Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - AAC Holdings, Inc. | aac-ex322_15.htm |
| EX-32.1 - EX-32.1 - AAC Holdings, Inc. | aac-ex321_14.htm |
| EX-31.2 - EX-31.2 - AAC Holdings, Inc. | aac-ex312_11.htm |
| EX-31.1 - EX-31.1 - AAC Holdings, Inc. | aac-ex311_7.htm |
| EX-23.1 - EX-23.1 - AAC Holdings, Inc. | aac-ex231_10.htm |
| EX-21.1 - EX-21.1 - AAC Holdings, Inc. | aac-ex211_17.htm |
| EX-10.38 - EX-10.38 - AAC Holdings, Inc. | aac-ex1038_1458.htm |
f
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
or
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-36643
AAC HOLDINGS, INC.
(Exact Name of Registrant as Specified in Its Charter)
|
Nevada |
|
35-2496142 |
|
(State or other jurisdiction of |
|
(I.R.S. Employer |
200 Powell Place
Brentwood, Tennessee 37027
(Address, including zip code, of registrant’s principal executive offices)
(615) 732-1231
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each Class |
|
Name of exchange on which registered |
|
Common Stock, $0.001 par value |
|
New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☒ |
|
|
|
|
|
|||
|
Non-accelerated filer |
|
☐ (Do not check if a smaller reporting company) |
|
Smaller reporting company |
|
☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of June 30, 2016, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant (based on the closing price of $22.82 on that date), was approximately $247,400,000. Common stock held by each officer and director and by each person known to the registrant who owned 5% or more of the outstanding common stock has been excluded in that such persons may be deemed affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 24, 2017, there were 23,690,436 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive proxy materials for its 2017 Annual Meeting of Stockholders are incorporated by reference into Part III hereof.
ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
2
Unless we indicate otherwise or the context requires, “we,” “our,” “us” and the “company” refer, prior to the Reorganization Transactions discussed below, to American Addiction Centers, Inc. and, after the Reorganization Transactions, to AAC Holdings, Inc., in each case together with their consolidated subsidiaries, respectively. The term “Holdings” refers to AAC Holdings, Inc. and the term “AAC” refers to American Addiction Centers, Inc.
Company Overview
We are a provider of inpatient and outpatient substance abuse treatment services for individuals with drug and alcohol addiction. In addition to our inpatient and outpatient treatment services, we perform drug testing and diagnostics laboratory services and provide physician services to our clients. As of December 31, 2016, we operated 12 residential substance abuse treatment facilities located throughout the United States, focused on delivering effective clinical care and treatment solutions across 1,140 beds, which includes 668 licensed detoxification beds, 18 standalone outpatient centers, and 202 sober living beds across three locations.
We are in the process of expanding Oxford Treatment Center, where we opened an additional 24 residential beds in September 2016 and currently anticipate completing an additional 24 residential beds and 48 sober living beds in the second quarter of 2017. In addition, we have leased one floor from New Orleans East Hospital in New Orleans, Louisiana, where we intend to operate 36 in-network beds to provide detoxification and residential treatment services. The beds are expected to be operational in the second quarter of 2017, subject to receiving licensure, and will be operated by Townsend’s clinical staff.
We are also an internet marketer in the addiction treatment industry operating a broad portfolio of internet assets that service millions of website visits each month. Through our websites such as Rehabs.com and Recovery.org, we serve families and individuals struggling with addiction and seeking treatment options through comprehensive online directories of treatment providers, treatment provider reviews, forums and professional communities. Recovery Brands also provides online marketing solutions to other treatment providers such as enhanced facility profiles, audience targeting, lead generation and tools for digital reputation management.
AAC Holdings, Inc. was incorporated as a Nevada corporation on February 12, 2014 for the purpose of acquiring the common stock of American Addiction Centers, Inc. and to engage in certain reorganization transactions in connection with the initial public offering of our common stock, which was completed in October 2014 (the “IPO”). For additional discussion of the reorganization transactions completed to facilitate our IPO, see Note 3. Reorganization Transactions to our audited financial statements included elsewhere in this report.
Business Strategy
We have developed our company and the American Addiction Centers national brand through substantial investment in our facilities, our clinical expertise, our professional staff and our national sales and marketing program. We seek to extend our position as a leading provider of treatment for drug and alcohol addiction by executing the following growth strategies:
|
|
• |
Improve census at existing facilities. We seek to improve census and client demand by increasing our client leads through our multi-faceted sales and marketing program, consisting of our national sales team, recommendations from alumni and healthcare professionals, internet, television and print advertising and potential client inquiries. By utilizing multiple sales and marketing channels, we generate significant inbound call volume from potential clients and the people close to them, and our consultative call center approach enables us to effectively identify and enroll qualified clients. |
|
|
• |
Expand capacity at existing residential and standalone outpatient facilities. When market conditions indicate, we anticipate selectively increasing our number of residential beds, expanding our clinical facility space, expanding our sober living bed capacity and hiring additional clinical staff to enable us to provide services to additional clients. During 2016, we increased the number of beds licensed for detoxification at Sunrise House, a New Jersey-based provider of substance abuse treatment and rehabilitation centers, to 29 beds from 18 beds and completed the addition of 24 residential beds at Oxford Treatment Center. |
Expand outpatient operations. We actively pursue opportunities to add outpatient centers to complement our broader network of residential treatment facilities. We believe expanding our reach by acquiring or developing premium outpatient facilities of a quality consistent with our inpatient services will further enhance our brand and our ability to provide a more comprehensive suite of services across the spectrum of care. Outpatient centers are expected to be an increasingly important source of leads for our residential programs as we believe a portion of clients receiving outpatient treatment will ultimately need a higher level of care. Moreover, we believe this will position us to better serve those clients whose payors require outpatient treatment as a prerequisite to any inpatient treatment. On April 1, 2016, we completed the acquisition of Wetsman Forensic Medicine, L.L.C. and its affiliates (d/b/a “Townsend”), which operates seven in-network outpatient centers throughout Louisiana (“the Townsend Acquisition”), and on May 3, 2016, we
3
completed the acquisition of Solutions Recovery, Inc., and its three in-network outpatient centers (collectively, “Solutions”), in Las Vegas, Nevada (the “Solutions Acquisition”).
We also provide sober living accommodations to clients completing treatment at lower levels of care. These sober living arrangements enable us to utilize existing beds for clients requiring higher levels of care while still providing an interim environment for clients transitioning from residential treatment centers to lower levels of care and eventually back to their former living arrangements. We anticipate that we will continue to expand our sober living accommodations to complement our expanding outpatient services. On April 18, 2016, we acquired a 100-bed hotel in Arlington, Texas that we are in the process of converting into sober living beds, and on May 3, 2016, as part of the Solutions Acquisition, we acquired a 116-bed hotel that we are converting into 100 sober living beds.
|
|
• |
Pursue de novo development of facilities. De novo development plays an important role in the growth of our facility base. Our de novo facility development consists of either building a new facility from the ground up or acquiring an existing facility with an alternative use and repurposing it as a substance abuse treatment facility. We have developed four full-service residential treatment facilities: Greenhouse, a former luxury spa in Dallas, Texas; Desert Hope, a former assisted living facility in Las Vegas, Nevada; River Oaks, a former adolescent behavioral facility in Riverview (Tampa area), Florida; and Laguna Treatment Hospital, a chemical dependency recovery hospital in Aliso Viejo, California, which is the first such hospital designation for an AAC treatment center and which began treating clients on June 16, 2016. We believe the success of these facilities provides us with the experience to develop additional premium facilities across the United States with comparable scale, capabilities and quality. |
Additionally, on February 24, 2015, we completed the acquisition of the Ringwood property, the site of a former convent on 96 acres in Ringwood, New Jersey, and in April 2016 began the development of a 150-bed residential treatment center at this location. The facility is expected to be completed in the first half of 2018 with a projected capital investment of approximately $25.0 million.
|
|
• |
Opportunistically pursue acquisitions of individual treatment facilities and multi-facility operations. We selectively seek opportunities to expand and diversify our geographic presence, service offerings and the portion of the population that can access our services based on their individual healthcare coverage through acquisitions. For example, all six acquisitions of treatment providers we have completed since the completion of our IPO have involved the acquisition of in-network providers. Over time, we plan to establish and maintain more of a balance between the number of in-network and out-of-network facilities/beds we operate and complement our commercial offerings with private-pay facilities as well. IBISWorld, an industry research organization, estimates that there were approximately 10,000 mental health and substance abuse treatment companies in operation in 2016, most of which are small, regional operations. We believe this high level of fragmentation presents us with the opportunity to acquire facilities or small providers and upgrade their treatment programs and facilities to improve client care and as a result improve our operating metrics. We believe that our brand recognition, marketing platform and referral network will enable us to improve census at acquired facilities. |
|
|
• |
Target complementary growth opportunities. There are additional growth opportunities that we may selectively pursue that are complementary to our current business. These may include, without limitation, providing pharmacy and laboratory services, expanding licensure of existing facilities, treating other mental health and wellness disorders, providing services to other substance abuse treatment providers and expanding other ancillary services. For example, our high complexity, mass spectrometry laboratory, Addiction Labs of America, is capable of providing full service clinical diagnostic testing (including toxicology, hematology, chemistry, infectious disease, hormones and genetics) in 44 states, including California, Florida, Louisiana, Mississippi, Nevada, New Jersey, Rhode Island and Texas, where we currently have operating facilities. On April 4, 2016, we expanded our laboratory services to include hematology testing across all of our existing facilities. Also during 2016, we began providing diagnostic laboratory services to clients of third-party addiction treatment providers. |
4
We provide quality, comprehensive and compassionate care to adults struggling with alcohol and/or drug abuse and dependence as well as co-occurring mental health issues. We maintain a research-based, disciplined treatment plan for all clients with schedules designed to engage the client in an enriched recovery experience. Our purpose and passion is to empower the individual, their families and the broader community through the promotion of optimal wellness of the mind, body and spirit.
Our curriculum, which is peer reviewed and research-based, has been recognized as one of our program strengths by the Commission on Accreditation of Rehabilitation Facilities, or CARF, a leader in the promotion and accreditation of quality, value and optimal outcomes of service. In particular, research studies show that certain aspects of our treatment programs, such as offering longer treatment stays, are effective for producing long-term recovery. In addition, we offer a variety of forms of therapy types and settings and related services that the National Institute on Drug Abuse has recognized as effective. We offer the following types of therapy: motivational interviewing, cognitive behavioral therapy, rational emotive behavior therapy, dialectical behavioral therapy, solution-focused therapy, eye movement desensitization and reprocessing and systematic family intervention. Our variety of therapy settings include individual, group and family therapies, recovery-oriented challenge therapies, expressive therapies (with a focus on music and art) and equine and trauma therapies.
Considering the high level of co-occurring substance abuse, mental health and medical conditions, we offer clients a spectrum of psychiatric, medical and wellness-focused services based upon individual needs as assessed through comprehensive evaluations at admission and throughout participation in the program. To maximize the likelihood of long-term recovery, all program levels provide clients access to the following services: assessment of individual substance abuse, mental health and medical history and physical within 72 hours of admission; psychiatric evaluations; psychological evaluations and services based on client needs; follow-up appointments with physicians and psychiatrists; medication monitoring; educational classes regarding health risks, nutrition, smoking cessation, HIV awareness, life skills, healthy nutritional programs and dietary plans; access to fitness facilities; interactive wellness activities such as swimming, basketball and yoga and structured daily schedules designed for restorative sleep patterns.
We emphasize clinical treatment, as well as the therapeutic value of overall physical and nutritional wellness. We are committed to providing fresh and nutritious meals throughout a client’s stay in order to promote healthy routines, beginning with diet and exercise. Some of our facilities offer comprehensive work-out facilities, and many locations offer various exercise classes and other amenities. We support long-term recovery for clients through research-based methodologies and individualized treatment planning while utilizing 12 step programs, which are a set of guiding principles outlining a course of action for recovery.
We believe we have a differentiated ability to manage dual diagnosis cases and coordinate treatment of individuals suffering from the common combination of mental illness and substance abuse simultaneously. These clients participate in education and discussion-oriented groups designed to provide information regarding the psychiatric disorders that co-occur with chemical dependency.
We place a strong emphasis on tracking client satisfaction scores in order to measure our client and staff interaction and overall outcome and reputation. In addition to client satisfaction surveys that we receive after a client’s discharge, we also solicit feedback during a client’s stay at our residential facilities. This allows us to further tailor an individual’s treatment plan to emphasize the programs that have been more impactful and helpful to a particular client.
We believe in tracking clinical outcomes. We have entered into a partnership with Centerstone Research Institute to conduct independent three-year longitudinal outcome studies on the effectiveness of our clinical approach. We are currently entering the third year of this arrangement and anticipate disseminating findings in 2017.
As detailed below, we offer a full spectrum of treatment services to clients, based upon individual needs as assessed through comprehensive evaluations at admission and throughout participation in the program. The assignment to, and frequency of, services corresponds to individualized treatment plans within the context of the level of care and treatment intensity level.
Detoxification: Detoxification is usually conducted at an inpatient facility for clients with physical or psychological dependence. Detoxification services are designed to clear toxins out of the body so that the body can safely adjust and heal itself after being dependent upon a substance. Clients are medically monitored 24 hours per day, seven days per week by experienced medical professionals who work to alleviate withdrawal symptoms through medication, as appropriate. We provide detoxification services for several substances including alcohol, sedatives and opiates.
Residential Treatment: Residential care is a structured treatment approach designed to prepare clients to return to the general community with a sober lifestyle, increased functionality and improved overall wellness. Treatment is provided on a 24 hours per day, seven days per week basis, and services generally include a minimum of two individual therapy sessions per week, regular group therapy, family therapy, didactic and psycho-educational groups, exercise (if cleared by medical staff), case management and recreational activities. Medical and psychiatric care is available to all clients, as needed, through our contracted professional physician groups.
Partial Hospitalization: Partial hospitalization is a structured program providing care at least five days a week for no fewer than six hours a day. This program is designed for clients who are stable enough physically and psychologically to participate in everyday activities but who still require a degree of medical monitoring. Services include a minimum of weekly individual therapy,
5
regular group therapy, family education and therapy, didactic and psycho-educational groups, exercise (if cleared by medical staff), case management and off-site recovery meetings and activities. Medical and psychiatric care is available to all clients, as needed, through our contracted professional physician groups.
Intensive Outpatient Services: Less intensive than the aforementioned levels of care, intensive outpatient services is a structured program providing care three days per week for three hours per day at a minimum. Designed as a “step down” from partial hospitalization, this program reinforces progress and assists in the attainment of sobriety, reduction of detrimental behaviors and improved overall wellness of clients while they integrate and interact in the community. Services include weekly individual therapy, group therapy, family education and therapy, didactic and psycho-educational groups, case management, off-site recovery meetings and activities and intensive transitional and aftercare planning.
Ancillary Services: In addition to our inpatient and outpatient treatment services, we provide medical monitoring for adherence to addiction treatment as well as clinical diagnostic laboratory services. We also provide physician services to our clients through our contracted professional physician groups. We believe toxicological monitoring of clients is an important component of substance abuse treatment. Clients are evaluated for illicit substances upon admission and thereafter on a random basis and as otherwise determined to be medically necessary by the treating physician. We conduct laboratory testing for our facilities using quantitative liquid chromatography time-of-flight mass spectrometry technology located in Brentwood, Tennessee as well as our laboratory in Slidell, Louisiana.
Sober Living Facilities: We provide sober living arrangements that serve as an interim environment for clients transitioning from residential treatment centers to lower levels of care and eventually back to their former living arrangements. Sober living facilities enable us to utilize existing beds for clients requiring higher levels of care, while still providing housing for clients completing outpatient treatment programs. We provide sober living arrangements to clients through our owned and leased properties in Arlington, Texas, Las Vegas, Nevada, Oxford, Mississippi, and through third party providers with whom we contract to provide sober living arrangements near our existing outpatient centers. We typically provide transportation between sober living housing and our outpatient centers. We expect that we will continue to rely on sober living facilities as a complement to our outpatient services.
6
The following table presents information about our network of substance abuse treatment facilities, including current facilities, facilities under development and properties under contract:
|
|
|
|
|
|
|
|
|
|
|
|
|
Real Property |
|
|
|
|
|
Facility |
|
|
|
Beds |
|
Services |
|
Owned / |
|
Facility Name |
|
Location |
|
Type(1) |
|
Current(2) |
|
Pending |
|
Provided(3) |
|
Lease |
|
California |
|
|
|
|
|
|
|
|
|
|
|
|
|
Forterus |
|
Temecula |
|
OON |
|
112(4) |
|
― |
|
DTX, RTC, PHP, IOP |
|
Leased |
|
San Diego Addiction Treatment Center |
|
San Diego |
|
OON |
|
36 |
|
― |
|
DTX, RTC, PHP, IOP |
|
Leased |
|
Laguna Treatment Hospital |
|
Aliso Viejo |
|
OON |
|
93 |
|
― |
|
DTX, RTC, PHP, IOP |
|
Owned |
|
Florida |
|
|
|
|
|
|
|
|
|
|
|
|
|
Recovery First |
|
Fort Lauderdale |
|
IN |
|
72(5) |
|
― |
|
DTX, RTC, PHP |
|
Owned / Leased |
|
Recovery First - West Palm(6) |
|
West Palm Beach |
|
IN |
|
65 |
|
― |
|
PHP, IOP |
|
Leased |
|
River Oaks |
|
Riverview (Tampa area) |
|
OON |
|
162 |
|
― |
|
DTX, RTC, PHP, IOP |
|
Owned |
|
Louisiana |
|
|
|
|
|
|
|
|
|
|
|
|
|
Townsend Treatment Center |
|
Lafayette |
|
IN |
|
32(7) |
|
― |
|
DTX, RTC, PHP, IOP |
|
Leased |
|
TBD |
|
New Orleans |
|
IN |
|
― |
|
36(8) |
|
DTX, RTC, PHP |
|
Leased |
|
Townsend Outpatient Centers |
|
Lafayette |
|
IN |
|
n/a(7) |
|
n/a |
|
IOP |
|
Leased |
|
Mississippi |
|
|
|
|
|
|
|
|
|
|
|
|
|
Oxford Treatment Center |
|
Etta |
|
OON |
|
100(9) |
|
24(9) |
|
DTX, RTC, PHP, IOP |
|
Owned |
|
Oxford Outpatient Center |
|
Oxford, Tupelo, and Olive Branch |
|
OON |
|
n/a |
|
n/a |
|
IOP |
|
Leased |
|
Resolutions Oxford |
|
Oxford |
|
n/a |
|
24 |
|
48(9) |
|
Sober Living |
|
Owned /Leased |
|
Nevada |
|
|
|
|
|
|
|
|
|
|
|
|
|
Desert Hope |
|
Las Vegas |
|
OON |
|
148 |
|
― |
|
DTX, RTC, PHP, IOP |
|
Owned |
|
Desert Hope Outpatient Center |
|
Las Vegas |
|
OON |
|
n/a |
|
n/a |
|
IOP |
|
Owned |
|
Solutions Treatment Center |
|
Las Vegas |
|
IN |
|
80(10) |
|
― |
|
DTX, RTC, PHP, IOP |
|
Owned |
|
Resolutions Las Vegas |
|
Las Vegas |
|
n/a |
|
114 |
|
14(11) |
|
Sober Living |
|
Owned / Leased |
|
New Jersey |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sunrise House |
|
Lafayette (New York City area) |
|
IN |
|
110(12) |
|
― |
|
DTX, RTC, PHP, IOP |
|
Owned |
|
Sunrise House Outpatient |
|
Lafayette |
|
IN |
|
n/a |
|
n/a |
|
IOP |
|
Leased |
|
TBD |
|
Ringwood (New York City area) |
|
OON |
|
― |
|
150(13) |
|
DTX, RTC, PHP, IOP |
|
Owned |
|
Rhode Island |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical Services of Rhode Island Outpatient |
|
Greenville, Portsmouth and South Kingstown |
|
IN |
|
n/a |
|
n/a |
|
IOP |
|
Leased |
|
Texas |
|
|
|
|
|
|
|
― |
|
|
|
|
|
Greenhouse |
|
Grand Prairie (Dallas area) |
|
OON |
|
130 |
|
― |
|
DTX, RTC, PHP, IOP |
|
Owned |
|
Greenhouse Outpatient Center |
|
Arlington (Dallas area) |
|
OON |
|
n/a |
|
n/a |
|
IOP |
|
Owned |
|
Resolutions Arlington |
|
Arlington (Dallas area) |
|
n/a |
|
64(14) |
|
60(14) |
|
Sober Living |
|
Owned / Leased |
|
Totals |
|
|
|
|
|
1,342 |
|
332 |
|
|
|
|
7
|
|
(1) |
Facility type reflects the primary payor type of the clients served at the facility: Out-of-network (OON) or in-network (IN). |
|
|
(2) |
Bed capacity reflected in the table represents total available beds. Actual capacity utilized depends on current staffing levels at each facility and may not equal total bed capacity at any given time. |
|
|
(3) |
DTX: Detoxification; RTC: Residential Treatment; PHP: Partial Hospitalization; IOP: Intensive Outpatient. |
|
|
(4) |
During 2016, we increased our capacity at Forterus from 107 beds to 112 beds. |
|
|
(5) |
During 2016, we increased our capacity at Recovery First from 63 beds to 72 beds. |
|
|
(6) |
On September 29, 2016, Singer Island transferred substantially all of its assets to Recovery First and now operates as Recovery First West Palm. In conjunction with this asset transfer, Recovery First West Palm will begin accepting clients with in-network benefits. |
|
|
(7) |
On April 1, 2016, we completed the Townsend Acquisition for $13.5 million in cash and $8.5 million in restricted shares of our common stock. |
|
|
(8) |
We have leased one floor from New Orleans East Hospital in New Orleans, Louisiana, where we intend to operate 36 in-network beds to provide detoxification and residential treatment services. The beds are expected to be operational in the second quarter of 2017, subject to receiving licensure, and will be operated by Townsend’s clinical staff. |
|
|
(9) |
On September 22, 2016, we opened an additional 24 residential beds at Oxford Treatment Center and currently anticipate opening an additional 24 beds licensed for detoxification and 48 sober living beds in the second quarter of 2017. |
|
|
(10) |
On May 3, 2016, we completed the Solutions Acquisition for $6.75 million in cash and $6.25 million in restricted shares of our common stock. |
|
|
(11) |
We are currently in the process of renovating Resolutions Las Vegas to accommodate an additional 14 sober living beds that we expect to complete by the end of the second quarter of 2017. |
|
|
(12) |
At the end of the second quarter of 2016, we increased our beds licensed for detoxification at Sunrise House from 18 to 29 beds. |
|
|
(13) |
We acquired this property on February 24, 2015 and have begun development of a residential treatment center. The facility is currently anticipated to be completed in the first half of 2018. |
|
|
(14) |
We are currently in the process of completing the conversion of Resolutions Arlington into sober living beds that will be used in support of the Greenhouse Outpatient Center. We began accepting sober living clients at the end of the third quarter of 2016 and currently have a total of 64 sober living beds. We currently anticipate completion of the remaining renovations by the end of the second quarter of 2017, which will result in an additional 60 sober living beds. |
Sales and Marketing
Sales and marketing supports the development of our brand and advances our comprehensive lead-generation platform. The primary sources of our new clients include:
|
|
• |
National Sales Force. We deploy and manage a sales force of over 65 representatives nationwide that focuses primarily on marketing to hospitals, other treatment facilities, employers, unions, alumni and employee assistance programs. In addition, our varied facilities located across the United States allow us to reach a broad audience of potential clients and their families and build a nationally recognized brand. This nationally branded, multi-channel approach has helped increase our number of admitted clients from 7,763 in 2015 to 11,733 in 2016, an increase of 51%. |
|
|
• |
Internet, Television and Print Advertising. Advertising through various media represents another important opportunity to obtain new clients as well as to develop our national brand. We maintain and run a series of television commercials that promote our facilities and overall capabilities and also maintain a strong presence on the internet. We operate a broad portfolio of internet assets that service millions of website visits each month. Through comprehensive online directories of treatment providers, treatment provider reviews, forums and professional communities, our addiction-related websites such as Rehabs.com and Recovery.org serve families and individuals struggling with addiction and seeking treatment options. Additionally, we continue to make advertising efforts in radio spots, newspaper articles, medical journals and other print media with the intent to build our integrated, national brand. |
|
|
• |
Recommendations by Alumni. We often receive new clients who were directly referred to our facilities by our alumni as well as their friends and families. As our national brand continues to grow and our business continues to increase, we believe our alumni will become an increasingly important source of business for us. |
Call Center Operations
We maintain a 24 hours per day, seven days per week call center currently staffed by over 130 employees. Our centralized call center is situated at our corporate headquarters in Brentwood, Tennessee, and focuses on enrolling clients identified by our sales and marketing activities into new client admissions. As part of its role, the call center team conducts benefits verification and handles initial communication with insurance companies, completes client assessments, begins the pre-certification process for treatment authorization, chooses the proper treatment facility for the client’s clinical and financial needs and assists clients with arrangements and logistics.
8
We are affiliated with a professional group in seven of the states in which we currently operate (the “Professional Groups”). These Professional Groups engage physicians and mid-level service providers and provide professional services to our clients through professional services agreements with each treatment facility. Under the professional services agreements, the Professional Groups also provide a physician to serve as medical director for the applicable facility. The Professional Groups either bill the payor for their services directly or are compensated by the treatment facility based on fair market value hourly rates. Each of the professional services agreements has a term of five years and will automatically renew for additional one year periods. For additional information related to the Professional Groups, see Note 2 to our consolidated financial statements included elsewhere in this report.
Competition
We believe we are one of the leading for-profit companies focused on substance abuse treatment in the United States. According to IBISWorld, approximately 77% of all substance abuse treatment clinics in the United States have a single location, and approximately 57% of all substance abuse treatment clinics have fewer than 20 employees. Many of the largest for-profit addiction treatment providers operate in the broader behavioral healthcare sector without focusing primarily on substance abuse. We believe our size and core focus on substance abuse treatment provide us with an advantage over competitors in terms of building our brand and marketing our platform to potential clients.
The market for mental health and substance abuse treatment facilities is highly fragmented with approximately 10,000 different companies providing services to the adult and adolescent population, of which only 35% are operated by for-profit organizations. Our residential treatment facilities compete with several national competitors and many regional and local competitors. Some of our competitors are government entities and supported by tax revenues, and others are non-profit entities that are primarily supported by endowments and charitable contributions. We do not receive financial support from these sources. Some larger companies in our industry compete with us on a national scale and offer substance abuse treatment services among other behavioral healthcare services. To a lesser extent, we also compete with other providers of substance abuse treatment services, including other inpatient behavioral healthcare facilities and general acute care hospitals.
We believe the primary competitive factors affecting our business include:
|
|
• |
quality of clinical programs and services; |
|
|
• |
reputation and brand recognition; |
|
|
• |
overall aesthetics of the facilities; |
|
|
• |
amenities offered to clients; |
|
|
• |
relationships with payors and referral sources; |
|
|
• |
sales and marketing capabilities; |
|
|
• |
information systems and proprietary data analytics; |
|
|
• |
senior management experience; and |
|
|
• |
national scope of operations. |
Regulatory Matters
Overview
Substance abuse treatment providers are regulated extensively at the federal, state and local levels. In order to operate our business and obtain reimbursement from third-party payors, we must obtain and maintain a variety of licenses, permits, certifications and accreditations. We must also comply with numerous other laws and regulations applicable to the conduct of business by substance abuse treatment providers. Our facilities are also subject to periodic on-site inspections by the agencies that regulate and accredit them in order to determine our compliance with applicable requirements.
The laws and regulations that affect substance abuse treatment providers are complex and change frequently. We must regularly review our organization and operations and make changes as necessary to comply with changes in the law or new interpretations of laws or regulations. In recent years, significant public attention has focused on the healthcare industry, including attention to the conduct of industry participants and the cost of healthcare services. Federal and state government agencies have heightened and coordinated civil and criminal enforcement efforts relating to the healthcare industry. The ongoing investigations relate to, among other things, various referral practices, cost reporting, billing practices, credit balances, physician ownership and joint ventures involving hospitals and other healthcare providers. We expect that healthcare costs and other factors will continue to encourage both the development of new laws and regulations and increased enforcement activity.
9
We believe we are in substantial compliance with all applicable laws and regulations and are not aware of any material pending or threatened investigations involving allegations of wrongdoing. Compliance with such laws and regulations may be subject to future government review and interpretation, as well as significant regulatory action including fines, penalties and exclusion from government health programs.
Licensure, Accreditation and Certification
All of our substance abuse treatment facilities are licensed under applicable state laws where licensure is required. Licensing requirements vary significantly depending upon the state in which a facility is located and the types of services provided. The types of licensed services that our facilities provide include medical detox, residential, partial hospitalization, intensive outpatient, outpatient treatment, ambulatory detox and community housing. In addition, our employed case managers, therapists, nurses and medical providers and technicians may be subject to individual state license requirements.
Our facilities that store and dispense controlled substances are required to register with the U.S. Drug Enforcement Administration, or DEA, and abide by DEA regulations regarding controlled substances. Each of our substance abuse treatment facilities has obtained or is in the process of obtaining accreditation from CARF and/or The Joint Commission, which are the primary accreditation bodies in the substance abuse treatment industry. This type of accreditation program is intended to improve the quality, safety, outcomes and value of healthcare services provided by accredited facilities. CARF and The Joint Commission require an initial application and completion of on-site surveys demonstrating compliance with accreditation requirements. Accreditation is granted for a specified period, typically ranging from one to three years, and renewals of accreditation require completion of a renewal application and an on-site renewal survey.
The Clinical Laboratory Improvement Amendments of 1988, or CLIA, regulates virtually all clinical laboratories by requiring that they be certified by the federal government and comply with various technical, operational, personnel and quality requirements intended to ensure that laboratory testing services are accurate, reliable and timely. Standards for testing under CLIA are based on the level of complexity of the tests performed by the laboratory. Each of our treatment facilities at which waived testing, such as point-of-care drug analysis, glucose monitoring, and pregnancy testing, is performed has a CLIA certificate of waiver.
Our Brentwood, Tennessee, clinical laboratory facility and our Slidell, Louisiana laboratory facility both perform high complexity testing, such as time-of-flight mass spectrometry. Both laboratories hold a CLIA certificate of accreditation, certifying them for complex testing, and are therefore required to meet more stringent requirements than laboratories performing less complex testing. We are regularly subject to survey and inspection to assess compliance with program standards. It is also accredited by COLA and participates in the College of American Pathologists (“CAP”) proficiency program.
CLIA does not preempt state laws that are more stringent than federal law. State laws may require additional personnel qualifications, quality control, record maintenance and/or proficiency testing. A number of states in which we operate have implemented their own regulatory and licensure requirements. In addition, some states require laboratories that solicit or test samples collected from individuals within that state to hold a laboratory license even though the laboratory does not have physical operations within the state. Our Brentwood laboratory facility is licensed as a medical reference laboratory by the state of Tennessee. It is also licensed in other states as required to process test samples originating from individuals within such states.
We believe that all of our facilities and programs are in substantial compliance with current applicable state and local licensure, certification and accreditation requirements. Periodically, state and local regulatory agencies as well as accreditation entities conduct surveys of our facilities and may find from time to time that a facility is not in full compliance with all of the accreditation standards. Upon receipt of any such finding, the facility timely submits a plan of correction and corrects any cited deficiencies.
FDA Laws and Regulations
The Food and Drug Administration (“FDA”) has regulatory responsibility over, among other areas, instruments, test kits, reagents and other devices used by clinical laboratories to perform diagnostic testing. A number of esoteric tests we develop internally are offered as laboratory developed tests (“LDTs”). The FDA has claimed regulatory authority over all LDTs but has stated that it exercises enforcement discretion for most LDTs performed by high complexity CLIA-certified laboratories. The FDA released draft guidance in 2014 that would increase regulation of LDTs but has indefinitely delayed finalizing the guidance.
Fraud, Abuse and Self-Referral Laws
We do not currently bill or accept payments from Medicare or Medicaid. Therefore, our operations are generally not impacted by the anti-kickback provisions of the Social Security Act, commonly known as the Anti-kickback Statute, or the federal prohibition on physician self-referrals, commonly referred to as the Stark Law. The Anti-kickback Statute prohibits the payment, receipt, offer or solicitation of remuneration of any kind in exchange for items or services that are reimbursed under federal healthcare programs. The Stark Law prohibits physicians from referring Medicare and Medicaid patients to healthcare providers that furnish certain designated health services, including laboratory services and inpatient and outpatient hospital services, if the physicians or their immediate family members have ownership interests in, or other financial arrangements with, the healthcare providers, unless an exception applies. Many states have anti-kickback and physician self-referral prohibitions similar to the federal statutes and regulations. Some of these state laws are drafted broadly to cover all payors (i.e., not restricted to Medicare and other federal healthcare programs), and they often lack interpretative guidance. A violation of these laws could result in a prohibition on billing
10
payors for such services or an obligation to refund amounts received, result in civil or criminal penalties and adversely affect the state license of any program or facility found to be in violation.
Federal prosecutors have broad authority to prosecute healthcare fraud. For example, federal law criminalizes the knowing and willful execution or attempted execution of a scheme or artifice to defraud any healthcare benefit program as well as obtaining by false pretenses any money or property owned by any healthcare benefit program. Federal law also prohibits embezzlement of healthcare funds, false statements relating to healthcare and obstruction of the investigation of criminal offenses. These federal criminal offenses are enforceable regardless of whether an entity or individual participates in the Medicare program or any other federal healthcare program.
False Claims
We are subject to state and federal laws that govern the submission of claims for reimbursement. These laws generally prohibit an individual or entity from knowingly and willfully presenting a claim (or causing a claim to be presented) for payment from Medicare, Medicaid or other third-party payors that is false or fraudulent. The standard for “knowing and willful” often includes conduct that amounts to a reckless disregard for whether accurate information is presented by claims processors. Penalties under these statutes include substantial civil and criminal fines, exclusion from the Medicare program and imprisonment.
One of the most prominent of these laws is the federal False Claims Act, or FCA, which may be enforced by the federal government directly or by a qui tam plaintiff (or whistleblower) on the government’s behalf. When a private plaintiff brings a qui tam action under the FCA, the defendant often will not be made aware of the lawsuit until the government commences its own investigation or determines whether it will intervene. When a defendant is determined by a court of law to be liable under the FCA, the defendant may be required to pay three times the amount of the alleged false claim, plus mandatory civil penalties of between $10,957 and $21,916 for each separate false claim, after taking into account 2017 updates to such penalties. These and certain other civil monetary penalties will increase annually based on updates to the consumer price index.
Many states have passed false claims acts similar to the FCA. Under these laws, the government may impose a penalty and recover damages, often treble damages, for knowingly submitting or participating in the submission of claims for payment that are false or fraudulent or which contain false or misleading information. These laws may be limited to specific programs (such as state workers’ compensation programs) or may apply to all payors. In many cases, alleged violations of these laws may be brought by a whistleblower who may be an employee, a referring physician, a competitor, a client or other individual or entity, and who may be eligible for a portion of any recovery. Further, like the federal law, state false claims act laws generally protect employed whistleblowers from retribution by their employers.
Although we believe that we have procedures in place to ensure the accurate completion of claims forms and requests for payment, the laws, regulations and standards defining proper billing, coding and claim submission are complex and have not been subjected to extensive judicial or agency interpretation. Billing errors can occur despite our best efforts to prevent or correct them, and we cannot assure you that the government or a payor will regard such errors as inadvertent and not in violation of the applicable false claims act laws or related statutes.
Privacy and Security Requirements
There are numerous federal and state regulations that address the privacy and security of client health information. In particular, federal regulations issued under the Drug Abuse Prevention, Treatment and Rehabilitation Act of 1979 strictly restrict the disclosure of client identifiable information related to substance abuse and apply to any of our facilities that receive any federal assistance, which is interpreted broadly to include facilities licensed, certified or registered by a federal agency. Further, the Health Insurance Portability and Accountability Act of 1996, or HIPAA privacy and security regulations, extensively regulate the use and disclosure of individually identifiable health information (known as “protected health information”) and require covered entities, which include most health providers, to implement and maintain administrative, physical and technical safeguards to protect the security of such information. Additional security requirements apply to electronic protected health information. These regulations also provide clients with substantive rights with respect to their health information.
The HIPAA privacy and security regulations also require our substance abuse treatment programs and facilities to impose compliance obligations by written agreement on certain contractors to whom our programs disclose client information known as “business associates.” Covered entities may be subject to penalties as a result of a business associate violating HIPAA privacy and security regulations if the business associate is found to be an agent of the covered entity. Business associates are also directly subject to liability under the HIPAA privacy and security regulations. In instances where our programs act as a business associate to a covered entity, there is the potential for additional liability beyond the program’s covered entity status.
Covered entities must report breaches of unsecured protected health information to affected individuals without unreasonable delay but not to exceed 60 days of discovery of the breach by a covered entity or its agents. Notification must also be made to the U.S. Department of Health and Human Services, or HHS, and, in certain situations involving large breaches, to the media. HHS is required to publish on its website a list of all covered entities that report a breach involving more than 500 individuals. All non-permitted uses or disclosures of unsecured protected health information are presumed to be breaches unless the covered entity or business associate establishes that there is a low probability the information has been compromised. Various state laws and regulations may also require
11
us to notify affected individuals in the event of a data breach involving individually identifiable information without regard to whether there is a low probability of the information being compromised.
After taking into account 2017 updates to penalty amounts, violations of the HIPAA privacy and security regulations may result in civil penalties of up to $55,910 per violation for a maximum civil penalty of $1,677,299 in a calendar year for violations of the same requirement. These penalties will increase annually based on updates to the consumer price index. HIPAA also provides for criminal penalties of up to $250,000 and ten years in prison, with the severest penalties for obtaining or disclosing protected health information with the intent to sell, transfer or use such information for commercial advantage, personal gain or malicious harm. In addition, state attorneys general may bring civil actions seeking either injunction or damages in response to violations of the HIPAA privacy and security regulations that threaten the privacy of state residents. HHS is required to impose penalties for violations resulting from willful neglect and to perform compliance audits.
Our programs remain subject to any privacy-related federal or state laws that are more restrictive than the HIPAA privacy and security regulations. These laws vary by state and could impose additional requirements and penalties. For example, some states impose strict restrictions on the use and disclosure of health information pertaining to mental health or substance abuse treatment. The Federal Trade Commission also uses its consumer protection authority to initiate enforcement actions in response to data breaches or other privacy or security lapses.
We enforce a health information privacy and security compliance plan, which we believe complies with the HIPAA privacy and security regulations and other applicable requirements. Compliance with federal and state privacy and security requirements has required and will continue to require us to expend significant resources.
