Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - CFO - Apyx Medical Corp | a201610-kexhibit322.htm |
| EX-32.1 - EXHIBIT 32.1 - CEO - Apyx Medical Corp | a201610-kexhibit321.htm |
| EX-31.2 - EXHIBIT 31.2 - CFO - Apyx Medical Corp | a201610-kexhibit312.htm |
| EX-31.1 - EXHIBIT 31.1 - CEO - Apyx Medical Corp | a201610-kexhibit311.htm |
| EX-23.1 - EXHIBIT 23.1 - CONSENT OF FRAZIER & DEETER, LLC - Apyx Medical Corp | a201610-kexhibit231.htm |
| EX-21.1 - EXHIBIT 21.1 - SUBSIDIARIES OF REGISTRANT - Apyx Medical Corp | a201610-kexhibit211.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016 |
or |
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _____ to _____ |
Commission File Number: 0-12183 |
 |
BOVIE MEDICAL CORPORATION |
(Exact name of registrant as specified in its charter) |
Delaware | 11-2644611 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
4 Manhattanville Road, Suite 106, Purchase, NY 10577
(Address of principal executive offices, zip code)
(914) 468-4009
(Issuer’s telephone number)
Securities registered pursuant to Section 12(b) of the Act:
Title of each Class | Name of each Exchange on which registered | |
Common Stock, $.001 Par Value | NYSE MKT Market | |
Securities registered under Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes: o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes: o No ý
Indicate by check mark whether the registrant (1) filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes: ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes: ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
Large accelerated filer | o | Accelerated filer | o | |
Non-accelerated filer | o | (Do not check if a smaller reporting company) | Smaller reporting company | ý |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes: o No ý
The aggregate market value of the common stock held by non-affiliates computed by reference to the price at which the stock was sold, or the average bid and asked prices of such stock on the NYSE MKT exchange, as of June 30, 2016, the registrant’s most recently completed second fiscal quarter, was approximately $44.4 million.
As of March 6, 2017, 30,859,753 shares of the registrant’s $0.001 par value common stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
December 31, 2016
Part I | Page | |||
Item 1 | ||||
Item 1A | ||||
Item 1B | ||||
Item 2 | ||||
Item 3 | ||||
Item 4 | ||||
Part II | ||||
Item 5 | ||||
Item 6 | ||||
Item 7 | ||||
Item 7A | ||||
Item 8 | ||||
Item 9 | ||||
Item 9A | ||||
Item 9B | ||||
Part III | ||||
Item 10 | ||||
Item 11 | ||||
Item 12 | ||||
Item 13 | ||||
Item 14 | ||||
Part IV | ||||
Item 15 | ||||
Signatures | ||||
i
BOVIE MEDICAL CORPORATION
Cautionary Notes Regarding “Forward-Looking” Statements
This report contains statements that we believe to be “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements give our current expectations or forecasts of future events. Forward-looking statements generally can be identified by the use of forward-looking terminology such as “may,” “will,” “expect,” “intend,” “estimate,” “anticipate,” “believe,” “project,” or “continue,” or similar words or the negative thereof. From time to time, we also may provide oral or written forward-looking statements in other materials we release to the public. Any or all of our forward-looking statements in this report and in any public statements we make could be materially different from actual results. They can be affected by assumptions we might make or by known or unknown risks or uncertainties. Consequently, we cannot guarantee any forward-looking statements. Investors are cautioned not to place undue reliance on any forward-looking statements. Investors should also understand that it is not possible to predict or identify all such factors and should not consider the risk factors discussed in Item 1A below to be a complete statement of all potential risks and uncertainties. Past performance is no guaranty of future results.
ii
BOVIE MEDICAL CORPORATION
PART I
ITEM 1. Business
General
Bovie Medical Corporation (“Company”, “Bovie Medical”, “we”, “us”, or “our”) was incorporated in 1982, under the laws of the State of Delaware and has its principal executive office at 4 Manhattanville Road, Suite 106, Purchase, NY 10577.
We are an energy-based medical device company specializing in developing, manufacturing and marketing a range of electrosurgical products and technologies, as well as related medical products used in doctor’s offices, surgery centers and hospitals worldwide. Our medical devices are marketed through Bovie’s own well-respected brands (Bovie®, IDS™, ICON™ and DERMTM) and on a private label basis to distributors throughout the world. The Company also leverages its expertise in the design, development and manufacturing of electrosurgical equipment by producing equipment for large, well-known medical device manufacturers through original equipment manufacturing (OEM) agreements, as well as start-up companies with the need for our energy based designs.
We are also the developer of J-Plasma; a patented helium-based plasma surgical product which we believe has the potential to be a transformational product for surgeons. J-Plasma has FDA clearance for the cutting, coagulation and ablation of soft tissue. The J-Plasma system consists of an electrosurgical generator unit (ESU), a handpiece and a supply of helium gas. Radiofrequency (RF) energy is delivered to the handpiece by the ESU and used to energize an electrode. When helium gas is passed over the energized electrode, a helium plasma is generated which allows for conduction of the RF energy from the electrode to the patient in the form of a precise helium plasma beam. The energy delivered to the patient via the helium plasma beam is very precise and cooler in temperature in comparison to other surgical energy modalities such as standard RF monopolar energy. J-Plasma has been the subject of ten white papers and has been cited therein for its clinical utility in gynecological and plastic surgery procedures.
Significant Subsidiaries
Aaron Medical Industries, Inc. is a wholly-owned Florida corporation based in Clearwater, Florida. It is principally engaged in the business of marketing our medical products.
Bovie Bulgaria, EOOD is a wholly-owned limited liability company incorporated under Bulgarian law, located in Sofia, Bulgaria. It is engaged in the business of engineering and manufacturing our electrosurgical and OEM products.
Industry
Healthcare reform has caused consolidation among providers, with hospitals merging, physician practices joining hospitals and institutions combining to form Accountable Care Organizations, to manage patients on an interdisciplinary basis. Although the medical device industry can be challenging and very competitive, we believe it will continue to have a positive, long-term growth trajectory with the number of surgical procedures performed increasing annually as a result of the aging “baby boomer” population and other healthcare trends. Additionally, we also anticipate a continued increase in minimally invasive surgical procedures due to ongoing advancements in technology coupled with continued overall pressure to reduce healthcare costs via a reduction in patient trauma and recovery time. Markets will also continue to provide growth opportunities for the medical device industry.
We believe that Bovie Medical has sustainable, competitive advantages in the medical device market for several reasons. We have a long history in electrosurgery. Our inspiration dates back to the first use of an electrosurgical generator in an operating room in the U.S. in 1926 where Dr. William T. Bovie was present. Thus, the Bovie name is recognized by surgeons the world over for having pioneered the electrosurgery field and is recognized for its outstanding product quality supported by strong engineering and research and development capabilities. This history equates to very strong recognition of the Bovie brand. We believe that our equipment and devices have and will continue to provide better experiences for patients at a lower cost to the healthcare system.
Business Strategy
Our objective is to achieve profitable, sustainable growth by increasing our market share in the advanced energy category, including the commercialization of products that have the potential to be transformational with respect to the results they produce for surgeons and patients. In order to achieve this objective, we plan to leverage our long history in the industry, along with the reputation for quality and reliability that the Bovie brand enjoys within the medical community. At the same time, we will expand our product offerings beyond radio frequency devices, move forward with research and developments projects aimed at creating value within our existing product portfolio and build our pipeline of new complementary products and utilize multiple channels to bring new and existing products to market.
1
BOVIE MEDICAL CORPORATION
We are working to build our position in advanced electrosurgical generators and disposables, which can be used in diversified niche markets with minimally invasive surgical instruments, while furthering our status as a pioneer in plasma technology and its various medical applications.
Our J-Plasma product initially received FDA clearance in 2012 and a CE mark in December, 2014, which enables us to sell the product in Europe. In 2014, we brought together a new management team and created and trained a direct sales force dedicated to J-Plasma. In 2015, we continued the commercialization process for J-Plasma with a multi-faceted strategy designed to accelerate adoption of the product. This strategy primarily involved deployment of a dedicated sales force, extending and customizing the J-Plasma product line and expanding the surgical specialties in which J-Plasma can become the “standard of care“ for certain procedures.
By the end of 2016, we had 17 field-based selling professionals and a network of 18 independent manufacturing representatives, resulting in a total sales force of 35. This is a hospital based selling organization with its focus on the use of J-Plasma for operating room procedures.
Additionally, in 2015, we launched six new J-Plasma hand piece configurations and the Bovie Ultimate generator, which combines J-Plasma functionality with standard electrosurgery modes in one generator. In 2016, we launched our Precise 360 hand piece, which has an angled and rotating tip that enables surgeons to access structures that are difficult to reach using a straight laparoscopic device. The Precise 360 Handpiece was named “Innovation of the Year” by the Society of Laparoendoscopic Surgeons (SLS) at their annual meeting in September 2016. The Bovie Ultimate Generator received a similar recognition in both 2015 and 2014. As a result of our sales, marketing and product development initiatives in 2016, we have significantly increased the number of surgeons using the product, gained approvals for the sale of J-Plasma from Hospital Value Analysis Committees and signed agreements for the use of J-Plasma by the members of Group Purchasing Organizations that serve the medical community.
In order to assist us in leveraging J-Plasma’s precision and effectiveness in multiple surgical specialties, we launched a Medical Advisory Board in 2015 which is currently comprised of surgeons who are recognized leaders in urology, cardiovascular and cardiothoracic surgery. In 2016, we added an additional surgeon to this board from the GYN surgical specialty.
We are continuing to make substantial investments in the development and marketing of our J-Plasma technology for the long term benefit of the Company and its stakeholders and this may adversely affect our short term profitability and cash flow, particularly over the next 12 to 24 months. While we believe that these investments have the potential to generate additional revenues and profits in the future, there can be no assurance that J-Plasma will be successful or that such future revenues and profitability will be realized. From June of 2010 through December 31, 2015, we invested approximately $11.0 million in the development and marketing of our J-Plasma technology and an additional approximately $8.1 million in 2016, bringing the total investment to approximately $19.1 million since inception.
Company Products
We group our products into three main categories: electrosurgery, battery-operated cauteries and other products. Information regarding sales by product categories and related percentages can be found in our annual and quarterly reports filed with the SEC. We manufacture and market various medical products, both under private label and the Bovie brands (Bovie, IDS, ICON and DERM), to distributors worldwide. Additionally, Bovie has original equipment manufacturing (OEM) agreements with other medical device manufacturers. These OEM and private label arrangements and our use of the Bovie brands enable us to gain greater market share for the distribution of our products.
Electrosurgery Products
Electrosurgery is our largest product line and includes desiccators, generators, electrodes, electrosurgical pencils and various ancillary disposable products. These electrosurgical products are used during surgical procedures in gynecology, urology, plastic surgery, dermatology, veterinary and other surgical markets for the cutting and coagulation of tissue. It is estimated that electrosurgery is used in 80% of all surgical procedures. Our electrosurgery products fall under two categories, monopolar and bipolar. Monopolar products require the use of a grounding pad attached to the patient for the return of the electrical current, while bipolar products consist of two electrodes; one for the inbound current and one for the return current and therefore do not require the use of a grounding pad.
2
BOVIE MEDICAL CORPORATION
DERM101 and DERM 102
These effective and economical 10 watt high frequency desiccators provide a low wattage platform for minor in-office skin procedures. We designed these products specifically for family practice physicians, pediatricians and other general practitioners, enabling them to perform simple skin procedures in their offices instead of referring the patient to a specialist saving the patient time and providing additional revenue generating procedures for the physician.
DERM A942™, Bantam|Pro A952™ and Specialist|Pro A1250S
Bovie’s line of electrosurgical generators has been recently updated to provide a more modern look for today’s physician office with added features.
The new Bovie DERM A942 is a low powered 40-watt high frequency desiccator designed primarily for the dermatology and family practice physicians. These units are used mainly for removing skin lesions and growths as well as for coagulation in office-based procedures. The A942 is an upgrade from the previous version A940.
The Bovie Bantam|Pro A952 is a 50-watt high frequency desiccator with the added feature of a cut capacity for outpatient surgical procedures. In effect, the Bovie Bantam|Pro is two independent surgical devices in one small package. The Bantam|Pro replaces the Aaron A950 but with the added feature of Bovie NEM (neutral electrode monitoring), a safety feature that reduces the potential for alternate site burns. This unit is designed mainly for use in doctors’ offices and is used in a broad range of specialties including dermatology, gynecology, family practice, urology, plastic surgery and ophthalmology.
The Bovie Specialist|Pro A1250S is a 120-watt multipurpose electrosurgery generator. The unit features monopolar and bipolar functions with pad sensing. This product is considered to be ideally suited for office-based procedures in the specialties of gynecology, plastic surgery and urology.
Bovie Surgicenter|Pro A2350 - IDS210, Bovie OR|Pro A3350 - IDS310 and IDS400
To address market demand for more powerful electrosurgical generators, Bovie developed 200, 300 and 400-watt multipurpose digital electrosurgery generators designed for the rapidly expanding surgi-center market and the hospital outpatient and inpatient markets. This equipment includes digital hardware that enables very high parallel data processing throughout the operation or procedure. All data is sampled and processed digitally. For the first time in electrosurgery, generators are able to measure tissue impedance in real time (5,000 times a second) thanks to the utilization of digital technology. The design of these units is based on a digital feedback system. By using dedicated digital hardware in place of a general purpose controller for processing data, our equipment enables the power to be adjusted as the impedance varies, to deliver a consistent clinical effect.
Bovie Surgicenter|Pro A2350 and IDS210 are 200-watt generators that have the capability to be used in the majority of procedures performed today in surgi-center or outpatient settings. Although 200 watts is adequate to do most procedures in the operating room, 300 watts is considered the standard and believed to be what most hospitals and surgi-centers will require. To meet this requirement, we developed Bovie OR|Pro A3350 - IDS310. The Bovie OR|Pro and IDS310 incorporate the best features of the IDS 300 and upgrade its capabilities by providing additional bipolar options, including the 225-watt Bovie bipolar and an auto bipolar feature. The 300 watt units also offer the capability to utilize two pencils with simultaneous activation in fulguration mode. In addition, these newer models meet new standards required to sell these products in many of the global markets. The Bovie IDS 400 is a 400-watt generator designed primarily for sale in markets outside of the United States. These units feature both monopolar and bipolar functions, have pad and tissue sensing and include nine blended cutting setting.
Electrosurgical Disposables
Resistick™ II
Resistick II is a trademarked and proprietary coating that is applied to stainless steel that resist eschar (scab or scar tissue caused by burning) during surgery. We have experienced strong demand for this product since its introduction in 2011 and it represents our continued expansion of the Bovie line of electrosurgical disposables.
3
BOVIE MEDICAL CORPORATION
Disposable Laparoscopic Electrodes
We have introduced a line of disposable laparoscopic electrodes in Resistick coated and stainless steel for use by physicians from a broad group of specialties including gynecology, general surgery and urology. These electrodes are offered in J-hook, L-hook, needle, ball and spatula design and have an adapter included which makes these laparoscopic electrodes usable with a 3/32“ or 4mm plug.
Cauteries
Battery Operated Cauteries
Battery operated cauteries were originally designed for precise hemostasis (to stop bleeding) in ophthalmology. The current use of cauteries has been substantially expanded to include a broad range of applications. Battery operated cauteries are primarily sterile one-time use products. We have continued to improve our offering and recently had a patent issued covering our snap design cautery. It features a switch mechanism that dramatically reduces the potential for accidental activation. We manufacture one of the broadest lines of cauteries in the world, including but not limited to, a line of replaceable battery and replaceable tip cauteries, which are popular in veterinary and overseas markets.
Other Products
Battery Operated Medical Lights
We manufacture and market a variety of specialty lighting instruments for use in ophthalmology as well as distribute specialty lighting instruments for general surgery, hip replacement surgery and for the placement of endotracheal tubes in emergency and surgical procedures. We also manufacture and market physicians’ office use penlights used in physician offices.
Nerve Locator Stimulator
We manufacture a nerve locator stimulator primarily used for identifying motor nerves in hand and facial reconstructive surgery. This instrument is a sterile, self-contained, battery-operated unit, for one time use.
Growth Products
During 2016, Growth Products consisted of the J-Plasma line of products. Bovie’s J-Plasma technology utilizes a helium ionization process producing a stable thin focused beam of ionized gas that can be controlled in a wide range of temperatures and intensities, providing the surgeon greater control and predictability with minimal thermal damage to surrounding tissue. The development of this helium plasma generator also includes the design of a new proprietary handpiece.
In March 2015, we launched The Bovie Ultimate™ generator. The Bovie Ultimate is a high frequency electrosurgical generator that can be used for delivery of RF energy and/or helium gas plasma to cut, coagulate and ablate soft tissue during open and laparoscopic surgical procedures. The generator offers users monopolar, bipolar and J-Plasma features in a single generator. It has both FDA clearance and CE Mark.
J-Plasma Disposable Portfolio
We offer different hand pieces for open and laparoscopic procedures. The helium-based plasma generated from these devices have been shown to cause less thermal damage to tissue than CO2 laser, argon plasma and RF energy products currently available on the market. The technology has a general indication and can be used for cutting, coagulating and ablating soft tissue. The two primary specialties that are targeted in phase one of the product launch are gynecology and plastic surgery. However, given the wide range of tissue applications for J-Plasma, we are now engaged in ongoing development to create products for urology, cardiovascular and cardiothoracic procedures. The advantages of helium plasma continue to be studied throughout the medical and scientific communities. We believe that surgical applications are just one area of opportunity for this technology.
In March 2016, we expanded our offering of laparoscopic hand pieces by introducing the J-Plasma Precise™ 360 configurations to the market. The four new J-Plasma Precise 360 configurations include two new lengths with either a blade or needle electrode with an angled tip that can be rotated 360 degrees by the user. These new configurations expand the procedure base for J-Plasma by providing surgeons with the tools they need to access additional anatomic locations.
4
BOVIE MEDICAL CORPORATION
Research and Development and New Products
Our research and development activities are an essential component of our efforts to develop innovative products for introduction in the marketplace to drive sales growth. We continue to emphasize the development of proprietary products and product improvements to complement and expand our existing product lines. In 2016, we spent approximately $2.6 million in R&D versus approximately $2.2 million during 2015, an increase of approximately 21.2%. Bovie products introduced to the market in 2016 as a result of our internal research and development opportunities include: J-Plasma Precise 360 hand pieces, the PlazXact™ arthroscopic ablator, the A952 and DERM 942 office based electrosurgical generators, the RF-1254 4MHz electrosurgical generator, and multiple new electrosurgical generators for OEM customers.
Sales & Marketing
The majority of our core products are marketed through medical distributors, which distribute to more than 6,000 hospitals and to doctors and other healthcare facilities. New distributors are contacted through responses to our advertising in international and domestic medical journals and our presence at domestic and international trade shows. International sales represented approximately 12.5% of total revenues in 2016, compared to approximately 16.9% in 2015. The decrease in international sales as a percentage of revenue is due to the discontinuation of certain international relationships and product offerings versus an increase in domestic sales and channel partnerships in Growth Products. Management estimates our products have been sold in more than 150 countries through local dealers coordinated by sales and marketing personnel at our Clearwater, Florida facility.
Competition
We compete with numerous manufacturers and distributors of medical supplies and devices, many of which are large and well-established. With the exception of J-Plasma and endoscopic instrumentation, which are sold directly to the end-user under our own brand, many of our products are private labeled. The majority of the products in our core business are sold through distributors under the Bovie label. The balance is private labeled for major distributors who sell it under their own name. By having private labeled and branded distribution, we are able to increase our position in the marketplace and compete with much larger organizations. While our private label customers distribute products through their internal sales force, the majority of our products are sold through distributors which increase our sales potential and help level the playing field relative to our large competitors that sell direct. Domestically, we continue to believe that we have a substantial market share in the field of electrosurgical generator manufacturing through our Bovie branded and OEM units.
Our main competitors in electrosurgical and accessory markets are Valleylab (a division of Medtronic), Conmed and Erbe Electromedizine. In the battery-operated cautery market, our main competitor is Beaver Visitec and in the endoscopic instrumentation market, it is Ethicon (a division of Johnson and Johnson) and Covidien Surgical Solutions. Currently, we are the only company with helium-based plasma and retractable blade products. However, there are argon plasma competitors and CO2 laser competitors for our target market. We believe our competitive position did not change in 2016.
Intellectual Property
We rely on our intellectual property that we have developed or acquired over the years including patents, trade secrets, technical innovations and various licensing agreements to provide our future growth and build our competitive position. We have been issued 33 patents in the United States and 19 foreign patents. We have 17 pending patent applications in the United States and 9 pending foreign applications. Our intellectual property portfolio for the technology and products related to Growth products is included in these totals and continues to grow. Specific to Growth products, we have been issued 15 U.S. and five foreign patents and we have 14 U.S. and six foreign applications pending. As we continue to expand our intellectual property portfolio we believe it is critical for us to continue to invest in filing patent applications to protect our technology, inventions and improvements. However, we can give no assurance that competitors will not infringe on our patent rights or otherwise create similar or non-infringing competing products that are technically patentable in their own right.
5
BOVIE MEDICAL CORPORATION
Manufacturing and Suppliers
We are committed to producing the most technically advanced and highest quality products of their kind available on the market. We manufacture the majority of our products on our premises in Clearwater, Florida and at our facility located in Sofia, Bulgaria, which are certified under the ISO international quality standards and are subject to continuing regulation and routine inspections by the FDA to ensure compliance with regulations relating to our quality system, medical device complaint reporting and adherence to FDA restrictions on promotion and advertising. In addition, we are subject to regulations under the Occupational Safety and Health Act, the Environmental Protection Act and other federal, state and local regulations.
During the fourth quarter of 2015, we acquired all of the outstanding shares of Bovie Bulgaria, EOOD. Bovie Bulgaria operates a 16,000 square foot ISO13485 certified and FDA registered manufacturing facility located in the capital city of Sofia, which houses manufacturing, development and assembly operations.
We also have collaborative arrangements with three foreign suppliers under which we request the development of certain items and components, which we purchase pursuant to purchase orders. Our purchase order commitments are never more than one year in duration and are supported by our sales forecasts.
Customers
We sell the majority of our current products through major distributors which include Cardinal Health, Independent Medical Co-Op Inc. (IMCO), McKesson Medical Surgical, Inc., Medline, National Distribution and Contracting Inc. (NDC) and Owens & Minor and have manufacturing agreements for private label of certain products with these and others.
During 2016, J-Plasma was named as an Innovative Technology by Vizient, the largest group purchasing organization (GPO) in the United States. In 2015, we signed long-term agreements with three GPO's and believe that partnering with GPO’s is critical to our sales efforts and J-Plasma commercialization efforts.
Backlog
The value of unshipped factory orders is not material.
Employees
At December 31, 2016, we had 217 full-time employees world-wide, of whom 6 were executive officers, 35 supervisory personnel, 17 sales personnel and 159 technical support, administrative and production employees. None of our current employees are covered by a collective bargaining agreement and we have never experienced a work stoppage. We consider our employee relations to be good.
6
BOVIE MEDICAL CORPORATION
ITEM 1A. Risk factors
In addition to risks and uncertainties in the ordinary course of business, important risk factors that may affect us are discussed below. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations.
Risks Related to Our Industry
The medical device industry is highly competitive and we may be unable to compete effectively.
The medical device industry is highly competitive. Many competitors in this industry are well-established, do a substantially greater amount of business and have greater financial resources and facilities than we do.
Domestically, we believe we rank third in the number of units sold in the field of electrosurgical generator manufacturing and we sell our products and compete with other manufacturers in various ways. In addition to advertising, attending trade shows and supporting our distribution channels, we strive to enhance product quality and functionality, improve user friendliness and expand product exposure.
We have also invested and continue to invest, substantial resources to develop and monetize our J-Plasma technology. If we are unable to gain acceptance in the marketplace of J-Plasma, our business and results of operations may be materially and adversely affected. From June of 2010 through December 31, 2016, we have invested approximately $19.1 million in the development and marketing of our J-Plasma technology.
We also compete by private labeling our products for major distributors under their label. This allows us to increase our position in the marketplace and thereby compete from two different approaches, our Bovie label and our customers’ private label. Our private label customers distribute our products under their name through their internal sales force. We believe our main competitors do not private label their products.
Lastly, at this time, we sell the majority of our products through distributors. Many of the companies we compete with sell direct, thus competing directly with distributors they sometimes use.
Our industry is highly regulated by the U.S. Food and Drug Administration and international regulatory authorities, as well as other governmental, state and federal agencies which have substantial authority to establish criteria which must be complied with in order for us to continue in operation.
United States
Our products and research and development activities are subject to regulation by the FDA and other regulatory bodies. FDA regulations govern, among other things, the following activities:
•Product development
•Product testing
•Product labeling
•Product storage
•Pre-market clearance or approval
•Advertising and promotion
•Product traceability and
•Product indications.
In the United States, medical devices are classified on the basis of control deemed necessary to reasonably ensure the safety and effectiveness of the device. Class I devices are subject to general controls. These controls include registration and listing, labeling, pre-market notification and adherence to the FDA Quality System Regulation. Class II devices are subject to general and special controls. Special controls include performance standards, post market surveillance, patient registries and FDA guidelines. Class III devices are those which must receive pre-market approval by the FDA to ensure their safety and effectiveness. Currently, we only manufacture Class I and Class II devices. Pre-market notification clearance must be obtained for some Class I and most Class II devices when the FDA does not require pre-market approval. All of our products have been cleared by, or are exempt from, the pre-market notification process.
