Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - Seagen Inc. | d481013dex322.htm |
| EX-32.1 - EX-32.1 - Seagen Inc. | d481013dex321.htm |
| EX-31.2 - EX-31.2 - Seagen Inc. | d481013dex312.htm |
| EX-31.1 - EX-31.1 - Seagen Inc. | d481013dex311.htm |
| EX-23.1 - EX-23.1 - Seagen Inc. | d481013dex231.htm |
| EX-21.1 - EX-21.1 - Seagen Inc. | d481013dex211.htm |
| EX-10.56 - EX-10.56 - Seagen Inc. | d481013dex1056.htm |
| EX-10.35 - EX-10.35 - Seagen Inc. | d481013dex1035.htm |
| EX-10.29 - EX-10.29 - Seagen Inc. | d481013dex1029.htm |
| EX-10.18 - EX-10.18 - Seagen Inc. | d481013dex1018.htm |
| EX-10.17 - EX-10.17 - Seagen Inc. | d481013dex1017.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 0-32405

Seattle Genetics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 91-1874389 | |
| (State or other Jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
21823 30th Drive SE
Bothell, WA 98021
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code: (425) 527-4000
Securities registered pursuant to Section 12(b) of the Act:
| Title of class |
Name of each exchange on which registered | |
| Common Stock, par value $0.001 | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☒ NO ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES ☒ NO ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☒ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☐ (Do not check if smaller reporting company) | Smaller reporting company | ☐ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $3,833,493,519 as of the last business day of the registrant’s most recently completed second fiscal quarter, based upon the closing sale price on The NASDAQ Global Select Market reported for such date. Excludes an aggregate of 45,611,169 shares of the registrant’s common stock held as of such date by officers, directors and stockholders that the registrant has concluded are or were affiliates of the registrant. Exclusion of such shares should not be construed to indicate that the holder of any such shares possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant or that such person is controlled by or under common control with the registrant.
There were 142,493,676 shares of the registrant’s Common Stock issued and outstanding as of February 16, 2017.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates information by reference from the registrant’s definitive proxy statement to be filed with the Securities and Exchange Commission pursuant to Regulation 14A, not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K, in connection with the Registrant’s 2017 Annual Meeting of Stockholders.
Table of Contents
SEATTLE GENETICS, INC.
FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2016
i
Table of Contents
PART I
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including those relating to future events or our future financial performance and financial guidance. In some cases, you can identify forward-looking statements by terminology such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “project,” “believe,” “estimate,” “predict,” “potential,” “intend” or “continue,” the negative of terms like these or other comparable terminology, and other words or terms of similar meaning in connection with any discussion of future operating or financial performance. These statements are only predictions. All forward-looking statements included in this Annual Report on Form 10-K are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements. Any or all of our forward-looking statements in this document may turn out to be wrong. Actual events or results may differ materially. Our forward-looking statements can be affected by inaccurate assumptions we might make or by known or unknown risks, uncertainties and other factors. We discuss many of these risks, uncertainties and other factors in this Annual Report on Form 10-K in greater detail under the heading “Item 1A—Risk Factors.” We caution investors that our business and financial performance are subject to substantial risks and uncertainties.
Overview
Seattle Genetics is a biotechnology company focused on the development and commercialization of targeted therapies for the treatment of cancer. Our marketed product ADCETRIS®, or brentuximab vedotin, is now approved by the United States Food and Drug Administration, or FDA, and the European Commission for three indications, encompassing several settings for the treatment of relapsed Hodgkin lymphoma and relapsed systemic anaplastic large cell lymphoma, or sALCL. ADCETRIS is commercially available in 66 countries around the world, including in the United States, Canada, members of the European Union and Japan. We are collaborating with Takeda Pharmaceutical Company Limited, or Takeda, to develop and commercialize ADCETRIS on a global basis. Under this collaboration, Seattle Genetics retains commercial rights for ADCETRIS in the United States and its territories and in Canada, and Takeda has commercial rights in the rest of the world.
Beyond our current labeled indications, we and Takeda have a broad development strategy for ADCETRIS evaluating its therapeutic potential in earlier lines of therapy for patients with Hodgkin lymphoma or mature T-cell lymphoma, or MTCL, also known as peripheral T-Cell lymphoma, or PTCL, including sALCL, and in other CD30-expressing malignancies. We and Takeda are currently conducting three phase 3 clinical trials of ADCETRIS: ALCANZA, ECHELON-1 and ECHELON-2. All of these trials are being conducted under Special Protocol Assessment, or SPA, agreements with the FDA and pursuant to scientific advice from the European Medicines Agency, or EMA. An SPA is an agreement with the FDA regarding the design of the clinical trial, including size and clinical endpoints, to support an efficacy claim in a new drug application or a Biologics License Application, or BLA, submission to the FDA if the trial achieves its primary endpoints. We plan to submit a supplemental Biologics License Application, or sBLA, to the FDA in mid-2017 to seek approval for a new indication in CD30-expressing relapsed CTCL. We have also completed enrollment of 1,334 patients in our ECHELON-1 trial and expect to report data in 2017. In November 2016, we completed enrollment of 452 patients in our ECHELON-2 trial, and expect to report data in 2018.
We are also advancing the development of SGN-CD33A, or vadastuximab talirine. A phase 3 clinical trial, called the CASCADE trial, was initiated in the second quarter of 2016 based on data from our phase 1 clinical trial for patients with acute myeloid leukemia, or AML. The CASCADE trial is evaluating SGN-CD33A in
1
Table of Contents
combination with hypomethylating agents, or HMAs, in previously untreated older patients with AML who are not candidates for intensive induction chemotherapy. We also have been evaluating SGN-CD33A in additional treatment settings, and overall, more than 300 patients have been treated with SGN-CD33A to date in clinical trials across these multiple treatment settings. On December 27, 2016, we announced that we had received notice from the FDA that a full clinical hold or partial clinical hold had been placed on several early stage trials of SGN-CD33A in AML to evaluate the potential risk of hepatotoxicity following adverse medical events, including fatal events. We are working diligently with the FDA to determine whether there is any association between hepatotoxicity and treatment with SGN-CD33A and to promptly identify appropriate measures for patient safety with the goal of addressing the FDA’s concerns.
In addition, in collaboration with Astellas Pharma, Inc., or Astellas, we are developing ASG-22ME, or enfortumab vedotin. We and Astellas are planning discussions with regulatory agencies during 2017 to advance the program into potential registrational trials in urothelial cancer patients, including patients who have been previously treated with a checkpoint inhibitor therapy.
Our clinical-stage pipeline also includes six other antibody-drug conjugate, or ADC, programs consisting of SGN-LIV1A, SGN-CD19A, or denintuzumab mafodotin, SGN-CD19B, SGN-CD123A, SGN-CD352A, and ASG-15ME, as well as two immuno-oncology agents, SEA-CD40, which is based on our sugar-engineered antibody, or SEA, technology, and SGN-2FF, which is a novel small molecule. In addition, we have multiple preclinical and research-stage programs that employ our proprietary technologies, including SGN-CD48A, a preclinical ADC that is a candidate for investigational new drug, or IND, submission in 2017.
We announced on February 10, 2017 that we had entered into a development and license agreement, or the Immunomedics License, with Immunomedics, Inc., or Immunomedics, pursuant to which, upon the terms and subject to the conditions set forth in the Immunomedics License, we would receive exclusive worldwide rights to develop, manufacture and commercialize sacituzumab govitecan, or IMMU-132. IMMU-132 is an ADC targeted to TROP-2, which is expressed in several solid tumors, and is in a pivotal phase 1/2 trial for patients with triple negative breast cancer, or TNBC, and is being investigated in other solid tumors. IMMU-132 received Breakthrough Therapy Designation, or BTD, from the FDA for the treatment of patients with TNBC who have failed prior therapies for metastatic disease. In connection with the closing of the transactions contemplated by the Immunomedics License, Immunomedics would receive an upfront payment of $250 million. In addition, Immunomedics would also be eligible to receive development, regulatory and sales-dependent milestone payments across multiple indications and geographical regions of up to a total maximum of approximately $1.7 billion, as well as royalties which are based on a percentage of annual net sales of the licensed products, if any, beginning in the teens and rising to twenty percent based on sales volume. The closing of the transactions contemplated by the Immunomedics License is subject to customary closing conditions, including the expiration of the applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, or the HSR Act, there being no pending court or administrative challenges to the Immunomedics License and there being no court or administrative orders blocking the closing. On February 20, 2017, Immunomedics and we entered into a letter agreement pursuant to which Immunomedics irrevocably waived to the extent applicable to Immunomedics the condition precedent to the closing and effectiveness of the Immunomedics License that there be no pending court or administrative challenges to the transaction. Additionally, under the terms of the Immunomedics License, Immunomedics had the right to continue discussions with a small number of parties that previously expressed interest in licensing IMMU-132 until 11:59 p.m. New York City time on February 19, 2017. If a third party had provided Immunomedics with a financially superior licensing offer, we would have had the right to match any such offer, and if we had decided not to match, Immunomedics would have had the right to accept the superior offer and terminate the Immunomedics License upon payment of a termination fee to us. We have not received notice from Immunomedics of any such third party offers during this limited time period, and on February 21, 2017, Immunomedics announced that it is subject to customary “no-shop” restrictions on its and its representatives’ ability to solicit, discuss or negotiate alternative licensing agreement proposals from third parties with regard to IMMU-132.
2
Table of Contents
On February 13, 2017, we were named a co-defendant in a lawsuit filed by venBio Select Advisors LLC, or venBio, in the Delaware Chancery Court against the members of the board of directors of Immunomedics pursuant to which, among other things, venBio seeks to enjoin the closing of the transactions contemplated by the Immunomedics License. As a result of the pending litigation challenging the transactions contemplated by the Immunomedics License, Immunomedics and we have committed to the Court not to close the transactions contemplated by the Immunomedics License prior to March 10, 2017. We cannot predict the timing or outcome of this lawsuit or the impact it may have on the Immunomedics License or the closing of the transactions contemplated by the Immunomedics License. See “License Agreements—Immunomedics License” below for more information.
We have collaborations for our ADC technology with a number of biotechnology and pharmaceutical companies, including AbbVie Biotechnology Ltd., or AbbVie; Bayer Pharma AG, or Bayer; Celldex Therapeutics, Inc., or Celldex; Genentech, Inc., a member of the Roche Group, or Genentech; GlaxoSmithKline LLC, or GSK; Pfizer, Inc., or Pfizer; and PSMA Development Company LLC, a subsidiary of Progenics Pharmaceuticals Inc., or Progenics. In addition, we have entered into a 50/50 co-development agreement with Agensys, Inc., an affiliate of Astellas, for the development of ADCs, including ASG-22ME. We also have an option for an ADC co-development agreement with Genmab A/S, or Genmab, and a collaboration with Unum Therapeutics, Inc., or Unum, to develop and commercialize novel antibody-coupled T-cell receptor, or ACTR, therapies incorporating our antibodies for the treatment of cancer.
Our Antibody-Drug Conjugate (ADC) Technologies
ADCETRIS and many product candidates in our pipeline of clinical-stage monoclonal antibody-based product candidates utilize our ADC technology. ADCs are monoclonal antibodies that are linked to cytotoxic or cell-killing agents. Our ADCs utilize monoclonal antibodies that internalize within target cells after binding to a specified cell-surface receptor. Enzymes present inside the cell catalyze the release of the cytotoxic agent from the monoclonal antibody, which then results in the desired activity, specific killing of the target cell.
A key component of our ADCs are the linkers that attach the cell-killing agent to the monoclonal antibody, which are designed to hold the cytotoxic agent to the monoclonal antibody until it binds to the cell surface receptor on the target cell and then to release the cytotoxic agent upon internalization within the target cell. This targeted delivery of the cell-killing agent is intended to maximize delivery of the cytotoxic agent to targeted cells while minimizing toxicity to normal tissues. Our ADCs use proprietary auristatins, which are microtubule disrupting agents, or pyrrolobenzodiazepine, or PBD, dimers, which are DNA cross-linkers, as cell-killing agents. The PBD dimer cell-killing agent is stably linked to an antibody using our proprietary, site-specific conjugation technology, resulting in uniform drug-loading of two PBD dimers per antibody. We call this engineered antibody an EC-mAb. In contrast to natural products that are often more difficult to produce and link to antibodies, the cytotoxic drugs used in our ADC’s are synthetically produced and easier to scale for manufacturing. ADCETRIS, SGN-CD33A (vadastuximab talirine), ASG-22ME (enfortumab vedotin), SGN-LIV1A, SGN-CD19A (denintuzumab mafodotin), SGN-CD19B, SGN-CD123A, SGN-CD352A, and ASG-15ME, each utilize our proprietary, auristatin-based or PBD-based ADC technologies. These technologies are also the basis of our corporate collaborations. In addition, we are advancing a preclinical product candidate, SGN-CD48A, which utilizes a novel linker technology, PEG-Glucuronide linker, attached to an auristatin. We own or hold exclusive or partially-exclusive licenses to multiple issued patents and patent applications covering our ADC technology. We continue to evaluate new linkers, antibody formats, and cell-killing agents for use in our ADC programs.
Our Sugar-Engineered Antibody (SEA) Technology
Our proprietary SEA technology is a method to selectively reduce fucose incorporation in monoclonal antibodies as they are produced in cell line-based manufacturing. We believe that this may result in increased effector function and antitumor activity. Our SEA technology is a novel approach to modify the activity of monoclonal antibodies that is complementary to our ADC technology.
3
Table of Contents
A key feature of our SEA technology is that no genetic modification of the antibody-producing cell line is necessary and standard cell culture conditions can be used while maintaining the underlying manufacturing processes, yields and product quality. We believe the SEA approach may be simpler and more cost-effective to implement as compared to existing technologies for enhancing antibody effector function, most of which require development of new cell lines.
SEA-CD40 is a clinical-stage non-fucosylated monoclonal antibody developed using SEA technology. Enhanced binding to effector cells results in crosslinking and activation of CD40 signaling in immune cells. We hypothesize that this increased stimulation of the patient’s own immune cells may result in meaningful antitumor activity. We are developing SEA-CD40 as a novel immuno-oncology agent. A phase 1 clinical trial of SEA-CD40 for solid tumors and hematologic malignancies is ongoing.
Other Technologies
In addition, we utilize other technologies designed to maximize antitumor activity and reduce toxicity of antibody-based therapies. Genetic engineering enables us to produce antibodies that are optimized for their intended uses. For ADCs, we screen and select antibodies that bind to antigens that are differentially expressed on tumor cells versus vital normal tissues, rapidly internalized within target cells and utilize native or engineered conjugation sites to optimize drug attachment. In some cases, we evaluate the use of our monoclonal antibodies and ADCs in combination with conventional chemotherapy and other anticancer agents, which may result in increased antitumor activity.
Our Strategy
Our strategy is to become a leading developer and marketer of targeted therapies for cancer. Key elements of our strategy are to:
| • | Successfully Execute our ADCETRIS Commercial Plan. An important near-term objective is to continue to execute our ADCETRIS commercial plan by driving market penetration and duration of therapy consistent with the current ADCETRIS label. We continue to focus our efforts on commercializing ADCETRIS in the United States and Canada through the coordinated efforts of our sales, marketing, reimbursement and market planning groups. In addition, as of January 31, 2017, ADCETRIS had received marketing authorizations in relapsed Hodgkin lymphoma and sALCL from regulatory authorities in 66 countries, and we are continuing to support Takeda’s efforts to obtain regulatory approvals and conduct commercial launches in additional countries worldwide. |
| • | Expand the Therapeutic Potential of ADCETRIS. We believe ADCETRIS may have applications in earlier lines of therapy for Hodgkin lymphoma and MTCL and in other types of CD30-expressing lymphomas. In this regard, during 2016 we reported data from the ALCANZA trial and intend to submit an sBLA to the FDA in mid-2017 to seek approval for a new indication in CD30-expressing relapsed CTCL. We also have ongoing clinical trials evaluating ADCETRIS in frontline therapy for Hodgkin lymphoma (the ECHELON-1 trial) and MTCL (the ECHELON-2 trial). Clinical trials are also being conducted by us, by our collaborators and as investigator sponsored trials in different CD30-expressing indications, including salvage therapy for patients with Hodgkin lymphoma prior to autologous hematopoietic stem cell transplant, or auto-HSCT, novel combinations of ADCETRIS plus immuno-oncology or other anticancer agents and in other areas of medical and scientific interest. |
| • | Advance Clinical Pipeline of Oncology Drugs. We are employing our clinical, development, regulatory and manufacturing expertise with the goal of advancing our clinical stage product candidates towards regulatory approval and commercialization on a global basis. Our efforts in this regard include: |
| • | Evaluate SGN-CD33A in AML and Myelodysplatic Syndrome Patient Populations. In the second quarter of 2016, we initiated the phase 3 CASCADE trial evaluating SGN-CD33A in combination with HMAs in previously untreated older patients with AML who are not candidates for intensive induction chemotherapy. We are also evaluating SGN-CD33A in additional treatment settings. On December 27, 2016, we announced that we had received notice from the FDA that a full clinical |
4
Table of Contents
| hold or partial clinical hold had been placed on several early stage trials of SGN-CD33A in AML to evaluate the potential risk of hepatotoxicity following adverse medical events, including fatal events. We are working diligently with the FDA to determine whether there is any association between hepatotoxicity and treatment with SGN-CD33A, and to promptly identify appropriate measures for patient safety with the goal of addressing the FDA’s concerns. No new studies of SGN-CD33A will be initiated unless and until the clinical holds are lifted. Our phase 3 CASCADE trial in older AML patients and our phase 1/2 trial in myelodysplastic syndrome, or MDS, are currently proceeding with enrollment. |
| • | Advance ASG-22ME into Registrational Trials for Urothelial Cancer. We and Astellas are planning discussions with regulatory agencies during 2017 to advance the program into potential registrational trials of ASG-22ME in urothelial cancer patients, including patients who have been previously treated with a checkpoint inhibitor therapy. |
| • | Global Expansion. We have established operations in Zug, Switzerland to support clinical trials, regulatory, medical affairs and manufacturing and future potential commercial activities for our pipeline. In 2017, we will continue to develop our European presence in support of our global expansion. |
| • | Continue to Develop our Other Pipeline Programs. We believe that it is important to maintain a diverse pipeline of product candidates to sustain our future growth. To accomplish this, we are continuing to advance the development of our other clinical product candidates, including SGN-LIV1A, SGN-CD19A, SEA-CD40, SGN-CD19B, SGN-CD123A, SGN-CD352A, SGN-2FF and ASG-15ME, as well as other preclinical and research-stage programs that employ our proprietary technologies. In addition, we have an ADC co-development agreement with Genmab that provides us with a future ADC product opportunity and we are co-developing several preclinical immuno-oncology programs with Unum Therapeutics. |
| • | Continue to Leverage our Industry-Leading ADC Technology. We have developed proprietary ADC technology designed to empower monoclonal antibodies. We are currently developing multiple product candidates that employ our ADC technology and we have also licensed this technology to biotechnology and pharmaceutical companies to generate collaboration revenues and funding, as well as potential milestones and potential future royalties. Presently, we have active ADC collaborations with AbbVie, Bayer, Celldex, Genentech, GSK, Pfizer, and Progenics, as well as ADC co-development agreements with Astellas and Genmab. These ADC collaboration and co-development agreements have generated over $350 million as of December 31, 2016 through a combination of upfront payments, research support, and other fees, milestone payments and equity purchases. |
| • | Support Future Growth of our Pipeline through Internal Research Efforts and Strategic In-Licensing. We have internal research programs directed toward identifying novel antigen targets, monoclonal antibodies and other targeting molecules, creating new antibody engineering techniques and developing new classes of stable linkers and cell-killing agents for our ADC technology. In addition, we supplement these internal efforts through ongoing initiatives to identify product candidates, products and technologies to in-license from biotechnology and pharmaceutical companies and academic institutions. In this regard, we recently announced our entry into the Immunomedics License, pursuant to which, if the transactions contemplated by the Immunomedics License are consummated, we would receive exclusive worldwide rights to develop, manufacture and commercialize IMMU-132. |
| • | Enter into Strategic Product Collaborations to Supplement our Internal Resources. We enter into collaborations to broaden and accelerate clinical trial development and potential commercialization of our product candidates. Collaborations can generate significant capital, supplement our own internal expertise in key areas such as manufacturing, regulatory affairs and clinical development, and provide us with access to our collaborators’ marketing, sales and distribution capabilities in specific territories. When establishing strategic collaborations, we seek beneficial financial terms and endeavor to retain significant product rights, particularly in the United States, Canada and Europe. |
5
Table of Contents
ADCETRIS and Product Candidate Development Pipeline
The following table summarizes our ADCETRIS and product candidate development pipeline:
| Name of Product or Product Candidate |
Description |
Commercial Rights |
Status | |||
| ADCETRIS® (brentuximab vedotin) | Anti-CD30 ADC | Seattle Genetics in United States and Canada; Takeda in rest of world | ADCETRIS has regular approval in the United States for the treatment of patients with (i) relapsed classical Hodgkin lymphoma or (ii) classical Hodgkin lymphoma at high risk of relapse or progression as post-autologous hematopoietic stem cell transplantation, or post-auto-HSCT consolidation. ADCETRIS also has accelerated approval in the United States for the treatment of patients with relapsed sALCL. In addition, ADCETRIS has approval with conditions in Canada for the treatment of patients with relapsed Hodgkin lymphoma or sALCL.
As of January 31, 2017, ADCETRIS had received marketing authorizations in relapsed Hodgkin lymphoma or relapsed sALCL from regulatory authorities in 66 countries. In particular, ADCETRIS has conditional marketing authorization in the European Union and marketing authorization in Japan for patients with relapsed Hodgkin lymphoma or relapsed sALCL. In January 2016, Takeda received an additional approval from the European Commission for retreatment of adult patients with relapsed or refractory Hodgkin lymphoma or sALCL who previously responded to ADCETRIS and who later relapse. In June 2016, the European Commission approved ADCETRIS for the treatment of adult patients with CD30-positive Hodgkin lymphoma at increased risk of relapse or progression following autologous stem cell transplant, or ASCT.
Ongoing trials of ADCETRIS include:
The ALCANZA phase 3 randomized trial for relapsed CD30-expressing CTCL patients, comparing ADCETRIS versus investigator’s choice of methotrexate or bexarotene. In 2016, we reported that the ALCANZA phase 3 trial met its primary endpoint. Based on the results of the trial, we expect to submit an sBLA to the FDA in mid- 2017 for approval of a new indication in CD30-expressing relapsed CTCL.
| |||
6
Table of Contents
| The ECHELON-1 phase 3 randomized trial ongoing for patients with newly diagnosed advanced stage classical Hodgkin lymphoma comparing adriamycin, bleomycin, vinblastine and dacarbazine, or ABVD, versus AVD plus ADCETRIS. We expect to report data in 2017.
| ||||||
| The ECHELON-2 phase 3 randomized trial ongoing for patients with newly diagnosed CD30-expressing MTCL, including sALCL, comparing cyclophosphamide, doxorubicin, vincristine and prednisone, or CHOP, versus CHP plus ADCETRIS. Enrollment was completed in November 2016 and we expect to report data in 2018.
| ||||||
| Phase 2 trial ongoing for patients age 60 or older with newly diagnosed Hodgkin lymphoma evaluating ADCETRIS as frontline monotherapy or in combination with other agents, including bendamustine or dacarbazine. In late 2016, the trial was amended to evaluate the combination of ADCETRIS and nivolumab.
Phase 1/2 second-line trial ongoing for patients with relapsed Hodgkin lymphoma evaluating ADCETRIS in combination with bendamustine.
Phase 2 trial ongoing for patients with relapsed or refractory CD30-expressing diffuse large B-cell lymphoma, or DLBCL, evaluating rituximab and bendamustine either with or without ADCETRIS.
Phase 1/2 trial ongoing for patients with relapsed or refractory Hodgkin lymphoma after failure of frontline therapy evaluating ADCETRIS in combination with nivolumab.
Phase 1/2 trial ongoing for patients with relapsed or refractory B-cell and T-cell non-Hodgkin lymphomas, including DLBCL, evaluating ADCETRIS in combination with nivolumab. In 2016, the trial was amended to also evaluate the combination in rare B-cell lymphomas.
Phase 2 monotherapy trial ongoing for patients with systemic lupus erythematosus.
| ||||||
| SGN-CD33A (vadastuximab talirine) | Anti-CD33 ADC | Seattle Genetics | Phase 3 CASCADE trial ongoing to evaluate SGN-CD33A in combination with HMAs in previously untreated older AML patients.
| |||
7
Table of Contents
| Phase 1/2 trial ongoing for patients with previously untreated MDS evaluating SGN-CD33A in combination with azacitidine.
Several early stage trials of SGN-CD33A are on a full or partial clinical hold. See “SGN-CD33A (Vadastuximab Talirine)” below for more information on these trials.
| ||||||
| ASG-22ME (enfortumab vedotin) | Anti-Nectin-4 ADC | 50: 50 co-development and commercialization with Astellas |
Phase 1 trial ongoing for Nectin-4-positive solid tumors, including urothelial cancers such as bladder cancer.
| |||
| SGN-LIV1A |
Anti-LIV-1 ADC | Seattle Genetics | Phase 1 trial ongoing for patients with LIV-1-positive metastatic breast cancer, in particular triple negative disease. In addition, the trial was expanded to include patients who are LIV-1 positive and HER2 positive to evaluate SGN-LIV1A in combination with trastuzumab.
| |||
| SGN-CD19A (denintuzumab mafodotin) | Anti-CD19 ADC | Seattle Genetics | Phase 2 randomized trial ongoing for patients with relapsed DLBCL evaluating SGN-CD19A in combination with rituximab, ifosfamide, carboplatin and etoposide, or RICE, to RICE alone.
Phase 2 randomized trial ongoing for patients with newly diagnosed DLBCL comparing a combination therapy that includes SGN-CD19A versus standard frontline therapy.
| |||
| SEA-CD40 |
Anti-CD40 SEA empowered antibody | Seattle Genetics | Phase 1 trial ongoing for patients with solid tumors. In 2016, the trial was amended to include patients with hematologic malignancies.
| |||
| SGN-CD19B |
Anti-CD19 ADC | Seattle Genetics | Phase 1 trial ongoing for patients with relapsed or refractory aggressive B-cell non-Hodgkin lymphoma.
| |||
| SGN-CD123A |
Anti-CD123 ADC | Seattle Genetics | Phase 1 trial ongoing for patients with relapsed or refractory AML.
| |||
| SGN-CD352A |
Anti-CD352 ADC | Seattle Genetics | Phase 1 trial ongoing for patients with relapsed or refractory multiple myeloma.
| |||
| SGN-2FF |
Small molecule | Seattle Genetics | Phase 1 trial ongoing for patients with advanced solid tumors.
| |||
| ASG-15ME |
Anti-SLITRK6 ADC | 50: 50 co-development and commercialization with Astellas |
Phase 1 trial ongoing for patients with urothelial cancer.
| |||
8
Table of Contents
ADCETRIS
ADCETRIS is an ADC comprised of an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a proprietary microtubule disrupting agent, monomethyl auristatin E, or MMAE. ADCETRIS employs a linker system that is designed to be stable in the bloodstream and to release MMAE upon internalization into CD30-expressing cells. We believe that the CD30 antigen is an attractive target for cancer therapy because it is expressed on multiple types of cancer, but has limited expression on normal tissues. We are collaborating with Takeda on the global development and commercialization of ADCETRIS. Under this collaboration, we retain commercial rights in the United States and Canada. Takeda has exclusive rights to commercialize ADCETRIS in the rest of the world. ADCETRIS has received regulatory approvals as follows:
| • | United States. ADCETRIS® (brentuximab vedotin) injection for intravenous infusion has received approval from the FDA for three indications: (1) regular approval for the treatment of patients with classical Hodgkin lymphoma after failure of auto-HSCT or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not auto-HSCT candidates, (2) regular approval for the treatment of classical Hodgkin lymphoma patients at high risk of relapse or progression as post-auto-HSCT consolidation, and (3) accelerated approval for the treatment of patients with sALCL after failure of at least one prior multi-agent chemotherapy regimen. The sALCL indication is approved under accelerated approval based on overall response rate. Continued approval for the sALCL indication is contingent upon verification and description of clinical benefit in confirmatory trials. |
| • | Canada. Health Canada has issued a Notice of Compliance with conditions, authorizing marketing of ADCETRIS for two lymphoma indications: (1) the treatment of patients with Hodgkin lymphoma after failure of ASCT, or after failure of at least two multi-agent chemotherapy regimens in patients who are not ASCT candidates, and (2) the treatment of patients with sALCL after failure of at least one multi-agent chemotherapy regimen. The indications for ADCETRIS were authorized based on promising response rates demonstrated in single-arm trials. |
| • | European Union. ADCETRIS was granted conditional marketing authorization by the European Commission in October 2012 for two indications: (1) for the treatment of adult patients with relapsed or refractory CD30-positive Hodgkin lymphoma following ASCT, or following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option, and (2) the treatment of adult patients with relapsed or refractory sALCL. The European Commission extended the current conditional approval of ADCETRIS and approved ADCETRIS for the treatment of adult patients with CD30-positive Hodgkin lymphoma at increased risk of relapse or progression following ASCT. |
| • | Worldwide. As of January 31, 2017, ADCETRIS is commercially available in 66 countries for relapsed or refractory Hodgkin lymphoma and relapsed or refractory sALCL. |
Required ADCETRIS Post-approval Clinical Study
ADCETRIS was granted approval for the treatment of patients with sALCL after failure of at least one prior multi-agent chemotherapy regimen under the FDA’s accelerated approval regulations, which allows the FDA to approve products for cancer or other serious or life-threatening illnesses based on surrogate endpoints or on a clinical endpoint other than survival or irreversible morbidity. Under the FDA’s accelerated approval regulations, we are subject to certain post-approval requirements pursuant to which we are conducting an additional confirmatory phase 3 trial to verify and describe the clinical benefit of ADCETRIS. In addition, we are subject to extensive ongoing obligations and continued regulatory review from the FDA and other applicable regulatory agencies, such as continued adverse event reporting requirements and the requirement to have our promotional materials pre-cleared by the FDA.
Successful completion of our AETHERA trial converted our accelerated approval to regular approval in the relapsed Hodgkin lymphoma indication. ECHELON-2 is a required post-approval study to convert the approval of ADCETRIS in the United States from accelerated approval to regular approval in its currently approved relapsed sALCL indication; however, the FDA has indicated that positive results from either the ECHELON-1 or
9
Table of Contents
the ECHELON-2 trial could form the basis for such a conversion. Post-approval clinical studies similar to those required by the FDA are required in many other countries, including in Canada and the European Union. The requirements of these post-approval clinical studies vary from country to country and may in some cases involve testing in addition to the post-approval studies required by the FDA.
Market Opportunities
According to the American Cancer Society, more than 8,500 cases of Hodgkin lymphoma were expected to be diagnosed in the United States during 2016, and an estimated 1,120 people were expected to die of the disease. Approximately 1,800 additional patients per year in the United States are diagnosed with sALCL, a type of MTCL that expresses the CD30 antigen. The use of combination chemotherapy as frontline therapy for malignant lymphomas has resulted in high remission rates; however, these frontline chemotherapy regimens have substantial associated toxicities and a significant number of lymphoma patients relapse and require additional treatments including other chemotherapy regimens and ASCT. For the reasons discussed in “Item 1A—Risk Factors”, we may not be able to obtain regulatory approvals to market ADCETRIS for frontline Hodgkin lymphoma or MTCL, or otherwise continue to expand its labeled indications of use.
Clinical Development Status and Plan
In collaboration with Takeda, we are pursuing a broad development strategy that includes clinical trials of ADCETRIS both as a single agent and in combination with standard therapies for CD30-expressing cancers. These ongoing clinical trials include:
Phase 3 Cutaneous T-Cell Lymphoma (ALCANZA). The ALCANZA trial is a randomized, open-label, phase 3 trial of ADCETRIS versus investigator’s choice of methotrexate or bexarotene in patients with CD30-expressing CTCL, including those with pcALCL or mycosis fungoides. The ALCANZA trial enrolled 131 patients and is being conducted under an SPA agreement with the FDA and also received EMA scientific advice. In August 2016, we reported that the ALCANZA phase 3 trial met its primary endpoint and showed that treatment with ADCETRIS resulted in a statistically significant improvement in the rate of objective response lasting at least four months (ORR4) versus the control arm as assessed by an independent review committee (p-value <0.0001). Additional data were reported at the December 2016 American Society of Hematology, or ASH, annual meeting showing that the median progression free survival, or PFS, in the ADCETRIS arm was 16.7 months compared to 3.5 months in the control arm (p-value <0.0001). The most common adverse events of any grade occurring in 15 percent or more of patients in the ADCETRIS and control arms were peripheral neuropathy (67 and six percent, respectively), nausea (36 and 13 percent, respectively), diarrhea (29 and six percent, respectively), fatigue (29 and 27 percent, respectively), vomiting (17 and five percent, respectively), alopecia (15 and three percent, respectively), pruritis (17 and 13 percent respectively), fever (17 and 18 percent, respectively), decreased appetite (15 and five percent, respectively) and hypertriglyceridemia (2 and 18 percent, respectively). We plan to submit a sBLA to the FDA in mid-2017 to seek approval for a new indication in CD30-expressing relapsed CTCL. ADCETRIS is included in National Comprehensive Cancer Network, or NCCN, treatment guidelines for patients with certain types of CTCL.
Phase 3 Frontline Hodgkin Lymphoma (ECHELON-1). We and Takeda have completed patient enrollment of 1,334 patients in a randomized, open-label, phase 3 trial investigating ADCETRIS plus AVD versus ABVD as frontline therapy in patients with advanced classical Hodgkin lymphoma, or the ECHELON-1 trial. The study enrolled patients who have histologically-confirmed diagnosis of Stage III or IV classical Hodgkin lymphoma and who have not been previously treated with systemic chemotherapy or radiotherapy. The primary endpoint of this trial is modified PFS per independent review facility assessment. Secondary endpoints include overall survival, complete remission rate and safety. The trial is being conducted under an SPA agreement with the FDA and also received scientific advice from the EMA. We expect to report data from this trial in 2017.
Data from a phase 1 trial in frontline Hodgkin lymphoma that evaluated ADCETRIS combined with ABVD or combined with AVD supported our decision to initiate the ECHELON-1 phase 3 trial. In November 2013, data
10
Table of Contents
from the phase 1 trial were published in the medical publication Lancet Oncology. Among the 25 evaluable patients in the ADCETRIS plus AVD cohorts, 24 patients who completed frontline therapy on study achieved a complete remission. Follow-up data were reported at the December 2014 ASH annual meeting showing that in the ADCETRIS plus AVD arm, three-year overall survival was 100 percent and three-year failure-free survival was 92 percent. In the ADCETRIS plus ABVD arm, three-year overall survival was 92 percent and three-year failure-free survival was 79 percent. Grade 3 or higher adverse events occurring in more than one patient overall noted in the ABVD and AVD cohorts, respectively, were neutropenia (80 percent, 77 percent), anemia (20 percent, 12 percent), febrile neutropenia (20 percent, 8 percent) and pulmonary toxicity (24 percent, 0 percent).
Phase 3 Frontline Mature T-Cell Lymphoma (ECHELON-2). We and Takeda have completed patient enrollment of 452 patients in a global randomized, double-blind, placebo-controlled multi-center phase 3 clinical trial known as ECHELON-2. This trial is evaluating ADCETRIS in combination with CHP versus CHOP for the treatment of newly diagnosed CD30-expressing MTCL patients, including patients with sALCL and other types of peripheral T-cell lymphomas. The primary endpoint of the trial is PFS per independent review facility assessment. Secondary endpoints include overall survival, complete remission rate and safety. We expect to report data in 2018. A molecular companion diagnostic test is being used in this trial to identify eligible patients based on CD30-expression. We are developing a companion diagnostic under a collaboration agreement with Ventana Medical Systems, or Ventana, and Takeda. The ECHELON-2 trial is being conducted under an SPA agreement with the FDA and also received scientific advice from the EMA. We are required to conduct this trial as part of our ADCETRIS post-marketing requirement for the relapsed sALCL indication, and the trial is designed to be confirmatory in the United States, Canada and Europe.
Data from a phase 1 trial that evaluated ADCETRIS plus chemotherapy for frontline sALCL, which was subsequently amended to include patients with any CD30-expressing MTCL, supported our decision to initiate the ECHELON-2 trial. Among the 26 patients who received the combination regimen of ADCETRIS plus CHP, 88 percent achieved a complete remission. At the December 2016 ASH annual meeting, follow-up data were reported showing that the estimated four-year PFS rate was 52 percent, with no patients receiving a consolidative stem cell transplant in first remission. The estimated four-year overall survival rate was 80 percent. There were no progression events or deaths in the trial since the previous presentation at the 2015 ASH annual meeting. The most common adverse events of any grade occurring in more than 30 percent of patients were nausea and peripheral sensory neuropathy (69 percent each), diarrhea (62 percent), fatigue (58 percent) and hair loss (54 percent). ADCETRIS is included in NCCN treatment guidelines for patients with relapsed CD30-expressing PTCL.
Frontline Therapy for Hodgkin Lymphoma Patients Age 60 and Over. In October 2012, we initiated a phase 2 clinical trial evaluating ADCETRIS monotherapy as a frontline therapy for patients age 60 or older with newly diagnosed Hodgkin lymphoma. The trial was subsequently amended to include the administration of ADCETRIS in combination with bendamustine or dacarbazine. The phase 2 open-label clinical trial is evaluating the activity and tolerability of ADCETRIS in patients age 60 or older with newly diagnosed Hodgkin lymphoma. The primary endpoint of the trial is the objective response rate, with key secondary endpoints of safety and tolerability, duration of response, complete remission rate and PFS. At the October 2016 International Symposium on Hodgkin Lymphoma, or ISHL, meeting, we presented updated data from this study. Of 21 evaluable patients in the dacarbazine combination arm, all patients (100 percent) had an objective response, including 62 percent with a complete remission. Of 17 evaluable patients in the bendamustine combination arm, all patients (100 percent) had an objective response, including 88 percent with a complete remission. The most common adverse events of any grade occurring in at least 25 percent of patients in the dacarbazine combination arm were peripheral sensory neuropathy (77 percent); constipation (45 percent); fatigue and nausea (41 percent each), peripheral edema (32 percent) and diarrhea (27 percent). The most common adverse events of any grade occurring in at least 25 percent of patients in the bendamustine combination arm were diarrhea (85 percent), nausea (65 percent), fatigue (50 percent), decreased appetite (45 percent) and peripheral sensory neuropathy and fever (40 percent each). Enrollment on the bendamustine arm was closed given the tolerability of the combination did not meet study goals for this fragile patient population. In 2016, the trial was amended to
11
Table of Contents
evaluate the combination of ADCETRIS and nivolumab. ADCETRIS monotherapy is included in NCCN guidelines for older patients with newly diagnosed Hodgkin lymphoma.
Relapsed or Refractory CD30-expressing DLBCL. In October 2015, we announced the initiation of a randomized phase 2 open-label, multi-center clinical trial of rituximab and bendamustine with or without ADCETRIS in relapsed or refractory patients with CD30-expressing DLBCL. The primary endpoint is to compare the objective response rates between the two study arms. Secondary endpoints include PFS, complete remission rate, duration of response and overall survival. ADCETRIS is included in NCCN guidelines for patients with second-line or beyond CD30-expressing DLBCL.
Combination trials with nivolumab (OPDIVO). In January 2015, we and BMS announced a clinical trial collaboration agreement to evaluate the investigational combination of ADCETRIS and BMS’ immunotherapy nivolumab in two phase 1/2 clinical trials. Nivolumab is a programmed death-1, or PD-1, immune checkpoint inhibitor that is designed to harness the body’s own immune system to help restore antitumor immune response.
One ongoing trial is evaluating the combination of ADCETRIS and nivolumab for patients with second line Hodgkin lymphoma. At the 2016 ASH annual meeting, data were reported from 42 patients with relapsed or refractory Hodgkin lymphoma after failure of frontline therapy who received the combination regimen. Patients were treated with up to four cycles of combination therapy. After completion of the fourth cycle of treatment, patients were eligible to undergo an ASCT. Of 29 response-evaluable patients, 90 percent had an objective response, including 62 percent with a complete metabolic response. The most common adverse events of any grade occurring prior to ASCT in more than 20 percent of patients were fatigue, nausea, infusion related reaction, or IRR, pruritus and rash. IRRs were observed in 38 percent of patients and most symptoms included flushing and nausea (14 percent each); chest discomfort, dyspnea, urticaria (12 percent each); cough and pruritis (10 percent each). The protocol was amended to require premedication with low-dose corticosteroids and antihistamine.
The second ongoing trial is evaluating the combination of ADCETRIS and nivolumab in patients with relapsed or refractory B-cell and T-cell non-Hodgkin lymphomas, including DLBCL. This trial has been subsequently amended to evaluate the combination in rare B-cell lymphomas, including gray zone and mediastinal B-cell lymphomas.
Investigator-Sponsored Trials. In addition to our corporate-sponsored trials, as of December 31, 2016, there were more than 35 reported investigator-sponsored trials of ADCETRIS in the United States. In addition, we and Takeda are reviewing proposals from multiple clinical investigators and cooperative groups in the United States, Canada and Europe about potential investigator-sponsored trials of ADCETRIS. The investigator-sponsored trials to date include the use of ADCETRIS in a number of malignant hematologic indications such as CTCL, DLBCL, untreated limited stage Hodgkin lymphoma, salvage therapy for patients with Hodgkin lymphoma prior to auto-HSCT and graft versus host disease. There are also numerous other investigator-sponsored trials for the use of ADCETRIS in other CD30-expressing and select CD30-undetectable settings, and in solid tumors such as mesothelioma and testicular germ cell tumors. One cooperative group investigator-sponsored trial is currently evaluating ADCETRIS with immuno-oncology compounds in Hodgkin lymphoma, and we expect additional investigator-sponsored trials might evaluate ADCETRIS in novel combination regimens.
SGN-CD33A (Vadastuximab Talirine)
SGN-CD33A is an ADC composed of an anti-CD33 monoclonal antibody linked to a potent PBD dimer using our proprietary ADC technology. We are developing this product candidate as a potential treatment of AML and MDS. SGN-CD33A targets CD33, a protein that is expressed on most AML cells. SGN-CD33A employs our newest proprietary ADC technology. This technology is comprised of a PBD dimer, which is a potent cell-killing agent that works by a different mechanism than auristatins, linked to an engineered antibody called EC-mAb, resulting in uniform drug-loading of two PBD dimers per antibody.
12
Table of Contents
On December 27, 2016, we announced that we had received notice from the FDA that a full clinical hold or partial clinical hold had been placed on several early stage trials of SGN-CD33A in AML to evaluate the potential risk of hepatotoxicity following adverse medical events, including fatal events. Our phase 1/2 trial of SGN-CD33A monotherapy in pre- and post- allogeneic transplant AML patients has been placed on full clinical hold. Additionally, clinical trials evaluating SGN-CD33A monotherapy, and including a subset of older AML patients in combination with HMAs, and SGN-CD33A combination treatment with 7+3 chemotherapy in newly diagnosed younger AML patients have been placed on partial clinical hold. Our phase 3 CASCADE trial in older AML patients and our phase 1/2 trial in MDS are proceeding with enrollment. No new studies of SGN-CD33A will be initiated unless and until the clinical holds are lifted. We are working diligently with the FDA to determine whether there is any association between hepatotoxicity and treatment with SGN-CD33A and to promptly identify appropriate measures for patient safety with the goal of addressing the FDA’s concerns.
In May 2016, we initiated a phase 3, randomized, double-blind, placebo-controlled, global clinical trial called CASCADE. The CASCADE trial is designed to evaluate if SGN-CD33A in combination with the HMAs azacitidine or decitabine can extend overall survival compared to either HMA alone in older patients with newly diagnosed AML. Patients will be randomized on a 1:1 ratio to be treated with an HMA plus SGN-CD33A or an HMA plus placebo. The secondary endpoints include the comparison of composite complete remission rate (complete remission and complete remission with incomplete hematologic recovery), event-free and leukemia-free survival, duration of response, safety, and 30- and 60-day mortality rates. Treatment and patient enrollment in this trial is ongoing.
Our decision to initiate the CASCADE trial was supported by interim data from a phase 1 open-label, multi-center, dose-escalation clinical trial of SGN-CD33A as monotherapy or in combination with other standard treatments. In addition, the trial was evaluating anti-leukemic activity, pharmacokinetics, PFS and overall survival in patients with CD33-positive AML. At the December 2016 ASH annual meeting, we reported updated data from a cohort of this trial that evaluated SGN-CD33A in combination with an HMA. Of 49 patients evaluable for response, 73 percent of patients achieved a complete remission or complete remission with incomplete platelet or neutrophil recovery. With a median follow-up of 14.7 months, median overall survival for all patients was 11.3 months and 28 percent of patients remained alive and on study as of last follow-up. The 30- and 60-day mortality rates were two and eight percent, with no treatment-related deaths occurring during that time. The most common Grade 3 or 4 treatment-emergent adverse events occurring in 20 percent or more of patients were thrombocytopenia, febrile neutropenia, anemia and neutropenia. The most common Grade 1 and 2 treatment-emergent adverse events occurring in 20 percent or more of patients were fatigue, nausea, constipation, peripheral edema and decreased appetite. In December 2016, this phase 1 trial was placed on a partial clinical hold by the FDA. Under the partial clinical hold, existing patients in the trial may continue treatment with SGN-CD33A following execution of an additional informed consent form, but no new patients may be enrolled in the trial.
In February 2016, we initiated a phase 1/2, open label, multi-center clinical trial of SGN-CD33A in combination with azacitidine in patients with previously untreated Intermediate-2 or high risk MDS. Phase 1 of the study will identify the recommended dose of SGN-CD33A when combined with azacitidine in this patient population. The phase 2 portion of the trial will be a randomized, double-blind, placebo-controlled study evaluating azacitidine with or without SGN-CD33A. The primary endpoint in phase 1 is determination of the recommended SGN-CD33A dose in combination with azacitidine. The primary endpoint in phase 2 is to compare the overall response rate between the two treatment arms. The secondary endpoints include evaluation of safety, best response, duration of response, PFS and overall survival. Treatment and patient enrollment in this trial is ongoing.
In December 2014, we initiated a phase 1b, open-label, multi-center, dose-escalation clinical trial designed to evaluate SGN-CD33A administered in combination with frontline standard of care regimens for induction (cytarabine and daunorubicin, also known as 7+3) for younger patients with newly diagnosed AML. The trial was also designed to evaluate SGN-CD33A in the consolidation setting for AML, both in combination with
13
Table of Contents
cytarabine and as a single-agent maintenance regimen. The primary endpoints are determination of the maximum tolerated dose and safety profile of SGN-CD33A. In addition, the trial was evaluating anti-leukemic activity, pharmacokinetics, PFS and overall survival. At the ASH 2016 annual meeting, we reported interim data showing that of 42 patients evaluable for response, 76 percent achieved a complete remission or complete remission with incomplete platelet or neutrophil recovery. Among patients who achieved a remission, 78 percent were negative for minimal residual disease. Median OS had not yet been reached. The 30-day mortality rate was two percent. The most common Grade 3 or 4 treatment-emergent adverse events occurring in 20 percent or more of patients were febrile neutropenia, thrombocytopenia, anemia and neutropenia. The most common Grade 1 and 2 treatment-emergent adverse events occurring in 20 percent or more of patients were nausea, diarrhea, constipation, hypokalemia and decreased appetite. In December 2016, this phase 1b trial was placed on partial clinical hold by the FDA. Under the partial clinical hold, existing patients in the trial may continue treatment with SGN-CD33A following execution of an additional informed consent form, but no new patients may be enrolled in the trial.
