Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 PRESS RELEASE - Ocera Therapeutics, Inc. | oceratoannounceadditionalr.htm |
| 8-K - 8-K COWEN CONFERENCE MARCH 8 2017 - Ocera Therapeutics, Inc. | ocera-8xkcowenconference38.htm |

NASDAQ: OCRX
M A R C H 2 0 1 7
37th Annual Cowen Healthcare Conference
The Boston Marriott Copley Place
March 6-8, 2017
A Liver Disease Medicines Company

2
Forward-Looking Statements
Certain statements in this presentation constitute “forward-looking statements” within the meaning of
the Securities Act of 1933, as amended (the “Securities Act”), and Securities Exchange Act of 1934, as
amended (“Exchange Act”), including, without limitation, all statements related to the OCR-002 clinical
development program, the timing of our planned meeting with the FDA, our ability to identify a path
forward for OCR-002, whether any future studies of OCR-002 will demonstrate similar results to our Phase
2b study, and the size of the potential market opportunity for OCR-002, and we intend these forward-
looking statements to be covered by the safe harbor provisions for forward-looking statements contained
in the Securities Act and the Exchange Act and are making this statement for purposes of complying with
those safe harbor provisions. These forward-looking statements reflect our current views about our plans,
intentions, expectations, strategies and prospects, which are based on the information currently available
to us and on assumptions we have made. Although we believe that our plans, intentions, expectations,
strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable,
we can give no assurance that the plans, intentions, expectations or strategies will be attained or
achieved. Furthermore, actual results may differ materially from those described in the forward-looking
statements and will be affected by a variety of risks and factors that are beyond our control, including our
ability to raise sufficient capital or consummate a strategic transaction to enable the continued
development of OCR-002, as well as those risks and uncertainties discussed under “Risk Factors” in our
Annual Report on form 10-K for the year ended December 31, 2015, and other risks detailed in our
subsequent filings with the SEC. All information in this presentation is as of the date of this presentation,
and we undertake no duty to update this information unless required by law.

3
Addressing a Serious Unmet Need
Alcohol Use
NASH / Fatty Liver
Hepatitis
Drug Exposure
Autoimmune Diseases
Diabetes
Obesity
Multiple Causes of Liver Disease (U.S.)
CHRONIC LIVER DISEASE
30-35M1
LIVER CIRRHOSIS
5.5M Million2
~200K Patients
Hospitalized
with OHE3
1 American Liver Foundation, Clinical Gastroenterology
and Hepatology, 2011;9:524-530 Zobair et al
2 Clin Liver Dis (2012) 73-89 Khungar et al
3 HCUP, Company estimate
OHE = Overt Hepatic Encephalopathy
Chronic HE
Patients2
AT RISK OF OHE
5.5M Million2

4
Disorientation
Impaired
Motor Skills
Personality
Changes
Stupor Coma Death
0 1 2 3 4
Hepatic Encephalopathy (HE):
Neurocognitive Disorder in Serious Liver Disease
Blood
Stream
Ammonia
Gut
Note: 0 to 4 as measured by West Haven Scale
Covert (CHE) Overt (OHE)
Elevated Ammonia Levels Drive HE