7
BOVIE MEDICAL CORPORATION
A pre-market approval application is required for most Class III devices. A pre-market approval application must be supported by valid scientific evidence to demonstrate the safety and effectiveness of the device. The pre-market approval application typically includes:
•Results of bench and laboratory tests, animal studies and clinical studies
•A complete description of the device and its components; and
• | A detailed description of the methods, facilities and controls used to manufacture the device and proposed labeling. |
The pre-market approval process can be expensive, uncertain and lengthy. A number of devices for which pre-market approval has been sought by other companies have never been approved for marketing.
International Regulation
To market products in the European Union, our products must bear the “CE” mark. Manufacturers of medical devices bearing the CE mark have gone through a conformity assessment process that assures that products are manufactured in compliance with a recognized quality system and to comply with the European Medical Devices Directive.
Each device that bears a CE mark has an associated technical documentation that includes a description of the following:
•Description of the device and its components,
•A Summary of how the device complies with the essential requirements of the medical devices directive,
•Safety (risk assessment) and performance of the device,
•Clinical evaluations with respect to the device,
•Methods, facilities and quality controls used to manufacture the device and
•Proposed labeling for the device.
Manufacturing and distribution of a device is subject to ongoing surveillance by the appropriate regulatory body to ensure continued compliance with quality system and reporting requirements.
We began CE marking of devices for sale in the European Union in 1999. In addition to the requirement to CE mark, each member country of the European Union maintains the right to impose additional regulatory requirements.
Outside of the European Union, regulations vary significantly from country to country. The time required to obtain approval to market products may be longer or shorter than that required in the United States or the European Union. Certain European countries outside of the European Union do recognize and give effect to the CE mark certification. We are permitted to market and sell our products in those countries.
If we are unable to successfully introduce new products or fail to keep pace with competitive advances in technology, our business, financial condition and results of operations could be adversely affected. In addition, our research and development efforts rely upon investments and alliances and we cannot guarantee that any previous or future investments or alliances will be successful.
Our research and development activities are an essential component of our efforts to develop new and innovative products for introduction in the marketplace. New and improved products play a critical role in our sales growth. We continue to place emphasis on the development of proprietary products, such as our J-Plasma technology, and product improvements to complement and expand our existing product lines. We maintain close working relationships with physicians and medical personnel in hospitals and universities who assist in product research and areas of development. Our research and development activities are primarily conducted internally and are expensed as incurred. These expenses include direct expenses for wages, materials and services associated with the development of our products net of any reimbursements from customers. Research and development expenses do not include any portion of general and administrative expenses. Our research and development activities are conducted at our Clearwater, Florida and Sofia, Bulgaria facilities. We expect to continue making future investments to enable us to develop and market new technologies and products to further our strategic objectives and strengthen our existing business. However, we cannot guarantee that any of our previous or future investments in both facilities will be successful or that our new products such as J-Plasma and PlazXact arthroscopic ablator, will gain market acceptance, the failure of which would have a material adverse effect on our business and results of operations.
8
BOVIE MEDICAL CORPORATION
The amount expended by us on research and development of our products during the years 2016, 2015 and 2014, totaled approximately $2.6 million, $2.2 million and $1.4 million, respectively. During the past three years, we invested substantial resources in the development and marketing of our Growth product technology. We have not incurred any direct costs relating to environmental regulations or requirements. For 2017, we expect to invest 6.5% to 7.0% of revenue for research and development activities.
Even if we are successful in developing and obtaining approval for our new product candidates, there are various circumstances that could prevent the successful commercialization of the products.
Our ability to successfully commercialize our products will depend on a number of factors, any of which could delay or prevent commercialization, including:
• | the regulatory approvals of our new products are delayed or we are required to conduct further research and development of our products prior to receiving regulatory approval; |
• | we are unable to build a sales and marketing group to successfully launch and sell our new products; |
• | we are unable to raise the additional funds needed to successfully develop and commercialize our products or acquire additional products for growth; |
• | we are required to allocate available funds to litigation matters; |
• | we are unable to manufacture the quantity of product needed in accordance with current good manufacturing practices to meet market demand, or at all; |
• | our product is determined to be ineffective or unsafe following approval and is removed from the market or we are required to perform additional research and development to further prove the safety and effectiveness of the product before re-entry into the market; |
• | competition from other products or technologies prevents or reduces market acceptance of our products; |
• | we do not have and cannot obtain the intellectual property rights needed to manufacture or market our products without infringing on another company’s patents; or |
• | we are unsuccessful in defending against patent infringement or other intellectual property rights, claims that could be brought against us, our products or technologies; |
The failure to successfully acquire or develop and commercialize new products will have a material and adverse effect on the future growth of our business, financial condition and results of operations.
Our international operations subject us to foreign currency fluctuations and other risks associated with operating in foreign countries.
We operate internationally and enter into transactions denominated in foreign currencies. To date, we have not hedged our exposure to changes in foreign currency exchange rates and as a result, we are subject to foreign currency transaction and translation gains and losses. We purchase goods and services in U.S. dollars and Euros. Foreign exchange risk is managed primarily by satisfying foreign denominated expenditures with cash flows or assets denominated in the same currency therefore we are subject to some foreign currency fluctuation risk. Our currency value fluctuations were not material for 2016. In addition, political changes or instability throughout the world could adversely affect our business internationally.
Our operations and cash flows may be adversely impacted by healthcare reform legislation.
The Patient Protection and Affordable Care Act and Health Care and Education Affordability Reconciliation Act were enacted into law in the U.S. in March 2010. Among other initiatives, this legislation imposes a 2.3% excise tax on domestic sales of class I, II and III medical devices beginning in 2013. The Consolidated Appropriations Act, 2016, signed into law on Dec. 18, 2015, includes a two year moratorium on the medical device excise tax imposed by Internal Revenue Code section 4191. Thus, the medical device excise tax does not apply to the sale of a taxable medical device by the manufacturer, producer, or importer of the device during the period beginning on Jan. 1, 2016 and ending on Dec. 31, 2017. Substantially all of our products are class I or class II medical devices and in 2016 we paid no federal excise tax and 2015 we paid $0.5 million. As approximately 87.5% of our 2016 sales were derived in the U.S., we cannot predict if any additional regulations will be implemented at the federal or state level, or the effect of any future legislation or regulation in the U.S. or internationally.
We are aware that the Affordable Care Act is under review by Congress and the potential impact of any actions, which may be taken by Congress is unknown.
9
BOVIE MEDICAL CORPORATION
Our operations may experience higher costs to produce our products as a result of changes in prices for oil, gasoline and other commodities.
We use plastics and other petroleum-based materials along with precious metals contained in electronic components as raw materials in many of our products. Prices of oil and gasoline also significantly affect our costs for freight and utilities. Oil, gasoline and precious metal prices are volatile and may increase, resulting in higher costs to produce and distribute our products. Due to the highly competitive nature of the healthcare industry and the cost-containment efforts of our customers we may be unable to pass along cost increases through higher prices. If we are unable to fully recover these costs through price increases or offset through other cost reductions, our results of operations could be materially and adversely affected.
Our manufacturing facilities are located in Clearwater, Florida and Sophia, Bulgaria and could be affected due to multiple weather risks. Specifically, in Clearwater, Florida from hurricanes, physical changes in the planet due to climate change and similar phenomena.
Our manufacturing facilities are located in Clearwater, Florida and Sophia, Bulgaria and could be affected by multiple weather risks. Most notably hurricanes in Clearwater, Florida. Although we carry property and casualty insurance and business interruption insurance, future possible disruptions of operations or damage to property, plant and equipment due to hurricanes or other weather risks could result in impaired production and affect our ability to meet our commitments to our customers and impair important business relationships, the loss of which could adversely affect our operations and profitability. We do however maintain a backup generator at our Clearwater facility and a disaster recovery plan is in place to help mitigate this risk.
We do not produce hazardous materials or emissions that would adversely impact the environment. We do however, have air conditioning units and consume electricity which could be impacted by climate change in the form of increased rates. However, we do not believe the increase in expense from any rate increases, as a percentage of sales, would be material in the near term.
Risks Relating to Our Business
We have historically done a substantial amount of business with seven of our top ten customers, who are also major distributors of our product, which as a group have produced substantial revenues for our Company. Loss of business from a major customer will likely materially and adversely affect our business.
We manufacture the majority of our products on our premises in Clearwater, Florida and in Sofia, Bulgaria. Labor-intensive sub-assemblies and labor-intensive products may be out-sourced to our specification. Although we sell through distributors, we market our products through national trade journal advertising, direct mail, distributor sales representatives and trade shows, under the Bovie name and private label. Major distributors include Cardinal Health, Independent Medical Co-Op Inc. (IMCO), McKesson Medical Surgical, Inc., Medline, National Distribution and Contracting Inc. (NDC) and Owens & Minor. If any of these distributor relationships are terminated or not replaced, our revenue from the territories served by these distributors could be adversely affected.
We are also dependent on OEM customers who have no legal obligation to purchase products from us. Should such customers fail to give us purchase orders for the product after development, our future business and value of related assets could be negatively affected. Furthermore, no assurance can be given that such customers will give sufficient high priority to our products. Finally, disagreements or disputes may arise between us and our customers, which could adversely affect production and sales of our products.
We rely on certain suppliers and manufacturers for raw materials and other products and are vulnerable to fluctuations in the availability and price of such products and services.
Fluctuations in the price, availability and quality of the raw materials we use in our manufacturing could have a negative effect on our cost of sales and our ability to meet the demands of our customers. Inability to meet the demands of our customers could result in the loss of future sales.
In addition, the costs to manufacture our products depend in part on the market prices of the raw materials used to produce them. We may not be able to pass along to our customers all or a portion of our higher costs of raw materials due to competitive and marketing pressures, which could decrease our earnings and profitability.
10
BOVIE MEDICAL CORPORATION
We also have collaborative arrangements with three key foreign suppliers under which we request the development of certain items and components and we purchase them pursuant to purchase orders. Our purchase order commitments are never more than one year in duration and are supported by our sales forecasts. The majority of our raw materials are purchased from sole-source suppliers. While we believe we could ultimately procure other sources for these components, should we experience any significant disruptions in this key supply chain, there are no assurances that we could do so in a timely manner which could render us unable to meet the demands of our customers, resulting in a material and adverse effect on our business and operating results.
If we are unable to protect our patents or other proprietary rights, or if we infringe on the patents or other proprietary rights of others, our competitiveness and business prospects may be materially damaged.
We have been issued 33 patents in the United States and 19 foreign patents. We have 17 pending patent applications in the United States and 9 pending foreign applications. Our intellectual property portfolio for the technology and products related to Growth products are included in these totals and continues to grow. Specific to Growth products, we have been issued 15 U.S. and 5 foreign patents and we have 14 U.S. and 6 foreign applications pending. We intend to continue to seek legal protection, primarily through patents, for our proprietary technology. Seeking patent protection is a lengthy and costly process and there can be no assurance that patents will be issued from any pending applications, or that any claims allowed from existing or pending patents will be sufficiently broad or strong to protect our proprietary technology. There is also no guarantee that any patents we hold will not be challenged, invalidated or circumvented, or that the patent rights granted will provide competitive advantages to us. Our competitors have developed and may continue to develop and obtain patents for technologies that are similar or superior to our technologies. In addition, the laws of foreign jurisdictions in which we develop, manufacture or sell our products may not protect our intellectual property rights to the same extent as do the laws of the United States.
Adverse outcomes in current or future legal disputes regarding patent and other intellectual property rights could result in the loss of our intellectual property rights, subject us to significant liabilities to third parties, require us to seek licenses from third parties on terms that may not be reasonable or favorable to us, prevent us from manufacturing, importing or selling our products, or compel us to redesign our products to avoid infringing third parties’ intellectual property. As a result, our product offerings may be delayed and we may be unable to meet customers’ requirements in a timely manner. Regardless of the merit of any related legal proceeding, we have incurred in the past and may be required to incur in the future substantial costs to prosecute, enforce or defend our intellectual property rights. Even in the absence of infringement by our products of third parties’ intellectual property rights, or litigation related to trade secrets, we have elected in the past and may in the future elect to enter into settlements to avoid the costs and risks of protracted litigation and the diversion of resources and management’s attention. However, if the terms of settlements entered into with certain of our competitors are not observed or enforced, we may suffer further costs and risks. Any of these circumstances could have a material adverse effect on our business, financial condition and resources or results of operations.
Our ability to develop intellectual property depends in large part on hiring, retaining and motivating highly qualified design and engineering staff with the knowledge and technical competence to advance our technology and productivity goals. To protect our trade secrets and proprietary information, generally we have entered into confidentiality agreements with our employees, as well as with consultants and other parties. If these agreements prove inadequate or are breached, our remedies may not be sufficient to cover our losses.
We have been and may in the future become subject to litigation proceedings that could materially and adversely affect our business.
Other Litigation
In addition to the litigation risks and proceedings mentioned below, from time to time we may become subject to legal claims or proceedings related to securities, employment, customer or third party contracts, environmental regulations, or other matters. The costs involved in defending these claims could be substantial, which have had an adverse effect on our profitability. In addition, if other claims are asserted against us, we may be required to defend against such claims, or deem it necessary or advisable to initiate a legal proceeding to protect our rights, the expense and distraction of such a claim or proceeding, whether or not resolved in our favor, could materially and adversely affect our business, financial condition and operating results. Further, if a claim or proceeding were resolved against us or if we were to settle any such dispute, we may be required to pay damages and costs or refrain from certain activities, any of which could have a material adverse impact on our business, financial condition and operating results.
11
BOVIE MEDICAL CORPORATION
Product Liability Litigation
Although we carry liability insurance, due to the nature of our products and their use by professionals, we are subject to litigation from persons who claim injury during medical procedures in hospitals, physician’s offices or in clinics and defending such litigation is expensive, disruptive, time consuming and could adversely affect our business. We currently maintain product liability insurance with combined coverage limits of $10 million on a claims-made basis. There is no assurance that this coverage will be adequate to protect us from any possible liabilities (individually or in the aggregate) we might incur in connection with the sale or testing of our products. In addition, we may need increased product liability coverage as additional products are commercialized. This insurance is expensive and in the future may not be available on acceptable terms, if at all.
While it is our policy not to promote off-label unapproved use of our products, and we train our personnel to caution against such use, if health care professionals in their discretion use our products in such a manner, we may be exposed to risk of litigation.
Intellectual Property Litigation or Trade Secrets
We have in the past, experienced certain allegations of infringement of intellectual property rights and use of trade secrets and may receive other such claims, with or without merit, in the future. Previously, claims of infringement of intellectual property rights have sometimes evolved into litigation against us and they may continue to do so in the future. It is inherently difficult to assess the outcome of litigation. Although we believe we have had adequate defenses to these claims and that the outcome of the litigation will not have a material adverse impact on our business, financial condition, or results of operations, there can be no assurances that we will prevail. Any such litigation could result in substantial cost to us, significantly reduce our cash resources and create a diversion of the efforts of our technical and management personnel, which could have a material and adverse effect on our business, financial condition and operating results. If we are unable to successfully defend against such claims, we could be prohibited from future sales of the allegedly infringing product or products, which could materially and adversely affect our future growth.
Our business is subject to the potential for defects or failures associated with our products which could lead to recalls or safety alerts and negative publicity.
Manufacturing flaws, component failures, design defects, off-label uses or inadequate disclosure of product-related information could result in an unsafe condition or the injury or death of a patient. These problems could lead to a recall of, or issuance of a safety alert relating to our products and result in significant costs and negative publicity. Due to the strong name recognition of our brands, an adverse event involving one of our products could result in reduced market acceptance and demand for all products within that brand and could harm our reputation and our ability to market our products in the future. In some circumstances, adverse events arising from or associated with the design, manufacture or marketing of our products could result in the suspension or delay of our current regulatory reviews of our applications for new product approvals. We also may undertake voluntarily to recall products or temporarily shut down certain production lines based on internal safety and quality monitoring and testing data. Any of the foregoing problems could disrupt our business and have a material adverse effect on our business, results of operations, financial condition and cash flows.
We have incurred and may in the future incur impairments to our long-lived assets.
We review our long-lived assets, including intangible assets, for impairment annually or more frequently if events or changes in circumstances indicate that the carrying amount of these assets may not be recoverable. Additionally, if in any period our stock price decreases to the point where our fair value, as determined by our market capitalization, is less than the book value of our assets, this could also indicate a potential impairment and we may be required to record an impairment charge in that period which could adversely affect our results of operations.
Our valuation methodology for assessing impairment requires management to make judgments and assumptions based on historical experience and to rely heavily on projections of future operating performance. We operate in highly competitive environments and projections of future operating results and cash flows may vary significantly from actual results. Additionally, if our analysis indicates potential impairment to a long-lived intangible asset, we may be required to record additional charges to earnings in our financial statements, which could negatively impact our results of operations.
12
BOVIE MEDICAL CORPORATION
Risks Related to Our Stock
The market price of our stock has been and may continue to be highly volatile.
Our common stock is listed on the NYSE MKT Market under the ticker symbol “BVX”. The market price of our stock has been and may continue to be highly volatile and announcements by us or by third parties may have a significant impact on our stock price. These announcements may include:
•our listing status on the NYSE MKT Market;
•our operating results falling below the expectations of public market analysts and investors;
•developments in our relationships with or developments affecting our major customers;
•negative regulatory action or regulatory non-approval with respect to our new products;
•government regulation, governmental investigations, or audits related to us or to our products;
•developments related to our patents or other proprietary rights or those of our competitors and
•changes in the position of securities analysts with respect to our stock.
The stock market has from time to time experienced extreme price and volume fluctuations, which have particularly affected the market prices for the medical technology sector companies and which have often been unrelated to their operating performance. These broad market fluctuations may adversely affect the market price of our common stock.
Historically, when the market price of a stock has been volatile, holders of that stock have often instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. The lawsuit could also divert the time and attention of our management.
In addition, future sales by existing stockholders, warrant holders receiving shares upon the exercise of warrants, or any new stockholders receiving our shares in any financing transaction may lower the price of our common stock, which could result in losses to our stockholders. Future sales of substantial amounts of common stock in the public market, or the possibility of such sales occurring, could adversely affect prevailing market prices for our common stock or our future ability to raise capital through an offering of equity securities. Substantially all of our common stock is freely tradable in the public market without restriction under the Securities Act, unless these shares are held by our “affiliates”, as that term is defined in Rule 144 under the Securities Act.
We have no present intention to pay dividends on our common stock and, even if we change that policy, we may be unable to pay dividends on our common stock.
We currently do not anticipate paying any dividends on our common stock in the foreseeable future. We currently intend to retain future earnings, if any, to finance operations and invest in our business. Any declaration and payment of future dividends to holders of our common stock will be at the discretion of our board of directors and will depend on many factors, including our financial condition, earnings, capital requirements, level of indebtedness, statutory and contractual restrictions applying to the payment of dividends and other considerations that our board of directors deems relevant.
If we change that policy and commence paying dividends, we will not be obligated to continue paying those dividends and our stockholders will not be guaranteed, or have contractual or other rights, to receive dividends. If we commence paying dividends in the future, our board of directors may decide, in its discretion, at any time, to decrease the amount of dividends, otherwise modify or repeal the dividend policy or discontinue entirely the payment of dividends. Under the Delaware law, our board of directors may not authorize the payment of a dividend unless it is either paid out of our statutory surplus.
The low trading volume of our common stock may adversely affect the price of our shares and their liquidity.
Although our common stock is listed on the NYSE MKT exchange, our common stock has experienced low trading volume. Limited trading volume may subject our common stock to greater price volatility and may make it difficult for investors to sell shares at a price that is attractive to them.
13
BOVIE MEDICAL CORPORATION
We may in the future seek to raise funds through equity offerings, which could have a dilutive effect on our common stock.
In the future we may determine to raise capital through offerings of our common stock, securities convertible into our common stock or rights to acquire these securities or our common stock. For instance, we are authorized to issue up to 40,000,000 shares of common stock and up to 10,000,000 shares of preferred stock, of which 3,588,139 shares have been designated as Series B Convertible Preferred Stock. The result of sales of such securities, or the conversion of the Series B Convertible Preferred Stock into shares of common stock, the exercise of warrants issued in connection with such offering or the triggering of anti-dilution provisions in such securities would ultimately be dilutive to our common stock by increasing the number of shares outstanding. We cannot predict the effect this dilution may have on the price of our common stock. In addition, the shares of preferred stock may have rights which are senior or superior to those of the common stock, such as rights relating to voting, the payment of dividends, redemption or liquidation.
Exercise of warrants and options issued by us will dilute the ownership interest of existing stockholders.
As of December 31, 2016, the warrants issued by us in December 2013 were exercisable for up to approximately 94,375 shares of our common stock, representing approximately 0.3% of our outstanding common stock.
As of December 31, 2016, our outstanding stock options to our employees, officers, directors and consultants amounted to approximately 3,752,209 shares of our common stock, representing approximately 12.2% of our outstanding common stock.
The exercise of some or all of our warrants and stock options will dilute the ownership interests of existing stockholders. Any sales in the public market of the common stock issuable upon such conversion or exercise could adversely affect prevailing market prices of our common stock.
ITEM 1B. Unresolved Staff Comments
There are no outstanding unresolved comments from the staff of the Securities and Exchange Commission.
ITEM 2. Properties
Bovie currently maintains a 60,000 square foot facility which consists of office, warehousing, manufacturing and research space located at 5115 Ulmerton Rd., Clearwater, Florida. Monthly principal and interest payments relating to the purchase of this facility are approximately $29,000 per month.
In October, 2015, through our Bulgaria acquisition, we acquired a lease for 16,000 square feet of office, warehousing and manufacturing facilities located in Sofia, Bulgaria. The rental cost of the facility is approximately $4,333 per month.
In March 2014, we signed a lease for offices located in Purchase, New York. The lease is for 3,650 square feet of office space with a monthly cost of approximately $9,277 per month. This facility presently houses our executive offices.
ITEM 3. Legal Proceedings
Not Applicable.
Other Matters
In the normal course of business, we are subject, from time to time, to legal proceedings, lawsuits and claims. Such matters are subject to many uncertainties and outcomes are not predictable with assurance. If any of these matters arise in the future, it could affect the operating results of any one or more quarters.
ITEM 4. Mine Safety Disclosures
Not Applicable.
14
BOVIE MEDICAL CORPORATION
PART II
ITEM 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock currently is traded on the NYSE MKT. The table shows the reported high and low bid prices for the common stock during each quarter of the last eight respective quarters. These prices do not represent actual transactions and do not include retail markups, markdowns or commissions.
2016 | 2015 | ||||||||||
High | Low | High | Low | ||||||||
4th Quarter | 5.55 | 3.50 | 2.19 | 1.77 | |||||||
3rd Quarter | 5.21 | 1.68 | 2.80 | 1.95 | |||||||
2nd Quarter | 1.97 | 1.56 | 3.59 | 2.35 | |||||||
1st Quarter | 2.44 | 1.60 | 4.01 | 2.20 | |||||||
On March 6, 2017, the closing bid for our common stock as reported by the NYSE MKT exchange was $3.14 per share. As of March 6, 2017, we had 605 stockholders of record. Since many stockholders choose to hold their shares under the name of their brokerage firm, we estimate that the actual number of stockholders was over 3,500 shareholders.
Securities Authorized for Issuance Under Equity Compensation Plans
Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) | Weighted Average Exercise Price of Outstanding Options, Warrants and Rights (b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) (c) | |||||||
Equity compensation plans approved by security holders | 2,019,922 | $ | 2.19 | 730,078 | |||||
Equity compensation plans not approved by security holders (1) | 1,732,287 | $ | 4.03 | — | |||||
Total | 3,752,209 | $ | 3.04 | 730,078 | |||||
Dividend Policy
We have never declared or paid any cash dividends on our common stock and we currently do not anticipate paying cash dividends in the foreseeable future. We currently expect to retain any future earnings to fund the operation and expansion of our business.
15
BOVIE MEDICAL CORPORATION
Five Year Performance Graph
The following line graph compares the cumulative total return of our common shares with the cumulative total return of the Standard & Poor’s Composite 500 Stock Index (the "S&P 500 Index") and the Standard & Poor's Composite 500 Healthcare Sector Index (the "S&P 500 Healthcare Index"). The line graph assumes, in each case, an initial investment of $100 on December 31, 2012, based on the market prices at the end of each fiscal year through and including December 31, 2016, and reinvestment of dividends.