In November 2015, we initiated a phase 1/2 clinical trial of SGN-CD33A in patients with relapsed or refractory AML. The trial was designed to evaluate SGN-CD33A monotherapy as a pre-conditioning regimen prior to an allogeneic stem cell transplant and also for use as maintenance therapy following transplant. In December 2016, this trial was placed on full clinical hold by the FDA. Under the full clinical hold, all patients in the trial were required to discontinue treatment with SGN-CD33A immediately and no new patients may be enrolled in the trial.
We may be unable to submit to the FDA all required clinical follow-up information to respond to the clinical holds. Even if we are able to provide such information, the FDA may not deem the information to be sufficient to lift any or all of the clinical holds. In addition, we may encounter delays in reaching an agreement with the FDA regarding the terms under which these trials may be resumed, or we may elect to discontinue one or more of these trials for safety or any other reasons. Furthermore, the FDA may require us to implement additional, potentially burdensome pharmacovigilance procedures or conduct additional pre-clinical studies before it will consider lifting the clinical holds, if at all. In addition, although the FDA has not placed a clinical hold on the CASCADE trial or our phase 1/2 trial in MDS and we continue to enroll patients in these trials, we cannot assure you that the FDA or another regulatory authority will not place a clinical hold on one or both of these trials. If we are unable to submit required information to the FDA in a timely manner, or at all; if the FDA does not lift the clinical holds in a timely manner, or at all; if the FDA does not permit us to initiate additional trials of SGN-CD33A and/or the FDA imposes clinical holds on the CASCADE trial or our phase 1/2 trial in MDS; or if there are additional safety results, including from our ongoing trials of SGN-CD33A, that alter the benefit-risk profile of SGN-CD33A or cause it to become unacceptable, we would be further delayed or prevented from advancing the clinical development of SGN-CD33A, which would adversely affect our business, results of operations and prospects.
ASG-22ME (Enfortumab Vedotin)
ASG-22ME is an ADC composed of an anti-Nectin-4 monoclonal antibody linked to a potent auristatin compound using our proprietary ADC technology. Nectin-4 is a novel target expressed in multiple cancers including urothelial cancers, such as bladder cancer, as well as ovarian and lung cancers. We are developing ASG-22ME as a potential treatment of solid tumors under our co-development collaboration with Astellas.
In October 2016, we reported interim data from a phase 1, open-label, dose-escalation, multi-center clinical trial of ASG-22ME at the European Society for Medical Oncology, or ESMO, annual meeting. Of the 49 metastatic urothelial cancer patients evaluable for response, 37 percent had an objective response, including two percent who achieved a complete response. The preliminary estimate of median PFS was 16.6 weeks. At the recommended phase 2 dose of 1.25 mg/kg, 17 patients were treated of which 59 percent had a partial response. In the 16 patients across dose levels who had previously been treated with checkpoint inhibitors, 38 percent achieved a partial response. Among the seven patients treated at the recommended phase 2 dose that had
14
Table of Contents
previously been treated with checkpoint inhibitors, 57 percent achieved a partial response. The most common treatment related adverse events of any grade occurring in 20 percent or more of patients were pruritis (31 percent), fatigue (30 percent), diarrhea (29 percent), nausea (28 percent), rash (26 percent) and alopecia (21 percent).
Based on these interim phase 1 data, we and Astellas are planning discussions with regulatory agencies during 2017 to advance the program into potential registrational trials of ASG-22ME in urothelial cancer patients, including patients who have been previously treated with a checkpoint inhibitor therapy.
SGN-LIV1A
SGN-LIV1A is an ADC composed of an anti-LIV-1 monoclonal antibody linked to a potent auristatin compound using our proprietary ADC technology, and is being developed as a potential treatment of LIV-1-positive metastatic breast cancer.
In October 2013 we initiated a phase 1, open-label, dose-escalation clinical trial to evaluate the safety and antitumor activity of SGN-LIV1A in patients with LIV-1-positive metastatic breast cancer. At the December 2016 San Antonio Breast Cancer Symposium annual meeting, updated interim data were reported showing that among 30 evaluable patients with triple negative disease, 37 percent achieved a partial response. The estimated median PFS for these patients was 12 weeks with seven patients remaining on treatment. The maximum tolerated dose was not reached among doses ranging from 0.5 to 2.8 mg/kg. For all patients in the study, the most common adverse events of any grade occurring in 20 percent or more of patients included fatigue (57 percent), nausea (53 percent), alopecia (42 percent), decreased appetite (34 percent) and constipation (32 percent). The incidence of grade 3/4 neutropenia at the 2.5 mg/kg dose was 50 percent. Two patients (seven percent) experienced febrile neutropenia, and there was one treatment-related death due to sepsis. Based on these safety data, a separate expansion cohort at 2.0 mg/kg is currently being evaluated. In addition, enrollment is ongoing for patients with HER2 positive breast cancer to evaluate SGN-LIV1A in combination with trastuzumab.
SGN-CD19A (Denintuzumab Mafodotin)
SGN-CD19A is an ADC composed of an anti-CD19 monoclonal antibody linked to a potent auristatin compound using our proprietary ADC technology. CD19 is a B-cell antigen that is expressed in non-Hodgkin lymphoma, chronic lymphocytic leukemia and acute lymphoblastic leukemia.
In October 2015, we announced the initiation of a phase 2 randomized, open-label, multi-center clinical trial of SGN-CD19A in combination with the second-line salvage regimen of rituximab, ifosfamide, carboplatin and etoposide, or RICE, for patients with relapsed or refractory DLBCL. The primary endpoint is to compare the complete remission rates between the two study arms. Secondary endpoints include safety of the combination regimen, PFS, overall survival and the number of patients who are able to undergo ASCT.
In October 2016, we announced the initiation of a phase 2 clinical trial of SGN-CD19A in patients with newly-diagnosed DLBCL. The trial will assess the activity and tolerability of adding SGN-CD19A to the standard frontline regimen, R-CHOP, as well as a modified regimen, R-CHP. The primary endpoints are to evaluate the complete remission rate and tolerability profile of the combinations. Secondary endpoints include event-free survival, PFS, overall survival and duration of response.
SEA-CD40
SEA-CD40 utilizes our novel proprietary SEA technology to produce a non-fucosylated antibody targeting CD40, which is believed to work as an immuno-oncology molecule, activating certain immune cells. It builds on our extensive experience targeting CD40. In February 2015, we announced initiation of a phase 1, open-label, multi-center, dose-escalation clinical trial of SEA-CD40 in patients with advanced solid tumors who have failed current standard of care treatments. Expansion cohorts are planned to evaluate SEA-CD40 across up to three
15
Table of Contents
cancer indications that will be determined based on data from the dose escalation portion of the study. The primary endpoints are determination of the maximum tolerated dose and safety profile of SEA-CD40. In addition, the trial will evaluate antitumor activity, pharmacokinetics and immunological pharmacodynamic effects. In 2016, the phase 1 trial was expanded to evaluate patients with hematologic malignancies.
SGN-CD19B
SGN-CD19B is a proprietary ADC composed of an anti-CD19 monoclonal antibody utilizing our proprietary EC-mAb technology linked to a potent PBD dimer. We are developing this product candidate as a potential treatment of non-Hodgkin lymphoma. In February 2016, we announced initiation of a phase 1, open-label, multi-center, dose-escalation clinical trial of SGN-CD19B for patients with relapsed or refractory DLBCL or grade 3 follicular lymphoma. The primary endpoints are the estimation of the maximum tolerated dose and evaluation of the safety of SGN-CD19B. In addition, the trial will evaluate antitumor activity, pharmacokinetics, objective response rate and PFS.
SGN-CD123A
SGN-CD123A is a proprietary ADC composed of an anti-CD123 monoclonal antibody utilizing our proprietary EC-mAb technology linked to a potent PBD dimer. We are developing this product candidate as a potential treatment of AML. In September 2016, we announced initiation of a phase 1, open-label, multi-center, dose-escalation clinical trial of SGN-CD123A for patients with relapsed or refractory AML. It will initially evaluate the maximum tolerated dose of SGN-CD123A, followed by an expansion cohort to further define safety and estimate anti-leukemic activity. In addition, the trial will assess pharmacokinetics, remission rate, duration of complete remission and overall survival.
SGN-CD352A
SGN-CD352A is a proprietary ADC composed of an anti-CD352 monoclonal antibody utilizing our proprietary EC-mAb technology linked to a potent PBD dimer. In January 2017, we announced initiation of a phase 1, open-label, multi-center, dose-escalation clinical trial of SGN-CD352A for patients with relapsed or refractory multiple myeloma. The trial will be conducted in two parts, with a dose escalation part to identify the maximum tolerated dose of SGN-CD352A followed by an expansion part to further define safety and antitumor activity.
SGN-2FF
SGN-2FF is a novel small molecule immuno-oncology agent. It is an oral agent that has been shown in preclinical models to inhibit fucosylation of proteins, which may make tumors more visible to the immune system, and thereby slow the growth and spread of cancer cells. In January 2017, we initiated an open-label, multi-center, dose-escalation clinical trial of SGN-2FF for patients with relapsed or refractory solid tumors, including non-small cell lung cancer.
ASG-15ME
ASG-15ME is an ADC composed of an anti-SLITRK6 monoclonal antibody linked to a potent auristatin compound using our proprietary ADC technology. We are developing ASG-15ME under our co-development collaboration with Astellas. A phase 1 clinical trial of ASG-15ME for the treatment of metastatic urothelial cancer, notably bladder cancer, was initiated in October 2013. This trial is evaluating the safety, tolerability, pharmacokinetic profile and antitumor activity of escalating doses of ASG-15ME. We and Astellas are focusing our development activities on ASG-22ME for metastatic urothelial cancer, while evaluating next development steps for ASG-15ME.
Research Programs
In addition to our pipeline of product candidates and antibody-based and SEA technologies, we have internal research programs directed toward developing new classes of potent, cell-killing agents and stable
16
Table of Contents
linkers, identifying novel antigen targets, monoclonal antibodies and other targeting molecules, and advancing our antibody engineering initiatives.
New Cell-Killing Agents. We continue to study new cell-killing agents that can be linked to antibodies, such as the auristatins and PBDs that we currently use in our ADC technology, and new classes of cell-killing agents.
New Stable Linkers. We are conducting research with the intent to develop new linker systems that are more stable in the bloodstream and more effective at releasing the cell-killing agent once inside targeted cancer cells.
Novel Monoclonal Antibodies and Antigen Targets. We are actively engaged in internal efforts to identify and develop monoclonal antibodies and other targeting molecules and ADCs with novel specificities and activities against selected antigen targets. We focus on antigen targets that are highly expressed on the surface of cancer cells that may serve as targets for monoclonal antibodies or ADCs. We may then create and screen panels of cancer-reactive monoclonal antibodies in our laboratories to identify those with the desired specificity. We supplement these internal efforts by evaluating opportunities to in-license targets and antibodies from academic groups and other biotechnology and pharmaceutical companies, such as our ongoing co-development collaboration with Genmab.
Antibody Engineering. We have substantial internal expertise in antibody engineering, both for antibody humanization and non-fucosylation, as well as engineering of antibodies to improve drug linkage sites for use with our ADC technology. By modifying the number and type of drug-linkage sites found on our antibodies, we believe that we can improve the robustness and cost-effectiveness of our manufacturing processes for conjugation of ADCs.
Research and Development Expense
Since inception, we have devoted a significant amount of resources to develop ADCETRIS, our product candidates and our antibody-based technologies. For the years ended December 31, 2016, 2015, and 2014, we recorded $379.3 million, $294.5 million, and $230.7 million, respectively, in research and development expenses.
Corporate Collaborations
We enter into collaborations with biotechnology and pharmaceutical companies to advance the development and commercialization of our product candidates and to supplement our internal pipeline. We seek collaborations that will allow us to retain significant future participation in product sales through either profit-sharing or royalties paid on net sales. We also license our ADC technology to collaborators to be developed with their own antibodies. These ADC collaborations benefit us in many ways, including generating cash flow and revenues that partially offset expenditures on our internal research and development programs, expanding our knowledge base regarding ADCs across multiple targets and antibodies provided by our collaborators and providing us with future pipeline opportunities through co-development or opt-in rights to new ADC product candidates.
Takeda ADCETRIS Collaboration
In December 2009, we entered into a collaboration agreement with Takeda to develop and commercialize ADCETRIS, under which Seattle Genetics retains commercial rights in the United States and its territories and in Canada, and Takeda and its Takeda affiliates have commercial rights in the rest of the world. As of December 31, 2016, we had received an upfront payment of $60 million and had achieved milestone payments totaling $70 million related to regulatory and commercial progress by Takeda. As of December 31, 2016, we were entitled to receive additional progress- and sales-dependent milestone payments of up to $165 million based on Takeda’s achievement of significant events under the collaboration in addition to tiered royalties with percentages starting in the mid-teens and escalating to the mid-twenties based on net sales of ADCETRIS within Takeda’s licensed
17
Table of Contents
territories. Takeda also bears a portion of third-party royalty costs owed on sales of ADCETRIS in its territory. We and Takeda equally co-fund the cost of development activities conducted under the collaboration. Although we are funding half of the development activities conducted under the collaboration, Takeda is responsible for the achievement of the progress- and sales-dependent milestone payments that we may receive. Either party may terminate the collaboration agreement if the other party materially breaches the agreement and such breach remains uncured. Takeda may terminate the collaboration agreement for any reason upon prior written notice to us and we may terminate the collaboration agreement in certain circumstances. The collaboration agreement can also be terminated by mutual written consent of the parties. If neither party terminates the collaboration agreement, then the agreement automatically terminates on the expiration of all payment obligations.
Astellas Co-Development Collaboration
In January 2007, we entered into an agreement with Astellas to jointly research, develop and commercialize ADCs for the treatment of several types of cancer. The collaboration encompasses combinations of our ADC technology with fully-human antibodies developed by Astellas to proprietary cancer targets.
Under the collaboration agreement, we and Astellas are co-funding all development and commercialization costs for ASG-22ME and ASG-15ME, and will share on a 50/50 basis in any profits that may come from these product candidates if successfully commercialized. Costs associated with co-development activities are included in research and development expense.
Astellas has the right to develop and commercialize certain other ADC product candidates on its own, subject to paying us annual maintenance fees, milestones, royalties and support fees for research and development services and material provided under the collaboration agreement. We are entitled to receive progress- and sales-dependent milestone payments of up to approximately $96 million based on Astellas’ achievement of significant events under the collaboration in addition to mid-single digit royalties on net sales of any of these other ADC product candidates by Astellas. Either party may opt out of co-development and profit-sharing in return for receiving milestones and royalties from the continuing party. Either party may terminate the collaboration agreement if the other party becomes insolvent or the other party materially breaches the agreement and such breach remains uncured. Subject to certain restrictions, either party may terminate the collaboration agreement for any reason upon prior written notice to the other party. The collaboration agreement can also be terminated by mutual written consent of the parties. If neither party exercises its option to terminate the collaboration agreement, then the agreement will automatically terminate on the later of: (a) the expiration of all payment obligations pursuant to the collaboration agreement, or (b) the day upon which we and Astellas cease to develop and commercialize products under the agreement.
Unum Therapeutics Collaboration
In June 2015, we entered into a strategic collaboration and license agreement with Unum to develop and commercialize novel ACTR therapies incorporating our antibodies for cancer. Unum’s proprietary ACTR technology enables programming of a patient’s T-cells to attack tumor cells when co-administered with tumor-specific therapeutic antibodies. Through our research and development in the field of ADCs, we have a substantial portfolio of cancer targets and tumor-specific monoclonal antibodies from which programs will be selected for the collaboration with Unum. Under the terms of the agreement, we and Unum will initially develop two ACTR product candidates that combine Unum’s ACTR technology with our antibodies, and we have an option to expand the collaboration to include a third ACTR product candidate upon payment of an additional fee. Unum is obligated to conduct preclinical research and clinical development activities through phase 1 clinical trials and we are obligated to provide funding for these activities. The agreement calls for us to work together to co-develop and jointly fund programs after phase 1 clinical trials unless either company opts out. We and Unum would co-commercialize any successfully developed product candidates and share any profits 50/50 on any co-developed programs in the United States. We retain exclusive commercial rights outside of the United States, paying Unum a royalty that is a high single digit to mid-teens percentage of ex-U.S. sales, if any. The potential future licensing and progress-dependent milestone payments to Unum under the collaboration may total up to
18
Table of Contents
$615 million across all three ACTR programs, payment of which is triggered by the achievement of development, regulatory and commercial milestones. As of the date of this filing, this agreement is not material to us.
Genmab Co-Development Collaboration
In September 2010, we entered into an ADC research collaboration agreement with Genmab. Under the agreement, Genmab has rights to utilize our ADC technology with its HuMax-TF antibody targeting the Tissue Factor, or TF, antigen, which is expressed on numerous types of solid tumors. Under this agreement, we received an upfront payment and have the right to exercise a co-development option for any resulting ADC products at the end of phase 1 clinical development. Genmab is responsible for research, manufacturing, preclinical development and phase 1 clinical trials under the collaboration. We receive research support payments for any assistance provided to Genmab. If we opt into the anti-TF ADC product at the end of phase 1, we and Genmab would co-develop and share all future costs and profits for the product on a 50/50 basis. If we do not opt in, then Genmab would pay us fees, milestones and mid-single digit royalties on worldwide net sales of the product. As of the date of this filing, this agreement is not material to us.
ADC Collaborations
We have active collaborations with a number of companies to allow them to use our proprietary ADC technology with their monoclonal antibodies. Under our ADC collaborations, which we enter into in the ordinary course of business, we receive or are entitled to receive upfront cash payments, progress-dependent milestones and single digit royalties on net sales of products incorporating our ADC technology, as well as annual maintenance fees and support fees for research and development services and materials provided under the agreements. Our ADC collaborators are responsible for development, manufacturing and commercialization of any ADC product candidates that result from the collaborations and are solely responsible for the achievement of any of the potential milestones under these collaborations.
19
Table of Contents
Our current ADC collaborations are at various stages of clinical and preclinical development. Our ability to generate significant future revenues from our current ADC collaboration agreements will largely depend on a product that incorporates our ADC technology entering late-stage clinical development and receiving marketing approval from the FDA at which point the milestone payments, royalties or other rights and benefits would become more substantial and material to our company. Below is a table setting forth our active ADC collaborations and current development status:
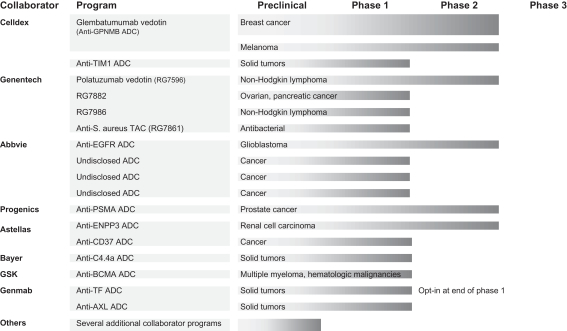
License Agreements
We have in-licensed antibodies, targets and enabling technologies from pharmaceutical and biotechnology companies and academic institutions for use in our pipeline programs and ADC technology, including the following:
Bristol-Myers Squibb License. In March 1998, we obtained rights to some of our technologies and product candidates, portions of which are exclusive, through a license agreement with Bristol-Myers Squibb. Through this license, we secured rights to use various targeting technologies. Under the terms of the license agreement, we are required to pay royalties in the low single digits on net sales of products, including ADCETRIS, which incorporate various technologies owned by Bristol-Myers Squibb.
University of Miami License. In September 1999, we entered into an exclusive license agreement with the University of Miami, Florida, covering an anti-CD30 monoclonal antibody that is the basis for the antibody component of ADCETRIS. Under the terms of this license, we made an upfront payment and are required to pay annual maintenance fees, progress-dependent milestone payments and royalties in the low single digits on net sales of products, including ADCETRIS, incorporating technology licensed from the University of Miami.
Immunomedics License. On February 10, 2017, we entered into the Immunomedics License. Pursuant to the Immunomedics License, Immunomedics granted us, subject to the terms and conditions of the Immunomedics License, exclusive worldwide rights to develop, manufacture and commercialize IMMU-132 and second generation ADCs targeting TROP-2 for all human therapeutics uses in any and all indications. IMMU-132 is an ADC targeted to TROP-2, which is expressed in several solid tumors, and is in a pivotal phase 1/2 trial for patients with TNBC, and is being investigated in other solid tumors. Under the Immunomedics License, we
20
Table of Contents
agreed to pay Immunomedics an up-front payment of $250 million following closing of the Immunomedics License. In addition, we agreed to pay development, regulatory and sales-dependent milestone payments to Immunomedics across multiple indications and geographic regions of up to a total maximum of approximately $1.7 billion. Immunomedics would also be eligible to receive royalties on worldwide annual net sales of licensed products at tiered royalty rate percentages beginning in the teens and rising to twenty percent, subject to customary reductions, during the royalty term specified in the Immunomedics License. We would bear the future costs of worldwide development and commercialization of licensed products, subject to specified exceptions. We agreed to use commercially reasonable efforts to develop IMMU-132. Following regulatory approval (and pricing and reimbursement approvals, as applicable) of any licensed product in any of the major market countries specified in the Immunomedics License, we agreed to use commercially reasonable efforts to commercialize such licensed product in each major market country where it has been approved. Under the Immunomedics License, Immunomedics would have the right to exercise a co-promotion option to provide up to 50% of the sales efforts for the commercialization of IMMU-132 in the United States, subject to certain parameters set forth in the Immunomedics License. We may terminate the Immunomedics License for convenience upon at least two hundred seventy (270) days prior written notice to Immunomedics. Either we or Immunomedics may terminate the Immunomedics License if the other party is in material breach of the Immunomedics License and fails to cure such breach within specified cure periods. Upon a termination of the Immunomedics License by us for convenience or by Immunomedics for our material breach, all licenses granted by Immunomedics to us terminate (other than specified exceptions), and all payment obligations that accrued prior to the date of such termination shall survive the termination, among other effects of termination. The Immunomedics License also provides us the right to terminate specified portions of the Immunomedics License in the event of certain fundamental breaches by Immunomedics that are not cured in accordance with specified cure periods and procedures. In connection with our entry into the Immunomedics License, we purchased 3,000,000 shares of Immunomedics common stock from Immunomedics at an aggregate purchase price of $14.7 million, and also received a warrant from Immunomedics to purchase up to 8,655,804 additional shares of Immunomedics common stock.
The closing of the transactions contemplated by the Immunomedics License is subject to customary closing conditions, including the expiration of the applicable waiting period under the HSR Act, there being no pending court or administrative challenges to the Immunomedics License and there being no court or administrative orders blocking the closing. On February 20, 2017, Immunomedics and we entered into a letter agreement pursuant to which Immunomedics irrevocably waived to the extent applicable to Immunomedics the condition precedent to the closing and effectiveness of the Immunomedics License that there be no pending court or administrative challenges to the transaction. Additionally, under the terms of the Immunomedics License, Immunomedics had the right to continue discussions with a small number of parties that previously expressed interest in licensing IMMU-132 until 11:59 p.m. New York City time on February 19, 2017. If a third party had provided Immunomedics with a financially superior licensing offer, we would have had the right to match any such offer, and if we had decided not to match, Immunomedics would have had the right to accept the superior offer and terminate the Immunomedics License upon payment of a termination fee to us. We have not received notice from Immunomedics of any such third party offers during this limited time period, and on February 21, 2017, Immunomedics announced that it is subject to customary “no-shop” restrictions on its and its representatives’ ability to solicit, discuss or negotiate alternative licensing agreement proposals from third parties with regard to IMMU-132. On February 13, 2017, we were named a co-defendant in a lawsuit filed by venBio in the Delaware Chancery Court against the members of the board of directors of Immunomedics pursuant to which, among other things, venBio seeks to enjoin the closing of the transactions contemplated by the Immunomedics License. As a result of the pending litigation challenging the transactions contemplated by the Immunomedics License, Immunomedics and we have committed to the Court not to close the transactions contemplated by the Immunomedics License prior to March 10, 2017. We cannot predict the timing or outcome of this lawsuit or the impact it may have on the Immunomedics License or the closing of the transactions contemplated by the Immunomedics License. However, it is possible that, in connection with this lawsuit, the Immunomedics License could be rescinded or reformed in a way that is disadvantageous to us, including by requiring us to increase the transaction consideration payable to Immunomedics under the Immunomedics License, or that otherwise adversely affects the anticipated benefits to us of the Immunomedics License.
21
Table of Contents
Patents and Proprietary Technology
Our owned and licensed patents and patent applications are directed to ADCETRIS, our product candidates, monoclonal antibodies, our ADC and SEA technologies and other antibody-based and/or enabling technologies. We commonly seek patent claims directed to compositions of matter, including antibodies, ADCs, and drug-linkers containing highly potent cell-killing agents, as well as methods of using such compositions. When appropriate, we also seek claims to related technologies, such as methods of using certain sugar analogs utilized in our SEA technology. For ADCETRIS and each of our product candidates, we have filed or expect to file multiple patent applications. We maintain patents and prosecute applications worldwide for technologies that we have out-licensed, such as our ADC technology. Similarly, for partnered products and product candidates, such as ADCETRIS, ASG-22ME, and ASG-15ME, we seek to work closely with our development partners to coordinate patent efforts, including patent application filings, prosecution, term extension, defense and enforcement. As ADCETRIS and our development product candidates advance through research and development, we seek to diligently identify and protect new inventions, such as combination therapies, improvements to methods of manufacturing, and methods of treatment. We also work closely with our scientific personnel to identify and protect new inventions that could eventually add to our development pipeline.
For ADCETRIS and our related ADC technology, we own nine patents in the United States and Europe that will expire between 2020 and 2031. For SGN-CD33A and our related ADC technology, we own, co-own or have licensed rights to six patents in the United States and Europe that will expire between 2027 and 2032. For ASG-22ME and our related ADC technology, we own, co-own or have licensed rights to nine patents in the United States and Europe that will expire between 2022 and 2031. Of these nine patents, we own or co-own seven patents and have licensed rights to two patents. For SGN-LIV1A and our related ADC technology, we own or have licensed rights to seven patents in the United States and Europe that will expire between 2020 and 2032. Of these seven patents, we own rights to five patents and have licensed rights to two patents. Of these six patents, we own or co-own four patents and have licensed rights to two patents. For SGN-CD19A and our related ADC technology, we own eleven patents in the United States and Europe that will expire between 2024 and 2029. For SEA-CD40 and our related SEA technology, we own or have licensed rights to eleven patents in the United States and Europe that will expire between 2020 and 2030. Of these eleven patents, we own eight patents and have licensed rights to three patents. For ASG-15ME and our related ADC technology, we own or co-own five patents in the United States and Europe that will expire between 2022 and 2033. In some cases, our U.S. patents may be eligible for patent term extension, and our European patents may be eligible for supplemental protection in one or more countries. The length of any such extension would vary by country.
Patents expire, on a country by country basis, at various times depending on various factors, including the filing date of the corresponding patent application(s), the availability of patent term extension and supplemental protection certificates and requirements for terminal disclaimers. Although we believe our owned and licensed patents and patent applications provide us with a competitive advantage, the patent positions of biotechnology and pharmaceutical companies can be uncertain and involve complex legal and factual questions. We and our corporate collaborators may not be able to develop patentable products or processes or obtain patents from pending patent applications. Even if patent claims are allowed, the claims may not issue. In the event of issuance, the patents may not be sufficient to protect the proprietary technology owned by or licensed to us or our corporate collaborators. Our or our collaborators’ current patents, or patents that issue on pending applications, may be challenged, invalidated, infringed or circumvented. In addition, changes to patent laws in the United States or other countries may limit our ability to defend or enforce our patents, or may applied retroactively to affect the term of our patents. Our patents have been and may in the future be challenged by third parties in post-issuance administrative proceedings or in litigation as invalid, not infringed or unenforceable under U.S. or foreign laws, or they may be infringed by third parties. As a result, we are or may be from time to time involved in the defense and enforcement of our patent or other intellectual property rights in a court of law, U.S. Patent and Trademark Office inter partes review or reexamination proceeding, foreign opposition proceeding or related legal and administrative proceeding in the United States and elsewhere. The costs of defending our patents or enforcing our proprietary rights in post-issuance administrative proceedings or litigation may be substantial and
22
Table of Contents
the outcome can be uncertain. An adverse outcome may allow third parties to use our proprietary technologies without a license from us or our collaborators. Our and our collaborators’ patents may also be circumvented, which may allow third parties to use similar technologies without a license from us or our collaborators.
Our commercial success depends significantly on our ability to operate without infringing patents and proprietary rights of third parties. Organizations such as pharmaceutical and biotechnology companies, universities and research institutions may have filed patent applications or may have been granted patents that cover technologies similar to the technologies owned, optioned by or licensed to us or to our collaborators. In addition, we are monitoring the progress of multiple pending patent applications of other organizations that, if granted, may require us to license or challenge their validity or enforceability in order to continue commercializing ADCETRIS or to commercialize our product candidates. Our challenges to patents of other organizations may not be successful, which may affect our ability to commercialize ADCETRIS or our product candidates. We cannot determine with certainty whether patents or patent applications of other parties may materially affect our or our collaborators’ ability to make, use or sell ADCETRIS or any other products.
We require our scientific personnel to maintain laboratory notebooks and other research records in accordance with our policies, which are designed to strengthen and support our intellectual property protection. In addition to our patented intellectual property, we also rely on trade secrets and other proprietary information, especially when we do not believe that patent protection is appropriate or can be obtained. Our policy is to require each of our employees, consultants and advisors to execute a proprietary information and inventions assignment agreement before beginning their employment, consulting or advisory relationship with us. These agreements provide that the individual must keep confidential and not disclose to other parties any confidential information developed or learned by the individual during the course of their relationship with us except in limited circumstances. These agreements also provide that we will own all inventions conceived or reduced to practice by the individual in the course of rendering services to us. Our agreements with collaborators require them to have a similar policy and agreements with their employees, consultants and advisors. Our policy and agreements and those of our collaborators may not sufficiently protect our confidential information, or third parties may independently develop equivalent information.
Government Regulation
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, pre-market approval, manufacture, marketing and distribution of biopharmaceutical products. These agencies and other regulatory agencies regulate research and development activities and the testing, approval, manufacture, quality control, safety, efficacy, labeling, storage, distribution, import, export, recordkeeping, advertising and promotion of products and product candidates. Failure to comply with applicable FDA or other requirements may result in Warning Letters, civil or criminal penalties, suspension or delays in clinical development, recall or seizure of products, partial or total suspension of production or withdrawal of a product from the market. The development and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all. We must obtain approval of our product candidates from the FDA before we can begin marketing them in the United States. Similar approvals are also required in other countries.
Product development and approval within this regulatory framework is uncertain, can take many years and requires the expenditure of substantial resources. The nature and extent of the governmental review process for our product candidates will vary, depending on the regulatory categorization of particular product candidates and various other factors.
The necessary steps before a new biopharmaceutical product may be sold in the United States ordinarily include:
| • | preclinical in vitro and in vivo tests, some of which must comply with Good Laboratory Practices, or GLP; |
23
Table of Contents
| • | submission to the FDA of an IND which must become effective before clinical trials may commence, and which must be updated at least annually with a report on development; |
| • | development of a drug formulation and manufacture of the drug product for clinical trials, and commercial sale, if approved; |
| • | completion of adequate and well controlled human clinical trials to establish the safety and efficacy of the product candidate for its intended use; |
| • | submission to the FDA of a marketing authorization application in the form of a BLA, which must be accompanied by a substantial user fee unless the fee is waived; |
| • | FDA pre-approval inspection of manufacturing facilities for current Good Manufacturing Practices, or GMP, compliance and FDA inspection of select clinical trial sites for Good Clinical Practice, or GCP, compliance; and |
| • | FDA review and approval of the marketing authorization application and product prescribing information prior to any commercial sale. |
The results of preclinical tests (which include laboratory evaluation as well as preclinical GLP studies to evaluate toxicity) for a particular product candidate, together with related manufacturing information and analytical data, and a clinical protocol are submitted as part of an IND to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30 day time period, raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. IND submissions may be authorized by the FDA, for example, to commence a clinical trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development. Further, an independent institutional review board, or IRB, for each medical center proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that center and it must monitor the study until completed. The FDA, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCP regulations and regulations for informed consent and privacy of individually-identifiable information.
Clinical trials generally are conducted in three sequential phases that may overlap or in some instances, be skipped. In phase 1, the initial introduction of the product into humans, the product candidate is tested to assess safety, metabolism, pharmacokinetics and pharmacological actions associated with increasing doses. Phase 2 usually involves trials in a limited patient population to evaluate the efficacy of the potential product for specific, targeted indications, determine dosage tolerance and optimum dosage and further identify possible adverse reactions and safety risks. Phase 3 and pivotal trials are undertaken to evaluate further clinical efficacy and safety often in comparison to standard therapies within a broader patient population, generally at geographically dispersed clinical sites. Phase 4, or post-marketing, trials may be required as a condition of commercial approval by the FDA and may also be voluntarily initiated by us or our collaborators. Since we received accelerated approval for ADCETRIS from the FDA for the relapsed sALCL indication, we are subject to certain post-approval requirements pursuant to which we are conducting an additional confirmatory phase 3 trial, the ECHELON-2 trial, to verify and describe the clinical benefit of ADCETRIS in the relapsed sALCL indication. Phase 1, phase 2 or phase 3 testing may not be completed successfully within any specific period of time, if at all, with respect to any of our product candidates. Similarly, suggestions of safety, tolerability or efficacy in earlier stage trials do not necessarily predict findings of safety and efficacy in subsequent trials. Furthermore, the FDA, an IRB or we may suspend a clinical trial at any time for various reasons, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical trials are subject to central registration and results reporting requirements, such as on www.clinicaltrials.gov.
The results of preclinical studies, pharmaceutical development and clinical trials, together with information on a product’s chemistry, manufacturing, and controls, are submitted to the FDA in the form of a BLA, for approval of the manufacture, marketing and commercial shipment of the pharmaceutical product. Data from
24
Table of Contents
clinical trials are not always conclusive and the FDA may interpret data differently than we or our collaborators interpret data. The FDA may also convene an Advisory Committee of external advisors to answer questions regarding the approvability and labeling of an application. The FDA is not obligated to follow the Advisory Committee’s recommendation. The submission of a BLA is required to be accompanied by a substantial user fee, with few exceptions or waivers. The user fee is administered under the Prescription Drug User Fee Act, or PDUFA, which sets goals for the timeliness of the FDA’s review. A standard review period is twelve months from submission of the application, while priority review is eight months from submission of the application. The testing and approval process is likely to require substantial time, effort and resources, and there can be no assurance that any approval will be granted on a timely basis, if at all. The FDA may deny review of an application by refusing to file the application or not approve an application by issuance of a complete response letter if applicable regulatory criteria are not satisfied, require additional testing or information, or require post-market testing and surveillance to monitor the safety or efficacy of the product. Approval may occur with significant Risk Evaluation and Mitigation Strategies, or REMS, that limit the clinical use in the prescribing information, distribution or promotion of a product. Accelerated approval of ADCETRIS for relapsed sALCL additionally requires the pre-submission of marketing materials to the FDA for the product until such time as the accelerated approval requirements have been fulfilled. Once an approval is issued, the FDA may require safety-related labeling changes or withdraw product approval if ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require further testing of ADCETRIS, including phase 4 clinical trials, and surveillance programs to monitor the safety of ADCETRIS, and the FDA has the power to prevent or limit further marketing of ADCETRIS based on the results of these post-marketing programs or other information.
Products manufactured or distributed pursuant to FDA approvals are subject to continuing regulation by the FDA, including manufacture, labeling, distribution, advertising, promotion, recordkeeping, annual product quality review and reporting requirements. Adverse event experience with the product must be reported to the FDA in a timely fashion and pharmacovigilance programs to proactively look for these adverse events are mandated by the FDA. Manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Following such inspections, the FDA may issue notices on Form FDA 483 and Warning Letters that could cause us to modify certain activities. A Form FDA 483 notice, if issued at the conclusion of an FDA inspection, can list conditions the FDA investigators believe may have violated cGMP or other FDA regulations or guidance. Failure to adequately and promptly correct the observations(s) can result in further regulatory enforcement action. In addition to Form FDA 483 notices and Warning Letters, failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as suspension of manufacturing, seizure of product, injunctive action or possible civil penalties. We cannot be certain that we or our present or future third-party manufacturers or suppliers will be able to comply with the cGMP regulations and other ongoing FDA regulatory requirements. If we or our present or future third-party manufacturers or suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, not approve our products, require us to recall a product from distribution or withdraw approval of the BLA for that product. Failure to comply with ongoing regulatory obligations can result in delay of approval or Warning Letters, product seizures, criminal penalties, and withdrawal of approved products, among other enforcement remedies.
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. These regulations include standards and restrictions for direct-to-consumer advertising, industry-sponsored scientific and educational activities, promotional activities involving the internet, and off-label promotion. While physicians may prescribe products for off label uses, manufacturers may only promote products for the approved indications and in accordance with the provisions of the approved label. The FDA has very broad enforcement authority under the Federal Food, Drug and Cosmetic Act, and failure to abide by these regulations can result in penalties, including the issuance of a Warning Letter directing entities to correct deviations from FDA standards, and state and federal civil and criminal investigations and prosecutions.
25
Table of Contents
FDA Regulation of Companion Diagnostics
ADCETRIS and certain of our product candidates may rely upon in vitro companion diagnostics for use in selecting the patients that we believe will respond to our therapeutics. If safe and effective use of a therapeutic product depends on an in vitro diagnostic, the FDA generally will require approval or clearance of the diagnostic at the same time that FDA approves the therapeutic product. This policy is described in an August 2014 FDA guidance document. The review of these in vitro companion diagnostics in conjunction with the review of our cancer treatments involves coordination of review by FDA’s Center for Drug Evaluation and Research and by FDA’s Center for Devices and Radiological Health. For example, we and Takeda have formed a collaboration with Ventana under which Ventana is working to develop, manufacture and commercialize a molecular companion diagnostic test with the goal of identifying patients who might respond to treatment with ADCETRIS based on CD30 expression levels in their tissue specimens, which companion diagnostic may be required by regulatory authorities to support regulatory approval of ADCETRIS in other CD30-expressing malignancies.
Regulation Outside of the United States
In addition to regulations in the U.S., we and our collaborators are and will be subject to regulations of other countries governing clinical trials and commercial sales, manufacturing and distribution of our products. We must obtain approval by the regulatory authorities of countries outside of the U.S. before we can commence clinical trials in such countries and approval of the regulators of such countries or economic areas, such as Canada, before we may market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Healthcare Regulation
Federal and state healthcare laws and regulations, including fraud and abuse and health information privacy and security laws and regulations, may also be applicable to our business. If we fail to comply with those laws, we could face substantial penalties and our business, results of operations, financial condition and prospects could be adversely affected. The healthcare laws and regulations that may affect our ability to operate include, without limitation, anti-kickback and false claims laws, data privacy and security laws, as well as transparency laws regarding payments or other items of value provided to healthcare providers.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, purchasing, leasing, ordering, or arranging for or recommending the purchase, lease, or order of any good, facility, item, or service reimbursable, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated. Additionally, the intent standard under the Anti-Kickback Statute was amended by the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, collectively PPACA, to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, PPACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
26
Table of Contents
The federal civil and criminal false claims laws, including the federal civil False Claims Act, prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment to or approval by the federal government, including the Medicare, and Medicaid programs, or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim or to avoid, decrease, or conceal an obligation to pay money to the federal government.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created additional federal criminal statutes that prohibit, among other actions, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program, including private third party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. Like the Anti-Kickback Statute, PPACA amended the intent standard for certain healthcare fraud under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations, imposes certain requirements on certain types of individuals and entities relating to the privacy and security of individually identifiable health information. Among other things, HITECH makes HIPAA’s security standards directly applicable to business associates, independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
The federal Physician Payments Sunshine Act, created under PPACA and its implementing regulations, requires certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program to annually report information related to certain payments or other transfers of value provided to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals, and to report annually certain ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately and completely the required information for all payments, transfers of value and ownership or investment interests may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an aggregate of $1 million per year for “knowing failures.” Covered manufacturers are required to submit reports on aggregate payment data to the Secretary of the U.S. Department of Health and Human Services on an annual basis.
Many states have similar statutes or regulations to the above federal laws and regulations that may be broader in scope than the aforementioned federal versions and apply regardless of payor, and many of which differ from each other in significant ways and may not have the same effect, further complicating compliance efforts. Additionally, our business operations in foreign countries and jurisdictions, including Canada and the European Union, may subject us to additional regulation.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to penalties, including potentially
27
Table of Contents
significant criminal and civil and/or administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Coverage and Reimbursement
Sales of ADCETRIS and any future products depend, in significant part, on the extent to which the costs of our products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. Patients who are prescribed treatment for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients and providers are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Pharmaceutical products are typically reimbursed based on FDA labeled indications, recognized compendia listings, available medical literature and determination of medical necessity.
Additionally, a third-party payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. Decisions regarding the extent of coverage and amount of reimbursement to be provided for each of our product candidates is individual to each insurer, can vary based on provider contract, and will be affected by state and federal laws providing for reimbursement formulas based on acquisition cost. Third party payors continue to work diligently to control their spending on prescription drugs and medical service. The containment of healthcare costs has become a priority of the U.S. government and abroad, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Payors, commercial and public in the U.S. and abroad, must review the therapeutics value of our products before extending coverage under their plans to reimburse our products If third-party payors do not find a product to be of therapeutic value, they may not cover it or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
Many of the patients in the U.S. who seek treatment with ADCETRIS may be eligible for Medicare or Medicaid benefits. The Medicare and Medicaid programs are administered by the Centers for Medicare and Medicaid Services, or CMS, and coverage and reimbursement for products and services under these programs are subject to changes in CMS regulations and interpretive policy determinations, in addition to statutory changes made by Congress. Federal budget decisions have and may result in reduced Medicare payment rates. In addition, as a condition of federal funds being made available to cover our products under Medicaid, we are required to participate in the Medicaid drug rebate program. The rebate amount under this program varies by quarter, and is based on pricing data we report to CMS. In addition, because we participate in the Medicaid drug rebate program, we must make ADCETRIS available to authorized users of the Federal Supply Schedule of the General Services Administration. This requires compliance with additional laws and requirements, including offering ADCETRIS at a reduced price to federal agencies including the United States Department of Veterans Affairs and United States Department of Defense, the Public Health Service and the Indian Health Service. We are also required to offer discounted pricing to certain eligible not for profit entities that are eligible for 340B pricing under the Public Health Services Act. Participation in these programs requires submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulations and the guidance governing such calculations is not always clear. Compliance with such requirements can require significant investment in
28
Table of Contents
personnel, systems and resources, but failure to properly calculate our prices, or offer required discounts or rebates could subject us to substantial criminal, civil and/or administrative penalties, as well as, administrative burdens and exclusion from or contract termination regarding these programs. The terms of these government programs could change in the future which may increase the discounts or rebates we are required to offer, possibly reducing the revenue derived from sales of ADCETRIS to these entities.
The requirements governing drug pricing vary widely from country to country. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products.
Healthcare Reform
PPACA substantially changes the way healthcare is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. PPACA aims to, among other things, expand coverage for the uninsured while at the same time containing overall healthcare costs. With regard to biopharmaceutical products, PPACA is expected to, among other things, expand and increase industry rebates for products covered under Medicaid programs and make changes to the coverage requirements under the Medicare Part D program. We cannot yet predict the full impact of PPACA at this time for many reasons including that many of its provisions require the promulgation of detailed implementing regulations, which has not yet occurred.
Many provisions of PPACA may impact the biopharmaceutical industry, including that in order for a biopharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the drug pricing program under the Public Health Services Act, or PHS. The required PHS discount on a given product is calculated based on the Average Manufacturers Price, or AMP, and Medicaid rebate amounts reported by the manufacturer. PPACA expanded the types of entities eligible to receive discounted PHS pricing, although, under the current state of the law, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted PHS pricing on orphan drugs when used for the orphan indication. In addition, as PHS drug pricing is determined based on AMP and Medicaid rebate data, revisions, including the recently published AMP rule, to the Medicaid rebate formula and AMP definition described above could cause the required PHS discount to increase.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of PPACA. In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, or the Budget Resolution, that authorizes the implementation of legislation that would repeal portions of PPACA. The Budget Resolution is not a law; however, it is widely viewed as the first step toward the passage of legislation that would repeal certain aspects of PPACA. Further, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under PPACA to waive, defer, grant exemptions from, or delay the implementation of any provision of PPACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. The potential impact of these efforts to repeal or defer and delay enforcement of PPACA on our business remains unclear. Congress also could consider subsequent legislation to replace elements of PPACA that are repealed. Because of the continued uncertainty about the implementation of the PPACA, including the potential for further legal challenges or repeal of PPACA, we cannot quantify or predict with any certainty the likely impact of the PPACA or its repeal on our business, prospects, financial condition or results of operations.
In addition, other legislative changes have been proposed and adopted since PPACA was enacted. The Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers, which went into effect in April 2013 and, following passage of the Bipartisan Budget Act of 2015, will remain in effect
29
Table of Contents
through 2025 unless additional congressional action is taken. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Further, the recently enacted Drug Supply Chain Security Act imposes on manufacturers of certain pharmaceutical products new obligations related to product tracking and tracing, among others, which will be phased in over several years beginning in 2015. Among the requirements of this new legislation, manufacturers subject to this federal law will be required to provide certain information regarding the drug product to individuals and entities to which product ownership is transferred, label drug product with a product identifier, and keep certain records regarding the drug product. The transfer of information to subsequent product owners by manufacturers will eventually be required to be done electronically. Covered manufacturers will also be required to verify that purchasers of the manufacturers’ products are appropriately licensed. Further, under this new legislation, covered manufacturers will have drug product investigation, quarantine, disposition, and notification responsibilities related to counterfeit, diverted, stolen, and intentionally adulterated products, as well as products that are the subject of fraudulent transactions or which are otherwise unfit for distribution such that they would be reasonably likely to result in serious health consequences or death.
Additionally, there has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
We cannot predict what healthcare reform initiatives may be adopted in the future. However, we anticipate that Congress, state legislatures, and third-party payors may continue to review and assess alternative healthcare delivery and payment systems and may in the future propose and adopt legislation or policy changes or implementations effecting additional fundamental changes in the healthcare delivery system. We also expect ongoing initiatives to increase pressure on drug pricing. We cannot assure you as to the ultimate content, timing, or effect of changes, nor is it possible at this time to estimate the impact of any such potential legislation; however, such changes or the ultimate impact of changes could negatively affect our revenue or sales of ADCETRIS or any future approved products.
