Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Audentes Therapeutics, Inc. | d355106d8k.htm |
Exhibit 99.1
|
|
Audentes Corporate Overview
Cowen and Company 37th Annual Health Care Conference March 7, 2017
Courageous Patients. Bold Effort.
|
|
Safe Harbor
Except for statements of historical fact, any information contained in this presentation may be a forward-looking statement that reflects the Company’s current views about future events and are subject to risks, uncertainties, assumptions and changes in circumstances that may cause events or the Company’s actual activities or results to differ significantly from those expressed in any forward-looking statement. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “plan,” “expect,” “estimate,” “anticipate,” “intend,” “goal,” “strategy,” “believe” and similar expressions and variations thereof. Forward-looking statements include statements regarding the Company’s business strategy; potential growth opportunities; clinical development activities; the timing and results of Investigational New Drug application and Clinical Trial Authorisation submissions; the timing and results of preclinical studies and clinical trials; the nature and timing of potential regulatory approvals; and the likelihood of future commercialization of product candidates. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements, and the timing and results of biotechnology development and potential regulatory approval is inherently uncertain. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading “Risk Factors” in documents the Company has filed with the SEC. These forward-looking statements speak only as of the date of this presentation, and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof.
Certain information contained in this presentation may be derived from information provided by industry sources. We believe such information is accurate and that the sources from which it has been obtained are reliable. However, we cannot guarantee the accuracy of, and have not independently verified, such information.
The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products.
2
|
|
Audentes Therapeutics
Developing gene therapy products to transform the lives of rare disease patients with limited or no treatment options
Rare disease and AAV gene therapy strategic focus Multi-product pipeline based on focused selection criteria Internal cGMP manufacturing provides core strategic advantage Proven leadership team with significant experience Preliminary clinical data from lead programs expected in 2017
3
|
|
Audentes Therapeutics
Experienced and Passionate Team
Matthew Patterson
President and Chief Executive Officer
Natalie Holles
Senior VP and Chief Operating Officer
Thomas Soloway
Senior VP and Chief Financial Officer
Suyash Prasad, M.D.
Senior VP and Chief Medical Officer
John Gray, Ph.D.
Senior VP, Research and Development
Mary Newman
Senior VP, Regulatory Affairs
David Nagler
Senior VP, Human Resources and Corporate Affairs
97 employees based in San Francisco Bay Area as of December 31, 2016
4
|
|
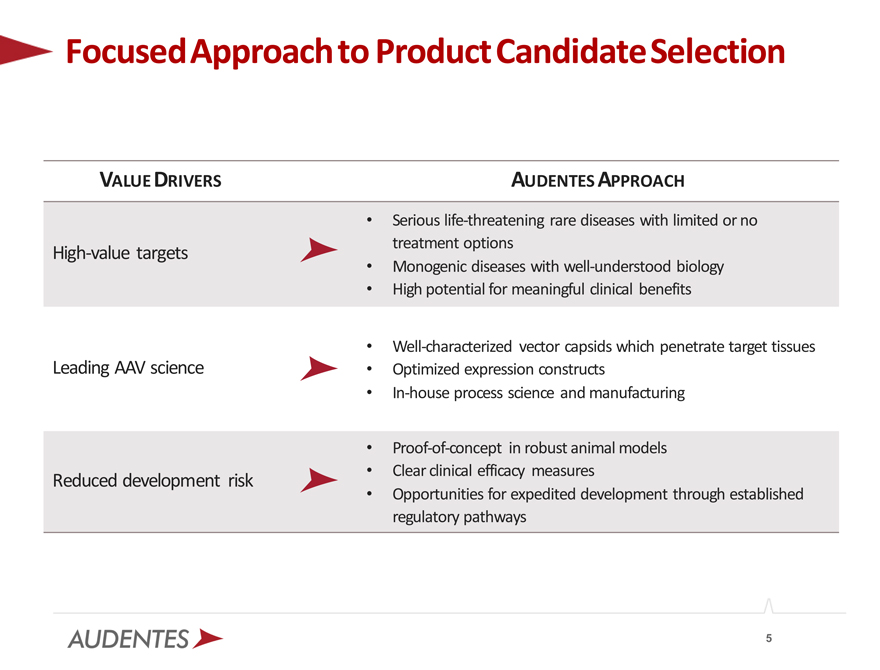
Focused Approach to Product Candidate Selection
VALUE DRIVERS AUDENTES APPROACH
Serious life-threatening rare diseases with limited or no
High-value targets treatment options
Monogenic diseases with well-understood biology
High potential for meaningful clinical benefits
Well-characterized vector capsids which penetrate target tissues Leading AAV science • Optimized expression constructs
In-house process science and manufacturing
Proof-of-concept in robust animal models Reduced development risk • Clear clinical efficacy measures
Opportunities for expedited development through established regulatory pathways
5
|
|
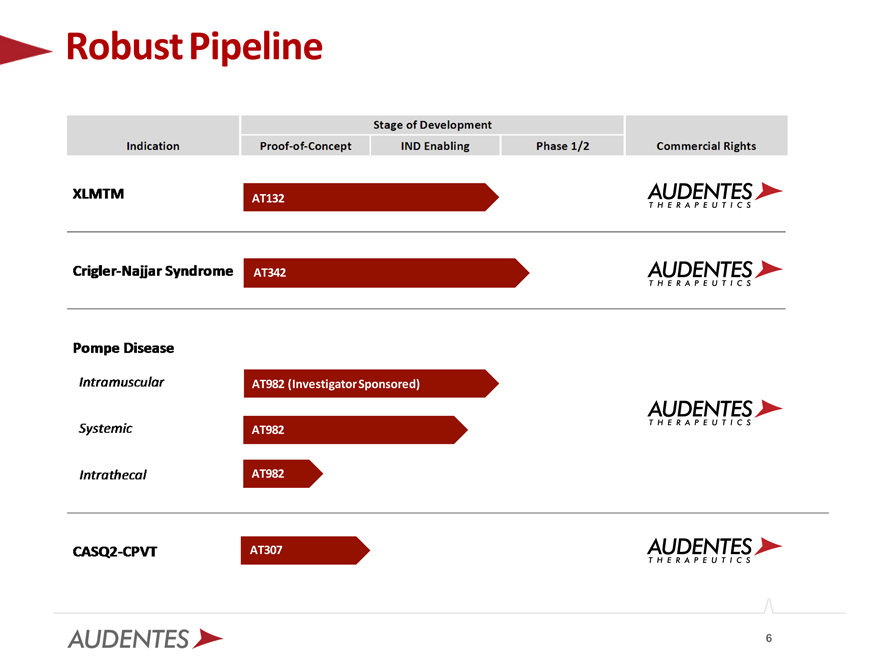
Robust Pipeline
AT132
AT342
AT982 (Investigator Sponsored)
AT982
AT982
AT307
6
|
|
Internal cGMP Manufacturing Capability
Internal cGMP manufacturing capability is a significant advantage in AAV field
Superior control over timelines, costs, and intellectual property Opportunity for rapid addition of new programs
Audentes facility established and on-line
38,000 square foot cGMP manufacturing facility in South San Francisco
2 x 500L bioreactor scale in first GMP suite
In-house QC lab for critical release assays
Additional capacity (~5,000L) possible in existing lease footprint
Planned to be capable of commercial production
7
|
|
Audentes cGMP Manufacturing
Leadership Position in AAV Production
Natural host for AAV
Mammalian cell- regulatory history with HEK293 Significant based production
Robust, industrial scale suspension culture system
Large-scale Facility qualified for cGMP manufacturing cGMP capacity
Production at 2 x 500L scale on-going to supply clinical trials
Continued process Next-generation production systems to optimize process yield and product quality improvements and Additional production suites expansion Larger scale bioreactors
Clinical AT132 and AT342 clinical manufacturing ongoing manufacturing status AT982 and AT307 planned to move in-house
8
|
|
AT132
X-LINKED MYOTUBULAR MYOPATHY
Courageous Patients. Bold Effort.
