Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - CYNOSURE INC | d289949dex322.htm |
| EX-32.1 - EX-32.1 - CYNOSURE INC | d289949dex321.htm |
| EX-31.2 - EX-31.2 - CYNOSURE INC | d289949dex312.htm |
| EX-31.1 - EX-31.1 - CYNOSURE INC | d289949dex311.htm |
| EX-23.1 - EX-23.1 - CYNOSURE INC | d289949dex231.htm |
| EX-21.1 - EX-21.1 - CYNOSURE INC | d289949dex211.htm |
| EX-10.31 - EX-10.31 - CYNOSURE INC | d289949dex1031.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 000-51623
Cynosure, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 04-3125110 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 5 Carlisle Road Westford, MA |
01886 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code
(978) 256-4200
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Name of each exchange on which registered | |
| Class A Common Stock, $0.001 par value | The Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ☒ Yes ☐ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ☒ | Accelerated filer | ☐ | Non-accelerated filer | ☐ | Smaller reporting company | ☐ | ||||||
| (Do not check if a smaller reporting company) | ||||||||||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, based on the last sale price for such stock on June 30, 2016: $1,141,835,641.
The number of shares outstanding of the registrant’s Class A common stock, as of February 21, 2017 was 24,351,449.
Portions of the registrant’s definitive Proxy Statement for its 2017 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
Table of Contents
| PART I | ||||||
| Item 1. |
4 | |||||
| Item 1A. |
29 | |||||
| Item 1B. |
48 | |||||
| Item 2. |
48 | |||||
| Item 3. |
48 | |||||
| Item 4. |
50 | |||||
| PART II | ||||||
| Item 5. |
51 | |||||
| Item 6. |
53 | |||||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
55 | ||||
| Item 7A. |
74 | |||||
| Item 8. |
75 | |||||
| Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
75 | ||||
| Item 9A. |
75 | |||||
| Item 9B. |
78 | |||||
| PART III | ||||||
| Item 10. |
79 | |||||
| Item 11. |
79 | |||||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
79 | ||||
| Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
79 | ||||
| Item 14. |
79 | |||||
| PART IV | ||||||
| Item 15. |
80 | |||||
| Item 16. |
80 | |||||
| 81 | ||||||
2
Table of Contents
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, which we refer to as this Annual Report, contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this Annual Report regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans, objectives of management and expected market growth are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
| • | the ability to achieve the projected benefits of the planned acquisition of our company by Hologic, Inc., or Hologic, including future financial and operating results, our or Hologic’s plans, and the ability to consummate the transactions contemplated by the agreement and plan of merger among us, Hologic and Minuteman Merger Sub, Inc. |
| • | our ability to identify and penetrate new markets for our products and technology; |
| • | our strategy of growing through acquisitions; |
| • | our ability to innovate, develop and commercialize new products; |
| • | our ability to obtain and maintain regulatory clearances; |
| • | our sales and marketing capabilities and strategy in the United States and internationally; |
| • | our intellectual property portfolio; and |
| • | our estimates regarding expenses, future revenues, capital requirements and needs for additional financing. |
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this Annual Report, particularly in Item 1A of this Annual Report, and in our other public filings with the Securities and Exchange Commission, or the SEC, that could cause actual results or events to differ materially from the forward-looking statements that we make.
You should read this Annual Report and the documents that we have filed as exhibits to this Annual Report completely and with the understanding that our actual future results may be materially different from what we expect. It is routine for internal projections and expectations to change as the year or each quarter in the year progresses, and therefore it should be clearly understood that the internal projections and beliefs upon which we base our expectations are made as of the date of this Annual Report and may change prior to the end of each quarter or the year. While we may elect to update forward-looking statements at some point in the future, we do not undertake any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise, except as required by law.
3
Table of Contents
PART I
| Item 1. | Business |
Overview
We develop, manufacture, and market aesthetic treatment systems that enable plastic surgeons, dermatologists and other medical practitioners to perform non-invasive and minimally invasive procedures to remove hair, treat vascular and benign pigmented lesions, remove multi-colored tattoos, revitalize the skin, reduce fat through laser lipolysis, reduce cellulite, clear nails infected by toe fungus, ablate sweat glands and improve women’s health. We also market radiofrequency, or RF, energy-sourced medical devices for precision surgical applications such as facial plastic and general surgery, gynecology, ear, nose, and throat procedures, ophthalmology, oral and maxillofacial surgery, podiatry and proctology.
Our product portfolio is composed of a broad range of energy sources including Alexandrite, diode, Nd: YAG, picosecond, pulse dye, Q-switched lasers, intense pulsed light and RF technology. We sell our products globally under the Cynosure, Palomar, ConBio and Ellman brand names through a direct sales force in the United States, Canada, France, Morocco, Germany, Spain, the United Kingdom, Australia, China, Japan and Korea, and through international distributors in over 120 other countries.
A key element of our business strategy is to launch innovative new products and technologies into high-growth aesthetic applications. Our research and development team builds on our existing broad range of laser, light-based technologies and other energies to develop new solutions and products to target unmet needs in significant aesthetic treatment markets. Innovation continues to be a strong contributor to our strength as we continue to add new products to our aesthetic treatment systems portfolio. Recent key innovations and acquisitions and significant partnerships have included:
| • | SculpSure® Laser System. We launched our SculpSure laser system for non-invasive fat reduction treatments in the second half of 2015. The SculpSure system requires the use of a Patented Applicator for Contouring, or PAC, to activate each applicator handpiece used in a treatment cycle. We offer PACs in “keys” containing 100 applicator uses. The SculpSure system is the first hyperthermic laser treatment system cleared by the U.S. Food and Drug Administration, or FDA, and has been cleared for marketing in the United Stated for the treatment of flanks and the abdomen. We have also received European Medical Device Directive certification from the European Notified Body, which allows us to place the “CE” Mark on the SculpSure system for distribution in the European Union and its member states. In December 2015, we received marketing authorization in Australia. In July 2016, we received clearance to market SculpSure in Canada and Korea. |
| • | Picosecond Technology Platform. In 2013, we launched our PicoSure® system, our picosecond laser technology platform using a 755nm wavelength for the treatment of tattoos and benign pigmented lesions. The PicoSure system was the first commercially available picosecond Alexandrite aesthetic laser platform. Picosecond lasers deliver pulses that are measured in trillionths of a second, in contrast with nanosecond technology, such as our MedLite® and RevLite™ products, which deliver pulses in billionths of a second. In February 2015, we received FDA clearance to market the PicoSure 532 nm wavelength, which we designed to more effectively treat red, yellow and orange tattoo ink colors, and we offer the wavelength as an upgrade to our current PicoSure customer base. In May 2016, we received FDA clearance to market the PicoSure 1064 nm wavelength, which we designed to more effectively treat black and other dark tattoo ink colors. |
| • | Establishment of Minimally Invasive Product Line. We have offered the SmartLipo® workstation, a minimally invasive aesthetic treatment system since 2006. |
| • | The SmartLipo system was the first FDA-cleared laser lipolysis system for use in a minimally invasive procedure for the removal of unwanted fat. Since the launch of the SmartLipo system, we have introduced two new workstations: the SmartLipo MPX workstation, which added a second |
4
Table of Contents
| wavelength laser, and the SmartLipo Triplex workstation, which added a third wavelength laser. Our MPX or MultiPlex™ technology enables the energy from two lasers to be blended during delivery by quickly following a pulse of energy from one laser with a pulse of energy from another laser, and our innovative SmartSense™ and ThermaGuide™ features provide for intelligent delivery of laser energy. In 2013, we launched our PrecisionTx™ system for minimally invasive removal of fat in small areas, such as the neck and jawline as well as for the ablation of axillary sweat glands. |
| • | In 2012, we received FDA clearance in the United States to sell and market our Cellulaze® system, the world’s first FDA-cleared minimally invasive aesthetic laser device for the treatment of cellulite. In 2014, we received additional international clearances for our Cellulaze system in Argentina, Mexico and Taiwan. |
| • | Ellman. In 2014, we diversified our technology base by acquiring substantially all of the assets of Ellman International, Inc., which we refer to as Ellman, a leading provider of advanced RF technology for precision surgical and aesthetic procedures. Ellman also offers a line of aesthetic lasers. |
| • | Palomar. In 2013, we acquired Palomar Medical Technologies, Inc., which we refer to as Palomar. The Palomar acquisition complemented and broadened our product lineup by adding the Icon® aesthetic system, which provides a comprehensive suite of the most popular treatments from hair removal to wrinkle reduction to scar and stretch mark treatment, the StarLux® laser and pulsed light system for hair removal, skin resurfacing and skin rejuvenation, and the Vectus® diode laser for high volume hair removal. |
| • | Significant Partnerships. We have entered into several distribution arrangements to expand and complement our product portfolio, including the following: |
| • | MonaLisa Touch®. In November 2014, we entered into an exclusive distribution agreement with El.En. S.p.A., or El.En., to market and distribute in North America the MonaLisa Touch product, a CO2 laser for gynecologic health for postmenopausal women, breast cancer survivors and women who have undergone hysterectomies and who may suffer from changes to their gynecologic health. The device received marketing clearance from the FDA in 2014, and we received a medical device license issued by Health Canada to market MonaLisa Touch for the treatment of symptoms related to Genitourinary Syndrome of Menopause, or GSM, including vaginal dryness, vaginal burning, vaginal itching, pain, dysuria and dyspareunia. In 2015, we and El.En. agreed to market and distribute the MonaLisa Touch laser under separate distribution agreements with our respective wholly-owned subsidiaries in the United Kingdom, Germany, and Spain. |
We offer the following flagship products (we use the term “flagship products” to refer to our leading products for a particular application):
| • | our Elite product line for hair removal and treatment of facial and leg veins and pigmentations; |
| • | our SmartLipo product line for LaserBodySculpting for the minimally invasive removal of unwanted fat; |
| • | our Cellulaze product line for the treatment of cellulite; |
| • | our Cynergy product line for the treatment of vascular lesions; |
| • | our MedLite C6 and RevLite product lines for the removal of benign pigmented lesions, as well as multi-colored tattoos; |
| • | our PicoSure product line for the treatment of tattoos, benign pigmented lesions, acne scars, fine lines and wrinkles; |
| • | our Icon aesthetic system for hair removal, wrinkle reduction and scar and stretch mark treatment; |
| • | our Vectus diode laser for high volume hair removal; |
5
Table of Contents
| • | our SculpSure hyperthermic laser treatment for LaserBodySculpting for non-invasive fat reduction; and |
| • | our MonaLisa Touch laser for women’s health. |
We have established ourselves as a leading provider of laser and light-based energy systems used for aesthetic treatment procedures. We plan to continue to enhance our existing product offerings and increase the leverage of our global distribution network through both internal research and development and the acquisition of complementary businesses, products or technologies, which may include small and substantial acquisitions, as well as joint ventures and other collaborative projects. We believe we have a disciplined acquisition strategy that focuses on complementary product offerings, integrated distribution networks, return on investment and other strategic benefits, and at any time we may be evaluating or in various stages of discussions with potential acquisition candidates. We also have a comprehensive post-acquisition strategic plan to facilitate the integration of companies and product lines that we may acquire.
As further described under Note 14 to our consolidated financial statements included in this Annual Report, on February 14, 2017, we entered into an Agreement and Plan of Merger, or the Merger Agreement, with Hologic, Inc., or Hologic, and Minuteman Merger Sub, Inc., a wholly-owned subsidiary of Hologic, or the Purchaser. Pursuant to the terms of Merger Agreement, the Purchaser commenced an all cash tender offer on February 22, 2017, or the Offer, for any (subject to the minimum condition) and all of our outstanding shares of Class A common stock, par value $0.001 per share, or the Shares, at a purchase price of $66.00 per Share, or the Offer Price, net to the seller in cash, without interest, subject to any required withholding of taxes. The Offer will remain open for a minimum of 20 business days from the date of commencement. Following the completion of the Offer, subject to the absence of injunctions or other legal restraints preventing the consummation of the Merger, the Purchaser will merge with and into us, with Cynosure surviving as a wholly-owned subsidiary of Hologic, which we refer to as the Merger, pursuant to the procedure provided for under Section 251(h) of the Delaware General Corporation Law, without any additional stockholder approvals. The Merger will be effected as soon as practicable following the time of purchase by the Purchaser of Shares validly tendered and not withdrawn in the Offer. Unless extended by Hologic in accordance with the Merger Agreement, the offering period for the Offer will expire at the end of the day, 12:00 Midnight, Eastern time, on Tuesday, March 21, 2017. The Merger Agreement contains customary termination rights for both us and Hologic, including, among others, for failure to consummate the Offer on or before August 14, 2017.
We expect to consummate the Merger in late March or April of 2017. However, we have prepared this Annual Report and the forward-looking statements contained in this Annual Report as if we were going to remain an independent company. If the Merger is consummated, many of the forward-looking statements contained in this Annual Report would no longer be applicable.
Corporate Information
We were incorporated under the laws of the State of Delaware in July 1991. Our principal executive offices are located at 5 Carlisle Road, Westford, Massachusetts 01886, and our telephone number is (978) 256-4200. We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and accordingly, file reports, proxy statements and other information with the SEC. Such reports, proxy statements and other information can be read and copied at the public reference facilities maintained by the SEC at the Public Reference Room, 100 F Street, NE, Room 1580, Washington, D.C. 20549. Information regarding the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a website (http://www.sec.gov) that contains material regarding issuers that file electronically with the SEC.
Our website address is www.cynosure.com and is included herein as an inactive textual reference only. The information on our website is not a part of this Annual Report. We make available free of charge on our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
6
Table of Contents
Industry
Aesthetic Market Opportunity
Medical Insight, an independent industry research and analysis firm, estimated that in 2016 total sales of products in the global aesthetic market exceeded $8 billion. Medical Insight believes that total sales of products in the global aesthetic market will increase 11% annually through 2020.
Key factors affecting growth rates in the markets for aesthetic treatment procedures and aesthetic laser equipment include:
| • | improvements in overall economic conditions and an expanding physician base; |
| • | the aging population of industrialized countries and the amount of discretionary income of the “baby boomer” demographic segment; |
| • | greater anticipated growth in Asian markets; |
| • | the desire of many individuals to improve their appearance; |
| • | the development of technology that allows for safe and effective aesthetic treatment procedures as well as advances in treatable conditions; |
| • | the impact of managed care and reimbursement on physician economics, which has motivated physicians to establish or seek to expand their elective aesthetic practices with procedures that are paid for directly by patients; and |
| • | reductions in cost per procedure, which has attracted a broader base of clients and patients for aesthetic treatment procedures. |
Non-Traditional Physician Customers
Aesthetic treatment procedures that use lasers and other light-based equipment have traditionally been performed by dermatologists and plastic surgeons. Based on published membership information from professional medical organizations, there are approximately 19,000 dermatologists and plastic surgeons in the United States. A broader group of physicians in the United States, including primary care physicians, obstetricians and gynecologists, have incorporated aesthetic treatment procedures into their practices. These non-traditional physician customers are largely motivated to offer aesthetic procedures by the potential for a reliable revenue stream that is unaffected by managed care and government payor reimbursement economics. We believe that there are approximately 100,000 of these potential non-traditional physician customers in the United States and Canada, representing a significant market opportunity to be addressed by suppliers of lasers and other light-based aesthetic equipment. Some physicians are electing to open medical spas, often adjacent to their conventional office space, where they perform aesthetic procedures in an environment designed to feel less like a health care facility.
The Structure of Skin and Conditions that Affect Appearance
The human skin consists of several layers. The epidermis is the outer layer and contains the cells that determine pigmentation, or skin color. The dermis is a thicker inner layer that contains hair follicles and large and small blood vessels. Beneath the dermis is a layer that contains subdermal fat. The dermis is composed of mostly collagen, which provides strength and flexibility to the skin.
The appearance of the skin may change over time due to a variety of factors, including age, sun damage, circulatory changes, deterioration of collagen and the human body’s diminished ability to repair and renew itself. These changes include:
| • | unwanted hair growth; |
7
Table of Contents
| • | uneven pigmentation; |
| • | wrinkles; |
| • | blood vessels and veins that are visible at the skin’s surface; |
| • | the appearance of cellulite and fat deposits; and |
| • | scarring. |
Changes to the skin caused by pigmentation are called pigmented lesions and are the result of the accumulation of excess melanin, the substance that gives skin its color. Pigmented lesions are characterized by the brown color of melanin and include freckles, solar lentigines, also known as sun spots or age spots, and café au lait birthmarks. Changes to the skin caused by abnormally large or numerous blood vessels located under the surface of the skin are called vascular lesions. Vascular lesions are characterized by blood vessels that are visible through the skin or that result in a red appearance of the skin. Vascular lesions may be superficial and shallow in the skin or deep in the skin. Shallow vascular lesions include small spider veins, port wine birthmarks, facial veins and rosacea, a chronic skin condition that causes rosy coloration and acne-like pimples on the face. Deep vascular lesions include large spider veins and leg veins.
People with undesirable skin conditions, unwanted hair growth or tattoos often seek aesthetic treatments, including treatments using non-invasive or minimally-invasive laser and light-based technologies.
Laser and Light-Based Aesthetic Treatments
A laser is a device that creates and amplifies a coherent beam of light generally of one wavelength or a narrow band of wavelengths. Lasers have been used for medical and aesthetic applications since the 1960s. Intense pulsed light technology was introduced in the 1990s and uses flashlamps, rather than lasers, to generate incoherent light of multiple wavelengths, often referred to as a broadband of wavelengths. Both lasers and intense pulsed light devices can emit high energy light over varying pulse durations or time intervals.
By producing intense bursts of concentrated light, lasers and other light-based technologies selectively target melanin in hair follicles and pigmented lesions, hemoglobin in blood vessels and vascular lesions, water surrounding collagen in or below the dermis, ink in tattoos or fat tissue below the dermis. When the target absorbs sufficient energy, it becomes heated and/or mechanically disrupted to cause a biological reaction useful for treatment. The degree to which energy is absorbed in the skin depends upon the skin structure targeted—e.g., hair follicle or blood vessel—and the skin type—e.g., light or dark. Different types of lasers and other light-based technologies are needed to effectively treat the spectrum of skin types and conditions. As a result, an active aesthetic practice may require multiple laser or other light-based systems in order to offer treatments to its entire client base.
Different types of lasers are currently used for a wide range of aesthetic treatments. Each type of laser operates at its own wavelength, measured in nanometers, which corresponds to a particular emission and color in the light spectrum. The most common lasers used for non-invasive aesthetic treatments are:
| • | Pulse dye lasers—may produce light of various wavelengths; our pulse dye laser produces an orange light that functions at a shallow penetration depth. |
| • | Alexandrite lasers—produce a near infrared invisible light that functions with high power at a deep penetration depth. |
| • | CO2 lasers—produce infrared invisible light that creates a deep ablation region. |
| • | Diode lasers—may produce light of various wavelengths; our diode lasers produce near infrared invisible light that functions at a deep penetration depth. |
| • | Erbium: glass lasers—produce a near infrared invisible light that functions at a deep penetration depth. |
8
Table of Contents
| • | Er:YAG lasers—produce a near infrared invisible light that creates a shallow ablation region. |
| • | Nd:YAG lasers—produce a near infrared invisible light that functions over a wide range of penetration depths or when frequency doubled produce a green light that functions at a shallow penetration depth. |
In addition to selecting the appropriate wavelength for a particular application, laser and other light-based treatments require an appropriate balance among three other parameters to optimize safety and effectiveness for aesthetic treatments:
| • | energy level—the amount of light emitted to heat the target; |
| • | pulse duration—the time interval over which the energy is delivered; and |
| • | spot size—the diameter of the energy beam. |
As a result of the wide variety of aesthetic treatments, patient skin types and users of technology, customer purchasing objectives for aesthetic treatment systems are diverse. We believe that as aesthetic spas and non-traditional physician customers play increasingly important roles as purchasers of aesthetic treatment systems, the market for these products will become even more diverse. Specifically, we expect that owners of different types of aesthetic treatment practices will place different emphases on various system attributes, such as breadth of treatment applications, return on investment, upgradeability and price. Accordingly, we believe that there is significant market opportunity for a company that tailors its product offerings to meet the needs of a wide range of market segments.
Our Solution
We offer tailored customer solutions to address the market for non-invasive and minimally invasive laser and light-based aesthetic treatment applications, as well as RF energy based surgical and aesthetic applications. These solutions include non-invasive and minimally invasive procedures to remove hair, treat vascular and benign pigmented lesions, remove multi-colored tattoos, revitalize the skin, reduce fat through laser lipolysis, reduce cellulite, clear nails infected by toe fungus, ablate sweat glands and improve women’s health. We believe our laser and other light-based and RF systems are reliable, user friendly and easily incorporated into both physician practices and medi-spas. We complement our product offerings with comprehensive and responsive service offerings, including assistance with training, aesthetic practice development consultation and product maintenance.
We believe that the following factors enhance our market position:
| • | Broad Technology Base. Our light-based products are based on a broad range of technologies and incorporate different types of lasers, such as Alexandrite, pulse dye, CO2, Erbium: glass, Er:YAG, Nd:YAG and diode, as well as intense pulsed light devices. We believe we are one of a few companies that currently offer aesthetic treatment systems using Alexandrite and pulse dye lasers, which are particularly well suited for some applications and skin types. The following table provides information regarding the principal energy sources used in laser and other light-based aesthetic treatments that we offer and the primary application of each of these energy sources. |
| Energy Source |
Type of Light/Wavelength |
Principal Applications | ||
| Pulse Dye Laser |
Visible light (Orange)(585/595 nm) |
Vascular lesions, including shallow and deep lesions | ||
| Alexandrite Laser |
Near infrared invisible light (755 nm) Long pulse (millisecond)
Short pulse (nanosecond)
Ultra short pulse (picosecond) |
Hair removal, particularly for light skin types Tattoo and benign pigmented lesions removal Tattoo and benign pigmented lesions removal | ||
9
Table of Contents
| Energy Source |
Type of Light/Wavelength |
Principal Applications | ||
|
CO2 Laser |
Infrared invisible light (10.6µm) |
Skin resurfacing Treatment of wrinkles and textural irregularities Women’s health | ||
| Diode Laser |
Near infrared invisible light (800/805/924/940/975/980 nm) |
Hair removal, particularly for light skin types Vascular lesions, particularly shallow lesions Temporary reduction in the appearance of cellulite Treatment of fat | ||
| Erbium: glass laser |
Near infrared invisible light (1540 nm) |
Skin resurfacing Treatment of surgical scars, acne scars and stretch marks | ||
| Er:YAG lasers |
Near infrared invisible light (2940 nm) |
Skin resurfacing Treatment of wrinkles and textural irregularities | ||
| Nd:YAG Laser |
Near infrared invisible light (1064/1320/1440 nm) Visible light (green)(532 nm) |
Hair removal, particularly for medium and dark skin types Vascular lesions, particularly deep lesions Treatment of fat and cellulite Tattoo and benign pigmented lesions removal | ||
| Intense Pulsed Light |
Visible/Near infrared invisible light (400-950 nm) |
Hair removal, all skin types Vascular lesions, particularly shallow lesions and benign pigmented lesions | ||
| Multiple Energy Source Workstations |
Multiple | Multiple | ||
| (incorporating two or more energy sources) |
||||
| • | Expansive Portfolio of Aesthetic Treatment Systems. We offer a variety of individual workstations tailored to specific high volume cosmetic applications to enable our customers to select the aesthetic treatment system best suited to their practice, business or clinical need. Our product portfolio includes single energy source systems as well as workstations that incorporate two or more different types of lasers or light-based technologies. By offering multiple technologies and system alternatives at a variety of price points, we seek to provide customers with tailored solutions that meet the specific needs of their practices while providing significant flexibility in their level of investment. |
| • | Energy-Sourced Systems. Our 2014 Ellman asset acquisition complemented and broadened our product line with the addition of multiple RF generators and single use electrodes for aesthetic and multi-specialty surgical indications such as facial plastic and general surgery, gynecology, ear, nose, and throat procedures, ophthalmology, oral and maxillofacial surgery, podiatry and proctology. |
| • | Upgrade Paths Within Product Families. We design our products to facilitate upgrading within product families. These upgrade paths provide our customers with the opportunity to add additional energy sources and handpieces, which provides our customers with technological flexibility as they expand their practices. |
10
Table of Contents
| • | Global Presence. We have offered our products in international markets for 25 years, with approximately 32% of our product revenue generated from product sales outside of North America in 2016. We target international markets through a direct sales force in Canada, France, Morocco, Germany, Spain, the United Kingdom, Australia, China, Japan and Korea and through international distributors in over 120 other countries. |
| • | Strong Reputation Established Over 25 Year History. We have been in the business of developing and marketing aesthetic treatment systems for 25 years. As a result of this history, we believe the Cynosure brand name is associated with a tradition of technological leadership. |
Our Business Strategy
Our goal is to become the worldwide leader in providing non-invasive and minimally invasive aesthetic treatment systems. The key elements of our two-pronged business strategy to achieve this goal are to:
| • | Launch Innovative New Products and Technologies into High-Growth Aesthetic Applications. Our research and development team builds on our existing broad range of laser and light-based technologies to develop new solutions and products to target unmet needs in significant aesthetic treatment markets. Innovation continues to be a strong contributor to our strength. |
| • | SculpSure® Laser System. In the second half of 2015, we commenced commercialization of our SculpSure laser system for non-invasive fat reduction treatments. The SculpSure system requires the use of a PAC to activate each applicator handpiece used in a treatment cycle. We offer PACs in “keys” containing 100 applicator uses. The SculpSure system is the first hyperthermic laser treatment system cleared by the FDA. In May 2015, we received FDA clearance to market the system for the treatment of flanks, and in July 2015 for the treatment of the abdomen. In September 2015, we received European Medical Device Directive certification from the European Notified Body, which allows us to place the “CE” Mark on the SculpSure system for distribution in the European Union and its member states. In December 2015, we received marketing authorization in Australia. In July 2016, we received marketing authorization in Canada and Korea. |
| • | In 2013, we launched our PicoSure system for the removal of tattoos and benign pigmented lesions. The PicoSure system, which is based on years of research and development effort and expense, was the first commercially available picosecond Alexandrite aesthetic laser platform using a 755 nm wavelength. We received FDA clearance to market the PicoSure laser for the removal of tattoos and benign pigmented lesions in 2012. We received marketing authorization for our PicoSure system in Canada and Australia in 2013, and in Korea and Taiwan in 2014. Our PicoSure FOCUS Lens Array microscopically concentrates the PicoSure laser pulse to a precise depth and exposes less than 10% of the skin to areas of high fluence while the surrounding skin is exposed to a low background fluence. We received FDA clearance to market PicoSure with the FOCUS Lens Array for the treatment of acne scars and wrinkles in 2014. In 2015, we received FDA clearance to market the PicoSure 532 nm wavelength, which we designed to more effectively treat red, yellow and orange tattoo ink colors, which we offer as an upgrade to our current PicoSure customer base. Further, we received clearance in 2015 from the China Food and Drug Administration to market the PicoSure 755 nm wavelength for tattoo removal. In May 2016, we received FDA clearance to market the PicoSure 1064 nm wavelength, which we designed to more effectively treat black and other dark tattoo ink. |
| • | In 2006, we expanded beyond our legacy non-invasive products by offering a minimally invasive aesthetic treatment system, the SmartLipo system. The SmartLipo system was the first FDA-cleared laser lipolysis system for use in a minimally invasive procedure for the removal of unwanted fat. Since the launch of the SmartLipo system in 2006, we have introduced new workstations; innovated MultiPlex technology, which enables the energy from two lasers to be |
11
Table of Contents
| blended during delivery by quickly following a pulse of energy from one laser with a pulse of energy from another laser, and also introduced SmartSense and ThermaGuide, our proprietary intelligent delivery systems. In 2011, we launched our Cellulaze Workstation, the world’s first aesthetic laser device for the treatment of cellulite, into the European community. In 2012, we received FDA clearance to sell and market the Cellulaze workstation in the United States. In 2013, we launched the PrecisionTx technology for minimally invasive removal of fat in small areas such as the neck and jawline as well as ablation of axillary sweat glands. |
| • | Expand Product Offerings Through Strategic Acquisitions and Significant Partnerships. We have enhanced our product offerings through acquisition of complementary businesses, products and technologies and intend to continue to do so. Such acquisitions may include small and substantial acquisitions, as well as joint ventures and other collaborative projects. |
| • | In November 2014, we entered into an exclusive distribution agreement with El.En. to market and distribute in North America the MonaLisa Touch product, a CO2 laser therapy for gynecologic health for postmenopausal women, breast cancer survivors and women who have undergone hysterectomies and who may suffer from changes to their gynecologic health. In addition, we received a medical device license issued by Health Canada to market MonaLisa Touch for the treatment of symptoms related to GSM, including vaginal dryness, vaginal burning, vaginal itching, pain, dysuria and dyspareunia. In the fourth quarter of 2015, we and El.En. agreed to market and distribute the MonaLisa Touch laser under separate distribution agreements with our respective wholly-owned subsidiaries in the United Kingdom, Germany, and Spain. |
| • | In September 2014, we acquired substantially all of the assets of Ellman, a leading provider of advanced RF technology for precision surgical and aesthetic procedures diversifying our laser aesthetic base business. Ellman also offers a line of aesthetic lasers. |
| • | In 2013, we acquired Palomar, which complemented and broadened our product lineup by adding the Icon Aesthetic System, the StarLux laser and pulsed light system and the Vectus diode laser. The Icon Aesthetic System provides a comprehensive suite of the most popular treatments from hair removal to wrinkle reduction to scar and stretch mark treatment, and the Vectus diode laser is a dedicated solution for high-volume hair removal. |
| • | In 2011, we expanded our product portfolio by acquiring substantially all of the assets of HOYA ConBio’s aesthetic laser business including the MedLite C6 and RevLite systems. |
| • | Offer a Full Range of Tailored Aesthetic Solutions. We believe that we have one of the broadest product portfolios in the industry, with multiple product offerings incorporating a range of laser and light sources at various price points across many aesthetic applications. Our approach is designed to allow our customers to select products that best suit their client base, practice size and the types of treatments that they wish to offer. This allows us to address the needs of the traditional physician customer market as well as the growing non-traditional physician customer market. Many of our newer products can be upgraded to systems with greater functionality as our customers’ practices expand. |
| • | Provide Comprehensive, Ongoing Customer Service. We support our customers with a worldwide service organization that includes 59 field service professionals in North America and 82 field service professionals outside of North America. Field service engineers install our products and respond rapidly to service calls to minimize disruption to our customers’ businesses. Most of our new products are modular in design to enable quick and efficient service and support. We maintain our service infrastructure with training and inventory hubs in Europe and the Asia/Pacific region. |
| • | Generate Additional Revenue from Existing Customer Base. We believe that there are opportunities for us to generate additional revenue from existing customers who are already familiar with our products for increasing treatment volumes or new treatment applications. We also expect that customers purchasing our new modular products will be candidates for technology upgrades to enhance the capabilities of their systems. In addition, several of our products contain consumable parts, and we generate additional revenue |
12
Table of Contents
| on sales of these consumable parts to our existing customers. As we continue to grow our service organization, we are seeking to increase the percentage of our customers that enter into service contracts, which would provide additional recurring customer revenue. |
Products
We offer a broad portfolio of light-based aesthetic treatment systems that address a wide variety of applications.
The following table provides information concerning our flagship products and their applications. We use the flagship designation for our products that are our leading products for a particular application.
| Hair Removal |
Vascular Lesions |
Skin Rejuve- nation(1) |
Benign Pigmented Lesions |
Treat Cellulite |
Scars | Tattoo Removal |
Anti- Aging |
Minimally Invasive LaserBody Sculpting for the Removal of Unwanted Fat |
Non-Invasive Removal of Unwanted Fat |
Women’s Health | ||||||||||||
| Flagship Products: |
||||||||||||||||||||||
| Elite Family |
Flagship | X | X | X | ||||||||||||||||||
| SmartLipo Family |
Flagship | |||||||||||||||||||||
| Cellulaze |
Flagship | |||||||||||||||||||||
| Cynergy |
X | Flagship | X | X | ||||||||||||||||||
| MedLite C6 /RevLite |
X | Flagship | X | X | ||||||||||||||||||
| PicoSure |
Flagship | Flagship | ||||||||||||||||||||
| Icon |
X | X | Flagship | X | Flagship | Flagship | ||||||||||||||||
| Vectus |
Flagship | |||||||||||||||||||||
| SculpSure |
Flagship | |||||||||||||||||||||
| MonaLisa Touch |
Flagship |
| (1) | We consider skin rejuvenation to be the treatment of shallow vascular lesions and benign pigmented lesions to rejuvenate the skin’s appearance. |
System Components
Each of our systems consists of a control console and one or more handpieces. The systems acquired from Ellman consist of RF-based control consoles where energy is transferred through a handpiece or electrode. Our control consoles are each comprised of a graphical user interface, control system software and high voltage electronics. Depending on the system, the laser or other light source may be within the control console or the handpiece. The graphical user interface allows the practitioner to set the appropriate laser or flashlamp parameters, such as energy and pulse duration, to meet the requirements of a particular application for each particular patient. The control system software communicates the operator’s instructions from the graphical user interface to the system’s components and manages system performance and calibration. For systems having multiple light sources within the control system, the graphical user interface allows the practitioner to change sources with the press of a button. For systems having light sources within handpieces, the graphical user interface automatically detects the connection of each handpiece and provides the appropriate display to the user.
The handpieces are used to deliver the light energy from the laser or other light source to the treatment area. For systems having the laser within the control console, the handpieces deliver the laser energy through a maneuverable optical fiber to the treatment area. For systems having the laser or flashlamp within the handpiece, the light energy is shaped through optical components before being delivered to the treatment area.
Several of our products use consumable parts. The PicoSure FOCUS Lens Array is a consumable micro lens array tip, which delivers the laser energy to the treatment area. The SmartLipo, Cellulaze and PrecisionTx systems use consumable laser fibers to deliver the laser energy directly to the treatment area. The surgical systems acquired from Ellman use consumable surgical electrodes and accessories. The SculpSure system requires the use of a PAC to activate each applicator handpiece used in a treatment cycle. We offer PACs in “keys” containing 100 applicator uses.
13
Table of Contents
For many applications, practitioners use cooling to protect the skin. The cooling system may be a separate system or integrated into the laser or intense pulsed light system itself. When not integrated, we offer our customers the SmartCool treatment cooling system, which we purchase from a third-party supplier and sell as a private label product under the SmartCool brand. The SmartCool product has nine variable settings and allows the practitioner to provide a continuous flow of chilled air before, during and after treatment to cool and comfort the patient’s skin. The SmartCool handpiece, which is specially designed for use with our laser systems, interlocks with the laser handpiece. In contrast to some competitive cooling systems, there are no disposable supplies required to use our integrated cooling systems or our SmartCool system.
Applications
Practitioners use our products to perform a variety of non-invasive procedures to remove hair, treat vascular and benign pigmented lesions, remove multi-colored tattoos, revitalize the skin, reduce fat through laser lipolysis, reduce cellulite, clear nails infected by toe fungus, ablate sweat glands and improve women’s health. Practitioners also use our products to perform minimally invasive procedures for the reduction of fat and the treatment of cellulite. The applications of our products are described below.
LaserBodySculpting ™ for the Removal of Unwanted Fat. The SmartLipo system was the first laser lipolysis system to offer a minimally invasive procedure for the removal of unwanted fat. LaserBodySculpting procedures with the SmartLipo system enables physicians to remove localized deposits of fat by inserting a small cannula, or metal tube, containing a laser fiber under the skin in direct contact with the treatment area. The laser energy causes the fat cells to rupture and melt. In addition, the laser energy promotes collagen shrinkage and causes a tissue tightening effect. LaserBodySculpting is a minimally invasive procedure; therefore, it can be performed under local anesthesia with minimal trauma in comparison to alternative liposuction procedures. We also offer our SmartLipo MPX system, which includes a second wavelength and features our patented MultiPlex technology, which enables the energy from two lasers to be blended during delivery by quickly following a pulse of energy from one laser with a pulse of energy from another laser, and also introduced in 2008 SmartSense and ThermaGuide our proprietary intelligent delivery systems. In addition, we sell our SmartLipo Triplex system, which includes a third wavelength. In 2013, we launched the PrecisionTx system for minimally invasive removal of fat in small areas such as the neck and jawline as well as ablation of axillary sweat glands. More recently, we launched in the second half of 2015 our SculpSure laser system, the world’s first FDA-cleared hyperthermic laser treatment for non-invasive lipolysis of the flanks and abdomen. Utilizing patented technology, SculpSure is designed to reduce fat non-invasively by eliminating subcutaneous fat cells. The versatile, hands-free device features a flexible applicator system to treat multiple anatomical areas of the body. SculpSure, which uses a 1060 nm diode laser, can treat an anatomical area in approximately 25 minutes. Patients are able to achieve desired results without downtime or surgery.
Cellulite. Cellulite is a deposit of fat that causes a dimple or other uneven appearance of the skin, typically around the thighs, hips and buttocks, more commonly found in women than men. According to published reports, an estimated 85% of women have some degree of cellulite. In 2011, we introduced our Cellulaze Cellulite Laser Workstation into the European community, and in 2012, we received FDA clearance to sell and market the product in the United States. The Cellulaze system was the world’s first minimally invasive surgical device designed to reduce cellulite by restoring the normal structure of the skin and underlying connective tissue. In the Cellulaze procedure, which is performed under a local anesthetic, the physician inserts a small cannula under the skin. Our SideLight 3D side-firing technology directs controlled, laser thermal energy to the treatment zones. The laser is designed to diminish the lumpy pockets of fat, release the areas of skin depression and increase the elasticity and thickness of the skin. Patients require just one treatment. Like the SmartLipo systems, the Cellulaze system incorporates the ThermaGuide intelligent delivery system which allows the physician to accurately monitor temperature and determine the treatment doses that will provide safe and more effective tissue tightening through tissue coagulation and maintain an even, controlled flow of laser energy.