5
1 HCUP Database
2 Clinical Gastroenterology and Hepatology 2012;10:1034–1041
HE: Large and Growing Healthcare Burden
Rising Hospitalizations1
(000s)
• Total national charges
related to HE: $7 Billion2
• HE hospitalizations
continue to grow despite
Rifaximin launch in 20101
• Rifaximin 2016 revenue:
$932 Million
• HE patient demographics
show increase in severity
of illness, elderly
population, and obesity as
comorbidities2
Rifaximin
approved for
Prevention of HE
April 2010
2006 2007 2008 2009 2010 2011 2012 2013 2014
105 107 106 110 120 133 141 148
156
83 104
180 196
252
275
331
380
436
Hepatic Coma
Encephalopathy
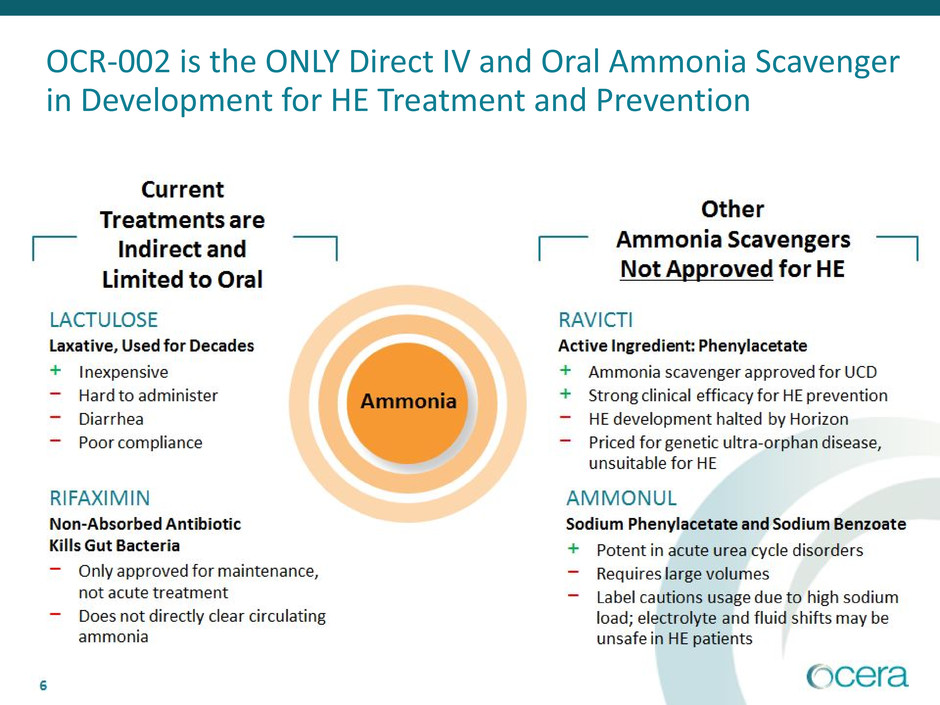
6
OCR-002 is the ONLY Direct IV and Oral Ammonia Scavenger
in Development for HE Treatment and Prevention

7
Significant Commercial Opportunity for
IV and Oral OCR-002
1 HCUP Database (includes ICD-9 codes 572.2/hepatic coma and 348.3/ encephalopathy NOS)

8
Valuable Commercial Estate
BROAD PATENTS
Composition
of Matter
to 2030 (not including
Hatch-Waxman
extension)
ORPHAN STATUS
in US for
hyperammonemia
and HE*
WORLDWIDE
RIGHTS
*Orphan Status also granted in EU for Acute Liver Failure (ALF)
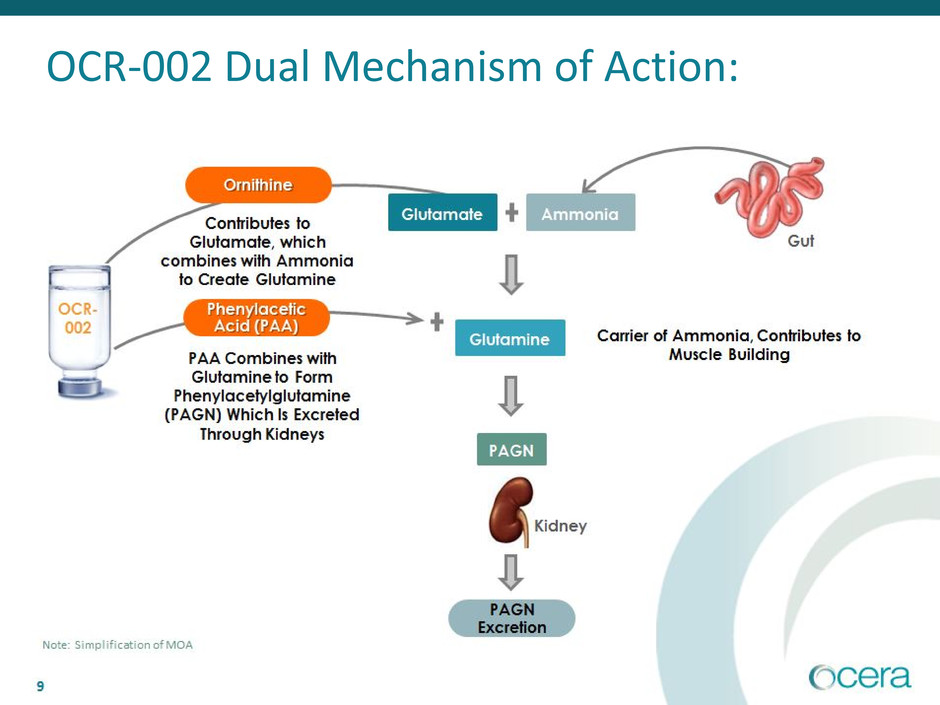
9
OCR-002 Dual Mechanism of Action:
Note: Simplification of MOA