Competition
The biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Many third parties compete with us in developing various approaches to treating cancer. They include pharmaceutical companies, biotechnology companies, academic institutions and other research organizations.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approval and marketing than we do. In addition, many of these competitors are active in seeking patent protection and licensing arrangements in anticipation of collecting royalties for use of technology that they have developed. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, as well as in acquiring technologies complementary to our programs.
With respect to ADCETRIS, there are several other FDA-approved drugs for the treatment of relapsed or refractory Hodgkin lymphoma or sALCL, including Bristol-Myers Squibb’s Opdivo for Hodgkin lymphoma after failure of auto-HSCT and ADCETRIS and Celgene’s Istodax and Spectrum Pharmaceuticals’ Folotyn for relapsed or refractory peripheral T-cell lymphoma. Merck is also developing a PD-1 inhibitor, pembrolizumab,
30
Table of Contents
and is conducting a clinical trial in relapsed or refractory classical Hodgkin lymphoma comparing pembrolizumab with ADCETRIS. In addition, we are aware of multiple investigational agents that are currently being studied, including Pfizer’s crizotinib, AbbVie’s ibrutinib, Kyowa’s mogamulizumab, and Gilead’s idelalisib, which, if successful, may compete with ADCETRIS in the future. Data have also been presented on several developing technologies, including bispecific antibodies and CAR modified T-cell therapies that may compete with ADCETRIS in the future. Further, there are many competing approaches used in the treatment of patients in ADCETRIS’ three approved indications, including auto-HSCT, conventional therapies, combination chemotherapy, clinical trials with experimental agents and single-agent regimens.
With respect to SGN-CD33A, there are several investigational agents that, if approved, could be competitive with our product candidate, including AbbVie’s venetoclax, which received breakthrough therapy designation from the FDA in January 2016 for use in combination with HMAs in treatment-naïve patients with AML who are not eligible for standard high-dose induction treatment, Jazz’s Vyxeos, Pfizer’s Mylotarg, Astex’s guadecitabine (SGI-110), and agents targeting biomarkers such as FLT3 and IDH1/2.
Many other pharmaceutical and biotechnology companies are developing and/or marketing therapies for the same types of cancer that our product candidates are designed and being developed to treat. For example, we believe that companies including AbbVie, ADC Therapeutics, Affimed, Agios, Amgen, Astellas, Bayer, Biogen, Bristol-Myers Squibb, Celgene, Eisai, Genentech, GSK, Gilead, ImmunoGen, Immunomedics, Infinity, Karyopharm, MedImmune, MEI Pharma, Merck, Novartis, Pfizer, Sanofi-Aventis, Spectrum Pharmaceuticals, Takeda, Teva, and Xencor are developing and/or marketing products or technologies that may compete with ours. In addition, our ADC collaborators may develop compounds utilizing our technology that may compete with product candidates that we are developing.
We are aware of other companies that have technologies that may be competitive with ours, including Astellas, AstraZeneca, Bristol-Myers Squibb, ImmunoGen, Immunomedics, MedImmune, Mersana, and Pfizer, all of which have ADC technology. ImmunoGen has several ADCs in development that may compete with our product candidates. ImmunoGen has also established partnerships with other pharmaceutical and biotechnology companies to allow those other companies to utilize ImmunoGen’s technology, including Sanofi-Aventis, Genentech, Novartis, Takeda and Lilly. We are also aware of a number of companies developing monoclonal antibodies directed at the same antigen targets or for the treatment of the same diseases as our product candidates. For example, we believe Bristol-Myers Squibb has an anti-CD30 antibody program that may be competitive with ADCETRIS, and Amgen and Xencor have anti-CD19 programs that may be competitive with our product candidates.
In addition, in the United States, the Biologics Price Competition and Innovation Act of 2009 created an abbreviated approval pathway for biological products that are demonstrated to be “highly similar” or “biosimilar” to or “interchangeable” with an FDA-approved biological product. This pathway allows competitors to reference the FDA’s prior approvals regarding innovative biological products and data submitted with a BLA to obtain approval of a biosimilar application 12 years after the time of approval of the innovative biological product. The 12-year exclusivity period runs from the initial approval of the innovator product and not from approval of a new indication. In addition, the 12-year exclusivity period does not prevent another company from independently developing a product that is highly similar to the innovative product, generating all the data necessary for a full BLA and seeking approval. Exclusivity only assures that another company cannot rely on the FDA’s prior approvals in approving a BLA for an innovator’s biological product to support the biosimilar product’s approval. Further, under the FDA’s current interpretation, it is possible that a biosimilar applicant could obtain approval for one or more of the indications approved for the innovator product by extrapolating clinical data from one indication to support approval for other indications. The FDA approved the first biosimilar product in the United States in May 2015. In the European Union, the European Commission has granted marketing authorizations for several biosimilars pursuant to a set of general and product class-specific guidelines for biosimilar approvals issued since 2005. We are aware of many pharmaceutical and biotechnology and other companies that are actively engaged in research and development of biosimilars or interchangeable products.
31
Table of Contents
With respect to our current and potential future product candidates, we believe that our ability to compete effectively and develop products that can be manufactured cost-effectively and marketed successfully will depend on our ability to:
| • | advance our technology platforms; |
| • | license additional technology; |
| • | complete clinical trials which position our products for regulatory and commercial success; |
| • | maintain a proprietary position in our technologies and products; |
| • | obtain required government and other public and private approvals on a timely basis; |
| • | attract and retain key personnel; |
| • | commercialize effectively; |
| • | obtain reimbursement for our products in approved indications; |
| • | comply with applicable laws, regulations and regulatory requirements and restrictions with respect to the commercialization of our products, including with respect to any changed or increased regulatory restrictions; and |
| • | enter into additional collaborations to advance the development and commercialization of our product candidates. |
Manufacturing
We do not currently have the internal ability to manufacture the drug products that we sell or need to conduct our clinical trials, and we therefore rely on corporate collaborators and contract manufacturing organizations to supply drug product for commercial supply and our IND-enabling studies and clinical trials. For the monoclonal antibody used in ADCETRIS, we have contracted with AbbVie for clinical and commercial supplies. For the drug linker used in ADCETRIS, we have contracted with Sigma Aldrich Fine Chemicals, or SAFC, for clinical and commercial supplies. We have multiple contract manufacturers for conjugating the drug linker to the antibody and producing the ADCETRIS product. For our ADC product candidates, multiple contract manufacturers, including AbbVie and SAFC, perform antibody and drug-linker manufacturing and several other contract manufacturers perform conjugation of the drug-linker to the antibody and fill/finish of the drug product. In addition, we rely on other third parties to perform additional steps in the manufacturing process, including shipping and storage of ADCETRIS and our product candidates.
We established our commercial scale supply chain for ADCETRIS prior to commercial launch. For the foreseeable future, we expect to continue to rely on contract manufacturers and other third parties to produce, vial and store sufficient quantities of ADCETRIS for use in our clinical trials and for commercial sale. In addition, we depend on outside vendors for the supply of raw materials used to produce ADCETRIS. For our pipeline programs, we believe that our existing supplies of drug product and our contract manufacturing relationships will be sufficient to accommodate clinical trials through phase 3. However, we may need to obtain additional manufacturing arrangements or increase our own manufacturing capability to meet our future commercial needs, both of which could require significant capital investment. In addition, we have committed to provide Takeda with their needs of certain parts of the ADCETRIS supply chain for a limited period of time, which may require us to arrange for additional manufacturing supply. We may also enter into collaborations with pharmaceutical or larger biotechnology companies to enhance the manufacturing capabilities for our product candidates.
AbbVie Biotechnology. In February 2004, we entered into a development and supply agreement with AbbVie (formerly a part of Abbott Laboratories) to manufacture developmental, clinical and commercial quantities of anti-CD30 monoclonal antibody, which is a component of ADCETRIS. The agreement generally
32
Table of Contents
provides for the supply by AbbVie and the purchase by us of such anti-CD30 monoclonal antibody. Under terms of the supply agreement, we may purchase a portion of our required anti-CD30 monoclonal antibody from a second source third-party supplier. We are required to make a minimum annual purchase. The anti-CD30 monoclonal antibody is purchased by us based upon a rolling forecast. The supply agreement will continue until 2025 with an automatic one-year term extension unless either party provides written termination notice to the other party. Either party has the right to terminate the supply agreement if the other party materially breaches its obligations thereunder.
SAFC. In December 2010, we entered into a commercial supply agreement with SAFC to manufacture commercial quantities of drug linker that is a component of ADCETRIS. The agreement generally provides for the supply by SAFC and the purchase by us of drug linker. Under terms of the supply agreement, we may purchase a portion of our required drug linker from a second source third-party supplier. We are required to make a minimum annual purchase. The drug linker is purchased by us based upon a rolling forecast. The supply agreement was made effective as of December 1, 2010 and will continue until the completion of the tenth contract year following the initial commercial order with automatic term extension unless either party provides written termination notice to the other party. Either party has the right to terminate the supply agreement if the other party materially breaches its obligations thereunder.
Commercial Operations and Information About Geographic Areas
We have allocated commercial resources, including sales, marketing, supply chain management and reimbursement capabilities, to commercialize ADCETRIS in the United States and Canada. We believe the U.S. and Canadian markets for ADCETRIS in the approved indications are addressable with a targeted sales and marketing organization, and we intend to continue promoting ADCETRIS ourselves in the United States and Canada for these and any additional indications we may obtain in the future. Takeda has commercial rights in the rest of the world. ADCETRIS was granted conditional marketing authorization in the European Union in October 2012 for patients with relapsed Hodgkin lymphoma or relapsed sALCL. As of January 31, 2017, we and Takeda had received marketing authorizations by regulatory authorities in 66 countries, and Takeda continues to pursue marketing authorizations in multiple other countries.
We sell ADCETRIS through a limited number of pharmaceutical distributors. Health care providers order ADCETRIS through these distributors. We receive orders from distributors and generally ship product directly to the health care provider. Three of our major distributors, together with entities under their common control—AmerisourceBergen Corporation, Cardinal Health, Inc., and McKesson Corporation—each accounted for 10% or more of our total revenue in 2016 and 2015, respectively. Our net product sales of ADCETRIS for the years ended December 31, 2016, 2015, and 2014, were $265.8 million, $226.1 million, and $178.2 million, respectively. Revenues generated outside the United States as determined by customer location were less than 10% of total revenues for the years ended December 31, 2016, 2015, and 2014. Substantially all of our long-lived assets are located in the United States.
Employees
As of December 31, 2016, we had 890 employees. Of these employees, 634 were engaged in or support research, development and clinical activities, 140 were in administrative and business related positions, and 116 were in sales and marketing. Each of our employees has signed confidentiality and inventions assignment agreements and none are covered by a collective bargaining agreement. We have never experienced employment-related work stoppages and consider our employee relations to be good.
Corporate Information
We were incorporated in Delaware on July 15, 1997. Our principal executive offices are located at 21823
30th Drive SE, Bothell, Washington 98021. Our telephone
number is (425) 527-4000. Seattle Genetics® and
 are our registered trademarks in the United States. All other trademarks, tradenames and service marks included in this Annual Report on Form 10-K are the property of their respective owners.
are our registered trademarks in the United States. All other trademarks, tradenames and service marks included in this Annual Report on Form 10-K are the property of their respective owners.
33
Table of Contents
We file electronically with the Securities and Exchange Commission our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available on our website at www.seattlegenetics.com, free of charge, through a hyperlink on our website, copies of these reports, as soon as reasonably practicable after electronically filing such reports with, or furnishing them to, the Securities and Exchange Commission. The information contained in, or that can be accessed through, our website is not part of, and is not incorporated into, this Annual Report on Form 10-K.
34
Table of Contents
You should carefully consider the following risk factors, in addition to the other information contained in this Annual Report on Form 10-K, including our condensed consolidated financial statements and related notes. If any of the events described in the following risk factors occurs, our business, operating results and financial condition could be seriously harmed. This Annual Report on Form 10-K also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described below and elsewhere in this Annual Report on Form 10-K.
Risks Related to Our Business
Our near-term prospects are substantially dependent on ADCETRIS. If we and/or Takeda are unable to effectively commercialize ADCETRIS for the treatment of patients in its approved indications and to continue to expand its labeled indications of use, our ability to generate significant revenue or potentially achieve profitability will be adversely affected.
ADCETRIS®, or brentuximab vedotin, is now approved by the United States Food and Drug Administration, or FDA, and the European Commission for three indications, encompassing several settings for the treatment of relapsed Hodgkin lymphoma and relapsed systemic anaplastic large cell lymphoma, or sALCL. ADCETRIS is our only product approved for marketing and our ability to generate revenue from product sales and potentially achieve profitability is substantially dependent on our continued ability to effectively commercialize ADCETRIS for the treatment of patients in its approved indications and our ability to continue to expand its labeled indications of use. We may not be able to fully realize the commercial potential of ADCETRIS for a number of reasons, including:
| • | we may not be able to obtain and maintain regulatory approvals to market ADCETRIS for any additional indications, including for relapsed cutaneous T-cell lymphoma, or CTCL, frontline Hodgkin lymphoma or frontline mature T-cell lymphoma, or MTCL, or to otherwise continue to expand its labeled indications of use; |
| • | negative or inconclusive results in our ECHELON-1 and ECHELON-2 phase 3 trials would negatively impact, or preclude altogether, our ability to obtain regulatory approval and commercialize ADCETRIS in the frontline Hodgkin lymphoma and frontline MTCL indications, respectively, either of which could limit our sales of, and the commercial potential of, ADCETRIS; |
| • | we may fail to obtain regulatory approval and commercialize ADCETRIS in the ALCANZA treatment setting notwithstanding the positive data we reported from our ALCANZA trial, which would also limit our sales of, and the commercial potential of, ADCETRIS; |
| • | results from our required post-approval study, the ECHELON-2 trial, may fail to verify the clinical benefit of ADCETRIS in relapsed sALCL, which could result in the withdrawal of approval of ADCETRIS in the relapsed sALCL indication and which could negatively impact our potential future product sales for the relapsed sALCL indication; |
| • | new competitive therapies, including immuno-oncology agents such as PD-1 inhibitors (e.g., nivolumab and pembrolizumab), have been approved by regulatory authorities (as in the case of nivolumab) or may be submitted in the near term to regulatory authorities for approval in ADCETRIS’ labeled indications in relapsed Hodgkin lymphoma, and these competitive products could negatively impact our commercial sales of ADCETRIS; |
| • | our commercial sales of ADCETRIS could be lower than our projections due to a lower market penetration rate, increased competition by alternative products or biosimilars, or a shorter duration of therapy in patients in ADCETRIS’ approved indications; |
| • | we may be unable to effectively commercialize ADCETRIS in any new indications for which we receive marketing approval; |
35
Table of Contents
| • | there may be additional changes to the label for ADCETRIS, including ADCETRIS’ boxed warning, that further restrict how we market and sell ADCETRIS, including as a result of data collected from our required post-approval study, or as the result of adverse events observed in that study or in other studies, including in the post-approval confirmatory studies that Takeda is required to conduct as a condition to the conditional marketing authorization of ADCETRIS by the European Commission or in investigator-sponsored studies; |
| • | we may not be able to establish or demonstrate in the medical community the safety and efficacy of ADCETRIS and its potential advantages over and side effects compared to existing and future therapeutics; |
| • | physicians may be reluctant to prescribe ADCETRIS until results from our required post-approval study are available or other long term efficacy and safety data exist; |
| • | the estimated incidence rate of new patients in ADCETRIS’ approved indications may be lower than our projections; |
| • | there may be adverse results or events reported in any of the clinical trials that we and/or Takeda are conducting or may in the future conduct for ADCETRIS; |
| • | we may be unable to continue to effectively market, sell and distribute ADCETRIS; |
| • | ADCETRIS may be impacted by adverse reimbursement and coverage policies from government and private payers such as Medicare, Medicaid, insurance companies, health maintenance organizations and other plan administrators, or may be subject to pricing pressures enacted by industry organizations or state and federal governments, including as a result of increased scrutiny over drug-pricing strategies by pharmaceutical companies or otherwise; |
| • | the relative price of ADCETRIS may be higher than alternative treatment options; |
| • | there may be changed or increased regulatory restrictions; |
| • | we may not have adequate financial or other resources to effectively commercialize ADCETRIS; and |
| • | we may not be able to obtain adequate commercial supplies of ADCETRIS to meet demand or at an acceptable cost. |
In December 2009, we entered into an agreement with Takeda to develop and commercialize ADCETRIS, under which we have commercial rights in the United States and its territories and Canada, and Takeda has commercial rights in the rest of the world. The success of this collaboration and the activities of Takeda will significantly impact the commercialization of ADCETRIS in countries other than the United States and in Canada. In October 2012, Takeda announced that it had received conditional marketing authorization for ADCETRIS from the European Commission for patients with relapsed Hodgkin lymphoma or relapsed sALCL, and has since obtained marketing approvals for ADCETRIS in many other countries. Conditional marketing authorization by the European Commission includes obligations to provide additional clinical data at a later stage to confirm the positive benefit-risk balance. In July 2016, Takeda announced that it had received marketing authorization for ADCETRIS from the European Commission for the treatment of adult patients with CD30-positive Hodgkin lymphoma at increased risk of relapse or progression following autologous stem cell transplant. We cannot control the amount and timing of resources that Takeda dedicates to the commercialization of ADCETRIS, or to its marketing and distribution, and our ability to generate revenues from ADCETRIS product sales by Takeda depends on Takeda’s ability to achieve market acceptance of, and to otherwise effectively market, ADCETRIS for its approved indications in Takeda’s territory.
We believe that the level of our ongoing ADCETRIS sales in the United States is largely attributable to the incidence flow of patients eligible for treatment with ADCETRIS. We also believe that the incidence rate of new patients in ADCETRIS’ approved indications is relatively low, particularly when compared to many other oncology indications. For these and other reasons, we expect that our ability to accelerate ADCETRIS sales
36
Table of Contents
growth, if at all, will depend primarily on our ability to continue to expand ADCETRIS’ labeled indications of use. Accordingly, we are exploring the use of ADCETRIS as a single agent and in combination therapy regimens earlier in the treatment of Hodgkin lymphoma and MTCL, including sALCL, and in a range of CD30-expressing hematologic malignancies, including CD30-expressing relapsed CTCL. This will continue to require additional time and investment in clinical trials and there can be no assurance that we and/or Takeda will obtain and maintain the necessary regulatory approvals to market ADCETRIS for any additional indications. In particular, negative or inconclusive results in our ECHELON-1 and ECHELON-2 trials would negatively impact, or preclude altogether, our ability to obtain regulatory approval in the frontline Hodgkin lymphoma and frontline MTCL, indications, respectively, either of which could limit our sales of, and the commercial potential of, ADCETRIS. For example, in accordance with the terms of their respective SPA, the ECHELON-1 and ECHELON-2 trials are designed to continue until a specified number of events designated for each trial have occurred (i.e. progressions or deaths). If events do not occur at the rate which was anticipated in one or both of these trials, the final data analysis for the applicable trial could be delayed beyond the predicted ranges. In addition, we may choose for a variety of reasons to complete the applicable trial with fewer events than planned. If we choose to complete a trial with fewer events than planned, the FDA may treat the applicable SPA as rescinded and the statistical power of that trial based on the original statistical analysis plan could be negatively impacted, making it more difficult for us to demonstrate that ADCETRIS is safe and effective and to successfully obtain regulatory approval for the specific indication studied in that trial. We may also undertake additional discussions with regulatory authorities or amend the protocol for one or both of these trials, and we cannot predict the outcome of those discussions or whether we would be able to reach agreement with the regulatory authorities. Should this situation occur, there could be a delay in our ability to obtain data from the applicable trial or impact the ability of the applicable trial to support approval of ADCETRIS for the specific indication studied in that trial.
In addition, although we reported in August 2016 that the ALCANZA trial evaluating ADCETRIS in patients with relapsed CTCL met its primary endpoint demonstrating a statistically significant improvement in the rate of objective response lasting at least four months and we plan to submit a supplemental Biologics License Application, or sBLA, to the FDA to seek approval for a new indication in CD30-expressing relapsed CTCL, there can be no assurance that the FDA will accept our planned sBLA for filing or that we will ultimately obtain approval of our planned sBLA in a timely manner or at all. Our failure to obtain regulatory approval and commercialize ADCETRIS in the ALCANZA treatment setting would also limit our sales of, and the commercial potential of, ADCETRIS. In addition, while ADCETRIS product sales grew from 2014 to 2015 and from 2015 to 2016, and our future plans assume that sales of ADCETRIS will increase, we cannot assure you that ADCETRIS sales will continue to grow or that we can maintain sales of ADCETRIS at or near current levels.
We and Takeda have formed a collaboration with Ventana Medical Systems, Inc., or Ventana, under which Ventana is working to develop, manufacture and commercialize a molecular companion diagnostic test with the goal of identifying patients who might respond to treatment with ADCETRIS based on CD30 expression levels in their tissue specimens. However, Ventana may not be able to successfully develop and obtain regulatory approval for a molecular companion diagnostic that may be required by regulatory authorities to support regulatory approval of ADCETRIS in other CD30-expressing malignancies in a timely manner or at all.
Even if we and Takeda receive the required regulatory approvals to market ADCETRIS for any additional indications or in additional jurisdictions, we and Takeda may not be able to effectively commercialize ADCETRIS, including for the reasons set forth above. Our ability to grow ADCETRIS product sales in future periods is also dependent on price increases and we periodically increase the price of ADCETRIS. Price increases on ADCETRIS and negative publicity regarding drug pricing and price increases generally, whether on ADCETRIS or products distributed by other pharmaceutical companies, could negatively affect market acceptance of, and sales of, ADCETRIS. In any event, we cannot assure you that price increases we have taken or may take in the future will not in the future negatively affect ADCETRIS sales.
37
Table of Contents
Reports of adverse events or safety concerns involving ADCETRIS or our product candidates could delay or prevent us from obtaining or maintaining regulatory approvals, or could negatively impact sales of ADCETRIS or the prospects for our product candidates.
Reports of adverse events or safety concerns involving ADCETRIS could interrupt, delay or halt clinical trials of ADCETRIS, including the ongoing FDA-required ADCETRIS post-approval confirmatory study as well as the post-approval confirmatory studies that Takeda is required to conduct as a condition to the conditional marketing authorization of ADCETRIS by the European Commission. For example, during 2013 concerns regarding pancreatitis caused an investigator conducting an independent study involving ADCETRIS to temporarily halt enrollment in the trial and to amend the eligibility criteria and monitoring for the trial. Subsequently, we have revised our prescribing information to add pancreatitis as a known adverse event. In addition, reports of adverse events or safety concerns involving ADCETRIS could result in regulatory authorities denying or withdrawing approval of ADCETRIS for any or all indications, including the use of ADCETRIS for the treatment of patients in its approved indications. There are no assurances that patients receiving ADCETRIS will not experience serious adverse events in the future. Further, there are no assurances that patients receiving ADCETRIS with co-morbid diseases not previously studied, such as autoimmune diseases, will not experience new or different serious adverse events in the future.
Adverse events may negatively impact the sales of ADCETRIS. We may be required to further update the ADCETRIS prescribing information, including boxed warnings, based on reports of adverse events or safety concerns or implement a Risk Evaluation and Mitigation Strategy, or REMS, which could adversely affect ADCETRIS’ acceptance in the market, make competition easier or make it more difficult or expensive for us to distribute ADCETRIS. For example, the prescribing information for ADCETRIS includes pancreatitis, impaired hepatic function, impaired renal function, pulmonary toxicity, and gastrointestinal complications as known adverse events as well as a boxed warning related to the risk that JC virus infection resulting in progressive multifocal leukoencephalopathy, or PML, and death can occur in patients receiving ADCETRIS. Further, based on the identification of future adverse events, we may be required to further revise the prescribing information, including ADCETRIS’ boxed warning, which could negatively impact sales of ADCETRIS or adversely affect ADCETRIS’ acceptance in the market.
Likewise, reports of adverse events or safety concerns involving ADCETRIS or our product candidates could interrupt, delay or halt clinical trials of such product candidates, or could result in our inability to obtain regulatory approvals for any of our product candidates. For example, on December 27, 2016, we announced that we had received notice from the FDA that a full clinical hold or partial clinical hold had been placed on several early stage trials of SGN-CD33A in AML to evaluate the potential risk of hepatotoxicity following adverse medical events, including fatal events. We may be unable to submit to the FDA all required clinical follow-up information to respond to the clinical holds. Even if we are able to provide such information, the FDA may not deem the information to be sufficient to lift any or all of the clinical holds. In addition, we may encounter delays in reaching an agreement with the FDA regarding the terms under which these trials may be resumed, or we may elect to discontinue one or more of these trials for safety or any other reasons. Furthermore, the FDA may require us to implement additional, potentially burdensome pharmacovigilance procedures or conduct additional pre-clinical studies before it will consider lifting the clinical holds, if at all. In addition, although the FDA has not placed a clinical hold on the CASCADE trial or our phase 1/2 trial in MDS and we continue to enroll patients in these trials, we cannot assure you that the FDA or another regulatory authority will not place a clinical hold on one or both of these trials. If we are unable to submit required information to the FDA in a timely manner, or at all; if the FDA does not lift the clinical holds in a timely manner, or at all; if the FDA does not permit us to initiate additional trials of SGN-CD33A and/or the FDA imposes clinical holds on the CASCADE trial or our phase 1/2 trial in MDS; or if there are additional safety results, including from our ongoing trials of SGN-CD33A, that alter the benefit-risk profile of SGN-CD33A or cause it to become unacceptable, we would be further delayed or prevented from advancing the clinical development of SGN-CD33A, which would adversely affect our business, results of operations and prospects.
38
Table of Contents
Concerns regarding the safety of ADCETRIS or our product candidates as a result of undesirable side effects identified during clinical testing or otherwise could cause the FDA to order us to cease further development or commercialization of ADCETRIS or the applicable product candidate. Undesirable side effects caused by ADCETRIS or our product candidates could also result in denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications, the requirement of additional trials or the inclusion of unfavorable information in our product labeling, and in turn delay or prevent us from commercializing ADCETRIS or the applicable product candidate. In addition, actual or potential drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete a trial for ADCETRIS or our product candidates or result in potential product liability claims. Any of these events could prevent us from developing or commercializing ADCETRIS or the particular product candidate, and could significantly harm our business, results of operations and prospects.
Even though we have obtained approval to market ADCETRIS in three indications, we are subject to extensive ongoing regulatory obligations and review, including post-approval requirements that could result in the withdrawal of ADCETRIS from the market for certain indications if such requirements are not met.
ADCETRIS is approved for treating patients in one indication, the relapsed sALCL indication, under accelerated approval regulations in the U.S. and approval with conditions in two indications in Canada, which allow for approval of products for cancer or other serious or life threatening illnesses based on a surrogate endpoint or on a clinical endpoint other than survival or irreversible morbidity. Under these types of approvals, we are subject to certain post-approval requirements pursuant to which we are conducting an additional confirmatory phase 3 trial to verify and describe the clinical benefit of ADCETRIS. Our failure to complete this required post-approval study, or to confirm a clinical benefit during this post-approval study, could result in the withdrawal of approval of ADCETRIS in the relapsed sALCL indication in the U.S. and both indications in Canada, which would seriously harm our business. In addition, under the FDA’s accelerated approval regulations, the labeling, packaging, adverse event reporting, storage, advertising and promotion of ADCETRIS for the treatment of patients with relapsed sALCL is subject to extensive regulatory requirements all of which entails significant expense and may limit our ability to commercialize ADCETRIS for the relapsed sALCL indication. Similarly, the conditional marketing authorization of ADCETRIS for two indications by the European Commission includes obligations to provide additional clinical data at a later time to confirm the results of the two pivotal studies. Takeda’s failure to provide these additional clinical data or to confirm the results of the pivotal studies, could result in the European Commission withdrawing approval of ADCETRIS in the European Union, which would negatively impact anticipated royalty revenue from ADCETRIS sales by Takeda in the European Union and could adversely affect our results of operations. In addition, we are subject to extensive ongoing obligations and continued regulatory review from applicable regulatory agencies with respect to any product for which we have obtained regulatory approval, including ADCETRIS in each of its approved indications, such as continued adverse event reporting requirements and the requirement to have some of our promotional materials pre-cleared by the FDA. There may also be additional post-marketing obligations, all of which may result in significant expense and limit our ability to commercialize ADCETRIS in the United States, Canada or potentially other jurisdictions.
We and the manufacturers of ADCETRIS are also required to comply with current Good Manufacturing Practices, or cGMP, regulations, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation. Further, regulatory agencies must approve these manufacturing facilities before they can be used to manufacture ADCETRIS, and these facilities are subject to ongoing regulatory inspections. In addition, regulatory agencies subject an approved product, its manufacturer and the manufacturer’s facilities to continual review and inspections, including periodic unannounced inspections. The subsequent discovery of previously unknown problems with ADCETRIS, including adverse events of unanticipated severity or frequency, or problems with the facilities where ADCETRIS is manufactured, may result in restrictions on the marketing of ADCETRIS, up to and including withdrawal of ADCETRIS from the market. If our manufacturing facilities or those of our suppliers fail to comply with applicable regulatory requirements, such noncompliance could result in regulatory action and additional costs to us.
39
Table of Contents
Failure to comply with applicable FDA and other regulatory requirements may subject us to administrative or judicially imposed sanctions, including:
| • | issuance of Form FDA 483 notices or Warning Letters by the FDA or other regulatory agencies; |
| • | imposition of fines and other civil penalties; |
| • | criminal prosecutions; |
| • | injunctions, suspensions or revocations of regulatory approvals; |
| • | suspension of any ongoing clinical trials; |
| • | total or partial suspension of manufacturing; |
| • | delays in commercialization; |
| • | refusal by the FDA to approve pending applications or supplements to approved applications submitted by us; |
| • | refusals to permit drugs to be imported into or exported from the United States; |
| • | restrictions on operations, including costly new manufacturing requirements; and |
| • | product recalls or seizures. |
The policies of the FDA and other regulatory agencies may change and additional government regulations may be enacted that could prevent or delay regulatory approval of ADCETRIS in any additional indications or further restrict or regulate post-approval activities. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are not able to maintain regulatory compliance, we or Takeda might not be permitted to market ADCETRIS and our business would suffer.
If we or our collaborators are not able to obtain or maintain required regulatory approvals, we or our collaborators will not be able to successfully commercialize ADCETRIS or our product candidates.
The research, testing, manufacturing, labeling, approval, selling, marketing and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. Neither we nor our collaborators are permitted to market our product candidates in the United States or foreign countries until we obtain marketing approval from the FDA or other foreign regulatory authorities, and we or our collaborators may never receive regulatory approval for the commercial sale of any of our product candidates. In addition, part of our strategy is to continue to explore the use of ADCETRIS earlier in the treatment of Hodgkin lymphoma and MTCL and in other CD30-expressing malignancies, including CTCL, and we are currently conducting multiple clinical trials for ADCETRIS. However, we and/or Takeda may be unable to obtain or maintain any regulatory approvals for the commercial sale of ADCETRIS for any additional indications. Obtaining marketing approval is a lengthy, expensive and uncertain process and approval is never assured, and we have only limited experience in preparing and submitting the applications necessary to gain regulatory approvals. Further, the FDA and other foreign regulatory agencies have substantial discretion in the approval process, and determining when or whether regulatory approval will be obtained for any product candidate we develop, including any regulatory approvals for the potential commercial sale of ADCETRIS in additional indications or in any additional territories. In this regard, even if we believe the data collected from clinical trials of ADCETRIS and our product candidates are promising, such data may not be sufficient to support approval by the FDA or any other foreign regulatory authority. In addition, the FDA or their advisors may disagree with our interpretations of data from preclinical studies and clinical trials. In this regard, based on the positive data we reported from the ALCANZA trial, we plan to submit an sBLA to the FDA to seek approval for a new indication in CD30-expressing relapsed CTCL. Even if the FDA accepts our planned sBLA for filing, the FDA may disagree with our interpretations of the data from the ALCANZA trial and/or may otherwise determine not to approve our planned sBLA in a timely manner
40
Table of Contents
or at all. Moreover, even though our ALCANZA, ECHELON-1 and ECHELON-2 trials are being conducted under SPA agreements with the FDA, this is not a guarantee or indication of approval, and we cannot be certain that the design of, or data collected from, any of our current or potential future clinical trials that were or are being conducted under SPAs with the FDA will be sufficient to support FDA approval. Further, an SPA agreement is not binding on the FDA if public health concerns unrecognized at the time the SPA agreement is entered into become evident, other new scientific concerns regarding product safety or efficacy arise, new drugs are approved in the same indication, or if we have failed to comply with the agreed upon trial protocols. In addition, an SPA agreement may be changed by us or the FDA on written agreement of both parties, and the FDA retains significant latitude and discretion in interpreting the terms of an SPA agreement and the data and results from the applicable clinical trial. For example, even though we believe that the data from the ALCANZA trial are supportive of approval of ADCETRIS in the ALCANZA treatment setting, our SPA with the FDA covering the ALCANZA trial is not a guarantee or indication of approval of ADCETRIS in the ALCANZA treatment setting (or in any other indication). Regulatory agencies also may approve a product candidate for fewer indications than requested or may grant approval subject to the performance of post-approval studies or REMS for a product candidate. Similarly, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of ADCETRIS in additional indications, including any indications in the ALCANZA treatment setting.
In addition, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols and/or related SPA agreements to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to institutional review boards, or IRBs, for reexamination, which may impact the costs, timing or successful completion of a clinical trial. In addition, as part of the U.S. Prescription Drug User Fee Act, or PDUFA, the FDA has a goal to review and act on a percentage of all regulatory submissions in a given time frame. However, the FDA does not always meet its PDUFA targeted action dates and if the FDA were to fail to meet a PDUFA targeted action date in the future for ADCETRIS or any of our product candidates, including in connection with our planned sBLA submission to seek approval to market ADCETRIS in the ALCANZA treatment setting, the commercialization of the affected product candidate or of ADCETRIS in any additional indications could be delayed or impaired. Due to these and other factors, ADCETRIS and our product candidates could take a significantly longer time to gain regulatory approvals than we expect or may never gain new regulatory approvals, which could delay or eliminate any potential product revenue from sales of our product candidates or of ADCETRIS in any additional indications, which could significantly delay or prevent us from achieving profitability.
The successful commercialization of ADCETRIS and product candidates will depend in part on the extent to which governmental authorities and health insurers establish adequate coverage and reimbursement levels and pricing policies.
Successful sales of ADCETRIS and any future products will depend, in part, on the extent to which coverage and reimbursement for our products will be available from government and health administration authorities, private health insurers and other third-party payors. To manage healthcare costs, many governments and third-party payors increasingly scrutinize the pricing of new products and require greater levels of evidence of favorable clinical outcomes and cost-effectiveness before extending coverage. In light of such challenges to prices, we cannot be sure that we will achieve and continue to have coverage available for ADCETRIS and any other product candidate that we commercialize and, if available, that the reimbursement rates will be adequate. If we are unable to obtain adequate levels of coverage and reimbursement for our product candidates, their marketability will be negatively and materially impacted.
Moreover, eligibility for coverage and reimbursement does not imply that a drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. In addition, obtaining and maintaining adequate coverage and reimbursement status is time-consuming and costly. Third-party payors may deny coverage and reimbursement status altogether of a given drug product, or cover the product but may also establish prices at levels that are too low to enable us to realize an appropriate return on our investment in product development. Further, one payor’s determination to provide coverage for a product does
41
Table of Contents
not assure that other payors will also provide coverage for the product. Because the rules and regulations regarding coverage and reimbursement change frequently, in some cases at short notice, even when there is favorable coverage and reimbursement, future changes may occur that adversely impact the favorable status.
The unavailability or inadequacy of third-party coverage and reimbursement could have a material adverse effect on the market acceptance of ADCETRIS and any of our future products and the future revenues we may expect to receive from those products. In addition, we are unable to predict what additional legislation or regulation relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future, or what effect such legislation or regulation would have on our business. Recent negative publicity regarding pharmaceutical prices and the results of the 2016 United States presidential and congressional elections create significant uncertainty regarding regulation of the healthcare industry and third party coverage and reimbursement.
Healthcare law and policy changes may have a material adverse effect on us.
In March 2010, the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively PPACA, became law in the United States. PPACA substantially changed the way healthcare is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. The provisions of PPACA of greatest importance to the pharmaceutical industry include increased Medicaid rebates, expanded Medicaid eligibility, extension of Public Health Service eligibility, annual fees payable by manufacturers and importers of branded prescription drugs, annual reporting of financial relationships with physicians and teaching hospitals, and a new Patient-Centered Outcomes Research Institute. Many of these provisions have had the effect of reducing the revenue generated by our sales of ADCETRIS and will have the effect of reducing any revenue generated by sales of any future commercial products we may have. Further, there have been judicial and Congressional challenges to certain aspects of PPACA In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, or the Budget Resolution, that authorizes the implementation of legislation that would repeal portions of PPACA. The Budget Resolution is not a law; however, it is widely viewed as the first step toward the passage of legislation that would repeal certain aspects of PPACA. Further, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under PPACA to waive, defer, grant exemptions from, or delay the implementation of any provision of PPACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Congress also could consider subsequent legislation to replace elements of PPACA that are repealed. Because of the continued uncertainty about the implementation of PPACA, including the potential for further legal challenges or repeal of PPACA, we cannot quantify or predict with any certainty the likely impact of the PPACA or its repeal on our business, prospects, financial condition or results of operations.
In addition, we anticipate that PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for ADCETRIS or any future approved product, which may harm our business. For example, increased discounts, rebates or chargebacks may be mandated by governmental or private insurers or fee caps and pricing pressures could be enacted by industry organizations or state and federal governments, any of which could significantly affect the revenue generated by sales of our products, including ADCETRIS. In addition, drug- pricing strategies by pharmaceutical companies have recently come under increased scrutiny. Specifically, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for drugs. We expect further federal and state proposals and healthcare reforms to continue to be proposed to control increasing healthcare costs and to control the rising cost of prescription drugs. These proposals, if implemented, could limit the price for ADCETRIS or any future approved products. Commercial opportunity could be negatively impacted by legislative action that controls pricing, mandates price negotiations, or increases government discounts and rebates. For example, in March 2016, the Centers for Medicare and Medicaid Services, or CMS, proposed to conduct a demonstration project that would reduce the Medicare
42
Table of Contents
payment rates for most Part B drugs for approximately half of all providers. If implemented, this project could potentially limit our ability to change pricing for ADCETRIS and all drugs reimbursed under Medicare Part B. Implementation could negatively impact future revenue from sales of ADCETRIS. We cannot predict how this initiative will be affected by the change in U.S. presidential administrations.
Also, price increases on ADCETRIS and negative publicity regarding drug pricing and price increases generally, whether on ADCETRIS or products distributed by other pharmaceutical companies, could negatively affect market acceptance of, and sales of, ADCETRIS. In addition, although ADCETRIS is approved in the European Union, Japan and other countries outside of the United States, government austerity measures or further healthcare reform measures and pricing pressures in other countries could adversely affect demand and pricing for ADCETRIS, which would negatively impact anticipated royalty revenue from ADCETRIS sales by Takeda.
Other legislative changes have also been proposed and adopted since PPACA was enacted. The Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes a 2% reduction in Medicare provider payments paid under Medicare Part B to physicians for physician-administered drugs, such as certain oral oncology drugs, which went into effect in April 2013 and, following passage of the Bipartisan Budget Act of 2015, will remain in effect through 2025 unless additional congressional action is taken. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. In addition, legislation has been proposed to shorten the period of biologic data and market exclusivity granted by the FDA. If such legislation is enacted, we may face competition from biosimilars of ADCETRIS or any future approved products earlier than otherwise would have occurred.
We cannot predict what healthcare reform initiatives may be adopted in the future. However, we anticipate that Congress, state legislatures, and third-party payors may continue to review and assess alternative healthcare delivery and payment systems and may in the future propose and adopt legislation or policy changes or implementations effecting additional fundamental changes in the healthcare delivery system. We also expect ongoing initiatives to increase pressure on drug pricing. We cannot assure you as to the ultimate content, timing, or effect of changes, nor is it possible at this time to estimate the impact of any such potential legislation; however, such changes or the ultimate impact of changes could negatively affect our revenue or sales of ADCETRIS or any future approved products.
Clinical trials are expensive and time consuming, may take longer than we expect or may not be completed at all, and their outcome is uncertain.
We are currently conducting multiple clinical trials for ADCETRIS and our product candidates and we plan to commence additional trials of ADCETRIS and our product candidates in the future. We are also conducting a phase 3 clinical trial for our product candidate SGN-CD33A, or the CASCADE trial, designed to evaluate SGN-CD33A in combination with hypomethylating agents, or HMAs, in previously untreated older AML patients. We initiated the CASCADE trial based on interim data presented at the December 2015 American Society of Hematology annual meeting from a cohort of a phase 1 trial that evaluated SGN-CD33A in combination with HMAs. While the interim phase 1 data may be promising, SGN-CD33A has not previously been evaluated in a randomized trial with other active agents and we cannot be certain that the design of, or data collected from, the CASCADE trial will be adequate to demonstrate the safety and efficacy of SGN-CD33A for the treatment of patients with AML, or will otherwise be sufficient to support FDA or any foreign regulatory approvals. In this regard, on December 27, 2016, we announced that we had received notice from the FDA that a full clinical hold or partial clinical hold had been placed on several early stage trials of SGN-CD33A in AML to evaluate the potential risk of hepatotoxicity following adverse medical events, including fatal events. We may be unable to submit to the FDA all required clinical follow-up information to respond to the clinical holds. Even if we are able
43
Table of Contents
to provide such information, the FDA may not deem the information to be sufficient to lift any or all of the clinical holds. In addition, we may encounter delays in reaching an agreement with the FDA regarding the terms under which these trials may be resumed, or we may elect to discontinue one or more of these trials for safety or any other reasons. Furthermore, the FDA may require us to implement additional, potentially burdensome pharmacovigilance procedures or conduct additional pre-clinical studies before it will consider lifting the clinical holds, if at all. In addition, although the FDA has not placed a clinical hold on the CASCADE trial or our phase 1/2 trial in MDS and we continue to enroll patients in these trials, we cannot assure you that the FDA or another regulatory authority will not place a clinical hold on one or both of these trials. If we are unable to submit required information to the FDA in a timely manner, or at all; if the FDA does not lift the clinical holds in a timely manner, or at all; if the FDA does not permit us to initiate additional trials of SGN-CD33A and/or the FDA imposes clinical holds on the CASCADE trial or our phase 1/2 trial in MDS; or if there are additional safety results, including from our ongoing trials of SGN-CD33A, that alter the benefit-risk profile of SGN-CD33A or cause it to become unacceptable, we would be further delayed or prevented from advancing the clinical development of SGN-CD33A, which would adversely affect our business, results of operations and prospects.
Each of our clinical trials requires the investment of substantial expense and time and the timing of the commencement, continuation and completion of these clinical trials may be subject to significant delays relating to various causes, including scheduling conflicts with participating clinicians and clinical institutions, difficulties in identifying and enrolling patients who meet trial eligibility criteria, failure of patients to complete the clinical trial, delays in accumulating the required number of clinical events for data analyses, delay or failure to obtain IRB approval to conduct a clinical trial at a prospective site, and shortages of available drug supply. For example, in accordance with the terms of their respective SPA, the ECHELON-1 and ECHELON-2 trials are designed to continue until a specified number of events designated for each trial have occurred (i.e. progressions or deaths). If events do not occur at the rate which was anticipated in one or both of these trials, the final data analysis for the applicable trial could be delayed beyond the predicted ranges. In addition, we may choose for a variety of reasons to complete the applicable trial with fewer events than planned. If we choose to complete a trial with fewer events than planned, the FDA may treat the applicable SPA as rescinded and/or the statistical power of that trial based on the original statistical analysis plan could be negatively impacted, making it more difficult for us to demonstrate that ADCETRIS is safe and effective and to successfully obtain regulatory approval for the specific indication studied in that trial. We may also undertake additional discussions with regulatory authorities or amend the protocol for one or both of these trials, and we cannot predict the outcome of those discussions or whether we would be able to reach agreement with the regulatory authorities. Should this situation occur, there could be a delay in our ability to obtain data from the applicable trial or impact the ability of the applicable trial to support approval of ADCETRIS for the specific indication studied in that trial.
Additionally, patient enrollment is a function of many factors, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the existence of competing clinical trials, perceived side effects and the availability of alternative or new treatments. Many of our future and ongoing ADCETRIS clinical trials are being or will be coordinated with Takeda, which may delay the commencement or affect the continuation or completion of these trials. From time to time, we have experienced enrollment-related delays in clinical trials and we will likely continue to experience similar delays in our current and future trials. We depend on medical institutions and clinical research organizations, or CROs, to conduct some of our clinical trials in compliance with Good Clinical Practice, or GCP, and to the extent they fail to enroll patients for our clinical trials, fail to conduct our trials in accordance with GCP, or are delayed for a significant time in achieving full enrollment, we may be affected by increased costs, program delays or both, which may harm our business. In addition, we conduct clinical trials in foreign countries which may subject us to further delays and expenses as a result of increased drug shipment costs, additional regulatory requirements and the engagement of foreign CROs, as well as expose us to risks associated with less experienced clinical investigators who are unknown to the FDA, different standards of medical care, and foreign currency transactions insofar as changes in the relative value of the U.S. dollar to the foreign currency where the trial is being conducted may impact our actual costs.
