes of this report, we use the term “assay(s)” to describe our existing diagnostic test product as well as our diagnostic test products under development. Our testing platform has the capability to identify up to 64 individual targets at one time. If the assay identifies one to three targets, we refer to them as low-plex tests, or tests, and if they identify four or more targets we refer to them as multi-plex panels, or panels.
We currently have four commercially available tests, our first for clostridium difficile, or C. diff , which received clearance from the United States Food and Drug Administration, or FDA, in April 2012, our second for Group B Strep, which received clearance from the FDA in April 2015 and launched commercially in June 2015, our third for Shiga Toxin Direct, or STEC, which received clearance from the FDA in March 2016 and launched commercially in August 2016 and the fourth Staphylococcus Identification and Resistance Blood Culture Panel, or Staph ID/R panel, which received FDA clearance in March 2016 and launched commercially in September 2016. Our customers consist of hospitals, clinics, laboratories and other healthcare providers in the United States, the European Union and New Zealand.
Molecular diagnostic testing generally reduces test time from days to hours compared to culture methods, and typically provides much more accurate results than non-molecular rapid assays. Culture testing utilizes a sample taken from a patient, which is incubated in a culture medium; the operator waits for the microorganisms, if there are any, to grow until they are in large enough quantities to be detected. This method can take days and in some cases requires highly trained laboratory technicians to perform the tests and interpret the results. The accuracy of culture-based methods has been shown to be lower than that of molecular-based approaches. For example, in a multi-arm, multicenter clinical study using our C. diff test, we increased detection sensitivity nearly 20% as compared to the culture-based arm. Molecular testing methods, like our system, utilize technologies to multiply the DNA from a small sample until it can be detected by an automated, visual system. A key difference between our system and other molecular systems is our use of a low-cost, but highly sensitive, semiconductor chip based detection system. This allows us to utilize existing components, for example digital camera components, to provide visual evidence of the result. This provides more accurate answers generally in hours and can be operated by technicians with less extensive training than is required for culture testing. We believe these advantages can lead to shortened hospital stays and improved patient outcomes, resulting in reduced costs for hospitals that implement molecular testing in their labs. We believe this improvement in the time to result and the quality of those results has led to a fast-growing market for molecular diagnostic systems at hospitals. We believe our system is well positioned to meet this need and attract new customers. As of December 31, 2016, we had 265 customers worldwide (244 in the United States and 21 in the rest of the world), who use an aggregate of 498 of our analyzers.
For additional information about our products, see “Our Assay Menu” below.
Our operations to date have been funded primarily through sales of capital stock, convertible notes, sale-leaseback transactions and cash from operations. We expect to continue developing additional diagnostic tests and conducting clinical trials over the next several years. In addition, while we expect our sales and marketing expenses will increase proportionately as we introduce the new tests, we believe commission expenses will decline as a percentage of revenue.
3
Our goal is to become the leading provider of sample-to-result, multiplex and low-plex molecular diagnostic testing in infectious disease by leveraging the strengths of our affordable diagnostic testing platform. We intend to expand the use of our platform by targeting small to medium sized hospitals in the United States with fewer than 400 beds. We believe that our low-cost platform will be attractive to these hospitals in particular, which may not otherwise have sufficient resources to justify the purchase of a molecular diagnostic sample-to-result solution. To achieve this objective, we intend to do the following:
|
|
• |
Leverage our Low-Cost Platform to Quickly Penetrate the Small and Medium Sized Hospital Market. We provide our customers with our analyzer at no cost and sell them the disposable, single-use diagnostic cartridges. This allows us to avoid the long sales cycle inherent in selling capital equipment and expand into hospitals that previously could not afford to implement a molecular diagnostic platform. |
|
|
• |
Accelerate the Growth of our U.S. Customer Base. We intend to expand our sales force to target small to medium hospitals in the United States. We anticipate that increasing our number of customers will drive sales of our diagnostic cartridges. We expect these sales will generate the majority of our revenue for the foreseeable future. |
|
|
• |
Expand our Menu of Molecular Diagnostic Assays. We intend to expand our assay menu to include additional assays for our platform that we believe will satisfy growing medical needs and present attractive commercial opportunities. For example, in 2014 we completed the clinical trials and filed the 510(k) application for our second test for Group B Strep, and in April 2015 we received FDA clearance for our Group B Strep test. In March 2016 we also received clearance for Staph ID/R and for Shiga toxin producing E. coli. In December 2016, we submitted a 510(k) application for our Stool Bacteria Pathogenic Panel (food-borne illness) to the FDA, and in January 2017, we submitted a 510(k) application to the FDA for our Bordatella Pertussis (whooping cough) test. We also have a pipeline of assays in late stage product development, including a test for Chlamydia-Gonorrhea, a pre-surgical screening test, and a panel for Candida blood infections. |
|
|
• |
Reduce our Cost of Sales through Automation and Volume Purchasing. We manufacture our proprietary diagnostic cartridges and analyzers at our manufacturing facility in Salt Lake City, Utah. We currently hand-build our diagnostic cartridges and purchase materials at a higher per unit cost due to low purchase volumes. We believe that investment in automation of portions of the manufacturing and assembly process and volume purchase pricing will significantly improve our gross margins and enhance our ability to provide a low-cost solution to customers. |
The Great Basin Platform
Our platform, which employs a combination of proprietary and patented technology, consists of a bench top analyzer with a touch screen (or, in early models, a laptop computer) into which our self-contained, single-use diagnostic cartridges are inserted. We believe that our platform is user friendly and intuitive and provides the hospital with the ability to perform molecular diagnostic assays in an efficient and cost-effective manner.
|
The Analyzer |
The Diagnostic Cartridge |
The User Placing the Diagnostic Cartridge into the Analyzer |
|
|
|
|
|
|
|
|
4
Once a patient specimen is received in the lab, a technician will typically perform no more than three to five steps of sample preparation before placing the sample in our disposable diagnostic cartridge and inserting it in the analyzer where the assay is run without further technician intervention. Our four FDA-cleared assays were rated by Clinical Laboratory Improvement Amendments of 1988, or CLIA, as moderately complex, which typically eliminates the need for highly-trained and expensive molecular technicians to run the assays, bringing additional cost benefit to our customers. We expect our future assays to be rated moderately complex or meet CLIA waiver criteria, which is reserved for assays that are simple and are judged by the FDA to present a low risk for erroneous results.
Our platform provides accurate results in 45 to 115 minutes depending on the assays. The speed of our assays allows for early diagnosis and treatment, which we believe can lead to better patient outcomes and reduced cost to the hospital.
We believe that our platform and related assays offer small-to-medium sized hospitals the following benefits:
|
|
• |
Ease of Use. Our platform is a sample-to-result system. Sample preparation can be completed in three to five steps that typically take no more than five minutes. Once the diagnostic cartridge is placed in the analyzer, the technician does not need to monitor the assay and can complete other unrelated tasks. The assay is complete in 45 to 115 minutes depending on the assay. |
|
|
• |
Cost Savings. We believe that our pricing strategy makes it possible for many small-to-medium sized hospitals that have cost constraints to adopt molecular testing. We provide the customer the use of our analyzer for no upfront charge, while we retain ownership. We then sell our assays to the hospital at a cost that we believe is similar to or less expensive than other molecular diagnostic solutions. This reduces the up-front cost for the customer, minimizes customer approval processes and accelerates adoption of our platform. |
|
|
• |
Versatile Platform with the Capability to Deliver a Broad Assay Menu. We believe our platform has broad potential application across a number of areas in molecular diagnostic testing for infectious disease, including the detection of pathogens from whole blood samples. The same analyzer can be utilized for all of our planned future assays. |
|
|
• |
Low-cost Low-plex tests. We believe our platform, including our low-cost chip detection and our single-use diagnostic cartridge, has a cost structure that will allow us to compete effectively in the market for low-plex tests with 1-3 answers like C. diff or pre-surgical and MRSA screening. We expect our low-plex tests like C. diff to drive system placements as hospitals convert from traditional testing methods. |
|
|
• |
Ability to Multiplex. Our platform has the technical ability to analyze up to 64 distinct targets in a single diagnostic panel, including controls. We refer to any test of more than four targets as a multi-plex panel. We believe that this capability will allow hospitals to test for multiple possible causes of an individual patient’s symptoms in a one-step detection process. This capability will reduce the time required for a laboratory to perform a diagnostic analysis that involves testing for multiple infectious disease pathogens. Without our platform, similar testing would require the hospital to run multiple, separate molecular or non-molecular diagnostic assays. |
Our Molecular Testing Process
In our molecular testing process, a clinician places a clinical specimen—processed or unprocessed—into the diagnostic cartridge (such as stool or blood), caps the cartridge and then places the diagnostic cartridge into the analyzer. The assay routine is initiated in the analyzer and starts a simple automated process. Within the instrument, mechanical valves are present to control the flow of fluid through the cartridge and to pierce the blister packs containing the required buffers, solutions and reagents to perform the selected assay. Low cost, reliable heaters are present for assay processing. Imaging occurs with a compact digital camera placed over a window in the cartridge above the chip. Proprietary software interprets the image and provides a clinical result to the laboratory.
5
The disposable diagnostic cartridge contains—in blister packs or freeze dried pellets—all of the reagents required to run the applicable assay. The three steps of a molecular assay (sample preparation, amplification, and detection) are performed in chambers present on the cartridge. All waste is collected in a chamber that is part of the diagnostic cartridge, significantly reducing the risk of lab contamination. After the assay is completed and the result is obtained, the diagnostic cartridge is disposed of with the hospital’s other medical waste.
To simplify processing within the analyzer, fluids are moved within the diagnostic cartridge through relatively large channels by exploiting pressure differences. Proprietary features have been designed into the diagnostic cartridge to allow for bubble-free fluid movement and sensor design permits accurate and precise volumetric delivery.
Our Assay Menu
We have received FDA clearance for four assays, C. diff , Group B Strep, Staph ID/R and E. coli and a CE mark for three assays, C. diff, Group B Strep and STEC. We began marketing the C. diff test in Europe in the first quarter of 2012 and in the United States in the fourth quarter of 2012. We received clearance from the FDA in April 2015 for our second diagnostic test for Group B Strep and launched this test commercially in June of 2015. We received clearance from the FDA in March of 2016 for STEC and launched this test commercially in August of 2016. We also received FDA clearance for our Staph ID/R trial in March of 2016 and launched this test commercially in September of 2016. We have two assays that have been submitted to the FDA for review, a test for pertussis and a food borne pathogen panel. Additionally, we have three other assays in various stages of product development: (i) a pre-surgical nasal screen for Staphylococcus aureus, or SA, (ii) a panel for candida blood infections and, (iii) a test for CT/NG.
Our Commercial Tests:
C. diff Test
C. diff infections are often life-threatening and can create a significant financial burden for hospitals. As a hospital-acquired infection, costs associated with the care of patients with C. diff are not covered by insurance or Medicaid/Medicare. An independent peer reviewed paper, published in the American Journal of Infection Control in 2012, highlights a significant reduction in C. diff infection rates when a hospital switched from culture to molecular testing — reducing cost and improving patient outcomes.
Our C. diff test is a rapid medical diagnostic test for the detection of C. diff , a gram-positive bacteria that causes severe diarrhea and other intestinal disorders. The test detects the presence of the C. diff toxin B gene, or tcdB gene, in the pathogenicity locus, or PaLOC region of C. diff , present in all known toxigenic strains, to diagnose the toxin in a patient’s stool. The test requires minimal sample preparation and can deliver results in 90 minutes. A swab from loose stool is placed into transfer solution and a portion of this solution is placed into the diagnostic cartridge. The diagnostic cartridge is then placed into the analyzer.
Group B Strep Test
Group B streptococcus, or Group B Strep, is a bacterium that colonizes in the warm moist areas of many humans. Harmless to healthy adults, it can be transmitted to a newborn during childbirth and is the single largest cause of meningitis in newborn infants. For this reason nearly every pregnant woman in the United States is tested for Group B Strep in the late third trimester. Historically this test was done by culture, but based on the recent introduction and sales of other Group B Strep molecular diagnostic tests, we believe labs are switching to molecular testing.
Our Group B Strep test is designed to detect Group B Strep from an anal/vaginal swab taken from a pregnant woman. Hospitals can use our test to identify Group B Strep colonization in pregnant women, who can then be treated with antibiotics to reduce the risk of transmission to the baby, reducing the risk of development of sepsis in the newborn. We received clearance from the FDA in April 2015 for our Group B Strep test and launched it commercially in June 2015.
Shiga Toxin Direct (STEC) Test
6
Escherichia coli ( E. coli ) bacteria normally live in the intestines of healthy people and animals. Most varieties of E. coli are harmless or cause relatively brief diarrhea, but a few strains, such as E. coli O157:H7, can cause severe abdominal cramps, bloody diarrhea and vomiting.
Our STEC Test is designed to be a rapid test that identifies Shiga toxin produced by E. coli , including E. coli O157 specifically which is the most serious type of E. coli contracted from contaminated food. We received FDA clearance for our STEC test in March 2016. We launched the product commercially in August of 2016.
Staphylococcus Identification and Resistance Blood Infection Panel
Staphylococcus aureus is a major cause of hospital and community-acquired infections and is associated with high rates of morbidity and mortality. Methicillin-resistant Staphylococcus aureus , or MRSA, is a potentially life-threatening infection that most frequently occurs in the hospital setting. Rapid diagnosis of Staph blood infections has been shown, in a report published in Clinical Practice in 2010, to save up to approximately $7,000 per patient and shorten the length of hospital stays by 6.2 days.
Our Staph ID/R panel is designed to be a multiplex panel to (i) identify species of Staphylococcus infections based on the detection of highly discriminatory and specific DNA sequences within a bacterial replication gene, (ii) detect the mecA gene, which confers drug resistance, directly from positive blood cultures and (iii) provide information on the antibiotic resistance profile of the bacteria. This test is designed to produce a result in less than one hour.
We received FDA clearance for our Staph ID/R panel in March 2016. We launched the product commercially in September of 2016.
Our Assays under FDA Review
Stool Bacterial Pathogenic Panel
According to the Agency for Healthcare Research and Quality, there were nearly five million U.S. hospital visits in 2010 for gastrointestinal distress that suggested food-borne illness. In 2010, inpatient costs attributable to patients suffering from gastrointestinal infections cost the healthcare system nearly $1.8 billion. One of the challenges faced by physicians assessing a patient with symptoms of gastrointestinal infection is determining the underlying cause.
We expect that our Stool Bacterial Pathogenic panel will detect the main causes of food poisoning. If approved, we believe that hospitals will be able to use our panel to identify the causative pathogen of food poisoning and provide appropriate treatment quickly, improving patient outcomes. Additionally, the results may be used to aid public health agencies to track causes of food poisoning outbreaks. We started the clinical trial in the second quarter of 2016 and filed a 510(k) application with the FDA in December of 2016. Our Stool Bacterial Pathogenic panel is not cleared by the FDA or available for commercial sale.
Bordatella Pertussis Test
Bordatella Pertussis, also known as whooping cough, is a highly contagious respiratory disease caused by the bacterium Bordetella pertussis. In 2012, there were over 48,000 cases reported in the United States and over 16 million worldwide. We started the clinical trial in the second quarter of 2016 and filed a 510(k) application with the FDA in January of 2017. Our Bordatella Pertussis test is not cleared by the FDA or available for commercial sale.
Our Assays in Development
Staphyloccocus aureus Pre-Surgical Nasal Screen
Staphyloccocus aureus (SA) often colonizes the nasal passages and other warm moist areas in healthy humans. Harmless in most circumstances, the colonization represents real risk to patients undergoing surgery. In fact
7
studies have shown the relative risk of post-surgical infection is up to eight and a half times greater in carriers of SA than in non-carriers.
We expect that our SA nasal screening test will be a rapid test for the presence of SA in the nasal passages of a pre-surgical patient. If approved, we believe that hospitals will be able to use our test to identify pre-surgical patients who are SA carriers and treat those patients with topical antibiotics, which has been shown in multiple peer-reviewed studies to significantly reduce the risk of post-surgical infection. Our SA pre-surgical nasal screen test is not cleared by the FDA or available for commercial sale.
Candida Blood Infections Panel
According to the United States Center for Disease Control there are 46,000 Candida blood infections annually in the U.S. Candida infections, although rare, have mortality rates as high as 40% if not diagnosed quickly. Our candida panel is a multiplex panel that will be designed to identify five species of Candida directly from positive blood cultures. We expect to complete product development in the first half of 2017. Our Candida Blood Infections Panel is not cleared by the FDA or available for commercial sale.
Chlamydia-Gonorrhea (CT/NG) Test
Our test for Chlamydia tracomatis and Neisseria gonorrhoeae (CT/NG) is designed to detect two significant sexually transmitted diseases. According to the CDC, there are over 20 million new CT infections each year in the U.S. and approximately 330,000 cases of NG. We expect to complete the pre-clinical development of our CT/NG test in the first half of 2017. Our CT/NG test is not cleared by the FDA or available for commercial sale.
Our Technology
Our proprietary and patented technology is based on detection of DNA targets on a coated silicon chip with results visible to the naked eye. DNA naturally forms a double-stranded structure, with each strand binding with high affinity and selectivity to a complementary strand. Our technology can detect DNA target strands of interest by attaching complementary strands of DNA to the chip surface, called capture probes, using our proprietary technology and processes. The capture probe can thereby immobilize the DNA target of interest. In order for the DNA target to be detected, it is labeled with biotin, a small molecule which can be used to create a signal in the diagnostic test. Biotin, immobilized onto the chip surface via DNA target hybridization to the DNA probe, will bind to a molecule which recognizes biotin and is conjugated to a signal generating enzyme, horseradish peroxidase, or HRP. Immobilized HRP can be reacted with a complex mixture to create a large colored product which deposits on the chip surface. This deposit causes the color of the chip surface to change. This color change is readily apparent to the naked eye as a dot in the vicinity of the DNA probe. In order to create tests with appropriate sensitivity for the given clinical indication, we can either amplify the quantity of DNA targets of interest or amplify the biotin-based signal. Each of these amplification approaches also serve to label DNA target(s) of interest with biotin.
Our Technology: Detection of On-chip Helicase Dependent Amplification (HDA) Reactions

8
Within the cartridge three distinct processes occur: sample processing, amplification, and detection. During sample processing, the specimen is treated to remove clinical matrices such as blood or feces that may interfere with assay results and is treated to break open cells to release potential DNA targets. Next, the sample is subjected to target amplification to create billions of copies of target DNA to improve assay sensitivity and to label each target with biotin. Finally, detection is triggered by hybridization of the biotin-labeled target DNA with capture probes on the chip surface. The chip is currently configured to hold as many as 64 probes, including controls, each of which can be configured to detect a different target DNA, enabling highly multiplexed testing. If signal amplification is used, a proprietary polymer is added to the detection reaction, which converts each target DNA associated biotin into 80 additional biotins to improve detection sensitivity. All waste generated from the assay is stored in a self-contained waste chamber which significantly reduces contamination risk.
Target Amplification. Helicase-dependent amplification, or HDA, is a process that utilizes an enzyme, helicase, to unwind double-stranded DNA to create two single strands through isothermal, or single temperature, amplification. Once this DNA is single-stranded, complementary DNA primers, which are short pieces of DNA that are complementary to the DNA target, can hybridize. Through the action of an enzyme, DNA polymerase, the DNA primers grow, or extend, to create a complementary strand of the DNA target, creating double-stranded DNA again. This process can be repeated and the degree of copying, or amplification, is exponential, so that billions of copies can be created. HDA is a method already commercially available for detection of DNA targets, however, it is a mistake-prone process. DNA primers can incorrectly hybridize to non-target DNA at lower temperatures and be copied, creating so-called primer artifacts, which leads to tests that are slow and poorly sensitive.
Our patented target amplification approach, termed blocked primer-mediated, helicase-dependent amplification, or bpHDA, utilizes blocked primers to enhance test speed and sensitivity. Blocked primers are DNA primers that contain a block at lower temperatures, where most incorrect hybridization events occur, preventing extension of the DNA primers or copying of the DNA target. As the temperature is raised, incorrect hybridization events are not stable and fall apart, but hybridization of DNA primers to complementary DNA targets is still very strong and an enzyme, RNase H, becomes active which can remove the block in blocked primers hybridized to DNA targets only. As a result, the accuracy of the process is dramatically improved, leading to faster and more sensitive tests. In order to label the DNA target for detection, each DNA primer has a biotin molecule attached to one end.
Our platform is also capable of performing polymerase chain reaction, or PCR, which is a widely used method of DNA amplification. Our Group B Strep test is our first commercial test to utilize PCR.
Signal Amplification. Our patented signal amplification approach, which we refer to as AMPED, utilizes a bridging molecule to connect the biotin label on each DNA target to a polymer containing 80 biotins, thereby amplifying the signal up to 80 times in our diagnostic tests. The AMPED polymer works in the presence of blood, mucus, and feces typically present in clinical samples, thereby simplifying sample processing. The AMPED signal amplification process takes approximately 20 to 30 minutes and is a more rapid approach for detection of DNA targets than target DNA amplification, which typically takes 45 to 120 minutes. The patented AMPED polymer is highly water soluble and stable and displays low non-specific binding properties, which are critical requirements for highly specific signal amplification approaches.
Based on papers published in peer-reviewed journals, we believe our AMPED detector to be among the most sensitive in the industry with a proven limit of detection of 20,000 DNA molecules. We believe this limit of detection will be more than adequate for us to develop assays focused on the nascent “direct from whole blood” market which we believe has the potential to be exceptionally disruptive by eliminating the need for culturing blood prior to testing. Currently patients suspected of having a blood infection (sepsis) have their blood drawn. That sample is then cultured for a day before testing, but published studies consistently show that treatment within 12 hours of symptoms has significant clinical benefit. Direct from whole blood testing has the potential to eliminate the need for culture, speeding diagnosis to under 12 hours, thus potentially improving patient morbidity and mortality.
Diagnostic Cartridges. Our patent-pending diagnostic cartridges are self-contained devices specifically configured for a given diagnostic assay. The diagnostic cartridge is injection molded and includes features such as reaction chambers, a waste chamber, and channels to direct the movement of fluids. The diagnostic cartridge also contains a coated silicon detection chip consisting of an array of up to 64 DNA probes, including controls. Integrated into the diagnostic cartridge are lance devices for the reagent blister packs and stirring devices. Reaction chambers and fluid
9
channels are covered with a clear thermoplastic to form liquid-tight features. All of the reagents necessary to perform the assay are stored within blister packs affixed to the cartridge, other than the target amplification reagents, which are stored as a freeze-dried pellet. The diagnostic cartridge utilizes patent-pending methods for controlling the flow of fluid and managing air to prevent bubbles. The diagnostic cartridge also contains bar coded information related to the test, including the cartridge lot number and expiration date.
Analyzer. The analyzer is an on-demand system controlled by an external touch screen or laptop. Each analyzer contains a module into which individual diagnostic cartridges are placed. Once a diagnostic cartridge has been loaded with a clinical specimen, the cartridge is inserted into the analyzer. The cartridge is then advanced onto a platform allowing access from above and below. The analyzer has three main sub-platforms to execute the diagnostic test: reagent flow control, thermal control, and the optical imaging platforms. To control reagent flow and delivery, valve actuators, which control fluid movements, and lance actuators, which lance blister packs, are located below the cartridge. Motors for mixing reactions and stepper motors, which serve to flatten blister packs and drive fluid into reagent channels, are located above the cartridge. Optical sensors located adjacent to the fluidic paths in the cartridge are used to determine fluidic movements. Compression valves are used to isolate regions of the cartridge for individual steps of the diagnostic assay. Multiple resistive heaters with thermocouple feedback are used to control temperature for each of the steps of the process. Once the test is complete a digital camera captures the resulting visible features. Processing and filtering techniques and multiple custom algorithms are used to determine test results, which are displayed on the touch screen or printed automatically.
The touch screen controls each analyzer, provides power and analyzes and stores data. Technicians can load patient identification numbers and reagent lot codes by using the included bar code scanner or the touch screen.
Advantages of Our Technology
Low Cost Design. Our technology was designed to use injection molded parts and filled blister packs can be produced in high volumes at relatively low cost. Production of the coated silicon chip uses existing semi-conductor processes and capital equipment. Our isothermal target amplification uses a relatively inexpensive heater which does not need to actively manage heat with coolers and fans. A low cost digital camera captures the visible results. Our proprietary DNA probe chemistry enables us to produce assays at relatively low cost.
Easy to Perform On-demand Testing. Our technology uses a sample-to-result approach. The customer loads a liquid clinical specimen into the diagnostic cartridge, closes it, inserts it into the analyzer and enters patient information to initiate each assay. The results are automatically generated with no user interpretation or intervention required. Hands-on time is less than 10 minutes for our C. diff test and we expect hands-on time to be generally less than 5 minutes for assays under development. Additionally, on-demand testing allows the technician to test samples as they come into the laboratory. Finally, the presence of comprehensive built-in controls means that the technician is not required to test external control samples to assure the quality of assay results. This allows laboratories to be more efficient with limited resources.
High Performance Assay Results. Our technology is capable of highly specific and sensitive detection of nucleic acids. For target DNA from multiple pathogen types, we can routinely detect fewer than three organisms using our bpHDA target amplification approach and have detected the identity of as few as 100 organisms using our AMPED signal amplification approach. Because the speed of bpHDA is only limited by the speed of the enzymes, we have demonstrated the ability to detect target DNA in less than 20 minutes of amplification time.
High Content Panels. Each of the 64 capture probes in our cartridge act independently to produce highly specific and sensitive detection for a given DNA target. The chip is optimized so that changes as small as a single base in DNA target sequence can be readily detected, allowing for detection of clinically meaningful mutations in DNA target samples. Our bpHDA technology is highly specific, allowing for simultaneous amplification of multiple DNA targets. The AMPED approach can be used to directly detect clinical specimens, thereby eliminating typical limitations of multiplexing. We expect the combination of highly multiplexed amplification and detection will allow for information-rich, multi-target panel results that allow clinicians to both rule-out and rule-in causes of disease. We believe this approach will lower testing costs and speed up the time to appropriate patient treatment, improving patient outcomes.
10
Ease of Development of New Assays. Early diagnostic test prototypes can be developed using our standard 96 well plates capable of processing 96 samples in a single experiment, rather than processing individual samples in the analyzer. We believe that this capability allows us to more easily develop and optimize new assays before transferring the design to the instrumented platform. Additionally, well-established software tools enable us to design and develop DNA probes and primers. The flexibility of the cartridge design allows for utilization of an efficient, low-cost approach for sample processing.
License Agreements
BioHelix. We hold non-exclusive licenses to key technologies from BioHelix related to isothermal amplification of nucleic acid targets, utilizing helicase-dependent amplification, or HDA. The term of this license agreement extends until the expiration of all the patents associated with the licensed patent rights, or until such time as we elect to terminate with 30 days’ notice. This license is limited to the fields of human diagnostic testing utilizing our solid chip surface detection and contains diligence and U.S. preference provisions. To date, these technologies have resulted in three issued U.S. patents, one issued European patent and one pending international patent family. In addition, these technologies may include related technologies that BioHelix may develop in the future. The BioHelix technologies are the basis of our nucleic acid amplification approach. In May of 2013, Quidel Corporation, a competitor of ours, purchased BioHelix. We pay a royalty fee for the licensing of this technology based on a percentage of our “Net Sales” of assays using these technologies (as defined in the license agreement).
IDT. In August 2010, we entered into a license agreement with Integrated DNA Technologies, or IDT, related to the use of blocked primers in combination with HDA. The term of this license agreement extends until the expiration of all the patents associated with the licensed patent rights, or until such time as we elect to terminate with 90 days’ notice. The license is exclusive to the fields of amplification utilizing HDA and detection of diagnostic targets in human in-vitro diagnostic testing, but is non-exclusive to all oncology and human papilloma virus targets or markers. These technologies have resulted in four pending U.S. patent applications and one issued European patent. We pay a royalty fee for the license of this technology based on a percentage of our “Net Sales” of products using these technologies (as defined in the license agreement).
Pursuant to the terms of these license agreements, we pay royalty fees in the aggregate equal to 14% of our worldwide “Net Sales” of those products that use these technologies (as defined and adjusted pursuant to the terms of the applicable license agreements). Our C. diff product is our only commercial product that uses these technologies. Our products under review by the FDA and all of our products under development do not use these technologies and will not be subject to royalty fees.
Market Opportunity
We believe the global market for molecular diagnostic testing is approximately $5.0 billion per year and will experience a growth rate of approximately 12% per year over the course of the next several years based on research published by outside market research firms. We believe our proprietary sample-to-result platform is best suited to address a subset of this market, including hospital-acquired infections and other infectious diseases.
|
|
• |
C. diff. According to the Agency for Healthcare Research and Quality, there are 347,000 cases of C. diff annually in the United States; |
|
|
• |
Group B Strep. According to the CDC, there were 4.0 million live births in the United States in 2012 and nearly every pregnant woman in the United States is tested for Group B Strep in the late third trimester; |
|
|
• |
Staphylococcus Identification and Resistance Panel. According to a market survey, there are 4.2 million positive blood cultures each year in the United States; |
|
|
• |
Shiga Toxin Producing E. coli. According to the Agency for Healthcare Research and Quality, there were nearly five million U.S. Hospital visits in 2010 for gastrointestinal distress that suggested food-borne illness and each of these patients could potentially be tested for STEC; |
11
|
|
• |
Stool Bacterial Pathogenic Panel. According to the Agency for Healthcare Research and Quality, there were nearly five million U.S. hospital visits in 2010 for gastrointestinal distress that suggested food-borne illness and each of these patients could potentially be tested for food borne pathogens. |
We anticipate that the market for the molecular diagnostic assays on which we are focused will increase significantly over the next several years. We believe that many factors are driving growth of this market, particularly the accelerating adoption of molecular testing inside the hospital micro-biology lab. Based on published research we believe that fewer than half of all hospitals are currently using molecular testing for their infectious disease testing. More importantly, we believe that a far smaller fraction of all testing done in hospital labs is molecular. We believe that as molecular testing becomes more cost effective, its advantages of faster time to result and higher sensitivity relative to legacy testing methods will lead more and more hospitals to convert to molecular testing.
Our diagnostic tests are currently sold in the United States, Europe and New Zealand. We currently utilize a direct sales and support team in the United States and in certain key European countries and New Zealand we utilize distributors, which we expect will be augmented by marketing partners and distributors in other strategic areas as we expand internationally.
According to the US Center for Disease Control, in 2011 there were approximately 5,700 hospitals in the United States, approximately 4,900 of which are under 400 beds and which we refer to as small to medium sized hospitals. According to outside research, fewer than half of those smaller hospitals have made the switch to molecular methods for diagnosing infectious disease. Based on our competitors’ public statements and published independent reports, we believe that 20% of small-to-medium sized hospitals have a sample to result molecular system. We believe these hospitals are excellent candidates for our molecular diagnostic systems. We believe that our platform will enable these hospitals—many of which could not previously afford more expensive or complex molecular diagnostic testing platforms—to modernize their laboratory testing and provide better patient care at an affordable cost.
The first step to acquiring a customer is an evaluation. During the evaluation period, potential customers utilize our platform alongside their current testing method (molecular or non-molecular) and at the end of the evaluation period determine if they are interested in switching to our platform, as evidenced by the purchase of our assays on a recurring basis, or by remaining with their current testing method. Our recent customer and evaluation history is as follows (excluding Staph ID/R and STEC diagnostic assays as they were in the beginning stages of the evaluation period):
|
|
|
Total U.S. Customers |
|
|
C. Diff Customers |
|
C. Diff Penetration |
|
Group B Strep Customers |
|
|
Group B Strep Penetration |
||
|
Third Quarter 2014 |
|
|
80 |
|
|
80 |
|
100% |
|
|
0 |
|
|
NA |
|
Fourth Quarter 2014 |
|
|
84 |
|
|
84 |
|
100% |
|
|
0 |
|
|
NA |
|
First Quarter 2015 |
|
|
101 |
|
|
101 |
|
100% |
|
|
0 |
|
|
NA |
|
Second Quarter 2015 |
|
|
115 |
|
|
115 |
|
100% |
|
|
0 |
|
|
NA |
|
Third Quarter 2015 |
|
|
143 |
|
|
142 |
|
99% |
|
|
25 |
|
|
17% |
|
Fourth Quarter 2015 |
|
|
186 |
|
|
185 |
|
99% |
|
|
47 |
|
|
25% |
|
First Quarter 2016 |
|
|
222 |
|
|
219 |
|
99% |
|
|
61 |
|
|
27% |
|
Second Quarter 2016 |
|
|
260 |
|
|
253 |
|
99% |
|
|
78 |
|
|
30% |
|
Third Quarter 2016 |
|
|
255 |
|
|
248 |
|
99% |
|
|
78 |
|
|
30% |
|
Fourth Quarter 2016 |
|
|
244 |
|
|
234 |
|
96% |
|
|
74 |
|
|
30% |
Research and Development
As of December 31, 2016 we had 50 employees focused on research and development. Our research and development expenditures were approximately $13.4 million and $8.5 million for the years ended
12
December 31, 2016 and 2015, respectively. The increase in research and development expenses from 2015 to 2016 was primarily due to our focus on growing our diagnostic assay pipeline. In the future we expect to continue to allocate resources to developing and obtaining regulatory approval for assays under development and expect to continue developing additional assays and conducting clinical trials. We will also allocate research and development resources to improve our product reliability and enhance our diagnostic cartridge manufacturing process.
In addition to the four assays that have received FDA approval, we have two assays submitted for FDA approval and four assays in late stage development. Our product offerings will include low-plex tests, multi-plex panels and direct from whole blood tests or panels.
Manufacturing
We manufacture our proprietary diagnostic cartridges and analyzers in Salt Lake City, Utah. We currently hand-build our diagnostic cartridges and purchase materials at higher per unit cost due to lower purchase volumes. We believe that investment in automation of portions of our manufacturing and assembly process and volume purchase pricing will significantly improve our gross margins and enhance our ability to provide a low cost solution to customers. We perform reagent formulation, diagnostic cartridge manufacturing and packaging of final components and diagnostic cartridges in accordance with applicable guidelines for medical device manufacturing. We currently lease approximately 33,000 square feet of manufacturing space, which we believe will be adequate to meet our manufacturing needs for the foreseeable future. The lease expires in April 2017 and has two options, each for a three year renewal term. We also rely on third party suppliers, including sole source suppliers in certain instances, for certain reagents used in our products and much of the disposable component molding for our test cartridges.
We have implemented a quality management platform designed to comply with FDA regulations and ISO standards governing diagnostic medical device products. These regulations carefully control the design, manufacture, testing and release of diagnostic testing products, as well as raw material receipt and control. We also have controlled methods for the consistent manufacturing of our proprietary diagnostic cartridge, and analyzers at our facility.
Sales and Marketing
Our current sales and marketing strategy is to both expand our footprint of installed hospitals and stand-alone laboratory sites in addition to increasing the number and volume of diagnostic assays utilized at our existing installed base of customers. Our assays are sold in the United States through a nine person direct sales force and a technical specialist service organization of five, which is supported by a centralized team of marketing, customer support, and technical support personnel.
Our sales representatives typically have experience in molecular diagnostic testing and a network of hospital contacts within their respective territories. We utilize our representatives’ knowledge along with market research databases and relationships with Group Purchasing Organizations (GPOs), Integrated Delivery Networks (IDNs) and other strategic partnerships to target and qualify prospects. We execute a variety of surveys and strategies to determine the buying criteria of the different customer segments we serve, and adapt our offering and strategies to best serve our target audience. To support our expanding molecular assay menu and the anticipated growth in our customer base, we continue to make investments in a variety of market outreach programs, campaigns and technologies.
In the United States, our sales process nearly always includes a customer evaluation period during which a site receives training on our platform and on the specific assay(s) they are evaluating. As part of the evaluation process, the evaluating site runs validations against their current method to assess accuracy, overall cost and workflow of the Great Basin platform. Upon successful validation and upon purchase of product, the evaluating entity becomes a customer. This process has taken between 25 and 64 days, depending on the product(s) being evaluated, from the time we install the analyzer at the evaluation site to acceptance as a customer. Our sales cycle has decreased over time due improvements we have made to the selling process and to higher market acceptance of molecular testing for infectious diseases. For example, for our customers
13
that run the C. diff test, during 2014 the sales cycle was 74 days, during the first half of 2015 the sales cycle was 65 days, during the second half of 2015 the sales cycle was 35 days and the sales cycle was 20 days by the end of 2016.
The analyzer is provided to the customers for their use with our assay but we retain ownership and control of the analyzer; we refer to our relationship with our customers as a vending machine model (or modified razor/razor blade). The customer buys our proprietary diagnostic cartridge from us and utilizes one disposable test cartridge each time an assay is run. Our revenue from U.S. customers is derived solely from the sales of assays.
We refer to the percentage of customers that elect to switch to our platform and purchase our diagnostic tests after receiving and evaluating our platform as our “win rate”. This is a metric that we use to determine our sales efficiency and our market acceptance. Our win rate calculation is determined without any minimum or recurring purchase threshold. Our win rates over various periods since we launched our first product commercially are as follows:
|
|
|
Number of Evaluations |
|
|
Win Rate |
|
||
|
Full Year 2013 |
|
|
86 |
|
|
|
69% |
|
|
Full Year 2014 |
|
|
49 |
|
|
|
76% |
|
|
Full Year 2015 |
|
|
125 |
|
|
|
85% |
|
|
1st Quarter 2016 |
|
|
40 |
|
|
|
85% |
|
|
2nd Quarter 2016 |
|
|
49 |
|
|
|
88% |
|
|
3rd Quarter 2016 |
|
|
24 |
|
|
|
79% |
|
|
4th Quarter 2016 |
|
|
7 |
|
|
|
86% |
|
|
Since Commercial Launch |
|
|
397 |
|
|
|
80% |
|
We offer our C. diff test and our platform for sale in the European Union and New Zealand through a network of distributors. Unlike the U.S. market, we sell the platform and assay to the distributor, who then sells them directly to the customer. There were no sales of the analyzer platform during the years ended December 31, 2016 and 2015.
Customers
For the year ended on December 31, 2016, we did not have any customer that represented more than 5% of our total revenue. For the year ended December 31, 2015 one customer, Vista Clinical Lab, represented 7.4% of our total revenue.
We define customers in terms of the number of customer sites actively reporting results using our platform. As of December 31, 2016, we had 265 customers worldwide (244 in the United States and 21 in the rest of the world), who use an aggregate of 498 analyzers.
Our U.S. customers represented approximately 99% of our total revenue for the year ended December 31, 2016 and approximately 98% of our total revenue for the year ended December 31, 2015. We do not enter into sales agreements with our U.S. customers and sell our products using purchase orders. We enter into distribution agreements with distribution partners for sales outside the U.S.
Competition
We primarily compete with other molecular diagnostic testing manufacturers, such as Cepheid, Meridian Bioscience, Inc., Nanosphere, Inc., Qiagen NV, Roche Diagnostics, Quidel Corporation, bioMerieux (which recently acquired Biofire Diagnostics, Inc.), T2 Biosystems, Becton, Dickinson and Company, GenMark Diagnostics, Inc., Hologic and others. We believe that we compete primarily on the basis of our platform’s speed, accuracy, ease of use and relatively low cost. We believe that the approval of additional assays will make our solution more attractive to our customers and prospective customers.
14
Many of our competitors have substantially greater financial, technical, research and other resources and larger, more established marketing, sales and distribution organizations than we do. Many of our competitors also offer broader product lines and have greater brand recognition than we do. Moreover, our existing and new competitors may make rapid technological developments that may result in our technologies and products becoming obsolete before we recover the expenses incurred to develop them or before they generate significant revenue.
Intellectual Property, Proprietary Technology
We integrate capabilities in platform design, development, production and DNA amplification technologies, along with design, development and manufacture of primers, probes, dyes, quenchers and other individual reagent components. We have and are continuing to develop our own proprietary intellectual property along with licensing specific third-party technologies.
We have an issued patent covering bpHDA, which is used in our C. diff test. bpHDA, or Blocked Primer Helicase Dependent Amplification, is our patented technology creating “target-dependent hot start” functionality in HDA amplification reactions. bpHDA utilizes a blocked primer technology such that amplification is not activated until the target analyte of interest is bound to the blocked primer at an elevated temperature used for HDA amplification, wherein the block is removed by a highly specific enzyme, allowing for amplification to proceed. We believe this approach significantly improves assay speed and limits of sensitivity such that single cells present in clinical specimens may be amplified to detectable levels in as few as 17 minutes. Multiplex product development is also simplified, allowing for more complex assays in a single reaction with up to four unique primer sets demonstrated to work since December 31, 2014.
(BP)-HDA: Great Basin Proprietary Hot Start Amplification Technology
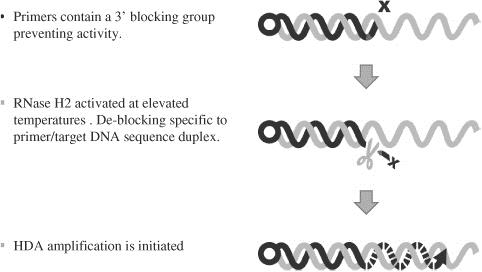
We also have an issued patent for our amplification method in the presence of the coated silicon chip, a method which we intend to use in each of our assays. We also have an issued patent for the AMPED signal amplification method, which we intend to utilize in assays currently under development. The issued patents described above were issued in the United States and each expires in 2029. Our issued patents are pending in Europe and Canada as well. All current maintenance fees payable regarding these issued patents have been paid.
Our competitive success will be affected in part by our continued ability to obtain and maintain patent protection for our inventions, technologies and discoveries, including intellectual property that includes technologies that we license. We have patents covering technologies of our own and have licensed technologies from others. Our pending patent applications may lack priority over applications submitted by third parties or may not result in the issuance of
15
patents. Even if issued, our patents may not be sufficiently broad to provide protection against competitors with similar technologies and may be challenged, invalidated or circumvented.
In addition to patents, we rely on a combination of trade secrets, copyright and trademark laws, nondisclosure agreements, licenses and other contractual provisions and technical measures to maintain and develop our competitive position with respect to intellectual property. Nevertheless, these measures may not be adequate to safeguard the technology underlying our products. For example, employees, consultants and others who participate in the development of our products may breach their agreements with us regarding our intellectual property, and we may not have adequate remedies for the breach. We also may not be able to effectively protect our intellectual property rights in some foreign countries, as many countries do not offer the same level of legal protection for intellectual property as the United States. Furthermore, for a variety of reasons, we may decide not to file for patent, copyright or trademark protection outside of the United States. Our trade secrets could become known through other unforeseen means. Notwithstanding our efforts to protect our intellectual property, our competitors may independently develop similar or alternative technologies or products that are equal or superior to our technology. Our competitors may also develop similar products without infringing on any of our intellectual property rights or design around our proprietary technologies. Furthermore, any efforts to enforce our proprietary rights could result in disputes and legal proceedings that could be costly and divert attention from our business. We could also be subject to third-party claims that we require additional licenses for our products, and such claims could interfere with our business. If our products infringe the intellectual property rights of others, we could face costly litigation, which could cause us to pay substantial damages and limit our ability to sell some or all of our products. Even if our products were determined not to infringe the intellectual property rights of others, we could incur substantial costs in defending any such claims.
We hold non-exclusive licenses to key technologies from BioHelix related to isothermal amplification of nucleic acid targets, utilizing helicase-dependent amplification, or HDA. This license is limited to the fields of human diagnostic testing utilizing our solid chip surface detection and contains diligence and U.S. preference provisions. To date, these technologies have resulted in three issued U.S. patents, one issued European patent and one pending international patent family. Additionally, these technologies may include related technologies that BioHelix may develop in the future. The BioHelix technologies are the basis of our DNA amplification approach. In August 2010, we entered into a license agreement with Integrated DNA Technologies, or IDT, related to the use of blocked primers. The license is exclusive to the fields of amplification utilizing HDA and detection of diagnostic targets in human in-vitro diagnostics, but is non-exclusive to all oncology and human papilloma virus targets or markers. These technologies have resulted in three pending U.S. patent applications and one issued European patent.
Government Regulation
The design, development, manufacture, testing and sale of our molecular diagnostic assays are subject to regulation by numerous governmental authorities, principally the FDA, and corresponding state and foreign regulatory agencies.
Regulation by the FDA
In the United States, the Food, Drug and Cosmetic Act, or FDCA, FDA regulations and other federal and state statutes and regulations govern, among other things, medical device design and development, pre-clinical and clinical testing, premarket clearance or approval, registration and listing, manufacturing, labeling, storage, advertising and promotion, sales and distribution, export and import, and post-market surveillance. The FDA regulates the design, manufacturing, servicing, sale and distribution of medical devices, including molecular diagnostic test kits and instrumentation platforms. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
Unless an exemption applies, each medical device we wish to distribute commercially in the United States will require marketing authorization from the FDA prior to distribution. The two primary types of FDA marketing authorization required applicable to a device are premarket notification, also called 510(k) clearance, and premarket approval, also called PMA. The type of marketing authorization required is generally linked to the classification of
16
the device. The FDA classifies medical devices into one of three classes (Class I, II or III) based on the degree of risk the FDA determines to be associated with a device and the level of regulatory control deemed necessary to ensure the device’s safety and effectiveness. Devices requiring fewer controls because they are deemed to pose lower risk are placed in Class I. Class I devices are deemed to pose the least risk and are subject only to general controls applicable to all devices, such as requirements for device labeling and adherence to the FDA’s current Good Manufacturing Practices and Quality Platform Requirements, as reflected in its Quality System Regulation, or QSR. Class II devices are intermediate risk devices that are subject to general controls and may also be subject to special controls such as performance standards, product-specific guidance documents, special labeling requirements, patient registries or post-market surveillance. Class III devices are high risk devices for which insufficient information exists to assure safety and effectiveness solely through general or special controls. Class III devices include life-sustaining, life-supporting or implantable devices, devices of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury.
Most Class I devices and some Class II devices are exempted by regulation from FDA’s premarket review requirement and can be commercialized without prior authorization from the FDA. Some Class I devices that have not been so exempted and most Class II devices are eligible for marketing through the 510(k) clearance process. By contrast, devices placed in Class III generally require PMA or 510(k) de novo clearance prior to commercial marketing.
Our Stool Bacterial Pathogen Panel and Group B Strep Test are both Class I devices and are eligible for marketing through the 510(k) clearance process. Our C. Diff test and Staphylococcus Identification and Resistance Panel are Class II devices and also are eligible for marketing through the 510(k) clearance process. Our two assays currently under FDA review have been submitted as Class II devices with a 501(k) application.
510(k) Clearance
To obtain 510(k) clearance for a medical device, an applicant must submit a premarket notification to the FDA demonstrating that the device is “substantially equivalent” to a device legally marketed in the United States that is not subject to PMA, commonly known as the “predicate device.” A device is substantially equivalent if, with respect to the predicate device, it has the same intended use and has either (i) the same technological characteristics or (ii) different technological characteristics and the information submitted demonstrates that the device is as safe and effective as a legally marketed device and does not raise different questions of safety or effectiveness. Demonstration of substantial equivalence may require clinical data. Although completion of the 510(k) review process is targeted for 90 days, these reviews typically take longer (e.g., up to 12 months or more) due to stoppage of the FDA review clock to address requests for additional information. Payment of a user fee is required for the FDA to initiate review of a 510(k) submission.
After a device has received 510(k) clearance for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, may require a new 510(k) clearance or PMA. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance; however, the FDA may review this determination to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
Before submitting a medical device for 510(k) clearance, a series of studies (e.g., method comparison, precision, reproducibility, interference and stability studies) must be conducted to characterize the performance of the test.
In addition, clinical studies may be required to validate these performance characteristics in a clinical setting as well as to ensure that the intended users can perform the test successfully.
Although clinical investigations of most devices are subject to the investigational device exemption, or IDE, requirements, clinical investigations of molecular diagnostic tests, including our assays and assays under development, are generally exempt from the IDE requirements. Thus, clinical investigations by intended users for intended uses of our assays generally do not require the FDA’s prior approval, provided the clinical evaluation
17
testing is non-invasive, does not require an invasive sampling procedure that presents a significant risk, does not intentionally introduce energy into the subject and is not used as a diagnostic procedure without confirmation by another medically established test or procedure. In addition, products must be appropriately labeled per FDA regulations to reflect the intended use of the product (e.g., for research use only or for investigational use only) and distribution controls must be established to assure that such products are distributed for those specified purposes.
PMA Applications
PMA applications must be supported by valid scientific evidence, which typically requires extensive performance data, including technical, pre-clinical, clinical and stability data, to demonstrate the safety and effectiveness of the device. A PMA application must also include a complete description of the device and its components, a detailed description of the methods, facilities and controls used to manufacture the device, and the proposed labeling. Payment of a user fee is required for FDA to initiate review of a PMA application.
During the PMA application review period, the FDA may request additional information or clarification of information provided in the application. In addition, the FDA will conduct a pre-approval inspection of the manufacturing facility or facilities to ensure compliance with the QSR, which requires manufacturers to follow design, testing, control, documentation and other quality assurance procedures.
Although FDA review of an initial PMA application is required by statute to take between six to ten months, these reviews typically take longer (e.g., up to 2 years) due to stoppage of the FDA review clock to address requests for additional information. The FDA can delay, limit or deny approval of a PMA application for many reasons, including:
|
|
• |
if it is not demonstrated that there is reasonable assurance that the device is safe or effective under the conditions of use prescribed, recommended or suggested in the proposed labeling; |
|
|
• |
if the data from pre-clinical studies and clinical trials may be insufficient to support approval; and |
|
|
• |
if the manufacturing process, methods, controls or facilities used for the manufacture, processing, packing or installation of the device do not meet applicable requirements. |
If the FDA evaluations of both the PMA application and the manufacturing facilities are favorable, the FDA will either issue an approval letter or an approvable letter, which may contain conditions that must be met in order to secure final approval of the PMA application. If the FDA’s evaluation of the PMA application or manufacturing facilities is not favorable, the FDA will deny approval of the application or issue a “not approvable” letter. A “not approvable” letter will outline the deficiencies in the application and, where practical, will identify what is necessary to make the application approvable. The FDA may also determine that additional studies (pre-clinical and/or clinical studies) are necessary, in which case the PMA may be delayed for several months or years while these studies are conducted and the subsequent amendment to the PMA application is submitted. Once granted, approval of the PMA application may be withdrawn by the FDA if compliance with post-approval requirements, conditions of approval or other regulatory standards are not maintained or problems are identified following initial marketing.