9
|
|
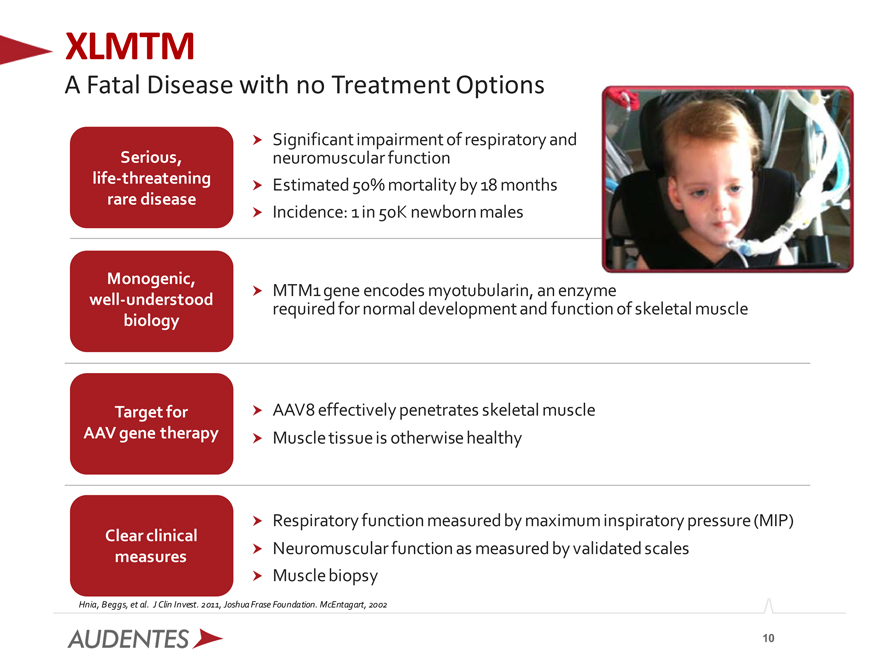
XLMTM
A Fatal Disease with no Treatment Options
Serious, life-threatening rare disease
Monogenic, well-understood biology
Target for AAV gene therapy
Clear clinical measures
Significant impairment of respiratory and neuromuscular function Estimated 50% mortality by 18 months Incidence: 1 in 50K newborn males
MTM1 gene encodes myotubularin, an enzyme required for normal development and function of skeletal muscle
AAV8 effectively penetrates skeletal muscle Muscle tissue is otherwise healthy
Respiratory function measured by maximum inspiratory pressure (MIP) Neuromuscular function as measured by validated scales Muscle biopsy
Hnia, Beggs, et al. J Clin Invest. 2011, Joshua Frase Foundation. McEntagart, 2002
10
|
|
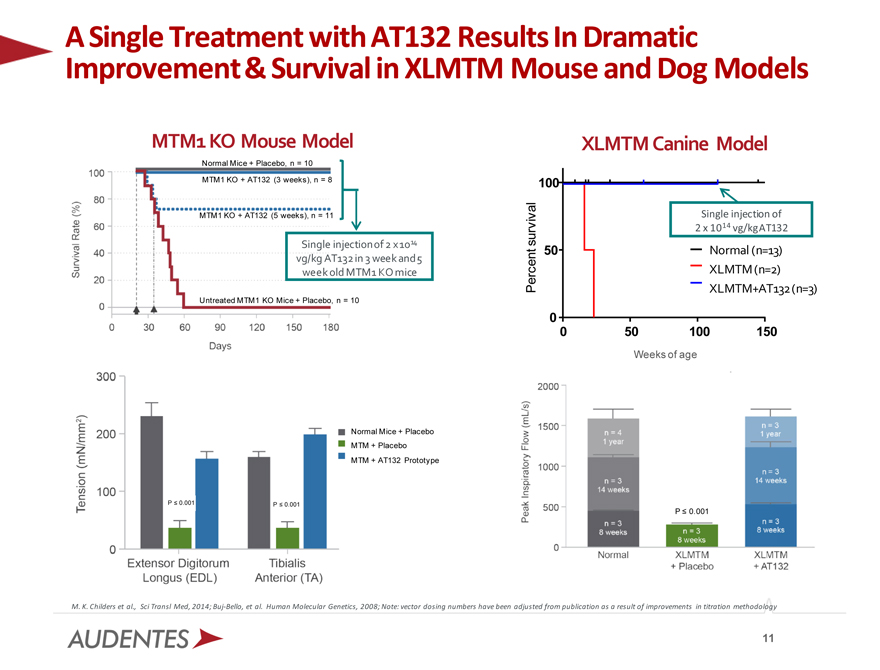
A Single Treatment with AT132 Results In Dramatic Improvement & Survival in XLMTM Mouse and Dog Models
MTM1 KO Mouse Model XLMTM Canine Model
Normal Mice + Placebo, n = 10
MTM1 KO + AT132 (3 weeks), n = 8 100
MTM1 KO + AT132 (5 weeks), n = 11 Single injection of
2 x 1014 vg/kg AT132
Single injection of 2 x 1014 survival
50 Normal (n=13)
vg/kg AT132 in 3 week and 5
week old MTM1 KO mice XLMTM (n=2)
Percent XLMTM+AT132 (n=3)
Untreated MTM1 KO Mice + Placebo, n = 10
0
0 50 100 150
Normal Mice + Placebo
MTM + Placebo
MTM + AT132 Prototype
P 0.001 P 0.001
P 0.001
M. K. Childers et al., Sci Transl Med, 2014; Buj-Bello, et al. Human Molecular Genetics, 2008; Note: vector dosing numbers have been adjusted from publication as a result of improvements in titration methodology
11
|
|
Treatment with AT132 Results in Dramatic Improvement and Survival in XLMTM Dogs
*Dogs 3, 4 and 5, the three dogs treated in the proof-of-concept canine study, achieved a statistically significant improvement in survival.