14
Table of Contents
Hair Removal. In a typical laser or pulsed light hair removal treatment the practitioner selects appropriate laser or pulsed light parameters based on the patient’s skin and hair types. If the system does not have integrated contact cooling in the handpiece, the practitioner often pre-cools the treatment area with cold air from the Zimmer SmartCool system. Next, the practitioner applies the handpiece to the target area. If the handpiece has integrated contact cooling, the skin is pre-cooled upon contact with the handpiece. The handpiece delivers laser or pulsed light energy to the skin and it is selectively absorbed by the target melanin in the hair follicle, destroying the hair follicle without harming the surrounding skin. We commercialized our first laser system for hair removal in 1997 and our first intense pulsed light system for hair removal and removal of pigmented lesions in 2001. Since then, we have launched several workstations including multiple light sources for the treatment of hair removal and various other skin conditions. Today, these systems include the Elite MPX and Icon systems. In 2012, we introduced the Vectus diode laser system for dedicated, high-volume hair removal. The Elite MPX workstation and Vectus system are our flagship products for hair removal. The Elite MPX workstation features a built-in Zimmer SmartCool skin cooling system which is integrated into a single compact model saving office space and reducing treatment time. Our Elite MPX and Apogee Elite products include two energy sources in one laser system: an Alexandrite laser, which is best suited for patients with light skin types, and an Nd:YAG laser, which is best suited for hair removal for patients with medium and dark skin types or tanned skin. The practitioner can switch between these two energy sources simply by pushing a button on the system console. Our Elite MPX allows the practitioner to blend the two energy sources for a customized treatment protocol. The Icon system provides multiple intense pulsed light handpieces for hair removal with integrated contact cooling, including the MaxR™ handpiece with large spot size and MaxRs™ handpiece with small spot size for use on all skin types and the MaxYs™ handpiece for hair removal on lighter skin types and removal of pigmented lesions. The Vectus diode laser features the largest spot size and most uniform beam profile available today and integrated contact cooling allowing providers to provide high-volume hair removal on a wide range of skin and hair types.
Treatment of Tattoos. In 2012, we received FDA clearance to market our PicoSure system, a picosecond laser technology platform, in the United States for removal of tattoos and benign pigmented lesions. We commenced commercialization of the PicoSure system in early 2013. Picosecond lasers deliver pulses that are measured in trillionths of a second in contrast with nanosecond technology, such as our MedLite and RevLite products, which deliver pulses in billionths of a second. The ultra-short pulses of the PicoSure laser provide both photothermal and photomechanical action. In clinical studies that we have conducted, the shorter pulse duration of the picosecond laser achieved increased efficiency in removing tattoo pigment, which we believe results in fewer treatments and better overall treatment outcomes than current laser technology. In October 2013, we launched the PicoSure FOCUS Lens Array for use with the PicoSure system to microscopically concentrate the PicoSure laser pulse to a precise depth and expose less than 10% of the skin to areas of high fluence while the surrounding skin is exposed to a low background fluence. In February 2015, we received FDA clearance to market the 532 nm wavelength for PicoSure, which we designed to more effectively treat red, yellow and orange tattoo ink colors and which we offer as an upgrade to our current PicoSure customer base. In May 2016, we received FDA clearance to market the 1064 nm wavelength for PicoSure, which we designed to more effectively treat black and other dark colored tattoo inks. The MedLite and RevLite systems provide frequency-doubled q-switched Nd:YAG laser energy for tattoo removal and can be used with the MultiLite Dye handpieces to provide 585 nm (yellow) and 650 nm (red) laser energy for full-color tattoo removal.
Anti-Aging. We believe the marketplace has moved to a less invasive approach for treating the indications of aging, including the treatment of wrinkles, pigmentation, redness and overall skin rejuvenation. Anti-aging treatments were historically performed by physicians who could only target one condition and one skin layer during each treatment. Previously, patients often faced longer, more painful procedures that penetrated deep into the dermal layers and could potentially damage healthy skin. Our PicoSure, Icon, Elite, MedLite and RevLite systems provide a non-ablative and micro-ablative treatment approach for wrinkles, skin texture, skin discoloration and skin tightening through tissue coagulation.
Treatment of Benign Pigmented Lesions. Given that the pigment associated with pigmented lesions is generally located close to the skin surface, practitioners generally do not pre-cool the target area. To treat
15
Table of Contents
pigmented lesions, the practitioners apply laser or intense pulsed light energy to the treatment area to damage or destroy the lesion. The lesion will then form a scab that sloughs off over time to reveal clearer skin beneath. Our PicoSure system delivers picosecond pulses of Alexandrite laser energy to remove benign pigmented lesions using both photothermal and photomechanical action. The PicoSure system may be used with the PicoSure FOCUS Lens Array to microscopically concentrate the PicoSure laser pulse to a precise depth and exposes less than 10% of the skin to areas of high fluence while the surrounding skin is exposed to low background fluence. Our MedLite and RevLite laser systems provide frequency-doubled Q-Switched Nd:YAG laser energy for removal of pigmented lesions. The MedLite provides a true flat-top beam profile for consistent results. The Icon system provides the LuxYs and LuxG pulsed light handpieces for the removal of pigmented lesions and the 1540 nm fractional laser for the removal of melasma. The broadband light of the LuxYs handpiece is optimally filtered to treat darker pigmented lesions. The Elite system provides 755 nm Alexandrite laser energy for the treatment of pigmented lesions.
Treatment of Vascular Lesions. To treat vascular lesions, the practitioner generally first pre-cools the target area, with the system handpiece or an external cooling system, and then uses the system handpiece to deliver laser or intense pulsed light energy to the treatment area to damage or destroy the blood vessels. One or more treatments may be required depending upon the type of lesion. Our flagship Icon system provides the MaxG™ intense pulsed light handpiece with Dynamic Spectrum Shifting™ and dual-band filters for more uniform heating across the entire diameter of larger vessels. For leg veins, the Icon system provides the Lux 1064+™ laser handpiece. The Elite system also provides 1064 nm laser energy for treatment of vascular lesions, both facial and leg veins.
Our Cynergy system is also used for the treatment of vascular lesions. The Cynergy system combines a pulse dye laser, which is best suited for treating shallow vascular lesions, such as port wine birthmarks, facial veins and rosacea, and an Nd:YAG laser, which is best suited for treating large or deep veins, such as leg veins. The practitioner can switch between these two energy sources simply by pressing a button on the system console. The Cynergy system also includes our patented MultiPlex technology that enables the energy from the two lasers to be blended during delivery by quickly following a pulse of energy from the pulse dye laser with a pulse of energy from the Nd:YAG laser. In addition to the Cynergy system, certain of our other systems can be used for the treatment of vascular lesions.
Treatment of Scars. Our PicoSure, Icon, MedLite and RevLite systems use short pulses of micro-fine laser light to reach deeply into the skin’s sub-layers, treating the support structure. The body’s natural healing process sweeps away older, damaged tissue and rebuilds it with fresh, new collagen and elastin to remove or reduce the appearance of the scar.
Axillary Gland Ablation. Historically, topical antiperspirants or oral medications have been recommended as the best treatment available. Our PrecisionTx system provides minimally invasive laser ablation of the axillary glands (glands in the armpit area).
Treatment of Onychomycosis. Onychomycosis is a condition marked by the growth of fungus under the nail. Fungi feed on keratin, the protein that makes up the hard surface of the toenails. The infected nail often turns darker in color, and debris may accumulate under the nail. As the infection continues, the nail either may crumble gradually and fall off or thicken. Our PinPointe FootLaser uses laser light to kill the fungus that lives in and under the nail without causing damage to the nail or the surrounding skin. The treatment typically takes 20 minutes with no downtime.
Women’s Health. GSM is a condition marked by the deterioration of the vaginal walls associated with the loss of estrogen due to aging, hormonal treatments for breast cancer, and other conditions affecting primarily postmenopausal women, breast cancer survivors and women who have undergone hysterectomies. The MonaLisa Touch delivers short CO2 ablative laser pulses to the vaginal wall, decreasing GSM symptoms such as vaginal dryness, soreness and itching as well as painful urination and intercourse. The MonaLisa Touch system is
16
Table of Contents
designed to stimulate and promote the regeneration of collagen fibers and the restoration of hydration and elasticity within the vaginal mucosa. The procedure, which can be administered in a doctor’s office, requires no anesthesia and has been performed on thousands of patients worldwide.
Sales and Marketing
We sell our aesthetic treatment systems to the traditional physician customer base of dermatologists and plastic surgeons as well as to non-traditional physician customers who are providing aesthetic services using laser and light-based technology. Non-traditional physician customers can include primary care physicians, obstetricians and gynecologists.
We target potential customers through office visits, trade shows and trade journals. We also conduct clinical workshops and webinars featuring recognized expert panelists and opinion leaders to promote existing and new treatment techniques using our products. We believe that these workshops and webinars enhance customer loyalty and provide us with new sales opportunities. We also use direct mail programs to target specific segments of the market that we seek to access, such as members of medical societies and attendees at meetings sponsored by medical societies or associations. We actively maintain a public relations program to promote coverage of our products on daytime television shows in the United States and Europe and we are active on popular social media outlets. In addition, our products are featured in several publications around the world.
We do not provide financing to our customers to purchase our products. If a potential customer requests financing, we refer the customer to third-party financing sources.
Physician Sales
We sell our products to physicians in North America through a direct sales force. Outside of North America, we sell our products to physicians through a direct sales force in France, Morocco, Germany, Spain, the United Kingdom, Australia, China, Japan and Korea and through independent distributors in over 120 other countries.
We conduct our own international sales and service operations through wholly owned subsidiaries in France, Morocco, Germany, Spain, the United Kingdom, the Netherlands, Australia, China, Japan, Korea and Mexico. We seek distributors in international markets where we do not believe that a direct sales presence is warranted or feasible. In those markets, we select distributors that have extensive knowledge of our industry and their local markets. Our distributors sell, install and service our products. We require our distributors to invest in service training and equipment, to stock and supply maintenance and service parts for our systems, to attend exhibitions and industry meetings and, in some instances, to commit to minimum sales amounts to gain or retain exclusivity. We have written distribution agreements with most of our third-party distributors. Generally, the written agreements with our distributors have terms of between one and two years.
See Note 6 to our consolidated financial statements included in this Annual Report for revenues by geographic region.
Service and Support
We support our customers with a range of services, including installation and product training, business and practice development consulting and product service and maintenance. In North America, our field service organization has 59 field service professionals. Outside of North America, we employ 82 field service professionals.
In connection with direct sales of our aesthetic treatment systems, we arrange for the installation of the system and initial product training. The installation is conducted by our field service engineers. The costs of installation and initial training for North American purchasers are all included in the purchase price of our systems. We also offer for an additional charge a more comprehensive package of services from pre-qualified third-party consultants. Our training and additional services are particularly appealing to the non-traditional physician customer and aesthetic spa segments of the market, which have less familiarity with the business aspects of laser and light-based aesthetic treatments than dermatologists and cosmetic surgeons.
17
Table of Contents
Within North America, we strive to respond to all service calls within 24 hours to minimize disruption of our customers’ businesses. We have designed our products in a modular fashion to enable quick and efficient service and support. Specifically, we build these products with several separate components that can easily be removed and replaced when the product is being serviced. We provide initial warranties on our products to cover parts and service, and we offer extended warranty packages that vary by type of product and level of service desired. Our base warranty typically covers parts and service for one year. We offer extended warranty arrangements through service contracts. We believe that we have a significant opportunity to increase our recurring customer revenues by increasing the percentage of our customers that enter into service contracts for our systems.
Research and Development
Our research and development team consists of 84 employees, including four physicists, with a broad base of experience in lasers and optoelectronics. Our research and development team works closely with opinion leaders and customers, both individually and through our sponsored seminars, to understand unmet needs and emerging applications in the field of aesthetic skin treatments and to innovate and develop new products and improvements to our existing products. They also conduct and coordinate clinical trials of our products. Our research and development team builds on the significant base of patented and proprietary intellectual property that we have developed in the fields of laser and other light-based technologies since our inception in 1991. From time to time, we may enter into collaborative research and development agreements to enhance our technology and develop new products.
Our research and development expenses were $29.0 million, $22.3 million and $22.0 million in the years ended December 31, 2016, 2015 and 2014, respectively.
Manufacturing and Raw Materials
We manufacture several of our products, including our Cynergy, Accolade, Elite MPX and PicoSure product families. We manufacture these products with components and subassemblies purchased from third-party suppliers. Accordingly, our manufacturing operations consist principally of assembly and testing of our systems and integration of our proprietary optics and software. We design the products that we manufacture so that they are built in a modular fashion. This approach enables us to manufacture and service our products more efficiently. We purchase many of our components and subassemblies from third-party manufacturers on an outsourced basis. We use one third-party to assemble and test many of the components and subassemblies for our Cynergy, Elite MPX and PicoSure product families.
For other products, such as the Elite family and SculpSure products, as well as the Icon and Vectus systems, we rely on third parties for manufacturing and testing. In addition, we depend on El.En. for the SLT II laser system that we integrate with our own proprietary software and delivery systems into our SmartLipo Triplex, Cellulaze and PrecisionTx systems.
We use Alexandrite rods in the lasers for our Elite and PicoSure systems. We depend exclusively on Northrop Grumman SYNOPTICS to supply the Alexandrite rods to us, and we are aware of no alternative supplier of Alexandrite rods meeting our quality standards. We offer our SmartCool cooling systems for use with our laser aesthetic treatment systems, and we depend exclusively on Zimmer Elektromedizin GmbH to supply SmartCool systems to us. We use diode laser bars from Coherent to manufacture our Vectus diode laser, and we use diode laser modules from Dilas to manufacture our SculpSure® laser system. Although alternative suppliers exist for the diode laser bars, they could take months to qualify and implement.
We do not have long-term contracts with our third-party manufacturers or sole source suppliers. We generally purchase components and subassemblies as well as our other supplies on a purchase order basis or with one year purchase agreements. If for any reason, our third-party manufacturers or sole source suppliers are not
18
Table of Contents
willing or able to provide us with components, subassemblies or supplies in a timely fashion, or at all, our ability to manufacture and sell many of our products could be impaired. To date, we have been able to obtain adequate outsourced manufacturing services and supplies of components from our third-party manufacturers and suppliers in a timely manner. We believe that over time alternative component and subassembly manufacturers and suppliers can be identified if our current third-party manufacturers and suppliers fail to fulfill our requirements.
We offer our MonaLisa Touch and PinPointe FootLaser systems pursuant to distribution agreements.
Backlog
We generally do not maintain a significant backlog. As a result, we do not believe that our backlog at any particular time is indicative of future sales levels.
El.En. Commercial Relationship
We have several distribution agreements with El.En. Under one of these agreements, we purchase from El.En. its SmartLipo MPX system and its proprietary SLT II laser system. The SLT II laser system is an essential component of our SmartLipo Triplex, Cellulaze, and PrecisionTx systems, which also incorporate our proprietary software and delivery systems. We have exclusive worldwide rights under this agreement to sell the SmartLipo MPX system and our products containing the SLT II laser system. Under another distribution agreement with El.En. and separate distribution agreements with certain of our wholly-owned subsidiaries in the United Kingdom, Germany and Spain, we purchase from El.En. its MonaLisa Touch laser system.
The prices at which we purchase these laser systems from El.En. are specified in the agreements; however, they may be changed by El.En. at its discretion upon 30 days’ notice. El.En. is required to provide us with training for the products we distribute under these agreements, as well as marketing and other sales support for such products as we and El.En. may agree. We are required to use commercially reasonable efforts to sell and promote our systems containing these laser systems, and we are responsible for obtaining and maintaining regulatory approvals for such products. The first distribution agreement has an initial term that expires in October 2019, and the second distribution agreement has an initial term that expires in November 2021. Each agreement automatically renews for additional one-year terms unless either party provides notice of termination at least six months prior to the expiration of the initial term or any subsequent renewal term. We or El.En. may terminate these agreements at any time based upon material uncured breaches by, or the insolvency of, the other party. In addition, El.En. may terminate each agreement if we do not meet annual minimum purchase obligations specified in the agreement and we may terminate if El.En. rejects a purchase order that is in line with our forecast.
Patents, Proprietary Technology and Trademarks
Our success depends in part on our ability to obtain and maintain proprietary protection for our products, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. We also rely on trademarks, trade secret and copyright laws and contractual restrictions to protect our proprietary technology. These legal protections afford only limited protection for our technology.
As of December 31, 2016, we were the sole owner of over 50 U.S. patents, as well as many U.S. pending applications and foreign patents and pending applications. We are also joint owners, including with El.En., of certain patents and pending applications. We are also licensed non-exclusively to certain patents owned by third parties and we have granted exclusive and non-exclusive licenses to third parties to certain of our patents,
19
Table of Contents
pending applications and know how. The term of individual patents depends upon the legal term for patents in the countries in which they are obtained. In most countries, including the United States, the patent term is 20 years from the earliest filing date of a non-provisional patent application. We expect our issued and exclusively licensed patents to expire at various dates that range from as early as February 2018 to as late as April 2031.
Prior to their expiration in the United States on February 1, 2015 and their foreign counterparts in several foreign jurisdictions on January 31, 2016, our Palomar subsidiary was the exclusive licensee of certain hair removal patents owned by Massachusetts General Hospital or MGH. Palomar has granted royalty bearing, non-exclusive sublicenses to those patents to third parties, including to us in 2006. We paid a royalty to MGH for sales of our hair removal products. Palomar was also the non-exclusive licensee of several other U.S. patents as well as corresponding foreign patents and pending applications owned by MGH, and Palomar was the joint owner with MGH and, in some cases, the exclusive licensee, of other U.S. patents as well as corresponding U.S. pending applications and foreign patents and pending applications. Palomar has also granted non-exclusive rights to other parties to other patents and received non-exclusive licenses to patents owned by other parties.
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. Our ability to maintain and solidify our proprietary position for our technology will depend on our success in obtaining effective patent claims and enforcing those claims once granted.
We do not know whether any of our patent applications or those patent applications that we license will result in the issuance of any patents. Our issued patents and those that may be issued in the future, or those licensed to us, may be challenged, invalidated or circumvented, which could limit our ability to stop competitors from marketing related products or shorten the term of patent protection that we may have for our products. In addition, the rights granted under any issued patents may not provide us with competitive advantages against competitors with similar technology. Furthermore, our competitors may independently develop similar technologies or duplicate any technology developed by us.
We seek to limit disclosure of our intellectual property by requiring employees, consultants and any third party with access to our proprietary information to execute confidentiality agreements with us and often agreements that include assignment of rights provisions to us. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our intellectual property may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants or contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. Due to rapid changes in technology, we believe that factors such as the technological and creative skills of our personnel, new product developments and enhancements to existing products are as important as the various legal protections of our technology to establishing and maintaining a leadership position.
Despite our efforts to protect our proprietary rights, unauthorized parties may attempt to copy aspects of our products or to obtain and use information that we regard as proprietary. Policing unauthorized use of our technology is difficult. Litigation may be necessary to enforce intellectual property rights, to protect our trade secrets, to determine the validity and scope of the proprietary rights of others or to defend against claims of infringement or invalidity. Any such resulting litigation, even if we are ultimately successful, could result in substantial costs and diversion of resources and could have a material adverse effect on our business, operating results and financial condition. There can be no assurance that our means of protecting proprietary rights will be adequate or that our competitors will not independently develop similar technology. Any failure by us to meaningfully protect our proprietary rights could have a material adverse effect on our business, operating results, and financial condition.
We use registered and common law trademarks on nearly all of our products and believe that having distinctive marks is an important factor in marketing our products. We have also registered some of our marks in
20
Table of Contents
a number of foreign countries. In addition, El.En. has registered the SmartLipo® and MonaLisa Touch marks in the United States. Although we have a foreign trademark registration program for selected marks, we may not be able to register or use such marks in each foreign country in which we seek registration.
Our management believes that none of our current products infringe upon valid claims of patents owned by third parties of which we are aware. However, there have been claims made against us and there can be no assurance that third parties will not make further claims of infringement with respect to our current or future products. Any such claims, with or without merit, could be time-consuming to defend, result in costly litigation, divert our attention and resources, cause product shipment delays or require us to enter into royalty or licensing agreements. Such royalty or licensing agreements, if required, may not be available on terms acceptable to us or at all. A successful claim of intellectual property infringement against us and our failure or inability to license the infringed technology or develop or license technology with comparable functionality could have a material adverse effect on our business, financial condition and operating results.
Competition
Our industry is subject to intense competition. Our products compete against laser and other energy-based products offered by public companies, such as Cutera, Syneron Medical and ZELTIQ Aesthetics (whose acquisition by Allergan is pending), as well as several smaller specialized private companies, such as Alma Lasers (acquired in May 2013 by Shanghai Fosun Pharmaceutical) and Lumenis. Some of these competitors have strong financial and human resources and have established reputations, as well as established worldwide distribution channels and sales and marketing capabilities. Additional competitors may enter the market, and we are likely to compete with new companies in the future. Our products also compete against non-laser and non-light-based medical products, such as BOTOX® and collagen injections, and surgical and non-surgical aesthetic procedures, such as face lifts, chemical peels, abdominoplasty, liposuction, microdermabrasion, sclerotherapy and electrolysis.
Competition among providers of aesthetic laser and other light-based products is characterized by significant research and development efforts and rapid technological progress. There are few barriers that would prevent new entrants or existing competitors from developing products that would compete directly with ours. There are many companies, both public and private, that are developing innovative devices that use both light-based and alternative technologies for aesthetic and medical applications. Accordingly, our success depends in part on developing and commercializing new and innovative applications of laser and other light-based technology and identifying new markets for and applications of existing products and technology.
To compete effectively, we have to demonstrate that our products are attractive alternatives to other devices and treatments by differentiating our products on the basis of performance, reputation, quality of customer support and price. Breadth of product offering is also important. We believe that we perform favorably with respect to each of these factors. However, we have encountered and expect to continue to encounter potential customers who, due to pre-existing relationships with our competitors, are committed to, or prefer the products offered by these competitors. Potential customers also may decide not to purchase our products, or to delay such purchases, based on a decision to recoup the cost of expensive products that they may have already purchased from our competitors. In addition, we expect that competitive pressures may result in price reductions and reduced margins over time for our products.
Government Regulation
Our products are medical devices that are subject to extensive regulation by government authorities in the United States and in other countries and jurisdictions, including the European Union, or EU. These governmental authorities regulate the marketing and distribution of medical devices in their respective jurisdictions. The regulations cover the entire life cycle of the product, including the research, development, testing, manufacture, quality control, packaging, storage, labeling, advertising and promotion of the devices. In addition, post-approval
21
Table of Contents
monitoring and reporting, as well as import and export of medical devices, are subject to various regulatory requirements. The processes for obtaining regulatory approvals or clearances in the United States and in foreign countries and jurisdictions, including the EU, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
Review and Clearance of Medical Devices in the United States
The FDA strictly regulates medical devices in the United States. Under the Federal Food, Drug and Cosmetic Act, or FDCA, a medical device is defined as an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part or accessory, which is, among other things: intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals; or intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. Unless an exemption applies, a new medical device may not be marketed in the United States unless it has been cleared by the FDA through filing of a 510(k) premarket notification, or 510(k), or approved by the FDA pursuant to a premarket approval application, or PMA. Both premarket notifications and premarket approval applications, when submitted to the FDA, must be accompanied by a user fee, unless exempt.
The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the FDA. Medical devices are classified into one of three classes depending on the level of control necessary to assure the safety and effectiveness of the device. Class I devices have the lowest level or risk associated with them, and are subject to general controls, including labeling, premarket notification and adherence to the QSR. Class II devices are subject to general controls and special controls, including performance standards. Class III devices, which have the highest level of risk associated with them, are subject to most of the aforementioned requirements as well as to premarket approval. Most Class I devices and some Class II devices are exempt from the 510(k) requirement, although manufacturers of these devices are still subject to registration, listing, labeling and QSR requirements.
In connection with its clearance or approval of device products, the FDA requires that we manufacture our products in accordance with its Quality System Regulation, or QSR. The QSR covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of our products. The FDA enforces the QSR through periodic announced and unannounced inspections. Our failure to maintain compliance with the QSR requirements could result in, among other things, the shutdown of, or restrictions on, our manufacturing operations and the recall or seizure of our products
All of our current products are Class II devices. To date, we have used exclusively the 510(k) premarket notification process to obtain regulatory clearance from the FDA for the marketing and sale of our products in the United States.
510(k) Premarket Notification
A 510(k) is a premarket submission made to the FDA to demonstrate that the proposed device to be marketed is at least as safe and effective (i.e., substantially equivalent) to another legally marketed device, or predicate device, that did not require premarket approval. In evaluating a 510(k), the FDA will determine whether the device has the same intended use as the predicate device, and (a) has the same technological characteristics as the predicate device, or (b) has different technological characteristics, and (1) the data supporting substantial equivalence contains information, including appropriate clinical or scientific data, if deemed necessary by the FDA, that demonstrates that the device is as safe and as effective as a legally marketed device, and (2) does not raise different questions of safety and effectiveness than the predicate device. Most 510(k)s do not require clinical data for clearance, but the FDA may request such data.
22
Table of Contents
The FDA seeks to review and act on a 510(k) within 90 days of submission, but it may take longer if the agency finds that it requires more information to review the 510(k). If the FDA concludes that a new device is not substantially equivalent to a predicate device, the new device will be classified in Class III and the manufacturer will be required to submit a PMA to market the product. With the enactment of the Food and Drug Administration Safety and Innovation Act, or the FDASIA, a de novo pathway is directly available for certain low to moderate risk devices that do not qualify for the 510(k) pathway due to the absence of a predicate device.
510(k) and Product Modifications
After a device receives 510(k) clearance, a modification that could affect its safety or effectiveness, or that would constitute a change in its intended use, will likely require a new clearance or approval. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. We have modified aspects of various products since receiving regulatory clearance and believe that new 510(k) clearances are not required for these modifications. The FDA’s position on when a device modification triggers the need to submit a new 510(k) has been evolving in recent years, and it is therefore difficult to predict whether the FDA will disagree with us. If the FDA disagrees with our determination not to seek a new 510(k) clearance, the FDA may retroactively require us to seek 510(k) clearance or premarket approval. The FDA could also require us, among other things, to cease marketing and distributing the modified device, and to recall any sold devices, until 510(k) clearance or premarket approval is obtained. Also, in these circumstances, we may be subject to significant regulatory fines or penalties.
Modifications to a 510(k)-cleared medical device may require the submission of another 510(k) or a PMA if the changes could significantly affect safety or effectiveness or constitute a major change in the intended use of the device. Modifications to a 510(k)-cleared device frequently require the submission of a traditional 510(k), but modifications meeting certain conditions may be candidates for FDA review under a Special 510(k). If a device modification requires the submission of a 510(k), but the modification does not affect the intended use of the device or alter the fundamental technology of the device, then summary information that results from the design control process associated with the cleared device can serve as the basis for clearing the application. A Special 510(k) allows a manufacturer to declare conformance to design controls without providing new data. When the modification involves a change in material, the nature of the “new” material will determine whether a traditional or Special 510(k) is necessary.
Premarket Approval Application
The PMA process for approval to market a medical device is more complex, costly and time consuming than the 510(k) clearance procedure. A PMA must be supported by extensive data, including technical, preclinical, clinical, manufacturing, control and labeling information, that demonstrate the safety and effectiveness of the device for its intended use. After a PMA is submitted, the FDA has 45 days to determine whether it is sufficiently complete to permit a substantive review. If the PMA is complete, the FDA will file the PMA. The FDA is subject to performance goal review times for PMAs and may issue a decision letter as a first action on a PMA within 180 days of filing, but if it has questions, it will likely issue a first major deficiency letter within 150 days of filing. It may also refer the PMA to an FDA advisory panel for additional review, and will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the QSR, either of which could extend the 180-day response target. In addition, the FDA may request additional information or request the performance of additional clinical trials before it will reconsider the approval of the PMA or as a condition of approval, in which case the trials must be completed after the PMA is approved.
If the FDA’s evaluations of both the PMA and the manufacturing facilities are favorable, the FDA will either issue an approval letter authorizing commercial marketing or an approvable letter that usually contains a number of conditions that must be met in order to secure final approval. If the FDA’s evaluations are not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. The agency may determine that additional clinical trials are necessary, in which case the PMA approval may be delayed while the trials are
23
Table of Contents
conducted and the data acquired are submitted in an amendment to the PMA. Even with additional trials, the FDA may not approve the PMA application. The PMA process, including the gathering of clinical and nonclinical data and the submission to and review by the FDA, can take several years, and the process can be expensive and uncertain. Moreover, even if the FDA approves a PMA, the agency can impose post-approval conditions that it believes necessary to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution. After approval of a PMA, a new PMA or PMA supplement may be required for a modification to the device, its labeling or its manufacturing process. None of our products are currently approved under a PMA approval.
Clinical Studies and the Investigational Device Exemption
When FDA clearance or approval of a device requires human clinical trials, and if the device presents a “significant risk,” as defined by the FDA, the FDA requires the device sponsor to file an investigational device exemption, or IDE, application with the FDA and obtain and IDE prior to commencing the human clinical trials. The sponsor must support the IDE application with appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. An IDE application is considered approved 30 days after it has been received by the FDA, unless the FDA otherwise informs the sponsor prior to 30 calendar days from the date of receipt, that the IDE is approved with conditions or disapproved. The sponsor also must obtain approval from the Institutional Review Board, or IRB, overseeing the clinical trial. The FDA, and the IRB at each institution at which a clinical trial is being performed may suspend or terminate a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable health risk.
For the purposes of determining whether an IDE is required, FDA has issued regulations that describe significant and nonsignificant risk device studies and the procedures for obtaining approval to begin the study differ accordingly. A significant risk device presents a potential for serious risk to the health, safety, or welfare of a subject. Significant risk devices are devices that are substantially important in diagnosing, curing, mitigating or treating disease or in preventing impairment to human health. Studies of devices that pose a significant risk require both FDA approval and an approval of an independent institutional review board, or IRB, prior to initiation of a clinical study. Nonsignificant risk devices are devices that do not pose a significant risk to the human subjects. A nonsignificant risk device study requires only IRB approval prior to initiation of a clinical study.
Clinical trials, including clinical trials that do not require prior IDE approval, must be conducted in accordance with the FDA’s IDE and other regulations, including, among other things, informed consent, monitoring and recordkeeping requirements. Clinical studies conducted on 510(k) cleared devices, when used or investigated in accordance with the labeling reviewed by the FDA, are exempt from most of the FDA’s IDE requirements. While we believe that a majority of our devices present only “non-significant” risks and, therefore, do not require IDE submission to the FDA, we have sought IDE approvals for certain study protocols in the past. We perform clinical trials to provide data to support the FDA clearance process for our products and for use in our sales and marketing efforts. Future clinical trials of our products may also require that we submit and obtain approval of an IDE from the FDA prior to commencing clinical trials.
Post-marketing restrictions and enforcement
After a device is placed on the market, numerous regulatory requirements apply. These include:
| • | establishment of registration and device listing; |
| • | the QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
| • | labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or “off-label” uses, and other requirements related to promotional activities; |
24
Table of Contents
| • | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; |
| • | corrections and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug, and Cosmetic Act that may present a risk to health; and |
| • | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
The FDA may require us to maintain a system for tracking our products through the chain of distribution to the patient level. The FDA has broad post-market and regulatory enforcement powers. We are also subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations. These inspections may include the manufacturing facilities of our subcontractors. Thus, we must continue to spend time, money and effort to maintain compliance.
The failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | repair, replacement, refunds, recall or seizure of our products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | refusing or delaying our requests for 510(k) clearance or premarket approval of new products or new intended uses; |
| • | withdrawing 510(k) clearance or premarket approvals that are already granted; and |
| • | criminal prosecution. |
The FDA also has the authority to require us to repair, replace or refund the cost of any medical device that we have manufactured or distributed.
FDA Regulation of Radiation-Emitting Products
The FDA regulates radiation emitting electronic products even when they are not intended to be used for medical purposes. X-rays, microwaves, radio waves, laser, visible light, sound, ultrasound, and ultraviolet light are a few examples of the many types of radiation that may be produced by an electronic product. Diagnostic X-ray systems, laser products, laser light shows, and microwave ovens are a few examples out of the many different electronic products that emit radiation subject to FDA regulation. Many radiation emitting electronic products are also medical devices. In those cases, the products must comply with two independent sets of regulations—radiation safety regulations that apply to radiation emitting electronic products, as well as medical device regulations that apply to all medical devices.
Under the Electronic Product Radiation Control provisions of the FDCA, the FDA has established regulations specifying electronic product performance standards covering several varieties of radiation emitting electronic products. Companies that manufacture or import electronic products subject to an FDA performance standard are required to submit various electronic product reports to the FDA to demonstrate that their products comply with the standard. The most important and basic of the reports is the Electronic Product Initial Report. When a manufacturer or importer submits an Electronic Product Initial Report to the FDA, the FDA reviews the report to ensure compliance with any applicable manufacturing and performance standards. The FDA then issues Accession Numbers to the manufacturer. The FDA and U.S. Customs require declaration of Accession Numbers
25
Table of Contents
on import forms when importing electronic products into the U.S. Unless exempted by the radiation safety regulations, a manufacturer or importer must also submit to the FDA follow-up reports for product updates or modifications, as well as an annual report for their radiation emitting electronic products. The radiation safety regulations provide specific certification and labeling requirements for electronic products. Labeling, which includes user manuals, must contain certain information, such as warnings, declarations and clear and concise instructions for use and service. The information must also be formatted in accordance with the regulations. The law and applicable federal regulations also require laser manufacturers to maintain manufacturing, testing and sales records, and report product defects. Various warning labels must be affixed and certain protective devices installed, depending on the class of the product.
Other Regulatory Requirements
Other laws that apply to our activities include the Patient Protection and Affordable Care Act (commonly referred to as the “Sunshine Act”). It sets forth reporting and disclosure requirements for “applicable manufacturers” of drugs, biological, medical devices and medical supplies with regard to payments or other transfers of value made to certain physicians and teaching hospitals. The final rules implementing the Sunshine Act are complex, ambiguous, and broad in scope. We are also subject to a wide range of federal, state and local laws and regulations, including those related to the environment, health and safety, land use and quality assurance. We believe that compliance with these laws and regulations as currently in effect will not have a material adverse effect on our capital expenditures, earnings and competitive and financial position. In 2013, most of the products and systems that we sell became subject to an excise tax on sales of certain medical devices in the United States after December 31, 2012 by the manufacturer, producer or importer in an amount equal to 2.3% of the sale price. This excise tax was suspended for the years 2016 and 2017. Under the law, additional charges, including warranties, may be deemed to be included in the sale price for purposes of determining the amount of the excise tax.
Healthcare Law and Regulation
Healthcare providers, physicians and third-party payors play a primary role in the recommendation and selection of medical devices for patients. Arrangements with providers, consultants, third-party payors and customers are subject to broadly applicable fraud and abuse, anti-kickback, false claims laws, reporting of payments to physicians and teaching physicians and patient privacy laws and regulations and other healthcare laws and regulations that may constrain business and/or financial arrangements. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, paying, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; |
| • | the federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, which prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false, fictitious or fraudulent or knowingly making, using or causing to made or used a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government. |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal laws that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their respective implementing regulations, including the Final Omnibus Rule published in January 2013, which impose obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
26
Table of Contents
| • | the federal false statements statute, which prohibits knowingly and willfully falsifying, concealing ·or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| • | the federal transparency requirements known as the federal Physician Payments Sunshine Act, under the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or the Affordable Care Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to the Centers for Medicare & Medicaid Services, or CMS, within the U.S. Department of Health and Human Services, information related to payments and other transfers of value made by that entity to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; and |
| • | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to healthcare items or services that are reimbursed by non-governmental third-party payors, including private insurers. |
State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Review and Approval of Medical Devices in the European Union
The European Union, or EU, has a coordinated system for the authorization of medical devices. The EU Medical Devices Directive (Council Directive 93/42/EEC, as amended) sets out the basic regulatory framework for medical devices in the European Union. In the EU our medical devices must comply with the Essential Requirements in Annex I to the EU Medical Devices Directive, which we refer to as the Essential Requirements. Compliance with these requirements is a prerequisite to be able to affix the Certificate of Conformity mark, or CE Mark, to our medical devices, without which they cannot be marketed or sold in the European Economic Area, or EEA. To demonstrate compliance with the Essential Requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low risk medical devices (Class I with no measuring function and which are not sterile), where the manufacturer can issue a CE Declaration of Conformity based on a self-assessment of the conformity of its products with the Essential Requirements, a conformity assessment procedure requires the intervention of a third-party organization designated by competent authorities of an EU country to conduct conformity assessments, which is referred to as a Notified Body. The Notified Body would typically audit and examine products’ technical file and the quality system for the manufacture, design and final inspection of the devices before issuing a CE Certificate of Conformity demonstrating compliance with the relevant Essential Requirements.
The method of assessing conformity varies depending on the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a Notified Body, an independent and neutral institution appointed by a country to conduct the conformity assessment. This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s device. An assessment by a Notified Body in one member state of the European Union or the European Free Trade Association is required in order for a manufacturer to distribute the product commercially throughout these countries. ISO 9001 and ISO 13845 certification are voluntary harmonized standards. Compliance establishes the presumption of conformity with the quality management system and compliance with the requirements of the Medical Device Directive permits our Notified Body to issue the CE mark for our products. In 2003, we received our certification for ISO 13485, which replaced our EN 46001 certification.