Mental Health Legislation and Reform Efforts
The regulatory framework in which we operate is constantly changing. We may be required to make operational changes to comply with and maximize opportunities created by new or revised laws and regulations. For example, the Mental Health Parity and Addiction Equity Act of 2008, or MHPAEA, is a federal parity law that requires large group health insurance plans that offer mental health and addiction coverage to provide that coverage on par with financial requirements and treatment limitations of coverage offered for other illnesses. The scope of coverage offered by health plans must comply with federal and state laws and must be consistent with generally recognized independent standards of current medical practice. The MHPAEA also contains a cost exemption that operates to temporarily exempt a group health plan from the MHPAEA’s requirements if compliance with the MHPAEA becomes too costly.
The 21st Century Cures Act (“Cures Act”), enacted in 2016, requires development of an action plan for enhanced enforcement of mental health parity requirements and additional compliance guidance for health plans regarding coverage under parity laws. Among other initiatives aimed at improving care for people with mental health and substance use disorders, the Cures Act includes provisions intended to increase the healthcare workforce dedicated to such treatment and expand programs that divert people with mental health and substance use disorders toward alternatives to incarceration. However, the impact of the Cures Act largely depends on its implementation by agencies, such as HHS, and on future appropriations by Congress.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, collectively known as the Affordable Care Act, resulted in extensive change to the healthcare industry, including reform of the health insurance market and adoption of a number of payment reform measures. The Affordable Care Act poses both opportunities and risks for us but, overall, the expansion of health insurance coverage under the law has been beneficial for the substance abuse treatment industry. However, the overall and continued impact of the Affordable Care Act is difficult to determine because of uncertainty around a number of factors, including the impact of the 2016 federal elections. The election of a Republican president and Republican control of both houses of Congress could result in the repeal of the Affordable Care Act or significant changes to the Affordable Care Act, its implementation and its interpretation.
Health Planning and Certificates of Need
The construction of new healthcare facilities, the expansion, transfer or change of ownership of existing facilities and the addition of new beds, services or equipment may be subject to state laws that require prior approval by state regulatory agencies under certificate of need (“CON”) laws. These laws generally require that a state agency determine the public need for construction or acquisition of facilities or the addition of new services. Review of CON applications and other healthcare planning initiatives may be lengthy and may require public hearings. Violations of these state laws may result in the imposition of civil sanctions or revocation of a facility’s license.
Other State Healthcare Laws
Most states have a variety of laws that may potentially impact our operations and business practices. For instance, many states in which our programs operate prohibit corporations (and other legal entities) from practicing medicine by employing physicians and certain non-physician practitioners. These prohibitions on the corporate practice of medicine impact how our programs structure their relationships with physicians and other affected non-physician practitioners. These arrangements, however, have typically not been vetted by either a court or the applicable regulatory body.
12
Similarly, many states prohibit physicians from sharing a portion of their professional fees with any other person or entity. These so-called fee-splitting prohibitions range from prohibiting arrangements resembling a kickback to broadly prohibiting percentage-based compensation and other variable compensation arrangements with physicians.
If our arrangements with physicians are found to violate a corporate practice of medicine prohibition or a state fee-splitting prohibition, our contractual arrangements with physicians in such states could be adversely affected, which, in turn, may adversely affect both our operations and profitability. Further, we could face sanctions for aiding and abetting the violation of the state’s medical practice act.
Local Land Use and Zoning
Municipal and other local governments also may regulate our treatment programs. Many of our facilities must comply with zoning and land use requirements in order to operate and many of our de novo acquisition targets will be contingent upon zoning and land use approvals. For example, local zoning authorities regulate not only the physical properties of a healthcare facility, such as its height and size, but also the location and activities of the facility. In addition, community or political objections to the placement of treatment facilities can result in delays in the land use permit process and may prevent the operation of facilities in certain areas.
Risk Management and Insurance
The healthcare industry in general continues to experience an increase in the frequency and severity of litigation and claims. Like other providers of healthcare-related services, we could be subject to claims that our services have resulted in injury to our clients or had other adverse effects. In addition, resident, visitor and employee injuries could also subject us to the risk of litigation. While we believe that quality care is provided to our clients and that we substantially comply with all applicable regulatory requirements, an adverse determination in a legal proceeding or government investigation could have a material adverse effect on our financial condition.
We maintain commercial insurance coverage on an occurrence basis for general and professional liability claims with no deductible, a primary $1.0 million per claim limit and an annual aggregate primary limit of $3.0 million with umbrella coverage for an aggregate $20.0 million limit.
Compliance Programs
Compliance with government rules and regulations is a significant concern throughout our industry, in part due to evolving interpretations of these rules and regulations. We seek to conduct our business in compliance with all statutes and regulations applicable to our operations. To this end, we have established a compliance program that reviews for regulatory compliance procedures, policies and facilities throughout our business. Our executive management team is responsible for the oversight and operation of our compliance program. We provide periodic and comprehensive training programs to our personnel, which are intended to promote the strict observance of our policies designed to ensure compliance with the statutes and regulations applicable to us.
On October 21, 2016, certain of our subsidiaries, AAC (formerly known as Forterus, Inc.), Forterus Health Care Services, Inc., and ABTTC, Inc. (the “Defendants”), agreed to the entry of a Permanent Injunction and Final Judgment (the “PIFJ”) with the Bureau of Medi-Cal Fraud and Elder Abuse of the Office of the Attorney General of the State of California (“BMFEA”). Pursuant to the terms of the PIFJ, we were required to, among other things, (i) institute a three-year compliance program (the “California Compliance Program”) with respect to our California facilities that includes maintaining or developing and implementing certain policies and procedures to promote each covered facility’s compliance with applicable statutes, regulations and the PIFJ, under the responsibility of our Chief Compliance Officer; (ii) establish a Compliance Committee composed of the Compliance Officer and senior personnel responsible for overseeing clinical operations to address issues raised by the Compliance Officer in connection with the Compliance Program and (iii) establish an oversight committee of the Board of Directors, or a committee of the Board of Directors, to review the adequacy and responsiveness of the California Compliance Program. In addition, for a period of 30 months following the effective date of the PIFJ, the Defendants shall retain a qualified independent monitor, appointed by BMFEA after consultation with the Defendants, to assess the effectiveness of the Defendants’ quality control systems and patient care.
Environmental, Health and Safety Matters
We are subject to various federal, state and local environmental laws that: (i) regulate certain activities and operations that may have environmental or health and safety effects, such as the handling, storage, transportation, treatment and disposal of medical and pharmaceutical waste products generated at our facilities, the presence of other hazardous substances in the indoor environment and protection of the environment and natural resources in connection with the development or construction of our facilities; (ii) impose liability for costs of cleaning up, and damages to natural resources from, past spills, waste disposals on and off-site or other releases of hazardous materials or regulated substances; and (iii) regulate workplace safety, including the safety of workers who may be exposed to blood-borne pathogens such as HIV, the hepatitis B virus and the hepatitis C virus. Our laboratory and some of our treatment facilities generate infectious or other hazardous medical waste due to the illness or physical condition of our clients and in connection with performing laboratory tests. The management of infectious medical waste is subject to regulation under various federal, state and local environmental laws, which establish management requirements for such waste. These requirements include record-keeping, notice and reporting obligations. Management believes that our operations are generally in compliance with
13
environmental and health and safety regulatory requirements or that any non-compliance will not result in a material liability or cost to achieve compliance. Historically, the costs of achieving and maintaining compliance with environmental laws and regulations at our facilities, including our laboratory, have not been material. However, we cannot assure you that future costs and expenses required for us to comply with any new, or changes in existing, environmental and health and safety laws and regulations or new or discovered environmental conditions will not have a material adverse effect on our business, financial condition or results of operations.
Employees
As of December 31, 2016, we employed approximately 2,100 people. During 2016, employees at Sunrise House voted to join the Health Professionals and Allied Employees (“HPAE”) labor union. None of our other employees are represented by a labor union or covered by a collective bargaining agreement. We believe that our employee relations are good.
Available Information
We file certain reports with the Securities and Exchange Commission (the “SEC”), including annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K. The public may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. We are an electronic filer, and the SEC maintains an Internet site at http://www.sec.gov that contains the reports, proxy and information statements and other information we file electronically. Our website address is www.americanaddictioncenters.org. We make available free of charge, through our website, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports filed or furnished pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC. Our website and the information contained therein or linked thereto are not intended to be incorporated into this Annual Report on Form 10-K.
Our actual operating results may differ materially from those described in forward-looking statements as a result of various factors, including but not limited to, those described below. You should carefully consider the following risk factors in addition to the other information included in this Annual Report on Form 10-K.
Risks Related to Our Business
Our revenues, profitability and cash flows could be materially adversely affected if we are unable to operate certain key treatment facilities, our corporate office or our laboratory facilities.
We derive a significant portion of our revenues from three treatment facilities located in Florida, Nevada and Texas. These treatment facilities accounted for 42.5% of our total revenues in 2016. It is likely that a small number of facilities will continue to contribute a significant portion of our total revenues in any given year for the foreseeable future. Additionally, we have a centralized corporate office that houses our accounting, billing and collections, information technology, marketing and call center departments and two high complexity laboratory facilities that conduct quantitative drug testing and other laboratory services. If any event occurs that would result in a complete or partial shutdown of any of these facilities or our centralized corporate office or two laboratory facilities, including, without limitation, any material changes in legislative, regulatory, economic, environmental or competitive conditions in these states or natural disasters such as hurricanes, earthquakes, tornadoes or floods or prolonged airline disruptions due to a natural disaster or for any reason, such event could lead to decreased revenues and/or higher operating costs, which could have a material adverse effect on our revenues, profitability and cash flows.
An increase in uninsured and underinsured clients or the deterioration in the collectability of the accounts of such clients could have a material adverse effect on our business, financial condition and results of operations.
Collection of receivables from third-party payors and clients is critical to our operating performance. Our primary collection risks are (i) the risk of overestimating our net revenues at the time of billing, which may result in us receiving less than the recorded receivable, (ii) the risk of non-payment as a result of commercial insurance companies denying claims, (iii) the risk that clients will fail to remit insurance payments to us when the commercial insurance company pays out-of-network claims directly to the client, (iv) resource and capacity constraints that may prevent us from handling the volume of billing and collection issues in a timely manner and (v) the risk of non-payment from uninsured or self-pay clients. Additionally, our ability to hire and retain experienced personnel also affects our ability to bill and collect accounts in a timely manner. We establish our provision for doubtful accounts based on the aging of the receivables and taking into consideration historical collection experience by facility, services provided, payor source and historical reimbursement rate, current economic trends and percentages applied to the accounts receivable aging categories. At December 31, 2016, our allowance for doubtful accounts represented approximately 24.4% of our accounts receivable balance as of such date, with two commercial payors each representing in excess of 10% of the accounts receivable balance as of December 31, 2016. We routinely review accounts receivable balances in conjunction with these factors and other economic conditions that might ultimately affect the collectability of the client accounts and make adjustments to our allowances as warranted. Significant changes in business operations, payor mix or economic conditions, including changes resulting from legislation or other health reform efforts, including efforts to repeal or significantly change the Affordable Care Act), could affect our collection of accounts receivable, cash flows and results of operations. In addition, increased client concentration in states that permit commercial insurance companies to pay
14
out-of-network claims directly to the client instead of us, such as California and Nevada, could adversely affect our collection of receivables. Increases in the growth of uninsured and underinsured clients or in our provision for doubtful accounts or unexpected changes in reimbursement rates by third-party payors could have a material adverse effect on our business, financial condition and results of operations.
We rely on our multi-faceted sales and marketing program to continuously attract and enroll clients in our network of facilities. Any disruption in our national sales and marketing program would have a material adverse effect on our business, financial condition and results of operations.
We believe our national sales and marketing program provides us with a competitive advantage compared to treatment facilities that primarily target local geographic areas and use fewer marketing channels to attract clients. If any disruption occurs in our national sales and marketing program for any reason or if we are unable to effectively attract and enroll new clients to our network of facilities, our ability to maintain census could be adversely affected, which would have a material adverse effect on our business, financial condition and results of operations.
In addition, our ability to grow or even to maintain our existing level of business depends significantly on our ability to establish and maintain close working and referral relationships with hospitals, other treatment facilities, employers, alumni, employee assistance programs and other referral sources. We have no binding commitments with any of these referral sources. We may not be able to maintain our existing referral relationships or develop and maintain new relationships in existing or new markets. If we lose existing relationships with our referral sources, the number of people to whom we provide services may decline, which may adversely affect our revenues. Also, if we fail to develop new referral relationships, our growth may be restrained.
We derive a significant portion of our revenues from providing services to clients covered by third-party payors who could reduce their reimbursement rates or otherwise restrain our ability to obtain, or provide services to, clients. This risk is heightened because we are generally an “out-of-network” provider.
Managed care organizations and other third-party payors pay for the services that we provide to many of our clients. For 2016, approximately 90% of our revenues were reimbursable by third-party payors, including amounts paid by such payors to clients, with the remaining portion payable directly by our clients. If any of these third-party payors reduce their reimbursement rates or elect not to cover some or all of our services, our business, financial condition and results of operations may be materially adversely affected.
In addition to limits on the amounts payors will pay for the services we provide to their members, controls imposed by third-party payors designed to reduce admissions and the length of stay for clients, including preadmission authorizations and utilization review, have affected and are expected to continue to affect our facilities. Utilization review entails the review of the admission and course of treatment of a client by third-party payors. Inpatient utilization, average lengths of stay and occupancy rates continue to be negatively affected by payor-required preadmission authorization and utilization review and by payor pressure to maximize outpatient and alternative healthcare delivery services for less acutely ill clients. Efforts to impose more stringent cost controls are expected to continue. Although we are unable to predict the effect these controls and changes will have on our operations, significant limits on the scope of services reimbursed and on reimbursement rates and fees could have a material adverse effect on our business, financial condition and results of operations. If the rates paid or the scope of laboratory or other substance abuse treatment services covered by third-party commercial payors are reduced, our business, financial condition and results of operations could be materially adversely affected.
For certain facilities, we are considered an “out-of-network” provider by the majority of third-party payors, and, therefore, we bill our full charges for services covered by such third-party payors. Third-party payors generally attempt to limit use of out-of-network providers by requiring clients to pay higher copayment and/or deductible amounts for out-of-network care. Additionally, third-party payors have become increasingly aggressive in attempting to minimize the use of out-of-network providers by disregarding the assignment of payment from clients to out-of-network providers (i.e., sending payments directly to clients instead to of out-of-network providers), capping out-of-network benefits payable to clients, waiving out-of-pocket payment amounts and initiating litigation against out-of-network providers for interference with contractual relationships, insurance fraud and violation of state licensing and consumer protection laws. If third-party payors impose further restrictions on out-of-network providers, our revenues could be threatened, forcing our facilities to participate with third-party payors and accept lower reimbursement rates compared to our historic reimbursement rates.
Third-party payors also are entering into sole source contracts with some healthcare providers, which could effectively limit our pool of potential clients. Moreover, third-party payors are beginning to carve out specific services, including substance abuse treatment and behavioral health services, and establish small, specialized networks of providers for such services at fixed reimbursement rates. Continued growth in the use of carve-out arrangements could materially adversely affect our business to the extent we are not selected to participate in such smaller specialized networks or if the reimbursement rate is not adequate to cover the cost of providing the service.
15
If we overestimate the reimbursement amounts that payors will pay us for services performed, it would increase our revenue adjustments, which could have a material adverse effect on our revenues, profitability and cash flows and lead to significant shifts in our results of operations from quarter to quarter that may make it difficult to project long-term performance.
We recognize revenues from commercial payors at the time services are provided based on our estimate of the amount that payors will pay us for the services performed. We estimate the net realizable value of revenues by adjusting gross client charges using our expected realization and applying this discount to gross client charges. A significant or sustained decrease in our collection rates could have a material adverse effect on our operating results. There is no assurance that we will be able to maintain or improve historical collection rates in future reporting periods.
Estimates of net realizable value are subject to significant judgment and approximation by management. It is possible that actual results could differ from the historical estimates management has used to help determine the net realizable value of revenues. If our actual collections either exceed or are less than the net realizable value estimates, we will record a revenue adjustment, either positive or negative, for the difference between our estimate of the receivable and the amount actually collected in the reporting period in which the collection occurred. A significant negative revenue adjustment could have a material adverse effect on our revenues, profitability and cash flows in the reporting period in which such adjustment is recorded. In addition, if we record a significant revenue adjustment, either positive or negative, in any given reporting period, it may lead to significant changes in our results from operations from quarter to quarter, which may limit our ability to make accurate long-term predictions about our future performance.
Certain third-party payors account for a significant portion of our revenues, and the reduction of reimbursement rates or coverage of services by any such payor could have a material adverse effect on our revenues, profitability and cash flows.
For the year ended December 31, 2016, approximately 10.5% of our revenue reimbursements came from Anthem Blue Cross Blue Shield of Florida, 10.4% came from Blue Cross Blue Shield of Texas, and 10.4% came from Aetna. No other payor accounted for more than 10% of our revenue reimbursements for the year ended December 31, 2016. For the year ended December 31, 2015, approximately 15.1% of our revenue reimbursements came from Anthem Blue Cross Blue Shield of Colorado, 12.5% came from Blue Cross Blue Shield of Texas, 11.5% came from Aetna, and 11.1% came from Blue Cross Blue Shield of California. No other payor accounted for more than 10% of our revenue reimbursements for the year ended December 31, 2015. If any of these or other third-party payors reduce their reimbursement rates for the services we provide or otherwise implement measures, such as specialized networks, that reduce the payments we receive, our revenues, profitability and cash flows could be materially adversely affected.
Our business depends on our information systems and our inability to effectively integrate, manage and keep secure our information systems could disrupt our operations and have a material adverse effect on our business.
Our business depends on effective and secure information systems that assist us in, among other things, admitting clients to our facilities, monitoring census and utilization, processing and collecting claims, reporting financial results, measuring outcomes and quality of care, managing regulatory compliance controls, and maintaining operational efficiencies. These systems include software developed in-house and systems provided by external contractors and other service providers. To the extent that these external contractors or other service providers become insolvent or fail to support the software or systems, our operations could be negatively affected. Our facilities also depend upon our information systems for electronic medical records, accounting, billing, collections, risk management, payroll and other information. If we experience a reduction in the performance, reliability, or availability of our information systems, our operations and ability to process transactions and produce timely and accurate reports could be adversely affected.
Our information systems and applications require continual maintenance, upgrading, and enhancement to meet our operational needs. Our acquisitions require transitions and integration of various information systems. We regularly upgrade and expand our information systems’ capabilities. If we experience difficulties with the transition and integration of information systems or are unable to implement, maintain, or expand our systems properly, we could suffer from, among other things, operational disruptions, regulatory problems, working capital disruptions and increases in administrative expenses.
In addition, we could be subject to a cyber-attack that bypasses our information technology security systems and other security incidents that result in security breaches, including the theft, loss or misappropriation of individually identifiable health information subject to HIPAA and other privacy and security laws, proprietary business information, or other confidential or personal data. Such an incident could disrupt our information technology business systems, and clinical operations, cause us to incur significant investigation and remediation expenses, and subject us to litigation, government inquiries, penalties and reputational damages. Information security and the continued development, maintenance and enhancement of our safeguards to protect our systems, data, software and networks is a priority for us. As security threats continue to evolve, we may be required to expend significant additional resources to modify and enhance our safeguards and investigate and remediate any information security vulnerabilities. If we are subject to cyber-attacks or security breaches, our business, financial condition and results of operations could be adversely impacted.
16
Further, our information systems are vulnerable to damage or interruption from fire, flood, natural disaster, power loss, telecommunications failure, break-ins and similar events. A failure to implement our disaster recovery plans or ultimately restore our information systems after the occurrence of any of these events could have a material adverse effect on our business, financial condition and results of operations. Because of the confidential health information that we store and transmit, loss of electronically-stored information for any reason could expose us to a risk of regulatory action, litigation, possible liability and loss.
Our acquisition strategy exposes us to a variety of operational, integration and financial risks, which may have a material adverse effect on our business, financial condition and results of operations.
A principal element of our business strategy is to grow by acquiring other companies and assets in the mental health and substance abuse treatment industry. We evaluate potential acquisition opportunities consistent with the normal course of our business. Our ability to complete acquisitions is subject to a number of risks and variables, including our ability to negotiate mutually agreeable terms with the counterparties, our ability to finance the purchase price and our ability to obtain any licenses or other approvals required to operate the assets to be acquired. We may not be successful in identifying and consummating suitable acquisitions, which may impede our growth and negatively affect our results of operations and may also require a significant amount of management resources. In addition, growth, especially rapid growth, through acquisitions exposes us to a variety of operational and financial risks. We summarize the most significant of these risks below.
Integration risks. We must integrate our acquisitions with our existing operations. This process involves various components of our business and the businesses we have acquired, including the following:
|
|
• |
physicians and employees who are not familiar with our operations; |
|
|
• |
clients who may elect to switch to another substance abuse treatment provider; |
|
|
• |
assignment or termination of material contracts, including commercial payor agreements; |
|
|
• |
regulatory compliance programs and state and federal licensing requirements; and |
|
|
• |
disparate operating, information and record keeping systems and technology platforms. |
The integration of acquisitions with our operations could be expensive, require significant attention from management, may impose substantial demands on our operations or other projects and may impose challenges on the combined business including, without limitation, consistencies in business standards, procedures, policies, business cultures and internal controls and compliance. In addition, certain acquisitions require a capital outlay, and the return we achieve on such invested capital may be less than the return that we could achieve on other projects or investments.
Benefits may not materialize. When evaluating potential acquisition targets, we identify potential synergies and cost savings that we expect to realize upon the successful completion of the acquisition and the integration of the related operations. We may, however, be unable to achieve or may otherwise never realize the expected benefits. Our ability to realize the expected benefits from potential cost savings and revenue improvement opportunities is subject to significant business, economic and competitive uncertainties and contingencies, many of which are beyond our control, such as changes to government regulation governing or otherwise impacting the substance abuse treatment and behavioral healthcare industries, reductions in reimbursement rates from third-party payors, operating difficulties, difficulties obtaining required licenses and permits, client preferences, changes in competition and general economic or industry conditions. If we do not achieve our expected results, it may adversely impact our results of operations.
Assumptions of unknown liabilities. Businesses that we acquire may have unknown or contingent liabilities, including, without limitation, liabilities for failure to comply with healthcare laws and regulations. Although we typically attempt to exclude significant liabilities from our acquisition transactions and seek indemnification from the sellers of such facilities for at least a portion of these matters, we may experience difficulty enforcing those indemnification obligations, or we may incur material liabilities for the past activities of acquired facilities. Such liabilities and related legal or other costs and/or resulting damage to a facility’s reputation could negatively impact our business.
Completing Acquisitions. Suitable acquisitions may not be accomplished due to unfavorable terms. Further, the cost of an acquisition could result in a dilutive effect on our results of operations, depending on various factors, including the amount paid for an acquired facility, the acquired facility’s results of operations, the fair value of assets acquired and liabilities assumed, effects of subsequent legislation and limits on reimbursement rate increases. In addition, we may have to pay cash, incur additional debt or issue equity securities to pay for any such acquisition, which could adversely affect our financial results, result in dilution to our existing stockholders, result in increased fixed obligations or impede our ability to manage our operations.
Managing growth. Some of the facilities we have acquired or may acquire in the future may have had significantly lower operating margins than the facilities we operated prior to the time of our acquisition thereof or had operating losses prior to such acquisition. If we fail to improve the operating margins of the facilities we acquire, operate such facilities profitably or effectively integrate the operations of acquired facilities, our results of operations could be negatively impacted.
17
Our level of indebtedness could adversely affect our ability to meet our obligations under our indebtedness, react to changes in the economy or our industry and to raise additional capital to fund our operations.
As of December 31, 2016, we had total debt of $189.1 million outstanding. We have historically relied on debt financing to fund our real estate development and our operating cash flow requirements, and we expect such debt financing needs to continue. A summary of the material terms of our indebtedness can be found in “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources.” Our level of indebtedness could have important consequences to our stockholders. For example, it could:
|
|
• |
make it more difficult for us to satisfy our obligations with respect to our indebtedness, resulting in possible defaults on, and acceleration of, such indebtedness; |
|
|
• |
increase our vulnerability to general adverse economic and industry conditions; |
|
|
• |
require us to dedicate a substantial portion of our cash flows from operations to payments on indebtedness, thereby reducing the availability of such cash flows to fund working capital, capital expenditures and other general corporate requirements or to carry out other aspects of our business; |
|
|
• |
limit our ability to obtain additional financing to fund future working capital, capital expenditures and other general corporate requirements or to carry out other aspects of our business; |
|
|
• |
limit our ability to make material acquisitions or take advantage of business opportunities that may arise; and |
|
|
• |
place us at a potential competitive disadvantage compared to our competitors that have less debt. |
Our operating flexibility is limited in significant respects by the restrictive covenants in our credit facility and subordinated debt and convertible notes, and we may be unable to comply with all covenants in the future.
On March 9, 2015, the Company entered into a five-year senior secured credit facility with Bank of America, N.A., as administrative agent for the lenders party thereto, as amended on July 13, 2016 and February 27, 2017 (the “2015 Credit Facility”). Our 2015 Credit Facility and subordinated debt and convertible notes impose restrictions that could impede our ability to enter into certain corporate transactions, as well as increases our vulnerability to adverse economic and industry conditions, by limiting our flexibility in planning for, and reacting to, changes in our business and industry. These restrictions limit our and our subsidiaries’ ability to, among other things:
|
|
• |
incur or guarantee additional debt; |
|
|
• |
pay dividends on our capital stock or redeem, repurchase, retire or otherwise acquire any of our capital stock; |
|
|
• |
make certain capital expenditures; |
|
|
• |
enter into leases; |
|
|
• |
make certain payments or investments; |
|
|
• |
create liens on our assets; |
|
|
• |
make any substantial change in the nature of our business as it is currently conducted; and |
|
|
• |
merge or consolidate with other companies or transfer all or substantially all of our assets. |
18
In addition, our 2015 Credit Facility requires us to meet certain financial covenants and may preclude additional borrowings. The restrictions may prevent us from taking actions that we believe would be in the best interests of our business and may make it difficult for us to successfully execute our business strategy or effectively compete with companies that are not similarly restricted. Our 2015 Credit Facility and subordinated debt and convertible notes also contain cross-default and cross-acceleration provisions, respectively, that would apply to each other and to any other material indebtedness we may have. We may also incur future debt obligations that might subject us to additional restrictive covenants that could affect our financial and operational flexibility. Our ability to comply with these restrictive covenants in future periods will largely depend on our ability to successfully implement our overall business strategy. We cannot assure you that we will be granted any waivers or amendments to the 2015 Credit Facility or under the subordinated debt and convertible notes if for any reason we are unable to comply with the terms of the 2015 Credit Facility and subordinated debt and convertible notes in the future. The breach of any of these covenants or restrictions could result in a default under the 2015 Credit Facility and/or subordinated debt and convertible notes, which could result in the acceleration of our debt. In the event of an acceleration of our debt, we could be forced to apply all available cash flows to repay such debt and could be forced into bankruptcy or liquidation.
We will need additional financing to execute our business plan and fund operations, which additional financing may not be available on reasonable terms or at all.
As of December 31, 2016, we had $51.1 million of working capital. Our acquisition and de novo development strategies will require substantial capital. During 2016, we completed two acquisitions with an aggregate cash purchase price of approximately $19.2 million. During the same period, we purchased a property for approximately $5.4 million.
To fund our acquisition and development strategies, we may consider raising additional funds through various financing sources, including the sale of our common or preferred stock and the procurement of commercial debt financing. However, there can be no assurance that such funds will be available on commercially reasonable terms, if at all. If such financing is not available on satisfactory terms, we may be unable to expand or continue our business as desired and operating results may be adversely affected. Any debt financing will increase expenses and must be repaid regardless of operating results and may involve restrictions limiting our operating flexibility. If we issue equity securities to raise additional funds, the percentage ownership of our existing stockholders will be reduced, and our stockholders may experience additional dilution in net book value per share.
Our ability to obtain needed financing may be impaired by such factors as the capital markets, both generally and specifically in our industry, which could impact the availability or cost of future financings. If the amount of capital we are able to raise from financing activities, together with our revenues from operations, is not sufficient to satisfy our capital needs, we may be required to decrease the pace of, or eliminate, our acquisition strategy and potentially reduce or even cease operations.
Our business may face significant risks with respect to future de novo expansion, including the time and costs of identifying new geographic markets, the ability to obtain necessary licensure and other zoning or regulatory approvals and significant start-up costs including advertising, marketing and the costs of providing equipment, furnishings, supplies and other capital resources.
As part of our growth strategy, we intend to develop new substance abuse treatment facilities in existing and new markets, either by building a new facility or by acquiring an existing facility with an alternative use and repurposing it as a substance abuse treatment facility. Such de novo expansion involves significant risks, including, but not limited to, the following:
|
|
• |
the time and costs associated with identifying locations in suitable geographic markets, which may divert management attention from existing operations; |
|
|
• |
the possibility of changes to comprehensive zoning plans or zoning regulations that imposes additional restrictions on use or requirements could impact our expansion into otherwise suitable geographic markets; |
|
|
• |
the need for significant advertising and marketing expenditures to attract clients; |
|
|
• |
our ability to provide each de novo facility with the appropriate equipment, furnishings, materials, supplies and other capital resources; |
|
|
• |
our ability to obtain licensure and accreditation, establish relationships with healthcare providers in the community and delays or difficulty in installing our operating and information systems; |
|
|
• |
the costs of evaluating new markets, hiring experienced local physicians, management and staff and opening new facilities, and the time lags between these activities and the generation of sufficient revenues to support the costs of the expansion; and |
|
|
• |
our ability to finance de novo expansion and possible dilution to our existing stockholders if our common stock is used as consideration. |
As a result of these and other risks, there can be no assurance that a de novo treatment facility will become profitable.
19
Our ability to maintain census and the average length of stay of our clients is dependent on a number of factors outside of our control, and if we are unable to maintain census, or if we experience a significant decrease in average length of stay, our business, results of operations and cash flows could be materially adversely affected.
Our revenues are directly impacted by our ability to maintain census and, to a lesser extent, the average length of stay of our clients. These metrics are dependent on a variety of factors, many of which are outside of our control, including the effectiveness of our sales and marketing efforts, our referral relationships, our staffing levels and facility capacity, the extent to which third-party payors require preadmission authorization or utilization review controls, competition in the industry and the decisions of our clients to seek and commit to treatment. A significant decrease in census or, to a lesser extent, average length of stay could materially adversely affect our revenues, profitability and cash flows due to lower reimbursements received and the additional resources required to collect accounts receivable and to maintain our existing level of business.
Given the client-driven nature of the substance abuse treatment sector, our business is dependent on clients seeking and committing to treatment. Although increased awareness and de-stigmatization of substance abuse treatment in recent years has resulted in more people seeking treatment, the decision of each client to seek treatment is ultimately discretionary. In addition, even after the initial decision to seek treatment, our adult clients may decide at any time to discontinue treatment and leave our facilities against the advice of our physicians and other treatment professionals. For this reason, among others, average length of stay can vary among periods without correlating to the overall operating performance of our business. Management does not view average length of stay as a key metric with respect to our operating performance; however, if clients or potential clients decide not to seek treatment or discontinue treatment early, census and average length of stay could decrease and, as a result, our business, financial condition and results of operations could be adversely affected.
As a provider of treatment services, we are subject to governmental investigations and potential claims and legal actions by clients, employees and others, which may increase our costs and have a material adverse effect on our business, financial condition and results of operations.
Given the addiction and mental health issues of clients and the nature of the services provided, the substance abuse treatment industry is heavily regulated by governmental agencies and involves significant risk of liability. We and others in our industry are exposed to the risk of governmental investigations and lawsuits or other claims against us and our physicians and other professionals arising out of our day to day business operations, including, without limitation, client treatment at our facilities and relationships with healthcare providers that may refer clients to us. Addressing any investigations, lawsuits or other claims may distract management and divert resources, even if we ultimately prevail. Regardless of the outcome of any such investigation, lawsuit or claim, the publicity and potential risks associated with the investigation, lawsuit or claim could negatively impact the perception of the Company by clients, investors or others. Fines, restrictions, penalties and damages imposed as a result of an investigation or a successful lawsuit or claim that is not covered by, or is in excess of, our insurance coverage may increase our costs and reduce our profitability. Our insurance premiums have increased year over year, and insurance coverage may not be available at a reasonable cost in the future, especially given the significant increase in insurance premiums generally experienced in the healthcare industry.
We are also subject to potential medical malpractice lawsuits and other legal actions in the ordinary course of business. Some of these actions may involve large claims as well as significant defense costs. We cannot predict the outcome of these lawsuits or the effect that findings in such lawsuits may have on us. All professional and general liability insurance we purchase is subject to policy limitations. We believe that, based on our past experience, our insurance coverage is adequate considering the claims arising from the operation of our facilities. While we continuously monitor our coverage, our ultimate liability for professional and general liability claims could change materially from our current estimates. If such policy limitations should be partially or fully exhausted in the future or if payments of claims exceed our estimates or are not covered by our insurance, it could have a material adverse effect on our financial condition and results of operations.
We operate in a highly competitive industry, and competition may lead to declines in client volumes and an increase in labor costs, which could have a material adverse effect on our business, financial condition and results of operations.
The substance abuse treatment industry is highly competitive, and competition among substance abuse treatment providers (including behavioral healthcare facilities) for clients has intensified in recent years. There are behavioral healthcare facilities that provide substance abuse and other mental health treatment services comparable to at least some of the services offered by our facilities in each of the geographical areas in which we operate. Some of our competitors are owned by tax-supported governmental agencies or by nonprofit corporations and may have certain financial advantages not available to us, including endowments, charitable contributions, tax-exempt financing and exemptions from sales, property and income taxes. If our competitors are better able to attract clients, expand services or obtain favorable participation agreements at their facilities, we may experience a decline in client volume, which could have a material adverse effect on our business, financial condition and results of operations.
Our operations depend on the efforts, abilities and experience of our management team, physicians and medical support personnel, including our nurses, mental health technicians, therapists and counselors. We compete with other healthcare providers in recruiting and retaining qualified management, physicians, nurses and other support personnel responsible for the daily operations of our facilities.
Increased labor union activity is another factor that could adversely affect our labor costs. A labor union, HPAE, represents
20
employees at Sunrise House. As a result, with respect to our Sunrise House facilities, we are subject to the risk of labor disputes, strikes, work stoppages and other labor-relations matters. Although we are not aware of any union organizing activity at any of our other facilities, we are unable to predict whether any such activity will take place in the future.
We depend heavily on key executives and other key management personnel, and the departure of one or more of our key executives could have a material adverse effect on our business, financial condition and results of operations.
The expertise and efforts of our key executives, including our chief executive officer, and other management personnel are critical to the success of our business. We do not currently have employment agreements or non-competition covenants with any of our key executives. The loss of the services of one or more of our key executives could significantly undermine our management expertise and our ability to provide efficient, quality healthcare services at our facilities. Furthermore, if one or more of our key executives were to terminate employment with us and engage in a competing business, we would be subject to increased competition, which could have a material adverse effect on our business, financial condition and results of operations.
Failure to adequately protect our trademarks and any other proprietary rights could have a material adverse effect on our business, financial condition and results of operations.
We maintain a trademark portfolio that we consider to be of significant importance to our business, and we may acquire additional trademarks or other proprietary rights in acquisitions that we pursue as part of our growth strategy. If the actions we take to establish and protect our trademarks and other proprietary rights are not adequate to prevent imitation of our services by others or to prevent others from seeking to block sales of our services as an alleged violation of their trademarks and proprietary rights, it may be necessary for us to initiate or enter into litigation in the future to enforce our trademark rights or to defend ourselves against claimed infringement of the rights of others. Any legal proceedings could result in an adverse determination that could have a material adverse effect on our business, financial condition and results of operations.
Failure to maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could have a material adverse effect on our business.
We are required to maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act in the course of preparing our consolidated financial statements. If we are unable to maintain effective internal control over financial reporting, we may be unable to report our financial information on a timely basis, may suffer adverse regulatory consequences or violations of NYSE listing rules. There could also be a negative reaction in the financial markets due to a loss of investor confidence in us and the reliability of our financial statements. Confidence in our financial statements is also likely to suffer if we report a material weakness in our internal control over financial reporting. In addition, we have incurred and will continue to incur incremental costs in order to improve our internal control over financial reporting and comply with Section 404 of the Sarbanes-Oxley Act, including increased auditing and legal fees.
Risks Related to Regulatory Matters
If we fail to comply with the extensive laws and government regulations impacting our industry, we could suffer penalties, be the subject of federal and state investigations or be required to make significant changes to our operations, which may reduce our revenues, increase our costs and have a material adverse effect on our business, financial condition and results of operations.
Healthcare service providers are required to comply with extensive and complex laws and regulations at the federal, state and local government levels relating to, among other things:
|
|
• |
licensure, certification and accreditation of substance abuse treatment services; |
|
|
• |
licensure, CLIA certification and accreditation of laboratory services; |
|
|
• |
handling, administration and distribution of controlled substances; |
|
|
• |
necessity and adequacy of care, quality of services, and qualifications of professional and support personnel; |
|
|
• |
referrals of clients and permissible relationships with physicians and other referral sources; |
|
|
• |
claim submission and collections, including penalties for the submission of, or causing the submission of, false, fraudulent or misleading claims and the failure to repay overpayments in a timely manner; |
|
|
• |
consumer protection issues and billing and collection of client-owed accounts issues; |
|
|
• |
privacy and security of health-related information, client personal information and medical records; |
|
|
• |
physical plant planning, construction of new facilities and expansion of existing facilities; |
21
|
|
• |
activities regarding competitors; |
|
|
• |
FDA laws and regulations related to drugs and medical devices; |
|
|
• |
operational, personnel and quality requirements intended to ensure that clinical testing services are accurate, reliable and timely; |
|
|
• |
health and safety of employees; |
|
|
• |
handling, transportation and disposal of medical specimens and infectious and hazardous waste; and |
|
|
• |
corporate practice of medicine, fee-splitting, self-referral and kickback prohibitions. |
Failure to comply with these laws and regulations could result in the imposition of significant civil or criminal penalties, loss of license or certification or require us to change our operations, which may have a material adverse effect on our business, financial condition and results of operations. Both federal and state government agencies as well as commercial payors have heightened and coordinated civil and criminal enforcement efforts as part of numerous ongoing investigations of healthcare organizations.
We endeavor to comply with all applicable legal and regulatory requirements, however, there is no guarantee that we will be able to adhere to all of the complex government regulations that apply to our business. We seek to structure all of our relationships with physicians to comply with applicable anti-kickback laws, physician self-referral laws, fee-splitting laws and state corporate practice of medicine prohibitions. We monitor these laws and their implementing regulations and implement changes as necessary. However, the laws and regulations in these areas are complex and often subject to varying interpretations. For example, if an enforcement agency were to challenge the compensation paid under our contracts with professional physician groups, we could be required to change our practices, face criminal or civil penalties, pay substantial fines or otherwise experience a material adverse effect as a result.
We may be required to spend substantial amounts to comply with legislative and regulatory initiatives relating to privacy and security of client health information.
