Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
[X]
ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE
ACT OF 1934
For the fiscal year ended December 31, 2016
or
[
]
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
For the transition period from ________ to _________
Commission file number: 0-53497
|
ADVANCED MEDICAL ISOTOPE
CORPORATION
|
|
(Exact
name of registrant as specified in its charter)
|
|
Delaware
|
|
80-0138937
|
|
(State
or other jurisdiction of
|
|
(I.R.S.
Employer
|
|
incorporation or
organization)
|
|
Identification
No.)
|
|
1021 N. Kellogg Street ● Kennewick, WA
99336
|
|
(Address of
principal executive offices) (Zip Code)
|
|
|
|
(509) 736-4000
|
|
Registrant’s
telephone number, including area code
|
Securities
registered pursuant to Section 12(b) of the Act: None
Securities
registered pursuant to Section 12(g) of the Act: Common Stock,
$0.001 Par Value
Indicate
by check mark if the registrant is a well-known seasoned issuer, as
defined in Rule 405 of the Securities Act. Yes [ ] No
[X]
Indicate
by check mark if the registrant is not required to file reports
pursuant to Section 13 or 15(d) of the Act. Yes [ ] No
[X]
Indicate
by check mark whether the registrant (1) has filed all reports
required to be filed by Section 13 or 15(d) of the Securities
Exchange Act of 1934 during the preceding 12 months (or for such
shorter period that the registrant was required to file such
reports), and (2) has been subject to such filing requirements for
the past 90 days. Yes [X] No [ ]
Indicate
by check mark whether the registrant has submitted electronically
and posted on its corporate Web site, if any, every Interactive
Data File required to be submitted and posted pursuant to Rule 405
of Regulation S-T during the preceding 12 months (or for such
shorter period that the registrant was required to submit and post
such files). Yes [X] No [ ]
Indicate
by check mark if disclosure of delinquent filers pursuant to Item
405 of Regulation S-K is not contained herein, and will not be
contained, to the best of registrant’s knowledge, in
definitive proxy or information statements incorporated by
reference in Part III of this Form 10-K or any amendment to this
Form 10-K. [ ]
Indicate
by check mark whether the registrant is a large accelerated filer,
an accelerated filer, a non-accelerated filer, or a smaller
reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in Rule
12b-2 of the Exchange Act. (Check one):
|
Large Accelerated Filer
|
[ ]
|
Accelerated Filer
|
[ ]
|
|
|
|
|
|
|
Non-Accelerated Filer
|
[ ]
|
Smaller Reporting Company
|
[X]
|
|
(Do
not check if a smaller reporting company)
|
|
|
|
Indicate
by check mark whether the registrant is a shell company (as defined
in Rule 12b-2 of the Act). Yes [ ] No
[X]
The
aggregate market value of the voting and non-voting common equity
held by non-affiliates computed by reference to the price at which
the common equity was last sold, or the average bid and asked price
of such common equity, as of the last business day of the
registrant’s most recently completed second fiscal quarter
was approximately $7,280,000. Shares of common stock held by each
executive officer and director and by each person who owns 10% or
more of the outstanding common stock of the registrant have been
excluded in that such persons may be deemed to be
affiliates. This determination of affiliate status is
not necessarily a conclusive determination for other
purposes. Without acknowledging that any individual
director of registrant is an affiliate, all directors have been
included as affiliates with respect to shares owned by
them.
As of
March 6, 2017, there were 37,541,697 shares of the
registrant’s Common Stock and 3,241,802 shares of the
registrant’s Series A Convertible Preferred Stock
outstanding.
Advanced Medical Isotope Corporation
Report on Form 10-K
TABLE OF CONTENTS
|
PART I.
|
|
|
|
|
|
|
|
|
|
Item
1.
|
Business
|
|
1
|
|
Item
1A.
|
Risk
Factors
|
|
10
|
|
Item
1B.
|
Unresolved Staff
Comments
|
|
19
|
|
Item
2.
|
Properties
|
|
19
|
|
Item
3.
|
Legal
Proceedings
|
|
19
|
|
Item
4.
|
Mine
Safety Disclosures
|
|
19
|
|
|
|
|
|
|
PART II.
|
|
|
|
|
|
|
|
|
|
Item
5.
|
Market
for Registrant’s Common Equity, Related Stockholder Matters
and Issuer Purchases of Equity Securities
|
|
20
|
|
Item
6.
|
Selected Financial
Data
|
|
22
|
|
Item
7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
|
22
|
|
Item
7A.
|
Quantitative and
Qualitative Disclosures About Market Risk
|
|
28
|
|
Item
8.
|
Financial
Statements and Supplementary Data
|
|
28
|
|
Item
9.
|
Changes In and
Disagreements With Accountants on Accounting and Financial
Disclosure
|
|
28
|
|
Item
9A.
|
Controls and
Procedures
|
|
29
|
|
Item
9B.
|
Other
Information
|
|
30
|
|
|
|
|
|
|
PART III.
|
|
|
|
|
|
|
|
|
|
Item
10.
|
Directors,
Executive Officers and Corporate Governance
|
|
30
|
|
Item
11.
|
Executive
Compensation
|
|
33
|
|
Item
12.
|
Security Ownership
of Certain Beneficial Owners and Management and Related Stockholder
Matters
|
|
36
|
|
Item
13.
|
Certain
Relationships and Related Transactions, and Director
Independence
|
|
38
|
|
Item
14.
|
Principal
Accountant Fees and Services
|
|
38
|
|
|
|
|
|
|
PART IV.
|
|||
|
|
|||
|
Item
15.
|
Exhibits and
Financial Statement Schedules
|
|
39
|
PART I
FORWARD LOOKING STATEMENTS
Except
for statements of historical fact, certain information described in
this Form 10-K report contains “forward-looking
statements” that involve substantial risks and
uncertainties. You can identify these statements by
forward-looking words such as “anticipate,”
“believe,” “could,” “estimate,”
“expect,” “intend,” “may,”
“should,” “will,” “would” or
similar words. The statements that contain these or
similar words should be read carefully because these statements
discuss the Company’s future expectations, including its
expectations of its future results of operations or financial
position, or state other “forward-looking”
information. Advanced Medical Isotope Corporation
believes that it is important to communicate its future
expectations to its investors. However, there may be
events in the future that the Company is not able to accurately
predict or to control. Further, the Company urges you to
be cautious of the forward-looking statements which are contained
in this Form 10-K report because they involve risks, uncertainties
and other factors affecting its operations, market growth, service,
products and licenses. The risk factors in the section
captioned “Risk Factors” in Item 1A of the
Company’s Form 10-K, as well as other cautionary language in
this Form 10-K report, describe such risks, uncertainties and
events that may cause the Company’s actual results and
achievements, whether expressed or implied, to differ materially
from the expectations the Company describes in its forward-looking
statements. The occurrence of any of the events
described as risk factors could have a material adverse effect on
the Company’s business, results of operations and financial
position.
In September of 2006, the Company began operating
as Advanced Medical Isotope Corporation (the
“Company”). The Company’s
predecessor was incorporated under the
laws of the state of Delaware in 1994. The Company has authorized
capital of 2,000,000,000 shares of common stock, $0.001 par value
per share, and 20,000,000 shares of preferred stock, $0.001 par
value per share.
Our
principal place of business is 1021 N Kellogg Street, Kennewick,
Washington, 99336. Our telephone number is (509) 736-4000. Our
corporate website address is http://www.isotopeworld.com. Our
common stock is currently listed for quotation on the OTC Pink
Marketplace under the symbol “ADMD.”
Overview
The
Company is a late stage radiation oncology medical device company
engaged in the development of its yttrium-90 based brachytherapy
device, RadioGel™, for the treatment of non-resectable
tumors. A prominent team of radiochemists, scientists and
engineers, collaborating with strategic partners, including
national laboratories, universities and private corporations, lead
the Company’s development efforts. The Company’s
overall vision is to globally empower physicians, medical
researchers and patients by providing them with new isotope
technologies that offer safe and effective treatments for
cancer.
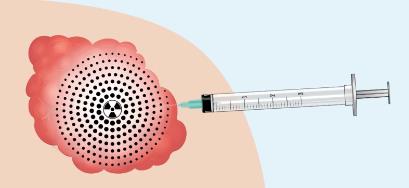
The Company’s current focus is on the
development of its RadioGel™ device. RadioGel™ is an
injectable particle-gel, for brachytherapy radiation treatment of
cancerous tumors in people and animals. RadioGel™ is
comprised of a hydrogel, or a substance that is liquid at room
temperature and then gels when reaching body temperature after
injection into a tumor. In the gel are small, one micron,
yttrium-90 phosphate particles (“Y-90”). Once injected, these inert particles are
locked in place inside the tumor by the gel, delivering a very high
local radiation dose. The radiation is beta, consisting of
high-speed electrons. These electrons only travel a short distance
so the device can deliver high radiation to the tumor with minimal
dose to the surrounding tissue. Optimally, patients can go home
immediately following treatment without the risk of radiation
exposure to family members. Since Y-90 has a half-life of 2.7 days,
the radioactively drops to 5% of its original value after ten
days.
The Company’s lead brachytherapy products,
including RadioGel™, incorporate patented technology
developed for Battelle Memorial Institute
(“Battelle”) at Pacific Northwest National Laboratory,
a leading research institute for government and commercial
customers. Battelle has granted the Company an exclusive license to
patents covering the manufacturing, processing and applications of
RadioGel™ (the “Battelle
License”). Other
intellectual property protection includes proprietary production
processes and trademark protection in 17 countries. The Company
plans to continue efforts to develop new refinements on the
production process, and the product and application hardware, as a
basis for future patents.
The Company is currently focusing on obtaining
approval from the Food and Drug Administration
(“FDA”) to market and sell RadioGel™ as a
Class II medical device. The Company first requested FDA approval
of RadioGel™ in June 2013, at which time the FDA classified
RadioGel™ as a medical device. The Company then followed with
a 510(k) submission which the FDA responded with a request for a
physician letter of substantial equivalence and a reformatted
510(k) summary, which the Company provided January 2014. In
February 2014 the FDA ruled the device as not substantially
equivalent due to a lack of predicate device and it was classified
to Class III. The Company is currently developing test plans to
address issues raised by the FDA in connection with the
Company’s previous submissions regarding RadioGel™,
including developing specific test plans and specific indication of
use. The Company intends to request FDA approval to apply
for de
novo classification of
RadioGel™, which would reclassify the device from a Class III
device to a Class II device, further simplifying the regulatory
path.
In previous FDA submittals, the Company proposed
applying RadioGel™ for a very broad range of cancer
therapies, referred to as Indication for Use. The FDA has requested
that the Company reduce its Indications for Use. To comply with
that request, the Company has expanded its Medical Advisory Board
(“MAB”) and engaged doctors from respected
hospitals who have evaluated the candidate cancer therapies based
on three criteria: (1) potential for FDA approval and successful
therapy; (2) notable advantage over current therapies; and (3)
probability of wide spread acceptance by the medical
community.
The
MAB selected eighteen applications for RadioGel™, each of
which meet the criteria described above. This large number confirms
the wide applicability of the device and defines the path for
future business growth. The Company intends to apply to the FDA for
a single Indication for Use, followed by subsequent applications
for additional Indications for Use. The initial application should
facilitate each subsequent application, and the testing for many of
the subsequent applications could be conducted in parallel,
depending on available resources.
The
MAB selected the treatment of basal cell and squamous cell skin
cancers for the first Indication for Use to be submitted to the
FDA. According to the American Cancer Society, one out of every
three new cancers diagnosed in the U.S. is a cancerous skin lesion
of this type, representing 5.5 million tumors diagnosed annually.
The MAB believes RadioGel™ has the potential to be the
preferred treatment in a reasonable number of cases in a very large
market.
The Company’s, IsoPet Solutions division was established in
May 2016 to focus on the veterinary oncology market. The Company
has engaged four different university veterinarian hospitals to
begin using RadioGel™ for treatment of four different cancer
types in dogs and cats. Washington State University Veterinary
Hospital has tested one cat to demonstrate the procedures and the
absence of any significant toxicity effect. The other three centers
are expected to begin therapy during the second quarter of 2017
after their internal administrative review process is
completed.
These
animal therapies will focus on creating labels that describe the
procedures in detail as a guide to future veterinarians. The labels
will be voluntarily submitted to the FDA for review. They will then
be used as data for future FDA applications in the medical sector
and as key intellectual property for licensing to private
veterinary clinics. In 2018, Dr. Alice Villalobos, the Chair of our
Veterinarian Advisory Board, will be the first licensee of these
therapies in her private clinic to demonstrate the business
model.
The
Company anticipates that future profit will be derived from direct
sales of RadioGel™ and related services, and from licensing
to private medical and veterinary clinics in the U.S. and
internationally.
Financing and Strategy
Research
and development of RadioGel™ and other products in the
Company’s brachytherapy product line has been funded with
proceeds from the sale of equity and debt securities as well as a
series of grants. The Company requires funding of approximately
$1.5 million annually to maintain current operating activities.
Over the next 12 to 24 months, the Company believes it will cost
approximately $5.0 million to $10.0 million to fund: (1) the FDA
approval process and initial deployment of RadioGel™ and
other brachytherapy products, and (2) initiate regulatory approval
processes outside of the United States. The continued deployment of
RadioGel™ and the Company’s other brachytherapy
products and a worldwide regulatory approval effort will require
additional resources and personnel. The principal variables in the
timing and amount of spending for the brachytherapy products in the
next 12 to 24 months will be the FDA’s classification of the
Company’s brachytherapy products, including RadioGel™,
as Class II or Class III devices (or otherwise) and any
requirements for additional studies which may possibly include
clinical studies. Thereafter, the principal variables in the amount
of the Company’s spending and its financing requirements
would be the timing of any approvals and the nature of the
Company’s arrangements with third parties for manufacturing,
sales, distribution and licensing of those products and the
products’ success in the U.S. and elsewhere. The Company
intends to fund its activities through strategic transactions such
as licensing and partnership agreements or additional capital
raises.
Following
receipt of required regulatory approvals and financing in the U.S.,
the Company intends to outsource material aspects of manufacturing,
distribution, sales and marketing. Outside of the U.S., the Company
intends to pursue licensing arrangements and/or partnerships to
facilitate its global commercialization strategy.
In the longer-term, subject to the Company receiving adequate
funding, regulatory approval for RadioGel™ and other
brachytherapy products, and thereafter being able to successfully
commercialize its brachytherapy products, the Company intends to
consider resuming research efforts with respect to other products
and technologies intended to help improve the diagnosis and
treatment of cancer and other illnesses
Based
on the Company’s financial history since inception, its
auditor has expressed substantial doubt as to the Company’s
ability to continue as a going concern. The Company has limited
revenue, nominal cash, and has accumulated deficits since
inception. If the Company cannot obtain sufficient additional
capital, the Company will be required to delay the implementation
of its business strategy and may not be able to continue
operations.
Product Features
The
Company’s RadioGel™ device has the following product
features:
●
Beta particles only travel a short distance so the
device can deliver high radiation to the tumor with minimal dose to
the nearby normal tissues. In
medical terms Y-90 beta emitter has a high efficacy
rate;
●
Benefitting
from the short penetration distance, the patient can go home
immediately with no fear of exposure to family members, and there
is a greatly reduced radiation risk to the doctor. A simple plastic
tube around the syringe, gloves and safety glasses are all that is
required. Other gamma emitting products require much more
protection;
●
A
2.7-day half-life means that only 5% of the radiation remains after
ten days. This is in contrast to the industry-standard gamma
irradiation product, which has a half-life of 17 days;
●
The
short half-life also means that any medical waste can be stored for
thirty days then disposed as normal hospital waste;
●
RadioGel™
can be administered with small diameter needles (27-gauge) so there
is minimal damage to the normal tissue. This is in contrast to the
injection of metal seeds, which does considerable damage;
and
●
After
about 120 days the gel resorbs by a normal biological cycle, called
the Krebs Cycle. The only remaining evidence of the treatment are
phosphate particles so small in diameter that it requires a
high-resolution microscope to find them. This is in contrast to
permanent presence of metal seeds.
Steps from Production to Therapy
Device Production
During
the next two years, the Company intends to outsource material
aspects of manufacturing and distribution. As future product volume
increases, the Company will reassess its make-buy decision on
manufacturing and will analyze the cost/benefit of a centrally
located facility.
Production of the Hydrogel
RadioGel™
is manufactured with a proprietary process under ventilated sterile
hood by following strict Good Laboratory Practices, or GLP,
procedures. It is made in large batches that are frozen for up to
three months. When the product is ready to ship, a small quantity
of the gel is dissolved in a sterile saline solution. It is then
passed through an ultra-fine filter to ensure
sterility.
Production of the Yttrium-90 Phosphate Particles
The
Y-90 particles are produced with simple ingredients via a
proprietary process, again following strict GLP procedures. They
are then mixed into a phosphate-buffered saline solution. They can
be produced in large batches for several shipments. The number of
particles per shipment is determined by the dose prescribed by the
doctor.
Shipment
RadioGel™
is shipped in two containers, one with a solution of the gel and
the other with a solution of the particles. Before shipment they
are subjected to sterility testing, again by strict procedures. The
vial with the Y-90 is put through a special radiation calibrator,
which measures beta particles. The vials can be shipped via FedEx
or UPS by following the proper protocols.
At the User
The
user receives the two vials. The solution containing the
RadioGel™ is mixed with the solution containing the Y-90
particles. This is then shaken to ensure homogeneity and withdrawn
into a syringe. The quantities that are mixed are calculated from
the information on the product label.

The
specific injection technique depends on the Indication for Use. For
small tumors, one centimeter in diameter or less, the cancer is
treated with a single injection. For larger tumors, the cancer is
treated with a series of small injections from the same
syringe.
Principal Markets
The
Company is currently pursuing two synergistic business sectors,
medical and veterinary, each of which are summarized
below.
Medical
Sector
Brachytherapy
is the use of radiation to destroy cancerous tumors by placing a
radiation source inside or next to the treatment area. According to
the 2014 MEDraysintell report, the global market for brachytherapy
reached US$ 680 million in 2013 and is projected to reach $2.4
billion by 2030. It is estimated that the U.S. market represents
approximately half of the global market. The Company believes there
are significant opportunities in prostate, breast, liver,
pancreatic, head and neck cancers. The 2014 U.S. estimated new
cases of cancer according to the American Cancer Society are
233,000 prostate, 235,030 breast, 31,190 liver, and 46,420
pancreas.
RadioGel™ is currently fully developed,
requiring only FDA approval before commercialization. The
Company has been seeking FDA approval of RadioGel™ for almost
four years. The principal issue preventing approval is that the
Company attempted to obtain regulatory approval for a broad range
of Indications for Use, including all non-resectable cancers,
without sufficient supporting data.
Building on the FDA’s ruling of
RadioGel™ as a device, the Company is currently developing
test plans to address issues raised in the Company’s prior
FDA submittal regarding RadioGel™. The Company intends to
request FDA approval to submit RadioGel™ for
de novo
classification, which would reclassify
the device from a Class III device to a Class II device, and
accelerate the regulatory approval
path.
After
analyzing the Company’s data and the last four years of
communication from the FDA, the Company has taken the following
steps:
1.
Under
new leadership, the Company is implementing all past
recommendations from the FDA. The Company intends to narrow the
Indications for Use, will provide test plans for FDA review to
respond to answer all previous FDA questions, and will request a
pre-submission meeting;
2.
Prepare a pre-submission request document and FDA
meeting request to obtain feedback on the test plans in order to
initiate testing, to present the proposed content for the final
application and to request permission to submit a
de
novo;
3.
Submit an Investigational Device Exemption
(“IDE”) to obtain permission to conduct human
clinical studies; and
4.
File a de novo or Pre-Market Approval
application.
The
critical path is the required testing – in vitro, animal
testing, human clinical studies – all of which is resource
dependent.
In previous submittals, the Company proposed
applying a very broad range of cancer therapies, referred to as
Indications for Use, to RadioGel™. The FDA has strongly
advised the Company to reduce its Indications for Use. To comply
with that request, the Company has expanded its MAB, consisting
of Drs. Barry D. Pressman (Chairman), Albert DeNittis,
Howard Sandler, and Darrell Fisher.
The MAB
evaluated the candidate cancer
therapies based on three criteria: (i) the potential for FDA
approval and successful therapy; (ii) notable advantages of
RadioGel™ over current therapies; and (iii) the likelihood
that RadioGel™ can be widely accepted by the medical
community and profitably commercialized.
The
MAB selected eighteen Indications for Use for RadioGel™, each
of which meets the above-mentioned criteria. These eighteen
Indications for Use are listed below. This large number confirms
the wide applicability of the device and defines the path for
future growth. The Company intends to apply to the FDA for a single
Indication for Use, followed by subsequent applications for
additional Indications for Use. The initial application should
facilitate each subsequent application, and the testing for many of
the subsequent applications could be conducted in parallel,
depending on available resources.
|
● Skin
cancer
● Involved
lymph nodes
● Bladder
● Liver
● Localized
prostate
● Pancreas
● Head
and neck (including sino-nasal and oropharyngeal)
● Ocular
melanoma
|
● Non-dendritic
brain
● Pediatric
cancers – several types
● Rectal
● Gynecological
● Spinal
● Recurrent
esophageal
● Breast
cancer resection cavity
● Anaplastic
thyroid
|
After
thorough review to prioritize indications, the MAB has selected
basal cell and squamous cell carcinoma (skin cancers) as the first
Indication for Use to be presented to the FDA. According to American Cancer Society, one out of
every three new cancers diagnosed in the U.S. is a cancerous skin
lesion of this type, representing 5.5 million tumors annually. The
MAB believes RadioGel™ will be the preferred treatment in a
reasonable number of cases in a very large
market.
Veterinary
Sector
There
are about 150 million pet dogs and cats in the United States.
Nearly one-half of dogs and one-third of cats are diagnosed with
cancer at some point in their lifetime. The Veterinary Oncology
& Hermatology Center in Norwalk, Connecticut, reports that
cancer is the number one natural cause of death in older cats and
dogs, accounting for nearly 50 percent of pet deaths each year. The
American Veterinary Medical Association reports that half of the
dogs ten years or older will die because of cancer. The National
Cancer Institute reports that about six million dogs are diagnosed
with cancer each year, translating to more than 16,000 a day. The
average cost of treating tumors in dogs using radiation is $5,000
to 7,000, according to petcarerx.com.
The Company’s IsoPet operating division
focuses on the veterinary oncology market. Dr. Alice
Villalobos, a founding member of the Veterinary Cancer Society and
the Chair of our Veterinary Medicine Advisory Board, has been
providing guidance to management regarding this
market.
Since
the FDA has classified RadioGel™ as a device, the Company
can implement future improvements in the product and the
application techniques to further benefit animals and to strengthen
our intellectual property, without any further FDA
approvals.
The
Company currently intends to utilize university veterinary
hospitals for therapy development, given that veterinary hospitals
offer superior and plentiful veterinarians and students, a large
number of animal patients, radioactive material handling licenses,
and are respected by private veterinary centers and
hospitals.
The Company has recently engaged four different
university veterinarian hospitals to begin using RadioGel™
for four different cancer types in dogs and cats. Each
veterinary hospital will focus on a different cancer
therapy:
●
Washington State
University – feline sarcoma, possibly followed by canine
sarcoma;
●
University of
Missouri – soft tissue carcinoma, possibly followed by
localized prostate;
●
University of
Florida – lymph nodes, followed by liver cancer;
and
●
Colorado State
University – oral squamous cancer.
Washington State University Veterinary Hospital
has tested one cat to demonstrate the procedures and the absence of
any significant toxicity effect. The others typically take
about two months to complete their internal review before they can
begin therapy. In each case, they will treat several animals with
the objective of creating a detailed therapy procedure, which will
be incorporated into product information for RadioGel™, known
as a Label. The Company will then voluntarily ask the FDA to review
this Label, as suggested by their guidance.
These
Labels will be used as part of our medical application to the FDA.
A number of these animal cancers also occur in humans, so there is
a direct benefit to our FDA applications when we get to that
particular Indication for Use.
The
Labels and the animal experience will also be used to transfer the
therapies to the private clinics and hospitals. Dr. Villalobos has
agreed that her private clinic will be the first to implement the
Company’s veterinary therapies, and the Company intends to
assist Dr. Villalobos to obtain the required radioactive material
handling license.
After
completing the first set of therapies, each university veterinary
hospital will select additional Indications for Use.
Competitors
The
Company competes in a market characterized by technological
innovation, extensive research efforts, and significant
competition.
The
pharmaceutical and biotechnology industries are intensely
competitive and subject to rapid and significant technological
changes. A number of companies are pursuing the development of
pharmaceuticals and products that target the same diseases and
conditions that our products target. We cannot predict with
accuracy the timing or impact of the introduction of potentially
competitive products or their possible effect on our sales. Certain
potentially competitive products to our products are possibly in
various stages of development. Also, there may be many ongoing
studies with currently marketed products and other developmental
products, which may yield new data that could adversely impact the
use of our products in their current and potential future
Indications for Use. The introduction of competitive products could
significantly reduce our sales, which, in turn would adversely
impact our financial and operating results.
There
are a wide variety of cancer treatments approved and marketed in
the U.S. and globally. General categories of treatment include
surgery, chemotherapy, radiation therapy and immunotherapy. These
products have a diverse set of success rates and side effects. The
Company’s products, including RadioGel™, fall into the
brachytherapy treatment category. There are a number of
brachytherapy devices currently marketed in the U.S. and globally.
The traditional iodine-125 (I-125) and palladium-103 (Pd-103)
technologies for brachytherapy are well entrenched with powerful
market players controlling the market. The industry-standard
I-125-based therapy was developed by Oncura, which is a unit of
General Electric Company. Additionally, C.R. Bard, a major industry
player competes in the I-125 brachytherapy marketplace. These
market competitors are also involved in the distribution of Pd-103
based products. Cs-131 brachytherapy products are sold by IsoRay.
