Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - Travere Therapeutics, Inc. | exhibit322-1231201610k.htm |
| EX-32.1 - EXHIBIT 32.1 - Travere Therapeutics, Inc. | exhibit321-1231201610k.htm |
| EX-31.2 - EXHIBIT 31.2 - Travere Therapeutics, Inc. | exhibit312-1231201610k.htm |
| EX-31.1 - EXHIBIT 31.1 - Travere Therapeutics, Inc. | exhibit311-1231201610k.htm |
| EX-23.1 - EXHIBIT 23.1 - Travere Therapeutics, Inc. | exhibit231-1231201610kxbdo.htm |
| EX-21.1 - EXHIBIT 21.1 - Travere Therapeutics, Inc. | exhibit211-1231201610k.htm |
| EX-10.34 - EXHIBIT 10.34 - Travere Therapeutics, Inc. | exhibit1034-1231201610k.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________
FORM 10-K
þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the Fiscal Year Ended December 31, 2016
¨ | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |
Commission File Number: 001-36257
RETROPHIN, INC.
(Exact Name of Registrant as specified in its Charter)
Delaware | 27-4842691 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
3721 Valley Centre Drive, Suite 200, San Diego CA | 92130 |
(Address of Principal Executive Offices) | (Zip code) |
760-260-8600
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of exchange on which registered |
Common Stock, par value $0.0001 per share | The NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. þ Yes ¨ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ¨ Yes þ No
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. þ Yes ¨ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). þ Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act):
Large Accelerated Filer þ | Accelerated Filer r | |
Non-Accelerated Filer r | Smaller Reporting Company r | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ¨ Yes þ No
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter. $576,730,241.
The number of shares of outstanding common stock, par value $0.0001 per share, of the Registrant as of February 28, 2017 was 38,093,583.
FORM 10-K REPORT INDEX
Page | ||
2
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Certain information contained in this Annual Report on Form 10-K of Retrophin, Inc., a Delaware corporation (the “Company”) include forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The statements herein which are not historical reflect our current expectations and projections about the Company’s future results, performance, liquidity, financial condition, prospects and opportunities and are based upon information currently available to the Company and management and is subject to its interpretation of what is believed to be significant factors affecting the businesses, including many assumptions regarding future events. Such forward-looking statements include statements regarding, among other things:
• | our ability to produce, sustain and expand sales of our products; |
• | our ability to develop, acquire and/or introduce new products; |
• | our projected future sales, profitability and other financial metrics; |
• | our future financing plans; |
• | our anticipated needs for working capital; |
• | the anticipated trends in our industry; |
• | acquisitions of other companies or assets that we might undertake in the future; |
• | our operations in the United States and abroad, and the domestic and foreign regulatory, economic and political conditions; and |
• | competition existing today or that will likely arise in the future. |
Forward-looking statements, which involve assumptions and describe our future plans, strategies and expectations, are generally identifiable by use of the words “may,” “should,” “expect,” “anticipate,” “estimate,” “believe,” “intend,” “seek,” or “project” or the negative of these words or other variations on these words or comparable terminology. Actual results, performance, liquidity, financial condition and results of operations, prospects and opportunities could differ materially from those expressed in, or implied by, these forward-looking statements as a result of various risks, uncertainties and other factors, including the ability to raise sufficient capital to continue the Company’s operations. Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in this Annual Report generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this Annual Report will in fact occur. Potential investors should not place undue reliance on any forward-looking statements. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason.
The specific discussions in this Annual Report about the Company include financial projections and future estimates and expectations about the Company’s business. The projections, estimates and expectations are presented in this Annual Report only as a guide about future possibilities and do not represent actual amounts or assured events. All the projections and estimates are based exclusively on the Company management’s own assessment of the business, the industry in which it works and the economy at large and other operational factors, including capital resources and liquidity, financial condition, fulfillment of contracts and opportunities. The actual results may differ significantly from the projections.
Potential investors should not make an investment decision based solely on the Company’s projections, estimates or expectations.
3
PART I
In this Annual Report on Form 10-K, unless the context requires otherwise, the terms “we”, “our”, “us”, “Retrophin” and the “Company” refer to Retrophin, Inc., a Delaware corporation, as well as our direct and indirect subsidiaries.
ITEM 1. BUSINESS
Those statements in the following discussion that are not historical in nature should be considered to be forward looking statements that are inherently uncertain. Actual results and the timing of the events may differ materially from those contained in these forward looking statements due to a number of factors, including those discussed in the “Cautionary Statement Regarding Forward-Looking Statements” and “Risk Factors” set forth elsewhere in this Annual Report.
Overview
We are a fully integrated biopharmaceutical company with approximately 135 employees headquartered in San Diego, California, dedicated to delivering life-changing therapies to people living with rare diseases who have few, if any, treatment options. Our approach centers on our pipeline, featuring clinical-stage assets and pre-clinical discovery programs targeting rare diseases with significant unmet medical needs. Our research and development efforts are supported by revenues from our marketed products, Chenodal®, Cholbam® and Thiola®. In addition we regularly evaluate and, where appropriate, act on opportunities to expand our product pipeline through licenses and acquisitions of products in areas that will serve patients with serious or rare diseases and that we believe offer attractive growth characteristics.
We currently have the following product candidates in clinical development:
Sparsentan (RE-021)
Sparsentan, also known as RE-021, is an investigational therapeutic agent which acts as both a potent angiotensin receptor blocker (“ARB”), as well as a selective endothelin receptor antagonist (“ERA”), with in vitro selectivity toward endothelin receptor type A. We have secured a license to sparsentan from Ligand Pharmaceuticals, Inc. and Bristol-Myers Squibb Company ("BMS") (who referred to it as DARA). We are developing sparsentan as a treatment for focal segmental glomerulosclerosis ("FSGS"), which is a leading cause of end-stage renal disease and nephrotic syndrome (“NS”).
Fosmetpantotenate (RE-024)
Fosmetpantotenate, also known as RE-024, a novel small molecule, is being developed as a potential treatment for pantothenate kinase-associated neurodegeneration (“PKAN”). PKAN is a genetic neurodegenerative disorder that is typically diagnosed in the first decade of life. Consequences of PKAN include dystonia, dysarthria, rigidity, retinal degeneration, and severe digestive problems.
We currently sell the following three products:
• | Chenodal® (chenodeoxycholic acid) is approved in the United States for the treatment of patients suffering from gallstones in whom surgery poses an unacceptable health risk due to disease or advanced age. Chenodal® has also been the standard of care for cerebrotendinous xanthomatosis (“CTX”) patients for more than three decades and the Company is currently pursuing adding this indication to the label. |
• | Cholbam® (cholic acid) is approved in the United States for the treatment of bile acid synthesis disorders due to single enzyme defects and is further indicated for adjunctive treatment of patients with peroxisomal disorders. |
• | Thiola® (tiopronin) is approved in the United States for the prevention of cystine (kidney) stone formation in patients with severe homozygous cystinuria. |
Our Strategy
Our goal is to become a leading biopharmaceutical company specializing in the development and commercialization of therapies that deliver significant value for patients with serious or rare diseases. In order to achieve our goal, we intend to:
• | Focus on developing products to treat rare diseases characterized by severe unmet medical needs. We focus on potentially transformational orphan drug candidates in order to leverage our development and commercialization capabilities in rare disease. We believe that drug development for orphan drug markets is particularly attractive because relatively small clinical trials can demonstrate the large clinical effects expected with transformational therapies. Furthermore, the regulatory and commercial models for orphan drugs are well established. Finally, we believe that our research, development, and commercialization capabilities are well suited to the orphan drug market and represent distinct competitive advantages. |
• | Develop a sustainable pipeline by employing disciplined decision criteria in the evaluation of potential in-licensing candidates. We seek to build a sustainable product pipeline by employing multiple therapeutic approaches and by developing or acquiring orphan drug candidates. We seek to augment our internally developed pipeline projects by selectively and strategically acquiring pipeline assets that will add value to the portfolio. We intend to mitigate risk by employing rigorous decision criteria, favoring drug candidates that have |
4
undergone at least some clinical study. Our decision to acquire rights to a drug candidate also depends on the scientific merits of the available clinical data; the identifiable orphan patient population; the economic terms of any proposed acquisition of rights; the projected amount of capital required to develop the drug candidate; and the economic potential of the drug candidate, should it be commercialized. We believe this strategy minimizes our clinical development risk and allows us to accelerate the development and potential commercialization of current and future drug candidates.
• | Evaluate the commercialization strategies on a product-by-product basis to maximize the value of each. As we move our drug candidates through development toward regulatory approval, we will evaluate several options for each drug candidate’s commercialization strategy. These options include utilizing or expanding our own internal sales force; entering into joint marketing partnerships with other pharmaceutical or biotechnology companies, whereby we jointly sell and market the product; and out-licensing our products, whereby other pharmaceutical or biotechnology companies sell and market our product and pay us a royalty on sales. Our decision will be made separately for each product and will be based on a number of factors including capital necessary to execute on each option, size of the market and terms of potential offers from other pharmaceutical and biotechnology companies. |
Our Product Candidates and Products on the Market
The following table summarizes the status of our product candidates, preclinical programs and products on the market, each of which is described in further detail below.
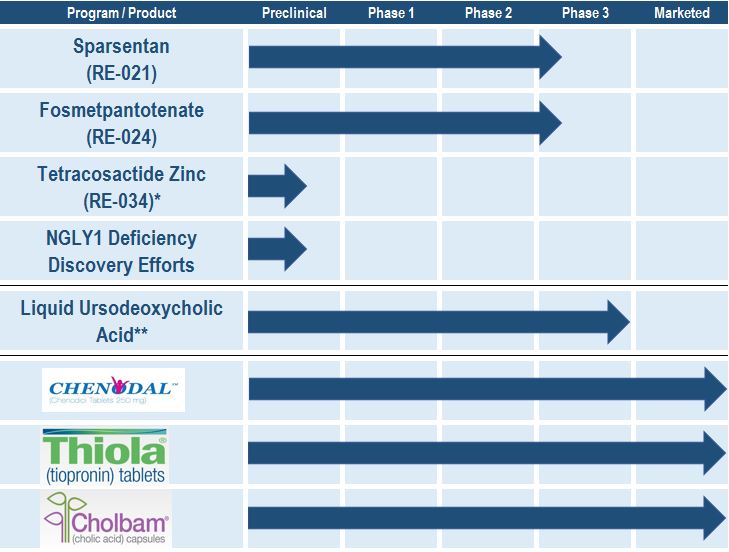
*Pursuing out licensing opportunities.
**Acquired rights in 2016; activities underway with the intention of making the liquid formulation commercially available in the United States.
5
Product Candidates:
Sparsentan (RE-021)
Sparsentan is an investigational therapeutic agent which acts as both a potent ARB, as well as a selective ERA, with in vitro selectivity toward endothelin receptor type A. We have secured a license to sparsentan from Ligand Pharmaceuticals, Inc. and Bristol-Myers Squibb Company (who referred to it as DARA). We are developing sparsentan as a treatment for FSGS, which is a leading cause of end-stage renal disease and NS. There are no FDA approved pharmacological treatments for FSGS and the off-label armamentarium is limited to ACE/ARBs, steroids, and immunosuppressant agents, which are effective in only a subset of patients. Every year approximately 5,400 patients are diagnosed with FSGS and we estimate that there are up to 40,000 FSGS patients in the United States with approximately half of them being candidates for sparsentan. Sparsentan was granted orphan drug designation in the United States and the European Union in January 2015 and November 2015, respectively. In the third quarter of 2016, we announced positive top-line data from the Phase 2 DUET study of sparsentan for the treatment of FSGS.
In early 2017, we had an End of Phase 2 meeting with the FDA regarding the regulatory pathway for sparsentan as a treatment for FSGS. Following the meeting and our receipt of confirmatory meeting minutes, we announced our plans to initiate a single Phase 3 clinical trial to serve as the basis of a New Drug Application ("NDA") filing for sparsentan for the treatment of FSGS. We expect that the trial will include an interim analysis of proteinuria as a surrogate endpoint and that if this interim analysis shows a substantial effect on proteinuria reduction, that the data could serve as a basis for accelerated approval of sparsentan for the treatment of FSGS pursuant to Subpart H of the FDA regulations. The confirmatory endpoint of the study would subsequently compare changes from baseline of estimated glomerular filtration rate, or eGFR. We are currently working with the FDA to finalize the study protocol and expect to initiate the trial in the second half of 2017.
Fosmetpantotenate (RE-024)
We are developing fosmetpantotenate, a novel small molecule, as a potential treatment for PKAN. PKAN is a genetic neurodegenerative disorder that is typically diagnosed in the first decade of life. Consequences of PKAN include dystonia, dysarthria, rigidity, retinal degeneration, and severe digestive problems. PKAN is estimated to affect up to 5,000 patients worldwide. There are currently no approved treatments for PKAN. Fosmetpantotenate (RE-024) is a phosphopantothenate replacement therapy that aims to restore levels of this key substrate in PKAN patients. Certain international health regulators have approved the initiation of dosing fosmetpantotenate (RE-024) in PKAN patients under physician-initiated studies in accordance with local regulations in their respective countries. We filed a U.S. IND for fosmetpantotenate (RE-024) with the FDA in the first quarter of 2015 to support the commencement of a company-sponsored Phase 1 study, which was successfully completed during 2015. The FDA granted fosmetpantotenate (RE-024) orphan drug designation in May 2015 and fast track designation in June 2015. In February 2016, we announced fosmetpantotenate (RE-024) was granted orphan drug designation by the European Commission. In November 2016, we announced that we had reached an agreement with the FDA under the Special Protocol Assessment (SPA) process for a Phase 3 clinical trial evaluating fosmetpantotenate (RE-024) for PKAN. We expect to begin dosing patients in this Phase 3 clinical trial in the coming months. We continue discussion with the EMA regarding the initiation of a potential registration-enabling efficacy trial in PKAN patients.
Tetracosactide Zinc (RE-034)
Tetracosactide zinc (RE-034) is a synthetic hormone analog of the first 24 amino acids of the 39 amino acids contained in adrenocorticotropic hormone ("ACTH") incorporated into a novel formulation developed by us. Tetracosactide zinc (RE-034) exhibits similar physiological actions as endogenous ACTH by binding to all five melanocortin receptors (pan-MCR), resulting in its anti-inflammatory and immunomodulatory effects. We have successfully manufactured tetracosactide zinc (RE-034) at proof-of-concept scale using a novel formulation process that allows modulation of the release of the active ingredient from the site of administration. We have decided to focus our further efforts on this program toward potential out-licensing opportunities.
NGLY1 Deficiency Discovery Efforts
We have entered into a research collaboration aimed at the discovery of a novel therapeutic for patients with NGLY1 deficiency, a rare genetic disorder. NGLY1 deficiency is believed to be caused by a deficiency in an enzyme called N-glycanase-1, which is encoded by the gene NGLY1. The condition is characterized by a variety of symptoms, including global developmental delay, movement disorder, seizures, and ocular abnormalities.
Liquid Ursodeoxycholic Acid
Liquid ursodeoxycholic acid ("L-UDCA") is a liquid formulation of ursodeoxycholic acid being developed for the treatment of a rare liver disease called primary biliary cholangitis ("PBC"). We obtained rights to L-UDCA during 2016 with the intention of making L-UDCA commercially available to the subset of PBC patients who have difficulty swallowing. There are no liquid formulations of ursodeoxycholic acid currently approved by the FDA.
Products on the Market:
Chenodal (chenodiol tablets)
Chenodal is a synthetic oral form of chenodeoxycholic acid, a naturally occurring primary bile acid synthesized from cholesterol in the liver, indicated for the treatment of radiolucent stones in well-opacifying gallbladders in patients in whom selective surgery would be undertaken except for the presence of increased surgical risk due to systemic disease or age.
6
Chenodal administration is known to reduce biliary cholesterol and the dissolution of radiolucent gallstones through suppression of hepatic synthesis of cholesterol, cholic acid and deoxycholic acid in the bile pool. Chenodal was first approved by the Food and Drug Administration (the "FDA") in 1983 for the management of gallstones but its marketing was later discontinued due to lack of commercial success. In 2009, Nexgen Pharma's Abbreviated New Drug Application ("ANDA") for Chenodal was approved by the FDA for the treatment of gallstones; Chenodal is manufactured for Manchester Pharmaceuticals LLC ("Manchester") under this ANDA. Manchester subsequently obtained orphan drug designation for Chenodal for the treatment of CTX, a rare autosomal recessive lipid storage disease, in 2010. Manchester was acquired by Retrophin in March 2014. For further discussion, see Note 3 of the Consolidated Financial Statements.
While Chenodal is not labeled for CTX, it has been used as the standard of care for over three decades. We are working to obtain FDA approval of Chenodal for the treatment of CTX. The prevalence of CTX is estimated in the literature to be as high as 1 in 70,000 in the overall population. Pathogenesis of CTX involves deficiency of the enzyme 27-hydroxylase (encoded by the gene CYP27A1), a rate-limiting enzyme in the synthesis of primary bile acids, including chenodeoxycholic acid ("CDCA"), from cholesterol. The disruption of primary bile acid synthesis in CTX leads to toxic accumulation of cholesterol and cholestanol in most tissues. Most patients present with intractable diarrhea, premature cataracts, tendon xanthomas, atherosclerosis, and cardiovascular disease in childhood and adolescence. Neurological manifestations of the disease, including dementia and cognitive and cerebellar deficiencies, emerge during late adolescence and adulthood. Oral administration of CDCA has been shown to normalize primary bile acid synthesis in patients with CTX.
Cholbam (cholic acid capsules)
The FDA approved Cholbam (cholic acid capsules) in March 2015, the first FDA approved treatment for pediatric and adult patients with bile acid synthesis disorders due to single enzyme defects, and for adjunctive treatment of patients with peroxisomal disorders (including Zellweger spectrum disorders). The effectiveness of Cholbam has been demonstrated in clinical trials for bile acid synthesis disorders and the adjunctive treatment of peroxisomal disorders. The estimated incidence of bile acid synthesis disorders due to single enzyme defects is 1 to 9 per million live births.
Kolbam, the branded name of Cholbam in Europe, is indicated in Europe for the treatment of inborn errors of primary bile acid synthesis, encompassing select single enzyme defects, in infants from one month of age for continuous lifelong treatment through adulthood.
Thiola (tiopronin tablets)
Thiola is approved by the FDA for the treatment of cystinuria, a rare genetic cystine transport disorder that causes high cystine levels in the urine and the formation of recurring kidney stones. The resulting long-term damage can cause loss of kidney function in addition to substantial pain and loss of productivity associated with renal colic and stone passage. The prevalence of cystinuria in the United States is estimated to be 10,000 to 12,000, indicating that there may be as many as 4,000 to 5,000 affected individuals with cystinuria in the United States that would be candidates for Thiola.
Competition
The pharmaceutical and biotechnology industries are intensely competitive and subject to rapid and significant technological change. Most of our competitors are larger than us and have substantially greater financial, marketing and technical resources than we have.
The development and commercialization of new products to treat orphan diseases is highly competitive, and we expect considerable competition from major pharmaceutical, biotechnology and specialty pharmaceutical companies. As a result, there are, and will likely continue to be, extensive research and substantial financial resources invested in the discovery and development of new orphan drug products.
We are a company with a limited history of operations. Many of our competitors have substantially more resources than we do, including both financial and technical. Our competition will be determined in part by the potential indications for which drugs are developed and ultimately approved by regulatory authorities. The speed with which we can develop products, complete pre-clinical testing, clinical trials, approval processes, and supply commercial quantities to market are expected to be important competitive factors. We expect that competition among products approved for sale will be based on various factors, including product efficacy, safety, reliability, availability, price, reimbursement, and patent position.
Fosmetpantotenate (RE-024)
There are currently no approved treatments for PKAN.
Tetracosactide Zinc (RE-034)
Questcor’s H.P. Acthar Gel (repository corticotropin injection) is a highly purified sterile preparation of the adrenocorticotropic hormone in 16% gelatin. Acthar is the only approved long-lasting ACTH medication in the U.S.
H.P. Acthar Gel is indicated for several diseases which would be a competitor for any indications we pursue.
Amphastar’s Cortrosyn® (cosyntropin) for injection use is a sterile Iyophilized powder in vials containing 0.25 mg of Cortrosyn® and 10 mg of mannitol. Cortrosyn® is indicated for the ACTH Stimulation Test which measures the ability of the adrenal cortex to respond to ACTH by producing cortisol appropriately. Administration is by intravenous or intramuscular injection. Currently, Cortrosyn is only approved as a diagnostic, not as a drug. Further, Cortrosyn is a short acting formulation of ACTH in contrast to Synacthen Depot and Acthar.
7
Liquid Ursodeoxycholic Acid
Also known as ursodiol or UDCA, ursodeoxycholic acid is a naturally occurring hydrophilic bile acid derived from cholesterol, which is indicated for the treatment of PBC and currently prescribed only in solid forms. Introducing a liquid formulation of ursodeoxycholic acid would provide healthcare professionals with a dosing alternative for patients who have difficulty swallowing tablets or capsules, and may facilitate increased compliance. Currently the only liquid form of UDCA is from compounding pharmacies who make it at a physician's request.
Chenodal
Statins lower cholesterol and have been studied as a treatment for CTX. However, statins deplete CoQ10 and thereby alter mitochondrial function, which is a theoretical concern because abnormal mitochondrial metabolism has been reported in CTX. Although data are sparse, statin monotherapy appears to have little or no benefit for CTX. However, statins may be useful for lowering cholestanol levels when combined with CDCA, and there is limited evidence that they provide additional clinical benefit over CDCA treatment alone.
Cholbam
There are currently no competitors in the United States.
Thiola
D-penicillamine is the only other prescription medication FDA approved for the treatment of cystinuria. D-penicillamine forms a penicillamine-cysteine disulfide complex that is 50 times more soluble than cystine. In uncontrolled trials and observational studies, penicillamine decreases stone size or dissolves stones in up to 75 percent of patients. The use of D-penicillamine is often limited by a relatively high incidence of side effects, such as fever, rash, abnormal taste, arthritis, leukopenia, aplastic anemia, hepatotoxicity, and pyridoxine (vitamin B6) deficiency. In addition, patients treated with penicillamine may develop proteinuria (usually due to membranous nephropathy), typically within the first 6 to 12 months of therapy, or, less commonly, crescentic glomerulonephritis. Given the high incidence of side effects, drug therapy may be discontinued once stones are no longer present. Additional courses can be given if stones recur. If penicillamine is to be used long term, pyridoxine supplementation (50 mg/day) is required.
Captopril is not FDA approved for the treatment of cystinuria but has been prescribed for patients with cystinuria. The proportion of orally administered captopril that appears in the urine is low. Thus, the doses of captopril required to reduce cystine excretion (more than 150 mg/day) may not be tolerated because of hypotension. In addition, the efficacy of captopril as a treatment for cystinuria remains unproven. Thus, its use is typically limited to patients who cannot tolerate other cystine-binding agents.
In 2016, Imprimis Pharmaceuticals, Inc., a specialty pharmaceutical company, announced plans to introduce a compounded form of tiopronin, the active ingredient in Thiola, in combination with potassium citrate. Compounded therapies are not subjected to the same level of safety and efficacy evaluation and may not offer the same therapeutic outcome for patients. There is no clinical data to support the compatibility of fixed dosing of tiopronin with potassium citrate. Fixed-dose combinations of therapies containing potassium are generally avoided due to the potential for fluctuations in serum potassium, which may cause serious adverse outcomes including cardiac events.
Revive Therapeutics is developing bucillamine, a dithiol derivative of tiopronin for the treatment of cystinuria. The FDA has granted orphan designation status for the use of the drug Bucillamine for the treatment of cystinuria.
Sparsentan
There are currently no products approved for FSGS in Europe or the United States. Generally, patients with primary FSGS are treated using glucocorticoids such as predinisone as initial therapy when proteinuria is >3.5 g/day and accompanied by hypoalbuminemia <3.5 g/dL (<35 g/L). Depending upon the response to and the toxicity from this therapy, the duration of prednisone therapy can vary from as short as 8 to 12 weeks to as long as one year. Some patients treated with glucocorticoids have only a transient remission or no remission whatsoever.
Acquisition of Liquid Ursodeoxycholic Acid (L-UDCA)
In June 2016, we entered into an asset purchase agreement with Asklepion Pharmaceuticals, LLC (“Asklepion”) to purchase Asklepion's rights, titles and ownership of a liquid formulation of ursodeoxycholic acid.
Acquisition of Cholbam (cholic acid)
In January 2015, we announced the signing of a definitive agreement under which we acquired the exclusive option to purchase from Asklepion all of Asklepion's rights, titles, and ownership of Cholbam (cholic acid) for the treatment of bile acid synthesis disorders, if approved by the FDA. In March 2015, the FDA approved Cholbam capsules and we then exercised our option and acquired from Asklepion all of Asklepion's rights, titles and ownership of Cholbam, including all related contracts, data assets, intellectual property, regulatory assets and a pediatric priority review voucher (the "PRV").
The total purchase price of the assets was $91.3 million. We paid Asklepion $33.4 million in cash, transferred 661,279 shares valued at $15.8 million and agreed to pay contingent consideration consisting of milestones and tiered royalties.
Sale of Asset to Sanofi
In July 2015, we sold and transferred the PRV to Sanofi for $245.0 million. $150.0 million was received upon closing, and $47.5 million is due on each of the first and second anniversaries of the closing. We received the first annual payment in July 2016 in accordance with the terms of the agreement.
8
Licenses and Royalties
Ligand License Agreement
In 2012, we entered into a license agreement with Ligand Pharmaceuticals, Inc., granting us a worldwide license for the development, manufacture and commercialization of sparsentan, which we are initially developing in connection with the treatment of FSGS. Under the license agreement, Ligand granted us a sublicense under certain of its patents and other intellectual property in connection with the development and commercialization of sparsentan. Under the license agreement, Ligand is obligated to transfer to us certain information, records, regulatory filings, materials and inventory controlled by Ligand and relating to or useful for developing sparsentan. We must use commercially reasonable efforts to develop and commercialize sparsentan in specified major market countries and other countries in which we believe it is commercially reasonable to develop and commercialize such products.
As consideration for the license, we are required to make payments upon the achievement of certain milestones, totaling up to $109.4 million. Should we commercialize sparsentan or any products containing any of the licensed compounds, we will be obligated to pay Ligand an escalating annual royalty between 15% and 17% of net sales of all such products. Through 2016, we have made milestone payments to Ligand of $2.6 million under the terms of the license agreement.
Under the terms of the license agreement, Bristol-Myers Squibb has a right of first negotiation and Ligand has a right of second negotiation with respect to any license arrangement for a licensed compound except to the extent such rights may be waived.
The license agreement will continue until neither party has any further payment obligations under the agreement and is expected to continue for approximately 10 to 20 years from the effective date. Ligand may terminate the license agreement due to (i) our insolvency, (ii) our material uncured breach of the agreement, (iii) our failure to use commercially reasonable efforts to develop and commercialize sparsentan as described above or (iv) certain other conditions. We may terminate the license agreement due to a material uncured breach of the agreement by Ligand.
Thiola License Agreement
In 2014, we entered into a license agreement with Mission Pharmacal Company ("Mission"), pursuant to which we obtained an exclusive, royalty-bearing license to market, sell and commercialize Thiola (Tiopronin) in the United States and Canada, and a non-exclusive license to use know-how relating to Thiola to the extent necessary to market Thiola.
We paid Mission an up-front license fee of $3.0 million and through June 30, 2024 will pay guaranteed minimum royalties during each calendar year the greater of $2.0 million or 20% of our net sales of Thiola.
In October 2015, the license agreement was amended to allow for us to secure enough active pharmaceutical ingredient ("API") to ensure an adequate level of safety stock to prevent an interruption in the supply of Thiola and to prepare for a reformulation development project.
In March 2016, the license agreement was amended to, among other things, include a new formulation development project for tiopronin tablets.
Intellectual Property
The proprietary nature of, and protection for, our product candidates and our discovery programs, processes and know-how are important to our business. We have sought patent protection in the United States and certain other jurisdictions for sparsentan, fosmetpantotenate (RE-024), tetracosactide zinc (RE-034) and certain other inventions to which we have rights, where available and when appropriate. Our policy is to pursue, maintain and defend patent rights, whether developed internally or licensed from third parties, and to protect the technology, inventions and improvements that are commercially important to the development of our business. We also rely on trade secrets relating to our proprietary technology that may be important to the development of our business.
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection for our current and future product candidates and the methods used to develop and manufacture them, as well as successfully defending these patents against third-party challenges. Our ability to stop third parties from making, using, selling, offering to sell, or importing our products depends on the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our product candidates, discovery programs and processes.
Sparsentan (RE-021)
Our patent portfolio for sparsentan is comprised of three distinct patent families, two of which are exclusively licensed from Ligand. One of the licensed patent families is owned by BMS, which exclusively licensed it to Ligand (the “BMS patent family”), and the other is owned by Ligand (the “Ligand patent family”). The third patent family is owned by Retrophin (the "Retrophin patent family").
The BMS patent family is directed to sparsentan and structural analogs thereof, and to pharmaceutical compositions containing sparsentan or a structural analog thereof. As of December 31, 2016, this patent family included three U.S. patents (U.S. Patent Nos. 6,638,937, which we refer to herein as the ‘937 patent; 6,835,741; and 6,852,745), of which one (U.S. Patent No. 6,638,937) claims sparsentan and pharmaceutical compositions that contain sparsentan. In addition, as of December 31, 2016, this patent family included a granted European patent and a granted Chinese patent. With the exception of the ‘937 patent, which the U.S. Patent and Trade Office ("USPTO") has determined is entitled to 175 days of patent term adjustment, we expect all U.S. and foreign patents in this patent family to expire in July 2019. In view of the USPTO determination that the ‘937 patent is entitled to 175 days of patent term adjustment, we expect the ‘937 patent to expire in December 2019.
9
The Ligand patent family is directed to methods of using sparsentan in the treatment of various diseases, including glomerulosclerosis. As of December 31, 2016, this patent family included an application pending in the United States (Application Serial No. 14/631,768, filed February 25, 2015), two pending European applications, one of which is allowed, and corresponding applications pending in China, Hong Kong and Japan. We expect any U.S. and foreign patents granted in this patent family to expire in March 2030.
The Retrophin patent family consists of two provisional patent applications filed in 2016.
It is possible, assuming that sparsentan achieves regulatory approval and depending upon the date of any such approval, that the term of the ‘937 patent may be extended up to a maximum of five additional years under the provisions of the Drug Price Competition and Patent Term Restoration Act of 1984, also referred to as the Hatch-Waxman Act. Patent term extension also may be available in certain foreign jurisdictions upon regulatory approval.
Fosmetpantotenate (RE-024)
Our patent portfolio covering compounds for the treatment of PKAN is comprised of four Retrophin-owned patent families. The first of these patent families includes patents and patent applications directed to fosmetpantotenate (RE-024) and structural analogs thereof, pharmaceutical compositions containing fosmetpantotenate (RE-024) or analogs thereof, and methods of using fosmetpantotenate (RE-024) or analogs thereof in the treatment of PKAN. As of December 31, 2016, this patent family included two U.S. patents (U.S. Patent No. 8,673,883, issued March 18, 2014, which we refer to herein as the ‘883 patent, and U.S. Patent No. 9,181,286, issued November 10, 2015), and one pending U.S. patent application (Application Serial No. 14/871,450, filed September 30, 2015). In addition, as of December 31, 2016 this patent family included a granted European patent, a granted Chinese patent and corresponding foreign patent applications pending in Australia, Brazil, Canada, China, Europe, Hong Kong, India, Japan, Korea, Mexico, and Russia. We expect the U.S. and foreign patents in this patent family to expire in April 2033.
Our second PKAN patent family is directed to a chemical genus that encompasses structural analogs of fosmetpantotenate (RE-024), but not fosmetpantotenate (RE-024) itself. As of December 31, 2016, this patent family was comprised of International Patent Application PCT/US2014/062451, filed October 27, 2014. We expect any U.S. or foreign patent granted from this patent family to expire in October 2034.
Our third PKAN patent family is directed to another chemical genus that encompasses structural analogs of fosmetpantotenate (RE-024), but not fosmetpantotenate (RE-024) itself. As of December 31, 2016, this patent family was comprised of an international patent application filed in 2016.
Our fourth PKAN patent family also is directed to a chemical genus that encompasses structural analogs of fosmetpantotenate (RE-024), but not fosmetpantotenate (RE-024) itself. As of December 31, 2016, this patent family was comprised of a U.S. provisional patent application filed in 2016.
It is possible, assuming that fosmetpantotenate (RE-024) achieves regulatory approval and depending upon the date of any such approval, that the term of the ‘883’ patent may be extended up to a maximum of five additional years under the provisions of the Hatch-Waxman Act. Patent term extension also may be available in certain foreign jurisdictions upon regulatory approval. Should we commercialize fosmetpantotenate (RE-024), we may be obligated to pay royalties of up to 5% of net sales of all such products.
Tetracosactide Zinc (RE-034)
Our patent portfolio for tetracosactide zinc (RE-034) is comprised of an international patent application filed in February 2015.
Regulatory Exclusivity
If we obtain marketing approval for sparsentan, fosmetpantotenate (RE-024), tetracosactide zinc (RE-034), or other drug candidates in the United States or in certain jurisdictions outside of the United States, we may be eligible for regulatory exclusivity. For example, in the U.S. an FDA approved product may be eligible to receive five years of new chemical entity exclusivity or, for drugs granted an orphan designation by the FDA, seven years of orphan drug exclusivity. In Europe a new drug product approved by the EMA may receive eight years of data exclusivity and up to 11 years of marketing exclusivity or, in the case of orphan drugs, ten years of data exclusivity. There can be no assurance that we will qualify for any such regulatory exclusivity, or that any such exclusivity will prevent competitors from seeking approval solely on the basis of their own studies. See “Government Regulation” below.
Chenodal
Chenodal received orphan drug designation in the U.S. for the treatment of CTX in 2010. Consequently, if Chenodal gains FDA approval for the treatment of CTX, we expect it will have 7 years of marketing exclusivity in the U.S. for that indication.
Cholbam (Kolbam)
Cholbam received orphan drug designation in the U.S. for the treatment for pediatric and adult patients with bile acid synthesis disorders due to single enzyme defects and for patients with peroxisomal disorders, and therefore is expected to have marketing exclusivity in the U.S. for these indications until March 2022.
Kolbam, the branded name of Cholbam in Europe, received marketing authorization in November 2015 from the EMA for the treatment of inborn errors of primary bile acid synthesis, encompassing select single enzyme defects. We expect Kolbam to have marketing exclusivity in Europe for these indications until September 2024.
Thiola
Thiola does not have regulatory exclusivity in the U.S.
10
Trademarks
Our trademark portfolio includes both Retrophin-owned and Retrophin-licensed trademarks and is comprised of various U.S. and foreign registered trademarks and pending trademark applications relating to our company name, our commercial products (Thiola, Chenodal and Cholbam/Kolbam), and two of our product candidates (i.e. sparsentan and L-UDCA).
More specifically, our trademark portfolio includes a registered U.S. trademark and U.S. and foreign trademark applications for the mark “RETROPHIN”, one U.S. trademark application directed to the Retrophin logo, one registered U.S. trademark and one registered Canadian trademark for the mark “CHENODAL”, one registered U.S. trademark directed to the Chenodal logo, one registered U.S. trademark for the mark “MANCHESTER PHARMACEUTICALS”, one U.S. trademark application for the mark “KEEP IT BELOW THE LINE”, a registered U.S. trademark and foreign trademark applications for the mark “CHOLBAM”, a registered European Community trademark for the mark “KOLBAM”, a registered U.S. trademark for the mark “TOTAL CARE HUB”, a U.S. trademark application directed to the Total Care Hub logo, a U.S. trademark application directed to a leaves logo, U.S. and foreign registered trademarks and pending applications related to sparsentan, and U.S. trademark applications relating to L-UDCA. In addition, under our license agreement with Mission we have an exclusive license to use Mission’s three registered U.S. trademarks and one registered Canadian trademark for the mark “THIOLA”.
Trade Secrets
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. We seek to protect our proprietary data and processes, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors, and partners. These agreements are designed to protect our proprietary information. We also seek to preserve the integrity and confidentiality of our data, trade secrets and know-how by maintaining physical security of our premises and physical and electronic security of our information technology systems. Trade secrets and know-how can be difficult to protect. Consequently, we anticipate that trade secrets and know-how will, over time, be disseminated within the industry through independent development, the publication of journal articles, and the movement of personnel skilled in the art from academic to industry scientific positions.
Manufacturing and Distribution
Nexgen Pharma manufactures Chenodal, New Zealand Pharma manufactures the active pharmaceutical ingredient for Cholbam, Patheon formulates and packages Cholbam, and Mission manufactures Thiola. Dohmen Life Sciences Services (“Dohmen”) is our distributor.
We intend to continue to use our financial resources to accelerate development of our drug candidates rather than diverting resources to establish our own manufacturing facilities. We intend to meet our pre-clinical and clinical trial manufacturing requirements by establishing relationships with third-party manufacturers and other service providers to perform these services for us.