44
Table of Contents
Clinical trials must be conducted in accordance with FDA or other applicable foreign government guidelines and are subject to oversight by the FDA, other foreign governmental agencies, the data safety monitoring boards for such trials and the IRBs for the institutions in which such trials are being conducted. In addition, clinical trials must be conducted with supplies of ADCETRIS or our product candidates produced under cGMP and other requirements in foreign countries, and may require large numbers of test patients. We, the FDA, other foreign governmental agencies or the applicable data safety monitoring boards and IRBs could delay, suspend, halt or modify our clinical trials of ADCETRIS or any of our product candidates, and we and/or the FDA could terminate or modify any related special protocol assessment, or SPA, agreements, for numerous reasons, including:
| • | ADCETRIS or the applicable product candidate may have unforeseen safety issues or adverse side effects, including fatalities, or a determination may be made that a clinical trial presents unacceptable health risks; |
| • | deficiencies in the conduct of the clinical trial, including failure to conduct the clinical trial in accordance with regulatory requirements, GCP or clinical protocols; |
| • | deficiencies in the clinical trial operations or trial sites resulting in the imposition of a clinical hold; |
| • | the time required to determine whether ADCETRIS or the applicable product candidate is effective may be longer than expected; |
| • | fatalities or other adverse events arising during a clinical trial due to medical problems that may not be related to clinical trial treatments; |
| • | ADCETRIS or the applicable product candidate may not appear to be more effective than current therapies; |
| • | the quality or stability of ADCETRIS or the applicable product candidate may fall below acceptable standards; |
| • | our inability to produce or obtain sufficient quantities of ADCETRIS or the applicable product candidate to complete the trials; |
| • | our inability to reach agreement on acceptable terms with prospective CROs and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| • | our inability to obtain IRB approval to conduct a clinical trial at a prospective site; |
| • | changes in governmental regulations or administrative actions that adversely affect our ability to continue to conduct or to complete clinical trials; |
| • | lack of adequate funding to continue the clinical trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional trials and studies and increased expenses associated with the services of our CROs and other third parties; |
| • | our inability to recruit and enroll patients to participate in clinical trials for reasons including competition from other clinical trial programs for the same or similar indications; |
| • | our inability to retain patients who have initiated a clinical trial but may be prone to withdraw due to side effects from the therapy, lack of efficacy or personal issues, or who are lost to further follow-up; or |
| • | our inability to ensure adequate statistical power to detect statistically significant treatment effects, whether through our inability to enroll or retain patients in trials or as a result of our determination to choose to complete trials with fewer events than planned. |
In addition, we may experience significant setbacks in advanced clinical trials, even after promising results in earlier trials, including unexpected adverse events that may occur when our product candidates are combined with other therapies. For example, on December 27, 2016, we announced that we had received notice from the
45
Table of Contents
FDA that a full clinical hold or partial clinical hold had been placed on several early stage trials of SGN-CD33A in AML to evaluate the potential risk of hepatotoxicity following adverse medical events, including fatal events. We may be unable to submit to the FDA all required clinical follow-up information to respond to the clinical holds, or even if we are able to provide such information, the FDA may not deem the information to be sufficient to lift any or all of the clinical holds. In addition, we may encounter delays in reaching an agreement with the FDA regarding the terms under which these trials may be resumed, or we may elect to discontinue one or more of these trials for safety or any other reasons. Furthermore, the FDA may require us to implement additional, potentially burdensome pharmacovigilance procedures or conduct additional pre-clinical studies before it will consider lifting the clinical holds, if at all. In addition, although the FDA has not placed a clinical hold on the CASCADE trial or our phase 1/2 trial in MDS and we continue to enroll patients in these trials, we cannot assure you that the FDA or another regulatory authority will not place a clinical hold on one or both of these trials. If we are unable to submit required information to the FDA in a timely manner, or at all; if the FDA does not lift the clinical holds in a timely manner, or at all; if the FDA does not permit us to initiate additional trials of SGN-CD33A and/or the FDA imposes clinical holds on the CASCADE trial or our phase 1/2 trial in MDS; or if there are additional safety results, including from our ongoing trials of SGN-CD33A, that alter the benefit-risk profile of SGN-CD33A or cause it to become unacceptable, we would be further delayed or prevented from advancing the clinical development of SGN-CD33A, which would adversely affect our business, results of operations and prospects.
Negative or inconclusive clinical trial results could adversely affect our ability to obtain regulatory approvals of our product candidates or to market ADCETRIS and/or expand ADCETRIS into other indications. In particular, negative or inconclusive results in our ECHELON-1 and ECHELON-2 trials would negatively impact or preclude altogether, our ability to obtain regulatory approval in the frontline Hodgkin lymphoma and frontline MTCL indications, respectively, either of which could limit our sales of, and the commercial potential of, ADCETRIS. Likewise, the imposition of a clinical hold on, or the termination of, or otherwise negative or inconclusive results in our CASCADE trial, would negatively impact or preclude altogether, our ability to obtain regulatory approval of SGN-CD33A. In addition, clinical trial results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. For example, although we reported that our ALCANZA trial met its primary endpoint and we plan to submit an sBLA to the FDA to seek approval for a new indication in CD30-expressing relapsed CTCL, regulatory agencies, including the FDA, or their advisors, may disagree with our interpretations of data from the ALCANZA trial and may not approve the expansion of ADCETRIS’ labeled indications of use based on the results of the ALCANZA trial or any other of our clinical trials. Adverse medical events during a clinical trial, including patient fatalities, such as those hepatotoxicity events and patient fatalities observed in certain of our early stage trials of SGN-CD33A resulting in the imposition by the FDA of clinical holds on those trials, could cause a trial to be redone or terminated, curtail or end the development of a product candidate, and may result in other negative consequences to us. Further, some of our clinical trials, including the CASCADE trial, are overseen by an independent data monitoring committee, or IDMC, and an IDMC may determine to delay or suspend one or more of these trials due to safety or futility findings based on events occurring during a clinical trial.
We face intense competition and rapid technological change, which may result in others discovering, developing or commercializing competing products before or more successfully than we do.
The biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Many third parties compete with us in developing various approaches to treating cancer. They include pharmaceutical companies, biotechnology companies, academic institutions and other research organizations.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approval and marketing than we do. In addition, many of these competitors are active in seeking patent protection and licensing arrangements in anticipation of collecting royalties for use of technology that they have developed. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative
46
Table of Contents
arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, as well as in acquiring technologies complementary to our programs.
With respect to ADCETRIS, there are several other FDA-approved drugs for the treatment of relapsed or refractory Hodgkin lymphoma or sALCL, including Bristol-Myers Squibb’s Opdivo for Hodgkin lymphoma after failure of auto-HSCT and ADCETRIS and Celgene’s Istodax and Spectrum Pharmaceuticals’ Folotyn for relapsed or refractory peripheral T-cell lymphoma. Merck is also developing a PD-1 inhibitor, pembrolizumab, and is conducting a clinical trial in relapsed or refractory classical Hodgkin lymphoma comparing pembrolizumab with ADCETRIS. In addition, we are aware of multiple investigational agents that are currently being studied, including Pfizer’s crizotinib, AbbVie’s ibrutinib, Kyowa’s mogamulizumab, and Gilead’s idelalisib, which, if successful, may compete with ADCETRIS in the future. Data have also been presented on several developing technologies, including bispecific antibodies and CAR modified T-cell therapies that may compete with ADCETRIS in the future. Further, there are many competing approaches used in the treatment of patients in ADCETRIS’ three approved indications, including auto-HSCT, conventional therapies, combination chemotherapy, clinical trials with experimental agents and single-agent regimens.
With respect to SGN-CD33A, there are several investigational agents that, if approved, could be competitive with our product candidate, including AbbVie’s venetoclax, which received breakthrough therapy designation from the FDA in January 2016 for use in combination with HMAs in treatment-naïve patients with AML who are not eligible for standard high-dose induction treatment, Jazz’s Vyxeos, Pfizer’s Mylotarg, Astex’s guadecitabine (SGI-110), and agents targeting biomarkers such as FLT3 and IDH1/2.
Many other pharmaceutical and biotechnology companies are developing and/or marketing therapies for the same types of cancer that our product candidates are designed and being developed to treat. For example, we believe that companies including AbbVie, ADC Therapeutics, Affimed, Agios, Amgen, Astellas, Bayer, Biogen, Bristol-Myers Squibb, Celgene, Eisai, Genentech, GSK, Gilead, ImmunoGen, Immunomedics, Infinity, Karyopharm, MedImmune, MEI Pharma, Merck, Novartis, Pfizer, Sanofi-Aventis, Spectrum Pharmaceuticals, Takeda, Teva, and Xencor are developing and/or marketing products or technologies that may compete with ours. In addition, our ADC collaborators may develop compounds utilizing our technology that may compete with product candidates that we are developing.
We are aware of other companies that have technologies that may be competitive with ours, including Astellas, AstraZeneca, Bristol-Myers Squibb, ImmunoGen, Immunomedics, MedImmune, Mersana and Pfizer, all of which have ADC technology. ImmunoGen has several ADCs in development that may compete with our product candidates. ImmunoGen has also established partnerships with other pharmaceutical and biotechnology companies to allow those other companies to utilize ImmunoGen’s technology, including Sanofi-Aventis, Genentech, Novartis, Takeda and Lilly. We are also aware of a number of companies developing monoclonal antibodies directed at the same antigen targets or for the treatment of the same diseases as our product candidates. For example, we believe Bristol-Myers Squibb has an anti-CD30 antibody program that may be competitive with ADCETRIS, and Amgen and Xencor have anti-CD19 programs that may be competitive with our product candidates.
In addition, in the United States, the Biologics Price Competition and Innovation Act of 2009 created an abbreviated approval pathway for biological products that are demonstrated to be “highly similar” or “biosimilar” to or “interchangeable” with an FDA-approved biological product. This pathway allows competitors to reference the FDA’s prior approvals regarding innovative biological products and data submitted with a BLA to obtain approval of a biosimilar application 12 years after the time of approval of the innovative biological product. The 12-year exclusivity period runs from the initial approval of the innovator product and not from approval of a new indication. In addition, the 12-year exclusivity period does not prevent another company from independently developing a product that is highly similar to the innovative product, generating all the data necessary for a full BLA and seeking approval. Exclusivity only assures that another company cannot rely on the FDA’s prior
47
Table of Contents
approvals in approving a BLA for an innovator’s biological product to support the biosimilar product’s approval. Further, under the FDA’s current interpretation, it is possible that a biosimilar applicant could obtain approval for one or more of the indications approved for the innovator product by extrapolating clinical data from one indication to support approval for other indications. The FDA approved the first biosimilar product in the United States in May 2015. In the European Union, the European Commission has granted marketing authorizations for several biosimilars pursuant to a set of general and product class-specific guidelines for biosimilar approvals issued since 2005. We are aware of many pharmaceutical and biotechnology and other companies that are actively engaged in research and development of biosimilars or interchangeable products.
It is possible that our competitors will succeed in developing technologies that are more effective than ADCETRIS, SGN-CD33A or our other product candidates or that would render our technology obsolete or noncompetitive, or will succeed in developing biosimilar or interchangeable products for ADCETRIS, SGN-CD33A or our other product candidates. We anticipate that we will face increased competition in the future as new companies enter our market and scientific developments surrounding biosimilars and other cancer therapies continue to accelerate. We cannot predict to what extent the entry of biosimilars or other competing products will impact potential future sales of ADCETRIS, SGN-CD33A or our other product candidates.
Our operating results are difficult to predict and may fluctuate. If our operating results are below the expectations of securities analysts or investors, the trading price of our stock could decline.
Our operating results are difficult to predict and may fluctuate significantly from quarter to quarter and year to year. In addition, although we provide sales guidance for ADCETRIS from time to time, you should not rely on ADCETRIS sales results in any period as being indicative of future performance. Such guidance is based on assumptions that may be incorrect or that may change from quarter to quarter. Sales of ADCETRIS have, on occasion, been below the expectations of securities analysts and investors and have been below prior period sales, and sales of ADCETRIS in the future may also be below prior period sales, our own guidance and/or the expectations of securities analysts and investors. To the extent that we do not meet our guidance or the expectations of analysts or investors, our stock price may be adversely impacted, perhaps significantly. We believe that our quarterly and annual results of operations may be affected by a variety of factors, including:
| • | customer ordering patterns for ADCETRIS, which may vary significantly from period to period; |
| • | the overall level of demand for ADCETRIS including the impact of any competitive or biosimilar products and the duration of therapy for patients receiving ADCETRIS; |
| • | the extent to which coverage and reimbursement for ADCETRIS is available from government and health administration authorities, private health insurers, managed care programs and other third-party payers; |
| • | changes in the amount of deductions from gross sales, including government-mandated rebates, chargebacks and discounts that can vary because of changes to the government discount percentage, including increases in the government discount percentage resulting from price increases we have taken or may take in the future, or due to different levels of utilization by entities entitled to government rebates and discounts and changes in patient demographics; |
| • | increases in the scope of eligibility for customers to purchase ADCETRIS at the discounted government price or to obtain government-mandated rebates on purchases of ADCETRIS; |
| • | changes in our cost of sales; |
| • | the incidence rate of new patients in ADCETRIS’ approved indications; |
| • | the timing, cost and level of investment in our sales and marketing efforts to support ADCETRIS sales; |
| • | the timing, cost and level of investment in our research and development and other activities involving ADCETRIS, SGN-CD33A and our other product candidates by us or our collaborators; |
| • | expenditures we will or may incur to develop and/or commercialize any additional products, product candidates, or technologies that we may develop, in-license, or acquire. |
48
Table of Contents
In addition, we have entered into licensing and collaboration agreements with other companies that include development funding and milestone payments to us, and we expect that amounts earned from our collaboration agreements will continue to be an important source of our revenues. Accordingly, our revenues will also depend on development funding and the achievement of development and clinical milestones under our existing collaboration and license agreements, including, in particular, our ADCETRIS collaboration with Takeda, as well as entering into potential new collaboration and license agreements. These upfront and milestone payments may vary significantly from quarter to quarter and any such variance could cause a significant fluctuation in our operating results from one quarter to the next.
Further, changes in our operations, such as increased development, manufacturing and clinical trial expenses in connection with our expanding pipeline programs, including several phase 3 trials, or our undertaking of additional programs, business activities or entry into strategic transactions, including potential additional acquisitions of products, technologies or businesses, such as our anticipated in-licensing of IMMU-132, may also cause significant fluctuations in our expenses. In addition, we measure compensation cost for stock-based awards made to employees at the grant date of the award, based on the fair value of the award, and recognize the cost as an expense over the employee’s requisite service period. As the variables that we use as a basis for valuing these awards change over time, including our underlying stock price, the magnitude of the expense that we must recognize may vary significantly. Additionally, we have implemented long-term incentive plans for our employees, and the incentives provided under these plans are contingent upon the achievement of certain regulatory milestones. Costs of performance-based compensation under our long-term incentive plans are not recorded as an expense until the achievement of the applicable milestones is deemed probable of being met, which may result in large fluctuations to the expense we must recognize in any particular period.
For these and other reasons, it is difficult for us to accurately forecast future sales of ADCETRIS, collaboration and license agreement revenues, royalty revenues, operating expenses or future profits or losses. As a result, our operating results in future periods could be below our guidance or the expectations of securities analysts or investors, which could cause the trading price of our common stock to decline, perhaps substantially.
We have a history of net losses. We expect to continue to incur net losses and may not achieve profitability for some time, if at all.
We have incurred substantial net losses in each of our years of operation. We have incurred these losses principally from costs incurred in our research and development programs and from our selling, general and administrative expenses. We expect to continue to spend substantial amounts on research and development, including amounts for conducting required post-approval and other clinical trials of, and seeking additional regulatory approvals for, ADCETRIS as well as commercializing ADCETRIS for the treatment of patients in its three approved indications. In addition, we expect to make substantial expenditures to further develop and potentially commercialize SGN-CD33A and our other product candidates. Likewise, in connection with our anticipated in-licensing of IMMU-132, we expect to incur substantial upfront and milestone payment obligations and to make to make substantial expenditures to potentially further develop and potentially commercialize IMMU-132. Accordingly, we expect to continue to incur net losses and may not achieve profitability for some time, if at all. Although we recognize revenue from ADCETRIS product sales and we continue to earn amounts under our collaboration agreements, our revenue and profit potential is unproven and our limited commercialization history makes our future operating results difficult to predict. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to achieve and sustain profitability, the market value of our common stock will likely decline.
49
Table of Contents
We depend on collaborative relationships with other companies to assist in the research and development of ADCETRIS and for the development and commercialization of product candidates utilizing or incorporating our technologies. If we are not able to locate suitable collaborators or if our collaborators do not perform as expected, this may negatively affect our ability to commercialize ADCETRIS, develop other product candidates and/or generate revenues through technology licensing, or may otherwise negatively affect our business.
We have established collaborations with third parties to develop and market ADCETRIS and some of our current and future product candidates. For example, we entered into a collaboration agreement with Takeda in December 2009 that granted Takeda rights to develop and commercialize ADCETRIS outside of the United States and Canada. In addition, we have entered into a 50/50 co-development agreement with Astellas for the development of ADCs, including ASG-22ME. We also have ADC collaborations with AbbVie, Bayer, Celldex, Genentech, GSK, Pfizer and Progenics, and an ADC co-development agreement with Genmab. In addition, we have entered into a collaboration agreement with Unum to develop and commercialize novel antibody-coupled T-cell receptor, or ACTR, therapies incorporating our antibodies for cancer. Our dependence on collaborative arrangements to assist in the development and commercialization of ADCETRIS and for the development and commercialization of product candidates utilizing or incorporating our technologies subjects us to a number of risks, including:
| • | we are not able to control the amount and timing of resources that our collaborators or co-development partners devote to the development or commercialization of products and product candidates utilizing or incorporating our technologies, or to their marketing and distribution; |
| • | disputes may arise between us and our collaborators or co-development partners that result in the delay or termination of the research, development or commercialization of the applicable products and product candidates or that result in costly litigation or arbitration that diverts management’s attention and resources; |
| • | with respect to collaboration and co-development arrangements under which we have an active role, such as our ADCETRIS collaboration and our 50/50 co-development agreement with Astellas, we may have differing opinions or priorities than our collaborators or co-development partners, or we may encounter challenges in joint decision making, which may result in the delay or termination of the research, development or commercialization of the applicable products and product candidates, including ADCETRIS and ASG-22ME; |
| • | our current and potential future collaborators and co-development partners may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| • | significant delays in the development of product candidates by current and potential collaborators and co-development partners could allow competitors to bring products to market before product candidates utilizing or incorporating our technologies are approved and impair the ability of current and potential future collaborators and co-development partners to effectively commercialize these product candidates; |
| • | our relationships with our collaborators and co-development partners may divert significant time and effort of our scientific staff and management team and require the effective allocation of our resources to multiple internal, collaborative and co-development projects; |
| • | our current and potential future collaborators and co-development partners may not be successful in their efforts to obtain regulatory approvals in a timely manner, or at all; |
| • | our current and potential future collaborators and co-development partners may receive regulatory sanctions relating to other aspects of their business that could adversely affect the development, approval or commercialization of the applicable products or product candidates; |
| • | our current and potential future collaborators and co-development partners may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
50
Table of Contents
| • | business combinations or significant changes in a collaborator’s or co-development partner’s business strategy may adversely affect such party’s willingness or ability to complete its obligations under any arrangement; |
| • | a collaborator or co-development partner could independently move forward with competing products, therapeutic approaches or technologies to develop treatments for the diseases targeted by us or our collaborators or co-development partners that are developed by such collaborator or co-development partner either independently or in collaboration with others, including our competitors; |
| • | our current and potential collaborators and co-development partners may experience financial difficulties; and |
| • | our collaborations and co-development agreements may be terminated, breached or allowed to expire, or our collaborators or co-development partners may reduce the scope of our agreements with them, which could have a material adverse effect on our financial position by reducing or eliminating the potential for us to receive technology access and license fees, milestones and royalties, and/or reimbursement of development costs, and which could require us to devote additional efforts and to incur the additional costs associated with pursuing internal development and commercialization of the applicable products and product candidates. |
If our collaborative or co-development arrangements are not successful as a result of any of the above factors, or any other factors, then our ability to advance the development and commercialization of the applicable products and product candidates and to otherwise generate revenue from these arrangements and to become profitable will be adversely affected, and our business and business prospects may be materially harmed. In particular, if Takeda were to terminate the ADCETRIS collaboration, we would not receive milestone payments, co-funded development payments or royalties for the sale of ADCETRIS outside the United States and Canada. As a result of such termination, we may have to engage another collaborator to complete the ADCETRIS development process and to commercialize ADCETRIS outside the United States and Canada, or to complete the development process and undertake commercializing ADCETRIS outside the United States and Canada ourselves, either of which could significantly delay the continued development and commercialization of ADCETRIS and increase our costs. In turn, this could significantly harm our financial position, adversely affect our stock price and require us to incur all the costs of developing and commercializing ADCETRIS, which are now being co-funded by Takeda.
In the future, we may not be able to locate third-party collaborators or co-development partners to develop and market products and product candidates utilizing or incorporating our technologies, and we may lack the capital and resources necessary to develop and market these products and product candidates alone.
We have engaged in, and may in the future engage in strategic transactions that increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities and subject us to other risks.
We actively evaluate various strategic transactions on an ongoing basis, including licensing or otherwise acquiring complementary products, technologies or businesses, and we recently announced our entry into the Immunomedics License pursuant to which, if consummated, we would be granted an exclusive worldwide license to IMMU-132. Any potential acquisitions or in-licensing transactions, such as our anticipated in-licensing of IMMU-132, may entail numerous risks, including but not limited to:
| • | risks associated with satisfying the closing conditions relating to such transactions and realizing their anticipated benefits; |
| • | increased operating expenses and cash requirements; |
| • | difficulty integrating acquired technologies, products, operations, and personnel with our existing business; |
51
Table of Contents
| • | diversion of management’s attention in connection with both negotiating the acquisition or license and integrating the business, technology or product; |
| • | retention of key employees; |
| • | uncertainties in our ability to maintain key business relationships of any acquired entities; |
| • | strain on managerial and operational resources; |
| • | difficulty implementing and maintaining effective internal control over financial reporting at businesses that we acquire, particularly if they are not located near our existing operations; |
| • | exposure to unforeseen liabilities of acquired companies or companies in which we invest; and |
| • | potential costly and time-consuming litigation, including stockholder lawsuits. |
As a result of these or other problems and risks, businesses, technologies or products we acquire or invest in or obtain licenses to may not produce the revenues, earnings or business synergies that we anticipated, acquired or licensed technologies may not result in regulatory approvals, and acquired or licensed products may not perform as expected. As a result, we may incur higher costs and realize lower revenues than we had anticipated. We cannot assure you that any acquisitions or investments we have made or may make in the future, including our anticipated in-licensing of IMMU-132, will be completed or that, if completed, the acquired business, licenses, investments, products, or technologies will generate sufficient revenue to offset the negative costs or other negative effects on our business. Failure to manage effectively our growth through acquisition or in-licensing transactions could adversely affect our growth prospects, business, results of operations, financial condition, and cash flow.
In addition, we may spend significant amounts, issue dilutive securities, assume or incur significant debt obligations, incur large one-time expenses and acquire intangible assets in connection with acquisitions and in-licensing transactions that could result in significant future amortization expense and write-offs. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or products that may be important to the development of our business. Even if appropriate opportunities are available, we may not be able to successfully identify them or we may not have the financial resources necessary to pursue them, and if pursued, we may be unable to structure and execute transactions in the anticipated timeframe, or at all. Other pharmaceutical companies, many of which may have substantially greater financial, marketing and sales resources, compete with us for these opportunities.
Even if we are able to successfully identify and acquire complementary products, technologies or businesses, we cannot assure you that we will be able to successfully manage the risks associated with integrating acquired products, technologies or businesses or the risks arising from anticipated and unanticipated problems in connection with an acquisition or in-licensing transaction. Further, while we seek to mitigate risks and liabilities of potential acquisitions and in-licensing transactions through, among other things, due diligence, there may be risks and liabilities that such due diligence efforts fail to discover, that are not disclosed to us, or that we inadequately assess. Any failure in identifying and managing these risks and uncertainties effectively, including in connection with the anticipated in-licensing of IMMU-132, would have a material adverse effect on our business. Additionally, we may not realize the anticipated benefits of such transactions, including the possibility that expected synergies and accretion will not be realized or will not be realized within the expected time frame.
Our current product candidates are in various stages of clinical development, and it is possible that none of these product candidates will ever become commercial products.
Our current product candidates are in various stages of clinical development, and other than SGN-CD33A, our product candidates are in relatively early stages of development. Our product candidates will require significant further development, financial resources and personnel to obtain regulatory approval and develop into commercially viable products, if at all. In 2016, we initiated the CASCADE trial, which is designed to evaluate SGN-CD33A in combination with HMAs in previously untreated older AML patients. SGN-CD33A has not
52
Table of Contents
previously been evaluated in a randomized trial with other active agents and we cannot be certain that the design of, or data collected from, the CASCADE trial will be adequate to demonstrate the safety and efficacy of SGN-CD33A for the treatment of patients with AML, or will otherwise be sufficient to support FDA or any foreign regulatory approvals. In addition, certain early stage trials of SGN-CD33A are currently under clinical holds to evaluate the potential risk of hepatotoxicity following adverse medical events, including fatal events. We may be unable to submit to the FDA all required clinical follow-up information to respond to the clinical holds. Even if we are able to provide such information, the FDA may not deem the information to be sufficient to lift any or all of the clinical holds. In addition, we may encounter delays in reaching an agreement with the FDA regarding the terms under which these trials may be resumed, or we may elect to discontinue one or more of these trials for safety or any other reasons. Furthermore, the FDA may require us to implement additional, potentially burdensome pharmacovigilance procedures or conduct additional pre-clinical studies before it will consider lifting the clinical holds, if at all. In addition, although the FDA has not placed a clinical hold on the CASCADE trial or our phase 1/2 trial in MDS and we continue to enroll patients in these trials, we cannot assure you that the FDA or another regulatory authority will not place a clinical hold on one or both of these trials. If we are unable to submit required information to the FDA in a timely manner, or at all; if the FDA does not lift the clinical holds in a timely manner, or at all; if the FDA does not permit us to initiate additional trials of SGN-CD33A and/or the FDA imposes clinical holds on the CASCADE trial or our phase 1/2 trial in MDS, or if there are additional safety results, including from our ongoing trials of SGN-CD33A that alter the benefit-risk profile of SGN-CD33A or cause it to become unacceptable, we would be further delayed or prevented from advancing the clinical development of SGN-CD33A, which would adversely affect our business, results of operations and prospects.
Currently, our other clinical-stage product candidates include seven ADC programs, which consist of ASG-22ME, SGN-LIV1A, SGN-CD19A, SGN-CD19B, SGN-CD123A, SGN-352A, and ASG-15ME, as well as two immuno-oncology agents, SEA-CD40, which is based on our sugar-engineered antibody, or SEA, technology, and SGN-2FF, which is a novel small molecule. If a product candidate fails at any stage of development or we otherwise determine to discontinue development of that product candidate, we will not have the anticipated revenues from that product candidate to fund our operations, and we may not receive any return on our investment in that product candidate. Moreover, we still have only limited data from our phase 1 trials of our product candidates. In this regard, preclinical studies and any encouraging or positive preliminary and interim data from our clinical trials of our product candidates may not necessarily be predictive of the results of ongoing or later clinical trials. Even if we are able to complete our planned clinical trials of our product candidates according to our current development timeline, the encouraging or positive results from clinical trials of our product candidates in earlier stage trials may not be replicated in subsequent clinical trial results. As a result, we may conduct lengthy and expensive clinical trials of our product candidates only to learn that a product candidate is not an effective treatment or is not superior to existing approved therapies, or has an unacceptable safety profile, which could prevent or significantly delay regulatory approval for such product candidate. Also, our later-stage clinical trials could differ in significant ways from earlier stage clinical trials, which could cause the outcome of the later-stage trials to differ from our earlier stage clinical trials. For instance, though we reported phase 1 interim efficacy data that supported our decision to initiate the CASCADE trial, we may not receive positive results for SGN-CD33A even if we complete the CASCADE trial. Differences in earlier and later stage clinical trials may include changes to inclusion and exclusion criteria, efficacy endpoints and statistical design. Many companies in the pharmaceutical and biotechnology industries, including us, have suffered significant setbacks in late-stage clinical trials after achieving encouraging or positive results in early-stage development. We cannot be certain that we will not face similar setbacks in our ongoing clinical trials, for instance, in the CASCADE trial. We have not yet completed any late-stage clinical trials for our current product candidates, and if we fail to produce positive results in our ongoing or planned clinical trials of any of our product candidates, the development timeline and regulatory approval and commercialization prospects for our product candidates, and, correspondingly, our business and financial prospects, would be materially adversely affected.
Due to the uncertain and time-consuming clinical development and regulatory approval process, we may not successfully develop any of our product candidates and it is possible that none of our current product candidates will ever become commercial products. In addition, we expect that much of our effort and many of our
53
Table of Contents
expenditures over the next few years will be devoted to the additional clinical development of and commercialization activities associated with ADCETRIS, which may restrict or delay our ability to develop our clinical and preclinical product candidates.
To date, we have depended on a small number of collaborators for a substantial portion of our revenue. The loss of any one of these collaborators or changes in their product development or business strategy could result in a material decline in our revenue.
We have collaborations with a limited number of companies. To date, a substantial portion of our revenue has resulted from payments made under agreements with our corporate collaborators, and although ADCETRIS sales currently comprise a greater proportion of our revenue, we expect that a portion of our revenue will continue to come from corporate collaborations. Even though we market ADCETRIS in the United States and Canada, our revenues still depend in part on Takeda’s ability and willingness to market ADCETRIS outside of the United States and Canada. The loss of our collaborators, especially Takeda, changes in product development or business strategies of our collaborators, or the failure of our collaborators to perform their obligations under their agreements with us for any reason, including paying license or technology fees, milestone payments, royalties or reimbursements, could have a material adverse effect on our financial performance. Payments under our existing and potential future collaboration agreements are also subject to significant fluctuations in both timing and amount, which could cause our revenue to fall below the expectations of securities analysts and investors and cause a decrease in our stock price.
We are dependent upon a small number of distributors for a significant portion of our net sales, and the loss of, or significant reduction or cancellation in sales to, any one of these distributors could adversely affect our operations and financial condition.
In the United States and Canada, we sell ADCETRIS through a limited number of pharmaceutical distributors. Customers order ADCETRIS through these distributors. We generally receive orders from distributors and ship product directly to the customer. We do not promote ADCETRIS to these distributors and they do not set or determine demand for ADCETRIS; however, our ability to effectively commercialize ADCETRIS will depend, in part, on the performance of these distributors. Although we believe we can find alternative distributors on relatively short notice, the loss of a major distributor could materially and adversely affect our results of operations and financial condition.
We currently rely on third-party manufacturers and other third parties for production of our drug products and our dependence on these manufacturers may impair the continued development and commercialization of ADCETRIS.
We do not currently have the internal ability to manufacture the drug products that we sell or need to conduct our clinical trials, and we therefore rely on corporate collaborators and contract manufacturing organizations to supply drug product for commercial supply and our IND-enabling studies and clinical trials. For the monoclonal antibody used in ADCETRIS, we have contracted with AbbVie for clinical and commercial supplies. For the drug linker used in ADCETRIS, we have contracted with Sigma Aldrich Fine Chemicals, or SAFC, for clinical and commercial supplies. We have multiple contract manufacturers for conjugating the drug linker to the antibody and producing the ADCETRIS product. For our ADC product candidates, multiple contract manufacturers, including AbbVie and SAFC, perform antibody and drug-linker manufacturing and several other contract manufacturers perform conjugation of the drug-linker to the antibody and fill/finish of the drug product. In addition, we rely on other third parties to perform additional steps in the manufacturing process, including shipping and storage of ADCETRIS and our product candidates. For the foreseeable future, we expect to continue to rely on contract manufacturers and other third parties to produce, vial and store sufficient quantities of ADCETRIS for use in our clinical trials and for commercial sale. If our contract manufacturers or other third parties fail to deliver ADCETRIS for clinical use or sale on a timely basis, with sufficient quality, and at commercially reasonable prices, and we fail to find replacement manufacturers or to develop our own manufacturing capabilities, we may be required to delay or suspend clinical trials or otherwise discontinue development, production and sale of ADCETRIS. Moreover, contract manufacturers have a limited number of
54
Table of Contents
facilities in which ADCETRIS can be produced and any interruption of the operation of those facilities due to events such as equipment malfunction or failure or damage to the facility by natural disasters or as the result of regulatory actions could result in the cancellation of shipments, loss of product in the manufacturing process, a shortfall in ADCETRIS supply, or the inability to sell our products in the U.S. or abroad. In addition, we have committed to provide Takeda with their needs of certain parts of the ADCETRIS supply chain for a limited period of time, which may require us to arrange for additional manufacturing supply. Moreover, we depend on outside vendors for the supply of raw materials used to produce ADCETRIS. If the third-party suppliers were to cease production or otherwise fail to supply us with quality raw materials and we were unable to contract on acceptable terms for these raw materials with alternative suppliers, our ability to have ADCETRIS manufactured to meet commercial and clinical requirements would be adversely affected.
We are subject to various state and federal laws and regulations, including healthcare laws and regulations, that may impact our business and could subject us to significant fines and penalties or other negative consequences.
Our operations may be directly or indirectly subject to various state and federal healthcare laws, including, without limitation, the federal Anti-Kickback Statute, federal civil and criminal false claims laws, HIPAA/HITECH, the federal civil monetary penalties statute, and the federal transparency requirements under the PPACA. These laws may impact, among other things, the sales, marketing and education programs for ADCETRIS.
The federal Anti-Kickback Statute prohibits persons and entities from knowingly and willingly soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. Additionally, PPACA amended the intent requirement of the federal Anti-Kickback Statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Penalties for violations of the federal Anti-Kickback Statute include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs.
The federal civil and criminal false claims laws, including the civil False Claims Act, prohibit, among other things, persons or entities from knowingly presenting, or causing to be presented, a false claim to, or the knowing use of false statements to obtain payment from or approval by the federal government, including the Medicare and Medicaid programs, or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim or to avoid, decrease, or conceal an obligation to pay money to the federal government. PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act. Suits filed under the civil False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, commonly known as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. Many pharmaceutical and other healthcare companies have recently been investigated or subject to lawsuits by whistleblowers and have reached substantial financial settlements with the federal government under the False Claims Act for a variety of alleged improper marketing or other activities, including providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees, grants, free travel, and other benefits to physicians to induce them to prescribe the company’s products; and inflating prices reported to private price publication services, which are used to set drug reimbursement rates under government healthcare programs.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing, or attempting to
55
Table of Contents
execute, a scheme to defraud any healthcare benefit program, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. Similar to the Anti-Kickback Statute, PPACA amended the intent requirement of the criminal healthcare fraud statutes such that a person or entity no longer needs to have actual knowledge of the statute or intent to violate it.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, governs certain types of individuals and entities with respect to the conduct of certain electronic healthcare transactions and imposes certain obligations with respect to the security and privacy of protected health information.
The federal civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
The federal transparency requirements under PPACA, the Physician Payments Sunshine Act, require certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program to annually report to the U.S. Department of Health and Human Services’ Centers for Medicare & Medicaid Services information related to payments and other transfers of value to physicians and teaching hospitals, and physician ownership and investment interests.
There are foreign and state law equivalents of these laws and regulations, such as anti-kickback, false claims, and data privacy and security laws, to which we are currently and/or may in the future, be subject. We may also be subject to state laws that require manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. Many of these state laws differ from each other in significant ways, thus complicating compliance efforts.
The FDA and other governmental authorities also actively investigate allegations of off-label promotion activities in order to enforce regulations prohibiting these types of activities. In recent years, private whistleblowers have also pursued False Claims Act cases against a number of pharmaceutical companies for causing false claims to be submitted as a result of off-label promotion. If we are found to have promoted an approved product, including ADCETRIS, for off-label uses we may be subject to significant liability, including civil and administrative financial penalties and other remedies as well as criminal financial penalties and other sanctions. Even when a company is not determined to have engaged in off-label promotion, the allegation from government authorities or market participants that a company has engaged in such activities could have a significant impact on the company’s sales, business and financial condition. The U.S. government has also required companies to enter into complex corporate integrity agreements and/or non-prosecution agreements that impose significant reporting and other burdens on the affected companies.
We are also subject to numerous other laws and regulations that are not specific to the healthcare industry. For instance, the U.S. Foreign Corrupt Practices Act, or FCPA, prohibits companies and individuals from engaging in specified activities to obtain or retain business or to influence a person working in an official capacity. Under the FCPA, it is illegal to pay, offer to pay, or authorize the payment of anything of value to any foreign government official, governmental staff members, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls.
The number and complexity of both U.S. federal and state laws continue to increase. In addition to enforcement by governmental agencies, we also expect a continuation of the trend of private plaintiff lawsuits
56
Table of Contents
against pharmaceutical manufacturers under the whistleblower provisions of the False Claims Act and state equivalents or other laws and regulations such as securities rules and the evolution of new theories of liability under those statutes. Government agencies will likely continue to intervene in such private whistleblower lawsuits and such intervention typically raises the company’s cost significantly. For example, federal enforcement agencies have recently scrutinized product and patient assistance programs, including manufacturer reimbursement support services as well as relationships with specialty pharmacies. Several investigations have resulted in government enforcement authorities intervening in related whistleblower lawsuits and obtaining significant civil and criminal settlements.
In order to comply with these laws, we have implemented a comprehensive compliance program to actively identify, prevent and mitigate risk through the implementation of compliance policies and systems and by promoting a culture of compliance. Although we take our obligation to maintain our compliance with these various laws and regulations seriously and our compliance program is designed to prevent the violation of these laws and regulations, we cannot guarantee that our compliance program will be sufficient or effective, that our employees will comply with our policies and that our employees will notify us of any violation of our policies, that we will have the ability to take appropriate and timely corrective action in response to any such violation, or that we will make decisions and take actions that will necessarily limit or avoid liability for whistleblower claims that individuals, such as employees or former employees, may bring against us or that governmental authorities may prosecute against us based on information provided by individuals. If we are found to be in violation of any of the laws and regulations described above or other applicable state and federal healthcare laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, disgorgement, contractual damages, reputational harm, imprisonment, diminished profits and future earnings, exclusion from government healthcare reimbursement programs, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and/or the curtailment or restructuring of our operations, any of which could have a material adverse effect on our business, results of operations and growth prospects. Any action against us for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal, state and foreign healthcare laws is costly and time-consuming for our management.
As we expand our operations internationally, we are subject to an increased risk of conducting activities in a manner that violates applicable anti-bribery or anti-corruption laws. We are also subject to foreign laws and regulations covering data privacy and the protection of health-related and other personal information. These laws and regulations could create liability for us or increase our cost of doing business, any of which could have a material adverse effect on our business, results of operations and growth prospects.
We are expanding our operations internationally, and we currently have subsidiaries in the U.K., Switzerland and Canada. Though we are at an early stage with our international expansion, our business activities outside of the United States are subject to the FCPA, which is described above, and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we currently and may in the future operate, including the U.K. Bribery Act. The U.K. Bribery Act prohibits giving, offering, or promising bribes to any person, including non-U.K. government officials and private persons, as well as requesting, agreeing to receive, or accepting bribes from any person. In addition, under the U.K. Bribery Act, companies which carry on a business or part of a business in the U.K. may be held liable for bribes given, offered or promised to any person, including non-U.K. government officials and private persons, by employees and persons associated with such company in order to obtain or retain business or a business advantage for such company. In the course of expanding our operations internationally, we will need to establish and expand business relationships with various third parties, such as independent contractors, distributors, vendors, advocacy groups and physicians, and we will interact more frequently with foreign officials, including regulatory authorities and physicians employed by state-run healthcare institutions who may be deemed to be foreign officials under the FCPA, U.K. Bribery Act or similar laws of other countries that may govern our activities. Any interactions with any such parties or individuals where compensation is provided that are found to be in violation of such laws could result in substantial fines and penalties and could materially harm our business. Furthermore, any finding of a violation
57
Table of Contents
under one country’s laws may increase the likelihood that we will be prosecuted and be found to have violated another country’s laws. If our business practices outside the United States are found to be in violation of the FCPA, U.K. Bribery Act or other similar laws, we may be subject to significant civil and criminal penalties which could have a material adverse effect on our business, results of operations and growth prospects. We are also subject to foreign laws and regulations covering data privacy and the protection of health-related and other personal information. In this regard, European Union member states and other foreign jurisdictions, including Switzerland, have adopted data protection laws and regulations which impose significant compliance obligations. Failure to comply with these laws could lead to government enforcement actions and significant penalties against us, which could have a material adverse effect on our business, results of operations and growth prospects.
Any failures or further setbacks in our ADC development program would negatively affect our business and financial position.
ADCETRIS and our SGN-CD33A, ASG-22ME, SGN-LIV1A, SGN-CD19A, SGN-CD19B, SGN-CD123A, SGN-CD352A, and ASG-15ME product candidates are all based on our ADC technology, which utilizes proprietary stable linkers and potent cell-killing synthetic agents. Our ADC technology is also the basis of our collaborations with AbbVie, Astellas, Bayer, Celldex, Genentech, GSK, Pfizer, and Progenics, and our co-development agreements with Takeda, Astellas, and Genmab. Although ADCETRIS has received marketing approval in the United States, Canada, the European Union, Japan and other countries, ADCETRIS is our first and only ADC product that has been approved for commercial sale in any jurisdiction. Any failures or further setbacks in our ADC development program, including adverse effects resulting from the use of this technology in human clinical trials and/or the imposition of additional clinical holds on our trials of SGN-CD33A or any of our other product candidates, could have a detrimental impact on the continued commercialization of ADCETRIS in its current or any potential future approved indications and on our internal product candidate pipeline, as well as our ability to maintain and/or enter into new corporate collaborations regarding our ADC technology, which would negatively affect our business and financial position.
We have been named a defendant in a purported securities class action lawsuit and a lawsuit in connection with the Immunomedics License. This, and potential similar or related litigation, could result in substantial damages and may divert management’s time and attention from our business.
On January 10, 2017, a purported securities class action lawsuit was commenced in the United States District Court for the Western District of Washington, naming as defendants us and certain of our officers. The lawsuit alleges material misrepresentations and omissions in public statements regarding our business, operational and compliance policies, violations by all named defendants of Section 10(b) of the Exchange Act, and Rule 10b-5 thereunder, as well as violations of Section 20(a) of the Exchange Act. The complaint seeks compensatory damages of an undisclosed amount. The plaintiff alleges, among other things, that we made false and/or misleading statements and/or failed to disclose that SGN-CD33A presents a significant risk of fatal hepatotoxicity and that we had therefore overstated the viability of SGN-CD33A as a treatment for AML. It is possible that additional suits will be filed, or allegations received from stockholders, with respect to these same matters and also naming us and/or our officers and directors as defendants.
In addition, on February 13, 2017, we were named a co-defendant in a lawsuit filed by venBio in the Delaware Chancery Court against the members of the board of directors of Immunomedics. The lawsuit, or the venBio lawsuit, alleges that the members of the Immunomedics board breached their fiduciary duties toward their stockholders by hastily licensing IMMU-132 to us. We are alleged to have aided and abetted the breach of fiduciary duties. Among other things, venBio seeks to enjoin the closing of the transactions contemplated by the Immunomedics License. As a result of the pending litigation challenging the transactions contemplated by the Immunomedics License, Immunomedics and we have committed to the Court not to close the transactions contemplated by the Immunomedics License prior to March 10, 2017. We cannot predict the timing or outcome of the venBio lawsuit or the impact it may have on the Immunomedics License or the closing of the transactions contemplated by the Immunomedics License. However, it is possible that, in connection with the venBio lawsuit, the Immunomedics License could be rescinded or reformed in a way that is disadvantageous to us, including by
58
Table of Contents
requiring us to increase the transaction consideration payable to Immunomedics under the Immunomedics License, or that otherwise adversely affects the anticipated benefits to us of the Immunomedics License.
We believe that we have meritorious defenses and intend to defend these lawsuits vigorously. These lawsuits and any other related lawsuits are subject to inherent uncertainties, and the actual costs to be incurred relating to the lawsuits will depend upon many unknown factors. The outcome of the litigation is necessarily uncertain, and we could be forced to expend significant resources in the defense of these suits, and we may not prevail. Monitoring and defending against legal actions is time-consuming for our management and detracts from our ability to fully focus our internal resources on our business activities, which could result in delays of our clinical trials or our development and commercialization efforts. In addition, we may incur substantial legal fees and costs in connection with the litigation. We are also generally obligated, to the extent permitted by law, to indemnify our current and former directors and officers who are named as defendants in these and similar lawsuits. We are not currently able to estimate the possible cost to us from this matter, as these lawsuits are currently at an early stage and we cannot be certain how long it may take to resolve these matters or the possible amount of any damages that we may be required to pay. We have not established any reserves for any potential liability relating to these lawsuits. It is possible that we could, in the future, incur judgments or enter into settlements of claims for monetary damages. Decisions adverse to our interests in these lawsuits could result in the payment of substantial damages, or possibly fines, and could have a material adverse effect on our cash flow, results of operations and financial position. Decisions adverse to our interests in the venBio lawsuit could also result in the termination of the Immunomedics License or otherwise frustrate our ability to, or increase our costs to, consummate the transactions contemplated by the Immunomedics License. In addition, the uncertainty of the currently pending litigation could lead to increased volatility in our stock price.
We may need to raise significant amounts of additional capital that may not be available to us.
We expect to make additional capital outlays and to increase operating expenditures over the next several years as we hire additional employees, support our preclinical development, manufacturing and clinical trial activities for ADCETRIS and our other pipeline programs, and expand internationally, as well as commercialize ADCETRIS and position ADCETRIS for potential additional regulatory approvals. In addition, we anticipate committing substantial capital resources to the transactions contemplated by the Immunomedics License and the anticipated transfer, integration and development activities related to IMMU-132, including with respect to our upfront and milestone payment obligations to Immunomedics. Our commitment of resources to the continuing development, regulatory and commercialization activities for ADCETRIS, the transactions contemplated by the Immunomedics License and the anticipated transfer, integration and development activities related to IMMU-132, and the research, continued development and manufacturing of our product candidates will likely require us to raise substantial amounts of additional capital. In addition, we actively evaluate various strategic transactions on an ongoing basis, including licensing or otherwise acquiring complementary products, technologies or businesses, such as our anticipated in-licensing of IMMU-132, and we may require significant additional capital in order to complete or otherwise provide funding for any additional acquisitions. We may seek additional funding through some or all of the following methods: corporate collaborations, licensing arrangements, and public or private debt or equity financings. We do not know whether additional capital will be available when needed, or that, if available, we will obtain financing on terms favorable to us or our stockholders. If we are unable to raise additional funds when we need them, we may be required to delay, reduce the scope of, or eliminate one or more of our development programs, which may adversely affect our business and operations. Our future capital requirements will depend upon a number of factors, including:
| • | the level of sales and market acceptance of ADCETRIS; |
| • | the rate of progress and cost of the confirmatory post-approval study that we are required to conduct as a condition to the FDA’s accelerated approval of ADCETRIS in the relapsed sALCL indication; |
| • | the time and costs involved in obtaining regulatory approvals of ADCETRIS in additional indications, if any; |
| • | the size, complexity, timing, progress and number of our clinical programs; |
59
Table of Contents
| • | the timing, receipt and amount of milestone-based payments or other revenue from our collaborations or license arrangements, including royalty revenue generated from commercial sales of ADCETRIS by Takeda; |
| • | the cost of establishing and maintaining clinical and commercial supplies of ADCETRIS; |
| • | the costs associated with acquisitions or licenses of additional technologies, products, or companies, as well as licenses we may need to commercialize our products, including the actual costs to us of our anticipated in-licensing of IMMU-132; |
| • | the terms and timing of any future collaborative, licensing and other arrangements that we may establish; |
| • | expenses associated with the pending and potential additional related purported securities class action lawsuits, as well as any other potential litigation; |
| • | the potential costs associated with international, state and federal taxes; and |
| • | competing technological and market developments. |
In addition, changes in our spending rate may occur that would consume available capital resources sooner, such as increased development, manufacturing and clinical trial expenses in connection with our expanding pipeline programs, including several phase 3 trials, or our undertaking of additional programs, business activities or entry into strategic transactions, including potential additional acquisitions of products, technologies or businesses such as our anticipated in-licensing of IMMU-132. To the extent that we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us.
During the past several years, domestic and international financial markets have experienced extreme disruption from time to time, including, among other things, high volatility and significant declines in stock prices and severely diminished liquidity and credit availability for both borrowers and investors. For example, in June 2016, the electorate in the U.K. voted in favor of leaving the European Union (commonly referred to as “Brexit”). The withdrawal of the U.K. from the European Union will take effect either on the effective date of the withdrawal agreement or, in the absence of agreement, two years after the United Kingdom provides a notice of withdrawal pursuant to the EU Treaty. The U.K. government has announced that it intends to deliver a notice of withdrawal by the end of March 2017. It is likely that the withdrawal of the U.K. from the European Union will involve a process of lengthy negotiations between the U.K and European Union member states to determine the future terms of the U.K.’s relationship with the European Union. This could lead to a period of considerable uncertainty, particularly in relation to global financial markets which in turn could adversely affect our ability to raise additional capital. Such adverse capital and credit market conditions could make it more difficult to obtain additional capital on favorable terms, or at all, which could have a material adverse effect on our business and growth prospects.