There are currently numerous legislative and regulatory initiatives at the federal and state levels addressing client privacy and security concerns. In particular, federal regulations issued under the Drug Abuse Prevention, Treatment and Rehabilitation Act of 1979 strictly restrict the disclosure of client identifiable information related to substance abuse. These requirements apply to any of our facilities that receive any federal assistance, which is interpreted broadly to include facilities licensed, certified or registered by a federal agency. In addition, the federal privacy and security regulations issued under HIPAA require our facilities to comply with extensive requirements on the use and disclosure of individually identifiable health information (known as “protected health information”) and to implement and maintain administrative, physical and technical safeguards to protect the security of such information. Additional security requirements apply to electronic protected health information. These regulations also provide clients with substantive rights with respect to their health information and impose substantial administrative obligations on our facilities, including the requirement to enter into written agreements with contractors, known as business associates, to whom our programs disclose protected health information. We may be subject to penalties as a result of a business associate violating HIPAA, if the business associate is found to be our agent. Covered entities must notify individuals, the U.S. Department of Health and Human Services, or HHS, and, in some cases, the media of breaches involving unsecured protected health information. HHS and state attorneys general are authorized to enforce these regulations. Violations of the HIPAA privacy and security regulations may result in significant civil and criminal penalties, and data breaches and other HIPAA violations may give rise to class action lawsuits by affected clients under state law.
Our programs remain subject to any privacy-related federal or state laws that are more restrictive than the HIPAA privacy and security regulations. These laws vary by state and could impose additional requirements and penalties. For example, some states impose strict restrictions on the use and disclosure of health information pertaining to mental health or substance abuse. Further, most states have enacted laws and regulations that require us to notify affected individuals in the event of a data breach involving individually identifiable information. In addition, the Federal Trade Commission may use its consumer protection authority to initiate enforcement actions in response to data breaches or other privacy or security lapses.
As public attention is drawn to issues related to the privacy and security of medical and other personal information, federal and state authorities may increase enforcement efforts, seek to impose harsher penalties as well as revise and expand laws or enact new laws concerning these topics. Compliance with current as well as any newly established provisions or interpretations of existing requirements will require us to expend significant resources. Increased focus on privacy and security issues by enforcement authorities may increase the overall risk that our substance abuse treatment facilities may be found lacking under federal and state privacy and security laws and regulations.
22
Our treatment facilities operate in an environment of increasing state and federal enforcement activity and private litigation targeted at healthcare providers.
Both federal and state government agencies have heightened and coordinated their civil and criminal enforcement efforts as part of numerous ongoing investigations of healthcare companies and various segments of the healthcare industry. These investigations relate to a wide variety of topics, including relationships with physicians, billing practices and use of controlled substances. The Affordable Care Act included an additional $350 million of federal funding over ten years to fight healthcare fraud, waste and abuse, including $10 million for each of federal fiscal years 2017 through 2020. From time to time, the HHS Office of Inspector General and the Department of Justice have established national enforcement initiatives that focus on specific billing practices or other suspected areas of abuse. Although we do not currently bill Medicare or Medicaid for substance abuse treatment services, there is a risk that specific investigation initiatives could be expanded to include our treatment facilities. In addition, increased government enforcement activities, even if not directed towards our treatment facilities, also increase the risk that our facilities, physicians and other clinicians furnishing services in our facilities, or our executives and directors, could be named as defendants in private litigation such as state or federal false claims act cases or consumer protection cases, or could become the subject of complaints at the various state and federal agencies that have jurisdiction over our operations. Any governmental investigations, private litigation or other legal proceedings involving any of our facilities, our executives or our directors, even if we ultimately prevail, could result in significant expense and could adversely affect our reputation or profitability. In addition, we may be required to make changes in our laboratory or other substance abuse treatment services as a result of an adverse determination in any governmental enforcement action, private litigation or other legal proceeding, which could materially adversely affect our business and results of operations.
Changes to federal, state and local regulations, as well as different or new interpretations of existing regulations, could adversely affect our operations and profitability.
Because our treatment programs and operations are regulated at federal, state and local levels, we could be affected by regulatory changes in different regional markets. Increases in the costs of regulatory compliance and the risks of noncompliance may increase our operating costs, and we may not be able to recover these increased costs, which may adversely affect our results of operations and profitability.
Many of the current laws and regulations are relatively new. Thus, we do not always have the benefit of significant regulatory or judicial interpretation of these laws and regulations. Evolving interpretations or enforcement of these laws and regulations could subject our current or past practices to allegations of impropriety or illegality or could require us to make changes in our treatment facilities, equipment, personnel, services or capital expenditure programs. A determination that we have violated these laws, or a public announcement that we are being investigated for possible violations of these laws, could adversely affect our business, operating results and overall reputation in the marketplace.
In addition, federal, state and local regulations may be enacted that impose additional requirements on our facilities. Adoption of legislation or the creation of new regulations affecting our facilities could increase our operating costs, restrain our growth, limit us from taking advantage of opportunities presented and could have a material adverse effect on our business, financial condition and results of operations. Adverse changes in existing comprehensive zoning plans or zoning regulations that impose additional restrictions on the use of, or requirements applicable to, our facilities may affect our ability to operate our existing facilities or acquire new facilities, which may adversely affect our results of operations and profitability.
We are subject to uncertainties regarding the direction and impact of healthcare reform efforts, particularly following the 2016 federal elections.
The healthcare industry is subject to changing political, regulatory, scientific, and technological changes, which have resulted and may continue to result in initiatives intended to reform the industry. For example, the 2016 federal elections, which resulted in the election of a Republican president and Republican majorities in both houses of Congress, increased the likelihood of repeal or significant changes to the Affordable Care Act. These elections may also impact how the executive branch implements the law and how the federal government responds to lawsuits challenging the law. As currently structured, the Affordable Care Act provides for increased access to coverage for healthcare and seeks to reduce healthcare-related expenses. For example, it requires all new small group and individual market health plans to cover ten essential health benefit categories, which include substance abuse addiction and mental health disorder services. We are unable to predict the full impact of the Affordable Care Act and related regulations or the impact of its repeal or modification on our operations in light of the uncertainty regarding whether or how the law will be changed, what alternative reforms, if any, may be enacted, or what other actions may be taken. Any government efforts related to health reform may have an adverse effect on our business, results of operations, cash flow, capital resources and liquidity. Moreover, the general uncertainty of health reform efforts, particularly if Congress elects to repeal provisions of the Affordable Care Act but delays the implementation of repeal or fails to enact replacement provisions at the time of repeal, may negatively impact our payment sources or demand for our services.
The expansion of health insurance coverage under the Affordable Care Act has been beneficial to the substance abuse treatment industry. This is due, in part, to higher demand for treatment services, which resulted from the requirement that small group and individual market plans comply with the requirements of the Mental Health Parity and Addiction Equity Act of 2008, which previously applied only to group health plans and group insurers. The 21st Century Cures Act requires development of an action plan
23
for enhanced enforcement of mental health parity requirements and additional guidance for health plans regarding compliance with parity laws. Increased demand for treatment services may bring new competitors to the market, some of which may be better capitalized and have greater market penetration than we do. In addition, we expect increased demand for substance abuse treatment services to increase the demand for case managers, therapists, medical technicians and others with clinical expertise in substance abuse treatment, which may make it more difficult to adequately staff our substance abuse treatment facilities and could significantly increase our costs in delivering treatment, which may adversely affect both our operations and profitability.
One of the many impacts of the Affordable Care Act and subsequent legislation has been a dramatic increase in payment reform efforts by federal and state government payors as well as commercial payors. These efforts take many forms, including the growth of Accountable Care Organizations, pay-for-performance bonus arrangements, partial capitation arrangements and the bundling of services into a single payment. One result of these efforts is that more risk of the overall cost of care is being transferred to providers. As institutional providers and their affiliated physicians assume more risk for the cost of care, we expect more services to be furnished within provider networks formed to accept these types of payment reform. Our ability to compete and to retain our traditional sources of clients may be adversely affected by our exclusion from such networks or our inability to be included in such networks.
Change of ownership or change of control requirements imposed by state and federal licensure and certification agencies as well as third-party payors may limit our ability to timely realize opportunities, adversely affect our licenses and certifications, interrupt our cash flows and adversely affect our profitability.
State licensure laws and many federal healthcare programs (where applicable) impose a number of obligations on healthcare providers undergoing a change of ownership or change of control transaction. These requirements may require new license applications as well as notices given a fixed number of days prior to the closing of affected transactions. These provisions require us to be proactive when considering both internal restructuring and acquisitions of third-party targets. Failure to provide such notices or to submit required paperwork can adversely affect licensure on a going forward basis, can subject the parties to penalties and can adversely affect our ability to operate our facilities.
Many third-party payor agreements, including government payor programs, also have change of ownership or change of control provisions. Such provisions generally include a prior notice provision as well as require the consent of the payor in order to continue the terms of the payor agreement. Abiding by the terms of such provisions may reopen pricing negotiations with third-party payors where the provider currently has favorable reimbursement terms as compared to the market. Failure to comply with the terms of such provisions can result in a breach of the underlying third-party payor agreement. Currently, we have few third-party payor agreements; however, as substance abuse treatment coverage and payment reform initiatives continue to expand, these types of provisions could have a significant impact on our ability to realize opportunities and could adversely affect our cash flows and profitability.
We could face risks associated with, or arising out of, environmental, health and safety laws and regulations.
We are subject to various federal, state and local laws and regulations that:
|
|
• |
regulate certain activities and operations that may have environmental or health and safety effects, such as the generation, handling and disposal of medical and pharmaceutical wastes; |
|
|
• |
impose liability for costs of cleaning up, and damages to natural resources from, past spills, waste disposals on and off-site and other releases of hazardous materials or regulated substances; and |
|
|
• |
regulate workplace safety. |
Compliance with these laws and regulations could increase our costs of operation. Violation of these laws may subject us to significant fines, penalties or disposal costs, which could negatively impact our results of operations, financial position or cash flows. We could be responsible for the investigation and remediation of environmental conditions at currently or formerly operated or leased sites, as well as for associated liabilities, including liabilities for natural resource damages, third-party property damage or personal injury resulting from lawsuits that could be brought by the government or private litigants relating to our operations, the operations of our facilities or the land on which our facilities are located. We may be subject to these liabilities regardless of whether we lease or own the facility, and regardless of whether such environmental conditions were created by us or by a prior owner or tenant, or by a third-party or a neighboring facility whose operations may have affected such facility or land, because liability for contamination under certain environmental laws can be imposed on current or past owners or operators of a site without regard to fault. We cannot assure you that environmental conditions relating to our prior, existing or future sites or those of predecessor companies whose liabilities we may have assumed or acquired will not have a material adverse effect on our business.
State efforts to regulate the construction or expansion of healthcare facilities could impair our ability to operate and expand our facilities.
The construction of new healthcare facilities, the expansion, transfer or change of ownership of existing facilities and the addition of new beds, services or equipment may be subject to state laws that require a determination of public need and prior
24
approval by state regulatory agencies under certificate of need (“CON”) laws. Review of CONs and other healthcare planning initiatives may be lengthy and may require public hearings. States in which we now or may in the future operate may require CONs under certain circumstances not currently applicable to us or may impose standards and other health planning requirements upon us. Violation of these state laws and our failure to obtain any necessary state approval could:
|
|
• |
result in our inability to acquire a targeted facility, complete a desired expansion or make a desired replacement; or |
|
|
• |
result in the revocation of a facility’s license or impose civil or criminal penalties on us, any of which could have a material adverse effect on our business, financial condition and results of operations. |
If we are unable to obtain required regulatory, zoning or other required approvals for renovations and expansions, our growth may be restrained and our operating results may be adversely affected. In the past, we have not experienced any material adverse effects from such requirements, but we cannot predict their future impact on our operations.
We may be unable to successfully implement the Compliance Program or timely implement recommendations made by the Compliance Officer in connection with the Compliance Program or otherwise comply with the Permanent Injunction and Final Judgment.
On October 21, 2016, the Defendants, agreed to the entry of the PIFJ with BMFEA relating to the criminal charges filed against the Defendants in connection with the death of a client in 2010 at one of our former locations. Pursuant to the terms of the PIFJ, we were required to, among other things, (i) institute the California Compliance Program with respect to our California facilities that includes maintaining or developing and implementing certain policies and procedures to promote each covered facility’s compliance with applicable statutes, regulations and the PIFJ, under the responsibility of our Chief Compliance Officer; (ii) establish a Compliance Committee composed of the Compliance Officer and senior personnel responsible for overseeing clinical operations to address issues raised by the Compliance Officer in connection with the Compliance Program and (iii) establish an oversight committee of the Board of Directors, or a committee of the Board of Directors, to review the adequacy and responsiveness of the California Compliance Program. In addition, for a period of 30 months following the effective date of the PIFJ, the Defendants shall retain a qualified independent monitor, appointed by BMFEA after consultation with the Defendants, to assess the effectiveness of the Defendants’ quality control systems and patient care.
We have incurred, and expect to continue to incur, significant costs in connection with the California Compliance Program and with the PIFJ. The Compliance Officer has completed the initial implementation of the California Compliance Program, but such efforts will be ongoing during the full term of the California Compliance Program. If we are not able to successfully fulfill our obligations under the California Compliance Program or timely implement recommendations made by the Compliance Officer in connection with the California Compliance Program, the BMFEA may pursue remedies under the PIFJ, including assessment of fines and civil and criminal actions. Should the BMFEA pursue remedies under the PIFJ, we could face significant fines and actions, which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Risks Related to Our Organization and Structure
We are a holding company with nominal net worth and will depend on dividends and distributions from our subsidiaries to pay dividends, if any.
AAC Holdings, Inc. is a holding company with nominal net worth. We do not conduct any business operations other than our investments in our subsidiaries. Our business operations are conducted primarily out of our direct operating subsidiary, AAC. As a result, our ability to pay dividends, if any, will be dependent upon cash dividends and distributions or other transfers to us from our subsidiaries, including AAC. Payments to us by our subsidiaries will be contingent upon their respective earnings and subject to any limitations on the ability of such entities to make payments or other distributions to us. In addition, our subsidiaries, including our direct operating subsidiary, AAC, are separate and distinct legal entities and have no obligation to make any funds available to us.
Our directors, executive officers and certain employees and their respective affiliates have substantial control over the company and could delay or prevent a change in corporate control.
Our directors, executive officers and certain employees owned, in the aggregate, approximately 53.4% of our outstanding common stock as of December 31, 2016. Michael T. Cartwright, our Chairman and Chief Executive Officer, and his affiliates owned approximately 21.7% of our common stock, and Jerrod N. Menz, our former President, a former member of our Board of Directors and an employee of the Company, and his affiliates owned approximately 19.0% of our common stock in each case as of December 31, 2016. As a result, these stockholders, acting together, have substantial control over the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these stockholders, acting together, will continue to have significant influence over the management and affairs of our company. Accordingly, this concentration of ownership may have the effect of:
|
|
• |
delaying, deferring or preventing a change in corporate control; |
|
|
• |
impeding a merger, consolidation, takeover or other business combination involving us; or |
25
|
|
• |
discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
Anti-takeover provisions in our articles of incorporation, bylaws and Nevada law could prevent or delay a change in control of our company.
Provisions in our articles of incorporation and amended and restated bylaws, which we refer to as our bylaws, may discourage, delay or prevent a merger, acquisition or change of control. These provisions could also discourage proxy contests and make it more difficult for stockholders to elect directors and take other corporate actions. These provisions:
|
|
• |
permit our Board of Directors to issue up to 5,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate, including the right to approve an acquisition or other change in our control; |
|
|
• |
provide that the authorized number of directors may be changed only by resolution of the Board of Directors; |
|
|
• |
provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
|
|
• |
provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner and also specify requirements as to the form and content of a stockholder’s notice; |
|
|
• |
provide that our stockholders may not take action by written consent, but may only take action at annual or special meetings of our stockholders; |
|
|
• |
do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); and |
|
|
• |
provide that special meetings of our stockholders may be called only by the Chairman of the Board of Directors, our Chief Executive Officer and the Board of Directors pursuant to a resolution adopted by a majority of the total number of authorized directors or the holders of a majority of the outstanding shares of voting stock. |
We are an emerging growth company, and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company” as defined under the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years following the completion of our IPO in October 2014, although we could lose that status sooner if our revenues exceed $1 billion, if we issue more than $1 billion in non-convertible debt in a three year period or if the market value of our common stock held by non-affiliates meets or exceeds $700 million as of any June 30th before that time, in which case we would no longer be an emerging growth company as of the following December 31st. If some investors find our common stock less attractive because we may rely on these exemptions, there may be a less active trading market for our common stock, and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period for implementing new or revised accounting standards and, therefore, will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies that are not emerging growth companies.
Item 1B. Unresolved Staff Comments.
None.
A listing of our owned and leased facilities is included in Item 1 of this report under the heading “Facilities.” Additionally, we lease approximately 102,000 square feet of office space located at 200 Powell Place, Brentwood, Tennessee for our corporate headquarters and call center. The initial term of the lease is for ten years, with one option to extend the lease for five years. We also
26
lease approximately 11,000 square feet of laboratory space in Brentwood, Tennessee to perform quantitative drug testing and other laboratory services that support our treatment facilities. The lease for our Brentwood, Tennessee laboratory space expires in May 2018. We also lease approximately 11,000 square foot laboratory space in Slidell, Louisiana that operates as the Townsend in-network laboratory. We believe that these facilities are in good condition and suitable for our present requirements.
From time to time, we may be engaged in various lawsuits and legal proceedings in the ordinary course of our business. The following legal proceedings are described in detail because, although they may not be required to be disclosed in this Part I, Item 3 under SEC rules, due to the nature of the business of the Company, we believe that the discussion of these open legal matters may provide useful information to security holders or other readers of this Annual Report on Form 10-K. Certain of the matters referenced below are also discussed in Note 16 to the Consolidated Financial Statements included in this Annual Report on Form 10-K.
State of California
On October 21, 2016, AAC (formerly known as Forterus, Inc.), Forterus Health Care Services, Inc., and ABTTC, Inc. (collectively, the “Defendants,” each of which is a direct or indirect subsidiary of Holdings) agreed to the entry of a Permanent Injunction and Final Judgment (the “PIFJ”) with the Bureau of Medi-Cal Fraud and Elder Abuse of the Office of the Attorney General of the State of California (“BMFEA”) relating to the criminal charges filed against the Defendants in connection with the death of a client in 2010 at one of the Company’s former locations. The PIFJ generally provides, among other things and subject to certain limitations, that the State of California (i) dismiss all criminal charges against the Defendants in connection with the case entitled People v. McCausland, et al. (Case No. SWF1501351) and not file or seek to file any other criminal charges against the Defendants in connection with the death of such client or in connection with such client’s admission or care; (ii) release all civil claims against the Defendants and their officers, directors and employees for conduct, acts or omissions arising out of or in connection with the quality of resident care or resident admissions and occurring prior to the effective date of the PIFJ at any California facility owned, licensed, operated, managed or controlled by Defendants, and (iii) release such parties from any civil false claims and claims for fraud (both statutory and common law) under California law.
Pursuant to the terms of the PIFJ, among other things and subject to certain limitations, Defendants shall (i) institute a three-year compliance program (the “Compliance Program”) with respect its California facilities that includes, to the extent not already established, that certain policies and procedures are maintained or developed and implemented to promote each covered facility’s compliance with applicable statutes, regulations and the PIFJ, under the responsibility of Holdings’ Chief Compliance Officer; (ii) establish a Compliance Committee composed of the Compliance Officer and senior personnel responsible for overseeing clinical operations to address issues raised by the Compliance Officer in connection with the Compliance Program; and (iii) establish an oversight committee of its Board of Directors or a committee of the Board of Directors, to review the adequacy and responsiveness of the Compliance Program. In addition, for a period of 30 months following the effective date of the PIFJ, the Defendants shall retain a qualified independent monitor, appointed by BMFEA after consultation with the Defendants, to assess the effectiveness of the Defendants’ quality control systems and patient care. The PIFJ also required that the Defendants pay $549,986 toward the costs of the investigation and $200,000 as a civil monetary penalty.
Kasper v. AAC Holdings, Inc. et al. and Tenzyk c. AAC Holdings, Inc. et al.
On August 24, 2015, a shareholder filed a purported class action in the United States District Court for the Middle District of Tennessee against the Company and certain of its current and former officers. The plaintiff generally alleges that the Company and certain of its current and former officers violated Sections 10(b) and/or 20(a) of the Securities Exchange Act of 1934 and Rule 10b-5 promulgated thereunder by making allegedly false and/or misleading statements and failing to disclose certain information. On September 14, 2015, a second class action against the same defendants asserting essentially the same allegations was filed in the same court. On October 26, 2015, the court entered an order consolidating these two described actions into one action. On February 29, 2016, the plaintiff filed a consolidated amended complaint. The Company intends to defend this action vigorously. At this time the Company cannot predict the results of litigation with certainty, and cannot estimate the amount or range of loss, if any.
Other
The New Jersey Department of Banking and Insurance (“NJ-DOBI”) and Leading Edge Recovery Center, LLC (“Leading Edge”), a former indirect operating subsidiary of American Addiction Centers, Inc. that was acquired in September 2012 and was subsequently closed in 2013, are negotiating a possible resolution to certain information provided to NJ-DOBI following Leading Edge’s recent claim settlement with Horizon Blue Cross Blue Shield of New Jersey. Based on these discussions, the Company has recorded an aggregate $350,000 reserve related to this matter.
The Company is aware of various other legal matters arising in the ordinary course of business. To cover these types of claims, the Company maintains insurance it believes to be sufficient for its operations, although some claims may potentially exceed the scope of coverage in effect. Plaintiffs in these matters may request punitive or other damages that may not be covered by insurance. After taking into consideration the evaluation of such matters by the Company’s legal counsel, the Company’s management believes the outcome of these matters will not have a material impact on the Company’s consolidated financial position, results of operations and cash flows.
Item 4. Mine Safety Disclosures
Not applicable.
27
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information and Price Range of Common Stock
Our common stock is listed for trading on the NYSE under the symbol “AAC.” The following table sets forth the high and low sale prices of our common stock as reported by the NYSE during the indicated quarters.
|
|
High |
|
|
Low |
|
||
|
2016 |
|
|
|
|
|
|
|
|
First Quarter |
$ |
24.11 |
|
|
$ |
14.36 |
|
|
Second Quarter |
$ |
22.91 |
|
|
$ |
17.70 |
|
|
Third Quarter |
$ |
24.38 |
|
|
$ |
16.51 |
|
|
Fourth Quarter |
$ |
19.98 |
|
|
$ |
6.01 |
|
|
|
|
|
|
|
|
|
|
|
2015 |
|
|
|
|
|
|
|
|
First Quarter |
$ |
38.43 |
|
|
$ |
23.94 |
|
|
Second Quarter |
$ |
45.48 |
|
|
$ |
30.00 |
|
|
Third Quarter |
$ |
46.60 |
|
|
$ |
15.09 |
|
|
Fourth Quarter |
$ |
28.72 |
|
|
$ |
18.25 |
|
On February 24, 2017, the closing price of our common stock on the NYSE was $7.58 per share. As of February 24, 2017, there were 417 holders of record of our common stock. This does not include the number of persons whose stock is in nominee or “street” name accounts through brokers.
Stock Performance Graph
The following graph compares the cumulative total return on our common stock during the period from October 2, 2014 (the day our common stock began trading on the NYSE) through December 31, 2016, with the cumulative total return of the S&P 500 Index and the S&P Health Care Index. The S&P 500 Index includes 500 companies representing all major industries. The S&P Health Care Index is a group of 59 companies involved in a variety of healthcare related businesses. The graph assumes $100 invested on October 2, 2014 in our common stock and in each index and assumes reinvestment of dividends, if any. Stock price performance shown in the graph is not necessarily indicative of future stock performance.
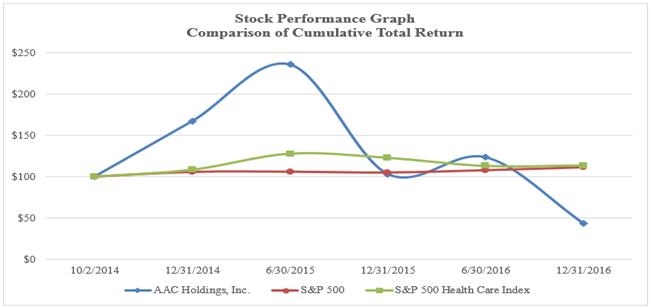
28
|
|
10/2/2014 |
|
12/31/2014 |
|
6/30/2015 |
|
12/31/2015 |
|
6/30/2016 |
|
12/31/2016 |
|
||||||
|
AAC Holdings, Inc. |
$ |
100.00 |
|
|
167.14 |
|
|
235.46 |
|
|
103.03 |
|
|
123.35 |
|
|
39.14 |
|
|
S&P 500 |
$ |
100.00 |
|
|
105.79 |
|
|
106.01 |
|
|
105.02 |
|
|
107.85 |
|
|
115.04 |
|
|
S&P 500 Health Care Index |
$ |
100.00 |
|
|
108.38 |
|
|
117.86 |
|
|
114.03 |
|
|
113.51 |
|
|
109.06 |
|
Dividend Policy
Holdings has never declared or paid cash dividends on our common stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business, and therefore, we do not anticipate paying cash dividends in the foreseeable future. Any future determination related to the payment of dividends will be made at the discretion of our Board of Directors and will depend on, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our Board of Directors may deem relevant.
Equity Compensation Plan Information
See Part III, “Item 12—Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” for information regarding securities authorized for issuance under our equity compensation plans.
Unregistered Sale of Equity Securities
None.
Issuer Purchases of Equity Securities by the Issuer and Affiliated Purchasers
During the three months ended December 31, 2016, the Company had no publicly announced plans or programs to repurchase shares. However, the Company withheld shares of Company common stock to satisfy employee minimum statutory tax withholding obligations payable upon the vesting of restricted stock, as follows:
|
|
Total number of shares purchased |
|
|
Aggregate price paid per share |
|
||
|
October 1, 2016 through October 31, 2016 |
|
5,159 |
|
|
$17.16 |
|
|
|
November 1, 2016 through November 30, 2016 |
|
— |
|
|
|
— |
|
|
December 1, 2016 through December 31, 2016 |
|
14,145 |
|
|
$7.24 |
|
|
29
Item 6. Selected Financial Data.
The following table presents our selected historical consolidated financial data as of the dates and for the periods indicated. Holdings was formed as a Nevada corporation on February 12, 2014, and acquired 93.6% of the outstanding shares of common stock of AAC on April 15, 2014 in connection with the Reorganization Transactions related to our IPO. As a result of the short-form merger completed in November 2014, AAC is a wholly-owned subsidiary of Holdings. Prior to the completion of the Reorganization Transactions, Holdings had not engaged in any business or other activities except in connection with its formation. Accordingly, all financial data herein relating to periods prior to the completion of the Reorganization Transactions is that of AAC and its consolidated subsidiaries.
The selected consolidated financial data below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and with our consolidated financial statements and notes thereto included elsewhere in this Annual Report on Form 10-K. The selected financial data in this section is not intended to replace our consolidated financial statements and the related notes. Our historical results are not necessarily indicative of results that may be expected in the future.
|
|
|
Year Ended December 31, |
|
|||||||||||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
2012 |
|
|||||
|
|
|
(Dollars in thousands, except share and per share amounts) |
|
|||||||||||||||||
|
Income Statement Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Client related revenue |
|
$ |
270,569 |
|
|
$ |
205,752 |
|
|
$ |
132,968 |
|
|
$ |
115,741 |
|
|
$ |
66,035 |
|
|
Other revenue |
|
|
9,201 |
|
|
|
6,509 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total revenue |
|
$ |
279,770 |
|
|
$ |
212,261 |
|
|
$ |
132,968 |
|
|
$ |
115,741 |
|
|
$ |
66,035 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Salaries, wages and benefits |
|
|
141,073 |
|
|
|
91,406 |
|
|
|
54,707 |
|
|
|
46,856 |
|
|
|
25,680 |
|
|
Client related services |
|
|
24,446 |
|
|
|
15,754 |
|
|
|
10,794 |
|
|
|
7,986 |
|
|
|
8,389 |
|
|
Provision for doubtful accounts |
|
|
21,485 |
|
|
|
18,113 |
|
|
|
11,391 |
|
|
|
10,950 |
|
|
|
3,344 |
|
|
Advertising and marketing |
|
|
18,275 |
|
|
|
20,821 |
|
|
|
15,683 |
|
|
|
13,493 |
|
|
|
8,667 |
|
|
Professional fees |
|
|
16,468 |
|
|
|
10,316 |
|
|
|
8,075 |
|
|
|
10,277 |
|
|
|
5,430 |
|
|
Other operating expenses |
|
|
29,627 |
|
|
|
22,708 |
|
|
|
13,518 |
|
|
|
11,615 |
|
|
|
6,384 |
|
|
Rentals and leases |
|
|
7,363 |
|
|
|
5,298 |
|
|
|
2,106 |
|
|
|
4,634 |
|
|
|
3,614 |
|
|
Litigation settlement |
|
|
1,292 |
|
|
|
2,379 |
|
|
|
487 |
|
|
|
2,588 |
|
|
|
— |
|
|
Restructuring |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
806 |
|
|
|
— |
|
|
Depreciation and amortization |
|
|
17,686 |
|
|
|
7,837 |
|
|
|
4,662 |
|
|
|
3,003 |
|
|
|
1,288 |
|
|
Acquisition-related expenses |
|
|
2,691 |
|
|
|
3,401 |
|
|
|
845 |
|
|
|
— |
|
|
|
— |
|
|
Total operating expenses |
|
|
280,406 |
|
|
|
198,033 |
|
|
|
122,268 |
|
|
|
112,208 |
|
|
|
62,796 |
|
|
(Loss) income from operations |
|
|
(636 |
) |
|
|
14,228 |
|
|
|
10,700 |
|
|
|
3,533 |
|
|
|
3,239 |
|
|
Interest expense, net |
|
|
8,175 |
|
|
|
3,607 |
|
|
|
1,872 |
|
|
|
1,390 |
|
|
|
980 |
|
|
Gain on contingent consideration |
|
|
(1,350 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Bargain purchase gain |
|
|
— |
|
|
|
(1,775 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Other (income) expense, net |
|
|
(500 |
) |
|
|
(725 |
) |
|
|
(93 |
) |
|
|
36 |
|
|
|
12 |
|
|
(Loss) income before income tax expense |
|
|
(6,961 |
) |
|
|
13,121 |
|
|
|
8,921 |
|
|
|
2,107 |
|
|
|
2,247 |
|
|
Income tax (benefit) expense |
|
|
(1,220 |
) |
|
|
4,780 |
|
|
|
2,555 |
|
|
|
615 |
|
|
|
1,148 |
|
|
Net (loss) income |
|
|
(5,741 |
) |
|
|
8,341 |
|
|
|
6,366 |
|
|
|
1,492 |
|
|
|
1,099 |
|
|
Less: net loss (income) attributable to noncontrolling interest |
|
|
5,152 |
|
|
|
2,833 |
|
|
|
1,182 |
|
|
|
(706 |
) |
|
|
405 |
|
|
Net (loss) income attributable to AAC Holdings, Inc. stockholders |
|
|
(589 |
) |
|
|
11,174 |
|
|
|
7,548 |
|
|
|
786 |
|
|
|
1,504 |
|
|
Deemed contribution-redemption of Series B Preferred Stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,000 |
|
|
|
— |
|
|
BHR Series A Preferred Unit dividend |
|
|
— |
|
|
|
(147 |
) |
|
|
(693 |
) |
|
|
— |
|
|
|
— |
|
|
Redemption of BHR Series A preferred Units |
|
|
— |
|
|
|
(534 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Net (loss) income available to AAC Holdings, Inc. common stockholders |
|
$ |
(589 |
) |
|
$ |
10,493 |
|
|
$ |
6,855 |
|
|
$ |
1,786 |
|
|
$ |
1,504 |
|
|
Earnings per share attributable to common stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings per common share |
|
$ |
(0.03 |
) |
|
$ |
0.49 |
|
|
$ |
0.41 |
|
|
$ |
0.13 |
|
|
$ |
0.12 |
|
|
Diluted earnings per common share |
|
$ |
(0.03 |
) |
|
$ |
0.48 |
|
|
$ |
0.41 |
|
|
$ |
0.12 |
|
|
$ |
0.12 |
|
|
Weighted-average common shares outstanding: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
22,718,117 |
|
|
|
21,605,037 |
|
|
|
16,557,655 |
|
|
|
13,855,797 |
|
|
|
12,208,160 |
|
|
Diluted |
|
|
22,718,117 |
|
|
|
21,661,259 |
|
|
|
16,619,180 |
|
|
|
14,291,937 |
|
|
|
12,363,164 |
|
30
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
3,964 |
|
|
$ |
18,750 |
|
|
$ |
48,540 |
|
|
$ |
2,012 |
|
|
$ |
740 |
|
|
Working capital |
|
$ |
51,137 |
|
|
$ |
52,187 |
|
|
$ |
63,153 |
|
|
$ |
1,220 |
|
|
$ |
3,190 |
|
|
Total assets |
|
$ |
383,884 |
|
|
$ |
316,049 |
|
|
$ |
145,952 |
|
|
$ |
81,638 |
|
|
$ |
53,598 |
|
|
Total debt, including current portion |
|
$ |
189,106 |
|
|
$ |
145,141 |
|
|
$ |
28,641 |
|
|
$ |
43,075 |
|
|
$ |
25,222 |
|
|
Total stockholders' equity, including noncontrolling interests |
|
$ |
154,788 |
|
|
$ |
136,488 |
|
|
$ |
95,141 |
|
|
$ |
11,883 |
|
|
$ |
4,678 |
|
31
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the federal securities laws. These forward-looking statements are made only as of the date of this Annual Report. In some cases, you can identify forward-looking statements by terms such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “may,” “potential,” “predicts,” “projects,” “should,” “will,” “would,” and similar expressions intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements may include information concerning Holdings’ (collectively with its subsidiaries; “AAC Holdings” or the “Company”) possible or assumed future results of operations, including descriptions of Holdings’ revenues, profitability, outlook and overall business strategy. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results and performance to be materially different from the information contained in the forward-looking statements. These risks, uncertainties and other factors include, without limitation: (i) our inability to operate our facilities; (ii) our reliance on our sales and marketing program to continuously attract and enroll clients; (iii) a reduction in reimbursement rates by certain third-party payors for inpatient and outpatient services and point-of-care and definitive lab testing; (iv) our failure to successfully achieve growth through acquisitions and de novo projects; (v) uncertainties regarding the timing of the closing of acquisitions; (vi) our failure to achieve anticipated financial results from contemplated acquisitions; (vii) the possibility that a governmental entity may prohibit, delay or refuse to grant approval for the consummation of an acquisition; (viii) a disruption in our ability to perform diagnostic drug testing services; (ix) maintaining compliance with applicable regulatory authorities, licensure and permits to operate our facilities and lab; (x) a disruption in our business and reputational and economic risks associated with the civil securities claims brought by shareholders; (xi) our inability to agree on conversion and other terms for the balance of convertible debt; (xii) our inability to meet our covenants in the loan documents; (xiii) our inability to obtain senior lender consent to exceed the current $50 million limit in unsecured subordinated debt; (xiv) our inability to integrate newly acquired facilities; and (xv) general economic conditions, as well as other risks discussed in the “Risk Factors” section of this Annual Report on Form 10-K, and other filings with the Securities and Exchange Commission. As a result of these factors, we cannot assure you that the forward-looking statements in this annual report will prove to be accurate. Investors should not place undue reliance upon forward-looking statements.
Overview
We are a provider of inpatient and outpatient substance abuse treatment services for individuals with drug and alcohol addiction. In addition to our inpatient and outpatient treatment services, we perform drug testing and diagnostics laboratory services and provide physician services to our clients. As of December 31, 2016, we operated 12 residential substance abuse treatment facilities located throughout the United States, focused on delivering effective clinical care and treatment solutions across 1,140 beds, which includes 668 licensed detoxification beds, 18 standalone outpatient centers, and 202 sober living beds across three locations.
We are in the process of expanding Oxford Treatment Center, where we opened an additional 24 residential beds in September 2016 and currently anticipate completing an additional 24 residential beds and 48 sober living beds in the second quarter of 2017. In addition, we have leased one floor from New Orleans East Hospital in New Orleans, Louisiana, where we intend to operate 36 in-network beds to provide detoxification and residential treatment services. The beds are expected to be operational in the second quarter of 2017, subject to receiving licensure, and will be operated by Townsend’s clinical staff.
We are also an internet marketer in the addiction treatment industry operating a broad portfolio of internet assets that service millions of website visits each month. Through our websites, such as Rehabs.com and Recovery.org, we serve families and individuals struggling with addiction and seeking treatment options through comprehensive online directories of treatment providers, treatment provider reviews, forums and professional communities. Recovery Brands also provides online marketing solutions to other treatment providers such as enhanced facility profiles, audience targeting, lead generation and tools for digital reputation management.
2016 Developments
Existing Facilities and Ancillary Services
On January 1, 2016, we increased the capacity at Forterus to 144 beds from 107 beds. During the third quarter of 2016, we decreased our capacity at Forterus from 144 beds to 112 beds.
On April 4, 2016, we expanded our laboratory services to include hematology testing across all of our existing facilities.
On May 31, 2016, we consolidated our outpatient services in New Jersey and relocated our Mountainside, New Jersey outpatient center to our existing outpatient center in Lafayette, New Jersey in order to gain additional operating efficiencies.
On June 14, 2016, we increased our beds licensed for detoxification at Sunrise House from 18 to 29 beds.
On June 16, 2016, we began treating clients at Laguna Treatment Hospital, a 93-bed chemical dependency recovery hospital in Aliso Viejo, California, which is the first such hospital designation for an AAC treatment center. The property was acquired by AAC in April 2015.
32
On September 22, 2016, we completed the addition of 24 residential beds at Oxford Treatment Center and currently expect to complete the construction of an additional 24 residential beds licensed for detoxification and an additional 48 sober living beds by the end of the first quarter of 2017.
On September 29, 2016, Singer Island transferred substantially all of its assets to Recovery First and now operates as Recovery First West Palm. In conjunction with this asset transfer, Recovery First West Palm will begin accepting clients with in-network benefits.
On December 13, 2016, we leased one floor from New Orleans East Hospital where we intend to operate 36 in-network beds to provide detoxification and residential treatment services. The beds are expected to be operational in the first half of 2017, subject to receiving licensure, and will be operated by Townsend’s clinical staff.
New Property Developments and Acquisitions
On April 1, 2016, we completed the Townsend Acquisition for $22.0 million, consisting of $13.5 million in cash and $8.5 million in restricted shares (447,369 shares) of common stock. The cash portion of the transaction was funded from borrowings pursuant to the Deerfield Facility (as later defined). In the Townsend Acquisition, we acquired a substance abuse treatment provider in Louisiana that operates seven in-network outpatient centers delivering intensive outpatient treatment as well as a 32-bed in-network facility located in Scott, Louisiana that has 20 beds licensed for detoxification and inpatient treatment. Townsend also operates an in-network lab that services these facilities. We are utilizing the in-network lab to continue servicing Townsend’s current lab customers and have begun servicing AAC’s in-network residential and outpatient facilities in Florida, New Jersey and Rhode Island.
