Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - Fulgent Genetics, Inc. | flgt-ex321_376.htm |
| EX-31.2 - EX-31.2 - Fulgent Genetics, Inc. | flgt-ex312_377.htm |
| EX-31.1 - EX-31.1 - Fulgent Genetics, Inc. | flgt-ex311_378.htm |
| EX-23.1 - EX-23.1 - Fulgent Genetics, Inc. | flgt-ex231_232.htm |
| EX-21.1 - EX-21.1 - Fulgent Genetics, Inc. | flgt-ex211_379.htm |
| EX-10.8 - EX-10.8 - Fulgent Genetics, Inc. | flgt-ex108_234.htm |
162
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-37894
FULGENT GENETICS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
81-2621304 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
4978 Santa Anita Avenue Temple City, CA |
91780 |
|
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (626) 350-0537
Securities registered pursuant to Section 12(b) of the Act:
|
|
||
|
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☐ NO ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES ☒ NO ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer”, and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
Non-accelerated filer |
|
☒ (Do not check if a smaller reporting company) |
|
Smaller reporting company |
|
☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). YES ☐ NO ☒
The registrant did not have a public float on June 30, 2016 (the last business day of its most recently completed second fiscal quarter) because there was no public market for the registrant’s common equity as of such date.
As of March 16, 2017, there were 17,676,256 outstanding shares of the registrant’s common stock.
Portions of the registrant’s definitive proxy statement for its 2017 annual meeting of stockholders are incorporated by reference into Part III of this report.
|
|
|
|
|
Page |
|
|
|
|
|
|
|
Item 1. |
|
|
1 |
|
|
Item 1A. |
|
|
20 |
|
|
Item 1B. |
|
|
41 |
|
|
Item 2. |
|
|
41 |
|
|
Item 3. |
|
|
41 |
|
|
Item 4. |
|
|
41 |
|
|
|
|
|
42 |
|
|
Item 5. |
|
|
42 |
|
|
Item 6. |
|
|
44 |
|
|
Item 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
46 |
|
Item 7A. |
|
|
60 |
|
|
Item 8. |
|
|
62 |
|
|
Item 9. |
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
|
62 |
|
Item 9A. |
|
|
62 |
|
|
Item 9B. |
|
|
62 |
|
|
|
|
|
63 |
|
|
Item 10. |
|
|
63 |
|
|
Item 11. |
|
|
63 |
|
|
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
63 |
|
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
63 |
|
Item 14. |
|
|
63 |
|
|
|
|
|
64 |
|
|
Item 15. |
|
|
64 |
|
|
|
|
64 |
i
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or Exchange Act. Forward-looking statements relate to future events or circumstances or our future performance and are based upon our current assumptions, expectations and beliefs concerning future developments and their potential effect on our business. The words “believe,” “may,” “will,” “potentially,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “plan,” “expect” and similar expressions that convey uncertainty of future events or outcomes identify forward-looking statements.
The forward-looking statements in this report include statements about, among other things:
|
|
• |
our operating performance, including our ability to achieve equal or higher levels of revenue and achieve or grow profitability; |
|
|
• |
the rate and degree of market acceptance and adoption of our tests and genetic testing generally and other anticipated trends in our industry; |
|
|
• |
our ability to continue to expand the number of genes covered by our tests and introduce other improvements to our tests; |
|
|
• |
our ability to grow and diversify our customer base and increase demand for our tests from existing and new customers; |
|
|
• |
our ability to maintain relationships with existing international customers and increase our global presence; |
|
|
• |
our ability to effectively manage any growth we may experience, including expanding our infrastructure and hiring additional skilled personnel in order to support any such growth; |
|
|
• |
our ability to comply with U.S. and foreign regulations applicable to our business and developments with respect to these regulations; |
|
|
• |
our sales and marketing plans, including our sales and marketing strategies and our expansion of our sales and marketing team; |
|
|
• |
our expectations regarding our ability to obtain and maintain protection of our trade secrets and other intellectual property rights and not infringe the rights of others; |
|
|
• |
our expectations regarding our future capital requirements and our ability to appropriately forecast and plan our expenses; and |
These forward-looking statements are subject to a number of risks and uncertainties, including those described under Item1A. “Risk Factors” and elsewhere in this report. Moreover, we operate in a competitive and rapidly evolving industry and new risks emerge from time to time. It is not possible for us to predict all of the risks we may face, nor can we assess the impact of all factors on our business or the extent to which any factor or combination of factors could cause actual results to differ materially from those described in or implied by any forward-looking statements we make. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this report may not occur and actual results could differ materially and adversely from those described in or implied by our forward-looking statements. Although we have based the forward-looking statements we make in this report on assumptions and expectations we believe to be reasonable, we cannot guarantee future results, levels of activity, performance or achievements. As a result, you should not rely upon forward-looking statements as predictions of future events and you should read this report with the understanding that our actual future results, levels of activity, performance and achievements may be materially different than what we
i
expect. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this report to conform these statements to actual results or to changes in our expectations.
We qualify all of our forward-looking statements by this cautionary note.
This report reflects the completion of the Reorganization, as defined and described below, on September 30, 2016. Pursuant to the Reorganization, Fulgent Therapeutics LLC became a wholly owned subsidiary of the registrant, Fulgent Genetics, Inc. As used in this report, unless the context otherwise requires, (i) the term “Fulgent LLC” refers to Fulgent Therapeutics LLC, (ii) the term “Fulgent Inc.” refers to Fulgent Genetics, Inc. and (iii) the terms “Fulgent,” the “company,” “we,” “us” and “our” refer, for periods prior to completion of the Reorganization, to Fulgent LLC and, for periods after completion of the Reorganization, to Fulgent Inc. and its consolidated subsidiaries after giving effect to the Reorganization. Following the Reorganization, Fulgent Inc. is a holding company with no material assets other than 100% of the equity interests in its subsidiaries, including Fulgent LLC, and Fulgent LLC is considered Fulgent Inc.’s predecessor for accounting purposes and its financial statements for all periods prior to completion of the Reorganization constitute Fulgent Inc.’s historical financial statements. In this report, Fulgent LLC’s equity interests are referred to as “units” and Fulgent LLC’s equity holders are referred to as “members.”
We own registered trademark rights to Fulgent™ and our company name. Any other service marks, trademarks and trade names appearing in this report are the property of their respective owners. We do not use the ™ symbol in each instance in which one of our common law trademarks appears in this report, but this should not be construed as any indication that we will not assert our rights thereto to the fullest extent under applicable law.
ii
Overview
We are a rapidly growing technology company with an initial focus on offering comprehensive genetic testing to provide physicians with clinically actionable diagnostic information they can use to improve the overall quality of patient care. We have developed a proprietary technology platform that integrates sophisticated data comparison and suppression algorithms, adaptive learning software, advanced genetic diagnostics tools and integrated laboratory processes. This platform allows us to offer a broad and flexible test menu and continually expand and improve our proprietary genetic reference library, while maintaining accessible pricing, high accuracy and competitive turnaround times. Combining next generation sequencing with our technology platform, we can perform full-gene sequencing with deletion/duplication analysis in single-gene tests, pre-established, multi-gene, disease-specific panels and customized panels that can be tailored to meet specific customer needs. We believe our test menu offers more genes for testing than our competitors in today’s market, which enables us to provide expansive options for test customization and clinically actionable results. After launching our first commercial genetic tests focused on rare pediatric diseases in 2013, our tests covered more than 1,000 genes in 100 panels by the first quarter of 2014 and more than 10,000 genes in over 170 panels by the end of 2015. Today, we have further expanded our test menu to offer approximately 18,000 single-gene tests and more than 300 panels that collectively test for approximately 7,700 genetic conditions, including various cancers, cardiovascular diseases, neurological disorders and pediatric tests.
Genetic testing has experienced significant growth in recent years. As this trend continues, we believe genetic testing will become a more accepted part of standard medical care and the knowledge of a person’s unique genetic makeup will begin to play a more important role in the practice of medicine. Genetic testing offers the possibility of early identification of a disease or a genetic predisposition to a disease. As a result, we believe widespread genetic testing could enable significant health improvements and healthcare cost reductions. Furthermore, we believe genetic testing and existing and future diagnostics tools will facilitate production of more comprehensive information that physicians can use to enhance disease prognosis and prediction, as well as for pharmacogenomic purposes. According to GrandView Research, the size of the global next generation sequencing, or NGS, genetic testing market, which includes pre-sequencing, sequencing and data analysis, is estimated to have been approximately $4.0 billion in 2016, including approximately $1.4 billion in the United States, and is expected to reach approximately $10.5 billion by 2022, including approximately $3.6 billion in the United States.
While adoption of genetic testing has increased in recent years, we believe widespread utilization has been limited because many tests are prohibitively expensive, are produced through inefficient processes and often do not result in clinically actionable data. Through our technology, we have developed genetic tests designed to address these limitations and provide a robust platform for future growth. The key features of our technology platform include: proprietary gene probes we develop and manufacture that are engineered to interact with our software; data comparison algorithms that allow for the efficient comparison of DNA sequences to publicly available databases and our proprietary reference library of genetic information; data suppression algorithms that reduce irrelevant noise in the genetic data we collect; adaptive learning software supporting our reporting systems; and integrated laboratory information management systems that allow us to efficiently manage workflow, monitor quality and ensure the fidelity of information generation and analytics for reporting. This technology platform allows us to deliver comprehensive, adaptable, clinically actionable and affordable genetic analysis while maintaining a low cost per billable test, enabling us to efficiently meet customer needs with the latest genes, panels and custom offerings. We believe our technology platform provides a sustainable competitive advantage in genetic testing today and as we implement new diagnostic tools in the future.
We believe we are well-positioned to succeed in today’s market because our technology platform has enabled us to develop a test menu that we believe produces more actionable results than available alternatives. A retrospective study conducted by the University of Southern California, or USC, Norris Comprehensive Cancer Center of 475 individuals with a personal or family history of cancer who had undergone a clinically indicated multi-gene panel test from one of six commercial laboratories found that multi-gene panel testing increases the yield of mutations detected and adds to the capability of providing individualized cancer risk assessment. Of the 17 Fulgent tests evaluated in the study, approximately 35% identified a genetic mutation compared to an average of approximately 17% of the other commercial laboratories’ tests included in the study. We believe our tests’ comprehensive data output and improved detection rate, both made possible by our expansive genetic coverage, provide physicians with information they can readily incorporate into treatment decisions for their patients, which we refer to as clinical actionability. In addition, our technology platform enables us to perform customized genetic tests using our expansive library of genes, and we believe this flexibility increases efficiency and the utility of the genetic data we produce. We have generated growing demand for our tests with relatively little marketing efforts to date, which we believe demonstrates the advantages of our offering compared to other available testing alternatives.
1
Our existing customer base consists primarily of hospitals and medical institutions, which are frequent and high-volume users of genetic tests. We believe our relationships with these customers provide an avenue for further growth as we seek to deepen these relationships and drive increased ordering. We believe the key to further penetrating our existing customer base and expanding into new customer markets is to continue to focus on delivering a superior test menu while maintaining affordable prices. In order to offer our customers affordable price points, we continue to enhance our technology platform to develop tests that we can perform at a low internal cost.
Our headquarters are located in Temple City, California, where we have our corporate offices and a laboratory where we receive tissue specimens and perform genetic tests. Our laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, accredited by the College of American Pathologists, or CAP, and licensed by the State of California Department of Public Health, or CA DPH. We offer tests at competitive prices, averaging approximately $1,461 per billable test delivered in 2016, and with competitive turnaround times. Our volume has grown rapidly since our commercial launch, with over 20,000 billable tests delivered to over 600 customers as of December 31, 2016. We delivered 12,507 billable tests in 2016, compared to 6,852 billable tests delivered in 2015 and 966 billable tests delivered in 2014, and we have experienced compound quarterly growth of 19% in the number of billable tests delivered from the first quarter of 2015 through the fourth quarter of 2016. Further, approximately 86% of our test billings that were generated and due in 2016 were paid during that period.
We have assembled a highly-qualified team of 70 employees as of March 1, 2017. Our team includes personnel with expertise in a number of fields important to our business, such as bioinformatics, genetics, software engineering, laboratory management and sales and marketing. We have relied upon this team to improve and support our proprietary technology platform and differentiated business model, which we believe have driven our commercial success to date and provide us with significant opportunity for future growth.
Genetic testing identifies mutations in genes or chromosomal abnormalities to confirm or rule out a suspected genetic condition or to evaluate a person’s likelihood of developing a genetic condition. For example, a person displaying symptoms of one or more conditions could use genetic testing to determine or confirm a diagnosis, which can be especially useful for conditions that are difficult to diagnose. Further, a person with a family history of a particular condition, such as breast cancer, could use genetic testing to predict the likelihood of developing the condition. For instance, a mutation in the BRCA1 gene indicates an estimated 84% cumulative risk of developing breast cancer by age 70. The results of genetic testing can also be used to improve the selection and implementation of drug treatment programs targeting specific diseases.
The availability and accessibility of genetic testing has grown significantly in recent years, due in large part to improvements in testing technologies that have driven costs down. The National Institutes of Health gene testing registry includes over 480 genetic testing laboratories and, as of February 16, 2017, genetests.org estimates that over 4,900 disorders can be identified via genetic testing. Due to the continued expansion of testing availability and accessibility, a growing and aging population and the increasing overall incidence of disease, among other factors, the global market for genetic testing is expected to grow significantly. According to GrandView Research, the size of the global NGS genetic testing market, which includes pre-sequencing, sequencing and data analysis, is estimated to have been approximately $4.0 billion in 2016, including approximately $1.4 billion in the United States, and is expected to reach approximately $10.5 billion by 2022, including approximately $3.6 billion in the United States.
The process for conducting a genetic test begins with the extraction of genomic DNA from a tissue specimen collected and provided by an ordering physician. The extracted DNA is then sequenced using various equipment and other tools depending on the nature of the test. For instance, tests relying upon next generation sequencing technology use NGS sequencers and associated reagents to sequence DNA. Additionally, gene probes are an important tool used in the sequencing process. A gene probe is a single strand of DNA that has a base sequence complementary to the base sequence of a targeted gene. During the sequencing process, gene probes are introduced and will bind to the complementary base sequence, identifying the presence and location of the gene. After the DNA is sequenced using all appropriate equipment and tools, the fully sequenced genes are analyzed in a process known as curation, in which every DNA sequence is aligned with a known reference sequence and differences between the DNA sequence and the reference sequence are identified. These differences, which represent potential genomic alterations, are then compared to publicly available sources and proprietary genetic libraries to identify pathogenic alterations associated with disease or disease risk. The data produced by this sequencing and analysis is then synthesized into a report that is delivered to the ordering physician.
The genetic testing market is characterized by several testing methods based on different techniques, including microarray-based tests and NGS tests. Microarray-based tests are used to measure the expression levels of large numbers of genes simultaneously. Although microarray technologies are older than NGS technologies, the market for these tests continues to be significant, totaling approximately $960 million, or 15.4% of the overall market, in 2014, according to GrandView Research. NGS technology, a relatively new genetic testing technique, has dramatically improved genetic testing by enabling millions of DNA fragments to be sequenced in
2
parallel. As the cost of NGS testing continues to decline and the performance of NGS testing continues to improve, the availability and demand for genetic tests is expected to continue to accelerate. Furthermore, with the innovations in genomic medicine in recent years and the expected further advances in this area in the near term, pharmacogenomics, the practice of selecting and implementing drug treatment programs based on genetic information, is expected to continue to grow.
While adoption of genetic testing has increased in recent years, we believe widespread utilization has been limited in large part because of certain barriers to adoption that exist in today’s market, including the challenges described below. As a result of these challenges, we believe a significant market exists for a genetic testing option that provides broad genetic coverage and the flexibility to customize tests for individual patient needs, while maintaining accuracy and affordability.
Prohibitive Cost of Some Genetic Tests
The price of a genetic test can range from $300 to more than $9,000, depending on the nature and complexity of the test, and the overall price increases if more than one test is necessary or if multiple family members must be tested to obtain a meaningful result. While the price of genetic testing has decreased over time, prices remain significant enough that many payors and physicians limit the scope of genetic tests to only those conditions for which the test has direct clinical application, rather than performing a more thorough genetic evaluation of a patient’s health.
In today’s market, third-party payors generally restrict the reimbursement of genetic testing to a limited subset of genetic tests and only for those patients that meet specific criteria. This lack of widespread favorable reimbursement policies has contributed to slower adoption of genetic testing by a broad market and has presented a challenge for genetic testing companies in building sustainable business models.
Limited Scope of Genetic Analysis
It is estimated that there are 10,000 human diseases that are caused by single-gene mutations within the human genome, which consists of approximately 25,000 genes. Genetic testing laboratories that offer tests covering a limited set of genes may not be capable of diagnosing or identifying a predisposition to a disease that is caused by mutations in genes that are not included in the gene set that is analyzed.
The genetic testing process can be inefficient due to sequential retesting that can involve multiple companies and continue for extended periods. UnitedHealth Group estimates that there are 1,000 to 1,300 available genetic tests; however, many of these tests are not sufficiently comprehensive in their gene coverage to identify genetic mutations. Additionally, many laboratories offer only a small subset of the available tests and a physician may be forced to submit specimens to multiple laboratories in order to obtain all of the desired genetic information for a patient. Moreover, many genetic tests are specific to a single disease, which has created a sequential retesting process—often called a diagnostic odyssey—in cases where initial tests return negative results or where patients require testing for more than one condition. These challenges are further exacerbated by long and unpredictable turnaround times associated with each test, which limit clinical applicability of genetic testing for patients in need of time-sensitive treatment.
Cumbersome and Time-Consuming Interpretation of Results
The scientific curation of individual genetic disorders, genes and variants is relatively new and rapidly evolving. Although genetic tests are available to assist in the diagnosis or treatment planning of thousands of disorders, the implications of gene mutations are subject to substantial uncertainty due to a number of factors. Genetic curation has historically been done manually through the review of information from the broader scientific community to understand the implications of variants that have been identified in a genetic test. This process is often performed through a time-consuming search of biomedical literature that does not have standard nomenclature or expression, is subject to individual interpretation of data from genetic analyses and literature and often includes outdated, incomplete or otherwise flawed information. As a result, functional predictions based on simple categorization of gene variations can be limited and interpretation of genetic test results can be cumbersome and time-consuming, especially when the scope of the test is narrowed to a few selected genes.
3
We have approached the competitive and operational challenges of our industry by building a multi-faceted technology platform. Through this technology-driven approach, we have developed a system of proprietary tools and processes that we believe will enable us to overcome many of the challenges facing our industry today. The key features of our technology platform include:
All genetic testing providers use gene probes in the sequencing process to extract and target specific genomic regions, and many companies obtain these probes from third-party suppliers. We have developed technologies to design and formulate proprietary gene probes that, when combined with our proprietary genetic reference library and publicly available genetic databases, support our ability to sequence DNA regions that we believe laboratories using commercial probes cannot and improve the detection rate of our test data. In turn, we believe this enables us to produce clinically actionable results physicians can use to improve care for their patients. Our proprietary gene probes are specifically engineered to generate genetic data that is optimized for our software, which enables us to rapidly incorporate new genes into our test menu, develop new panels of disease-specific tests and customize tests for our customers. Moreover, once we develop a probe for a new gene, we can efficiently reproduce, validate and assure the quality of that probe under CLIA and CAP guidelines, which allows us to continuously and rapidly expand our library of genetic content while increasing the breadth of our test menu. Additionally, we believe our probes more effectively enrich the targeted genes to improve the quality of the sequenced data we produce.
We have developed proprietary data comparison and data suppression algorithms to improve and simplify the curation process by highlighting identified pathogenic mutations. Our advanced data comparison algorithms measure DNA sequences from patient specimens against genetic data available from the broader scientific community and our own proprietary reference library of genetic information, which enables us to rapidly and effectively detect pathogenic mutations. Our advanced data suppression algorithms reduce irrelevant noise in the genetic data we analyze to improve the efficiency and speed of our data analysis while reducing the need for manual curation.
We have developed software that automatically incorporates the data from each completed test into our expansive genetic reference library, enabling it to continuously evolve and support the improvement of our gene probes. This software leverages the capabilities of our gene probes to improve the speed and effectiveness of curation and reporting. Our adaptive learning software also communicates with our integrated laboratory systems, which leads to increasing efficiency and effectiveness.
Proprietary Laboratory Information Management Systems
We have developed proprietary laboratory information management systems that are highly integrated with our laboratory processes and adaptive learning software. These systems provide the backbone by which we efficiently manage workflow, monitor quality and ensure the fidelity of information generation and analytics for reporting to our customers. The result is a highly connected platform that allows us to process tests and information in an efficient manner. Our talented team of software engineers continuously iterates with our laboratory and customer-facing personnel to improve the efficiencies of these systems.
The benefits provided by our technology platform include the following:
We have developed various proprietary technologies that improve our laboratory efficiency and reduce the costs we incur to perform our tests. Our technology platform enables us to perform each test and deliver its results at a lower internal cost than many of our competitors, averaging approximately $537 per billable test delivered in 2016. This low cost per billable test allows us to maintain affordable pricing for our customers, averaging approximately $1,461 per billable test delivered in 2016, which we believe encourages repeat ordering from existing customers and attracts new customers. We believe our low cost per billable test will also facilitate the process for establishing coverage and reimbursement from third-party payors at a level adequate for us to achieve profitability with this payor group.
4
We offer single-gene tests on approximately 18,000 genes, which, to our knowledge, is thousands more than any of our competitors’ portfolios. We believe the breadth of genes in our portfolio allows us to provide more comprehensive genetic information and improves our variant detection rate, which can increase the clinical actionability of the data we produce. The breadth of genes in our portfolio also allows us to provide a flexible and customizable test menu for our customers that can reduce sequential retesting. We offer single-gene tests on all of the genes in our portfolio, as well as deletion/duplication analysis and site-specific tests. If customers desire a broader test, we offer more than 300 pre-established, multi-gene panels that focus on various genetic conditions. These panels can be adjusted up or down to include more or fewer genes, or customers can design their own panels to their exact specifications. We also offer clinical and full gene exome testing options. We offer our tests at different price points and turnaround times depending on the size and complexity of the test, which increases optionality for our customers. We believe the flexibility of our offering improves the efficiency and utility of the data output by our tests and decreases overall customer costs. We also offer our customers access to our highly qualified genetic counselors and laboratory experts to assist in interpreting the data we provide, which further increases the utility of our test results for ordering physicians.
The benefit of including multiple genes on a single panel was recently discussed in a study published in 2016 by the USC Norris Comprehensive Cancer Center in Cancer Genetics. The study retrospectively evaluated 475 individuals with a personal or family history of cancer who had undergone a clinically indicated multi-gene panel test of six to 110 genes from one of the following six commercial laboratories: Myriad Genetics (n=354), Ambry Genetics (n=100), Fulgent (n=17), University of Washington Genetics Laboratory (n=2), City of Hope Molecular Diagnostics (n=1) and Baylor Genetics Laboratory (n=1). The study concluded that multi-gene panel testing increases the yield of mutations detected and adds to the capability of providing individualized cancer risk assessment. More specifically, the study reported that deleterious mutations were identified in 15.6% of patients tested on a variety of multi-gene panels, which included 8.6% of patients who would not have a mutation detected if a targeted gene-by-gene-approach had been used. The study also presented evidence that, as the number of genes on a panel increased, a higher proportion of panels identified a mutation. The Fulgent panels evaluated in the study contained over 100 genes compared to less than 30 genes in the next largest panel. Additionally, approximately 35% of our panels identified a genetic mutation, and in comparison, the test with the next highest percentage of detected mutations identified mutations in approximately 17% of its tests.
Expansive and Growing Genetic Library
Using our proprietary gene probes and testing processes, we are able to capture large amounts of genetic information on each test we perform—oftentimes more than is ordered for the test—without an incremental increase in our costs. Through this data collection process, we have developed a proprietary reference library of expansive genetic information. This reference library is automatically curated by our adaptive learning software and supplemented with manual curation by our team of highly trained professionals, which adds to and improves upon the information available in public genetic databases. This software allows us to leverage publicly available information from the broader scientific community with our internally developed reference library to develop what we believe is a more reliable catalog of genetic information and to accelerate, standardize and improve our reporting process.
We aim to be a leading provider of genetic information and other diagnostic tools to physicians for disease prediction and prognosis, as well as for pharmacogenomic purposes. Our strategy for long-term growth is to focus on the following key drivers of our business:
Our existing customer base consists primarily of hospitals and medical institutions, which are frequent and high-volume users of genetic tests. We believe we must expand our customer base laterally and vertically to achieve our desired growth.
We are seeking to grow our customer base laterally by continuing to acquire new hospital and medical institution customers and expand into additional customer groups, such as individual physicians and other practitioners, as well as research institutions. To this end, we have made efforts to diversify our customer base beyond hospitals by establishing a vendor code with a national clinical laboratory that orders our tests to fulfill some of the genetic testing orders it receives from certain U.S. government agencies and contracting with a regional hospital network within the U.S. Army to provide genetic tests for members and their families. We have also pursued relationships with payors, including Medicare, some state Medicaid programs and third-party payors, in order to obtain coverage and reimbursement for our tests to make them accessible to more individual physicians. To achieve further lateral customer growth, we plan to continue to increase our direct sales force and to invest in our sales and marketing efforts, including continued efforts to obtain coverage and adequate reimbursement for our tests.
5
Our vertical customer growth strategy focuses on more deeply penetrating our relationships with existing customers to increase the volume of tests they order from us. We plan to achieve this vertical customer growth by continuing to broaden our test menu and by educating the medical community about the benefits of our genetic tests and genetic testing in general.
We intend to continue to expand our test menu to include more options and to cover more genes. For example, we recently launched our first Focus and Comprehensive panels, which are designed to offer customers an efficient ordering process for comprehensive and customizable tests at an attractive price. Our first Focus and Comprehensive panels are focused on oncology, and we intend to launch additional panels targeting other areas, including cardiology, pediatrics and prenatal, all of which represent large genetic testing markets in which we believe our comprehensive and flexible tests will be competitive. Further, we recently launched a new chromosomal test that is designed to use NGS technology to detect copy number variants with similar or improved results as compared to microarray-based genomic tests, which we anticipate will expand our potential customer market to include users of these tests. We believe offering a broad and flexible test menu will appeal to potential customers and increase our revenue potential.
Increase the Global Presence of Our Business
Approximately $10.0 million, $4.5 million and $638,000 of our revenue came from non-U.S. customers in 2016, 2015 and 2014, respectively, and of this, approximately $3.8 million, $2.7 million and $194,000 in the respective periods came from customers located in Canada. We aim to increase this volume in the near term, from customers in Canada and other geographic markets, including Asia and Europe. To this end, we received revenues of $3.2 million from tests ordered by customers in China in 2016 and we are working with Xi Long USA, Inc., or Xi Long, a large stockholder of our company, to develop a strategic commercial relationship to pursue additional customers in China, which we expect to be finalized in the first half of 2017. Although the terms of this relationship have not been finalized, we anticipate that this relationship, if it proceeds, could expand our long-term opportunities to address the genetic testing market in Asia. We believe there is a large potential for growth of genetic testing in many international markets due to the presence of high unmet diagnostic and predictive testing needs, rapidly rising healthcare expenditures and patient awareness of NGS technologies. We plan to engage distributors or establish other types of arrangements, such as joint ventures or other partner relationships, in an effort to expand our presence and test volume in new geographic markets.
Maintain Our Low-Cost Operations
Our low costs for each test we perform allow us to provide customers with clinically actionable genetic information at an accessible price. In order to maintain the low costs we incur to perform our tests and, in turn, the affordability of our tests for our customers, we plan to continue to improve our internal processes, increase their scalability and implement additional automation procedures to further increase efficiencies. As our business grows, we believe our investment in these processes and procedures will allow us to achieve further cost advantages in our specimen collection, genetic testing, report preparation and customer service functions.
Develop Relationships with Payors by Focusing on Established Genetic Testing Markets
In order to effectively market our tests to non-hospital customers, we intend to pursue coverage and adequate levels of reimbursement from third-party payors. To this end, we have contracted with a regional physician services organization and a national health insurance company to become an in-network provider and enrolled as a supplier with Medicare and 10 state Medicaid programs, which means that we have agreed with these payors to provide certain of our tests at negotiated rates. As part of our strategy for obtaining adequate reimbursement for our tests, we intend to increase our focus on established genetic testing markets, including primarily oncology, cardiology, pediatrics and prenatal. We believe this approach will enable us to develop relationships with third-party payors in connection with tests for which coverage and reimbursement are well-established, which we anticipate will allow us to demonstrate the benefits of our platform and improve the reimbursement profile for many of the other genetic conditions covered by our broad test offering. Further, we believe our low cost per billable test will enhance our ability to compete effectively in, and our flexibility in approaching, the third-party payor market.
Pursue Additional Opportunities in Pharmacogenomics and Drug Discovery
We plan to pursue relationships with pharmaceutical companies to deepen our opportunities in pharmacogenomics and drug discovery. We expect that we will attract pharmaceutical partners with our comprehensive reference library of genetic information, which allows us to aggregate the role genetic variations play in diseases and drug responses. We believe pharmaceutical companies could use our reference library to enhance clinical trial design, identify novel gene targets, support precision medicine strategies and improve existing or develop new targeted drug therapies.
6
In addition, we are building relationships with research institutions, which use genetic tests to find unknown genetic disease relationships, learn how genes work, advance current knowledge about genetic conditions and for other research purposes. Like hospitals, research institutions can be frequent and high-volume users of genetic tests, and we believe these users represent a potentially large customer market for our tests.
Leverage Our Technology Platform into Other Diagnostic Modalities
We believe genetic testing and other existing and future diagnostic tools will facilitate production of more comprehensive information to physicians, enabling enhanced disease prognosis and prediction and pharmacogenomic advances. We have constructed our technology platform to be highly adaptive and scalable, which could allow us to apply it to other types of diagnostic tools in the future. We could use these tools to analyze other components of biology in addition to DNA, which may include RNA, proteins and metabolic systems. By utilizing a complement of diagnostic tools with our highly adaptive technology platform, we believe we will be able to develop new tests in the future that further enhance our offering. We may also seek to expand our business through opportunistic acquisitions, investments, collaborations or other strategic relationships in order to enhance our tests, enter new geographical or other markets or leverage our existing capabilities, among other things.
Our offering consists of the following types of full-gene sequencing and deletion/duplication analysis:

Our customers have a high degree of choice when selecting a test from our menu. A customer may select a single-gene test of any of the approximately 18,000 genes in our portfolio. A customer may also select one of our more than 300 panels, which are designed to test for particular genes and mutations within these genes that relate to a wide range of conditions and diseases. For example, our Focus and Comprehensive oncology panels test 29 genes and 122 genes, respectively, that relate to various cancers. We also offer whole exome and clinical exome panel tests, which test all genes included in our portfolio and up to 4,810 genes located in the exome, respectively, and produce results that we combine with the individual’s unique clinical presentation and family history to enhance the clinical relevance of the results. Our whole exome and clinical exome tests also include the option for Trio testing, which involves sequencing the genes of a patient’s parents and is thought to enhance the utility of the test results. In addition, we offer whole genome testing, which determines and tests the complete DNA sequence of a genome at a single time. We also provide known mutation testing, which can be used to target familial specific or other desired mutations, as well as repeat expansion testing, which tests for a particular type of mutation known as “copy choice” DNA replication. Importantly, all of our pre-established panels are completely customizable, offering customers the ability to add up to 20 additional genes to, or remove any number of genes from, any of these panels when ordering, at no additional cost.
Since inception, we have sold our tests to over 600 total customers. We typically consider each single billing and paying unit to be an individual customer, even though the unit may represent multiple physicians and healthcare providers ordering tests. We have primarily sold our tests to hospitals, including children’s hospitals, and medical institutions. We have approached the genetic testing market with a focus on these customers in part because they are frequent and high-volume users of genetic tests. We believe this customer base provides a meaningful opportunity for further growth by vertically deepening these relationships to drive increased ordering. Additionally, collection of billings from these institutional customers is more attainable than other types of customers in today’s reimbursement environment. Approximately 86% of our test billings that were generated and due in 2016 were paid during that period. In addition, we believe hospitals and medical institutions are early adopters of NGS technology and could influence broader clinical acceptance of genetic testing as a predictive, diagnostic and pharmacogenomic tool due to their influential position in the medical community. As a result, we have pursued and attained as customers many hospitals and medical institutions that we
7
believe are recognized leaders in the medical field. In 2016, Dongyang People’s Hospital (“Customer A”) and Kaiser Permanente (“Customer B”) each contributed 15% of our total revenue, and Kaiser Permanente also contributed 19% and 11% of our total revenue in 2015 and 2014, respectively. The following is a representative list of some of our other top 50 hospital and medical institution and children’s hospital customers by number of billable tests delivered in 2016:
|
|
|
|
|
|
||
|
Dartmouth-Hitchcock Medical Center Loma Linda University Medical Center LSU Health Sciences Center Shreveport |
|
Children’s Hospital of Orange County Cincinnati Children’s Hospital Medical |
We intend to continue to expand our reach laterally to include new customer groups, such as individual physicians and other practitioners, as well as research institutions, as we increase our focus on our sales and marketing activities, including our efforts to obtain coverage and adequate reimbursement for our tests. To this end, we have made efforts to diversify our customer base beyond hospitals by establishing a vendor code with a national clinical laboratory that orders our tests to fulfill some of the genetic testing orders it receives from certain U.S. government agencies, contracting with a regional hospital network within the U.S. Army to provide genetic tests for members and their families. We have also pursued relationships with payors, including Medicare, some state Medicaid programs and third-party payors, in order to obtain coverage and reimbursement for our tests to make them accessible to more individual physicians.
Additionally, much of our business to date has been from non-U.S. customers, with approximately 55%, 47% and 50% of our total revenue generated from sales to non-U.S. customers in 2016, 2015 and 2014, respectively. We intend to further grow our non-U.S. customer base and the volume of tests ordered from non-U.S. customers in the near-term, from customers in Canada and other geographic markets, including Asia and Europe. To this end, we started to receive revenues from tests ordered by customers in China in 2016 and we are working with Xi Long to develop a strategic commercial relationship in order to further pursue our opportunities in this market, although this relationship has not been, and may never be, finalized or successful. We intend to pursue international distributor relationships or establish other types of arrangements, such as joint ventures or other partner relationships, to cover new geographic markets and expand our international customer base.
Generally, our customers can be divided into three categories based on the party from which we receive payment for our tests: hospitals and medical institutions; patients and third-party payors. Hospitals and medical institutions are responsible for paying for the vast majority of the tests we have delivered since our inception. We bill these organizations for our tests and they are responsible for paying us directly and either billing their patients separately or obtaining reimbursement from third-party payors in connection with a patient’s diagnosis related group. A small percentage of our customers are patients, whose physicians order our tests and the patients elect to pay for the tests themselves with out-of-pocket payments. Third-party payors, which consist of private health insurers and the Centers for Medicare and Medicaid Services, or CMS, have been responsible for paying for a small number of the tests we have delivered to date; however, as we seek to expand our customer base to include more individual practitioners, we expect this category of payors will be responsible for many of the tests we deliver to these customers.
Third-party payors require us to identify the test for which we are seeking reimbursement using a Current Procedural Terminology, or CPT, code set maintained by the American Medical Association, or AMA. Where we offer a multi-gene panel and there is no CPT code for the full panel but the panel includes a gene for which the AMA has an established CPT code, we identify the test provided under that CPT code when billing a third-party payor for that test. In cases where there is not a specific CPT code, our
8
test may be billed under a miscellaneous code for an unlisted molecular pathology procedure. Because this miscellaneous code does not describe a specific service, the insurance claim must be examined to determine what service was provided, whether the service was appropriate and medically necessary, and whether payment should be rendered, which may require a letter of medical necessity from the ordering physician. Given the changing CPT coding environment and our development of relationships with third-party payors, we expect that our practices regarding billing these payors will evolve in the future.
We currently operate with a lean sales team consisting of sales and marketing experts who are highly trained and educated about the complexities of our tests. Because our sales and marketing personnel serve as a primary interface between our company and many of our customers, we believe the power of this team is directly correlated to its breadth and depth of understanding of our technologies, our offering and the advantages of each. As a result, we expect to invest time and capital in aggressively growing our sales force and delivering rigorous training to these personnel. Our sales and marketing team consisted of 16 individuals as of March 1, 2017 and we plan to continue to increase this number, with the goal of more than doubling the size of our sales and marketing team in the next 12 months. We have experienced our sales to date largely through organic growth of our customer base and in spite of our small marketing presence, which we believe demonstrates the value of our tests and the power of word-of-mouth communication among current and potential future customers as a marketing tool.
Our sales and marketing strategy is designed to expand our brand awareness, laterally grow our customer base and vertically penetrate our relationships with existing customers by educating the medical community, including existing and potential future customers, about the benefits and the full scale of our offering. Our marketing activities include targeted marketing initiatives, such as working with medical professional societies to promote awareness of the benefits of our tests and genetic testing in general, presenting at medical conferences and scientific meetings and pursuing publication in medical and scientific journals. In addition, we conduct email advertising campaigns to existing and potential future customers when we want to send a specific message about our company and our brand, including, for instance, when we launch new tests or new test options, such as our Focus and Comprehensive oncology panel tests launched in the first half of 2016, and when we add new genes to our test menu.
Our sales and marketing strategy is also focused on offering differentiated and highly available customer service resources, which we believe is an important factor in maintaining and deepening our customer relationships. Genetic tests are highly complex by nature and we recognize that our customers may want to discuss with us available testing options, specimen collection requirements, expected turnaround times, the cost of the test and the clinical reports we produce. As a result, we offer comprehensive customer service designed to enable efficient ordering and increase the accessibility of our clinical reports. We strive to answer phone calls directed to our customer service team with a person, not an auto-attendant, and to provide physicians with the answers they need on their first contact with us, including, as needed, access to our licensed and qualified laboratory directors who review and approve each report we produce. Additionally, all of the reports we produce are accessible by our customers online via an encrypted web portal, allowing our customers flexibility in viewing their reports and seamless access to our customer service resources.
We rely on a limited number of suppliers, and, in some cases, sole suppliers, for certain laboratory reagents, sequencers and other equipment and materials that we use in our laboratory operations. We rely on Illumina, Inc. as the sole supplier of our next generation sequencers and associated reagents and as the sole provider of maintenance and repair services for these sequencers. Our laboratory operations would be interrupted if we encounter delays or difficulties in securing these reagents, sequencers or other equipment or materials or if we need a substitute or replacement for any of our suppliers and are not able to locate and make arrangements with an acceptable substitute or replacement.
Our competitors include dozens of companies focused on molecular genetic testing services, including specialty and reference laboratories that offer traditional single-gene and multi-gene tests. Principal competitors include companies such as Ambry Genetics, Inc.; Counsyl; Foundation Medicine, Inc.; GeneDx, a subsidiary of OPKO Health, Inc.; Invitae Corporation; Myriad Genetics, Inc.; and Pathway Genomics Corporation, as well as other commercial and academic laboratories. In addition, other established and emerging healthcare, information technology and service companies may develop and sell competitive tests, which may include informatics, analysis, integrated genetic tools and services for health and wellness.
