Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - GERON CORP | a2230923zex-32_2.htm |
| EX-32.1 - EX-32.1 - GERON CORP | a2230923zex-32_1.htm |
| EX-31.2 - EX-31.2 - GERON CORP | a2230923zex-31_2.htm |
| EX-31.1 - EX-31.1 - GERON CORP | a2230923zex-31_1.htm |
| EX-23.1 - EX-23.1 - GERON CORP | a2230923zex-23_1.htm |
| EX-12.1 - EX-12.1 - GERON CORP | a2230923zex-12_1.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | ||
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the Fiscal Year Ended December 31, 2016 |
||
or |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to . |
||
Commission File Number: 0-20859
GERON CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
75-2287752 (I.R.S. Employer Identification No.) |
|
149 Commonwealth Drive, Suite 2070, Menlo Park, CA (Address of principal executive offices) |
94025 (Zip Code) |
Registrant's telephone number, including area code: (650) 473-7700
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
|---|---|---|
| Common Stock, $0.001 par value | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer", "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer ý | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No ý
The aggregate market value of voting and non-voting common equity held by non-affiliates of the registrant was approximately $424,378,000 based upon the closing price of the registrant's common stock on June 30, 2016 on the Nasdaq Global Select Market. The calculation of the aggregate market value of voting and non-voting common equity held by non-affiliates of the registrant excludes shares of common stock held by each officer, director and stockholder that the registrant concluded were affiliates on that date. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 21, 2017, there were 159,158,636 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE:
Document
|
Form 10-K Parts |
|
|---|---|---|
Portions of the Registrant's definitive proxy statement for the 2017 annual meeting of stockholders to be filed pursuant to Regulation 14A within 120 days of the Registrant's fiscal year ended December 31, 2016 |
III |
In this report, unless otherwise indicated or the context otherwise requires, "Geron," "the registrant," "we," "us," and "our" refer to Geron Corporation, a Delaware corporation.
2
Forward-Looking Statements
This annual report on Form 10-K, including "Business" in Part I, Item 1 and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in Part II, Item 7, contains forward-looking statements that involve risks and uncertainties, as well as assumptions that, if they never materialize or prove incorrect, could cause the results of Geron Corporation, or Geron or the Company, to differ materially from those expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. In some cases, forward-looking statements can be identified by the use of terminology such as "may," "expects," "plans," "intends," "will," "should," "projects," "believes," "predicts," "anticipates," "estimates," "potential," or "continue" or the negative thereof or other comparable terminology. The risks and uncertainties referred to above include, without limitation, risks related to our dependence on Janssen Biotech, Inc., or Janssen, for the development, regulatory approval, manufacture and commercialization of imetelstat, uncertainty of clinical trial results or regulatory approvals or clearances, the future development of imetelstat, including any future efficacy or safety results that cause the benefit-risk profile of imetelstat to become unacceptable, our ability to identify and acquire and/or in-license other oncology products, product candidates, programs or companies to grow and diversify our business, our need for additional capital to support the development and commercialization of imetelstat in collaboration with Janssen and to otherwise grow our business, enforcement of our patent and proprietary rights, potential competition and other risks that are described herein and that are otherwise described from time to time in our Securities and Exchange Commission reports including, but not limited to, the factors described in Part I, Item 1A, "Risk Factors," of this annual report on Form 10-K. Geron assumes no obligation for and except as required by law, disclaims any obligation to update these forward-looking statements to reflect future information, events or circumstances.
Calculation of Aggregate Market Value of Non-Affiliate Shares
For purposes of calculating the aggregate market value of shares of our common stock held by non-affiliates as set forth on the cover page of this annual report on Form 10-K, we have assumed that all outstanding shares are held by non-affiliates, except for shares held by each of our executive officers, directors and 5% or greater stockholders. In the case of 5% or greater stockholders, we have not deemed such stockholders to be affiliates unless there are facts and circumstances which would indicate that such stockholders exercise any control over our Company. These assumptions should not be deemed to constitute an admission that all executive officers, directors and 5% or greater stockholders are, in fact, affiliates of our Company, or that there are no other persons who may be deemed to be affiliates of our Company. Further information concerning shareholdings of our executive officers, directors and principal stockholders is incorporated by reference in Part III, Item 12 of this annual report on Form 10-K.
Company Overview
We are a biopharmaceutical company that currently supports the clinical stage development of a telomerase inhibitor, imetelstat, in hematologic myeloid malignancies, by Janssen Biotech, Inc., or Janssen. Early clinical data, including molecular responses in essential thrombocythemia, or ET, and remission responses, including reversal of bone marrow fibrosis, in myelofibrosis, or MF, suggest imetelstat may have disease-modifying activity by inhibiting the progenitor cells of the malignant clones for the underlying diseases.
3
On November 13, 2014, we entered into a collaboration and license agreement, or the Collaboration Agreement, pursuant to which we granted Janssen the exclusive rights to develop and commercialize imetelstat worldwide for all indications in oncology, including hematologic myeloid malignancies, and all other human therapeutic uses. The Collaboration Agreement became effective on December 15, 2014, and we received $35 million from Janssen as an upfront payment. Additional consideration under the Collaboration Agreement includes potential payments of up to an aggregate maximum total of $900 million for the achievement of development, regulatory and commercial milestones, as well as royalties on worldwide net sales of imetelstat.
Under the Collaboration Agreement, Janssen is wholly responsible for developing, manufacturing, seeking regulatory approval for, and commercialization of, imetelstat worldwide. To that end, Janssen is currently conducting two clinical trials of imetelstat: IMbark, a Phase 2 trial in MF, in which the first patient was dosed in September 2015; and IMerge, a Phase 2/3 trial in myelodysplastic syndromes, or MDS, in which the first patient was dosed in January 2016.
In September 2016, Janssen completed planned internal reviews of data from both IMbark and IMerge. Following these internal reviews, Janssen determined to continue the trials in order to obtain additional and more mature data. In the first quarter of 2017, Janssen initiated the process for the second internal data reviews for both IMbark and IMerge, which will include comprehensive analyses encompassing safety, efficacy, pharmacokinetic and pharmacodynamic data, as well as other exploratory assessments, such as cytogenetic and molecular data. We expect the outcomes from the second internal data reviews, regulatory considerations and the totality of other program information, including the evolving treatment landscapes in MF and MDS, to inform Janssen's decisions regarding future development plans for imetelstat. Following the second internal data reviews, we expect Janssen could decide to resume, modify or terminate IMbark, IMerge, the imetelstat program or the Collaboration Agreement. We expect Janssen's decision-making regarding IMbark, IMerge, the imetelstat program or the Collaboration Agreement to occur in the second quarter of 2017. Janssen's decisions regarding future development plans for imetelstat, if any, may be subject to subsequent regulatory feedback. While patients remaining in the treatment phase of IMbark and IMerge continue to be dosed with imetelstat, new patient enrollment in both trials currently is suspended until completion of the second internal data reviews.
We had approximately $129.1 million in cash and investments as of December 31, 2016. To grow and diversify our business, we plan to continue our business development efforts to identify, and seek to acquire and/or in-license other oncology product candidates, programs or companies. Acquisition or in-licensing opportunities that we may pursue could materially affect our liquidity and capital resources and may require us to incur indebtedness or seek equity capital, or both.
Telomerase: Scientific Rationale
Telomeres and Telomerase in Normal Development
In the human body, normal growth and maintenance of tissues occurs by cell division. However, most cells are only able to divide a limited number of times, and this number of divisions is regulated by telomere length. Telomeres are repetitions of a deoxyribonucleic acid, or DNA, sequence located at the ends of chromosomes. They act as protective caps to maintain stability and integrity of the chromosomes, which contain the cell's genetic material. Normally, every time a cell divides, the telomeres shorten. Eventually, they shrink to a critically short length, and as a result, the cell either dies by apoptosis or stops dividing and senesces.
Telomerase is a naturally occurring enzyme that maintains telomeres and prevents them from shortening during cell division in cells, such as stem cells that must remain immortalized to support normal health. Telomerase consists of at least two essential components: a ribonucleic acid, or RNA, template (hTR), which binds to the telomere, and a catalytic subunit (hTERT) with reverse
4
transcriptase activity, which adds a specific DNA sequence to the chromosome ends. The 2009 Nobel Prize for Physiology and Medicine was awarded to Drs. Elizabeth H. Blackburn, Carol W. Greider and Jack Szostak, former Geron collaborators, for the discovery of how chromosomes are protected by both telomeres and telomerase.
Telomerase is active during embryonic development, enabling the rapid cell division that supports normal growth. During the latter stages of human fetal development and in adulthood, telomerase is repressed in most cells, and telomere length gradually decreases during a lifetime. In tissues that have a high turnover throughout life, such as blood and gut, telomerase can be transiently upregulated in progenitor cells to enable controlled, self-limited proliferation to replace cells lost through natural cell aging processes. As the progeny of progenitor cells mature, telomerase is downregulated and telomeres shorten with cell division, preventing uncontrolled proliferation.
Telomeres and Telomerase in Cancer
Telomerase is upregulated in many tumor progenitor cells, enabling the continued and uncontrolled proliferation of the malignant cells that drive tumor growth and progression. Telomerase expression has been found to be present in approximately 90% of biopsies taken from a broad range of human cancers. Our nonclinical studies, in which the telomerase gene was artificially introduced and expressed in normal cells grown in culture, have suggested that telomerase does not itself cause a normal cell to become malignant. Instead, the sustained upregulation of telomerase enables tumor cells to maintain telomere length, providing them with the capacity for limitless proliferation. We believe that the sustained upregulation of telomerase is critical for tumor progression as it enables malignant progenitor cells to acquire cellular immortality and avoid apoptosis, or cell death.
Telomerase Inhibition: Inducing Cancer Cell Death
We believe that inhibiting telomerase may be an attractive approach to treating cancer because it may limit the proliferative capacity of malignant cells. We and others have observed in various in vitro and rodent tumor models that inhibiting telomerase results in telomere shortening and arrests uncontrolled malignant cell proliferation and tumor growth. In vitro studies have suggested that tumor cells with short telomeres may be especially sensitive to the anti-proliferative effects of inhibiting telomerase. Our nonclinical data also suggest that inhibiting telomerase is particularly effective at limiting the proliferation of malignant progenitor cells, which have high levels of telomerase and are believed to be important drivers of tumor growth and progression.
Imetelstat: The First Telomerase Inhibitor to Advance to Clinical Development
Imetelstat is a lipid conjugated 13-mer oligonucleotide that we designed to be complementary to and bind with high affinity to the RNA template of telomerase, thereby directly inhibiting telomerase activity. Imetelstat does not elicit its effect through an antisense inhibition of protein translation. The compound has a proprietary thio-phosphoramidate backbone, which is designed to provide resistance to the effect of cellular nucleases, thus conferring improved stability in plasma and tissues, as well as improved binding affinity to its target. To improve the ability of imetelstat to penetrate cellular membranes, we conjugated the oligonucleotide to a lipid group. Imetelstat's IC50, or half maximal inhibitory concentration, is 0.5 - 10 nM in cell free assays. Single-dose kinetics in patients has shown dose-dependent increases in exposure to imetelstat, with a plasma half-life, which is the time it takes for the concentration or amount of imetelstat to be reduced by half, ranging from 4 - 5 hours. Data from animal studies and clinical trials have suggested that the residence time of imetelstat in bone marrow is long, with 0.19 - 0.51 µM observed at 41 - 45 hours after a 7.5 mg/kg dose in patients. Imetelstat also has been shown in nonclinical studies to exhibit relatively preferential inhibition of the clonal proliferation of malignant progenitor cells compared to normal progenitors. For these reasons, imetelstat has been studied as a treatment for malignant diseases.
5
Imetelstat is the first telomerase inhibitor to advance to clinical development. The Phase 1 trials that we completed evaluated the safety, tolerability, pharmacokinetics and pharmacodynamic effects of imetelstat. We established doses and dosing schedules that were tolerable and achieved target exposures in patients that were consistent with those required for efficacy in animal models. The dose-limiting toxicities were thrombocytopenia, or reduced platelet count, and neutropenia, or reduced neutrophil count. Following intravenous administration of imetelstat using tolerable dosing regimens, clinically relevant and significant inhibition of telomerase activity was observed in various types of tissue in which telomerase activity is measurable, including normal bone marrow hematopoietic cells, malignant plasma cells, hair follicle cells and peripheral blood mononuclear cells.
Disease Characteristics of Hematologic Malignancies
Hematologic malignancies, or blood cancers, are classified according to the predominant location of the malignancy. A hematologic myeloid malignancy is a cancer that occurs in the precursor cells to red blood cells, platelets and white blood cells such as granulocytes. Examples include acute myelogenous leukemia, chronic myelogenous leukemia, MDS and the myeloproliferative neoplasms, such as ET, polycythemia vera and MF. These are different from lymphocytic malignancies which typically occur in the lymphoid lineage that includes white blood cells, such as T lymphocytes and B lymphocytes. Examples of lymphoid malignancies include acute lymphoblastic leukemia, chronic lymphocytic leukemia, lymphomas and multiple myeloma.
Many hematologic myeloid malignancies, such as ET, MF, and MDS, have been shown to arise from malignant progenitor cells in the bone marrow that express higher telomerase activity and have shorter telomeres when compared to normal healthy cells.
Unmet Medical Need in Myelofibrosis
MF, a type of myeloproliferative neoplasm, is a chronic blood cancer in which abnormal or malignant precursor cells in the bone marrow proliferate rapidly, causing scar tissue, or fibrosis, to form. As a result, normal blood production in the bone marrow is impaired and may shift to other organs such as the spleen and liver, which can cause them to enlarge substantially. People with MF may have abnormally low or high numbers of circulating red blood cells, white blood cells or platelets, and abnormally high numbers of immature cells in the blood or bone marrow. MF patients can also suffer from debilitating constitutional symptoms, such as drenching night sweats, fatigue, severe itching, or pruritus, abdominal pain, fever and bone pain. The estimated prevalence of MF in the United States, or U.S., is approximately 13,000 patients, with an annual incidence of approximately 3,000 patients. Up to 20% of patients with MF develop acute myeloid leukemia, or AML.
Approximately 70% of MF patients are classified as having intermediate-2 or high risk disease, as defined by the Dynamic International Prognostic Scoring System Plus, or DIPSS Plus, described in a 2011 Journal of Clinical Oncology article. There is currently only one targeted drug therapy, ruxolitinib, approved by the U.S. Food and Drug Administration, or FDA, and other health authorities for treating these MF patients. Currently, no drug therapy is approved for those patients who fail or no longer respond to that treatment, and median survival for such MF patients is only approximately seven months, representing a significant unmet medical need.
Inadequacy of Conventional Treatments in Myelodysplastic Syndromes
MDS are a group of blood disorders in which the proliferation of malignant progenitor cell clones in the bone marrow results in disordered and ineffective production of the myeloid lineage, which includes red blood cells, white blood cells and platelets. In MDS, bone marrow and peripheral blood cells may have abnormal, or dysplastic, cell morphology. MDS is frequently characterized clinically by severe anemia, or low red cell counts and low hemoglobin. In addition, other peripheral cytopenias, or
6
low numbers of white blood cells and platelets, may cause life-threatening infections and bleeding. Transformation to AML occurs in up to 30% of MDS cases and results in poorer overall survival.
MDS is the most common of the myeloid malignancies. There are approximately 60,000 people in the United States living with the disease and approximately 12,000 reported new cases of MDS in the United States every year. MDS is primarily a disease of the elderly, with median age at diagnosis around 70 years. The majority of patients, approximately 70%, fall into what are considered to be the lower risk groups at diagnosis, according to the International Prognostic Scoring System, or IPSS, that takes into account the presence of a number of disease factors, such as cytopenias and cytogenetics, to assign relative risk of progression to AML and overall survival.
Chronic anemia is the predominant clinical problem in patients who have lower risk MDS. Many of these patients become dependent on red blood cell transfusions, which can lead to elevated levels of iron, which the body has no normal way to eliminate, in the blood and other tissues. Iron overload is a potentially dangerous condition. Studies in patients with MDS have shown that iron overload resulting from regular red blood cell transfusions is associated with a poorer overall survival and a higher risk of developing AML.
Developing Imetelstat to Treat Hematologic Myeloid Malignancies
Proof-of-Concept of Imetelstat's Disease-Modifying Potential
Early clinical data, including molecular responses observed in a Phase 2 clinical trial of imetelstat in patients with ET, or the ET Trial, and partial or complete remissions, that included reversal of bone marrow fibrosis in patients with MF, observed in a pilot study of imetelstat conducted at Mayo Clinic, or the Pilot Study, suggest imetelstat may exhibit disease-modifying activity by inhibiting the proliferation of malignant progenitor cell clones. These data were published in two separate articles in the September 3, 2015 issue of The New England Journal of Medicine. In addition, the Pilot Study included a cohort of patients who had a sub-type of MDS referred to as refractory anemia with ring sideroblasts, or RARS. A small number of these patients achieved a period of red blood cell transfusion independence longer than eight weeks when treated with imetelstat. These data were published in the March 2016 online issue of Blood Cancer Journal.
Reported adverse events, or AEs, and laboratory investigations associated with imetelstat in the ET Trial and the Pilot Study included cytopenias, gastrointestinal symptoms, constitutional symptoms, and hepatic biochemistry abnormalities. Dose-limiting toxicities, such as profound and prolonged thrombocytopenia and neutropenia, and other safety issues, including death, were observed in the ET Trial and the Pilot Study. In those trials, such myelosuppression was managed by dose holds and modification rules.
In March 2014, the FDA issued a full clinical hold on our Investigational New Drug application, or IND, for imetelstat, citing a lack of evidence of reversibility of hepatotoxicity, concern regarding a risk of chronic liver injury, and a lack of adequate follow-up in patients who had hepatotoxic effects. Follow-up safety data showed that liver function test, or LFT, abnormalities resolved to normal or baseline during or after withdrawal of imetelstat treatment in most patients. We submitted a complete response to the FDA, and the FDA lifted the clinical hold in October 2014. Also in March 2014, we were informed by Mayo Clinic that the investigator's IND for the Pilot Study was placed on partial clinical hold by the FDA due to a safety signal of hepatotoxicity and that it was unknown if this hepatotoxicity was reversible. The investigator submitted a complete response to the FDA, and the partial hold was removed by the FDA in June 2014.
7
Imetelstat Clinical Trials Initiated Under the Collaboration with Janssen
Under the Collaboration Agreement (as described below), Janssen is conducting two clinical trials of imetelstat: IMbark and IMerge.
| |
|
|
|
|
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | | | | | | | | | | | | |
|
Study Name | |||||||||||
| | | | | | | | | | | | | |
|
IMbark | IMerge | ||||||||||
| | | | | | | | | | | | | |
|
Indication |
Myelofibrosis | Myelodysplastic Syndromes | |||||||||
| | | | | | | | | | | | | |
|
Patient Population |
DIPSS intermediate-2 or high risk MF patients who have relapsed after or are refractory to janus kinase, or JAK, inhibitor treatment | Transfusion dependent patients with IPSS low or intermediate-1 risk MDS who have relapsed after or are refractory to prior erythropoiesis-stimulating agent, or ESA, treatment | |||||||||
| | | | | | | | | | | | | |
|
Design |
Phase 2, open-label, randomized to two dosing arms, single-blinded | Phase 2/3: Part 1 is Phase 2, open-label, single-arm; Part 2 is Phase 3 double-blind, randomized, placebo-controlled | |||||||||
| | | | | | | | | | | | | |
|
Primary Endpoint |
Spleen response rate and symptom response rate (co-primary endpoints) | Red blood cell transfusion-independence, or RBC-TI, rate ³8 weeks | |||||||||
| | | | | | | | | | | | | |
|
Selected Secondary Endpoints |
Complete remission or partial remission rate, clinical improvement rate, anemia, spleen and symptom responses, and safety | RBC-TI rate ³24 weeks, time to and duration of RBC-TI, hematologic improvement rate, complete remission or partial remission rate, red blood cell transfusion requirement, myeloid growth factor use, patients' quality of life, overall survival assessment and time to progression to AML | |||||||||
| | | | | | | | | | | | | |
|
Initiated (First Patient Dosed) |
September 2015 | January 2016 | |||||||||
| | | | | | | | | | | | | |
|
First Internal Data Review |
Completed in September 2016 (see below) | Completed in September 2016 (see below) | |||||||||
| | | | | | | | | | | | | |
|
Second Internal Data Review |
Process initiated by Janssen in the first quarter of 2017 | Process initiated by Janssen in the first quarter of 2017 | |||||||||
| | | | | | | | | | | | | |
IMbark
IMbark was initiated to assess the efficacy, safety and tolerability of two dose levels of single-agent imetelstat in patients with MF. The trial was originally designed to enroll approximately 200 patients, including approximately 100 patients per dosing arm, with DIPSS intermediate-2 or high risk MF who have relapsed after or are refractory to JAK inhibitor treatment. At the time of enrollment, patients must have measurable splenomegaly and symptoms of MF. Patients were assigned randomly on a blinded basis in a 1:1 ratio to one of two dosing arms—9.4 mg/kg every three weeks or 4.7 mg/kg every three weeks. Dose reductions for adverse events are allowed and follow protocol-specified algorithms.
8
The co-primary efficacy endpoints for the trial are spleen response rate and symptom response rate. Spleen response rate is defined as the percentage of patients who achieve ³35% reduction in spleen volume from baseline at the Week 24 visit, as measured by imaging scans and assessed at a central imaging facility and by an Independent Review Committee. Symptom response rate is defined as the percentage of patients who have ³50% reduction in Total Symptom Scores from baseline at the Week 24 visit, based on patient-reported outcomes on a modified Myelofibrosis Symptom Assessment Form version 2.0 electronic diary. Secondary efficacy endpoints include the number of patients achieving complete remission, or CR, or partial remission, or PR, clinical improvement, or CI, and anemia, spleen and symptom responses as assessed using the modified 2013 International Working Group for Myeloproliferative Neoplasms Research and Treatment, or IWG-MRT criteria, described in a 2013 Blood article. These secondary endpoints will be assessed at the time of the primary efficacy analysis. Exploratory endpoints include cytogenetic and molecular responses, as well as leukemia-free survival.
Safety outcomes are monitored throughout the trial and include enhanced data collection and reporting for adverse events of interest, including hepatobiliary-associated laboratory findings and hepatic adverse events.
IMbark is being conducted at multiple medical centers across North America, Europe and Asia. Study design information for IMbark, including patient eligibility criteria, and clinical sites is posted on clinicaltrials.gov.
Outcomes of First Internal Data Review of IMbark
To inform an assessment of the appropriate dose and schedule of imetelstat for relapsed or refractory MF patients in IMbark, in September 2016, Janssen completed a planned internal review of safety, efficacy and pharmacokinetic data from 20 patients from each dosing arm who had been followed on the trial for at least 12 weeks. Based on this first internal review at the 12-week time point, the following were determined by Janssen:
- •
- The safety profile was consistent with the previous adverse events observed in the ET Trial and the Pilot Study. No new safety signals were
identified.
- •
- Activity in the 4.7 mg/kg dosing arm did not warrant further investigation of that dose, and this arm has been closed to new patient
enrollment.
- •
- The 9.4 mg/kg dosing arm warranted further investigation because encouraging trends in the efficacy data were observed. However, new patient enrollment to this arm was suspended because an insufficient number of patients met the protocol defined interim efficacy criteria at 12 weeks to confirm dosing.
Current Status of IMbark
Patients remaining in the treatment phase who were originally enrolled in the 4.7 mg/kg dosing arm may continue to receive imetelstat at that dose or, at the investigator's discretion may receive imetelstat at an increased dose of 9.4 mg/kg. Dosing continues for those patients remaining in the treatment phase who were enrolled in the 9.4 mg/kg dosing arm to obtain additional and more mature data.
We expect Janssen to conduct a second internal data review to assess potential future development, if any, of imetelstat in MF, as well as to assess whether 9.4 mg/kg is the appropriate starting dose and is sufficiently efficacious and safe for the IMbark patient population. In the first quarter of 2017, Janssen initiated the process for the second internal data review for IMbark, which will include comprehensive analyses encompassing safety, efficacy, pharmacokinetic and pharmacodynamic data, as well as other exploratory assessments, such as cytogenetic and molecular data, from patients
9
enrolled in the 9.4 mg/kg dosing arm who have been followed for at least 24 weeks, consistent with the co-primary efficacy endpoints. Following the second internal review, we expect Janssen could decide to resume enrollment in the 9.4 mg/kg dosing arm, modify the trial, close the trial, discontinue development of imetelstat in MF, or terminate the Collaboration Agreement. We expect the outcomes from the second internal data reviews for both IMbark and IMerge, regulatory considerations and the totality of other program information, including the evolving treatment landscapes in MF and MDS, to inform Janssen's decisions regarding future development plans for imetelstat. We expect Janssen's decision-making regarding IMbark, the imetelstat program and the Collaboration Agreement to occur in the second quarter of 2017. Janssen's decisions regarding future development plans for imetelstat, if any, may be subject to subsequent regulatory feedback.
The protocol-specified primary analysis of the co-primary efficacy endpoints in IMbark is planned to occur after all patients (i.e., planned to be approximately 100 enrolled and treated patients on the 9.4 mg/kg dosing arm) have been followed for at least 24 weeks. Due to the current suspension of new patient enrollment in IMbark, the timing of the protocol-specified primary analysis for the trial is uncertain and may be substantially delayed due to numerous factors, including whether Janssen resumes patient enrollment in the trial, or may not occur at all if IMbark is terminated early based on preliminary data, safety concerns or for any other reason.
IMerge
IMerge was initiated to evaluate imetelstat in transfusion dependent patients with IPSS low or intermediate-1 risk MDS who have relapsed after or are refractory to prior treatment with an ESA.
IMerge was designed as a two-part clinical trial and a total of approximately 200 patients are expected to be enrolled. Part 1 of the trial is designed as a Phase 2, open-label, single-arm design to assess the efficacy and safety of imetelstat. Part 1 of IMerge is fully enrolled with approximately 30 patients, who will all receive imetelstat and be followed for safety, hematologic improvement and reduction in transfusion requirement. Before proceeding to Part 2, the data from Part 1 must support a positive assessment of the benefit-risk profile of imetelstat in these patients. Part 2 of the trial is planned as a Phase 3 double-blind, randomized, placebo-controlled design to compare the efficacy of imetelstat against placebo. Under the original design of IMerge, if the trial proceeds to Part 2, approximately 170 patients are expected to be enrolled in Part 2, and such patients will be assigned randomly, in a 2:1 ratio, to receive either imetelstat or placebo.
The inclusion criteria for both parts of the trial require that at the time of enrollment each patient must be red blood cell transfusion-dependent. Imetelstat in Parts 1 and 2, or placebo in Part 2, will be administered as an intravenous infusion. The planned starting dose of imetelstat will be 7.5 mg/kg every four weeks and may be escalated according to certain protocol-specified conditions. Dose reductions for adverse events may follow protocol-specified algorithms. All patients may receive supportive care, including transfusions or myeloid growth factors, as needed per investigator discretion and according to local standard practices.
The primary efficacy endpoint of IMerge is designed to be the percentage of patients, defined by the trial protocol, who achieve red blood cell transfusion-independence lasting at least eight consecutive weeks at any time after entry to the trial. A primary efficacy analysis is planned to occur 12 months after the last patient is enrolled.
Secondary efficacy endpoints include the proportion of patients achieving red blood cell transfusion-independence lasting at least 24 weeks, the time to and duration of red blood cell transfusion-independence, the proportion of patients achieving hematologic improvement, the proportion of patients achieving CR or PR according to the 2006 International Working Group, or IWG, criteria for MDS, the proportion of patients requiring red blood cell transfusions and the amount, the proportion of patients requiring the use of myeloid growth factors and the dose, as well as
10
assessments of the change in the patients' quality of life using several different validated instruments. Patients will be followed by Janssen for an assessment of overall survival and time to progression to AML. These secondary endpoints are expected to be assessed at the time of the primary efficacy analysis.
Safety outcomes are monitored throughout the trial and include enhanced data collection and reporting for adverse events of interest, including hepatobiliary-associated laboratory findings and hepatic adverse events.
IMerge is being conducted at multiple medical centers across North and South America, Europe and Asia. Study design information for IMerge, including patient eligibility criteria, is posted on clinicaltrials.gov.
Outcomes of First Internal Data Review in IMerge
In September 2016, Janssen completed an initial internal review of efficacy, safety and pharmacokinetic data in a subset of patients in Part 1 of IMerge and determined that emerging safety and efficacy in IMerge were consistent with data reported from the Pilot Study conducted in MDS-RARS patients. Janssen also determined to continue IMerge unmodified.
Current Status of IMerge
Part 1 of IMerge has been fully enrolled and patients remaining in the treatment phase continue to be dosed with imetelstat. As originally designed, further patient enrollment is suspended until a decision has been made to move forward with Part 2 of the trial. We expect clinical data from Part 1 of IMerge to be presented at a medical conference to be determined in the future.
We expect Janssen to conduct a second internal data review for IMerge to evaluate potential future development, if any, of imetelstat in MDS, as well as to determine whether to move forward to Part 2 of IMerge. In the first quarter of 2017, Janssen initiated the process for the second internal data review for IMerge, which will include comprehensive analyses encompassing safety, efficacy, pharmacokinetic and pharmacodynamic data, as well as other exploratory assessments, such as cytogenetic and molecular data, from all patients in Part 1. Following the second internal review, we expect Janssen could decide to move forward with Part 2 of IMerge, modify the trial, close the trial, discontinue development of imetelstat in MDS, or terminate the Collaboration Agreement. We expect the outcomes from the second internal data reviews for both IMbark and IMerge, regulatory considerations and the totality of other program information, including the evolving treatment landscapes in MF and MDS, to inform Janssen's decisions regarding future development plans for imetelstat. We expect Janssen's decision-making regarding IMerge, the imetelstat program and the Collaboration Agreement to occur in the second quarter of 2017. Janssen's decisions regarding future development plans for imetelstat, if any, may be subject to subsequent regulatory feedback.
Intellectual Property
Intellectual property, including patent protection, is very important to our business. We file patent applications in the United States and other jurisdictions, and we also rely on trade secret protection and contractual arrangements to protect aspects of our business. An enforceable patent with appropriate claim coverage can provide an advantage over competitors who may seek to employ similar approaches to develop therapeutics, and so the future commercial success of imetelstat in collaboration with Janssen, and therefore our future success, will be in part dependent on our intellectual property strategy. The information provided in this section should be reviewed in the context of the section entitled "Risks Related to Protecting Our Intellectual Property" under Item 1A, "Risk Factors".
11
The development of biotechnology products, including imetelstat, typically includes the early development of a technology, followed by rounds of increasingly focused innovation around a product opportunity, including identification and definition of a specific product candidate and uses thereof, manufacturing processes, product formulation and administration methods. The result of this process is that biotechnology products are often protected by several families of patent filings that are filed at different times during product development and cover different aspects of the product. Consequently, earlier filed, broad technology patents will usually expire ahead of patents covering later developments such as product formulations, so that patent expirations on a product may span several years. Patent coverage may also vary from country to country based on the scope of available patent protection. There are also opportunities to obtain an extension of patent coverage for a product in certain countries, which adds further complexity to the determination of patent life.
We endeavor to monitor worldwide patent filings by third parties that are relevant to our business. Based on this monitoring, we may determine that an action is appropriate to protect our business interests. Such actions may include negotiating patent licenses where appropriate, filing oppositions against a patent, filing a request for post grant review against a patent or filing a request for the declaration of an interference with a patent application or issued patent.
Imetelstat
We own issued patents in the United States, Europe and other countries related to imetelstat. Composition of matter patents generally provide the most material coverage, and therefore may convey competitive advantages. Because imetelstat is still under development, subsequent innovation and associated patent filings may provide additional patent coverage with later expiration dates. Examination of overseas patent applications typically lags behind U.S. examination particularly where cases are filed first in the United States. It may be possible to obtain patent term extensions of some patents in some countries for claims covering imetelstat which could further extend the patent term.
Product Candidate
|
U.S. Patent Status / Expiration Date |
Europe Patent Status / Expiration Date |
Japan Patent Status / Expiration Date |
|||
|---|---|---|---|---|---|---|
Imetelstat (composition of matter) |
Issued / 2025 | Issued / 2020* | Issued / 2024 |
- *
- An additional composition of matter patent application for imetelstat has been filed that, if issued, would provide European patent protection until 2024. In addition, we have received patent coverage in Europe until 2029 for the use of imetelstat for the treatment of cancer utilizing certain dosing regimens.
Our patent rights relating to imetelstat include those covering composition claims to the drug molecule and related nucleic acid telomerase inhibiting molecules, as well as reagents useful in manufacturing processes for the drug, and method of treatment and kit claims, certain of which are co-owned with other entities. Our patent rights for imetelstat and related products whose mechanism of action is telomerase inhibition have been exclusively licensed (even as to us) to Janssen for all human disorders or medical conditions. In addition, certain of our patent rights for measuring the length of telomeres in cells have been non-exclusively licensed to Janssen.
Under the terms of the Collaboration Agreement with Janssen, we remain responsible for prosecuting, at Janssen's direction, the patents exclusively licensed to Janssen, with costs shared between us and Janssen on a 50/50 basis. For intellectual property developed under the Collaboration Agreement, the party having sole ownership interest in such intellectual property will be responsible for prosecuting any such patents, with Janssen bearing all of the patent costs for such intellectual property solely owned by Janssen and for intellectual property either jointly owned or solely owned by us such patent costs to be shared between the parties on a 50/50 basis.
12
Telomerase
Our patent rights relating to telomerase that cover technologies such as polynucleotides that encode a catalytic protein component (hTERT) of human telomerase, antibodies or antigen binding fragments that specifically bind to hTERT, and methods of drug screening using cells expressing hTERT are co-owned with and in-licensed exclusively from the University of Colorado. A patent for identifying inhibitors of telomerase activity is co-owned and in-licensed from the University of Texas Southwestern Medical Center and the University of California. We expect the last of these patent rights relating to telomerase to expire during 2019. See Item 1A, "Risk Factors" for additional information regarding our patent rights relating to telomerase.
Licensing
Collaboration and License Agreement with Janssen
On November 13, 2014, we entered into the Collaboration Agreement, pursuant to which we granted to Janssen the exclusive rights to develop and commercialize imetelstat worldwide for all indications in oncology, including hematologic myeloid malignancies, and all other human therapeutic uses. The Collaboration Agreement became effective on December 15, 2014, and we received $35 million from Janssen as an upfront payment. Additional consideration under the Collaboration Agreement includes potential payments of up to an aggregate maximum total of $900 million for the achievement of development, regulatory and commercial milestones, as well as royalties on worldwide net sales.
Under the Collaboration Agreement, Janssen is wholly responsible for developing, manufacturing, seeking regulatory approval for, and commercialization of, imetelstat worldwide. To that end, Janssen is currently conducting two clinical trials, IMbark and IMerge. We are contributing 50% of the development costs for these clinical trials, which Janssen is solely conducting.
Following the protocol-specified primary analysis of IMbark or a certain time period after the initiation of the first Phase 3 MF study, if any, Janssen must notify us of their decision, or a Continuation Decision, as to whether they elect to maintain the license rights granted to them under the Collaboration Agreement and continue to advance the development of imetelstat in any indication. In the event that IMbark is terminated early, or placed on clinical hold or suspended by a regulatory authority for an extended period of time, then Janssen must instead notify us of their Continuation Decision by the date that is approximately 24 months after the initiation of IMerge.
In the event that Janssen notifies us of an affirmative Continuation Decision, we then would have an option, or the U.S. Opt-In Rights, to share further U.S. development and promotion costs, including our share of development costs incurred to date by Janssen beyond IMbark or IMerge, in exchange for higher tiered royalty rates and higher future development and regulatory milestone payments if imetelstat is successfully developed and approved. If we exercise the U.S. Opt-In Rights, then we and Janssen would share U.S. development and promotion costs beyond IMbark and IMerge on a 20/80 basis (Geron 20%, Janssen 80%), we would receive a $65 million milestone payment, or the Continuation Fee, at the time of an affirmative Continuation Decision, and would be eligible to receive additional potential payments of up to $470 million for the achievement of certain development and regulatory milestones, up to $350 million for the achievement of certain sales milestones, and tiered royalties ranging from a mid-teens up to a low twenties percentage rate on worldwide net sales of imetelstat in any countries where regulatory exclusivity exists or there are valid claims under the patent rights exclusively licensed to Janssen. In addition, if we exercise the U.S. Opt-In Rights, we then would also have a separate co-promotion option, or the U.S. Co-Promotion Option, to provide 20% of the U.S. selling effort with our sales force personnel, in lieu of funding 20% of U.S. promotion costs, upon regulatory approval and commercial launch of imetelstat in the United States. Such co-promotion would be conducted under a Janssen prepared promotion plan, and in accordance with a co-promotion
13
agreement to be agreed by us and Janssen at the time of our exercise of the U.S. Co-Promotion Option. We would be responsible for all costs associated with establishing and maintaining our sales force in any conduct of such co-promotion. All product sales would be booked by Janssen. If we do not exercise the U.S. Opt-In Rights, then all further development and promotion costs beyond IMbark or IMerge would be borne by Janssen, we would receive the $65 million Continuation Fee at the time of an affirmative Continuation Decision plus a $70 million payment for Janssen's retention of full U.S. rights to imetelstat, and would be eligible to receive additional potential payments of up to $415 million for the achievement of certain development and regulatory milestones, up to $350 million for the achievement of certain sales milestones, and tiered royalties ranging from a double-digit up to a mid-teens percentage rate on worldwide net sales of imetelstat in any countries where regulatory exclusivity exists or there are valid claims under the patent rights exclusively licensed to Janssen.
Under the terms of the Collaboration Agreement, we and Janssen have created a joint governance structure, including joint development and steering committees and working groups, to oversee and manage worldwide regulatory, development and manufacturing work under the joint clinical development plan and promotional activities (assuming we exercise the U.S. Opt-In Rights) for imetelstat, with Janssen responsible for the operational execution of those activities. In addition, both we and Janssen may propose to the joint development committee imetelstat development for any new indications not then provided for in the joint clinical development plan and if we and Janssen agree such development should be conducted outside of the joint clinical development plan, both we and Janssen would be entitled to independently undertake such development at the developing party's own cost, subject to the other party's obligation to provide reimbursement for its specified portion of the development costs plus a premium following marketing approval of imetelstat in such newly proposed indication as a result of such independent development. In the event that we do not exercise the U.S. Opt-In Rights following Janssen's affirmative Continuation Decision, the joint governance structure under the Collaboration Agreement would be dissolved, a joint oversight committee would monitor the progress of the collaboration, and we would have no further rights to conduct any independent imetelstat development.
After an affirmative Continuation Decision by Janssen, the Collaboration Agreement would remain in effect until the expiration of the last-to-expire patent or the royalty obligations on sales of imetelstat cease, unless terminated earlier. If Janssen does not effect an affirmative Continuation Decision, then the Collaboration Agreement would terminate and all rights to the imetelstat program would revert to us. Janssen may terminate the Collaboration Agreement at any time for convenience or due to a safety-related concern. If a notice of termination from Janssen occurs, we would be entitled to certain continued operational support from Janssen and cost-sharing under various circumstances and all rights to the imetelstat program would revert to us. The information provided in this section should be reviewed in the context of the section entitled "Risks Related to Our Business" under Item 1A, "Risk Factors".
License Agreement with Janssen Pharmaceuticals
On September 15, 2016, we entered into a license agreement, or the License Agreement, with Janssen Pharmaceuticals, Inc., or Janssen Pharmaceuticals, an affiliate of Janssen, whereby we granted to Janssen Pharmaceuticals an exclusive worldwide license under our proprietary patents for the research, development and commercialization of products based on specialized oligonucleotide backbone chemistry and novel amidates for ribonucleic acid interference, or RNAi, for the prevention, treatment and/or diagnosis of any and all human disorders, excluding cancers originating from the blood or bone marrow. In connection with the License Agreement, we also granted to Janssen Pharmaceuticals a non-exclusive worldwide license under our patent rights covering the synthesis of monomers, which are the building blocks of oligonucleotides, necessary for the research, development and commercialization of the oligonucleotides. These non-exclusively licensed patent rights are licensed
14
exclusively to Janssen under the Collaboration Agreement for the imetelstat program. The License Agreement with Janssen Pharmaceuticals expressly excludes products whose predominant or primary mechanism of action is telomerase inhibition and is subject to the rights and licenses granted to Janssen under the Collaboration Agreement. Under the License Agreement, we received a non-refundable upfront payment of $5 million from Janssen Pharmaceuticals. We are also eligible to receive additional potential development and regulatory milestone payments of up to an aggregate maximum total of $75 million and tiered royalties in the low single digit percentage range on worldwide net sales of each licensed product commercialized under the License Agreement. Earned royalties would be subject to royalty stacking in the event that Janssen Pharmaceuticals is obligated to make royalty payments to any third party having patent claims covering a licensed product as a composition of matter. Under the terms of the License Agreement, we remain responsible for prosecution and maintenance of the patent rights under the License Agreement, with reasonable input provided by Janssen Pharmaceuticals. The costs of prosecution and maintenance of patent rights under the License Agreement will be shared 50/50 by the parties. The License Agreement will remain in effect until the expiration of the last-to-expire patent, unless terminated earlier. The License Agreement may be terminated by either us or Janssen Pharmaceuticals in the event of uncured material breach or insolvency proceeding by the other party. Janssen Pharmaceuticals also may terminate the License Agreement at will upon prior written notice to us. In the event of early termination, all licenses granted to Janssen Pharmaceuticals would terminate.