Post-approval modifications to the manufacturing process, labeling, device specifications, materials or design of a Class III device may require approval of a PMA supplement. PMA supplements require submission of technical data to support implementation of the proposed change to the Class III device. Payment of a user fee is required for FDA to initiate review of a PMA supplement.
Regulation after FDA Clearance or Approval
Any devices we manufacture or distribute pursuant to clearance or approval by the FDA are subject to comprehensive and continuing regulation by the FDA and certain state agencies. We are required to adhere to applicable regulations setting forth detailed GMP requirements, as set forth in the QSR, which includes testing, control and documentation requirements. Non-compliance with these standards can result in fines, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, refusal of the government to grant 510(k) clearance or PMA of devices, withdrawal of marketing approvals and criminal prosecutions. We have
18
designed and implemented quality platform processes within our manufacturing facilities in order to comply with FDA’s GMP requirements.
Because we are a medical device manufacturer, we must also comply with the FDA’s medical device reporting requirements whenever there is evidence that reasonably suggests that one of our products may have caused or contributed to a death or serious injury. We must also report any incident in which our product has malfunctioned if that malfunction would likely cause or contribute to a death or serious injury if it were to recur.
Labeling, advertising, and promotional activities are subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission. Medical devices approved or cleared by the FDA may not be promoted for unapproved or uncleared uses, otherwise known as “off-label” promotion. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability, including substantial monetary penalties and criminal prosecution. We have implemented quality platform processes and advertising/promotional policies designed to comply with these requirements.
Our facilities are also subject to periodic inspection by the FDA and foreign regulatory agencies for, among other things, conformance to the FDA’s QSR and current Good Manufacturing Practice requirements, as well as applicable foreign or international standards. The results of these inspections can include inspectional observations, which are recorded on FDA Form 483, regarding potential violations of the FDCA and related laws, warning letters, or other forms of enforcement. On February 27, 2013, the FDA issued a Form 483 after inspecting our manufacturing facility in Salt Lake City, Utah. The Form 483 included 17 observations of non-compliance with FDA’s requirements. These observations included the material finding of the FDA’s inspection which were noted in the Form 483 were that we did not have appropriate environmental testing to ensure that our contamination controls were adequate and our design history file was not complete. The FDA’s observations listed on a Form 483 do not constitute a final determination that we were in violation of any law or regulation and in response to the Form 483 we took corrective actions to address all 17 observations, including revising manufacturing and quality procedures, training personnel, and reconfiguring our existing manufacturing facilities, and informed the FDA that all observations had been resolved in a final update letter on February 7, 2014. We received a letter from the FDA, dated July 22, 2014, informing us that the inspection is closed. We do not anticipate the FDA to take further action or provide further notice with regard to this matter.
International Regulations
International sales of medical devices are subject to foreign governmental regulations, which vary substantially from country to country. The time required to obtain certification or approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ.
The European Union, or EU, which consists of 25 member countries, has adopted numerous directives and has promulgated voluntary standards regulating the design, manufacture, labeling, clinical trials and adverse event reporting for medical devices. Devices that comply with the requirements of relevant directives will be entitled to bear CE conformity marking, and, accordingly, can be commercially distributed throughout the member states of the European Union and other countries that comply with or mirror these directives. The method for assessing conformity varies depending upon the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a notified body, which is an independent and neutral institution appointed by a country to conduct the conformity assessment. This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s device. Such an assessment in one country within the EU is required for a manufacturer to commercially distribute the product throughout the EU. In addition, compliance with voluntary harmonizing standards ISO 9001 and ISO 13845 issued by the International Organization for Standards establishes the presumption of conformity with the essential requirements for a CE marking. In order to maintain CE Mark certification, we are subject to both announced and unannounced inspections by the notified body. Significant changes to the device or manufacturer’s quality system are also subject to approval by the notified body. Reporting of certain adverse events and product recalls to the national health authorities is required for compliance with the directive. Member states may also require registration of devices and translation of labeling into national languages.
19
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances. Some of these laws require us to obtain licenses or permits to conduct our operations. We have numerous policies and quality platform procedures in place to ensure compliance with these laws and to minimize the risk of occupational exposure to hazardous materials. We do not expect the operations of our products to produce significant quantities of hazardous or toxic waste or radiation that would require the use of extraordinary disposal practices. Although the costs to comply with these applicable laws and regulations have not been material, we cannot predict the impact on our business of new or amended laws or regulations or any changes in the way existing and future laws and regulations are interpreted or enforced, nor can we ensure we will be able to obtain or maintain any required licenses or permits.
Export Regulations
Medical devices that are legally marketed in the United States may be exported anywhere in the world without prior FDA notification or approval. Devices that have not been approved or cleared in the United States must follow the export provisions of the FDCA. Depending on which section of the FDCA we may export under, we may need to request an export permit letter or export certificate, or we may need to submit a simple notification. Export certificates may be requested by foreign customers or foreign governments to provide proof of the products’ status as regulated by the FDA. The export certificate is prepared by FDA and contains information about a product’s regulatory or marketing status in the United States.
Clinical Laboratory Improvement Amendments of 1988 (“CLIA”)
The use of our assays is also affected by CLIA, and related federal and state regulations, which provide for regulation of laboratory testing. Any customers using our assays for clinical use in the United States will be regulated under CLIA, which establishes quality standards for all laboratory testing to ensure the accuracy, reliability and timeliness of patient test results regardless of where the test was performed. In particular, these regulations mandate that clinical laboratories must be certified by the federal government or a federally approved accreditation agency, or must be located in a state that has been deemed exempt from CLIA requirements because the state has in effect laws that provide for requirements equal to or more stringent than CLIA requirements. Moreover, these laboratories must meet quality assurance, quality control and personnel standards, and they must undergo proficiency testing and inspections. The CLIA standards applicable to clinical laboratories are based on the complexity of the method of testing performed by the laboratory, which range from “waived” to “moderate complexity” to “high complexity.” We expect our future assays to all be rated moderately complex or meet the CLIA waiver criteria for tests that are simple and are judged by the FDA to process a low risk for erroneous results.
Foreign Government Regulation
Although it is not a current focus we intend to market our products in European and other select international markets in the future. The regulatory pre-market requirements for molecular devices vary from country to country. Some countries impose product standards, packaging requirements, labeling requirements and import restrictions on devices. Each country has its own tariff regulations, duties and tax requirements. Failure to comply with applicable foreign regulatory requirements may subject us to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. For products sold in the European Economic Area, we have self- declared a Declaration of Conformity under the relevant sections of the applicable European Community standards and other normative documents.
Fraud and Abuse Regulations
We are subject to numerous federal and state healthcare anti-fraud laws, including the federal anti-kickback statute and False Claims Act that are intended to reduce waste, fraud and abuse in the healthcare industry. These laws are broad and subject to evolving interpretations. They prohibit many arrangements and practices that are lawful in industries other than healthcare, including certain payments for consulting and other personal services, some discounting arrangements, the provision of gifts and business courtesies, the furnishing of free supplies and services,
20
and waivers of payments. In addition, many states have enacted or are considering laws that limit arrangements between medical device manufacturers and physicians and other healthcare providers and require significant public disclosure concerning permitted arrangements. These laws are vigorously enforced against medical device manufacturers and have resulted in manufacturers paying significant fines and penalties and being subject to stringent corrective action plans and reporting obligations. We believe that we operate our business within the requirements of these laws. A failure to comply with these laws could require us to expend significant resources on investigation, remediation and monetary penalties. We must, and do, operate our business within the requirements of these laws and, if we are ever accused of violating them, we could be forced to expend significant resources on investigation, remediation and monetary penalties.
Employees
As of December 31, 2016, we had 190 full-time employees located in Salt Lake City, Utah and other locations throughout the United States. We also contract for hire with two outside consultants and contractors. On February 10, 2017, we implemented a workforce reduction plan constituting approximately 26% of our workforce, resulting in the elimination of 49 employees nationwide.
Available Information
We are required to file annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and other information with the SEC. We are subject to the informational requirements of the Exchange Act and file or furnish reports, proxy statements, and other information with the SEC. Copies of these materials, filed by us with the SEC, are available free of charge on our website at www.gbscience.com. These filings are available as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. You can also obtain copies of these materials by visiting the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549, by calling the SEC at 1-800-SEC-0330, or by accessing the SEC’s website at www.sec.gov. The information on, or that may be accessed through, these websites is not incorporated into this filing and should not be considered a part of this filing.
An investment in our securities involves a high degree of risk. Before you invest in our securities, you should give careful consideration to the following risk factors, in addition to the other information included in this annual report, including our financial statements and related notes, before deciding whether to invest in our securities. The occurrence of any of the adverse developments described in the following risk factors could materially and adversely harm our business, financial condition, results of operations or prospects. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Relating to Our Financial Position and Need for Additional Capital
We expect that we will need substantial additional funding to expand our commercialization efforts for our C. diff and Group B Strep assay and other new assays.
Molecular diagnostic development, which includes research and development, pre-clinical and human clinical trials, is a time-consuming and expensive process that takes years to complete. We expect that our expenses will increase substantially as we move new assays through human clinical trials, seek regulatory approvals, and pursue development of additional innovations. If we obtain marketing approval for the diagnostic tests that we develop, license, or acquire, we expect to incur significant commercialization expenses related to regulatory compliance requirements, sales and marketing, manufacturing, and distribution. Net loss for the years ended December 31, 2016 and 2015 was approximately $89.1 million and $57.9 million, respectively. As of December 31, 2016, we had an accumulated deficit of $211.1 million. As discussed in Note 3 to the audited financial statements, our recurring operating losses from operations and our need for additional sources of capital to fund our ongoing operations raise substantial doubt about our ability to continue as a going concern. We expect to continue to incur operating losses for the foreseeable future, and we anticipate these losses will increase as we continue our development and commercialization of our platform, and seek regulatory approval for additional assays. Accordingly, our ability to
21
continue as a going concern depends on our ability to obtain additional financing to fund our operations, and there can be no assurance that additional financing will be available to us or that such financing, if available, will be available on favorable terms. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
We expect that we will need additional funding to manufacture analyzers to be used by potential customers during the sales evaluation phase.
Our potential customers evaluate the performance of our products through the use of analyzers that we manufacture and provide at no cost. Our ability to grow our customer base depends upon our ability to obtain additional financing to fund the manufacturing of analyzers to deliver to such potential customers.
Our inability to raise capital on acceptable terms in the future may cause us to delay, diminish, or curtail certain operational activities, including research and development activities, clinical trials, sales and marketing, and other operations, in order to reduce costs and sustain the business, and such inability would have a material adverse effect on our business and financial condition.
We expect capital outlays and operating expenditures to increase over the next several years as we work to expand our commercial activities, expand our development activities, conduct clinical trials, expand manufacturing operations and expand our infrastructure. We may need to raise additional capital to, among other things:
|
|
• |
fund clinical trials and pre-clinical trials for our assays under development as requested or required by regulatory agencies; |
|
|
• |
sustain commercialization of our C. diff, Group B Strep, e. coli, and Staph ID/R assays and assays under development or review by the FDA; |
|
|
• |
continue the commercial launch and sale of our Staph ID/R assay; |
|
|
• |
expand and automate our manufacturing capabilities and reduce our cost of sales; |
|
|
• |
increase our sales and marketing efforts to drive market adoption and address competitive developments; |
|
|
• |
finance capital expenditures and our general and administrative expenses; |
|
|
• |
develop new assays; |
|
|
• |
maintain, expand and protect our intellectual property portfolio; |
|
|
• |
add operational, financial and management information systems; and |
|
|
• |
hire additional research and development, quality control, scientific, and general and administrative personnel. |
Our present and future funding requirements will depend on many factors, including but not limited to:
|
|
• |
the progress and timing of our clinical trials; |
|
|
• |
the level of research and development investment required to maintain and improve our technology position; |
|
|
• |
the cost of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights, if any; |
|
|
• |
our efforts to acquire or license complementary technologies or acquire complementary businesses; |
|
|
• |
changes in product development plans needed to address any difficulties in commercialization or changing market conditions; |
|
|
• |
competing technological and market developments; |
|
|
• |
changes in regulatory policies or laws that may affect our operations; and |
|
|
• |
changes in physician acceptance or medical society recommendations that may affect commercial efforts. |
22
We may not be able to continue to operate as a going concern.
Our independent registered public accounting firm has included an explanatory paragraph relating to our ability to continue as a going concern in its report on our audited financial statements. Our ability to continue as a going concern is contingent upon, among other factors, the release of restricted cash related to our convertible notes upon satisfaction of the conditions set forth in the terms of the convertible notes, refinance or existing debt arrangements or obtaining alternate financing. We cannot provide any assurance that we will be able to raise additional capital. If we are unable to secure additional capital, refinance our existing debt or gain access to the cash in the restricted accounts, we may be required to curtail our business plans and initiatives and take additional measures to reduce costs in order to conserve our cash in amounts sufficient to sustain operations and meet our obligations.
We have a history of highly dilutive financings and reverse stock splits which amplifies the dilutive effect of these financings on our stockholders. Any investment in our common stock will likely be highly diluted through the future conversion of our outstanding derivative securities and our likely future capital raising efforts.
In the past 12 months, the Company has conducted three (3) stock splits to address the Company’s need to issue shares of common stock to raise capital to fund ongoing operations. These stock splits were as follows:
|
Date |
|
Reverse Split Ratio |
|
March 30, 2016 |
|
35 to 1 |
|
September 16, 2016 |
|
80 to 1 |
|
December 28, 2016 |
|
300 to 1 |
|
Cumulative Ratio |
|
840,000 to 1 |
The multiple stock splits permitted the Company to issue a large number of shares of common stock pursuant to issued and outstanding convertible securities as well as conduct several public offerings to raise additional capital for operations. The conversion of these derivative securities resulted in the substantial dilution of stockholders over the course of the last 12 months. The following table sets forth the number of post-split shares that were issued upon conversion of these derivative securities, the post-split average conversion price, the number of pre-split shares (being the number of share equivalents prior to the three reverse stock splits, calculated by multiplying post-split shares by the cumulative ratio of 840,000 to 1) and the pre-split equivalent conversion prices.
|
|
|
|
|
|
|
Post-Split |
|
|
Pre-Split |
|
|
Pre-Split |
|
|||
|
|
|
|
|
|
|
Average |
|
|
Equivalent |
|
|
Equivalent |
|
|||
|
|
|
Post-Split |
|
|
Conversion |
|
|
Shares Issued |
|
|
Conversion |
|
||||
|
Security |
|
Shares Issued |
|
|
Price |
|
|
(millions) |
|
|
Price |
|
||||
|
Series C Warrants |
|
|
90 |
|
|
$ |
112,392,000 |
|
|
|
76 |
|
|
$ |
133.80 |
|
|
2015 Convertible Notes |
|
|
325,121 |
|
|
$ |
42.00 |
|
|
|
273,102 |
|
|
$ |
0.00 |
|
|
Series F Preferred Stock |
|
|
438,759 |
|
|
$ |
6.00 |
|
|
|
368,558 |
|
|
$ |
0.00 |
|
|
2016 Convertible Notes |
|
|
1,485,139,803 |
|
|
$ |
0.00 |
|
|
|
1,247,517,435 |
|
|
$ |
0.00 |
|
|
Total |
|
|
1,485,903,773 |
|
|
$ |
0.00 |
|
|
|
1,248,159,169 |
|
|
$ |
0.00 |
|
The large number of pre-split equivalent shares of common stock issued is due primarily to three factors: (1) the conversion price of the 2015 Notes being tied to a discount to the market price on the date of the installment payments, (2) the continued decline in the price of the Company’s shares of common stock following each of the reverse splits (3) the conversion of a large number of Series F Preferred Stock at the current post conversion price of $0.002 per $1,000 principal amount of each share of Series F Preferred Stock and (4) the conversion price of the 2016 Notes being tied to a discount to the market price on the date of conversion. Following each reverse split, the issuance of common stock pursuant to these derivative securities and other market factors resulted in a rapid decline in the market price of our common stock. This decline in market price resulted in a greater number of shares of common stock being issued to settle conversion of these derivative securities which was amplified over time by the cumulative effective of the reverse split ratios.
23
In order to maintain flexibility to settle our obligations under our convertible notes issued on July 1, 2016 (the “2016 Notes”), and our Series F Preferred Stock through the issuance of shares and to permit the Company to have available additional shares of common stock to pursue further financing for the Company as needed, our board of directors has unanimously determined that it is in the best interests of the Company and our stockholders to amend the Company’s Certificate of Incorporation to effect the a reverse stock split at a ratio between 1-for-1,700 and 1-for-2,000 (the “2017 Reverse Stock Split”) and to the effect the Share Increase (the “Authorized Share Increase”).
At a special meeting of stockholders held on March 9, 2017, our stockholders (i) approved a reverse split of our common stock at a ratio between 1-for-1,700 and 1-for-2,000, to be effective upon a date on or prior to May 31, 2017, such ratio and date to be determined by our Board and (ii) approved the increase to our authorized common stock from 1,500,000,000 shares to 3,000,000,000 shares.
It is anticipated that the Company will issue a majority of those shares of common stock primarily as follows (i) to settle conversion of the Company’s outstanding Series F Preferred Stock, (ii) to settle conversion of the Company’s outstanding $32.2 million of 2016 Notes outstanding as of March 17, 2017 and (iii) to raise additional capital through one or more offerings of equity or equity-linked securities.
Both the Series F Preferred Stock (beginning July 2017) and the 2016 Notes have conversion prices that are tied to a discount to the Company’s recent average trading price. As a result, if after the 2017 Reverse Stock Split is effective and the stock price again declines, the number of post-split shares that must be issued to settle these securities will increase and the pre-split equivalent number of shares will increase rapidly due to the large proposed ratios of 1,700-for-1 and 2,000-for-1. The dilution to current stockholders will be substantial; similar to the effect of past stock splits.
Our board of directors believes that the 2017 Reverse Stock Split could also facilitate our efforts to raise capital to fund our operations. We will need to raise additional capital and may elect to do so through the issuance of equity or convertible debt securities. The 2017 Reverse Stock Split would reduce the number of shares of common stock outstanding without reducing the total number of authorized shares of common stock. As a result, we would have a larger number of authorized but unissued shares from which to issue additional shares of common stock, or securities convertible or exercisable into shares of common stock, in equity or convertible debt financing transactions. As of the date hereof, we have no specific plans, arrangements or understandings, whether written or oral, with respect to the increase in shares available for issuance as a result of the proposed reverse stock split or the increase in authorized shares.
Raising additional capital will cause dilution to our existing stockholders, and restrict our operations or require us to relinquish certain intellectual property rights.
We will seek additional capital through a combination of public and private equity offerings, debt financings, strategic partnerships and alliances, licensing arrangements and grants. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing stockholders may be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our stockholders. Debt and receivables financings may be coupled with an equity component, such as warrants to purchase shares, which could also result in dilution of our existing stockholders’ ownership. The incurrence of indebtedness would result in increased fixed payment obligations and could also result in certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us. A failure to obtain adequate funds may cause us to curtail certain operational activities, including research and development, regulatory trials, sales and marketing, and manufacturing operations, in order to reduce costs and sustain the business, and would have a material adverse effect on our business and financial condition.
24
The issuance of shares of our common stock pursuant to the Series D warrants, 2016 Notes and related Series H warrants and the Series F Preferred Stock will result in significant dilution to our stockholders.
At a special meeting of our shareholders held on March 9, 2017, our shareholders approved a reverse split of our common stock at a ratio between 1-for-1,700 and 1-for-2,000, to be effective upon a date on or prior to May 31, 2017, such ratio and date to be determined by our Board. This reverse split of our common stock will mean that our stockholders will experience significant dilution as a result of shares of common stock issued pursuant to our outstanding 2016 Notes, Series F Preferred Stock, Series D Warrants and Series H warrants. Under the Series D warrants, 2016 Notes and related Series H warrants and Series F Preferred Stock, we are required to have reserved or have designated for future issuance a number of shares of common stock necessary to effect the conversion of such convertible notes and preferred stock and the exercise of the Series D warrants and Series H warrants, subject to potential future anti-dilution adjustments.
The price at which the Company will convert the 2016 Notes installment amounts is equal to the lowest of (x) $0.50 per share, subject to adjustment as provided therein, (y) 85% of the lower of (I) the lowest volume weighted average price of the common stock and (II) the lowest closing bid price of the common stock, in each case, during the five (5) consecutive trading day period ending on, and including, the trading day on which the holder delivers a conversion notice to the Company (such trading prices to be appropriately adjusted for any share dividend, share split, share combination, reclassification or similar transaction during such five (5) consecutive trading day period), and (z) 85% of the volume weighted average price of the common stock during the period beginning at 9:30:01 a.m., New York time (or such other time as the principal market publicly announces the official opening of trading), and ending at 1:00:00 p.m., New York time, on the delivery date of the applicable conversion notice; provided however, during the period through and including January 24, 2017 to February 15, 2017, the conversion price shall not be lowered to an amount lower than 90% of the volume weighted average price of the common stock on the delivery date of the applicable conversion notice.
For conversions at the election of the holder that are not subject to a floor price pursuant to the terms of the 2016 Notes as originally issued, and at the current conversion price of $0.00068 per share as of March 17, 2017 for such conversions, the $32.2 million in principal amount of 2016 Notes would be convertible into 47.4 billion shares of our common stock. For conversions in relation to the 2016 Notes amortization payments on the convertible notes, if the stock price continues to decline, the number of pre-split equivalent shares of common stock issued could be significantly higher than 47.4 billion shares. If we don’t have sufficient authorized capital to issue these shares we would need to seek another reverse stock split which would result in further substantial dilution to shareholders, an increase in our authorized share capital or settle the 2016 Notes in cash, if available.
Further, we issued Series D and 2015 Subordination warrants issuable to acquire 17.1% of our issued and outstanding shares of common stock on a fully-diluted basis, which were subject to a one time reset on December 31, 2016 to 17.1% of our fully diluted shares of common stock on that date. As of March 17, 2017, following such adjustment, the Series D and 2015 Subordination warrants were exercisable to acquire 2,432,599 shares of common stock at an exercise price of $0.00068 per share, subject to adjustment for subsequent issuances.
Further in connection with the 2016 Note, the Series H warrants that were issued in July 2016 became exercisable as of January 1, 2017. The Series H warrants are exercisable into 2,346 shares of common stock at the current exercise price of $0.00068 per share, subject to adjustment for certain subsequent issuances.
The Series F Preferred Stock is currently convertible at the election of the holder into shares of our common stock at a conversion price equal to $0.00068, subject to adjustments. From and after July 3, 2017, the Series F Preferred Stock shall be convertible at a conversion price equal to 85% of the arithmetic average, in each case of the lower of (i) the three lowest daily weighted average prices of the our common stock during the twenty (20) consecutive trading day period ending on the trading day immediately preceding the date of determination and (iii) the weighted average price of the our common stock on the trading day immediately preceding the date of determination. On November 3, 2016, 2,098 Series F Preferred shares were mandatorily converted into 349,667 shares of our common stock at a conversion price of $6.00 per share. On November 3, 2018, so long as no Triggering Event then exists, any remaining Preferred Shares then outstanding shall be converted into shares of our common stock at a conversion
25
price of $6.00 per share. In each case, shares of our common stock will be held in abeyance to the extent necessary to satisfy limitations on beneficial ownership as described in the Certificate of Designations for the Series F Preferred Stock. As of March 17, 2017 we had converted 2,576 Series F Preferred Shares into 429,334 shares of common stock. As of March 17, 2017, based on 5,860 shares of Series F Preferred Stock issued and outstanding, at a conversion price of $0.00068 per share, we could potentially issue up to 8.6 billion shares of our common stock on conversion of Series F Preferred Stock. If the stock price declines following the Reverse Stock Split, the number of pre-split equivalent shares of Common Stock issued could be significantly higher than 8.6 billion.
Due to the variable nature of the adjustments of the 2016 Notes and Series F Preferred Stock conversion prices and the formula which sets certain conversion prices of these securities based on a discount to the then current market price, the Company could issue substantially more shares of common stock upon conversion of these securities than anticipated above. This is especially the case when the Company conducts reverse stock splits which may increase the per share price in the market but the price may subsequently drop below the immediate post-split price. Although we have the option to settle the principal payments on the 2016 Notes in cash and certain conversion and exercise restrictions are placed upon the holders of the 2016 Notes, Series F Preferred Stock, Series D Warrants and Series H Warrants, the issuance of material amounts of common stock by us would cause our stockholders to experience significant dilution in their investment in the Company.
We may be at risk of securities class action litigation.
We may be at risk of securities class action litigation. This risk is especially relevant for us due to our dependence on positive clinical trial outcomes and regulatory approvals of our diagnostic tests. In the past, life science companies have experienced significant stock price volatility, particularly when associated with binary events such as clinical trials and product approvals. Additionally, due to our price volatility and our high demand for cash to fund operations, we have had to conduct a number of reverse stock splits and highly dilutive financings to continue as a going concern which exposes us to additional risk of securities class action litigation. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business and results in a decline in the market price of our common stock. If such lawsuits were successful we may not be able to pay awarded damages and we may be forced into bankruptcy which would likely result in the complete loss of your investment.
Market and economic conditions may negatively impact our business, financial condition and share price.
In recent years, concerns over inflation, energy costs, geopolitical issues, the U.S. mortgage market and a declining real estate market, unstable global credit markets and financial conditions, and volatile oil prices have led to periods of significant economic instability, diminished liquidity and credit availability, declines in consumer confidence and discretionary spending, diminished expectations for the global economy and expectations of slower global economic growth going forward, increased unemployment rates, and increased credit defaults. Our general business strategy may be adversely affected by any such economic downturns, volatile business environments and unstable or unpredictable economic and market conditions. If these conditions occur, deteriorate or do not improve, it may make any necessary debt or equity financing more difficult to complete, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance, and share price and could require us to delay or abandon development or commercialization plans. In addition, there is a risk that one or more of our current and future service providers, manufacturers, suppliers, hospitals and other medical facilities, our third party payors, and other partners could be negatively affected by these difficult economic times, which could adversely affect our ability to attain our operating goals on schedule and on budget or meet our business and financial objectives.
Our ability to use our net operating loss carryforwards is limited.
As of December 31, 2016, we had federal income tax net operating loss, or NOL, carryforwards of approximately $46.7 million and state income tax NOL carryforwards of approximately $41.6 million. These NOL carryforwards, if not previously used, will begin to expire in 2023. During 2015 we experienced a shift in our stock ownership within the meaning of Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, or under
26
applicable state tax laws that currently subject our NOL carryforwards to an annual limitation. Accordingly, we were required to write off $23.2 million of net operating loss carryforwards. If we experience a shift in our stock ownership and earn net taxable income in the future, the limitations on our ability to use our NOL carryforwards to reduce U.S. federal and state tax liabilities will result in increased future tax liability to us.
We have identified a material weakness in our internal control over financial reporting that, if not properly remediated, could result in material misstatements in our financial statements in future periods.
Management identified the following material weakness that continues to be a material weakness as of December 31, 2016:
|
|
• |
We did not design and maintain effective controls to analyze complex and non-routine transactions or adequately review the accounting and/or disclosure for these transactions. The nature of our financing agreements increases the complexity of our accounting for potential fair value liabilities. As we enter into additional equity or debt financing transactions, which may have contractual provisions different from those of our existing financing instruments, the accounting and valuation of these financial instruments may become increasingly complicated. This additional complexity could increase the chance that we experience additional errors in the future, particularly because we have a material weakness in our internal control. |
Because of the material weakness described above, management concluded that the Company did not maintain effective internal control over financial reporting as of December 31, 2016, based on criteria in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
With respect to the remediation of the material weakness, we have hired additional accounting and IT personnel to help improve our segregation of duties. We continue to engage third-party consultants to assist us with our evaluation of complex technical accounting matters, including the valuation and accounting for our complex derivative agreements. We also continue to engage consultants to advise us on making further improvements to our internal control over financial reporting. We believe that these additional resources will enable us to broaden the scope and quality of our controls relating to the oversight and review of financial statements and our application of relevant accounting policies. These remediation efforts are still in process and have not yet been completed. We cannot assure you that the steps taken will remediate such weakness, nor can we be certain of whether additional actions will be required or the costs of any such actions.
We may need to take additional measures to fully mitigate these issues, and the measures we have taken, and expect to take, to improve our internal controls may not be sufficient to address the issues identified, to ensure that our internal controls are effective or to ensure that the identified material weaknesses or significant deficiencies or other material weaknesses or deficiencies will not result in a material misstatement of our annual or interim financial statements. In addition, other material weaknesses or deficiencies may be identified in the future. If we are unable to correct material weaknesses or deficiencies in internal controls in a timely manner, our ability to record, process, summarize and report financial information accurately and within the time periods specified in the rules and forms of the SEC will be adversely affected, and could result in material misstatements in our financial statements in future periods. This failure could negatively affect the market price and trading liquidity of our common stock, cause investors to lose confidence in our reported financial information, subject us to civil and criminal investigations and penalties, and generally materially and adversely impact our business and financial condition.
Risks Related to the Convertible Note Financings
Our obligations to the holders of our 2016 Notes are secured by a security interest in substantially all of our assets, so if we default on those obligations, the convertible note holders could foreclose on our assets.
Our obligations under the 2016 Notes and the transaction documents relating to those convertible notes are secured by a security interest in substantially all of our assets. As a result, if we default under our obligations under the 2016
27
Notes or the transaction documents, the holders of such convertible notes, acting through their appointed agent, could foreclose on their security interests and liquidate some or all of these assets, which would harm our business, financial condition and results of operations and could require us to reduce or cease operations.
The holders of the 2016 Notes have certain additional rights upon an event of default under such convertible notes which could harm our business, financial condition and results of operations and could require us to reduce or cease or operations.
Under the 2016 Notes, the holders have certain rights upon an event of default. Such rights include (i) the remaining principal amount of the convertible notes bearing interest at a rate of 10% per annum, (ii) during the event of default the conversion price being adjusted to the lowest of (a) the conversion price then in effect, (b) 75% of the lowest weighted average price of the common stock during the 30 consecutive trading day period ending on the trading day immediately preceding the date of the event of default conversion and (c) 75% of the weighted average price of the common stock on the date of the applicable event of default conversion, and (iii) the holder having the right to demand redemption of all or a portion of the convertible notes, as described below. At any time after certain notice requirements for an event of default are triggered, a holder of convertible notes may require us to redeem all or any portion of the convertible note by delivering written notice. Each portion of the convertible note subject to redemption would be redeemed by us in cash by wire transfer of immediately available funds at a price equal to the greater of (x) 125% of the conversion amount being redeemed and (y) the product of (A) the conversion amount being redeemed and (B) the quotient determined by dividing (I) the greatest closing price of the shares of common stock during the period beginning on the date immediately preceding such event of default and ending on the date the holder delivers the redemption notice, by (II) the lowest conversion price in effect during such period. We may not have sufficient funds to settle the redemption price and, as described above, this could trigger rights under the security interest granted to the holders and result in the foreclosure of their security interests and liquidation of some or all of our assets.
The exercise of any of these rights upon an event of default could substantially harm our financial condition and force us to reduce or cease operations.
The Company is in default as of February 28, 2017 on the dollar value of daily trading and stock price requirement in the equity conditions under the 2016 Notes. If the Company does not obtain a waiver or an amendment to equity conditions to lower the stock price requirement, then the Company will not be able to convert amortization payments under the 2016 Notes and the restrictions on cash held in the Company’s restricted accounts will not be released, either of which will harm our financial condition, could force us to declare bankruptcy and could result in the loss of your entire investment.
The release of restrictions on cash held in the Company’s restricted accounts on certain dates under the terms of the 2016 Notes depends upon the Company’s satisfaction of certain equity conditions as set forth in the 2016 Notes. If the Company does not meet these equity conditions, then the cash will not become available to the Company. Two of those equity conditions are that the Company’s shares of common stock have a dollar value of daily trading volume of at least $800,000 and a 5-day weighted average price of $31,200 (adjusted for the Company’s reverse stock splits) during certain equity condition measurement periods. The Company obtained a waiver on January 9, 2017, to these equity conditions which extended through February 28, 2017 and has now expired. As of March 17, 2017, the Company’s common stock closed at $0.001 per share and the dollar value of daily trading volume was $17,172. If the Company does not receive a new waiver or an amendment to the 2016 Notes which amends these equity conditions, then the Company will not be able to convert amortization payments under the 2016 Notes, which would require settlement of the payments in cash. Further, the Company would not meet the requirements to receive the release of the restrictions on cash in the restricted accounts. If the Company does not have sufficient cash to pay the amortization payments when due and payable under the 2016, then an Event of Default (as defined in the 2016 Notes) would occur and the holders of the 2016 Notes would be able to exercise their rights under the 2016 Notes, including but not limited to demanding immediate payment of the entire aggregate principal amount of the 2016 Notes and exercising rights under the security agreement over the Company’s assets.
28
Further, if the Company doesn’t receive the release of restrictions on cash in the restricted accounts, then the Company likely would need to raise more capital to continue as a going concern. Any of the above events would substantially harm our financial position and could result in the Company declaring bankruptcy.
Risk Related to Our Series F Preferred Stock
Holders of our Series F Preferred Stock have certain additional rights upon the occurrence of certain triggering events which could harm our business, financial condition and results of operations and could require us to reduce or cease or operations.
Under the Certificate of Designations for the Series F Preferred Stock, the holders will have certain rights upon a “Triggering Event” (as defined in the Certificate of Designations for the Series F Preferred Stock). Such rights include (i) the remaining principal amount of the Series F Preferred Stock bearing interest at a rate of 10% per annum, (ii) during the Triggering Event the conversion price being adjusted to the lowest of (a) the conversion price then in effect, (b) 75% of the lowest weighted average price of the our common stock during the 30 consecutive trading day period ending on the trading day immediately preceding the date of the Triggering Event conversion and (c) 75% of the weighted average price of the our common stock on the date of the applicable Triggering Event conversion, and (iii) the holder having the right to demand redemption of all or any number of the preferred shares.
At any time after the earlier of the holder’s receipt of a notice of an Triggering Event and the holder becoming aware of an Triggering Event and ending on the 15th trading day after the later of (x) the date such Triggering Event is cured and (y) the holder’s receipt of an Triggering Event notice, the holder may require the Company to redeem all or any number of the preferred shares at a price equal to the greater of (x) 125% of the conversion amount being redeemed and (y) the product of (A) the conversion amount being redeemed and (B) the quotient determined by dividing (I) the greatest closing price of the shares of our common stock during the period beginning on the date immediately preceding such Triggering Event and ending on the date the holder delivers the redemption notice, by (II) the lowest conversion price in effect during such period.
“Triggering Event” includes, but is not limited to (and subject to the ability to cure in certain instances): (i) (A) the suspension from trading for more than an aggregate of ten (10) trading days in any 365-day period or (B) the failure of the our common stock to be listed on an eligible market; (ii) the Company’s (i) failure to convert the Series F Preferred Shares or related warrants by delivery of the required number of shares of our common stock within five (5) trading days after the applicable conversion or exercise date, (ii) notice of its intention not to comply with a request for conversion or exercise of the Series F Preferred Stock or related warrants or (iii) the Company fails to have sufficient authorized shares to convert the Series F Preferred Stock and related warrants in full for 75 consecutive days. (iii) any payment failure; (iv) any default under, redemption of or acceleration prior to maturity of more than $100,000, individually or in the aggregate, of Indebtedness of the Company or any of its subsidiaries; (v) certain bankruptcy events; (vii) a final judgment or judgments for the payment of money aggregating in excess of $250,000, individually or in the aggregate, are rendered against the Company or any of its subsidiaries and which judgments are not, within sixty (60) days after the entry thereof, bonded, discharged or stayed pending appeal, or are not discharged within seventy-five (75) days after the expiration of such stay; provided, however, that any judgment which is covered by insurance or an indemnity from a credit worthy party shall not be included in calculating the $250,000 amount set forth above so long as the Company provides each holder a written statement from such insurer or indemnity provider (which written statement shall be reasonably satisfactory to such holder) to the effect that such judgment is covered by insurance or an indemnity and the Company will receive the proceeds of such insurance or indemnity within forty-five (45) days of the issuance of such judgment; (viii) breaches of representations, warranties and covenants in the Certificate of Designations for the Series F Preferred Stock and related transaction documents; or (ix) the Company’s failure for any reason after the date that is six (6) months immediately following the issuance date to satisfy the current public information requirement under Rule 144(c) of the Securities Act.
The triggering and exercise of any of these rights could substantially harm our financial condition and force us to reduce or cease operations.
Holders of our Series F Preferred Stock have voting rights on an as converted basis. These rights permit the holders of the Series F Preferred Stock to vote up to certain percentage of our outstanding voting securities.
29
The holders of the Series F Preferred Stock may have interests in matters brought before the shareholders that are different than holders of common stock. The holders of Series F Preferred Stock have the right to vote with holders of shares of our common stock, voting together as one class on all matters, with each share of Series F Preferred share entitling the holder thereof to cast that number of votes per share as is equal to the aggregate number of stock of our common stock into which it is then convertible (without regard to any limitations on conversion set forth in the Certificate of Designations for the Series F Preferred Stock including without limitation, the maximum percentage and/or the failure to have a sufficient number of shares of common stock reserved or available for issuance pursuant to the Certificate of Designations for the Series F Preferred Stock) using the record date for determining the stockholders of the Company eligible to vote on such matters as the date as of which the conversion price is calculated; provided, that no holder (together with such holder’s attribution parties) shall be permitted to have a number of votes in excess of such aggregate number of votes granted to the holders of 9.99% of the shares our common stock then outstanding (including any votes with respect to any shares of our common stock and preferred stock beneficially owned by the holder or such holder’s attribution parties).
The holders of the Series F Preferred Stock may have interests in matters brought before the stockholders that are different than the interests of holders of our common stock. Specifically, the holders of our Series F Preferred Stock are also holders of our 2016 Notes and their interests in relation to stockholder matters that affect the 2016 Notes will be different than the interests of holders of our common stock. While the Series F Preferred Stock holders do not act as a group, in the instances where their interests are aligned, their ability to cast votes on an as converted basis may affect the outcome of any stockholder votes on such matters.
Risks Related to Owning our Common Stock and Other Securities
The price of our common stock may fluctuate substantially.
The market price of our common stock has been and may continue to be subject to wide fluctuation in response to various factors, some of which are beyond our control. Some factors that may cause the market price of our common stock to fluctuate, in addition to the other risks mentioned in this “Risk Factors” section and elsewhere in this report, are:
|
|
• |
sales of our common stock by our stockholders, executives, and directors; |
|
|
• |
conversions of the 2016 Notes into shares of common stock and subsequent sales of such shares by such holders; |
|
|
• |
volatility and limitations in trading volumes of our shares of common stock or units; |
|
|
• |
fluctuations in our results of operations; |
|
|
• |
our ability to enter new markets; |
|
|
• |
actual or unanticipated fluctuations in our annual and quarterly financial results; |
|
|
• |
our ability to obtain financings to continue and expand our commercial activities, expand our manufacturing operations, conduct and complete research and development activities including, but not limited to, our human clinical trials, and other business activities; |
|
|
• |
our ability to secure resources and the necessary personnel to continue and expand our commercial activities, develop additional assays, conduct clinical trials and gain approval for our additional assays on our desired schedule; |
|
|
• |
commencement, enrollment or results of our clinical trials of our assays or any future clinical trials we may conduct; |
|
|
• |
changes in the development status of our assays; |
|
|
• |
any delays or adverse developments or perceived adverse developments with respect to the FDA’s review of our planned clinical trials; |
30
|
|
• |
any delay in our submission for studies or test approvals or adverse regulatory decisions, including failure to receive regulatory approval for our assays; |
|
|
• |
our announcements or our competitors’ announcements regarding new assays, enhancements, significant contracts, acquisitions or strategic investments; |
|
|
• |
unanticipated safety concerns related to our assays; |
|
|
• |
failures to meet external expectations or management guidance; |
|
|
• |
changes in our capital structure or dividend policy, including as a result of future issuances of securities and sales of large blocks of common stock by our stockholders; |
|
|
• |
our cash position; |
|
|
• |
announcements and events surrounding financing efforts, including debt and equity securities; |
|
|
• |
our inability to enter into new markets or develop new assays; |
|
|
• |
reputational issues; |
|
|
• |
competition from existing technologies and assays or new technologies and assays that may emerge; |
|
|
• |
announcements of acquisitions, partnerships, collaborations, joint ventures, new assays, capital commitments, or other events by us or our competitors; |
|
|
• |
changes in general economic, political and market conditions in any of the regions in which we conduct our business; |
|
|
• |
changes in industry conditions or perceptions; |
|
|
• |
changes in valuations of similar companies or groups of companies; |
|
|
• |
analyst research reports, recommendations and changes in recommendations, price targets and withdrawals of coverage; |
|
|
• |
departures and additions of key personnel; |
|
|
• |
disputes and litigations related to intellectual properties, proprietary rights and contractual obligations; |
|
|
• |
changes in applicable laws, rules, regulations, or accounting practices and other dynamics; |
|
|
• |
announcements or actions taken by our principal stockholders; and |
|
|
• |
other events or factors, many of which may be out of our control. |
In addition, if any of the market for stocks in our industry or industries related to our industry, or the stock market in general, experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition and results of operations. If any of the foregoing occurs, it could cause our stock price to fall and may expose us to lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
Certain of our outstanding warrants and convertible securities have anti-dilution provisions triggered by the issuance of shares of common stock and securities exercisable for shares of our common stock at prices below the then current exercise prices for such warrants, pursuant to which the exercise price of such warrants or conversion price of such convertible securities will be adjusted downward and could make it more likely that such warrants are exercised or such convertible securities are converted and dilute our current stockholders.
The exercise price for each of the Class A Warrants, Class B Warrants, Series D Warrants, Series G Warrants, Series H Warrants, certain other warrants, 2015 Subordination Warrants and 2016 Subordination Warrants and the conversion price of the Series F Preferred Stock and 2016 Notes is subject to adjustment in the event we issue common stock or securities convertible into common stock at a price lower than the then-current exercise price or conversion price, as the case may be. The exercise price of the Series B Warrants is subject to adjustment in the
31
event we issue common stock or securities convertible into common stock at a price lower than the current market price.
If we issue shares of common stock or securities exercisable or convertible for shares of common stock that trigger these provisions, then the exercise price for these warrants and conversion price for the 2016 Notes and the Series F Preferred Stock will be reduced according to their provisions, in most cases, to the per share price of the triggering transaction. A reduction in the exercise price of these warrants and other securities will make it more likely that they are exercised or converted resulting in further dilution to our then current stockholders.
Broker-dealers may be discouraged from effecting transactions in our shares of common stock because they are considered a penny stock and are subject to the penny stock rules.
Our shares of common stock are currently considered a “penny stock.” The SEC has adopted Rule 15g-9 which generally defines “penny stock” to be any equity security that has a market price (as defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. The shares of common stock are covered by the penny stock rules, which impose additional sales practice requirements on broker-dealers who sell to persons other than established customers and “accredited investors.” The term “accredited investor” refers generally to institutions with assets in excess of $5,000,000 or individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouse. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document in a form prepared by the SEC, which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer’s confirmation. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for the shares of common stock. Consequently, these penny stock rules may affect the ability of broker-dealers to trade in the shares of common stock.
Future sales and issuances of our common stock or rights to purchase common stock could result in additional dilution of the percentage ownership of our stockholders and could cause our share price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations, including expanding research and development, funding clinical trials, purchasing of capital equipment, hiring new personnel, commercializing our diagnostic tests, and continuing activities as an operating public company. To the extent we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. We may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights superior to our existing stockholders.
We do not intend to pay cash dividends on our shares of common stock so any returns will be limited to the value of our shares.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to stockholders will therefore be limited to the increase, if any, of our share price.
We are an “emerging growth company” and will be able to avail ourselves of reduced disclosure requirements applicable to emerging growth companies, which could make our common stock less attractive to investors.
32
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. Investors may find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of our IPO; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission.
We have elected to use the extended transition periods for complying with new or revised accounting standards.
We have elected to use the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date we (i) are no longer an emerging growth company or (ii) affirmatively and irrevocably opt out of the extended transaction period provided in Section 7(a)(2)(B). As a result, our financial statements may not be comparable to those of companies that comply with public company effective dates.
Our management is required to devote substantial time to compliance initiatives.
As a public company, we incur significant legal, accounting and other expenses that we did not incur as a newly formed entity. The Sarbanes-Oxley Act, as well as rules subsequently implemented by the Securities and Exchange Commission, have imposed various new requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Our management and other personnel devote a substantial amount of time to these new compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time consuming and costly. We expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage.
Provisions of our Seventh Amended and Restated Certificate of Incorporation, as amended, our Amended and Restated Bylaws and Delaware law could make an acquisition of our Company, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove the current members of our board of directors and management.
Certain provisions of our Seventh Amended and Restated Certificate of Incorporation, as amended, or our Certificate, and our Amended and Restated Bylaws (our “Bylaws”), could discourage, delay or prevent a merger, acquisition or other change of control that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove members of our board of directors. These provisions also could limit the price that investors might be willing to pay in the future for our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions:
|
|
• |
establish a classified board of directors, such that not all members of the board of directors may be elected at one time; |
33
|
|
• |
require that stockholder actions must be effected at a duly called stockholder meeting or by written consent of the stockholders if such action has been earlier approved by the board of directors; |
|
|
• |
establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings; |
|
|
• |
limit who may call stockholder meetings; and |
|
|
• |
require the approval of the holders of at least sixty percent of the outstanding shares of our capital stock entitled to vote in order to amend certain provisions of our Certificate and at least two-thirds of the outstanding voting stock to amend certain provisions of our Bylaws. |
In addition, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of the voting rights on our common stock, from merging or combining with us for a prescribed period of time.
Risks Related to Our Business and Industry
We have a limited commercial history upon which to base our prospects, have not generated profits and do not expect to generate profits for the foreseeable future. We may never achieve or sustain profitability.
We began operations in January 2005, and we have a limited operating history. We have not earned significant revenue to-date, and do not expect to earn significant revenue in the near future. We had a net loss for the years ended December 31, 2016 and 2015 of approximately $89.1 million and $57.9 million, respectively. As of December 31, 2016, we had an accumulated deficit of $211.1 million. Investors should be aware of the difficulties normally encountered by a new enterprise, many of which are beyond our control, including substantial risks and expenses in the course of developing new diagnostic tests, establishing or entering new markets, organizing operations and marketing procedures. The likelihood of our success must be considered in light of these risks, expenses, complications and delays, and the competitive environment in which we operate. There is, therefore, nothing at this time upon which to base an assumption that our business plan will prove successful, and we may not be able to generate significant revenue, raise additional capital or operate profitably. We will continue to encounter risks and difficulties frequently experienced by early commercial stage companies, including scaling up our infrastructure and headcount, and may encounter unforeseen expenses, difficulties or delays in connection with our growth. In addition, as a result of the start-up nature of our business, we can be expected to continue to sustain substantial operating expenses without generating sufficient revenues to cover expenditures. As discussed in Note 3 to the audited financial statements, our recurring operating losses from operations and our need for additional sources of capital to fund our ongoing operations raise substantial doubt about our ability to continue as a going concern. Any investment in our Company is therefore highly speculative and could result in the loss of your entire investment.
Our near-term success is dependent upon our ability to expand our customer base.
Our current customer base is composed of hospitals and testing laboratories that use our C. diff, Group B Strep and other assays. Our success will depend, in part, upon our ability to expand our customer base and increase revenue by adding new products. Attracting new customers requires substantial time and expense. Any failure to expand our existing customer base would adversely affect our operating results. Many factors could affect the market acceptance and commercial success of our assays, including:
|
|
• |
our ability to convince our potential customers of the advantages and economic value of our analyzers and assays over competing technologies and diagnostic assays; |
|
|
• |
the breadth of our assay menu relative to competitors; |
|
|
• |
changes to policies, procedures or currently accepted best practices in clinical diagnostics; |
34
|
|
• |
the extent and success of our marketing and sales efforts; |
|
|
• |
our ability to manufacture analyzers for use by potential customers during the sales evaluation phase; and |
|
|
• |
our ability to manufacture our commercial diagnostic cartridges and meet demand in a timely fashion. |
If we cannot successfully develop, maintain, commercialize, or obtain regulatory approvals for new and existing diagnostic assays, our financial results will be harmed and our ability to compete will be harmed.
Our financial performance depends in part upon our ability to successfully develop and market new assays in a rapidly changing technological and economic environment, and to maintain and successfully commercialize previously cleared assays. If we fail to successfully introduce new assays or do not maintain approval for previously FDA-cleared assays, we could lose customers and market share. We could also lose market share if our competitors introduce new assays or technologies that render our assays less competitive or obsolete. In addition, delays in the introduction of new assays due to regulatory, developmental or other obstacles could negatively impact our revenue and market share, as well as our earnings. Factors that can influence our ability to introduce new assays, the timing associated with new product approvals and commercial success of these assays include:
|
|
• |
the scope of and progress made in our research and development activities; |
|
|
• |
our ability to successfully initiate and complete clinical trial studies; |
|
|
• |
timely expansion of our menu of assays; |
|
|
• |
the results of clinical trials needed to support any regulatory approvals of our assays; |
|
|
• |
our ability to obtain and maintain requisite FDA or other regulatory clearances or approvals for our assays on a timely basis; |
|
|
• |
demand for the new assays we introduce; |
|
|
• |
product offerings from our competitors; and |
|
|
• |
the functionality of new assays that address market requirements and customer demands. |
We are subject to many laws and governmental regulations and any adverse regulatory action may materially adversely affect our financial condition and business operations.
Our assays for C. diff, Group B Strep, Staph ID/R and STEC and any assays that we develop and commercialize in the future are subject to regulation by numerous government agencies, including the FDA and comparable foreign agencies. To varying degrees, each of these agencies requires us to comply with laws and regulations governing the development, testing, manufacturing, labeling, marketing and distribution of our assays. In particular, FDA regulations govern activities such as product development, product testing, product labeling, product storage, premarket clearance or approval, manufacturing, advertising, promotion, product sales, reporting of certain product failures and distribution. Our assays will require 510(k) clearance from the FDA prior to marketing. Clinical trials are required to support a 510(k) submission.