Additionally, participants in closely related markets, such as prenatal testing and clinical trial or companion diagnostic testing, could converge on offerings that are competitive with the type of tests we perform. Instances where potential competitors are aligned with key suppliers or are themselves suppliers could provide such potential competitors with significant advantages. Further, hospitals,
9
research institutions and eventually individual physicians and other practitioners may also seek to perform at their own facilities the type of genetic testing we would otherwise perform for them. In this regard, continued development of, and associated decreases in the cost of, equipment, reagents and other materials and databases and genetic data interpretation services may enable broader direct participation in genetic testing and analysis and drive down the use of third-party testing companies such as ours. Moreover, the biotechnology and genetic testing fields continue to undergo significant consolidation, permitting larger clinical laboratory service providers to increase cost efficiencies and service levels, resulting in more intense competition.
We believe the principal competitive factors in our market are:
We believe we compare favorably with our competitors on the basis of these factors. However, many of our existing and potential future competitors have longer operating histories, larger customer bases, greater brand recognition and market penetration, substantially greater financial, technological and research and development resources and selling and marketing capabilities and considerably more experience dealing with third-party payors. As a result, they may be able to respond more quickly to changes in customer requirements or preferences, devote greater resources to the development, promotion and sale of their tests, devote more resources to and obtain more favorable results from third-party payors regarding coverage and reimbursement for their offerings, adopt more aggressive pricing policies for their tests, secure supplies from vendors on more favorable terms or devote substantially more resources to infrastructure and systems development. In addition, competitors may be acquired by, receive investments from or enter into other commercial relationships with larger, well-established and well-financed companies as use of NGS for clinical diagnosis and preventative care increases. Further, companies or governments that effectively control access to genetic testing through umbrella contracts or regional preferences could promote our competitors or prevent us from performing certain tests in certain territories. We may not be able to compete effectively against these organizations.
We have assembled a highly-qualified team with expertise in a number of fields important to our business, such as bioinformatics, genetics, software engineering, laboratory management and sales and marketing, and including 33 employees with a PhD or other advanced degree as of March 1, 2017. We rely upon this team to conduct all of our research and development activities, including efforts to develop and curate our expansive library of genetic information and further expand our technology platform. Our research and development expenses were $3.6 million, $4.4 million and $521,000 in 2016, 2015 and 2014, respectively.
We rely on a combination of unregistered intellectual property rights, including trade secrets, common law trademarks and customary contractual protections, to protect our core technology and intellectual property.
We rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain and develop the competitive position afforded by many of our laboratory, analytic and business practices. For example, significant elements of our genetic tests and our testing procedures, including aspects of specimen preparation, bioinformatics algorithms and related processes and software, are based on unpatented trade secrets and know-how. We try to protect trade secrets and know-how by taking reasonable steps to keep them confidential, including entering into nondisclosure and confidentiality agreements with parties
10
who have access to them, such as our employees and certain third parties, and entering into invention assignment agreements with our employees and consultants that obligate them to assign to us any inventions developed in the course of their work for us.
We own registered trademark rights under applicable U.S. and foreign law to distinguish and/or protect our tests and our brand, including our company name. We also own a registered service mark in the United States for our company logo.
As a clinical laboratory, we are required to hold certain federal licenses, certifications and permits to conduct our business. In 1988, Congress passed CLIA, which establishes quality standards for all laboratory testing designed to ensure the accuracy, reliability and timeliness of patient test results. Our Temple City, California laboratory is CLIA-certified and accredited by CAP, a CLIA-approved accrediting organization.
Under CLIA, a laboratory is any facility that performs laboratory testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease or the impairment or assessment of health. CLIA requires that we hold a certificate applicable to the type of laboratory examinations we perform and that we comply with various standards with respect to personnel qualifications, facility administration, proficiency testing, quality control and assurance and inspections. Laboratories must register and list their tests with CMS, the agency that oversees CLIA, and CLIA compliance and certification is a prerequisite to be eligible to bill government payors and many private payors for our tests. CLIA is user-fee funded, such that all costs of administering the program must be covered by the regulated facilities, including certification and survey costs.
We are subject to survey and inspection every two years to assess compliance with CLIA’s program standards, and we may be subject to additional unannounced inspections. Our CLIA certification was last renewed October 23, 2015. If our clinical reference laboratory is found to be out of compliance with CLIA requirements at any of these inspections, we may be subject to sanctions such as suspension, limitation or revocation of our CLIA certificate, a directed plan of correction, on-site monitoring, civil monetary penalties, civil injunctive suits, criminal penalties, exclusion from the Medicare and Medicaid programs and significant adverse publicity.
In addition to CLIA requirements, we elect to participate in the accreditation program of CAP. CMS has deemed CAP standards to be equally or more stringent than CLIA regulations and has approved CAP as a recognized accrediting organization. Inspection by CAP is performed in lieu of inspection by CMS for CAP-accredited laboratories. Because we are accredited by the CAP Laboratory Accreditation Program, we are deemed to also comply with CLIA.
State and Foreign Laboratory Licensure
Under CLIA, states may adopt laboratory regulations that are more stringent than those under federal law, and a number of states have implemented their own more stringent laboratory regulatory requirements. State laws may require that laboratory personnel meet certain qualifications, specify certain quality control procedures or facility requirements or prescribe record maintenance requirements.
We are required to maintain a license to conduct testing in the State of California. California laws establish standards for day-to-day operations of our laboratory in Temple City, including with respect to the training and skills required of personnel, quality control and proficiency testing requirements. If our clinical reference laboratory is out of compliance with California standards, the CA DPH may suspend, restrict or revoke our license to operate our clinical reference laboratory, assess substantial civil money penalties or impose specific corrective action plans. Any such actions could materially affect our business. We maintain a current license in good standing with CA DPH.
Additionally, several states require the licensure of out-of-state laboratories that accept specimens from those states and/or receive specimens from laboratories in those states. Our laboratory holds the required out-of-state laboratory licenses to perform testing on specimens from Florida, Maryland and Pennsylvania. In addition to having a laboratory license in New York, our laboratory is required to obtain approval on a test-specific basis by the New York State Department of Health before specific testing is performed on specimens from New York. Because our licensure application is currently pending in New York, we are currently prohibited from performing tests on specimens from New York until our license is approved.
11
Other states may adopt similar licensure requirements in the future, which could require us to modify, delay or discontinue our operations in such jurisdictions. If we identify any other state with such requirements or if we are contacted by any other state advising us of such requirements, we intend to follow instructions from the state regulators as to how to comply with such requirements.
We are also subject to regulation in foreign jurisdictions, which we expect will increase as we seek to expand international utilization of our tests or if jurisdictions in which we pursue operations adopt new or modified licensure requirements. Foreign licensure requirements could require review and modification of our tests in order to offer them in certain jurisdictions or could impose other limitations, such as restrictions on the transport of human blood or other tissue necessary for us to perform our tests that may limit our ability to make our tests available outside of the United States on a broad scale.
Pursuant to its authority under the federal Food, Drug, and Cosmetic Act, or FDC Act, the U.S. Food and Drug Administration, or FDA, has jurisdiction over medical devices, which are defined to include, among other things, in vitro diagnostic products, or IVDs, used for clinical purposes. The tests that we offer are IVDs. The laws and regulations governing the marketing of IVDs are evolving, extremely complex, and in many instances there are no significant regulatory or judicial interpretations of these laws and regulations. The FDA regulates, among other things, the research, testing, manufacturing, safety, labeling, storage, recordkeeping, premarket clearance or approval, marketing and promotion and sales and distribution of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. In addition, the FDA regulates the import and export of medical devices.
The FDC Act classifies medical devices into one of three categories based on the risks associated with the device and the level of control necessary to provide reasonable assurance of safety and effectiveness. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices or devices deemed not substantially equivalent to a previously 510(k) cleared device, are categorized as Class III. These devices typically require submission and approval of a premarket approval application, or PMA. Devices deemed to pose lower risk are categorized as either Class I or II, which requires the manufacturer to submit to the FDA a 510(k) premarket notification submission requesting clearance of the device for commercial distribution in the United States. Some low-risk devices are exempted from this requirement. When a 510(k) premarket notification submission is required, the manufacturer must submit to the FDA a premarket notification submission demonstrating that the device is “substantially equivalent” to: (i) a device that was legally marketed prior to May 28, 1976, for which PMA approval is not required, (ii) a legally marketed device that has been reclassified from Class III to Class II or Class I, or (iii) another legally marketed, similar device that has been cleared through the 510(k) clearance process.
After the FDA permits a device to enter commercial distribution, numerous regulatory requirements apply. These include: the Quality System Regulation, which requires manufacturers to follow elaborate design, testing, control, documentation and other quality assurance procedures during the manufacturing process; labeling regulations; the FDA’s general prohibition against promoting products for unapproved or “off-label” uses; and the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. The FDA has broad post-market and regulatory and enforcement powers. Failure to comply with the applicable U.S. medical device regulatory requirements could result in, among other things, warning letters, fines, injunctions, consent decrees, civil penalties, repairs, replacements, refunds, recalls or seizures of products, total or partial suspension of production, the FDA’s refusal to grant future premarket clearances or approvals, withdrawals or suspensions of current product applications, and criminal prosecution.
Although the FDA has statutory authority to assure that medical devices, including IVDs, are safe and effective for their intended uses, the FDA has historically exercised its enforcement discretion and not enforced applicable provisions of the FDC Act and regulations with respect to laboratory developed tests, or LDTs, which are a subset of IVDs that are intended for clinical use and designed, manufactured and used within a single laboratory. We believe our tests fall within the definition of an LDT. As a result, we believe our diagnostic tests are not currently subject to the FDA’s enforcement of its medical device regulations and the applicable FDC Act provisions.
Even though we commercialize our tests as LDTs, our tests may in the future become subject to more onerous regulation by the FDA. Pursuant to the Food and Drug Administration Safety and Innovation Act of 2012, the FDA notified Congress on July 31, 2014 that the FDA intended to issue in 60 days the Framework Guidance and the Notification Guidance. On October 3, 2014, the FDA issued the anticipated Framework Guidance and Notification Guidance. The Framework Guidance states that the FDA intends to modify its policy of enforcement discretion with respect to LDTs in a risk-based manner consistent with the existing classification of medical devices. Thus, the FDA plans to begin to enforce its medical device requirements, including premarket submission requirements, for LDTs that have historically been marketed without FDA premarket review and oversight. The FDA states its intention in the Framework Guidance to require registration or listing and adverse event reporting six months after the Framework Guidance is finalized and to publish general LDT classification guidance within 24 months of the date on which the Framework
12
Guidance is finalized. According to the Framework Guidance, the FDA intends to enforce premarket review requirements in a risk-based, phased-in manner, starting with the highest risk LDTs beginning 12 months after the Framework Guidance is finalized, followed by other high risk LDTs in the next four years, and then moderate risk LDTs in the four years after that. Generally, for each category of LDTs, the FDA intends to continue exercising enforcement discretion pending the FDA’s review and consideration of the premarket submissions for devices that are already in use at the time—so long as premarket submissions are timely made. However, for certain categories of the highest risk LDTs (specifically, (i) LDTs with the same intended use as a cleared or approved companion diagnostic; (ii) LDTs with the same intended use as an FDA-approved Class III medical device; and (iii) certain LDTs for determining the safety or efficacy of blood or blood products), the FDA intends to begin enforcing premarket review requirements immediately upon publication of the finalized Framework Guidance for all new LDTs in those categories.
If and when the Framework Guidance and Notification Guidance are finalized, or if the FDA disagrees with our assessment that our tests fall within the definition of an LDT, we could for the first time be subject to enforcement of regulatory requirements such as registration and listing requirements, medical device reporting requirements and quality control requirements. Any new FDA enforcement policies affecting LDTs may result in increased regulatory burdens on our ability to continue marketing our tests and to develop and introduce new tests in the future. Additionally, if and when the FDA begins to actively enforce its premarket submission regulations with respect to LDTs generally or our tests in particular, we may be required to obtain premarket clearance for our tests under Section 510(k) of the FDC Act or approval of a PMA. The process for submitting a 510(k) premarket notification and receiving FDA clearance usually takes from three to 12 months, but it can take significantly longer and clearance is never guaranteed. The process for submitting and obtaining FDA approval of a PMA generally takes from one to three years or even longer and approval is not guaranteed. PMA approval typically requires extensive clinical data and can be significantly longer, more expensive and more uncertain than the 510(k) clearance process. If premarket review is required for some or all of our tests, the FDA could require that we stop selling our products pending clearance or approval and conduct clinical testing prior to making submissions to FDA to obtain premarket clearance or approval. The FDA could also require that we label our tests as investigational or limit the labeling claims we are permitted to make.
While there is also the risk that the FDA does not consider our tests to be LDTs, the Framework Guidance states that, in the interest of ensuring continuity in the testing market and avoiding disruption of access to tests marketed as LDTs that do not meet the FDA’s definition of LDTs, the FDA intends to apply the same risk-based framework described in the Framework Guidance to any IVD that is offered as an LDT by a CLIA-certified laboratory.
Additionally, the FDA has recently solicited public input and published two draft guidance documents relating to FDA oversight of NGS-based tests. The two draft guidance documents on NGS-based tests describe the FDA’s current thinking and proposed approach regarding the possible use of FDA-recognized standards to support analytical validity, and public human genetic variant databases to support clinical validity, of these tests. While it appears that the FDA is striving to provide a flexible pathway to device clearance or approval for manufacturers seeking to market NGS-based tests, it is unknown how the FDA may regulate such tests in the future and what testing and data may be required to support such clearance or approval. If premarket review is required for some or all of our tests and the FDA requires more extensive testing such as clinical trials, for example, we could experience significantly increased development costs and delay.
The FDA enforces its medical device requirements by various means, including inspection and market surveillance. If the FDA finds a violation, it can institute a wide variety of enforcement actions, ranging from an Untitled Letter or Warning Letter to more severe sanctions, such as: fines, injunctions and civil penalties; recall or seizure of products; operating restrictions, partial suspension or total shutdown of production; and criminal prosecution.
Legislative proposals addressing the FDA’s oversight of LDTs have been introduced by Congress in the past and we expect that new legislative proposals may be introduced from time to time in the future. The likelihood that Congress will pass such legislation and the extent to which such legislation may affect the FDA’s plans to enforce its medical device requirements with respect to certain LDTs is difficult to predict at this time. If the FDA ultimately lifts its policy of enforcement discretion over LDTs and begins to enforce its medical device requirements with respect to LDTs, our tests may be subject to additional regulatory requirements imposed by the FDA, the nature and extent of which would depend upon applicable final guidance or regulation by the FDA or instruction by Congress. Failure to comply with any applicable FDA requirements could trigger a range of enforcement actions by the FDA, including warning letters, civil monetary penalties, injunctions, criminal prosecution, recall or seizure, operating restrictions, partial suspension or total shutdown of operations and denial of or challenges to applications for clearance or approval, as well as significant adverse publicity.
13
Third-party payors, including private insurers and CMS, require genetic testing companies to identify each test for which reimbursement is sought using a CPT code set maintained by the AMA. These CPT codes in their current form are not readily applied to many of the genetic tests we conduct. For example, for many of our multi-gene panels, there may not be an appropriate CPT code for any genes in a panel, in which case our test would be billed under a miscellaneous code for an unlisted molecular pathology procedure. Because these miscellaneous codes do not describe a specific service, the insurance claim would need to be examined to determine the service that was provided, whether the service was appropriate and medically necessary and whether payment should be rendered. This process can require a letter of medical necessity from the ordering physician and it can result in a delay in processing the claim, a lower reimbursement amount or denial of the claim.
In September 2014, the AMA published new CPT codes for genomic sequencing procedures that are effective for dates of service on or after January 1, 2015. These include genomic sequencing procedure codes for certain multi-gene panel tests. In a final determination under the Medicare Clinical Laboratory Fee Schedule, or CLFS, published in November 2014, CMS set the 2015 payment rate for these codes using the gap-fill process. Under the gap-fill process, local Medicare Administrative Contractors, or MACs, establish rates for the codes that each MAC believes meet the criteria for Medicare coverage and considering laboratory charges and discounts to charges, resources, amounts paid by other payors for the tests and amounts paid by the MAC for similar tests. In 2015, gap-filled payment rates were established for some, but not all, of the published codes for genomic sequencing procedures. For the codes for which local gap-filled rates were established in 2015, a national limitation amount for Medicare was established for 2016. For the codes for which local gap-filled rates were not established in 2015, associated procedures are priced by the local MACs in 2016 if an individual MAC determines that such codes should be covered. Where available, the national limitation amount serves as a cap on the Medicare and Medicaid payment rates for a test procedure, which may not be adequate for all of the procedures covered by the applicable codes, including our tests to the extent we are required to report them under these codes.
In April 2014, Congress passed the Protecting Access to Medicare Act of 2014, or PAMA, which included substantial changes to the way in which clinical laboratory services will be paid under Medicare. Under PAMA, laboratories that receive the majority of their Medicare revenue from payments made under the CLFS or the Physician Fee Schedule are required to report to CMS, beginning in 2017 and every three years thereafter (or annually for “advanced diagnostic laboratory tests”), private payor payment rates and volumes for their tests. Laboratories that fail to report the required payment information may be subject to substantial civil monetary penalties. We do not believe that our tests meet the current definition of advanced diagnostic laboratory tests, and therefore we believe we will be required to report private payor rates for our tests every three years. As required under PAMA, CMS will use the rates and volumes reported by laboratories to develop Medicare payment rates for laboratory tests equal to the volume-weighted median of the private payor payment rates for the tests. On June 23, 2016, CMS published the final rule implementing the reporting and rate-setting requirements under PAMA.
As set forth under PAMA, for tests furnished on or after January 1, 2018, Medicare payments for clinical diagnostic laboratory tests will be paid based upon these reported private payor rates. For clinical diagnostic laboratory tests that are assigned a new or substantially revised CPT code, initial payment rates will be assigned by the gap-fill methodology, as under prior law. Initial payment rates for new advanced diagnostic laboratory tests will be based on the actual list charge for the laboratory test.
The payment rates calculated under PAMA are set to be effective starting January 1, 2018. Any reductions to payment rates resulting from the new methodology are limited to 10% per test per year in each of the years 2018 through 2020 and to 15% per test per year in each of the years 2021 through 2023.
PAMA codifies Medicare coverage rules for laboratory tests by requiring any local coverage determination to be made following the local coverage determination process. PAMA also authorizes CMS to consolidate coverage policies for clinical laboratory tests among one to four laboratory-specific MACs. These same contractors may also be designated to process claims if CMS determines that such a model is appropriate. It is unclear whether CMS will proceed with contractor consolidation under this authorization.
PAMA also authorizes the adoption of new, temporary billing codes and/or unique test identifiers for FDA-cleared or approved tests as well as advanced diagnostic laboratory tests. The AMA’s CPT Editorial Panel has approved a proposal to create a new section of billing codes to facilitate implementation of this section of PAMA. At this time, it is unclear whether or when the new section of billing codes will be implemented, nor is it clear if or how these codes would apply to our tests.
14
Under the administrative simplification provisions of the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the federal Health Information Technology for Economic and Clinical Health Act, or HITECH, the U.S. Department of Health and Human Services, or HHS, has issued regulations that establish uniform standards governing the conduct of certain electronic healthcare transactions and requirements for protecting the privacy and security of protected health information, or PHI, used or disclosed by most healthcare providers and other covered entities and their respective business associates, including subcontractors of business associates. The following four principal regulations with which we are required to comply have been issued in final form under HIPAA and HITECH: privacy regulations, security regulations, the breach notification rule and standards for electronic transactions, which establish standards for common healthcare transactions.
The privacy regulations of HIPAA and HITECH cover the use and disclosure of PHI by covered entities and business associates, which include subcontractors that create, receive, maintain or transmit PHI on behalf of a business associate. A subcontractor means any person to whom a business associate delegates a function, activity or service, other than in the capacity of the business associate’s workforce. As a general rule, a covered entity or business associate may not use or disclose PHI except as permitted under the privacy regulations of HIPAA and HITECH. The privacy regulations also set forth certain rights of an individual with respect to his or her PHI maintained by a covered entity or business associate, including the right to access or amend certain records containing his or her PHI or to request restrictions on the use or disclosure of his or her PHI.
Covered entities and business associates must also comply with the security regulations of HIPAA and HITECH, which establish requirements for safeguarding the confidentiality, integrity and availability of electronic PHI. In addition, HITECH established, among other things, certain breach notification requirements with which covered entities and business associates must comply. In particular, a covered entity must notify any individual whose unsecured PHI is breached according to the specifications set forth in the breach notification rule. A covered entity must also notify the Secretary of HHS and, under certain circumstances, the media.
There are significant civil and criminal fines and other penalties that may be imposed for violating HIPAA. A covered entity or business associate is also liable for civil monetary penalties for a violation that is based on an act or omission of any of its agents, including a downstream business associate, as determined according to the federal common law of agency. Penalties for failure to comply with a requirement of HIPAA and HITECH vary significantly depending on the failure and include civil monetary penalties of up to $1.5 million per violation of the same requirement per calendar year. A single breach incident can result in violations of multiple requirements, resulting in potential penalties in excess of $1.5 million. Additionally, a person who knowingly obtains or discloses individually identifiable health information in violation of HIPAA may face a criminal penalty of up to $50,000 and up to one year of imprisonment. These criminal penalties increase if the wrongful conduct involves false pretenses or the intent to sell, transfer or use identifiable health information for commercial advantage, personal gain or malicious harm. Further, to the extent that we submit electronic healthcare claims and payment transactions that do not comply with the electronic data transmission standards established under HIPAA and HITECH, payments to us may be delayed or denied.
The HIPAA privacy, security, and breach notification regulations establish a uniform federal “floor,” but do not supersede state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing PHI or insofar as such state laws apply to personal information that is broader in scope than PHI as defined under HIPAA. Massachusetts, for example, has a state law that protects the privacy and security of personal information of Massachusetts residents.
Numerous other federal, state and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of patient health information. In addition, Congress and some states are considering new laws and regulations that further protect the privacy and security of medical records or medical information. With the recent increase in publicity regarding data breaches resulting in improper dissemination of consumer information, many states have passed laws regulating the actions that a business must take if it experiences a data breach, such as prompt disclosure to affected customers. Generally, these laws are limited to electronic data and make some exemptions for smaller breaches. Congress has also been considering similar federal legislation relating to data breaches. The Federal Trade Commission and states’ Attorneys General have also brought enforcement actions and prosecuted some data breach cases as unfair and/or deceptive acts or practices under the Federal Trade Commission Act. In addition to data breach notification laws, some states have enacted statutes and rules requiring businesses to reasonably protect certain types of personal information they hold or to otherwise comply with certain specified data security requirements for personal information. We intend to continue to comprehensively protect all personal information and to comply with all applicable laws regarding the protection of such information.
15
We are also subject to foreign privacy laws in the jurisdictions in which we sell our tests. The interpretation, application and interplay of consumer and health-related data protection laws in the United States, Europe and elsewhere are often uncertain, contradictory and in flux. For example, a new General Data Protection Regulation, or GDPR, and Cybersecurity Directive have been enacted in the European Union and will come into full effect in May 2018. These texts will introduce many changes to privacy and security in the European Union, including stricter rules on consent and security duties for critical industries, including for the health sector. The interpretation of some rules is still unclear, and some requirements may be completed by national legislation. This makes it difficult to assess the impact of these new data protection laws on our business at this time. More generally, foreign laws and interpretations governing data privacy and security are constantly evolving and it is possible that laws may be interpreted and applied in a manner that is inconsistent with our current practices, in which case we could be subject to government-imposed fines or orders requiring that we change our practices. These fines can be very high. For instance, the GDPR introduces fines of up to approximately $22 million or 4% of a group’s worldwide annual turnover for certain infringements. In addition, privacy regulations differ widely from country to country.
In the United States, we must comply with various fraud and abuse laws and we are potentially subject to regulation by various federal, state and local authorities, including CMS, other divisions of HHS (such as the Office of Inspector General), the U.S. Department of Justice, individual U.S. Attorney offices within the Department of Justice and state and local governments. We also may be subject to foreign fraud and abuse laws.
Anti-Kickback and Fraud Statutes
In the United States, the federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, overtly or covertly, in cash or in kind, in order to induce or in return for the referral of an individual for the furnishing of or arranging for the furnishing of, purchasing, leasing, ordering or arranging for or recommending purchasing, leasing or ordering of any good, facility, service or item for which payment may be made in whole or in part by a federal healthcare program. Courts have stated that a financial arrangement may violate the Anti-Kickback Statute if any one purpose of the arrangement is to encourage patient referrals or other federal healthcare program business, regardless of whether there are other legitimate purposes for the arrangement. The definition of “remuneration” has been broadly interpreted to include anything of value, including gifts, discounts, credit arrangements, payments of cash, consulting fees, waivers of co-payments, ownership interests and providing anything at less than its fair market value. The Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements within the healthcare industry, although it does contain several exceptions. HHS has issued a series of regulatory “safe harbors,” which set forth certain provisions that, if met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. Although full compliance with the statutory exceptions or regulatory safe harbors ensures against prosecution under the federal Anti-Kickback Statute, the failure of a transaction or arrangement to fit within a specific statutory exception or regulatory safe harbor does not necessarily mean that the transaction or arrangement is illegal or that prosecution under the Anti-Kickback Statute will be pursued. Furthermore, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Penalties for violations of the Anti-Kickback Statute are severe and include imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. In addition, a violation of the federal Anti-Kickback Statute can serve as a basis of liability under the federal False Claims Act (described below). Many states also have anti-kickback statutes, some of which may apply to items or services reimbursed by any third-party payor, including commercial insurers.
There are also U.S. federal laws related to healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government payor programs such as the Medicare and Medicaid programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. A violation of this statute is also a felony and may result in fines, imprisonment or exclusion from government payor programs.
Another development affecting the healthcare industry is the increased enforcement of the federal False Claims Act and, in particular, actions brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal government payor program. The qui tam provisions of the False Claims Act allow a private individual to bring
16
actions on behalf of the federal government alleging that the defendant has defrauded the federal government by submitting a false claim to the federal government and permit such individuals to share in any amounts paid by the entity to the government in fines or settlement. In addition, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, which we collectively refer to as the Affordable Care Act, establishes a requirement for providers and suppliers to report and return any overpayments received from government payors under the Medicare and Medicaid programs within 60 days of identification. Failure to identify and return such overpayments exposes the provider or supplier to False Claims Act liability. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties ranging from $5,500 to $11,000 for each false claim.
In addition, various states have enacted false claim laws analogous to the federal False Claims Act, although many of these state laws apply where a claim is submitted to any third-party payor and not merely a government payor program.
The federal Civil Monetary Penalties Law imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or for a claim that is false or fraudulent. This law also prohibits the offering or transfer of remuneration to a Medicare or state healthcare program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of items or services reimbursable by Medicare or a state healthcare program, unless an exception applies.
Physician Referral Prohibitions
The U.S. federal law directed at “self-referrals,” commonly known as the “Stark Law,” prohibits a physician from making referrals for certain designated health services, including laboratory services, that are covered by the Medicare program, to an entity with which the physician or an immediate family member has a direct or indirect financial relationship, unless an exception applies. The prohibition also extends to payment for any services referred in violation of the Stark Law. A physician or entity that engages in a scheme to circumvent the Stark Law’s referral prohibition may be fined up to $100,000 for each such arrangement or scheme. In addition, any person who presents or causes to be presented a claim to the Medicare program in violation of the Stark Law is subject to civil monetary penalties of up to $15,000 per service, an assessment of up to three times the amount claimed and possible exclusion from participation in federal healthcare programs. The Stark Law is a strict liability statute, meaning that a physician’s financial relationship with a laboratory must meet an exception under the Stark Law or the referrals are prohibited. Thus, unlike the Anti-Kickback Statute’s safe harbors, if a laboratory’s financial relationship with a referring physician does not meet the requirements of a Stark Law exception, then the physician is prohibited from making Medicare and Medicaid referrals to the laboratory and any such referrals will result in overpayments to the laboratory and subject the laboratory to the Stark Law’s penalties.
Many states have comparable laws that are not limited to Medicare referrals. The Stark Law also prohibits state receipt of federal Medicaid matching funds for services furnished pursuant to a prohibited referral, but this provision of the Stark Law has not been implemented by regulations. In addition, some courts have held that the submission of claims to Medicaid that would be prohibited as self-referrals under the Stark Law for Medicare could implicate the False Claims Act.
The Affordable Care Act, among other things, imposed new reporting requirements on manufacturers of certain devices, drugs and biologics for certain payments and transfers of value by them and in some cases their distributors to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Because we manufacture our own LDTs solely for use by or within our own laboratory, we believe we are exempt from these reporting requirements. We may become subject to such reporting requirements, however, if the FDA requires us to obtain premarket clearance or approval for our tests.
We are subject to U.S. Foreign Corrupt Practices Act, or FCPA, which prohibits companies and their intermediaries from making payments in violation of law to non-U.S. government officials for the purpose of obtaining or retaining business or securing any other improper advantage. The sale of our tests internationally demands a high degree of vigilance in maintaining, implementing and enforcing a policy against participation in corrupt activity. Other U.S. companies in the medical device and pharmaceutical fields have faced substantial monetary fines and criminal penalties under the FCPA for allowing their agents to deviate from appropriate practices in doing business with non-U.S. government officials.
17
We are also subject to similar anti-bribery laws in the foreign jurisdictions in which we operate. In Europe, various countries have adopted anti-bribery laws providing for severe consequences, in the form of criminal penalties and/or significant fines for individuals and/or companies committing a bribery offence. For instance, in the United Kingdom, under the Bribery Act of 2010, which became effective in July 2011, a bribery occurs when a person offers, gives or promises to give a financial or other advantage to induce or reward another individual to improperly perform certain functions or activities, including any function of a public or private nature. Bribery of foreign public officials also falls within the scope of the Bribery Act of 2010. Under the new regime, an individual found in violation of the Bribery Act of 2010 faces imprisonment of up to 10 years and could be subject to an unlimited fine, as could commercial organizations for failure to prevent bribery.
In March 2010, the Affordable Care Act was enacted in the United States. The Affordable Care Act made a number of substantial changes to the way healthcare is financed both by governmental and private insurers. For example, the Affordable Care Act requires each medical device manufacturer to pay a sales tax equal to 2.3% of the price for which such manufacturer sells its medical devices. The medical device tax has been suspended for 2016 and 2017, but is scheduled to return beginning in 2018. It is unclear at this time when, or if, the provision of our LDTs will trigger the medical device tax if the FDA ends its policy of general enforcement discretion and regulates certain LDTs as medical devices, and it is possible that this tax will apply to some or all of our existing tests or tests we may develop in the future. Additionally, the Affordable Care Act establishes an Independent Payment Advisory Board, or IPAB, to propose reductions to payments in order to reduce the per capita rate of growth in Medicare spending if expenditures exceed certain targets. The expenditure targets for IPAB proposals have not been exceeded at this time, and it is unclear when such targets may be exceeded in the future, when any IPAB-proposed reductions to payments could take effect and how any such reductions would affect reimbursement payments for our tests. The Affordable Care Act also contains a number of other provisions, including provisions governing enrollment in federal and state healthcare programs, reimbursement matters and fraud and abuse, which we expect will impact our industry and our operations in ways that we cannot currently predict. Following the results of the 2016 U.S. presidential election and in light of the policies of the current administration, which has threatened to repeal the Affordable Care Act, there is uncertainty regarding the continued effect or the Affordable Care Act in its current form.
Corporate Practice of Medicine
Numerous states have enacted laws prohibiting business corporations, such as us, from practicing medicine and employing or engaging physicians to practice medicine, generally referred to as the prohibition against the corporate practice of medicine. These laws are designed to prevent interference in the medical decision-making process by anyone who is not a licensed physician. For example, California’s Medical Board has indicated that determining the appropriate diagnostic tests for a particular condition and taking responsibility for the ultimate overall care of a patient, including providing treatment options available to the patient, would constitute the unlicensed practice of medicine if performed by an unlicensed person. Violation of these corporate practice of medicine laws may result in civil or criminal fines, as well as sanctions imposed against the business corporation and/or the professional through licensure proceedings. Typically such laws are only applicable to entities with a physical presence in the applicable state.
Environmental and Other Regulatory Requirements
Our laboratory is subject to federal, state and local laws and regulations relating to the use, storage, handling and disposal of regulated medical waste, hazardous waste and biohazardous waste, including chemicals, biological agents and compounds, blood and other tissue specimens. Typically, we use outside vendors to dispose of such waste that are licensed or otherwise qualified to handle and dispose of the waste. However, many of these laws and regulations provide for strict liability, holding a party potentially liable without regard to fault or negligence. As a result, we could be held liable for damages and fines if our, or others’, business operations result in contamination of the environment or individual exposure to hazardous substances. Our costs for complying with these laws and regulations cannot be predicted at this time and will depend upon, among other things, the amount and nature of waste we produce (which will depend in part on the number of tests we perform) and the terms we negotiate with our waste disposal vendors.
Our operations are also subject to extensive requirements established by the U.S. Occupational Safety and Health Administration relating to workplace safety for healthcare employees, including requirements to develop and implement programs to protect workers from exposure to blood-borne pathogens by preventing or minimizing any exposure through needle stick or similar penetrating injuries.
Reporting Segment and Geographical Information
We operate in one reportable business segment. See Note 7, Reporting Segment and Geographic Information, to our consolidated financial statements for information about revenue attributable to customers and long-lived assets located in the United
18
States and other regions. We are subject to risks attendant to our foreign operations, which are discussed in this report under “Item 1A. Risk Factors.”
We believe growing and retaining a strong team is crucial to our success. As of March 1, 2017, we had 70 employees engaged in bioinformatics, genetics, software engineering, laboratory management, sales and marketing and corporate and administrative activities. None of our employees are represented by a labor union or covered by collective bargaining agreements and we believe our relationship with our employees is good.
We were incorporated in Delaware on May 13, 2016. We are the holding company of Fulgent LLC, which was initially formed in June 2011 as a California corporation and converted to a California limited liability company in September 2012. On September 30, 2016, Fulgent LLC became our wholly owned subsidiary in a transaction we refer to as the Reorganization, in which the holders of all equity interests in Fulgent LLC immediately prior to the Reorganization became all of our stockholders immediately following the Reorganization.
Our initial operations focused on Fulgent LLC’s former pharmaceutical business, or the Pharma Business, and in 2013 we commenced the genetic testing business we are currently pursuing. In October 2015, we recapitalized Fulgent LLC to establish two series of units, with the Class D units (consisting of Class D-1 preferred, Class D-2 preferred and Class D common units) having economic rights based on the genetic testing business we are currently pursuing and the Class P units (consisting of the Class P preferred and Class P common units) having economic rights based on the Pharma Business. On April 4, 2016, Fulgent LLC separated the Pharma Business from our genetic testing business in a transaction we refer to as the Pharma Split-Off. The operating results of the Pharma Business have been reported as discontinued operations for all periods in our consolidated financial statements included in this report.
Our headquarters and laboratory are located at 4978 Santa Anita Avenue, Temple City, California 91780, and our telephone number is (626) 350-0537. Our website address is www.fulgentgenetics.com. The information contained on or that can be accessed through our website is not part of and is not incorporated into this report by this reference.
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended, or JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are applicable generally to other public companies. We will remain an emerging growth company until December 31, 2021, unless our gross revenue exceeds $1.0 billion in any fiscal year before that date, we issue more than $1.0 billion of non-convertible debt in any three-year period before that date or the market value of our common stock held by non-affiliates exceeds $700 million as of the last business day of the second fiscal quarter of any fiscal year before that date.
We file reports with the Securities and Exchange Commission, or the SEC, and make available, free of charge, on or through our website, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy and information statements and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
19
Investing in our common stock involves a high degree of risk. Before making any investment decision with respect to our common stock, you should carefully consider the risks described below and all of the other information included in this report and the other filings we make with the SEC. We believe the risks and uncertainties described below are the most significant we face. The occurrence of any of these risks could harm our business, financial condition, results of operations, prospects and reputation and could cause the trading price of our common stock to decline. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business.
Business and Strategy Risks
Our industry is subject to rapidly changing technology and new and increasing amounts of scientific data, and if we fail to keep pace with these technological advances, we may be unable to compete effectively and our business and prospects could suffer.
In recent years, there have been numerous advances in the analysis of large amounts of genomic information and the role of genetics and gene variants in disease diagnosis and treatment. Our industry has been, and we believe will continue to be, characterized by rapid technological change, increasing amounts of data, frequent introductions of new genetic tests and evolving industry standards, all of which could make our tests obsolete if we are not able to enhance our technologies and tests faster and better than our competitors to maintain our competitive advantage. Our future success will depend on our ability to keep pace with the evolving needs of our customers in a timely and cost-effective manner and to pursue new market opportunities that develop as a result of technological and scientific advances. If we are not able to keep pace with technological advances and increased customer expectations that develop as a result of these advances, we may be unable to sustain or grow our business and our future operations and prospects could suffer.
We are an early-stage company with a limited operating history, which may expose us to enhanced risks and increase the difficulty of evaluating our business and prospects.
We began operations in May 2012 and commercially launched our first genetic tests in 2013. As a result, we have only a limited operating history upon which you can evaluate our business and prospects. Our revenue growth may not continue or increase, we may not achieve profitability and, if we achieve profitability, we may not be able to sustain it. Our limited operating history makes it difficult to evaluate our current business and inhibits our ability to forecast our future operating results, including revenue, cash flows and movement toward profitability. Our limited operating history makes it difficult to determine if these fluctuations reflect seasonality in our performance or are the result of other events. We have encountered and will continue to encounter risks and uncertainties frequently experienced by growing companies in the life sciences and technology industries, such as risks related to an evolving and unpredictable industry and business model, management of growth and other uncertainties described in this report. If our assumptions regarding these risks and uncertainties are incorrect or these risks and uncertainties change due to changes in our markets, or if we do not address these risks successfully, our operating and financial results could differ materially from our expectations and our business could suffer.
We have a history of losses, and we may never be able to achieve or sustain profitability.
We have a history of losses. For 2016, 2015 and 2014, we recorded a loss from continuing operations of $5.4 million, $5.0 million and $990,000, respectively. To date, we have generated limited revenue, and we may never achieve revenue sufficient to offset our expenses and achieve or sustain profitability. In addition, we may continue to incur losses in the future, particularly as we focus on growing our business and operations and experience expected increases in expenses related to this growth. Our prior losses and any future losses have had and will continue to have an adverse effect on our stockholders’ equity and working capital. Our failure to achieve and sustain profitability in the future would negatively affect our business, financial condition, results of operations and cash flows, and could cause the market price of our common stock to decline.
Sales to a small number of customers have represented a significant portion of our revenue in certain periods, and the loss of, or a significant reduction in sales to, any one of these customers could materially harm our business.