Several Y-90 therapies have been FDA approved including SIR-Spheres
by Sirtex, TheraSphere by Biocompatibles UK and Zevalin by Spectrum
Pharmaceuticals.
Raw Materials
The
Company currently manufactures research quantities of brachytherapy
products, including RadioGel™, or components of these
products. The Company obtains supplies, hardware, handling
equipment and packaging from several different U.S. and foreign
suppliers. Some of the products the Company currently manufactures
or intends to manufacture require purchasing raw materials from a
limited number of suppliers, many of which are international
suppliers.
Customers
The
Company anticipates that potential customers for our potential
brachytherapy products likely would include those institutions and
individuals that currently purchase brachytherapy products or other
oncology treatment products.
Government
Regulation
The
Company's present and future intended activities in the
development, manufacturing and sale of cancer therapy products,
including RadioGel™, are subject to extensive laws,
regulations, regulatory approvals and guidelines. Within the U.S.,
the Company's therapeutic radiological devices must comply with the
U.S. Federal Food, Drug and Cosmetic Act, enforced by the FDA. The
Company is also required to adhere to applicable FDA Quality System
Regulations, also known as the Good Manufacturing Practices, which
include extensive record keeping and periodic inspections of
manufacturing facilities.
In the
U.S., the FDA regulates, among other things, new product clearances
and approvals to establish the safety and efficacy of these
products. We are also subject to other federal and state laws and
regulations, including the Occupational Safety and Health Act and
the Environmental Protection Act.
The
Federal Food, Drug, and Cosmetic Act and other federal statutes and
regulations govern or influence the research, testing, manufacture,
safety, labeling, storage, record keeping, approval, distribution,
use, reporting, advertising and promotion of such products.
Noncompliance with applicable requirements can result in civil
penalties, recall, injunction or seizure of products, refusal of
the government to approve or clear product approval applications,
disqualification from sponsoring or conducting clinical
investigations, preventing us from entering into government supply
contracts, withdrawal of previously approved applications, and
criminal prosecution.
In the
United States, a manufacturer of medical devices and devices
utilizing radioactive byproduct material are subject to extensive
regulation by not only federal governmental authorities, such as
the FDA, but also by state and local governmental authorities, such
as the Washington State Department of Health, to ensure such
devices are safe and effective. In Washington State, the Department
of Health, by agreement with the federal Nuclear Regulatory
Commission (“NRC”), regulates the possession,
use, and disposal of radioactive byproduct material as well as the
manufacture of radioactive sealed sources to ensure compliance with
state and federal laws and regulations.
Moreover, any use,
management, and disposal of certain radioactive substances and
wastes are subject to regulation by several federal and state
agencies depending on the nature of the substance or waste
material.
Employees
As
of December 31, 2016, the Company had three full-time personnel.
The Company utilizes several independent contractors to assist with
its operations. The Company does not have a collective bargaining
agreement with any of its personnel, and believes its relations
with its personnel are good.
Available Information
The Company prepares and files annual reports on
Form 10-K, quarterly reports on Form 10-Q, current reports on Form
8-K and certain other information with the United States Securities
and Exchange Commission (the “SEC”). Persons may read and copy any materials
the Company files with the SEC at the SEC’s public reference
room at 100 F Street, NE, Washington D.C. 20549, on official
business days during the hours of 10 a.m. to 3 p.m. Eastern Time.
Information may be obtained on the operation of the public
reference room by calling the SEC at 1-800-SEC-0330. The SEC
maintains an Internet site that contains reports, proxy and
information statements, and other information regarding issuers
that file electronically with the SEC at http://www.sec.gov.
Moreover, the Company maintains a website at
http://www.isotopeworld.com that contains important information
about the Company, including biographies of key management
personnel, as well as information about the Company’s
business. This information is publicly available and is updated
regularly. The content on any website referred to in this Form 10-K
report is not incorporated by reference into this Form 10-K report,
unless (and only to the extent) expressly so stated
herein.
ITEM 1A. RISK FACTORS.
Investing in our common stock involves a high degree of risk. You
should carefully consider the risks described below, as well as the
other information in this Form 10-K, including our consolidated
financial statements and the related notes and
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations,” before deciding whether
to invest in our securities. The occurrence of any of the events or
developments described below could harm our business, financial
condition, operating results, and growth prospects. In such an
event, the market price of our common stock could decline, and you
may lose all or part of your investment. Additional risks and
uncertainties not presently known to us or that we currently deem
immaterial also may impair our business
operations.
RISKS ASSOCIATED WITH THE COMPANY’S BUSINESS
Our independent registered public accounting firms’ reports
on its financial statements questions the Company’s ability
to continue as a going concern.
The
Company’s independent registered public accounting
firms’ reports on the Company’s financial statements
for the years ended December 31, 2016 and 2015 express doubt about
the Company’s ability to continue as a going
concern. The reports include an explanatory paragraph
stating that the Company has suffered recurring losses, used
significant cash in support of its operating activities and, based
on its current operating levels, require additional capital or
significant restructuring to sustain its operation for the
foreseeable future. There is no assurance that the Company will be
able to obtain sufficient additional capital to continue its
operations and to alleviate doubt about its ability to continue as
a going concern. If the Company obtains additional financing, such
funds may not be available on favorable terms and likely would
entail considerable dilution to existing shareholders. Any debt
financing, if available, may involve restrictive covenants that
restrict its ability to conduct its business. It is
extremely remote that the Company could obtain any financing on any
basis that did not result in considerable dilution for
shareholders. Inclusion of a “going concern
qualification” in the report of its independent accountants
or in any future report may have a negative impact on its ability
to obtain debt or equity financing and may adversely impact its
stock price.
A combination of our current financial condition and the
FDA’s determinations to date regarding our brachytherapy
products raise material concerns about ability to continue as a
going concern.
The
Company will not be able to continue as a going concern unless the
Company obtains financing. Depending upon the amount of financing,
if any, the Company is able to obtain, the Company may not receive
adequate funds to continue the approval process for RadioGel™
or other brachytherapy products with the FDA.
The Company has
generated operating losses since inception, which are expected to
continue, and has increasing cash requirements, which it may be
unable to satisfy.
The
Company has generated material operating losses since
inception. The Company has had recurring net losses
since inception which has resulted in an accumulated deficit of
$57,869,440 as of December 31, 2016, including a net loss of
$9,854,895 for the year ended December 31, 2016 and a net income of
$6,232,686 for the year ended December 31,
2015. Historically, the Company has relied upon investor
funds to maintain its operations and develop its
business. The Company needs to raise additional capital
within the next quarter from investors for working capital as well
as business expansion, and there is no assurance that additional
investor funds will be available on terms acceptable to the
Company, or at all. If the Company is unable to unable
to obtain additional financing to meet its working capital
requirements, the Company likely would cease
operations.
The
Company requires funding of at least $1.5 million per year to
maintain current operating activities. Over the next 12 to 24
months, the Company believes it will cost approximately $5.0
million to $10.0 million to fund: (1)
the FDA approval process and initial deployment of the
brachytherapy products and (2)
initiate regulatory approval processes outside of the United
States. The continued deployment of the brachytherapy products and
a worldwide regulatory approval effort will require additional
resources and personnel. The principal variables in the
timing and amount of spending for the brachytherapy products in the
next 12 to 24 months will be the FDA’s classification of the
Company’s brachytherapy products as Class II or Class III
devices (or otherwise) and any requirements for additional studies,
which may possibly include clinical studies. Thereafter,
the principal variables in the amount of the Company’s
spending and its financing requirements would be the timing of any
approvals and the nature of the Company’s arrangements with
third parties for manufacturing, sales, distribution and licensing
of those products and the products’ success in the U.S. and
elsewhere. The Company intends to fund its activities through
strategic transactions such as licensing and partnership agreements
or additional capital raises.
Recent
economic events, including the inherent instability in global
capital markets, as well as the lack of liquidity in the capital
markets, could adversely impact the Company’s ability to
obtain financing and its ability to execute its business
plan.
The Company has a limited operating history, which may make it
difficult to evaluate its business and prospects.
The
Company has a limited operating history upon which one can base an
evaluation of its business and prospects. As a company
in the development stage, there are substantial risks,
uncertainties, expenses and difficulties to which its business is
subject. To address these risks and uncertainties, the
Company must do the following:
●
successfully
develop and execute the business strategy;
●
respond to
competitive developments; and
●
attract,
integrate, retain and motivate qualified personnel.
There
is no assurance that the Company will achieve or maintain
profitable operations or that the Company will obtain or maintain
adequate working capital to meet its obligations as they become
due. The Company cannot be certain that its business
strategy will be successfully developed and implemented or that the
Company will successfully address the risks that face its
business. In the event that the Company does not
successfully address these risks, its business, prospects,
financial condition, and results of operations could be materially
and adversely affected.
The Company’s new products are regulated and require
appropriate clearances and approvals to be marketed in the U.S. and
globally.
There
is no assurance the FDA or other global regulatory authorities will
grant the Company permission to market the Company’s
products, including the Company’s brachytherapy Y-90
RadioGel™ device, Y-90 Fast-Resorbable Polymer Seeds and Y-90
Polymer Topical Paste.
The Company has been working with the FDA to
obtain clearance for its brachytherapy Y-90 RadioGel™ device,
but no assurances have been received. On December 23,
2014, the Company announced that it submitted
a de
novo to the FDA for
marketing clearance for its patented Y-90 RadioGel™ device
pursuant to Section 513(f)(2) of the U.S. Food, Drug and Cosmetic
Act (the “Act”). In June 2015 the FDA notified the
Company the de novo was not granted. In February 2014, the FDA
found the same device under Section 510(k) of the Act not
substantially equivalent, and concluded that the device is
classified by statute as a Class III medical device, unless the
device is reclassified. By filing the de novo, the Company is seeking reclassification of the
product to Class II. If the de novo is granted, as a regulatory matter, the
device could be immediately marketed in the United States, though,
as a practical matter, the Company would have to secure funding and
commercial arrangements before marketing could commence. If
the de
novo is declined and if
the Company obtains funding to permit it to continue operations,
the Company will explore steps toward seeking approval for the
device as a Class III medical device. Generally, the time period
and cost of seeking approval as a Class III medical device is
materially greater than the time period and cost of seeking
approval as a Class II medical device. If the Company
seeks approval as a Class III device, human clinical trials will be
necessary. Generally, human trials for Class III
products are larger, of longer duration and more costly than those
for Class II devices. If human clinical trials are
necessary, there will be additional cost and time to reach
marketing clearance or approval. Unless the Company
obtains sufficient funding it will be unable to do the foregoing
activities. There can be no assurance that the product
will be approved as either a Class II or Class III device by the
FDA even if additional data is provided. There can be no assurance
that the Company will receive FDA approval, or the timing
thereof.
If the Company is successful in increasing the size of its
organization, the Company may experience difficulties in managing
growth.
The
Company is a small organization with a minimal number of
employees. If the Company is successful, it may
experience a period of significant expansion in headcount,
facilities, infrastructure and overhead and further expansion may
be required to address potential growth and market
opportunities. Any such future growth will impose
significant added responsibilities on members of management,
including the need to improve the Company’s operational and
financial systems and to identify, recruit, maintain and integrate
additional managers. The Company’s future
financial performance and its ability to compete effectively will
depend, in part, on the ability to manage any future growth
effectively.
The Company’s business is dependent upon the continued
services of the Company’s Chief Executive Officer, Michael
Korenko. Should the Company lose the services of Mr. Korenko,
the Company’s operations will be negatively
impacted.
The
Company’s business is dependent upon the expertise of its
Chief Executive Officer, Michael Korenko. Mr. Korenko is essential
to the Company’s operations. Accordingly, an investor must
rely on Mr. Korenko’s management decisions that will continue
to control the Company’s business affairs. The Company does
not maintain key man insurance on Mr. Korenko’s life. The
loss of the services of Mr. Korenko would have a material adverse
effect upon the Company’s business.
The Company is heavily dependent on consultants for many of the
services necessary to continue operations. The loss of
any of these consultants could have a material adverse effect on
the Company’s business, results of operations and financial
condition.
The
Company’s success is heavily dependent on the continued
active participation of certain consultants and collaborating
scientists. Certain key employees and consultants have
no written employment contracts. Loss of the services of any one or
more of its consultants could have a material adverse effect upon
the Company’s business, results of operations and financial
condition.
If the Company is unable to hire and retain additional qualified
personnel, the business and financial condition may
suffer.
The
Company’s success and achievement of its growth plans depend
on its ability to recruit, hire, train and retain highly qualified
technical, scientific, regulatory and managerial employees,
consultants and advisors. Competition for qualified
personnel among pharmaceutical and biotechnology companies is
intense, and an inability to attract and motivate additional highly
skilled personnel required for the expansion of the Company’s
activities, or the loss of any such persons, could have a material
adverse effect on its business, results of operations and financial
condition.
The Company’s revenues have historically been derived from
sales made to a small number of customers. The Company
has discontinued prior operations related to its core business. To
succeed, we will need to recommence our operations and achieve
sales to a materially larger number of customers.
During
2014, the Company ceased all previous manufacturing and sales
activities. Our sales for the year ended December 31, 2016 and 2015
consisted of only consulting revenue. The Company’s
consulting revenues for the years ended December 31, 2016 and 2015
were made to one customer, and those sales constituted 100.0% of
total revenues for those years. At such time as the Company
recommences active operations, no assurances can be given that the
Company will be successful in commercializing its products or
expanding the number of customers purchasing its products and
services.
Many of the
Company’s competitors have greater resources and experience
than the Company has.
Many
of the Company’s competitors have greater financial
resources, longer history, broader experience, greater name
recognition, and more substantial operations than the Company has,
and they represent substantial long-term competition for
us. The Company’s competitors may be able to
devote more financial and human resources than the Company can to
research, new product development, regulatory approvals, and
marketing and sales. The Company’s competitors may
develop or market products that are viewed by customers as more
effective or more economical than the Company’s
products. There is no assurance that the Company will be
able to compete effectively against current and future competitors,
and such competitive pressures may adversely affect the
Company’s business and results of operations.
The Company’s future revenues depend upon acceptance of its
current and future products in the markets in which they
compete.
The
Company’s future revenues depend upon receipt of financing,
regulatory approval and the successful production, marketing, and
sales of the various isotopes the Company might market in the
future. The rate and level of market acceptance of each
of these products, if any, may vary depending on the perception by
physicians and other members of the healthcare community of its
safety and efficacy as compared to that of any competing products;
the clinical outcomes of any patients treated; the effectiveness of
its sales and marketing efforts in the United States, Europe, Far
East, Middle East, and Russia; any unfavorable publicity concerning
its products or similar products; the price of the Company’s
products relative to other products or competing treatments; any
decrease in current reimbursement rates from the Centers for
Medicare and Medicaid Services or third-party payers; regulatory
developments related to the manufacture or continued use of its
products; availability of sufficient supplies to either purchase or
manufacture its products; its ability to produce sufficient
quantities of its products; and the ability of physicians to
properly utilize its products and avoid excessive levels of
radiation to patients. Any material adverse developments
with respect to the commercialization of any such products may
adversely affect revenues and may cause the Company to continue to
incur losses in the future.
The Company will in the future rely heavily on a limited number of
suppliers.
Some
of the products the Company might market and components thereof are
currently available only from a limited number of suppliers,
several of which are international suppliers. Failure to
obtain deliveries from these sources could have a material adverse
effect on the Company’s ability to operate.
The Company may incur material losses and costs as a result of
product liability claims that may be brought against
it.
The
Company faces an inherent business risk of exposure to product
liability claims in the event that products supplied by the Company
fail to perform as expected or such products result, or is alleged
to result, in bodily injury. Any such claims may also
result in adverse publicity, which could damage the Company’s
reputation by raising questions about the safety and efficacy of
its products, and could interfere with its efforts to market its
products. A successful product liability claim against
the Company in excess of its available insurance coverage or
established reserves may have a material adverse effect on its
business. Although the Company currently maintains
liability insurance in amounts it believes are commercially
reasonable, any product liability the Company may incur may exceed
its insurance coverage.
The Company is subject to the risk that certain third parties may
mishandle the Company’s products.
If
the Company markets products, the Company likely will rely on third
parties, such as commercial air courier companies, to deliver the
products, and on other third parties to package the products in
certain specialized packaging forms requested by
customers. The Company thus would be subject to the risk
that these third parties may mishandle its product, which could
result in material adverse effects, particularly given the
radioactive nature of some of the products.
The Company’s operations expose it to the risk of material
environmental liabilities.
The
Company is subject to potentially material liabilities related to
the remediation of environmental hazards and to personal injuries
or property damages that may be caused by hazardous substance
releases and exposures. The Company is subject to
various federal, state, local and foreign government requirements
regulating the discharge of materials into the environment or
otherwise relating to the protection of the
environment. These laws and regulations can impose
substantial fines and criminal sanctions for violations, and can
require installation of costly equipment or operational changes to
limit emissions and/or decrease the likelihood of accidental
hazardous substance releases. The Company expects to
incur capital and operating costs to comply with these laws and
regulations. In addition, changes in laws, regulations
and enforcement of policies, the discovery of previously unknown
contamination or new technology or information related to
individual sites, or the imposition of new clean-up requirements or
remedial techniques may require the Company to incur costs in the
future that would have a negative effect on its financial condition
or results of operations. Operational hazards could
result in the spread of contamination within the Company’s
facility and require additional funding to correct.
The Company is subject to uncertainties regarding reimbursement for
use of its products.
Hospitals
and freestanding clinics may be less likely to purchase the
Company’s products if they cannot be assured of receiving
favorable reimbursement for treatments using its products from
third-party payers, such as Medicare and private health insurance
plans. Third-party payers are increasingly challenging
the pricing of certain medical services or devices, and there is no
assurance that they will reimburse the Company’s customers at
levels sufficient for it to maintain favorable sales and price
levels for the Company’s products. There is no
uniform policy on reimbursement among third-party payers, and there
is no assurance that the Company’s products will continue to
qualify for reimbursement from all third-party payers or that
reimbursement rates will not be reduced. A reduction in
or elimination of third-party reimbursement for treatments using
the Company’s products would likely have a material adverse
effect on the Company’s revenues.
The Company’s future growth is largely dependent upon its
ability to develop new technologies that achieve market acceptance
with appropriate margins.
The
Company’s business operates in global markets that are
characterized by rapidly changing technologies and evolving
industry standards. Accordingly, future growth rates
depends upon a number of factors, including the Company’s
ability to (i) identify emerging technological trends in the
Company’s target end-markets, (ii) develop and maintain
competitive products, (iii) enhance the Company’s products by
adding innovative features that differentiate the Company’s
products from those of its competitors, and (iv) develop,
manufacture and bring products to market quickly and
cost-effectively. The Company’s ability to develop
new products based on technological innovation can affect the
Company’s competitive position and requires the investment of
significant resources. These development efforts divert
resources from other potential investments in the Company’s
business, and they may not lead to the development of new
technologies or products on a timely basis or that meet the needs
of the Company’s customers as fully as competitive
offerings. In addition, the markets for the
Company’s products may not develop or grow as it currently
anticipates. The failure of the Company’s
technologies or products to gain market acceptance due to more
attractive offerings by the Company’s competitors could
significantly reduce the Company’s revenues and adversely
affect the Company’s competitive standing and
prospects.
The Company may rely on third parties to represent it locally in
the marketing and sales of its products in international markets
and its revenue may depend on the efforts and results of those
third parties.
The
Company’s future success may depend, in part, on its ability
to enter into and maintain collaborative relationships with one or
more third parties, the collaborator’s strategic interest in
the Company’s products and the Company’s products under
development, and the collaborator’s ability to successfully
market and sell any such products. The Company intends
to pursue collaborative arrangements regarding the marketing and
sales of its products; however, it may not be able to establish or
maintain such collaborative arrangements, or if it is able to do
so, the Company’s collaborators may not be effective in
marketing and selling its products. To the extent that
the Company decides not to, or is unable to, enter into
collaborative arrangements with respect to the sales and marketing
of its products, significant capital expenditures, management
resources and time will be required to establish and develop an
in-house marketing and sales force with technical
expertise. To the extent that the Company depends on
third parties for marketing and distribution, any revenues received
by the Company will depend upon the efforts and results of such
third parties, which may or may not be successful.
The Company may pursue strategic acquisitions that may have an
adverse impact on its business.
Executing
the Company’s business strategy may involve pursuing and
consummating strategic transactions to acquire complementary
businesses or technologies. In pursuing these strategic
transactions, even if the Company does not consummate them, or in
consummating such transactions and integrating the acquired
business or technology, the Company may expend significant
financial and management resources and incur other significant
costs and expenses. There is no assurance that any
strategic transactions will result in additional revenues or other
strategic benefits for the Company’s business. The
Company may issue the Company’s stock as consideration for
acquisitions, joint ventures or other strategic transactions, and
the use of stock as purchase consideration could dilute the
interests of its current stockholders. In addition, the
Company may obtain debt financing in connection with an
acquisition. Any such debt financing may involve
restrictive covenants relating to capital-raising activities and
other financial and operational matters, which may make it more
difficult for the Company to obtain additional capital and pursue
business opportunities, including potential
acquisitions. In addition, such debt financing may
impair the Company’s ability to obtain future additional
financing for working capital, capital expenditures, acquisitions,
general corporate or other purposes, and a substantial portion of
cash flows, if any, from the Company’s operations may be
dedicated to interest payments and debt repayment, thereby reducing
the funds available to the Company for other purposes.
The Company will need to hire additional qualified accounting
personnel in order to remediate a material weakness in its internal
control over financial accounting, and the Company will need to
expend any additional resources and efforts that may be necessary
to establish and to maintain the effectiveness of its internal
control over financial reporting and its disclosure controls and
procedures.
As
a public company, the Company is subject to the reporting
requirements of the Securities Exchange Act of 1934, as amended,
and the Sarbanes-Oxley Act of 2002. The Company’s
management is required to evaluate and disclose its assessment of
the effectiveness of the Company’s internal control over
financial reporting as of each year-end, including disclosing any
“material weakness” in the Company’s internal
control over financial reporting. A material weakness is
a control deficiency, or combination of control deficiencies, that
results in more than a remote likelihood that a material
misstatement of the annual or interim financial statements will not
be prevented or detected. As a result of its assessment,
management has determined that there is a material weakness due to
the lack of segregation of duties and, due to this material
weakness, management concluded that, as of December 31, 2016 and
2015, the Company’s internal control over financial reporting
was ineffective. This material weakness was first identified in the
Company’s Form 10-K/A amended annual report for the year
ended December 31, 2008. This material weakness has the potential
of adversely impacting the Company’s financial reporting
process and the Company’s financial
reports. Because of this material weakness, management
also concluded that the Company’s disclosure controls and
procedures were ineffective as of December 31, 2016 and
2015. The Company needs to hire additional
qualified accounting personnel in order to resolve this material
weakness. The Company also will need to expend any
additional resources and efforts that may be necessary to establish
and to maintain the effectiveness of the Company’s internal
control over financial reporting and disclosure controls and
procedures.
The Company may be unable to make timely license and patent
payments
Patent
costs associated with existing and new technology are
significant. Existing patent and license fees must be
paid for the Company to maintain rights to the
technology. The Company would forfeit its exclusive
rights to licensed technologies without paying patent and rights
fees in a timely fashion. There is no assurance of
sufficient capital to meet ongoing legal costs associated with the
patent costs for the Company’s technology.
The Company’s patented or other technologies may infringe on
other patents, which may expose it to costly
litigation.
It
is possible that the Company’s patented or other technologies
may infringe on patents or other rights owned by
others. The Company may have to alter its products or
processes, pay licensing fees, defend infringement actions or
challenge the validity of the patents in court, or cease activities
altogether because of patent rights of third parties, thereby
causing additional unexpected costs and delays to the
Company. Patent litigation is costly and time consuming,
and the Company may not have sufficient resources to pursue such
litigation. If the Company does not obtain a license
under such patents, if it is found liable for infringement, or if
it are not able to have such patents declared invalid, the Company
may be liable for significant money damages, may encounter
significant delays in bringing products to market or may be
precluded from participating in the manufacture, use or sale of
products or methods of treatment requiring such
licenses.
Protecting the Company’s intellectual property is critical to
its innovation efforts.
The
Company owns or has a license to use several U.S. and foreign
patents and patent applications, trademarks and
copyrights. The Company’s intellectual property
rights may be challenged, invalidated or infringed upon by third
parties, or it may be unable to maintain, renew or enter into new
licenses of third party proprietary intellectual property on
commercially reasonable terms. In some non-U.S.
countries, laws affecting intellectual property are uncertain in
their application, which can adversely affect the scope or
enforceability of the Company’s patents and other
intellectual property rights. Any of these events or
factors could diminish or cause the Company to lose the competitive
advantages associated with the Company’s intellectual
property, subject the Company to judgments, penalties and
significant litigation costs, or temporarily or permanently disrupt
its sales and marketing of the affected products or
services.
The Company may not be able to protect its trade secrets and other
unpatented proprietary technology, which could give competitors an
advantage.
The
Company relies upon trade secrets and other unpatented proprietary
technology. The Company may not be able to adequately
protect its rights with regard to such unpatented proprietary
technology, or competitors may independently develop substantially
equivalent technology. The Company seeks to protect
trade secrets and proprietary knowledge, in part through
confidentiality agreements with its employees, consultants,
advisors and collaborators. Nevertheless, these
agreements may not effectively prevent disclosure of the
Company’s confidential information and may not provide the
Company with an adequate remedy in the event of unauthorized
disclosure of such information, and as a result the
Company’s competitors could gain a competitive
advantage.