We may be unable to obtain marketing clearance for our assays in development. If such approval is obtained, it may:
|
|
• |
take a significant amount of time; |
|
|
• |
require the expenditure of substantial resources; |
|
|
• |
involve stringent clinical and pre-clinical testing; |
|
|
• |
involve modifications, repairs, or replacements of our assays; and/or |
|
|
• |
result in limitations on the proposed uses of our assays. |
Our facilities are subject to periodic inspection by the FDA and foreign regulatory agencies and conformance to the FDA’s Quality System Regulation (the “QSR”) and current Good Manufacturing Practice requirements, as well as applicable foreign or international standards. The results of these inspections can include inspectional observations
35
regarding potential violations of the Food, Drug and Cosmetic Act (the “FDCA”) and related laws, warning letters, restrictions on medical device sales and other forms of enforcement.
Since 2009, the FDA has significantly increased its oversight of companies subject to its regulations, including medical device companies, by hiring new investigators and stepping up inspections of manufacturing facilities. The FDA has recently also significantly increased the number of warning letters issued to companies. If the FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our medical devices are ineffective or pose an unreasonable health risk, the FDA could ban such medical devices, detain or seize adulterated or misbranded medical devices, order a recall, repair, replacement, or refund of such devices, refuse to grant pending pre-market approval applications or require certificates of foreign governments for exports, and/or require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health. The FDA may also impose operating restrictions on a company-wide basis, enjoin and restrain certain violations of applicable law pertaining to medical devices and assess civil or criminal penalties against our officers, employees or us. The FDA may also recommend prosecution to the U.S. Department of Justice. Any adverse regulatory action, depending on its magnitude, may restrict us from effectively marketing and selling our diagnostic tests.
Foreign governmental regulations have become increasingly stringent and more common, and we may become subject to more rigorous regulation by foreign governmental authorities in the future. Penalties for a company’s non-compliance with foreign governmental regulation could be severe, including revocation or suspension of a company’s business license and criminal sanctions. Any domestic or foreign governmental law or regulation imposed in the future may have a material adverse effect on us.
Our current and potential customers in the United States and elsewhere may also be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens and diminished profits and future earnings.
The life sciences industry is highly competitive and subject to rapid technological change. If our competitors and potential competitors develop superior assays and technologies, our competitive position and results of operations would suffer.
We face intense competition from a number of companies that offer assays in our target markets, many of which have substantially greater financial resources and larger, more established marketing, sales and service operations than we do. The life sciences industry is characterized by rapid and continuous technological innovation. We may need to develop new technologies for our existing product and our assays to be competitive. One or more of our current or future competitors could render our existing products or assays under development obsolete or uneconomical by technological advances. We may also encounter other problems in the process of delivering new assays to the marketplace, such as problems related to FDA clearance or regulations, design, development or manufacturing of such assays, and as a result we may be unsuccessful in selling such assays. Our future success depends on our ability to compete effectively against current technologies, as well as to respond effectively to technological advances by developing and marketing assays that are competitive in the continually changing technological landscape.
If our assays do not perform as expected or the reliability of the technology on which our assays are based is questioned, we could experience delayed or reduced market acceptance of our assays, increased costs and damage to our reputation.
Our success depends on the market’s confidence that we can provide reliable, high-quality analyzers and diagnostic cartridges. We believe that customers in our target markets are likely to be particularly sensitive to product defects and errors. Our reputation and the public image of our assays or technologies may be impaired if our assays fail to perform as expected or our assays are perceived as difficult to use. Despite quality control testing, defects or errors could occur in our assays or technologies.
36
In the future, if our assays experience a material defect or error, this could result in loss or delay of revenues, delayed market acceptance, product recalls, damaged reputation, diversion of development resources, legal claims, increased insurance costs or increased service and warranty costs, any of which could harm our business. Such defects or errors could also prompt us to amend certain warning labels or narrow the scope of the use of our assays, either of which could hinder our success in the market. Even after any underlying concerns or problems are resolved, any widespread concerns regarding our technology or any manufacturing defects or performance errors in our assays could result in lost revenue, delayed market acceptance, damaged reputation, increased service and warranty costs and claims against us.
If our international distributor relationships are not successful, our ability to market and sell our assays will be harmed and our financial performance will be adversely affected.
Outside of the United States, we depend on relationships with distributors for the marketing and sales of our assays in various geographic regions, and we have a limited ability to influence their efforts. Relying on distributors for our sales and marketing could harm our business for various reasons, including:
|
|
• |
agreements with distributors may terminate prematurely due to disagreements or may result in litigation between the partners; |
|
|
• |
our distributors may not devote sufficient resources to the sale of our assays; |
|
|
• |
our distributors may be unsuccessful in marketing our assays; and |
|
|
• |
we may not be able to negotiate future distributor agreements on acceptable terms. |
If any of our products, or the malfunctioning of our products, causes or contributes to a death or a serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA medical device reporting regulations, medical device manufacturers are required to report to the FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device were to recur. If we fail to report these events to the FDA within the required timeframes, or at all, FDA could take enforcement action against us. Any such adverse event involving our assays could also result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
Our assays may in the future be subject to product recalls. A recall of our products, either voluntarily or at the direction of the FDA or another governmental authority, including a third-country authority, or the discovery of serious safety issues with our products, could have a significant adverse impact on us.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. The FDA requires that certain classifications of recalls be reported to the FDA within ten working days after the recall is initiated. A government-mandated or voluntary recall by us or one of our international distributors could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our assays would divert managerial and financial resources and have an adverse effect on our reputation, results of operations and financial condition, which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be subject to liability claims, be required to bear other costs, or take other actions that may have a negative impact
37
on our future sales and our ability to generate profits. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA or another third-country competent authority. We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the FDA or another third-country competent authority. If the FDA disagrees with our determinations, it could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they occur. We are also required to follow detailed recordkeeping requirements for all Company-initiated medical device corrections and removals. In addition, in December 2012, the FDA issued a draft guidance intended to assist the FDA and industry in distinguishing medical device recalls from product enhancements. Per the guidance, if any change or group of changes to a device that addresses a violation of the FDCA, that change would generally constitute a medical device recall and require submission of a recall report to the FDA.
If we become subject to claims relating to improper handling, storage or disposal of hazardous materials, we could incur significant cost and time to comply.
Our research and development processes involve the controlled storage, use and disposal of hazardous materials, including biological hazardous materials. We are subject to foreign, federal, state and local regulations governing the use, manufacture, storage, handling and disposal of materials and waste products. We may incur significant costs complying with both existing and future environmental laws and regulations. In particular, we are subject to regulation by the Occupational Safety and Health Administration, or OSHA, and the Environmental Protection Agency, or EPA, and to regulation under the Toxic Substances Control Act and the Resource Conservation and Recovery Act in the United States. OSHA or the EPA may adopt additional regulations in the future that may affect our research and development programs. The risk of accidental contamination or injury from hazardous materials cannot be eliminated completely. In the event of an accident, we could be held liable for any damages that result, and any liability could exceed the limits or fall outside the coverage of our workers’ compensation insurance. We may not be able to maintain insurance on acceptable terms, if at all.
Our diagnostic cartridges have not been manufactured on a high volume scale and are subject to unforeseen scale-up risks.
Although we have developed a process to manufacture diagnostic cartridges for our current volume of sales, there can be no assurance that we can manufacture our diagnostic cartridges at a scale that is adequate for our future commercial needs. We may face significant or unforeseen difficulties in manufacturing our diagnostic cartridges, including but not limited to:
|
|
• |
technical issues relating to manufacturing components of our diagnostic cartridges on a high volume commercial scale at reasonable cost, and in a reasonable time frame; |
|
|
• |
difficulty meeting demand or timing requirements for orders due to excessive costs or lack of capacity for part or all of an operation or process; |
|
|
• |
lack of skilled labor or unexpected increases in labor costs needed to produce or maintain our analyzers or perform certain required operations; |
|
|
• |
changes in government regulations or in quality or other requirements that lead to additional manufacturing costs or an inability to supply product in a timely manner, if at all; and |
|
|
• |
increases in raw material or component supply cost or an inability to obtain supplies of certain critical supplies needed to complete our manufacturing processes. |
These and other difficulties may only become apparent when scaling up to the manufacturing process of our diagnostic cartridges to a more substantive commercial scale. If our diagnostic cartridges cannot be manufactured in sufficient commercial quantities or manufacturing is delayed, our future prospects could be significantly impacted and our financial prospects would be materially harmed.
We or our suppliers may experience development or manufacturing problems or delays that could limit the growth of our revenue or increase our losses.
38
We may encounter unforeseen situations in the manufacturing of our diagnostic cartridges that could result in delays or shortfalls in our production. Our suppliers may also face similar delays or shortfalls. In addition, our or our suppliers’ production processes may have to change to accommodate any significant future expansion of our manufacturing capacity, which may increase our or our suppliers’ manufacturing costs, delay production of our diagnostic cartridges, reduce our product gross margin and adversely impact our business. If we are unable to satisfy demand for our diagnostic cartridges by successfully manufacturing and shipping our diagnostic cartridges in a timely manner, our revenue could be impaired, market acceptance for our assays could be adversely affected and our customers might instead purchase our competitors’ assays. In addition, developing manufacturing procedures for assays under development may require developing specific production processes for those assays. Developing such processes could be time consuming and any unexpected difficulty in doing so can delay the introduction of a product.
We are dependent on single source suppliers for some of the components and materials used in our assays, and supply chain interruptions could negatively impact our operations and financial performance.
Our assays are manufactured by us and we obtain supplies from a limited number of suppliers. In some cases, critical components required to manufacture our assays may only be available from a sole supplier or limited number of suppliers, any of whom would be difficult to replace. The supply of any of our manufacturing materials may be interrupted because of poor vendor performance or other events outside our control, which may require us, among other things, to identify alternate vendors and result in lost sales and increased expenses. Even if the manufacturing materials that we source are available from other parties, the time and effort involved in validating the new supplies and obtaining any necessary regulatory approvals for substitutes could impede our ability to replace such components in a timely manner or at all.
We expect to rely on third parties to conduct studies of our assays under development that will be required by the FDA or other regulatory authorities and those third parties may not perform satisfactorily.
We do not have the ability to independently conduct the field trial studies or other studies that may be required to obtain FDA and other regulatory clearances or approvals for our assays. Accordingly, we expect to rely on third parties, such as independent testing laboratories and hospitals, to conduct such studies. Our reliance on these third parties will reduce our control over these activities. These third-party contractors may not complete activities on schedule or conduct studies in accordance with regulatory requirements or our study design. We cannot control whether they devote sufficient time, skill and resources to our studies. Our reliance on third parties that we do not control will not relieve us of any applicable requirement to prepare, and ensure compliance with, various procedures required under good clinical practices. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our studies may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for additional assays.
Any clinical trials that we may conduct may not begin on time, or at all, may not be completed on schedule, or at all, or may be more expensive than we expect, which could prevent or delay regulatory approval of our assays or impair our financial position.
The commencement or completion of any clinical trials that we may conduct may be delayed or halted for numerous reasons, including, but not limited to, the following:
|
|
• |
the FDA or other regulatory authorities suspend or place on hold a clinical trial, or do not approve a clinical trial protocol or a clinical trial; |
|
|
• |
the data and safety monitoring committee or applicable hospital institutional ethics review board recommends that a trial be placed on hold or suspended; |
|
|
• |
fewer patients meet our clinical study criteria and our enrollment rate is lower than we expected; |
39
|
|
• |
clinical trial sites decide not to participate or cease participation in a clinical trial; |
|
|
• |
third-party clinical investigators do not perform our clinical trials on schedule or consistent with the clinical trial protocol and good clinical practices, or other third-party organizations do not perform data collection and analysis in a timely or accurate manner; |
|
|
• |
we fail regulatory inspections of our manufacturing facilities requiring us to undertake corrective action or suspend or terminate our clinical trials; |
|
|
• |
interim results of the clinical trial are inconclusive or negative; |
|
|
• |
pre-clinical or clinical data are interpreted by third parties in unanticipated ways; or |
|
|
• |
our trial design is inadequate to demonstrate safety and/or efficacy. |
Our clinical trial costs will increase if we have material delays in those trials or if we need to perform more or larger trials than planned. Adverse events during a clinical trial could cause us to repeat a trial, terminate a trial or cancel an entire program. Should our clinical development plan be delayed, this could have a material adverse effect on our operations and financial condition.
Product liability claims could adversely impact our financial condition and our earnings and impair our reputation.
Our business exposes us to potential product liability risks that are inherent in the design, manufacture and marketing of medical devices. Device failures, manufacturing defects, design flaws, or inadequate disclosure of product-related risks or product-related information with respect to our assays could result in an unsafe condition regarding, injury to, or death of, a patient. The occurrence of such a problem could result in product liability claims or a recall of, or safety alert relating to, one or more of our assays. Product liability claims or product recalls in the future, regardless of their ultimate outcome, could have a material adverse effect on our business and reputation and on our ability to attract and retain customers for our assays.
Healthcare policy changes, including U.S. healthcare reform legislation signed in 2010, may have a material adverse effect on us.
In March 2010, the Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010, or PPACA, were signed into law. The legislation imposes a 2.3% excise tax on medical device manufacturers. This significant tax burden on our industry could have a material, negative impact on our results of operations and our cash flows. Other elements of this legislation, such as comparative effectiveness research, an independent payment advisory board, payment system reforms, including shared savings pilots, and other provisions, could meaningfully change the way healthcare is developed and delivered, and may materially impact numerous aspects of our business.
In addition, we expect that the new presidential administration and U.S. Congress will seek to modify, repeal, or otherwise invalidate all, or certain provisions of, the Affordable Care Act. Since taking office, President Trump has continued to support the repeal of all or portions of the Affordable Care Act. In January 2017, the House and Senate passed a budget resolution that authorizes congressional committees to draft legislation to repeal all or portions of the Affordable Care Act and permits such legislation to pass with a majority vote in the Senate. President Trump has also recently issued an executive order in which he stated that it is his administration’s policy to seek the prompt repeal of the Affordable Care Act and directed executive departments and federal agencies to waive, defer, grant exemptions from, or delay the implementation of the provisions of the Affordable Care Act to the maximum extent permitted by law. There is still uncertainty with respect to the impact President Trump’s administration and the U.S. Congress may have, if any, and any changes will likely take time to unfold, and could have an impact on coverage and reimbursement for healthcare items and services covered by plans that were authorized by the Affordable Care Act. However, we cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us.
40
Adverse changes in reimbursement policies and procedures by payors may impact our ability to market and sell our assays that are subject to reimbursement.
Healthcare costs have risen significantly over the past decade, and there have been and continue to be proposals by legislators, regulators and third-party payors to decrease costs. Third-party payors are increasingly challenging the prices charged for medical products and services and instituting cost containment measures to control or significantly influence the purchase of medical products and services. For example, the PPACA, among other things, reduced and/or limited Medicare reimbursement to certain providers. The Budget Control Act of 2011, as amended by subsequent legislation, further reduces Medicare’s payments to providers by 2 percent through fiscal year 2024. These reductions may reduce providers’ revenues or profits, which could affect their ability to purchase new technologies. Furthermore, the healthcare industry in the United States has experienced a trend toward cost containment as government and private insurers seek to control healthcare costs by imposing lower payment rates and negotiating reduced contract rates with service providers. Legislation could be adopted in the future that limits payments for our products from governmental payors. In addition, commercial payors such as insurance companies, could adopt similar policies that limit reimbursement for medical device manufacturers’ products. Therefore, we cannot be certain that those of our assays that will be subject to reimbursement will be reimbursed at a cost-effective level. We face similar risks relating to adverse changes in reimbursement procedures and policies in other countries where we market or intend to market our assays. Reimbursement and healthcare payment systems vary significantly among international markets. Our inability to obtain international reimbursement approval, or any adverse changes in the reimbursement policies of foreign payors, could negatively affect our ability to sell our assays and have a material adverse effect on our business and financial condition.
Consolidation in the healthcare industry could have an adverse effect on our revenues and results of operations.
Many healthcare industry companies, including healthcare systems, are consolidating to create new companies with greater market power. As the healthcare industry consolidates, competition to provide goods and services to industry participants will become more intense. These industry participants may try to use their market power to negotiate price concessions or reductions for diagnostic tests. If we are forced to reduce our prices because of consolidation in the healthcare industry, our projected revenues would decrease and our earnings, financial condition, and/or cash flows would suffer.
If we or our distributors do not comply with the U.S. federal and state fraud and abuse laws, including anti-kickback laws for any products approved in the U.S., or with similar foreign laws where we market our products, we could face significant liability.
There are numerous United States federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback laws, false claims, and physician transparency laws. Our relationships with physicians and surgeons, hospitals and our independent distributors are subject to scrutiny under these laws. Violations of these laws are punishable by criminal and civil sanctions, including significant fines, damages and monetary penalties and in some instances, imprisonment and exclusion from participation in federal and state healthcare programs, including the Medicare, Medicaid and Veterans Administration health programs.
Healthcare fraud and abuse regulations are complex, and even minor irregularities can potentially give rise to claims that a statute or prohibition has been violated. The laws that may affect our ability to operate include:
|
|
• |
the federal healthcare program Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving, or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any good or service for which payment may be made under federal healthcare programs such as Medicare and Medicaid; |
41
|
|
• |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology and Clinical Health Act of 2009, which, among other things, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
|
|
• |
HIPAA also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services; |
|
|
• |
the Federal Trade Commission Act and similar laws regulating advertisement and consumer protections; |
|
|
• |
the federal Foreign Corrupt Practices Act of 1997, which makes it illegal to offer or provide money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage; and |
|
|
• |
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians. Some states, such as California, Massachusetts, Nevada, and Vermont mandate implementation of commercial compliance programs and/or impose restrictions on device manufacturer marketing practices and tracking and reporting of gifts, compensation and other remuneration to physicians. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from federal healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. To enforce compliance with the federal laws, the U.S. Department of Justice, or DOJ, has recently increased its scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Dealing with investigations can be time and resource consuming and can divert management’s attention from the business. In addition, settlements with the DOJ or other law enforcement agencies have forced healthcare providers to agree to additional onerous compliance and reporting requirements as part of a consent decree or corporate integrity agreement. Any violations of these laws, or any action against us for violation of these laws, even if we successfully defend against it, could have a material adverse effect on our reputation, business and financial condition.
Many foreign countries have enacted similar laws addressing fraud and abuse in the healthcare sector. The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance requirements in multiple jurisdictions increases the possibility that we may run afoul of one or more of the requirements.
The implementation of the reporting and disclosure obligations of the Physician Payments Sunshine Act/Open Payments provisions of the Health Care Reform Law could adversely affect our business upon commercialization of our product in the U.S.
The federal Physician Payments Sunshine Act, being implemented as the Open Payments Program, requires device manufacturers to engage in extensive tracking of payments and other transfers of value made to physicians and teaching hospitals, as well as physician ownership and investment interests, and public reporting of such data to the
42
Centers for Medicare and Medicaid Services annually. Although we have and expect to continue to have substantially compliant programs and controls in place to comply with the Physician Payments Sunshine Act requirements and similar state and foreign laws, our compliance with the Physician Payments Sunshine Act and similar state and foreign transparency laws imposes additional costs on us. Additionally, failure to comply with the Physician Payment Sunshine Act or similar state or foreign laws may subject us to monetary penalties.
Our ability to compete depends on our ability to attract and retain talented employees.
Our future success depends on our ability to identify, attract, train, integrate and retain highly qualified technical, development, sales and marketing, managerial and administrative personnel. Competition for highly skilled individuals is extremely intense and we face difficulty identifying and hiring qualified personnel in many areas of our business. We may not be able to hire and retain such personnel at compensation levels consistent with our existing compensation and salary structure. Many of the companies with which we compete for hiring experienced employees have greater resources than we have. If we fail to identify, attract, train, integrate and retain highly qualified and motivated personnel, our reputation could suffer and our business, financial condition and results of operations could be adversely affected.
Our future success also depends on the continued service and performance of our senior management team. The replacement of members of our senior management team likely would involve significant time and costs, and the loss of any these individuals may delay or prevent the achievement of our business objectives.
Changes in tax laws or exposure to additional income tax liabilities could have a material impact on our financial condition and results of operations.
We are subject to income taxes as well as non-income based taxes in both the United States and various foreign jurisdictions. Changes in existing tax laws, treaties, regulations or policies or the interpretation or enforcement thereof, or the enactment or adoption of new tax laws, treaties, regulations or policies could materially impact our effective tax rate.
If we do not achieve, sustain or successfully manage our anticipated growth, our business and prospects will be harmed.
If we are unable to obtain or sustain adequate revenue growth, our financial results could suffer. Furthermore, significant growth will place strains on our management and our operational and financial systems and processes and our operating costs may escalate even faster than planned. If we cannot effectively manage our expanding operations and our costs, we may not be able to grow effectively or we may grow at a slower pace. Additionally, if we do not successfully forecast the timing of regulatory authorization for our additional tests, marketing and subsequent demand for our diagnostic tests or manage our anticipated expenses accordingly, our operating results will be harmed.
Other companies or institutions have commercial assays or may develop and market novel or improved methods for infectious disease diagnostics, which may make our diagnostic platform less competitive or obsolete.
The market for diagnostics is large and established, and our competitors may possess significantly greater financial resources and have larger development and commercialization capabilities than we do. We may be unable to compete effectively against these competitors either because their diagnostic platforms are superior or because they may have more expertise, experience, financial resources or stronger business relationships.
Demand for our assays depends in part on the operating budgets and hospital-acquired infection rates of our customers, a reduction in which could limit demand for our assays and adversely affect our business.
In the near term, we expect that our revenue will be derived primarily from sales of our C. diff, Group B Strep, Staph ID/R and STEC diagnostic assays to hospitals. The demand for our assays will depend in part upon the prevalence
43
of C. diff and Group B Strep at the hospitals of these customers and impacted by other factors beyond our control, such as:
|
|
• |
global macroeconomic conditions; |
|
|
• |
|
total bed days; |
|
|
• |
changes in the regulatory environment; |
|
|
• |
differences in budgetary cycles; |
|
|
• |
market-driven pressures to consolidate operations and reduce costs; and |
|
|
• |
market acceptance of new technologies. |
Our operating results may fluctuate due to reductions and delays in expenditures by our customers. Any decrease in our customers’ budgets or expenditures, or in the size, scope or frequency of operating expenditures, could materially and adversely affect our business, operating results and financial condition.
New technologies, techniques or assays could emerge that might offer better combinations of price and performance than our current C. diff, Group B Strep, Staph ID/R and e. coli assays or future assays and analyzers.
It is critical to our success that we anticipate changes in technology and customer requirements and to successfully introduce, on a timely and cost-effective basis, new, enhanced and competitive technologies that meet the needs of current and prospective customers. If we do not successfully innovate and introduce new technology into our product lines or manage the transitions to new product offerings, our revenues, results of operations and business will be adversely impacted. Competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards or customer requirements. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved diagnostic tests and as new companies enter the market with new technologies.
Due to our fixed overhead costs and the depreciation of our analyzers at customer sites included in cost of sales and the costs associated with our current hand-build cartridge manufacturing process we have in the past experienced substantial negative gross margins. We will need to increase our sales volumes significantly and automate our cartridge manufacturing process in order to achieve profitability.
We had negative gross margins of 164.5% and 124.7% during the years ended December 31, 2016 and 2015, respectively. The components of our cost of sales include cost of materials, supplies, labor for manufacturing, equipment and facility expenses associated with manufacturing. Facility expenses include allocated overhead comprised of rent, equipment depreciation and utilities. Due to our fixed overhead costs we will continue to experience negative gross margins unless and until we are able to significantly increase our sales volume. In addition, we currently hand-build our diagnostic cartridges. We are working to automate portions of our manufacturing and assembly process, which we believe will reduce our cartridge manufacturing costs. However, there is no assurance that we will be successful in automating our manufacturing process, and our failure to do so will materially limit our ability to reduce our cost of sales in the future.
If we experience a significant disruption in our information technology systems or if we fail to implement new systems and software successfully, our business could be adversely affected.
We depend on information systems to manufacture products, process orders, manage inventory, process shipments to customers and respond to customer inquiries. If we were to experience a prolonged disruption in the information technology systems that involve our interactions with customers and suppliers, it could result in the loss of sales and customers, which could adversely affect our business.
44
Risks Related to Intellectual Property
The extent to which we can protect our business and technologies through intellectual property rights that we own, acquire or license is uncertain.
We employ a variety of proprietary and patented technologies and methods in connection with the assays that we sell or are developing. We license some of these technologies from third parties. We cannot provide any assurance that the intellectual property rights that we own or license provide effective protection from competitive threats or that we would prevail in any litigation in which our intellectual property rights are challenged. In addition, we may not be successful in obtaining new proprietary or patented technologies or methods in the future, whether through acquiring ownership or through licenses from third parties.
Our currently pending or future patent applications may not result in issued patents, and we cannot predict how long it may take for a patent to issue on any of our pending patent applications, assuming a patent does issue.
Other parties may challenge patents issued or exclusively licensed to us, or courts or administrative agencies may hold our patents or the patents we license on an exclusive basis to be invalid or unenforceable. We may not be successful in defending challenges made against our patents and other intellectual property rights. Any third-party challenge to any of our patents could result in the unenforceability or invalidity of some or all of the claims of such patents and could be time consuming and expensive.
The extent to which the patent rights of life sciences companies effectively protect their diagnostic tests and technologies is often highly uncertain and involves complex legal and factual questions for which important legal principles remain unresolved.
No consistent policy regarding the proper scope of allowable claims of patents held by life sciences companies has emerged to date in the United States. Various courts, including the U.S. Supreme Court, have rendered decisions that impact the scope of patentability of certain inventions or discoveries relating to diagnostic tests or genomic diagnostic testing. These decisions generally stand for the proposition that inventions that recite laws of nature are not themselves patentable unless they have sufficient additional features that provide practical assurance that the processes are genuine inventive applications of those laws rather than patent drafting efforts designed to monopolize a law of nature itself. What constitutes a “sufficient” additional feature for this purpose is uncertain. Although we do not generally rely on gene sequence patents, this evolving case law in the United States may adversely impact our ability to obtain new patents and may facilitate third-party challenges to our existing owned and exclusively licensed patents.
We cannot predict the breadth of claims that may be allowed or enforced in patents we own or in those to which we have exclusive license rights. For example:
|
|
• |
the inventor(s) named in one or more of our patents or patent applications might not have been the first to have made the relevant invention; |
|
|
• |
the inventor (or his assignee) might not have been the first to file a patent application for the claimed invention; |
|
|
• |
others may independently develop similar or alternative diagnostic tests and technologies or may successfully replicate our product and technologies; |
|
|
• |
it is possible that the patents we own or in which have exclusive license rights may not provide us with any competitive advantages or may be challenged by third parties and found to be invalid or unenforceable; |
|
|
• |
any patents we obtain or exclusively license may expire before, or within a limited time period after, the assays and services relating to such patents are commercialized; |
|
|
• |
we may not develop or acquire additional proprietary assays and technologies that are patentable; and |
45
|
|
• |
others may acquire patents that could be asserted against us in a manner that could have an adverse effect on our business. |
Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property rights.
On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted, redefine prior art, may affect patent litigation and switch the U.S. patent system from a “first-to-invent” system to a “first-to-file” system. Under a first-to-file system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor had made the invention earlier. The U.S. Patent and Trademark Office, or USPTO, recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, including the first-to-file provisions in particular, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned and licensed patent applications and the enforcement or defense of issued patents that we own or license, all of which could have a material adverse effect on our business and financial condition.
Patent applications in the United States and many foreign jurisdictions are not published until at least eighteen months after filing and it is possible for a patent application filed in the United States to be maintained in secrecy until a patent issues on the application. In addition, publications in the scientific literature often lag behind actual discoveries. We therefore cannot be certain that others have not filed patent applications that cover inventions that are the subject of pending applications that we own or exclusively license or that we were the first to invent the technology (if filed prior to the Leahy-Smith Act) or first to file (if filed after the Leahy-Smith Act). Our competitors may have filed, and may in the future file, patent applications covering technology that is similar to or the same as our technology. Any such patent application may have priority over patent applications that we own and, if a patent issues on such patent application, we could be required to obtain a license to such patent in order to carry on our business. If another party has filed a U.S. patent application covering an invention that is similar to, or the same as, an invention that we own, we may have to participate in an interference or other proceeding in the USPTO or a court to determine priority of invention in the United States, for applications and patents made prior to the enactment of the Leahy-Smith Act. For applications and patents made following the enactment of the Leahy-Smith Act, we may have to participate in a derivation proceeding to resolve disputes relating to inventorship. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in our inability to obtain or retain any U.S. patent rights with respect to such invention.
In addition, the laws of foreign jurisdictions may not protect our rights to the same extent as the laws of the United States. For example, European patent law restricts the patentability of methods of treatment of the human body more than U.S. law does. Publications of discoveries in scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions. Moreover, the USPTO might require that the term of a patent issuing from a pending patent application be disclaimed and limited to the term of another patent that is commonly owned or names a common inventor. As a result, the issuance, scope, validity, term, enforceability and commercial value of our patent rights are highly uncertain.
The patent prosecution process is expensive and time-consuming, is highly uncertain and involves complex legal and factual questions. Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and product candidates. We seek to protect our proprietary
46
position by filing in the United States and in certain foreign jurisdictions patent applications related to our novel technologies and product candidates that are important to our business.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. In addition, we may not pursue or obtain patent protection in all major markets. Moreover, in some circumstances, we may not have the right to control the preparation, filing or prosecution of patent applications, or to maintain the patents, covering technology that we license from third parties. In some circumstances, our licensors may have the right to enforce the licensed patents without our involvement or consent, or to decide not to enforce or to allow us to enforce the licensed patents. Therefore, these patents and patent applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. If any of our licensors fail to maintain such patents, or lose rights to those patents, the rights that we have licensed may be reduced or eliminated and our right to develop and commercialize any of our product candidates that are the subject of such licensed rights could be adversely affected.
Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. In particular, during prosecution of any patent application, the issuance of any patents based on the application may depend upon our ability to generate additional nonclinical or clinical data that support the patentability of our proposed claims. We may not be able to generate sufficient additional data on a timely basis, or at all. Moreover, changes in either the patent laws or interpretation of the patent laws in the United States or other countries may diminish the value of our patents or narrow the scope of our patent protection.
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the USPTO, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings or other patent office proceedings or litigation, in the United States or elsewhere, challenging our patent rights or the patent rights of others. An adverse determination in any such submission or proceeding could reduce the scope of, or invalidate, our patent rights; allow third parties to commercialize our technology or products and compete directly with us, without payment to us; or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our owned and licensed patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Obtaining and maintaining our patent protection depends upon compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent prosecution process and following the issuance of a patent. There are situations in which noncompliance with these requirements can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case if our patent were in force.
Our intellectual property rights may not be sufficient to protect our competitive position and to prevent others from manufacturing, using or selling competing assays.
The scope of our owned and exclusively licensed intellectual property rights may not be sufficient to prevent others from manufacturing, using or selling competing assays. Competitors could purchase our product and attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies and thereby avoid infringing our intellectual property rights. If our intellectual property is not sufficient to
47
effectively prevent our competitors from developing and selling similar diagnostic tests, our competitive position and our business could be adversely affected.
Our platform depends on certain technologies that are licensed to us. We do not control these technologies and any loss of our rights to them could prevent us from manufacturing our assays.
We rely on licenses to various proprietary technologies that are material to our business, including the development of certain future assays. We have entered into non-exclusive licenses with Biohelix Corp., or Biohelix, a subsidiary of Quidel Corporation and a license with Integrated DNA Technologies, Inc. that has certain exclusive and non-exclusive fields. Our rights to use these technologies will be subject to the continuation of and our compliance with the terms of those licenses.
We may become involved in disputes relating to our intellectual property rights, and may need to resort to litigation in order to defend and enforce our intellectual property rights.
Extensive litigation regarding patents and other intellectual property rights has been common in the medical diagnostic testing industry. Litigation may be necessary to assert infringement claims, protect trade secrets or know-how and determine the enforceability, scope and validity of certain proprietary rights. Litigation may even be necessary to resolve disputes of inventorship or ownership of proprietary rights. The defense and prosecution of intellectual property lawsuits, USPTO interference or derivation proceedings and related legal and administrative proceedings (e.g., a re-examination) in the United States and internationally involve complex legal and factual questions. As a result, such proceedings are costly and time consuming to pursue, and their outcome is uncertain.
Even if we prevail in such a proceeding in which we assert our intellectual property rights against third parties, the remedy we obtain may not be commercially meaningful or adequately compensate us for any damages we may have suffered. If we do not prevail in such a proceeding, our patents could potentially be declared to be invalid, unenforceable or narrowed in scope, or we could otherwise lose valuable intellectual property rights. Similar proceedings involving the intellectual property we exclusively license could also have an impact on our business. Further, if any of our other owned or exclusively licensed patents are declared invalid, unenforceable or narrowed in scope, our competitive position could be adversely affected.
We could face claims that our activities or the manufacture, use or sale of our assays infringe the intellectual property rights of others, which could cause us to pay damages or licensing fees and limit our ability to sell some or all of our assays and services.
Our research, development and commercialization activities may infringe or be claimed to infringe patents or other intellectual property rights owned by other parties of which we may be unaware because the relevant patent applications may have been filed but not yet published. Certain of our competitors and other companies have substantial patent portfolios, and may attempt to use patent litigation as a means to obtain a competitive advantage or to extract licensing revenue. In addition to patent infringement claims, we may also be subject to other claims relating to the violation of intellectual property rights, such as claims that we have misappropriated trade secrets or infringed third party trademarks. The risks of being involved in such litigation may also increase as we gain greater visibility as a public company and as we gain commercial acceptance of our diagnostic tests and move into new markets and applications for our assays.
Regardless of merit or outcome, our involvement in any litigation, interference or other administrative proceedings could cause us to incur substantial expense and could significantly divert the efforts of our technical and management personnel. Any public announcements related to litigation or interference proceedings initiated or threatened against us could cause our share price to decline. An adverse determination, or any actions we take or agreements we enter into in order to resolve or avoid disputes, may subject us to the loss of our proprietary position or to significant liabilities, or require us to seek licenses that may include substantial cost and ongoing royalties. Licenses may not be available from third parties, or may not be obtainable on satisfactory terms. An adverse determination or a failure to obtain necessary licenses may restrict or prevent us from manufacturing and selling our diagnostic tests and offering our services. These outcomes could materially harm our business, financial condition and results of operations.
48
We may not be able to adequately protect our intellectual property outside of the United States.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to medical devices, diagnostic testing and biotechnology, which could make it difficult for us to stop the infringement of our patents and for licensors, if they were to seek to do so, to stop infringement of patents that are licensed to us. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business. Additionally, prosecuting and maintaining intellectual property (particularly patent) rights are very costly endeavors, and for these and other reasons we may not pursue or obtain patent protection in all major markets. We do not know whether legal and government fees will increase substantially and therefore are unable to predict whether cost may factor into our global intellectual property strategy.
In addition to the risks associated with patent rights, the laws in some foreign jurisdictions may not provide protection for our trade secrets and other intellectual property. If our trade secrets or other intellectual property are misappropriated in foreign jurisdictions, we may be without adequate remedies to address these issues. Additionally, we also rely on confidentiality and assignment of invention agreements to protect our intellectual property in foreign jurisdictions. These agreements may provide for contractual remedies in the event of misappropriation, but we do not know to what extent, if any, these agreements, and any remedies for their breach, will be enforced by a foreign court. If our intellectual property is misappropriated or infringed upon and an adequate remedy is not available, our future prospects will likely diminish. The sale of diagnostic tests that infringe our intellectual property rights, particularly if such diagnostic tests are offered at a lower cost, could negatively impact our ability to achieve commercial success and may materially and adversely harm our business.
Our failure to secure trademark registrations could adversely affect our business and our ability to market our assays and product candidates.
Our trademark applications in the United States and any other jurisdictions where we may file may not be allowed for registration, and our registered trademarks may not be maintained or enforced. During trademark registration proceedings, we may receive rejections. Although we are given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in corresponding foreign agencies, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our applications and/or registrations, and our applications and/or registrations may not survive such proceedings. Failure to secure such trademark registrations in the United States and in foreign jurisdictions could adversely affect our business and our ability to market our diagnostic tests and product candidates.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information, or the misappropriation of the intellectual property we regard as our own.
We rely on trade secrets to protect our proprietary know how and technological advances, particularly where we do not believe patent protection is appropriate or obtainable. Nevertheless, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, third party contractors, third party collaborators and other advisors to protect our trade secrets and other proprietary information. These agreements generally require that the other party to the agreement keep confidential and not disclose to third parties all confidential information developed by us or made known to the other party by us during the course of the other party’s relationship with us. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to seek to pursue a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be
49
unpredictable. Further, courts outside the United States may be less willing to protect trade secrets. In addition, others may independently discover our trade secrets and proprietary information and therefore be free to use such trade secrets and proprietary information. Costly and time consuming litigation could be necessary to enforce and determine the scope of our proprietary rights. In addition, our trade secrets and proprietary information may be misappropriated as a result of breaches of our electronic or physical security systems in which case we may have no legal recourse. Failure to obtain, or maintain, trade secret protection could enable competitors to use our proprietary information to develop assays that compete with our assays or cause additional, material adverse effects upon our competitive business position.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is common in our industry, we employ individuals who were previously employed at other companies in our industry or in related industries, including our competitors or potential competitors. We may be subject to claims that we or these employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None
Our current headquarters and manufacturing facility are both located in Salt Lake City, Utah. We have signed a lease for approximately 35,540 square feet of office space in various suites located at 420 E. South Temple, Salt Lake City, UT 84111 for use as our executive offices and labs. Base rent payments due under the lease are expected to be approximately $3,454,611 in the aggregate over the term of the lease of 69 months that began on December 1, 2015. The Company leases approximately 33,000 square feet of manufacturing and lab space located in Salt Lake City, Utah pursuant to two lease agreements totaling $25,926 in base rent per month. The leases expire on April 30, 2017 and each have two options, with each option for a three-year renewal period. We believe that our facilities are adequate for our current needs.
On April 5, 2016 and May 31, 2016, Great Basin Scientific, Inc., received notices from the Utah Labor Commission, Occupational Safety and Health Division (ULC) and/or the Occupational Safety and Health Administration (OSHA) that former employee Christina Steele filed a claim alleging retaliation in violation of the Utah Occupational Safety and Health Act as well as the Corporate and Criminal Fraud Accountability Act of 2002, the Sarbanes-Oxley Act and the Occupational Safety and Health Act, among other claims relating to her employment. Ms. Steele alleges that Great Basin retaliated against her by terminating her employment after she allegedly acted as a whistleblower by allegedly raising concerns with management. Ms. Steele seeks lost wages, future wages, consequential losses, emotional distress damages, interest, fees and costs. The OSHA charge remains under investigation.
On June 15, 2016, Ms. Steele also filed a complaint against Great Basin Scientific, Inc. in the United States District Court for the District of Utah alleging retaliation in violation of the False Claims Act based on similar alleged facts. Ms. Steele seeks back pay, special damages, consequential damages, compensatory damages, interest, fees and costs. On August 15, 2016, Great Basin Scientific, Inc. filed a motion to dismiss Ms. Steele’s claims. On November 21, 2016, the United States District Court for the District of Utah granted the Company's motion to dismiss and dismissed Ms. Steele's claims with prejudice. Judgement was entered in favor of Great Basin Scientific, Inc. on November 28, 2016. Ms. Steele has appealed the court’s order to the United States
50
Court of Appeals for the 10th Circuit. The court has not yet set a briefing schedule but it is unlikely that the 10th Circuit will issue a decision in this case until at least the fall of 2017.
The Company asserts that the claims are without merit and that the employee resigned and was not terminated.
We are not currently a party to any other material pending legal proceeding or regulatory or government investigations. We may become involved in litigation from time to time relating to claims arising in the ordinary course of our business.
ITEM 4. MINE SAFETY DISCLOSURES.
Not Applicable
51
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Between October 9, 2014 and October 11, 2016, our common stock was traded on the NASDAQ Capital Market. Since October 11, 2016, our common stock is quoted on the OTCQB under the symbol “GBSN.”
The following table sets forth the high and low sales prices of our common stock, as reported by the NASDAQ Capital Market and the high and low bid prices of our common stock, as reported by the OTCQB for the periods indicated.
Over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
|
|
|
2015 |
|
|||||
|
|
|
High |
|
|
Low |
|
||
|
First Quarter |
|
$ |
212,688,302 |
|
|
$ |
74,592,101 |
|
|
Second Quarter |
|
$ |
307,440,454 |
|
|
$ |
127,008,202 |
|
|
Third Quarter |
|
$ |
161,280,252 |
|
|
$ |
2,268,000 |
|
|
Fourth Quarter |
|
$ |
13,507,200 |
|
|
$ |
777,003 |
|
|
|
|
2016 |
|
|||||
|
|
|
High |
|
|
Low |
|
||
|
First Quarter |
|
$ |
798,003 |
|
|
$ |
88,200 |
|
|
Second Quarter |
|
$ |
188,400 |
|
|
$ |
38,400 |
|
|
Third Quarter |
|
$ |
42,240 |
|
|
$ |
660 |
|
|
Fourth Quarter (through October 10, 2016) |
|
$ |
695.10 |
|
|
$ |
17.10 |
|
|
Fourth Quarter (October 11 through December 31, 2016) |
|
$ |
23.40 |
|
|
$ |
1.09 |
|
|
|
|
|
|
|
|
|
|
|
As of March 17, 2017, there were approximately 475 stockholders of record of our common stock. This number excludes stockholders whose stock is held in nominee or street name by brokers.
On March 17, 2017, the closing price of our common stock was $0.001 per share.
Dividends
We have not paid any cash dividends on our equity security and our Board has no present intention of declaring any cash dividends. We are prohibited from paying any dividends pursuant to the terms in the 2016 Notes. In addition, we intend to retain any future earnings and do not expect to pay cash dividends on our common stock for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our board of directors and will be dependent on a number of factors, including our earnings, capital requirements and overall financial condition.
Purchases of Equity Securities by the Company and Affiliates
There were no purchases of our equity securities by us or any of our affiliates during the year ended December 31, 2016.
52
Unregistered Sales of Securities
All unregistered sales of equity securities during the period covered by this annual report on Form 10-K were previously disclosed in our current reports on Form 8-K or quarterly reports on Form 10-Q.
Equity Compensation Plan Information
See the discussion at Item 12 below.
ITEM 6. SELECTED FINANCIAL DATA.
As a smaller reporting company, we have elected not to provide the disclosure required by this item.
53
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing elsewhere in this annual report. Except for historical information contained herein, the following discussion and analysis contains forward-looking statements which are subject to known and unknown risks, uncertainties and other factors that may cause our actual results to differ materially from those expressed or implied by such forward-looking statements. We discuss such risks, uncertainties and other factors throughout this report and specifically under Item 1A of Part I of this report, “Risk Factors”.
Overview of Our Business
We are a molecular diagnostic testing company. We are focused on improving patient care through the development and commercialization of our patented, low-cost, molecular diagnostic platform for testing for infectious disease, especially hospital-acquired infections. We believe our platform has the ability to transform molecular testing for infectious diseases at small to medium sized hospitals by providing an affordable solution that meets the rapidly evolving needs of patients and providers.
We believe there is a fast-growing market for molecular diagnostic systems being purchased by hospital microbiology labs to replace culture and other legacy testing formats. We believe our platform is well positioned to meet this need. Our platform provides results in 45 to 115 minutes depending on the test. Molecular testing generally reduces test time from days to hours, and provides more accurate results, which we believe leads to shortened hospital stays and improved patient outcomes, all of which leads to reduced cost for hospitals that implement molecular testing in their labs.
Our platform is an automated molecular diagnostic system, consisting of an analyzer and associated assay cartridge. Our platform utilizes a sample-to-result format, which means that once a patient specimen is received, it undergoes limited processing before it is placed in the analyzer where the assay is run without further technician intervention. This reduces assay complexity and eliminates the need for highly trained and expensive molecular technicians to run the tests. Our platform is designed to enable simple, rapid and cost-effective analysis of multiple pathogens from a single clinical sample, which will allow small to medium sized community hospitals that traditionally could not afford more expensive or complex molecular diagnostic testing platforms to modernize their laboratory testing and provide better patient care at an affordable cost.
In November 2012, we launched our first FDA-cleared test for C. diff, a bacteria that causes life-threatening gastrointestinal distress in hospital patients. In addition to our C. diff assay, we have three additional FDA-cleared tests, Group B Strep assay for which we received FDA clearance in April 2015 and launched commercially in June 2015, Shiga toxin producing E. coli assay, or STEC, for which we received FDA clearance in March 2016 and launched commercially in August 2016, and Staph ID/R (“SIDR”) assay for which we received FDA clearance in March of 2016 and commercially launched on September 30, 2016. Additionally, we have completed clinical trials and submitted 510(k) applications to the FDA for two assays—a food borne pathogens panel and a test for Bordatella pertussis (whooping cough). We expect a decision from the FDA on these two applications during the first half of 2017. We also have three other assays in various stages of product development: (i) a test for Chlamydia-Gonorrhea, (ii) a pre-surgical nasal screen for Staphylococcus aureus, and (iii) a panel for Candida blood infections.
We currently sell our diagnostic test cartridge in the United States through a direct sales force and we use distributors in the European Union and New Zealand. As of December 31, 2016, we had 265 customers worldwide (244 in the United States and 21 in the rest of the world), who use an aggregate of 498 of our analyzers. Our easy to use platform allows small to medium sized hospitals that we believe could not previously afford more expensive or complex molecular diagnostic systems to modernize their laboratory testing and provide better patient care at an affordable cost.
Since inception, we have incurred net losses from operations each year and we expect to continue to incur losses for the foreseeable future. Our losses attributable to operations for the fiscal year ending December 31, 2016 and 2015
54
were approximately $89.1 million and $57.9 million, respectively. As of December 31, 2016, we had an accumulated deficit of $211.1 million.
Financial Operations Overview
Revenue
We derive our revenue from the sale of single use assays sold through our dedicated sales force in the United States, and in the European Union and New Zealand through a network of distributors. Revenue is recognized when all four of the following criteria are met: (1) persuasive evidence that an arrangement exists; (2) delivery of the products has occurred; (3) the selling price of the product is fixed or determinable; and (4) collectability of that price is reasonably assured. Change in title to the product and recognition of revenue from sales of the assays occurs at the time of shipment.
We believe our revenue from the sale of our assays will increase as we expand our sales and marketing efforts, and as we introduce new assays into the market. We expect that our revenue will continue to be primarily attributable to sales of our assays in the United States.
Our material increases in revenues since the inception of our business have been attributable to increases in the volume of goods being sold as a result of increases in the number of customers. As of December 31, 2016, we have four commercial products, our C. diff assay, our Group B Strep assay, our Staph ID/R panel and our Shiga Toxin Direct Test. The average price has not materially changed since their commercial releases.
Cost of Sales
The components of our cost of sales include cost of materials, supplies, labor for manufacturing and support personnel, equipment, and facility expenses associated with manufacturing. We depreciate the cost of each analyzer that is at a customer site over a period of 5 years on a straight line basis, and include that cost in cost of sales. We perform all of our manufacturing activities at our facility located in Salt Lake City, Utah. Facility expenses include allocated overhead comprised of rent, equipment depreciation, and utilities. We expect our cost of sales in absolute dollars to increase, as the number of diagnostic cartridges we manufacture increases. However, we also expect that as assay volumes increase we will realize manufacturing efficiencies, which would result in a decrease in our cost of sales as a percentage of revenue. We also license certain technologies for our C. diff assay, which are described elsewhere in this report. Pursuant to the terms of these license agreements, we pay royalty fees in the aggregate equal to 14% of our worldwide “Net Sales” of those products that use these technologies (as defined and adjusted pursuant to the terms of the applicable license agreements).
Research and Development
All research and development costs, including those funded by third parties, are expensed as incurred. Research and development costs consist of engineering, product development, clinical trials, test-part manufacturing, testing, developing and validating the manufacturing process, manufacturing, facility, and regulatory-related costs. Research and development costs also include employee cash compensation, employee and non-employee stock-based compensation, supplies and materials, consultant services, and travel related to research activities.
In 2015 and 2016 we incurred additional research and development costs as we continued to develop new assays, and as we advanced the development of our product candidates, including our Group B Strep, Staph ID/R, and Shiga toxin producing e. coli assays. In particular, we conducted clinical trials for our Staph ID/R, Shiga toxin producing e. coli, food borne pathogen, and Bordatella pertussis (whopping cough) product candidates, which increased our research and development expenses. Additionally, we have three other assays in various stages of product development: (i) a panel for Candida blood infections, (ii) a test for Chlamydia-Gonorrhea, and (iii) a pre-surgical nasal screen for Staphylococcus aureus. We plan to continue the development of these products and conduct clinical trials in 2017 and 2018.
55
Sales and marketing expenses primarily consist of salaries, benefits, and other related costs, including stock-based compensation, for personnel employed in sales, marketing, and training. In addition, our sales and marketing expenses include commissions and bonuses paid to our sales representatives, generally based on the number of new customers obtained and other objectives attained.
We expect our sales and marketing expenses to increase proportionately as we introduce new assays, such as our food borne pathogen panel and Bordatella pertussis (whopping cough), if cleared by the FDA, and will seek to enhance, if necessary, our commercial infrastructure and marketing efforts. Additionally, we expect commissions to continue to increase in absolute terms over time, but to decline as a percentage of revenue.
General and Administrative Expenses
General and administrative expenses primarily consist of salaries, benefits, and other related costs, including stock-based compensation, for certain members of our executive team and other personnel employed in finance, legal, compliance, administrative, information technology, customer service, executive, and human resource departments. General and administrative expenses include allocated facility expenses, related travel expenses, and professional fees for accounting and legal services.
We expect our general and administrative expenses will increase due to costs associated with our public and private funding efforts as we continue to raise capital for our business needs. Our expenses related to compliance with the rules and regulations of the Securities and Exchange Commission, as well as investor relations activities and other administrative and professional services, will continue to increase as we grow our business.
Interest Income
Interest income and other income primarily consist of interest earned on our cash.
Interest Expense
Interest expense consists of interest on capital leases, notes payable, letters of credit, as well as the amortization of debt discounts. In certain debt transactions that have components required to be measured at fair value, the excess of the fair value over the proceeds received is recorded as an interest expense.