On April 18, 2016, we acquired Resolutions Arlington, a 100-room hotel in Arlington, Texas, for $5.35 million in cash and are currently in the process of converting the property into sober living beds that will be used in support of the Greenhouse Outpatient Center. The acquisition was funded from borrowings on the Deerfield Facility. We began accepting sober living clients at the end of the third quarter of 2016 and currently have a total of 64 sober living beds at Resolutions Arlington. We currently anticipate completion of the remaining renovations in the second quarter of 2017 which will result in 60 additional sober living beds.
In April 2016, we began the development of a 150-bed residential treatment center at the site of a former convent on 96 acres in Ringwood, New Jersey. The facility is expected to be completed in the first half of 2018 with a projected capital investment of approximately $25.0 million.
On May 3, 2016, we completed the Solutions Recovery Acquisition for an aggregate $13.0 million, consisting of $6.75 million in cash and $6.25 million of restricted shares (309,871 shares) of common stock (the “Solutions Acquisition”). The cash portion of the purchase price was funded from borrowings pursuant to the Deerfield Facility. Solutions provides detoxification, residential and intensive outpatient treatment as well as sober living services in the greater Las Vegas area. The acquisition included:
|
|
• |
124 Sober Living Beds |
|
|
• |
80 Licensed In-Network Detox, Residential and Halfway House Beds |
|
|
• |
Two Licensed, In-Network Outpatient Centers |
We are currently in the process of renovating the sober living services we acquired in conjunction with the Solutions Acquisition to accommodate an additional 14 sober living beds that we currently expect to complete in the second quarter of 2017.
Financing
In connection with the second quarter 2016 acquisitions of Solutions, Townsend and the hotel in Arlington, Texas, we drew down an aggregate $25.0 million of unsecured subordinated debt under the Deerfield Facility. The unsecured subordinated debt bears interest at an annual rate of 12.0% and matures on October 2, 2020. The $25.0 million of unsecured subordinated debt can be repaid under certain conditions without penalty prior to October 2, 2017.
On July 13, 2016, we amended our 2015 Credit Facility, which increased the current borrowing capacity from $121.3 million to $171.3 million, consisting of a $50.0 million revolving credit facility and a $121.3 million term loan. The 2015 Credit Facility is scheduled to mature in March 2020 and bears interest at LIBOR plus a margin between 2.25% and 3.75% or a base rate plus a margin between 1.25% and 2.75%, in each case depending on our leverage ratio. The 2015 Credit Facility has an accordion feature that provides for an additional $75.0 million of borrowing capacity, subject to certain consents and conditions, including obtaining additional commitments from lenders.
On February 27, 2017, we amended our 2015 Credit Facility to, among other things, provide for certain modifications to the terms of our Credit Agreement, dated as of March 19, 2015, as amended from time to time (the “2015 Credit Agreement”), including the following: (i) extend the maximum Consolidated Total Leverage Ratio (as defined in the 2015 Credit Agreement) of 4:25:1.00 through the measurement period ending September 30, 2017; and (ii) amend the definition of Applicable Margin (as defined in the 2015 Credit Agreement) to add an additional pricing level of 3.75% for Eurodollar Rate Loans and Letter of Credit Fee, 2.75% for Base Rate Loans and 0.60% for Commitment Fee (as all such terms are defined in the 2015 Credit Agreement), which will be applicable when the Consolidated Total Leverage Ratio is equal to or exceeds 4.00:1.00 at the end of the applicable measuring period (the “New Pricing Level”) and to provide that the Applicable Rate (as defined in the 2015 Credit Agreement) be set at the New
33
Pricing Level from the date of such amendment until the first business day following the date we deliver our next Compliance Certificate (as defined in the 2015 Credit Agreement). The amendment also provided for additional Adjusted EBITDA (as defined in the 2015 Credit Agreement) add backs under its covenant calculation to account for its February 2017 reduction in workforce.
For additional discussion, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financing Relationships.”
Components of Results of Operations
Client Related Revenue. Our client related revenue primarily consists of service charges related to providing addiction treatment and related services, including the collection and laboratory testing of urine for controlled substances. We recognize revenues at the estimated net realizable value in the period in which services are provided. For the years ended December 31, 2016 and 2015, approximately 90% of our client related revenues were reimbursable by commercial payors, including amounts paid by such payors to clients, with the remaining revenues payable directly by our clients. Given the scale and nationwide reach of our network of substance abuse treatment facilities, we generally have the ability to serve clients located across the country from any of our facilities, which allows us to operate our business and analyze revenue on a system-wide basis rather than focusing on any individual facility.
For the year ended December 31, 2016, approximately 10.5% of our client related revenue reimbursements came from Anthem Blue Cross Blue Shield of Florida, 10.4% came from Blue Cross Blue Shield of Texas, and 10.4% came from Aetna. No other payor accounted for more than 10% of our client related revenue reimbursements for the year ended December 31, 2016. For the year ended December 31, 2015, approximately 15.1% of our client related revenue reimbursements came from Anthem Blue Cross Blue Shield of Colorado, 12.5% came from Blue Cross Blue Shield of Texas, 11.5% came from Aetna and 11.1% came from Blue Cross Blue Shield of California. No other payor accounted for more than 10% of our client related revenue reimbursements for the year ended December 31, 2015.
The following table summarizes the composition of our client related revenues for detoxification and residential treatment services, partial hospitalization and intensive outpatient treatment services, and point-of-care drug testing and diagnostic laboratory services, and professional groups and other ancillary services for the years ended December 31, 2016 and 2015:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Detoxification and residential treatment services |
|
|
39% |
|
|
|
32% |
|
|
Partial hospitalization and intensive outpatient treatment services |
|
|
36% |
|
|
|
38% |
|
|
Point-of-care drug testing and definitive laboratory services |
|
|
24% |
|
|
|
28% |
|
|
Professional groups and other ancillary services |
|
|
1% |
|
|
|
2% |
|
The increase in detoxification and residential treatment services as a percentage of client related revenues and the decrease in partial hospitalization and intensive outpatient treatment services as a percentage of client related revenues was primarily related to the 39% increase in licensed detoxification beds during 2016 as a result of acquisitions and the opening of Laguna Treatment Hospital. As of December 31, 2016, we had 668 licensed detoxification beds compared to 482 licensed detoxification beds as of December 31, 2015.
The decrease in point-of-care drug testing and diagnostic laboratory services as a percentage of client related revenues in 2016 compared to 2015 was primarily related to a combination of a decrease in the number of tests performed on a per client basis and a decline in reimbursement rates for point-of-care testing and diagnostic laboratory services. We currently expect a continued decline in reimbursement rates and revenues for point-of-care testing and diagnostic laboratory services during 2017. However, we also currently anticipate that the declines in revenues from point-of-care testing and diagnostic laboratory services may be partially offset by providing laboratory services to clients of third party providers.
We recognize revenues from commercial payors at the time services are provided based on our estimate of the amount that payors will pay us for the services performed. We estimate the net realizable value of revenues by adjusting gross client charges using our expected realization and applying this discount to gross client charges. Our expected realization is determined by management after taking into account the type of services provided and the historical collections received from the commercial payors, on a per facility basis, compared to the gross client charges billed.
Our accounts receivable primarily consists of amounts due from commercial payors. The client self-pay portion is usually collected upon admission and in limited circumstances the client will make a deposit and negotiate the remaining payments as part of the services. We do not recognize revenue for any amounts not collected from the client in either of these situations. From time to time, we may provide free care to a limited number of clients, which we refer to as scholarships. We do not recognize revenues for scholarships provided. Included in the aging of accounts receivable are amounts for which the commercial insurance company paid out-of-network claims directly to the client and for which the client has yet to remit the insurance payment to us (which we refer to as “paid to client”). Such amounts paid to clients continue to be reflected in our accounts receivable aging as amounts due from commercial payors. Accordingly, our accounts receivable aging does not provide for the distinct identification of paid to client
34
receivables. Also included in the aging of accounts receivable are amounts where we have received a partial payment from the commercial insurance company and are continuing to pursue additional collections for the estimated remaining balance outstanding.
Other Revenue. Our other revenue consists of service charges from the delivery of quality targeted leads to behavioral and mental health service businesses through our operating subsidiary Referral Solutions Group, LLC (“RSG”) and its wholly owned subsidiary, Recovery Brands, LLC (“Recovery Brands”), which was acquired July 2, 2015 (the “RSG Acquisition”), and diagnostic laboratory services provided to clients of third-party addiction treatment providers. Revenue is recognized when persuasive evidence of an arrangement exists, services have been rendered, the fee for services is fixed or determinable and collectability of the fee is reasonably assured.
Operating Expenses. Our operating expenses are primarily impacted by nine categories of expenses: salaries, wages and benefits; client related services; provision for doubtful accounts; advertising and marketing; professional fees; other operating expenses; rentals and leases; depreciation and amortization; and acquisition-related expenses.
|
|
• |
Salaries, wages and benefits. We employ a variety of staff related to providing client care, including case managers, therapists, medical technicians, housekeepers, cooks and drivers, among others. Our clinical salaries, wages and benefits expense is largely driven by the total number of beds in our facilities, our average daily residential census and the number of our outpatient visits. We also employ a professional sales force and staff a centralized call center. Our corporate staff includes accounting, billing and finance professionals, marketing and human resource personnel, IT staff and senior management. |
|
|
• |
Client related services. Client related services consist of physician and medical services as well as client meals, pharmacy, travel, and various other expenses associated with client treatment. Client related services are significantly influenced by our average daily residential census. |
|
|
• |
Provision for doubtful accounts. The provision for doubtful accounts represents the expense associated with management’s best estimate of accounts receivable that could become uncollectible in the future. We establish our provision for doubtful accounts based on the aging of the receivables, historical collection experience by facility, services provided, payor source and historical reimbursement rate, current economic trends and percentages applied to the accounts receivable aging categories. As of December 31, 2016, substantially all of accounts receivable aged greater than 360 days were fully reserved in our consolidated financial statements. In assessing the adequacy of the allowance for doubtful accounts, we rely on the results of detailed reviews of historical write-offs and recoveries on a twelve-month basis (the hindsight analysis) as a primary source of information to utilize in estimating the collectability of our accounts receivable. We supplement this hindsight analysis with other analytical tools, including, but not limited to, historical trends in cash collections compared to net revenues less bad debt and days sales outstanding. |
|
|
• |
Advertising and marketing. We promote our treatment facilities through a variety of channels including television advertising, internet search engines and Yellow Page advertising, among others. While we do not compensate our referral sources for client referrals, we do have arrangements with multiple marketing channels that we pay on a performance basis (i.e., pay per click or pay per inbound call). We also host and attend industry conferences. Our advertising and marketing efforts and expense is largely driven by the total number of available beds in our facilities. |
|
|
• |
Professional fees. Professional fees consist of various professional services used to support primarily corporate related functions. These services include client billings and collections, accounting related fees for financial statement audits and tax preparation and legal fees for, among other matters, employment, compliance and general corporate matters. These fees also include information technology, consulting, payroll fees and national medical director fees. |
|
|
• |
Other operating expenses. Other operating expenses consists primarily of utilities, insurance, telecom, travel and repairs and maintenance expenses, and is significantly influenced by the total number of beds in our facilities and our average daily residential census. |
|
|
• |
Rentals and leases. Rentals and leases mainly consist of properties under various equipment and operating leases, which includes space required to perform client services and space for administrative facilities. |
|
|
• |
Depreciation and amortization. Depreciation and amortization represents the ratable use of our capitalized property and equipment, including assets under capital leases, over the estimated useful lives of the assets, and amortizable intangible assets, which mainly consist of trademark-related intangibles and non-compete agreements. |
|
|
• |
Acquisition-related expenses. Acquisition-related expenses consist primarily of professional fees and travel costs associated with our acquisition activities. |
35
Key Drivers of Our Results of Operations. Our results of operations and financial condition are affected by numerous factors, including those described under “Risk Factors” and those described below:
|
|
• |
Average Daily Residential Census. We refer to the average number of clients to whom we are providing services at our residential facilities on a daily basis over a specific period as our “average daily residential census.” Our revenues are directly impacted by our average daily residential census, which fluctuates based on the effectiveness of our sales and marketing efforts, total number of beds, the number of client admissions and discharges in a period, average length of stay, and the ratio of clinical staff to clients. |
|
|
• |
Average Daily Residential Revenue and Average Net Daily Residential Revenue. Our average daily residential revenue is a per census metric equal to our total residential revenues for a period divided by our average daily residential census for the same period divided by the number of days in the period. Our average net daily residential revenue is a per census metric equal to our total residential revenues less provision for doubtful accounts for a period divided by our average daily residential census for the same period divided by the number of days in the period. The key drivers of average daily residential revenue and average net daily residential revenue include the mix of services and level of care that we provide to our clients during the period and payor mix. We provide a broad continuum of services including detoxification, residential treatment, partial hospitalization and intensive outpatient care, with detoxification resulting in the highest daily charges and intensive outpatient care resulting in the lowest daily charges. We also generate revenues from point-of care drug testing, diagnostic laboratory services, professional groups and other ancillary services associated with serving our clients. We tend to experience higher margins from our point-of-care drug testing, which is conducted on-site at our treatment facilities, and our diagnostic laboratory services, which are conducted at our centralized laboratory facility in Brentwood, Tennessee, than we do from other services. |
|
|
• |
Outpatient Visits. Our outpatient visits represents the total number of outpatient visits at our standalone outpatient centers during the period. Our revenues are directly impacted by our outpatient visits, which fluctuates based on our sales and marketing efforts, utilization review and the average length of stay. |
|
|
• |
Expense Management. Our profitability is directly impacted by our ability to manage our expenses, most notably salaries, wages and benefits and advertising and marketing costs, and to adjust accordingly based upon our capacity. |
|
|
• |
Billing and Collections. Our revenues and cash flow are directly impacted by our ability to properly verify our clients’ insurance benefits, obtain authorization for levels of care, properly submit insurance claims and manage collections. |
36
The following table presents our consolidated income statements for the periods indicated (dollars in thousands):
|
|
|
Year Ended December 31, |
|
|||||||||||||||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||||||||||||||
|
|
|
Amount |
|
|
% |
|
|
Amount |
|
|
% |
|
|
Amount |
|
|
% |
|
||||||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Client related revenue |
|
$ |
270,569 |
|
|
|
96.7 |
|
|
$ |
205,752 |
|
|
|
96.9 |
|
|
$ |
132,968 |
|
|
|
100.0 |
|
|
Other revenue |
|
|
9,201 |
|
|
|
3.3 |
|
|
|
6,509 |
|
|
|
3.1 |
|
|
|
— |
|
|
|
— |
|
|
Total revenue |
|
$ |
279,770 |
|
|
|
100.0 |
|
|
$ |
212,261 |
|
|
|
100.0 |
|
|
$ |
132,968 |
|
|
|
100.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Salaries, wages and benefits |
|
|
141,073 |
|
|
|
50.4 |
|
|
|
91,406 |
|
|
|
43.1 |
|
|
|
54,707 |
|
|
|
41.1 |
|
|
Client related services |
|
|
24,446 |
|
|
|
8.7 |
|
|
|
15,754 |
|
|
|
7.4 |
|
|
|
10,794 |
|
|
|
8.1 |
|
|
Provision for doubtful accounts |
|
|
21,485 |
|
|
|
7.7 |
|
|
|
18,113 |
|
|
|
8.5 |
|
|
|
11,391 |
|
|
|
8.6 |
|
|
Advertising and marketing |
|
|
18,275 |
|
|
|
6.5 |
|
|
|
20,821 |
|
|
|
9.8 |
|
|
|
15,683 |
|
|
|
11.8 |
|
|
Professional fees |
|
|
16,468 |
|
|
|
5.9 |
|
|
|
10,316 |
|
|
|
4.9 |
|
|
|
8,075 |
|
|
|
6.1 |
|
|
Other operating expenses |
|
|
29,627 |
|
|
|
10.6 |
|
|
|
22,708 |
|
|
|
10.7 |
|
|
|
13,518 |
|
|
|
10.2 |
|
|
Rentals and leases |
|
|
7,363 |
|
|
|
2.6 |
|
|
|
5,298 |
|
|
|
2.5 |
|
|
|
2,106 |
|
|
|
1.6 |
|
|
Litigation settlement |
|
|
1,292 |
|
|
|
0.5 |
|
|
|
2,379 |
|
|
|
1.1 |
|
|
|
487 |
|
|
|
0.4 |
|
|
Depreciation and amortization |
|
|
17,686 |
|
|
|
6.3 |
|
|
|
7,837 |
|
|
|
3.7 |
|
|
|
4,662 |
|
|
|
3.5 |
|
|
Acquisition-related expenses |
|
|
2,691 |
|
|
|
1.0 |
|
|
|
3,401 |
|
|
|
1.6 |
|
|
|
845 |
|
|
|
0.6 |
|
|
Total operating expenses |
|
|
280,406 |
|
|
|
100.2 |
|
|
|
198,033 |
|
|
|
93.3 |
|
|
|
122,268 |
|
|
|
92.0 |
|
|
(Loss) income from operations |
|
|
(636 |
) |
|
|
(0.2 |
) |
|
|
14,228 |
|
|
|
6.7 |
|
|
|
10,700 |
|
|
|
8.0 |
|
|
Interest expense, net |
|
|
8,175 |
|
|
|
2.9 |
|
|
|
3,607 |
|
|
|
1.7 |
|
|
|
1,872 |
|
|
|
1.4 |
|
|
Gain on contingent consideration |
|
|
(1,350 |
) |
|
|
(0.5 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Bargain purchase gain |
|
|
— |
|
|
|
— |
|
|
|
(1,775 |
) |
|
|
(0.8 |
) |
|
|
— |
|
|
|
— |
|
|
Other income, net |
|
|
(500 |
) |
|
|
(0.2 |
) |
|
|
(725 |
) |
|
|
(0.3 |
) |
|
|
(93 |
) |
|
|
(0.1 |
) |
|
(Loss) income before income tax expense |
|
|
(6,961 |
) |
|
|
(2.5 |
) |
|
|
13,121 |
|
|
|
6.2 |
|
|
|
8,921 |
|
|
|
6.7 |
|
|
Income tax (benefit) expense |
|
|
(1,220 |
) |
|
|
(0.4 |
) |
|
|
4,780 |
|
|
|
2.3 |
|
|
|
2,555 |
|
|
|
1.9 |
|
|
Net (loss) income |
|
|
(5,741 |
) |
|
|
(2.1 |
) |
|
|
8,341 |
|
|
|
3.9 |
|
|
|
6,366 |
|
|
|
4.8 |
|
|
Less: net loss attributable to noncontrolling interest |
|
|
5,152 |
|
|
|
1.8 |
|
|
|
2,833 |
|
|
|
1.3 |
|
|
|
1,182 |
|
|
|
0.9 |
|
|
Net (loss) income attributable to AAC Holdings, Inc. stockholders |
|
|
(589 |
) |
|
|
(0.2 |
) |
|
|
11,174 |
|
|
|
5.3 |
|
|
|
7,548 |
|
|
|
5.7 |
|
|
BHR Series A Preferred Unit dividend |
|
|
— |
|
|
|
— |
|
|
|
(147 |
) |
|
|
(0.1 |
) |
|
|
(693 |
) |
|
|
(0.5 |
) |
|
Redemption of BHR Series A Preferred Units |
|
|
— |
|
|
|
— |
|
|
|
(534 |
) |
|
|
(0.3 |
) |
|
|
— |
|
|
|
— |
|
|
Net (loss) income available to AAC Holdings, Inc. common stockholders |
|
$ |
(589 |
) |
|
|
(0.2 |
) |
|
$ |
10,493 |
|
|
|
4.9 |
|
|
$ |
6,855 |
|
|
|
5.2 |
|
Comparison of Year Ended December 31, 2016 to Year Ended December 31, 2015
Client Related Revenue
Client related revenues increased $64.8 million, or 31.5%, to $270.6 million for the year ended December 31, 2016 from $205.8 million for the year ended December 31, 2015. Revenues were positively impacted by our 2015 and 2016 acquisitions and de novo facilities as well as an increase in average daily residential census and outpatient visits at our 18 standalone outpatient centers, partially offset by a decrease in average daily residential revenue.
Our average daily residential census increased by 45.6% to 818 clients for the year ended December 31, 2016 from 562 clients for the year ended December 31, 2015. The increase in the residential average daily census was primarily driven by the acquisitions completed in 2015 and 2016. Our bed capacity at our residential treatment facilities increased 27.1% to 1,140 beds at December 31, 2016 from 897 beds at December 31, 2015.
Our average daily residential revenue decreased 14.9% to $800 for the year ended December 31, 2016 from $940 for the year ended December 31, 2015. The decline in the average daily residential revenue was significantly impacted by a greater percentage of client related revenues being derived from in-network facilities/beds during the year ended December 31, 2016 as compared to December 31, 2015. Our average daily residential revenue derived from in-network facilities/beds is generally lower than our average daily residential revenue derived from out-of-network facilities/beds. For the year ended December 31, 2016 approximately 16.4% of
37
our client related revenue was derived from in-network facilities/beds versus 6.3% for the year ended December 31, 2015. We currently expect that the percentage of our client related revenue derived from in-network facilities/beds will increase during 2017 primarily as a result of a full year impact of the Townsend Acquisition and Solutions Acquisition which are principally in-network facilities. Townsend was acquired in April 2016 and Solutions was acquired in May of 2016.
Outpatient visits increased 281.8% to 49,173 for the year ended December 31, 2016 from 12,879 for the year ended December 31, 2015. The increase in outpatient visits is primarily related to an increase in the number of standalone outpatient centers we operate. At December 31, 2016, we operated 18 standalone outpatient centers versus nine standalone outpatient centers at December 31, 2015.
Partially offsetting the increase in client related revenue as a result of increases in average daily census and outpatient visits is a decline in revenue from point-of-care testing and diagnostic laboratory services. As a percentage of client related revenues, point-of-care drug testing and diagnostic laboratory services were 24% and 28% for the year ended December 31, 2016 and 2015, respectively. The decline as a percentage of client related revenues was related to a combination of a decrease in the number of tests performed on a per client basis and a decline in reimbursement rates for point-of-care testing and diagnostic laboratory services. As a percentage of client related revenues, point-of-care drug testing and diagnostic laboratory services were 20% for the quarter ended December 31, 2016. We currently anticipate continued declines in reimbursement rates for revenue from point-of-care testing and diagnostic laboratory services. However, we also currently anticipate that the declines in revenues from point-of-care testing and diagnostic laboratory services may be partially offset by providing laboratory services to clients of third party providers.
Other revenue increased $2.7 million, or 41.4%, to $9.2 million for the year ended December 31, 2016 from $6.5 million for the year ended December 31, 2015. Our other revenue primarily consists of service charges from the delivery of quality targeted leads to behavioral and mental health service businesses through our operating subsidiary RSG and diagnostic laboratory services provided to clients of third-party addiction treatment providers.
Salaries, Wages and Benefits
Salaries, wages and benefits increased $49.7 million, or 54.3%, to $141.1 million for the year ended December 31, 2016 from $91.4 million for the year ended December 31, 2015. The increase in salaries and wages was primarily impacted by growth in our residential facilities and our standalone outpatient centers. Our number of employees increased by approximately 500 employees, or 31.3%, to approximately 2,100 employees at December 31, 2016 from approximately 1,600 employees at December 31, 2015. Also contributing to the increase are increased employee health insurance costs. Health insurance costs increased $5.7 million for the year ended December 31, 2016. There was also an increase in stock based compensation of $3.0 million to $8.8 million for the year ended December 31, 2016 compared to $5.8 million for the year ended December 31, 2015. As a percentage of revenues, salaries, wages and benefits were 50.4% of total revenues for the year ended December 31, 2016 compared to 43.1% of total revenues for the year ended December 31, 2015. The increase in salaries, wages and benefits as a percentage of revenues is primarily related to the increases in health insurance costs and stock based compensation noted above. Also contributing to the increase in salaries, wages and benefits as a percentage of revenues is the previously noted decline point-of-care testing and diagnostic laboratory services revenue where we tend to experience lower labor costs.
Client Related Services
Client related services expenses increased $8.7 million, or 55.2%, to $24.4 million for the year ended December 31, 2016 from $15.8 million for the year ended December 31, 2015. The increase in expense was primarily related to the growth in the average daily residential census to 818 for the year ended December 31, 2016 from 562 for the year ended December 31, 2015. As a percentage of revenues, client related services expenses were 8.7% of total revenues for the year ended December 31, 2016 compared to 7.4% of total revenues for the year ended December 31, 2015.
Provision for Doubtful Accounts
The provision for doubtful accounts increased $3.4 million, or 18.6%, to $21.5 million for the year ended December 31, 2016 from $18.1 million for the year ended December 31, 2015. The increase in the provision for doubtful accounts was primarily related to the increase in total revenues. As a percentage of revenues, the provision for doubtful accounts was 7.7% of total revenues for the year ended December 31, 2016 compared to 8.5% of total revenues for the year ended December 31, 2015.
We establish our provision for doubtful accounts based on the aging of the receivables and taking into consideration historical collection experience by facility, services provided, payor source and historical reimbursement rate, current economic trends and percentages applied to the accounts receivable aging categories. As of December 31, 2016, substantially all accounts receivable aged greater than 360 days were fully reserved in our consolidated financial statements. In assessing the adequacy of the allowance for doubtful accounts, we rely on the results of detailed reviews of historical write-offs and recoveries (the hindsight analysis) as a primary source of information to utilize in estimating the collectability of our accounts receivable. We perform the hindsight analysis utilizing twelve-month accounts receivable collection, write-off and recovery data. We supplement this hindsight analysis with other
38
analytical tools, including, but not limited to, historical trends in cash collections compared to net revenues less bad debt and days sales outstanding.
The following table presents a summary of our aging of accounts receivable as of December 31, 2016 and 2015:
|
|
|
Current |
|
31-180 Days |
|
Over 180 Days |
|
Total |
|
||||
|
December 31, 2016 |
|
|
26.5 |
% |
|
36.1 |
% |
|
37.4 |
% |
|
100.0 |
% |
|
December 31, 2015 |
|
|
25.7 |
% |
|
43.0 |
% |
|
31.3 |
% |
|
100.0 |
% |
The aging of our accounts receivable continue to be negatively impacted by increased documentation requests from commercial payors prior to payment and slower collections related to laboratory services. Our days sales outstanding was 111 days as of and for the quarter ended December 21, 2016 compared with 96 days as of and for the quarter ended December 31, 2015.
Advertising and Marketing
Advertising and marketing expenses decreased $2.5 million, or 12.2%, to $18.3 million for the year ended December 31, 2016 from $20.8 million for the year ended December 31, 2015. The decrease in advertising and marketing expense was primarily driven by an increase in the number of in-network residential beds, which generally have a lower advertising and marketing expense on a per admission basis than out-of-network residential beds and efficiencies gained through the RSG Acquisition and Taj Media, a premier digital marketing agency with significant experience in the substance abuse treatment industry (the “Taj Media Acquisition”) in July 2015. As a percentage of revenues, advertising and marketing expenses were 6.5% of total revenues for the year ended December 31, 2016 compared to 9.8% of total revenues for the year ended December 31, 2015.
Professional Fees
Professional fees increased $6.2 million, or 59.6%, to $16.5 million for the year ended December 31, 2016 compared to $10.3 million for the year ended December 31, 2015. As a percentage of revenues, professional fees were 5.9% of total revenues for the year ended December 31, 2016 compared to 4.9% of total revenues for the year ended December 31, 2015. The increase in professional fees was primarily related to approximately $7.4 million in professional fees associated with certain litigation costs in California incurred for the year ended December 31, 2016 as compared to $3.1 million incurred for the year ended December 31, 2015. See Note 16 to the consolidated financial statements for further discussion and “Litigation Settlement” below.
Other Operating Expenses
Other operating expenses increased $6.9 million, or 30.5%, to $29.6 million for the year ended December 31, 2016 from $22.7 million for the year ended December 31, 2015. The increase was primarily the result of additional operating expenses associated with the acquisitions completed in 2015 and 2016, the opening of River Oaks in October 2015, and the opening of Laguna Treatment Hospital in June 2016. As a percentage of revenues, other operating expenses were 10.6% of total revenues for the year ended December 31, 2016 compared to 10.7% of total revenues for the year ended December 31, 2015.
Rentals and Leases
Rentals and leases increased $2.1 million, or 39.0%, to $7.4 million for the year ended December 31, 2016 from $5.3 million for the year ended December 31, 2015. As a percentage of revenues, rentals and leases were 2.6% of total revenues for the year ended December 31, 2016 compared to 2.5% of total revenues for the year ended December 31, 2015. The increase was primarily the result of increased rent as a result of the acquisitions completed in 2015 and 2016 and the lease associated with our new corporate headquarters and call center beginning in June 2015.
Litigation Settlement
Litigation settlement expense decreased $1.1 million, or 45.7%, to $1.3 million for the year ended December 31, 2016 from $2.4 million for the year ended December 31, 2015. Litigation settlement expense for the year ended December 31, 2016, includes approximately $750,000 related to the State of California litigation settlement and approximately $350,000 related to the New Jersey Department of Banking and Insurance matter related to reserves established for the settlement of the Horizon Blue Cross Blue Shield of New Jersey complaint in October 2015. For further discussion of significant legal matters, see Note 16 to the Company’s consolidated financial statements included in this annual report.
Depreciation and Amortization
Depreciation and amortization expense increased $9.8 million, or 125.7%, to $17.7 million for the year ended December 31, 2016 from $7.8 million for the year ended December 31, 2015. Depreciable and amortizable assets were $109.1 million and $88.4 million as of December 31, 2016 and 2015, respectively. As a percentage of revenues, depreciation and amortization expense was 6.3% of total revenues for the year ended December 31, 2016, compared to 3.7% of total revenues for the year ended December 31, 2015. The increase in depreciation and amortization expense is primarily related to additions of property and equipment and intangible assets as a result of the 2015 and 2016 acquisitions, a full year of depreciation related to River Oaks, which was opened in October 2015, and the opening of Laguna Treatment Hospital in June 2016.
39
Acquisition-related expense decreased $0.7 million, or 20.9%, to $2.7 million for the year ended December 31, 2016 from $3.4 million for the year ended December 31, 2015. The decrease in acquisition-related expense for the year ended December 31, 2016 was primarily related to professional fees and travel costs associated with our acquisition activity in fiscal 2016 as compared to 2015. As a percentage of revenues, acquisition-related expense was 1.0% of total revenues for the year ended December 31, 2016, compared to 1.6% of total revenues for the year ended December 31, 2015.
Interest Expense
Interest expense increased $4.6 million, or 126.6%, to $8.2 million for the year ended December 31, 2016 compared to $3.6 million for the year ended December 31, 2015. The increase in interest expense was primarily the result of an increase in outstanding debt and in interest rates. Outstanding debt at December 31, 2016 was approximately $189.1 million compared to $145.1 million at December 31, 2015. Our weighted average interest rate on outstanding debt at December 31, 2016 was 5.0% compared to 3.0% at December 31, 2015. As a percentage of revenues, interest expense was 2.9% of total revenues for the year ended December 31, 2016 compared to 1.7% of total revenues for the year ended December 31, 2015.
Gain on Contingent Consideration
Gain on contingent consideration was $1.4 million for the year ended December 31, 2016. The gain on contingent consideration is directly attributable to the Townsend Acquisition in April 2016, as it pertains to 2016 results against certain performance measures specified in the purchase agreement.
Bargain Purchase Gain
Bargain purchase gain was $1.8 million for the year ended December 31, 2015. There was no bargain purchase gain for the year ended December 31, 2016. The bargain purchase gain is directly attributable to our acquisition of the Sunrise House in October 2015. As the fair value of the net acquired assets exceeded the consideration paid for the Sunrise House, a bargain purchase gain was recognized in the fourth quarter of 2015.
Income Tax Expense
For the year ended December 31, 2016, income tax benefit was $1.2 million, reflecting an effective tax rate of 17.5%, compared to $4.8 million income tax expense, reflecting an effective tax rate of 36.4%, for the year ended December 31, 2015. The decrease in income tax expense and the change in the effective tax rate is primarily related the tax treatment of stock-compensation.
Net Loss Attributable to Noncontrolling Interest
For the year ended December 31, 2016, net loss attributable to noncontrolling interest was $5.2 million compared to net loss attributable to noncontrolling interest of $2.8 million for the year ended December 31, 2015, representing a $2.3 million change. The increase in the net loss attributable to noncontrolling interest is primarily related to an expansion of our professional groups (that are consolidated as VIEs) as a result of the acquisitions completed during 2016 and 2015 and the opening of Laguna Treatment Hospital in June 2016 and River Oaks in October 2015.
Comparison of Year ended December 31, 2015 to Year ended December 31, 2014
Client Related Revenue
Client related revenues increased $72.8 million, or 54.7%, to $205.8 million for the year ended December 31, 2015 from $133.0 million for the year ended December 31, 2014. Revenues were positively impacted by an increase in average daily residential census, outpatient visits at our nine standalone outpatient centers, and an increase in high complexity lab testing.
Our average daily residential census increased by 42.6% to 562 clients for the year ended December 31, 2015 from 396 clients for the year ended December 31, 2014. The increase in average daily residential census was driven by the 60 bed expansion of Greenhouse in July 2014, the 31 bed expansion at Forterus in January 2015, the Recovery First Acquisition, which added 56 beds, in February 2015, the Oxford Centre Acquisition, which added 76 beds, in August 2015, the Sunrise House Acquisition, which added 110 beds in October 2015, and the opening of River Oaks, which added 162 beds, in October 2015.
With the opening of the Desert Hope Outpatient Center in January 2015, the opening of the Greenhouse Outpatient Center in April 2015, the CSRI Acquisition in April 2015 (which added three standalone outpatient centers), the Oxford Centre Acquisition in August (which added three standalone outpatient centers), and the Sunrise House Acquisition in October 2015 (which added one standalone outpatient center), we now operate nine standalone outpatient centers. We had 12,879 outpatient visits at our nine standalone outpatient centers for the year ended December 31, 2015.
As a percentage of client related revenues, point-of-care drug testing, diagnostic laboratory services, professional groups and other ancillary services were 30% and 26% for the years ended December 31, 2015 and 2014, respectively. We experienced a decline of 14% in point-of-care drug testing, diagnostic laboratory services, professional groups and other ancillary services as a percentage of client related revenues for the six months ended December 31, 2015 compared to the six months ended June 30, 2015. Point-of-care
40
drug testing, diagnostic laboratory services, professional groups and other ancillary services as a percentage of client related revenues for the six months ended December 31, 2015 was 24% compared to 38% for the six months ended June 30, 2015. The decline as a percentage of client related revenues was related to a combination of a decrease in the number of tests performed on a per client basis and a decline in reimbursement rates for point-of-care testing and diagnostic laboratory services during the second half of 2015.
Other Revenue
Other revenue increased $6.5 million for the year ended December 31, 2015 compared to zero for the year ended December 31, 2014. Other revenue consists of service charges from the delivery of quality targeted leads to behavioral and mental health service businesses obtained through phone calls from online inquiries and click-throughs from our suite of websites and network ad campaigns through RSG, which we acquired in July 2015.
Salaries, Wages and Benefits
Salaries, wages and benefits increased $36.7 million, or 67.1%, to $91.4 million for the year ended December 31, 2015 from $54.7 million for the year ended December 31, 2014. The increase in salaries and wages was primarily impacted by growth in our residential facilities and our standalone outpatient centers. Our number of employees increased by approximately 720 employees, or 93%, to approximately 1,600 employees at December 31, 2015, from approximately 880 employees at December 31, 2014. Also contributing to the increase was an increase in stock based compensation of $2.7 million to $5.8 million for the year ended December 31, 2015 from $3.1 million for the year ended December 31, 2014. As a percentage of revenues, salaries, wages and benefits were 43.1% of total revenues for the year ended December 31, 2015 compared to 41.1% of total revenues for the year ended December 31, 2014.
Client Related Services
Client related services expenses increased $5.0 million, or 46.3%, to $15.8 million for the year ended December 31, 2015 from $10.8 million for the year ended December 31, 2014. The increase in expense was primarily related to increases in clinician fees paid as a result of the increase in average daily residential census to 562 for the year ended December 31, 2015 from 396 for the year ended December 31, 2014. As a percentage of revenues, client related services expenses were 7.4% of total revenues for the year ended December 31, 2015 compared to 8.1% of total revenues for the year ended December 31, 2014. The decline in client related services expenses as a percentage of revenues was primarily related to increased revenues for high complexity lab testing for our facilities in Florida and California and increased other revenues which do not require client related services.
Provision for Doubtful Accounts
The provision for doubtful accounts increased $6.7 million, or 58.8%, to $18.1 million for the year ended December 31, 2015 from $11.4 million for the year ended December 31, 2014. The increase in the provision for doubtful accounts was primarily related to the 54.7% increase in client related revenues during 2015. Also contributing to the increase was an increase in the aging of our accounts receivable as of December 31, 2015 primarily due to acquisitions, a conversion to new lab billing software in the third and fourth quarters and ICD-10 conversion. As a percentage of revenues, the provision for doubtful accounts was 8.5% of total revenues for the year ended December 31, 2015 compared to 8.6% of total revenues for the year ended December 31, 2014.
We establish our provision for doubtful accounts based on the aging of the receivables and taking into consideration historical collection experience by facility, services provided, payor source and historical reimbursement rate, current economic trends and percentages applied to the accounts receivable aging categories. As of December 31, 2015, all accounts receivable aged greater than 360 days were fully reserved in our consolidated financial statements. In assessing the adequacy of the allowance for doubtful accounts, we rely on the results of detailed reviews of historical write-offs and recoveries (the hindsight analysis) as a primary source of information to utilize in estimating the collectability of our accounts receivable. We perform the hindsight analysis utilizing rolling twelve-month accounts receivable collection, write-off and recovery data. We supplement this hindsight analysis with other analytical tools, including, but not limited to, historical trends in cash collections compared to net revenues less bad debt and days sales outstanding.
The following table presents a summary of our aging of accounts receivable as of December 31, 2015 and 2014:
|
|
|
Current |
|
31-180 Days |
|
Over 180 Days |
|
Total |
|
||||
|
December 31, 2015 |
|
|
25.7 |
% |
|
43.0 |
% |
|
31.3 |
% |
|
100.0 |
% |
|
December 31, 2014 |
|
|
28.9 |
% |
|
47.0 |
% |
|
24.1 |
% |
|
100.0 |
% |
Advertising and Marketing
Advertising and marketing expenses increased $5.1 million, or 32.5%, to $20.8 million for the year ended December 31, 2015 from $15.7 million for the year ended December 31, 2014. The increase in advertising and marketing expense was primarily driven by the continued expansion of our national advertising and marketing programs, including the acquisitions of RSG and Taj Media in July 2015. As a percentage of revenues, advertising and marketing expenses were 9.8% of total revenues for the year ended December 31, 2015 compared to 11.8% of total revenues for the year ended December 31, 2014. The decrease in advertising and
41
marketing expenses as a percentage of revenue is partially attributable to efficiencies gained through the RSG Acquisition and the Taj Media Acquisition.