We rely on license agreements for certain aspects of ADCETRIS and our ADC technology. Failure to maintain these license agreements or to secure any required new licenses could prevent us from continuing to develop and commercialize ADCETRIS and our product candidates.
We have entered into agreements with third-party commercial and academic institutions to license technology for use in ADCETRIS and our ADC technology. Currently, we have license agreements with Bristol-Myers Squibb and the University of Miami, among others. In addition to royalty provisions, some of these license agreements contain diligence and milestone-based termination provisions, in which case our failure to meet any agreed upon royalty or diligence requirements or milestones may allow the licensor to terminate the agreement. Many of our license agreements grant us exclusive licenses to the underlying technologies. If our licensors terminate our license agreements or if we are unable to maintain the exclusivity of our exclusive license agreements, we may be unable to continue to develop and commercialize ADCETRIS or our product candidates.
60
Table of Contents
Further, we have had in the past, and may in the future have, disputes with our licensors, which may impact our ability to develop and commercialize ADCETRIS or our product candidates or require us to enter into additional licenses. An adverse result in potential future disputes with our licensors may impact our ability to develop and commercialize ADCETRIS and our product candidates, or may require us to enter into additional licenses or to incur additional costs in litigation or settlement. In addition, continued development and commercialization of ADCETRIS and our product candidates will likely require us to secure licenses to additional technologies. We may not be able to secure these licenses on commercially reasonable terms, if at all.
If we are unable to enforce our intellectual property rights or if we fail to sustain and further build our intellectual property rights, we may not be able to successfully commercialize ADCETRIS or future products and competitors may be able to develop competing therapies.
Our success depends, in part, on obtaining and maintaining patent protection and successfully enforcing these patents and defending them against third-party challenges in the United States and other countries. We own multiple U.S. and foreign patents and pending patent applications for our technologies. We also have rights to issued U.S. patents, patent applications, and their foreign counterparts, relating to our monoclonal antibody, linker and drug-based technologies. Our rights to these patents and patent applications are derived in part from worldwide licenses from third parties. In addition, we have licensed certain of our U.S. and foreign patents and patent applications to third parties.
The standards that the U.S. Patent and Trademark Office and foreign patent offices use to grant patents are not always applied predictably or uniformly and can change. Consequently, our pending patent applications may not be allowed and, if allowed, may not contain the type and extent of patent claims that will be adequate to conduct our business as planned. Additionally, any issued patents we currently own or obtain in the future may have a shorter patent term than expected or may not contain claims that will permit us to stop competitors from using our technology or similar technology or from copying our products. Similarly, the standards that courts use to interpret patents are not always applied predictably or uniformly and may evolve, particularly as new technologies develop. In addition, changes to patent laws in the United States or other countries may be applied retroactively to affect the validation enforceability, or term of our patent. For example, the U.S. Supreme Court has recently modified some legal standards applied by the U.S. Patent and Trademark Office in examination of U.S. patent applications, which may decrease the likelihood that we will be able to obtain patents and may increase the likelihood of challenges to patents we obtain or license. In addition, changes to the U.S. patent system have come into force under the Leahy-Smith America Invents Act, or the America Invents Act, including changes from a “first-to-invent” system to a “first to file” system, changes to examination of U.S. patent applications and changes to the processes for challenging issued patents. These changes include provisions that affect the way patent applications are being filed, prosecuted and litigated. For example, the America Invents Act enacted proceedings involving post-issuance patent review procedures, such as inter partes review, or IPR, and post-grant review and covered business methods. These proceedings are conducted before the Patent Trial and Appeal Board, or PTAB, of the U.S. Patent and Trademark Office, or USPTO. Each proceeding has different eligibility criteria and different patentability challenges that can be raised. In this regard, the IPR process permits any person (except a party who has been litigating the patent for more than a year) to challenge the validity of some patents on the grounds that it was anticipated or made obvious by prior art. As a result, non-practicing entities associated with hedge funds, pharmaceutical companies who may be our competitors and others have challenged certain valuable pharmaceutical U.S. patents based on prior art through the IPR process. A decision in such a proceeding adverse to our interests could result in the loss of valuable patent rights which would have a material adverse effect on our business, financial condition, results of operations and growth prospects. In any event, the America Invents Act and any other potential future changes to the U.S. patent system could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We rely on trade secrets and other proprietary information where we believe patent protection is not appropriate or obtainable. However, trade secrets and other proprietary information are difficult to protect. We
61
Table of Contents
have taken measures to protect our unpatented trade secrets and know-how, including the use of confidentiality and assignment of inventions agreements with our employees, consultants and certain contractors. It is possible, however, that these persons may breach the agreements or that our competitors may independently develop or otherwise discover our trade secrets or other proprietary information. Our research collaborators may publish confidential data or other restricted information to which we have rights. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborations, then our ability to receive patent protection or protect our proprietary information may be impaired.
We may incur substantial costs and lose important rights or may not be able to continue to commercialize ADCETRIS or to commercialize any of our product candidates that may be approved for commercial sale as a result of litigation or other proceedings relating to patent and other intellectual property rights, and we may be required to obtain patent and other intellectual property rights from others.
We may face potential lawsuits by companies, academic institutions or others alleging infringement of their intellectual property. Because patent applications can take a few years to publish, there may be currently pending applications of which we are unaware that may later result in issued patents that adversely affect the continued commercialization of ADCETRIS or future commercialization of our product candidates in development. In addition, we are monitoring the progress of multiple pending patent applications of other organizations that, if granted, may require us to license or challenge their enforceability in order to continue commercializing ADCETRIS or to commercialize our product candidates that may be approved for commercial sale. Our challenges to patents of other organizations may not be successful, which may affect our ability to commercialize ADCETRIS or our product candidates. As a result of the patent infringement lawsuits that have been filed or may be filed against us in the future by third parties alleging infringement by us of patent or other intellectual property rights, we may be required to pay substantial damages, including lost profits, royalties, treble damages, attorneys’ fees and costs, for past infringement if it is ultimately determined that our products infringe a third party’s intellectual property rights. Even if infringement claims against us are without merit, the results may be unpredictable. In addition, defending lawsuits takes significant time, may be expensive and may divert management’s attention from other business concerns. Further, we may be stopped from developing, manufacturing or selling our products until we obtain a license from the owner of the relevant technology or other intellectual property rights, or be forced to undertake costly design-arounds, if feasible. If such a license is available at all, it may require us to pay substantial royalties or other fees.
We are or may be from time to time involved in the defense and enforcement of our patent or other intellectual property rights in a court of law, USPTO interference, IPR, post-grant review or reexamination proceeding, foreign opposition proceeding or related legal and administrative proceeding in the United States and elsewhere. In addition, if we choose to go to court to stop a third party from infringing our patents, that third party has the right to ask the court to rule that these patents are invalid, not infringed and/or should not be enforced. Under the America Invents Act, a third party may also have the option to challenge the validity of certain patents at the PTAB, whether they are accused of infringing our patents or not, and certain entities associated with hedge funds, pharmaceutical companies and other entities have challenged valuable pharmaceutical patents through the IPR process. These lawsuits and administrative proceedings are expensive and consume time and other resources, and we may not be successful in these proceedings or in stopping infringement. In addition, there is a risk that a court will decide that these patents are not valid or not infringed or otherwise not enforceable, or that the PTAB will decide that certain patents are not valid or should have a shorter term, and that we do not have the right to stop a third party from using the patented subject matter. Successful challenges to our patent or other intellectual property rights through these proceedings could result in a loss of rights in the relevant jurisdiction and may allow third parties to use our proprietary technologies without a license from us or our collaborators, which may also result in loss of future royalty payments. Furthermore, if such challenges to our rights are not resolved promptly in our favor, our existing business relationships may be jeopardized and we could be delayed or prevented from entering into new collaborations or from commercializing potential products, which could adversely affect our business and results of operations. In addition, we may challenge the patent or other intellectual property rights of third parties and if we are unsuccessful in actions we bring against the rights of such parties, through litigation or otherwise, and it is
62
Table of Contents
determined that we infringe the intellectual property rights of such parties, we may be prevented from commercializing potential products in the relevant jurisdiction, or may be required to obtain licenses to those rights or develop or obtain alternative technologies, any of which could harm our business.
If we lose our key personnel or are unable to attract and retain additional qualified personnel, our future growth and ability to compete would suffer.
We are highly dependent on the efforts and abilities of the principal members of our senior management. Additionally, we have scientific personnel with significant and unique expertise in monoclonal antibodies, ADCs and related technologies. The loss of the services of any one of the principal members of our managerial or scientific staff may prevent us from achieving our business objectives.
In addition, the competition for qualified personnel in the biotechnology field is intense, and our future success depends upon our ability to attract, retain and motivate highly skilled scientific, technical and managerial employees. In order to commercialize ADCETRIS, we have been required to expand our workforce, particularly in the areas of manufacturing, clinical trials management, regulatory affairs, business development, sales and marketing. These activities required the addition of new personnel, including sales and marketing management, and the development of additional expertise by existing management personnel. We continue to face intense competition for qualified individuals from numerous pharmaceutical and biotechnology companies, as well as academic and other research institutions. To the extent we are not able to retain these individuals on favorable terms or attract any additional personnel that may be required, our business may be harmed.
If we are unable to manage our growth, our business, financial condition, results of operations and prospects may be adversely affected.
We have experienced and expect to continue to experience significant growth in the number of our employees and in the scope of our operations. This growth places significant demands on our management, operational and financial resources, and our current and planned personnel, systems, procedures and controls may not be adequate to support our growth. To effectively manage our growth, we must continue to improve existing, and implement new, operational and financial systems, procedures and controls and must expand, train and manage our growing employee base, and there can be no assurance that we will effectively manage our growth without experiencing operating inefficiencies or control deficiencies. We expect that we may need to increase our management personnel to oversee our expanding operations, and recruiting and retaining qualified individuals is difficult. In addition, the physical expansion of our operations may lead to significant costs and may divert our management and capital resources. If we are unable to manage our growth effectively, or are unsuccessful in recruiting qualified management personnel, our business, financial condition, results of operations and prospects may be adversely affected.
Product liability and product recalls could harm our business, and we may not be able to obtain adequate insurance to protect us against product liability losses.
The current and future use of ADCETRIS by us and our corporate collaborators in clinical trials and the sale of ADCETRIS, expose us to product liability claims. These claims have and may in the future be made directly by consumers or healthcare providers or indirectly by pharmaceutical companies, our corporate collaborators or others selling such products. We may experience substantial financial losses in the future due to product liability claims. We have obtained product liability coverage, including coverage for human clinical trials and product sold commercially. However, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against all losses. If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured amounts, our assets may not be sufficient to cover such claims and our business operations could be impaired.
Product recalls may be issued at our discretion, or at the discretion of government agencies and other entities that have regulatory authority for pharmaceutical sales. Any recall of ADCETRIS could materially adversely affect our business by rendering us unable to sell ADCETRIS for some time and by adversely affecting our reputation.
63
Table of Contents
Risks associated with operating in foreign countries could materially adversely affect our business.
We are expanding our operations internationally, and we currently have subsidiaries in the U.K., Switzerland and Canada. Consequently, we are, and will continue to be, subject to risks related to operating in foreign countries. Risks associated with conducting operations in foreign countries include:
| • | diverse regulatory, financial and legal requirements, and any future changes to such requirements, in one or more countries where we are located or do business; |
| • | adverse tax consequences, including changes in applicable tax laws and regulations; |
| • | applicable trade laws, tariffs, export quotas, custom duties or other trade restrictions and any changes to them; |
| • | economic weakness, including inflation, or political or economic instability in particular foreign economies and markets; |
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations incident to doing business or operating in another country; |
| • | liabilities for activities of, or related to, our international operations; |
| • | workforce uncertainty in countries where labor unrest is more common than in the United States; and |
| • | laws and regulations relating to data security and the unauthorized use of, or access to, commercial and personal information. |
For example, since a significant proportion of the regulatory framework in the U.K. is derived from European Union directives and regulations, Brexit could materially change the regulatory regime applicable to our operations and those of our collaborators, including with respect to marketing authorizations for ADCETRIS and our product candidates. We may also face new regulatory costs and challenges as result of Brexit that could have a material adverse effect on our operations. Depending on the terms of Brexit, the U.K. could lose the benefits of global trade agreements negotiated by the European Union on behalf of its members, which may result in increased trade barriers which could make our doing business in Europe more difficult. In addition, currency exchange rates for the British Pound and the Euro with respect to each other and the U.S. dollar have already been affected by Brexit. Should this foreign exchange volatility continue, it could cause volatility in our quarterly financial results. In any event, we cannot predict to what extent these changes will impact our business or results of operations, or our ability to conduct operations in Europe.
These and other risks described elsewhere in these risk factors associated with expanding our international operations could materially adversely affect our business.
Our operations involve hazardous materials and are subject to environmental, health and safety controls and regulations.
We are subject to environmental, health and safety laws and regulations, including those governing the use of hazardous materials, and we spend considerable time complying with such laws and regulations. Our business activities involve the controlled use of hazardous materials and although we take precautions to prevent accidental contamination or injury from these materials, we cannot completely eliminate the risk of using these materials. In the event of an accident or environmental discharge, we may be held liable for any resulting damages, which may materially harm our business, financial condition and results of operations.
If any of our facilities are damaged or our clinical, research and development or other business processes are interrupted, our business could be seriously harmed.
We conduct most of our business in a limited number of facilities in a single geographical location in Bothell, Washington. Damage or extended periods of interruption to our corporate, development or research
64
Table of Contents
facilities due to fire, natural disaster, power loss, communications failure, unauthorized entry or other events could cause us to cease or delay development of some or all of our product candidates or interrupt the sales process for ADCETRIS. Although we maintain property damage and business interruption insurance coverage on these facilities, our insurance might not cover all losses under such circumstances and our business may be seriously harmed by such delays and interruption.
If we experience a significant disruption in our information technology systems or breaches of data security, our business could be adversely affected.
We rely on information technology systems to keep financial records, capture laboratory data, maintain clinical trial data and corporate records, communicate with staff and external parties and operate other critical functions. Our information technology systems are potentially vulnerable to disruption due to breakdown, malicious intrusion and computer viruses or other disruptive events including but not limited to natural disaster. If we were to experience a prolonged system disruption in our information technology systems or those of certain of our vendors, it could delay or negatively impact the development and commercialization of ADCETRIS and our product candidates, which could adversely impact our business. Although we maintain offsite back-ups of our data, if operations at our facilities were disrupted, it may cause a material disruption in our business if we are not capable of restoring function on an acceptable timeframe. In addition, our information technology systems are potentially vulnerable to data security breaches—whether by employees or others—which may expose sensitive data to unauthorized persons. Such data security breaches could lead to the loss of trade secrets or other intellectual property, or could lead to the public exposure of personal information (including sensitive personal information) of our employees, customers and others, any of which could have a material adverse effect on our business, financial condition and results of operations. Moreover, a security breach or privacy violation that leads to disclosure or modification of, personally identifiable information, could harm our reputation, compel us to comply with federal and/or state breach notification laws, subject us to mandatory corrective action, require us to verify the correctness of database contents and otherwise subject us to liability under laws and regulations that protect personal data, resulting in increased costs or loss of revenue. In addition, a data security breach could result in loss of clinical trial data or damage to the integrity of that data. If we are unable to prevent such security breaches or privacy violations or implement satisfactory remedial measures, our operations could be disrupted, and we may suffer loss of reputation, financial loss and other negative consequences because of lost or misappropriated information. In addition, these breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above.
Increasing use of social media could give rise to liability.
We are increasingly relying on social media tools as a means of communications. To the extent that we continue to use these tools as a means to communicate about ADCETRIS and our product candidates or about the diseases that ADCETRIS and our product candidates are intended to treat, there are significant uncertainties as to either the rules that apply to such communications, or as to the interpretations that health authorities will apply to the rules that exist. As a result, despite our efforts to comply with applicable rules, there is a significant risk that our use of social media for such purposes may cause us to nonetheless be found in violation of them. Such uses of social media could have a material adverse effect on our business, financial condition and results of operations.
Legislative actions and new accounting pronouncements are likely to impact our future financial position or results of operations.
Future changes in financial accounting standards may cause adverse, unexpected revenue fluctuations and affect our financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with frequency in the past and are expected to occur again in the future and as a result we may be required to make changes in our accounting policies. Those changes could adversely affect our reported revenues and expenses, future profitability or financial position. Compliance with new regulations regarding corporate governance and public disclosure may result in additional expenses.
65
Table of Contents
For example, in May 2014, the Financial Accounting Standards Board, or FASB, issued an Accounting Standards Update entitled “ASU 2014-09, Revenue from Contracts with Customers” which will replace the existing revenue recognition guidance in U.S. GAAP when it becomes effective for us on January 1, 2018. Our preliminary assessment of this new standard is that it will generally not change the way in which we recognize product revenue from sales of ADCETRIS. However, we expect that sales-based royalties will be recorded in the period of the related sale based on estimates, rather than recording them as reported by the customer. In addition, the achievement of development milestones under our collaborations will be recorded in the period their achievement becomes probable, which may result in their recognition earlier than under current accounting principles. We are continuing to evaluate the impact of the new standard on all of our revenues, including those mentioned above, and our assessments may change in the future based on our continuing evaluation. In any event, the application of existing or future financial accounting standards, particularly those relating to the way we account for revenues and costs, could have a significant impact on our reported results. In addition, compliance with new regulations regarding corporate governance and public disclosure may result in additional expenses. As a result, we intend to invest all reasonably necessary resources to comply with evolving standards, and this investment may result in increased general and administrative expenses and a diversion of management time and attention from science and business activities to compliance activities.
Risks Related to the Immunomedics License
Failure to consummate the transactions contemplated by the Immunomedics License could negatively impact our stock price and our future business and financial results.
We announced on February 10, 2017 that we had entered into the Immunomedics License pursuant to which, upon the terms and subject to the conditions set forth in the Immunomedics License, we would receive exclusive worldwide rights to develop and commercialize IMMU-132. The closing of the transactions contemplated by the Immunomedics License, which has not yet occurred, is subject to customary closing conditions, including expiration of the applicable waiting period under the HSR Act, there being no pending court or administrative challenges to the Immunomedics License and there being no court or administrative orders blocking the closing. On February 20, 2017, Immunomedics and we entered into a letter agreement pursuant to which Immunomedics irrevocably waived to the extent applicable to Immunomedics the condition precedent to the closing and effectiveness of the Immunomedics License that there be no pending court or administrative challenges to the transaction. Additionally, under the terms of the Immunomedics License, Immunomedics had the right to continue discussions with a small number of parties that previously expressed interest in licensing IMMU-132 until 11:59 p.m. New York City time on February 19, 2017. If a third party had provided Immunomedics with a financially superior licensing offer, we would have had the right to match any such offer, and if we had decided not to match, Immunomedics would have had the right to accept the superior offer and terminate the Immunomedics License upon payment of a termination fee to us. We have not received notice from Immunomedics of any such third party offers during this limited time period, and on February 21, 2017, Immunomedics announced that it is subject to customary “no-shop” restrictions on its and its representatives’ ability to solicit, discuss or negotiate alternative licensing agreement proposals from third parties with regard to IMMU-132. On February 13, 2017, we were named a co-defendant in a lawsuit filed by venBio in the Delaware Chancery Court against the members of the board of directors of Immunomedics, pursuant to which, among other things, venBio seeks to enjoin the closing of the transactions contemplated by the Immunomedics License. venBio has also initiated a proxy contest with respect the Immunomedics annual meeting to be held on March 3, 2017, seeking to have its nominees elected to the Immunomedics board of directors in lieu of Immunomedics’ management nominees. As a result of the pending litigation challenging the transactions contemplated by the Immunomedics License, Immunomedics and we have committed to the Court not to close the transactions contemplated by the Immunomedics License prior to March 10, 2017. We cannot predict the timing or outcome of the venBio lawsuit or the impact it may have on the Immunomedics License or the closing of the transactions contemplated by the Immunomedics License. Likewise, we cannot predict the effect a potential change in the board of directors of Immunomedics could have on our ability to consummate the transactions contemplated by the Immunomedics License. However, it is possible that, in connection with the venBio lawsuit and/or the proxy contest, the Immunomedics License could be rescinded or reformed in a way
66
Table of Contents
that is disadvantageous to us, including by requiring us to increase the transaction consideration payable to Immunomedics under the Immunomedics License, or that otherwise adversely affects the anticipated benefits to us of the Immunomedics License. If the transactions contemplated by the Immunomedics License are not consummated, our ongoing business may be adversely affected and, without realizing any of the benefits of having consummated the transactions contemplated by the Immunomedics License, we will be subject to a number of risks, including the following:
| • | the current price of our common stock may reflect a market assumption that the closing of the transactions contemplated by the Immunomedics License will occur, such that a failure to complete such transactions could result in a decline in our stock price; |
| • | we have made an equity investment of approximately $14.7 million in Immunomedics (which investment may never result in a financial return to us) in contemplation of securing exclusive development and commercialization rights to IMMU-132 without realizing any of the benefits of securing such rights; |
| • | matters relating to the Immunomedics License have required and will continue to require substantial commitments of time and resources by our management and other employees, which could otherwise have been devoted to other opportunities that may have been beneficial to us; |
| • | we may be required to reimburse Immunomedics for certain expenses incurred by Immunomedics in connection with the Immunomedics License if it is not consummated; and |
| • | we may incur significant costs and potential additional harms in connection with the lawsuit brought by venBio. |
We also could be subject to additional litigation related to any failure to consummate the transactions contemplated by the Immunomedics License or to perform our obligations under the Immunomedics License. If the transactions contemplated by the Immunomedics License are not consummated, these risks may materialize and may adversely affect our business, financial results and stock price.
Obtaining required approvals necessary to satisfy the conditions to the closing of the transactions contemplated by the Immunomedics License and the effect of the venBio lawsuit and the outcome of the Immunomedics proxy contest may delay or prevent completion of the closing of the transactions contemplated by the Immunomedics License, result in additional expenditures of money and resources and/or reduce or eliminate the anticipated benefits to us of the Immunomedics License.
The closing of the transactions contemplated by the Immunomedics License is subject to customary closing conditions. These closing conditions include, among others, the expiration of the applicable waiting period under the HSR Act, compliance with antitrust-related filing requirements in certain other countries, there being no pending court or administrative challenges to the Immunomedics License and there being no court or administrative orders blocking the closing. On February 20, 2017, Immunomedics and we entered into a letter agreement pursuant to which Immunomedics irrevocably waived to the extent applicable to Immunomedics the condition precedent to the closing and effectiveness of the Immunomedics License that there be no pending court or administrative challenges to the transaction. Additionally, under the terms of the Immunomedics License, Immunomedics had the right to continue discussions with a small number of parties that previously expressed interest in licensing IMMU-132 until 11:59 p.m. New York City time on February 19, 2017. If a third party had provided Immunomedics with a financially superior licensing offer, we would have had the right to match any such offer, and if we had decided not to match, Immunomedics would have had the right to accept the superior offer and terminate the Immunomedics License upon payment of a termination fee to us. We have not received notice from Immunomedics of any such third party offers during this limited time period, and on February 21, 2017, Immunomedics announced that it is subject to customary “no-shop” restrictions on its and its representatives’ ability to solicit, discuss or negotiate alternative licensing agreement proposals from third parties with regard to IMMU-132. The governmental agencies from which the parties will seek approvals have broad discretion in administering the governing regulations. As a condition to their approval, agencies may impose
67
Table of Contents
requirements, limitations or costs or require divestitures or place restrictions on the conduct of our IMMU-132 activities after the closing. These requirements, limitations, costs, divestitures or restrictions could jeopardize or delay the closing of the transactions contemplated by the Immunomedics License or may reduce the anticipated benefits to us of the Immunomedics License. If we and Immunomedics agree to any material requirements, limitations, costs or restrictions in order to obtain any approvals required to consummate the transactions contemplated by the Immunomedics License, these requirements, limitations, costs or restrictions could adversely affect the anticipated benefits of the Immunomedics License. In addition, it is possible that, in connection with the venBio lawsuit and/or the proxy contest, the Immunomedics License could be rescinded or reformed in a way that is disadvantageous to us, including by requiring us to increase the transaction consideration payable to Immunomedics under the Immunomedics License, or that otherwise adversely affects the anticipated benefits to us of the Immunomedics License. Any of these factors could result in a failure to consummate these transactions or have a material adverse effect on our business and results of operations.
We may not be able to successfully integrate IMMU-132 or any other potential future product candidates we may acquire or license rights to, or we may otherwise fail to realize their full potential.
We actively evaluate various strategic transactions on an ongoing basis, including licensing or otherwise acquiring complementary products, technologies or businesses, and we recently announced our entry into the Immunomedics License with Immunomedics pursuant to which, if consummated, we would be granted an exclusive worldwide license to IMMU-132. However, we cannot ensure that we would be able to manage the risks associated with the transfer of development, regulatory and manufacturing activities for IMMU-132 from Immunomedics to us or manage the risks associated with integrating IMMU-132 into our existing business and infrastructure. We may encounter unexpected difficulties during the transfer, integration or further development of IMMU-132, any of which may cause us to expend greater funds and efforts or may slow, delay or limit the progress of IMMU-132’s development. Unexpected difficulties may further be disruptive to our ongoing development and commercialization efforts, put a strain on our existing personnel, infrastructure and business and divert management’s time and attention. As a result of these or other problems and risks, even if the transactions contemplated by the Immunomedics License are consummated, we may never receive regulatory approval for IMMU-132, we may not realize the full potential of IMMU-132 or we may never generate significant value or revenues from IMMU-132.
In addition, the market price of our common stock may decline if the integration or further development of IMMU-132 is unsuccessful, takes longer than expected or fails to result in benefits to the extent anticipated by financial analysts or investors, or the effect of our license of IMMU-132 on our financial position and results of operations is otherwise not consistent with the expectations of financial analysts or investors.
Risks Related to Our Common Stock
Our stock price is volatile and our shares may suffer a decline in value.
The market price of our stock has in the past been, and is likely to continue in the future to be, very volatile. During the year ended December 31, 2016, our closing stock price fluctuated between $26.87 and $73.71 per share. As a result of fluctuations in the price of our common stock, you may be unable to sell your shares at or above the price you paid for them. The market price of our common stock may be subject to substantial volatility in response to many risk factors listed in this section, and others beyond our control, including:
| • | the level of ADCETRIS sales in the United States, Canada, the European Union, Japan and other countries in which Takeda has received approval by relevant regulatory authorities; |
| • | announcements regarding the results of discovery efforts and preclinical, clinical and commercial activities by us, or those of our competitors; |
| • | announcements of FDA or foreign regulatory approval or non-approval of ADCETRIS, or specific label indications for or restrictions, warnings or limitations in its use, or delays in the regulatory review or approval process, including in connection with our planned sBLA submission to the FDA to seek approval of ADCETRIS in the ALCANZA treatment setting; |
68
Table of Contents
| • | announcements regarding the results of the clinical trials we and/or Takeda are conducting or may in the future conduct for ADCETRIS, including our ECHELON-1 and ECHELON-2 phase 3 trials; |
| • | announcements regarding, or negative publicity concerning, adverse events associated with the use of ADCETRIS or our product candidates, including announcements similar to our December 2016 announcement that the FDA had placed a clinical hold or a partial clinical hold on several early stage trials of SGN-CD33A in AML to evaluate the potential risk of hepatotoxicity following adverse medical events, including fatal events; |
| • | issuance of new or changed analysts’ reports and recommendations regarding us or our competitors; |
| • | termination of or changes in our existing collaborations or licensing arrangements, especially our ADCETRIS collaboration with Takeda or establishment of new collaborations or licensing arrangements; |
| • | announcements regarding the Immunomedics License, or the termination of or other changes to the Immunomedics License and the related lawsuit brought by venBio; |
| • | our entry into additional material strategic transactions including licensing or acquisition of products, businesses or technologies; |
| • | actions taken by regulatory authorities with respect to our product candidates, our clinical trials or our regulatory filings, including the imposition or lifting of clinical trial holds on trials of SGN-CD33A and our product candidates; |
| • | our raising of additional capital and the terms upon which we may raise any additional capital; |
| • | market conditions for equity investments in general, or the biotechnology or pharmaceutical industries in particular; |
| • | developments or disputes concerning our proprietary rights; |
| • | developments regarding the pending and potential additional related purported securities class action lawsuits, as well as any other potential litigation; |
| • | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| • | changes in government regulations; and |
| • | economic or other external factors. |
The stock markets in general, and the markets for biotechnology and pharmaceutical stocks in particular, have historically experienced significant volatility that has often been unrelated or disproportionate to the operating performance of particular companies. For example, negative publicity regarding drug pricing and price increases by pharmaceutical companies has negatively impacted, and may continue to negatively impact, the markets for biotechnology and pharmaceutical stocks. Likewise, as a result of Brexit and/or significant changes in U.S. social, political, regulatory and economic conditions or in laws and policies governing foreign trade and health care spending and delivery, including the potential repeal and/or replacement of all or portions of PPACA or greater restrictions on free trade stemming from Trump Administration policies, the financial markets could experience significant volatility that could also negatively impact the markets for biotechnology and pharmaceutical stocks. These broad market fluctuations have adversely affected and may in the future adversely affect the trading price of our common stock.
In the past, class action or derivative litigation has often been instituted against companies whose securities have experienced periods of volatility in market price. In this regard, we have become, and may in the future again become, subject to claims and litigation alleging violations of the securities laws or other related claims, which could harm our business and require us to incur significant costs. The pending purported securities class action lawsuit and any additional lawsuits brought against us could result in substantial costs, which would hurt our financial condition and results of operations and divert management’s attention and resources, which could result in delays of our clinical trials or our development and commercialization efforts.
69
Table of Contents
Substantial future sales of shares of our common stock or equity-related securities could cause the market price of our common stock to decline.
Sales of a substantial number of shares of our common stock into the public market, including sales by members of our management or board of directors or entities affiliated with such members, could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock and could impair our ability to raise capital through the sale of additional equity or equity-related securities. We are unable to predict the effect that such sales may have on the prevailing market price of our common stock. As of February 16, 2017, we had 142,493,676 shares of common stock outstanding, all of which shares are eligible for sale in the public market, subject in some cases to the volume limitations and manner of sale and other requirements under Rule 144. In addition, we may issue a substantial number of shares of our common stock or equity- related securities, including convertible debt, to meet our capital needs, including in connection with funding acquisition or licensing opportunities, capital expenditures or product development costs, which issuances could be substantially dilutive and could adversely affect the market price of our common stock. Likewise, future issuances by us of our common stock upon the exercise, conversion or settlement of equity-based awards or other equity-related securities would dilute existing stockholders’ ownership interest in our company and any sales in the public market of these shares, or the perception that these sales might occur, could also adversely affect the market price of our common stock.
Moreover, we have in the past and may in the future grant rights to some of our stockholders that require us to register the resale of our common stock or other securities on behalf of these stockholders and/or facilitate public offerings of our securities held by these stockholders, including in connection with potential future acquisition or capital-raising transactions. For example, in connection with our September 2015 public offering of common stock, we entered into a registration rights agreement with entities affiliated with Baker Bros. Advisors LP, or the Baker Entities, that together, based on information available to us, collectively beneficially owned approximately 32.1% of our common stock as of February 16, 2017. Under the registration rights agreement, if at any time and from time to time the Baker Entities demand that we register their shares of our common stock for resale under the Securities Act of 1933, we would be obligated to effect such registration. On October 12, 2016, pursuant to the registration rights agreement, we registered for resale, from time to time, up to 44,059,594 shares of our common stock held by the Baker Entities. Our registration obligations under the registration rights agreement cover all shares now held or hereafter acquired by the Baker Entities, will continue in effect for up to ten years, and include our obligation to facilitate certain underwritten public offerings of our common stock by the Baker Entities in the future. If the Baker Entities, by its exercise of these registration and/or underwriting rights in the future, or otherwise, sell a large number of our shares, or the market perceives that the Baker Entities intend to sell a large number of our shares, including in connection with our October 2016 registration of shares held by the Baker Entities for resale, this could adversely affect the market price of our common stock. We have also filed registration statements to register the sale of our common stock reserved for issuance under our equity incentive and employee stock purchase plans. Accordingly, these shares will be able to be freely sold in the public market upon issuance as permitted by any applicable vesting requirements.
Our existing stockholders have significant control of our management and affairs.
Our executive officers and directors and holders of greater than five percent of our outstanding voting stock, together with entities that may be deemed affiliates of, or related to, such persons or entities, beneficially owned approximately 68.1% of our voting power as of February16, 2017. As a result, these stockholders, acting together, are able to control our management and affairs and matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, such as mergers, consolidations or the sale of substantially all of our assets. Consequently, this concentration of ownership may have the effect of delaying, deferring or preventing a change in control, including a merger, consolidation, takeover or other business combination involving us or discourage a potential acquirer from making a tender offer or otherwise attempting to obtain control, which might affect the market price of our common stock.
70
Table of Contents
Anti-takeover provisions could make it more difficult for a third party to acquire us.
Our Board of Directors has the authority to issue up to 5,000,000 shares of preferred stock and to determine the price, rights, preferences, privileges and restrictions, including voting rights, of those shares without any further vote or action by the stockholders, which authority could be used to adopt a “poison pill” that could act to prevent a change of control of Seattle Genetics that has not been approved by our Board of Directors. The rights of the holders of common stock may be subject to, and may be adversely affected by, the rights of the holders of any preferred stock that may be issued in the future. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change of control of Seattle Genetics without further action by the stockholders and may adversely affect the voting and other rights of the holders of common stock. Further, certain provisions of our charter documents, including provisions eliminating the ability of stockholders to take action by written consent and limiting the ability of stockholders to raise matters at a meeting of stockholders without giving advance notice, may have the effect of delaying or preventing changes in control or management of Seattle Genetics, which could have an adverse effect on the market price of our stock. In addition, our charter documents provide for a classified board, which may make it more difficult for a third party to gain control of our Board of Directors. Similarly, state anti-takeover laws in Delaware and Washington related to corporate takeovers may prevent or delay a change of control of Seattle Genetics.
Item 1B. Unresolved Staff Comments
None.
Our headquarters are in Bothell, Washington, where we lease six buildings totaling approximately 355,000 square feet of office space that we use for laboratory, discovery, research and development and general and administrative purposes. All of our leases include renewal options.
We believe that our facilities are currently adequate to meet our needs.
On January 10, 2017, a putative securities class action complaint was filed against Seattle Genetics, Inc., our chief executive officer, Clay Siegall, and our chief financial officer, Todd Simpson, in the United States District Court for the Western District of Washington under the following captions: Samit Patel, etc., v. Seattle Genetics, Inc., et al., No. C17-41. The putative class is composed of all purchasers of our securities between October 27, 2016 and December 26, 2016, inclusive. The complaint alleges material misrepresentations and omissions in public statements regarding our business, operational and compliance policies, violations by all named defendants of Section 10(b) of the Exchange Act, and Rule 10b-5 thereunder, as well as violations of Section 20(a) of the Exchange Act. The plaintiff alleges, among other things, that we made false and/or misleading statements and/or failed to disclose that SGN-CD33A presents a significant risk of fatal hepatotoxicity and that we had therefore overstated the viability of SGN-CD33A as a treatment for AML. The complaint seeks compensatory damages of an undisclosed amount. We believe that we have meritorious defenses and intend to defend this lawsuit vigorously.
On February 13, 2017, we were named a co-defendant in a lawsuit filed by venBio against the members of the board of directors of Immunomedics, or the venBio lawsuit. The venBio lawsuit was filed in the Court of Chancery of the State of Delaware under the caption venBio v. Goldenberg et. al. and alleges that the members of the Immunomedics board breached their fiduciary duties toward their stockholders by hastily licensing IMMU-132 to us. We are alleged to have aided and abetted the breach of fiduciary duties. Among other things, venBio seeks to enjoin the closing of the transactions contemplated by the Immunomedics License. As a result of the pending litigation challenging the transactions contemplated by the Immunomedics License, Immunomedics and we have committed to the Court not to close the transactions contemplated by the Immunomedics License prior to March 10, 2017.
71
Table of Contents
We do not believe it is feasible to predict or determine the outcome or resolution of these lawsuits, or to estimate the amount of, or potential range of, loss with respect to these lawsuits. In addition, the timing of the final resolution of these lawsuits is uncertain. As a result of these lawsuits, we will incur litigation expenses and may incur indemnification expenses, and potential resolutions of these lawsuits could include a settlement requiring payments by us. Those expenses could have a material impact on our financial position, results of operations, and cash flows.
In addition, from time to time in the ordinary course of business we become involved in various lawsuits, claims and proceedings relating to the conduct of our business, including those pertaining to the defense and enforcement of our patent or other intellectual property rights. These proceedings are costly and time consuming. Successful challenges to our patent or other intellectual property rights through these proceedings could result in a loss of rights in the relevant jurisdiction and may allow third parties to use our proprietary technologies without a license from us or our collaborators.
Item 4. Mine Safety Disclosures
Not applicable.
72
Table of Contents
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Price Range of Our Common Stock
Our common stock is traded on the NASDAQ Global Select Market under the symbol “SGEN.” As of February 16, 2017, there were 142,493,676 shares of our common stock outstanding, which were held by approximately 70 holders of record of our common stock. On February 16, 2017, the closing price of our common stock as reported on the NASDAQ Global Select Market was $65.30 per share.
The following table sets forth, for the periods indicated, the reported high and low sales prices per share of our common stock as reported on the NASDAQ Global Select Market, as applicable:
| High | Low | |||||||
| 2015 |
||||||||
| First Quarter |
$ | 39.98 | $ | 30.05 | ||||
| Second Quarter |
49.84 | 32.68 | ||||||
| Third Quarter |
52.33 | 35.94 | ||||||
| Fourth Quarter |
46.74 | 36.85 | ||||||
| 2016 |
||||||||
| First Quarter |
$ | 44.45 | $ | 26.02 | ||||
| Second Quarter |
44.07 | 32.40 | ||||||
| Third Quarter |
57.23 | 39.38 | ||||||
| Fourth Quarter |
75.36 | 47.29 | ||||||
| 2017 |
||||||||
| First Quarter (through February 16, 2017) |
$ | 66.64 | $ | 50.65 | ||||
Dividend Policy
We have not paid any cash dividends on our common stock since our inception. We do not intend to pay any cash dividends in the foreseeable future, but intend to retain all earnings, if any, for use in our business operations.
Sales of Unregistered Securities and Issuer Repurchases of Securities
There were no unregistered sales of equity securities by us during the year ended December 31, 2016. In addition, we did not repurchase any of our equity securities during the fourth quarter of 2016.
73
Table of Contents
Stock Performance Graph
We show below the cumulative total return to our stockholders during the period from December 31, 2011 through December 31, 2016 in comparison to the cumulative return on the NASDAQ Pharmaceutical Index, the NASDAQ Composite Index and the NASDAQ Biotechnology Index during that same period. The results assume that $100 was invested on December 31, 2011 in our common stock and each of the indexes listed above, including reinvestment of dividends, if any.
| Years ended | ||||||||||||||||||||||||
| 12/11 | 12/12 | 12/13 | 12/14 | 12/15 | 12/16 | |||||||||||||||||||
| Seattle Genetics, Inc. |
100.00 | 138.62 | 238.65 | 192.22 | 268.50 | 315.70 | ||||||||||||||||||
| NASDAQ Composite |
100.00 | 116.41 | 165.47 | 188.69 | 200.32 | 216.54 | ||||||||||||||||||
| NASDAQ Pharmaceutical |
100.00 | 136.13 | 229.92 | 296.47 | 308.15 | 243.63 | ||||||||||||||||||
| NASDAQ Biotechnology |
100.00 | 134.68 | 232.37 | 307.67 | 328.76 | 262.08 | ||||||||||||||||||
This information under “Stock Performance Graph” is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference in any filing of Seattle Genetics, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K and irrespective of any general incorporation language in those filings.
74
Table of Contents
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with our consolidated financial statements and notes to our consolidated financial statements and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained elsewhere in this Annual Report on Form 10-K. The selected Consolidated Statements of Comprehensive Loss data for the years ended December 31, 2016, 2015, and 2014, and Consolidated Balance Sheet data as of December 31, 2016 and 2015 have been derived from our audited financial statements appearing elsewhere in this Annual Report on Form 10-K. The selected Consolidated Statements of Comprehensive Loss data for the years ended December 31, 2013 and 2012 and Consolidated Balance Sheet data as of December 31, 2014, 2013, and 2012 have been derived from our audited financial statements that are not included in this Annual Report on Form 10-K. Historical results are not necessarily indicative of future results.
| Years ended December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (in thousands, except for per share amounts) | ||||||||||||||||||||
| Consolidated Statements of Comprehensive Loss Data: |
||||||||||||||||||||
| Revenues: |
||||||||||||||||||||
| Net product sales |
$ | 265,766 | $ | 226,052 | $ | 178,198 | $ | 144,665 | $ | 138,200 | ||||||||||
| Collaboration and license agreement revenues |
84,926 | 69,770 | 68,556 | 106,781 | 67,547 | |||||||||||||||
| Royalty revenues |
67,455 | 40,980 | 40,004 | 17,818 | 5,065 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenues |
418,147 | 336,802 | 286,758 | 269,264 | 210,812 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Costs and expenses: |
||||||||||||||||||||
| Cost of sales |
28,168 | 24,476 | 17,513 | 13,759 | 11,546 | |||||||||||||||
| Cost of royalty revenues |
14,149 | 12,964 | 11,545 | 7,385 | 1,923 | |||||||||||||||
| Research and development |
379,308 | 294,529 | 230,743 | 218,627 | 170,297 | |||||||||||||||
| Selling, general and administrative |
139,247 | 125,783 | 104,320 | 92,354 | 84,300 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(142,725 | ) | (120,950 | ) | (77,363 | ) | (62,861 | ) | (57,254 | ) | ||||||||||
| Investment and other income, net |
2,614 | 464 | 1,222 | 341 | 3,472 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (140,111 | ) | $ | (120,486 | ) | $ | (76,141 | ) | $ | (62,520 | ) | $ | (53,782 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per share—basic and diluted |
$ | (1.00 | ) | $ | (0.93 | ) | $ | (0.62 | ) | $ | (0.51 | ) | $ | (0.46 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Shares used in computation of net loss per share—basic and diluted |
140,746 | 129,184 | 123,408 | 121,575 | 117,851 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| December 31, | ||||||||||||||||||||
| 2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents and investment securities |
$ | 618,974 | $ | 712,711 | $ | 313,413 | $ | 374,267 | $ | 364,258 | ||||||||||
| Working capital |
586,132 | 636,793 | 282,093 | 338,058 | 340,283 | |||||||||||||||
| Total assets |
838,396 | 895,095 | 458,965 | 483,898 | 471,422 | |||||||||||||||
| Stockholders’ equity |
634,087 | 685,911 | 210,834 | 230,185 | 226,148 | |||||||||||||||
75
Table of Contents
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Forward-Looking Statements
The following discussion of our financial condition and results of operations contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including those relating to future events or our future financial performance and financial guidance. In some cases, you can identify forward-looking statements by terminology such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “project,” “believe,” “estimate,” “predict,” “potential,” “intend” or “continue,” the negative of terms like these or other comparable terminology, and other words or terms of similar meaning in connection with any discussion of future operating or financial performance. These statements are only predictions. All forward-looking statements included in this Annual Report on Form 10-K are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements. Any or all of our forward-looking statements in this document may turn out to be wrong. Actual events or results may differ materially. Our forward-looking statements can be affected by inaccurate assumptions we might make or by known or unknown risks, uncertainties and other factors. We discuss many of these risks, uncertainties and other factors in this Annual Report on Form 10-K in greater detail under the heading “Item 1A—Risk Factors.” We caution investors that our business and financial performance are subject to substantial risks and uncertainties.
Overview
Seattle Genetics is a biotechnology company focused on the development and commercialization of targeted therapies for the treatment of cancer. Our marketed product ADCETRIS®, or brentuximab vedotin, is now approved by the United States Food and Drug Administration, or FDA, and the European Commission for three indications, encompassing several settings for the treatment of relapsed Hodgkin lymphoma and relapsed systemic anaplastic large cell lymphoma, or sALCL. ADCETRIS is commercially available in 66 countries around the world, including in the United States, Canada, members of the European Union and Japan. We are collaborating with Takeda Pharmaceutical Company Limited, or Takeda, to develop and commercialize ADCETRIS on a global basis. Under this collaboration, Seattle Genetics retains commercial rights for ADCETRIS in the United States and its territories and in Canada, and Takeda has commercial rights in the rest of the world.
Beyond our current labeled indications, we and Takeda have a broad development strategy for ADCETRIS evaluating its therapeutic potential in earlier lines of therapy for patients with Hodgkin lymphoma or mature T-cell lymphoma, or MTCL, also known as peripheral T-Cell lymphoma, or PTCL, including sALCL, and in other CD30-expressing malignancies. We and Takeda are currently conducting three phase 3 clinical trials of ADCETRIS: ALCANZA, ECHELON-1 and ECHELON-2. All of these trials are being conducted under Special Protocol Assessment, or SPA, agreements with the FDA and pursuant to scientific advice from the European Medicines Agency, or EMA. An SPA is an agreement with the FDA regarding the design of the clinical trial, including size and clinical endpoints, to support an efficacy claim in a new drug application or a Biologics License Application, or BLA, submission to the FDA if the trial achieves its primary endpoints. We plan to submit a supplemental Biologics License Application, or sBLA, to the FDA in mid-2017 to seek approval for a new indication in CD30-expressing relapsed CTCL. We have also completed enrollment of 1,334 patients in our ECHELON-1 trial and expect to report data in 2017. In November 2016, we completed enrollment of 452 patients in our ECHELON-2 trial, and expect to report data in 2018.
We are also advancing the development of SGN-CD33A, or vadastuximab talirine. A phase 3 clinical trial, called the CASCADE trial, was initiated in the second quarter of 2016 based on data from our phase 1 clinical trial for patients with acute myeloid leukemia, or AML. The CASCADE trial is evaluating SGN-CD33A in combination with hypomethylating agents, or HMAs, in previously untreated older patients with AML who are
76
Table of Contents
not candidates for intensive induction chemotherapy. We also have been evaluating SGN-CD33A in additional treatment settings, and overall, more than 300 patients have been treated with SGN-CD33A to date in clinical trials across these multiple treatment settings. On December 27, 2016, we announced that we had received notice from the FDA that a full clinical hold or partial clinical hold had been placed on several early stage trials of SGN-CD33A in AML to evaluate the potential risk of hepatotoxicity following adverse medical events, including fatal events. We are working diligently with the FDA to determine whether there is any association between hepatotoxicity and treatment with SGN-CD33A and to promptly identify appropriate measures for patient safety with the goal of addressing the FDA’s concerns.
In addition, in collaboration with Astellas Pharma, Inc., or Astellas, we are developing ASG-22ME, or enfortumab vedotin. We and Astellas are planning discussions with regulatory agencies during 2017 to advance the program into potential registrational trials in urothelial cancer patients, including patients who have been previously treated with a checkpoint inhibitor therapy.