The composition of our customer base can fluctuate from period to period, as our key customers experience volatility in their genetic testing demand. In certain periods, a small number of customers has accounted for a significant portion of our revenue. For instance, in 2016, Customers A and B each contributed 15% of our total revenue, and Customer B also contributed 19% and 11% of our total revenue in 2015 and 2014, respectively. Generally, we do not have long-term purchase agreements with any of our customers and, as result, any or all of them could decide at any time to discontinue, decrease or delay their orders from us. In addition, the prices that our customers pay for our products could be subject to fluctuations. Further, the failure of any one of our key customers to pay on a timely basis would negatively impact our cash flows from operations. Our ability to maintain or increase sales to our key customers
20
depends on a variety of factors, including the other risk factors discussed in this report, many of which are beyond our control. Because of these and other factors, sales to these customers may not continue and the amount of such sales may not reach or exceed historical levels in any future period. The loss of any of our key customers or a reduction in orders or difficulties collecting payments from any of them could significantly reduce our revenue and adversely affect our operating results.
If we are not able to grow and diversify our customer base and increase demand for our tests from existing and new customers, our commercial success would be limited.
To achieve our anticipated revenue growth, we must increase test volume by further penetrating our existing hospital and medical institution customers. In addition, we must grow our customer base beyond hospitals and medical institutions and into additional customer groups, such as individual physicians, other practitioners and research institutions. To this end, we have made efforts to diversify our customer base beyond hospitals by establishing a vendor code with a national clinical laboratory that orders our tests to fulfill some of the genetic testing orders it receives from certain U.S. government agencies and contracting with a regional hospital network within the U.S. Army to provide genetic tests for members and their families. We have also pursued relationships with payors, including Medicare, some state Medicaid programs and third-party payors, in order to obtain coverage and reimbursement for our tests to make them accessible to more individual physicians. Establishing these relationships means that we have agreed with the applicable payor, laboratory or other customer to provide certain of our tests at negotiated rates, but, except for our relationship with the hospital network within the U.S. Army, which involves a minimum commitment of approximately $400,000 of our tests annually over a three-year period, none of these relationships obligate any party to order our tests at any agreed volume or frequency, or at all. Further, our relationship with the hospital network within the U.S. Army is subject to unique risks associated with government contracts, including cancellation if adequate appropriations for subsequent performance periods are not made and modification or termination at their convenience and without prior notice. As a result, these relationships, or any similar relationships we may establish in the future, may not amount to meaningful increases in our customer base, the number of billable tests we deliver or our total revenue, or improve our ability to achieve profitability. We may not succeed in facilitating the clinical acceptance and adoption of our tests needed to achieve the increased volumes and customer growth we expect. Because detailed genetic data from tests such as ours have only recently become available at relatively affordable prices, the pace and degree of market acceptance and adoption of these tests is uncertain.
We may fail to expand our customer base and grow our volume of tests delivered for a variety of reasons, including, among others:
|
• |
the genetic testing market generally, and particularly the market for NGS genetic tests, is relatively new and may not grow as predicted or may decline; |
|
• |
our efforts to improve our existing tests and develop and launch new tests may be unsuccessful; |
|
• |
we may not be able to convince additional hospitals and medical institutions or additional customer groups, such as individual physicians, other practitioners and research institutions, of the utility of our tests and their potential advantages over existing and new alternatives; |
|
• |
our efforts to increase our sales force and expand our marketing efforts may fail; |
|
• |
we may be unsuccessful in demonstrating the benefits of our broad and customizable test menu; |
|
• |
genetic testing is expensive and many existing and potential new customers may be sensitive to pricing, particularly if we are not able to maintain low prices relative to our competitors; |
|
• |
potential new customers, particularly individual physicians and other practitioners, may not adopt our tests if coverage and adequate reimbursement are not available; |
|
• |
negative publicity or regulatory investigations into the actions of companies within our industry could raise doubts about the legitimacy of diagnostics technologies generally, and could result in scrutiny of diagnostic activities by the FDA or other applicable government agencies; and |
|
• |
our competitors could introduce new tests that cover more genes or that provide more accurate or reliable results at the same or a lower price than ours. |
If we are unable to address these and other risks associated with growing our customer base and deepening our relationships with existing customers, we may not achieve our anticipated growth in billable tests and our results of operations would be adversely impacted.
21
We face intense competition, which is likely to intensify further as existing competitors devote additional resources to, and new participants enter, the market, and if we cannot compete successfully, we may be unable to increase our revenue or achieve or grow profitability.
With the development of NGS, the clinical genetic testing market has become increasingly competitive, and we expect this competition to further intensify in the future. We face competition from a variety of sources, including, among others:
|
• |
dozens of companies focused on molecular genetic testing services, including specialty and reference laboratories that offer traditional single-gene and multi-gene tests, such as Ambry Genetics, Inc.; Counsyl Inc.; Foundation Medicine, Inc.; GeneDx, a subsidiary of OPKO Health, Inc.; Invitae Corporation; Myriad Genetics, Inc.; and Pathway Genomics Corporation, as well as other commercial and academic laboratories; and |
|
• |
established and emerging healthcare, information technology and service companies that may develop and sell competitive tests, which may include informatics, analysis, integrated genetic tools and services for health and wellness. |
Additionally, participants in closely related markets, such as prenatal testing and clinical trial or companion diagnostic testing, could converge on offerings that are competitive with the type of tests we perform. Instances where potential competitors are aligned with key suppliers or are themselves suppliers could provide such potential competitors with significant advantages. Further, hospitals, research institutions and eventually individual physicians and other practitioners may also seek to perform at their own facilities the type of genetic testing we would otherwise perform for them. In this regard, continued development of, and associated decreases in the cost of, equipment, reagents and other materials and databases and genetic data interpretation services may enable broader direct participation in genetic testing and analysis and drive down the use of third-party testing companies such as ours. Moreover, the biotechnology and genetic testing fields continue to undergo significant consolidation, permitting larger clinical laboratory service providers to increase cost efficiencies and service levels, resulting in more intense competition.
Many of our existing and potential future competitors have longer operating histories, larger customer bases, greater brand recognition and market penetration, substantially greater financial, technological and research and development resources and selling and marketing capabilities, and considerably more experience dealing with third-party payors. As a result, they may be able to respond more quickly to changes in customer requirements or preferences, devote greater resources to the development, promotion and sale of their tests, devote more resources to and obtain more favorable results from third-party payors regarding coverage and reimbursement for their offerings, adopt more aggressive pricing policies for their tests, secure supplies from vendors on more favorable terms or devote substantially more resources to infrastructure and systems development. We may not be able to compete effectively against these organizations.
Additionally, increased competition and cost-saving initiatives on the part of government entities and other third-party payors could result in pricing pressures, which could harm our sales or ability to gain market share and achieve profitability. In addition, competitors may be acquired by, receive investments from or enter into other commercial relationships with larger, well-established and well-financed companies as use of NGS for clinical diagnosis and preventative care increases. Further, companies or governments that effectively control access to genetic testing through umbrella contracts or regional preferences could promote our competitors or prevent us from performing certain tests in certain territories. If we are unable to compete successfully against current and future competitors, we may be unable to increase market acceptance and sales volume of our tests, which could prevent us from increasing our revenue or achieving or growing profitability.
We will need to invest in and expand our infrastructure and hire additional skilled personnel in order to support our anticipated growth, and our failure to effectively manage any future growth could jeopardize our business.
To increase the volume of tests that we offer and deliver, we must invest in our infrastructure, including our testing capacity and information systems, enterprise software systems, customer service, billing and collections systems and processes and internal quality assurance programs, in the near term. We will also need to invest in hiring additional skilled personnel, including biostatisticians, geneticists, software engineers, laboratory directors and specialists, sales and marketing experts and other scientific, technical and managerial personnel to market, process, interpret and validate the quality of results of our genetic tests and otherwise manage our operations. For example, before we deliver a report for any of our genetic tests, the results summarized in the report must be reviewed and approved by a licensed and qualified laboratory director. We currently have only one such laboratory director with all of the required licenses, Dr. Han Lin Gao, who conducts this review and approval for each test we deliver. We are in the process of licensing additional laboratory directors to assist Dr. Gao, and we may need to hire more laboratory directors in the future to further scale our business. If we fail to hire additional qualified personnel or otherwise develop our infrastructure sufficiently in advance of demand or if we fail to generate demand commensurate with our level of investment in our infrastructure, our business, prospects, financial condition and results of operations could be adversely affected. Additionally, although we do not presently have plans to acquire new or expand our existing laboratory space, we may need to do so in the future as our volumes increase, and any need to obtain an additional facility or replace our existing facility with a larger one would involve significant challenges.
22
The time and resources required to implement new systems, to add and train additional skilled personnel and to acquire or expand laboratory space as needed are uncertain. Any future growth we may experience could create a strain on our organizational, administrative and operational infrastructure, including laboratory operations, quality control, customer service, sales and marketing and management. We may not be able to maintain the quality of or expected turnaround times for our tests or satisfy customer demand if and when it grows. Our ability to effectively manage any growth we experience will also require us to continue to improve our laboratory and other operational, financial and management systems and controls and our reporting processes and procedures, which we may not be able to do.
We have limited experience marketing and selling our tests and our commercial success will depend in part upon our ability to grow our sales and marketing team and generate sales using this relatively small internal and developing team.
We have limited experience marketing and selling our tests, which we began selling in 2013. We may not be able to market or sell our existing tests or any future tests we may develop in order to drive demand sufficient to support our planned growth. We currently sell our tests in the United States through a small internal sales force and outside the United States through one internal sales person and we have historically relied significantly on organic growth and word-of-mouth among our customers to generate interest in our tests. Our ability to maintain and grow sales volume in the future will depend in large part upon our ability to develop and substantially expand our sales team and to increase the scope of our marketing efforts. We intend to aggressively build our sales and marketing team in the near term in order to pursue expansion of our customer base and growth in the volume of tests ordered, with the goal of more than doubling the size of our sales and marketing team in the next 12 months. This expansion will involve significant time and expense. Additionally, we may not be able to attract and hire the qualified personnel we need to grow our sales and marketing team as quickly as we intend for various reasons, including intense competition in our industry for qualified personnel. Even if we are able to further develop our sales and marketing team, we have limited experience managing a sales and marketing team and it may not be successful in growing our customer base or increasing penetration into our existing customers.
In addition, our future sales will depend in large part upon our ability to expand our brand awareness, laterally grow our customer base and vertically penetrate our relationships with existing customers by educating the medical community, including existing and potential future customers, about the benefits and the full scale of our offering. We also intend to obtain publication of scientific and medical results in peer-reviewed journals and make presentations at leading industry conferences. We have limited experience with this type of activity and we may not be successful in implementing these initiatives. If we are not able to drive sufficient levels of revenue using our sales and marketing strategies to support our planned growth, our business and results of operations would be negatively affected. Additionally, if we are not able to obtain sufficient clinical information in support of our tests, third-party payors could designate our tests as experimental or investigational and decline to cover and reimburse our tests as a result of such designation.
We also intend to increase our focus on growing our international sales and customer base. Outside the United States, we use and intend to continue to use one internal sales person, and we may also engage distributors or establish other types of arrangements, such as joint ventures or other partner relationships, to assist with sales, logistics, education and customer support in the future. To this end, we are working with Xi Long, a large stockholder of our company, to develop a strategic commercial relationship to pursue additional customers in China, which we expect to be finalized in the first half of 2017. We anticipate that this relationship, if it proceeds, could expand our long-term opportunities to address the genetic testing market in Asia; however, the terms of this relationship have not been finalized and we may never be successful in forming this relationship or realizing any of the benefits we anticipate from it. We believe identifying, qualifying and engaging distributors or other partners with local industry experience and knowledge will be necessary to effectively market and sell our tests outside the United States. We may not be successful in finding, attracting and retaining qualified distributors or other partners or we may not be able to enter into arrangements covering desired territories on favorable terms. Sales practices utilized by distributors or other partners that are locally acceptable may not comply with sales practices or standards required under U.S. laws that apply to us, which could subject us to additional compliance risks. If our sales and marketing efforts are not successful outside the United States, we may not achieve significant market acceptance for our tests in international markets, which could materially and adversely impact our business operations.
If we are sued for product or professional liability, we could face substantial liabilities that exceed our resources.
Our business depends upon our ability to provide reliable and accurate test results that incorporate rapidly evolving information about the role of genes and gene variants in disease and clinically relevant outcomes associated with those variants. Hundreds of genes can be implicated in some disorders and overlapping networks of genes and symptoms can be implicated in multiple conditions. As a result, a substantial amount of judgment is required in order to interpret the results of each test we perform and produce a report summarizing these results. Errors, such as failures to detect genomic variants with high accuracy, or mistakes, such as failures to completely and correctly identify the significance of gene variants, could subject us to product liability or professional liability claims. A product liability or professional liability claim against us could result in substantial damages and be costly and time-consuming to defend. Although we maintain liability insurance, including for errors and omissions, our insurance may not fully protect us from the financial impact of defending against these types of claims or any judgments, fines or settlement costs arising out of any such claims.
23
Any liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing adequate insurance coverage in the future. Additionally, any liability lawsuit could damage our reputation or force us to suspend sales of our tests. The occurrence of any of these events could have a material adverse effect on our business, reputation and results of operations.
Our ability to achieve profitability depends upon our ability to collect payment for the tests we deliver to hospitals and medical institutions, which we may not be able to do successfully.
We are currently, and we have been since starting our genetic testing business, focused primarily on providing our tests to hospitals and medical institutions. These customers are typically able to pay for the cost of our tests using funds reimbursed in connection with a patient’s diagnosis related group, or DRG. However, our ability to collect payment for the tests we perform is subject to a number of risks, many of which are not within our control, including risks of default or bankruptcy by the party responsible for payment and other risks associated with payment collection generally. Further, healthcare policy changes that influence the way healthcare is financed or other changes in the market that impact payment rates by institutional or non-institutional customers could affect our collection rates. For example, because reimbursement under a DRG is typically provided at a fixed amount intended to cover all services provided to the patient, the cost of our tests may be viewed to limit the profitability of the billing institution. If we are unable to convince hospitals and medical institutions of the value and benefit provided by our tests, or if the amount reimbursed under these DRG codes was decreased, these customers may slow, or stop altogether, their purchasing of our tests. Moreover, our ability to collect payment for our tests in a timely manner or at all may decline to the extent we expand our business into new customer groups, including individual physicians and other practitioners, from which collection rates may be lower than hospitals and medical institutions.
If third-party payors do not provide coverage and adequate reimbursement for our tests, our commercial success could be limited.
Coverage and reimbursement by third-party payors, including managed care organizations, private health insurers and government healthcare programs, such as Medicare and Medicaid, for the types of genetic tests we perform can be limited and uncertain. Although our existing customer base consists primarily of hospitals and medical institutions, from which we typically receive direct payment for ordered tests, we believe our potential for future success is dependent upon our ability to attract new customer groups, including individual physicians and other practitioners. These practitioners may not order our tests unless third-party payors cover and provide adequate reimbursement for a substantial portion of the price of our tests. If we are not able to obtain coverage and an acceptable level of reimbursement for our tests from third-party payors, there would typically be a greater co-insurance or co-payment requirement from the patient for whom the test is ordered or the patient may be forced to pay the entire cost of the test out-of-pocket, which could dissuade practitioners from ordering our tests and, if ordered, could result in delay in or decreased likelihood of our collection of payment, whether from patients or from third-party payors. We believe our ability to increase the number of tests we sell and our revenue will depend upon our success in achieving broad coverage and reimbursement for our tests from third-party payors.
Coverage and reimbursement by a third-party payor may depend on a number of factors, including a payor’s determination that a test is appropriate, medically necessary and cost-effective. Each payor makes its own decision as to whether to establish a policy or enter into a contract to cover our tests and the amount it will reimburse for a test, and seeking a determination by a payor to cover our tests and the amount it will reimburse for our tests would likely be made on an indication-by-indication basis. In addition, the coding procedure used by all third-party payors with respect to establishing payment rates for various procedures, including our tests, is complex, does not currently adapt well to the genetic tests we perform and may not enable coverage and adequate reimbursement rates for our tests. As a result, obtaining approvals from third-party payors to cover our tests and establishing adequate reimbursement levels is an unpredictable, challenging, time-consuming and costly process, and we may never be successful.
To date, we have contracted with a regional physician services organization and a national health insurance company to become an in-network provider. We have also enrolled as a supplier in the Medicare program and in 10 state Medicaid programs, but we have not obtained any coverage, pricing or reimbursement approvals from payors in any countries outside of the United States. Although becoming an in-network provider or enrolling as a supplier means that we have agreed with these payors to provide certain of our tests at negotiated rates, it does not obligate any physicians to order our tests or guarantee that we will receive reimbursement for our tests from these or any other payors at adequate levels. Thus, these payor relationships, or any similar relationships we may establish in the future, may not result in acceptable levels of reimbursement for our tests or meaningful increases in our physician customer base or the number of billable tests we sell to physicians. We expect to focus on increasing coverage and reimbursement for our current tests and any future tests we may develop. We believe it may take several years to achieve coverage and adequate contracted reimbursement with third-party payors. However, we cannot predict whether, under what circumstances, or at what payment levels payors will cover and reimburse for our tests. If we fail to establish and maintain broad coverage and reimbursement for our tests, our ability to generate increased revenue and grow our test volume and customer base could be limited and our future prospects and our business could suffer.
24
If our sole laboratory facility becomes inoperable, if we are forced to vacate the facility or if we are unable to obtain additional laboratory space as and when needed, we would be unable to perform our tests and our business would be harmed.
We perform all of our tests at a single laboratory in Temple City, California. Our laboratory facility could be damaged or rendered inoperable by natural or man-made disasters, including earthquakes, floods, fires and power outages, which could render it difficult or impossible for us to perform our tests for some period of time. The inability to perform our tests or the backlog that could develop if our laboratory is inoperable for even a short period of time could result in the loss of customers or harm to our reputation. Although we maintain insurance for damage to our property and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, if at all.
Further, if we need to move to a different facility or locate additional laboratory space as our business grows, we may have difficulty locating suitable space in a timely manner, on reasonable terms or at all, and even if acceptable space was available, it would be challenging, time-consuming and expensive to obtain or transfer the licensure and accreditation required for a commercial laboratory like ours and the equipment we use to perform our tests. These challenges could be amplified if we or our partners seek to procure laboratory space outside the United States as we seek to expand our international operations. If we are unable to obtain or are delayed in obtaining new laboratory space as needed, we may not be able to provide existing tests or develop and launch new tests, which could result in harm to our business, reputation, financial condition and results of operations.
Ethical, legal and social concerns related to the use of genetic information could reduce demand for our tests.
Genetic testing has raised ethical, legal and social issues regarding privacy and the appropriate uses of the resulting information. Government authorities could, for social or other purposes, limit or regulate the use of genetic information or genetic testing or prohibit testing for genetic predisposition to certain conditions, particularly for those that have no known cure. Similarly, these concerns may lead patients to refuse to use or physicians to be reluctant to order genetic tests, even if permissible. These and other ethical, legal and social concerns may limit market acceptance and adoption of our tests or reduce the potential markets for our tests, any of which could have an adverse effect on our business, financial condition and results of operations.
We rely on a limited number of suppliers and, in some cases, a sole supplier, for some of our laboratory instruments and materials and we may not be able to find replacements or immediately transition to alternative suppliers if necessary.
We rely on a limited number of suppliers, or, in the case of Illumina, Inc., a sole supplier, for certain laboratory substances used in the chemical reactions incorporated into our processes, which we refer to as reagents, as well as for the sequencers and various other equipment and materials that we use in our laboratory operations. We do not have long-term agreements with any of our suppliers and, as a result, they could cease supplying these materials and equipment to us at any time or fail to provide us with sufficient quantities of materials that meet our specifications. Our laboratory operations would be interrupted if we encounter delays or difficulties in securing these reagents, sequencers or other equipment or materials or if we need a substitute for any of our suppliers and are not able to locate and make arrangements with an acceptable substitute. Any such interruption could significantly affect our business, financial condition, results of operations and reputation. We rely on Illumina as the sole supplier of the next generation sequencers and associated reagents we use to perform our genetic tests and as the sole provider of maintenance and repair services for these sequencers. Any disruption in Illumina’s operations could impact our supply chain and laboratory operations as well as our ability to conduct our tests.
We believe there are only a few other manufacturers that are currently capable of supplying and servicing the equipment necessary for our laboratory operations, including sequencers and various associated reagents. Transitioning to a new supplier would be time-consuming and expensive, could result in interruptions in or otherwise affect the performance specifications of our laboratory operations or could require that we revalidate our tests. In addition, the use of equipment or materials provided by a replacement supplier could require us to alter our laboratory operations and procedures. In the case of obtaining an alternative supplier for Illumina, replacement sequencers and associated reagents that meet our quality control and performance requirements may not be available on reasonable terms, in a timely manner or at all. If we encounter delays or difficulties in securing, reconfiguring or revalidating the equipment and reagents we require for our tests, our business, financial condition, results of operations and reputation would be adversely affected.
We rely on a third-party for certain portions of our billing and collection processing, which is complex and time-consuming, and any delay in transmitting and collecting claims could have an adverse effect on our future revenue.
We have engaged a third-party service provider for certain claims processing, billing and collection functions. Billing for our tests is complex, time-consuming and expensive. Depending on the billing arrangement and applicable law, we plan to bill various payors, including customers directly in the case of our hospital and medical institution customers, as well as Medicare, Medicaid, insurance companies and patients, all of which may have different billing requirements. We may face increased risk in our collection
25
efforts due to the complexities of these billing requirements, including long collection cycles, which could adversely affect our business, results of operations and financial condition.
Several factors make the billing process complex, including:
|
• |
differences between the list price for our tests and the reimbursement rates of payors; |
|
• |
compliance with complex federal and state regulations related to billing government healthcare programs, including Medicare and Medicaid; |
|
• |
disputes among payors as to which party is responsible for payment; |
|
• |
differences in coverage among payors and the effect of patient co-payments or co-insurance; |
|
• |
differences in information and billing requirements among payors; |
|
• |
incorrect or missing billing information; and |
|
• |
the resources required to manage the billing and claims appeals process. |
Even though we have engaged a third party to assist with some of these billing and collections functions, we will still need to make significant efforts and expend substantial resources to develop systems and procedures to handle these aspects of our business, which will become increasingly important as we focus on establishing coverage and reimbursement policies with third-party payors. As a result, these billing complexities, along with the related uncertainty in obtaining payment for our tests, could negatively affect our revenue and cash flow, our ability to achieve profitability and the consistency and comparability of our results of operations. In addition, if claims for our tests are not submitted to payors on a timely basis, or if we are required to switch to a different provider to handle our processing and collections functions, it could have an adverse effect on our revenue and our business.
We are exposed to additional business, regulatory, political, operational, financial and economic risks related to our international operations.
Our existing customer base includes international customers, many of which are based in Canada. Approximately $10.0 million, $4.5 million and $638,000 of our revenue came from non-U.S. customers in 2016, 2015 and 2014, respectively, and of this, approximately $3.8 million, $2.7 million and $194,000 in the respective periods came from customers located in Canada. Our business strategy includes plans to increase this volume in the near term, from customers in Canada and other geographic markets, including Asia and Europe. To this end, we recorded revenues of $3.2 million from tests ordered by customers in China in 2016 and we are working with Xi Long to develop a strategic commercial relationship in order to further pursue our opportunities in this market, although this relationship has not been, and may never be, finalized or successful. We may also enter into new geographic markets and increase our presence in existing foreign markets by engaging distributors or joining with other partners to conduct physician outreach activities and develop and expand payor relationships outside of the United States.
Doing business internationally involves a number of risks, including, among others:
|
• |
multiple, conflicting and evolving laws and regulations, such as privacy regulations, tax laws, employment laws, regulatory requirements and other government approvals, permits and licenses; |
|
• |
limits on our ability to penetrate international markets if we do not conduct our tests locally, including local legal and regulatory requirements that would force us to build additional laboratories or engage in joint ventures or other partner relationships in order to offer our tests in certain countries; |
|
• |
failure by us or any distributors or other partners we may engage in the future to obtain regulatory approvals for the use of our tests in various countries; |
|
• |
complexities and difficulties in obtaining protection for and enforcing our intellectual property; |
|
• |
difficulties in staffing and managing foreign operations; |
|
• |
complexities associated with managing multiple payor coverage and reimbursement regimes, government payors or patient self-pay systems; |
26
|
• |
natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; and |
|
• |
regulatory and compliance risks that relate to prohibiting bribery and maintaining accurate information and control over activities that may fall within the purview of applicable anti-bribery laws. |
Any of these factors could significantly harm our existing relationships with international customers or derail our international expansion plans, which would cause our revenue and results of operations to suffer.
We may not be successful in developing and marketing new tests, which could negatively impact our performance and prospects.
We believe our future success will depend upon our ability to continue to expand our test offering and develop and sell new tests. For instance, in 2016 we launched a new chromosomal test called CNV+ that is designed to use NGS technology to detect copy number variants with similar or improved results as compared to microarray-based genomic tests. We expect these tests will target customers that are already using microarray-based testing; however, these tests may not be accepted as a replacement for microarray-based tests and they may not be adopted by these customers or at all. In addition, we expect to offer somatic testing for certain cancers in the near future. We may not be successful in launching or marketing these or any other new tests we may develop or, if we are successful, the demand for our other tests could decrease or may not continue to increase at historical rates due to sales of the new tests.
Our pipeline of new tests is in various stages of development and will be time-consuming and costly to fully develop and introduce, as development and marketing of new tests requires us to conduct research and development and further develop and scale our laboratory processes and infrastructure to be able to analyze increasing amounts of more diverse data. Further, we may be unable to discover or develop new tests for a variety of reasons, including failure of any proposed test to perform as expected, lack of validation or reference data for the test or failure to demonstrate the utility of the test. Further, any new test we are able to develop may not be launched in a timely manner, meet applicable regulatory standards, successfully compete with other technologies and available tests, avoid infringing the proprietary rights of others, achieve coverage and adequate reimbursement from third-party payors, be capable of performance at commercial levels and at reasonable costs, be successfully marketed or achieve sufficient market acceptance for us to recoup our time and capital investment in the development of the test. Any failure to successfully develop and sell new tests could negatively impact our ability to attract and retain customers and our revenue and prospects.
Actual or attempted security breaches, loss of data and other disruptions could compromise sensitive information related to our business or to patients or prevent us from accessing critical information, any of which could expose us to liability and adversely affect our business and our reputation.
In the ordinary course of our business, we and a third-party billing and collections provider that we have engaged generate, collect and store sensitive data, including PHI, personally identifiable information, intellectual property and proprietary information and other business-critical information, such as research and development data, commercial information and business and financial information. We manage and maintain the data we generate, collect and store utilizing a combination of on-site systems and managed data center systems. We also communicate sensitive patient data when we deliver reports summarizing test results to our customers, which we deliver via our online encrypted web portal, encrypted email or fax or overnight courier. We face a number of risks related to protecting this information, including loss of access, inappropriate disclosure, unauthorized modification and inability to adequately implement protective controls.
The secure processing, storage, maintenance and transmission of this critical information are vital to our operations and business strategy and we devote significant resources to protecting the confidentiality and integrity of this information. Although we have implemented security measures designed to protect sensitive information from unauthorized access, use or disclosure, our information technology and infrastructure and that of our third-party billing and collections provider could fail, be inadequate or vulnerable to attacks by hackers or viruses or be breached due to employee error, malfeasance or other disruptions. A breach or interruption could compromise our information systems and the information we store could be accessed by unauthorized parties, manipulated, publicly disclosed, lost or stolen. Any such unauthorized access, manipulation, disclosure or other loss of information could result in legal claims or proceedings and could result in liability or penalties under federal and state laws that protect the privacy of personal information, discussed below under “We are subject to broad legal requirements regarding the information we test and analyze and any failure to comply with these requirements could result in harsh penalties, damage our reputation and materially harm our business.” Additionally, unauthorized access, manipulation, loss or dissemination could significantly damage our reputation and disrupt our operations, including our ability to perform our tests, analyze and provide test results, bill customers or other payors, process claims for reimbursement, provide customer service, conduct research and development activities, collect, process, and
27
prepare company financial information, conduct education and outreach activities and manage the administrative aspects of our business, any of which could adversely affect our business.
The loss of any member of our senior management team could adversely affect our business.
Our success depends in large part upon the skills, experience and performance of our executive management team and others in key leadership positions, especially Ming Hsieh, our founder and Chief Executive Officer, and Dr. Gao, our Chief Scientific Officer and Lab Director. The continued efforts of these persons will be critical to us as we continue to develop our technologies and test processes and focus on growing our business. If we lose one or more key executives, we could experience difficulties maintaining our operations, including delivering reports to customers after review and approval by a licensed and qualified laboratory director, competing effectively, advancing our technologies, developing new tests and implementing our business strategies. All of our executives and employees, including Mr. Hsieh and Dr. Gao, are at-will, which means that either we or the executive or employee may terminate their employment at any time. We do not carry key man insurance for any of our executives or other employees. In addition, we do not have a long-term retention agreement in place with any of our executives or key employees.
We rely on highly skilled personnel in a broad array of disciplines, and if we are unable to hire, retain or motivate these individuals, we may not be able to maintain the quality of our tests or grow effectively.
Our performance, including our research and development programs and laboratory operations, largely depends upon our continued ability to identify, hire, train, motivate and retain highly skilled personnel for all areas of our organization, including biostatisticians, geneticists, software engineers, laboratory directors and specialists, sales and marketing experts and other scientific, technical and managerial personnel. Competition in our industry for qualified executives and other employees is intense and we may not be able to attract or retain the qualified personnel we need to execute our business plan due to high levels of competition for these personnel among our competitors, other life science businesses, universities and public and private research institutions. In addition, our compensation arrangements may not be successful in attracting new employees and retaining and motivating our existing employees. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that could adversely affect our ability to expand our business and support our clinical laboratory operations and our sales and marketing and research and development efforts, which would negatively affect our prospects for future growth and success.
Our inability to obtain additional capital in the future when needed and on acceptable terms may limit our ability to execute our business plans.
We expect our capital expenditures and operating expenses to increase over the next several years as we expand our infrastructure, sales and marketing and other commercial operations and research and development activities. We may seek to raise additional capital through securities offerings, credit facilities or other debt financings, asset sales or collaborations or licensing arrangements. Additional funding may not be available to us when needed, on acceptable terms or at all. If we raise funds by issuing equity securities, our existing stockholders could experience substantial dilution. Additionally, any preferred equity securities we issue could provide for rights, preferences or privileges senior to those of our common stock, and our issuance of any additional equity securities, or the possibility of such an issuance, could cause the market price of our common stock to decline. The terms of debt securities issued or borrowings, if available, could impose significant restrictions on our operations, such as limitations on our ability to incur additional debt or issue additional equity, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely affect our ability to conduct our business, and would result in increased fixed payment obligations. In the event that we seek to sell assets or enter into collaborations or licensing arrangements to raise capital, we may be required to accept unfavorable terms or relinquish or license to a third party our rights to important or valuable technologies or tests we may otherwise seek to develop ourselves. Moreover, we may incur substantial costs in pursuing future capital, including investment banking, legal and accounting fees, printing and distribution expenses and other costs. If we are not able to secure additional funding when needed and on reasonable terms, we may be forced to delay, reduce the scope of or eliminate one or more sales and marketing initiatives, research and development programs or other growth plans or strategies. In addition, we may be forced to work with a partner on one or more aspects of our tests or market development programs or initiatives, which could lower the economic value of these tests, programs or initiatives to our company. Any such outcome could significantly harm our business, performance and prospects.
We may acquire businesses or assets, form joint ventures, make investments in other companies or technologies or establish other strategic relationships that could harm our operating results, dilute our stockholders’ ownership or cause us to incur debt or significant expense.
As part of our business strategy, we may pursue acquisitions of complementary businesses or assets, investments in other companies, technology licensing arrangements, joint ventures or strategic relationships, including partnerships with pharmaceutical companies to further develop our pharmacogenomics opportunities. As an organization, we have limited experience with respect to acquisitions, investments or the formation of strategic relationships or joint ventures. If we make acquisitions in the future, we may
28
not be able to successfully integrate the acquired businesses or technologies into our existing business, we could assume unknown or contingent liabilities and we could be forced to record significant write-offs or incur debt as a result of the acquisitions, any of which could harm our operating results. Further, integration of an acquired business or technology could require management and capital resources that otherwise would be available for ongoing development of our existing business or pursuit of other opportunities. If we pursue partnerships with pharmaceutical companies, our ability to establish and maintain these partnerships could be challenging due to several factors, including competition with other genetic testing companies and internal and external constraints placed on pharmaceutical organizations that limit the number and type of relationships they can establish with companies like ours. Moreover, we may not be able to identify or complete any acquisition, investment, technology license, joint venture or strategic relationship in a timely manner, on a cost-effective basis or at all, and we may not realize the anticipated benefits of any such transaction sufficiently to recoup our costs.
To finance any acquisitions, investments, joint ventures or strategic relationships, we may seek to raise additional funds through securities offerings, credit facilities, asset sales or collaborations or licensing arrangements. Each of these methods of fundraising is subject to a variety of risks, including those discussed above under “Our inability to obtain additional capital when needed and on acceptable terms in the future may limit our ability to execute our business plan.” Further, additional funds may not be available when needed, on acceptable terms or at all. We may also seek to fund these transactions with issuances of our capital stock, even if the price of our common stock is low or volatile, which would involve risks associated with capital-raising equity offerings, including dilution to existing stockholders and the possible decline of the market price of our common stock. Any inability to fund acquisitions, investments or strategic relationships could cause us to forfeit opportunities that we believe to be promising or valuable, which could harm our prospects.
We depend on our information technology systems and any failure of these systems, due to hardware or software failures, delays in operation, failures to implement new or enhanced systems or cybersecurity breaches, could harm our business.
We depend on information technology and telecommunications systems for significant elements of our operations, such as our laboratory information management systems, including test validation, specimen tracking and quality control, our bioinformatics analytical software systems, our expansive reference library of information relating to genetic variants and their role in disease, personal information storage, maintenance and transmission, our customer-facing web-based software and customer service, our report production systems and our billing and reimbursement, research and development, scientific and medical data analysis and general administrative activities, including our disclosure controls, internal control over financial reporting and other public reporting functions. In addition, our third-party service providers depend upon technology and telecommunications systems in order to provide the contracted services for us. Now that we are a public company, we expect to expand and strengthen a number of enterprise software systems that affect a broad range of business processes and functions, including for example, systems handling human resources, financial and other disclosure controls and reporting, customer relationship management, regulatory compliance, security controls and other infrastructure operations.
Information technology and telecommunications systems are vulnerable to disruption and damage from a variety of sources, including power outages and other telecommunications or network failures, natural disasters, the outbreak of war or acts of terrorism. Moreover, despite network security and back-up measures, our servers and other electronic systems are potentially vulnerable to cybersecurity breaches, such as physical or electronic break-ins, computer viruses and similar disruptive events. Despite the precautionary measures we have taken to detect and prevent or solve problems that could affect our information technology and telecommunications systems, there may be failures or significant downtime of these systems or those used by our third-party service providers. Any such failure or downtime could prevent us from conducting tests, preparing and providing reports to customers, billing payors, handling customer inquiries, conducting research and development activities, maintaining our financial and disclosure controls and other reporting functions and managing the administrative aspects of our business. Moreover, any such failure or downtime could force us to transfer data collection operations to an alternate provider of server-hosting services, which could involve significant costs and could result in further delays in our ability to conduct tests, deliver reports to our customers and otherwise manage our operations. Any such disruption or loss of information technology or telecommunications systems on which critical aspects of our operations depend could have a material adverse effect on our business and our reputation.
Additionally, as our business grows, we will need to continually improve and expand the scope of our technology systems in order to maintain adequate systems for the scale of our operations. Any failure to make such improvements or any significant delay in the planned delivery of new systems or system enhancements could render our systems obsolete or inadequate, in which case our service to our customers and our other business activities could suffer and we could be more vulnerable to electronic breaches from outside sources.
Although we carry property and business interruption insurance, the coverage may not be adequate to compensate for all losses that may occur in the event of any system failures.
29
We rely on commercial courier delivery services to transport specimens to our laboratory facility in a timely and cost-efficient manner, and if these delivery services are disrupted, our business would be harmed.
Our business depends on our ability to quickly and reliably deliver test results to our customers. Specimens are typically received within days for analysis at our Temple City, California laboratory. Disruptions in delivery service, whether due to labor disruptions, bad weather, natural disaster, terrorist acts or threats or for other reasons could adversely affect specimen integrity and our ability to process specimens in a timely manner and otherwise service our customers, and ultimately our reputation and our business. In addition, if we are unable to continue to obtain expedited delivery services on commercially reasonable terms, our operating results may be adversely affected.
Regulatory Risks
Any changes in laws, regulations or the enforcement discretion of the FDA with respect to the marketing of diagnostic products, or violations of laws or regulations by us, could adversely affect our business, prospects, results of operations or financial condition.
The laws and regulations governing the marketing of diagnostic products are evolving, extremely complex and in many instances there are no significant regulatory or judicial interpretations of these laws and regulations. Pursuant to its authority under the FDC Act, the FDA has jurisdiction over medical devices, including our tests. Among other things, pursuant to the FDC Act and its implementing regulations, the FDA regulates the research, testing, manufacturing, safety, labeling, storage, recordkeeping, premarket clearance or approval, marketing and promotion, and sales and distribution of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. In addition, the FDA regulates the import and export of medical devices.
Although the FDA has statutory authority to assure that medical devices, including our tests, are safe and effective for their intended uses, the FDA has historically exercised its enforcement discretion and not enforced applicable provisions of the FDC Act and regulations with respect to LDTs, which are a particular type of medical device. We believe our tests are LDTs. As a result, we believe our tests are not currently subject to the FDA’s enforcement of its medical device regulations and the applicable FDC Act provisions.
Even though we commercialize our tests as LDTs, our tests may in the future become subject to more onerous regulation by the FDA. For example, the FDA may disagree with our assessment that our tests fall within the definition of an LDT and seek to regulate our tests as medical devices. Moreover, the FDA has issued certain guidance stating that it intends to modify its policy of enforcement discretion with respect to LDTs and begin to enforce its medical device requirements, including premarket submission requirements, for LDTs that have historically been marketed without FDA premarket review and oversight. Subsequently, the FDA solicited public input and published two draft guidance documents relating to FDA oversight of NGS-based tests. These two draft guidance documents describe the FDA’s thinking and proposed approach regarding the possible use of FDA-recognized standards to support analytical validity, and public human genetic variant databases to support clinical validity, of these tests. Until the FDA finalizes its guidance regarding LDTs and NGS-based tests, it is unknown how the FDA may regulate our tests in the future and what testing and data may be required to support any required clearance or approval.