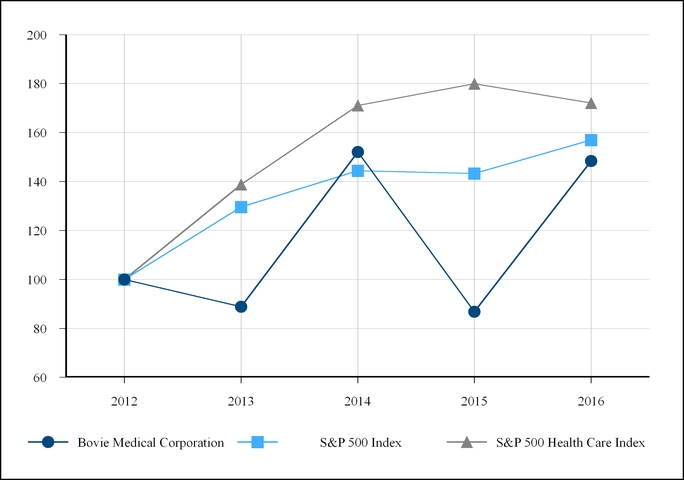
December 31, | ||||||||||||||
2012 | 2013 | 2014 | 2015 | 2016 | ||||||||||
Bovie Medical Corporation | 100.00 | 88.84 | 152.06 | 86.77 | 148.34 | |||||||||
S&P 500 Index | 100.00 | 129.60 | 144.36 | 143.31 | 156.97 | |||||||||
S&P 500 Health Care Index | 100.00 | 138.74 | 171.07 | 179.98 | 172.13 | |||||||||
16
BOVIE MEDICAL CORPORATION
ITEM 6. Selected Financial Data
The following selected consolidated financial data (presented in thousands, except per share amounts and employee data) are derived from our consolidated financial statements. This data should be read in conjunction with the consolidated financial statements and notes thereto and with Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
Sales | $ | 36,627 | $ | 29,520 | $ | 27,681 | $ | 23,660 | $ | 27,671 | |||||||||
Cost of sales | 18,712 | 16,963 | 18,689 | 14,462 | 16,338 | ||||||||||||||
Gross profit | 17,915 | 12,557 | 8,992 | 9,198 | 11,333 | ||||||||||||||
Other costs and expenses: | |||||||||||||||||||
Research and development | 2,618 | 2,160 | 1,416 | 1,260 | 1,329 | ||||||||||||||
Professional services | 1,486 | 1,484 | 1,016 | 1,835 | 1,439 | ||||||||||||||
Salaries and related costs | 9,038 | 7,482 | 5,723 | 3,992 | 3,178 | ||||||||||||||
Selling, general and administrative | 8,565 | 8,417 | 6,686 | 5,777 | 4,341 | ||||||||||||||
Total other costs and expenses | 21,707 | 19,543 | 14,841 | 12,864 | 10,287 | ||||||||||||||
(Loss) income from operations | (3,792 | ) | (6,986 | ) | (5,849 | ) | (3,666 | ) | 1,046 | ||||||||||
Interest expense, net | (158 | ) | (158 | ) | (151 | ) | (237 | ) | (232 | ) | |||||||||
Investor warrants issuance cost | — | — | — | (664 | ) | — | |||||||||||||
Fee associated with refinance | — | — | — | (543 | ) | — | |||||||||||||
Change in fair value of derivative liabilities, net | 64 | 1,799 | (7,285 | ) | (842 | ) | 20 | ||||||||||||
Total other (expense) income, net | (94 | ) | 1,641 | (7,436 | ) | (2,286 | ) | (212 | ) | ||||||||||
(Loss) income before income taxes | (3,886 | ) | (5,345 | ) | (13,285 | ) | (5,952 | ) | 834 | ||||||||||
Income tax expense (benefit) | 64 | 25 | 3,997 | (1,613 | ) | 217 | |||||||||||||
Net (loss) income | $ | (3,950 | ) | $ | (5,370 | ) | $ | (17,282 | ) | $ | (4,339 | ) | $ | 617 | |||||
Accretion on convertible preferred stock | — | (222 | ) | (932 | ) | (39 | ) | — | |||||||||||
Gain on conversion of warrants and preferred shares, net | — | 13,956 | — | — | — | ||||||||||||||
Deemed dividend on conversion beneficial conversion feature | — | — | — | (2,616 | ) | — | |||||||||||||
Net (loss) income attributable to common shareholders | $ | (3,950 | ) | $ | 8,364 | $ | (18,214 | ) | $ | (6,994 | ) | $ | 617 | ||||||
(Loss) income per share attributable to common shareholders | |||||||||||||||||||
Basic | $ | (0.14 | ) | $ | 0.34 | $ | (1.03 | ) | $ | (0.40 | ) | $ | 0.04 | ||||||
Diluted | $ | (0.15 | ) | $ | 0.24 | $ | (1.03 | ) | $ | (0.40 | ) | $ | 0.03 | ||||||
Balance Sheet Information: | |||||||||||||||||||
Cash and restricted cash | $ | 15,235 | $ | 12,644 | $ | 6,632 | $ | 7,924 | $ | 4,162 | |||||||||
Working capital | $ | 21,267 | $ | 17,921 | $ | 11,599 | $ | 16,910 | $ | 14,322 | |||||||||
Total assets | $ | 35,110 | $ | 31,448 | $ | 24,833 | $ | 33,176 | $ | 28,183 | |||||||||
Long-term liabilities | $ | 3,615 | $ | 3,923 | $ | 16,373 | $ | 8,934 | $ | 3,366 | |||||||||
Total stockholders' equity | $ | 26,223 | $ | 23,404 | $ | 1,504 | $ | 19,071 | $ | 22,895 | |||||||||
17
BOVIE MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis in conjunction with our financial statements and related notes contained elsewhere in this report. This discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of a variety of factors discussed in this report and those discussed in other documents we file with the SEC. In light of these risks, uncertainties and assumptions, readers are cautioned not to place undue reliance on such forward-looking statements. These forward-looking statements represent beliefs and assumptions as of the date of this report. While we may elect to update forward-looking statements and at some point in the future, we specifically disclaim any obligation to do so, even if our estimates change. Past performance does not guarantee future results.
Executive Level Overview
We are an energy-based medical device company specializing in developing, manufacturing and marketing a range of electrosurgical products and technologies, as well as related medical products used in doctor’s offices, surgery centers and hospitals worldwide. Our medical products include a wide range of devices including electrosurgical generators and accessories, cauteries, medical lighting, nerve locators and other products.
We internally divide our operations into three product lines; electrosurgery products, battery-operated cauteries and other products. The electrosurgical line includes electrosurgical products which include desiccators, generators, electrodes, electrosurgical pencils and various ancillary disposable products. These products are used in surgery for the cutting and coagulation of tissue. Battery-operated cauteries are used for precise hemostasis (to stop bleeding) in ophthalmology and in other fields. Our other revenues are derived from nerve locators, disposable and reusable penlights, medical lighting, license fees, development fees and other miscellaneous income.
The majority of our core products are marketed through medical distributors, which distribute to more than 6,000 hospitals, and to doctors and other healthcare facilities. New distributors are contacted through responses to our advertising in international and domestic medical journals and our presence at domestic and international trade shows. International sales represented approximately 12.5% of total revenues in 2016, 16.9% in 2015 and 15.8% in 2014. Management estimates our products have been sold in more than 150 countries through local dealers coordinated by sales and marketing personnel at the Clearwater, Florida facility.
On November 10, 2016, we entered into an underwriting agreement (the “Underwriting Agreement”) with certain selling stockholders of the Company (the “Selling Stockholders”) and Piper Jaffray & Co. (the “Underwriter”) relating to public offerings of our common stock, par value $0.001 per share at a public offering price of $4.00 per share. We made a primary offering of 1,625,000 shares and a secondary offering of 1,625,000 shares by the Selling Stockholders.
Our net proceeds from the sale of the shares, after deducting the Underwriter’s discounts and commissions and estimated offering expenses payable by us, were approximately $5.8 million. The offerings closed on November 16, 2016.
In March 2015, we completed an underwritten public offering of approximately 5,219,000 shares of common stock, par value $0.001 per share at a price to the public of $2.50 per share, resulting in net proceeds of approximately $11.5 million, after deducting underwriting discounts and commissions and offering expenses. We used the proceeds from the offering for operating costs, capital expenditures and for general corporate purposes, including working capital. Craig-Hallum Capital Group LLC (“Craig-Hallum”) acted as the sole managing underwriter for the offering.
Concurrently with the March 2015 offering, we completed the transactions contemplated under an exchange agreement with certain investors (the “Investors”) with respect to which Great Point Partners, LLC acts as investment manager. Pursuant to the terms of the exchange agreement, we issued 3,588,139 shares of our Series B Convertible Preferred Stock (the “Series B Preferred Stock”) in exchange for 3,500,000 shares of our Series A 6% Convertible Preferred Stock and warrants to purchase up to 5,250,000 shares of our common stock in the aggregate which were previously issued in conjunction with the sale of our Series A 6% Convertible Preferred Stock to the Investors in a December 13, 2013 offering, as well as accrued and unpaid preferred dividends. At December 31, 2016, the outstanding Series B Preferred Stock is convertible into an aggregate of 1,951,278 shares of our common stock.
In October, 2015 we entered into and consummated a Share Purchase Agreement whereby we acquired all of the outstanding equity interests of Bovie Bulgaria EOOD, a limited liability company incorporated under Bulgarian law.
18
BOVIE MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Our business is generally not seasonal in nature. Our international sales as a percentage of total revenue decreased to 12.5% in 2016 from 16.9% in 2015; the decrease in international sales as a percentage of revenue is due to the discontinuation of certain international relationships and product offerings versus an increase in domestic sales and channel partnerships in Growth Products.
During 2016, we continued our full scale commercialization efforts for J-Plasma. We have a direct sales force of 17 field-based selling professionals and a network of 18 independent manufacturing representatives, resulting in a total sales force of 35. This selling organization is focused on the use of J-Plasma for operating room procedures. In addition, we have invested in training programs and marketing-related activities to support accelerated adoption of J-Plasma.
We strongly encourage investors to visit our website: www.boviemedical.com to view the most current news and to review our filings with the Securities and Exchange Commission.
Results of Operations
Sales
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2016 | 2015 | Change | 2015 | 2014 | Change | |||||||||||||||
Sales by Product Line | |||||||||||||||||||||
Electrosurgical | $ | 20,901 | $ | 17,558 | 19.0 | % | $ | 17,558 | $ | 16,706 | 5.1 | % | |||||||||
Cauteries | 7,101 | 6,886 | 3.1 | % | 6,886 | 6,896 | (0.1 | )% | |||||||||||||
Other | 8,625 | 5,076 | 69.9 | % | 5,076 | 4,079 | 24.4 | % | |||||||||||||
Total | $ | 36,627 | $ | 29,520 | 24.1 | % | $ | 29,520 | $ | 27,681 | 6.6 | % | |||||||||
Sales by Domestic and International | |||||||||||||||||||||
Domestic | $ | 32,050 | $ | 24,540 | 30.6 | % | $ | 24,540 | $ | 23,313 | 5.3 | % | |||||||||
International | 4,577 | 4,980 | (8.1 | )% | 4,980 | 4,368 | 14.0 | % | |||||||||||||
Total | $ | 36,627 | $ | 29,520 | 24.1 | % | $ | 29,520 | $ | 27,681 | 6.6 | % | |||||||||
Sales by Operating Segment | |||||||||||||||||||||
Core | $ | 27,808 | $ | 26,098 | 6.6 | % | $ | 26,098 | $ | 24,322 | 7.3 | % | |||||||||
OEM | 5,328 | 2,116 | 151.8 | % | 2,116 | 3,150 | (32.8 | )% | |||||||||||||
Growth | 3,491 | 1,306 | 167.3 | % | 1,306 | 209 | 524.9 | % | |||||||||||||
Total | $ | 36,627 | $ | 29,520 | 24.1 | % | $ | 29,520 | $ | 27,681 | 6.6 | % | |||||||||
Overall sales increased by 24.1% or approximately $7.1 million for the year ended December 31, 2016 when compared with 2015. The increase in electrosurgical sales was mainly attributable to an increase in sales of generators of $2.9 million and electrodes of $0.4 million. Other product sales improved due to increases in Growth products of $2.2 million and OEM related products of $0.3 million. OEM related products included a subsequently discontinued pilot program for demonstration product to Hologic for $0.6 million and Arteriocyte for $0.2 million. Additionally, sales increased in coloscope products of $0.2 million, additional sales to distributors of $0.1 million and miscellaneous products for $0.5 million.
Although we have only one reporting segment, beginning in 2014, management began analyzing revenue and other operating metrics across three operating categories. Core product revenue, which consists of our brand name electrosurgical devices and accessories, cauteries, penlights, lighting, colposcopes and other similar products, increased 6.6% or approximately $1.7 million for the year ended December 31, 2016 when compared with 2015. The OEM product line consists of proprietary products designed specifically for third party equipment manufacturers; revenue for this product line increased 151.8% or approximately $3.2 million when compared to 2015. Growth product sales were $3.5 million, an increase of approximately 167.3% when compared to 2015.
Overall sales increased by 6.6%, or approximately $1.8 million, for the year ended December 31, 2015 when compared with 2014. The increase in sales was mainly attributable to an increase in Growth product sales of approximately $1.1 million, electrodes of $0.4 million, lighting of $0.4 million, other products of $0.4 million and a decrease of sales discounts for $0.3 million. Generator sales declined $0.8 million in 2015 due to the expiration of existing OEM contracts.
19
BOVIE MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Our ten largest customers accounted for approximately 54.4%, 58.3% and 61.0% of net revenues for the years ended December 31, 2016, 2015 and 2014, respectively. In 2016, McKesson accounted for 15.9% and National Distribution & Contracting Inc. accounted for 9.8% of our sales. In 2015, McKesson accounted for 18.6% and National Distribution & Contracting Inc. accounted for 13.3% of our sales. In 2014, National Distribution & Contracting Inc. accounted for 13.9% of our sales.
Gross Profit
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2016 | 2015 | Change | 2015 | 2014 | Change | |||||||||||||||
Cost of sales | $ | 18,712 | $ | 16,963 | 10.3 | % | $ | 16,963 | $ | 18,689 | (9.2 | )% | |||||||||
Percentage of revenue | 51.1 | % | 57.5 | % | 57.5 | % | 67.5 | % | |||||||||||||
Gross profit | $ | 17,915 | $ | 12,557 | 42.7 | % | $ | 12,557 | $ | 8,992 | 39.6 | % | |||||||||
Percentage of revenue | 48.9 | % | 42.5 | % | 6.4 | % | 42.5 | % | 32.5 | % | 10.0 | % | |||||||||
Our gross profit margin as a percentage of sales increased by 6.4% or approximately $5.4 million during the year ended December 31, 2016 compared with 2015. The increase was driven by higher margins in Growth and OEM products, comparatively. Additionally margins improved due to decreases in the warranty expense partially offset by the expensing of product molds no longer used in production.
Our gross profit margin as a percentage of sales increased by 10.0% or approximately $3.6 million during the year ended December 31, 2015 compared with 2014. This comparable increase was attributable to excess and obsolete inventory write downs of approximately $2.0 million in 2014, which reduced margins in that year.
We do not anticipate any material impact to our gross profit, material costs, or other costs as a result of the effect of inflation or any material impact of changing prices on net revenue.
Other Costs and Expenses
Research and development
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2016 | 2015 | Change | 2015 | 2014 | Change | |||||||||||||||
Research and Development expense | $ | 2,618 | $ | 2,160 | 21.2 | % | $ | 2,160 | $ | 1,416 | 52.5 | % | |||||||||
Percentage of revenue | 7.1 | % | 7.3 | % | 7.3 | % | 5.1 | % | |||||||||||||
Our expenditures for R&D related activities increased by 21.2% or approximately $0.5 million for the year ended December 31, 2016 compared with 2015. This was mainly caused by increased labor and material costs of approximately $0.4 million and an increase in consulting costs of approximately $0.1 million as we continue to accelerate our R&D product pipeline.
Our expenditures for R&D related activities increased by 52.5% or approximately $0.7 million for the year ended December 31, 2015 compared with 2014. This was mainly caused by increased labor and material costs of approximately $0.6 million and an increase in consulting costs of approximately $0.1 million.
20
BOVIE MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Professional services
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2016 | 2015 | Change | 2015 | 2014 | Change | |||||||||||||||
Professional services expense | $ | 1,486 | $ | 1,484 | 0.1 | % | $ | 1,484 | $ | 1,016 | 46.0 | % | |||||||||
Percentage of revenue | 4.1 | % | 5.0 | % | 5.0 | % | 3.7 | % | |||||||||||||
Professional services expenses increased 0.1% for the year ended December 31, 2016 compared with 2015. Legal fees decreased over the prior year by approximately $0.1 million due to the acquisition of Bovie Bulgaria and the public offering, which closed in March 2015, partially offset by an increase of $0.1 million in consulting fees.
Professional services costs increased 46.0% or approximately $0.5 million for the year ended December 31, 2015 compared with 2014. Accounting and auditing fees incurred in connection with our acquisition of Bovie Bulgaria increased approximately $0.1 million compared with 2014. Legal fees increased over the prior year by approximately $0.1 million due to the acquisition of Bovie Bulgaria and the public offering, which closed in March 2015. We experienced an increase of approximately $0.2 million related to investor relations and other professional services of $0.1 million compared to 2014.
Salaries and related costs
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2016 | 2015 | Change | 2015 | 2014 | Change | |||||||||||||||
Salaries and related expenses | $ | 9,038 | $ | 7,482 | 20.8 | % | $ | 7,482 | $ | 5,723 | 30.7 | % | |||||||||
Percentage of revenue | 24.7 | % | 25.3 | % | 25.3 | % | 20.7 | % | |||||||||||||
During 2016, salaries and related expenses increased approximately 20.8% or approximately $1.6 million compared to the prior year. The increase was attributable to $0.6 million for incentive compensation and $0.3 million related to direct sales force and associated management. Additionally, accounting and marketing each accounted for $0.2 million and regulatory, customer service and human resources of $0.1 million each.
During 2015, salaries and related expenses increased approximately 30.7% or approximately $1.8 million compared to the prior year. The increase was attributable to our hiring of management and production staff and the Growth product direct sales force and incentive compensation.
Selling, general and administrative expenses
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2016 | 2015 | Change | 2015 | 2014 | Change | |||||||||||||||
SG&A Expense | $ | 8,565 | $ | 8,417 | 1.8 | % | $ | 8,417 | $ | 6,686 | 25.9 | % | |||||||||
Percentage of revenue | 23.4 | % | 28.5 | % | 28.5 | % | 24.2 | % | |||||||||||||
Selling, general and administrative expense increased by 1.8% or approximately $0.1 million for the year ended December 31, 2016 compared with 2015. We experienced increases in sales commissions of approximately $1.0 million offset by decreases in ACA excise taxes of $0.4 million, sales related travel and entertainment costs of approximately $0.2 million, reduction in the bad debt reserve of $0.2 million and general insurance of $0.1 million.
Selling, general and administrative expense increased by 25.9% or approximately $1.7 million for the year ended December 31, 2015 compared with 2014. We experienced increases in advertising, marketing and trade show costs of approximately $0.5 million related largely to the branding and marketing of Growth products, sales commissions of approximately $0.5 million resulting from the increase in sales, an increase in general insurance of $0.2 million, sales related travel and entertainment costs of approximately $0.7 million, which was expected as we expanded our direct sales force and territory footprint and utilities, rents, maintenance, office and computer supplies of approximately $0.2 million, offset by decreases in professional services of $0.2 million and other costs of $0.2 million.
21
BOVIE MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Other Income (Expense), net
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2016 | 2015 | Change | 2015 | 2014 | Change | |||||||||||||||
Interest expense, net | $ | (158 | ) | $ | (158 | ) | — | % | $ | (158 | ) | $ | (151 | ) | 4.6 | % | |||||
Percentage of revenue | (0.4 | )% | (0.5 | )% | (0.5 | )% | (0.5 | )% | |||||||||||||
Change in fair value of derivative liabilities, net | $ | 64 | $ | 1,799 | (96.4 | )% | $ | 1,799 | $ | (7,285 | ) | (124.7 | )% | ||||||||
Percentage of revenue | 0.2 | % | 6.1 | % | 6.1 | % | (26.3 | )% | |||||||||||||
Interest expense, net
Total net interest expense was flat for the year ended December 31, 2016 as compared with 2015.
Total net interest expense increased by 4.6% for the year ended December 31, 2015 as compared with 2014.
Change in fair value of liabilities, net
On December 13, 2013, we entered into a securities purchase agreement pursuant to which we issued 3,500,000 shares of our newly designated Series A 6% Convertible Preferred Stock with a stated value of $2.00 per share and 5,250,000 warrants to purchase our common stock, at an exercise price of $2.387 per share. We also issued 525,000 warrants to the placement agent, of which 94,375 remain outstanding as of December 31, 2016. The warrants are accounted for as derivative financial instruments at fair value and are re-valued each period.
On March 17, 2015, we completed transactions contemplated under an exchange agreement (the “Exchange Agreement”) entered into on March 11, 2015 with certain investors (the “Investors”) with respect to which Great Point Partners, LLC acts as investment manager. Pursuant to the terms of the Exchange Agreement, we issued 3,588,139 shares of our Series B Convertible Preferred Stock (the “Series B Preferred Stock”) in exchange for 3,500,000 shares of our Series A 6% Convertible Preferred Stock and warrants to purchase up to 5,250,000 shares of our common stock in the aggregate which were previously issued in conjunction with the sale of our Series A 6% Convertible Preferred Stock to the Investors in a December 13, 2013 offering, as well as accrued and unpaid preferred dividends. The Series B Preferred Stock issued at that time was convertible into an aggregate of 7,176,298 shares of our common stock, upon the terms set forth in the Certificate of Designation. During the year ended December 31, 2016, the holders of Series B Preferred Stock exercised their conversion rights on 1,000,000 shares of Series B Preferred Stock. The remaining Series B Stock at December 31, 2016 is convertible into an aggregate of 1,951,278 shares of our common stock.
At December 31, 2016, the placement agent warrants were valued at $0.2 million and we recognized a net gain of $64,000.
At December 31, 2015, the placement agent warrants were valued at $0.3 million and we recognized a net gain of $1.8 million.
Income Taxes
The income tax provision is related to foreign and certain state income taxes. We have recorded a full valuation allowance against the net deferred tax assets with a finite life. A valuation allowance is required to be provided to reduce the deferred tax assets to a level which, more likely than not, will be realized. Management evaluated the positive and negative evidence in determining the realizability of the net deferred tax asset. In determining the need for valuation allowance, we reviewed historic operating results, updated 2016 actual results, as well as future income forecasts based on the projections, management concluded that it was not more likely than not that the Company should realize its net deferred tax assets through future operating results and the reversal of taxable temporary differences. If in the future we determine that we will be able to realize any of the net deferred tax assets, we will make an adjustment to the valuation allowance, which would increase our income in the period that the determination is made.
22
BOVIE MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Liquidity and Capital Resources
Our working capital at December 31, 2016 was approximately $21.3 million compared with $17.9 million at December 31, 2015. Accounts receivable days sales outstanding were 44 days and 34 days at December 31, 2016 and 2015, respectively. The number of days sales in inventory, which is the total inventory available for production divided by the 12-month average cost of materials, decreased 17 days to 160 days equating to an inventory turn ratio of 1.99 at December 31, 2016 from 177 days and an inventory turn ratio of 1.85 at December 31, 2015. The lower number of days sales in inventory which translated into a higher inventory turnover rate is mainly due to an increase in sales.
For the year ended December 31, 2016, net cash used in operating activities was approximately $2.8 million compared with net cash used by operating activities of approximately $5.8 million in 2015. The change was mainly attributable to the increase of stock based compensation of $0.8 million and the change in working capital of $0.7 million.
Net cash used in investing activities was approximately $0.3 million for the year ended December 31, 2016 compared to net cash used in investing activities was approximately $0.9 million during 2015. The change was due mainly to the acquisition of Bovie Bulgaria in 2015 and a reduction in purchases of equipment, molds and test fixtures in 2016.
Cash provided by financing activities of approximately $5.8 million during the year ended December 31, 2016, attributable to the proceeds from the public offering and the exercise of warrants, compared to cash provided by similar financing activities of approximately $12.8 million during year ended December 31, 2015.
On June 28, 2016, the Company entered into a transaction with Bank of Tampa, a Florida banking corporation (“Lender”) wherein Lender amended the terms of a mortgage loan (“the Loan”) originally executed on March 20, 2014 with a principal amount of $3,592,000. The Initial Maturity Date of the Loan was extended to July 20, 2019 from March 19, 2017, and the Extended Maturity Date was amended to July 20, 2024 from March 20, 2022. In addition, the Lender released as collateral to the Loan, the Company’s working capital accounts in exchange for a negative covenant limited to $2,000,000 of the aggregate indebtedness secured by these accounts.
The obligations under the Loan are secured by a first mortgage and security interest in the Company’s Clearwater, Florida facility. In addition, the Company has pledged an interest in a certificate of deposit in the amount of $779,000 as additional collateral. The amount of the additional collateral required declines on a pro rata basis as principal is paid.
Borrowings under the Loan bear interest at LIBOR plus 3.5%, with a fixed monthly principal payment of $19,956. The interest rate at December 31, 2016 was 4.272%.
The Loan documents contain customary financial covenants, including a covenant that the Company maintains a minimum liquidity of $750,000. Should we desire to extend the Loan beyond July 20, 2019, we must maintain a Debt Service Coverage Ratio for each of the preceding four quarters of not less than 1.0 to 1.0.
Approximate future expected principal and interest payments under the Loan agreement are as follows as of December 31, 2016:
(In thousands) | |||
2017 | $ | 247 | |
2018 | 247 | ||
2019 | 2,541 | ||
Total | $ | 3,035 | |
At December 31, 2016, we had purchase commitments for inventories totaling approximately $4.4 million, substantially all of which is expected to be purchased by the end of 2017.
We may choose to access the capital markets to raise additional capital. To facilitate this and to allow us to quickly react should we so decide, we filed a shelf registration statement with the Securities and Exchange Commission in December 2014.
23
BOVIE MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Critical Accounting Estimates
In preparing the consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP), we have adopted various accounting policies. Our most significant accounting policies are disclosed in Note 2 to the consolidated financial statements.