Our clinical-stage pipeline also includes six other antibody-drug conjugate, or ADC, programs consisting of SGN-LIV1A, SGN-CD19A, or denintuzumab mafodotin, SGN-CD19B, SGN-CD123A, SGN-CD352A, and ASG-15ME, as well as two immuno-oncology agents, SEA-CD40, which is based on our sugar-engineered antibody, or SEA, technology, and SGN-2FF, which is a novel small molecule. In addition, we have multiple preclinical and research-stage programs that employ our proprietary technologies, including SGN-CD48A, a preclinical ADC that is a candidate for investigational new drug, or IND, submission in 2017.
We announced on February 10, 2017 that we had entered into a development and license agreement, or the Immunomedics License, with Immunomedics, Inc., or Immunomedics, pursuant to which, upon the terms and subject to the conditions set forth in the Immunomedics License, we would receive exclusive worldwide rights to develop, manufacture and commercialize sacituzumab govitecan (IMMU-132). IMMU-132 is an ADC targeted to TROP-2, which is expressed in several solid tumors, and is in a pivotal phase 1/2 trial for patients with triple negative breast cancer, or TNBC, and is being investigated in other solid tumors. IMMU-132 received Breakthrough Therapy Designation, or BTD, from the FDA for the treatment of patients with TNBC who have failed prior therapies for metastatic disease. In connection with the closing of the transactions contemplated by the Immunomedics License, Immunomedics would receive an upfront payment of $250 million. In addition, Immunomedics would also be eligible to receive development, regulatory and sales-dependent milestone payments across multiple indications and geographical regions of up to a total maximum of approximately $1.7 billion, as well as royalties which are based on a percentage of annual net sales of the licensed products, if any, beginning in the teens and rising to twenty percent based on sales volume. The closing of the transactions contemplated by the Immunomedics License is subject to customary closing conditions, including the expiration of the applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, or the HSR Act, there being no pending court or administrative challenges to the Immunomedics License and there being no court or administrative order blocking the closing. On February 20, 2017, Immunomedics and we entered into a letter agreement pursuant to which Immunomedics irrevocably waived to the extent applicable to Immunomedics the condition precedent to the closing and effectiveness of the Immunomedics License that there be no pending court or administrative challenges to the transaction. Additionally, under the terms of the Immunomedics License, Immunomedics had the right to continue discussions with a small number of parties that previously expressed interest in licensing IMMU-132 until 11:59 p.m. New York City time on February 19, 2017. If a third party had provided Immunomedics with a financially superior licensing offer, we would have had the right to match any such offer, and if we had decided not to match, Immunomedics would have had the right to accept the superior offer and terminate the Immunomedics License upon payment of a termination fee to us. We have not received notice from Immunomedics of any such third party offers during this limited time period, and on February 21, 2017, Immunomedics announced that it is subject to customary “no-shop” restrictions on its and its representatives’ ability to solicit, discuss or negotiate alternative licensing agreement proposals from third parties with regard to IMMU-132. On February 13, 2017, we were named a co-defendant in a lawsuit filed by venBio Select Advisors LLC, or venBio, in the Delaware Chancery Court against the members of the board of directors of Immunomedics pursuant to which, among other things, venBio seeks to enjoin the closing of the
77
Table of Contents
transactions contemplated by the Immunomedics License. As a result of the pending litigation challenging the transactions contemplated by the Immunomedics License, Immunomedics and we have committed to the Court not to close the transactions contemplated by the Immunomedics License prior to March 10, 2017. We cannot predict the timing or outcome of this legal proceeding or the impact it may have on the Immunomedics License or the closing of the transactions contemplated by the Immunomedics License. However, it is possible that, in connection with the venBio lawsuit, the Immunomedics License could be rescinded or reformed in a way that is disadvantageous to us, including by requiring us to increase the transaction consideration payable to Immunomedics under the Immunomedics License, or that otherwise adversely affects the anticipated benefits to us of the Immunomedics License. See “License Agreements—Immunomedics License” in Item 1—Business for more information.
We have collaborations for our ADC technology with a number of biotechnology and pharmaceutical companies, including AbbVie Biotechnology Ltd., or AbbVie; Bayer Pharma AG, or Bayer; Celldex Therapeutics, Inc., or Celldex; Genentech, Inc., a member of the Roche Group, or Genentech; GlaxoSmithKline LLC, or GSK; Pfizer, Inc., or Pfizer; and PSMA Development Company LLC, a subsidiary of Progenics Pharmaceuticals Inc., or Progenics. In addition, we have entered into a 50/50 co-development agreement with Agensys, Inc., an affiliate of Astellas, for the development of ADCs, including ASG-22ME. We also have an option for an ADC co-development agreement with Genmab A/S, or Genmab, and a collaboration with Unum Therapeutics, Inc., or Unum, to develop and commercialize novel antibody-coupled T-cell receptor, or ACTR, therapies incorporating our antibodies for the treatment of cancer.
Our ongoing research, development and commercial activities, together with our anticipated transfer, integration and development activities related to IMMU-132, will require substantial amounts of capital and may not ultimately be successful. In addition, we may encounter unexpected difficulties during our anticipated transfer, integration and development activities related to IMMU-132, any of which may cause us to expend greater funds and efforts or may slow, delay or limit the progress of IMMU-132’s development. Over the next several years, we expect that we will incur substantial expenses, primarily as a result of activities related to the commercialization of ADCETRIS, the continued development of ADCETRIS and SGN-CD33A and the anticipated development of IMMU-132 under the Immunomedics License. Our other product candidates are in relatively early stages of development; SGN-CD33A, our other product candidates and IMMU-132 will require significant further development, financial resources and personnel to pursue and obtain regulatory approval and develop into commercially viable products, if at all. In addition, SGN-CD33A is our only product candidate in late stage clinical development and if we are unable to resolve the clinical holds on our SGN-CD33A trials or to otherwise advance the development of SGN-CD33A, or if we fail to produce positive results in the CASCADE trial, the commercialization prospects for SGN-CD33A, as well as our business and financial prospects, would be materially adversely affected. In addition, despite the substantial commitments of time and resources by our management and other employees already incurred in connection with the Immunomedics License as well as our equity investment of approximately $14.7 million in Immunomedics, we may be unable to consummate the transactions contemplated the Immunomedics License, whether as a result of the venBio lawsuit or otherwise, in which case we will not realize any of the benefits of the licensing of IMMU-132. Likewise, it is possible that, in connection with the venBio lawsuit and/or the proxy contest that venBio has initiated with respect to the election of directors at the Immunomedics annual meeting, the Immunomedics License could be reformed in a way that is disadvantageous to us, including by requiring us to increase the transaction consideration payable to Immunomedics under the Immunomedics License, or that otherwise adversely affects the anticipated benefits to us of the Immunomedics License. Our commitment of resources to the continuing development, regulatory and commercialization activities for ADCETRIS, the research, continued development and manufacturing of our product candidates, the transactions contemplated by the Immunomedics License, including the related upfront and milestone payments provided for under the Immunomedics License, and the anticipated transfer, integration and development activities related to IMMU-132, will likely require us to raise substantial amounts of additional capital and our operating expenses will fluctuate as a result of such activities. In addition, we may incur significant milestone payment obligations to certain of our licensors, including to Immunomedics if the
78
Table of Contents
transactions contemplated by the Immunomedics License are comsummated, as our product candidates progress through clinical trials towards potential commercialization.
We recognize revenue from ADCETRIS product sales in the United States and Canada. Our future ADCETRIS product sales are difficult to accurately predict from period to period. In this regard, our product sales have varied, and may continue to vary, significantly from period to period and may be affected by a variety of factors. Such factors include the incidence rate of new patients in ADCETRIS’ approved indications, customer ordering patterns, the overall level of demand for ADCETRIS, the duration of therapy for patients receiving ADCETRIS, and the extent to which coverage and reimbursement for ADCETRIS is available from government and other third-party payers. Obtaining and maintaining appropriate coverage and reimbursement for ADCETRIS is increasingly challenging due to, among other things, the attention being paid to healthcare cost containment and other austerity measures in the U.S. and worldwide, as well as increasing legislative and enforcement interest in the United States with respect to pharmaceutical drug pricing practices. We anticipate that healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for ADCETRIS. We also anticipate that Congress, state legislatures, and third-party payors may continue to review and assess alternative healthcare delivery and payment systems and may in the future propose and adopt legislation or policy changes or implementations effecting additional fundamental changes in the healthcare delivery system, any of which could negatively affect our revenue or sales of ADCETRIS (or any future approved products). We also believe that the level of our current ADCETRIS sales in the United States has been attributable to the incidence flow of patients eligible for treatment with ADCETRIS, which can vary significantly from period to period. Moreover, we believe that the incidence rate in ADCETRIS’ approved indications is relatively low, particularly when compared to many other oncology indications. For these and other reasons, we expect that our ability to accelerate ADCETRIS sales growth, if at all, will depend primarily on our ability to continue to expand ADCETRIS’ labeled indications of use, particularly with respect to the frontline Hodgkin lymphoma and frontline MTCL indications. Our efforts to continue to expand ADCETRIS’ labeled indications of use will continue to require additional time and investment in clinical trials to complete and may not be successful. Our ability to successfully commercialize ADCETRIS and to continue to expand its labeled indications of use are subject to a number of risks and uncertainties, including those discussed in Part I, Item 1A of this Annual Report on Form 10-K. In particular, negative or inconclusive results in our ECHELON-1 and ECHELON-2 trials would negatively impact, or preclude altogether, our ability to obtain regulatory approval in the frontline Hodgkin lymphoma and frontline MTCL indications, respectively, either of which could limit our sales of, and the commercial potential of, ADCETRIS. In addition, although we reported in August 2016 that the ALCANZA trial evaluating ADCETRIS in patients with relapsed CTCL met its primary endpoint demonstrating a statistically significant improvement in the rate of objective response lasting at least four months and we plan to submit an sBLA to the FDA to seek approval for a new indication in CD30-expressing relapsed CTCL, there can be no assurance that the FDA will accept our planned sBLA for filing or that we will ultimately obtain approval of our planned sBLA in a timely manner or at all. Our failure to obtain regulatory approval and commercialize ADCETRIS in the ALCANZA treatment setting would also limit our sales of, and the commercial potential of, ADCETRIS. We also expect that amounts earned from our collaboration agreements, including royalties, will continue to be an important source of our revenues and cash flows. These revenues will be impacted by future development funding and the achievement of development, clinical and commercial success by our collaborators under our existing collaboration and license agreements, including our ADCETRIS collaboration with Takeda, as well as by entering into potential new collaboration and license agreements. Our results of operations may vary substantially from year to year and from quarter to quarter and, as a result, we believe that period to period comparisons of our operating results may not be meaningful and should not be relied upon as being indicative of our future performance.
Financial summary
Total revenues increased to $418.1 million in 2016, compared to $336.8 million in 2015. This increase was primarily driven by ADCETRIS net product sales that increased 18% to $265.8 million in 2016 as compared to
79
Table of Contents
$226.1 million in 2015, and by royalty revenues that increased 65% to $67.5 million in 2016 as compared to $41.0 million in 2015. During 2016, royalty revenues included a one-time $20.0 million milestone payment triggered by Takeda exceeding $200 million in annual net sales of ADCETRIS in its territory during 2015. Total costs and expenses increased 23% to $560.9 million in 2016, compared to $457.8 million in 2015. This primarily reflects increases in our investment in SGN-CD33A and increased ADCETRIS collaboration activities for product supply to Takeda, as well as investment in our growing pipeline of preclinical and clinical-stage programs. As of December 31, 2016, we had $619.0 million in cash, cash equivalents and investments, and $634.1 million in total stockholders’ equity.
Critical Accounting Policies
The preparation of financial statements in accordance with generally accepted accounting principles, or GAAP, requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosures of contingent assets and liabilities. We believe the following critical accounting policies describe the more significant judgments and estimates used in the preparation of our financial statements.
Revenue Recognition. Our revenues are comprised of ADCETRIS net product sales, amounts earned under our collaboration and licensing agreements and royalties. Revenue recognition is predicated upon persuasive evidence of an agreement existing, delivery of products or services being rendered, amounts payable being fixed or determinable, and collectibility being reasonably assured.
Net product sales
We sell ADCETRIS through a limited number of pharmaceutical distributors. Customers order ADCETRIS through these distributors and we typically ship product directly to the customer. We record product sales when title and risk of loss pass, which generally occurs upon delivery of the product to the customer. Product sales are recorded net of estimated government-mandated rebates and chargebacks, distribution fees, estimated product returns and other deductions. These are generally referred to as gross-to-net deductions. Accruals are established for these deductions and actual amounts incurred are offset against applicable accruals. We reflect these accruals as either a reduction in the related account receivable from the distributor, or as an accrued liability depending on the nature of the sales deduction. Sales deductions are based on our estimates that consider payer mix in target markets and our experience to date. These estimates involve a substantial degree of judgment.
Government-mandated rebates and chargebacks: We have entered into a Medicaid Drug Rebate Agreement with the Centers for Medicare & Medicaid Services. This agreement provides for a rebate to participating states based on covered purchases of ADCETRIS. Medicaid rebates are invoiced to us by the various state Medicaid programs. We estimate Medicaid rebates based on a variety of factors, including our experience to date. We also have completed our Federal Supply Schedule, or FSS, agreement under which certain U.S. government purchasers receive a discount on eligible purchases of ADCETRIS. We have entered into a Pharmaceutical Pricing Agreement with the Secretary of Health and Human Services which enables certain entities that qualify for government pricing under the Public Health Services Act, or PHS, to receive discounts on their qualified purchases of ADCETRIS. Under these agreements, distributors process a chargeback to us for the difference between wholesale acquisition cost and the applicable discounted price. As a result of our direct-ship distribution model, we can identify the entities purchasing ADCETRIS and this information enables us to estimate expected chargebacks for FSS and PHS purchases based on each entity’s eligibility for the FSS and PHS programs. We also review actual rebate and chargeback information to further refine these estimates.
Distribution fees, product returns and other deductions: Our distributors charge a volume-based fee for distribution services that they perform for us. We allow for the return of product that is within 30 days of its expiration date or that is damaged. We estimate product returns based on our experience to date. In addition, we consider our direct-ship distribution model, our belief that product is not typically held in the distribution channel, and the expected rapid use of the product by healthcare providers. We provide financial assistance to
80
Table of Contents
qualifying patients that are underinsured or cannot cover the cost of commercial coinsurance amounts through SeaGen Secure. SeaGen Secure is available to patients in the U.S. and its territories who meet various financial and treatment need criteria. Estimated contributions for commercial coinsurance under SeaGen Secure are deducted from gross sales and are based on an analysis of expected plan utilization. These estimates are adjusted as necessary to reflect our actual experience.
Collaboration and license agreement revenues
Our proprietary ADC technologies are the basis of our ADC collaborations that we have entered into in the ordinary course of business with a number of biotechnology and pharmaceutical companies. Under these ADC collaboration agreements, we grant our collaborators research and commercial licenses to our technology and typically provide technology transfer services, technical advice, supplies and services for a period of time.
If there are continuing performance obligations, we use a time-based proportional performance model to recognize revenue over our performance period for the related agreement. Collaboration and license agreements are evaluated to determine whether the multiple elements and associated deliverables can be considered separate units of accounting. To date, the pre-commercial deliverables under our collaboration and license agreements have not qualified as separate units of accounting. The assessment of multiple element arrangements requires judgment in order to determine the appropriate point in time, or period of time, that revenue should be recognized. We believe that the development period in each agreement is a reasonable estimate of the performance obligation period of such agreement. Accordingly, all amounts received or due, including any upfront payments, maintenance fees, development and regulatory milestone payments and reimbursement payments, are recognized as revenue over the performance obligation periods of each agreement. These performance obligation periods typically range from one to three years. The agreements with Takeda Pharmaceutical Company Limited, or Takeda, and Genentech, Inc., a member of the Roche Group, or Genentech, have performance obligation periods of ten and seventeen years, respectively. All of the remaining performance obligation periods for our active collaborations are currently expected to be completed in three years or less. When no performance obligations are required of us, or following the completion of the performance obligation period, such amounts are recognized as revenue when collectibility is reasonably assured. Generally, all amounts received or due other than sales-based milestones and royalties are classified as collaboration and license agreement revenues as they are earned. Sales-based milestones and royalties are recognized as royalty revenue as they are reported to us.
Our collaboration and license agreements include contractual milestones. Generally, the milestone events contained in our collaboration and license agreements coincide with the progression of the collaborators’ product candidates from development to regulatory approval and then to commercialization.
Development milestones in our collaborations may include the following types of events:
| • | Designation of a product candidate or initiation of pre-clinical studies. Our collaborators must undertake significant pre-clinical research and studies to make a determination of the suitability of a product candidate and the time from those studies or designation to initiation of a clinical trial may take several years. |
| • | Initiation of a phase 1 clinical trial. Generally, phase 1 clinical trials may take one to two years to complete. |
| • | Initiation of a phase 2 clinical trial. Generally, phase 2 clinical trials may take one to three years to complete. |
| • | Initiation of a phase 3 clinical trial. Generally, phase 3 clinical trials may take two to six years to complete. |
81
Table of Contents
Regulatory milestones in our collaborations may include the following types of events:
| • | Filing of regulatory applications for marketing approval such as a BLA in the United States or a Marketing Authorization Application in Europe. Generally, it may take up to twelve months to prepare and submit regulatory filings. |
| • | Receiving marketing approval in a major market, such as in the United States, Europe, Japan or other significant countries. Generally it may take up to three years after a marketing application is submitted to obtain approval for marketing and pricing from the applicable regulatory agency. |
Commercialization milestones in our collaborations may include the following types of events:
| • | First commercial sale in a particular market, such as in the United States, Europe, Japan or other significant countries. |
| • | Product sales in excess of a pre-specified threshold. The amount of time to achieve this type of milestone depends on several factors, including, but not limited to, the dollar amount of the threshold, the pricing of the product, market penetration of the product and the rate at which customers begin using the product. |
Our ADC collaborators are solely responsible for the development of their product candidates and the achievement of milestones in any of the categories identified above is based solely on the collaborators’ efforts.
In the case of our ADCETRIS collaboration with Takeda, we may be involved in certain development activities; however, the achievement of development, regulatory and commercial milestone events under the agreement is primarily based on activities undertaken by Takeda.
The process of successfully developing a product candidate, obtaining regulatory approval and ultimately commercializing a product candidate is highly uncertain and the attainment of any milestones is therefore uncertain and difficult to predict. In addition, since we do not take a substantive role or control the research, development or commercialization of any products generated by our ADC collaborators, we are not able to reasonably estimate when, if at all, any milestone payments or royalties may be payable to us by our ADC collaborators. As such, the milestone payments associated with our ADC collaborations involve a substantial degree of uncertainty and risk that they may never be received. Similarly, even in those collaborations where we may have an active role in the development of the product candidate, such as our ADCETRIS collaboration with Takeda, the attainment of a milestone is based on the collaborator’s activities and is generally outside our direction and control.
We generally invoice our collaborators and licensees on a monthly or quarterly basis, or upon the completion of the effort or achievement of a milestone, based on the terms of each agreement. Any deferred revenue arising from amounts received in advance of the culmination of the earnings process is recognized as revenue in future periods when the applicable revenue recognition criteria have been met. Deferred revenue expected to be recognized within the next twelve months is classified as a current liability.
Royalty revenues and cost of royalty revenues
Royalty revenues primarily reflect royalties paid to us by Takeda under the ADCETRIS collaboration. These royalties include commercial sales-based milestones and sales royalties. The royalty rate paid by Takeda is calculated as a percentage of Takeda’s net sales of ADCETRIS, ranges from the mid-teens to the mid-twenties depending on sales volumes, and resets annually. Takeda bears a portion of third-party royalty costs owed on sales of ADCETRIS in its territory. This amount is also included in our royalty revenues. Cost of royalty revenues reflect amounts owed to our third-party licensors related to the sale of ADCETRIS in Takeda’s territory. These amounts are recognized in the quarter in which Takeda reports its sales activity to us, which is the quarter following the related sales. Royalty revenues also include certain amounts earned in connection with our ADC collaborations.
82
Table of Contents
Investments. We have investments in debt securities in accordance with our investment policy. We classify our investments as available-for-sale, which are reported at estimated fair value with the related unrealized gains and losses included in accumulated other comprehensive loss in stockholders’ equity. Realized gains and losses and declines in value of investments judged to be other-than-temporary are included in investment and other income, net. The fair value of our investments is subject to volatility. Declines in the fair value of our investments judged to be other-than-temporary could adversely affect our future operating results. We estimate fair values in accordance with a hierarchy prescribed by GAAP. This hierarchy prioritizes the inputs and assumptions used, and the valuation techniques used to measure fair value.
Accrued Liabilities. As part of the process of preparing financial statements, we are required to estimate accrued liabilities. This process involves identifying services that have been performed on our behalf and estimating the level of services performed and the associated costs incurred for such services where we have not yet been invoiced or otherwise notified of actual cost. We record these estimates in our consolidated financial statements as of each balance sheet date. Examples of estimated accrued liabilities include fees due to contract research organizations and other costs in conjunction with clinical trials, fees due in conjunction with manufacturing ADCETRIS and our product candidates, third-party royalties that accrue on our sales of ADCETRIS and professional service fees, among other items.
In accruing service fees, we estimate the time period over which services will be provided and the level of effort in each period. If the actual timing of the provision of services or the level of effort varies from the estimate, we will adjust the accrual accordingly. In the event that we do not identify costs that have been incurred or we under or overestimate the level of services performed or the costs of such services, our actual liabilities would differ from such estimates. The date on which some services commence, the level of services performed on or before a given date and the cost of such services are often subjective determinations. We make judgments based upon the facts and circumstances known to us at the time and in accordance with GAAP.
Research and Development. Research and development expenses consist of salaries, benefits and other headcount related costs of our research and development staff, preclinical activities, clinical trials, lab supplies, drug manufacturing costs for our product candidates and for ADCETRIS when used in research and clinical trials, contract and outside service fees, and facilities and overhead expenses. Clinical trial expenses are a significant component of research and development expenses, and we outsource a significant portion of these costs to third parties. Our third-party clinical trial expenses include investigator fees, site costs, clinical research organization costs, the cost for other comparative or companion drugs used in the conduct of clinical trials, and costs for central laboratory testing and data management. Research and development activities are expensed as incurred. Costs associated with activities performed under research and development co-development collaborations are reflected in research and development expense. Non-refundable advance payments for goods or services that will be used or rendered for future research and development activities are capitalized and recognized as expense as the related goods are delivered or the related services are performed. Technology in-licensing fees, including milestones and maintenance fees, and other costs to acquire technologies for product candidates that have not yet received regulatory approval that are utilized in research and development and that are not expected to have alternative future use are expensed when incurred.
Share-based Compensation. Share-based compensation cost is based on the fair value of the award on the date of grant. We use the Black-Scholes option pricing model to determine the fair value of options on the date of grant which requires certain estimates to be made by management, including the expected forfeiture rate and expected term of the options. We also make decisions regarding the method of calculating the expected stock price volatility and the risk free interest rate used in the model. Fluctuations that affect these estimates could have an impact on the resulting compensation cost. We recognize this estimated fair value over the vesting period of the arrangement using the graded-vesting attribution method for stock options which vest ratably over the vesting period. For performance-based stock options, we recognized this estimated fair value over the service period of the award when we believe vesting of the performance-based stock options is considered probable. Once vesting of performance-based stock options is considered probable, we record compensation expense based on the
83
Table of Contents
portion of the service period elapsed to date, with a cumulative catch-up, net of estimated forfeitures, and recognize remaining compensation expense, if any, over the remaining estimated service period.
The fair value of each restricted stock unit, or RSU, equals the closing price of our common stock on the date of grant. RSUs granted to date vest 100% at a single point in time. We therefore amortize the value of RSUs, net of estimated forfeitures, to expense on a straight-line basis over the vesting period of the award.
Long-term Incentive Plans. We have long term incentive plans which provide eligible employees with the opportunity to receive performance-based incentive compensation comprised of cash and stock options or restricted stock units. The payment of cash and the grant or vesting of equity are contingent upon the achievement of pre-determined regulatory milestones. We record compensation expense over the estimated service period for a milestone when we believe the milestone is considered probable, which we assess at each reporting date. Once a milestone is considered probable, we record compensation expense based on the portion of the service period elapsed to date with respect to that milestone, with a cumulative catch-up, net of estimated forfeitures, and recognize any remaining compensation expense, if any, over the remaining estimated service period.
Income Taxes. We have net deferred tax assets which are fully offset by a valuation allowance due to our determination that it is more likely than not that the deferred assets will not be realized. We believe that a full valuation allowance is appropriate as we have a history of net operating losses. In the event we were to determine that we would be able to realize our net deferred tax assets in the future, an adjustment to the valuation allowance would be made, a portion of which would increase income (or decrease losses) in the period in which such a determination was made. We follow the guidance related to accounting for uncertainty in income taxes, which requires the recognition of an uncertain tax position when it is more likely than not to be sustainable upon audit by the applicable taxing authority.
Inventories. We consider regulatory approval of product candidates to be uncertain. Accordingly, we charge manufacturing costs to research and development expense until such time as a product has received regulatory approval for commercial sale. We began capitalizing ADCETRIS production costs into inventory following its accelerated approval by the FDA in 2011. ADCETRIS inventory that is deployed into clinical, research or development use is removed from inventory and charged to research and development expense when it is no longer available for use in commercial sales. Production costs for our other product candidates continue to be charged to research and development expense.
We value our inventories at the lower of cost or market value. Cost is determined on a specific identification basis. Inventory includes the cost of materials, third-party contract manufacturing and overhead associated with the production of ADCETRIS. We would write-down inventory cost to net realizable value if we were to determine that we had any excess, obsolete or unsalable inventory.
Loss Contingencies. We are involved in various legal proceedings in the normal course of our business. A loss contingency is recorded if it is probable that an asset has been impaired or a liability has been incurred and the amount of the loss can be reasonably estimated. We evaluate, among other factors, the probability of an unfavorable outcome and our ability to make a reasonable estimate of the amount of the ultimate loss. Loss contingencies that we determine to be reasonably possible, but not probable, are disclosed but not recorded. Changes in these estimates could materially affect our financial position and results of operations. Legal fees incurred as a result of our involvement in legal proceedings are expensed as incurred.
On January 10, 2017, a putative securities class action complaint was filed against Seattle Genetics, Inc., our chief executive officer, Clay Siegall, and our chief financial officer, Todd Simpson, in the United States District Court for the Western District of Washington under the following captions: Samit Patel, etc., v. Seattle Genetics, Inc., et al., No. C17-41. The putative class is composed of all purchasers of our securities between October 27, 2016 and December 26, 2016, inclusive. The complaint alleges material misrepresentations and omissions in
84
Table of Contents
public statements regarding our business, operational and compliance policies, violations by all named defendants of Section 10(b) of the Exchange Act, and Rule 10b-5 thereunder, as well as violations of Section 20(a) of the Exchange Act. The complaint seeks compensatory damages of an undisclosed amount.
On February 13, 2017, we were named a co-defendant in a lawsuit filed by venBio against the members of the board of directors of Immunomedics, or the venBio lawsuit. The venBio lawsuit was filed in the Court of Chancery of the State of Delaware under the caption venBio v. Goldenberg et. al. and alleges that the members of the Immunomedics board breached their fiduciary duties toward their stockholders by hastily licensing IMMU-132 to us. We are alleged to have aided and abetted the breach of fiduciary duties. Among other things, venBio seeks to enjoin the closing of the transactions contemplated by the Immunomedics License. As a result of the pending litigation challenging the transactions contemplated by the Immunomedics License, Immunomedics and we have committed to the Court not to close the transactions contemplated by the Immunomedics License prior to March 10, 2017.
We do not believe it is feasible to predict or determine the outcome or resolution of these lawsuits, or to estimate the amount of, or potential range of, loss with respect to these lawsuits. In addition, the timing of the final resolution of these lawsuits uncertain. As a result of these lawsuits, we will incur litigation expenses and may incur indemnification expenses, and potential resolutions of these lawsuits could include settlements requiring payments by us. Those expenses could have a material impact on our financial position, results of operations, and cash flows.
On an ongoing basis, we evaluate our estimates, including those related to revenue recognition, investments, accrued expenses, research and development, share-based compensation, income taxes, inventories and loss contingencies. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form our basis for making judgments about the carrying values of assets and liabilities and the reported amounts of revenues and expenses that are not readily apparent from other sources. Actual results may differ from those estimates under different assumptions and conditions.
Results of Operations
Years Ended December 31, 2016, 2015, and 2014
Net product sales
We sell ADCETRIS in the U.S. and Canada. Our net product sales were as follows ($ in thousands):
| 2016 | 2015 | 2014 | Annual percentage change |
|||||||||||||||||
| 2016/2015 | 2015/2014 | |||||||||||||||||||
| Net product sales |
$ | 265,766 | $ | 226,052 | $ | 178,198 | 18 | % | 27 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Net product sales increased in 2016 and 2015 as compared to prior years due to an increase in sales volume and, to a lesser extent, from the effect of price increases. The increases in sales volume in 2016 and 2015 were primarily driven by increased use of ADCETRIS across multiple lines of therapy for the treatment of Hodgkin lymphoma, sALCL and for the treatment of other CD30 expressing malignancies. Additionally, our 2016 sales volume includes a full year of ADCETRIS sales for the post-autologous hematopoietic stem cell transplantation, or auto-HSCT, consolidation indication for which we received FDA approval in the third quarter of 2015.
ADCETRIS received approval from the FDA in 2011 for the treatment of patients with classical Hodgkin lymphoma after failure of auto-HSCT or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not auto-HSCT candidates, and for the treatment of patients with sALCL, after failure of at least one prior multi-agent chemotherapy regimen. In August 2015, ADCETRIS was approved by the FDA for the treatment of patients with classical Hodgkin lymphoma at high risk of relapse or progression as post-auto-HSCT consolidation.
85
Table of Contents
We expect continued growth in ADCETRIS sales in 2017 as compared to 2016. Our ability to accelerate the rate of ADCETRIS sales growth in future periods, if at all, will be primarily dependent on our ability to continue to expand ADCETRIS’ labeled indications of use. Our efforts to expand ADCETRIS’ labeled indications of use will continue to require additional time and investment in clinical trials to complete and may not be successful.
We record product sales net of estimated government-mandated rebates and chargebacks, distribution fees, product returns and other deductions. These are generally referred to as gross-to-net deductions. Gross-to-net deductions, net of related payments and credits, are summarized as follows:
| December 31, 2016 | December 31, 2015 | December 31, 2014 | ||||||||||||||||||||||||||||||||||
| Rebates & chargebacks |
Distribution fees, product returns and other |
Total | Rebates & chargebacks |
Distribution fees, product returns and other |
Total | Rebates & chargebacks |
Distribution fees, product returns and other |
Total | ||||||||||||||||||||||||||||
| Balance, beginning of year |
$ | 7,111 | $ | 2,359 | $ | 9,470 | $ | 5,268 | $ | 1,618 | $ | 6,886 | $ | 4,525 | $ | 1,523 | $ | 6,048 | ||||||||||||||||||
| Provision related to current year sales |
74,075 | 6,522 | 80,597 | 48,214 | 5,391 | 53,605 | 31,541 | 4,370 | 35,911 | |||||||||||||||||||||||||||
| Adjustments for prior period sales |
(1,043 | ) | (141 | ) | (1,184 | ) | (1,065 | ) | 34 | (1,031 | ) | (913 | ) | (62 | ) | (975 | ) | |||||||||||||||||||
| Payments/credits for current year sales |
(65,598 | ) | (4,733 | ) | (70,331 | ) | (42,656 | ) | (4,070) | (46,726 | ) | (28,038 | ) | (3,753 | ) | (31,791 | ) | |||||||||||||||||||
| Payments/credits for prior year sales |
(5,045 | ) | (809 | ) | (5,854 | ) | (2,650 | ) | (614) | (3,264 | ) | (1,847 | ) | (460 | ) | (2,307 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balance, end of year |
$ | 9,500 | $ | 3,198 | $ | 12,698 | $ | 7,111 | $ | 2,359 | $ | 9,470 | $ | 5,268 | $ | 1,618 | $ | 6,886 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
Mandatory government discounts are the most significant component of our total gross to net deductions and the discount percentage has been increasing. These discount percentages increased during 2016 and 2015 as a result of price increases we instituted that exceeded the rate of inflation, and to a lesser extent in 2016, as a result of an increase in the proportion of our sales eligible for government mandated rebates or chargebacks. Generally, the change in government prices is limited to the rate of inflation. Distribution fees, product returns and other gross to net deductions were virtually unchanged as a percentage of our gross sales among the three years presented above. We expect future gross-to-net deductions to fluctuate based on the volume of purchases eligible for government mandated discounts and rebates, as well as changes in the discount percentage which is impacted by potential future price increases, the rate of inflation, and other factors. We implemented a price increase at the beginning of 2017 and, as a result of this price increase, and a recent increase in the percentage of our gross sales that are eligible for government mandated rebates and chargebacks we expect gross-to-net deductions to increase in 2017. In recent months there has been extensive discussion in the United States about expanding government discount programs, including allowing Medicare to negotiate drug prices, and pressure on pharmaceutical drug pricing is expected to increase. If government discounted programs are expanded or discounts increased as a result of changes in regulations in the United States, our gross to net deductions will increase and our net sales will be negatively impacted.
86
Table of Contents
Collaboration and license agreement revenues
Collaboration and license agreement revenues reflect amounts earned under product collaborations and ADC collaboration and co-development agreements. These revenues reflect the earned portion of payments received by us for technology access and maintenance fees, milestone payments and reimbursement payments for research and development support that we provide to our collaborators. Collaboration and license agreement revenues are summarized by collaborator as follows:
| Collaboration and license agreement revenues by |
Annual percentage change |
|||||||||||||||||||
| 2016 | 2015 | 2014 | 2016/2015 | 2015/2014 | ||||||||||||||||
| Takeda |
$ | 44,384 | $ | 17,234 | $ | 31,787 | 158 | % | (46 | %) | ||||||||||
| AbbVie |
25,676 | 31,055 | 14,851 | (17 | %) | 109 | % | |||||||||||||
| Genentech |
4,324 | 9,110 | 7,791 | (53 | %) | 17 | % | |||||||||||||
| Other |
10,542 | 12,371 | 14,127 | (15 | %) | (12 | %) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 84,926 | $ | 69,770 | $ | 68,556 | 22 | % | 2 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Collaboration revenues from Takeda fluctuate based on changes in the earned portion of reimbursement funding under the ADCETRIS collaboration, which are influenced by the activities each party is performing under the collaboration agreement at a given time. For example, when Takeda’s level of spending on clinical collaboration activities increases above our own, our earned portion of reimbursement funding generally decreases. Additionally, we receive reimbursement for the cost of drug product supplied to Takeda for its use, the timing of which fluctuates based on Takeda’s product supply needs. The earned portion of reimbursement funding fluctuates based upon how much drug product Takeda has purchased from us in a given period.
The increase in the earned portion of reimbursement funding in 2016 primarily reflects a decrease in clinical trial costs related to activity performed by Takeda as the ALCANZA and ECHELON-1 studies advanced, and an increase in drug product supply activities to Takeda. The reduction in the earned portion of reimbursement funding in 2015 occurred as clinical trial activity performed by Takeda for the ALCANZA and ECHELON-1 studies increased significantly, resulting in net reimbursement payments by us to Takeda in 2015.
Revenues from AbbVie decreased during 2016 as compared to 2015 primarily as a result of a decrease in the earned portion of milestone payments achieved in 2015. Revenues from AbbVie increased during 2015 as compared to 2014 primarily as a result of the earned portion of milestone payments achieved upon AbbVie’s commencement of clinical trials for multiple product targets, the completion of a contract amendment in 2015 that expanded the scope of our ADC collaboration with AbbVie, and increased reimbursements for support activities provided by us to AbbVie in 2015.
Changes in revenues recognized from our Genentech and other collaboration agreements, which include our ADC collaborations and our co-development collaborations, reflect the timing of development milestones and licensing fees.
Our collaboration and license agreement revenues are impacted by the term and duration of our collaboration agreements and by progress-dependent milestones, annual maintenance fees and reimbursement of materials and support services. Collaboration and license agreement revenues may vary substantially from year to year and quarter to quarter depending on the progress made by our collaborators with their product candidates, the level of support we provide to our collaborators, specifically to Takeda under our ADCETRIS collaboration, the timing of milestones achieved and our ability to enter into additional collaboration and co-development agreements. We expect our collaboration and license agreement revenues in 2017 to be consistent with 2016. We have a significant balance of deferred revenue, representing prior payments from our collaborators that have not yet been recognized as revenue. This deferred revenue will be recognized as revenue in future periods using a time-based approach as we fulfill our performance obligations.
87
Table of Contents
Collaboration Agreements
Takeda
Our ADCETRIS collaboration with Takeda provides for the global co-development of ADCETRIS by the companies and the commercialization of ADCETRIS by Takeda in its territory. We received an upfront payment and have received and are entitled to receive progress-dependent milestone payments based on Takeda’s achievement of certain events related to ADCETRIS development. Additionally, the companies equally co-fund the cost of selected development activities conducted under the collaboration. We recognize as collaboration revenue the upfront payment, progress-dependent development and regulatory milestone payments, and net development cost reimbursement payments from Takeda over the ten-year development period of the collaboration, which began in December 2009. When the performance of development activities under the collaboration results in us making a reimbursement payment to Takeda, the effect is to reduce the amount of collaboration revenue that we record. We also receive reimbursement for the cost of drug product supplied to Takeda for its use and, in some cases, pay Takeda for drug product they supply to us. The earned portion of net collaboration payments is reflected as a component of collaboration and license agreement revenues.
As of December 31, 2016, total future potential milestone payments to us under the ADCETRIS collaboration could total approximately $165 million. Of the remaining amount, up to approximately $7 million relates to the achievement of development milestones, up to approximately $118 million relates to the achievement of regulatory milestones and up to approximately $40 million relates to the achievement of commercial milestones. As of December 31, 2016, $70 million in milestones had been achieved as a result of regulatory and commercial progress by Takeda.
Astellas
We entered into an agreement with Agensys, subsequently acquired by Astellas, to jointly research, develop and commercialize ADCs for the treatment of several types of cancer. The collaboration encompasses combinations of our ADC technology with fully-human antibodies developed by Astellas to proprietary cancer targets.
Under the collaboration agreement, we and Astellas are co-funding all development and commercialization costs for ASG-22ME and ASG-15ME, and will share in any profits that may come from these product candidates if successfully commercialized on a 50/50 basis. Costs associated with co-development activities are included in research and development expense.
Astellas is developing another ADC product candidate on its own, subject to paying us annual maintenance fees, milestones, royalties and support fees for research and development services and material provided under the collaboration agreement. Amounts received for this product candidate being developed solely by Astellas are recognized as revenue.
Unum Therapeutics
We have a strategic collaboration and license agreement with Unum to develop and commercialize novel ACTR therapies incorporating our antibodies for cancer. We and Unum will initially develop two ACTR product candidates that combine Unum’s ACTR technology with our antibodies, and we have an option to expand the collaboration to include a third ACTR product candidate upon payment of an additional fee. Unum is obligated to conduct preclinical research and clinical development activities through phase 1 clinical trials and we are obligated to provide funding for these activities. The agreement calls for us to work together to co-develop and jointly fund programs after phase 1 clinical trials unless either company opts out. Costs associated with co-development activities are included in research and development expense.
We and Unum would co-commercialize any successfully developed product candidates and share any profits 50/50 on any co- developed programs in the United States. We retain exclusive commercial rights outside
88
Table of Contents
of the United States, paying Unum a royalty that is a high single digit to mid-teens percentage of ex-U.S. sales, if any. The potential future licensing and progress-dependent milestone payments to Unum under the collaboration may total up to $615 million across all three ACTR programs, payment of which is triggered by the achievement of development, regulatory and commercial milestones.
ADC Collaboration Agreements
We have other active collaborations with a number of companies to allow them to use our proprietary ADC technology. Under our ADC collaborations, which we have entered into in the ordinary course of business, we typically receive or are entitled to receive upfront cash payments, progress-dependent milestones and royalties on net sales of products incorporating our ADC technology, as well as annual maintenance fees and support fees for research and development services and materials provided under the agreements. These amounts are recognized as revenue as they are realized, or over the performance obligation period of the agreements during which we provide limited support to the collaborator. Our ADC collaborators are responsible for development, manufacturing and commercialization of any ADC product candidates that result from the collaborations and are solely responsible for the achievement of the potential milestones under these collaborations.
As of December 31, 2016, our ADC collaborations and co-development agreements had generated more than $350 million, primarily in the form of upfront payments. Total milestone payments to us under our current ADC collaboration and co-development agreements could total up to approximately $3.3 billion if all potential product candidates achieved all of their milestone events. Of this amount, approximately $0.5 billion relates to the achievement of development milestones, approximately $1.3 billion relates to the achievement of regulatory milestones and approximately $1.5 billion relates to the achievement of commercial milestones. Since we do not control the research, development or commercialization of any of the products that would generate these milestones, we are not able to reasonably estimate when, if at all, any milestone payments or royalties may be payable by our collaborators. Successfully developing a product candidate, obtaining regulatory approval and ultimately commercializing it is a significantly lengthy and highly uncertain process which entails a significant risk of failure. In addition, business combinations, changes in a collaborator’s business strategy and financial difficulties or other factors could result and have resulted in a collaborator abandoning or delaying development of its product candidates. As such, the milestone payments associated with our ADC collaborations and co-development agreements involve a substantial degree of risk and may never be received. Accordingly, we do not expect, and investors should not assume, that we will receive all of the potential milestone payments described above and it is possible that we may never receive any significant milestone payments under these agreements.
Royalty Revenues and Cost of Royalty Revenues
Royalty revenues primarily reflect royalties paid to us by Takeda under the ADCETRIS collaboration. These royalties include commercial sales-based milestones and sales royalties, which are based on a percentage of Takeda’s net sales at rates that range from the mid-teens to the mid-twenties based on sales volume. Takeda bears a portion of third-party royalty costs owed on sales of ADCETRIS in its territory. This amount is included in our royalty revenues. Cost of royalty revenues reflect amounts owed to our third-party licensors related to the sale of ADCETRIS in Takeda’s territory.
Our royalty revenues and cost of royalty revenues were as follows ($ in thousands):
| Annual percentage change |
||||||||||||||||||||
| 2016 | 2015 | 2014 | 2016/2015 | 2015/2014 | ||||||||||||||||
| Royalty revenues |
$ | 67,455 | $ | 40,980 | $ | 40,004 | 65 | % | 2 | % | ||||||||||
| Cost of royalty revenues |
14,149 | 12,964 | 11,545 | 9 | % | 12 | % | |||||||||||||
Royalty revenues increased in 2016 and 2015 as compared to the prior year amounts primarily as a result of an increase in sales volumes of ADCETRIS by Takeda in its territory. Royalty revenues in 2016 and 2014
89
Table of Contents
included a $20 million and a $5 million milestone payment, respectively, related to Takeda achieving one-time sales-based milestone targets. Royalty revenues also increased in both periods as a result of regulatory approvals of ADCETRIS in additional countries, as well as increases in the royalty rate based on sales volumes. Takeda’s international sales of ADCETRIS are converted to U.S. dollars for purposes of determining the amount of royalties payable to us and therefore, our royalty revenues are subject to foreign exchange rate volatility. The U.S. dollar strengthened during 2016 and 2015, which limited to some extent the rate of royalty growth year over year.
Cost of royalty revenues fluctuates based on the amount of net sales of ADCETRIS by Takeda in its territories.
We expect that royalty revenues will decrease in 2017 as compared to 2016 primarily as a result of the 2016 royalty revenues including a $20 million one-time sales milestone triggered by Takeda’s exceeding $200 million in annual sales for the first time. We expect cost of royalty revenues to increase in 2017 primarily due to anticipated increases in sales volumes in Takeda’s territory.
Cost of Sales
ADCETRIS cost of sales includes manufacturing costs of product sold, third-party royalty costs, amortization of technology license costs, and distribution and other costs.
| 2016 | 2015 | 2014 | Annual percentage change |
|||||||||||||||||
| 2016/2015 | 2015/2014 | |||||||||||||||||||
| Cost of sales |
$ | 28,168 | $ | 24,476 | $ | 17,513 | 15 | % | 40 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Cost of sales increased during 2016 as compared to 2015 primarily due to increased sales volumes. Cost of sales increased in 2015 as compared to 2014 primarily due to increased sales volumes and, to a lesser extent, due to a higher average cost of product sold as remaining pre-commercial ADCETRIS inventory was fully depleted in the fourth quarter of 2015.We began capitalizing ADCETRIS manufacturing costs as inventory following the accelerated approval of ADCETRIS by the FDA in 2011. The cost of product manufactured prior to FDA approval was expensed as research and development expense as incurred and was combined with other research and development expenses. Certain components manufactured prior to FDA approval, and therefore expensed, continued to provide some cost benefit through the end of 2015. We expect cost of sales to increase in 2017, primarily due to anticipated increases in sales volumes.
Research and development
Our research and development expenses are summarized as follows:
| Research and development ($ in thousands) |
Annual percentage change |
|||||||||||||||||||
| 2016 | 2015 | 2014 | 2016/2015 | 2015/2014 | ||||||||||||||||
| Research (1) |
$ | 62,071 | $ | 77,215 | $ | 41,190 | (20 | %) | 87 | % | ||||||||||
| Development and contract manufacturing |
143,920 | 93,734 | 83,326 | 54 | % | 12 | % | |||||||||||||
| Clinical (1) |
173,317 | 123,580 | 106,227 | 40 | % | 16 | % | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total research and development expenses |
$ | 379,308 | $ | 294,529 | $ | 230,743 | 29 | % | 28 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | The cost of pharmacology and toxicology studies from the 2015 and 2014 periods have been reclassified from Clinical to Research to conform to our fiscal 2016 classification in the above table. |
Research expenses include personnel, occupancy and laboratory expenses, technology access fees associated with the discovery and identification of new monoclonal antibodies and related technologies, and the
90
Table of Contents
development of novel classes of stable linkers and cell-killing agents for our ADC technology. Research expenses also include research activities associated with our product candidates, such as preclinical translational biology and in vitro and in vivo studies, and investigational new drug, or IND-enabling pharmacology and toxicology studies. The decrease in research expenses in 2016 as compared to 2015 is primarily the result of a $25.0 million technology access fee related to the initiation of our collaboration agreement with Unum in June of 2015, offset partially by increased staffing, facilities and other costs to support our growing pipeline of product candidates, as well as increases in technology access fees paid and cost reimbursements to Unum under our collaboration agreement. The increase in research expenses in 2015 as compared to 2014 reflects increases in staffing costs and discovery activities in support of our growing pipeline of product candidates, and a $25.0 million technology access fee related to our collaboration agreement with Unum.
Development and contract manufacturing expenses include personnel and occupancy expenses, external contract manufacturing costs for the scale up and pre-approval manufacturing of drug product used in research and our clinical trials, and costs for drug product supplied to our collaborators. Development and contract manufacturing expenses also include quality control and assurance activities, and storage and shipment of our product candidates. The increase in development and contract manufacturing expenses in 2016 as compared to 2015 resulted primarily from an increase in drug product supplied to Takeda under the ADCETRIS collaboration, and to a lesser extent, increases in staffing and other costs to support our growing pipeline of product candidates and cost reimbursements to Astellas under our collaboration. The increase in development and contract manufacturing expenses in 2015 as compared to 2014 was primarily the result of increased staffing, facilities and other costs to support our growing pipeline of product candidates.