Even after we receive a CE Certificate of Conformity enabling us to affix the CE Mark on a product and to sell our product in the EEA countries, a Notified Body or a competent authority may require post-marketing studies of our product. Failure to comply with such requirements in a timely manner could result in the withdrawal of our CE Certificate of Conformity and the recall or withdrawal of our product from the market in
27
Table of Contents
the EU. Moreover, each CE Certificate of Conformity is valid for a maximum of five years, but more commonly three years. At the end of each period of validity we are required to apply to the Notified Body for a renewal of the CE Certificate of Conformity. There may be delays in the renewal of the CE Certificate of Conformity or the Notified Body may require modifications to our products or to the related Technical Files before it agrees to issue the new CE Certificate of Conformity.
In addition, we must inform the Notified Body that carried out the conformity assessment of the medical devices we market or sell in the EEA of any planned substantial changes to our devices that could affect compliance with the Essential Requirements or the devices’ intended purpose. The Notified Body will then assess the changes and verify whether they affect the products’ conformity with the Essential Requirements or the conditions for the use of the devices. If the assessment is favorable, the Notified Body will issue a new CE Certificate of Conformity or an addendum to the existing CE Certificate of Conformity attesting compliance with the Essential Requirements. If it is not, we may not be able to continue to market and sell the product in the EEA.
On September 26, 2012, the European Commission adopted a package of legislative proposals designed to replace the existing regulatory framework for medical devices in the EU. These proposals provide for a revision of the current regulatory framework for medical devices in the EU to strengthen patient safety, transparency and product traceability. The proposals, for instance, include reinforced rules governing clinical evaluation throughout the life of the device, improved traceability of devices in the supply chain, including a phased and risk-based introduction of unique device identification, or UDI, improved market surveillance and vigilance, as well as better co-ordination between national regulators, increased powers for Notified Bodies to undertake unannounced inspections and strengthened supervision of Notified Bodies by member states. The European Commission’s proposals may undergo significant amendments as they are reviewed by the European Council and European Parliament as part of the EU legislative process. If and when adopted, the proposed new legislation may prevent or delay the EU approval or clearance of our products under development or may impact our ability to modify our currently EU approved or cleared products on a timely basis and impose additional costs relating to clinical evaluation, vigilance and product traceability.
Marketing and Sales Considerations in the EU
In the EU, medical devices may be promoted only for the intended purpose for which the devices have been CE Marked. Failure to comply with this requirement could lead to the imposition of penalties by the competent authorities of the EU Member States. The penalties could include warnings, orders to discontinue the promotion of the medical device, seizure of the promotional materials and fines. Promotional materials must also comply with various laws and codes of conduct developed by medical device industry bodies in the EU governing promotional claims, comparative advertising, advertising of medical devices reimbursed by the national health insurance systems and advertising to the general public.
Product Vigilance and Post-approval Monitoring in the EU
Additionally, all manufacturers placing medical devices into the market in the EU are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the competent authority in whose jurisdiction the incident occurred. In the EU, manufacturers must comply with the EU Medical Device Vigilance System. Under this system, incidents must be reported to the relevant authorities of the EU countries, and manufacturers are required to take field safety corrective actions, or FSCAs, to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market.
Employees
As of December 31, 2016, we had 982 employees, including 439 employees in sales and marketing functions, 84 employees in research, development and engineering functions, 347 employees in manufacturing and service functions and 112 employees in general and administrative functions. We believe that our future success will depend in part on our continued ability to attract, hire and retain qualified personnel. None of our employees are represented by a labor union, and we believe our employee relations are good.
28
Table of Contents
| Item 1A. | Risk Factors |
Except for the important factors relating to consummation of the Merger, the following important factors have been prepared as if our company were remaining independent. The following important factors, among others, could cause our business, financial condition, results of operations and cash flows to be materially adversely affected. This section contains forward-looking statements. You should refer to the explanation of the qualifications and limitations on forward-looking statements beginning on page 3.
Risks Related to the Merger
The announcement and pendency of Hologic’s proposed acquisition of our company pursuant to a tender offer and subsequent merger with a wholly owned direct subsidiary of Hologic could have an adverse effect on our stock price and/or our business, financial condition, results of operations or business prospects.
The announcement and pendency of the Offer and Merger could disrupt our business in the following ways, among others:
| • | third parties may determine to delay or defer purchase decisions with regard to our products or terminate and/or attempt to renegotiate their relationships with us as a result of the Offer and Merger, whether pursuant to the terms of their existing agreements with us or otherwise; |
| • | the attention of our management may be directed toward the completion of the Offer and Merger and related matters, and their focus may be diverted from the day-to-day business operations of our company, including from other opportunities that might otherwise be beneficial to us; and |
| • | current and prospective employees may experience uncertainty regarding their future roles with us (and, if the Merger is completed, Hologic), which might adversely affect our ability to retain, recruit and motivate key personnel and may adversely affect the focus of our employees on development and sales of our products. |
Any of the above matters could adversely affect our stock price or harm our financial condition, results of operations or business prospects.
If the proposed Merger is not consummated, our business and stock price could be adversely affected.
The consummation of the transactions contemplated by the Merger Agreement is subject to the satisfaction or waiver of certain conditions set forth in the Merger Agreement. We expect to close the Merger in late March or April of 2017, subject to the satisfaction or waiver of these conditions. If the Merger is delayed or otherwise not consummated within the contemplated time periods or at all, we could suffer a number of consequences that may adversely affect our business, results of operations and stock price, including the following:
| • | we have incurred and expect to continue to incur significant expenses related to the proposed Merger. These Merger-related expenses include investment banking fees and legal and other professional fees. These fees will be payable by us even if the Merger does not close; |
| • | we would be obligated to pay Hologic a $57.7 million termination fee in connection with the termination of the Merger Agreement in certain circumstances; |
| • | our customers, prospective customers and investors in general may view the failure to consummate the Merger as a poor reflection on our business or prospects; |
| • | certain of our suppliers, distributors, and other business partners may seek to change or terminate their relationships with us as a result of the proposed Merger; and |
| • | the market price of our common stock may decline, particularly to the extent that the current market price reflects a market assumption that the proposed Merger will be completed. |
29
Table of Contents
Our executive officers and directors may have interests that are different from, or in addition to, those of our stockholders generally.
Our executive officers and directors may have interests in the Offer and Merger that are different from, or are in addition to, those of our stockholders generally. These interests include direct or indirect ownership of our common stock, stock options, restricted stock units and performance-based stock units, and the receipt of change in control or other retention or severance payments in connection with the proposed transactions.
The Merger Agreement contains provisions that could discourage or make it difficult for a third party to acquire our company prior to the completion of the Offer and the Merger.
The Merger Agreement contains provisions that make it difficult for us to entertain a third-party proposal for an acquisition of our company. These provisions include our agreement not to solicit or initiate any additional discussions with third parties regarding other proposals for our acquisition, as well as restrictions on our ability to respond to such proposals, subject to fulfillment of certain fiduciary requirements of our board of directors. The Merger Agreement also contains certain termination rights, including, under certain circumstances, a requirement for us to pay to Hologic a termination fee of approximately $57.7 million.
These provisions might discourage an otherwise-interested third party from considering or proposing an acquisition of our company, even one that may be deemed of greater value to our stockholders than the Hologic offer. Furthermore, even if a third party elects to propose an acquisition, our obligation to pay a termination fee may result in that third party’s offering of a lower value to our stockholders than such third party might otherwise have offered.
A lawsuit has been filed, and additional lawsuits may be filed, against us and the members of our board of directors arising out of our acquisition by Hologic, which may delay or prevent the proposed transaction.
A putative stockholder class action complaint has been filed against us and our board of directors in connection with the transactions contemplated by the Merger Agreement. We and our directors believe the lawsuit is without merit; however, the outcome of such litigation is uncertain. Regardless of the outcome of this lawsuit, it could delay or prevent our acquisition by Hologic, divert the attention of our management and employees from our day-to-day business and otherwise adversely affect us financially.
Risks Related to Our Business and Industry
We have incurred net losses in prior periods.
While we were profitable in 2016, we incurred losses in the first quarter of 2015 and for the full year 2013. If we are unable to maintain profitability, the market value of our stock may decline, and an investor could lose all or a part of their investment.
If there is not sufficient consumer demand for the procedures performed with our products, practitioner demand for our products could decline, which would adversely affect our operating results.
The aesthetic laser and light-based treatment system industry in which we operate is particularly vulnerable to economic trends. Most procedures performed using our aesthetic treatment systems are elective procedures that are not reimbursable through government or private health insurance. The cost of these elective procedures must be borne by the patient. As a result, the decision to undergo a procedure that uses our products may be influenced by the cost.
Consumer demand, and therefore our business, is sensitive to a number of factors that affect consumer spending, including political and macroeconomic conditions, health of credit markets, disposable consumer income levels, consumer debt levels, interest rates, consumer confidence and other factors. For example, consumer demand for the procedures performed with our products, and practitioner demand for our products, decreased dramatically during 2009 as a result of turmoil in the financial markets, which contributed to a significant decrease in our total product revenues during that year. If there is not sufficient consumer demand for the procedures performed with our products, practitioner demand for our products would decline, and our business would suffer.
30
Table of Contents
Our financial results may fluctuate from quarter to quarter, which makes our results difficult to predict and could cause our results to fall short of expectations.
Our financial results may fluctuate as a result of a number of factors, many of which are outside of our control. For these reasons, comparing our financial results on a period-to-period basis may not be meaningful, and our past results should not be relied upon as an indication of our future performance. Our future quarterly and annual expenses as a percentage of our revenues may be significantly different from those we have recorded in the past or which we expect for the future. Our financial results in some quarters may fall below our expectations or the expectations of market analysts or investors. Any of these events could cause our stock price to fall. Each of the risk factors listed in this “Risk Factors” section, and the following factors, may adversely affect our financial results:
| • | our inability to introduce new products to the market in a timely fashion, or at all; |
| • | our inability to quickly address and resolve reliability issues in our products and/or meet warranty and service obligations to our customers; |
| • | continued availability of attractive equipment leasing terms for our customers, which may be negatively influenced by interest rate increases or lack of available credit; |
| • | increases in the length of our sales cycle; and |
| • | reductions in the efficiency of our manufacturing processes. |
In addition, we may be subject to seasonal fluctuations in our results of operations, because our customers may be more likely to make equipment purchasing decisions near year-end, and because practitioners may be less likely to make purchasing decisions in the summer months.
Our competitors may prevent us from achieving further market penetration or improving operating results.
Competition in the aesthetic device industry is intense. Our products compete against products offered by public companies, such as Cutera, Syneron Medical, and ZELTIQ Aesthetics (whose acquisition by Allergan is pending), as well as several smaller specialized private companies, such as Alma Lasers (acquired in 2013 by Shanghai Fosun Pharmaceutical) and Lumenis. Additional competitors may enter the market, and we are likely to compete with new companies in the future.
We also face competition against non-laser and non-light-based medical products, such as BOTOX® and collagen injections, and surgical and non-surgical aesthetic procedures, such as face lifts, chemical peels, abdominoplasty, liposuction, microdermabrasion, sclerotherapy and electrolysis. We may also face competition from manufacturers of pharmaceutical and other products that have not yet been developed. As a result of competition with these companies, products and procedures, we could experience loss of market share and decreasing revenue as well as reduced prices and profit margins, any of which would harm our business and operating results.
Our ability to compete effectively depends upon our ability to distinguish our company and our products from our competitors and their products. Factors affecting our competitive position include:
| • | product performance, reliability and design; |
| • | ability to sell products tailored to meet the applications needs of clients and patients; |
| • | quality of customer support; |
| • | product pricing; |
| • | product safety; |
| • | sales, marketing and distribution capabilities; |
31
Table of Contents
| • | success and timing of new product development and introductions; and |
| • | intellectual property protection. |
We face exposure to credit risk of customers.
In the event of deterioration of general business conditions or the availability of credit, the financial strength and stability of our customers and potential customers may deteriorate over time, which may cause them to cancel or delay their purchase of our products. In addition, we may be subject to increased risk of non-payment of our accounts receivables. We may also be adversely affected by bankruptcies or other business failures of our customers and potential customers. A significant delay in the collection of funds or a reduction of funds collected may impact our liquidity or result in bad debts.
If we do not continue to develop and commercialize new products and identify new markets for our products and technology, we may not remain competitive, and our revenues and operating results could suffer.
The aesthetic laser and light-based treatment system industry is subject to continuous technological development and product innovation. If we do not continue to innovate and develop new products and applications, our competitive position will likely deteriorate as other companies successfully design and commercialize new products and applications. Accordingly, our success depends in part on developing or acquiring new and innovative applications of laser and other light-based technology and identifying new markets for and applications of existing products and technology. If we are unable to develop and commercialize new products, identify and acquire complementary businesses, products or technologies, and identify new markets for our products and technology, our product and technology offerings could become obsolete and our revenues and operating results could be adversely affected.
To remain competitive, we must:
| • | develop or acquire new technologies that either add to or significantly improve our current products; |
| • | convince our target practitioner customers that our new products or product upgrades would be attractive revenue-generating additions to their practices; |
| • | sell our products to non-traditional customers, including primary care physicians, gynecologists and other specialists; |
| • | identify new markets and emerging technological trends in our target markets and react effectively to technological changes; |
| • | preserve goodwill and brand value with customers; and |
| • | maintain effective sales and marketing strategies. |
If our new products do not gain market acceptance, our revenues and operating results could suffer, and our newer generation product sales could cause earlier generation product sales to suffer.
The commercial success of the products and technology we develop will depend upon the acceptance of these products by providers of aesthetic procedures and their patients and clients. It is difficult for us to predict how successful recently introduced products, or products we are currently developing, will be over the long term. If the products we develop do not gain market acceptance or meet customer expectations, our revenues and operating results could suffer.
We expect that many of the products we develop will be based upon new technologies or new applications of existing technologies. It may be difficult for us to achieve market acceptance of some of our products, particularly the first products that we introduce to the market based on new technologies or new applications of existing technologies.
32
Table of Contents
As we introduce new technologies to the market, our earlier generation product sales could suffer, which may result in write-offs of those earlier generation products.
If demand for our aesthetic treatment systems by physician customers does not increase, our revenues will suffer and our business will be harmed.
We market our aesthetic treatment systems to physicians and other practitioners. We believe, and our growth expectations assume, that we and other companies selling lasers and other light-based aesthetic treatment systems have not fully penetrated these markets and that we will continue to receive a significant percentage of our revenues from selling to these markets. If our expectations as to the size of these markets and our ability to sell our products to participants in these markets are not correct, our revenues will suffer and our business will be harmed.
We sell our products and services through subsidiaries and distributors in numerous international markets. Our operating results may suffer if we are unable to manage our international operations effectively.
We sell our products and services through subsidiaries and distributors in over 120 foreign countries, and we therefore are subject to risks associated with having international operations. We derived 32%, 39%, and 48% of our product revenues from sales outside North America for the years ended December 31, 2016, 2015, and 2014, respectively.
Our international sales are subject to a number of risks, including:
| • | foreign certification and regulatory requirements; |
| • | difficulties in staffing and managing our foreign operations; |
| • | import and export controls; and |
| • | political and economic instability. |
If we are unsuccessful at managing these risks, our results of operations may be adversely affected.
We may incur foreign currency conversion charges as a result of changes in currency exchange rates, which could cause our operating results to suffer.
The U.S. dollar is our functional currency. Although we sell our products and services through subsidiaries and distributors in over 120 foreign countries, approximately 39% and 46% of our revenues outside of North America for the years ended December 31, 2016 and 2015, respectively, were denominated in or linked to the U.S. dollar. Substantially all of our remaining revenues and all of our operating costs outside of North America are recognized in euros, British pounds, Moroccan dirham, Japanese yen, Chinese yuan, South Korean won and Australian dollars. We have not historically engaged in hedging activities relating to our non-U.S. dollar operations. Fluctuations in exchange rates between the currencies in which such revenues are realized or costs are incurred and the U.S. dollar may have a material adverse effect on our results of operations and financial condition. For the year ended December 31, 2016 we incurred a loss on foreign currency of $0.8 million, which is recorded as other expense, net within our consolidated statement of operations. This was primarily due to the strengthening of the U.S. dollar against the euro, British pound, Korean won and Chinese yuan.
We may not receive revenues from our current research and development efforts for several years, if at all.
Investment in product development often involves a long payback cycle and risks associated with new technology. For example, our SculpSure laser system, which we launched in 2015, was in development for five years. Our PicoSure laser system, which we launched in 2013, was in development for several years. We have made and expect to continue making significant investments in research and development and related product
33
Table of Contents
opportunities. Accelerated product introductions and short product life cycles require high levels of expenditures for research and development that could adversely affect our operating results if not offset by revenue increases. We believe that we must continue to dedicate a significant amount of resources to our research and development efforts to maintain our competitive position. However, we may not generate anticipated revenues from these investments for several years, if at all.
Because we do not require training for users of our non-invasive products, and we sell these products to non-physicians, there exists an increased potential for misuse of these products, which could harm our reputation and our business.
Federal regulations allow us to sell our products to or on the order of practitioners licensed by law to use or order the use of a prescription device. The definition of “licensed practitioners” varies from state to state. As a result, our products may be purchased or operated by physicians with varying levels of training and, in many states, by non-physicians, including nurse practitioners, chiropractors and technicians. Outside the United States, many jurisdictions do not require specific qualifications or training for purchasers or operators of our products. We do not supervise the procedures performed with our products, nor can we require that direct medical supervision occur. We and our distributors offer product training sessions, but neither we nor our distributors require purchasers or operators of our non-invasive products to attend training sessions. The lack of required training and the purchase and use of our non-invasive products by non-physicians may result in product misuse and adverse treatment outcomes, which could harm our reputation and expose us to costly product liability litigation.
We may be unable to attract and retain management and other personnel we need to succeed.
Our success depends on the services of our senior management and other key research and development, manufacturing, sales and marketing employees. The loss of the services of one or more of these employees could have a material adverse effect on our business. We consider retaining Michael R. Davin, our chief executive officer, key to our efforts to develop, sell and market our products and remain competitive. We have entered into an employment agreement with Mr. Davin; however, the employment agreement is terminable by him on short notice and may not ensure his continued service with our company. Our future success will depend in large part upon our ability to attract, retain and motivate highly skilled employees. We cannot be certain that we will be able to do so.
We may seek to acquire companies or technologies, which could disrupt our ongoing business, divert the attention of our management and employees and adversely affect our results of operations.
We may, from time to time, evaluate potential strategic acquisitions of other complementary businesses, products or technologies, as well as consider joint ventures and other collaborative projects. We may not be able to identify suitable future acquisition candidates, consummate acquisitions on favorable terms or complete otherwise favorable acquisitions because of antitrust or other regulatory concerns. We cannot be certain that the acquisitions we have completed, including our 2014 acquisition of substantially all of the assets of Ellman International, Inc., or any future acquisitions that we may make, will enhance our products or strengthen our competitive position. In particular, we may encounter difficulties assimilating or integrating the acquired businesses, technologies, products, personnel or operations of the acquired companies, and in retaining and motivating key personnel from these businesses. The integration of these businesses may not result in the realization of the full benefits of synergies, cost savings, innovation and operational efficiencies that may be possible from this integration and these benefits may not be achieved within a reasonable period of time.
Our stock price has fluctuated substantially, and we expect it will continue to do so.
Our Class A common stock price has fluctuated substantially in recent years, and we expect it will continue to do so. From January 1, 2012 through February 21, 2017, our Class A common stock has traded as high as
34
Table of Contents
$66.50 per share and as low as $11.64 per share. The stock market in general has experienced extreme volatility that has often been unrelated to the operating performance of particular companies. The market price for our Class A common stock may be influenced by many factors, including:
| • | the success of competitive products or technologies; |
| • | regulatory developments in the United States and foreign countries; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | the recruitment or departure of key personnel; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | activism by any single large stockholder or combination of stockholders; |
| • | securities or other litigation; |
| • | market conditions in our industry and issuance of new or changed securities analysts’ reports or recommendations; |
| • | purchases or sales of our stock by our officers and directors; |
| • | repurchases of shares of our Class A common stock; and |
| • | general economic, industry and market conditions. |
In addition, if the stock market in general experiences a loss of investor confidence, the trading price of our Class A common stock could decline for reasons unrelated to our business, financial condition or results of operations.
Our common stock could be further diluted by the conversion of outstanding options, restricted stock units and performance-based stock units.
In the past, we have issued and still have outstanding convertible securities in the form of options, restricted stock units and performance-based stock units. We may continue to issue options, restricted stock units, performance-based stock units and other equity rights as compensation for services and incentive compensation for our employees, directors and consultants or others who provide services to us. We have a substantial number of shares of common stock reserved for issuance upon the conversion and exercise of these securities. Such a conversion would dilute our stockholders and could adversely affect the market price of our common stock.
We may not be able to successfully continue collecting licensing royalties.
Since 2013, portions of our revenues have consisted of royalties from sub-licensing patents, including patents licensed to us on an exclusive basis by MGH. These patents expired in the United States on February 1, 2015 and their foreign counterparts expired in several foreign jurisdictions on January 31, 2016. Though we receive royalty revenues on other patents, our revenues have decreased as a result of the expiration of the MGH patents because we will no longer receive any royalties from such patents, and such license revenues may not be replaced.
We face risks associated with product warranties.
We could incur substantial costs as a result of product failures for which we are responsible under warranty obligations.
If we are unable to protect our information technology infrastructure against service interruptions, data corruption, cyber-based attacks or network security breaches, our reputation, business and operating results may suffer.
We rely on information technology networks and systems, including the Internet, to process and transmit sensitive electronic information and to manage or support a variety of business processes and activities, including
35
Table of Contents
procurement and supply chain, manufacturing, distribution, and invoicing and collection of payments for our products. We use enterprise information technology systems to record, process, and summarize financial information and results of operations for internal reporting purposes and to comply with regulatory financial reporting, legal, and tax requirements. Our information technology systems, some of which are managed by third parties, are susceptible to damage, disruptions or shutdowns due to failures during the process of upgrading or replacing software, databases or components thereof, power outages, hardware failures, computer viruses, attacks by computer hackers, telecommunication failures, user errors or catastrophic events. If our information technology systems are damaged, disrupted or shutdown and we are unable to effectively resolve the issues in a timely manner, our reputation, business and operating results may suffer.
Similar security risks exist with respect to our business partners and the third-party vendors that we rely on for aspects of our information technology support services and administrative functions. As a result, we are subject to the risk that the activities of our business partners and third-party vendors may adversely affect our business even if the attack or breach does not directly impact our information technology systems.
Risks Related to Our Reliance on Third Parties
If we fail to obtain key components of our products from our sole source or limited source suppliers or service providers, our ability to manufacture and sell our products would be impaired and our business could be materially harmed.
We depend on sole or limited suppliers of certain components and systems that are critical to the products that we manufacture and sell, and to which the significant majority of our revenues are attributable. We depend on a single contract manufacturer in the United States for the subassembly of certain products that we manufacture and sell, and for the manufacturing of certain products that we sell, and to which a significant portion of our revenues are attributable to these products. We depend on El.En. for the SLT II laser system that we integrate with our own proprietary software and delivery systems into our SmartLipo Triplex, Cellulaze, and PrecisionTx systems. We also depend on El.En. for the MonaLisa Touch laser system. For further information regarding our distribution agreements with El.En., please see the discussion on page 19 of this Annual Report. We use Alexandrite rods to manufacture the lasers for our Elite and PicoSure products and Nd:YAG rods to manufacture the lasers for our RevLite / MedLite C6 products. We depend exclusively on Northrop Grumman SYNOPTICS to supply both the Alexandrite and Nd:YAG rods to us, and we are aware of no alternative supplier of Alexandrite rods meeting our quality standards. We offer our SmartCool treatment cooling systems for use with our laser aesthetic treatment systems, and we depend exclusively on Zimmer Elektromedizin GmbH to supply SmartCool systems to us. In addition, one third-party supplier assembles and tests many of the components and subassemblies for our Elite and Cynergy product families. We use diode laser bars from Coherent to manufacture our Vectus diode laser, and we use diode laser modules from Dilas to manufacture our SculpSure® laser system. Although alternative suppliers exist for the diode laser subassemblies and diode laser bars, they could take months to qualify and implement.
Other than with El.En., we do not have long-term arrangements with any of our suppliers or our contract manufacturer for the supply of these components or systems or with the assembly and test service provider referenced above, but instead purchase from them on a purchase order basis or with one year purchase agreements. Northrop Grumman SYNOPTICS, Zimmer Elektromedizin, IPG Photonics, Dilas and Coherent are not required, and may not be able or willing, to meet our future requirements at current prices, or at all.
Under our agreements with El.En. and our purchase order arrangements with our other suppliers and service providers, we are vulnerable to supply shortages and cessations and price fluctuations with respect to these critical components and systems and services. Such shortages or cessations could occur either as a result of breach by El.En. or us of our distribution agreements, or as a result of other types of business decisions made by El.En. or other suppliers and service providers. Any extended interruption in our supplies of these components or systems or in the assembly and test services could materially harm our business.
36
Table of Contents
We rely on third-party distributors to market, sell and service a significant portion of our products. If these distributors do not commit the necessary resources to effectively market, sell and service our products or if our relationships with these distributors are disrupted, our business and operating results may be harmed.
In the United States, Canada, France, Morocco, Germany, Spain, the United Kingdom, Australia, China, Japan and Korea, we sell our products through our internal sales organization. Outside of these markets, we sell our products through third-party distributors. Our sales and marketing success in these other markets depends on these distributors, in particular their sales and service expertise and relationships with the customers in the marketplace. Sales of our aesthetic treatment systems by third-party distributors represented 12%, 17% and 21% of our product revenue in 2016, 2015 and 2014, respectively.
We do not control our distributors, and these parties may not be successful in marketing our products. These parties may fail to commit the necessary resources to market and sell our products to the level of our expectations. Currently, we have written distributor agreements in place with most of our third-party distributors. We cannot be sure that our distributors will agree with our interpretation of the terms of the agreements or that we will receive payments under the agreements. The third-party distributors with which we do not have written distributor agreements may also disagree with the terms of our relationship. Our distributors may terminate their relationships with us and stop selling and servicing our products with little or no notice. If current or future third-party distributors or other parties that sell our products do not perform adequately, or if we fail to maintain our existing relationships with these parties or fail to recruit and retain distributors in particular geographic areas, our revenue from international sales may be adversely affected and our operating results could suffer.
Risks Related to Our Relationship with El.En. and Our Corporate Structure
El.En. and its subsidiaries market and sell products that compete with our products, and any increased competition from El.En. could have a material adverse effect on our business.
El.En. is a leading laser manufacturer in Europe and a leading light-based medical device manufacturer worldwide. El.En. and its subsidiaries develop and produce laser systems with scientific, industrial, commercial and medical applications. Under exclusive distribution agreements with El.En., we purchase from El.En. its proprietary SmartLipo MPX system and its SLT II laser system. The SLT II laser system is an essential component of our SmartLipo Triplex and Cellulaze systems, which also incorporate our proprietary software and delivery systems. We also depend on El.En. for the MonaLisa Touch laser system. For further information regarding our distribution agreements with El.En., please see the discussion on page 19 of this Annual Report.
El.En. markets, sells, promotes and licenses other products that compete with our products, both in North America and elsewhere throughout the world and our agreements with El.En. do not prevent El.En. from competing with us by selling products that we purchased in the past from El.En., including earlier generation SmartLipo systems. Our business could be materially and adversely affected by increased competition from El.En.
Provisions in our corporate charter documents and under Delaware law may delay or prevent attempts by our stockholders to change our management and hinder efforts to acquire a controlling interest in us.
Several provisions of our certificate of incorporation and bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which our stockholders might otherwise receive a premium for their shares. These provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions include:
| • | the classification of the members of our board of directors until our 2019 annual meeting of stockholders, at which point the full board of directors will be declassified and each director will be elected on an annual basis; |
37
Table of Contents
| • | limitations on the removal of our directors until our 2019 annual meeting of stockholders, after which our directors may be removed with or without cause by the affirmative vote of the holders of a majority of our shares of capital stock entitled to vote; |
| • | advance notice requirements for stockholder proposals and nominations; |
| • | the inability of stockholders to act by written consent or to call special meetings; and |
| • | the ability of our board of directors to designate the terms of and issue new series of preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors. |
Absent approval of our board of directors, our bylaws may only be amended or repealed by the affirmative vote of the holders of at least 75% of the voting power of our shares of capital stock entitled to vote.
In addition, Section 203 of the Delaware General Corporation Law prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person which together with its affiliates owns or within the last three years has owned 15% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Accordingly, Section 203 may discourage, delay or prevent a change in control of our company.
Risks Related to Intellectual Property
If we infringe or are alleged to infringe intellectual property rights of third parties, our business could be adversely affected.
Our products may infringe, or it may be claimed that they infringe, patents or patent applications under which we do not hold licenses or other rights. Third parties may own or control these patents and patent applications in the United States and abroad. These third parties could bring claims against us that would cause us to incur substantial expenses and, if successfully asserted against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay manufacturing or sales of the product that is the subject of the suit.
As a result of patent infringement claims, or in order to avoid potential claims, we may choose or be required to seek a license from the third party and be required to pay license fees or royalties or both, as we did in a 2006 patent license agreement with Palomar Medical Technologies, Inc. Such licenses may not be available on acceptable terms, or at all. Even if we were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be forced to cease some aspect of our business operations if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms. This could harm our business significantly.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in our industry. In addition to infringement claims against us, we may become a party to other types of patent litigation and other proceedings, including reexamination proceedings, inter partes or post-grant review or interference proceedings declared by the U.S. Patent and Trademark Office and opposition proceedings in the European Patent Office, regarding intellectual property rights with respect to our products and technology. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
38
Table of Contents
If we are unable to obtain or maintain intellectual property rights relating to our technology and products, the commercial value of our technology and products will be adversely affected and our competitive position could be harmed.
Our success and ability to compete depends in part upon our ability to obtain protection in the United States and other countries for our products by establishing and maintaining intellectual property rights relating to or incorporated into our technology and products. We own numerous patents and patent applications in the United States and corresponding patents and patent applications in many foreign jurisdictions. We do not know how successful we would be in any instance in which we asserted our patents against suspected infringers. Our pending and future patent applications may not issue as patents or, if issued, may not issue in a form that would be advantageous to us. Even if issued, our patents may be challenged, narrowed, invalidated or circumvented, which could limit our ability to stop competitors from marketing similar products or limit the length of term of patent protection we may have for our products. Changes in either patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection.
We jointly own patents and patent applications with third parties. Our ability to exploit or enforce these patent rights, or to prevent the third party from granting licenses to others with respect to these patent rights, may be limited in some circumstances.
We jointly own certain patents and patent applications with third parties. In the absence of an agreement with each co-owner of jointly owned patent rights, we will be subject to default rules pertaining to joint ownership. Some countries require the consent of all joint owners to exploit, license or assign jointly owned patents, and if we are unable to obtain that consent from the joint owners, we may be unable to exploit the invention or to license or assign our rights under these patents and patent applications in those countries.
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely upon unpatented proprietary technology, processes and know-how. We generally seek to protect this information in part by confidentiality agreements with our employees, consultants and third parties. These agreements may be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently developed by competitors.
Risks Related to Government Regulation and Other Legal Compliance Matters
If we fail to obtain and maintain necessary FDA clearances and approvals for our products and indications or if clearances and approvals for future products and indications are delayed or not issued, our business would be harmed.
Our products are classified as medical devices and are subject to extensive regulation by the U.S. Food and Drug Administration, or FDA, and other federal, state and local authorities. These regulations relate to manufacturing, design, labeling, sale, promotion, distribution, importing and exporting and shipping of our products. In the United States, before we can market a new medical device, or a new use of, or claim for, an existing product, we must first receive either 510(k) clearance or premarket approval from the FDA, unless an exemption applies. Both of these processes can be expensive and lengthy and entail significant user fees, unless exempt. The FDA’s 510(k) clearance process often takes from three to 12 months. The FDA may require us to withdraw an application if they cannot make a determination that the device is substantially equivalent according to the regulations. In such situations, we may re-submit the 510(k) application with additional data for reconsideration or we may determine not to commercialize the device or the new indication for use. The process of obtaining premarket approval is much more costly and uncertain than the 510(k) clearance process. It generally takes from one to three years, or even longer, from the time the premarket approval application, or PMA, is submitted to the FDA until an approval is obtained.
39
Table of Contents
In order to obtain premarket approval and, in some cases, a 510(k) clearance, a product sponsor must conduct well controlled clinical trials designed to test the safety and effectiveness of the product. Conducting clinical trials generally entails a long, expensive and uncertain process that is subject to delays and failure at any stage. The data obtained from clinical trials may be inadequate to support approval or clearance of a submission. In addition, the occurrence of unexpected findings in connection with clinical trials may prevent or delay obtaining approval or clearance. If we conduct clinical trials, they may be delayed or halted, or be inadequate to support approval or clearance, for numerous reasons, including:
| • | the FDA, other regulatory authorities or an institutional review board may place a clinical trial on hold; |
| • | patients may not enroll in clinical trials, or patient follow-up may not occur, at the rate we expect; |
| • | patients may not comply with trial protocols; |
| • | institutional review boards and third-party clinical investigators may delay or reject our trial protocol; |
| • | third-party clinical investigators may decline to participate in a trial or may not perform a trial on our anticipated schedule or consistent with the clinical trial protocol, good clinical practices, or other FDA requirements; |
| • | third-party organizations may not perform data collection and analysis in a timely or accurate manner; |
| • | regulatory inspections of our clinical trials or manufacturing facilities may, among other things, require us to undertake corrective action or suspend or terminate our clinical trials, or invalidate our clinical trials; |
| • | changes in governmental regulations or administrative actions; and |
| • | the interim or final results of the clinical trials may be inconclusive or unfavorable as to safety or effectiveness. |
Medical devices may be marketed only for the indications for which they are approved or cleared. The FDA may not approve or clear indications that are necessary or desirable for successful commercialization. Indeed, the FDA may refuse our requests for 510(k) clearance or premarket approval of new products, new intended uses or modifications to existing products. Our clearances can be revoked if safety or effectiveness problems develop.
In addition, if the FDA requires us to go through a lengthier, more rigorous examination for products or modifications to products than we expect, or at a particular time in the future had expected, our product introductions or modifications could be delayed or canceled, which could negatively affect our ability to generate sales, or cause sales at such time to decline. For example, the FDA could demand that we obtain a PMA prior to marketing certain of our products in cases in which we did not expect to have to obtain a PMA. If we were to determine that a product we plan to market is subject to an exemption from premarket review, the FDA could disagree with our determination and could require us to submit a 510(k) or PMA in order to continue marketing the product.
Finally, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions that may prevent or delay approval or clearance of products we are developing or impact our ability to modify any of our products for which we receive regulatory clearance or approval in the future on a timely basis. Any change in the laws or regulations that govern the clearance and approval processes relating to the products we are developing could make it more difficult and costly to obtain clearance or approval for such products, or to produce, market and distribute products for which we receive regulatory approval or clearance in the future.
40
Table of Contents
After clearance or approval of our products, we are subject to continuing regulation by the FDA, and if we fail to comply with FDA regulations, or other regulations to which we are subject, our business could suffer.
Even after clearance or approval of a product, we are subject to continuing regulation by the FDA, including the requirements that our facility be registered and our devices be listed with the agency. We are subject to Medical Device Reporting regulations, which require us to report to the FDA if our products may have caused or contributed to a death or serious injury or malfunction in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur. We must report corrections and removals to the FDA where the correction or removal was initiated to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug, and Cosmetic Act, or FDCA, caused by the device that may present a risk to health, and maintain records of other corrections or removals. The FDA closely regulates promotion and advertising and our promotional and advertising activities could come under scrutiny. If the FDA objects to our promotional and advertising activities or finds that we failed to submit reports under the Medical Device Reporting regulations, for example, the FDA may allege our activities resulted in violations.
Violations of the FDCA and other laws and regulations to which we are subject may lead to investigations by the FDA, Department of Justice, or DOJ, and state Attorneys General. In addition, later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| • | restrictions on such products, manufacturers or manufacturing processes; |
| • | restrictions on the labeling or marketing of a product; |
| • | restrictions on product distribution or use of a product; |
| • | requirements to conduct post-marketing studies or clinical trials; |
| • | warning letters or untitled letters; |
| • | withdrawal of the products from the market; |
| • | refusing or delaying our requests for 510(k) clearance or premarket approval of new products or new intended uses; |
| • | fines, restitution or disgorgement of profits or revenues; |
| • | suspension or withdrawal of marketing approvals; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusal to permit the import or export of our products; |
| • | repair, replacement, refunds, recalls or seizure or detention of our products; |
| • | consent decrees; |
| • | injunctions or the imposition of civil or criminal penalties; |
| • | damage to relationships with any potential contributors; |
| • | unfavorable press coverage and damage to our reputation; or |
| • | litigation involving patients using our products. |
Non-compliance with European Union requirements can also result in significant financial penalties.
We may be subject to enforcement action if we engage in improper marketing or promotion of any of our products for which we receive regulatory clearance or approval. Any such enforcement action could result in significant fines, costs and penalties and could result in damage to our reputation.
Our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of unapproved use. Use of a device outside its cleared or
41
Table of Contents
approved diseases or conditions is known as “off-label” use. Despite our efforts to comply with such laws and regulations, physicians may use our products for which we receive regulatory clearance or approval off-label, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. If the FDA determines that our promotional materials or other product labeling constitute promotion of an unapproved, or off-label, use, it could request that we modify our materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, recall, injunction, seizure, civil fine or criminal penalties.
Other federal, state and foreign regulatory agencies, including the U.S. Federal Trade Commission, have issued guidelines and regulations that govern how we will be required to promote products for which we receive regulatory clearance or approval, including how we may use endorsements and testimonials. If our promotional materials are inconsistent with these guidelines or regulations, we could be subject to enforcement actions, which could result in significant fines, costs and penalties. Our reputation could also be damaged and the adoption of our products could be impaired. In addition, the off-label use of our products may increase the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention, result in substantial damage awards against us and harm our reputation.