Gain (Loss) on Exchange and Issuance of Warrants
The exchange and issuance of warrants during 2016 resulted in a gain on the exchange of Series E Warrants, partially offset by a loss on the issuance of our Series G Warrants. During 2015, we also exchanged our Series C Warrants for a convertible note payable and our Series D Warrants. This was considered an extinguishment of a liability, and resulted in a net loss comprising the difference between the fair value of the warrants extinguished and the fair value of the consideration provided.
Loss on Extinguishment of Debt
The Company’s senior secured convertible note issued in 2015 contained a conversion feature whereby installment payments could be made if the Company issued shares of common stock to the noteholders. Because this feature was separate from the note itself, when such conversions occurred they were deemed extinguishments of debt for accounting purposes. During 2016, the Company issued shares of common stock in various installments to pay down the note. In November 2016, the noteholders agreed to extinguish the remaining balance of the 2015 note in exchange for a new class of Series F Convertible Preferred Stock. A total loss on the extinguishment of debt was recorded to account for all these transactions. The loss amounts were equal to the fair market values of the common and preferred shares issued, offset by the principal amounts of the notes either paid down or extinguished, the related remaining debt discount associated with the principal amounts, the related derivative liability amounts also reduced, and the fair value of an embedded conversion feature inherent in the preferred shares.
56
Change in Fair Value Liabilities
We have issued certain common stock warrants that contain a price adjustment clause which states that in the event the Company issues common stock for a price less than the exercise price of warrants, the exercise price will be reduced to the issuance price of the common stock. We have also issued convertible notes payable that contain a conversion feature that could result in modification of the conversion price to issue a variable amount of additional common shares. We have determined that the warrants are accounted for as a derivative liability, and the conversion feature of the convertible notes is an embedded derivative. These derivatives are recorded at fair value measured at the transaction date and again at each reporting period. In addition, we have issued Series F Preferred Stock that is required to be recorded as a liability on the accompanying balance sheet as it predominantly represents an unconditional obligation to issue a variable number of common shares for a fixed amount and are recorded at fair value at the transaction date and again at each reporting period. Any difference in fair value of these instruments between the transaction date and future reporting periods must be recognized in earnings for the period.
Results of Operations
Comparison of the Years Ended December 31, 2016 and 2015
The following table sets forth our results of operations for the years ended December 31, 2016 and December 31, 2015:
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Revenue |
|
$ |
3,048,126 |
|
|
$ |
2,142,040 |
|
|
Cost of sales |
|
|
8,061,382 |
|
|
|
4,813,415 |
|
|
Gross loss |
|
|
(5,013,256 |
) |
|
|
(2,671,375 |
) |
|
Operating Expenses |
|
|
|
|
|
|
|
|
|
Research and development |
|
|
13,406,370 |
|
|
|
8,485,668 |
|
|
Selling and marketing |
|
|
6,859,323 |
|
|
|
5,007,320 |
|
|
General and administrative |
|
|
8,801,997 |
|
|
|
6,241,433 |
|
|
Total operating expenses |
|
|
29,067,690 |
|
|
|
19,734,421 |
|
|
Loss from operations |
|
|
(34,080,946 |
) |
|
|
(22,405,796 |
) |
|
Interest expense |
|
|
(167,466,170 |
) |
|
|
(11,757,445 |
) |
|
Interest income |
|
|
7,399 |
|
|
|
18,193 |
|
|
Gain (loss) on extinguishment of warrants |
|
|
3,374,752 |
|
|
|
(4,038,063 |
) |
|
Loss on extinguishment of debt |
|
|
(24,172,736 |
) |
|
|
— |
|
|
Change in fair value liabilities |
|
|
133,191,183 |
|
|
|
(19,714,808 |
) |
|
Provision for income taxes |
|
|
(1,750 |
) |
|
|
(1,250 |
) |
|
Net loss |
|
$ |
(89,148,268 |
) |
|
$ |
(57,899,169 |
) |
Revenue
|
|
|
Years Ended December 31, |
|
|
Increase |
|
||||||||||
|
|
|
2016 |
|
|
2015 |
|
|
$ |
|
|
% |
|
||||
|
C. diff |
|
$ |
2,584,376 |
|
|
$ |
2,075,240 |
|
|
$ |
509,136 |
|
|
|
25 |
% |
|
Group B Strep |
|
|
410,770 |
|
|
|
66,800 |
|
|
|
343,970 |
|
|
|
515 |
% |
|
STEC |
|
|
24,730 |
|
|
|
— |
|
|
|
24,730 |
|
|
|
— |
|
|
SIDR |
|
|
28,250 |
|
|
|
— |
|
|
|
28,250 |
|
|
|
— |
|
|
|
|
$ |
3,048,126 |
|
|
$ |
2,142,040 |
|
|
$ |
906,086 |
|
|
|
42 |
% |
Revenue increased 42% for the year ended December 31, 2016, as compared to the year ended December 31, 2015. Revenue derived from the sale of STEC and SIDR assays began since the products were commercially launched in August and September of 2016. The 42% increase in total sales was attributable to the number of U.S. customers
57
increasing to 244 by December 31, 2016, as compared to 186 at the end of December 31, 2015. In addition, the number of analyzers placed with customers rose to 498 at December 31, 2016, as compared to 428 at the end of December of the prior year. During 2016, the Company was intensely focused on building its customer base and placing additional analyzers in the field. While the increase in customers and analyzers was not always directly proportional to the increase in the Company’s traditional C. diff sales, the Company anticipates selling higher quantities of existing assays, as well as new assays to this larger customer group once the additional products come to market.
Cost of Sales
|
|
|
Years ended December 31, |
|
|
|
|
|
|
|
|
|
|||||||||||||
|
|
|
2016 |
|
|
2015 |
|
|
Increase |
|
|||||||||||||||
|
|
|
|
|
|
|
% of |
|
|
|
|
|
|
% of |
|
|
|
|
|
|
|
|
|
||
|
|
|
Expense |
|
|
revenue |
|
|
Expense |
|
|
revenue |
|
|
$ |
|
|
% |
|
||||||
|
Materials and direct labor |
|
$ |
2,859,370 |
|
|
|
94% |
|
|
$ |
1,721,563 |
|
|
|
80% |
|
|
$ |
1,137,807 |
|
|
|
66% |
|
|
Indirect labor, overhead, and royalties |
|
|
3,766,259 |
|
|
|
123% |
|
|
|
2,170,544 |
|
|
|
101% |
|
|
|
1,595,715 |
|
|
|
74% |
|
|
Analyzer depreciation |
|
|
1,435,753 |
|
|
|
47% |
|
|
|
921,308 |
|
|
|
43% |
|
|
|
514,445 |
|
|
|
56% |
|
|
|
|
$ |
8,061,382 |
|
|
|
264% |
|
|
$ |
4,813,415 |
|
|
|
225% |
|
|
$ |
3,247,967 |
|
|
|
67% |
|
The overall cost of direct labor increased as a percentage of revenue due to the hiring of production workers during 2016 for additional daily shifts in anticipation of doubling the number of products produced. We brought to market both the STEC and Staph ID/R assays in a relatively short period of time during the second half of 2016. Sales of these new products only began in August and September of 2016, respectively, and their impact on 2016’s total revenue and gross margins was minimal. Accordingly, we don’t expect to see additional revenue and gross margin potential until 2017. Similar to direct labor costs, indirect labor and overhead increased as a percentage of revenue due to anticipated demand for the new assays, along with underutilization of capacity in our analyzer manufacturing group. During periods when analyzers are being manufactured, the group’s labor and overhead costs are allocated to the cost of the analyzers produced and expensed eventually through depreciation. Otherwise, the group’s costs are expensed directly to cost of sales during the period incurred. Depreciation of analyzers also increased since the number of analyzers placed in the field grew from 428 at December 31, 2015, to 498 at December 31, 2016.
Operating Expenses
|
|
|
Years ended |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
December 31, |
|
|
Increase |
|
||||||||||
|
|
|
2016 |
|
|
2015 |
|
|
$ |
|
|
% |
|
||||
|
Research and development |
|
$ |
13,406,370 |
|
|
$ |
8,485,668 |
|
|
$ |
4,920,702 |
|
|
|
58% |
|
|
Selling and marketing |
|
|
6,859,323 |
|
|
|
5,007,320 |
|
|
|
1,852,003 |
|
|
|
37% |
|
|
General and administrative |
|
|
8,801,997 |
|
|
|
6,241,433 |
|
|
|
2,560,564 |
|
|
|
41% |
|
|
|
|
$ |
29,067,690 |
|
|
$ |
19,734,421 |
|
|
$ |
9,333,269 |
|
|
|
47% |
|
Research and Development
Research and development expenses increased 58% during the year ended December 31, 2016, as compared to the prior year, due to an increase of $1.1 million for hiring new developers, scientists, and recruiting costs, an increase of $3.3 million for additional costs for clinical trials, experimental assays, and outside consultants, all related to the development of new products, and a net increase of all other research and development expenses aggregating to $489,392.
Selling and Marketing
The 2016 increase in sales and marketing expenses as compared to 2015 was due primarily to $1.5 million in higher salaries, travel, and promotional assay costs resulting from an increase in sales and marketing personnel and their increased efforts to add customers and sell additional existing and newly-developed assays. These higher expenses
58
included increased commissions related to the increase in new customers, and were also the result of efforts to place additional analyzers in the field. All other expenses increased $360,630.
General and Administrative
The increase in general and administrative expenses for 2016 compared to 2015 resulted from an increase of $811,131 related to the hiring of additional accounting, regulatory, human resource, and IT personnel, as well as $335,225 in increased rent expense, all to accommodate growth experienced during 2016 and growth anticipated in the future. In addition, there was $1.5 million in increased legal and consulting fees incurred during 2016 because of increased financing and financial restructuring activities. There was also $82,139 in additional corporate registration and property tax expenses, a decrease of $612,376 in share-based compensation, and $425,027 in all other general and administrative expenses.
Interest Expense
Interest expense for the year ended December 31, 2016 increased by $155.7 million, from $11.8 million to $167.5 million, as compared to the year ended December 31, 2015.
The difference was due mainly to the $107.8 million increase in the loss on the issuance of the 2016 convertible note as compared to the loss on the 2015 convertible note issued at the end of the previous year, and $47.3 million in debt discount amortization on both convertible notes during 2016 which we didn’t have during 2015. Other interest expense increased $651,107.
Net Gain (Loss) on Exchange and Issuance of Warrants
The exchange and issuance of warrants during the year ended December 31, 2016 resulted in a net gain of $3.4 million, which comprised a gain on the exchange of Series E Warrants in the amount of $4.1 million, partially offset by a loss on the issuance of our Series G Warrants in the amount of $0.7 million.
The loss on extinguishment of warrants in the amount of $4.0 million for the year ended December 31, 2015 was due to the extinguishment of 1.05 million Series C Warrants for $2.1 million in convertible notes payable and associated Series D Warrants. The fair value of the convertible note and warrants issued as consideration in the exchange was $6.4 million, which was offset by $2.3 million, the fair value of the Series C Warrants.
Loss on Extinguishment of Debt
The Company’s senior secured convertible note issued in 2015 contained a conversion feature whereby installment payments could be made if the Company issued shares of common stock to the noteholders. Because this feature was separate from the note itself, when such conversions occurred they were deemed extinguishments of debt for accounting purposes. During 2016, the Company issued shares of common stock in various installments to pay down the note. In November 2016, the noteholders agreed to extinguish the remaining balance of the 2015 note in exchange for a new class of Series F Convertible Preferred Stock. A total loss on the extinguishment of debt in the amount of $24.2 million was recorded to account for all these transactions. The loss amounts were equal to the fair market values of the common and preferred shares issued, offset by the principal amounts of the notes either paid down or extinguished, the related remaining debt discount associated with the principal amounts, the related derivative liability amounts also reduced, and the fair value of an embedded conversion feature inherent in the preferred shares.
Change in Fair Value Liabilities
The change in fair value of the derivative liability resulted in a net gain in earnings of $133.2 million for the year ended December 31, 2016, as compared to a net loss in earnings of $19.7 million for the year ended December 31, 2015. During 2016, we had a decrease in the fair value of our conversion feature and warrants associated with the 2015 and 2016 Notes in the amount of $125.9 million and a net decrease in the fair value of all other fair value liabilities in the amount of $21.8 million. These decreases were the result of the decrease in the value of our common stock during the year.
59
Liquidity and Capital Resources
We have funded our operations to date primarily with net proceeds from our IPO, our follow-on public offerings, cash exercises of warrants, sales of our preferred stock, issuances of convertible notes, and revenues from operations. At December 31, 2016, we had unrestricted cash of $1.0 million, and restricted cash of $59.4 million. The restricted cash is related to 2016 Notes we entered into in July of 2016.
|
|
• |
On December 28, 2015, we entered into a securities purchase agreement to issue $22.1 million of 2015 Notes and related Series D Warrants and Subordination Warrants to purchase common stock. We received $18.4 million in total proceeds, of which $13.8 million was placed in restricted accounts and $4.6 million was immediately available to the Company. |
|
|
• |
In February 2016, we completed a follow-on public offering, whereby 39,200,000 units were sold at a price of $0.16 per unit for net proceeds of $5.0 million after deducting underwriting commissions and offering costs. Pursuant to the sale of the units, the Company issued 47 shares of common stock and 58,800,000 Series E Warrants. The Series E Warrants were exercisable into 70 shares of common stock at $210,000 per share. |
|
|
• |
In June 2016, we completed another follow-on public offering, whereby 3,160,000 units were sold at a price of $1.90 per unit for net proceeds of $5.3 million after deducting underwriting commissions and offering costs. Pursuant to the sale of the units, the Company issued 132 shares of common stock and 3,160,000 Series G Warrants. The Series G Warrants were initially exercisable into 132 shares of common stock at $45,600 per share. |
|
|
• |
In July 2016, we entered into a securities purchase agreement to issue $75 million of 2016 Notes and related Series H Warrants and Subordination Warrants to purchase common stock. We received $68 million in total proceeds, of which $62 million was placed in restricted accounts and $6 million was immediately available to the Company. |
|
|
• |
By November 2016, we had withdrawn from restricted accounts all of the remaining $13.8 million related to our 2015 Notes. |
|
|
• |
In December 2016, the holders of our July 2016 notes voluntarily removed restrictions on the use of $2.6 million of the funds. In January and February of 2017, the noteholders were issued 1.486 billion shares of common stock pursuant to the terms of the elective conversion provisions of the note agreement, which reduced the principal amount of the notes by $3.9 million. In January and February of 2017, the noteholders voluntarily removed restrictions on $2.4 million of restricted funds. Also in February 2017, the Company and the noteholders agreed to reduce the convertible notes by $38.9 million in exchange for the return of $38.9 million of restricted funds. In March 2017, the noteholders voluntarily removed restrictions on an additional $1.1 million of restricted funds. |
We have limited liquidity, and have not yet established a stabilized source of revenue sufficient to cover operating costs based on our current estimated burn rate. In order to finance the continued growth in product sales, to invest in further product development and to otherwise satisfy obligations as they mature, we will need to seek additional financing through the issuance of common stock, preferred stock, and convertible or non-convertible debt financing. Any additional equity financing, if available to us, may not be available on favorable terms, will most likely be dilutive to our current stockholders, and debt financing, if available, may involve restrictive covenants. If we are unable to access additional funds when needed, we will not be able to continue the development of our molecular diagnostic platform, our diagnostic tests or we could be required to delay, scale back or eliminate some or all of our development programs and other operations. Any of these events could have an adverse impact on our business, financial condition and prospects.
Our independent registered public accounting firm has included an explanatory paragraph raising substantial doubt about our ability to continue as a going concern in its report on our audited financial statements. We may be unable to continue to operate without the threat of liquidation for the foreseeable future unless we raise additional capital.
In February 2017, we filed a registration statement with the Securities and Exchange Commission for an offering of equity securities, which we anticipate closing by the end of March 2017; however, we can provide no assurance that any such offering will be successful or on terms favorable to the Company or our stockholders. In the event we
60
complete such offering, along with additional funds to be withdrawn from our restricted cash accounts, if we are able to meet the conditions of the 2016 Note or if we are able to negotiate the releases, we believe we will have sufficient cash to fund our planned operations through much of calendar year 2017. If we are unable to successfully gain access to the cash in the restricted accounts, we will need to raise additional capital early in the first half of 2017. We also anticipate we will need to seek additional financing prior to the end of the calendar year to continue funding our operations for the remainder of 2017 and into 2018. If the cash in the restricted accounts is available, we will have capital to fund our operations for at least the next 12 months.
Summary Statement of Cash Flows for the Years Ended December 31, 2016 and 2015
The following table summarizes our cash flows for the periods indicated:
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Cash used in operating activities |
|
$ |
(31,654,390 |
) |
|
$ |
(20,669,754 |
) |
|
Cash used in investing activities |
|
|
(4,372,880 |
) |
|
|
(4,792,987 |
) |
|
Cash provided by financing activities |
|
|
32,253,766 |
|
|
|
28,232,677 |
|
|
Net increase (decrease) in cash |
|
$ |
(3,773,504 |
) |
|
$ |
2,769,936 |
|
Cash Flows from Operating Activities
Cash used in operating activities for the year ended December 31, 2016 was $31.7 million. The net loss of $89.1 million was offset by non-cash items of $46.5 million for the convertible note debt discount amortization, $119.2 million for the loss on the issuance of convertible note, $24.2 million for the loss on the extinguishment of debt, $2.6 million for the increase in depreciation and amortization, and $148,762 in all other non-cash items. These non-cash increases were partially offset by a $133.2 million change in the fair value of the derivative liability, and $3.4 million for a non-cash net gain on the exchange and issuance of warrants. The change in operating assets and liabilities resulted in a net provision of cash of $1.4 million, primarily due to a rise in accounts payable and accrued liabilities attributable to the launch of new products and sales increases, partially offset by related decreases in accounts receivable, inventory, and vendor prepayments. As of December 31, 2016, 82% of accounts receivable was less than 60 days old.
Cash used in operating activities for 2015 was $20.7 million. The net loss of $57.9 million was partially offset by non-cash items of $19.7 million for change in fair value of derivative liability, $10.6 million in interest from issuance of the convertible note, $4.0 million in a loss on extinguishment of warrants, $1.6 million for depreciation and amortization, $612,006 of warrant issuance costs, and $232,174 in other non-cash items. The change in operating assets and liabilities offset the cash used in operating activities by $426,096, primarily due to an increase of $602,056 in accounts payable, and an increase of $700,790 in accrued liabilities partially offset by a $676,048 increase in inventory and a $200,702 increase in accounts receivables and prepaid and other assets. As of December 31, 2015, 72% of accounts receivable was less than 60 days old.
Cash Flows from Investing Activities
Cash used in investing activities was $4.4 million for 2016 and is due to $3.3 million for the cost of the construction of our analyzer equipment and $1.1 million of capital expenditures for acquisition of property and equipment.
Cash used in investing activities was $4.8 million for 2015 and is due to $3.2 million for the cost of the construction of our analyzer equipment, and $1.6 million of capital expenditures for acquisition of property and equipment.
Cash Flows from Financing Activities
Cash provided by financing activities for 2016 of $32.3 million was from the proceeds of $10.6 million from the issuance of units in follow-on offerings, proceeds of $21.8 million from the issuance of convertible notes payable and associated releases of restricted cash, and proceeds of $1.4 million from exercise of warrants. These amounts
61
were offset by $1.3 million in payments of capital lease obligations, $314,879 paid to settle warrant exercises, and $5,693 in payments on notes payable.
Cash provided by financing activities for 2015 of $28.2 million was from the proceeds of $21.9 million from the issuance of units in a follow-on offering, proceeds of $4.1 million from the issuance of convertible notes payable, proceeds of $3.2 million from exercise of warrants and $250,000 in other financing activities, offset by $947,423 in payments of capital lease obligations, and $299,994 in payments on notes payable.
Satisfaction of our cash obligations for the next 12 months and our ability to continue as a going concern
Our financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the liquidation of liabilities in the ordinary course of business. At December 31, 2016, we had unrestricted and restricted cash amounts of $1.0 million and $59.4 million, respectively. We have incurred substantial losses from operations and negative operating cash flows which raise substantial doubt about our ability to continue as a going concern. We sustained net losses for the years ended December 31, 2016 and 2015 of $89.1 million and $57.9 million, respectively. We have an accumulated deficit of $211.1 million as of December 31, 2016. During the year ended December 31, 2016, cash used for operations was $31.7 million. Whether and when we can attain profitability and positive cash flows from operations is uncertain.
We intend to continue to develop our products and expand our customer base, but we do not have sufficient realized revenues or operating cash flows in order to finance these activities internally. As a result, we have obtained and intend to continue to obtain financing in order to fund our working capital and development needs. In February 2016, we obtained financing by completing a follow-on offering for net proceeds of $5.0 million, and in June 2016, we completed another follow-on offering for net proceeds of $5.3 million. In July 2016, we signed and issued convertible notes for net proceeds of $68 million, of which a net $5.4 million was immediately available and the remaining proceeds were placed in the Company’s restricted accounts. Through early March 2017, we withdrew from restricted accounts all of the remaining $13.8 million related to our December 2015 convertible notes, and withdrew $6.1 million from the restricted accounts related to our July 2016 convertible notes.
As mentioned above, in order to continue operations through the end of 2017, we will need to obtain additional financing. In February 2017, we filed a registration statement with the Securities and Exchange Commission for an offering of equity securities, which we anticipate closing by the end of March 2017; however, we can provide no assurance that any such offering will be successful or on terms favorable to the Company or our stockholders. In the event we complete such offering, along with additional funds to be withdrawn from our restricted cash accounts, if we are able to meet the conditions of the 2016 Note or if we are able to negotiate the releases, we believe we will have sufficient cash to fund our planned operations through much of calendar year 2017. If we are unable to successfully gain access to the cash in the restricted accounts, we will need to raise additional capital early in the first half of 2017. We also anticipate we will need to seek additional financing prior to the end of the calendar year to continue funding our operations for the remainder of 2017 and into 2018. If the cash in the restricted accounts is available, we will have capital to fund our operations for at least the next 12 months.
In order to continue operations through the end of 2017, we will need to obtain additional financing. In February 2017, we filed a registration statement with the Securities and Exchange Commission for an offering of equity securities, which we anticipate closing by the end of March 2017. Utilizing those proceeds, along with additional funds to be withdrawn from our restricted cash accounts, if we are able to meet the conditions of the 2016 Note or if we are able to negotiate the releases, we believe we will have sufficient cash to fund our planned operations through much of calendar year 2017. If we are unable to successfully gain access to the cash in the restricted accounts, we will need to raise additional capital early in the second half of 2017. However, we anticipate we will need to seek additional financing prior to the end of the calendar year to continue funding our operations for the remainder of 2017 and into 2018.
Since inception, we have been able to meet our short-term funding needs through private placements of convertible preferred securities, an initial public offering (“IPO”), follow-on public offerings, issuances of convertible debt and the sale and leaseback of analyzers used to report test results. We will continue to seek funding through the issuance of additional equity securities, debt financing, the sale and leaseback of analyzers, or a combination of these items. Any proceeds received from these items could provide the needed funds for continued operations and development programs. We can provide no assurance that we will be able to obtain the additional financing that we need to
62
alleviate doubt about our ability to continue as a going concern. If we are able to obtain sufficient proceeds from additional financing, we cannot be certain that this additional financing will be available on acceptable terms. To the extent we raise additional funds by issuing equity securities, our stockholders will likely experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact our ability to conduct business. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. If we are unable to obtain additional financings, the impact on our operations will be material and adverse.
Critical Accounting Policies and Estimates
The preparation of the financial statements requires us to make assumptions, estimates and judgments that affect the reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities as of the date of the financial statements, and the reported amounts of revenue and expenses during the reporting periods. Certain of our more critical accounting policies require the application of significant judgment by management in selecting the appropriate assumptions for calculating financial estimates. By their nature, these judgments are subject to an inherent degree of uncertainty. On an ongoing basis, we evaluate our judgments, including those related to inventories, receivables, recoverability of long-lived assets, and the fair value of our preferred and common stock and related instruments. We use historical experience and other assumptions as the basis for our judgments and making these estimates. Because future events and their effects cannot be determined with precision, actual results could differ significantly from these estimates. Any changes in those estimates will be reflected in our financial statements as they occur. While our significant accounting policies are more fully described in the footnotes to our financial statements included elsewhere in this Form 10-K, we believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results. The critical accounting policies addressed below reflect our most significant judgments and estimates used in the preparation of our financial statements.
As an emerging growth company, we have elected to opt-in to the extended transition period for new or revised accounting standards. As a result, our financial statements may not be comparable to those of companies that comply with public company effective dates.
Revenue Recognition
We derive our revenue from the sale of single use assays sold through our dedicated sales force in the United States, and through a network of distributors in the European Union and New Zealand. Revenue is recognized when all four of the following criteria are met: (1) persuasive evidence that an arrangement exists; (2) delivery of the products has occurred; (3) the selling price of the product is fixed or determinable; and (4) collectability of that price is reasonably assured. Change in title to the product and recognition of revenue from sales of assays occurs at the time of shipment.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are generated from the sale of assays to end users in the United States and to a network of distributors outside the United States. These accounts receivable are recorded at the invoiced amount, net of allowances for doubtful amounts. We routinely review outstanding accounts receivable balances for estimated uncollectible accounts and establish or adjust the allowances for doubtful accounts receivable using the specific identification method and record a reserve for amounts not expected to be fully recovered. Actual balances are not applied against the reserve until substantially all collection efforts have been exhausted. We do not have customer acceptance provisions, but we provide our customers a limited right of return for defective assays.
63
Inventories are stated at the lower of cost or market with cost determined according to the average cost method. Manufactured inventory consists of raw material, direct labor and manufacturing overhead cost components. We review the components of our inventory on a regular basis for excess and obsolete inventory, and make appropriate adjustments when necessary. We have made adjustments to, and it is reasonably possible that we may be required to make further adjustments to, the carrying value of inventory in future periods.
Long-Lived Assets
Long-lived tangible assets, including property and equipment, and definite-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of the asset may not be recoverable. We regularly evaluate whether events or circumstances have occurred that indicate possible impairment and rely on a number of factors, including expected future operating results, business plans, economic projections, and anticipated future cash flows. We use an estimate of the future undiscounted net cash flows and comparisons to like-kind assets, as appropriate, of the related asset over the remaining life in measuring whether the assets are recoverable. Measurement of the amount of impairment, if any, is based upon the difference between the asset’s carrying value and estimated fair value. Fair value is determined through various valuation techniques, including cost-based, market and income approaches as considered necessary. We amortize intangible assets on a straight-line basis over their estimated useful lives.
Our long-lived assets include our analyzers used by hospitals in the United States to run the assays they buy from us. There are no contractual terms with respect to the usage of our analyzers by our customers. Hospitals are under no contractual commitment to use our analyzers. We maintain ownership of these analyzers and, when requested, we can remove the analyzers from the customer’s site. We do not currently charge for the use of our analyzers and there are no minimum purchase commitments of our assays. As our analyzer is used numerous times over several years, often by many different customers, analyzers are capitalized as property and equipment once they have been placed in service. Once placed in service, analyzers are carried at cost, less accumulated depreciation. Depreciation is computed using the straight-line method based on average estimated useful lives. The estimated useful life of our analyzers is determined based on a variety of factors including in reference to associated product life cycles, and average 5 years. As analyzers are integral to the performance of our diagnostic tests, depreciation of analyzers is recognized as a cost of sales.
Analyzers used outside the United States are sold to the customer and the sale is accounted for as a sale of fixed assets. Since inception, the Company has not focused nor placed significant emphasis on developing international markets for the Company’s product. The Company has never had an international sales force and has never manufactured analyzers specifically for international markets. On occasion, small, international sales opportunities have come along through international distributors. The analyzers that were sold to them were part of the fixed asset pool of analyzers the Company has, and many of these specific analyzers had been previously placed at customer locations within the United States. Sale of the fixed asset analyzers in these limited international opportunities have not been based on established product price listings as no such listing exists or has been publicly marketed to customers; instead, the final sales price has been a negotiated amount based on the sale of a functioning fixed asset analyzer, whether or not that analyzer was previously used at another customer site. Similar to other fixed asset sales, there were no stated or implied warranties or other continuing service requirements made with the sale of these assets. For these limited situations, management has elected to sell the fixed asset analyzers as opposed to placing them with international customers (thereby not retaining title over the analyzers) as it would be impractical for us to retain ownership due to, among other reasons, the Company lacking the necessary personnel needed to service international customers, the need to comply with the additional laws and regulations of countries outside the United States to which the Company is not currently subject, and the added costs to recover, reconfigure, ship and redeploy fixed asset analyzers that have been used internationally. During the years ended December 31, 2016 and 2015 there were no analyzers sold to international distributors.
Fair Value Instruments
The Company accounts for derivative instruments under the provisions of Accounting Standards Codification (“ASC”) 815, Derivatives and Hedging. ASC 815 requires the Company to record derivative instruments at their
64
fair value. Changes in the fair value of derivatives are recognized in earnings. As a result of certain terms, conditions and features included in our convertible notes and certain common stock purchase warrants granted by the Company, those securities are required to be accounted for as derivatives at estimated fair value, with changes in fair value recognized in earnings.
The Company accounts for other fair value liability instruments under the provisions of ASC 480 Distinguishing Liabilities from Equity. ASC 480 requires to record certain liabilities at their fair value. Changes in the fair value of these liabilities are recognized in earnings. As a result of certain terms, conditions and features included in the Series F Preferred Stock issued by the Company, it is required to be accounted for as a liability at estimated fair value, with changes in fair value recognized in earnings.
Income Taxes
We are required to determine the aggregate amount of income tax expense or loss based upon tax statutes in jurisdictions in which we conduct business. In making these estimates, we adjust our results determined in accordance with generally accepted accounting principles for items that are treated differently by the applicable taxing authorities. Deferred tax assets and liabilities resulting from these differences are reflected on our balance sheet for temporary differences in loss and credit carryforwards that will reverse in subsequent years. We also establish a valuation allowance against deferred tax assets when it is more likely than not that some or all of the deferred tax assets will not be realized. Valuation allowances are based, in part, on predictions that management must make as to our results in future periods. The outcome of events could differ over time which would require that we make changes in our valuation allowance.
The tax effects from an uncertain tax position can be recognized in the financial statements only if the position is more likely than not of being sustained if the position were to be challenged by a taxing authority. We examined the tax positions taken in tax returns and determined that there are no uncertain tax positions. As a result, we recorded no uncertain tax liabilities in our balance sheet.
Stock Based Compensation
We measure and recognize compensation expense for stock options granted to our employees and directors, based on the estimated fair value of the award on the grant date. Since our IPO, our board of directors determines the fair value of each share of underlying common stock based on the closing price of our common stock on the date of grant. We use the Black-Scholes valuation model to estimate the fair value of stock option awards. The fair value is recognized as expense, net of estimated forfeitures, over the requisite service period, which is generally the vesting period of the respective award on a straight-line basis.
Emerging Growth Company Status
We qualify as an “emerging growth company” as defined in Section 101 of the Jumpstart our Business Startups Act (“JOBS Act”) as we do not have more than $1,000,000,000 in annual gross revenue and did not have such amount as of December 31, 2016, being the last day of our prior fiscal year.
We may lose our status as an emerging growth company on the last day of our fiscal year during which (i) our annual gross revenue exceeds $1,000,000,000 or (ii) we issue more than $1,000,000,000 in non-convertible debt in a three-year period. We will lose our status as an emerging growth company if at any time we are deemed to be a large accelerated filer. We will lose our status as an emerging growth company on the last day of our fiscal year following the fifth anniversary of the date of the first sale of common equity securities pursuant to an effective registration statement.
65
As an emerging growth company under the JOBS Act, we have elected to opt in to the extended transition period for complying with new or revised standards pursuant to Section 107(b) of the Act.
As an emerging growth company, we are exempt from Section 404(b) of the Sarbanes-Oxley Act of 2002 and Section 14A(a) and (b) of the Securities Exchange Act of 1934. Such sections are provided below:
|
|
• |
Section 404(b) of the Sarbanes-Oxley Act of 2002 requires a public company’s auditor to attest to, and report on, management's assessment of its internal controls. |
|
|
• |
Sections 14A(a) and (b) of the Exchange Act, implemented by Section 951 of the Dodd-Frank Act, require companies to hold shareholder advisory votes on executive compensation and golden parachute compensation. |
As long as we qualify as an emerging growth company, we will not be required to comply with the requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002 and Section 14A (a) and (b) of the Exchange Act, as amended (the (“Exchange Act”).
Off-Balance Sheet Arrangements
We currently do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
As a smaller reporting company, we have elected not to provide the disclosure required by this item.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
Our financial statements required by this item are set forth immediately following the signature page to this annual report on Form 10-K beginning on page F-1 and are incorporated herein by reference. As a smaller reporting company, we have elected not to provide the disclosure regarding supplementary data required by this item.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES.
None.
66
ITEM 9A. CONTROLS AND PROCEDURES.
Internal Control Over Financial Reporting
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal financial officer), evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2016 pursuant to Rule 13a-15(b) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
We have carried out an evaluation under the supervision and with the participation of our management, of the effectiveness of the design and operation of our disclosure controls and procedures. As described below, management has identified a material weakness in our internal control over financial reporting related to properly identifying and accounting for transactions of a complex or non-routine nature. As a result of this material weakness, the Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective as of December 31, 2016.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined by Rule 13a-15(f) under the Exchange Act). In assessing the effectiveness of our internal control over financial reporting as of December 31, 2016, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework).
Based on this assessment as of December 31, 2016, our chief executive officer and chief financial officer concluded that our internal control over financial reporting was not effective as of December 31, 2016 due to the material weakness described below.
Material Weakness
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
We previously reported a material weakness in internal control over financial reporting as of December 31, 2015. This material weakness has not been fully remediated and as a result, as of December 31, 2016, we continued to have deficiencies in our internal control over financial reporting related to properly identifying and accounting for transactions of a complex or non-routine nature. The nature of our financing agreements increases the complexity of our accounting for potential fair value liabilities. As we enter into additional equity or debt financing transactions, which may have contractual provisions different from those of our existing financing instruments, the accounting and valuation of these financial instruments may become increasingly complicated. This additional complexity could increase the chance that we experience additional errors in the future, particularly because we have a material weakness in our internal control.
We continue to take steps to remediate the underlying causes of the material weakness. During 2016 we hired additional accounting and IT personnel to help improve our segregation of duties. We continue to engage third-party consultants to assist us with our evaluation of complex technical accounting matters, including the valuation and accounting for our complex derivative agreements. We also continue to engage consultants to advise us on
67
making further improvements to our internal control over financial reporting. We believe that these additional resources will enable us to broaden the scope and quality of our controls relating to the oversight and review of financial statements and our application of relevant accounting policies. These remediation efforts are still in process and have not yet been completed. Because of this material weakness, there is heightened risk that a material misstatement of our annual or quarterly financial statements will not be prevented or detected.
Limitations of the Effectiveness of Disclosure Controls and Procedures and Internal Control over Financial Reporting
Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls or internal control over financial reporting will prevent all errors or all instances of fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and any design may not succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures. Because of the inherent limitation of a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2016 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
68
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Directors
Set forth below is a list of the names, ages as of December 31, 2016 and positions, and a brief account of the business experience of the individuals who serve as our executive officers and directors as of the date of this report.
|
Name |
|
Age |
|
Position |
|
Ryan Ashton |
|
56 |
|
President, Chief Executive Officer and Director |
|
Robert Jenison |
|
50 |
|
Chief Technology Officer and Senior Vice President of Research |
|
Jeffrey Rona |
|
48 |
|
Chief Financial Officer |
|
David Spafford |
|
56 |
|
Director and Executive Chairman |
|
Kirk Calhoun |
|
72 |
|
Director |
|
Ronald Labrum |
|
60 |
|
Director |
|
Sam Chawla |
|
42 |
|
Director |
Ryan Ashton — President, Chief Executive Officer and Director. Mr. Ashton joined us in January 2005 and has served as our President, Chief Executive Officer and a Director since then. Prior to joining us, from 2001 to 2005 he served as the CEO of Printelligent Corporation. From 1999 to 2001 he served as the Vice President of Sales and Marketing at Inari Inc., a venture-funded technology start-up. Prior to that, Mr. Ashton was hired, in 1989, as a marketing manager of Megahertz Corporation, a manufacturer of communications products for mobile computing, and by 1991 he was responsible for all sales and marketing for Megahertz Corporation. By the time his tenure with Megahertz Corporation ended when it was sold to U.S. Robotics in 1995, he was serving as Senior Vice President, Sales and Marketing.
We believe that Mr. Ashton possesses attributes that qualify him to serve as a member of our board of directors, including his depth of operating, strategic, transactional and senior management experience, in addition to his intimate knowledge of our Company, as he has been the CEO since 2005, overseeing the development of the technology and the commercialization of our first product.
Robert Jenison — Chief Technology Officer and Senior Vice President of Research. Mr. Jenison has served as Chief Technology Officer since 2006. From 1999 – 2006, Mr. Jenison was Associate Director, R&D of Thermo BioStar where he was responsible for establishing a molecular diagnostic testing business. From 1992 – 1999, Mr. Jenison was a Senior Research Associate at Nexstar Pharmaceuticals responsible for aptamer development. From 1990 – 1992, Mr. Jenison was a Scientist at ISIS Pharmaceuticals. From 1989 – 1990, Mr. Jenison was a Research Associate at Research Institute, Scripps Clinic. Mr. Jenison received a B.A.Sc. in Chemistry and Biochemistry from the University of California, San Diego.
Jeffrey A. Rona — Chief Financial Officer. Mr. Rona has served as the Chief Financial Officer since October 2014. Prior to that he was a financial consultant to Great Basin during 2013 and has served as the Managing Director of Rona Capital, LLC, a life sciences-focused transactional advisory consultancy, since 2011. From 2006 to 2011, Mr. Rona was the Chief Business Officer of GlobeImmune a private life sciences company. Prior to that, from 2003 to 2006, Mr. Rona was the Chief Financial Officer for AlgoRx Pharmaceuticals, a private life sciences company that was merged into a publicly traded company, Corgentech Inc. Mr. Rona was in the Investment Banking Department at UBS Warburg, a global securities and investment banking firm, from 2000 to 2002. From 1998 to 2000, Mr. Rona served as the Director of Finance and Corporate Development at Antigenics Inc., a life sciences company that went public in 2000, and Mr. Rona was responsible for managing the IPO process. In 1998, Mr. Rona was employed by Carr & Company, a private equity firm. From 1990 – 1997, Mr. Rona was with Coopers and Lybrand and its wholly owned subsidiary Coopers & Lybrand Securities, serving in a variety of capacities. Mr. Rona received a B.S. in Accounting from Case Western Reserve University in 1990. Mr. Rona is a trustee of the PKD Foundation (Polysystic Kidney Disease), a not-for-profit foundation.
69
David Spafford — Director and Executive Chairman. Mr. Spafford is a founding investor of Great Basin and has served as Chairman of our board of directors since its inception. Mr. Spafford was a co-founder, director and senior executive officer of Megahertz Corporation. Megahertz Corporation completed an initial
public offering in 1993 and was acquired by U.S. Robotics in 1995 in a transaction valued at approximately $450 million. Since 1994 Mr. Spafford has focused on angel investing and philanthropic work.
We believe Mr. Spafford possesses specific attributes that qualify him to serve as a member of our board of directors and as our Executive Chairman, including the depth of his sales and marketing and operating experience and his intimate knowledge of our business as a founder, investor and member of the board of directors since our inception.
Kirk Calhoun — Director . Mr. Calhoun became a director at our annual meeting of stockholders on May 27, 2015. Mr. Calhoun is a Certified Public Accountant (non-practicing) with a background in auditing and accounting. Mr. Calhoun joined Ernst & Young LLP, a public accounting firm, in 1965 and served as a partner of the firm from 1975 until his retirement in 2002. Mr. Calhoun currently serves on the board and audit committee of Ryerson Holding Corporation (NYSE: RYI) and NanHealth, Inc ((OTC: NH). Mr. Calhoun has served previously on the boards and audit committees of six public companies in the pharmaceutical industry up until the dates of their respective sales, including Abraxis Bioscience, Inc., Myogen, Inc., Aspreva Pharmaceuticals Company, Replidyne, Inc. and Adams Respiratory Therapeutics, Inc. Mr. Calhoun also currently serves on the boards of three private companies, including NeuroSigma, Inc., a developer of products that treat major neurological and neuropsychiatric disorders such as post traumatic stress disorder (PTSD), epilepsy and depression. Mr. Calhoun received a B.S. in Accounting from the University of Southern California.
We believe that Mr. Calhoun brings to the board of directors experience and skills in finance, management and corporate governance, developed over his career in public accounting and through his service as an audit committee financial expert on various public company boards and his service as a director of other life sciences companies. Mr. Calhoun was recommended for the board of directors by members of the board of directors based on these qualifications.
Sam Chawla — Director . Mr. Chawla became a director in October 2014. Mr. Chawla is a Portfolio Manager of Perceptive Advisors LLC, an investment fund focused on the healthcare sector. Mr. Chawla leads Perceptive’s Credit Opportunities Fund. Prior to joining Perceptive Advisors in 2013, Mr. Chawla was a Managing Director at UBS Securities LLC in the Global Healthcare Group, where he led origination and execution of financing and advisory assignments for healthcare companies, with a focus on the diagnostics sector. Mr. Chawla’s investment banking experience centered on strategic advisory, including M&A buy-side and sell-side assignments, and financial advisory, including equity and debt capital raises, for both public and private healthcare companies. Prior to joining UBS in September 2010, Mr. Chawla was a Director (from January 2009 to September 2010) and a Vice President (from July 2007 to January 2009) in the Healthcare Investment Banking Group of Credit Suisse LLC, which he originally joined as an investment banker in 2002. Mr. Chawla also worked at Bloomberg L.P. and Pelican Life Sciences. Mr. Chawla received an M.B.A. from Georgetown University and a B.A. in Economics from Johns Hopkins University. Mr. Chawla is also a director of VBI Vaccines Inc. (NASDAQ:VBIV).
Mr. Chawla brings to the board of directors significant investment banking, mergers and acquisitions, financing and advisory expertise focusing on the healthcare sector, particularly in the diagnostic laboratory industry. Mr. Chawla’s experience and knowledge in these areas are important to the board of directors’ ability to help guide us in evaluating optimal short and long term strategic plans as well as providing insight and guidance in pursing growth through strategic opportunities.
Ronald Labrum — Director . Mr. Labrum became a director of the board of directors in October 2014. Since 2012, Mr. Labrum has served as an operating partner of Linden Capital Partners, a private equity group focused on leveraged investments in middle market healthcare and life science companies. From 2007 until 2012, Mr. Labrum served as the Chief Executive Officer of Fenwal, Inc., a provider of products and technologies that support and improve blood collection, processing and transfusion medicine. From 2004 to 2006, Mr. Labrum served as the Chief Executive Officer of Cardinal Health, Inc.’s Healthcare Supply Chain Services, which
70
includes medical products distribution, pharmaceutical distribution, nuclear pharmacy services and the specialty distribution businesses of Cardinal Health, Inc. During 2004, Mr. Labrum served as Chairman and Chief Executive Officer of Integrated Provider Solutions and Cardinal Health — International, both divisions of Cardinal Health, Inc. Prior to 2004, Mr. Labrum served as executive vice president of Cardinal Health, Inc. and Group President of the Medical Products and Services segment.
Mr. Labrum joined Cardinal Health in 1999 with the acquisition of Allegiance Healthcare Corporation, originally American Hospital Supply Corp., where he was president of Allegiance Manufacturing and Distribution. Mr. Labrum is also a director of Aptalis Pharma Inc. and Procure Treatment Centers, Inc., which are both privately held companies, and Wright Medical Group, Inc. (NASDAQ:WMGI).
Mr. Labrum possesses attributes that qualify him to serve as a member of our board of directors including his significant management and operating experience within the healthcare sector and specifically within the diagnostic sector.
Arrangements between Officers and Directors
To our knowledge, there is no arrangement or understanding between any of our officers and any other person, including directors, pursuant to which the officer or director was selected to serve as an officer or director.
Family Relationships
There are no family relationships among any of our directors or executive officers.
Legal Proceedings
No director or officer of the Company is a party adverse to the Company or any of its subsidiaries, or has a material interest adverse to the Company or any of its subsidiaries. To the best of our knowledge, none of our directors or executive officers has, during the past ten years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K.
Code of Business Conduct and Ethics
Our Board of Directors has adopted a written Code of Business Conduct and Ethics applicable to our employees, officers and directors, including those officers responsible for financial reporting. The Code of Business Conduct and Ethics is available on our website at www.gbscience.com. If the Company amends the Code of Business Conduct and Ethics or grants a waiver, including an implicit waiver, from the Code of Business Conduct and Ethics, the Company will disclose the information on its internet website. The waiver information will remain on the website for at least twelve months after the initial disclosure of such waiver.
Audit Committee and Audit Committee Financial Experts
We have a standing audit committee and such committee is in compliance with Rule 10A-3 of the Exchange Act comprised of Mr. Calhoun (Chair), Mr. Chawla and Mr. Labrum. Our board of directors has determined that Mr. Calhoun and Mr. Labrum are audit committee financial experts, as defined by the rules of the Securities and Exchange Commission, and both satisfy the financial sophistication requirements of applicable NASDAQ rules.
Recommendations to the Board
The Nominating and Corporate Governance Committee will consider recommendations for director nominees made by stockholders and others. For consideration by the Committee, the nominating stockholder or other person must provide the Corporate Secretary, Jeffery Rona, at the Company’s principal offices with information about the nominee, including the detailed background of the suggested candidate.
No stockholder or stockholders holding 5% or more of the Company’s outstanding stock, either individually or in aggregate, has recommended a nominee for election to the Board as of the date of this filing.
71
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act, as amended, requires our officers, directors, and persons who beneficially own more than 10% of our common stock, to file reports of ownership and changes in ownership with the SEC Such officers, directors and 10% Stockholders are also required by SEC rules to furnish us with copies of all Section 16(a) forms that they file.
Based solely on our review of the copies of such forms received by us, or written representations from certain reporting persons, the Company believes that during fiscal year ended December 31, 2016, the filing requirements applicable to our officers, directors and greater than 10% percent beneficial owners were complied with.
ITEM 11. EXECUTIVE COMPENSATION
Executive Officers
Summary Compensation Table
The following table sets forth the compensation paid or accrued during the fiscal years ended December 31, 2015 and December 31, 2016 to our Chief Executive Officer and our other executive officers. We collectively refer to these officers as our “named executive officers”.
|
Name and Principal Position |
|
Year |
|
Salary ($) |
|
|
Bonus ($) |
|
|
Option Awards ($) |
|
All Other Compensation ($) |
|
|
Total ($) |
|
||
|
Ryan Ashton |
|
2016 |
|
425,000 |
(1) |
|
— |
|
|
— |
|
16,153 |
(4) |
|
|
441,153 |
|
|
|
President, Chief Executive Officer and Director |
|
2015 |
|
425,000 |
(1) |
|
|
255,000 |
|
|
— |
|
14,654 |
(5) |
|
|
694,654 |
|
|
Robert Jenison |
|
2016 |
|
250,000 |
(2) |
|
— |
|
|
— |
|
23,210 |
(6) |
|
|
273,210 |
|
|
|
Chief Technology Officer |
|
2015 |
|
250,000 |
(2) |
|
|
75,000 |
|
|
— |
|
21,072 |
(7) |
|
|
346,072 |
|
|
Jeffrey Rona |
|
2016 |
|
325,000 |
(3) |
|
— |
|
|
— |
|
23,210 |
(8) |
|
|
348,210 |
|
|
|
Chief Financial Officer |
|
2015 |
|
325,000 |
(3) |
|
|
97,500 |
|
|
— |
|
21,072 |
(9) |
|
|
443,572 |
|
|
(1) |
Consists of base salary of $425,000. |
|
(2) |
Consists of base salary of $250,000. |
|
(3) |
Consists of base salary of $325,000. |
|
(4) |
Consists of medical insurance payments of $14,552, dental insurance payments of $1,442 and vision insurance payments of $160. |
|
(5) |
Consists of medical insurance payments of $13,417 and dental insurance payments of $1,237. |
|
(6) |
Medical insurance payments of $21,513, dental insurance payments of $1,442 and vision insurance payments of $255. |
|
(7) |
Consists of medical insurance payments of $19,835 and dental insurance payments of $1,237. |
|
(8) |
Consists of medical insurance payments of $21,513, dental insurance payments of $1,442 and vision insurance payments of $255. |
|
(9) |
Consists of medical insurance payments of $19,835 and dental insurance payments of $1,237. |
Narrative to Summary Compensation Table
Our board of directors periodically reviews and, when appropriate, adjusts the compensation of management. Our management is eligible to participate in any other compensation or benefit plans, as approved by the board of directors, made available to our other employees, including without limitation, stock option plans, health insurance plans, and 401(k) plans. Discretionary cash bonuses are not based upon set targets or criteria, but are awarded at the Board’s complete discretion based on the Board review of the Company’s performance at the end of the year, including but not limited to, a review of the achievement of development and marketing goals, revenues and stock performance. In 2015, the Board granted discretionary cash bonuses to the executive
72
officers and the executive chairman. The bonuses were based on the Board’s assessment of the Company’s achievements during the preceding fiscal year. The Board, without Mr. Ashton or Mr. Spafford, voted and agreed upon the amount and timing of the cash bonuses. There were no discretionary cash bonuses awarded in 2016.
The Board did not issue any equity compensation in 2015 or 2016. The option pool did not contain a material number of shares available for issue, therefore, the Board decided not to issue any equity compensation.
Employment, Change in Control and Severance Disclosure
We have entered into employment agreements with Ryan Ashton, Jeffrey Rona and Robert Jenison. These employment agreements provide for at-will employment and set forth each officer’s initial base salary, initial equity grant amount and eligibility for employee benefits. In addition, each of our named executive officers has executed a form of our standard confidential information and invention assignment agreement. The key terms of these employment agreements are described below. Other than the employment agreements described
below, we have not entered into any arrangements providing for payments or benefits in connection with the resignation, severance, retirement or other termination of any of our named executive officers, changes in their compensation or a change in control.
Ryan Ashton
We have entered into an employment agreement with Mr. Ashton to be our Chief Executive Officer and a member of our board of directors. The employment agreement provides for “at-will” employment and sets forth his initial annual base salary of $425,000 and his eligibility to participate in our employee benefit plans and programs, as in effect from time to time. Mr. Ashton would also be entitled to severance in the event of a termination without cause or a constructive termination within one year of a change of control in an amount equal to two times Mr. Ashton’s annual base salary at the time of such termination, which amount would be paid in a lump sum within 60 days of such termination. In addition, upon such termination we would also pay to Mr. Ashton an amount equal to 100% of his target employee bonus in equal monthly installments over the six-month period following such termination.