Professional Fees
Professional fees were $10.3 million for the year ended December 31, 2015 compared to $8.1 million for the year ended December 31, 2014. The increase in professional fees was primarily related to approximately $3.1 million of professional fees incurred during the second half of 2015 associated with certain litigation in California as partially offset by a decrease in professional fees related to the elimination of customer and billing collection fees as a result of the CRMS Acquisition in April 2014.
Other Operating Expenses
Other operating expenses increased $9.2 million, or 68.1%, to $22.7 million for the year ended December 31, 2015 from $13.5 million for the year ended December 31, 2014. The increase was primarily the result of additional operating expenses associated with the expansions and acquisitions that occurred in fiscal 2015. As a percentage of revenues, other operating expenses were 10.7% of total revenues for the year ended December 31, 2015 compared to 10.2% of total revenues for the year ended December 31, 2014.
Rentals and Leases
Rentals and leases increased $3.2 million, or 152.4%, to $5.3 million for the year ended December 31, 2015 from $2.1 million for the year ended December 31, 2014. As a percentage of revenues, rentals and leases were 2.5% of total revenues for the year ended December 31, 2015 compared to 1.6% of total revenues for the year ended December 31, 2014. The increase was primarily the result of increased rent as a result of the bed expansion at Forterus in January 2015, the Recovery First Acquisition in February 2015, the lease associated with our new corporate headquarters and call center beginning in June 2015, and the acquisitions of RSG and Taj Media in July 2015.
Litigation Settlement
Litigation settlement expense increased $1.9 million to $2.4 million for the year ended December 31, 2015 from $0.5 million for the year ended December 31, 2014. During 2015, we recognized $2.2 million in litigation settlement expense related to reserves established for the Horizon matter, which was settled in October 2015. For further discussion of significant legal matters, see Note 16 to the Company’s consolidated financial statements included in this annual report.
Depreciation and Amortization
Depreciation and amortization expense increased $3.1 million, or 66.0%, to $7.8 million for the year ended December 31, 2015 from $4.7 million for the year ended December 31, 2014. As a percentage of revenues, depreciation and amortization expense was 3.7% of total revenues for the year ended December 31, 2015, compared to 3.5% of total revenues for the year ended December 31, 2014. The increase in depreciation and amortization expense was primarily attributable to additions of property and equipment and intangible assets as a result of recent acquisitions and facility expansions.
Acquisition-related Expense
Acquisition-related expense increased $2.6 million, or 302.5%, to $3.4 million for the year ended December 31, 2015 from $0.8 million for the year ended December 31, 2014. The acquisition-related expense for the year ended December 31, 2015 was primarily related to professional fees and travel costs associated with our acquisition activity in fiscal 2015, during which we completed or announced eight acquisitions. As a percentage of revenues, acquisition-related expense was 1.6% of total revenues for the year ended December 31, 2015, compared to 0.6% of total revenues for the year ended December 31, 2014.
Interest Expense
Interest expense was $3.6 million for the year ended December 31, 2015 compared to $1.9 million for the year ended December 31, 2014. The increase in interest expense was primarily the result of an increase in outstanding debt as partially offset by a reduction in interest rates. Outstanding debt at December 31, 2015 was approximately $145.1 million compared to $28.6 million at December 31, 2014. The interest rate on the $118.9 million outstanding under the 2015 Credit Facility was 3.33% at December 31, 2015 and the interest rate outstanding on the $25.0 million of 2015 Subordinated Convertible Debt was 2.5% at December 31, 2015.
As a percentage of revenues, interest expense was 1.7% of total revenues for the year ended December 31, 2015 compared to 1.4% of total revenues for the year ended December 31, 2014.
Bargain Purchase Gain
Bargain purchase gain was $1.8 million for the year ended December 31, 2015 compared to zero for the year ended December 31, 2014. The bargain purchase gain is directly attributable to our acquisition of the Sunrise House in October 2015. As the fair value of the net acquired assets exceeded the consideration paid for the Sunrise House, a bargain purchase gain was recognized in the fourth quarter of 2015.
42
For the year ended December 31, 2015, income tax expense was $4.8 million, reflecting an effective tax rate of 36.4%, compared to $2.6 million, reflecting an effective tax rate of 28.6%, for the year ended December 31, 2014. The prior year effective tax rate included a 4.2% benefit related to the release of a valuation allowance and a 3.2% benefit related to income taxed directly to flow-through owners of BHR prior to the acquisition of BHR on April 15, 2014.
Net Loss Attributable to Noncontrolling Interest
For the year ended December 31, 2015, net loss attributable to noncontrolling interest was $2.8 million compared to net loss attributable to noncontrolling interest of $1.2 million for the year ended December 31, 2014, representing a $1.6 million change. The net loss attributable to noncontrolling interest is directly related to our consolidated variable interest entity (“VIE”). During the year ended December 31, 2014, we consolidated one real estate VIE, BHR, through April 15, 2014 at which point it became a wholly owned subsidiary; and five professional group VIEs. During the year ended December 31, 2015, noncontrolling interest was comprised of six professional group VIEs.
Liquidity and Capital Resources
General
Our primary sources of liquidity are net cash generated from operations, borrowings under our 2015 Credit Facility, the issuance of subordinated convertible debt under the Deerfield Facility, and proceeds from issuances of our common stock. We have also utilized operating lease transactions with respect to commercial properties primarily to perform client services and provide space for administrative facilities. We expect that our future funding for working capital needs, capital expenditures, long-term debt repayments and other financing activities will continue to be provided from some or all of these sources. Our future liquidity could be impacted by a decrease in our net cash generated from operations due to a decrease in payments from commercial insurance companies or our ability to access capital markets, which may be restricted as a result of our leverage capacity, existing or future debt agreements credit ratings, and general market conditions.
We anticipate that our current level of cash on hand, internally generated cash flows and our revolver will be sufficient to fund our anticipated working capital needs, debt service and repayment obligations and interest and maintenance capital expenditures for at least the next twelve months. However, to the extent we pursue acquisitions or facility expansions in the future, we may need to access additional capital resources to fund such activities.
Cash Flow Analysis
Our cash flows are summarized as follows (in thousands):
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Provided by operating activities |
|
$ |
143 |
|
|
$ |
6,193 |
|
|
$ |
8,038 |
|
|
Used in investing activities |
|
|
(56,454 |
) |
|
|
(143,402 |
) |
|
|
(19,521 |
) |
|
Provided by financing activities |
|
|
41,525 |
|
|
|
107,419 |
|
|
|
58,011 |
|
|
Net (decrease) increase in cash and cash equivalents |
|
|
(14,786 |
) |
|
|
(29,790 |
) |
|
|
46,528 |
|
|
Cash and cash equivalents at end of period |
|
|
3,964 |
|
|
|
18,750 |
|
|
|
48,540 |
|
Net Cash Provided by Operating Activities
Cash provided by operating activities was $0.1 million for the year ended December 31, 2016, compared to $6.2 million for the year ended December 31, 2015. Cash flows provided by operations for the year ended December 31, 2016 was primarily related to adjustments to net loss for non-cash expenses (e.g., provision for doubtful accounts, depreciation and amortization, equity compensation, etc.). Approximately $4.0 million of increased cash outflows was related to certain litigation in California. Working capital totaled $51.1 million at December 31, 2016 and $52.2 million at December 31, 2015.
Cash provided by operating activities was $6.2 million for the year ended December 31, 2015, compared to $8.0 million for the year ended December 31, 2014. Cash flows for operations for the year ended December 31, 2015 were positively impacted by net income, adjusted for non-cash expenses, as well as the timing of accounts payable and accrued liabilities for the year ended December 31, 2015 as compared to 2014. These increases were more than offset by an increase in accounts receivable of $30.9 million for the year ended December 31, 2015 as compared to the year ended December 31, 2014, and by approximately $6.6 million of increased cash outflows related to certain litigation in California, of which $3.1 million was expensed during 2015. The increase in accounts receivable was primarily related to an increase in revenues in 2015 as compared to 2014. Also contributing to the increase in accounts receivable were increases related to billing and collection delays associated with our acquisitions and conversion to new lab billing software in the second half of 2016. Working capital totaled $52.2 million at December 31, 2015 and $63.2 million at
43
December 31, 2014. The decrease of $9.7 million was primarily attributable to funding of acquisitions and de novo development projects.
Net Cash Used in Investing Activities
Cash used in investing activities was $56.5 million for the year ended December 31, 2016, a decrease of $86.9 million compared to cash used in investing activities of $143.4 million for the year ended December 31, 2015. Cash used in investing activities for the year ended December 31, 2016 was primarily related to $12.5 million in cash for the Townsend Acquisition on April 1, 2016, $5.4 million for the acquisition of a 100-room hotel in Arlington, Texas that is being converted into sober living beds to be used in support of the Greenhouse Outpatient Center, $6.7 million related to the Solutions Acquisition, $8.9 million related to the Oxford Treatment Center, $3.5 million related to the Ringwood property, $2.2 million related to the development of Laguna Treatment Hospital, with the remaining amount related to continuing costs associated with our expansion projects at existing facilities and other de novo projects.
Cash used in investing activities was $143.4 million for the year ended December 31, 2015, an increase of $123.9 million compared to cash used in investing activities of $19.5 million for the year ended December 31, 2014. The increase was primarily related to the business acquisitions, net of cash acquired, and de novo development activity during 2015, including $35.0 million for The Oxford Centre, $32.2 million for RSG, $13.5 million for the purchase of a property in Aliso Viejo, California, $13.0 million for Recovery First, $10.2 million for the development of River Oaks, $6.6 million for Sunrise House, $6.4 million for the purchase of a property in Ringwood, New Jersey, $2.2 million for Taj Media, and $0.6 million for CSRI, with the remaining amount primarily related to continuing costs associated with our de novo projects and completion of our new corporate headquarters.
Net Cash Provided by Financing Activities
Cash provided by financing activities was $41.5 million for the year ended December 31, 2016, a decrease of $65.9 million compared to cash provided by financing activities of $107.4 million for the year ended December 31, 2015. Cash provided by financing activities for the year ended December 31, 2016 was primarily related to $25.0 million of additional unsecured subordinated debt under the Deerfield Facility that was used primarily to fund the acquisitions of Townsend, the 100-room hotel in Arlington, Texas and the Solutions Acquisition and $20.0 million in borrowings under the revolver of the 2015 credit facility that was used primarily to fund our expansion projects at existing facilities and de novo projects.
Cash provided by financing activities was $107.4 million for the year ended December 31, 2015, an increase of $49.4 million compared to cash provided by financing activities of $58.0 million for the year ended December 31, 2014. The increase was primarily related to net proceeds from the 2015 Credit Facility of $120.8 million and $24.2 million from the financing transaction with Deerfield, and partially offset by the redemption of the BHR Series A Preferred Units of $8.5 million, repayments of long-term debt and capital leases of $27.6 million and repayments of subordinated notes payable of $1.0 million.
Financing Relationships
2015 Credit Facility
For discussion of our 2015 Credit Facility and a summary of its terms, see Note 9 to the accompanying consolidated financial statements. As of December 31, 2016 under our 2015 Credit Facility, we had $21.0 million outstanding under our revolver, $118.8 million outstanding on our term loan and $2.5 million in standby letters of credit issued for various corporate purposes.
On July 13, 2016, we amended our 2015 Credit Facility, which increased the then current borrowing capacity from $121.3 million to $171.3 million, consisting of a $50.0 million revolving credit facility and a $121.3 million term loan. The amendment also allowed for the inclusion of certain expenses associated with the California litigation in the definition of Consolidated EBITDA, as defined in the 2015 Credit Facility, for purposes of calculating debt covenants.
On February 27, 2017, we amended our 2015 Credit Facility to, among other things, provide for certain modifications to the terms of our Credit Agreement, dated as of March 19, 2015, as amended from time to time (the “2015 Credit Agreement”), including the following: (i) extend the maximum Consolidated Total Leverage Ratio (as defined in the 2015 Credit Agreement) of 4:25:1.00 through the measurement period ending September 30, 2017; and (ii) amend the definition of Applicable Margin (as defined in the 2015 Credit Agreement) to add an additional pricing level of 3.75% for Eurodollar Rate Loans and Letter of Credit Fee, 2.75% for Base Rate Loans and 0.60% for Commitment Fee (as all such terms are defined in the 2015 Credit Agreement), which will be applicable when the Consolidated Total Leverage Ratio is equal to or exceeds 4.00:1.00 at the end of the applicable measuring period (the “New Pricing Level”) and to provide that the Applicable Rate (as defined in the 2015 Credit Agreement) be set at the New Pricing Level from the date of such amendment until the first business day following the date we deliver our next Compliance Certificate (as defined in the 2015 Credit Agreement). The amendment also provided for additional Adjusted EBITDA (as defined in the 2015 Credit Agreement) add backs under its covenant calculation to account for its February 2017 reduction in workforce.
The 2015 Credit Facility is scheduled to mature in March 2020 and bears interest at LIBOR plus a margin between 2.25% to 3.75% or a base rate plus a margin between 1.25% and 2.75%, in each case depending on the Company’s leverage ratio. The 2015 Credit Facility has an accordion feature that provides for an additional $75.0 million of borrowing capacity under the credit facility, subject to certain consents and conditions, including obtaining additional commitments from lenders.
44
On October 2, 2015, we completed the closing of two financing facilities with affiliates of Deerfield. The capital commitment consists of $25.0 million of subordinated convertible debt and up to $25.0 million of unsecured subordinated debt, together with an incremental facility of up to an additional $50.0 million of subordinated convertible debt (subject to certain conditions) (collectively, the “Deerfield Facility”). We drew down $25.0 million of subordinated convertible debt at closing, and we used the proceeds to fund acquisitions, de novo projects and other corporate purposes. The $25.0 million of subordinated convertible debt bears interest at an annual rate of 2.50% and matures on September 30, 2021. The $25.0 million of subordinated convertible debt funded at closing is convertible into shares of our common stock at $30.00 per share.
In the second quarter of 2016, we borrowed an aggregate of $25.0 million of unsecured subordinated debt that bears interest at an annual rate of 12.0% and matures on October 2, 2020. The $25.0 million of unsecured subordinated debt can be repaid under certain conditions without penalty prior to October 2, 2017. The proceeds of the $25.0 million of unsecured subordinated debt were used to the fund the cash portion of the Townsend Acquisition and Solutions Acquisition as well as the acquisition of the 100-room hotel in Arlington, Texas.
Related Party Notes Payable
At December 31, 2015, we had outstanding notes payables of $1.2 million resulting from the seller financing of the TSN Acquisition. On February 29, 2016, we paid in full the outstanding balance, including principal of $1.2 million and accrued interest of $0.2 million.
Capital Lease Obligations
We have capital leases with third party leasing companies for equipment and office furniture. The capital leases bear interest at rates ranging from 2.71% to 11.58% and have maturity dates through September 2019. Total obligations under capital leases at December 31, 2016 were $1.7 million, of which $0.7 million was included in the current portion of long-term debt.
Contractual Obligations
The following table sets forth information regarding our contractual obligations as of December 31, 2016:
|
|
|
Payments due by period: |
|
|||||||||||||||||
|
|
|
(in thousands) |
|
|||||||||||||||||
|
|
|
|
|
|
|
Less than |
|
|
1 to 3 |
|
|
3 to 5 |
|
|
More than |
|
||||
|
Contractual Obligations |
|
Total |
|
|
1 year |
|
|
years |
|
|
years |
|
|
5 years |
|
|||||
|
Senior secured loans(1) |
|
$ |
155,255 |
|
|
$ |
15,132 |
|
|
$ |
140,123 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Subordinated debt(2) |
|
|
68,416 |
|
|
|
3,625 |
|
|
|
10,875 |
|
|
|
53,916 |
|
|
|
— |
|
|
Capital lease obligations(3) |
|
|
1,845 |
|
|
|
820 |
|
|
|
1,025 |
|
|
|
— |
|
|
|
— |
|
|
Operating lease obligations |
|
|
41,607 |
|
|
|
6,518 |
|
|
|
15,341 |
|
|
|
12,006 |
|
|
|
7,742 |
|
|
Employment separation agreements(4) |
|
|
833 |
|
|
|
833 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
267,956 |
|
|
$ |
26,928 |
|
|
$ |
167,364 |
|
|
$ |
65,922 |
|
|
$ |
7,742 |
|
|
|
(1) |
Amounts include required principal and interest payments. The estimated interest payments assume no change in LIBOR, or the base rate as defined in the Company’s 2015 Credit Facility, as of December 31, 2016. Also included in the estimated interest payments is the effect of two interest rate swap agreements with notional amounts at December 31, 2016 of $7.3 million and $10.8 million, bearing interest at 4.21% and 4.73%, with maturity dates of May 2018 and August 2019, respectively. |
|
(2) Amounts include required principal and interest payments.
|
|
(3) |
Includes future cash payments, including interest, due under our capital lease arrangements. |
|
|
|
(4) |
Amount includes $550,000 related to the Separation Agreement with Ms. Henderson-Grice. Refer to Note 16 for further discussion of the Separation Agreement. |
|
Consolidation of VIEs
The Professional Groups engage physicians and mid-level service providers and provide professional services to our clients through professional services agreements with each treatment facility. Under the professional services agreements, the Professional Groups also provide a physician to serve as medical director for the applicable facility. The Professional Groups either bill the payor for their services directly or are compensated by the treatment facility based on fair market value hourly rates. Each of the professional services agreements has a term of five years and will automatically renew for additional one-year periods.
We provided the initial working capital funding in connection with the formation of the Professional Groups and recorded a receivable. We make additional advances to the Professional Groups during periods in which there is a shortfall between revenues collected by the Professional Groups from the treatment facilities and payors, on the one hand, and the Professional Group’s contracting expenses and payroll requirements, on the other hand, thereby increasing the balance of the receivable. Excess cash flow
45
of the Professional Groups is repaid to us, resulting in a decrease in the receivable. The Professional Groups are obligated to repay these funds and are charged commercially reasonable interest. We had receivables from the Professional Groups at December 31, 2016. The receivables due to us from the Professional Groups are eliminated in consolidation as the Professional Groups are VIEs of which we are the primary beneficiary.
AAC has entered into written management services agreements with each of the Professional Groups under which AAC provides management and other administrative services to the Professional Groups. These services include billing, collection of accounts receivable, accounting, management and human resource functions and setting policies and procedures. Pursuant to the management services agreements, the Professional Groups’ monthly revenues will first be applied to the payment of operating expenses consisting of refunds or rebates owed to clients or payors, compensation expenses of the physicians and other service providers, lease payments, professional and liability insurance premiums and any other costs or expenses incurred by AAC for the benefit of the Professional Groups and, thereafter, to the payment to AAC of a management fee equal to 20% of the Professional Groups’ gross collected monthly revenues. As described above, AAC also provides financial support to each Professional Group on an as-needed basis to cover any shortfall between revenues collected by such Professional Groups from the treatment facilities and payors and the Professional Group’s contracting expenses and payroll requirements. Through these arrangements, we are directing the activities that most significantly impact the financial results of the respective Professional Groups; however, treatment decisions are made solely by licensed healthcare professionals employed or engaged by the Professional Groups as required by various state laws. Based on our ability to direct the activities that most significantly impact the financial results of the Professional Groups, provide necessary funding and the obligation and likelihood of absorbing all expected gains and losses, we have determined that we are the primary beneficiary, and, therefore, consolidate the six Professional Groups as VIEs.
Off Balance Sheet Arrangements
We have entered into various non-cancelable operating leases expiring through June 2025. Commercial properties under operating leases primarily include space required to perform client services, sober living accommodations for our clients, and space for administrative facilities. Rent expense was $7.4 million and $5.3 million for the years ended December 31, 2016 and 2015, respectively.
Critical Accounting Policies
Our consolidated financial statements have been prepared in accordance with GAAP. In preparing our consolidated financial statements, we are required to make certain estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses included in the financial statements. Estimates are based on historical experience and other available information, the results of which form the basis of such estimates. While we believe our estimation processes are reasonable, actual results could differ from our estimates. The following accounting policies are considered critical to our operating performance and involve subjective and complex assumptions and assessments.
Revenue Recognition
We provide services to our clients in both inpatient and outpatient treatment settings. Revenues are recognized when services are performed at the estimated net realizable value amount from clients, third-party payors and others for services provided. We receive the majority of payments from commercial payors at out-of-network rates. Client service revenues are recorded at established billing rates less adjustments to estimate net realizable value. Adjustments are recorded to state client service revenues at the amount expected to be collected for the service provided based on historic adjustments for out-of-network services not under contract. Provisions for estimated third party payor reimbursements are provided in the period related services are rendered and adjusted in future periods when actual reimbursements are received.
Prior to admission, insurance coverage, as applicable, is verified and the client self-pay amount is determined. The client self-pay portion is generally collected upon admission. In some instances, clients will pay out-of-pocket as services are provided or will make a deposit and negotiate the remaining payments as part of the services. These out-of-pocket payments are included in accrued liabilities in the accompanying consolidated balance sheets and revenues related to these payments are deferred and recognized over the period services are provided. We do not recognize revenue for any amounts not collected from the client. From time to time, we may provide scholarships to a limited number of clients. We do not recognize revenues for scholarships provided.
We recognize revenues from commercial payors at the time services are provided based on our estimate of the amount that payors will pay us for the services performed. We estimate the net realizable value of revenues by adjusting gross client charges using our expected realization and applying this discount to gross client charges. Our methodology related to our net realizable value is designed to react to potential changes in reimbursements by facility, by type of service and by payor. Management adjusts the expected realization discount, on a per facility basis, to reflect a historical analysis of reimbursement data by facility in addition to considering the type of services provided, the payors and the gross client charge rates by facility.
Estimates of net realizable value are subject to significant judgment and approximation by management. It is possible that actual results could differ from the historical estimates management has used to help determine the net realizable value of revenues. If our actual collections either exceed or are less than the net realizable value estimates, we will record a revenue adjustment, either
46
positive or negative, for the difference between our estimate of the receivable and the amount actually collected in the reporting period in which the collection occurred.
Our other revenue consists of service charges from the delivery of quality targeted leads to behavioral and mental health service businesses through our operating subsidiary RSG, which was acquired on July 2, 2015, and diagnostic laboratory services provided to clients of third-party addiction treatment providers. Revenue is recognized when persuasive evidence of an arrangement exists, services have been rendered, the fee for services is fixed or determinable, and collectability of the fee is reasonably assured.
Allowance for Doubtful Accounts
Accounts receivable primarily consist of amounts due from third-party commercial payors and clients and we record accounts receivable net of contractual discounts. Our ability to collect outstanding receivables is critical to our results of operations and cash flows. Accounts receivable are reported net of an allowance for doubtful accounts, which is management’s best estimate of accounts receivable that could become uncollectible in the future. Accordingly, the accounts receivable reported in our consolidated financial statements are recorded at the net amount expected to be received. Our primary collection risks are (i) the risk of overestimating our net revenues at the time of billing that may result in us receiving less than the recorded receivable, (ii) the risk of non-payment as a result of commercial insurance companies denying claims, (iii) the risk that clients will fail to remit insurance payments to us when the commercial insurance company pays out-of-network claims directly to the client, (iv) resource and capacity constraints that may prevent us from handling the volume of billing and collection issues in a timely manner and (v) the risk of non-payment from uninsured clients. In evaluating the collectability of accounts receivable and evaluating the adequacy of our allowance for doubtful accounts, management considers a number of factors, including historical experience, the age of the accounts and current economic trends. We continually monitor our accounts receivable balances and utilize retrospective reviews and cash collection data to support our estimates of the allowance for doubtful accounts. Estimates of our allowance for doubtful accounts are determined on a quarterly basis and adjusted monthly thereafter based on actual collections. If actual future collections are less favorable than those projected by management, additional allowances for uncollectible accounts may be required. There can be no guarantee that we will continue to experience the same collection rates that we have experienced in the past. We do not believe that there are any significant concentrations of revenues from any particular payor that would subject us to significant credit risks in the event a payor becomes unwilling or unable to pay claims.
Goodwill and Intangible Assets
Goodwill represents the excess of the purchase price over the fair value of the identifiable net assets acquired. Goodwill and intangible assets with indefinite lives are not amortized, but instead tested for impairment at least annually or whenever events or changes in circumstances indicate the carrying value may not be recoverable. We have no intangible assets with indefinite useful lives other than goodwill. We consider the following to be important factors that could trigger an impairment review: significant underperformance relative to historical or projected future operating results; identification of other impaired assets within a reporting unit; significant adverse changes in business climate or regulations; significant changes in senior management; significant changes in the manner of use of the acquired assets or the strategy for our overall business; and significant negative industry or economic trends.
Goodwill is assessed for impairment using a fair value approach at the reporting unit level. The goodwill impairment test is a two-step process, if necessary. The provisions for the accounting standard of goodwill provide an entity with the option to assess qualitative factors to determine whether the existence of events or circumstances leads to the determination that it is more likely than not that the fair value of a reporting unit is less than its carrying amount. This qualitative assessment is referred to as a “step zero” approach. If, based on the qualitative factors, an entity determines it is not more likely than not that the fair value of a reporting unit is less than its carrying value, the entity may skip the two-step impairment test required by accounting guidance. If an entity determines otherwise or, at the option of the entity, if a step zero is not performed, step one of the two-step impairment test is required. Under step one, the fair value of the reporting unit is compared with its carrying value (including goodwill). If the fair value of the reporting unit is less than its carrying value, an indication of goodwill impairment exists for the reporting unit and the entity must perform step two of the impairment test (measurement). Under step two, an impairment loss is recognized for any excess of the carrying amount of the reporting unit’s goodwill over the implied fair value of that goodwill. The implied fair value of goodwill is determined by allocating the fair value of the reporting unit in a manner similar to a purchase price allocation and the residual fair value after this allocation is the implied fair value of the reporting unit goodwill. Fair value of the reporting unit is determined using a discounted cash flow analysis. If the fair value of the reporting unit exceeds its carrying value, step two does not need to be performed. Impairment shall be recognized to the extent that the carrying amount of goodwill exceeds its implied fair value. In performing step one of the goodwill impairment test, we compare the carrying amount of the reporting unit to the estimated fair value.
In assessing the recoverability of goodwill, we consider historical results, current operating trends and results, and make estimates and assumptions about revenues, margins and discount rates based on our budgets, business plans, economic projections and anticipated future cash flows. Each of these factors contains inherent uncertainties, and management exercises substantial judgment and discretion in evaluating and applying these factors.
47
The annual goodwill impairment test is performed as of December 31 of each year, utilizing the two-step test. We concluded that the carrying value of the reporting unit as of December 31, 2016 did not exceed its fair value, and thus no indication of impairment was present.
Accounting for Income Taxes
We account for income taxes in accordance with ASC 740, Income Taxes. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
Under ASC 740, the effect on the deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is provided for significant deferred tax assets when it is more likely than not that such assets will not be recovered.
Our practice is to recognize interest and/or penalties related to uncertain income tax positions in income tax expense.
Stock-Based Compensation Expense
We measure compensation expense for all stock-based awards at fair value on the date of grant and recognize compensation expense over the service period for the awards expected to vest.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Our interest expense is sensitive to changes in market interest rates. With respect to our interest-bearing liabilities, our long-term debt outstanding at December 31, 2016 consisted of $139.8 million of variable rate debt with interest based on LIBOR plus an applicable margin. In July 2014, we entered into two interest rate swap agreements to mitigate our exposure to interest rate risks. As of December 31, 2016, the interest rate swap agreements had notional amounts of $10.8 million and $7.3 million which fix the interest rates over the life of the respective swap agreement at 4.73% and 4.21%, respectively. A hypothetical 1% increase in interest rates would decrease our pre-tax income and cash flows by approximately $1.2 million on an annual basis based upon our borrowing level at December 31, 2016.
Item 8. Financial Statements and Supplementary Data.
Information with respect to this Item is contained in our consolidated financial statements beginning on Page F-1 of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
Not applicable.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this report, our management conducted an evaluation, with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Report of Management on Internal Control Over Financial Reporting for the Fiscal Year Ended December 31, 2016
Our management is responsible for establishing and maintaining effective internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an assessment of the effectiveness of our internal control over financial reporting based on the framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on our assessment under the framework in Internal Control — Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2016.
48
This annual report does not include an attestation report from our registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by our registered public accounting firm pursuant to rules of the SEC that permit emerging growth companies, which we are, to provide only management's report in this annual report.
Changes in Internal Control Over Financial Reporting
There have been no changes in our internal control over financial reporting during the fourth quarter ended December 31, 2016, that have materially affected, or are reasonably likely to materially affect our internal control over financial reporting.
Not applicable.
49
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this Item is incorporated by reference to information set forth under the captions “Corporate Governance,” “Management” and “General Information - Section 16(a) Beneficial Ownership Reporting Compliance” in our definitive proxy statement for our 2017 Annual Meeting of Stockholders scheduled to be held on or about May 16, 2017, which we intend to file within 120 days after our fiscal year end.
We have adopted a Code of Business Conduct and Ethics that applies to all of our directors, officers and employees and a Code of Ethics for Senior Financial Officers. These documents, as well as the charters of the Nominating and Corporate Governance Committee, Audit Committee and the Compensation Committee, are available on the Investor Relations section of our website at www.americanaddictioncenters.com under the captions “About Us,” “Investor Relations,” “Corporate Profile” and “Governance Documents.” Upon the written request of any person, we will furnish, without charge, a copy of any of these documents. Requests should be directed to AAC Holdings, Inc., 200 Powell Place, Brentwood, Tennessee 37027, Attention: Kathryn Sevier Phillips, General Counsel and Secretary. We intend to disclose any amendments to our Code of Ethics and any waiver from a provision of our code, as required by the SEC, on our website.
Item 11. Executive Compensation
The information required by this Item is incorporated by reference to information set forth under the captions “Executive Compensation” and “Corporate Governance – Director Compensation” in our definitive proxy statement for our 2017 Annual Meeting of Stockholders scheduled to be held on or about May 16, 2017, which we intend to file within 120 days after our fiscal year end.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item is incorporated by reference to information set forth under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in our definitive proxy statement for our 2017 Annual Meeting of Stockholders scheduled to be held on or about May 16, 2017, which we intend to file within 120 days after our fiscal year end.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is incorporated by reference to information set forth under the captions “Certain Relationships and Related Person Transactions” and “Corporate Governance” in our definitive proxy statement for our 2017 Annual Meeting of Stockholders scheduled to be held on or about May 16, 2017, which we intend to file within 120 days after our fiscal year end.
Item 14. Principal Accounting Fees and Services
The information required by this Item is incorporated by reference to information set forth under the proposal “Ratification of Appointment of Independent Registered Public Accounting Firm” in our definitive proxy statement for our 2017 Annual Meeting of Stockholders scheduled to be held on or about May 16, 2017, which we intend to file within 120 days after our fiscal year end.
50
Item 15. Exhibits and Financial Statement Schedules.
(a) The following documents are filed as part of this Annual Report on Form 10-K:
|
|
1. |
Consolidated Financial Statements: |
The consolidated financial statements required to be included in Part II, Item 8, Financial Statements and Supplementary Data, begin on Page F-1 and are submitted as a separate section of this report.
|
|
2. |
Financial Statement Schedules: |
All schedules are omitted because they are not applicable or are not required, or because the required information is included in the consolidated financial statements or notes in this report.
|
|
3. |
Exhibits: |
The exhibits required by Item 601 of Regulation S-K are listed in the Exhibit Index and incorporated by reference herein.
None.
51
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
AAC Holdings, Inc.
Brentwood, Tennessee
We have audited the accompanying consolidated balance sheets of AAC Holdings, Inc. as of December 31, 2016 and 2015 and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of AAC Holdings, Inc. at December 31, 2016 and 2015, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
/s/ BDO USA, LLP
Nashville, Tennessee
March 7, 2017
F-2
(Dollars in thousands)
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
Assets |
|
|||||||
|
Current assets |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
3,964 |
|
|
$ |
18,750 |
|
|
Accounts receivable, net of allowances |
|
|
87,334 |
|
|
|
60,934 |
|
|
Prepaid expenses and other current assets |
|
|
5,181 |
|
|
|
6,840 |
|
|
Total current assets |
|
|
96,479 |
|
|
|
86,524 |
|
|
Property and equipment, net |
|
|
141,307 |
|
|
|
109,724 |
|
|
Goodwill |
|
|
134,396 |
|
|
|
108,722 |
|
|
Intangible assets, net |
|
|
10,356 |
|
|
|
9,470 |
|
|
Deferred tax assets |
|
|
598 |
|
|
|
— |
|
|
Other assets |
|
|
748 |
|
|
|
1,609 |
|
|
Total assets |
|
$ |
383,884 |
|
|
$ |
316,049 |
|
|
|
|
|||||||
|
Liabilities and Stockholders’ Equity |
|
|||||||
|
Current liabilities |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
9,155 |
|
|
$ |
7,878 |
|
|
Accrued liabilities |
|
|
26,742 |
|
|
|
21,653 |
|
|
Current portion of long-term debt |
|
|
9,445 |
|
|
|
3,611 |
|
|
Current portion of long-term debt – related party |
|
|
— |
|
|
|
1,195 |
|
|
Total current liabilities |
|
|
45,342 |
|
|
|
34,337 |
|
|
Deferred tax liabilities |
|
|
— |
|
|
|
1,195 |
|
|
Long-term debt, net of current portion |
|
|
179,661 |
|
|
|
140,335 |
|
|
Other long-term liabilities |
|
|
4,093 |
|
|
|
3,694 |
|
|
Total liabilities |
|
|
229,096 |
|
|
|
179,561 |
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity |
|
|
|
|
|
|
|
|
|
Common stock, $0.001 par value: 70,000,000 shares authorized, 23,673,907 and 22,813,809 shares issued and outstanding at December 31, 2016 and 2015, respectively |
|
|
24 |
|
|
|
23 |
|
|
Additional paid-in capital |
|
|
145,963 |
|
|
|
121,923 |
|
|
Retained earnings |
|
|
19,119 |
|
|
|
19,708 |
|
|
Total stockholders’ equity |
|
|
165,106 |
|
|
|
141,654 |
|
|
Noncontrolling interest |
|
|
(10,318 |
) |
|
|
(5,166 |
) |
|
Total stockholders’ equity including noncontrolling interest |
|
|
154,788 |
|
|
|
136,488 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
383,884 |
|
|
$ |
316,049 |
|
See accompanying notes to consolidated financial statements.
F-3
CONSOLIDATED STATEMENTS OF OPERATIONS
(Dollars in thousands, except share and per share amounts)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
Client related revenue |
|
$ |
270,569 |
|
|
$ |
205,752 |
|
|
$ |
132,968 |
|
|
Other revenue |
|
|
9,201 |
|
|
|
6,509 |
|
|
|
— |
|
|
Total revenue |
|
$ |
279,770 |
|
|
$ |
212,261 |
|
|
$ |
132,968 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Salaries, wages and benefits |
|
|
141,073 |
|
|
|
91,406 |
|
|
|
54,707 |
|
|
Client related services |
|
|
24,446 |
|
|
|
15,754 |
|
|
|
10,794 |
|
|
Provision for doubtful accounts |
|
|
21,485 |
|
|
|
18,113 |
|
|
|
11,391 |
|
|
Advertising and marketing |
|
|
18,275 |
|
|
|
20,821 |
|
|
|
15,683 |
|
|
Professional fees |
|
|
16,468 |
|
|
|
10,316 |
|
|
|
8,075 |
|
|
Other operating expenses |
|
|
29,627 |
|
|
|
22,708 |
|
|
|
13,518 |
|
|
Rentals and leases |
|
|
7,363 |
|
|
|
5,298 |
|
|
|
2,106 |
|
|
Litigation settlement |
|
|
1,292 |
|
|
|
2,379 |
|
|
|
487 |
|
|
Depreciation and amortization |
|
|
17,686 |
|
|
|
7,837 |
|
|
|
4,662 |
|
|
Acquisition-related expenses |
|
|
2,691 |
|
|
|
3,401 |
|
|
|
845 |
|
|
Total operating expenses |
|
|
280,406 |
|
|
|
198,033 |
|
|
|
122,268 |
|
|
(Loss) income from operations |
|
|
(636 |
) |
|
|
14,228 |
|
|
|
10,700 |
|
|
Interest expense, net (including change in fair value of interest rate swaps of ($180), $431, and $33, respectively) |
|
|
8,175 |
|
|
|
3,607 |
|
|
|
1,872 |
|
|
Gain on contingent consideration |
|
|
(1,350 |
) |
|
|
— |
|
|
|
— |
|
|
Bargain purchase gain |
|
|
— |
|
|
|
(1,775 |
) |
|
|
— |
|
|
Other income, net |
|
|
(500 |
) |
|
|
(725 |
) |
|
|
(93 |
) |
|
(Loss) income before income tax expense |
|
|
(6,961 |
) |
|
|
13,121 |
|
|
|
8,921 |
|
|
Income tax (benefit) expense |
|
|
(1,220 |
) |
|
|
4,780 |
|
|
|
2,555 |
|
|
Net (loss) income |
|
|
(5,741 |
) |
|
|
8,341 |
|
|
|
6,366 |
|
|
Less: net loss attributable to noncontrolling interest |
|
|
5,152 |
|
|
|
2,833 |
|
|
|
1,182 |
|
|
Net (loss) income attributable to AAC Holdings, Inc. stockholders |
|
|
(589 |
) |
|
|
11,174 |
|
|
|
7,548 |
|
|
BHR Series A Preferred Unit dividends |
|
|
— |
|
|
|
(147 |
) |
|
|
(693 |
) |
|
Redemption of BHR Series A Preferred Units |
|
|
— |
|
|
|
(534 |
) |
|
|
— |
|
|
Net (loss) income available to AAC Holdings, Inc. common stockholders |
|
$ |
(589 |
) |
|
$ |
10,493 |
|
|
$ |
6,855 |
|
|
Basic earnings per common share |
|
$ |
(0.03 |
) |
|
$ |
0.49 |
|
|
$ |
0.41 |
|
|
Diluted earnings per common share |
|
$ |
(0.03 |
) |
|
$ |
0.48 |
|
|
$ |
0.41 |
|
|
Weighted-average common shares outstanding: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
22,718,117 |
|
|
|
21,605,037 |
|
|
|
16,557,655 |
|
|
Diluted |
|
|
22,718,117 |
|
|
|
21,661,259 |
|
|
|
16,619,180 |
|
See accompanying notes to consolidated financial statements.