Other License Agreements
In addition to the above agreements, we have also granted licenses to a number of other organizations in the ordinary course of our business to utilize aspects of our technologies to develop and commercialize products outside of the imetelstat program. These include:
- •
- licenses to several biotechnology and pharmaceutical companies to use or commercialize telomerase immortalized cells in drug discovery
research;
- •
- licenses to several companies to sell antibodies specific to telomerase for research purposes;
- •
- licenses to several companies to develop and commercialize reagent kits, or to provide services, for the measurement of telomere length or
telomerase activity for research purposes;
- •
- a license to a company to develop and commercialize a particular telomerase-based technology for cancer detection; and
- •
- a license to a company for the development of cancer immunotherapies for veterinary applications.
See Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations—Revenues" for a further discussion of revenues from our license agreements. We expect revenues under our license agreements related to our telomerase technology to decline significantly in the coming years, and to be eliminated by the end of 2019, due to upcoming patent expirations on such technology.
Concentration of Revenues
In 2016, we recognized revenue from the $5 million upfront payment we received from Janssen Pharmaceuticals upon our delivery of the license rights and transfer of certain know-how under the license we granted to Janssen Pharmaceuticals in September 2016. The upfront payment from Janssen Pharmaceuticals represented 81% of our 2016 revenues. In 2015, we recognized revenue from the $35 million upfront payment we received from Janssen in December 2014 upon delivery of the imetelstat license rights and completion of our performance of the technology transfer-related activities to Janssen as outlined under the Collaboration Agreement. The upfront payment from Janssen
15
represented 96% of our 2015 revenues. In 2014, the majority of our revenues were from license fees and royalties under licenses granted to several biotechnology and pharmaceutical companies to use telomerase immortalized cells in drug discovery research and for drug discovery applications. Two customers accounted for approximately 31% of our 2014 revenues. We operate in one operating segment and have operations solely in the United States. All of our long-lived assets are maintained in the United States. Information regarding total revenues, net loss and total assets is set forth in our financial statements included in Item 8 of this annual report on Form 10-K.
Research and Development
Our research and development costs were $18.0 million, $17.8 million and $20.7 million for the years ended December 31, 2016, 2015 and 2014, respectively. See Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations—Research and Development Expenses" for additional detail regarding our research and development activities.
Manufacturing
A typical sequence of steps in the manufacture of imetelstat drug product includes the following key components:
- •
- starting materials, which are well-defined raw materials that are used to make bulk drug substance;
- •
- bulk drug substance, which is the active pharmaceutical ingredient in a drug product that provides pharmacological activity or other direct
effect in the treatment of disease; and
- •
- final drug product, which is the finished dosage form that contains the drug substance that is shipped to the clinic for patient treatment.
In accordance with the Collaboration Agreement, Janssen is now responsible for the manufacture and management of the supply of imetelstat on a global basis for clinical trials and, after any regulatory approval, all commercial activities. Consequently, we are, and expect to remain, dependent on Janssen to appropriately supply imetelstat and other clinical trial materials. Currently, third-party contractors perform certain process development and other technical and scientific work with respect to imetelstat, as well as supply starting materials and manufacturing drug substance and drug product. Janssen does not have direct control over third-party personnel or operations. These third-party contractors, and/or any other contractors that Janssen may rely upon for the manufacture and/or supply of imetelstat, typically complete their services on a proposal by proposal basis under master supply agreements and may need to make substantial investments to enable sufficient capacity increases and cost reductions, and to implement those regulatory and compliance standards necessary for successful Phase 3 clinical trials and commercial production. These third-party contractors, and/or any other contractors that Janssen may rely upon for the manufacture and/or supply of imetelstat, may not be able to achieve such capacity increases, cost reductions, or regulatory and compliance standards, and even if they do, such achievements may not be at a commercially reasonable cost. Neither we nor Janssen have any long-term commitments or commercial supply agreements with any of the third-party contractors for imetelstat. The information provided in this section should be reviewed in the context of the section entitled "Risks Related to Our Business" under Item 1A, "Risk Factors".
Consultants
We have consulting agreements with drug development professionals, clinicians and regulatory experts with experience in numerous fields, including oncology and drug regulations. We retain each consultant according to the terms of a consulting agreement. Under such agreements, we pay them a consulting fee and reimburse them for out-of-pocket expenses incurred in performing their services for us. In addition, some consultants hold options to purchase our common stock, subject to the vesting requirements contained in the consulting agreements. Our consultants may be employed by other entities and therefore may have commitments to their employer, or may have other consulting or advisory agreements that may limit their availability to us.
16
Competition
The pharmaceutical and biotechnology industries are intensely competitive. Other pharmaceutical and biotechnology companies and research organizations currently engage in or have in the past engaged in efforts related to the biological mechanisms related to imetelstat, including the study of telomeres, telomerase, our proprietary oligonucleotide chemistry, and the research and development of therapies for the treatment of hematologic myeloid malignancies. In addition, other products and therapies that could directly compete with imetelstat currently exist or are being developed by pharmaceutical and biopharmaceutical companies and by academic institutions, government agencies and other public and private research organizations.
Many companies are developing alternative therapies to treat hematologic myeloid malignancies. For example, if approved for commercial sale for the treatment of MF, imetelstat would compete against Incyte Corporation's ruxolitinib, or Jakafi®, which is orally administered. In clinical trials, Jakafi® reduced spleen size, abdominal discomfort, early satiety, bone pain, night sweats and itching in MF patients. Recently, there have also been reports of overall survival benefit as well as improvement in bone marrow fibrosis from Jakafi® treatment. Other treatment modalities for MF include hydroxyurea for the management of splenomegaly, leukocytosis, thrombocytosis and constitutional symptoms; splenectomy and splenic irradiation for the management of splenomegaly and co-existing cytopenias, or low blood cell counts; chemotherapy and pegylated interferon. Drugs for the treatment of MF-associated anemia include erythropoiesis-stimulating agents, androgens, danazol, corticosteroids, thalidomide and lenalidomide. There are other investigational treatments for MF further along in development than imetelstat, such as pacritinib by CTI Biopharma Corporation, or CTI Biopharma, and momelotinib by Gilead Sciences, Inc., or Gilead, which have reported results from Phase 3 clinical trials, and other inhibitors of the JAK-STAT pathway, such as NS-018 by NS Pharma, Inc., as well as several investigational treatments in early phase testing such as histone deacetylase inhibitors, interleukin-3 receptor targeted agents, inhibitors of heat shock protein 90, hypomethylating agents, PI3 Kinase and mTOR inhibitors, anti-fibrosis antibodies, hedgehog and SMO inhibitors, PIM kinase inhibitors, IAP inhibitors, anti-LOX2 inhibitors, recombinant pentraxin 2 protein, KIP-1 activators, TGF-beta superfamily inhibitors, FLT inhibitors, and other tyrosine kinase inhibitors.
If approved for commercial sale for the treatment of lower risk MDS, imetelstat would compete against a number of treatment options, including erythropoiesis stimulating agents and other hematopoietic growth factors; immunomodulators, such as lenalidomide by Celgene Corporation, or Celgene; hypomethylating agents, such as azacitidine by Celgene and decitabine by Janssen; in addition to investigational treatments that may be further along in development than imetelstat, such as oral versions of azacitidine; histone deacetylase inhibitors; TGF-beta superfamily inhibitors, such as luspatercept by Acceleron Pharma, Inc., or Acceleron, in collaboration with Celgene; PI3 Kinase inhibitors; aminopeptidase inhibitors, such as tosedostat by CTI Biopharma; TLR2-specific antibodies; anti-CD33 antibodies; Fas ligand inhibitors; and JAK-STAT pathway inhibitors.
Independently, Janssen is developing therapies for hematologic malignancies, including AML, MDS, multiple myeloma and ABC-subtype diffuse large B-cell lymphoma. Molecular and cellular pathways of interest include:
- •
- cell surface targets for immune-directed therapy;
- •
- immune checkpoint inhibition;
- •
- leukemia stem cells;
- •
- pathway addiction (genetic alterations, cell-type specific pathways);
- •
- conditional sensitivity (stress, protein-producing tumors);
- •
- targeting of T-cells and natural killer "NK" cells to tumors;
17
- •
- identification of novel tumor-specific antigens; and
- •
- progression from early MDS to AML and cancer interception.
Success by Janssen in any of these approaches may compete with imetelstat or render imetelstat obsolete or noncompetitive, which could lead to a decision by Janssen to discontinue the imetelstat program and terminate the Collaboration Agreement, which would materially and adversely affect our business and business prospects and might cause us to cease operations.
Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. We anticipate increased competition in the future as new companies explore treatments for hematologic myeloid malignancies, which may significantly impact the commercial viability of imetelstat. Academic institutions, government agencies and other public and private research organizations may also conduct research, seek patent protection and establish collaborative arrangements for research, clinical development and marketing of products similar to imetelstat. These companies and institutions compete with us in recruiting and retaining qualified development and management personnel as well as in acquiring technologies complementary to the imetelstat program.
In addition to the above factors, imetelstat will face competition based on:
- •
- product efficacy and safety;
- •
- convenience of product administration;
- •
- cost of manufacturing;
- •
- the timing and scope of regulatory consents;
- •
- status of reimbursement coverage;
- •
- price; and
- •
- patent position, including potentially dominant patent positions of others.
As a result of the foregoing, competitors may develop more commercially desirable or affordable products than imetelstat, or achieve earlier patent protection or product commercialization than us or Janssen. Competitors have developed, or are in the process of developing, technologies that are, or in the future may be, competitive to imetelstat. Some of these products may have an entirely different approach or means of accomplishing therapeutic effects similar or superior to those that may be demonstrated by imetelstat. Competitors may develop products that are safer, more effective, or less costly than imetelstat, or more convenient to administer to patients and, therefore, present a serious competitive threat to imetelstat. In addition, competitors may price their products below what Janssen may determine to be an acceptable price for imetelstat, may receive better third-party payor coverage and/or reimbursement, or may be more cost-effective than imetelstat. Such competitive products or activities by competitors may render imetelstat obsolete, which may cause Janssen to terminate the Collaboration Agreement, which would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
Government Regulation
Regulation by governmental authorities in the United States and other countries is a significant factor in the development, manufacture and marketing of imetelstat, which is being developed in collaboration with Janssen. We anticipate that imetelstat will require regulatory approval by governmental agencies prior to commercialization. In particular, potential human therapeutic products, such as imetelstat, are subject to rigorous preclinical and clinical testing and other approval procedures of the FDA and similar regulatory authorities in European and other countries. Various governmental
18
statutes and regulations also govern or influence testing, manufacturing, safety, labeling, storage, import, export, distribution and recordkeeping related to such products and their marketing. In collaboration with Janssen, the process of obtaining these approvals and the subsequent compliance with appropriate statutes and regulations require the expenditure of substantial time and money, and there can be no guarantee that approvals will be granted. The information provided in this section should be reviewed in the context of the section entitled "Risks Related to Clinical and Commercialization Activities" under Item 1A, "Risk Factors".
United States Food and Drug Administration Regulatory Approval Process
Prior to commencement of clinical trials involving humans, preclinical testing of new pharmaceutical products is generally conducted on animals in the laboratory to evaluate the potential efficacy and safety of a product candidate. The results of these trials are submitted to the FDA as part of an Investigational New Drug, or IND, application, which must become effective before clinical testing in humans can begin. The FDA can place an IND on clinical hold at any time, which prevents the conduct of clinical trials under the IND until safety concerns are addressed by the IND sponsor to the FDA's satisfaction. Typically, clinical evaluation involves a time consuming and costly three phase trial process. In Phase 1, clinical trials are conducted with a small number of healthy volunteers or patients afflicted with a specific disease to assess safety and to evaluate the pattern of drug distribution and metabolism within the body. In Phase 2, clinical trials are conducted with groups of patients afflicted with a specific disease in order to determine preliminary efficacy, optimal dosages and expanded evidence of safety. The Phase 2 trials can be conducted comparing the investigational treatment to a comparator arm, or not. If used, a comparator usually includes standard of care therapy. Safety and efficacy data from Phase 2 clinical trials, even if favorable, may not provide sufficient rationale for proceeding to a Phase 3 clinical trial. In Phase 3, large scale, multi-center, comparative trials are conducted with patients afflicted with a target disease to provide sufficient data to demonstrate the efficacy and safety required by the FDA. The FDA closely monitors the progress of each of the three phases of clinical testing and may, at its discretion, re-evaluate, alter, suspend, or terminate the trials. Human clinical trials must be conducted in compliance with Good Clinical Practice regulations and applicable laws, with the oversight of Institutional Review Boards for the protection of human subjects. The manufacture of drug product candidates is subject to requirements that drugs be manufactured, packaged and labeled in conformity with current Good Manufacturing Practices and applicable laws.
The results of the preclinical and clinical testing of drugs and complete manufacturing information are submitted to the FDA in the form of a New Drug Application, or NDA, for review and for approval prior to commencement of commercial sales. Submission of an NDA requires the payment of a substantial user fee to the FDA, which may be waived in certain cases. In responding to an NDA submission, the FDA may approve the drug for commercialization, impose limitations on its indications for use and labeling, including in the form of Risk Evaluation and Mitigation Strategies or may issue a complete response letter. Even if an NDA is approved, its sponsor is subject to ongoing and pervasive regulatory compliance requirements.
European and Other Regulatory Approval Process
Prior to initiating clinical trials in a region outside of the United States, a clinical trial application must be submitted and reviewed by the appropriate regulatory authority regulating the country in which the trial will be conducted. Whether or not FDA clearance or approval has been obtained, approval of a product by comparable regulatory authorities in Europe and other countries is necessary prior to commencement of marketing the product in such countries. The regulatory authorities in each country may impose their own requirements and may refuse to grant an approval, or may require additional data before granting it, even though the relevant product has been cleared or approved by the FDA or
19
another authority. As with the FDA, the regulatory authorities in the European Union, or EU, and other developed countries have lengthy approval processes for pharmaceutical products. The process for gaining approval in particular countries varies, but generally follows a similar sequence to that described for FDA approval. In Europe, the European Medicine Agency, or EMA, and the European Committee for Proprietary Medicinal Products, or CPMP, provide a mechanism for EU member states to exchange information on all aspects of product licensing. The EU has established the EMA for the evaluation of medical products, with both a centralized procedure with which the marketing authorization is recognized in all EU member states and a decentralized procedure, the latter being based on the principle of licensing within one member country followed by mutual recognition by the other member countries.
Orphan Drug Designation
For a drug to qualify for orphan drug designation by the FDA, both the drug and the disease or condition must meet certain criteria specified in the Orphan Drug Act, or ODA, and FDA's implementing regulations. Orphan drug designation is granted by the FDA's Office of Orphan Drug Products in order to support development of medicines for underserved or rare diseases and patient populations that affect fewer than 200,000 people in the United States or, if the disease or condition affects more than 200,000 individuals annually in the United States, if there is no reasonable expectation that the cost of developing and making the drug would be recovered from sales in the United States. Orphan drug designation qualifies the sponsor of the drug for various development incentives of the ODA, including, if regulatory approval is received, the potential for seven years of market exclusivity with certain limited exceptions and certain tax credits for qualified clinical testing. A marketing application for a prescription drug product that has received orphan drug designation is not subject to a prescription drug user fee unless the application includes an indication for a disease or condition other than the rare disease or condition for which the drug was granted orphan drug designation. The granting of orphan drug designation does not alter the standard regulatory requirements and process for obtaining marketing approval. The safety and effectiveness of a drug must be established through adequate and well-controlled studies. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition.
On June 11, 2015 and December 23, 2015, the FDA granted orphan drug designation to imetelstat for the treatment of MF and MDS, respectively.
Orphan drug designation by the European Commission provides regulatory and financial incentives for companies to develop and market therapies that treat a life-threatening or chronically debilitating condition affecting no more than five in 10,000 persons in the EU, and where no satisfactory treatment is available. In the EU, orphan drug designation also entitles a party to financial incentives such as reduction of fees or fee waivers, as well as protocol assistance from the EMA during the product development phase, and direct access to the centralized authorization procedure. In addition, ten years of market exclusivity is granted following drug product approval, meaning that another application for marketing authorization of a later similar medicinal product for the same therapeutic indication will generally not be approved in the EU. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable to not justify maintenance of market exclusivity.
On November 15, 2015, the EMA granted orphan drug designation to imetelstat for the treatment of MF.
20
Fraud and Abuse, Data Privacy and Security, and Transparency Laws and Regulations
We may also be subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which we conduct our business. These additional healthcare regulations could affect our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security, and physician payment sunshine laws.
The federal Anti-Kickback Statute makes it illegal for any person or entity, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully, directly or indirectly, solicit, receive, offer, or pay any remuneration that is intended to induce the referral of business, including the purchase, order, or lease of any good, facility, item or service for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. The term "remuneration" has been broadly interpreted to include anything of value. Several courts have interpreted the statute's intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated. The Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act, collectively the Affordable Care Act or ACA, among other things, amended the intent requirement of the federal Anti-Kickback Statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate, in order to commit a violation.
Federal false claims and false statement laws, including the federal civil False Claims Act and whistleblower or qui tam actions, prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, for payment to, or approval by, federal programs, including Medicare and Medicaid, claims for items or services, including drugs, that are false or fraudulent or not provided as claimed. Entities can be held liable under these laws if they are deemed to "cause" the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers, promoting a product off-label, or for providing medically unnecessary services or items.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created additional federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations, require certain types of individuals and entities to protect the privacy, security, and electronic exchange of certain patient data.
The federal Physician Payments Sunshine Act requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children's Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services, or CMS, information related to payments or other transfers of value made to physicians and teaching hospitals, and applicable manufacturers and applicable group purchasing organizations to report annually to CMS ownership and investment interests held by the physicians and their immediate family members.
Analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services
21
reimbursed by non-governmental third party payors, including private insurers. Additionally, we may be subject to state laws that require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government. Further, we may be subject to state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians, other healthcare providers and healthcare entities, or marketing expenditures, as well as state and foreign laws governing the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. If our operations are found to be in violation of any of these federal, state or foreign laws or regulations, we may be subject to penalties, including without limitation, administrative or civil penalties, imprisonment, damages, fines, disgorgement, individual imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws or the curtailment or restructuring of our operations.
Reimbursement and Health Reform
Significant uncertainty exists as to the coverage and reimbursement status of any product candidate that receives regulatory approval. In the United States and markets in other countries, sales of imetelstat, if approved for commercial sale, will depend, in part, on the extent to which third-party payors provide coverage and establish adequate reimbursement levels for imetelstat.
In the United States, third-party payors include federal and state healthcare programs, government authorities, private managed care providers, private health insurers and other organizations. There has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare, and reform government program reimbursement methodologies for drugs. Further, third-party payors are increasingly challenging the price, examining the medical necessity and reviewing the cost-effectiveness of medical drug products and medical services, in addition to questioning their safety and efficacy. Such payors may limit coverage to specific drug products on an approved list, also known as a formulary, which might not include all of the FDA-approved drugs for a particular indication. Janssen may need to conduct expensive pharmaco-economic studies in order to demonstrate the medical necessity and cost-effectiveness of imetelstat, in addition to the costs required to obtain the FDA approvals. Nonetheless, imetelstat may not be considered medically necessary or cost-effective.
Moreover, the process for determining whether a third-party payor will provide coverage for a drug product may be separate from the process for setting the price of a drug product or for establishing the reimbursement rate that such a payor will pay for the drug product. A payor's decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor's determination to provide coverage for a drug product does not assure that other payors will also provide coverage for the drug product. Adequate third-party reimbursement may not be available to enable Janssen to maintain price levels sufficient to realize an appropriate return on Janssen's and our investment in imetelstat.
The United States and some foreign jurisdictions are considering or have enacted legislative and regulatory proposals to contain healthcare costs, as well as to improve quality and expand access. For example, in March 2010, the ACA, was signed into law that included a number of provisions of importance to the biopharmaceutical industry. In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, or the Budget Resolution, that authorizes the implementation of legislation that would repeal portions of the ACA. Further, on January 20, 2017, President Trump
22
signed an Executive Order directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Congress also could consider subsequent legislation to replace elements of the ACA that are repealed.
We expect that other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and lower reimbursement, and in additional downward pressure on the price that may be charged for imetelstat.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. For example, in August 2011, the Budget Control Act of 2011 was enacted, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee on Deficit Reduction did not achieve a targeted deficit reduction of at least $1.2 trillion for fiscal years 2012 through 2021, triggering the legislation's automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect beginning on April 1, 2013 and, following passage of the Bipartisan Budget Act of 2015, will stay in effect through 2025 unless additional Congressional action is taken. Additionally, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals and imaging centers.
Executive Officers of the Company
The following table sets forth certain information with respect to our executive officers as of January 31, 2017:
Name
|
Age | Position | |||
|---|---|---|---|---|---|
John A. Scarlett, M.D. |
65 | President and Chief Executive Officer | |||
Olivia K. Bloom |
48 | Executive Vice President, Finance, Chief Financial Officer and Treasurer | |||
Melissa A. Kelly Behrs |
53 | Executive Vice President, Business Development and Portfolio & Alliance Management | |||
Andrew J. Grethlein, Ph.D. |
52 | Executive Vice President, Development and Technical Operations | |||
Stephen N. Rosenfield, J.D. |
67 | Executive Vice President, General Counsel and Corporate Secretary | |||
John A. Scarlett, M.D., has served as our Chief Executive Officer and a director since September 2011 and President since January 2012. Dr. Scarlett has served as a director for Chiasma, Inc., a biopharmaceutical company focused on transforming injectable drugs into oral medications, since February 2015 and CytomX Therapeutics, Inc., a biopharmaceutical company focused on developing antibody therapeutics for the treatment of cancer, since June 2016. Prior to joining Geron, Dr. Scarlett served as President, Chief Executive Officer and a member of the board of directors of Proteolix, Inc., a privately held, oncology-oriented biopharmaceutical company, from February 2009 until its acquisition by Onyx Pharmaceuticals, Inc., an oncology-oriented biopharmaceutical company, in November 2009. From February 2002 until its acquisition by Ipsen, S.A. in October 2008, Dr. Scarlett served as the Chief Executive Officer and a member of the board of directors of Tercica, Inc., an endocrinology-oriented biopharmaceutical company, and also as its President from February 2002 through February 2007. From March 1993 to May 2001, Dr. Scarlett served as President and Chief Executive Officer of Sensus Drug Development Corporation. In 1995, he co-founded Covance Biotechnology Services, Inc., a contract biopharmaceutical manufacturing operation, and served as a member of its board of
23
directors from inception to 2000. From 1991 to 1993, Dr. Scarlett headed the North American Clinical Development Center and served as Senior Vice President of Medical and Scientific Affairs at Novo Nordisk Pharmaceuticals, Inc., a wholly owned subsidiary of Novo Nordisk A/S. Dr. Scarlett received his B.A. degree in chemistry from Earlham College and his M.D. from the University of Chicago, Pritzker School of Medicine.
Olivia K. Bloom has served as our Executive Vice President, Finance since February 2014, Chief Financial Officer since December 2012 and Treasurer since February 2011. Ms. Bloom previously served as our Senior Vice President, Finance from December 2012 to February 2014, Chief Accounting Officer from September 2010 to December 2012 and Vice President, Finance from January 2007 to December 2012. Ms. Bloom joined the Company in 1994 as a Senior Financial Analyst and from 1996 to 2011 served as our Controller. Prior to Geron, Ms. Bloom started her career in public accounting at KPMG Peat Marwick and became a Certified Public Accountant in 1994. Ms. Bloom graduated Phi Beta Kappa with a B.S. in Business Administration from the University of California at Berkeley.
Melissa A. Kelly Behrs has served as our Executive Vice President, Business Development and Portfolio & Alliance Management, since July 2014. Prior to that she was our Executive Vice President, Portfolio and Alliance Management since February 2014 and she was our Senior Vice President, Portfolio and Alliance Management from September 2012 to February 2014. Ms. Behrs joined Geron in November 1998 as Director of Corporate Development. Since then, she has served in various managerial positions, including General Manager, R&D Technologies; Vice President, Corporate Development; Senior Vice President, Therapeutic Development, Oncology; and Senior Vice President, Strategic Portfolio Management. From 1990 to 1998, Ms. Behrs worked at Genetics Institute, Inc., a biotechnology research and development company, serving initially as Assistant Treasurer and then as Associate Director of Preclinical Operations where she was responsible for all business development, regulatory, and project management activities for the Preclinical Development function. Ms. Behrs received a B.S. from Boston College and an M.B.A. from Babson College.
Andrew J. Grethlein, Ph.D., has served as our Executive Vice President, Development and Technical Operations, since July 2014. He joined Geron in September 2012 as our Executive Vice President, Technical Operations. Prior to joining Geron, Dr. Grethlein was Executive Vice President and Chief Operating Officer for Inspiration Biopharmaceuticals, a biopharmaceutical company, from January 2010 to September 2012. From October 2008 until January 2010, Dr. Grethlein was Senior Vice President of Biotechnology and Portfolio Management Team Leader for Hematology at Ipsen S.A., a global specialty pharmaceutical company. His responsibilities at Ipsen included planning and execution of worldwide strategy for product and portfolio development in the hematologic therapeutic area. From 2003 to 2008, Dr. Grethlein served as Senior Vice President of Pharmaceutical Operations at Tercica, Inc., an endocrinology-oriented biopharmaceutical company, where he was a member of the senior executive team that governed corporate strategy, business planning and company operations, and had responsibility for all manufacturing and quality functions. Before joining Tercica, Dr. Grethlein served in various positions at Elan Corporation, a biotechnology company, from 1997 to 2003, including as Senior Director, South San Francisco Pharmaceutical Operations. From 1995 to 1997, Dr. Grethlein served as Manager, Biologics Development and Manufacturing, for Athena Neurosciences, Inc., a pharmaceutical company. Prior to this, he served in various engineering positions for the Michigan Biotechnology Institute, a nonprofit technology research and business development corporation. Dr. Grethlein received his A.A. degree in liberal arts from Simon's Rock Early College, his B.S. in biology from Bates College, and his M.S. and Ph.D. in chemical engineering from Michigan State University.
24
Stephen N. Rosenfield, J.D., has served as our Executive Vice President, General Counsel and Corporate Secretary since February 2012, General Counsel and Secretary since January 2012 and Secretary since October 2011. From July 2009 to December 2016, Mr. Rosenfield served as a consultant to private companies. From June 2004 until June 2009, Mr. Rosenfield held several positions at Tercica, Inc., an endocrinology-oriented biopharmaceutical company, and through its acquisition by Ipsen, S.A. in October 2008, including Executive Vice President, General Counsel and Secretary. Prior to joining Tercica, Mr. Rosenfield served as the Executive Vice President of Legal Affairs, General Counsel and Secretary of InterMune, Inc., a biotechnology company focused in pulmonology and fibrotic diseases. Prior to joining InterMune, Mr. Rosenfield was an attorney at Cooley LLP, an international law firm, where he served as outside counsel for biotechnology and technology clients. Mr. Rosenfield received a B.S. from Hofstra University and a J.D. from Northeastern University School of Law.
Employees
As of December 31, 2016, we had 15 full-time and three part-time employees. Two of our employees hold Ph.D. degrees and seven hold other advanced degrees. Of this current total workforce, two employees were engaged in, or directly supported, our research and development activities, and 16 employees were engaged in business development, legal, finance and administration. None of our employees are covered by a collective bargaining agreement; nor have we experienced work stoppages. We consider relations with our employees to be good.
Corporate Information
Geron Corporation was incorporated in the State of Delaware on November 28, 1990.
Available Information
Our internet address is www.geron.com. Information included on our website is not part of this annual report on Form 10-K. We make available free of charge on our website our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the United States Securities and Exchange Commission, or the SEC. In addition, copies of our annual reports are available free of charge upon written request. The SEC also maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is www.sec.gov.
Our business is subject to various risks and uncertainties that may have a material adverse effect on our business, financial condition or results of operations. You should carefully consider the risks and uncertainties described below, together with all of the other information included in this annual report on Form 10-K. Our business faces significant risks and uncertainties, and those described below may not be the only risks and uncertainties we face. Additional risks and uncertainties not presently known to us or that we currently believe are immaterial may also significantly impair our business, financial condition or results of operations. If any of these risks or uncertainties occur, our business, financial condition or results of operations could suffer, the market price of our common stock could decline and you could lose all or part of your investment in our common stock.
25
We have exclusively outlicensed imetelstat, which was our sole product candidate, to Janssen. We are wholly dependent upon our collaborative relationship with Janssen to further develop, manufacture and commercialize imetelstat. If Janssen fails to perform as required by the Collaboration Agreement or abandons the imetelstat program, the potential for us to generate future revenues from milestone payments and royalties from imetelstat would be significantly reduced, the development and/or commercialization of imetelstat could be terminated or substantially delayed, and our business would be severely harmed.
Under the terms of the Collaboration Agreement, we and Janssen have created a joint governance structure, including joint committees and working groups, to oversee and manage worldwide regulatory, development, manufacturing and commercialization activities for imetelstat; however, Janssen is solely responsible for the operational execution of those activities. Accordingly, the timely and successful completion by Janssen of those activities will significantly affect the timing and amount of any revenues from milestone payments and royalties we may receive under the Collaboration Agreement, and these activities will be influenced by, among other things, the efforts and allocation of resources by Janssen, none of which we control. If Janssen does not perform in the manner we expect or fulfill its responsibilities in a timely manner, including with respect to conducting future additional or further data reviews of IMbark or IMerge, or at all, the clinical development, manufacturing, regulatory approval and/or commercialization of imetelstat could be delayed or terminated, and it could become necessary for us to assume responsibility for the clinical development, manufacturing, regulatory approval and/or commercialization of imetelstat at our own expense. Accordingly, there can be no assurance that any of the development, regulatory or sales milestones under the Collaboration Agreement will be achieved or that we will receive any future milestone or royalty payments under the Collaboration Agreement.
In addition, because Janssen is solely responsible for the operational execution of worldwide regulatory, development, manufacturing and commercialization activities related to imetelstat, we are solely dependent on Janssen to provide us with timely and accurate information concerning these activities as well as information about the costs incurred under the Collaboration Agreement. If we do not receive accurate information from Janssen in a timely manner, or at all, regarding these activities, including, for example, plans for, and enrollment of, and efficacy and safety results from, clinical trials of imetelstat, then the timeliness and accuracy of our public disclosures, as well as our governance-related decision-making regarding these activities, may be adversely affected.
Our collaboration with Janssen may be unsuccessful due to other factors, including the following:
- •
- Janssen may choose to terminate the Collaboration Agreement for any reason;
- •
- Janssen may provide a negative Continuation Decision and halt its development of imetelstat, in which case we would receive no further payments
from Janssen under the Collaboration Agreement;
- •
- Janssen may believe that any preliminary or final results of IMbark and/or IMerge, including in connection with the second internal data
reviews of these trials, are negative under the criteria set forth in the respective protocols, are inconclusive, or do not otherwise demonstrate adequate efficacy or clinical benefit to warrant
further development or commercialization of imetelstat by Janssen, which would likely result in a termination of the Collaboration Agreement by Janssen at any time, or a negative Continuation
Decision;
- •
- Janssen may observe safety issues in either IMbark and/or IMerge, or any potential future clinical trials of imetelstat, which may result in a termination of the Collaboration Agreement by Janssen at any time, or a negative Continuation Decision;
26
- •
- Janssen may choose not to develop and commercialize imetelstat in certain, or any, markets or for one or more indications, if at all;
- •
- Janssen may take considerably more time advancing imetelstat through the clinical and regulatory process than we currently anticipate, which
could materially delay the achievement of milestones and, consequently the receipt of milestone payments from Janssen, and ultimately, any royalties we might receive on worldwide net sales of
imetelstat;
- •
- in the event of a dispute between us and Janssen regarding Janssen's performance under the Collaboration Agreement, it may be difficult or
impossible for us to prove that Janssen breached its obligations under the Collaboration Agreement, including the obligation to use "commercially reasonable efforts" with regard to the development,
regulatory approval, manufacture and commercialization of imetelstat under the Collaboration Agreement;
- •
- Janssen may not dedicate the resources necessary to carry imetelstat through clinical development or may not obtain the necessary regulatory
approvals for imetelstat, and this would delay or preclude the achievement of development, regulatory or sales milestones under the Collaboration Agreement;
- •
- Janssen may be unable to obtain regulatory clearance to develop or commercialize imetelstat for sale in the United States and other countries,
in a timely manner, or at all, or such regulatory clearance may be revoked or put on hold by governmental or regulatory authorities in any jurisdiction;
- •
- Janssen's ability to develop, manufacture and commercialize imetelstat may be delayed or substantially impacted if we are unable to provide to
Janssen in a timely manner, or at all, data or results from studies of imetelstat conducted by us and others prior to the Collaboration Agreement, or other information, related to imetelstat that may
be requested by Janssen;
- •
- subject to our election of the U.S. Co-Promotion Option, Janssen will be responsible for all aspects of the commercialization of imetelstat
worldwide, including pricing decisions which would affect the royalties on worldwide net sales we could receive;
- •
- Janssen may change the focus of its commercialization efforts or prioritize other programs more highly and, accordingly, reduce the efforts and
resources allocated to imetelstat, which might delay or halt the commercialization of imetelstat, and would have the direct effect of reducing our royalties or share of potential co-promotion
activities since the extent of our U.S. Co-Promotion Option is limited to a percentage of overall promotion activities under the Collaboration Agreement;
- •
- Janssen may fail to manufacture or supply sufficient quantities of imetelstat or other clinical trial materials for use in current and/or
planned clinical trials, which could delay, suspend or stop any imetelstat clinical activities;
- •
- Janssen may fail to develop a commercially viable formulation or manufacturing process for imetelstat, and may fail to manufacture or supply
sufficient quantities of imetelstat for commercial use, if approved, which would result in lost sales revenue and reduced royalties for us;
- •
- Janssen may not comply with all applicable regulatory requirements or may fail to report safety data from clinical trials of imetelstat in accordance with all applicable regulatory requirements, which could delay, suspend or stop clinical activities of imetelstat being performed by Janssen or by us; and
27
- •
- if Janssen is acquired by a third party during the term of our collaboration with Janssen, the acquirer may have different strategic priorities that could cause it to terminate the Collaboration Agreement or reduce its commitment to our collaboration.
If our collaboration with Janssen is unsuccessful as a result of any of the above factors, or any other factors, then Janssen may terminate the Collaboration Agreement or cease its efforts to develop, manufacture or commercialize imetelstat, and we would not be eligible for any further payments from Janssen under the Collaboration Agreement, which would adversely impact our financial results, business and business prospects, and the future of imetelstat, and could cause us to cease operations.
Clinical development involves a lengthy and expensive process with uncertain outcomes. Current clinical trials of imetelstat being conducted by Janssen, including IMbark, IMerge and the Pilot Study, and potential future clinical trials of imetelstat, may fail to adequately demonstrate the safety and efficacy of imetelstat, which would prevent or delay regulatory approval and commercialization, negatively affect our ability to earn revenues from milestone payments or royalties under the Collaboration Agreement with Janssen and could result in termination of the Collaboration Agreement by Janssen.
Before regulatory approvals for the commercial sale of imetelstat can be obtained, clinical testing must be conducted to show that imetelstat is both safe and effective for use in each target indication. Such clinical testing is expensive, can take many years to complete, and is inherently uncertain. Failure can occur at any time during clinical testing. Most product candidates that commence clinical trials are never approved as commercial products.
The clinical development of imetelstat will be influenced by results from current clinical trials being conducted by Janssen, including IMbark, IMerge and the Pilot Study, and potential future clinical trials of imetelstat. The advancement of current clinical trials of imetelstat and commencement of potential future clinical trials of imetelstat could be delayed or abandoned for a variety of reasons, including as a result of failures or delays by Janssen in:
- •
- obtaining or maintaining regulatory clearance to continue to conduct current clinical trials being conducted by Janssen, including IMbark,
IMerge and the Pilot Study, or obtaining or maintaining regulatory clearance to commence, conduct or continue to conduct potential future clinical trials of imetelstat, in a timely manner, or at all,
in the United States or other countries;
- •
- maintaining the INDs for imetelstat without such INDs being placed on full or partial clinical hold by the FDA;
- •
- properly designing, enrolling, conducting or completing IMbark, IMerge and potential future clinical trials of imetelstat, and promptly or
adequately reporting data from such trials;
- •
- demonstrating sufficient safety and efficacy of imetelstat in IMbark, IMerge and potential future clinical trials without safety issues, side
effects or dose-limiting toxicities, including any additional or more severe safety issues in addition to those that have been observed to date in previous or ongoing clinical trials related to
imetelstat, whether or not in the same indications or therapeutic areas;
- •
- properly conducting and/or completing the Pilot Study and promptly or adequately reporting data from such trial;
- •
- obtaining or accessing necessary clinical data in accordance with appropriate clinical or quality practices to ensure complete data sets;
- •
- responding to safety or futility findings by the data review committees of current clinical trials, including IMbark, IMerge and the Pilot Study, and potential future clinical trials of imetelstat, based on emerging data occurring during such clinical trials, such as significant systemic or organ
28
- •
- manufacturing sufficient quantities of imetelstat or other clinical trial materials in a manner that meets the quality standards of the FDA and
other regulatory authorities, and responding to any disruptions to drug supply, clinical trial materials or quality issues that may arise;
- •
- ensuring the ability to manufacture imetelstat at acceptable costs for potential Phase 3 clinical trials and commercialization;
- •
- obtaining sufficient quantities of any study-related treatments, materials (including comparator products, placebo or combination therapies) or
ancillary supplies;
- •
- obtaining acceptance by regulatory authorities of manufacturing changes, as well as subsequently implementing such manufacturing changes
successfully;
- •
- complying with current and future regulatory requirements, policies or guidelines, including domestic and international laws and regulations
pertaining to fraud and abuse, transparency, and the privacy and security of health information;
- •
- reaching agreement on acceptable terms and on a timely basis, if at all, with collaborators and vendors located in the United States or foreign
jurisdictions, including contract research organizations, laboratory service providers and clinical trial sites, on all aspects of clinical development;
- •
- obtaining timely review and approval by regulatory authorities of protocol amendments which may be sought for IMbark, IMerge and potential
future clinical trials of imetelstat; and
- •
- obtaining institutional review board or ethics committee approval of clinical trial protocols or protocol amendments, including the recent protocol amendment for IMbark, to conduct clinical trials at existing or prospective clinical trial sites.
toxicities, including hepatotoxicity, fatal bleeding, or other safety issues, including patient injury or death, resulting in an unacceptable benefit-risk profile;
Failures or delays with respect to any of these events could adversely affect Janssen's ability to maintain or successfully complete any current clinical trials of imetelstat or to initiate potential future clinical trials of imetelstat, which could increase development costs, delay the timing of the Continuation Decision from Janssen, impair our ability to earn revenues from milestone payments or royalties under the Collaboration Agreement or cause Janssen to terminate the Collaboration Agreement, any of which could severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
For example, to inform an assessment of the appropriate dose and schedule of imetelstat for relapsed or refractory MF patients in IMbark, in the third quarter of 2016, Janssen conducted a planned internal interim review of safety, efficacy and pharmacokinetic data from 20 patients from each dosing arm who had been followed on the trial for at least 12 weeks. Based on this first internal review at the 12-week time point, Janssen determined to close the 4.7 mg/kg dosing arm to new patient enrollment and to suspend enrollment in the 9.4 mg/kg dosing arm because an insufficient number of patients met the protocol defined interim efficacy criteria at 12 weeks.
In the first quarter of 2017, Janssen initiated the process to conduct a second internal data review of IMbark that is expected to include patients enrolled in the 9.4 mg/kg dosing arm as a starting dose who have been followed for at least 24 weeks. It is possible that, as a result of the second internal review, Janssen could decide to terminate the trial and/or the Collaboration Agreement altogether. Even if Janssen decides to continue the trial, either by resuming enrollment in the 9.4 mg/kg dosing arm, with or without changes to the dosing regimen, or adding a new dosing arm or otherwise, the development of imetelstat could be substantially delayed.
29
Due to the current suspension of enrollment in IMbark, the timing of the protocol-specified primary analysis for the trial is now uncertain and may be substantially delayed due to numerous factors, including whether patient enrollment resumes in the trial, or the protocol-specified primary analysis may not occur at all if IMbark is terminated early based on preliminary data, safety concerns or for any other reason, any of which could increase our development costs, delay the timing of the Continuation Decision from Janssen, impair our ability to earn revenues from milestone payments or royalties under the Collaboration Agreement or cause Janssen to terminate the Collaboration Agreement, which would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
If Janssen encounters difficulties enrolling or retaining patients in current or potential future clinical trials of imetelstat, clinical development and commercialization activities could be delayed or otherwise adversely affected, which could cause Janssen to terminate the Collaboration Agreement, and our business would be severely harmed.
The timely completion of a clinical trial in accordance with its protocol depends, among other things, on the ability to enroll a sufficient number of patients who remain in the study until its conclusion. Janssen may experience difficulties in patient enrollment or retention in IMbark and IMerge, or potential future clinical trials of imetelstat for a variety of reasons. The enrollment and retention of patients depends on many factors, including:
- •
- the patient eligibility criteria in the protocol;
- •
- the size of the patient population required for analysis of the trial's primary endpoint;
- •
- the proximity of patients to study sites;
- •
- the design of the trial;
- •
- Janssen's ability to recruit and retain clinical trial investigators with the appropriate competencies and experience;
- •
- clinicians' and patients' perceptions as to the potential advantages of imetelstat in relation to other available therapies, including any new
drugs that may be approved for the indications being investigated;
- •
- the ability to obtain and maintain patient consents; and
- •
- the risk that patients enrolled in the clinical trial will drop out of the trial before completion due to lack of efficacy, side effects, enrollment suspension, investigator decision, or personal issues.