Jeffrey Rona
We have entered into an employment agreement with Mr. Rona to be our Chief Financial Officer. The employment agreement provides for “at-will” employment and sets forth his initial annual base salary of $325,000 and his eligibility to participate in our employee benefit plans and programs, as in effect from time to time. Mr. Rona would also be entitled to severance in the event of a termination without cause or a constructive termination within one year of a change of control in an amount equal to one half of Mr. Rona’s annual base salary at the time of such termination if Mr. Rona were terminated prior to completing six months of service to us or a full year’s base salary if Mr. Rona were terminated after providing at least six months of service to us, which amount would be paid in a lump sum within 60 days of such termination. In addition, upon such termination we would also pay to Mr. Rona an amount equal to 50% of his target employee bonus in equal monthly installments over the six-month period following such termination.
Robert Jenison
We have entered into an employment agreement Mr. Jenison to be our Chief Technology Officer and Senior Vice President of Research. The employment agreement provides for “at-will” employment and sets forth his initial annual base salary of $250,000 and his eligibility to participate in our employee benefit plans and programs, as in effect from time to time. Mr. Jenison would also be entitled to severance in the event of a termination without cause or a constructive termination within one year of a change of control in an amount equal to Mr. Jenison’s annual base salary at the time of such termination, which amount would be paid in a lump sum within 60 days of such termination. In addition, upon such termination we would also pay to Mr. Jenison an amount equal to 50% of his target employee bonus in equal monthly installments over the six-month period following such termination.
73
Outstanding Equity Awards as of December 31, 2016
The following sets forth information concerning the number and value of common stock of unexercised options held by each named executive officer as of December 31, 2016.
|
|
|
OPTION AWARDS |
|
||||||||||||||||
|
Name |
|
Number of Securities Underlying Unexercised Options Exercisable (#) |
|
|
Number of Securities Underlying Unexercised Options Unexercisable (#) |
|
|
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
|
|
Option Exercise Price ($ Millions) (1) |
|
|
Option Expiration Date |
|
||||
|
Ryan Ashton |
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
$ |
176.4 |
|
|
2/14/2020 |
(2) |
|
|
|
|
1 |
|
|
|
1 |
|
|
|
— |
|
|
$ |
100.8 |
|
|
4/11/2024 |
(3) |
|
Jeffrey Rona |
|
|
1 |
|
|
|
1 |
|
|
|
— |
|
|
$ |
352.8 |
|
|
10/5/2014 |
(4) |
|
Robert Jenison |
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
$ |
176.4 |
|
|
7/22/2017 |
(5) |
|
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
$ |
176.4 |
|
|
11/7/2017 |
(6) |
|
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
$ |
176.4 |
|
|
2/14/2020 |
(7) |
|
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
$ |
176.4 |
|
|
7/26/2016 |
(8) |
|
|
|
|
1 |
|
|
|
1 |
|
|
|
— |
|
|
$ |
100.8 |
|
|
4/11/2014 |
(9) |
|
(1) |
(1) |
The option exercise prices in this table reflect the option exercise prices per share as of December 31, 2016. |
|
|
(2) |
This award vested ¼ of the total underlying shares on February 15, 2011 and 1/48 of the total underlying shares at the end of each month for the remaining 36 months commencing with the first month following the first anniversary of the grant date. |
|
|
(3) |
This award vested ¼ of the total underlying shares on April 14, 2015, and 1/48 of the total underlying shares at the end of each month for the remaining 36 months commencing with the first month following the first anniversary of the grant date. |
|
|
(4) |
This award vested ¼ of the total underlying shares on the day it was issued, and 1/48 of the total underlying shares at the end of each month for the remaining 36 months commencing with the first month following the grant date. |
|
|
(5) |
This award vested ¼ of the total underlying shares on each of January 26, 2008, 2009, 2010 and 2011. |
|
|
(6) |
This award vested ¼ of the total underlying shares on each of November 7, 2008, 2009, 2010 and 2011. |
|
|
(7) |
This award vested ¼ of the total underlying shares on February 15, 2011 and 1/48 of the total underlying shares at the end of each month for the remaining 36 months commencing with the first month following the first anniversary of the grant date. |
|
|
(8) |
This award vested ¼ of the total underlying shares on each of July 26, 2007, 2008, 2009 and 2010. |
|
|
(9) |
This award vested ¼ of the total underlying shares on April 14, 2015, and 1/48 of the total underlying shares at the end of each month for the remaining 36 months commencing with the first month following the first anniversary of the grant date. |
74
Members of our board of directors who are our employees do not receive any fees for their service on our board of directors or for their service as a chair or committee member. Ryan Ashton is our only employee director. Our non-employee directors earned the following compensation for their service during our fiscal year ended December 31, 2016:
|
Name |
|
Fees Earned or Paid in Cash ($) |
|
|
|
Stock Awards ($) |
|
|
Option Awards ($) |
|
|
Non-Equity Incentive Plan Compensation ($) |
|
|
|
All Other Compensation ($) |
|
|
Total ($) |
|
||||||
|
David Spafford |
|
|
250,000 |
|
|
|
|
— |
|
|
|
— |
|
|
|
22,074 |
|
(1) |
|
|
15,000 |
|
(2) |
|
287,074 |
|
|
Ronald Labrum |
|
|
50,000 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
50,000 |
|
|
Sam Chawla |
|
|
53,000 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
53,000 |
|
|
Kirk Calhoun |
|
|
53,000 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
53,000 |
|
|
(1) |
Consists of medical insurance payments of $21,513 and dental insurance payments of $561. |
|
(2) |
Consists of a club dues payment of $15,000. |
The following are the compensatory arrangements for our non-employee directors:
|
Director Position |
|
Annual Payment |
|
|
|
Executive Chairman Retainer |
|
$ |
180,000 |
|
|
Director Retainer paid to all directors |
|
$ |
35,000 |
|
|
Lead Director Supplement |
|
$ |
35,000 |
|
|
Audit Committee Chair |
|
$ |
10,000 |
|
|
Audit Committee Member |
|
$ |
5,000 |
|
|
Compensation Committee Chair |
|
$ |
10,000 |
|
|
Compensation Committee Member |
|
$ |
5,000 |
|
|
Nominating and Corporate Governance Committee Chair |
|
$ |
5,000 |
|
|
Nominating and Corporate Governance Committee Member |
|
$ |
3,000 |
|
75
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Shares of common stock that may be acquired by an individual or group within 60 days of March 17, 2017, pursuant to the exercise of options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Percentage of ownership is based on 1,485,904,493 shares of common stock outstanding as of March 17, 2017.
Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information provided to us by such stockholders. Unless otherwise indicated, the address for each director and executive officer listed is: c/o Great Basin Scientific, Inc., 420 E. South Temple, Suite 520, Salt Lake City, UT 84111.
|
Name of Beneficial Owner |
|
Number of Common Share Equivalents Beneficially Owned |
|
|
Percent |
|
|
Named Executive Officers and Directors: |
|
|
|
|
|
|
|
Ryan Ashton (1) |
|
|
2 |
|
|
* |
|
David Spafford (2) |
|
|
71,145 |
|
|
* |
|
Robert Jenison (3) |
|
|
1 |
|
|
* |
|
Jeffrey Rona (4) |
|
|
3 |
|
|
* |
|
Sam Chawla (5) |
|
|
4 |
|
|
* |
|
Ron Labrum (6) |
|
|
4 |
|
|
* |
|
Kirk Calhoun (7) |
|
|
1 |
|
|
* |
|
All Executive Officers and Directors as a Group (7 Persons) |
|
|
71,160 |
|
|
* |
|
|
|
|
|
|
|
|
|
* |
Represents less than 1% of the outstanding shares of Common Stock |
|
(1) |
Represents 1 share of common stock and options and warrants to purchase 1 share of common stock that are currently exercisable or exercisable within 60 days after March 17, 2017. |
|
(2) |
Represents (i) 1 share of common stock, warrants for 1 share of common stock owned by Mr. Spafford and options to purchase 1 share of common stock that are currently exercisable or exercisable within 60 days after March 17, 2017; (ii) shares owned by Spring Forth Investments LLC, an entity controlled by Mr. Spafford, which owns 21,360 shares of Series E Convertible Preferred Stock, convertible into 1 share of common stock, 86 Class A warrants to purchase 1 share of common stock and 86 Class B warrants to purchase 1 share of common stock and 35,565 Series D warrants convertible into 35,565 shares of common stock; (iii) 1 share of common stock owned by DSM Ventures, an entity controlled by Mr. Spafford; (iv) 1 share of common stock, Class A warrants to purchase 1 share of common stock and Class B warrants to purchase 1 share of common stock and warrants to purchase 1 share of common stock owned by DRS, LLC, an entity controlled by Mr. Spafford; (v) shares owned by Craig F. McCullough, Trustee, SBS Charitable Remainder Trust U/A/D November 27, 1995, a trust affiliated with Mr. Spafford, which owns 1 share of common stock and warrants to purchase 1 share of common stock; (vi) shares owned by Craig F. McCullough, Trustee, DRS Charitable Remainder Trust U/A/D May 5, 1993, a trust affiliated with Mr. Spafford, which owns 1 share of common stock and warrants to purchase 1 share of common stock; (vii) shares owned by Bourne Spafford Charitable Trust U/A/D May 15, 1995, a trust affiliated with Mr. Spafford, which owns 1 share of common stock and the Utah Autism Foundation a not-for-profit for which Mr. Spafford is a Trustee, owns 35,564 Series D warrants convertible into 35,564 shares of common stock. |
|
(3) |
Represents options to purchase 1 share of common stock that are currently exercisable or exercisable within 60 days after March 17, 2017. |
|
(4) |
Represents warrants to purchase 1 share of common stock, Class A Warrants to purchase 1 share of common stock, Class B Warrants to purchase 1 share of common stock and options to purchase 1 share of common stock exercisable within 60 days after March 17, 2017. |
76
|
(6) |
Represents options to purchase 1 share of common stock that are currently exercisable or exercisable within 60 days after March 17, 2017, 1 share of common stock, Class A Warrants to purchase 1 share of common stock and Class B Warrants to purchase 1 share of common stock. |
|
(7) |
Represents options to purchase 1 share of common stock that are exercisable or exercisable within 60 days after March 17, 2017. |
We are not, to the best of our knowledge, directly or indirectly owned or controlled by another corporation or foreign government.
Five Percent Stockholders:
|
Owner |
|
Title of Class |
|
Address of Beneficial Owner |
|
Amount and nature of beneficial ownership (Common Stock) |
|
|
Percent of Class (Common Stock) |
|
|
Amount and Nature of Beneficial Ownership (Series F Preferred Stock) |
|
|
Percent of Class (Series F Preferred Stock) |
|
||||
|
Hudson Bay Master Fund Ltd. (1) |
|
common stock and Series F Preferred Stock |
|
777 Third Avenue, 30th Floor New York, NY 10017 Attn: George Antonopoulos |
|
|
163,268,085 |
|
|
|
9.99% |
|
|
|
2,574 |
|
|
|
43.90% |
|
|
CVI Investments, Inc. (2) |
|
common stock and Series F Preferred Stock |
|
101 California Street, Suite 3250 San Francisco, CA 94111 Attention: Martin Kobinger |
|
|
163,268,085 |
|
|
|
9.99% |
|
|
273 |
|
|
|
4.70% |
|
|
|
Empery Asset Management L.P. (3) |
|
common stock and Series F Preferred Stock |
|
1 Rockefeller Plaza, Suite 1205 New York, NY 10020 AttnL Ryan M. Lane |
|
|
163,268,085 |
|
|
|
9.99% |
|
|
|
1,202 |
|
|
|
20.50% |
|
|
Sabby Healthcare Master Fund, Ltd. (4) |
|
common stock and Series F Preferred Stock |
|
10 Mountainview Road, Suite 205 Upper Saddle River, NJ 07458 Attention: Rob Grundstein |
|
|
163,268,085 |
|
|
|
9.99% |
|
|
|
1,407 |
|
|
|
24.00% |
|
|
Alto Opportunity Master Fund Ltd. (5) |
|
common stock and Series F Preferred Stock |
|
1180 Avenue of Americas, Suite 1940 New York, NY 10036 Attention: Robin Shah |
|
|
163,268,085 |
|
|
|
9.99% |
|
|
404 |
|
|
|
6.90% |
|
|
77
|
(2) |
Heights Capital Management, Inc., the authorized agent of CVI Investments, Inc. (“CVI”), has discretionary authority to vote and dispose of the shares held by CVI and may be deemed to be the beneficial owner of these shares. Martin Kobinger, in his capacity as Investment Manager of HeightsCapital Management, Inc., may also be deemed to have investment discretion and voting power over the shares held by CVI. Mr. Kobinger disclaims any such beneficial ownership of the shares. CVI Investments, Inc. is affiliated with one or more FINRA member. Amount beneficially owned includes 163,268,085 shares of common stock issuable upon conversion of the Convertible Series F Preferred Stock or the 2016 Note within 60 days of March 17, 2017, both of which are subject to beneficial ownership limitations on conversion of 9.99% of the total issued and outstanding immediately following conversion. Amount of Series F Preferred Stock includes 273 shares of Series F Preferred Stock which votes on an as-converted basis into shares of common stock, subject to limitation per holder of 9.99% of the total issued and outstanding voting shares, including any common stock beneficially owned. |
|
(3) |
Empery Asset Management LP, the authorized agent of Empery Asset Master Ltd (“EAM”), Empery Tax Efficient, LP (“ETE”) and Empery Tax Efficient II, LP (“ETE II”), has discretionary authority to vote and dispose of the shares held by these funds and may be deemed the beneficial owner of these shares. Martin Hoe and Ryan Lane, in their capacity as investment managers of Empery Asset Management LP, may also be deemed to have investment discretion and voting power over the shares held by these funds. These funds, Mr. Hoe and Mr. Lane each disclaim any beneficial ownership of these shares. Amount beneficially owned includes 163,268,085 shares of common stock issuable upon conversion of the Convertible Series F Preferred Stock or the 2016 Note within 60 days of March 17, 2017, both of which are subject to beneficial ownership limitations on conversion of 9.99% of the total issued and outstanding immediately following conversion. Amount of Series F Preferred Stock includes 1,202 shares of Series F Preferred Stock which votes on an as-converted basis into shares of common stock, subject to limitation per holder of 9.99% of the total issued and outstanding voting shares, including any common stock beneficially owned. |
|
(4) |
Sabby Management, LLC serves as the investment manager of Sabby Healthcare Master Fund, Ltd. Hal Mintz is the manager of Sabby Management, LLC. Each of Sabby Management, LLC and Hal Mintz disclaims beneficial ownership over the securities, except to the extent of its pecuniary interest therein. Amount beneficially owned includes 163,268,085 shares of common stock issuable upon conversion of the Convertible Series F Preferred Stock or the 2016 Note within 60 days of March 17, 2017, both of which are subject to beneficial ownership limitations on conversion of 9.99% of the total issued and outstanding immediately following conversion. Amount of Series F Preferred Stock includes 1,407 shares of Series F Preferred Stock which votes on an as-converted basis into shares of common stock, subject to limitation per holder of 9.99% of the total issued and outstanding voting shares, including any common stock beneficially owned. |
|
(5) |
Tenor Capital Management Company, L.P., the investment manager to Alto Opportunity Master Fund, SPC, has discretionary authority to vote and dispose of the shares held by Alto Opportunity Master Fund, SPC and may be deemed to be the beneficial owner of these shares. Robin Shah, in his capacity as partner of Tenor Capital Management Company, L.P., may also be deemed to have investment discretion and voting power over the shares held by Alto Opportunity Master Fund, SPC.. Amount beneficially owned includes 163,268,085 shares of common stock issuable upon conversion of the Convertible Series F Preferred Stock or the 2016 Note within 60 days of March 17, 2017, both of which are subject to beneficial ownership limitations on conversion of 9.99% of the total issued and outstanding immediately following conversion. Amount of Series F Preferred Stock includes 404 shares of Series F Preferred Stock which votes on an as-converted basis into shares of common stock, subject to limitation per holder of 9.99% of the total issued and outstanding voting shares, including any common stock beneficially owned |
Change in Control
The Company is not aware of any arrangement that might result in a change in control in the future. The Company has no knowledge of any arrangements, including any pledge by any person of our securities, the operation of which may at a subsequent date result in a change in the Company’s control.
78
Equity Compensation Plan Information
The following table sets forth information as of December 31, 2016 relating to all of our equity compensation plans:
|
Plan Category |
|
(a) Number of Shares to be Issued upon Exercise of Outstanding Options |
|
|
|
(b) Weighted- average Exercise Price of Outstanding Options |
|
|
|
(c) Number of Securities Remaining Available for Future Issuance under Equity Compensation Plans (Excluding Securities Referenced in Column (a)) |
|
|
|||
|
Equity compensation plans approved by stockholders |
|
70 |
|
(1) |
|
$145.0 million |
|
(2) |
|
|
— |
|
(3) |
||
|
Equity compensation plans not approved by Stockholders |
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
Total |
|
70 |
|
(1) |
|
$145.0 million |
|
(2) |
|
|
— |
|
(3) |
||
|
(1) |
Includes options outstanding under our 2014 Incentive Plan and 2014 Stock Option Plan |
|
(2) |
Represents weighted-average exercise price per share of common stock acquirable upon exercise of outstanding stock options. |
|
(3) |
Stock option awards may no longer be granted under the 2014 Incentive Plan, the 2014 Stock Option Plan or 2006 Stock Option Plan. |
On December 11, 2015 we completed a one-for-sixty reverse stock split, on March 30, 2016 we completed a one-for-35 reverse stock split, on September 16, 2016 we completed a one-for-eighty reverse stock split and on December 28, 2016 we completed a one-for-three hundred reverse stock split. Proportional adjustments were made to the number of securities to be issued upon exercise of outstanding options.
The Great Basin Scientific, Inc. 2014 Omnibus Incentive Plan
In connection with our IPO, we adopted our Omnibus Plan, which was approved by our board of directors and stockholders. Our stockholders also approved an amendment to our Omnibus Plan on June 2, 2015. The compensation committee of our board of directors (also referred to herein as the “committee”) has the authority to administer the Omnibus Plan and has full power and authority to determine when and to whom awards will be granted, and the type, amount, form of payment and other terms and conditions of each award, consistent with the provisions of the Omnibus Plan. Subject to the provisions of the Omnibus Plan, the committee may amend or waive the terms and conditions, or accelerate the exercisability, of an outstanding award. The committee has authority to interpret the Omnibus Plan and establish rules and regulations for the administration of the Omnibus Plan. In addition, the Omnibus Plan provides that our board of directors may generally exercise the powers of the committee at any time. Any employee, officer, non-employee directors, consultant, independent contractor or advisor providing services to us or any of our affiliate or any such person to whom an offer of employment or engagement with us or any of our affiliates is extended, who is selected by the committee, is eligible to receive awards under the Omnibus Plan.
The aggregate number of shares of common stock that may be issued under all stock-based awards made under the Omnibus Plan is 19 shares, which adjusts automatically on the first day of each fiscal quarter to an amount equal to the greater of (i) fifteen percent (15%) of the number of shares of common stock outstanding on the last day of the immediately preceding fiscal quarter or (ii) 1 share, provided that this amount cannot exceed 19 shares. Any shares of our common stock subject to any award that is terminated or forfeited without delivery of any shares will be available for future awards under the Omnibus Plan. The shares of common stock issuable under the Omnibus Plan may be drawn from shares of authorized but unissued common stock or from shares of common stock that we acquire. No eligible person may be granted any stock options, stock appreciation rights or performance awards denominated in shares of our common stock, for more than 120 shares of our common stock in the aggregate in any calendar year.
79
If the committee or our board of directors determines that any dividend or other distribution (whether in the form of cash, shares of our common stock, other securities or other property), recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, repurchase or exchange of shares of our common stock or other securities, issuance of warrants or other rights to purchase shares of our common stock or other securities or other event identified by the committee as affecting shares of our common stock such that an adjustment is necessary or appropriate in order to prevent dilution or enlargement of the benefits or potential benefits intended to be made available under the Omnibus Plan, then the committee or the board of directors shall, in such manner as it may deem equitable, adjust any or all of (i) the number and type of shares of common stock (or other securities or other property) that thereafter may be made the subject of awards, (ii) the number and type of shares of common stock (or other securities or other property) subject to outstanding awards, (iii) the purchase price or exercise price with respect to any award and (iv) the share limitations contained in the Omnibus Plan.
Under the Omnibus Plan, the committee is permitted and authorized to make the following grants to all eligible persons:
|
|
• |
Stock Options. The committee may grant stock options to any person eligible under the Omnibus Plan, including options intended to qualify as incentive stock options, as defined in Section 422 of the Code. The holder of an option will be entitled to purchase a number of shares of our common stock at a specified exercise price during a specified time period, all as determined by the committee. The exercise price of an option may not be less than 100% of the fair market value of our common stock on the date of grant, or in the case of incentive stock options, 110% of the fair market value of our common stock with respect to holders of more than 10% of our common stock. The fair market value of our common stock will be the closing sale price as quoted on The NASDAQ Capital Market on the date of grant. The Omnibus Plan permits payment of the exercise price to be made by cash, shares of our common stock, other securities, other awards or other property. The shares subject to each option will generally vest in one or more installments over a specified period of service measured from the grant date. |
|
|
• |
Stock Appreciation Rights. The holder of a stock appreciation right (a “SAR”) is entitled to receive the excess of the fair market value (calculated as of the exercise date or, at the committee’s discretion, as of any time during a specified period before or after the exercise date) of a specified number of shares of our common stock over the grant price of the SAR, as determined by the committee, paid solely in shares of common stock. SARs vest and become exercisable in accordance with a vesting schedule established by the committee. |
|
|
• |
Restricted Stock and Restricted Stock Units. The holder of restricted stock will own shares of our common stock subject to restrictions imposed by the committee (including, for example, restrictions on transferability or on the right to vote the restricted shares or to receive any dividends with respect to the shares) for a specified time period determined by the committee. The restrictions, if any, may lapse or be waived separately or collectively, in installments or otherwise, as the committee may determine. The holder of restricted stock units will have the right, subject to any restrictions imposed by the committee, to receive shares of our common stock at some future date determined by the committee. |
|
|
• |
Performance Awards. Performance awards give participants the right to receive payments in cash, stock or property based solely upon the achievement of certain performance goals during a specified performance period. Subject to the terms of the Omnibus Plan, the performance goals to be achieved during any performance period, the length of any performance period, the amount of any performance award granted, the amount of any payment or transfer to be made pursuant to any performance award and any other terms and conditions of any performance award is determined by the committee. No eligible person may be granted performance awards in excess of shares of our common stock (subject to adjustment in the event of a stock split or similar event) in the aggregate in any taxable year. |
|
|
• |
Dividend Equivalents. The committee may grant dividend equivalents under which the participant is entitled to receive payments (in cash, shares of common stock, other securities, other awards or other property as determined in the discretion of the committee) equivalent to the amount of cash dividends paid by us to holders of shares of common stock with respect to a number of shares of common stock determined by the committee. |
80
The term of awards will not be longer than ten years, or in the case of incentive stock options, longer than five years with respect to holders of more than 10% of our common stock. The committee may permit accelerated vesting of an award upon the occurrence of certain events, including a change in control, regardless of whether the award is assumed, substituted or otherwise continued in effect by the successor corporation. The acceleration of vesting in the event of a change in the ownership or control may be seen as an anti-takeover provision and may have the effect of discouraging a merger proposal, a takeover attempt or other efforts to gain control of us.
Awards under the Omnibus Plan may be subject to performance goals, including revenue, cash flow, gross profit, earnings before interest and taxes, earnings before interest, taxes, depreciation and amortization, net earnings, earnings per share, margins (including one or more of gross, operating and net income margins), returns (including one or more of return on assets, equity, investment, capital, revenue and total stockholder return), stock price, economic value added, working capital, market share, cost reductions, workforce satisfaction and diversity goals, employee retention, customer satisfaction, completion of key projects and strategic plan development and implementation. The goals may reflect absolute entity or business unit performance or a relative comparison to the performance of a peer group of entities or other external measure of the selected performance criteria.
Unless earlier discontinued or terminated by the board of directors, the Omnibus Plan will expire on the tenth anniversary of the Omnibus Plan’s effective date. No awards may be made after that date. However, unless otherwise expressly provided in the Omnibus Plan or an applicable award agreement, any award granted under the Omnibus Plan prior to expiration may extend beyond the end of such period through the award’s normal expiration date. Our board of directors may amend, suspend or terminate the Omnibus Plan at any time, provided that our board of directors will get stockholder approval when necessary to not violate the rules of The NASDAQ Capital Market, to increase the number of shares of common stock authorized under the Omnibus Plan, to reprice options or SARs, to permit the grant of options or SARs with an exercise price less than the fair market value of the common stock, to prevent the grant of options or SARs that would qualify under Section 162(m) of the Code or increase the maximum term permitted for options and SARs as specified in the Omnibus Plan. The committee may not amend an outstanding award in a manner that adversely affects the holder of the award without the holder’s consent.
The Great Basin Scientific, Inc. 2014 Stock Option Plan
The Great Basin Scientific, Inc. 2014 Stock Option Plan, which we refer to as the 2014 Stock Option Plan, was adopted by our board of directors on April 18, 2014 and approved by our stockholders on April 21, 2014 and became effective on April 18, 2014. The compensation committee of our board of directors has authority to administer the 2014 Stock Option Plan and will have full power and authority to determine when and to whom awards will be granted, and the type, amount, form of payment and other terms and conditions of each award, consistent with the provisions of the 2014 Stock Option Plan. Subject to the provisions of the 2014 Stock Option Plan, the compensation committee may amend or waive the terms and conditions, or accelerate the exercisability, of an outstanding award. The compensation committee has authority to interpret the 2014 Stock Option Plan and establish rules and regulations for the administration of the 2014 Stock Option Plan. In addition, our board of directors may generally exercise the powers of the compensation committee at any time. Any employee, officer, consultant, independent contractor or director providing services to us or any of our affiliates, who is selected by the compensation committee, is eligible to receive awards under the 2014 Stock Option Plan.
Any shares of common stock that are used by a participant as full or partial payment to us of the purchase price relating to an award, or in connection with the satisfaction of tax obligations relating to an award, shall again be available for granting awards (other than incentive stock options) under the 2014 Stock Option Plan. Additionally, shares of our common stock subject to any award that are terminated or forfeited without delivery of any shares will be available for future awards under the 2014 Stock Option Plan. The shares of common stock issuable under the 2014 Stock Option Plan may be drawn from shares of authorized but unissued common stock or from shares of common stock that we acquire. No eligible person may be granted any award or awards under the 2014 Stock Option Plan, the value of which award or awards is based solely on an increase in the value of shares of common stock after the date of grant of such award or awards, and which is intended to represent “qualified performance
81
based compensation” with the meaning of Section 162(m) of Code, for more than shares of our common stock (subject to adjustment in the event of a stock split or similar corporate event), in the aggregate in any taxable year.
If the compensation committee determines that any dividend or other distribution (whether in the form of cash, shares of our common stock, other securities or other property), recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, repurchase or exchange of shares of our common stock or other securities, issuance of warrants or other rights to purchase shares of our common stock or other securities or other event identified by the compensation committee as affecting shares of our common stock such that an adjustment is necessary or appropriate in order to prevent dilution or enlargement of the benefits or potential benefits intended to be made available under the 2014 Stock Option Plan, then the compensation committee shall, in such manner as it may deem equitable, adjust any or all of (i) the number and type of shares of common stock (or other securities or other property) that thereafter may be made the subject of awards, (ii) the number and type of shares of common stock (or other securities or other property) subject to outstanding awards, (iii) the purchase price or exercise price with respect to any award and (iv) the share limitations contained in the 2014 Stock Option Plan.
Under our 2014 Stock Option Plan, the compensation committee is permitted and authorized to make the following grants to all eligible persons:
|
|
• |
Stock Options. The compensation committee may grant stock options to officers and other employees intended to qualify as incentive stock options, as defined in Section 422 of the Code, and may also grant options to employees, consultants, independent contractors and directors that do not qualify as incentive stock options. The holder of an option will be entitled to purchase a number of shares of our common stock at a specified exercise price during a specified time period, all as determined by the compensation committee. The exercise price of an option may not be less than 100% of the fair market value of our common stock on the date of grant, or in the case of incentive stock options, 110% of the fair market value of our common stock with respect to holders of more than 10% of our common stock. The fair market value of our common stock will be the closing sale price as quoted on The NASDAQ Capital Market on the date of grant. The 2014 Stock Option Plan permits payment of the exercise price to be made by cash, shares of our common stock, other securities, other awards or other property. The shares subject to each option will generally vest in one or more installments over a specified period of service measured from the grant date. No employee may be granted stock options to the extent the aggregate fair market value (determined as of the time each option is granted) of the common stock with respect to which any such options are exercisable would exceed $100,000. |
The term of awards will not be longer than ten years, or in the case of incentive stock options, longer than five years with respect to holders of more than 10% of our common stock. The compensation committee may permit accelerated vesting of an award upon the occurrence of certain events, including a change in control, regardless of whether the award is assumed, substituted or otherwise continued in effect by the successor corporation. The acceleration of vesting in the event of a change in the ownership or control may be seen as an anti-takeover provision and may have the effect of discouraging a merger proposal, a takeover attempt or other efforts to gain control of us.
Awards under the 2014 Stock Option Plan may be subject to performance goals, including revenue, cash flow, gross profit, earnings before interest and taxes, earnings before interest, taxes, depreciation and amortization, net earnings, earnings per share, margins (including one or more of gross, operating and net income margins), returns (including one or more of return on assets, equity, investment, capital, revenue and total stockholder return), stock price, economic value added, working capital, market share, cost reductions, workforce satisfaction and diversity goals, employee retention, customer satisfaction, completion of key projects and strategic plan development and
82
implementation. The goals may reflect absolute entity or business unit performance or a relative comparison to the performance of a peer group of entities or other external measure of the selected performance criteria.
Unless earlier discontinued or terminated by the board of directors, the 2014 Stock Option Plan will expire on April 17, 2024. No awards may be made after that date. However, unless otherwise expressly provided in an applicable award agreement, any award granted under the 2014 Stock Option Plan prior to expiration may extend beyond the end of such period through the award’s normal expiration date. Our board of directors may amend, suspend or terminate the 2014 Stock Option Plan at any time, provided that our board of directors will get stockholder approval when necessary to not violate the rules of The NASDAQ Capital Market, to allow the grant of incentive stock options, to increase the number of shares of common stock authorized under the 2014 Stock Option Plan, to grant or reprice options or SARs with an exercise price less than the fair market value of the common stock, or to prevent the grant of options or SARs that would qualify under Section 162(m) of the Code. The compensation committee may not amend an outstanding award in a manner that adversely affects the holder of the award without the holder’s consent.
We do not intend to make any future stock options grants under the 2014 Stock Option Plan, as all future grants will be made pursuant to the Omnibus Plan.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Since January 1, 2015, we were a participant in certain transactions and relationships with related persons as more fully described below.
Convertible Notes and Related Warrants
We have debt obligations to certain persons in connection with convertible notes with related persons as described below. Each of these notes converts to shares of common stock as set forth below.
In July 2014, we entered into a note agreement for $500,000 with Spring Forth Investments, LLC a company owned by Mr. David Spafford, a director. The original maturity date for the note was July 18, 2015, which we extended to July 18, 2016 by giving notice and paying an extension fee of $10,000. We extended the maturity date again to July 18, 2017 without an extension fee. The note pays interest at an annual rate of 20% and is paid monthly. We prepaid the last three months of interest for a total of $25,000 at the time of issuance of the note. We paid $66,667 and $100,000 in interest to Spring Forth Investments, LLC for the years ended December 31, 2016 and 2015, respectively. In conjunction with issuing the note, we issued to Spring Forth Investments 20,000 Series D Units. The proceeds from the financing were used for general working capital purposes.
In February 2015, we entered into another loan agreement for $250,000 with Spring Forth Investments, LLC. The loan had an interest rate of 12% per year and matured the earlier of (i) 90 days from the date of the loan agreement, or (ii) five days after we closed a registered public offering of our securities. In April 2015, we paid the principal amount together with accrued interest in the amount of $4,192 and a termination fee of $12,500.
In relation to the 2015 Note financing on December 30, 2015, the Utah Autism Foundation, an entity for which Mr. Spafford serves on the Board of Trustees and as a Founder Trustee (the “Foundation”), and Spring Forth Investments LLC agreed to enter into subordination agreements with the collateral agent in the 2015 Note financing whereby each agreed to subordinate their debt to the notes issued in the convertible note financing. As consideration for their agreement we issued to them 2015 Subordination Warrants exercisable for 2 shares of common stock. The 2015 Subordination Warrants included an adjustment provision which provides that the number of common shares the 2015 Subordination Warrants are exercisable into will increase on December 31, 2016 to be 0.5% of the sum of the number of shares actually outstanding on December 31, 2016 plus the number of shares of common stock deemed to be outstanding pursuant to all outstanding options, warrants or convertible securities of the Company. Accordingly, on December 31, 2016, we adjusted the 2015 Subordination Warrants such that they are exercisable into 71,131 shares of common stock. All other terms remain the same as the original 2015 Subordination Warrant.
83
In relation to the 2016 Note financing on July 1, 2016, the Foundation and Spring Forth Investments LLC agreed to enter into subordination agreements with the collateral agent in the 2016 Note financing whereby each agreed to subordinate their debt to the notes issued in the convertible note financing. As consideration for their agreement, we issued to the entities warrants exercisable for 70 shares of common stock.
Master Lease Agreement with Onset and Related Warrants and Letters of Credit
We entered into a Master Lease Agreement to provide for the sale-leaseback of molecular diagnostic analyzers. We have completed two lease schedules under this lease agreement: Lease Schedule 001 dated October 16, 2013, amended December 10, 2013, for the sale of 125 molecular diagnostic analyzers for a purchase price of $2,500,000, which are being leased back for 36 monthly payments of $74,875 and Lease Schedule 002 dated March 14, 2014, amended March 18, 2014, for the sale of 75 molecular diagnostic analyzers for a purchase price of $1,500,000, which are being leased back for 24 monthly payments of $64,665. At the end of the lease term of Schedule 001, the lease will automatically renew for twelve additional months at the current monthly rate unless we give written notice 150 days prior to the end of the lease. If timely notice is given we have the opportunity to: 1) repurchase the analyzers for a purchase price determined by lessor not to exceed forty percent of the original costs; or 2) terminate the lease, return the property and enter into a new lease with new property that replaces the property of the old lease. Both we and the lessor will have the right to reject any terms of option 1 or 2 and if rejected, the 12 month extension will apply. Schedule 002 includes similar end of term options as found in Schedule 001, except that if we give timely notice, we have the option to purchase the analyzers at a price to be determined by lessor and us. Schedule 002 also includes a provision that during the first 14 months of the base period of the Schedule, provided there is no event of default and if we complete a successful capital raise, then lessor will use commercially acceptable best efforts to rewrite this Schedule 002 at more favorable terms. We are accounting for these transactions as a capital lease sale-leaseback in accordance with ASC 840 “Leases.”
Our obligations pursuant to the sale-leaseback agreement are secured by letters of credit obtained by Spring Forth Investments, LLC, an entity controlled by David Spafford, and the Foundation in an aggregate amount of $3,000,000. These letters of credit were issued by a bank for the benefit of the lessor. Pursuant to three reimbursement agreements we entered into with those entities in connection with the letters of credit, we have agreed to pay each of them 10% interest per annum on the total amount of the letter of credit. As of March 17, 2017, we have paid $664,500 in interest on the letters of credit. Under the reimbursement agreements, we are also obligated to reimburse those third parties for any draws made under the letters of credit. As of March 17, 2017, no draws on either letter of credit had taken place. Our obligations under the reimbursement agreements are secured by a security interest in all of our assets pursuant to security agreements effective the dates of the respective lease schedule.
Ryan Ashton, our chief executive officer, and David Spafford, one of our directors, each personally guaranteed our obligations under the sale-lease agreement. These guarantees cover “the full amount of liability for any amounts due” from us to the lessor under the lease agreement. On November 25, 2013, we issued Mr. Ashton warrants to purchase 1 share of common stock and Mr. Spafford warrants to purchase 1 share of common stock, each in compensation for their personal guarantees of our obligations under the lease agreement, with an exercise price of $100.8 million per share.
The foregoing constitutes a summary of the material terms of the lease documents and is qualified in its entirety by the full text of the lease documents, copies of which are filed as exhibits to this report.
84
On February 16, 2010 we entered into a voting agreement under which certain holders of our preferred stock, including entities affiliated with certain of our directors, have agreed to vote in a certain way on certain matters, including with respect to the election of directors. In connection with the issuance of Series D Preferred Stock, the Voting Agreement was amended and restated on April 21, 2014 and July 30, 2014. Pursuant to the July 2014 amendment and restatement, (i) the parties agreed to vote their shares to set the size of the board of directors at five directors, (ii) the holders of common stock, voting as a separate class, elect one director to our board of directors (initially Ryan Ashton), and (iii) for so long as Hitachi owns 5% of the issued and outstanding shares of our capital stock, Hitachi will be entitled to elect one director. Upon the closing of our IPO, the board of directors election voting provisions contained in the voting agreement terminated; however, pursuant to the terms of the Series D Stock Purchase Agreement and our Certificate, Hitachi will continue to be entitled to elect one director.
Investor Rights Agreement
On February 16, 2010, we entered into an investor rights agreement with the holders of our outstanding Series A Preferred Stock, including entities affiliated with certain of our directors. On November 26, 2013, the investor rights agreement was amended in connection with the issuance of Series C Preferred Stock and Series C-1 Preferred Stock. On April 21, 2014, the investor rights agreement was amended in connection with the issuance of Series D Preferred Stock and on July 30, 2014 the investor rights agreement and was further amended in connection with the issuance of additional shares of Series D Preferred Stock. In connection with our IPO, each share of Series A, Series C, Series C-1 and Series D Preferred Stock was converted into one share of our common stock.
Transactions with Certain Securityholders
On February 19, 2016, the Company entered into subscription agreements with certain investors (the “February Investors”) relating to the sale and issuance by the Company of 39,200,000 Units (the “February Units”), at a price of $0.16 per February Unit. The Company issued 47 shares of common stock and Series E Warrants to purchase 71 shares of common stock. The Company received gross proceeds from the February 2016 Offering of approximately $6.3 million and net proceeds, after deducting agent’s fees and offering expenses, of approximately $5.9 million. In relation to the February 2016 Offering, two of the Investors were Hudson Bay Master Fund Ltd. (“Hudson Bay”) and Empery Asset Management LP (through Empery Tax Efficient, LP, Empery Tax Efficient II, LP and Empery Asset Master Fund, Ltd.) (“Empery”). Both Hudson Bay and Empery participated on the same terms as other February Investors in the public offering. In connection with the offering, Hudson Bay purchased 6.25 million February Units for gross proceeds to the Company of $1 million and Empery (though its three entities) purchased an aggregate of 9.375 million February Units for gross proceeds to the Company of $1.5 million. Both Hudson Bay and Empery were beneficial owners of 9.99% of our issued and outstanding common stock on the date of the transaction due to their ownership of 2015 Notes purchased on December 30, 2015.
On April 4, 2016, the Company entered into certain warrant exchange agreements each by and between us and a holder of our outstanding Series E Warrants, pursuant to which we and each such holder agreed to exchange outstanding Series E Warrants for shares of common stock. All of the issued and outstanding Series E Warrants were exchanged for 28 shares of common stock. As holders of Series E Warrants, both Hudson Bay and Empery participated in the exchange. Hudson Bay exchanged 6,250,000 Series E Warrants for 5 shares of common stock and Empery exchanged 9,375,000 Series E Warrants for 8 shares of common stock. Both Hudson Bay and Empery were beneficial owners of 9.99% of our issued and outstanding common stock on the date of the transaction due to their ownership of 2015 Notes purchased on December 30, 2015 and the Series E Warrants purchased in the February 2016 Offering.
On May 26, 2016, the Company entered into subscription agreements with certain investors (the “May Investors”) relating to the sale and issuance by the Company of up to 3,160,000 Units (the “May 2016 Units”), at a price of $1.90 per May 2016 Unit. The Company issued 163 shares of common stock and Series H Warrants to purchase 163 shares of common stock. The Company received gross proceeds from the offering of the May Units of approximately $6 million and net proceeds, after deducting agent’s fees and offering expenses, of approximately $5.6 million. In relation to the May 2016 Offering, two of the Investors were Hudson Bay and Empery (through Empery Tax Efficient, LP, Empery Tax Efficient II, LP and Empery Asset Master Limited). Both Hudson Bay and Empery participated on the same terms as other May Investors in the
85
public offering. In connection with the offering, Hudson Bay purchased 395,000 May Units for gross proceeds to the Company of approximately $0.75 million and Empery (though its three entities) purchased an aggregate of 523,315 May Units for gross proceeds to the Company of approximately $0.99 million. Both Hudson Bay and Empery were beneficial owners of 9.99% of our issued and outstanding common stock on the date of the transaction due to their ownership of 2015 Notes purchased on December 30, 2015.
On June 29, 2016, the Company entered into the 2016 SPA with certain investors named on the Schedule of Buyers attached to the 2016 SPA (each a “2016 Note Buyer”) pursuant to which the Company issued, on July 1, 2016, $75 million in principal face amount of 2016 Notes and related Series H warrants. The Buyers purchased the 2016 Notes and related Series H warrants through payment of cash at a discount by paying $906.67 for each $1,000 in principal face amount of 2016 Notes and related Series H Warrants. The 2016 Notes do not bear any ordinary interest. The Company received total gross proceeds of $68 million from the issuance of the 2016 Notes and related Series H Warrants. In relation to the issuance of the 2016 Notes, two of the 2016 Note Buyers were Hudson Bay and Empery (through Empery Tax Efficient, LP, Empery Tax Efficient II, LP and Empery Asset Master Limited). Both Hudson Bay and Empery participated on the same terms as other 2016 Note Buyers in the offering. In connection with the offering, Hudson Bay purchased approximately $57.8 million aggregate principal amount of 2016 Notes and approximately 43.36 million Series H warrants for gross proceeds to the Company of approximately $52.4 million and Empery (though its three entities) purchased an aggregate of approximately $4.4 million aggregate principal amount of 2016 Notes and approximately 3.3 million Series H warrants for gross proceeds to the Company of approximately $4 million. Both Hudson Bay and Empery were beneficial owners of 9.99% of our issued and outstanding common stock on the date of the transaction due to their ownership of 2015 Notes purchased on December 30, 2015 and Series G warrants. The largest amount of debt owned under the 2016 Notes to Hudson Bay since issuance is approximately $52.4 million. The largest amount of debt owed under the 2016 Notes to Empery since issuance is approximately $4.4 million. As of March 17, 2017, we have issued 1,153,596,582 shares of common stock to Hudson Bay upon conversion of principal in the amount of $3,854,077. As of March 17, 2017 we have issued 87,403,614 shares to Empery upon conversion of principal in the amount of $308,519. No interest has been paid as the Notes do not bear interest. See “Description of Certain Indebtedness — 2016 Convertible Note Financing” for more details regarding the 2016 Note terms and conditions.
On November 3, 2016, the Company exchanged all of the remaining 2015 Notes outstanding, approximately $8.4 million in aggregate principal amount thereof, for 8,436 shares of a new class of Series F Convertible Preferred Stock, par value $0.001 per share, pursuant to a certificate of designations, preferences and rights of the Series F Preferred Stock. As holders of the 2015 Notes, Hudson Bay and Empery participated in the exchange. Hudson Bay exchanged $3.5 million aggregate principal amount of 2015 Notes for 3,538 Series F Preferred Stock and Empery (through its three entities) exchanged $1.8 million of aggregate principal amount of 2015 Notes for 1,757 Series F Preferred Stock. Both Hudson Bay and Empery were beneficial owners of 9.99% of our issued and outstanding common stock on the date of the transaction due to their ownership of 2016 Notes purchased on December 30, 2015 and Series G warrants.
Other Relationships
Sandra Nielsen, who became the domestic partner of Ryan Ashton in 2012, is employed by us as our Senior Vice President of Sales, Marketing and Human Resources. In 2015, Ms. Nielsen received a salary of $205,000, commissions of $201,576 and a bonus of $61,500. In 2016, Ms. Nielsen received a salary of $216,667 and commissions of $148,439.
Indemnification Provisions
On February 27, 2017 we entered into indemnification agreements with each of our directors and officers. These indemnification agreements, and our Certificate and Bylaws, require us to indemnify our directors and officers to the fullest extent permitted by Delaware law.
Policies and Procedures for Transactions with Related Persons
Our Audit Committee is responsible for reviewing any potential conflict of interest situations, on an ongoing basis, any future proposed transaction, or series of transactions, with related persons, and either approve or disapprove each reviewed transaction or series of related transactions with related persons.
86
We have adopted a written policy and procedures with respect to related person transactions, which includes specific provisions for the approval of related person transactions. Pursuant to this policy, related person transactions include a transaction, arrangement or relationship or series of similar transactions, arrangements or relationships, in which we and certain enumerated related persons participate, the amount involved exceeds the lesser of $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years, and the related person has a direct or indirect material interest.
If a related person transaction is identified, such transaction must be reviewed and approved or ratified by our Audit Committee. If it is impracticable for our Audit Committee to review such transaction, the transaction will be reviewed by the chair of our Audit Committee, whereupon the chair of our Audit Committee will report to the Audit Committee the approval or disapproval of such transaction.
In reviewing and approving related person transactions, the Audit Committee, or its chair, considers all information that the Audit Committee, or its chair, believes to be relevant and important to a review of the transaction. The Audit Committee or its chair, as the case may be, approves only those related person transactions that are determined to be in, or not inconsistent with, our best interests and that of our stockholders, taking into account all available relevant facts and circumstances available to the Audit Committee or the chair. These facts and circumstances will typically include, but not be limited to, the benefits of the transaction to us; the impact on a director’s independence in the event the related person is a director, an immediate family member of a director or an entity in which a director is a partner, stockholder or executive officer; the availability of other sources for comparable products or services; the terms of the transaction; and the terms of comparable transactions that would be available to unrelated third parties or to employees generally. No member of the Audit Committee will participate in any review, consideration or approval of any related person transaction with respect to which the member or any of his or her immediate family members is the related person.
Director Independence
The board of directors has determined that Mr. Calhoun, Mr. Labrum and Mr. Chawla are independent directors as defined under Section 5605(a)(2) of The NASDAQ Stock Market Rules.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Principal Accountant Fees and Services
The following table presents fees for professional services rendered by our principal accountants over the last two fiscal years for the audit of the Company’s annual financial statements and review of financial statements included in the Company’s Forms 10-Q, and fees billed for other services. BDO USA, LLP was our principal accountant as of December 31, 2016 and Mantyla McReynolds, LLC was our principal accountant as of December 31, 2015.
|
|
|
2016 |
|
|
2015 |
|
||
|
Audit Fees (1) |
|
$ |
349,831 |
|
|
$ |
360,696 |
|
|
All Other Fees |
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
349,831 |
|
|
$ |
360,696 |
|
|
(1) |
Audit Fees consist of fees for the audit of the Company’s annual financial statements included in Form 10-K and services in connection with the Company’s various statutory and regulatory filings. Audit fees also include fees related to the reviews of interim financial information included in Forms 10-Q and for consent or comfort letter procedures performed in conjunction with the Company filing a registration statement or completing financial transactions during the respective fiscal years. |
Pre-approval Policies
Our policy has been for the Audit Committee to pre-approve all audit, audit-related and non-audit services performed by our independent auditors and to subsequently review the actual fees and expenses paid to our independent auditors. Accordingly, the Audit Committee pre-approved all audit, audit-related and non-audit services performed by our independent auditors and subsequently reviewed the actual fees and expenses paid to Mantyla
87
McReynolds, LLC and BDO USA, LLP. The Audit Committee has determined that the fees paid to Mantyla McReynolds, LLC and BDO USA, LLP for services are compatible with maintaining Mantyla McReynolds, LLC’s and BDO USA, LLP’s independence as our auditors.