F-4
Consolidated Statements of Stockholders’ Equity
(In thousands, except share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Paid-in |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital/ |
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
|
|
|
|
||
|
|
|
Common Stock – |
|
|
Common Stock – |
|
|
|
|
|
|
|
|
|
|
(Distributions |
|
|
|
|
|
|
|
|
|
|
Stockholders’ |
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
American Addiction Centers, Inc. |
|
|
AAC Holdings, Inc. |
|
|
|
|
|
|
|
|
|
|
in Excess of |
|
|
|
|
|
|
|
|
|
|
Equity (Deficit) |
|
|
Non- |
|
|
Total |
|
||||||||||||||
|
|
|
Shares |
|
|
|
|
|
|
Shares |
|
|
|
|
|
|
|
|
|
|
Subscription |
|
|
Paid-in |
|
|
Treasury |
|
|
Retained |
|
|
of American |
|
|
Controlling |
|
|
Stockholders’ |
|
|||||||||
|
|
|
Outstanding |
|
|
Amount |
|
|
Outstanding |
|
|
Amount |
|
|
Subscribed |
|
|
Receivable |
|
|
Capital) |
|
|
Stock |
|
|
Earnings |
|
|
AAC Holdings, Inc. |
|
|
Interests |
|
|
Equity (Deficit) |
|
||||||||||||
|
Balance at December 31, 2013 |
|
|
3,199,869 |
|
|
$ |
3 |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
100 |
|
|
$ |
(58 |
) |
|
$ |
9,449 |
|
|
$ |
(3,671 |
) |
|
$ |
2,360 |
|
|
$ |
8,183 |
|
|
$ |
3,700 |
|
|
$ |
11,883 |
|
|
Common stock issued |
|
|
741,322 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
(100 |
) |
|
|
58 |
|
|
|
6,117 |
|
|
|
— |
|
|
|
— |
|
|
|
6,076 |
|
|
|
— |
|
|
|
6,076 |
|
|
Exercise of common stock warrants |
|
|
112,658 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
72 |
|
|
|
— |
|
|
|
— |
|
|
|
72 |
|
|
|
— |
|
|
|
72 |
|
|
Common stock granted and issued under stock incentive plan |
|
|
111,676 |
|
|
|
— |
|
|
|
158,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,745 |
|
|
|
— |
|
|
|
— |
|
|
|
1,745 |
|
|
|
— |
|
|
|
1,745 |
|
|
Dividends to mezzanine noncontrolling interests |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(61 |
) |
|
|
(61 |
) |
|
Distribution to noncontrolling interest holders, net |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(915 |
) |
|
|
(915 |
) |
|
Redemption of common stock |
|
|
(14,318 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(5,594 |
) |
|
|
(116 |
) |
|
|
— |
|
|
|
(5,710 |
) |
|
|
— |
|
|
|
(5,710 |
) |
|
Private share exchange |
|
|
(4,151,207 |
) |
|
|
(4 |
) |
|
|
14,618,886 |
|
|
|
15 |
|
|
|
— |
|
|
|
— |
|
|
|
4,892 |
|
|
|
3,787 |
|
|
|
— |
|
|
|
8,690 |
|
|
|
1,694 |
|
|
|
10,384 |
|
|
BHR acquisition |
|
|
— |
|
|
|
— |
|
|
|
820,124 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
(1,217 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1,216 |
) |
|
|
(3,661 |
) |
|
|
(4,877 |
) |
|
CRMS acquisition |
|
|
— |
|
|
|
— |
|
|
|
234,324 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,000 |
|
|
|
— |
|
|
|
— |
|
|
|
2,000 |
|
|
|
— |
|
|
|
2,000 |
|
|
Common stock issued |
|
|
— |
|
|
|
— |
|
|
|
5,250,000 |
|
|
|
5 |
|
|
|
— |
|
|
|
— |
|
|
|
68,866 |
|
|
|
— |
|
|
|
— |
|
|
|
68,871 |
|
|
|
— |
|
|
|
68,871 |
|
|
Short-form merger |
|
|
— |
|
|
|
— |
|
|
|
293,040 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,908 |
|
|
|
— |
|
|
|
— |
|
|
|
1,908 |
|
|
|
(1,908 |
) |
|
|
— |
|
|
Dividends BHR Series A Preferred Units |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(693 |
) |
|
|
(693 |
) |
|
|
— |
|
|
|
(693 |
) |
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
7,548 |
|
|
|
7,548 |
|
|
|
(1,182 |
) |
|
|
6,366 |
|
|
Balance at December 31, 2014 |
|
|
— |
|
|
$ |
— |
|
|
|
21,374,374 |
|
|
$ |
21 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
88,238 |
|
|
$ |
— |
|
|
$ |
9,215 |
|
|
$ |
97,474 |
|
|
$ |
(2,333 |
) |
|
$ |
95,141 |
|
|
Common stock granted and issued under stock incentive plan, net of forfeitures |
|
|
— |
|
|
|
— |
|
|
|
806,892 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
5,375 |
|
|
|
— |
|
|
|
|
|
|
|
5,376 |
|
|
|
|
|
|
|
5,376 |
|
|
Excess tax benefit from equity awards |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
33 |
|
|
|
— |
|
|
|
|
|
|
|
33 |
|
|
|
|
|
|
|
33 |
|
|
Effect of employee stock purchase plan |
|
|
— |
|
|
|
— |
|
|
|
12,637 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
554 |
|
|
|
— |
|
|
|
|
|
|
|
554 |
|
|
|
|
|
|
|
554 |
|
|
BHR Series A Preferred Unit dividends |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(147 |
) |
|
|
(147 |
) |
|
|
|
|
|
|
(147 |
) |
|
Redemption of Series A BHR Preferred Units |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(534 |
) |
|
|
(534 |
) |
|
|
|
|
|
|
(534 |
) |
|
Acquisition of Clinical Services of Rhode Island, Inc. |
|
|
— |
|
|
|
— |
|
|
|
42,460 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,343 |
|
|
|
— |
|
|
|
|
|
|
|
1,343 |
|
|
|
|
|
|
|
1,343 |
|
|
Acquisition of Referral Solutions Group, LLC |
|
|
— |
|
|
|
— |
|
|
|
540,193 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
24,173 |
|
|
|
— |
|
|
|
|
|
|
|
24,174 |
|
|
|
|
|
|
|
24,174 |
|
|
Acquisition of Taj Media, LLC |
|
|
— |
|
|
|
— |
|
|
|
37,253 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,667 |
|
|
|
— |
|
|
|
|
|
|
|
1,667 |
|
|
|
|
|
|
|
1,667 |
|
|
Acquisition of marketing intangibles |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
540 |
|
|
|
— |
|
|
|
|
|
|
|
540 |
|
|
|
|
|
|
|
540 |
|
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
11,174 |
|
|
|
11,174 |
|
|
|
(2,833 |
) |
|
|
8,341 |
|
|
Balance at December 31, 2015 |
|
|
— |
|
|
$ |
— |
|
|
|
22,813,809 |
|
|
$ |
23 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
121,923 |
|
|
$ |
— |
|
|
$ |
19,708 |
|
|
$ |
141,654 |
|
|
$ |
(5,166 |
) |
|
$ |
136,488 |
|
F-5
See accompanying notes to consolidated financial statements.
F-6
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Dollars in thousands)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income |
|
$ |
(5,741 |
) |
|
$ |
8,341 |
|
|
$ |
6,366 |
|
|
Adjustments to reconcile net income to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for doubtful accounts |
|
|
21,485 |
|
|
|
18,113 |
|
|
|
11,391 |
|
|
Depreciation and amortization |
|
|
17,686 |
|
|
|
7,837 |
|
|
|
4,662 |
|
|
Equity compensation |
|
|
8,823 |
|
|
|
5,757 |
|
|
|
1,802 |
|
|
Loss on disposal of property and equipment |
|
|
163 |
|
|
|
365 |
|
|
|
— |
|
|
Gain on contingent consideration |
|
|
(1,350 |
) |
|
|
— |
|
|
|
— |
|
|
Bargain purchase gain |
|
|
— |
|
|
|
(1,775 |
) |
|
|
— |
|
|
Accretion of BHR Series A Preferred Units |
|
|
— |
|
|
|
— |
|
|
|
66 |
|
|
Amortization of debt issuance costs |
|
|
633 |
|
|
|
261 |
|
|
|
32 |
|
|
Deferred income taxes |
|
|
(1,793 |
) |
|
|
962 |
|
|
|
(1,374 |
) |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(45,838 |
) |
|
|
(46,097 |
) |
|
|
(15,155 |
) |
|
Prepaid expenses and other assets |
|
|
2,510 |
|
|
|
(1,924 |
) |
|
|
190 |
|
|
Accounts payable |
|
|
824 |
|
|
|
5,061 |
|
|
|
106 |
|
|
Accrued liabilities |
|
|
3,135 |
|
|
|
9,463 |
|
|
|
(320 |
) |
|
Other long term liabilities |
|
|
(394 |
) |
|
|
(171 |
) |
|
|
272 |
|
|
Net cash provided by operating activities |
|
|
143 |
|
|
|
6,193 |
|
|
|
8,038 |
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment |
|
|
(37,304 |
) |
|
|
(51,525 |
) |
|
|
(15,584 |
) |
|
Issuance of notes and other receivables - related parties |
|
|
— |
|
|
|
— |
|
|
|
(488 |
) |
|
Collection of notes and other receivables - related parties |
|
|
— |
|
|
|
— |
|
|
|
738 |
|
|
Acquisition of subsidiaries, net of cash acquired |
|
|
(18,825 |
) |
|
|
(89,985 |
) |
|
|
(3,483 |
) |
|
Escrow funds held on acquisition |
|
|
(325 |
) |
|
|
(1,100 |
) |
|
|
(500 |
) |
|
Purchase of intangible assets |
|
|
— |
|
|
|
(540 |
) |
|
|
— |
|
|
Purchase of other assets, net |
|
|
— |
|
|
|
(252 |
) |
|
|
(204 |
) |
|
Net cash used in investing activities |
|
|
(56,454 |
) |
|
|
(143,402 |
) |
|
|
(19,521 |
) |
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds (payments) from revolving line of credit, net |
|
|
22,000 |
|
|
|
47,000 |
|
|
|
(12,550 |
) |
|
Proceeds from long-term debt |
|
|
27,500 |
|
|
|
100,218 |
|
|
|
4,053 |
|
|
Payments on long-term debt and capital leases |
|
|
(6,210 |
) |
|
|
(27,572 |
) |
|
|
(5,742 |
) |
|
Repayment of long-term debt — related party |
|
|
(1,195 |
) |
|
|
(542 |
) |
|
|
(2,601 |
) |
|
Repayment of subordinated notes payable |
|
|
— |
|
|
|
(945 |
) |
|
|
— |
|
|
Payment of debt issuance costs |
|
|
(570 |
) |
|
|
(2,211 |
) |
|
|
— |
|
|
Repurchase of common stock |
|
|
— |
|
|
|
— |
|
|
|
(5,710 |
) |
|
Proceeds from sale of common stock — initial public offering |
|
|
— |
|
|
|
— |
|
|
|
69,518 |
|
|
Proceeds from sale of common stock — private placement |
|
|
— |
|
|
|
— |
|
|
|
6,089 |
|
|
Proceeds from sale of BHR Series A Preferred Units |
|
|
— |
|
|
|
— |
|
|
|
8,203 |
|
|
Redemption of BHR Series A Preferred Units |
|
|
— |
|
|
|
(8,529 |
) |
|
|
(1,825 |
) |
|
Dividends paid |
|
|
— |
|
|
|
— |
|
|
|
(509 |
) |
|
Distributions to noncontrolling interest |
|
|
— |
|
|
|
— |
|
|
|
(915 |
) |
|
Net cash provided by financing activities |
|
|
41,525 |
|
|
|
107,419 |
|
|
|
58,011 |
|
|
Net change in cash and cash equivalents |
|
|
(14,786 |
) |
|
|
(29,790 |
) |
|
|
46,528 |
|
|
Cash and cash equivalents, beginning of period |
|
|
18,750 |
|
|
|
48,540 |
|
|
|
2,012 |
|
|
Cash and cash equivalents, end of period |
|
$ |
3,964 |
|
|
$ |
18,750 |
|
|
$ |
48,540 |
|
See accompanying notes to audited consolidated financial statements.
F-7
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Dollars in thousands)
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
||||||
|
Cash and cash equivalents paid for: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest, net of capitalized interest |
|
$ |
4,933 |
|
|
$ |
3,404 |
|
|
$ |
1,743 |
|
|
Income taxes, net of refunds |
|
$ |
2,626 |
|
|
$ |
1,250 |
|
|
$ |
4,156 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental information on non-cash investing and financing transactions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
2016 Acquisitions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase Price, including contingent consideration |
|
$ |
33,930 |
|
|
$ |
1,100 |
|
|
$ |
— |
|
|
Buyer common stock issued |
|
|
(15,105 |
) |
|
|
— |
|
|
|
— |
|
|
Cash paid for acquisition |
|
$ |
18,825 |
|
|
$ |
1,100 |
|
|
$ |
— |
|
|
2015 Acquisitions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase Price |
|
$ |
— |
|
|
$ |
117,168 |
|
|
$ |
— |
|
|
Buyer common stock issued |
|
|
— |
|
|
|
(27,183 |
) |
|
|
— |
|
|
Cash paid for acquisition |
|
$ |
— |
|
|
$ |
89,985 |
|
|
$ |
— |
|
|
2014 Acquisitions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase Price |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
14,259 |
|
|
Assumption of debt |
|
|
— |
|
|
|
— |
|
|
|
(1,759 |
) |
|
Buyer common stock issued |
|
|
— |
|
|
|
— |
|
|
|
(9,000 |
) |
|
Cash paid for acquisition |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
3,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition of equipment through capital lease |
|
$ |
1,807 |
|
|
$ |
291 |
|
|
$ |
614 |
|
|
Accrued purchase of property and equipment |
|
$ |
2,650 |
|
|
$ |
1,487 |
|
|
$ |
— |
|
See accompanying notes to audited consolidated financial statements.
F-8
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Description of Business
AAC Holdings, Inc. (collectively with its subsidiaries, the “Company” or “Holdings”), was incorporated on February 12, 2014 for the purpose of acquiring all the common stock of American Addiction Centers, Inc. (“AAC”) and to engage in certain reorganization transactions as more fully described in Note 3. The Company is headquartered in Brentwood, Tennessee and provides inpatient and outpatient substance abuse treatment services for individuals with drug and alcohol addiction. In addition to the Company’s inpatient and outpatient substance abuse treatment services, the Company performs drug testing and diagnostic laboratory services and provides physician services to clients. At December 31, 2016, the Company, through its subsidiaries, operated 12 residential substance abuse treatment facilities located throughout the United States, and 18 standalone outpatient centers, and 202 sober living beds across three locations.
The Company is in the process of expanding Oxford Treatment Center, where it opened an additional 24 residential beds in September 2016 and currently anticipates completing an additional 24 residential beds and 48 sober living beds in the second quarter of 2017. In addition, the Company has leased one floor from New Orleans East Hospital in New Orleans, Louisiana, where it intends to operate 36 in-network beds to provide detoxification and residential treatment services. The beds are expected to be operational in the second quarter of 2017, subject to receiving licensure, and will be operated by Townsend’s clinical staff.
The Company is also an internet marketer in the addiction treatment industry operating a broad portfolio of internet assets that service millions of website visits each month. Through the acquisition (the “RSG Acquisition”) of Referral Solutions Group, LLC (“RSG”) in July 2015, and its wholly owned subsidiary Recovery Brands, LLC (“Recovery Brands”), a leading publisher of addiction related websites such as Rehabs.com and Recovery.org, the Company serves families and individuals struggling with addiction and seeking treatment options through comprehensive online directories of treatment providers, treatment provider reviews, forums and professional communities. Recovery Brands also provides online marketing solutions to other treatment providers such as enhanced facility profiles, audience targeting, lead generation and tools for digital reputation management.
2. Basis of Presentation
Principles of Consolidation
The Company conducts its business through limited liability companies and C-corporations, each of which is a direct or indirect wholly owned subsidiary of the Company. The accompanying consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries, and the accounts of variable interest entities (“VIEs”) in which the Company is the primary beneficiary, which include certain professional groups through rights granted to the Company by contract to manage and control the business of such professional groups. All intercompany transactions and balances have been eliminated in consolidation.
The Private Share Exchange (defined below) between the Company and AAC’s stockholders (as discussed in Note 3) was accounted for similar to a common control transaction resulting in the assets, liabilities, and equity of AAC being carried over at their historical bases. At the time of the Private Share Exchange, Holdings was a shell company that had not conducted any business and had no material assets or liabilities. As such, the historical financial statements presented for periods prior to the Private Share Exchange represent the historical results of operations of AAC.
During the year ended December 31, 2014, the Company consolidated one real estate VIE, Behavioral Healthcare Realty, LLC (“BHR”) through April 15, 2014, at which point BHR was acquired by the Company and became a wholly owned subsidiary of the Company (see Note 3 for further discussion). At the time of the BHR Acquisition, BHR leased two treatment facilities to the Company under long-term triple net leases and was renovating and constructing additional treatment facilities that it planned to lease to the Company. The Company was the primary beneficiary as a result of its guarantee of BHR’s debt prior to the BHR Acquisition. The Company also consolidated six professional groups (“Professional Groups”) that constituted VIEs as of December 31, 2015 and seven Professional Groups that constituted VIEs as of December 31, 2016. The Professional Groups are responsible for the supervision and delivery of medical services to the Company’s clients. The Company provides management services to the Professional Groups. Based on the Company’s ability to direct the activities that most significantly impact the economic performance of the Professional Groups, provide necessary funding to the Professional Groups and the obligation and likelihood of absorbing all expected gains and losses of the Professional Groups, the Company has determined that it is the primary beneficiary of these Professional Groups.
The accompanying consolidated balance sheets include assets of $1.4 million as of both December 31, 2016 and 2015, respectively, and liabilities of $0.7 million and $0.6 million, respectively, related to the VIEs. The accompanying consolidated income statements include net loss attributable to noncontrolling interest of $5.2 million, $2.8 million and $1.2 million related to the VIEs for the years ended December 31, 2016, 2015 and 2014, respectively.
The accompanying consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make
F-9
estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
3. Reorganization Transactions
On April 15, 2014, the Company completed the following transactions which were all completed substantially concurrently (collectively, the “Reorganization Transactions”):
|
|
• |
A voluntary private share exchange with certain stockholders of AAC, whereby holders representing over 93.6% of the outstanding shares of common stock of AAC exchanged their shares on a one-for-one basis for shares of the Company’s common stock (the “Private Share Exchange”); |
|
|
• |
The acquisition of all of the outstanding common membership interests of BHR, an entity controlled by related parties, which through its subsidiaries owns properties located in Florida, Nevada and Texas, in exchange for $3.0 million in cash, the assumption of a $1.8 million term loan and 820,124 shares of the Company’s common stock (the “BHR Acquisition”); and |
|
|
• |
The acquisition of all of the outstanding membership interests of Clinical Revenue Management Services, LLC (“CRMS”), an entity controlled by related parties, which provides client billing and collection services for the Company, in exchange for $0.5 million in cash and 234,324 shares of the Company’s common stock. |
As a result of the foregoing transactions, the Company owned (i) over 93.6% of the outstanding common stock of AAC, (ii) 100% of the outstanding common membership interests in BHR, and (iii) 100% of the outstanding membership interests in CRMS. To help fund or facilitate these transactions, the following additional financing transactions were undertaken in 2014 prior to or in connection with the aforementioned transactions: (i) AAC sold 741,322 shares of its common stock in a private placement to certain accredited investors from February 2014 through April 2014, with net proceeds of $6.0 million, (ii) BHR sold 8.5 Series A Preferred Units in a private placement to certain accredited investors in January and February 2014 with net proceeds of $0.4 million (See Note 10), (iii) BHR redeemed 36.5 Series A Preferred Units from certain accredited investors in April 2014 (See Note 10) and (iv) BHR sold 160 new Series A Preferred Units in a private placement to an accredited investor in April 2014 with net proceeds of $7.8 million (see Note 10).
Initial Public Offering and Short-Form Merger
On October 7, 2014, the Company completed an initial public offering (“IPO”) of 5,750,000 shares of its common stock at a public offering price of $15.00 per share, which included the exercise in full of the underwriters’ option to purchase an additional 250,000 shares from the Company and 500,000 shares from certain stockholders. Net proceeds to the Company from the IPO were approximately $68.8 million, after deducting underwriting discounts and offering costs.
On November 10, 2014, the Company completed a subsidiary short-form merger with AAC and a wholly-owned merger subsidiary whereby the legacy holders of AAC common stock who did not participate in the Private Share Exchange received 1.571119 shares of Holdings common stock for each share of AAC common stock owned at the effective time of the merger (for an aggregate of approximately 293,040 shares of Holdings common stock). Upon completion of the short-form merger, Holdings owned 100% of the outstanding shares AAC. The Private Share Exchange was accounted for similar to a common control transaction resulting in the assets, liabilities and equity of AAC being carried over at their historical bases. Shares of AAC common stock that were not exchanged remained in mezzanine equity or stockholders’ equity until the completion of the short-form merger in November 2014. The short-form merger was accounted for as an equity transaction in accordance with ASC 810, Consolidation.
4. Summary of Significant Accounting Policies
Use of Estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses at the date and for the periods that the consolidated financial statements are prepared. On an ongoing basis, the Company evaluates its estimates, including those related to insurance adjustments, provisions for doubtful accounts, goodwill and intangible assets, long-lived assets, deferred revenues and income taxes. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances. Actual results could materially differ from those estimates.
General and Administrative Costs
The majority of the Company’s expenses are “cost of revenue” items. Costs that could be classified as general and administrative expenses include the Company’s corporate overhead costs, which were $74.8 million, $48.2 million, and $25.3 million for the years ended December 31, 2016, 2015, and 2014, respectively.
F-10
The Company provides services to its clients in both inpatient and outpatient treatment settings. Client related revenues are recognized when services are performed at estimated net realizable value from clients, third-party payors and others for services provided. The Company receives the majority of payments from commercial payors at out-of-network rates. Client related revenues are recorded at established billing rates less adjustments to estimate net realizable value. Adjustments are recorded to state client service revenues at the amount expected to be collected for the service provided based on historic adjustments for out-of-network services not under contract. Prior to admission, each client’s insurance is verified and the client self-pay amount is determined. The client self-pay portion is generally collected upon admission. In some instances, clients will pay out-of-pocket as services are provided or will make a deposit and negotiate the remaining payments as part of the services. These out-of-pocket payments are included in accrued liabilities in the accompanying consolidated balance sheets and revenues related to these payments are deferred and recognized over the period services are provided. From time to time, scholarships may be provided to a limited number of clients. The Company does not recognize revenues for care provided via scholarships.
For the year ended December 31, 2016, approximately 10.5% of the Company’s revenue reimbursements came from Blue Cross Blue Shield of Florida, 10.4% came from Blue Cross Blue Shield of Texas, and 10.4% came from Aetna. No other payor accounted for more than 10% of the Company’s revenue reimbursements for the year ended December 31, 2016.
For the year ended December 31, 2015, approximately 15.1% of the Company’s revenues were reimbursed by Anthem Blue Cross Blue Shield of Colorado; 12.5% by Blue Cross Blue Shield of Texas; 11.5% by Aetna, and 11.1% were reimbursed by Blue Cross Blue Shield of California. No other payor accounted for more than 10% of revenue reimbursements for the year ended December 31, 2015.
For the year ended December 31, 2014, approximately 18.1% of the Company’s revenue reimbursements came from Anthem Blue Cross Blue Shield of Colorado, 13.3% came from Blue Cross Blue Shield of Texas, 12.9% came from Aetna, 10.5% came from Blue Cross Blue Shield of California, and 10.5% came from United Behavioral Health. No other payor accounted for more than 10% of the Company’s revenue reimbursements for the year ended December 31, 2014.
Other Revenue
The Company’s other revenue consists of service charges from the delivery of quality targeted leads to behavioral and mental health service businesses through the Company’s operating subsidiary Referral Solutions Group, LLC, which was acquired on July 2, 2015, and diagnostic laboratory services provided to clients of third-party addiction treatment providers. Revenue is recognized when persuasive evidence of an arrangement exists, services have been rendered, the fee for services is fixed or determinable, and collectability of the fee is reasonably assured.
Allowance for Contractual and Other Discounts
The Company derives the majority of its revenues from commercial payors at out-of-network rates. Management estimates the allowance for contractual and other discounts based on its historical collection experience. The services authorized and provided and related reimbursement are often subject to interpretation and negotiation that could result in payments that differ from the Company’s estimates.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable primarily consists of amounts due from third-party payors (non-governmental) and is recorded net of contractual discounts. The Company’s ability to collect outstanding receivables is critical to its results of operations and cash flows. Accounts receivable is reported net of an allowance for doubtful accounts, which is management’s best estimate of accounts receivable that could become uncollectible in the future. Accordingly, accounts receivable reported in the Company’s consolidated financial statements is recorded at the net amount expected to be received. The Company’s primary collection risks are (i) the risk of overestimating net revenues at the time of billing that may result in the Company receiving less than the recorded receivable, (ii) the risk of non-payment as a result of commercial insurance companies denying claims, (iii) the risk that clients will fail to remit insurance payments to the Company when the commercial insurance company pays out-of-network claims directly to the client and (iv) resource and capacity constraints that may prevent the Company from handling the volume of billing and collection issues in a timely manner, (v) the risk that clients do not pay the Company for their self-pay balance (including co-pays, deductibles and any portion of the claim not covered by insurance) and (vi) the risk of non-payment from uninsured clients. The Company’s allowance for doubtful accounts is based on historical experience, but management also takes into consideration the age of accounts, creditworthiness and current economic trends when evaluating the adequacy of the allowance for doubtful accounts. Approximately $10.4 million of the accounts receivable, net the of the allowance for doubtful accounts, at December 31, 2016 includes accounts where the Company has received a partial payment from a commercial insurance company and the Company is continuing to pursue additional collections for the balance that the Company estimates remains outstanding. An account is written off only after the Company has pursued collection efforts or otherwise determines an account to be uncollectible.
At December 31, 2016, 11.6% of accounts receivable was from Anthem Blue Cross Blue Shield of Colorado and 10.3% was from Anthem Blue Cross Blue Shield of California. At December 31, 2015, 22.4% of accounts receivable was from Anthem Blue
F-11
Cross Blue Shield of Colorado and 15.0% was from Blue Cross Blue Shield of California. No other payor accounted for more than 10% of accounts receivable at December 31, 2016 or 2015.
A summary of activity in the Company’s allowance for doubtful accounts is as follows (in thousands):
|
Balance at December 31, 2013 |
|
$ |
13,320 |
|
|
Additions charged to provision for doubtful accounts |
|
|
11,391 |
|
|
Accounts written off, net of recoveries |
|
|
(16,243 |
) |
|
Balance at December 31, 2014 |
|
$ |
8,468 |
|
|
Additions charged to provision for doubtful accounts |
|
|
18,113 |
|
|
Accounts written off, net of recoveries |
|
|
(9,704 |
) |
|
Balance at December 31, 2015 |
|
$ |
16,877 |
|
|
Additions charged to provision for doubtful accounts |
|
|
21,485 |
|
|
Accounts written off, net of recoveries |
|
|
(10,234 |
) |
|
Balance at December 31, 2016 |
|
$ |
28,128 |
|
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less when purchased to be cash equivalents.
Property and Equipment
Property and equipment are stated at cost or at acquisition date fair value for assets obtained in business combinations, net of accumulated depreciation and amortization. Expenditures for maintenance and repairs are charged to expense as incurred. The Company capitalizes interest on construction projects and such interest is included in the cost of the related asset. Assets held for development are classified as construction in progress and the Company does not depreciate these assets until they are placed in service. Leasehold improvements are amortized over their estimated useful lives or the remaining lease period, whichever is less. Assets under capital leases are amortized over the lease term or in the event of transfer of ownership at the end of the lease over the economic life of the leased asset. Amortization expense related to assets under capital lease is included with depreciation and amortization expense in the consolidated statements of operations. Depreciation is calculated using the straight-line method over the estimated economic useful lives of the assets, as follows:
|
|
|
Range of Lives |
|
Computer software and equipment |
|
3 years |
|
Buildings |
|
36 years |
|
Furniture, fixtures and equipment |
|
5 years |
|
Vehicles |
|
5 years |
|
Equipment under capital lease |
|
3-5 years |
|
Leasehold improvements |
|
Life of the asset or lease, |
|
|
|
whichever is less |
Goodwill and Intangible Assets
The Company has only one operating segment, substance abuse/behavioral healthcare treatment services, for segment reporting purposes. The substance abuse/behavioral healthcare treatment services operating segment represents one reporting unit for purposes of the Company’s goodwill impairment test. Goodwill represents the excess of the purchase price over the fair value of the identifiable net assets acquired. Goodwill and intangible assets with indefinite lives are not amortized, but instead are tested for impairment at least annually or whenever events or changes in circumstances indicate the carrying value may not be recoverable. If the carrying value of goodwill exceeds its implied fair value, an impairment loss is recorded. The Company’s annual impairment tests of goodwill and other indefinite-lived intangibles in 2016 and 2015 resulted in no impairment charges. The Company has no intangible assets with indefinite useful lives other than goodwill.
The Company’s other intangible assets principally relate to trademarks, marketing intangibles, non-compete agreements, and leasehold interests acquired during business combinations. Trademarks and marketing intangibles are amortized over a period of ten years, non-compete agreements are amortized over the term of the agreements, and leasehold interests are amortized over the remaining life of the leases.
Long-Lived Asset Impairment
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net undiscounted cash flows expected to be generated by the asset. Impairment is measured by the amount by which
F-12
the carrying value of the assets exceeds the fair value of the assets. The Company did not identify any indicators of impairment during the years ended December 31, 2016 and 2015.
Accrued Liabilities
The Company’s accrued liabilities, reflected as a current liability in the accompanying consolidated balance sheets, consist of the following (in thousands):
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Accrued payroll liabilities |
|
|
13,501 |
|
|
$ |
10,090 |
|
|
Accrued litigation settlement |
|
|
962 |
|
|
|
800 |
|
|
Accrued legal fees |
|
|
915 |
|
|
|
479 |
|
|
Income taxes payable |
|
|
244 |
|
|
|
2,527 |
|
|
Accrued property, plant, and equipment |
|
|
2,650 |
|
|
|
1,487 |
|
|
Other |
|
|
8,470 |
|
|
|
6,270 |
|
|
Total accrued liabilities |
|
$ |
26,742 |
|
|
$ |
21,653 |
|
Segments
The focus of all Company operations is centered on a single service, substance abuse/behavioral healthcare treatment. As such, the Company has one operating segment. The Company is organized and operates as one reportable segment, consisting of various treatment facilities located in the United States. The treatment facilities operate in the same industry and have similar economic characteristics, services and clients. Management has the ability to direct and serve clients in any of these facilities, which allows management to operate the Company’s business and analyze its revenues on a system-wide basis rather than focusing on any individual facility. The Company’s chief operating decision maker evaluates performance and manages resources based on the results of the consolidated operations as a whole.
Advertising Expenses
Advertising costs are expensed as the related activity occurs.
Stock-Based Compensation
The Company accounts for stock-based compensation to employees using a fair-value based method for costs related to all share-based payments. Prior to the Company’s stock being traded in an active market, the Company estimated the fair value of employee restricted stock awards on the date of grant based on the appraised fair value. The fair value of the portion of the award that is ultimately expected to vest is recognized as expense on a straight-line basis over the requisite service periods in the Company’s consolidated statements of operations.
Earnings Per Share
Basic and diluted earnings per common share are calculated based on the weighted-average number of common shares outstanding in each period and dilutive stock options, non-vested shares, convertible debt securities, and warrants, to the extent such securities have a dilutive effect on earnings per share using the treasury stock method.
Income Taxes
The Company accounts for income taxes using the asset and liability method. Under the asset and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
The effect on the deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is provided for significant deferred tax assets when it is more likely than not that such assets will not be recovered.
The Company’s practice is to recognize interest and/or penalties related to uncertain income tax positions in income tax expense.
For the years ended December 31, 2016, 2015 and 2014, the Company had no accrued interest or penalties related to income tax matters.
VIEs included in the accompanying consolidated financial statements consist of various corporations and a partnership. As discussed in Note 3, the Company acquired BHR on April 15, 2014, prior to that date, BHR was a partnership for income tax purposes. Partnerships are characterized as flow through entities for federal and certain state income tax purposes, taxes for the VIEs
F-13
that are considered partnerships are not recorded in the accompanying consolidated financial statements, except for certain state taxes imposed at the entity level. Taxes that are imposed on the owners of these partnerships are not included in the accompanying consolidated financial statements. Taxes attributable to BHR after the acquisition date are included in these consolidated financial statements.
Fair Value Measurements
Fair value, for financial reporting purposes, is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants.
Disclosure is required about how fair value was determined for assets and liabilities and following a hierarchy for which these assets and liabilities must be grouped, based on significant levels of inputs as follows: Level 1—quoted prices in active markets for identical assets or liabilities; Level 2—quoted prices in active markets for similar assets and liabilities and inputs that are observable for the asset or liability; or Level 3—unobservable inputs for the asset or liability, such as discounted cash flow models or valuations. The determination of where assets and liabilities fall within this hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
Comprehensive Income
As of December 31, 2016, 2015, and 2014, the Company did not have any components of other comprehensive income. As such, comprehensive income equaled net income for each of the periods presented in the accompanying consolidated statements of operations.
Recent Pronouncements
In January 2017, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) 2017-04, “Simplifying the Test for Goodwill Impairment” (“ASU 2017-04”). The new guidance eliminates the requirement to calculate the implied fair value of goodwill (i.e., Step 2 of the current goodwill impairment test) to measure a goodwill impairment charge. Instead, entities will record an impairment charge based on the excess of a reporting unit’s carrying amount over its fair value (i.e., measure the charge based on the current Step 1). ASU 2017-04 is effective for annual and any interim impairment tests for periods beginning after December 15, 2019. The Company is currently evaluating the provisions of ASU 2017-04 to determine the impact on the Company’s financial statements.
In August 2016, the FASB issued (“ASU”) No. 2016-15, “Statement of Cash Flows: Classification of Certain Cash Receipts and Cash Payments” (“ASU 2016-15”). Among other clarifications, ASU 2016-15 clarifies certain items, including the classification of payments for debt prepayment or debt extinguishment costs, including third-party costs, premiums paid, and other fees paid to lenders that are directly related to the debt prepayment or debt extinguishment, excluding accrued interest, which will now be included in the Financing Activities section in the Statements of Cash Flows. The new standard is effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. The adoption of ASU 2016-15 is not expected to have a material impact on the Company’s results of operations.
In June 2016, the FASB issued ASU 2016-13, “Financial Instruments-Credit Losses: Measurement of Credit Losses on Financial Instruments,” which updates the impairment model for most financial assets and certain other instruments. For trade receivables and other instruments, entities will be required to use an updated forward-looking expected loss model that generally will result in the earlier recognition of allowances for losses. The new standard is effective for annual reporting periods beginning after December 15, 2019, including interim periods within those years, with early adoption permitted only as of annual reporting periods beginning after December 15, 2018. The Company is currently evaluating the provisions of ASU 2016-13 to determine the impact on the Company’s financial statements.
In March 2016, the FASB issued ASU No. 2016-09, “Compensation-Stock Compensation: Improvements to Employee Share-Based Payment Accounting” (“ASU 2016-09”). ASU 2016-09 requires that all excess tax benefits and tax deficiencies resulting from share-based payments be recognized as income tax expense or benefit in the income statement, which eliminates the accounting for additional paid-in capital pools. ASU 2016-09 also allows companies to make an entity-wide policy election to either estimate the number of stock-based awards that are expected to vest or account for forfeitures as they occur. With regards to the Statement of Cash Flows, both inflows and outflows of cash related to excess tax benefits will be classified as operating activities, whereas prior to this update, excess tax benefits as cash inflows were to be classified as financing activities. Also, cash paid by an employer when directly withholding shares for tax-withholding purposes (“net share settlement”) will now be classified as a financing activity. The new standard is effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The Company is in the early stages of evaluating the impact that adoption of this new standard will have on its consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02, “Leases”. The new standard establishes a right-of-use (ROU) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those
F-14
fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. The Company anticipates that the adoption of ASU 2016-2 will result in an increase in both total assets and total liabilities. The Company is continuing to evaluate the impact that adoption of this standard will have on its consolidated financial statements.
In November 2015, the FASB issued ASU 2015-17, “Income Taxes: Balance Sheet Classification of Deferred Taxes” (“ASU 2015-17”). ASU 2015-17 requires deferred tax assets and liabilities to be netted and presented as a single non-current amount in the balance sheet, rather than separating them into current and non-current amounts. The Company prospectively adopted the provisions of ASU 2015-17 during the fourth quarter of 2015. Prior periods were not retrospectively adjusted. The adoption of ASU 2015-17 had no impact on the Company’s results of operations or cash flows.
In September 2015, the FASB issued ASU 2015-16, “Business Combinations: Simplifying the Accounting for Measurement-Period Adjustments” (“ASU 2015-16”). ASU 2015-16 eliminates the requirement for an acquirer to retrospectively adjust its financial statements for changes to provisional amounts that are identified during the measurement-period following the consummation of a business combination. Instead, ASU 2015-16 requires these types of adjustments to be made during the reporting period in which they are identified and would require additional disclosure or separate presentation of the portion of the adjustment that would have been recorded in the previously reported periods as if the adjustment to the provisional amounts had been recognized as of the acquisition date. ASU 2015-16 is effective prospectively for fiscal years beginning after December 15, 2015, including interim periods within those years, with earlier application permitted for financial statements that have not been issued. The adoption of ASU 2015-16 did not have a material impact on the Company’s results of operations.
In April 2015, the FASB issued ASU No. 2015-05, “Intangibles – Goodwill and Other – Internal-Use Software.” The update was effective for financial statements issued for fiscal years beginning after December 15, 2015, including interim periods within those fiscal years, with early adoption permitted. ASU 2015-05 provides guidance for customer’s accounting for fees paid in a cloud computing arrangement. If a cloud computing arrangement includes a software license, the customer should account for the software license element of the arrangement consistent with the acquisition of other software licenses. If the cloud computing arrangement does not include a software license, the customer should account for the arrangement as a service contract. An entity can elect to adopt the amendments either prospectively to all arrangements entered into or materially modified after the effective date or retrospectively. The adoption of ASU 2015-05 on January 1, 2016 did not have a material impact on the Company’s consolidated financial statements.
In March 2015, the FASB issued ASU No. 2015-03, “Simplifying the Presentation of Debt Issuance Costs”. The update is effective for financial statements issued for fiscal years beginning after December 15, 2015, and those interim periods within those fiscal years, with early adoption permitted. The update requires debt issuance costs related to a note to be reported in the balance sheet as a direct deduction from the face amount of that note on a retrospective basis upon adoption. The Company elected to early adopt the revised guidance and presented $1.9 million of deferred debt issuance costs associated with the Company’s 2015 Credit Facility (as later defined) net of the debt balance at December 31, 2015 (see Note 9).