In addition, IMbark and IMerge compete, and potential future clinical trials of imetelstat will compete, with other clinical trials for product candidates that are in the same therapeutic areas with imetelstat, and this competition will reduce the number and type of patients available to enroll in the imetelstat clinical trials. Since the number of qualified clinical investigators is limited, IMbark and IMerge are being conducted, and potential future clinical trials of imetelstat are expected to be conducted, at the same clinical trial sites that competitors use, which will reduce the number of patients who are available for the imetelstat clinical trials at such clinical trial sites. Moreover, because imetelstat represents a departure from more commonly used methods for cancer treatment, potential patients and their doctors may be inclined to use conventional therapies, rather than enroll patients into the imetelstat clinical trials.
Delays in patient enrollment or the inability to retain or treat patients could result in increased costs, lead to incomplete data sets or adversely affect the timing or outcome of IMbark and IMerge, or potential future clinical trials of imetelstat, which could prevent completion of these trials and adversely affect the clinical development and commercialization of imetelstat, either of which would delay the
30
timing of the Continuation Decision from Janssen or could cause Janssen to terminate the Collaboration Agreement. Such occurrences would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
Imetelstat may cause, or have attributed to it, undesirable or unintended side effects or other characteristics that delay or prevent the commencement and/or completion of clinical trials for imetelstat, delay or prevent its regulatory approval, or limit its commercial potential, which in each case could cause Janssen to terminate the Collaboration Agreement and which in turn would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
Imetelstat may cause, or have attributed to it, undesirable or unintended side effects or other characteristics adversely affecting its safety or efficacy that could delay or prevent the commencement and/or completion of current or potential future clinical trials for imetelstat. In our Phase 1 clinical trials of imetelstat, we observed dose-limiting toxicities, including thrombocytopenia, when imetelstat was used as a single agent, and neutropenia when imetelstat was used in combination with paclitaxel, as well as a low incidence of severe infusion reactions. In our Phase 2 clinical trials of imetelstat in ET, multiple myeloma, and solid tumors, we observed hematologic toxicities as well as gastrointestinal events, infections, muscular and joint pain, fatigue and infusion reactions. In addition, in our Phase 2 clinical trials of imetelstat, we observed liver function test, or LFT, abnormalities, the clinical significance and long-term consequences of which are currently undetermined. In the ET Trial, one patient died of bleeding esophageal varices, a complication of chronic liver disease, for which imetelstat initially could not be excluded as a causative agent but which was later determined by the investigator to be unrelated to imetelstat. In the Pilot Study, cytopenias have been the primary dose-limiting toxicity reported to date, consistent with our observations in previous Geron-sponsored imetelstat studies. However, during the Pilot Study, more persistent and profound cytopenias, particularly thrombocytopenia, were observed with imetelstat administered on a weekly basis. This included one case of febrile neutropenia after prolonged myelosuppression with intracranial hemorrhage resulting in patient death, which was assessed as possibly related to imetelstat by the investigator. In addition, adverse events previously observed in the ET Trial and the Pilot Study, such as LFT abnormalities, profound and prolonged thrombocytopenia and neutropenia, bleeding events, and other safety issues, including death, have also been observed by Janssen in IMbark and IMerge. If patients in current or potential future clinical trials of imetelstat experience similar or more severe adverse events, including LFT abnormalities, or severe hepatic, hemorrhagic, or new or unusual adverse events, the INDs for imetelstat may again be placed on clinical hold, as it was in March 2014, and Janssen may be delayed or precluded from further developing imetelstat.
Serious adverse events observed in clinical trials could delay or prevent any regulatory approvals of imetelstat or could hinder or prevent market acceptance of imetelstat, which could cause Janssen to delay the timing of the Continuation Decision, terminate the Collaboration Agreement or cause Janssen to limit its commercialization of imetelstat to certain indications. Such occurrences would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
If Janssen does not elect to continue the development of imetelstat in a timely manner, or at all, our business and business prospects would be severely harmed, and we might cease operations.
Under the terms of the Collaboration Agreement, Janssen is not obligated to make any additional payments to us until it makes an affirmative Continuation Decision following the protocol-specified primary analysis of IMbark, or, if IMbark is terminated early, or placed on clinical hold or suspended by a regulatory authority for an extended period of time, within approximately 24 months after the initiation of IMerge. The timing of Janssen's Continuation Decision also affects the timing and our opportunity to make our decision regarding our U.S. Opt-In Rights, as well as our election, if we
31
exercise our U.S. Opt-In Rights, of our U.S. Co-Promotion Option. If IMbark is terminated early, placed on clinical hold or suspended by a regulatory authority for an extended period of time, or is otherwise unsuccessful, Janssen may delay the timing of the Continuation Decision or provide a negative Continuation Decision, in which case, the Collaboration Agreement would terminate, we would not be eligible for any further payments from Janssen under the Collaboration Agreement and our financial results, business and business prospects, and the future of imetelstat, would be severely and adversely affected, which might cause us to cease operations.
In addition, Janssen may terminate the Collaboration Agreement at any time for convenience. If Janssen terminates the Collaboration Agreement, then, depending on the timing of such event:
- •
- we would no longer have the right to receive any milestone payments or royalties under the Collaboration Agreement;
- •
- the development of imetelstat would be significantly delayed or terminated;
- •
- we would bear all of the risks and costs related to any further clinical development, manufacturing, regulatory approval and commercialization
of imetelstat;
- •
- we would need to raise additional capital if we were to choose to pursue imetelstat development on our own, or we would need to establish
alternative collaborations with third parties, which might not be possible in a timely manner, or at all, or might not be possible on terms acceptable to us, in which case it would likely be necessary
for us to limit the size or scope of the imetelstat development program or to seek additional funding by other means to accommodate the increased expenditures; and
- •
- we would need to hire additional qualified employees to support the development and commercialization of imetelstat, which may take significant amounts of time, may not be feasible, and which would increase our need for additional funding.
Any termination of the Collaboration Agreement by Janssen at any time would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
Failure by Janssen to manufacture or provide adequate clinical and commercial quantities of imetelstat on a timely basis, or at all, would result in a delay of clinical trials or regulatory approvals, or lost sales, and our business and business prospects could be severely harmed.
In accordance with the Collaboration Agreement, Janssen is responsible for the manufacture and management of the supply of imetelstat on a global basis for all clinical trials and commercial activities. Consequently, we are, and expect to remain, dependent on Janssen to appropriately supply imetelstat and other clinical trial materials. The process of manufacturing imetelstat is complex and subject to several risks, including:
- •
- the ability to scale-up and attain sufficient production yields with appropriate quality control and quality assurance;
- •
- reliance on third-party manufacturers and suppliers;
- •
- supply chain issues, including the timely availability and shelf life requirements of raw materials and other supplies;
- •
- shortage of qualified personnel; and
- •
- compliance with regulatory requirements, which are less well-defined for oligonucleotide products than for small molecule drugs and vary in each country where imetelstat might be sold or used.
32
As a result of these risks, Janssen may not perform as agreed or may default in its obligations to supply imetelstat or other clinical trial materials for clinical trials and/or commercial activities. Janssen also may fail to deliver the required quantities of imetelstat or other clinical trial materials on a timely basis, or at required or applicable quality standards. Any such failure by Janssen could delay current and/or potential future clinical trials and any applications for regulatory approval and therefore delay the payment of potential milestone payments to us, or, if approved for commercial sale, could impair Janssen's ability to meet the market demand for imetelstat and therefore result in decreased sales and reduced royalties for us which would severely and adversely affect our financial results, business and business prospects, and might cause us to cease operations.
If Janssen makes an affirmative Continuation Decision under the Collaboration Agreement, our decision to exercise our U.S. Opt-In Rights must thereafter be made within a short timeframe and, as a result, we may be required to invest substantial capital based on limited clinical data and information.
If Janssen makes an affirmative Continuation Decision under the Collaboration Agreement, we must decide whether to elect to exercise our U.S. Opt-In Rights within a short timeframe following such a decision, and although we expect to receive information from Janssen regarding data from IMbark and IMerge, proposed future clinical development plans and costs for imetelstat, estimates in timing for commercializing imetelstat and related promotional activities, and a calculation of our share of development costs incurred to date by Janssen that we will be required to reimburse if we exercise our U.S. Opt-In Rights, we will be required to rapidly decide whether to make a substantial capital investment in imetelstat prior to the conclusion of any Phase 3 registration-enabling clinical trial. Accordingly, if we exercise our U.S. Opt-In Rights and imetelstat were to become unsuccessful in any Phase 3 registration-enabling clinical trial or were to fail to receive regulatory approval, we would not receive any financial return on this substantial capital investment. Such an occurrence would negatively impact our financial condition and results of operations, and might cause us to cease operations.
We may not be able to successfully identify and acquire and/or in-license other oncology products, product candidates, programs or companies to grow and diversify our business, and, even if we are able to do so, we may not be able to successfully manage the risks associated with integrating any such products, product candidates, programs or companies into our business or we may otherwise fail to realize the anticipated benefits of these licenses or acquisitions.
We have exclusively outlicensed imetelstat, which was our sole product candidate, to Janssen. Accordingly, we are relying exclusively upon our collaborative relationship with Janssen to further develop, manufacture and commercialize imetelstat. To grow and diversify our business, we plan to continue our business development efforts to identify and seek to acquire and/or in-license other oncology products, product candidates, programs or companies. Such efforts have not yet resulted in any transaction, and may never result in a transaction. Future growth through acquisition or in-licensing will depend upon the availability of suitable products, product candidates, programs or companies for acquisition or in-licensing on acceptable prices, terms and conditions. Even if appropriate opportunities are available, we may not be able to acquire rights to them on acceptable terms, or at all. The competition to acquire or in-license rights to promising products, product candidates, programs and companies is fierce, and many of our competitors are large, multinational pharmaceutical and biotechnology companies with considerably more financial, development and commercialization resources, personnel, and experience than we have. In order to compete successfully in the current business climate, we may have to pay higher prices for assets than may have been paid historically, which may make it more difficult for us to realize an adequate return on any acquisition. In addition, even if we succeed in identifying promising products, product candidates, programs or companies, we may not have the ability to develop, obtain regulatory approval for and commercialize such opportunities, or the financial resources necessary to pursue them.
33
Even if we are able to successfully identify and acquire or in-license new products, product candidates, programs or companies, we may not be able to successfully manage the risks associated with integrating any products, product candidates, programs or companies into our business or the risks arising from anticipated and unanticipated problems in connection with an acquisition or in-licensing. Further, while we seek to mitigate risks and liabilities of potential acquisitions through, among other things, due diligence, there may be risks and liabilities that such due diligence efforts fail to discover, that are not disclosed to us, or that we inadequately assess. Any failure in identifying and managing these risks and uncertainties effectively would have a material adverse effect on our business. In any event, we may not be able to realize the anticipated benefits of any acquisition or in-licensing for a variety of reasons, including the possibility that a product candidate fails to advance to clinical development, proves not to be safe or effective in clinical trials, a product fails to reach its forecasted commercial potential or the integration of a product, product candidate, program or company gives rise to unforeseen difficulties and expenditures. Any failure in identifying and managing these risks and uncertainties would have a material adverse effect on our business.
In addition, acquisitions create other uncertainties and risks, particularly when the acquisition takes the form of a merger or other business consolidation. We may encounter unexpected difficulties, or incur unexpected costs, in connection with transition activities and integration efforts, which include:
- •
- high acquisition costs;
- •
- the need to incur substantial debt or engage in dilutive issuances of equity securities to pay for acquisitions;
- •
- the potential disruption of our historical business and our activities under the Collaboration Agreement;
- •
- the strain on, and need to expand, our existing operational, technical, financial and administrative infrastructure;
- •
- our lack of experience in late-stage product development and commercialization;
- •
- the difficulties in assimilating employees and corporate cultures;
- •
- the difficulties in hiring qualified personnel and establishing necessary development and/or commercialization capabilities;
- •
- the failure to retain key management and other personnel;
- •
- the challenges in controlling additional costs and expenses in connection with and as a result of the acquisition;
- •
- the need to write down assets or recognize impairment charges;
- •
- the diversion of our management's attention to integration of operations and corporate and administrative infrastructures; and
- •
- any unanticipated liabilities for activities of or related to the acquired business or its operations, products or product candidates.
If we fail to integrate or otherwise manage an acquired business successfully and in a timely manner, resulting operating inefficiencies could increase our costs more than we planned, could negatively impact the market price of our common stock and could otherwise distract us from execution of our strategy. Failure to maintain effective financial controls and reporting systems and procedures could also impact our ability to produce timely and accurate financial statements.
In addition, the Collaboration Agreement with Janssen prohibits us from commercializing, under the intellectual property we have licensed exclusively to Janssen, any substance whose identified or
34
known mechanism of action is telomerase inhibition. Further, if we exercise our U.S. Co-Promotion Option under the Collaboration Agreement, we will be required to certify to Janssen at the time of exercising our U.S. Co-Promotion Option that we are not marketing or promoting, and have no right to market or promote, any such products for any oncology indication. Our right to co-promote in the United States may be terminated by Janssen if we develop or commercialize a product for treating an oncology indication that acts through the same mechanism of action as imetelstat or that is substitutable for imetelstat. Accordingly, our Collaboration Agreement with Janssen could adversely affect our ability to acquire or in-license, or to research, develop or market, promising products, product candidates or programs.
We may be unable to successfully retain key personnel to support our collaboration with Janssen or to manage any future growth.
Under the terms of the Collaboration Agreement, we and Janssen have created a joint governance structure, including joint committees and working groups, to manage worldwide regulatory, development, manufacturing and commercialization activities for imetelstat, and we have ongoing responsibilities to oversee and participate in the collaboration with Janssen. In addition, we remain responsible for prosecuting, at Janssen's direction, the patents we exclusively licensed to Janssen, and have sole responsibility for those patents that were non-exclusively licensed to Janssen. If we are unable to successfully retain, motivate and incentivize our personnel, our ability to support the Collaboration Agreement with Janssen could be impaired, and our business and the price of our common stock would be adversely impacted.
In addition, our future growth and success depend to a significant extent on the skills, experience and efforts of our executive officers and key members of our staff. We face intense competition for qualified individuals from numerous pharmaceutical, biopharmaceutical and biotechnology companies, as well as academic and other research institutions. The previous restructurings we implemented, as well as the fact that we exclusively outlicensed imetelstat, which was our sole product candidate, to Janssen, and the uncertainties regarding our ability to diversify our business could have an adverse impact on our ability to retain and recruit qualified personnel or we may incur unanticipated inefficiencies caused by our reduced personnel resources. We may be unable to retain our current personnel or attract or assimilate other highly qualified management and development personnel in the future on acceptable terms. The loss of any or all of these individuals could harm our business and could impair our ability to support our collaboration with Janssen or to support future growth.
We are involved in securities-related legal actions that are expensive and time consuming, and, if resolved adversely, could harm our business, financial condition, or results of operations.
Securities-related class action lawsuits and/or derivative lawsuits have often been brought against companies which experience volatility in the market price of their securities. This risk is especially relevant for us because biotechnology and biopharmaceutical companies often experience significant stock price volatility in connection with their product development programs.
We and certain of our officers have been named as defendants in two purported class action securities lawsuits filed in the United States District Court for the Northern District of California, or the California District Court, as well as a third securities lawsuit, not styled as a class action, which was originally filed in the United States District Court for the Southern District of Mississippi, but subsequently transferred to the California District Court. These three cases, or the Class Action Lawsuits, which are based on the same factual background, have been consolidated for all purposes, and are currently stayed to enable the parties to seek to resolve them. On November 23, 2016, the parties signed a Memorandum of Understanding that set forth material deal points of resolving the Class Action Lawsuits. On December 13, 2016, the parties filed a notice of settlement, which the California District Court signed on December 16, 2016, staying the Class Action Lawsuits pending final
35
approval of a settlement. Final settlement of the Class Action Lawsuits is contingent upon, among other things, approval by the California District Court. Such approval, if any, is not expected until the end of 2017.
Certain of our officers and directors were also named as defendants in four purported stockholder derivative lawsuits, two of which were filed in the Superior Court of California for the County of San Mateo, or the San Mateo County Court, and two of which were filed in the California District Court. These cases were all based on the same factual background and were consolidated in their respective courts. On July 22, 2016, the parties entered into a stipulation to settle all claims in each of the foregoing derivative lawsuits in exchange for our agreement to (i) implement certain corporate governance refinements, and (ii) instruct our insurer to pay in full the plaintiffs' attorneys a total of $950,000. On August 19, 2016, the San Mateo County Court preliminarily approved the proposed settlement. On November 18, 2016, the San Mateo County Court issued an order granting final approval of the settlement and dismissing with prejudice the two derivative lawsuits filed in the San Mateo County Court. On December 6, 2016, the California District Court issued an order dismissing with prejudice the two derivative lawsuits filed in the California District Court. Accordingly, as of December 6, 2016, all of the derivative lawsuits have been fully and finally settled and dismissed.
For a more complete discussion of the Class Action Lawsuits and the derivative lawsuits, see the section entitled "Legal Proceedings" under Item 3.
It is possible that additional suits will be filed, or allegations received from stockholders naming us and/or our officers and directors as defendants with respect to these same or other matters, including, for example, allegations related to the duration and nature of follow-up conducted by Janssen or us of patients enrolled in current and potential future clinical trials of imetelstat, the nature and timing of our disclosures related to efficacy or safety data observed in current and potential future clinical trials of imetelstat, or serious adverse events encountered in current and potential future clinical trials of imetelstat. We could be forced to expend significant resources in the implementation of the corporate governance refinements required by the terms of the settlement for the derivative lawsuits, or in the settlement or defense of the Class Action Lawsuits or any additional lawsuits, and we may not prevail in such lawsuits. We have not established any reserve for any potential liability relating to any additional lawsuits. It is possible that we could, in the future, incur judgments or enter into settlements of claims for monetary damages. A decision adverse to our interests in any such lawsuit, or in similar or related litigation, could result in the payment of substantial damages, or possibly fines, and could have a material adverse effect on our cash flow, results of operations and financial condition.
We may be subject to litigation that will be costly to defend or pursue and uncertain in its outcome.
Our business may bring us into conflict with our licensees, licensors, or others with whom we have contractual or other business relationships, or with our competitors or others whose interests differ from ours. If we are unable to resolve those conflicts on terms that are satisfactory to all parties, we may become involved in litigation brought by or against us.
For example, as a result of possible disagreements with Janssen, we may become involved in litigation or arbitration, which would be time-consuming and expensive. Possible disagreements with Janssen could include disagreements regarding the development and/or commercialization of imetelstat, interpretation of the Collaboration Agreement and ownership of proprietary rights. In addition, in certain circumstances we may believe that a particular milestone under the Collaboration Agreement has been achieved, and Janssen may disagree with our belief. In that case, receipt of that milestone payment may be delayed or may never be received, which would adversely affect our financial condition and may require us to adjust our operating plans. While the Collaboration Agreement provides for a joint governance structure to oversee and manage worldwide regulatory, development, manufacturing and commercialization activities for imetelstat, Janssen generally will, subject to limited exceptions,
36
have the deciding vote in the event of any disagreement. In any event, the joint governance structure contemplated by the Collaboration Agreement will be dissolved in the event that Janssen makes a Continuation Decision, but we do not exercise our U.S. Opt-In Rights, which would preclude our ability to participate in any further decision-making for imetelstat. Reliance on a joint governance structure also subjects us to the risk that changes in key Janssen management personnel that are members of the various joint committees may materially and adversely affect the functioning of these committees, which could significantly delay or preclude imetelstat development and/or commercialization.
Monitoring, initiating and defending against legal actions is time-consuming for our management, likely to be expensive and may detract from our ability to fully focus our internal resources on our business activities. In addition, despite the availability of insurance, we may incur substantial legal fees and costs in connection with litigation. Lawsuits are subject to inherent uncertainties, and defense and disposition costs depend upon many unknown factors. Lawsuits could result in judgments against us that require us to pay damages, enjoin us from certain activities, or otherwise negatively affect our legal or contractual rights, which could have a significant adverse effect on our business. In addition, the inherent uncertainty of such litigation could lead to increased volatility in our stock price and a decrease in the value of our stockholders' investment in our common stock.
We may also be subject to litigation arising from completed strategic transactions or if the results of our business and collaboration activities are not successful.
On November 13, 2014, we announced that we had entered into the Collaboration Agreement with Janssen to develop and commercialize imetelstat worldwide. We may face litigation arising from or related to the Collaboration Agreement or the transactions contemplated thereby, including if we are unable to generate substantial value under the Collaboration Agreement with Janssen or such collaboration is otherwise unsuccessful.
The Collaboration Agreement could result in litigation arising out of any claims that our stockholders suffered financial losses due to the transaction, the approval of our stockholders was required under applicable law or otherwise should have been obtained prior to the completion of the transaction, or that our officers and directors breached their fiduciary duties in connection with the approval and completion of the transaction. Although we believe that stockholder approval was not required under applicable law in order to complete our transaction with Janssen, and therefore we neither sought nor intend to seek such stockholder approval, it is possible that persons who were stockholders at the time of the applicable transaction may claim that their approval was required, in which case litigation could follow, which could result in substantial damages to us and/or could negatively affect our rights and obligations, or result in the termination of, the Collaboration Agreement.
Likewise, our stockholders may believe that the financial and other terms of the Collaboration Agreement are not favorable to either us or our stockholders, including any belief that the potential payments we may receive under the Collaboration Agreement are inadequate. Litigation brought by our stockholders challenging the validity of, or financial losses resulting from, these transactions could also result in claims against us by Janssen, and the Collaboration Agreement provides for indemnification by us of Janssen against all losses and expenses relating to breaches of our representations, warranties and covenants in the Collaboration Agreement, which could expose us to further financial obligations and damages. The occurrence of any one or more of the above could have a significant adverse impact on our business and financial condition.
In addition, if the results of our business and collaboration activities are not successful, including without limitation, if our Collaboration Agreement with Janssen is terminated or is otherwise unsuccessful, then our stock price would decline significantly, and future litigation may result. A
37
decision adverse to our interests in any such lawsuits could result in the payment of substantial damages by us, and could have a material adverse effect on our cash flow, results of operations and financial condition or could otherwise severely harm our business.
RISKS RELATED TO CLINICAL AND COMMERCIALIZATION ACTIVITIES
The research and development of imetelstat is subject to numerous risks and uncertainties.
The science and technology of telomere biology, telomerase and our proprietary oligonucleotide chemistry are relatively new. There is no precedent for the successful commercialization of a therapeutic product candidate based on these technologies. Significant research and development activities will be necessary to further develop imetelstat, which was our sole product candidate that we have exclusively outlicensed to Janssen, which may take years to accomplish, if at all.
Because of the significant scientific, regulatory and commercial challenges that must be overcome to successfully research, develop and commercialize imetelstat, the development of imetelstat in hematologic myeloid malignancies, including MF and MDS, or any other indications, may be delayed or abandoned, even after significant resources have been expended on it. Examples of such decisions include the discontinuation of our Phase 2 clinical trial of imetelstat in metastatic breast cancer in September 2012, the discontinuation our development of imetelstat in solid tumors with short telomeres in April 2013, and Janssen's determinations in the third quarter of 2016 to close the 4.7 mg/kg dosing arm in IMbark to new patient enrollment and to suspend enrollment in the 9.4 mg/kg dosing arm in IMbark because an insufficient number of patients in the 9.4 mg/kg dosing arm met the protocol defined interim efficacy criteria at 12 weeks. Any further delay, suspension or abandonment of the development of imetelstat in hematologic myeloid malignancies would have a material adverse effect on our collaboration with Janssen, which could result in the termination of the Collaboration Agreement. Any of these events would have severe adverse effects on the future of imetelstat and our business prospects and likely result in the failure of our business.
Success in early clinical trials may not be predictive or indicative of results in current ongoing clinical trials or potential future clinical trials. Likewise, preliminary data from clinical trials should be considered carefully and with caution since the final data may be materially different from the preliminary data, particularly as more patient data become available.
A number of new drugs and biologics have shown promising results in preclinical studies and initial clinical trials, but subsequently fail to establish sufficient safety and efficacy data to obtain necessary regulatory approvals to initiate commercial sale of a drug. There is typically an extremely high rate of attrition from the failure of product candidates proceeding through clinical trials. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval. Product candidates in later stages of clinical trials may fail to show the desired benefit-risk profile despite having progressed through preclinical studies and initial clinical trials.
Data from our preclinical studies and Phase 1 and Phase 2 clinical trials of imetelstat, including the Pilot Study, as well as the results of the initial internal interim data reviews conducted by Janssen in the third quarter of 2016 for IMbark and IMerge, should not be relied upon as predictive or indicative of future clinical results, including any final results in the Pilot Study, IMbark or IMerge or the results in potential subsequent or larger-scale clinical trials of imetelstat. The results we obtained from the ET Trial and the Pilot Study and the results obtained by Janssen in the initial internal interim data reviews conducted by Janssen for IMbark and IMerge, as well as any future results that may be obtained by Janssen from IMbark and IMerge, may not predict the future therapeutic benefit of imetelstat, if any, in hematologic myeloid malignancies, including MF and MDS. In addition, the known LFT abnormalities and dose-limiting toxicities associated with imetelstat, such as profound and
38
prolonged thrombocytopenia and neutropenia and other safety issues, including death, that have been observed in both previous Geron-sponsored clinical trials and investigator-sponsored clinical trials, including in the Pilot Study, have also been observed in the internal interim data reviews conducted by Janssen of IMbark and IMerge. Since remaining patients previously enrolled in the Pilot Study, as well as patients currently enrolled in IMbark and IMerge, continue to receive imetelstat, safety data continue to be generated, and additional and updated data may materially change the overall conclusions from the preliminary data reported for the Pilot Study, as well as determinations made by Janssen based on its initial internal reviews of data for IMbark and IMerge. If additional, new or more severe adverse events occur in current imetelstat clinical trials, including the Pilot Study, IMbark and IMerge, such trials could be discontinued by Janssen or the IND for imetelstat may again be placed on clinical hold, as it was in March 2014. Also, the criteria used to assess efficacy in the Pilot Study have not been validated for clinical use and may not be considered by the FDA or other regulatory authorities to be accurate predictors of efficacy for different endpoints that may be required by the FDA or other regulatory authorities for Phase 3 clinical trials.
In addition, from time-to-time, preliminary or interim data from current clinical trials, such as the ongoing Pilot Study, IMbark and IMerge, or potential future clinical trials may be reported or announced by Janssen, its investigators, or us. For example, preliminary results of the Pilot Study were presented by the investigator at the American Society of Hematology, or ASH, annual meeting in December 2013, and updated by the investigator at ASH in December 2014, and preliminary data were reported by the investigator from a cohort of MDS patients in the Pilot Study in December 2015. Since such data are preliminary, the final data from any protocol-specified primary analysis which may be conducted, or any future analyses of the Pilot Study, IMbark or IMerge, or potential future clinical trials of imetelstat, may be materially different. Material adverse differences in the final data, compared to preliminary or interim data, from the Pilot Study, IMbark or IMerge, or potential future clinical trials of imetelstat, could result in a decision by Janssen to discontinue the imetelstat program, delay its Continuation Decision, or terminate the Collaboration Agreement, which would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations. Even if final safety and/or efficacy data from the Pilot Study, IMbark or IMerge or potential future clinical trials of imetelstat, are positive, significant additional clinical testing will be necessary to advance the future development of imetelstat in hematologic myeloid malignancies, including MF or MDS. Preliminary or interim results from the Pilot Study, IMbark or IMerge reported by us, Janssen or by the investigator in the Pilot Study may not be reproduced in any potential future clinical trials of imetelstat, and thus should not be relied upon as indicative of future clinical results of imetelstat in MF, MDS or in any other hematologic myeloid malignancy. Preliminary or interim data should be considered carefully and with caution.
Clinical trials of imetelstat may not uncover all possible adverse effects that patients may experience from imetelstat treatment.
Clinical trials by their nature examine the effect of a potential therapy in a sample of the potential future patient population. As such, clinical trials conducted with imetelstat, to date and in the future, may not uncover all possible adverse events that patients treated with imetelstat may experience. Because previously enrolled patients who remain on study continue to receive imetelstat in the Pilot Study, additional or more severe toxicities or safety issues, including additional serious adverse events and clinically significant LFT abnormalities, may be observed in the Pilot Study as patient treatment continues and more data become available. Likewise, additional or more severe toxicities or safety issues, including additional serious adverse events and clinically significant LFT abnormalities, may be observed in IMbark and IMerge, particularly given the larger target patient enrollment in those studies. Since IMbark, IMerge and the Pilot Study are ongoing studies in which additional data are being generated, the benefit-risk profile of imetelstat will continue to be assessed, including the risk of hepatotoxicity, severe cytopenias, fatal bleeding and any other severe adverse effects that may be
39
associated with life-threatening clinical outcomes. If such toxicities or other safety issues in any clinical trial of imetelstat result in an unacceptable benefit-risk profile, then:
- •
- the commencement, continuation and/or completion of any current ongoing clinical trials, including IMbark, IMerge and the Pilot Study, or
potential future clinical trials would likely be delayed, for example by being placed on a clinical hold, halted or prohibited; or
- •
- additional, unexpected clinical trials or preclinical studies may be required to be conducted.
The occurrence of any of these events could cause Janssen to delay its Continuation Decision, or abandon the development of imetelstat entirely and terminate the Collaboration Agreement. Any termination of the Collaboration Agreement by Janssen would have a severe adverse effect on our results of operations, financial condition, business prospects and the future of imetelstat, any of which might cause us to cease operations.
Obtaining regulatory clearances and approvals to develop and market imetelstat in the United States and other countries is a costly and lengthy process, and we cannot predict whether or when regulatory authorities will permit additional imetelstat development or approve imetelstat for commercial sale.
Federal, state and local governments in the United States and governments in other countries have significant regulations in place that govern drug research and development and may prevent us, in collaboration with Janssen, from successfully conducting development efforts or from commercializing imetelstat. Imetelstat must receive all relevant regulatory approvals before it may be marketed in the United States or other countries. Obtaining regulatory approval is a lengthy, expensive and uncertain process. For example in June 2016, the electorate in the United Kingdom voted in favor of exiting the European Union. Although the impact of the withdrawal of the United Kingdom from the European Union will not be known for some time, this could lead to a period of considerable uncertainty in relation to the regulatory process in Europe, which could result in a delay in the review of regulatory submissions made in Europe by biotechnology and pharmaceutical companies, and could also lead to less efficient, more expensive, and potentially lengthier regulatory review processes for companies, including us and Janssen, who may seek to obtain regulatory approval for drug products in the European Union or the United Kingdom. Likewise, the new Trump Administration has and will appoint and employ many new secretaries, directors and the like into positions of authority in the U.S. federal government dealing with the pharmaceutical and healthcare industries that may potentially have a negative impact on the prices and the regulatory pathways for pharmaceuticals. Such changes could adversely affect and/or delay the ability of Janssen to obtain approval of, and market and sell, imetelstat in the United States. In addition, because imetelstat involves the application of new technologies and a new therapeutic approach, it may be subject to substantial additional review by various government regulatory authorities, and, as a result, the process of obtaining regulatory approvals for imetelstat may proceed more slowly than for product candidates based upon more conventional technologies, and any approval that may be received could limit the use of imetelstat. We do not expect imetelstat to be approved for commercial sale for many years, if at all.
Prior to initiating potential future clinical trials of imetelstat, clinical trial protocols must be submitted to the FDA or regulatory authorities in other countries. Questions or comments regarding protocols for potential future clinical trials of imetelstat from these agencies that must be addressed would likely delay further clinical development of imetelstat which could cause Janssen to terminate the Collaboration Agreement, which would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations. Similarly, questions or comments from regulatory authorities on any protocol amendments of IMbark or IMerge, could potentially delay the timing of any Continuation Decision by Janssen.
Before Janssen can seek to obtain regulatory approval for the commercial sale of imetelstat, multiple clinical trials, including larger-scale Phase 3 clinical trials, will need to be conducted to
40
demonstrate that imetelstat is safe and effective for use in a diverse population. If imetelstat cannot be developed in potential future clinical trials, including Phase 3 clinical trials, our Collaboration Agreement with Janssen will be negatively impacted and likely be terminated altogether, which would have severe adverse effects on our business and business prospects, and might result in the failure of our business.
If the interpretation by us or Janssen of safety and efficacy data obtained from preclinical and clinical studies varies from interpretations by the FDA or regulatory authorities in other countries, this would likely delay, limit or prevent further development and approval of imetelstat which may cause Janssen to terminate the Collaboration Agreement. For example, the FDA and regulatory authorities in other countries may require more or different data than what has been generated from our preclinical studies and previous or ongoing clinical trials, such as IMbark, IMerge or the Pilot Study. In addition, delays or rejections of regulatory approvals, or limitations in marketing authorizations, may be encountered as a result of changes in the regulatory environment or regulatory policy during the period of product development and/or the period of review of any application for regulatory approval for imetelstat.
The benefit-risk profile of imetelstat will also affect the assessment by the FDA and regulatory authorities in other countries of the drug's cost-effectiveness and/or marketability, which assessment could prevent or limit its approval for marketing and successful commercial use. If regulatory submissions requesting approval to market imetelstat are submitted, the FDA and regulatory authorities in other countries may conclude that the overall benefit-risk profile of imetelstat treatment does not merit approval of imetelstat for marketing or further development for any indication. Any of these events could cause Janssen to terminate the Collaboration Agreement, which would severely harm our business and business prospects, and might cause us to cease operations.
Delays in obtaining regulatory clearances and approvals or limitations in the scope of such clearances or approvals could:
- •
- significantly harm the commercial potential of imetelstat;
- •
- impose costly procedures upon future development activities;
- •
- diminish any competitive advantages that may have been available; or
- •
- adversely limit the amount of, or affect our ability to receive, any milestone payments or royalties under the Collaboration Agreement with Janssen.
Even if the necessary time and resources are committed by Janssen, the required regulatory clearances and approvals may not be obtained for imetelstat. Further, if regulatory clearances and approvals are obtained to commence commercial sales of imetelstat, they may impose significant limitations on the indicated uses or other aspects of the product label for which imetelstat can be marketed. An approval might also be contingent on the performance of costly additional post-marketing clinical trials. The occurrence of any of these events could limit the potential commercial use of imetelstat, and therefore delay the payment of potential milestone payments to us, or, if approved for commercial sale, could reduce the market demand for imetelstat and therefore result in decreased sales and reduced royalties for us under the Collaboration Agreement. Occurrence of any of these events could negatively impact our collaboration with Janssen or cause Janssen to terminate the Collaboration Agreement, which would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
41
Although orphan drug designation has been granted to imetelstat for the treatment of MF and MDS, these designations may not be maintained, which would eliminate the benefits associated with orphan drug designation, including the potential for market exclusivity, which would likely result in potential sales revenue for imetelstat, if any, to be reduced, and would likely harm our business and business prospects.
Although the FDA granted orphan drug designation to imetelstat in June 2015 for the treatment of MF and for the treatment of MDS in December 2015, and the European Medicines Agency, or EMA, granted it in November 2015 for the treatment of MF, Janssen may not be the first to obtain marketing approval of a product candidate for the orphan-designated indication due to the uncertainties associated with developing pharmaceutical products. In addition, exclusive marketing rights in the United States or the European Union, if granted, may be limited if Janssen seeks approval for an indication broader than the orphan-designated indication or such marketing exclusivity may be lost if the FDA or the EMA later determines that the request for orphan drug designation was materially defective, or if Janssen is unable to ensure and provide sufficient quantities of imetelstat to meet the needs of patients with the rare disease or condition. Further, even if Janssen obtains orphan drug exclusivity for imetelstat, that exclusivity may not effectively protect imetelstat from competition because different drugs with different active moieties can be approved for the same condition. Even after an orphan drug product is approved, the FDA or EMA can subsequently approve a different drug with the same active moiety for the same condition if the FDA or EMA concludes that the later drug is safer, more effective, or makes a major contribution to patient care. Occurrence of any of these events could result in decreased sales and reduced royalties for us, and may harm our business and business prospects. In addition, orphan drug designation will neither shorten the development time nor regulatory review time for imetelstat, and does not give imetelstat any advantage in the regulatory review or approval process.
Failure to achieve continued compliance with government regulation could delay or halt commercialization of imetelstat, which we have exclusively outlicensed to Janssen.
Approved products and their manufacturers are subject to continual review, and discovery of previously unknown problems with a product or its manufacturer may result in restrictions on the product or manufacturer, including import restrictions, seizure and withdrawal of the product from the market. If approved for commercial sale, future sales of imetelstat will be subject to government regulation related to numerous matters, including the processes of:
- •
- manufacturing;
- •
- advertising and promoting;
- •
- selling and marketing;
- •
- labeling; and
- •
- distribution.
If, and to the extent that, we are or Janssen is unable to comply with these regulations, our ability to earn potential milestone payments and royalties from worldwide net sales of imetelstat would be materially and adversely impacted.
Failure to comply with regulatory requirements can result in severe civil and criminal penalties, including but not limited to:
- •
- recall or seizure of products;
- •
- injunctions against the import, manufacture, distribution, sales and/or marketing of products; and
- •
- criminal prosecution.
42
The imposition of any of these penalties or other commercial limitations could negatively impact our collaboration with Janssen or cause Janssen to terminate the Collaboration Agreement, either of which would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
Any development activities conducted by Janssen under a Janssen Independent Development Plan, or IDP, may create significant reimbursement obligations for us, which could result in reduced cash inflow from future milestone payments and royalties until we have fully paid our reimbursement obligations under the Collaboration Agreement.
Under the Collaboration Agreement, Janssen may conduct certain development activities for imetelstat under a Janssen IDP, if we and Janssen agree that such activities should be performed outside of the mutually agreed global clinical development plan. Although Janssen would bear all of the costs for such Janssen IDP, if we exercised our U.S. Opt-In Rights and if any data from a Janssen IDP supports approval by a regulatory authority in the United States or other countries, then we would be required to reimburse Janssen for our share of the costs of that Janssen IDP plus a premium pursuant to the terms of the Collaboration Agreement. This cost reimbursement is payable as a lump sum up to a certain threshold upon receipt of regulatory approval for the Janssen IDP. Any remaining amounts in excess of the threshold are payable in installments by offsetting milestone payments or royalties received by us over a certain period of time, at which time any remaining reimbursement amount would be payable in a lump sum. This payment mechanism could result in reduced cash inflow from future milestone payments and royalties, which would adversely affect our results of operations and financial condition.
Under the Collaboration Agreement, if we develop imetelstat independently under our own IDP, the success of that IDP depends on our ability to provide adequate financial and technical resources.
Under the Collaboration Agreement, we may conduct certain development activities for imetelstat under a Geron IDP if we and Janssen agree that such activities should be performed outside of the mutually agreed global clinical development plan. In the event we conduct any clinical activities under a Geron IDP, we will be responsible for paying all of the development costs for the Geron IDP. Because the outcome of any clinical activities and/or regulatory approval process is highly uncertain, we cannot reasonably estimate whether any Geron IDP activities we may undertake will succeed. Since we are only eligible for reimbursement from Janssen for their share of the Geron IDP costs plus a premium if any data from a Geron IDP supports approval by a regulatory authority in the United States or other countries, we may not recoup our investment in any Geron IDP, which could adversely affect our financial condition. In addition, we may need additional capital to support any Geron IDP activities and we cannot assure you that our existing capital resources, future interest income, potential milestone payments and royalties under the Collaboration Agreement and potential future sales of our common stock will be sufficient to fund these future activities. If sufficient capital is not available, we may be unable to pursue activities under a Geron IDP, which could adversely affect our business.
To execute activities under a Geron IDP, we likely would be required to collaborate with contract research organizations, investigators, academic institutions, vendors, clinical trial sites, scientific consultants and others. We would be dependent upon the ability of these parties to perform their responsibilities reliably. In addition, we would have limited control over the activities of these organizations, investigators, scientific consultants and vendors. Except as otherwise required by our agreements with them, we could expect only limited amounts of their time to be dedicated to our activities. If any of these third parties were unable or refused to contribute to projects on which we needed their help, our ability to conduct activities under a Geron IDP could be significantly harmed. Also, if the performance of these services is not of the highest quality, does not achieve necessary regulatory compliance standards, or if such organization or vendor stops or delays its performance for
43
any reason, it would impair and delay our ability to report data from clinical activities under a Geron IDP which would, in turn, hinder our ability to make the necessary representations or provide the necessary information to regulatory authorities, if at all. As a result, we may not obtain regulatory approval and receive any reimbursement from Janssen for their share of the costs for the Geron IDP, which could adversely affect our business and financial condition.
If third parties that manufacture imetelstat fail to perform as needed, then the clinical and commercial supply of imetelstat will be limited.