In the EU, our medical devices may be promoted only for the intended purpose for which the devices have been CE marked. Failure to comply with this requirement could lead to the imposition of penalties by the competent authorities of the EU Member States. The penalties could include warnings, orders to discontinue the promotion of the medical device, seizure of the promotional materials and fines. The promotional materials related to any of our products that may be CE marked in the future must also comply with various laws and codes of conduct developed by medical device industry bodies in the EU governing promotional claims, comparative advertising, advertising of medical devices reimbursed by the national health insurance systems and advertising to the general public. If we do not comply with these laws and industry codes, we could be subject to penalties which could include significant fines. Our reputation could also be damaged and the adoption of our products after receipt of a CE mark could be impaired.
We have modified some of our products without FDA clearance. Modifications to any FDA-cleared or approved products may require new regulatory clearances or approvals or require us to recall or cease marketing such products until clearances or approvals are obtained.
We have made modifications to our devices in the past and may make additional modifications in the future that we believe do not or will not require additional clearances or approvals. Any modification to one of our 510(k)-cleared products that would constitute a major change in its intended use or any change that could significantly affect the safety or effectiveness of the device would require us to obtain a new 510(k) marketing clearance and may even, in some circumstances, require the submission of a PMA, if the change raises complex or novel scientific issues or the product has a new intended use. We may be required to submit extensive pre-clinical and clinical data depending on the nature of the changes. We may not be able to obtain additional 510(k) clearances or premarket approvals for modifications to, or additional diseases or conditions for, any products for which we receive regulatory clearance or approval in a timely fashion, or at all. Delays in obtaining future clearances or approvals would adversely affect our ability to introduce enhanced products in a timely manner, which in turn would harm our revenue and operating results.
The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) submission in the first instance, but the FDA may review any manufacturer’s decision. If the FDA disagrees with our determination and requires us to seek new 510(k) clearances or PMA approval for modifications to our previously-cleared products for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or distribution of the products or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties.
Furthermore, potential changes to the 510(k) program may make it more difficult for us to make modifications to any of our then-previously-cleared products, by either imposing more strict requirements on
42
Table of Contents
when a new 510(k) clearance for a modification to a previously-cleared product must be submitted or applying more onerous review criteria to such submissions. In 2011, respectively, the FDA issued draft guidance documents addressing when to submit a new 510(k) clearance due to modifications to 510(k)-cleared products and the criteria for evaluating substantial equivalence. The 2011 draft guidance was ultimately withdrawn as the result of the Food and Drug Administration Safety and Innovation Act. As a result, the FDA’s original guidance document regarding 510(k) modifications, which dates back to 1997, remains in place. However, the FDA may seek to issue new guidance on product modifications. Any efforts to do so could result in a more rigorous review process and make it more difficult to obtain clearance for device modifications.
If we or our contract manufacturers fail to comply with ongoing FDA and EU or other foreign regulatory authority requirements concerning manufacturing operations and laser performance standards, our products could be subject to additional restrictions and withdrawal from the market, which would harm our business.
We are currently required to demonstrate and maintain compliance with the FDA’s Quality System Regulation, or QSR. The QSR is a complex regulatory scheme that covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of our products. Because our products involve the use of lasers, our products also are covered by a performance standard for lasers set forth in FDA regulations. The laser performance standard imposes specific record keeping, reporting, product testing and product labeling requirements. These requirements include affixing warning labels to laser products as well as incorporating certain safety features in the design of laser products. In the EU countries, compliance with harmonized standards is also recommended as this is interpreted as a presumption of conformity with the relevant Essential Requirements. The FDA enforces the QSR and laser performance standards through periodic unannounced inspections. We have been, and anticipate in the future being, subject to such inspections.
These FDA regulations and EU standards cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of any products for which we may obtain regulatory clearance or approval. Compliance with applicable regulatory requirements is subject to continual review and is monitored rigorously through periodic inspections by the FDA. Compliance with harmonized standards in the EU is also subject to regular review through the conduct of inspection by Notified Bodies or other certification bodies. If we, or our contract manufacturers, fail to adhere to QSR requirements in the United States or other harmonized standards in the EU, this could delay production of our products and lead to fines, difficulties in obtaining regulatory clearances or CE Certificates of Conformity, recalls, enforcement actions, including injunctive relief or consent decrees, or other consequences, which could, in turn, have a material adverse effect on our financial condition or results of operations.
The FDA audits compliance with the QSR through periodic announced and unannounced inspections of manufacturing and other facilities. The failure by us or any of our contract manufacturers to comply with applicable statutes and regulations administered by the FDA, or harmonized standards in the EU, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in various enforcement actions, including a public warning letter, a shutdown of or restrictions on our manufacturing operations, delays in approving or clearing a product, refusal to permit the import or export of our products, a recall or seizure of our products, fines, injunctions, civil or criminal penalties, or other sanctions, such as those described in the preceding paragraphs, any of which could cause our business and operating results to suffer.
If any of our products for which we receive regulatory clearance or approval cause or contribute to a death or a serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions, which could harm our business.
Under the FDA medical device reporting, or MDR, regulations, medical device manufacturers are required to report to the FDA information that a device has or may have caused or contributed to a death or serious injury
43
Table of Contents
or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or a similar device of such manufacturer were to recur. The decision to file an MDR involves a judgment by us as the manufacturer. If and when we make such decisions in the future, the FDA may not agree with our decisions. If we fail to report MDRs to the FDA within the required timeframes, or at all, or if the FDA disagrees with any of our determinations regarding the reportability of certain events, the FDA could take enforcement actions against us, which could have an adverse impact on our reputation and financial results.
Additionally, all manufacturers placing medical devices in the market in the EU are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the competent authority in whose jurisdiction the incident occurred. In the EU, we must comply with the EU Medical Device Vigilance System. Under this system, incidents must be reported to the relevant authorities of the EU countries, and manufacturers are required to take Field Safety Corrective Actions, or FSCAs, to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. An incident is defined as any malfunction or deterioration if the characteristics or performance of a device, as well as any inadequacy in the labeling or the instructions for use which, directly or indirectly, might lead to or might have led to the death of a patient or user or of other persons or to a serious deterioration in their state of health. An FSCA may include the recall, modification, exchange, destruction or retrofitting of the device. FSCAs must be communicated by the manufacturer or its European Authorized Representative to its customers and to the end users of the device through Field Safety Notices.
Any such safety issues involving our products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
Any of the products for which we receive regulatory clearance or approval may be subject to product recalls. A recall of such products, either voluntarily or at the direction of the FDA or another governmental authority, or the discovery of serious safety issues with our future products, could have a significant adverse impact on our business.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. The authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. The FDA requires that certain classifications of recalls be reported to the FDA within 10 working days after the recall is initiated. A government-mandated or voluntary recall by us, or any distributor we may have in the future, could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing errors, design or labeling defects or other deficiencies and issues.
We are also required to follow detailed recordkeeping requirements for all company-initiated medical device corrections and removals and to report such corrective and removal actions to the FDA if they are carried out in response to a risk to health and have not otherwise been reported under the MDR regulations. We may initiate voluntary recalls involving our products for which we receive regulatory clearance or approval in the future that we determine do not require notification to the FDA. If the FDA disagrees with our determinations, the FDA could require us to report those actions as recalls. Recalls of any of our products for which we receive regulatory clearance or approval in the future would divert managerial and financial resources and have an adverse effect on our reputation, results of operations and financial condition, which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be subject to enforcement actions or liability claims, any may be required to bear other costs, or take other actions that may have a negative impact on our future sales and our ability to generate profits.
44
Table of Contents
If we fail to comply with state laws and regulations, or if state laws or regulations change, our business could suffer.
In addition to FDA regulations, most of our products are also subject to state regulations relating to their sale and use. These regulations are complex and vary from state to state, which complicates monitoring compliance. In addition, these regulations are in many instances in flux. For example, federal regulations allow our prescription products to be sold to or on the order of “licensed practitioners,” that is, practitioners licensed by law to use or order the use of a prescription device. Licensed practitioners are defined on a state-by-state basis. As a result, some states permit non-physicians to purchase and operate our products, while other states do not. Additionally, a state could change its regulations at any time to prohibit sales to particular types of customers. We believe that, to date, we have sold our prescription products only to licensed practitioners. However, our failure to comply with state laws or regulations and changes in state laws or regulations may adversely affect our business.
We, or our distributors, may be unable to obtain or maintain foreign regulatory qualifications or approvals for our current or future products and indications, which could harm our business.
Sales of our products outside the United States are subject to foreign regulatory requirements that vary widely from country to country. In many countries, our third-party distributors are responsible for obtaining and maintaining regulatory approvals for our products. We do not control our third-party distributors, and they may not be successful in obtaining or maintaining these regulatory approvals.
Complying with foreign regulatory requirements can be an expensive and time consuming process, and approval is not certain. The time required to obtain foreign clearances or approvals may be longer than that required for FDA clearance or approval, and requirements for such clearances or approvals may differ significantly from FDA requirements. Foreign regulatory authorities may not clear or approve our products for the same indications cleared or approved by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA clearance or approval in addition to other risks. Although we or our distributors have obtained regulatory approvals in the European Union and other countries outside the United States for many of our products, we or our distributors may be unable to maintain regulatory qualifications, clearances or approvals in these countries or obtain qualifications, clearances or approvals in other countries. If we are not successful in doing so, our business will be harmed. We may also incur significant costs in attempting to obtain and in maintaining foreign regulatory clearances, approvals or qualifications. Foreign regulatory agencies, as well as the FDA, periodically inspect manufacturing facilities both in the United States and abroad. If we experience delays in receiving necessary qualifications, clearances or approvals to market our products outside the United States, or if we fail to receive those qualifications, clearances or approvals, or if we fail to comply with other foreign regulatory requirements, we and our distributors may be unable to market our products or enhancements in international markets effectively, or at all. Additionally, the imposition of new requirements may significantly affect our business and our products. We may not be able to adjust to such new requirements, which may adversely affect our business.
Federal regulatory reforms may adversely affect our ability to sell our products profitably.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of a device. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. For example, the FDA proposed changing its standards for determining when a medical device modification must receive premarket clearance or approval. Although Congress objected to these revised standards, it is possible that the FDA will seek to implement these or similar changes in the future.
The Patient Protection and Affordable Care Act includes reporting and disclosure requirements, commonly referred to as the “Sunshine Act”, for “applicable manufacturers” of drugs, biological, medical devices and
45
Table of Contents
medical supplies with regard to payments or other transfers of value made to certain physicians and teaching hospitals. The final rules implementing the Sunshine Act are complex, ambiguous, and broad in scope. Sales related to the Ellman surgical product line are subject to the reporting requirements under the Sunshine Act. We believe that sales related to our aesthetic product lines fall under a reporting exception under this law and, accordingly, reporting related to those sales is not required. With the new U.S. presidential administration and Congress, there will likely be additional legislative changes, including the possible repeal and replacement of certain provisions of the Sunshine Act, but it is unclear if and when such legislation would be enacted, what it would provide and what impact it would have on, among other things, reporting and disclosure requirements.
There are a number of federal and state laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated patient privacy rules under the Health Insurance Portability and Accountability Act of 1996, or HIPAA. These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. If we or any of our service providers are found to be in violation of the promulgated patient privacy rules under HIPAA, we could be subject to civil or criminal penalties, which could increase our liabilities, harm our reputation and have a material adverse effect on our business, financial condition and operating results. Similarly, failure to comply with the European Union’s requirements regarding the protection of personal information can also lead to significant penalties and sanctions.
Our compliance with applicable legal and regulatory requirements is, and will continue to be, costly and time consuming. It is impossible to predict whether other legislative changes will be enacted or government regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be. If we are found to be in violation of any of this or any other law, we may be subject to penalties, including fines.
In addition, most of the products and systems that we sell became subject in 2013 to an excise tax on sales of certain medical devices in the United States after December 31, 2012 by the manufacturer, producer or importer in an amount equal to 2.3% of the sale price. Under the law, additional charges, including warranties, may be deemed to be included in the sale price for purposes of determining the amount of the excise tax. Although this excise tax has been suspended for 2016 and 2017, we cannot predict whether it will be reinstated, and if it is, we believe it could harm our future sales and profitability.
Risks Related to Litigation
If our past fax practices are shown to have violated the Telephone Consumer Protection Act, that could have a material impact on our results of operations, cash flows and financial condition.
In the past, we have used faxes to disseminate information about our clinical workshops to large numbers of customers and potential customers. Under the federal Telephone Consumer Protection Act, or TCPA, recipients of noncompliant fax advertisements are entitled to $500 per violation, and that amount may be increased up to $1,500 for knowing or willful violations.
In July 2016, we were sued in federal court by ARcare, Inc., or ARcare, individually and as putative representative of a purported class under the TCPA. The plaintiff alleges that we violated the TCPA by sending fax advertisements that did not comply with statutory and Federal Communications Commission requirements that senders provide recipients with certain information about how to opt out from receiving faxed advertisements in the future, and by sending unsolicited fax advertisements.
We answered the complaint and denied liability, and in January 2017, we entered into an agreement to settle the lawsuit. The settlement agreement was subject to court approval and, if approved, would have given
46
Table of Contents
Cynosure a full release from the settlement class concerning the conduct alleged in the complaint. However, the court overseeing the matter subsequently expressed concern about aspects of the settlement agreement, and in February 2017, we and ARcare agreed in principle to new terms to be incorporated into a new settlement agreement. The new settlement agreement will be subject to further negotiation, and thereafter the settlement agreement will be subject to court approval. As with the initial settlement, if approved, we would receive a full release from the settlement class concerning the conduct alleged in the complaint.
Litigation is subject to numerous uncertainties and we are unable to predict the ultimate outcome of this matter. Even if we prevail in or are able to settle this lawsuit, other claims may be brought against us alleging past violations of the TCPA. Moreover, the amount of any potential liability in connection with other possible lawsuits will depend, to a large extent, on whether a settlement class is certified in the ARcare matter, which we cannot predict at this time. We have tendered a claim with respect to the ARcare lawsuit to our general liability insurance carrier and the carrier has indicated that coverage is unlikely for this claim.
We may be required to pay damages in respect of this lawsuit or other possible future lawsuits arising out of our past fax transmissions, any of which could materially and adversely affect our results of operations, cash flows and financial condition. Regardless of the outcome, this lawsuit or other possible future lawsuits may cause us to incur significant legal expenses and divert the attention of our management and key personnel from our business operations.
Product liability and business liability suits could be brought against us due to defective design, material or workmanship or due to misuse of our products. These lawsuits could be expensive and time consuming and result in substantial damages to us and increases in our insurance rates.
If our products are defectively designed, manufactured or labeled, contain defective components or are misused, we may become subject to substantial and costly litigation by our customers or their patients or clients. Misusing our products or failing to adhere to operating guidelines for our products can cause severe burns or other significant damage to the eyes, skin or other tissue. If our products fail to function properly, we may be required to conduct product recalls and our customers may lose the ability to treat their patients or clients resulting in a loss of business for our customers. We are routinely involved in claims related to the use of our products. Product liability and business liability claims could divert management’s attention from our core business, be expensive to defend and result in sizable damage awards against us. Our current insurance coverage may not apply or may not be sufficient to cover these claims, and the coverage we have is subject to deductibles for which we are responsible. Moreover, in the future, we may not be able to obtain insurance in amount or scope sufficient to provide us with adequate coverage against potential liabilities. Any product liability or other claims brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing continuing coverage, could harm our reputation in the industry and reduce product sales. We would need to pay any losses in excess of our insurance coverage out of cash reserves, harming our financial condition and adversely affecting our operating results.
Employment related lawsuits could be brought against us for improper termination of employment, sexual harassment, hostile work environment and other claims. These lawsuits could be expensive and time consuming and result in substantial damages to us and increases in our insurance rates.
If we terminate employment for improper reasons or fail to provide an appropriate work environment or it is alleged that we did so, we may become subject to substantial and costly litigation by our former and current employees. We are routinely involved in claims related to improper termination and other claims. Such claims could divert management’s attention from our core business, be expensive to defend and result in sizable damage awards against us. Our current insurance coverage may not apply or may not be sufficient to cover these claims, and the coverage we have is subject to deductibles for which we are responsible. Moreover, in the future, we may not be able to obtain insurance in amount or scope sufficient to provide us with adequate coverage against potential liabilities. Any employment related claims brought against us, with or without merit, could increase our
47
Table of Contents
employment law insurance rates or prevent us from securing continuing coverage, could harm our reputation in the industry and reduce product sales. We would need to pay any losses in excess of our insurance coverage out of cash reserves, harming our financial condition and adversely affecting our operating results.
| Item 1B. | Unresolved Staff Comments |
None.
| Item 2. | Properties |
We lease a 150,000 square foot facility in Westford, Massachusetts which houses our executive offices and our manufacturing, research and development and warehouse operations. The lease on this facility expires in May 2028. We also lease a 19,000 square foot facility in Westford, Massachusetts which houses additional warehouse operations. The lease on this facility expires in June 2024. In addition, we lease a 44,000 square foot facility in Hicksville, New York which houses our manufacturing and warehouse operations for our Ellman product line. The lease on the Hicksville facility expires in June 2020. Lastly, we lease an aggregate of approximately 70,000 square feet of space at 19 other locations in Europe and the Asia/Pacific region, which we use for sales and service purposes.
| Item 3. | Legal Proceedings |
PicoSure Litigation
On June 26, 2015, a plaintiff, LDGP, LLC d/b/a Hartsough Dermatology, individually and on behalf of a putative class, filed a complaint against us in the U.S. District Court for the Northern District of Illinois—Western Division seeking monetary damages, injunctive relief, costs and attorneys’ fees. The plaintiff filed an amended complaint on November 9, 2015, which added three new plaintiffs. The amended complaint alleges that we falsely represented that the PicoSure laser removes and eliminates tattoos and difficult colors, and alleges violations of several state consumer fraud laws, breach of warranty, common law fraud and negligent misrepresentation. It seeks to assert claims on behalf of all entities in the United States who purchased a PicoSure laser, except those located in Louisiana. On December 7, 2015, we filed our answer and motion for partial judgment on the pleadings regarding several counts in the amended complaint. On August 15, 2016, the court granted our motion in connection with the negligent misrepresentation count, and denied it with respect to the breach of contract claim. We believe that we have meritorious defenses to the individual claims of the named plaintiffs, and we believe that the putative class claims are without merit.
SmartLipo Litigation
On May 2, 2016, Susan Lovell Ficken and certain other named plaintiffs, individually and on behalf of a putative class, filed a complaint against us in Missouri state court seeking monetary and punitive damages, injunctive relief, costs and attorneys’ fees. The matter was removed to the U.S. District Court for the Western District of Missouri—Central Division on June 3, 2016. The amended complaint, filed on June 27, 2016, alleges that the SmartLipo laser was unlawfully marketed by us and third parties, giving rise to alleged violations of the Missouri Merchandising Practices Act. It seeks to assert claims on behalf of all individuals who purchased a SmartLipo procedure in Missouri. On July 11, 2016, we filed a motion to dismiss the entire action. In response, on September 2, 2016, plaintiffs filed a second amended complaint with the same material allegations but that added a named plaintiff in an attempt to defeat our statute of limitations defense. We filed a motion to dismiss the second amended complaint on September 16, 2016. That motion has been fully briefed, and we are awaiting the Court’s ruling. In the interim, we are engaged in discovery. In accordance with the requirements imposed upon us by Western District of Missouri’s mandatory mediation and assessment program, we participated in mediation on February 15, 2017 which did not result in a resolution of the matter. We believe the claims are without merit, and that we have meritorious defenses.
48
Table of Contents
TCPA Litigation
On July 27, 2016, ARcare, Inc., individually and as putative representative of a purported nationwide class, filed a complaint against us in the U.S. District Court for the District of Massachusetts. The plaintiff alleges that we violated the federal Telephone Consumer Protection Act, or TCPA, by sending fax advertisements that did not comply with statutory and Federal Communications Commission, or FCC, requirements that senders provide recipients with certain information about how to opt out from receiving faxed advertisements in the future, and by sending unsolicited fax advertisements. The complaint seeks damages, declaratory and injunctive relief, and attorneys’ fees on behalf of a purported class of all recipients of purported fax advertisements that the plaintiff alleges did not receive an adequate opt-out notice. The TCPA provides for statutory damages of $500 per violation and gives courts the discretion to increase that amount up to $1,500 for knowing and willful violations. We estimate the aggregate number of faxes sent in the four years prior to the lawsuit to be approximately 835,000. On September 30, 2016, we answered the complaint and denied liability. On September 7, 2016, the plaintiff sent a demand letter seeking a class settlement for statutory damages under Massachusetts General Laws, Chapter 93A § 9, and on October 7, 2016, we responded denying any liability under Chapter 93A, but offering the plaintiff statutory damages under Chapter 93A on an individual basis. To date, the plaintiff has not moved to amend the complaint to assert a claim under Chapter 93A. On October 12, 2016, the court agreed to the parties’ request to stay the case until January 11, 2017 to allow the parties to pursue mediation. In January 2017, Cynosure and ARcare entered into an agreement to settle the lawsuit. The settlement agreement was subject to court approval and, if approved, would have given Cynosure a full release from the settlement class concerning the conduct alleged in the complaint, in exchange for which Cynosure would have paid settlement compensation between $6.5 million and $16.0 million depending on the number of class members to file a valid claim. The court overseeing the matter subsequently expressed concern about aspects of the settlement agreement, and in February 2017, Cynosure and ARcare agreed in principle to new terms to be incorporated into a new settlement agreement that is subject to further negotiation, and thereafter will be subject to court approval. As with the initial settlement, if approved, we would pay a fixed settlement compensation amount of $8.5 million and receive a full release from the settlement class concerning the conduct alleged in the complaint.
In connection with the original January 2017 settlement agreement, we recorded a $7.2 million charge in the fourth quarter of 2016 in connection with the settlement agreement, including a $6.5 million estimated settlement plus and expenses associated with this matter. As a result of the new settlement terms, we subsequently increased that charge by $2.0 million, also effective in the fourth quarter of 2016, to a total of $9.2 million. This $9.2 million represents our best estimate of the total settlement expense to be incurred in connection with the ARcare matter.
Merger Litigation
On February 24, 2017, a putative stockholder class action complaint, captioned Joel Rosenfeld IRA v. Cynosure, Inc., et al., Civ. Action No. 17-10309 (D. Mass.), or the Rosenfeld Action, was filed in the U.S. District Court for the District of Massachusetts in connection with the Offer and the Merger, naming as defendants us and each member of our board of directors. The complaint in the Rosenfeld Action, among other things, criticizes the proposed transaction price as inadequate and alleges that we and our board of directors omitted certain allegedly material information from the Solicitation/Recommendation Statement on Schedule 14D-9 in violation of the Exchange Act and related SEC regulations. The alleged omissions relate to (i) certain of our projections, (ii) certain data and inputs underlying the financial valuation analyses, and (iii) the background of the merger process.
The plaintiff in the Rosenfeld Action seeks an order (i) maintaining the action as a class action, certifying the plaintiff as the class representative, and certifying the plaintiff’s counsel as class counsel, (ii) preliminarily and permanently enjoining defendants and all persons acting in concert with them from proceeding with, consummating, or closing the Offer or the Merger, (iii) in the event defendants consummate the Offer or the Merger, rescinding it and setting it aside or awarding rescissory damages, and (iv) awarding appropriate costs, attorneys’ fees, and experts’ fees. We and our directors believe this lawsuit is without merit.
49
Table of Contents
In addition to the matters discussed above, from time to time, we are subject to various claims, lawsuits, disputes with third parties, investigations and pending actions involving various allegations against us incident to the operation of its business, principally product liability. Each of these other matters is subject to various uncertainties, and it is possible that some of these other matters may be resolved unfavorably to us. We establish accruals for losses that management deems to be probable and subject to reasonable estimate. We believe that the ultimate outcome of these other matters will not have a material adverse impact on our consolidated financial position, results of operations or cash flows.
| Item 4. | Mine Safety Disclosures |
None.
50
Table of Contents
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Price of and Dividends on Our Common Stock and Related Stockholder Matters.
Our Class A common stock trades on The Nasdaq Global Market under the symbol “CYNO.” The following table sets forth, for the periods indicated, the high and low sales prices of our Class A common stock on The Nasdaq Global Market.
| High | Low | |||||||
| Fiscal Year Ended December 31, 2015 |
||||||||
| First quarter |
$ | 31.83 | $ | 27.02 | ||||
| Second quarter |
$ | 40.52 | $ | 29.76 | ||||
| Third quarter |
$ | 42.97 | $ | 29.00 | ||||
| Fourth quarter |
$ | 45.05 | $ | 29.60 | ||||
| Fiscal Year Ended December 31, 2016 |
||||||||
| First quarter |
$ | 44.77 | $ | 32.90 | ||||
| Second quarter |
$ | 53.33 | $ | 42.80 | ||||
| Third quarter |
$ | 55.94 | $ | 47.70 | ||||
| Fourth quarter |
$ | 53.06 | $ | 39.90 | ||||
On February 21, 2017, the closing price per share of our Class A common stock was $66.10, as reported on The Nasdaq Global Market. The number of record holders of our Class A common stock as of February 21, 2017 was 607.
In February 2016, we announced that our board of directors has authorized the repurchase of up to $35 million of our Class A common stock, from time to time, on the open market or in privately negotiated transactions under a new stock repurchase program. This program will terminate on February 1, 2018. During the year ended December 31, 2016, we did not repurchase any shares of our common stock under this program.
We have never paid or declared any cash dividends on our common stock. We currently intend to retain our earnings, if any, to finance the growth and development of our business. Payment of future dividends, if any, will be at the discretion of our board of directors.
At December 31, 2016, our total cash, cash equivalents and short and long-term marketable securities balance was $237.8 million. We did not sell any unregistered securities during the period covered by this Annual Report.
The graph below shows the cumulative total stockholder return of an investment of $100 (and the reinvestment of any dividends thereafter) on December 31, 2011 (the last trading day for the year ended December 31, 2011) in our Class A common stock, the Nasdaq U.S. Index and the Nasdaq Medical Devices, Instruments, Supplies, Manufacturers and Distributors Index. Our stock price performance shown in the graph below is not indicative of future stock price performance.
51
Table of Contents
The following performance graph and related information shall not be deemed to be “soliciting material” or “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or Exchange Act, except to the extent that we specifically incorporate it by reference into such filing.
Comparison of 5 Year Cumulative Total Return
Among Cynosure, Inc., NASDAQ U.S. Index and
the NASDAQ Medical Dev/Ins/Sup Manufacturers & Distributors Index
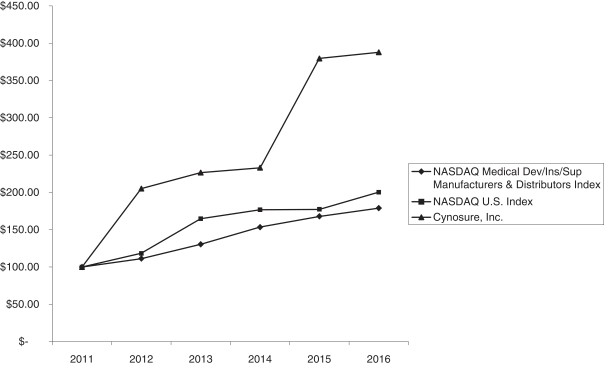
| Name |
12/31/2011 | 12/31/2012 | 12/31/2013 | 12/31/2014 | 12/31/2015 | 12/31/2016 | ||||||||||||||||||
| NASDAQ Medical Dev/Ins/Sup Manufacturers & Distributors Index |
$ | 100.00 | $ | 111.32 | $ | 130.46 | $ | 153.48 | $ | 167.82 | $ | 178.95 | ||||||||||||
| NASDAQ U.S. Index |
$ | 100.00 | $ | 118.31 | $ | 164.73 | $ | 176.47 | $ | 177.32 | $ | 200.39 | ||||||||||||
| Cynosure, Inc. |
$ | 100.00 | $ | 205.02 | $ | 226.53 | $ | 233.16 | $ | 379.85 | $ | 387.76 | ||||||||||||
52
Table of Contents
| Item 6. | Selected Consolidated Financial Data |
The following selected consolidated financial data should be read in conjunction with our consolidated financial statements and the related notes which are included elsewhere in this Annual Report and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this Annual Report. The consolidated statements of operations data for the years ended December 31, 2016, 2015 and 2014 and the consolidated balance sheet data as of December 31, 2016 and 2015 are derived from our audited consolidated financial statements, which are included elsewhere in this Annual Report. The consolidated statement of operations data for the years ended December 31, 2013 and 2012 and the consolidated balance sheet data as of December 31, 2014, 2013 and 2012 are derived from our audited consolidated financial statements, which are not included in this Annual Report. Our historical results for any prior period are not necessarily indicative of results to be expected for any future period.
| Year Ended December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (In thousands, except per share data) | ||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||
| Revenues |
$ | 433,532 | $ | 339,462 | $ | 292,369 | $ | 226,010 | $ | 153,493 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cost of revenues |
178,221 | 145,928 | 127,131 | 95,730 | 64,567 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
255,311 | 193,534 | 165,238 | 130,280 | 88,926 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Sales and marketing |
153,853 | 111,506 | 88,564 | 65,211 | 48,220 | |||||||||||||||
| Research and development |
28,973 | 22,343 | 22,033 | 17,473 | 12,972 | |||||||||||||||
| Amortization of intangible assets acquired |
2,741 | 2,990 | 2,961 | 2,114 | 1,368 | |||||||||||||||
| General and administrative |
42,404 | 30,374 | 30,420 | 51,309 | 14,233 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
227,971 | 167,213 | 143,978 | 136,107 | 76,793 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
27,340 | 26,321 | 21,260 | (5,827 | ) | 12,133 | ||||||||||||||
| Interest (expense) income, net |
(1,563 | ) | (1,683 | ) | (1,446 | ) | (23 | ) | 60 | |||||||||||
| Other (expense) income, net |
(834 | ) | (1,440 | ) | (1,476 | ) | 313 | 532 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) before provision (benefit) for income taxes |
24,943 | 23,198 | 18,338 | (5,537 | ) | 12,725 | ||||||||||||||
| Provision (benefit) for income taxes (1) |
10,742 | 7,391 | (13,000 | ) | (3,890 | ) | 1,764 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
$ | 14,201 | $ | 15,807 | $ | 31,338 | $ | (1,647 | ) | $ | 10,961 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic net income (loss) per share |
$ | 0.61 | $ | 0.71 | $ | 1.44 | $ | (0.09 | ) | $ | 0.83 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted net income (loss) per share |
$ | 0.60 | $ | 0.70 | $ | 1.41 | $ | (0.09 | ) | $ | 0.79 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic weighted average common shares outstanding |
23,304 | 22,286 | 21,824 | 19,325 | 13,189 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted weighted average common shares outstanding |
23,706 | 22,658 | 22,195 | 19,325 | 13,792 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
53
Table of Contents
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents, marketable securities, investments and related financial instruments |
$ | 237,783 | $ | 182,760 | $ | 133,375 | $ | 129,092 | $ | 146,745 | ||||||||||
| Working capital |
233,176 | 182,090 | 144,568 | 152,362 | 148,615 | |||||||||||||||
| Total assets |
586,507 | 534,610 | 456,077 | 406,189 | 233,930 | |||||||||||||||
| Capital lease obligation, net of current portion |
18,707 | 17,372 | 16,088 | 14,957 | 432 | |||||||||||||||
| Retained earnings |
69,982 | 55,781 | 39,974 | 8,636 | 10,283 | |||||||||||||||
| Total stockholders’ equity |
455,494 | 404,402 | 358,112 | 328,352 | 197,507 | |||||||||||||||
| (1) | Provision (benefit) for income taxes for the year ended December 31, 2014 includes an income tax benefit of $19.9 million resulting from the release of substantially all of the valuation allowance maintained against our net U.S. deferred tax asset. |
54
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes and other financial data included elsewhere in this Annual Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should review Item 1A of this Annual Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Company Overview
We develop, manufacture, and market aesthetic treatment systems that enable plastic surgeons, dermatologists and other medical practitioners to perform non-invasive and minimally invasive procedures to remove hair, treat vascular and benign pigmented lesions, remove multi-colored tattoos, revitalize the skin, reduce fat through laser lipolysis, reduce cellulite, clear nails infected by toe fungus, ablate sweat glands and improve women’s health. We also market radiofrequency, or RF, energy-sourced medical devices for precision surgical applications such as facial plastic and general surgery, gynecology, ear, nose, and throat procedures, ophthalmology, oral and maxillofacial surgery, podiatry and proctology.
Our product portfolio is composed of a broad range of energy sources including Alexandrite, diode, Nd: YAG, picosecond, pulse dye, Q-switched lasers, intense pulsed light and RF technology. We sell our products globally under the Cynosure, Palomar, ConBio and Ellman brand names through a direct sales force in the United States, Canada, France, Morocco, Germany, Spain, the United Kingdom, Australia, China, Japan and Korea, and through international distributors in over 120 other countries.
A key element of our business strategy is to launch innovative new products and technologies into high-growth aesthetic applications. Our research and development team builds on our existing broad range of laser, light-based technologies and other energies to develop new solutions and products to target unmet needs in significant aesthetic treatment markets. Innovation continues to be a strong contributor to our strength as we continue to add new products to our aesthetic treatment systems portfolio.
As further described under Note 14 to our consolidated financial statements included in this Annual Report, on February 14, 2017, we entered into an Agreement and Plan of Merger, or the Merger Agreement, with Hologic, Inc., or Hologic, and Minuteman Merger Sub, Inc., a wholly-owned subsidiary of Hologic, or the Purchaser. Pursuant to the terms of Merger Agreement, the Purchaser commenced an all cash tender offer on February 22, 2017, or the Offer, for any (subject to the minimum condition) and all of our outstanding shares of Class A common stock, par value $0.001 per share, or the Shares, at a purchase price of $66.00 per Share, or the Offer Price, net to the seller in cash, without interest, subject to any required withholding of taxes. The Offer will remain open for a minimum of 20 business days from the date of commencement. Following the completion of the Offer, subject to the absence of injunctions or other legal restraints preventing the consummation of the Merger, the Purchaser will merge with and into us, with Cynosure surviving as a wholly-owned subsidiary of Hologic, which we refer to as the Merger, pursuant to the procedure provided for under Section 251(h) of the Delaware General Corporation Law, without any additional stockholder approvals. The Merger will be effected as soon as practicable following the time of purchase by the Purchaser of Shares validly tendered and not withdrawn in the Offer. Unless extended by Hologic in accordance with the Merger Agreement, the offering period for the Offer will expire at the end of the day, 12:00 Midnight, Eastern time, on Tuesday, March 21, 2017. The Merger Agreement contains customary termination rights for both us and Hologic, including, among others, for failure to consummate the Offer on or before August 14, 2017.
We expect to consummate the Merger in late March or April of 2017. However, we have prepared this Annual Report and the forward-looking statements contained in this Annual Report as if we were going to remain an independent company. If the Merger is consummated, many of the forward-looking statements contained in this Annual Report would no longer be applicable.
55
Table of Contents
Revenues
We generate revenues primarily from sales of our non-invasive and minimally invasive laser and light-based aesthetic treatment systems, as well as RF energy based surgical and aesthetic systems, which we refer to collectively as our product sales. We also generate revenues from sales of parts and accessories, from services, including product warranty revenues, and from royalty payments received from our licensees. Our business model generally does not involve separate contracts entered into at or near the same time; nor does our business model involve incremental future discounts offered to the same customer. We do not offer a right of cancellation, termination, refund or return. During the year ended December 31, 2016, we derived approximately 83% of our revenues from product sales and 17% of our revenues from the sale of parts, accessories, service and royalties. During the year ended December 31, 2015, we derived approximately 81% of our revenues from product sales and 19% of our revenues from the sale of parts, accessories, service and royalties.
We recognize revenue from sales of our products, parts and accessories in accordance with the Accounting Standards Codification, or ASC, Revenue Recognition Topic 605-10-S99. We recognize revenue from sales of our products, parts and accessories when title and risk of ownership has been transferred, which is upon shipment, provided there are no uncertainties regarding customer acceptance, and the following factors are present:
| • | persuasive evidence of an arrangement; |
| • | the fee is fixed or determinable; and |
| • | collectability of the related receivable is reasonably assured. |
Revenues from the sale of services and extended warranty contracts are deferred and recognized on a straight-line basis over the contract period as services are provided.
We recognize royalty revenues when we can reliably estimate such amounts and collectability is reasonably assured. As such, we recognize royalty revenues in the quarter reported to us by our licensees, or one quarter following the quarter in which sales by our licensees occurred. Royalty revenues also include amounts due from settlements with licensees for back-owed royalties from prior periods. These settlement amounts are considered revenue, when collectability is reasonably assured, because they constitute our ongoing major or central operations.
Multiple-element arrangements are evaluated in accordance with ASC 605-25, Multiple-Element Arrangements. We determined that our multiple-element arrangements are generally comprised of the following elements that are recognized as separate units of accounting: products, accessories, extended warranties and installation and training services. Installation and training services are not essential to the functionality of the aesthetic treatment system. We allocate revenue among the elements based upon their vendor specific objective evidence of fair value, or VSOE. If VSOE does not exist, we may use third-party evidence of fair value, or TPE, to determine the relative selling price of each element. If neither VSOE nor TPE exists, we may use management’s best estimate of selling price, or BESP, of each element to determine the relative selling price. Presently there are no elements for which we are utilizing TPE or BESP.