10
OCR-002 (Ornithine Phenylacetate)
Validated Ammonia Scavenger Designed to Treat HE
• IV easy to administer in hospitalized patients
▪ Peripheral IV line, low infusion volume and neutral pH
▪ Rapid onset
▪ No sodium load or electrolyte disturbances
• Oral formulation for chronic care patients
▪ Prevention of HE via ammonia-scavenging has been clinically
established
▪ Provides continuity of care for patients at home

STOP-HE Phase 2b Study
OCR-002 IV for Overt HE

12
STOP-HE Phase 2b: OCR-002 IV for Overt HE
*As scored by a modified version of the West Haven Scale
20g 15g 10g
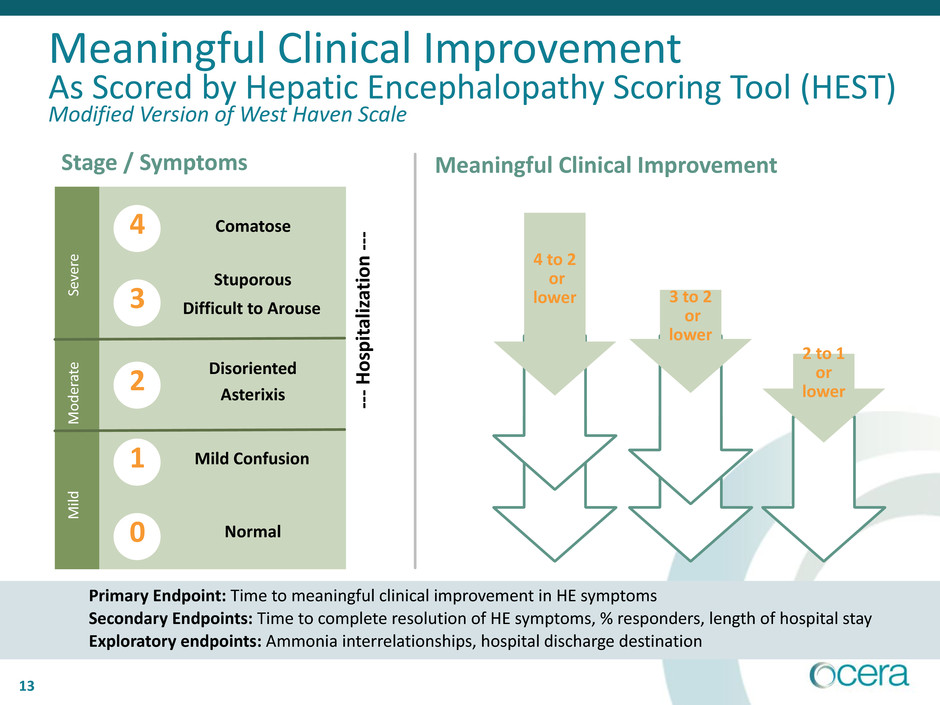
13
Primary Endpoint: Time to meaningful clinical improvement in HE symptoms
Secondary Endpoints: Time to complete resolution of HE symptoms, % responders, length of hospital stay