Currently, third-party contractors perform certain process development or other technical and scientific work with respect to imetelstat, as well as supply starting materials and manufacture drug substance and drug product. Janssen, which is responsible for the manufacture and management of the supply of imetelstat on a global basis for clinical trials and, after any regulatory approval, all commercial activities, currently relies on these third-party contractors to produce and deliver sufficient quantities of imetelstat and other clinical trial materials to support clinical trials on a timely basis and to comply with applicable regulatory requirements. Janssen does not have direct control over these third-party personnel or operations. Reliance on these third-party manufacturers is subject to several risks, including:
- •
- being unable to identify suitable third-party manufacturers, because the number of potential manufacturers is limited and regulatory
authorities may require significant activities to validate and qualify any replacement manufacturer, which could involve new testing and compliance inspections;
- •
- being unable to contract with third-party manufacturers on acceptable terms, or at all;
- •
- the inability of third-party manufacturers to timely formulate and manufacture imetelstat or to produce imetelstat in the quantities or of the
quality required to meet clinical and commercial needs;
- •
- decisions by third-party manufacturers to exit the contract manufacturing business during the time required to supply clinical trials or to
successfully produce, store and distribute products;
- •
- compliance by third-party manufacturers with current Good Manufacturing Practice, or cGMP, standards mandated by the FDA and state agencies and
other government regulations corresponding to foreign regulatory authorities;
- •
- breach or termination of manufacturing contracts;
- •
- capacity limitation and scheduling imetelstat as a priority in contracted facilities; and
- •
- natural disasters that affect contracted facilities.
Each of these risks could lead to delays or shortages in drug supply, or the inability to manufacture drug supply necessary for preclinical and clinical activities, and commercialization. In addition, any decision by Janssen to self-manufacture imetelstat, change third-party contractor manufacturers or make changes to manufacturing processes, product vial size or packaging, or formulations for imetelstat, could result in manufacturing delays. Manufacturing delays could adversely impact the completion of current clinical trials, such as IMbark and IMerge, or the initiation of potential future clinical trials, which may cause Janssen to terminate the Collaboration Agreement or delay the timing of any Continuation Decision that Janssen could provide to us, either of which would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
In addition, current third-party contractors and/or any other contractors utilized by Janssen may need to make substantial investments to enable sufficient capacity increases and cost reductions, and to
44
implement those regulatory and compliance standards necessary for successful Phase 3 clinical trials and commercial production of imetelstat. These third-party contractors may not be able to achieve such capacity increases, cost reductions, or regulatory and compliance standards, and even if they do, such achievements may not be at commercially reasonable costs. Janssen currently does not have any long-term commitments or commercial supply agreements with any of the third-party contractors for imetelstat, and changing manufacturers may be prolonged and difficult due to inherent technical complexities and because the number of potential manufacturers is limited. It may be difficult or impossible for Janssen to find a replacement manufacturer on acceptable terms, or at all.
It may not be possible to manufacture imetelstat at costs or scales necessary to conduct clinical trials or potential future commercialization activities.
Oligonucleotides are relatively large molecules produced using complex chemistry, and the cost of manufacturing an oligonucleotide like imetelstat is greater than the cost of making typical small-molecule drugs. Therefore, imetelstat for clinical use is more expensive to manufacture than most other treatments currently available today or that may be available in the future. Similarly, the cost of manufacturing imetelstat for commercial use will need to be significantly lower than current costs in order for imetelstat to become a commercially successful product. Janssen may not be able to achieve sufficient scale increases or cost reductions necessary for successful commercial production of imetelstat. Failure to achieve necessary cost reductions could result in decreased sales and reduced royalties for us, could negatively impact our collaboration with Janssen or could cause Janssen to terminate the Collaboration Agreement, any of which would materially and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
We have not yet negotiated our agreement with Janssen specifying all of the terms for our co-promotion of imetelstat should we exercise our U.S. Co-Promotion Option. In addition, we do not have a sales force and may not develop an effective one, if at all.
Pursuant to the Collaboration Agreement with Janssen, we have a U.S. Co-Promotion Option if we exercise our U.S. Opt-In Rights. Assuming we exercise the U.S. Co-Promotion Option, we can elect to provide 20% of the U.S. imetelstat selling effort with Geron sales force personnel, in lieu of funding 20% of U.S. promotion costs upon regulatory approval and commercial launch of imetelstat in the United States. While the Collaboration Agreement includes the material terms of our U.S. Co-Promotion Option, we and Janssen mutually agreed to negotiate a separate agreement specifying detailed activities and responsibilities with respect to the marketing and co-promotion of imetelstat following our election to exercise our U.S. Co-Promotion Option. We will need to negotiate this separate agreement with Janssen and, as a result, Janssen may impose restrictions or additional obligations on us, including financial obligations. Any restrictions or additional obligations may restrict our co-promotion activities or involve more significant financial or other obligations than we currently anticipate. In addition, we have no sales experience as a company, and there are risks involved with establishing our own sales force capabilities, including:
- •
- incurring substantial expenditures to develop a sales force and function;
- •
- exposure to unforeseen costs and expenses; and
- •
- being unable to effectively recruit, train or retain sales personnel.
Accordingly, we may be unable to establish our own sales force, which would delay or preclude us from participating in co-promoting imetelstat in the United States. In addition, because of our current lack of expertise in sales operations, any sales force we establish may not be effective, or may be less effective than any sales force that Janssen utilizes to promote imetelstat. In such event, the commercialization of imetelstat may be adversely affected, since we would be wholly reliant on
45
Janssen's sales efforts, and this could materially and adversely affect any sales milestone or royalties we may receive under the Collaboration Agreement.
The Collaboration Agreement limits our ability to transfer our U.S. Co-Promotion Option to a potential acquirer.
Although the Collaboration Agreement permits us to be acquired by any company, our right to transfer our U.S. Co-Promotion Option in the case of an acquisition, merger, consolidation, share exchange, business combination, recapitalization, sale of a majority of assets or similar transaction is limited, and subject to Janssen's sole discretion under certain circumstances. If we are acquired outside of such limited circumstances, then we may not be able to transfer the U.S. Co-Promotion Option to such acquirer as part of the acquisition. This limiting provision may discourage potential acquisition bids for us or lower our value, thus preventing holders of our common stock from benefiting from what they may believe are the positive aspects of an acquisition, including the potential realization of a higher rate of return on their investment from this type of transaction.
We may not be able to obtain or maintain sufficient insurance on commercially reasonable terms or with adequate coverage against potential liabilities in order to protect ourselves against product liability claims.
Our business exposes us to potential product liability risks that are inherent in the testing, manufacturing and marketing of human therapeutic and diagnostic products. We may become subject to product liability claims if the use of imetelstat is alleged to have injured patients, including any injuries alleged to arise from any hepatotoxicity or hemorrhagic event associated with the use of imetelstat. We currently have limited clinical trial liability insurance, and we may not be able to maintain this type of insurance for any clinical trials, including clinical trials that we may conduct under a Geron IDP or in collaboration with Janssen under the Collaboration Agreement. In addition, product liability insurance is becoming increasingly expensive. Being unable to obtain or maintain product liability insurance in the future on acceptable terms or with adequate coverage against potential liabilities could have a material adverse effect on our business.
RISKS RELATED TO PROTECTING OUR INTELLECTUAL PROPERTY
We remain responsible for prosecuting, at Janssen's direction, the patents we have exclusively licensed to Janssen. The success of our collaboration with Janssen will depend on our ability to protect our technologies and imetelstat through patents and other intellectual property rights.
Protection of our proprietary technology is critically important to our business, especially with respect to our collaboration with Janssen. Our success will depend in part on our ability to obtain, enforce and extend our patents and maintain trade secrets, both in the United States and in other countries. Our patents may be challenged, invalidated or circumvented, and our patent rights may not provide proprietary protection or competitive advantages to us or Janssen. In the event that we are unsuccessful in obtaining and enforcing our patents and other intellectual property rights, the value of our technologies and imetelstat will be adversely affected, and we or Janssen may not be able or willing to further develop or commercialize imetelstat. Loss or impairment of our intellectual property related to imetelstat might delay or halt ongoing or potential future clinical trials of imetelstat and any applications for regulatory approval, and therefore delay or halt the payment of potential milestone payments to us under the Collaboration Agreement. Further, if imetelstat is approved for commercial sale, such events could impair Janssen's ability to sell imetelstat and therefore result in decreased sales and reduced royalties for us. Occurrence of any of these events could negatively impact our collaboration with Janssen or cause Janssen to terminate the Collaboration Agreement, which would materially and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
46
The patent positions of pharmaceutical and biopharmaceutical companies, including ours, are highly uncertain and involve complex legal and technical questions. In particular, legal principles for biotechnology and pharmaceutical patents in the United States and in other countries are evolving, and the extent to which we will be able to obtain patent coverage to protect our technologies and imetelstat, or enforce or defend issued patents, is uncertain.
A number of significant changes to U.S. patent law occurred when the Leahy-Smith America Invents Act, or the AIA, was signed into law on September 16, 2011. These include provisions that affect the way patent applications are prosecuted and may affect patent litigation. Many of the substantive changes to patent law associated with the AIA, and in particular, the first to file provisions, became effective on March 16, 2013. For example, under the AIA, patent rights are awarded to the first inventor to file a patent application with respect to a particular invention. Since the publication of discoveries in scientific or patent literature tends to lag behind actual discoveries by at least several months and sometimes several years, the persons or entities that we name as inventors in our patents and patent applications may not have been the first to invent the inventions disclosed in the patent applications or patents, or the first to file patent applications for these inventions. As a result, we may not be able to obtain patents for discoveries that we otherwise would consider patentable and that we consider to be extremely significant to the future success of imetelstat. Thus, our ability to protect our patentable intellectual property depends, in part, on our ability to be the first to file patent applications with respect to our inventions or any joint inventions that we may develop with Janssen. Delay in the filing of a patent application for any purpose, including further development or refinement of an invention, may result in the risk of loss of patent rights. The AIA and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Among some of the other changes introduced by the AIA are changes that limit where a patentee may file a patent infringement suit and changes providing opportunities for third parties to challenge any issued patent in the U.S. Patent and Trademark Office, or the Patent Office. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard necessary to invalidate a patent claim in Patent Office proceedings compared to the evidentiary standard in U.S. federal court, a third party could potentially provide evidence in a Patent Office proceeding sufficient for the Patent Office to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party could attempt to use the Patent Office procedures to invalidate patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Significant impairment of our imetelstat patent rights would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and could cause Janssen to terminate the Collaboration Agreement, which might cause us to cease operations.
U.S. court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. For example, on June 13, 2013, the U.S. Supreme Court, or the Court, issued a decision in Association for Molecular Pathology v. Myriad Genetics, Inc. holding that claims to isolated genomic DNA were not patentable subject matter, but claims to complementary DNA, or cDNA, molecules were patentable subject matter. On March 20, 2012, in Mayo Collaborative Services, DBA Mayo Medical Laboratories, et al. v. Prometheus Laboratories, Inc., the Court held that several claims drawn to measuring drug metabolite levels from patient samples and correlating them to drug doses were not patentable subject matter. In addition, court rulings in cases such as BRCA1- & BRCA2-Based Hereditary Cancer Test Patent Litig. and Promega Corp. v. Life Technologies Corp. have also narrowed the scope of patent protection available in certain circumstances. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events may have created uncertainty with respect to the value of certain patents we have previously obtained or in-licensed.
47
Depending on decisions by the U.S. federal courts and the Patent Office, the interpretation of laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents. Occurrence of these events could significantly impair our imetelstat patent rights which would have a material adverse effect on our business and could cause Janssen to terminate the Collaboration Agreement, which might cause us to cease operations.
Challenges to our patent rights would result in costly and time-consuming legal proceedings that could prevent or limit development of imetelstat.
Our patents may be challenged through administrative or judicial proceedings, which could result in the loss of important patent rights. For example, where more than one party seeks U.S. patent protection for the same technology, the Patent Office may declare an interference proceeding in order to ascertain the party to which the patent should be issued. Patent interferences are typically complex, highly contested legal proceedings, subject to appeal. They are usually expensive and prolonged, and can cause significant delay in the issuance of patents. Our pending patent applications, or our issued patents, may be drawn into interference proceedings or be challenged through post-grant review procedures or litigation, any of which could delay or prevent the issuance of patents, or result in the loss of issued patent rights.
Under the AIA, interference proceedings between patent applications filed on or after March 16, 2013 have been replaced with other types of proceedings, including derivation proceedings. The AIA also includes post-grant review procedures subjecting U.S. patents to post-grant review procedures similar to European oppositions, such as inter partes review, or IPR, covered business method post-grant reviews and other post-grant reviews. U.S. patents owned or licensed by us may therefore be subject to post-grant review procedures, as well as other forms of review and re-examination. In addition, the IPR process under the AIA permits any person, whether they are accused of infringing the patent at issue or not, to challenge the validity of certain patents. As a result, entities associated with hedge funds have challenged valuable pharmaceutical patents through the IPR process.
Certain jurisdictions, such as Europe, New Zealand and Australia, permit oppositions to be filed against granted patents or patents proposed to be granted. Under the Collaboration Agreement, Janssen could commercialize imetelstat internationally if approved by regulatory authorities for commercial sale. Therefore, securing both proprietary protection and freedom to operate outside of the United States is important to the Collaboration Agreement with Janssen and our business. Opposition proceedings require significant time and costs, and if we are unable to commit these types of resources to protect our imetelstat patent rights, we and/or Janssen could be prevented or limited in the development and commercialization of imetelstat.
As more groups become engaged in scientific research and product development in the areas of telomerase biology, the risk of our patents, or patents that we have in-licensed, being challenged through patent interferences, derivation proceedings, IPRs, post-grant proceedings, oppositions, re-examinations, litigation or other means will likely increase. Challenges to our patents through these procedures would be extremely expensive and time-consuming, even if the outcome was favorable to us. An adverse outcome in a patent dispute could severely harm our collaboration with Janssen or cause Janssen to terminate the Collaboration Agreement, or could otherwise have a material adverse effect on our business, and might cause us to cease operations, by:
- •
- causing us to lose patent rights in the relevant jurisdiction(s);
- •
- subjecting us to litigation, or otherwise preventing Janssen or us from commercializing imetelstat in the relevant jurisdiction(s);
- •
- requiring Janssen or us to obtain licenses to the disputed patents;
48
- •
- forcing Janssen or us to cease using the disputed technology; or
- •
- requiring Janssen or us to develop or obtain alternative technologies.
We or Janssen may be subject to infringement claims that are costly to defend, and as to which we may be obligated to indemnify Janssen or obtain unblocking licenses, and such claims may limit our or Janssen's ability to use disputed technologies and prevent us or Janssen from pursuing research, development, manufacturing or commercialization of imetelstat.
The commercial success of imetelstat will depend upon our and Janssen's ability to research, develop, manufacture, market and sell imetelstat without infringing or otherwise violating the intellectual property and other proprietary rights of third parties. There is considerable intellectual property litigation in the biotechnology and pharmaceutical industries, and many pharmaceutical companies, including potential competitors, have substantial patent portfolios. In the event our technologies infringe the rights of others or require the use of discoveries and technologies controlled by third parties, we or Janssen may be prevented from pursuing research, development, manufacturing or commercialization of imetelstat, or may be required to obtain licenses to those patents or other proprietary rights or develop or obtain alternative technologies. For example, we are aware that certain third parties have or may be prosecuting patents and patent estates that may relate to imetelstat, and while we believe these patents will expire before imetelstat is commercialized and/or that these patents are invalid and/or would not be infringed by the manufacture, use or sale of imetelstat, it is possible that the owner(s) of these patents will assert claims against us and/or Janssen in the future. Under the Collaboration Agreement, we are obligated under certain circumstances to indemnify Janssen from any claim of infringement of the patent rights of third parties in Janssen's development, manufacture or commercialization of imetelstat, or to obtain unblocking licenses from such third parties, at our cost.
Since we cannot be aware of all intellectual property rights potentially relating to imetelstat and its uses, we do not know with certainty that imetelstat, or the intended commercialization thereof, does not and will not infringe or otherwise violate any third party's intellectual property. Any infringement claims against us or Janssen would likely be expensive to resolve, and the cost of any indemnification of Janssen or unblocking license that we could be required to obtain under the Collaboration Agreement is unpredictable and could be significant. If we or Janssen are unable to resolve an infringement claim successfully, we or Janssen could be subject to an injunction which would prevent us or Janssen from commercializing imetelstat, and could also require us or Janssen to pay substantial damages. In addition to infringement claims, in the future we or Janssen may also be subject to other claims relating to intellectual property, such as claims that we or Janssen have misappropriated the trade secrets of third parties. Provided that Janssen continues to progress the development of imetelstat, we expect to see more efforts by others to obtain patents that are positioned to cover imetelstat. Our success therefore depends significantly on our and Janssen's ability to operate without infringing patents and the proprietary rights of others.
We or Janssen may become aware of discoveries and technologies controlled by third parties that are advantageous to developing or manufacturing imetelstat. Under such circumstances, we or Janssen may initiate negotiations for licenses to other technologies as the need or opportunity arises. We or Janssen may not be able to obtain a license to a technology required for the research, development, manufacture or commercialization of imetelstat on commercially favorable terms, or at all, or such licenses may be terminated on certain grounds, including as a result of our or Janssen's failure to comply with the obligations under such licenses. If we or Janssen do not obtain a necessary license or if such a license is terminated, we or Janssen may need to redesign such technologies or obtain rights to alternative technologies, which may not be possible, and even if possible, could cause delays in the development efforts for imetelstat and could increase the development and/or production costs of imetelstat. In cases where we or Janssen are unable to license necessary technologies, we and/or Janssen could be subject to litigation and prevented from researching, developing, manufacturing or
49
commercializing imetelstat, and in certain circumstances we may be required to indemnify Janssen for infringement claims arising from Janssen's research, development, manufacture or commercialization of imetelstat, which could materially and adversely impact our business. Failure by us or Janssen to obtain rights to alternative technologies or a license to any technology that may be required to research, develop, manufacture or commercialize imetelstat would delay potential future clinical trials of imetelstat and any applications for regulatory approval and therefore delay the payment of potential milestone payments to us, or, if imetelstat is approved for commercial sale, could impair Janssen's ability to sell imetelstat and therefore result in decreased sales and reduced royalties for us. Occurrence of any of these events could negatively impact our collaboration with Janssen or cause Janssen to terminate the Collaboration Agreement, which would materially and adversely affect our business, and might cause us to cease operations.
We may become involved in disputes with Janssen or any past or future collaborator(s) over intellectual property inventorship or ownership, and publications by us or Janssen, or by investigators, scientific consultants, research collaborators or others could impair our ability to obtain patent protection or protect our proprietary information, which, in either case, could have a significant impact on our business.
Inventions discovered under research, material transfer or other such collaborative agreements, including our Collaboration Agreement with Janssen, may become jointly owned by us and the other party to such agreements in some cases and the exclusive property of either party in other cases. Under some circumstances, it may be difficult to determine who invents and owns a particular invention, or whether it is jointly owned, and disputes can arise regarding inventorship and ownership of those inventions. These disputes could be costly and time-consuming and an unfavorable outcome could have a significant adverse effect on our business if we were not able to protect or license rights to these inventions. In addition, clinical trial investigators, scientific consultants and research collaborators generally have contractual rights to publish data and other proprietary information, subject to review by us and/or Janssen. Publications by us or Janssen, or by investigators, scientific consultants, previous employees, research collaborators or others, either with permission or in contravention of the terms of their agreements or otherwise, may impair the ability to obtain patent protection or protect proprietary information which would have a material adverse effect on our business and could cause Janssen to terminate the Collaboration Agreement, which might cause us to cease operations.
Much of the information and know-how that is critical to our business is not patentable, and we may not be able to prevent others from obtaining this information and establishing competitive enterprises.
We sometimes rely on trade secrets to protect our proprietary technology, especially in circumstances in which we believe patent protection is not appropriate or available. We attempt to protect our proprietary technology in part by confidentiality agreements with our employees, consultants, collaborators and contractors. We cannot provide assurance that these agreements will not be breached, that we would have adequate remedies for any breach, or that our trade secrets will not otherwise become known or be independently discovered by competitors, any of which would harm our business significantly.
In May 2016, the Defend Trade Secrets Act of 2016, or the DTSA, was enacted, providing a federal cause of action for misappropriation of trade secrets. Under the DTSA, an employer may not collect enhanced damages or attorney fees from an employee or contractor in a trade secret dispute brought under the DTSA, unless certain advanced provisions are observed. We cannot provide assurance that our existing agreements with employees and contractors contain notice provisions that would enable us to seek enhanced damages or attorneys' fees in the event of any dispute for misappropriation of trade secrets brought under the DTSA.
50
Significant disruptions of information technology systems, including cloud-based systems, or breaches of data security could adversely affect our business.
Our business is increasingly dependent on critical, complex and interdependent information technology systems, including cloud-based systems, to support business processes as well as internal and external communications. Our computer systems are potentially vulnerable to breakdown, malicious intrusion and computer viruses that may result in the impairment of key business processes. Such disruptions and breaches of security could have a material adverse effect on our business, financial condition and results of operations.
In addition, our data security and information technology systems are potentially vulnerable to data security breaches—whether by employees or others—that may expose sensitive data to unauthorized persons. Such data security breaches could lead to the loss of trade secrets or other intellectual property, or could lead to the public disclosure of sensitive clinical or commercial data, and the exposure of personally identifiable information (including sensitive personal information) of our employees, collaborators, clinical trial patients and others. A data security breach or privacy violation that leads to disclosure or modification of or prevents access to patient information, including personally identifiable information or protected health information, could result in increased costs or loss of revenue as a result of:
- •
- harm to our reputation;
- •
- additional compliance obligations under federal and/or state breach notification laws;
- •
- requirements for mandatory corrective action to be taken by us; and
- •
- requirements to verify the correctness of database contents and otherwise subject us to liability under laws and regulations that protect personal data.
If we are unable to prevent such data security breaches or privacy violations or implement satisfactory remedial measures, our operations could be disrupted, and we may suffer loss of reputation, financial loss and other regulatory penalties because of lost or misappropriated information, including sensitive patient data. In addition, these breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above. Moreover, the prevalent use of mobile devices that access confidential information increases the risk of data security breaches, which could lead to the loss of confidential information, trade secrets or other intellectual property. While we have implemented security measures to protect our data security and information technology systems, such measures may not prevent such events.
RISKS RELATED TO OUR FINANCIAL POSITION AND NEED FOR ADDITIONAL FINANCING
Although we reported a small profit for the year ending December 31, 2015, we have a history of losses and anticipate continued future losses, and our continued losses could impair our ability to sustain operations.
Until 2015, we had never been profitable and we had incurred operating losses every year since our operations began in 1990. While we were profitable in 2015 due to the recognition of revenue in connection with the upfront payment from Janssen under the Collaboration Agreement, we expect to incur additional operating losses and, as clinical development activities for imetelstat continue under our Collaboration Agreement with Janssen, our operating losses may increase in size. As of December 31, 2016, our accumulated deficit was approximately $957.9 million. Losses have resulted principally from costs incurred in connection with our research and development activities and from general and administrative costs associated with our operations.
Substantially all of our revenues to date have been payments under collaborative agreements and milestones, royalties and other revenues from our licensing arrangements. Any revenues generated from our licensing arrangements or ongoing collaborative agreements, including the Collaboration
51
Agreement with Janssen, may not be sufficient alone to sustain our operations. For example, we expect revenues under our license agreements related to our telomerase technology to decline significantly in the coming years, and to be eliminated by the end of 2019, due to upcoming patent expirations on such technology. In addition, there can be no assurance that we will receive any milestone payments or royalties from Janssen in the future. We may be unsuccessful in entering into any new corporate collaboration, partnership or license agreements that result in revenues, or existing collaborative agreements or license arrangements, such as the Collaboration Agreement with Janssen, may be terminated or expire.
We also expect to experience negative cash flow for the foreseeable future as we fund our operations and capital expenditures. This will result in decreases in our working capital, total assets and stockholders' equity, which may not be offset by milestone payments or royalties from Janssen or by future financings. We will need to generate significant revenues to achieve consistent future profitability. We may not be able to generate these revenues under the Collaboration Agreement with Janssen through milestone payments or royalties, and we may never achieve consistent future profitability. Even if we do become profitable in the future, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to achieve consistent future profitability could negatively impact the market price of our common stock and our ability to sustain operations.
We may require additional capital to support development and commercialization of imetelstat in collaboration with Janssen and to otherwise grow our business, and our ability to obtain the necessary funding is uncertain.
We may need additional capital resources in order to support development and commercialization of imetelstat, especially if we elect to exercise our U.S. Opt-In Rights and U.S. Co-Promotion Option under the Collaboration Agreement and potentially independently pursue imetelstat development under our own IDP, and to otherwise support the future growth of our business through the potential acquisition and/or in-licensing of other oncology products, product candidates, programs or companies. We cannot assure you that our existing capital resources, future interest income, potential milestone payments and royalties under the Collaboration Agreement with Janssen and potential future sales of our common stock, including pursuant to our At Market Issuance Sales Agreement, or 2015 Sales Agreement, with MLV & Co. LLC, or MLV, will be sufficient to fund future planned activities. The timing and degree of any future capital requirements will depend on many factors, including:
- •
- the accuracy of the assumptions underlying our estimates for our capital needs;
- •
- in the event that Janssen provides an affirmative Continuation Decision to us, whether we then elect our U.S. Opt-In Rights to share further
U.S. development and promotion costs for imetelstat beyond IMbark or IMerge under the Collaboration Agreement, including our share of development costs incurred to date by Janssen that we will be
required to reimburse if we exercise our U.S. Opt-In Rights;
- •
- to the extent permitted under the Collaboration Agreement, whether we independently pursue imetelstat development under our own IDP;
- •
- our potential reimbursement obligations to Janssen if any data from a Janssen IDP support approval by regulatory authorities in the United
States or other countries;
- •
- the achievement of development, regulatory and commercial milestones resulting in the payment to us from Janssen under the Collaboration
Agreement and the timing of receipt of such payments, if any;
- •
- changes or delays in Janssen's development plans for imetelstat, including changes which may result from interim data reviews or any future
clinical holds on any INDs for imetelstat;
- •
- Janssen's ability to meaningfully reduce manufacturing costs of imetelstat;
52
- •
- the progress, timing, magnitude, scope and costs of clinical development, manufacturing and commercialization of imetelstat, including the
number of indications being pursued, subject to clearances and approvals by the FDA and other regulatory authorities;
- •
- the time and costs involved in obtaining regulatory clearances and approvals in the United States and in other countries;
- •
- Janssen's ability to successfully market and sell imetelstat, upon regulatory approval or clearance, in the United States and other countries;
- •
- if we exercise our U.S. Opt-In Rights, our decision to also exercise our U.S. Co-Promotion Option, including the costs and timing of building a
U.S. sales force;
- •
- the sales price and availability of adequate third-party reimbursement for imetelstat;
- •
- the timing, receipt and amount of royalties under the Collaboration Agreement on worldwide net sales of imetelstat, upon regulatory approval or
clearance, if any;
- •
- the cost of acquiring and/or in-licensing other oncology products, product candidates, programs or companies, if any;
- •
- the timing, receipt and amount of any milestone payments or royalties on sales of any products licensed under the License Agreement by Janssen
Pharmaceuticals, upon regulatory approval or clearance, if any;
- •
- expenses associated with the pending class action securities lawsuits, as well as any other litigation; and
- •
- the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims.
In addition, changes in our business may occur that would consume available capital resources sooner than we expect. If our existing capital resources, future interest income, and potential milestone payments and royalties under the Collaboration Agreement with Janssen are insufficient to meet future capital requirements, we will need to raise additional capital to fund our operations.
Further, if the Collaboration Agreement is terminated, including as a result of Janssen's failure to provide an affirmative Continuation Decision to us, or for any other reason, we would not receive any milestone payments or royalties under the Collaboration Agreement, and then, depending on the timing of such event, we would be required to fund all clinical development, manufacturing and commercial activities for imetelstat should we elect to continue the development of imetelstat ourselves, which would require us to raise additional capital or establish alternative collaborations with third-party collaboration partners, which may not be possible. If the Collaboration Agreement is terminated and we are unable to raise additional capital or establish alternative collaborations with third-party collaboration partners for imetelstat, the development of imetelstat would be discontinued, which might cause us to cease operations. Additional financing through public or private equity financings, including pursuant to our 2015 Sales Agreement with MLV, capital lease transactions or other financing sources may not be available on acceptable terms, or at all. We may raise equity capital at a stock price or on other terms that could result in substantial dilution of ownership for our stockholders. The receptivity of the public and private equity markets to proposed financings is substantially affected by the general economic, market and political climate and by other factors which are unpredictable and over which we have no control. In this regard, continued volatility and instability in the global financial markets and political climate could adversely affect our ability to raise additional funds through financings and the terms upon which we may raise those funds.
53
Our ability to raise additional funds will be severely impaired in the event of:
- •
- changes or delays in Janssen's development plans for imetelstat, including as a result of any future clinical holds on any IND for imetelstat
or interim data reviews;
- •
- a failure to show adequate safety or efficacy of imetelstat in current or potential future clinical trials, which may result in a decision by
Janssen to delay or discontinue further development of imetelstat; or
- •
- a termination of the Collaboration Agreement or if our collaboration with Janssen is otherwise unsuccessful.
If sufficient capital is not available, we may be unable to fulfill our funding obligations under the Collaboration Agreement with Janssen, resulting in our breach of the Collaboration Agreement, which could lead to Janssen paying lower milestone payments and lower royalties to us under a reduced royalty tier. This would have a material adverse effect on our results of operations and financial condition.
Moreover, in order to grow and diversify our business, we plan to continue our business development efforts to identify and seek to acquire and/or in-license other oncology products, product candidates, programs or companies. Acquisition or in-licensing opportunities that we may pursue could materially affect our liquidity and capital resources and may require us to incur indebtedness, seek equity capital or both. In addition, there can be no assurance that sufficient additional capital would be available to us in order to pursue any of these opportunities.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an "ownership change," generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation's ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes (such as research tax credits) to offset its post-change taxable income or taxes may be limited. Changes in our stock ownership, some of which are outside of our control, may have resulted or could in the future result in an ownership change. If a limitation were to apply, utilization of a portion of our domestic net operating loss and tax credit carryforwards could be limited in future periods. In addition, a portion of the carryforwards may expire before being available to reduce future income tax liabilities which could adversely impact our financial position.
RISKS RELATED TO OUR COMMON STOCK AND FINANCIAL REPORTING
Historically, our stock price has been extremely volatile.
Historically, our stock price has been extremely volatile. Between January 1, 2007 and December 31, 2016, our stock has traded as high as $9.85 per share and as low as $0.91 per share. Between January 1, 2014 and December 31, 2016, the price has ranged between a high of $5.92 per share and a low of $1.39 per share. The significant market price fluctuations of our common stock have been due to and may in the future be influenced by a variety of factors, including:
- •
- not receiving timely regulatory clearances or approvals in any jurisdiction, whether within or outside of the United States, including, if we,
Janssen or future investigators do not obtain regulatory clearance to commence, conduct or continue clinical trials of imetelstat in MF, MDS or any additional hematologic myeloid malignancies in a
timely manner or at all, or to amend any clinical trial protocol with respect to the conduct of a current clinical trial;
- •
- developments in our collaboration with Janssen, including the termination, modification or amendment of the Collaboration Agreement, or disputes regarding the collaboration;
54
- •
- announcements regarding the research and development of imetelstat, including results of, delays in, discontinuation of, or modifications to
any clinical trials of imetelstat, and investor perceptions thereof;
- •
- announcements regarding the safety of imetelstat, including announcements similar to our March 2014 announcements that the FDA had placed a
full clinical hold on our IND for imetelstat and a partial clinical hold on the investigator's IND for the Pilot Study due to safety concerns;
- •
- announcements regarding plans to discontinue any imetelstat clinical trials, including IMbark or IMerge;
- •
- announcements regarding regulatory developments concerning imetelstat;
- •
- the experimental nature of imetelstat;
- •
- perception by our stockholders about the adequacy of potential payments we may receive under the Collaboration Agreement;
- •
- the demand in the market for our common stock;
- •
- announcements of technological innovations, new commercial products, or clinical progress or lack thereof by us, our collaborators, licensees,
partners or our competitors;
- •
- fluctuations in our operating results;
- •
- our declining cash balance as a result of operating losses;
- •
- general market conditions or market conditions relating to the biopharmaceutical and pharmaceutical industries;
- •
- announcements concerning imetelstat proprietary rights;
- •
- comments by securities analysts;
- •
- large stockholders exiting their position in our common stock;
- •
- announcements of or developments concerning pending and/or potential future litigation;
- •
- the issuance of common stock to partners, vendors or investors to raise additional capital or to acquire other oncology products, product
candidates, programs or companies; and
- •
- the occurrence of any other risks and uncertainties discussed under the heading "Risk Factors."
Stock prices and trading volumes for many biopharmaceutical companies fluctuate widely for a number of reasons, including factors which may be unrelated to their businesses or results of operations, such as media coverage, statements made on message boards and social media forums, legislative and regulatory measures and the activities of various interest groups or organizations. In addition to the risk factors described in this section, overall market volatility, as well as general domestic or international economic, market and political conditions, could materially and adversely affect the market price of our common stock and the return on your investment.
If we fail to continue to meet the listing standards of NASDAQ, our common stock may be delisted, which could have a material adverse effect on the liquidity of our common stock.
Our common stock is currently traded on the Nasdaq Global Select Market. The NASDAQ Stock Market LLC has requirements that a company must meet in order to remain listed on NASDAQ. In particular, NASDAQ rules require us to maintain a minimum bid price of $1.00 per share of our common stock. If the closing bid price of our common stock were to fall below $1.00 per share for 30 consecutive trading days or we do not meet other listing requirements, we would fail to be in compliance with NASDAQ's listing standards. There can be no assurance that we will continue to meet
55
the minimum bid price requirement, or any other requirement in the future. If we fail to meet the minimum bid price requirement, The NASDAQ Stock Market LLC may initiate the delisting process with a notification letter. If we were to receive such a notification, we would be afforded a grace period of 180 calendar days to regain compliance with the minimum bid price requirement. In order to regain compliance, shares of our common stock would need to maintain a minimum closing bid price of at least $1.00 per share for a minimum of 10 consecutive trading days. In addition, we may be unable to meet other applicable NASDAQ listing requirements, including maintaining minimum levels of stockholders' equity or market values of our common stock, in which case our common stock could be delisted. If our common stock were to be delisted, the liquidity of our common stock would be adversely affected and the market price of our common stock could decrease.
The sale of a substantial number of shares may adversely affect the market price of our common stock.
The sale of a substantial number of shares of our common stock in the public market, or the perception that such sales could occur, could significantly and negatively affect the market price of our common stock. As of December 31, 2016, we had 300,000,000 shares of common stock authorized for issuance and 159,158,636 shares of common stock outstanding. In addition, we had reserved 30,148,830 shares of our common stock for future issuance pursuant to our option and equity incentive plans and outstanding warrants as of December 31, 2016. Issuing additional shares could negatively affect the market price of our common stock and the return on your investment.
Future sales of our common stock, including pursuant to our 2015 Sales Agreement with MLV, or the issuance of common stock to satisfy our current or future cash payment obligations or to acquire technology, property, or other businesses, could cause immediate dilution and adversely affect the market price of our common stock. In addition, under the universal shelf registration statement filed by us in August 2015 and declared effective by the SEC in September 2015, we may sell any combination of common stock, preferred stock, debt securities and warrants in one or more offerings, up to a cumulative value of $250 million. The sale or issuance of our securities, as well as the existence of outstanding options and shares of common stock reserved for issuance under our option and equity incentive plans and outstanding warrants, also may adversely affect the terms upon which we are able to obtain additional capital through the sale of equity securities, which could negatively affect the market price of our common stock and the return on your investment.
Our undesignated preferred stock may inhibit potential acquisition bids; this may adversely affect the market price of our common stock and the voting rights of holders of our common stock.
Our certificate of incorporation provides our board of directors with the authority to issue up to 3,000,000 shares of undesignated preferred stock and to determine or alter the rights, preferences, privileges and restrictions granted to or imported upon these shares without further vote or action by our stockholders. The issuance of shares of preferred stock may delay or prevent a change in control transaction without further action by our stockholders. As a result, the market price of our common stock may be adversely affected.
In addition, if in the future, we issue preferred stock that has preference over our common stock with respect to the payment of dividends or upon our liquidation, dissolution or winding up, or if we issue preferred stock with voting rights that dilute the voting power of our common stock, the rights of holders of our common stock or the market price of our common stock could be adversely affected.
56
Provisions in our charter, bylaws and Delaware law may inhibit potential acquisition bids for us, which may prevent holders of our common stock from benefiting from what they believe may be the positive aspects of acquisitions and takeovers.
Provisions of our charter documents and bylaws may make it substantially more difficult for a third party to acquire control of us and may prevent changes in our management, including provisions that:
- •
- prevent stockholders from taking actions by written consent;
- •
- divide the board of directors into separate classes with terms of office that are structured to prevent all of the directors from being elected
in any one year; and
- •
- set forth procedures for nominating directors and submitting proposals for consideration at stockholders' meetings.
Provisions of Delaware law may also inhibit potential acquisition bids for us or prevent us from engaging in business combinations. In addition, we have severance agreements with several employees and a company-wide severance plan, either of which could require a potential acquirer to pay a higher price. Either collectively or individually, these provisions may prevent holders of our common stock from benefiting from what they may believe are the positive aspects of acquisitions and takeovers, including the potential realization of a higher rate of return on their investment from these types of transactions.
We do not intend to pay cash dividends on our common stock in the foreseeable future.
We do not anticipate paying cash dividends on our common stock in the foreseeable future. Any payment of cash dividends will depend upon our financial condition, results of operations, capital requirements and other factors and will be at the discretion of our board of directors.
Failure to achieve and maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 could have a material adverse effect on our business and stock price.
Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, requires that we establish and maintain an adequate internal control structure and procedures for financial reporting. Our annual reports on Form 10-K must contain an annual assessment by management of the effectiveness of our internal control over financial reporting and must include disclosure of any material weaknesses in internal control over financial reporting that we have identified. In addition, our independent registered public accounting firm must provide an opinion annually on the effectiveness of our internal control over financial reporting.
The requirements of Section 404 are ongoing and also apply to future years. We expect that our internal control over financial reporting will continue to evolve as our business develops. Although we are committed to continue to improve our internal control processes and we will continue to diligently and vigorously review our internal control over financial reporting in order to ensure compliance with Section 404 requirements, any control system, regardless of how well designed, operated and evaluated, can provide only reasonable, not absolute, assurance that its objectives will be met. Therefore, we cannot be certain that material weaknesses or significant deficiencies will not exist or otherwise be discovered in the future. If material weaknesses or other significant deficiencies occur, such weaknesses or deficiencies could result in misstatements of our results of operations, restatements of our financial statements, a decline in our stock price, or other material adverse effects on our business, reputation, results of operations, financial condition or liquidity.
57
RISKS RELATED TO COMPETITIVE FACTORS
Competitors may develop technologies that are superior to or more cost-effective than ours, which may significantly impact the commercial viability of imetelstat, which could cause Janssen to terminate the Collaboration Agreement and damage our ability to sustain operations.
The pharmaceutical and biotechnology industries are intensely competitive. Other pharmaceutical and biotechnology companies and research organizations currently engage in or have in the past engaged in efforts related to the biological mechanisms related to imetelstat, the study of telomeres, telomerase, or our proprietary oligonucleotide chemistry, and the research and development of therapies for the treatment of hematologic myeloid malignancies. In addition, other products and therapies that could directly compete with imetelstat currently exist or are being developed by pharmaceutical and biopharmaceutical companies and by academic institutions, government agencies and other public and private research organizations.
Many companies are developing alternative therapies to treat hematologic myeloid malignancies. For example, if approved for commercial sale for the treatment of MF, imetelstat would compete against Incyte Corporation's ruxolitinib, or Jakafi®, which is orally administered. In clinical trials, Jakafi® reduced spleen size, abdominal discomfort, early satiety, bone pain, night sweats and itching in MF patients. Recently, there have also been reports of overall survival benefit as well as improvement in bone marrow fibrosis from Jakafi® treatment. Other treatment modalities for MF include hydroxyurea for the management of splenomegaly, leukocytosis, thrombocytosis and constitutional symptoms; splenectomy and splenic irradiation for the management of splenomegaly and co-existing cytopenias, or low blood cell counts; chemotherapy and pegylated interferon. Drugs for the treatment of MF-associated anemia include erythropoiesis-stimulating agents, androgens, danazol, corticosteroids, thalidomide and lenalidomide. There are other investigational treatments for MF further along in development than imetelstat, such as pacritinib by CTI Biopharma Corporation, or CTI Biopharma, and momelotinib by Gilead Sciences, Inc., or Gilead, which have reported results from Phase 3 clinical trials, and other inhibitors of the JAK-STAT pathway, such as NS-018 by NS Pharma, Inc., as well as several investigational treatments in early phase testing such as histone deacetylase inhibitors, interleukin-3 receptor targeted agents, inhibitors of heat shock protein 90, hypomethylating agents, PI3 Kinase and mTOR inhibitors, anti-fibrosis antibodies, hedgehog and SMO inhibitors, PIM kinase inhibitors, IAP inhibitors, anti-LOX2 inhibitors, recombinant pentraxin 2 protein, KIP-1 activators, TGF-beta superfamily inhibitors, FLT inhibitors, and other tyrosine kinase inhibitors.