General economic conditions in markets in which the Company does
business can impact the demand for the Company’s goods and
services. Decreased demand for the Company’s products and
services could have a negative impact on its financial performance
and cash flow.
Demand
for the Company’s products and services, in part, depends on
the general economic conditions affecting the countries and
industries in which the Company does business. A
downturn in economic conditions in a country or industry that the
Company serves may adversely affect the demand for the
Company’s products and services, in turn negatively impacting
the Company’s operations and financial
results. Further, changes in demand for the
Company’s products and services can magnify the impact of
economic cycles on the Company’s
businesses. Unanticipated contract terminations by
current customers can negatively impact operations, financial
results and cash flow. The Company’s earnings,
cash flow and financial position are exposed to financial market
risks worldwide, including interest rate and currency exchange rate
fluctuations and exchange rate
controls. Fluctuations in domestic and world
financial markets could adversely affect interest rates and impact
the Company’s ability to obtain credit or attract
investors.
The Company is subject to extensive government regulation in
jurisdictions around the world in which it does business.
Regulations address, among other things, environmental compliance,
import/export restrictions, healthcare services, taxes and
financial reporting, and those regulations can significantly
increase the cost of doing business, which in turn can negatively
impact operations, financial results and cash flow.
If
the Company is successful in developing manufacturing capability,
the Company will be subject to extensive government regulation and
intervention both in the U.S. and in all foreign jurisdictions in
which it conducts business. Compliance with applicable
laws and regulations will result in higher capital expenditures and
operating costs, and changes to current regulations with which the
Company complies can necessitate further capital expenditures and
increases in operating costs to enable continued
compliance. Additionally, from time to time, the Company
may be involved in proceedings under certain of these laws and
regulations. Foreign operations are subject to political
instabilities, restrictions on funds transfers, import/export
restrictions, and currency fluctuation.
Volatility in raw material and energy costs, interruption in
ordinary sources of supply, and an inability to recover from
unanticipated increases in energy and raw material costs could
result in lost sales or could increase significantly the cost of
doing business.
Market
and economic conditions affecting the costs of raw materials,
utilities, energy costs, and infrastructure required to provide for
the delivery of the Company’s products and services are
beyond the Company’s control. Any disruption or
halt in supplies, or rapid escalations in costs, could adversely
affect the Company’s ability to manufacture products or to
competitively price the Company’s products in the
marketplace. To date, the ultimate impact of energy
costs increases have been mitigated through price increases or
offset through improved process efficiencies; however, continuing
escalation of energy costs could have a negative impact upon the
Company’s business and financial performance.
RISKS RELATED TO THE COMPANY’S COMMON STOCK
The Company’s common stock is listed on the OTC Bulletin
Board. Failure to develop or maintain a more active trading market
may negatively affect the value of the Company’s common
stock, may deter some potential investors from purchasing the
Company’s common stock or other equity securities, and may
make it difficult or impossible for stockholders to sell their
shares of common stock.
The
Company’s average daily volume of shares traded for the years
ended December 31, 2016 and 2015 was 113,034 and 403,927,
respectively. Failure to develop or maintain an active trading
market may negatively affect the value of the Company’s
common stock, may make some potential investors unwilling to
purchase the Company’s common stock or equity securities that
are convertible into or exercisable for the Company’s common
stock, and may make it difficult or impossible for the
Company’s stockholders to sell their shares of common stock
and recover any part of their investment.
The Company’s outstanding securities, the stock or securities
that it may become obligated to issue under existing agreements,
and certain provisions of those securities, may cause immediate and
substantial dilution to existing stockholders and may make it more
difficult to raise additional equity capital.
The
Company had 37,541,697 shares of common stock outstanding on March
6, 2017. The Company also had outstanding on that date
derivative securities consisting of options, warrants, and
convertible notes that if they had been exercised and converted in
full on March 6, 2017, would have resulted in the issuance of up to
11,637,245 additional shares of common stock. The
issuance of shares upon the exercise of options or the conversion
of convertible notes may result in substantial dilution to each
stockholder by reducing that stockholder’s percentage
ownership of the Company’s total outstanding common
stock. Additionally, the Company has outstanding notes
that if not prepaid by specific dates entitle the holder to convert
the principal and accrued interest into common stock at 60% of the
lowest trading price during the previous thirty day trading period
of the Company’s common stock prior to conversion as provided
in the notes. See Note 11 of the footnotes to the Consolidated
Financial Statements for the years ended December 31, 2016 and 2015
beginning on page F-1 of this report regarding the equity issuable
upon conversion. The issuance of some or all of those warrants and
any exercise of those warrants will have the effect of further
diluting the percentage ownership of the Company’s other
stockholders. That agreement also provides for stock compensation
for consulting services. The existence and terms of these
derivative securities and other obligations may make it more
difficult for the Company to raise additional capital through the
sale of stock or other equity securities.
Future sales of the Company’s stock, including sales
following exercise or conversion of derivative securities, or the
perception that such sales may occur, may depress the price of
common stock and could encourage short sales.
The
sale or availability for sale of substantial amounts of the
Company’s shares in the public market, including shares
issuable upon exercise of options or warrants or upon the
conversion of convertible securities, or the perception that such
sales may occur, may adversely affect the market price of the
Company’s common stock. Any decline in the price
of the Company’s common stock may encourage short sales,
which could place further downward pressure on the price of the
Company’s common stock.
The Company’s stock price is likely to be
volatile.
For
the year ended December 31, 2016, the reported low closing price
for the Company’s common stock was $0.07 per share, and the
reported high closing price was $0.96 per share. For the
year ended December 31, 2015, the reported low closing price for
the Company’s common stock was $0.03 per share, and the
reported high closing price was $0.49 per share. There
is generally significant volatility in the market prices, as well
as limited liquidity, of securities of early stage companies,
particularly early stage medical product
companies. Contributing to this volatility are various
events that can affect the Company’s stock price in a
positive or negative manner. These events include, but
are not limited to: governmental approvals, refusals to approve,
regulations or other actions; market acceptance and sales growth of
the Company’s products; litigation involving the Company or
the Company’s industry; developments or disputes concerning
the Company’s patents or other proprietary rights; changes in
the structure of healthcare payment systems; departure of key
personnel; future sales of its securities; fluctuations in its
financial results or those of companies that are perceived to be
similar to us; investors’ general perception of us; and
general economic, industry and market conditions. If any
of these events occur, it could cause the Company’s stock
price to fall, and any of these events may cause the
Company’s stock price to be volatile.
The Company’s common stock is subject to the “Penny
Stock” rules of the SEC and the trading market in its
securities is limited, which makes transactions in its common stock
cumbersome and may reduce the value of an investment in the
Company’s stock.
The
Securities and Exchange Commission has adopted Rule 3a51-1 which
establishes the definition of a “penny stock,” for the
purposes relevant to us, as any equity security that has a market
price of less than $5.00 per share or with an exercise price of
less than $5.00 per share, subject to certain
exceptions. For any transaction involving a penny stock,
unless exempt, Rule 15g-9 requires that a broker or dealer approve
a person’s account for transactions in penny stocks and that
the broker or dealer receive from the investor a written agreement
to the transaction, setting forth the identity and quantity of the
penny stock to be purchased.
In
order to approve a person’s account for transactions in penny
stocks, the broker or dealer must obtain financial information and
investment experience and objectives of the person and must make a
reasonable determination that the transactions in penny stocks are
suitable for that person and that the person has sufficient
knowledge and experience in financial matters to be capable of
evaluating the risks of transactions in penny stocks.
The
broker or dealer must also deliver, prior to any transaction in a
penny stock, a disclosure schedule prescribed by the SEC relating
to the penny stock market, which sets forth the basis on which the
broker or dealer made the suitability determination, and that the
broker or dealer received a signed, written agreement from the
investor prior to the transaction.
Generally, brokers may be less willing to execute
transactions in securities subject to the “penny stock”
rules. This may make it more difficult for investors to
dispose of the Company’s common stock and may cause a decline
in the market value of its stock.
Disclosure
also has to be made about the risks of investing in penny stocks in
both public offerings and in secondary trading and about the
commissions payable to both the broker-dealer and the registered
representative, current quotations for the securities and the
rights and remedies available to an investor in cases of fraud in
penny stock transactions. Finally, monthly statements
have to be sent disclosing recent price information for the penny
stock held in the account and information on the limited market in
penny stocks.
As a result of the Company issuing preferred stock, the rights of
holders of the Company’s common stock and the value of the
Company’s common stock may be adversely
affected.
The Company’s
board of directors is authorized to issue classes or series of
preferred stock, without any action on the part of the
stockholders. The Company’s board of directors
also has the power, without stockholder approval, to set the terms
of any such classes or series of preferred stock, including voting
rights, dividend rights and preferences over the common stock with
respect to dividends or upon the liquidation, dissolution or
winding-up of its business, and other terms. The Company
has issued preferred stock that has a preference over the common
stock with respect to the payment of dividends or upon liquidation,
dissolution or winding-up, and with respect to voting rights.
In accordance with that and with the issuance of preferred stock
the voting rights the common stockholders voting rights have been
diluted and it is possible that the rights of holders of the common
stock or the value of the common stock have been adversely
affected.
The Company does not expect to pay any dividends on common stock
for the foreseeable future.
The Company has not paid any cash dividends on its
common stock to date and does not anticipate it will pay cash
dividends on its common stock in the foreseeable
future. Accordingly, stockholders must be prepared to rely
on sales of their common stock after price appreciation to earn an
investment return, which may never occur. Any
determination to pay dividends in the future will be made at the
discretion of the Company’s board of directors and will
depend on the Company’s results of operations, financial
conditions, contractual restrictions, restrictions imposed by
applicable law, and other factors that the Company’s board
deems relevant.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
This
item is not applicable to the Company because the Company is a
smaller reporting company as defined by Rule 12b-2 under the
Securities Exchange Act of 1934.
ITEM 2. PROPERTIES.
The
Company is headquartered in Kennewick, Washington and maintains a
lease at a cost of $1,500 per month from an entity controlled by
Carlton M. Cadwell, Chairman of the Company’s Board of
Directors and one of the Company’s significant shareholders.
This lease initially expired in December 2014, however the Company
continues to rent the space on a month-to-month basis.
ITEM 3. LEGAL PROCEEDINGS.
On
March 6, 2015, Robert and Maribeth Myers filed a lawsuit against
the Company and BancLeasing, Inc., owner of a linear accelerator
and other equipment leased by the Company, in the Superior Court of
the State of Washington, in and for Benton County (Case No.
15-2-0054101), asserting various claims related to the
Company’s five-year lease of production center space owned by
Mr. and Mrs. Myers. The Company subsequently filed counterclaims
against Mr. and Mrs. Myers, BancLeasing and Washington Trust Bank,
alleging misapplication of lease payments to the principal loan
amount for a linear accelerator and other equipment stored on the
production center property, as well as certain building
improvements made by the Company. During 2016, the Company entered
into a Settlement Agreement with Robert and Maribeth Myers,
pursuant to which the Company agreed to pay a settlement amount of
$438,830 in exchange for the release of all claims related to the
matter, which amount was paid by the Company during the year ended
December 31, 2016.
In 2016, the
Company was awarded in the Superior Court of the State of
Washington a total sum of $527,876 against BancLeasing. The Company
is pursuing its options for collection of the awarded amount,
however there can be no assurance as to any eventual
collection.
ITEM 4. MINE SAFETY DISCLOSURES.
Not
applicable.
PART
II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED
STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY
SECURITIES.
Market Information
The
Company’s common stock is traded on the OTC Pink Marketplace
under the symbol “ADMD.” The following table
sets forth, in U.S. dollars the high and low closing prices for
each of the calendar quarters indicated, as reported by the OTC
Pink Marketplace for the past two fiscal years. The
prices in the table may not represent actual transactions and do
not include retail markups, markdowns or commissions. All prices
listed below reflect the Company’s 1:100 reverse stock split
implemented on October 7, 2016.
|
|
High
|
Low
|
|
2016
|
|
|
|
Quarter ended
December 31
|
$0.24
|
$0.07
|
|
Quarter ended
September 30
|
$0.40
|
$0.14
|
|
Quarter ended June
30
|
$0.96
|
$0.20
|
|
Quarter ended March
31
|
$0.96
|
$0.07
|
|
|
|
|
|
2015
|
|
|
|
Quarter ended
December 31
|
$0.48
|
$0.11
|
|
Quarter ended
September 30
|
$0.49
|
$0.05
|
|
Quarter ended June
30
|
$0.19
|
$0.03
|
|
Quarter ended March
31
|
$0.27
|
$0.03
|
Holders
As of
March 6, 2017 there were 37,541,697 shares of common
stock outstanding and approximately 200 stockholders of
record.
Dividend Policy
The
Company has not paid any cash dividends on its common stock to date
and do not anticipate it will pay cash dividends on its common
stock in the foreseeable future. The payment of
dividends in the future will be contingent upon revenues and
earnings, if any, capital requirements, and its general financial
condition. The payment of any dividends will be within
the discretion of the board of directors. It is the
present intention of the board of directors to retain all earnings,
if any, for use in the business operations. Accordingly,
the board does not anticipate declaring any dividends on its common
stock in the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation
Plans
The
following table sets forth information as of December 31, 2016 with
respect to the Company’s equity compensation plans previously
approved by stockholders and equity compensation plans not
previously approved by stockholders.
|
|
|
Equity Compensation Plan Information
|
|
|||||||||
|
|
|
|
|
|||||||||
|
Plan Category
|
|
Number of securities to be issued upon exercise of
outstanding
options, warrants
and rights
|
|
|
Weighted-average
exercise price of
outstanding options,
warrants and rights
|
|
|
Number of securities remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a))
|
|
|||
|
|
|
(a)
|
|
|
(b)
|
|
|
(c)
|
|
|||
|
Equity
compensation plans approved by stockholders
|
|
|
-
|
|
|
$
|
-
|
|
|
|
3,993,868
|
|
|
Equity
compensation plans not approved by stockholders
|
|
|
5,135,000
|
|
|
$
|
0.15
|
|
|
|
-
|
|
|
Total
|
|
|
5,135,000
|
(1)
|
|
$
|
0.15
|
(1)
|
|
|
-
|
|
(1)
In
addition to the 2015 Plan (defined below), the Company has
individual compensation arrangements under which equity securities
are authorized for issuance in exchange for consideration in the
form of goods or services of certain individuals.
2015 Omnibus Securities and Incentive Plan
In October 2015,
our Board of Directors and stockholders approved the adoption of
the 2015 Omnibus Securities and Incentive Plan (the
“2015 Plan”).
The 2015 Plan authorizes an aggregate number of shares of common
stock for issuance to all employees of the Company or any
subsidiary of the Company, any non-employee director, consultants
and independent contractors of the Company or any subsidiary, and
any joint venture partners (including, without limitation,
officers, directors and partners thereof) of the Company or any
subsidiary. The aggregate number of shares that may be issued under
the Plan shall not exceed twenty percent (20%) of the issued and
outstanding shares of common stock on an as converted primary basis
on a rolling basis. For calculation purposes, the As Converted
Primary Shares shall include all shares of common stock and all
shares of common stock issuable upon the conversion of outstanding
preferred stock and other convertible securities, but shall not
include any shares of common stock issuable upon the exercise of
options, warrants and other convertible securities issued pursuant
to the 2015 Plan. As of December 31, 2016 the Converted Primary
Shares calculation results in 9,835,788 aggregate shares that may
be issued under the 2015 Plan. The 2015 Plan is administered by the
Company’s Compensation Committee, who may issue awards in the
form of stock options and/or restricted stock awards. As of
December 31, 2016, no awards have been issued pursuant to the 2015
Plan.
Recent Sales of Unregistered Securities
Below
is a description of all unregistered securities issued by the
Company during and subsequent to the quarter ended December 31,
2016, through the date of this report. Each of the issuances
identified below were issued in transactions exempt from
registration under the Securities
Act of 1933, as amended, in reliance on Section 3(a)(9) and/or 4(2)
thereof.
Issuances During the Quarter Ended December 31, 2016
During
October, November and December 2016, the Company issued 347,400
shares of Series A Convertible Preferred Stock (“Series A Preferred”) as a
commitment fee payable on $547,500 in convertible
debt.
During
November 2016, the Company issued 563,523 shares of common stock in
exchange for $126,640 of convertible debt, and $25,300 of accrued
interest.
During
November 2016, the Company issued 52,000 shares of Series A
Preferred in exchange for $93,011 of convertible debt, and $23,462
of accrued interest.
During
October 2016, the Company issued 150,000 shares of Series A
Preferred as payment for certain consulting services.
During the months
of October, November and December 2016, the Company issued
11,180,289 shares of common stock upon conversion of 1,118,024
shares of Series A Preferred.
Issuances Subsequent to December 31, 2016
During January and
February, and through March 6, 2017, the Company issued 5,517,900
shares of common stock upon conversion of 531,790 shares of Series
A Preferred.
During
February 2017, the Company issued 280,000 shares of common stock in
exchange for $140,000 of accounts payable.
ITEM 6. SELECTED FINANCIAL DATA.
This
item is not applicable to the Company because the Company is a
smaller reporting company as defined by Rule 12b-2 under the
Securities Exchange Act of 1934, as amended.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS.
The following discussion and analysis is intended as a review of
significant factors affecting the Company’s financial
condition and results of operations for the periods
indicated. The discussion should be read in conjunction
with the Company’s financial statements and the notes
presented herein. In addition to historical information,
the following Management’s Discussion and Analysis of
Financial Condition and Results of Operations contains
forward-looking statements that involve risks and uncertainties.
The Company’s actual results could differ significantly from
those anticipated in these forward-looking statements as a result
of the risk factors set forth above in Item 1A and other factors
discussed in this Annual Report on Form 10-K.
Results of Operations
Comparison for the Year Ended December 31, 2016 and December 31,
2015
The
following table sets forth information from the Company’s
statements of operations for the years ended December 31, 2016
and 2015.
|
|
Year
Ended
December
31,
2016
|
Year
Ended
December
31,
2015
|
|
Revenues
|
$8,108
|
$24,108
|
|
|
|
|
|
Operating
expense
|
4,444,578
|
2,065,371
|
|
|
|
|
|
Operating
loss
|
(4,436,470)
|
(2,041,263)
|
|
|
|
|
|
Non-operating
income (expense)
|
(5,418,425)
|
8,273,949
|
|
|
|
|
|
Net income
(loss)
|
$(9,854,895)
|
$6,232,686
|
Revenue
Consulting revenue
was $8,108 and $24,108 for the years ended December 31, 2016 and
2015, respectively. Consulting revenue consists of
providing the Company with assistance in strategic targetry
services, and research into production of radiophamaceuticals and
the operations of radioisotope production facilities.
Operating Expense
Operating expense
for the twelve months ended December 31, 2016 and 2015 consists of
the following:
|
|
Twelve months
ended December
31,
2016
|
Twelve months
ended December
31,
2015
|
|
Cost of
materials
|
$-
|
$474
|
|
Sales and marketing
expense
|
284,138
|
-
|
|
Depreciation and
amortization expense
|
2,947
|
5,672
|
|
Professional
fees
|
2,068,796
|
741,375
|
|
Stock options and
warrants granted
|
675,324
|
80,635
|
|
Payroll
expense
|
652,877
|
679,259
|
|
Research and
development
|
328,026
|
149,650
|
|
General and
administrative expense
|
432,470
|
408,306
|
|
|
$4,444,578
|
$2,065,371
|
Operating expense
for the twelve months ended December 31, 2016 and 2015 was
$4,444,578 and $2,065,371, respectively. The increase in operating
expense from 2015 to 2016 is largely attributable to professional
fees ($2,068,796 for the twelve months ended December 31, 2016
versus $741,375 for the twelve months ended December 31, 2015). The
increase in professional fees was due to hiring consultants to
assist in raising capital to pay off debt and for operating
expenses.
The
increase in operating expense from 2015 to 2016 can also be
attributed to research and development ($328,026 for the twelve
months ended December 31, 2016 versus $149,650 for the twelve
months ended December 31, 2015), and stock options granted and
warrant expense ($675,324 for the twelve months ended December 31,
2016 versus $80,635 for the twelve months ended December 31,
2015).
Non-Operating Income (Expense)
Non-Operating
income (expense) for the twelve months ended December 31, 2016 and
2015 consists of the following:
|
|
Twelve months
ended December
31,
2016
|
Twelve months
ended December
31,
2015
|
|
Interest
expense
|
$(6,259,467)
|
$(3,196,153)
|
|
Net gain (loss) on
settlement of debt
|
3,108,342
|
3,562,067
|
|
Recognized income
from grants
|
21,010
|
21,010
|
|
Gain (loss) on
derivative liability
|
(2,244,353)
|
7,887,025
|
|
Loss on impaired
assets
|
(43,957)
|
-
|
|
|
$(5,418,425)
|
$8,273,949
|
Non-operating
income (expense) for the twelve months ended December 31, 2016
varied from the twelve months ended December 31, 2015 primarily due
to the difference in the gain (loss) on derivative liability of
$10,131,378 (a $7,887,025 gain on derivative liability in 2015
versus a $2,244,353 loss on derivative liability in 2016).
Additionally, there was an increase in interest expense of
$3,063,314, attributable to loan fees incurred ($5,098,094 for the
twelve months ended December 31, 2016 versus $536,347 for the
twelve months ended December 31, 2015).
Income from Grants
On
September 1, 2015, the Company received notification that it had
been awarded from Washington State University $42,019 grant funds
from the sub-award project entitled “Optimized Injectable
Radiogels for High-dose Therapy of Non-Resectable Solid
Tumors”. The Company received $21,010 of the total grant
award in December 2015 and $21,009 of the total grant award in
October 2016.
Net Loss
The
Company’s net income (loss) for the twelve months ended
December 31, 2016 and 2015 was $(9,854,895) and $6,232,686,
respectively, as a result of the items described
above.
Liquidity and Capital Resources
At
December 31, 2016, the Company had negative working capital of
$3,022,417, as compared to $9,755,128 at December 31,
2015. During the twelve months ended December 31, 2016,
the Company experienced negative cash flow from operations of
$1,926,713 and it expended $0 for investing activities while adding
$1,775,570 of cash flows from financing activities. As
of December 31, 2016, the Company had $0 commitments for capital
expenditures.
Cash
used in operating activities increased from $1,193,105 for the
twelve-month period ending December 31, 2015 to $1,926,713 for
the twelve-month period ending December 31, 2016. Cash
used in operating activities was primarily a result of the
Company’s non-cash items, such as loss from operations, loss
on preferred and common stock and stock options and warrants issued
for services and other expenses, offset by the net gain and the
gain realized from derivative liabilities and settlement of debt,
preferred stock converted to common stock, and the penalties
resulting from short term debt. Cash provided from financing
activities increased from $1,371,934 for the twelve month period
ending December 31, 2015 to $1,775,570 for the twelve month period
ending December 31, 2016. The increase in cash provided from
financing activities was primarily a result of increase in proceeds
from related party notes, shareholder advances, and sale of
preferred shares.
The
Company has generated material operating losses since inception.
The Company had a net income of $6,232,686 for the twelve months
ended December 31, 2015, and a net loss of $9,854,895 for the
twelve months ended December 31, 2016. The Company expects to
continue to experience net operating losses. Historically, the
Company has relied upon investor funds to maintain its operations
and develop the Company’s business. The Company anticipates
raising additional capital within the next twelve months for
working capital as well as business expansion, although the Company
can provide no assurance that additional capital will be available
on terms acceptable to the Company, if at all. If the Company is
unable to obtain additional financing to meet its working capital
requirements, it may have to curtail its business or cease all
operations.
The
Company requires funding of at least $1.5 million per year to
maintain current operating activities. Over the next 12 to 24
months, the Company believes it will cost approximately $5.0
million to $10.0 million to fund: (1) the FDA approval process and
initial deployment of RadioGel™ and other brachytherapy
products and (2) initiate regulatory approval processes outside of
the United States. The continued deployment of the Company’s
brachytherapy products, including RadioGel™, and a worldwide
regulatory approval effort will require additional resources and
personnel. The principal variables in the timing and
amount of spending for the brachytherapy products in the next 12 to
24 months will be the FDA’s classification of the
Company’s brachytherapy products as Class II or Class III
devices (or otherwise) and any requirements for additional studies,
which may possibly include clinical studies. Thereafter,
the principal variables in the amount of the Company’s
spending and its financing requirements would be the timing of any
approvals and the nature of the Company’s arrangements with
third parties for manufacturing, sales, distribution and licensing
of those products and the products’ success in the U.S. and
elsewhere. The Company intends to fund its activities through
strategic transactions such as licensing and partnership agreements
or additional capital raises.
Although the
Company is seeking to raise additional capital and has engaged in
numerous discussions with investment bankers and investors, the
Company has not received firm commitments for the required funding.
Based upon its discussions, the Company anticipates that if the
Company is able to obtain the funding required to retire
outstanding debt, pay past due payables and maintain its current
operating activities, that the terms thereof will be materially
dilutive to existing shareholders.
Recent
geopolitical events, including the inherent instability and
volatility in global capital markets, as well as the lack of
liquidity in the capital markets, could impact the Company’s
ability to obtain financing and its ability to execute its business
plan.
Contractual Obligations (payments due by period as of December 31,
2016)
|
Contractual
Obligation
|
Total
Payments
Due
|
Less
than
1
Year
|
1-3
Years
|
3-5
Years
|
More
than
5
Years
|
|
License Agreement
with Battelle Memorial Institute
|
$100,000
|
$25,000
|
$25,000
|
$25,000
|
$
25,000 per year
|
|
Corporate Office
Lease – begins January 1, 2014
|
$18,000
|
$18,000
|
$-
|
$-
|
$-
|
In
January 2014, the Company entered into a new 12-month lease for its
corporate offices for a monthly rent of $1,500 from an entity
controlled by Carlton M. Cadwell, a significant shareholder and a
Director of the Company. The Company continued to rent this
facility in 2015 and 2016 on a month-to-month basis. The Company
incurred $18,000 rent expense for this facility for each of the
twelve months ended December 31, 2016 and 2015.