Exploratory endpoints: Ammonia interrelationships, hospital discharge destination
Meaningful Clinical Improvement
As Scored by Hepatic Encephalopathy Scoring Tool (HEST)
Modified Version of West Haven Scale
Stage / Symptoms Meaningful Clinical Improvement
4 to 2
or
lower 3 to 2
or
lower
2 to 1
or
lower
--- Hospi
tali
za
tion --
-Comatose
Stuporous
Disoriented
Difficult to Arouse
Asterixis
Mild Confusion
Normal
Mode
ra
te
Mil
d
Se
ve
re
0
1
2
3
4
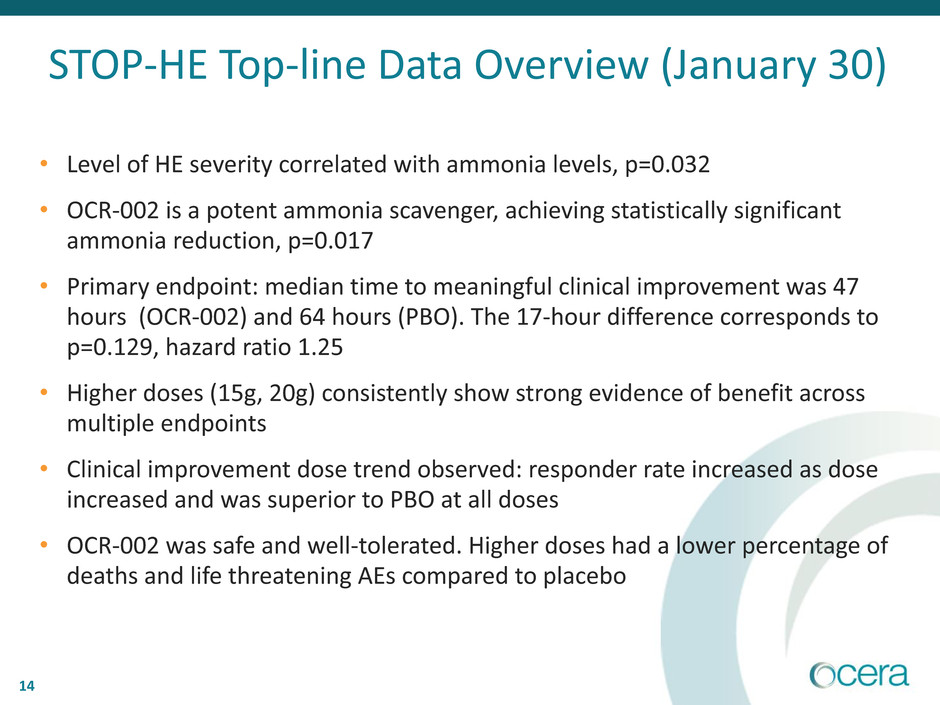
14
• Level of HE severity correlated with ammonia levels, p=0.032
• OCR-002 is a potent ammonia scavenger, achieving statistically significant
ammonia reduction, p=0.017
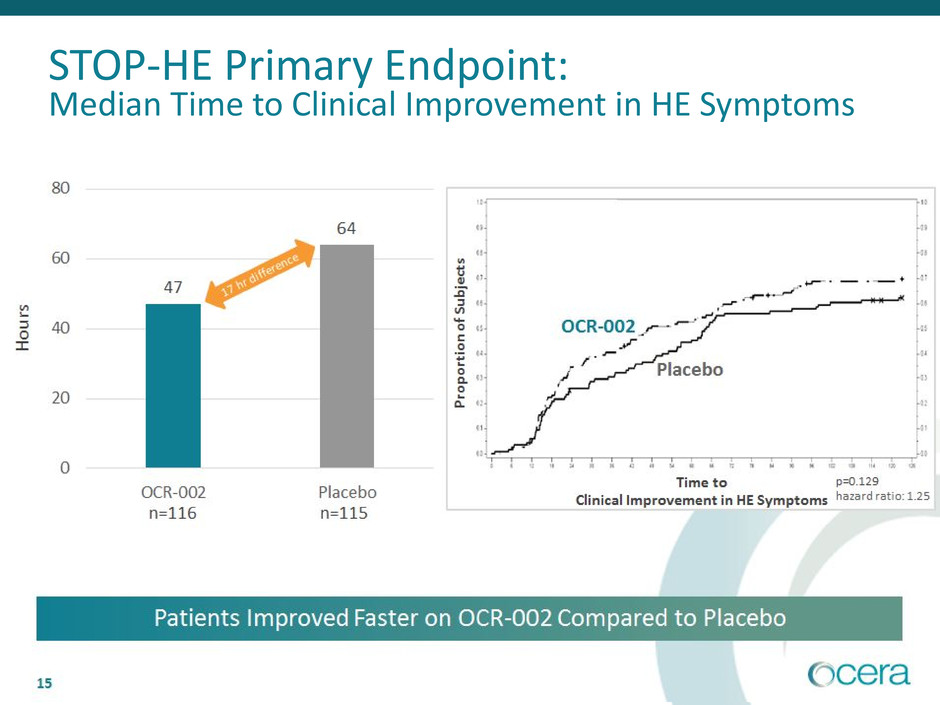
• Primary endpoint: median time to meaningful clinical improvement was 47