If approved for commercial sale for the treatment of lower risk MDS, imetelstat would compete against a number of treatment options, including erythropoiesis stimulating agents and other hematopoietic growth factors; immunomodulators, such as lenalidomide by Celgene Corporation, or Celgene; hypomethylating agents, such as azacitidine by Celgene and decitabine by Janssen; in addition to investigational treatments that may be further along in development than imetelstat, such as oral versions of azacitidine; histone deacetylase inhibitors; TGF-beta superfamily inhibitors, such as luspatercept by Acceleron, in collaboration with Celgene; PI3 Kinase inhibitors; aminopeptidase inhibitor, such as tosedostat by CTI Biopharma; TLR2-specific antibodies; anti-CD33 antibodies; Fas ligand inhibitors; and JAK-STAT pathway inhibitors.
Independently, Janssen is developing therapies for hematologic malignancies, including AML, MDS, multiple myeloma and ABC-subtype diffuse large B-cell lymphoma. Molecular and cellular pathways of interest include:
- •
- cell surface targets for immune-directed therapy;
- •
- immune checkpoint inhibition;
- •
- leukemia stem cells;
58
- •
- pathway addiction (genetic alterations, cell-type specific pathways);
- •
- conditional sensitivity (stress, protein-producing tumors);
- •
- targeting of T-cells and natural killer "NK" cells to tumors;
- •
- identification of novel tumor-specific antigens; and
- •
- progression from early MDS to AML and cancer interception.
Success by Janssen in any of these approaches may compete with imetelstat or render imetelstat obsolete or noncompetitive, which could lead to a decision by Janssen to discontinue the imetelstat program and terminate the Collaboration Agreement, which would materially and adversely affect our business and business prospects and might cause us to cease operations.
Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. We anticipate increased competition in the future as new companies explore treatments for hematologic myeloid malignancies, which may significantly impact the commercial viability of imetelstat. Academic institutions, government agencies and other public and private research organizations may also conduct research, seek patent protection and establish collaborative arrangements for research, clinical development and marketing of products similar to imetelstat. These companies and institutions compete with us in recruiting and retaining qualified development and management personnel as well as in acquiring technologies complementary to the imetelstat program.
In addition to the above factors, imetelstat will face competition based on:
- •
- product efficacy and safety;
- •
- convenience of product administration;
- •
- cost of manufacturing;
- •
- the timing and scope of regulatory consents;
- •
- status of reimbursement coverage;
- •
- price; and
- •
- patent position, including potentially dominant patent positions of others.
As a result of the foregoing, competitors may develop more commercially desirable or affordable products than imetelstat, or achieve earlier patent protection or product commercialization than us or Janssen. Competitors have developed, or are in the process of developing, technologies that are, or in the future may be, competitive to imetelstat. Some of these products may have an entirely different approach or means of accomplishing therapeutic effects similar or superior to those that may be demonstrated by imetelstat. Competitors may develop products that are safer, more effective, or less costly than imetelstat, or more convenient to administer to patients and, therefore, present a serious competitive threat to imetelstat. In addition, competitors may price their products below what Janssen may determine to be an acceptable price for imetelstat, may receive better third-party payor coverage and/or reimbursement, or may be more cost-effective than imetelstat. Such competitive products or activities by competitors may render imetelstat obsolete, which may cause Janssen to terminate the Collaboration Agreement, which would severely and adversely affect our financial results, business and business prospects, and the future of imetelstat, and might cause us to cease operations.
59
To be successful, imetelstat must be accepted by the health care community, which can be very slow to adopt or unreceptive to new technologies and products.
If approved for marketing, imetelstat may not achieve market acceptance since hospitals, physicians, patients or the medical community in general may decide not to accept and utilize imetelstat. If approved for commercial sale, imetelstat will compete with a number of conventional and widely accepted drugs and therapies manufactured and marketed by major pharmaceutical companies. The degree of market acceptance of imetelstat will depend on a number of factors, including:
- •
- the clinical indications for which imetelstat is approved;
- •
- the establishment and demonstration to the medical community of the clinical efficacy and safety of imetelstat;
- •
- the ability to demonstrate that imetelstat is superior to alternatives on the market at the time;
- •
- the ability to establish in the medical community the potential advantage of imetelstat over alternative treatment methods, including with
respect to cost and route of administration;
- •
- the label and promotional claims allowed by the FDA or other regulatory authorities for imetelstat, if any;
- •
- the timing of market introduction of imetelstat as well as competitive products;
- •
- the effectiveness of sales, marketing and distribution support for imetelstat;
- •
- the availability of adequate coverage, reimbursement and pricing by government and third-party payors; and
- •
- the willingness of patients to pay out-of-pocket in the absence of coverage by third-party payors, including governmental authorities.
The established use of conventional products competitive with imetelstat may limit or preclude the potential for imetelstat to receive market acceptance upon any commercialization. Janssen may be unable to demonstrate any pharmacoeconomic advantage for imetelstat compared to established or standard-of-care therapies, or newly developed therapies, for hematologic myeloid malignancies. Third-party payors may decide that any potential improvement that imetelstat may provide to clinical outcomes in hematologic myeloid malignancies is not adequate to justify the costs of treatment with imetelstat. If third-party payors do not view imetelstat as offering a better balance between clinical benefit and treatment cost compared to standard-of-care therapies or other treatment modalities currently in development, imetelstat may not be commercially viable. If the health care community does not accept imetelstat for any of the foregoing reasons, or for any other reason, our ability to earn potential milestone payments and royalties under the Collaboration Agreement with Janssen would be negatively impacted and our business and business prospects would be severely and adversely affected.
If we fail to comply with federal and state healthcare laws, including fraud and abuse, transparency, and health information privacy and security laws, we could face substantial penalties and our business, results of operations, financial condition and prospects could be adversely affected.
Our business operations and current and future arrangements with investigators, healthcare professionals, consultants, third-party payors and customers, may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations. These laws may constrain the business or financial arrangements and relationships through which we conduct our operations, including how we
60
research, market, sell and distribute any product of ours for which marketing approval is obtained. Such laws include:
- •
- the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering,
receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or
recommendation of, any good or service for which payment may be made under a federal healthcare program such as Medicare and Medicaid;
- •
- the federal false claims and civil monetary penalties laws, including the civil False Claims Act and its qui
tam or whistleblower provisions, which impose criminal and civil penalties against individuals or entities for, among other things, knowingly presenting, or causing to be
presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
- •
- the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other
things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
- •
- HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, which
imposes obligations, including mandatory contractual terms, on certain types of individuals and entities with respect to safeguarding the privacy, security and transmission of individually
identifiable health information;
- •
- the federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which
payment is available under Medicare, Medicaid or the Children's Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services, or CMS,
information related to payments or other transfers of value made to physicians and teaching hospitals, and applicable manufacturers and applicable group purchasing organizations to report annually to
CMS ownership and investment interests held by the physicians and their immediate family members; and
- •
- analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians, other healthcare providers, and healthcare entities, or marketing expenditures; and state and foreign laws governing the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Efforts to ensure that our current and future business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law involving applicable healthcare laws. If our operations are found to be in violation of any of these or any other health regulatory laws that may apply to us, we may be subject to significant penalties, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgement, individual imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and curtailment of our operations, any of which could
61
adversely affect our ability to operate our business and our results of operations. Defending against any such actions can be costly, time-consuming and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
If acceptable prices or adequate reimbursement for imetelstat is not obtained, the use of imetelstat could be severely limited.
The ability to successfully commercialize imetelstat will depend significantly on obtaining acceptable prices and the availability of coverage and adequate reimbursement to the patient from third-party payors. Government authorities and other third-party payors, such as private health insurers and health maintenance organizations, determine which medications they will cover and establish reimbursement levels. Assuming Janssen obtains coverage for imetelstat by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. If approved for commercial sale, patients are unlikely to use imetelstat unless coverage is provided and reimbursement is adequate to cover all or a significant portion of the cost of imetelstat. Therefore, coverage and adequate reimbursement is critical to new product acceptance.
Government authorities and other third-party payors are developing increasingly sophisticated methods of controlling healthcare costs, such as by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices as a condition of coverage, are using restrictive formularies and preferred drug lists to leverage greater discounts in competitive classes, and are challenging the prices charged for medical products. Further, no uniform policy requirement for coverage and reimbursement for drug products exists among third-party payors in the United States. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require Janssen to provide scientific and clinical support for the use of imetelstat to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
We cannot be sure that coverage and reimbursement will be available for imetelstat, if approved for commercial sale, and, if reimbursement is available, what the level of reimbursement will be. Coverage and reimbursement may impact the demand for, or the price of, any product candidate for which marketing approval is obtained. If coverage and reimbursement are not available or reimbursement is available only to limited levels, Janssen may not successfully commercialize imetelstat, even if marketing approval is obtained.
There may also be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively known as the Affordable Care Act, or ACA, became law and substantially changed the way healthcare will be financed by both governmental and private insurers, and significantly impacted the pharmaceutical industry. The ACA contains a number of provisions that may have a significant impact on our business.
While the Court upheld the constitutionality of most elements of the ACA in June 2012 and upheld the ACA against challenges to nationwide tax subsidies in July 2015, other judicial and Congressional challenges against the ACA are likely to be brought in the future. In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, or the Budget Resolution, that authorizes the implementation of legislation that would repeal portions of the ACA. Further, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with
62
authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Congress also could consider subsequent legislation to replace elements of the ACA that are repealed. We cannot assure that the ACA, as currently enacted or as amended in the future, or any legislation that may replace the ACA, will not adversely affect our business and financial results and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. For example, in August 2011, the Budget Control Act of 2011 was enacted, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee on Deficit Reduction did not achieve a targeted deficit reduction of at least $1.2 trillion for fiscal years 2012 through 2021, triggering the legislation's automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect beginning on April 1, 2013 and, following passage of the Bipartisan Budget Act of 2015, will stay in effect through 2025 unless additional Congressional action is taken. Further, the American Taxpayer Relief Act of 2012, signed into law in January 2013, among other things, also reduced Medicare payments to certain providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
In the future, we anticipate additional proposals relating to the reform of the U.S. healthcare system, some of which could further limit the prices, or the amounts of reimbursement available for imetelstat. There has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the price of drugs under Medicare, and reform government program reimbursement methodologies for drugs. If future legislation were to impose direct governmental price controls and access restrictions, it could have a significant adverse impact on our business and financial results. Managed care organizations, as well as Medicaid and other government agencies, continue to seek price discounts. Some states have implemented, and other states are considering, price controls or patient access constraints under the Medicaid program, and some states are considering price-control regimes that would apply to broader segments of their populations that are not Medicaid-eligible. Due to the volatility in the current economic and market dynamics, we are unable to predict the impact of any unforeseen or unknown legislative, regulatory, payor or policy actions, which may include cost containment and healthcare reform measures. Such policy actions could have a material adverse impact on the potential royalties under the Collaboration Agreement with Janssen on worldwide net sales of imetelstat, if approved.
Cost control initiatives also could decrease the price that Janssen may receive for imetelstat in the future. If imetelstat is not considered cost-effective or adequate third-party reimbursement for the users of imetelstat cannot be obtained, then Janssen may be unable to maintain price levels sufficient to realize an appropriate return on the investment in imetelstat, which would have a material adverse effect on our ability to earn potential milestone payments and royalties under the Collaboration Agreement, or could cause Janssen to terminate the Collaboration Agreement. Any of these events would severely and adversely affect our financial results, business and business prospects, and might cause us to cease operations.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
63
In September 2015, we amended the lease agreement for our premises at 149 Commonwealth Drive, Menlo Park, California to extend the lease term through January 2018 and reduce the space leased by us to approximately 14,500 square feet of office space effective February 2016. Our amended lease at 149 Commonwealth Drive includes an option to extend the lease for one additional period of two years. We believe that our facilities are adequate to meet our requirements for the near term.
On March 14, 2014, the first of two substantially similar purported class action securities lawsuits, or the Class Action Lawsuits, was filed in the United States District Court for the Northern District of California, or the California District Court, naming as defendants us and certain of our officers. The second such lawsuit was filed on March 28, 2014. On June 6, 2014, a securities lawsuit, not styled as a class action, was filed in the United States District Court for the Southern District of Mississippi, or the Mississippi District Court, naming as defendants us and certain of our officers. This lawsuit was based on the same factual background as the Class Action Lawsuits.
On June 30, 2014, the Class Action Lawsuits were consolidated for all purposes into a single case, and on November 4, 2014, the securities lawsuit filed in the Mississippi District Court was transferred and also consolidated into the Class Action Lawsuits. The California District Court appointed a lead plaintiff and lead counsel for the Class Action Lawsuits on June 30, 2014. On September 19, 2014, the lead plaintiff filed his consolidated amended complaint which alleges violations of the Securities Exchange Act of 1934 in connection with allegedly false and misleading statements made by us, including that we allegedly failed to disclose facts related to the occurrence of persistent low-grade liver function test, or LFT, abnormalities observed in the Phase 2 clinical trial of imetelstat in essential thrombocythemia, or the ET Trial, and the potential risk of chronic liver injury following long-term exposure to imetelstat. The consolidated amended complaint seeks unspecified damages and an award of reasonable costs and expenses, including attorneys' fees. We filed our motion to dismiss the consolidated amended complaint on November 18, 2014. On April 10, 2015, the California District Court granted our motion to dismiss with respect to some of the allegedly false and misleading statements made by us and denied our motion to dismiss with respect to other allegedly false and misleading statements made by us. On May 22, 2015, we filed our answer to the consolidated amended complaint.
On September 9, 2016, the California District Court signed an order temporarily staying the Class Action Lawsuits to enable the parties to seek to resolve the Class Action Lawsuits. On November 23, 2016, the parties signed a Memorandum of Understanding that set forth material deal points of resolving the Class Action Lawsuits. On December 13, 2016, the parties filed a notice of settlement, which the California District Court signed on December 16, 2016, staying the Class Action Lawsuits pending final approval of a settlement. Final settlement of the Class Action Lawsuits is contingent upon, among other things, approval by the California District Court. Such approval, if any, is not expected until the end of 2017.
On April 21, 2014, a stockholder purporting to act on our behalf filed a derivative lawsuit in the Superior Court of California for the County of San Mateo, or the San Mateo County Court, against certain of our officers and directors. On June 26, 2015 and June 29, 2015, respectively, substantially similar purported stockholder derivative lawsuits were filed in the California District Court, and on August 25, 2016 another similar purported stockholder derivative lawsuit was filed in the San Mateo County Court. The two derivative cases filed in the California District Court were consolidated on August 17, 2015, and the two derivative cases filed in the San Mateo County Court were consolidated on September 25, 2015. All of these lawsuits alleged breaches of fiduciary duties by the defendants and other violations of law, and generally that the defendants caused or allowed the dissemination of
64
allegedly false and misleading statements related to the ET Trial. On July 22, 2016, a stipulation of settlement and a motion of preliminary approval was filed in the San Mateo County Court to settle all claims in each of the foregoing derivative lawsuits in exchange for our agreement to (i) implement certain corporate governance refinements and (ii) instruct our insurer to pay in full the plaintiffs' attorneys a total of $950,000. On August 19, 2016, the San Mateo County Court preliminarily approved the proposed settlement. On November 18, 2016, the San Mateo County Court issued an order granting final approval of the settlement and dismissing with prejudice the two derivative cases filed in the San Mateo County Court. On December 6, 2016, the California District Court issued an order dismissing with prejudice the two derivative lawsuits filed in the California District Court. Accordingly, as of December 6, 2016, all of the derivative lawsuits have been fully and finally settled and dismissed. As of December 31, 2016, we have implemented the corporate governance refinements specified in the stipulation of settlement and our insurers have paid the settlement amount in full to the plaintiffs' attorneys.
It is possible that additional lawsuits will be filed, or allegations will be made by stockholders, with respect to these same or other matters and also naming us and/or our officers and directors as defendants. The Class Action Lawsuits and any other lawsuits are subject to inherent uncertainties, and the actual defense and disposition costs will depend upon many unknown factors. Monitoring, initiating and defending against legal actions is time-consuming for our management, likely to be expensive and may detract from our ability to fully focus our internal resources on our business activities. In addition, despite the availability of insurance, we may incur substantial legal fees and costs in connection with litigation and such amounts could be material to our financial statements. We could be forced to expend significant resources in the implementation of the corporate governance refinements required by the terms of the settlement for the derivative lawsuits, or in the settlement or defense of the Class Action Lawsuits or any other lawsuits, and we may not prevail in such lawsuits. We have not established any reserve for any potential liability relating to any additional lawsuits. Lawsuits could result in judgments against us that require us to pay damages, enjoin us from certain activities, or otherwise negatively affect our legal or contractual rights, which could have a significant adverse effect on our business. In addition, the inherent uncertainty of such litigation could lead to increased volatility in our stock price and a decrease in the value of our stockholders' investment in our common stock.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
65
ITEM 5. MARKET FOR THE REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is quoted on the Nasdaq Global Select Market under the symbol GERN. The high and low intraday sales prices as reported by the Nasdaq Global Select Market of our common stock for each of the quarters in the years ended December 31, 2016 and 2015 were as follows:
| |
High | Low | |||||
|---|---|---|---|---|---|---|---|
Year ended December 31, 2016: |
|||||||
First quarter |
$ | 4.77 | $ | 2.30 | |||
Second quarter |
$ | 3.35 | $ | 2.42 | |||
Third quarter |
$ | 3.13 | $ | 1.84 | |||
Fourth quarter |
$ | 2.45 | $ | 1.81 | |||
Year ended December 31, 2015: |
|||||||
First quarter |
$ | 4.49 | $ | 2.70 | |||
Second quarter |
$ | 4.47 | $ | 3.52 | |||
Third quarter |
$ | 4.67 | $ | 2.60 | |||
Fourth quarter |
$ | 5.30 | $ | 2.60 | |||
As of February 21, 2017, there were approximately 603 stockholders of record of our common stock. This number does not include "street name" or beneficial holders, whose shares are held of record by banks, brokers and other financial institutions. We are engaged in a highly dynamic industry, which often results in significant volatility of our common stock price. On February 21, 2017, the closing sales price for our common stock was $2.20 per share.
Dividend Policy
We have never paid cash dividends on our capital stock and do not anticipate paying cash dividends in the foreseeable future, but intend to retain our capital resources for reinvestment in our business. Any future determination to pay cash dividends will be at the discretion of the board of directors and will be dependent upon our financial condition, results of operations, capital requirements and other factors our board of directors deems relevant.
Performance Measurement Comparison(1)
The following graph compares total stockholder returns of Geron Corporation for the last five fiscal years beginning December 30, 2011 to two indices: the Nasdaq CRSP Total Return Index for the Nasdaq Stock Market-U.S. Companies, or the Nasdaq-US, and the Nasdaq Biotech Index, or the Nasdaq-Biotech. The total return for our stock and for each index assumes the reinvestment of dividends, although we have never declared cash dividends on Geron stock, and is based on the returns of the component companies weighted according to their capitalizations as of the end of each quarterly period. The Nasdaq-US tracks the aggregate price performance of equity securities of U.S. companies traded on the Nasdaq Global Select Market, or NGSM. The Nasdaq-Biotech, which is calculated and supplied by Nasdaq, represents biotechnology companies trading on Nasdaq under the Standard Industrial Classification (SIC) Code No. 283 Drugs main category (2833—Medicinals & Botanicals, 2834—Pharmaceutical Preparations, 2835—Diagnostic Substances, 2836—Biological Products). Geron common stock trades on the NGSM and is a component of both the Nasdaq-US and the Nasdaq-Biotech. The stockholder return shown in the graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
66
Comparison of Five Year Cumulative Total Return on Investment Among
Geron Corporation, the Nasdaq-US Index and the Nasdaq-Biotech Index(2)
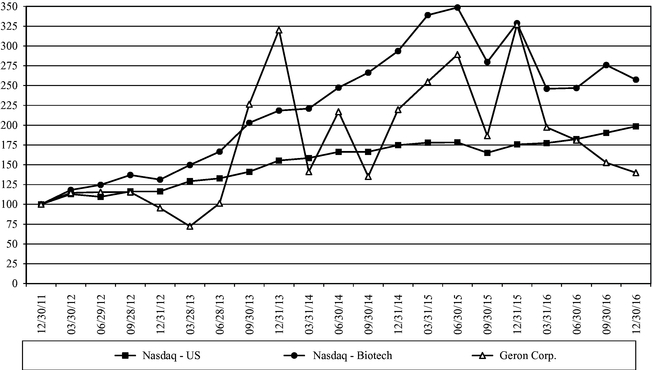
- (1)
- This
Section is not "soliciting material," is not deemed "filed" with the SEC and is not to be incorporated by reference in any filing of Geron Corporation under the
Securities Act of 1933, as amended, or the Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
- (2)
- Shows the cumulative total return on investment assuming an investment of $100 in each of Geron, the Nasdaq-US and the Nasdaq-Biotech on December 30, 2011. The cumulative total return on Geron stock has been computed based on a price of $1.48 per share, the price at which Geron common stock closed on December 30, 2011.
Recent Sales of Unregistered Securities
During the year ended December 31, 2016, there were no unregistered sales of equity securities by us.
67
ITEM 6. SELECTED FINANCIAL DATA
The following selected consolidated financial data should be read together with our audited financial statements and accompanying notes and "Management's Discussion and Analysis of Financial Condition and Results of Operations" appearing elsewhere in this annual report on Form 10-K. The selected consolidated financial data in this section is not intended to replace our financial statements and the accompanying notes. Our historical results are not necessarily indicative of our future results.
| |
Year Ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||
| |
(In thousands, except share and per share data) |
|||||||||||||||
Consolidated Statements of Operations Data: |
||||||||||||||||
Revenues: |
||||||||||||||||
Collaboration revenue(1) |
$ | — | $ | 35,000 | $ | — | $ | — | $ | — | ||||||
License fees and royalties(2) |
6,162 | 1,371 | 1,153 | 1,283 | 2,709 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total revenues |
6,162 | 36,371 | 1,153 | 1,283 | 2,709 | |||||||||||
Operating expenses: |
||||||||||||||||
Research and development |
18,047 | 17,831 | 20,707 | 23,155 | 51,368 | |||||||||||
Restructuring charges(3) |
— | 1,306 | — | 1,462 | 2,702 | |||||||||||
General and administrative |
18,761 | 17,793 | 16,758 | 15,624 | 20,397 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total operating expenses |
36,808 | 36,930 | 37,465 | 40,241 | 74,467 | |||||||||||
| | | | | | | | | | | | | | | | | |
Loss from operations |
(30,646 | ) | (559 | ) | (36,312 | ) | (38,958 | ) | (71,758 | ) | ||||||
Unrealized gain (loss) on derivatives |
— | 16 | 351 | (316 | ) | 13 | ||||||||||
Interest and other income |
1,192 | 677 | 373 | 951 | 3,097 | |||||||||||
Interest and other expense |
(83 | ) | (88 | ) | (82 | ) | (56 | ) | (233 | ) | ||||||
| | | | | | | | | | | | | | | | | |
Net (loss) income |
$ | (29,537 | ) | $ | 46 | $ | (35,670 | ) | $ | (38,379 | ) | $ | (68,881 | ) | ||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Net (loss) income per share: |
||||||||||||||||
Basic |
$ | (0.19 | ) | $ | 0.00 | $ | (0.23 | ) | $ | (0.30 | ) | $ | (0.54 | ) | ||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Diluted |
$ | (0.19 | ) | $ | 0.00 | $ | (0.23 | ) | $ | (0.30 | ) | $ | (0.54 | ) | ||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Shares used in computing net (loss) income per share: |
||||||||||||||||
Basic |
159,045,644 | 158,036,162 | 153,540,341 | 128,380,800 | 126,941,024 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Diluted |
159,045,644 | 162,663,894 | 153,540,341 | 128,380,800 | 126,941,024 | |||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- In November 2014, we entered into a collaboration and license agreement, or the Collaboration Agreement, pursuant to which we granted to Janssen Biotech Inc., or Janssen, the exclusive rights to develop and commercialize imetelstat worldwide for all indications in oncology, including hematologic myeloid malignancies, and all other human therapeutic uses. The Collaboration Agreement became effective in December 2014 and we received $35 million from Janssen as an upfront payment, which was classified as deferred revenue on our balance sheet as of December 31, 2014. Upon delivery of the imetelstat license rights and completion of our performance of the technology transfer-related activities to Janssen as outlined under the Collaboration Agreement, we fully recognized the $35 million upfront payment as collaboration revenue in the third quarter of 2015.
68
- (2)
- In
September 2016, we entered into a license agreement, or License Agreement, with Janssen Pharmaceuticals, Inc., or Janssen Pharmaceuticals, pursuant to
which we granted to Janssen Pharmaceuticals an exclusive worldwide license under our proprietary patents for the research, development and commercialization of products based on specialized
oligonucleotide backbone chemistry and novel amidates for ribonucleic acid interference, or RNAi, for the prevention, treatment and/or diagnosis of any and all human disorders, excluding cancers
originating from the blood or bone marrow, and products whose predominant or primary mechanism of action is telomerase inhibition. In accordance with the terms of the License Agreement, we received
$5 million from Janssen Pharmaceuticals as an upfront payment, which we recognized as license fee revenue in the third quarter of 2016 upon our delivery of the license rights and transfer of
know-how to Janssen Pharmaceuticals under the License Agreement.
- (3)
- In March 2015, we implemented an organizational resizing which resulted in aggregate restructuring charges of approximately $1.3 million in 2015. All actions associated with this restructuring were completed in 2015, and we do not anticipate incurring any further charges in connection with this restructuring. See Note 6 on Restructuring in Notes to Financial Statements of this annual report on Form 10-K.
In April 2013, we discontinued our discovery research programs and companion diagnostics program based on telomere length and closed our research laboratory facility located at 200 Constitution Drive, Menlo Park, California. In connection with this restructuring, we incurred aggregate restructuring charges of approximately $1.4 million in 2013. All actions associated with this restructuring were completed in 2013, and we do not anticipate incurring any further charges in connection with this restructuring.
In December 2012, we discontinued development of GRN1005, a product candidate that we previously exclusively in-licensed. In connection with this restructuring, we incurred aggregate restructuring charges of approximately $2.8 million, of which $2.7 million was recorded in 2012 and $92,000 was recorded in 2013. All actions associated with this restructuring were completed in 2013, and we do not anticipate incurring any further charges in connection with this restructuring.
| |
December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (In thousands) |
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||
Consolidated Balance Sheets Data: |
||||||||||||||||
Cash, restricted cash, cash equivalents and marketable securities |
$ | 129,067 | $ | 146,700 | $ | 170,639 | $ | 66,019 | $ | 96,329 | ||||||
Working capital |
108,243 | 109,258 | 111,607 | 59,470 | 84,269 | |||||||||||
Total assets |
130,249 | 148,760 | 172,511 | 67,344 | 99,801 | |||||||||||
Accumulated deficit |
(957,924 | ) | (928,387 | ) | (928,433 | ) | (892,763 | ) | (854,384 | ) | ||||||
Total stockholders' equity |
122,380 | 142,126 | 130,712 | 59,757 | 85,653 | |||||||||||
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
The following discussion should be read in conjunction with the section entitled "Business" in Part I, Item 1 and the audited financial statements and notes thereto included in Part II, Item 8 of this annual report on Form 10-K.
We are a biopharmaceutical company that currently supports the clinical stage development of a telomerase inhibitor, imetelstat, in hematologic myeloid malignancies, by Janssen Biotech, Inc., or Janssen. Early clinical data, including molecular responses in essential thrombocythemia, or ET, and remission responses, including reversal of bone marrow fibrosis, in myelofibrosis, or MF, suggest
69
imetelstat may have disease-modifying activity by inhibiting the progenitor cells of the malignant clones for the underlying diseases.
In November 2014, we entered into a collaboration and license agreement, or the Collaboration Agreement, pursuant to which we granted Janssen the exclusive rights to develop and commercialize imetelstat worldwide for all indications in oncology, including hematologic myeloid malignancies, and all other human therapeutic uses. The Collaboration Agreement became effective in December 2014 and we received $35 million from Janssen as an upfront payment. Additional consideration under the Collaboration Agreement includes potential payments of up to an aggregate maximum total of $900 million for the achievement of development, regulatory and sales milestones, as well as royalties on worldwide net sales of imetelstat.
Under the Collaboration Agreement, Janssen is wholly responsible for the development, manufacturing, seeking regulatory approval for and commercialization of, imetelstat worldwide. To that end, Janssen is currently conducting two clinical trials of imetelstat: IMbark, a Phase 2 trial in MF, in which the first patient was dosed in September 2015; and IMerge, a Phase 2/3 trial in myelodysplastic syndromes, or MDS, in which the first patient was dosed in January 2016. We are contributing 50% of the development costs for these studies, which Janssen is solely conducting. For a further discussion of the Collaboration Agreement, see Note 4 on License Agreements in Notes to Financial Statements of this annual report on Form 10-K.
In September 2016, Janssen completed planned internal reviews of data from both IMbark and IMerge. Following these internal reviews, Janssen determined to continue the trials in order to obtain additional and more mature data. In the first quarter of 2017, Janssen initiated the process for the second internal data reviews for both IMbark and IMerge, which will include comprehensive analyses encompassing safety, efficacy, pharmacokinetic and pharmacodynamic data, as well as other exploratory assessments, such as cytogenetic and molecular data. We expect the outcomes from the second internal data reviews, regulatory considerations and the totality of other program information, including the evolving treatment landscapes in MF and MDS, to inform Janssen's decisions regarding future development plans for imetelstat. Following the second internal data reviews, we expect Janssen could decide to resume, modify or terminate IMbark, IMerge, the imetelstat program or the Collaboration Agreement. We expect Janssen's decision-making regarding IMbark, IMerge, the imetelstat program or the Collaboration Agreement to occur in the second quarter of 2017. Janssen's decisions regarding future development plans for imetelstat, if any, may be subject to subsequent regulatory feedback. While patients remaining in the treatment phase of IMbark and IMerge continue to be dosed with imetelstat, new patient enrollment in both trials currently is suspended until completion of the second internal data reviews.
We had approximately $129.1 million in cash and investments as of December 31, 2016. To grow and diversify our business, we plan to continue our business development efforts to identify, and seek to acquire and/or in-license other oncology product candidates, programs or companies. Acquisition or in-licensing opportunities that we may pursue could materially affect our liquidity and capital resources and may require us to incur indebtedness or seek equity capital, or both. While we reported a small profit for the year ended December 31, 2015 due to our recognition of revenue in connection with the upfront payment from Janssen under the Collaboration Agreement, until 2015 we had never been profitable. We have incurred significant net losses since our inception in 1990, resulting principally from costs incurred in connection with our research and development activities and from general and administrative costs associated with our operations. As of December 31, 2016, we had an accumulated deficit of $957.9 million. Since our inception, we primarily have financed our operations through the sale of equity securities, interest income on our marketable securities and payments we received under our collaborative and licensing arrangements.
70
Substantially all of our revenues to date have been payments under collaborative agreements, and milestones, royalties and other revenues from our licensing arrangements. We currently have no source of product revenue. The significance of future losses, future revenues and any potential future profitability will depend primarily on whether Janssen continues to develop and advance imetelstat and the clinical and commercial success of imetelstat, which would result in potential future revenues to us in the form of milestone payments and royalties under the Collaboration Agreement, and whether we in-license or acquire other oncology products, product candidates, programs or companies in order to grow and diversify our business. There can be no assurance that we will receive any milestone payments or royalties from Janssen in the future, or at all. In addition, if Janssen does not perform in the manner we expect or fulfill its responsibilities in a timely manner, including with respect to conducting future additional or further data reviews of IMbark or IMerge, or at all, the clinical development, manufacturing, regulatory approval and/or commercialization of imetelstat could be delayed or terminated, and it could become necessary for us to assume responsibility for the clinical development, manufacturing, regulatory approval and/or commercialization of imetelstat at our own expense. In any event, imetelstat will require significant additional clinical testing prior to possible regulatory approval in the United States and other countries, and we do not expect imetelstat to be commercially available for many years, if at all.
Critical Accounting Policies and Estimates
Our financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. Note 1 of Notes to Financial Statements describes the significant accounting policies used in the preparation of our financial statements. Certain of these significant accounting policies are considered to be critical accounting policies, as defined below.
A critical accounting policy is defined as one that is both material to the presentation of our financial statements and requires management to make difficult, subjective or complex judgments that could have a material effect on our financial condition and results of operations. Specifically, critical accounting estimates have the following attributes: (i) we are required to make assumptions about matters that are highly uncertain at the time of the estimate; and (ii) different estimates we could reasonably have used, or changes in the estimate that are reasonably likely to occur, would have a material effect on our financial condition or results of operations.
Estimates and assumptions about future events and their effects cannot be determined with certainty. We base our estimates on historical experience and on various other assumptions believed to be applicable and reasonable under the circumstances. These estimates may change as new events occur, as additional information is obtained and as our operating environment changes. These changes historically have been minor and have been included in the financial statements as soon as they became known. Based on a critical assessment of our accounting policies and the underlying judgments and uncertainties affecting the application of those policies, management believes that our financial statements are stated fairly in accordance with accounting principles generally accepted in the United States, and meaningfully present our financial condition and results of operations.
71
We believe the following critical accounting policies reflect our more significant estimates and assumptions used in the preparation of our financial statements:
Fair Value of Financial Instruments
We categorize financial instruments recorded at fair value on our balance sheets based upon the level of judgment associated with inputs used to measure their fair value. The categories are as follows:
Level 1—Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date. An active market for the asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis.
Level 2—Inputs (other than quoted market prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument's anticipated life.
Level 3—Inputs reflect management's best estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model.
A financial instrument's categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. Following is a description of the valuation methodologies used for financial instruments measured at fair value on our balance sheets, including the category for such financial instruments.
Financial instruments classified as Level 1 include money market funds and certificates of deposit, representing 9% of our total financial instruments classified as assets measured at fair value as of December 31, 2016. Financial instruments classified as Level 2 include U.S. government-sponsored enterprise securities, commercial paper and corporate notes, representing 91% of our total financial instruments classified as assets measured at fair value as of December 31, 2016. The price for each security at the measurement date is derived from various sources. Periodically, we assess the reasonableness of these sourced prices by comparing them to the prices provided by our portfolio managers from broker quotes as well as reviewing the pricing methodologies used by our portfolio managers. Historically, we have not experienced significant deviation between the sourced prices and our portfolio managers' prices.
For a further discussion regarding fair value measurements, see Note 2 on Fair Value Measurements in Notes to Financial Statements of this annual report on Form 10-K.
Revenue Recognition
We recognize revenue for each unit of accounting when all of the following criteria have been met: (a) persuasive evidence of an arrangement exists, (b) delivery has occurred or services have been rendered, (c) the seller's price to the buyer is fixed or determinable, and (d) collectability is reasonably assured. Amounts received prior to satisfying these revenue recognition criteria are recorded as deferred revenue. Amounts expected to be recognized as revenue within the 12 months following the balance sheet date are classified as current deferred revenue. Amounts not expected to be recognized as revenue within the 12 months following the balance sheet date are classified as noncurrent deferred revenue.
Since our inception, substantially all of our revenues have been generated from license and collaboration agreements. Economic terms in these agreements may include non-refundable upfront license payments in cash or equity securities, option payments in cash or equity securities, cost reimbursements, cost-sharing arrangements, milestone payments, royalties on future sales of products,
72
or any combination of these items. In applying the appropriate revenue recognition guidance related to these agreements, we first assess whether the arrangement contains multiple elements. In this evaluation, we consider: (i) the deliverables included in the arrangement and (ii) whether the individual deliverables represent separate units of accounting or whether they must be accounted for as a combined unit of accounting. This evaluation involves subjective determinations and requires us to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that: (i) the delivered item(s) has value to the customer on a standalone basis, and (ii) if the arrangement includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in our control. In assessing whether an item has standalone value, we consider factors such as the research, manufacturing and commercialization capabilities of the collaboration partner or licensee and the availability of the associated expertise in the general marketplace. In addition, we consider whether the collaboration partner or licensee can use the other deliverable(s) for their intended purpose without the receipt of the remaining element(s), whether the value of the deliverable is dependent on the undelivered item(s) and whether there are other vendors that can provide the undelivered element(s).
Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method. We then apply the applicable revenue recognition criteria noted above to each of the separate units of accounting in determining the appropriate period and pattern of recognition. We determine how to allocate arrangement consideration to identified units of accounting based on the selling price hierarchy provided under relevant accounting guidance. The estimated fair value of deliverables under the arrangement may be derived using a best estimate of selling price if vendor-specific-objective evidence and third-party evidence are not available.
Upfront non-refundable signing, license or non-exclusive option fees are recognized as revenue: (i) when rights to use the intellectual property have been delivered, if the license has standalone value from the other deliverables to be provided under the agreement, or (ii) over the term of the agreement if we have continuing performance obligations, as the arrangement would be accounted for as a single unit of accounting. When payments are received in equity securities, we do not recognize any revenue unless such securities are determined to be realizable in cash.
At the inception of an arrangement that includes milestone payments, we assess whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether: (i) the consideration is commensurate with either the performance to achieve the milestone or the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the performance to achieve the milestone, (ii) the consideration relates solely to past performance and (iii) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We consider various factors, such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone and the level of effort and investment required to achieve the respective milestone, in making this assessment. There is considerable judgment involved in determining whether a milestone satisfies all of the criteria required to conclude that a milestone is substantive. Milestone payments for milestones that are considered substantive would be recognized as revenue in their entirety upon successful accomplishment of the milestone, assuming all other revenue recognition criteria are met. Milestone payments for milestones that are not considered substantive would be recognized as revenue over the remaining period of performance, assuming all other revenue recognition criteria are met.
Royalties are recognized as earned in accordance with contract terms when royalties from licensees can be reasonably estimated and collectability is reasonably assured. If royalties cannot be reasonably estimated or collectability of a royalty amount is not reasonably assured, royalties are recognized as revenue when the cash is received. Revenue from commercial milestone payments is accounted for as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
73
Cost-sharing expenses are recorded as earned or owed based on the performance requirements by both parties under the respective contracts. For arrangements in which we and our collaboration partner in the agreement are exposed to significant risks and rewards depending on the commercial success of the activity, we recognize payments between the parties on a net basis and record such amounts as a reduction or addition to research and development expense. For arrangements in which we have agreed to perform certain research and development services for our collaboration partner and are not exposed to significant risks and rewards that depend on the commercial success of the activity, we recognize the respective cost reimbursements as revenue under the collaborative agreement as the related research and development services are rendered.
Revenue recognition for licenses and collaboration agreements requires significant judgment. We evaluate the deliverables under an arrangement and estimate the fair value of those deliverables. We also assess the substantive nature of milestones. Our assessments and estimates are based on contractual terms, historical experience and general industry practice. Revisions in these values or estimations have the effect of increasing or decreasing license fee or collaboration revenue in the period of revision. As of December 31, 2016, we have not made any revisions to revenue recognition estimates and we do not expect revisions to currently active agreements in the future.
Clinical Trial Accruals
Prior to our collaboration with Janssen for imetelstat, substantial portions of our preclinical studies and all of our clinical trials were performed by third-party contract research organizations, or CROs, and other vendors. We accrued expenses for these activities based upon the estimated amount of work completed on each study. For our clinical trial expenses, the significant factors used in estimating accruals included the number of patients enrolled, the number of active clinical sites and the duration for which the patients had been enrolled in the study. For the clinical development activities being conducted by Janssen under the Collaboration Agreement, we monitor patient enrollment levels and related activities to the extent possible through discussions with Janssen personnel and base our estimates on the best information available at the time. However, additional information may become available to us which would allow us to make a more accurate estimate in future periods. In this event, we may be required to record adjustments to research and development expenses in future periods when the actual level of activity becomes more certain.
Valuation of Stock-Based Compensation
We measure and recognize compensation expense for all share-based payment awards to our employees and directors, including stock options, restricted stock awards and employee stock purchases related to our Employee Stock Purchase Plan, or ESPP, based on estimated grant-date fair values for these instruments. The grant-date fair value of share-based payment awards is amortized over the vesting period of the awards using a straight-line method and reduced for estimated forfeitures. We use the Black Scholes option-pricing model to estimate the grant-date fair value of our stock options and employee stock purchases.
Option-pricing valuation model assumptions, such as expected volatility, expected term and risk-free interest rate, impact the fair value estimate. Expected volatilities are based on historical volatilities of our stock since traded options on Geron common stock do not correspond to option terms and trading volume of options is limited. The expected term of options represents the period of time that options granted are expected to be outstanding. In deriving this assumption, we review actual historical exercise and post-vesting cancellation data and the remaining outstanding options not yet exercised or cancelled. The expected term of employees' purchase rights under our ESPP is equal to the purchase period. The risk-free interest rate is based on the U.S. Zero Coupon Treasury Strip Yields for the expected term in effect on the date of grant. Forfeiture rates are estimated based on historical
74
data and are adjusted, if necessary, over the requisite service period based on the extent to which actual forfeitures differ, or are expected to differ, from their estimate.
Prior to 2012, we granted restricted stock awards to employees and non-employee directors with service-based vesting schedules that generally vested annually over four years. The grant-date fair value for service-based restricted stock awards was determined using the fair value of our common stock on the date of grant. The grant-date fair value was amortized as stock-based compensation expense over the requisite service period of the award, which was generally the vesting period, on a straight-line basis and was reduced for estimated forfeitures, as applicable.
Compensation expense recognized for share-based payment awards to employees and directors was $8.2 million, $8.4 million and $7.7 million for the years ended December 31, 2016, 2015 and 2014, respectively. As of December 31, 2016, total compensation cost related to unvested share-based payment awards not yet recognized, net of estimated forfeitures, was $10.1 million, which is expected to be recognized over the next 24 months on a weighted-average basis.