Should any of our drug candidates obtain marketing approval, we anticipate establishing relationships with third-party manufacturers and other service providers in connection with the commercial production of our products. We have some flexibility in securing other manufacturers to produce our drug candidates; however, our alternatives may be limited due to proprietary technologies or methods used in the manufacture of some of our drug candidates.
Sales and Marketing
During fiscal 2016, we continued to utilize our specialty sales force to market our products. In order to commercialize our clinical drug candidates if and when they are approved for sale in the United States or elsewhere, we will need to increase our marketing, sales and distribution capabilities.
Commercialization
Through deep understanding of patient and healthcare provider needs, we believe we are able to:
• | serve patients living with rare disease that have limited treatment options; |
• | drive optimum performance of our marketed products; |
• | educate and train healthcare providers about our products and the diseases for which they are approved to treat; |
• | support access to and reimbursement coverage for our products in the U.S. without significant restrictions; and |
• | minimize the number of patients who discontinue treatment or have low compliance with our products by providing patients with support services and disease education, to the extent and in the manner permitted under applicable laws, to help them maximize the benefits of treatment. |
Our U.S. commercial initiatives are designed to support patients living with rare diseases and clinicians treating these patients. We believe that it is possible to commercialize our products in the U.S. with a relatively small specialty sales force. The primary call points for Thiola include urologists and nephrologists. The primary call points for Cholbam are gastroenterologists, hepatologists, and metabolic specialists. We do not promote Chenodal with our sales force.
Our sales force is differentiated by its high level of experience, averaging more than 15 years in pharmaceutical sales including over five years of experience in rare disease. Our commercial management and operations team has an average of more than 15 years of pharmaceutical experience focused on specialty and rare disease.
11
Our small marketing team, supported by third-party agencies with rare disease experience, drives our commercialization and disease awareness efforts in the U.S. and countries where our products may be approved or available through named patient sales. Specifically, we implement a variety of marketing programs to educate physicians, including direct-to-physician contact by sales representatives, peer-to-peer educational programs, and participation in targeted medical convention programs.
We distribute our products through one direct to patient pharmacy, Dohmen, who also provides our comprehensive patient support services (i.e, the Total Care Hub). This patient support program (for all U.S. commercial products) includes a case-managed approach to patient education, insurance verification and reimbursement support, co-pay and other financial assistance for eligible patients, monitoring and support of adherence, and 24/7 access to pharmacist counseling.
Outside the U.S., including in the EU, we plan to hire local distributors and certain field based personnel as necessary to conduct permitted commercial activities. Our near-term efforts are focused on securing pricing and reimbursement approval for Kolbam.
Medical Affairs
We have a medical affairs team in the U.S. which supports independent medical education programs and investigator-initiated studies by providing education and financial grants in a number of medical and disease-related areas. The responsibilities of medical affairs personnel also include providing education through the dissemination of medical information and publications, providing support in connection with our post-approval clinical commitments, and assisting in organizing scientific and medical advisory boards to obtain input from experts and practitioners on medical topics relevant to our products and diseases.
Government Regulation
Regulation by government authorities in the United States and foreign countries is a significant factor in the development, manufacture and marketing of our proposed products and in our ongoing research and product development activities. All of our products will require regulatory approval by government agencies prior to commercialization. In particular, human therapeutic products are subject to rigorous preclinical studies and clinical trials and other approval procedures of the FDA and similar regulatory authorities in foreign countries. Various federal and state statutes and regulations also govern or influence testing, manufacturing, safety, labeling, storage and record-keeping related to such products and their marketing. The process of obtaining these approvals and the subsequent compliance with appropriate federal and state statutes and regulations require the expenditure of substantial time and financial resources.
FDA Drug Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act, and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications, or NDAs, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution.
We cannot market a drug product candidate in the United States until the drug has received FDA approval. The steps required before a drug may be marketed in the United States generally include the following:
• | completion of extensive pre-clinical laboratory tests, animal studies, and formulation studies in accordance with the FDA’s GLP regulations; |
• | submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin; |
• | performance of adequate and well-controlled human clinical trials in accordance with Good Clinical Practices ("GCP") requirements to establish the safety and efficacy of the drug for each proposed indication; |
• | submission to the FDA of an NDA after completion of all pivotal clinical trials; |
• | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the active pharmaceutical ingredient, or API, and finished drug product are produced and tested to assess compliance with current Good Manufacturing Practices ("cGMPs"); and |
• | FDA review and approval of the NDA prior to any commercial marketing or sale of the drug in the United States. |
Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
Preclinical tests include laboratory evaluation of product chemistry, formulation and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls and a proposed clinical trial protocol. Long term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin. If the FDA raises concerns or questions
12
about the conduct of the trial, such as whether human research subjects will be exposed to an unreasonable health risk, the IND sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted in compliance with federal regulations, including GCP requirements, as well as under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval at each site at which the clinical trial will be conducted. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess pharmacological actions, side effects associated with increasing doses and, if possible, early evidence on effectiveness. Phase 2 usually involves trials in a limited patient population to determine metabolism, pharmacokinetics, the effectiveness of the drug for a particular indication, dosage tolerance and optimum dosage, and to identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 clinical trials, also called pivotal trials, are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In most cases the FDA requires two adequate and well controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 clinical trial with other confirmatory evidence may be sufficient in rare instances where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the United States. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls. The cost of preparing and submitting an NDA is substantial. The submission of most NDAs is additionally subject to a substantial application user fee, and the manufacturer and/or sponsor under an approved NDA are also subject to annual product and establishment user fees. These fees are typically increased annually.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of NDAs. Most such applications for standard review drug products are reviewed within 10 to 12 months; most applications for priority review drugs are reviewed in six to eight months. Priority review can be applied to drugs to treat serious conditions that the FDA determines offer significant improvement in safety or effectiveness. The review process for both standard and priority review may be extended by the FDA for three additional months to consider certain late-submitted information, or information intended to clarify information already provided in the submission.
The FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an advisory committee—typically a panel that includes clinicians and other experts—for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCPs. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with cGMPs is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require risk evaluation and mitigation strategies ("REMS") to ensure that the benefits of the drug outweigh the potential risks. REMS can include a medication guide, a communication plan for healthcare professionals and elements to assure safe use, such as special training and certification requirements for individuals who prescribe or dispense the drug, requirements that patients enroll in a registry and other measures that the FDA deems necessary to assure the safe use of the drug. The requirement for REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs. Such supplements are typically reviewed within 10 months of receipt.
13
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition—generally a disease or condition that affects fewer than 200,000 individuals in the U.S. Orphan drug designation must be requested before submitting an NDA. After the FDA confers orphan drug status, the generic identity of the drug and its potential orphan indication are disclosed publicly by the FDA. Orphan drug designation in and of itself does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. The first NDA applicant to receive FDA approval for a particular active ingredient to treat a particular indication with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the U.S. for that product, for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. Prior to FDA approval, orphan designation provides incentives for sponsors including tax credits for clinical research expenses, the opportunity to obtain government grant funding to support clinical research, and an exemption from FDA user fees.
Fast Track Designation
Fast track is a process designed by the FDA to facilitate the development of drugs to treat serious conditions through expediting their review. The purpose is to get important new drugs to patients earlier. Fast Track addresses a broad range of serious conditions. Determining whether a condition is serious is a matter of judgment, but generally is based on whether the drug will have an impact on such factors as survival, day-to-day functioning, or the likelihood that the condition, if left untreated, will progress from a less severe condition to a more serious one.
A drug that receives Fast Track designation is eligible for some or all of the following:
• | more frequent meetings with FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval; |
• | more frequent written communication from FDA about such things as the design of the proposed clinical trials and use of biomarkers; |
• | eligibility for Accelerated Approval and Priority Review, if relevant criteria are met; and |
• | rolling Review, which means that a drug company can submit completed sections of its Biologic License Application (BLA) or NDA for review by FDA, rather than waiting until every section is completed before the entire application can be reviewed. BLA or NDA review usually does not begin until the drug company has submitted the entire application to the FDA. |
Once a drug receives Fast Track designation, early and frequent communication between the FDA and a drug company is encouraged throughout the entire drug development and review process. The frequency of communication assures that questions and issues are resolved quickly, often leading to earlier drug approval and access by patients.
Accelerated Approval
Under the FDA’s accelerated approval regulations, FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A drug candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow FDA to withdraw the drug from the market on an expedited basis. All promotional materials for drug candidates approved under accelerated regulations are subject to prior review by FDA.
The Hatch-Waxman Amendments: Orange Book Listing
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant’s product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an ANDA. An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing to be therapeutically equivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct, or submit results of, pre-clinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The ANDA applicant may also elect to submit a section viii statement certifying that its proposed ANDA label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. If the applicant does not challenge the listed patents, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
14
A certification that the new product will not infringe the already approved product’s listed patents, or that such patents are invalid, is called a Paragraph IV certification. If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA applicant.
The ANDA application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the referenced product has expired.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet and social media. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, REMS, surveillance to monitor the effects of an approved product, or restrictions on the distribution or use of the product. In addition, quality-control, drug manufacture, packaging and labeling procedures must continue to conform to cGMPs after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality-control to maintain compliance with cGMPs. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or failure to comply with regulatory requirements, may result in, among other things:
• | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
• | fines, warning letters or holds on post-approval clinical trials; |
• | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product approvals; |
• | product seizure or detention, or refusal to permit the import or export of products; or |
• | injunctions or the imposition of civil or criminal penalties. |
Pricing and Reimbursement
A portion of our end-user demand for our drugs comes from patients covered under Medicaid, Medicare and other federal and state government-related programs such as TRICARE and the Department of Veterans Affairs, or the VA. As required by Federal regulations, we will provide rebates and discounts in connection with these programs.
Our commercial success depends in significant part on the extent to which coverage and adequate reimbursement for these products will be available from third-party payers, including government health administration authorities, private health insurers and other organizations. Third-party payers determine which medications they will cover and establish reimbursement levels. Even if a third-party payer covers a particular product, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. Patients who are prescribed medications for the treatment of their conditions, and their prescribing physicians, generally rely on third-party payers to reimburse all or part of the costs associated with their prescription drugs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover all or a significant portion of the cost of our products. Therefore, coverage and adequate reimbursement is critical to product acceptance.
Government authorities and other third-party payers are developing increasingly sophisticated methods of controlling healthcare costs, such as by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payers are requiring that drug companies provide them with predetermined discounts from list prices as a condition of coverage, are using restrictive formularies and preferred drug lists to leverage greater discounts in competitive classes, and are challenging the prices charged for medical products. Third party payers also are carefully evaluating the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy, which may require us to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of our products. Further, no uniform policy requirement for coverage and reimbursement for drug products exists among third-party payers in the United States. Therefore, coverage and reimbursement for drug products can differ significantly from payer to payer. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payer separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
In addition, it is possible that future legislation in the United States and other jurisdictions could be enacted which could potentially impact the coverage and reimbursement rates for our products and also could further impact the levels of discounts and rebates paid to federal and state government entities. Any legislation that impacts these areas could impact, in a significant way, our ability to generate revenues from sales of products that, if successfully developed, we bring to market.
There have been a number of enacted or proposed legislative and regulatory changes affecting the healthcare system and pharmaceutical industry that could affect our commercial success. For example, in March 2010, President Obama signed into law the Patient Protection and Affordable Care
15
Act, as amended by the Health Care and Education Reconciliation Act of 2010, (collectively, the “PPACA”) a law intended to, among other things, broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers and impose additional health policy reforms. In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, or the Budget Resolution, that authorizes the implementation of legislation that would repeal portions of the PPACA. The Budget Resolution is not a law, however, it is widely viewed as the first step toward the passage of legislation that would repeal certain aspects of the PPACA. Further, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the PPACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the PPACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Congress also could consider subsequent legislation to replace elements of the PPACA that are repealed.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. For example, in August 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee on Deficit Reduction did not achieve a targeted deficit reduction of at least $1.2 trillion for fiscal years 2012 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect beginning on April 1, 2013 and will stay in effect through 2024 unless additional Congressional action is taken. Additionally, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals and imaging centers.
Moreover, the Drug Supply Chain Security Act imposes new obligations on manufacturers of pharmaceutical products related to product tracking and tracing. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We are not sure whether additional legislative changes will be enacted, or whether the current regulations, guidance or interpretations will be changed, or what the impact of such changes on our business, if any, may be.
There has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and numerous proposed bills at both the state and federal levels designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, expedite generic competition, review the relationship between pricing and manufacturer patient programs, institute drug reimportation, and reform government program reimbursement methodologies for drugs. We expect that the PPACA, as well as other federal and state healthcare reform measures that have been and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any of our products, and could seriously harm our future revenues.
In addition, it is possible that future legislation in the United States and other jurisdictions could be enacted which could potentially impact the reimbursement rates for the products we are developing and may develop in the future and also could further impact the levels of discounts and rebates paid to federal and state government entities. Any legislation that impacts these areas could impact, in a significant way, our ability to generate revenues from sales of products that, if successfully developed, we bring to market.
Health Care Regulatory Laws
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain business practices in the pharmaceutical industry in recent years. These laws include, without limitation, anti-kickback statutes and false claims laws, data privacy and security laws, and transparency laws regarding payments or other items of value provided to healthcare providers.
The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce; or in return for; purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. This statute has been interpreted broadly to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers, and formulary managers, among others, on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration that may induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the anti-kickback statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the federal anti-kickback statute has been violated. Additionally, the PPACA amended the federal anti-kickback statute to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the PPACA codified case law that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
Federal false claims laws, including the civil False Claims Act, prohibit any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. This includes claims made to programs where the federal government reimburses, such as Medicaid, as well as programs where the federal government is a direct purchaser, such as when it purchases off the Federal Supply Schedule. The False Claims Act contains qui tam provisions, which allow a private individual, or relator, to bring a civil action on behalf of the federal government alleging that the defendant submitted a false claim to the federal government, and to share in any monetary recovery. In recent years, the number of suits brought by private individuals has increased dramatically. For example, pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly inflating drug prices they report to
16
pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate federal false claims laws.
The U.S. Foreign Corrupt Practices Act, and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. Our policies mandate compliance with these anti-bribery laws. We operate in parts of the world that have experienced governmental corruption to some degree and in certain circumstances, strict compliance with antibribery laws may conflict with local customs and practices or may require us to interact with doctors and hospitals, some of which may be state controlled, in a manner that is different than in the United States. We cannot assure you that our internal control policies and procedures will protect us from reckless or criminal acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our business and result in criminal or civil penalties or remedial measures, any of which could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common stock to decline.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payers, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the federal anti-kickback statute, the PPACA amended the intent standard for certain healthcare fraud provisions under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Additionally, the civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
Also, many states have similar fraud and abuse statutes or regulations, including state anti-kickback and false claims laws, that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payer.
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their respective implementing regulations, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH, through its implementing regulations, makes certain of HIPAA’s privacy and security standards directly applicable to business associates, defined as a person or organization, other than a member of a covered entity’s workforce, that creates, receives, maintains or transmits protected health information for or on behalf of a covered entity for a function or activity regulated by HIPAA.
Additionally, the federal Physician Payments Sunshine Act within the PPACA, and its implementing regulations, require that certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to annually report information related to certain payments or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members. Certain states also require implementation of commercial compliance programs and compliance with the pharmaceutical industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government, or otherwise restrict payments or the provision of other items of value that may be made to healthcare providers and other potential referral sources; impose restrictions on marketing practices; require the registration of sales representatives; or require drug manufacturers to track and report information related to payments, gifts and other items of value to physicians and other healthcare providers. We implemented compliance with the Sunshine Act in the first quarter of 2015, as was required.
In addition, several states now require prescription drug companies to report expenses relating to the marketing and promotion of drug products and to report gifts and payments to health care providers and entities in these states. Other states prohibit various other marketing-related activities. Still other states require the posting of information relating to clinical studies and their outcomes. Certain states, such as California and Connecticut, require manufacturers to implement compliance programs and/or marketing codes. Other states, such as Massachusetts and Vermont, impose restrictions on manufacturer marketing practices and require tracking and reporting of gifts, compensation, and other remuneration to healthcare professionals and entities. Several additional states are considering similar proposals. Compliance with these laws is difficult and time consuming, and companies that do not comply with these state laws face civil penalties.
If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to significant penalties, including imprisonment, criminal fines, civil monetary penalties, administrative penalties, disgorgement, and exclusion from participation in federal healthcare programs, contractual damages, injunctions, recall or seizure of products, total or partial suspension of production, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries before we can commence clinical trials and approval of foreign countries or economic areas, such as the European Union, before we may market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
17
Other Laws and Regulatory Processes
We are subject to a variety of financial disclosure and securities trading regulations as a public company in the United States, including laws relating to the oversight activities of the Securities and Exchange Commission (“SEC”), and NASDAQ rules under which our stock is listed. In addition, the Financial Accounting Standards Board (“FASB”), the SEC, and other bodies that have jurisdiction over the form and content of our accounts, our financial statements and other public disclosure are constantly discussing and interpreting proposals and existing pronouncements designed to ensure that companies best display relevant and transparent information relating to their respective businesses.
Our present and future business has been and will continue to be subject to various other laws and regulations. Various laws, regulations and recommendations relating to safe working conditions, laboratory practices, the experimental use of animals, and the purchase, storage, movement, import and export and use and disposal of hazardous or potentially hazardous substances used in connection with our research work are or may be applicable to our activities. Certain agreements entered into by us involving exclusive license rights or acquisitions may be subject to national antitrust regulatory control, the effect of which cannot be predicted. The extent of government regulation which might result from future legislation or administrative action, cannot accurately be predicted.
Available Information
Our website address is www.retrophin.com. We post links on our website to the following filings as soon as reasonably practicable after they are electronically filed with or furnished to the SEC: annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements, and any amendments to those reports filed or furnished pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended. All such filings are available through our website free of charge. Our filings may also be read and copied at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. The SEC also maintains an internet site at www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
ITEM 1A. RISK FACTORS
Table of Contents
Our business, as well as an investment in our common stock, is highly speculative in nature and involves a high degree of risk. Our securities should be purchased only by persons who can afford to lose their entire investment. Carefully consider the risks and uncertainties described below together with all of the other information included herein, including the financial statements and related notes, before deciding to invest in our common stock. If any of the following risks actually occur, they could adversely affect our business, prospects, financial condition and results of operations. In such event(s), the market price of our common stock could decline and result in a loss of part or all of your investment. Accordingly, prospective investors should carefully consider, along with other matters referred to herein, the following risk factors in evaluating our business before purchasing any shares of our common stock.
Risks Related to the Commercialization of Our Products
The commercial success of Chenodal, Cholbam and Thiola depends on them being considered to be effective drugs with advantages over other therapies.
The commercial success of our products Chenodal, Cholbam and Thiola depends on them being considered to be effective drugs with certain advantages over other therapies. A number of factors, as discussed in greater detail below, may adversely impact the degree of acceptance of these products, including their efficacy, safety, price and benefits over competing therapies, as well as the reimbursement policies of third-party payers, such as government and private insurance plans.
If unexpected adverse events are reported in connection with the use of any of these products, physician and patient acceptance of the product could deteriorate and the commercial success of such product could be adversely affected. We are required to report to the FDA events associated with our products relating to death or injury. Adverse events could result in additional regulatory controls, such as a requirement for costly post-approval clinical studies or revisions to our approved labeling which could limit the indications or patient population for a product or could even lead to the withdrawal of a product from the market.
If physicians, patients and third-party payers do not accept our products, we may be unable to generate significant revenues.
Our drugs may not gain or maintain market acceptance among physicians and patients. Effectively marketing our products and any of our drug candidates, if approved, requires substantial efforts, both prior to launch and after approval. Physicians may elect not to prescribe our drugs, and patients may elect not to request or take them, for a variety of reasons including:
• | lower demonstrated efficacy, safety and/or tolerability compared to other drugs; |
• | prevalence and severity of adverse side-effects; |
• | lack of cost-effectiveness; |
• | lack of coverage and adequate reimbursement availability from third-party payers; |
• | a decision to wait for the approval of other therapies in development that have significant perceived advantages over our drug; |
18
• | convenience and ease of administration; |
• | other potential advantages of alternative treatment methods; and |
• | ineffective marketing and/or distribution support. |
If our drugs fail to achieve or maintain market acceptance, we will not be able to generate significant revenues.
Changes in reimbursement practices of third-party payers could affect the demand for our products and the prices at which they are sold.
The business and financial condition of healthcare-related businesses will continue to be affected by efforts of governments and third-party payers to contain or reduce the cost of healthcare through various means. In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval for sparsentan, fosmetpantotenate (RE-024) and L-UDCA, or any other product candidate that we develop, restrict or regulate post-approval activities and affect our ability to profitably sell sparsentan, fosmetpantotenate (RE-024) and L-UDCA or any other product candidate for which we obtain marketing approval.
Our products are sold to patients whose healthcare costs are met by third-party payers, such as government programs, private insurance plans and managed-care programs. These third-party payers are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for medical products and services. Levels of reimbursement, if any, may be decreased in the future, and future healthcare reform legislation, regulations or changes to reimbursement policies of third party payers may otherwise adversely affect the demand for and price levels of our products, which could have a material adverse effect on our sales and profitability.
Economic, social, and congressional pressure may result in individuals and government entities increasingly seeking to achieve cost savings through mechanisms that limit coverage or payment for our products. For example, state Medicaid programs are increasingly requesting manufacturers to pay supplemental rebates and requiring prior authorization for use of drugs. Managed care organizations continue to seek price discounts and, in some cases, to impose restrictions on the coverage of particular drugs. Government efforts to reduce Medicaid expenses may lead to increased use of managed care organizations by Medicaid programs. This may result in managed care organizations influencing prescription decisions for a larger segment of the population and a corresponding constraint on prices and reimbursement for our products.
We may not be able to rely on orphan drug exclusivity for Cholbam/Kolbam or any of our products.
Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for relatively small patient populations as orphan drugs. We have obtained orphan designation for Cholbam/Kolbam in the United States and the European Union. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, that product is entitled to a period of marketing exclusivity, which precludes the applicable regulatory authority from approving another marketing application for the same drug for the same indication for that time period. The applicable period is seven years in the United States and ten years in Europe. Even though we have been awarded orphan drug exclusivity for Cholbam in the United States, we may not be able to maintain it. For example, if a competitive product that contains the same active moiety and treats the same disease as our product is shown to be clinically superior to our product, any orphan drug exclusivity we have obtained will not block the approval of such competitive product and we may effectively lose orphan drug exclusivity. Similarly, if a competitive product that contains the same active moiety and treats the same disease as our product candidate is approved for orphan drug exclusivity before our product candidate, we may not be able to obtain approval for our product candidate until the expiration of the competitive product’s orphan drug exclusivity unless our product candidate is shown to be clinically superior to the competitive product.
Additional competitors could enter the market, including with generic versions of our products, and sales of our affected products may decline materially.
Under the Hatch-Waxman Amendments of the Federal Food, Drug, and Cosmetic Act (the "FDC Act"), a pharmaceutical manufacturer may file an ANDA, seeking approval of a generic copy of an approved innovator product or an NDA under Section 505(b)(2) that relies on the FDA’s prior findings of safety and effectiveness in approving the innovator product. A Section 505(b)(2) NDA may be for a new or improved version of the original innovator product. The Hatch-Waxman Amendments also provide for certain periods of regulatory exclusivity, which preclude FDA approval (or in some circumstances, FDA acceptance) of an ANDA or Section 505(b)(2) NDA. In addition, the FDC Act provides, subject to certain exceptions, a period during which an FDA-approved drug may be afforded orphan drug exclusivity. In addition to the benefits of regulatory exclusivity, an innovator NDA holder may have patents claiming the active ingredient, product formulation or an approved use of the drug, which would be listed with the product in the FDA publication, “Approved Drug Products with Therapeutic Equivalence Evaluations,” known as the “Orange Book.” If there are patents listed in the Orange Book, a generic or Section 505(b)(2) applicant that seeks to market its product before expiration of the patents must include in the ANDA what is known as a “Paragraph IV certification,” challenging the validity or enforceability of, or claiming non-infringement of, the listed patent or patents. Notice of the certification must be given to the innovator, too, and if within 45 days of receiving notice the innovator sues to enforce its patents, approval of the ANDA is stayed for 30 months, or as lengthened or shortened by the court.
Chenodal and Thiola are subject to immediate competition from compounded and generic entrants, as the ANDA and NDA for these drug products have no remaining patent or nonpatent exclusivity. If a generic version is approved, sales of our product would be negatively impacted, which could have a material adverse impact on our sales and profitability.
We are dependent on third parties to manufacture and distribute our pharmaceutical products who may not fulfill their obligations.
We have no manufacturing capabilities and rely on third party manufacturers who are sole source suppliers for manufacturing of Chenodal, Thiola, and Cholbam. The facilities used by our third party manufacturers must be approved by the FDA, or in the case of Kolbam in the European Union, the European Medicines Agency. Our dependence on third parties for the manufacture of our products may harm our profit margin on the sale of products
19
and our ability to deliver products on a timely and competitive basis. If our third party manufacturers are unable to manufacture to specifications or in compliance with applicable regulatory requirements, our ability to commercialize our products will be adversely impacted and could affect our ability to gain market acceptance for our products and negatively impact our revenues.
We currently have no in-house distribution channels for Chenodal, Thiola or Cholbam and we are dependent on a third-party distributor, Dohmen Life Sciences Services, to distribute such products. We rely on this distributor for all of our proceeds from sales of Chenodal, Thiola and Cholbam in the United States. The outsourcing of our distribution function is complex, and we may experience difficulties that could reduce, delay or stop shipments of such products. If we encounter such distribution problems, and we are unable to quickly enter into a similar agreement with another distributor on substantially similar terms, distribution of Chenodal, Thiola and/or Cholbam could become disrupted, resulting in lost revenues, provider dissatisfaction, and/or patient dissatisfaction.
Governments outside the United States tend to impose strict price controls and reimbursement approval policies, which may adversely affect our prospects for generating revenue.
In some countries, particularly European Union countries, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time (6 to 12 months or longer) after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost effectiveness of our product candidate to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our prospects for generating revenue outside of the United States, if any, could be adversely affected and our business may suffer.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our product candidates, we may be unable to generate product revenue outside of the United States.
Risks Related to the Development of our Product Candidates
Our clinical trials may fail to demonstrate the safety and efficacy of our product candidates which could prevent or significantly delay their regulatory approval.
Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and initial results from a clinical trial do not necessarily predict final results. There can be no assurance that the positive results from the DUET study of sparsentan will be repeated in the Phase 3 clinical trial or that the favorable responses we have seen with the physician-initiated treatment of RE-024 in PKAN patients outside the United States will translate to positive data in the Phase 3 clinical trial of RE-024. We cannot assure that any future clinical trials of sparsentan and/or RE-024 will ultimately be successful.
Before obtaining regulatory approval to conduct clinical trials of our product candidates, we must conduct extensive preclinical tests to demonstrate the safety of our product candidates in animals. Preclinical testing is expensive, difficult to design and implement, and can take many years to complete. A failure of one or more of our preclinical studies can occur at any stage of testing.
We will only obtain regulatory approval to commercialize a product candidate if we can demonstrate to the satisfaction of the FDA, and in the case of foreign commercialization, to the applicable foreign regulatory authorities, in well-designed and conducted clinical trials, that our product candidates are safe and effective and otherwise meet the appropriate standards required for approval for a particular indication.
We recently announced plans to initiate a single Phase 3 clinical trial to serve as the basis for an NDA filing for sparsentan for the treatment of FSGS. Although we received feedback from the FDA at an End of Phase 2 meeting during which the FDA communicated that it was open to accepting a substantial treatment effect on proteinuria in this trial as a basis for accelerated approval pursuant to Subpart H of the FDA regulations, there can be no guarantee that the data generated from such interim analysis will be sufficient to serve as the basis for an NDA filing or accelerated approval. In addition, as part of the protocol design for the trial, we will need to conduct sufficient statistical modeling on, and reach agreement with the FDA regarding, the magnitude of proteinuria reduction that would need to be shown to ensure we are able to verify the anticipated benefit (preservation of eGFR) in the post-marketing confirmatory portion of the trial. There is no guarantee that we will be able to satisfactorily conduct such modeling or reach agreement with the FDA on these matters. Furthermore, even if sparsentan is granted accelerated approval under Subpart H, there can be no assurance that the post-marketing confirmatory portion of the trial will support full approval of sparsentan as a treatment for FSGS.
Although we have obtained a Special Protocol Assessment ("SPA") from the FDA for a planned Phase 3 clinical trial of RE-024 for the treatment of PKAN, this agreement does not guarantee any particular outcome from regulatory review. The SPA is intended to provide assurance that if the agreed upon clinical trial protocols are followed and the clinical trial endpoints are achieved, the data may serve as the primary basis for an efficacy claim in support of an NDA. However, a SPA is not a guarantee of an approval of a product candidate or any permissible claims about the product candidate. In particular, a SPA agreement is not binding on the FDA if previously unrecognized public health concerns arise during the performance of the clinical trial, if other new scientific concerns regarding product candidate safety or efficacy arise or if the sponsoring company fails to comply with the agreed upon clinical trial protocols. Moreover, a SPA does not address all of the variables and details that may go into planning for or conducting a clinical trial, and changes in the protocol for a clinical trial can invalidate a SPA or require that the FDA agree in writing to the modified protocol. In addition, while a SPA addresses the requirements for submission of an NDA, the results of the related clinical trial may not support FDA approval. There can be no assurance that the planned Phase 3 clinical trial for RE-024 will demonstrate that RE-024 is safe and effective for treating PKAN or that the data obtained from any such clinical trials will support an application for approval by the FDA.
20
Clinical trials can be lengthy, complex and extremely expensive processes with uncertain results. Our product candidates are intended to treat FSGS and PKAN, each of which is a rare disease. Given that these development candidates are still undergoing required testing, we may not be able to initiate or continue clinical trials if we are unable to locate a sufficient number of eligible patients willing and able to participate in the clinical trials required by the FDA or foreign regulatory agencies. Our inability to enroll a sufficient number of patients for any of our current or future clinical trials would result in significant delays or may require us to abandon one or more clinical trials altogether.
We may experience numerous unforeseen events during, or as a result of, preclinical testing and the clinical trial process that could delay or prevent our ability to obtain regulatory approval or commercialize our product candidates, including:
• | our preclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical testing or clinical trials or we may abandon projects that we expect to be promising; |
• | regulators may require us to conduct studies of the long-term effects associated with the use of our product candidates; |
• | regulators or institutional review boards may not authorize us to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
• | the FDA or any non-United States regulatory authority may impose conditions on us regarding the scope or design of our clinical trials or may require us to resubmit our clinical trial protocols to institutional review boards for re-inspection due to changes in the regulatory environment; |
• | the number of patients required for our clinical trials may be larger than we anticipate or participants may drop out of our clinical trials at a higher rate than we anticipate; |
• | our third-party contractors or clinical investigators may fail to comply with regulatory requirements or fail to meet their contractual obligations to us in a timely manner; |
• | we might have to suspend or terminate one or more of our clinical trials if we, regulators or institutional review boards determine that the participants are being exposed to unacceptable health risks; |
• | regulators or institutional review boards may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements; |
• | the cost of our clinical trials may be greater than we anticipate; |
• | the supply or quality of our product candidates or other materials necessary to conduct our clinical trials may be insufficient or inadequate or we may not be able to reach agreements on acceptable terms with prospective clinical research organizations; and |
• | the effects of our product candidates may not be the desired effects or may include undesirable side effects or the product candidates may have other unexpected characteristics. |
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete our clinical trials or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
• | be delayed in obtaining, or may not be able to obtain, marketing approval for one or more of our product candidates; |
• | obtain approval for indications that are not as broad as intended or entirely different than those indications for which we sought approval; and |
• | have the product removed from the market after obtaining marketing approval. |
Our product development costs will also increase if we experience delays in testing or approvals. We do not know whether any preclinical tests or clinical trials will be initiated as planned, will need to be restructured or will be completed on schedule, if at all. Significant preclinical or clinical trial delays also could shorten the patent protection period during which we may have the exclusive right to commercialize our product candidates. Such delays could allow our competitors to bring products to market before we do and impair our ability to commercialize our products or product candidates.
In addition, we depend on independent clinical investigators and contract research organizations (“CROs”) to conduct our clinical trials under agreements with us. The CROs play a significant role in the conduct of our clinical trials. Failure of the CROs to meet their obligations could adversely affect clinical development of our product candidates. The independent clinical investigators are not our employees and we cannot control the timing or amount of resources they devote to our studies. If their performance is substandard, it could delay or prevent approval of our FDA applications.
FDA approval for a product requires substantial or extensive preclinical and clinical data and supporting documentation. The approval process may take years and may involve on-going requirements as well as post marketing obligations. For example, we have certain post marketing requirements and commitments associated with Cholbam. FDA approval once obtained, may be withdrawn. If the regulatory approval for Thiola, Chenodal and/or Cholbam are withdrawn for any reason, it would have a material adverse impact on our sales and profitability. Further, we face risks relating to the post marketing obligations and commercial acceptance of Cholbam, which was approved by the FDA on March 17, 2015.
21
We face substantial risks related to the commercialization of our product candidates.
We have invested a significant portion of our efforts and financial resources in the development and acquisition of our most advanced product candidates, sparsentan, fosmetpantotenate (RE-024) and L-UDCA. Our ability to generate product revenue from these development stage compounds, which we do not expect will occur for at least the next several years, if ever, may depend heavily on the successful development and commercialization of these product candidates. The successful commercialization of our future product candidates will depend on several factors, including the following:
• | obtaining supplies of sparsentan, fosmetpantotenate (RE-024) and subsequent product candidates for completion of our clinical trials on a timely basis; |
• | successful completion of pre-clinical and clinical studies; |
• | with respect to L-UDCA, our ability to complete the activities necessary to submit an NDA; |
• | obtaining marketing approvals from the FDA and similar regulatory authorities outside the United States; |
• | establishing commercial-scale manufacturing arrangements with third-party manufacturers whose manufacturing facilities are operated in compliance with cGMP regulations; |
• | launching commercial sales of the product, whether alone or in collaboration with others; |
• | acceptance of the product by patients, the medical community and third-party payers; |
• | reimbursement from medical, medicaid or private payers; |
• | competition from other companies; |
• | successful protection of our intellectual property rights from competing products in the United States and abroad; and |
• | a continued acceptable safety and efficacy profile of our product candidates following approval. |
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval or commercialization.
Undesirable side effects caused by our product candidates could interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications, and in turn prevent us from commercializing our product candidates and generating revenues from their sale.
In addition, if any of our product candidates receive marketing approval and we or others later identify undesirable side effects caused by the product:
• | regulatory authorities may require the addition of restrictive labeling statements; |
• | regulatory authorities may withdraw their approval of the product; and |
• | we may be required to change the way the product is administered or conduct additional clinical trials. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase the costs and expenses of commercializing the product candidate, which in turn could delay or prevent us from generating significant revenues from its sale or adversely affect our reputation.
We may not be able to obtain orphan drug exclusivity for our product candidates. If our competitors are able to obtain orphan drug exclusivity for their products that are the same drug as our product candidates, we may not be able to have competing products approved by the applicable regulatory authority for a significant period of time.
Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for relatively small patient populations as orphan drugs. Although we have obtained orphan designation for sparsentan and fosmetpantotenate (RE-024), there can be no assurance that there will be any benefits associated with such designation.
Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, that product is entitled to a period of marketing exclusivity, which precludes the applicable regulatory authority from approving another marketing application for the same drug for the same indication for that time period. The applicable period is seven years in the United States and ten years in Europe. Even if we have orphan drug exclusivity, we may not be able to maintain it. For example, if a competitive product that contains the same active moiety and treats the same disease as our product candidate is shown to be clinically superior to our product candidate, any orphan drug exclusivity we have obtained will not block the approval of such competitive product and we may effectively lose what had previously been orphan drug exclusivity. Similarly, if a competitive product that contains the same active moiety and treats the same disease as our product candidate is approved before our product candidate is approved, we may not be able to obtain approval for our product candidate until the expiration of the competitive product’s orphan drug exclusivity unless our product candidate is shown to be clinically superior to the competitive product.
Risks Related to our Products and Product Candidates
Our products may not achieve or maintain expected levels of market acceptance or commercial success.
22
The success of our products is dependent upon achieving and maintaining market acceptance. Commercializing products is time consuming, expensive and unpredictable. There can be no assurance that we will be able to, either by ourselves or in collaboration with our partners or through our licensees, successfully commercialize new products or current products or gain market acceptance for such products. New product candidates that appear promising in development may fail to reach the market or may have only limited or no commercial success.
Further, the discovery of significant problems with a product similar to one of our products that implicate (or are perceived to implicate) an entire class of products could have an adverse effect on sales of the affected products. Accordingly, new data about our products, or products similar to our products, could negatively impact demand for our products due to real or perceived side effects or uncertainty regarding efficacy and, in some cases, could result in product withdrawal.