If the FDA begins to enforce its medical device requirements for LDTs or if the FDA disagrees with our assessment that our tests are LDTs, we could for the first time be subject to enforcement of a variety of regulatory requirements, including registration and listing, medical device reporting and quality control, and we could be required to obtain premarket clearance or approval for our existing tests and any new tests we may develop, which may force us to cease marketing our tests until we obtain the required clearance or approval. The premarket review process can be lengthy, expensive, time-consuming and unpredictable. Further, obtaining pre-market clearance may involve, among other things, successfully completing clinical trials. Clinical trials require significant time and cash resources and are subject to a high degree of risk, including risks of experiencing delays, failing to complete the trial or obtaining unexpected or negative results. If we are required to obtain premarket clearance or approval and/or conduct premarket clinical trials, our development costs could significantly increase, our introduction of any new tests we may develop may be delayed and sales of our existing tests could be interrupted or stopped. Any of these outcomes could reduce our revenue or increase our costs and materially adversely affect our business, prospects, results of operations or financial condition. Moreover, any cleared or approved labeling claims may not be consistent with our current claims or adequate to support continued adoption of and reimbursement for our tests. For instance, if we are required by the FDA to label our tests as investigational, or if labeling claims the FDA allows us to make are limited, order levels may decline and reimbursement may be adversely affected. As a result, we could experience significantly increased development costs and a delay in generating additional revenue from our existing tests or from tests we may develop.
In addition, while we qualify all materials used in our products in accordance with CLIA regulations and guidelines, the FDA could promulgate regulations or guidance documents impacting our ability to purchase materials necessary for the performance of our tests. If any of the reagents we obtain from suppliers and use in our tests are affected by future regulatory actions, our business could
30
be adversely affected, including by increasing the cost of testing or delaying, limiting or prohibiting the purchase of reagents necessary to perform testing with our products.
Failure to comply with any applicable FDA requirements could trigger a range of enforcement actions by the FDA, including warning letters, civil monetary penalties, injunctions, criminal prosecution, recall or seizure, operating restrictions, partial suspension or total shutdown of operations and denial of or challenges to applications for clearance or approval, as well as significant adverse publicity.
If we fail to comply with applicable federal, state, local and foreign laboratory licensing requirements, we could lose the ability to perform our tests or experience disruptions to our business.
We are subject to CLIA, a federal law that establishes quality standards for all laboratory testing and is intended to ensure the accuracy, reliability and timeliness of patient results. CLIA requires that we hold a certificate specific to the laboratory examinations we perform and that we comply with various standards with respect to personnel qualifications, facility administration, proficiency testing, quality control, quality assurance and inspections. CLIA certification is required in order for us to be eligible to bill federal and state healthcare programs, as well as many private third-party payors, for our tests. We have obtained CLIA certification to conduct our tests at our laboratory in Temple City, California. To renew this certification, we are subject to survey and inspection every two years and we may be subject to additional unannounced inspections.
We are also required to maintain a license to conduct testing in the State of California. California laws establish standards for day-to-day operation of our clinical reference laboratory in Temple City, including with respect to the training and skills required of personnel, quality control and proficiency testing requirements. In addition, certain other states require us to maintain out-of-state laboratory licenses or obtain approval on a test-specific basis to perform testing on specimens from these states. Additional states could adopt similar licensure requirements in the future, which could require us to modify, delay or discontinue our operations in such jurisdictions. We are also subject to regulation in foreign jurisdictions, which we expect will increase as we seek to expand international utilization of our tests or if jurisdictions in which we pursue operations adopt new or modified licensure requirements. Foreign licensure requirements could require review and modification of our tests in order to offer them in certain jurisdictions or could impose other limitations, such as restrictions on the transport of human blood or other tissue necessary for us to perform our tests that may limit our ability to make our tests available outside of the United States. Additionally, complying with licensure requirements in new jurisdictions may be expensive, time-consuming and subject us to significant and unanticipated delays.
Failure to comply with applicable clinical laboratory licensure requirements could result in a range of enforcement actions, including license suspension, limitation or revocation, directed plan of correction, onsite monitoring, civil monetary penalties, civil injunctive suits, criminal sanctions and exclusion from the Medicare and Medicaid programs, as well as significant adverse publicity. Any sanction imposed under CLIA, its implementing regulations or state or foreign laws or regulations governing clinical laboratory licensure, or our failure to renew our CLIA certificate or any other required local, state or foreign license or accreditation, could have a material adverse effect on our business, financial condition and results of operations. In such case, even if we were able to bring our laboratory back into compliance, we could incur significant expenses and lose revenue while doing so.
In addition to CLIA requirements, we elect to participate in CAP accreditation. CMS has deemed CAP standards to be equally or more stringent than CLIA regulations and has approved CAP as a recognized accrediting organization. Inspection by CAP is performed in lieu of inspection by CMS for CAP-accredited laboratories. Because we are accredited by the CAP Laboratory Accreditation Program, we are deemed to also comply with CLIA. While not required to operate a CLIA-certified laboratory, many private insurers require CAP accreditation as a condition to contracting with clinical laboratories to cover their tests. In addition, some countries outside the United States require CAP accreditation as a condition to permitting clinical laboratories to test samples taken from their citizens. Failure to maintain CAP accreditation could have a material adverse effect on the sales of our tests and the results of our operations.
We are subject to broad legal requirements regarding the information we test and analyze and any failure to comply with these requirements could result in harsh penalties, damage our reputation and materially harm our business.
Our business is subject to federal and state laws that protect the privacy and security of personal health information, including HIPAA, HITECH and similar state laws.
Numerous other federal, state and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of patient health information. In addition, new laws and regulations that further protect the privacy and security of medical records or medical information are regularly considered by federal and state governments. Further, with the recent increase in publicity regarding data breaches resulting in improper dissemination of consumer information, federal and state governments have passed or are considering laws regulating the actions that a business must take if it experiences a data breach, such as prompt disclosure to affected customers. The Federal Trade Commission and states’ Attorneys General have also brought enforcement actions and prosecuted some data breach cases as unfair and/or deceptive acts or practices
31
under the Federal Trade Commission Act. In addition to data breach notification laws, some states have enacted statutes and rules requiring businesses to reasonably protect certain types of personal information they hold or to otherwise comply with certain specified data security requirements for personal information. We intend to continue to comprehensively protect all personal information and to comply with all applicable laws regarding the protection of such information.
Any failure to implement appropriate security measures to protect the confidentiality and integrity of personal information or any breach or other failure of these systems resulting in the unauthorized access, manipulation, disclosure or loss of this information could result in our noncompliance with these laws. Penalties for failure to comply with a requirement of HIPAA and HITECH vary significantly depending on the failure and could include civil monetary or criminal penalties.
In addition, the interpretation, application and interplay of consumer and health-related data protection laws in the United States, Europe and elsewhere are often uncertain, contradictory and in flux. As a result, it is possible that laws may be interpreted and applied in a manner that is inconsistent with our current practices. Moreover, these laws and their interpretations are constantly evolving and they may become more stringent over time. Complying with these laws or any new laws or interpretations of their application could involve significant time and substantial costs or require us to change our business practices and compliance procedures in a manner adverse to our business. We may not be able to obtain or maintain compliance with the diverse privacy and security requirements in all of the jurisdictions in which we currently or plan to do business, and failure to comply with any of these requirements could result in civil or criminal penalties, harm our reputation and materially adversely affect our business.
We conduct business in a heavily regulated industry. Complying with the numerous statutes and regulations pertaining to our business is expensive and time-consuming, and any failure by us, our consultants or commercial partners to comply could result in substantial penalties.
Our industry is heavily regulated, and the regulatory environment in which we operate could change significantly and adversely in the future. Our operations are subject to extensive federal, state, local and foreign laws and regulations, all of which are subject to change. These laws and regulations currently include, among others:
|
• |
the FDA’s enforcement discretion with respect to LDTs; |
|
• |
CLIA’s and CAP’s regulation of our laboratory activities; |
|
• |
federal and state laws and standards affecting reimbursement by government payors, including certain coding requirements to obtain reimbursement and certain changes to the payment mechanism for clinical laboratory services resulting from PAMA; |
|
• |
HIPAA and HITECH, which establish comprehensive federal standards with respect to the privacy and security of PHI, and requirements for the use of certain standardized electronic transactions with respect to transmission of such information; |
|
• |
state laws governing the maintenance of personally identifiable information of state residents, including medical information, and which impose varying breach notification requirements, some of which allow private rights of action by individuals for violations and also impose penalties for such violations; |
|
• |
the federal Anti-Kickback Statute, which generally prohibits knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, in return for or to induce a person to refer to an individual any good, facility, item or service that is reimbursable under a federal healthcare program; |
|
• |
the federal Stark Law, which generally prohibits a physician from making a referral for certain designated health services covered by the Medicare program, including laboratory and pathology services, if the physician or an immediate family member has a financial relationship with the entity providing the designated health services; |
|
• |
the federal false claims laws, which generally impose liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government; |
|
• |
the federal Civil Monetary Penalties Law, which generally prohibits, among other things, the offering or transfer of remuneration to a Medicare or state healthcare program beneficiary if it is likely to influence the beneficiary’s selection of a particular provider, practitioner or supplier of services reimbursable by Medicare or a state healthcare program; |
|
• |
the Affordable Care Act, which, among other things, establishes a requirement for providers and suppliers to report and return any overpayments received from government payors under the Medicare and Medicaid programs; |
|
• |
other federal and state fraud and abuse laws, such as anti-kickback laws, prohibitions on self-referral, fee-splitting restrictions, insurance fraud laws, anti-markup laws, prohibitions on the provision of tests at no or discounted cost to induce physician or patient adoption and false claims acts, some of which may extend to services reimbursable by any third-party payor, including private insurers; |
32
|
• |
the federal Physician Sunshine Payment Act and various state laws on reporting relationships with healthcare providers and customers, which could be determined to apply to our LDTs; |
|
• |
the prohibition on reassignment of Medicare claims; |
|
• |
state laws that prohibit other specified practices, such as billing physicians for tests that they order, waiving coinsurance, copayments, deductibles and other amounts owed by patients, business corporations practicing medicine or employing or engaging physicians to practice medicine and billing a state Medicaid program at a price that is higher than what is charged to one or more other payors; |
|
• |
the FCPA and other applicable anti-bribery laws; |
|
• |
federal, state and local regulations relating to the handling and disposal of regulated medical waste, hazardous waste and biohazardous waste and workplace safety for healthcare employees; and |
|
• |
similar foreign laws and regulations that apply to us in the countries in which we operate or may operate in the future. |
The growth of our business generally and our intent to grow our international business, as well as our use of consultants and commercial partners may increase the potential of violating these laws. Our risk of violating these or other laws and regulations is further increased because of the lack of their complete interpretation by applicable regulatory authorities or the courts, and their provisions are thus open to a variety of interpretations.
We have adopted policies and procedures designed to comply with these laws and regulations and, in the ordinary course of our business, we conduct internal reviews of our compliance with these laws. Our compliance is also subject to review by applicable government agencies. It is not always possible to identify and deter misconduct by employees, distributors, consultants and commercial partners, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from government investigations or other actions or lawsuits stemming from a failure to comply with applicable laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and harm our reputation. If our operations, including the conduct of our employees, distributors, consultants and commercial partners, are found to be in violation of any of these laws and regulations, we may be subject to applicable penalties associated with the violation, including administrative, civil and criminal penalties, damages, fines, individual imprisonment, exclusion from participation in federal healthcare programs, refunding of payments received by us and curtailment or cessation of our operations. Any of these consequences could seriously harm our business and our financial results.
Healthcare policy changes, including recently enacted legislation reforming the U.S. healthcare system, could cause significant harm to our business, operations and financial condition.
The Affordable Care Act made a number of substantial changes to the way healthcare is financed both by governmental and private insurers. For example, the Affordable Care Act requires each medical device manufacturer to pay a sales tax on the medical devices it sells. It is unclear at this time when, or if, the provision of our LDTs will trigger the medical device tax if the FDA ends its policy of general enforcement discretion and regulates certain LDTs as medical devices, and it is possible that this tax will apply to some or all of our existing tests or tests we may develop in the future. Additionally, the Affordable Care Act introduces mechanisms to reduce the per capita rate of growth in Medicare spending if expenditures exceed certain targets. Any such reductions could affect reimbursement payments for our tests. The Affordable Care Act also contains a number of other provisions, including provisions governing enrollment in federal and state healthcare programs, reimbursement matters and fraud and abuse, which we expect will impact our industry and our operations in ways that we cannot currently predict.
In April 2014, Congress passed PAMA, which included substantial changes to the way in which clinical laboratory services will be paid under Medicare. Under PAMA, certain clinical laboratories are required to periodically report to CMS private payor payment rates and volumes for their tests. Laboratories that fail to report the required payment information may be subject to substantial civil monetary penalties. As required under PAMA, CMS will use the rates and volumes reported by laboratories to develop Medicare payment rates for laboratory tests equal to the volume-weighted median of the private payor payment rates for the tests. The impact of this new payment system on rates for our tests, including any current or future tests we may develop, is uncertain.
We cannot predict whether or when these or other recently enacted healthcare initiatives will be implemented at the federal or state level or how any such legislation or regulation may affect us. For instance, the payment reductions imposed by the Affordable Care Act and the changes to reimbursement amounts paid by Medicare for tests such as ours based on the procedure set forth in PAMA, could limit the prices we will be able to charge or the amount of available reimbursement for our tests, which would reduce our revenue. Additionally, these healthcare policy changes could be amended or additional healthcare initiatives could be implemented in the future. For instance, there is uncertainty regarding the continued effect or the Affordable Care Act in its current form following
33
the results of the 2016 U.S. presidential election and in light of the policies of the current administration, which has threatened to repeal the Affordable Care Act. Further, the impact on our business of the expansion of the federal and state governments’ role in the U.S. healthcare industry generally including the social, governmental and other pressures to reduce healthcare costs while expanding individual benefits, is uncertain. Any future changes or initiatives could have a materially adverse effect on our business, financial condition, results of operations and cash flows.
If we use hazardous materials in a manner that causes contamination or injury, we could be liable for resulting damages.
Our activities require the use of regulated medical waste, hazardous waste and biohazardous waste, including chemicals, biological agents and compounds, blood and other tissue specimens. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have secured. Additionally, we are subject on an ongoing basis to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we typically use outside vendors to dispose of such waste that are licensed or otherwise qualified to handle and dispose of the waste, applicable laws and regulations may hold us liable for damages and fines as a result of others’ actions should contamination of the environment or individual exposure to hazardous substances occur. The cost of compliance with these laws and regulations could become significant and our failure to comply could result in substantial fines or other consequences, any of which could negatively affect our operating results and significantly harm our reputation.
We could be adversely affected by violations of the FCPA and other anti-bribery laws.
Our international operations are subject to various anti-bribery laws, including the FCPA and similar anti-bribery laws in the non-U.S. jurisdictions in which we operate. The FCPA prohibits companies and their intermediaries from offering, making, or authorizing improper payments to non-U.S. or foreign officials for the purpose of obtaining or retaining business or securing any other improper advantage. These laws are complex and far-reaching in nature, and we may be required in the future to alter one or more of our practices to be in compliance with these laws or any changes to these laws or their interpretation.
We currently engage in significant business outside of the United States, and we plan to increase our international operations in the future. These operations could involve dealings with governments, foreign officials and state-owned entities, such as government hospitals, outside of the United States. In addition, we may engage distributors, partners or third-party intermediaries, such as representatives, contractors, and agents, to assist with promotion and sale of our tests abroad and to obtain necessary permits, licenses, and other regulatory approvals. Any such third parties could be deemed to be our agents and we could be held responsible for any corrupt or other illegal activities of our employees or these third parties, even if we do not explicitly authorize or have actual knowledge of such activities. We have instituted policies, procedures, and internal controls reasonably designed to promote compliance with the FCPA and other anti-corruption laws and we exercise a high degree of vigilance in maintaining, implementing and enforcing these policies and controls. However, these policies and controls could be circumvented or ignored and they cannot guarantee compliance with these laws and regulations. Any violations of these laws or allegations of such violations could disrupt our operations, involve significant management distraction, involve significant costs and expenses, including legal fees, and harm our reputation. Additionally, other U.S. companies in the medical device and pharmaceutical fields have faced substantial fines and criminal penalties in the recent past for violating the FCPA, and we could also incur these types of penalties, including criminal and civil penalties, disgorgement, and other remedial measures, if we violate the FCPA or other applicable anti-bribery laws. Any of these outcomes could result in a material adverse effect on our business, prospects, financial condition, or results of operations.
Our services present the potential for embezzlement, identity theft or other similar illegal behavior by our employees, consultants or commercial partners.
Our operations involve the use and disclosure of personal and business information that could be used to impersonate third parties or otherwise gain access to their data or funds. If any of our employees, consultants or commercial partners takes, converts or misuses such funds, documents or data, we could be liable for damages, and our business reputation could be damaged or destroyed.
Intellectual Property Risks
We currently own no patent applications related to our technology platform and rely upon trade secret protection, non-disclosure agreements and invention assignment agreements to protect our proprietary information, which may not be effective to protect our proprietary technologies and other information.
We currently rely upon trade secret protection, non-disclosure agreements and invention assignment agreements with our employees, consultants and third-parties to protect our confidential and proprietary information. Although our competitors have utilized and are expected to continue to utilize similar methods and have aggregated and are expected to continue to aggregate similar
34
libraries of genetic testing information, our success will depend upon our ability to develop proprietary methods and libraries and to defend any advantages afforded to us by such methods and libraries relative to our competitors. If we do not protect our intellectual property adequately, competitors may be able to use our proprietary technologies and information and thereby erode any competitive advantages they provide us.
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies are effectively maintained as trade secrets. We expect to rely primarily upon trade secret and proprietary know-how protection for our confidential and proprietary information and we have taken security measures to protect this information. These measures, however, may not provide adequate protection for our trade secrets, know-how or other confidential information. Among other things, we seek to protect our trade secrets and other confidential information by entering into confidentiality agreements with employees, consultants and other third parties. These confidentiality agreements may not sufficiently safeguard our trade secrets and confidential information and may not provide adequate remedies in the event of unauthorized use or disclosure of such information. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive and time-consuming and the outcome could be unpredictable. In addition, trade secrets or other confidential information could otherwise become known or be independently developed by others in a manner that could prevent legal recourse by us. If any of our trade secrets or other confidential or proprietary information were to be disclosed or misappropriated or if any such information was independently developed by a competitor, our competitive position could be harmed and our business could suffer.
Litigation or other proceedings or third-party claims of intellectual property infringement or misappropriation could require us to spend significant time and money and could prevent us from selling our tests.
Our commercial success will depend in part upon our ability to avoid infringement of patents and other proprietary rights owned by third parties, including the intellectual property rights of competitors. There are numerous U.S. and foreign patents and pending patent applications and other intellectual property rights that cover technologies relevant to genetic testing and that are owned by third parties. We may be unaware of patents or other intellectual property rights that a third-party might assert are infringed by our business and there may be patent applications that, if issued, could be asserted against us. As a result, our existing or future operations may be found or alleged to infringe existing or future patents or other intellectual property rights of others. As we continue to sell our existing tests, launch new tests and enter new markets, competitors may claim that our tests infringe or misappropriate their intellectual property rights as part of strategies designed to impede our successful entry into new markets.
If a patent infringement or misappropriation of intellectual property suit were brought against us, we could be forced to discontinue or delay our development or sales of any tests or other activities that are the subject of the suit while it is pending. Additionally, defense of these claims, regardless of merit, could cause us to incur substantial expenses, be a substantial diversion of our management and other employee resources and significantly harm our reputation. In the event of a successful claim of infringement against us, we may be forced to pay substantial damages, including treble damages and attorneys’ fees if we are found to have willfully infringed patents, obtain one or more licenses, which may not be available when needed, on commercially reasonable terms or at all, pay royalties, which may be substantial, or redesign any infringing tests or other activities, which may be impossible or require substantial time and monetary expenditure. Further, third parties making claims against us for infringement or misappropriation of their patents or other intellectual property rights could seek and obtain injunctive or other equitable relief, which, if granted, could prohibit us from performing our tests. Any of these outcomes could delay our introduction of new tests, significantly increase our costs or prevent us from conducting certain of our essential activities, which could materially adversely affect our ability to operate and grow our business.
Developments in patent law could have a negative impact on our business.
From time to time, the U.S. Supreme Court, other federal courts, the U.S. Congress or the U.S. Patent and Trademark Office, or USPTO, may change the standards of patentability, and any such changes could have a negative impact on our business.
Three cases involving diagnostic method claims and “gene patents” have recently been decided by the Supreme Court. In March 2012, the Supreme Court issued a decision in Mayo Collaborative v. Prometheus Laboratories, or Prometheus, a case involving patent claims directed to optimizing the amount of drug administered to a specific patient, holding that the applicable patents’ claims failed to incorporate sufficient inventive content above and beyond mere underlying natural correlations to allow the claimed processes to qualify as patent-eligible processes that apply natural laws. In June 2013, the Supreme Court decided Association for Molecular Pathology v. Myriad Genetics, or Myriad, a case challenging the validity of patent claims relating to the breast cancer susceptibility genes BRCA1 and BRCA2, holding that isolated genomic DNA that exists in nature, such as the DNA constituting the BRCA1 and BRCA2 genes, is not patentable subject matter, but that cDNA, which is an artificial construct created from RNA transcripts of genes, may be patent eligible. In June 2014, the Supreme Court decided Alice Corporation Pty. Ltd. v. CLS Bank International, or Alice, which affirmed the Prometheus and Myriad decisions and provided additional interpretation.
35
If we make efforts to seek patent protection for our technology and tests, these efforts may be negatively impacted by the Prometheus, Myriad and Alice decisions, rulings in other cases or guidance or procedures issued by the USPTO. However, we cannot fully predict the impact of the Prometheus, Myriad and Alice decisions on the ability of genetic testing, biopharmaceutical or other companies to obtain or enforce patents relating to DNA, genes or genomic-related discoveries in the future, as the contours of when claims reciting laws of nature, natural phenomena or abstract ideas may meet patent eligibility requirements are not clear and may take years to develop via interpretation at the USPTO and in the courts. There are many previously issued patents claiming nucleic acids and diagnostic methods based on natural correlations that issued before these recent Supreme Court decisions and, although many of these patents may be invalid under the standards set forth in these decisions, these patents are presumed valid and enforceable until they are successfully challenged, and third parties holding these patents could allege that we infringe or request that we obtain a license under these patents. Whether based on patents issued prior to or after these Supreme Court decisions, we could be forced to defend against claims of patent infringement or obtain license rights, if available, under these patents. In particular, although the Supreme Court has held in Myriad that isolated genomic DNA is not patent-eligible subject matter, third parties could allege that our activities infringe other classes of gene-related patent claims. There are numerous risks associated with any patent infringement claim against us, which are discussed above under “—Litigation or other proceedings or third-party claims of intellectual property infringement or misappropriation could require us to spend significant time and money and could prevent us from selling our tests.”
In addition, the Leahy-Smith America Invents Act, or America Invents Act, which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged and changes to the way patent applications are disputed during the examination process. These changes may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. The USPTO has developed new regulations and procedures to govern the full implementation of the America Invents Act, but the impact of the America Invents Act on the cost of prosecuting any patent applications we may file, our ability to obtain patents based on our discoveries if we pursue them and our ability to enforce or defend any patents that may issue remains unclear.
These and other substantive changes to U.S. patent law could affect our susceptibility to patent infringement claims and our ability to obtain any patents we may pursue and, if obtained, to enforce or defend them, any of which could have a material adverse effect on our business.
We may not be able to enforce our intellectual property rights outside the United States.
The laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the United States and many companies have encountered significant challenges in establishing and enforcing their proprietary rights outside the United States. These challenges can be caused by the absence of rules and methods for the establishment and enforcement of intellectual property rights in certain jurisdictions. In addition, the legal systems of some countries, particularly developing countries, do not favor the enforcement of certain intellectual property protection, especially relating to healthcare. These aspects of many foreign legal systems could make it difficult for us to stop the misappropriation of our intellectual property rights. Moreover, changes in the law and legal decisions by courts in foreign countries could affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property rights. As a result, our efforts to protect and enforce our intellectual property rights in foreign countries may ultimately prove to be inadequate, in which case our ability to grow our business and our revenue and prospects could be materially harmed.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We employ individuals who were previously employed at universities, biometric solution or genetic testing, diagnostic or other healthcare companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees or consultants have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third-party. Further, we may become subject to ownership disputes in the future arising from, for example, conflicting obligations of consultants or others who are involved in developing our technology and other parties’ intellectual property. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, we could be subject to monetary damages and the loss of valuable intellectual property rights or personnel. Even if we are successful in defending against any such claims, litigation could result in substantial costs, distract management and other employees and damage our reputation.
36
We will incur increased costs and demands as a result of compliance with laws and regulations applicable to public companies.
As a public company, we will experience significant additional demands that we did not experience as a private company. For example, the Sarbanes-Oxley Act of 2002, as amended, or Sarbanes-Oxley Act, and related and other rules implemented by the SEC and The NASDAQ Stock Market LLC, or NASDAQ, impose a number of requirements on public companies, including with respect to corporate governance practices. For instance, as a result of becoming a public company, a majority of our directors are required to be independent and we are required to maintain audit and compensation committees comprised solely of independent directors, maintain a variety of corporate governance policies, adopt and maintain policies regarding internal controls and disclosure controls and procedures and prepare reports on internal controls over financial reporting. Until completion of the Reorganization, we operated without a board of directors under the direction of the Manager of Fulgent LLC, Mr. Hsieh. Further, the SEC and other regulators have continued to adopt new rules and regulations and make additional changes to existing regulations that require our compliance, including pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act, or Dodd-Frank Act, which was enacted in July 2010. There are significant corporate governance and executive compensation-related disclosure provisions in the Dodd-Frank Act that require the SEC to adopt additional rules and regulations in these areas.
Moreover, the rules and regulations applicable to public companies will substantially increase our legal, accounting and financial compliance costs. For instance, we will need to hire additional personnel for, and devote more resources to, our financial reporting function. Additionally, if we continue to grow as anticipated, we will need to implement new and more sophisticated financial and accounting systems and adopt additional procedures for financial reporting in order to meet our obligations as a public company. Any transition of accounting systems can be expensive and can result in delays in our ability to process and report transactions in a timely manner. Our management and other personnel will need to devote a substantial amount of attention to maintaining our compliance with these obligations, which could be time-consuming and expensive. If these requirements divert the attention of our management and personnel from other aspects of our business or if they require substantial costs that we cannot afford, they could have a material adverse effect on our business, financial condition and results of operations. We also expect that, as a public company, it will be more expensive for us to attract and compensate qualified directors and officers and obtain adequate director and officer liability insurance.
If we are unable to maintain effective internal control over financial reporting, investors could lose confidence in the accuracy and completeness of our reported financial information and the market price of our common stock could decline.
As a public company, we are required to maintain internal control over financial reporting and to report any material weaknesses in such internal controls. Section 404 of the Sarbanes-Oxley Act requires that we evaluate and determine the effectiveness of our internal control over financial reporting and, beginning with our annual report for the year ended December 31, 2017, provide a management report on our internal control over financial reporting. If we have a material weakness in our internal control over financial reporting, we may not detect errors on a timely basis and our financial statements may be materially misstated. We have only started to implement the systems and processes necessary to perform the evaluation needed to comply with Section 404 of the Sarbanes-Oxley Act. We will need to maintain and enhance these systems, processes and controls as we grow and we may need to hire additional personnel and devote more resources to our financial reporting function in order to do so.
During the process of evaluating our internal controls, if we identify one or more material weaknesses, our management will be unable to conclude that our internal control over financial reporting is effective. Moreover, when we are no longer an emerging growth company, our independent registered public accounting firm will be required to issue an attestation report on the effectiveness of our internal control over financial reporting. Even if our management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm may conclude that there are material weaknesses with respect to our internal controls or the level at which our internal controls are documented, designed, implemented or reviewed.
If we are unable to conclude that our internal control over financial reporting is effective or, when we are no longer an emerging growth company, our auditors were to express an adverse opinion on the effectiveness of our internal control over financial reporting because one or more material weaknesses had been identified or if internal control deficiencies result in the restatement of our financial results, investors could lose confidence in the accuracy and completeness of our financial disclosures and the price of our common stock could decline.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
As a result of becoming a public company, we are now subject to the periodic reporting and other requirements of the Exchange Act. As a result, we have implemented disclosure controls and procedures designed to provide reasonable assurance that information we must disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. However, any disclosure
37
controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple errors or mistakes. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. As a result, because of these inherent limitations in our control system, misstatements or omissions due to error or fraud may occur and may not be detected, which could result in failures to file required reports in a timely manner and filing reports containing incorrect information. Any of these outcomes could result in SEC enforcement actions, monetary fines or other penalties, damage to our reputation and harm to our financial condition.
We are an emerging growth company and may elect to comply with reduced public company reporting requirements available to emerging growth companies, which could make our common stock less attractive to investors.
We are an emerging growth company, as defined in the JOBS Act. We will remain an emerging growth company until December 31, 2021, unless our gross revenue exceeds $1.0 billion in any fiscal year before that date, we issue more than $1.0 billion of non-convertible debt in any three-year period before that date or the market value of our common stock held by non-affiliates exceeds $700 million as of the last business day of the second fiscal quarter of any fiscal year before that date. As an emerging growth company, we are eligible for certain exemptions from various reporting requirements applicable to certain other public companies, including exemption from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced financial statement and other financial disclosures in registration statements we file, reduced disclosure obligations regarding executive compensation and exemption from the requirements of holding a nonbinding advisory vote on executive compensation and obtaining stockholder approval of any golden parachute payments not previously approved. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and having reduced disclosure obligations regarding executive compensation. We have relied on many of these exemptions to date and investors may find our common stock less attractive if we choose to continue to rely on any of these exemptions, in which case there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the Securities Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption and, as a result, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Common Stock Risks
An active, liquid trading market for our common stock may never develop, which could make it difficult for you to sell your shares of our common stock.
Prior to the completion of our initial public offering on October 4, 2016, no public market for shares of our common stock existed. An active trading market for our shares may never develop or, if developed, may not be sustained. Further, Mr. Hsieh, our founder and Chief Executive Officer, beneficially owns approximately 43.9% of our outstanding voting equity as of the completion of our initial public offering. As a result, fewer shares are actively traded in the public market, which reduces the liquidity of the market for our common stock. The lack of an active trading market could impair your ability to sell your shares at the time you wish to sell them or at a price you consider reasonable. Further, an inactive trading market may impair our ability to raise capital in the future by selling shares of our common stock and may impair our ability to enter into strategic relationships or acquire companies or technologies using shares of our common stock as consideration.
Our common stock is listed on the NASDAQ Global Market under the symbol “FLGT.” If we fail to satisfy the continued listing standards of NASDAQ, however, we could be de-listed, which would negatively impact the price of our common stock.
The price of our common stock may be volatile and you could lose all or part of your investment.
The trading price of our common stock has experienced, and may continue to experience, wide fluctuations and significant volatility. This volatility may be exacerbated by the relatively small and illiquid market for our shares since the completion of our initial public offering on October 4, 2016. Other factors that may contribute to this volatility may include, among others:
|
• |
actual or anticipated fluctuations in our operating results; |
|
• |
competition from existing tests or new tests that may emerge; |
38
|
• |
announcements by us or our competitors of significant acquisitions, investments, strategic relationships, joint ventures, collaborations or capital commitments; |
|
• |
failure to meet or exceed financial estimates and projections of the investment community or guidance that we provide to the public; |
|
• |
issuance of new or updated research or reports by securities analysts or changed recommendations for our common stock; |
|
• |
the timing and amount of our investments in the growth of our business; |
|
• |
disputes or other developments with respect to our or others’ intellectual property rights; |
|
• |
actual or anticipated changes in laws or regulations applicable to our business or our tests; |
|
• |
additions or departures of key management or other personnel; |
|
• |
changes in coverage and reimbursement by current or potential payors; |
|
• |
inability to obtain additional funding, as and when needed; |
|
• |
product liability claims or other litigation; |
|
• |
sales of our common stock by us or our stockholders; |
|
• |
general economic, political, industry and market conditions, including factors not directly related to our operating performance or the operating performance of our competitors, such as increased uncertainty in the U.S. healthcare regulatory environment following the results of the 2016 U.S. presidential election; and |
|
• |
the other risk factors discussed in this report. |
In addition, the stock market in general, and the market for stock of companies in the life sciences and technology industries in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of specific companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. In addition, in the past, following periods of volatility in the overall market and the market price of a particular company’s securities, securities class action litigation has often been instituted against the company. This type of litigation, if instituted against us, could result in substantial costs and a diversion of our management’s attention and resources.
Our principal stockholders and management own a significant percentage of our capital stock and are able to exert significant control over matters subject to stockholder approval.
As of March 1, 2017, our executive officers, directors, holders of 5% or more of our outstanding voting equity and their respective affiliates beneficially owned approximately 70.2% of our outstanding voting equity and Mr. Hsieh, our founder and Chief Executive Officer, beneficially owns approximately 43.9% of our outstanding voting equity. As a result, these stockholders have the ability to control matters submitted to our stockholders for approval, including elections of directors, amendments to our organizational documents or approval of any merger, sale of assets or other major corporate transaction. This concentration of ownership may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders, as the interests of these stockholders may not coincide with your interests or the interests of other stockholders and they may act in a manner that advances their best interests and not necessarily those of all stockholders. Further, this concentration of ownership could adversely affect the prevailing market price for our common stock.
Sales of a substantial number of shares of our common stock in the public market, or the perception that such sales could occur, could cause the price of our common stock to fall.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that such sales are pending or could occur, could reduce the market price of our common stock. As of March 16, 2017, we had 17,676,256 outstanding shares of common stock. Of these shares, approximately 3,830,000 shares of our common stock are freely tradable without restriction in the public market. The remaining outstanding shares of our common stock are restricted from sale until March 27, 2017 pursuant to the terms of certain lock-up agreements entered into in connection with our initial public offering and/or are held by our “affiliates,” as that term is defined in the Securities Act, and constitute restricted securities under the Securities Act. Generally, restricted securities may not be sold in the public market unless the sale is registered under the Securities Act or an exemption from registration is available.
Moreover, Xi Long, which holds an aggregate of 2,025,623 shares of our common stock, has the right, subject to certain conditions, to include its shares in registration statements we may file for ourselves or other stockholders and to require us to file registration statements covering its shares following May 16, 2019. We have also registered the shares of our common stock that we
39
may issue under our 2016 Omnibus Incentive Plan, or the 2016 Plan, totaling 591,112 shares subject to options outstanding as of December 31, 2016 and 1,482,727 additional shares reserved for issuance under the 2016 Plan. As a result, these shares will be freely tradable in the public market upon issuance, subject to volume and manner of sale limitations applicable to affiliates and any other legal and contractual limitations.
Future issuances of our common stock or rights to purchase our common stock, including pursuant to our equity incentive plans, could result in additional dilution to the percentage ownership of our stockholders and could cause the price of our common stock to fall.
To raise capital in the future, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities, our then-existing stockholders could be materially diluted by such issuances and new investors could gain rights, preferences and privileges senior to the holders of our common stock, which could cause the price of our common stock to decline.
We do not intend to pay dividends on our common stock, so any returns will be limited to the value of our common stock.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business. As a result, we do not anticipate declaring or paying any cash dividends or other distributions for the foreseeable future. Further, if we were to enter into a credit facility or issue debt securities or preferred equity securities in the future, we may be contractually restricted from paying dividends. If we do not pay dividends, our common stock may be less valuable because stockholders must rely on sales of their common stock after price appreciation, which may never occur, to realize any future gains on their investment.
If securities or industry analysts do not publish research or reports about our business or if they issue an adverse or misleading opinion regarding our common stock, our stock price and trading volume could decline.
If a trading market for our common stock develops, that trading market will be influenced to some extent by the research and reports that industry or securities analysts publish about us or our business. We have only recently obtained research coverage by securities and industry analysts. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, we could lose visibility in the financial markets, which could cause the price and trading volume of our common stock to decline. Further, if any of these analysts issues an adverse or misleading opinion regarding us, our business model, our industry or our stock performance or if our operating results fail to meet analyst expectations, the price of our common stock could significantly decline.
Provisions in our charter documents and Delaware law could discourage, delay or prevent a change in control of our company or changes in our management and depress the market price of our common stock.
Our certificate of incorporation and bylaws contain provisions that could depress the market price of our common stock by acting to discourage, delay or prevent a change in control of our company or changes in our management that the stockholders of our company may deem advantageous. These provisions, among other things:
|
• |
authorize our board of directors to issue, without further action by our stockholders, up to 1,000,000 shares of undesignated or “blank check” preferred stock; |
|
• |
prohibit stockholder action by written consent, thus requiring all stockholder actions to be taken at a duly noticed and held meeting of our stockholders; |
|
• |
specify that special meetings of our stockholders can be called only by our board of directors, the Chairman of our board of directors or our President, thereby eliminating the ability of our stockholders to call special meetings; |
|
• |
permit only the board of directors to establish the number of directors and fill vacancies on the board of directors, except as may be required by law; |
|
• |
permit the board of directors to amend our bylaws, subject to the power of our stockholders to repeal any such amendment; |
|
• |
do not permit cumulative voting on the election of directors; and |
|
• |
establish advance notice requirements for stockholders to propose nominees for election as directors or matters to be acted upon at annual meetings of stockholders. |
In addition, we are subject to Section 203 of the Delaware General Corporation Law, or DGCL, which may discourage, delay or prevent a change in control of our company. Section 203 imposes certain restrictions on mergers, business combinations and other transactions between us and holders of 15% or more of our common stock.
40
Holders of our common stock could be adversely affected if we issue preferred stock.
Pursuant to our certificate of incorporation, our board of directors is authorized to issue up to 1,000,000 shares of preferred stock without any action on the part of our stockholders. Our board of directors will also have the power, without stockholder approval, to set the terms of any series of preferred stock that may be issued, including voting rights, dividend rights, preferences over our common stock with respect to dividends or in the event of a dissolution, liquidation or winding up and other terms. In the event that we issue preferred stock in the future that has preferences over our common stock with respect to payment of dividends or upon our liquidation, dissolution or winding up, or if we issue preferred stock that is convertible into our common stock at greater than a one-to-one ratio, the voting and other rights of the holders of our common stock or the market price of our common stock could be adversely affected.
Our certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees.
Our certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for:
|
• |
any derivative action or proceeding brought on our behalf; |
|
• |
any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or to our stockholders; |
|
• |
any action asserting a claim against us arising pursuant to any provision of the DGCL, our certificate of incorporation or our bylaws; or |
|
• |
any action asserting a claim against us governed by the internal affairs doctrine. |
Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and consented to this provision of our certificate of incorporation. This choice-of-forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. Alternatively, if a court were to find these provisions of our certificate of incorporation inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business, financial condition or results of operations.
Item 1B. Unresolved Staff Comments.
None.
Our corporate headquarters and laboratory operations are located in Temple City, California, where we lease and occupy approximately 12,000 square feet of office and laboratory space under leases that will expire in March, April and July 2018. The Company has options to renew for two or three years. We use these facilities for all of our laboratory testing and management activities and certain research and development, administrative and other functions. We also lease approximately 650 square feet of office space near Atlanta, Georgia under a lease that will expire in August 2017, where we conduct certain research and development, customer service, report generation and other administrative functions, although no laboratory activities occur at this facility. We believe our existing facilities are adequate for our current and expected near-term needs and additional space would be available on commercially reasonable terms if required.
From time to time, we may be involved in legal proceedings arising in the ordinary course of our business. We are not presently a party, and our properties are not currently subject, to any legal proceedings that, in the opinion of management, would have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us due to defense and settlement costs, diversion of management resources, negative publicity and reputational harm, among other factors.