88
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
|
(a) |
Financial Statements: |
See “Index to Financial Statements” set forth on page F-1.
|
(b) |
Financial Statement Schedules: |
All schedules for which provision is made in the applicable accounting requirements of the Securities and Exchange Commission are not required or the required information has been included within the financial statements or the notes thereto.
|
(c) |
Exhibits: |
|
Exhibit No. |
Description |
||||||
|
|
|
|
|||||
|
3.1* |
|
Seventh Amended and Restated Certificate of Incorporation of Great Basin Scientific, Inc., as amended. |
|||||
|
|
|
||||||
|
3.2 |
|
Amended and Restated Bylaws of Great Basin Scientific, Inc. (2) |
|||||
|
|
|
||||||
|
4.1 |
|
Specimen certificate evidencing shares of common stock (2) |
|||||
|
|
|
||||||
|
4.2 |
|
Amended and Restated Voting Agreement dated as of July 30, 2014 (1) |
|||||
|
|
|
||||||
|
4.3 |
|
Third Amended and Restated Investor Rights Agreement dated as of April 21, 2014 (1) |
|||||
|
|
|
||||||
|
4.4 |
|
Form of Second Amended and Restated Series C Warrant (8) |
|||||
|
|
|
||||||
|
4.5 |
|
Form of Warrant to Purchase common stock (4) |
|||||
|
|
|
||||||
|
4.6 |
|
Form of Warrant to Purchase common stock or Preferred Stock (4) |
|||||
|
|
|
||||||
|
4.7 |
|
Form of Warrant to Purchase common stock (4) |
|||||
|
|
|
||||||
|
4.8 |
|
Form of Series A Warrant (3) |
|||||
|
|
|
|
|||||
|
4.9 |
|
Form of Series B Warrant (3) |
|||||
|
|
|
||||||
|
4.10 |
|
Amended and Restated Form of Series C Warrant (amended and restated as of June 23, 2015) (7) |
|||||
|
|
|
||||||
|
4.11 |
|
Form of Unit Purchase Option issued in connection with the Registrant’s follow-on offering (5) |
|||||
|
|
|
||||||
|
4.12 |
|
Form of Representative’s Warrant issued in connection with the Registrant’s initial public offering (3) |
|||||
|
|
|
||||||
|
4.13 |
|
Form of Senior Secured Convertible Note, filed as Exhibit A to the Securities Purchase Agreement (9) |
|||||
|
|
|
||||||
|
4.14 |
|
Form of Series D Warrant, filed as Exhibit B to the Securities Purchase Agreement (9) |
|||||
|
|
|
||||||
|
4.15 |
|
Form of Series E Warrant (16) |
|||||
|
|
|
|
|||||
|
4.16 |
|
Form of Prefunded Series F Warrant (12) |
|||||
|
|
|
|
|||||
|
4.17 |
|
Form of Class A Warrant to Purchase common stock issued to investors pursuant to the Series D Purchase Agreement (1) |
|||||
|
|
|
||||||
|
4.18 |
|
Form of Class B Warrant to Purchase common stock issued to investors pursuant to Series D Purchase Agreement (1) |
|||||
|
|
|
|
|||||
|
4.19 |
|
Form of Series G Warrant (22) |
|||||
|
|
|
|
|||||
89
90
|
Exhibit No. |
Description |
|||||
|
|
|
|
||||
|
|
Employment Agreement with Ryan Ashton (2) |
|||||
|
|
|
|
||||
|
10.21# |
|
Employment Agreement with Jeffrey A. Rona (2) |
||||
|
|
|
|||||
|
10.22# |
|
Employment Agreement with Robert Jenison (2) |
||||
|
|
|
|||||
|
10.23 |
|
Lease Agreement between JTM, Inc. and Great Basin, dated April 26, 2010, as amended by that certain amendment dated July 1, 2012 (South Side Lease) (1) |
||||
|
|
|
|||||
|
10.24 |
|
Lease Agreement between JTM, Inc. and Great Basin, dated September 6, 2012 (North Side Lease) (1) |
||||
|
|
|
|||||
|
10.25 |
|
Loan and Issuance Agreement by and between Great Basin and Spring Forth Investments, LLC, dated July 18, 2014 (1) |
||||
|
|
|
|||||
|
10.26 |
|
Promissory Note dated July 18, 2014 in favor of Spring Forth Investments, LLC (1) |
||||
|
|
|
|||||
|
10.27 |
|
Loan Agreement between Great Basin and Spring Forth Investments, LLC, dated February 12, 2015 (5) |
||||
|
|
|
|||||
|
10.28 |
|
Form of Second Amendment Agreement dated December 7, 2015 (8) |
||||
|
|
|
|||||
|
10.29 |
|
Securities Purchase Agreement dated December 28, 2015 (9) |
||||
|
|
|
|||||
|
10.30 |
|
Registration Rights Agreement, filed as Exhibit C to the Securities Purchase Agreement (9) |
||||
|
|
|
|||||
|
10.31 |
|
Pledge and Security Agreement, filed as Exhibit D to the Securities Purchase Agreement (9) |
||||
|
|
|
|||||
|
10.32 |
|
Amendment Agreement to Securities Purchase Agreement between the Company and certain holders of its Notes and Series D warrants, dated February 8, 2016 (10) |
||||
|
|
|
|||||
|
10.33 |
|
Settlement Agreement between the Company and Dawson James dated February 8, 2016 (11) |
||||
|
|
|
|||||
|
10.34 |
|
Consulting Agreement between the Company and Dawson James dated February 8, 2016 (11) |
||||
|
|
|
|||||
|
10.35 |
|
Form of Amendment Agreement to the Registration Rights Agreement between the Company and certain holders of its convertible notes and Series D warrants, dated February 13, 2016 (13) |
||||
|
|
|
|||||
|
10.36 |
|
Placement Agent Agreement by and between the Company and Roth Capital Partners, LLC for February 2016 Unit offering, dated February 19, 2016 (16) |
||||
|
|
|
|||||
|
10.37 |
|
Office Lease between Bay Pacific East South Temple, LLC and the Registrant executed August 25, 2015 (14) |
||||
|
|
|
|||||
|
10.38 |
|
First Amendment to Lease between Bay Pacific East South Temple, LLC and the Registrant (14) |
||||
|
|
|
|||||
|
10.39 |
|
Form of Amendment Agreement for Series C Warrants (15) |
||||
|
|
|
|
||||
|
10.40 |
|
Form of Subscription Agreement for February 2016 Unit Offering (16) |
||||
|
|
|
|
||||
|
10.41 |
|
Form of Second Amendment Agreement to the Registration Rights Agreement between the Company and certain holders of its convertible notes and Series D warrants, dated February 29, 2016 (17) |
||||
|
|
|
|||||
|
10.42 |
|
Second Amendment to Lease between Bay Pacific East South Temple, LLC and Great Basin Scientific, Inc. (18) |
||||
|
|
|
|||||
|
10.43 |
|
Form of Series E Warrant Exchange Agreement (19) |
||||
|
|
|
|||||
|
10.44 |
|
Form of Third Amendment Agreement to Registration Rights Agreement (20) |
||||
|
|
|
|||||
|
10.45 |
|
Form of Waiver Agreement with subscribers to December 2015 offering, dated May 23, 2016 (21) |
||||
|
|
|
|||||
|
10.46 |
|
Placement Agent Agreement by and between the Company and Roth Capital Partners, LLC for June 2016 Unit offering, dated May 26, 2016 (22) |
||||
91
|
Exhibit No. |
Description |
|||||
|
|
|
|
||||
|
|
|
|||||
|
10.47 |
|
Form of Subscription Agreement for June 2016 Unit offering (22) |
||||
|
|
|
|||||
|
10.48 |
|
Securities Purchase Agreement dated June 29, 2016 (23) |
||||
|
|
|
|
||||
|
10.49 |
|
Form of Pledge and Security Agreement, filed as Exhibit C to the Securities Purchase Agreement (23) |
||||
|
|
|
|
||||
|
10.50 |
|
Form of Waiver Agreement with subscribers to December 2015 offering, dated June 28, 2016 (23) |
||||
|
|
|
|
||||
|
10.51 |
|
Form of Waiver Agreement with subscribers to June 2016 Unit offering, dated June 28, 2016 (23) |
||||
|
|
|
|
||||
|
10.52 |
|
Amendment to Spring Forth Promissory Note, dated July 18, 2016 (25) |
||||
|
|
|
|
||||
|
10.53 |
|
Form of Waiver Agreement with subscribers to December 2015 offering, dated August 17, 2016 (26) |
||||
|
|
|
|
||||
|
10.54 |
|
Form of Waiver Agreement with subscribers to July 2016 offering, dated August 17, 2016 (26) |
||||
|
|
|
|
||||
|
10.55 |
|
Form of Leak-Out Agreement (27) |
||||
|
|
|
|
||||
|
10.56 |
|
Form of Exchange Agreement with subscribers to December 2015 offering, dated October 2, 2016 (28) |
||||
|
|
|
|
||||
|
10.57 |
|
Form of Amended Leak-Out Agreement (29) |
||||
|
|
|
|
||||
|
10.58 |
|
Form of Amended and Restated Exchange Agreement with subscribers to December 2015 offering, dated November 2, 106 (30) |
||||
|
|
|
|
||||
|
10.59 |
|
Form of Fourth Amendment to Registration Rights Agreement (30) |
||||
|
|
|
|
||||
|
10.60 |
|
Form of Waiver Agreement with subscribers to July 2016 offering, dated December 2, 2016 (31) |
||||
|
|
|
|
||||
|
16.1 |
|
Mantyla McReynolds, LLC Letter of Concurrence (24) |
||||
|
|
|
|||||
|
23.1* |
|
Consent of BDO USA, LLP, independent registered public accounting firm |
||||
|
|
|
|||||
|
23.2* |
|
Consent of Mantyla McReynolds, LLC, independent registered public accounting firm |
||||
|
|
|
|
||||
|
24.1* |
|
Power of Attorney (contained on signature page hereto) |
||||
|
|
|
|
||||
|
31.1* |
|
Certification of Principal Executive Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
||||
|
|
|
|
||||
|
31.2* |
|
Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
||||
|
|
|
|||||
|
32.1** |
|
Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
||||
|
|
|
|||||
|
32.2** |
|
Certification of Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
||||
|
|
|
|||||
|
101.INS* |
|
XBRL Instance Document |
||||
|
|
|
|||||
|
101.SCH* |
|
XBRL Taxonomy Extension Schema Linkbase Document |
||||
92
|
Exhibit No. |
Description |
|||||
|
|
|
|
||||
|
|
|
|||||
|
101.CAL* |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
||||
|
|
|
|||||
|
101.DEF* |
|
XBRL Taxonomy Extension Definition Linkbase Document |
||||
|
|
|
|||||
|
101.LAB* |
|
XBRL Taxonomy Extension Label Linkbase Document |
||||
|
|
|
|||||
|
101.PRE* |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
||||
|
* |
Filed herewith |
|
** |
Furnished herewith |
|
@ |
Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a grant of confidential treatment from the SEC |
|
# |
Management contract or compensatory plan or arrangement |
|
(1) |
Filed as an exhibit to Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-197954) filed with the SEC on August 20, 2014, and incorporated herein by reference |
|
(2) |
Filed as an exhibit to Amendment No. 2 to the Registrant’s Registration Statement on Form S-1 (File No. 333-197954) filed with the SEC on September 8, 2014, and incorporated herein by reference |
|
(3) |
Filed as an exhibit to Amendment No. 3 to the Registrant’s Registration Statement on Form S-1 (File No. 333-197954) filed with the SEC on September 23, 2014, and incorporated herein by reference |
|
(4) |
Filed as an exhibit to Amendment No. 4 to the Registrant’s Registration Statement on Form S-1 (File No. 333-197954) filed with the SEC on September 24, 2014, and incorporated herein by reference |
|
(5) |
Filed as an exhibit to Amendment No. 2 to the Registrant’s Registration Statement on Form S-1 (File No. 333-201596) filed with the SEC on February 24, 2015, and incorporated herein by reference |
|
(6) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-3662) filed with the SEC on June 2, 2015, and incorporated herein by reference |
|
(7) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-3662) filed with the SEC on June 23, 2015, and incorporated herein by reference |
|
(8) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on December 7, 2015 and incorporated herein by reference |
|
(9) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on December 28, 2015 and incorporated herein by reference |
|
(10) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on February 8, 2016 and incorporated herein by reference |
|
(11) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on February 8, 2016 and incorporated by reference |
|
(12) |
Filed as an exhibit to Amendment No. 3 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207761) filed with the SEC on February 11, 2016 and incorporated by reference |
|
(13) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on February 16, 2016 and incorporated herein by reference |
|
(14) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on August 28, 2015 and incorporated herein by reference |
|
(15) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on September 21, 2015 and incorporated herein by reference |
|
(16) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on February 19, 2016 and incorporated herein by reference |
|
(17) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on February 29, 2016 and incorporated herein by reference |
|
(18) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on March 16, 2016 and incorporated herein by reference |
|
(19) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on April 4, 2016 and incorporated herein by reference |
|
(20) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on May 12, 2016 and incorporated herein by reference |
93
|
(21) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on May 24, 2016 and incorporated herein by reference |
|
(22) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on May 26, 2016 and incorporated herein by reference |
|
(23) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on June 29, 2016 and incorporated herein by reference |
|
(24) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on July 19, 2016 and incorporated herein by reference |
|
(25) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on August 4, 2016 and incorporated herein by reference |
|
(26) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on August 17, 2016 and incorporated herein by reference |
|
(27) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on September 20, 2016 and incorporated herein by reference |
|
(28) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on October 3, 2016 and incorporated herein by reference |
|
(29) |
Filed as an exhibit to the Registrant’s Current Report on Form 8-K (File No. 001-36662) filed with the SEC on October 17, 2016 and incorporated herein by reference |
None
94
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
Great Basin Scientific, Inc. |
|
|
|
|
|
|
Date: March 22, 2017 |
By: |
/s/ Ryan Ashton |
|
|
|
Ryan Ashton |
|
|
|
President and Chief Executive Officer
|
|
|
|
|
|
Date: March 22, 2017 |
By: |
/s/ Jeffrey A. Rona |
|
|
|
Jeffrey A. Rona |
|
|
|
Chief Financial Officer
|
POWER OF ATTORNEY
Each person whose signature appears below constitutes and appoints Ryan Ashton and Jeffrey A. Rona, and each of them, as his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this annual report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitutes, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
|
Name |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ David Spafford |
|
Director and Executive Chairman |
|
March 22, 2017 |
|
David Spafford |
|
|
||
|
|
|
|
|
|
|
/s/ Kirk K. Calhoun |
|
Director |
|
March 22, 2017 |
|
Kirk K. Calhoun |
|
|
||
|
|
|
|
|
|
|
/s/ Ronald K. Labrum |
|
Director |
|
March 22, 2017 |
|
Ronald K. Labrum |
|
|
||
|
|
|
|
|
|
|
/s/ Sam Chawla |
|
Director |
|
March 22, 2017 |
|
Sam Chawla |
|
|
||
|
|
|
|
|
|
|
/s/ Ryan Ashton |
|
President, Chief Executive Officer and Director (Principal Executive Officer) |
|
March 22, 2017 |
|
Ryan Ashton |
|
|
||
|
|
|
|
|
|
|
/s/ Jeffrey A. Rona |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
March 22, 2017 |
|
Jeffrey A. Rona |
|
|
95
|
Great Basin Scientific, Inc. Audited Financial Statements: |
|
|
|
|
|
F-2 |
|
|
|
|
|
F-4 |
|
|
|
|
|
Statements of Operations for the Years Ended December 31, 2016 and 2015 |
F-5 |
|
|
|
|
Statements of Stockholders’ Deficit for the Years Ended December 31, 2016 and 2015 |
F-6 |
|
|
|
|
Statements of Cash Flows for the Years Ended December 31, 2016 and 2015 |
F-7 |
|
|
|
|
F-8 |
F-1
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Great Basin Scientific, Inc.
Salt Lake City, Utah
We have audited the accompanying balance sheet of Great Basin Scientific, Inc. (“Company”) as of December 31, 2016 and the related statements of operations, stockholders’ deficit, and cash flows for the year then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Great Basin Scientific, Inc. at December 31, 2016, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 3 to the financial statements, the Company has incurred substantial losses from operations, has negative operating cash flows and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ BDO USA, LLP
Salt Lake City, Utah
March 22, 2017
F-2
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Great Basin Scientific, Inc.
Salt Lake City, Utah
We have audited the accompanying balance sheet of Great Basin Scientific, Inc. as of December 31, 2015 and the related statements of operations, stockholders’ deficit, and cash flows for the year then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Great Basin Scientific, Inc. at December 31, 2015, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 3 to the financial statements, the Company has incurred substantial losses from operations causing negative working capital and negative operating cash flows. These issues raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Mantyla McReynolds, LLC
Salt Lake City, Utah
March 1, 2016
F-3
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
1,014,255 |
|
|
$ |
4,787,759 |
|
|
Restricted cash |
|
|
47,066,313 |
|
|
|
13,800,000 |
|
|
Accounts receivable, net |
|
|
479,394 |
|
|
|
411,390 |
|
|
Inventory |
|
|
1,421,572 |
|
|
|
1,133,142 |
|
|
Prepaid and other current assets |
|
|
950,694 |
|
|
|
564,910 |
|
|
Total current assets |
|
|
50,932,228 |
|
|
|
20,697,201 |
|
|
Restricted cash, net of current portion |
|
|
12,344,039 |
|
|
|
— |
|
|
Intangible assets, net |
|
|
42,586 |
|
|
|
119,171 |
|
|
Property and equipment, net |
|
|
10,078,484 |
|
|
|
7,741,991 |
|
|
Total assets |
|
$ |
73,397,337 |
|
|
$ |
28,558,363 |
|
|
Liabilities and Stockholders' Deficit |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
3,855,997 |
|
|
$ |
2,432,459 |
|
|
Accrued expenses |
|
|
6,275,808 |
|
|
|
1,313,149 |
|
|
Current portion of notes payable |
|
|
— |
|
|
|
5,693 |
|
|
Current portion of convertible notes payable |
|
|
60,000,000 |
|
|
|
16,575,000 |
|
|
Notes payable - related party |
|
|
500,000 |
|
|
|
500,000 |
|
|
Current portion of capital lease obligations |
|
|
865,049 |
|
|
|
1,305,426 |
|
|
Total current liabilities |
|
|
71,496,854 |
|
|
|
22,131,727 |
|
|
Convertible notes payable, net of current portion and debt discount |
|
|
15,000,000 |
|
|
|
2,177,657 |
|
|
Capital lease obligations, net of current portion |
|
|
55,912 |
|
|
|
851,410 |
|
|
Derivative liability, net of current portion |
|
|
36,344,180 |
|
|
|
26,592,532 |
|
|
Series F convertible preferred stock |
|
|
5,655,006 |
|
|
|
— |
|
|
Other long term liabilities |
|
|
831,678 |
|
|
|
— |
|
|
Total liabilities |
|
|
129,383,630 |
|
|
|
51,753,326 |
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
Stockholders' deficit: |
|
|
|
|
|
|
|
|
|
Preferred stock, $.001 par value, 5,000,000 shares authorized; Series E convertible preferred stock; 74,380 and 2,860,200 shares authorized, respectively; 74,380 and 88,347 shares issued and outstanding, respectively |
|
|
74 |
|
|
|
88 |
|
|
Common stock, $.0001 par value: 1,500,000,000 and 200,000,000 shares authorized; 764,690 and 489 shares issued and outstanding, respectively |
|
|
76 |
|
|
|
— |
|
|
Additional paid-in capital |
|
|
155,065,690 |
|
|
|
98,708,814 |
|
|
Accumulated deficit |
|
|
(211,052,133 |
) |
|
|
(121,903,865 |
) |
|
Total stockholders' deficit |
|
|
(55,986,293 |
) |
|
|
(23,194,963 |
) |
|
Total liabilities and stockholders' deficit |
|
$ |
73,397,337 |
|
|
$ |
28,558,363 |
|
The accompanying notes are an integral part of these financial statements
F-4
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Revenues |
|
$ |
3,048,126 |
|
|
$ |
2,142,040 |
|
|
Cost of sales |
|
|
8,061,382 |
|
|
|
4,813,415 |
|
|
Gross loss |
|
|
(5,013,256 |
) |
|
|
(2,671,375 |
) |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Research and development |
|
|
13,406,370 |
|
|
|
8,485,668 |
|
|
Selling and marketing |
|
|
6,859,323 |
|
|
|
5,007,320 |
|
|
General and administrative |
|
|
8,801,997 |
|
|
|
6,241,433 |
|
|
Total operating expenses |
|
|
29,067,690 |
|
|
|
19,734,421 |
|
|
Loss from operations |
|
|
(34,080,946 |
) |
|
|
(22,405,796 |
) |
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
Interest expense |
|
|
(167,466,170 |
) |
|
|
(11,757,445 |
) |
|
Interest income |
|
|
7,399 |
|
|
|
18,193 |
|
|
Gain (loss) on exchange and issuance of warrants |
|
|
3,374,752 |
|
|
|
(4,038,063 |
) |
|
Loss on extinguishment of debt |
|
|
(24,172,736 |
) |
|
|
— |
|
|
Change in fair value liabilities |
|
|
133,191,183 |
|
|
|
(19,714,808 |
) |
|
Total other income (expense) |
|
|
(55,065,572 |
) |
|
|
(35,492,123 |
) |
|
Loss before provision for income taxes |
|
|
(89,146,518 |
) |
|
|
(57,897,919 |
) |
|
Provision for income taxes |
|
|
(1,750 |
) |
|
|
(1,250 |
) |
|
Net loss |
|
$ |
(89,148,268 |
) |
|
$ |
(57,899,169 |
) |
|
Net loss per common share - basic and diluted |
|
$ |
(798.17 |
) |
|
$ |
(121,636.91 |
) |
|
Weighted average common shares - basic and diluted |
|
|
111,691 |
|
|
|
476 |
|
The accompanying notes are an integral part of these financial statements
F-5
STATEMENTS OF STOCKHOLDERS’ DEFICIT
For the Years Ended December 31, 2015 and 2016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
Total |
|
||
|
|
|
Preferred Stock |
|
|
Common Stock |
|
|
Paid-In |
|
|
Accumulated |
|
|
Stockholders' |
|
|||||||||||||
|
|
|
Shares |
|
|
Par Value |
|
|
Shares |
|
|
Par Value |
|
|
Capital |
|
|
Deficit |
|
|
Deficit |
|
|||||||
|
Balance - December 31, 2014 |
|
|
— |
|
|
$ |
— |
|
|
|
474 |
|
|
$ |
— |
|
|
$ |
55,996,146 |
|
|
$ |
(64,004,696 |
) |
|
$ |
(8,008,550 |
) |
|
Issuance of preferred stock and warrants |
|
|
2,724,000 |
|
|
|
2,724 |
|
|
|
— |
|
|
|
— |
|
|
|
54,489 |
|
|
|
— |
|
|
|
57,213 |
|
|
Cash exercise of Series A common stock warrants |
|
|
— |
|
|
|
— |
|
|
|
1 |
|
|
|
— |
|
|
|
2,252,020 |
|
|
|
— |
|
|
|
2,252,020 |
|
|
Cash exercise of Series C common stock warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
979,200 |
|
|
|
— |
|
|
|
979,200 |
|
|
Cash exercise of unit purchase option |
|
|
14,750 |
|
|
|
15 |
|
|
|
— |
|
|
|
— |
|
|
|
162,235 |
|
|
|
— |
|
|
|
162,250 |
|
|
Cashless exercise of Class A & B warrants |
|
|
— |
|
|
|
— |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Cashless exercise of Series C warrants |
|
|
— |
|
|
|
— |
|
|
|
12 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
Employee stock option expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
110,124 |
|
|
|
— |
|
|
|
110,124 |
|
|
Conversion of preferred stock into common stock |
|
|
(2,650,403 |
) |
|
|
(2,651 |
) |
|
|
1 |
|
|
|
— |
|
|
|
2,651 |
|
|
|
— |
|
|
|
— |
|
|
Derivative liability on warrants issued and exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
39,151,949 |
|
|
|
— |
|
|
|
39,151,949 |
|
|
Net loss year to date |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(57,899,169 |
) |
|
|
(57,899,169 |
) |
|
Balance - December 31, 2015 |
|
|
88,347 |
|
|
|
88 |
|
|
|
489 |
|
|
|
- |
|
|
|
98,708,814 |
|
|
|
(121,903,865 |
) |
|
|
(23,194,963 |
) |
|
Issuance of common stock and warrants |
|
|
— |
|
|
|
— |
|
|
|
210 |
|
|
|
— |
|
|
|
428 |
|
|
|
— |
|
|
|
428 |
|
|
Exchange of Series E warrants for common stock |
|
|
— |
|
|
|
— |
|
|
|
28 |
|
|
|
— |
|
|
|
2,659,155 |
|
|
|
— |
|
|
|
2,659,155 |
|
|
Cash exercise of unit purchase option |
|
|
121,450 |
|
|
|
122 |
|
|
|
— |
|
|
|
— |
|
|
|
1,335,828 |
|
|
|
— |
|
|
|
1,335,950 |
|
|
Cashless exercise of Series C warrants settled in stock |
|
|
— |
|
|
|
— |
|
|
|
79 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Cashless exercise of Series C warrants settled in cash |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(314,879 |
) |
|
|
— |
|
|
|
(314,879 |
) |
|
Exercise of Series G warrants for cash |
|
|
— |
|
|
|
— |
|
|
|
4 |
|
|
|
— |
|
|
|
113,900 |
|
|
|
— |
|
|
|
113,900 |
|
|
Employee stock option expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
136,060 |
|
|
|
— |
|
|
|
136,060 |
|
|
Exchange of convertible note for preferred stock |
|
|
2,096 |
|
|
|
2 |
|
|
|
— |
|
|
|
— |
|
|
|
3,143,998 |
|
|
|
— |
|
|
|
3,144,000 |
|
|
Conversions of preferred stock into common stock |
|
|
(137,513 |
) |
|
|
(138 |
) |
|
|
438,759 |
|
|
|
44 |
|
|
|
147,994 |
|
|
|
— |
|
|
|
147,900 |
|
|
Conversions of convertible note into common stock |
|
|
— |
|
|
|
— |
|
|
|
325,121 |
|
|
|
32 |
|
|
|
36,631,116 |
|
|
|
— |
|
|
|
36,631,148 |
|
|
Derivative liability on warrants issued and exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
12,503,276 |
|
|
|
— |
|
|
|
12,503,276 |
|
|
Net loss year to date |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(89,148,268 |
) |
|
|
(89,148,268 |
) |
|
Balance - December 31, 2016 |
|
|
74,380 |
|
|
$ |
74 |
|
|
|
764,690 |
|
|
$ |
76 |
|
|
$ |
155,065,690 |
|
|
$ |
(211,052,133 |
) |
|
$ |
(55,986,293 |
) |
The accompanying notes are an integral part of these financial statements
F-6
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(89,148,268 |
) |
|
$ |
(57,899,169 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
2,634,372 |
|
|
|
1,612,086 |
|
|
Bad debt expense |
|
|
12,702 |
|
|
|
— |
|
|
Change in fair value liabilities |
|
|
(133,191,183 |
) |
|
|
19,714,808 |
|
|
Loss on issuance of convertible note as interest |
|
|
119,185,886 |
|
|
|
10,594,182 |
|
|
Loss on extinguishment of debt |
|
|
24,172,736 |
|
|
|
— |
|
|
Net gain on exchange and issuance of warrants |
|
|
(3,374,752 |
) |
|
|
4,038,063 |
|
|
Employee stock compensation |
|
|
136,060 |
|
|
|
110,124 |
|
|
Warrant issuance and modifications |
|
|
— |
|
|
|
612,006 |
|
|
Debt discount amortization |
|
|
46,529,237 |
|
|
|
122,050 |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
Increase in accounts receivable |
|
|
(80,706 |
) |
|
|
(143,905 |
) |
|
Increase in inventory |
|
|
(288,430 |
) |
|
|
(676,048 |
) |
|
Increase in prepaid and other assets |
|
|
(385,784 |
) |
|
|
(56,797 |
) |
|
Increase in accounts payable |
|
|
879,403 |
|
|
|
602,056 |
|
|
Increase in accrued liabilities |
|
|
1,264,337 |
|
|
|
700,790 |
|
|
Net cash used in operating activities |
|
|
(31,654,390 |
) |
|
|
(20,669,754 |
) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
Acquisition of property and equipment |
|
|
(1,161,125 |
) |
|
|
(1,566,044 |
) |
|
Construction of analyzer instruments |
|
|
(3,211,755 |
) |
|
|
(3,226,943 |
) |
|
Net cash used in investing activities |
|
|
(4,372,880 |
) |
|
|
(4,792,987 |
) |
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
Proceeds from exercise of warrants |
|
|
1,449,850 |
|
|
|
3,161,220 |
|
|
Proceeds from issuance of convertible notes payable |
|
|
5,407,772 |
|
|
|
4,135,000 |
|
|
Proceeds from follow-on offering |
|
|
10,631,377 |
|
|
|
21,933,874 |
|
|
Proceeds from issuance of notes payable - related party |
|
|
— |
|
|
|
250,000 |
|
|
Proceeds from release of restricted cash |
|
|
16,396,214 |
|
|
|
— |
|
|
Payment of cash settlement for warrant exercises |
|
|
(314,879 |
) |
|
|
— |
|
|
Principal payments of capital leases |
|
|
(1,310,875 |
) |
|
|
(947,423 |
) |
|
Principal payments of notes payable |
|
|
(5,693 |
) |
|
|
(49,994 |
) |
|
Principal payments of notes payable -related party |
|
|
— |
|
|
|
(250,000 |
) |
|
Net cash provided by financing activities |
|
|
32,253,766 |
|
|
|
28,232,677 |
|
|
Net increase (decrease) in cash |
|
|
(3,773,504 |
) |
|
|
2,769,936 |
|
|
Cash, beginning of the period |
|
|
4,787,759 |
|
|
|
2,017,823 |
|
|
Cash, end of the period |
|
$ |
1,014,255 |
|
|
$ |
4,787,759 |
|
|
Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
|
Interest paid |
|
$ |
1,778,831 |
|
|
$ |
1,055,255 |
|
|
Income taxes paid |
|
$ |
1,750 |
|
|
$ |
1,250 |
|
|
Supplemental schedule of non-cash investing and financing activities: |
|
|
|
|
|
|
|
|
|
Conversion of preferred stock to common stock |
|
$ |
36 |
|
|
$ |
2,651 |
|
|
Conversion of note payable to preferred stock |
|
$ |
3,144,000 |
|
|
$ |
— |
|
|
Assets acquired through capital leases |
|
$ |
80,138 |
|
|
$ |
— |
|
|
Offering costs incurred but unpaid |
|
$ |
281,188 |
|
|
$ |
235,020 |
|
|
Property and equipment included in accounts payable |
|
$ |
446,400 |
|
|
$ |
226,214 |
|
|
Cashless exercise of warrants |
|
$ |
- |
|
|
$ |
1,011 |
|
|
Change in derivative liability from exercised and issued warrants and convertible notes |
|
$ |
12,503,276 |
|
|
$ |
54,883,264 |
|
The accompanying notes are an integral part of these financial statements
F-7
NOTE 1 DESCRIPTION OF BUSINESS
Great Basin Scientific, Inc. (the “Company”) (d.b.a., Great Basin Corporation) is a Delaware corporation headquartered in Salt Lake City, Utah. The Company was originally incorporated as Diagnostic Micro Arrays, Inc., a Nevada corporation, on June 27, 2003. The Company changed its name to Great Basin Scientific, Inc. on April 19, 2006. On August 12, 2008, the Company took steps to change its corporate domicile from Nevada to Delaware by forming Great Basin Scientific, Inc., a Delaware corporation, and on August 29, 2008, Great Basin Scientific, Inc., a Nevada corporation, was merged with and into Great Basin Scientific, Inc., a Delaware corporation, wherein the Delaware corporation was the sole surviving entity.
The Company is a molecular diagnostic testing company focused on the development and commercialization of its patented, molecular diagnostic platform designed to test for infectious disease, especially hospital-acquired infections. The Company believes that laboratories in small to medium sized hospitals, those under 400 beds, are in need of simpler and more affordable molecular diagnostic testing methods. The Company markets a system that combines both affordability and ease-of-use, when compared to other commercially available molecular testing methods, which it believes will accelerate the adoption of molecular testing in small to medium sized hospitals. The system includes an analyzer, which is provided for our customers’ use without charge in the United States, and a diagnostic cartridge, which is sold to our customers. The testing platform has the capability to identify up to 64 individual targets at one time. If the test identifies one to three targets, they are referred to as low-plex tests, or tests, and if they identify four or more targets they are referred to as multi-plex panels, or panels. The Company currently has four commercially available tests, the first for clostridium difficile, or C. diff, which received clearance from the Food and Drug Administration, or FDA, in April 2012, the second for Group B Strep, which received clearance from the FDA in April 2015 and launched commercially in June 2015, the third for Shiga Toxin producing E. coli or STEC, which received clearance from the FDA in March 2016 and launched commercially in August 2016 and the fourth for Staphylococcus Identification and Resistance Panel, or Staph ID/R panel, which received FDA clearance in March 2016 and launched commercially in September 2016. Our customers consist of hospitals, clinics, laboratories and other healthcare providers in the United States, the European Union and New Zealand.
NOTE 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
These financial statements have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) and in accordance with generally accepted accounting principles in the United States of America (“GAAP”) and reflect the financial position, results of operations and cash flows of the Company.
F-8
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and the accompanying notes. Such estimates include the warranty reserve, accounts receivable and inventory reserves, intangible assets and other long lived assets, legal and regulatory contingencies, income taxes, share based arrangements, the derivative liability and others. These estimates and assumptions are based on management’s best estimates and judgments. Actual amounts and results could differ from those estimates.
Cash and Cash Equivalents
The Company considers highly liquid investments with insignificant interest rate risk and original maturities to the Company of three months or less to be cash equivalents. Cash equivalents consist primarily of interest and non-interest bearing bank accounts held in checking, savings and money market accounts. These assets are generally available on a daily or weekly basis and are highly liquid in nature. If the balances are greater than $250,000, the Company does not have FDIC coverage on the entire amount of bank deposits.
Restricted Cash
Cash and cash equivalents that are restricted as to withdrawal or use under the terms of certain contractual agreements are recorded as restricted cash on our balance sheet. On December 30, 2015, the Company entered into a Securities Purchase Agreement pursuant to which the Company issued $22.1 million senior secured convertible notes (the “2015 Notes”) and received $18.4 million in cash proceeds. Under the terms of the 2015 Notes, at closing the Company received an initial tranche of $4.6 million for immediate use by the Company for general corporate purposes. The remaining cash proceeds of $13.8 million was restricted as of December 31, 2015 and subject to an account control agreement. The restrictions were removed and became available to the Company pursuant to the terms of the 2015 Notes at various dates throughout 2016. On July 1, 2016 the Company entered into a Securities Purchase Agreement pursuant to which the Company issued $75 million in senior secured convertible notes (the “2016 Notes”) and received $68.0 million in cash proceeds. Under the terms of the 2016 Notes, at closing the Company received an initial tranche of $6.0 million for immediate use for general corporate purposes. The remaining cash proceeds of $62.0 million were restricted and subject to an account control agreement. The release of restrictions on cash held under the terms of the 2016 Notes depends upon the Company’s satisfaction of certain equity conditions that include the common stock listed on an eligible market, stock trading volume, share price and other requirements. As of December 31, 2016, cash in the amount of $59.4 million from the 2016 Notes is still being held in restricted accounts and will be released to the Company at future dates pursuant to the terms of the 2016 Notes (see NOTE 8 CONVERTIBLE NOTES PAYABLE). The restricted cash is deposited in an account that is not FDIC insured.
Accounts Receivable
Accounts receivable are generated from the sale of single use diagnostic test cartridges to end users in the United States and to a network of distributors outside the United States. These accounts receivable are recorded at the invoiced amount, net of allowances for doubtful amounts. The Company routinely reviews outstanding accounts receivable balances for estimated uncollectible accounts and establishes or adjusts the allowances for doubtful accounts receivable using the specific identification method and records a reserve for amounts not expected to be fully recovered. Actual balances are not applied against the reserve until substantially all collection efforts have been exhausted. The Company does not have customer acceptance provisions, but it does provide its customers a limited right of return for defective diagnostic test cartridges.
The allowance for doubtful accounts at December 31, 2016 and 2015 was $25,169 and $16,892, respectively.
F-9
Inventories are stated at the lower of cost or market with cost determined according to the average cost method. Manufactured inventory consists of raw material, direct labor and manufacturing overhead cost components. The Company reviews the components of its inventory on a regular basis for excess and obsolete inventory and makes appropriate adjustments when necessary. Inventories consisted of the following at December 31, 2016 and 2015:
|
|
|
December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Raw materials |
|
$ |
1,076,764 |
|
|
$ |
758,870 |
|
|
Work-in-process |
|
|
274,741 |
|
|
|
277,827 |
|
|
Finished goods |
|
|
70,067 |
|
|
|
96,445 |
|
|
Total inventories |
|
$ |
1,421,572 |
|
|
$ |
1,133,142 |
|
Property and Equipment
Property and equipment is recorded at cost and depreciated over the estimated useful lives of the assets (which range from three to ten years) using the straight-line method. Amortization of leasehold improvements is computed on the straight-line method over the shorter of the lease term or estimated useful lives of the assets. The analyzers that the Company manufactures and retains title over are placed with customers and are recorded in property and equipment under “Analyzers.” The materials used for the manufacture of the analyzers are recorded in property and equipment under “Construction in progress.” Major renewals and betterments are capitalized and depreciated over their estimated useful lives while minor expenditures for maintenance and minor repairs are charged to operations as incurred.
Intangible Assets
The Company records its intangible assets at cost which consist of two licensing and royalty agreements for certain intellectual property rights used in the development and manufacture of our products. These intangible assets are being amortized over an estimated useful life of seven years from the date that the technology licenses became effective. As of December 31, 2016 and 2015, intangible assets totaled $42,586 and $119,171 valued at cost of $600,000 less accumulated amortization of $557,414 and $480,829, respectively. The Company recorded amortization associated with these agreements of $76,585 and $97,407 for the years ended December 31, 2016 and 2015, respectively. The intangible assets will be fully amortized during the year ended December 31, 2017.
Impairment of Long Lived Assets
Long-lived tangible assets, including property and equipment, and definite-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of the asset may not be recoverable. The Company regularly evaluates whether events or circumstances have occurred that indicate possible impairment and relies on a number of factors, including expected future operating results, business plans, economic projections, FDA approvals and anticipated future cash flows. The Company uses an estimate of the future undiscounted net cash flows and comparisons to like-kind assets, as appropriate, of the related asset over the remaining life in measuring whether the assets are recoverable. Measurement of the amount of impairment, if any, is based upon the difference between the asset’s carrying value and estimated fair value. Fair value is determined through various valuation techniques, including cost-based, market and income approaches as considered necessary.
F-10
Fair Value Liability Instruments
The Company accounts for derivative instruments under the provisions of Accounting Standards Codification (“ASC”) 815 Derivatives and Hedging. ASC 815 requires the Company to record derivative instruments at their fair value. Changes in the fair value of derivatives are recognized in earnings. As a result of certain terms, conditions and features included in certain common stock warrants granted by the Company as well as the conversion features in the convertible notes, those provisions are required to be accounted for as derivatives at estimated fair value, with changes in fair value recognized in earnings (see NOTE 12 FAIR VALUE LIABILITIES).
The Company accounts for other fair value liability instruments under the provisions of ASC 480 Distinguishing Liabilities from Equity. ASC 480 requires to record certain liabilities at their fair value. Changes in the fair value of these liabilities are recognized in earnings. As a result of certain terms, conditions and features included in the Series F Preferred Stock issued by the Company, it is required to be accounted for as a liability at estimated fair value, with changes in fair value recognized in earnings (see NOTE 10 COMMON AND PREFERRED STOCK).
Fair Value of Financial Instruments
The Company measures at fair value certain of its financial and non-financial assets and liabilities by using a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, essentially an exit price, based on the highest and best use of the asset or liability. The levels of the fair value hierarchy are:
Level 1—Quoted market prices in active markets for identical assets or liabilities;
Level 2—Significant other observable inputs (e.g. quoted prices for similar items in active markets, quoted prices for identical or similar items in markets that are not active, inputs other than quoted prices that are observable, such as interest rate and yield curves, and market-corroborated inputs); and
Level 3—Unobservable inputs in which there is little or no market data, which require the reporting unit to develop its own assumptions.
The internal models used to determine fair value for these Level 3 instruments use certain significant unobservable inputs and their use requires determination of relevant inputs and assumptions. Accordingly, changes in these unobservable inputs may have a significant impact on fair value. Such inputs include risk free interest rate, expected average life, expected dividend yield, and expected volatility. These Level 3 liabilities would decrease (increase) in value based upon an increase (decrease) in risk free interest rate and expected dividend yield. Conversely, the fair value of these Level 3 liabilities would generally increase (decrease) in value if the expected average life or expected volatility were to increase (decrease).
F-11
The Company derives its product revenue from the sale of single use diagnostic test cartridges sold through our dedicated sales force, except in the European Union where the Company sells through a network of distributors. Product revenue is recognized when all four of the following criteria are met: (1) persuasive evidence that an arrangement exists; (2) delivery of the products has occurred; (3) the selling price of the product is fixed or determinable; and (4) collectability of that price is reasonably assured. Change in title to the product and recognition of revenue from sales of diagnostic test cartridges occurs at the time of shipment. Shipping and handling fees and related freight costs and supplies for test kits are billed to customers. Additional costs associated with shipping products to customers are included as a component of cost of sales.
Research and Development Costs
Research and development costs are charged to operations as incurred. Research and development costs include, among other things, salaries and wages for research scientists and staff (including stock-based compensation), materials and supplies used in the development of new products, developing and validating the manufacturing process, costs for clinical trials, and costs for research and development facilities and equipment.
Stock Based Compensation
The Company has accounted for stock-based compensation under the provisions of ASC 718 Compensation—Stock Compensation. This standard requires the Company to record an expense associated with the fair value of stock-based compensation over the requisite service period. The Company uses the Black-Scholes option valuation model to calculate the value of options at the date of grant. Option pricing models require the input of highly subjective assumptions, including the estimated fair value of the Company’s common stock on the date of grant, the expected term of the stock option, and the expected price volatility of the Company’s common stock over the period equal to the expected term of the grant. Changes in these assumptions can materially affect the fair value estimate. The Company estimates forfeitures at the date of grant and revises the estimates, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Financial Instruments and Concentration of Credit Risk
The Company’s financial instruments include cash and cash equivalents, accounts receivable, accounts payable and convertible notes payable. The carrying amount of cash and cash equivalents, accounts receivable and accounts payable approximate fair value because of their immediate or short-term maturities. The carrying amount of convertible notes payable on the balance sheet approximates fair value.
All of the Company’s accounts receivable result from sales in the normal course of business to its customers primarily throughout the United States. The Company attempts to limit its credit risk by performing credit evaluations of new customers and maintaining adequate allowances for potential credit losses. As of December 31, 2016, no one customer had over 10% of the accounts receivable balance. As of December 31, 2015, 17% of the accounts receivable balance resulted from one customer. Allowances for bad debt in the amount of $25,169 and $16,892 were recorded against accounts receivable for the years ended December 31, 2016 and 2015, respectively. Bad debt expense in the amount of $12,702 and $0 was recorded for the years ended December 31, 2016 and 2015, respectively. The Company cannot ensure that such losses will not be realized in the future.
The Company’s customers consist of hospitals, clinics, laboratories and other healthcare providers in the United States, the European Union and New Zealand. For the years ended December 31, 2016 and 2015, there were no customers that accounted for more than 10% of revenues.
F-12
The Company accounts for income taxes under Financial Accounting Standards Board (“FASB”) ASC 740, “Income Taxes”. Deferred income tax assets and liabilities are determined based upon differences between the financial reporting and tax basis of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company has incurred significant losses that have created a deferred tax asset. Accounting standards require the consideration of a valuation allowance for deferred tax assets if it is “more likely than not” that some component or all of the benefits of deferred tax assets will not be realized. The Company has considered the facts and circumstances surrounding the future realization of the deferred tax asset and determined that a full valuation allowance should be recorded.
The tax effects from an uncertain tax position can be recognized in the financial statements only if the position is more likely than not of being sustained if the position were to be challenged by a taxing authority. The Company has examined the tax positions taken in its tax returns and determined that there are no uncertain tax positions. As a result, the Company has recorded no uncertain tax liabilities in its balance sheet.
Loss per Common Share
Basic loss per share (“EPS”) is computed by dividing net loss, less cumulative preferred stock dividends for the period, including undeclared or unpaid cumulative dividends (the numerator) by the weighted average number of common shares outstanding for the period (the denominator). Diluted EPS is computed by dividing net loss by the weighted average number of common shares and potential common shares outstanding (if dilutive) during each period. Potential common shares include convertible preferred stock, stock options and warrants. The number of potential common shares outstanding is computed using the treasury stock method.
As the Company has incurred losses for the years ended December 31, 2016 and 2015, the potentially dilutive shares are anti-dilutive and are thus not added into the loss per share calculations. As of December 31, 2016 and 2015, there were 15,912,142 and 33 potentially dilutive shares, respectively.
Reverse Stock Split
On December 11, 2015, the Company effected a reverse stock split of the Company’s common stock whereby each sixty shares of common stock was replaced with one share of common stock (with no fractional shares issued). The par value of the common stock was adjusted from $0.001 per share to $0.0001 per share as a result of the reverse stock split. The authorized shares of the common stock were not adjusted. On March 30, 2016, the Company effected a reverse stock split of the Company’s common stock whereby each thirty-five shares of common stock were replaced with one share of common stock (with no fractional shares issued). On September 16, 2016, the Company effected a reverse stock split of the Company’s common stock whereby each eighty shares of common stock were replaced with one share of common stock (with no fractional shares issued). The par value and the number of authorized shares of the common stock were not adjusted. On December 28, 2016, the Company effected a reverse stock split of the Company’s common stock whereby each three hundred shares of common stock were replaced with one share of common stock (with no fractional shares issued). The par value and the number of authorized shares of the common stock was not adjusted as a result of the reverse stock split. However, the number of authorized shares was increased to 1,500,000,000 as a result of an amendment to the Certificate of Incorporation. All common share and per share amounts for all periods presented in these financial statements have been adjusted retroactively to reflect these reverse stock splits. The quantity of Series E Preferred Stock and all warrants and employee and other options were not included in the reverse stock splits and their outstanding quantities have not been adjusted. However, the conversion and exchange ratios were adjusted for the effect of the reverse stock splits such that upon conversion each 50.4 million shares of Series E Preferred Stock will be converted into four shares of common stock and each 50.4 million of Class A, Class B, Series B, common warrants and options will be exercisable into one share of common stock. The Series G, Series H and 2016 Subordination Warrants conversion ratio has been adjusted such that each 24,000 of the Series G, Series H and 2016 Subordination Warrants will now be exercisable into one share of common stock. The Series D and 2015 Subordination Warrants conversion ratio was also adjusted for the effect of the reverse splits, but pursuant to the December 31, 2016 adjustment provision in the warrant agreement the conversion ratio was reset such that each one of the Series D and Subordination Warrants will
F-13
be exercisable into one share of common stock. Please see NOTE 11 WARRANTS for further explanation and detail on each type of warrant.
Reclassification
Certain items on the 2015 Balance Sheet have been reclassified to conform to the presentation of the 2016 Balance Sheet. When a company issues convertible debt and the conversion option is bifurcated as a derivative liability, the conversion option should be combined with the debt on the company’s balance sheet. The Company combined the derivative liability of the embedded conversion feature of the 2016 Notes in the 2016 Balance Sheet presentation. Accordingly the presentation on the 2015 Balance Sheet was reclassified such that the current portion of convertible notes payable, net of discount was increased to $16,575,000 and convertible notes payable, net of current portion and debt discount was increased to $2,177,657. There was a corresponding decrease in the derivative liability in the amount of $16,588,940, and an increase in total current liabilities of $14,936,283. No other balance sheet items were impacted by this presentation correction. The Company has evaluated the effect of the incorrect presentation, both qualitatively and quantitatively, and concluded that it did not have a material impact on the 2015 financial statements.
New Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) that are adopted by the Company as of the specified effective date. If not discussed, management believes that the impact of recently issued standards, which are not yet effective, will not have a material impact on the Company’s financial statements upon adoption. The Company has elected to use the extended transition period provided in JOBS Act for complying with new or revised accounting standards that have different effective dates for public and private companies. The new accounting pronouncements below indicate the public company transition dates, however, the Company has not yet decided whether to early adopt these standards.
In November 2016, the FASB issued Accounting Standards Update (“ASU”) 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash. Historically, there has been a diversity in practice in how changes in restricted cash are presented and classified in the statement of cash flows. The amendments in this update require that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The amendments in this update are effective for public entities for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted. The Company notes that this guidance applies to its reporting requirements at present, and will implement the new guidance beginning in 2018.
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows (Topic 230). The objective of this update is to add or clarify guidance on the classification of certain cash receipts and payments in the statement of cash flows. This ASU is effective for fiscal years beginning after December 15, 2017, including interim periods within those annual periods and is to be applied utilizing a retrospective approach. Early adoption is permitted. The Company is currently evaluating the new guidance to determine the impact it may have on its consolidated financial statements and related disclosures.
In March 2016, the FASB issued ASU 2016-09, Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. The objective of this update is to simplify several aspects of the accounting for employee share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. This ASU is effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The Company has adopted this standard and the effects are reflected in its financial statements and related disclosures.
In February 2016, the FASB issued ASU No. 2016-02, Leases, which requires recognition of leased assets and liabilities on the balance sheet and disclosing key information about leasing arrangements. This update is effective for annual periods and interim periods with those periods beginning after December 15, 2018. The Company has approximately $3 million of operating lease obligations as of December 31, 2016 (see Note 6) and upon adoption of
F-14
this standard it will record a right-of-use asset and lease liability for present value of these leases. However, the statement of operations recognition of lease expenses is not expected to change from the current methodology.
In July 2015, the FASB issued ASU 2015-11, Simplifying the Measurement of Inventory, that simplifies the subsequent measurement of inventories by replacing the current lower of cost or market test with a lower of cost or net realizable value test. The ASU is effective for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption is permitted. The Company has evaluated the effects this standard will have on its financial statements and related disclosures, and concluded that upon adoption it will have little or no impact.
In April 2015, the FASB issued ASU No. 2015-03, Interest – Imputation of Interest, Simplifying the Presentation of Debt Issuance Cost. This standard provides guidance on the balance sheet presentation for debt issuance costs and debt discounts and debt premiums. To simplify the presentation of debt issuance costs, this standard requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability. This ASU is effective for fiscal years beginning after December 15, 2015. The Company has adopted this standard, and concluded a prior period reclassification was unnecessary. The effects of adopting the standard are reflected in its financial statements and related disclosures.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers, which supersedes nearly all existing revenue recognition guidance under GAAP. The core principle is that a company should recognize revenue when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled to those goods or services. ASU 2014-09 defines a five-step process to achieve this core principle and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing GAAP. In April 2016, the FASB issued ASU No. 2016-10, Identifying Performance Obligations and Licensing, which clarified various aspects of the core principle in ASU No. 2014-09 pertaining to identifying promised goods and services. In May 2016, the FASB issued ASU No. 2016-12, Narrow-Scope Improvements and Practical Expedients, which clarified certain consideration collectability requirements described in ASU No. 2014-09. In December 2016, the FASB issued ASU No. 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers, which addressed several narrow aspects of the guidance included in ASU No. 2014-09. All four standards were originally effective for annual periods beginning after December 15, 2016, and interim periods therein, and were to be applied either retrospectively to each period presented or as a cumulative-effect adjustment as of the date of adoption. In August 2015, the FASB deferred the effective date of ASU 2014-09 to fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. The Company is currently in the process of determining the impact of adoption of the provisions of ASU 2014-09, ASU 2016-10, ASU 2016-12, and ASU 2016-20 on its financial statements.