12
|
|
Treatment with AT132 Results in Rapid Improvement in XLMTM Dog Treated After Symptom Onset
13
|
|
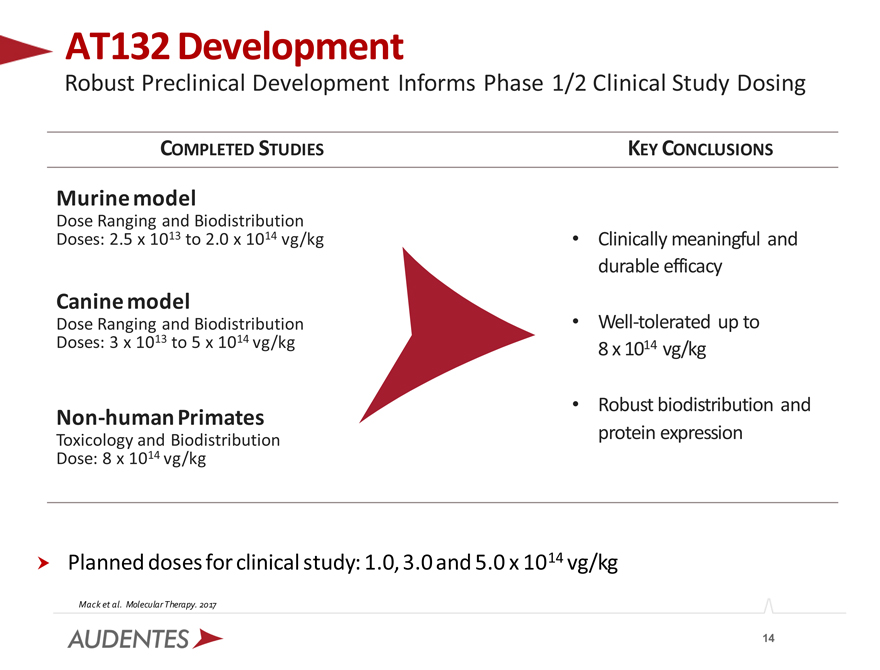
AT132 Development
Robust Preclinical Development Informs Phase 1/2 Clinical Study Dosing
COMPLETED STUDIES
Murine model
Dose Ranging and Biodistribution Doses: 2.5 x 1013 to 2.0 x 1014 vg/kg
Canine model
Dose Ranging and Biodistribution Doses: 3 x 1013 to 5 x 1014 vg/kg
Non-human Primates
Toxicology and Biodistribution Dose: 8 x 1014 vg/kg
KEY CONCLUSIONS
Clinically meaningful and durable efficacy
Well-tolerated up to 8 x 1014 vg/kg
Robust biodistribution and protein expression
Planned doses for clinical study: 1.0, 3.0 and 5.0 x 1014 vg/kg
Mack et al. Molecular Therapy. 2017
14
|
|
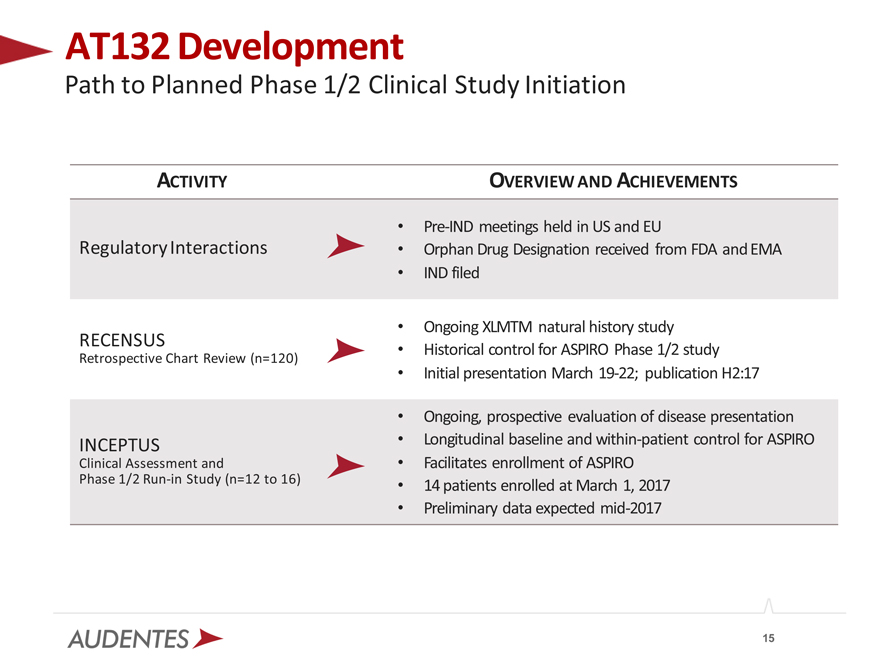
AT132 Development
Path to Planned Phase 1/2 Clinical Study Initiation
ACTIVITY
Regulatory Interactions
RECENSUS
Retrospective Chart Review (n=120)
INCEPTUS
Clinical Assessment and
Phase 1/2 Run-in Study (n=12 to 16)
OVERVIEW AND ACHIEVEMENTS
Pre-IND meetings held in US and EU
Orphan Drug Designation received from FDA and EMA IND filed
Ongoing XLMTM natural history study Historical control for ASPIRO Phase 1/2 study Initial presentation March 19-22; publication H2:17
Ongoing, prospective evaluation of disease presentation Longitudinal baseline and within-patient control for ASPIRO Facilitates enrollment of ASPIRO
14 patients enrolled at March 1, 2017 Preliminary data expected mid-2017
15
|
|
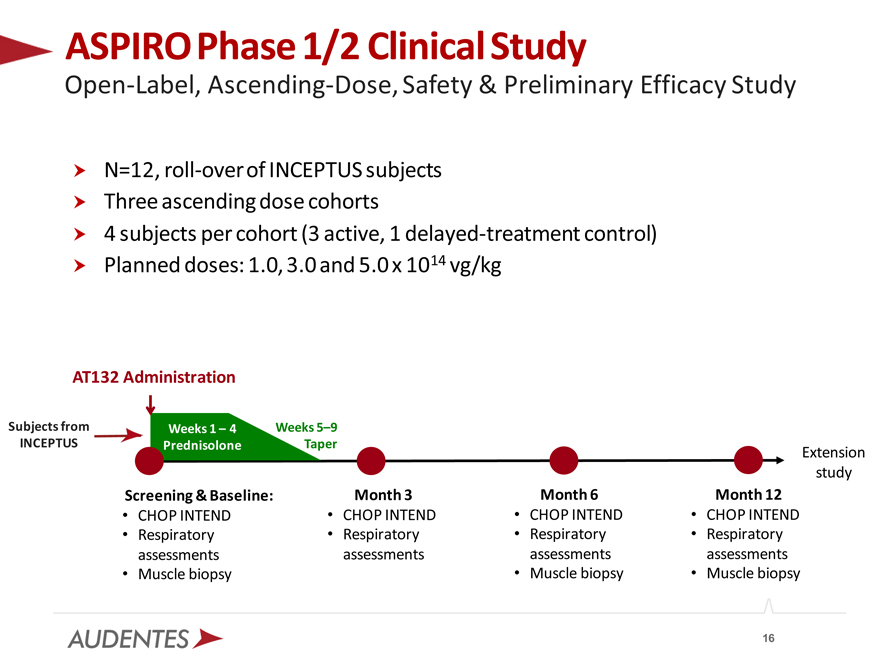
N=12, roll-over of INCEPTUS subjects Three ascending dose cohorts
4 subjects per cohort (3 active, 1 delayed-treatment control) Planned doses: 1.0, 3.0 and 5.0 x 1014 vg/kg
ASPIRO Phase 1/2 Clinical Study
Open-Label, Ascending-Dose, Safety & Preliminary Efficacy Study
AT132 Administration
Subjects from Weeks 1 – 4 Weeks 5–9
INCEPTUS Prednisolone Taper
Screening & Baseline:
CHOP INTEND Respiratory assessments Muscle biopsy