Clinical expenses include personnel, travel, occupancy costs, and external clinical trial costs including costs for clinical sites, clinical research organizations, central laboratories, data management, contractors and regulatory activities associated with conducting human clinical trials. The increases in clinical expenses in both 2016 and 2015 as compared to prior years reflect increased clinical trial activity for our product candidates, primarily SGN-CD33A, and to a lesser extent, increases in compensation, facilities and other employee related costs as a result of increased staffing levels to support the clinical development of our product candidates.
We utilize our employee and infrastructure resources across multiple development projects as well as our discovery and research programs directed towards identifying monoclonal antibodies and new classes of stable linkers and cell-killing agents for our ADC program. We track human resource efforts expended on many of our programs for purposes of billing our collaborators for time incurred at agreed upon rates and for resource planning. We do not account for actual costs on a project-by-project basis as it relates to our infrastructure, facility, employee and other indirect costs. We do, however, separately track significant third-party costs including clinical trial costs, manufacturing costs and other contracted service costs on a project-by-project basis.
91
Table of Contents
The following table shows expenses incurred for research, contract manufacturing of our product candidates and clinical and regulatory services provided by third parties as well as pre-commercial milestone payments for in-licensed technology for ADCETRIS and certain of our clinical-stage product candidates. The table also presents other third-party costs and overhead consisting of personnel, facilities and other indirect costs not directly charged to these development programs.
| Product candidates ($ in thousands) |
Annual percentage change |
(5
years) January 1, 2012 to December 31, 2016 |
||||||||||||||||||||||
| 2016 | 2015 | 2014 | 2016/2015 | 2015/2014 | ||||||||||||||||||||
| ADCETRIS (brentuximab vedotin) |
$ | 73,623 | $ | 50,965 | $ | 53,473 | 44 | % | (5 | %) | $ | 290,620 | ||||||||||||
| SGN-CD33A (vadastuximab talirine) |
49,387 | 15,769 | 7,371 | 213 | % | 114 | % | 87,811 | ||||||||||||||||
| SGN-CD19A (denintuzumab mafodotin) |
7,696 | 5,356 | 7,276 | 44 | % | (26 | %) | 30,154 | ||||||||||||||||
| ASG-22ME (enfortumab vedotin) |
5,607 | 2,618 | 2,100 | 114 | % | 25 | % | 18,751 | ||||||||||||||||
| Other clinical stage programs |
19,437 | 17,875 | 13,490 | 9 | % | 33 | % | 65,858 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total third-party costs |
155,750 | 92,583 | 83,710 | 68 | % | 11 | % | 493,194 | ||||||||||||||||
| Other costs and overhead |
223,558 | 201,946 | 147,033 | 11 | % | 37 | % | 800,310 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total research and development |
$ | 379,308 | $ | 294,529 | $ | 230,743 | 29 | % | 28 | % | $ | 1,293,504 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Third-party costs for ADCETRIS increased during 2016 as compared to 2015 primarily as a result of an increase in drug product supplied to Takeda, and to a lesser extent, third-party clinical trial costs as we evaluated the use of ADCETRIS in other lines of therapy. Third-party costs for ADCETRIS decreased during 2015 as compared to 2014 primarily due to a decrease in drug product supplied to Takeda. The cost of drug product supplied to Takeda is charged to research and development expense. We are reimbursed for the drug product, which is included as a component of collaboration revenue.
Third-party costs for SGN-CD33A increased during 2016 and 2015 as compared to prior years due to increases in clinical trial costs and drug supply activities for both ongoing and potential additional clinical trials. In 2016, we initiated a phase 3 clinical trial of SGN-CD33A called the CASCADE trial, and continued planning for potential additional phase 1 and 2 trials.
Third-party costs for SGN-19A increased during 2016 as compared to 2015 due to an increase in clinical trial costs as we continued to make progress in our clinical trials in DLBCL. Third party costs decreased in 2015 as compared to 2014 due to the timing of drug supply activities.
Third-party costs for ASG-22ME increased during 2016 as compared to 2015 due to an increase in drug supply activities as we prepare to initiate additional clinical trials in 2017. The modest change in third party costs between 2014 and 2015 reflects increasing activity on this project.
The increase in third-party costs of our other clinical stage programs during 2016 and 2015 reflects the continued investment in our other pipeline products, which are primarily in phase 1 clinical trials. The development costs for our product candidates typically accelerate in preparation for an IND submission to the FDA and then decrease until the subsequent clinical trials commence. During 2016, we advanced SGN-CD19B, SGN-CD123A, and SGN-CD352A into phase 1 clinical trials. During 2015, we advanced SEA-CD40 into phase 1 clinical trials.
Other costs and overhead include third-party costs of our other preclinical programs, including our strategic collaboration with Unum, and costs associated with personnel and facilities. The increases in 2016 and 2015
92
Table of Contents
primarily reflect increases in staffing levels and the expansion of our facilities to accommodate our growth. Additionally, these costs increased during 2015 due to a $25.0 million technology access fee related to our collaboration agreement with Unum.
Our expenditures on our ADCETRIS clinical development program and on our current and future preclinical and clinical development programs are subject to numerous uncertainties in timing and cost to completion. In order to advance our product candidates toward commercialization, the product candidates are tested in numerous preclinical safety, toxicology and efficacy studies. We then conduct clinical trials for those product candidates that take several years or more to complete. The length of time varies substantially based upon the type, complexity, novelty and intended use of a product candidate. Likewise, in order to expand ADCETRIS’ labeled indications of use, we are required to conduct additional extensive clinical studies. The cost of clinical trials may vary significantly over the life of a project as a result of a variety of factors, including:
| • | the number of patients required in our clinical trials; |
| • | the length of time required to enroll trial participants; |
| • | the number and location of sites included in the trials; |
| • | the costs of producing supplies of the product candidates needed for clinical trials and regulatory submissions; |
| • | the safety and efficacy profile of the product candidate; |
| • | the use of clinical research organizations to assist with the management of the trials; and |
| • | the costs and timing of, and the ability to secure, regulatory approvals. |
Reports of adverse events or safety concerns involving ADCETRIS and our product candidates can interrupt, delay or halt clinical trials of ADCETRIS, including the ADCETRIS post-approval confirmatory study that is required as a condition to our relapsed sALCL approval in the United States, or clinical trials of our other product candidates. In this regard, in December 2016, several trials of SGN-CD33A in AML patients were placed on full or partial clinical holds. No new studies of SGN-CD33A will be initiated unless and until the clinical holds are lifted, and these delays could increase the cost of conducting clinical trials. Our other ongoing trials of SGN-CD33A, the phase 3 CASCADE trial in older AML patients and phase 1/2 trial in myelodysplastic syndrome, are proceeding with enrollment.
Our strategy has included entering into collaborations with third parties. In these situations, the preclinical development or clinical trial process for a product candidate and the estimated completion date are largely under the control of that third party and not under our control. We cannot forecast with any degree of certainty which of our product candidates will be subject to future collaborations or how such arrangements would affect our development plans or capital requirements.
We anticipate that our total research and development expenses in 2017 will increase compared to 2016 due to increased costs for the development of our product candidates, primarily SGN-CD33A, ASG-22ME, SGN-CD19A, and SGN-LIV1A. If the transactions contemplated by the Immunomedics License are consummated as anticipated, we expect total research and development expenses to potentially increase significantly as a result of the upfront and other milestone payments and development costs that we would undertake as part of the Immunomedics License. Certain ADCETRIS development activities, including some clinical studies, will be conducted by Takeda, the costs of which are not reflected in our research and development expenses. Because of these and other factors, expenses will fluctuate based upon many factors, including the degree of collaborative activities, timing of manufacturing campaigns, numbers of patients enrolled in our clinical trials and the outcome of each clinical trial event.
The risks and uncertainties associated with our research and development projects are discussed more fully in Item 1A—Risk Factors. As a result of the uncertainties discussed above, we are unable to determine, with any
93
Table of Contents
degree of certainty, the duration and completion costs of our research and development projects, anticipated completion dates or when and to what extent we will receive cash inflows from the commercialization and sale of ADCETRIS in any additional approved indications or of any of our product candidates.
Selling, general and administrative
| Selling, general and administrative ($ in thousands) |
Annual percentage change |
|||||||||||||||||||
| 2016 | 2015 | 2014 | 2016/2015 | 2015/2014 | ||||||||||||||||
| Selling, general and administrative |
$ | 139,247 | $ | 125,783 | $ | 104,320 | 11 | % | 21 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Selling, general and administrative expenses in 2016 primarily reflect increases in costs for staffing to support our continued growth. Selling, general and administrative expenses in 2015 primarily reflect increases in commercial activities in support of ADCETRIS, and to a lesser extent, increases in costs for staffing and legal matters.
We anticipate that selling, general and administrative expenses will increase in 2017 compared to 2016 as we continue our commercial activities in support of the commercialization of ADCETRIS, as well as our support of general operations. If the transactions contemplated by the Immunomedics License are consummated as anticipated, we expect selling, general and administrative expenses to increase further.
Investment and other income, net
| Investment and other income, net ($ in thousands) |
||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Total |
$ | 2,614 | $ | 464 | $ | 1,222 | ||||||
|
|
|
|
|
|
|
|||||||
Investment and other income, net reflects amounts earned on our investments in U.S. Treasury securities. Investment income increased during 2016 as compared to 2015 due to higher average investment balances following our public offering in September 2015, which resulted in net proceeds to us of approximately $526.6 million. Investment income in 2014 was higher than in 2015. This reflects a gain on the sale of a security sold in 2014.
Liquidity and capital resources
| December 31, | ||||||||||||
| Selected balance sheet and cash flow data ($ in thousands) |
2016 | 2015 | 2014 | |||||||||
| Cash, cash equivalents and investments |
$ | 618,974 | $ | 712,711 | $ | 313,413 | ||||||
| Working capital |
586,132 | 636,793 | 282,093 | |||||||||
| Stockholders’ equity |
634,087 | 685,911 | 210,834 | |||||||||
| Years ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Cash provided by (used in): |
||||||||||||
| Operating activities |
$ | (96,971 | ) | $ | (133,203 | ) | $ | (59,999 | ) | |||
| Investing activities |
68,193 | (375,850 | ) | 36,622 | ||||||||
| Financing activities |
35,196 | 554,381 | 16,188 | |||||||||
The changes in net cash used in operating activities are primarily related to our net loss, working capital fluctuations and changes in our non-cash expenses, all of which are highly variable. The changes in cash provided by (used in) investing activities reflect differences between the proceeds received from sale and
94
Table of Contents
maturity of our investments and amounts reinvested. Cash provided by financing activities includes proceeds from stock option exercises and our employee stock purchase plan for all years presented, and for 2015 includes $526.6 million in net proceeds from our underwritten public offering in September 2015.
We have primarily financed the majority of our operations through the issuance of equity securities, by amounts received pursuant to product collaborations, our ADC collaborations and through collections from commercial sales of ADCETRIS. To a lesser degree, we have also financed our operations through royalty revenues and interest earned on cash, cash equivalents and investment securities. These financing and revenue sources have historically allowed us to maintain adequate levels of cash and investments.
Our cash, cash equivalents, and investments are held in a variety of non-interest bearing bank accounts and interest-bearing instruments subject to investment guidelines allowing for holdings in U.S. government and agency securities, corporate securities, taxable municipal bonds, commercial paper and money market accounts. Our investment portfolio is structured to provide for investment maturities and access to cash to fund our anticipated working capital needs. However, if our liquidity needs should be accelerated for any reason in the near term, or investments do not pay at maturity, we may be required to sell investment securities in our portfolio prior to their scheduled maturities, which may result in a loss. As of December 31, 2016, we had $589.0 million held in cash or investments scheduled to mature within the next twelve months.
At our currently planned spending rates we believe that our financial resources, together with product and royalty revenues from sales of ADCETRIS and the fees, milestone payments and reimbursements we expect to receive under our existing collaboration and license agreements, will be sufficient to fund our operations for at least the next twelve months, including with respect to the upfront payment we would be required to make to Immunomedics if the transactions contemplated by the Immunomedics License are consummated on the currently-agreed upon terms. Changes in our spending rate may occur that would consume available capital resources sooner, such as increased development, manufacturing and clinical trial expenses in connection with our expanding pipeline programs, including several phase 3 trials, or our undertaking of additional programs, business activities, or entry into strategic transactions, including potential additional acquisitions of products, technologies or businesses such as our anticipated in-licensing of IMMU-132. In addition, we do not currently have sufficient capital to fund all of the potential milestone payments to Immunomedics and to Unum under our agreements with those companies. Accordingly, we may be required to, or may otherwise determine to, raise additional capital to fund those obligations or otherwise in connection with our entry into the Immunomedics License and/or our collaboration agreement with Unum. Further, in the event of a termination of the ADCETRIS collaboration agreement with Takeda, we would not receive development cost sharing payments or milestone payments or royalties for the development or sale of ADCETRIS in Takeda’s territory, and we would be required to fund all ADCETRIS development and commercial activities. Any of these factors could lead to a need for us to raise additional capital.
We expect to make additional capital outlays and to increase operating expenditures over the next several years as we hire additional employees, support our preclinical development, manufacturing and clinical trial activities for ADCETRIS and our other pipeline programs, and expand internationally, as well as commercialize ADCETRIS and position ADCETRIS for potential additional regulatory approvals. In addition, we anticipate committing substantial capital resources to the transactions contemplated by the Immunomedics License and the anticipated transfer, integration and development activities related to IMMU-132, including with respect to our potential upfront and milestone payment obligations to Immunomedics. Our commitment of resources to the continuing development, regulatory and commercialization activities for ADCETRIS, the transactions contemplated by the Immunomedics License and the anticipated transfer, integration and development activities related to IMMU-132, and the research, continued development and manufacturing of our product candidates will likely require us to raise substantial amounts of additional capital. In addition, we actively evaluate various strategic transactions on an ongoing basis, including licensing or otherwise acquiring complementary products, technologies or businesses, such as our anticipated in-licensing of IMMU-132, and we may require significant additional capital in order to complete or otherwise provide funding for any additional acquisitions. We may seek
95
Table of Contents
additional funding through some or all of the following methods: corporate collaborations, licensing arrangements and public or private debt or equity financings. We do not know whether additional capital will be available when needed, or that, if available, we will obtain financing on terms favorable to us or our stockholders. If we are unable to raise additional funds when we need them, we may be required to delay, reduce the scope of, or eliminate one or more of our development programs, which may adversely affect our business and operations.
Commitments
The following table reflects our future minimum contractual commitments as of December 31, 2016 (in thousands):
| Total | 2017 | 2018 | 2019 | 2020 | 2021 | Thereafter | ||||||||||||||||||||||
| Operating leases |
$ | 26,189 | $ | 6,995 | $ | 4,706 | $ | 2,500 | $ | 2,575 | $ | 2,653 | $ | 6,760 | ||||||||||||||
| Tenant improvements |
11,477 | 11,477 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||
| Manufacturing, license & collaboration agreements |
266,939 | 71,318 | 29,644 | 26,554 | 25,165 | 25,988 | 88,270 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total |
$ | 304,605 | $ | 89,790 | $ | 34,350 | $ | 29,054 | $ | 27,740 | $ | 28,641 | $ | 95,030 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
We have entered into leases for our office and laboratory facilities expiring in 2018 through 2024 that contain rate escalations and options for us to extend the leases. Operating lease obligations in the table above do not assume the exercise by us of any extension options.
Manufacturing, license and collaboration agreement commitments include non-cancellable obligations under our manufacturing, license and collaboration agreements. A substantial portion of the minimum payments under manufacturing, license and collaboration agreements represents contractual obligations related to manufacturing our product candidates for use in our clinical trials and for commercial operations in the case of ADCETRIS.
Some of our manufacturing, license and collaboration agreements provide for periodic maintenance fees over specified time periods, as well as payments by us upon the achievement of development and regulatory milestones. Some of our licensing agreements obligate us to pay royalties from the low single digit to mid-teens based on net sales of products utilizing licensed technology. Such royalties are dependent on future product sales and are not provided for in the table above as they are dependent on events that have not yet occurred. Future milestone payments for research and pre-clinical stage development programs have not been included in the above table as the event triggering such payment or obligation has not yet occurred. The above table also excludes up to $1.9 billion in upfront and potential future milestone payments currently provided for under the Immunomedics License, up to $615.0 million in potential future milestone payments to Unum under our collaboration agreement with Unum and up to approximately $96.8 million in potential future milestone payments to other third parties under license agreements for our clinical-stage development programs. These milestone payments generally become due and payable only upon the achievement of certain developmental, clinical, regulatory and/or commercial milestones. These contingent payments have not been included in the above table as the event triggering such payment or obligation has not yet occurred. No milestones were paid to Unum during 2016 or 2015; however, we made an upfront payment of $25.0 million to Unum and a $5.0 million equity investment in Unum in connection with our entering into the Unum collaboration in June 2015. Milestone payments under license agreements for clinical-stage development programs through December 31, 2016 have totaled $14.6 million.
Recent accounting pronouncements
In May 2014, the Financial Accounting Standards Board, or FASB, issued an Accounting Standards Update entitled “ASU 2014-09, Revenue from Contracts with Customers.” The standard requires entities to recognize
96
Table of Contents
revenue through an evaluation that includes identification of the contract, identification of the performance obligations, determination of the transaction price, allocation of the transaction price to the performance obligations, and recognition of revenue as the entity satisfies the performance obligations. In August 2015, FASB issued an Accounting Standards Update entitled “ASU 2015-14, Revenue from Contracts with Customers: Deferral of the Effective Date”, which defers the effective date of ASU 2014-09 to our fiscal year beginning January 1, 2018. The FASB has continued to issue accounting standards updates to clarify and provide implementation guidance related to Revenue from Contracts with Customers, including “ASU 2016-08, Revenue from Contract with Customers: Principal versus Agent Considerations”, “ASU 2016-10, Revenue from Contracts with Customers: Identifying Performance Obligations and Licensing”, “ASU 2016-12, Revenue from Contracts with Customers: Narrow-Scope Improvements and Practical Expedients” and “ASU 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers.” Our preliminary assessment of this new standard is that it will generally not change the way in which we recognize product revenue from sales of ADCETRIS. However, we expect that sales-based royalties will be recorded in the period of the related sale based on estimates, rather than as reported by the customer. In addition, the achievement of development milestones under our collaborations will be recorded in the period their achievement becomes probable, which may result in their recognition earlier than under current accounting principles. The new standard also requires more extensive disclosures related to revenue recognition, particularly in quarterly financial statements. We will adopt the standard on January 1, 2018 and intend to use the modified retrospective method of adoption. We are continuing to evaluate the impact of the standard on all of our revenues, including those mentioned above, and our assessments may change in the future based on our ongoing evaluation.
In January 2016, FASB issued an Accounting Standards Update entitled “ASU 2016-01, Financial Instruments: Overall.” The standard addresses certain aspects of recognition, measurement, presentation and disclosure of financial instruments. The standard will become effective for us beginning January 1, 2018. We are currently evaluating the guidance to determine the potential impact on our financial condition, results of operations and cash flows, and financial statement disclosures.
In February 2016, FASB issued an Accounting Standards Update entitled “ASU 2016-02, Leases.” The standard requires entities to recognize in the consolidated balance sheet a liability to make lease payments and a right-of-use asset representing its right to use the underlying asset for the lease term. The standard will become effective for us beginning January 1, 2019, with early adoption permitted. We are currently evaluating the guidance to determine the potential impact on our financial condition, results of operations and cash flows, and financial statement disclosures.
In March 2016, FASB issued an Accounting Standard Update entitled “ASU 2016-09, Compensation – Stock Compensation.” The standard is intended to simplify certain elements of accounting for share-based payment transactions, including the income tax impact, classification of awards as either equity or liabilities, and classification on the statement of cash flows. In addition, the standard allows an entity-wide accounting policy election to either estimate the number of awards that are expected to vest, as currently required, or account for forfeitures when they occur. We have elected to continue estimating the number of awards that are expected to vest. We will adopt the standard as of January 1, 2017. Since we have incurred net losses since our inception and maintain a full valuation allowance on our net deferred tax assets, the adoption is not expected to have a material impact on our financial condition, results of operations and cash flows. Upon implementing the new standard, the amount of deferred tax assets disclosed, prior to any related valuation allowance, will increase due to the change in the manner of accounting for the income tax benefit of the excess tax deduction over financial statement expense for share-based compensation. Currently, we maintain a valuation allowance that fully offsets our deferred tax assets.
In October 2016, FASB issued an Accounting Standard Update entitled “ASU 2016-16, Accounting for Income Taxes: Intra-Entity Asset Transfers of Assets Other than Inventory”. The standard is intended to simplify the accounting for intercompany sales of assets other than inventory. Under current GAAP, the tax effects of intra-entity asset transfers are deferred until the transferred asset is sold to a third party or otherwise recovered
97
Table of Contents
through use. Under the new guidance, a reporting entity would recognized the tax expense from the sale of the asset in the seller’s jurisdiction when the transfer occurs, even though the pre-tax effects of that transaction are eliminated in consolidation. Any deferred tax asset that arises in the buyer’s jurisdiction would also be recognized at the time of the transfer. The standard will become effective for us beginning on January 1, 2018. We are currently evaluating the new standard; however, since we have incurred net losses since our inception and maintain a full valuation allowance on our net deferred tax assets, the adoption is not expected to have a material impact on our financial condition, results of operations and cash flows, or financial statement disclosures.
In June 2016, FASB issued an Accounting Standard Update entitled “ASU 2016-13, Financial Instruments: Credit Losses”. The objective of the standard is to provide information about expected credit losses on financial instruments at each reporting date, and to change how other than temporary impairments on investments securities are recorded. The standard will become effective for us beginning on January 1, 2020 with early adoption permitted. We are currently evaluating the guidance to determine the potential impact on our financial condition, results of operations and cash flows, and financial statement disclosures.
In January 2017, FASB issued an Accounting Standard Update entitled, “ASU 2017-01, Business Combinations: Clarifying the Definition of a Business.” The objective of the standard is to clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions of assets or businesses. We have elected to early adopt this standard on a prospective basis as of January 1, 2017. The adoption of this standard did not have a material impact on our financial condition, results of operations and cash flows, or financial statement disclosures.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio. We do not have any derivative financial instruments in our investment portfolio. We currently have holdings in U.S. Treasury securities. A summary of our investment securities follows (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Short-term investments |
$ | 480,313 | $ | 547,396 | ||||
| Long-term investments |
29,988 | 63,060 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 510,301 | $ | 610,456 | ||||
|
|
|
|
|
|||||
We have estimated the effect on our investment portfolio of a hypothetical increase in interest rates by one percent to be a reduction of $2.4 million in the fair value of our investments as of December 31, 2016. In addition, a hypothetical decrease of 10% in the effective yield of our investments would reduce our expected investment income by less than $0.4 million over the next twelve months based on our investment balance at December 31, 2016.
Foreign Currency Risk
Most of our revenues and expenses are denominated in U.S. dollars and as a result, we have not experienced significant foreign currency transaction gains and losses to date. Our commercial sales in Canada are denominated in Canadian Dollars. We also had other transactions denominated in foreign currencies during the year ended December 31, 2016, primarily related to contract manufacturing and ex-U.S. clinical trial activities, and we expect to continue to do so. Our primary exposure is to fluctuations in the Euro, British Pound, Canadian Dollar and Swiss Franc. We do not anticipate that foreign currency transaction gains or losses will be significant at our current level of operations. However, transaction gains or losses may become significant in the future as we continue to expand our operations internationally. We have not engaged in foreign currency hedging to date; however, we may do so in the future.
98
Table of Contents
Item 8. Financial Statements and Supplementary Data
Index to Financial Statements
| Page | ||||
| 100 | ||||
| 101 | ||||
| 102 | ||||
| 103 | ||||
| 104 | ||||
| 105 | ||||
99
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Seattle Genetics, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of comprehensive loss, stockholders’ equity and cash flows present fairly, in all material respects, the financial position of Seattle Genetics, Inc. and its subsidiaries at December 31, 2016 and 2015 and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO. The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Seattle, Washington
February 21, 2017
100
Table of Contents
Consolidated Balance Sheets
(In thousands, except par value)
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Assets |
||||||||
| Current assets |
||||||||
| Cash and cash equivalents |
$ | 108,673 | $ | 102,255 | ||||
| Short-term investments |
480,313 | 547,396 | ||||||
| Accounts receivable, net |
61,928 | 52,930 | ||||||
| Inventories |
68,124 | 56,963 | ||||||
| Prepaid expenses and other current assets |
15,610 | 11,515 | ||||||
|
|
|
|
|
|||||
| Total current assets |
734,648 | 771,059 | ||||||
| Property and equipment, net |
62,870 | 49,598 | ||||||
| Long-term investments |
29,988 | 63,060 | ||||||
| Other non-current assets |
10,890 | 11,378 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 838,396 | $ | 895,095 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities |
||||||||
| Accounts payable and accrued liabilities |
$ | 120,669 | $ | 88,031 | ||||
| Current portion of deferred revenue |
27,847 | 46,235 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
148,516 | 134,266 | ||||||
|
|
|
|
|
|||||
| Long-term liabilities |
||||||||
| Deferred revenue, less current portion |
53,006 | 71,249 | ||||||
| Deferred rent and other long-term liabilities |
2,787 | 3,669 | ||||||
|
|
|
|
|
|||||
| Total long-term liabilities |
55,793 | 74,918 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity |
||||||||
| Preferred stock, $0.001 par value, 5,000 shares authorized; none issued |
0 | 0 | ||||||
| Common stock, $0.001 par value, 250,000 shares authorized; 142,193 shares issued and outstanding at December 31, 2016 and 139,674 shares issued and outstanding at December 31, 2015 |
142 | 140 | ||||||
| Additional paid-in capital |
1,701,048 | 1,613,383 | ||||||
| Accumulated other comprehensive loss |
(63 | ) | (683 | ) | ||||
| Accumulated deficit |
(1,067,040 | ) | (926,929 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
634,087 | 685,911 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 838,396 | $ | 895,095 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
101
Table of Contents
Consolidated Statements of Comprehensive Loss
(In thousands, except per share amounts)
| Years ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Revenues |
||||||||||||
| Net product sales |
$ | 265,766 | $ | 226,052 | $ | 178,198 | ||||||
| Collaboration and license agreement revenues |
84,926 | 69,770 | 68,556 | |||||||||
| Royalty revenues |
67,455 | 40,980 | 40,004 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
418,147 | 336,802 | 286,758 | |||||||||
|
|
|
|
|
|
|
|||||||
| Costs and expenses |
||||||||||||
| Cost of sales |
28,168 | 24,476 | 17,513 | |||||||||
| Cost of royalty revenues |
14,149 | 12,964 | 11,545 | |||||||||
| Research and development |
379,308 | 294,529 | 230,743 | |||||||||
| Selling, general and administrative |
139,247 | 125,783 | 104,320 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total costs and expenses |
560,872 | 457,752 | 364,121 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(142,725 | ) | (120,950 | ) | (77,363 | ) | ||||||
| Investment and other income, net |
2,614 | 464 | 1,222 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (140,111 | ) | $ | (120,486 | ) | $ | (76,141 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share—basic and diluted |
$ | (1.00 | ) | $ | (0.93 | ) | $ | (0.62 | ) | |||
|
|
|
|
|
|
|
|||||||
| Shares used in computation of net loss per share—basic and diluted |
140,746 | 129,184 | 123,408 | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive income (loss): |
||||||||||||
| Net loss |
$ | (140,111 | ) | $ | (120,486 | ) | $ | (76,141 | ) | |||
| Other comprehensive income (loss): |
||||||||||||
| Foreign currency translation gain (loss) |
4 | (12 | ) | (0 | ) | |||||||
| Unrealized gain (loss) on securities available for sale |
616 | (642 | ) | (18 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total other comprehensive income (loss) |
620 | (654 | ) | (18 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (139,491 | ) | $ | (121,140 | ) | $ | (76,159 | ) | |||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
102
Table of Contents
Consolidated Statements of Stockholders’ Equity
(In thousands)
|
Common stock |
Additional paid-in capital |
Accumulated other comprehensive income (loss) |
Accumulated deficit |
Total stockholders’ equity |
||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Balances at December 31, 2013 |
122,615 | $ | 123 | $ | 960,375 | $ | (11 | ) | $ | (730,302 | ) | $ | 230,185 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
0 | 0 | 0 | 0 | (76,141 | ) | (76,141 | ) | ||||||||||||||||
| Other comprehensive loss |
0 | 0 | 0 | (18 | ) | 0 | (18 | ) | ||||||||||||||||
| Issuance of common stock for employee stock purchase plan |
150 | 0 | 4,939 | 0 | 0 | 4,939 | ||||||||||||||||||
| Stock option exercises |
886 | 1 | 11,249 | 0 | 0 | 11,250 | ||||||||||||||||||
| Restricted stock vested during the period, net |
322 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Share-based compensation |
0 | 0 | 40,619 | 0 | 0 | 40,619 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balances at December 31, 2014 |
123,973 | 124 | 1,017,182 | (29 | ) | (806,443 | ) | 210,834 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
0 | 0 | 0 | 0 | (120,486 | ) | (120,486 | ) | ||||||||||||||||
| Other comprehensive loss |
0 | 0 | 0 | (654 | ) | 0 | (654 | ) | ||||||||||||||||
| Issuance of common stock for employee stock purchase plan |
201 | 0 | 5,317 | 0 | 0 | 5,317 | ||||||||||||||||||
| Stock option exercises |
1,502 | 2 | 22,444 | 0 | 0 | 22,446 | ||||||||||||||||||
| Restricted stock vested during the period, net |
535 | 1 | (1 | ) | 0 | 0 | 0 | |||||||||||||||||
| Issuance of common stock |
13,463 | 13 | 526,605 | 0 | 0 | 526,618 | ||||||||||||||||||
| Share-based compensation |
0 | 0 | 41,836 | 0 | 0 | 41,836 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balances at December 31, 2015 |
139,674 | 140 | 1,613,383 | (683 | ) | (926,929 | ) | 685,911 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
0 | 0 | 0 | 0 | (140,111 | ) | (140,111 | ) | ||||||||||||||||
| Other comprehensive income |
0 | 0 | 0 | 620 | 0 | 620 | ||||||||||||||||||
| Issuance of common stock for employee stock purchase plan |
203 | 0 | 5,686 | 0 | 0 | 5,686 | ||||||||||||||||||
| Stock option exercises |
1,778 | 1 | 29,509 | 0 | 0 | 29,510 | ||||||||||||||||||
| Restricted stock vested during the period, net |
538 | 1 | (1 | ) | 0 | 0 | 0 | |||||||||||||||||
| Share-based compensation |
0 | 0 | 52,471 | 0 | 0 | 52,471 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balances at December 31, 2016 |
142,193 | $ | 142 | $ | 1,701,048 | $ | (63 | ) | $ | (1,067,040 | ) | $ | 634,087 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
103
Table of Contents
Consolidated Statements of Cash Flows
(In thousands)
| Years ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (140,111 | ) | $ | (120,486 | ) | $ | (76,141 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities |
||||||||||||
| Share-based compensation |
52,471 | 41,836 | 40,619 | |||||||||
| Depreciation and amortization |
18,034 | 14,505 | 12,490 | |||||||||
| Amortization of premiums, accretion of discounts and gain (loss) on investments |
4,746 | 2,846 | (150 | ) | ||||||||
| Deferred rent and other long-term liabilities |
(882 | ) | (921 | ) | (734 | ) | ||||||
| Changes in operating assets and liabilities |
||||||||||||
| Accounts receivable, net |
(8,998 | ) | (13,682 | ) | (9,740 | ) | ||||||
| Inventories |
(11,161 | ) | (13,512 | ) | (16,378 | ) | ||||||
| Prepaid expenses and other assets |
(4,378 | ) | (1,952 | ) | (5,232 | ) | ||||||
| Accounts payable and accrued liabilities |
29,939 | 6,539 | 18,448 | |||||||||
| Deferred revenue |
(36,631 | ) | (48,376 | ) | (23,181 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(96,971 | ) | (133,203 | ) | (59,999 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Investing activities |
||||||||||||
| Purchases of securities available for sale |
(603,772 | ) | (754,663 | ) | (451,274 | ) | ||||||
| Proceeds from maturities of securities available for sale |
699,800 | 367,200 | 504,100 | |||||||||
| Proceeds from sales of securities available for sale |
0 | 30,005 | 972 | |||||||||
| Purchases of property and equipment |
(27,835 | ) | (13,392 | ) | (17,176 | ) | ||||||
| Purchase of cost-method investment |
(0 | ) | (5,000 | ) | 0 | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
68,193 | (375,850 | ) | 36,622 | ||||||||
|
|
|
|
|
|
|
|||||||
| Financing activities |
||||||||||||
| Net proceeds from issuance of common stock |
0 | 526,618 | 0 | |||||||||
| Proceeds from exercise of stock options and employee stock purchase plan |
35,196 | 27,763 | 16,188 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
35,196 | 554,381 | 16,188 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
6,418 | 45,328 | (7,189 | ) | ||||||||
| Cash and cash equivalents at beginning of year |
102,255 | 56,927 | 64,116 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of year |
$ | 108,673 | $ | 102,255 | $ | 56,927 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
104
Table of Contents
Notes to Consolidated Financial Statements
1. Organization and Business
Organization
The Company is a biotechnology company focused on the development and commercialization of targeted therapies for the treatment of cancer. The Company’s marketed product ADCETRIS®, or brentuximab vedotin, is now approved for three indications, encompassing several settings for the treatment of relapsed Hodgkin lymphoma and relapsed systemic anaplastic large cell lymphoma, or sALCL, in 66 countries around the world, including the United States, Canada, members of the European Union and Japan. The Company also has multiple clinical and pre-clinical product candidates in development utilizing the Company’s proprietary antibody drug conjugate, or ADC, technology as well as other technologies.
Capital Requirements
To execute the Company’s growth plans, it may need to seek additional funding through public or private financings, including debt or equity financings, and through other means, including collaborations and license agreements. If the Company cannot maintain adequate funds, it may be required to delay, reduce the scope of or eliminate one or more of its development programs. Additional financing may not be available when needed, or if available, the Company may not be able to obtain financing on favorable terms.
2. Summary of Significant Accounting Policies
Basis of presentation
The accompanying consolidated financial statements reflect the accounts of Seattle Genetics, Inc. and its wholly-owned subsidiaries (collectively “Seattle Genetics” or the “Company”). The consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP. All significant intercompany transactions and balances have been eliminated. Management has determined that the Company operates in one segment: the development and sale of pharmaceutical products on its own behalf or in collaboration with others. Substantially all of the Company’s assets and revenues are related to operations in the United States; however, the Company also has subsidiaries in the United Kingdom, Switzerland and Canada.
Use of estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash and cash equivalents
The Company considers all highly liquid investments with maturities of three months or less at the date of acquisition to be cash equivalents.
Non-cash investing activities
The Company had $8.1 million and $5.4 million of accrued capital expenditures as of December 31, 2016 and December 31, 2015, respectively. Accrued capital expenditures have been treated as a non-cash investing activity and, accordingly, have not been included in the statement of cash flows until such amounts have been paid in cash.
Investments
The Company classifies its securities as available-for-sale, which are reported at estimated fair value with unrealized gains and losses included in accumulated other comprehensive loss in stockholders’ equity. Realized
105
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
gains, realized losses and declines in the value of securities judged to be other-than-temporary, are included in investment and other income, net. The cost of investments for purposes of computing realized and unrealized gains and losses is based on the specific identification method. Amortization of premiums and accretion of discounts are included in investment and other income, net. Interest and dividends earned on all securities are included in investment and other income, net. The Company classifies investments maturing within one year of the reporting date, or where management’s intent is to use the investments to fund current operations or to make them available for current operations as short-term investments.
If the estimated fair value of a security is below its carrying value, the Company evaluates whether it is more likely than not that it will sell the security before its anticipated recovery in market value and whether evidence indicating that the cost of the investment is recoverable within a reasonable period of time outweighs evidence to the contrary. The Company also evaluates whether or not it intends to sell the investment. If the impairment is considered to be other-than-temporary, the security is written down to its estimated fair value. In addition, the Company considers whether credit losses exist for any securities. A credit loss exists if the present value of cash flows expected to be collected is less than the amortized cost basis of the security. Other-than-temporary declines in estimated fair value and credit losses are charged against investment and other income, net.
Fair value of financial instruments
The recorded amounts of certain financial instruments, including cash and cash equivalents, interest receivable, accounts receivable, accounts payable and accrued liabilities approximate fair value due to their relatively short maturities. Investments that are classified as available-for-sale are recorded at fair value. The fair value for securities held is determined using quoted market prices, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency.
Inventories
The Company considers regulatory approval of product candidates to be uncertain. Accordingly, it charges manufacturing costs to research and development expense until such time as a product has received regulatory approval for commercial sale. Production costs for the Company’s marketed product, ADCETRIS, are capitalized into inventory. ADCETRIS inventory that is deployed for clinical, research or development use is charged to research and development expense when it is no longer available for commercial sales. Production costs for the Company’s other product candidates continue to be charged to research and development expense.
The Company values its inventories at the lower of cost or market value. Cost is determined on a specific identification basis. Inventory includes the cost of materials, third-party contract manufacturing and overhead associated with the production of ADCETRIS. In the event that the Company identifies excess, obsolete or unsalable inventory, its value is written down to net realizable value.
Property and equipment
Property and equipment are stated at cost and are depreciated using the straight-line method over the estimated useful lives of the assets, which are generally as follows:
| Years | ||||
| Laboratory equipment |
5 | |||
| Furniture and fixtures |
5 | |||
| Computers, software and office equipment |
3 | |||
Leasehold improvements are amortized over the shorter of the remaining term of the applicable lease or the useful life of the asset. Gains and losses from the disposal of property and equipment are reflected in the
106
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
consolidated statement of comprehensive loss at the time of disposition and have not been significant. Expenditures for additions and improvements to the Company’s facilities are capitalized and expenditures for maintenance and repairs are charged to expense as incurred. Concessions received by the Company in connection with leases, including tenant improvement allowances and prorated rent, are included in deferred rent and other long-term liabilities and recognized as a reduction in rent expense over the term of the applicable lease.
Other non-current assets
Included in other non-current assets are intangible assets resulting from milestone payments that became due upon the approval of ADCETRIS related to certain in-licensed technology. Intangible assets are amortized to cost of sales over the estimated life of the related licenses which range from six to ten years.
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Intangible assets |
$ | 5,650 | $ | 5,650 | ||||
| Less: accumulated amortization |
(4,115 | ) | (3,343 | ) | ||||
|
|
|
|
|
|||||
| Total |
$ | 1,535 | $ | 2,307 | ||||
|
|
|
|
|
|||||
Amortization expenses on intangible assets was $0.8 million for each of the years ended December 31, 2016, 2015, and 2014, respectively. Assuming no changes in the cost basis of intangible assets, the estimated aggregate amortization for the next five years will total $1.5 million.
Other non-current assets also include a $5.0 million non-controlling investment in a privately-held company that is accounted for under the cost method of accounting. The Company periodically evaluates the carrying value of the investment if significant adverse events or circumstances indicate an impairment in value.
Impairment of long-lived assets
The Company assesses the impairment of long-lived assets, primarily property and equipment and intangible assets, included in other non-current assets, whenever events or changes in business circumstances indicate that the carrying amounts of the assets may not be fully recoverable. When such events occur, management determines whether there has been an impairment in value by comparing the asset’s carrying value with its fair value, as measured by the anticipated undiscounted net cash flows of the asset. If an impairment in value exists, the asset is written down to its estimated fair value. The Company has not recognized any impairment losses through December 31, 2016 as there have been no events warranting an impairment analysis. The Company’s long-lived assets are primarily located in the United States.
Revenue Recognition
The Company’s revenues are comprised of ADCETRIS net product sales, amounts earned under its collaboration and licensing agreements and royalties. Revenue recognition is predicated upon persuasive evidence of an agreement existing, delivery of products or services being rendered, amounts payable being fixed or determinable, and collectibility being reasonably assured.
Net product sales
The Company sells ADCETRIS through a limited number of pharmaceutical distributors in the U.S. and Canada. Customers order ADCETRIS through these distributors and the Company typically ships product directly to the customer. The Company records product sales when title and risk of loss pass, which generally occurs upon delivery of the product to the customer. Product sales are recorded net of estimated government-
107
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
mandated rebates and chargebacks, distribution fees, estimated product returns and other deductions. Accruals are established for these deductions and actual amounts incurred are offset against applicable accruals. The Company reflects these accruals as either a reduction in the related account receivable from the distributor, or as an accrued liability depending on the nature of the sales deduction. Sales deductions are based on management’s estimates that consider payer mix in target markets and experience to date. These estimates involve a substantial degree of judgment.
Government-mandated rebates and chargebacks: The Company has entered into a Medicaid Drug Rebate Agreement, or MDRA, with the Centers for Medicare & Medicaid Services. This agreement provides for a rebate based on covered purchases of ADCETRIS. Medicaid rebates are invoiced to the Company by the various state Medicaid programs. The Company estimates Medicaid rebates based on a variety of factors, including its experience to date. The Company has also completed a Federal Supply Schedule, or FSS, agreement under which certain U.S. government purchasers receive a discount on eligible purchases of ADCETRIS. The Company has entered into a Pharmaceutical Pricing Agreement with the Secretary of Health and Human Services, which enables certain entities that qualify for government pricing under the Public Health Services Act, or PHS, to receive discounts on their qualified purchases of ADCETRIS. Under these agreements, distributors process a chargeback to the Company for the difference between wholesale acquisition cost and the applicable discounted price. As a result of the Company’s direct-ship distribution model, it can determine the entities purchasing ADCETRIS and this information enables the Company to estimate expected chargebacks for FSS and PHS purchases based on each entity’s eligibility for the FSS and PHS programs. The Company also reviews historical rebate and chargeback information to further refine these estimates.
Distribution fees, product returns and other deductions: The Company’s distributors charge a volume-based fee for distribution services that they perform for the Company. The Company allows for the return of product that is within 30 days of its expiration date or that is damaged. The Company estimates product returns based on its experience to date. In addition, the Company considers its direct-ship distribution model, its belief that product is not typically held in the distribution channel, and the expected rapid use of the product by healthcare providers. The Company provides financial assistance to qualifying patients that are underinsured or cannot cover the cost of commercial coinsurance amounts through SeaGen Secure. SeaGen Secure is available to patients in the U.S. and its territories who meet various financial and treatment need criteria. Estimated contributions for commercial coinsurance under SeaGen Secure are deducted from gross sales and are based on an analysis of expected plan utilization. These estimates are adjusted as necessary to reflect the Company’s actual experience.
The Company has developed a proprietary technology for linking cytotoxic agents to monoclonal antibodies called antibody-drug conjugates, or ADCs. This proprietary technology is the basis of ADC collaborations that the Company has entered into in the ordinary course of its business with a number of biotechnology and pharmaceutical companies. Under these ADC collaboration agreements, the Company grants its collaborators research and commercial licenses to the Company’s technology and provides technology transfer services, technical advice, supplies and services for a period of time.
If there are continuing performance obligations, the Company uses a time-based proportional performance model to recognize revenue over the Company’s performance period for the related agreement. Collaboration and license agreements are evaluated to determine whether the multiple elements and associated deliverables can be considered separate units of accounting. To date, the pre-commercial deliverables under the Company’s collaboration and license agreements have not qualified as separate units of accounting. The assessment of multiple element arrangements requires judgment in order to determine the appropriate point in time, or period of time, that revenue should be recognized. The Company believes that the development period in each agreement
108
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
is a reasonable estimate of the performance obligation period of such agreement. Accordingly, all amounts received or due, including any upfront payments, maintenance fees, development and regulatory milestone payments and reimbursement payments, are recognized as revenue over the performance obligation periods of each agreement. These performance obligation periods typically range from one to three years. The agreements with Takeda Pharmaceutical Company Limited, or Takeda, and Genentech, Inc., a member of the Roche Group, or Genentech, have performance obligation periods of ten and seventeen years, respectively. All of the remaining performance obligation periods for our active collaborations are currently expected to be completed in three years or less. When no performance obligations are required of the Company, or following the completion of the performance obligation period, such amounts are recognized as revenue when collectibility is reasonably assured. Generally, all amounts received or due other than sales-based milestones and royalties are classified as collaboration and license agreement revenues as they are earned. Sales-based milestones and royalties are recognized as royalty revenue as they are reported to the Company.
The Company’s collaboration and license agreements include contractual milestones. Generally, the milestone events contained in the Company’s collaboration and license agreements coincide with the progression of the collaborators’ product candidates from development, to regulatory approval and then to commercialization and fall into the following categories.
Development milestones in the Company’s collaborations may include the following types of events:
| • | Designation of a product candidate or initiation of preclinical studies. The Company’s collaborators must undertake significant preclinical research and studies to make a determination of the suitability of a product candidate and the time from those studies or designation to initiation of a clinical trial may take several years. |
| • | Initiation of a phase 1 clinical trial. Generally, phase 1 clinical trials may take one to two years to complete. |
| • | Initiation of a phase 2 clinical trial. Generally, phase 2 clinical trials may take one to three years to complete. |
| • | Initiation of a phase 3 clinical trial. Generally, phase 3 clinical trials may take two to six years to complete. |
Regulatory milestones in the Company’s collaborations may include the following types of events:
| • | Filing of regulatory applications for marketing approval such as a Biologics License Application in the United States or a Marketing Authorization Application in Europe. Generally, it may take up to twelve months to prepare and submit regulatory filings. |
| • | Receiving marketing approval in a major market, such as in the United States, Europe, Japan or other significant countries. Generally it may take up to three years after a marketing application is submitted to obtain approval for marketing and pricing from the applicable regulatory agency. |
Commercialization milestones in the Company’s collaborations may include the following types of events:
| • | First commercial sale in a particular market, such as in the United States, Europe, Japan or other significant countries. |
| • | Product sales in excess of a pre-specified threshold. The amount of time to achieve this type of milestone depends on several factors, including, but not limited to, the dollar amount of the threshold, the pricing of the product, market penetration of the product and the rate at which customers begin using the product. |
109
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
The Company’s ADC collaborators are solely responsible for the development of their product candidates and the achievement of milestones in any of the categories identified above is based solely on the collaborators’ efforts.
In the case of the Company’s ADCETRIS collaboration with Takeda, the Company may be involved in certain development activities; however, the achievement of milestone events under the agreement is primarily based on activities undertaken by Takeda.
The process of successfully developing a product candidate, obtaining regulatory approval and ultimately commercializing a product candidate is highly uncertain and the attainment of any milestones is therefore uncertain and difficult to predict. In addition, since the Company does not take a substantive role or control the research, development or commercialization of any products generated by its ADC collaborators, the Company is not able to reasonably estimate when, if at all, any milestone payments or royalties may be payable to the Company by its ADC collaborators. As such, the milestone payments associated with its ADC collaborations involve a substantial degree of uncertainty and risk that they may never be received. Similarly, even in those collaborations where the Company may have an active role in the development of the product candidate, such as the Company’s ADCETRIS collaboration with Takeda, the attainment of a milestone is based on the collaborator’s activities and is generally outside the direction and control of the Company.