NOTE 3 GOING CONCERN
The Company’s audited financial statements have been prepared on a going concern basis which contemplates the realization of assets and the liquidation of liabilities in the ordinary course of business. The Company has incurred substantial losses from operations and negative operating cash flows which raise substantial doubt about the Company’s ability to continue as a going concern. The Company sustained a net loss for the year ended December 31, 2016 of $89.1 million and a net loss for the year ended December 31, 2015 of $57.9 million, and has an accumulated deficit of $211.1 million as of December 31, 2016. We have limited liquidity and have not yet established a stabilized source of revenue sufficient to cover operating costs and development needs. Accordingly, our continuation as a going concern is dependent upon our ability to generate greater revenue through increased sales and/or our ability to raise additional funds through the capital markets. Whether and when the Company can attain profitability and positive cash flows from operations or obtain additional financing is uncertain.
The Company has been able to obtain financing in order to fund its short term working capital and development needs. In February 2016, the Company obtained financing by completing a follow-on offering for net proceeds of $5.0 million. In May 2016, holders of the 2015 Notes voluntarily agreed to remove restrictions on the Company’s use of $2.0 million previously funded to the Company and authorized the release of those funds from the restricted cash accounts of the Company. In June 2016, the Company obtained additional financing by completing another follow-on offering for net proceeds of $5.3 million. In July 2016, the Company issued the 2016 Notes and received $68.0 million in total gross proceeds, of which $5.4 million in net proceeds was immediately available to the
F-15
Company and $62.0 million was placed in restricted accounts. In September, October and November 2016, holders of the 2015 Notes voluntarily agreed to remove restrictions on the Company’s use of $4.7 million, $3.5 million, and $3.6 million, respectively, of funds previously funded to the Company and authorized the release of those funds from the restricted cash accounts of the Company. In December 2016, holders of the 2016 Notes voluntarily agreed to remove restrictions on the Company’s use of $2.6 million previously funded to the Company and authorized the release of those funds from the restricted cash accounts of the Company. As of December 31, 2016, cash in the amount of $59.4 million is still being held in restricted accounts and will be released to the Company at future dates pursuant to the terms of the 2016 Notes. Subsequent to December 31, 2016, the holders of 2016 Notes voluntarily agreed to remove restrictions on the Company’s use of an aggregate of approximately $3.5 million in cash previously funded to the Company and authorized the release of those funds from the restricted accounts. In addition, the Company and the holders of 2016 Notes entered into an agreement, pursuant to which the Company agreed to redeem $38.9 million of the 2016 Notes held by each of the holders for an aggregate redemption price of $38.9 million, which will satisfy such redemption note in full. The Company paid the redemption price for the redemption notes from cash held in the restricted accounts of the Company. As of March 17, 2016, cash in the amount of $17.0 million is still being held in the restricted accounts.
The Company will continue to seek funding through the issuance of additional equity securities or debt financing, or a combination of the two. Any proceeds received from these items could provide the needed funds for continued operations and development programs. The Company can provide no assurance that it will be able to obtain sufficient additional financing that it needs to alleviate doubt about its ability to continue as a going concern. If the Company is able to obtain sufficient additional financing proceeds, the Company cannot be certain that this additional financing will be available on acceptable terms. To the extent the Company raises additional funds by issuing equity securities or convertible debt, the Company’s stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact the Company’s ability to conduct business. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. If the Company is unable to obtain additional financings, the impact on the Company’s operations will be material and adverse.
NOTE 4 PROPERTY AND EQUIPMENT
Property and equipment consisted of the following at December 31, 2016 and December 31, 2015:
|
|
|
December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Construction in progress |
|
$ |
1,544,839 |
|
|
$ |
680,679 |
|
|
Analyzers |
|
|
7,867,406 |
|
|
|
5,045,481 |
|
|
Computers and office equipment |
|
|
846,482 |
|
|
|
462,441 |
|
|
Machinery and equipment |
|
|
3,013,376 |
|
|
|
2,372,556 |
|
|
Leasehold improvements |
|
|
494,014 |
|
|
|
393,271 |
|
|
Furniture and fixtures |
|
|
128,210 |
|
|
|
72,618 |
|
|
Equipment under capital lease |
|
|
2,175,476 |
|
|
|
2,148,476 |
|
|
|
|
|
16,069,803 |
|
|
|
11,175,522 |
|
|
Less: accumulated depreciation and amortization |
|
|
(5,991,319 |
) |
|
|
(3,433,531 |
) |
|
Total property and equipment, net |
|
$ |
10,078,484 |
|
|
$ |
7,741,991 |
|
The total expense for depreciation of fixed assets and amortization of leasehold improvements was $2,557,787 and $1,514,679 for the years ended December 31, 2016 and 2015, respectively. Of this amount $562,364 and $597,236 was for depreciation of equipment under capital leases for the year ended December 31, 2016 and 2015, respectively.
F-16
Accrued liabilities consisted of the following as of December 31, 2016 and 2015:
|
|
|
December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Accrued payroll |
|
$ |
1,398,288 |
|
|
$ |
1,094,666 |
|
|
Royalties |
|
|
167,240 |
|
|
|
75,642 |
|
|
Accrued interest |
|
|
16,114 |
|
|
|
44,291 |
|
|
Accrued consulting fees |
|
|
150,000 |
|
|
|
- |
|
|
Accrued property and use tax |
|
|
756,348 |
|
|
|
10,905 |
|
|
Accrued placement fees on convertible notes |
|
|
4,480,163 |
|
|
|
- |
|
|
Other |
|
|
139,333 |
|
|
|
87,645 |
|
|
Total accrued liabilities |
|
$ |
7,107,486 |
|
|
$ |
1,313,149 |
|
NOTE 6 LEASE COMMITMENTS
Capital Leases
The Company has entered into two lease agreements for the sale-leaseback of molecular diagnostic analyzers. The first agreement was entered into in November 2013 and provided for the sale of 125 molecular diagnostic analyzers for a sales price of $2,500,000, which are being leased back for a base period of thirty-six monthly payments of $74,875. The second agreement was entered into in April 2014 for the sale of 75 molecular diagnostic analyzers for a sales price of $1,500,000, which are being leased back for a base period of twenty-four monthly payments of $64,665. At the end of each lease term, the leases automatically renewed for twelve additional months at the current monthly rate. As such, the Company is amortizing the capital leases over a forty-eight month period for the first agreement and a thirty-six month period for the second agreement. The Company’s obligations under the lease agreements are secured by a $3,000,000 letter of credit. The Letter of Credit was issued by a bank at the behest of a non-profit foundation and Spring Forth Investments LLC both of which are related parties through Mr. David Spafford, a director of the Company. The Company is obligated to reimburse the non-profit foundation and Spring Forth Investments LLC for any draws made under the Letter of Credit. The lease agreement is also secured by personal guarantees from Mr. Ryan Ashton, the Chief Executive Officer of the Company, and Mr. Spafford (See Note 14 RELATED PARTY TRANSACTIONS). The lease is accounted for as a capital lease sale-leaseback transaction in accordance with ASC 840, “Leases”. The Company has also entered into a capital lease agreement for research and development equipment valued at $75,000. The lease was entered into in July 2016 for a sixty month period with monthly payments in the amount of $1,444.
Annual future minimum lease payments of capital leases for the next five years are as follows:
|
Years ended December 31, |
|
|
|
|
|
2017 |
|
$ |
940,307 |
|
|
2018 |
|
|
17,325 |
|
|
2019 |
|
|
17,325 |
|
|
2020 |
|
|
17,325 |
|
|
2021 |
|
|
10,106 |
|
|
Total capital lease payments |
|
|
1,002,388 |
|
|
Less amount representing interest |
|
|
(81,427 |
) |
|
Total future minimum lease payments |
|
|
920,961 |
|
|
Less current portion of capital leases |
|
|
(865,049 |
) |
|
Long term portion of capital leases |
|
$ |
55,912 |
|
Operating leases
The Company leases approximately 33,000 square feet of manufacturing and lab space located in Salt Lake City, Utah pursuant to two lease agreements totaling $25,926 in base rent per month. The leases expire on April 30, 2017
F-17
and each have two options, with each option for a three-year renewal period. We also lease approximately 35,540 square feet of office space located at another location in Salt Lake City, Utah for use as our executive offices and labs. Base rent payments due under the lease are expected to be approximately $3,454,611 in the aggregate over the term of the lease of 69 months that began on December 1, 2015. The Company also leases certain office equipment such as copiers and printers under operating lease agreements that expire at various dates.
Amounts charged to expense under operating leases were $885,602 and $323,175 for the years ended December 31, 2016 and 2015, respectively.
Operating lease commitments for the next five years are as follows:
|
Years ended December 31, |
|
|
|
|
|
2017 |
|
$ |
750,225 |
|
|
2018 |
|
|
638,985 |
|
|
2019 |
|
|
637,663 |
|
|
2020 |
|
|
636,211 |
|
|
2021 |
|
|
417,850 |
|
|
Total operating lease commitments |
|
$ |
3,080,934 |
|
NOTE 7 NOTES PAYABLE
The Company purchased certain machinery and equipment under two note payable agreements with aggregate monthly payments of $4,489, an interest rate between 10.0% and 15.2% and a maturity of January and February 2016. The outstanding principal as of December 31, 2015 was $5,693 which was subsequently paid in 2016.
NOTE 8 CONVERTIBLE NOTES PAYABLE
December 2015 Note Transaction
On December 30, 2015, the Company entered into a Securities Purchase Agreement (“SPA”) with certain investors pursuant to which it agreed to issue $22.1 million in senior secured convertible notes (“2015 Notes”) and Series D Warrants (further described below). The 2015 Notes were convertible into shares of Common Stock at a conversion price that was subject to adjustment for certain dilutive events. $20 million of the notes were issued for cash proceeds totaling $18.4 million with an original issue discount in the amount of $1.6 million which is equal to sixteen (16) months of simple interest at a rate of six percent (6.0%) per annum on the aggregate principal of the 2015 Notes (assuming, that the entire aggregate original principal amount remains outstanding through the maturity date). $2.1 million of the 2015 Notes were issued to extinguish 1,050,000 outstanding Series C Warrants at an extinguish value of $2.00 per warrant. The 2015 Notes were senior secured obligations of the Company and ranked senior to all outstanding and future indebtedness of the Company. They were secured by a first priority perfected security interest (subject to permitted liens as defined in the 2015Notes) in all of the current and future assets of the Company. The 2015 Notes contained standard and customary events of default and the entire principal balance was subject to the default and redemption provisions contained in the 2015 Notes, regardless of whether or not any of the proceeds were released from the Company’s restricted accounts.
In connection with the issuance of the 2015 Notes under the SPA, the Company issued Series D Warrants (the “Series D Warrants”), exercisable to acquire 8 shares of Common Stock, subject to a one time adjustment on December 31, 2016 under the terms of the Series D Warrants (see NOTE 11 WARRANTS). Each Series D Warrant is exercisable by the holder beginning six months after December 30, 2015 and continuing for a period five years thereafter. The exercise price of each Series D Warrant is subject to adjustments for certain dilutive events.
Under the terms of the 2015 Notes, at closing the Company received an initial tranche of $4.6 million for immediate use for general corporate purposes. The remaining cash proceeds of $13.8 million was held in a restricted account to be released to the Company in subsequent equal tranches subject to certain equity conditions and pursuant to the terms of the 2015 Notes. As of December 31, 2016 all the funds in the restricted accounts had been released to the Company.
F-18
The dilutive events that provide for the adjustment of the conversion price of the 2015 Notes and the exercise price of the Series D Warrants relate to any transaction in which the Company issues or is deemed to have issued shares of common stock for consideration per share less than the conversion price then in effect.
2015 Notes of $20 Million Issued for Cash
$20 million of the 2015 Notes were issued for cash proceeds of $18.4 million with an original issue discount in the amount of $1.6 million. In addition, the Company incurred debt issuance costs in the amount of $568,685. The conversion feature in the 2015 Notes represents an embedded derivative that requires bifurcation due to the ratchet provision described above related to the conversion feature. The provisions in the Series D Warrants also require the Company to account for the warrants as derivative liabilities. The original issue discount, the fair value of the embedded conversion feature, the fair value of the Series D Warrants and the debt issuance costs are all together considered the debt discount. Any excess of the total debt discount and the face value of the convertible notes are immediately recorded to interest expense in the statement of operations.
The initial fair value of the embedded conversion feature on the $20 million portion of the 2015 Notes were valued using a binomial model with Monte Carlo simulation, resulting in a fair value of $14,788,365. The initial fair value of the Series D Warrants related to the $20 million note was also valued using a binomial model with Monte Carlo simulation, resulting in a fair value of $13,637,132. The Company recorded a charge to interest expense in the amount of $10,594,182 in the statement of operations for the year ended December 31, 2015, representing the excess of the total debt discount over the face value of the convertible notes. The Company recorded a debt discount in the amount of $20 million which will be amortized over the life of the note using the effective interest method. As of December 31, 2015, $63,717 of the debt discount had been amortized to interest expense with the remainder being amortized or extinguished during 2016.
2015 Notes of $2.1 Million Issued for Series C Warrants
$2.1 million of the 2015 Notes and related Series D Warrants were issued to extinguish 1,050,000 outstanding Series C Warrants. Since the Series C Warrants were derivative liabilities at the time of the transaction, the Company has accounted for this as an extinguishment of liabilities. Accordingly, all consideration issued to extinguish the liabilities were recorded at their fair value on the date of the extinguishment and the liabilities extinguished were removed at their carrying value. Since the liabilities extinguished were derivative liabilities, their carrying value is continuously adjusted to equal their fair value. The fair value of the Series C Warrants that were extinguished was calculated using the predetermined inputs to Black Scholes formula as defined in the Series C Warrant, resulting in a fair value of $2,340,240 for the extinguished warrants. The initial fair value of the embedded conversion feature on the $2.1 million portion of the 2015 Notes was valued using a binomial model with Monte Carlo simulation, resulting in a fair value of $1,865,729. The initial fair value of the Series D Warrants related to the $2.1 million note was also valued using a binomial model with Monte Carlo simulation, resulting in a fair value of $2,412,574. The host debt instrument’s fair value was deemed to be $2.1 million. The Company recorded a loss in the amount of $4,038,063 in its statement of operations for the year ended December 31, 2015, representing the excess of the consideration provided over the liability extinguished.
Although the embedded conversion feature is bifurcated from the 2015 Notes for measurement purposes, the embedded derivative is combined with the 2015 Notes for presentation purposes on the balance sheet. The following table summarizes the balance sheet presentation of convertible notes outstanding at December 31, 2015:
|
Convertible notes payable, principal |
|
$ |
22,100,000 |
|
|
Debt discounts |
|
|
(19,936,283 |
) |
|
Conversion feature derivative liability |
|
|
16,588,940 |
|
|
Net convertible note payable |
|
|
18,752,657 |
|
|
Less current portion |
|
|
16,575,000 |
|
|
Convertible notes payable, long term |
|
$ |
2,177,657 |
|
F-19
Pursuant to the terms of the 2015 Notes, the Company was to make amortization payments with respect to the 2015 Notes in twelve equal installments beginning April 29, 2016 (each, an “Installment Date”). On each installment date, assuming certain equity conditions were met, the installment payment would automatically be converted into shares of Common Stock at a conversion rate defined in the agreement. As of April 29, 2016, the Company was not able to bring a registration statement effective covering the resale of the shares of common stock issuable under the terms of the 2015 Notes and therefore did not satisfy the equity conditions under the 2015 Notes to permit settlement of installment payments through conversion into shares of common stock. The holders of the 2015 Notes deferred the three installment payments due in April 2016, May 2016 and June 2016, respectively to the installment payment with a due date of July 29, 2016. In May 2016, holders of the 2015 Notes voluntarily agreed to remove restrictions on the Company’s use of $2.0 million previously funded to the Company and authorized the release of those funds from the restricted cash accounts of the Company. During the period from July 2016 through October 2016, approximately $13.7 million of installment payments (through the conversion into 325,374 shares of common stock) were made bringing the principal note balance of the 2015 Notes down to approximately $8.4 million. During this period, holders of the 2015 Notes voluntarily agreed to remove restrictions on the Company’s use of $8.2 million previously funded to the Company and authorized the release of those funds from the restricted cash accounts of the Company. Given the conversion feature is bifurcated from the host instrument, conversions are deemed to be extinguishments for accounting purposes and accordingly, a loss on extinguishment of debt in the amount of $20,975,024 was recognized during the year ended December 31, 2016.
A summary of the conversions accounted as extinguishments for the year ended December 31, 2016 is as follows:
|
Fair value of common stock issued |
|
$ |
36,631,149 |
|
|
Less: |
|
|
|
|
|
2015 Note principal extinguished |
|
|
13,666,887 |
|
|
Debt discount related to extinguished 2015 Note |
|
|
(3,529,806 |
) |
|
Derivative liability extinguished |
|
|
5,519,044 |
|
|
Loss on extinguishment of debt |
|
$ |
20,975,024 |
|
|
On November 3, 2016, the Company exchanged the remaining 2015 Notes outstanding principal of approximately $8.4 million for 8,436 shares of a new class of Series F Convertible Preferred Stock (the “Series F Preferred Stock”) (see NOTE 10 COMMON AND PREFERRED STOCK). The Company accounted for this exchange as an extinguishment of debt transaction in accordance with ASC 470 which states that for extinguishments of debt, the difference between the reacquisition price and the net carrying amount of the debt being extinguished should be recognized as a gain or loss during the period in which the debt is extinguished. Since the 2015 Notes had a bifurcated embedded conversion feature, the Company considered the exchange as the extinguishment of two liabilities for accounting purposes – the host 2015 Notes and the embedded conversion feature. The 2015 Notes were considered extinguished at their carrying value of $7,160,290 calculated by subtracting the unamortized debt discount of $1,272,823 from the remaining principal amount of $8,433,113. The embedded conversion feature is a derivative liability and is carried at fair value. The fair value of the embedded conversion feature at November 3, 2016 was determined to be $1,562,998 (see NOTE 12 FAIR VALUE LIABILITIES). The reacquisition price is the fair value in the amount of $12,071,249 for Series F Preferred Stock issued in the exchange (see NOTE 10 COMMON AND PREFERRED STOCK and NOTE 12 FAIR VALUE LIABILITIES). The difference between the carrying value of the host 2015 Notes and embedded conversion feature and the fair value of the Series F Preferred Stock was $3,347,971 and recognized as a loss on extinguishment of debt during the year ended December 31, 2016 as calculated below: |
|
Fair value of Series F preferred stock issued |
|
$ |
12,071,249 |
|
|
Less: |
|
|
|
|
|
Principal amount on 2015 Notes |
|
|
8,433,113 |
|
|
Fair value of embedded conversion feature |
|
|
1,562,988 |
|
|
Unamortized debt discount on 2015 Notes |
|
|
(1,272,823 |
) |
|
Loss on extinguishment of debt |
|
$ |
3,347,971 |
|
F-20
On July 1, 2016, the Company entered into a Securities Purchase Agreement (“July SPA”) with certain investors pursuant to which it agreed to issue $75 million in senior secured convertible notes (“2016 Notes”) and Series H Warrants (further described below). The 2016 Notes were convertible into 1,563 shares of Common Stock at a price equal to $48,000.00 per share, subject to adjustment for certain dilutive events. The 2016 Notes were issued for cash proceeds totaling $68.0 million with an original issue discount in the amount of $7.0 million with no stated interest rate. The 2016 Notes are senior secured obligations of the Company and will rank senior to all outstanding and future indebtedness of the Company. They are secured by a first priority perfected security interest (subject to the priority interest of the 2015 Notes and permitted liens as defined in the 2016 Notes) in all of the current and future assets of the Company. The 2016 Notes contain standard and customary events of default and the entire principal balance is subject to the default and redemption provisions contained in the 2016 Notes, regardless of whether or not any of the proceeds have been released from the Company’s restricted accounts.
In connection with the issuance of the 2016 Notes under the July SPA, the Company issued Series H Warrants (the “Series H Warrants”), exercisable to acquire 2,346 shares of Common Stock and Subordination Warrants (the “2016 Subordination Warrants”), exercisable to acquire 71 shares of Common Stock (see NOTE 11 WARRANTS). The Series H and 2016 Subordination Warrants become exercisable by the holder beginning six months after July 1, 2016 and continues for a period five years thereafter. The Series H and 2016 Subordination Warrants also have a provision that adjusts the exercise price upon certain dilutive events. As of December 31, 2016, pursuant to the terms of the warrant agreement, the exercise price of the Series H and 2016 Subordination Warrants are such that the exercise of 24,000 warrants with an aggregate exercise price of $6.00 will result in the issuance of one share of common stock.
The Company has agreed to make amortization payments with respect to the 2016 Notes in fifteen (15) equal installments beginning January 30, 2017. On each installment date, assuming certain equity conditions are met, the installment payment shall automatically be converted into shares of Common Stock at a conversion rate defined in the agreement.
Under the terms of the 2016 Notes, at closing the Company received an initial tranche of $6.0 million for immediate use for general corporate purposes. The remaining cash proceeds of $62 million are being held in restricted accounts and will be released to the Company from the restricted accounts in subsequent equal tranches subject to certain equity conditions. In December 2016, the noteholders voluntarily removed restrictions on the Company’s use of an aggregate of approximately $2.6 million previously funded to the Company and authorized the release of those funds from the restricted accounts of the Company. As of December 31, 2016, the remaining cash in the amount of $59.4 million is still being held in restricted accounts and will be released to the Company subject to certain equity conditions and the terms of the 2016 Notes. Subsequent to December 31, 2016, the holders of 2016 Notes voluntarily agreed to remove restrictions on the Company’s use of an aggregate of approximately $3.5 million in cash previously funded to the Company and authorized the release of those funds from the restricted accounts. In addition, the Company and the holders of 2016 Notes entered into an agreement, pursuant to which the Company agreed to redeem $38.9 million of the 2016 Notes held by each of the holders for an aggregate redemption price of $38.9 million, which will satisfy such redemption note in full. The Company paid the redemption price for the redemption notes from cash held in the restricted accounts of the Company. As of the date of the note redemption, cash in the amount of $17.0 million remained in the restricted accounts.
The Company determined the conversion feature in the 2016 Notes represents an embedded derivative that requires bifurcation due to the ratchet provision described above related to the conversion feature. The provisions in the Series H and 2016 Subordination Warrants also require the Company to account for the warrants as derivative liabilities. The initial fair value of the embedded conversion feature on the 2016 Notes was valued using a binomial model with Monte Carlo simulation, resulting in a fair value of $80,599,528. The initial fair value of the Series H Warrants and 2016 Subordination Warrants was also valued using a binomial model with Monte Carlo simulation, resulting in a fair value of $101,644,520. The original issue discount, the fair value of the embedded conversion feature, the fair value of the Series H Warrants and 2016 Subordination Warrants and the debt issuance costs are all together considered the debt discount. Any excess of the total debt discount over the face value of the convertible notes are immediately recorded to interest expense in the statement of operations. The Company recorded a charge to interest expense in the amount of $119,185,886 in the statement of operations for the year ended December 31,
F-21
2016, representing the excess of the total debt discount over the face value of the convertible notes. The Company recorded a debt discount in the amount of $75.0 million which is being amortized over the life of the note using the effective interest method. For the year ended December 31, 2016, $31,395,583 of the debt discount has been amortized to interest expense.
As of December 31, 2016, the 2016 Notes are convertible at the option of the holder at $6.00 per share. The Company has a conversion right related to the required installment payments where the Company can convert the installments payments (subject to a floor of $1.00) at: (a) the prevailing holder conversion price; (b) 80% of the arithmetic average of the 3 lowest volume weighted average price (VWAP) days in the prior 20 days; or (c) the weighted average value of the common stock on the trading day preceding the installment payment date. Both the conversion right of the holder and the Company is subject to a reset clause if the Company issues or sells common stock at a lower price than the applicable conversion rate at such time (not subject to the $1.00 floor). At December 31, 2016, the most advantageous conversion term is a conversion price of $1.43 which would convert the note into 52,473,973 shares of common stock.
Although the embedded conversion feature is bifurcated from the 2016 Notes for measurement purposes, the embedded derivative is combined, only to extent of the face value of the note, with the 2016 Notes for presentation purposes on the balance sheet. The remaining amount of the embedded conversion feature derivative liability in the amount of $32,222,344 is included in long-term derivative liability in the balance sheet. The following table summarizes the balance sheet presentation of the 2016 Notes outstanding at December 31, 2016:
|
Convertible notes payable, principal |
|
$ |
75,000,000 |
|
|
Debt discounts |
|
|
(43,604,417 |
) |
|
Conversion feature derivative liability |
|
|
43,604,417 |
|
|
Net convertible note payable |
|
|
75,000,000 |
|
|
Less current portion |
|
|
(60,000,000 |
) |
|
Convertible notes payable, long term |
|
$ |
15,000,000 |
|
The following summarizes by year the future principal payments on the 2016 Convertible Note as of December 31, 2016:
|
Years ended December 31, |
|
|
|
|
|
2017 |
|
$ |
60,000,000 |
|
|
2018 |
|
|
15,000,000 |
|
|
Total future principal payments |
|
$ |
75,000,000 |
|
NOTE 9 NOTES PAYABLE—RELATED PARTY
In July 2014, the Company entered into a note agreement for $500,000 with Spring Forth Investments, LLC a company owned by Mr. David Spafford, a director. The original maturity date for the note was July 18, 2015, which was extended by the Company to July 18, 2016 by giving notice and paying an extension fee of $10,000. It was extended again by the Company to July 18, 2017. The note pays interest at an annual rate of 20% and is paid monthly. The Company prepaid the last three months of interest for a total of $25,000 at the time of issuance of the note. The Company paid $66,667 and $100,000 in interest to Spring Forth Investments, LLC for the years ended December 31, 2016 and 2015, respectively.
In February 2015, the Company entered into another loan agreement for $250,000 with Spring Forth Investments, LLC. The loan had an interest rate of twelve percent (12%) per year and matured the earlier of (i) 90 days from the date of the loan agreement, or (ii) five days after the closing of a registered public offering of securities of the Company. In April 2015, the Company paid off the note along with the accrued interest in the amount of $4,192 and a termination fee of $12,500.
F-22
NOTE 10 COMMON AND PREFERRED STOCK
Common Stock
On December 28, 2016 the Company increased the authorized shares of common stock to 1,500,000,000 at a par value of $0.0001 per share. The Company had 200,000,000 shares of common stock authorized at a par value of $0.0001 per share as of December 31, 2015. As of December 31, 2016 and 2015 there were 764,690 and 489 shares of common stock issued and outstanding, respectively.
During the year ended December 31, 2015, the Company issued 1 share of common stock pursuant to the cash exercise of 1,074,082 Series A Warrants for total net proceeds of $2,252,020. In conjunction with the exercise of the Series A Warrants, 1,074,082 Series B Warrants to purchase shares of common stock were also issued.
During the year ended December 31, 2015, the Company issued 1 share of common stock pursuant to the conversion of 2,650,403 shares of Series E Convertible preferred stock at a conversion ratio of 50.4 million preferred shares to 4 common shares.
During the year ended December 31, 2015, the Company issued 1 share of common stock pursuant to the cashless exercise of both 508,641 Class A Warrants and 334,889 Class B Warrants.
During the year ended December 31, 2015, the Company issued 12 shares of common stock pursuant to the exercise of 15,630,027 Series C Warrants. Of these, 11 shares of common stock were issued as a result of the cashless exercise of 15,246,027 Series C Warrants and 1 share of common stock was issued as a result of the cash exercise of 384,000 Series C Warrants for total net proceeds of $979,200.
During the year ended December 31, 2016, the Company issued 64 shares of common stock pursuant to the cashless exercise of 5,091,815 Series C Warrants.
During the year ended December 31, 2016, the Company issued 15 shares of common stock pursuant to the cash exercise of 121,540 Underwriter Unit Purchase Options at an exercise price of $11.00 per option for total proceeds of $1,335,950. Upon exercise of these options, 121,450 shares of Series E Preferred Stock were issued and immediately converted into 1 share of common stock and 972,320 Series C Warrants were issued and immediately exercised pursuant to the cashless exercise provision into 14 shares of common stock.
During the year ended December 31, 2016, the Company issued 1 share of common stock pursuant to the conversion of 13,967 shares of Series E Preferred Stock (see NOTE 10 COMMON AND PREFERRED STOCK).
On February 24, 2016, the Company completed a public offering of 39.2 million Units (the “February 2016 Unit Offering”) and received approximately $5.0 million of net proceeds. Pursuant to the sale of the units, the Company issued 47 shares of common stock and 58,800,000 Series E Warrants. The Series E Warrants were exercisable into 70 shares of common stock at $210,000 per share. The Series E Warrants expire six years from the date of grant, were not exercisable for one year and which exercise was subject to a shareholder vote and an increase in the number of authorized shares of common stock the Company can issue. The proceeds from this offering were not recorded as paid in capital since the fair value of the Series E Warrants were in excess of the cash received.
On April 7, 2016, the Company entered into certain warrant exchange agreements (the “Exchange Agreements”), each by and between the Company and a holder of its outstanding Series E Warrants, pursuant to which the Company and each such holder agreed to exchange all outstanding Series E Warrants for shares of common stock of the Company. Pursuant to the Exchange Agreements, the Company issued 28 shares of common stock of the Company in exchange for the surrender by the holders to the Company of 58,800,000 Series E Warrants exercisable to acquire approximately 70 shares of common stock of the Company (representing an exchange ratio of one share of common stock for each 2.5 shares of common stock underlying the surrendered Series E Warrants). The surrendered Series E Warrants were immediately cancelled by the Company.
On June 1, 2016, the Company completed a public offering of 3,160,000 units (the “June 2016 Unit Offering”) and received approximately $5.3 million of net proceeds. Pursuant to the sale of the units, the Company issued 163 shares of common stock and 3,160,000 Series G Warrants. The Series G Warrants were initially exercisable into
F-23
163 shares of common stock at $45,600 per share, subject to adjustments and expire five years from the date of grant. The proceeds from this offering were not recorded as paid in capital since the fair value of the Series E Warrants were in excess of the cash received.
On July 11, 2016, the Company issued 4 shares of common stock pursuant to the exercise of 85,000 Series G Warrants for cash in the amount of $113,900 or $28,475 per share.
During the year ended December 31, 2016, holders of the 2015 Notes submitted notices to convert payments on the 2015 Notes into shares of the Company’s common stock (the “Conversions”). In connection with the Conversions, the Company issued 325,121 shares of common stock upon the conversion of $13,666,887 principal amount of 2015 Notes at a weighted average conversion price of $42.04 per share (see NOTE 8 CONVERTIBLE NOTES PAYABLE).
During the year ended December 31, 2016 the Company issued 438,759 shares of common stock for the mandatory conversion of 2,096 shares and the voluntary conversion of 480 of Series F Preferred Stock at a conversion price of $6.00 per share. The Series F Preferred Stock have a stated value of $1,000 per share.
Preferred Stock
The Company had 5,000,000 shares of preferred stock authorized at a par value of $0.001 per share as of December 31, 2016 and 2015. As of December 31, 2016 there are 74,380 shares of Series E Preferred Stock (recorded in equity) and 5,860 shares of Series F Preferred Stock (recorded as a liability) issued and outstanding. There were 88,347 shares of Series E Preferred Stock issued and outstanding as of December 31, 2015. The preferred stock may be issued from time to time by the board of directors as shares of one or more classes or series with authority to fix the designation and relative powers including voting powers, preferences, rights, qualifications, limitations, and restrictions relating to the shares of each class or series.
Series E Preferred Stock
In February 2015 the Company initiated a Units Offering (the “February 2015 Units Offering”) whereby the Company sold 2,724,000 units at a price of $8.80 per unit for net proceeds of $21.8 million after deducting underwriting commissions and offering costs. Each unit consisted of one share of our Series E Convertible Preferred Stock and eight Series C Warrants (the “Units”).
The original terms of the Units provided that shares of Series E Convertible Preferred Stock and the Series C Warrants would automatically separate on August 25, 2015. In June 2015, the terms of the Series E Convertible Preferred Stock and Series C Warrants were each modified to allow for an optional early separation and conversion upon the cash exercise of all eight of the Series C Warrants within the Unit.
In June 2015, 48,000 of the Units were separated early pursuant to the optional early separation resulting in the exercise of 384,000 Series C Warrants into 1 share of common stock for cash proceeds of $979,200. On August 25, 2015 the remaining 2,676,000 Units separated into 2,676,000 shares of Series E Convertible Preferred Stock and 21,408,000 Series C Warrants.
The Series E Convertible Preferred Stock has no voting rights. An amendment to the terms of the Series E Convertible Preferred Stock only requires the vote of the holders of Series E Convertible Preferred Stock. With respect to payment of dividends and distribution of assets upon liquidation or dissolution or winding up of the Company, the Series E Preferred Stock shall rank equal to the common stock of the Company. No sinking fund has been established for the retirement or redemption of the Convertible Preferred Stock. As such, the Series E Convertible Preferred Stock is not subject to any restriction on the repurchase or redemption of shares by the Company due to an arrearage in the payment of dividends or sinking fund installments. The Series E Convertible Preferred Stock also has no liquidation rights or preemption rights, and there are no special classifications of our Board of Directors related to the Series E Convertible Preferred Stock.
During the year ended December 31, 2015, 14,750 Underwriter Purchase Options were exercised for cash in the amount of $162,250 or $11.00 per option. Pursuant to the exercise of these options, 14,750 shares of Series E Convertible Preferred Stock and 118,000 Series C Warrants were issued.
F-24
During the year ended December 31, 2015, 2,650,403 shares of Series E Convertible Preferred Stock were converted into 1 share of common stock. As of December 31, 2015, 88,347 shares of Series E Convertible Preferred Stock remain outstanding and were convertible into 101 shares of common stock.
During the year ended December 31, 2016, 13,967 shares of Series E Preferred Stock were converted into 1 share of common stock. On November 2, 2016, the Company filed a Certificate of Designation for Series E Preferred Stock to the Certificate of Incorporation. The Certificate of Designation reduced, the number of authorized Series E Preferred Shares from 2,860,200 Series E Preferred Shares to 74,380 Series E Preferred Shares, the number of Series E Preferred Shares issued and outstanding as of November 2, 2016. As of December 31, 2016, there are 74,380 shares of Series E Preferred Stock issued and outstanding that are convertible into 100 shares of common stock.
Series F Preferred Stock
On November 3, 2016, the Company amended its Certificate of Incorporation to create a new class of Series F Preferred Stock of the Company. Each Preferred Share shall have a stated value of $1,000 and a par value of $0.001 per share. The Series F Preferred Shares rank senior to the common stock and shall be entitled to dividends, on an as converted basis, with the holders of our common stock, but will not accrue additional dividends unless certain events as defined in the Series F Preferred Stock has occurred and is continuing, in which case dividends will accrue at a default rate of 10% per annum. The holders of Preferred Shares shall have the right to vote with holders of shares of our common stock, voting together as one class on all matters, with each Preferred Share entitling the holder thereof to cast that number of votes per share on an as converted basis.
On November 3, 2016, the Company issued 8,436 shares of Series F Preferred Stock for the exchange of $8,433,113 in remaining principal on the 2015 Notes (See NOTE 8 CONVERTIBLE NOTES PAYABLE). Of these, 2,096 were immediately mandatorily converted into 349,333 shares of common stock at a conversion price of $6.00 per share. Due to the limitations on beneficial ownership, some shares of our common stock were held in abeyance. All shares were delivered in November 2016. The remaining Series F Preferred Stock is initially convertible at the election of the holder into shares of our common stock at a conversion price equal to $6.00. From and after July 3, 2017, the Series F Preferred Stock shall be convertible at a conversion price equal to 85% of the arithmetic average, in each case of the lower of (i) the three lowest daily weighted average prices of the our common stock during the twenty (20) consecutive trading day period ending on the trading day immediately preceding the date of determination and (iii) the weighted average price of the our common stock on the trading day immediately preceding the date of determination. On November 3, 2018, so long as certain events do not exist, any remaining Series F Preferred Stock then outstanding shall be converted into shares of our common stock at a conversion price of $6.00 per share. In each case, the exercise price is subject to certain adjustments upon the occurrence of certain dilutive events, including the issuance of certain options or convertible securities, and upon the occurrence of certain corporate events, including stock splits and dividends. At any time after the issue of the Series F Preferred Stock, so long as there has been no failure of the equity during the applicable measurement periods, the Company shall have the right to redeem all, but not less than all, of the conversion amount then remaining under the Preferred Shares at a price equal to the greater of (x) 125% of the conversion amount being redeemed and (y) the product of (A) the conversion amount being redeemed and (B) the quotient determined by dividing (I) the greatest closing price of the shares of our common stock during the period beginning on the date immediately preceding the Company’s notice of redemption and ending on the Company redemption date, by (II) the lowest conversion price in effect during such period.
The Company evaluated the Series F Preferred Stock to determine the proper accounting under ASC 480 – Distinguishing Liabilities from Equity. Due to the differing nature of the contractual terms between the mandatorily converted and remaining portions of the Series F Preferred Stock, the Company analyzed them separately.
The conversion terms of a portion of Series F Preferred Stock were contractually overridden by the exchange agreement wherein both parties agreed that 2,096 shares would be mandatorily converted into common stock immediately. Thus, in true economic substance, it was an immediate mandatory conversion which is akin to direct issuance of common stock. The Company has determined that ASC 480 does not apply to these instruments as they are only redeemable into common shares and there are no mandatory redemption features into cash or other assets that are certain to occur and accordingly has accounted for these as equity instruments. The Company also evaluated
F-25
any embedded conversion feature of the mandatory instrument under ASC 815 – Derivative and Hedging. The Company determined that the embedded conversion feature is clearly and closely related and no further evaluation is required. The Company is applying the extinguishment method of accounting to the 2015 Notes which requires these shares, as part of the reacquisition price, to be initially valued at fair value. The Company determined fair value of the mandatorily converted Series F Preferred Stock to be $3,144,000, which was the fair value of the common stock on the conversion day multiplied by the number of shares of common stock issued pursuant to the conversion. As mentioned above, these have all been converted and there are no mandatorily convertible Series F Preferred Stock outstanding as of December 31, 2016.
The Company has concluded that the remaining Series F Preferred Stock are within the scope of ASC 480 as they predominantly represent an unconditional obligation to issue a variable number of common shares for a fixed monetary amount. Accordingly, they will be accounted for as liabilities in the financial statements and measured initially and subsequently at fair value with any change in fair value to be recorded in earnings. The Company determined the fair value of the non-mandatory Series F Preferred Stock at issuance on November 3, 2016 to be $8,927,249 which was included as part of the reacquisition price of the 2015 Notes.
During December 2016, 480 shares of Series F Preferred Stock were converted at the option of the holder into 80,000 shares of common stock at a conversion price of $6.00 per share. As of December 31, 2016, there are 5,860 shares of Series F Preferred Stock outstanding convertible into 976,667 shares of common stock. The Company is accounting for these as a liability on the financial statements with a period end fair value of $5,655,006 (see NOTE 12 FAIR VALUE LIABILITIES).
NOTE 11 WARRANTS
As of December 31, 2015, the Company had 13,219,597 warrants outstanding to purchase 160 shares of common stock.
The following table outlines the warrants outstanding and exercisable as of December 31, 2015. All warrants have been accounted for as derivative liabilities (see NOTE 12 FAIR VALUE LIABILITIES):
|
Warrants |
|
Outstanding and Exercisable |
|
|
Total Shares of Common Stock Underlying the Warrant |
|
|
Aggregate Exercise Price for One Common Share ($ millions) |
|
Expiration |
||
|
Class A |
|
|
1,532,598 |
|
|
|
48 |
|
|
$1.6 |
|
April 2021 - July 2021 |
|
Class B |
|
|
1,310,956 |
|
|
|
29 |
|
|
$1.6 |
|
April 2021 - July 2021 |
|
Series B |
|
|
1,074,082 |
|
|
|
34 |
|
|
$441.0 |
|
March 2021 - July 2021 |
|
Series C |
|
|
5,229,973 |
|
|
|
13 |
|
|
$128.5 |
|
January 2017 |
|
Series D |
|
|
3,503,116 |
|
|
|
8 |
|
|
$1.6 |
|
June 2021 |
|
2015 Subordination |
|
|
105,516 |
|
|
|
2 |
|
|
$1.6 |
|
June 2021 |
|
Common |
|
|
463,356 |
|
|
|
26 |
|
|
$100.8 - $1,612.0 |
|
April 2016 - July 2021 |
|
Total Warrants |
|
|
13,219,597 |
|
|
|
160 |
|
|
|
|
|
F-26
The following table outlines the warrants outstanding as of December 31, 2016. All warrants have been accounted for as derivative liabilities (see NOTE 12 FAIR VALUE LIABILITIES):
|
Warrants |
|
Outstanding |
|
|
Total Shares of Common Stock Underlying the Warrant |
|
|
Aggregate Exercise Price for One Common Share |
|
Expiration |
||
|
Class A |
|
|
1,532,598 |
|
|
|
48 |
|
|
$6.00 |
|
April 2021 - July 2021 |
|
Class B |
|
|
1,310,956 |
|
|
|
29 |
|
|
$6.00 |
|
April 2021 - July 2021 |
|
Series B |
|
|
1,074,082 |
|
|
|
34 |
|
|
$27.5 million |
|
March 2021 - July 2021 |
|
Series D |
|
|
2,361,468 |
|
|
|
2,361,468 |
|
|
$6.00 |
|
June 2021 |
|
2015 Subordination |
|
|
71,131 |
|
|
|
71,131 |
|
|
$6.00 |
|
June 2021 |
|
Series G |
|
|
3,075,000 |
|
|
|
159 |
|
|
$6.00 |
|
June 2021 |
|
Series H |
|
|
56,250,000 |
|
|
|
2,346 |
|
|
$6.00 |
|
December 2021 |
|
2016 Subordination |
|
|
1,687,500 |
|
|
|
71 |
|
|
$6.00 |
|
December 2021 |
|
Common |
|
|
372,331 |
|
|
|
19 |
|
|
$6.00 - $1,612.8 million |
|
February 2017 - July 2021 |
|
Total Warrants |
|
|
67,735,066 |
|
|
|
2,435,305 |
|
|
|
|
|
All Warrants with the exception of the Series H and 2016 Subordination Warrants are exercisable as of December 31, 2016. All Warrants require derivative liability treatment due to the Company not having sufficient authorized shares to settle these warrants all in shares.
Class A Warrants and Class B Warrants
During the year ended December 31, 2015, 508,641 Class A Warrants were exercised pursuant to the cashless exercise provision of the warrant resulting in the issuance of 1 share of common stock and 334,889 Class B Warrants were exercised pursuant to the cashless exercise provision of the warrant resulting in the issuance of 1 share of common stock. The Company did not have any issuances or exercises of the Class A Warrants or Class B Warrants during the year ended December 31, 2016. These warrants include a provision which provides that their exercise prices be adjusted in connection with certain equity issuances by the Company. During the year ended December 31, 2016 there were numerous adjustments to the exercise prices. The exercise prices per share of common stock as of December 31, 2016 is shown in the table above.
Series A Warrants
During the year ended December 31, 2015, 1,074,082 Series A Warrants were exercised into 1 share of common stock resulting in net cash proceeds of $2,252,020. The Series A Warrants expired on October 15, 2015 and all remaining 248,418 outstanding Series A Warrants expired unexercised.
Series B Warrants
During the year ended December 31, 2015, the Company issued 1,074,082 Series B Warrants pursuant to the exercise of 1,074,082 Series A Warrants. The Company did not have any issuances or exercises of the Series B Warrants during the year ended December 31, 2016. These warrants include a provision which provides that their exercise prices be adjusted in connection with certain equity issuances by the Company. During the year ended December 31, 2016 there were numerous adjustments to the exercise prices. The exercise price per share of common stock as of December 31, 2016 is shown in the table above.
Series C Warrants
In connection with the February 2015 Units Offering, the Company issued Series C Warrants to purchase 26 shares of common stock as part of the Units sold in the follow-on offering (see NOTE 10 COMMON AND PREFERRED STOCK) with and exercise price of $2.1 million per share and expire in five years. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends,
F-27
stock splits, reorganizations or similar events affecting our common stock and the exercise price. The Series C Warrants have a cashless exercise provision where in lieu of payment of the exercise price in cash, the holder may receive, at the Company’s discretion, either a cash payment of a predetermined Black Scholes Value of the number of shares the holder elects to exercise, or a number of shares of the Company’s common stock determined according to a cashless exercise formula using the predetermined Black Scholes Value. On December 11, 2015, an amendment was made to the Series C Warrants to require that all warrants be exercised within 25 trading days or be subject to a mandatory exercise provision.
In June 2015, 48,000 of the Units were separated early pursuant to the optional early separation as 384,000 Series C Warrants were exercised into 1 share of common stock resulting in cash proceeds of $979,200. On August 25, 2015 the remaining 2,676,000 Units separated into 2,676,000 shares of Series E Convertible Preferred Stock and 21,408,000 Series C Warrants.
From August 25, 2015 through December 31, 2015, 15,128,027 Series C Warrants were exercised pursuant to the cashless exercise provision resulting in the issuance of 12 shares of Common Stock.
During the year ended December 31, 2015, 14,750 Underwriter Purchase Options were exercised for cash in the amount of $162,250. Upon the exercise of these options, 118,000 Series C Warrants were issued and immediately exercised pursuant to the cashless exercise provision resulting in the issuance of 1 share of common stock.
As of December 31, 2015, 5,229,973 Series C Warrants remained outstanding. Had the cashless exercise provision been exercised by all holders of our Series C Warrants at December 31, 2015, the Company would have had to either pay $11.7 million in cash or issue 12 shares of common stock. The number of shares of common stock that would be required to satisfy the cashless exercise provision increases as the price of the Company’s stock decreases and decreases as the price of the Company’s stock increases.
During the year ended December 31, 2016, 5,229,973 Series C Warrants were exercised pursuant to the cashless exercise provision. The Company settled 5,091,815 of the Series C Warrant exercises through the issuance of 64 shares of common stock and the Company settled 138,158 of the Series C Warrant exercises with cash in the amount of $314,879. On January 21, 2016 all outstanding Series C Warrants were mandatorily exercised utilizing the cashless provision of the warrants and the corresponding shares of common stock issued. As of December 31, 2016 there are 47,528 Series C Warrant certificates that have yet to be delivered to the Company representing 1 share of common stock.
Series D Warrants
In connection with the issuance of the 2015 Notes, the Company issued Series D Warrants (the “Series D Warrants”), exercisable to acquire 8 shares of Common Stock. Each Series D Warrant is exercisable by the holder beginning six months after December 30, 2015 and continuing for a period five years thereafter. The Series D Warrants are exercisable on a cashless basis. As of December 31, 2015, each Series D Warrant was exercisable at an exercise price of $1.6 million per share of common stock, subject to adjustments for certain dilutive events.
The Series D Warrants included an adjustment provision which provides that the number of common shares the Series D Warrants are exercisable into will increase on December 31, 2016 to be 16.6% of the sum of the number of shares actually outstanding on December 31, 2016 plus the number of shares of common stock deemed to be outstanding pursuant to all outstanding options, warrants or convertible securities of the Company. Accordingly, the Company adjusted the Series D Warrants such that they are exercisable into 2,361,468 shares of common stock as of December 31, 2016. All other terms remain the same as the original Series D Warrant. The Series D Warrants include a provision which provides that their exercise price be adjusted in connection with certain equity issuances by the Company. During the year ended December 31, 2016 there were numerous adjustments to the exercise prices. The exercise price per share of common stock for the Series D Warrants as of December 31, 2016 is shown in the table above.
2015 Subordination Warrants
The 2015 Subordination Warrants were issued to Spring Forth Investments LLC and Utah Autism Foundation in relation to their agreement to enter into subordination agreements with the collateral agent in the Note Financing
F-28
whereby each agreed to subordinate their debt to the Notes issued in the Note Financing. The Subordination Warrants have the same general material terms and conditions of the Series D Warrants. The 2015 Subordination Warrants were exercisable for 2 shares of common stock at December 31, 2015. Each Subordination Warrant is exercisable by the holder beginning six months after December 30, 2015 and continuing for a period five years thereafter. Each 2015 Subordination Warrant was initially exercisable at a price of $1.6 million per share, subject to adjustments for certain dilutive events (same as the Series D Warrants).
The 2015 Subordination Warrants included an adjustment provision which provides that the number of common shares the 2015 Subordination Warrants are exercisable into will increase on December 31, 2016 to be 0.5% of the sum of the number of shares actually outstanding on December 31, 2016 plus the number of shares of common stock deemed to be outstanding pursuant to all outstanding options, warrants or convertible securities of the Company. Accordingly, the Company adjusted the 2015 Subordination Warrants such that they are exercisable into 71,131 shares of common stock as of December 31, 2016. All other terms remain the same as the original 2015 Subordination Warrant. The 2015 Subordination Warrants include a provision which provides that their exercise price be adjusted in connection with certain equity issuances by the Company. During the year ended December 31, 2016 there were numerous adjustments to the exercise prices. The exercise price per share of common stock for the 2015 Subordination Warrants as of December 31, 2016 is shown in the table above.
Series E Warrants
In connection with the February 2016 Unit offering the Company issued Series E Warrants to purchase 70 shares of common stock as part of the units sold in the offering (see NOTE 10 COMMON AND PREFERRED STOCK). The Series E Warrants expire six years from the date of grant, were not exercisable for one year and which exercise was subject to a shareholder vote and an increase in the number of authorized shares of common stock the Company can issue. On April 7, 2016, the Company entered into certain warrant exchange agreements (the “Exchange Agreements”), each by and between the Company and a holder of its outstanding Series E Warrants, pursuant to which the Company and each such holder agreed to exchange outstanding Series E Warrants for shares of common stock of the Company. Pursuant to the Exchange Agreements, the Company issued 28 shares of common stock of the Company in exchange for the surrender by the holders to the Company of all 58,800,000 Series E Warrants exercisable to acquire approximately 70 shares of common stock of the Company (representing an exchange ratio of one share of common stock for each 2.5 shares of common stock underlying the surrendered Series E Warrants). The surrendered Series E Warrants were immediately cancelled by the Company (see NOTE 10 COMMON AND PREFERRED STOCK).
Series G Warrants
In connection with the June 2016 Unit Offering, the Company issued Series G Warrants to purchase 163 shares of common stock as part of the units sold in the offering (see NOTE 10 COMMON AND PREFERRED STOCK). The Series G Warrants expire 5 years after the date of issuance. On July 11, 2016, 85,000 of the Series G Warrants were exercised for cash in the amount of $113,900. Pursuant to the exercise of these warrants, the Company issued 4 shares of common stock. The Series G Warrants include a provision which provides that their exercise price be adjusted in connection with certain equity issuances by the Company. During the year ended December 31, 2016 there were numerous adjustments to the exercise prices. The exercise price per share of common stock for the Series G Warrants as of December 31, 2016 is shown in the table above.