hours (OCR-002) and 64 hours (PBO). The 17-hour difference corresponds to
p=0.129, hazard ratio 1.25
• Higher doses (15g, 20g) consistently show strong evidence of benefit across
multiple endpoints
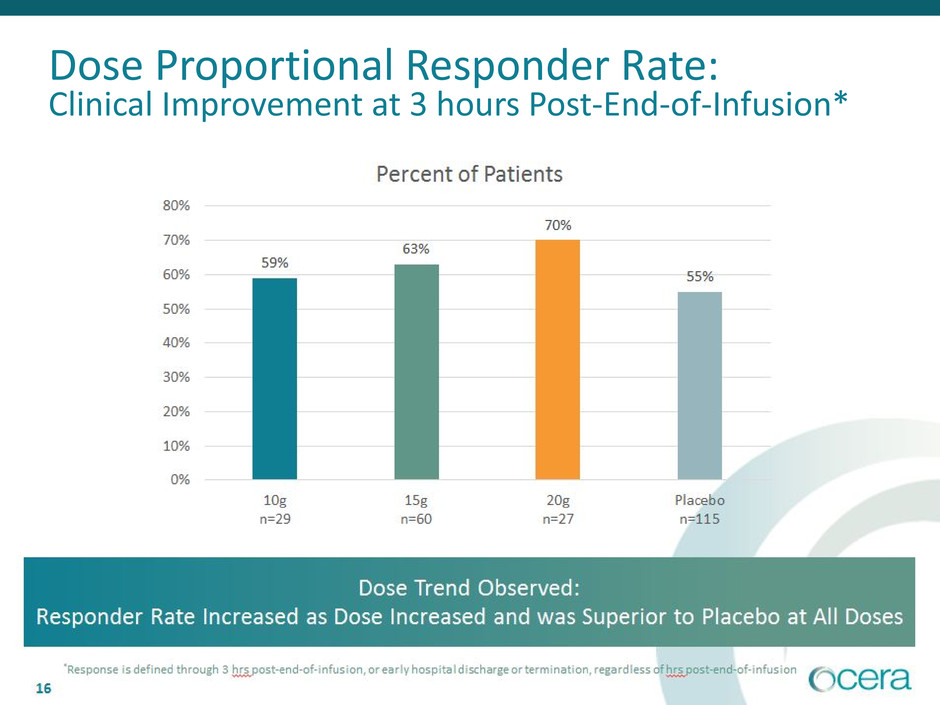
• Clinical improvement dose trend observed: responder rate increased as dose
increased and was superior to PBO at all doses
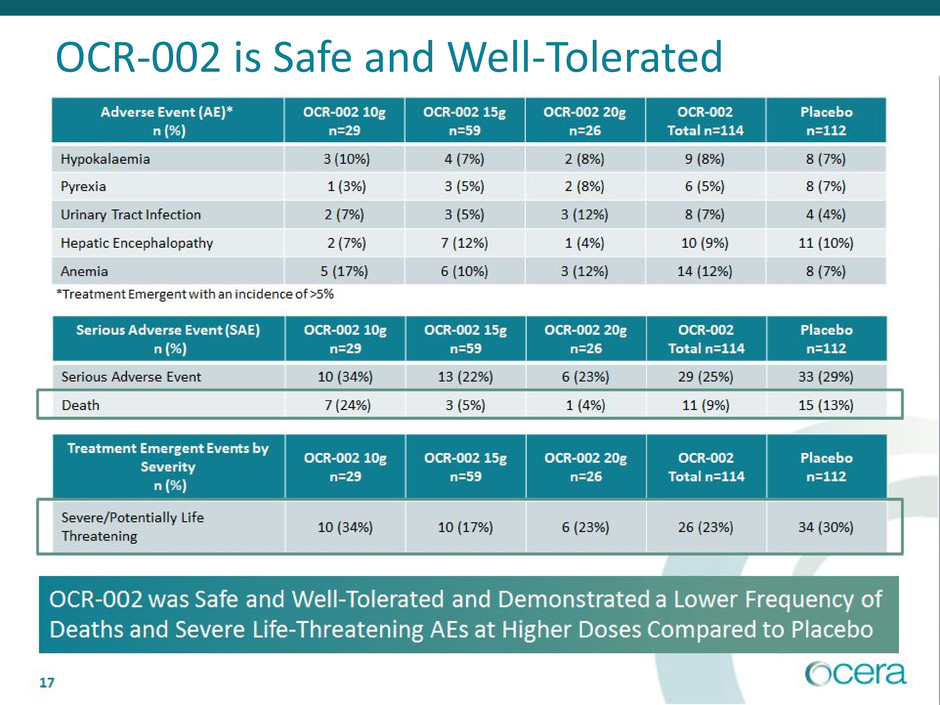
• OCR-002 was safe and well-tolerated. Higher doses had a lower percentage of
deaths and life threatening AEs compared to placebo
STOP-HE Top-line Data Overview (January 30)
15
STOP-HE Primary Endpoint:
Median Time to Clinical Improvement in HE Symptoms
16
Dose Proportional Responder Rate:
Clinical Improvement at 3 hours Post-End-of-Infusion*
17
OCR-002 is Safe and Well-Tolerated