Off-Balance Sheet Arrangements
The
Company does not have any off balance sheet arrangements that are
reasonably likely to have a current or future effect on the
Company’s financial condition, revenues, results of
operations, liquidity or capital expenditures.
Accounting Policies
Use
of Estimates
The
preparation of financial statements in accordance with generally
accepted accounting principles requires management to make
estimates and assumptions that affect the reported amounts of
assets and liabilities and the disclosure of contingent assets and
liabilities at the date of financial statements and the reported
amounts of revenues and expenses during the reporting
period. Actual results could differ from those
estimates.
Fixed
Assets
Fixed
assets are carried at the lower of cost or net realizable value.
Production equipment with a cost of $2,500 or greater and other
fixed assets with a cost of $1,500 or greater are capitalized.
Major betterments that extend the useful lives of assets are also
capitalized. Normal maintenance and repairs are charged to expense
as incurred. When assets are sold or otherwise disposed of, the
cost and accumulated depreciation are removed from the accounts and
any resulting gain or loss is recognized in
operations.
Depreciation is
computed using the straight-line method over the following
estimated useful lives:
|
Production
equipment:
|
3 to 7
years
|
|
Office
equipment:
|
2 to 5
years
|
|
Furniture
and fixtures:
|
2 to 5
years
|
Leasehold
improvements and capital lease assets are amortized over the
shorter of the life of the lease or the estimated life of the
asset.
Management of the
Company reviews the net carrying value of all of its equipment on
an asset by asset basis whenever events or changes in circumstances
indicate that its carrying amount may not be recoverable. These
reviews consider the net realizable value of each asset, as
measured in accordance with the preceding paragraph, to determine
whether impairment in value has occurred, and the need for any
asset impairment write-down.
License
Fees
License
fees are stated at cost, less accumulated amortization.
Amortization of license fees is computed using the straight-line
method over the estimated economic useful life of the
asset.
The
Company periodically reviews the carrying values of capitalized
license fees and any impairments are recognized when the expected
future operating cash flows to be derived from such assets are less
than their carrying value.
Patents
and Intellectual Property
The Company had a
total $35,482 of capitalized patents and intellectual property
costs at December 31, 2015 for the patent rights in the area of a
Brachytherapy seed with a Fast-dissolving Matrix for Optimized
Delivery of Radionuclides. Effective December 31, 2016 the Company
agreed to terminate this non-utilized patent license for which the
$35,482 of capitalized patent and intellectual costs applied and
therefore the Company wrote off $35,482 of capitalized costs in the
twelve months ending December 31, 2016.
While
patents are being developed or pending, they are not being
amortized. Management has determined that the economic
life of the patents to be ten years and amortization, over such
10-year period and on a straight-line basis will begin once the
patents have been issued and the Company begins utilization of the
patents through production and sales, resulting in
revenues.
The
Company evaluates the recoverability of intangible assets,
including patents and intellectual property on a continual basis.
Several factors are used to evaluate intangibles, including, but
not limited to, management’s plans for future operations,
recent operating results and projected and expected undiscounted
future cash flows.
Revenue
Recognition
The
Company recognized revenue related to product sales when (i)
persuasive evidence of the arrangement exists, (ii) shipment has
occurred, (iii) the fee is fixed or determinable, and (iv)
collectability is reasonably assured.
Revenue
for the fiscal years ended December 31, 2016 and 2015 consisted of
consulting revenue. The Company recognizes revenue as consulting
services have been performed. Prepayments, if any, received from
customers prior to the services being performed are recorded as
deferred revenue. In these cases, when the services are performed,
the amount recorded as deferred revenue is recognized as revenue.
The Company does not accrue for sales returns and other allowances
as it has not experienced any returns or other
allowances.
Income
from Grants and Deferred Income
Government grants
are recognized when all conditions of such grants are fulfilled or
there is reasonable assurance that they will be fulfilled. The
Company has chosen to recognize income from grants as it incurs
costs associated with those grants, and until such time as it
recognizes the grant as income those funds received will be
classified as deferred income on the balance sheet.
Net
Income (Loss) Per Share
The
Company accounts for its income (loss) per common share by
replacing primary and fully diluted earnings per share with basic
and diluted earnings per share. Basic earnings/loss per share is
computed by dividing income (loss) available to common stockholders
(the numerator) by the weighted-average number of common shares
outstanding (the denominator) for the period, and does not include
the impact of any potentially dilutive common stock equivalents.
The computation of diluted earnings per share is similar to basic
earnings per share, except that the denominator is increased to
include the number of additional common shares that would have been
outstanding if potentially dilutive common shares had been
issued.
Research
and Development Costs
Research and
developments costs, including salaries, research materials,
administrative expenses and contractor fees, are charged to
operations as incurred. The cost of equipment used in research and
development activities which has alternative uses is capitalized as
part of fixed assets and not treated as an expense in the period
acquired. Depreciation of capitalized equipment used to perform
research and development is classified as research and development
expense in the year computed.
Income
Taxes
To
address accounting for uncertainty in tax positions, the Company
clarifies the accounting for income taxes by prescribing a minimum
recognition threshold that a tax position is required to meet
before being recognized in the financial statements. The Company
also provides guidance on de-recognition, measurement,
classification, interest, and penalties, accounting in interim
periods, disclosure and transition.
The
Company files income tax returns in the U.S. federal jurisdiction,
and Delaware.
Interest costs and
penalties related to income taxes, if any, will be classified as
interest expense and general and administrative costs,
respectively, in the Company’s financial statements. For the
years ended December 31, 2016 and 2015, the Company did not
recognize any interest or penalty expense related to income taxes.
The Company believes that it is not reasonably possible for the
amounts of unrecognized tax benefits to significantly increase or
decrease within the next 12 months.
Fair
Value of Financial Instruments
The
Company adopted ASC Topic 820 (originally issued as SFAS 157,
“Fair Value
Measurements”) as of January 1, 2008 for financial
instruments measured as fair value on a recurring basis. ASC Topic
820 defines fair value, established a framework for measuring fair
value in accordance with accounting principles generally accepted
in the United States and expands disclosures about fair value
measurements.
Fair
value is defined as the price that would be received to sell an
asset or paid to transfer a liability in an orderly transaction
between market participants at the measurement date. ASC Topic 820
established a three-tier fair value hierarchy which prioritizes the
inputs used in measuring fair value. The hierarchy gives the
highest priority to unadjusted quoted prices in active markets for
identical assets or liabilities (level 1 measurements) and the
lowest priority to unobservable inputs (level 3 measurements).
These tiers include:
-
Level 1, defined as
observable inputs such as quoted prices for identical instruments
in active markets;
-
Level 2, defined as
inputs other than quoted prices in active markets that are either
directly or indirectly observable such as quoted prices for similar
instruments in active markets or quoted prices for identical or
similar instruments in markets that are not active;
and
-
Level 3, defined as
unobservable inputs in which little or no market data exists,
therefore requiring an entity to develop its own assumptions, such
as valuations derived from valuation techniques in which one or
more significant inputs or significant value drivers are
unobservable.
Stock-Based
Compensation
The
Company recognizes in the financial statements compensation related
to all stock-based awards, including stock options, based on their
estimated grant-date fair value. The Company has estimated expected
forfeitures and is recognizing compensation expense only for those
awards expected to vest. All compensation is recognized by the time
the award vests.
The
Company accounts for equity instruments issued in exchange for the
receipt of goods or services from non-employees. Costs are measured
at the fair market value of the consideration received or the fair
value of the equity instruments issued, whichever is more reliably
measurable. The value of equity instruments issued for
consideration other than employee services is determined on the
earlier of the date on which there first exists a firm commitment
for performance by the provider of goods or services or on the date
performance is complete. The Company recognizes the fair
value of the equity instruments issued that result in an asset or
expense being recorded by the company, in the same period(s) and in
the same manner, as if the Company has paid cash for the goods or
services.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET
RISK.
This
item is not applicable to the Company because the Company is a
smaller reporting company as defined by Rule 12b-2 under the
Securities Exchange Act of 1934.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
All
financial information required by this Item is included on the
pages immediately following the Index to Financial Statements
appearing on page F-1, and is hereby incorporated by
reference.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING
AND FINANCIAL DISCLOSURE.
On,
August 18, 2016 (the "Resignation
Date"), Haynie & Company, Salt Lake City, Utah, resigned
as the Company’s independent registered public accounting
firm. On September 1, 2016, the Company engaged Fruci &
Associates II, PLLC (“Fruci”), as its new independent
registered public accounting firm. The change of the
Company’s independent registered public accounting firm from
Haynie & Company to Fruci was approved unanimously by our Board
of Directors.
The
report of Haynie & Company on the Company’s financial
statements for the fiscal year ended December 31, 2015 did not
contain an adverse or disclaimer of opinion and were not qualified
or modified as to uncertainty, audit scope, or accounting
principles, except the report did contain an explanatory paragraph
related to the Company’s ability to continue as a going
concern.
During
the two most recent fiscal years and through the Resignation Date,
there were (i) no disagreements between the Company and Haynie
& Company or Fruci on any matter of accounting principles or
practices, financial statement disclosure, or auditing scope or
procedures, which disagreement, if not resolved to the satisfaction
of Haynie & Company and Fruci, would have caused Haynie &
Company or Fruci to make reference thereto in their reports on the
consolidated financial statements for such years, and (ii) with the
exception of material weaknesses related to our internal control
over financial reporting, no “reportable events” as
that term is defined in Item 304(a)(1)(v) of Regulation
S-K.
ITEM 9A. CONTROLS AND PROCEDURES.
Disclosure Controls and Procedures
Based
on an evaluation as of the date of the end of the period covered by
this report, the Company’s Chief Executive Officer and Chief
Financial Officer conducted an evaluation of the effectiveness of
the design and operation of the Company’s disclosure controls
and procedures, as required by Exchange Act Rule
13a-15. Based on that evaluation, the Company’s
Chief Executive Officer and Chief Financial Officer concluded that,
because of the disclosed material weaknesses in the Company’s
internal control over financial reporting, the Company’s
disclosure controls and procedures were ineffective as of the end
of the period covered by this report to ensure that information
required to be disclosed by the Company in the reports that the
Company files or submits under the Exchange Act is recorded,
processed, summarized and reported within the time periods
specified by the SEC’s rules and forms.
Disclosure controls
and procedures are controls and other procedures that are designed
to ensure that information required to be disclosed in the
Company’s reports filed or submitted under the Exchange Act
is recorded, processed, summarized and reported, within the time
periods specified in the SEC’s rules and
forms. Disclosure controls and procedures include,
without limitation, controls and procedures designed to ensure that
information required to be disclosed in the Company’s reports
filed under the Exchange Act is accumulated and communicated to
management, including the Company’s Chief Executive Officer
and the Company’s Chief Financial Officer, to allow timely
decisions regarding required disclosure.
Management’s Annual Report on Internal Control Over Financial
Reporting
Management is
responsible for establishing and maintaining adequate internal
control over financial reporting as defined in Exchange Act Rule
13a-15(f). Management conducted an evaluation of the
effectiveness of the internal control over financial reporting as
of December 31, 2016, using the criteria established in
Internal Control –
Integrated Framework issued by the Committee of Sponsoring
Organizations of the Treadway Commission (“COSO”). Because of its
inherent limitations, internal control over financial reporting may
not prevent or detect misstatements. Also, projections
of any evaluation of effectiveness to future periods are subject to
the risk that controls may become inadequate because of changes in
conditions, or that the degree of compliance with the policies or
procedures may deteriorate.
A
material weakness is a control deficiency, or combination of
control deficiencies, that results in more than a remote likelihood
that a material misstatement of the annual or interim financial
statements will not be prevented or detected. As a
result of management’s assessment, management has determined
that there are material weaknesses due to the lack of segregation
of duties and, due to the limited resources based on the size of
the Company. Due to the material weaknesses management concluded
that as of December 31, 2016, the Company’s internal control
over financial reporting was ineffective. In order to
address and resolve the weaknesses, the Company will endeavor to
locate and appoint additional qualified personnel to the board of
directors and pertinent officer positions as the Company’s
financial means allow. To date, the Company’s
limited financial resources have not allowed the Company to hire
the additional personnel necessary to address the material
weaknesses.
Management’s Annual Report on Internal Control Over Financial
Reporting
This
annual report does not include an attestation report of the
Company’s registered public accounting firm regarding
internal control over financial reporting. Management’s
report was not subject to attestation by the Company’s
registered public accounting firm pursuant to temporary rules of
the Securities and Exchange Commission that permit the Company to
provide only management’s report in this annual
report.
Changes in Internal Control Over Financial Reporting
There
have been no changes in the Company’s internal control over
financial reporting that occurred during the Company’s last
fiscal quarter (the Company’s fourth fiscal quarter in the
case of an annual report) that has materially affected, or is
reasonably likely to materially affect, the Company’s
internal control over financial reporting.
The
term “internal control over financial reporting” is
defined as a process designed by, or under the supervision of, the
registrant’s principal executive and principal financial
officers, or persons performing similar functions, and effected by
the registrant’s board of directors, management and other
personnel, to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally
accepted accounting principles and includes those policies and
procedures that:
(a)
Pertain to the
maintenance of records that in reasonable detail accurately and
fairly reflect the transactions and dispositions of the assets of
the registrant;
(b)
Provide reasonable
assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally
accepted accounting principles, and that receipts and expenditures
of the registrant are being made only in accordance with
authorizations of management and directors of the registrant;
and
(c)
Provide reasonable
assurance regarding prevention or timely detection of unauthorized
acquisition, use or disposition of the registrant’s assets
that could have a material effect on the financial
statements.
ITEM 9B. OTHER INFORMATION.
None.
PART
III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE.
The
Company’s current directors and executive officers are as
follows:
|
NAME
|
|
AGE
|
|
POSITION
|
|
Michael
K. Korenko
|
|
71
|
|
Interim
President and Chief Executive Officer
|
|
L.
Bruce Jolliff
|
|
67
|
|
Chief
Financial Officer
|
|
Carlton
M. Cadwell
|
|
72
|
|
Chairman
of the Board and Secretary
|
|
Thomas
J. Clement
|
|
60
|
|
Director
|
|
James
C. Katzaroff
|
|
60
|
|
Director
|
Term
of Office
All of
the Company’s directors hold office until the next annual
meeting of the stockholders or until their successors is elected
and qualified. The Company’s executive officers
are appointed by the Company’s board of directors and hold
office until their resignation, removal, death or
retirement.
Background
and Business Experience
The
business experience during the past five years of each of the
Company’s directors and executive officers is as
follows:
Dr. Michael K.
Korenko, interim
President and Chief Executive Officer of the Company since December
2016, joined the Company as an Advisor to the Board of the Company
during 2009 and served as member of the Board from May 2009 to March 2010. Dr.
Korenko has also served on the Hanford Advisory Board since 2009.
Dr. Korenko served as Business Development Manager for
Curtiss-Wright from 2006 to 2009, as Chief Operating Officer for
Curtiss-Wright from 2000 to 2005 and was Executive Vice President
of Closure for Safe Sites of Colorado at Rocky Flats from 1994 to
2000. Dr. Korenko served as Vice President of Westinghouse from
1987 to 1994 and was responsible for the 300 and 400 areas,
including the Fast Flux Testing Facility
(“FFTF”)
and all engineering, safety analysis, and projects for the
Hanford site. Dr. Korenko is the author of 28 patents and has
received many awards, including the National Energy Resources
Organization Research and Development Award, the U.S. Steelworkers
Award for Excellence in Promoting Safety, and the Westinghouse
Total Quality Award for Performance Manager of the Year. Dr.
Korenko has a Doctor of Science from MIT, was a NATO Postdoctoral
Fellow at Oxford University, and was selected as a White House
Fellow for the Department of Defense, reporting to Secretary Cap
Weinberger.
Carlton M.
Cadwell, Chairman of
the Board and Secretary since December 2016, joined the Company as
a director in 2006. Dr. Cadwell brings over 30 years of
experience in business management, strategic planning, and
implementation. He co-founded Cadwell Laboratories, Inc.
in 1979 and has served as its President since its
inception. Cadwell Laboratories, Inc. is a major
international provider of neurodiagnostic medical devices. After
receiving his bachelor’s degree from the University of Oregon
in 1966 and a doctoral degree from the University of Washington in
1970, he began his career serving in the United States Army as a
dentist for three years. From 1973 to 1980, Dr. Cadwell
practiced dentistry in private practice and since has started
several businesses.
Mr.
Cadwell brings to the Board over ten years of service on the Board
and over forty-five years of experience as a successful
entrepreneur, as well as medical expertise.
Leonard Bruce
Jolliff, the Chief
Financial Officer, joined the Company as chief financial officer in
2006. For nine years prior to joining the Company, Mr.
Jolliff was a sole practitioner in the role of CFO for Hire and as
a Forensic Accountant, working with companies ranging from Fortune
500 to small family operations. Mr. Jolliff is a CPA and
a member of the Washington Society of CPAs. He is also a
CFE and a member of the Association of Certified Fraud
Examiners.
Mr. Jolliff has
held CFO and Controller positions in an array of industries and has
worked as a CPA in public practice.
James C.
Katzaroff, a director,
was the Chief Executive Officer of the Company from its inception
in 2006 to December 2016. Mr. Katzaroff is the founder of the
Company. Initially a financial consultant with Wall Street
firms Bateman Eichler, Smith Barney and EF Hutton, Mr. Katzaroff
has been responsible for senior-level corporate strategy, fostering
investment banking relationships, and served as a senior financial
advisor for numerous start-ups and development-stage
companies. From 1998 to 2016, Mr. Katzaroff held senior
positions including Chief Executive Officer, Chief Financial
Officer, Senior Vice President of Finance, Senior Vice President,
and Corporate Secretary of Telemac Corporation, an international
communications company active in the wireless telephony
market. In 2001 he became Chairman and CEO of Apogee
Biometrics, and in 2004 became President of Manakoa Services
Corporation, serving as its interim CEO. He holds a
Bachelor’s Degree in Business Economics from the University
of California, Santa Barbara, and has completed advanced management
courses at the University of Washington.
Mr.
Katzaroff brings to the Board experience working with Wall Street
banking firms as well as experience in numerous start-ups and
development-stage companies.
Thomas J.
Clement, a director,
joined the Company as a director in 2013. Mr. Clement has over 30
years experience in product development engineering, engineering
management, and senior management. He has participated in two
Company startups through full production. He was responsible for
development of seven novel medical devices through commercial
launch; one device was the leading royalty generator for the
University of Washington for nearly ten years, and in another,
generated revenues of more than $140 million for a leading medical
device company. Mr. Clement since 2013
has held the position of Founder and CEO of Aquaduct Critical Care,
Inc, a private, pre-revenue medical device
company.
Mr. Clement brings to the Board previous
experience in bringing two startups from development through
production as well as substantial experience in the development of
medical devices.
Identification of Significant Employees and
Consultants
David J. Swanberg, M.S.,
P.E. Chief Technical Officer, has over 30 years’
experience in radiochemical processing, medical isotope production,
nuclear waste management, materials science, regulatory affairs,
and project management. He has worked in diverse organizations
ranging from small start-up businesses to corporations with
multi-billion dollar annual revenues. He previously served as
Executive Vice President of Operations for IsoRay Medical Inc.
managing day-to-day operations, R&D, and New Product
Development. Mr. Swanberg was a co-founder of IsoRay and led the
initial Cs-131 brachytherapy seed product development, FDA 510(k)
submission/clearance, and NRC Sealed Source review and
registration. He led the radiation dosimetry evaluations to meet
American Association of Physicists in Medicine guidelines and is a
current member of the AAPM. Mr. Swanberg served on the IsoRay Board
of Directors and participated in several capital financing rounds
totaling over $30.0 million. He holds a BA in Chemistry from Bethel
University (MN) and an MS in Chemical Engineering from Montana
State University. He has numerous technical publications and holds
several patents.
Fu-Min Su,
Ph.D. was appointed as the Company’s Chief
RadioChemist and Radiation Safety Officer in 2007. With
over 20 years experience in medical isotope R&D and
manufacturing, Dr. Su is also experienced in the area of
coordinating and conducting clinical trials. He has
worked as a senior scientist for a several bio-technology firms,
including NeoRx Corporation from 1987 through
1998, Nycomed-Amersham Imaging in 1999, Bristol-Myers Squibb
from 2000 to 2006, and Cellectar, LLC in 2007, during which time he
developed various radiopharmaceuticals, isotope production methods
and generator systems. Dr. Su has authored a number of
scientific papers, and has written numerous abstracts for the
Journal of Nuclear Medicine. He also holds several
patents relating to radionuclide production and
preparation. Dr. Su received his Ph.D. from the
University of Washington.
Alan E. Waltar,
Ph.D., Chairman of the
Company’s Medical Advisory Board. Dr.
Waltar served as director of Nuclear Energy for the Pacific
Northwest National Laboratory (“PNNL”) in Richland, Washington.
Since 2004, he has continued his affiliation with PNNL as a senior
advisor. Waltar’s other professional appointments include
director of international programs at Advanced Nuclear Medical
Systems; manager of various fast reactor safety and fuels
organizations of Westinghouse Hanford Company; and as professor and
department head of Nuclear Engineering at Texas A&M University.
His other teaching experience includes stints at the Joint Center
for Graduate Study in Richland Washington, the University of
Virginia, and Los Alamos National Laboratory. Formerly the
president of the American Nuclear Society, Dr. Waltar has served on
a number of international nuclear science and radiation panels,
societies, and committees. He is the author of three books: Fast
Breeder Reactors, America the Powerless: Facing Our Nuclear Energy
Dilemma, and Radiation and Modern Life: Fulfilling Marie
Curie’s Dream, and has penned over 70 open literature
papers.
Dr.
Waltar earned his M.S. in Nuclear Engineering from M.I.T. and his
PhD in Engineering Science from the University of California,
Berkeley.
Nigel R. Stevenson,
Ph.D., is a world renowned expert in the production of
medical isotopes. He holds a Ph.D. in Nuclear Physics from the
University of London and has directed many corporate innovations
for imaging and therapeutic nuclide agents. For the past five
years he has served as Chief Operating Officer for Clear Vascular
Inc. and was previously Chief Operating Officer of Trace Life
Sciences, which produced a range of medical radiochemicals and
radiopharmaceuticals. Prior to this, he had been VP Production and
Research for Theragenics Corp. and directed operations in Atlanta
for the world’s largest cyclotron facility (14 cyclotrons)
that produced brachytherapy seeds. Dr. Stevenson was
also Head of Isotope Production and Research at TRIUMF (Canadian
National Accelerator Laboratory) where he managed the production of
medical radioisotopes for MDS Nordion.
Donald A. Ludwig,
Ph.D., Special
Projects Manager and IsoPet Business Development Manager. Dr.
Ludwig is an expert in particle accelerator applications in
radiation therapy, nuclear medicine and radioisotope
production. Since 1988 he has served as an advisor to numerous
entities in the field, both domestic and foreign. Among these
are the Atomic Energy of Canada, the U. S. Department of Energy
Labs at Los Alamos, Berkeley, Fermi, Hanford and Oak Ridge, the
Israel Atomic Energy Agency, the Australian Nuclear Science and
Technology Organization, the Kurchatov Russian Research Institute
in Moscow and the Bhabha Atomic Research Center in Mumbai,
India. He holds a Ph.D. from UCLA in Medical Physics as well
as an MS in Nuclear Physics from Cal Tech, a BS in Physics from the
U. S. Military Academy at West Point and an MBA in Theoretical
Marketing from the University of Southern California.
Section 16(a) Beneficial Ownership Reporting
Compliance
Section
16(a) of the Securities Exchange Act of 1934 requires the
Company’s executive officers, directors and persons who own
more than 10% of the Company’s common stock to file with the
SEC initial reports of beneficial ownership on Form 3, changes in
beneficial ownership on Form 4, and an annual statement of
beneficial ownership on Form 5. Such executive officers,
directors and greater than 10% stockholders are required by SEC
rules to furnish the Company with copies of all such forms that
they have filed.
Based
solely on its review of such forms filed with the SEC and received
by the Company and representations from certain reporting persons,
the Company believes that each of its executive officers and
directors failed to report at least one transaction during the year
ended December 31, 2016.
Code of Ethics
The
Company’s Board of Directors has not adopted a code of ethics
that applies to the principal executive officer, principal
financial officer, principal accounting officer or controller, or
persons performing similar functions, because of the
Company’s limited number of executive officers and employees
that would be covered by such a code and the Company’s
limited financial resources. The Company anticipates
that it will adopt a code of ethics after it increases the number
of executive officers and employees and obtain additional financial
resources.
Audit Committee and Audit Committee Financial Expert
As of
the date of this report, the Company has not established an audit
committee, and therefore, the Company’s board of directors
performs the functions that customarily would be undertaken by an
audit committee. The Company’s board of directors
during 2016 was comprised of three directors, two of whom the
Company has determined satisfied the general independence standards
of the NASDAQ listing requirements. See Item 13 of this
Form 10-K.
The
Company’s Board of Directors has determined that none of its
current members qualifies as an “audit committee financial
expert,” as defined by the rules of the SEC. In
the future, the Company intends to establish board committees and
to appoint such persons to those committees as are necessary to
meet the corporate governance requirements imposed by a national
securities exchange, although it is not required to comply with
such requirements until the Company elects to seek listing on a
national securities exchange.
ITEM 11. EXECUTIVE COMPENSATION.