Our current products and any products that we bring to the market, including sparsentan, fosmetpantotenate (RE-024) and L-UDCA if they receive marketing approval,may not gain market acceptance by physicians, patients, third-party payers, and others in the medical community. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and we may not become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
• | the prevalence and severity of any side effects, including any limitations or warnings contained in a product’s approved labeling; |
• | the efficacy and potential advantages over alternative treatments; |
• | the pricing of our product candidates; |
• | relative convenience and ease of administration; |
• | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
• | the strength of marketing and distribution support and timing of market introduction of competitive products; |
• | publicity concerning our products or competing products and treatments; and |
• | sufficient third-party insurance coverage or reimbursement. |
Even if a potential or current product displays a favorable efficacy and safety profile in preclinical and clinical trials, market acceptance of the product will not be known until after it is launched. Our efforts to educate patients, the medical community, and third-party payers on the benefits of our product may require significant resources and may never be successful. Such efforts to educate the marketplace may require more resources than are required by the conventional marketing technologies employed by our competitors.
If the market opportunities for our products and product candidates are smaller than we believe they are, our revenues may be adversely affected and our business may suffer.
Certain of the diseases that our current and future product candidates are being developed to address, such as FSGS and PKAN, are relatively rare. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates, may not be accurate.
Currently, most reported estimates of the prevalence of FSGS and PKAN are based on studies of small subsets of the population of specific geographic areas, which are then extrapolated to estimate the prevalence of the diseases in the broader world population. As new studies are performed the estimated prevalence of these diseases may change. There can be no assurance that the prevalence of FSGS and PKAN in the study populations accurately reflect the prevalence of these diseases in the broader world population. If our estimates of the prevalence of FSGS or PKAN or of the number of patients who may benefit from treatment with sparsentan and fosmetpantotenate (RE-024) prove to be incorrect, the market opportunities for our product candidates may be smaller than we believe they are, our prospects for generating revenue may be adversely affected and our business may suffer.
We do not currently have patent protection for certain of our products and product candidates. If we are unable to obtain and maintain protection for the intellectual property relating to our technology and products, the value of our technology and products will be adversely affected.
Our success will depend in large part on our ability to obtain and maintain protection in the United States and other countries for the intellectual property covering, or incorporated into, our technology and products. The patent situation in the field of biotechnology and pharmaceuticals generally is highly uncertain and involves complex legal, technical, scientific and factual questions. We may not be able to obtain additional issued patents relating to our technology or products. Even if issued, patents issued to us or our licensors may be challenged, narrowed, invalidated, held to be unenforceable or circumvented, which could limit our ability to stop competitors from marketing similar products or reduce the term of patent protection we may have for our products. Changes in either patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection. fosmetpantotenate (RE-024) is covered by our U.S. Patent No. 8,673,883, which was granted in 2014 and expires in 2033. In addition, our U.S. Patent No. 9,181,286, which was granted on November 10, 2015 and expires in 2033, covers the use of fosmetpantotenate (RE-024) for the treatment of PKAN. Sparsentan is covered by U.S. Patent No. 6,638,937, which expires in 2019 and to which we have an exclusive license. Our RE-034 formulation is covered by a PCT patent application we filed in February 2016, which claims priority to a U.S. provisional patent application we filed in February 2015.
For products we develop based on a new chemical entity not previously approved by the FDA, we expect that in addition to the protection afforded by our patent filings that we will be able to obtain either five years regulatory exclusivity via the provisions of the FDC Act or seven years regulatory
23
exclusivity via the orphan drug provisions of the FDC Act. In addition, we may be able to obtain up to five years patent term extension (to compensate for regulatory approval delay) for a patent covering such a product.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
• | we or our licensors were the first to make the inventions covered by each of our pending patent applications; |
• | we or our licensors were the first to file patent applications for these inventions; |
• | others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
• | any patents issued to us or our licensors that provide a basis for commercially viable products will provide us with any competitive advantages or will not be challenged by third parties; |
• | we will develop additional proprietary technologies that are patentable; |
• | we will file patent applications for new proprietary technologies promptly or at all; |
• | the claims we make in our patents will be upheld by patent offices in the United States and elsewhere; |
• | our patents will not expire prior to or shortly after commencing commercialization of a product; and |
• | the patents of others will not have a negative effect on our ability to do business. |
We have negotiated a license agreement with Ligand Pharmaceuticals for the rights to sparsentan which we are initially developing for the treatment of FSGS. Further, this license subjects us to various commercialization, reporting and other obligations. If we were to default on our obligations, we could lose our rights to sparsentan. We cannot be certain when or if we will file for patent protection for different indications for sparsentan, if we would be successful in obtaining these patents, or if we would be able to enforce these patents. If we are unsuccessful in obtaining additional patents covering the use of sparsentan for treating FSGS, we may not be able to stop competitors from marketing sparsentan following the latter of expiration of our sparsentan composition of matter patent (i.e. U.S. Patent No. 6,638,937) and expiration of the regulatory exclusivity afforded to sparsentan upon NDA approval.
Our patents also may not afford us protection against competitors with similar technology. Because patent applications in the United States and many other jurisdictions are typically not published until 18 months after filing, or in some cases not at all, and because publications of discoveries in the scientific literature often lag behind the actual discoveries, neither we nor our licensors can be certain that we or they were the first to make the inventions claimed in our or their issued patents or pending patent applications, or that we or they were the first to file for protection of the inventions set forth in these patent applications. If a third party has also filed a United States patent application prior to the effective date of the relevant provisions of the America Invents Act (i.e. before March 16, 2013) covering our product candidates or a similar invention, we may have to participate in an adversarial proceeding, known as an interference, declared by the USPTO to determine priority of invention in the United States. The costs of these proceedings could be substantial and it is possible that our efforts could be unsuccessful, resulting in a loss of our United States patent position.
We cannot assure you that third parties will not assert patent or other intellectual property infringement claims against us with respect to technologies used in our products. If patent infringement suits were brought against us, we may be unable to commercialize some of our products which could severely harm our business. Litigation proceedings, even if not successful, could result in substantial costs and harm our business.
We expect to rely on orphan drug status to develop and commercialize certain of our product candidates, but our orphan drug designations may not confer marketing exclusivity or other expected commercial benefits.
We expect to rely on orphan drug exclusivity for sparsentan and fosmetpantotenate (RE-024) and potential future product candidates that we may develop. Orphan drug status currently confers seven years of marketing exclusivity in the United States under the FDC Act, and up to ten years of marketing exclusivity in Europe for a particular product in a specified indication. The FDA and EMA have granted orphan designation for sparsentan and fosmetpantotenate (RE-024) for the treatment of FSGS and PKAN, respectively. While we have been granted these orphan designations, we will not be able to rely on these designations to exclude other companies from manufacturing or selling these molecules for the same indication beyond these time frames. Furthermore, any marketing exclusivity in Europe can be reduced from ten years to six years if the initial designation criteria have significantly changed since the market authorization of the orphan product.
For any product candidate for which we have been granted orphan drug designation in a particular indication, it is possible that another company also holding orphan drug designation for the same product candidate will receive marketing approval for the same indication before we do. If that were to happen, our applications for that indication may not be approved until the competing company's period of exclusivity expires. Even if we are the first to obtain marketing authorization for an orphan drug indication in the United States, there are circumstances under which a competing product may be approved for the same indication during the seven-year period of marketing exclusivity, such as if the later product is shown to be clinically superior to our orphan product, or if the later product is deemed a different product than ours. Further, the seven-year marketing exclusivity would not prevent competitors from obtaining approval of the same product candidate as ours for indications other than those in which we have been granted orphan drug designation, or for the use of other types of products in the same indications as our orphan product.
Any drugs we develop may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, thereby harming our business.
In March 2010, President Obama signed the PPACA, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries,
24
impose new taxes and fees on the health industry and impose additional health policy reforms. The PPACA revised the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the law imposes a significant annual fee on companies that manufacture or import certain branded prescription drug products. It is likely the PPACA, or a replacement of it under the current administration, will continue to put pressure on pharmaceutical pricing.
If we are unable to obtain coverage and adequate reimbursement from governments or third-party payers for any products that we may develop or if we are unable to obtain acceptable prices for those products, our prospects for generating revenue and achieving profitability will suffer.
Our prospects for generating revenue and achieving profitability will depend heavily upon the availability of coverage and adequate reimbursement for the use of our approved product candidates from governmental and other third-party payers, both in the United States and in other markets. Reimbursement by a third-party payer may depend upon a number of factors, including the third-party payer’s determination that use of a product is:
• | a covered benefit under its health plan; |
• | safe, effective and medically necessary; |
• | appropriate for the specific patient; |
• | cost-effective; and |
• | neither experimental nor investigational. |
Obtaining reimbursement approval for a product from each government or other third-party payer is a time consuming and costly process that could require us to provide supporting scientific, clinical and cost effectiveness data for the use of our products to each payer. We may not be able to provide data sufficient to gain acceptance with respect to reimbursement or we might need to conduct post-marketing studies in order to demonstrate the cost-effectiveness of any future products to such payers’ satisfaction. Such studies might require us to commit a significant amount of management time and financial and other resources. Even when a payer determines that a product is eligible for reimbursement, the payer may impose coverage limitations that preclude payment for some uses that are approved by the FDA or non-United States regulatory authorities. Also prior authorization for a product may be required. In addition, there is a risk that full reimbursement may not be available for high-priced products. Moreover, eligibility for coverage does not imply that any product will be reimbursed in all cases or at a rate that allows us to make a profit or even cover our costs. Interim payments for new products, if applicable, may also not be sufficient to cover our costs and may not be made permanent. A primary trend in the United States healthcare industry and elsewhere is toward cost containment. We expect recent changes in the Medicare program and increasing emphasis on managed care to continue to put pressure on pharmaceutical product pricing.
Further, there has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices, including several recent United States Congressional inquiries and proposed bills at both the state and federal levels designed to, among other things, increase drug pricing transparency, expedite generic competition, review relationships between pricing and manufacturer patient assistance programs, and reform government program drug reimbursement methodologies. Any reduction in reimbursement from Medicare, Medicaid or other government-funded programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our drugs. Additionally, we are currently unable to predict what additional legislation or regulation, if any, relating to the healthcare industry may be enacted in the future or what effect recently enacted federal legislation or any such additional legislation or regulation would have on our business.
We face potential product liability exposure far in excess of our limited insurance coverage.
The use of any of our potential products in clinical trials, and the sale of any approved products, may expose us to liability claims. These claims might be made directly by consumers, health care providers, pharmaceutical companies or others selling our products. We have obtained limited product liability insurance coverage for our clinical trials in the amount of $10 million per occurrence and $10 million in the aggregate. However, our insurance may not reimburse us or may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. We intend to expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for product candidates in development, but we may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. On occasion, juries have awarded large judgments in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us would decrease our cash reserves and could cause our stock price to fall.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do. Our operating results will suffer if we fail to compete effectively.
Several of our competitors have substantially greater financial, research and development, distribution, manufacturing and marketing experience and resources than we do and represent substantial long-term competition for us. Other companies may succeed in developing and marketing products that are more effective and/or less costly than any products that may be developed and marketed by us, or that are commercially accepted before any of our products. Factors affecting competition in the pharmaceutical and drug industries vary, depending on the extent to which a competitor is able to achieve a competitive advantage based on its proprietary technology and ability to market and sell drugs. The industry in which we compete is characterized by extensive research and development efforts and rapid technological progress. Although we believe that our orphan drug status for Cholbam and proprietary position with respect to sparsentan and fosmetpantotenate (RE-024) may give us a competitive advantage, new developments are expected to continue and there can be no assurance that discoveries by others will not render such potential products noncompetitive.
25
Our competitive position also depends on our ability to enter into strategic alliances with one or more large pharmaceutical and contract manufacturing companies, attract and retain qualified personnel, develop effective proprietary products, implement development and marketing plans, obtain patent protection, secure adequate capital resources and successfully sell and market our approved products. There can be no assurance that we will be able to successfully achieve all of the foregoing objectives.
Use of third parties to manufacture and distribute our products and product candidates may increase the risk that we will not have sufficient quantities of our product and product candidates or such quantities at an acceptable cost, and clinical development and commercialization of our product and product candidates could be delayed, prevented or impaired.
We do not own or operate manufacturing facilities for clinical or commercial production of our products. We have limited personnel with experience in drug manufacturing and we lack the resources and the capabilities to manufacture any of our product candidates on a clinical or commercial scale. We outsource all manufacturing and packaging of our preclinical, clinical, and commercial products to third parties. The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling up initial production and in maintaining required quality control. These problems include difficulties with production costs and yields and quality control, including stability of the product candidate.
We do not currently have any agreements with third-party manufacturers for the long-term commercial supply of any of our development stage product candidates. We may be unable to enter into agreements for commercial supply with third-party manufacturers, or may be unable to do so on acceptable terms. Even if we enter into these agreements, the manufacturers of each product candidate will be single source suppliers to us for a significant period of time. Reliance on third-party manufacturers entails risks to which we may not be subject if we manufactured our product candidates or products ourselves, including:
• | reliance on the third party for regulatory compliance and quality assurance; |
• | limitations on supply availability resulting from capacity and scheduling constraints of the third parties; |
• | impact on our reputation in the marketplace if manufacturers of our products fail to meet the demands of our customers; |
• | the possible breach of the manufacturing agreement by the third party because of factors beyond our control; and |
• | the possible termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that is costly or inconvenient for us. |
The failure of any of our contract manufacturers to maintain high manufacturing standards could result in injury or death of clinical trial participants or patients using products. Such failure could also result in product liability claims, product recalls, product seizures or withdrawals, delays or failures in testing or delivery, cost overruns or other problems that could seriously harm our business or profitability.
Our contract manufacturers will be required to adhere to FDA regulations setting forth cGMP. These regulations cover all aspects of the manufacturing, testing, quality control and recordkeeping relating to our product candidates and any products that we may commercialize. Our manufacturers may not be able to comply with cGMP regulations or similar regulatory requirements outside the United States. Our manufacturers are subject to unannounced inspections by the FDA, state regulators and similar regulators outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approval of our product candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect regulatory approval and supplies of our product candidates.
Our product and any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that are both capable of manufacturing for us and willing to do so. If the third parties that we engage to manufacture products for our developmental or commercial products should cease to continue to do so for any reason, we likely would experience interruptions in cash flows and/or delays in advancing our clinical trials while we identify and qualify replacement suppliers, and we may be unable to obtain replacement supplies on terms that are favorable to us. Later relocation to another manufacturer will also require notification, review and other regulatory approvals from the FDA and other regulators and will subject our production to further cost and instability in the availability of our product candidates. In addition, if we are not able to obtain adequate supplies of our product candidates, or the drug substances used to manufacture them, it will be more difficult for us to sell our products and to develop our product candidates. This could greatly reduce our competiveness.
Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins and our ability to develop product candidates and commercialize any products that obtain regulatory approval on a timely and competitive basis.
Materials necessary to manufacture our products and product candidates may not be available on commercially reasonable terms, or at all, which may delay the development and commercialization of our products and product candidates.
We rely on the manufacturers of our products and product candidates to purchase from third-party suppliers the materials necessary to produce the compounds for our preclinical and clinical studies and rely on these other manufacturers for commercial distribution if we obtain marketing approval for any of our product candidates. Suppliers may not sell these materials to our manufacturers at the time we need them or on commercially reasonable terms and all such prices are susceptible to fluctuations in price and availability due to transportation costs, government regulations, price controls, and changes in economic climate or other foreseen circumstances. We do not have any control over the process or timing of the acquisition of these materials by our manufacturers. Moreover, we currently do not have any agreements for the commercial production of these materials. If our manufacturers are
26
unable to obtain these materials for our preclinical and clinical studies, product testing and potential regulatory approval of our product candidates would be delayed, significantly impacting our ability to develop our product candidates. If our manufacturers or we are unable to purchase these materials after regulatory approval has been obtained for our product candidates, the commercial launch of our product candidates would be delayed or there would be a shortage in supply, which would materially affect our ability to generate revenues from the sale of our product candidates.
Risks Related to Our Business
Our limited operating history makes it difficult to evaluate our future prospects, and our profitability in the future is uncertain.
We face the problems, expenses, difficulties, complications and delays, many of which are beyond our control, associated with any business in its early stages and have a limited operating history on which an evaluation of our prospects can be made. Such prospects should be considered in light of the risks, expenses and difficulties frequently encountered in the establishment of a business in a new industry, characterized by a number of market entrants and intense competition, and in the shift from development to commercialization of new products based on innovative technologies.
We have experienced significant growth in the number of our employees and the scope of our operations. We began 2014 with 26 employees and currently have approximately 135 employees, having added sales and marketing, compliance and legal functions in addition to expansion of all functions to support a commercial organization. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability on the part of our management to manage growth could delay the execution of our business plans or disrupt our operations.
Factors that may inhibit our efforts to commercialize our products without strategic partners or licensees include:
• | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
• | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe our products; |
• | the lack of complementary products to be offered by our sales personnel, which may put us at a competitive disadvantage against companies with broader product lines; |
• | unforeseen costs associated with expanding our own sales and marketing team for new products or with entering into a partnering agreement with an independent sales and marketing organization; and |
• | efforts by our competitors to commercialize competitive products. |
Moreover, though we generate revenues from product sales arrangements, we may incur significant operating losses over the next several years. Our ability to achieve profitable operations in the future will depend in large part upon successful in-licensing of products approved by the FDA, selling and manufacturing these products, completing development of our products, obtaining regulatory approvals for these products, and bringing these products to market. The likelihood of the long-term success of our company must be considered in light of the expenses, difficulties and delays frequently encountered in the development and commercialization of new drug products, competitive factors in the marketplace, as well as the regulatory environment in which we operate.
In addition, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors.
We will likely experience fluctuations in operating results and could incur substantial losses.
We expect that our operating results will vary significantly from quarter-to-quarter and year-to-year as a result of investments in research and development, specifically our clinical and preclinical development activities. We have not completed development of any drugs and we anticipate that our expenses will increase substantially as we:
• | continue the open label portion of DUET and conduct the planned Phase 3 trial of sparsentan; |
• | continue our ongoing clinical development of fosmetpantotenate (RE-024) for the treatment of PKAN; |
• | complete requirements necessary for an NDA filing of L-UDCA; |
• | continue the research and development of additional product candidates; |
• | expand our sales and marketing infrastructure to commercialize our current products and any new products for which we may obtain regulatory approval; and |
• | expand operational, financial, and management information systems and personnel, including personnel to support product development efforts and our obligations as a public company. |
To attain and sustain profitability, we must succeed in developing and commercializing drugs with significant market potential. This will require us to be successful in a range of challenging activities, including the discovery of product candidates, successful completion of preclinical testing and clinical trials of our product candidates, obtaining regulatory approval for these product candidates and manufacturing, marketing and selling those products for which we may obtain regulatory approval. We are only in the preliminary stages of these activities. We may not be successful enough in these activities to generate revenues that are substantial enough to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become or remain profitable could depress the market price of our common stock
27
and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations. A decline in the market price of our common stock may also cause a loss of a part or all of your investment.
Negative publicity regarding any of our products could impair our ability to market any such product and may require us to spend time and money to address these issues.
If any of our products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to consumers and/or subject to FDA enforcement action, our ability to successfully market and sell our products could be impaired. Because of our dependence on patient and physician perceptions, any adverse publicity associated with illness or other adverse effects resulting from the use or misuse of our products or any similar products distributed by other companies could limit the commercial potential of our products and expose us to potential liabilities.
We may not have sufficient insurance to cover our liability in any current or future litigation claims either due to coverage limits or as a result of insurance carriers seeking to deny coverage of such claims.
We face a variety of litigation-related liability risks. Our certificate of incorporation, bylaws, other applicable agreements, and/or Delaware law require us to indemnify (and advance expenses to) our current and past directors and officers and employees from reasonable expenses related to the defense of any action arising from their service to us, including circumstances under which indemnification is otherwise discretionary. While our directors and officers are included in a director and officer liability insurance policy, which covers all our directors and officers in some circumstances, our insurance coverage does not cover all of our indemnification obligations and may not be adequate to cover any indemnification or other claims against us. In addition, the underwriters of our present coverage may seek to avoid coverage in certain circumstances based upon the terms of the respective policies. If we incur liabilities that exceed our coverage under our directors and officers insurance policy or incur liabilities not covered by our insurance, we would have to self-fund any indemnification amounts owed to our directors and officers and employees in which case our results of operations and financial condition could be materially adversely affected. Further, if D&O insurance becomes prohibitively expensive to maintain in the future, we may be unable to renew such insurance on economic terms or unable renew such insurance at all. The lack of D&O insurance may make it difficult for us to retain and attract talented and skilled directors and officers to serve our company, which could adversely affect our business
We may need substantial funding and may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect our general and research and development expenses to increase in connection with our ongoing and planned activities, particularly as we conduct Phase 3 clinical trials of sparsentan and RE-024, complete requirements for filings of L-UDCA, and conduct any other later-stage clinical trials of our product candidates. In addition, subject to obtaining regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales and marketing, securing commercial quantities of product from our manufacturers, and product distribution. We currently have no additional commitments or arrangements for any additional financing to fund the research and development and commercial launch of our product candidates.
Management believes our ability to continue our operations depends on our ability to sustain and grow revenue, results of operations and our ability to access capital markets when necessary to accomplish our strategic objectives. Management believes that we may incur losses in the immediate future. For the twelve months ended December 31, 2016, we generated a positive cash flow from operations; however, we expect that our operating results will vary significantly from quarter-to-quarter and year-to-year as a result of investments in research and development, specifically our clinical and preclinical development activities. We expect to finance our cash needs from cash on hand and results of operations, and depending on results of operations we may either need additional equity or debt financing, or need to enter into strategic alliances on products in development to continue our operations until we can achieve sustained profitability and positive cash flows from operating activities. Additional funds may not be available to us when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to reduce or eliminate research development programs or commercial efforts.
Our future capital requirements will depend on many factors, including:
• | the progress and results of our pre-clinical and clinical studies of sparsentan and fosmetpantotenate (RE-024) and other drug candidates; |
• | the costs, timing and outcome of regulatory review of our product candidates; |
• | the number and development requirements of other product candidates that we pursue; |
• | the costs of commercialization activities, including product marketing, sales and distribution; |
• | the emergence of competing technologies and other adverse market developments; |
• | the costs of preparing, filing and prosecuting patent applications and maintaining, enforcing and defending intellectual property related claims; |
• | the extent to which we acquire or invest in businesses, products and technologies; and |
• | our ability to establish collaborations and obtain milestone, royalty or other payments from any such collaborators. |
The market price for shares of our common stock may be volatile and purchasers of our common stock could incur substantial losses.
The price of our stock is likely to be volatile. The stock market in general, and the market for biotechnology companies in particular, have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. The market price for our common stock may be influenced by many factors, including:
28
• | results of clinical trials of our product candidates or those of our competitors; |
• | our entry into or the loss of a significant collaboration; |
• | regulatory or legal developments in the United States and other countries, including changes in the health care payment systems; |
• | our ability to obtain and maintain marketing approvals from the FDA or similar regulatory authorities outside the United States; |
• | variations in our financial results or those of companies that are perceived to be similar to us; |
• | changes in the structure of healthcare payment systems; |
• | market conditions in the pharmaceutical and biotechnology sectors and issuance of new or changed securities analysts’ reports or recommendations; |
• | general economic, industry and market conditions; |
• | results of clinical trials conducted by others on drugs that would compete with our product candidates; |
• | developments or disputes concerning patents or other proprietary rights; |
• | public concern over our product candidates or any products approved in the future; |
• | litigation; |
• | communications from government officials regarding health care costs or pharmaceutical pricing; |
• | future sales or anticipated sales of our common stock by us or our stockholders; and |
• | the other factors described in this “Risk Factors” section. |
In addition, the stock markets, and in particular, the NASDAQ Global Market, have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many pharmaceutical companies. The realization of any of the above risks or any of a broad range of other risks, including those described in these “Risk Factors” could have a dramatic and material adverse impact on the market price of our common stock.
We may be unable to successfully integrate new products or businesses we may acquire.
We intend to expand our product pipeline by pursuing acquisition of pharmaceutical products. If an acquisition is consummated, the integration of the acquired business, product or other assets into our company may also be complex and time- consuming and, if such businesses, products and assets are not successfully integrated, we may not achieve the anticipated benefits, cost-savings or growth opportunities. Potential difficulties that may be encountered in the integration process include the following:
• | integrating personnel, operations and systems, while maintaining focus on producing and delivering consistent, high quality products; |
• | coordinating geographically dispersed organizations; |
• | distracting employees from operations; |
• | retaining existing customers and attracting new customers; and |
• | managing inefficiencies associated with integrating the operations of the Company. |
Furthermore, these acquisitions and other arrangements, even if successfully integrated, may fail to further our business strategy as anticipated, expose us to increased competition or challenges with respect to our products or geographic markets, and expose us to additional liabilities associated with an acquired business, product, technology or other asset or arrangement. Any one of these challenges or risks could impair our ability to realize any benefit from our acquisitions or arrangements after we have expended resources on them.
If we are unable to maintain an effective and specialized sales force, we will not be able to commercialize our products successfully.
In order to successfully commercialize our products, we have built a specialized sales force. Factors that may hinder our ability to successfully market and commercially distribute our products include:
• | inability of sales personnel to obtain access to or convince adequate numbers of physicians to prescribe our products; |
• | inability to recruit, retain and effectively manage adequate numbers of effective sales personnel; |
• | lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies that have more extensive product lines; and |
• | unforeseen delays, costs and expenses associated with maintaining our sales organization. |
If we are unable to maintain our sales force for our products, we may not be able to generate sufficient product revenue.
We will need to continue to expend significant time and resources to train our sales forces to be credible, persuasive and compliant in discussing our products with the specialists treating the patients indicated under the product’s label. In addition, if we are unable to effectively train our sales force and
29
equip them with effective marketing materials our ability to successfully commercialize our products could be diminished, which would have a material adverse effect on our business, results of operations and financial condition.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
Our business exposes us to potential liability risks inherent in the research, development, manufacturing and marketing of pharmaceutical products. If any of our product candidates in clinical trials or marketed products harm people we may be subject to costly and damaging product liability claims. We have clinical trial insurance and commercial product liability coverage. However, this insurance may not be adequate to cover all claims. We may be exposed to product liability claims and product recalls, including those which may arise from misuse or malfunction of, or design flaws in, such products, whether or not such problems directly relate to the products and services we have provided. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
• | decreased demand for any product candidates or products that we may develop; |
• | damage to our reputation; |
• | regulatory investigations that could require costly recalls or product modifications; |
• | withdrawal of clinical trial participants; |
• | costs to defend the related litigation; |
• | substantial monetary awards to trial participants or patients, including awards that substantially exceed our product liability insurance, which we would then be required to pay from other sources, if available, and would damage our ability to obtain liability insurance at reasonable costs, or at all, in the future; |
• | loss of revenue; |
• | the diversion of management’s attention from managing our business; and |
• | the inability to commercialize any products that we may develop. |
A successful product liability claim or a series of claims brought against us could cause our stock price to fall and, if judgments exceed our insurance coverage, could decrease our available cash and adversely affect our business.
We are involved in various litigation matters, any of which could result in substantial costs, divert management's attention and otherwise have a material adverse effect on our business, operating results or financial condition.
We are involved in various litigation matters, each described in Note 11 of the Consolidated Financial Statements included in this report. Although we intend to vigorously defend any claims for which we have been named as a defendant, there is no guarantee that we will be successful and we may have to pay damages awards or otherwise may enter into settlement arrangements in connection with such claims. Any such payments or settlement arrangements could have material adverse effects on our business, operating results or financial condition. Even if the pending claims are not successful, litigation with respect to such claims could result in substantial costs and significant adverse impact on our reputation and divert management's attention and resources, which could have a material adverse effect on our business, operating results or financial condition.
We are subject to significant ongoing regulatory obligations and oversight, which may result in significant additional expense and may limit our commercial success.
We are subject to significant ongoing regulatory obligations, such as safety reporting requirements and additional post-marketing obligations, including regulatory oversight of the promotion and marketing of our products. In addition, the manufacture, quality control, labeling, packaging, safety surveillance, adverse event reporting, storage and recordkeeping for our products are subject to extensive and ongoing regulatory requirements. If we become aware of previously unknown problems with any of our products, a regulatory agency may impose restrictions on our products, our contract manufacturers or us. If we, our products and product candidates, or the manufacturing facilities for our products and product candidates fail to comply with applicable regulatory requirements, a regulatory agency, including the FDA, may send enforcement letters, mandate labeling changes, suspend or withdraw regulatory approval, suspend any ongoing clinical trials, refuse to approve pending applications or supplements filed by us, suspend or impose restrictions on manufacturing operations, request a recall of, seize or detain a product, seek criminal prosecution or an injunction, or impose civil or criminal penalties or monetary fines. In such instances, we could experience a significant drop in the sales of the affected products, our product revenues and reputation in the marketplace may suffer, and we could become the target of lawsuits.
We are also subject to regulation by national, regional, state and local agencies, including but not limited to the FDA, Centers for Medicare and Medicaid Services, Department of Justice, the Federal Trade Commission, the Office of Inspector General of the U.S. Department of Health and Human Services and other regulatory bodies. The FDC Act, Social Security Act, Public Health Service Act and other federal and state statutes and regulations govern to varying degrees the research, development, manufacturing and commercial activities relating to prescription pharmaceutical products, including preclinical testing, clinical research, approval, production, labeling, sale, distribution, post-market surveillance, advertising, dissemination of information, promotion, marketing, and pricing to government purchasers and government health care programs. Our manufacturing partners are subject to many of the same requirements.
30
Companies may not promote drugs for “off-label” uses-that is, uses that are not described in the product’s labeling and that differ from those approved by the FDA or other applicable regulatory agencies. A company that is found to have improperly promoted off-label uses may be subject to significant liability, including civil and administrative remedies as well as criminal sanctions. In addition, management’s attention could be diverted from our business operations and our reputation could be damaged.
The federal health care program Anti-Kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any health care item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted broadly to apply to arrangements that pharmaceutical companies have with prescribers, purchasers and formulary managers, among others. Further, the PPACA, among other things, amends the intent requirement of the federal anti-kickback statute so that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act. Although there are a number of statutory exceptions and regulatory safe harbors under the federal anti-kickback statute protecting certain common manufacturer business arrangements and activities from prosecution, the exceptions and safe harbors are drawn narrowly and an arrangement must meet all of the conditions specified in order to be fully protected from scrutiny under the federal anti-kickback statute. We seek to comply with the exceptions and safe harbors whenever possible, but our practices, such as our patient assistance programs and prompt pay discounts with certain customers, may not in all cases meet all of the criteria for protection from anti-kickback liability and may be subject to scrutiny.
The federal false claims laws, including the Federal False Claims Act, prohibit any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Many pharmaceutical and other health care companies have been investigated and have reached substantial financial settlements with the federal government under the Federal False Claims Act for a variety of alleged marketing activities, including providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees, grants, free travel, and other benefits to physicians to induce them to prescribe the company’s products; and inflating prices reported to private price publication services, which may be used by states to set drug payment rates under government health care programs. Companies have been prosecuted for causing false claims to be submitted because of the marketing of their products for unapproved uses. Pharmaceutical and other health care companies have also been prosecuted on other legal theories of Medicare and Medicaid fraud.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. It is not clear whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of any Retrophin products, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject Retrophin to more stringent product labeling and post-marketing testing and other requirements.
Many states also have statutes or regulations similar to the federal Anti-Kickback Statute and False Claims Act and civil monetary penalty laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, which apply regardless of the payer. In addition, several states require pharmaceutical companies to implement compliance programs or marketing codes as does the U.S. Department of Health and Human Services
We also could become subject to government investigations and related subpoenas. Such subpoenas are often associated with previously filed qui tam actions, or lawsuits filed under seal under the Federal False Claims Act. Qui tam actions are brought by private plaintiffs suing on behalf of the federal government for alleged violations of the Federal False Claims Act. The time and expense associated with responding to such subpoenas, and any related qui tam or other actions, may be extensive, and we cannot predict the results of our review of the responsive documents and underlying facts or the results of such actions. Responding to government investigations, defending any claims raised, and any resulting fines, restitution, damages and penalties, settlement payments or administrative actions, as well as any related actions brought by stockholders or other third parties, could have a material impact on our reputation, business and financial condition and divert the attention of our management from operating our business.
The number and complexity of both federal and state laws continues to increase, and additional governmental resources are being added to enforce these laws and to prosecute companies and individuals who are believed to be violating them. In particular, the PPACA includes a number of provisions aimed at strengthening the government’s ability to pursue anti-kickback and false claims cases against pharmaceutical manufacturers and other healthcare entities, including substantially increased funding for healthcare fraud enforcement activities, enhanced investigative powers, amendments to the federal False Claims Act that make it easier for the government and whistleblowers to pursue cases for alleged kickback and false claim violations and, for payments made on or after August 1, 2013, public reporting of payments by pharmaceutical manufacturers to physicians and teaching hospitals nationwide. While it is too early to predict the full effect these changes will have on our business, we anticipate that government scrutiny of pharmaceutical sales and marketing practices will continue for the foreseeable future and subject us to the risk of further government investigations and enforcement actions. Responding to a government investigation or enforcement action would be expensive and time-consuming, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
The U.S. Foreign Corrupt Practices Act, and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. Our policies mandate compliance with these anti-bribery laws. We operate in parts of the world that have experienced governmental corruption to some degree and in certain circumstances, strict compliance with antibribery laws may conflict with local customs and practices or may require us to interact with doctors and hospitals, some of which may be state controlled, in a manner that is different than in the United States. We cannot assure you that our internal control policies and procedures will protect us from reckless or criminal acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our
31
business and result in criminal or civil penalties or remedial measures, any of which could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common stock to decline.
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. The Federal Health Insurance Portability and Accountability Act of 1996 ("HIPAA"), as amended by the Health Information Technology for Economic and Clinical Health Act ("HITECH"), and their respective implementing regulations, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information.
Additionally, the federal Physician Payments Sunshine Act within the PPACA, and its implementing regulations, require that certain manufacturers of drugs, devices, biologicals and medical supplies to report annually information related to certain payments or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members. Moreover, the Drug Supply Chain Security Act imposes new obligations on manufacturers of pharmaceutical products related to product tracking and tracing. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We are not sure whether additional legislative changes will be enacted, or whether the current regulations, guidance or interpretations will be changed, or what the impact of such changes on our business, if any, may be. Several states now require pharmaceutical companies to report their expenses relating to the marketing and promotion of pharmaceutical products in those states and to report gifts and payments to certain individual health care providers in those states. Some of these states also prohibit certain marketing-related activities, including the provision of gifts, meals, and other items to certain health care providers.
If we or any of our partners fail to comply with applicable regulatory requirements, we or they could be subject to a range of regulatory actions that could affect our or our partners' ability to commercialize our products and could harm or prevent sales of the affected products, or could substantially increase the costs and expenses of commercializing and marketing our products. Any threatened or actual government enforcement action could also generate adverse publicity and require that we devote substantial resources that could otherwise be used in other aspects of our business. Compliance with applicable federal and state laws is difficult and time consuming, and companies that violate them may face substantial penalties. The potential sanctions include criminal fines, civil monetary penalties, administrative penalties, disgorgement, exclusion from participation in federal health care programs, imprisonment, injunctions, recall or seizure of products, and other sanctions. Because of the breadth of these laws, it is possible that some of our business activities could be subject to challenge under one or more of these laws. Such a challenge, irrespective of the underlying merits of the challenge or the ultimate outcome of the matter, could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
If we are not able to obtain and maintain required regulatory approvals, we will not be able to commercialize our products, and our ability to generate revenue will be materially impaired.
Our product candidates, once approved, and the activities associated with their manufacture, marketing, distribution, and sales are subject to extensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Failure to adhere to regulations set out by these bodies for one or more of our commercial products could prevent us from commercializing the product candidate in the jurisdiction of the regulatory authority. We have only limited experience in meeting the regulatory requirements incumbent on the sale of drugs in the United States and elsewhere, and expect to rely on third-parties to assist us in these processes. If these third parties fail to adequately adhere to the regulations governing drug distribution and promotion we may be unable to sell our products, which could have a material effect on our ability to generate revenue.
Our product candidates and the activities associated with their development and commercialization, including testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Failure to obtain regulatory approval for a product candidate will prevent us from commercializing the product candidate in the jurisdiction of the regulatory authority. We have only limited experience in filing and prosecuting the applications necessary to obtain regulatory approvals and expect to rely on third-party contract research organizations to assist us in this process.
Securing FDA approval requires the submission of extensive preclinical and clinical data and supporting information to the FDA for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing FDA approval also requires the submission of information about the product manufacturing process to, and successful inspection of manufacturing facilities by, the FDA. Our future products may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining regulatory approval or prevent or limit commercial use.
Our product candidates may fail to obtain regulatory approval for many reasons, including:
• | our failure to demonstrate to the satisfaction of the FDA or comparable regulatory authorities that a product candidate is safe and effective for a particular indication; |
• | the results of clinical trials may not meet the level of statistical significance required by the FDA or comparable regulatory authorities for approval; |
• | our inability to demonstrate that a product candidate’s benefits outweigh its risks; |
• | our inability to demonstrate that the product candidate presents an advantage over existing therapies; |
• | the FDA’s or comparable regulatory authorities’ disagreement with the manner in which we interpret the data from preclinical studies or clinical trials; |
32
• | failure of the third-party manufacturers with which we contract for clinical or commercial supplies to satisfactorily complete an FDA pre-approval inspection of the facility or facilities at which the product is manufactured to assess compliance with the FDA’s cGMP regulations to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
• | a change in the approval policies or regulations of the FDA or comparable regulatory authorities or a change in the laws governing the approval process. |
The process of obtaining regulatory approvals is expensive, often takes many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Changes in regulatory approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application may cause delays in the approval or rejection of an application. The FDA and non-United States regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post approval commitments that render the approved product not commercially viable. Any FDA or other regulatory approval of our product candidates, once obtained, may be withdrawn, including for failure to comply with regulatory requirements or if clinical or manufacturing problems follow initial marketing.