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires us to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Our estimates and assumptions, including those related to inventories, intangible assets, property, plant and equipment, legal proceedings, research and development, warranty obligations, product liability, fair valued liabilities, sales returns and discounts, stock based compensation and income taxes are updated as appropriate, which in most cases is at least quarterly. We base our estimates on historical experience, or various assumptions that are believed to be reasonable under the circumstances and the results form the basis for making judgments about the reported values of assets, liabilities, revenues and expenses. Actual results may materially differ from these estimates.
Estimates are considered to be critical if they meet both of the following criteria: (1) the estimate requires assumptions about material matters that are uncertain at the time the accounting estimates are made and (2) other materially different estimates could have been reasonably made or material changes in the estimates are reasonably likely to occur from period to period. Our critical accounting estimates include the following:
Inventory reserves
We maintain a reserve for excess and obsolete inventory resulting from the potential inability to sell our products at prices in excess of current carrying costs. The markets in which we operate are highly competitive, with new products and surgical procedures introduced on an ongoing basis. Such marketplace changes may cause our products to become obsolete. We make estimates regarding the future recoverability of the costs of these products and record a provision for excess and obsolete inventories based on historical experience and expected future trends. If actual product life cycles, product demand or acceptance of new product introductions are less favorable than projected by management, additional inventory write-downs may be required, which would unfavorably affect future operating results.
Long-lived assets
We review long-lived assets which are held and used, including property and equipment and intangible assets, for impairment whenever changes in circumstances indicate that the carrying amount of the assets may not be recoverable. Such evaluations compare the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset over its expected useful life and are significantly impacted by estimates of future prices and volumes for our products, capital needs, economic trends and other factors that are inherently difficult to forecast. If the asset is considered to be impaired, we record an impairment charge equal to the amount by which the carrying value of the asset exceeds its fair value determined by either a quoted market price, if any, or a value determined by utilizing a discounted cash flow technique.
Derivative liabilities valued at fair value
We generally do not use derivative financial instruments to hedge exposures to cash-flow risks or market-risks. However, certain financial instruments, such as warrants, which are indexed to our common stock, are classified as liabilities when either (a) the holder possesses rights to net-cash settlement or (b) physical or net-share settlement is not within our control. In such instances, net-cash settlement is assumed for financial accounting and reporting purposes, even when the terms of the underlying contracts do not provide for net-cash settlement. Such financial instruments are initially recorded and continuously carried, at fair value.
Determining the fair value of these instruments involves judgment and the use of certain relevant assumptions including, but not limited to, interest rate risk, historical volatility and stock price, estimated life of the derivative, anti-dilution provisions and conversion/redemption privileges. The use of different assumptions or changes in those assumptions could have a material effect on the estimated fair value amounts.
24
BOVIE MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Stock-based Compensation
Under our stock option plan, options to purchase common shares of the Company may be granted to key employees, officers and directors of the Company by the Board of Directors. The Company accounts for stock options in accordance with FASB ASC Topic 718-10, Compensation-Stock Compensation, with compensation expense amortized over the vesting period based on the trinomial lattice option-pricing model fair value on the grant date, which includes a number of estimates that affect the amount of our expense.
Litigation Contingencies
From time to time, we are exposed to claims and litigation arising in the ordinary course of business or otherwise and use various methods to resolve these matters in a manner that we believe serves the best interest of the Company and our stockholders. There can be no assurance these actions or other third party assertions will be resolved without costly litigation, or in a manner that is not adverse to our financial position. We do not believe that any of the currently identified claims or litigation matters will have a material adverse impact on our results of operations, cash flows or financial condition. However, given uncertainties associated with any litigation, if our assessments prove to be wrong, or if additional information becomes available such that we estimate that there is a possible loss or possible range of loss associated with these contingencies, then we would record the minimum estimated liability, which could materially impact our results of operations, financial position and cash flows.
Income Taxes
The provision for income taxes includes federal, foreign, state and local income taxes currently payable and those deferred because of temporary differences between the financial statement and tax bases of assets and liabilities. Deferred tax assets or liabilities are computed based on the difference between the financial statement and income tax bases of assets and liabilities using enacted marginal tax rates. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. Deferred income tax expenses or credits are based on the changes in the asset or liability from period to period.
We have net operating loss and tax credit carry forwards available in certain jurisdictions to reduce future taxable income. Future tax benefits for net operating loss and tax credit carry forwards are recognized to the extent that realization of these benefits is considered more likely than not. This determination is based on the expectation that related operations will be sufficiently profitable or various tax, business and other planning strategies will enable us to utilize the operating loss and tax credit carry forwards. We cannot be assured that we will be able to realize these future tax benefits or that future valuation allowances will not be required. To the extent that available evidence raises doubt about the realization of a deferred income tax asset, a valuation allowance is established.
It is our policy to provide for uncertain tax positions and the related interest and penalties based upon management’s assessment of whether a tax benefit is more likely than not to be sustained upon examination by tax authorities. To the extent that the probable tax outcome of these uncertain tax positions changes, such changes in estimate will impact the income tax provision in the period in which such determination is made. At December 31, 2016, we believe we have appropriately accounted for any unrecognized tax positions. To the extent we prevail in matters for which a liability for an unrecognized tax benefit is established or we are required to pay amounts in excess of the liability, our effective tax rate in a given financial statement period may be affected.
Since inception, we have been subject to tax by both federal and state taxing authorities. Until the respective statutes of limitations expire (which may be as much as 20 years while we have unused NOL’s), we are subject to income tax audits in the jurisdictions in which we operate.
Inflation
Inflation has not materially impacted the operations of our Company.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements at this time.
25
BOVIE MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Recent Accounting Pronouncements
See Note 10 of the Notes to Consolidated Financial Statements.
ITEM 7A. Quantitative and Qualitative Disclosures about Market Risk
Our short-term investments consist of cash, cash equivalents and overnight investments. As such, we do not believe we are exposed to significant interest rate risk. The primary objective of our investment activities is to preserve principal while at the same time maximizing yields without significantly increasing risk. To achieve this objective, we invest in highly liquid overnight money market investments. If a 10% change in interest rates were to have occurred on December 31, 2016, this change would not have had a material effect on the fair value of our investment portfolio as of that date.
26
BOVIE MEDICAL CORPORATION
ITEM 8. Financial Statements and Supplementary Data
INDEX TO FINANCIAL INFORMATION
Page | |
27
[LETTER HEAD OF FRAZIER & DEETER, LLC]
REPORT OF INDEPENDENT REGISTERED CERTIFIED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Bovie Medical Corporation
Purchase, New York
We have audited the accompanying consolidated balance sheets of Bovie Medical Corporation and subsidiaries (the "Company") as of December 31, 2016 and 2015 and the related consolidated statements of operations, stockholders’ equity, and cash flows for the years ended December 31, 2016, 2015 and 2014. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2016 and 2015, and the results of its operations and its cash flows for the years ended December 31, 2016, 2015 and 2014 in conformity with accounting principles generally accepted in the United States of America.
/s/ Frazier & Deeter, LLC
Frazier & Deeter, LLC
Tampa, FL
March 10, 2017
28
(In thousands, except share and per share data)
December 31, 2016 | December 31, 2015 | ||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 14,456 | $ | 11,805 | |||
Restricted cash | 779 | 839 | |||||
Trade accounts receivable, net | 4,733 | 2,925 | |||||
Inventories, net | 6,158 | 5,957 | |||||
Prepaid expenses and other current assets | 413 | 516 | |||||
Total current assets | 26,539 | 22,042 | |||||
Property and equipment, net | 6,449 | 6,810 | |||||
Brand name and trademark | 1,510 | 1,510 | |||||
Purchased technology and license rights, net | 215 | 323 | |||||
Goodwill | 185 | 185 | |||||
Deposits | 109 | 123 | |||||
Deferred tax asset | — | 25 | |||||
Other assets | 103 | 430 | |||||
Total assets | $ | 35,110 | $ | 31,448 | |||
LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 1,606 | $ | 1,214 | |||
Accrued payroll | 419 | 321 | |||||
Accrued vacation | 404 | 228 | |||||
Current portion of mortgage note payable | 239 | 239 | |||||
Accrued and other liabilities | 2,604 | 2,119 | |||||
Total current liabilities | 5,272 | 4,121 | |||||
Mortgage note payable, net of current portion | 2,694 | 2,934 | |||||
Note payable | 140 | 140 | |||||
Deferred rents | 14 | 18 | |||||
Deferred tax liability | 564 | 564 | |||||
Derivative liabilities | 203 | 267 | |||||
Total liabilities | 8,887 | 8,044 | |||||
Commitments and Contingencies (see Notes 9 and 11) | |||||||
Series A 6% convertible preferred stock, par value $0.001; 3,500,000 shares authorized, zero issued and outstanding as of December 31, 2016 and December 31, 2015 | — | — | |||||
STOCKHOLDERS' EQUITY | |||||||
Series B convertible preferred stock, $0.001 par value; 3,588,139 authorized and 975,639 issued and outstanding as of December 31, 2016 and 3,588,139 authorized and 1,975,639 issued and outstanding as of December 31, 2015, respectively | 1 | 2 | |||||
Common stock, $0.001 par value; 40,000,000 shares authorized; 31,002,832 issued and 30,859,753 outstanding as of December 31, 2016 and 27,194,251 issued and 27,051,172 outstanding as of December 31, 2015, respectively | 31 | 27 | |||||
Additional paid-in capital | 49,625 | 42,859 | |||||
Accumulated deficit | (23,434 | ) | (19,484 | ) | |||
Total stockholders' equity | 26,223 | 23,404 | |||||
Total liabilities and stockholders' equity | $ | 35,110 | $ | 31,448 | |||
The accompanying notes are an integral part of the consolidated financial statements.
29
(In thousands, except per share data)
Year Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Sales | $ | 36,627 | $ | 29,520 | $ | 27,681 | |||||
Cost of sales | 18,712 | 16,963 | 18,689 | ||||||||
Gross profit | 17,915 | 12,557 | 8,992 | ||||||||
Other costs and expenses: | |||||||||||
Research and development | 2,618 | 2,160 | 1,416 | ||||||||
Professional services | 1,486 | 1,484 | 1,016 | ||||||||
Salaries and related costs | 9,038 | 7,482 | 5,723 | ||||||||
Selling, general and administrative | 8,565 | 8,417 | 6,686 | ||||||||
Total other costs and expenses | 21,707 | 19,543 | 14,841 | ||||||||
Loss from operations | (3,792 | ) | (6,986 | ) | (5,849 | ) | |||||
Interest expense, net | (158 | ) | (158 | ) | (151 | ) | |||||
Change in fair value of derivative liabilities, net | 64 | 1,799 | (7,285 | ) | |||||||
Total other (expense) income, net | (94 | ) | 1,641 | (7,436 | ) | ||||||
Loss before income taxes | (3,886 | ) | (5,345 | ) | (13,285 | ) | |||||
Income tax expense | 64 | 25 | 3,997 | ||||||||
Net loss | $ | (3,950 | ) | $ | (5,370 | ) | $ | (17,282 | ) | ||
Accretion on convertible preferred stock | — | (222 | ) | (932 | ) | ||||||
Gain on conversion of warrants and preferred shares, net | — | 13,956 | — | ||||||||
Net (loss) income attributable to common shareholders | $ | (3,950 | ) | $ | 8,364 | $ | (18,214 | ) | |||
(Loss) income per share attributable to common shareholders | |||||||||||
Basic | $ | (0.14 | ) | $ | 0.34 | $ | (1.03 | ) | |||
Diluted | $ | (0.15 | ) | $ | 0.24 | $ | (1.03 | ) | |||
Weighted average number of shares outstanding - basic | 27,433 | 24,333 | 17,756 | ||||||||
Weighted average number of shares outstanding - dilutive | 27,449 | 27,747 | 17,756 | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
30
(In thousands, except share amounts)
Preferred Stock | Common Stock | ||||||||||||||||||||||||
Shares | Par Value | Shares | Par Value | Additional Paid-In Capital | Accumulated Deficit | Total | |||||||||||||||||||
Balance December 31, 2013 | — | $ | — | 17,684 | $ | 18 | $ | 28,687 | $ | (9,634 | ) | $ | 19,071 | ||||||||||||
Options exercised | — | — | 107 | — | 232 | — | 232 | ||||||||||||||||||
Warrants exercised | — | — | 112 | — | 237 | — | 237 | ||||||||||||||||||
Stock based compensation | — | — | — | — | 388 | — | 388 | ||||||||||||||||||
Stock swap to acquire options | — | — | (51 | ) | — | (210 | ) | — | (210 | ) | |||||||||||||||
Accretion on convertible preferred stock | — | — | — | — | — | (932 | ) | (932 | ) | ||||||||||||||||
Net loss | — | — | — | — | — | (17,282 | ) | (17,282 | ) | ||||||||||||||||
Balance December 31, 2014 | — | $ | — | 17,852 | $ | 18 | $ | 29,334 | $ | (27,848 | ) | $ | 1,504 | ||||||||||||
Options exercised | — | — | 98 | — | 220 | — | 220 | ||||||||||||||||||
Warrants exercised | — | — | 739 | — | 1,519 | — | 1,519 | ||||||||||||||||||
Issuance of common stock | — | — | 5,219 | 5 | 11,526 | — | 11,531 | ||||||||||||||||||
Conversion of Series A preferred stock and common warrants to Series B preferred stock | 3,588 | 4 | — | — | (40 | ) | 13,956 | 13,920 | |||||||||||||||||
Conversion of Series B convertible preferred to common stock | (1,612 | ) | (2 | ) | 3,225 | 4 | (2 | ) | — | — | |||||||||||||||
Stock based compensation | — | — | — | — | 575 | — | 575 | ||||||||||||||||||
Stock swap to acquire options and warrants | — | — | (81 | ) | — | (273 | ) | — | (273 | ) | |||||||||||||||
Accretion on convertible preferred stock | — | — | — | — | — | (222 | ) | (222 | ) | ||||||||||||||||
Net loss | — | — | — | — | — | (5,370 | ) | (5,370 | ) | ||||||||||||||||
Balance December 31, 2015 | 1,976 | $ | 2 | 27,052 | $ | 27 | $ | 42,859 | $ | (19,484 | ) | $ | 23,404 | ||||||||||||
Options exercised | — | — | 36 | — | 130 | — | 130 | ||||||||||||||||||
Warrants exercised | — | — | 293 | — | 698 | — | 698 | ||||||||||||||||||
Issuance of common stock | — | — | 1,625 | 2 | 5,828 | — | 5,830 | ||||||||||||||||||
Conversion of Series B convertible preferred to common stock | (1,000 | ) | (1 | ) | 2,000 | 2 | (1 | ) | — | — | |||||||||||||||
Stock based compensation | — | — | — | — | 809 | — | 809 | ||||||||||||||||||
Stock swap to acquire options and warrants | — | — | (146 | ) | — | (698 | ) | — | (698 | ) | |||||||||||||||
Net loss | — | — | — | — | — | (3,950 | ) | (3,950 | ) | ||||||||||||||||
Balance December 31, 2016 | 976 | $ | 1 | 30,860 | $ | 31 | $ | 49,625 | $ | (23,434 | ) | $ | 26,223 | ||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
31
(In thousands)
Year Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Cash flows from operating activities | |||||||||||
Net loss | $ | (3,950 | ) | $ | (5,370 | ) | $ | (17,282 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
Depreciation and amortization | 734 | 812 | 876 | ||||||||
Provision for inventory obsolescence | 178 | 157 | 733 | ||||||||
Gain on disposal of property and equipment, net | 21 | 21 | 14 | ||||||||
Stock based compensation | 809 | 575 | 388 | ||||||||
Change in fair value of derivative liabilities | (64 | ) | (1,799 | ) | 7,285 | ||||||
Provision for allowance for doubtful accounts | 74 | (59 | ) | (93 | ) | ||||||
Provision (benefit) for deferred taxes | 25 | (25 | ) | 3,975 | |||||||
Changes in current assets and liabilities: | |||||||||||
Trade receivables | (1,884 | ) | (874 | ) | 90 | ||||||
Prepaid expenses | 103 | 286 | (259 | ) | |||||||
Inventories | (379 | ) | (102 | ) | 1,955 | ||||||
Deposits and other assets | 341 | 228 | 952 | ||||||||
Accounts payable | 392 | (189 | ) | 494 | |||||||
Accrued and other liabilities | 763 | 553 | 455 | ||||||||
Net cash used in operating activities | (2,837 | ) | (5,786 | ) | (417 | ) | |||||
Cash flows from investing activities | |||||||||||
Purchases of property and equipment | (286 | ) | (421 | ) | (630 | ) | |||||
Acquisition of Bovie Bulgaria, net of cash acquired | — | (500 | ) | — | |||||||
Net cash used in investing activities | (286 | ) | (921 | ) | (630 | ) | |||||
Cash flows from financing activities | |||||||||||
Proceeds from stock options/warrants exercised | 124 | 1,427 | 259 | ||||||||
Change in restricted cash | 60 | 60 | (899 | ) | |||||||
Proceeds from (repayment of) mortgage note payable | (240 | ) | (239 | ) | 3,173 | ||||||
Proceeds from issuance of common shares, net | 5,830 | 11,531 | — | ||||||||
Repayment of industrial revenue bonds | — | — | (3,257 | ) | |||||||
Repurchase of warrants | — | — | (420 | ) | |||||||
Net cash provided by (used in) financing activities | 5,774 | 12,779 | (1,144 | ) | |||||||
Net change in cash and cash equivalents | 2,651 | 6,072 | (2,191 | ) | |||||||
Cash and cash equivalents, beginning of period | 11,805 | 5,733 | 7,924 | ||||||||
Cash and cash equivalents, end of period | $ | 14,456 | $ | 11,805 | $ | 5,733 | |||||
Cash paid for: | |||||||||||
Interest paid, net | $ | 158 | $ | 158 | $ | 151 | |||||
Non cash investing activities: | |||||||||||
Note payable for acquisitions | $ | — | $ | 140 | $ | — | |||||
The accompanying notes are an integral part of the consolidated financial statements.
32
NOTE 1. DESCRIPTION OF BUSINESS
Bovie Medical Corporation (“Bovie”) was incorporated in 1982, under the laws of the State of Delaware and is a medical device company engaged in the manufacturing and marketing of electrosurgical devices. Our medical products include a wide range of devices including electrosurgical generators and accessories, cauteries, medical lighting, nerve locators and other products.
NOTE 2. SIGNIFICANT ACCOUNTING POLICIES
Consolidated Financial Statements
The accompanying consolidated financial statements include the accounts of Bovie and its wholly owned subsidiaries, Aaron Medical Industries, Inc., Bovie Bulgaria, EOOD, BVX Holdings LLC and Bovie Holdings, Inc., (collectively, the “Company” or “we”, “our” or “us”). All intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates in the Preparation of Financial Statements
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements. The reported amounts of revenues and expenses during the reporting period may be affected by the estimates and assumptions we are required to make.
Cash and Cash Equivalents
Holdings of highly liquid investments with original maturities of three months or less are considered to be cash equivalents.
Fair Values of Financial Instruments and Concentration of Credit Risk
The carrying amounts of our financial instruments included in current assets and liabilities approximate fair value due to their short term nature. In addition, we believe the book values of our mortgage payable and capital lease payable approximates their fair values as the terms of such obligations approximate the terms at which similar types of borrowing arrangements could be currently obtained.
Financial instruments, which potentially subject us to significant concentrations of credit risk, consist primarily of cash and cash equivalents and trade accounts receivable. With respect to cash, we frequently maintain cash and cash equivalent balances in excess of federally insured limits. We have not experienced any losses in such accounts.
Derivative Financial Instruments
We generally do not use derivative financial instruments to hedge exposures to cash-flow risks or market risks. However, certain financial instruments, such as warrants, which are indexed to our common stock, are classified as liabilities when either (a) the holder possesses rights to net-cash settlement or (b) physical or net-share settlement is not within our control. In such instances, net-cash settlement is assumed for financial accounting and reporting purposes, even if the terms of the underlying contracts do not always provide for net-cash settlement. Such financial instruments are initially recorded and continuously carried, at fair value.
Determining the fair value of these instruments involves judgment and the use of certain relevant assumptions including, but not limited to, interest rate risk, historical volatility and stock price, estimated life of the derivative, anti-dilution provisions and conversion/redemption privileges. The use of different assumptions or changes in those assumptions could have a material effect on the estimated fair value amounts.
33
Accounts Receivable and Allowance for Doubtful Accounts
Our credit terms for our billings range from net 10 days to net 60 days, depending on the customer agreement. Accounts receivable are determined to be past due if payments are not made in accordance with such agreements and an allowance is generally recorded for accounts that become three months past due, or sooner if there are other indicators that the receivables may not be recovered. Customary collection efforts are initiated and receivables are written off when we determine they are not collectible and abandon these collection efforts. We gave negotiated sales volume discounts, which amounted to approximately $0.6 million, $0.3 million and $0.6 million for the years ended December 31, 2016, 2015 and 2014, respectively. Sales are reported net of all discounts.
We evaluate the allowance for doubtful accounts on a regular basis for adequacy based upon our periodic review of the collectability of the receivables in light of historical experience, adverse situations that may affect our customers’ ability to pay, estimated value of any underlying collateral and prevailing economic conditions. This evaluation is inherently subjective, as it requires estimates that are susceptible to significant revision as more information becomes available. Management believes that the allowances for doubtful accounts of approximately $0.1 million and $0.2 million at December 31, 2016 and 2015, respectively, are, or were, adequate to provide for possible bad debts.
With respect to receivables, our ten largest customers accounted for approximately 42.9% and 48.0% of trade receivables as of December 31, 2016 and 2015, respectively and 54.4%, 58.3% and 61.0% of net revenues for the years ended December 31, 2016, 2015 and 2014, respectively. In 2016, McKesson accounted for 15.9% and National Distribution & Contracting Inc. accounted for 9.8% of our sales. In 2015, McKesson accounted for 18.6% and National Distribution & Contracting Inc. accounted for 13.3% of our sales. In 2014, National Distribution & Contracting Inc. accounted for 13.9% of our sales. No other customer accounted for more than ten percent of our sales in 2014.
Inventories and Repair Parts
Inventories are stated at the lower of cost or market. Cost is determined on a first in, first out basis. Finished goods and work-in-process inventories include material, labor and overhead costs. Factory overhead costs are allocated to inventory manufactured in-house based upon labor hours.
We monitor usage reports to determine if the carrying value of any items should be adjusted due to lack of demand for the item and adjust the inventory for estimated obsolescence or unusable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required.
Inventories consisted of the following:
(In thousands) | December 31, 2016 | December 31, 2015 | |||||
Raw materials | $ | 4,521 | $ | 5,110 | |||
Finished goods | 3,048 | 2,080 | |||||
Gross inventories | 7,569 | 7,190 | |||||
Less: reserve for obsolescence | (1,411 | ) | (1,233 | ) | |||
Inventories, net | $ | 6,158 | $ | 5,957 | |||
The Company recorded changes in excess and obsolete inventory totaling approximately $0.2 million, $0.2 million and $2.0 million during 2016, 2015 and 2014, respectively. The change in 2014 was related to management updates to the commercial plan, book to physical inventory adjustments at both the Clearwater facility and consignments at foreign suppliers and other adjustments.
Property and Equipment
Property and equipment are recorded at cost. Depreciation and amortization are provided for using the straight-line method over the estimated useful lives of the assets. The amortization of leasehold improvements is based on the shorter of the lease term or the life of the improvement. Betterments and large improvements, which extend the life of the asset, are capitalized, whereas maintenance and repairs and small improvements are expensed as incurred. The estimated useful lives are: machinery and equipment, 3-10 years; buildings, 39 years; molds, 7-15 years and furniture and fixtures, 5-10 years.
34
Intangible Assets
Intangible assets consist of licenses, purchased technology and brand name and trademarks. The licenses and purchased technology are being amortized by the straight-line method over a 5-17 year period commencing with the date they were placed in service.
Brand name and trademark qualifies as an indefinite-lived intangible asset and is not subject to amortization. Intangibles with indefinite lives are analyzed for impairment annually or more frequently if events and circumstances indicate that the asset may be impaired. If impaired, an impairment loss is recognized in an amount equal to the excess of the asset’s carrying value over its fair value. Management concluded that the assigned value at December 31, 2016 of approximately $1.5 million was not impaired and is reasonable.
Other Long-Lived Assets
We review other long-lived assets for recoverability if events or changes in circumstances indicate that the assets may have been impaired. This circumstance exists when the carrying amount of the asset exceeds the sum of the undiscounted cash flows expected to result from its use and eventual disposition. In those cases an impairment loss is recognized to the extent that the assets’ carrying amount exceeds its fair value. Any impairment losses are not restored in the future if the fair value increases. At December 31, 2016, we believe the remaining carrying values of our long-lived assets are recoverable.
Revenue Recognition
Revenue is recognized when title has been transferred to the customer, which is generally at the time of shipment or receipt by customer for FOB destination terms. The following policies apply to our major categories of revenue transactions:
• | The majority of our sales to customers are evidenced by firm purchase orders. Generally, title and the risks and rewards of ownership are transferred to the customer when the product is shipped. Payment by the customer is due under fixed payment terms. |
• | Product returns are only accepted at our discretion and in accordance with our “Returned Goods Policy”. Historically, the level of product returns has not been significant. We accrue for sales returns, rebates and allowances based upon an analysis of historical customer returns and credits, rebates, discounts and current market conditions. |
• | Our terms of sale to customers generally do not include any obligations to perform future services. Limited warranties are generally provided for sales and provisions for warranty are provided at the time of product sale based upon an analysis of historical data. |
• | Amounts billed to customers related to shipping and handling charges are included in sales. Shipping and handling costs included in cost of sales were approximately $0.2 million, $0.1 million and $0.1 million in 2016, 2015 and 2014, respectively. |
Advertising Costs
All advertising costs are expensed as incurred. The amounts of advertising costs were approximately $0.7 million, $0.5 million and $0.5 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Stock-Based Compensation
We account for stock-based compensation in accordance with FASB ASC Topic 718, Compensation-Stock Compensation. FASB ASC 718 requires recognizing compensation costs for all share-based payment awards made to employees and directors based upon the awards’ grant date fair value. The standard covers employee stock options, restricted stock and other equity awards. For stock options, we use a trinomial lattice option-pricing model to estimate the grant date fair value of stock option awards and recognize compensation cost on a straight-line basis over the awards’ vesting periods.