VSOE is based on the relative sales price based on separate stand-alone sales of our elements. Revenues associated with products and accessories are recognized when shipped. Accessories are predominantly shipped with the aesthetic treatment system when sold with the product. Revenues associated with extended warranties are deferred and recognized ratably over the extended warranty period, which is generally one year. The relative sales prices of extended warranties are based on the actual pricing of service contracts purchased on a standalone basis at a later time, normally at the end of the standard warranty period. Due to the type of revenue arrangements and structure of our contracts, we refer to guidance on separately priced extended warranties within ASC 605-20, Services. Under ASC 605-20, we defer the selling price of the separately priced extended warranty at the inception of the arrangement, and allocate the remainder of the arrangement consideration among the remaining elements on their VSOE of fair value in accordance with ASC 605-25.
56
Table of Contents
We sell our products globally under the Cynosure, Palomar, ConBio and Ellman brand names through a direct sales force in the United States, Canada, France, Morocco, Germany, Spain, the United Kingdom, Australia, China, Japan and Korea, and use distributors to sell our products in other countries where we do not have a direct presence. During the years ended December 31, 2016 and 2015, we derived 32% and 38% of our total revenues, respectively, from sales outside North America. As of December 31, 2016, we had 177 sales employees covering North America and 54 sales employees in France, Morocco, Germany, Spain, the United Kingdom, Australia, China, Japan and Korea. We utilize a global distribution network covering over 120 countries.
The following table provides revenue data by geographical region for the years ended December 31, 2016, 2015 and 2014:
| Percentage of Revenues | ||||||||||||
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Region |
||||||||||||
| North America |
68 | % | 62 | % | 54 | % | ||||||
| Europe |
10 | 12 | 17 | |||||||||
| Asia/Pacific |
19 | 21 | 22 | |||||||||
| Other |
3 | 5 | 7 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
100 | % | 100 | % | 100 | % | ||||||
|
|
|
|
|
|
|
|||||||
See Note 6 to our consolidated financial statements included in this Annual Report for revenues and asset data by geographic region.
Cost of Revenues
Our cost of revenues consists primarily of material, labor and manufacturing overhead expenses and includes the cost of components and subassemblies supplied by third-party suppliers. Cost of revenues also includes royalties incurred on certain products sold by us and our licensees, costs incurred in connection with our efforts to litigate or settle additional third-party license agreements, amortization expense related to developed technology and patents intangible assets, service and warranty expenses, as well as salaries and personnel-related expenses, including stock-based compensation, for our operations management team, purchasing and quality control.
Sales and Marketing Expenses
Our sales and marketing expenses consist primarily of salaries, commissions and other personnel-related expenses, including stock-based compensation, for employees engaged in sales, marketing and support of our products, trade show, promotional and public relations expenses and management and administration expenses in support of sales and marketing. We expect our sales and marketing expenses to increase in absolute dollars but remain consistent as a percentage of revenues in 2017.
Research and Development Expenses
Our research and development expenses consist of salaries and other personnel-related expenses, including stock-based compensation, for employees primarily engaged in research, development and engineering activities, materials used and other overhead expenses incurred in connection with the design and development of our products and, from time to time, expenses associated with collaborative research and development agreements that we may enter into. We expense all of our research and development costs as incurred. We expect our research and development expenses to increase in absolute dollars and as a percentage of revenues in 2017.
57
Table of Contents
Amortization of Intangible Assets Acquired
Amortization of intangible assets acquired consists of amortization expense related to customer relationships and trade name intangible assets.
General and Administrative Expenses
Our general and administrative expenses consist primarily of salaries and other personnel-related expenses, including stock-based compensation for executives, accounting and administrative personnel, professional fees and other general corporate expenses. We expect our general and administrative expenses to decrease in absolute dollars and as a percentage of revenues in 2017.
Interest Expense, net
Interest expense, net consists primarily of interest charges on capital lease obligations and the license transfer agreement assumed in the Ellman asset acquisition. Interest earned on our short and long-term marketable securities consists of state and municipal bonds, and U.S. government agencies and treasuries. We expect interest expense to remain consistent in 2017 as compared to 2016.
Other Expense, net
Other expense, net consists primarily of foreign currency remeasurement gains or losses and other miscellaneous income and expense items.
Provision for Income Taxes
We account for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted rates in effect for the year in which those temporary differences are expected to be recovered or settled. A deferred tax asset is established for the expected future benefit of net operating loss carryforward and credit carryforwards.
A full valuation allowance is maintained on the net deferred tax assets of our subsidiary in Mexico. Valuation allowances are provided if, based on the weight of available evidence, it is more-likely-than-not that some portion or all of the deferred tax assets will not be realized. We consider several sources of taxable income in making our valuation allowance assessments including taxable income in carryback years, future reversals of existing taxable temporary differences, tax planning strategies and forecasted future income. We will continue to monitor the need for valuation allowances in each jurisdiction, and may adjust our positions in the future based on actual results. We account for uncertain tax positions following the provisions of ASC, 740, Accounting for Income Taxes, or ASC 740. ASC 740 clarifies the accounting for income taxes, by prescribing a minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. ASC 740 also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
58
Table of Contents
Results of Operations
Years Ended December 31, 2016 and 2015
The following table contains selected statements of operations data, which serve as the basis of the discussion of our results of operations for the years ended December 31, 2016 and 2015:
| Year Ended December 31, 2016 |
Year Ended December 31, 2015 |
Change 2015 to 2016 |
||||||||||||||||||||||
| Amount | As a % of Total Revenues |
Amount | As a % of Total Revenues |
$ Change | % Change | |||||||||||||||||||
| (Dollars in thousands) | ||||||||||||||||||||||||
| Product revenues |
$ | 357,968 | 83 | % | $ | 276,085 | 81 | % | $ | 81,883 | 30 | % | ||||||||||||
| Parts, accessories, service and royalty revenues |
75,564 | 17 | 63,377 | 19 | 12,187 | 19 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total revenues |
433,532 | 100 | 339,462 | 100 | 94,070 | 28 | ||||||||||||||||||
| Cost of revenues |
178,221 | 41 | 145,928 | 43 | 32,293 | 22 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Gross profit |
255,311 | 59 | 193,534 | 57 | 61,777 | 32 | ||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Sales and marketing |
153,853 | 35 | 111,506 | 33 | 42,347 | 38 | ||||||||||||||||||
| Research and development |
28,973 | 7 | 22,343 | 6 | 6,630 | 30 | ||||||||||||||||||
| Amortization of intangible assets acquired |
2,741 | 1 | 2,990 | 1 | (249 | ) | (8 | ) | ||||||||||||||||
| General and administrative |
42,404 | 10 | 30,374 | 9 | 12,030 | 40 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
227,971 | 53 | 167,213 | 49 | 60,758 | 36 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Income from operations |
27,340 | 6 | 26,321 | 8 | 1,019 | 4 | ||||||||||||||||||
| Interest expense, net |
(1,563 | ) | — | (1,683 | ) | (1 | ) | 120 | 7 | |||||||||||||||
| Other expense, net |
(834 | ) | — | (1,440 | ) | — | 606 | 42 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Income before provision for income taxes |
24,943 | 6 | 23,198 | 7 | 1,745 | 8 | ||||||||||||||||||
| Provision for income taxes |
10,742 | 3 | 7,391 | 2 | 3,351 | 45 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income |
$ | 14,201 | 3 | % | $ | 15,807 | 5 | % | $ | (1,606 | ) | (10 | %) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Revenues
Total revenues for the year ended December 31, 2016 increased by $94.1 million, or 28%, to $433.5 million, as compared to the year ended December 31, 2015 revenues of $339.5 million (in thousands, except for percentages):
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2016 | 2015 | |||||||||||||||
| Product sales in North America |
$ | 242,351 | $ | 168,650 | $ | 73,701 | 44 | % | ||||||||
| Product sales outside North America |
115,617 | 107,435 | 8,182 | 8 | ||||||||||||
| Global parts, accessories, service and royalty sales |
75,564 | 63,377 | 12,187 | 19 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Revenues |
$ | 433,532 | $ | 339,462 | $ | 94,070 | 28 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The change in total revenues was attributable to a number of factors:
| • | Revenues from the sale of products in North America increased $73.7 million, or 44%, from the 2015 period, attributable primarily to sales of SculpSure, to which we have increased dedicated sales and marketing resources since its September 2015 launch, and, to a lesser extent, increased unit sales of |
59
Table of Contents
| certain other existing products. For the period, revenues from North American product sales of SculpSure exceeded the amount of the aggregate $73.7 million increase. These increases were partially offset by a decline in the number of units sold in certain other products, as a result of normal shifts in product mix, timing of order qualification and fulfillment, and certain products nearing the end of their product life cycle. Average selling prices for our products remained consistent with the 2015 period. |
| • | Revenues from the sale of products outside of North America increased $8.2 million, or 8%, from the 2015 period. Product sales from our Asia Pacific subsidiaries increased 33% from the 2015 period following regulatory approval, and the resulting commencement of sales, of PicoSure and Icon in China in September 2015 and November 2016, respectively, and SculpSure in Australia and in South Korea in December 2015 and July 2016, respectively. This was offset by a 9% decrease in third-party distributor product sales from the 2015 period due to economic weakness in certain third party distributor territories. Third-party distributor product sales increased 15% for the three months ended December 31, 2016 as compared to three months ended December 31, 2015. |
| • | Revenues from the sale of parts, accessories, services and royalties increased $12.2 million, or 19%, from the 2015 period primarily due to increases in our sales of SculpSure PACs, which were launched in September 2015. Revenues from our services business, including repairs and contracts, increased over the 2015 period in part due to our expanded install base. These increases were partially offset by the final $3.0 million settlement payment received from Tria Beauty, Inc. in the 2015 period and the expiration of MGH royalty agreements in the 2016 period. |
Cost of Revenues
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2016 | 2015 | |||||||||||||||
| Cost of revenues (dollars in thousands) |
$ | 178,221 | $ | 145,928 | $ | 32,293 | 22 | % | ||||||||
| Cost of revenues (as a percentage of total revenues) |
41 | % | 43 | % | ||||||||||||
Total cost of revenues increased $32.3 million, or 22%, to $178.2 million for the 2016 period, as compared to $145.9 million for the 2015 period. The increase was primarily associated with our 28% increase in total revenues in the 2016 period compared to the 2015 period. Total cost of revenues as a percentage of total revenues decreased for the 2016 period as compared to the 2015 period due to a higher percentage of product revenue from our distribution in North America, where average selling prices tend to be higher, and increases in our sales of SculpSure PACs.
Sales and Marketing
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2016 | 2015 | |||||||||||||||
| Sales and marketing (dollars in thousands) |
$ | 153,853 | $ | 111,506 | $ | 42,347 | 38 | % | ||||||||
| Sales and marketing (as a percentage of total revenues) |
35 | % | 33 | % | ||||||||||||
Sales and marketing expenses increased $42.3 million, or 38%, to $153.9 million for the 2016 period, as compared to $111.5 million for the 2015 period. The increase was due to an increase of $16.3 million in payroll and payroll related expenses as we expanded our sales and marketing efforts for SculpSure, an increase of $14.6 million from expenses related to workshops, trade shows, advertising and other marketing efforts and an increase of $11.4 million in commission expense as a result of increased product sales. Our total sales and marketing expenses for the 2016 period increased as a percentage of total revenues to 35% as compared to the 2015 period, primarily due to the sales and marketing costs associated with SculpSure, which was launched in September 2015.
60
Table of Contents
Research and Development
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2016 | 2015 | |||||||||||||||
| Research and development (dollars in thousands) |
$ | 28,973 | $ | 22,343 | $ | 6,630 | 30 | % | ||||||||
| Research and development (as a percentage of total revenues) |
7 | % | 6 | % | ||||||||||||
Research and development expenses increased $6.6 million, or 30%, to $29.0 million for the 2016 period, as compared to $22.3 million for the 2015 period. The increase was due to increases in personnel costs of $3.9 million and professional services costs of $2.7 million related to research and development projects to expand our product offerings for new aesthetic indications and treatment areas. Our total research and development expenses as a percentage of total revenues increased in the 2016 period as compared to the 2015 period primarily due to the increased research and development costs in 2016 to expand our product offerings.
Amortization of Intangible Assets Acquired
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2016 | 2015 | |||||||||||||||
| Amortization of intangible assets acquired (dollars in thousands) |
$ | 2,741 | $ | 2,990 | $ | (249 | ) | (8 | %) | |||||||
| Amortization of intangible assets acquired (as a percentage of total revenues) |
1 | % | 1 | % | ||||||||||||
Amortization of intangible assets acquired decreased $0.2 million, or 8%, for the 2016 period as compared to the 2015 period. Amortization of intangible assets acquired as a percentage of total revenues remained consistent for the 2016 and 2015 periods.
General and Administrative
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2016 | 2015 | |||||||||||||||
| General and administrative (dollars in thousands) |
$ | 42,404 | $ | 30,374 | $ | 12,030 | 40 | % | ||||||||
| General and administrative (as a percentage of total revenues) |
10 | % | 9 | % | ||||||||||||
General and administrative expenses increased $12.0 million, or 40%, to $42.4 million for the 2016 period, as compared to $30.4 million for the 2015 period. The increase is due to increases in payroll, legal and administrative expenses of $10.6 million, which includes a $9.2 million charge in the fourth quarter of 2016 in connection with the ARcare settlement agreement and expenses associated with the ARcare matter, as well as an increase of $1.4 million in stock-based compensation expense from the performance-based stock unit awards during the 2016 period. Our total general and administrative expenses as a percentage of total revenues increased in the 2016 period as compared to the 2015 period primarily due to the costs incurred associated with the ARcare matter.
Interest Expense, net
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2016 | 2015 | |||||||||||||||
| Interest expense, net (dollars in thousands) |
$ | (1,563 | ) | $ | (1,683 | ) | $ | 120 | 7 | % | ||||||
61
Table of Contents
The decrease in interest expense, net was primarily due to an increase in interest income from investments resulting from increased cash invested in interest-bearing securities during the 2016 period as compared to the 2015 period.
Other Expense, net
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2016 | 2015 | |||||||||||||||
| Other expense, net (dollars in thousands) |
$ | (834 | ) | $ | (1,440 | ) | $ | 606 | 42 | % | ||||||
The change in other expense, net was primarily as a result of decreased net foreign currency remeasurement losses in the 2016 period as compared to the 2015 period.
Provision for Income Taxes
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2016 | 2015 | |||||||||||||||
| Provision for income taxes (dollars in thousands) |
$ | 10,742 | $ | 7,391 | $ | 3,351 | 45 | % | ||||||||
| Provision as a percentage of income before provision for income taxes |
43 | % | 32 | % | ||||||||||||
The provision for income taxes results from a combination of the activities of our U.S. entities and foreign subsidiaries. During the 2016 period, we recorded an income tax provision of $10.7 million, representing an effective tax rate of 43%. The difference between the U.S. federal statutory tax rate and the effective tax rate during the 2016 period was predominantly related to tax expense associated with non-deductible expenses (primarily related to disallowed officers’ compensation), partially offset by benefits associated with the jurisdictional mix of worldwide earnings, the federal research credit and the domestic manufacturing deduction. During the 2015 period, we recorded an income tax provision of $7.4 million, representing an effective tax rate of 32%. The difference between the U.S. federal statutory tax rate and the effective tax rate during the 2015 period was primarily attributable to tax benefits associated with federal and state research credits, the release of valuation allowance against certain foreign deferred tax assets and the domestic manufacturing deduction, partially offset by the impact of non-deductible expenses.
62
Table of Contents
Years Ended December 31, 2015 and 2014
The following table contains selected statements of operations data, which serves as the basis of the discussion of our results of operations for the years ended December 31, 2015 and 2014:
| Year Ended December 31, 2015 |
Year Ended December 31, 2014 |
Change 2014 to 2015 |
||||||||||||||||||||||
| Amount | As a % of Total Revenues |
Amount | As a % of Total Revenues |
$ Change | % Change | |||||||||||||||||||
| (Dollars in thousands) | ||||||||||||||||||||||||
| Product revenues |
$ | 276,085 | 81 | % | $ | 236,878 | 81 | % | $ | 39,207 | 17 | % | ||||||||||||
| Parts, accessories, service and royalty revenues |
63,377 | 19 | 55,491 | 19 | 7,886 | 14 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total revenues |
339,462 | 100 | 292,369 | 100 | 47,093 | 16 | ||||||||||||||||||
| Cost of revenues |
145,928 | 43 | 127,131 | 43 | 18,797 | 15 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Gross profit |
193,534 | 57 | 165,238 | 57 | 28,296 | 17 | ||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Sales and marketing |
111,506 | 33 | 88,564 | 30 | 22,942 | 26 | ||||||||||||||||||
| Research and development |
22,343 | 6 | 22,033 | 8 | 310 | 1 | ||||||||||||||||||
| Amortization of intangible assets acquired |
2,990 | 1 | 2,961 | 1 | 29 | 1 | ||||||||||||||||||
| General and administrative |
30,374 | 9 | 30,420 | 11 | (46 | ) | — | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
167,213 | 49 | 143,978 | 50 | 23,235 | 16 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Income from operations |
26,321 | 8 | 21,260 | 7 | 5,061 | 24 | ||||||||||||||||||
| Interest expense, net |
(1,683 | ) | (1 | ) | (1,446 | ) | — | (237 | ) | (16 | ) | |||||||||||||
| Other expense, net |
(1,440 | ) | — | (1,476 | ) | (1 | ) | 36 | 2 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Income before provision (benefit) for income taxes |
23,198 | 7 | 18,338 | 6 | 4,860 | 27 | ||||||||||||||||||
| Provision (benefit) for income taxes |
7,391 | 2 | (13,000 | ) | (5 | ) | 20,391 | 157 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income |
$ | 15,807 | 5 | % | $ | 31,338 | 11 | % | $ | (15,531 | ) | (50 | )% | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Revenues
Total revenues for the year ended December 31, 2015 increased by $47.1 million, or 16%, to $339.5 million, as compared to the year ended December 31, 2014 revenues of $292.4 million (in thousands, except for percentages):
| Year
Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2015 | 2014 | |||||||||||||||
| Product sales in North America |
$ | 168,650 | $ | 123,403 | $ | 45,247 | 37 | % | ||||||||
| Product sales outside North America |
107,435 | 113,475 | (6,040 | ) | (5 | ) | ||||||||||
| Global parts, accessories, service and royalty sales |
63,377 | 55,491 | 7,886 | 14 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Revenues |
$ | 339,462 | $ | 292,369 | $ | 47,093 | 16 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The change in total revenues was attributable to a number of factors:
| • | Revenues from the sale of products in North America increased by approximately $45.2 million, or 37%, from the 2014 period, primarily due to a full year of MonaLisa Touch sales, strong sales of PicoSure and Icon, and the launch of SculpSure in the 2015 period. Average selling prices for our products remained consistent with the 2014 period. |
63
Table of Contents
| • | Revenues from the sale of products outside of North America decreased by approximately $6.0 million, or 5%, from the 2014 period, primarily due to unfavorable exchange rates affecting our European subsidiaries and a lower number of units sold by our European and Middle East distributors due to economic instability in these regions. |
| • | Revenues from the sale of parts, accessories, services and royalties increased by approximately $7.9 million, or 14%, from the 2014 period, primarily due to a full year of accessories revenues attributable to products acquired from Ellman, sales of the PicoSure FOCUS Lens Array and the inclusion of SculpSure accessories sales in the 2015 period. |
Cost of Revenues
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2015 | 2014 | |||||||||||||||
| Cost of revenues (dollars in thousands) |
$ | 145,928 | $ | 127,131 | $ | 18,797 | 15 | % | ||||||||
| Cost of revenues (as a percentage of total revenues) |
43 | % | 43 | % | ||||||||||||
Total cost of revenues increased $18.8 million, or 15%, to $145.9 million for the 2015 period, as compared to $127.1 million for the 2014 period. The increase was primarily associated with our 16% increase in total revenues in the 2015 period as compared to the 2014 period. Total cost of revenues as a percentage of total revenues remained consistent for the 2015 and 2014 periods.
Sales and Marketing
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2015 | 2014 | |||||||||||||||
| Sales and marketing (dollars in thousands) |
$ | 111,506 | $ | 88,564 | $ | 22,942 | 26 | % | ||||||||
| Sales and marketing (as a percentage of total revenues) |
33 | % | 30 | % | ||||||||||||
Sales and marketing expenses increased $22.9 million, or 26%, to $111.5 million for the 2015 period as compared to $88.6 million for the 2014 period. The increase was primarily due to an increase in payroll and payroll related expenses of $10.3 million as we expanded our direct sales force related to the launch of new products, an increase in commission expense of $5.0 million from increased revenues, marketing costs of $2.3 million in the 2015 period primarily associated with the launch of SculpSure and an increase of $1.2 million from a full year of Ellman’s sales and marketing expenses. Our total sales and marketing expenses for the 2015 period increased as a percentage of total revenues to 33% as compared to the 2014 period, primarily due to the additional sales and marketing costs associated with the launch of SculpSure.
Research and Development
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2015 | 2014 | |||||||||||||||
| Research and development (dollars in thousands) |
$ | 22,343 | $ | 22,033 | $ | 310 | 1 | % | ||||||||
| Research and development (as a percentage of total revenues) |
6 | % | 8 | % | ||||||||||||
Research and development expenses increased $0.3 million, or 1%, to $22.3 million for the 2015 period compared to $22.0 million for the 2014 period. The increase was primarily due to research and development costs associated with SculpSure in the 2015 period, as well as the inclusion in the 2015 period of a full year of Ellman’s research and development expenses. Our total research and development expenses for the 2015 period decreased as a percentage of total revenues as compared to the 2014 period due to improved operating leverage.
64
Table of Contents
Amortization of Intangible Assets Acquired
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2015 | 2014 | |||||||||||||||
| Amortization of intangible assets acquired (dollars in thousands) |
$ | 2,990 | $ | 2,961 | $ | 29 | 1 | % | ||||||||
| Amortization of intangible assets acquired (as a percentage of total revenues) |
1 | % | 1 | % | ||||||||||||
Amortization of intangible assets acquired increased $29,000, or 1%, for the 2015 period as compared to the 2014 period. Amortization of intangible assets acquired as a percentage of total revenues remained consistent for the 2015 and 2014 periods.
General and Administrative
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2015 | 2014 | |||||||||||||||
| General and administrative (dollars in thousands) |
$ | 30,374 | $ | 30,420 | $ | (46 | ) | — | % | |||||||
| General and administrative (as a percentage of total revenues) |
9 | % | 11 | % | ||||||||||||
General and administrative expenses decreased $46,000 for the 2015 period as compared the 2014 period. The decrease is primarily due to $3.6 million less in costs associated with acquisitions, partially offset by increased headcount and administrative expenses during the 2015 period as compared to the 2014 period. Excluding acquisition costs, our total general and administrative expenses as a percentage of total revenues remained consistent.
Interest Expense, net
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2015 | 2014 | |||||||||||||||
| Interest expense, net (dollars in thousands) |
$ | (1,683 | ) | $ | (1,446 | ) | $ | (237 | ) | (16 | )% | |||||
The increase in interest expense, net was due to an increase in interest expense associated with the capital lease of our U.S. operating facility in Westford, Massachusetts, as well as a full year of interest charges included in the 2015 period on the license transfer agreement acquired in the 2014 Ellman asset acquisition.
Other Expense, net
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2015 | 2014 | |||||||||||||||
| Other expense, net (dollars in thousands) |
$ | (1,440 | ) | $ | (1,476 | ) | $ | 36 | 2 | % | ||||||
Other expense, net decreased $36,000, or 2%, for the 2015 period as compared to the 2014 period. Other expense, net is comprised primarily of foreign currency remeasurement losses incurred by our foreign subsidiaries primarily due to the strengthening of the U.S. dollar against the euro, British pound, Japanese yen, Australian dollar and Chinese yuan.
65
Table of Contents
Provision (Benefit) for Income Taxes
| Year Ended December 31, |
$ Change | % Change | ||||||||||||||
| 2015 | 2014 | |||||||||||||||
| Provision (benefit) for income taxes (dollars in thousands) |
$ | 7,391 | $ | (13,000 | ) | $ | 20,391 | 157 | % | |||||||
| Provision (benefit) as a percentage of income before provision (benefit) for income taxes |
32 | % | (71 | )% | ||||||||||||
The provision for income taxes results from a combination of the activities of our U.S. entities and foreign subsidiaries. In 2015, we recorded an income tax provision of $7.4 million representing an effective tax rate of 32%. In 2014, we recorded an income tax benefit of $13.0 million representing an effective tax rate of negative 71%. The year-over-year increase in our tax provision is primarily attributable to the release of substantially all of the valuation allowance maintained against our U.S. net deferred tax asset in 2014, resulting in a tax benefit of $19.0 million. In 2015 we recorded a tax benefit of $0.7 million for the release of a valuation allowance previously maintained against the net deferred tax assets of Palomar Japan K.K. and Palomar Germany. In 2014, we recorded a tax benefit of $0.9 million related to the release of valuation allowances against the net deferred tax assets of Cynosure Japan, Palomar Australia and Palomar Spain. The effective tax rate of 32% differs from the 35% federal statutory rate primarily due to a 3% decrease attributable to the valuation allowance release.
Liquidity and Capital Resources
We require cash to pay our operating expenses, invest in research and development projects, make capital expenditures, fund acquisitions and pay our long-term liabilities. We have funded our operations through cash generated from our operations and proceeds from public offerings of our Class A common stock.
Our cash, cash equivalents and marketable securities balance increased by $55.0 million as of December 31, 2016 from December 31, 2015. At December 31, 2016, our cash, cash equivalents and short and long-term marketable securities were $237.8 million. Our cash and cash equivalents of $153.6 million are highly liquid investments with maturities of 90 days or less at date of purchase and consist of cash in operating accounts and investments in money market funds. Our short-term marketable securities of $46.5 million consist of investments in various state and municipal governments, and U.S. government agencies and treasuries, all of which mature by December 15, 2017. Our long-term marketable securities of $37.6 million consist of investments in various state and municipal governments, and U.S. government agencies and treasuries, all of which mature by November 16, 2018.
At December 31, 2016, $17.3 million of cash and cash equivalents was held by our foreign subsidiaries, which we expect will be used primarily to fund working capital requirements, service existing obligations and invest in efforts to secure future business. We have provided U.S. deferred income taxes on approximately $0.6 million of unremitted earnings at our foreign subsidiaries at December 31, 2016, which are not deemed to be indefinitely reinvested, as they are not necessary to fund existing business needs of our subsidiaries. There has not been a material change in the amount of unremitted earnings on which deferred taxes have been provided as of December 31, 2016.
Our future capital requirements depend on a number of factors, including the rate of market acceptance of our current and future products, the resources we devote to developing and supporting our products and continued progress of our research and development of new products. During the years ended December 31, 2016 and 2015, we purchased $13.5 million and $10.8 million, respectively, of property and equipment. During the years ended December 31, 2016 and 2015, we transferred $6.5 million and $6.6 million, respectively, of demonstration equipment to fixed assets. We expect that our capital expenditures during the next 12 months will decrease compared to the 2016 period.
66
Table of Contents
In February 2016, we announced that our board of directors has authorized the repurchase of up to $35 million of our Class A common stock, from time to time, on the open market or in privately negotiated transactions under a new stock repurchase program. This program will terminate on February 1, 2018. During the year ended December 31, 2016, we did not repurchase any shares of our common stock under this program.
We believe that our current cash, cash equivalents and short and long-term marketable securities, as well as cash generated from operations, will be sufficient to meet our anticipated cash needs for working capital and capital expenditures for at least the next 12 months.
Under the terms and prior to the termination of the Merger Agreement, we must receive Hologic’s approval of specified expenditures above specified dollar amounts, as set forth in the Merger Agreement.
Cash Flows
Net cash provided by operating activities was $45.0 million for the year ended December 31, 2016, and resulted primarily from net income of $14.2 million and $30.8 million in depreciation and amortization and stock-based compensation expense. Net changes in working capital items increased cash from operating activities by approximately $0.5 million, primarily related to an increase in accrued expenses and a decrease in prepaid expenses, partially offset by decreases in deferred revenue and accounts payable. Net cash used in investing activities was $26.2 million for the year ended December 31, 2016, which consisted primarily of $64.6 million in purchases of marketable securities and $13.5 million in purchases of property and equipment, offset by $51.9 million in proceeds from the sales and maturities of marketable securities. Net cash provided by financing activities during the year ended December 31, 2016 was $26.3 million, primarily related to the proceeds from stock option exercises.
Net cash provided by operating activities was $37.7 million for the year ended December 31, 2015, and resulted primarily from net income of $15.8 million and $26.9 million in depreciation and amortization and stock-based compensation expense. Net changes in working capital items decreased cash from operating activities by approximately $4.1 million, primarily related to increases in inventory and prepaid expenses, partially offset by increases in deferred revenue, accounts payable and accrued expenses. Net cash used in investing activities was $29.1 million for the year ended December 31, 2015, which consisted primarily of $63.0 million in purchases of marketable securities and $10.8 million in purchases of property and equipment, partially offset by $44.8 million in proceeds from the sales and maturities of marketable securities. Net cash provided by financing activities during the year ended December 31, 2015 was $24.2 million, primarily related to the proceeds from stock option exercises.
Net cash provided by operating activities was $42.1 million for the year ended December 31, 2014, and resulted primarily from net income of $31.3 million and $24.9 million in depreciation and amortization and stock-based compensation expense offset by $15.8 million in changes in deferred income taxes. Net changes in working capital items decreased cash from operating activities by approximately $0.5 million, primarily related to increases in inventory and accounts receivable, partially offset by increases to accounts payable and accrued expenses. Net cash used in investing activities was $53.7 million for the year ended December 31, 2014, which consisted primarily of $56.9 million in purchases of marketable securities, $15.8 million in additions of fixed assets and $13.2 million in cash paid for the Ellman asset acquisition, partially offset by $32.4 million in proceeds from the sales and maturities of marketable securities. Net cash used in financing activities during the year ended December 31, 2014 was $7.0 million, primarily related to $15.6 million in repurchases of common stock offset by $8.2 million in proceeds from stock option exercises.
67
Table of Contents
Contractual Obligations
Our significant outstanding contractual obligations relate to our capital leases from our facilities leases, including the buildings portion of our U.S. operating facility, and equipment financings. Our leases are non-cancellable and typically contain renewal options. Certain leases contain rent escalation clauses for which we recognize the expense on a straight-line basis. We have summarized in the table below our fixed contractual cash obligations as of December 31, 2016.
| Total | Less Than One Year |
One to Three Years |
Three to Five Years |
More than Five Years |
||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Capital lease obligations, including interest |
$ | 34,091 | $ | 2,960 | $ | 5,685 | $ | 5,714 | $ | 19,732 | ||||||||||
| Operating leases |
10,156 | 2,220 | 3,627 | 1,379 | 2,930 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations |
$ | 44,247 | $ | 5,180 | $ | 9,312 | $ | 7,093 | $ | 22,662 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
In the event we terminate the Merger Agreement, we have agreed to pay Hologic a termination fee of $57.7 million under specified circumstances, including (i) a termination by us to accept a superior proposal (as defined in the Merger Agreement), (ii) a termination by Hologic if our board of directors changes its recommendation in respect of the Offer or (iii) if we receive an alternative acquisition proposal (as defined in the Merger Agreement) prior to termination of the Merger Agreement and the Merger Agreement is terminated (a) by either party because the outside date (as defined in the Merger Agreement) occurs or (b) by Hologic upon a breach by us of our covenants in a manner that would lead to the failure of a closing condition and, within 12 months after such termination, we enter into a definitive agreement to consummate, or consummate, any alternative acquisition proposal.
Purchase Commitments
We have entered into two distribution agreements with a contract manufacturer that expire in October 2019 and November 2021. Each agreement automatically renews for additional one-year terms unless either party provides notice of termination at least six months prior to the expiration of the initial term or any subsequent renewal term. The distribution agreements have annual minimum purchase obligations and may be terminated by the contract manufacturer if we do not meet the annual minimum purchase obligations. The future annual minimum purchase obligations can be reduced if we exceed the purchase limits in a given year as stated in the distribution agreement.
Off Balance Sheet Arrangements
Since inception, we have not engaged in any off balance sheet financing activities.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations set forth above are based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles, or U.S. GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. On an ongoing basis, we evaluate our estimates and judgments, including those described below. We base our estimates on historical experience and on various assumptions that we believe to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities, and the reported amounts of revenues and expenses, that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies require significant judgment and estimates by us in the preparation of our financial statements.
68
Table of Contents
Revenue Recognition and Deferred Revenue
We generate revenues primarily from our product sales. We also generate revenues from sales of parts and accessories, from services, including product warranty revenues, and from royalty payments received from our licensees. Our business model generally does not involve separate contracts entered into at or near the same time; nor does our business model involve incremental future discounts offered to the same customer. We do not offer a right of cancellation, termination, refund or return. During the year ended December 31, 2016, we derived approximately 83% of our revenues from product sales and 17% of our revenues from the sales of parts, accessories, service and royalties. During the year ended December 31, 2015, we derived approximately 81% of our revenues from sales of our products and 19% of our revenues from parts, accessories, service and royalty revenues.
We recognize revenue from sales of our products, parts and accessories in accordance with the ASC Revenue Recognition Topic 605-10-S99. We recognize revenue from sales of our products, parts and accessories when title and risk of ownership has been transferred, which is upon shipment, provided there are no uncertainties regarding customer acceptance, and the following factors are present:
| • | persuasive evidence of an arrangement; |
| • | the fee is fixed or determinable; and |
| • | collectability of the related receivable is reasonably assured. |
Revenues from the sale of services and extended warranty contracts are deferred and recognized on a straight-line basis over the contract period as services are provided.
We recognize royalty revenues when we can reliably estimate such amounts and collectability is reasonably assured. As such, we recognize royalty revenues in the quarter reported to us by our licensees, or one quarter following the quarter in which sales by our licensees occurred. Royalty revenues also include amounts due from settlements with licensees for back-owed royalties from prior periods. These settlement amounts are considered revenue, when collectability is reasonably assured, because they constitute our ongoing major or central operations.
Multiple-element arrangements are evaluated in accordance with ASC 605-25, Multiple-Element Arrangements. We determined that our multiple-element arrangements are generally comprised of the following elements that are recognized as separate units of accounting: products, accessories, extended warranties and installation and training services. Installation and training services are not essential to the functionality of the aesthetic treatment system. We allocate revenue among the elements based upon their VSOE. If VSOE does not exist, we may use TPE to determine the relative selling price of each element. If neither VSOE nor TPE exists, we may use BESP of each element to determine the relative selling price. Presently there are no elements for which we are utilizing TPE or BESP.
VSOE is based on the relative sales price based on separate stand-alone sales of our elements. Revenues associated with products and accessories are recognized when shipped. Accessories are predominantly shipped with the aesthetic treatment system when sold with the product. Revenues associated with extended warranties are deferred and recognized ratably over the extended warranty period, which is generally one year. The relative sales prices of extended warranties are based on the actual pricing of service contracts purchased on a standalone basis at a later time, normally at the end of the standard warranty period. Due to the type of revenue arrangements and structure of our contracts, we refer to guidance on separately priced extended warranties within ASC 605-20, Services. Under ASC 605-20, we defer the selling price of the separately priced extended warranty at the inception of the arrangement, and allocate the remainder of the arrangement consideration among the remaining elements on their VSOE of fair value in accordance with ASC 605-25.
69
Table of Contents
Cost of Revenues
Our cost of revenues consists primarily of material, labor and manufacturing overhead expenses and includes the cost of components and subassemblies supplied by third-party suppliers. Cost of revenues also includes royalties incurred on certain products sold by us and our licensees, costs incurred in connection with our efforts to litigate or settle additional third-party license agreements, amortization expense related to developed technology and patents intangible assets, service and warranty expenses, as well as salaries and personnel-related expenses, including stock-based compensation, for our operations management team, purchasing and quality control.
Concentration of Credit Risk
The Financial Accounting Standards Board, or FASB, requires disclosure of any significant off-balance-sheet and credit risk concentrations. Financial instruments that subject us to credit risk consist primarily of cash and cash equivalents, short and long-term marketable securities and accounts receivable. We place cash and cash equivalents and short and long-term marketable securities in established financial institutions. We have no significant off-balance-sheet risk or concentration of credit risk, such as foreign exchange contracts, options contracts, or other foreign hedging arrangements. Our accounts receivable balance, net of allowance for doubtful accounts, was $42.1 million as of December 31, 2016, compared to $42.0 million as of December 31, 2015. The allowance for doubtful accounts was $3.3 million as of December 31, 2016, compared to $3.2 million as of December 31, 2015. We maintain an allowance for doubtful accounts based upon the aging of our receivable balances, known collectability issues and our historical experience with losses. We work to mitigate bad debt exposure through our credit evaluation policies, reasonably short payment terms and geographical dispersion of sales. Our revenues include export sales to foreign companies located principally in Europe, the Asia/Pacific region and the Middle East. We obtain letters of credit for foreign sales that we consider to be at risk.
Inventories and Allowance for Excess and Obsolescence
We state all inventories at the lower of cost or market value, determined on a first-in, first-out method. We monitor standard costs on a monthly basis and update them annually and as necessary to reflect changes in raw material costs and labor and overhead rates. Our inventory balance was $76.0 million as of December 31, 2016, compared to $79.8 million as of December 31, 2015. The decrease in inventory relates to improved inventory management in 2016.
We provide inventory allowances when conditions indicate that the selling price could be less than cost due to physical deterioration, usage, obsolescence, reductions in estimated future demand and reductions in selling prices. We balance the need to maintain strategic inventory levels with the risk of obsolescence due to changing technology and customer demand levels. Unfavorable changes in market conditions may result in a need for additional inventory reserves that could adversely impact our gross margins. Conversely, favorable changes in demand could result in higher gross margins when we sell products. Our inventory allowance was $5.4 million as of December 31, 2016, compared to $5.9 million as of December 31, 2015.