Our internal computer systems, or those of our CROs or other contractors and vendors who host our applications or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
Despite the implementation of security measures, our internal computer systems and those of our CROs and other contractors vendors who host our applications and consultants are vulnerable to damage or disruption from computer viruses, software bugs, unauthorized access including cyber-attack, natural disasters, terrorism, war, and telecommunication, equipment and electrical failures. While we have not, to our knowledge, experienced any significant system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our programs. For example, the loss of clinical trial data from completed or ongoing clinical trials for any of our product candidates could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure or theft of confidential or proprietary information, we could incur liability, the further development of our product candidates could be delayed, our competitive position could be compromised, or our business reputation could be harmed.
We face risks related to research and the ability to develop new drugs.
Our growth and survival depends on our ability to consistently discover, develop and commercialize new products and find new and improve on existing technology and platforms. As such, if we fail to make sufficient investments in research, be attentive to consumer needs or do not focus on the most advanced technology, our current and future products could be surpassed by more effective or advanced products of other companies.
Risks Related to our Indebtedness and Investments
Our indebtedness could adversely affect our financial condition.
As of December 31, 2016, we had approximately $46.0 million of total debt outstanding, classified as long term. The total debt outstanding relates to a Note Purchase Agreement dated May 29, 2014 for the private placement of $46.0 million aggregate senior secured notes (the “Notes”). As a result of our indebtedness, a portion of our cash flow will be required to pay interest and principal on the Notes if the Notes are not converted to shares of common stock prior to maturity. We may not generate sufficient cash flow from operations or have future borrowings available to enable us to repay our indebtedness or to fund other liquidity needs.
Our indebtedness pursuant to the Notes could have important consequences. For example, it could:
• | make it more difficult for us to satisfy our obligations with respect to any other debt we may incur in the future; |
• | increase our vulnerability to general adverse economic and industry conditions; |
• | require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness and related interest, thereby reducing the availability of our cash flow to fund working capital, capital expenditures and other general corporate purposes; |
• | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
• | increase our cost of borrowing; |
• | place us at a competitive disadvantage compared to our competitors that may have less debt; and |
• | limit our ability to obtain additional financing for working capital, capital expenditures, acquisitions, debt service requirements or general corporate purposes. |
We expect to use cash flow from operations and outside financings to meet our current and future financial obligations, including funding our operations, debt service and capital expenditures. Our ability to make these payments depends on our future performance, which will be affected by financial, business, economic and other factors, many of which we cannot control. Our business may not generate sufficient cash flow from operations in the future, which could result in our being unable to repay indebtedness, or to fund other liquidity needs. If we do not generate sufficient cash from operations, we may be forced to reduce or delay our business activities and capital expenditures, sell assets, obtain additional debt or equity capital or restructure
33
or refinance all or a portion of our debt, including the Notes, on or before maturity. We cannot make any assurances that we will be able to accomplish any of these alternatives on terms acceptable to us, or at all. In addition, the terms of existing or future indebtedness may limit our ability to pursue any of these alternatives.
A default under the Notes may have a material adverse effect on our financial condition.
If an event of default under the Notes occurs, the principal amount of the Notes, plus accrued and unpaid interest (including additional interest, if any) may be declared immediately due and payable, subject to certain conditions set forth in the indenture governing such notes. Events of default include, but are not limited to:
• | failure to pay (for more than 30 days) interest when due; |
• | failure to pay principal when due; |
• | failure to deliver shares of common stock upon conversion of a Note; |
• | failure to provide notice of a fundamental change; |
• | acceleration on our other indebtedness in excess of $10 million (other than indebtedness that is non-recourse to us); or |
• | certain types of bankruptcy or insolvency involving us. |
Accordingly, the occurrence of a default under the Notes, unless cured or waived, may have a material adverse effect on our results of operations.
The Notes are structurally subordinated to all obligations of our subsidiaries.
The Notes are our obligations and are structurally subordinated to all indebtedness and other obligations, including trade payables, of our subsidiaries. The effect of this structural subordination is that, in the event of a bankruptcy, liquidation, dissolution, reorganization or similar proceeding involving a subsidiary which is not a guarantor of the Notes, the assets of the affected entity could not be used to pay noteholders until after all other claims against that subsidiary, including trade payables, have been fully paid.
Provisions of the Notes could discourage an acquisition of us by a third party.
Certain provisions of the Notes could make it more difficult or more expensive for or prevent a third party to acquire us. Upon the occurrence of certain transactions constituting a fundamental change, holders of the Notes will have the right, at their option, to require us to repurchase all of their Notes or any portion of the principal amount of such Notes in integral multiples of $1,000. We may also be required to increase the conversion rate for conversions in connection with certain fundamental changes.
Conversion of the Notes may dilute the ownership interest of existing stockholders, including holders who had previously converted their Notes.
To the extent we issue shares of common stock upon conversion of the Notes, the conversion of some or all of the Notes will dilute the ownership interests of existing stockholders. Any sales in the public market of shares of the common stock issuable upon such conversion could adversely affect prevailing market prices of shares of our common stock. In addition, the existence of the Notes may encourage short selling by market participants because the conversion of the Notes could depress the price of shares of our common stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
We lease the following locations to conduct our business:
Location | Address | Lease Expiration | Square Feet |
San Diego, California (corporate headquarters) | 3721 Valley Centre Drive, Suite 200 | July 31, 2024 | 23,107 |
Cambridge, Massachusetts | 141 Portland Street | July 31, 2017 | 15,887 |
We believe these facilities are adequate to conduct our business.
ITEM 3. LEGAL PROCEEDINGS
The information required by this Item is incorporated herein by reference to Notes to the audited Consolidated Financial Statements-Note 11 Commitments and Contingencies: Legal Proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
34
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is listed for quotation on the NASDAQ Global Market under the trading symbol “RTRX” and is part of the NASDAQ Biotechnology Index (NASDAQ: NBI).
As of February 28, 2017, the last reported sale price of our Common Stock as reported by the NASDAQ was $21.27. The following table sets forth the high and low sales prices for our common stock for each full quarterly period within the two most recent fiscal years as reported by the NASDAQ.
Quarter Ending | High | Low | ||||||
Fiscal Year 2016 | ||||||||
First Quarter | $ | 19.24 | $ | 11.60 | ||||
Second Quarter | $ | 19.32 | $ | 13.31 | ||||
Third Quarter | $ | 24.57 | $ | 15.88 | ||||
Fourth Quarter | $ | 24.20 | $ | 16.07 | ||||
Fiscal Year 2015 | ||||||||
First Quarter | $ | 24.71 | $ | 11.87 | ||||
Second Quarter | $ | 34.68 | $ | 21.12 | ||||
Third Quarter | $ | 37.04 | $ | 18.34 | ||||
Fourth Quarter | $ | 23.04 | $ | 17.20 | ||||
As of February 28, 2017, we had approximately 199 holders of record of our common stock.
Performance Graph
The following is not deemed “filed” with the Securities and Exchange Commission and is not to be incorporated by reference into any filing we make under the Securities Act of 1933, as amended, whether made before or after the date hereof and irrespective of any general incorporation by reference language in such filing.
Our common stock is traded on the NASDAQ Global Market and is a component of both the NASDAQ Composite Index and the NASDAQ Biotechnology Index. The total return for our common stock and for each index assumes the reinvestment of dividends, although dividends have never been declared on our common stock, and is based on the returns of the component companies weighted according to their capitalizations as of the end of each monthly period. The NASDAQ-Composite tracks the aggregate price performance of equity securities of companies traded on the NASDAQ National Market. The NASDAQ Biotechnology Index contains securities and tracks the aggregate price performance of equity securities of NASDAQ-listed companies classified according to the Industry Classification Benchmark as either Biotechnology or Pharmaceuticals which also meet other eligibility criteria. The comparisons shown in the graph are based upon historical data and we caution that the stock price performance shown in the graph is not indicative of, nor intended to forecast, the potential future performance of our stock.
35
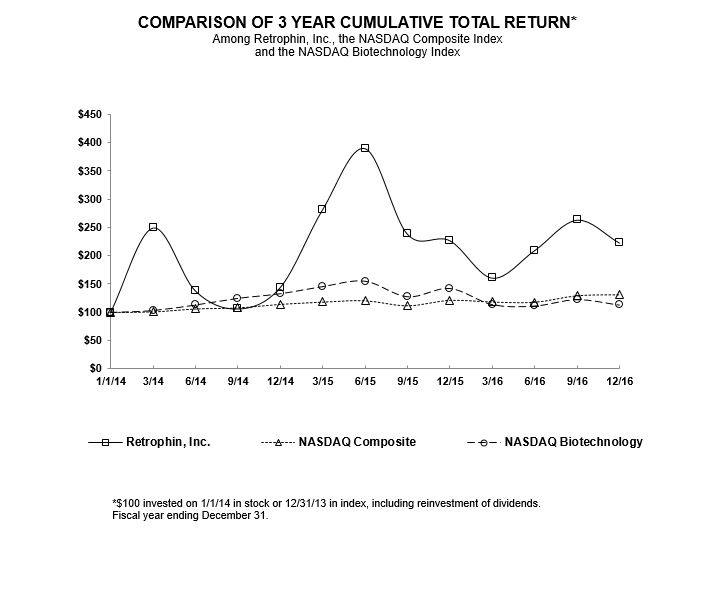
Dividends
Since inception we have not paid any dividends on our common stock. We currently do not anticipate paying any cash dividends in the foreseeable future on our common stock. Although we intend to retain our earnings, if any, to finance the exploration and growth of our business, our Board of Directors will have the discretion to declare and pay dividends in the future. Payment of dividends in the future will depend upon our earnings, capital requirements and other factors which our Board of Directors may deem relevant.
ITEM 6. SELECTED FINANCIAL DATA
The following table presents selected historical financial data of the Company for the periods indicated. The selected historical financial information is derived from the audited Consolidated Financial Statements of the Company referred to under Item 8 of this Annual Report on Form 10-K, and previously published historical financial statements. The following selected financial data should be read in conjunction with Item 7 - Management’s Discussion and Analysis of Financial Condition and Results of Operations, and the Company’s Consolidated Financial Statements, including the notes thereto, included elsewhere herein.
Selected historical financial data (in thousands, except share and per share amounts):
36
For the year ended December 31, | |||||||||||||||||||
Consolidated Statement of Operations: | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||
Net product sales | $ | 133,591 | $ | 99,892 | $ | 28,203 | $ | — | $ | — | |||||||||
Total operating expenses | 191,805 | 150,640 | 108,011 | 24,773 | 30,257 | ||||||||||||||
Operating loss | (58,214 | ) | (50,748 | ) | (79,808 | ) | (24,773 | ) | (30,257 | ) | |||||||||
Total other income (expenses), net | 632 | 156,215 | (33,590 | ) | (9,776 | ) | (87 | ) | |||||||||||
Income (Loss) before benefit for income taxes | (57,582 | ) | 105,467 | (113,398 | ) | (34,549 | ) | (30,344 | ) | ||||||||||
Income tax benefit | 9,679 | 11,770 | 2,460 | (76 | ) | — | |||||||||||||
Net income (loss) | $ | (47,903 | ) | $ | 117,237 | $ | (110,938 | ) | $ | (34,625 | ) | $ | (30,344 | ) | |||||
Per Share Data: | |||||||||||||||||||
Net Income (loss) per common share, basic | $ | (1.29 | ) | $ | 3.49 | $ | (4.43 | ) | $ | (2.44 | ) | $ | (8.29 | ) | |||||
Net Income (loss) per common share, diluted | $ | (1.29 | ) | $ | 3.17 | $ | (4.43 | ) | $ | (2.44 | ) | $ | (8.29 | ) | |||||
Weighted average common shares outstanding, basic | 36,997,865 | 33,560,249 | 25,057,509 | 14,205,264 | 3,662,114 | ||||||||||||||
Weighted average common shares outstanding, diluted | 38,288,012 | 37,581,439 | 25,057,509 | 14,205,264 | 3,662,114 | ||||||||||||||
As of December 31, | |||||||||||||||||||
Balance Sheet data: | 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||
Cash, cash equivalents and marketable securities | $ | 255,873 | $ | 229,604 | $ | 27,760 | $ | (6,130 | ) | $ | 11,388 | ||||||||
Working capital (deficit) | 249,090 | 214,951 | (70,205 | ) | (29,064 | ) | (57,966 | ) | |||||||||||
Total assets | 525,282 | 512,264 | 134,973 | 20,499 | 2,391 | ||||||||||||||
Long-term debt | 44,422 | 43,766 | 42,790 | — | — | ||||||||||||||
Total stockholders’ equity (deficit) | $ | 307,767 | $ | 299,971 | $ | (37,251 | ) | $ | (19,667 | ) | $ | (3,408 | ) | ||||||
Note: Cash dividends were not paid during the above periods.
37
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our audited Consolidated Financial Statements, including the notes thereto.
Overview
We are a fully integrated biopharmaceutical company with approximately 135 employees headquartered in San Diego, California, dedicated to delivering life-changing therapies to people living with rare diseases who have few, if any, treatment options.
Research and Development Programs:
Sparsentan (RE-021)
Sparsentan is an investigational therapeutic agent which acts as both a potent ARB, as well as a selective ERA, with in vitro selectivity toward endothelin receptor type A. We have secured a license to sparsentan from Ligand Pharmaceuticals, Inc. and Bristol-Myers Squibb Company (who referred to it as DARA). We are developing sparsentan as a treatment for FSGS, which is a leading cause of end-stage renal disease and NS. There are no FDA approved pharmacological treatments for FSGS and the off-label armamentarium is limited to ACE/ARBs, steroids, and immunosuppressant agents, which are effective in only a subset of patients. Every year approximately 5,400 patients are diagnosed with FSGS and we estimate that there are up to 40,000 FSGS patients in the United States with approximately half them being candidates for sparsentan. Sparsentan was granted orphan drug designation in the United States and the European Union in January 2015 and November 2015, respectively. In the third quarter of 2016, we announced positive top-line data from the Phase 2 DUET study of sparsentan for the treatment of FSGS.
In early 2017, we had an End of Phase 2 meeting with the FDA regarding the regulatory pathway for sparsentan as a treatment for FSGS. Following the meeting and our receipt of confirmatory meeting minutes, we announced our plans to initiate a single Phase 3 clinical trial to serve as the basis of an NDA filing for sparsentan for the treatment of FSGS. We expect that the trial will include an interim analysis of proteinuria as a surrogate endpoint and that if this interim analysis shows a substantial effect on proteinuria reduction, that the data could serve as a basis for accelerated approval of sparsentan for the treatment of FSGS pursuant to Subpart H of the FDA regulations. The confirmatory endpoint of the study would subsequently compare changes from baseline of estimated glomerular filtration rate, or eGFR. We are currently working with the FDA to finalize the study protocol and expect to initiate the trial in the second half of 2017.
Fosmetpantotenate (RE-024)
We are developing fosmetpantotenate, a novel small molecule, as a potential treatment for PKAN. PKAN is a genetic neurodegenerative disorder that is typically diagnosed in the first decade of life. Consequences of PKAN include dystonia, dysarthria, rigidity, retinal degeneration, and severe digestive problems. PKAN is estimated to affect up to 5,000 patients worldwide. There are currently no viable treatment options for patients with PKAN. Fosmetpantotenate (RE-024) is a phosphopantothenate replacement therapy that aims to restore levels of this key substrate in PKAN patients. Certain international health regulators have approved the initiation of dosing fosmetpantotenate (RE-024) in PKAN patients under physician-initiated studies in accordance with local regulations in their respective countries. We filed a U.S. IND for fosmetpantotenate (RE-024) with the FDA in the first quarter of 2015 to support the commencement of a company-sponsored Phase 1 study, which was successfully completed during 2015. The FDA granted fosmetpantotenate (RE-024) orphan drug designation in May 2015 and fast track designation in June 2015. In February, 2016, we announced fosmetpantotenate (RE-024) was granted orphan drug designation by the European Commission. In November, 2016, we announced that we had reached an agreement with the FDA under the Special Protocol Assessment (SPA) process for a Phase 3 clinical trial evaluating fosmetpantotenate (RE-024) for PKAN. We expect to begin dosing patients in this Phase 3 clinical trial in the coming months. We continue discussion with the EMA regarding the initiation of a potential registration-enabling efficacy trial in PKAN patients.
Tetracosactide Zinc (RE-034)
Tetracosactide zinc (RE-034) is a synthetic hormone analog of the first 24 amino acids of the 39 amino acids contained in adrenocorticotropic hormone ("ACTH") incorporated into a novel formulation developed by us. Tetracosactide zinc (RE-034) exhibits similar physiological actions as endogenous ACTH by binding to all five melanocortin receptors (pan-MCR), resulting in its anti-inflammatory and immunomodulatory effects. We have successfully manufactured tetracosactide zinc (RE-034) at proof-of-concept scale using a novel formulation process that allows modulation of the release of the active ingredient from the site of administration. We have decided to focus our further efforts on this program toward potential out-licensing opportunities.
NGLY1 Deficiency Discovery Efforts
We have entered into a research collaboration aimed at the discovery of a novel therapeutic for patients with NGLY1 deficiency, a rare genetic disorder. NGLY1 deficiency is believed to be caused by a deficiency in an enzyme called N-glycanase-1, which is encoded by the gene NGLY1. The condition is characterized by a variety of symptoms, including global developmental delay, movement disorder, seizures, and ocular abnormalities.
Liquid Ursodeoxycholic Acid
38
Liquid ursodeoxycholic acid ("L-UDCA") is a liquid formulation of ursodeoxycholic acid being developed for the treatment of a rare liver disease called primary biliary cholangitis ("PBC"). We obtained L-UDCA during 2016 with the intention of making L-UDCA commercially available to the subset of PBC patients who have difficulty swallowing. There are no liquid formulations of ursodeoxycholic acid currently approved by the FDA.
We currently sell the following three products:
Chenodal (chenodiol tablets)
Chenodal is a synthetic oral form of chenodeoxycholic acid, a naturally occurring primary bile acid synthesized from cholesterol in the liver, indicated for the treatment of radiolucent stones in well-opacifying gallbladders in patients in whom selective surgery would be undertaken except for the presence of increased surgical risk due to systemic disease or age.
Chenodal administration is known to reduce biliary cholesterol and the dissolution of radiolucent gallstones through suppression of hepatic synthesis of cholesterol, cholic acid and deoxycholic acid in the bile pool. Chenodal was first approved by the Food and Drug Administration (the "FDA") in 1983 for the management of gallstones but its marketing was later discontinued due to lack of commercial success. In 2009, Nexgen Pharma's ANDA for Chenodal was approved by the FDA for the treatment of gallstones; Chenodal is manufactured for Manchester under this ANDA. Manchester subsequently obtained orphan drug designation for Chenodal for the treatment of CTX, a rare autosomal recessive lipid storage disease, in 2010. Manchester was acquired by Retrophin in March 2014. For further discussion, see Note 3 of the Consolidated Financial Statements.
While Chenodal is not labeled for CTX, it has been used as the standard of care for over three decades. We are working to obtain FDA approval of Chenodal for the treatment of CTX. The prevalence of CTX is estimated in the literature to be as high as 1 in 70,000 in the overall population.
Cholbam (cholic acid capsules)
In March of 2015, we announced that the FDA approved Cholbam capsules, the first FDA approved treatment for pediatric and adult patients with bile acid synthesis disorders due to single enzyme defects, and for adjunctive treatment of patients with peroxisomal disorders (including Zellweger spectrum disorders). The effectiveness of Cholbam has been demonstrated in clinical trials for bile acid synthesis disorders and the adjunctive treatment of peroxisomal disorders. The estimated incidence of bile acid synthesis disorders due to single enzyme defects is 1 to 9 per million live births.
Thiola (tiopronin tablets)
Thiola is approved by the FDA for the treatment of cystinuria, a rare genetic cystine transport disorder that causes high cystine levels in the urine and the formation of recurring kidney stones. The resulting long-term damage can cause loss of kidney function in addition to substantial pain and loss of productivity associated with renal colic and stone passage. The prevalence of cystinuria in the United States is estimated to be 10,000 to 12,000, indicating that there may be as many as 4,000 to 5,000 affected individuals with cystinuria in the United States that would be candidates for Thiola.
Financial Overview
Research and Development Costs
Research and development costs include expenses related to sparsentan, fosmetpantotenate (RE-024) and our other pipeline programs. We expense all research and development costs as they are incurred. Our research and development costs are comprised of salaries and bonuses, benefits, non-cash share based compensation, license fees, milestones under license agreements, costs paid to third-party contractors to perform research, conduct clinical trials, and develop drug materials and delivery devices, and associated overhead expenses and facilities costs. Reimbursed research and development costs under collaborative arrangements are recorded as a reduction to research and development costs. We charge direct internal and external program costs to the respective development programs. We also incur indirect costs that are not allocated to specific programs because such costs benefit multiple development programs and allow us to increase our pharmaceutical development capabilities. These consist of internal shared resources related to the development and maintenance of systems and processes applicable to all of our programs.
At any point in time, we typically have various early stage research and drug discovery projects. Our internal resources, employees and infrastructure are not directly tied to any one research or drug discovery project and are typically deployed across multiple projects. As such, we do not maintain information regarding costs incurred for these early stage research and drug discovery programs on a project-specific basis.
We routinely engage vendors and service providers for scientific research, clinical trial, regulatory compliance, manufacturing and other consulting services. We also make grants to research and non-profit organizations to conduct research which may lead to new intellectual properties that we may subsequently license under separately negotiated license agreements. Such grants may be funded in lump sums or installments.
The following table summarizes our research and development expenses during the years ended December 31, 2016, 2015 and 2014. The internal costs include personnel, facility costs, and discovery and research related activities associated with our pipeline. The external program costs reflect external costs attributable to our clinical development candidates and preclinical candidates selected for further development. Such expenses
39
primarily include third-party contract costs relating to clinical trial activities, nonclinical studies and manufacturing.
For the Year Ended December 31, | |||||||||||
(in thousands) | |||||||||||
2016 | 2015 | 2014 | |||||||||
External service provider costs: | |||||||||||
Sparsentan | $ | 21,064 | $ | 11,179 | $ | 7,449 | |||||
Fosmetpantotenate (RE-024) | 12,625 | 7,631 | 11,175 | ||||||||
Tetracosactide zinc (RE-034) | 331 | 357 | 3,237 | ||||||||
Syntocinon | — | — | 3,353 | ||||||||
Other product candidates | 1,076 | 696 | 1,829 | ||||||||
General | 10,958 | 6,754 | 7,077 | ||||||||
Total external service provider costs: | 46,054 | 26,617 | 34,120 | ||||||||
Internal personnel costs: | 24,799 | 23,809 | 13,675 | ||||||||
Total research and development | $ | 70,853 | $ | 50,426 | $ | 47,795 | |||||
We expect our research and development expenses to increase during fiscal 2017 as we focus on clinical trials for our key product candidates, advance our discovery research projects into the preclinical stage and continue our early stage research. The process of conducting preclinical studies and clinical trials necessary to obtain regulatory approval is costly and time consuming. We may never succeed in achieving marketing approval for any of our product candidates. The probability of success of each product candidate may be affected by numerous factors, including preclinical data, clinical data, competition, manufacturing capability and commercial viability.
Most of our product development programs are in clinical trials which are highly uncertain and may not result in approved products. Completion dates and completion costs can vary significantly for each product candidate and are difficult to project. Given the uncertainty associated with clinical trial enrollments and the risks inherent in the development process, we are unable to determine the duration and completion costs of current or future clinical trials of our product candidates or if and to what extent we will generate revenues, if any, from the commercialization and sale of any of our product candidates.
Selling, General and Administrative
Selling, general and administrative expenses consist of salaries and bonuses, benefits, non-cash share based compensation, professional fees, rent, depreciation and amortization, travel, insurance, business development, sales and marketing programs, and other operating expenses.
Other Income/Expenses
Other income/expenses consist of the change in fair value of derivative financial instruments, litigation settlement gain, interest income and expense, finance expense, bargain purchase gain, loss on the extinguishment of debt, debt early payment penalty, gain on sale of assets, and miscellaneous other income/expenses.
License Agreements
Ligand License Agreement
In 2012, we entered into a license agreement with Ligand Pharmaceuticals, granting us a worldwide license for the development, manufacture and commercialization of sparsentan, which we are initially developing in connection with the treatment of FSGS. Under the license agreement, Ligand granted us a sublicense under certain of its patents and other intellectual property in connection with the development and commercialization of sparsentan. Under the license agreement, Ligand is obligated to transfer to us certain information, records, regulatory filings, materials and inventory controlled by Ligand and relating to or useful for developing sparsentan. We must use commercially reasonable efforts to develop and commercialize sparsentan in specified major market countries and other countries in which we believe it is commercially reasonable to develop and commercialize such products.
As consideration for the license, we are required to make payments upon the achievement of certain milestones, totaling up to $109.4 million. Should we commercialize sparsentan or any products containing any of the licensed compounds, we will be obligated to pay Ligand an escalating annual royalty between 15% and 17% of net sales of all such products. Through 2016, we made milestone payments to Ligand of $2.6 million under the license agreement.
Under the terms of the license agreement, Bristol-Myers Squibb has a right of first negotiation and Ligand has a right of second negotiation with respect to any license arrangement for a licensed compound, except to the extent such rights may be waived.
The license agreement will continue until neither party has any further payment obligations under the agreement and is expected to continue for approximately 10 to 20 years from the effective date. Ligand may terminate the license agreement due to (i) our insolvency, (ii) our material uncured breach of the agreement, (iii) our failure to use commercially reasonable efforts to develop and commercialize sparsentan as described above or (iv) certain other conditions. We may terminate the license agreement due to a material uncured breach of the agreement by Ligand.
40
Thiola License Agreement
In 2014, we entered into a license agreement with Mission Pharmacal Company ("Mission"), pursuant to which we obtained an exclusive, royalty-bearing license to market, sell and commercialize Thiola (Tiopronin) in the United States and Canada, and a non-exclusive license to use know-how relating to Thiola to the extent necessary to market Thiola.
We paid Mission an up-front license fee of $3.0 million and through June 30, 2024 will pay guaranteed minimum royalties during each calendar year the greater of $2.0 million or 20% of our net sales of Thiola.
Other Matters
Investigation and Impact on Financial Statements
See Note 15 of Consolidated Financial Statements for discussion.
Stock Option Accounting
See Note 12 of Consolidated Financial Statements for discussion.
Results of Operations for the Years Ended December 31, 2016, 2015 and 2014
Net Product Sales
The following table provides information regarding net product sales (in thousands):
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||||
2016 | 2015 | Change | 2015 | 2014 | Change | ||||||||||||||||||
Net product sales | $ | 133,591 | $ | 99,892 | $ | 33,699 | $ | 99,892 | $ | 28,203 | $ | 71,689 | |||||||||||
Net product sales for the years ended December 31, 2016, 2015 and 2014 were $133.6 million, $99.9 million and $28.2 million, respectively, and consisted of sales of Thiola, Chenodal and Cholbam in 2016, sales of Thiola, Chenodal, Cholbam and Vecamyl in 2015 and sales of Thiola, Chenodal and Vecamyl in 2014, less allowances for government rebates and patient assistance programs.
The increase in net product sales for the year ended December 31, 2016 as compared to the same period in 2015, is due to a full year of sales for Cholbam, and increased patient counts for all products.
The increase in net product sales for the year ended December 31, 2015 as compared to the same period in 2014, is due to a full year of sales for Chenodal and Thiola, increased growth of Thiola sales due to new patient starts, and the addition of Cholbam to our product group in April 2015.
We use a direct-to-patient distributor. Under this distribution model, we record revenues when the distributor ships products to customers and such customers take title of the product.
Operating Expenses
The following table provides information regarding operating expenses (in thousands):
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||||
2016 | 2015 | Change | 2015 | 2014 | Change | ||||||||||||||||||
Cost of goods sold | $ | 4,554 | $ | 2,185 | $ | 2,369 | $ | 2,185 | $ | 571 | $ | 1,614 | |||||||||||
Research and development | 70,853 | 50,426 | 20,427 | 50,426 | 47,795 | 2,631 | |||||||||||||||||
Selling, general and administrative | 92,803 | 79,541 | 13,262 | 79,541 | 59,645 | 19,896 | |||||||||||||||||
Legal fee settlement | 5,212 | — | 5,212 | — | — | — | |||||||||||||||||
Change in fair value of contingent consideration | 18,383 | 13,778 | 4,605 | 13,778 | — | 13,778 | |||||||||||||||||
Impairment of intangible assets | — | 4,710 | (4,710 | ) | 4,710 | — | 4,710 | ||||||||||||||||
$ | 191,805 | $ | 150,640 | $ | 41,165 | $ | 150,640 | $ | 108,011 | $ | 42,629 | ||||||||||||
2016 versus 2015 results
Operating expenses for the year ended December 31, 2016, were $191.8 million compared to $150.6 million for the year ended December 31, 2015, an increase of $41.2 million. The operating expenses increase is attributable to an increase in our research and development expenses of $20.4 million, an increase in selling, general and administrative expenses of $13.3 million, a change in fair value of contingent consideration of $4.6 million, the accrual of legal settlement expense of $5.2 million, and increased cost of goods sold, offset by lower impairment expenses of $4.7 million.
Research and development costs increased by $20.4 million due to increases in clinical trials for sparsentan and fosmetpantotenate (RE-024) and non-clinical studies to support the clinical trials.
41
Selling, general and administrative expenses increased by $13.3 million due to a full year of Cholbam intangible asset amortization, a year-over-year increase in Thiola intangible asset amortization, increases for salary and benefits including stock compensation, and a full year of commercial support for Cholbam.
Legal fee settlement expense of $5.2 million relates to amounts we agreed to advance for legal fees to our former Chief Executive Officer in defense of litigation for his actions while holding that title. See Note 11 to the Consolidated Financial Statements for further discussion.
Change in the fair value of contingent consideration of $4.6 million is due to changes in revenue forecasts, discount factors and timing of payments (in thousands):
2016 | 2015 | Variance | |||||||||
Chenodal | $ | 15,743 | $ | 9,115 | $ | 6,628 | |||||
Cholbam | 4,940 | 4,663 | 277 | ||||||||
L-UDCA | (2,300 | ) | — | (2,300 | ) | ||||||
Total | $ | 18,383 | $ | 13,778 | $ | 4,605 | |||||
Cost of goods sold increased by $2.4 million due to increased product sales, increased inventory reserves, and a one time termination fee from a distribution partner.
Impairment of intangible assets decreased by $4.7 million resulting from the 2015 write off of the Carbetocin asset. We noted no indications for impairment on any of our intangible assets in 2016.
2015 versus 2014 results
Our operating expenses for the year ended December 31, 2015 were $150.6 million compared to $108.0 million for the year ended December 31, 2014, an increase of $42.6 million. The operating expenses increase is attributable to an increase in our research and development expenses of $2.6 million and an increase in selling general and administrative expenses of $19.9 million, a change in valuation of contingent consideration of $13.8 million, and impairment of intangible assets of $4.7 million.
The increase in research and development costs of $2.6 million is primarily due to an increase of $10.1 million from headcount and compensation related to the hiring of critical regulatory and development expertise. This was offset by the divestiture of Syntocinon, the timing of fosmetpantotenate (RE-024) preclinical studies, and lower spending on tetracosactide zinc (RE-034).
The increase in selling, general and administrative expenses of $19.9 million is primarily due to an increase in sales and marketing personnel and associated expenses of $6.4 million to support our commercialization efforts, stock compensation increases of $5.5 million and amortization from intangible assets of $8.0 million.
In addition, we incurred charges of $13.8 million and $4.7 million in operating expenses related to the revaluation of contingent consideration liabilities for the products Chenodal and Cholbam, and the write-off of intangible assets related to Carbetocin as we elected not to pursue internal development of the asset, respectively.
Other Income/Expenses
The following table provides information regarding Other Income (Expenses) (in thousands):
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||||
2016 | 2015 | Change | 2015 | 2014 | Change | ||||||||||||||||||
Litigation settlement gain | $ | — | $ | 15,500 | $ | (15,500 | ) | $ | 15,500 | $ | — | $ | 15,500 | ||||||||||
Other income (expense), net | (264 | ) | (296 | ) | 32 | (296 | ) | 2,352 | (2,648 | ) | |||||||||||||
Interest expense, net | (759 | ) | (7,748 | ) | 6,989 | (7,748 | ) | (7,435 | ) | (313 | ) | ||||||||||||
Debt early payment penalty | — | (2,250 | ) | 2,250 | (2,250 | ) | — | (2,250 | ) | ||||||||||||||
Loss on extinguishment of debt | — | (4,151 | ) | 4,151 | (4,151 | ) | — | (4,151 | ) | ||||||||||||||
Finance expense | — | (600 | ) | 600 | (600 | ) | (4,721 | ) | 4,121 | ||||||||||||||
Change in fair value of derivative instruments | 1,655 | (33,307 | ) | 34,962 | (33,307 | ) | (23,786 | ) | (9,521 | ) | |||||||||||||
Gain on sale of assets | — | 140,004 | (140,004 | ) | 140,004 | — | 140,004 | ||||||||||||||||
Bargain purchase gain | — | 49,063 | (49,063 | ) | 49,063 | — | 49,063 | ||||||||||||||||
$ | 632 | $ | 156,215 | $ | (155,583 | ) | $ | 156,215 | $ | (33,590 | ) | $ | 189,805 | ||||||||||
Other income for the year ended December 31, 2016 was $0.6 million compared to other income of $156.2 million for the year ended December 31, 2015, which represents a decrease of $155.6 million. The change was primarily attributable to one time events in 2015 such as the gain on the sale of the Pediatric PRV to Sanofi, the bargain purchase gain on the Cholbam acquisition and the litigation settlement gain, offset by the change in fair value of derivative instruments, driven by changes in our stock price.
Other income for the year ended December 31, 2015 was $156.2 million compared to other expense of $33.6 million for the year ended December 31, 2014, which represents a variance of $189.8 million. The change was primarily attributable to the gain on the sale of the Pediatric PRV to Sanofi, the
42
bargain purchase gain on the Cholbam acquisition and the litigation settlement gain, offset by the change in fair value of derivative instruments, driven by changes in our stock price.
Income Tax Benefit (Provision):
We follow ASC 740, Income Taxes, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the asset will not be realized.
The standard addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740, we may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the tax authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. ASC 740 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures. Our policy is to record estimated interest and penalty related to the underpayment of income taxes or unrecognized tax benefits as a component of its income tax provision.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, except for operating leases.
Liquidity and Capital Resources
We believe that our available cash and short-term investments as of the date of this filing will be sufficient to fund our anticipated level of operations in the near term. Management believes that our operating results will vary from quarter to quarter and year to year depending upon various factors including revenues, general and administrative expenses, and research and development expenses.
For the years ended December 31, 2016 and 2015 we had the following financial performance (in thousands):
December 31, 2016 | December 31, 2015 | ||||||
Revenue | $ | 133,591 | $ | 99,892 | |||
Net Income (Loss) | (47,903 | ) | 117,237 | ||||
Cash & Cash Equivalents | 41,002 | 37,805 | |||||
Short Term Investments | 214,871 | 191,799 | |||||
Accumulated Deficit | (113,056 | ) | (65,153 | ) | |||
Stockholders' Equity | 307,767 | 299,971 | |||||
Net Working Capital | $ | 249,090 | $ | 214,951 | |||
Net Working Capital Ratio | 3.99 | 3.47 | |||||
Assets sold to Sanofi
On July 2, 2015, we sold and transferred the Pediatric PRV to Sanofi for $245.0 million. $150.0 million was received upon closing, and $47.5 million is due on each of the first and second anniversaries of the closing. In accordance with U.S. GAAP, we recorded the future short term and long term receivables at their present value of $46.2 million and $44.9 million, respectively, at the date of the sale. The gain from the sale of the asset was approximately $140.0 million, net of $4.9 million in fees contractually due as part of the Cholbam acquisition. The first annual payment was received on July 1, 2016 in accordance with the terms of the sale agreement.
Borrowings
Convertible Notes Payable
On May 29, 2014, we entered into a note purchase agreement relating to a private placement by us of $46.0 million aggregate principal senior convertible notes due 2019 (the “Notes”) which are convertible into shares of our common stock at an initial conversion price of $17.41 per share. The conversion price is subject to customary anti-dilution protection. The Notes bear interest at a rate of 4.5% per annum, payable semiannually in arrears on May 15 and November 15 of each year, beginning on November 15. The Notes mature on May 30, 2019 unless earlier converted or repurchased in accordance with the terms. The aggregate carrying value of the Notes on their issuance was $43.0 million, which was net of the $3.0 million debt discount.