Summary Compensation Table
The
following table sets forth the compensation paid to the
Company’s Chief Executive Officer and those executive
officers that earned in excess of $100,000 during the twelve month
periods ended December 31, 2016 and 2015 (collectively, the
“Named Executive
Officers”):
|
Name and Principal Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards ($)
|
Option Awards
($)
|
Total
($)
|
|
|
|
|
|
|
|
|
|
Dr. Michael K.
Korenko
|
2016
|
$- (1)
|
$-
|
$-
|
$-
|
$-
|
|
Interim CEO and
President
|
2015
|
$- (1)
|
$-
|
$-
|
$-
|
$-
|
|
|
|
|
|
|
|
|
|
James C.
Katzaroff
|
2016
|
$250,000(2)
|
$-
|
$-
|
$408,165(3)
|
$658,165
|
|
Former CEO and
Chairman
|
2015
|
$250,000(4)
|
$-
|
$-
|
$-
|
$250,000
|
|
|
|
|
|
|
|
|
|
L. Bruce
Jolliff
|
2016
|
$180,000
|
$-
|
|
$110,389(5)
|
$290,389
|
|
CFO
|
2015
|
$180,000(6)
|
$11,357(7)
|
$-
|
$-
|
$190,357
|
(1)
Mr.
Korenko began serving as our interim CEO and President on December
14, 2016 and, as such, did not receive any compensation during 2016
or 2015.
(2)
Mr.
Katzaroff resigned as Chief Executive Officer in December
2016.
(3)
In June
2016, Mr. Katzaroff received 100,000 three-year common stock
options at $1.00, and 1,000,000 five year common stock options at
$0.50. Additionally Mrs. Katzaroff received 100,000, five-year
common stock options at $0.50, vested equally over 24
months.
(4)
Of this
amount, $150,000 was exchanged for 100,000 shares of Series A
Preferred and $75,144 was not paid in 2015, but was accrued as of
December 31, 2015.
(5)
In June
2016, Mr. Jolliff received 240,000, five year common stock options
at $0.50 and another 240,000, five year common stock options at
$0.50 vesting equally over 24 months.
(6)
Of this
amount $112,500 was exchanged for 75,000 shares of Series A
Preferred.
(7)
Mr.
Jolliff received the additional $11,357 in 2015 to compensate for
additional duties performed that were not originally
contemplated.
Narrative Disclosure to Summary Compensation Table
L. Bruce Jolliff. Mr. Jolliff has a May
2007 employment agreement with the Company that provides for a
salary of $100,000 per year, which amount was increased on January
1, 2012 to $156,000, and again adjusted beginning January 1, 2013
to $180,000. In 2015, Mr. Jolliff received $67,500, and exchanged
$112,500 for 75,000 Series A Preferred shares. In 2016, Mr. Jolliff
received $96,721 and an additional $83,279 was accrued as of
December 31, 2016. The Company may terminate the agreement without
cause at any time upon 30 days’ written
notice. Upon termination, the Company will pay Mr.
Jolliff a severance allowance of two month’s
salary.
James C.
Katzaroff. The Company’s
former Chief Executive Officer, James C. Katzaroff, did not have a
written employment agreement and, therefore, no contracted amount
or schedule of pay. However, his annual compensation was $250,000
and, accordingly, accruals were made for his compensation in 2016
and 2015 to bring his recorded salary to $250,000. In
2016, Mr. Katzaroff received $43,928 and an additional $206,072 was
accrued as of December 31, 2016. In 2015, Mr. Katzaroff received
$24,856, exchanged $150,000 for 100,000 shares of Series A
Preferred, and an additional $75,144 was accrued as of December 31,
2015.
The
Company paid bonuses to certain employees based on their
performance, the Company’s need to retain such employees, and
funds available. All bonus payments were approved by the
Company’s Board of Directors.
Outstanding Equity Awards at Fiscal Year-End Table
The
following table sets forth all outstanding equity awards held by
the Company’s Named Executive Officers as of the end of last
fiscal year.
|
|
Option
Awards
|
|||
|
Name
|
Number of
Securities Underlying Unexercised Options(#)
Exercisable
|
Number of
Securities Underlying Unexercised Options (#)
Unexercisable
|
Option
Exercise
Price
($)
|
Option
Exercise
Date
|
|
James C.
Katzaroff
|
32,500
|
-
|
$15.00
|
2/11/23
|
|
James C.
Katzaroff
|
100,000
|
-
|
$1.00
|
6/21/19
|
|
James C.
Katzaroff
|
1,000,000
|
-
|
$0.50
|
6/21/21
|
|
L. Bruce
Jolliff
|
480,000
|
176,548
|
$0.50
|
6/21/21
|
Employment Agreement with Significant Employee
Employment
Agreement with Dr. Fu Min-Su
In
January 2008, the Company entered into a five-year employment
agreement with Dr. Fu Min-Su pursuant to which the Company agreed
to pay Dr. Fu Min-Su an annual salary equal to $90,000, which was
increased to $95,000 on January 1, 2010, to $110,000 on January 1,
2011, and to $125,000 effective June 1, 2016.
In
the event the
employment is terminated by the Company without cause, by Dr. Fu
Min-Su for good reason or a change in control, the Company will
have to provide Dr. Fu Min-Su with one month of his base salary and
any portion of an annual bonus allocated by the Board of Directors,
disability and other welfare plan benefits for a period
of one year from the date of termination and pro-rated vesting of
all outstanding
options, stock grants, shares of restricted stock and any other
equity incentive compensation; provided, that the stock options
shall be exercisable only until the earlier to occur of (i) two
years from the date of the termination, or (ii) the date the option
would have otherwise expired if Dr. Fu Min-Su had not
terminated employment.
During
the term of the employment agreement, including any extension
thereof, and for a period of one year thereafter, Dr. Fu Min-Su
shall not provide services that he provides for the Company for a
business in the production, import for resale, and distribution of
radioisotopes for use in the medical industries.
Compensation of Directors
During
the year ended December 31, 2016, the Company’s non-employee
directors were not paid any compensation.
The
following table sets forth, for each of the Company’s
non-employee directors who served during 2016, the aggregate number
of stock awards and the aggregate number of stock option awards
that were outstanding as of December 31, 2016:
|
|
Outstanding
|
Outstanding
|
|
|
Stock
|
Stock
|
|
Name
|
Awards (#)
|
Options (#)
|
|
Carlton M.
Cadwell
|
-
|
100,000
|
|
Thomas J.
Clement
|
-
|
100,000
|
During June 2016,
the Company granted to Messrs. Cadwell and Clement options to
purchase 100,000 shares of common stock at an exercise price of
$1.00 per share. The options are fully vested and expire June 21,
2019.
There
are no employment contracts or compensatory plans or arrangements
with respect to any director that would result in payments by the
Company to such person because of his or her resignation as a
director or any change in control of the
Company.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND
MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Beneficial Ownership of The Company’s Common
Stock
The
following table sets forth, as of March 6, 2017, the number of
shares of common stock beneficially owned by the following persons:
(i) all persons the Company knows to be beneficial owners of at
least 5% of the Company’s common stock, (ii) the
Company’s directors, (iii) the Company’s executive
officers, both of whom are named in the Summary Compensation Table
above, and (iv) all current directors and executive officers as a
group.
As of
March 6, 2017, there were 37,541,697 shares outstanding and up to
11,637,245 shares issuable upon exercise of outstanding options,
warrants, and conversion of outstanding convertible securities,
assuming exercise and conversion occurred as of that date, for a
total of 49,178,942 shares.
|
Name
and Address of Beneficial Owner(1)
|
Amount and Nature of Beneficial
Ownership(2)
|
Percent of Class
|
|
Cadwell Family
Irrevocable Trust
|
169,839
|
0.5%
|
|
|
|
|
|
Carlton M. Cadwell
(3)
|
1,268,844
|
3.4%
|
|
|
|
|
|
James C. Katzaroff
(4)
|
1,409,860
|
3.7%
|
|
|
|
|
|
Thomas J. Clement
(3)
|
100,000
|
0.3%
|
|
|
|
|
|
L. Bruce Jolliff
(5)
|
635,034
|
1.7%
|
|
|
|
|
|
All Current
Directors and Executive Officers as a group (5
individuals)
|
3,583,577
|
7.1%
|
(1)
The
address of each of the beneficial owners above is c/o Advanced
Medical Isotope Corporation, 1021 N. Kellogg Street, Kennewick, WA
99336, except that the address of the Cadwell Family Irrevocable
Trust (the “Cadwell
Trust ”) is 909 North Kellogg Street, Kennewick,
WA 99336.
(2)
In
determining beneficial ownership of the Company’s common
stock as of a given date, the number of shares shown includes
shares of common stock which may be acquired upon exercise of
options or conversion of convertible securities within 60 days of
that date. In determining the percent of common stock owned by a
person or entity on February 1, 2017, (a) the numerator
is the number of shares of the class beneficially owned by such
person or entity, including shares which may be acquired within 60
days on exercise of options and conversion of convertible
securities, and (b) the denominator is the sum of (i) the
total shares of common stock outstanding on February 1, 2017, and
(ii) the total number of shares that the beneficial owner may
acquire upon conversion of the convertible securities and upon
exercise of the options. Subject to community property laws where
applicable, the Company believes that each beneficial owner has
sole power to vote and dispose of its shares, except that Mr.
Cadwell under the terms of the Cadwell Trust does not have or share
voting or investment power over the shares beneficially owned by
the Cadwell Trust.
(3)
Includes 100,000
shares each issuable under options to Mr. Cadwell and Mr.
Clements.
(4)
Includes 1,232,500
shares issuable under options held by Mr. Katzaroff.
(5)
Includes 480,000
shares issuable under options held by Mr. Jolliff.
Beneficial Ownership of The Company’s Preferred
Stock
The
following table sets forth, as of March 6, 2017, the
number of shares of preferred stock beneficially owned by the
following persons: (i) all persons the Company known to be
beneficial owners of at least 5% of the Company’s preferred
stock, (ii) the Company’s directors, (iii) the
Company’s executive officers, both of whom are named in the
Summary Compensation Table above, and (iv) all current directors
and executive officers as a group.
|
Name and Address of Beneficial Owner
(1)
|
Amount and Nature of Beneficial
Ownership (2)
|
Percent of Class
|
|
Cadwell Family
Irrevocable Trust
|
148,309
|
4.5%
|
|
|
|
|
|
Carlton M.
Cadwell
|
908,910
|
27.6%
|
|
|
|
|
|
James C.
Katzaroff
|
-
|
-%
|
|
|
|
|
|
Thomas J.
Clement
|
-
|
-%
|
|
|
|
|
|
L. Bruce
Jolliff
|
114,700
|
3.5%
|
|
|
|
|
|
All Current
Directors and Executive Officers as a group (5
individuals)
|
1,171,919
|
35.5%
|
(1)
The
address of each of the beneficial owners above is c/o Advanced
Medical Isotope Corporation, 1021 N. Kellogg Street, Kennewick, WA
99336, except that the address of the Cadwell Family Irrevocable
Trust (the “Cadwell
Trust ”) is 909 North Kellogg Street, Kennewick,
WA 99336.
(2)
In
determining beneficial ownership of the Company’s common
stock as of a given date, the number of shares shown includes
shares of common stock which may be acquired upon exercise of
options or conversion of convertible securities within 60 days of
that date. In determining the percent of common stock owned by a
person or entity on February 13, 2017, (a) the numerator
is the number of shares of the class beneficially owned by such
person or entity, including shares which may be acquired within 60
days on exercise of options and conversion of convertible
securities, and (b) the denominator is the sum of (i) the
total shares of common stock outstanding on February 13, 2017, and
(ii) the total number of shares that the beneficial owner may
acquire upon conversion of the convertible securities and upon
exercise of the options. Subject to community property laws where
applicable, the Company believes that each beneficial owner has
sole power to vote and dispose of its shares, except that Mr.
Cadwell under the terms of the Cadwell Trust does not have or share
voting or investment power over the shares beneficially owned by
the Cadwell Trust.
Changes in Control
The
Company does not know of any arrangements, including any pledges of
the Company’s securities that may result in a change in
control of the Company.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND
DIRECTOR INDEPENDENCE.
Indebtedness from Related Parties
Beginning in
December, 2008, the Company has obtained financing from Carlton M.
Cadwell, one of our directors and a beneficial owner of more than
10% of the Company’s common stock, in transactions which
involved the Company’s issuance of convertible notes and
common stock. On September 4, 2015, the Company exchanged
$1,414,100 of convertible notes plus $810,538 of accrued interest
into 148,311 shares of Series A Preferred and another $2,224,466 of
convertible notes plus $889,838 of accrued interest into 207,620
shares of Series A Preferred. Additionally, the Company exchanged
the remaining $906,572 of convertible notes plus $148,960 accrued
interest into a $1,055,532 demand note, 8% interest rate, due on
demand at any time after March 31, 2017. Such note was converted
into 73,546 shares of Series A Preferred on May 19, 2016. At
December 31, 2016 Mr. Cadwell has an aggragate total of $332,195 in
promissory notes.
Independent Directors
The
Company’s common stock is traded on the OTC Pink Marketplace,
which does not impose any independence requirements on the board of
directors or the board committees of the companies whose stock is
traded on that market. The Company has decided to adopt
the independence standards of the NASDAQ listing rules in
determining whether the Company’s directors are
independent. Generally, under those rules a director
does not qualify as an independent director if the director or a
member of the director’s immediate family has had in the past
three years certain relationships or affiliations with the Company,
the Company’s auditors, or other companies that do business
with the Company. The Company’s board of directors
has determined that Mr. Cadwell and Mr. Clement each qualified as
an independent director under those NASDAQ rules, and accordingly,
each would have been qualified under those rules to serve on a
compensation committee or a nominating committee, if the Company
had established such committees of the Company’s Board of
Directors. Mr. Katzaroff is not an independent director due to his
employment by the Company as an executive officer.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
Audit Fees
The aggregate fees
incurred by the Company’s principal accountant for the audit
of the Company’s annual financial statements, review of
financial statements included in the quarterly reports and other
fees that are normally provided by the accountant in connection
with statutory and regulatory filings or engagements for the fiscal
years ended December 31, 2016 and 2015 were $13,500, all of which
was paid to Fruci & Associates II, PLLC, and $156,000 ($71,000
was to HJ & Associates, LLC and $85,000 was to Haynie &
Company), respectively.
Tax
Fees
The aggregate fees
billed for professional services rendered by principal accountant
for tax compliance, tax advice and tax planning during the fiscal
years ended December 31, 2016 and 2015 were $0, all of which was
paid to Fruci & Associates II, PLLC, and $3,000, respectively,
all of which was paid to HJ & Associates, LLC. These fees
related to the preparation of federal income tax
returns.
All Other Fees
There
were no other fees billed for products or services
provided by the Company’s principal accountant during the
fiscal years ended December 31, 2016 and 2015.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
(a)
Documents filed as part of this Report.
1.
Financial Statements. The
Advanced Medical Isotope Corporation Balance Sheets as of
December 31, 2016 and 2015, the Statements of Operations for
the years ended December 31, 2016 and 2015, the Statements of
Changes in Stockholders’ Equity (Deficit) for the years ended
December 31, 2016 and December 31, 2015, and the Statements of
Cash Flows for the years ended December 31, 2016 and 2015,
together with the notes thereto and the reports of Haynie &
Company and with Fruci & Associates II as required by
Item 8 are included in this 2016 Annual Report on Form 10-K as
set forth in Item 8 above.
2.
Financial Statement Schedules. All financial
statement schedules have been omitted since they are either not
required or not applicable, or because the information required is
included in the financial statements or the notes
thereto.
3.
Exhibits. The following exhibits are either filed as a part
hereof or are incorporated by reference. Exhibit numbers
correspond to the numbering system in Item 601 of Regulation
S-K. Exhibits 10.3, 10.5, 10.7 and 10.10 relate to
compensatory plans included or incorporated by reference as
exhibits hereto.
| Exhibit |
|
|
| Number |
|
Description
|
|
3.1
|
|
Certificate
of Incorporation of Savage Mountain Sports Corporation, dated
January 11, 2000 (incorporated by reference to Exhibit 3.1 to the
Company’s Registration Statement on Form 10-12G (File No.
000-53497) filed on November 12, 2008).
|
|
3.2
|
|
By-Laws
(incorporated by reference to Exhibit 3.2 to the Company’s
Registration Statement on Form 10-12G (File No. 000-53497) filed on
November 12, 2008).
|
|
3.3
|
|
Articles
and Certificate of Merger of HHH Entertainment Inc. and Savage
Mountain Sports Corporation, dated April 3, 2000 (incorporated by
reference to Exhibit 3.3 to the Company’s Registration
Statement on Form 10-12G (File No. 000-53497) filed on November 12,
2008).
|
|
3.4
|
|
Articles
and Certificate of Merger of Earth Sports Products Inc. and Savage
Mountain Sports Corporation, dated May 11, 2000 (incorporated by
reference to Exhibit 3.4 to the Company’s Registration
Statement on Form 10-12G (File No. 000-53497) filed on November 12,
2008).
|
|
3.5
|
|
Certificate
of Amendment of Certificate of Incorporation changing the name of
the Company to Advanced Medical Isotope Corporation, dated May 23,
2006 (incorporated by reference to Exhibit 3.5 to the
Company’s Registration Statement on Form 10-12G (File No.
000-53497) filed on November 12, 2008).
|
|
3.6
|
|
Certificate
of Amendment of Certificate of Incorporation increasing authorized
capital dated September 26, 2006 (incorporated by reference to
Exhibit 3.6 to the Company’s Registration Statement on Form
10-12G (File No. 000-53497) filed on November 12,
2008).
|
|
3.7
|
|
Certificate
of Amendment to the Certificate of Incorporation increasing
authorized common stock and authorizing preferred stock, dated May
18, 2011 (incorporated by reference to Exhibit 3.1 to the
Company’s Current Report on Form 8-K filed on May 18,
2011).
|
|
3.8
|
|
Certificate
of Amendment to the Certificate of Incorporation authorizing a
series of Preferred Stock to be named “Series A Convertible
Preferred Stock”, consisting of 2,500,000 shares, which
series shall have specific designations, powers, preferences and
relative and other special rights, qualifications, limitations and
restrictions as outlined in the Certificate of Designations, filed
June 30, 2015 (incorporated by reference to Exhibit
3.4).
|
|
3.9
|
|
Certificate
of Amendment to the Certificate of Incorporation increasing the
authorized series of “Series A Convertible Preferred
Stock” to 5,000,000 shares, filed June 31, 2016 (incorporated
by reference to Exhibit 3.5).
|
|
10.1
|
|
Agreement
and Plan of Reorganization, dated as of December 15, 1998, by and
among HHH Entertainment, Inc. and Earth Sports Products, Inc.
(incorporated by reference to Exhibit 10.1 to the Company’s
Registration Statement on Form 10-12G (File No. 000-53497) filed on
November 12, 2008).
|
|
10.2
|
|
Agreement
and Plan of Merger of HHH Entertainment, Inc. and Savage Mountain
Sports Corporation, dated as of January 6, 2000 (incorporated by
reference to Exhibit 10.2 to the Company’s Registration
Statement on Form 10-12G (File No. 000-53497), filed on November
12, 2008).
|
|
10.3
|
|
Agreement
and Plan of Acquisition by and between Neu-Hope Technologies, Inc.,
UTEK Corporation and Advanced Medical Isotope Corporation, dated
September 22, 2006 (incorporated by reference to Exhibit 10.4 to
the Company’s Registration Statement on Form 10-12G (File No.
000-53497), filed on November 12, 2008).
|
|
10.4
|
|
Employment
Agreement dated May 16, 2007 with Leonard Bruce Jolliff
(incorporated by reference to Exhibit 10.5 to the Company’s
Registration Statement on Form 10-12G (File No. 000-53497), filed
on November 12, 2008).
|
|
10.5
|
|
Agreement
and Plan of Acquisition by and between Isonics Corporation and
Advanced Medical Isotope Corporation dated June 13, 2007
(incorporated by reference to Exhibit 10.6 to the Company’s
Registration Statement on Form 10-12G (File No. 000-53497), filed
on November 12, 2008).
|
|
10.6
|
|
Employment
Agreement dated January 15, 2008 with Dr. Fu-Min Su (incorporated
by reference to Exhibit 10.7 to the Company’s Registration
Statement on Form 10-12G (File No. 000-53497), filed on November
12, 2008).
|
|
10.7
|
|
Master
Lease Agreement dated September 20, 2007 between BancLeasing, Inc.
and Advanced Medical Isotope Corporation, and related documents
(incorporated by reference to Exhibit 10.8 to the Company’s
Annual Report on Form 10-K/A filed on December 2,
2011).
|
|
10.8
|
|
Lease
Agreement dated July 17, 2007 between Robert L. and Maribeth F.
Myers and Advanced Medical Isotope Corporation (incorporated by
reference to Exhibit 10.9 to the Company’s Annual Report on
Form 10-K/A filed on December 2, 2011).
|
|
10.9
|
|
Form of
Non-Statutory Stock Option Agreement (incorporated by reference to
Exhibit 10.1 to the Company’s Current Report on Form 8-K
filed on March 15, 2012).
|
|
10.10
|
|
Promissory
Note dated December 16, 2008 between Advanced Medical Isotope
Corporation and Carlton M. Cadwell (incorporated by reference to
Exhibit 10.11 to the Company’s Annual Report on Form 10-K
filed on March 3, 2012).
|
|
10.11
|
|
Memorandum
of Agreement for Strategic Relationship dated August 19, 2011
between Advanced Medical Isotope Corporation and Spivak Management
Inc. (incorporated by reference to Exhibit 10.12 to the
Company’s Annual Report on Form 10-K filed on March 3,
2012).
|
|
10.12
|
|
2015
Omnibus Securities and Incentive Plan (incorporated by reference to
Exhibit 10.12 to the Company’s Annual Report on Form 10-K,
filed May 25, 2016).
|
|
31.1*
|
|
Certification of Chief Executive Officer pursuant to Sec. 302 of
the Sarbanes-Oxley Act of 2002 (4)
|
|
31.2*
|
|
Certification of Chief Financial Officer pursuant to Sec. 302 of
the Sarbanes-Oxley Act of 2002 (4)
|
|
32.1*
|
|
Certification of Chief Executive Officer and Chief Financial
Officer pursuant to 18 U.S.C. Section 1350 (4)
|
|
101.INS*
|
|
XBRL Instance Document
|
|
101.SCH*
|
|
XBRL
Taxonomy Extension Schema
|
|
101.CAL*
|
|
XBRL
Taxonomy Extension Calculation Linkbase
|
|
101.DEF*
|
|
XBRL
Taxonomy Extension Definition Linkbase
|
|
101.LAB*
|
|
XBRL
Taxonomy Extension Label Linkbase
|
|
101.PRE*
|
|
XBRL
Taxonomy Extension Presentation Linkbase
|
* Filed
herewith.
SIGNATURES
Pursuant to the
requirements of Section 13 or 15(d) of the Securities Exchange
Act of 1934, the Registrant has duly caused this report to be
signed on its behalf by the undersigned, thereunto duly
authorized.
|
|
ADVANCED
MEDICAL ISOTOPE CORPORATION
|
|
|
|
|
|
|
Date:
March 8, 2017
|
By:
|
/s/ Michael K. Korenko
|
|
|
Name:
|
Michael
K. Korenko
|
|
|
Title:
|
Chief
Executive Officer
|
Pursuant to the
requirements of the Securities Exchange Act of 1934, this report
has been signed below by the following persons on behalf of the
Registrant and in the capacities and on the dates
indicated.
|
Date:
March 8, 2017
|
By:
|
/s/ Michael K. Korenko
|
|
|
Name:
|
Michael K.
Korenko
|
|
|
Title:
|
Chief
Executive Officer
(Principal
Executive Officer)
|
|
|
|
|
|
Date:
March 8, 2017
|
By:
|
/s/ L. Bruce Jolliff
|
|
|
Name:
|
L.
Bruce Jolliff
|
|
|
Title:
|
Chief
Financial Officer
(Principal
Financial and Accounting Officer)
|
|
|
|
|
|
Date:
March 8, 2017
|
By:
|
/s/ Carlton M. Cadwell
|
|
|
Name:
|
Carlton
M. Cadwell
|
|
|
Title:
|
Secretary
and Chairman of the Board
|
Advanced Medical Isotope Corporation
Index to Financial Statements
|
|
|
Pages
|
|
|
|
|
|
Report
of Independent Registered Public Accounting Firm for
2016
|
|
F-1
|
|
|
|
|
|
Report
of Independent Registered Public Accounting Firm for
2015
|
|
F-2
|
|
|
|
|
|
Financial Statements:
|
|
|
|
|
|
|
|
Consolidated
Balance Sheets as of December 31, 2016 and 2015
|
|
F-3
|
|
|
|
|
|
Consolidated
Statements of Operations for the years ended December 31, 2016 and
2015
|
|
F-4
|
|
|
|
|
|
Consolidated
Statement of Changes in Stockholders’ Equity (Deficit) for
the years ended December 31, 2016 and 2015
|
|
F-5
|
|
|
|
|
|
Consolidated
Statements of Cash Flow for the years ended December 31, 2016
and 2015
|
|
F-6
|
|
|
|
|
|
Notes
to Consolidated Financial Statements
|
|
F-7
|
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Board of Directors and
Stockholders
of Advanced Medical Isotope Corporation
We have
audited the accompanying consolidated balance sheet of Advanced
Medical Isotope Corporation as of December 31, 2016, and the
related consolidated statement of operations, stockholders’
equity (deficit), and cash flows for the year ended December 31,
2016. Advanced Medical Isotope Corporation’s management is
responsible for these consolidated financial statements. Our
responsibility is to express an opinion on these financial
statements based on our audit.