Intangible Assets
We capitalize and include in intangible assets the costs of developed technology and patents, customer relationships, trade names and business licenses acquired in a business combination or asset acquisition. Intangible assets are recorded at fair value and stated net of accumulated amortization and impairments. We amortize our intangible assets that have finite lives using either the straight-line or accelerated method, based on the useful life of the asset over which it is expected to be consumed utilizing expected undiscounted future cash flows. Amortization is recorded over the estimated useful lives ranging from five to 23 years. We evaluate the realizability of our definite lived intangible assets whenever events or changes in circumstances or business conditions indicate that the carrying value of these assets may not be recoverable based on expectations of future undiscounted cash flows for each asset group. If the carrying value of an asset or asset group exceeds its
70
Table of Contents
undiscounted cash flows, we estimate the fair value of the assets, generally utilizing a discounted cash flow analysis based on the present value of estimated future cash flows to be generated by the assets using a risk-adjusted discount rate. To estimate the fair value of the assets, we use market participant assumptions pursuant to ASC 820, Fair Value Measurements. If the estimate of an intangible asset’s remaining useful life is changed, we will amortize the remaining carrying value of the intangible asset prospectively over the revised useful life.
Goodwill
Goodwill represents the excess of the purchase price over the fair value of assets acquired and liabilities assumed in a business combination. We do not amortize our goodwill, but instead test for impairment at least annually and more frequently whenever events or changes in circumstances indicate that the fair value of the asset may be less than its carrying value of the asset. Our annual test for impairment occurs on the first day of our fourth quarter.
We have adopted ASU 2011-08 Intangibles—Goodwill and Other, an amendment to ASC 350, which updates how an entity evaluates its goodwill for impairment. The guidance provides entities an option to perform a “qualitative” assessment to determine whether further impairment testing is necessary. If further testing is required, the test for impairment continues with the two step process. The first step compares the carrying amount of the reporting unit to its estimated fair value (Step 1). To the extent that the carrying value of the reporting unit exceeds its estimated fair value, a second step is performed, wherein the reporting unit’s carrying value is compared to the implied fair value (Step 2). To the extent that the carrying value exceeds the implied fair value, impairment exists and must be recognized.
We have concluded that Cynosure, Inc. represents one reporting unit for goodwill impairment testing and we have performed a qualitative assessment on that reporting unit. As a result of our assessment, we determined that goodwill is not impaired as of December 31, 2016.
Product Warranty Costs and Provisions
We typically provide a one-year system and labor warranty on end-user sales of our aesthetic treatment systems. Distributor sales of our aesthetic treatment systems generally include a warranty on systems only. These one-year warranty obligations are limited to the repair or replacement of parts and materials which prove to be defective, and do not contain any service performance obligations. We estimate and provide for future costs for initial product warranties at the time revenue is recognized. We base product warranty costs on related material costs, technical support labor costs and overhead. We provide for the estimated cost of product warranties by considering historical material, labor and overhead expenses and applying the experience rates to the outstanding warranty period for products sold. As we sell new products to our customers, we must exercise considerable judgment in estimating the expected failure rates and warranty costs. If actual product failure rates, material usage, service delivery costs or overhead costs differ from our estimates, we would be required to revise our estimated warranty liability.
Fair Value of Financial Instruments
ASC 820, Fair Value Measurements Topic, defines fair value, establishes a framework for measuring fair value under U.S. GAAP and enhances disclosures about fair value measurements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes the following fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:
| • | Level 1—Quoted prices in active markets for identical assets or liabilities. |
71
Table of Contents
| • | Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable markets data for substantially the full term of the assets or liabilities. |
| • | Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
Capital Lease Obligations
In November 2013, we entered into a Fifth Amendment to the lease dated January 31, 2005, or the Lease, related to our headquarters located at 5 Carlisle Road, Westford, Massachusetts, pursuant to which the term of the Lease was extended to May 2028 (with two five-year extension options) and the leased premises were expanded to include additional space at the current location as well as space in the adjacent building, 3 Carlisle Road, Westford, Massachusetts. In April and June 2016, we executed additional amendments to the Lease, and these amendments became effective in October 2016. These amendments, among other things, extended the term of the Lease for an additional year, provided additional rental space and require additional base rent of $5.7 million over the remaining life of the Lease.
ASC 840, Leases, establishes the framework for accounting for the Lease Amendment. In accordance with ASC 840, we are accounting for the land portion of the leased premises as an operating lease. The buildings at 5 Carlisle Road and 3 Carlisle Road have met the criteria for capital lease accounting under ASC 840-10-25-1, and thus we are accounting for the buildings portion of the leased premises as capital leases and incurring interest charges accordingly. The expansion of the leased premises, as well as improvements to the leased premises, are recorded on the balance sheet as leasehold improvements within property, plant and equipment. Assets under capital leases and leasehold improvements related to the Lease Amendment are amortized using the straight-line method over the respective lease term.
Stock-Based Compensation
We follow the fair value recognition provisions of ASC 718, Stock Compensation Topic. This guidance requires share-based payments to employees, including grants of employee stock options and restricted stock units, or RSUs, to be recognized in the statement of operations based on their fair values at the date of grant. The fair value of performance-based stock units is determined based on the fair market value of our common stock on the vest dates. ASC 718 requires companies to utilize an estimated forfeiture rate when calculating the expense for the period. Accordingly, we review our actual forfeiture rates periodically and align our stock compensation expense with the share-based payments that are vesting.
We use the Black-Scholes option pricing model to estimate the fair value of stock options. This option-pricing model requires the input of various subjective assumptions, including the option’s expected life and the price volatility of the underlying stock. Our estimated expected stock price volatility is based on our own historic volatility. We believe this is more reflective and a better indicator of the expected future volatility, than using an average of a comparable market index or of a comparable company in the same industry. We did not grant any options during the year ended December 31, 2016. Our expected term of options granted during the years ended December 31, 2015 and 2014 represents the weighted average period of time that options granted are expected to be outstanding giving consideration to vesting schedules and our historical exercise patterns. The risk-free rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The dividend yield of zero is based on the fact that we have never paid cash dividends and have no present intention to pay cash dividends.
We account for transactions in which services are received from non-employees in exchange for equity instruments based on the fair value of such services received or of the equity instruments issued, whichever is more reliably measured, in accordance with ASC 718 and the Equity Topic, ASC 505.
72
Table of Contents
Income Taxes
We provide for income taxes in accordance with ASC 740. ASC 740 recognizes tax assets and liabilities for the cumulative effect of all temporary differences between the financial statement carrying amounts and the tax basis of assets and liabilities, and are measured using the enacted tax rates that will be in effect when these differences are expected to reverse. Valuation allowances are provided if, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. We consider several sources of taxable income in making our valuation allowance assessments including taxable income in carryback years, future reversals of existing taxable temporary differences, tax planning strategies and forecasted future income.
We evaluate at the end of each reporting period whether some or all of the undistributed earnings of our foreign subsidiaries are permanently reinvested. We recognize deferred income tax liabilities to the extent that management asserts that undistributed earnings of our foreign subsidiaries are not permanently reinvested or will not be permanently reinvested in the future. At December 31, 2016, management has determined that a portion of our undistributed earnings are not indefinitely reinvested and recorded a $0.1 million deferred tax liability. Changes in facts and circumstances that may impact our indefinite reinvestment assertion include a change in the estimated capital needs of our foreign subsidiaries or changes in our corporate liquidity requirements. Such changes in the future could result in our management determining that some or all of such undistributed earnings are no longer permanently reinvested.
We account for uncertain tax positions following the provisions of ASC 740. ASC 740 clarifies the accounting for income taxes, by prescribing a minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. ASC 740 also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Recent Accounting Pronouncements
In May 2014, the FASB issued guidance codified in ASC 606, Revenue Recognition—Revenue from Contracts with Customers, which amends the guidance in ASC 605, Revenue Recognition. This new revenue standard creates a single source of revenue guidance for all companies in all industries and is more principles-based than the current revenue guidance. The new guidance must be adopted using either a full retrospective approach for all periods presented in the period of adoption or a modified retrospective approach. ASC 606 will be effective for public entities for interim and annual reporting periods beginning after December 15, 2017. We have prepared an initial assessment of the potential effects of ASC 606. Based on the initial assessment, we anticipate that the new standard will not impact the recognition of product sales given their point of sale nature. We are still in the process of evaluating our arrangements with distributors as well as those related to service contracts and extended warranties under ASC 606 and anticipate the impact will not be significant, however, final conclusions will not be determined until we complete our analysis. We expect to adopt ASC 606 using the modified retrospective method. Should the adoption of ASC 606 under the modified retrospective method result in the deferral of revenue previously recognized, or the recognition of revenue previously deferred under ASC 605 beyond January 1, 2018, we would record a cumulative catch-up adjustment at January 1, 2018.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements—Going Concern, which requires management of public and private companies to evaluate whether there is substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. Our evaluation should be based on relevant conditions and events that are known and reasonably knowable at the date that the financial statements are issued. If conditions or events raise substantial doubt about an entity’s ability to continue as a going concern, and substantial doubt is not alleviated after consideration of management’s plans, an entity should include a statement in the footnotes indicating that there is substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. The new standard is effective for annual periods ending after December 15, 2016, and for
73
Table of Contents
annual periods and interim periods thereafter. We have adopted ASU 2014-15 and have assessed our ability to continue as a going concern. As of December 31, 2016, we have concluded that substantial doubt about our ability to continue as a going concern does not exist.
In July 2015, the FASB issued ASU 2015-11, Simplifying the Measurement of Inventory. ASU 2015-11 simplifies the subsequent measurement of inventory by replacing the lower of cost or market test with a lower of cost and net realizable value test. The guidance applies only to inventories for which cost is determined by methods other than last-in first-out and the retail inventory method. The guidance is effective for public business entities for fiscal years beginning after December 15, 2016. We are currently evaluating the impact of the provisions of ASU 2015-11.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes the existing guidance for lease accounting, Leases (Topic 840). ASU 2016-02 requires lessees to recognize leases on their balance sheets, and leaves lessor accounting largely unchanged. The amendments in this ASU are effective for fiscal years beginning after December 15, 2018 and interim periods within those fiscal years. Early application is permitted for all entities. ASU 2016-02 requires a modified retrospective approach for all leases existing at, or entered into after, the date of initial application, with an option to elect to use certain transition relief. We are currently evaluating the impact of this new standard on our consolidated financial statements.
In March 2016, the FASB issued ASU 2016-09, Compensation—Stock Compensation (Topic 718): Improvement to Employee Share-Based Payment Accounting. The new standard contains several amendments that will simplify the accounting for employee share-based payment transactions, including the accounting for income taxes, forfeitures, statutory tax withholding requirements, classification of awards as either equity or liabilities, and classification on the statement of cash flows. The changes in the new standard eliminate the accounting for excess tax benefits to be recognized in additional paid-in capital and tax deficiencies recognized either in the income tax provision or in addition paid-in capital. The new standard is effective for public business entities for fiscal years beginning after December 15, 2016 with early adoption permitted. ASU 2016-09 requires a modified retrospective approach, with a cumulative-effect adjustment to opening retained earnings for any outstanding liability awards that qualify for the classification. We are currently evaluating the impact of this new standard on our consolidated financial statements.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
The following discussion about our market risk disclosures involves forward-looking statements. Actual results could differ materially from those projected in the forward-looking statements. We are exposed to market risk related to changes in interest rates and foreign currency exchange rates. We do not use derivative financial instruments.
Interest Rate Sensitivity. We maintain an investment portfolio consisting mainly of money market funds, state and municipal government obligations and U.S. government agencies and treasuries. The securities, other than money market funds and certain U.S. government agencies and treasuries, are classified as available-for-sale and consequently are recorded on the balance sheet at fair value with unrealized gains and losses reported as a separate component of accumulated other comprehensive loss. All investments mature by November 16, 2018. These available-for-sale securities are subject to interest rate risk and will fall in value if market interest rates increase, which could result in a realized loss if we are forced to sell an investment before its scheduled maturity. We currently have the ability and intent to hold our fixed income investments until maturity. We do not utilize derivative financial instruments to manage our interest rate risks.
74
Table of Contents
The following table provides information about our investment portfolio in available-for-sale debt securities. For investment securities, the table presents principal cash flows (in thousands) and weighted average interest rates by expected maturity dates.
| December 31, 2016 | Future Maturities in 2017 |
Future Maturities in 2018 |
||||||||||
| Investments (at fair value) |
$ | 84,158 | $ | 46,530 | $ | 37,628 | ||||||
| Weighted average interest rate |
0.69 | % | 0.58 | % | 0.82 | % | ||||||
Foreign Currency Exchange. A portion of our operations is conducted through operations in countries other than the United States. Revenues from our international operations that were recorded in U.S. dollars represented approximately 39% of our total international revenues during the year ended December 31, 2016. Substantially all of the remaining 61% were sales in euros, British pounds, Moroccan dirham, Japanese yen, Chinese yuan, South Korean won and Australian dollars. Since we conduct our business in U.S. dollars, our main exposure, if any, results from changes in the exchange rate between these currencies and the U.S. dollar. Our functional currency is the U.S. dollar. Our policy is to reduce exposure to exchange rate fluctuations by having most of our assets and liabilities, as well as most of our revenues and expenditures, in U.S. dollars, or U.S. dollar linked. We have not historically engaged in hedging activities relating to our non-U.S. dollar operations. We sell inventory to our subsidiaries in U.S. dollars. These amounts are recorded at our local subsidiaries in local currency rates in effect on the transaction date. Therefore, we may be exposed to exchange rate fluctuations that occur while the debt is outstanding which we recognize as unrealized gains and losses in our statements of operations. Upon settlement of these debts, we may record realized foreign exchange gains and losses in our statements of operations. We may incur negative foreign currency conversion charges as a result of changes in currency exchange rates.
| Item 8. | Financial Statements and Supplementary Data |
All financial statements and schedules required to be filed hereunder are included beginning on page F-1 and are incorporated in this Annual Report by reference.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer (our principal executive and principal financial officers, respectively), evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2016. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2016, our chief executive officer and chief financial officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
75
Table of Contents
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the fourth quarter of the year ended December 31, 2016 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management’s Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Internal control over financial reporting includes those policies and procedures that: 1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; 2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and 3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, a system of internal control over financial reporting can provide only reasonable assurance and may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2016. In making its assessment, management used the criteria set forth in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations (COSO) of the Treadway Commission (2013 framework). A “material weakness” is a control deficiency (within the meaning of Public Company Accounting Oversight Board Auditing Standard No. 5), or combination of control deficiencies, that result in there being more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis by employees in the normal course of their assigned functions. Based on management’s assessment, management determined that we maintained effective internal control over financial reporting as of December 31, 2016 based on the COSO criteria.
Our internal control over financial reporting as of December 31, 2016 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in its report below.
76
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
Cynosure, Inc.:
We have audited Cynosure, Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Cynosure Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on Cynosure, Inc.’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Cynosure, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Cynosure, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2016 and our report dated February 28, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
February 28, 2017
77
Table of Contents
| Item 9B. | Other Information |
None.
78
Table of Contents
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by this item with respect to our directors and executive officers will be contained in our 2017 Proxy Statement under the captions “PROPOSAL 1—ELECTION OF TWO CLASS III DIRECTORS” and “EXECUTIVE COMPENSATION” and is incorporated in this Annual Report by reference.
The information required by this item with respect to Section 16(a) beneficial ownership reporting compliance will be contained in our 2017 Proxy Statement under the caption “SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE” and is incorporated in this Annual Report by reference.
The information required by this item with respect to corporate governance matters will be contained in our 2017 Proxy Statement under the caption “CORPORATE GOVERNANCE” and is incorporated in this Annual Report by reference.
Code of Ethics
We have adopted a code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Copies of our code of conduct are available without charge upon written request directed to Corporate Secretary, Cynosure, Inc., 5 Carlisle Road, Westford, Massachusetts 01886. We have posted a current copy of the code on the “Corporate Governance” section of the “Investors” page of our website, www.cynosure.com. In addition, we intend to post on our website all disclosures that are required by law or NASDAQ listing requirements concerning any amendments to, or waivers from, any provision of the code.
| Item 11. | Executive Compensation |
The information required by this item will be contained in our 2017 Proxy Statement under the captions “DIRECTOR COMPENSATION,” “COMPENSATION DISCUSSION AND ANALYSIS” and “EXECUTIVE COMPENSATION” and is incorporated in this Annual Report by reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item with regard to security ownership of certain beneficial owners and management will be contained in our 2017 Proxy Statement under the caption “SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT” and is incorporated in this Annual Report by reference.
The information required by this item with regard to securities authorized for issuance under equity compensation plans will be contained in our 2017 Proxy Statement under the caption “EXECUTIVE COMPENSATION—Securities Authorized for Issuance under our Equity Compensation Plans” and is incorporated in this Annual Report by reference.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item will be contained in our 2017 Proxy Statement under the captions “RELATED-PERSON TRANSACTIONS” and “CORPORATE GOVERNANCE” and is incorporated in this Annual Report by reference.
| Item 14. | Principal Accountant Fees and Services |
The information required by this item will be contained in our 2017 Proxy Statement under the caption “PROPOSAL 4—RATIFICATION OF THE SELECTION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM” and is incorporated in this Annual Report by reference.
79
Table of Contents
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
| (a) |
1. Financial Statements. The financial statements and notes thereto annexed to this Annual Report begin on page F-1. | |
| 2. Financial Statement Schedules. All other supplemental schedules are omitted because of the absence of conditions under which they are required or because the required information is given in the financial statements or notes thereto. | ||
| 3. Exhibits. The Exhibit Index annexed to this Annual Report, and immediately preceding the exhibits, is incorporated by reference. | ||
| Item 16. | Form 10-K Summary |
None.
80
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CYNOSURE, INC. | ||
| By: |
/s/ MICHAEL R. DAVIN | |
| Michael R. Davin | ||
| President, Chief Executive Officer and Chairman of the Board of Directors | ||
| Date: February 28, 2017 | ||
Pursuant to the requirements of the Securities Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ MICHAEL R. DAVIN Michael R. Davin |
President, Chief Executive Officer and Chairman of the Board of Directors (Principal Executive Officer) |
February 28, 2017 | ||
| /s/ STEPHEN J. WEBBER Stephen J. Webber |
Executive Vice President, Chief Financial Officer, Chief Accounting Officer and Treasurer (Principal Financial Officer and Principal Accounting Officer) |
February 28, 2017 | ||
| /s/ BRIAN M. BAREFOOT Brian M. Barefoot |
Director |
February 28, 2017 | ||
| /s/ ETTORE V. BIAGIONI Ettore V. Biagioni |
Director |
February 28, 2017 | ||
| /s/ WILLIAM O. FLANNERY William Flannery |
Director |
February 28, 2017 | ||
| /s/ MARINA HATSOPOULOS Marina Hatsopoulos |
Director |
February 28, 2017 | ||
| /s/ THOMAS H. ROBINSON Thomas H. Robinson |
Director |
February 28, 2017 | ||
81
Table of Contents
CYNOSURE, INC.
| Consolidated Financial Statements of Cynosure, Inc. |
||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 |
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Cynosure, Inc.:
We have audited the accompanying consolidated balance sheets of Cynosure, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of Cynosure, Inc.’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Cynosure, Inc. at December 31, 2016 and 2015, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Cynosure, Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 28, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston, Massachusetts
February 28, 2017
F-2
Table of Contents
CYNOSURE, INC.
(In thousands, except par value data)
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 153,625 | $ | 108,587 | ||||
| Short-term marketable securities |
46,530 | 35,412 | ||||||
| Accounts receivable, net of allowance of $3,301 and $3,181 in 2016 and 2015, respectively |
42,092 | 42,012 | ||||||
| Inventories |
75,972 | 79,768 | ||||||
| Prepaid expenses and other current assets |
20,358 | 21,356 | ||||||
|
|
|
|
|
|||||
| Total current assets |
338,577 | 287,135 | ||||||
| Property and equipment, net |
47,950 | 39,706 | ||||||
| Long-term marketable securities |
37,628 | 38,761 | ||||||
| Goodwill |
105,752 | 105,807 | ||||||
| Intangibles, net |
35,707 | 44,317 | ||||||
| Deferred tax asset |
19,834 | 17,882 | ||||||
| Other assets |
1,059 | 1,002 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 586,507 | $ | 534,610 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 24,860 | $ | 33,885 | ||||
| Accrued expenses |
64,760 | 45,616 | ||||||
| Deferred revenue |
14,915 | 24,803 | ||||||
| Capital lease obligations |
866 | 741 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
105,401 | 105,045 | ||||||
| Capital lease obligations, net of current portion |
18,707 | 17,372 | ||||||
| Deferred revenue, net of current portion |
988 | 903 | ||||||
| Other noncurrent liabilities |
5,917 | 6,888 | ||||||
| Commitments and Contingencies (Note 12) |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.001 par value Authorized—5,000 shares as of December 31, 2016 and 2015 Issued—no shares as of December 31, 2016 and 2015 |
— | — | ||||||
| Class A and Class B common stock, $0.001 par value |
||||||||
| Authorized—61,500 Class A shares and no Class B shares at December 31, 2016 Authorized—61,500 Class A shares and 8,500 Class B shares at December 31, 2015 |
||||||||
| Issued—25,316 Class A shares and no Class B shares at December 31, 2016 Issued—24,327 Class A shares and no Class B shares at December 31, 2015 |
25 | 24 | ||||||
| Additional paid-in capital |
426,014 | 387,161 | ||||||
| Retained earnings |
69,982 | 55,781 | ||||||
| Accumulated other comprehensive loss |
(7,423 | ) | (5,460 | ) | ||||
| Treasury stock, 1,628 Class A shares, at cost |
(33,104 | ) | (33,104 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
455,494 | 404,402 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 586,507 | $ | 534,610 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
CYNOSURE, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Product revenues |
$ | 357,968 | $ | 276,085 | $ | 236,878 | ||||||
| Parts, accessories, service and royalty revenues |
75,564 | 63,377 | 55,491 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
433,532 | 339,462 | 292,369 | |||||||||
| Cost of revenues |
178,221 | 145,928 | 127,131 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
255,311 | 193,534 | 165,238 | |||||||||
| Operating expenses: |
||||||||||||
| Sales and marketing |
153,853 | 111,506 | 88,564 | |||||||||
| Research and development |
28,973 | 22,343 | 22,033 | |||||||||
| Amortization of intangible assets acquired |
2,741 | 2,990 | 2,961 | |||||||||
| General and administrative |
42,404 | 30,374 | 30,420 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
227,971 | 167,213 | 143,978 | |||||||||
|
|
|
|
|
|
|
|||||||
| Income from operations |
27,340 | 26,321 | 21,260 | |||||||||
| Interest expense, net |
(1,563 | ) | (1,683 | ) | (1,446 | ) | ||||||
| Other expense, net |
(834 | ) | (1,440 | ) | (1,476 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Income before provision (benefit) for income taxes |
24,943 | 23,198 | 18,338 | |||||||||
| Provision (benefit) for income taxes |
10,742 | 7,391 | (13,000 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net income |
$ | 14,201 | $ | 15,807 | $ | 31,338 | ||||||
|
|
|
|
|
|
|
|||||||
| Basic net income per share |
$ | 0.61 | $ | 0.71 | $ | 1.44 | ||||||
|
|
|
|
|
|
|
|||||||
| Diluted net income per share |
$ | 0.60 | $ | 0.70 | $ | 1.41 | ||||||
|
|
|
|
|
|
|
|||||||
| Basic weighted average common shares outstanding |
23,304 | 22,286 | 21,824 | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted weighted average common shares outstanding |
23,706 | 22,658 | 22,195 | |||||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
CYNOSURE, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Net income |
$ | 14,201 | $ | 15,807 | $ | 31,338 | ||||||
|
|
|
|
|
|
|
|||||||
| Other comprehensive loss components: |
||||||||||||
| Cumulative translation adjustment |
(1,882 | ) | (1,558 | ) | (2,318 | ) | ||||||
| Unrealized loss on marketable securities, net of taxes |
(81 | ) | (39 | ) | (46 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total other comprehensive loss |
(1,963 | ) | (1,597 | ) | (2,364 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive income |
$ | 12,238 | $ | 14,210 | $ | 28,974 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
CYNOSURE, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except par value data)
| Class A and
B Common Stock |
Additional Paid-In Capital |
Retained Earnings |
Accumulated Other Comprehensive Loss |
Class A and
B Treasury Stock |
Total Stockholders’ Equity |
|||||||||||||||||||||||||||
| Shares | $0.001 Par Value |
Shares | Cost | |||||||||||||||||||||||||||||
| Balance at December 31, 2013 |
22,633 | $ | 23 | $ | 338,742 | $ | 8,636 | $ | (1,499 | ) | (886 | ) | $ | (17,550 | ) | $ | 328,352 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Stock-based compensation expense |
— | — | 7,114 | — | — | — | — | 7,114 | ||||||||||||||||||||||||
| Exercise of stock options and vesting of restricted stock units |
620 | — | 8,153 | — | — | — | — | 8,153 | ||||||||||||||||||||||||
| Tax benefit from stock-based compensation expense in excess of book deductions |
— | — | 1,073 | — | — | — | — | 1,073 | ||||||||||||||||||||||||
| Repurchase of common stock |
— | — | — | — | — | (742 | ) | (15,554 | ) | (15,554 | ) | |||||||||||||||||||||
| Net income |
— | — | — | 31,338 | — | — | — | 31,338 | ||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | (2,318 | ) | — | — | (2,318 | ) | ||||||||||||||||||||||
| Unrealized loss on marketable securities, net of taxes |
— | — | — | — | (46 | ) | — | — | (46 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2014 |
23,253 | $ | 23 | $ | 355,082 | $ | 39,974 | $ | (3,863 | ) | (1,628 | ) | $ | (33,104 | ) | $ | 358,112 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Stock-based compensation expense |
— | — | 7,652 | — | — | — | — | 7,652 | ||||||||||||||||||||||||
| Exercise of stock options and vesting of restricted stock units |
1,074 | 1 | 20,162 | — | — | — | — | 20,163 | ||||||||||||||||||||||||
| Tax benefit from stock-based compensation expense in excess of book deductions |
— | — | 4,265 | — | — | — | — | 4,265 | ||||||||||||||||||||||||
| Net income |
— | — | — | 15,807 | — | — | — | 15,807 | ||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | (1,558 | ) | — | — | (1,558 | ) | ||||||||||||||||||||||
| Unrealized loss on marketable securities, net of taxes |
— | — | — | — | (39 | ) | — | — | (39 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2015 |
24,327 | $ | 24 | $ | 387,161 | $ | 55,781 | $ | (5,460 | ) | (1,628 | ) | $ | (33,104 | ) | $ | 404,402 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Stock-based compensation expense |
— | — | 10,112 | — | — | — | — | 10,112 | ||||||||||||||||||||||||
| Exercise of stock options and vesting of restricted stock units |
989 | 1 | 25,185 | — | — | — | — | 25,186 | ||||||||||||||||||||||||
| Tax benefit from stock-based compensation expense in excess of book deductions |
— | — | 3,556 | — | — | — | — | 3,556 | ||||||||||||||||||||||||
| Net income |
— | — | — | 14,201 | — | — | — | 14,201 | ||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | (1,882 | ) | — | — | (1,882 | ) | ||||||||||||||||||||||
| Unrealized loss on marketable securities, net of taxes |
— | — | — | — | (81 | ) | — | — | (81 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2016 |
25,316 | $ | 25 | $ | 426,014 | $ | 69,982 | $ | (7,423 | ) | (1,628 | ) | $ | (33,104 | ) | $ | 455,494 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
CYNOSURE, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Operating activities: |
||||||||||||
| Net income |
$ | 14,201 | $ | 15,807 | $ | 31,338 | ||||||
| Reconciliation of net income to net cash from operating activities: |
||||||||||||
| Depreciation and amortization |
20,664 | 19,291 | 17,546 | |||||||||
| Stock-based compensation |
10,110 | 7,646 | 7,113 | |||||||||
| Loss on disposal of fixed assets |
465 | 60 | 217 | |||||||||
| Noncash interest expense on capital lease obligations |
1,880 | 1,727 | 1,495 | |||||||||
| Noncash interest expense on license transfer agreement |
187 | 197 | 67 | |||||||||
| Deferred income taxes |
(1,965 | ) | (885 | ) | (15,786 | ) | ||||||
| Net accretion of marketable securities |
2,526 | 2,266 | 1,675 | |||||||||
| Tax benefit from stock option exercises |
(3,556 | ) | (4,265 | ) | (1,073 | ) | ||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(944 | ) | (752 | ) | (4,864 | ) | ||||||
| Inventories |
(3,843 | ) | (27,410 | ) | (8,257 | ) | ||||||
| Net book value of demonstration inventory sold |
1,097 | 845 | 963 | |||||||||
| Prepaid expenses and other current assets |
4,304 | (7,927 | ) | 1,183 | ||||||||
| Accounts payable |
(8,943 | ) | 13,136 | 5,714 | ||||||||
| Accrued expenses |
19,430 | 3,963 | 3,331 | |||||||||
| Deferred revenue |
(9,641 | ) | 14,145 | 1,402 | ||||||||
| Other noncurrent liabilities |
(938 | ) | (119 | ) | 42 | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash from operating activities |
45,034 | 37,725 | 42,106 | |||||||||
| Investing activities: |
||||||||||||
| Purchases of property and equipment |
(13,465 | ) | (10,787 | ) | (15,778 | ) | ||||||
| Proceeds from the sales and maturities of marketable securities |
51,926 | 44,763 | 32,400 | |||||||||
| Purchases of marketable securities |
(64,560 | ) | (63,033 | ) | (56,948 | ) | ||||||
| Cash paid for acquisitions, net of cash received |
— | — | (13,235 | ) | ||||||||
| Increase in other assets |
(78 | ) | (61 | ) | (117 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash from investing activities |
(26,177 | ) | (29,118 | ) | (53,678 | ) | ||||||
| Financing activities: |
||||||||||||
| Excess tax benefit on options exercised |
3,556 | 4,265 | 1,073 | |||||||||
| Repurchases of common stock |
— | — | (15,554 | ) | ||||||||
| Proceeds from stock option exercises |
25,186 | 20,163 | 8,153 | |||||||||
| Payments on capital lease obligation |
(2,474 | ) | (213 | ) | (688 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash from financing activities |
26,268 | 24,215 | (7,016 | ) | ||||||||
| Effect of exchange rate changes on cash and cash equivalents |
(87 | ) | 634 | 64 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
45,038 | 33,456 | (18,524 | ) | ||||||||
| Cash and cash equivalents, beginning of year |
108,587 | 75,131 | 93,655 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of year |
$ | 153,625 | $ | 108,587 | $ | 75,131 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental cash flow information: |
||||||||||||
| Cash paid for interest |
$ | 18 | $ | 19 | $ | 19 | ||||||
|
|
|
|
|
|
|
|||||||
| Cash paid for income taxes |
$ | 8,576 | $ | 3,670 | $ | 2,899 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental noncash investing and financing activities: |
||||||||||||
| Transfer of demonstration equipment from inventory to fixed assets |
$ | 6,493 | $ | 6,586 | $ | 4,135 | ||||||
|
|
|
|
|
|
|
|||||||
| Assets acquired under capital lease |
$ | 2,058 | $ | 357 | $ | 239 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Table of Contents
CYNOSURE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of the Business
Cynosure, Inc. (“Cynosure” or “the Company”) develops, manufactures and markets aesthetic treatment systems that enable plastic surgeons, dermatologists and other medical practitioners to perform non-invasive and minimally invasive procedures to remove hair, treat vascular and benign pigmented lesions, remove multi-colored tattoos, revitalize the skin, reduce fat through laser lipolysis, reduce cellulite, clear nails infected by toe fungus, ablate sweat glands and improve women’s health. Cynosure also markets radiofrequency (“RF”) energy sourced medical devices for precision surgical applications such as facial plastic and general surgery, gynecology, ear, nose, and throat procedures, ophthalmology, oral and maxillofacial surgery, podiatry and proctology. Cynosure sells its products through a direct sales force in the United States, Canada, France, Morocco, Germany, Spain, the United Kingdom, Australia, China, Japan and Korea and through international distributors in over 120 other countries. Cynosure is a Delaware corporation, incorporated on July 10, 1991, located in Westford, Massachusetts.
As further described under Note 14, Subsequent Events, below, on February 14, 2017, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Hologic, Inc. (“Hologic”) and Minuteman Merger Sub, Inc., a wholly-owned subsidiary of Hologic (the “Purchaser”). Pursuant to the terms of Merger Agreement, the Purchaser commenced an all cash tender offer on February 22, 2017 (the “Offer”) for any (subject to the minimum condition) and all of Cynosure’s outstanding shares of Class A common stock, par value $0.001 per share (the “Shares”), at a purchase price of $66.00 per Share (the “Offer Price”), net to the seller in cash, without interest, subject to any required withholding of taxes. The Offer will remain open for a minimum of 20 business days from the date of commencement. Following the completion of the Offer, subject to the absence of injunctions or other legal restraints preventing the consummation of the Merger, the Purchaser will merge with and into Cynosure, with Cynosure surviving as a wholly-owned subsidiary of Hologic (the “Merger”), pursuant to the procedure provided for under Section 251(h) of the Delaware General Corporation Law, without any additional stockholder approvals. The Merger will be effected as soon as practicable following the time of purchase by the Purchaser of Shares validly tendered and not withdrawn in the Offer. Unless extended by Hologic in accordance with the Merger Agreement, the offering period for the Offer will expire at the end of the day, 12:00 Midnight, Eastern time, on Tuesday, March 21, 2017. The Company expects the Merger to close in late March or April of 2017. The Merger Agreement contains customary termination rights for both Cynosure and Hologic, including, among others, for failure to consummate the Offer on or before August 14, 2017.
2. Summary of Significant Accounting Policies
Significant accounting policies followed in the preparation of these consolidated financial statements are as follows:
Management Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (“U.S. GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and the related disclosures at the date of the financial statements and during the reporting period. Components particularly subject to estimation include the allowance for doubtful accounts, inventory reserves, reserve for sales returns, intangible assets, impairment analysis of goodwill and intangibles, deferred tax assets, liabilities and valuation allowances, fair value of stock options and investments and accrued warranties. On an ongoing basis, management evaluates its estimates. Actual results could differ from these estimates.
F-8
Table of Contents
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Cynosure, Inc. and its wholly-owned subsidiaries: Cynosure Securities Corporation, Palomar Medical Products, LLC, Palomar Medical Technologies, LLC, Cynosure Mexico, Cynosure GmBH, S.A.R.L. Cynosure France, Cynosure Maroc SARL, Cynosure UK LTD, Cynosure Spain S.L., Cynosure B.V., Cynosure K.K, Suzhou Cynosure Medical Devices Company Ltd., Cynosure Korea Limited, Cynosure Pty Ltd and Cynosure Portugal. All intercompany balances and transactions have been eliminated.
Reclassification
Certain amounts in prior years’ investing activities within the consolidated statements of cash flows have been reclassified to conform to the current year’s presentation.
Cash, Cash Equivalents, Short and Long-Term Marketable Securities
Cynosure considers all short-term, highly liquid investments with original maturities at the time of purchase of 90 days or less to be cash equivalents. Cynosure accounts for short and long-term marketable securities as available-for-sale in accordance with Accounting Standards Codification (“ASC”) 320, Investments—Debt and Equity Securities Topic. Under ASC 320, securities purchased to be held for indefinite periods of time and not intended at the time of purchase to be held until maturity are classified as available-for-sale securities. ASC 320 requires Cynosure to recognize all marketable securities on the consolidated balance sheets at fair value. Cynosure’s marketable securities are stated at fair value based on quoted market prices. Adjustments to the fair value of marketable securities that are classified as available-for-sale are recorded as increases or decreases, net of income taxes, within accumulated other comprehensive loss in shareholders’ equity. The amortized cost of marketable debt securities is adjusted for amortization of premiums and discounts to maturity computed under the effective interest method. The cost of securities sold is determined by the specific identification method. Cynosure continually evaluates whether any marketable securities have been impaired and, if so, whether such impairment is temporary or other than temporary.
Fair Value of Financial Instruments
Cynosure’s financial instruments consist of cash, cash equivalents, short and long-term marketable securities, accounts receivable and capital leases. The rate implicit within Cynosure’s capital lease obligations approximates market interest rates. Cynosure’s estimate of fair value for financial instruments, other than marketable securities, which are carried at fair value, approximates their carrying value at December 31, 2016 and 2015.
ASC 820, Fair Value Measurement Topic, applies to all financial assets and financial liabilities that are being measured and reported on a fair value basis, establishes a framework for measuring fair value of assets and liabilities and expands disclosures about fair value measurements.
Concentration of Credit Risk
Financial instruments that subject Cynosure to credit risk consist primarily of cash and cash equivalents, short and long-term marketable securities and accounts receivable. Cynosure places cash and cash equivalents and short and long-term marketable securities in established financial institutions. Cynosure has no significant off-balance-sheet risk or concentration of credit risk, such as foreign exchange contracts, options contracts, or other foreign hedging arrangements. Cynosure’s accounts receivable balance, net of allowance for doubtful accounts, was $42.1 million as of December 31, 2016, compared to $42.0 million as of December 31, 2015. The allowance for doubtful accounts as of December 31, 2016 and 2015 was $3.3 million and $3.2 million, respectively. Cynosure maintains an allowance for doubtful accounts based upon the aging of its receivable balances, known collectability issues and Cynosure’s historical experience with losses. Cynosure works to mitigate bad debt exposure through its credit evaluation policies, reasonably short payment terms and geographical dispersion of sales. Losses from bad debt have historically been within management’s estimates.
F-9
Table of Contents
Cynosure’s revenue includes export sales to foreign companies located principally in Europe, the Asia/Pacific region and the Middle East. Cynosure obtains letters of credit for foreign sales that the Company considers to be at risk.