Month 3
CHOP INTEND Respiratory assessments
Month 6
CHOP INTEND Respiratory assessments Muscle biopsy
Month 12
CHOP INTEND Respiratory assessments Muscle biopsy
Extension study
16
|
|
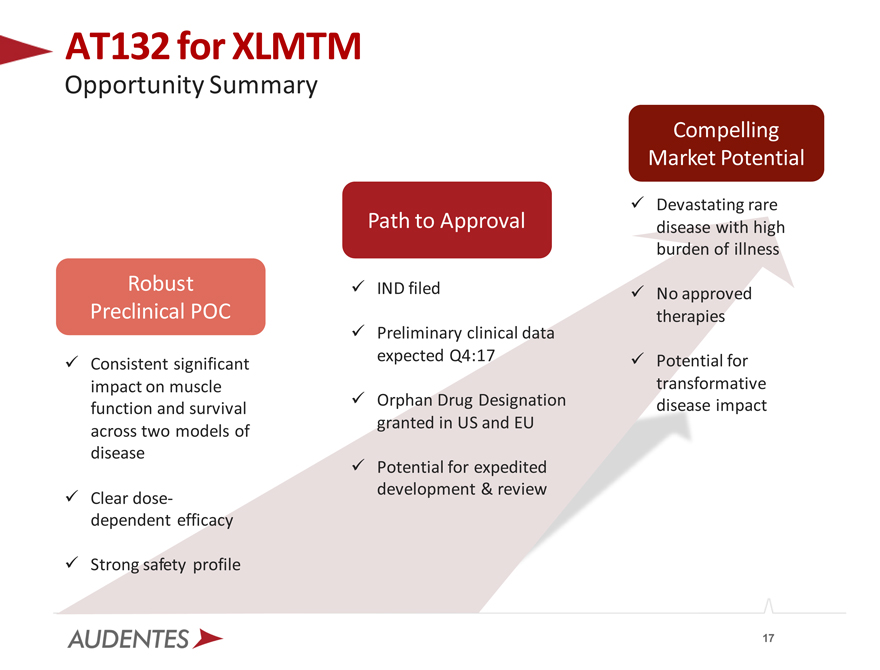
AT132 for XLMTM
Opportunity Summary
Robust Preclinical POC
Consistent significant impact on muscle function and survival across two models of disease
Clear dose-dependent efficacy
Strong safety profile
Path to Approval
IND filed
Preliminary clinical data expected Q4:17
Orphan Drug Designation granted in US and EU
Potential for expedited development & review
Compelling Market Potential
Devastating rare disease with high burden of illness
No approved therapies
Potential for transformative disease impact
17
|
|
AT342
CRIGLER-NAJJAR SYNDROME
Courageous Patients. Bold Effort. 18
18
|
|
Crigler-Najjar Syndrome
A Rare Disease that Leads to Neurological Damage and Death
Serious, life-threatening rare disease
Significant bilirubin accumulation that can lead to irreversible neurological damage and death Current liver transplant treatments: > 12 hours/day of phototherapy, Estimated incidence of 1 in 1 million newborns
Monogenic, well-understood biology
UGT1A1 gene encodes enzyme required for bilirubin metabolism and excretion
Target for AAV gene therapy
AAV8 effectively penetrates the liver Liver tissue is otherwise healthy
Clear clinical measures
Serum bilirubin Time on phototherapy
Bortolussi et. al, Human Gene Therapy, 2014; Orphanet.com
19
|
|
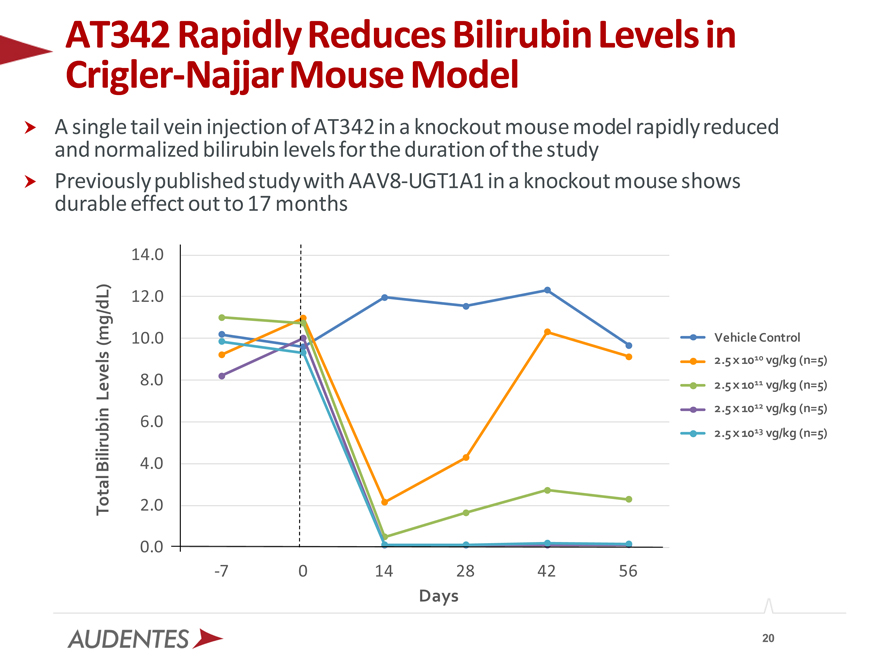
AT342 Crigler-Najjar Rapidly Mouse Reduces Model Bilirubin Levels in
A and single normalized tail vein bilirubin injection levels of AT342 for the in aduration knockout of mouse the study model rapidly reduced Previously durable effect published out to 17 study months with AAV8-UGT1A1 in a knockout mouse shows
14.0 12.0
(mg/dL) 10.0 Vehicle Control
2.5 x 1010 vg/kg (n=5) Levels 8.0 2.5 x 1011 vg/kg (n=5)