We evaluate the assumptions used in estimating grant-date fair values of our share-based payment awards by reviewing current trends in comparison to historical data on an annual basis. We have not revised the methods by which we derive assumptions in order to estimate grant-date fair values of our share-based payment awards. If factors change and we employ different assumptions in future periods, the stock-based compensation expense that we record for share-based payment awards to employees and directors may differ significantly from what we have recorded in the current period.
For our non-employee stock-based awards, the measurement date on which the fair value of the stock-based award is calculated is equal to the earlier of: (i) the date at which a commitment for performance by the counterparty to earn the equity instrument is reached or (ii) the date at which the counterparty's performance is complete. We recognized stock-based compensation expense of $104,000, $311,000 and $94,000 for the vested portion of the fair value of non-employee stock options and restricted stock awards in our statements of operations for the years ended December 31, 2016, 2015 and 2014, respectively.
Results of Operations
Our results of operations have fluctuated from period to period and may continue to fluctuate in the future, based primarily upon the progress of research and development efforts in collaboration with Janssen and whether we are able to acquire and/or in-license other oncology products, product candidates, programs or companies in order to grow and diversify our business. Results of operations for any period may be unrelated to results of operations for any other period. Thus, historical results should not be viewed as indicative of future operating results. For example, in 2015 we reported net income for the first time due to recognition of revenue in the third quarter of 2015 in connection with the upfront payment from Janssen under the Collaboration Agreement. However, we expect to incur operating losses in the future as clinical development activities for imetelstat continue under our Collaboration Agreement with Janssen, and our operating losses may increase in size. We are subject to risks common to companies in our industry and at our stage of development, including, but not limited to, risks inherent in research and development efforts, our dependence on Janssen for the development, regulatory approval, manufacture and commercialization of imetelstat, uncertainty of preclinical and clinical trial results or regulatory approvals or clearances, the future development of imetelstat, including any future efficacy or safety results that may cause the benefit-risk profile of imetelstat to become unacceptable, need for future capital, enforcement of our patent and proprietary rights, reliance upon our collaborators, licensees, investigators and other third parties, and potential competition. In order for imetelstat to be commercialized, we are wholly dependent on Janssen to conduct preclinical tests and clinical trials to demonstrate the safety and efficacy of imetelstat, obtain regulatory approvals or clearances and enter into manufacturing, distribution and marketing
75
arrangements, as well as obtain market acceptance. We do not expect to receive royalties based on sales of imetelstat for many years, if at all.
Revenues
Collaboration Revenue
Upon the effectiveness of the Collaboration Agreement with Janssen in December 2014, we received $35 million as an upfront payment, which we classified as deferred revenue upon receipt. We determined delivery of the imetelstat license rights granted by us to Janssen, together with our performance of the technology transfer-related activities outlined in the Collaboration Agreement, represented the sole non-contingent deliverable associated with the upfront payment. Therefore, we accounted for our delivery of the imetelstat license rights and our performance of the technology transfer-related activities as a single unit of accounting. During the third quarter of 2015, we completed performance of the technology transfer-related activities to Janssen as outlined under the Collaboration Agreement. Combining this performance with the delivery of the imetelstat license rights, we fully recognized the $35 million upfront payment from Janssen as collaboration revenue on our statements of operations during the third quarter of 2015. No further payments have been made by Janssen to us in 2016. Any future collaboration revenue is substantially dependent on Janssen's ability to successfully develop and commercialize imetelstat in accordance with the Collaboration Agreement. See further discussion of revenue recognition for the Collaboration Agreement in Note 4 on License Agreements in Notes to Financial Statements of this annual report on Form 10-K.
License Fees and Royalties
In addition to the Collaboration Agreement with Janssen for imetelstat, we have entered into several license or collaboration agreements with companies involved with oncology, diagnostics, research tools and biologics production, whereby we have granted certain rights to our non-imetelstat related technologies. In connection with these agreements, we are eligible to receive license fees, option fees, milestone payments and royalties on future sales of products, or any combination thereof. We recognized license fee revenues of $5.6 million, $722,000 and $738,000 in 2016, 2015 and 2014, respectively, related to our various agreements. The increase in license fee revenues in 2016 compared to 2015 primarily reflects the full recognition of an upfront payment of $5 million from Janssen Pharmaceuticals under the License Agreement that was executed in September 2016 related to license rights to commercialize products based on specialized oligonucleotide backbone chemistry and novel amidates for RNAi for the prevention, treatment and/or diagnosis of any and all human disorders, excluding cancers originating from the blood or bone marrow, and products whose predominant or primary mechanism of action is telomerase inhibition. The decrease in license fee revenues in 2015 compared to 2014 primarily reflects the receipt of higher license payments in 2014 for research licenses related to our hTERT technology.
We determined delivery of the license rights granted by us to Janssen Pharmaceuticals under the License Agreement, together with the transfer of certain know-how necessary for the research, development and commercialization of products under the License Agreement, represented the sole non-contingent deliverable under the License Agreement. Accordingly, we accounted for our delivery of the license rights and transfer of know-how under the License Agreement as a single unit of accounting. Since we completed the delivery of the license rights and transfer of know-how to Janssen Pharmaceuticals under the License Agreement during the third quarter of 2016, we fully recognized the upfront payment from Janssen Pharmaceuticals as license fee revenue on our statements of operations in the third quarter of 2016. Since the research and development of the technology we licensed to Janssen Pharmaceuticals under the License Agreement is at a very early stage, we do not expect significant milestone payments under the License Agreement unless and until a product that utilizes or incorporates the licensed technology enters clinical development and receives marketing approval,
76
which may take many years, if ever. As such, future revenues under the License Agreement involve a substantial degree of uncertainty and risk that they may never be realized. See further discussion of revenue recognition for the License Agreement in Note 4 on License Agreements in Notes to Financial Statements of this annual report on Form 10-K.
We recognized royalty revenues of $537,000, $649,000 and $415,000 in 2016, 2015 and 2014, respectively, on product sales of telomerase detection and telomere measurement kits to the research-use-only market, cell-based research products and nutritional products. The decrease in royalty revenues in 2016 compared to 2015 primarily reflects lower product sales by our licensees. The increase in royalty revenues in 2015 compared to 2014 primarily reflects higher product sales by our licensees.
Future license fee and royalty revenues are dependent on additional agreements being signed, if any, current agreements being maintained and the underlying patent rights for the licenses remaining active. We expect revenues under our license agreements related to our hTERT technology to decline significantly in the coming years, and to be eliminated by the end of 2019, due to upcoming patent expirations on such technology. Current revenues may not be predictive of future revenues.
Research and Development Expenses
During the years ended December 31, 2016, 2015 and 2014, imetelstat was the sole research and development program we supported. For the imetelstat research and development program, we incur direct external, personnel related and other research and development costs. For the years ended December 31, 2016 and 2015, direct external expenses primarily consisted of our proportionate share of research and development costs incurred by Janssen under the Collaboration Agreement. For the year ended December 31, 2014, direct external expenses primarily consisted of costs paid to outside parties to perform laboratory studies, develop manufacturing processes and manufacture raw materials and clinical trial drug materials, conduct and manage clinical trials, and provide advice and consultation for scientific and clinical strategies. Personnel related expenses primarily consist of salaries and wages, stock-based compensation, payroll taxes and benefits for Geron employees involved with ongoing research and development efforts. Other research and development expenses primarily consist of research related overhead associated with allocated expenses for rent and maintenance of facilities and other supplies.
Research and development expenses were $18.0 million, $17.8 million and $20.7 million for the years ended December 31, 2016, 2015 and 2014, respectively. The increase in research and development expenses in 2016 compared to 2015 primarily reflects the net result of higher direct external costs for our proportionate share of clinical development expenses under the Collaboration Agreement with Janssen, partially offset by reduced personnel related expenses and research related overhead as a result of the March 2015 restructuring and lower direct external expenses for the manufacturing of imetelstat drug product. The decrease in research and development expenses in 2015 compared to 2014 primarily reflects the net result of lower personnel related expenses and research related overhead as a result of the March 2015 restructuring and lower direct external expenses for the manufacturing of imetelstat drug product, partially offset by higher direct external costs for our proportionate share of clinical development expenses under the Collaboration Agreement with Janssen.
77
Research and development expenses for the years ended December 31, 2016, 2015 and 2014 were as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (In thousands) |
2016 | 2015 | 2014 | |||||||
Direct external research and development expenses: |
||||||||||
Clinical program: Imetelstat |
$ | 14,695 | $ | 9,574 | $ | 8,901 | ||||
Personnel related expenses |
2,729 | 6,478 | 9,674 | |||||||
All other research and development expenses |
623 | 1,779 | 2,132 | |||||||
| | | | | | | | | | | |
Total |
$ | 18,047 | $ | 17,831 | $ | 20,707 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
At this time, we cannot provide reliable estimates of how much time or investment will be necessary to commercialize imetelstat. For a more complete discussion of the risks and uncertainties associated with the development of imetelstat in collaboration with Janssen, see the sub-sections entitled "Risks Related to Our Business" and "Risks Related to Clinical and Commercialization Activities" in Part I, Item 1A entitled "Risk Factors" and elsewhere in this annual report on Form 10-K.
Restructuring Charges
In March 2015, in connection with projected reduced operational demands as a result of the Collaboration Agreement with Janssen, we implemented an organizational resizing which resulted in aggregate restructuring charges of approximately $1.3 million in 2015 related to one-time termination benefits, including $307,000 of non-cash stock-based compensation expense relating to the extension of the post-termination exercise period for certain stock options previously granted to employees affected by the restructuring. All actions associated with this restructuring were completed in 2015, and we do not anticipate incurring any further charges in connection with this restructuring. See Note 6 on Restructuring in Notes to Financial Statements of this annual report on Form 10-K for further discussion of the restructuring charges.
General and Administrative Expenses
General and administrative expenses were $18.8 million, $17.8 million and $16.8 million for the years ended December 31, 2016, 2015 and 2014, respectively. The increase in general and administrative expenses in 2016 compared to 2015 and 2015 compared to 2014 primarily reflects the net result of higher non-cash stock-based compensation expense and an increased allocation of facilities and other overhead costs to general and administrative activities, partially offset by lower consulting and legal costs. We expect general and administrative expenses to remain consistent in 2017.
Unrealized Gain on Derivatives
Non-employee options classified as derivative liabilities are marked to fair value at each financial reporting date with any resulting changes in fair value being recorded as unrealized gain (loss) in the statements of operations. We incurred unrealized gains on derivatives of $16,000 and $351,000 for the years ended December 31, 2015 and 2014, respectively, which reflected a decline in the fair value of non-employee options classified as derivative liabilities. No comparable amount was incurred in 2016 as all non-employee options classified as derivative liabilities expired unexercised during the first quarter of 2015.
78
Interest and Other Income
Interest income was $1.2 million, $677,000 and $373,000 for the years ended December 31, 2016, 2015 and 2014, respectively. The increase in interest income in 2016 compared to 2015 primarily reflects higher yields on our marketable securities portfolio. The increase in interest income in 2015 compared to 2014 primarily reflects the increase in our marketable securities portfolio as a result of the receipt of the $35 million upfront payment from Janssen in December 2014 upon the effectiveness of the Collaboration Agreement and higher yields on our marketable securities portfolio. Interest earned in future periods will depend on the size of our marketable securities portfolio and prevailing interest rates.
Other income of $16,000 for the year ended December 31, 2016 reflects gains on sales of excess equipment. No other income was recognized for the years ended December 31, 2015 and 2014.
Interest and Other Expense
Interest and other expense was $83,000, $88,000 and $82,000 for the years ended December 31, 2016, 2015 and 2014, respectively. Interest and other expense primarily reflects bank charges related to our cash operating accounts and marketable securities portfolio.
Liquidity and Capital Resources
As of December 31, 2016, we had cash, restricted cash, cash equivalents and marketable securities of $129.1 million, compared to $146.7 million at December 31, 2015 and $170.6 million at December 31, 2014. The decrease in cash, restricted cash, cash equivalents and marketable securities in 2016 and 2015 was the result of cash being used for operations. We expect to experience negative cash flow and to incur significant and increasing operating expenses for the foreseeable future as the development of imetelstat continues in collaboration with Janssen. We estimate that our existing capital resources and future interest income will be sufficient to fund our current level of operations through at least the next 12 months. However, we may use our available capital resources sooner than we anticipate. For example, in order to grow and diversify our business, we plan to continue our business development efforts to identify and seek to acquire and/or in-license other oncology products, product candidates, programs or companies. Acquisition or in-licensing opportunities that we may pursue could materially affect our liquidity and capital resources and may require us to incur indebtedness, seek equity capital or both. In addition, there can be no assurance that sufficient additional capital would be available to us in order to pursue any of these opportunities.
We have an investment policy to invest our cash in liquid, investment grade securities, such as interest-bearing money market funds, certificates of deposit, municipal securities, U.S. government and agency securities, corporate notes and commercial paper. Our investment portfolio does not contain securities with exposure to sub-prime mortgages, collateralized debt obligations, asset-backed securities or auction rate securities and, to date, we have not recognized any other-than-temporary impairment charges on our marketable securities or any significant changes in aggregate fair value that would impact our cash resources or liquidity. To date, we have not experienced lack of access to our invested cash and cash equivalents; however, access to our invested cash and cash equivalents may be impacted by adverse conditions in the financial and credit markets.
In August 2015, we entered into an At Market Issuance Sales Agreement, or 2015 Sales Agreement, with MLV & Co. LLC, or MLV, which provides that, upon the terms and subject to the conditions and limitations set forth in the 2015 Sales Agreement, we may elect to issue and sell shares of our common stock having an aggregate offering price of up to $50 million from time to time through MLV as our sales agent. We are not obligated to make any sales of common stock under the 2015 Sales Agreement. To date, we have not sold any common stock pursuant to the 2015 Sales Agreement. The 2015 Sales Agreement will expire in August 2018 unless extended by the parties.
79
We may need additional capital resources in order to support development and commercialization of imetelstat, especially if we elect to exercise certain options under the Collaboration Agreement and potentially independently pursue imetelstat development under our own independent development plan, or IDP, under the Collaboration Agreement, and to otherwise support the future growth of our business through the potential acquisition and/or in-licensing of other oncology products, product candidates, programs or companies. We cannot assure you that our existing capital resources, future interest income, potential milestone payments and royalties under the Collaboration Agreement with Janssen, and potential future sales of our common stock, including pursuant to our 2015 Sales Agreement with MLV, will be sufficient to fund future planned activities. The timing and degree of any future capital requirements will depend on many factors, including:
- •
- the accuracy of the assumptions underlying our estimates for our capital needs;
- •
- in the event that Janssen provides an affirmative Continuation Decision to us, whether we then elect our U.S. Opt-In Rights to share further
U.S. development and promotion costs for imetelstat beyond IMbark or IMerge under the Collaboration Agreement, including our share of development costs incurred to date by Janssen that we will be
required to reimburse if we exercise our U.S. Opt-In Rights;
- •
- to the extent permitted under the Collaboration Agreement, whether we independently pursue imetelstat development under our own IDP;
- •
- our potential reimbursement obligations to Janssen if any data from a Janssen IDP support approval by regulatory authorities in the United
States or other countries;
- •
- the achievement of development, regulatory and commercial milestones resulting in the payment to us from Janssen under the Collaboration
Agreement and the timing of receipt of such payments, if any;
- •
- changes or delays in Janssen's development plans for imetelstat, including changes which may result from interim data reviews or any future
clinical holds on any investigational new drug applications, or INDs, for imetelstat;
- •
- Janssen's ability to meaningfully reduce manufacturing costs of imetelstat;
- •
- the progress, timing, magnitude, scope and costs of clinical development, manufacturing and commercialization of imetelstat, including the
number of indications being pursued, subject to clearances and approvals by the U.S. Food and Drug Administration, or the FDA, and other regulatory authorities;
- •
- the time and costs involved in obtaining regulatory clearances and approvals in the United States and in other countries;
- •
- Janssen's ability to successfully market and sell imetelstat, upon regulatory approval or clearance, in the United States and other countries;
- •
- if we exercise our U.S. Opt-In Rights, our decision to also exercise our U.S. Co-Promotion Option, including the costs and timing of building a
U.S. sales force;
- •
- the sales price and availability of adequate third-party reimbursement for imetelstat;
- •
- the timing, receipt and amount of royalties under the Collaboration Agreement on worldwide net sales of imetelstat, upon regulatory approval or
clearance, if any;
- •
- the cost of acquiring and/or in-licensing other oncology products, product candidates, programs or companies, if any;
80
- •
- the timing, receipt and amount of any milestone payments or royalties on sales of any products licensed under the License Agreement by Janssen
Pharmaceuticals, upon regulatory approval or clearance, if any;
- •
- expenses associated with the pending class action securities lawsuits, as well as any other litigation; and
- •
- the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims.
In addition, changes in our business may occur that would consume available capital resources sooner than we expect. If our existing capital resources, future interest income, and potential milestone payments and royalties under the Collaboration Agreement with Janssen are insufficient to meet future capital requirements, we will need to raise additional capital to fund our operations. Further, if the Collaboration Agreement is terminated, including as a result of Janssen's failure to provide an affirmative Continuation Decision to us, or for any other reason, we would not receive any milestone payments or royalties under the Collaboration Agreement, and then, depending on the timing of such event, we would be required to fund all clinical development, manufacturing and commercial activities for imetelstat should we elect to continue the development of imetelstat ourselves, which would require us to raise additional capital or establish alternative collaborations with third-party collaboration partners, which may not be possible. If the Collaboration Agreement is terminated and we are unable to raise additional capital or establish alternative collaborations with third-party collaboration partners for imetelstat, the development of imetelstat would be discontinued, which might cause us to cease operations. Additional financing through public or private equity financings, including pursuant to our 2015 Sales Agreement with MLV, capital lease transactions or other financing sources may not be available on acceptable terms, or at all. We may raise equity capital at a stock price or on other terms that could result in substantial dilution of ownership for our stockholders. The receptivity of the public and private equity markets to proposed financings is substantially affected by the general economic, market and political climate and by other factors which are unpredictable and over which we have no control. In this regard, continued volatility and instability in the global financial markets and political climate could adversely affect our ability to raise additional funds through financings and the terms upon which we may raise those funds.
Our ability to raise additional funds will be severely impaired in the event of:
- •
- changes or delays in Janssen's development plans for imetelstat, including as a result of any future clinical holds on any IND for imetelstat
or interim data reviews;
- •
- a failure to show adequate safety or efficacy of imetelstat in current or potential future clinical trials, which may result in a decision by
Janssen to delay or discontinue further development of imetelstat; or
- •
- a termination of the Collaboration Agreement or if our collaboration with Janssen is otherwise unsuccessful.
If sufficient capital is not available, we may be unable to fulfill our funding obligations under the Collaboration Agreement with Janssen, resulting in our breach of the Collaboration Agreement, which could lead to Janssen paying lower milestone payments and lower royalties to us under a reduced royalty tier. This would have a material adverse effect on our results of operations and financial condition.
Moreover, in order to grow and diversify our business, we plan to continue our business development efforts to identify and seek to acquire and/or in-license other oncology products, product candidates, programs or companies. Acquisition or in-licensing opportunities that we may pursue could materially affect our liquidity and capital resources and may require us to incur indebtedness, seek equity capital or both. In addition, there can be no assurance that sufficient additional capital would be available to us in order to pursue any of these opportunities.
81
Cash Flows from Operating Activities
Net cash used in operations was $18.4 million and $24.2 million in 2016 and 2015, respectively. Net cash provided by operations was $9.4 million in 2014. The decrease in net cash used in operations in 2016 compared to 2015 primarily reflects the net result of the receipt of the $5 million upfront payment from Janssen Pharmaceuticals under the License Agreement that was executed in September 2016, reduced costs for the manufacturing of imetelstat drug product and lower personnel related expenses as a result of the restructuring announced in March 2015, partially offset by higher payments to Janssen in 2016 under the cost-sharing arrangement for imetelstat clinical development as outlined under the Collaboration Agreement. The increase in net cash used in operations in 2015 compared to 2014 primarily reflects the receipt of the $35 million upfront payment from Janssen in December 2014 upon the effectiveness of the Collaboration Agreement.
Cash Flows from Investing Activities
Net cash provided by investing activities was $8.8 million and $73,000 in 2016 and 2015, respectively. Net cash used in investing activities was $77.9 million in 2014. The increase in net cash provided by investing activities in 2016 compared to 2015 and 2015 compared to 2014 primarily reflects a higher rate of maturities than purchases of marketable securities in 2016 and 2015, respectively.
For the three years ended December 31, 2016, we have purchased approximately $278,000 in property and equipment, none of which was financed through equipment financing arrangements.
Cash Flows from Financing Activities
Net cash provided by financing activities in 2016, 2015 and 2014 was $1.2 million, $2.6 million and $98.4 million, respectively. The decrease in net cash provided by financing activities in 2016 compared to 2015 primarily reflects the receipt of higher cash proceeds in 2015 from the exercise of outstanding stock options under our equity plans and the receipt of cash proceeds of approximately $881,000 in March 2015 from the exercise of warrants to purchase 235,000 shares of our common stock at an exercise price of $3.75 per share. The decrease in net cash provided by financing activities in 2015 compared to 2014 primarily reflects the receipt of net cash proceeds of approximately $96.8 million, after deducting the underwriting discount and offering expenses payable by us, from the underwritten public offering of 25,875,000 shares of our common stock at a public offering price of $4.00 per share that we completed in February 2014.
Significant Cash and Contractual Obligations
As of December 31, 2016, our contractual obligations for the next five years and thereafter were as follows:
| |
Payments Due by Period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Contractual Obligations(1)
|
Total | Less Than 1 Year |
1 - 3 Years | 4 - 5 Years | After 5 Years |
|||||||||||
| |
(In thousands) |
|||||||||||||||
Equipment lease |
$ | 10 | $ | 10 | $ | — | $ | — | $ | — | ||||||
Operating lease(2) |
716 | 661 | 55 | — | — | |||||||||||
License fees(3) |
285 | 60 | 120 | 30 | 75 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total contractual cash obligations |
$ | 1,011 | $ | 731 | $ | 175 | $ | 30 | $ | 75 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- This table does not include payments under our severance plan if there were a change in control of Geron or severance payments to employees in the event of an involuntary termination. In
82
addition, this table does not include any royalty obligations under our license agreements as the timing and likelihood of such payments are not known.
- (2)
- In
September 2015, we amended the lease agreement for our premises at 149 Commonwealth Drive, Menlo Park, California to extend the lease term through January 2018.
Our amended lease at 149 Commonwealth Drive includes an option to extend the lease for one additional period of two years. Operating lease obligations in the table above do not assume the exercise by
us of the option to extend the lease or any right of termination.
- (3)
- License fees are comprised of minimum annual license payments under our existing license agreements with several universities and companies for the right to use intellectual property related to technologies that we have in-licensed.
Off-Balance Sheet Arrangements
We have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The following discussion about our market risk disclosures contains forward-looking statements. Actual results could differ materially from those projected in the forward-looking statements. We are exposed to credit risk and interest rate risk. We do not use derivative financial instruments for speculative or trading purposes.
Credit Risk. We currently place our cash, restricted cash, cash equivalents and marketable securities with four financial institutions in the United States. Deposits with banks may exceed the amount of insurance provided on such deposits. While we monitor the cash balances in our operating accounts and adjust the cash balances as appropriate, these cash balances could be impacted if the underlying financial institutions fail or could be subject to other adverse conditions in the financial markets. Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash equivalents and marketable securities. Cash equivalents and marketable securities currently consist of money market funds, U.S. government-sponsored enterprise securities, commercial paper and corporate notes. Our investment policy, approved by the audit committee of our board of directors, limits the amount we may invest in any one type of investment issuer, thereby reducing credit risk concentrations. We limit our credit and liquidity risks through our investment policy and through regular reviews of our portfolio against our policy. To date, we have not experienced any loss or lack of access to cash in our operating accounts or to our cash equivalents and marketable securities in our investment portfolio. The effect of a hypothetical decrease of 10% in the average yield earned on our cash equivalents and marketable securities would have resulted in an immaterial decrease in our interest income for the year ended December 31, 2016.
Interest Rate Risk. The primary objective of our investment activities is to manage our marketable securities portfolio to preserve principal and liquidity while maximizing the return on the investment portfolio through the full investment of available funds without significantly increasing risk. To achieve this objective, we primarily invest in widely diversified investments with fixed interest rates, which carry a degree of interest rate risk. Fixed rate securities may have their fair value adversely impacted due to a rise in interest rates. Due in part to these factors, our future interest income may fall short of expectations due to changes in market conditions and in interest rates or we may suffer losses in principal if forced to sell securities which may have declined in fair value due to changes in interest rates.
The fair value of our cash equivalents and marketable securities at December 31, 2016 was $127.2 million. These investments include $11.2 million of cash equivalents which are due in less than
83
90 days, $102.0 million of short-term investments which are due in less than one year and $14.0 million of long-term investments which are due in one to two years. In accordance with our investment policy, which is approved by our audit committee, we primarily invest in marketable securities with at least an investment grade rating to minimize interest rate and credit risk as well as to provide for an immediate source of funds. Although changes in interest rates may affect the fair value of the marketable securities portfolio and cause unrealized gains or losses, such gains or losses would not be realized unless the investments are sold. Due to the nature of our investments, which are primarily money market funds, U.S. government-sponsored enterprise securities, commercial paper and corporate notes, we have concluded that there is no material interest rate risk exposure and a hypothetical movement of 1% in market interest rates would not have a significant impact on the total realized value of our portfolio.
84
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The following financial statements and the related notes thereto, of Geron Corporation and the Report of Independent Registered Public Accounting Firm, Ernst & Young LLP, are filed as a part of this annual report on Form 10-K.
85
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Geron Corporation
We have audited the accompanying balance sheets of Geron Corporation as of December 31, 2016 and 2015, and the related statements of operations, comprehensive loss, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Geron Corporation at December 31, 2016 and 2015, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Geron Corporation's internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 1, 2017 expressed an unqualified opinion thereon.
/s/
Ernst & Young LLP
Redwood City, California
March 1, 2017
86
GERON CORPORATION
BALANCE SHEETS
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | |||||
| |
(In thousands, except share and per share data) |
||||||
ASSETS |
|||||||
Current assets: |
|||||||
Cash and cash equivalents |
$ | 12,810 | $ | 21,248 | |||
Restricted cash |
268 | 267 | |||||
Marketable securities |
102,035 | 92,524 | |||||
Interest and other receivables |
475 | 1,206 | |||||
Prepaid assets |
524 | 647 | |||||
| | | | | | | | |
Total current assets |
116,112 | 115,892 | |||||
Noncurrent marketable securities |
13,954 | 32,661 | |||||
Property and equipment, net |
183 | 207 | |||||
| | | | | | | | |
|
$ | 130,249 | $ | 148,760 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY |
|||||||
Current liabilities: |
|||||||
Accounts payable |
$ | 225 | $ | 160 | |||
Accrued compensation and benefits |
2,843 | 3,026 | |||||
Accrued collaboration charges |
3,367 | 2,328 | |||||
Accrued liabilities |
1,434 | 1,120 | |||||
| | | | | | | | |
Total current liabilities |
7,869 | 6,634 | |||||
Commitments and contingencies |
|||||||
Stockholders' equity: |
|||||||
Preferred stock, $0.001 par value; 3,000,000 shares authorized; no shares issued and outstanding at December 31, 2016 and 2015 |
— | — | |||||
Common stock, $0.001 par value; 300,000,000 shares authorized; 159,158,636 and 158,781,359 shares issued and outstanding at December 31, 2016 and 2015, respectively |
159 | 159 | |||||
Additional paid-in capital |
1,080,198 | 1,070,567 | |||||
Accumulated deficit |
(957,924 | ) | (928,387 | ) | |||
Accumulated other comprehensive loss |
(53 | ) | (213 | ) | |||
| | | | | | | | |
Total stockholders' equity |
122,380 | 142,126 | |||||
| | | | | | | | |
|
$ | 130,249 | $ | 148,760 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See accompanying notes.
87
GERON CORPORATION
STATEMENTS OF OPERATIONS
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||||||
| |
(In thousands, except share and per share data) |
|||||||||
Revenues: |
||||||||||
Collaboration revenue |
$ | — | $ | 35,000 | $ | — | ||||
License fees and royalties |
6,162 | 1,371 | 1,153 | |||||||
| | | | | | | | | | | |
Total revenues |
6,162 | 36,371 | 1,153 | |||||||
Operating expenses: |
||||||||||
Research and development |
18,047 | 17,831 | 20,707 | |||||||
Restructuring charges |
— | 1,306 | — | |||||||
General and administrative |
18,761 | 17,793 | 16,758 | |||||||
| | | | | | | | | | | |
Total operating expenses |
36,808 | 36,930 | 37,465 | |||||||
| | | | | | | | | | | |
Loss from operations |
(30,646 | ) | (559 | ) | (36,312 | ) | ||||
Unrealized gain on derivatives |
— | 16 | 351 | |||||||
Interest and other income |
1,192 | 677 | 373 | |||||||
Interest and other expense |
(83 | ) | (88 | ) | (82 | ) | ||||
| | | | | | | | | | | |
Net (loss) income |
$ | (29,537 | ) | $ | 46 | $ | (35,670 | ) | ||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Net (loss) income per share: |
||||||||||
Basic |
$ | (0.19 | ) | $ | 0.00 | $ | (0.23 | ) | ||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Diluted |
$ | (0.19 | ) | $ | 0.00 | $ | (0.23 | ) | ||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Shares used in computing net (loss) income per share: |
||||||||||
Basic |
159,045,644 | 158,036,162 | 153,540,341 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Diluted |
159,045,644 | 162,663,894 | 153,540,341 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes.
88
GERON CORPORATION
STATEMENTS OF COMPREHENSIVE LOSS
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||||||
| |
(In thousands) |
|||||||||
Net (loss) income |
$ | (29,537 | ) | $ | 46 | $ | (35,670 | ) | ||
Net unrealized gain (loss) on marketable securities |
160 | (129 | ) | (70 | ) | |||||
| | | | | | | | | | | |
Comprehensive loss |
$ | (29,377 | ) | $ | (83 | ) | $ | (35,740 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes.
89
GERON CORPORATION
STATEMENTS OF STOCKHOLDERS' EQUITY
| |
Common Stock | |
|
Accumulated Other Comprehensive Loss |
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional Paid-In Capital |
Accumulated Deficit |
Total Stockholders' Equity |
||||||||||||||||
| |
Shares | Amount | |||||||||||||||||
| |
(In thousands, except share data) |
||||||||||||||||||
Balances at December 31, 2013 |
130,677,949 | $ | 131 | $ | 952,403 | $ | (892,763 | ) | $ | (14 | ) | $ | 59,757 | ||||||
Net loss |
— | — | — | (35,670 | ) | — | (35,670 | ) | |||||||||||
Other comprehensive loss |
— | — | — | — | (70 | ) | (70 | ) | |||||||||||
Issuance of common stock in connection with public offering, net of issuance costs of $6,695 |
25,875,000 | 26 | 96,779 | — | — | 96,805 | |||||||||||||
Stock-based compensation related to issuance of common stock and options in exchange for services |
71,239 | — | 253 | — | — | 253 | |||||||||||||
Issuance of common stock upon net exercise of warrants |
168,039 | — | — | — | — | — | |||||||||||||
Issuances of common stock under equity plans, net of cancellations of non-vested restricted stock |
564,950 | — | 1,555 | — | — | 1,555 | |||||||||||||
Stock-based compensation for equity-based awards to employees and directors |
— | — | 7,658 | — | — | 7,658 | |||||||||||||
401(k) contribution |
72,694 | — | 424 | — | — | 424 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2014 |
157,429,871 | 157 | 1,059,072 | (928,433 | ) | (84 | ) | 130,712 | |||||||||||
Net income |
— | — | — | 46 | — | 46 | |||||||||||||
Other comprehensive loss |
— | — | — | — | (129 | ) | (129 | ) | |||||||||||
Stock-based compensation related to issuance of common stock and options in exchange for services |
18,077 | — | 364 | — | — | 364 | |||||||||||||
Issuance of common stock upon exercise of warrants |
235,000 | 1 | 880 | — | — | 881 | |||||||||||||
Issuances of common stock under equity plans, net of cancellations of non-vested restricted stock |
1,098,411 | 1 | 1,693 | — | — | 1,694 | |||||||||||||
Stock-based compensation for equity-based awards to employees and directors |
— | — | 8,397 | — | — | 8,397 | |||||||||||||
401(k) contribution |
— | — | 161 | — | — | 161 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2015 |
158,781,359 | 159 | 1,070,567 | (928,387 | ) | (213 | ) | 142,126 | |||||||||||
Net loss |
— | — | — | (29,537 | ) | — | (29,537 | ) | |||||||||||
Other comprehensive income |
— | — | — | — | 160 | 160 | |||||||||||||
Stock-based compensation related to issuance of common stock and options in exchange for services |
21,541 | — | 156 | — | — | 156 | |||||||||||||
Issuances of common stock under equity plans |
355,736 | — | 1,169 | — | — | 1,169 | |||||||||||||
Stock-based compensation for equity-based awards to employees and directors |
— | — | 8,245 | — | — | 8,245 | |||||||||||||
401(k) contribution |
— | — | 61 | — | — | 61 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2016 |
159,158,636 | $ | 159 | $ | 1,080,198 | $ | (957,924 | ) | $ | (53 | ) | $ | 122,380 | ||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
See accompanying notes.
90
GERON CORPORATION
STATEMENTS OF CASH FLOWS
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||||||
| |
(In thousands) |
|||||||||
Cash flows from operating activities: |
||||||||||
Net (loss) income |
$ | (29,537 | ) | $ | 46 | $ | (35,670 | ) | ||
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: |
||||||||||
Depreciation and amortization |
81 | 56 | 47 | |||||||
Accretion and amortization on investments, net |
552 | 2,098 | 2,889 | |||||||
(Gain) loss on sales/retirement of property and equipment |
(16 | ) | — | 3 | ||||||
Loss on sales of marketable securities |
— | 1 | — | |||||||
Stock-based compensation for services by non-employees |
156 | 364 | 253 | |||||||
Stock-based compensation for employees and directors |
8,245 | 8,397 | 7,658 | |||||||
Amortization related to 401(k) contributions |
61 | 161 | 111 | |||||||
Unrealized gain on derivatives |
— | (16 | ) | (351 | ) | |||||
Changes in assets and liabilities: |
||||||||||
Interest and other receivables |
731 | (243 | ) | (399 | ) | |||||
Prepaid assets |
123 | 89 | (72 | ) | ||||||
Deposits and other assets |
— | — | 5 | |||||||
Accounts payable |
65 | (873 | ) | (364 | ) | |||||
Accrued compensation and benefits |
(183 | ) | (1,187 | ) | 486 | |||||
Accrued collaboration charges |
1,039 | 2,328 | — | |||||||
Accrued liabilities |
314 | (417 | ) | (246 | ) | |||||
Deferred revenue |
— | (35,000 | ) | 35,000 | ||||||
| | | | | | | | | | | |
Net cash (used in) provided by operating activities |
(18,369 | ) | (24,196 | ) | 9,350 | |||||
Cash flows from investing activities: |
||||||||||
Restricted cash transfer |
(1 | ) | (1 | ) | 529 | |||||
Purchases of property and equipment |
(57 | ) | (90 | ) | (131 | ) | ||||
Proceeds from sales of property and equipment |
16 | — | — | |||||||
Purchases of marketable securities |
(129,250 | ) | (206,459 | ) | (190,263 | ) | ||||
Proceeds from sales/calls of marketable securities |
— | 4,242 | 10,549 | |||||||
Proceeds from maturities of marketable securities |
138,054 | 202,381 | 101,412 | |||||||
| | | | | | | | | | | |
Net cash provided by (used in) investing activities |
8,762 | 73 | (77,904 | ) | ||||||
Cash flows from financing activities: |
||||||||||
Proceeds from issuance of common stock, net of issuance costs |
1,169 | 2,575 | 98,360 | |||||||
| | | | | | | | | | | |
Net cash provided by financing activities |
1,169 | 2,575 | 98,360 | |||||||
| | | | | | | | | | | |
Net (decrease) increase in cash and cash equivalents |
(8,438 | ) | (21,548 | ) | 29,806 | |||||
Cash and cash equivalents, at beginning of year |
21,248 | 42,796 | 12,990 | |||||||
| | | | | | | | | | | |
Cash and cash equivalents, at end of year |
$ | 12,810 | $ | 21,248 | $ | 42,796 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes.
91
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization
Geron Corporation, or we or Geron, was incorporated in the State of Delaware on November 28, 1990. We are a biopharmaceutical company that currently supports the clinical stage development of a telomerase inhibitor, imetelstat, in hematologic myeloid malignancies, by Janssen Biotech, Inc., or Janssen. Principal activities to date have included obtaining financing, securing operating facilities and conducting research and development. In November 2014, we entered into an exclusive collaboration and license agreement, or the Collaboration Agreement, with Janssen to develop and commercialize imetelstat worldwide for all indications in oncology, including hematologic myeloid malignancies, and all other human therapeutic uses. Under the Collaboration Agreement, Janssen is wholly responsible for the worldwide development, manufacturing, seeking regulatory approval for and commercialization of, imetelstat, which was our sole product candidate.
Net Income (Loss) Per Share
Basic net income (loss) per share is calculated by dividing net income (loss) by the weighted-average number of shares of common stock outstanding during the periods presented, without consideration of common stock equivalents. Diluted net income per share is calculated by adjusting the weighted-average number of shares of common stock outstanding for the dilutive effect of common stock equivalents outstanding for the periods presented, as determined using the treasury-stock method. Potential dilutive securities primarily consist of outstanding stock options, restricted stock awards and warrants to purchase our common stock. For periods in which we have incurred a net loss, common stock equivalents outstanding for the periods presented, as determined using the treasury-stock method, are excluded, as their effect would be anti-dilutive, resulting in the same number of shares being used for the calculation of basic and diluted net loss per share. For all periods presented in the accompanying statements of operations, the net income (loss) applicable to common stockholders is equal to the reported net income (loss).
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (In thousands, except share and per share data) |
2016 | 2015 | 2014 | |||||||
Net (loss) income |
$ | (29,537 | ) | $ | 46 | $ | (35,670 | ) | ||
Weighted-average shares: |
||||||||||
Basic |
159,045,644 | 158,036,162 | 153,540,341 | |||||||
Effect of dilutive securities: |
||||||||||
Stock options and restricted stock awards |
— | 4,627,732 | — | |||||||
| | | | | | | | | | | |
Diluted |
159,045,644 | 162,663,894 | 153,540,341 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Net (loss) income per share: |
||||||||||
Basic |
$ | (0.19 | ) | $ | 0.00 | $ | (0.23 | ) | ||
Diluted |
$ | (0.19 | ) | $ | 0.00 | $ | (0.23 | ) | ||
Because we were in a net loss position for 2016 and 2014, 3,023,520 and 3,072,340 common stock equivalents, respectively, related to outstanding stock options, restricted stock awards and warrants (as determined using the treasury-stock method at the estimated average market value) were excluded from the diluted net loss per share calculation as their effect would have been anti-dilutive. In addition for 2016, 2015 and 2014, 11,352,766, 9,375,851 and 9,113,088 potentially dilutive securities, respectively,
92
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
were excluded from the treasury-stock method and calculation of diluted net income (loss) per share as their effect would have been anti-dilutive.
Reclassifications
We have reclassified certain prior period financial statement amounts to conform to current period presentation. On the balance sheets and statements of cash flows, amounts in "Accrued restructuring charges" have been reclassified to "Accrued compensation and benefits".
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. On a regular basis, management evaluates these estimates and assumptions. Actual results could differ from those estimates.
Fair Value of Financial Instruments
Cash Equivalents and Marketable Securities
We consider all highly liquid investments with an original maturity of three months or less to be cash equivalents. We are subject to credit risk related to our cash equivalents and marketable securities. We place our cash and cash equivalents in money market funds and cash operating accounts. Our marketable securities include U.S. government-sponsored enterprise securities, commercial paper and corporate notes with original maturities ranging from four to 24 months.
We classify our marketable securities as available-for-sale. We record available-for-sale securities at fair value with unrealized gains and losses reported in accumulated other comprehensive income (loss) in stockholders' equity. Realized gains and losses are included in interest and other income and are derived using the specific identification method for determining the cost of securities sold and have been insignificant to date. Dividend and interest income are recognized when earned and included in interest and other income in our statements of operations. We recognize a charge when the declines in the fair values below the amortized cost basis of our available-for-sale securities are judged to be other-than-temporary. We consider various factors in determining whether to recognize an other-than-temporary charge, including whether we intend to sell the security or whether it is more likely than not that we would be required to sell the security before recovery of the amortized cost basis. Declines in market value associated with credit losses judged as other-than-temporary result in a charge to interest and other income. Other-than-temporary charges not related to credit losses are included in accumulated other comprehensive income (loss) in stockholders' equity. We have not recorded any other-than-temporary impairment charges on our available-for-sale securities for the years ended December 31, 2016, 2015 and 2014. See Note 2 on Fair Value Measurements.
Fair Value of Derivatives
Non-employee options classified as derivative liabilities are marked to fair value at each financial reporting date with any resulting changes in fair value being recorded in the statements of operations as unrealized gain (loss) on derivatives. The non-employee options continue to be reported as a derivative liability until such time as the instruments are exercised or expire, at which time these
93
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
instruments are marked to fair value and reclassified from liabilities to stockholders' equity. As of March 31, 2015, all non-employee options classified as derivative liabilities expired unexercised.