35
Litigation Contingencies
From time to time, we are exposed to claims and litigation arising in the ordinary course of business or otherwise and use various methods to resolve these matters in a manner that we believe serves the best interest of the Company and our stockholders. There can be no assurance these actions or other third party assertions will be resolved without costly litigation, or in a manner that is not adverse to our financial position. We do not believe that any of the currently identified claims or litigation matters will have a material adverse impact on our results of operations, cash flows or financial condition. However, given uncertainties associated with any litigation, if our assessments prove to be wrong, or if additional information becomes available such that we estimate that there is a possible loss or possible range of loss associated with these contingencies, then we would record the minimum estimated liability, which could materially impact our results of operations, financial position and cash flows.
Tax Effects of Stock-Based Compensation
We will only recognize a tax benefit from windfall tax deductions for stock-based awards in additional paid-in capital if an incremental tax benefit is realized after all other tax attributes currently available have been utilized.
Net Loss Per Common Share
We compute basic earnings (loss) attributable to common shareholders per share by dividing net income (loss) attributable to common shareholders by the weighted average number of common shares outstanding for the reporting period. Diluted earnings (loss) per share attributable to common shareholders gives effect to all potential dilutive shares outstanding during the period. The number of dilutive shares is calculated using the treasury method which reduces the effective number of shares by the amount of shares we could purchase with the proceeds of assumed exercises. In 2015 and 2016, the diluted net income per share calculated included the dilutive effect of the employee stock options, warrants and the conversion of Series B Preferred Stock because their effects were dilutive.
In 2014, the net loss per share calculated when including the dilutive effect of the employee stock options and warrants are excluded from diluted net loss per common share calculations as of such dates because they are anti-dilutive and results in basic and diluted loss per share to be equivalent.
Research and Development Costs
With the exception of development costs that are purchased from another enterprise and have alternative future use, research and development expenses are charged to operations as incurred. We have expended approximately $2.6 million and $2.2 million and $1.4 million for the years ended 2016, 2015 and 2014 respectively.
Research and Development Costs for Others
For research and development activities that are partially or completely funded by other parties and when the obligation is incurred solely to perform contractual services, expenses are charged to cost of sales and all revenues resulting from such activities are shown as sales.
Income Taxes
We utilize the liability method of accounting for income taxes as set forth in FASB ASC 740. Under the liability method, deferred taxes are determined based on the temporary differences between the financial statement and tax basis of assets and liabilities using tax rates expected to be in effect during the years in which the basis differences reverse. Management evaluated the positive and negative evidence in determining the realizability of the net deferred tax asset. In determining the need for valuation allowance, we reviewed historic operating results, updated 2016 actual results, as well as future income forecasts based on the projections, management concluded that it was not more likely that the Company should realize its net deferred tax assets through future operating results and the reversal of taxable temporary differences.
If in the future we determine that we will be able to realize any of the net deferred tax assets, we will make adjustment to the valuation allowance, which would increase our income in the period that the determination is made.
36
We assess our income tax positions and record tax benefits for all years subject to examination based upon our evaluation of the facts, circumstances and information available as of the reporting date. For those tax positions where there is a greater than 50% likelihood that a tax benefit will be sustained, we have recorded the largest amount of tax benefit that may potentially be realized upon ultimate settlement with a taxing authority that has full knowledge of all relevant information. For those income tax positions where there is less than 50% likelihood that a tax benefit will be sustained, no tax benefit has been recognized in the financial statements.
NOTE 3. ACQUISITION OF BOVIE BULGARIA
On October 20, 2015 (the “Effective Date”), the Company and Nikolay Shilev entered into and consummated a Share Purchase Agreement (the “Purchase Agreement”) whereby the Company acquired all of the outstanding equity interests of Bovie Bulgaria EOOD, a limited liability company incorporated under Bulgarian law (“Bovie Bulgaria”). Pursuant to the terms of the Purchase Agreement, the Company agreed to pay Mr. Shilev approximately $559,000 payable as follows: (i) $419,000 payable within three business days after the effective registration of the Company as the sole shareholder of Bovie Bulgaria and (ii) $140,000 payable on the five year anniversary of the Effective Date.
In conjunction with the execution and consummation of the Purchase Agreement, the Company caused Bovie Bulgaria to enter into a Management Agreement with Mr. Shilev (the “Management Agreement”). Pursuant to the terms of the Management Agreement: (i) Mr. Shilev shall be engaged by the Company for a period of five years; (ii) the Company agreed to pay Mr. Shilev an annual base salary of $141,250; (iii) Mr. Shilev shall be entitled to, subject to certain limitations, an annual performance based bonus equal to twenty percent of Mr. Shilev’s base salary; (iv) as an inducement to enter into the Management Agreement, the Company awarded Mr. Shilev a restricted stock grant of 225,922 shares of the Company’s common stock, with such restricted stock vesting ratably over a five year period and subject to forfeiture upon Mr. Shilev’s Management Agreement being terminated for Cause or without “Good Reason” (as each is defined in the Management Agreement); and (v) the Company agreed to provide severance payments in the event of certain termination events as set forth in the Management Agreement.
The table below summarizes the preliminary purchase price and the preliminary fair values of the assets acquired and liabilities assumed at the acquisition date of October 20, 2015:
(In thousands) | |||
Cash and cash equivalents | $ | 59 | |
Inventories, net | 285 | ||
Prepaid expenses and other current assets | 1 | ||
Property and equipment, net | 167 | ||
Goodwill | 185 | ||
Deferred income tax assets, net | 25 | ||
Deposits, net of current portion | 8 | ||
Accounts payable | (150 | ) | |
Accrued and other liabilities | (21 | ) | |
Value of consideration paid | $ | 559 | |
NOTE 4. TRADE ACCOUNTS RECEIVABLE
Trade accounts receivable consisted of the following:
(In thousands) | December 31, 2016 | December 31, 2015 | |||||
Trade accounts receivable | $ | 4,851 | $ | 3,117 | |||
Less: allowance for doubtful accounts | (118 | ) | (192 | ) | |||
Trade accounts receivable, net | 4,733 | 2,925 | |||||
37
NOTE 5. PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consisted of the following:
(In thousands) | December 31, 2016 | December 31, 2015 | |||||
Land | $ | 1,600 | $ | 1,600 | |||
Machinery and equipment | 3,775 | 3,933 | |||||
Building and improvements | 4,237 | 4,200 | |||||
Furniture and fixtures | 2,194 | 2,237 | |||||
Leasehold improvements | 12 | 12 | |||||
Molds | 1,900 | 1,701 | |||||
Total property, plant and equipment | 13,718 | 13,683 | |||||
Less: accumulated depreciation | (7,269 | ) | (6,873 | ) | |||
Net property, plant and equipment | $ | 6,449 | $ | 6,810 | |||
Total depreciation expense was $0.6 million, $0.7 million and $0.7 million for the years ended December 31, 2016, 2015 and 2014, respectively. Depreciation expense is included primarily within cost of goods sold in the consolidated statements of operations.
NOTE 6. INTANGIBLE ASSETS
Intangible assets consisted of the following:
(In thousands) | December 31, 2016 | December 31, 2015 | |||||
Brand name and trademark (life indefinite) | $ | 1,510 | $ | 1,510 | |||
Purchased technology (5-17 year lives) | $ | 1,441 | $ | 1,441 | |||
Less: accumulated amortization | (1,226 | ) | (1,118 | ) | |||
Purchased technology, net | $ | 215 | $ | 323 | |||
Goodwill | $ | 185 | $ | 185 | |||
With respect to our trademark and brand name, we continue to market products, release new products and product extensions and maintain and promote these trademarks and brand name in the marketplace through legal registration and such methods as advertising, medical education and trade shows. It is our belief that these trademarks and brand names will generate cash flow for an indefinite period of time. Therefore, we believe our trademarks and brand name intangible assets are not impaired. Goodwill results from our acquisition of Bovie Bulgaria, EOOD.
Amortization of intangible assets was $0.1 million for the years ended December 31, 2016, 2015 and 2014. Amortization expense is classified within selling, general and administration expenses in the consolidated statements of operations.
Amortization expense amounts for the next three years are expected to be approximately $0.1 million for 2017 through 2019.
38
NOTE 7. EARNINGS PER SHARE
We compute basic earnings per share (“basic EPS”) by dividing the net income or loss by the weighted average number of common shares outstanding for the reporting period. Diluted earnings per share (“diluted EPS”) gives effect to all dilutive potential shares outstanding. The following table provides the computation of basic and diluted earnings per share.
Year Ended December 31, | |||||||||||
(in thousands, except per share data) | 2016 | 2015 | 2014 | ||||||||
Numerator: | |||||||||||
Net (loss) income available to common shareholders | $ | (3,950 | ) | $ | 8,364 | $ | (18,214 | ) | |||
Effect of dilutive securities: | |||||||||||
Derivative liability - warrants | (64 | ) | (1,799 | ) | — | ||||||
Accretion on convertible preferred stock | — | 222 | — | ||||||||
Numerator for dilutive (loss) income per common share | (4,014 | ) | 6,787 | (18,214 | ) | ||||||
Denominator: | |||||||||||
Weighted average shares used to compute basic (loss) income per common share | 27,433 | 24,333 | 17,756 | ||||||||
Effect of dilutive securities: | |||||||||||
Derivative liability - warrants | 16 | 28 | — | ||||||||
Convertible preferred stock | — | 3,137 | — | ||||||||
Stock options | — | 249 | — | ||||||||
Denominator for dilutive (loss) income per common share | 27,449 | 27,747 | 17,756 | ||||||||
Basic (loss) income per common share | $ | (0.14 | ) | $ | 0.34 | $ | (1.03 | ) | |||
Diluted (loss) income per common share | $ | (0.15 | ) | $ | 0.24 | $ | (1.03 | ) | |||
For the year ended December 31, 2016, warrants to purchase approximately 16,000 shares of common stock and approximately $64,000 of the gain on the fair market valuation of the derivative liabilities were included in the computation of diluted earnings per share because their effects were dilutive.
For the year ended December 31, 2015, warrants to purchase approximately 28,000 shares of common stock and approximately $1.8 million of the gain on the fair market valuation of the derivative liabilities, options to purchase approximately 249,000 shares of common stock and the conversion of Series B Preferred Stock into approximately 3,137,000 shares of common stock were included in the computation of diluted earnings per share because their effects were dilutive.
For the year ended December 31, 2014, options and warrants to purchase approximately 6,500,000 shares of common stock were excluded in the computation of diluted earnings per share because their effects were anti-dilutive.
NOTE 8. CAPITAL STOCK
Common Stock - We are authorized to issue 40,000,000 shares of common stock. Holders of common stock are entitled to one vote for each share held of record on each matter submitted to a vote of shareholders. Holders of our common stock do not have a cumulative voting right, which means that the holders of more than one half of our outstanding shares of common stock, subject to the rights of the holders of preferred stock, can elect all of our directors, if they choose to do so. In this event, the holders of the remaining shares of common stock would not be able to elect any directors. Subject to the prior rights of any class or series of preferred stock which may from time to time be outstanding, if any, holders of common stock are entitled to receive ratably, dividends when, as and if declared by our Board of Directors out of funds legally available for that purpose and, upon our liquidation, dissolution, or winding up, are entitled to share ratably in all assets remaining after payment of liabilities and payment of accrued dividends and liquidation preferences on the preferred stock, if any. Holders of common stock have no preemptive rights and have no rights to convert their common stock into any other securities. The outstanding common stock is duly authorized and validly issued, fully-paid and non-assessable. Except as otherwise required by Delaware law and subject to the rights of the holders of
39
preferred stock, all stockholder action is taken by the vote of a majority of the outstanding shares of common stock present at a meeting of shareholders at which a quorum consisting of a majority of the outstanding shares of common stock is present in person or by proxy. Shares repurchased are held as treasury shares and used for general corporate purposes including, but not limited to, satisfying obligations under our employee benefit plans. Treasury stock is recorded at cost.
On November 10, 2016, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with certain selling stockholders of the Company (the “Selling Stockholders”) and Piper Jaffray & Co. (the “Underwriter”) relating to public offerings of the Company's common stock, par value $0.001 per share at a public offering price of $4.00 per share. The Company made a primary offering of 1,625,000 shares and a secondary offering of 1,625,000 shares by the Selling Stockholders.
The net proceeds from the sale of the shares, after deducting the Underwriter’s discounts and commissions and estimated offering expenses payable, were approximately $5.8 million. The offerings closed on November 16, 2016.
The shares were offered and sold by the Company pursuant to a prospectus dated December 16, 2014 and a prospectus supplement filed with the Securities and Exchange Commission (the “SEC”) on November 10, 2016, which are part of the effective shelf registration statement on Form S-3 (File No. 333-200986) filed with the SEC on December 15, 2014. The shares were offered and sold by the Selling Stockholders pursuant to a prospectus dated April 24, 2015 and a prospectus supplement filed with the SEC on November 10, 2016, which are part of the effective registration statement on Form S-3 (File No. 333-203422) filed with the SEC on April 15, 2015.
Preferred Stock - We are authorized to issue 10,000,000 shares of preferred stock, par value $0.001 per share. We may issue preferred stock in one or more series and having the rights, privileges and limitations, including voting rights, conversion rights, liquidation preferences, dividend rights and preferences and redemption rights, as may from time to time be determined by our Board of Directors. Preferred stock may be issued in the future in connection with acquisitions, financings, or other matters, as our Board of Directors deems appropriate. In the event that we determine to issue any shares of preferred stock, a certificate of designation containing the rights, privileges and limitations of this series of preferred stock will be filed with the Secretary of State of the State of Delaware. The effect of this preferred stock designation power is that our Board of Directors alone, subject to Federal securities laws, applicable blue sky laws and Delaware law, may be able to authorize the issuance of preferred stock which could have the effect of delaying, deferring, or preventing a change in control of our company without further action by our shareholders and may adversely affect the voting and other rights of the holders of our common stock. The issuance of preferred stock with voting and conversion rights may also adversely affect the voting power of the holders of our common stock, including the loss of voting control to others.
Series B Convertible Preferred Stock – On March 16, 2015, the Company filed a Certificate to Set Forth Designations, Voting Powers, Preferences, Limitations, Restrictions and Relative Rights (the “Certificate of Designations”) of its Series B Convertible Preferred Stock with the Secretary of State of the State of Delaware to amend our articles of incorporation. The Certificate of Designations sets forth the rights, preferences and privileges of the Series B Preferred Stock. As provided in our articles of incorporation, the filing of the Certificate of Designations was approved by our Board of Directors. The following is a summary of the rights, privileges and preferences of the Series B Preferred Stock:
Number of Shares: The number of shares of Preferred Stock designated as Series B Preferred Stock are 3,588,139.
Conversion: The Series B Preferred Stock are convertible at the option of the holder, into common stock at a conversion ratio of one (1) share of Series B Preferred to two (2) shares of Common Stock, subject to adjustments for stock dividends, splits, combinations and similar events as described in the form of Certificate of Designations.
Dividends: The Series B Preferred Stock is not entitled to receive any special dividend.
Voting Rights: Except as described in the Certificate of Designations, holders of the Series B Preferred Stock will vote together with holders of the Company common stock on all matters, on an as-converted to common stock basis and not as a separate class or series (subject to limited exceptions).
Series A 6% Convertible Preferred Stock - During December 2013, our Board of Directors approved a Certificate of Designation of Preferences, Rights and Limitations of Series A 6% Convertible Preferred Stock, which Certificate was filed with the Secretary of State of the State of Delaware on December 13, 2013. These shares were exchanged for shares of Series B Preferred Stock issued in connection with the March 2015 Exchange Agreement and canceled. The following is a summary of the rights, privileges and preferences of the Series A Preferred Stock:
40
Number of Shares. The number of shares of Preferred Stock designated as Series A Preferred Stock was 3.5 million (which shall not be subject to increase without the written consent of all of the holders of the Series A Preferred Stock).
Stated Value: The initial Stated Value of each share of Series A Preferred Stock is $2.00 (as adjusted pursuant to the Certificate of Designations).
Conversion: The Series A Preferred Stock was convertible at the option of the holder, into common stock on a one-for-one basis, subject to adjustments for stock dividends, splits, combinations and similar events as described in the form of Certificate of Designations. In addition, the Company had the right to require the holders to convert to common stock under certain enumerated circumstances.
Redemption: At any time after the 48 month anniversary of the date of issuance of the Series A Preferred Stock, each share of Series A Preferred Stock was redeemable at the option of the holder thereof, for an amount equal to the Stated Value (the “Redemption Amount”). The Company would have paid the Redemption Amount as follows: (i) one third of such amount not later than five business days following the applicable Redemption Date (as defined in the Certificate of Designations); (ii) one third of such amount one year following the applicable Redemption Date; and (iii) one third of such amount two years following the applicable Redemption Date; provided, however, that if the applicable Redemption Date is a date following the eighty fourth (84th) anniversary of the issuance of the Series A Preferred Stock, the entire redemption amount shall be payable in one single payment.
Dividends: Dividends would have accrued on each share of Series A Preferred Stock at the rate of 6% of the stated value per year, compounded annually, whether or not declared. The holders of the Series A Preferred Stock, following notice, had the right to be paid an amount equal to one third of all accrued and unpaid dividends on the following dates: (i) the 48th month following the issuance of the Series A Preferred Stock; (ii) the 60th month following the issuance of the Series A Preferred Stock and (iii) the 72nd month following the issuance of the Series A Preferred Stock.
Voting Rights: Except as described in the Certificate of Designations, holders of the Series A Preferred Stock voted together with holders of the Company common stock on all matters, on an as-converted to common stock basis and not as a separate class or series (subject to limited exceptions).
Liquidation Preferences. In the event of any liquidation or winding up of the Company prior to and in preference to any Junior Securities (including common stock), the holders of the Series A Preferred Stock were entitled to receive in preference to the holders of the Company common stock a per share amount equal to the Stated Value (as adjusted pursuant to the Certificate of Designations).
NOTE 9. CONVERTIBLE PREFFERED STOCK AND WARRANTS
2013 Financing
On December 13, 2013, the Company entered into a securities purchase agreement with certain investors for the private placement, for aggregate gross proceeds of $7.0 million, of 3,500,000 shares of the Company’s newly-designated Series A 6% Convertible Preferred Stock (the “Series A Preferred Stock” – see Note 6) and warrants to purchase 5,250,000 shares of our common stock at an exercise price of $2.387 per share.
The shares of Series A Preferred Stock, which had a stated liquidation value of $2.00, were convertible at any time, at the option of the holder, into shares of common stock on a one-for-one basis and vote with the shares of common stock on an as-converted basis. The holders of the Series A Preferred Stock could request redemption of their shares at their stated value of $2.00 per share, beginning on December 13, 2017. The Series A Preferred Stock accrued dividends at the rate of 6% per annum, whether or not declared by the Board of Directors.
The Warrants could have been exercised at any time on or after June 13, 2014 and expire on June 13, 2019. They could have been exercised on a cashless basis and contain customary anti-dilution protection in the event of stock splits, stock dividends or similar events.
In connection with the placement of the Series A Preferred Stock and warrants, we also issued warrants to purchase 525,000 shares of our common stock, with the same terms as the investor warrants, to the placement agent and paid cash fees to the placement agent of $420,000, equal to six percent of the purchase price paid by the investors in the offering. We also incurred other cash fees related to the offering of $202,145.
41
The warrants contain a provision that may require net cash settlement in the event that there is a Fundamental Transaction (contractually defined as a merger, sale of substantially all assets, tender offer or share exchange). Because of this contingent redemption provision, the warrants require liability classification in accordance with FASB ASC 480-10, Distinguishing Liabilities from Equity and do not meet all of the established criteria for equity classification in FASB ASC 815-40, Derivatives and Hedging – Contracts in Entity’s Own Equity. Accordingly, the warrants are recorded as derivative liabilities at fair value. Changes in the fair value of the warrants are charged or credited to income each period.
The warrants issued to the investors and to the placement agent were valued using a trinomial lattice model, because that model embodies all of the relevant assumptions that address the features underlying these instruments. Significant assumptions used in the model included the market price of our common stock on the date of valuation, an expected dividend yield of zero, the remaining period to the expiration date of the warrants, expected volatility of our common stock over the remaining life of the warrants of 48.75% estimated based on a review of our historical volatility and risk-free rates of return based on constant maturity rates published by the U.S. Federal Reserve, applicable to the remaining life of the warrants. At December 31, 2015, all of the investor warrants had been exercised for Series B Preferred stock.
The Company and the investors also executed a Registration Rights Agreement whereby the Company agreed to register the shares of common stock issuable upon conversion of the Series A Preferred Stock and upon exercise of the Warrants, as well as the common stock underlying the warrants issued to the placement agent. Pursuant to the terms of the Registration Rights Agreement, the Company agreed to file a registration statement within thirty days of the closing date and was required to obtain the effectiveness of such registration statement within ninety days of its filing.
In the event that the required filing and effectiveness dates were not met or if the registration statement, once effective, failed to remain effective for a continuous period of 30 days or for a cumulative total of 60 days in any 12 month period, then the Company would have been required to pay to each investor an amount in cash, as liquidated damages and not as a penalty, equal to 1% of the aggregate purchase price paid by such investor for its Preferred Stock and Warrants; and on each monthly anniversary of each such event (if the applicable event has not been cured by such date) until the applicable event was cured, a further 1% of the purchase price, subject to a maximum payment of 10% of the purchase price. The required registration statement was filed on January 10, 2014 and became effective on January 28, 2014. Accordingly, the Company was not required to pay any liquidated damages to the investors, unless the registration statement failed to remain continuously effective for the periods specified by the Registration Rights Agreement. The Company does not presently anticipate being required to make any such payments.
The gross proceeds of the offering of $7.0 million were first allocated to the fair value of the warrants issued to the investors, with the balance of the proceeds allocated to the Series A Preferred Stock. The aggregate costs of the offering of $1.1 million, including the cash fees paid of $622,145 and the fair value of the placement agent warrants of $438,375, were allocated between the Series A Preferred Stock and the warrants based on the gross proceeds allocated to each instrument, as follows:
Proceeds Allocated | Expenses Allocated | ||||||
Series A Preferred Stock | $ | 2,616,250 | $ | 396,369 | |||
Investor Warrants | 4,383,750 | 664,151 | |||||
$ | 7,000,000 | $ | 1,060,520 | ||||
Because the warrants are recorded as a liability at fair value, the portion of the expenses allocated to the warrants was expensed and is included in other income (expense) section in our Statement of Operations.
In accordance with FASB ASC 470-20-25-5, the company recognized a beneficial conversion feature related to the Series A Preferred Stock, The beneficial conversion feature, which was limited to $2.6 million, the proceeds initially allocated to the Series A Preferred Stock, was credited to additional paid-in capital. Because the Series A Preferred Stock is not mandatorily redeemable but can be immediately converted by the holder, the discount recognized by the allocation of proceeds to the beneficial conversion feature was immediately accreted and recognized as a dividend to the preferred shareholders, in accordance with FASB ASC 470-20-35-7c.
42
Because the holders of the Series A Preferred Stock could have requested redemption on or after December 13, 2017, the preferred stock has conditions for its redemption that are not within the control of the Company. Accordingly, the carrying amount of the Series A Preferred Stock of $2.6 million, net of the expenses allocated to the preferred stock of $0.4 million, was recorded outside of stockholders’ equity, as mezzanine equity, in accordance with FASB ASC 480-10-S99. The net carrying amount of the Series A Preferred Stock was accreted to its redemption value over the four year period to when the holders may request redemption, using an effective interest method. For the year ended December 31, 2015, additional accretion of $221,902 was recognized. During 2015, the Series A Preferred Stock and certain related common warrants were exchanged for Series B Preferred Stock. As a result the net carrying amount of the Series A Preferred Stock at December 31, 2016 and 2015 was $0.
The warrants are valued using a trinomial lattice model. Significant assumptions used in the model at December 31, 2016 included the market price of our common stock, an expected dividend yield of zero, the remaining period to the expiration date of the warrants, expected volatility of our common stock over the remaining life of the warrants of 2.5 years, estimated based on a review of our historical volatility of 82.470% and risk-free rates of return of 1.470% based on constant maturity rates published by the U.S. Federal Reserve, applicable to the remaining life of the warrants. We also take into consideration a probability assumption for anti-dilution. At December 31, 2016 and December 31, 2015, the fair value of the remaining 94,375 placement agent warrants was approximately $0.2 million and $0.3 million, respectively.
On March 31, 2014, the Company entered into an agreement with an existing warrant holder pursuant to which the Company repurchased warrants exercisable into 142,857 shares of Common Stock for an aggregate purchase price of $0.4 million.