On June 30, 2014, we issued 401,047 shares of Common Stock to the holders of the Notes and such Noteholders granted us a release of certain claims they may have had in connection with our sale of the Notes or certain statements made by us in connection with such sale due to our former Chief Executive Officer’s violation of his lockup agreement. We recorded the value of these shares on the date of issuance as finance expense. The amount was classified as other expense in our Consolidated Financial Statements for the year ended December 31, 2014.
As of December 31, 2016 all $46 million of principle senior convertible notes is outstanding.
Credit Facility
43
In June 2014, we entered into a $45 million Credit Agreement (the “Credit Facility”) which bore interest at an annual rate of (i) the Adjusted LIBOR Rate (as such term is defined in the Credit Facility) plus 10.00% or (ii) in certain circumstances, the Base Rate (as such term is defined in the Credit Agreement) plus 9.00%. The Credit Facility contained certain covenants, including those limiting us and our subsidiaries' abilities to incur indebtedness, incur liens, sell or acquire assets or businesses, change the nature of their businesses, engage in transactions with related parties, make certain investments or pay dividends. In addition, the Credit Facility required us and our subsidiaries to meet certain financial quarterly requirements.
On January 12, 2015, we entered into Amendment No. 3 (“Amendment No. 3”) to the Credit Agreement in which we obtained a commitment letter from Athyrium Capital Management, LLC and Perceptive Credit Opportunities Fund, LP (collectively, the “Lenders”), our existing lenders, providing a commitment for a senior secured incremental term loan under our existing term loan facility in an aggregate principal amount of $30 million (the “Incremental Loan”), which could have been drawn down at our option to finance the acquisition of assets.
As consideration for the commitment letter for the Incremental Loan, we made a cash payment to the Lenders and issued the Lenders warrants initially exercisable to purchase up to an aggregate of 125,000 shares of our common stock. We recorded a charge of $1.1 million in interest expense.
On July 1, 2015, we paid $47.3 million as payment in full for all principal and accrued interest under the Credit Facility, which included $45.0 million of principal balance, $2.3 million in prepayment premiums for early payment penalty, and an immaterial amount of interest accrued through the settlement date, as required by the terms of the Credit Agreement. Upon receipt of this final payment, the liens and security interests granted pursuant to the Credit Agreement and the documents executed and delivered pursuant thereto or in connection therewith were automatically and irrevocably released and terminated.
Interest Expense
Total interest expense, net, recognized for the years ended December 31, 2016, 2015 and 2014 was $0.8 million, $7.7 million and $7.4 million, respectively.
Equity Offering
In March 2015, we completed a public offering of 7,866,000 shares of common stock at a price of $19.00 per share. We received net proceeds from the offering of $140.0 million, after deducting underwriting fees and other offering costs of $9.5 million.
On January 9, 2014, we completed a public offering of 4,705,882 shares of common stock at a price of $8.50 per share. We received net proceeds from the offering of $36.8 million, after deducting the underwriting fees and other offering costs of $3.2 million.
License Agreement Obligations
See discussion above under the header "License Agreements".
Funding Requirements
We believe that our available cash and short-term investments as of the date of this filing will be sufficient to fund our anticipated level of operations for the near term. This belief is based on many factors, however, some factors are beyond our control. Factors affecting our financing requirements include, but are not limited to:
• | revenue growth of our marketed products; |
• | the rate of progress and cost of our clinical trials, preclinical studies and other discovery and research and development activities; |
• | the timing of, and costs involved in, seeking and obtaining marketing approvals for our products, and in maintaining quality systems standards for our products; |
• | our ability to manufacture sufficient quantities of our products to meet expected demand; |
• | the costs of preparing, filing, prosecuting, maintaining and enforcing any patent claims and other intellectual property rights, litigation costs and the results of litigation; |
• | our ability to enter into collaboration, licensing or distribution arrangements and the terms and timing of these arrangements; |
• | the potential need to expand our business, resulting in additional payroll and other overhead expenses; |
• | the potential acquisition or in-licensing of other products or technologies; and |
• | the emergence of competing technologies or other adverse market or technological developments. |
Future capital requirements will also depend on the extent to which we acquire or invest in additional complementary businesses, products and technologies.
44
Cash Flows
The following table summarizes our cash flows for the periods set forth below (in thousands):
2016 | 2015 | 2014 | |||||||||
Net cash used in operating activities | $ | (1,596 | ) | $ | (554 | ) | $ | (45,850 | ) | ||
Net cash provided by (used in) investing activities | 10,370 | (80,602 | ) | (37,263 | ) | ||||||
Net cash provided by (used in) financing activities | (5,694 | ) | 100,767 | 95,320 | |||||||
Net increase in cash | 3,080 | 19,611 | 12,207 | ||||||||
Effect of exchange rate changes on cash | 117 | (10 | ) | — | |||||||
Cash & cash equivalents, beginning of period | 37,805 | 18,204 | 5,997 | ||||||||
Cash & cash equivalents, end of period | $ | 41,002 | $ | 37,805 | $ | 18,204 | |||||
Management considers marketable securities to be available to fund current operations, and they are classified as available for sale and included within current assets in our Consolidated Balance Sheets. Therefore, cash on hand includes marketable securities and is considered to be $255.9 million.
Cash Flows from Operating Activities
Operating activities used $1.6 million during the year ended December 31, 2016 compared to $0.6 million used for the year ended December 31, 2015. After excluding one time items and non-cash adjustments, the variance is primarily due to changes in operating assets offset by increased operating expenses exceeding increased product sales.
Operating activities used $0.6 million of cash during the year ended December 31, 2015 compared to $45.9 million used for the year ended December 31, 2014. The variance of $45.3 million was the result of an increase in revenue of $71.7 million, offset by a decrease in operating assets of $25.3 million.
Cash Flows from Investing Activities
Cash provided by investing activities for the year ended December 31, 2016 was $10.4 million compared to cash used of $80.6 million for the year ended December 31, 2015. The variance of $91.0 million was primarily driven by assets received from the sale of the pediatric PRV and a portion of the funds obtained from the 2015 equity offering being invested in marketable securities in 2015. In 2016 we reinvested the proceeds from matured marketable securities as well as increased the total investments with the funds received from the note receivable payment.
Cash used in investing activities for the year ended December 31, 2015 was $80.6 million compared to $37.3 million used for the year ended December 31, 2014. The variance of $43.3 million was primarily the result of cash used in the purchase of marketable securities of $198.5 million in 2015, partially offset by cash received from the divestiture of assets of $148.4 million.
Cash Flows from Financing Activities
For the year ended December 31, 2016, cash used in financing activities was $5.7 million compared to cash provided of $100.8 million during the year ended December 31, 2015. The variance is due to the issuance of common stock of $140.0 million, net of fees, offset by the cash used to pay down debt of $45.0 million in 2015, with an increase in payments for contingent consideration of $8.4 million. The remaining variance of $3.1 million is due to variances between years in cash provided by warrant and option exercises, offset by payments of other liabilities.
For the year ended December 31, 2015, cash provided by financing activities was $100.8 million compared to cash provided of $95.3 million during the year ended December 31, 2014. The variance of $5.5 million was primarily a result of the issuance of common stock of $140.0 million, net of fees, offset by the cash used to pay down debt of $45.0 million. The cash provided in 2014 was the result of the issuance of common stock of $40.0 million, net of fees, net proceeds from the Credit Agreement of $42.4 million, and net proceeds from the note purchase agreement of $43.0 million, offset by the pay down of the Manchester Note payable of $31.3 million.
Contractual Commitments
See Note 11 to the Consolidated Financial Statements for discussion.
Critical Accounting Policies and Estimates
See Note 2 to the Consolidated Financial Statements for discussion.
Recently Issued Accounting Pronouncements
See Note 2 to the Consolidated Financial Statements for discussion.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our primary exposure to market risk is related to changes in interest rates. As of December 31, 2016, we had cash equivalents and marketable securities of approximately $215.9 million, consisting of money market funds, U.S. backed entity debt, corporate debt and commercial paper. This exposure to
45
market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments are in short-term marketable securities. Our marketable securities are subject to interest rate risk and will fall in value if market interest rates increase. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, a one percent change in interest rates would have approximately a $2.1 million impact on our investments. We carry our investments based on publicly available information. We do not currently have any hard to value investment securities or securities for which a market is not readily available or active.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The Consolidated Financial Statements and supplementary data of Retrophin, Inc. required by this Item are described in Item 15 of this Annual Report on Form 10-K and are presented beginning on page F-1.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the timelines specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objectives, and in reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As required by SEC Rule 13a-15(b), we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the year covered by this report. Based on the foregoing, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Report on Internal Control Over Financial Reporting
Internal control over financial reporting refers to the process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
(1) Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
(2) Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and
(3) Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of such limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk. Management is responsible for establishing and maintaining adequate internal control over financial reporting for the company.
Management has used the framework set forth in the report entitled Internal Control-Integrated Framework (2013 framework) published by the Committee of Sponsoring Organizations of the Treadway Commission, known as COSO, to evaluate the effectiveness of our internal control over financial reporting. Based on this assessment, our Chief Executive Officer and Chief Financial Officer concluded that our internal control over financial reporting was effective as of December 31, 2016. BDO USA, LLP, our independent registered public accounting firm, has issued an attestation report on our internal control over financial reporting as of December 31, 2016, which is included herein.
46
Changes In Internal Control Over Financial Reporting
There have not been any changes in our internal control over financial reporting during the year ended December 31, 2016 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
47
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Retrophin, Inc.
San Diego, California
We have audited Retrophin, Inc. and its subsidiaries’ (the “Company”) internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Retrophin Inc. and its subsidiaries’ management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying, “Item 9A, Management’s Report on Internal Control Over Financial Reporting.” Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Retrophin, Inc. and its subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Retrophin, Inc. and its subsidiaries as of December 31, 2016 and 2015, and the related consolidated statements of operations and comprehensive income (loss), stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2016 and our report dated March 1, 2017 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
New York, NY
March 1, 2017
48
ITEM 9B. OTHER INFORMATION
Executive Compensation
On February 27, 2017, the Compensation Committee (the “Compensation Committee”) of the Board of Directors (the “Board”) of the Company approved the following 2016 performance-based bonuses for the Company’s executive officers pursuant to the Company’s 2016 Executive Officer Annual Bonus Plan (taking into account the prior recommendation of the independent members of the Board):
• | Stephen Aselage, the Company’s Chief Executive Officer, was granted a performance-based cash bonus equal to $263,415; |
• | Laura Clague, the Company’s Chief Financial Officer, was granted a performance-based cash bonus equal to $157,165; and |
• | Neil McFarlane, the Company’s Chief Operating Officer, was granted a performance-based cash bonus equal to $84,115 (pro-rated amount for partial year of service). |
Additionally, on February 27, 2017, the Compensation Committee approved the following annual base salary increases for the Company’s executive officers (taking into account the prior recommendation of the independent members of the Board):
• | Stephen Aselage had his annual base salary increased to $560,000; |
• | Laura Clague had her annual base salary increased to $385,000; and |
• | Neil McFarlane had his annual base salary increased to $482,125. |
Elizabeth Reed, the Company’s Senior Vice President & General Counsel, and William Rote, the Company’s Senior Vice President, Research & Development, joined the Company in 2017 and therefore were not eligible for a 2016 cash bonus or a 2017 annual base salary increase.
2017 Executive Officer Annual Bonus Plan
On February 27, 2017, the Compensation Committee approved the adoption of the 2017 Retrophin, Inc. Executive Officer Annual Bonus Plan (the “Bonus Plan”) for the Company’s executive officers.
Each participant in the Bonus Plan has been assigned a target bonus percentage of such participant’s current base salary for 2017. Pursuant to the terms of the Bonus Plan, the target bonus percentage is set at 60% of base salary for the Chief Executive Officer and 50% of base salary for the other executive officers.
The amounts payable to each participant under the Bonus Plan will be based entirely on the determination by the Compensation Committee or the Board of the achievement by the Company of corporate performance goals. Depending on actual corporate performance during 2017, the Compensation Committee or the Board may, in their sole discretion, determine a goal achievement percentage under the Bonus Plan within a range between 0% and 150%.
A participant’s bonus under the Bonus Plan will be equal to his or her annual base salary, multiplied by his or her target bonus percentage, multiplied by the goal achievement percentage determined by the Compensation Committee or the Board. However, no payments will be made pursuant to the Bonus Plan in the event that the Compensation Committee or the Board determines that less than 50% of the corporate performance goals are achieved.
The corporate performance goals under the Bonus Plan for 2017 relate to (i) total revenues, (ii) progress of the sparsentan Phase 3 clinical trial, (iii) progress of the RE-024 Phase 3 clinical trial, (iv) CMC/manufacturing objectives for the Company’s development and commercial stage programs, (v) business development objectives, (vi) objectives related to the investment community, and (vii) internal operational objectives.
The foregoing description of the terms of the Bonus Plan is qualified in its entirety by reference to the Bonus Plan, a copy of which is attached hereto as Exhibit 10.34 and is incorporated herein by reference.
49
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the Securities and Exchange Commission within 120 days of December 31, 2016. Such information is incorporated herein by reference.
Item 11. EXECUTIVE COMPENSATION
Information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the Securities and Exchange Commission within 120 days of December 31, 2016. Such information is incorporated herein by reference.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the Securities and Exchange Commission within 120 days of December 31, 2016. Such information is incorporated herein by reference.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the Securities and Exchange Commission within 120 days of December 31, 2016. Such information is incorporated herein by reference.
Item 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the Securities and Exchange Commission within 120 days of December 31, 2016. Such information is incorporated herein by reference.
50
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The financial statements at page F-1 are incorporated by reference to a part of this Annual Report on Form 10-K.
Financial statement schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto.
(b) Exhibits: The exhibits to this report are listed in the exhibit index below.
Exhibit No. | Description | |
2.1 | Membership Interest Purchase Agreement, dated as of March 26, 2014, by and among Retrophin, Inc., on the one hand, and Loring Creek Holdings LLC, Lloyd Glenn and Matthias Kurth, on the other hand (incorporated by reference to Exhibit 10.2 to Company’s Current Report on Form 8-K, filed with the Securities and Exchange Commission (the “SEC”) on March 31, 2014). | |
2.2 | Asset Purchase Agreement, dated May 22, 2015, by and between Retrophin, Inc. and Sanofi (incorporated by reference to Exhibit 2.1 to the Company's Current report on Form 8-K filed with the SEC on May 27, 2015). | |
3.1 | Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.1 to Amendment No. 2 to the Company’s General Form for Registration of Securities on Form 10-12G, filed with the SEC on October 28, 2010). | |
3.2 | Certificate of Amendment of Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K, filed with the SEC on June 11, 2015). | |
3.3 | Amended and Restated Bylaws of the Company (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K, filed with the SEC on June 11, 2015). | |
4.1 | Form of Warrant Certificate, dated June 30, 2014, issued to the Lenders under the Credit Agreement (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, filed with the SEC on July 7, 2014). | |
4.2 | Form of Warrant issued to the purchasers in the private placement of 3,045,929 shares of common stock, dated February 14, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, filed with the SEC on February 19, 2013). | |
4.3 | Form of Common Stock Purchase Warrant, dated August 15, 2013, issued to the purchasers of securities in the private placement of the Company closed on August 15, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K, filed with the SEC on August 20, 2013). | |
4.4 | Form of Note Purchase Agreement for principal senior convertible notes with an interest rate of 4.50% due 2019 (“2019 Notes”), dated May 29, 2014, by and among the Company and the investors identified therein (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on June 4, 2014). | |
4.5 | Form of Indenture for 2019 Notes, dated May 30, 2014 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K, filed with the SEC on June 4, 2014). | |
4.6 | Form of Note for 2019 Notes, dated May 30, 2014 (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K, filed with the SEC on May 29, 2014). | |
4.7 | Registration Rights Agreement, dated February 12, 2013, by and among the Company and the February 2013 Purchasers (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K, filed with the SEC on February 19, 2013). | |
4.8 | Registration Rights Agreement, dated August 15, 2013, by and among the Company and the August 2013 Purchasers (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K, filed with the SEC on August 20, 2013). | |
4.9 | First Amendment to Registration Rights Agreement, dated August 14, 2013, by and among the Company and the purchasers signatory thereto (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K, filed with the SEC on August 20, 2013). | |
4.10 | Form of Indenture for Senior Debt Securities (incorporated by reference to Exhibit 4.10 to the Company’s Registration Statement on Form S-8, filed with the SEC on September 9, 2014). | |
4.11 | Form of Indenture for Subordinated Debt Securities (incorporated by reference to Exhibit 4.11 to the Company’s Registration Statement on Form S-8, filed with the SEC on September 9, 2014). | |
10.1 | Separation Agreement and Release, dated September 15, 2014, by and between Retrophin, Inc. and Marc Panoff (incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K, filed with the SEC on September 16, 2014). | |
10.2 | Form of Credit Agreement, dated as of June 30, 2014, among Retrophin, Inc., the lenders from time to time party thereto and U.S. Bank National Association, as Administrative Agent and Collateral Agent (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on November 13, 2014). | |
10.3 | Form of Guarantee and Collateral Agreement, dated as of June 30, 2014, among Retrophin, Inc., the Guarantors from time to time party thereto and U.S. Bank National Association, as Collateral Agent (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K, filed with the SEC on July 7, 2014). | |
10.4 | Amendment No. 1 to Credit Agreement, dated July 16, 2014, among Retrophin, Inc., the lenders from time to time party thereto and U.S. Bank National Association, as Administrative Agent and Collateral Agent (incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on November 13, 2014). | |
10.5 | Amendment No. 2 to Credit Agreement, dated November 13, 2014, among Retrophin, Inc., the lenders from time to time party thereto and U.S. Bank National Association, as Administrative Agent and Collateral Agent (incorporated by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on November 13, 2014). | |
10.6 | License Agreement, dated May 29, 2014, by and among Retrophin, Inc. and Mission Pharmacal Company (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on June 4, 2014). | |
10.7 | First Amendment to Trademark License and Supply Agreement, effective as of July 28, 2014, by and between Mission Pharmacal Company and Retrophin, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on July 29, 2014). | |
10.8 | International Rights Purchase Agreement, dated as of March 26, 2014, by and between Manchester Pharmaceuticals LLC and Retrophin Therapeutics International, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on March 31, 2014). | |
51
10.9 | Secured Promissory Note, dated March 26, 2014, made by Retrophin, Inc. in favor of Loring Creek Holdings LLC, Lloyd Glenn and Matthias Kurth (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K, filed with the SEC on March 31, 2014). | |
10.10 | Membership Interest Pledge Agreement, dated as of March 26, 2014, by and between Retrophin, Inc., on the one hand, and Loring Creek Holdings LLC, Lloyd Glenn and Matthias Kurth, on the other hand (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K, filed with the SEC on March 31, 2014). | |
10.11 | Security Agreement, dated as of March 26, 2014, by and between Manchester Pharmaceuticals LLC, on the one hand, and Loring Creek Holdings LLC, Lloyd Glenn and Matthias Kurth, on the other hand. (incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K, filed with the SEC on March 31, 2014). | |
10.12+ | Sublicense Agreement, dated February 16, 2012, by and among Ligand Pharmaceuticals Incorporated, a Delaware corporation, Pharmacopeia, Inc., a Delaware limited liability company, and Retrophin, LLC, a Delaware limited liability company (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on December 19, 2012). | |
10.13† | Employment Agreement, dated March 2, 2015, by and between Retrophin, Inc. and Laura M. Clague (incorporated by reference to Exhibit 10.25 to the Company’s Annual Report on Form 10-K, filed with the SEC on March 11, 2015). | |
10.14† | Employment Agreement, dated March 2, 2015, by and between Retrophin, Inc. and Stephen Aselage (incorporated by reference to Exhibit 10.27 to the Company’s Annual Report on Form 10-K, filed with the SEC on March 11, 2015). | |
10.15 | Summary Separation Proposal, dated October 13, 2014, by and between Retrophin, Inc. and Martin Shkreli (incorporated by reference to Exhibit 10.28 to the Company’s Annual Report on Form 10-K, filed with the SEC on March 11, 2015). | |
10.16 | Retrophin, Inc. 2014 Incentive Compensation Plan as amended (incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K, filed with the SEC on February 9, 2015). | |
10.17 | Retirement and Transition Agreement, dated February 1, 2016, by and between Retrophin, Inc. and Margaret Valeur-Jensen, Ph.D. (incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K, filed with the SEC on February 2, 2016). | |
10.18+ | Amendment No. 4 to Sublicense Agreement dated as of September 17, 2015, between Retrophin, Inc. and Ligand Pharmaceuticals Incorporated (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q/A, filed with the SEC on December 22, 2015). | |
10.19 | Addendum to Trademark License and Supply Agreement, dated October 19, 2015, by and between to Company and Mission Pharmacal (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on November 6, 2015). | |
10.20 | Asset Purchase Agreement dated as of January 9, 2015, between Retrophin, Inc. and Turing Pharmaceuticals AG (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 11, 2015). | |
10.21 | Asset Purchase Agreement dated as of February 12, 2015, among Retrophin, Inc., Manchester Pharmaceuticals LLC and Turing Pharmaceuticals AG (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 11, 2015). | |
10.22 | Asset Purchase Agreement dated as of February 12, 2015, between Retrophin, Inc. and Turing Pharmaceuticals AG (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 11, 2015). | |
10.23 | Amendment No. 3 to Credit Agreement dated January 12, 2015, among Retrophin, Inc., the lenders from time to time thereto and U.S. Bank National Association, as administrative agent and collateral agent (incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 11, 2015). | |
10.24+ | Amendment No. 3 to Sublicense Agreement dated as of February 27, 2015, between Retrophin, Inc. and Ligand Pharmaceuticals Incorporated (incorporated by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 11, 2015). | |
10.25+ | Asset Purchase Agreement dated January 10, 2015 by and between Retrophin, Inc. and Asklepion Pharmaceuticals, LLC (incorporated by reference to Exhibit 10.6 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 11, 2015). | |
10.26 | Amendment No. 4 to Credit Agreement, dated March 24, 2015, among Retrophin, Inc., the lenders from time to time thereto and U.S. Bank National Association, as administrative agent and collateral agent (incorporated by reference to Exhibit 10.7 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 11, 2015). | |
10.27 | Purchase Agreement dated as of February 12, 2015 among Retrophin Inc., Manchester Pharmaceuticals LLC and Waldun Pharmaceuticals LLC (incorporated by reference to Exhibit 10.9 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 11, 2015). | |
10.28† | 2016 Retrophin, Inc. Executive Officer Annual Bonus Plan (incorporated by reference to Exhibit 99.2 to the Company’s Current Report on Form 8-K, filed with the SEC on February 25, 2016). | |
10.29† | Employment Agreement, dated August 15, 2016, by and between Retrophin, Inc. and Neil McFarlane (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on November 4, 2016). | |
10.30+ | Amendment One to the Third Amendment to Trademark License and Supply Agreement, dated September 12, 2016, by and between the Company and Mission Pharmacal Company (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on November 4, 2016). | |
10.31+ | Third Amendment to Trademark License and Supply Agreement dated as of March 17, 2016, between Retrophin Inc. and Mission Pharmacal. (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 5, 2016). | |
10.32+ | Asset Purchase Agreement dated as of June 9, 2016 between Retrophin, Inc. and Asklepion Pharmaceuticals, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on August 4, 2016). | |
10.33 | Retrophin, Inc. 2015 Equity Incentive Plan, as amended (incorporated by reference to Exhibit 99.1 to the Company's current report on Form 8-K, filed with the SEC on May 19, 2016 | |
10.34† | 2017 Retrophin, Inc. Executive Officer Annual Bonus Plan | |
21.1 | List of subsidiaries of the Company. | |
23.1 | Consent of BDO USA, LLP. | |
24.1 | Power of Attorney (see signature page hereto). | |
31.1 | Chief Executive Officer's Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
31.2 | Chief Financial Officer's Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
32.1 | Chief Executive Officer’s Certification pursuant to Section 906 of Sarbanes Oxley Act of 2002. | |
32.2 | Chief Financial Officer’s Certification pursuant to Section 906 of Sarbanes Oxley Act of 2002. | |
101.INS | XBRL Instance Document. | |
101.SCH | XBRL Taxonomy Extension Schema Document. | |
52
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document. | |
101.PRE | Taxonomy Extension Presentation Linkbase Document. | |
+ | We have received confidential treatment of certain portions of this agreement, which have been omitted and filed separately with the SEC pursuant to Rule 406 under the Securities Act of 1933, as amended, or Rule 24b-2 of the Securities Exchange Act of 1934, as amended. | |
† | Indicates management contract or compensatory plan. | |
53
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: March 1, 2017 | RETROPHIN, INC. | |
By: | /s/ Stephen Aselage | |
Name: Stephen Aselage | ||
Title: Chief Executive Officer | ||
By: | /s/ Laura Clague | |
Name: Laura Clague | ||
Title: Chief Financial Officer | ||
POWER OF ATTORNEY
Know all men by these presents, that each person whose signature appears below constitutes and appoints Stephen Aselage and Laura Clague, and each of them, as his attorneys-in-fact and agents, each with power of substitution in any and all capacities, to sign any amendments to this annual report on Form 10-K, and to file the same with exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that the attorney-in-fact or his substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
Signature | Title | Date | ||
/s/ Stephen Aselage | Chief Executive Officer and Director (Principal Executive Officer) | March 1, 2017 | ||
Stephen Aselage | ||||
/s/ Laura Clague | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | March 1, 2017 | ||
Laura Clague | ||||
/s/ Timothy Coughlin | Director | |||
Timothy Coughlin | March 1, 2017 | |||
/s/ Cornelius Golding | Director | |||
Cornelius Golding | March 1, 2017 | |||
/s/ Jeffrey A. Meckler | Director | |||
Jeffrey A. Meckler | March 1, 2017 | |||
/s/ Gary Lyons | Director | |||
Gary Lyons | March 1, 2017 | |||
/s/ John Kozarich | Director | |||
John Kozarich | March 1, 2017 | |||
/s/ Roy D. Baynes | Director | |||
Roy D. Baynes | March 1, 2017 | |||
54
RETROPHIN, INC. AND SUBSIDIARIES
INDEX TO FINANCIAL STATEMENTS
Page | |
Financial Statements | |
F-1
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Retrophin, Inc.
San Diego, CA
We have audited the accompanying consolidated balance sheets of Retrophin, Inc. and its subsidiaries (the “Company”) as of December 31, 2016 and 2015 and the related consolidated statements of operations and comprehensive income (loss), stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Retrophin, Inc. and its subsidiaries at December 31, 2016 and 2015, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Retrophin Inc. and its subsidiaries’ internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 1, 2017 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
New York, NY
March 1, 2017
F-2
RETROPHIN, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
December 31, 2016 | December 31, 2015 | ||||||
Assets | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 41,002 | $ | 37,805 | |||
Marketable securities | 214,871 | 191,799 | |||||
Accounts receivable, net | 18,510 | 12,458 | |||||
Inventory, net | 2,826 | 2,536 | |||||
Prepaid expenses and other current assets | 4,831 | 2,378 | |||||
Prepaid taxes | 3,463 | 8,107 | |||||
Note receivable, current | 46,849 | 46,849 | |||||
Total current assets | 332,352 | 301,932 | |||||
Property and equipment, net | 2,587 | 428 | |||||
Other assets | 7,364 | 1,859 | |||||
Intangible assets, net | 182,043 | 161,536 | |||||
Goodwill | 936 | 936 | |||||
Note receivable, long-term | — | 45,573 | |||||
Total assets | $ | 525,282 | $ | 512,264 | |||
Liabilities and Stockholders' Equity | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 7,522 | $ | 7,639 | |||
Accrued expenses | 33,308 | 23,820 | |||||
Guaranteed minimum royalty, short term | 2,000 | 2,000 | |||||
Other current liabilities | 1,842 | 958 | |||||
Business combination-related contingent consideration | 16,150 | 13,754 | |||||
Derivative financial instruments, warrants | 22,440 | 38,810 | |||||
Total current liabilities | 83,262 | 86,981 | |||||
Convertible debt | 44,422 | 43,766 | |||||
Other noncurrent liabilities | 4,010 | 3,066 | |||||
Guaranteed minimum royalty, long term | 8,068 | 8,885 | |||||
Business combination-related contingent consideration, less current portion | 71,328 | 45,267 | |||||
Deferred income tax liability, net | 6,425 | 24,328 | |||||
Total liabilities | 217,515 | 212,293 | |||||
Stockholders' Equity: | |||||||
Preferred stock Series A $0.001 par value; 20,000,000 shares authorized; 0 issued and outstanding as of December 31, 2016 and 2015, respectively | — | — | |||||
Common stock $0.0001 par value; 100,000,000 shares authorized; 37,906,669 and 36,465,853 issued and outstanding as of December 31, 2016 and 2015, respectively | 4 | 4 | |||||
Additional paid-in capital | 421,309 | 365,802 | |||||
Accumulated deficit | (113,056 | ) | (65,153 | ) | |||
Accumulated other comprehensive loss | (490 | ) | (682 | ) | |||
Total stockholders' equity | 307,767 | 299,971 | |||||
Total liabilities and stockholders' equity | $ | 525,282 | $ | 512,264 | |||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
RETROPHIN, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
(In thousands, except share and per share amounts)
Years Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Net product sales | $ | 133,591 | $ | 99,892 | $ | 28,203 | |||||
Operating expenses: | |||||||||||
Cost of goods sold | 4,554 | 2,185 | 571 | ||||||||
Research and development | 70,853 | 50,426 | 47,795 | ||||||||
Selling, general and administrative | 92,803 | 79,541 | 59,645 | ||||||||
Legal fee settlement | 5,212 | — | — | ||||||||
Change in fair value of contingent consideration | 18,383 | 13,778 | — | ||||||||
Impairment of intangible assets | — | 4,710 | — | ||||||||
Total operating expenses | 191,805 | 150,640 | 108,011 | ||||||||
Operating loss | (58,214 | ) | (50,748 | ) | (79,808 | ) | |||||
Other Income (expense), net: | |||||||||||
Litigation settlement gain | — | 15,500 | — | ||||||||
Other income (expense), net | (264 | ) | (296 | ) | 2,352 | ||||||
Interest expense, net | (759 | ) | (7,748 | ) | (7,435 | ) | |||||
Debt early payment penalty | — | (2,250 | ) | — | |||||||
Loss on extinguishment of debt | — | (4,151 | ) | — | |||||||
Finance expense | — | (600 | ) | (4,721 | ) | ||||||
Change in fair value of derivative instruments | 1,655 | (33,307 | ) | (23,786 | ) | ||||||
Gain on sale of assets | — | 140,004 | — | ||||||||
Bargain purchase gain | — | 49,063 | — | ||||||||
Total other income (expense), net | 632 | 156,215 | (33,590 | ) | |||||||
Income (loss) before benefit for income taxes | (57,582 | ) | 105,467 | (113,398 | ) | ||||||
Income tax benefit | 9,679 | 11,770 | 2,460 | ||||||||
Net income (loss) | $ | (47,903 | ) | $ | 117,237 | $ | (110,938 | ) | |||
Net income (loss) per common share: | |||||||||||
Basic | $ | (1.29 | ) | $ | 3.49 | $ | (4.43 | ) | |||
Diluted | $ | (1.29 | ) | $ | 3.17 | $ | (4.43 | ) | |||
Weighted average common shares outstanding: | |||||||||||
Basic | 36,997,865 | 33,560,249 | 25,057,509 | ||||||||
Diluted | 38,288,012 | 37,581,439 | 25,057,509 | ||||||||
Comprehensive income (loss): | |||||||||||
Net income (loss) | $ | (47,903 | ) | $ | 117,237 | $ | (110,938 | ) | |||
Foreign currency translation gain (loss) | 93 | (40 | ) | — | |||||||
Unrealized gain (loss) on sale of marketable securities | 99 | (4,927 | ) | 4,396 | |||||||
Comprehensive income (loss) | $ | (47,711 | ) | $ | 112,270 | $ | (106,542 | ) | |||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
RETROPHIN, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (DEFICIT)
(In thousands, except share amounts)
Common Stock | Common Stock in Treasury | Additional Paid in Capital | Accumulated Other Comprehensive Income (Loss) | Accumulated Deficit | Total Stockholders' Equity (Deficit) | ||||||||||||||||||||||||
Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||
BALANCE - DECEMBER 31, 2013 | 18,546,363 | $ | 2 | (130,790 | ) | $ | (957 | ) | $ | 49,635 | $ | (110 | ) | $ | (68,237 | ) | $ | (19,667 | ) | ||||||||||
Share based payments | 730,774 | — | — | — | 16,639 | — | — | 16,639 | |||||||||||||||||||||
Kyalin payments | 96,628 | — | — | — | 1,000 | — | — | 1,000 | |||||||||||||||||||||
Issuance of common stock in connection with January 2014 public offering at $8.50 per share, net of fees of $3.2 million | 4,705,882 | 1 | — | — | 36,835 | — | — | 36,836 | |||||||||||||||||||||
Exercise of warrants and reclassification of derivative liability | 1,947,377 | — | — | — | 31,762 | — | — | 31,762 | |||||||||||||||||||||
August 2013 private placement settlement | — | — | — | — | 272 | — | — | 272 | |||||||||||||||||||||
Treasury stock | — | — | (248,801 | ) | (2,258 | ) | — | — | — | (2,258 | ) | ||||||||||||||||||
Issuance of common stock to convertible debt holders | 401,047 | — | — | — | 4,708 | — | — | 4,708 | |||||||||||||||||||||
Unrealized gain/(loss) on marketable securities | — | — | — | — | — | 4,395 | — | 4,395 | |||||||||||||||||||||
Net loss | — | — | — | — | — | — | (110,938 | ) | (110,938 | ) | |||||||||||||||||||
BALANCE - DECEMBER 31, 2014 | 26,428,071 | $ | 3 | (379,591 | ) | $ | (3,215 | ) | $ | 140,851 | $ | 4,285 | $ | (179,175 | ) | $ | (37,251 | ) | |||||||||||
Share based payments | — | — | — | — | 25,900 | — | — | 25,900 | |||||||||||||||||||||
Vesting of stock for accrued severance | — | — | — | — | 2,126 | — | — | 2,126 | |||||||||||||||||||||
Issuance of common stock in connection with March 2015 public offering at $19.00 per share, net of fees of $9 million | 7,866,000 | 1 | — | — | 139,986 | — | — | 139,987 | |||||||||||||||||||||
Exercise of warrants and reclassification of derivative liability | 870,306 | — | — | — | 28,012 | — | — | 28,012 | |||||||||||||||||||||
Retirement of treasury stock | (379,591 | ) | — | 379,591 | 3,215 | — | — | (3,215 | ) | — | |||||||||||||||||||
Unrealized gain/(loss) on marketable securities | — | — | — | — | — | (4,927 | ) | — | (4,927 | ) | |||||||||||||||||||
Foreign currency translation adjustments | — | — | — | — | — | (40 | ) | — | (40 | ) | |||||||||||||||||||
Option inducement liability reversal and adjustments | — | — | — | — | 3,840 | — | — | 3,840 | |||||||||||||||||||||
Issuance of common shares under the equity incentive plan | 1,019,788 | — | — | — | 6,818 | — | — | 6,818 | |||||||||||||||||||||
Shares issued in connection with Cholbam acquisition | 661,279 | — | — | — | 15,844 | — | — | 15,844 | |||||||||||||||||||||
Excess tax benefits of stock option exercises | — | — | — | — | 2,425 | — | — | 2,425 | |||||||||||||||||||||
Net income | — | — | — | — | — | — | 117,237 | 117,237 | |||||||||||||||||||||
BALANCE - DECEMBER 31, 2015 | 36,465,853 | $ | 4 | — | $ | — | $ | 365,802 | $ | (682 | ) | $ | (65,153 | ) | $ | 299,971 | |||||||||||||
Share based payments | — | — | — | — | 29,102 | — | — | 29,102 | |||||||||||||||||||||
Legal fee settlement-short swing profit recovery | — | — | — | — | 2,025 | — | — | 2,025 | |||||||||||||||||||||
Exercise of warrants and reclassification of derivative liability | 898,633 | — | — | — | 20,720 | — | — | 20,720 | |||||||||||||||||||||
Unrealized gain/(loss) on marketable securities | — | — | — | — | — | 99 | — | 99 | |||||||||||||||||||||
Foreign currency translation adjustments | — | — | — | — | 3 | 93 | — | 96 | |||||||||||||||||||||
Issuance of common shares under the equity incentive plan | 542,183 | — | — | — | 4,016 | — | — | 4,016 | |||||||||||||||||||||
Tax shortfall from stock option exercises | — | — | — | — | (359 | ) | — | — | (359 | ) | |||||||||||||||||||
Net loss | — | — | — | — | — | — | (47,903 | ) | (47,903 | ) | |||||||||||||||||||
BALANCE - DECEMBER 31, 2016 | 37,906,669 | $ | 4 | — | $ | — | $ | 421,309 | $ | (490 | ) | $ | (113,056 | ) | $ | 307,767 | |||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-5
RETROPHIN, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
For the year ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Cash Flows From Operating Activities: | |||||||||||
Net income (loss) | $ | (47,903 | ) | $ | 117,237 | $ | (110,938 | ) | |||
Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
Depreciation and amortization | 16,135 | 13,392 | 5,401 | ||||||||
Realized (gain) loss on marketable securities | (8 | ) | 293 | (2,349 | ) | ||||||
Gain upon divestiture of Pediatric Priority Review Voucher | — | (140,004 | ) | — | |||||||
Gain upon divestiture of assets to Turing Pharmaceuticals | — | (914 | ) | — | |||||||
Deferred income tax | (22,661 | ) | (15,573 | ) | (2,460 | ) | |||||
Settlement expense | 5,212 | — | 6,018 | ||||||||
Loss on extinguishment of debt | — | 4,151 | — | ||||||||
Impairment of intangible assets | — | 4,710 | — | ||||||||
Loss on disposal of fixed assets | 62 | 112 | — | ||||||||
Derivative financial instruments, warrants, issued, recorded in interest expense | — | 1,050 | — | ||||||||
Accretion on notes receivable | (1,927 | ) | (1,267 | ) | — | ||||||
Accretion on Contingent Consideration | 1,976 | 2,461 | — | ||||||||
Amortization of debt discount and deferred financing costs | 656 | 1,340 | 1,084 | ||||||||
Amortization of premiums on investments | 1,097 | 398 | — | ||||||||
Non-cash financing cost | — | — | 4,708 | ||||||||
Loss on impairment of cost method purchase | — | — | 400 | ||||||||
Share based compensation | 29,102 | 25,900 | 15,900 | ||||||||
Legal accrual reversal | (2,967 | ) | — | — | |||||||
Bargain purchase gain | — | (49,063 | ) | — | |||||||
Change in estimated fair value of contingent consideration, net of payments | 18,383 | 13,778 | — | ||||||||
Payments from Change in Fair Value of Contingent Consideration | (2,571 | ) | (490 | ) | — | ||||||
Change in estimated fair value of liability classified warrants | (1,655 | ) | 33,307 | 23,786 | |||||||
Changes in operating assets and liabilities, net of business acquisitions: | |||||||||||
Accounts receivable | (6,090 | ) | (4,504 | ) | (7,959 | ) | |||||
Inventory | (306 | ) | (1,174 | ) | (282 | ) | |||||
Prepaid expenses and other current assets | (2,447 | ) | (966 | ) | 237 | ||||||
Prepaid income taxes | 4,644 | (8,107 | ) | — | |||||||
Accounts payable and accrued expenses | 9,672 | 3,379 | 20,604 | ||||||||
Net cash used in operating activities | (1,596 | ) | (554 | ) | (45,850 | ) | |||||
Cash Flows From Investing Activities: | |||||||||||
Purchase of fixed assets | (1,428 | ) | (22 | ) | (663 | ) | |||||
Purchase of intangible assets | (10,496 | ) | (7,008 | ) | (3,301 | ) | |||||
Security deposits | (115 | ) | — | (93 | ) | ||||||
Proceeds from the sale/maturity of marketable securities | 159,520 | 9,977 | 6,493 | ||||||||
Purchase of marketable securities | (184,111 | ) | (198,530 | ) | (10,149 | ) | |||||
Proceeds from securities sold, not yet purchased | — | — | 7,500 | ||||||||
Securities sold, not yet purchased | — | — | (7,500 | ) | |||||||
Proceeds from the maturity of notes receivable | 47,500 | — | — | ||||||||
Cash received upon sale of assets, net | — | 148,411 | — | ||||||||
Cash paid for investment | — | — | (400 | ) | |||||||
Cash paid upon acquisition, net of cash acquired | (500 | ) | (33,430 | ) | (29,150 | ) | |||||
Net cash provided by (used in) investing activities | 10,370 | (80,602 | ) | (37,263 | ) | ||||||
Cash Flows From Financing Activities: | |||||||||||
Payment of acquisition-related contingent consideration | (12,356 | ) | (3,938 | ) | (1,163 | ) | |||||
Payment of other liability | (1,000 | ) | (2,000 | ) | (500 | ) | |||||
Payment of guaranteed minimum royalty | (2,000 | ) | (2,000 | ) | — | ||||||
Proceeds from credit agreement | — | — | 42,366 | ||||||||
Proceeds from Note Purchase Agreement | — | — | 42,924 | ||||||||
Proceeds from exercise of warrants | 6,005 | 4,475 | 8,398 | ||||||||
Proceeds from exercise of stock options | 4,016 | 6,818 | — | ||||||||
Repayment of Manchester note payable | — | — | (31,283 | ) | |||||||
Excess tax benefit (shortfall) related to stock compensation | (359 | ) | 2,425 | — | |||||||
Proceeds received from issuance of common stock | — | 149,487 | 40,000 | ||||||||
Financing costs from issuance of common stock | — | (9,500 | ) | (3,165 | ) | ||||||
F-6
Repayment of credit facility | — | (45,000 | ) | — | |||||||
Purchase of treasury stock, at cost | — | — | (2,257 | ) | |||||||
Net cash provided by (used in) financing activities | (5,694 | ) | 100,767 | 95,320 | |||||||
Effect of exchange rate changes on cash | 117 | (10 | ) | — | |||||||
Net increase in cash and cash equivalents | 3,197 | 19,601 | 12,207 | ||||||||
Cash and cash equivalents, beginning of year | 37,805 | 18,204 | 5,997 | ||||||||
Cash and cash equivalents, end of year | $ | 41,002 | $ | 37,805 | $ | 18,204 | |||||
Supplemental Disclosure of Cash Flow Information: | |||||||||||
Cash paid for interest | $ | 2,070 | $ | 5,838 | $ | 4,080 | |||||
Cash paid for income taxes | $ | 7,933 | $ | 9,610 | $ | 5 | |||||
Non-cash Investing and financing activities: | |||||||||||
Short swing profit judgment offset with settlement expense accrual | $ | 2,025 | $ | — | $ | — | |||||
Reclassification of derivative liability to equity due to exercise of warrants | $ | 14,715 | $ | 23,537 | $ | 23,365 | |||||
Accrued royalty in excess of minimum payable to the sellers of Thiola | $ | 11,206 | $ | 8,219 | $ | — | |||||
Present value of contingent consideration payable upon acquisition related to L-UDCA | $ | 25,000 | $ | — | $ | — | |||||
Present value of contingent consideration payable upon acquisition relate to Cholbam | $ | — | $ | 42,010 | $ | — | |||||
Shares issued in connection with Cholbam acquisition | $ | — | $ | 15,844 | $ | — | |||||
Present value of contingent consideration payable upon acquisition related to Chenodal | $ | — | $ | — | $ | 12,800 | |||||
Present value of guaranteed minimum royalty payable to sellers of Thiola | $ | — | $ | — | $ | 11,850 | |||||
Note payable entered into upon acquisition of Manchester Pharmaceuticals, LLC. | $ | — | $ | — | $ | 31,283 | |||||
Purchase of Kyalin in exchange for future consideration | $ | — | $ | — | $ | 1,000 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
RETROPHIN, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. DESCRIPTION OF BUSINESS
Organization and Description of Business
Retrophin, Inc. (“we”, “our”, “us”, “Retrophin” and the “Company”) refers to Retrophin, Inc., a Delaware corporation, as well as our direct and indirect subsidiaries. Retrophin is a fully integrated biopharmaceutical company headquartered in San Diego, California focused on the development, acquisition and commercialization of therapies for the treatment of serious or rare diseases. We regularly evaluate and, where appropriate, act on opportunities to expand our product pipeline through licenses and acquisitions of products in areas that will serve patients with serious or rare diseases and that we believe offer attractive growth characteristics.