STOP-HE Phase 2b Study
Subsequent Analysis

19
Summary of Subsequent Analyses
• Ammonia reduction correlates with clinical improvement (p=0.0006)
• Dose proportional response and PK data indicate some patients were under-
dosed
• Earlier timing of drug administration and efficacy assessment is important:
▪ Patients who improve within 48 hours are discharged earlier than patients who
do not improve within 48 hours
▪ Patients on OCR-002 more likely to respond within 48 hours compared to placebo
(p=0.026)
• Pre-defined measures of improvement were statistically significant: ammonia
reduction (p=0.017), Physician overall evaluation (p= 0.026)
• OCR-002 appears to have an equal or better benefit-risk ratio than rifaximin,
which is not indicated for overt HE but widely used in the hospital

20
Ammonia Reduction Correlates with Clinical Improvement
(change in ammonia from baseline)
Clinical Improvement Positively Correlated with Ammonia Reduction, p=0.0006*
*ANCOVA, 2 sided
Ammonia l
ev
el µ
g/m
L
Clinical Outcome
No Improvement Improvement

21
OCR-002 Has a Dose-Proportional Reduction in
Ammonia and Does So Faster than Placebo
p=0.028
hazard ratio: 1.69
p=0.017
OCR-002 Superior to PBO in Both Ammonia Parameters, p=0.017 and p=0.028

22
Not all Patients Achieved Target Exposure
Target
Range
Possible Change to Dosing Regimen Under Evaluation

23
Timing of Outcome Measurement:
OCR-002 was Statistically Significantly Better than PBO at 48 Hours
• Most HE patients eventually respond to SOC; goal of study was to shorten time to response
• Patients received SOC immediately; OCR-002 patients waited >12 hours
• OCR-002 superior to PBO at 48 hours, p=0.026
• Patients on OCR-002 had a higher response rate at 48 hours vs PBO (51% and 37%,
respectively) and responders left hospital ~1.5 days earlier
• We believe future studies should begin OCR-002 administration earlier in admission and
measure efficacy at an earlier timepoint
24
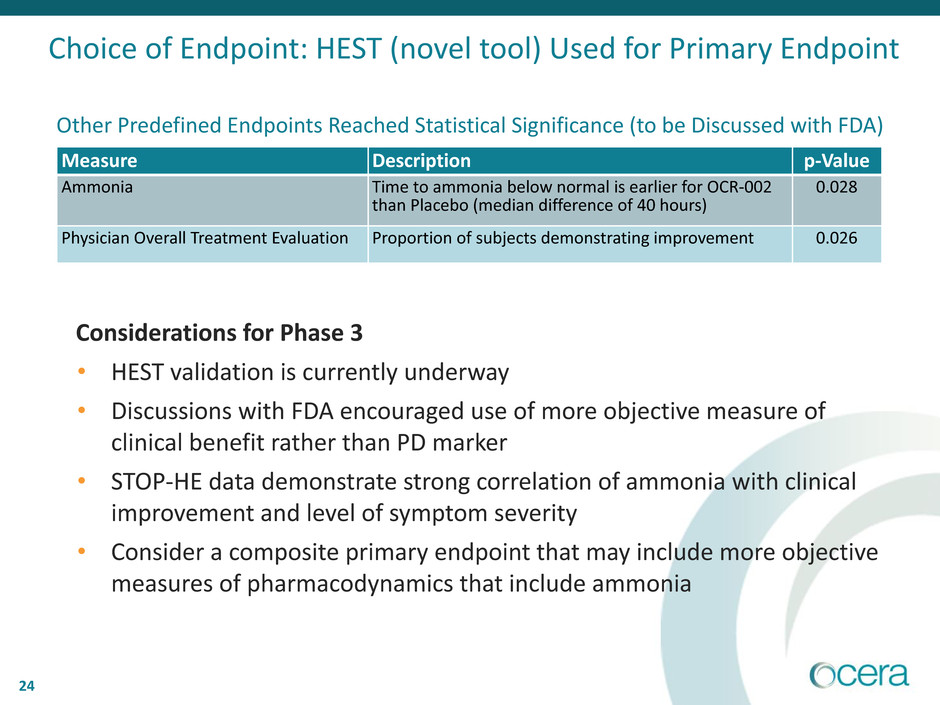
Measure Description p-Value
Ammonia Time to ammonia below normal is earlier for OCR-002
than Placebo (median difference of 40 hours)
0.028
Physician Overall Treatment Evaluation Proportion of subjects demonstrating improvement 0.026
Choice of Endpoint: HEST (novel tool) Used for Primary Endpoint
Considerations for Phase 3
• HEST validation is currently underway
• Discussions with FDA encouraged use of more objective measure of
clinical benefit rather than PD marker
• STOP-HE data demonstrate strong correlation of ammonia with clinical
improvement and level of symptom severity
• Consider a composite primary endpoint that may include more objective
measures of pharmacodynamics that include ammonia
Other Predefined Endpoints Reached Statistical Significance (to be Discussed with FDA)