Item 4. Mine Safety Disclosures.
Not applicable.
41
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
On September 29, 2016, our common stock was listed for trading on the NASDAQ Global Market under the symbol “FLGT.” There was no public market for our common stock prior to September 29, 2016. The following table sets forth the high and low sales prices of our common stock as reported on the NASDAQ Global Market for the periods presented.
|
|
High |
|
|
Low |
|
||
|
2016: |
|
|
|
|
|
|
|
|
Quarter ended September 30, 2016 |
$ |
9.22 |
|
|
$ |
9.18 |
|
|
Quarter ended December 31, 2016 |
|
11.79 |
|
|
|
8.14 |
|
Holders of Common Stock
As of March 16, 2017, there were 9 holders of record of our common stock, plus an indeterminate number of additional stockholders whose shares of our common stock are held on their behalf by brokerage firms or other agents.
Dividend Policy
We anticipate that any future earnings will be retained to finance continuing development of our business. Accordingly, we do not anticipate paying dividends on our common stock in the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our Board of Directors and will depend upon our results of operation, financial condition and other factors as the Board of Directors, in its discretion, deems relevant.
Stock Performance Graph
The following performance graph shall not be deemed to be “soliciting material” or "filed" for purposes of Section 18 of the Exchange Act, or incorporated by reference into any filing under the Securities Act or the Exchange Act, unless it is specifically incorporated by reference into any such filing. The graph is required by applicable SEC rules and is not intended to forecast or be indicative of possible future performance of our common stock.
The following graph illustrates a comparison of the total cumulative stockholder return on our common stock since September 29, 2016, the first date on which our common stock began trading on a national securities exchange, to the following two indices: (i) the NASDAQ Composite Index (symbol: ^IXIC) and (ii) the NASDAQ Biotechnology Index (symbol: ^NBI). The graph assumes an initial investment of $100 on September 29, 2016 and reinvestment of all dividends. No cash dividends have been declared on our common stock since September 29, 2016.
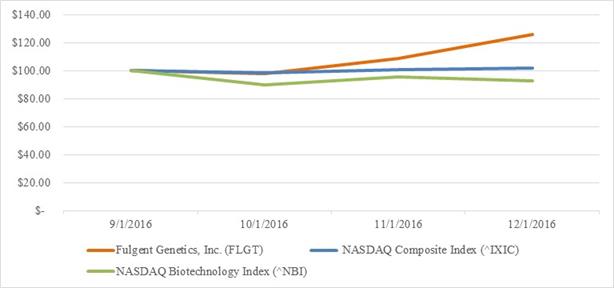
42
Recent Sales of Unregistered Securities
The descriptions below set forth information regarding all securities issued and sold by Fulgent LLC in 2016 that were not registered under the Securities Act and that have not been described in a previously filed quarterly report on Form 10-Q or current report on Form 8-K, as well as the consideration, if any, received for such securities. None of these transactions involved any public offering of securities based on the specific facts of each transaction described below. As a result, we believe each transaction was exempt from the registration requirements of the Securities Act as described below.
On January 27, 2016, we issued 2,500,000 Class D voting common units to a member of our management team as an inducement to entering into employment with us, which upon completion of the Reorganization, became 328,947 shares of our common stock. This issuance was exempt from the registration requirements of the Securities Act in reliance upon Section 4(a)(2) thereof because it did not involve any public offering of securities based on the following facts: no underwriters, underwriting discounts or commissions were involved in the transaction; no general solicitation was used; the recipient of the securities represented his intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and had adequate access through their relationship with us to information about us; and all of the securities were issued as restricted securities for purposes of the Securities Act.
On April 4, 2016, Fulgent LLC separated the Pharma Business, which was conducted through Fulgent LLC’s former subsidiary, Fulgent Pharma LLC, or Fulgent Pharma, in the Pharma Split-Off. Prior to the Pharma Split-Off, Fulgent LLC had two series of units, with the Class D units (consisting of Class D-1 preferred, Class D-2 preferred and Class D common units) having economic rights based on the genetic testing business we are currently pursuing and the Class P units (consisting of the Class P preferred and Class P common units) having economic rights based on the Pharma Business. At the time of the Pharma Split-Off, Fulgent LLC had seven members and 10 option holders, each of which had provided services to Fulgent Pharma and had a pre-existing relationship with Fulgent LLC and Fulgent Pharma and access to information about each company. To effect the Pharma Split-Off, Fulgent LLC redeemed each member’s Class P preferred and common units, distributed to each such member substantially identical units of Fulgent Pharma and caused Fulgent Pharma to assume all then-outstanding options to acquire Class P common units. This issuance was exempt from the registration requirements of the Securities Act in reliance upon Section 4(a)(2) thereof because it did not involve any public offering of securities based on the following facts: no underwriters, underwriting discounts or commissions were involved in the transaction; no general solicitation was used; the recipients of the securities represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and had adequate access through their relationship with Fulgent Pharma and its management to information about Fulgent Pharma; and all of the securities were issued as restricted securities for purposes of the Securities Act.
On May 17, 2016, we sold 5,131,579 Class D-2 preferred units to Xi Long for an aggregate purchase price of $15.2 million. On May 17, 2016, we exchanged for Class D-2 preferred units, on a one-for-one basis, an aggregate of 10,263,158 Class D-1 preferred and Class D common units acquired by Xi Long from our other members on May 13, 2016. Upon completion of the Reorganization, all such Class D-2 preferred units became 2,025,623 shares of our common stock. These issuances were exempt from the registration requirements of the Securities Act in reliance upon Section 4(a)(2) thereof and Regulation D thereunder because it did not involve any public offering of securities based on the following facts: no underwriters, underwriting discounts or commissions were involved in the transaction; no general solicitation was used; the recipient of the securities represented its intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and had adequate access to information about us; and all of the securities were issued as restricted securities for purposes of the Securities Act.
Between January 1, 2016 and June 30, 2016, we issued options to acquire an aggregate of 324,000 Class D non-voting common units subject to exercise prices of $0.38 and $12.31 per unit. These issuances were exempt from the registration requirements of the Securities Act in reliance upon Rule 701 thereunder because the securities were issued under written compensatory plans intended to comply with Rule 701 and the recipients of these securities were bona fide service providers to us at the time of grant.
Use of Proceeds from Registered Securities
On October 4, 2016, we completed the initial public offering of our common stock, or the IPO, in which we issued and sold an aggregate of 4,830,000 shares of common stock (including 630,000 shares issued and sold on October 7, 2016 pursuant to the underwriters’ exercise in full of their option to purchase additional shares) at a public offering price of $9.00 per share. We received net proceeds of approximately $36.0 million, after deducting underwriting discounts and commissions and offering expenses paid or payable by us of approximately $4.4 million. The shares issued and sold in the IPO were registered under the Securities Act on a registration statement on Form S-1 (File No. 333-213469), as amended, or the Registration Statement, and the final prospectus dated September 28, 2016 included in the Registration Statement, or the Prospectus.
43
The net proceeds from the IPO are invested in short term, investment-grade, interest-bearing securities such as money market accounts, certificates of deposit, commercial paper and guaranteed obligations of the U.S. government. There has been no material change in the planned use of proceeds from the IPO from that described in the Prospectus.
Item 6. Selected Financial Data.
The following selected consolidated financial data should be read in conjunction with “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements included under “Item 8. Financial Statements and Supplementary Data.” The selected consolidated balance sheet data as of December 31, 2016 and 2015 and the selected consolidated statement of operations data for each of the three years in the period ended December 31, 2016, have been derived from our consolidated financial statements, which are included under “Item 8. Financial Statements and Supplementary Data.” The selected consolidated balance sheet data as of December 31, 2014 have been derived from our consolidated financial statements not included in this report. Historical results are not indicative of the results to be expected in the current period or in future periods.
|
|
Year Ended December 31, |
|
|||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
(in thousands) |
|
|||||||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
$ |
18,276 |
|
|
$ |
9,576 |
|
|
$ |
1,278 |
|
|
Cost of revenue(1) |
|
6,722 |
|
|
|
5,069 |
|
|
|
936 |
|
|
Gross profit |
|
11,554 |
|
|
|
4,507 |
|
|
|
342 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development(1) |
|
3,558 |
|
|
|
4,431 |
|
|
|
521 |
|
|
Selling and marketing(1) |
|
2,469 |
|
|
|
2,670 |
|
|
|
581 |
|
|
General and administrative(1) |
|
4,609 |
|
|
|
2,418 |
|
|
|
230 |
|
|
Total operating expenses |
|
10,636 |
|
|
|
9,519 |
|
|
|
1,332 |
|
|
Operating income (loss) |
|
918 |
|
|
|
(5,012 |
) |
|
|
(990 |
) |
|
Interest and other income (expense) |
|
(5,386 |
) |
|
|
27 |
|
|
|
0 |
|
|
Income (loss) before income taxes |
|
(4,468 |
) |
|
|
(4,985 |
) |
|
|
(990 |
) |
|
Provision for income taxes |
|
920 |
|
|
|
— |
|
|
|
— |
|
|
Income (loss) from continuing operations |
|
(5,388 |
) |
|
|
(4,985 |
) |
|
|
(990 |
) |
|
Income (loss) from discontinued operations |
|
41 |
|
|
|
(3,329 |
) |
|
|
(3,293 |
) |
|
Net income (loss) |
$ |
(5,347 |
) |
|
$ |
(8,314 |
) |
|
$ |
(4,283 |
) |
|
Basic and diluted loss per common share: |
|
|
|
|
|
|
|
|
|
|
|
|
Continuing operations—common stock |
$ |
(1.00 |
) |
|
$ |
(0.61 |
) |
|
* |
|
|
|
Weighted-average common stock/unit—outstanding—basic and diluted |
|
13,710 |
|
|
|
11,842 |
|
|
* |
|
|
* Basic and diluted loss per common share was calculated prospectively from the date the Class D common units were issued in the Recapitalization in October 2015. See Note 1, Overview and Basis of Presentation, and Note 12, Income (Loss) per Share, to our consolidated financial statements for additional information.
(1) Includes equity-based compensation expense as follows:
|
|
Year Ended December 31, |
|
|||||
|
|
2016 |
|
|
2015 |
|
||
|
|
(in thousands) |
|
|||||
|
Equity-Based Compensation Data: |
|
|
|
|
|
|
|
|
Cost of revenue |
$ |
754 |
|
|
$ |
1,673 |
|
|
Research and development |
|
1,161 |
|
|
|
3,241 |
|
|
Selling and marketing |
|
454 |
|
|
|
1,569 |
|
|
General and administrative |
|
2,285 |
|
|
|
1,673 |
|
|
Total equity-based compensation |
$ |
4,654 |
|
|
$ |
8,156 |
|
44
|
Year Ended December 31, |
|
||||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
(in thousands) |
|
|||||||||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
$ |
7,897 |
|
|
$ |
489 |
|
|
$ |
172 |
|
|
Short-term investments in marketable securities |
$ |
12,971 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Long-term investments in marketable securities |
$ |
25,597 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Total assets |
$ |
58,040 |
|
|
$ |
5,832 |
|
|
$ |
2,120 |
|
|
Total liabilities |
$ |
3,561 |
|
|
$ |
686 |
|
|
$ |
436 |
|
|
Total stockholders'/members' equity |
$ |
54,479 |
|
|
$ |
5,146 |
|
|
$ |
1,684 |
|
45
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
On September 30, 2016, Fulgent Therapeutics LLC became a wholly owned subsidiary of Fulgent Genetics, Inc. in a transaction we refer to as the Reorganization. As used in the following discussion and analysis, unless the context otherwise requires, (i) the term “Fulgent LLC” refers to Fulgent Therapeutics LLC, (ii) the term “Fulgent Inc.” refers to Fulgent Genetics, Inc. and (iii) the terms “Fulgent,” the “company,” “we,” “us” and “our” refer, for periods prior to completion of the Reorganization, to Fulgent LLC and, for periods after completion of the Reorganization, to Fulgent Inc. and its consolidated subsidiaries after giving effect to the Reorganization. Following the Reorganization, Fulgent Inc. is a holding company with no material assets other than 100% of the equity interests in its subsidiaries, including Fulgent LLC, and Fulgent LLC is considered Fulgent Inc.’s predecessor for accounting purposes and its financial statements for all periods prior to completion of the Reorganization constitute Fulgent Inc.’s historical financial statements.
The following discussion and analysis of our financial condition and results of operations should be read together with our consolidated financial statements and related notes included in this report.
The following discussion and analysis contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements are statements other than historical facts and relate to future events or circumstances or our future performance, and they are based upon our current assumptions, expectations and beliefs concerning future developments and their potential effect on our business. The forward-looking statements in this discussion and analysis include statements about, among other things, our future financial and operating performance, future cash flows and liquidity, our strategies for growth and anticipated trends in our business and industry. These forward-looking statements are subject to a number of risks and uncertainties, including, among others, those described under “Item 1A. Risk Factors” in this report. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this discussion and analysis may not occur and actual results could differ materially and adversely from those described in or implied by our forward-looking statements. As a result, you should not rely upon forward-looking statements as predictions of future events and you should read this discussion and analysis with the understanding that our actual future results, levels of activity, performance and achievements may be materially different than what we expect. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this report to conform these statements to actual results or to changes in our expectations.
We are a rapidly growing technology company with an initial focus on offering comprehensive genetic testing to provide physicians with clinically actionable diagnostic information they can use to improve the overall quality of patient care. We have developed a proprietary technology platform that integrates sophisticated data comparison and suppression algorithms, adaptive learning software, advanced genetic diagnostics tools and integrated laboratory processes. This platform allows us to offer a broad and flexible test menu and continually expand and improve our proprietary genetic reference library, while maintaining accessible pricing, high accuracy and competitive turnaround times. We believe our test menu offers more genes for testing than our competitors in today’s market, which enables us to provide expansive options for test customization and clinically actionable results. Our current test menu includes approximately 18,000 single-gene tests and more than 300 pre-established, multi-gene, disease-specific panels that collectively test for approximately 7,700 genetic conditions, including various cancers, cardiovascular diseases, neurological disorders and pediatric tests.
Our existing customer base consists primarily of hospitals and medical institutions, which are frequent and high-volume users of genetic tests and which typically pay us directly for our tests. We have generated growing demand for our tests with relatively little marketing efforts to date, which we believe demonstrates the advantages of our offering compared to other available testing alternatives. We offer tests at competitive prices, averaging approximately $1,461 per billable test delivered in 2016, and at a lower cost to us than many of our competitors, totaling approximately $537 per billable test delivered in 2016. Our volume has grown rapidly since our commercial launch, with over 20,000 billable tests delivered to over 600 total customers as of December 31, 2016. We delivered 12,507 billable tests in 2016, compared to 6,852 billable tests delivered in 2015 and 966 billable tests delivered in 2014. We recorded revenue and loss from continuing operations of $18.3 million and $5.4 million, respectively, in 2016, $9.6 million and $5.0 million, respectively, in 2015, and $1.3 million and $990,000, respectively, in 2014.
46
Factors Affecting Our Performance
Genetic testing has experienced significant growth in recent years. As this trend continues, we believe genetic testing will become a more accepted part of standard medical care and the knowledge of a person’s unique genetic makeup will begin to play a more important role in the practice of medicine. The advent of next generation sequencing, or NGS, technology, a relatively new genetic testing technique that enables millions of DNA fragments to be sequenced in parallel, has dramatically lowered the cost and improved the quality of genetic testing, contributing to increased adoption generally and increased volumes for our tests. According to GrandView Research, the size of the global NGS genetic testing market, which includes pre-sequencing, sequencing and data analysis, is estimated to have been approximately $4.0 billion in 2016, including approximately $1.4 billion in the United States, and is expected to reach approximately $10.5 billion by 2022, including approximately $3.6 billion in the United States.
While adoption of genetic testing has increased in recent years, we believe widespread utilization has been limited in large part because of certain barriers to adoption that exist in today’s market. Among these barriers are that genetic testing can be prohibitively expensive, only a limited number of genetic tests are currently reimbursable, certain genetic conditions cannot be diagnosed due to the limited scope of genetic analysis, genetic testing can be an inefficient process and the interpretation of genetic results can be cumbersome and time-consuming. We believe a significant market exists for a genetic testing option that provides broad genetic coverage and the flexibility to customize tests for individual patient needs, while maintaining accuracy and affordability, and we believe the proprietary technology platform we have developed will enable us to overcome many of the challenges facing our industry today.
Number of Billable Tests Delivered
Our performance is closely correlated with the number of tests for which we bill our customers, which we refer to as billable tests. The number of billable tests we deliver in a period depends on a number of factors, including the other factors affecting our performance discussed in this discussion and analysis. We believe the number of billable tests delivered in any period is an important indicator of the performance of our business. We have experienced compound quarterly growth of 19% in the number of billable tests delivered from the first quarter of 2015 through the fourth quarter of 2016.
Our existing customer base consists primarily of hospitals and medical institutions, which are frequent and high-volume users of genetic tests. Additionally, collection of billings from these institutional customers is more attainable than other types of customers in today’s reimbursement environment. Approximately 86% of our test billings that were generated and due in 2016 were paid during that period. In certain periods, a small number of customers have accounted for a significant portion of our revenue. For instance, in 2016, Customers A and B each contributed 15% of our total revenue, and Customer B contributed 19% and 11% of our total revenue in 2015 and 2014, respectively. Generally, we do not have long-term purchase agreements with any of our customers and, as result, any or all of them could decide at any time to increase, decrease or delay their orders from us. We are focused on more deeply penetrating our relationships with existing customers, including these key customers, to increase the volume of tests they order from us.
In addition, we are seeking to grow our customer base by continuing to acquire new hospital and medical institution customers and expand into additional customer groups, such as individual physicians and other practitioners, as well as research institutions. To this end, we have made efforts to diversify our customer base beyond hospitals by establishing a vendor code with a national clinical laboratory that orders our tests to fulfill some of the genetic testing orders it receives from certain U.S. government agencies, contracting with a regional hospital network within the U.S. Army to provide genetic tests for members and their families. We have also pursued relationships with payors, including Medicare, some state Medicaid programs and third-party payors, in order to obtain coverage and reimbursement for our tests to make them accessible to more individual physicians. Other than our relationship with the hospital network within the U.S. Army, which involves a minimum commitment of approximately $400,000 of our tests annually over a three-year period, none of these relationships obligate any party to order our tests at any agreed volume or frequency, or at all. However, we believe our ability to establish these types of relationships and relationships with other new customers and achieve increased sales to existing customers are significant indicators of the potential for growth of our business.
We believe the key to further penetrating our existing customer base and expanding into new customer markets is to continue to focus on delivering a superior test menu while maintaining affordable prices. In order to offer our customers affordable price points, we continue to improve our technology platform to develop tests that we can perform at a low internal cost. In addition, we believe an increased focus on sales and marketing will facilitate customer growth. To that end, we plan to invest in our sales and marketing function in the near-term, with the goal of more than doubling the size of our sales and marketing team in the next 12 months.
47
Ability to Maintain Our Broad and Flexible Test Menu
We believe the number of genes that we incorporate into our test menu provides a meaningful competitive advantage. We believe the breadth of genes in our portfolio allows us to provide more comprehensive genetic information and improves our variant detection rate, which can increase the clinical actionability of the data we produce. The breadth of genes in our portfolio also allows us to provide a flexible and customizable test menu for our customers. We believe that our ability to continue to offer more genes than our competitors could be a key contributor to the rate of growth of our business.
We have developed various proprietary technologies that improve our laboratory efficiency and reduce the costs we incur to perform our tests, including our proprietary gene probes, data algorithms, adaptive learning software and genetic reference library. This technology platform enables us to perform each test and deliver its results at a lower cost to us than many of our competitors, totaling approximately $537 per billable test delivered in 2016. This low cost per billable test allows us to maintain affordable pricing for our customers, averaging approximately $1,461 per billable test delivered in 2016, which we believe encourages repeat ordering from existing customers and attracts new customers. We believe this low internal cost is a key contributor to our ability to grow our business and drive profitability.
We intend to continue to expand our test menu to include more options and to cover more genes. For example, we intend to expand our offering of oncology, cardiology, pediatrics and prenatal test panels, which represent large genetic testing markets in which we believe our comprehensive and flexible tests will be competitive. For instance, we recently launched a new chromosomal test called CNV+ that is designed to use NGS technology to detect copy number variants with similar or improved results as compared to microarray-based genomic tests. In addition, we expect to offer somatic testing for certain cancers in the near future. We also believe there is a large potential for growth of genetic testing in many international markets due to the presence of high unmet diagnostic and predictive testing needs, rapidly rising healthcare expenditures and patient awareness of next generation sequencing technologies. We plan to engage distributors or establish other types of arrangements, such as joint ventures or other partner relationships, in an effort to expand our presence and test volume in new geographic markets. To this end, we received revenues of $3.2 million from tests ordered by customers in China in 2016 and we are working with Xi Long USA, Inc., or Xi Long, a large stockholder of our company, to develop a strategic commercial relationship to pursue additional customers in China, which we expect to be finalized in the first half of 2017. Although the terms of this relationship have not been finalized, we anticipate that this relationship, if it proceeds, could expand our long-term opportunities to address the genetic testing market in Asia. We believe expanding our test menu and our geographic presence will appeal to a broader base of potential customers and increase our revenue potential.
Success Obtaining Reimbursement
In today’s market, third-party payors generally restrict the reimbursement of genetic testing to a limited subset of genetic tests and only for those patients that meet specific criteria. This lack of widespread favorable reimbursement policies has presented a challenge for genetic testing companies in building sustainable business models. As part of our strategy for growth, we intend to pursue coverage and reimbursement from third-party payors at a level adequate for us to achieve profitability with this payor group. To this end, we have contracted with a regional physician services organization and a national health insurance company to become an in-network provider and enrolled as a supplier with Medicare and 10 state Medicaid programs, which means that we have agreed with these payors to provide certain of our tests at negotiated rates. Although this does not guarantee that we will receive reimbursement for our tests from these or any other payors at adequate levels, we believe our low cost per billable test will enhance our ability to compete effectively in the third-party payor market and our flexibility in establishing additional relationships with third-party payors. Our level of success in obtaining and maintaining adequate coverage and reimbursement from third-party payors for our testing services will, we believe, be a key factor in the rate of growth of our business over the long term.
In October 2015 and January 2016, our predecessor, Fulgent LLC granted awards of fully vested equity to employees and non-employees. The equity-based compensation expense associated with these awards was recorded in full in the period in which the awards were granted. As a result, there was a substantial increase in cost of revenue in the quarter ended December 31, 2015 and in operating expenses in the quarters ended December 31, 2015 and March 31, 2016. We do not intend to make additional awards of fully vested equity and, as a result, we do not expect that we will experience similar levels of equity-based compensation expense in future periods. Generally, we record equity-based compensation over the requisite service period from the grant date of the applicable award.
48
In 2015 and 2016, Fulgent LLC issued options that were not exercisable, whether or not vested, until the earlier of a liquidity event or an incorporation of Fulgent LLC, each as defined in Fulgent LLC’s equity incentive plan under which the awards were granted. An incorporation was deemed to have occurred upon completion of the Reorganization on September 30, 2016, at which time the options became immediately exercisable, to the extent vested. As a result, no expense was recorded for these options prior to their exercisability, and a cumulative expense of $1.1 million for the requisite service period related to these options was recorded during the period in which the Reorganization occurred. Generally, we record equity-based compensation expense for option awards over the requisite service period. As of December 31, 2016, we have no outstanding equity-based awards whose exercisability is subject to this type of performance condition.
In 2015 and 2016, Fulgent LLC granted awards of units that constitute profits interests, which we sometimes refer to simply as profits interests. Profits interests are a type of equity-based award containing a participation threshold, or a profits interest threshold, that entitled the recipient of the award to participate in the value of Fulgent LLC only to the extent it appreciated from and after the grant date of the award. Pursuant to the determination of the Mr. Hsieh in his capacity as the Manager of Fulgent LLC prior to the Reorganization, the participation thresholds applicable to profits interests (i) were ignored and not applied in calculating the number of shares of our common stock that were issued in exchange for such units in the Reorganization, and (ii) did not carry over to such shares of our common stock. As a result, the holders of Fulgent LLC’s profits interests received shares of our common stock in the Reorganization at the same ratio as the holders of Fulgent LLC’s units that were not subject to such profits interest thresholds. Ignoring all profits interest thresholds upon the conversion of these profits interests into shares of our common stock resulted in an equity-based compensation expense of $1.4 million that we recorded in the period in which the Reorganization occurred.
In May 2016, Fulgent LLC completed a transaction with Xi Long and certain members of Fulgent LLC. In this transaction, (i) Xi Long acquired 4,618,421 Class D-1 preferred units and 5,644,737 Class D common units from certain existing members of Fulgent LLC for an aggregate purchase price of approximately $12.0 million, which units were required to be redeemed by Fulgent LLC in exchange for its issuance to Xi Long of an equivalent number of Class D-2 preferred units, and (ii) Fulgent LLC sold an additional 5,131,579 Class D-2 preferred units to Xi Long for gross proceeds of approximately $15.2 million. Fulgent LLC incurred issuance costs of $185,000 for the transaction, resulting in net proceeds to Fulgent LLC of approximately $15.0 million. As a result of the transaction, Xi Long acquired an aggregate of 15,394,737 Class D-2 preferred units for an aggregate purchase price of approximately $27.2 million, even though, at issuance, the fair value of 15,394,737 Class D-2 preferred units as evidenced by Fulgent LLC’s then most recent third-party valuation was approximately $32.6 million. The $5.5 million difference between the fair value of, and the aggregate consideration paid by Xi Long for, the Class D-2 preferred units issued in the transaction was not attributable to any stated rights or privileges. Rather Fulgent LLC, Xi Long and the members of Fulgent LLC that were party to the transaction determined to complete the transaction in line with their discussions, notwithstanding that the fair value of the Class D-2 preferred units as evidenced by Fulgent LLC’s third-party valuation had increased from the time these discussions were initiated to the time the transaction was completed. The $5.5 million difference was determined to be a cost of completing the transaction with Xi Long and was recorded as an expense in 2016 in our consolidated statements of operations.
Business Risks and Uncertainties
Our business and prospects are exposed to numerous risks and uncertainties. For more information, see “Item 1A. Risk Factors” in this report.
Prior to April 4, 2016, Fulgent LLC conducted the following two lines of business: the genetic testing business we are currently pursuing, which Fulgent LLC conducted directly; and our former pharmaceutical business, or the Pharma Business, which was conducted through Fulgent LLC’s former subsidiary, Fulgent Pharma LLC, or Fulgent Pharma. Prior to April 4, 2016, all of Fulgent LLC’s equity interests were separated into two series of units based on these two lines of business, with the Class D units (consisting of Class D-1 preferred, Class D-2 preferred and Class D common units) having economic rights based on the genetic testing business we are currently pursuing and the Class P units (consisting of the Class P preferred and Class P common units) having economic rights based on the Pharma Business. On April 4, 2016, Fulgent LLC separated the Pharma Business from the genetic testing business we are currently pursuing in a transaction we refer to as the Pharma Split-Off. To effect the Pharma Split-Off, Fulgent LLC redeemed each member’s Class P preferred and common units, distributed to each such member substantially identical units of Fulgent Pharma and caused Fulgent Pharma to assume all then-outstanding options to acquire Class P common units.
The operating results of the Pharma Business have been reported as discontinued operations for all periods presented in the consolidated financial statements included in this report. In 2016, 2015 and 2014, we recorded an income (loss) from discontinued operations of $41,000, $(3.3) million and $(3.3) million, respectively.
49
On September 30, 2016, our predecessor Fulgent LLC became our wholly owned subsidiary upon completion of the Reorganization.
Prior to the Reorganization, among other things:
|
|
• |
Fulgent Inc. did not conduct any activities other than activities incidental to its formation and preparation for our initial public offering. |
Upon completion of the Reorganization, among other things:
|
|
• |
each outstanding 7.6 units of Fulgent LLC were cancelled in exchange for one share of our common stock; |
After completion of the Reorganization, we continue to exist as a holding company, with no material assets other than 100% of the equity interests in Fulgent LLC and our other subsidiaries. We consolidate the financial results of Fulgent LLC, and the historical financial statements of Fulgent LLC are our historical financial statements.
We generate revenue from sales of our genetic tests. We recognize revenue upon delivery of a report to the ordering physician based on the established billing rate less contractual and other adjustments to arrive at the amount that we expect to collect. We generally bill directly to a hospital, medical institution or research institution customer or to a patient, a third-party payor or a combination of the patient and third-party payor.
Cost of revenue reflects the aggregate costs incurred in delivering test results and consists of: personnel costs, including salaries, employee benefit costs, bonuses and equity-based compensation expenses; costs of laboratory supplies; depreciation of laboratory equipment; amortization of leasehold improvements and allocated overhead, including rent and utilities. Costs associated with performing tests are recorded as tests are processed. We expect cost of revenue to generally increase as we increase the number of tests we deliver.
Our operating expenses are classified into the following three categories: research and development; selling and marketing; and general and administrative. For each category, the largest component is personnel costs, which include salaries, employee benefit costs, bonuses and equity-based compensation expenses.
Research and Development Expenses
Research and development expenses represent costs incurred to develop our technology and future tests. These costs consist of personnel costs, laboratory supplies, consulting costs and allocated overhead, including rent and utilities. We expense all research and development costs in the periods in which they are incurred. We expect our research and development expenses will increase in absolute dollars in future periods as we continue to invest in research and development.
50
Selling and Marketing Expenses
Selling and marketing expenses consist of personnel costs, customer service expenses, direct marketing expenses, educational and promotional expenses, market research and analysis and allocated overhead, including rent and utilities. We expense all selling and marketing costs as incurred. We expect our selling and marketing costs will continue to increase in absolute dollars, primarily driven by our efforts to expand our sales and marketing team, increase our presence within and outside the United States and expand our brand awareness and customer base through targeted marketing initiatives.
General and Administrative Expenses
General and administrative expenses include executive, finance and accounting, legal and human resources functions. These expenses consist of personnel costs, audit and legal expenses, consulting costs and allocated overhead, including rent and utilities. We expense all general and administrative expenses as incurred. We expect our general and administrative expenses will increase as we scale our operations. We also expect to incur additional general and administrative expenses as a result of completing our initial public offering and operating as a public company, including expenses related to compliance with the rules and regulations of the Securities and Exchange Commission and the NASDAQ Stock Market, additional insurance expenses, investor relations activities and other administration and professional services.
Provision for (Benefit from) Income Taxes
Provision for income taxes consists of U.S. federal and state income taxes. To date, we have not had significant U.S. federal and state income taxes because of the status of our predecessor Fulgent LLC as a pass-through entity for tax purposes. As a result, for all periods prior to the Reorganization, all taxable income or loss and tax credits generally were reflected in the personal income tax returns of the Fulgent LLC’s members and no provision for federal and state income taxes was provided in our consolidated financial statements. We became a taxable entity upon completion of the Reorganization on September 30, 2016.
We record a valuation allowance when it is more likely than not that some portion or all of a deferred tax asset will not be realized. In making such a determination, we consider all the available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, and ongoing prudent and feasible tax planning strategies, to assess the amount of the valuation allowance. When we establish or reduce the valuation allowance against the deferred tax assets, our provision for income taxes will increase or decrease, respectively, in the period in which such a determination is made.
51
Comparison of Results of Operations in 2014, 2015 and 2016
The following table summarizes the results of our continuing operations for each of the periods presented:
|
|
Year Ended December 31, 2016 |
|
|
$ Change from 2015 |
|
|
% Change from 2015 |
|
|
Year Ended December 31, 2015 |
|
|
$ Change from 2014 |
|
|
% Change from 2014 |
|
|
Year Ended December 31, 2014 |
|
|||||||
|
Statement of Operations Data: |
(dollars in thousands) |
|
|||||||||||||||||||||||||
|
Revenue |
$ |
18,276 |
|
|
$ |
8,700 |
|
|
|
91% |
|
|
$ |
9,576 |
|
|
$ |
8,298 |
|
|
|
649% |
|
|
$ |
1,278 |
|
|
Cost of revenue |
|
6,722 |
|
|
|
1,653 |
|
|
|
33% |
|
|
|
5,069 |
|
|
|
4,133 |
|
|
|
442% |
|
|
|
936 |
|
|
Gross profit |
|
11,554 |
|
|
|
7,047 |
|
|
|
156% |
|
|
|
4,507 |
|
|
|
4,165 |
|
|
|
1218% |
|
|
|
342 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
3,558 |
|
|
|
(873 |
) |
|
|
(20)% |
|
|
|
4,431 |
|
|
|
3,910 |
|
|
|
750% |
|
|
|
521 |
|
|
Selling and marketing |
|
2,469 |
|
|
|
(201 |
) |
|
|
(8)% |
|
|
|
2,670 |
|
|
|
2,089 |
|
|
|
360% |
|
|
|
581 |
|
|
General and administrative |
|
4,609 |
|
|
|
2,191 |
|
|
|
91% |
|
|
|
2,418 |
|
|
|
2,188 |
|
|
|
951% |
|
|
|
230 |
|
|
Total operating expenses |
|
10,636 |
|
|
|
1,117 |
|
|
|
12% |
|
|
|
9,519 |
|
|
|
8,187 |
|
|
|
615% |
|
|
|
1,332 |
|
|
Operating income (loss) |
|
918 |
|
|
|
5,930 |
|
|
|
118% |
|
|
|
(5,012 |
) |
|
|
(4,022 |
) |
|
|
(406)% |
|
|
|
(990 |
) |
|
Interest and other income (expense) |
|
(5,386 |
) |
|
|
(5,413 |
) |
|
* |
|
|
|
27 |
|
|
|
27 |
|
|
* |
|
|
|
— |
|
||
|
Income (loss) before income taxes |
|
(4,468 |
) |
|
|
517 |
|
|
|
10% |
|
|
|
(4,985 |
) |
|
|
(3,995 |
) |
|
|
(404)% |
|
|
|
(990 |
) |
|
Provision for income taxes |
|
920 |
|
|
|
920 |
|
|
* |
|
|
|
— |
|
|
|
— |
|
|
* |
|
|
|
— |
|
||
|
Income (loss) from continuing operations |
$ |
(5,388 |
) |
|
$ |
(403 |
) |
|
|
(8)% |
|
|
$ |
(4,985 |
) |
|
$ |
(3,995 |
) |
|
|
(404)% |
|
|
$ |
(990 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Operating Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Billable tests |
|
12,507 |
|
|
|
|
|
|
|
83% |
|
|
|
6,852 |
|
|
|
|
|
|
|
609% |
|
|
|
966 |
|
* Percentage not meaningful.
Revenue
Revenue increased $8.7 million, or 91%, from $9.6 million in 2015 to $18.3 million in 2016. The increase in revenue was primarily due to an increased number of billable tests delivered, which increased from 6,852 in 2015 to 12,507 in 2016. The increase in the number of billable tests delivered between periods was primarily attributable to the expansion of our test menu, including single-gene tests and multi-gene panels, and an increase in sales to our existing customers, combined with growth in the genetic testing market and increased physician awareness and acceptance of genetic tests generally. The average price of the billable tests we delivered remained relatively consistent between periods, but increased slightly from $1,398 in 2015 to $1,461 in 2016, largely due to our ability to offer more complex and customized tests. Additionally, revenue from non-U.S. customers increased $5.5 million, from $4.5 million in 2015 to $10.0 million in 2016. The increase in revenue from non-U.S. customers was primarily attributable to our commencement of sales to customers in China in 2016, which contributed $3.2 million in revenue, and an increase of $1.1 million in revenue from customers in Canada. Further, research revenue related to gene sequencing was $1.0 million in 2016 and we did not have any research revenue in 2015.
Revenue increased $8.3 million, or 649%, from $1.3 million in 2014 to $9.6 million in 2015. The increase in revenue was primarily due to an increased number of billable tests delivered, which increased from 966 in 2014 to 6,852 in 2015. The increase in the number of billable tests delivered between periods was primarily attributable to the expansion of our test menu, including single-gene tests and multi-gene panels, and an increase in sales to our existing customers, combined with growth in the genetic testing market and increased physician awareness and acceptance of genetic tests generally. The average price of the billable tests we delivered increased slightly in 2015 compared to 2014. Revenue from non-U.S. customers accounted for 50% and 47% of total revenue in 2014 and 2015, respectively.
Cost of Revenue
Cost of revenue increased $1.7 million, or 33%, from $5.1 million in 2015 to $6.7 million in 2016. The increase was primarily due to increases of $992,000 in reagents and supplies expenses related to an increase in the number of billable tests delivered, $898,000 in personnel costs related to increased headcount, $411,000 in depreciation costs due an increasing amount of medical lab equipment purchased to expand our capacity and throughput, and $259,000 in allocated facilities expenses, partially offset by a decrease of $919,000 in equity-based compensation expense due in part to grants of fully vested equity-based awards in 2015 that did not occur in 2016. Our gross profit increased $7.0 million between periods, primarily due to increased revenue, and our cost of
52
revenue as a percent of revenue, or gross margin, increased from 47.1% to 63.2% between periods, primarily due to the decrease in equity-based compensation expense.
Cost of revenue increased $4.1 million, or 442%, from $936,000 in 2014 to $5.1 million in 2015. The increase was primarily due to increases of $1.7 million in equity-based compensation expense, which relates to grants of fully vested equity-based awards in 2015, $1.2 million in reagents and supplies expenses related to the increase in the number of billable tests delivered, $687,000 in personnel costs related to increased headcount, and $233,000 in depreciation costs due to an increasing amount of medical lab equipment purchased to expand our capacity and throughput. Our gross profit increased $4.2 million between periods, primarily due to increased revenue, and our gross margin increased from 26.8% to 47.1% between periods, primarily due to lower costs per billable test resulting from economies of scale.
Research and Development
Research and development expenses decreased $873,000, or 20%, from $4.4 million in 2015 to $3.6 million in 2016. The decrease was primarily due to a decrease of $2.1 million in equity-based compensation expense due in part to grants of fully vested equity-based awards in 2015 that did not occur in 2016, partially offset by increases of $638,000 in personnel costs related to increased headcount, $418,000 in reagents and supplies expenses related to increased headcount performing more research and development activities, and $116,000 in allocated facilities expenses.
Research and development expenses increased $3.9 million, or 750%, from $521,000 in 2014 to $4.4 million in 2015. The increase was primarily due to increases of $3.2 million in equity-based compensation expense, which relates to grants of fully vested equity-based awards in 2015, and $546,000 in personnel costs related to increased headcount.
Selling and Marketing
Selling and marketing expenses decreased $201,000, or 8%, from $2.7 million in 2015 to $2.5 million in 2016. The decrease was primarily due to a decrease of $1.1 million in equity-based compensation expense due in part to grants of fully vested equity-based awards in 2015 that did not occur in 2016, partially offset by increases of $247,000 in personnel costs related to increased headcount, $230,000 in travel expenses related to increased headcount, $188,000 in consulting fees to train sales personnel and obtain new contracts, and $155,000 in marketing costs related to our targeted marketing initiatives.