2.5 x 1012 vg/kg (n=5)
6.0
2.5 x 1013 vg/kg (n=5)
Bilirubin 4.0 Total 2.0
0.0
-7 0 14 28 42 56
Days
20
|
|
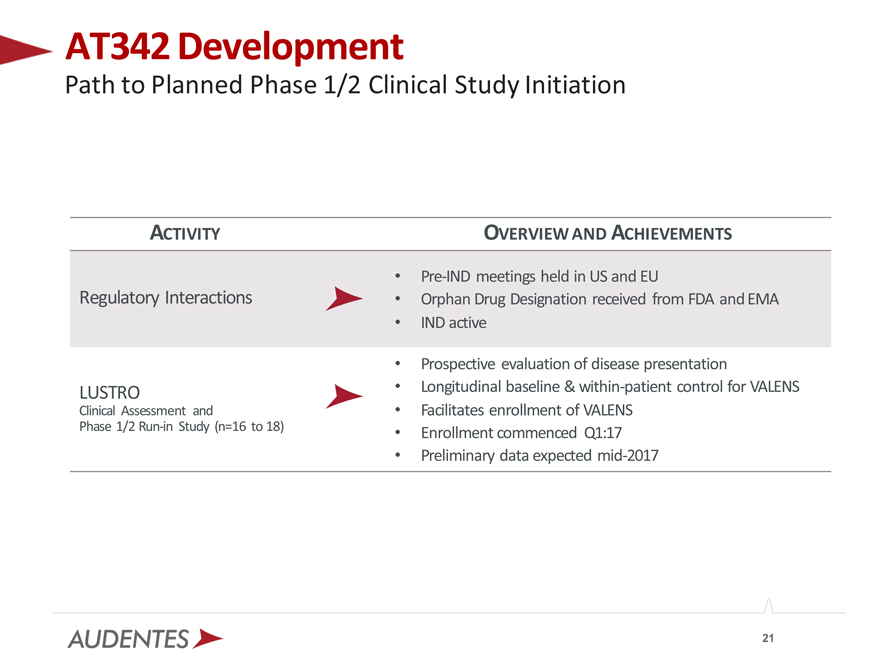
AT342 Development
Path to Planned Phase 1/2 Clinical Study Initiation
ACTIVITY OVERVIEW AND ACHIEVEMENTS
Pre-IND meetings held in US and EU
Regulatory Interactions Orphan Drug Designation received from FDA and EMA
IND active
Prospective evaluation of disease presentation
LUSTRO Longitudinal baseline & within-patient control for VALENS Clinical Assessment and Facilitates enrollment of VALENS
Phase 1/2 Run-in Study (n=16 to 18) Enrollment commenced Q1:17
Preliminary data expected mid-2017
21
|
|
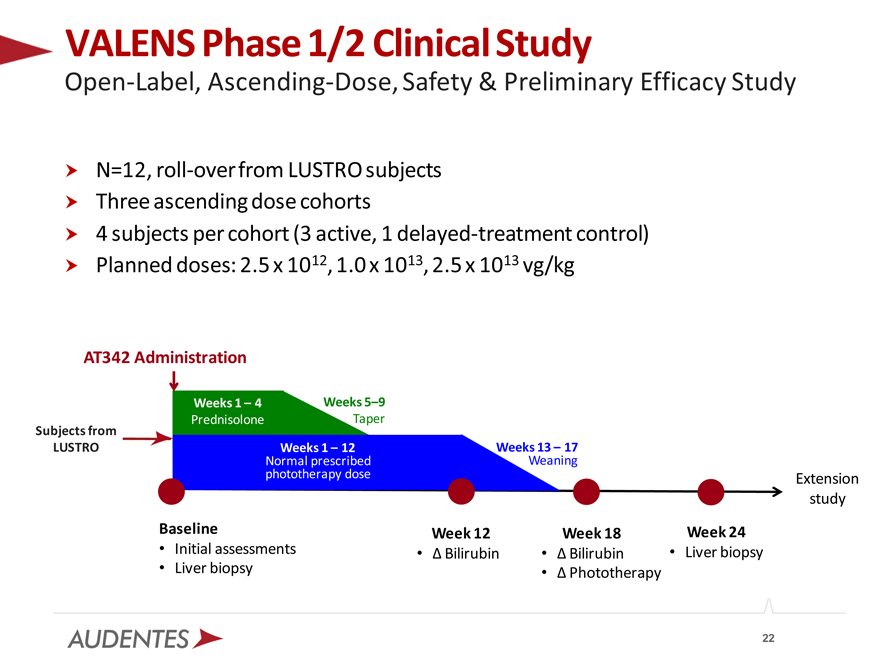
VALENS Phase 1/2 Clinical Study
Open-Label, Ascending-Dose, Safety & Preliminary Efficacy Study
N=12, roll-over from LUSTRO subjects Three ascending dose cohorts
4 subjects per cohort (3 active, 1 delayed-treatment control) Planned doses: 2.5 x 1012, 1.0 x 1013, 2.5 x 1013 vg/kg
AT342 Administration
Weeks 1 – 4 Weeks 5–9
Prednisolone Taper
Subjects from
LUSTRO Normal Weeks prescribed 1 – 12 Weeks Weaning 13 – 17 phototherapy dose Extension
study
Baseline Week 12 Week 18 Week 24
Initial assessments Bilirubin Bilirubin Liver biopsy
Liver biopsy Phototherapy
22
|
|
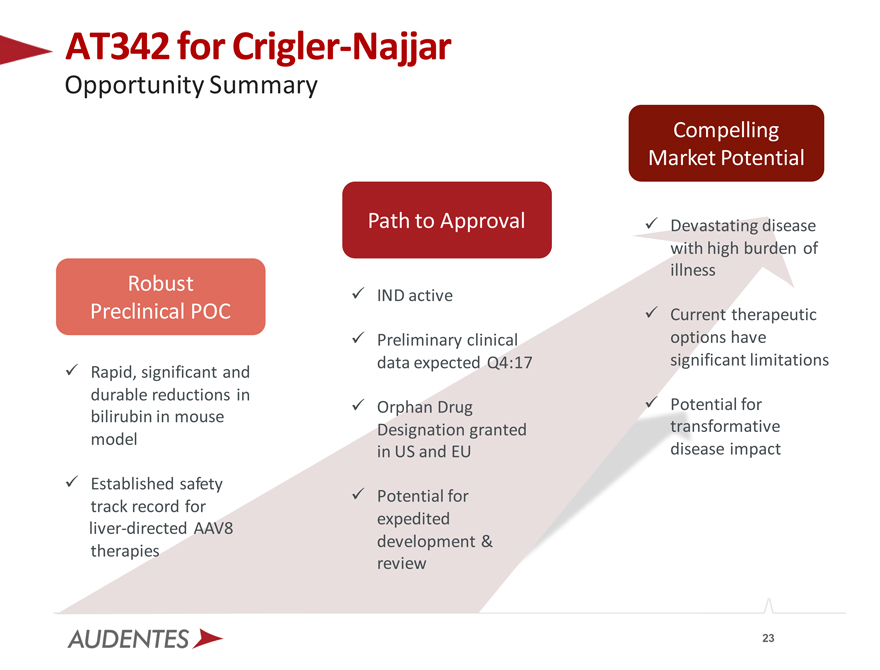
AT342 for Crigler-Najjar
Opportunity Summary
Robust Preclinical POC
Rapid, significant and durable reductions in bilirubin in mouse model
Established safety track record for liver-directed AAV8 therapies
Path to Approval
IND active
Preliminary clinical data expected Q4:17
Orphan Drug Designation granted in US and EU
Potential for expedited development & review
Compelling Market Potential
Devastating disease with high burden of illness
Current therapeutic options have significant limitations
Potential for transformative disease impact
23
|
|
AT982 POMPE DISEASE
Courageous Patients. Bold Effort.