No customer accounted for 10% or greater of revenue during 2016, 2015 or 2014. No customer accounted for 10% or greater of accounts receivable as of December 31, 2016 or 2015. Accounts receivable allowance activity consisted of the following for the years ended December 31:
| 2016 | 2015 | 2014 | ||||||||||
| (In thousands) | ||||||||||||
| Balance at beginning of year |
$ | 3,181 | $ | 2,949 | $ | 1,803 | ||||||
| Additions |
1,606 | 2,599 | 2,373 | |||||||||
| Deductions |
(1,486 | ) | (2,367 | ) | (1,227 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Balance at end of year |
$ | 3,301 | $ | 3,181 | $ | 2,949 | ||||||
|
|
|
|
|
|
|
|||||||
Inventory
Cynosure states all inventories at the lower of cost or market, determined on a first-in, first-out method. Inventory includes material, labor and overhead and consists of the following:
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| (In thousands) | ||||||||
| Raw materials |
$ | 19,448 | $ | 22,569 | ||||
| Work in process |
3,258 | 3,317 | ||||||
| Finished goods |
53,266 | 53,882 | ||||||
|
|
|
|
|
|||||
| $ | 75,972 | $ | 79,768 | |||||
|
|
|
|
|
|||||
Included in finished goods are lasers used for demonstration purposes. Cynosure’s policy is to include demonstration lasers as inventory for a period of up to one year. As finished goods inventory, demonstration systems remain sellable while in inventory, are kept in proper working order and may be sold or transferred to other warehouse locations. Similar to any other finished goods in inventory, Cynosure accounts for such demonstration inventory in accordance with the policy for excess and obsolescence review of its entire inventory. The majority of Cynosure’s demonstration system sales occur during the first year. Sales beyond the first year are infrequent. If not sold after a period of one year, demonstration systems are transferred to fixed assets and continuously used by representatives to foster sales. Cynosure places a three-year useful life on each demonstration system classified as a fixed asset, which reflects the reduction in value and benefits realized from its use over time.
Cynosure’s excess and obsolescence reserve policy is to establish inventory reserves when conditions exist that suggest that inventory may be in excess of anticipated demand or is obsolete based upon assumptions about future demand for products and market conditions. Cynosure regularly evaluates the ability to realize the value of inventory based on a combination of factors including the following: historical usage rates, forecasted sales or usage, product end of life dates, estimated current and future market values and new product introductions.
Cynosure purchases raw material components as well as certain finished goods from sole source suppliers. A delay in the production capabilities of these vendors could cause a delay in Cynosure’s manufacturing, and a possible loss of revenues, which would adversely affect operating results.
Property and Equipment
Property and equipment are recorded at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Assets under capital leases and leasehold
F-10
Table of Contents
improvements are amortized using the straight-line method over the shorter of the estimated useful life of the asset or the respective lease term. Included in property and equipment are certain lasers that are used for demonstration purposes. Maintenance and repairs are charged to expense as incurred. Cynosure continually evaluates whether events or circumstances have occurred that indicate that the estimated remaining useful life of its long-lived assets may warrant revision or that the carrying value of these assets may be impaired. Cynosure evaluates the realizability of its long-lived assets based on profitability and cash flow expectations for the related asset. Any write-downs are treated as permanent reductions in the carrying amount of the assets. Based on this evaluation, Cynosure believes that, as of each of the balance sheet dates presented, none of Cynosure’s long-lived assets were impaired.
Intangible Assets
Cynosure capitalizes and includes in intangible assets the costs of developed technology and patents, customer relationships, trade names and business licenses acquired in a business combination or asset acquisition. Intangible assets are recorded at fair value and stated net of accumulated amortization and impairments. Cynosure amortizes its intangible assets that have finite lives using either the straight-line or accelerated method, based on the useful life of the asset over which it is expected to be consumed utilizing expected undiscounted future cash flows. Amortization is recorded over the estimated useful lives ranging from five to 23 years. Cynosure evaluates the realizability of its definite lived intangible assets whenever events or changes in circumstances or business conditions indicate that the carrying value of these assets may not be recoverable based on expectations of future undiscounted cash flows for each asset group. If the carrying value of an asset or asset group exceeds its undiscounted cash flows, Cynosure estimates the fair value of the assets, generally utilizing a discounted cash flow analysis based on the present value of estimated future cash flows to be generated by the assets using a risk-adjusted discount rate. To estimate the fair value of the assets, Cynosure uses market participant assumptions pursuant to ASC 820, Fair Value Measurements. If the estimate of an intangible asset’s remaining useful life is changed, Cynosure will amortize the remaining carrying value of the intangible asset prospectively over the revised useful life.
Goodwill
Goodwill represents the excess of the purchase price over the fair value of assets acquired and liabilities assumed in a business combination. Cynosure does not amortize its goodwill, but instead tests for impairment at least annually and more frequently whenever events or changes in circumstances indicate that the fair value of the asset may be less than its carrying value of the asset. Cynosure’s annual test for impairment occurs on the first day of its fourth quarter.
Cynosure has adopted Accounting Standards Update (“ASU”) 2011-08 Intangibles—Goodwill and Other, an amendment to ASC 350, which updates how an entity evaluates its goodwill for impairment. The guidance provides entities an option to perform a “qualitative” assessment to determine whether further impairment testing is necessary. If further testing is required, the test for impairment continues with the two step process. The first step compares the carrying amount of the reporting unit to its estimated fair value (Step 1). To the extent that the carrying value of the reporting unit exceeds its estimated fair value, a second step is performed, wherein the reporting unit’s carrying value is compared to the implied fair value (Step 2). To the extent that the carrying value exceeds the implied fair value, impairment exists and must be recognized.
Cynosure has one reporting unit for goodwill impairment testing and has performed a qualitative assessment on that reporting unit. As a result of this assessment, the Company determined that goodwill is not impaired as of December 31, 2016 and 2015.
Revenue Recognition and Deferred Revenue
Cynosure generate revenues primarily from sales of its non-invasive and minimally invasive laser and light-based aesthetic treatment systems, as well as RF energy based surgical and aesthetic systems, which Cynosure refers to collectively as its product sales. Cynosure also generates revenues from sales of parts and accessories,
F-11
Table of Contents
from services, including product warranty revenues, and from royalty payments received from its licensees. Cynosure’s business model generally does not involve separate contracts entered into at or near the same time; nor does its business model involve incremental future discounts offered to the same customer. Cynosure does not offer a right of cancellation, termination, refund or return.
Cynosure recognizes revenue from sales of its products, parts and accessories in accordance with the ASC Revenue Recognition Topic 605-10-S99. Cynosure recognizes revenue from sales of its products, parts and accessories when title and risk of ownership has been transferred, which is upon shipment, provided there are no uncertainties regarding customer acceptance, and the following factors are present:
| • | persuasive evidence of an arrangement; |
| • | the fee is fixed or determinable; and |
| • | collectability of the related receivable is reasonably assured. |
Revenues from the sale of services and extended warranty contracts are deferred and recognized on a straight-line basis over the contract period as services are provided.
Cynosure recognizes royalty revenues when it can reliably estimate such amounts and collectability is reasonably assured. As such, Cynosure recognizes royalty revenues in the quarter reported to it by its licensees, or one quarter following the quarter in which sales by its licensees occurred. Royalty revenues also include amounts due from settlements with licensees for back-owed royalties from prior periods. These settlement amounts are considered revenue, when collectability is reasonably assured, because they constitute Cynosure’s ongoing major or central operations.
Multiple-element arrangements are evaluated in accordance with ASC 605-25, Multiple-Element Arrangements. Cynosure determined that its multiple-element arrangements are generally comprised of the following elements that are recognized as separate units of accounting: products, accessories, extended warranties and installation and training services. Installation and training services are not essential to the functionality of the aesthetic treatment system. Cynosure allocates revenue among the elements based upon their vendor specific objective evidence of fair value (“VSOE”). If VSOE does not exist, Cynosure may use third-party evidence of fair value (“TPE”), to determine the relative selling price of each element. If neither VSOE nor TPE exists, Cynosure may use management’s best estimate of selling price (“BESP”) of each element to determine the relative selling price. Presently there are no elements for which Cynosure is utilizing TPE or BESP.
VSOE is based on the relative sales price based on separate stand-alone sales of Cynosure’s elements. Revenues associated with products and accessories are recognized when shipped. Accessories are predominantly shipped with the aesthetic treatment system when sold with the product. Revenues associated with extended warranties are deferred and recognized ratably over the extended warranty period, which is generally one year. The relative sales prices of extended warranties are based on the actual pricing of service contracts purchased on a standalone basis at a later time, normally at the end of the standard warranty period. Due to the type of revenue arrangements and structure of its contracts, Cynosure refers to guidance on separately priced extended warranties within ASC 605-20, Services. Under ASC 605-20, Cynosure defers the selling price of the separately priced extended warranty at the inception of the arrangement, and allocates the remainder of the arrangement consideration among the remaining elements on their VSOE of fair value in accordance with ASC 605-25.
In accordance with the provisions of ASC 605-45, Revenue Recognitions Topic—Principal Agent Considerations, Cynosure records shipping and handling costs billed to its customers as a component of revenue, and the underlying expense as a component of cost of revenue. Shipping and handling costs included as a component of revenue totaled approximately $1.9 million, $1.4 million and $1.0 million for the years ended December 31, 2016, 2015 and 2014, respectively. Shipping and handling costs included as a component of cost of revenue totaled $2.0 million, $1.7 million and $1.1 million for the years ended December 31, 2016, 2015 and 2014, respectively.
F-12
Table of Contents
Cynosure collects sales tax from its customers on product sales for which the customer is not tax exempt and remits such taxes to the appropriate governmental authorities. Cynosure presents its sales taxes on a net basis; therefore, these taxes are excluded from revenues.
Cost of Revenues
Cynosure’s cost of revenues consist primarily of material, labor and manufacturing overhead expenses and includes the cost of components and subassemblies supplied by third-party suppliers. Cost of revenues also includes royalties incurred on certain products sold by Cynosure and its licensees, costs incurred in connection with Cynosure’s efforts to litigate or settle additional third-party license agreements, amortization expense related to developed technology and patents intangible assets, service and warranty expenses, as well as salaries and personnel-related expenses, including stock-based compensation, for Cynosure’s operations management team, purchasing and quality control.
Product Warranty Costs
Cynosure typically provides a one-year system and labor warranty on end-user sales of lasers. Distributor sales of lasers generally include a one-year warranty on systems only. These one-year warranty obligations are limited to the repair or replacement of parts and materials which prove to be defective, and do not contain any service performance obligations. Estimated future costs for initial product warranties are provided for at the time of revenue recognition and recorded as cost of revenues within Cynosure’s consolidated statement of operations. The following table sets forth activity in the accrued warranty account, which is a component of accrued expenses in the consolidated balance sheets:
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (In thousands) | ||||||||||||
| Balance at beginning of year |
$ | 8,841 | $ | 8,118 | $ | 6,651 | ||||||
| Warranty provision related to new sales |
18,603 | 13,754 | 15,104 | |||||||||
| Costs incurred |
(16,004 | ) | (13,031 | ) | (13,637 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Balance at end of year |
$ | 11,440 | $ | 8,841 | $ | 8,118 | ||||||
|
|
|
|
|
|
|
|||||||
Research and Development
Research and development costs consist of salaries and other personnel-related expenses, including stock-based compensation, for employees primarily engaged in research, development and engineering activities and materials used and other overhead expenses incurred in connection with the design and development of Cynosure’s products and from time to time expenses associated with collaborative research agreements that the Company may enter into. These costs are expensed as incurred.
Advertising Costs
Cynosure expenses advertising costs as incurred. Advertising costs totaled $3.5 million, $1.0 million and $1.2 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Foreign Currency Translation
The financial statements of Cynosure’s foreign subsidiaries are translated from local currency into U.S. dollars using the current exchange rate at the balance sheet date for assets and liabilities, and the average exchange rate prevailing during the period for revenue and expenses. The functional currency for Cynosure’s foreign subsidiaries is considered to be the local currency for each entity and, accordingly, translation
F-13
Table of Contents
adjustments for these subsidiaries are included in accumulated other comprehensive loss within stockholders’ equity. Certain intercompany and third-party foreign currency-denominated transactions generated foreign currency remeasurement losses of approximately $0.8 million, $1.8 million and $1.6 million during 2016, 2015 and 2014, respectively, which are included in other expense, net, in the consolidated statements of operations.
Accumulated Other Comprehensive Loss
Changes to accumulated other comprehensive loss during the year ended December 31, 2016 were as follows (in thousands):
| Unrealized Loss on Marketable Securities, net of taxes |
Translation Adjustment |
Accumulated Other Comprehensive Loss |
||||||||||
| Balance—December 31, 2015 |
$ | (49 | ) | $ | (5,411 | ) | $ | (5,460 | ) | |||
| Current period other comprehensive loss |
(81 | ) | (1,882 | ) | (1,963 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Balance—December 31, 2016 |
$ | (130 | ) | $ | (7,293 | ) | $ | (7,423 | ) | |||
|
|
|
|
|
|
|
|||||||
Stock-Based Compensation
Cynosure follows the fair value recognition provisions of ASC 718, Stock Compensation Topic. This guidance requires share-based payments to employees, including grants of employee stock options and restricted stock units (“RSUs”), to be recognized in the statements of operations based on their fair values at the date of grant. The fair value of performance-based stock units (“PSUs”) is determined based on the fair market value of Cynosure’s common stock on the vest dates. Cynosure expenses the fair value of share-based payments over the service period. ASC 718 requires companies to utilize an estimated forfeiture rate when calculating the expense for the period. Accordingly, Cynosure reviews its actual forfeiture rates and periodically aligns its stock compensation expense with the share-based payments that are vesting. Cynosure recorded stock-based compensation expense of $10.1 million, $7.6 million and $7.1 million for the years ended December 31, 2016, 2015 and 2014, respectively. As of December 31, 2016 and 2015, Cynosure had $31,000 and $29,000, respectively, of stock-based compensation expense capitalized as a part of inventory.
Total stock-based compensation expense was recorded to cost of revenues and operating expenses based upon the functional responsibilities of the individual holding the respective share-based payments, as follows:
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (In thousands) | ||||||||||||
| Cost of revenues |
$ | 463 | $ | 344 | $ | 292 | ||||||
| Sales and marketing |
2,774 | 2,059 | 1,934 | |||||||||
| Research and development |
1,356 | 1,167 | 1,007 | |||||||||
| General and administrative |
5,517 | 4,076 | 3,880 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total stock-based compensation expense |
$ | 10,110 | $ | 7,646 | $ | 7,113 | ||||||
|
|
|
|
|
|
|
|||||||
As of December 31, 2016, there was $16.3 million of unrecognized compensation expense related to non-vested share awards that is expected to be recognized on a straight-line basis over a weighted average period of 1.6 years. Cash received from option exercises was $25.2 million, $20.2 million and $8.2 million during the years ended December 31, 2016, 2015 and 2014, respectively.
Cynosure did not grant any stock options during the year ended December 31, 2016. Cynosure granted 455,999 and 854,180 stock options during the years ended December 31, 2015 and 2014, respectively. Cynosure
F-14
Table of Contents
uses the Black-Scholes option pricing model to determine the weighted average fair value of options. The weighted average fair value of the options granted during the years ended December 31, 2015 and 2014 was $12.42 and $10.50, respectively, using the following assumptions:
| Years Ended December 31, | ||||
| 2015 | 2014 | |||
| Risk-free interest rate |
1.39% - 1.75% | 1.41% - 1.80% | ||
| Expected dividend yield |
— | — | ||
| Expected term |
4.8 years | 4.6 years | ||
| Expected volatility |
41% - 43% | 43% - 44% | ||
Option-pricing models require the input of various subjective assumptions, including the option’s expected life and the price volatility of the underlying stock. Cynosure’s estimated expected stock price volatility is based on its own historical volatility for the 2015 and 2014 periods. Cynosure’s expected term of options granted during the years ended December 31, 2015 and 2014 represents the weighted average period of time that options granted are expected to be outstanding giving consideration to vesting schedules and Cynosure’s historical exercise patterns. The risk-free rate for the expected term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The dividend yield of zero is based on the fact that Cynosure has never paid cash dividends and has no present intention to pay cash dividends.
Cynosure has been increasingly using RSUs as an equity incentive award for employees. Cynosure granted 173,113 and 86,618 RSUs to employees during the years ended December 31, 2016 and 2015, respectively, at the fair market value of its common stock on the date of grant and which vest annually over a three-year period. Fair market value was determined using the closing price of Cynosure’s common stock on the date of grant. Cynosure is recognizing related compensation expense, net of estimated forfeitures, on a straight-line basis over the three-year period.
Cynosure granted 25,425 and 16,685 RSUs to non-employee directors during the years ended December 31, 2016 and 2015, respectively, at a fair market value of its common stock on the date of grant and which vest quarterly over a one-year period. Fair market value was determined using the closing price of Cynosure’s common stock on the date of grant. Cynosure is recognizing related compensation expense, net of estimated forfeitures, on a straight-line basis over the one-year period.
Cynosure implemented a PSU equity compensation program in 2016. Cynosure granted PSUs in February 2016 to executive-level employees that vest annually over a three-year period based on the achievement of performance goals (determined by the compensation committee of the board of directors in its sole discretion) and continued performance of services. If Cynosure’s consolidated cumulative revenue and/or EBITDA, as defined in each respective award agreement, exceeds defined thresholds for the one, two and three-year periods beginning on January 1, 2016 and ending December 31, 2016, December 31, 2017 and December 31, 2018, respectively, the PSUs vest on the date which is the earlier of (i) the financial earnings release for the year following the year in which the performance period ended or (ii) a determination by the compensation committee of the board of directors that the performance goal has been achieved. Cynosure recognizes compensation expense for performance goals when the probability of achieving such goals is considered probable and is recognizing related compensation expense over the period from the date of grant through the expected vest dates. Each recipient of the PSUs is eligible to receive between zero and 200% of the target number of shares of Cynosure’s common stock at the end of the one, two and three-year periods, provided that the performance goals have been achieved and the recipient has continued performing services for Cynosure. For the year ended December 31, 2016, 16,188 shares were awarded due to the achievement of performance goals for the one-year period then ended. The actual number of shares earned upon vesting will range from zero shares up to a maximum of 52,888 shares if the 200% target is achieved applicable to these PSUs for the two-year period ending December 31, 2017, and the actual number of shares earned upon vesting will range from zero shares up to a maximum of 24,052 shares for the three-year period ending December 31, 2018. Fair value of the PSUs is
F-15
Table of Contents
determined based on the fair market value of Cynosure’s common stock at each reporting period until each performance goal is achieved. Cynosure reevaluates at each reporting period whether the performance goals are probable of achievement and, if at any point in time, Cynosure believes that achieving a performance goal is not probable, it will stop recognizing the related compensation expense and will adjust the previously recognized compensation expense prospectively.
Cynosure granted 62,893 PSUs in December 2016 to one executive-level employee that vest annually over a three-year period based on achievement of performance goals, at the discretion of the compensation committee of the board of directors, for the first year, and then based on continued performance of services over the remainder of the three-year period. If Cynosure’s adjusted income from operations, as defined in the award agreement, exceeds defined thresholds for the one-year period beginning on January 1, 2017 and ending December 31, 2017, the PSUs will vest upon the determination by the compensation committee of the board of directors that the performance goal has been achieved. This determination will be made promptly once Cynosure’s consolidated statements of operations are finalized for the year ending December 31, 2017. Cynosure recognizes compensation expense for performance goals when the probability of achieving such goals is considered probable and is recognizing related compensation expense over the period from the date of grant through the expected vest dates. Fair value of the PSUs is determined based on the fair market value of Cynosure’s common stock at each reporting period end until each performance goal is achieved. Cynosure reevaluates at each reporting period whether the performance goals are probable of achievement and, if at any point in time, Cynosure believes that achieving a performance goal is not probable, it will stop recognizing the related compensation expense and will adjust the previously recognized compensation expense prospectively.
Interest Expense, net
Interest expense consists primarily of interest charges on the capital lease of Cynosure’s U.S. operating facility, license transfer agreement acquired from Ellman International, Inc. (“Ellman”) and interest earned on Cynosure’s short and long-term marketable securities consisting of state and municipal bonds, and U.S. government agencies and treasuries. Cynosure expects interest expense to remain consistent in 2017 as compared to 2016.
Income Taxes
Cynosure provides for income taxes in accordance with ASC 740, Accounting for Income Taxes (“ASC 740”). ASC 740 recognizes tax assets and liabilities for the cumulative effect of all temporary differences between the financial statement carrying amounts and the tax basis of assets and liabilities, and are measured using the enacted tax rates that will be in effect when these differences are expected to reverse. Valuation allowances are provided if, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Cynosure considers several sources of taxable income in making its valuation allowance assessments including taxable income in carryback years, future reversals of existing taxable temporary differences, tax planning strategies and forecasted future income.
Cynosure evaluates at the end of each reporting period whether some or all of the undistributed earnings of its foreign subsidiaries are permanently reinvested. Cynosure recognizes deferred income tax liabilities to the extent that management asserts that undistributed earnings of its foreign subsidiaries are not permanently reinvested or will not be permanently reinvested in the future. Cynosure’s position is based upon several factors including the Company’s evaluation of its subsidiaries’ financial requirements, the short term and long term operational and fiscal objectives of the Company, and the tax consequences associated with the repatriation of earnings.
Cynosure accounts for uncertain tax positions following the provisions of ASC 740. ASC 740 clarifies the accounting for income taxes, by prescribing a minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. ASC 740 also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
F-16
Table of Contents
Net Income per Share
Basic net income per share is determined by dividing net income by the weighted average common shares outstanding during the period. Diluted net income per share is determined by dividing net income by the diluted weighted average shares outstanding during the period. Diluted weighted average shares reflect the dilutive effect, if any, of common stock options, RSUs and PSUs based on the treasury stock method.
The reconciliation of basic and diluted weighted average shares outstanding for the years ended December 31, 2016, 2015 and 2014 is as follows (in thousands, except per share data):
| Years Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Net income |
$ | 14,201 | $ | 15,807 | $ | 31,338 | ||||||
|
|
|
|
|
|
|
|||||||
| Basic weighted average common shares outstanding |
23,304 | 22,286 | 21,824 | |||||||||
| Weighted average common stock equivalents |
402 | 372 | 371 | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted weighted average common shares outstanding |
23,706 | 22,658 | 22,195 | |||||||||
|
|
|
|
|
|
|
|||||||
| Basic net income per share |
$ | 0.61 | $ | 0.71 | $ | 1.44 | ||||||
|
|
|
|
|
|
|
|||||||
| Diluted net income per share |
$ | 0.60 | $ | 0.70 | $ | 1.41 | ||||||
|
|
|
|
|
|
|
|||||||
For the years ended December 31, 2016, 2015 and 2014 approximately 0.1 million shares, 1.0 million shares and 1.3 million shares, respectively, of Cynosure’s Class A common stock issuable pursuant to options and RSUs were excluded from the calculation of diluted weighted average common shares outstanding as their effect was antidilutive.
Recent Accounting Pronouncements
In May 2014, the FASB issued guidance codified in ASC 606, Revenue Recognition—Revenue from Contracts with Customers, which amends the guidance in ASC 605, Revenue Recognition. This new revenue standard creates a single source of revenue guidance for all companies in all industries and is more principles-based than the current revenue guidance. The new guidance must be adopted using either a full retrospective approach for all periods presented in the period of adoption or a modified retrospective approach. ASC 606 will be effective for public entities for interim and annual reporting periods beginning after December 15, 2017. Cynosure has prepared an initial assessment of the potential effects of ASC 606. Based on the initial assessment, Cynosure anticipates that the new standard will not impact the recognition of product sales given their point of sale nature. Cynosure is still in the process of evaluating its arrangements with distributors as well as those related to service contracts and extended warranties under ASC 606 and anticipates the impact will not be significant, however, final conclusions will not be determined until Cynosure completes their analysis. Cynosure expects to adopt ASC 606 using the modified retrospective method. Should the adoption of ASC 606 under the modified retrospective method result in the deferral of revenue previously recognized, or the recognition of revenue previously deferred under ASC 605 beyond January 1, 2018, Cynosure would record a cumulative catch-up adjustment at January 1, 2018.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements—Going Concern, which requires management of public and private companies to evaluate whether there is substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. Management’s evaluation should be based on relevant conditions and events that are known and reasonably knowable at the date that the financial statements are issued. If conditions or events raise substantial doubt about an entity’s ability to continue as a going concern, and substantial doubt is not alleviated after consideration of management’s plans, an entity should include a statement in the footnotes indicating that there is
F-17
Table of Contents
substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. The new standard is effective for annual periods ending after December 15, 2016, and for annual periods and interim periods thereafter. Cynosure has adopted ASU 2014-15 and has assessed its ability to continue as a going concern. As of December 31, 2016, Cynosure has concluded that substantial doubt about its ability to continue as a going concern does not exist.
In July 2015, the FASB issued ASU 2015-11, Simplifying the Measurement of Inventory. ASU 2015-11 simplifies the subsequent measurement of inventory by replacing the lower of cost or market test with a lower of cost and net realizable value test. The guidance applies only to inventories for which cost is determined by methods other than last-in first-out and the retail inventory method. The guidance will be effective for public business entities for fiscal years beginning after December 15, 2016. Cynosure is currently evaluating the impact of the provisions of ASU 2015-11.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes the existing guidance for lease accounting, Leases (Topic 840). ASU 2016-02 requires lessees to recognize leases on their balance sheets, and leaves lessor accounting largely unchanged. The amendments in this ASU are effective for fiscal years beginning after December 15, 2018 and interim periods within those fiscal years. Early application is permitted for all entities. ASU 2016-02 requires a modified retrospective approach for all leases existing at, or entered into after, the date of initial application, with an option to elect to use certain transition relief. Cynosure is currently evaluating the impact of this new standard on its consolidated financial statements.
In March 2016, the FASB issued ASU 2016-09, Compensation—Stock Compensation (Topic 718): Improvement to Employee Share-Based Payment Accounting. The new standard contains several amendments that will simplify the accounting for employee share-based payment transactions, including the accounting for income taxes, forfeitures, statutory tax withholding requirements, classification of awards as either equity or liabilities, and classification on the statement of cash flows. The changes in the new standard eliminate the accounting for excess tax benefits to be recognized in additional paid-in capital and tax deficiencies recognized either in the income tax provision or in addition paid-in capital. The new standard is effective for public business entities for fiscal years beginning after December 15, 2016 with early adoption permitted. ASU 2016-09 requires a modified retrospective approach , with a cumulative-effect adjustment to opening retained earnings for any outstanding liability awards that qualify for the classification. Cynosure is currently evaluating the impact of this new standard on its consolidated financial statements.
3. Fair Value
U.S. GAAP establishes a framework for measuring fair value under generally accepted accounting principles and enhances disclosures about fair value measurements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes the following fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:
| • | Level 1—Quoted prices in active markets for identical assets or liabilities. |
| • | Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable markets data for substantially the full term of the assets or liabilities. |
| • | Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
F-18
Table of Contents
The following table represents Cynosure’s fair value hierarchy for its financial assets (cash equivalents and marketable securities) measured at fair value as of December 31, 2016 (in thousands):
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Money market funds(1) |
$ | 6,196 | $ | — | $ | — | $ | 6,196 | ||||||||
| State and municipal bonds |
— | 56,859 | — | 56,859 | ||||||||||||
| Treasuries and government agencies |
— | 27,299 | — | 27,299 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 6,196 | $ | 84,158 | $ | — | $ | 90,354 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The following table represents Cynosure’s fair value hierarchy for its financial assets (cash equivalents and marketable securities) measured at fair value as of December 31, 2015 (in thousands):
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Money market funds(1) |
$ | 5,785 | $ | — | $ | — | $ | 5,785 | ||||||||
| State and municipal bonds |
— | 59,786 | — | 59,786 | ||||||||||||
| Treasuries and government agencies |
— | 14,387 | — | 14,387 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 5,785 | $ | 74,173 | $ | — | $ | 79,958 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Included in cash and cash equivalents at December 31, 2016 and 2015. |
4. Short and Long-Term Marketable Securities
Cynosure’s available-for-sale securities at December 31, 2016 consisted of approximately $84.2 million in investments in debt securities consisting of state and municipal bonds, treasuries and government agencies. All investments in available-for-sale securities are recorded at fair market value, with any unrealized gains and losses reported as a separate component of accumulated other comprehensive loss.
As of December 31, 2016, Cynosure’s marketable securities consist of the following (in thousands):
| Market Value |
Amortized Cost |
Unrealized Gains |
Unrealized Losses |
|||||||||||||
| Available-for-Sale Securities: |
||||||||||||||||
| Short-term marketable securities: |
||||||||||||||||
| State and municipal bonds |
$ | 40,495 | $ | 40,542 | $ | — | $ | (47 | ) | |||||||
| Treasuries and government agencies |
6,035 | 6,038 | — | (3 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total short-term marketable securities |
$ | 46,530 | $ | 46,580 | $ | — | $ | (50 | ) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Long-term marketable securities: |
||||||||||||||||
| State and municipal bonds |
$ | 16,364 | $ | 16,448 | $ | — | $ | (84 | ) | |||||||
| Treasuries and government agencies |
21,264 | 21,334 | — | (70 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total long-term marketable securities |
$ | 37,628 | $ | 37,782 | $ | — | $ | (154 | ) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total available-for-sale securities |
$ | 84,158 | $ | 84,362 | $ | — | $ | (204 | ) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total marketable securities |
$ | 84,158 | ||||||||||||||
|
|
|
|||||||||||||||
F-19
Table of Contents
As of December 31, 2015, Cynosure’s marketable securities consist of the following (in thousands):
| Market Value |
Amortized Cost |
Unrealized Gains |
Unrealized Losses |
|||||||||||||
| Available-for-Sale Securities: |
||||||||||||||||
| Short-term marketable securities: |
||||||||||||||||
| State and municipal bonds |
$ | 33,914 | $ | 33,923 | $ | — | $ | (9 | ) | |||||||
| Treasuries and government agencies |
1,498 | 1,500 | — | (2 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total short-term marketable securities |
$ | 35,412 | $ | 35,423 | $ | — | $ | (11 | ) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Long-term marketable securities: |
||||||||||||||||
| State and municipal bonds |
$ | 25,872 | $ | 25,916 | $ | 1 | $ | (45 | ) | |||||||
| Treasuries and government agencies |
12,889 | 12,914 | — | (25 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total long-term marketable securities |
$ | 38,761 | $ | 38,830 | $ | 1 | $ | (70 | ) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total available-for-sale securities |
$ | 74,173 | $ | 74,253 | $ | 1 | $ | (81 | ) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total marketable securities |
$ | 74,173 | ||||||||||||||
|
|
|
|||||||||||||||
As of December 31, 2016, Cynosure’s available-for-sale debt securities mature as follows (in thousands):
| Maturities | ||||||||||||||||
| Total | Less Than One Year | One to Five Years | More than five years | |||||||||||||
| State and municipal bonds |
$ | 56,859 | $ | 40,495 | $ | 16,364 | $ | — | ||||||||
| Treasuries and government agencies |
27,299 | 6,035 | 21,264 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total available-for-sale debt securities |
$ | 84,158 | $ | 46,530 | $ | 37,628 | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
5. Goodwill and Other Intangible Assets
Changes to goodwill during the year ended December 31, 2016 were as follows (in thousands):
| Balance—December 31, 2015 |
$ | 105,807 | ||
| Translation adjustment |
(55 | ) | ||
|
|
|
|||
| Balance—December 31, 2016 |
$ | 105,752 | ||
|
|
|
Other intangible assets consist of the following at December 31, 2016 and December 31, 2015 (in thousands):
| Developed Technology & Patents |
Business Licenses |
Customer Relationships |
Trade Names |
Other | Total | |||||||||||||||||||
| December 31, 2016 |
||||||||||||||||||||||||
| Cost |
$ | 27,950 | $ | 284 | $ | 16,395 | $ | 18,370 | $ | 1,347 | $ | 64,346 | ||||||||||||
| Translation adjustment |
— | — | (43 | ) | — | — | (43 | ) | ||||||||||||||||
| Accumulated amortization |
(18,579 | ) | (146 | ) | (6,095 | ) | (3,406 | ) | (370 | ) | (28,596 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2016 |
$ | 9,371 | $ | 138 | $ | 10,257 | $ | 14,964 | $ | 977 | $ | 35,707 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| December 31, 2015 |
||||||||||||||||||||||||
| Cost |
$ | 29,240 | $ | 384 | $ | 19,718 | $ | 18,390 | $ | 1,353 | $ | 69,085 | ||||||||||||
| Translation adjustment |
— | — | (42 | ) | — | — | (42 | ) | ||||||||||||||||
| Accumulated amortization |
(14,055 | ) | (232 | ) | (7,799 | ) | (2,518 | ) | (122 | ) | (24,726 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2015 |
$ | 15,185 | $ | 152 | $ | 11,877 | $ | 15,872 | $ | 1,231 | $ | 44,317 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
F-20
Table of Contents
Amortization expense for the years ended December 31, 2016, 2015 and 2014 was $8.6 million, $9.2 million and $8.6 million, respectively. Cynosure has approximately $56,000 of indefinite-life intangible assets that are included in other intangible assets in the table above. As of December 31, 2016, amortization expense on existing intangible assets for the next five years and beyond is as follows (in thousands):
| 2017 |
$ | 6,624 | ||
| 2018 |
5,108 | |||
| 2019 |
3,373 | |||
| 2020 |
2,923 | |||
| 2021 |
2,449 | |||
| 2022 and thereafter |
15,174 | |||
|
|
|
|||
| Total |
$ | 35,651 | ||
|
|
|
6. Segment and Geographic Information
In accordance with ASC 280, Segment Reporting Topic, operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions how to allocate resources and assess performance. Cynosure’s chief decision-maker, as defined under ASC 280, is a combination of the Chief Executive Officer and the Chief Financial Officer. Cynosure views its operations and manages its business as one segment, aesthetic treatment products and services.
The following table represents total revenue by geographic destination:
| Year Ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| (In thousands) | ||||||||||||
| United States |
$ | 283,137 | $ | 202,485 | $ | 148,803 | ||||||
| Europe |
41,900 | 40,942 | 50,513 | |||||||||
| Asia/Pacific |
80,789 | 70,233 | 64,651 | |||||||||
| Other |
27,706 | 25,802 | 28,402 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 433,532 | $ | 339,462 | $ | 292,369 | |||||||
|
|
|
|
|
|
|
|||||||
Total assets by geographic area are as follows:
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| (In thousands) | ||||||||
| United States |
$ | 549,490 | $ | 497,007 | ||||
| Europe |
19,662 | 22,204 | ||||||
| Asia/Pacific |
23,554 | 21,139 | ||||||
| Eliminations |
(6,199 | ) | (5,740 | ) | ||||
|
|
|
|
|
|||||
| $ | 586,507 | $ | 534,610 | |||||
|
|
|
|
|
|||||
F-21
Table of Contents
Long-lived assets (property and equipment only) by geographic area are as follows:
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| (In thousands) | ||||||||
| United States |
$ | 42,908 | $ | 35,185 | ||||
| Europe |
1,485 | 1,641 | ||||||
| Asia/Pacific |
3,557 | 2,880 | ||||||
|
|
|
|
|
|||||
| $ | 47,950 | $ | 39,706 | |||||
|
|
|
|
|
|||||
No individual country within Europe or Asia/Pacific represented greater than 10% of total revenue, assets or long-lived assets for any period presented.
7. Balance Sheet Accounts
Property and Equipment
Property and equipment consists of the following at December 31:
| Estimated Useful Life (Years) |
2016 Cost |
2015 Cost |
||||||||||
| (In thousands) | ||||||||||||
| Equipment |
3-5 | $ | 13,783 | $ | 11,216 | |||||||
| Furniture and fixtures |
3-5 | 3,077 | 2,470 | |||||||||
| Computer equipment and software |
3 | 7,233 | 4,902 | |||||||||
| Leased equipment |
3-4 | 1,181 | 1,324 | |||||||||
| Leased buildings |
15 | 15,224 | 13,327 | |||||||||
| Leasehold improvements |
5 | 13,061 | 13,106 | |||||||||
| Demonstration equipment |
3 | 23,821 | 22,572 | |||||||||
| Construction in-progress |
11,055 | 4,163 | ||||||||||
|
|
|
|
|
|||||||||
| 88,435 | 73,080 | |||||||||||
| Less: Accumulated depreciation and amortization |
(40,485 | ) | (33,374 | ) | ||||||||
|
|
|
|
|
|||||||||
| $ | 47,950 | $ | 39,706 | |||||||||
|
|
|
|
|
|||||||||
Depreciation expense relating to property and equipment was $12.1 million, $10.1 million and $8.9 million for the years ended December 31, 2016, 2015 and 2014, respectively. As of December 31, 2016 and 2015, the cost of assets recorded under capitalized leases was approximately $16.4 million and $14.7 million, respectively, and the related accumulated amortization was approximately $3.8 million and $2.9 million, respectively. Amortization expense of assets recorded under capitalized leases is included as a component of depreciation expense.
F-22
Table of Contents
Accrued Expenses
Accrued expenses consist of the following at December 31:
| 2016 | 2015(1) | |||||||
| (In thousands) | ||||||||
| Accrued payroll and payroll taxes |
$ | 10,159 | $ | 7,296 | ||||
| Accrued employee benefits |
2,653 | 2,241 | ||||||
| Accrued warranty costs |
11,440 | 8,841 | ||||||
| Accrued commissions |
10,740 | 8,251 | ||||||
| Accrued income, value-added and sales taxes |
6,629 | 7,562 | ||||||
| ARcare settlement agreement charge |
9,209 | — | ||||||
| Accrued other |
13,930 | 11,425 | ||||||
|
|
|
|
|
|||||
| $ | 64,760 | $ | 45,616 | |||||
|
|
|
|
|
|||||
| (1) | Certain amounts in the 2015 column have been reclassified to conform to 2016 presentation. |
Other Noncurrent Liabilities
Other noncurrent liabilities consist of the following at December 31:
| 2016 | 2015 | |||||||
| (In thousands) | ||||||||
| Noncurrent deferred rent |
$ | 543 | $ | 451 | ||||
| Noncurrent tenant improvement allowances |
2,170 | 2,432 | ||||||
| License transfer agreement |
3,204 | 4,005 | ||||||
|
|
|
|
|
|||||
| $ | 5,917 | $ | 6,888 | |||||
|
|
|
|
|
|||||
8. Stockholders’ Equity
Common Stock Authorized
Cynosure has a single-class common stock capital structure consisting of $0.001 par value Class A common stock. Cynosure has authorized 61,500,000 shares of $0.001 par value Class A common stock.