We
conducted our audit in accordance with the standards of the Public
Company Accounting Oversight Board (United States). Those standards
require that we plan and perform the audit to obtain reasonable
assurance about whether the consolidated financial statements are
free of material misstatement. The company is not required to have,
nor were we engaged to perform, an audit of its internal control
over financial reporting. Our audit included consideration of
internal control over financial reporting as a basis for designing
audit procedures that are appropriate in the circumstances, but not
for the purpose of expressing an opinion on the effectiveness of
the company’s internal control over financial reporting.
Accordingly, we express no such opinion. An audit also includes
examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements, assessing the accounting
principles used and significant estimates made by management, as
well as evaluating the overall financial statement presentation. We
believe that our audit provides a reasonable basis for our
opinion.
In our
opinion, the consolidated financial statements referred to above
present fairly, in all material respects, the financial position of
Advanced Medical Isotope Corporation as of December 31, 2016, and
the results of its operations and its cash flows for the year ended
December 31, 2016, in conformity with accounting principles
generally accepted in the United States of America.
The
accompanying consolidated financial statements have been prepared
assuming the Company will continue as a going concern. As discussed
in Note 1 to the consolidated financial statements, the Company has
a history of recurring losses, has limited cash, resources, and,
based upon current operating levels, its viability is dependent
upon its ability to meet future financing requirements or
restructuring to sustain its operations. These issues raise
substantial doubt about the Company’s ability to continue as
a going concern. Management’s plans in regard to these
matters are also described in Note 1. The consolidated financial
statements do not include any adjustments that might result from
the outcome of this uncertainty.
|
/s/Fruci &
Associates II, PLLC
|
|
|
|
|
|
Fruci
& Associates II, PLLC
Spokane,
WA
|
|
|
|
|
|
March
8, 2017
|
|
|
|
|
Report of Independent Registered Public Accounting
Firm
To the
Board of Directors and Shareholders
Advanced
Medical Isotope Corporation
We have
audited the accompanying balance sheet of Advanced Medical Isotope
Corporation as of December 31, 2015, and the related statements of
operations, stockholders’ equity (deficit), and cash flow for
the year then ended. These financial statements are the
responsibility of the Company’s management. Our
responsibility is to express an opinion on these financial
statements based on our audit.
We
conducted our audit in accordance with the standards of the Public
Company Accounting Oversight Board (United States). Those standards
require that we plan and perform the audit to obtain reasonable
assurance about whether the financial statements are free of
material misstatement. The Company is not required to have, nor
were we engaged to perform, an audit of its internal control over
financial reporting. Our audit included consideration of internal
control over financial reporting as a basis for designing audit
procedures that are appropriate in the circumstances, but not for
the purpose of expressing an opinion on the effectiveness of the
Company’s internal control over financial reporting.
Accordingly, we express no such opinion. An audit also includes
examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements, assessing the accounting
principles used and significant estimates made by management, as
well as evaluating the overall financial statement presentation. We
believe that our audit provides a reasonable basis for our
opinion.
In our
opinion, the financial statements referred to above present fairly,
in all material respects, the financial position of Advanced
Medical Isotope Corporation as of December 31, 2015, and the
results of its operations and its cash flows for the year then
ended, in conformity with U.S. generally accepted accounting
principles.
The
accompanying financial statements have been prepared assuming that
the Company will continue as a going concern. As discussed in Note
1 to the financial statements, the Company has suffered recurring
losses, used significant cash in support of its operating
activities, and, based upon current operating levels, requires
additional capital or significant restructuring to sustain its
operations for the foreseeable future. These issues raise
substantial doubt about the Company’s ability to continue as
a going concern. Management’s plans in regard to these
matters are also described in Note 1. The financial statements do
not include any adjustments that might result from the outcome of
this uncertainty.
Haynie
& Company
Salt
Lake City, Utah
May 25,
2016
Advanced Medical Isotope Corporation
Consolidated Balance Sheets
|
|
December
31,
|
|
|
|
2016
|
2015
|
|
ASSETS
|
|
|
|
|
|
|
|
Current
assets:
|
|
|
|
Cash
|
$27,889
|
$179,032
|
|
Prepaid
expenses
|
11,990
|
26,211
|
|
Inventory
|
-
|
8,475
|
|
Total
current assets
|
39,879
|
213,718
|
|
|
|
|
|
Fixed
assets, net of accumulated depreciation
|
1,473
|
4,420
|
|
|
|
|
|
Other
assets:
|
|
|
|
Patents
and intellectual property
|
-
|
35,482
|
|
Deposits
|
644
|
644
|
|
Total
other assets
|
644
|
36,126
|
|
|
|
|
|
Total
assets
|
$41,996
|
$254,264
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
Current
liabilities:
|
|
|
|
Accounts payable
and accrued expenses
|
$1,137,086
|
$1,182,112
|
|
Related
party accounts payable
|
109,718
|
102,647
|
|
Accrued
interest payable
|
114,755
|
229,246
|
|
Payroll
liabilities payable
|
499,502
|
298,900
|
|
Convertible notes
payable, net
|
544,508
|
1,788,384
|
|
Derivative
liability
|
324,532
|
4,235,016
|
|
Related
party promissory note
|
332,195
|
1,280,450
|
|
Liability for lack
of authorized shares
|
-
|
852,091
|
|
Total
current liabilities
|
3,062,296
|
9,968,846
|
|
|
|
|
|
Total
liabilities
|
3,062,296
|
9,968,846
|
|
|
|
|
|
Commitments and contingencies
|
|
|
|
|
|
|
|
MEZZANINE EQUITY
|
|
|
|
Preferred stock,
$.001 par value, 20,000,000 shares of authorized preferred stock,
$.001 par value, 5,000,000 Series A shares
authorized; 3,773,592 and 1,627,000 - shares issued and
outstanding, respectively
|
14,144,571
|
4,617,052
|
|
Total
mezzanine equity
|
14,144,571
|
4,617,052
|
|
Stockholders’
equity (deficit):
|
|
|
|
Common
stock, $.001 par value; 2,000,000,000 shares authorized; 31,743,797
and 19,969,341 shares issued and outstanding,
respectively
|
31,744
|
19,969
|
|
Paid in
capital
|
40,672,825
|
33,662,942
|
|
Accumulated
deficit
|
(57,869,440)
|
(48,014,545)
|
|
Total
stockholders’ equity (deficit)
|
(17,164,871)
|
(14,331,634)
|
|
|
|
|
|
Total
liabilities and stockholders’ equity (deficit)
|
$41,996
|
$254,264
|
The
accompanying notes are an integral part of these consolidated
financial statements.
Advanced Medical Isotope Corporation
Consolidated Statements of Operations
|
|
Year
ended
|
|
|
|
December
31,
|
|
|
|
2016
|
2015
|
|
Consulting
revenues
|
$8,108
|
$24,108
|
|
|
|
|
|
Operating
expenses
|
|
|
|
Cost of
materials
|
-
|
474
|
|
Sales and marketing
expenses
|
284,138
|
-
|
|
Depreciation and
amortization expense
|
2,947
|
5,672
|
|
Professional
fees
|
2,068,796
|
741,375
|
|
Stock based
compensation
|
675,324
|
80,635
|
|
Payroll
expenses
|
652,877
|
679,259
|
|
Research and
development
|
328,026
|
149,650
|
|
General and
administrative expenses
|
432,470
|
408,306
|
|
Total operating
expenses
|
4,444,578
|
2,065,371
|
|
|
|
|
|
Operating
loss
|
(4,436,470)
|
(2,041,263)
|
|
|
|
|
|
Non-operating
income (expense):
|
|
|
|
Interest
expense
|
(6,259,467)
|
(3,196,153)
|
|
Gain on settlement
of debt
|
3,108,342
|
3,562,067
|
|
Grant
income
|
21,010
|
21,010
|
|
Gain (loss) on
derivative liability
|
(2,244,353)
|
7,887,025
|
|
Loss on impaired
assets
|
(43,957)
|
-
|
|
|
|
|
|
Non-operating
income (expense), net
|
(5,418,425)
|
8,273,949
|
|
|
|
|
|
Income (loss)
before income taxes
|
(9,854,895)
|
6,232,686
|
|
|
|
|
|
Income tax
provision
|
-
|
-
|
|
|
|
|
|
Net income
(loss)
|
$(9,854,895)
|
$6,232,686
|
|
|
|
|
|
Earnings (loss) per
common share
|
$(0.46)
|
$0.34
|
|
|
|
|
|
Weighted average
number of common shares outstanding
|
21,497,069
|
18,505,467
|
The
accompanying notes are an integral part of these consolidated
financial statements.
Advanced
Medical Isotope Corporation
Consolidated Statements of Changes in Stockholders’ Equity
(Deficit)
|
|
Common
Stock
|
Paid
in
|
Accumulated
|
|
|
|
|
Shares
|
Amount
|
Capital
|
Deficit
|
Total
|
|
Balances
at December 31, 2014
|
17,053,825
|
17,054
|
34,068,009
|
(54,247,231)
|
(20,162,168)
|
|
|
|
|
|
|
|
|
Common stock issued
for:
|
|
|
|
|
|
|
Exercise
of options and warrants
|
1,995,000
|
1,995
|
(1,995)
|
-
|
-
|
|
Loan fees on
convertible debt
|
43,326
|
43
|
6,344
|
-
|
6,387
|
|
Conversion of
debt
|
920,516
|
920
|
108,892
|
-
|
109,812
|
|
Classified to
liability due to lack of authorized shares
|
(43,326)
|
(43)
|
(598,943)
|
-
|
(598,986)
|
|
Options issued for
services
|
-
|
-
|
80,635
|
-
|
80,635
|
|
Net
income
|
-
|
-
|
-
|
6,232,686
|
6,232,686
|
|
|
|
|
|
|
|
|
Balances
at December 31, 2015
|
19,969,341
|
$19,969
|
$33,662,942
|
$(48,014,545)
|
$(14,331,634)
|
|
|
|
|
|
|
|
|
Common stock issued
for:
|
|
|
|
|
|
|
Exercise
of options and warrants
|
30,644
|
31
|
(31)
|
-
|
-
|
|
Settlement of
debt
|
563,523
|
564
|
70,299
|
-
|
70,863
|
|
Conversion of
preferred stock
|
11,180,289
|
11,180
|
4817024
|
-
|
4,828,204
|
|
Classified to
liability due to lack of authorized shares
|
-
|
-
|
852,092
|
-
|
852,092
|
|
Options and
warrants issued for services
|
-
|
-
|
1,270,499
|
-
|
1,270,499
|
|
Net
loss
|
-
|
-
|
-
|
(9,854,895)
|
(9,854,895)
|
|
|
|
|
|
|
|
|
Balances
at December 31, 2016
|
31,743,797
|
31,744
|
40,672,825
|
(57,869,440)
|
(17,164,871)
|
The
accompanying notes are an integral part of these consolidated
financial statements.
Advanced Medical Isotope Corporation
Consolidated Statements of Cash Flows
|
|
Year
ended December 31,
|
|
|
|
2016
|
2015
|
|
CASH
FLOW FROM OPERATING ACTIVITIES:
|
|
|
|
Net income
(loss)
|
$(9,854,895)
|
$6,232,686
|
|
Adjustments to
reconcile net income (loss) to net cash used by operating
activities:
|
|
|
|
Depreciation of
fixed assets
|
2,947
|
4,333
|
|
Amortization of
licenses and intangible assets
|
-
|
1,339
|
|
Amortization of
convertible debt discount
|
604,042
|
1,080,771
|
|
Amortization of
prepaid expenses paid with stock
|
(2,500)
|
-
|
|
Amortization of
debt issuance costs
|
-
|
13,917
|
|
(Gain) loss on
derivative liability
|
2,244,353
|
(7,887,025)
|
|
(Gain) on
settlement of debt
|
(3,108,342)
|
(3,562,067)
|
|
Loss on impaired
assets
|
43,957
|
-
|
|
Preferred stock
issued for services
|
1,003,814
|
334,880
|
|
Preferred stock
issued for loan fees
|
162,456
|
527,325
|
|
Stock options and
warrants issued for services
|
1,270,499
|
80,635
|
|
New derivatives
recorded as loan fees
|
4,935,638
|
-
|
|
Penalties on notes
payable
|
-
|
1,148,997
|
|
Changes in
operating assets and liabilities:
|
|
|
|
Prepaid
expenses
|
16,721
|
(4,501)
|
|
Accounts
payable
|
102,954
|
151,607
|
|
Payroll
liabilities
|
200,602
|
252,240
|
|
Accrued
interest
|
451,041
|
431,758
|
|
|
|
|
|
Net cash used by
operating activities
|
(1,926,713)
|
(1,193,105)
|
|
|
|
|
|
CASH
FLOWS FROM INVESTING ACTIVITIES
|
-
|
-
|
|
|
|
|
|
CASH
FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
Principal payments
on capital lease
|
-
|
(39,481)
|
|
Proceeds from
convertible note
|
1,273,534
|
1,666,415
|
|
Proceeds from
related party notes
|
723,308
|
-
|
|
Proceeds from
shareholder advances
|
132,533
|
-
|
|
Proceeds from
warrant exercised for preferred stock
|
250
|
-
|
|
Proceeds from sale
of preferred stock
|
70,000
|
-
|
|
Payments on
convertible debt
|
(419,055)
|
-
|
|
Payments on
shareholder advances
|
(5,000)
|
-
|
|
Payments on short
term debt
|
-
|
(255,000)
|
|
|
|
|
|
Net cash provided
by financing activities
|
1,775,570
|
1,371,934
|
|
|
|
|
|
Net increase in
cash
|
(151,143)
|
178,829
|
|
Cash, beginning of
period
|
179,032
|
203
|
|
|
|
|
|
CASH,
END OF PERIOD
|
$27,889
|
$179,032
|
|
|
|
|
|
Supplemental
disclosures of cash flow information:
|
|
|
|
Cash paid for
interest
|
$105,758
|
$9,920
|
|
Cash paid for
income taxes
|
$-
|
$-
|
The
accompanying notes are an integral part of these consolidated
financial statements.
Advanced Medical Isotope Corporation
Notes to Consolidated Financial Statements
For the years ended December 31, 2016 and 2015
NOTE 1: ORGANIZATION & BASIS OF PRESENTATION
Business Overview
In September of 2006, the Company began operating as Advanced
Medical Isotope Corporation (the “Company”). The Company’s
predecessor was incorporated under the
laws of the state of Delaware in 1994. The Company has authorized
capital of 2,000,000,000 shares of common stock, $0.001 par value
per share, and 20,000,000 shares of preferred stock, $0.001 par
value per share.
Our principal place of business is 1021 N Kellogg Street,
Kennewick, Washington, 99336. Our telephone number is (509)
736-4000. Our corporate website address
is http://www.isotopeworld.com. Our common stock is currently
listed for quotation on the OTC Pink Marketplace under the symbol
“ADMD.”
The Company is a late stage radiation oncology medical device
company engaged in the development of its yttrium-90 based
brachytherapy device RadioGel™ for the treatment of
non-resectable tumors. A prominent team of radiochemists,
scientists and engineers, collaborating with strategic partners,
including national laboratories, universities and private
corporations, lead the Company’s development efforts. The
Company’s overall vision is to globally empower physicians,
medical researchers and patients by providing them with new isotope
technologies that offer safe and effective treatments for
cancer.
The Company’s current focus is on the development of its
RadioGel™ device. RadioGel™ is an injectable
particle-gel, for brachytherapy radiation treatment of cancerous
tumors in people and animals. RadioGel™ is comprised of a
hydrogel, or a substance that is liquid at room temperature and
then gels when reaching body temperature after injection into a
tumor. In the gel are small, one micron, yttrium-90 phosphate
particles (“Y-90”). Once injected, these inert particles are
locked in place inside the tumor by the gel, delivering a very high
local radiation dose. The radiation is beta, consisting of
high-speed electrons. These electrons only travel a short distance
so the device can deliver high radiation to the tumor with minimal
dose to the surrounding tissue. Optimally, patients can go home
immediately following treatment without the risk of radiation
exposure to family members. Since Y-90 has a half-life of 2.7 days,
the radioactively drops to 5% of its original value after ten
days.
The Company’s lead brachytherapy products, including
RadioGel™, incorporate patented technology developed for
Battelle Memorial Institute (“Battelle”) at Pacific Northwest National Laboratory,
a leading research institute for government and commercial
customers. Battelle has granted the Company an exclusive license to
patents covering the manufacturing, processing and applications of
RadioGel™ (the “Battelle
License”). Other
intellectual property protection includes proprietary production
processes and trademark protection in 17 countries. The Company
plans to continue efforts to develop new refinements on the
production process, and the product and application hardware, as a
basis for future patents.
The Company is currently focusing on obtaining approval from the
Food and Drug Administration (“FDA”) to market and sell RadioGel™ as a
Class II medical device. The Company first requested FDA approval
of RadioGel™ in June 2013, at which time the FDA classified
RadioGel™ as a medical device. The Company is currently
developing test plans to address issues raised by the FDA in
connection with the Company’s previous submissions regarding
RadioGel™.The Company intends to request FDA approval to
apply for de novo classification of RadioGel™, which would
reclassify the device from a Class III device to a Class II device,
further simplifying the regulatory path.
In previous FDA submittals, the Company proposed applying
RadioGel™ for a very broad range of cancer therapies,
referred to as Indication for Use. The FDA has requested that the
Company reduce its Indications for Use. To comply with that
request, the Company has expanded its Medical Advisory Board
(“MAB”) and engaged doctors from respected
hospitals who have evaluated the candidate cancer therapies based
on three criteria: (1) potential for FDA approval and successful
therapy; (2) notable advantage over current therapies; and (3)
probability of wide spread acceptance by the medical
community.
The MAB selected eighteen applications for RadioGel™, each of
which meet the criteria described above. This large number confirms
the wide applicability of the device and defines the path for
future business growth. The Company intends to apply to the FDA for
a single Indication for Use, followed by subsequent applications
for additional Indications for Use. The initial application should
facilitate each subsequent application, and the testing for many of
the subsequent applications could be conducted in parallel,
depending on available resources.
The MAB selected the treatment of basal cell and squamous cell skin
cancers for the first Indication for Use to be submitted to the
FDA. According to the American Cancer Society, one out of every
three new cancers diagnosed in the U.S. is a cancerous skin lesion
of this type, representing 5.5 million tumors diagnosed annually.
The MAB believes RadioGel™ has the potential to be the
preferred treatment in a reasonable number of cases in a very large
market.
The Company’s, IsoPet Solutions division was established in
May 2016 to focus on the veterinary oncology market. The Company
has engaged four different university veterinarian hospitals to
begin using RadioGel™ for treatment of four different cancer
types in dogs and cats. Washington State University Veterinary
Hospital has tested one cat to demonstrate the procedures and the
absence of any significant toxicity effect. The other three centers
are expected to begin therapy during the second quarter of 2017
after their internal administrative review process is
completed.
These animal therapies will focus on creating labels that describe
the procedures in detail as a guide to future veterinarians. The
labels will be voluntarily submitted to the FDA for review. They
will then be used as data for future FDA applications in the
medical sector and as key intellectual property for licensing to
private veterinary clinics. In 2018, Dr. Alice Villalobos, the
Chair of our Veterinarian Advisory Board, will be the first
licensee of these therapies in her private clinic to demonstrate
the business model.
The Company anticipates that future profit will be derived from
direct sales of RadioGel™ and related services, and from
licensing to private medical and veterinary clinics in the U.S. and
internationally.
Going Concern
The
accompanying financial statements have been prepared on a going
concern basis, which contemplates the realization of assets and
satisfaction of liabilities in the normal course of
business. As shown in the accompanying financial
statements, the Company has suffered recurring losses and used
significant cash in support of its operating activities and the
Company’s cash position is not sufficient to support the
Company’s operations. Research
and development of the Company’s brachytherapy product line
has been funded with proceeds from the sale of equity and debt
securities as well as a series of grants. The Company requires
funding of approximately $1.5 million annually to maintain current
operating activities. Over the next 12 to 24 months, the Company
believes it will cost approximately $5.0 million to $10.0 million
to fund: (1) the FDA approval process and initial deployment of the
brachytherapy products, and (2) initiate regulatory approval
processes outside of the United States. The continued deployment of
the brachytherapy products and a worldwide regulatory approval
effort will require additional resources and personnel. The
principal variables in the timing and amount of spending for the
brachytherapy products in the next 12 to 24 months will be the
FDA’s classification of the Company’s brachytherapy
products as Class II or Class III devices (or otherwise) and any
requirements for additional studies which may possibly include
clinical studies. Thereafter, the principal variables in the amount
of the Company’s spending and its financing requirements
would be the timing of any approvals and the nature of the
Company’s arrangements with third parties for manufacturing,
sales, distribution and licensing of those products and the
products’ success in the U.S. and elsewhere. The Company
intends to fund its activities through strategic transactions such
as licensing and partnership agreements or additional capital
raises.
Following receipt of required regulatory approvals and financing,
in the U.S., the Company intends to outsource material aspects of
manufacturing, distribution, sales and marketing. Outside of the
U.S., the Company intends to pursue licensing arrangements and/or
partnerships to facilitate its global commercialization
strategy.
In the longer-term, subject to the Company receiving adequate
funding, regulatory approval for RadioGel™ and other
brachytherapy products, and thereafter being able to successfully
commercialize its brachytherapy products, the Company intends to
consider resuming research efforts with respect to other products
and technologies intended to help improve the diagnosis and
treatment of cancer and other illnesses
Based on the Company’s financial history since inception, its
auditor has expressed substantial doubt as to the Company’s
ability to continue as a going concern. The Company has limited
revenue, nominal cash, and has accumulated deficits since
inception. If the Company cannot obtain sufficient additional
capital, the Company will be required to delay the implementation
of its business strategy and may not be able to continue
operations.
As of
December 31, 2016 the Company has $27,889 cash on hand. There are
currently commitments to vendors for products and services
purchased, plus, the employment agreements of the CFO and other
employees of the Company and the Company’s current lease
commitments that will necessitate liquidation of the Company if it
is unable to raise additional capital. The current level of cash is
not enough to cover the fixed and variable obligations of the
Company.
Assuming
the Company is successful in the Company’s sales/development
effort, it believes that it will be able to raise additional funds
through strategic agreements or the sale of the Company’s
stock to either current or new stockholders. There is no guarantee
that the Company will be able to raise additional funds or to do so
at an advantageous price.
The
financial statements do not include any adjustments relating to the
recoverability and classification of liabilities that might be
necessary should the Company be unable to continue as a going
concern. The Company’s continuation as a going
concern is dependent upon its ability to generate sufficient cash
flow to meet its obligations on a timely basis and ultimately to
attain profitability. The Company plans to seek
additional funding to maintain its operations through debt and
equity financing and to improve operating performance through a
focus on strategic products and increased efficiencies in business
processes and improvements to the cost structure. There
is no assurance that the Company will be successful in its efforts
to raise additional working capital or achieve profitable
operations. The financial statements do not include any
adjustments that might result from the outcome of this
uncertainty.
NOTE 2: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The
preparation of financial statements in accordance with generally
accepted accounting principles requires management to make
estimates and assumptions that affect the reported amounts of
assets and liabilities and the disclosure of contingent assets and
liabilities at the date of financial statements and the reported
amounts of revenues and expenses during the reporting
period. Actual results could differ from those
estimates.
Principles of Consolidation
The accompanying consolidated financial statements include the
accounts of Advanced Medical Isotope Corporation
and its wholly-owned subsidiary
IsoPet Solutions Corporation (“Iso-Pet”). All significant inter-company balances and
transactions have been eliminated in
consolidation.
Financial Statement Reclassification
Certain account balances from prior periods have been reclassified
in these audited consolidated financial statements so as to conform
to current period classifications.
Cash Equivalents
For the
purposes of the statement of cash flows, the Company considers all
highly liquid debt instruments purchased with an original maturity
of three months or less to be cash equivalents.
Inventory
Inventory
is reported at the lower of cost or market, determined using the
first-in, first-out basis, or net realizable value. All inventories
consist of finished goods. The Company had no raw materials or work
in process. The Company had been carrying inventory consisting of
two bottles of O-18 water for a value of $8,475. The Company
determined this water was no longer usable and wrote off the $8,475
value as of December 31, 2016.
Fair Value of Financial Instruments
Fair
Value of Financial Instruments, requires disclosure of the fair
value information, whether or not recognized in the balance sheet,
where it is practicable to estimate that value. As of December 31,
2016 and December 31, 2015, the balances reported for cash, prepaid
expenses, accounts receivable, accounts payable, and accrued
expenses, approximate the fair value because of their short
maturities.
Fair
value is defined as the price that would be received to sell an
asset or paid to transfer a liability in an orderly transaction
between market participants at the measurement date. ASC Topic 820
established a three-tier fair value hierarchy which prioritizes the
inputs used in measuring fair value. The hierarchy gives the
highest priority to unadjusted quoted prices in active markets for
identical assets or liabilities (level 1 measurements) and the
lowest priority to unobservable inputs (level 3 measurements).
These tiers include:
Level
1, defined as observable inputs such as quoted prices for identical
instruments in active markets;
Level
2, defined as inputs other than quoted prices in active markets
that are either directly or indirectly observable such as quoted
prices for similar instruments in active markets or quoted prices
for identical or similar instruments in markets that are not
active; and
Level
3, defined as unobservable inputs in which little or no market data
exists, therefore requiring an entity to develop its own
assumptions, such as valuations derived from valuation techniques
in which one or more significant inputs or significant value
drivers are unobservable.