24
|
|
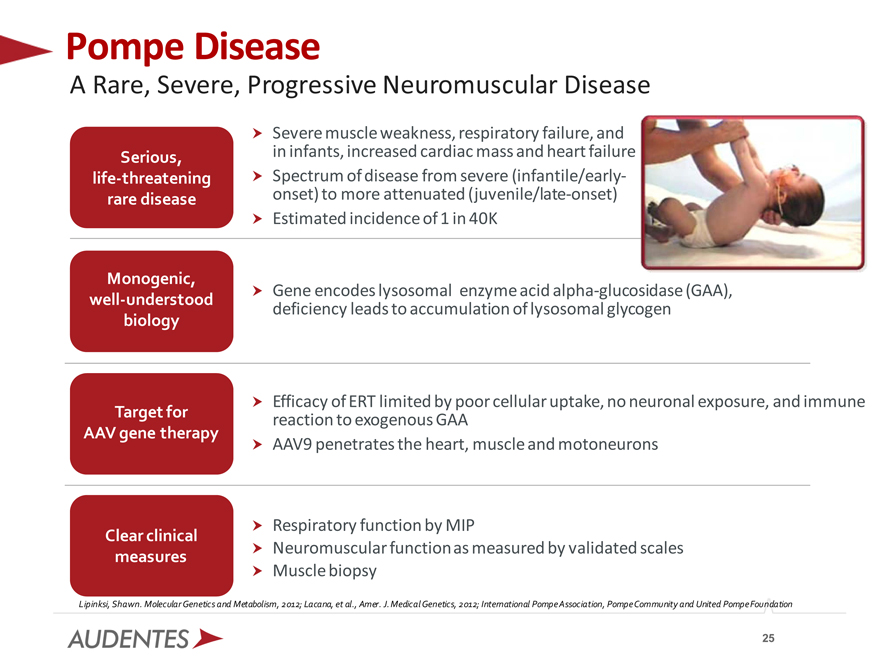
Pompe Disease
A Rare, Severe, Progressive Neuromuscular Disease
Severe muscle weakness, respiratory failure, and Serious, in infants, increased cardiac mass and heart failure life-threatening Spectrum of disease from severe (infantile/early-rare disease onset) to more attenuated (juvenile/late-onset) Estimated incidence of 1 in 40K
Monogenic,
Gene encodes lysosomal enzyme acid alpha-glucosidase (GAA), well-understood deficiency leads to accumulation of lysosomal glycogen biology
Efficacy of ERT limited by poor cellular uptake, no neuronal exposure, and immune Target for reaction to exogenous GAA
AAV gene therapy
AAV9 penetrates the heart, muscle and motoneurons
Respiratory function by MIP
Clear clinical
Neuromuscular function as measured by validated scales measures
Muscle biopsy
Lipinksi, Shawn. Molecular Genetics and Metabolism, 2012; Lacana, et al., Amer. J. Medical Genetics, 2012; International Pompe Association, Pompe Community and United Pompe Foundation
25
|
|
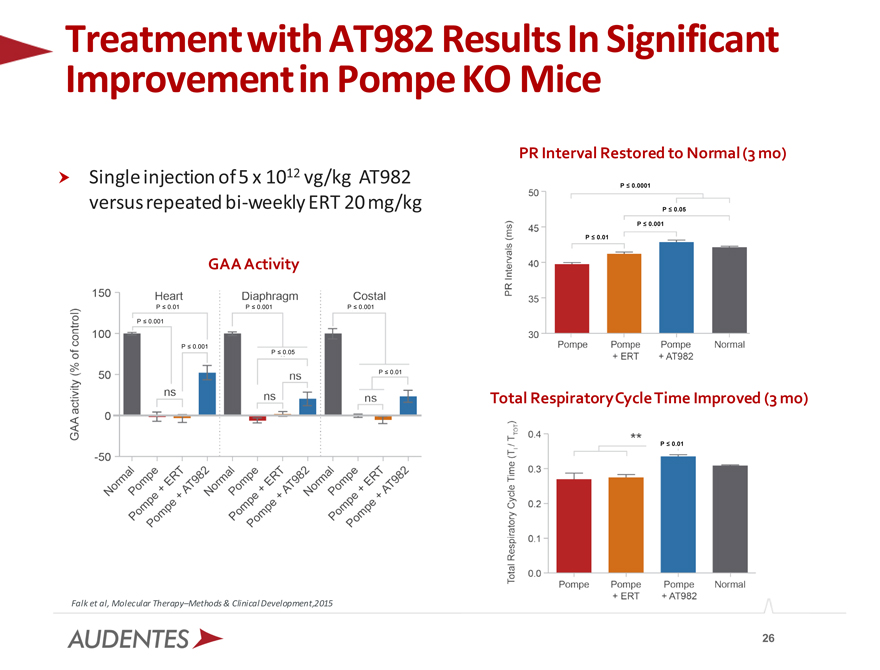
Improvement Treatment with in AT982 Pompe Results KO Mice In Significant
Single injection of 5 x 1012 vg/kg AT982 versus repeated bi-weekly ERT 20 mg/kg
PR Interval Restored to Normal (3 mo)
P 0.0001
P 0.05
P 0.001 P 0.01
GAA Activity
P 0.01 P 0.001 P 0.001
P 0.001
P 0.001 P 0.05
P 0.01
Falk et al, Molecular Therapy–Methods & Clinical Development,2015
Total Respiratory Cycle Time Improved (3 mo)
P 0.01
26
|
|
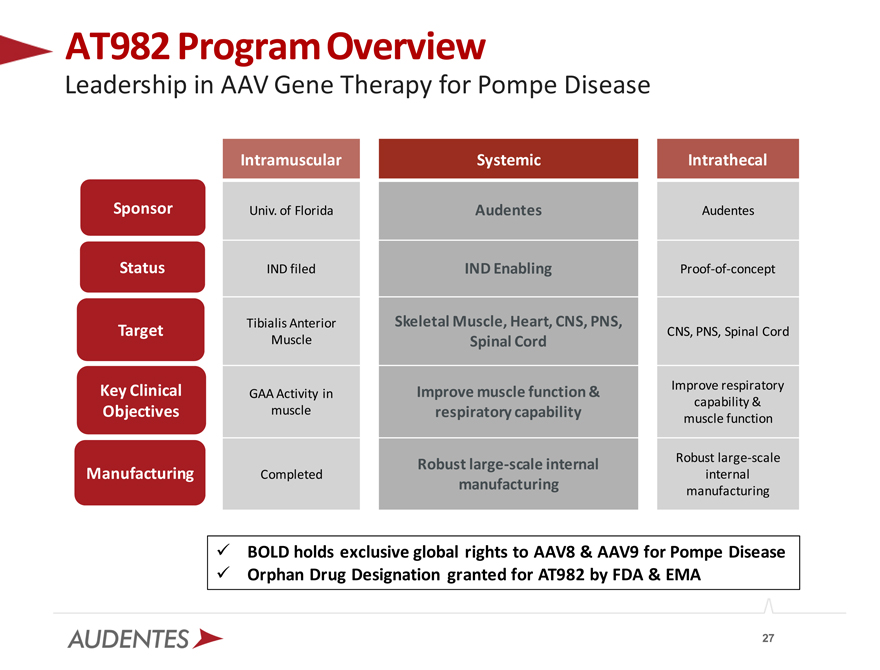
AT982 Program Overview
Leadership in AAV Gene Therapy for Pompe Disease
Sponsor Status Target
Key Clinical Objectives
Manufacturing
Intramuscular Systemic Intrathecal
Univ. of Florida Audentes Audentes
IND filed IND Enabling Proof-of-concept
Tibialis Anterior Skeletal Muscle, Heart, CNS, PNS,
CNS, PNS, Spinal Cord
Muscle Spinal Cord
Improve muscle function & Improve respiratory
GAA Activity in capability &
muscle respiratory capability
muscle function
Robust large-scale internal Robust large-scale
Completed internal