Selling and marketing expenses increased $2.1 million, or 360%, from $581,000 in 2014 to $2.7 million in 2015. The increase was primarily due to increases of $1.6 million in equity-based compensation expense, which relates to grants of fully vested equity-based awards in 2015, and $402,000 in personnel costs related to increased headcount.
General and Administrative
General and administrative expenses increased $2.2 million, or 91%, from $2.4 million in 2015 to $4.6 million in 2016. The increase was primarily due to increases of $748,000 in accounting fees related to services performed in connection with being a public company, $720,000 in personnel costs related to increased headcount, $612,000 in equity-based compensation expense due to increased headcount and grants of fully vested equity-based awards in 2016, $129,000 in insurance costs due to additional directors and officers policies and other policies, $160,000 in depreciation costs related to leasehold improvements, and $106,000 in computer expenses related to an increase in equipment purchases due to increased headcount, partially offset by decreases of $374,000 in allocated facilities and $122,000 in professional service fees including legal fees.
General and administrative expenses increased $2.2 million, or 951%, from $230,000 in 2014 to $2.4 million in 2015. The increase was primarily due to increases of $1.7 million in equity-based compensation expense, which relates to grants of fully vested equity-based awards in 2015, $200,000 in professional service fees, and $131,000 in personnel costs related to increased headcount.
Interest and Other Income (Expense)
Interest and other expense was $(5.4) million for 2016, compared to income of $27,000 for 2015. The expense in the 2016 period related to the difference between the fair value and the effective issuance price of the Class D-2 preferred units we issued in the Xi Long financing in May 2016, which was determined to be a cost of completing the transaction with Xi Long and was recorded as an expense in our consolidated statements of operations. Interest income was not significant in 2015 or 2014.
53
Provision for income taxes in 2016 increased $920,000, from $0 in 2015. There was no income tax provision in 2014. The increase was due to the status of our predecessor Fulgent LLC as a pass-through entity for tax purposes prior to our company becoming a taxable entity upon completion of the Reorganization. This change in tax status on September 30, 2016 resulted in $417,000 income tax expense related to the recognition of a net state deferred tax asset of $86,000 and a net federal deferred tax liability of $503,000 representing the temporary differences in existence on September 30, 2016 between the tax basis of the Company’s assets and liabilities and the amount reported in the financial statements. The temporary differences in existence as of September 30, 2016 are primarily from depreciation and equity-based compensation. As of December 31, 2016, the net state deferred tax asset was $54,000 and net federal deferred tax liability was $243,000.
Liquidity and Capital Resources
We had $7.9 million and $489,000 in cash and cash equivalents as of December 31, 2016 and 2015, respectively, and $38.6 million in marketable securities, consisting of corporate bonds, as of December 31, 2016 and no marketable securities as of December 31, 2015.
Since inception, our operations have been financed primarily by our founder, Ming Hsieh, and, in recent periods, by cash from our operations and equity financings. In May 2016, we closed the Xi Long financing for net proceeds to us of approximately $15.0 million, and in October 2016, we closed the initial public offering of our common stock, in which we issued an aggregate of 4,830,000 shares of our common stock and received net proceeds of approximately $36.0 million, after deducting underwriting discounts and commissions and offering expenses paid or payable by us.
Our primary uses of cash are to fund our operations as we continue to grow and invest in our business. Cash used to fund operating expenses is impacted by the timing of our payment of expenses, as reflected in the changes in our outstanding accounts payable and accrued expenses. In addition, in September 2016, we distributed $4.6 million to Mr. Hsieh in his capacity as a member of Fulgent LLC as a return of capital contribution, and in November 2016, we paid $1.3 million in tax distributions to the former members of Fulgent LLC based on the income tax liabilities of such former members attributable to Fulgent LLC’s 2016 net taxable income through the date of the Reorganization.
We believe that our existing cash, along with cash from our operations and proceeds from our equity financings, will be sufficient to meet our anticipated cash requirements for at least the next 12 months. Much of the losses we incurred in 2016 were attributable to non-cash charges for equity-based compensation expense associated with the grant of a fully vested equity-based award and the recognition of previously unrecognized compensation expense related to stock options upon the satisfaction of a performance condition and the modification of profits interest awards in the Reorganization, as well as for other expense associated with the difference between the fair value and the effective issuance price of the units we issued to Xi Long in May 2016. Thus, in spite of the losses we recorded, cash provided by continuing operations has been positive since 2015 and has significantly contributed to our ability to meet our liquidity needs, including our ability to pay capital expenditures. Additionally, if our business continues to grow as we anticipate and we are able to achieve increased efficiencies and economies of scale in line with this growth, we expect that increased revenue will increase our ability to rely on cash from our operations to support our business in future periods, even if our expenses also increase as a result of the growth of our business. As a result, we anticipate that cash from our operations will play a meaningful role in our ability to meet our liquidity requirements and pursue our business plans and strategies in the next 12 months and in the longer term.
However, our expectations regarding the cash to be provided by our operations and our cash needs in future periods could be wrong, in which case we may require additional financing to support our operations, as we do not presently have any commitments for future capital. Further, even if our liquidity expectations are correct, we may seek to raise additional capital through securities offerings, credit facilities or other debt financings, asset sales or collaborations or licensing arrangements. Additional funding may not be available to us when needed, on acceptable terms or at all. If we raise funds by issuing equity securities, our existing stockholders could experience substantial dilution. Additionally, any preferred equity securities we issue could provide for rights, preferences or privileges senior to those of our common stock and our issuance of any additional equity securities, or the possibility of such an issuance, could cause the market price of our common stock to decline. The terms of debt securities issued or borrowings, if available, could impose significant restrictions on our operations, such as limitations on our ability to incur additional debt or issue additional equity, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely affect our ability to conduct our business, and would result in increased fixed payment obligations. In the event that we seek to sell assets or enter into collaborations or licensing arrangements to raise capital, we may be required to accept unfavorable terms or relinquish or license to a third party our rights to important or valuable technologies or tests we may otherwise seek to develop ourselves. Moreover, we may incur substantial costs in pursuing future capital, including investment banking, legal and accounting
54
fees, printing and distribution expenses and other costs. If we are not able to secure additional funding when needed and on reasonable terms, we may be forced to delay, reduce the scope of or eliminate one or more sales and marketing initiatives, research and development programs or other growth plans or strategies. In addition, we may be forced to work with a partner on one or more aspects of our tests or market development programs or initiatives, which could lower the economic value of these tests, programs or initiatives to our company. Any such outcome could significantly harm our business, performance and prospects.
The following table summarizes cash flows from continuing operations for each of the periods presented:
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
||||
|
|
|
(in thousands) |
|
|||||||||
|
Cash provided by (used in) operating activities |
|
$ |
4,436 |
|
|
$ |
2,026 |
|
|
$ |
(1,084 |
) |
|
Cash used in investing activities |
|
$ |
(42,795 |
) |
|
$ |
(2,030 |
) |
|
$ |
(731 |
) |
Operating Activities
Cash provided by operating activities in 2016 was $4.4 million. The difference between net loss and cash provided by operating activities for the period was primarily due to the effect of a $5.5 million non-cash charge associated with the difference between the fair value and the effective issuance price of the units we issued to Xi Long, which was recorded as other expense, and the effect of $4.7 million of non-cash equity-based compensation charges associated with the grant of a fully vested equity-based award and the recognition of previously unrecognized compensation expense related to stock options upon the satisfaction of a performance condition and the modification of profits interest awards in the Reorganization. Cash provided by operating activities increased primarily due to a $1.1 million increase in accounts payable, which resulted from purchases of medical lab equipment and reagents, partially offset by the negative effect of a $2.3 million increase in accounts receivable related to increased revenue, a $381,000 increase in other current assets due to prepaid insurance and maintenance on equipment and a $372,000 decrease in accrued liabilities due to payroll liabilities and customer deposits.
Cash provided by operating activities in 2015 was $2.0 million. The difference between net loss and cash provided by operating activities for the period was primarily due to the effect of $8.2 million of non-cash equity-based compensation charges associated with fully vested equity-based awards granted in October 2015. Cash provided by operating activities was negatively affected by a $1.8 million increase in accounts receivable related to increased revenue.
Cash used in operating activities in 2014 was $1.1 million, which primarily resulted from a net loss of $1.0 million and a $416,000 increase in accounts receivable related to increased revenue.
Cash used in investing activities in 2016 was $42.8 million, which primarily related to purchases of $39.0 million of marketable securities consisting of debt securities and certificates of deposit and $3.8 million of fixed assets consisting mainly of medical laboratory equipment, computer hardware, software and leasehold improvements. Our purchases of medical laboratory equipment have been increasing to expand our capacity and throughput.
Cash used in investing activities in 2015 was $2.0 million, which primarily related to purchases of DNA sequencing equipment and reagent kits.
Cash used in investing activities in 2014 was $731,000, which primarily related to purchases of fixed assets consisting mainly of medical laboratory equipment and leasehold improvements.
55
The following table summarizes cash flows from discontinued operations for each of the periods presented:
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
||||
|
|
|
(in thousands) |
|
|||||||||
|
Cash provided (used in) operating activities |
|
$ |
(31 |
) |
|
$ |
(2,995 |
) |
|
$ |
(3,313 |
) |
|
Cash used in investing activities |
|
$ |
— |
|
|
$ |
(175 |
) |
|
$ |
(49 |
) |
Financing Activities
The following table summarizes cash flows from financing activities from continuing and discontinued operations for each of the periods presented:
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
||||
|
|
|
(in thousands) |
|
|||||||||
|
Cash provided by financing activities |
|
$ |
45,789 |
|
|
$ |
3,500 |
|
|
$ |
4,000 |
|
Cash provided by financing activities in 2016 was $45.8 million, which consists of net proceeds of approximately $36.0 million received from our initial public offering completed in October 2016 and$15.2 million from our issuance of Class D-2 preferred units to Xi Long in May 2016, partially offset by a $4.6 million return of capital contribution to Mr. Hsieh and a $1.3 million in tax distributions to the former members of Fulgent LLC based on the income tax liabilities of such former members attributable to Fulgent LLC’s 2016 net taxable income through the date of the Reorganization.
Cash provided by financing activities in 2015 and 2014 was $3.5 million and $4.0 million, respectively. All cash provided by financing activities in 2015 and 2014 represents capital contributions received from Mr. Hsieh.
The following table summarizes our contractual obligations as of December 31, 2016:
|
|
|
Payments Due by Period |
|
|||||||||||||||||
|
|
Total |
|
|
Less than 1 Year |
|
|
1 - 3 Years |
|
|
3 - 5 Years |
|
|
More than 5 Years |
|
||||||
|
|
|
(in thousands) |
|
|||||||||||||||||
|
Operating lease obligations |
|
$ |
332 |
|
|
$ |
252 |
|
|
$ |
80 |
|
|
$ |
- |
|
|
$ |
- |
|
|
Purchase obligations |
|
$ |
2,177 |
|
|
$ |
2,177 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
Total |
|
$ |
2,509 |
|
|
$ |
2,429 |
|
|
$ |
80 |
|
|
$ |
- |
|
|
$ |
- |
|
The Company subleases certain of its headquarters facilities to the Sino-American Cancer Foundation. The Company recognized $28,300 and $11,500 in 2016 and 2015, respectively, as consideration for this sublease. Dr. Yun Yen, who is a member of our board of directors and a stockholder, serves as the President and Chairman of the Board for the Sino-American Cancer Foundation. See Note 14, Related Party, to our consolidated financial statements for additional information.
Critical Accounting Policies and Use of Estimates
This discussion and analysis is based on our consolidated financial statements, which have been prepared in accordance with generally accepted accounting principles in the United States of America, or U.S. GAAP. The preparation of consolidated financial statements in accordance with U.S. GAAP requires management to make certain estimates, judgments and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenue and expenses during the reporting periods. These estimates, judgments and assumptions are based on historical data and experience available at the date of the consolidated financial statements, as well as various other factors management believes to be reasonable under the circumstances. Actual results could differ from these estimates.
56
While our significant accounting policies are described in more detail in the notes to the consolidated financial statements included in this report, we believe the accounting policies discussed below used in the preparation of our financial statements require the most significant estimates, judgments and assumptions.
We generate revenue from sales of our genetic tests. We currently receive payments from: hospitals and medical institutions with which we have direct-bill relationships; research institutions; individual patients and third-party payors.
We recognize revenue when all of the following criteria are met: (i) persuasive evidence of an arrangement exists; (ii) delivery has occurred; (iii) the fee is fixed or determinable and (iv) collectability is reasonably assured. Criterion (i) is satisfied when we have an arrangement or contract in place. Criterion (ii) is satisfied when we deliver a report to the ordering physician or test results to the research institution. Determination of criteria (iii) and (iv) are based on management’s judgments regarding whether the fee is fixed or determinable, and whether the collectability of the fee is reasonably assured. We recognize revenue on a cash basis when we cannot conclude that either criterion (iii) or (iv) has been met.
Our test results are delivered electronically, and as such there are no shipping and handling fees incurred by us or billed to customers. Our sales are typically exempt from state sales taxation due to the nature of the results delivered. As a result, we do not charge customers state sales tax.
We have included equity-based compensation expense as part of cost of revenue and operating expenses in our consolidated statements of operations as follows:
|
|
Year Ended December 31, |
|
|||||
|
2016 |
|
|
2015 |
|
|||
|
|
(in thousands) |
|
|||||
|
Cost of revenue |
$ |
754 |
|
|
$ |
1,673 |
|
|
Research and development |
|
1,161 |
|
|
|
3,241 |
|
|
Selling and marketing |
|
454 |
|
|
|
1,569 |
|
|
General and administrative |
|
2,285 |
|
|
|
1,673 |
|
|
Total |
$ |
4,654 |
|
|
$ |
8,156 |
|
We also recorded equity-based compensation expense of $0, $120,000 and $0 related to the Pharma Business in 2016, 2015 and 2014, respectively, which amounts are recorded in discontinued operations for the respective periods.
We account for equity-based compensation arrangements with our employees, consultants and non-employee directors using a fair value method, which requires us to recognize compensation expense for costs related to all equity-based awards. Prior to the Reorganization, our equity-based awards included fully vested equity-based awards, including common units subject to profits interest thresholds, and option and RSU awards subject to time-based vesting and, for option awards, exercisability restrictions until a liquidity event or incorporation of Fulgent LLC, each as defined in the equity incentive plan under which the awards were granted. For purposes of these option awards, an incorporation was deemed to have occurred upon completion of the Reorganization, at which time the options became immediately exercisable, to the extent vested. Following the Reorganization, our equity-based awards have included option and restricted stock unit, or RSU, awards subject to time-based vesting. The fair value method requires us to estimate the fair value of equity-based awards to employees and non-employees on the date of grant, and we have utilized the Black-Scholes option-pricing model to make this estimation. The fair value is then recognized as equity-based compensation expense over the requisite service period, which is typically the vesting period, of the award. For fully vested equity-based awards, the entire fair value is recognized as equity-based compensation expense in the period the award is granted. Equity-based awards granted to non-employees are subject to periodic remeasurement over their vesting term.
The Black-Scholes option-pricing model requires the input of subjective assumptions, including the expected term of the option or other award, risk-free interest rates, assumed dividend yield of the underlying equity, expected volatility of the price of the underlying equity and the fair value of the underlying equity, as follows:
57
|
|
• |
Risk-Free Interest Rate. We determine the risk-free interest rate by using the equivalent to the expected term based on the U.S. Treasury yield curve in effect as of the date of grant. |
|
|
• |
Dividend Yield. The assumed dividend yield is based on our expectation that we will not pay dividends in the foreseeable future, which is consistent with its history of not paying dividends. |
We did not grant any equity-based awards prior to October 2015. For the years ended December 31, 2016 and 2015, we estimated the fair value of options and awards subject to profits interest thresholds granted prior to the Reorganization at their respective grant dates using the following assumptions:
|
|
|
Year Ended December 31, |
|
|||||||||||||
|
|
2016 |
|
|
2015 |
|
|||||||||||
|
|
|
Employee |
|
|
Non-Employee |
|
|
Employee |
|
|
Non-Employee |
|
||||
|
Expected term (in years) |
|
6.1 |
|
|
10 |
|
|
6.1 |
|
|
10 |
|
||||
|
Risk-free interest rates |
|
|
1.4% |
|
|
|
1.6% |
|
|
|
1.6% |
|
|
|
2.3% |
|
|
Dividend yield |
|
0 |
|
|
0 |
|
|
0 |
|
|
0 |
|
||||
|
Expected volatility |
|
|
95.6% |
|
|
|
96.9% |
|
|
|
86.0% |
|
|
|
94.8% |
|
Profits Interests:
|
|
|
|
|
|
|
|
Year Ended December 31, 2015 |
|
||
|
|
|
Employee(1) |
|
|
|
Expected term (in years) |
|
2 |
|
|
|
Risk-free interest rates |
|
|
0.6% |
|
|
Dividend yield |
|
0 |
|
|
|
Expected volatility |
|
|
68.1% |
|
(1) We did not grant any awards subject to profits interest thresholds to non-employees during the year ended December 31, 2015.
We did not grant any equity-based awards subject to profit interest thresholds during the year ended December 31, 2016.
There is a high degree of subjectivity involved when using option-pricing models to estimate equity-based compensation. There is not currently a market-based mechanism or other practical application to verify the reliability and accuracy of the estimates stemming from these valuation models, nor is there a means to compare and adjust the estimates to actual values. Although the fair value of equity-based awards is determined using an option-pricing model, this value may not be indicative of the fair value that would be observed in a market transaction between a willing buyer and willing seller. If factors change and different assumptions are used when valuing our options or other equity-based awards, our equity-based compensation expense could be materially different in the future.
58
Determination of the Fair Value on Grant Dates
Historically, for all periods prior to our initial public offering, the fair value of the common units underlying our equity-based awards were estimated on each grant date by our Manager. In determining fair value, our Manager considered valuations prepared by an independent third party in a manner consistent with the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation, also known as the Practice Aid. In conducting the valuations, we considered all objective and subjective factors that we believed to be relevant in each valuation conducted, including management’s best estimate of our business condition, prospects and operating performance at each valuation date. Within the valuations, a range of factors, assumptions and methodologies were used. The significant factors included:
|
|
• |
the fact that we were a privately held company with illiquid securities; |
|
|
• |
our stage of commercialization; |
|
|
• |
the likelihood of achieving a liquidity event for our equity, such as an initial public offering, given prevailing market conditions; |
|
|
• |
our historical operating results; |
|
|
• |
valuations of comparable public companies; |
|
|
• |
our discounted future cash flows, based on our projected operating results; and |
|
|
• |
our capital structure, including the rights and preferences of our various classes of equity. |
There are significant judgments and estimates inherent in these valuations. These judgments and estimates include assumptions regarding our future operating performance, stage of commercial growth, average selling price, continued penetration into hospital and medical institution customers, reimbursement from third-party payors, the timing of a potential initial public offering or other liquidity event and the determination of the appropriate valuation method at each valuation date. If we had made different assumptions, our equity-based compensation expense, income (loss) applicable to common unitholders and income (loss) per unit applicable to common unitholders could have been materially different.
The valuations utilized the market approach, the income approach or a combination of both. The market approach and the income approach are both acceptable valuation methods in accordance with the Practice Aid. There are three general methodologies under the market approach:
|
|
• |
Guideline Company Method. This method involves the identification and analysis of publicly traded companies that are comparable to the subject company. Pricing multiples of the publicly traded companies are applied to representative financial metrics of the subject company. |
|
|
• |
Similar Transaction Method. This method includes the identification of transactions in which the targets are comparable to the subject company. This method can also include identification of transactions completed by the most likely buyers in the subject company’s industry. Transaction multiples from the identified transactions are applied to the representative financial metrics of the subject company. |
|
|
• |
Precedent Transaction Method. By considering the sale price of equity in a recent financing, the equity value can be “backsolved” using an option-pricing model that gives consideration to a company’s capitalization structure and rights of preferred and common equity holders. |
Under the income approach, enterprise value can be estimated using the discounted cash flow, or DCF, method, which assumes:
|
|
• |
a business is worth today what it can generate in future cash to its owners; |
|
|
• |
cash received today is worth more than an equal amount of cash received in the future; and |
|
|
• |
future cash flows can be reasonably estimated. |
The DCF analysis is comprised of the sum of the present value of two components: discrete period projected cash flows and a residual or terminal value.
Additionally, each valuation reflects a marketability discount, resulting from the illiquidity of our common units prior to completion of our initial public offering.
As provided in the Practice Aid, there are several approaches for allocating enterprise value of a privately held company among the securities held in a complex capital structure. The possible methodologies include the probability-weighted expected return method, or PWERM, the option-pricing method, or OPM, the current-value method or a hybrid of the PWERM and the OPM, which
59
is referred to as the hybrid method. Under the PWERM, equity is valued based upon the probability-weighted present value of expected future returns, considering various future outcomes available to us, as well as the rights of each class of equity. The OPM treats common equity and preferred equity as call options on the enterprise’s value. The exercise prices associated with these call options vary according to the liquidation preference of the preferred equity, the preferred equity conversion price, the exercise prices of common equity options and other features of a company’s equity capital structure. The current-value method, which is generally only used for early stage companies, is based on first determining enterprise value using a market, income or asset-based approach, and then allocating that value to the preferred equity-based on its liquidation preference or conversion value, whichever would be greater.
The valuation of Class D common units related to awards of Class D common units and options to acquire Class D common units granted in 2015 incorporated the income approach (Gordon Growth Analysis) and the market approach (Guideline Public Company Method) in determining value, and we applied 50% weight to each approach, applying a 35% discount for lack of marketability. For the valuation of Class D common units related to awards of Class D common units and options to acquire Class D common units granted in 2016 prior to completion of our initial public offering, we incorporated the PWERM and utilized the market approach (Precedent Transactions Method) incorporating the Xi Long financing, applying a 20% discount for lack of marketability.
The valuation of Class P units related to awards of Class P units and options to acquire Class P units granted in the year ended December 31, 2015 incorporated the market approach (Precedent Transactions Method), utilizing OPM to backsolve.
After completion of our initial public offering on October 4, 2016, the fair value of shares of our common stock underlying stock option grants is determined by our board of directors or the compensation committee thereof based on the closing price of our common stock on the date of grant as reported by the NASDAQ Global Market.
Recent Accounting Pronouncements
See Note 2, Summary of Significant Accounting Policies, to our consolidated financial statements regarding recent accounting pronouncements.
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable to public companies that are not emerging growth companies, including an extended transition period to comply with new or revised accounting standards applicable to public companies. We have chosen to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable. See Note 2, Summary of Significant Accounting Policies, to our consolidated financial statements regarding recent accounting pronouncements. We would cease to be an emerging growth company on the date that is the earliest of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (ii) December 31, 2020; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the Securities and Exchange Commission, that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to various market risks in the ordinary course of our business. As of December 31, 2016, we had $7.9 million of cash and cash equivalents, which consists of bank deposits and money market funds, and $38.6 million in marketable securities, which consist of corporate bonds. Such interest-bearing instruments carry a degree of risk; however, we have not been exposed to, nor do we anticipate being exposed to, material risks due to changes in interest rates. A hypothetical 10% change in interest rates during any of the periods presented in this report would not have had a material impact on our financial results.
Revenue from sales outside of the United States represented 55% of our revenue in 2016. Currently, our revenue-producing transactions are primarily denominated in U.S. dollars; however, as we continue to expand internationally, our results of operations
60
and cash flows may increasingly become subject to fluctuations due to changes in foreign currency exchange rates. In periods when the U.S. dollar declines in value as compared to foreign currencies in which we incur expenses, our foreign-currency based expenses will increase when translated into U.S. dollars. In addition, future fluctuations in the value of the U.S. dollar may affect the price at which we sell our tests outside the United States. To date, our foreign currency risk has been minimal and we have not historically hedged our foreign currency risk, although we may consider doing so in the future.
61
Item 8. Financial Statements and Supplementary Data.
The information required by this Item 8 is included immediately following the signature page to this report on pages F-1 through F-25 and is incorporated herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
As required by Rule 13a-15(b) under the Exchange Act, our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2016. Disclosure controls and procedures are controls and other procedures of a company that are designed to ensure that information required to be disclosed by the company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate, to allow timely decisions regarding required disclosure. Based on the evaluation of our disclosure controls and procedures as of December 31, 2016, our Chief Executive Officer and Chief Financial Officer have concluded that, as of December 31, our disclosure controls and procedures were effective.
Management recognizes that any controls and procedures, no matter how well-designed and operated, can provide only reasonable assurance of achieving their objectives, and management necessarily applies its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Management’s Annual Report on Internal Control over Financial Reporting
This report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of our independent registered public accounting firm due to transition periods established by SEC rules and the JOBS Act applicable to newly public companies and emerging growth companies.
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting during the quarter ended December 31, 2016, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Pursuant to the approval of our board of directors, we will hold our first annual meeting of stockholders as a publicly traded company, or the 2017 Annual Meeting, on May 16, 2017. Pursuant to the advance notice requirements in our bylaws and applicable SEC requirements, including Rule 14a 8 under the Exchange Act, any stockholder proposals sought to be brought before our 2017 Annual Meeting must be submitted in a written notice that is delivered to or mailed and received at the address of our principal executive offices no later than the close of business on March 30, 2017, which is the 15th day following this public announcement of the date of the 2017 Annual Meeting. Any such notice must also comply with all other requirements of Rule 14a 8 under the Exchange Act, including delivery of proof of ownership of our common stock in accordance with Rule 14a 8(b)(2), and must set forth, as to each proposal the stockholder seeks to bring before the 2017 Annual Meeting, all of the information required by our bylaws.
62
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2017 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2016.
Item 11. Executive Compensation.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2017 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2016.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2017 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2016.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2017 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2016.
Item 14. Principal Accounting Fees and Services.
The information required by this item is incorporated by reference to the definitive proxy statement for our 2017 annual meeting of stockholders or an amendment to this report, in either case to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2016.
63
Item 15. Exhibits, Financial Statement Schedules.
(a)(1)Consolidated Financial Statements.
The following financial statements are included immediately following the signature page hereof and are filed as part of this report:
(a)(2)Financial Statement Schedules.
All financial statement schedules have been omitted, as they are not required, not applicable, or the required information is otherwise included.
(a)(3)Exhibits.
The information required by this Item 15(a)(3) is set forth on the Exhibit Index immediately following the last page of this report and is incorporated herein by reference.
We have elected not to provide summary information.
64
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
FULGENT GENETICS, INC. |
|
|
|
|
|
|
|
Date: March 16, 2017 |
|
By: |
/s/ Ming Hsieh |
|
|
|
|
Ming Hsieh |
|
|
|
|
President, Chief Executive Officer |
POWER OF ATTORNEY
IN WITNESS WHEREOF, each person whose signature appears below constitutes and appoints Ming Hsieh and Paul Kim as his true and lawful agent, proxy and attorney-in-fact, each acting alone, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to (i) act on and sign any amendments to this report, with exhibits thereto and other documents in connection therewith, (ii) act on and sign such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith, and in each case file the same with the SEC, hereby approving, ratifying and confirming all that such agent, proxy and attorney-in-fact or any of his substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
|
Name and Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Ming Hsieh |
|
President, Chief Executive Officer and Chairman of the Board |
|
March 16, 2017 |
|
Ming Hsieh |
|
(principal executive officer) |
|
|
|
|
|
|
|
|
|
/s/ Paul Kim |
|
Chief Financial Officer |
|
March 16, 2017 |
|
Paul Kim |
|
(principal financial and accounting officer) |
|
|
|
|
|
|
|
|
|
/s/John Bolger |
|
Director |
|
March 16, 2017 |
|
John Bolger |
|
|
|
|
|
|
|
|
|
|
|
/s/ James J. Mulay (Mulé) |
|
Director |
|
March 16, 2017 |
|
James J. Mulay (Mulé) |
|
|
|
|
|
|
|
|
|
|
|
/s/ Yun Yen |
|
Director |
|
March 16, 2017 |
|
Yun Yen |
|
|
|
|
65
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
Fulgent Genetics, Inc.
Temple City, California
We have audited the accompanying consolidated balance sheets of Fulgent Genetics, Inc. and its subsidiaries (the “Company”) as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive loss, stockholders’/members’ equity, and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of Fulgent Genetics, Inc. and its subsidiaries at December 31, 2016 and 2015, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
/s/ DELOITTE & TOUCHE LLP
Los Angeles, California
March 16, 2017
F-2
CONSOLIDATED FINANCIAL STATEMENTS
FULGENT GENETICS, INC.
(in thousands, except par value data)
|
|
December 31, |
|
||||
|
|
2016 |
|
2015 |
|
||
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
|
Cash and cash equivalents |
$ |
7,897 |
|
$ |
489 |
|
|
Marketable securities |
|
12,971 |
|
|
— |
|
|
Trade accounts receivable, net of allowance for doubtful accounts of $151 and $75, as of December 31, 2016 and 2015, respectively |
|
4,364 |
|
|
2,118 |
|
|
Other current assets |
|
906 |
|
|
314 |
|
|
Current assets of discontinued operations |
|
— |
|
|
9 |
|
|
Total current assets |
|
26,138 |
|
|
2,930 |
|
|
Marketable securities, long term |
|
25,597 |
|
|
— |
|
|
Fixed assets, net |
|
6,234 |
|
|
2,469 |
|
|
Deferred tax asset |
|
54 |
|
|
— |
|
|
Other long-term assets |
|
17 |
|
|
— |
|
|
Non-current assets of discontinued operations |
|
— |
|
|
433 |
|
|
|
|
31,902 |
|
|
2,902 |
|
|
Total assets |
$ |
58,040 |
|
$ |
5,832 |
|
|
Liabilities and Stockholders’/ Members’ Equity |
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
Accounts payable |
$ |
2,756 |
|
$ |
314 |
|
|
Accrued liabilities |
|
436 |
|
|
199 |
|
|
Income tax payable |
|
124 |
|
|
— |
|
|
Current liabilities of discontinued operations |
|
— |
|
|
173 |
|
|
Total current liabilities |
|
3,316 |
|
|
686 |
|
|
Other long-term liabilities |
|
2 |
|
|
— |
|
|
Deferred tax liability |
|
243 |
|
|
— |
|
|
Total liabilities |
|
3,561 |
|
|
686 |
|
|
Commitments and contingencies (Note 8) |
|
|
|
|
|
|
|
Stockholders’/ members’ equity |
|
|
|
|
|
|
|
Units 56,000 Class D and 51,000 Class P preferred units authorized, issued and outstanding, 44,000 Class D and 49,000 Class P common units authorized and 34,000 Class D and 45,000 Class P common units issued and outstanding, at December 31, 2015 (Note 9) |
|
— |
|
|
58,306 |
|
|
Common stock, $0.0001 par value per share, 200,000 shares authorized, 17,676 shares issued and outstanding at December 31, 2016 |
|
2 |
|
|
— |
|
|
Preferred stock, $0.0001 par value per share, 1,000 shares authorized, no shares issued or outstanding at December 31, 2016 |
|
— |
|
|
— |
|
|
Additional paid-in capital |
|
109,734 |
|
|
|
|
|
Accumulated other comprehensive loss |
|
(103 |
) |
|
— |
|
|
Accumulated deficit |
|
(55,154 |
) |
|
(53,160 |
) |
|
Total stockholders’/ members’ equity |
|
54,479 |
|
|
5,146 |
|
|
Total liabilities and stockholders’/ members’ equity |
$ |
58,040 |
|
$ |
5,832 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Consolidated Statements of Operations
(in thousands, except per share data)
|
|
Year Ended December 31, |
|
|||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Revenue |
$ |
18,276 |
|
|
$ |
9,576 |
|
|
$ |
1,278 |
|
|
Cost of revenue |
|
6,722 |
|
|
|
5,069 |
|
|
|
936 |
|
|
Gross profit |
|
11,554 |
|
|
|
4,507 |
|
|
|
342 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
3,558 |
|
|
|
4,431 |
|
|
|
521 |
|
|
Selling and marketing |
|
2,469 |
|
|
|
2,670 |
|
|
|
581 |
|
|
General and administrative |
|
4,609 |
|
|
|
2,418 |
|
|
|
230 |
|
|
Total operating expenses |
|
10,636 |
|
|
|
9,519 |
|
|
|
1,332 |
|
|
Operating income (loss) |
|
918 |
|
|
|
(5,012 |
) |
|
|
(990 |
) |
|
Interest and other income (expense) |
|
(5,386 |
) |
|
|
27 |
|
|
|
— |
|
|
Income (loss) before income taxes |
|
(4,468 |
) |
|
|
(4,985 |
) |
|
|
(990 |
) |
|
Provision for income taxes |
|
920 |
|
|
|
— |
|
|
|
— |
|
|
Income (loss) from continuing operations |
|
(5,388 |
) |
|
|
(4,985 |
) |
|
|
(990 |
) |
|
Income (loss) from discontinued operations |
|
41 |
|
|
|
(3,329 |
) |
|
|
(3,293 |
) |
|
Net income (loss) |
$ |
(5,347 |
) |
|
$ |
(8,314 |
) |
|
$ |
(4,283 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted income (loss) per common share: |
|
|
|
|
|
|
|
|
|
|
|
|
Continuing operations—common stock |
$ |
(1.00 |
) |
|
$ |
(0.61 |
) |
|
* |
|
|
|
Continuing operations: |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average common shares—outstanding—basic and diluted |
|
13,710 |
|
|
|
11,842 |
|
|
|
|
|
* Basic and diluted income (loss) per common share was calculated prospectively from the date the Class D common units were issued in the Recapitalization in October 2015. See Note 1, Overview and Basis of Presentation, and Note 12, Income (Loss) per Share, to these consolidated financial statements for additional information.
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Consolidated Statements of Comprehensive Loss
(in thousands)
|
|
Year Ended December 31, |
|
|||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Net loss |
$ |
(5,347 |
) |
|
$ |
(8,314 |
) |
|
$ |
(4,283 |
) |
|
Other comprehensive loss, net of tax: |
|
|
|
|
|
|
|
|
|
|
|
|
Net unrealized loss on marketable securities |
|
(103 |
) |
|
|
— |
|
|
|
— |
|
|
Comprehensive loss |
$ |
(5,450 |
) |
|
$ |
(8,314 |
) |
|
$ |
(4,283 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Consolidated Statements of Stockholders’/Members’ Equity
(in thousands)
|
|
|
Members' Equity |
|
|
Stockholders' Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
Units |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Additional Paid-In Capital |
|
|
Accumulated Other comprehensive Loss |
|
|
Accumulated Deficit |
|
|
Total Equity |
|
||||||||
|
Balance at December 31, 2013 |
|
|
1 |
|
|
$ |
8,000 |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
$ |
(6,033 |
) |
|
$ |
1,967 |
|
|
Capital contribution |
|
|
|
|
|
|
4,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,000 |
|
|
Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4,283 |
) |
|
|
(4,283 |
) |
|
Balance at December 31, 2014 |
|
|
1 |
|
|
$ |
12,000 |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
(10,316 |
) |
|
$ |
1,684 |
|
|
Capital contribution |
|
|
|
|
|
|
3,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,500 |
|
|
Recapitalization and deemed distribution |
|
|
153,999 |
|
|
|
34,530 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(34,530 |
) |
|
|
— |
|
|
Equity-based compensation |
|
|
32,000 |
|
|
|
8,276 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8,276 |
|
|
Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(8,314 |
) |
|
|
(8,314 |
) |
|
Balance at December 31, 2015 |
|
|
186,000 |
|
|
$ |
58,306 |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
(53,160 |
) |
|
$ |
5,146 |
|
|
Split-off of Pharma business |
|
|
(96,000 |
) |
|
|
(12,390 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11,900 |
|
|
|
(490 |
) |
|
Issuance of Class D-2 preferred units (net of $185 issuance costs) |
|
|
15,395 |
|
|
|
32,452 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32,452 |
|
|
Repurchase and retirement of Class D-1 preferred units |
|
|
(4,618 |
) |
|
|
(1,663 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1,663 |
) |
|
Deemed dividend on retirement of Class D-1 preferred units |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(3,727 |
) |
|
|
(3,727 |
) |
|
Repurchase and retirement of Class D common units |
|
|
(5,645 |
) |
|
|
(1,767 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4,820 |
) |
|
|
(6,587 |
) |
|
Equity-based compensation (Pre-Reorganization) |
|
|
2,500 |
|
|
|
2,978 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,978 |
|
|
Distribution to Class D-1 preferred unitholder |
|
|
— |
|
|
|
(4,592 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4,592 |
) |
|
Tax distribution to Class D common and preferred unitholders |
|
|
|
|
|
|
(1,253 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1,253 |
) |
|
Reorganization (Note 1) |
|
|
(97,632 |
) |
|
|
(72,071 |
) |
|
|
12,846 |
|
|
|
2 |
|
|
|
72,069 |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
Equity-based compensation (Post-Reorganization) |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
1,676 |
|
|
|
|
|
|
|
|
|
|
|
1,676 |
|
|
Issuance of stock in initial public offering, net of offering costs |
|
|
|
|
|
|
|
|
|
|
4,830 |
|
|
|
|
|
|
|
35,989 |
|
|
|
|
|
|
|
|
|
|
|
35,989 |
|
|
Other comprehensive loss, net |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(103 |
) |
|
|
|
|
|
|
(103 |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(5,347 |
) |
|
|
(5,347 |
) |
|
Balance at December 31, 2016 |
|
|
— |
|
|
$ |
— |
|
|
|
17,676 |
|
|
$ |
2 |
|
|
$ |
109,734 |
|
|
$ |
(103 |
) |
|
$ |
(55,154 |
) |
|
$ |
54,479 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Consolidated Statements of Cash Flows
(in thousands)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Cash flow from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(5,347 |
) |
|
$ |
(8,314 |
) |
|
$ |
(4,283 |
) |
|
Income (loss) from discontinued operations |
|
|
41 |
|
|
|
(3,329 |
) |
|
|
(3,293 |
) |
|
Loss from continuing operations |
|
|
(5,388 |
) |
|
|
(4,985 |
) |
|
|
(990 |
) |
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity-based compensation |
|
|
4,654 |
|
|
|
8,156 |
|
|
|
— |
|
|
Depreciation and amortization |
|
|
1,170 |
|
|
|
575 |
|
|
|
196 |
|
|
Gain on disposal of fixed assets |
|
|
— |
|
|
|
(20 |
) |
|
|
— |
|
|
Provision for bad debt |
|
|
76 |
|
|
|
48 |
|
|
|
33 |
|
|
Deferred income taxes |
|
|
246 |
|
|
|
— |
|
|
|
— |
|
|
Amortization of premium on marketable securities |
|
|
63 |
|
|
|
— |
|
|
|
— |
|
|
Fair value adjustment recorded upon issuance of D-2 preferred units |
|
|
5,472 |
|
|
|
— |
|
|
|
— |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(2,322 |
) |
|
|
(1,779 |
) |
|
|
(416 |
) |
|
Other current assets |
|
|
(381 |
) |
|
|
(163 |
) |
|
|
(138 |
) |
|
Accounts payable |
|
|
1,094 |
|
|
|
132 |
|
|
|
150 |
|
|
Income taxes payable |
|
|
124 |
|
|
|
— |
|
|
|
0 |
|
|
Accrued liabilities |
|
|
(372 |
) |
|
|
62 |
|
|
|
81 |
|
|
Cash provided by (used in) continuing operations |
|
|
4,436 |
|
|
|
2,026 |
|
|
|
(1,084 |
) |
|
Cash used in discontinued operations |
|
|
(31 |
) |
|
|
(2,995 |
) |
|
|
(3,313 |
) |
|
Net cash provided by (used in) operating activities |
|
|
4,405 |
|
|
|
(969 |
) |
|
|
(4,397 |
) |
|
Cash flow from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from disposal of fixed assets |
|
|
10 |
|
|
|
70 |
|
|
|
— |
|
|
Purchases of fixed assets |
|
|
(3,788 |
) |
|
|
(2,100 |
) |
|
|
(731 |
) |
|
Purchase of marketable securities |
|
|
(39,017 |
) |
|
|
— |
|
|
|
— |
|
|
Cash used in continuing operations |
|
|
(42,795 |
) |
|
|
(2,030 |
) |
|
|
(731 |
) |
|
Cash used in discontinued operations |
|
|
— |
|
|
|
(175 |
) |
|
|
(49 |
) |
|
Net cash used in investing activities |
|
|
(42,795 |
) |
|
|
(2,205 |
) |
|
|
(780 |
) |
|
Cash flow from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock, net of offering costs |
|
|
36,790 |
|
|
|
— |
|
|
|
— |
|
|
Cash distributed in split-off of Pharma business |
|
|
(159 |
) |
|
|
— |
|
|
|
— |
|
|
Capital contributions |
|
|
— |
|
|
|
3,500 |
|
|
|
4,000 |
|
|
Distribution to Class D-1 preferred unitholder |
|
|
(4,592 |
) |
|
|
— |
|
|
|
— |
|
|
Issuance of Class D-2 preferred units, net of issuance costs |
|
|
26,980 |
|
|
|
— |
|
|
|
— |
|
|
Repurchase and retirement of Class D-1 preferred and Class D common units |
|
|
(11,977 |
) |
|
|
— |
|
|
|
— |
|
|
Tax distribution to Class D common and preferred unitholders |
|
|
(1,253 |
) |
|
|
— |
|
|
|
— |
|
|
Net cash provided by financing activities |
|
|
45,789 |
|
|
|
3,500 |
|
|
|
4,000 |
|
|
Net increase (decrease) in cash |
|
|
7,399 |
|
|
|
326 |
|
|
|
(1,177 |
) |
|
Cash balance at beginning of period (including $9 and $0 at January 1, 2016 and 2015, respectively, from discontinued operations) |
|
498 |
|
|
|
172 |
|
|
|
1,349 |
|
|
|
Cash balance at end of period (including $0 and $9 at December 31, 2016 and 2015, respectively, from discontinued operations) |
$ |
7,897 |
|
|
$ |
498 |
|
|
$ |
172 |
|
|
|
Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Income taxes paid |
|
$ |
550 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Supplemental disclosures of non-cash investing and financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Recapitalization |
|
$ |
— |
|
|
$ |
34,530 |
|
|
$ |
— |
|
|
Fixed assets included in accounts payable |
|
$ |
1,173 |
|
|
$ |
17 |
|
|
$ |
— |
|
|
Initial public offering costs included in accounts payable and accrued expenses |
|
$ |
801 |
|
|
$ |
— |
|
|
$ |
— |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Notes to Consolidated Financial Statements
Note 1. Overview and Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Nature of the Business
Fulgent Genetics, Inc., together with its subsidiaries (collectively referred to as the “Company,” unless otherwise noted or the context otherwise requires), is a rapidly growing technology company with an initial focus on offering comprehensive genetic testing to provide physicians with clinically actionable diagnostic information they can use to improve the overall quality of patient care (the “Diagnostics business”). The Company has developed a proprietary technology platform that allows it to offer a broad and flexible test menu and continually expand and improve its proprietary genetic reference library. The Company’s current test menu offers single-gene tests and pre-established, multi-gene, disease-specific panels that collectively test for many genetic conditions, including various cancers, cardiovascular diseases, neurological disorders and pediatric tests. The Company’s existing customer base consists primarily of hospitals and medical institutions, which are frequent and high-volume users of genetic tests and which typically pay the Company directly for its tests.