The
Company measures certain financial instruments at fair value on a
recurring basis. Assets and liabilities measured at fair value on a
recurring basis were calculated using the Black-Scholes pricing
model and are as follows at December 31, 2016:
|
|
Total
|
Level
1
|
Level
2
|
Level
3
|
|
Assets:
|
|
|
|
|
|
Total Assets
Measured at Fair Value
|
$-
|
$-
|
$-
|
$-
|
|
|
|
|
|
|
|
Liabilities:
|
|
|
|
|
|
Derivative
Liability
|
324,532
|
-
|
-
|
324,532
|
|
Total Liabilities
Measured at Fair Value
|
$324,532
|
$-
|
$-
|
$324,532
|
Assets
and liabilities measured at fair value on a recurring basis are as
follows at December 31, 2015:
|
|
Total
|
Level
1
|
Level
2
|
Level
3
|
|
Assets
|
|
|
|
|
|
Total Assets
Measured at Fair Value
|
$-
|
$-
|
$-
|
$-
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
Liability for lack
of authorized shares
|
852,091
|
-
|
-
|
852,091
|
|
Derivative
Liability
|
4,235,016
|
-
|
-
|
4,235,016
|
|
Total Liabilities
Measured at Fair Value
|
$5,087,107
|
$-
|
$-
|
$5,087,107
|
Fixed Assets
Fixed
assets are carried at the lower of cost or net realizable value.
Production equipment with a cost of $2,500 or greater and other
fixed assets with a cost of $1,500 or greater are capitalized.
Major betterments that extend the useful lives of assets are also
capitalized. Normal maintenance and repairs are charged to expense
as incurred. When assets are sold or otherwise disposed of, the
cost and accumulated depreciation are removed from the accounts and
any resulting gain or loss is recognized in
operations.
Depreciation
is computed using the straight-line method over the following
estimated useful lives:
|
Production
equipment:
|
3 to 7
years
|
|
Office
equipment:
|
2 to 5
years
|
|
Furniture
and fixtures:
|
2 to 5
years
|
Leasehold
improvements and capital lease assets are amortized over the
shorter of the life of the lease or the estimated life of the
asset.
Management
of the Company reviews the net carrying value of all of its
equipment on an asset by asset basis whenever events or changes in
circumstances indicate that its carrying amount may not be
recoverable. These reviews consider the net realizable value of
each asset, as measured in accordance with the preceding paragraph,
to determine whether impairment in value has occurred, and the need
for any asset impairment write-down.
License Fees
License
fees are stated at cost, less accumulated amortization.
Amortization of license fees is computed using the straight-line
method over the estimated economic useful life of the
assets.
The
Company made a $10,000 investment in 2010 for an exclusive license
with Battelle Memorial Institute regarding its technology for the
production of a Brachytherapy seed. In August 2010 the Company
entered into a License Agreement for the Patent Rights in the area
of a Brachytherapy seed with a Fast-dissolving Matrix for Optimized
Delivery of Radionuclides. This Agreement calls for a $10,000
nonrefundable fee upon execution, a royalty agreement on sales and
on funds received from any sublicenses. The $10,000 nonrefundable
fee paid upon execution was capitalized as License Fees and is
amortized on the straight-line basis over a three-year life.
Additionally the Agreement calls for a minimum annual
fee.
The
Company agreed for a mutual termination of this license effective
December 31, 2016 and that the $25,000 minimum annual fee for 2016
due January 2017 would not be owed. The Company was carrying
$35,482 in patent costs relating to this license, which was written
off as impaired assets during the twelve months ended December 31,
2016. The Company still has a $7,026 balance owed for patent
expense accrued during 2016.
|
Calendar
Year
|
Minimum
Royalties per Calendar Year
|
|
|
2010
|
$-
|
|
|
2011
|
$-
|
|
|
2012
|
$2,500
|
|
|
2013
|
$5,000
|
(1)
|
|
2014
|
$7,500
|
(2)
|
|
2015
|
$10,000
|
(3)
|
|
2016 and each
calendar year thereafter
|
$25,000
|
(4)
|
|
|
|
|
|
(1) Paid February,
2014
|
|
|
|
(2) Paid February,
2015
|
|
|
|
(3) Paid February,
2016
|
|
|
|
(4) No longer owed
due to termination of license agreement effective December 31,
2016
|
|
|
The
Company made a $5,000 investment in February 2011 for a one-year
option agreement to negotiate an exclusive license agreement with
Battelle Memorial Institute regarding its patents for the
production of RadioGel™. This option agreement
calls for a $5,000 upfront fee for the option, which expired
February 2012 and was fully expensed in the twelve months ended
December 31, 2011. Effective March 2012, the Company entered
into an exclusive license agreement with Battelle Memorial
Institute regarding the use of its patented RadioGel™
technology. This license agreement calls for a $17,500
nonrefundable license fee and a royalty based on a percent of gross
sales for licensed products sold; the license agreement also
contains a minimum royalty amount to be paid each year starting
with 2013.
|
Calendar
Year
|
Minimum
Royalties per Calendar Year
|
|
|
2012
|
$-
|
|
|
2013
|
$5,000
|
(1)
|
|
2014
|
$7,500
|
(2)
|
|
2015
|
$10,000
|
(3)
|
|
2016
|
$10,000
|
(4)
|
|
|
|
|
|
2017 and each
calendar year thereafter
|
$25,000
|
|
|
(1) Paid February,
2014
|
|
|
|
(2) Paid February,
2015
|
|
|
|
(3) Paid February,
2016
|
|
|
|
(4) $5,399 was paid
January 2017 and the remaining $4,601 was paid February
2017.
|
|
|
The
Company periodically reviews the carrying values of capitalized
license fees and any impairments are recognized when the expected
future operating cash flows to be derived from such assets are less
than their carrying value.
Amortization
is computed using the straight-line method over the estimated
useful live of three years. Amortization of license fees was $0,
and $1,339 for the years ended December 31, 2016, and 2015,
respectively.
Patents and Intellectual Property
The
Company had a total $35,482 of capitalized patents and intellectual
property costs at December 31, 2015 for the patent rights in the
area of a Brachytherapy seed with a Fast-dissolving Matrix for
Optimized Delivery of Radionuclides. Effective December 31, 2016
the Company agreed to terminate this non-utilized patent license
for which the $35,482 of capitalized patent and intellectual costs
applied and therefore the Company wrote off $35,482 of capitalized
costs in the twelve months ending December 31, 2016.
Revenue Recognition
The
Company recognized revenue related to product sales when (i)
persuasive evidence of the arrangement exists, (ii) shipment has
occurred, (iii) the fee is fixed or determinable, and (iv)
collectability is reasonably assured.
Revenue
for the fiscal year ended December 31, 2016 and December 31, 2015
consisted of consulting revenue. The Company recognizes revenue
once an order has been received and shipped to the customer or
services have been performed. Prepayments, if any, received from
customers prior to the time products are shipped are recorded as
deferred revenue. In these cases, when the related products are
shipped, the amount recorded as deferred revenue is recognized as
revenue. The Company does not accrue for sales returns and other
allowances as it has not experienced any returns or other
allowances.
Income from Grants and Deferred Income
Government
grants are recognized when all conditions of such grants are
fulfilled or there is reasonable assurance that they will be
fulfilled. The Company has chosen to recognize income from grants
as it incurs costs associated with those grants, and until such
time as it recognizes the grant as income those funds received will
be classified as deferred income on the balance sheet.
On
September 1, 2015, the Company received notification it had been
awarded from Washington State University, $42,019 grant funds from
the sub-award project entitled “Optimized Injectable
Radiogels for High-dose Therapy of Non-Resectable Solid
Tumors”. The Company received $21,010 and $21,009 of the
grand award in the twelve months ended December 31, 2016 and 2015,
respectively.
Earnings (Loss) Per Share
The
Company accounts for its earnings (loss) per common share by
replacing primary and fully diluted earnings per share with basic
and diluted earnings per share. Basic earnings (loss) per share is
computed by dividing income (loss) available to common stockholders
(the numerator) by the weighted-average number of common shares
outstanding (the denominator) for the period, and does not include
the impact of any potentially dilutive common stock equivalents
since the impact would be anti-dilutive. The computation of diluted
earnings per share is similar to basic earnings per share, except
that the denominator is increased to include the number of
additional common shares that would have been outstanding if
potentially dilutive common shares had been issued. However, since
the Company does not have sufficient authorized shares of common
stock, we did not include these in our calculation.
Securities,
all of which represent common stock equivalents, that could be
dilutive in the future as of December 31, 2016 and 2015, are as
follows:
|
|
December
31,
2016
|
December
31,
2015
|
|
Convertible
debt
|
6,441,644
|
7,848,421
|
|
Preferred
stock
|
37,735,920
|
16,270,000
|
|
Common stock
options
|
2,402,500
|
51,350
|
|
Common stock
warrants
|
3,579,505
|
6,791,003
|
|
Total potential
dilutive securities
|
50,159,569
|
30,960,774
|
Research and Development Costs
Research
and developments costs, including salaries, research materials,
administrative expenses and contractor fees, are charged to
operations as incurred. The cost of equipment used in research and
development activities which has alternative uses is capitalized as
part of fixed assets and not treated as an expense in the period
acquired. Depreciation of capitalized equipment used to perform
research and development is classified as research and development
expense in the year computed.
The
Company incurred $328,026 and $149,650 research and development
costs for the years ended December 31, 2016, and 2015,
respectively, all of which were recorded in the Company’s
operating expenses noted on the income statements for the years
then ended.
Advertising and Marketing Costs
Advertising
and marketing costs are expensed as incurred except for the cost of
tradeshows which are deferred until the tradeshow occurs. Tradeshow
expenses incurred and not expensed as of the years ended December
31, 2016, and 2015 were $0 and $14,800, respectively. During the
twelve months ended December 31, 2016 and 2015, the Company
incurred $284,138 and $0, respectively, in advertising and
marketing costs.
Shipping and Handling Costs
Shipping
and handling costs are expensed as incurred and included in cost of
materials.
Legal Contingencies
In the
ordinary course of business, the Company is involved in legal
proceedings involving contractual and employment relationships,
product liability claims, patent rights, and a variety of other
matters. The Company records contingent liabilities resulting from
asserted and unasserted claims against it, when it is probable that
a liability has been incurred and the amount of the loss is
reasonably estimable. The Company discloses contingent liabilities
when there is a reasonable possibility that the ultimate loss will
exceed the recorded liability. Estimated probable losses require
analysis of multiple factors, in some cases including judgments
about the potential actions of third-party claimants and courts.
Therefore, actual losses in any future period are inherently
uncertain. Currently, the Company does not believe any probable
legal proceedings or claims will have a material impact on its
financial position or results of operations. However, if actual or
estimated probable future losses exceed the Company’s
recorded liability for such claims, it would record additional
charges as other expense during the period in which the actual loss
or change in estimate occurred.
There
had been an ongoing dispute with the landlord, Rob and Maribeth
Myers, regarding the production center rent. During
2016, the Company reached a Settlement Agreement with regards to
this dispute resulting in a payment of $438,830 for rent, interest,
and costs.
There
is an ongoing dispute with BancLeasing and Washington Trust Bank
regarding application of lease payments to the principal loan
amount for the linear accelerator, and the Company believed it
overpaid by approximately $300,000. In 2016 the Company was awarded
in the Superior Court of the State of Washington a total sum of
$527,876 against BancLeasing. The Company is pursuing its options
for collection of the awarded amount, however there can be no
assurance as to any eventual collection.
Income Taxes
To
address accounting for uncertainty in tax positions, the Company
clarifies the accounting for income taxes by prescribing a minimum
recognition threshold that a tax position is required to meet
before being recognized in the financial statements. The Company
also provides guidance on de-recognition, measurement,
classification, interest, and penalties, accounting in interim
periods, disclosure and transition.
The
Company files income tax returns in the U.S. federal jurisdiction,
and Delaware. The Company did not have any tax expense
for the years ended December 31, 2016 and 2015. The
Company did not have any deferred tax liability or asset on its
balance sheet on December 31, 2016 and 2015.
Interest
costs and penalties related to income taxes, if any, will be
classified as interest expense and general and administrative
costs, respectively, in the Company’s financial statements.
For the years ended December 31, 2016 and 2015, the Company did not
recognize any interest or penalty expense related to income taxes.
The Company believes that it is not reasonably possible for the
amounts of unrecognized tax benefits to significantly increase or
decrease within the next 12 months.
Stock-Based Compensation
The
Company recognizes in the financial statements compensation related
to all stock-based awards, including stock options, based on their
estimated grant-date fair value. The Company has estimated expected
forfeitures and is recognizing compensation expense only for those
awards expected to vest. All compensation is recognized by the time
the award vests.
The
Company accounts for equity instruments issued in exchange for the
receipt of goods or services from non-employees. Costs are measured
at the fair market value of the consideration received or the fair
value of the equity instruments issued, whichever is more reliably
measurable. The value of equity instruments issued for
consideration other than employee services is determined on the
earlier of the date on which there first exists a firm commitment
for performance by the provider of goods or services or on the date
performance is complete. The Company recognizes the fair
value of the equity instruments issued that result in an asset or
expense being recorded by the Company, in the same period(s) and in
the same manner, as if the Company has paid cash for the goods or
services.
Recent Accounting Pronouncements
There
are no recently issued accounting pronouncements that the Company
believes are applicable or would have a material impact on the
financial statements of the Company.
Reclassifications
Certain
prior-year amounts have been reclassified to conform with current
year’s presentation.
NOTE 3: FIXED ASSETS
Fixed
assets consist of the following at December 31, 2016 and
2015:
|
|
December
31,
2016
|
December
31,
2015
|
|
Production
equipment
|
$15,182
|
$1,938,532
|
|
Building
|
-
|
446,772
|
|
Leasehold
improvements
|
-
|
3,235
|
|
Office
equipment
|
14,594
|
32,769
|
|
|
29,776
|
2,421,308
|
|
Less accumulated
depreciation
|
(28,303)
|
(2,416,888)
|
|
|
$1,473
|
$4,420
|
Depreciation
expense for the above fixed assets for the years ended December 31,
2016 and 2015, respectively, was $2,947 and $4,332.
During
the year ended December 31, 2016, the Company abandoned production
equipment, building, leasehold improvements, and office equipment
with an original cost totaling $1,016,532 that had been located in
the production center. Additionally the Company removed the linear
accelerator form the production center and has placed the equipment
into storage. The $1,375,000 original cost of the linear
accelerator was also written off during the year ended December 31,
2016. All of the equipment written off during the year ended
December 31, 2016 had been fully depreciated.
NOTE
4: INTANGIBLE ASSETS
Intangible
assets consist of the following at December 31, 2016 and December
31, 2015:
|
|
December
31,
2016
|
December
31,
2015
|
|
License
Fee
|
$112,500
|
$112,500
|
|
Less accumulated
amortization
|
(112,500)
|
(112,500)
|
|
|
-
|
-
|
|
Patents and
intellectual property
|
-
|
35,482
|
|
|
$-
|
$35,482
|
Amortization
expense for the above intangible assets for the years ended
December 31, 2016 and 2015, respectively, was $0 and
$1,339.
NOTE 5: DEBT ISSUANCE
COSTS
During
the years ending December 31, 2016 and 2015, the Company paid $0
and $5,000, respectively, in cash for debt
arrangements.
The
Company amortizes debt issuance costs on a straight-line basis over
the life of the debt arrangements and recognized $0, and $18,917,
during the years ending December 31, 2016, and 2015,
respectively.
During
the twelve months ending December 31, 2016 and 2015 the Company had
the following activity in their debt issuance cost
account:
|
Debt issuance costs
at December 31, 2014
|
$13,917
|
|
Cash paid as fees
for debt arrangements
|
5,000
|
|
Amortization of
debt issuance costs
|
(18,917)
|
|
Debt issuance costs
at December 31, 2015
|
-
|
|
Cash paid as fees
for debt arrangements
|
-
|
|
Amortization of
debt issuance costs
|
-
|
|
Debt issuance costs
at December 31, 2016
|
$-
|
NOTE 6: RELATED PARTY TRANSACTIONS
Related Party Promissory Notes
As of
December 31, 2016 and 2015, the Company had the following related
party promissory notes outstanding:
|
|
December 31,
2016
|
December 31,
2015
|
||
|
|
Principal
(net)
|
Accrued
Interest
|
Principal
(net)
|
Accrued
Interest
|
|
September 2015
$1,055,535 Note, 8% interest, due on demand after March
2017
|
$-
|
$-
|
$1,055,535
|
27,299
|
|
September 2015
$142,415 Note, 10% interest, due May 2016
|
-
|
-
|
142,415
|
4,669
|
|
October 2015
$82,500 Note, 10% interest due October 2016
|
82,500
|
10,239
|
82,500
|
1,966
|
|
February 2016
$50,000 Note, 10% interest, due February
2017
|
50,000
|
4,192
|
-
|
-
|
|
May 2016
$109,695 Note, 10% interest, due July 2017
|
109,695
|
7,093
|
-
|
-
|
|
May 2016
$90,000 Note, 10% interest, due May 2017
|
90,000
|
5,425
|
-
|
-
|
|
Total Convertible
Notes Payable, Net
|
$332,195
|
$26,949
|
$1,280,450
|
$33,934
|
During
the year ending December 31, 2016, the Company received proceeds
from new related party promissory notes of $249,695 and recorded
conversions of $1,197,950 for related party note
principal.
During
the year ending December 31, 2015 the Company received proceeds
from new related party promissory notes of $224,915. Additionally,
in September of 2015 multiple convertible notes were rolled in
together for a total new non-convertible promissory note of
$1,055,532.
Related Party Convertible Notes Payable
During
the year ending December 31, 2016, the Company received proceeds
from new related party convertible notes of $473,613 and, at
inception, issued 37,906 shares of Series A Convertible Preferred
Stock (“Series A
Preferred”) as loan fees valued at $29,156. Each of
the Company’s related party convertible notes have a
conversion rate that is variable, therefore the Company has
accounted for such conversion features as derivative instruments
(see Note 9). As a result of recording the preferred stock and the
derivative liabilities at note inception, the Company increased the
debt discount recorded on their convertible notes by $473,613 and
recorded $1,912,587 in additional interest expense. During the year
ending December 31, 2016, the Company recorded amortization of
$25,000 on their related party convertible note debt discounts,
recorded conversions of $473,613 for related party note principal,
and recorded $448,613 of debt discount write-offs for early debt
conversion. As a result of all related party convertible notes
being converted during the year ending December 31, 2016, the
related party convertible notes payable balance was $0 as of
December 31, 2016.
During
the year ending December 31, 2015, the Company received proceeds
from new related party convertible notes of $156,500. Of that
$156,500, $113,500 was convertible at a fixed rate and $43,000 was
convertible at a variable rate.
The new
related party convertible notes of $113,500 were convertible into
the Company’s common stock at $0.001 per share. The
convertible notes were issued with 43,326 shares of common stock
(see Note 11), therefore the Company recorded debt discounts at
inception of $4,896 to allocate the convertible note proceeds to
the common stock. In September of 2015, the $113,500 of new
convertible notes were rolled in together with multiple other notes
for a total new promissory note of $1,055,532 which was not
convertible. The $4,896 of debt discounts were fully amortized
prior to the convertible notes being rolled into the promissory
note.
The new
related party convertible notes of $43,000 were issued with 3,440
shares of Series A preferred stock as loan fees valued at $17,401
(see Note 11). Each of these related party convertible notes have a
conversion rate that is variable, therefore the Company has
accounted for such conversion features as derivative instruments
(see Note 9). As a result of recording the preferred stock and the
derivative liabilities at note inception, the Company increased the
debt discount recorded on these convertible notes by $43,000 and
recorded $17,401 in additional interest expense. During the year
ending December 31, 2016 the Company recorded amortization of
$43,000 on these related party convertible note debt discounts and
recorded conversions of $43,000 for related party note
principal.
During
the year ending December 31, 2015 the Company recorded additional
conversions of $3,638,566 of note principal and amortization of
$6,328 of debt discounts for related party convertible notes that
had been outstanding as of December 31, 2014.
As a
result of all related party convertible notes being rolled into a
non-convertible promissory note or converted during the year ending
December 31, 2015 the related party convertible notes payable
balance was $0 as of December 31, 2015.
Preferred Shares Issued to Officers
During
2016, the Company issued 10,000 shares of its Series A Preferred to
its CFO, representing $54,152, for services (see Note
11).
During
2015, the Company issued the CEO and CFO a combined total of
175,000 shares of Series A Preferred in exchange for a combined
total of $262,500 of accrued and unpaid wages (see Note
11).
Rent Expenses
On July
17, 2007, the Company entered into a five-year lease for its
production center from a less than 5% shareholder. Subsequent to
July 31, 2012, the Company was renting this space on a
month-to-month basis at $11,904 per month. Effective January 1,
2015, the Company’s lease was terminated. There has been an
ongoing dispute with the landlord, Rob and Maribeth Myers,
regarding the production center rent. During 2016, the
Company reached a Settlement Agreement with regards to this dispute
resulting in a payment of $438,830 for rent, interest, and
costs.
The
Company rents office space from a significant shareholder and
director of the Company on a month-to-month basis with a monthly
payment of $1,500.
Rental
expense for the years ended December 31, 2016 and 2015 consisted of
the following:
|
|
Year ended
December 31, 2016
|
Year ended
December 31, 2015
|
|
Office and
warehouse space
|
$40,000
|
$155,306
|
|
Corporate
office
|
18,000
|
18,000
|
|
Total Rental
Expense
|
$58,000
|
$173,306
|
NOTE 7: CAPITAL LEASE OBLIGATIONS
During
September 2007, the Company obtained two master lease agreements
for $1,875,000 and $631,000, with interest on both leases accruing
at 8.6% annually, secured by equipment and personal guarantee of
two of the major stockholders. These long-term agreements shall be
deemed capital lease obligations for purposes of financial
statement reporting. The purpose of the lease is to acquire a
Pulsar 10.5 PET Isotope Production System plus ancillary
equipment.
This
capital lease and its resulting obligation is recorded at an amount
equal to the present value at the beginning of the lease term of
minimum lease payments during the lease term, excluding any portion
of the payments representing taxes to be paid by the Company. This
amount does not exceed the fair value of the leased property at the
lease inception, so the recorded amount is the fair
value.
The
lease requires the Company to maintain a minimum debt service
coverage ratio of 1:1 measured at fiscal year-end. Non-compliance
with this provision shall constitute a default and guarantors must
contribute capital sufficient to fund any deficit in the debt
service coverage ratio.
The
Company fully paid this capital lease obligations during the twelve
months ending December 31, 2015; however there is an ongoing
dispute with the lessor as the Company believes it has overpaid its
lease obligations.
NOTE 8: CONVERTIBLE NOTES PAYABLE
As of
December 31, 2016 and 2015, the Company had the following
convertible notes outstanding:
|
|
December 31,
2016
|
December 31,
2015
|
||
|
|
Principal
(net)
|
Accrued
Interest
|
Principal
(net)
|
Accrued
Interest
|
|
July and August
2012 $1,060,000 Notes convertible into common stock at $4.60 per
share, 12% interest, due December 2013 and January
2014
|
$95,000
|
$50,365
|
$165,000
|
69,712
|
|
January 2014
$50,000 Note convertible into common stock at a variable conversion
price, 8% interest, due January 2015
|
-
|
-
|
50,000
|
7,682
|
|
January 2014
$55,500 Note convertible into common stock at a variable
conversion price, 10% interest, due October 2014
|
-
|
-
|
10,990
|
5,457
|
|
February 2014
$46,080 Note convertible into common stock at a variable conversion
price, 10% interest, due February 2015
|
-
|
-
|
-
|
2,358
|
|
February 2014
$27,800 Note convertible into common stock at a variable conversion
price, 10% one-time interest, due February 2015
|
-
|
-
|
20,000
|
-
|
|
March 2014 $50,000
Note convertible into common stock at a variable conversion price,
10% interest, due March 2015
|
-
|
-
|
36,961
|
6,572
|
|
March 2014
$165,000 Note convertible into common stock at a variable
conversion price, 10% interest, due April 2015
|
-
|
-
|
61,301
|
24,109
|
|
April 2014
$32,000 Note convertible into common stock at a variable
conversion price, 10% interest, due April 2015
|
-
|
-
|
22,042
|
3,034
|
|
April 2014 $46,080
Note convertible into common stock at a variable conversion price,
10% interest due April 2015
|
-
|
-
|
5,419
|
4,608
|
|
May 2014
$55,000 Note convertible into common stock at a variable
conversion price, 12% interest, due May 2015
|
-
|
-
|
25,000
|
1,836
|
|
June 2014 $28,800
Note convertible into common stock at a variable conversion price,
10% interest due June 2015
|
-
|
-
|
28,800
|
2,880
|
|
June 2014 $40,000
Note convertible into common stock at a variable conversion price,
10% interest, due June 2015
|
-
|
-
|
40,000
|
6,049
|
|
June 2014 $40,000
Note convertible into common stock at a variable conversion price,
10% interest, due June 2015
|
-
|
-
|
38,689
|
5,851
|
|
June 2014 $56,092
Note convertible into common stock at a variable conversion price,
16% interest, due July 2015
|
-
|
-
|
56,092
|
13,462
|
|
July 2014 $37,500
Note convertible into common stock at a variable conversion price,
12% interest, due July 2015
|
-
|
-
|
37,015
|
6,377
|
|
August 2014 $36,750
Note convertible into common stock at a variable conversion price,
10% interest, due April 2015
|
-
|
-
|
36,750
|
5,538
|
|
August 2014
$33,500 Note convertible into common stock at a variable
conversion price, 4% interest, due February 2015
|
-
|
-
|
33,500
|
-
|
|
September 2014
$37,500 Note convertible into common stock at a variable conversion
price, 12% interest, due September 2015
|
-
|
-
|
36,263
|
5,576
|
|
May through October
2015 $605,000 Notes convertible into preferred stock at $1 per
share, 8-10% interest, due September 30, 2015
|
-
|
17,341
|
-
|
18,264
|
|
October through
December 2015 $613,000 Notes convertible into preferred stock at $1
per share, 8% interest, due June 30, 2016, net of debt discount of
$0 and $560,913, respectively
|
-
|
5,953
|
52,087
|
2,519
|
|
January through
March 2016 $345,000 Notes convertible into preferred stock at $1
per share, 8% interest, due June 30, 2016
|
-
|
696
|
-
|
-
|
|
November 2016
$979,162 Notes convertible into common stock at a variable
conversion price, 10% interest, due May 2017, net of debt discounts
of $540,720 and $0, respectively
|
438,442
|
12,397
|
-
|
-
|
|
Penalties on
notes in default
|
11,066
|
-
|
1,032,475
|
-
|
|
Total Convertible
Notes Payable, Net
|
$544,508
|
$86,752
|
$1,788,384
|
$191,884
|
During
the year ending December 31, 2016, the Company received proceeds
from new convertible notes of $1,273,534, obtained advances from
shareholders of $127,533 that were reclassified into convertible
notes payable, and reclassified $455,150 of accrued interest into
convertible notes payable. The Company recorded original issue
discounts and loan fees on new convertible notes of $193,831 and
$6,000, respectively, which also increased the debt discounts
recorded on the convertible notes. The Company recorded $419,055 of
payments on their convertible notes, conversions of $1,444,950 of
convertible note principal, a total gain on settlement of
$1,456,113 representing the write-off of convertible note
principal, and $814,625 of debt discount write-offs for early debt
conversion or extinguishment. Each of the Company’s
convertible notes have a conversion rate that is variable. The
Company therefore has accounted for such conversion features as
derivative instruments (see Note 9). As a result of recording
derivative liabilities at note inception, the Company increased the
debt discount recorded on their convertible notes by $809,835
during the year ending December 31, 2016. The Company also recorded
amortization of $579,042 on their convertible note debt discounts.