Reconciliation of changes in fair value
Certain assets and liabilities that are measured at fair value on a recurring basis are measured in accordance with FASB ASC Topic 820-10-05, Fair Value Measurements. FASB ASC Topic 820-10-05 defines fair value, establishes a framework for measuring fair value and expands the disclosure requirements regarding fair value measurements for financial assets and liabilities as well as for non-financial assets and liabilities that are recognized or disclosed at fair value on a recurring basis in the financial statements.
The statement requires fair value measurement be classified and disclosed in one of the following three categories:
Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
Level 2: Quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability; and
Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e., supported by little or no market activity).
Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to their fair value measurement. Our derivative financial instruments that are measured at fair value on a recurring basis are all measured at fair value using Level 3 inputs. Level 3 inputs are unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
43
The following represents a reconciliation of the changes in fair value of warrants measured at fair value using Level 3 inputs during the year ended December 31, 2016:
(in thousands) | 2013 Placement Agent Warrants | ||
Balance, December 31, 2015 | $ | 267 | |
Exercise of warrants | (698 | ) | |
Change in fair value | 634 | ||
Balance December 31, 2016 (1) | $ | 203 | |
(1) | The warrants are valued using a trinomial lattice valuation methodology because that model embodies all of the relevant assumptions that address the features underlying these instruments. Significant assumptions used in the model at December 31, 2016 included the market price of our common stock, an expected dividend yield of zero, the remaining period to the expiration date of the warrants, expected volatility of our common stock over the remaining life of the warrants of 2.5 years, estimated based on a review of our historical volatility of 82.470% and risk-free rates of return of 1.470% for the 2013 warrants based on constant maturity rates published by the U.S. Federal Reserve, applicable to the remaining life of the warrants. We also take into consideration a probability assumption for anti-dilution. |
NOTE 10. RECENT ACCOUNTING PRONOUNCEMENTS
In August 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-15, Classification of Certain Cash Receipts and Cash Payments. The new guidance clarifies the classification of certain cash receipts and cash payments in the statement of cash flows, including debt prepayment or extinguishment costs, settlement of contingent consideration arising from a business combination, insurance settlement proceeds, and distributions from certain equity method investees. The new standard is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. Early adoption is permitted. The amendment is not expected to have a material impact on our financial condition or results of operations.
In March 2016, FASB issued ASU No. 2016-09 Compensation-Stock Compensation - (Topic 718) Improvements to employee share-based payments accounting as part of simplicity initiatives. This update involve several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. Some of the areas for simplification apply only to nonpublic entities. For us, the amendments in this Update are effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. The Company is currently assessing the impact the adoption of ASU 2016-09 will have on its consolidated financial statements.
In February 2016, FASB issued ASU No. 2016-02, Leases (Topic 842). The core principle of Topic 842 is that a lessee should recognize the assets and liabilities that arise from leases. ASU 2016-02 is effective for public companies for annual reporting periods beginning after December 15, 2018, and interim periods within those fiscal years. The guidance may be adopted prospectively or retrospectively and early adoption is permitted. The Company is currently assessing the impact the adoption of ASU 2016-02 will have on its consolidated financial statements.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers, which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. ASU No. 2014-09 will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. The new standard is effective for us on January 1, 2018. Early application is not permitted. The standard permits the use of either the retrospective or cumulative effect transition method. We are currently evaluating the effect that ASU No. 2014-09 will have on our consolidated financial statements and related disclosures.
No other new accounting pronouncement issued or effective during the fiscal year had or is expected to have a material impact on our consolidated financial statements or disclosures.
44
NOTE 11. LONG TERM DEBT
On June 28, 2016, the Company entered into a transaction with Bank of Tampa, a Florida banking corporation (“Lender”) wherein Lender amended the terms of a mortgage loan (“the Loan”) originally executed on March 20, 2014 with a principal amount of $3,592,000. The Initial Maturity Date of the Loan was extended to July 20, 2019 from March 19, 2017, and the Extended Maturity Date was amended to July 20, 2024 from March 20, 2022. In addition, the Lender released as collateral to the Loan, the Company’s working capital accounts in exchange for a negative covenant limited to $2,000,000 of the aggregate indebtedness secured by these accounts.
The obligations under the Loan are secured by a first mortgage and security interest in the Company’s Clearwater, Florida facility. In addition, the Company has pledged an interest in a certificate of deposit in the amount of $779,000 as additional collateral. The amount of the additional collateral required declines on a pro rata basis as principal is paid.
Borrowings under the Loan bear interest at LIBOR plus 3.5%, with a fixed monthly principal payment of $19,956. The interest rate at December 31, 2016 was 4.272%.
The Loan documents contain customary financial covenants, including a covenant that the Company maintains a minimum liquidity of $750,000. Should we desire to extend the Loan beyond July 20, 2019, we must maintain a Debt Service Coverage Ratio for each of the preceding four quarters of not less than 1.0 to 1.0.
Our future contractual obligations for agreements with initial terms greater than one year are as follows:
(In thousands) | Long-term debt | ||
2017 | $ | 239 | |
2018 | 239 | ||
2019 | 2,455 | ||
Total | $ | 2,933 | |
NOTE 12. TAXES AND NET OPERATING LOSS CARRYFORWARDS
Deferred income taxes reflect the impact of temporary differences between the amount of assets and liabilities recognized for financial reporting purposes and such amounts recognized for income tax purposes. The tax effects of these temporary differences representing the components of deferred tax assets (liabilities) were as follows:
(In thousands) | December 31, 2016 | December 31, 2015 | |||||
Deferred tax assets: | |||||||
Loss and credit carry-forwards | $ | 9,169 | $ | 6,578 | |||
Stock-based compensation | 519 | 283 | |||||
Inventory Reserve | 534 | 466 | |||||
Other | 263 | 273 | |||||
Total deferred tax assets | 10,485 | 7,600 | |||||
Valuation allowance | (10,185 | ) | (7,404 | ) | |||
Total deferred tax assets, net of valuation allowance | 300 | 196 | |||||
Deferred tax liabilities: | |||||||
State taxes (capital) | (19 | ) | (10 | ) | |||
Property and equipment | (459 | ) | (413 | ) | |||
Intangibles | (386 | ) | (337 | ) | |||
Total deferred tax liabilities | (864 | ) | (760 | ) | |||
Net deferred tax liabilities | $ | (564 | ) | $ | (564 | ) | |
45
We consider all positive and negative evidence regarding the realization of deferred tax assets, including past operating results and future sources of taxable income. U.S. net operating losses will begin to expire in years beginning in 2019.
We assess the financial statement impact of an uncertain tax position taken or expected to be taken on an income tax return at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized in the financial statements unless it is more likely than not of being sustained. All of our positions arise from taxable temporary differences and, as such, the liability has been recognized in the net deferred tax asset, current and non-current items to which they relate.
Below is a reconciliation of the statutory federal income tax rate to our effective tax rate:
Year Ended December 31, | ||||||||
2016 | 2015 | 2014 | ||||||
Federal tax provision | 34.0 | % | 34.0 | % | 34.0 | % | ||
State taxes (net of federal benefit) | 3.7 | % | 2.4 | % | 1.4 | % | ||
Warrant gains | 31.4 | % | (11.1 | )% | (18.8 | )% | ||
Valuation allowance | (71.8 | )% | (26.3 | )% | (45.3 | )% | ||
Other | 1.5 | % | 0.6 | % | (1.6 | )% | ||
(1.2 | )% | (0.4 | )% | (30.3 | )% | |||
NOTE 13. RETIREMENT PLAN
The Company provides a tax-qualified profit-sharing retirement plan under section 401(k) of the Internal Revenue Code for the benefit of eligible employees with an accumulation of funds for retirement on a tax-deferred basis and provides for annual discretionary contribution to individual trust funds.
All employees are eligible to participate. The employees may make voluntary contributions to the plan up to the maximum percentage allowed by the Internal Revenue Code. Vesting in employee matching contributions is graded and depends on the years of service. After three years from their date of hire, the employees are 100% vested. The Company makes matching contributions of 50% of the employee contributions up to a total of 3% of participant payroll. Matching contributions made by the Company totaled $0.3 million, $0.2 million and $0.1 million for the years ended December 31, 2016, 2015 and 2014, respectively.
NOTE 14. RELATED PARTY TRANSACTIONS
Research and Development Consulting Services
Our policy is that employees, non-employees and third parties must obtain authorization from the appropriate department executive manager, for any business relationship or proposed business transaction in which they or an immediate family member has a direct or indirect interest, or from which they or an immediate family member may derive a personal benefit (a “related party transaction”). The maximum dollar amount of related party transactions that may be approved as described above in this paragraph in any calendar year is $120,000. Any related party transactions that would bring the total value of such transactions to greater than $120,000 must be referred to the Audit Committee to determine the procedure for approval and then have the recommendations presented to the Board of Directors for approval.
Several relatives of Nikolay Shilev, Bovie Bulgaria’s Managing Director, are considered related parties. Teodora Shileva, Mr. Shilev’s spouse is an employee of the company working in the Accounting department. Antoaneta Dimitrova Shileva-Toromanova, Mr. Shilev’s sister is the Manager of Production and Human Resources. Svetoslav Shilev, Mr. Shilev’s son is an Engineer in the Quality Assurance department.
46
A relative of Moshe Citronowicz, Bovie’s Senior Vice President, is considered a related party. Arik Zoran is a consultant of the Company doing business as AR Logic, Inc., a consulting firm owned by Arik Zoran, Mr. Citronowicz’s brother. On March 1, 2013 the Company amended the Consulting Services Agreement dated January 2011, extending the term of the existing agreement until December 31, 2014. The agreement shall automatically renew for additional one year periods, unless either party gives written notice of its desire not to renew at least one year prior to the expiration of the initial Term or renewal term. The agreement with AR Logic provides for a separate hourly based fee structure for consulting related to new projects. AR Logic has a royalty contract with us related to the creation and design of proprietary technology that is used in some of our generators. AR Logic was paid consulting fees of approximately $0.2 million, $0.3 million and $0.2 million during 2016, 2015 and 2014, respectively.
A second relative of Mr. Citronowicz is considered a related party. Yechiel Tsitrinovich is also a brother of Mr. Citronowicz and acts as a consultant to the Company related to research and development of certain products. Mr. Tsitrinovich has a royalty contract with us related to the creation and design of a proprietary technology that is used in some of our generators. Mr. Tsitrinovich was paid a combination of consulting fees and royalties on previous product designs approximately $0.1 million per year for 2016, 2015 and 2014.
NOTE 15. OTHER COMMITMENTS AND CONTINGENCIES
Property and Rental Agreements
In March 2014, we signed a lease for offices located in Purchase, New York. The lease is for 3,650 square feet of office space with a monthly cost of approximately $9,277 per month.
In October 2015, pursuant to our acquisition of Bovie Bulgaria, we are obligated to pay $4,333 per month for the lease expiring on December 4, 2021.
The following is a schedule of approximate future minimum lease payments under operating leases as of December 31, 2016:
(In thousands) | |||
2017 | $ | 168 | |
2018 | 171 | ||
2019 | 114 | ||
2020 | 55 | ||
2021 | 55 | ||
Total | $ | 563 | |
Rent expense for the each year ended December 31, 2016, 2015 and 2014 approximated $0.2 million.
Purchase Commitments
At December 31, 2016, we had purchase commitments for inventories totaling approximately $4.4 million, substantially all of which is expected to be purchased by the end of 2017.
NOTE 16. STOCK OPTIONS
On October 30, 2007, our stockholders approved and the Board of Directors adopted an amendment to the 2003 Executive and Employee Stock Option Plan (the “Plan”) to increase the maximum aggregate number of shares of common stock reserved for issuance under the Plan from 1.2 million shares (already reserved against outstanding options) to 1.7 million shares. Except for the increase in the number of shares covered by the Plan, the Plan remained otherwise unchanged. In 2001, the Board of Directors adopted the 2001 Executive and Employee Stock Option Plan which reserved for issuance 1.2 million stock options. Stock options typically have a ten-year life and currently vest over a seven year period.
In July of 2012, the stockholders approved the 2012 Share Incentive Plan covering a total of 750,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2016 approximately 37,000 remain to be issued in this plan.
47
In July of 2015, the stockholders approved the 2015 Executive and Employee Stock Option Plan covering a total of 2,000,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2016 approximately 693,078 remain to be issued in this plan.
The status of our stock options and stock awards are summarized as follows:
Number of options | Weighted average exercise price | |||||
Outstanding at December 31, 2014 | 2,864,189 | $ | 3.69 | |||
Granted | 830,922 | 2.21 | ||||
Exercised | (97,500 | ) | 2.25 | |||
Canceled and forfeited | (466,164 | ) | 3.57 | |||
Outstanding at December 31, 2015 | 3,131,447 | $ | 3.38 | |||
Granted | 810,762 | 1.87 | ||||
Exercised | (36,250 | ) | 3.62 | |||
Canceled and forfeited | (153,750 | ) | 3.69 | |||
Outstanding at December 31, 2016 | 3,752,209 | $ | 3.04 | |||
Exercisable at December 31, 2016 | 2,006,702 | $ | 3.79 | |||
Number of options | Weighted average grant date fair value | |||||
Non-vested at December 31, 2015 | 1,707,411 | $ | 2.63 | |||
Granted | 810,762 | 0.89 | ||||
Vested | (618,917 | ) | 1.08 | |||
Forfeited | (153,750 | ) | 1.75 | |||
Non-vested at December 31, 2016 | 1,745,506 | 1.25 | ||||
Common shares required to be issued upon the exercise of stock options and warrants would be issued from our authorized and unissued shares. We calculated the fair value of issued options utilizing a trinomial lattice with an expected life calculated via the simplified method as we do not have sufficient history to determine actual expected life.
2016 Grants | 2015 Grants | 2014 Grants | |||||||||
Option value | $0.80 | - | $0.91 | $0.90 | - | $1.84 | $3.50 | - | $4.30 | ||
Risk-free rate | 1.5% | - | 1.8% | 0.2% | - | 1.6% | 0.6% | ||||
Expected dividend yield | —% | —% | —% | ||||||||
Expected volatility | 49.5% | - | 50.3% | 53.0% | - | 54.0% | 53.0% | - | 54.0% | ||
Expected term (in years) | 6 | 4 | - | 10 | 4 | - | 10 | ||||
As of December 31, 2016, the aggregate intrinsic value of all stock options outstanding and expected to vest was approximately $4,150,334 and the aggregate intrinsic value of currently exercisable stock options was approximately $1,574,318. The intrinsic value of each option share is the difference between the fair market value of our common stock and the exercise price of such option share to the extent it is “in-the-money”. Aggregate intrinsic value represents the value that would have been received by the holders of in-the-money options had they exercised their options on the last trading day of the year and sold the underlying shares at the closing stock price on such day. The intrinsic value calculation is based on the $3.59 closing stock price of our common stock on December 31, 2016, the last trading day of 2016. The total number of in-the-money options outstanding and exercisable as of December 31, 2016 was approximately 1,211,014.
48
As of December 31, 2015, the aggregate intrinsic value of all stock options outstanding and expected to vest was approximately $102,992 and the aggregate intrinsic value of currently exercisable stock options was approximately $11,250. The intrinsic value of each option share is the difference between the fair market value of our common stock and the exercise price of such option share to the extent it is “in-the-money”. Aggregate intrinsic value represents the value that would have been received by the holders of in-the-money options had they exercised their options on the last trading day of the year and sold the underlying shares at the closing stock price on such day. The intrinsic value calculation is based on the $2.10 closing stock price of our common stock on December 31, 2015, the last trading day of 2015. The total number of in-the-money options outstanding and exercisable as of December 31, 2015 was approximately 410,715.
The total intrinsic value of options exercised during the years ended December 31, 2016, 2015 and 2014 was approximately $119,026, $51,575 and $229,135, respectively. Intrinsic value of exercised shares is the total value of such shares on the date of exercise less the cash received from the option holder to exercise the options. The total cash proceeds received from the exercise of stock options was approximately $12,300 and $209,250 and $87,475 for the years ended December 31, 2016, 2015 and 2014, respectively.
The total fair value of options granted during the years ended December 31, 2016, 2015 and 2014 was approximately $1,516,125, $932,771 and $1,107,180, respectively. The total fair value of option shares vested during the years ended December 31, 2016, 2015 and 2014, was approximately $612,464, $412,638 and $637,000, respectively.
During the year ended December 31, 2016, we issued 9,614 common shares in exchange for 36,250 non-employee stock options and 26,636 common shares (via stock swaps). Net proceeds from the issuance of common shares along with the shares received in the stock swap exercises were approximately $12,300 for the year ended December 31, 2016.
During the year ended December 31, 2015, we issued 33,520 common shares in exchange for 114,500 non-employee stock options and 80,980 common shares (via stock swaps).
During the year ended December 31, 2014, we issued 73,699 common shares in exchange for 107,000 employee and non-employee stock options and 33,301 common shares (via stock swaps). Net proceeds from the issuance of common shares along with the shares received in the stock swap exercises were approximately $80,475 for the year ended December 31, 2014.
As of December 31, 2016, there was approximately $1.2 million of total unrecognized stock-based compensation cost, related to unvested stock options granted under the Amended Plan. This cost is expected to be recognized over a weighted-average period of approximately 4 years.
Allocation of stock based compensation expense was as follows:
Year Ended December 31, | |||||||||||
(In thousands) | 2016 | 2015 | 2014 | ||||||||
Cost of sales | $ | 2 | $ | 3 | $ | 8 | |||||
Research and development | 27 | 39 | 51 | ||||||||
Salaries and related costs | 780 | 526 | 329 | ||||||||
Total | $ | 809 | $ | 568 | $ | 388 | |||||
49
NOTE 17. GEOGRAPHIC AND SEGMENT INFORMATION
International sales in 2016, 2015 and 2014 were 12.5%, 16.9% and 15.8% of sales, respectively. Substantially all of these sales are denominated in U.S. dollars.
Although we have only one reporting segment, beginning in 2014, Management began analyzing revenue and other operating metrics across three operating segments.
Year Ended December 31, | |||||||||||
(In thousands) | 2016 | 2015 | 2014 | ||||||||
Sales by Product Line | |||||||||||
Electrosurgical | $ | 20,901 | $ | 17,558 | $ | 16,706 | |||||
Cauteries | 7,101 | 6,886 | 6,896 | ||||||||
Other | 8,625 | 5,076 | 4,079 | ||||||||
Total | $ | 36,627 | $ | 29,520 | $ | 27,681 | |||||
Sales by Domestic and International | |||||||||||
Domestic | $ | 32,050 | $ | 24,540 | $ | 23,313 | |||||
International | 4,577 | 4,980 | 4,368 | ||||||||
Total | $ | 36,627 | $ | 29,520 | $ | 27,681 | |||||
Sales by Operating Segment | |||||||||||
Core | $ | 27,808 | $ | 26,098 | $ | 24,322 | |||||
OEM | 5,328 | 2,116 | 3,150 | ||||||||
Growth | 3,491 | 1,306 | 209 | ||||||||
Total | $ | 36,627 | $ | 29,520 | $ | 27,681 | |||||
NOTE 18. SUPPLEMENTAL UNAUDITED QUARTERLY FINANCIAL INFORMATION
The following table sets forth certain unaudited quarterly data for each of the four quarters in the years ended December 31, 2016 and 2015, respectively. The data has been derived from the Company’s unaudited consolidated financial statements that, in management’s opinion, include all adjustments (consisting of normal recurring adjustments) necessary for a fair presentation of such information when read in conjunction with the Consolidated Financial Statements and Notes thereto. The results of operations for any quarter are not necessarily indicative of the results of operations for any future period.
(In thousands, except per share data) | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||||
Year ended December 31, 2016 | |||||||||||||||
Sales | $ | 7,775 | $ | 9,295 | $ | 10,063 | $ | 9,494 | |||||||
Gross profit | 3,323 | 4,700 | 5,062 | 4,830 | |||||||||||
Net loss attributable to common shareholders | (1,944 | ) | (519 | ) | (964 | ) | (523 | ) | |||||||
Income (loss) per basic share | $ | (0.07 | ) | $ | (0.02 | ) | $ | (0.04 | ) | $ | (0.02 | ) | |||
Year ended December 31, 2015 | |||||||||||||||
Sales | $ | 6,128 | $ | 7,274 | $ | 7,823 | $ | 8,295 | |||||||
Gross profit | 2,674 | 3,140 | 3,229 | 3,514 | |||||||||||
Net income (loss) attributable to common shareholders | 12,858 | (1,497 | ) | (1,587 | ) | (1,410 | ) | ||||||||
Income (loss) per basic share | $ | 0.69 | $ | (0.06 | ) | $ | (0.06 | ) | $ | (0.05 | ) | ||||
50
BOVIE MEDICAL CORPORATION
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
There were no disagreements with our current accountants on accounting and financial disclosures.
ITEM 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We have carried out an evaluation, under the supervision of and with the participation of our management, including our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended), as of December 31, 2016. Based upon that evaluation, our CEO and CFO concluded that, as of the end of that period, our disclosure controls and procedures are effective in providing reasonable assurance that (a) the information required to be disclosed by us in the reports that we filed or submitted under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (b) such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives and our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Changes in Internal Control over Financial Reporting
Section 404 of the Sarbanes-Oxley Act of 2002 requires us to evaluate annually the effectiveness of our internal controls over financial reporting as of the end of each fiscal year and to include a management report assessing the effectiveness of our internal control over financial reporting in all annual reports. There were no changes in our internal control over financial reporting during the quarter ended December 31, 2016 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act as a process designed by, or under the supervision of, a company’s principal executive and principal financial officers and effected by a company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that:
• | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
• | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
• | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2016. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control — Integrated Framework (2013). Based on our assessment, our management has concluded that, as of December 31, 2016, our internal control over financial reporting was effective.
51
BOVIE MEDICAL CORPORATION
ITEM 9B. Other Information
None.
52
BOVIE MEDICAL CORPORATION
Part III
ITEM 10. Directors, Executive Officers and Corporate Governance
BACKGROUND AND EXPERIENCE OF DIRECTORS
When considering whether directors and nominees have the experience, qualifications, attributes or skills, taken as a whole, to enable the Board of Directors (“Board”) to satisfy its oversight responsibilities effectively in light of the Company’s business and structure, the Governance and Nominating Committee focused primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth immediately below. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of our business. As more specifically described in such person’s individual biographies set forth below, our directors possess relevant and industry-specific experience and knowledge in the medical, engineering and business fields, as the case may be, which we believe enhances the Board’s ability to oversee, evaluate and direct our overall corporate strategy. The Governance and Nominating Committee annually reviews and makes recommendations to the Board regarding the composition and size of the Board so that the Board consists of members with the proper expertise, skills, attributes and personal and professional backgrounds needed by the Board, consistent with applicable regulatory requirements.
The Governance and Nominating Committee believes that all directors, including nominees, should possess the highest personal and professional ethics, integrity and values and be committed to representing the long-term interests of our stockholders. The Governance and Nominating Committee will consider criteria including the nominee’s current or recent experience as a senior executive officer, whether the nominee is independent, as that term is defined in existing independence requirements of the NYSE MKT Market and the Securities and Exchange Commission, the business, scientific or engineering experience currently desired on the Board, geography, the nominee’s industry experience and the nominee’s general ability to enhance the overall composition of the Board.
The Governance and Nominating Committee does not have a formal policy on diversity; however, in recommending directors, the Board and the Committee consider the specific background and experience of the Board members and other personal attributes in an effort to provide a diverse mix of capabilities, contributions and viewpoints which the Board believes enables it to function effectively as the Board of Directors of a company with our size and nature of business.
Directors serve for one-year terms and are elected at the annual stockholders’ meeting. Set forth below is information regarding the executive officers, directors and key employees of Bovie Medical Corporation as of March 10, 2017.
Name | Position | Director Since | ||
Andrew Makrides | Executive Chairman of the Board | December 1982 | ||
Robert L. Gershon | Chief Executive Officer and Director | December 2013 | ||
J. Robert Saron | President, Chief Sales and Marketing Officer and Director | August 1988 | ||
Jay D. Ewers | Chief Financial Officer, Treasurer and Secretary | N/A | ||
Jack McCarthy | Chief Commercialization Officer | N/A | ||
Moshe Citronowicz | Senior Vice President | N/A | ||
Lawrence J. Waldman | Director | March 2011 | ||
Michael Geraghty | Director | March 2011 | ||
John Andres | Director | July 2014 | ||
Charles T. Orsatti | Director | May 2015 (1) | ||
Scott Davidson | Director | July 2016 (2) | ||
(1) | Mr. Orsatti tendered his resignation from the board effective on December 31, 2016. |
(2) | Mr. Davidson tendered his resignation from the board effective on December 19, 2016. |
53
BOVIE MEDICAL CORPORATION
Andrew Makrides, Esq. age 75, Executive Chairman of the Board of Directors, received a Bachelor of Arts degree in Psychology from Hofstra University and a Juris Doctor Degree from Brooklyn Law School. He is a member of the Bar of the State of New York and practiced law from 1968 until joining Bovie Medical Corporation as a co-founder and Executive Vice President and director, in 1982. Mr. Makrides became President of the Company in 1985 and the CEO in December 1998 and has served as such until March 18, 2011 at which point he relinquished his position as President, but remained CEO until December 2013. Mr. Makrides employment contract expired December 31, 2016. Mr. Makrides has over 30 years of executive experience in the medical industry.