manufacturing manufacturing
BOLD holds exclusive global rights to AAV8 & AAV9 for Pompe Disease Orphan Drug Designation granted for AT982 by FDA & EMA
27
|
|
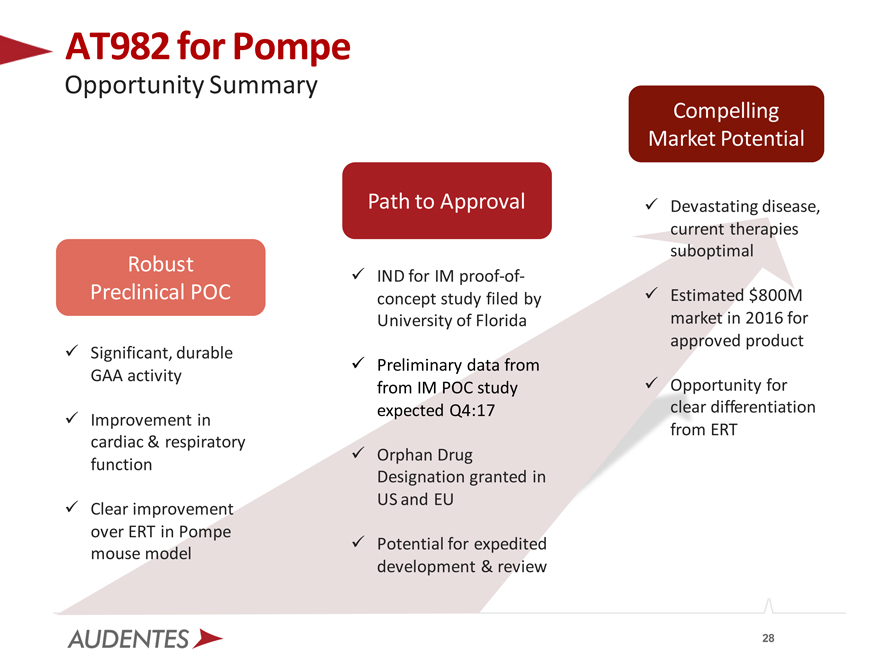
AT982 for Pompe
Opportunity Summary
Robust Preclinical POC
Significant, durable GAA activity
Improvement in cardiac & respiratory function
Clear improvement over ERT in Pompe mouse model
Path to Approval
IND for IM proof-of-concept study filed by University of Florida
Preliminary data from from IM POC study expected Q4:17
Orphan Drug
Designation granted in US and EU
Potential for expedited development & review
Compelling Market Potential
Devastating disease, current therapies suboptimal
Estimated $800M market in 2016 for approved product
Opportunity for clear differentiation from ERT
28
|
|
CATECHOLAMINERGIC AT307 POLYMORPHIC VENTRICULAR TACHYCARDIA (CPVT)
Courageous Patients. Bold Effort. 29
29
|
|
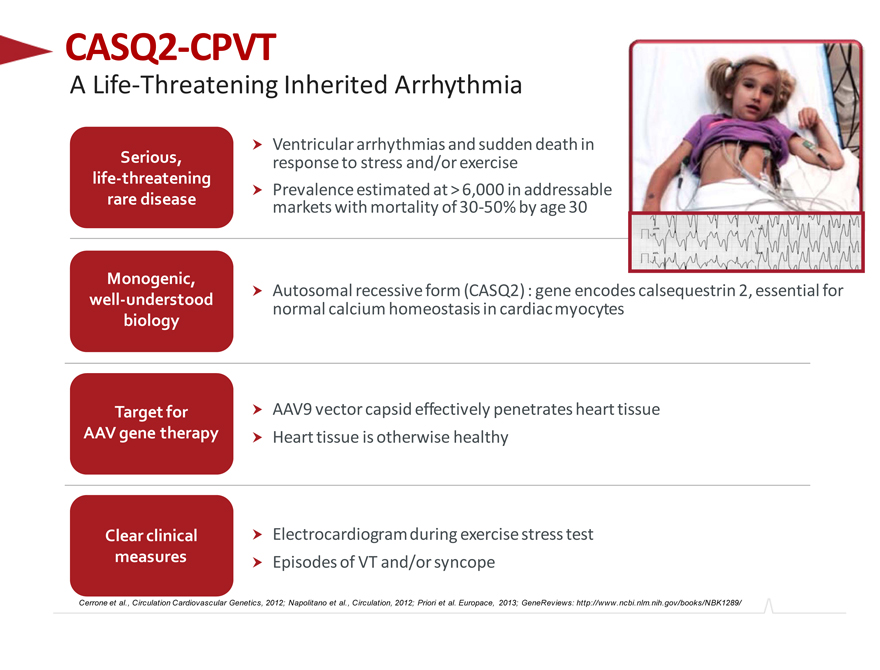
CASQ2-CPVT
A Life-Threatening Inherited Arrhythmia
Ventricular arrhythmias and sudden death in Serious, response to stress and/or exercise life-threatening
Prevalence estimated at > 6,000 in addressable rare disease markets with mortality of 30-50% by age 30
Monogenic,
Autosomal recessive form (CASQ2) : gene encodes calsequestrin 2, essential for well-understood normal calcium homeostasis in cardiac myocytes biology
Target for AAV9 vector capsid effectively penetrates heart tissue AAV gene therapy Heart tissue is otherwise healthy
Clear clinical Electrocardiogram during exercise stress test measures Episodes of VT and/or syncope
Cerrone et al., Circulation Cardiovascular Genetics, 2012; Napolitano et al., Circulation, 2012; Priori et al. Europace, 2013; GeneReviews: http://www.ncbi.nlm.nih.gov/books/NBK1289/
THERAPEUTICS CORPORATE PRESENTATION 30
|
|
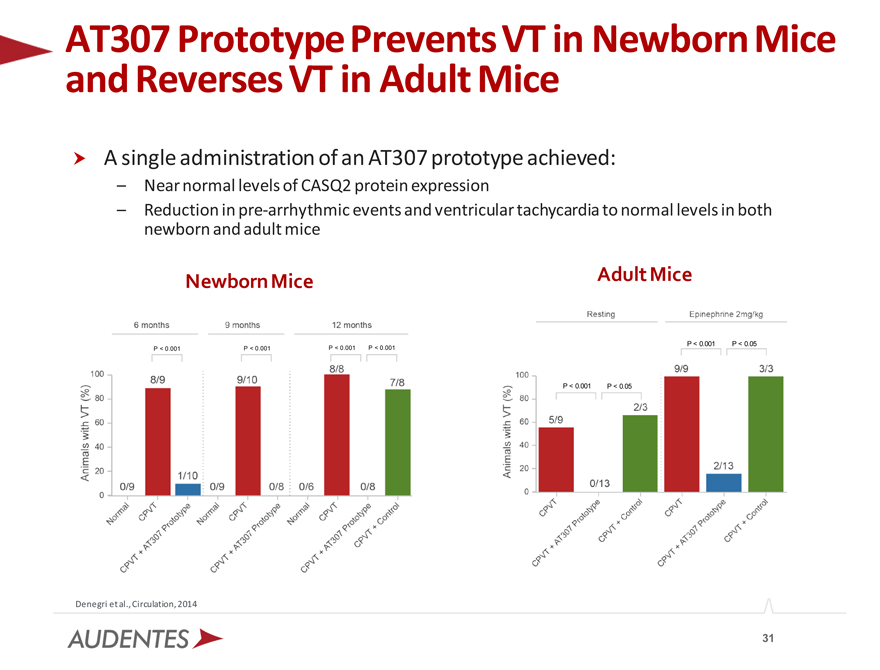
AT307 and Reverses Prototype VT in Prevents Adult Mice VT in Newborn Mice
A single administration of an AT307 prototype achieved:
– Near normal levels of CASQ2 protein expression