We are currently developing the following pipeline products:
Sparsentan is an investigational therapeutic agent which acts as both a potent ARB, as well as a selective ERA, with in vitro selectivity toward endothelin receptor type A. The Company has secured a license to sparsentan from Ligand Pharmaceuticals, Inc. and Bristol-Myers Squibb Company (who referred to it as DARA). The Company is developing sparsentan as a treatment for FSGS, which is a leading cause of end-stage renal disease and NS. Sparsentan was granted orphan drug designation in the United States and the European Union in January 2015 and November 2015, respectively. In the third quarter of 2016, the Company announced positive top-line data from the Phase 2 DUET study of sparsentan for the treatment of FSGS. In early 2017, we had an End of Phase 2 meeting with the FDA regarding the regulatory pathway for sparsentan as a treatment for FSGS. Following the meeting and our receipt of confirmatory meeting minutes, we announced our plans to initiate a single Phase 3 clinical trial to serve as the basis of a New Drug Application ("NDA") filing for sparsentan for the treatment of FSGS.
Fosmetpantotenate, also known as RE-024, a novel small molecule, is being developed as a potential treatment for pantothenate kinase-associated neurodegeneration (“PKAN”). PKAN is a genetic neurodegenerative disorder that is typically diagnosed in the first decade of life. Consequences of PKAN include parkinsonism, dystonia, and other severe systemic manifestations. Fosmetpantotenate (RE-024) is a phosphopantothenate replacement therapy that aims to restore levels of this key substrate in PKAN patients. Certain international health regulators have approved the initiation of dosing fosmetpantotenate (RE-024) in PKAN patients under physician-initiated studies in accordance with local regulations in their respective countries. The Company filed a U.S. IND for fosmetpantotenate (RE-024) with the FDA in the first quarter of 2015 to support the commencement of a Company-sponsored Phase 1 study, which was successfully completed during 2015. The FDA granted fosmetpantotenate (RE-024) orphan drug designation in May 2015 and fast track designation in June 2015. In February, 2016, the Company announced fosmetpantotenate (RE-024) was granted orphan drug designation by the European Commission. In November, 2016, the Company announced that it had reached an agreement with the FDA under the Special Protocol Assessment (SPA) process for a Phase 3 clinical trial evaluating fosmetpantotenate (RE-024) for PKAN. The Company expect to begin dosing patients in this Phase 3 clinical trial in the coming months. The Company continues discussions with the EMA regarding the initiation of a potential registration-enabling efficacy trial in PKAN patients.
Tetracosactide zinc (RE-034) is a synthetic hormone analog of the first 24 amino acids of the 39 amino acids contained in adrenocorticotropic hormone ("ACTH") incorporated into a novel formulation developed by us. Tetracosactide zinc (RE-034) exhibits similar physiological actions as endogenous ACTH by binding to all five melanocortin receptors (pan-MCR), resulting in its anti-inflammatory and immunomodulatory effects. The Company successfully manufactured tetracosactide zinc (RE-034) at proof-of-concept scale using a novel formulation process that allows modulation of the release of the active ingredient from the site of administration. The Company decided to focus our further efforts on this program toward an external partnership and out-licensing opportunities.
Liquid ursodeoxycholic acid ("L-UDCA") is a liquid formulation of ursodeoxycholic acid being developed for the treatment of a rare liver disease called primary biliary cholangitis ("PBC"). We obtained L-UDCA during 2016 with the intention of making L-UDCA commercially available to the subset of PBC patients who have difficulty swallowing.
We currently sell the following three products:
• | Chenodal (chenodiol tablets) is approved in the United States for the treatment of patients suffering from gallstones in whom surgery poses an unacceptable health risk due to disease or advanced age. Chenodal has been the standard of care for CTX patients for more than three decades and the Company is currently pursuing adding this indication to the label. |
• | Cholbam (cholic acid capsules) is approved in the United States for the treatment of bile acid synthesis disorders due to single enzyme defects and is further indicated for adjunctive treatment of patients with peroxisomal disorders. |
• | Thiola (tiopronin tablets) is approved in the United States for the prevention of cystine (kidney) stone formation in patients with severe homozygous cystinuria. |
F-8
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
A summary of the significant accounting policies applied in the preparation of the accompanying consolidated financial statements follows:
Principles of Consolidation
The consolidated financial statements represent the consolidation of the accounts of the Company and its subsidiaries in conformity with accounting principles generally accepted in the United States ("U.S. GAAP"). All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
In preparing financial statements in conformity with U.S. GAAP, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of expenses during the reporting period. Due to inherent uncertainty involved in making estimates, actual results reported in future periods may be affected by changes in these estimates. On an ongoing basis, the Company evaluates its estimates and assumptions. These estimates and assumptions include revenue recognition, valuing equity securities in share-based payments, estimating fair value of equity instruments recorded as derivative liabilities, estimating the fair value of net assets acquired in business combinations, estimating the useful lives of depreciable and amortizable assets, goodwill impairment, estimating of contingent consideration, estimating of valuation allowances and uncertain tax positions, and estimating the fair value of long-lived assets to assess whether impairment charges may apply.
Revenue Recognition
Product sales for the year ended December 31, 2016 consisted of sales of Chenodal, Cholbam and Thiola. Product sales for the year ended December 31, 2015 consisted of sales of Chenodal, Cholbam, Thiola and Vecamyl (divested in 2015). Product sales for the year ended December 31, 2014 consisted of sales of Chenodal, Thiola and Vecamyl. Revenue from product sales is recognized when persuasive evidence of an arrangement exists, title to product and associated risk of loss have passed to the customer, the price is fixed or determinable, collection from the customer is reasonably assured, the Company has no further performance obligations, and returns can be reasonably estimated. The Company sells in the United States and Canada through a direct-to-patient distributor. Under this distribution model, the Company records revenues when customers take title of the product.
The Company sells Kolbam internationally, and these revenues are immaterial when taken in consideration of the financial statements as a whole.
Revenue from product sales is recorded net of applicable provisions for rebates under government programs (including Medicaid), prompt pay discounts, and other sales-related deductions. We review our estimates of rebates and other applicable provisions each period and record any necessary adjustments in the current period.
Deductions from Revenue
Government Rebates and Chargebacks: The Company estimates the rebates that we will be obligated to provide to government programs and deducts these estimated amounts from our gross product sales at the time the revenues are recognized. Allowances for government rebates and discounts are established based on actual payer information, which is reasonably estimated at the time of delivery, and the government-mandated discounts applicable to government-funded programs. Rebate discounts are included in other current liabilities in the accompanying consolidated balance sheets.
Prompt Pay Discounts: The Company offers discounts to certain customers for prompt payments. The Company accrues for the estimated prompt pay discount based on the gross amount of each invoice for those customers at the time of sale.
Product Returns: Consistent with industry practice, the Company offers its customers a limited right to return product purchased directly from the Company, which is principally based upon the product’s expiration date. Generally, shipments are only made upon a patient prescription thus returns are minimal.
Research and Development Costs
Research and development includes expenses related to RE-021, fosmetpantotenate (RE-024) and our other pipeline programs. We expense all research and development costs as they are incurred. Our research and development costs are comprised of salaries and bonuses, benefits, non-cash share based compensation, license fees, milestones under license agreements, costs paid to third-party contractors to perform research, conduct clinical trials, and develop drug materials and delivery devices, and associated overhead expenses and facilities costs. Reimbursed research and development costs under collaborative arrangements are recorded as a reduction to research and development costs. We charge direct internal and external program costs to the respective development programs. We also incur indirect costs that are not allocated to specific programs because such costs benefit multiple development programs and allow us to increase our pharmaceutical development capabilities. These consist of internal shared resources related to the development and maintenance of systems and processes applicable to all of our programs. Clinical trial costs are a significant component of research and development expenses and include costs associated with third-party contractors, and clinical research organizations (“CRO’s"). Invoicing from third-party contractors for services performed can lag several months. We accrue the costs of services rendered in connection with third-party contractor activities based on our estimate of management fees, and costs associated with site monitoring and data management.
F-9
Employee Stock-Based Compensation
The Company recognizes all employee share-based compensation as a cost in the financial statements. Equity-classified awards principally related to stock options, restricted stock units (“RSUs”) and performance stock units ("PSUs"), are measured at the grant date fair value of the award. The Company determines grant date fair value of stock option awards using the Black-Scholes option-pricing model. The fair value of RSUs are determined using the closing price of the Company’s common stock on the grant date. For service based vesting grants, expense is recognized over the requisite service period based on the number of options or shares expected to ultimately vest. For PSUs, expense is recognized over the implicit service period, assuming vesting is probable. No expense is recognized for PSUs if it is not probable the vesting criteria will be satisfied. Forfeitures are estimated at the date of grant and revised when actual or expected forfeiture activity differs materially from original estimates.
Vesting Term | |
Stock Options | 1 to 3 years |
Restricted Stock Units | 1 to 3 years |
Earnings (Loss) Per Share
We calculate our basic earnings per share by dividing net income by the weighted average number of shares outstanding during the period. The diluted earnings per share computation includes the effect, if any, of shares that would be issuable upon the exercise of outstanding stock options, derivative liability, convertible debt and RSUs, reduced by the number of shares which are assumed to be purchased by the Company from the resulting proceeds at the average market price during the year, when such amounts are dilutive to the earnings per share calculation.
Cash and Cash Equivalents
We consider all highly liquid marketable securities with an original maturity of three months or less to be cash equivalents. Due to the short-term maturity of such investments, the carrying amounts are a reasonable estimate of fair value.
Marketable Securities
The Company accounts for marketable securities held as “available-for-sale” in accordance with ASC 320, “Investments Debt and Equity Securities” (“ASC 320”). The Company classifies these investments as current assets and carries them at fair value. Unrealized gains and losses are recorded as a separate component of stockholders’ equity as accumulated other comprehensive loss. Realized gains or losses on marketable security transactions are reported in the Statements of Operations and Comprehensive Income (Loss). Marketable securities are maintained at one financial institution and are governed by the Company’s investment policy as approved by our Board of Directors.
Trade and Notes Receivable
Trade Receivables, Net
Trade accounts receivable are recorded net of allowances for prompt payment and doubtful accounts. Estimates for allowances for doubtful accounts are determined based on existing contractual obligations, historical payment patterns and individual customer circumstances. The allowance for doubtful accounts was $0.3 million and $0.0 million at December 31, 2016 and 2015, respectively. For the years ended December 31, 2016 and 2015, bad debt expense recorded in the Statement of Operations and Comprehensive Income (Loss) is approximately $0.2 million and less than $0.1 million, respectively. There was no bad debt expense recorded in the Statement of Operations and Comprehensive Income (Loss) for the year ended December 31, 2014.
Notes Receivable
Notes receivable arose from the sale of the pediatric priority review voucher (the "PRV"). On July 2, 2015, the Company sold and transferred the PRV to Sanofi for $245.0 million. $150.0 million was received upon closing, and $47.5 million is due on each of the first and second anniversaries of the closing. In accordance with U.S. GAAP, the Company recorded the future short term and long term notes receivable at their present value of $46.2 million and $44.9 million, respectively, at the date of the sale using a discount rate of 2.8%. The accretion on the notes receivables totaled $1.9 million and is recorded in interest expense, net, in the consolidated statements of operations and comprehensive income (loss) for 2016. The first annual payment was received on July 1, 2016 in accordance with the terms of the sale agreement. As of December 31, 2016, the present value of the remaining receivable is $46.8 million and is recorded in current assets. As of December 31, 2015 the present value of the receivables was $46.8 million and $45.6 million which were recorded as current and long term assets respectively. The Company noted no indications for impairment as of December 31, 2016 and 2015.
Inventories and Related Reserves
Inventories, which are recorded at the lower of cost or market, include materials, labor, and other direct and indirect costs and are valued using the first-in, first-out method. The Company periodically analyzes its inventory levels to identify inventory that may expire prior to expected sale or has a cost basis in excess of its estimated realizable value, and writes down such inventory as appropriate. In addition, the Company's products are subject to strict quality control and monitoring which the Company’s manufacturers perform throughout their manufacturing process. The Company does not directly manufacture any product. The Company has single suppliers for products Chenodal and Thiola, and prospectively arranges for manufacture from contract service providers for its product Cholbam. The inventory reserve was $0.6 million and $0.3 million at December 31, 2016 and 2015, respectively.
F-10
Inventory, net of reserve, consisted of the following at December 31, 2016 and 2015 (in thousands):
December 31, 2016 | December 31, 2015 | ||||||
Raw material | $ | 1,336 | $ | 289 | |||
Finished goods | 1,490 | 2,247 | |||||
Total inventory | $ | 2,826 | $ | 2,536 | |||
Property and Equipment, net
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is computed using the straight-line method over the related estimated useful lives as presented in the table below. Significant additions and improvements are capitalized, while repairs and maintenance are charged to expense as incurred. Property and equipment purchased for specific research and development projects with no alternative use is expensed as incurred.
The major classifications of property and equipment, including their respective expected useful lives, consists of the following:
Computer equipment | 3 years |
Furniture and fixtures | 7 years |
Leasehold improvements | Shorter of length of lease or life of the asset |
Intangible Assets, Net
Our intangible assets consist of licenses, purchased technology and acquired in-process research and development (IPR&D). Intangible assets with definite lives are amortized on a straight-line basis over their estimated useful lives and are reviewed periodically for impairment.
Intangible assets related to IPR&D projects are considered to be indefinite-lived until the completion or abandonment of the associated research and development efforts. During the period the assets are considered indefinite-lived, they will not be amortized but will be tested for impairment. If and when development is complete, which generally occurs when regulatory approval to market a product is obtained, the associated assets are deemed finite-lived and are amortized over a period that best reflects the economic benefits provided by these assets.
Goodwill
Goodwill represents the excess of purchase price over fair value of net assets acquired in a business combination and is not amortized. Goodwill is subject to impairment testing at least annually or when a triggering event occurs that could indicate a potential impairment. The Company has one segment and one reporting unit and as such reviews goodwill for impairment at the consolidated level.
For the years ended December 31, 2016, 2015 and 2014 there were no impairments to goodwill.
Impairment of Long-Lived Assets
Our long-lived assets are primarily comprised of intangible assets and property and equipment. We evaluate our finite-lived intangible assets and property and equipment, for impairment whenever events or changes in circumstances indicate the carrying value of an asset or group of assets may not be recoverable. If these circumstances exist, recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset group to future undiscounted net cash flows expected to be generated by the asset group. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets.
In addition, indefinite-lived intangible assets, comprised of IPR&D, are reviewed for impairment annually and whenever events or changes in circumstances indicate that it is more likely than not that the asset is impaired by comparing the fair value to the carrying value of the asset.
For the year ended December 31, 2015 the Company wrote off the intangible asset related to Carbetocin and recorded a loss of $4.7 million. There were no impairments related to intangible assets in the years ended December 31, 2016 or 2014.
Contingent Consideration
We record contingent consideration resulting from a business combination at its fair value on the acquisition date. On a quarterly basis, we revalue these obligations and record increases or decreases from their fair value as an adjustment to operating earnings. Changes to contingent consideration obligations can result from changes to discount rates, accretion of the liability due to the passage of time, changes in revenue forecasts and changes in our estimates of the likelihood or timing of achieving commercial milestones.
Income Taxes
The Company follows ASC 740, Income Taxes, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the asset will not be realized.
F-11
The standard addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the tax authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. ASC 740 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures. The Company’s policy is to record estimated interest and penalty related to the underpayment of income taxes or unrecognized tax benefits as a component of its income tax provision.
Reclassifications
Certain reclassifications have been made to the prior year financial statements in order to conform to the current year’s presentation.
Patents
The Company expenses external costs, such as filing fees and associated attorney fees, incurred to obtain issued patents and patent applications pending. The Company also expenses costs associated with maintaining and defending patents subsequent to their issuance in the period incurred.
Derivative Financial Instruments, Warrants
The Company does not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks. However, certain warrants to purchase common stock that do not meet the requirements for classification as equity, in accordance with the Derivatives and Hedging Topic of the ASC, are classified as liabilities.The Company’s warrants are classified as liability instruments due to an anti-dilution provision that provides for a reduction to the exercise price of the warrants if the Company issues additional equity or equity linked instruments in the future at an effective price per share less than the exercise price then in effect. The derivative instrument was initially recorded at its fair value and is then revalued at each reporting date, with changes in the fair value reported in the consolidated statements of operations and comprehensive income (loss).
Treasury Stock
The Company records treasury stock at the cost to acquire it and includes treasury stock as a component of stockholders’ equity until it is retired.
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies. Unless otherwise discussed, the Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on its consolidated financial position or results of operations upon adoption.
In May 2014, the Financial Accounting Standard Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers. Under the new standard, revenue is recognized at the time a good or service is transferred to a customer for the amount of consideration for which the entity expects to be entitled for that specific good or service. Entities may use a full retrospective approach or report the cumulative effect as of the date of adoption. On July 9, 2015, the FASB voted to defer the effective date by one year to December 15, 2017 for interim and annual reporting periods beginning after that date. Early adoption of ASU 2014-09 is permitted but not before the original effective date (annual periods beginning after December 15, 2016). The Company does not expect the new standard to change the timing for recognition of revenue from product sales, but it is still reviewing the standard and its effects on disclosures and other matters.
In February 2016, the FASB issued Accounting Standards Update No. 2016-02, Leases. The new standard establishes a right-of-use (ROU) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available.The Company is in the process of evaluating the impact of this guidance on its consolidated financial statements and related disclosures; however, based on the Company's current operating leases, it is expected to have a material impact to the Consolidated Balance Sheet by increasing assets and liabilities.
In April 2016, the FASB issued ASU No. 2016-09, Compensation —Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. Specifically, the ASU requires all excess tax benefits and tax deficiencies (including tax benefits of dividends on share-based payment awards) to be recognized as income tax expense or benefit in the income statement. The tax effects of exercised or vested awards should be treated as discrete items in the reporting period in which they occur. An entity also should recognize excess tax benefits, and assess the need for a valuation allowance, regardless of whether the benefit reduces taxes payable in the current period. That is, off balance sheet accounting for net operating losses stemming from excess tax benefits would no longer be required and instead such net operating losses would be recognized when they arise. Existing net operating losses that are currently tracked off balance sheet would be recognized, net of a valuation allowance if required, through an adjustment to opening retained earnings in the period of adoption. Entities will no longer need to maintain and track an “APIC pool.” The ASU also requires excess tax benefits to be classified along with other income tax cash flows as an operating activity in the statement of cash flows. In addition, the ASU elevates the statutory tax withholding threshold to qualify for equity classification up to the maximum statutory tax rates in the applicable jurisdiction(s). The ASU also clarifies that cash paid by an employer when directly withholding shares for tax withholding purposes should be classified as a financing activity. The ASU provides an optional accounting policy election (with limited exceptions), to be applied on an entity-wide
F-12
basis, to either estimate the number of awards that are expected to vest (consistent with GAAP) or account for forfeitures when they occur. The ASU is effective for public business entities for annual periods beginning after December 15, 2016, and interim periods within those annual periods. Upon adoption, all of the tax effects related to share-based payments at settlement (or expiration) will be recorded through the income statement. In 2016 and 2015, the Company would have recorded a tax expense of $0.4 million and a tax benefit of $2.4 million, respectively. In addition the Company will adopt accounting for forfeitures as actuals rates in the period they occur.
In June 2016, the FASB issued No. 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. Topic 326 amends guidance on reporting credit losses for assets held at amortized cost basis and available for sale debt securities. For assets held at amortized cost basis, Topic 326 eliminates the probable initial recognition threshold in current GAAP and, instead, requires an entity to reflect its current estimate of all expected credit losses. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial assets to present the net amount expected to be collected. For available for sale debt securities, credit losses should be measured in a manner similar to current GAAP, however Topic 326 will require that credit losses be presented as an allowance rather than as a write-down. This Accounting Standards Update affects entities holding financial assets and net investment in leases that are not accounted for at fair value through net income. The amendments affect loans, debt securities, trade receivables, net investments in leases, off balance sheet credit exposures, reinsurance receivables, and any other financial assets not excluded from the scope that have the contractual right to receive cash. This update is effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. As of December 31, 2016, the Company holds $214.9 million in available for sale debt securities that are affected by this ASU. If adopted as of December 31, 2016, this would not have a material impact on financial statements.
In October 2016, the FASB issued No. 2016-16, Income Taxes (Topic 740): Intra-Entity Transfers of Assets Other Than Inventory. The new guidance changes the accounting for income tax effects of intra-entity transfers of assets other than inventory. Under the new guidance, the selling (transferring) entity is required to recognize a current tax expense or benefit upon transfer of the asset. Similarly, the purchasing (receiving) entity is required to recognize a deferred tax asset or deferred tax liability, as well as the related deferred tax benefit or expense, upon receipt of the asset. The new guidance will be effective for the Company starting in fiscal year 2018 on a modified retrospective basis, and early adoption is permitted. As of December 31, 2016, the Company has recorded a Prepaid Tax Asset of $4.9 million related to an Intra-Entity Transfer. Upon adoption, the Company will reverse the balance in its Prepaid Tax Asset account as a charge to retained earnings. The Company is currently evaluating whether to adopt the new guidance early.
In January 2017, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update (“ASU”) 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business. The new guidance dictates that, when substantially all of the fair value of the gross assets acquired (or disposed of) is concentrated in a single identifiable asset or a group of similar identifiable assets, it should be treated as an acquisition or disposal of an asset. The guidance will be effective for the fiscal year beginning on January 1, 2018, including interim periods within that year (early adoption is permitted). The Company is currently evaluating the potential effect of the guidance on its consolidated financial statements.
NOTE 3. BUSINESS COMBINATION AND DIVESTITURE OF ASSETS
Acquisition of Liquid Ursodeoxycholic Acid (L-UDCA)
On June 20, 2016, the Company announced the signing of a definitive agreement to purchase the rights, titles, licenses and ownership of L-UDCA from Asklepion Pharmaceuticals, LLC ("Asklepion").
The acquisition was accounted for under the purchase method of accounting in accordance with Accounting Standard Codification ("ASC") 805. The fair value of assets acquired and liabilities assumed was based upon an independent third-party valuation and the Company’s estimates. Critical estimates in valuing certain intangible assets include but are not limited to future expected cash flows from acquired product rights for L-UDCA, licenses, trade names and developed technologies, present value and discount rates. Management’s estimates of fair value are based upon assumptions believed to be reasonable, but which are inherently uncertain and unpredictable and, as a result, actual results may differ from estimates.
The purchase included $25.5 million for an intangible asset with a definite life related to product rights in the U.S. The useful life related to the acquired product rights is expected to be approximately 17 years once the NDA is approved by the FDA. Until approval, the asset is considered IPR&D with an indefinite life and is not amortized.
The contingent consideration of $25.0 million (present value) recorded during the period ended June 30, 2016, is related to an agreement to pay an additional cash amount in the form of milestones and sales royalties through 2035. The accrued contingent consideration was recorded as a liability at acquisition-date fair value using the income approach with an assumed discount rate of 12.0% over the applicable term. The undiscounted amount the Company could pay as contingent consideration under the agreement is up to $70.3 million.
F-13
The purchase price allocation of $25.5 million as of the acquisition completion date of June 16, 2016 is as follows (in thousands):
Cash paid upon consummation | $ | 500 | |
Present value of contingent consideration | 25,000 | ||
Total purchase price | $ | 25,500 | |
Fair Value of Assets Acquired and Liabilities Assumed | |||
Acquired product rights: L-UDCA (intangible asset) | $ | 25,500 | |
Total purchase price | $ | 25,500 | |
Unaudited pro forma information for the transaction is not presented, because the effects of such transaction are considered immaterial to the Company.
Acquisition of Cholbam (cholic acid)
On January 12, 2015, the Company announced the signing of a definitive agreement under which it acquired the exclusive right to purchase from Asklepion, all worldwide rights, titles, and ownership of Cholbam (cholic acid) for the treatment of bile acid synthesis disorders, if approved by the FDA. Under the terms of the agreement, the Company paid Asklepion an upfront payment of $5.0 million and agreed to pay milestones based on FDA approval and net product sales, plus tiered royalties on future net sales of Cholbam.
On March 18, 2015, the Company announced that the FDA had approved Cholbam capsules, the first FDA approved treatment for pediatric and adult patients with bile acid synthesis disorders due to single enzyme defects, and for patients with peroxisomal disorders (including Zellweger spectrum disorders). As a result of the approval, the Company exercised its right to purchase from Asklepion all worldwide rights, titles, and ownership of Cholbam and related assets. The FDA also granted Asklepion a Rare Pediatric Disease Priority Review Voucher ("Pediatric PRV"), awarded to encourage development of new drugs and biologics for the prevention and treatment of rare pediatric diseases. A Pediatric PRV is transferable and provides the bearer with FDA priority review classification for a new drug application. The Pediatric PRV was transferred to Retrophin under the original terms of the agreement with Asklepion.
On March 31, 2015, the Company completed its acquisition from Asklepion of all worldwide rights, titles and ownership of Cholbam, including all related contracts, data assets, intellectual property, regulatory assets and the Pediatric PRV, in exchange for a cash payment of $28.4 million, in addition to approximately 661,279 shares of the Company’s common stock (initially valued at $9 million at the time of the definitive agreement with Asklepion, and $15.8 million at the acquisition completion date). The Company is also required to pay contingent consideration consisting of milestones and tiered royalties with a present value of $39.1 million.
The original asset value of the Pediatric PRV was recognized at $96.3 million. In this valuation process, we considered various factors which included data from recent sales of similar vouchers. The consideration paid to Asklepion did not value the Pediatric PRV because the issuance of a Pediatric PRV is extremely rare. Therefore when the FDA granted the Pediatric PRV with the Cholbam approval, a bargain purchase gain resulted.
The acquisition was accounted for under the purchase method of accounting in accordance with ASC 805. The fair value of assets acquired and liabilities assumed was based upon an independent third-party valuation and the Company’s estimates. Critical estimates in valuing certain intangible assets include but are not limited to future expected cash flows from acquired product rights-Cholbam, Pediatric PRV, trade names and developed technologies, present value and discount rates. Management’s estimates of fair value are based upon assumptions believed to be reasonable, but which are inherently uncertain and unpredictable and, as a result, actual results may differ from estimates.
The purchase included $83.2 million of intangible assets with definite lives related to product rights with values of $75.9 million for the U.S. and $7.3 million for the international rights. The useful lives related to the acquired product rights are expected to be approximately 10 years.
The contingent consideration of $39.1 million recorded during the year ended December 31, 2015 is related to an agreement to pay an additional cash amount based on the product performance through 2025. The accrued contingent consideration was recorded as a liability at acquisition-date fair value using the income approach with assumed discount rates of 19.0% over the applicable term. The undiscounted amount the Company could pay as contingent consideration under the agreement is up to $78.4 million.
Service fees with a net present value of $2.9 million were recorded during the year ended December 31, 2015. The net present value is based upon $4.0 million in total payments over a four year period starting as of the acquisition date.
As part of the business combination the Company recorded a deferred tax liability of $39.9 million. The deferred tax liability is derived from the difference in the Company's book basis and tax basis in the assets acquired of $88.5 million. Our tax rate utilized is 45.4%.
F-14
The purchase price allocation of $91.3 million as of the acquisition completion date of March 31, 2015 is as follows (in thousands):
Cash paid upon consummation | $ | 33,430 | |
Present value of contingent consideration and service fees | 42,010 | ||
Fair Value of 661,279 shares issued to Asklepion | 15,844 | ||
Total Purchase Price | $ | 91,284 | |
Fair Value of Assets Acquired and Liabilities Assumed | |||
Acquired product rights-Cholbam (Intangible Asset) | $ | 83,200 | |
Pediatric Priority Review Voucher | 96,250 | ||
Inventory | 777 | ||
Deferred tax liability | (39,880 | ) | |
Total Allocation of Purchase Price | 140,347 | ||
Bargain Purchase Gain | (49,063 | ) | |
Total Purchase Price | $ | 91,284 | |
Acquisition of Manchester Pharmaceuticals LLC
On March 26, 2014 (the “Manchester Closing Date”), the Company acquired 100% of the outstanding membership interests of Manchester. Under the terms of the agreement, the Company paid $29.2 million upon consummation of the transaction, of which $3.2 million was paid by Retrophin Therapeutics International LLC, an indirect wholly owned subsidiary, for rights to use the trade name outside of the United States. Acquisition costs amounted to approximately $0.3 million and were recorded as selling, general, and administrative expense in the 2014 Consolidated Financial Statements. The Company entered into a promissory note with Manchester for $33 million which was discounted to $31.3 million to be paid in three equal installments of $11 million within three, six, and nine months after the Manchester Closing Date. On June 30, 2014, the Company paid the sellers of Manchester $33 million in full satisfaction of the outstanding amount owed.
In addition, the Company agreed to make contractual payments based on 10% of net sales of the products Chenodal and Vecamyl to the former members of Manchester. Additional contingent payments will be made based on 5% of net sales from any new products derived from Chenodal and Vecamyl. Business combination-related contingent consideration estimated at $12.8 million will be revalued at each reporting period and any change in valuation will be recorded in the Company’s statement of operations.
The acquisition was accounted for under the purchase method of accounting in accordance with ASC 805, with the excess purchase price over the fair market value of the assets acquired and liabilities assumed allocated to goodwill. Based on the purchase price allocation, the purchase price of $73.2 million resulted in goodwill of $0.9 million which is primarily attributed to the synergies expected to arise after the acquisition. The $0.9 million of goodwill resulting from the acquisition is deductible for income tax purposes.
Critical estimates in valuing certain intangible assets include but are not limited to future expected cash flows from customer relationships and developed technology, present value and discount rates. Management’s estimates of fair value are based an independent third-party valuation. These assumptions are believed to be reasonable, but which are inherently uncertain and unpredictable and, as a result, actual results may differ from estimates.