Robert L. Gershon, age 50, Chief Executive Officer and Director, has over 28 years of healthcare industry experience. Under his leadership Bovie Medical grew revenue approximately 55% and achieved 3 year 15.7% CAGR. On the operations side he ran the largest sales and marketing business at Covidien. With over $1B in P&L responsibility he consistently led an organization of over 600 people to double-digit revenue growth outpacing market category growth and capturing significant market share points during challenging healthcare economic conditions. He also was VP of sales and marketing at Henry Schein ($1.4B shared P&L for medical division/$115M full P&L for dialysis division) and earlier in his career spent over 13 years as a healthcare consultant for Booz Allen Hamilton, KPMG and two boutique consultancies where his practice focused on strategic planning, business development and mergers and acquisitions. Mr. Gershon received an MBA from J.L. Kellogg Graduate School of Management at Northwestern University and a BSBA degree from American University.
J. Robert Saron, age 64, President, Chief Sales and Marketing Officer and Director, holds a Bachelor degree in Social and Behavioral Science from the University of South Florida. From 1988 to present Mr. Saron has served as a director of the Company. Mr. Saron has previously served as both director and president of the Health Care Manufacturing Management Council. In 2011 Mr. Saron received the Leonard Berke Achievement award for ethics, mentoring, marketing skill, industry knowledge, contributions to the industry and contributions to HMMC. He currently serves as a director of the Health Industry Distributors Association Education Foundation. Mr. Saron received the Health Industry Distributors Association’s highest award in 2008, the Industry Award of Distinction (renamed the John F. Sasen Leadership Award) and in February 2013 was inducted into the Medical Distribution Hall of Fame. Mr. Saron’s employment contract extends to December 31, 2017. Mr. Saron brings over 39 years of executive marketing and distribution experience in the medical industry.
Jay D. Ewers, CPA, age 56, Chief Financial Officer, Treasurer and Secretary, has more than 30 years of accounting experience, having held financial executive positions in corporations ranging from early stage to high profile public companies with global operations in the medical equipment, manufacturing and semiconductor industries. Mr. Ewers joined the company as Corporate Controller in June, 2014. From 2004 to 2014, Mr. Ewers worked in private practice providing accounting and advisory services to both publicly traded and privately-held companies. Mr. Ewers received his CPA license in 1987 and is a certified internal auditor.
Jack McCarthy, age 50, has served as our Chief Commercialization Officer since March 2014. Mr. McCarthy has 23 years of sales and marketing experience of which the last 16 years has been spent in the healthcare industry. Most recently, he served as Vice President of Sales and Marketing for US Healthcare at Z-Medica. Prior to that, Mr. McCarthy spent 15 years with Covidien, in positions of increasing responsibility where he was charged with achieving sales, marketing and business development goals. His most recent position at Covidien was as Area Sales Vice President for the Endo Mechanical Intelligent Device franchise, where he managed a team of 50 sales professionals. Mr. McCarthy is a graduate of Loyola University in Baltimore, Maryland, were he obtained a BA degree in Marketing in 1988 and an MBA in Marketing in 1990.
Moshe Citronowicz, age 64, Senior Vice President came to the United States in 1978 and has worked in a variety of manufacturing and high technology industries. In October 1993, Mr. Citronowicz joined the Company as Vice President of Operations and served as our Chief Operating Officer until November 2011. Currently, he is serving as the Senior Vice President. Mr. Citronowicz’s employment contract extends to December 31, 2017.
Lawrence J. Waldman, CPA, age 70, has served as a director since 2011 and is currently the Chair of our audit committee and Lead Independent Director of the Board. Mr. Waldman has over thirty-five years of experience in public accounting. Mr. Waldman currently serves as a senior advisor to First Long Island Investors, LLC, an investment and wealth management firm since May 2016. Prior to that Mr. Waldman served as an advisor to the accounting firm of EisnerAmper LLP, where he was previously the Partner-in-Charge of Commercial Audit Practice Development for Long Island since September 2011. Prior to joining EisnerAmper LLP, Mr. Waldman was the Partner-in-Charge of Commercial Audit Practice Development for Holtz Rubenstein Reminick, LLP from July 2006 to August 2011. Mr. Waldman was the Managing Partner of the Long Island office of KPMG LLP from 1994 through 2006, the accounting firm where he began his career in 1972. Mr. Waldman was elected to the Board of Directors of Comtech Telecommunications Corp. in August of 2015 and since December 2015, serves as Chair of its audit committee. In October 2016, Mr. Waldman was appointed and subsequently in December 2016 elected to the Board of Directors of CVD Equipment Corporation, and serves as a member of the audit committee and Chair of the compensation committee. Mr. Waldman serves as a
54
BOVIE MEDICAL CORPORATION
member of the Board of Directors of Northstar/RXR Metro Income Fund, a non-traded Real Estate Investment Trust and has served as a member of its audit committee since 2014. Mr. Waldman also serves as a member of the State University of New York’s Board of Trustees and as chair of its audit committee. Mr. Waldman is also the Chair of the Supervisory Committee of Bethpage Federal Credit Union. He previously served as the Chairman of the Board of Trustees of the Long Island Power Authority and as Chair and a member of the finance and audit committee of its Board of Trustees. Mr. Waldman meets the definition of a financial expert as defined by the SEC and NYSE MKT.
Michael Geraghty, age 70, has served as a director since March 2011 and was previously employed as the President of Global Sales at Optos, Inc., a developer and manufacturer of retinal imaging devices for screening, detection and diagnosis of eye related conditions. From 2005 through 2008, he was the President of International Sales at Gyrus Acmi where he first started in 2000 as Senior Vice President of Sales for Gyrus Medical. Prior to this, Mr. Geraghty was the Vice President of Sales and Marketing for Everest Medical, Inc. and before that was the Director of Marketing for Advanced Products at Arthrocare Corporation. Mr. Geraghty specializes in building independent direct sales teams in the medical device industry and has extensive domestic and international sales and marketing experience. He received his bachelor’s degree from St. Mary’s University and graduate degree in Executive Sales Management from the University of Minnesota.
John Andres, age 59, serves as Vice Chairman of the Board and has over thirty years of experience in the medical device industry. Since April, 2004, Mr. Andres has been a private consultant, doing business through John C. Andres, LLC, specializing in patent/business strategy development and execution. He also is a partner of Hawk Healthcare, LLC, which provides strategic transaction management to private individuals and companies.
In 2004, Mr. Andres helped found K2M, Inc. (KTWO) and from 2004 until 2010 served as a member of the Board of Directors of K2M, Inc. Prior to 2004, Mr. Andres held various legal and strategic business development positions at the Surgical Division of Tyco Healthcare Group, LLP, now Medtronic (NYSE: MDT) and its predecessor, United States Surgical Corporation. Before joining U.S. Surgical, Mr. Andres worked at the New York law firm of Morgan & Finnegan. He received his Associate of Applied Science degree from Rochester Institute of Technology, his Bachelor of Arts degree from Lehigh University and his Juris Doctor from Pace University School of Law.
Involvement in Certain Legal Proceedings
None
Independent Board Members
During 2016, the Board had four independent members, John Andres, Charles Orsatti, Michael Geraghty and Lawrence J. Waldman, who meet the existing independence requirements of the NYSE MKT Market and the Securities and Exchange Commission and represent a majority of the board.
Board Leadership
The independent directors appointed Lawrence J. Waldman as the Lead Independent Director. The Lead Independent Director is appointed by the Board and is responsible for coordinating the activities of the independent directors and coordinating with the Chief Executive Officer of the Company to set agendas for Board meetings and chair executive sessions of the independent directors. The Lead Independent Director is also responsible for meeting, from time to time, with the Company’s Compensation Committee to discuss the Chief Executive Officer’s performance.
The Company’s Corporate Governance Policies also contain several features which the company believes will ensure that the Board maintains effective and independent oversight of management, including the following:
• | Executive sessions without management and non-independent directors present are a standing Board agenda item. Executive sessions of the independent directors are held at any time requested by an independent director and, in any event, are held in connection with at least 100% of regularly scheduled Board meetings. |
• | The Board regularly meets in executive session with Mr. Gershon without other members of management present. |
• | All Board committee members are independent directors. The committee chairs have authority to hold executive sessions without management and non-independent directors present. |
55
BOVIE MEDICAL CORPORATION
The Board has no formal policy with respect to separation of the positions of Chairman and CEO or with respect to whether the Chairman should be a member of management or an independent director and believes that these are matters that should be discussed and determined by the Board from time to time. On December 13, 2013 Andrew Makrides resigned from his position as Chief Executive Officer of the Company and Robert Gershon was appointed as Chief Executive Officer of the Company. Mr. Gershon is tasked with the responsibility of implementing our corporate strategy, we believe he is best suited for leading discussions, at the Board level, regarding performance relative to our corporate strategy and this discussion accounts for a significant portion of the time devoted at our Board meetings.
Board Evaluations
The Board has adopted a policy to evaluate its performance and effectiveness as well as that of the three standing committees on an annual basis. The purpose of the evaluation is to track progress in certain areas targeted for improvement from year to year and to identify ways to enhance the Board’s effectiveness. As part of the evaluation, each Director may complete a written questionnaire developed by the Governance and Nominating Committee to provide feedback on the effectiveness of the Board, the Committees, as well as each individual Director’s own contributions. The collective ratings and comments of the Directors are compiled and then presented to the Governance and Nominating Committee and to the full Board for discussion and action as necessary.
Risk Management
The Board believes that risk management is an important component of the Company’s corporate strategy. While we assess specific risks at our committee levels, the Board, as a whole, oversees our risk management process and discusses and reviews with management major policies with respect to risk assessment and risk management. The Board is regularly informed through its interactions with management and committee reports about risks we face in the course of our business. Our Audit Committee also takes an active role in risk assessment and risk management.
Audit Committee
The Audit Committee assists the Board in its general oversight of our financial reporting, internal controls and audit functions and is directly responsible for the appointment, compensation and oversight of the work of our independent registered public accounting firm. The Audit Committee reviews and discusses with management and our independent accountants the annual audited and quarterly financial statements (including the disclosures under “Management’s Discussion and Analysis of Financial Condition and Results of Operations”), reviews the integrity of the financial reporting processes, both internal and external, reviews the qualifications, performance and independence of our independent accountants and prepares the Audit Committee Report included in this Annual Report on Form 10-K in accordance with rules and regulations of the Securities and Exchange Commission. The Audit Committee has the power to investigate any matter brought to its attention within the scope of its duties. It also has the authority to retain counsel and advisors to fulfill its responsibilities and duties. The Audit Committee also acts as a qualified legal compliance committee.
During 2016, our Audit Committee consisted of four independent members of the Board of Directors, Lawrence J. Waldman, John Andres, Charles Orsatti and Scott Davidson. As a smaller reporting company, we are required to have at least two independent members comprising our Audit Committee in accordance with Rule 10A-3 of the Securities Exchange Act of 1934 and the rules of the NYSE MKT Exchange. During 2016, Mr. Waldman served as the Audit Committee Chairman and financial expert. The Audit Committee meets as often as it determines necessary but not less frequently than once every fiscal quarter. During 2016, the Audit Committee met five times.
Governance and Nominating Committee
The Governance and Nominating Committee is responsible for matters relating to the corporate governance of our company and the nomination of members of the board and committees thereof. During 2016, our Governance and Nominating Committee consisted of three independent members of the Board of Directors, John Andres who serves as Chairman, Lawrence J. Waldman and Michael Geraghty. The Governance and Nominating Committee meets as often as it determines necessary, but not less than once a year. During 2016, Governance and Nominating Committee met two times.
56
BOVIE MEDICAL CORPORATION
Compensation Committee
The Compensation Committee is responsible for overseeing our compensation and employee benefit plans (including those involving the issuance of our equity securities) and practices, including formulating, evaluating and approving the compensation of our executive officers and reviewing and recommending to the full Board of Directors the compensation of our Chief Executive Officer. During 2016, our Compensation Committee consisted of four independent members of the Board of Directors, Charles Orsatti who served as Chairman, John Andres, Lawrence J. Waldman and Scott Davidson. The Compensation Committee meets as often as it determines necessary, but not less than once a year. During 2016, the Compensation Committee met four times.
Code of Ethics
On March 30, 2004 Bovie adopted a Code of Ethics for executive employees.
A copy of the code of ethics which expressly includes the CEO and CFO, is available on our website at http://boviemed.com/financials/Bovie_Code_of_Business_Conduct_and_Ethics_v2.pdf
ITEM 11. Executive Compensation Discussion and Analysis
General Compensation Philosophy
The primary objective of our compensation program for employees, including our compensation program for executive officers, is to attract, retain and motivate qualified individuals and reward them in a manner that is fair to all stockholders. We strive to provide incentives for every employee that rewards them for their contribution to the Company.
Our compensation program is designed to be competitive with other employment opportunities and to align the interests of all employees, including executive officers, with the long-term interests of our stockholders. Historically, for our executive officers, we link a much higher percentage of total compensation to incentive compensation such as stock based compensation than we do for other employees.
With these objectives in mind, our Board has built executive and non-executive compensation programs that consist of three principal elements - base salary, performance bonuses and grants of stock options and/or shares of restricted stock.
To understand the competitiveness of compensation arrangements provided to our named executive officers, in 2014 the Compensation Committee engaged Pearl Meyer & Partners to perform a competitive assessment of base salaries, bonuses for on-target performance and grants of equity incentives. In 2016, Pearl Meyer & Partners updated the competitive frame of reference for the study to consist of the following group of pre-selected companies that were of comparable size and operated in our industry category.
Avinger, Inc. | Esko Bionics Holdings, Inc. | IRIDEX Corporation |
AxoGen, Inc. | Fonar Corporation | Misonix, Inc. |
BIOLASE, Inc | iCAD, Inc. | Retractable Technologies, Inc. |
Cogentix Medical, Inc. | Invuity, Inc. | Utah Medical Products Inc. |
Cutera, Inc. | IRadimed Corporation | |
In addition to the peer group, Pearl Meyer referenced industry-specific, size-adjusted market survey data where appropriate.
The results of the survey confirmed that, consistent with our desired philosophy, our compensation arrangements were competitive with the marketplace, with some variation by individual.
57
BOVIE MEDICAL CORPORATION
Compensation Program
Base Salary
We pay base salaries to our Named Executive Officers (as defined below) in order to provide a consistent, minimum level of pay that sustained individual performance warrants. We also believe that a competitive annual base salary is important to attract and retain an appropriate caliber of talent for each position over time.
The annual base salaries of our Named Executive Officers are determined by our Compensation Committee and approved by the Board of Directors. All salary decisions are based on each Named Executive Officer’s level of responsibility, experience and recent and past performance, as determined by the Compensation Committee. The Compensation Committee benchmarks base salaries using a major independent consulting firm and using their recommendations and other information the Committee evaluates and establishes the base compensation for our named executives.
Performance Bonus
The second component of executive compensation is performance bonuses which are earned when defined metrics are achieved.
For 2016, the Company established a combination of financial, operational and personal objectives as the broad criteria that would determine annual performance bonus amounts for the year.
(In millions) | Threshold | Target | Achievement | Overall Weight | Achievement | Calculation | ||||||||||||
J Plasma | 2.3 | 3.1 | 3.5 | 35 | % | 150 | % | 53 | % | |||||||||
Total Revenue Excluding J-Plasma | 28.0 | 32.0 | 33.0 | 20 | % | 125 | % | 25 | % | |||||||||
Operating Loss | (4.1 | ) | (3.7 | ) | (3.7 | ) | 20 | % | 100 | % | 20 | % | ||||||
Total Cash Balance | 5.0 | 6.6 | 14.5 | 10 | % | 200 | % | 20 | % | |||||||||
MBO | 1.0 | 1.0 | 1.0 | 15 | % | 100 | % | 15 | % | |||||||||
Total | 100 | % | 133 | % | ||||||||||||||
After careful review and consideration of the measures that comprise the 2016 bonus, the Compensation Committee approved the following performance bonuses:
Name | Bonus | |||
Robert L. Gershon | $ | 243,224 | ||
Andrew Makrides | $ | 100,322 | ||
Jay D. Ewers | $ | 109,392 | ||
J. Robert Saron | $ | 148,456 | ||
Moshe Citronowicz | $ | 99,612 | ||
Jack McCarthy | $ | 133,773 | ||
Total | $ | 834,779 | ||
Stock Options
The third component of executive compensation is equity grants which have mainly come in the form of stock options. We believe that equity ownership in our Company is important to provide our Named Executive Officers with long-term incentives to better align interests of executives with the interests of stockholders and build value for our stockholders. In addition, the equity compensation is designed to attract and retain the executive management team. Stock options have value only if the stock price increases over time and, therefore, provide executives with an incentive to build Bovie’s value. This characteristic ensures that the Named Executive Officers have a meaningful portion of their compensation tied to future stock price increases and rewards management for long-term strategic planning through the resulting enhancement of the stock price.
58
BOVIE MEDICAL CORPORATION
Stock option awards to Named Executive Officers are entirely discretionary. The CEO recommends to the Compensation Committee awards for Named Executive Officers other than himself. The Compensation Committee considers this recommendation along with the prior contribution of these individuals and their expected future contributions to our growth. The Committee formulates and presents its recommended allocation of stock option awards to the Board of Directors for approval. The Compensation Committee then would make an independent determination on CEO stock option awards, again formulating and presenting its recommendation for the allocation of stock option awards to the Board of Directors for approval. The Board of Directors approves, rejects, or, if necessary, modifies the Committee’s recommendations.
Perquisites and Other Benefits
Our Named Executive Officers are eligible for the same health and welfare programs and benefits as the rest of our employees in their respective locations. In addition, our CEO, Chairman of the Board, President and Chief Sales and Marketing Officer, Chief Financial Officer, Chief Commercialization Officer and Senior Vice President each receive an automobile allowance.
Our Named Executive Officers are entitled to participate in and receive employer contributions to Bovie’s 401(k) Savings Plan. For more information on employer contributions to the 401(k) Savings Plan see the Summary Compensation Table and its footnotes.
Tax and Accounting Considerations
Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”), places a limit of $1.0 million on the amount of compensation that we may deduct as a business expense in any year with respect to each of our most highly paid executives unless, among other things, such compensation is performance-based and has been approved by stockholders. The non-performance-based compensation paid to our executive officers for the 2016 fiscal year did not exceed the $1.0 million limit per executive officer. Accounting considerations also play an important role in the design of our executive compensation program. Accounting rules, such as FASB ASC Topic 718-10-10, Share-Based Payment, require us to expense the cost of our stock option grants which reduces the amount of our reported profits. Because of option expensing and the impact of dilution on our stockholders, we pay close attention to the number and value of the shares underlying stock options we grant.
59
BOVIE MEDICAL CORPORATION
Compensation of Named Executive Officers
The following table sets forth the compensation paid to each of our Named Executive Officers for the three years ended December 31, 2016 for services to our Company in all capacities:
Summary Compensation Table | ||||||||||||||||||||||||||||||||||
Name and Principal Position | Year | Salary | Bonus ($) | Stock Awards ($) | Option Awards ($) (1) | Non-Equity Incentive Plan Compensation Earnings ($) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) (3) | Total ($) | |||||||||||||||||||||||||
Robert L. Gershon | 2016 | $ | 365,750 | $ | 293,724 | $ | — | $ | 65,625 | $ | — | $ | — | $ | 30,201 | $ | 755,300 | |||||||||||||||||
CEO and Director | 2015 | $ | 350,000 | $ | 180,000 | $ | — | $ | — | $ | — | $ | — | $ | 30,201 | $ | 560,201 | |||||||||||||||||
2014 | $ | 350,000 | $ | 175,500 | $ | — | $ | — | $ | — | $ | — | $ | 19,720 | $ | 545,220 | ||||||||||||||||||
J. Robert Saron | 2016 | $ | 318,917 | $ | 148,956 | $ | — | $ | 32,375 | $ | — | $ | — | $ | 24,383 | $ | 524,631 | |||||||||||||||||
President, Chief Sales & | 2015 | $ | 305,184 | $ | 79,543 | $ | — | $ | — | $ | — | $ | — | $ | 24,383 | $ | 409,110 | |||||||||||||||||
Marketing Officer & Director | 2014 | $ | 317,949 | $ | 79,917 | $ | — | $ | — | $ | — | $ | — | $ | 16,317 | $ | 414,183 | |||||||||||||||||
Jack McCarthy | 2016 | $ | 287,375 | $ | 134,273 | $ | — | $ | 32,375 | $ | — | $ | — | $ | 29,922 | $ | 483,945 | |||||||||||||||||
Chief Commercialization | 2015 | $ | 275,000 | $ | 74,500 | $ | — | $ | — | $ | — | $ | — | $ | 29,922 | $ | 379,422 | |||||||||||||||||
Officer | 2014 | $ | 201,469 | $ | 100,500 | $ | — | $ | 336,540 | $ | — | $ | — | $ | 12,420 | $ | 650,929 | |||||||||||||||||
Andrew Makrides | 2016 | $ | 215,515 | $ | 100,822 | $ | — | $ | — | $ | — | $ | — | $ | 18,621 | $ | 334,958 | |||||||||||||||||
Executive Chairman | 2015 | $ | 238,620 | $ | 56,318 | $ | — | $ | — | $ | — | $ | — | $ | 18,621 | $ | 313,559 | |||||||||||||||||
of the Board | 2014 | $ | 238,620 | $ | 56,582 | $ | — | $ | — | $ | — | $ | — | $ | 18,574 | $ | 313,776 | |||||||||||||||||
Jay D. Ewers* | 2016 | $ | 235,000 | $ | 109,892 | $ | — | $ | — | $ | — | $ | — | $ | 10,608 | $ | 355,500 | |||||||||||||||||
Chief Financial Officer, | 2015 | $ | 171,456 | $ | 65,255 | $ | — | $ | 70,655 | (2) | $ | — | $ | — | $ | 32,185 | $ | 339,551 | ||||||||||||||||
Treasurer and Secretary | 2014 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||||
Moshe Citronowicz | 2016 | $ | 213,990 | $ | 100,112 | $ | — | $ | 32,375 | $ | — | $ | — | $ | 22,066 | $ | 368,543 | |||||||||||||||||
Senior Vice President | 2015 | $ | 204,775 | $ | 53,537 | $ | — | $ | — | $ | — | $ | — | $ | 22,066 | $ | 280,378 | |||||||||||||||||
2014 | $ | 229,978 | $ | 53,788 | $ | — | $ | — | $ | — | $ | — | $ | 16,437 | $ | 300,203 | ||||||||||||||||||
* Assumed role as CFO on October 1, 2015.
(1) | These columns represent the grant date fair value of the awards as calculated in accordance with FASB ASC 718 (Stock Compensation). Pursuant to SEC rule changes effective February 28, 2010, we are required to reflect the total grant date fair values of the option grants in the year of grant, rather than the portion of this amount that was recognized for financial statement reporting purposes in a given fiscal year which was required under the prior SEC rules, resulting in a change to the amounts reported in prior Annual Reports. |
(2) | On October 14, 2015, a total of 65,000 options were granted to Mr. Ewers with a fair value of $1.087 per option. |
60
BOVIE MEDICAL CORPORATION
(3) | The amounts for 2016 include compensation under the following plans and programs: |
R.L. Gershon | J.R. Saron | J.J. McCarthy | A. Makrides | J.D. Ewers | M. Citronowicz | |||||||||||||||||||
Car Allowance | $ | 6,000 | $ | 6,000 | $ | 6,000 | $ | 6,000 | $ | 6,000 | $ | 6,000 | ||||||||||||
Life insurance premiums | 512 | 512 | 512 | 512 | 512 | 512 | ||||||||||||||||||
Health insurance premiums | 17,064 | 10,828 | 17,064 | 9,125 | — | 10,828 | ||||||||||||||||||
Employer 401(k) contribution | 6,625 | 7,043 | 6,346 | 2,984 | 4,096 | 4,726 | ||||||||||||||||||
Total | $ | 30,201 | $ | 24,383 | $ | 29,922 | $ | 18,621 | $ | 10,608 | $ | 22,066 | ||||||||||||
Amounts in the table above are pro-rated where applicable.
Employment Agreements and Potential Payments Upon Termination or Change in Control
At December 31, 2016, we were obligated under five employment agreements which have expiration dates between December 2016 and December 2017.
Name | Contract Expiration Date | |
Robert L. Gershon | N/A(1) | |
J. Robert Saron | December, 2017 | |
Moshe Citronowicz | December, 2017 | |
Jack McCarthy | N/A(1) | |
Jay D. Ewers | N/A(1) | |
(1) | Employment contracts were revised to remove a date certain for the conclusion of such term and provide for the Executives to remain employed by the Company until such time as their employment is terminated pursuant to the terms of their Employment Agreement. |
Approximate future minimum payments under these agreements are as follows as of December 31, 2016:
(In thousands) | |||
2017 | $ | 1,453 | |
2018 | — | ||
Total | $ | 1,453 | |
Employment contracts, other than for Messrs. Gershon and Ewers, contain an automatic extension for a period of one year after the initial term unless we provide the executives with appropriate 60 days written notice pursuant to the contracts. The employment agreements provide, among other things, that the executive may be terminated as follows:
(a) | Upon the death of the executive, in which case the executive’s estate shall be paid the basic annual compensation due the employee pro-rated through the date of death. |
(b) | By the resignation of the executive at any time upon at least thirty (30) days prior written notice to Bovie in which case Bovie shall be obligated to pay the employee the basic annual compensation due him pro-rated to the effective date of termination. |
(c) | By Bovie, “for cause” if during the term of the employment agreement the employee violates the non-competition provisions of his employment agreement, or is found guilty in a court of law of any crime of moral turpitude in which case the contract would be terminated and provisions for future compensation forfeited. |
(d) | By Bovie, without cause, with the majority approval of the Board of Directors, for Mr. Makrides, Mr. Gershon, Mr. Saron, Mr. McCarthy, Mr. Ewers and Mr. Citronowicz at any time upon at least thirty (30) days prior written notice to the executive. In this case Bovie shall be obligated to pay the executive compensation in effect at such time, including all bonuses, accrued or prorated and expenses up to the date of termination. Thereafter for Messrs. Makrides, Saron and Citronowicz, Bovie shall pay the executive three times the salary in effect at the time of termination payable in one lump sum. |
61
BOVIE MEDICAL CORPORATION
(e) | If Bovie fails to meet its obligations to the executive on a timely basis, or if there is a change in the control of Bovie, the executive may elect to terminate his employment agreement. Upon any such termination or breach of any of its obligations under the employment agreement, Bovie shall pay Mr. Makrides, Mr. Saron and Mr. Citronowicz a lump sum severance equal to three times the annual salary and bonus in effect the month preceding such termination or breach as well as any other sums which may be due under the terms of the employment agreement up to the date of termination. Mr. Gershon, Mr. Ewers and Mr. McCarthy shall be paid two times their annual salary and bonus in effect the month preceding such termination or breach as well as any other sums which may be due under the terms of their respective employment agreement up to the date of termination. |
There are no other employment contracts that have non-cancelable terms in excess of one year.