25
Role of Rifaximin was Explored
OCR-002 without rifaximin
vs. PBO without rifaximin
OCR-002 vs. PBO
irrespective of rifaximin
147 patients (78 OCR-002, 69 Placebo) received rifaximin during therapy or entered hospital on rifaximin
31% of patients were on rifaximin at admission
STOP-HE Without Rifaximin Would Have Met Primary Endpoint with High
Statistical Significance, p=0.004
26
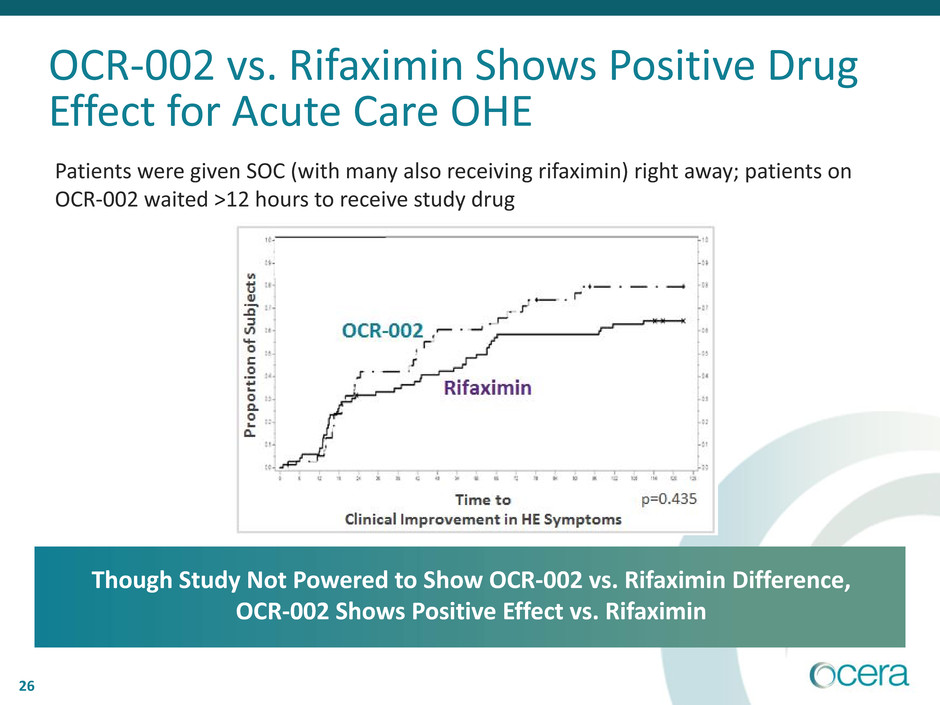
OCR-002 vs. Rifaximin Shows Positive Drug
Effect for Acute Care OHE
Patients were given SOC (with many also receiving rifaximin) right away; patients on
OCR-002 waited >12 hours to receive study drug
Though Study Not Powered to Show OCR-002 vs. Rifaximin Difference,
OCR-002 Shows Positive Effect vs. Rifaximin