– Reduction in pre-arrhythmic events and ventricular tachycardia to normal levels in both newborn and adult mice
Adult Mice Newborn Mice
P < 0.001 P < 0.05
P < 0.001 P < 0.001 P < 0.001 P < 0.001
P < 0.001 P < 0.05
Denegri et al., Circulation, 2014
31
|
|
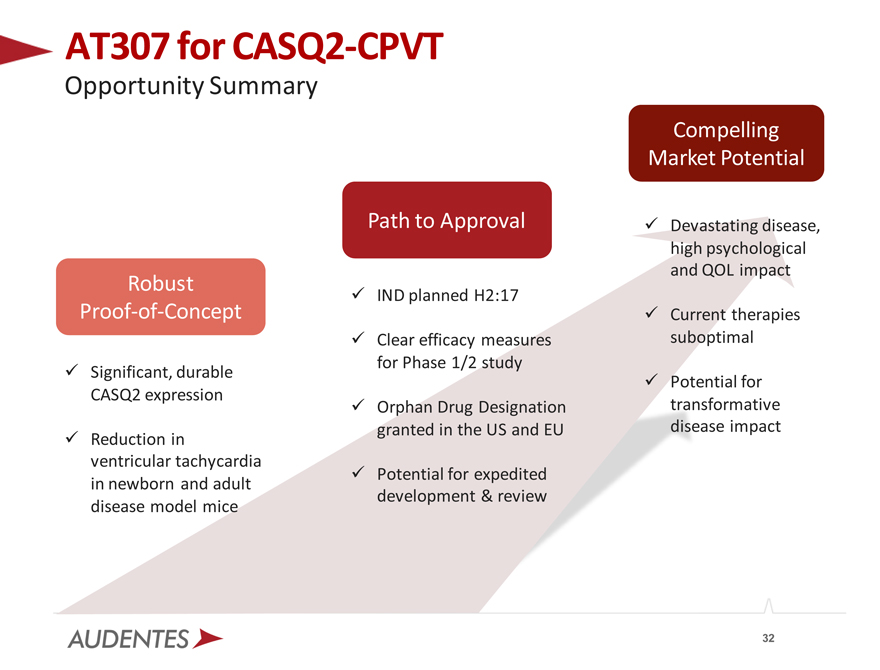
AT307 for CASQ2-CPVT
Opportunity Summary
Path to Approval
Robust
IND planned H2:17
Proof-of-Concept
Clear efficacy measures for Phase 1/2 study Significant, durable CASQ2 expression Orphan Drug Designation granted in the US and EU Reduction in ventricular tachycardia Potential for expedited in newborn and adult development & review disease model mice
Compelling Market Potential
Devastating disease, high psychological and QOL impact
Current therapies suboptimal
Potential for transformative disease impact
32
|
|
FINANCIAL OVERVIEW
Courageous Patients. Bold Effort.
33
|
|
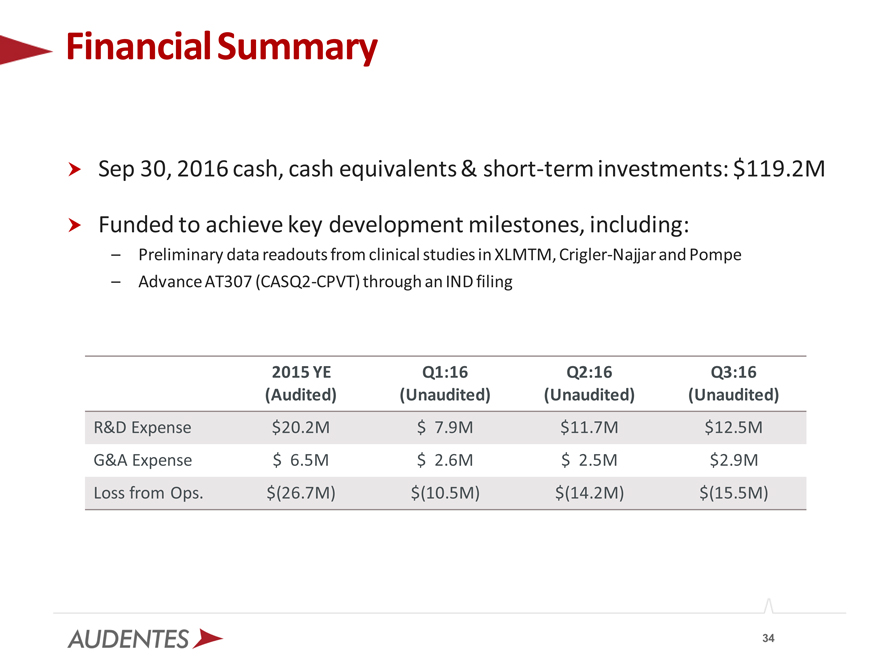
Financial Summary
Sep 30, 2016 cash, cash equivalents & short-term investments: $119.2M
Funded to achieve key development milestones, including:
– Preliminary data readouts from clinical studies in XLMTM, Crigler-Najjar and Pompe
– Advance AT307 (CASQ2-CPVT) through an IND filing
2015 YE Q1:16 Q2:16 Q3:16 (Audited) (Unaudited) (Unaudited) (Unaudited)
R&D Expense $20.2M $ 7.9M $11.7M $12.5M G&A Expense $ 6.5M $ 2.6M $ 2.5M $2.9M Loss from Ops. $(26.7M) $(10.5M) $(14.2M) $(15.5M)
34
|
|
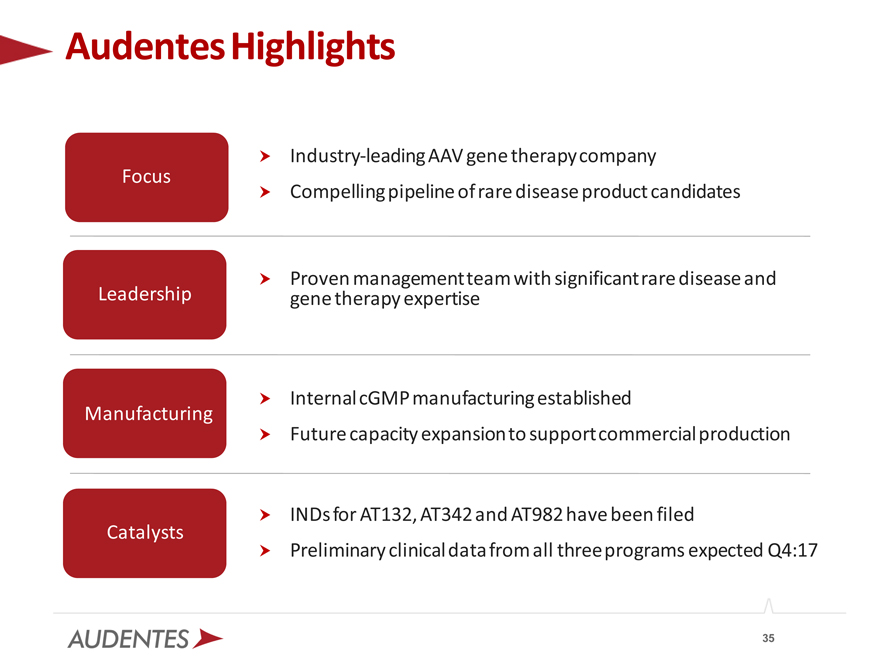
Audentes Highlights
Industry-leading AAV gene therapy company Focus
Compelling pipeline of rare disease product candidates
Proven management team with significant rare disease and Leadership gene therapy expertise
Internal cGMP manufacturing established Manufacturing Future capacity
expansion to support commercial production
? INDs for AT132, AT342 and AT982 have been filed Catalysts
? Preliminary clinical data from all three programs expected Q4:17
35
|
|
Audentes Corporate Overview
Cowen and Company 37th Annual Health Care Conference March 7, 2017
Courageous Patients. Bold Effort.