Options Exercises During Fiscal 2016
The following table sets forth information with respect to our named executive officers concerning the exercises of stock options during fiscal 2016.
Exercise of Equity Based Awards
Name (a) | Number of Shares Acquired on Exercise (b) | Value Realized on Exercise ($) (c) | ||
None | ||||
Outstanding Equity Awards
The following table presents information with respect to each unexercised stock option held by our Named Executive Officers as of December 31, 2016:
Name | # of Securities Underlying Unexercised Options (# Exercisable) | # of Securities Underlying Unexercised Options (# Unexercisable) | Weighted Average Option Exercise Price ($/Sh) | Option Expiration Range After Grant Date | ||||||||||
Andrew Makrides | 24,000 | 6,000 | $ | 2.54 | 7/12/2022 | |||||||||
J. Robert Saron | 18,000 | 49,000 | $ | 2.13 | 7/12/2022 | - | 3/16/2026 | |||||||
Moshe Citronowicz | 18,000 | 49,000 | $ | 2.13 | 7/12/2022 | - | 3/16/2026 | |||||||
Robert L. Gershon | 375,000 | 450,000 | $ | 2.06 | 12/13/2023 | - | 3/16/2026 | |||||||
Jack McCarthy | 106,500 | 143,500 | $ | 3.59 | 3/31/2024 | - | 3/16/2026 | |||||||
Jay D. Ewers | 25,000 | 75,000 | $ | 2.66 | 6/30/2024 | - | 10/14/2025 | |||||||
62
BOVIE MEDICAL CORPORATION
Compensation of Non-Employee Directors
The following is a table showing the director compensation for the year ended December 31, 2016:
Name (a) | Fees Earned Or Paid In Cash ($) (b) | Stock Awards ($) (c) | Option Awards *** ($) (d)(1) | Non-Equity Incentive Plan Compensation ($) (e) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) (I) | All Other Compensation ($) (g) | Total ($) (h) | |||||||||||||||||||||
Lawrence J. Waldman | $ | 70,000 | $ | — | $ | 10,906 | $ | — | $ | — | $ | — | $ | 80,906 | ||||||||||||||
Michael Geraghty | $ | 27,500 | $ | — | $ | 10,906 | $ | — | $ | — | $ | — | $ | 38,406 | ||||||||||||||
John Andres | $ | 52,750 | $ | — | $ | 10,906 | $ | — | $ | — | $ | — | $ | 63,656 | ||||||||||||||
Charles T. Orsatti | $ | 51,250 | $ | — | $ | 10,906 | $ | — | $ | — | $ | — | $ | 62,156 | ||||||||||||||
Scott Davidson | $ | 18,250 | $ | — | $ | 10,906 | $ | — | $ | — | $ | — | $ | 29,156 | ||||||||||||||
*** These columns represent the grant date fair value of the awards as calculated in accordance with FASB ASC 718 (Stock Compensation).
(1) | On July 16, 2015, 12,000 ten year stock options with an exercise price of $1.80 and calculated option fair value of $0.909 were granted to each member of the Board. |
In 2016, our Board of Directors consisted of Robert Gershon, J. Robert Saron, Andrew Makrides, John Andres, Larry Waldman, Michael Geraghty, Charles Orsatti, and Scott Davidson. Mr. Davidson tendered his resignation from the board December 19, 2016, and Mr. Orsatti tendered his resignation from the board effective on December 31, 2016.
In 2003, the Board of Directors adopted and stockholders approved Bovie’s 2003 Executive and Employee Stock Option Plan covering a total of 1,200,000 shares of common stock issuable upon exercise of options to be granted under the Plan.
On October 30, 2007, stockholders approved and the Board of Directors adopted an amendment to the 2003 Executive and Employee Stock Option Plan to increase the maximum aggregate number of shares of common stock reserved for issuance under the 2003 Plan from 1.2 million shares (already reserved against outstanding options) to 1.7 million shares, or an increase of 500,000 shares of common stock for future issuance pursuant to the terms of the plan. Except for the increase in the number of shares covered by the plan, the plan remains otherwise unchanged from its present status. In 2011, the Board of Directors granted 25,000 options to purchase a like number of shares of common stock.
In July of 2012, the stockholders approved the 2012 Executive and Employee Stock Option Plan covering a total of 750,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2016 approximately 37,000 remain to be issued in this plan.
In July of 2015 the stockholders approved the 2015 Executive and Employee Stock Option Plan covering a total of 2,000,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2016 approximately 693,078 remain to be issued in this plan.
There have been no changes in the pricing of any options previously or currently awarded.
Compensation Committee Interlocks and Insider Participation
The Compensation Committee of the Board of Directors is responsible for determining the compensation of executive officers of the Company, as well as compensation awarded pursuant to the Company’s equity incentive plans.
In 2016, our Compensation Committee consisted of three independent members of the Board of Directors, Charles Orsatti who served as Chairman, John Andres and Lawrence J. Waldman.
63
BOVIE MEDICAL CORPORATION
No member of the Compensation Committee is or has been an officer or employee of the Company or any of its subsidiaries. In addition, no member of the Compensation Committee had any relationships with the Company or any other entity that require disclosure under the proxy rules and regulations promulgated by the SEC.
COMPENSATION COMMITTEE REPORT
Our Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis contained in this Annual Report on Form 10-K with management. Based on our Compensation Committee’s review of and the discussions with management with respect to the Compensation Discussion and Analysis, our Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in our Proxy Statement and in this Annual Report on Form 10-K for the fiscal year ended December 31, 2016 for filing with the SEC. During the majority of 2016, our Compensation Committee consisted of three independent members of the Board of Directors, Charles Orsatti, who served as Chairman, John Andres and Lawrence J. Waldman.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Equity Compensation Plan Information
See “ITEM 5. Market for Registrant’s Common Equity and Related Stockholder Matters”.
Security Ownership of Certain Beneficial Owners
The following table sets forth certain information as of March 6, 2017 with respect to the beneficial ownership of the Company’s common stock by its executive officers, directors, all persons known by the Company to be the beneficial owners of more than 5% of its outstanding shares and by all officers and directors as a group.
Number of Shares | |||||||||||
Name and Address | Title | Owned (i) | Nature of Ownership | Percentage of Ownership (i) | |||||||
Great Point Partners, LLC | Common | 3,084,268 | (ii) | Beneficial | 9.985 | % | |||||
165 Mason Street 3rd Floor | |||||||||||
Greenwich, CT 06830 | |||||||||||
William Weeks Vanderfelt | Common | 2,273,249 | Beneficial | 7.4 | % | ||||||
Coralis 44, Azzuri Village 44 | |||||||||||
Roches Noires, 31201 Mauritius | |||||||||||
Cortina Asset Management, LLC | Common | 1,581,239 | Beneficial | 5.1 | % | ||||||
825 North Jefferson, Suite 400 | |||||||||||
Milwaukee, WI 53202 | |||||||||||
Andrew Makrides | Common | 635,972 | (iii) | Beneficial | 2.1 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Robert L. Gershon | Common | 405,000 | (iv) | Beneficial | 1.3 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
64
BOVIE MEDICAL CORPORATION
J. Robert Saron | Common | 423,940 | (v) | Beneficial | 1.4 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Moshe Citronowicz | Common | 444,504 | (vi) | Beneficial | 1.4 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
John Andres | Common | 19,167 | (vii) | Beneficial | 0.1 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Jay D. Ewers | Common | 25,000 | (viii) | Beneficial | 0.1 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Jack McCarthy | Common | 222,250 | (ix) | Beneficial | 0.7 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Michael Geraghty | Common | 44,524 | (x) | Beneficial | 0.1 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Lawrence J. Waldman | Common | 86,524 | (xi) | Beneficial | 0.3 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Officers and Directors as a group (9 persons) | 2,306,881 | (xii) | 7.3 | % | |||||||
(i) | Based on 30,859,753 outstanding shares of Common Stock and 3,752,209 outstanding options to acquire a like number of shares of Common Stock as of March 6, 2017, of which officers and directors owned a total of 716,715 options and 1,590,166 shares at March 6, 2017. We have calculated the percentage on the basis of the amount of outstanding securities plus, for each person or group, any securities that person or group has current or future right to acquire pursuant to options, warrants, conversion privileges or other rights. |
(ii) | Consists of (i) 3,055,000 shares of Common Stock owned collectively by Biomedical Value Fund, LP ("BVF"), Biomedical Offshore Value Fund, Ltd. ("BOVF"), Biomedical Institutional Value Fund, LP ("BIVF"), Class D Series of GEF-PS, LP ("GEF-PS"), and WS Investments, II, LLC ("WS"). The shares of common stock are owned of record as follows: BVF: 1,444,921; BOVF: 808,323; BIVF: 371,588; GEF-PS: 379,021; WS: 51,147. Does not include: (i) 975,639 shares of Series B preferred stock convertible into 1,951,278 common shares, collectively owned by each of BVF, BOVF, BIVF, GEF-PS, and WS. The provisions of such preferred stock restrict the conversion of such preferred stock to the extent that, after giving effect to such conversion, the holder of the preferred stock and its affiliates and any other person or entities with which such holder would constitute a group would beneficially own in excess of 9.985% of the number of shares of Common Stock of the Issuer outstanding immediately after giving effect to such conversion or exercise (the "Ownership Cap"). Therefore, the reporting persons could be deemed to beneficially own such number of shares underlying such preferred stock as would result in total beneficial ownership by such reporting persons up to the Ownership Cap. |
65
BOVIE MEDICAL CORPORATION
(iii) | Includes 611,972 shares and 24,000 vested options out of a total of 30,000 ten year options owned by Mr. Makrides to purchase shares of Common Stock of the Company at an exercise price of $2.54. These options vest equally over a four year period. |
(iv) | Includes 30,000 shares and 375,000 vested options out of a total of 825,000 ten year options owned by Mr. Gershon to purchase shares of Common Stock of the Company at an exercise price of $2.06. These options vest equally over a four year period. |
(v) | Includes 405,940 shares and 18,000 vested options out of a total of 67,000 ten year options owned by Mr. Saron to purchase shares of Common Stock of the Company at an exercise price of $2.13. These options vest equally over a four year period. |
(vi) | Includes 426,504 shares and 18,000 vested options out of a total of 67,000 ten year options owned by Mr. Citronowicz to purchase shares of Common Stock of the Company at an exercise price of $2.13. These options vest equally over a four year period. |
(vii) | Includes 19,167 vested options out of a total of 34,500 ten year options owned by Mr. Andres to purchase shares of Common Stock of the Company at an exercise price of $2.59. These options vest equally over a four year period. |
(viii) | Includes 25,000 vested options out of a total of 100,000 ten year options owned by Mr. Ewers to purchase shares of Common Stock of the Company at an exercise price of $2.66. These options vest equally over a four year period. |
(ix) | Includes 115,750 shares and 106,500 vested options out of a total of 250,000 ten year options owned by Mr. McCarthy to purchase shares of Common Stock of the Company at an exercise price of $3.59. These options vest equally over a four year period. |
(x) | Includes 44,524 vested options out of a total of 62,000 ten year options owned by Mr. Geraghty to purchase shares of Common Stock of the Company at an exercise price of $2.69. These options vest equally over a four year period. |
(xi) | Includes 86,524 vested options out of a total of 113,000 ten year options owned by Mr. Waldman to purchase shares of Common Stock of the Company at an exercise price of $2.58. These options vest equally over a four year period. |
(xii) | Includes 716,715 vested ten year options out of a total of 1,548,500 ten year outstanding options and 1,590,166 shares owned by all Executive Officers and directors as a group. The last date options can be exercised is July 28, 2016. |
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires our officers and directors and persons who own more than ten percent of a registered class of our equity securities, to file reports of ownership and changes in ownership with the Securities and Exchange Commission. Officers, directors and greater than ten-percent shareholders (the “Reporting Persons”) are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file.
To the Company’s knowledge, based solely on its review of the copies of such reports received or written representations from certain Reporting Persons that no other reports were required, the Company believes that during its fiscal year ended December 31, 2016 all filing requirements applicable to the Reporting Persons were timely met.
ITEM 13. Certain Relationships and Related Transactions and Director Independence
Certain Relationships and Related Transactions
Our policy is that employees, non-employees and third parties must obtain authorization from the appropriate department executive manager, for any business relationship or proposed business transaction in which they or an immediate family member has a direct or indirect interest, or from which they or an immediate family member may derive a personal benefit (a “related party transaction”). The maximum dollar amount of related party transactions that may be approved as described above in this paragraph in any calendar year is $120,000. Any related party transactions that would bring the total value of such transactions to greater than $120,000 must be referred to the Audit Committee to determine the procedure for approval and then have the recommendations presented to the Board of Directors for approval.
Several relatives of Nikolay Shilev, Bovie Bulgaria’s Managing Director, are considered related parties. Teodora Shileva, Mr. Shilev’s spouse is an employee of the company working in the Accounting department. Antoaneta Dimitrova Shileva-Toromanova, Mr. Shilev’s sister is the Manager of Production and Human Resources. Svetoslav Shilev, Mr. Shilev’s son is an Engineer in the Quality Assurance department.
66
BOVIE MEDICAL CORPORATION
A relative of Moshe Citronowicz, Bovie’s Senior Vice President, is considered a related party. Arik Zoran is a consultant of the Company doing business as AR Logic, Inc., a consulting firm owned by Arik Zoran, Mr. Citronowicz’s brother. On March 1, 2013 the Company amended the Consulting Services Agreement dated January 2011, extending the term of the existing agreement until December 31, 2014. The agreement shall automatically renew for additional one year periods, unless either party gives written notice of its desire not to renew at least one year prior to the expiration of the initial Term or renewal term. The agreement with AR Logic provides for a separate hourly based fee structure for consulting related to new projects. AR Logic has a royalty contract with us related to the creation and design of proprietary technology that is used in some of our generators. AR Logic was paid consulting fees of approximately $0.2 million, $0.3 million and $0.2 million during 2016, 2015 and 2014, respectively.
A second relative of Mr. Citronowicz is considered a related party. Yechiel Tsitrinovich is also a brother of Mr. Citronowicz and acts as a consultant to the Company related to research and development of certain products. Mr. Tsitrinovich has a royalty contract with us related to the creation and design of a proprietary technology that is used in some of our generators. Mr. Tsitrinovich was paid a combination of consulting fees and royalties on previous product designs approximately $0.1 million per year for 2016, 2015 and 2014.
Independent Board Members
The Board currently has three independent members, John Andres, Michael Geraghty and Lawrence J. Waldman who meet the existing independence requirements of the NYSE MKT Market and the Securities and Exchange Commission.
ITEM 14. Principal Accountant Fees and Services
The following table sets forth the aggregate fees billed to us by our current accountants, Frazier & Deeter, LLC:
Year Ended December 31, | |||||||
(In thousands) | 2016 | 2015 | |||||
Audit fees (1) | $ | 173 | $ | 141 | |||
Non-Audit fees: | |||||||
Audit related fees (2) | 3 | 17 | |||||
Tax fees (3) | — | — | |||||
All other fees (4) | — | — | |||||
Total fees billed | $ | 176 | $ | 158 | |||
(1) | Audit fees consist of fees billed for professional services rendered for the audit of Bovie’s annual financial statements and reviews of its interim consolidated financial statements included in quarterly reports and other services related to statutory and regulatory filings or engagements. |
(2) | Audit related fees consist of fees billed for assurance and related services that are reasonably related to the performance of the audit or reviews of Bovie’s consolidated financial statements and are not reported under “Audit Fees”. |
(3) | Tax fees consist of fees billed for professional services rendered for tax compliance and tax advice (domestic and international). These services include assistance regarding federal, state and international tax compliance, acquisitions and international tax planning. |
(4) | All other fees consist of fees for products and services other than the services reported above. |
67
BOVIE MEDICAL CORPORATION
PART IV
ITEM 15. Exhibits and Financial Statement Schedules
(a)(1) | LISTING OF FINANCIAL STATEMENTS | Page | ||
The following consolidated financial statements of the Company are included in Item 8 of this Report: | ||||
Consolidated Balance Sheets at December 31, 2016 and 2015 | ||||
Consolidated Statements of Operations for the years ended December 31, 2016, 2015 and 2014 | ||||
Consolidated Statement of Changes in Stockholders’ Equity for the years ended December 31, 2016, 2015 and 2014 | ||||
Consolidated Statements of Cash Flows for the years ended December 31, 2016, 2015 and 2014 | ||||
Notes to Consolidated Financial Statements | ||||
Supplemental unaudited quarterly financial information | ||||
(a)(2) | FINANCIAL STATEMENT SCHEDULES | |||
All financial statement schedules have been omitted, since the required information is not applicable or is not present in amounts sufficient to require submission of the schedule, or because the information required is included in the consolidated financial statements and notes thereto included in this Report. | ||||
(a)(3) EXHIBITS
1.1 | Underwriting Agreement dated 3/12/15 between Bovie Medical Corporation and Craig Hallum Capital Group, LLC (Incorporated by reference to Exhibit 2.1 to Form 8-K filed on March 12, 2015) | |
1.2 | Underwriting Agreement, dated November 10, 2016, by and among Bovie Medical Corporation, the Selling Stockholders named therein, and Piper Jaffray & Co. (Incorporated by reference to Exhibit 1.1 to Form 8-K filed on November 14, 2016) | |
3.1 | Articles of Incorporation of the Registrant (Incorporated by reference to the Registrant’s report on Form 10-K/A filed on March 31, 2011) | |
3.2 | By laws of the Registrant (Incorporated by reference to the Registrant’s report on Form 10-K/A filed on March 31, 2011) | |
3.3 | Certificate of Designation of Preferences, Rights and Limitations of Series A 6% Convertible Preferred Stock of Bovie Medical Corporation (Incorporated by reference to the Registrant’s report on Form 8-K filed December 16, 2013) | |
3.4 | Certificate of Designation of Series B Preferred Stock (Incorporated by reference to Exhibit 3.1 on Form 8-K filed on March 11, 2015. | |
10.1 | Employment Agreement dated May 5, 2014 between Bovie Medical Corporation and Peter L. Donato (Incorporated by reference to the Registrant’s report on Form 8-K filed May 8, 2014) | |
10.2 | Loan Agreement (Incorporated by reference to the Registrant’s report on Form 8-K filed on March 24, 2014) | |
10.3 | Mortgage, Security agreement, Financial Statement and Assignment (Incorporated by reference to the Registrant’s report on Form 8-K filed on March 24, 2014) | |
10.4 | Promissory Note (Incorporated by reference to the Registrant’s report on Form 8-K filed on March 24, 2014) | |
10.5 | Assignment of Rents, Leases and Profits and Contracts (Incorporated by reference to the Registrant’s report on Form 8-K filed March 24, 2014) | |
10.6 | Security Agreement (Incorporated by reference to the Registrant’s report on Form 8-K filed on March 24, 2014) | |
10.7 | Environmental Indemnity Agreement (Incorporated by reference to the Registrant’s report on Form 8-K filed on March 24, 2014.) | |
10.8 | Exchange Agreement (Incorporated by reference to Exhibit 10.1 on Form 8-K filed on March 11, 2015) | |
10.9 | Registration Rights Agreement (Incorporated by reference to Exhibit 10.2 on Form 8-L filed on March 11, 2015) | |
10.10 | Amendment to Jay D. Ewers Employment Agreement dated August 6, 2015 (Incorporated by reference to Exhibit 10.2 to Form 8-K Filed on August 12, 2015) | |
68
BOVIE MEDICAL CORPORATION
10.11 | Amendment to Robert L. Gershon Employment Agreement dated October 14, 2015 (Incorporated by reference to Exhibit 10.2 to Form 8-K Filed on October 19, 2015) | |
10.12 | Amendment to Jay D. Ewers Employment Agreement dated October 14, 2015 (Incorporated by reference to Exhibit 10.3 to Form 8-K Filed on October 19, 2015) | |
10.13 | Share Purchase Agreement (Incorporated by reference to Exhibit 10.1 to Form 8-K filed on October 23, 2015) | |
10.14 | Management Agreement (Incorporated by reference to Exhibit 10.2 on Form 8-K filed on October 23, 2015) | |
10.15 | Restricted Stock Agreement (Incorporated by reference to Exhibit 10.3 to Form 8-K filed on October 23, 2015) | |
10.16 | Jack McCarthy Employment Agreement, dated March 31, 2014 (Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Quarter ended March 31, 2014 and filed on May 15, 2014) | |
10.17 | Amendment to Jack McCarthy Employment Agreement, dated April 20, 2016 (Incorporated by reference to Exhibit 10.2 on Form 8-K filed on April 21, 2016) | |
10.18 | First Amendment to Loan Agreement, dated June 28, 2016, between the Company and Bank of Tampa, a Florida banking corporation (Incorporated by reference to Exhibit 10.1 on Form 8-K filed on July 5, 2016) | |
10.19 | Amended and Restated Promissory Note (Incorporated by reference to Exhibit 10.2 on Form 8-K filed on July 5, 2016) | |
10.20 | Mortgage and Other Loan Documents Extension & Modification (Incorporated by reference to Exhibit 10.3 on Form 8-K filed on July 5, 2016) | |
14.1 | Bovie Medical Corporation Code of Ethics (Incorporated by reference to the Registrant’s report on Form 10-K/A filed March 31, 2011) | |
21.1* | List of Subsidiaries | |
23.1* | Consent of Frazier & Deeter, LLC | |
31.1* | Certification pursuant to Section 302 of Sarbanes-Oxley Act of 202 | |
31.2* | Certification pursuant to Section 302 of Sarbanes-Oxley Act of 202 | |
32.1* | Certification pursuant to Section 906 of Sarbanes-Oxley Act of 202 | |
32.2* | Certification pursuant to Section 906 of Sarbanes-Oxley Act of 202 | |
99.1 | Stipulation of Settlement, dated June 26, 2014 (Incorporated by reference to the Registrant’s report on Form 8-K filed July 2, 2014) | |
99.2 | Amended order preliminarily approving Derivative Settlement and Providing Notice dated July 7, 2014 (Incorporated by reference to the Registrant’s report on Form 8-K filed July 16, 2014) | |
99.3 | Notice of Proposed Settlement of Derivative Action (Incorporated by reference to the Registrant’s report on Form 8-K filed July 16, 2014) | |
99.4 | Summary Notice of Proposed Settlement of Derivative Action (Incorporated by reference to the Registrant’s report on Form 8-K filed July 16, 2014) | |
101.INS** | XBRL Instance Document | |
101.SCH** | XBRL Taxonomy Extension Schema Document | |
101.CAL** | XBRL Taxonomy Extension Calculation Linkbase Document | |
101.DEF** | XBRL Taxonomy Extension Definition Linkbase Document | |
101.LAB** | XBRL Taxonomy Extension Label Linkbase Document | |
101.PRE** | XBRL Taxonomy Extension Label Presentation Document | |
* Filed herewith.
** XBRL (Extensible Business Reporting Language) information is furnished and not filed or a part of registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended and otherwise is not subject to liability under these sections.
69
BOVIE MEDICAL CORPORATION
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in Clearwater, Florida on March 10, 2017.
Bovie Medical Corporation | |||
By: | /s/ Robert L. Gershon | ||
Robert L. Gershon | |||
Chief Executive Officer and Director | |||
(Principal Executive Officer) | |||
By: | /s/ Jay D. Ewers | ||
Jay D. Ewers | |||
Chief Financial Officer, | |||
Treasurer and Secretary | |||
(Principal Financial Officer) | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Name | Title | Date | ||
Directors: | ||||
/s/ ANDREW MAKRIDES | Executive Chairman of the Board | March 10, 2017 | ||
Andrew Makrides | ||||
/s/ ROBERT L. GERSHON | Chief Executive Officer and Director | March 10, 2017 | ||
Robert L. Gershon | ||||
/s/ JAY D. EWERS | Chief Financial Officer, Treasurer and Secretary | March 10, 2017 | ||
Jay D. Ewers | ||||
/s/ J. ROBERT SARON | President, Chief Sales and Marketing Officer and Director | March 10, 2017 | ||
J. Robert Saron | ||||
/s/ JOHN ANDRES | Director | March 10, 2017 | ||
John Andres | ||||
/s/ LAWRENCE J. WALDMAN | Director | March 10, 2017 | ||
Lawrence J. Waldman | ||||
/s/ MICHAEL GERAGHTY | Director | March 10, 2017 | ||
Michael Geraghty | ||||
70