27
OCR-002 May Be More Appropriate than
Rifaximin in Treating Acute Episodes of Overt HE
• Rifaximin, an antibiotic to reduce the recurrence of acute HE episodes, is not
indicated for overt HE, but is widely used in acute care settings
• Rifaximin label cautions use in severe liver impairment and cites potential for
21-fold increase in systemic exposure in Child-Pugh C patients (70% in the
study)
• Serious treatment-emergent infections were disproportionately observed in
the rifaximin group
• Safety and efficacy profile observed to date of OCR-002 IV make it well-suited
for acute treatment of overt HE patients, especially those with severe liver
impairment
28
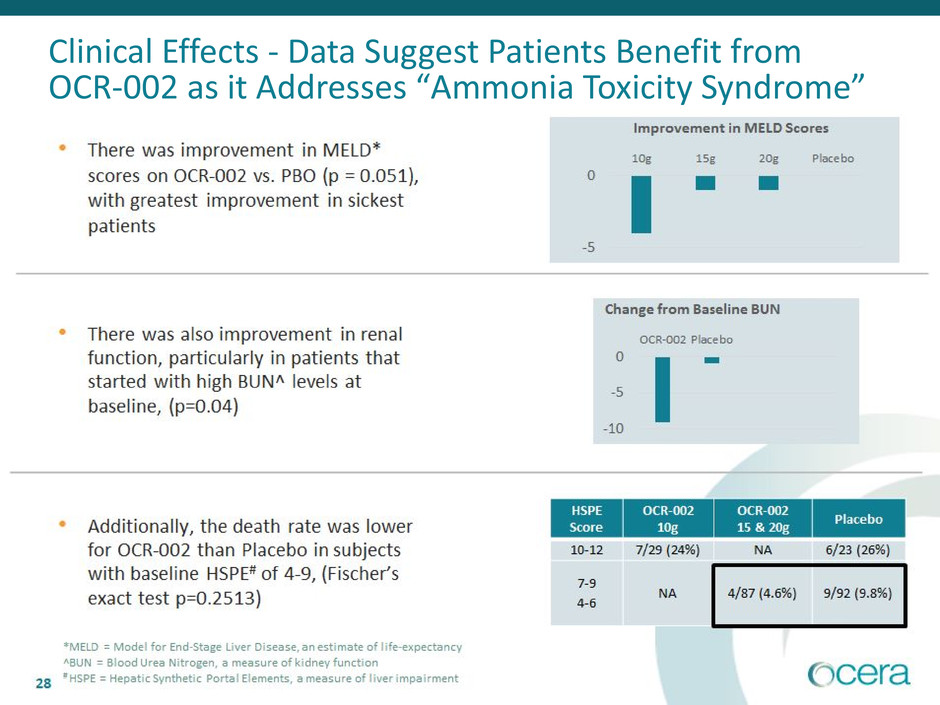
Clinical Effects - Data Suggest Patients Benefit from
OCR-002 as it Addresses “Ammonia Toxicity Syndrome”
*MELD = Model for End-Stage Liver Disease, an estimate of life-expectancy
^BUN = Blood Urea Nitrogen, a measure of kidney function
# HSPE = Hepatic Synthetic Portal Elements, a measure of liver impairment

29
Data Suggest OCR-002 has Potential Broad Application
1. Hospital-based Acute Care for HE
◦ Acute therapy, rapidly reduce ammonia; effect seen early
◦ Dose without rifaxamin as patients presenting in hospital have
moderate to severe liver disease, in whom rifaxamin must be used
with caution, nor is it indicated for acute episodes of overt HE
2. Step Down Therapy
◦ Continued administration following initial ammonia reduction to
allow “inflammatory cascade”* from ammonia to reduce (Child-
Pugh B and/or Child-Pugh C patients)
3. Chronic Care Outpatient Use
◦ Maintain remission of HE for patients at home and/or rescue
therapy for patients at risk of developing therapy
◦ May allow for reduction of lactulose use
IV infusion
Oral
*Wright, et al “Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension” Metabolic Brain
Disorder 2007 Dec:22(3-4):375-88

30
Wealth of Data Supports Promise of OCR-002

31
Next Steps