The purchase included $72 million of intangible assets with definite lives related to product rights, trade names, and customer relationships with values of $71.4 million, $0.2 million, and $0.4 million, respectively. The useful lives related to the acquired product rights, trade names, and customer relationships are approximately 16, 1 and 10 years, respectively. Under the terms of the agreement, the sellers agreed to indemnify the Company for uncertain tax liabilities, any breach of any representation or warranty the sellers made to the purchaser, failure of the sellers to perform any covenants or obligations made to the purchaser, and third party claims relating to the operation of the Company and events occurring prior to the Manchester Closing Date. As of December 31, 2014, the Company recorded an indemnification asset with a corresponding liability in the amount of $1.5 million related to uncertain tax liabilities.
F-15
The purchase price allocation of $73.2 million as of the Manchester Closing Date was as follows (in thousands):
Cash paid upon consummation, net | $ | 29,150 | |
Secured promissory note | 31,283 | ||
Fair value of business combination-related contingent consideration | 12,800 | ||
Total purchase price | $ | 73,233 | |
Prepaid expenses | $ | 116 | |
Inventory | 517 | ||
Product rights | 71,372 | ||
Trade names | 175 | ||
Customer relationship | 403 | ||
Goodwill | 936 | ||
Other asset | 1,522 | ||
Accounts payable and accrued expenses | (286 | ) | |
Other liability | (1,522 | ) | |
Total allocation of purchase price consideration | $ | 73,233 | |
Divestiture of Assets:
Sale of Assets to Sanofi
The FDA granted Asklepion a Pediatric PRV, awarded to encourage development of new drugs and biologics for the prevention and treatment of rare pediatric diseases. A Pediatric PRV is transferable and provides the bearer with FDA priority review classification for a new drug application. The Pediatric PRV was transferred to the Company under the terms of the asset purchase agreement between the Company and Asklepion dated January 12, 2015, pursuant to which the Company acquired Cholbam.
On July 2, 2015, the Company sold and transferred the Pediatric PRV to Sanofi for $245.0 million. $150.0 million was received upon closing, and $47.5 million is due on each of the first and second anniversaries of the closing. In accordance with U.S. GAAP, the Company recorded the future short term and long term notes receivable at their present value of $46.2 million and $44.9 million, respectively, at the date of the sale using a discount rate of 2.8%. The gain from the sale of the asset was approximately $140.0 million, net of $4.9 million in fees contractually due as part of the Cholbam acquisition. The first annual payment was received on July 1, 2016 in accordance with the terms of the sale agreement.
Sale of Assets to Turing Pharmaceuticals
On October 13, 2014, the Company entered into a binding Summary Separation Proposal with its then-current Chief Executive Officer. Among other things, the Summary Separation Proposal set forth the terms for the sale of the Company’s Vecamyl, Syntocinon and ketamine licenses and assets to Turing Pharmaceuticals, a company controlled by the former Chief Executive Officer.
On January 9, 2015, the Company entered into a purchase agreement with Turing Pharmaceuticals pursuant to which the Company sold Turing Pharmaceuticals the Sold Assets for a purchase price of $1.0 million, and pursuant to which Turing Pharmaceuticals also assumed all future liabilities related to the Sold Assets.
On February 13, 2015, the Sellers entered into a purchase agreement with Waldun, pursuant to which the Sellers sold Waldun the Vecamyl Product Rights for a purchase price of $0.7 million. Waldun in turn sold the Vecamyl Product Rights to Turing Pharmaceuticals. In connection therewith, on February 13, 2015, the Company, together with Manchester, entered into an asset purchase agreement with Turing Pharmaceuticals, pursuant to which the Company sold Turing Pharmaceuticals the Inventory for a purchase price of $0.3 million, and pursuant to which Turing Pharmaceuticals also assumed certain liabilities related to the Vecamyl Product Rights and the Inventory.
On February 13, 2015, the Company entered into an asset purchase agreement with Turing Pharmaceuticals pursuant to which the Company sold Turing Pharmaceuticals its Oxytocin Assets, including related inventory, for a purchase price of $1.1 million, and pursuant to which Turing Pharmaceuticals also assumed certain liabilities related to the Oxytocin Assets.See Note 16 for further discussion
The effect on the Statement of Operations and Comprehensive Income (Loss) for 2015 is a gain of approximately $0.9 million.
NOTE 4. MARKETABLE SECURITIES
The Company's marketable securities as of December 31, 2016 and 2015 were comprised of available-for-sale marketable securities which are carried at fair value, with the unrealized gains and losses reported in accumulated other comprehensive income (loss). The amortized cost of debt securities in this category are adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion is included in interest income. Realized gains and losses and declines in value judged to be other-than-temporary, if any, on available-for-sale securities are included in other income or expense. The cost of securities sold is based on the specific identification method. Interest and dividends on securities classified as available-
F-16
for-sale are included in interest income. All available-for-sale securities are classified as current assets, even if the maturity when acquired by the Company is greater than 1 year due to the possibility of liquidation within the next 12 months.
Marketable securities consist of the following (in thousands):
As of December 31, | |||||||
2016 | 2015 | ||||||
Marketable Securities: | |||||||
Commercial paper | 30,303 | 31,864 | |||||
Corporate debt securities | 134,570 | 125,547 | |||||
Securities of government sponsored entities | 49,998 | 34,388 | |||||
Total Marketable Securities: | $ | 214,871 | $ | 191,799 | |||
The following is a summary of short-term marketable securities classified as available-for-sale as of December 31, 2016 (in thousands):
Contractual Maturity (in years) | Amortized Cost | Unrealized Gains | Unrealized Losses | Aggregate Estimated Fair Value | |||||||||||||
Marketable Securities: | |||||||||||||||||
Commercial paper | Less than 1 | $ | 30,330 | $ | — | $ | (27 | ) | $ | 30,303 | |||||||
Corporate debt securities | Less than 1 | 64,794 | 7 | (91 | ) | 64,710 | |||||||||||
Securities of government-sponsored entities | Less than 1 | 19,500 | — | (10 | ) | 19,490 | |||||||||||
Total maturity less than 1 year | 114,624 | 7 | (128 | ) | 114,503 | ||||||||||||
Corporate debt securities | 1 to 2 | 70,207 | — | (347 | ) | 69,860 | |||||||||||
Securities of government-sponsored entities | 1 to 2 | 30,583 | — | (75 | ) | 30,508 | |||||||||||
Total maturity 1 to 2 years | 100,790 | — | (422 | ) | 100,368 | ||||||||||||
Total available-for-sale securities | $ | 215,414 | $ | 7 | $ | (550 | ) | $ | 214,871 | ||||||||
The following is a summary of short-term marketable securities classified as available-for-sale as of December 31, 2015 (in thousands):
Contractual Maturity (in years) | Amortized Cost | Unrealized Gains | Unrealized Losses | Aggregate Estimated Fair Value | |||||||||||||
Marketable Securities: | |||||||||||||||||
Commercial paper | Less than 1 | $ | 31,899 | $ | 6 | $ | (41 | ) | $ | 31,864 | |||||||
Corporate debt securities | Less than 1 | 43,464 | — | (78 | ) | 43,386 | |||||||||||
Total maturity less than 1 year | 75,363 | 6 | (119 | ) | 75,250 | ||||||||||||
Corporate debt securities | 1 to 2 | 82,557 | — | (396 | ) | 82,161 | |||||||||||
Securities of government-sponsored entities | 1 to 2 | 34,522 | 2 | (136 | ) | 34,388 | |||||||||||
Total maturity 1 to 2 years | 117,079 | 2 | (532 | ) | 116,549 | ||||||||||||
Total available-for-sale securities | $ | 192,442 | $ | 8 | $ | (651 | ) | $ | 191,799 | ||||||||
During 2016, 2015 and 2014, the Company recognized a gain of less than $0.1 million, a loss of $0.3 million and a gain of $2.3 million on marketable securities, respectively. The Company had proceeds from the sale or maturity of marketable securities of $159.5 million, $10.0 million and $6.5 million for 2016, 2015 and 2014, respectively.
The primary objective of the Company’s investment portfolio is to enhance overall returns while preserving capital and liquidity. The Company’s investment policy limits interest-bearing security investments to certain types of instruments issued by institutions with primarily investment grade credit ratings and places restrictions on maturities and concentration by asset class and issuer.
The Company reviews the available-for-sale investments for other-than-temporary declines in fair value below cost basis each quarter and whenever events or changes in circumstances indicate that the cost basis of an asset may not be recoverable. This evaluation is based on a number of factors, including the length of time and the extent to which the fair value has been below the cost basis and adverse conditions related specifically to the security, including any changes to the credit rating of the security, and the intent to sell, or whether the Company will more likely than not be required to sell the security before recovery of its amortized cost basis. The assessment of whether a security is other-than-temporarily impaired could change in the future due to new developments or changes in assumptions related to any particular security. As of December 31, 2016 and 2015, the Company believed the cost basis for available-for-sale investments were recoverable in all material respects. For both December 31, 2016 and 2015, any investments in an unrealized loss position for longer than 12 months are immaterial.
NOTE 5. DERIVATIVE FINANCIAL INSTRUMENTS
F-17
Since 2013, the Company has issued 5 tranches of common stock purchase warrants to secure financing, remediate covenant violations related to the Credit Facility (See Note. 10) and provide consideration for Credit Facility amendments.
The Company accounts for derivative financial instruments in accordance with ASC 815-40, “Derivative and Hedging – Contracts in Entity’s Own Equity” (“ASC 815-40”), in which instruments which do not have fixed settlement provisions are deemed to be derivative instruments. The Company’s warrants are classified as liability instruments due to an anti-dilution provision that provides for a reduction to the exercise price of the warrants if the Company issues additional equity or equity linked instruments in the future at an effective price per share less than the exercise price then in effect.
Issuances
2016
None.
2015
On January 12, 2015, the Company entered into Amendment No. 3 to the Credit Facility discussed in Note 10, in which the Company obtained a commitment letter from Athyrium Capital Management, LLC and Perceptive Credit Opportunities Fund, LP (collectively, the “ Lenders”), the Company’s existing lenders, providing a commitment for a senior secured incremental term loan under the Company’s existing term loan facility in an aggregate principal amount of $30 million, which could have been drawn down at the Company’s option to finance the acquisition of the assets of Asklepion.
As consideration for the commitment letter for the Incremental Loan, the Company made a cash payment to the Lenders and issued the Lenders warrants initially exercisable to purchase up to an aggregate of 125,000 shares of the Company’s common stock. The Company recorded $1.05 million of interest expense related to the warrants upon issuance.
The Company calculated the fair value of the warrants using the Monte Carlo Simulation utilizing the following assumptions as of the grant date of the warrants:
Risk free rate | 1.39 | % | |
Expected volatility | 85 | % | |
Expected life (in years), represents the weighted average period until next liquidity event | 0.3 | ||
Expected dividend yield | — | ||
Exercise Price | $ | 13.25 | |
2014
In connection with the execution of the Credit Facility, the Company issued warrants to the lenders under the Credit Facility, initially exercisable to purchase up to an aggregate of 337,500 shares of common stock of the Company. The Warrants will be exercisable in whole or in part, at an initial exercise price per share of $12.76 per share, which is subject to weighted-average anti-dilution protections. The Warrants may be exercised at any time upon the election of the holder, beginning on the date of issuance and ending on the fifth anniversary of the date of issuance.
The total grant date fair value of the Warrants was $2.5 million, was recorded as a derivative liability, and is included in the debt discount to the Note Payable in the Consolidated Balance Sheets.
The Company calculated the fair value of the warrants using the Binomial Lattice pricing model using the following assumptions as of the grant date of the Warrants:
Risk free rate | 1.62 | % | |
Expected volatility | 85 | % | |
Expected life (in years), represents the weighted average period until next liquidity event | 0.36 | ||
Expected dividend yield | — | ||
Exercise Price | $ | 12.76 | |
On November 13, 2014, the Company entered into Amendment No. 2 to the Credit Facility which allowed the Company to be in compliance with certain covenants as of September 30, 2014. In addition certain covenants related to the 4th quarter of fiscal 2014 and 2015 were amended. As compensation for Amendment No. 2, the Company agreed to issue additional warrants to the lenders, initially exercisable to purchase an aggregate of 300,000 shares of common stock of the Company which were valued at $2.2 million as of November 13, 2014, with an exercise price of $9.96 per share, and was recorded in change in fair value of derivative instruments in the 2014 Consolidated Statements of Operations.
Re-measurement
The warrants are re-measured at each balance sheet date based on estimated fair value. Changes in estimated fair value are recorded as non-cash valuation adjustments within other income (expenses) in the Company’s accompanying Consolidated Statements of Operations. The Company
F-18
recorded a change in the estimated fair value of warrants of a gain of $1.7 million, loss of $33.3 million, and loss of $23.8 million during the years ended December 31, 2016, 2015 and 2014, respectively.
The Company calculated the fair value of the warrants using the Monte Carlo Simulation as of December 31, 2016 and 2015, using the following assumptions:
As of | |||||||
December 31, 2016 | December 31, 2015 | ||||||
Fair value of common stock | $ | 18.93 | $ | 19.29 | |||
Remaining Life (in years) of the Warrants | 1.2 - 3.0 years | 2.1 – 4.0 years | |||||
Risk-free interest rate | .89 - 1.48% | 1.11 – 1.59% | |||||
Expected volatility | 55 - 75% | 75 – 85% | |||||
Dividend yield | — | — | |||||
Expected volatility is based on analysis of the Company’s volatility. The risk free interest rate is based on the U.S. Treasury security rates for the remaining term of the warrants at December 31, 2016 and 2015.
The following tables presents the Company’s derivative warrant issuances and balances outstanding during the years ended December 31, 2016 and 2015:
Weighted Average | ||||||||||
Warrants | Exercise Price | Grant Date Fair Value | ||||||||
Outstanding at December 31, 2014 | 3,421,355 | $ | 6.43 | $ | 3.79 | |||||
Issued | 125,000 | 13.25 | 8.40 | |||||||
Canceled | — | — | — | |||||||
Exercised | 880,807 | 5.35 | 3.23 | |||||||
Outstanding at December 31, 2015 | 2,665,548 | $ | 7.05 | $ | 4.20 | |||||
Issued | — | — | — | |||||||
Canceled | — | — | — | |||||||
Exercised | 898,643 | 6.68 | 4.85 | |||||||
Outstanding at December 31, 2016 | 1,766,905 | $ | 7.23 | $ | 3.87 | |||||
The following information applies to derivative warrants outstanding at December 31, 2016:
Exercise Price | Number of Warrants | Weighted Average Remaining Contractual Life (years) | Number Exercisable | |||||||
$ | 3.60 | 168,336 | 1.12 | 168,336 | ||||||
$ | 6.00 | 1,219,402 | 1.62 | 1,219,402 | ||||||
$ | 12.76 | 337,500 | 2.49 | 337,500 | ||||||
$ | 13.25 | 41,667 | 3.03 | 41,667 | ||||||
The total intrinsic value of derivative warrants outstanding and exercisable as of December 31, 2016 was $20.7 million. The Company’s closing stock price was $18.93 on December 31, 2016.
NOTE 6. FAIR VALUE MEASUREMENTS
Financial Instruments and Fair Value
The Company accounts for financial instruments in accordance with ASC 820, “Fair Value Measurements and Disclosures” (“ASC 820”). ASC 820 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy under ASC 820 are described below:
Level 1 – Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
Level 2 – Quoted prices in markets that are not active or financial instruments for which all significant inputs are observable, either directly or indirectly; and
Level 3 – Prices or valuations that require inputs that are both significant to the fair value measurement and unobservable.
In estimating the fair value of the Company’s derivative liabilities, the Company used the Monte Carlo Simulation as of December 31, 2016 and 2015. Based on the fair value hierarchy, the Company classified the derivative liability within Level 3.
F-19
In estimating the fair value of the Company’s contingent consideration, the Company used the comparable uncontrolled transaction (“CUT”) method for royalty payments based on projected revenues. Based on the fair value hierarchy, the Company classified contingent consideration within Level 3 because valuation inputs are based on projected revenues discounted to a present value.
Financial instruments with carrying values approximating fair value include cash and cash equivalents, accounts receivable, notes receivable, deposits on lease agreements, and accounts payable, due to their short term nature.
The following table presents the Company’s asset and liabilities that are measured and recognized at fair value on a recurring basis classified under the appropriate level of the fair value hierarchy as of December 31, 2016 (in thousands):
As of December, 2016 | Fair Value Hierarchy at December 31, 2016 | ||||||||||||||
Total carrying and estimated fair value | Quoted prices in active markets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) | ||||||||||||
Asset: | |||||||||||||||
Cash and Cash Equivalents | $ | 41,002 | $ | 39,929 | $ | 1,073 | $ | — | |||||||
Marketable securities, available-for-sale | 214,871 | — | 214,871 | — | |||||||||||
Total | $ | 255,873 | $ | 39,929 | $ | 215,944 | $ | — | |||||||
Liabilities: | |||||||||||||||
Derivative liability related to warrants | $ | 22,440 | $ | — | $ | — | $ | 22,440 | |||||||
Business combination-related contingent consideration | 87,478 | — | — | 87,478 | |||||||||||
Total | $ | 109,918 | $ | — | $ | — | $ | 109,918 | |||||||
The following table presents the Company’s asset and liabilities that are measured and recognized at fair value on a recurring basis classified under the appropriate level of the fair value hierarchy as of December 31, 2015 (in thousands):
As of December, 2015 | Fair Value Hierarchy at December 31, 2015 | ||||||||||||||
Total carrying and estimated fair value | Quoted prices in active markets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) | ||||||||||||
Asset: | |||||||||||||||
Cash and Cash Equivalents | $ | 37,805 | $ | 31,055 | $ | 6,750 | $ | — | |||||||
Marketable securities, available-for-sale | 191,799 | — | 191,799 | — | |||||||||||
Total | $ | 229,604 | $ | 31,055 | $ | 198,549 | $ | — | |||||||
Liabilities: | |||||||||||||||
Derivative liability related to warrants | $ | 38,810 | $ | — | $ | — | $ | 38,810 | |||||||
Business combination-related contingent consideration | 59,021 | — | — | 59,021 | |||||||||||
Total | $ | 97,831 | $ | — | $ | — | $ | 97,831 | |||||||
The following table sets forth a summary of changes in the estimated fair value of the Company’s Level 3 derivative liability for years ended December 31, 2016 and 2015 (in thousands):
Fair Value Measurements of Common Stock Warrants Using Significant Unobservable Inputs (Level 3) | |||||||
2016 | 2015 | ||||||
Balance at January 1, | $ | 38,810 | $ | 27,990 | |||
Issuance of common stock warrants | — | 1,050 | |||||
Reclassification of derivative liability to equity upon exercise of warrants | (14,715 | ) | (23,537 | ) | |||
Change in estimated fair value of liability classified warrants | (1,655 | ) | 33,307 | ||||
Balance at December 31, | $ | 22,440 | $ | 38,810 | |||
F-20
The following table sets forth a summary of changes in the estimated fair value of the Company's Level 3 business combination-related contingent consideration for the years ended December 31, 2016 and 2015 (in thousands):
Fair Value Measurements of Acquisition-Related Contingent Consideration (Level 3) | |||||||
2016 | 2015 | ||||||
Balance at January 1, | $ | 59,021 | $ | 11,637 | |||
Present value of contingent consideration upon acquisition related to a business combination | 25,000 | 39,107 | |||||
Increase from revaluation of contingent consideration | 18,383 | 13,778 | |||||
Decrease of contingent consideration, asset divestiture | — | (604 | ) | ||||
Contractual Payments | (12,826 | ) | (3,938 | ) | |||
Contractual Payments accrued at December 31 | (1,988 | ) | (959 | ) | |||
Foreign currency impact | (112 | ) | — | ||||
Balance at December 31, | $ | 87,478 | $ | 59,021 | |||
NOTE 7. INTANGIBLE ASSETS
Amortizable intangible assets
Ligand License Agreement
In fiscal 2013, the Company entered into a $2.5 million agreement with Ligand Pharmaceuticals Incorporated for a worldwide sublicense to develop, manufacture and commercialize a drug technology compound including RE-021 or sparsentan (the “Ligand License Agreement”). The cost of the Ligand License Agreement, which is presented net of amortization in the accompanying Consolidated Balance Sheet in intangible assets, net, is being amortized to research and development on a straight-line basis through September 30, 2023. As consideration for the license, we are required to make substantial payments upon the achievement of certain milestones, totaling up to $109.4 million. Through 2016, we have made milestone payments to Ligand of $2.6 million under the terms of the license agreement. Should we commercialize sparsentan or any products containing related compounds, we will be obligated to pay to Ligand an escalating annual royalty between 15% and 17% of net sales of all such products.
In September 2015, the Ligand License Agreement was amended to facilitate sub-licensing in Asia-Pacific. As consideration for the amendment the Company paid $1.0 million.
Carbetocin Technology
In September 2015, the Company wrote-off the entire value of intangible assets related to Carbetocin. The write-off was deemed appropriate as the Company elected not to pursue any internal development of the asset and attempts to divest it were unsuccessful. The total charge of $4.7 million was included in operating expenses on the Consolidated Statement of Operations and Comprehensive Income (Loss).
Manchester Pharmaceuticals LLC
The Company acquired intangible assets with finite lives related to the Chenodal product rights, trade names, and customer relationships with the values of $67.8 million, $0.2 million, and $0.4 million, respectively. The useful lives related to the acquired product rights, trade names, and customer relationships are expected to be approximately 16, 1 and, 10 years, respectively. Amortization of product rights, trade names and customer relationships are being recorded in selling, general and administrative expense over their respective lives.
In 2015, the Company divested the assets related to Vecamyl, valued at $3.6 million, to Turing Pharmaceuticals.
Thiola License Agreement
In 2014, the Company entered into a license agreement with Mission Pharmacal, in which the Company obtained an exclusive, royalty-bearing license to market, sell and commercialize Thiola (Tiopronin) in the United States and Canada, and a non-exclusive license to use know-how relating to Thiola to the extent necessary to market Thiola. The initial term of the license is 10 years and will automatically renew thereafter for periods of one year.
The Company paid Mission an up-front license fee of $3 million and will pay guaranteed minimum royalties during each calendar year the greater of $2 million or twenty percent (20%) of the Company’s net sales of Thiola through June 30, 2024. The present value of guaranteed minimum royalties payable using a discount rate of approximately 11% based on the Company’s then borrowing rate is $10.1 million and $10.9 million as of December 31, 2016 and 2015, respectively. As of December 31, 2016, the guaranteed minimum royalty current and long term liability is approximately $2.0 million and $8.1 million, respectively, and is recorded as guaranteed minimum royalty in the Consolidated Balance Sheet. As of December 31, 2015, the guaranteed minimum royalty current and long term liability is approximately $2.0 million and $8.9 million, respectively, and is recorded as guaranteed minimum royalty in the Consolidated Balance Sheet. The Company has capitalized $35.3 million related to the Thiola asset which consists of the up-front license fee, professional fees, present value of the guaranteed minimum royalties and any additional payments through 2016 in excess of minimum royalties. There are 7.5 years remaining in the initial term of the license agreement.
F-21
Cholbam (Kolbam) Asset Purchase
On March 31, 2015, the Company completed its acquisition from Asklepion of all worldwide rights, titles and ownership of Cholbam, including all related contracts, data assets, intellectual property, regulatory assets and the PRV. The Company capitalized $75.9 million and $7.3 million for the U.S. and International economic interest, respectively.
L-UDCA
On June 20, 2016, the Company announced the signing of a definitive agreement to purchase the rights, titles, and ownership of L-UDCA from Asklepion. The purchase included $25.5 million for an intangible asset with a definite life related to product rights for the U.S. The useful life related to the acquired product rights is expected to be approximately 17 years once the NDA is approved by the FDA. Until approval, the asset is considered IPR&D with an indefinite life and is not amortized.
Amortizable intangible assets as of December 31, 2016 (in thousands):
Useful Life | Gross Carrying Amount | Accumulated Amortization | Net Book Value | |||||||||
Chenodal Product Rights | 16 | $ | 67,849 | $ | (11,738 | ) | $ | 56,111 | ||||
Thiola License | 10 | 35,339 | (5,818 | ) | 29,521 | |||||||
Economic Interest - U.S. revenue Cholbam | 10 | 75,900 | (13,320 | ) | 62,580 | |||||||
Economic Interest - International revenue Cholbam | 10 | 7,074 | (1,241 | ) | 5,833 | |||||||
Economic Interest - L-UDCA (acquired IPR&D) | Indefinite | 25,500 | — | 25,500 | ||||||||
Ligand License | 11 | 3,300 | (1,093 | ) | 2,207 | |||||||
Manchester Customer Relationships | 10 | 403 | (112 | ) | 291 | |||||||
Manchester Trade Name | 1 | 175 | (175 | ) | — | |||||||
Total | $ | 215,540 | $ | (33,497 | ) | $ | 182,043 | |||||
Amortizable intangible assets as of December 31, 2015 (in thousands):
Useful Life | Gross Carrying Amount | Accumulated Amortization | Net Book Value | |||||||||
Chenodal Product Rights | 16 | $ | 67,849 | $ | (7,489 | ) | $ | 60,360 | ||||
Thiola License | 10 | 24,133 | (2,793 | ) | 21,340 | |||||||
Economic Interest - U.S. revenue Cholbam | 10 | 75,900 | (5,715 | ) | 70,185 | |||||||
Economic Interest - International revenue Cholbam | 10 | 7,336 | (552 | ) | 6,784 | |||||||
Ligand License | 11 | 3,300 | (765 | ) | 2,535 | |||||||
Manchester Customer Relationships | 10 | 403 | (71 | ) | 332 | |||||||
Manchester Trade Name | 1 | 175 | (175 | ) | — | |||||||
Total | $ | 179,096 | $ | (17,560 | ) | $ | 161,536 | |||||
The following table summarizes amortization expense for the twelve months ended December 31, 2016, 2015 and 2014 (in thousands):
2016 | 2015 | 2014 | |||||||||
Research and development | $ | 328 | $ | 697 | $ | 823 | |||||
Selling, general and administrative | 15,665 | 12,534 | 4,455 | ||||||||
Total amortization expense | $ | 15,993 | $ | 13,231 | $ | 5,278 | |||||
As of December 31, 2016, amortization expense (excluding infinite lived IPR&D) for the next five years is expected to be as follows (in thousands):
2017 | $ | 16,879 | |
2018 | 16,879 | ||
2019 | 16,879 | ||
2020 | 16,879 | ||
2021 | 16,879 | ||
Thereafter | 72,148 | ||
Total | $ | 156,543 | |
F-22
NOTE 8. ACCRUED EXPENSES
Accrued expenses consist of the following at December 31, 2016 and 2015 (in thousands):
2016 | 2015 | ||||||
Compensation related costs | $ | 7,441 | $ | 7,143 | |||
Research and development | 7,311 | 4,281 | |||||
Government rebate reserves | 6,967 | 3,158 | |||||
Selling, general and administrative | 3,333 | 3,586 | |||||
Royalty/contingent consideration | 5,766 | 4,688 | |||||
Miscellaneous | 2,490 | 964 | |||||
$ | 33,308 | $ | 23,820 | ||||
NOTE 9. RELATED PARTY TRANSACTIONS
On October 13, 2014, the Company entered into a binding Summary Separation Proposal with its then-current Chief Executive Officer. Among other matters, the Summary Separation Proposal set forth a summary of the terms for the sale of the Company’s Vecamyl, Syntocinon and ketamine licenses and assets to Turing Pharmaceuticals, a company controlled by the former Chief Executive Officer.
On January 9, 2015, the Company entered into a purchase agreement with Turing Pharmaceuticals pursuant to which the Company sold Turing Pharmaceuticals the sold assets for a purchase price of $1.0 million, and pursuant to which Turing Pharmaceuticals also assumed all future liabilities related to the sold assets.
On February 13, 2015, the Sellers entered into a purchase agreement with Waldun, pursuant to which the Sellers sold Waldun the Vecamyl Product Rights for a purchase price of $0.7 million. Waldun in turn sold the Vecamyl Product Rights to Turing Pharmaceuticals. In connection therewith, on February 13, 2015, the Company, together with Manchester, entered into an asset purchase agreement with Turing Pharmaceuticals, pursuant to which the Company sold Turing Pharmaceuticals the Inventory for a purchase price of $0.3 million, and pursuant to which Turing Pharmaceuticals also assumed certain liabilities related to the Vecamyl Product Rights and the Inventory.
On February 13, 2015, the Company entered into an asset purchase agreement with Turing Pharmaceuticals pursuant to which the Company sold Turing Pharmaceuticals its Oxytocin Assets, including related inventory, for a purchase price of $1.1 million, and pursuant to which Turing Pharmaceuticals also assumed certain liabilities related to the Oxytocin Assets.
The total impact to the Statement of Operations and Comprehensive Income (Loss) related to the divestitures for 2015 was a gain of $0.9 million.
NOTE 10. NOTES PAYABLE
Convertible Notes Payable
On May 29, 2014, the Company entered into a Note Purchase Agreement relating to a private placement by the Company of $46 million aggregate principal senior convertible notes due 2019 (the “Notes”) which are convertible into shares of the Company’s common stock at an initial conversion price of $17.41 per share. The conversion price is subject to customary anti-dilution protection. The Notes bear interest at a rate of 4.5% per annum, payable semiannually in arrears on May 15 and November 15 of each year, beginning on November 15, 2014. The Notes mature on May 30, 2019 unless earlier converted or repurchased in accordance with the terms. The aggregate carrying value of the Notes on their issuance was $43 million, which was net of the $3 million debt discount.
On June 30, 2014, the Company issued 401,047 shares of common stock to the holders of the Notes and such noteholders granted the Company a release of certain claims they may have had in connection with the Company's sale of the Notes or certain statements made by the Company in connection with such sale. The Company recorded finance expense as other expense in the amount of $4.7 million for the year ended December 31, 2014 based on the fair market value of the stock on the date of issuance in relation to the shares issued.
As of December 31, 2016 the fair value of a share of common stock was $18.93, exceeding the initial conversion price per share of the notes. If the debt holders were to convert the Company would be required to issue 2,642,160 shares of common stock assuming that no fundamental change in the Company has occurred. The Company has reserved sufficient shares of its common stock to satisfy the conversion requirements related to the Notes. As of December 31, 2016, the convert value exceeded the carrying value by approximately $5.5 million.
In estimating the fair value of the Company’s convertible debt, the Company performed an analysis on the straight-debt portion, excluding the conversion feature and the conversion feature. To estimate the fair value of conversion feature, the Company used the Monte Carlo Simulation as of December 31, 2016. To estimate the fair value of straight-debt portion, excluding the conversion feature, the Company discounted to present value the scheduled coupon payments and principal repayment, using an appropriate fair market yield. Based on the fair value hierarchy, the Company classified the derivative liability within Level 3. As of December 31, 2016 the fair value of the debt is estimated at $64.6 million.
F-23
The net carrying amount of the Notes consists of the following (in thousands):
December 31, | |||||||
2016 | 2015 | ||||||
Aggregate principle amount of Notes | $ | 46,000 | $ | 46,000 | |||
Unamortized debt discount and debt issuance costs | (1,578 | ) | (2,234 | ) | |||
$ | 44,422 | $ | 43,766 | ||||
Credit Facility
In June 2014, the Company entered into a $45 million Credit Agreement (“Credit Facility”) which bore interest at an annual rate of (i) the Adjusted LIBOR Rate (as such term was defined in the Credit Facility) plus 10.00% or (ii) in certain circumstances, the Base Rate (as such term was defined in the Credit Agreement) plus 9.00% and was payable quarterly. The Credit Facility contained certain financial and non-financial covenants.
In connection with the execution of the Credit Facility, the Company issued warrants (the “Warrants”) to the lenders under the Credit Facility, initially exercisable to purchase up to an aggregate of 337,500 shares of common stock of the Company. The Warrants will be exercisable in whole or in part, at an initial exercise price per share of $12.76 per share, which is subject to weighted-average anti-dilution protections. The Warrants may be exercised at any time upon the election of the holder, beginning on the date of issuance and ending on the fifth anniversary of the date of issuance. The issuance of the Warrants was not registered under the Securities Act of 1933, as amended (the “Securities Act”), as such issuance was exempt from registration under Section 4(2) of the Securities Act.
The total grant date fair value of the Warrants was $2.5 million, was recorded as a derivative liability, and was included in the debt discount to the Note Payable in the 2014 Consolidated Balance Sheets.
The Company calculated the fair value of the warrants using the Binomial Lattice pricing model using the following assumptions as of the grant date of the Warrants:
Risk free rate | 1.62 | % | |
Expected volatility | 85 | % | |
Expected life (in years), represents the weighted average period until next liquidity event | 0.36 | ||
Expected dividend yield | — | ||
Exercise Price | $ | 12.76 | |
In November 2014, the Company entered into Amendment No. 2 (“Amendment No. 2”) to the Credit Facility which allowed the Company to be in compliance with certain covenants as of September 30, 2014. In addition certain covenants related to the fourth quarter of fiscal 2014 and 2015 were amended. As compensation for Amendment No. 2, the Company agreed to issue additional warrants to Athyrium Capital Management, LLC and Perceptive Credit Opportunities Fund, LP (collectively, the “Lenders”), initially exercisable to purchase an aggregate of 300,000 shares of common stock of the Company which were valued at $2.2 million and recorded in change in fair value of derivative instruments in the 2014 Consolidated Statements of Operations.
On January 12, 2015, the Company entered into Amendment No. 3 (“Amendment No. 3”) to the Credit Facility in which the Company obtained a commitment letter from the Lenders, providing a commitment for a senior secured incremental term loan under the Company’s existing term loan facility in an aggregate principal amount of $30.0 million, which could have been drawn down at the Company’s option to finance the acquisition of the Cholbam assets from Asklepion.
As consideration for Amendment No. 3, the Company made a $0.6 million cash payment to the Lenders, recorded in finance expense in the Consolidated Statements of Operations, and issued the Lenders warrants initially exercisable to purchase up to an aggregate of 125,000 shares of the Company’s common stock which were valued at $1.1 million on January 12, 2015 and were recorded in interest expense in the Consolidated Statements of Operations. Due to the closing of its public offering on March 24, 2015, the Company received cash proceeds of $140.0 million, after deducting underwriting fees and other offering costs, which the Company used to make the $27.0 million payment due to Asklepion upon the closing of the Company’s acquisition of the Cholbam assets, and as a result, the Company did not utilize the commitment from the Lenders.
On July 1, 2015, the Company paid off the Credit Facility in its entirety including a prepayment premium of $2.3 million, and incurred an additional charge of $4.2 million, included in other expenses on the Company's Consolidated Statement of Operations and Comprehensive Income (Loss), for the write-off of the debt discount and equity issuances for the Credit Facility
Note Payable - Manchester Pharmaceuticals, LLC
On March 26, 2014, upon the acquisition of Manchester, the Company entered into a note payable in the amount of $33 million. The note is non-interest bearing and therefore the Company recorded the loan at present value of $31.3 million using the effective interest rate of approximately 11%, which was the Company’s current borrowing rate. The note was due and payable in three consecutive payments, each in the amount of $11 million payable on June 26, 2014, September 26, 2014, and December 12, 2014 (the maturity date). On June 30, 2014, the Company paid off the note in its entirety. The Company accelerated interest expense in the amount of $1.7 million for the difference between the present value of the loan, and the loan balance paid was recorded in interest income (expense), net for the year ended December 31, 2014.
F-24
Interest Expense
Total interest expense, net, recognized for the years ended December 31, 2016, 2015 and 2014 was $0.8 million, $7.7 million and $7.4 million, respectively.
NOTE 11. COMMITMENTS AND CONTINGENCIES
Leases and Sublease Agreements
Facilities | Base Rent | Lease Expiration | Comments | |||
Occupied Locations | ||||||
Corporate Headquarters San Diego CA | $1.1 million | July 2024 | Occupied in December 2016 | |||
Cambridge MA | October 2017 | Subleased space for less than one year | ||||
Vacated Locations | ||||||
San Diego CA | 0.5 | December 2017 | Available for sublease | |||
New York NY | 0.5 | November 2018 | Available for sublease | |||
Carlsbad CA | June 2017 | Sublet through expiration | ||||
Contractual Commitments
The following table summarizes our principal contractual commitments, excluding open orders that support normal operations, as of December 31, 2016 (in thousands):
Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | |||||||||||||||
Operating leases | $ | 10,861 | $ | 2,052 | $ | 2,870 | $ | 2,491 | $ | 3,448 | |||||||||
Note payable, including contractual interest | 66,003 | 4,070 | 52,933 | 4,000 | 5,000 | ||||||||||||||
Sales support services | 3,054 | 416 | 833 | 833 | 972 | ||||||||||||||
Product supply contracts | 1,994 | 1,515 | 479 | — | — | ||||||||||||||
Purchase order commitments | 2,741 | 2,591 | 150 | — | — | ||||||||||||||
$ | 84,653 | $ | 10,644 | $ | 57,265 | $ | 7,324 | $ | 9,420 | ||||||||||
Legal Proceedings
On September 19, 2014, purported shareholders of the Company sued Martin Shkreli, the Company’s former Chief Executive Officer, in federal court in the Southern District of New York (Donoghue v. Retrophin, Inc., Case No. 14-cv-7640). The Company was a nominal defendant in this action. The plaintiffs sought, on behalf of the Company, disgorgement of short-swing profits from Mr. Shkreli under section 16(b) of the Securities Exchange Act of 1934 (15 U.S.C. 78(p)(b)). The Court approved a settlement between the parties, under which Mr. Shkreli was obligated to pay $2.025 million to the Company and an additional $0.6 million to Plaintiffs to compensate them for their legal fees. Mr. Shkreli defaulted on the judgment and the Company and the Plaintiffs took steps to collect it. The Company did not record anything related to the judgment on its financial statements for 2015. In November 2016, the Company and Mr. Shkreli entered into a binding term sheet with respect to a settlement, under which the $2.025 million judgment against Mr. Shkreli would offset and satisfy certain existing legal fees that Mr. Shkreli claimed should be advanced to him by the Company, as described more fully below. That offset has now taken place and this matter has concluded.