Revenue Recognition
We recognize revenue for each unit of accounting when all of the following criteria have been met: (a) persuasive evidence of an arrangement exists, (b) delivery has occurred or services have been rendered, (c) the seller's price to the buyer is fixed or determinable, and (d) collectability is reasonably assured. Amounts received prior to satisfying these revenue recognition criteria are recorded as deferred revenue. Amounts expected to be recognized as revenue within the 12 months following the balance sheet date are classified as current deferred revenue. Amounts not expected to be recognized as revenue within the 12 months following the balance sheet date are classified as noncurrent deferred revenue.
License and/or Collaboration Agreements
In addition to the Collaboration Agreement (which is more fully described in Note 4 on License Agreements), we have entered into several license or collaboration agreements with various oncology, diagnostics, research tools and biologics production companies. Economic terms in these agreements may include non-refundable upfront license payments in cash or equity securities, option payments in cash or equity securities, cost reimbursements, cost-sharing arrangements, milestone payments, royalties on future sales of products, or any combination of these items. In applying the appropriate revenue recognition guidance related to these agreements, we first assess whether the arrangement contains multiple elements. In this evaluation, we consider: (i) the deliverables included in the arrangement and (ii) whether the individual deliverables represent separate units of accounting or whether they must be accounted for as a combined unit of accounting. This evaluation involves subjective determinations and requires us to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. Deliverables are considered separate units of accounting provided that: (i) the delivered item(s) has value to the customer on a standalone basis, and (ii) if the arrangement includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in our control. In assessing whether an item has standalone value, we consider factors such as the research, manufacturing and commercialization capabilities of the collaboration partner or licensee and the availability of the associated expertise in the general marketplace. In addition, we consider whether the collaboration partner or licensee can use the other deliverable(s) for their intended purpose without the receipt of the remaining element(s), whether the value of the deliverable is dependent on the undelivered item(s) and whether there are other vendors that can provide the undelivered element(s).
Arrangement consideration that is fixed or determinable is allocated among the separate units of accounting using the relative selling price method. We then apply the applicable revenue recognition criteria noted above to each of the separate units of accounting in determining the appropriate period and pattern of recognition. We determine how to allocate arrangement consideration to identified units of accounting based on the selling price hierarchy provided under relevant accounting guidance. The estimated fair value of deliverables under the arrangement may be derived using a best estimate of selling price if vendor-specific-objective evidence and third-party evidence are not available.
94
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Upfront non-refundable signing, license or non-exclusive option fees are recognized as revenue: (i) when rights to use the intellectual property have been delivered, if the license has standalone value from the other deliverables to be provided under the agreement, or (ii) over the term of the agreement if we have continuing performance obligations, as the arrangement would be accounted for as a single unit of accounting. When payments are received in equity securities, we do not recognize any revenue unless such securities are determined to be realizable in cash.
At the inception of an arrangement that includes milestone payments, we assess whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether: (i) the consideration is commensurate with either the performance to achieve the milestone or the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the performance to achieve the milestone, (ii) the consideration relates solely to past performance and (iii) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We consider various factors, such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone and the level of effort and investment required to achieve the respective milestone, in making this assessment. There is considerable judgment involved in determining whether a milestone satisfies all of the criteria required to conclude that a milestone is substantive. Milestone payments for milestones that are considered substantive would be recognized as revenue in their entirety upon successful accomplishment of the milestone, assuming all other revenue recognition criteria are met. Milestone payments for milestones that are not considered substantive would be recognized as revenue over the remaining period of performance, assuming all other revenue recognition criteria are met.
Royalties are recognized as earned in accordance with contract terms when royalties from licensees can be reasonably estimated and collectability is reasonably assured. If royalties cannot be reasonably estimated or collectability of a royalty amount is not reasonably assured, royalties are recognized as revenue when the cash is received. Revenue from commercial milestone payments is accounted for as royalties and recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met.
Cost-sharing expenses are recorded as earned or owed based on the performance requirements by both parties under the respective contracts. For arrangements in which we and our collaboration partner in the agreement are exposed to significant risks and rewards that depend on the commercial success of the activity, we recognize payments between the parties on a net basis and record such amounts as a reduction or addition to research and development expense. For arrangements in which we have agreed to perform certain research and development services for our collaboration partner and are not exposed to significant risks and rewards that depend on the commercial success of the activity, we recognize the respective cost reimbursements as revenue under the collaborative agreement as the related research and development services are rendered.
Restricted Cash
Restricted cash consists of funds maintained in a separate certificate of deposit account for credit card purchases.
95
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Research and Development Expenses
Research and development expenses consist of expenses incurred in identifying, developing and testing product candidates resulting from our independent efforts as well as efforts associated with collaborations. These expenses include, but are not limited to, in-process research and development acquired in an asset acquisition and deemed to have no alternative future use, payroll and personnel expense, lab supplies, preclinical studies, clinical trials, including support for investigator-sponsored clinical trials, raw materials to manufacture clinical trial drugs, manufacturing costs for research and clinical trial materials, sponsored research at other labs, consulting, costs to maintain technology licenses, our proportionate share of research and development costs under cost-sharing arrangements with collaboration partners and research-related overhead. Research and development costs are expensed as incurred, including costs incurred under our collaboration and/or license agreements.
Clinical Trial Costs
Prior to our collaboration with Janssen for imetelstat, substantial portions of our preclinical studies and all of our clinical trials were performed by third-party contract research organizations, or CROs, and other vendors. We accrued expenses for these activities based upon the estimated amount of work completed on each study. For our clinical trial expenses, the significant factors used in estimating accruals included the number of patients enrolled, the number of active clinical sites and the duration for which the patients had been enrolled in the study. For the clinical development activities being conducted by Janssen under the Collaboration Agreement, we monitor patient enrollment levels and related activities to the extent possible through discussions with Janssen personnel and base our estimates on the best information available at the time. However, additional information may become available to us which would allow us to make a more accurate estimate in future periods. In this event, we may be required to record adjustments to research and development expenses in future periods when the actual level of activity becomes more certain.
Depreciation and Amortization
We record property and equipment at cost and calculate depreciation using the straight-line method over the estimated useful lives of the assets, generally four years. Leasehold improvements are amortized over the shorter of the estimated useful life or remaining term of the lease.
Stock-Based Compensation
We maintain various stock incentive plans under which stock options and restricted stock awards are granted to employees, directors and consultants. We also have an employee stock purchase plan for all eligible employees. We recognize stock-based compensation expense on a straight-line basis over the requisite service period, which is generally the vesting period. For additional information, see Note 8 on Stockholders' Equity.
Stock Options and Employee Stock Purchase Plan
We grant service-based stock options under our equity plans to employees, directors and consultants. The vesting period for employee options is generally four years. We use the Black Scholes option-pricing model to estimate the grant-date fair value of our stock options and employee stock plan purchases. The determination of fair value for these stock-based awards on the date of grant using the
96
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Black Scholes option-pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. For additional information, see Note 8 on Stockholders' Equity.
Restricted Stock Awards
Prior to 2012, we granted restricted stock awards to employees and non-employee directors with service-based vesting schedules that generally vested annually over four years. The fair value for service-based restricted stock awards was determined using the fair value of our common stock on the date of grant. The fair value was amortized as stock-based compensation expense over the requisite service period of the award, which was generally the vesting period, on a straight-line basis and was reduced for estimated forfeitures, as applicable.
Non-Employee Stock-Based Awards
For our non-employee stock-based awards, the measurement date on which the fair value of the stock-based award is calculated is equal to the earlier of: (i) the date at which a commitment for performance by the counterparty to earn the equity instrument is reached or (ii) the date at which the counterparty's performance is complete. We recognize stock-based compensation expense for the fair value of the vested portion of non-employee stock-based awards in our statements of operations.
Accumulated Other Comprehensive Loss
Accumulated other comprehensive loss includes certain changes in stockholders' equity which are excluded from net income (loss). Accumulated other comprehensive loss on our balance sheets as of December 31, 2016 and 2015 is solely comprised of net unrealized losses on marketable securities.
Income Taxes
We maintain deferred tax assets and liabilities that reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes and are subject to tests of recoverability. Our deferred tax assets include net operating loss carryforwards, research credits and capitalized research and development. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Our net deferred tax asset has been fully offset by a valuation allowance because of our history of losses. Any potential accrued interest and penalties related to unrecognized tax benefits would be recorded as income tax expense.
Concentrations of Customers and Suppliers
The majority of our revenues was earned in the United States. Approximately 81% of our 2016 revenues represented an upfront payment from Janssen Pharmaceuticals, Inc., or Janssen Pharmaceuticals, in connection with a License Agreement signed in September 2016. Approximately 96% of our 2015 revenues represented an upfront payment from Janssen under the imetelstat Collaboration Agreement. Two other customers accounted for approximately 31% of our 2014 revenues.
97
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
In accordance with the Collaboration Agreement, Janssen is now responsible for the manufacture and management of the supply of imetelstat on a global basis for clinical trials and, after any regulatory approval, all commercial activities. Janssen contracts third-party manufacturers to produce GMP-grade drugs for preclinical and clinical studies. Janssen also contracts for starting materials to supply those manufacturers and for its own use. Certain development and clinical activities may be delayed if Janssen is unable to obtain sufficient quantities of starting materials or GMP-grade drugs from current third-party suppliers or other third-party sources.
Segment Information
Our executive management team represents our chief decision maker. We view our operations as a single segment, the development of therapeutic products for oncology. As a result, the financial information disclosed herein materially represents all of the financial information related to our principal operating segment.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update No. 2014-09, or ASU 2014-09, which creates Accounting Standards Codification Topic 606, Revenue from Contracts with Customers, or Topic 606, and supersedes the revenue recognition requirements in Accounting Standards Codification Topic 605, Revenue Recognition, including most industry-specific revenue recognition guidance throughout the Industry Topics of the Codification. In summary, the core principle of Topic 606 is to recognize revenue when promised goods or services are transferred to customers in an amount that reflects the consideration that is expected to be received for those goods or services. Companies are allowed to select between two transition methods: (1) a full retrospective transition method with the application of the new guidance to each prior reporting period presented, or (2) a retrospective transition method that recognizes the cumulative effect on prior periods at the date of adoption together with additional footnote disclosures. The amendments in ASU 2014-09 are effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period. Earlier application is permitted only as of annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. In March, April, May and December 2016, the FASB issued Accounting Standards Update No. 2016-08 (Topic 606), Revenue From Contracts With Customers: Principal vs. Agent Considerations, or ASU 2016-08, Accounting Standards Update No. 2016-10 (Topic 606), Revenue From Contracts with Customers: Identifying Performance Obligations and Licensing, or ASU 2016-10, Accounting Standards Update No. 2016-12 (Topic 606), Revenue From Contracts with Customers: Narrow-Scope Improvements and Practical Expedients, or ASU 2016-12, and Accounting Standards Update No. 2016-20 (Topic 606), Revenue from Contracts with Customers: Technical Corrections and Improvements to Topic 606, or ASU 2016-20, respectively, to provide supplemental adoption guidance and clarification to ASU 2014-09. We do not plan to early adopt these standards.
We currently anticipate adopting ASU 2014-09 and its related supplemental guidance using the full retrospective transition method to restate each prior reporting period presented in our financial statements. While we are continuing to assess the effect of this new standard, we have not identified any material differences in the accounting treatment under ASU 2014-09 compared to the current accounting treatment for the Collaboration Agreement with Janssen, which is currently the most material agreement to our financial statements. However, such assessment is preliminary and subject to
98
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
change. Our analysis of other agreements could identify material changes from the current accounting treatment. ASU 2014-09 differs from the current accounting standard in many respects, such as in the accounting for variable consideration, including milestone payments. Under our current accounting policy, we recognize milestone revenue using the milestone method specified in ASC 605-28, Milestone Method of Revenue Recognition, which generally results in the recognition of milestone payments as revenue in the period that the milestone is achieved. However, under the new accounting standard, it is possible to start to recognize milestone revenue before the milestone is achieved, subject to management's assessment of whether it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
The new revenue standard is principle based and interpretation of those principles may vary from company to company based on their unique circumstances. It is possible that interpretation, industry practice, and guidance may evolve as companies and the accounting profession work to implement this new standard. We are still in the process of evaluating the effect of the new standard on our historical financial statements. While we have not completed our evaluation, we currently believe the impact to revenue and expense recognized will not be material to any of the years presented. As we complete our evaluation of this new standard, new information may arise that could change our current understanding of the impact to revenue and expense recognized. Additionally, we will continue to monitor industry activities and any additional guidance provided by regulators, standards setters, or the accounting profession and adjust our assessment and implementation plans accordingly.
In November 2015, the FASB issued Accounting Standards Update No. 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes, or ASU 2015-17. Current generally accepted accounting principles require an entity to separate deferred income tax liabilities and assets into current and noncurrent amounts in a classified statement of financial position. To simplify the presentation of deferred income taxes, ASU 2015-17 requires that deferred tax liabilities and assets be classified as noncurrent in a statement of financial position. The current requirement that deferred tax liabilities and assets of a tax paying component of an entity be offset and presented as a single amount is not affected by the amendments in this update. The amendments in this update are effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods. We plan to adopt ASU 2015-17 commencing in 2017 and do not expect the adoption of this guidance to have any impact on our financial statements and related disclosures.
In February 2016, the FASB issued Accounting Standards Update No. 2016-02, Leases (Topic 842), or ASU 2016-02. ASU 2016-02 requires an entity to recognize a right-of-use asset and lease liability for all leases with terms of more than 12 months. Recognition, measurement and presentation of expenses will depend on classification as a finance or operating lease. Certain quantitative and qualitative disclosures about leasing arrangements also are required. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early adoption is permitted. The updated guidance requires a modified retrospective adoption. We are currently evaluating the impact that the adoption of ASU 2016-02 will have on our financial statements and related disclosures and have not made any decision regarding the timing of adoption.
In March 2016, the FASB issued Accounting Standards Update No. 2016-09, Improvements to Employee Share-Based Payment Accounting, or ASU 2016-09, which amends Accounting Standards Codification Topic 718, Compensation—Stock Compensation. ASU 2016-09 simplifies several aspects of
99
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities and classification on the statement of cash flows. ASU 2016-09 is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods and early adoption is permitted. We plan to adopt ASU 2016-09 commencing in 2017 and do not expect the adoption of this guidance to have a material impact on our financial statements and related disclosures.
In June 2016, the FASB issued Accounting Standard Update No. 2016-13, Measurement of Credit Losses on Financial Instruments, or ASU 2016-13. ASU 2016-13 amends the guidance for measuring and recording credit losses on financial assets measured at amortized cost by replacing the "incurred loss" model with an "expected loss" model. Accordingly, these financial assets will be presented at the net amount expected to be collected. ASU 2016-13 also requires that credit losses related to available-for-sale debt securities be recorded through an allowance for such losses rather than reducing the carrying amount under the current other-than-temporary-impairment model. ASU 2016-13 is effective for interim and annual periods beginning after December 15, 2019. Early adoption is permitted for interim and annual periods beginning after December 15, 2018. We are currently evaluating the impact of the adoption of ASU 2016-13 on our financial statements and related disclosures and have not made any decision regarding the timing of adoption.
In August 2016, the FASB issued Accounting Standards Update No. 2016-15, Classification of Certain Cash Receipts and Cash Payments, or ASU 2016-15, to clarify how certain cash receipts and cash payments are presented and classified in the statement of cash flows. ASU 2016-15 is effective for annual periods beginning after December 15, 2017, and interim periods within those annual periods and early adoption is permitted. ASU 2016-15 must be applied retrospectively to each period presented. We do not plan to early adopt ASU 2016-15. We are currently evaluating the impact of the adoption of ASU 2016-15 on our financial statements and related disclosures.
In November 2016, the FASB issued Accounting Standards Update No. 2016-18, Statement of Cash Flows (Topic 230) Restricted Cash, or ASU 2016-18, to address the diversity in practice in the classification and presentation of changes in restricted cash on the statement of cash flows. ASU 2016-18 is effective for annual periods beginning after December 15, 2017, and interim periods within those annual periods. Early adoption is permitted, including adoption in an interim period which would require any adjustments to be reflected as of the beginning of the annual period that includes that interim period. ASU 2016-18 must be applied using a retrospective transition method to each period presented. We do not plan to early adopt ASU 2016-18. We are currently evaluating the impact of the adoption of ASU 2016-18 on our financial statements and related disclosures.
With the exception of the standards discussed above, there have been no new accounting pronouncements not yet effective that have significance, or potential significance, to our financial statements.
100
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
2. FAIR VALUE MEASUREMENTS
We categorize financial instruments recorded at fair value on our balance sheets based upon the level of judgment associated with inputs used to measure their fair value. The categories are as follows:
|
Level 1—Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date. An active market for the asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. |
|
|
Level 2—Inputs (other than quoted market prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument's anticipated life. |
|
|
Level 3—Inputs reflect management's best estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model. |
A financial instrument's categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. Below is a description of the valuation methodologies used for financial instruments measured at fair value on our balance sheets, including the category for such financial instruments.
Cash Equivalents and Marketable Securities Available-for-Sale
Certificates of deposit and money market funds are categorized as Level 1 within the fair value hierarchy as their fair values are based on quoted prices available in active markets. U.S. government-sponsored enterprise securities, commercial paper and corporate notes are categorized as Level 2 within the fair value hierarchy as their fair values are estimated by using pricing models, quoted prices of securities with similar characteristics or discounted cash flows.
101
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
2. FAIR VALUE MEASUREMENTS (Continued)
Cash equivalents, restricted cash and marketable securities by security type at December 31, 2016 were as follows:
| (In thousands) |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Included in cash and cash equivalents: |
|||||||||||||
Money market funds |
$ | 11,193 | $ | — | $ | — | $ | 11,193 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Restricted cash: |
|||||||||||||
Certificate of deposit |
$ | 268 | $ | — | $ | — | $ | 268 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Marketable securities: |
|||||||||||||
Government-sponsored enterprise securities (due in less than one year) |
$ | 5,000 | $ | — | $ | (3 | ) | $ | 4,997 | ||||
Government-sponsored enterprise securities (due in one to two years) |
12,500 | — | (42 | ) | 12,458 | ||||||||
Commercial paper (due in less than one year) |
31,024 | 50 | (5 | ) | 31,069 | ||||||||
Corporate notes (due in less than one year) |
66,012 | 4 | (47 | ) | 65,969 | ||||||||
Corporate notes (due in one to two years) |
1,506 | — | (10 | ) | 1,496 | ||||||||
| | | | | | | | | | | | | | |
|
$ | 116,042 | $ | 54 | $ | (107 | ) | $ | 115,989 | ||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Cash equivalents, restricted cash and marketable securities by security type at December 31, 2015 were as follows:
| (In thousands) |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Included in cash and cash equivalents: |
|||||||||||||
Money market funds |
$ | 4,577 | $ | — | $ | — | $ | 4,577 | |||||
Government-sponsored enterprise securities |
1,999 | — | — | 1,999 | |||||||||
Commercial paper |
7,599 | — | — | 7,599 | |||||||||
Corporate notes |
5,002 | — | (1 | ) | 5,001 | ||||||||
| | | | | | | | | | | | | | |
|
$ | 19,177 | $ | — | $ | (1 | ) | $ | 19,176 | ||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Restricted cash: |
|||||||||||||
Certificate of deposit |
$ | 267 | $ | — | $ | — | $ | 267 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Marketable securities: |
|||||||||||||
Government-sponsored enterprise securities (due in one to two years) |
$ | 10,007 | $ | — | $ | (57 | ) | $ | 9,950 | ||||
Commercial paper (due in less than one year) |
27,661 | 49 | (2 | ) | 27,708 | ||||||||
Corporate notes (due in less than one year) |
64,892 | 1 | (77 | ) | 64,816 | ||||||||
Corporate notes (due in one to two years) |
22,837 | — | (126 | ) | 22,711 | ||||||||
| | | | | | | | | | | | | | |
|
$ | 125,397 | $ | 50 | $ | (262 | ) | $ | 125,185 | ||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
102
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
2. FAIR VALUE MEASUREMENTS (Continued)
Cash equivalents and marketable securities with unrealized losses at December 31, 2016 and 2015 were as follows:
| |
Less Than 12 Months | 12 Months or Greater | Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (In thousands) |
Estimated Fair Value |
Gross Unrealized Losses |
Estimated Fair Value |
Gross Unrealized Losses |
Estimated Fair Value |
Gross Unrealized Losses |
|||||||||||||
As of December 31, 2016: |
|||||||||||||||||||
Government-sponsored enterprise securities (due in less than one year) |
$ | 4,997 | $ | (3 | ) | $ | — | $ | — | $ | 4,997 | $ | (3 | ) | |||||
Government-sponsored enterprise securities (due in one to two years) |
12,458 | (42 | ) | — | — | 12,458 | (42 | ) | |||||||||||
Commercial paper (due in less than one year) |
8,365 | (5 | ) | — | — | 8,365 | (5 | ) | |||||||||||
Corporate notes (due in less than one year) |
39,218 | (37 | ) | 6,944 | (10 | ) | 46,162 | (47 | ) | ||||||||||
Corporate notes (due in one to two years) |
1,496 | (10 | ) | — | — | 1,496 | (10 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
|
$ | 66,534 | $ | (97 | ) | $ | 6,944 | $ | (10 | ) | $ | 73,478 | $ | (107 | ) | ||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
As of December 31, 2015: |
|||||||||||||||||||
Government-sponsored enterprise securities (due in one to two years) |
$ | 9,950 | $ | (57 | ) | $ | — | $ | — | $ | 9,950 | $ | (57 | ) | |||||
Commercial paper (due in less than one year) |
7,834 | (2 | ) | — | — | 7,834 | (2 | ) | |||||||||||
Corporate notes (due in less than one year) |
61,006 | (71 | ) | 6,301 | (7 | ) | 67,307 | (78 | ) | ||||||||||
Corporate notes (due in one to two years) |
22,711 | (126 | ) | — | — | 22,711 | (126 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
|
$ | 101,501 | $ | (256 | ) | $ | 6,301 | $ | (7 | ) | $ | 107,802 | $ | (263 | ) | ||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The gross unrealized losses related to government-sponsored enterprise securities, commercial paper and corporate notes as of December 31, 2016 and 2015 were due to changes in interest rates. We determined that the gross unrealized losses on our cash equivalents and marketable securities as of December 31, 2016 and 2015 were temporary in nature. We review our investments quarterly to identify and evaluate whether any investments have indications of possible impairment. Factors considered in determining whether a loss is temporary include the length of time and extent to which the fair value has been less than the cost basis and whether we intend to sell the security or whether it is more likely than not that we would be required to sell the security before recovery of the amortized cost basis. We currently do not intend to sell these securities before recovery of their amortized cost bases.
103
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
2. FAIR VALUE MEASUREMENTS (Continued)
Fair Value on a Recurring Basis
The following table presents information about our financial instruments that are measured at fair value on a recurring basis as of December 31, 2016 and indicates the fair value category assigned.
| |
Fair Value Measurements at Reporting Date Using | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
|
|||||||||
| (In thousands) |
Level 1 | Level 2 | Level 3 | Total | |||||||||
Assets |
|||||||||||||
Money market funds(1) |
$ | 11,193 | $ | — | $ | — | $ | 11,193 | |||||
Government-sponsored enterprise securities(2)(3) |
— | 17,455 | — | 17,455 | |||||||||
Commercial paper(2) |
— | 31,069 | — | 31,069 | |||||||||
Corporate notes(2)(3) |
— | 67,465 | — | 67,465 | |||||||||
| | | | | | | | | | | | | | |
Total |
$ | 11,193 | $ | 115,989 | $ | — | $ | 127,182 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The following table presents information about our financial instruments that are measured at fair value on a recurring basis as of December 31, 2015 and indicates the fair value category assigned.
| |
Fair Value Measurements at Reporting Date Using | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
|
|||||||||
| (In thousands) |
Level 1 | Level 2 | Level 3 | Total | |||||||||
Assets |
|||||||||||||
Money market funds(1) |
$ | 4,577 | $ | — | $ | — | $ | 4,577 | |||||
Government-sponsored enterprise securities(1)(3) |
— | 11,949 | — | 11,949 | |||||||||
Commercial paper(1)(2) |
— | 35,307 | — | 35,307 | |||||||||
Corporate notes(1)(2)(3) |
— | 92,528 | — | 92,528 | |||||||||
| | | | | | | | | | | | | | |
Total |
$ | 4,577 | $ | 139,784 | $ | — | $ | 144,361 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- (1)
- Included
in cash and cash equivalents on our balance sheets.
- (2)
- Included
in current portion of marketable securities on our balance sheets.
- (3)
- Included in noncurrent portion of marketable securities on our balance sheets.
Credit Risk
We currently place our cash, restricted cash, cash equivalents and marketable securities with four financial institutions in the United States. Generally, these deposits may be redeemed upon demand and therefore, bear minimal risk. Deposits with banks may exceed the amount of insurance provided on such deposits. Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash equivalents and marketable securities. Cash equivalents and marketable securities currently consist of money market funds, U.S. government-sponsored enterprise securities, commercial
104
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
2. FAIR VALUE MEASUREMENTS (Continued)
paper and corporate notes. Our investment policy, approved by the audit committee of our board of directors, limits the amount we may invest in any one type of investment issuer, thereby reducing credit risk concentrations.
3. PROPERTY AND EQUIPMENT
Property and equipment, stated at cost, is comprised of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| (In thousands) |
2016 | 2015 | |||||
Furniture and computer equipment |
$ | 1,268 | $ | 1,224 | |||
Lab equipment |
12 | 130 | |||||
Leasehold improvements |
111 | 98 | |||||
| | | | | | | | |
|
1,391 | 1,452 | |||||
Less accumulated depreciation and amortization |
(1,208 | ) | (1,245 | ) | |||
| | | | | | | | |
|
$ | 183 | $ | 207 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
4. LICENSE AGREEMENTS
Janssen Biotech, Inc. Collaboration and License Agreement
On November 13, 2014, we and Janssen entered into the Collaboration Agreement under which we granted to Janssen exclusive worldwide rights to develop and commercialize imetelstat for all human therapeutic uses, including hematologic myeloid malignancies. Upon the effectiveness of the Collaboration Agreement, we received $35,000,000 from Janssen as an upfront payment, which we classified as deferred revenue upon receipt.
Under the Collaboration Agreement, Janssen is wholly responsible for the development, manufacturing, seeking regulatory approval for and commercialization of, imetelstat worldwide. To that end, Janssen is currently proceeding with the development of imetelstat with two clinical trials: a Phase 2 trial in myelofibrosis, referred to as IMbark, and a Phase 2/3 trial in myelodysplastic syndromes, referred to as IMerge. Development costs for IMbark and IMerge are being shared between us and Janssen on a 50/50 basis. Additionally, under the terms of the Collaboration Agreement, we remain responsible for prosecuting, at Janssen's direction, the patents licensed to Janssen at the time we entered into the Collaboration Agreement, with costs shared between us and Janssen on a 50/50 basis. The cost-sharing arrangement with Janssen began in January 2015. As of December 31, 2016, accrued collaboration charges of $3,367,000 on our balance sheet represent the net amount owed to Janssen for our proportionate share of research and development costs incurred by Janssen under the Collaboration Agreement for the three months ended December 31, 2016.
Following the protocol-specified primary analysis of IMbark or a certain time period after the initiation of the first Phase 3 myelofibrosis study, if any, Janssen must notify us of their decision, or a Continuation Decision, as to whether they elect to maintain the license rights granted to them under the Collaboration Agreement and continue to advance the development of imetelstat in any indication. In the event that IMbark is terminated early, or placed on clinical hold or suspended by a regulatory
105
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
4. LICENSE AGREEMENTS (Continued)
authority for an extended period of time, then Janssen must instead notify us of their Continuation Decision by the date that is approximately 24 months after the initiation of IMerge.
In the event that Janssen provides an affirmative Continuation Decision, we then would have an option, or the U.S. Opt-In Rights, to share further U.S. development and promotion costs, including our share of development costs incurred to date by Janssen beyond IMbark or IMerge, in exchange for higher tiered royalty rates and higher future development and regulatory milestone payments if imetelstat is successfully developed and approved. If we exercise the U.S. Opt-In Rights, then we and Janssen would share U.S. development and promotion costs beyond IMbark and IMerge on a 20/80 basis (Geron 20%, Janssen 80%), we would receive a $65,000,000 milestone payment, or the Continuation Fee, at the time of an affirmative Continuation Decision, and would be eligible to receive additional potential payments of up to $470,000,000 for the achievement of certain development and regulatory milestones, up to $350,000,000 for the achievement of certain sales milestones, and tiered royalties ranging from a mid-teens up to low twenties percentage rate on worldwide net sales of imetelstat in any countries where regulatory exclusivity exists or there are valid claims under the patent rights exclusively licensed to Janssen. In addition, if we exercise the U.S. Opt-In Rights, we then would also have a separate option, or the U.S. Co-Promotion Option, to provide 20% of the U.S. selling effort with our sales force personnel, in lieu of funding 20% of U.S. promotion costs, upon regulatory approval and commercial launch of imetelstat in the United States. Such co-promotion would be conducted under a Janssen prepared promotion plan, and in accordance with a co-promotion agreement to be agreed by the parties at the time of our exercise of the U.S. Co-Promotion Option. We would be responsible for all costs associated with establishing and maintaining our sales force in any conduct of such co-promotion. All product sales would be booked by Janssen. If we do not exercise the U.S. Opt-In Rights, then all further development and promotion costs beyond IMbark and IMerge would be borne by Janssen, we would receive the $65,000,000 Continuation Fee at the time of an affirmative Continuation Decision plus a $70,000,000 payment, or the Full U.S. Rights Fee, for Janssen's retention of full U.S. rights to imetelstat, and would be eligible to receive additional potential payments of up to $415,000,000 for the achievement of certain development and regulatory milestones, up to $350,000,000 for the achievement of certain sales milestones, and tiered royalties ranging from a double-digit up to mid-teens percentage rate on worldwide net sales of imetelstat in any countries where regulatory exclusivity exists or there are valid claims under the patent rights exclusively licensed to Janssen.
Under the terms of the Collaboration Agreement, we and Janssen have created a joint governance structure, including joint development and steering committees and working groups, to oversee and manage worldwide regulatory, development and manufacturing work under the joint clinical development plan and promotional activities (assuming we exercise the U.S. Opt-In Rights) for imetelstat, with Janssen responsible for the operational execution of those activities. In addition, both we and Janssen may propose to the joint development committee imetelstat development for any new indications not then provided for in the joint clinical development plan and if we and Janssen agree such development should be conducted outside of the joint clinical development plan, both we and Janssen would be entitled to independently undertake such development at the developing party's own cost, subject to the other party's obligation to provide reimbursement for its specified portion of the development costs plus a premium following marketing approval of imetelstat in such newly proposed indication as a result of such independent development. In the event that we do not exercise the U.S. Opt-In Rights following an affirmative Continuation Decision by Janssen, if any, the joint governance
106
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
4. LICENSE AGREEMENTS (Continued)
structure under the Collaboration Agreement would be dissolved, a joint oversight committee would monitor the progress of the collaboration, and we would have no further rights to conduct any independent imetelstat development.
After an affirmative Continuation Decision by Janssen, the Collaboration Agreement would remain in effect until the expiration of the last-to-expire patent or the royalty obligations on sales of imetelstat cease, unless terminated earlier. If Janssen does not effect an affirmative Continuation Decision, then the Collaboration Agreement would terminate and all rights to the imetelstat program would revert to us. Janssen may terminate the Collaboration Agreement at any time for convenience or due to a safety-related concern. If a notice of termination from Janssen occurs, we would be entitled to certain continued operational support and cost sharing under various circumstances and all rights to the imetelstat program would revert to us.
The terms of the Collaboration Agreement contain multiple deliverables, which included at inception: (i) exclusive worldwide rights to develop and commercialize imetelstat for all indications, (ii) transfer of know-how and intellectual property, including our obligation to procure supply for manufacturing imetelstat for up to nine months after the effective date of the Collaboration Agreement, (iii) participation on the joint committees and working groups and (iv) potential participation in promoting imetelstat in the United States, if approved for commercial sale. We concluded the license for exclusive worldwide rights to develop and commercialize imetelstat has standalone value to Janssen based on the technical and financial resources of Janssen, including Janssen's drug development experience, sizeable employee base with specific experience in hematologic malignancies, and sufficient capital to independently develop imetelstat on a global basis. Since Janssen has final decision-making authority in the event a unanimous decision cannot be reached by the joint committees, we determined our participation on the joint committees does not represent a non-contingent deliverable under the Collaboration Agreement. In addition, we determined our potential participation in promoting imetelstat in the United States does not represent a non-contingent deliverable because such participation is uncertain and dependent on imetelstat being approved for commercial sale, which is not within our control. Accordingly, we determined delivery of the license rights granted by us to Janssen, together with our performance of certain technology transfer-related activities under the Collaboration Agreement, represents the sole non-contingent deliverable under the Collaboration Agreement associated with the upfront payment. Therefore, we accounted for our delivery of the imetelstat license rights and our performance of the technology transfer-related activities as a single unit of accounting. During the third quarter of 2015, we completed performance of the technology transfer-related activities to Janssen as outlined under the Collaboration Agreement. Combining this performance with the delivery of the imetelstat license rights, we fully recognized the $35,000,000 upfront payment from Janssen as collaboration revenue on our statements of operations in the third quarter of 2015.
We have determined that each of the additional potential milestone payments to us under the Collaboration Agreement, including: (i) the Continuation Fee at the time of an affirmative Continuation Decision, (ii) the Full U.S. Rights Fee, if we do not exercise the U.S. Opt-In Rights and (iii) payments based on the achievement of certain development, regulatory or sales milestones, represent substantive milestones. Consequently, we will recognize revenue for these payments in their entirety upon successful accomplishment of the respective milestone. Royalties on future product sales of imetelstat, if successfully commercialized under the Collaboration Agreement, will be recognized as revenue when earned.
107
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
4. LICENSE AGREEMENTS (Continued)
Janssen Pharmaceuticals, Inc. License Agreement
On September 15, 2016, we entered into a license agreement, or License Agreement, with Janssen Pharmaceuticals whereby we granted to Janssen Pharmaceuticals an exclusive worldwide license, or the Exclusive License, under our proprietary patents for the research, development and commercialization of products based on specialized oligonucleotide backbone chemistry and novel amidates for ribonucleic acid interference, or RNAi, for the prevention, treatment and/or diagnosis of any and all human disorders, excluding cancers originating from the blood or bone marrow, and products whose predominant or primary mechanism of action is telomerase inhibition.
In addition to the Exclusive License, we granted to Janssen Pharmaceuticals a non-exclusive worldwide license, or the Non-Exclusive License, under our patents covering the synthesis of monomers, which are the building blocks of oligonucleotides, and certain know-how necessary for the research, development and commercialization of products under the Exclusive License. The patent rights under the Non-Exclusive License are also licensed exclusively to Janssen under the Collaboration Agreement, as described in the section above titled "Janssen Biotech, Inc. Collaboration and License Agreement", for the development and commercialization of imetelstat, and the License Agreement with Janssen Pharmaceuticals expressly excludes, and is subject to, the rights and licenses granted to Janssen under the Collaboration Agreement.
Under the terms of the License Agreement, Janssen Pharmaceuticals, at its sole expense, is required to use reasonable efforts to perform research, development and commercialization activities to obtain at least one licensed product to be researched, developed and commercialized under the License Agreement. We remain responsible for prosecuting the patent rights under the Exclusive License, with reasonable input provided by Janssen Pharmaceuticals, and the costs for such prosecution will be shared between us and Janssen Pharmaceuticals on a 50/50 basis. In addition, we remain responsible for prosecuting the patent rights under the Non-Exclusive License, as set forth under the terms of the Collaboration Agreement with Janssen.
Under the terms of the License Agreement, we received $5,000,000 from Janssen Pharmaceuticals as a non-refundable upfront payment. We are also eligible to receive additional potential payments of up to an aggregate maximum total of $75,000,000 for the achievement of certain development and regulatory milestones and tiered royalties in the low single digit percentage range on worldwide net sales of each licensed product commercialized under the License Agreement in any countries where there are valid claims under the patent rights licensed to Janssen Pharmaceuticals.
The License Agreement will remain in effect until the expiration of the last-to-expire patent, unless terminated earlier. Janssen Pharmaceuticals may also terminate the License Agreement at will upon prior written notice to us. In the event of an early termination of the License Agreement, all licenses to Janssen Pharmaceuticals would terminate.
The terms of the License Agreement contain multiple deliverables, which included at inception the transfer of: (i) license rights under the Exclusive License and (ii) license rights and certain know-how under the Non-Exclusive License. We concluded the License Agreement has standalone value to Janssen Pharmaceuticals based on Janssen Pharmaceuticals' technical and financial resources, including drug development experience, sizeable employee base with specific knowledge of oligonucleotide chemistry, and sufficient capital to independently research, develop and commercialize products under the License Agreement on a global basis. Accordingly, we have determined delivery of the license rights
108
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
4. LICENSE AGREEMENTS (Continued)
granted by us to Janssen Pharmaceuticals under the Exclusive License and Non-Exclusive License, together with the transfer of certain know-how under the Non-Exclusive License, represents the sole non-contingent deliverable under the License Agreement associated with the upfront payment. Therefore, we accounted for our delivery of the license rights and transfer of know-how under the License Agreement as a single unit of accounting. During the third quarter of 2016, we completed the delivery of the license rights and transfer of know-how to Janssen Pharmaceuticals under the License Agreement. Accordingly, we fully recognized the $5,000,000 upfront payment from Janssen Pharmaceuticals as license fee revenue on our statements of operations in the third quarter of 2016.
We have determined that each of the additional potential development and regulatory milestone payments to us under the License Agreement represent substantive milestones. Consequently, we will recognize revenue for these payments in their entirety upon successful accomplishment of the respective milestone. Royalties on potential future product sales under the License Agreement will be recognized as revenue when earned.
5. ACCRUED LIABILITIES
Accrued liabilities consisted of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| (In thousands) |
2016 | 2015 | |||||
Professional legal and accounting fees |
$ | 350 | $ | 481 | |||
Clinical trial costs |
723 | 375 | |||||
Other |
361 | 264 | |||||
| | | | | | | | |
|
$ | 1,434 | $ | 1,120 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
6. RESTRUCTURING
With projected reduced operational demands as a result of the Collaboration Agreement with Janssen, on March 3, 2015, we announced an organizational resizing to reduce our workforce. For the year ended December 31, 2015, we recorded restructuring charges of approximately $1,306,000, net of non-cash adjustments, related to one-time termination benefits. These charges included $307,000 of non-cash stock-based compensation expense relating to the extension of the post-termination exercise period for certain stock options previously granted to employees affected by the restructuring. The restructuring resulted in aggregate cash expenditures of approximately $988,000 after adjustments and non-cash credits. All actions associated with this restructuring were completed in 2015, and we do not anticipate incurring any further charges in connection with this restructuring. As of December 31, 2016, we had no remaining obligations related to the restructuring.
7. COMMITMENTS AND CONTINGENCIES
Securities and Derivative Lawsuits
We and certain of our officers have been named as defendants in two purported class action securities lawsuits filed in the United States District Court for the Northern District of California, or the California District Court, as well as a third securities lawsuit, not styled as a class action, which was originally filed in the United States District Court for the Southern District of Mississippi, and
109
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
7. COMMITMENTS AND CONTINGENCIES (Continued)
subsequently transferred to the California District Court. These three cases, or the Class Action Lawsuits, which are based on the same factual background, have been consolidated for all purposes, and are currently stayed to enable the parties to seek to resolve them. On November 23, 2016, the parties signed a Memorandum of Understanding that set forth material deal points of resolving the Class Action Lawsuits. On December 13, 2016, the parties filed a notice of settlement, which the California District Court signed on December 16, 2016, staying the Class Action Lawsuits pending final approval of a settlement. Final settlement of the Class Action Lawsuits is contingent upon, among other things, approval by the California District Court. Such approval, if any, is not expected until the end of 2017.
Certain of our officers and directors were also named as defendants in four purported stockholder derivative lawsuits, two of which were filed in the Superior Court of California for the County of San Mateo, or the San Mateo County Court, and two of which were filed in the California District Court. These cases were all based on the same factual background and were consolidated in their respective courts. On July 22, 2016, the parties entered into a stipulation to settle all claims in each of the foregoing derivative lawsuits in exchange for our agreement to (i) implement certain corporate governance refinements, and (ii) instruct our insurer to pay in full the plaintiffs' attorneys a total of $950,000. On August 19, 2016, the San Mateo County Court preliminarily approved the proposed settlement. On November 18, 2016, the San Mateo County Court issued an order granting final approval of the settlement and dismissing with prejudice the two derivative lawsuits filed in the San Mateo County Court. On December 6, 2016, the California District Court issued an order dismissing with prejudice the two derivative lawsuits filed in the California District Court. Accordingly, as of December 6, 2016, all of the derivative lawsuits have been fully and finally settled and dismissed. As of December 31, 2016, we have implemented the corporate governance refinements specified in the stipulation of settlement and our insurers have paid the settlement amount in full to the plaintiffs' attorneys.
For a more complete discussion of the Class Action Lawsuits and the derivative lawsuits, see the section entitled "Legal Proceedings" under Item 3 of this annual report on Form 10-K.