Background and Reorganization
The Company was incorporated in the State of Delaware on May 13, 2016. On August 2, 2016, pursuant to the approval of the board of directors of the Company, the Company changed its name from Fulgent Diagnostics, Inc. to Fulgent Genetics, Inc. On September 30, 2016, the Company completed a reorganization pursuant to which Fulgent Therapeutics LLC, a California limited liability company (referred to, together with its former subsidiary unless otherwise noted or the context otherwise requires, as “Fulgent LLC”), became a wholly owned subsidiary of the Company (the “Reorganization”). Prior to the Reorganization, the Company had no material assets and had not conducted any activities other than those incidental to its incorporation and preparation for the initial public offering of its common stock. Following the Reorganization, the Company is a holding company with no material assets other than 100% of the equity interests in Fulgent LLC and its other subsidiaries, and Fulgent LLC is considered the Company’s predecessor for accounting purposes and its financial statements for all periods prior to completion of the Reorganization constitute the Company’s historical financial statements.
For purposes of these notes and the accompanying consolidated financial statements: (i) Fulgent LLC’s operating agreement, as amended from time to time, is referred to as the “Operating Agreement;” (ii) Fulgent LLC’s equity holders are referred to as “members;” (iii) Fulgent LLC’s authorized, issued and outstanding equity interests prior to the Reorganization are referred to as “units” and consisted of Class D common units and Class D-1 and Class D-2 preferred units; (iv) certain of Fulgent LLC’s Class D common units outstanding prior to the Reorganization constituted profits interests (which are sometimes referred to simply as “profits interests”), which are a type of equity-based award containing a participation threshold (which is sometimes referred to as a “profits interest threshold”) that entitled the recipient of the award to participate in the value of Fulgent LLC only to the extent it appreciated from and after the grant date of the award; and (v) prior to the Reorganization, Fulgent LLC was managed by its Manager, Ming Hsieh, who was also Fulgent LLC’s controlling equity holder. In the Reorganization, each outstanding 7.6 units of Fulgent LLC were cancelled in exchange for one share of the Company’s common stock, such that (i) all outstanding Class D common units of Fulgent LLC (including profits interests) were cancelled in exchange for an aggregate of 4,059,900 shares of the Company’s common stock; (ii) all outstanding Class D-1 preferred units of Fulgent LLC were cancelled in exchange for an aggregate of 6,760,733 shares of the Company’s common stock; (iii) all outstanding Class D-2 preferred units were cancelled in exchange for an aggregate of 2,025,623 shares of the Company’s common stock; (iv) all outstanding options to acquire common units of Fulgent LLC were cancelled in exchange for equivalent options to acquire up to an aggregate of 591,112 shares of the Company’s common stock, and all such options became immediately exercisable to the extent vested; and (v) all outstanding restricted share units relating to common units of Fulgent LLC were cancelled in exchange for equivalent restricted stock units (“RSUs”) relating to 65,789 shares of the Company’s common stock. The Reorganization was accounted for as a common control transaction and no gain or loss was recorded.
Recapitalization and Discontinued Operations
The Company historically conducted two lines of business: the Diagnostics business, which the Company conducted directly and which is the only business it is presently pursuing, and its former pharmaceutical business (the “Pharma business”), which was conducted by the Company directly until the creation of its wholly owned subsidiary, Fulgent Pharma LLC (“Fulgent Pharma”), in 2015, after which the Pharma business was conducted by Fulgent Pharma.
F-8
In October 2015, the Company was recapitalized by canceling the then-existing Class A and Class B units and authorizing and issuing equity interests separated into two series based on these two lines of business (the “Recapitalization”). The holders of the Company’s Class D preferred units and Class D voting and non-voting common units had economic rights based on the Diagnostics business, and the holders of the Company’s Class P preferred units and Class P voting and non-voting common units had economic rights based on the Pharma business. The Class D and Class P units that were created by the Recapitalization, sometimes referred to as “tracking” units, were intended to “track,” or reflect, the relative performance of the Diagnostics business and the Pharma business, respectively. After the Recapitalization, there was no single security that represented the performance of the Company as a whole.
In the Recapitalization, the holders of Class A units received both Class D and Class P preferred units and the holders of Class B units received both Class D and Class P common units. All of the Class D common units issued in the Recapitalization were subject to a profits interest threshold. In the Recapitalization, the number of units and ownership interests held by each equity holder changed and the nature of the units changed from units that track the performance of the Company as a whole to units that track the separate businesses. As a result, the Recapitalization was accounted for as the extinguishment of Class A and Class B units and the issuance of Class D and Class P preferred and common units, based on the Company’s application of the qualitative approach. The Class D and Class P preferred and common units were recorded at their fair value with the difference between the fair value and carrying value of $34.5 million being recorded as a deemed distribution to Class A and Class B units attributable to the period prior to the issuance of the Class D and Class P units.
In April 2016, Fulgent LLC’s Operating Agreement was amended and restated to provide for the distribution of Fulgent Pharma in full redemption and cancellation of Fulgent LLC’s former Class P preferred and common units (collectively, the “Class P units”). On April 4, 2016, the Company completed the split-off of Fulgent Pharma and the Pharma business by redeeming all of the then-outstanding Class P units, distributing to each holder of Class P units substantially identical shares of Fulgent Pharma and causing Fulgent Pharma to assume all then-outstanding options to purchase Class P units. All Class P units were immediately cancelled upon redemption. The split-off of the Pharma business was a pro-rata distribution to all of the holders of Class P units, but did not involve the holders of Fulgent LLC’s Class D common or preferred units. The Manager and controlling equity holder of Fulgent LLC prior to the Reorganization, Mr. Hsieh, is the Manager and controlling equity holder of Fulgent Pharma. As a result, the split-off of the Pharma business was accounted for as a common control transaction and the recorded amount of Fulgent Pharma’s net assets was transferred to the holders of Class P units and no gain or loss was recorded.
The split-off of the Pharma business is presented as discontinued operations in the accompanying consolidated financial statements for all periods presented. Significant asset and liability categories of the Pharma business are disclosed on the accompanying consolidated balance sheets. Significant assets and liabilities of the discontinued operations consist of fixed assets and accounts payable.
The major components of statements of operations data comprising the income (loss) from discontinued operations are as follows:
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
350 |
|
|
$ |
2,217 |
|
|
$ |
3,013 |
|
|
General and administrative |
|
|
9 |
|
|
|
1,112 |
|
|
|
280 |
|
|
Total operating expenses |
|
|
359 |
|
|
|
3,329 |
|
|
|
3,293 |
|
|
Operating Income (loss) |
|
|
(359 |
) |
|
|
(3,329 |
) |
|
|
(3,293 |
) |
|
Other income |
|
|
400 |
|
|
|
— |
|
|
|
— |
|
|
Income (loss) |
|
$ |
41 |
|
|
$ |
(3,329 |
) |
|
$ |
(3,293 |
) |
Initial Public Offering
On October 4, 2016, the Company completed the initial public offering of its common stock (the “IPO”), in which it issued and sold an aggregate of 4,830,000 shares of common stock (including 630,000 shares issued and sold on October 7, 2016 pursuant to the underwriters’ exercise in full of their option to purchase additional shares) at a public offering price of $9.00 per share. The Company received net proceeds of approximately $36.0 million, after deducting underwriting discounts and commissions of $3.0 million and other offering expenses paid or payable by the Company of approximately $4.4 million. The shares issued and sold in the IPO were registered under the Securities Act of 1933, as amended, on a registration statement on Form S-1 (File No. 333- 213469), as amended (the “Registration Statement”).
F-9
Note 2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make certain estimates, judgments and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenue and expenses during the reporting periods. These estimates, judgments and assumptions are based on historical data and experience available at the date of the accompanying consolidated financial statements, as well as various other factors management believes to be reasonable under the circumstances. Actual results could differ from these estimates.
On an on-going basis, management evaluates its estimates, primarily those related to: (i) revenue recognition criteria, (ii) accounts receivable and allowances for doubtful accounts, (iii) the useful lives of fixed assets, (iv) estimates of tax liabilities and (v) the valuation of equity-based awards.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents. Cash and cash equivalents include cash held in banks and money market accounts. Cash equivalents are stated at fair value.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are stated at the amount the Company expects to collect. The Company performs credit evaluations of its customers and generally does not require collateral. The Company establishes an allowance for doubtful accounts based upon factors surrounding the credit risk of specific customers, historical trends and other information that assists in management’s evaluation. The Company writes off accounts receivable following a review by management and a determination that the receivable is uncollectible.
A roll-forward of the activity in the Company’s allowance for doubtful accounts is as follows:
|
|
December 31, |
|
|||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
(in thousands) |
|
|||||||||
|
Allowance for doubtful accounts at beginning of year |
$ |
75 |
|
|
$ |
27 |
|
|
$ |
— |
|
|
Bad debt expense |
|
103 |
|
|
|
48 |
|
|
|
33 |
|
|
Deductions |
|
(27 |
) |
|
|
— |
|
|
|
(6 |
) |
|
Allowance for doubtful accounts at end of year |
$ |
151 |
|
|
$ |
75 |
|
|
$ |
27 |
|
Marketable Securities
All marketable securities, which consist of debt securities, have been classified as “available for sale” and are carried at fair value. Unrealized gains and losses, net of any related tax effects, are excluded from earnings and are included in other comprehensive loss and reported as a separate component of stockholders’ equity until realized. Realized gains and losses and declines in value judged to be other than temporary, if any, on marketable securities are included in other income (expense), net. The cost of any marketable securities sold is based on the specific-identification method. The amortized cost of marketable securities is adjusted for amortization of premiums and accretion of discounts to maturity. Interest on marketable securities is included in interest income. In accordance with the Company’s investment policy, management invests to diversify credit risk and only invests in securities with high credit quality, including U.S. government securities.
The Company regularly evaluates whether declines in the fair value of its investments below their cost are other than temporary. The evaluation includes consideration of the cause of the impairment, including the creditworthiness of the security issuers, the number of securities in an unrealized loss position, the severity and duration of the unrealized losses, whether the Company has the intent to sell the securities, and whether it is more likely than not that the Company will be required to sell the securities before the recovery of their amortized cost basis. If the Company determines that the decline in fair value of an investment is below its accounting basis and this decline is other than temporary, the Company would reduce the carrying value of the security it holds and
F-10
record a loss for the amount of such decline. The Company has not recorded any realized losses or declines in value judged to be other than temporary on its investments.
Fair Value of Financial Instruments
The Company's financial instruments consist principally of cash and cash equivalents, marketable securities, accounts receivable and accounts payable. The carrying amounts of certain of these financial instruments, including cash and cash equivalents, accounts receivable and accounts payable, approximate fair value due to their short maturities. Fair value of marketable securities is disclosed in Note 4, Fair Value Measurements, to our consolidated financial statements.
Concentrations of Credit Risk, Customers and Suppliers
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash, accounts receivable and marketable securities, which consist of debt securities, and cash equivalents. As of December 31, 2016, substantially all of the Company’s cash and cash equivalents were deposited in accounts at financial institutions, and amounts may exceed federally insured limits. Management believes that the Company is not exposed to significant credit risk due to the financial strength of the depository institutions in which its cash and cash equivalents are held.
For the year ended December 31, 2016, four customers comprised more than 10% of total accounts receivable as follows:
|
|
December 31, |
|
|||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Customer A |
|
12% |
|
|
* |
|
|
* |
|
||
|
Customer B |
|
12% |
|
|
|
10% |
|
|
|
10% |
|
|
Customer C |
|
11% |
|
|
* |
|
|
* |
|
||
|
Customer D |
|
11% |
|
|
* |
|
|
* |
|
||
* Less than 10%
For the year ended December 31, 2016, Customers A and B each comprised 15% of total revenue, and Customer B also comprised 19% and 11% of our total revenue in 2015 and 2014, respectively.
Revenue from the U.S. government was less than 10% of total revenue in 2016, 2015 and 2014.
The Company relies on a limited number of suppliers and, in some cases, sole suppliers, for some of its laboratory instruments and materials and it may not be able to find replacements or immediately transition to alternative suppliers if necessary. The Company uses a single supplier for certain laboratory substances used in the chemical reactions incorporated into its processes, referred to as reagents, as well as for sequencers and various other equipment and materials that it uses in its laboratory operations. The Company’s laboratory operations would be interrupted if it encounters delays or difficulties in securing these reagents, sequencers or other equipment or materials or if it needs a substitute or replacement for any of its suppliers and is not able to locate and make arrangements with an acceptable substitute or replacement. The Company believes there are only a few other manufacturers that are currently capable of supplying and servicing the equipment necessary for its laboratory operations, including sequencers and various associated reagents.
Fixed Assets
Fixed assets are recorded at cost, net of accumulated depreciation and amortization. Depreciation is recorded using the straight-line method over the estimated useful lives of the assets, which is generally between three and five years. Leasehold improvements are capitalized and amortized over the shorter of their expected lives or the applicable lease term, including renewal options, if available. Major replacements and improvements are capitalized, while general repairs and maintenance are expensed as incurred.
Software for Internal Use
The Company capitalizes certain costs incurred to purchase computer software for internal use. These costs include purchased software packages for Company use. Capitalized computer software costs are amortized over the estimated useful life of the computer software, which is generally three years. Internally developed software costs are capitalized after management has committed to funding the project, it is probable that the project will be completed and the software will be used for its intended function. Costs that do not meet that criteria and costs incurred on projects in the preliminary and post-implementation phases are expensed as incurred.
F-11
Impairment of Long-Lived Assets
The Company evaluates the carrying amount of its long-lived assets whenever events or changes in circumstances indicate that the assets may not be recoverable. An impairment loss would be recognized when estimated future cash flows expected to result from the use of an asset and its eventual disposition is less than the carrying amount of the asset. To date, there have been no such impairment losses.
Reporting Segment and Geographic Information
Reporting segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company’s chief operating decision maker is its Chief Executive Officer. The Company views its operations and manages its business in one reporting segment.
Revenue Recognition
The Company generates revenue from sales of its genetic tests. The Company currently receives payments from: hospitals and medical institutions with which it has direct-bill relationships; research institutions; individual patients and third-party payors.
The Company recognizes revenue when all of the following criteria are met: (i) persuasive evidence of an arrangement exists; (ii) delivery has occurred; (iii) the fee is fixed or determinable and (iv) collectability is reasonably assured. Criterion (i) is satisfied when the Company has an arrangement or contract in place. Criterion (ii) is satisfied when the Company delivers a report to the ordering physician or test results to the research institution. Determination of criteria (iii) and (iv) are based on management’s judgments regarding whether the fee is fixed or determinable, and whether the collectability of the fee is reasonably assured. The Company recognizes revenue on a cash basis when it cannot conclude that either criterion (iii) or (iv) has been met.
The Company’s test results are delivered electronically, and as such there are no shipping and handling fees incurred by it or billed to customers. The Company’s sales are typically exempt from state sales taxation due to the nature of the results delivered. As a result, the Company currently does not charge customers state sales tax and continues to assess.
Deferred Revenue
Payments received in advance of services rendered are recorded as deferred revenue and are subsequently recognized as revenue in the period in which the applicable revenue recognition criteria, as described above, are met. Deferred revenue consists primarily of revenue from tests performed for customers that have a limited time period following an initial order to request certain follow-up tests at no additional charge.
Overhead Expenses
The Company allocates overhead expenses, such as rent and utilities, to cost of revenue and operating expense categories based on headcount. As a result, an overhead expense allocation is reflected in cost of revenue and each operating expense category.
Cost of Revenue
Cost of revenue reflects the aggregate costs incurred in delivering test results and consists of: personnel costs, including salaries, employee benefit costs, bonuses and equity-based compensation expenses; costs of laboratory supplies; depreciation of laboratory equipment; amortization of leasehold improvements and allocated overhead. Costs associated with performing tests are recorded as tests are processed.
Research and Development Expenses
Research and development expenses represent costs incurred to develop the Company’s technology and future tests. These costs consist of: personnel costs, including salaries, employee benefit costs, bonuses and equity-based compensation expenses; laboratory supplies; consulting costs and allocated overhead. The Company expenses all research and development costs in the periods in which they are incurred.
F-12
Selling and Marketing Expenses
Selling and marketing expenses consist of: personnel costs, including salaries, employee benefit costs, bonuses and equity-based compensation expenses; customer service expenses; direct marketing expenses; educational and promotional expenses; market research and analysis and allocated overhead. The Company expenses all selling and marketing costs as incurred.
General and Administrative Expenses
General and administrative expenses include executive, finance and accounting, legal and human resources functions. These expenses consist of: personnel costs, including salaries, employee benefit costs, bonuses and equity-based compensation expenses; audit and legal expenses; consulting costs and allocated overhead. The Company expenses all general and administrative expenses as incurred.
Income Taxes
Income taxes are accounted for under the asset and liability method. The Company provides for federal, state and foreign income taxes currently payable, as well as for taxes deferred due to timing differences between reporting income and expenses for financial statement purposes versus tax purposes. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted income tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect of a change in income tax rates is recognized as income or expense in the period that includes the enactment date.
The Company recognizes the effect of income tax positions only if those positions are more likely than not to be sustained. Recognized income tax positions are measured at the largest amount with a greater than 50% likelihood of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. For income tax positions where it is not more likely than not that a tax benefit will be sustained, the Company does not recognize a tax benefit in its consolidated financial statements. The Company records interest and penalties related to uncertain tax positions, if applicable, as a component of income tax expense.
Our predecessor, Fulgent LLC, is organized as a limited liability company and its members elected to have Fulgent LLC treated as a partnership for income tax purposes. As a result, for all periods prior to the Reorganization, all taxable income or loss and tax credits of the Company generally were reflected in the personal income tax returns of Fulgent LLC’s members, and no provision for federal and state income taxes was provided in the accompanying consolidated financial statements. The Company became a taxable entity upon completion of the Reorganization on September 30, 2016.
Equity-Based Compensation
The Company’s employee equity-based awards result in a cost that is measured at fair value on an award’s grant date. Equity-based compensation costs are reflected in the accompanying statements of operations based upon the award recipient’s role within the Company. The Company grants options and RSUs to its employees that generally vest upon the satisfaction of service period criteria typically up to four years. Additionally, the options typically have a contractual term of 10 years. The Company records equity-based compensation expense on stock options and RSUs. Expense for stock options is based on the grant date fair value of the equity-based awards using the accelerated attribution method. Expense for RSUs is based on the grant date fair value of the equity-based awards recognized ratably over the requisite vesting period of the awards.
Transactions with non-employees in which goods or services are the consideration received for the grant of equity-based awards are accounted for based on the fair value of the consideration received or the fair value of the equity-based award, whichever is more reliably measurable. The measurement date of the fair value of the equity-based award is the earlier of the date on which the counterparty’s performance is complete or the date on which it is probable that performance will occur. The Company records equity-based compensation expense on stock options based on the measurement date fair value of the equity-based awards using the accelerated attribution method. Expense for RSU is based on the measurement date fair value of the equity-based awards recognized ratably over the requisite vesting period of the awards.
Prior to the Reorganization, all option awards granted to employees and non-employees were also subject to a performance condition, such that they did not become exercisable until the occurrence of a qualifying liquidity event or incorporation, each as defined in the 2015 Plan (as defined in Note 10 below). Such an incorporation was deemed to have occurred upon completion of the Reorganization, at which time the options became immediately exercisable, to the extent vested. As a result, prior to the Reorganization, no equity-based compensation expense had been recognized relating to the Company’s options, and at the time of the
F-13
Reorganization, the Company recognized equity-based compensation expense based on the grant date fair value of all outstanding option awards using the accelerated attribution method.
Prior to the Reorganization, the Company also granted awards of Class D and Class P common units to employees and non-employees, some of which were subject to profits interest thresholds. These legally outstanding units allowed the holder to participate along with other unitholders in distributions only after the designated profits interest threshold amounts were met. These units were immediately vested as of the applicable grant date. The Company has recognized compensation cost relating to unit awards, including those subject to profit interest thresholds, based on the fair value of the awards. In the Reorganization, all outstanding Class D common units were converted into shares of the Company’s common stock at a ratio of 7.6 units for one share of common stock. Class P options were not exercisable, whether or not vested, until the earlier of a liquidity event or an incorporation. These options were all assumed by Fulgent Pharma as part of the split-off of the Pharma business and will not result in any recognition of expense by the Company.
Comprehensive Loss
Comprehensive loss is comprised of net loss and other comprehensive loss. Other comprehensive loss consists of unrealized loss on marketable securities. The Company did not have reclassifications from other comprehensive loss to the loss during the year ended December 31, 2016.
Basic and Diluted Net Loss per Share
Basic net loss per common share is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period. Diluted net loss per common share is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common shares and dilutive common share equivalents outstanding during the period. Because the Company has reported a net loss attributable to common stockholders for all periods presented, diluted net loss per common share is the same as basic net loss per common share for these periods.
Emerging Growth Company
Pursuant to the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), a company constituting an “emerging growth company” is, among other things, entitled to rely upon certain reduced reporting requirements. The Company is an emerging growth company, but has irrevocably elected not to take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards. As a result, the Company will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for public companies that are not emerging growth companies.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (Topic 606), which outlines a comprehensive new revenue recognition model designed to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In March 2016, the FASB issued ASU No. 2016-08, Revenue from Contracts with Customers (Topic 606)-Principal versus Agent Considerations (Reporting Revenue Gross versus Net), which further clarifies the implementation guidance on principal versus agent considerations contained in ASU 2014-09. In April 2016, the FASB issued ASU No. 2016-10, Revenue from Contracts with Customers (Topic 606)-Identifying Performance Obligations and Licensing, which further clarifies the implementation guidance relating to identifying performance obligations and the licensing implementation guidance. In May 2016, the FASB issued ASU No. 2016-12, Revenue from Contracts with Customers (Topic 606)-Narrow-Scope Improvements and Practical Expedients, which further clarifies guidance on collectability, noncash consideration, presentation of sales tax, practical expedients and transition. In December 2016, the FASB issued ASU No. 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers, which makes minor corrections or minor improvements to the codification that are not expected to have a significant effect on current accounting practice or create a significant administrative cost to most entities. These standards, pursuant to ASU No. 2015-14, Revenue from Contracts with Customers-Deferral of the Effective Date (Topic 606) issued by the FASB in August 2015, will be effective for annual periods (including interim periods) beginning after December 15, 2017. The Company has commenced its assessment of these new standards, developed a project plan to guide the implementation, and is evaluating the impact these new standards will have on its consolidated financial statements. The standard can be applied either retrospectively to each period presented or as a cumulative effect adjustment as of the date of adoption. The Company plans to implement these standards effective January 1, 2018 and anticipates adopting based on the modified retrospective method.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities, which addresses certain aspects of recognition, measurement, presentation,
F-14
and disclosure of financial instruments, including a provision that requires equity investments (except for investments accounted for under the equity method of accounting) to be measured at fair value, with changes in fair value recognized in current earnings. The ASU is effective for the Company in the first quarter of 2018, with early adoption permitted. The Company is currently evaluating the effect this ASU will have on its consolidated financial statements and related disclosures.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842). The update is aimed at making leasing activities more transparent and comparable, and requires substantially all leases be recognized by lessees on their balance sheet as a right-of-use asset and corresponding lease liability, including leases currently accounted for as operating leases. This guidance will become effective for interim and annual reporting periods beginning with the year ending December 31, 2019. The standard requires the use of a modified retrospective transition approach for existing leases. Early adoption is permitted. The Company is currently evaluating the effect this ASU will have on its consolidated financial statements and related disclosures.
In March 2016, the FASB issued ASU No. 2016-09, Stock Compensation (Topic 718); Improvements to Employee Share-Based Payment Accounting. The new guidance simplifies several aspects of the accounting for share-based payment transactions including the income tax consequences, classification of awards as either equity or liabilities, policy election to account for forfeitures as they occur rather than on an estimated basis and classification on the statement of cash flows. The ASU is effective for annual periods beginning after December 15, 2016, including interim periods within that annual period. Early adoption is permitted. The Company elected to early adopt this ASU and has elected to account for forfeitures as they occur.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments-Credit Losses: Measurement of Credit Losses on Financial Instruments. ASU 2016-13 replaces the incurred loss impairment methodology in current U.S. GAAP with a methodology that reflects expected credit losses. The update is intended to provide financial statement users with more decision-useful information about the expected credit losses on financial instruments and other commitments to extend credit held by a reporting entity at each reporting date. The standard will be effective for annual reporting periods beginning after December 15, 2019, including interim periods within those reporting periods. Early adoption is permitted. The Company has not yet evaluated the effect this ASU will have on its consolidated financial statements and related disclosures.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230). The standard clarifies the way certain cash receipts and cash payments are classified with the objective of reducing the existing diversity in practice. The standard is effective for fiscal years and interim periods beginning after December 15, 2017. Early adoption is permitted for all periods beginning after December 15, 2016. The Company is currently evaluating the effect this ASU will have on its consolidated financial statements and related disclosures.
In December 2016, the FASB issued ASU 2016-19, Technical Corrections and Improvements, which clarifies and removes inconsistencies in key areas of U.S. GAAP and is effective immediately. The Company adopted this guidance in December 2016 and there was no impact on its consolidated financial statements.
The Company’s marketable securities consisted of the following:
|
|
December 31, 2016 |
|
|||||||||||||
|
|
Amortized Cost Basis |
|
|
Unrealized Gains |
|
|
Unrealized Losses |
|
|
Aggregate Fair Value |
|
||||
|
|
(in thousands) |
|
|||||||||||||
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market accounts |
$ |
1,580 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,580 |
|
|
Corporate debt securities |
|
38,728 |
|
|
|
3 |
|
|
|
(163 |
) |
|
|
38,568 |
|
|
Less: Cash equivalents |
|
(1,580 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1,580 |
) |
|
Total marketable securities |
$ |
38,728 |
|
|
$ |
3 |
|
|
$ |
(163 |
) |
|
$ |
38,568 |
|
The Company did not hold any marketable securities as of December 31, 2015. As of December 31, 2016, the contractual maturities of the Company’s marketable securities less than one year were $13.0 million and the contractual maturities of the Company’s marketable securities greater than one year and less than five years were $25.6 million. Management determined that the gross unrealized losses of $163,000 on the Company’s marketable securities as of December 31, 2016 were temporary in nature. The Company currently does not intend to sell these securities prior to maturity and does not consider these investments to be other-than-temporarily impaired as of December 31, 2016. There were no sales of marketable securities in any of the periods presented.
F-15
Note 4. Fair Value Measurements
The authoritative guidance on fair value measurements establishes a framework with respect to measuring assets and liabilities at fair value on a recurring basis and non-recurring basis. Under the framework, fair value is defined as the exit price, or the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants, as of the measurement date. The framework also establishes a three-tier hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability and are developed based on market data obtained from sources independent of our Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability and are developed based on the best information available in the circumstances. The hierarchy consists of the following three levels:
|
Level 1: |
Inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity can access at the measurement date. |
|
Level 2: |
Inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly. |
|
Level 3: |
Inputs are unobservable inputs for the asset or liability. |
The following table presents information about our financial assets measured at fair value on a recurring basis as of December 31, 2016, based on the three-tier fair value hierarchy:
|
|
Fair Value Measurements at December 31, 2016 |
|
|||||||||||||
|
|
Total |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
|
|
(in thousands) |
|
|||||||||||||
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate debt securities |
$ |
38,568 |
|
|
$ |
— |
|
|
$ |
38,568 |
|
|
$ |
— |
|
|
Money market accounts |
|
1,580 |
|
|
|
1,580 |
|
|
|
— |
|
|
|
— |
|
|
Total marketable securities |
$ |
40,148 |
|
|
$ |
1,580 |
|
|
$ |
38,568 |
|
|
$ |
— |
|
The Company’s Level 1 assets include money market instruments and are valued based upon observable market prices. Level 2 assets consist of marketable investment securities consisting of corporate bonds. Level 2 securities are valued based upon observable inputs that include reported trades, broker/dealer quotes, bids and offers. As of December 31, 2016, the Company had no investments that were measured using unobservable (Level 3) inputs.
There were no transfers between fair value measurement levels during the year ended December 31, 2016.
Gross unrealized gains or losses for cash equivalents and marketable securities as of December 31, 2016 were not material. As of December 31, 2016 there were no securities that were in an unrealized loss position for more than 12 months.
F-16
Major classes of fixed assets consisted of the following:
|
|
|
December 31, |
|
|||||
|
|
Useful Lives |
2016 |
|
|
2015 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Computer hardware |
3 Years |
$ |
1,293 |
|
|
$ |
601 |
|
|
Computer software |
3 Years |
|
354 |
|
|
|
176 |
|
|
Machinery and equipment |
5 Years |
|
177 |
|
|
|
210 |
|
|
Medical lab equipment |
5 Years |
|
4,678 |
|
|
|
2,016 |
|
|
General equipment |
3 Years |
|
59 |
|
|
|
59 |
|
|
Furniture and fixtures |
5 Years |
|
86 |
|
|
|
51 |
|
|
Leasehold improvements |
Shorter of lease term or estimated useful life |
|
520 |
|
|
|
256 |
|
|
Assets not yet placed in service |
|
|
1,114 |
|
|
|
— |
|
|
Total |
|
|
8,281 |
|
|
|
3,369 |
|
|
Less: Accumulated depreciation |
|
|
(2,047 |
) |
|
|
(900 |
) |
|
Property and equipment, net |
|
$ |
6,234 |
|
|
$ |
2,469 |
|
Depreciation expense on fixed assets totaled $1.2 million, $575,000 and $196,000 for the years ended December 31, 2016, 2015 and 2014, respectively.
Note 6. Other Current Assets
Other current assets consisted of the following:
|
|
December 31, |
|
|||||
|
|
2016 |
|
|
2015 |
|
||
|
|
(in thousands) |
|
|||||
|
Reagents |
$ |
322 |
|
|
$ |
212 |
|
|
Prepaid expenses |
|
375 |
|
|
|
65 |
|
|
Marketable securities interest receivable |
|
209 |
|
|
|
— |
|
|
Payroll tax refund |
|
— |
|
|
|
37 |
|
|
Total |
$ |
906 |
|
|
$ |
314 |
|
Reagents are used for DNA sequencing applications in the Company’s DNA sequencing equipment.
Note 7. Reporting Segment and Geographic Information
The Company views its operations and manages its business in one reporting segment. All long-lived assets were located in the United States during the years ended December 31, 2016, 2015 and 2014.Revenue by region was as follows:
|
|
Year Ended December 31, |
|
|||||||||
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
(in thousands) |
|
|||||||||
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
United States |
$ |
8,258 |
|
|
$ |
5,084 |
|
|
$ |
640 |
|
|
Foreign: |
|
|
|
|
|
|
|
|
|
|
|
|
Canada |
|
3,798 |
|
|
|
2,658 |
|
|
|
194 |
|
|
China |
|
3,241 |
|
|
|
— |
|
|
|
— |
|
|
Other Countries |
|
2,979 |
|
|
|
1,834 |
|
|
|
444 |
|
|
Total |
$ |
18,276 |
|
|
$ |
9,576 |
|
|
$ |
1,278 |
|
F-17
Note 8. Commitments and Contingencies
Operating Leases
The Company has commitments under various non-cancelable operating leases with varying terms through July 2018. The Company has options to renew for two or three years. Future minimum payments under non-cancelable operating leases as of December 31, 2016 are as follows:
|
|
|
Minimum Payments |
|
|
|
Year ending December 31, |
|
(in thousands) |
|
|
|
2017 |
|
$ |
252 |
|
|
2018 |
|
|
80 |
|
|
2019 |
|
|
— |
|
|
2020 |
|
|
— |
|
|
Thereafter |
|
|
— |
|
|
Total minimum lease payments |
|
$ |
332 |
|
The Company’s headquarters is located in Temple City, California, which is comprised of various corporate offices and a laboratory certified under the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”), accredited by the College of American Pathologists (“CAP”) and licensed by the State of California Department of Public Health (“CA DPH”). A second office located in Atlanta, Georgia is used for certain research and development, customer service, report generation and other administrative functions.
Rent expense for the years ended December 31, 2016, 2015 and 2014 was approximately $204,000, $158,000 and $64,000, respectively.
Purchase Obligations
As of December 31, 2016, the Company had purchase obligations of $2.2 million for reagents and equipment.
Contingencies
From time to time, the Company may be subject to legal proceedings and claims arising in the ordinary course of business. Management does not believe that the outcome of any of these matters will have a material effect on the Company’s consolidated financial position, results of operations or cash flows.
Note 9. Stockholders’/Members’ Equity
Xi Long Financing
In May 2016, Fulgent LLC completed a transaction with Xi Long USA, Inc. (“Xi Long”), an independent investor, and certain members of Fulgent LLC. In this transaction, (i) Xi Long acquired 4,618,421 Class D-1 preferred units and 5,644,737 Class D common units from certain existing members of Fulgent LLC for an aggregate purchase price of $12.0 million, which units were required to be redeemed by Fulgent LLC in exchange for its issuance to Xi Long of an equivalent number of Class D-2 preferred units, and (ii) Fulgent LLC sold an additional 5,131,579 Class D-2 preferred units to Xi Long for gross proceeds of $15.2 million. Fulgent LLC incurred issuance costs of $185,000 for the transaction, resulting in net proceeds to Fulgent LLC of $15.0 million. Fulgent LLC immediately cancelled the redeemed Class D common and Class D-1 preferred units upon completion of the transaction. All Class D-2 preferred units issued to Xi Long in the transaction were converted into an aggregate of 2,025,623 shares of the Company’s common stock upon completion of the Reorganization.
This transaction was accounted for as: (i) the retirement of the redeemed Class D common units, (ii) the extinguishment of the redeemed Class D-1 preferred units, with the excess of the consideration transferred over the related carrying amount recorded as a deemed dividend in the amount of $3.7 million, and (iii) the issuance of 15,394,737 Class D-2 preferred units for $32.6 million. As a result of the transaction, Xi Long acquired an aggregate of 15,394,737 Class D-2 preferred units for an aggregate purchase price of $27.2 million, even though, at issuance, the fair value of 15,394,737 Class D-2 preferred units as evidenced by Fulgent LLC’s then most recent third-party valuation was $32.6 million. The $5.5 million difference between the fair value of, and the aggregate consideration paid by Xi Long for, the Class D-2 preferred units issued in the transaction was not attributable to any stated rights or privileges. Rather, Fulgent LLC, Xi Long and the members of Fulgent LLC that were party to the transaction determined to complete the transaction in line with their discussions, notwithstanding that the fair value of the Class D-2 preferred units as evidenced by Fulgent LLC’s third-party valuation had increased from the time these discussions were initiated to the time the transaction was
F-18
completed. The $5.5 million difference was determined to be a cost of completing the transaction with Xi Long and was recorded as an expense in the accompanying consolidated statement of operations.
Reorganization
Upon completion of the Reorganization on September 30, 2016, all of Fulgent LLC’s outstanding units were cancelled in exchange for shares of the Company’s common stock, at a ratio of 7.6 units of Fulgent LLC cancelled in exchange for each one share of the Company’s common stock.