Series H Warrants
In connection with the issuance of the 2016 Notes, the Company issued 56,250,000 Series H Warrants exercisable for 2,346 shares of common stock. Each Series H Warrant will be exercisable by the holder beginning six months after the date of issuance and continuing for a period five years thereafter. The Series H Warrants include a provision which provides that their exercise price be adjusted in connection with certain equity issuances by the Company. During the year ended December 31, 2016 there were numerous adjustments to the exercise prices. The exercise price per share of common stock for the Series H Warrants as of December 31, 2016 is shown in the table above.
2016 Subordination Warrants
In consideration of the Utah Autism Foundation and Spring Forth Investments LLC entering into subordination agreements in connection with the 2016 Notes, the Company has agreed to issue to the entities warrants exercisable for 70 shares of common stock (the “2016 Subordination Warrants”). The 2016 Subordination Warrants have the
F-29
same material terms and conditions as the Series H Warrants. Each 2016 Subordination Warrant will be exercisable by the holder beginning six months after the date of issuance and continuing for a period five years thereafter. The 2016 Subordination Warrants include a provision which provides that their exercise price be adjusted in connection with certain equity issuances by the Company. During the year ended December 31, 2016 there were numerous adjustments to the exercise prices. The exercise price per share of common stock for the 2016 Subordination Warrants as of December 31, 2016 is shown in the table above.
Common Warrants
For the year ended December 31, 2015, the Company granted 25,000 Common Stock warrants exercisable into 1 share of common stock to a consultant of the Company. The warrants are fully vested, have an exercise price of $129.0 million per share and expire in August 2020. The Company recorded an expense in the amount of $54,489 on the date of grant which represents the fair value of the warrants. The Company estimates the fair value of the warrants at grant date using a Black-Scholes valuation model. The estimates in the Black-Scholes option-pricing model are based, in part, on assumptions, including a stock price volatility of 127.37%, the warrant life of 5 years, a risk free rate of 1.53%, the fair value of $153.60 of the equity stock underlying the option and the aggregate exercise price of $153.60 for one share of common stock. During the year ended December 31, 2016 there were 91,025 Common Warrants exercisable into 7 shares of common stock that expired without being exercised. There were no other issuances or exercises of Common Warrants during the year ended December 31, 2016. Certain Common Warrants include a provision which provides that the exercise price of these certain Common Warrants will be adjusted in connection with certain equity issuances by the Company. During the year ended December 31, 2016 there were numerous adjustments to the exercise prices of these certain Common Warrants. As of December 31, 2016, the exercise price certain Common Warrants is $6.00 per share of common stock.
Underwriters’ Unit Purchase Option
In connection with the February 2015 Units Offering, the Company issued to the representative of the underwriters’ a Unit Purchase Option (“Option”) to purchase a number of our Units equal to an aggregate of 5% of the Units sold or 136,200 Units. The purchase option has an exercise price equal to 125% of the public offering price of the Units or $11.00, and the units may be exercised on a cashless basis and will expire 5 years from the date of issue. Each Unit consists of one share of Series E Convertible Preferred Stock and eight Series C Warrants. During the year ended December 31, 2015, 14,750 Underwriter Purchase Options were exercised for cash in the amount of $162,250. Pursuant to the exercise of these options, 14,750 shares of Series E Convertible Preferred Stock were issued and immediately converted into 1 share of common stock and 118,000 Series C Warrants were issued and immediately exercise pursuant to the cashless exercise provision into 1 share of common stock. As of December 31, 2015, 121,450 Unit Purchase Options remained outstanding. During the year ended December 31, 2016, 121,450 Underwriters’ Unit Purchase Options were exercised for cash in the amount of $1,335,950. Pursuant to the exercise of these options, 121,450 shares of Series E Convertible Preferred Stock were issued and immediately converted into 1 share of common stock and 972,320 Series C Warrants were issued and immediately exercised pursuant to the cashless exercise provision of the Series C Warrants into 15 shares of common stock. There are no outstanding Underwriters’ Unit Purchase Options as of December 31, 2016.
F-30
The following table summarizes the common stock warrant activity during the years ended December 31, 2016 and 2015:
|
|
|
Common Stock Warrants |
|
|
Weighted Average Exercise Price Per Share $ |
|
|
Weighted Average Remainder Contractual Term in Years |
|
|||
|
As of December 31, 2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants outstanding as of January 1, 2015 |
|
|
5,447,940 |
|
|
|
3,502,800 |
|
|
|
4.9 |
|
|
Granted |
|
|
26,617,714 |
|
|
|
2,276,400 |
|
|
|
4.3 |
|
|
Exercised |
|
|
(17,547,639 |
) |
|
|
2,074,800 |
|
|
|
4.0 |
|
|
Expired |
|
|
(1,298,418 |
) |
|
|
2,083,200 |
|
|
|
3.4 |
|
|
Warrants outstanding as of December 31, 2015 |
|
|
13,219,597 |
|
|
|
2,276,400 |
|
|
|
4.7 |
|
|
As of December 31, 2016: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants outstanding as of January 1, 2016 |
|
|
13,219,597 |
|
|
|
2,276,400 |
|
|
|
4.7 |
|
|
Granted |
|
|
119,897,500 |
|
|
|
5,623 |
|
|
|
5.7 |
|
|
Exercised |
|
|
(5,314,973 |
) |
|
|
2,050,268 |
|
|
|
4.1 |
|
|
Extinguished |
|
|
(59,976,033 |
) |
|
|
201,371 |
|
|
|
5.9 |
|
|
Expired |
|
|
(91,025 |
) |
|
|
504,000,000 |
|
|
|
- |
|
|
Warrants outstanding as of December 31, 2016 |
|
|
67,735,066 |
|
|
|
2,758 |
|
|
|
4.9 |
|
NOTE 12 FAIR VALUE LIABILITIES
The liability for our instruments classified as fair value liabilities are recorded at fair value at inception and subsequently re-measured to fair value as long as such instruments are classified as fair value liabilities. Changes in the fair value of these liabilities are included as a component of Other income (expense) and has no effect on the Company’s cash flows. The valuation methodology used varies by instrument and includes a modified Black-Scholes option valuation model utilizing the fair value of underlying common stock and a binomial model with Monte Carlo simulation. The Company has determined the fair value measurements to be a level 3 measurement (see NOTE 2 SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES).
Class A Warrants, Class B Warrants, Series A Warrants and Certain Common Warrants
Our Class A Warrants, Class B Warrants, Series A Warrants and certain common warrants, have an exercise price adjustment provision that in the event the Company sells shares of any additional stock, subject to certain exceptions, at a price per share less than the original exercise price of the respective warrant, the exercise price shall be adjusted to a price equal to the price paid per share for such additional stock. Such exercise price adjustments prohibit the Company from being able to conclude that the warrants are indexed to the Company’s own stock. Accordingly, these warrants are accounted for as derivative liabilities and are recorded at fair value at each reporting date with the change in fair value being recorded in earnings for the period.
For the year ended December 31, 2015 the fair value of these warrants was calculated using a modified Black-Scholes option valuation model utilizing the fair value of underlying common stock. Black-Scholes has inherent limitations for use in the case of a warrant with a price protection provision, since the model is designed to be used when the inputs to the model are static throughout the life of a security. Due to the significant variance between the fair market value of the stock and the exercise price, the Black-Scholes option-pricing model resulted in a total fair value fair value of these warrants in the amount of $61,941 at December 31, 2015.
For the year ended December 31, 2016 due to the multiple reverse stock splits and decline in stock price the fair value of Class A, Class B and common warrants was deemed to be negligible at December 31, 2016.
F-31
Series C Warrants and Unit Purchase Option
Our Series C Warrants contain a cashless exercise provision using a predetermined Black Scholes Value. Such provision, if exercised by the holder, would require the Company to settle these warrants, at its option, either by cash payment or the granting of a variable number of common shares. This provision results in the potential for the Company to either have to net cash settle the warrant or potentially issue an indeterminate number of common shares which prohibits the Company from being able to conclude that the warrants are indexed to the Company’s own stock. Accordingly, the warrants and the unit purchase option are accounted for as derivative liabilities and are recorded at fair value at each reporting date with the change in fair value being recorded in earnings for the period.
For the year ended December 31, 2015 the Series C Warrants and Unit Purchase Option have predetermined inputs to be used in the Black Scholes formula for the determination of the fair value of the warrants and options which the Company used for the calculation of the fair value of the Series C Warrants and Unit Purchase Option at December 31, 2015. The contractual inputs to the Black-Scholes formula are a stock price volatility of 135.00%, the warrant life of 5 years, a risk free rate of 1.61%, the fair value of $128.5 million per share of the equity stock underlying the option and the exercise price of $2.1 million per share of common stock. The total fair value of these warrants and option at December 31, 2015 was $12,404,503.
All of the remaining Series C Warrants were exercised on a cashless basis and the Unit Purchase Option was exercised for cash during the year ended December 31, 2016. The fair value of the warrants and options have predetermined inputs to be used in the Black Scholes formula for the determination of the fair value of the warrants and options which the Company used for the calculation of the fair value of the Series C Warrants and Unit Purchase Option at the exercises dates during the year ended December 31, 2016. The contractual inputs to the Black-Scholes formula are a stock price volatility of 135.00%, the warrant life of 5 years, a risk free rate of 1.61%, the fair value of between $99,120 and $792,123 per share of the equity stock underlying the option and the exercise price of $1.9 million per share of common stock. The total fair value of these warrants and option at their exercise date during the year ended December 31, 2016 was $12,384,852.
2015 Notes Conversion Feature
The 2015 Notes contain provisions that protect holders from future issuances of the Company’s common stock at prices below such convertible notes’ respective conversion price. These provisions could result in modification of the conversion price due to a future equity offering and as such the conversion feature cannot be considered indexed to the Company’s own stock. The note also provides that the Company will repay the principal amount at an initial conversion rate subject to certain adjustment with a floor price. These features represent an embedded derivative that requires bifurcation and was recorded at fair value at issuance and again at December 31, 2015 with the change in fair value being recorded in earnings for the period. At issuance on December 30, 2015 the Company determined the fair value of the conversion feature to be $16.7 million. At December 31, 2015 the Company determined the fair value of the conversion feature to be $16.6 million. Although the embedded conversion feature is bifurcated from the 2015 Notes for measurement purposes, the embedded derivative is combined with the 2015 Notes for presentation purposes on the balance sheet. The following assumptions were used as inputs to the binomial model with Monte Carlo simulation to reflect different scenarios where reset may be triggered in 2016:
|
Trading price of common stock on measurement date |
|
$789,600 - $924,000 |
|
Conversion price (1) |
|
$730,800 - $865,200 |
|
Risk free interest rate (2) |
|
0.86% |
|
Conversion notes lives in years |
|
1.33 |
|
Expected volatility (3) |
|
215% |
|
Expected dividend yield (4) |
|
— |
|
Expected probability of shareholder approval (5) |
|
85% |
|
(1) |
The conversion price of the convertible notes was calculated based on the formula in the Notes agreement as of the respective measurement date |
|
(2) |
The risk-free interest rate was determined by management using the 1.5-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
F-32
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
|
(5) |
Management has estimated a probability of 85% that shareholder approval will be obtained for the removal of the 19.9% conversion cap. This is based on past shareholder voting history and discussions with current shareholders and consultants. |
As discussed in NOTE 8 CONVERTIBLE NOTES PAYABLE, approximately $13.7 million of the 2015 Note was converted into shares of common stock during the year ended December 31, 2016. This resulted in an extinguishment of the derivative liability of approximately $5.5 million which was used in the calculation of the loss on extinguishment of debt. In order to appropriately calculate the extinguishment expense, the derivative liability was marked to fair value at each extinguishment date during the period. The fair value of the derivative was calculated at the various extinguishment dates using a modified binomial model to reflect different scenarios where reset may be triggered using the following range of assumptions: The fair value of the derivative was calculated at the various extinguishment dates using a modified binomial model to reflect different scenarios where reset may be triggered using the following range of assumptions:
|
Trading price of common stock on measurement date |
|
$9.00 - $37,200 |
|
Conversion price (1) |
|
$7.45 - $29,280 |
|
Risk free interest rate (2) |
|
0.29% - 0.52% |
|
Conversion notes lives in years |
|
0.48 - 0.81 |
|
Expected volatility (3) |
|
224.7 – 244.8% |
|
Expected dividend yield (4) |
|
— |
|
(1) |
The conversion price was calculated based on the formula in the 2015 Notes agreement as of the respective measurement dates. |
|
(2) |
The risk-free interest rate was determined by management using the average of the 6 month and 1-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
On November 3, 2016 the remaining $8.4 million of the 2015 Note was exchanged for Series F Preferred Stock. This exchange resulted in the extinguishment of the derivative liability of approximately $1.6 million which was used in the calculation of the loss on extinguishment of debt. In order to appropriately calculate the extinguishment expense, the derivative liability was marked to fair value on the exchange date. The fair value of the derivative was calculated at November 3, 2016 using a modified binomial model to reflect different scenarios where reset may be triggered using the following of assumptions:
|
Trading price of common stock on measurement date |
|
$ |
9.00 |
|
|
Conversion price (1) |
|
$ |
7.74 |
|
|
Risk free interest rate (2) |
|
|
0.52 |
% |
|
Conversion notes lives in years |
|
|
0.48 |
|
|
Expected volatility (3) |
|
|
244.3 |
% |
|
Expected dividend yield (4) |
|
|
— |
|
|
(1) |
The conversion price was calculated based on the formula in the 2015 Notes agreement as of the respective measurement date |
|
(2) |
The risk-free interest rate was determined by management using the average of the 6 month and 1-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
F-33
Series D Warrants and 2015 Subordination Warrants
In connection with the issuance of convertible notes on December 30, 2015, the Company issued Series D Warrants to acquire 8 shares of common stock. In addition, the Company issued 2015 Subordination Warrants to acquire 2 shares of common stock. The Company has determined that the provisions contained in the Series D Warrants and the Subordination Warrants could result in modification of the warrants exercise price resulting in a variable number of additional common shares that could be issued. These warrants also contained a provision for a one-time adjustment at December 31, 2016 such that the number of shares exercisable pursuant to the Series D Warrants shall equal 16.6% and the number of shares exercisable pursuant to the 2015 Subordination Warrants shall equal 0.5% of the number of shares of common stock actually outstanding or deemed to be outstanding on December 31, 2016 (see NOTE 8 CONVERTIBLE NOTES PAYABLE).
These provisions represent a derivative liability that requires recording at fair value at issuance and again at December 31, 2015 with the change in fair value being recorded in earnings for the period. At issuance on December 30, 2015 the Company determined the fair value of the Series D Warrants and 2015 Subordination Warrants to be $16.6 million. At December 31, 2015 the Company determined the fair value of the Series D Warrants and 2015 Subordination Warrants to be $14.1 million using a binomial model with Monte Carlo simulation to reflect different scenarios where reset may be triggered and to project the range of the additional shares to be issued on December 31, 2016 due to the 16.6% and 0.5% requirement using the following assumptions:
|
Trading price of common stock on measurement date |
|
$789,600 - $924,000 |
|
Conversion price (1) |
|
1,554,000 |
|
Risk free interest rate (2) |
|
1.80% |
|
Warrant lives in years |
|
5.50 |
|
Expected volatility (3) |
|
215% |
|
Expected dividend yield (4) |
|
— |
|
(1) |
The exercise price of the Series D Warrants calculated by 120% of the arithmetic average of five weighted average price of the common stock on the five consecutive trading days prior to issuance date on December 30, 2015. |
|
(2) |
The risk-free interest rate was determined by management using the 5-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
On December 31, 2016 pursuant to the terms of the Series D Warrants and 2015 Subordination Warrants, the Company adjusted the Series D Warrants such that they are exercisable into 2,361,468 shares of common stock and adjusted the 2015 Subordination Warrants such that they are exercisable into 71,131 shares of common stock. All other terms remain the same as the original Series D Warrants and 2015 Subordination Warrants.
The Company determined the fair value of the Series D Warrants and 2015 Subordination Warrants to be $4.1 million at December 31, 2016 using a binomial model with a Monte Carlo simulation to reflect different scenarios where reset may be triggered and to project the range of the additional shares to be issued on December 31, 2016 using the following assumptions:
|
Trading price of common stock on measurement date |
|
$ |
1.71 |
|
|
Exercise price (1) |
|
$ |
6.00 |
|
|
Risk free interest rate (2) |
|
|
1.93 |
% |
|
Warrant lives in years |
|
|
4.50 |
|
|
Expected volatility (3) |
|
|
240.9 |
% |
|
Expected dividend yield (4) |
|
|
— |
|
|
(1) |
The exercise price of the Series D and Subordination Warrants was calculated based on the terms in the warrant agreement. |
F-34
|
(2) |
The risk-free interest rate was determined by management using the 5-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
Series E Warrants
In connection with the February 2016 Unit Offering, the Company issued Series E Warrants to purchase 70 shares of common stock as part of the units sold in the offering (see NOTE 10 COMMON AND PREFERRED STOCK). The Series E Warrants contain a provision that for one year from issuance the exercise price per share will adjust if the Company has certain equity issuances for consideration per share that is less than the current exercise price of the Series E Warrants. In addition, these warrants contain a provision for a one-time adjustment one year from date of issuance, to the number of warrants issued. The Company has determined that the provisions contained in the Series E Warrants could result in modification of the exercise price due to a future equity offering resulting in a variable number of additional common shares that could be issued. This prohibits the company from being able to conclude that the warrants are indexed to the Company’s own stock. Accordingly, the warrants represent a derivative liability that requires recording at fair value at issuance and again at each reporting period with the change in fair value being recorded in earnings for the period.
On April 7, 2016, the Company entered into certain warrant exchange agreements (the “Exchange Agreements”), each by and between the Company and a holder of its outstanding Series E Warrants, pursuant to which the Company and each such holder agreed to exchange outstanding Series E Warrants for shares of common stock of the Company. Pursuant to the Exchange Agreements, the Company issued 28 shares of common stock of the Company in exchange for the surrender by the holders to the Company of all 58,800,000 Series E Warrants exercisable to acquire approximately 70 shares of common stock of the Company (representing an exchange ratio of one share of common stock for each 2.5 shares of common stock underlying the surrendered Series E Warrants). The surrendered Series E Warrants were immediately cancelled by the Company (see NOTE 10 COMMON AND PREFERRED STOCK).
The Company determined the fair value of the Series E Warrants to be $6,800,927 at April 7, 2016 using a binomial model with a Monte Carlo simulation model using the following assumptions:
|
|
|
April 7, 2016 |
|
|
|
Trading price of common stock on measurement date |
|
$ |
98,160 |
|
|
Exercise price (1) |
|
$ |
96,240 |
|
|
Risk free interest rate (2) |
|
|
1.30 |
% |
|
Warrant lives in years |
|
|
5.89 |
|
|
Expected volatility (3) |
|
|
228.1 |
% |
|
Expected dividend yield (4) |
|
|
— |
|
|
(1) |
The exercise price of the Series E Warrants was calculated based on the terms in the warrant agreement. |
|
(2) |
The risk-free interest rate was determined by management using an average of the 5-year and 7-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
F-35
Since the Series E Warrants were derivative liabilities at the time of the transaction, the Company has accounted for the exchange as an extinguishment of a liability. Accordingly, all consideration issued to extinguish the liability was recorded at fair value on the date of the extinguishment and the liability extinguished was removed at its carrying value. Since the liabilities extinguished were derivative liabilities, their carrying value is continuously adjusted to equal their fair value. The difference between the fair value of the liability extinguished and the fair value of the consideration provided on April 7, 2016 was recorded as a gain in the statement of operations as follows:
|
Fair value of Series E Warrants exchanged |
|
$ |
6,800,927 |
|
|
Fair value of common stock issued |
|
|
2,659,154 |
|
|
Gain on exchange of warrants |
|
$ |
4,141,773 |
|
Series G Warrants
In connection with the June 2016 Unit Offering, the Company issued Series G Warrants to purchase 163 shares of common stock as part of the units sold in the offering (see NOTE 10 COMMON AND PREFERRED STOCK). The Series G Warrants contain a provision that the exercise price per share will adjust if the Company has certain equity issuances for consideration per share that is less than the current exercise price of the Series G Warrants. The Company has determined that the provisions contained in the Series G Warrants could result in modification of the exercise price due to a future equity offering resulting in a variable number of additional common shares that could be issued. This prohibits the Company from being able to conclude that the warrants are indexed to the Company’s own stock. Accordingly, the warrants represent a derivative liability that requires recording at fair value at issuance and again at each reporting period with the change in fair value being recorded in earnings for the period.
The fair value of the Series G Warrants on June 1, 2016 using the Black Scholes valuation method was $6.0 million using the following assumptions:
|
|
|
|
|
|
|
Trading price of common stock on measurement date |
|
$ |
20,265 |
|
|
Exercise price (1) |
|
$ |
19,950 |
|
|
Risk free interest rate (2) |
|
|
1.39 |
% |
|
Term |
|
|
5.00 |
|
|
Expected volatility (3) |
|
|
227.5 |
% |
|
Expected dividend yield |
|
|
— |
|
|
(1) |
The exercise price of the Series G Warrants was calculated based on the terms in the warrant agreement. |
|
(2) |
The risk-free interest rate was determined by management using an average of the 5-year and 7-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
The fair value of the warrants is in excess of the proceeds received and the Company is required to record the fair value over the net proceeds received as a loss in earnings. Therefore, at inception, the loss recorded in earnings is calculated as follows:
|
Net proceeds received |
|
$ |
5,268,030 |
|
|
Less: |
|
|
|
|
|
Par value of common stock issued |
|
|
(316 |
) |
|
Fair value Series G Warrants issued |
|
|
(6,034,735 |
) |
|
Loss on issuance |
|
$ |
(767,021 |
) |
On July 11, 2016, 85,000 Series G Warrants were exercised for cash in the amount of $113,900 resulting in the issuance of 4 shares of common stock. These warrants are required to be recorded at fair value at the transaction
F-36
date with any change in the fair value from the previous period being recorded in earnings for the period. This revaluing is necessary as derivatives are required to be subsequently measured at fair value under ASC 815.The Company determined the fair value of the 85,000 of the Series G Warrants exercised to be $118,424 at the transaction date of July 11, 2016 resulting in a gain from the change in fair value to be recorded in the statement of operations in the amount of $30,547.
The fair value of the 85,000 Series G Warrants exercised for cash was calculated using a Black Scholes model with the following inputs:
|
|
|
July 11, 2016 |
|
|
|
Trading price of common stock on measurement date |
|
$ |
33,840 |
|
|
Exercise price (1) |
|
$ |
32,160 |
|
|
Risk free interest rate (2) |
|
|
1.03 |
% |
|
Warrant lives in years |
|
|
4.89 |
|
|
Expected volatility (3) |
|
|
225.8 |
% |
|
Expected dividend yield (4) |
|
|
— |
|
|
(1) |
The exercise price of the Series G Warrants was calculated based on the terms in the warrant agreement. |
|
(2) |
The risk-free interest rate was determined by management using the average of the 5-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
The Company determined the fair value of the remaining 3,075,000 Series G Warrants to be $272 on December 31, 2016 using a Black Scholes valuation model with the following assumptions:
|
|
|
December 31, 2016 |
|
|
|
Trading price of common stock on measurement date |
|
$ |
1.71 |
|
|
Exercise price (1) |
|
$ |
6.00 |
|
|
Risk free interest rate (2) |
|
|
1.93 |
% |
|
Warrant lives in years |
|
|
4.41 |
|
|
Expected volatility (3) |
|
|
240.9 |
% |
|
Expected dividend yield (4) |
|
|
— |
|
|
(1) |
The exercise price of the Series G Warrants as defined in the warrant agreement at June 1, 2016. The reset provision at July 1, 2016 that was known at June 30, 2016. |
|
(2) |
The risk-free interest rate was determined by management using the 5-year Treasury Bill as of the respective measurement date. |
F-37
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
Convertible Notes Conversion Feature – 2016 Notes
The 2016 Notes contain provisions that protect holders from future issuances of the Company’s common stock at prices below such convertible notes’ respective conversion price. These provisions could result in modification of the conversion price due to a future equity offering and as such the conversion feature cannot be considered indexed to the Company’s own stock. The 2016 Notes also provide that the Company will repay the principal amount at an initial conversion rate subject to certain adjustments. These features represent an embedded derivative that requires bifurcation and are recorded at fair value at each reporting period with the change in fair value being recorded in earnings for the period.
The Company determined the fair value of the conversion feature to be $80.6 million and $75.8 million, at inception (July 1, 2016) and December 31, 2016, respectively. Although the embedded conversion feature is bifurcated from the 2016 Notes for measurement purposes, the embedded derivative is combined, only to extent of the face value of the note, with the 2016 Notes for presentation purposes on the balance sheet. The Company determined fair value using a modified binomial model to reflect different scenarios where reset may be triggered using the following assumptions:
|
|
|
July 1, 2016 |
|
|
December 31, 2016 |
|
||
|
Trading price of common stock on measurement date |
|
$ |
42,480 |
|
|
$ |
1.71 |
|
|
Exercise price (1) |
|
$ |
32,160 |
|
|
$ |
1.49 |
|
|
Risk free interest rate (2) |
|
|
0.59 |
% |
|
|
0.97 |
% |
|
Term |
|
|
1.84 |
|
|
|
1.33 |
|
|
Expected volatility (3) |
|
|
228.1 |
% |
|
|
240.9 |
% |
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
(1) |
The conversion price was calculated based on the formula in the 2016 Notes agreement as of the respective measurement date |
|
(2) |
The risk-free interest rate was determined by management using the average of the 1-year and 2-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
Series H Warrants and 2016 Subordination Warrants
In connection with the issuance of the 2016 Notes, the Company issued Series H Warrants to acquire 2,346 shares of common stock and 2016 Subordination Warrants to acquire 71 shares of common stock. The Series H Warrants and 2016 Subordination Warrants contain provisions that will adjust the exercise price upon certain equity issuances. The Company has determined that the provisions contained in the Series H Warrants and the 2016 Subordination Warrants could result in modification of the exercise price due to future equity offerings resulting in a variable number of additional common shares that could be issued. This prohibits the company from being able to conclude that the warrants are indexed to the Company’s own stock. Accordingly, the warrants represent a derivative liability that requires recording at fair value at each reporting period with the change in fair value being recorded in earnings for the period.
F-38
The Company determined the fair value of the Series H Warrants and 2016 Subordination Warrants to be $101.6 million and $4,089 at inception (July 1, 2016) and December 31, 2016, respectively. The Company determined the fair value using a modified binomial model to reflect different scenarios where reset may be triggered using the following assumptions:
|
|
|
July 1, 2016 |
|
|
December 31, 2016 |
|
||
|
Trading price of common stock on measurement date |
|
$ |
42,480 |
|
|
$ |
1.71 |
|
|
Exercise price (1) |
|
$ |
49,920 |
|
|
$ |
6.00 |
|
|
Risk free interest rate (2) |
|
|
1.01 |
% |
|
|
1.93 |
% |
|
Term |
|
|
5.50 |
|
|
|
5.00 |
|
|
Expected volatility (3) |
|
|
228.1 |
% |
|
|
240.9 |
% |
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
(1) |
The exercise price of the Series H and Subordination Warrants was calculated based on the terms in the warrant agreement. |
|
(2) |
The risk-free interest rate was determined by management using the 5-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
F-39
On November 3, 2016, the Company issued 8,436 shares of Series F Preferred Stock for the exchange of $8,433,113 in remaining principal on the 2015 Notes (See NOTE 8 CONVERTIBLE NOTES PAYABLE). Of these, 2,096 shares were immediately mandatorily converted into 349,333 shares of common stock at a conversion price of $6.00 per share. The Company has concluded that the remaining non-mandatory 6,340 shares of Series F Preferred Stock are within the scope of ASC 480 as they predominantly represent an unconditional obligation to issue a variable number of common shares for a fixed monetary amount. Accordingly, they will be accounted for as liabilities in the financial statements and measured initially and subsequently at fair value with any change in fair value to be recorded in earnings. The Company determined the fair value of the non-mandatory Series F Preferred Stock at issuance on November 3, 2016 to be $8,927,249 which was included as part of the reacquisition price of the 2015 Notes. During December 2016, 480 shares of Series F Preferred Stock were converted at the option of the holder into 80,000 shares of common stock at a conversion price of $6.00 per share. The Company determined the fair value of these shares of Series F Preferred Stock to be $298,159 in the aggregate on the respective conversion dates. As of December 31, 2016, there are 5,860 shares of Series F Preferred Stock outstanding convertible into 976,667 shares of common stock. The Company is accounting for these as a liability on the financial statements with a period end fair value of $5,655,006.
The Company used the following assumptions for the fair value calculations of non-mandatory shares of Series F Preferred Stock shares using the modified binomial model to reflect different scenarios where reset may be triggered:
|
|
|
July 1, 2016 |
|
|
December 2016 |
|
|
December 31, 2016 |
|
|||
|
Trading price of common stock on measurement date |
|
$ |
9.00 |
|
|
$1.84 - $1.85 |
|
|
$ |
1.71 |
|
|
|
Exercise price (1) |
|
$ |
6.00 |
|
|
$1.59 - $1.60 |
|
|
$ |
1.52 |
|
|
|
Risk free interest rate (2) |
|
|
0.81 |
% |
|
1.22 - 1.26% |
|
|
|
1.20 |
% |
|
|
Term |
|
|
2.00 |
|
|
|
1.84 |
|
|
|
1.84 |
|
|
Expected volatility (3) |
|
|
244.3 |
% |
|
|
240.9 |
% |
|
|
240.9 |
% |
|
Expected dividend yield (4) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
(1) |
The conversion price of the Series F was calculated based off the lower of the fixed conversion rate of $6.00 (split adjusted) and 85% of the lower of the (1) 3 lowest VWAP days in the past 20 trading days and (2) the VWAP of the preceding day. |
|
(2) |
The risk-free interest rate was determined by management using the 2-year Treasury Bill as of the respective measurement date. |
|
(3) |
The volatility factor was estimated by using the historical volatilities of the Company’s trading history. |
|
(4) |
Management determined the dividend yield to be 0% based upon its expectation that it will not pay dividends for the foreseeable future. |
F-40
The Company classifies assets and liabilities measured at fair value in their entirety based on the lowest level of input that is significant to their fair value measurement. No financial assets were measured on a recurring basis at December 31, 2016 and December 31, 2015. The following tables set forth the financial liabilities measured at fair value on a recurring basis by level within their fair value hierarchy at December 31, 2016 and December 31, 2015:
|
|
|
Fair Value Measurement at December 31, 2016 |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
Fair value liability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series F preferred stock |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
5,655,006 |
|
|
$ |
5,655,006 |
|
|
Common stock warrants |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
4,121,836 |
|
|
$ |
4,121,836 |
|
|
Conversion feature of 2016 Notes |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
75,826,761 |
|
|
$ |
75,826,761 |
|
|
Total fair value liabilities |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
85,603,603 |
|
|
$ |
85,603,603 |
|
|
|
|
Fair Value Measurement at December 31, 2015 |
|
|||||||||||||
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
|
Fair value liability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock warrants |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
26,592,532 |
|
|
$ |
26,592,532 |
|
|
Conversion feature of 2015 Notes |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
16,588,940 |
|
|
$ |
16,588,940 |
|
|
Total fair value liabilities |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
43,181,472 |
|
|
$ |
43,181,472 |
|
The following summarizes the total change in the value of the fair value Level 3 liabilities during the year ended December 31, 2015:
|
|
|
Common Stock Warrants |
|
|
Conversion Feature of Notes |
|
|
Series F Preferred Stock |
|
|
Total |
|
||||
|
As of December 31, 2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at January 1, 2015 |
|
$ |
9,998,636 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
9,998,636 |
|
|
Issuance of warrants, options and convertible note |
|
|
39,372,885 |
|
|
|
16,654,094 |
|
|
|
— |
|
|
|
56,026,979 |
|
|
Exercise and expiration of warrants and unit purchase option |
|
|
(42,558,951 |
) |
|
|
— |
|
|
|
— |
|
|
|
(42,558,951 |
) |
|
Change in fair value of warrants, options and conversion feature |
|
|
19,779,962 |
|
|
|
(65,154 |
) |
|
|
— |
|
|
|
19,714,808 |
|
|
Balance at December 31, 2015 |
|
$ |
26,592,532 |
|
|
$ |
16,588,940 |
|
|
$ |
— |
|
|
$ |
43,181,472 |
|
The following table reconciles the Level 3 fair value liabilities to the derivative liability on the balance sheet at December 31, 2015:
|
|
|
Common Stock Warrants |
|
|
Conversion Feature of Notes |
|
|
Total Derivative Liability |
|
|||
|
As of December 31, 2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value Level 3 liabilities |
|
$ |
26,592,532 |
|
|
$ |
16,588,940 |
|
|
$ |
43,181,472 |
|
|
Portion of derivative liability combined with convertible note |
|
|
— |
|
|
|
(16,588,940 |
) |
|
|
(16,588,940 |
) |
|
Derivative liability on balance sheet at December 31, 2015 |
|
$ |
26,592,532 |
|
|
$ |
— |
|
|
$ |
26,592,532 |
|
F-41
The following summarizes the total change in the value of the fair value Level 3 liabilities during the year ended December 31, 2016:
|
|
|
Common Stock Warrants |
|
|
Conversion Feature of Notes |
|
|
Series F Preferred Stock |
|
|
Total |
|
||||
|
As of December 31, 2016: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at January 1, 2016 |
|
$ |
26,592,532 |
|
|
$ |
16,588,940 |
|
|
$ |
— |
|
|
$ |
43,181,472 |
|
|
Issuance of convertible note, warrants and preferred stock |
|
|
112,770,932 |
|
|
|
80,599,528 |
|
|
|
12,071,249 |
|
|
|
205,441,709 |
|
|
Exercise and expiration of warrants, options, convertible notes,and preferred stock |
|
|
(19,304,203 |
) |
|
|
(7,082,033 |
) |
|
|
(3,442,159 |
) |
|
|
(29,828,395 |
) |
|
Change in fair value of warrants, options, conversion feature and preferred stock |
|
|
(115,937,425 |
) |
|
|
(14,279,674 |
) |
|
|
(2,974,084 |
) |
|
|
(133,191,183 |
) |
|
Balance at December 31, 2016 |
|
$ |
4,121,836 |
|
|
$ |
75,826,761 |
|
|
$ |
5,655,006 |
|
|
$ |
85,603,603 |
|
The following table reconciles the Level 3 fair value liabilities to the derivative liability on the balance sheet at December 31, 2016:
|
|
|
Common Stock Warrants |
|
|
Conversion Feature of Notes |
|
|
Total Derivative Liability |
|
|||
|
As of December 31, 2016: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value Level 3 liabilities |
|
$ |
4,121,836 |
|
|
$ |
75,826,761 |
|
|
$ |
79,948,597 |
|
|
Portion of derivative liability combined with convertible note |
|
|
— |
|
|
|
(43,604,417 |
) |
|
|
(43,604,417 |
) |
|
Derivative liability on balance sheet at December 31, 2016 |
|
$ |
4,121,836 |
|
|
$ |
32,222,344 |
|
|
$ |
36,344,180 |
|
NOTE 13 EMPLOYEE STOCK OPTIONS
The Company has three stock based employee compensation plans, the 2006 Stock Option Plan, the 2014 Stock Option Plan, and the Omnibus Plan pursuant to which certain employees and non-employee directors have been granted options to purchase common stock. The Company had 738,534 employee stock options exercisable into 70 shares of stock outstanding as of December 31, 2016 and 792,534 employee stock options exercisable into 88 shares of stock outstanding as of December 31, 2015. All options vest in installments over a three to four year period and expire ten years from the date of grant.
There were no employee stock options issued during the year ended December 31, 2016. For the year ended December 31, 2015, the Company awarded 117,500 common stock options exercisable into 3 shares of stock under the Omnibus Plan to certain employees and non-employee directors with an exercise price of $2.56 per option that expire in ten years and vest over a three and four year period. The Company determined the value of the 117,500 options granted during the year ended December 31, 2015 to be $268,202 of which $29,309 was expensed in 2015 with the remainder to be expensed over the vesting term of the options.
F-42
The following is the weighted average of the assumptions used in calculating the fair value of the options granted in 2015 using the Black-Scholes method:
|
Fair market value of one share of common stock |
|
$129 million |
|
|
|
Aggregate exercise price of 60 options |
|
$129 million |
|
|
|
Risk free rate |
|
|
1.71 |
% |
|
Dividend yield |
|
|
— |
% |
|
Expected volatility |
|
|
127.52 |
% |
|
Expected term |
|
6.14 years |
|
|
The following table summarizes the Company’s total employee stock option activity for the years ended December 31, 2016 and 2015:
|
|
|
Options |
|
|
Total Shares of Common Stock Underlying the Options |
|
|
Weighted Average Exercise Price for One Common Share (millions) |
|
|
Weighted Average Remaining Contractual Term in Years |
|
|
Intrinsic Value $ |
|
|||||
|
As of December 31, 2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options outstanding as of January 1, 2015 |
|
|
703,034 |
|
|
|
99 |
|
|
$ |
150.2 |
|
|
|
8.8 |
|
|
|
— |
|
|
Granted |
|
|
117,500 |
|
|
|
3 |
|
|
$ |
129.0 |
|
|
|
9.6 |
|
|
|
— |
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
|
— |
|
|
Forfeited/expired |
|
|
(28,000 |
) |
|
|
(14 |
) |
|
$ |
260.1 |
|
|
|
8.6 |
|
|
|
— |
|
|
Options outstanding as of December 31, 2015 |
|
|
792,534 |
|
|
|
88 |
|
|
$ |
143.1 |
|
|
|
8.0 |
|
|
|
— |
|
|
As of December 31, 2016: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
Options outstanding as of January 1, 2016 |
|
|
792,534 |
|
|
88 |
|
|
$ |
143.1 |
|
|
|
8.0 |
|
|
|
— |
|
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Forfeited/expired |
|
|
(54,000 |
) |
|
|
(18 |
) |
|
$ |
118.5 |
|
|
|
7.8 |
|
|
|
— |
|
|
Options outstanding as of December 31, 2016 |
|
|
738,534 |
|
|
|
70 |
|
|
$ |
145.0 |
|
|
|
7.0 |
|
|
|
— |
|
Outstanding and exercisable stock options as of December 31, 2016 and 2015 are as follows:
|
|
|
Options Outstanding |
|
|
Options Exercisable |
|
||||||||||||||||||
|
|
|
Number of Options Outstanding |
|
|
Remaining Life (Years) |
|
|
Exercise Price per Share of Stock ($ millions) |
|
|
Number of Options Exercisable |
|
|
Exercise Price |
|
|
Intrinsic Value |
|
||||||
|
December 31, 2015 |
|
|
792,534 |
|
|
|
8.0 |
|
|
$ |
143.1 |
|
|
|
328,445 |
|
|
$ |
154.7 |
|
|
$ |
— |
|
|
December 31, 2016 |
|
|
738,534 |
|
|
|
7.0 |
|
|
$ |
145.0 |
|
|
|
472,824 |
|
|
$ |
152.8 |
|
|
$ |
— |
|
The estimated fair value of the Company stock options, less expected forfeitures, is amortized over the options vesting period on the straight-line basis. The Company recognized the following equity-based compensation expenses during the twelve months ended December 31, 2016 and 2015:
|
|
|
December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Stock based compensation expense |
|
$ |
136,060 |
|
|
$ |
110,123 |
|
As of December 31, 2016 and 2015, there were $272,847 and $408,907 of total unrecognized compensation cost with a remaining vesting period of 1.71 and 2.71 years, respectively.
F-43
NOTE 14 RELATED PARTY TRANSACTIONS
The Company’s obligations pursuant to its sale-leaseback agreements described in NOTE 6 LEASE COMMITMENTS are secured by letters of credit (Letters of Credit) in an aggregate amount of $3,000,000. The Letters of Credit were issued by a bank at the behest of a non-profit foundation (the “Foundation”) and Spring Forth Investments. Mr. Spafford, one of our directors, and his wife, Susan Spafford, have been designated by the Foundation as “Founding Trustees” under its bylaws and have authority to control certain activities of the Foundation. The Company is obligated to reimburse the Foundation and Spring Forth Investments for any draws made under the Letters of Credit pursuant to two reimbursement agreements between the Company and the Foundation and Spring Forth Investments dated October 30, 2013. The Company has agreed to pay each entity 10% interest per annum on the total amount of the letter of credit. Our obligations under the reimbursement agreements are secured by a security interest in all of our assets pursuant to a Security Agreement dated October 30, 2013. As of December 31, 2016, no draws on the line of credit had taken place. The Company paid $226,667 and $250,000 in interest to the entities during the year ended December 31, 2016 and 2015, respectively.
In relation to the 2015 Note financing on December 30, 2015, the Foundation and Spring Forth Investments agreed to enter into subordination agreements with the collateral agent in the 2015 Note financing whereby each agreed to subordinate their debt to the notes issued in the convertible note financing. As consideration for their agreement the Company issued them 2015 Subordination Warrants exercisable for 2 shares of common stock. The 2015 Subordination Warrants included an adjustment provision which provides that the number of common shares the 2015 Subordination Warrants are exercisable into will increase on December 31, 2016 to be 0.5% of the sum of the number of shares actually outstanding on December 31, 2016 plus the number of shares of common stock deemed to be outstanding pursuant to all outstanding options, warrants or convertible securities of the Company. Accordingly, on December 31, 2016 the Company adjusted the 2015 Subordination Warrants such that they are exercisable into 71,131 shares of common stock. All other terms remain the same as the original 2015 Subordination Warrant.
In relation to the 2016 Note financing on July 1, 2016, the Foundation and Spring Forth Investments agreed to enter into subordination agreements with the collateral agent in the 2016 Note financing whereby each agreed to subordinate their debt to the notes issued in the convertible note financing. As consideration for their agreement, the Company has agreed to issue to the entities warrants exercisable for 70 shares of common stock (see NOTE 11 WARRANTS).
NOTE 15 INCOME TAXES
The Company utilizes the asset and liability approach to measuring deferred tax assets and liabilities based on temporary differences existing at each balance sheet date using currently enacted tax rates in accordance with FASB ASC 740. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment.
The income tax expense for the years ended December 31, 2016 and 2015 consists of the following:
|
|
|
2016 |
|
|
2015 |
|
||
|
Current |
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
— |
|
|
$ |
— |
|
|
State and Local |
|
|
1,750 |
|
|
|
1,250 |
|
|
|
|
|
1,750 |
|
|
|
1,250 |
|
|
Deferred |
|
|
|
|
|
|
|
|
|
Federal |
|
|
— |
|
|
|
— |
|
|
State and Local |
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
$ |
1,750 |
|
|
$ |
1,250 |
|
F-44
The components of the Company’s deferred tax assets for the years ended December 31, 2016 and 2015 are as follows:
|
|
|
2016 |
|
|
2015 |
|
||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
Net operating losses |
|
|
17,720,179 |
|
|
|
4,263,605 |
|
|
Depreciation and amortization |
|
|
(4,742 |
) |
|
|
(182,903 |
) |
|
Allowance for doubtful accounts |
|
|
9,681 |
|
|
|
6,324 |
|
|
Accrued vacation |
|
|
161,374 |
|
|
|
112,892 |
|
|
Accrued personal property tax |
|
|
3,152 |
|
|
|
4,083 |
|
|
Other |
|
|
4,292 |
|
|
|
1,651 |
|
|
Total deferred tax assets |
|
|
17,893,936 |
|
|
|
4,205,652 |
|
|
Less: Valuation allowance |
|
|
(17,893,936 |
) |
|
|
(4,205,652 |
) |
|
Net deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
The following is a reconciliation of the reported amount of income tax expense (benefit) for the years ended December 31, 2016 and 2015 to the amount of income tax expenses that would result from applying the statutory rate to pretax income.
|
|
|
2016 |
|
|
2015 |
|
||
|
Benefit for income taxes computed at federal statutory rate |
|
$ |
(30,309,817 |
) |
|
$ |
(19,685,292 |
) |
|
State income taxes, net of federal tax benefit |
|
|
(3,977,787 |
) |
|
|
(1,998,974 |
) |
|
Non-deductible expenses |
|
|
20,524,787 |
|
|
|
12,902,916 |
|
|
NOL write off due to Section 382 limitation |
|
|
— |
|
|
|
23,200,232 |
|
|
Increase (decrease) in valuation allowance |
|
|
13,688,236 |
|
|
|
(14,277,865 |
) |
|
Other, net |
|
|
76,331 |
|
|
|
(139,767 |
) |
|
Provision for income taxes |
|
$ |
1,750 |
|
|
$ |
1,250 |
|
|
Effective tax rate |
|
|
(0.01 |
)% |
|
|
(0.01 |
)% |
As of December 31, 2016 the Company has generated operating losses. As a result the Company has recorded a full valuation allowance against its net deferred tax assets as of December 31, 2016 and 2015. The valuation allowance increased by $13,688,236 during the tax year ended December 31, 2016.
During the prior year ended December 31, 2015, the Company had a change of ownership for Internal Revenue Code purposes. The amount of the net operating losses for federal and state purposes was reduced to the amount that can be used considering those limitations. The amount presented is reduced based on the section 382 limitation and the carryforward period as provided by the federal and state tax laws.
As of December 31, 2016 and 2015, the Company has a net operating loss carry forwards for Federal income tax purposes of $46.7 million and $11.5 million, respectively, which expire in varying amounts during the tax years 2023 and 2035. The Company has net operating loss carry forwards for State income tax purposes of $41.6 million and $10.5 million which expire in varying years from 2025 to 2035.
Under FASB ASC 740, tax benefits are recognized only for tax positions that are more likely than not to be sustained upon examination by tax authorities. The amount recognized is measured as the largest amount of benefit that is greater than 50 percent likely to be realized upon ultimate settlement. Unrecognized tax benefits are tax benefits claimed in the Company’s tax returns that do not meet these recognition and measurement standards. As of December 31, 2016 and 2015, the Company has no liabilities for unrecognized tax benefits.
The Company’s policy is to recognize potential interest and penalties accrued related to unrecognized tax benefits within income tax expense. For the years ended December 31, 2016, and 2015, the Company did not recognize any interest or penalties in its statement of operations, nor did it have any interest or penalties accrued in its balance sheet at December 31, 2016 and 2015 relating to unrecognized tax benefits.
F-45
The tax years 2013-2016 remain open to examination for federal income tax purposes and by the other major taxing jurisdictions to which the Company is subject.
NOTE 16 LEGAL PROCEEDINGS
On April 5, 2016 and May 31, 2016, Great Basin Scientific, Inc., received notices from the Utah Labor Commission, Occupational Safety and Health Division (ULC) and/or the Occupational Safety and Health Administration (OSHA) that former employee Christina Steele filed a claim alleging retaliation in violation of the Utah Occupational Safety and Health Act as well as the Corporate and Criminal Fraud Accountability Act of 2002, the Sarbanes-Oxley Act and the Occupational Safety and Health Act, among other claims relating to her employment. Ms. Steele alleges that Great Basin retaliated against her by terminating her employment after she allegedly acted as a whistleblower by allegedly raising concerns with management. Ms. Steele seeks lost wages, future wages, consequential losses, emotional distress damages, interest, fees and costs. The OSHA charge remains under investigation.
On June 15, 2016, Ms. Steele also filed a complaint against Great Basin Scientific, Inc. in the United States District Court for the District of Utah alleging retaliation in violation of the False Claims Act based on similar alleged facts. Ms. Steele seeks back pay, special damages, consequential damages, compensatory damages, interest, fees and costs. On August 15, 2016, Great Basin Scientific, Inc. filed a motion to dismiss Ms. Steele’s claims. On November 21, 2016, the United States District Court for the District of Utah granted the Company's motion to dismiss and dismissed Ms. Steele's claims with prejudice. Judgement was entered in favor of Great Basin Scientific, Inc. on November 28, 2016. Ms. Steele has appealed the court’s order to the United States Court of Appeals for the 10th Circuit. The court has not yet set a briefing schedule but it is unlikely that the 10th Circuit will issue a decision in this case until at least the fall of 2017.
The Company asserts that the claims are without merit and that the employee resigned and was not terminated.
We are not currently a party to any other material pending legal proceeding or regulatory or government investigations. We may become involved in litigation from time to time relating to claims arising in the ordinary course of our business.
NOTE 17 GEOGRAPHIC INFORMATION
The Company has both domestic (U.S.) and international customers for its products. Sales for the years ended December 31, 2016 and 2015 were as follows:
|
|
|
2016 |
|
|
2015 |
|
||
|
Domestic sales |
|
$ |
3,010,371 |
|
|
$ |
2,096,825 |
|
|
International sales |
|
|
37,755 |
|
|
|
45,215 |
|
|
Total sales |
|
$ |
3,048,126 |
|
|
$ |
2,142,040 |
|
NOTE 18 SUBSEQUENT EVENTS
In January and February 2017 holders of the 2016 Notes were issued shares of the Company’s common stock in connection with conversions at the election of such holders pursuant to the terms of the 2016 Notes. In connection with the conversions, the Company issued 1,485,139,803 shares of Common Stock. As per the terms of the 2016 Notes, the Conversion Shares immediately reduced the principal amount outstanding of the 2016 Notes by $3.9 million based upon a conversion price between $0.044 and $0.00068 per share.
In January and February 2017 the holders of 2016 Notes voluntarily agreed to remove restrictions on the Company’s use of an aggregate of approximately $3.5 million in cash previously funded to the Company and authorized the release of those funds from the restricted accounts of the Company for each 2016 Note holder in accordance with that certain Master Control Account Agreement previously entered into by and among the Company, UBS Financial Services Inc. and the collateral agent.
F-46
In February 2017, the Company and the holders of 2016 Notes entered into an agreement, pursuant to which the Company agreed to redeem $38.9 million of the 2016 Notes held by each of the holders for an aggregate redemption price of $38.9 million, which will satisfy such redemption note in full. The Company paid the redemption price for the redemption notes from cash held in the restricted accounts of the Company.
At a Special Meeting of Stockholders held on March 9, 2017, our stockholders (i) approved a reverse split of our common stock at a ratio between 1-for-1,700 and 1-for-2,000, to be effective upon a date on or prior to May 31, 2017, such ratio and date to be determined by our Board and (ii) approved the increase to our authorized common stock from 1,500,000,000 shares to 3,000,000,000 shares.
F-47