Lastly, the Company issued 347,400 shares of Series A Preferred as
loan fees with their new convertible notes. The Company therefore
increased their convertible note debt discount by $363,807, which
represented the portion of the convertible note proceeds that were
allocated to preferred stock.
During
the year ending December 31, 2015, the Company received proceeds
from new convertible notes of $1,285,000, recorded default penalties on new
convertible notes of $116,521, and recorded original issue
discounts of $5,000, which also increased the debt discounts
recorded on the convertible notes. The Company recorded $255,000 of
payments on their convertible notes conversions of $584,700 of
convertible note principal, a total loss on settlement of $967,038
representing the write-off of convertible note principal, and
$3,035 of debt discount write-offs for early debt conversion or
extinguishment. Each of the Company’s convertible notes have
a conversion rate that is variable. The Company therefore has
accounted for such conversion features as derivative instruments
(see Note 9). As a result of recording derivative liabilities at
note inception, the Company has increased the debt discount
recorded on their convertible notes by $1,285,000 during the year
ending December 31, 2015. The Company also recorded amortization of
$1,031,443 on their convertible note debt discounts.
NOTE 9: DERIVATIVE LIABILITY
During
the years ending December 31, 2016 and 2015, the Company had the
following activity in their derivative liability
account:
|
|
Warrants
|
Conversion
Feature
|
Total
|
|
Derivative
Liability at December 31, 2014
|
1,263,929
|
10,238,451
|
11,502.380
|
|
Derivative
Liability Recorded on New Instruments
|
-
|
698,705
|
698,705
|
|
Elimination of
Liability on Conversion
|
-
|
(79,044)
|
(79,044)
|
|
Change in Fair
Value
|
(549,528)
|
(7,337,497)
|
(7,887,025)
|
|
Derivative
Liability at December 31, 2015
|
714,401
|
3,520,615
|
4,235,016
|
|
Derivative
Liability Recorded on New Instruments
|
-
|
6,150,026
|
6,150,026
|
|
Elimination of
Liability on Conversion
|
(1,266,795)
|
(10,381,138)
|
(11,647,933)
|
|
(Gain) on
Settlement of Debt
|
-
|
(656,930)
|
(656,930)
|
|
Change in Fair
Value
|
552,452
|
1,691,901
|
(2,244,353)
|
|
Derivative
Liability at December 31, 2016
|
58
|
324,474
|
324,532
|
The
Company uses the Black-Scholes pricing model to estimate fair value
for those instruments convertible into common stock at inception,
at conversion date, and at each reporting date. During
the year ending December 31, 2016, and 2015, the Company used the
following assumptions in their Black-Scholes model:
|
|
|
December
31, 2016
|
|
|
December
31, 2015
|
|
||||||||||
|
|
|
Warrants
|
|
|
Conversion
Feature
|
|
|
Warrants
|
|
|
Conversion
Feature
|
|
||||
|
Risk-free
interest rate
|
|
|
0.46% - 1.38%
|
|
|
|
0.12% -
0.85%
|
|
|
|
0.65% - 1.31%
|
|
|
|
0.02% -
0.25%
|
|
|
Expected
life in years
|
|
|
0.53 -
4.08
|
|
|
|
0.01 -
1.00
|
|
|
|
0.70 -
4.00
|
|
|
|
0.07 -
0.94
|
|
|
Dividend
yield
|
|
|
0%
|
|
|
|
0%
|
|
|
|
0%
|
|
|
|
0%
|
|
|
Expected
volatility
|
|
|
156.29% - 273.70%
|
|
|
|
115.46% - 239.93%
|
|
|
|
197.83% - 263.33%
|
|
|
|
279.01% - 555.60%
|
|
NOTE 10: INCOME TAXES
Deferred
taxes are provided on a liability method whereby deferred tax
assets are recognized for deductible temporary differences and
operating loss and tax credit carry-forwards and deferred tax
liabilities are recognized for taxable temporary differences.
Temporary differences are the differences between the reported
amounts of assets and liabilities and their tax bases. Deferred tax
assets are reduced by a valuation allowance when, in the opinion of
management, it is more likely than not that some portion or all of
the deferred tax assets will not be realized. Deferred tax assets
and liabilities are adjusted for the effects of changes in tax laws
and rates on the date of enactment.
Net
deferred tax assets consist of the following components as of
December 31, 2016 and 2015:
|
|
December
31,
2016
|
December
31,
2015
|
|
Deferred tax
assets:
|
|
|
|
Net operating loss
carryover
|
$8,854,900
|
$8,007,900
|
|
Depreciation
|
-
|
247,900
|
|
Related party
accrual
|
179,400
|
113,400
|
|
Capital Loss
Carryover
|
5,500
|
5,500
|
|
Deferred tax
liabilities
|
|
|
|
Depreciation
|
(197,200)
|
-
|
|
Valuation
allowance
|
(8,842,600)
|
(8,374,700)
|
|
Net deferred tax
asset
|
$-
|
$-
|
|
Net deferred tax
asset$
|
-
|
$-
|
The
income tax provision differs from the amount of income tax
determined by applying the U.S. Federal income tax rate to pretax
income from continuing operations for the years ended December 31,
2016 and 2015 due to the following:
|
|
December
31,
2016
|
December
31,
2015
|
|
Book income
(loss)
|
$(3,350,700)
|
$2,119,100
|
|
Grant
income
|
(7,100)
|
(7,100)
|
|
Depreciation
|
(25,300)
|
(41,500)
|
|
Intangible asset
impairment
|
12,100
|
-
|
|
Related party
accrual
|
65,900
|
(516,100)
|
|
Meals and
entertainment
|
1,600
|
1,600
|
|
Stock for
services
|
341,300
|
113,900
|
|
Options
expense
|
229,600
|
9,700
|
|
Non-cash interest
expense
|
1,976,300
|
581,500
|
|
Other
non-deductable expenses
|
(90,600)
|
(3,112,100)
|
|
Valuation
allowance
|
846,900
|
851,000
|
|
Income tax
expense
|
$-
|
$-
|
At
December 31, 2016, the Company had net operating loss carryforwards
of approximately $26,043,700 that may be offset against future
taxable income from the year 2017 through 2036.
Due to
the change in ownership provisions of the Tax Reform Act of 1986,
net operating loss carryforwards for Federal income tax reporting
purposes are subject to annual limitations. Should a
change in ownership occur, net operating loss carryforwards may be
limited as to use in future years.
Topic
740 provides guidance on the accounting for uncertainty in income
taxes recognized in a company’s financial statements. Topic
740 requires a company to determine whether it is more likely than
not that a tax position will be sustained upon examination based
upon the technical merits of the position. If the
more-likely-than-not threshold is met, a company must measure the
tax position to determine the amount to recognize in the financial
statements. At the adoption date of January 1, 2007, the Company
had no unrecognized tax benefit, which would affect the effective
tax rate if recognized.
The
Company includes interest and penalties arising from the
underpayment of income taxes in the statements of operations in the
provision for income taxes. As of December 31, 2016, the Company
had no accrued interest or penalties related to uncertain tax
positions.
The
Company files income tax returns in the U.S. federal jurisdiction.
The Company is located in the state of Washington and Washington
state does not require the filing of income taxes. With few
exceptions, the Company is no longer subject to U.S. federal, state
and local, or non-U.S. income tax examinations by tax authorities
for years before 2013.
NOTE 11: STOCKHOLDERS’ EQUITY
As of
December 31, 2016 and 2015, the Company has 20,000,000 shares of
Series A Preferred authorized with a par value of $0.001. The
Company’s Board of Directors is authorized to provide for the
issuance of shares of preferred stock in one or more series, to
establish the number of shares in each series, and to determine the
designations, preferences and rights through a resolution of the
Board of Directors.
Effective
June of 2015, the Board of Directors designated the Series A
Preferred as a new series of preferred stock. As of December 31,
2016 and 2015, the Company has 5,000,000 shares of Series A
Preferred authorized, with a par value of $0.001, and as of
December 31, 2016 and 2015, the Company has 3,773,592 and 1,627,000
shares issued and outstanding, respectively. Each Series A
Preferred share is convertible into shares of the Company’s
common stock at a conversion price of $0.50 per share, subject to
adjustment. Each holder of Series A Preferred is entitled to the
equivalent of five votes for every conversion share, where the
conversion shares are the number of common stock the Series A
Preferred would be convertible into. The holders of the Series A
Preferred have a liquidation preference equal to $5.00 per
share.
The
Company has 2,000,000,000 shares of common stock authorized, with a
par value of $0.001, and as of December 31, 2016 and 2015, the
Company has 31,743,797 and 19,969,341 shares issued and
outstanding, respectively.
As of
December 31, 2015, there was an insufficient amount of the
Company’s authorized common stock to satisfy the potential
number of shares that would be required to satisfy the outstanding
options, warrants and convertible debt into common stock. As a
result, the Company recorded a liability in the amount of $852,091,
offset by $852,091 of equity for the year ending December 31, 2015.
Effective October 2016, the Company filed a Certificate of
Amendment to perform a 1:100 reverse stock split which eliminated
the shortage of sufficient authorized common shares. Additionally,
the Company removed the $852,091 liability for lack of authorized
shares and increased paid in capital. The Company’s financial
statements have been retroactively adjusted for all periods
presented to reflect the reverse stock split.
Common Stock Issued for the Exercise of Options and
Warrants
During
2015, the Company issued 1,995,000 shares of common stock for
cashless warrants exercise.
During
2016, the Company issued 30,644 shares of common stock for cashless
warrants exercise.
Common Stock Issued for Loan Fees on Convertible Debt
During
the year ending December 31, 2016, the Company did not issue any
common stock for loan fees.
During
the year ending December 31, 2015, the Company issued 43,326 shares
of common stock for loan fees when entering into related party
convertible notes. The shares were valued at $6,387, of which
$4,896 was allocated to a debt discount (see Note 6) and $1,491 was
recorded as interest expense.
Common Stock Issued for Settlement and Conversion of
Debt
During
2016, the Company issued 563,523 shares of common stock in
conjunction with the settlement of convertible debt that was paid
in cash. The shares were valued at $70,863 and were recognized as a
loss on the settlement of debt.
During
2015, the Company issued 920,516 shares of common stock valued at
$109,812 in exchange for convertible debt raised in 2014 resulting
in a reduction in debt of $31,721, a reduction in derivative
liability of $79,044, with an offset of $3,035 to debt discount and
a $2,083 gain on extinguishment of debt.
Common Stock Issued for Conversion of Preferred Stock
During
2016, the Company issued 11,180,289 shares of common stock valued
at $1,590,801 in exchange for 1,118,024 shares of Series A
Preferred valued at $4,828,204, resulting in an increase in
retained earnings of $3,237,403.
Common
Stock Options
The
following schedule summarizes the changes in the Company’s
stock options:
|
|
|
|
Weighted
|
|
Weighted
|
|
|
Options Outstanding
|
Average
|
|
Average
|
|
|
|
Number
|
Exercise
|
Remaining
|
Aggregate
|
Exercise
|
|
|
Of
|
Price
|
Contractual
|
Intrinsic
|
Price
|
|
|
Shares
|
Per
Share
|
Life
|
Value
|
Per
Share
|
|
|
|
|
|
|
|
|
Balance at
December 31, 2014
|
87,850
|
$9.00-15.00
|
3.42
years
|
$-
|
$14.00
|
|
|
|
|
|
|
|
|
Options
granted
|
-
|
$-
|
-
|
|
$-
|
|
Options
exercised
|
-
|
$-
|
-
|
|
$-
|
|
Options
expired
|
(36,500)
|
$9.00-21.00
|
-
|
|
$12.00
|
|
|
|
|
|
|
|
|
Balance at
December 31, 2015
|
51,350
|
$12.00-15.00
|
7.12
years
|
$-
|
$15.00
|
|
|
|
|
|
|
|
|
Options
granted
|
2,370,000
|
$0.50-1.00
|
-
|
|
$0.62
|
|
Options
exercised
|
-
|
$-
|
-
|
|
$-
|
|
Options
expired
|
(18,850)
|
$0.12-0.15
|
-
|
|
$14.84
|
|
|
|
|
|
|
|
|
Balance at
December 31, 2016
|
2,402,500
|
$0.50-15
|
4.05
years
|
$-
|
$0.81
|
|
|
|
|
|
|
|
|
Exercisable at
December 31, 2016
|
1,939,062
|
$0.50-15
|
3.95
years
|
$-
|
$0.88
|
During
the year ending December 31, 2016 and 2015, the Company recognized
$675,324 and $80,635, respectively, worth of stock based
compensation related to the vesting of it stock
options.
Common Stock Warrants
The
following schedule summarizes the changes in the Company’s
common stock warrants:
|
|
|
|
Weighted
|
|
Weighted
|
|
|
|
Warrants Outstanding
|
Average
|
|
Average
|
||
|
|
Number
|
Exercise
|
Remaining
|
Aggregate
|
Exercise
|
|
|
|
Of
|
Price
|
Contractual
|
Intrinsic
|
Price
|
|
|
|
Shares
|
Per
Share
|
Life
|
Value
|
Per
Share
|
|
|
|
|
|
|
|
|
|
|
Balance at
December 31, 2014
|
23,107,701
|
$0.01-25
|
2.86
years
|
$-
|
$1
|
|
|
|
|
|
|
|
|
|
|
Warrants
granted
|
312,500
|
$0.10-0.15
|
2.94
years
|
|
$0.13
|
|
|
Warrants
exercised
|
(2,856,041)
|
$0.30
|
-
|
|
$0.30
|
|
|
Warrants adjusted
(1)
|
(13,601,951)
|
$0.10-0.15
|
-
|
|
0.01
|
|
|
Warrants
expired/cancelled
|
(158,706)
|
$6.00-25.00
|
-
|
|
$8.76
|
|
|
|
|
|
|
|
|
|
|
Balance at
December 31, 2015
|
6,803,503
|
$0.1-10
|
1.90
years
|
$2,052,699
|
$0.81
|
|
|
|
|
|
|
|
|
|
|
Warrants
granted
|
233,334
|
$0.40
|
1.71
years
|
|
$0.34
|
|
|
Warrants
exercised
|
(202,500)
|
$0.10
|
|
-
|
|
$0.10
|
|
Warrants
expired/cancelled
|
(3,254,832)
|
$0.01-6.00
|
|
-
|
|
$0.13
|
|
|
|
|
|
|
|
|
|
Balance at
December 31, 2016
|
3,579,505
|
$0.01-10
|
0.52
years
|
$749
|
$4.45
|
|
|
|
|
|
|
|
|
|
|
Exercisable at
December 31, 2016
|
3,579,505
|
$0.01-10
|
0.52
years
|
$749
|
$4.45
|
|
(1)
Based upon Delaware
law and on the terms and conditions set forth in the applicable
warrant agreement, any adjustments to the warrants were limited to
a floor price of $0.001. Pursuant to the defective warrant exercise
notice using an exercise price below $0.001, the Company issued at
total of 1,473,777 shares of common stock to the warrant holders,
which the Company believes are voidable, and also recorded
16,009,450 warrants outstanding to the holder on the
Company’s financial statements for the year ended December
31, 2014, and for the periods ending March 31 and June 30,
2015. Management believes that the warrants were recorded in
error during the periods presented, and has recorded the revised
number of warrants outstanding at September 30, 2015 at 2,407,500,
which reflects the number of shares of common stock purchase
warrants outstanding and exercisable under the terms of the
warrants at an exercise price of $0.001 per share. This net
adjustment of 13,601,951 warrants has been reflected in the
schedule for the twelve months ending December 31, 2015. The
Company reached a Settlement Agreement in 2016 with this warrant
holder to cancel all the remaining 2,407,500 warrants in exchange
for 50,000 shares of Series A Preferred.
During
the year ending December 31, 2016 and 2015, the Company recognized
$595,175 and $0, respectively, worth of expense related to warrants
granted for services.
Preferred Stock Issued for Cash
During
2016, the Company issued 46,666 shares of Series A Preferred for
$70,000 cash.
Preferred Stock Issued for Exercise of Warrants
During
2016, the Company issued 250,000 shares of Series A Preferred for
$250 for the exercise of warrants.
Preferred Stock Issued for Warrants Surrendered
During
2016, the Company issued 62,854 shares of Series A Preferred,
representing $407,973, in exchange for the surrender of 2,407,500
warrants. This resulted in the Company writing off $1,179,710 worth
of derivative liabilities and recognizing a gain on debt
extinguishment of $771,737.
Preferred Stock Issued for Services
During
2016, the Company issued 218,000 shares of its Series A Preferred,
representing $942,644, for services valued at $949,661, therefore
recognizing $7,018 of a gain on settlement of debt. During 2016,
the Company issued 10,000 shares of its Series A Preferred to its
CFO, representing $54,152, for services (see Note 6).
During
2015, the Company issued 100,000 shares of its Series A Preferred,
representing $334,880, for services.
Preferred Stock Issued for Loan Fees
During
2016, the Company issued 30,000 shares of its Series A Preferred,
valued at $162,456, as a finders fee and issued 37,906 shares of
its Series A Preferred, valued at $29,156 (see Note 6), as loan
fees on related party convertible notes. The Company also issued
347,400 shares of its Series A Preferred, valued at $363,807, as
loan fees on convertible notes recorded as debt discount (see Note
8), and issued 47,840 shares of its Series A Preferred, valued at
$39,904, as loan fees on convertible notes recorded as derivative
liabilities.
During
2015, the Company issued 133,833 shares of its Series A Preferred,
valued at $527,325, as loan fees on convertible notes and issued
3,440 shares of its Series A Preferred, valued at $17,401 (see Note
6), as loan fees on related party convertible notes.
Preferred Stock Issued for Debt Extinguishment
During
2016, the Company issued 215,961 shares of Series A Preferred,
valued at $1,401,760, in exchange for $1,197,950 of related party
debt, and $59,671 of accrued interest (see Note 6). This resulted
in the Company recognizing $144,139 as a loss on debt
extinguishment.
During
2016, the Company issued 473,830 shares of Series A Preferred,
valued at $2,330,876, in exchange for $473,613 of related party
convertible debt, and $0 of accrued interest (see Note 6). This
resulted in the Company writing off $2,330,154 in derivative
liabilities, $448,613 in debt discounts, and recognizing $24,278 as
a gain on debt extinguishment.
During
2016, the Company issued 1,460,600 shares of Series A Preferred,
valued at $8,552,746 in exchange for $1,444,950 of convertible
debt, and $50,711 of accrued interest (see Note 8). This resulted
in the Company writing off $8,138,070 in derivative liabilities,
$814,625 in debt discounts, and recognizing $266,358 as a gain on
debt extinguishment.
In
September 2015, the Company issued 148,310 shares of Series A
Preferred in exchange for $1,414,100 of related party debt plus
$810,538 of accrued interest and 207,620 shares of Series A
preferred stock in exchange for $2,224,466 of related party debt
plus $889,838 of accrued interest, to the major shareholder and
director of the Company (see Note 6).
In
September 2015, the Company issued 43,000 shares of Series A
Preferred in exchange for $43,000 of related party convertible debt
raised during 2015 (see Note 6).
In
November and December 2015, the Company issued 99,000 shares of
Series A Preferred in exchange for $22,700 of convertible debt,
$9,812 of accrued interest, and extension of a due date for a
payment on a debt settled. In September 2015 the Company issued
552,000 shares of Series A Preferred in exchange for $552,000 of
convertible debt raised during 2015. In October 2015 the Company
issued 10,000 shares of Series A Preferred in exchange for $10,000
of convertible debt raised during 2015 (see Note 8).
Preferred Stock Issued for Accounts Payable and Accrued
Liabilities
In
December 2015, the Company issued 100,000 and 75,000 shares of
Series A Preferred to the CEO and CFO, in exchange for $150,000 and
$112,500, respectively, of accrued payroll (see Note 6). In
December 2015, the Company issued 246,797 shares of Series A
Preferred in exchange for $370,197 of accounts
payable.
NOTE 12: CONCENTRATIONS OF CREDIT AND OTHER RISKS
Accounts Receivable
The
Company had one customer that represented 100% of the
Company’s total revenues for each of the years ended December
31, 2016 and 2015. The customer that represented 100% of the
Company’s total revenue for the years ended December 31, 2016
and 2015, and accounted for 100% of the consulting revenue for that
year. The Company had no net accounts receivable balance at
December 31, 2016 and 2015.
The
loss of a significant customer representing the percentage of total
revenue as represented for the years ended December 31, 2016 and
2015 would have a temporary adverse effect on the Company’s
revenue, which would continue until the Company located new
customers to replace them.
The
Company routinely assesses the financial strength of its customers
and provides an allowance for doubtful accounts as necessary. As of
December 31, 2016 and 2015, the Company had no allowance or bad
debt expense recorded.
NOTE 13: SUPPLEMENTAL CASH FLOW INFORMATION
During
the year ended December 31, 2016, the Company had the following
non-cash investing and financing activities:
-
Issued 30,644
shares of common stock valued at $0 for the issuance of cashless
warrants.
-
Decreased related
party notes by $1,197,950, decrease related party convertible notes
by $473,613, offset by a decrease $448,613 in debt discount
decreased convertible notes payable by $1,444,950, offset by a
decrease $814,624 in debt discount, decreased accrued interest by
$110,382, decreased derivative liabilities by $10,468,224, and
increased Series A Preferred by $12,431,882 due to 2,150,391 shares
issued in conjunction with the settlement of convertible
notes.
-
Increased
convertible notes payable and decreased accrued interest by
$455,150 for the reclassification of accrued interest to
principal.
-
Issued 62,854
shares of Series A Preferred, valued at $407,973, which decreased
derivative liabilities by $1,179,710 and for which a gain on debt
extinguishment was recorded for $771,737.
-
Increased
derivative liabilities for $1,283,448 to record a debt discount on
related party convertible notes of $473,613 and a debt discount on
convertible notes of $809,835.
-
Increased paid in
capital and decreased liability for lack of authorized shares for
$852,092.
-
Increased
convertible notes payable and decreased loan from shareholder by
$127,533 to roll proceeds from shareholder advances to a formal
convertible note payable.
-
Issued 433,146
shares of Series A Preferred, valued at $432,866, for loan fees
that increased the convertible note debt discount by $363,807 and
increased derivative liabilities by $69,059.
-
Issued 11,180,289
shares of common stock valued at $1,590,801 in exchange for
1,118,024 shares of Series A Preferred valued at $4,828,204,
resulting in an increase in retained earnings of
$3,237,403.
During
the year ended December 31, 2015, the Company had the following
non-cash investing and financing activities:
-
Issued 1,995,000
shares of common stock valued at $0 for the issuance of cashless
warrants.
-
Issued 43,326
shares of common stock as loan fees on convertible
debt.
-
Issued 108,833
shares of preferred stock as loan fees on convertible
debt.
-
Issued 920,516
shares of common stock for an extinguishment of $31,721 worth of
principal on convertible notes payable $0 worth of accrued
interest, $79,044 worth of derivative liabilities and $3,035 worth
of debt discount.
-
Issued 74,000
shares of preferred stock for an extinguishment of $22,700 worth of
principal on convertible notes payable $0 worth of accrued
interest, $0 worth of derivative liabilities and $0 worth of debt
discount.
-
Issued 960,929
shares of preferred stock valued at $2,201,963 in exchange for
$4,243,566 of notes.
-
Issued 421,797
shares of preferred stock valued at $1,358,726 in exchange for
$370,197 of accounts payable and $262,500 of accrued
payroll.
-
Issued 25,000
shares of preferred stock as loan extension fees.
NOTE 14: SUBSEQUENT EVENTS
In
January, February and through March 6, 2017, the Company issued
5,517,900 shares of common stock for 531,790 shares of Series A
Preferred.
In
February 2017, the Company issued 280,000 shares of common stock
shares in exchange for $140,000 of accounts payable.
In
March 2017, the Company received $25,000 as a shareholder
loan.
The Company has evaluated subsequent events pursuant to ASC Topic
855 and has determined that there are no additional subsequent
events to disclose.
F-27