The following is a summary of the units of Fulgent LLC that were cancelled in exchange for shares of the Company’s common stock:
|
|
Pre-Reorganization |
|
|
|
|
(in thousands) |
|
|
|
Class D-1 preferred units |
|
51,382 |
|
|
Class D-2 preferred units |
|
15,395 |
|
|
Class D common units |
|
2,500 |
|
|
Class D common units — profit interests |
|
28,355 |
|
|
Total |
|
97,632 |
|
The units set forth in the table above were cancelled in exchange for an aggregate of 12,846,256 shares of the Company’s common stock. The members’ equity balance of $72.0 million was reclassified into common stock and additional paid-in capital in the accompanying consolidated balance sheet as of September 30, 2016.
Certificate of Incorporation
In accordance with the Company’s certificate of incorporation, the Company is authorized to issue 200,000,000 shares of common stock, with a par value of $0.0001 per share, and 1,000,000 shares of preferred stock, with a par value of $0.0001 per share. Holders of the Company’s common stock are entitled to one vote for each share held on all matters submitted to a vote of its stockholders. Holders of the Company’s common stock have no cumulative voting rights. Further, as of December 31, 2016, holders of the Company’s common stock have no preemptive, conversion, redemption or subscription rights and there are no sinking fund provisions applicable to the Company’s common stock. Upon liquidation, dissolution or winding-up of the Company, holders of the Company’s common stock are entitled to share ratably in all assets remaining after payment of all liabilities and the liquidation preferences of any outstanding shares of preferred stock. Subject to preferences that may be applicable to any outstanding shares of preferred stock, holders of the Company’s common stock are entitled to receive dividends, if any, as may be declared from time to time by the Company’s board of directors. As of December 31, 2016, there were no outstanding shares of preferred stock.
Distributions
In the year ended December 31, 2016, the Company made distributions as follows: (i) the Company paid $1.3 million in tax distributions to the former members of Fulgent LLC based on the income tax liabilities of such former members attributable to Fulgent LLC’s 2016 net taxable income through the date of the Reorganization, and (ii) the Company paid $4.6 million to Mr. Hsieh in his capacity as a member of Fulgent LLC as a return of capital contribution. The Company made no distributions in the year ended December 31, 2015.
Note 10. Equity-Based Compensation
Equity-based compensation expense for awards granted to employees is measured based on the fair value of the award on the grant date and recognized in the Company’s consolidated statements of operations over the period during which the employee is required to perform services in exchange for the award (generally the vesting period of the award). Compensation expense for awards with both a service and performance condition is recognized over the period required to achieve both conditions using the accelerated attribution method. The Company estimates the fair value of stock options using the Black-Scholes option valuation model. The Company measures the fair value of RSUs and share awards based on the fair value of the underlying shares on the date of grant. For awards of Fulgent LLC units subject to a profits interest threshold that were granted before the Reorganization, the fair value was measured using the Black-Scholes option valuation model.
Prior to the Reorganization, the Company’s employees and other service providers were granted awards under the Fulgent Therapeutics LLC Amended and Restated 2015 Equity Incentive Plan (the “2015 Plan”), which provided for the issuance of equity-based awards to eligible employees, directors and consultants. These awards generally consisted of options, RSUs and units subject to
F-19
a profits interest threshold. Options granted under the 2015 Plan typically vested over four years and expired 10 years from the date of grant, and were not exercisable, whether or not vested, until the earlier of a liquidity event or incorporation, each as defined in the 2015 Plan. Because the options were subject to both a service condition (as set forth in their vesting schedules) and a performance condition (the occurrence of a qualifying liquidity event or incorporation), no equity-based compensation expense was recognized for these options until the performance condition was deemed to have been satisfied. RSUs granted under the 2015 Plan typically vested over four years. Awards of units subject to profits interest thresholds were typically fully vested at the date of grant.
In connection with the Reorganization, the Company approved its 2016 Omnibus Incentive Plan (the “2016 Plan”), which provides for the issuance of up to an aggregate of 2,038,480 shares of the Company’s common stock pursuant to awards granted to eligible employees, directors and consultants. The vesting period, contractual life and other material terms and conditions of awards granted under the 2016 Plan are generally not significantly different from the terms and conditions of awards granted under the 2015 Plan.
Additionally, at the effective time of the Reorganization:
|
|
• |
The 2015 Plan was terminated and no additional awards have been or will be granted thereunder. |
|
|
• |
Each outstanding option to purchase 7.6 Class D common units of Fulgent LLC was cancelled in exchange for an equivalent option granted under the 2016 Plan to purchase one share of the Company’s common stock. The new options are subject to the same vesting schedule and other material terms and conditions as the cancelled options. The Reorganization was considered an incorporation pursuant to the terms of the 2015 Plan and the performance condition applicable to all options was deemed to have been satisfied. As a result, all of the options became immediately exercisable, to the extent vested, upon completion of the Reorganization. This satisfaction of the performance condition resulted in a cumulative equity-based compensation expense of $1.1 million for the requisite service period related to these options, which the Company recorded in the period in which the Reorganization occurred. The remaining unrecognized equity-based compensation expense related to the options is being recorded over the remaining requisite vesting period of the awards using the accelerated attribution method. |
|
|
• |
Each outstanding RSU relating to 7.6 Class D common units of Fulgent LLC was cancelled in exchange for an equivalent RSU granted under the 2016 Plan relating to one share of the Company’s common stock. The new RSUs are subject to the same vesting schedule and other material terms and conditions as the cancelled RSUs. Equity-based compensation expense for RSUs is recognized ratably over the requisite vesting period of the awards. |
|
|
• |
Pursuant to the determination of Mr. Hsieh in his capacity as the Manager of Fulgent LLC prior to the Reorganization, the participation thresholds applicable to all profits interests (i) were ignored and not applied in calculating the number of shares of the Company’s common stock that were issued in exchange for such units in the Reorganization, and (ii) did not carry over to such shares of the Company’s common stock. As a result, the holders of profits interests received shares of the Company’s common stock in the Reorganization at the same ratio, 7.6 units-to-one share, as the holders of Fulgent LLC’s units that were not subject to profits interest thresholds. Ignoring all profits interest thresholds upon the conversion of Fulgent LLC’s profits interests into shares of the Company’s common stock resulted in an equity-based compensation expense of $1.4 million that the Company recorded in the period in which the Reorganization occurred. |
The Company has included equity-based compensation expense as part of cost of revenue and operating expenses in the accompanying consolidated statements of operations as follows:
|
|
Year Ended December 31, |
|
|||||
|
|
2016 |
|
|
2015 |
|
||
|
|
(in thousands) |
|
|||||
|
Cost of revenue |
$ |
754 |
|
|
$ |
1,673 |
|
|
Research and development |
|
1,161 |
|
|
|
3,241 |
|
|
Selling and marketing |
|
454 |
|
|
|
1,569 |
|
|
General and administrative |
|
2,285 |
|
|
|
1,673 |
|
|
Total |
$ |
4,654 |
|
|
$ |
8,156 |
|
Equity-based compensation expense of $0 and $120,000 recorded in the years ended December 31, 2016 and 2015, respectively, was related to the Pharma business and is included in discontinued operations.
F-20
The below discussions of equity-based award activity, including all share numbers and weighted-average exercise prices, have been adjusted to give retroactive effect to the Reorganization as if it occurred at the beginning of each period presented, with the exception of Class P unit options and Class P unit awards which are not subject to the effect of the Reorganization.
Option Awards
The following table summarizes activity for options to acquire shares of the Company’s common stock in the years ended December 31, 2016 and 2015:
|
|
|
Shares Available for Grant (in thousands) |
|
|
Number of Shares Subject to Options (in thousands) |
|
|
Weighted- Average Exercise Price |
|
|
Weighted- Average Remaining Contractual Life (in years) |
|
|
Aggregate Intrinsic Value (in thousands) |
|
|||||
|
Balance at December 31, 2014 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Authorized |
|
|
1,974 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
(274 |
) |
|
|
274 |
|
|
$ |
0.38 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Canceled |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2015 |
|
|
1,700 |
|
|
|
274 |
|
|
$ |
0.38 |
|
|
|
9.8 |
|
|
$ |
645 |
|
|
Authorized |
|
|
64 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
(324 |
) |
|
|
324 |
|
|
$ |
1.18 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Canceled |
|
|
42 |
|
|
|
(42 |
) |
|
$ |
0.38 |
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2016 |
|
|
1,482 |
|
|
|
556 |
|
|
$ |
0.85 |
|
|
|
9.0 |
|
|
$ |
5,976 |
|
|
Exercisable as of December 31, 2016 |
|
|
|
|
|
|
103 |
|
|
$ |
0.38 |
|
|
|
8.8 |
|
|
$ |
1,149 |
|
The weighted-average grant date fair value of options to acquire shares of Company’s common stock granted in the years ended December 31, 2016 and 2015 was $7.94 and $2.51, respectively. The remaining unrecognized compensation expense related to these options of $1.6 million for the year ended December 31, 2016, is expected to be recognized over a weighted-average period of 2.9 years.
Share and RSU Awards
The following table shows grants of share awards and grants of restricted stock units during the years ended December 31, 2016 and 2015:
|
|
Year Ended December 31, |
|
|||||||||||||
|
|
2016 |
|
|
2015 |
|
||||||||||
|
|
Employee |
|
|
Non-Employee |
|
|
Employee |
|
|
Non-Employee |
|
||||
|
|
(in thousands) |
|
|||||||||||||
|
Share Awards |
|
329 |
|
|
|
— |
|
|
|
3,421 |
|
|
|
— |
|
|
Restricted Stock Units |
|
364 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Share Awards
In the year ended December 31, 2016, one employee was granted a share award, which was recorded based on the estimated fair value of shares of common stock on the grant date and resulted in equity-based compensation expense of $1.6 million. These shares were granted outside of the 2015 Plan, were immediately vested and were not subject to a profits interest threshold.
In the year ended December 31, 2015, Fulgent LLC granted awards subject to profits interest thresholds, which were fully vested as of the grant date and could have been repurchased in whole or in part by Fulgent LLC at any time during the nine-month period following the termination of the holder’s continuous service. Fulgent LLC’s repurchase right would terminate if not timely exercised by Fulgent LLC and upon the effective date of a registration statement of Fulgent LLC or a successor entity filed under the Securities Act of 1933, as amended, which occurred in connection with the Company’s IPO. The participation threshold for each of the awards granted during the year ended December 31, 2015 was $0.3617 per share, although, as described above, such thresholds were ignored in the Reorganization. Of the awards granted during the period, all were granted under the 2015 Plan except for an award
F-21
of 2,105,263 shares granted to an employee. These awards were legally outstanding equity of Fulgent LLC as of their respective grant dates that allowed the holder to participate in distributions upon exceeding the designated thresholds. These awards were accounted for at fair value and were considered equity instruments as of their respective grant dates.
RSU Awards
RSUs are share awards that entitle the holder to receive freely tradable shares of the Company’s common stock upon vesting. The fair value of RSUs is based upon the closing sales price of the Company’s common stock on the grant date. RSUs granted to employees generally vest over a four year period. The RSUs granted in the year ended December 31, 2016 are recorded based on the fair value of shares of common stock on the grant date, which resulted in equity-based compensation expense of $3.5 million to be recognized over four years. As of December 31, 2016, $192,000 has been recognized and the remaining unrecognized compensation expense of $3.3 million related to these RSUs is expected to be recognized over a weighted-average period of 3.8 years.
No RSU awards were granted prior to the year ended December 31, 2016. The following table summarizes activity for RSUs relating to shares of the Company’s common stock in the year ended December 31, 2016:
|
|
|
Number of Shares (in thousands) |
|
|
Weighted-Average Grant-Date Fair Value |
|
||
|
Balance at December 31, 2015 |
|
|
— |
|
|
|
— |
|
|
Granted |
|
|
364 |
|
|
$ |
9.69 |
|
|
Vested and settled |
|
|
— |
|
|
|
— |
|
|
Forfeited |
|
|
(2 |
) |
|
$ |
9.02 |
|
|
Balance at December 31, 2016 |
|
|
362 |
|
|
$ |
9.69 |
|
Class P Unit Options and Class P Unit Awards
Prior to the split-off of the Pharma business, Fulgent LLC granted awards of options to acquire Class P common units and awards of Class P units subject to profits interest thresholds.
The following table summarizes activity for options to acquire Class P common units in the year ended December 31, 2015:
|
|
|
Number of Units Subject to Options (in thousands) |
|
|
Weighted- Average Exercise Price Per Unit |
|
|
Weighted- Average Remaining Contractual Life (in years) |
|
|
Aggregate Intrinsic Value |
|
||||
|
Outstanding as of December 31, 2014 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Granted |
|
|
1,810 |
|
|
$ |
0.04 |
|
|
|
9.8 |
|
|
$ |
— |
|
|
Exercised |
|
|
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
Forfeited/canceled |
|
|
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
Outstanding as of December 31, 2015 |
|
|
1,810 |
|
|
$ |
0.04 |
|
|
|
9.8 |
|
|
$ |
— |
|
|
Vested and expected to vest as of December 31, 2015 |
|
|
1,810 |
|
|
$ |
0.04 |
|
|
|
9.8 |
|
|
$ |
— |
|
The weighted average grant date fair value of options to acquire Class P common units granted in the year ended December 31, 2015 was $0.04. The options were not exercisable, whether or not vested, until the earlier of a liquidity event or an incorporation, each as defined in the Plan, which, as of December 31, 2015, were not probable. As of December 31, 2015, the remaining unrecognized compensation expense related to these options was $64,000. These options were all assumed by Fulgent Pharma as part of the split-off of the Pharma business and did not result in any recognition of expense by the Company.
There were no grants of Class P unit awards prior to the year ended December 31, 2015.
The following tables show grants of Class P unit awards, all of which were subject to profits interest thresholds, during the year ended December 31, 2015:
|
|
|
Employee |
|
|
Non-Employee |
|
||
|
|
|
(in thousands) |
|
|||||
|
Profits Interests |
|
|
4,500 |
|
|
|
1,500 |
|
F-22
In the year ended December 31, 2015, Fulgent LLC granted awards subject to profits interest thresholds, which were fully vested as of the grant date and could have been repurchased in whole or in part by Fulgent LLC at any time during the nine-month period following the termination of the holder’s continuous service. Fulgent LLC’s repurchase right would terminate if not timely exercised by Fulgent LLC and upon the effective date of a registration statement of Fulgent LLC or a successor entity filed under the Securities Act of 1933, as amended. The participation threshold for each of the awards granted during the year ended December 31, 2015 was $0.0287 per unit for the Class P units. Of the awards granted during the period, all were granted under the 2015 Plan. The weighted average grant date fair value of Class P common units granted in the year ended December 31, 2015 was $0.02. These awards were legally outstanding equity of Fulgent LLC as of their respective grant dates that allowed the holder to participate in distributions upon exceeding the designated thresholds. These awards were accounted for at fair value and were considered equity instruments as of their respective grant dates. These units were all assumed by Fulgent Pharma as part of the split-off of the Pharma business and do not represent any equity interest in Fulgent LLC or the Company.
Fair Value Assumptions
Option Awards to Employees
The following table sets forth weighted-average assumptions used to estimate the fair value of options to acquire shares of the Company’s common stock (or, prior to the Reorganization, options to acquire common units) granted to employees during the years ended December 31, 2016 and 2015:
|
|
Year Ended December 31, |
|
|||||
|
|
2016 |
|
|
2015 |
|
||
|
Expected term (in years) |
|
6.1 |
|
|
|
6.1 |
|
|
Risk-free interest rates |
|
1.4 |
% |
|
|
1.6 |
% |
|
Dividend yield |
|
— |
|
|
|
— |
|
|
Expected volatility |
|
95.6 |
% |
|
|
86.0 |
% |
These assumptions and estimates are as follows:
|
|
• |
Expected Term. The expected term represents the period that the Company’s equity-based awards are expected to be outstanding. The Company determines the expected term assumption based on the vesting terms, exercise terms and contractual terms of the options, and, in the case of equity-based awards subject to a profits interest threshold granted before the Reorganization, based on the estimated time to liquidity. |
|
|
• |
Risk-Free Interest Rate. The Company determines the risk-free interest rate by using the equivalent to the expected term based on the U.S. Treasury yield curve in effect as of the date of grant. |
|
|
• |
Dividend Yield. The assumed dividend yield is based on the Company’s expectation that it will not pay dividends in the foreseeable future, which is consistent with its history of not paying dividends. |
|
|
• |
Expected Volatility. The Company does not have sufficient history to estimate the volatility of the price of its common equity or the expected term of its options. The Company calculates expected volatility based on historical volatility data of a representative group of companies that are publicly traded. The Company selected representative companies with comparable characteristics to it, including risk profiles and position within the industry, and with historical equity price information sufficient to meet the expected term of the equity-based awards. The Company computes the historical volatility of this selected group using the daily closing prices for the selected companies’ equity during the equivalent period of the calculated expected term of its equity-based awards. The Company will continue to use the representative group volatility information until the historical volatility of its equity is relevant to measure expected volatility for future option grants. |
|
|
• |
Forfeiture Rate. The Company has early adopted ASU No. 2016-09, Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting, and it has elected to account for forfeitures as they occur. |
Option Awards to Non-Employees
Equity-based compensation expense related to options granted to non-employees is recognized as the options are earned. The fair value of the options is more reliably measurable than the fair value of the services received.
F-23
The fair value of non-employee options is calculated at each reporting date, using the Black-Scholes option-pricing model, until the award vests or there is a substantial incentive for the non-employee not to perform the required services.
The following table sets forth the weighted-average assumptions used to estimate the fair value of options to acquire shares of the Company’s common stock (or, prior to the Reorganization, options to acquire common units) granted to non-employees during the years ended December 31, 2016 and 2015:
|
|
Year Ended December 31, |
|
|||||
|
|
2016 |
|
|
2015 |
|
||
|
Expected term (in years) |
|
10 |
|
|
|
10 |
|
|
Risk-free interest rates |
|
1.6 |
% |
|
|
2.3 |
% |
|
Dividend yield |
|
— |
|
|
|
— |
|
|
Expected volatility |
|
96.9 |
% |
|
|
94.8 |
% |
The following table sets forth the weighted-average assumptions used to estimate the fair value of options to acquire Class P common units granted to non-employees during the year ended December 31, 2015:
|
|
Year Ended December 31, |
|
|
|
|
2015 |
|
|
|
Expected term (in years) |
|
10 |
|
|
Risk-free interest rates |
|
2.3 |
% |
|
Dividend yield |
|
— |
|
|
Expected volatility |
|
98.0 |
% |
There were no options to acquire Class P common units granted to non-employees during the year ended December 31, 2016.
Unit Awards to Employees and Non-Employees
The fair value of the unit awards is more reliably measurable than the fair value of the services received for awards granted to non-employees. The fair value of awards subject to profits interest thresholds was calculated at each reporting date using the Black-Scholes option-pricing model.
The following table sets forth weighted-average assumptions used to estimate the fair value of Class D and Class P common unit awards subject to profits interest thresholds granted to employees and non-employees during the year ended December 31, 2015 (no such Class D awards were granted to non-employees during such period and no such awards were granted to employees or non-employees during the year ended December 31, 2016):
|
|
|
Class D |
|
|
Class P |
|
||
|
Employee: |
|
|
|
|
|
|
|
|
|
Expected term (in years) |
|
|
2 |
|
|
|
2 |
|
|
Risk-free interest rates |
|
|
0.6 |
% |
|
|
0.6 |
% |
|
Dividend yield |
|
|
0 |
|
|
|
0 |
|
|
Expected volatility |
|
|
68.1 |
% |
|
|
75.8 |
% |
|
Non—Employee: |
|
|
|
|
|
|
|
|
|
Expected term (in years) |
|
* |
|
|
|
2 |
|
|
|
Risk-free interest rates |
|
* |
|
|
|
0.6 |
% |
|
|
Dividend yield |
|
* |
|
|
|
0 |
|
|
|
Expected volatility |
|
* |
|
|
|
75.6 |
% |
|
* No awards granted
Determination of the Fair Value on Grant Dates
Historically, for all periods prior to the Company’s initial public offering, the fair value of the common units underlying Fulgent LLC’s equity-based awards was estimated on each grant date by Fulgent LLC’s Manager. In determining fair value, the Manager considered valuations prepared by an independent third party in a manner consistent with the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held Company Equity Securities Issued as Compensation, also known as the Practice Aid. In conducting the valuations, Fulgent LLC considered all objective and subjective factors that it believed to be relevant in each valuation conducted, including management’s best estimate of Fulgent LLC’s business condition, prospects and operating
F-24
performance at each valuation date. Within the valuations, a range of factors, assumptions and methodologies were used. The significant factors included:
|
|
• |
the likelihood of achieving a liquidity event for Fulgent LLC’s equity, such as an initial public offering, given prevailing market conditions; |
|
|
• |
Fulgent LLC capital structure, including the rights and preferences of its various classes of equity. |
There are significant judgments and estimates inherent in these valuations. These judgments and estimates include assumptions regarding Fulgent LLC’s future operating performance, stage of commercial growth, average selling price, continued penetration into hospital and medical institution customers, reimbursement from third-party payors, the timing of a potential initial public offering or other liquidity event and the determination of the appropriate valuation method at each valuation date. If Fulgent LLC had made different assumptions, its equity-based compensation expense, income (loss) applicable to common unitholders and income (loss) per unit applicable to common unitholders could have been materially different.
The valuations utilized the market approach, the income approach or a combination of both. The market approach and the income approach are both acceptable valuation methods in accordance with the Practice Aid. There are three general methodologies under the market approach:
Under the income approach, enterprise value can be estimated using the discounted cash flow (“DCF”) method, which assumes:
The DCF analysis is comprised of the sum of the present value of two components: discrete period projected cash flows and a residual or terminal value.
Additionally, each valuation reflects a marketability discount, resulting from the illiquidity of Fulgent LLC’s common units prior to completion of the Company’s initial public offering.
As provided in the Practice Aid, there are several approaches for allocating enterprise value of a privately held company among the securities held in a complex capital structure. The possible methodologies include the probability-weighted expected return method (“PWERM”), the option-pricing method (“OPM”), the current-value method or a hybrid of the PWERM and the OPM, which is referred to as the hybrid method. Under the PWERM, equity is valued based upon the probability-weighted present value of expected future returns, considering various future outcomes available to us, as well as the rights of each class of equity. The OPM treats common equity and preferred equity as call options on the enterprise’s value. The exercise prices associated with these call options vary according to the liquidation preference of the preferred equity, the preferred equity conversion price, the exercise prices of common equity options and other features of a company’s equity capital structure. The current-value method, which is generally only used for early stage companies, is based on first determining enterprise value using a market, income or asset-based approach,
F-25
and then allocating that value to the preferred equity-based on its liquidation preference or conversion value, whichever would be greater.
The valuation of Class D common units related to awards of Class D common units and options to acquire Class D common units granted in 2015 incorporated the income approach (Gordon Growth Analysis) and the market approach (Guideline Public Company Method) in determining value, and Fulgent LLC applied 50% weight to each approach, applying a 35% discount for lack of marketability. For the valuation of Class D common units related to awards of Class D common units and options to acquire Class D common units granted in 2016 prior to completion of the Company’s initial public offering, Fulgent LLC incorporated the PWERM and utilized the market approach (Precedent Transactions Method) incorporating the Xi Long financing, applying a 20% discount for lack of marketability.
The valuation of Class P units related to awards of Class P units and options to acquire Class P units granted in the year ended December 31, 2015 incorporated the market approach (Precedent Transactions Method), utilizing OPM to backsolve.
After completion of the Company’s initial public offering on October 4, 2016, the fair value of shares of its common stock underlying stock option grants is determined by its board of directors or the compensation committee thereof based on the closing price of its common stock on the date of grant as reported by the NASDAQ Global Market.
Note 11. Income Taxes
Provision for income taxes consists of U.S. federal and state income taxes. A deferred tax liability is recognized for all taxable temporary differences, and a deferred tax asset is recognized for all deductible temporary differences, operating losses and tax credit carryforwards. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
Prior to the Reorganization, Fulgent LLC was organized as a limited liability company and its members elected to have Fulgent LLC treated as a partnership for income tax purposes. All taxable income or loss and tax credits generally were reflected in the personal income tax returns of the Fulgent LLC’s members. Accordingly, no provision for federal and state income taxes was provided in the accompanying consolidated financial statements prior to the Reorganization.
Upon completion of the Reorganization, the Company converted from a pass-through entity for tax purposes to a taxable entity. The change in tax status resulted in $417,000 income tax expense related to the recognition of a net state deferred tax asset of $86,000 and a net federal deferred tax liability of $503,000, which represents the temporary differences in existence on September 30, 2016 between the tax basis of the Company’s assets and liabilities and the amount reported in the financial statements. As of December 31, 2016, the net state deferred tax asset was $54,000 and the net federal deferred tax liability was $243,000.
Income tax expense consisted of the following:
|
|
Year Ended December 31, 2016 |
|
|
|
|
(in thousands) |
|
|
|
Current: |
|
|
|
|
Federal |
$ |
616 |
|
|
State |
|
58 |
|
|
Total Current |
|
674 |
|
|
Deferred: |
|
|
|
|
Federal |
|
295 |
|
|
State |
|
(49 |
) |
|
Change in valuation allowance |
|
— |
|
|
Total Deferred |
|
246 |
|
|
Total income tax expense |
$ |
920 |
|
F-26
Reconciliation of the difference between the federal statutory income tax rate and the effective income tax rate for the year ended December 31, 2016 is as follows:
|
|
|
Year Ended December 31, 2016 |
|
|
|
Tax provision at federal statutory rate |
|
|
34.00 |
% |
|
State franchise tax, net of federal income tax benefit |
|
|
1.98 |
% |
|
Other |
|
|
0.16 |
% |
|
LLC income not subject to corporate taxes |
|
|
-37.23 |
% |
|
Recognition of pre-merger temporary differences |
|
|
-19.51 |
% |
|
Tax provision |
|
|
-20.60 |
% |
The following table summarizes the elements of the deferred tax assets (liabilities) at December 31, 2016:
|
|
Year Ended December 31, 2016 |
|
|
|
|
(in thousands) |
|
|
|
Deferred tax assets |
|
|
|
|
Accrued vacation and other accrued expenses |
$ |
69 |
|
|
Provision for bad debts |
|
54 |
|
|
Equity-based compensation |
|
603 |
|
|
Unrealized loss on investments |
|
58 |
|
|
State income taxes |
|
20 |
|
|
Total deferred tax assets |
$ |
804 |
|
|
Deferred tax liabilities |
|
|
|
|
Depreciation |
$ |
993 |
|
|
Total deferred tax liabilities |
|
993 |
|
|
Net deferred tax liabilities |
$ |
(189 |
) |
Uncertain Tax Positions
The Company is subject to income taxation by the United States government and certain states in which the Company's activities give rise to an income tax filing requirement. The Company does not have income tax filing requirements in any foreign jurisdiction, nor are any taxes withheld from income taxes withheld from foreign sales. As of December 31, 2016, there were no pending tax audits in any jurisdiction.
There were no interest or penalties accrued at December 31, 2016.
While the Company believes it has adequately provided for all tax positions, amounts asserted by taxing authorities could differ from the Company's accrued positions. Accordingly, additional provisions on federal, state and foreign tax-related matters could be recorded in future periods as revised estimates are settled or otherwise resolved.
F-27
Note 12. Income (Loss) per Share
Income (loss) per share for the years ended December 31, 2016 and 2015 was computed as if the Reorganization, including the issuance of one share of common stock in exchange for the cancellation of every 7.6 Class D common and preferred units of Fulgent LLC, had occurred subsequent to the Recapitalization, which occurred in October 2015, with the exception of Class P units which are not subject to the effect of the Reorganization. Income (loss) per share prior to the Recapitalization in October 2015 is not presented. The following is a reconciliation of the denominators of the basic and diluted (loss) per share computations:
|
|
|
Years Ended |
|
|||||||||||||||||||||
|
|
|
2016 |
|
|
2015 |
|
||||||||||||||||||
|
|
|
Continuing Operations |
|
|
Discontinued Operations |
|
|
Total |
|
|
Continuing Operations |
|
|
Discontinued Operations |
|
|
Total |
|
||||||
|
|
|
(in thousands, except per share data) |
|
|||||||||||||||||||||
|
Loss for the period from October 16, 2015 through December 31, 2015 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
(7,239 |
) |
|
$ |
(896 |
) |
|
$ |
(8,135 |
) |
|
Income (loss) |
|
$ |
(5,388 |
) |
|
$ |
41 |
|
|
$ |
(5,347 |
) |
|
$ |
(7,239 |
) |
|
$ |
(896 |
) |
|
$ |
(8,135 |
) |
|
Deemed dividend on redemption of Class D-1 preferred unit |
|
$ |
(3,727 |
) |
|
|
— |
|
|
$ |
(3,727 |
) |
|
|
— |
|
|
|
— |
|
|
$ |
0 |
|
|
Distribution to Class D-1 preferred unitholder |
|
$ |
(4,592 |
) |
|
|
— |
|
|
$ |
(4,592 |
) |
|
|
— |
|
|
|
— |
|
|
$ |
0 |
|
|
Income (loss) allocable to common shareholders |
|
$ |
(13,707 |
) |
|
$ |
41 |
|
|
$ |
(13,666 |
) |
|
$ |
(7,239 |
) |
|
$ |
(896 |
) |
|
$ |
(8,135 |
) |
|
Income (loss) allocated to common shareholders |
|
$ |
(13,707 |
) |
|
|
|
|
|
|
|
|
|
$ |
(7,239 |
) |
|
|
|
|
|
|
|
|
|
Income (loss) allocated to Class P common units |
|
|
— |
|
|
$ |
18 |
|
|
|
|
|
|
|
— |
|
|
$ |
(896 |
) |
|
|
|
|
|
Income (loss) allocated to Class P preferred units |
|
|
— |
|
|
$ |
23 |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Weighted-average common shares—outstanding, basic and diluted |
|
|
13,710 |
|
|
|
— |
|
|
|
|
|
|
|
11,842 |
|
|
|
— |
|
|
|
|
|
|
Weighted-average Class P common units - profit interests - outstanding, basic and diluted |
|
|
— |
|
|
|
10,123 |
|
|
|
|
|
|
|
— |
|
|
|
5,796 |
|
|
|
|
|
|
Weighted-average Class P preferred units outstanding, basic and diluted |
|
|
— |
|
|
|
13,238 |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Income (loss) per common share from continuing operations, basic and diluted |
|
$ |
(1.00 |
) |
|
|
— |
|
|
|
|
|
|
$ |
(0.61 |
) |
|
|
— |
|
|
|
|
|
|
Income per Class P common unit - profit interests, basic and diluted |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
$ |
(0.15 |
) |
|
|
|
|
|
Income per Class P preferred unit, basic and diluted |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
On April 4, 2016, Fulgent LLC completed the split-off of the Pharma business. The financial condition and results of the Pharma business are included in the accompanying consolidated financial statements as discontinued operations for all periods presented, and the weighted-average Class P units related to the Pharma business were computed through the separation date of April 4, 2016.
The Class P common and preferred units had the right to participate in earnings and distributions of Fulgent LLC but were not obligated to fund losses. As a result, in periods of net loss, Fulgent LLC allocated losses to the holders of its common units subject to profits interest thresholds, as they were determined to be the most subordinate unit.
The following securities have been excluded from the calculation of diluted income (loss) per share for all periods presented because their effect would have been anti-dilutive:
|
|
Years Ended |
|
|||||||||||||
|
|
2016 |
|
|
2015 |
|
||||||||||
|
|
Continuing Operations |
|
|
Discontinued Operations |
|
|
Continuing Operations |
|
|
Discontinued Operations |
|
||||
|
|
(in thousands) |
|
|||||||||||||
|
Options |
|
490 |
|
|
|
— |
|
|
|
274 |
|
|
|
— |
|
|
RSUs |
|
79 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Class P unit options |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,810 |
|
The anti-dilutive shares disclosed above were calculated using the treasury stock method. During the years ended December 31, 2016 and 2015, the Company had options and RSUs that were excluded from the weighted average share calculation for continuing operations due to the Company’s net loss positions. During the year period ended December 31, 2015, the Company had Class P
F-28
common unit options that were excluded from the weighted average share calculation for discontinued operations because the units were contingently exercisable.
Note 13. Retirement Plans
The Company offers a 401(k) retirement savings plan (the “401(k) Plan”) for its employees, including its executive officers, who satisfy certain eligibility requirements. The Internal Revenue Code of 1986, as amended, allows eligible employees to defer a portion of their compensation, within prescribed limits, on a pre-tax basis through contributions to the 401(k) Plan. The Company matches contributions to the 401(k) Plan based on the amount of salary deferral contributions the participant makes to the 401(k) Plan. The Company will match up to 3% of an employee’s compensation that the employee contributes to his or her 401(k) Plan account. Total Company matching contributions to the 401(k) Plan were $86,000, $37,000 and $21,000 in the years ended December 31, 2016, 2015 and 2014, respectively.
Note 14. Related Party
Dr. Yun Yen, who is a member of the Company’s Board of Directors and a stockholder, serves as the President and Chairman of the Board for the Sino-American Cancer Foundation (the “Foundation”) and served as the President for the University Taipei Medical University (the “University”), from August 1, 2011 through July 31, 2016 and currently serves as an honorary Chairman of the Board for the University.
The Company performs research testing services for the Foundation. The Company recognized $150,000 during the year ended December 31, 2016, as consideration for such services. No services were rendered to the Foundation in 2015 and 2014. Additionally, the Company subleases certain of its headquarters facilities to the Foundation. The Company recognized $28,000 and $11,500 in the years ended December 31, 2016 and 2015, respectively, as consideration for such sublease.
The Company performs genetic sequencing services for the University. The Company recognized $353,000 and $13,000 as consideration for such services in the years ended December 31, 2016 and 2015, respectively.
As of December 31, 2016 and 2015, $0 and $0 was due from the Foundation and $29,550 and $0 was due from the University, respectively.
During 2014 and 2015, the Pharma business incurred expenses to ANP Technologies, Inc. (“ANP”) totaling $1 million and $800,000, respectively, for services related to patented nanoencapsulation technology and other drug-related services in the oncology drug area and related expense is recorded in discontinued operations. The Company did not incur any business expenses with ANP during the year ended December 31, 2016. The Chief Executive Officer of ANP is a shareholder of the Company and the Company’s Chief Executive Officer is a shareholder of ANP.
F-29
Note 15. Selected Quarterly Financial Data (Unaudited)
The tables below set forth the Company’s quarterly consolidated statements of operations data for the eight quarters ended December 31, 2016. In the opinion of management, this quarterly data has been prepared on the same basis as the consolidated financial statements and includes all adjustments, consisting of normal recurring adjustments, necessary for a fair presentation of the results of operations for the periods presented. See Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in this report for descriptions of the effects of any extraordinary, unusual or infrequently occurring items recognized in any of the periods covered by this data. The results for any one quarter are not indicative of the results to be expected in the current period or any future period.
|
|
Three Months Ended |
|
|||||||||||||||||||||||||||||
|
|
Dec. 31, 2016 |
|
|
Sept. 30, 2016 |
|
|
June 30, 2016 |
|
|
Mar. 31, 2016 |
|
|
Dec. 31, 2015 |
|
|
Sept. 30, 2015 |
|
|
June 30, 2015 |
|
|
Mar. 31, 2015 |
|
||||||||
|
|
(in thousands) |
|
|||||||||||||||||||||||||||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
$ |
5,854 |
|
|
$ |
5,011 |
|
|
$ |
3,971 |
|
|
$ |
3,440 |
|
|
$ |
2,901 |
|
|
$ |
2,905 |
|
|
$ |
2,182 |
|
|
$ |
1,588 |
|
|
Cost of revenue |
|
1,863 |
|
|
|
2,143 |
|
|
|
1,411 |
|
|
|
1,304 |
|
|
|
2,726 |
|
|
|
918 |
|
|
|
772 |
|
|
|
653 |
|
|
Gross profit |
|
3,991 |
|
|
|
2,868 |
|
|
|
2,560 |
|
|
|
2,136 |
|
|
|
175 |
|
|
|
1,987 |
|
|
|
1,410 |
|
|
|
935 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
818 |
|
|
|
1,523 |
|
|
|
656 |
|
|
|
561 |
|
|
|
3,650 |
|
|
|
312 |
|
|
|
252 |
|
|
|
217 |
|
|
Selling and marketing |
|
798 |
|
|
|
893 |
|
|
|
477 |
|
|
|
301 |
|
|
|
1,913 |
|
|
|
280 |
|
|
|
243 |
|
|
|
234 |
|
|
General and administrative |
|
1,116 |
|
|
|
1,147 |
|
|
|
457 |
|
|
|
1,889 |
|
|
|
1,956 |
|
|
|
215 |
|
|
|
168 |
|
|
|
79 |
|
|
Total operating expenses |
|
2,732 |
|
|
|
3,563 |
|
|
|
1,590 |
|
|
|
2,751 |
|
|
|
7,519 |
|
|
|
807 |
|
|
|
663 |
|
|
|
530 |
|
|
Operating income (loss) |
|
1,259 |
|
|
|
(695 |
) |
|
|
970 |
|
|
|
(615 |
) |
|
|
(7,344 |
) |
|
|
1,180 |
|
|
|
747 |
|
|
|
405 |
|
|
Interest and other income (expense) |
|
57 |
|
|
|
5 |
|
|
|
(5,462 |
) |
|
|
13 |
|
|
|
7 |
|
|
|
- |
|
|
|
- |
|
|
|
20 |
|
|
Income (loss) before income taxes |
|
1,316 |
|
|
|
(690 |
) |
|
|
(4,492 |
) |
|
|
(602 |
) |
|
|
(7,337 |
) |
|
|
1,180 |
|
|
|
747 |
|
|
|
425 |
|
|
Provision for income taxes |
|
503 |
|
|
|
417 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Net income (loss) |
$ |
813 |
|
|
$ |
(1,107 |
) |
|
$ |
(4,492 |
) |
|
$ |
(602 |
) |
|
$ |
(7,337 |
) |
|
$ |
1,180 |
|
|
$ |
747 |
|
|
$ |
425 |
|
|
Net income (loss) per common share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
$ |
0.05 |
|
|
$ |
(0.44 |
) |
|
$ |
(5.49 |
) |
|
$ |
(0.02 |
) |
|
$ |
(0.61 |
) |
|
* |
|
|
* |
|
|
* |
|
|||
|
Diluted |
$ |
0.05 |
|
|
$ |
(0.44 |
) |
|
$ |
(5.49 |
) |
|
$ |
(0.02 |
) |
|
$ |
(0.61 |
) |
|
* |
|
|
* |
|
|
* |
|
|||
*Basic and diluted income (loss) per common share was calculated prospectively from the date the Class D common units were issued in the Recapitalization in October 2015. See Note 1, Overview and Basis of Presentation, and Note 12, Income (Loss) per Share, to these consolidated financial statements for additional information.
F-30
|
* |
Filed herewith. |
|
† |
Furnished herewith. |
|
# |
Management contract or compensatory plan, contract or arrangement. |