As of December 31, 2016, there were 25,316,116 shares of Class A common stock issued.
The rights, preferences and privileges of Class A common stock are as follows:
Voting Rights
The holders of Class A common stock will be entitled to one vote per share with respect to each matter presented to Cynosure stockholders on which the holders of common stock are entitled to vote.
Conversion
Cynosure’s Class A common stock is not convertible into any other shares of Cynosure’s capital stock.
Dividends
Subject to preferences that may apply to any shares of preferred stock outstanding at the time, the holders of Class A common stock shall be entitled to share equally, on a per share basis, in any dividends that Cynosure’s board of directors may determine to issue from time to time.
F-23
Table of Contents
Liquidation Rights
In the event of Cynosure’s liquidation or dissolution, the holders of Class A common stock shall be entitled to share equally, on a per share basis, in all assets remaining after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock.
Preferred Stock
Cynosure has authorized 5,000,000 shares of $0.001 par value preferred stock. The Company’s board of directors has full authority to issue this stock and to fix the voting powers, preference rights, qualifications, limitations, or restrictions thereof, including dividend rights, conversion rights, redemption privileges and liquidation preferences and the number of shares constituting any series or designation of such series.
Treasury Stock
In February 2016, Cynosure announced that its board of directors has authorized the repurchase of up to $35 million of its Class A common stock, from time to time, on the open market or in privately negotiated transactions under a new stock repurchase program. This program will terminate on February 1, 2018. During the year ended December 31, 2016, Cynosure did not repurchase any shares of its common stock under this program.
9. Stock-Based Compensation
2005 Stock Incentive Plan
In August 2005, the Company’s board of directors adopted the 2005 Stock Incentive Plan (the “2005 Plan”), which was approved by Cynosure’s stockholders in December 2005. The 2005 Plan provides for the grant of incentive stock options (“ISOs”), as well as nonstatutory options, RSUs and PSUs. The board of directors administers the 2005 Plan and has sole discretion to grant options to purchase shares of Cynosure’s common stock, RSUs and PSUs.
The board of directors determines the term of each option, RSU and PSU, option price, number of shares for which each option, RSU and PSU is granted, whether restrictions would be imposed on the shares subject to options and the rate at which each option is exercisable. The exercise price for options granted is determined by the board of directors, except that for ISOs, the exercise price could not be less than the fair market value per share of the underlying common stock on the date granted (110% of fair market value for ISOs granted to holders of more than 10% of the voting stock of Cynosure). The term of the options, RSUs and PSUs is set forth in the applicable option agreement, except that in the case of ISOs, the option term cannot exceed ten years. At December 31, 2016 the number of shares of Class A common stock reserved for issuance under the 2005 Plan is 5,588,369 shares. Options granted under the 2005 Plan vest either (i) over a 36-month period at the rate of 8.33% each quarter until fully vested or (ii) over a vesting period determined by the board of directors. RSUs granted to employees under the 2005 Plan vest over a 36-month period at the rate of 33% each year until fully vested, and RSUs granted to non-employee directors under the 2005 Plan vest over a 12-month period at the rate of 25% each quarter until fully vested. PSUs granted to employees under the 2005 Plan vest annually over a three-year period based on continued performance of services and the achievement of performance goals. As of December 31, 2016, there were 608,688 shares available for future grant under the 2005 Plan.
F-24
Table of Contents
Stock Options
Stock option activity under the 2005 Plan is as follows:
| Number of Options |
Exercise
Price Range |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Life |
Aggregate Intrinsic Value (in thousands) |
||||||||||||||||
| Vested |
1,157,284 | $ | 6.78 - 41.24 | $ | 24.84 | $ | 22,950 | |||||||||||||
| Unvested |
689,958 | 18.94 - 41.24 | 30.18 | 10,000 | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Outstanding, December 31, 2015 |
1,847,242 | $ | 6.78 - 41.24 | $ | 26.83 | 7.33 years | $ | 32,950 | ||||||||||||
| Exercised |
(924,614 | ) | 6.78 - 41.24 | 27.24 | 18,428 | |||||||||||||||
| Forfeited |
(29,278 | ) | 11.02 - 41.24 | 31.73 | 414 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Outstanding, December 31, 2016 |
893,350 | $ | 6.78 - 41.21 | $ | 26.26 | 6.35 years | $ | 17,278 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Vested |
645,412 | 6.78 - 41.21 | 24.48 | 5.81 years | 13,629 | |||||||||||||||
| Unvested |
247,938 | 18.94 - 41.00 | 30.88 | 7.75 years | 3,649 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Vested or expected to vest, December 31, 2016 |
881,201 | 6.78 - 41.21 | 26.19 | 6.33 years | 17,100 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Exercisable, December 31, 2016 |
645,412 | $ | 6.78 - 41.21 | $ | 24.48 | 5.81 years | $ | 13,629 | ||||||||||||
Restricted Stock Units
RSU activity under the 2005 Plan is as follows:
| Number of RSUs |
Grant Date
Fair Value Range |
Weighted- Average Grant Date Fair Value |
Weighted- Average Remaining Contractual Life |
Aggregate Intrinsic Value (in thousands) |
||||||||||||||||
| Vested |
— | $ | — | $ | — | — | $ | — | ||||||||||||
| Unvested |
124,627 | 30.51 - 35.37 | 30.60 | 5,567 | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Outstanding, December 31, 2015 |
124,627 | $ | 30.51 - 35.37 | $ | 30.60 | 8.90 years | $ | 5,567 | ||||||||||||
| Granted |
198,538 | 36.24 - 52.78 | 39.06 | 9,053 | ||||||||||||||||
| Vested |
(64,806 | ) | 29.40 - 47.03 | 34.12 | 2,481 | |||||||||||||||
| Forfeited |
(19,880 | ) | 30.51 - 36.24 | 34.47 | 907 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Outstanding, December 31, 2016 |
238,479 | $ | 29.40 - 52.78 | $ | 36.36 | 8.43 years | $ | 10,875 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Vested |
— | — | — | — | — | |||||||||||||||
| Unvested |
238,479 | 29.40 - 52.78 | 36.36 | 8.43 years | 10,875 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Vested or expected to vest, December 31, 2016 |
236,134 | $ | 29.40 - 52.78 | $ | 36.29 | 8.43 years | $ | 10,768 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Exercisable, December 31, 2016 |
— | $ | — | $ | — | — | $ | — | ||||||||||||
The following table summarizes the RSU grant and unrecognized compensation expense as of December 31, 2016. RSUs granted to employees vest annually over a three-year period; Cynosure recognizes the related compensation expense on a straight-line basis over the three-year period. RSUs granted to non-employee directors vest quarterly over a one-year period; Cynosure recognizes the related compensation expense on a straight-line basis over the one-year period.
| Number of
RSUs Granted |
Unrecognized Stock-based Compensation at December 31, 2016 (in thousands) |
Weighted Average Period for Recognition of Unrecognized Compensation |
||||||||||
| As of December 31, 2016 |
390,181 | $ | 8,669 | 2.0 years | ||||||||
|
|
|
|
|
|
|
|||||||
F-25
Table of Contents
Performance- Based Stock Units
PSU activity under the 2005 Plan is as follows:
| Number of PSUs(1) |
Weighted Average Award-Issuance Fair Value |
|||||||
| Outstanding, December 31, 2015 |
— | $ | — | |||||
| Granted |
180,855 | 40.23 | ||||||
| Vested |
(16,188 | ) | 36.24 | |||||
| Forfeited |
(24,834 | ) | 36.24 | |||||
|
|
|
|||||||
| Outstanding, December 31, 2016 |
139,833 | $ | 41.39 | |||||
|
|
|
|||||||
| (1) | Number of PSUs listed at the maximum amounts. Pursuant to the terms of the Merger Agreement, each then-outstanding Cynosure PSU award will vest and be cancelled and converted into the right to receive the Offer Price in respect of the maximum number of Shares underlying such PSU award. |
10. Income Taxes
Income before income tax provision (benefit) consists of the following:
| 2016 | 2015 | 2014 | ||||||||||
| (In thousands) | ||||||||||||
| Domestic |
$ | 22,388 | $ | 20,667 | $ | 15,424 | ||||||
| Foreign |
2,555 | 2,531 | 2,914 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 24,943 | $ | 23,198 | $ | 18,338 | ||||||
|
|
|
|
|
|
|
|||||||
The provision (benefit) for income taxes consists of:
| 2016 | 2015 | 2014 | ||||||||||
| (In thousands) | ||||||||||||
| Current: |
||||||||||||
| Federal |
$ | 9,531 | $ | 6,133 | $ | 734 | ||||||
| State |
1,751 | 1,404 | 577 | |||||||||
| Foreign |
1,854 | 920 | 1,306 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current |
13,136 | 8,457 | 2,617 | |||||||||
| Deferred: |
||||||||||||
| Federal |
(558 | ) | 701 | (13,032 | ) | |||||||
| State |
(1,054 | ) | (1,234 | ) | (1,398 | ) | ||||||
| Foreign |
(782 | ) | (533 | ) | (1,187 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total deferred |
(2,394 | ) | (1,066 | ) | (15,617 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| $ | 10,742 | $ | 7,391 | $ | (13,000 | ) | ||||||
|
|
|
|
|
|
|
|||||||
F-26
Table of Contents
A reconciliation of the federal statutory rate to Cynosure’s effective tax rate is as follows for the years ended December 31:
| 2016 | 2015 | 2014 | ||||||||||
| Income tax provision at federal statutory rate: |
35.0 | % | 35.0 | % | 35.0 | % | ||||||
| (Decrease) increase in tax resulting from - |
||||||||||||
| State taxes, net of federal benefit |
2.1 | 0.2 | 0.8 | |||||||||
| Nondeductible expenses |
2.6 | 1.5 | 6.7 | |||||||||
| Tax-exempt interest income |
(0.2 | ) | — | (0.2 | ) | |||||||
| Effect of foreign taxes |
(0.9 | ) | (1.4 | ) | (0.6 | ) | ||||||
| Stock-based compensation |
0.3 | 0.1 | 0.1 | |||||||||
| Research and development credit |
(4.2 | ) | (2.4 | ) | (2.7 | ) | ||||||
| Change in uncertain tax positions |
— | — | (0.3 | ) | ||||||||
| Change in valuation allowance |
0.1 | (3.0 | ) | (108.8 | ) | |||||||
| Change in control payments |
— | — | 1.6 | |||||||||
| Unremitted earnings |
(1.0 | ) | 1.4 | — | ||||||||
| Domestic manufacturing deduction |
(1.5 | ) | (2.0 | ) | — | |||||||
| Non-deductible officer compensation |
9.1 | 2.7 | 0.5 | |||||||||
| Other |
1.7 | (0.2 | ) | (3.0 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Effective income tax rate |
43.1 | % | 31.9 | % | (70.9 | )% | ||||||
|
|
|
|
|
|
|
|||||||
In 2016, Cynosure recorded an income tax provision of $10.7 million, representing an effective tax rate of 43.1%. The effective tax rate, in comparison to the U.S. statutory rate of 35%, is favorably impacted by the jurisdictional mix of worldwide earnings, the domestic manufacturing deduction and federal research credits. The effective tax rate is unfavorably impacted by non-deductible expenses (primarily related to disallowed officers’ compensation). The year-over-year increase in the effective tax rate is primarily due to the increase in non-deductible expenses and the impact of a release of valuation allowance against certain foreign deferred tax assets during 2015.
Significant components of Cynosure’s deferred tax assets and liabilities as of December 31, 2016 and 2015 are as follows:
| 2016 | 2015 | |||||||
| (In thousands) | ||||||||
| Deferred tax assets: |
||||||||
| Accrued expenses and reserves |
$ | 13,576 | $ | 9,002 | ||||
| Domestic net operating loss & tax credit carry-forwards |
8,858 | 12,500 | ||||||
| Foreign net operating loss carry-forwards |
1,216 | 1,851 | ||||||
| Stock-based compensation |
3,707 | 5,561 | ||||||
| Capital leases |
7,435 | 6,694 | ||||||
| Other deferred tax assets |
3,281 | 3,105 | ||||||
|
|
|
|
|
|||||
| Gross deferred tax assets |
$ | 38,073 | $ | 38,713 | ||||
| Valuation allowance |
(277 | ) | (251 | ) | ||||
|
|
|
|
|
|||||
| Total deferred tax assets (after valuation allowance) |
$ | 37,796 | $ | 38,462 | ||||
| Long-term deferred tax liabilities: |
||||||||
| Intangible assets |
$ | (10,444 | ) | $ | (12,733 | ) | ||
| Fixed assets |
(7,101 | ) | (6,782 | ) | ||||
| Other deferred tax liabilities |
(417 | ) | (1,065 | ) | ||||
|
|
|
|
|
|||||
| Total long-term deferred tax liabilities |
$ | (17,962 | ) | $ | (20,580 | ) | ||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | 19,834 | $ | 17,882 | ||||
|
|
|
|
|
|||||
F-27
Table of Contents
Cynosure’s valuation allowance increased by $26,000 during the 2016 period, which is due to an increase in tax attributes that the Company believes are not more-likely-than-not realizable. The Company has considered several sources of taxable income in making its valuation allowance assessment including taxable income in carryback years, future reversals of existing taxable temporary differences, tax planning strategies and forecasted future income. As of December 31, 2016, a full valuation allowance is maintained on the net deferred tax assets of its subsidiary in Mexico.
At December 31, 2016, Cynosure has domestic federal net operating loss carryforwards of approximately $7.6 million, federal tax credit carryforwards of $4.9 million and state tax credit carryforwards of $3.3 million that are available to reduce future taxable income. Utilization of the federal net operating losses and federal tax credits are subject to an annual limitation due to the ownership change limitations set forth under Internal Revenue Code Sections 381, 382 and 383. At December 31, 2016, none of the federal net operating loss carryforwards, none of the federal tax credit carryforwards and $0.2 million of the state tax credit carryforwards relate to excess stock based compensation tax benefits. The Company will record the $0.2 million of off balance sheet state tax credits as a deferred tax asset with an offset to retained earnings upon the adoption of ASU 2016-09. The federal net operating losses begin to expire in 2033. The federal and state tax credits begin to expire in 2018.
At December 31, 2016, Cynosure has foreign net operating losses of approximately $4.2 million in Germany, Mexico, Japan, France, and Spain that are available to reduce future income. Foreign net operating losses in Germany and France do not expire. Mexican net operating losses will begin to expire in 2019, Japanese net operating losses will begin to expire in 2021 and Spanish net operating losses will begin to expire in 2029.
As of December 31, 2016, the Company has provided a deferred tax liability of $0.1 million associated with U.S. income taxes and foreign withholding taxes on approximately $0.6 million of unremitted earnings which are not indefinitely invested. The Company has not provided U.S. income taxes or foreign withholding taxes on unremitted foreign earnings of approximately $19.7 million as such amounts are considered to be indefinitely reinvested in the business. The accumulated earnings in the foreign subsidiaries are primarily utilized to fund working capital requirements as Cynosure’s subsidiaries continue to expand their operations. Due to the complexities of the U.S. tax law, including the effect of the U.S. foreign tax credits, it is not practicable to estimate the amount of income taxes payable on the unremitted earnings that are considered indefinitely reinvested.
ASC 740 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements by prescribing a minimum recognition threshold and measurement of a tax position taken or expected to be taken in a tax return.
The aggregate changes in gross unrecognized tax benefits during the years ended December 31, 2016, 2015 and 2014 were as follows (in thousands):
| 2016 | 2015 | 2014 | ||||||||||
| Balance at beginning of year |
$ | 810 | $ | 810 | $ | 1,701 | ||||||
| Decreases for acquired tax positions within measurement window |
— | — | (815 | ) | ||||||||
| Decreases for tax positions taken in prior periods |
— | — | (76 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Balance at end of year |
$ | 810 | $ | 810 | $ | 810 | ||||||
|
|
|
|
|
|
|
|||||||
At December 31, 2016, 2015, and 2014, Cynosure had gross tax-effected unrecognized tax benefits of $0.8 million of which the entire amount, if recognized, would favorably impact the effective tax rate. Cynosure classifies interest and penalties related to income taxes as a component of its provision for income taxes, and the amount of interest and penalties recorded as of December 31, 2016 and 2015 in the statements of operations and balance sheet was immaterial. Cynosure does not expect any material changes in the amounts of unrecognized tax benefits over the next 12 months.
F-28
Table of Contents
Cynosure files income tax returns in the U.S. federal jurisdiction, and in various state and foreign jurisdictions. Cynosure is no longer subject to U.S. federal tax examinations for years prior to 2013. With few exceptions, Cynosure is no longer subject to U.S. state and local income tax examinations by tax authorities for years before 2012. Additionally, certain non-U.S. jurisdictions are no longer subject for income tax examinations by tax authorities for years before 2012.
11. 401(k) Plan
Cynosure sponsors the Cynosure 401(k) defined contribution plan. Participation in the plan is available to all employees of Cynosure who meet certain eligibility requirements. The 401(k) plan is qualified under Section 401(k) of the Internal Revenue Code, and is subject to contribution limitations as set annually by the Internal Revenue Service. Employer matching contributions are at Cynosure’s discretion. Cynosure’s contributions to this plan totaled approximately $1.9 million, $1.3 million and $0.9 million for the years ended December 31, 2016, 2015 and 2014, respectively.
12. Commitments and Contingencies
Lease Commitments
Cynosure leases the land portion of its U.S. operating facility and certain U.S. and foreign facilities under non-cancelable operating lease agreements expiring through May 2028. These leases are non-cancellable and typically contain renewal options. Certain leases contain rent escalation clauses for which Cynosure recognizes the expense on a straight-line basis. Rent expense for the years ended December 31, 2016, 2015 and 2014 was approximately $2.3 million, $2.1 million and $3.2 million, respectively. The 2014 period rent expense includes rent expense on the former Palomar headquarters, which Cynosure occupied through July 2014.
Cynosure leases the buildings portion of its U.S. operating facility and certain equipment and vehicles under capital lease agreements with payments due through May 2028. Commitments under Cynosure’s lease arrangements are as follows as of December 31, 2016 (in thousands):
| Operating Leases |
Capital Leases |
|||||||
| 2017 |
$ | 2,220 | $ | 2,960 | ||||
| 2018 |
1,966 | 2,875 | ||||||
| 2019 |
1,661 | 2,810 | ||||||
| 2020 |
817 | 2,791 | ||||||
| 2021 |
562 | 2,923 | ||||||
| Thereafter |
2,930 | 19,732 | ||||||
|
|
|
|
|
|||||
| Total minimum lease payments |
$ | 10,156 | $ | 34,091 | ||||
|
|
|
|
|
|||||
| Less amount representing interest |
(14,518 | ) | ||||||
|
|
|
|||||||
| Present value of obligations under capital leases |
$ | 19,573 | ||||||
| Current portion of capital lease obligations |
866 | |||||||
|
|
|
|||||||
| Capital lease obligations, net of current portion |
$ | 18,707 | ||||||
|
|
|
|||||||
Purchase Commitments
Cynosure has entered into two distribution agreements with a contract manufacturer that expire in October 2019 and November 2021. Each agreement automatically renews for additional one-year terms unless either party provides notice of termination at least six months prior to the expiration of the initial term or any subsequent renewal term. The distribution agreements have annual minimum purchase obligations and may be
F-29
Table of Contents
terminated by the contract manufacturer if Cynosure does not meet the annual minimum purchase obligations. The future annual minimum purchase obligations can be reduced if Cynosure exceeds the purchase limits in a given year as stated in the distribution agreement.
Contingencies
Cynosure continually assesses litigation to determine if an unfavorable outcome would lead to a probable loss or reasonably possible loss, which could be estimated. In accordance with the Financial Accounting Standards Board’s (FASB) guidance on accounting for contingencies, Cynosure accrues for all direct costs associated with the estimated resolution of contingencies at the earliest date at which it is deemed probable that a liability has been incurred and the amount of such liability can be reasonably estimated. If the estimate of a probable loss is a range and no amount within the range is more likely, Cynosure accrues the minimum amount of the range. In cases where Cynosure believes that a reasonably possible loss exists, Cynosure discloses the facts and circumstances of the litigation, including an estimable range, if possible.
On July 27, 2016, ARcare, Inc., individually and as putative representative of a purported nationwide class, filed a complaint against Cynosure in the U.S. District Court for the District of Massachusetts. The plaintiff alleges that Cynosure violated the federal Telephone Consumer Protection Act (TCPA) by sending fax advertisements that did not comply with statutory and Federal Communications Commission (FCC) requirements that senders provide recipients with certain information about how to opt out from receiving faxed advertisements in the future, and by sending unsolicited fax advertisements. The complaint seeks damages, declaratory and injunctive relief, and attorneys’ fees on behalf of a purported class of all recipients of purported fax advertisements that the plaintiff alleges did not receive an adequate opt-out notice. The TCPA provides for statutory damages of $500 per violation and gives courts the discretion to increase that amount up to $1,500 for knowing and willful violations. Based on preliminary estimates, Cynosure believes the aggregate number of faxes sent in the four years prior to the lawsuit to be approximately 835,000. On September 30, 2016, Cynosure answered the complaint and denied liability. On September 7, 2016, the plaintiff sent a demand letter seeking a class settlement for statutory damages under Massachusetts General Laws, Chapter 93A § 9, and on October 7, 2016, Cynosure responded denying any liability under Chapter 93A, but offering the plaintiff statutory damages under Chapter 93A on an individual basis. To date, the plaintiff has not moved to amend the complaint to assert a claim under Chapter 93A. Cynosure and the plaintiff have agreed to explore a possible class settlement of the litigation in a mediation. On October 12, 2016, the court allowed the parties’ request to stay the case until January 11, 2017 while they pursued mediation. In January 2017, Cynosure and ARcare entered into an agreement to settle the lawsuit. The settlement agreement was subject to court approval, and, if approved, would have given Cynosure a full release from the settlement class concerning the conduct alleged in the complaint, in exchange for which Cynosure would have paid settlement compensation between $6.5 million and $16.0 million depending on the number of class members to file a valid claim. The court overseeing the matter subsequently expressed concern about aspects of the settlement agreement, and in February 2017, Cynosure and ARcare agreed in principle to new terms to be incorporated into a new settlement agreement that is subject to further negotiation, and thereafter will be subject to court approval. As with the initial settlement, if approved, Cynosure would pay a fixed settlement compensation amount of $8.5 million and would receive a full release from the settlement class concerning the conduct alleged in the complaint.
The terms of the original January 2017 settlement indicated that it was probable that a liability would be incurred and that it could be reasonably estimated. In connection with the original January 2017 settlement agreement, Cynosure recorded a $7.2 million charge in the fourth quarter of 2016 in connection with the settlement agreement, including a $6.5 million estimated settlement plus expenses associated with this matter. As a result of the new settlement terms, Cynosure subsequently increased that charge by $2.0 million, also effective in the fourth quarter of 2016, to a total of $9.2 million. This $9.2 million represents Cynosure’s best estimate of the total settlement expense to be incurred in connection with the ARcare matter.
F-30
Table of Contents
13. Summary Selected Quarterly Financial Data (Unaudited)
The following table sets forth certain unaudited consolidated quarterly statements of operations data for the eight quarters ended December 31, 2016. This information is unaudited, but in the opinion of management, it has been prepared on the same basis as the audited consolidated financial statements and all necessary adjustments, consisting only of normal recurring adjustments, have been included in the amounts stated below to state fairly the unaudited consolidated quarterly results of operations. The results of operations for any quarter are not necessarily indicative of the results of operations for any future period.
| Quarter Ended | ||||||||||||||||
| March 31, 2016 |
June 30, 2016 |
Sept. 30, 2016 |
Dec. 31, 2016 |
|||||||||||||
| (In thousands, except per share data) | ||||||||||||||||
| Revenues |
$ | 94,664 | $ | 110,343 | $ | 106,386 | $ | 122,139 | ||||||||
| Gross profit |
$ | 55,420 | $ | 65,057 | $ | 62,810 | $ | 72,024 | ||||||||
| Income from operations |
$ | 4,451 | $ | 10,500 | $ | 8,162 | $ | 4,227 | ||||||||
| Net income |
$ | 2,847 | $ | 6,340 | $ | 4,163 | $ | 851 | ||||||||
| Basic net income per share |
$ | 0.13 | $ | 0.27 | $ | 0.18 | $ | 0.04 | ||||||||
| Diluted net income per share |
$ | 0.12 | $ | 0.27 | $ | 0.17 | $ | 0.04 | ||||||||
| Quarter Ended | ||||||||||||||||
| March 31, 2015 |
June 30, 2015 |
Sept. 30, 2015 |
Dec. 31, 2015 |
|||||||||||||
| (In thousands, except per share data) | ||||||||||||||||
| Revenues |
$ | 74,912 | $ | 83,694 | $ | 78,414 | $ | 102,442 | ||||||||
| Gross profit |
$ | 42,773 | $ | 47,356 | $ | 44,405 | $ | 59,000 | ||||||||
| Income from operations |
$ | 2,053 | $ | 7,641 | $ | 4,835 | $ | 11,792 | ||||||||
| Net income (loss) |
$ | (8 | ) | $ | 5,358 | $ | 3,229 | $ | 7,228 | |||||||
| Basic net income (loss) per share |
$ | (0.00 | ) | $ | 0.24 | $ | 0.14 | $ | 0.32 | |||||||
| Diluted net income (loss) per share |
$ | (0.00 | ) | $ | 0.24 | $ | 0.14 | $ | 0.31 | |||||||
14. Subsequent Events
On February 14, 2017, Cynosure and Hologic entered into the Merger Agreement. Pursuant to the terms of Merger Agreement, the Purchaser commenced the Offer on February 22, 2017 for any (subject to the minimum condition) and all of the Shares at the Offer Price, net to the seller in cash, without interest, subject to any required withholding of taxes. The Offer will remain open for a minimum of 20 business days from the date of commencement.
The obligation of the Purchaser to purchase Shares tendered in the Offer is subject to customary closing conditions, including (i) Shares having been validly tendered and not withdrawn that represent at least a majority of the total number of Shares then outstanding, (ii) the expiration or termination of applicable waiting periods under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, (iii) approval from the German Federal Cartel Office under the German Act Against Restraints of Competition having been obtained, (iv) the absence of injunctions or other legal restraints preventing the consummation of the Offer or the Merger, as defined below, (v) the accuracy of representations and warranties made by Cynosure in the Merger Agreement, (vi) compliance by Cynosure with its covenants in the Merger Agreement, and (vii) other conditions set forth in Annex I to the Merger Agreement. The consummation of the Offer is not subject to any financing conditions.
Following the completion of the Offer, subject to the absence of injunctions or other legal restraints preventing the consummation of the Merger, the Purchaser will merge with and into Cynosure, with Cynosure surviving as a wholly-owned subsidiary of Hologic, pursuant to the procedure provided for under Section 251(h) of the Delaware General Corporation Law, without any additional stockholder approvals. The Merger will be effected as soon as practicable following the time of purchase by the Purchaser of Shares validly tendered and not withdrawn in the Offer.
F-31
Table of Contents
At the effective time of the Merger (the “Effective Time”), each issued and outstanding Share (other than Shares owned by (i) Cynosure, Hologic, the Purchaser, or any other subsidiary of Hologic, which Shares will be cancelled and will cease to exist, (ii) any subsidiary of Cynosure, which Shares will be converted into such number of shares of common stock of the surviving corporation so as to maintain relative ownership percentages or (iii) stockholders who validly exercise appraisal rights under Delaware law with respect to such Shares) will be converted into the right to receive an amount in cash equal to the Offer Price, without interest, subject to any required withholding taxes.
Pursuant to the terms of the Merger Agreement, as of immediately prior to the Effective Time, (i) each then-outstanding Cynosure stock option will vest in full and be cancelled and converted into a right to receive the Offer Price (less the applicable exercise price) in respect of each Share underlying such stock option, (ii) each then outstanding Cynosure restricted stock unit award will vest and be cancelled and converted into the right to receive the Offer Price in respect of each Share underlying such restricted stock unit award, and (iii) each then-outstanding Cynosure PSU award will vest and be cancelled and converted into the right to receive the Offer Price in respect of the maximum number of Shares underlying such PSU award.
In the Merger Agreement, Cynosure has agreed, among other things, (i) to use commercially reasonable efforts to conduct its business in the ordinary course during the period between the execution of the Merger Agreement and the consummation of the Merger; (ii) subject to certain customary exceptions set forth in the Merger Agreement to permit Cynosure’s board of directors to comply with its fiduciary duties, to recommend that Cynosure’s stockholders accept the Offer and tender their shares pursuant to the Offer; and (iii) not to solicit alternative acquisition proposals and to certain restrictions on its ability to respond to any such proposals. The Merger Agreement also contains customary representations, warranties and covenants of Cynosure, Hologic and the Purchaser.
Cynosure expects the Merger to close in late March or April of 2017. The Merger Agreement contains customary termination rights for both Cynosure and Hologic, including, among others, for failure to consummate the Offer on or before August 14, 2017.
F-32
Table of Contents
EXHIBIT INDEX
| Exhibit Number |
Description | |
| 2.1 | Amended and Restated Agreement and Plan of Merger, dated as of May 15, 2013, among the Company, Commander Acquisition, LLC and Palomar Medical Technologies, Inc. (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed May 16, 2013) | |
| 2.2 | Asset Purchase Agreement, dated as of September 5, 2014, among the Company, Ellman International, Inc., Ellman Holdings, Inc., and Ellman Holding Corporation (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed September 8, 2014) | |
| 2.3** | Agreement and Plan of Merger, dated as of February 14, 2017, by and among the Company, Hologic, Inc. and Minuteman Merger Sub, Inc. (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed February 14, 2017) | |
| 3.1 | Second Restated Certificate of Incorporation of the Company (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed May 16, 2016) | |
| 3.2 | Amended and Restated By-laws of the Company (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed May 16, 2016) | |
| 3.2 | Amendment to Amended and Restated By-laws of the Company (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed February 14, 2017) | |
| 4.1 | Specimen certificate evidencing shares of Class A common stock (Incorporated by reference to the exhibits to Amendment No. 1 of the Company’s Registration Statement on Form S-1 filed November 3, 2005 (333-127463)) | |
| 10.1* | 2004 Stock Option Plan, as amended (Incorporated by reference to the exhibits to the Company’s Registration Statement on Form S-1 filed August 11, 2005 (333-127463)) | |
| 10.2* | Amended and Restated 2005 Stock Incentive Plan (Incorporated by reference to the exhibits to the Company’s Quarterly Report on Form 10-Q filed August 9, 2013) | |
| 10.3* | Employment Agreement, dated December 15, 2008, between the Company and Michael Davin (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed December 19, 2008) | |
| 10.4* | First Amendment to Employment Agreement, dated December 20, 2010, between the Company and Michael Davin (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed December 21, 2010) | |
| 10.5* | Employment Agreement, dated December 15, 2008, between the Company and Douglas Delaney (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed December 19, 2008) | |
| 10.6† | Distribution Agreement, effective as of October 26, 2012, between the Company and El.En. S.p.A. (Incorporated by reference to the exhibits to the Company’s Annual Report on Form 10-K filed March 8, 2013) | |
| 10.7 | Lease, dated January 31, 2005, between Glenborough Fund V, Limited Partnership and the Company, as amended (Incorporated by reference to the exhibits to the Company’s Annual Report on Form 10-K filed March 7, 2012) | |
| 10.8 | Non-Exclusive Patent License, dated November 6, 2006, between Palomar Medical Technologies, Inc. and the Company (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed November 7, 2006) | |
| 10.9* | Employment Agreement, dated December 15, 2008, between the Company and Timothy W. Baker (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed December 19, 2008) | |
Table of Contents
| Exhibit Number |
Description | |
| 10.10* | Second Amendment to Employment Agreement, entered into as of July 20, 2011, by and between the Company and Michael R. Davin (Incorporated by reference to the exhibits to the Company’s Annual Report on Form 10-K filed March 13, 2015) | |
| 10.11* | Third Amendment to Employment Agreement, entered into as of November 5, 2013, by and between the Company and Michael R. Davin (Incorporated by reference to the exhibits to the Company’s Quarterly Report on Form 10-Q filed November 12, 2013) | |
| 10.12* | First Amendment to Employment Agreement, entered into as of November 5, 2013, by and between the Company and Timothy W. Baker (Incorporated by reference to the exhibits to the Company’s Quarterly Report on Form 10-Q filed November 12, 2013) | |
| 10.13* | First Amendment to Employment Agreement, entered into as of November 7, 2013, by and between the Company and Douglas J. Delaney (Incorporated by reference to the exhibits to the Company’s Quarterly Report on Form 10-Q filed November 12, 2013) | |
| 10.14* | Employment Agreement, dated March 17, 2003, between the Company and Joseph P. Caruso (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed March 18, 2013) | |
| 10.15 | Purchase and Sale Agreement, dated November 25, 2013, Palomar Medical Technologies, LLC and Network Drive Owner, LLC (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed December 2, 2013) | |
| 10.16 | Fourth Amendment to the Lease, dated December 20, 2012, between the Company and Glenborough Westford Center, LLC (Incorporated by reference to the exhibits to the Company’s Annual Report on Form 10-K filed March 13, 2015) | |
| 10.17 | Fifth Amendment to Lease, dated November 18, 2013, between the Company and Glenborough Westford Center, LLC (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed November 22, 2013) | |
| 10.18* | Separation Agreement, effective September 6, 2013, between the Company and Palomar Medical Technologies, LLC and Joseph P. Caruso (Incorporated by reference to the exhibits to the Company’s Annual Report on Form 10-K filed March 17, 2014) | |
| 10.19* | Form of Nonstatutory Stock Option Agreement under 2005 Stock Incentive Plan (Incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed August 3, 2006) | |
| 10.20* | Form of Incentive Stock Option Agreement under 2005 Stock Incentive Plan (Incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed August 3, 2006) | |
| 10.21* | Form of Restricted Stock Unit Agreement under 2005 Stock Incentive Plan (Incorporated by reference to the exhibits to the Company’s Annual Report on Form 10-K filed March 13, 2015) | |
| 10.22 | Sixth Amendment to Lease, dated April 16, 2015, between the Company and Glenborough Westford Center, LLC (Incorporated by reference to the exhibits to the Company’s Quarterly Report on Form 10-Q filed May 7, 2015) | |
| 10.23* | Non-Equity Incentive Plan (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed February 5, 2016) | |
| 10.24* | Form of Performance-Based Stock Unit Award under 2005 Stock Incentive Plan (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed February 5, 2016) | |
| 10.25 | Seventh Amendment to Lease, dated April 29, 2016, between the Company and Curo Westford, LLC (Incorporated by reference to the exhibits to the Company’s Quarterly Report on Form 10-Q filed May 10, 2016) | |
Table of Contents
| Exhibit Number |
Description | |
| 10.26 | Extension of Dates in Seventh Amendment to Lease, dated June 30, 2016, between the Company and Curo Westford, LLC (Incorporated by reference to the exhibits to the Company’s Quarterly Report on Form 10-Q filed August 5, 2016) | |
| 10.27† | Exclusive Distribution Agreement, dated November 14, 2014, between the Company and El.En. S.p.A. (Incorporated by reference to the exhibits to the Company’s Quarterly Report on Form 10-Q filed August 5, 2016) | |
| 10.28 | Restated and Amended External Manufacturing Agreement, dated April 25, 2016, between the Company and Columbia Electrical Contractors, Inc. (Incorporated by reference to the exhibits to the Company’s Quarterly Report on Form 10-Q filed August 5, 2016) | |
| 10.29 | Offer Letter, dated September 1, 2016 between the Company and Stephen J. Webber (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed September 13, 2016) | |
| 10.30 | Consulting Agreement, dated September 12, 2016, between the Company and Timothy W. Baker (Incorporated by reference to the exhibits to the Company’s Current Report on Form 8-K filed September 13, 2016) | |
| 10.31 | Lease, dated January 18, 2017, between the Company and Ryan Development, LLC | |
| 21.1 | Subsidiaries of the Company | |
| 23.1 | Consent of Ernst & Young LLP | |
| 31.1 | Certification of the Principal Executive Officer | |
| 31.2 | Certification of the Principal Financial Officer | |
| 32.1 | Certification of the Principal Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2 | Certification of the Principal Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101 | The following materials from the Cynosure, Inc. Annual Report on Form 10-K for the year ended December 30, 2016, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets at December 31, 2016 and December 31, 2015, (ii) Consolidated Statements of Operations for the years ended December 31, 2016, 2015 and 2014, (iii) Consolidated Statements of Comprehensive Income for the years ended December 31, 2016, 2015 and 2014, (iv) Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2016, 2015 and 2014, (v) Consolidated Statements of Cash Flows for the years ended December 31, 2016, 2015 and 2014, and (vi) Notes to Consolidated Financial Statements. | |
| ** | Schedules have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The Company hereby undertakes to furnish supplemental copies of any of the omitted schedules upon request by the Securities and Exchange Commission. |
| * | Management contract or compensation plan or arrangement required to be filed as an exhibit pursuant to Item 15(b) of Form 10-K. |
| † | Confidential treatment has been granted as to certain portions, which portions have been omitted and filed separately with the Securities and Exchange Commission. |