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2014-09, “Revenue from Contracts with Customers”, which outlines a single comprehensive model for recognizing revenue and supersedes most existing revenue recognition guidance, including guidance specific to the healthcare industry. Updates to this standard have been issued subsequent to the initial ASU 2014-09. Companies across all industries will use a new five-step model to recognize revenue from customer contracts. The new standard, which replaces nearly all existing GAAP revenue recognition guidance, will require significant management judgment in addition to changing the way many companies recognize revenue in their financial statements. The standard is effective for public entities for annual and interim periods beginning after December 15, 2017, with early adoption permitted for annual periods beginning after December 15, 2016. As the Company progresses with its implementation efforts to adopt ASU 2014-09, management continues to evaluate and refine its estimates of the anticipated impact the new standard will have on its revenue recognition policies, procedures, financial position, results of operations, cash flows and financial disclosures. Specifically, the Company is continuing to evaluate the impact the adoption will have on the presentation of the provision for doubtful accounts in the consolidated statements of operations and whether some or all of the provision for doubtful accounts will continue to be presented as a separate line item or will be recorded as a direct reduction to revenues.
5. Earnings Per Share
Basic EPS is computed by dividing net income attributable to common stockholders by the weighted-average number of shares of common stock outstanding for the period. Common shares outstanding include both the common shares classified as mezzanine equity and those classified as equity.
F-15
For the calculation of diluted EPS, net income attributable to common stockholders for basic EPS is adjusted by the effect of dilutive securities, including awards under stock-based payment arrangements, and outstanding convertible debt securities. Diluted EPS attributable to common stockholders is computed by dividing net income attributable to common stockholders by the weighted-average number of fully diluted common shares outstanding during the period.
The following tables reconcile the numerator and denominator used in the calculation of basic and diluted EPS for years ended December 31, 2016, 2015 and 2014 (in thousands except share and per share amounts):
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Numerator |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income attributable to AAC Holdings, Inc. |
|
$ |
(589 |
) |
|
$ |
11,174 |
|
|
$ |
7,548 |
|
|
Less: Series A Preferred Unit dividends |
|
|
— |
|
|
|
(147 |
) |
|
|
(693 |
) |
|
Less: Redemption of BHR Series A Preferred Units |
|
|
— |
|
|
|
(534 |
) |
|
|
— |
|
|
Net (loss) income available to common shares |
|
$ |
(589 |
) |
|
$ |
10,493 |
|
|
$ |
6,855 |
|
|
Denominator |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares outstanding – basic |
|
|
22,718,117 |
|
|
|
21,605,037 |
|
|
|
16,557,655 |
|
|
Dilutive securities |
|
|
— |
|
|
|
56,222 |
|
|
|
61,525 |
|
|
Weighted-average shares outstanding – diluted |
|
|
22,718,117 |
|
|
|
21,661,259 |
|
|
|
16,619,180 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings per share |
|
$ |
(0.03 |
) |
|
$ |
0.49 |
|
|
$ |
0.41 |
|
|
Diluted earnings per share |
|
$ |
(0.03 |
) |
|
$ |
0.48 |
|
|
$ |
0.41 |
|
The Company has included common stock that is classified as mezzanine equity in the denominator for basic and diluted EPS calculations.
The Company had 25,181 dilutive shares for the year ended December 31, 2016 that are not included in the earnings per share calculation above, because to do so would be anti-dilutive for the period presented.
6. Acquisitions
2016 Acquisitions
On April 1, 2016, the Company acquired certain assets of Wetsman Forensic Medicine, L.L.C. (d/b/a Townsend) and its affiliates (“Townsend”), a substance abuse treatment provider in Louisiana that operated seven in-network outpatient centers delivering intensive outpatient treatment as well as a 32-bed in-network facility located in Scott, Louisiana, of which 20 beds are licensed for detoxification and inpatient treatment, and an in-network lab that services these facilities (the “Townsend Acquisition”), for total consideration of $13.5 million in cash and 447,369 in restricted shares of AAC Holdings’ common stock. The purchase agreement includes provisions for contingent consideration of up to $2.0 million, consisting of cash and shares of AAC Holdings’ common stock currently held in escrow, if certain financial targets for the year ending December 31, 2016 are met. The Company determined the fair value of the contingent consideration at the acquisition date to be $1.4 million, which was included in the purchase price and allocated to the net assets acquired and liabilities assumed accordingly. The $0.6 million difference between the contingent consideration and fair value was recorded as a contingent consideration asset on the acquisition date. The purchase price was based upon arms-length negotiations between the Company and Townsend, an unrelated third party, that resulted in a premium to the fair value of the net assets acquired and, correspondingly, the recognition of goodwill. The amount recorded for goodwill is consistent with the Company’s intentions for the acquisition and is deductible for income tax purposes. As of December 31, 2016, the Company measured the contingent consideration requirements in accordance with the purchase agreement noting that the required financial targets for the year ended December 31, 2016 were not met. As such, the Company recorded a gain during the period for the $1.4 million of contingent consideration.
On May 3, 2016, the Company acquired certain assets of Solutions Recovery, Inc., its affiliates and associated real estate assets (collectively, “Solutions”) for total consideration of $6.8 million in cash and 309,871 of restricted shares of AAC Holdings’ common stock (the “Solutions Acquisition”). Solutions provides detoxification, residential and intensive outpatient treatment as well as sober living services in the greater Las Vegas, Nevada area. The purchase price was based upon arms-length negotiations between the Company and Solutions, an unrelated third party, that resulted in a premium to the fair value of the net assets acquired and, correspondingly, the recognition of goodwill. The amount recorded for goodwill is consistent with the Company’s intentions for the acquisition and is deductible for income tax purposes.
Both the acquisitions of Townsend and Solutions (collectively “2016 Acquisitions”) were accounted for as business combinations in accordance with FASB ASC 805, Business Combinations. The Company recorded each transaction based upon the fair value of the consideration paid. This consideration was allocated to the assets acquired and liabilities assumed at the
F-16
corresponding acquisition dates, based on their fair values. The fair values of assets acquired and liabilities assumed, at the corresponding acquisition dates, were as follows (in thousands):
|
|
|
Townsend |
|
|
Solutions |
|
|
Total |
|
|||
|
Acquisition date |
|
April 1, 2016 |
|
|
May 3, 2016 |
|
|
|
|
|
||
|
Accounts receivable |
|
|
1,149 |
|
|
|
898 |
|
|
|
2,047 |
|
|
Property and equipment |
|
|
282 |
|
|
|
5,220 |
|
|
|
5,502 |
|
|
Goodwill |
|
|
19,695 |
|
|
|
5,912 |
|
|
|
25,607 |
|
|
Intangible assets |
|
|
1,200 |
|
|
|
1,228 |
|
|
|
2,428 |
|
|
Total assets acquired |
|
|
22,326 |
|
|
|
13,258 |
|
|
|
35,584 |
|
|
Accrued liabilities |
|
|
364 |
|
|
|
190 |
|
|
|
554 |
|
|
Net assets acquired |
|
$ |
21,962 |
|
|
$ |
13,068 |
|
|
$ |
35,030 |
|
|
Total purchase price of acquisition |
|
$ |
21,962 |
|
|
$ |
13,068 |
|
|
$ |
35,030 |
|
2015 Acquisitions
During 2015, the Company acquired certain assets of the following entities, collectively referred to as the “2015 Acquisitions”. The 2015 Acquisitions were accounted for as business combinations in accordance with FASB ASC 805, Business Combinations. The Company recorded each transaction based upon the fair value of the consideration paid. The purchase price for each acquisition was based upon arms-length negotiations that resulted in a premium to the fair value of the net assets acquired and, correspondingly, the recognition of goodwill. The purchase price of each acquisition was allocated to the assets acquired and liabilities assumed at the corresponding acquisition dates, based on their fair values. The fair values of assets acquired and liabilities assumed, at the corresponding acquisition dates, were as follows (in thousands):
|
|
|
Recovery First |
|
|
CSRI |
|
|
RSG |
|
|
Taj Media |
|
|
Oxford |
|
|
Sunrise House |
|
|
Total |
|
|||||||
|
Acquisition date |
|
February 20, 2015 |
|
|
April 17, 2015 |
|
|
July 2, 2015 |
|
|
July 2, 2015 |
|
|
August 10, 2015 |
|
|
October 1, 2015 |
|
|
|
|
|
||||||
|
Cash and cash equivalents |
|
$ |
— |
|
|
$ |
27 |
|
|
$ |
231 |
|
|
$ |
45 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
303 |
|
|
Accounts receivable |
|
|
750 |
|
|
|
158 |
|
|
|
546 |
|
|
|
24 |
|
|
|
1,930 |
|
|
|
758 |
|
|
|
4,166 |
|
|
Prepaid expenses and other assets |
|
|
— |
|
|
|
6 |
|
|
|
— |
|
|
|
5 |
|
|
|
— |
|
|
|
— |
|
|
|
11 |
|
|
Property and equipment |
|
|
1,416 |
|
|
|
3 |
|
|
|
15 |
|
|
|
34 |
|
|
|
4,706 |
|
|
|
6,604 |
|
|
|
12,778 |
|
|
Other assets |
|
|
— |
|
|
|
— |
|
|
|
22 |
|
|
|
8 |
|
|
|
— |
|
|
|
113 |
|
|
|
143 |
|
|
Goodwill |
|
|
10,574 |
|
|
|
1,857 |
|
|
|
51,946 |
|
|
|
3,844 |
|
|
|
27,866 |
|
|
|
|
|
|
|
96,087 |
|
|
Intangible assets |
|
|
300 |
|
|
|
— |
|
|
|
4,470 |
|
|
|
— |
|
|
|
680 |
|
|
|
940 |
|
|
|
6,390 |
|
|
Total assets acquired |
|
|
13,040 |
|
|
|
2,051 |
|
|
|
57,230 |
|
|
|
3,960 |
|
|
|
35,182 |
|
|
|
8,415 |
|
|
|
119,878 |
|
|
Accrued liabilities |
|
|
40 |
|
|
|
44 |
|
|
|
576 |
|
|
|
53 |
|
|
|
182 |
|
|
|
40 |
|
|
|
935 |
|
|
Net assets acquired |
|
|
13,000 |
|
|
|
2,007 |
|
|
|
56,654 |
|
|
|
3,907 |
|
|
|
35,000 |
|
|
|
8,375 |
|
|
|
118,943 |
|
|
Bargain purchase gain(1) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,775 |
) |
|
|
(1,775 |
) |
|
Total purchase price of acquisition |
|
$ |
13,000 |
|
|
$ |
2,007 |
|
|
$ |
56,654 |
|
|
$ |
3,907 |
|
|
$ |
35,000 |
|
|
$ |
6,600 |
|
|
$ |
117,168 |
|
|
(1) |
The $1.8 million of assets in excess of the purchase price was recorded as a bargain purchase gain and is included in “Bargain purchase gain” in the consolidated statements of operations. Prior to recording this gain, the Company reassessed its identification of assets acquired and liabilities assumed, including the use of independent valuation experts to assist the Company in appraising the personal property, real property and intangible assets acquired. The Company believes there were several factors that contributed to this transaction resulting in a bargain purchase gain, including historical losses incurred by the business. |
Acquisition-related costs for the acquisitions were expensed in acquisition-related expenses in the consolidated statements of operations.
F-17
The consolidated statements of operations for the year ended December 31, 2016 included revenue of $17.1 million and income before income taxes of $3.4 million for the 2016 Acquisitions. The following presents the unaudited pro forma revenues and income before taxes of the combined entity as if the 2016 Acquisitions and 2015 Acquisitions occurred on January 1, 2015 and 2014, respectively (in thousands):
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Revenues |
|
$ |
287,720 |
|
|
$ |
257,554 |
|
|
(Loss) income before income tax expense |
|
$ |
(4,720 |
) |
|
$ |
18,647 |
|
7. Property and Equipment, net
Property and equipment consisted of the following (in thousands):
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
Land |
|
$ |
14,705 |
|
|
$ |
13,380 |
|
|
Buildings and improvements |
|
|
103,742 |
|
|
|
77,412 |
|
|
Equipment and software |
|
|
25,206 |
|
|
|
16,149 |
|
|
Construction in progress |
|
|
27,841 |
|
|
|
17,424 |
|
|
Total property and equipment |
|
|
171,494 |
|
|
|
124,365 |
|
|
Less accumulated depreciation |
|
|
(30,187 |
) |
|
|
(14,641 |
) |
|
Property and equipment, net |
|
$ |
141,307 |
|
|
$ |
109,724 |
|
Depreciation expense was $16.1 million and $6.9 million for the year ended December 31, 2016 and 2015, respectively.
On April 18, 2016, the Company acquired a 100-bed hotel in Arlington, Texas for $5.4 million in cash that is being converted to sober living beds.
8. Goodwill and Intangible Assets
The Company’s goodwill balance was $134.4 million and $108.7 million as of December 31, 2016 and 2015, respectively. The increase in goodwill relates to the acquisitions noted below and as discussed in Note 6.
|
Balance at December 31, 2015 |
|
$ |
108,722 |
|
|
Townsend Acquisition |
|
|
19,695 |
|
|
Solutions Acquisition |
|
|
5,912 |
|
|
Oxford Acquisition |
|
|
67 |
|
|
Balance at December 31, 2016 |
|
$ |
134,396 |
|
Other identifiable intangible assets and related accumulated amortization consisted of the following as of December 31, 2016 and 2015 (in thousands):
|
|
|
Gross Carrying Value |
|
|
Accumulated Amortization |
|
||||||||||
|
|
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
||||
|
|
|
2016 |
|
|
2015 |
|
|
2016 |
|
|
2015 |
|
||||
|
Trademarks |
|
$ |
5,322 |
|
|
$ |
4,052 |
|
|
$ |
1,454 |
|
|
$ |
955 |
|
|
Non-compete agreements |
|
|
1,587 |
|
|
|
1,257 |
|
|
|
1,139 |
|
|
|
838 |
|
|
Marketing intangibles |
|
|
5,651 |
|
|
|
5,651 |
|
|
|
920 |
|
|
|
355 |
|
|
Leasehold interests |
|
|
1,498 |
|
|
|
670 |
|
|
206 |
|
|
|
34 |
|
|
|
Other |
|
|
51 |
|
|
|
51 |
|
|
|
34 |
|
|
|
29 |
|
|
|
|
$ |
14,109 |
|
|
$ |
11,681 |
|
|
$ |
3,753 |
|
|
$ |
2,211 |
|
F-18
Changes to the carrying value of identifiable intangible assets during the year ended December 31, 2016 were as follows (in thousands):
|
Balance at December 31, 2015 |
|
$ |
9,470 |
|
|
Amortization expense |
|
|
(1,542 |
) |
|
Townsend Acquisition |
|
|
1,200 |
|
|
Solutions Acquisition |
|
|
1,228 |
|
|
Balance at December 31, 2016 |
|
$ |
10,356 |
|
On April 17, 2015, the Company acquired certain marketing assets with a value of $1.1 million for cash consideration of $0.5 million and 17,110 shares of the Company’s common stock issued on January 1, 2016, with an estimated fair value of $0.5 million at the date of acquisition.
The weighted-average amortization periods of the acquired intangible assets are as follows:
|
|
|
Weighted - Average Amortization Period (in Years) |
|
Trademarks |
|
10 |
|
Non-compete agreements |
|
5 |
|
Marketing intangibles |
|
10 |
|
Leasehold interests |
|
10 |
|
Other |
|
5 |
At December 31, 2016, all intangible assets are amortized using a straight-line method. The following table presents amortization expense expected to be recognized during fiscal years subsequent to December 31, 2016 (in thousands):
|
Year ended December 31, |
|
|
|
|
|
2017 |
|
$ |
1,527 |
|
|
2018 |
|
|
1,358 |
|
|
2019 |
|
|
1,322 |
|
|
2020 |
|
|
1,318 |
|
|
2021 |
|
|
1,267 |
|
|
Thereafter |
|
|
3,564 |
|
|
Total |
|
$ |
10,356 |
|
9. Notes Payable and Revolving Line of Credit
A summary of the Company’s debt obligations is as follows (in thousands):
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2016 |
|
|
2015 |
|
||
|
Non-related party debt: |
|
|
|
|
|
|
|
|
|
Senior secured loans |
|
$ |
139,750 |
|
|
$ |
120,125 |
|
|
Subordinated debt |
|
|
50,000 |
|
|
|
25,000 |
|
|
Unamortized debt issuance costs |
|
|
(2,386 |
) |
|
|
(1,949 |
) |
|
Capital lease obligations |
|
|
1,742 |
|
|
|
770 |
|
|
Total non-related party debt |
|
|
189,106 |
|
|
|
143,946 |
|
|
Less current portion |
|
|
(9,445 |
) |
|
|
(3,611 |
) |
|
Total non-related party debt, long-term |
|
$ |
179,661 |
|
|
$ |
140,335 |
|
|
Related party debt: |
|
|
|
|
|
|
|
|
|
Acquisition-related debt |
|
$ |
— |
|
|
$ |
1,195 |
|
|
Less current portion |
|
|
— |
|
|
|
(1,195 |
) |
|
Total related party debt, long-term |
|
$ |
— |
|
|
$ |
— |
|
F-19
On March 9, 2015, the Company entered into a five-year senior secured credit facility (the “2015 Credit Facility”) with Bank of America, N.A., as administrative agent for the lenders party thereto. The 2015 Credit Facility initially consisted of a $50.0 million revolving credit facility and a $75.0 million term loan. The Company incurred approximately $2.0 million in debt issuance costs related to underwriting and other professional fees, of which approximately $1.1 million related to the revolving credit loan and approximately $0.9 million related to the term loan. The Company deferred these costs over the term of the 2015 Credit Facility. The Company used the proceeds to re-pay $24.9 million of certain existing indebtedness, fund acquisitions and de novo projects and for general corporate purposes. On June 16, 2015, the Company amended the 2015 Credit Facility to remove from the definition of “change of control” what is often referred to as a “dead hand proxy put” provision.
On July 13, 2016, the Company increased its 2015 Credit Facility to $171.3 million, consisting of a $50.0 million revolving credit facility and a $121.3 million term loan. The facility is scheduled to mature in March 2020 and bears interest at LIBOR plus a margin between 2.25% to 3.75% or a base rate plus a margin between 1.25% and 2.75%, in each case depending on the Company’s leverage ratio. The facility has an accordion feature that provides for an additional $75.0 million of borrowing capacity under the credit facility, subject to certain consents and conditions, including obtaining additional commitments from lenders. As of December 31, 2016, the balance on the term loan was $118.8 million, and the balance on the revolving credit facility was $21.0 million.
On February 27, 2017, the Company amended its 2015 Credit Facility, to, among other things, provide for certain modifications to the terms of the 2015 Credit Agreement, dated as of March 19, 2015, as amended from time to time (the “2015 Credit Agreement”), including the following: (i) extend the maximum Consolidated Total Leverage Ratio (as defined in the 2015 Credit Agreement) of 4:25:1.00 through the measurement period ending September 30, 2017; and (ii) amend the definition of Applicable Margin (as defined in the 2015 Credit Agreement) to add an additional pricing level of 3.75% for Eurodollar Rate Loans and Letter of Credit Fee, 2.75% for Base Rate Loans and 0.60% for Commitment Fee (as all such terms are defined in the 2015 Credit Agreement), which will be applicable when the Consolidated Total Leverage Ratio is equal to or exceeds 4.00:1.00 at the end of the applicable measuring period (the “New Pricing Level”) and to provide that the Applicable Rate (as defined in the 2015 Credit Agreement) be set at the New Pricing Level from the date of such amendment until the first business day following the date the Company delivers its next Compliance Certificate (as defined in the 2015 Credit Agreement). The amendment also provided for additional Adjusted EBITDA (as defined in the 2015 Credit Agreement) add backs under its covenant calculation to account for its February 2017 reduction in workforce.
The 2015 Credit Facility requires quarterly term loan principal repayments for the outstanding term loan of $2.3 million for March 31, 2017 to December 31, 2017, $3.9 million from March 31, 2018 to December 31, 2018, and $4.7 million from March 31, 2019 to December 31, 2019, with the remaining principal balance of the term loan due on the maturity date of March 9, 2020. Repayment of the revolving loan is due on the maturity date of March 9, 2020. The 2015 Credit Facility generally requires quarterly interest payments, and limits our ability to pay dividends.
Borrowings under the 2015 Credit Facility are guaranteed by the Company and each of its subsidiaries and are secured by a lien on substantially all of the Company’s and its subsidiaries’ assets. Borrowings under the 2015 Credit Facility bear interest at a rate tied to the Company’s Consolidated Total Leverage Ratio (defined as Consolidated Funded Indebtedness to Consolidated EBITDA, in each case as defined in the 2015 Credit Facility, as amended). Eurodollar Rate Loans with respect to the 2015 Credit Facility bear interest at the Applicable Rate plus the Eurodollar Rate (each as defined in the 2015 Credit Facility, as amended) (based upon the LIBOR Rate (as defined in the 2015 Credit Facility, as amended) prior to commencement of the interest rate period). Base Rate Loans with respect to the 2015 Credit Facility bear interest at the Applicable Rate plus the highest of (i) the federal funds rate plus 0.50%, (ii) the prime rate and (iii) the Eurodollar Rate plus 1.0% (the interest rate at December 31, 2016 was 4.25%). In addition, the Company is required to pay a commitment fee on undrawn amounts under the revolving loan of the 2015 Credit Facility of 0.35% to 0.60% depending on the Company’s Consolidated Total Leverage Ratio (the commitment fee rate at December 31, 2016 was 0.50%). The Applicable Rates and the unused commitment fees of the 2015 Credit Facility, after the February 27, 2017 amendment, are based upon the following tiers:
|
Pricing Tier |
|
Consolidated Total Leverage Ratio |
|
Eurodollar Rate Loans |
|
|
Base Rate Loans |
|
|
Commitment Fee |
|
|||
|
1 |
|
> 4.00:1.00 |
|
|
3.75 |
% |
|
|
2.75 |
% |
|
|
0.60 |
% |
|
2 |
|
> 3.50:1.00 but < 4.00:1.00 |
|
|
3.25 |
% |
|
|
2.25 |
% |
|
|
0.50 |
% |
|
3 |
|
> 3.00:1.00 but < 3.50:1.00 |
|
|
3.00 |
% |
|
|
2.00 |
% |
|
|
0.45 |
% |
|
4 |
|
> 2.50:1.00 but < 3.00:1.00 |
|
|
2.75 |
% |
|
|
1.75 |
% |
|
|
0.40 |
% |
|
5 |
|
> 2.00:1.00 but < 2.50:1.00 |
|
|
2.50 |
% |
|
|
1.50 |
% |
|
|
0.35 |
% |
|
6 |
|
< 2.00:1.00 |
|
|
2.25 |
% |
|
|
1.25 |
% |
|
|
0.35 |
% |
The 2015 Credit Facility requires the Company to comply with customary affirmative, negative and financial covenants, including a Consolidated Fixed Charge Coverage Ratio, Consolidated Total Leverage Ratio and a Consolidated Senior Secured Leverage Ratio (each as defined in the 2015 Credit Facility, as amended). The Company may be required to pay all of its indebtedness
F-20
immediately if the Company defaults on any of the financial or other restrictive covenants contained in the 2015 Credit Facility. The financial covenants, after the February 27, 2017 amendment, include maintenance of the following:
|
|
• |
Fixed Charge Coverage Ratio may not be less than 1.50:1.00 as of the end of any fiscal quarter. |
|
|
• |
Consolidated Total Leverage Ratio: may not be greater than the following levels as of the end of each fiscal quarter: |
|
Measurement Period Ending |
|
Maximum Consolidated Total Leverage Ratio |
|
December 31, 2016 |
|
4.25:1.00 |
|
March 31, 2017 |
|
4.25:1.00 |
|
June 30, 2017 |
|
4.25:1.00 |
|
September 30, 2017 |
|
4.25:1.00 |
|
December 31, 2017 and each fiscal quarter thereafter |
|
4.00:1.00 |
|
|
• |
Consolidated Senior Secured Leverage Ratio may not be greater than the following levels as of the end of each fiscal quarter: |
|
Measurement Period Ending |
|
Maximum Consolidated Senior Secured Leverage Ratio |
|
December 31, 2016 |
|
3.75:1.00 |
|
March 31, 2017 and each fiscal quarter thereafter |
|
3.50:1.00 |
At December 31, 2016, the Company was in compliance with all applicable covenants under the 2015 Credit Facility.
In 2015, the Company incurred approximately $1.4 million in debt issuance costs related to underwriting and other professional fees, and deferred these costs over the term of the 2015 Credit Facility.
As of December 31, 2016, total borrowings under the $50.0 million revolver portion of the 2015 Credit Facility were $21.0 million and $2.5 million in standby letters of credit issued for various corporate purposes resulting in $26.5 million available to the Company.
2015 Subordinated Debt
On October 2, 2015, the Company entered into two financing facilities with affiliates of Deerfield Management Company, L.P. (“Deerfield”). The financing facilities consist of $25.0 million of subordinated convertible debt and up to $25.0 million of unsecured subordinated debt, together with an incremental facility of up to an additional $50.0 million of subordinated convertible debt (subject to certain conditions) (the “Deerfield Facility”). The Company issued $25.0 million of subordinated convertible debt at closing and used the proceeds to fund acquisitions, its de novo projects and for general corporate purposes. The $25.0 million of subordinated convertible debt bears interest at an annual rate of 2.50% and matures on September 30, 2021. The $25.0 million of subordinated convertible debt funded at closing is convertible into shares of the Company’s common stock at $30.00 per share. In the second quarter of 2016, the Company issued $25.0 million of the unsecured subordinated debt and used the proceeds to fund the acquisitions of Townsend, the hotel in Arlington, Texas and Solutions. The unsecured subordinated debt bears interest at an annual rate of 12.0% and matures on October 2, 2020.
The Company incurred approximately $1.4 million in debt issuance costs related to underwriting and other professional fees and deferred these costs over the term of the debt.
As of December 31, 2016, both the $25.0 million of subordinated convertible debt, bearing interest at 2.5%, and the $25.0 million of unsecured subordinated debt, bearing interest at 12.0%, were outstanding.
Acquisition Related Debt
At December 31, 2015, the Company had outstanding notes payables of $1.2 million resulting from the seller financing of the acquisition of certain assets of AJG Solutions and its subsidiaries and the equity of B&B Holdings INTL LLC (collectively, the “TSN Acquisition”). On February 29, 2016, the Company paid in full the outstanding balance, including principal of $1.2 million and accrued interest of $0.2 million.
Interest Rate Swap Agreements
In July 2014, the Company entered into two interest rate swap agreements to mitigate its exposure to fluctuations in interest rates. The interest rate swap agreements have notional amounts of $7.3 million and $10.8 million which fix the interest rates over the life of the respective swap agreement at 4.21% and 4.73%, and mature in May 2018 and August 2019, respectively. The notional
F-21
amounts of the swap agreements represent amounts used to calculate the exchange of cash flows and are not the Company’s assets or liabilities. The interest payments under these agreements are settled on a net basis. The Company has not designated the interest rate swaps as cash flow hedges and therefore the changes in the fair value of the interest rate swaps are included within interest expense in the consolidated statements of operations.
The fair value of the interest rate swaps at December 31, 2016 and 2015 represented a liability of $284,000 and $464,000, respectively, and is reflected in other long-term liabilities on the consolidated balance sheets. Refer to Note 15 for further discussion of fair value of the interest rate swap agreements. The Company’s credit risk related to these agreements is considered low because the swap agreements are with a creditworthy financial institution.
A summary of future maturities of long-term debt, as of December 31, 2016, is as follows (in thousands):
|
Years ending December 31, |
|
Non-Related Party |
|
|
Capital Lease Obligations |
|
|
Total |
|
|||
|
2017 |
|
$ |
9,375 |
|
|
$ |
774 |
|
|
$ |
10,149 |
|
|
2018 |
|
|
15,625 |
|
|
|
716 |
|
|
|
16,341 |
|
|
2019 |
|
|
18,750 |
|
|
|
252 |
|
|
|
19,002 |
|
|
2020 |
|
|
96,000 |
|
|
|
— |
|
|
|
96,000 |
|
|
2021 |
|
|
25,000 |
|
|
|
— |
|
|
|
25,000 |
|
|
Thereafter |
|
|
25,000 |
|
|
|
— |
|
|
|
25,000 |
|
|
Total |
|
$ |
189,750 |
|
|
$ |
1,742 |
|
|
$ |
191,492 |
|
|
Interest on outstanding amounts |
|
|
33,921 |
|
|
|
103 |
|
|
|
34,024 |
|
|
Total, including interest |
|
$ |
223,671 |
|
|
$ |
1,845 |
|
|
$ |
225,516 |
|
10. Mezzanine Equity
BHR Series A Preferred
On April 15, 2014, BHR sold 160 units of Series A Preferred Units, valued at $50,000 per unit, with proceeds to BHR of $7.8 million, net of issuance costs of $0.2 million. The issuance costs were being amortized over a 36-month period, the first date the holder could put the shares back to the Company. See Note 3 for a complete disclosure of the major components of this transaction and the related Series A Preferred Units. On February 25, 2015, the Company exercised its call provision and redeemed 100% of the outstanding Series A Preferred units for a total redemption price of approximately $8.6 million which included $0.2 million for the 3.0% call premium and $0.4 million for unpaid preferred returns.
11. Stockholders’ Equity
Common Stock
Holders of the Company’s common stock are entitled to one vote for each share held of record on all matters on which stockholders may vote. Certain restrictions imposed by the Company’s various debt instruments limit the Company’s ability to pay dividends.
In February and March 2014, AAC received proceeds of $4.2 million, net of $12,500 in issuance costs, from the sale of 516,625 shares of its common stock at $8.12 per share, which the Company’s management determined to be fair value, in an exempt common stock offering. Included within the total shares sold in the Company’s 2014 private placement were 61,563 shares sold to directors of the Company, 12,313 shares sold to the Company’s General Counsel and Secretary, 6,156 shares sold to the Company’s then current Chief Operating Officer and 3,078 shares sold to one of the Company’s Vice Presidents. The share price was based, in part, on an independent valuation analysis obtained in December 2013.
On April 11, 2014, AAC granted 77,765 shares of restricted common stock to its General Counsel and Secretary under the Company’s 2007 Stock Incentive Plan (the “Incentive Plan”), of which 38,883 shares vested immediately with the remaining 38,882 shares vesting on April 10, 2015. The fair value on the award date was $8.12 per share, as determined by the Company’s management. As a result of the award, AAC recorded $0.3 million of compensation expense, $0.2 million of additional compensation expense to satisfy the employee’s personal tax obligation related to the vesting of the grant during the second quarter of 2014, and $0.3 million ratably over the one-year vesting period. Additionally, on April 11, 2014, AAC granted 4,744 shares of its common stock to a non-executive employee. AAC recorded $39,000 of compensation expense and $30,000 of additional compensation expense to satisfy the employee’s personal tax obligation related to the stock grant during the second quarter of 2014.
On April 17, 2014, AAC redeemed a total of 14,318 shares of its common stock at $8.12 per share, which the Company’s management estimates to be fair value, for an aggregate redemption price of $0.1 million.
F-22
In connection with the issuance of subordinated notes in 2012, AAC issued detachable warrants to the lenders to purchase a total of 112,658 shares of common stock at $0.64 per share. The warrants were exercisable at any time up to their expiration on March 31, 2022. In March 2014, 106,728 of the outstanding warrants were exercised and a total of 106,728 shares of AAC common stock were issued to the exercising warrant holders, including 23,717 shares to a Company director. In April 2014, the remaining outstanding warrants for the purchase of 5,930 shares of AAC common stock were exercised.
In connection with the 2013 exempt offering of AAC common stock, a Company employee subscribed for 19,090 shares of common stock at $5.24 per share, which the Company’s management estimated to be fair value. As consideration for the shares, the employee issued to the Company a subscription note receivable in the amount of $0.1 million. The Company forgave this subscription note receivable over a 12-month period ending on July 1, 2014. During the year ended December 31, 2014, the Company recorded $58,000, respectively, in compensation expense related to this forgiveness.
Stock Split
On September 18, 2014, a 1.571119-for-1 stock split in the form of a stock dividend was effected. The common share and per share amounts included in the consolidated financial statements have been adjusted to reflect the stock split for all periods presented.
Initial Public Offering and Short-Form Merger
On October 7, 2014, the Company completed an IPO of 5,750,000 shares of its common stock at a public offering price of $15.00 per share, which included the exercise in full of the underwriters’ option to purchase an additional 250,000 shares from the Company and 500,000 shares from certain stockholders. Net proceeds to the Company from the IPO were approximately $68.8 million, after deducting underwriting discounts and offering costs.
On November 10, 2014, the Company completed a subsidiary short-form merger with AAC and a wholly-owned merger subsidiary whereby the legacy holders of AAC common stock who did not participate in the Private Share Exchange received 1.571119 shares of Holdings common stock for each share of AAC common stock owned at the effective time of the merger (for an aggregate of approximately 293,040 shares of Holdings common stock). Upon completion of the short-form merger, Holdings owned 100% of the outstanding shares of AAC. The short-form merger was accounted for as an equity transaction in accordance with ASC 810, Consolidation.
12. Stock Based Compensation
2014 Equity Incentive Plan
The Company adopted the 2014 Equity Incentive Plan (“2014 Incentive Plan”) in 2014. An aggregate of 1,571,120 shares of common stock are reserved for issuance pursuant to the 2014 Incentive Plan. The Incentive Plan is administered by the Board of Directors, which determines, subject to the provisions of the 2014 Incentive Plan, the employees, directors or consultants to whom incentives are awarded. As of December 31, 2016, 564,654 shares of common stock were available for issuance pursuant to the 2014 Incentive Plan.
On October 7, 2014, the Company granted a total of 158,000 shares of restricted common stock under the 2014 Incentive Plan. The shares vest annually over a period of four years from issuance.
On January 7, 2015, the Company granted a total of 400,000 shares of restricted common stock under the 2014 Incentive Plan. The shares vest quarterly over a period of three years.
On January 8, 2015, the Company granted 2,544 shares of fully vested common stock to each of its five non-employee directors. The Company recognized $0.4 million of compensation expense for the year ended December 31, 2015, as a result of these grants.
On July 9, 2015, the Company granted 3,174 shares of restricted common stock under the 2014 Incentive Plan. Of these shares, 75% vested immediately, with the remaining amount vesting quarterly over a one year period.
On November 23, 2015, the Company granted 405,000 shares of restricted common stock under the 2014 Incentive Plan. The shares vest quarterly over a period of three years.
On January 13, 2016, the Company granted 30,000 shares of fully vested common stock to each of its five non-employee directors. Additionally, on January 13, 2016, the Company issued 110,000 shares of restricted common stock under the 2014 Incentive Plan which vest quarterly over a three year period.
For the year ended December 31, 2016, the Company withheld 65,089 common shares for minimum statutory tax purposes. There were no common shares withheld for minimum statutory tax purposes for the year ended December 31, 2015.
The Company recognized $8.8 million, $5.8 million, and $1.8 million in stock based compensation expense for the years ended December 31, 2016, 2015 and 2014, respectively. As of December 31, 2016, there was $11.9 million of unrecognized compensation expense related to unvested restricted stock grants, which is expected to be recognized over the remaining weighted average vesting period of 1.7 years.
F-23
A summary of share activity under the Incentive Plan is set forth below:
|
|
|
|
|
|
|
Weighted- |
|
|
|
|
|
|
|
|
|
average Grant |
|
|
|
|
|
Shares |
|
|
Date Fair Value |
|
||
|
Unvested at December 31, 2014 |
|
|
196,882 |
|
|
$ |
18.53 |
|
|
Granted |
|
|
825,227 |
|
|
|
26.27 |
|
|
Vested |
|
|
(226,755 |
) |
|
|
27.34 |
|
|
Forfeitures |
|
|
(18,335 |
) |
|
|
25.31 |
|
|
Unvested at December 31, 2015 |
|
|
777,019 |
|
|
|
24.99 |
|
|
Granted |
|
|
140,000 |
|
|
|
16.24 |
|
|
Vested |
|
|
(361,507 |
) |
|
|
23.64 |
|
|
Forfeitures |
|
|
(33,337 |
) |
|
|
23.89 |
|
|
Unvested at December 31, 2016 |
|
|
522,175 |
|
|
$ |
23.22 |
|
Employee Stock Purchase Plan
On May 19, 2015, the Company’s shareholders approved the Company’s Employee Stock Purchase Plan (“ESPP”), which was adopted by the Board of Directors in the fourth quarter of 2014. The ESPP enables eligible employees to purchase shares of the Company’s common stock through a payroll deduction during certain option periods, generally commencing on January 1 and July 1 of each year and ending on June 30 and December 31 of each year. On the exercise date (the last trading day of each option period), the cumulative amount deducted from each participant’s salary during that option period will be used to purchase the maximum number of shares of the Company’s common stock at a purchase price equal to the lesser of (i) 85% of the closing market price of the Company’s common stock as quoted on the New York Stock Exchange on the exercise date or (ii) 85% of the closing market price of the Company’s common stock as quoted on the New York Stock Exchange on the grant date, subject to certain limitations and restrictions.
In July 2015, the Company issued 12,637 shares of the Company’s common stock in connection with employee deductions contributed in the January 1, 2015 through June 30, 2015 ESPP option period.
In January 2016, the Company issued 15,445 shares of the Company’s common stock in connection with employee deductions contributed in the July 1, 2015 through December 31, 2015 ESPP option period.
In July 2016, the Company issued 28,729 shares of the Company’s common stock in connection with employee deductions contributed in the January 1, 2016 through June 30, 2016 ESPP option period.
At December 31, 2016 and 2015, the Company recorded a liability of $0.3 million and $0.2 million, respectively, related to employee deductions contributed during the July 1, 2016 and December 31, 2016 and the July 1, 2015 through December 31, 2015 periods, respectively.
For the year ended December 31, 2016 and 2015, the Company recognized $0.3 million and $0.2 million of compensation expense related to the ESPP, respectively.
On January 10, 2017, the Company issued 50,362 shares of the Company’s common stock in connection with employee deductions of $0.4 million contributed in the July 1, 2016 through December 31, 2016 ESPP option period.
13. Qualified 401(k) Savings Plan
The Company has a qualified 401(k) savings plan (the “Plan”) which provides for eligible employees (as defined) to make voluntary contributions to the Plan. The Company makes contributions to the Plan based upon the participants’ level of participation, which is fully vested at the time of contribution. For each of the years ended December 31, 2016 and 2015, the Company contributions under this Plan were $0.8 million.