It is possible that additional lawsuits will be filed, or allegations will be made by stockholders, with respect to these same or other matters and also naming us and/or our officers and directors as defendants. The Class Action Lawsuits and any other lawsuits are subject to inherent uncertainties, and the actual defense and disposition costs will depend upon many unknown factors. Monitoring, initiating and defending against legal actions is time-consuming for our management, likely to be expensive and may detract from our ability to fully focus our internal resources on our business activities. In addition, despite the availability of insurance, we may incur substantial legal fees and costs in connection with litigation and such amounts could be material to our financial statements. We could be forced to expend significant resources in the implementation of the corporate governance refinements required by the terms of the settlement for the derivative lawsuits, or in the settlement or defense of the Class Action Lawsuits or any other lawsuits, and we may not prevail in such lawsuits. We have not established any reserve for any potential liability relating to any additional lawsuits.
Indemnifications to Officers and Directors
Our corporate bylaws require that we indemnify our officers and directors, as well as those who act as directors and officers of other entities at our request, against expenses, judgments, fines,
110
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
7. COMMITMENTS AND CONTINGENCIES (Continued)
settlements and other amounts actually and reasonably incurred in connection with any proceedings arising out of their services to Geron. In addition, we have entered into separate indemnification agreements with each of our directors and officers which provide for indemnification of these directors and officers under similar circumstances and under additional circumstances. The indemnification obligations are more fully described in our bylaws and the indemnification agreements. We purchase standard insurance to cover claims or a portion of the claims made against our directors and officers. Since a maximum obligation is not explicitly stated in our bylaws or in our indemnification agreements and will depend on the facts and circumstances that arise out of any future claims, the overall maximum amount of the obligations cannot be reasonably estimated.
Operating Lease Commitment
On September 15, 2015, we amended the lease agreement for our premises at 149 Commonwealth Drive, Menlo Park, California, to extend the lease term from February 2016 through January 2018. As of December 31, 2016, operating lease obligations under the amended lease agreement include aggregate future minimum payments of approximately $716,000. Rent expense under our operating leases was approximately $708,000, $878,000 and $936,000 for the years ended December 31, 2016, 2015 and 2014, respectively.
Severance Plan
We have an Amended and Restated Severance Plan, or Severance Plan, that applies to all employees that are not subject to performance improvement plans, and provides for, among other benefits: (i) a severance payment upon a Change of Control Triggering Event and Separation from Service and (ii) a severance payment for each non-executive employee upon a Non-Change of Control Triggering Event and Separation from Service. As defined in the Severance Plan, a Change of Control Triggering Event and Separation from Service requires a "double trigger" where: (i) an employee is terminated by us without cause in connection with a change of control or within 12 months following a change of control provided, however, that if an employee is terminated by us in connection with a change of control but immediately accepts employment with our successor or acquirer, the employee will not be eligible for the benefits outlined in the Severance Plan, (ii) an employee resigns because in connection with a change of control, the offered terms of employment (new or continuing) by us or our successor or acquirer within 30 days after the change of control results in a material change in the terms of employment, or (iii) after accepting (or continuing) employment with us after a change of control, an employee resigns within 12 months following a change of control due to a material change in the terms of employment. Under the Severance Plan, a Non-Change of Control Triggering Event and Separation from Service is defined as an event where a non-executive employee is terminated by us without cause. Severance payments range from two to 18 months of base salary, depending on the employee's position with us, payable in a lump sum payment. The Severance Plan also provides that the provisions of employment agreements entered into between us and executive or non-executive employees supersede the provisions of the Severance Plan. As of December 31, 2016, all our executive officers have employment agreements with provisions that may provide greater severance benefits than those in the Severance Plan.
111
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
8. STOCKHOLDERS' EQUITY
Sales Agreement
On August 28, 2015, we entered into an At Market Issuance Sales Agreement, or the 2015 Sales Agreement, with MLV & Co. LLC, or MLV, pursuant to which we may elect to issue and sell shares of our common stock having an aggregate offering price of up to $50,000,000 from time to time into the open market at prevailing prices through MLV as our sales agent. We will pay MLV an aggregate commission rate equal to up to 3.0% of the gross proceeds of the sales price per share for common stock sold through MLV under the 2015 Sales Agreement. Pursuant to the 2015 Sales Agreement, sales of common stock will be made in such quantities and on such minimum price terms as we may set from time to time. We are not obligated to make any sales of common stock under the 2015 Sales Agreement. As of December 31, 2016, we had not sold any common stock pursuant to the 2015 Sales Agreement. The 2015 Sales Agreement will expire in August 2018 unless extended by the parties.
Public Offering
On February 4, 2014, we completed an underwritten public offering of 25,875,000 shares of our common stock at a public offering price of $4.00 per share, resulting in net cash proceeds of approximately $96,805,000 after deducting the underwriting discount and offering expenses payable by us.
Warrants
In connection with each disbursement under a previous loan agreement with the California Institute for Regenerative Medicine, or CIRM, we were obligated to issue to CIRM a warrant to purchase Geron common stock. Such warrants and the underlying common stock were unregistered. We have no further obligations to issue any additional warrants to CIRM. As of December 31, 2016, a warrant to purchase 537,893 shares of our common stock remained outstanding. The warrant was issued to CIRM in August 2011 at an exercise price of $3.98 per share and expires in August 2021.
In December 2014, CIRM exercised a warrant to purchase 461,382 shares of our common stock utilizing the net exercise provision in the warrant resulting in the issuance of 168,039 shares of our common stock. On March 31, 2015, a warrant to purchase 235,000 shares of our common stock was exercised at an exercise price of $3.75 per share. We received cash proceeds of approximately $881,000 from the exercise of this warrant.
Equity Plans
2002 Equity Incentive Plan
The 2002 Equity Incentive Plan, or 2002 Plan, expired in May 2012. Upon the adoption of the 2011 Incentive Award Plan in May 2011 (see below), no further grants of options or stock purchase rights were made from the 2002 Plan. Options granted under the 2002 Plan expire no later than ten years from the date of grant. Option exercise prices were equal to 100% of the fair market value of the underlying common stock on the date of grant. Service-based stock options under the 2002 Plan generally vested over a period of four years from the date of the option grant. Other stock awards (restricted stock awards and restricted stock units) had variable vesting schedules which were determined by our board of directors on the date of grant. All outstanding awards granted under the
112
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
8. STOCKHOLDERS' EQUITY (Continued)
2002 Plan remain subject to the terms of the 2002 Plan and the individual award agreements thereunder.
2011 Incentive Award Plan
In May 2011, our stockholders approved the adoption of the 2011 Incentive Award Plan, or 2011 Plan. Our board of directors administers the 2011 Plan. The 2011 Plan provides for grants to employees (including officers and employee directors) of either incentive stock options or nonstatutory stock options and stock purchase rights to employees (including officers and employee directors) and consultants (including non-employee directors). As of December 31, 2016, an aggregate of 9,570,535 shares of our common stock were available for future grants of equity awards under the 2011 Plan. Pursuant to the terms of the 2011 Plan, any shares subject to outstanding stock options or outstanding unvested restricted stock awards originally granted under the 2002 Plan that expire or terminate for any reason prior to exercise or settlement or are forfeited because of the failure to meet a contingency or condition required to vest such shares shall become available for issuance under the 2011 Plan. Options granted under the 2011 Plan expire no later than ten years from the date of grant. Option exercise prices shall be equal the fair market value of the underlying common stock on the date of grant. If, at the time we grant an option, the optionee directly or by attribution owns stock possessing more than 10% of the total combined voting power of all classes of our stock, the option exercise price shall be at least 110% of the fair market value of the underlying common stock and shall not be exercisable more than five years after the date of grant.
We grant service-based stock options to employees under our 2011 Plan that generally vest over a period of four years from the date of the option grant. Other stock awards (restricted stock awards and restricted stock units) have variable vesting schedules as determined by our board of directors on the date of grant.
Under certain circumstances, options may be exercised prior to vesting, subject to our right to repurchase shares subject to such option at the exercise price paid per share. Our repurchase rights would generally terminate on a vesting schedule identical to the vesting schedule of the exercised option. During 2016, we have not repurchased any shares under the 2011 Plan. As of December 31, 2016, we have no shares outstanding subject to repurchase.
As of December 31, 2016, our Non-Employee Director Compensation Policy adopted by our board of directors in March 2014 and amended by our board of directors in February 2016 provides for the automatic grant to non-employee directors of the following types of equity awards under the 2011 Plan:
First Director Option. Each person who becomes a non-employee director, whether by election by our stockholders or by appointment by our board of directors to fill a vacancy, will automatically be granted an option to purchase 100,000 shares of common stock, or First Director Option, on the date such person first becomes a non-employee director. The First Director Option vests annually over three years upon each anniversary date of appointment to our board of directors.
Subsequent Director Option. Each non-employee director (other than any director receiving a First Director Option on the date of the annual meeting) will automatically be granted a subsequent option to purchase 50,000 shares of common stock, a Subsequent Director Option, on the date of the annual meeting of stockholders in each year during such director's service on our board of directors. The Subsequent Director Option vests in full on the earlier of: (i) the date of the next annual meeting of our stockholders or (ii) the first anniversary of the date of grant.
113
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
8. STOCKHOLDERS' EQUITY (Continued)
2006 Directors' Stock Option Plan
The 2006 Directors' Stock Option Plan, or 2006 Directors Plan, was terminated by our board of directors and replaced by the 2011 Plan in March 2014. No further grants of options were made from the 2006 Directors Plan upon the 2006 Directors Plan's termination. All outstanding awards granted under the 2006 Directors Plan remain subject to the terms of the 2006 Directors Plan and the individual award agreements made thereunder.
The options granted to non-employee directors under the 2006 Directors Plan were nonstatutory stock options, and they expire no later than ten years from the date of grant. The option exercise price was equal to the fair market value of the underlying common stock on the date of grant. The First Director Option granted to non-employee directors under the 2006 Directors Plan vested annually over three years upon each anniversary date of appointment to the board of directors. The Subsequent Director Option granted to non-employee directors on the date of the annual meeting of stockholders in each year during such director's service on our board of directors under the 2006 Directors Plan vested one year from the date of grant.
Aggregate option and award activity for the 2002 Plan, 2011 Plan and 2006 Directors Plan is as follows:
| |
|
Outstanding Options | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Shares Available For Grant |
Number of Shares |
Weighted Average Exercise Price Per Share |
Weighted Average Remaining Contractual Life (In years) |
Aggregate Intrinsic Value (In thousands) |
|||||||
Balance at December 31, 2015 |
12,054,916 | 17,205,683 | $3.29 | |||||||||
Options granted |
(2,834,000 | ) | 2,834,000 | $2.56 | ||||||||
Awards granted |
(21,541 | ) | — | $ — | ||||||||
Options exercised |
— | (328,486 | ) | $1.50 | ||||||||
Options cancelled/forfeited |
371,160 | (585,910 | ) | $5.34 | ||||||||
| | | | | | | | | | | | | |
Balance at December 31, 2016 |
9,570,535 | 19,125,287 | $3.15 | 6.65 | $3,816 | |||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Options exercisable at December 31, 2016 |
14,074,457 | $2.99 | 6.05 | $3,718 | ||||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Options fully vested and expected to vest at December 31, 2016 |
18,694,300 | $3.14 | 6.60 | $3,815 | ||||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
The aggregate intrinsic value in the preceding table represents the total intrinsic value, based on Geron's closing stock price of $2.07 per share as of December 30, 2016, which would have been received by the option holders had all the option holders exercised their options as of that date.
We have not granted any options with an exercise price below or greater than the fair market value of our common stock on the date of grant in 2016, 2015 or 2014. As of December 31, 2016, 2015 and 2014, there were 14,074,457, 11,356,232 and 9,129,576 exercisable options outstanding at weighted average exercise prices per share of $2.99, $2.98 and $3.12, respectively.
114
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
8. STOCKHOLDERS' EQUITY (Continued)
The total pretax intrinsic value of stock options exercised during 2016, 2015 and 2014 was $595,000, $2,398,000 and $989,000, respectively. Cash received from the exercise of options in 2016, 2015 and 2014 totaled approximately $493,000, $2,205,000 and $1,286,000, respectively.
Information about stock options outstanding as of December 31, 2016 is as follows:
| |
Options Outstanding | ||||||
|---|---|---|---|---|---|---|---|
Exercise Price Range
|
Number of Shares |
Weighted Average Exercise Price Per Share |
Weighted Average Remaining Contractual Life (In years) |
||||
$1.10 - $1.51 |
5,668,162 | $1.45 | 5.89 | ||||
$1.55 - $2.54 |
5,017,031 | $2.27 | 7.25 | ||||
$2.74 - $5.01 |
6,347,008 | $4.55 | 7.28 | ||||
$5.09 - $9.32 |
2,093,086 | $5.59 | 5.36 | ||||
| | | | | | | | |
$1.10 - $9.32 |
19,125,287 | $3.15 | 6.65 | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Aggregate restricted stock activity for the 2011 Plan is as follows:
| |
Number of Shares |
Weighted Average Grant Date Fair Value Per Share |
Weighted Average Remaining Contractual Term (In years) |
||||
|---|---|---|---|---|---|---|---|
Non-vested restricted stock at December 31, 2015 |
500 | $4.65 | 0.10 | ||||
Granted |
21,541 | $2.44 | |||||
Vested |
(22,041 | ) | $2.49 | ||||
| | | | | | | | |
Non-vested restricted stock at December 31, 2016 |
— | $— | — | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The weighted average grant date fair value of restricted stock granted during the years ended December 31, 2016, 2015 and 2014 was $2.44, $3.75 and $2.67 per share, respectively. The total fair value of restricted stock that vested during 2016, 2015 and 2014 was $54,000, $275,000 and $782,000, respectively.
Employee Stock Purchase Plan
In March 2014, our board of directors adopted the 2014 Employee Stock Purchase Plan, or 2014 Purchase Plan. The 2014 Purchase Plan was approved by our stockholders in May 2014. The 2014 Purchase Plan replaced the 1996 Employee Stock Purchase Plan, or 1996 Purchase Plan, which was terminated effective as of the date the 2014 Purchase Plan was approved by our stockholders. Under the 2014 Purchase Plan, we are authorized to sell to eligible employees up to an aggregate of 1,000,000 shares of Geron common stock. As of December 31, 2016, an aggregate of 84,885 shares of our common stock have been issued under the 2014 Purchase Plan since its adoption.
The 2014 Purchase Plan is comprised of a series of offering periods, each with a maximum duration (not to exceed 12 months) with new offering periods commencing on January 1st and July 1st of each year. The date an employee enters the offering period will be designated as the entry date for purposes of that offering period. An employee may participate only in one offering period at a
115
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
8. STOCKHOLDERS' EQUITY (Continued)
time. Each offering period consists of two consecutive purchase periods of six months' duration, with the last day of such period designated a purchase date.
Under the terms of the 2014 Purchase Plan, employees can choose to have up to 10% of their annual salary withheld to purchase our common stock. An employee may not make additional payments into such account or increase the withholding percentage during the offering period.
The purchase price per share at which common stock is purchased by the employee on each purchase date within the offering period is equal to 85% of the lower of (i) the fair market value per share of Geron's common stock on the employee's entry date into that offering period or (ii) the fair market value per share of Geron's common stock on the purchase date. If the fair market value of Geron's common stock on the purchase date is less than the fair market value at the beginning of the offering period, a new 12 month offering period will automatically begin on the first business day following the purchase date with a new fair market value.
Stock-Based Compensation for Employees and Directors
We measure and recognize compensation expense for all share-based payment awards made to employees and directors, including employee stock options, restricted stock awards and employee stock purchases, based on grant-date fair values for these instruments. We use the Black Scholes option-pricing model to estimate the grant-date fair value of our stock options and employee stock purchases. The fair value for service-based restricted stock awards is determined using the fair value of our common stock on the date of grant.
As stock-based compensation expense recognized in the statements of operations for the years ended December 31, 2016, 2015 and 2014 is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures but at a minimum, reflects the grant-date fair value of those awards that actually vested in the period. Forfeitures have been estimated at the time of grant based on historical data and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
We recognize stock-based compensation expense on a straight-line basis over the requisite service period, which is generally the vesting period. The following table summarizes the stock-based compensation expense related to stock options, restricted stock awards and employee stock purchases for the years ended December 31, 2016, 2015 and 2014 which was allocated as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (In thousands) |
2016 | 2015 | 2014 | |||||||
Research and development |
$ | 1,275 | $ | 2,139 | $ | 2,545 | ||||
Restructuring charges |
— | 307 | — | |||||||
General and administrative |
6,970 | 5,951 | 5,113 | |||||||
| | | | | | | | | | | |
Stock-based compensation expense included in operating expenses |
$ | 8,245 | $ | 8,397 | $ | 7,658 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Stock-based compensation expense also has been recognized for the modification of the post-termination exercise period for certain stock options previously granted to employees affected by
116
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
8. STOCKHOLDERS' EQUITY (Continued)
the March 2015 restructuring, which has been included in restructuring charges in our statements of operations. See Note 6 on Restructuring for further discussion of the restructuring.
The fair value of stock options granted in 2016, 2015 and 2014 has been estimated at the date of grant using the Black Scholes option-pricing model with the following assumptions:
| |
2016 | 2015 | 2014 | |||
|---|---|---|---|---|---|---|
Dividend yield |
0% | 0% | 0% | |||
Expected volatility range |
0.888 to 0.890 | 0.874 to 0.884 | 0.898 to 0.922 | |||
Risk-free interest rate range |
1.21% to 1.38% | 1.68% to 1.71% | 1.64% to 1.92% | |||
Expected term |
5.5 yrs | 5.5 yrs | 5.5 yrs |
The fair value of employee stock purchases in 2016, 2015 and 2014 has been estimated using the Black Scholes option-pricing model with the following assumptions:
| |
2016 | 2015 | 2014 | |||
|---|---|---|---|---|---|---|
Dividend yield |
0% | 0% | 0% | |||
Expected volatility range |
0.641 to 0.684 | 0.654 to 1.392 | 0.835 to 1.666 | |||
Risk-free interest rate range |
0.28% to 0.45% | 0.11% to 0.28% | 0.06% to 0.15% | |||
Expected term range |
6 mos to 12 mos | 6 mos to 12 mos | 6 mos to 12 mos |
Dividend yield is based on historical cash dividend payments and Geron has paid no cash dividends to date. The expected volatility range is based on historical volatilities of our stock since traded options on Geron common stock do not correspond to option terms and the trading volume of options is limited. The risk-free interest rate range is based on the U.S. Zero Coupon Treasury Strip Yields for the expected term in effect on the date of grant for an award. The expected term of options is derived from actual historical exercise and post-vesting cancellation data and represents the period of time that options granted are expected to be outstanding. The expected term of employees' purchase rights is equal to the purchase period.
Based on the Black Scholes option-pricing model, the weighted average estimated fair value of stock options granted during the years ended December 31, 2016, 2015 and 2014 was $1.83, $3.06 and $3.57 per share, respectively. The weighted average estimated fair value of employees' purchase rights for the years ended December 31, 2016, 2015 and 2014 was $1.01, $1.64 and $2.10 per share, respectively. As of December 31, 2016, total compensation cost related to unvested share-based payment awards not yet recognized, net of estimated forfeitures, was $10,128,000, which is expected to be recognized over the next 24 months on a weighted-average basis.
401(k) Plan Matching Contributions
We sponsor a defined-contribution savings plan under Section 401(k) of the Internal Revenue Code covering all full-time U.S. employees, or the Geron 401K Plan. Participating employees may contribute up to the annual Internal Revenue Service contribution limit. The Geron 401K Plan also permits us to provide discretionary matching and profit sharing contributions. Prior to 2014, our board of directors approved matching contributions for the Geron 401K Plan in our common stock, which vested ratably over four years for each year of service completed by our employees, commencing from the date of hire.
117
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
8. STOCKHOLDERS' EQUITY (Continued)
Stock-Based Compensation to Service Providers
We grant stock options and restricted stock awards to consultants from time to time in exchange for services performed for us. In general, the stock options and restricted stock awards vest over the contractual period of the consulting arrangement. We granted stock options to purchase 75,000 shares of our common stock to consultants in 2014. The fair value of stock options and restricted stock awards held by consultants is recorded as operating expenses over the vesting term of the respective equity awards. In addition, we will record any increase in the fair value of the stock options and restricted stock awards as the respective equity award vests. We recorded stock-based compensation expense of $104,000, $311,000 and $94,000 for the vested portion of the fair value of stock options and restricted stock awards held by consultants in 2016, 2015 and 2014, respectively.
We have also issued common stock to non-employee directors, consultants and vendors. For stock issuances where services are to be performed for us, we record a prepaid asset equal to the fair market value of the shares on the date of issuance and amortize the fair value of the shares to our operating expenses on a pro-rata basis as services are performed or goods are received. For stock issuances where services have been performed for us, we record the fair market value of the shares on the date of issuance to offset the amounts owed. In 2016, 2015 and 2014, we issued 21,541, 18,077 and 71,239 shares of common stock, respectively, in exchange for goods or services. In 2016, 2015 and 2014, we recognized approximately $52,000, $53,000 and $158,000, respectively, of expense in connection with stock grants to non-employee directors, consultants and vendors.
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance as of December 31, 2016 is as follows:
Outstanding stock options |
19,125,287 | |||
Options and awards available for grant |
9,570,535 | |||
Employee stock purchase plan |
915,115 | |||
Warrant outstanding |
537,893 | |||
| | | | | |
Total |
30,148,830 | |||
| | | | | |
| | | | | |
| | | | | |
118
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
9. INCOME TAXES
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets are as follows:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | |||||
| |
(In thousands) |
||||||
Net operating loss carryforwards |
$ | 280,400 | $ | 276,100 | |||
Research credits |
29,800 | 25,000 | |||||
Capitalized research and development |
300 | 1,400 | |||||
License fees |
200 | 300 | |||||
Other—net |
11,500 | 9,800 | |||||
| | | | | | | | |
Total deferred tax assets |
322,200 | 312,600 | |||||
Valuation allowance for deferred tax assets |
(322,200 | ) | (312,600 | ) | |||
| | | | | | | | |
Net deferred tax assets |
$ | — | $ | — | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
We record net deferred tax assets to the extent we believe these assets will more likely than not be realized. In making such determination, we consider all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax planning strategies and recent financial performance. Forming a conclusion that a valuation allowance is not required is difficult when there is negative evidence such as cumulative losses in recent years. Because of our history of losses, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by $9,600,000 for the year ended December 31, 2016 and decreased by $2,300,000 and $3,300,000 during the years ended December 31, 2015 and 2014, respectively. Approximately $6,400,000 of the valuation allowance for deferred tax assets relates to benefits of stock option deductions which, when recognized, will be allocated directly to contributed capital. No income tax benefit was realized from stock options exercised in 2016.
As of December 31, 2016, we had domestic federal net operating loss carryforwards of approximately $778,000,000 expiring at various dates beginning in 2018 through 2036 and state net operating loss carryforwards of approximately $293,000,000 expiring at various dates beginning in 2017 through 2036, if not utilized. We also had federal research and development tax credit carryforwards of approximately $21,100,000 expiring at various dates beginning in 2018 through 2036, if not utilized. Our state research and development tax credit carryforwards of approximately $13,300,000 carry forward indefinitely.
Due to the change of ownership provisions of the Tax Reform Act of 1986, utilization of a portion of our domestic net operating loss and tax credit carryforwards may be limited in future periods. Further, a portion of the carryforwards may expire before being applied to reduce future income tax liabilities.
We adopted the provision of the standard for accounting for uncertainties in income taxes on January 1, 2007. Upon adoption, we recognized no material adjustment in the liability for unrecognized tax benefits. At December 31, 2016, we had approximately $14,700,000 of unrecognized tax benefits, none of which would currently affect our effective tax rate if recognized due to our deferred tax assets being fully offset by a valuation allowance.
119
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
9. INCOME TAXES (Continued)
A reconciliation of the beginning and ending amounts of unrecognized tax benefits is as follows (in thousands):
Balance as of December 31, 2015 |
$ | 20,100 | ||
Decrease related to prior year tax positions |
(7,500 | ) | ||
Increase related to current year tax positions |
2,100 | |||
| | | | | |
Balance as of December 31, 2016 |
$ | 14,700 | ||
| | | | | |
| | | | | |
| | | | | |
If applicable, we would classify interest and penalties related to uncertain tax positions in income tax expense. Through December 31, 2016, there has been no interest expense or penalties related to unrecognized tax benefits.
We do not currently expect any significant changes to unrecognized tax benefits during the fiscal year ended December 31, 2017. In certain cases, our uncertain tax positions are related to tax years that remain subject to examination by the relevant tax authorities. Tax years for which we have carryforward net operating loss and credit attributes remain subject to examination by federal and most state tax authorities.
10. STATEMENTS OF CASH FLOWS DATA
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||||||
| |
(In thousands) |
|||||||||
Supplemental operating activities: |
||||||||||
Issuance of common stock for 401(k) matching contributions |
$ | — | $ | — | $ | 313 | ||||
Reclassification between deposits and other current assets |
$ | — | $ | — | $ | 190 | ||||
Supplemental investing activities: |
||||||||||
Net unrealized gain (loss) on marketable securities |
$ | 160 | $ | (129 | ) | $ | (70 | ) | ||
We have not made any cash payments for taxes or interest for the years ended December 31, 2016, 2015 and 2014.
120
GERON CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
11. SELECTED QUARTERLY FINANCIAL INFORMATION (UNAUDITED)
| |
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands, except per share amounts) |
||||||||||||
Year Ended December 31, 2016: |
|||||||||||||
Revenues(1) |
$ | 749 | $ | 211 | $ | 5,108 | $ | 94 | |||||
Operating expenses |
9,826 | 9,122 | 8,985 | 8,875 | |||||||||
Net loss |
(8,842 | ) | (8,637 | ) | (3,576 | ) | (8,482 | ) | |||||
Basic and diluted net loss per share |
$ | (0.06 | ) | $ | (0.05 | ) | $ | (0.02 | ) | $ | (0.05 | ) | |
Year Ended December 31, 2015: |
|||||||||||||
Revenues(2) |
$ | 537 | $ | 251 | $ | 35,363 | $ | 220 | |||||
Operating expenses |
9,993 | 9,730 | 8,343 | 8,864 | |||||||||
Net (loss) income |
(9,315 | ) | (9,356 | ) | 27,185 | (8,468 | ) | ||||||
Basic and diluted net (loss) income per share |
$ | (0.06 | ) | $ | (0.06 | ) | $ | 0.17 | $ | (0.05 | ) | ||
- (1)
- The
third quarter of 2016 includes the full recognition of the $5,000,000 upfront payment from Janssen Pharmaceuticals as license fee revenue. See Note 4 on
License Agreements.
- (2)
- The third quarter of 2015 includes the full recognition of the $35,000,000 upfront payment from Janssen as collaboration revenue. See Note 4 on License Agreements.
Basic and diluted net income (loss) per share are computed independently for each of the quarters presented. Therefore, the sum of the quarters may not be equal to the full year net income (loss) per share amounts.
121
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
(I) Evaluation of Disclosure Controls and Procedures
We have carried out an evaluation under the supervision and with the participation of management, including our Chief Executive Officer and our Chief Financial Officer, of our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this annual report on Form 10-K. Based on that evaluation, our Chief Executive Officer and our Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2016.
In designing and evaluating disclosure controls and procedures, our management recognizes that any system of controls, however well designed and operated, can provide only reasonable assurance, and not absolute assurance, that the desired control objectives of the system are met. In addition, the design of any control system is based in part upon certain assumptions about the likelihood of future events. Because of these and other inherent limitations of control systems, there can be no assurance that any design will succeed in achieving its stated goals in all future circumstances. Accordingly, our disclosure controls and procedures are designed to provide reasonable, not absolute, assurance that the objectives of our disclosure control system are met and, as set forth above, our Chief Executive Officer and our Chief Financial Officer have concluded, based on their evaluation as of the end of the period covered by this annual report on Form 10-K, that our disclosure controls and procedures were effective to provide reasonable assurance that the objectives of our disclosure control system were met.
(II) Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2016 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
122
(III) Management's Report on Internal Control over Financial Reporting
Internal control over financial reporting refers to the process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
- (1)
- Pertain
to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
- (2)
- Provide
reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted
accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
- (3)
- Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Management is responsible for establishing and maintaining an adequate internal control over financial reporting for us. Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of such limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework set forth in "Internal Control—Integrated Framework" issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on our evaluation under the framework set forth in "Internal Control—Integrated Framework," our management concluded that our internal control over financial reporting was effective as of December 31, 2016. The effectiveness of our internal control over financial reporting as of December 31, 2016 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which is included herein.
123
(IV) Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Geron Corporation
We have audited Geron Corporation's internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Geron Corporation's management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Geron Corporation maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016 based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Geron Corporation as of December 31, 2016 and 2015, and the related statements of operations, comprehensive loss, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2016 of Geron Corporation and our report dated March 1, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Redwood
City, California
March 1, 2017
ITEM 9B. OTHER INFORMATION
None.
124
PART III
Certain information required by Part III is omitted from this annual report on Form 10-K because we will file with the U.S. Securities and Exchange Commission a definitive proxy statement pursuant to Regulation 14A in connection with the solicitation of proxies for Geron's Annual Meeting of Stockholders expected to be held in May 2017, or the Proxy Statement, not later than 120 days after the end of the fiscal year covered by this annual report on Form 10-K, and certain information included therein is incorporated herein by reference.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Identification of Directors and Nominees for Director
The information required by this item concerning our directors and nominees for director is incorporated by reference from the section captioned "Proposal 1: Election of Directors" contained in our Proxy Statement.
Identification of Executive Officers
The information required by this item concerning our executive officers is set forth in Part I, Item 1 of this annual report on Form 10-K.
Code of Ethics
We have adopted a Code of Conduct with which every person who works for Geron, including our board of directors, is expected to comply. The Code of Conduct is publicly available on our website under the Investor Relations section at www.geron.com. This website address is intended to be an inactive, textual reference only; none of the material on this website is part of this annual report on Form 10-K. If any substantive amendments are made to the Code of Conduct or any waiver granted, including any implicit waiver, from a provision of the Code to our Chief Executive Officer, Chief Financial Officer or Corporate Controller, we will disclose the nature of such amendment or waiver on that website or in a report on Form 8-K.
Copies of the Code of Conduct will be furnished without charge to any person who submits a written request directed to the attention of our Corporate Secretary, at our offices located at 149 Commonwealth Drive, Suite 2070, Menlo Park, California, 94025.
Section 16(a) Compliance
Information concerning Section 16(a) beneficial ownership reporting compliance is incorporated by reference from the section captioned "Section 16(a) Beneficial Ownership Reporting Compliance" contained in the Proxy Statement.
Certain Corporate Governance Matters
The information required by this item concerning our audit committee, audit committee financial expert and procedures by which stockholders may recommend nominees to our board of directors, may be found under the sections captioned "Board Leadership and Governance" and "Other Matters" contained in the Proxy Statement.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item is incorporated by reference from the sections captioned "Compensation Discussion and Analysis," "Compensation Committee Report," "Executive
125
Compensation Tables," "Compensation of Directors" and "Compensation Committee Interlocks and Insider Participation" contained in the Proxy Statement.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is incorporated by reference from the sections captioned "Equity Compensation Plans" and "Security Ownership of Certain Beneficial Owners and Management" contained in the Proxy Statement.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item is incorporated by reference from the sections captioned "Proposal 1: Election of Directors" and "Certain Transactions" contained in the Proxy Statement.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item is incorporated by reference from the section captioned "Principal Accountant Fees and Services" contained in the Proxy Statement.
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
- (a)
- (1) Financial Statements
Included in Part II, Item 8 of this Report:
- (2)
- Financial Statement Schedules
Financial statement schedules are omitted because they are not required or the information is disclosed in the financial statements listed in Item 15(a)(1) above.
- (3)
- Exhibits
See Exhibit Index.
None.
126
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| GERON CORPORATION | ||||
Date: March 1, 2017 |
By: |
/s/ OLIVIA K. BLOOM OLIVIA K. BLOOM Executive Vice President, Finance, Chief Financial Officer and Treasurer |
||
127
KNOW BY ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints, jointly and severally, John A. Scarlett, M.D., and Olivia K. Bloom, and each one of them, attorneys-in-fact for the undersigned, each with the power of substitution, for the undersigned in any and all capacities, to sign any and all amendments to this annual report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his or her substitutes, may do or cause to be done by virtue hereof.
IN WITNESS WHEREOF, each of the undersigned has executed this Power of Attorney as of the date indicated opposite his/her name.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ JOHN A. SCARLETT JOHN A. SCARLETT |
President, Chief Executive Officer and Director (Principal Executive Officer) |
March 1, 2017 | ||
/s/ OLIVIA K. BLOOM OLIVIA K. BLOOM |
Executive Vice President, Finance, Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) |
March 1, 2017 |
||
/s/ DANIEL M. BRADBURY DANIEL M. BRADBURY |
Director |
March 1, 2017 |
||
/s/ KARIN EASTHAM KARIN EASTHAM |
Director |
March 1, 2017 |
||
/s/ HOYOUNG HUH HOYOUNG HUH |
Director |
March 1, 2017 |
||
/s/ V. BRYAN LAWLIS V. BRYAN LAWLIS |
Director |
March 1, 2017 |
||
/s/ SUSAN M. MOLINEAUX SUSAN M. MOLINEAUX |
Director |
March 1, 2017 |
||
/s/ ROBERT J. SPIEGEL ROBERT J. SPIEGEL |
Director |
March 1, 2017 |
128
| |
|
Incorporation by Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description |
Exhibit Number |
Filing |
Filing Date |
File No. |
||||||
2.1 |
Asset Contribution Agreement by and among Geron Corporation, BioTime, Inc. and Asterias Biotherapeutics, Inc. (formerly known as BioTime Acquisition Corporation) | 2.1 | 8-K | January 8, 2013 | 000-20859 | ||||||
3.1 |
Restated Certificate of Incorporation | 3.3 | 8-K | May 18, 2012 | 000-20859 | ||||||
3.2 |
Certificate of Amendment of the Restated Certificate of Incorporation | 3.1 | 8-K | May 18, 2012 | 000-20859 | ||||||
3.3 |
Amended and Restated Bylaws of Registrant | 3.1 | 8-K | March 19, 2010 | 000-20859 | ||||||
4.1 |
Form of Common Stock Certificate | 4.1 | 10-K | March 15, 2013 | 000-20859 | ||||||
4.2 |
Form of 2011 Warrant | Attachment to 10.1 | 10-Q | November 3, 2011 | 000-20859 | ||||||
10.1 |
Form of Indemnification Agreement | 10.1 | 10-K | March 7, 2012 | 000-20859 | ||||||
10.2 |
Amended and Restated 2002 Equity Incentive Plan* | 4.1 | S-8 | June 4, 2010 | 333-167349 | ||||||
10.3 |
Form of Stock Option Agreement under 2002 Equity Incentive Plan* | 10.6 | 10-K | March 15, 2013 | 000-20859 | ||||||
10.4 |
Amended and Restated 2006 Directors' Stock Option Plan* | 10.5 | 10-Q | November 7, 2013 | 000-20859 | ||||||
10.5 |
2011 Incentive Award Plan* | 10.1 | 8-K | May 16, 2011 | 000-20859 | ||||||
10.6 |
Form of Stock Option Agreement under 2011 Incentive Award Plan* | 10.11 | 10-K | March 15, 2013 | 000-20859 | ||||||
10.7 |
Form of Restricted Stock Award Agreement under 2011 Incentive Award Plan* | 10.12 | 10-K | March 15, 2013 | 000-20859 | ||||||
10.8 |
Form of Non-Employee Director Stock Option Agreement under 2011 Incentive Award Plan* | 10.2 | 10-Q | May 7, 2015 | 000-20859 | ||||||
10.9 |
2014 Employee Stock Purchase Plan* | 10.1 | 8-K | May 23, 2014 | 000-20859 | ||||||
10.10 |
Non-Employee Director Compensation Policy, as amended* | 10.30 | 10-K | March 10, 2016 | 000-20859 | ||||||
10.11 |
Amended and Restated Severance Plan, effective as of May 23, 2013* | 10.1 | 8-K | May 24, 2013 | 000-20859 | ||||||
10.12 |
Employment agreement between the Registrant and John A. Scarlett, M.D., effective as of September 29, 2011* | 10.2 | 10-Q | November 3, 2011 | 000-20859 | ||||||
| |
|
Incorporation by Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description |
Exhibit Number |
Filing |
Filing Date |
File No. |
||||||
10.13 |
First Amendment to Employment Agreement between the Registrant and John A. Scarlett, M.D., effective as of February 11, 2014* | 10.5 | 8-K | February 14, 2014 | 000-20859 | ||||||
10.14 |
Employment agreement between the Registrant and Stephen N. Rosenfield, effective as of February 16, 2012* | 10.32 | 10-K | March 7, 2012 | 000-20859 | ||||||
10.15 |
First Amendment to Employment Agreement between the Registrant and Stephen N. Rosenfield, effective as of September 24, 2013* | 10.4 | 8-K | September 27, 2013 | 000-20859 | ||||||
10.16 |
Employment agreement between the Registrant and Andrew J. Grethlein, effective as of September 17, 2012* | 10.2 | 10-Q | November 2, 2012 | 000-20859 | ||||||
10.17 |
First Amendment to Employment Agreement between the Registrant and Andrew J. Grethlein, effective as of February 11, 2014* | 10.4 | 8-K | February 14, 2014 | 000-20859 | ||||||
10.18 |
Employment agreement between the Registrant and Olivia K. Bloom, effective as of December 7, 2012* | 10.26 | 10-K | March 15, 2013 | 000-20859 | ||||||
10.19 |
First Amendment to Employment Agreement between the Registrant and Olivia K. Bloom, effective as of September 24, 2013* | 10.2 | 8-K | September 27, 2013 | 000-20859 | ||||||
10.20 |
Second Amendment to Employment Agreement between the Registrant and Olivia K. Bloom, effective as of February 11, 2014* | 10.1 | 8-K | February 14, 2014 | 000-20859 | ||||||
10.21 |
Employment agreement between the Registrant and Melissa A. Kelly Behrs, effective as of January 31, 2013* | 10.28 | 10-K | March 15, 2013 | 000-20859 | ||||||
10.22 |
First Amendment to Employment Agreement between the Registrant and Melissa A. Kelly Behrs, effective as of September 24, 2013* | 10.1 | 8-K | September 27, 2013 | 000-20859 | ||||||
10.23 |
Second Amendment to Employment Agreement between the Registrant and Melissa A. Kelly Behrs, effective as of February 11, 2014* | 10.2 | 8-K | February 14, 2014 | 000-20859 | ||||||
10.24 |
† | California Institute for Regenerative Medicine Notice of Loan Award | 10.1 | 10-Q | November 3, 2011 | 000-20859 | |||||
| |
|
Incorporation by Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description |
Exhibit Number |
Filing |
Filing Date |
File No. |
||||||
10.25 |
† | Office Lease Agreement by and between the Registrant and Exponent Realty, LLC, effective as of February 29, 2012 | 10.36 | 10-K/A | March 27, 2012 | 000-20859 | |||||
10.26 |
Fifth Amendment to Office Lease Agreement by and between the Registrant and Exponent Realty, LLC, effective as of September 15, 2015 | 10.1 | 8-K | September 18, 2015 | 000-20859 | ||||||
10.27 |
At Market Issuance Sales Agreement, dated August 28, 2015, by and between the Registrant and MLV & Co. LLC | 10.1 | 8-K | August 28, 2015 | 000-20859 | ||||||
10.28 |
† | Collaboration and License Agreement by and between the Registrant and Janssen Biotech, Inc., dated November 13, 2014 | 10.36 | 10-K | March 11, 2015 | 000-20859 | |||||
10.29 |
† | License Agreement by and between the Registrant and Janssen Pharmaceuticals Inc., dated September 15, 2016 | 10.1 | 10-Q | November 3, 2016 | 000-20859 | |||||
12.1 |
Computation of Ratio of Earnings to Fixed Charges | ||||||||||
23.1 |
Consent of Independent Registered Public Accounting Firm | ||||||||||
24.1 |
Power of Attorney (see signature page) | ||||||||||
31.1 |
Certification of Chief Executive Officer pursuant to Form of Rule 13a-14(a), as Adopted Pursuant to Section 302(a) of the Sarbanes-Oxley Act of 2002, dated March 1, 2017 | ||||||||||
31.2 |
Certification of Chief Financial Officer pursuant to Form of Rule 13a-14(a), as Adopted Pursuant to Section 302(a) of the Sarbanes-Oxley Act of 2002, dated March 1, 2017 | ||||||||||
| |
|
Incorporation by Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description |
Exhibit Number |
Filing |
Filing Date |
File No. |
||||||
32.1 |
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, dated March 1, 2017** | ||||||||||
32.2 |
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, dated March 1, 2017** | ||||||||||
101 |
The following materials from the Registrant's annual report on Form 10-K for the year ended December 31, 2016, formatted in Extensible Business Reporting Language (XBRL) include: (i) Balance Sheets as of December 31, 2016 and 2015, (ii) Statements of Operations, Comprehensive Loss, Stockholders' Equity and Cash Flows for each of the three years in the period ended December 31, 2016, and (iii) Notes to Financial Statements | ||||||||||
- †
- Confidential
treatment has been granted for certain portions of this exhibit. Omitted information has been filed separately with the Securities and
Exchange Commission.
- *
- Management
contract or compensation plan or arrangement.
- **
- The certifications attached as Exhibits 32.1 and 32.2 that accompany this annual report on Form 10-K, are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of this Form 10-K), irrespective of any general incorporation language contained in such filing.