In January 2015, the Company received a subpoena relating to a criminal investigation by the U.S. Attorney for the Eastern District of New York. The subpoena requested information regarding, among other things, the Company’s relationship with Mr. Shkreli and individuals or entities that had been investors in investment funds previously managed by Mr. Shkreli. The Company has been informed that it is not a target of the U.S. Attorney’s investigation, and is cooperating with the investigation. On December 17, 2015, an indictment against the Company’s former Chief Executive Officer, Martin Shkreli, and its former outside counsel, Evan Greebel, was unsealed in the United States District Court for the Eastern District of New York. A superseding indictment reflecting additional charges was filed on June 3, 2016. The Company has also been cooperating with a parallel investigation by the U.S. Securities and Exchange Commission (the “SEC”). On December 17, 2015, the SEC filed a civil complaint against Mr. Shkreli, Mr. Greebel, MSMB Capital Management LLC, and MSMB Healthcare Management LLC in the United States District Court for the Eastern District of New York. In connection with these proceedings, Mr. Shkreli, as well as a number of other current and former directors, officers, and employees, sought advancement of their legal fees from the Company. Mr. Shkreli, in particular, claimed that the Company owes him in excess of $5 million in legal fees that he has incurred defending these actions. The Company disputed its obligation to pay the amount in full. In November 2016, the Company and Mr. Shkreli entered into a binding term sheet with respect to a settlement under which the Company would advance $2.8 million in legal fees to Mr. Shkreli’s counsel, representing a portion of the existing legal fees related to these proceedings that Mr. Shkreli claims should be advanced. The Company would also undertake an obligation to advance an additional $2 million in future legal fees in the event the matter proceeds to trial. The Company has now advanced $2.8 million ($1.8 million of which occurred in 2016) in pre-trial fees to Mr. Shkreli's counsel. At present, the Company has been reimbursed approximately $0.7 million of this amount from its directors’ and officers’ insurance carriers, and expects to receive payments for a portion of the remaining amount. The total amount it will receive from the insurance carriers is not currently
F-25
estimable. In addition, the legal fees the Company has advanced will be subject to reimbursement by Mr. Shkreli under Delaware law in the event it is ultimately determined that Mr. Shkreli is not entitled to be indemnified by the Company in these proceedings.
On August 17, 2015, the Company filed a lawsuit in federal district court for the Southern District of New York against Martin Shkreli, asserting that he breached his fiduciary duty of loyalty during his tenure as the Company’s Chief Executive Officer and a member of its Board of Directors (Retrophin, Inc. v. Shkreli, 15-CV-06451(NRB)). On August 19, 2015, Mr. Shkreli served a demand for JAMS arbitration on Retrophin, claiming that Retrophin had breached his December 2013 employment agreement. In response to Mr. Shkreli’s arbitration demand, the Company asserted counterclaims in the arbitration that are substantially similar to the claims it previously asserted in the federal lawsuit against Mr. Shkreli. The parties have selected an arbitration panel. On Mr. Shkreli’s application, and with the Company’s consent, the federal Court granted a stay of the federal lawsuit pending a determination by the arbitration panel whether the Company’s counterclaims would be litigated in the arbitration, as the Company is seeking. On April 22, 2016, the arbitration panel granted the parties’ request for a stay of the proceedings pending settlement discussions. In connection with these proceedings, Mr. Shkreli sought advancement of his legal fees from the Company relating to his defense of the Company’s claims against him. Mr. Shkreli claimed that he has to date incurred in excess of $2.8 million in fees, including fees incurred in negotiating with the Company over advancement. The Company disputed its obligation to pay the amount in full. In November 2016, the Company and Mr. Shkreli entered into a binding term sheet with respect to a settlement under which the significant majority of the existing legal fees related to these proceedings that Mr. Shkreli claimed should be advanced would be offset and satisfied by the $2.025 million judgment against Mr. Shkreli in the Donoghue v. Retrophin, Inc. case described above. The Company would also advance $0.4 million in legal fees to Mr. Shkreli’s counsel, a portion of which represented additional legal fees related to these proceedings that Mr. Shkreli claims should be advanced. In accordance with the Term Sheet, the Donoghue settlement was offset, and the Company paid the $0.4 million to Mr. Shkreli's counsel. The legal fees the Company has advanced will be subject to reimbursement by Mr. Shkreli under Delaware law in the event it is ultimately determined that Mr. Shkreli is not entitled to be indemnified by the Company in these proceedings.
The Company will also be subject to additional obligations when the litigation resumes, as well as advancement obligations in the interim.
Through December 31, 2016, the Company has expensed $5.2 million and paid $2.2 million under the settlement and subsequently received $0.7 million in reimbursement from directors’ and officers’ insurance carriers. Both the settlement and the reimbursement are recorded in selling, general and administrative expenses within the Consolidated Statement of Operations and Comprehensive Income. The Company expects to receive additional payment from its insurance carriers for a portion of the legal fees advanced, however the Company has not yet recorded any amounts for such payments as the amount it will receive from the insurance carriers is not currently estimable.
From time to time the Company is involved in legal proceedings arising in the ordinary course of business. The Company believes there is no litigation pending that could have, individually or in the aggregate, a material adverse effect on its results of operations or financial condition.
NOTE 12. STOCKHOLDERS’ EQUITY / DEFICIT
Common Stock
The Company is currently authorized to issue up to 100,000,000 shares of $0.0001 par value common stock. All issued shares of common stock are entitled to vote on a 1 share/1 vote basis.
Preferred Stock
The Company is currently authorized to issue up to 20,000,000 shares of $0.001 par value preferred stock, of which 1,000 shares are designated Class "A" Preferred shares, $0.001 par value. Class A Preferred Shares are not entitled to interest, have certain liquidation preferences, special voting rights and other provisions. No preferred stock has been issued to date.
Public Offering - 2015
On March 24, 2015, the Company completed a public offering of 7,866,000 shares of common stock at a price of $19.00 per share. The Company received net proceeds from the offering of $140.0 million after deducting underwriting fees and other offering costs of $9.5 million. The shares of common stock were offered by the Company pursuant to a shelf registration statement that was declared effective by the SEC on March 13, 2015.
2014 Incentive Compensation Plan
On May 9, 2014, the Company’s stockholders approved the 2014 Incentive Compensation Plan (the "Plan"). The Plan authorizes the granting of stock options, stock appreciation rights, restricted stock and restricted stock units, deferred stock, performance units and annual incentive awards covering up to 3.0 million shares of the Company’s common stock. In a special shareholder meeting held February 3, 2015, the Company’s shareholders ratified an incremental 1,928,000 shares of common stock and 230,000 restricted shares of common stock for issuance under the Plan. These shares were granted to employees between February 24, 2014 and August 18, 2014.
2015 Equity Incentive Plan
On June 8, 2015, the Company's stockholders approved the 2015 Equity Incentive Plan (the "2015 Plan"). The 2015 Plan is intended as the successor to and continuation of the Plan. Stockholders approved 1.4 million new shares to be issued under the 2015 Plan, in addition to 0.6 million unallocated shares remaining available for issuance under the Plan that were added to the 2015 Plan.
F-26
On May 18, 2016, the Company's stockholders approved an amendment to the 2015 Equity Incentive Plan (the "Amended 2015 Plan"). The amendment provides for an additional 1.6 million new shares to be issued under the Amended 2015 Plan, in addition to 0.7 million unallocated shares remaining available for issuance. The amendment also includes a provision where on or after March 21, 2016, the number of shares available for issuance under the Amended 2015 Plan will be reduced by one share for each share subject to a stock option or stock appreciation right and by 2.0 shares for each share subject to any other type of stock award issued pursuant to the Amended 2015 Plan, and any such shares will return to the share reserve at the same rates upon cancellation or other forfeiture of such awards or shares.
Stock Options
The fair values of stock option grants during the year ended December 31, 2016, 2015 and 2014 were calculated on the date of grant using the Black-Scholes option pricing model, except for options granted for market and revenue performance criteria. Compensation expense is recognized over the period of service, generally the vesting period. During the year ended December 31, 2016, 1,687,250 stock options were granted by the Company. The following weighted average assumptions were used in the Black-Scholes options pricing model to estimate the fair value of stock options for the specified reporting periods:
Twelve Months Ended December 31, | ||||||||
2016 | 2015 | 2014 | ||||||
Risk free rate | 1.20 | % | 1.53 | % | 1.55 | % | ||
Expected volatility | 68 | % | 83 | % | 85 | % | ||
Expected life (in years) | 5.8 | 5.8 | 5.8 | |||||
Expected dividend yield | — | — | — | |||||
The risk-free interest rate was based on rates established by the Federal Reserve. The Company’s expected volatility was based on analysis of the Company’s volatility, as well as the volatilities of guideline companies. The expected life of the Company’s options was determined using the simplified method as a result of limited historical data regarding the Company’s activity. The dividend yield is based upon the fact that the Company has not historically paid dividends, and does not expect to pay dividends in the foreseeable future.
The following table summarizes our stock option activity and related information for the year ended December 31, 2016:
Weighted Average | |||||||||||||
Shares Underlying Options | Exercise Price | Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | ||||||||||
Exercisable at December 31, 2015 | 2,036,906 | $ | 12.55 | 8.34 | $ | 15,582 | |||||||
Outstanding at December 31, 2015 | 5,665,584 | $ | 17.05 | 8.75 | $ | 31,542 | |||||||
Granted | 1,687,250 | $ | 16.73 | — | — | ||||||||
Forfeited and expired | (541,416 | ) | 22.19 | — | — | ||||||||
Exercised | (380,848 | ) | 10.55 | — | 2,873 | ||||||||
Outstanding at December 31, 2016 | 6,430,570 | $ | 16.91 | 7.64 | $ | 30,088 | |||||||
Exercisable at December 31, 2016 | 3,793,017 | $ | 14.94 | 6.82 | $ | 23,358 | |||||||
The following table summarizes our stock option activity and related information for the year ended December 31, 2015:
Weighted Average | |||||||||||||
Shares Underlying Options | Exercise Price | Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | ||||||||||
Exercisable at December 31, 2014 | 1,225,833 | $ | 9.73 | 7.96 | $ | 3,395 | |||||||
Outstanding at December 31, 2014 | 4,892,208 | $ | 10.93 | 8.57 | $ | 8,353 | |||||||
Granted | 2,285,000 | $ | 27.15 | — | — | ||||||||
Forfeited and expired | (970,170 | ) | 14.91 | — | — | ||||||||
Exercised | (541,454 | ) | 13.10 | — | 7,230 | ||||||||
Outstanding at December 31, 2015 | 5,665,584 | $ | 17.05 | 8.75 | $ | 31,542 | |||||||
Exercisable at December 31, 2015 | 2,036,906 | $ | 12.55 | 8.34 | $ | 15,582 | |||||||
F-27
The following table summarizes our stock option activity and related information for the year ended December 31, 2014:
Weighted Average | |||||||||||||
Shares Underlying Options | Exercise Price | Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | ||||||||||
Exercisable at December 31, 2013 | 172,667 | $ | 7.85 | 9.86 | $ | 14,333 | |||||||
Outstanding at December 31, 2013 | 1,721,000 | $ | 7.66 | 9.89 | $ | 172,000 | |||||||
Granted | 4,168,000 | $ | 12.11 | — | — | ||||||||
Forfeited and expired | (977,625 | ) | 10.27 | — | — | ||||||||
Exercised | (19,167 | ) | 5.16 | — | — | ||||||||
Outstanding at December 31, 2014 | 4,892,208 | $ | 10.93 | 8.57 | $ | 8,353 | |||||||
Exercisable at December 31, 2014 | 1,225,833 | $ | 9.73 | 7.96 | $ | 3,395 | |||||||
The weighted average grant date fair value of options granted was $10.09, $19.02, and $8.56 during the years ended December 31, 2016, 2015 and 2014, respectively. The aggregate intrinsic value for outstanding options is calculated as the difference between the exercise price of the underlying awards and the quoted price of the Company’s common stock as of December 31, 2016 of $18.93. Unrecognized compensation cost associated with unvested stock options amounts to $32.2 million as of December 31, 2016, which will be expensed over a weighted average remaining vesting period of 1.8 years.
Restricted Shares
As of December 31, 2016, there was approximately $4.4 million of unrecognized compensation cost related to restricted shares granted. This amount is expected to be recognized over a weighted average period of 1 year.
The following table summarizes our restricted stock award activity for the year ended December 31, 2016:
Number of shares | Weighted Average Grant Date Fair Value | |||||
Unvested December 31, 2014 | 691,668 | $ | 10.83 | |||
Granted | 273,000 | 26.97 | ||||
Vested | (478,334 | ) | 11.56 | |||
Forfeited/cancelled | (56,668 | ) | 13.97 | |||
Unvested December 31, 2015 | 429,666 | 20.38 | ||||
Granted | 245,000 | 17.52 | ||||
Vested | (161,335 | ) | 16.76 | |||
Forfeited/cancelled | (105,585 | ) | 21.19 | |||
Unvested December 31, 2016 | 407,746 | $ | 19.88 | |||
Share Based Compensation
Total non-cash stock-based compensation expense consisted of the following for the years ended December 31, 2016, 2015 and 2014 (in thousands):
Twelve Months Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
Selling, general and administrative expenses | $ | 18,614 | $ | 16,483 | $ | 10,940 | |||||
Research and development expenses | 10,488 | 9,417 | 4,960 | ||||||||
Total | $ | 29,102 | $ | 25,900 | $ | 15,900 | |||||
Exercise of Warrants
During the twelve months ended December 31, 2016, the Company issued 898,633 shares of common stock upon the exercise of warrants for cash received by the Company in the amount of $6.0 million. The Company reclassified $14.7 million derivative liability as equity for the value of these warrants on the date of exercise. The warrants were revalued immediately prior to exercise and the change in the fair value of $2.9 million was recorded as other expense in the Consolidated Financial Statements of the Company.
During the twelve months ended December 31, 2015, the Company issued 870,306 shares of common stock upon the exercise of warrants for cash received by the Company in the amount of $4.5 million. The Company reclassified $23.5 million derivative liability as equity for the value of these warrants on the date of exercise. The warrants were revalued immediately prior to exercise and the change in the fair value of $2.8 million was recorded as other expense in the Consolidated Financial Statements of the Company.
F-28
During the twelve months ended December 31, 2014, the Company issued 1,947,377 shares of common stock upon the exercise of warrants for cash received by the Company in the amount of $8.4 million. The Company reclassified $23.4 million derivative liability as equity for the value of these warrants on the date of exercise. The warrants were revalued immediately prior to exercise and the change in the fair value of the warrants was recorded as other expense in the Consolidated Financial Statements of the Company.
Treasury Stock
In the fourth quarter of 2013, the Company repurchased 130,790 shares of its common stock for an aggregate purchase price of $957,272. The Company currently recognizes such repurchased common stock as treasury stock.
During the year ended December 31, 2014, the Company repurchased 248,801 shares of its common stock for an aggregate purchase price of $2.3 million. The Company recognizes repurchased common stock as treasury stock.
In March 2015 the Company retired 379,591 shares of its common stock held as treasury stock. This was the entire holding of treasury stock. No shares were repurchased during the year.
NOTE 13. EARNINGS (LOSS) PER SHARE
Basic earnings (loss) per share (“EPS”) represents net income (loss) attributable to common shareholders divided by the weighted average number of common shares outstanding during the measurement period. Diluted EPS represents net income attributable to common shareholders divided by the weighted average number of common shares outstanding during the measurement period while also giving effect to all potentially dilutive common shares that were outstanding during the period using the treasury stock method.
Basic and diluted EPS is calculated as follows (net income amounts are stated in thousands):
For the year ended December 31, | ||||||||||||||||||||||||||||||||
2016 | 2015 | 2014 | ||||||||||||||||||||||||||||||
Shares | Net Income | EPS | Shares | Net Income | EPS | Shares | Net Income | EPS | ||||||||||||||||||||||||
Basic Earnings per Share | 36,997,865 | $ | (47,903 | ) | $ | (1.29 | ) | 33,560,249 | $ | 117,237 | $ | 3.49 | 25,057,509 | $ | (110,938 | ) | $ | (4.43 | ) | |||||||||||||
Dilutive shares related to warrants | 1,290,147 | — | — | — | — | — | ||||||||||||||||||||||||||
Change in fair value of derivative instruments | — | (1,655 | ) | — | — | — | — | |||||||||||||||||||||||||
Convertible Debt | — | — | 2,642,160 | 1,881 | — | — | ||||||||||||||||||||||||||
Restricted Stock | — | — | 290,966 | — | — | — | ||||||||||||||||||||||||||
Stock Options | — | — | 1,088,064 | — | — | — | ||||||||||||||||||||||||||
Dilutive Earnings per Share | 38,288,012 | $ | (49,558 | ) | $ | (1.29 | ) | 37,581,439 | $ | 119,118 | $ | 3.17 | 25,057,509 | $ | (110,938 | ) | $ | (4.43 | ) | |||||||||||||
For the years ended December 31, 2016, 2015 and 2014, the following shares were excluded because they were anti-dilutive:
For the year ended December 31, | ||||||||
2016 | 2015 | 2014 | ||||||
Convertible Debt | 2,642,160 | — | — | |||||
Restricted Stock | 444,942 | 22,069 | — | |||||
Options | 6,286,584 | 1,049,375 | 1,132,500 | |||||
Warrants | — | 2,665,548 | 3,083,855 | |||||
Total Anti-Dilutive Shares | 9,373,686 | 3,736,992 | 4,216,355 | |||||
NOTE 14. INCOME TAXES
For financial reporting purposes, net income before income taxes includes the following components (in thousands):
Year Ended December 31, | |||||||||||
2016 | 2015 | 2014 | |||||||||
United States | $ | (52,750 | ) | $ | 107,038 | $ | (112,558 | ) | |||
Foreign | (4,832 | ) | (1,571 | ) | (840 | ) | |||||
Total | $ | (57,582 | ) | $ | 105,467 | $ | (113,398 | ) | |||
F-29
The components of the provision (benefit) for income taxes, in the Consolidated Statement of Operations are as follows (in thousands):
2016 | 2015 | 2014 | |||||||||
Current | |||||||||||
Federal | $ | 13,137 | $ | 2,094 | $ | — | |||||
State | (155 | ) | 1,709 | — | |||||||
12,982 | 3,803 | — | |||||||||
Deferred | |||||||||||
Federal | (18,814 | ) | (8,296 | ) | (1,886 | ) | |||||
State | (3,847 | ) | (7,277 | ) | (574 | ) | |||||
(22,661 | ) | (15,573 | ) | (2,460 | ) | ||||||
Total tax benefit | $ | (9,679 | ) | $ | (11,770 | ) | $ | (2,460 | ) | ||
The following is a reconciliation of the statutory federal income tax rate to the Company’s effective tax rate expressed as a percentage of income (loss) before income taxes:
2016 | 2015 | 2014 | ||||||
Statutory rate - federal | (35.00 | )% | 35.00 | % | (35.00 | )% | ||
State taxes, net of federal benefit | (3.16 | )% | 1.53 | % | (6.77 | )% | ||
Change in FV of derivative liability (warrants) | 1.10 | % | 10.89 | % | 7.40 | % | ||
Stock Based Compensation | — | % | — | % | 5.51 | % | ||
Bargain purchase gain | — | % | (16.04 | )% | — | % | ||
Other permanent differences | 2.05 | % | 3.68 | % | — | % | ||
Tax credits | (1.58 | )% | (7.85 | )% | — | % | ||
Return to provision adjustments and other true-ups | (1.15 | )% | (10.40 | )% | — | % | ||
Other | 3.09 | % | (0.79 | )% | — | % | ||
Change in valuation allowance | 16.30 | % | (27.02 | )% | 26.63 | % | ||
Income tax benefit | (18.35 | )% | (11.00 | )% | (2.23 | )% | ||
The significant components of the Company’s deferred tax assets and liabilities as of December 31, 2016 and 2015 are as follows (in thousands):
2016 | 2015 | ||||||
Deferred Tax Assets: | |||||||
Net operating loss | $ | 1,832 | $ | 2,870 | |||
Research and development tax credits | 60 | — | |||||
Contingent consideration | 32,792 | 21,575 | |||||
Other accrued expenses | 4,621 | 3,160 | |||||
Stock based compensation | 18,520 | 9,484 | |||||
Other | 30 | — | |||||
57,855 | 37,089 | ||||||
Deferred Tax Liabilities: | |||||||
Intangible assets | (34,153 | ) | (25,124 | ) | |||
Deferred gain on installment sale | (14,547 | ) | (29,095 | ) | |||
Tax basis depreciation less than book depreciation | — | (218 | ) | ||||
(48,700 | ) | (54,437 | ) | ||||
Net deferred tax assets (liabilities) before valuation allowance | 9,155 | (17,348 | ) | ||||
Valuation allowance | (15,580 | ) | (6,980 | ) | |||
Total deferred tax liability | $ | (6,425 | ) | $ | (24,328 | ) | |
The Company has established a partial valuation allowance against its U.S. federal and state deferred tax assets due to the uncertainty surrounding the realization of such assets in future periods. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which temporary differences become deductible. Management considers the scheduled reversal of deferred liabilities and tax planning strategies in making this assessment and evaluates the recoverability of the deferred tax assets as of each reporting date. At such time as it is determined that it is more likely than not that deferred assets are realizable, the valuation allowance will be reduced accordingly and recorded as a tax benefit.
Based on the scheduled reversal of deferred tax liabilities, a portion of the Company's deferred tax liabilities cannot be used as a source of income to support the recoverability of the deferred tax assets. Accordingly, the Company has recorded a net deferred tax liability as of December 31, 2016.
F-30
The Company has recorded a valuation allowance of $15.6 million as of December 31, 2016 to reflect the estimated amount of deferred tax assets that may not be realized. The Company increased its valuation allowance by $8.6 million for the year ended December 31, 2016.
At December 31, 2016, the Company had available unused U.S. federal net operating loss (“NOL”) carryforwards of $5.2 million and a minimal amount of state NOL carryforwards, all of which are fully offset by a valuation allowance. The U.S. federal NOL carryforwards will expire beginning in 2030. In addition, at December 31, 2016, the Company had federal research and development tax credit carryforwards of $61,000 that begin to expire in 2036 unless utilized. The Company has international subsidiaries whose operations are not material for the year ended December 31, 2016.
The Company accounts for uncertain tax benefits in accordance with the provisions of ASC 740-10 of the Accounting for Uncertainty in Income Taxes. As of December 31, 2016 and 2015 the Company had recorded an indemnification asset with a corresponding liability in the amount of $1.5 million for an uncertain tax position related to the acquisition of Manchester Pharmaceuticals, LLC. The Company is indemnified with respect to the liability.
The Company does not anticipate that the amount of unrecognized tax benefits as of December 31, 2016 will change materially within the following 12 months.
A reconciliation of the Company's unrecognized tax benefits for the years 2016 and 2015 is provided in the following table (in thousands):
2016 | 2015 | ||||||
Balance as of January 1: | $ | 3,324 | $ | 1,500 | |||
Increase in current period positions | — | 1,424 | |||||
Decrease in prior period positions | (1,824 | ) | — | ||||
Increase in prior period positions | — | 400 | |||||
Balance as of December 31: | $ | 1,500 | $ | 3,324 | |||
The Company files income tax returns in the U.S. federal jurisdiction and various state and local jurisdictions. The Company’s income tax returns are open to examination by federal, state and foreign tax authorities, generally for the years ended December 31, 2013 and later. The Company is currently under income tax audit examination for our United States federal income tax return for 2014. At this time, the Company does not anticipate that the conclusion of the audit will have a material effect on the financial statements.
The Company’s policy is to record estimated interest and penalties related to the underpayment of income taxes or unrecognized tax benefits as a component of its income tax provision. During the years ended 2016, 2015 and 2014, the Company did not recognize any interest or penalties in its statements of operations and there were no accruals recorded for interest or penalties at December 31, 2016 and 2015.
NOTE 15. INVESTIGATIONAL MATTERS
Investigation and Impact on Financial Statements
In September 2014, the Company’s board of directors requested that its outside legal counsel conduct an investigation into various matters related to the former Chief Executive Officer of the Company. In January 2015, our board of directors appointed an Oversight Committee to oversee and direct the investigation and make findings and decisions related to the investigation. As a result of the investigation, the Oversight Committee determined that, throughout 2013 and 2014, the former Chief Executive Officer engaged in a series of transactions (the “Prior Transactions”), which involved individuals and entities that had been investors in investment funds previously managed by the former Chief Executive Officer (the “MSMB Entities”), pursuant to which assets of the Company were misappropriated.
As a result of the Prior Transactions the financial statements contained in the Company’s Form 10-Q for the three months ended September 30, 2013 (the “2013 Q3 Form 10-Q”), the Company’s Form 10-K for the year ended December 31, 2013 (the “2013 Form 10-K”) and the Company’s Forms 10-Q for the quarters ended March 31, 2014, June 30, 2014 and September 30, 2014 (the “2014 Forms 10-Q”) contained errors related to the reporting of certain consulting agreements entered into as part of the Prior Transactions, the predominant purpose of which appears to have been to settle and release claims against the MSMB Entities or the former Chief Executive Officer personally.
On February 19, 2015, our board of directors concluded that as a result of the errors related to such consulting agreements, the financial statements contained in the 2013 Q3 Form 10-Q and the 2013 Form 10-K should no longer be relied upon. Accordingly, the Quarterly Report on Form 10-Q for the quarter ending September 30, 2013 and the Annual Report on Form 10-K for the year ended December 31, 2013 were amended and filed with the SEC in July 2015.
Stock Option Accounting
The Company held a Special Meeting of Stockholders on February 3, 2015, at which its stockholders voted to approve a proposal ratifying the prior issuance of stock options to purchase 1,928,000 shares of common stock and 230,000 restricted shares of common stock granted to employees between February 24, 2014 and August 18, 2014 (the “Ratified Equity Grants”). The 2014 Forms 10-Q contained errors related to the non-cash compensation expense recognized in connection with the Ratified Equity Grants, because the grant/measurement date of the Ratified Equity Grants for financial accounting purposes did not occur until their ratification in 2015.
The Company previously accounted for the Ratified Equity Awards as if a grant/measurement date for financial accounting purposes had occurred upon their issuance date, and recognized compensation expense for such Ratified Equity Awards based on the grant/measurement date value,
F-31
which is amortized ratably to compensation expense and additional paid-in capital over the applicable service periods. The Company should have accounted for the Ratified Equity Awards as equity grants without a grant/measurement date, which are accounted for as “liability awards”, with compensation expense and an offsetting compensation liability recorded over the term of the award, and the liability award revalued at each reporting period based on changes in the Company’s stock price until it is ratified.
The Company believes that the errors in the 2014 Forms 10-Q related to the non-cash compensation expense recognized in connection with the Ratified Equity Grants do not cause the financial statements included within the 2014 Forms 10-Q to be misleading, and therefore such financial statements can still be relied upon. The Company corrected such errors, including any related disclosures, in its 2014 Annual Report on Form 10-K, and restated those quarters in 2015 Form 10-Q filings.
On February 27, 2015, the Company received a Public Letter of Reprimand from NASDAQ (the “Letter of Reprimand”), in accordance with Nasdaq Listing Rule 5810(c)(4). The Letter of Reprimand communicates NASDAQ’s belief that the interests of the Company’s shareholders were not materially adversely affected by the matters described above, and while not having been cured, the violation described above was remediated to the extent possible. Accordingly, NASDAQ does not believe that the delisting of the Company’s securities is an appropriate sanction, but rather, the circumstances warranted the issuance of the Letter of Reprimand. The issuance of the Letter of Reprimand completed NASDAQ’s review of the matters described above.
NOTE 16. SEVERANCE AGREEEMENTS
On September 15, 2014, the Company entered into a separation agreement and release (the “Separation Agreement”) with Marc Panoff, the Company’s Chief Financial Officer, pursuant to which Mr. Panoff’s employment with the Company was terminated effective as of February 28, 2015. Under the terms of the Separation Agreement, Mr. Panoff will be entitled to receive: (i) severance payments equal to six months of his current base salary; (ii) 100% of his target bonus for 2014; (iii) accelerated vesting of 81,333 shares of restricted common stock of the Company; and (iv) benefits under the Company’s benefit plans, subject to the terms of each such plan. In conjunction with the Separation Agreement, the Company had initially recorded and accrued $0.1 million of severance expense through September 30, 2014 in connection with Mr. Panoff’s severance which was to be expensed ratably over the service period from September 15, 2014 through February 28, 2015. During the 4th quarter of 2014, the Company determined that Mr. Panoff’s service to the Company was substantially completed prior to December 31, 2014 and as a result recorded the remaining unamortized severance expense related to his separation agreement of $1.1 million in the 4th quarter of fiscal 2014 in selling, general and administrative in the Consolidated Statements of Operations. Mr. Panoff’s target bonus which was included as part of his severance agreement was recognized ratably over the course of the fiscal year ended December 31, 2014.
On October 13, 2014, Martin Shkreli resigned as a member of the Board and as an employee of the Company, and from any and all other positions that he held with the Company. On October 13, 2014, the Company entered into a resignation letter with Mr. Shkreli (“Separation Agreement”). As part of Mr. Shkreli’s Separation Agreement, Mr. Shkreli received cash severance, unpaid bonus and health insurance coverage, 12 months of continued vesting of time based stock options and no vesting of performance based stock options. Pursuant to the Separation Agreement, Mr. Shkreli’s market and performance based stock options were forfeited. As a result, the Company recorded compensation expense in the amount of $0.5 million relating to Mr. Shkreli’s cash severance, unpaid bonus and health insurance coverage and compensation expense of $1.1 million related to the accelerated vesting of Mr. Shkreli’s time based stock options.
On October 13, 2014, the Company signed a Letter of Intent for the terms for the sale of the Company’s Vecamyl, Syntocinon and ketamine licenses and assets to Turing Pharmaceuticals AG (“Turing Pharmaceuticals”), which includes an up-front payment to the Company of $3.0 million and the assumption of certain liabilities including license fees and royalties (the “Sale Transaction”). Martin Shkreli, the Company’s former Chief Executive Officer and Director, is the Chief Executive Officer of Turing Pharmaceuticals. The closing of the Sale Transaction was subject to various conditions, including the negotiation and execution of a binding definitive agreement between the Company and Turing Pharmaceuticals and the receipt of necessary third party consents. In connection with the Letter of Intent with Martin Shkreli, the Company recorded severance expense and accrued severance expense of $2.9 million as of and for the year ended December 31, 2014 which is the difference between of the net book value of the assets to be sold, the $3.0 million expected upfront payment, and $3.0 million of liabilities expected to be assumed.
As both transactions were contemplated simultaneously, they were both considered in calculating the respective severance expense related to Mr. Shkreli’s termination. The full amount of the severance was recorded as of September 30, 2014 as that was the date that the Board replaced Martin Shkreli as Chief Executive Officer of the Company until a formal separation agreement could be finalized. As of September 30, 2014, it was deemed to be probable and estimable that Mr. Shkreli would enter into a separation agreement that would entitle him to severance benefits. Therefore the estimated severance that was booked as of the end of the third quarter is based on the best estimate currently available and the full severance amount was recorded as of September 30, 2014 as Mr. Shkreli was not required to perform any future service for the Company. For the year ended December 31, 2014, the Company recorded a total of $4.5 million severance expense in connection with Mr. Shkreli’s Separation Agreement which was recorded in selling, general and administrative expenses in the Consolidated Statements of Operations.
On January 9, 2015, the Company entered into an Asset Purchase Agreement (the “Purchase Agreement”) with Turing Pharmaceuticals pursuant to which the Company sold Turing Pharmaceuticals its ketamine licenses and assets (the “Ketamine Assets”) for a purchase price of $1.0 million. Turing Pharmaceuticals also assumed all future liabilities related to the Ketamine Assets.
On February 13, 2015, the Company, its wholly-owned subsidiary Manchester and its other wholly-owned subsidiary Retrophin Therapeutics International, LLC (collectively, the “Sellers”), entered into a Purchase Agreement with Waldun, pursuant to which the Sellers sold Waldun their product rights to mecamylamine hydrochloride (also referred to as Vecamyl) (the “Vecamyl Product Rights”) for a purchase price of $0.7 million. Waldun in turn sold the Vecamyl Product Rights to Turing Pharmaceuticals. In connection therewith, on February 13, 2015, the Company and Manchester entered into an Asset
F-32
Purchase Agreement with Turing Pharmaceuticals, pursuant to which the Company and Manchester sold Turing Pharmaceuticals their Vecamyl inventory for a purchase price of $0.3 million. Turing Pharmaceuticals also assumed certain liabilities related to the Vecamyl Product Rights and inventory.
Additionally, on February 13, 2015, the Company entered into an Asset Purchase Agreement with Turing Pharmaceuticals pursuant to which the Company sold Turing Pharmaceuticals its Syntocinon licenses and assets, including related inventory, for a purchase price of $1.1 million. Turing Pharmaceuticals also assumed certain liabilities related to the Syntocinon licenses and assets.
NOTE 17. RETIREMENT PLAN
The Company has a 401(k) defined contribution savings plan for the benefit of all eligible employees. Employer matching contributions were $0.5 million for the year ended December 31, 2016. There were no employer contributions for the years ended December 31, 2015 and 2014.
NOTE 18. QUARTERLY FINANCIAL INFORMATION (UNAUDITED)
The following table presents selected consolidated statements of operations data for each quarter for the fiscal years ended December 31, 2016 and 2015 (unaudited, in thousands, except for per share data):
Fourth Quarter | Third Quarter | Second Quarter | First Quarter | ||||||||||||
For the year ended December 31, 2016: | |||||||||||||||
Net product sales | $ | 37,327 | $ | 33,945 | $ | 33,311 | $ | 29,008 | |||||||
Total operating expenses | 55,549 | 54,317 | 44,690 | 37,249 | |||||||||||
Operating loss | (18,222 | ) | (20,372 | ) | (11,379 | ) | (8,241 | ) | |||||||
Total other income (expense), net1 | (5,935 | ) | 10,274 | 9,416 | (14,387 | ) | |||||||||
Income (loss) before provision for income taxes | (12,287 | ) | (30,646 | ) | (20,795 | ) | 6,146 | ||||||||
Income tax benefit (provision) | 3,684 | (6,467 | ) | 7,392 | 5,070 | ||||||||||
Net income (loss) | $ | (8,603 | ) | $ | (37,113 | ) | $ | (13,403 | ) | $ | 11,216 | ||||
Net income (loss) per common share | |||||||||||||||
Basic | $ | (0.23 | ) | $ | (1.00 | ) | $ | (0.37 | ) | $ | 0.31 | ||||
Diluted | $ | (0.39 | ) | $ | (1.00 | ) | $ | (0.37 | ) | $ | (0.08 | ) | |||
For the year ended December 31, 2015: | |||||||||||||||
Net product sales | $ | 30,447 | $ | 28,005 | $ | 24,068 | $ | 17,372 | |||||||
Total operating expenses | 45,651 | 48,501 | 31,012 | 25,476 | |||||||||||
Operating loss | (15,204 | ) | (20,496 | ) | (6,944 | ) | (8,104 | ) | |||||||
Total other income (expense), net1 | 2,210 | 164,835 | 2 | (18,568 | ) | 7,738 | |||||||||
Income (loss) before provision for income taxes | (12,994 | ) | 144,339 | (25,512 | ) | (366 | ) | ||||||||
Income tax benefit (provision) | 10,525 | (38,761 | ) | (15 | ) | 40,021 | |||||||||
Net income (loss) | $ | (2,469 | ) | $ | 105,578 | $ | (25,527 | ) | $ | 39,655 | |||||
Net income (loss) per common share | |||||||||||||||
Basic | $ | (0.07 | ) | $ | 2.95 | $ | (0.73 | ) | $ | 1.46 | |||||
Diluted | $ | (0.14 | ) | $ | 1.78 | $ | (0.73 | ) | $ | 1.32 | |||||
1The Company has experienced large changes in its stock price which directly effects the fair value of derivative instruments issued by the Company. These changes in fair value are charged to other income (expense) which correspondingly incurs larges variance from period to period. See Note 5 and 6 to the Consolidated Financial Statements for further discussion.
2On July 2, 2015, the Company sold and transferred the Pediatric PRV to Sanofi for $245.0 million. The gain from the sale of the asset was approximately $140.0 million . See Note 3 to the Consolidated Financial Statements for further details.
F-33