F-24
14. Income Taxes
Income tax expense consisted of the following for the years ended December 31, 2016, 2015 and 2014:
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Current |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
(197 |
) |
|
$ |
3,580 |
|
|
$ |
3,555 |
|
|
State |
|
|
1,042 |
|
|
|
285 |
|
|
|
477 |
|
|
Total current tax expense |
|
|
845 |
|
|
|
3,865 |
|
|
|
4,032 |
|
|
Deferred: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
|
(1,282 |
) |
|
|
1,052 |
|
|
|
(1,214 |
) |
|
State |
|
|
(783 |
) |
|
|
(137 |
) |
|
|
(263 |
) |
|
Total deferred tax expense |
|
|
(2,065 |
) |
|
|
915 |
|
|
|
(1,477 |
) |
|
Total income taxes, net of federal tax benefit |
|
$ |
(1,220 |
) |
|
$ |
4,780 |
|
|
$ |
2,555 |
|
The company’s effective income tax rate for the years ended December 31, 2016, 2015 and 2014 reconciles with the federal statutory rate as follows:
|
|
|
Year Ended December 31, |
|
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|
|||
|
Federal statutory rate |
|
|
35.0 |
|
% |
|
35.0 |
|
% |
|
35.0 |
|
% |
|
State income taxes, net of federal tax benefit |
|
|
3.2 |
|
|
|
3.5 |
|
|
|
2.2 |
|
|
|
Non-deductible expenses |
|
|
(2.2 |
) |
|
|
2.7 |
|
|
|
1.9 |
|
|
|
Stock compensation adjustment |
|
|
(15.8 |
) |
|
|
— |
|
|
|
— |
|
|
|
Return to provision adjustment |
|
|
(2.6 |
) |
|
|
— |
|
|
|
— |
|
|
|
Change in valuation allowance |
|
|
(0.1 |
) |
|
|
(0.3 |
) |
|
|
(4.2 |
) |
|
|
Benefit from tax deductible dividends |
|
|
— |
|
|
|
(1.9 |
) |
|
|
(2.9 |
) |
|
|
State tax credits |
|
|
— |
|
|
|
(2.7 |
) |
|
|
— |
|
|
|
Income taxed directly to flow-through owners of BHR |
|
|
— |
|
|
|
— |
|
|
|
(3.2 |
) |
|
|
Other differences |
|
|
— |
|
|
|
0.1 |
|
|
|
(0.2 |
) |
|
|
Effective income tax rate on income before taxes |
|
|
17.5 |
|
% |
|
36.4 |
|
% |
|
28.6 |
|
% |
The difference between the Company’s effective tax rate for 2016 and the federal statutory rate is 17.5%, which is primarily due to the tax treatment of stock compensation. For tax purposes, the deductible amount is determined by the stock price as of the vesting date.
Deferred income tax assets (liabilities) are comprised of the following at December 31, 2016 and 2015:
|
|
|
Year Ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
Employee compensation |
|
$ |
2,628 |
|
|
$ |
1,642 |
|
|
Operating loss carryforwards |
|
|
1,462 |
|
|
|
1,060 |
|
|
Accrued litigation |
|
|
213 |
|
|
|
— |
|
|
Accounts receivable |
|
|
542 |
|
|
|
310 |
|
|
Tax credits |
|
|
178 |
|
|
|
329 |
|
|
Acquisition related costs |
|
|
1,891 |
|
|
|
1,254 |
|
|
Other |
|
|
363 |
|
|
|
903 |
|
|
Valuation allowances |
|
|
(623 |
) |
|
|
(615 |
) |
|
Total deferred tax assets |
|
$ |
6,654 |
|
|
$ |
4,883 |
|
|
Property, equipment and amortization |
|
|
(209 |
) |
|
|
(3,036 |
) |
|
Goodwill and other intangible property |
|
|
(5,368 |
) |
|
|
(2,435 |
) |
|
Other |
|
|
(479 |
) |
|
|
(607 |
) |
|
Total deferred tax liabilities |
|
$ |
(6,056 |
) |
|
$ |
(6,078 |
) |
|
Net deferred tax assets (liabilities) |
|
$ |
598 |
|
|
$ |
(1,195 |
) |
F-25
At December 31, 2016, the Company had $2 million in state net operating losses which expire between 2027 and 2036 and $0.2 million in state tax credits, which expire in 2029.
At December 31, 2016, the Company had approximately $0.7 million in federal net operating losses attributable to the VIEs which will expire between 2032 and 2034. In addition, the Company had $1.2 million in state net operating losses which expire between 2027 and 2034 and $0.5 million in state tax credits, which expire in 2029.
The Company’s valuation allowance of $0.6 million is related to state NOLs, which are limited due to apportionable income to certain jurisdictions.
The Company had no uncertain tax positions as of December 31, 2016 and 2015, respectively. Generally, for federal and state purposes, the Company's 2013 through 2016 tax years remain open for examination by tax authorities. Additionally, any net operating losses that were generated in prior years and utilized in these years may also be subject to examination by the IRS. The Internal Revenue Service is currently conducting a routine examination of the Company’s 2013 and 2014 tax returns. The results of such examination and impact on the Company’s results of operation are not known at this time. The Company has not been notified of any state income tax examinations.
15. Fair Value of Financial Instruments
The carrying amounts reported at December 31, 2016 and 2015 for cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, accounts payable and accrued liabilities approximate fair value because of the short-term maturity of these instruments and are categorized as Level 1 within the GAAP fair value hierarchy. The fair value of the Company’s revolving line of credit is categorized as Level 2. The carrying amount of the Company’s debt approximates fair value because interest rates approximate the current rates available to the Company.
The Company has debt with variable and fixed interest rates. The fair value of debt with fixed interest rates was determined using the quoted market prices of debt instruments with similar terms and maturities, which are considered Level 2 inputs. The fair value of debt with variable interest rates was also measured using other Level 2 inputs, including good faith estimates of the market value for the particular debt instrument, which represent the amount an independent market participant would provide, based upon market observations and other factors relevant under the circumstances. The carrying value of such debt approximated its estimated fair value at December 31, 2016 and 2015.
The Company has entered into interest rate swap agreements to manage exposure to fluctuations in interest rates. Fair value of the interest rate swaps is determined using a pricing model based on published interest rates and other observable market data. The fair value was determined after considering the potential impact of collateralization, adjusted to reflect both the Company’s own nonperformance risk and the respective counterparty’s nonperformance risk. The fair value measurement of interest rate swaps utilizes Level 2 inputs. At each of December 31, 2016 and 2015, the fair value of the interest rate swaps represented a liability of $0.3 million and $0.5 million, respectively. Refer to Note 9 for further discussion of the interest rate swap agreements.
Intangible assets are measured at fair value on a non-recurring basis. These assets are classified in Level 3 of the fair value hierarchy. Goodwill and other indefinite-lived intangibles are tested for impairment at least annually, or more frequently if circumstances indicate that the carrying amount exceeds fair value.
The Company estimates the fair values of goodwill and other indefinite-lived intangibles utilizing multiple measurement techniques. The estimation is primarily determined based on an estimate of future cash flows (income approach) discounted at a market derived weighted-average cost of capital. The income approach has been determined to be the most representative of fair value because the Company’s equity does not have an active trading market. Other unobservable inputs used in these valuations include management’s cash flow projections and estimated terminal growth rates. The valuation of indefinite-lived intangible assets also includes an unobservable input for royalty rate, which is based on rates used by comparable industries.
The useful lives of definite-lived intangible assets (customer relationships) are evaluated whenever events or circumstances warrant a revision to the remaining amortization period. The fair value of definite-lived intangible assets is based on estimated cash flows from the future use of the asset, discounted at a market derived weighted-average cost of capital.
No impairment charges were recorded related to goodwill or other intangible assets for the years ended December 31, 2016, 2015, and 2014.
Long-lived assets are measured at fair value on a non-recurring basis and are classified in Level 3 of the fair value hierarchy. The fair value is estimated utilizing unobservable inputs, including appraisals on real estate as well as evaluations of the marketability and potential relocation of other assets in similar condition and similar market areas. The Company analyzes long-lived assets on an annual basis for any triggering events that would necessitate an impairment test. No impairment charges were recorded for the years ended December 31, 2016, 2015, and 2014.
F-26
16. Commitments and Contingencies
Operating Leases
The Company has entered into various operating leases expiring through June 2025. Commercial properties under operating leases primarily include space required to perform client services and space for administrative facilities. Rent expense was $7.4 million, $5.3 million and $2.1 million for the years ended December 31, 2016, 2015 and 2014, respectively.
The future minimum lease payments under non-cancelable operating leases with remaining terms of one or more years as of December 31, 2016 consisted of the following (in thousands):
|
Years ending December 31, |
Annual Payment |
|
|
|
2017 |
$ |
6,518 |
|
|
2018 |
|
5,562 |
|
|
2019 |
|
5,127 |
|
|
2020 |
|
4,652 |
|
|
2021 |
|
4,331 |
|
|
Thereafter |
|
15,417 |
|
|
Total |
$ |
41,607 |
|
The Company recognizes rent expense on a straight line basis with the difference between rent expense and rent paid recorded as deferred rent. Such amount is included in accrued liabilities in the consolidated balance sheets.
Litigation
State of California
On October 21, 2016, American Addiction Centers, Inc. (formerly known as Forterus, Inc.), Forterus Health Care Services, Inc., and ABTTC, Inc. (collectively, the “Defendants,” each of which is a direct or indirect subsidiary of AAC Holdings) agreed to the entry of a Permanent Injunction and Final Judgment (the “PIFJ”) with the Bureau of Medi-Cal Fraud and Elder Abuse of the Office of the Attorney General of the State of California (“BMFEA”) relating to the criminal charges filed against the Defendants in connection with the death of a client in 2010 at one of the Company’s former locations. The PIFJ generally provides, among other things and subject to certain limitations, that the State of California (i) dismiss all criminal charges against the Defendants in connection with the case entitled People v. McCausland, et al. (Case No. SWF1501351) and not file or seek to file any other criminal charges against the Defendants in connection with the death of such client or in connection with such client’s admission or care; (ii) release all civil claims against the Defendants and their officers, directors and employees for conduct, acts or omissions arising out of or in connection with the quality of resident care or resident admissions and occurring prior to the effective date of the PIFJ at any California facility owned, licensed, operated, managed or controlled by Defendants and (iii) release such parties from any civil false claims and claims for fraud (both statutory and common law) under California law.
Pursuant to the terms of the PIFJ, among other things and subject to certain limitations, Defendants shall (i) institute a three-year compliance program (the “Compliance Program”) with respect its California facilities that includes, to the extent not already established, that certain policies and procedures are maintained or developed and implemented to promote each covered facility’s compliance with applicable statutes, regulations and the PIFJ, under the responsibility of AAC Holdings’ Chief Compliance Officer; (ii) establish a Compliance Committee composed of the Compliance Officer and senior personnel responsible for overseeing clinical operations to address issues raised by the Compliance Officer in connection with the Compliance Program and (iii) establish an oversight committee of its board of directors (the “Board”), or a committee of the Board, to review the adequacy and responsiveness of the Compliance Program. In addition, for a period of 30 months following the effective date of the PIFJ, the Defendants shall retain a qualified independent monitor, appointed by BMFEA after consultation with the Defendants, to assess the effectiveness of the Defendants’ quality control systems and patient care. The PIFJ also provides that the Defendants will pay $549,986 toward the costs of the investigation and $200,000 as a civil monetary penalty, the total of which was accrued for and paid in full in 2016 and is included in litigation settlement on the statement of operations.
With respect to the individual defendants, the California Attorney General’s office elected not to re-try Kristopher McCausland, and all criminal charges against Mr. McCausland were dismissed. All felony charges were also dismissed against Jerrod Menz and James Fent, and their criminal cases were resolved pursuant to their agreements not to contest misdemeanor charges, which will ultimately be eligible for dismissal upon their satisfying certain conditions required by the court.
Shareholder Litigation
On August 24, 2015, a shareholder filed a purported class action in the United States District Court for the Middle District of Tennessee against the Company and certain of its current and former officers (Kasper v. AAC Holdings, Inc. et al. and Tenzyk c. AAC
F-27
Holdings, Inc. et al.). The plaintiff generally alleges that the Company and certain of its current and former officers violated Sections 10(b) and/or 20(a) of the Securities Exchange Act of 1934 and Rule 10b-5 promulgated thereunder by making allegedly false and/or misleading statements and failing to disclose certain information. On September 14, 2015, a second class action against the same defendants asserting essentially the same allegations was filed in the same court. On October 26, 2015, the court entered an order consolidating these two described actions into one action. On April 14, 2016, the Company and the individual defendants filed a motion to dismiss the complaint for failure to state a claim. On July 1, 2016, the court denied the motion to dismiss. In a related matter, on November 28, 2015, a shareholder filed a derivative action on behalf of AAC Holdings, Inc. in the Eighth Judicial District Court for Clark County, Nevada (Bushansky v. Jerrod N. Menz et al.) against AAC’s board of directors and certain of its officers alleging that these directors and officers breached their fiduciary duties and engaged in mismanagement and illegal conduct. On January 19, 2016, the Court entered an Order staying this litigation pending the earlier of the close of discovery in the related securities class action pending in Tennessee or the deadline for appealing any dismissal of the securities class action. The Company intends to defend these actions vigorously. At this time, the Company cannot predict the results of litigation with certainty and cannot estimate the amount or range of loss, if any.
New Jersey Department of Banking and Insurance
The New Jersey Department of Banking and Insurance (“NJ-DOBI”) and Leading Edge Recovery Center, LLC (“Leading Edge”), a former indirect operating subsidiary of American Addiction Centers, Inc. that was acquired in September 2012 and was subsequently closed in 2013, are negotiating a possible resolution to certain information provided to NJ-DOBI following Leading Edge’s recent claim settlement with Horizon Blue Cross Blue Shield of New Jersey. Based on these discussions, the Company has recorded an aggregate $350,000 reserve related to this matter and included in litigation settlement on the statement of operations.
Horizon Blue Cross Blue Shield of New Jersey v. Avee Laboratories et al.
On September 4, 2013, Horizon Blue Cross Blue Shield of New Jersey (“Horizon”) filed an amended complaint in the Superior Court of New Jersey against several defendants, including Leading Edge Recovery Center, LLC, one of the Company’s subsidiaries. Leading Edge Recovery Center, LLC formerly operated a drug and alcohol treatment facility in New Jersey. Horizon alleges the defendants submitted and caused others to submit unnecessary drug tests in violation of New Jersey law and is seeking recovery for monetary and treble damages. The parties have reached a confidential settlement in this matter and consider it closed. The Company recognized $2.2 million of expense in litigation settlement related to this matter during 2015. Upon execution of the settlement, the Company made a payment of $1.2 million and agreed to pay $0.1 million per month until the full settlement amount has been paid in full. The settlement was paid in full during 2016, resulting in no remaining balance outstanding as of December 31, 2016, compared to $0.8 million as of December 31, 2015.
Bevell Settlement
On February 3, 2014, AAC filed an action against James D. Bevell in the U.S. District Court in the Middle District of Tennessee, alleging breach of contract and tortious interference with business practices arising out of Mr. Bevell’s breach of his non-compete agreements. Mr. Bevell is the former Chief Innovation Officer of AAC. On July 16, 2014, Mr. Bevell filed an action, for which an amended complaint was filed on August 15, 2014, in the Chancery Court for the State of Tennessee in Williamson County which alleged the defendants breached fiduciary duties owed to Mr. Bevell and breached the Agreement Among Stockholders entered into in connection with the TSN Acquisition. On August 15, 2014, AAC, entered into settlement agreements to resolve all outstanding disputes among the parties (the “Bevell Settlement”). Pursuant to the terms of the Bevell Settlement, the Company agreed to pay Mr. Bevell the sum of approximately $7.6 million. In addition, pursuant to the terms of the Bevell Settlement, the Company eliminated the debt payable to Mr. Bevell of $1.9 million and Mr. Bevell surrendered 698,259 shares ($5.7 million) of AAC common stock that were subsequently cancelled. There was no impact to the Company’s consolidated statement of operations as a result of the Bevell Settlement.
Other
The Company is aware of various other legal matters arising in the ordinary course of business. To cover these types of claims, the Company maintains insurance it believes to be sufficient for its operations, although some claims may potentially exceed the scope of coverage in effect. Plaintiffs in these matters may request punitive or other damages that may not be covered by insurance. After taking into consideration the evaluation of such matters by the Company’s legal counsel, the Company’s management believes the outcome of these matters will not have a material impact on the Company’s consolidated financial position, results of operations and cash flows.
Other Commitments and Contingencies
Effective February 3, 2017, Candance Henderson-Grice, resigned from her then current positions as Chief Operating Officer of Holdings and as Chief Operating Officer of AAC. In connection with her departure and effective as of February 3, 2017, AAC entered into a Separation Agreement and Release (the “Separation Agreement”) with Ms. Henderson-Grice. Ms. Henderson-Grice will receive $550,000, which will be divided and payable in equal payments in accordance with AAC’s normal payroll schedule ending on December 25, 2017, and continuation of health benefits coverage through December 31, 2017.
F-28
The Company is a party to certain placement agreements with Vaco, LLC (“Vaco”). One of the Company’s directors, who is also a stockholder, is an executive officer and an equity owner of Vaco. Vaco provides the Company with accounting professionals and other staff, either on a temporary or permanent basis. Vaco is typically paid 25% of each employee’s first year salary as a placement fee or paid an hourly rate for temporary professional services. Total payments and expense recognized related to this agreement were $0.2 million for the year ended December 31, 2016 and $0.1 million for both years ended December 31, 2015, and 2014, respectively.
An entity beneficially owned by Mr. Cartwright, the Company’s Chief Executive Officer, owns an airplane that the Company uses for business purposes in the course of its operations pursuant to a written lease agreement. The Company pays an hourly rate for use of the airplane as well as fuel and certain maintenance costs. For the years ended December 31, 2016 and 2015, the Company made aggregate payments to the related entity for use of the airplane of approximately $0.9 million and $1.0 million, respectively.
The Company utilized a construction company owned by its Vice President of Development as a general contractor for various 2016 and 2015 construction projects. The Company reimbursed the construction company at cost for any expenses it incurred in serving as its general contractor which totaled approximately $0.8 million and $7.7 million for years ended December 31, 2016 and 2015, respectively.
18. Quarterly Information (Unaudited)
The tables below present summarized unaudited quarterly results of operations for the years ended December 31, 2016 and 2015. Management believes that all necessary adjustments have been included in the amounts stated below for a fair presentation of the results of operations for the periods presented when read in conjunction with the Company’s consolidated financial statements for the years ended December 31, 2016 and 2015. Results of operations for a particular quarter are not necessarily indicative of results of operations for an annual period and are not predictive of future periods.
|
|
|
Quarter Ended |
|
|||||||||||||
|
|
|
March 31, |
|
|
June 30, |
|
|
September 30, |
|
|
December 31, |
|
||||
|
|
|
(In thousands except per share amounts) |
|
|||||||||||||
|
2016: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
65,348 |
|
|
$ |
71,542 |
|
|
$ |
70,528 |
|
|
$ |
72,352 |
|
|
Net loss |
|
$ |
(269 |
) |
|
$ |
(158 |
) |
|
$ |
(4,011 |
) |
|
$ |
(1,303 |
) |
|
Net income (loss) available to AAC Holdings, Inc. common stockholders |
|
$ |
586 |
|
|
$ |
872 |
|
|
$ |
(2,525 |
) |
|
$ |
478 |
|
|
Basic net income (loss) per share |
|
$ |
0.03 |
|
|
$ |
0.04 |
|
|
$ |
(0.11 |
) |
|
$ |
0.02 |
|
|
Diluted net income (loss) per share |
|
$ |
0.03 |
|
|
$ |
0.04 |
|
|
$ |
(0.11 |
) |
|
$ |
0.02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
42,823 |
|
|
$ |
53,784 |
|
|
$ |
57,372 |
|
|
$ |
58,282 |
|
|
Net income (loss) |
|
$ |
2,119 |
|
|
$ |
5,116 |
|
|
$ |
1,744 |
|
|
$ |
(638 |
) |
|
Net income available to AAC Holdings, Inc. common stockholders |
|
$ |
2,038 |
|
|
$ |
5,555 |
|
|
$ |
2,452 |
|
|
$ |
448 |
|
|
Basic net income per share |
|
$ |
0.10 |
|
|
$ |
0.26 |
|
|
$ |
0.11 |
|
|
$ |
0.02 |
|
|
Diluted net income per share |
|
$ |
0.10 |
|
|
$ |
0.26 |
|
|
$ |
0.11 |
|
|
$ |
0.02 |
|
F-29
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
AAC Holdings, Inc. |
||
|
|
|
|
|
By: |
|
/s/ Michael T. Cartwright |
|
|
|
Michael T. Cartwright |
|
|
|
Chief Executive Officer and Chairman |
Dated: March 7, 2017
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
||
|
/s/ Michael T. Cartwright |
|
Chief Executive Officer and Chairman |
|
March 7, 2017 |
|
Michael T. Cartwright |
|
(Principal Executive Officer) |
|
|
|
|
|
|
||
|
/s/ Kirk R. Manz |
|
Chief Financial Officer |
|
March 7, 2017 |
|
Kirk R. Manz |
|
(Principal Financial Officer) |
|
|
|
|
|
|
||
|
/s/ Andrew W. McWilliams |
|
Chief Accounting Officer |
|
March 7, 2017 |
|
Andrew W. McWilliams |
|
(Principal Accounting Officer) |
|
|
|
|
|
|
||
|
/s/ Darrell S. Freeman, Sr. |
|
Lead Independent Director |
|
March 7, 2017 |
|
Darrell S. Freeman, Sr. |
|
|
|
|
|
|
|
|
||
|
/s/ Jerry D. Bostelman |
|
Director |
|
March 7, 2017 |
|
Jerry D. Bostelman |
|
|
|
|
|
|
|
|
||
|
/s/ Lucius E. Burch, III |
|
Director |
|
March 7, 2017 |
|
Lucius E. Burch, III |
|
|
|
|
|
|
|
|
||
|
/s/ David C. Kloeppel |
|
Director |
|
March 7, 2017 |
|
David C. Kloeppel |
|
|
|
|
|
|
|
|
||
|
/s/ Richard E. Ragsdale |
|
Director |
|
March 7, 2017 |
|
Richard E. Ragsdale |
|
|
|
|
|
Exhibit No. |
Description |
|
2.1† |
Agreement and Plan of Merger by and among AAC Holdings, Inc., American Addiction Centers, Inc. and AAC Merger Sub, Inc., dated as of October 30, 2014 (previously filed as Exhibit 2.1 to the Registration Statement on Form S-4 (Registration No. 333-199749), filed on October 31, 2014 and incorporated herein by reference). |
|
2.2† |
Contribution Agreement by and among AAC Holdings, Inc., Michael T. Cartwright, Jerrod N. Menz and Kirk R. Manz, dated as of April 15, 2014 (previously filed as Exhibit 2.1 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on July 11, 2014 and incorporated herein by reference). |
|
2.3† |
Contribution Agreement by and among Tina Cartwright, Victoria Menz, AAC Holdings, Inc. and, solely for the purposes of Section 4.6, Clinical Revenue Management Services, LLC, dated as of April 15, 2014 (previously filed as Exhibit 2.2 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on July 11, 2014 and incorporated herein by reference). |
|
2.4† |
Asset and Equity Purchase Agreement by and among American Addiction Centers, Inc., AJG Solutions, Inc., Member Assistance Solutions, LLC, James D. Bevell, Jr., and Michael Blackburn, dated as of August 31, 2012 (previously filed as Exhibit 2.3 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on July 11, 2014 and incorporated herein by reference). |
|
2.5† |
Asset Purchase Agreement by and among American Addiction Centers, Inc., AAC Florida Acquisition Sub, LLC (n/k/a Recovery First of Florida, LLC) and Recovery First, Inc., dated as of December 15, 2014 (previously filed as Exhibit 2.1 to the Current Report on Form 8-K (File No. 001-36643), filed on February 24, 2015 and incorporated herein by reference). |
|
2.6† |
Amendment to the Asset Purchase Agreement, dated February 17, 2015, by and among American Addiction Centers, Inc., AAC Florida Acquisition Sub, LLC (n/k/a Recovery First of Florida, LLC), The Academy Real Estate, LLC and Recovery First, Inc. (previously filed as Exhibit 2.2 to the Current Report on Form 8-K (File No. 001-36643), filed on February 24, 2015 and incorporated herein by reference). |
|
2.7† |
Purchase and Sale Agreement by and among AAC Holdings, Inc., Behavioral Healthcare Realty, LLC and FiftyNine Palms, Inc., dated as of January 28, 2015 (previously filed as Exhibit 2.3 to the Quarterly Report on Form 10-Q (File No. 001-36643), filed on May 5, 2015 and incorporated herein by reference). |
|
2.8† |
First Amendment to Purchase and Sale Agreement, by and among Zareen Faiz, BHR Aliso Viejo Real Estate, LLC, and PHL Care, Inc., dated as of March 27, 2015 (previously filed as Exhibit 2.4 to the Quarterly Report on Form 10-Q (File No. 001-36643), filed on May 5, 2015 and incorporated herein by reference). |
|
2.9† |
Securities Purchase Agreement, dated July 2, 2015, by and among AAC Holdings, Inc., American Addiction Centers, Inc., Sober Media Group, LLC, Sellers’ Representative and the direct and indirect owners of Referral Solutions Group, LLC (previously filed as Exhibit 2.1 to the Current Report on Form 8-K (File No. 001-36643), filed on July 8, 2015 and incorporated herein by reference). |
|
2.10† |
Asset Purchase Agreement, dated May 8, 2015, by and among American Addiction Centers, Inc., Oxford Treatment Center, LLC, The Oxford Centre, Inc. and River Road Management, LLC (previously filed as Exhibit 2.1 to the Quarterly Report on Form 10-Q (File No. 001-36643), filed on August 3, 2015 and incorporated herein by reference). |
|
2.11† |
Amendment to the Asset Purchase Agreement, dated August 10, 2015, by and among American Addiction Centers, Inc., Oxford Treatment Center, LLC, BHR Oxford Real Estate, LLC, The Oxford Centre, Inc. and River Road Management, LLC (previously filed as Exhibit 2.2 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on August 12, 2015 and incorporated herein by reference). |
|
2.12† |
Asset Purchase Agreement, dated December 10, 2015, by and among AAC Holdings, Inc., American Addiction Centers, Inc., Townsend Treatment Center, LLC, the Sellers party thereto, the Members party thereto and the Sellers’ Representative party thereto (previously filed as Exhibit 2.12 to the Company’s Annual Report on Form 10-K (File No. 001-36643), filed on March 9, 2016 and incorporated herein by reference). |
|
2.13† |
Amendment to the Asset Purchase Agreement, dated March 30, 2016, by and among AAC Holdings, Inc., American Addiction Centers, Inc., Townsend Treatment Center, LLC, Sagenex Diagnostics Laboratory, LLC, Rush Medical – Lafayette, LLC, Houma Treatment Center, L.L.C. and the Sellers’ Representative party thereto (previously filed as Exhibit 2.2 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on April 5, 2016 and incorporated herein by reference). |
|
Articles of Incorporation of AAC Holdings, Inc. (previously filed as Exhibit 3.1 to Amendment No. 2 to Registration Statement on Form S-1 (Registration No. 333-197383), filed on September 10, 2014 and incorporated herein by reference). |
|
|
3.2 |
Amended and Restated Bylaws of AAC Holdings, Inc. (previously filed as Exhibit 4.2 to the Registration Statement on Form S-8 (Registration No. 333-199161), filed on October 3, 2014 and incorporated herein by reference). |
|
4.1 |
Form of Certificate of Common Stock of AAC Holdings, Inc. (previously filed as Exhibit 4.1 to Amendment No. 1 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on August 15, 2014 and incorporated herein by reference). |
|
10.1+ |
AAC Holdings, Inc. 2014 Equity Incentive Plan (previously filed as Exhibit 10.3 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on July 11, 2014 and incorporated herein by reference). |
|
10.2+ |
Form of Restricted Share Award under the AAC Holdings, Inc. 2014 Equity Incentive Plan (previously filed as Exhibit 10.4 to Amendment No. 3 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on September 22, 2014 and incorporated herein by reference). |
|
10.3+ |
Form of Restricted Share Award Agreement under the AAC Holdings, Inc. 2014 Equity Incentive Plan (previously filed as Exhibit 10.1 to the Current Report on Form 8-K (File No. 001-36643), filed on January 9, 2015 and incorporated herein by reference). |
|
10.4+ |
Form of Non-Employee Director Award Agreement under the AAC Holdings, Inc. 2014 Equity Incentive Plan (previously filed as Exhibit 10.2 to the Current Report on Form 8-K (File No. 001-36643), filed on January 9, 2015 and incorporated herein by reference). |
|
10.5+ |
AAC Holdings, Inc. 2016 Annual Bonus Plan (previously filed as Exhibit 10.1 to the Current Report on Form 8-K (File No. 001-36643), filed on February 26, 2016 and incorporated herein by reference). |
|
10.6+ |
Form of Director Indemnification Agreement (previously filed as Exhibit 10.6 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on July 11, 2014 and incorporated herein by reference). |
|
10.7 |
Form of Management Services Agreement by and among American Addiction Centers, Inc. and each professional physician group (previously filed as Exhibit 10.38 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on July 11, 2014 and incorporated herein by reference). |
|
10.8 |
Professional Services Agreement by and among San Diego Addiction Treatment Center, Inc. and San Diego Professional Group, P.C., dated as of August 5, 2014 (previously filed as Exhibit 10.39 to Amendment No. 1 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on August 15, 2014 and incorporated herein by reference). |
|
10.9 |
Professional Services Agreement by and among Forterus Health Care Services, Inc. and San Diego Professional Group, P.C., dated as of August 5, 2014 (previously filed as Exhibit 10.40 to Amendment No. 1 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on August 15, 2014 and incorporated herein by reference). |
|
10.10 |
Professional Services Agreement by and among Singer Island Recovery Center LLC and Palm Beach Professional Group, Professional Corporation, dated as of August 5, 2014 (previously filed as Exhibit 10.41 to Amendment No. 1 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on August 15, 2014 and incorporated herein by reference). |
|
10.11 |
Professional Services Agreement by and among Concorde Treatment Center, LLC d/b/a Desert Hope Center and Las Vegas Professional Group – Calarco, P.C., dated as of August 5, 2014 (previously filed as Exhibit 10.43 to Amendment No. 1 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on August 15, 2014 and incorporated herein by reference). |
|
10.12 |
Professional Services Agreement by and among Greenhouse Treatment Center, LLC d/b/a The Greenhouse and Grand Prairie Professional Group, P.A., dated as of August 5, 2014 (previously filed as Exhibit 10.45 to Amendment No. 1 to the Registration Statement on Form S-1 (Registration No. 333-197383), filed on August 15, 2014 and incorporated herein by reference). |
|
10.13 |
Professional Services Agreement by and among Oxford Treatment Center, LLC and Oxford Professional Group, P.C., dated as of August 10, 2015 (previously filed as Exhibit 10.16 to the Company’s Annual Report on Form 10-K (File No. 001-36643), filed on March 9, 2016 and incorporated herein by reference). |
|
Professional Services Agreement by and between AAC Florida Acquisition Sub, LLC d/b/a Recovery First and Palm Beach Professional Group, Professional Corporation, dated as of February 20, 2015 (previously filed as Exhibit 10.17 to the Company’s Annual Report on Form 10-K (File No. 001-36643), filed on March 9, 2016 and incorporated herein by reference). |
|
|
10.15 |
Professional Services Agreement by and between AAC Las Vegas Outpatient Center, LLC d/b/a Desert Hope Outpatient Center and Las Vegas Professional Group – Calarco, P.C., dated as of January 8, 2015 (previously filed as Exhibit 10.18 to the Company’s Annual Report on Form 10-K (File No. 001-36643), filed on March 9, 2016 and incorporated herein by reference). |
|
10.16 |
Professional Services Agreement by and between AAC Dallas Outpatient Center, LLC d/b/a Greenhouse Outpatient Center and Grand Prairie Professional Group, P.A., dated as of February 18, 2015 (previously filed as Exhibit 10.19 to the Company’s Annual Report on Form 10-K (File No. 001-36643), filed on March 9, 2016 and incorporated herein by reference). |
|
10.17 |
Professional Services Agreement by and between River Oaks Treatment Center, LLC and Palm Beach Professional Group, Professional Corporation, dated as of October 1, 2015 (previously filed as Exhibit 10.20 to the Company’s Annual Report on Form 10-K (File No. 001-36643), filed on March 9, 2016 and incorporated herein by reference). |
|
10.18 |
Credit Agreement dated as of March 9, 2015 among AAC Holdings, Inc., certain Subsidiaries of the Borrower party thereto, Bank of America, N.A., as Administrative Agent, Swingline Lender and L/C Lender and SunTrust Bank, as Syndication Agent, Raymond James Bank, N.A. and BMO Harris Bank N.A., as Co-Documentation Agents and the other lenders party thereto (previously filed as Exhibit 10.27 to the Annual Report on Form 10-K (File No. 001-36643), filed on March 11, 2015 and incorporated herein by reference). |
|
10.19 |
First Amendment to Credit Agreement dated as of June 16, 2015, by and among AAC Holdings, Inc., the Guarantors, the Lenders party thereto and Bank of America, N.A., as Administrative Agent, Swingline Lender and L/C Issuer (previously filed as Exhibit 10.1 to the Current Report on Form 8-K (File No. 001-36643), filed on June 19, 2015 and incorporated herein by reference). |
|
10.20 |
Second Amendment to Credit Agreement dated as of October 2, 2015, by and among AAC Holdings, Inc., certain Guarantors party thereto, the Lenders party thereto and Bank of America, N.A., as Administrative Agent, Swingline Lender and L/C Issuer (previously filed as Exhibit 10.6 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on October 7, 2015 and incorporated herein by reference). |
|
10.21 |
Third Amendment to Credit Agreement dated as of July 13, 2016, by and among AAC Holdings, Inc., the Guarantors party thereto, the Lenders party thereto and Bank of America, N.A., as Administrative Agent, Swingline Lender and L/C Issuer (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on July 15, 2016 and incorporated herein by reference). |
|
10.22 |
Fourth Amendment to Credit Agreement dated as of February 27, 2017, by and among AAC Holdings, Inc., the Guarantors party thereto, the Lenders party thereto and Bank of America, N.A., as Administrative Agent, Swingline Lender and L/C Issuer (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on February 28, 2017 and incorporated herein by reference). |
|
10.23 |
Office Space Lease by and between CV Brentwood Properties, LLC and American Addiction Centers, Inc. (previously filed as Exhibit 10.1 to the Current Report on Form 8-K (File No. 001-36643), filed on January 12, 2015 and incorporated herein by reference). |
|
10.24 |
Guaranty of Lease by and between AAC Holdings, Inc. and CV Brentwood Properties, LLC (previously filed as Exhibit 10.2 to the Current Report on Form 8-K (File No. 001-36643), filed on January 12, 2015 and incorporated herein by reference). |
|
10.25 |
Facility Agreement, dated as of October 2, 2015, by and among AAC Holdings, Inc., Deerfield Private Design Fund III, L.P., Deerfield Partners, L.P. and Deerfield International Master Fund, L.P. (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on October 7, 2015 and incorporated herein by reference). |
|
10.26 |
Form of Convertible Note (previously filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on October 7, 2015 and incorporated herein by reference). |
|
10.27 |
Form of Acquisition Note (previously filed as Exhibit 10.3 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on October 7, 2015 and incorporated herein by reference). |
|
Guaranty, dated as of October 2, 2015, by each Guarantor in favor of Deerfield Private Design Fund III, L.P., Deerfield Partners, L.P. and Deerfield International Master Fund, L.P. (previously filed as Exhibit 10.4 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on October 7, 2015 and incorporated herein by reference). |
|
|
10.29 |
Registration Rights Agreement, dated as of October 2, 2015, by and among AAC Holdings, Inc., Deerfield Private Design Fund III, L.P., Deerfield Partners, L.P. and Deerfield International Master Fund, L.P. (previously filed as Exhibit 10.5 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on October 7, 2015 and incorporated herein by reference). |
|
10.30 |
Non-Exclusive Aircraft Lease Agreement, dated as of November 1, 2015, by and between AMC, Inc. and American Addiction Centers, Inc. (previously filed as Exhibit 10.7 to the Company’s Quarterly Report on Form 10-Q (File No. 001-36643), filed on November 10, 2015 and incorporated herein by reference).
|
|
10.31 |
Note Modification Agreement, dated as of August 31, 2015, by and between American Addiction Centers, Inc. and Michael Blackburn (previously filed as Exhibit 10.8 to the Company’s Quarterly Report on Form 10-Q (File No. 001-36643), filed on November 10, 2015 and incorporated herein by reference). |
|
10.32 |
Senior Subordinated Note, dated April 1, 2016, payable to Deerfield International Master Fund, L.P. (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on April 5, 2016 and incorporated herein by reference).
|
|
10.33 |
Senior Subordinated Note, dated April 1, 2016, payable to Deerfield Private Design Fund III, L.P. (previously filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on April 5, 2016 and incorporated herein by reference).
|
|
10.34 |
Senior Subordinated Note, dated April 1, 2016, payable to Deerfield Partners, L.P. (previously filed as Exhibit 10.3 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on April 5, 2016 and incorporated herein by reference).
|
|
10.35 |
Senior Subordinated Note, dated April 15, 2016, payable to Deerfield Private Design Fund III, L.P. (previously filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on April 20, 2016 and incorporated herein by reference).
|
|
10.36 |
Senior Subordinated Note, dated April 15, 2016, payable to Deerfield International Master Fund, L.P. (previously filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on April 20, 2016 and incorporated herein by reference).
|
|
10.37 |
Senior Subordinated Note, dated April 15, 2016, payable to Deerfield Partners, L.P. (previously filed as Exhibit 10.3 to the Company’s Current Report on Form 8-K (File No. 001-36643), filed on April 20, 2016 and incorporated herein by reference).
|
|
10.38+* |
Separation and Agreement and Release, dated February 3, 2017, by and between American Addiction Centers, Inc. and Candance Henderson-Grice.
|
|
21.1* |
List of subsidiaries |
|
23.1* |
Consent of BDO USA, LLP |
|
31.1* |
Certification of Chief Executive Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
31.2* |
Certification of Chief Financial Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
32.1** |
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
32.2** |
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
101.INS* |
XBRL Instance Document. |
|
XBRL Taxonomy Extension Schema Document. |
|
|
101.CAL* |
XBRL Taxonomy Calculation Linkbase Document. |
|
101.DEF* |
XBRL Taxonomy Extension Definition Linkbase Document. |
|
101.LAB* |
XBRL Taxonomy Labels Linkbase Document. |
|
101.PRE* |
XBRL Taxonomy Presentation Linkbase Document. |
|
* |
Filed herewith. |
|
† |
Schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. AAC Holdings, Inc. hereby undertakes to furnish supplementally copies of any of the omitted schedules and exhibits upon request by the Securities and Exchange Commission. |
|
+ |
Denotes a management contract or compensatory plan or arrangement. |
|
** |
The certifications attached as Exhibit 32.1 and Exhibit 32.2 that accompany this Annual Report on Form 10-K are deemed furnished and not filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of AAC Holdings, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K, irrespective of any general incorporation language contained in such filing. |