The Company generally invoices its collaborators and licensees on a monthly or quarterly basis, or upon the completion of the effort or achievement of a milestone, based on the terms of each agreement. Deferred revenue arises from amounts received in advance of the culmination of the earnings process and is recognized as revenue in future periods when the applicable revenue recognition criteria have been met. Deferred revenue expected to be recognized within the next twelve months is classified as a current liability.
Royalty revenues and cost of royalty revenues
Royalty revenues primarily reflect amounts earned under the ADCETRIS collaboration with Takeda. These royalties include sales royalties, which are based on a percentage of Takeda’s net sales at rates that range from the mid-teens to the mid-twenties based on sales volume, and commercial sales-based milestones. Takeda bears a portion of third-party royalty costs owed on its sales of ADCETRIS. This amount is included in royalty revenue in the Company’s consolidated financial statements. Cost of royalty revenues reflects amounts owed to the Company’s third party licensors related to Takeda’s sales of ADCETRIS. These amounts are recognized in the quarter in which Takeda reports its sales activity to the Company, which is the quarter following the related sales. Royalty revenues also include amounts earned in connection with the Company’s ADC collaborations.
Research and development expenses
Research and development, or R&D, expenses consist of salaries, benefits and other headcount related costs of the Company’s R&D staff, preclinical activities, clinical trials and related manufacturing costs, lab supplies, contract and outside service fees and facilities and overhead expenses for research, development and preclinical studies focused on drug discovery, development and testing. Clinical trial expenses are a significant component of research and development expenses, and the Company outsources a significant portion of these costs to third parties. Third party clinical trial expenses include investigator fees, site costs, clinical research organization costs, and costs for central laboratory testing and data management. R&D activities are expensed as incurred. In-licensing fees, milestones, maintenance fees and other costs to acquire technologies that are utilized in R&D for product candidates that have not yet received regulatory approval, and that are not expected to have alternative future use are expensed when incurred. Costs associated with activities performed under co-development collaborations are reflected in R&D expense. Non-refundable advance payments for goods or services that will
110
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
be used or rendered for future R&D activities are capitalized and recognized as expense as the related goods are delivered or the related services are performed. This results in the temporary deferral of recording expense for amounts incurred for research and development activities from the time payments are made until the time goods or services are provided.
Advertising
Advertising costs are expensed as incurred. The Company incurred $12.9 million, $16.4 million, and $10.1 million in advertising expense during 2016, 2015, and 2014, respectively.
Concentration of credit risk
Cash, cash equivalents and investments are invested in accordance with the Company’s investment policy. The policy includes guidelines for the investment of cash reserves and is reviewed periodically to minimize credit risk. Most of the Company’s investments are not federally insured. The Company has accounts receivable from the sale of ADCETRIS from a small number of distributors. The Company does not require collateral on amounts due from its distributors or its collaborators and is therefore subject to credit risk. The Company has not experienced any significant credit losses to date as a result of credit risk concentration and does not consider an allowance for doubtful accounts to be necessary.
Major customers
The Company sells ADCETRIS through a limited number of distributors. Certain of these distributors, together with entities under their common control, each individually accounted for greater than 10% of total revenues and greater than 10% of accounts receivable as noted below. In addition, one of the Company’s collaborators accounted for greater than 10% of total revenues as noted below. Revenues generated outside the United States, as determined by customer location, were less than 10% of total revenues for all years presented.
The following table presents each major distributor or collaborator that comprised more than 10% of total revenue in the periods presented:
| Percent of total revenues for the years ended December 31, |
||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Distributor A |
22 | % | 24 | % | 22 | % | ||||||
| Distributor B |
19 | % | 21 | % | 20 | % | ||||||
| Distributor C |
17 | % | 18 | % | 16 | % | ||||||
| Collaborator A |
27 | % | 17 | % | 25 | % | ||||||
The following table presents each major distributor that accounted for more than 10% of accounts receivable as of the dates presented:
| Percent of total accounts receivable at December 31, |
||||||||
| 2016 | 2015 | |||||||
| Distributor A |
34 | % | 34 | % | ||||
| Distributor B |
26 | % | 29 | % | ||||
| Distributor C |
26 | % | 27 | % | ||||
111
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Major suppliers
The use of a relatively small number of contract manufacturers to supply drug product necessary for the Company’s commercial operations and clinical trials creates a concentration of risk for the Company. While primarily one source of supply is utilized for certain components of ADCETRIS and each of the Company’s product candidates, other sources are available should the Company need to change suppliers. The Company also endeavors to maintain reasonable levels of drug supply for its use. A change in suppliers, however, could cause a delay in delivery of drug product which could result in the interruption of commercial operations or clinical trials. Such an event would adversely affect the Company’s business.
Income taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Deferred tax assets and liabilities are determined based on the differences between the financial statement and tax bases of assets and liabilities using tax rates in effect for the year in which the differences are expected to reverse. The Company has provided a full valuation allowance against its deferred tax assets for all periods presented. A valuation allowance is recorded when it is more likely than not that the net deferred tax asset will not be realized. The Company follows the guidance related to accounting for uncertainty in income taxes, which requires the recognition of an uncertain tax position when it is more likely than not to be sustainable upon audit by the applicable taxing authority.
Share-based compensation
The Company uses the graded-vesting attribution method for recognizing compensation expense for its stock options and the straight-line method for recognizing compensation expense for its restricted stock units (“RSUs”). Compensation expense is recognized on awards ultimately expected to vest and reduced for forfeitures that are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. For performance-based stock options, the Company will record compensation expense over the estimated service period once the achievement of the performance-based milestone is considered probable. At each reporting date, the Company assesses whether achievement of a milestone is considered probable, and if so, records compensation expense based on the portion of the service period elapsed to date with respect to that milestone, with a cumulative catch-up, net of estimated forfeitures. The Company will recognize remaining compensation expense with respect to a milestone, if any, over the remaining estimated service period.
Long-term incentive plans
In May and July of 2016, the Company established two Long-Term Incentive Plans, or the First LTIP and the Second LTIP, respectively. The First LTIP provides eligible employees with the opportunity to receive a performance-based incentive comprised of a cash payment and a stock option. The Second LTIP provides eligible employees with the opportunity to receive a performance-based incentive comprised of a cash payment and a restricted stock unit award. The payment of the cash, commencement of the vesting of the stock options, and grant and commencement of vesting of the restricted stock units under the LTIPs are contingent upon the achievement of pre-determined regulatory milestones. The Company will record compensation expense for the LTIPs over the estimated service period for each milestone once the achievement of the milestone is considered probable in accordance with the provisions of ASC 450, Contingencies. At each reporting date, the Company assesses whether achievement of a milestone is considered probable, and if so, records compensation expense based on the portion of the service period elapsed to date with respect to that milestone, with a cumulative catch-up, net of estimated forfeitures. The Company will recognize remaining compensation expense with respect to a milestone, if any, over the remaining estimated service period. As of December 31, 2016, the estimated value to be delivered in cash and stock options for employees currently eligible under the First LTIP was approximately
112
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
$20.8 million. As of December 31, 2016, the estimated value to be delivered in cash and restricted stock units for employees currently eligible under the Second LTIP was approximately $20.6 million. No compensation expense has been recorded to date under the LTIPs as the conditions for recognizing expense have not yet been met. The total potential value to be delivered under the LTIPs is expected to change in the future for several reasons, including the addition of more eligible employees to the LTIPs.
Comprehensive loss
Comprehensive loss is the change in stockholders’ equity from transactions and other events and circumstances other than those resulting from investments by stockholders and distributions to stockholders. The Company’s comprehensive loss is comprised of net loss, unrealized gains and losses on available-for-sale investments, and foreign currency translation adjustments.
Legal matters
The Company is involved in various legal proceedings in the normal course of its business. Legal fees incurred as a result of the Company’s involvement in legal procedures are expensed as incurred.
The Company is a named defendant in a securities class action complaint filed on January 10, 2017 seeking compensatory damages of an undisclosed amount. The lawsuit alleges material misrepresentations and omissions in public statements regarding the Company’s business, operational and compliance policies, violations by all named defendants of Section 10(b) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and Rule 10b-5 thereunder, as well as violations of Section 20(a) of the Exchange Act. The Company is also named as co-defendant in a lawsuit filed by venBio Select Advisors, LLC, or venBio, in the Delaware Chancery Court on February 13, 2017, against the members of the board of directors of Immunomedics, Inc., or Immunomedics. The lawsuit alleges that the members of the Immunomedics board have breached their fiduciary duties toward their stockholders by hastily licensing IMMU-132 to the Company. The Company is alleged to have aided and abetted the breach of fiduciary duties. Among other things, venBio seeks to enjoin closing of the transactions contemplated by the development and license agreement the Company entered into with Immunomedics with respect to the in-licensing of IMMU-132, or the Immunomedics License. As a result of the pending litigation challenging the transactions contemplated by the Immunomedics License, the Company and Immunomedics have committed to the court not to close the transactions contemplated by the Immunomedics License prior to March 10, 2017.
The Company does not believe it is feasible to predict or determine the outcome or resolution of these lawsuits, or to estimate the amount of, or potential range of, loss with respect to these lawsuits. In addition, the timing of the final resolution of these lawsuits is uncertain. As a result of these lawsuits, the Company will incur litigation expenses and may incur indemnification expenses, and potential resolutions of these lawsuits could include settlements requiring payments by the Company. Those expenses could have a material impact on the Company’s financial position, results of operations, and cash flows.
Arizona State University and related entities filed patent infringement lawsuits in the United States and in Italy against the Company, and in Italy and France against Takeda, the Company’s licensee of rights to ADCETRIS outside the U.S. and Canada, concerning certain patents owned by Arizona State University. In August 2015, the lawsuit in the United States against the Company was dismissed by the U.S. District Court of Arizona. In September 2015, the Company, Takeda and Arizona State University and their related entities entered into an agreement to fully and finally settle all remaining claims and disputes between the parties throughout the world, including proceedings asserting European patent rights owned by Arizona State University.
113
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Certain risks and uncertainties
The Company’s revenues are derived from ADCETRIS sales and royalties and from collaboration and license agreements. ADCETRIS is the Company’s only product available for sale and is subject to regulation by the FDA in the United States and other regulatory agencies outside the United States as well as competition by other pharmaceutical companies. The Company’s collaboration and license agreement revenues are derived from a relatively small number of agreements. Each of these agreements can be terminated by the Company’s collaborators at their discretion. The Company is also subject to risks common to companies in the pharmaceutical industry, including risks and uncertainties related to commercial success and acceptance of ADCETRIS and the Company’s potential future products by patients, physicians and payers, competition from other products, regulatory approvals, regulatory requirements and protection of intellectual property. Also, drug development is a lengthy process characterized by a relatively low rate of success. The Company may commit substantial resources toward developing product candidates that never result in further development, achieve regulatory approvals or achieve commercial success. Likewise, the Company has committed and expects to continue to commit substantial resources towards additional clinical development of ADCETRIS in an effort to continue to expand ADCETRIS’ labeled indications of use, and there can be no assurance that the Company and/or Takeda will obtain and maintain the necessary regulatory approvals to market ADCETRIS for any additional indications.
Guarantees
In the normal course of business, the Company indemnifies its directors, certain employees and other parties, including distributors, collaboration partners, lessors and other parties that perform certain work on behalf of, or for the Company or take licenses to the Company’s technologies. The Company has agreed to hold these parties harmless against losses arising from the Company’s breach of representations or covenants, intellectual property infringement or other claims made against these parties in performance of their work with the Company. These agreements typically limit the time within which the party may seek indemnification by the Company and the amount of the claim. It is not possible to prospectively determine the maximum potential amount of liability under these indemnification agreements. Further, each potential claim would be based on the unique facts and circumstances of the claim and the particular provisions of each agreement.
Net loss per share
Basic and diluted net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding during the period. The Company excluded all RSUs and options to purchase common stock from the calculation of basic and diluted net loss per share as such securities are anti-dilutive for all periods presented.
The following table presents the weighted-average shares that have been excluded from the number of shares used to calculate basic and diluted net loss per share (in thousands):
| Years ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Stock options and RSUs |
12,987 | 11,953 | 11,868 | |||||||||
|
|
|
|
|
|
|
|||||||
Recent accounting pronouncements
In May 2014, the Financial Accounting Standards Board, or FASB, issued an Accounting Standards Update entitled “ASU 2014-09, Revenue from Contracts with Customers.” The standard requires entities to recognize revenue through an evaluation that includes identification of the contract, identification of the performance obligations, determination of the transaction price, allocation of the transaction price to the performance
114
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
obligations, and recognition of revenue as the entity satisfies the performance obligations. In August 2015, FASB issued an Accounting Standards Update entitled “ASU 2015-14, Revenue from Contracts with Customers: Deferral of the Effective Date”, which defers the effective date of ASU 2014-09 to the Company’s fiscal year beginning January 1, 2018. The FASB has continued to issue accounting standards updates to clarify and provide implementation guidance related to Revenue from Contracts with Customers, including “ASU 2016-08, Revenue from Contract with Customers: Principal versus Agent Considerations”, “ASU 2016-10, Revenue from Contracts with Customers: Identifying Performance Obligations and Licensing”, “ASU 2016-12, Revenue from Contracts with Customers: Narrow-Scope Improvements and Practical Expedients” and “ASU 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers.” The Company’s preliminary assessment of this new standard is that it will generally not change the way in which the Company recognizes product revenue from sales of ADCETRIS. However, the Company expects that sales-based royalties will be recorded in the period of the related sale based on estimates, rather than recording them as reported by the customer. In addition, the achievement of development milestones under the Company’s collaborations will be recorded in the period their achievement becomes probable, which may result in their recognition earlier than under current accounting principles. The new standard also requires more extensive disclosures related to revenue recognition, particularly in quarterly financial statements. The Company will adopt the standard on January 1, 2018 and intends to use the modified retrospective method of adoption. The Company is continuing to evaluate the impact of the standard on all of its revenues, including those mentioned above, and its assessments may change in the future based on its ongoing evaluation.
In January 2016, FASB issued an Accounting Standards Update entitled “ASU 2016-01, Financial Instruments: Overall.” The standard addresses certain aspects of recognition, measurement, presentation and disclosure of financial instruments. The standard will become effective for the Company beginning January 1, 2018. The Company is currently evaluating the guidance to determine the potential impact on its financial condition, results of operations and cash flows, and financial statement disclosures.
In February 2016, FASB issued an Accounting Standards Update entitled “ASU 2016-02, Leases.” The standard requires entities to recognize in the consolidated balance sheet a liability to make lease payments and a right-of-use asset representing its right to use the underlying asset for the lease term. The standard will become effective for the Company beginning January 1, 2019, with early adoption permitted. The Company is currently evaluating the guidance to determine the potential impact on its financial condition, results of operations and cash flows, and financial statement disclosures.
In March 2016, FASB issued an Accounting Standard Update entitled “ASU 2016-09, Compensation – Stock Compensation.” The standard is intended to simplify certain elements of accounting for share-based payment transactions, including the income tax impact, classification of awards as either equity or liabilities, and classification on the statement of cash flows. In addition, the standard allows an entity-wide accounting policy election to either estimate the number of awards that are expected to vest, as currently required, or account for forfeitures when they occur. The Company has elected to continue estimating the number of awards that are expected to vest. The Company will adopt the standard as of January 1, 2017. Since the Company has incurred net losses since its inception and maintains a full valuation allowance on its net deferred tax assets, the adoption is not expected to have a material impact on the Company’s financial condition, results of operations and cash flows. Upon implementing the new standard, the amount of deferred tax assets disclosed, prior to any related valuation allowance, will increase due to the change in the manner of accounting for the income tax benefit of the excess tax deduction over financial statement expense for share-based compensation. Currently, the Company maintains a valuation allowance that fully offsets its deferred tax assets.
In October 2016, FASB issued an Accounting Standard Update entitled “ASU 2016-16, Accounting for Income Taxes: Intra-Entity Asset Transfers of Assets Other than Inventory”. The standard is intended to simplify
115
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
the accounting for intercompany sales of assets other than inventory. Under current GAAP, the tax effects of intra-entity asset transfers are deferred until the transferred asset is sold to a third party or otherwise recovered through use. Under the new guidance, a reporting entity would recognized the tax expense from the sale of the asset in the seller’s jurisdiction when the transfer occurs, even though the pre-tax effects of that transaction are eliminated in consolidation. Any deferred tax asset that arises in the buyer’s jurisdiction would also be recognized at the time of the transfer. The standard will become effective for the Company beginning on January 1, 2018. The Company is currently evaluating the new standard; however, since the Company has incurred net losses since its inception and maintains a full valuation allowance on its net deferred tax assets, the adoption is not expected to have a material impact on the Company’s financial condition, results of operations and cash flows, or financial statement disclosures.
In June 2016, FASB issued an Accounting Standard Update entitled “ASU 2016-13, Financial Instruments: Credit Losses”. The objective of the standard is to provide information about expected credit losses on financial instruments at each reporting date, and to change how other than temporary impairments on investments securities are recorded. The standard will become effective for the Company beginning on January 1, 2020 with early adoption permitted. The Company is currently evaluating the guidance to determine the potential impact on its financial condition, results of operations and cash flows, and financial statement disclosures.
In January 2017, FASB issued an Accounting Standard Update entitled, “ASU 2017-01, Business Combinations: Clarifying the Definition of a Business.” The objective of the standard is to clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions of assets or businesses. The Company has elected to early adopt this standard on a prospective basis as of January 1, 2017. The adoption of this standard did not have a material impact on the Company’s financial condition, results of operations and cash flows, or financial statement disclosures.
3. Investments
Investments consisted of available-for-sale securities as follows (in thousands):
| Amortized cost |
Gross unrealized gains |
Gross unrealized losses |
Fair value |
|||||||||||||
| December 31, 2016 |
||||||||||||||||
| U.S. Treasury securities |
$ | 510,356 | $ | 68 | $ | (123 | ) | $ | 510,301 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Contractual Maturities |
||||||||||||||||
| Due in one year or less |
$ | 229,856 | $ | 229,864 | ||||||||||||
| Due in one to two years |
280,500 | 280,437 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Total |
$ | 510,356 | $ | 510,301 | ||||||||||||
|
|
|
|
|
|||||||||||||
| Amortized cost |
Gross unrealized gains |
Gross unrealized losses |
Fair value |
|||||||||||||
| December 31, 2015 |
||||||||||||||||
| U.S. Treasury securities |
$ | 611,128 | $ | 1 | $ | (673 | ) | $ | 610,456 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Contractual Maturities |
||||||||||||||||
| Due in one year or less |
$ | 532,823 | $ | 532,418 | ||||||||||||
| Due in one to two years |
78,305 | 78,038 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Total |
$ | 611,128 | $ | 610,456 | ||||||||||||
|
|
|
|
|
|||||||||||||
116
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Investments are presented in the accompanying consolidated balance sheets as follows (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Short-term investments |
$ | 480,313 | $ | 547,396 | ||||
| Long-term investments |
29,988 | 63,060 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 510,301 | $ | 610,456 | ||||
|
|
|
|
|
|||||
The aggregate estimated fair value of the Company’s investments with unrealized losses was as follows (in thousands):
| Period of continuous unrealized loss | ||||||||||||||||
| 12 Months or less | Greater than 12 months | |||||||||||||||
| Fair value |
Gross unrealized losses |
Fair value |
Gross unrealized losses |
|||||||||||||
| December 31, 2016 |
||||||||||||||||
| U.S. Treasury securities |
$ | 200,327 | $ | (123 | ) | $ | NA | $ | NA | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| December 31, 2015 |
||||||||||||||||
| U.S. Treasury securities |
$ | 605,457 | $ | (673 | ) | $ | NA | $ | NA | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
4. Fair Value
The Company holds short-term and long-term available-for-sale securities that are measured at fair value which is determined on a recurring basis according to a fair value hierarchy that prioritizes the inputs and assumptions used, and the valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are described as follows:
| Level 1: |
Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities. | |
| Level 2: |
Quoted prices in markets that are not active or financial instruments for which all significant inputs are observable, either directly or indirectly. | |
| Level 3: |
Prices or valuations that require inputs that are both significant to the fair value measurement and unobservable. |
The determination of a financial instrument’s level within the fair value hierarchy is based on an assessment of the lowest level of any input that is significant to the fair value measurement. The Company considers observable data to be market data which is readily available, regularly distributed or updated, reliable and verifiable, not proprietary, and provided by independent sources that are actively involved in the relevant market.
117
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Level 1 investments, which include investments that are valued based on quoted market prices in active markets, consisted of U.S. Treasury securities. The Company did not hold any Level 2 or 3 investments as of December 31, 2016 or 2015 and did not transfer any investments in or out of Levels 1, 2 and 3 during the years ended December 31, 2016 or 2015.
The following table presents the Company’s available-for-sale securities by level within the fair value hierarchy (in thousands):
| Fair value measurement using: | ||||||||||||||||
| Quoted prices in active markets for identical assets (Level 1) |
Other observable inputs (Level 2) |
Significant unobservable inputs (Level 3) |
Total | |||||||||||||
| As of December 31, 2016 |
||||||||||||||||
| Short-term investments—U.S. Treasury securities |
$ | 480,313 | $ | 0 | $ | 0 | $ | 480,313 | ||||||||
| Long-term investments—U.S. Treasury securities |
29,988 | 0 | 0 | 29,988 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 510,301 | $ | 0 | $ | 0 | $ | 510,301 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Fair value measurement using: | ||||||||||||||||
| Quoted prices in active markets for identical assets (Level 1) |
Other observable inputs (Level 2) |
Significant unobservable inputs (Level 3) |
Total | |||||||||||||
| As of December 31, 2015 |
||||||||||||||||
| Short-term investments—U.S. Treasury securities |
$ | 547,396 | $ | 0 | $ | 0 | $ | 547,396 | ||||||||
| Long-term investments—U.S. Treasury securities |
63,060 | 0 | 0 | 63,060 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 610,456 | $ | 0 | $ | 0 | $ | 610,456 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
5. Inventories
The following table presents the Company’s inventories of ADCETRIS (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Raw materials |
$ | 62,516 | $ | 50,501 | ||||
| Work in process |
8 | 1,693 | ||||||
| Finished goods |
5,600 | 4,769 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 68,124 | $ | 56,963 | ||||
|
|
|
|
|
|||||
118
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
6. Property and equipment
Property and equipment consisted of the following (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Leasehold improvements |
$ | 77,133 | $ | 59,025 | ||||
| Laboratory equipment |
37,639 | 32,471 | ||||||
| Computers, software and office equipment |
15,076 | 10,700 | ||||||
| Furniture and fixtures |
6,598 | 6,157 | ||||||
|
|
|
|
|
|||||
| 136,446 | 108,353 | |||||||
| Less: accumulated depreciation and amortization |
(73,576 | ) | (58,755 | ) | ||||
|
|
|
|
|
|||||
| Total |
$ | 62,870 | $ | 49,598 | ||||
|
|
|
|
|
|||||
Depreciation and amortization expenses on property and equipment totaled $17.3 million, $13.7 million, and $11.7 million for the years ended December 31, 2016, 2015, and 2014, respectively. Leasehold improvements included $17.8 million and $9.6 million of construction in process at December 31, 2016 and December 31, 2015, respectively, related to facility improvements.
7. Accounts payable and accrued liabilities
Accounts payable and accrued liabilities consisted of the following (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Employee compensation and benefits |
$ | 29,670 | $ | 24,829 | ||||
| Trade accounts payable |
29,005 | 20,786 | ||||||
| Clinical trial and related costs |
21,006 | 17,142 | ||||||
| Contract manufacturing |
22,008 | 12,780 | ||||||
| Third-party royalties and government rebates |
12,351 | 9,678 | ||||||
| Other |
6,629 | 2,816 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 120,669 | $ | 88,031 | ||||
|
|
|
|
|
|||||
8. Income taxes
The Company’s deferred tax assets primarily consist of net operating loss, or NOL, carryforwards, deferred revenue, capitalized research and development expense and tax credit carryforwards. Realization of deferred tax assets is dependent upon a number of factors, including future earnings, the timing and amount of which is uncertain. Accordingly, the deferred tax assets have been fully offset by a valuation allowance. At December 31, 2016, the Company has gross federal NOL carryforwards of $600.8 million expiring from 2021 to 2036 if not utilized, gross state NOL carryforwards of $199.3 million, gross foreign NOL carryforwards of $19.0 million and tax credit carryforwards of $140.4 million expiring from 2021 to 2036.
Utilization of the NOL and tax credit carryforwards may be subject to a substantial annual limitation in the event of a change in ownership as set forth in Section 382 of the Internal Revenue Code of 1986, as amended. The Company has evaluated ownership changes through the year ended December 31, 2014 and believes that it is likely that utilization of its NOLs should not be limited under Section 382 as of December 31, 2014. It is possible that there may be a change in ownership after this date, which would limit the Company’s ability to utilize its NOL. Any limitation may result in the expiration of the NOL and tax credit carryforwards before utilization.
119
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
The Company’s book income (loss) by jurisdiction consisted of the following (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| US |
$ | (66,215 | ) | $ | (95,860 | ) | ||
| Foreign |
(73,896 | ) | (24,626 | ) | ||||
|
|
|
|
|
|||||
| Total |
$ | (140,111 | ) | $ | (120,486 | ) | ||
|
|
|
|
|
|||||
The Company’s net deferred tax assets consisted of the following (in thousands):
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Deferred tax assets |
||||||||
| Net operating loss carryforwards |
$ | 150,465 | $ | 145,595 | ||||
| Foreign net operating loss |
1,702 | 145 | ||||||
| Tax credit carryforwards |
124,396 | 75,270 | ||||||
| Deferred revenue |
30,731 | 42,563 | ||||||
| Share-based compensation |
33,041 | 29,310 | ||||||
| Capitalized research and development |
12,578 | 6,806 | ||||||
| Depreciation and amortization |
8,970 | 6,103 | ||||||
| Other |
19,468 | 16,375 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
381,351 | 322,167 | ||||||
| Less: valuation allowance |
(381,351 | ) | (322,167 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
The tax credit carry forward increase in 2016 is a result of the completion of a tax credit study during the year. Increases in the valuation allowance were $59.2 million in 2016 and $4.5 million in 2015.
A reconciliation of the federal statutory income tax rate to the effective income tax rate is as follows:
| Years ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Statutory federal income tax rate |
(35 | %) | (35 | %) | (35 | %) | ||||||
| Tax credits |
(25 | ) | (13 | ) | (10 | ) | ||||||
| Foreign rate differential |
16 | 5 | — | |||||||||
| State income taxes and other |
4 | 2 | (4 | ) | ||||||||
| Valuation allowance |
40 | 41 | 49 | |||||||||
|
|
|
|
|
|
|
|||||||
| Effective tax rate |
0 | % | 0 | % | 0 | % | ||||||
|
|
|
|
|
|
|
|||||||
The foreign rate differential in the table above reflects the effect of operations in jurisdictions with tax rates that differ from the rate in the United States. This primarily results from the Company’s operations in Switzerland which began in 2015. At December 31, 2016, unremitted earnings of the Company’s foreign subsidiaries, which were insignificant, will be retained indefinitely by the foreign subsidiaries for continuing investment. If foreign earnings were to be repatriated to the United States, the Company could be subject to additional U.S. federal income taxes.
120
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
The financial statement recognition of the benefit for a tax position is dependent upon the benefit being more likely than not to be sustainable upon audit by the applicable taxing authority. If this threshold is met, the tax benefit is then measured and recognized at the largest amount that is greater than 50% likely of being realized upon ultimate settlement. A reconciliation of the beginning and ending amount of unrecognized tax benefits for the years ended December 31, 2016, 2015 and 2014 is as follows (in thousands):
| Years ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Balance as of January 1 |
$ | 0 | $ | 0 | $ | 0 | ||||||
| Increase related to prior year tax positions |
12,631 | 0 | 0 | |||||||||
| Increase related to current year tax positions |
3,392 | 0 | 0 | |||||||||
| Lapses of statute of limitations |
0 | 0 | 0 | |||||||||
|
|
|
|
|
|
|
|||||||
| Balance at December 31 |
$ | 16,023 | $ | 0 | $ | 0 | ||||||
|
|
|
|
|
|
|
|||||||
The Company does not anticipate any significant changes to its unrecognized tax positions or benefits during the next twelve months. Interest and penalties related to the settlement of uncertain tax positions, if any, will be reflected in income tax expense. Tax years 2001to 2016 remain subject to future examination for federal income taxes.
9. Collaboration and license agreements
The Company has entered into various product, collaboration and license agreements with pharmaceutical and biotechnology companies. Revenues recognized under these agreements were as follows (in thousands):
| Years ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Takeda |
$ | 44,384 | $ | 17,234 | $ | 31,787 | ||||||
| AbbVie |
25,676 | 31,055 | 14,851 | |||||||||
| Genentech |
4,324 | 9,110 | 7,791 | |||||||||
| Other |
10,542 | 12,371 | 14,127 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 84,926 | $ | 69,770 | $ | 68,556 | ||||||
|
|
|
|
|
|
|
|||||||
Takeda ADCETRIS Collaboration
The ADCETRIS collaboration provides for the global co-development of ADCETRIS and the commercialization of ADCETRIS by Takeda in its territory. Under this collaboration, the Company has retained commercial rights for ADCETRIS in the United States and its territories and in Canada, and Takeda has commercial rights in the rest of the world. Additionally, the companies equally co-fund the cost of development activities conducted under the collaboration. In Japan, Takeda is solely responsible for development costs. Costs incurred by the Company associated with co-development activities performed under this collaboration are included in research and development expense in the accompanying consolidated statements of comprehensive loss.
The Company recognizes as collaboration revenue the upfront payment, progress-dependent development and regulatory milestone payments, and net development cost reimbursement payments from Takeda over the ten-year development period of the collaboration which began in December 2009. When the performance of development activities under the collaboration results in the Company making a reimbursement payment to Takeda, the effect is to reduce the amount of collaboration revenue recorded by the Company. The Company also
121
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
receives reimbursement for the cost of drug product supplied to Takeda for its use and, in some cases, pays Takeda for drug product they supply to the Company. The earned portion of net collaboration payments is reflected as a component of collaboration revenue.
The Company also receives royalties based on a percentage of Takeda’s net sales of ADCETRIS in its territory ranging from the mid-teens to the mid-twenties based on sales volume and sales-based milestones. Takeda also bears a portion of third-party royalty costs owed on its sales of ADCETRIS which is included as a component of the Company’s royalty revenue. Either party may terminate the collaboration agreement if the other party materially breaches the agreement and such breach remains uncured. Takeda may terminate the collaboration agreement for any reason upon prior written notice to the Company. The collaboration agreement can also be terminated by mutual written consent of the parties. If neither party terminates the collaboration agreement, then the agreement automatically terminates on the expiration of all payment obligations.
Astellas Co-Development Collaboration
The Company has entered into an agreement with Astellas to jointly research, develop and commercialize ADCs for the treatment of certain types of cancer. The agreement encompasses combinations of the Company’s ADC technology with fully-human antibodies developed by Astellas to proprietary cancer targets. Under this collaboration, Astellas conducted research and development aimed at identifying product candidates for multiple designated antigens and is now conducting clinical trials on various ADC product candidates.
The Company and Astellas are co-funding all development and commercialization costs for ASG-22ME and ASG-15ME, and will share in any profits that may come from these product candidates if successfully commercialized on a 50/50 basis. Either party may opt out of co-development and profit-sharing in return for receiving milestones and royalties from the continuing party.
Costs associated with co-development activities performed under this collaboration are included in research and development expense in the accompanying consolidated statements of comprehensive loss. The Astellas collaboration agreement defines a mechanism for calculating the costs of co-development activities and for reimbursing the other party in order to maintain an equal sharing of development costs. Third-party costs are billed at actual cost and internal labor and support costs are billed at a contractual rate. The following table summarizes research and development expenses incurred by the Company and funding provided to Astellas under the collaboration to achieve equal cost sharing.
| Years ended December 31, | ||||||||||||
| 2016 | 2015 | 2014 | ||||||||||
| Research and development expense using contractual rates |
$ | 2,947 | $ | 539 | $ | 275 | ||||||
| Co-development funding paid to Astellas |
12,043 | 5,545 | 3,785 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 14,990 | $ | 6,084 | $ | 4,060 | ||||||
|
|
|
|
|
|
|
|||||||
The agreement also allows Astellas to develop and commercialize another ADC product candidates on its own, subject to paying the Company annual maintenance fees, milestones, royalties and support fees for research and development services and material provided under the agreement. Amounts received for the product candidate being developed solely by Astellas are recognized in revenues as they become due.
Unum Therapeutics Collaboration
In June 2015, the Company entered into a strategic collaboration and license agreement with Unum Therapeutics, Inc., or Unum, to develop and commercialize novel antibody-coupled T-cell receptor, or ACTR,
122
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
therapies for cancer. Under the terms of the agreement, the Company made an upfront payment of $25.0 million and an equity investment of $5.0 million in Unum. The agreement provides for the Company and Unum to initially develop two ACTR products incorporating the Company’s antibodies, and the Company has an option to expand the collaboration to include a third ACTR product upon payment of an additional fee. Unum is conducting preclinical research and clinical development activities through phase 1 clinical trials and the Company is providing funding for these activities. The agreement calls for the Company and Unum to work together to co-develop and jointly fund programs after phase 1 clinical trials unless either company opts out.
The Company and Unum would co-commercialize any successfully developed product candidates and share any profits 50/50 on any co-developed programs in the United States. The Company retains exclusive commercial rights outside of the United States, paying Unum a royalty that is a high single digit to mid-teens percentage of ex-U.S. sales. The potential future licensing and progress-dependent milestone payments to Unum under the collaboration may total up to $615 million across all three ACTR programs, payment of which is triggered by the achievement of development, regulatory and commercial milestones.
Costs associated with co-development activities performed under this collaboration are included in research and development expense in the accompanying consolidated statements of comprehensive loss. The following table summarizes third party research and development expenses incurred by the Company and funding provided to Unum under the collaboration.
| Years ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Co-development funding paid to Unum |
$ | 3,243 | $ | 569 | ||||
| Third party research and development expenses incurred |
2,086 | 0 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 5,329 | $ | 569 | ||||
|
|
|
|
|
|||||
ADC collaboration agreements
The Company has entered into collaborations for its ADC technology with a number of biotechnology and pharmaceutical companies. Under the ADC collaborations, which the Company has entered into in the ordinary course of business, the Company has granted research and commercial licenses to use its technology in conjunction with the collaborator’s technology. The Company also has agreed to conduct limited development activities and to provide other materials, supplies and services to its ADC collaborators during the performance obligation period of the collaboration. The Company receives upfront cash payments, progress- and sales-dependent milestones for the achievement by its collaborators of certain events, annual maintenance fees and support fees for research and development services and materials provided under the agreements. The Company is also entitled to receive royalties on net sales of any resulting products incorporating its ADC technology. The Company’s ADC collaborators are solely responsible for research, product development, manufacturing and commercialization of all products under these collaborations
10. License agreements
The Company has in-licensed antibodies, targets and enabling technologies from pharmaceutical and biotechnology companies and academic institutions for use in ADCETRIS, its pipeline programs and ADC technology, including the following:
Bristol-Myers Squibb. The Company has obtained rights to some of its technologies and product candidates, portions of which are exclusive, through a license agreement with Bristol-Myers Squibb. Through this license, the Company secured rights to monoclonal antibody-based cancer targeting technologies, including
123
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
patents, monoclonal antibodies, chemical linkers, including the linker used in ADCETRIS and other product candidates, a ribosome-inactivating protein and enabling technologies. Under the terms of the license agreement, the Company is required to pay a low single-digit royalty on net sales of products, including ADCETRIS, that incorporate patented technology licensed from Bristol-Myers Squibb.
University of Miami. The Company has entered into an exclusive license agreement with the University of Miami, Florida, covering an anti-CD30 monoclonal antibody that is the basis for the antibody component of ADCETRIS. Under the terms of this license, the Company made an upfront payment and progress-dependent milestone payments. The Company is obligated to pay annual maintenance fees and a low single-digit royalty on net sales of products, including ADCETRIS, incorporating technology licensed from the University of Miami.
Other Licenses. The Company has other non-exclusive licenses to other technology used in ADCETRIS that require the Company to pay a low single-digit royalty on net sales of ADCETRIS.
11. Commitments and contingencies
Commitments. The Company is obligated to make future minimum payments under five operating leases for approximately 355,000 square feet of space used for general office and research and development purposes. The leases expire between 2018 through 2024 and include options to renew at the then fair market rental for the facilities. The lease agreements typically contain scheduled rent increases and provide for tenant improvement allowances. Accordingly, the Company has recorded a deferred rent liability of $2.2 million at December 31, 2016, and $3.2 million at December 31, 2015. This deferred rent liability is amortized over the term of the related lease. Assuming the Company does not exercise any extensions, future minimum lease payments under all noncancelable operating leases are set forth below.
In addition, the Company has certain noncancelable obligations under other agreements, including supply agreements relating to the manufacture of ADCETRIS and the Company’s product candidates which contain annual minimum purchase commitments and other firm commitments when a binding forecast is provided. As of December 31, 2016, the Company’s future obligations related to its supply and other agreements are as follows (in thousands):
| Leases | Other Agreements |
|||||||
| Years ending December 31, |
||||||||
| 2017 |
$ | 6,995 | $ | 82,795 | ||||
| 2018 |
4,706 | 29,644 | ||||||
| 2019 |
2,500 | 26,554 | ||||||
| 2020 |
2,575 | 25,165 | ||||||
| 2021 |
2,653 | 25,988 | ||||||
| Thereafter |
6,760 | 88,270 | ||||||
|
|
|
|
|
|||||
| $ | 26,189 | $ | 278,416 | |||||
|
|
|
|
|
|||||
Rent expense attributable to noncancelable operating leases totaled approximately $5.6 million, $4.1 million, and $3.6 million for the years ended December 31, 2016, 2015, and 2014, respectively.
Noncancelable obligations under other agreements do not include payments that are contingent upon achievement of certain progress-dependent milestones, as well as the payment of royalties based on net sales of commercial products. These amounts have been excluded from the table because the events triggering the obligations have not yet occurred.
124
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
12. Stockholders’ equity
Common stock
In September 2015, the Company completed an underwritten public offering of 13,463,415 shares of its common stock at a public offering price of $41.00 per share. The offering resulted in net proceeds to the Company of approximately $526.6 million, after deducting underwriting discounts and commissions and other offering expenses.
At December 31, 2016, shares of common stock reserved for future issuance are as follows (in thousands):
| Stock options and RSUs outstanding |
13,480 | |||
| Shares available for future grant under the 2007 Equity Incentive Plan |
5,513 | |||
| Employee stock purchase plan shares available for future issuance |
761 | |||
|
|
|
|||
| Total |
19,754 | |||
|
|
|
13. Share-based compensation
2007 Equity Incentive Plan
In 2007, the Company adopted the 2007 Equity Incentive Plan, or the 2007 Plan, that provides for the issuance of the Company’s common stock to employees, including officers, directors and consultants of the Company and its affiliates. The 2007 Plan was amended and restated in May 2016 to reserve an additional 6,000,000 shares thereunder, such that an aggregate of 27,000,000 shares of the Company’s common stock were authorized for issuance under the 2007 Plan at December 31, 2016. Under the 2007 Plan, the Company may issue stock options (including incentive stock options and nonstatutory stock options), restricted stock, RSUs, stock appreciation rights and other similar types of awards (including awards, such as RSUs, that do not require the awardee to pay any amount in connection with receiving the shares or that have an exercise or purchase price that is less than the grant date fair market value of the Company’s stock). No awardee may be granted, in any calendar year under the 2007 Plan, options or stock awards covering more than 1,000,000 shares. The 2007 Plan was also amended and restated in May 2014 to extend its term through May 2024 unless it is terminated earlier pursuant to its terms.
Restricted stock grants are awards of a specific number of shares of the Company’s common stock. RSUs represent a promise to deliver shares of the Company’s common stock, or an amount of cash or property equal to the value of the underlying shares, at a future date. Stock appreciation rights are rights to receive cash and/or shares of the Company’s common stock based on the amount by which the exercise date fair market value of a specific number of shares exceeds the grant date fair market value of the exercised portion of the stock appreciation right. The Company has only issued options to purchase shares of common stock and RSUs under the 2007 Plan.
Incentive stock options under the 2007 Plan may be granted only to employees of the Company or its subsidiaries. The exercise price of an incentive stock option or a nonstatutory stock option may not be less than 100% of the fair market value of the common stock on the date the option is granted and the options have a maximum term of ten years from the date of grant. In the case of options granted to holders of more than 10% of the voting power of the Company, the exercise price may not be less than 110% of the fair market value of the common stock on the date the option is granted and the term of the option may not exceed five years. The Company may grant options with exercise prices lower than the fair market value of its common stock on the date of grant in connection with an acquisition by the Company of another company. Options become exercisable in whole or in part from time to time as determined by the Board of Directors, which administers the 2007 Plan.
125
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Generally, options granted to employees under the 2007 Plan vest 25% one year after the beginning of the vesting period and thereafter ratably each month over the following thirty-six months. RSUs granted to employees vest 100% on the third anniversary of the beginning of the vesting period. Option and RSU grants to independent members of the Company’s board of directors vest over one year. Performance-based options granted under the 2007 Plan pursuant to the First LTIP commence vesting upon the achievement of a regulatory milestone, and vest 25% each year over four years beginning one year after the achievement of the milestone. The Equity Plan provides for (i) the full acceleration of vesting of equity awards, including stock options and RSUs, upon a change in control (as defined in the 2007 Plan) if the successor company does not assume, substitute or otherwise replace the stock awards upon the change in control; and (ii) the full acceleration of vesting of any equity awards, including stock options and RSUs, if at the time of, immediately prior to or within twelve months after a change in control of the Company, the holder of such equity awards is involuntarily terminated without cause or is constructively terminated by the successor company that assumed, substituted or otherwise replaced such stock awards in connection with the change in control.
Each equity award agreement under the 2007 Plan contains provisions regarding (i) the number of shares subject to the equity award, (ii) the purchase or exercise price of the shares, if any, and the means of payment for the shares, (iii) in the case of stock options, the type of option and term of the option; (iv) the performance criteria (including qualifying performance criteria), if any, and level of achievement versus these criteria that will determine the number of shares granted, issued, retainable and vested, as applicable, (v) such terms and conditions on the grant, issuance, vesting and forfeiture of the shares, as applicable, as may be determined from time to time by the plan administrator, (vi) restrictions on the transferability of the equity award or the shares, and (vii) such further terms and conditions, in each case not inconsistent with the 2007 Plan, as may be determined from time to time by the plan administrator; provided, however, that each stock award must have a minimum vesting period of one year from the date of grant.
Share-based compensation
The Company recorded total share based compensation cost of $52.5 million, $41.8 million, and $40.6 million for the years ended December 31, 2016, 2015, and 2014, respectively. No tax benefit was recognized related to share-based compensation cost since the Company has not reported taxable income to date and has established a full valuation allowance to offset all of the potential tax benefits associated with its deferred tax assets. During 2016, 2015, and 2014, $1.0 million, $1.4 million, and $1.4 million of share based compensation costs were included in production overhead used in the determination of inventory cost, respectively.
Valuation assumptions
The Company calculates the fair value of each option award on the date of grant using the Black-Scholes option pricing model. The following weighted-average assumptions were used for the periods indicated:
| 2007 Plan Years ended December 31, |
Employee Stock Purchase Plan Years ended December 31, |
|||||||||||||||||||||||
| 2016 | 2015 | 2014 | 2016 | 2015 | 2014 | |||||||||||||||||||
| Risk-free interest rate |
1.3 | % | 1.5 | % | 1.7 | % | 0.35 | % | 0.1 | % | 0.1 | % | ||||||||||||
| Expected lives in years |
6.5 | 5.5 | 5.5 | 0.5 | 0.5 | 0.5 | ||||||||||||||||||
| Expected dividends |
0 | % | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | ||||||||||||
| Expected volatility |
44 | % | 42 | % | 44 | % | 46 | % | 42 | % | 41 | % | ||||||||||||
The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for the expected life of the award. The Company’s computation of expected life was determined based on its historical experience with similar awards, giving consideration to the contractual terms of the share-based awards, vesting
126
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
schedules and expectations of future employee behavior. A forfeiture rate is estimated at the time of grant to reflect the amount of awards that are granted, but are expected to be forfeited by the award holder prior to vesting. The estimated forfeiture rate applied to these amounts is derived from historical stock award forfeiture behavior. The Company has never paid cash dividends and does not currently intend to pay cash dividends, thus has assumed a 0% dividend yield. The Company’s computation of expected volatility is based on the historical volatility of the Company’s stock price. Determination of all of these assumptions involves management’s best estimates at the time, which impact the fair value of the awards calculated under the Black-Scholes methodology, and ultimately the expense that will be recognized over the life of the award.
Stock option activity
A summary of stock option activity, excluding performance-based stock options, is as follows:
| Shares | Weighted- average exercise price per share |
Weighted-average remaining contractual term (in years) |
Aggregate intrinsic value (in thousands) |
|||||||||||||
| Balances at December 31, 2015 |
10,572,663 | $ | 25.28 | |||||||||||||
|
|
|
|
|
|||||||||||||
| Granted |
1,984,175 | 44.79 | ||||||||||||||
| Exercised |
(1,778,056 | ) | 16.60 | |||||||||||||
| Forfeited/expired |
(182,462 | ) | 40.15 | |||||||||||||
|
|
|
|
|
|||||||||||||
| Balances at December 31, 2016 |
10,596,320 | $ | 30.14 | 6.23 | $ | 240,143 | ||||||||||
|
|
|
|
|
|||||||||||||
| Expected to vest |
10,264,488 | $ | 29.70 | 6.13 | $ | 237,036 | ||||||||||
| Options exercisable |
6,966,828 | $ | 23.46 | 4.82 | $ | 204,236 | ||||||||||
The weighted average grant-date fair values of options granted with exercise prices equal to market were $18.20, $15.84, and $18.24, for the years ended December 31, 2016, 2015, and 2014, respectively.
The aggregate intrinsic value in the table above is calculated as the difference between the exercise price of the underlying options and the quoted price of the Company’s common stock for all options that were in-the-money at December 31, 2016. The aggregate intrinsic value of options exercised was $61.4 million during 2016, $36.2 million during 2015, and $27.7 million during 2014, determined as of the date of option exercise. As of December 31, 2016, there was approximately $33.9 million of total unrecognized compensation cost related to unvested option arrangements, as adjusted for expected forfeitures, granted under the 2007 Plan. That cost is expected to be recognized over a weighted-average period of 1.43 years. The Company utilizes newly issued shares to satisfy option exercises.
A summary of performance-based stock option activity for performance-based options granted under the 2007 Plan pursuant to the First LTIP plan is as follows:
| Shares | Weighted- average exercise price per share |
Weighted-average remaining contractual term (in years) |
Aggregate intrinsic value (in thousands) |
|||||||||||||
| Balances at December 31, 2015 |
0 | $ | 0 | |||||||||||||
|
|
|
|
|
|||||||||||||
| Granted |
834,211 | 34.20 | ||||||||||||||
| Exercised |
(0 | ) | 0 | |||||||||||||
| Forfeited/expired |
(29,810 | ) | 34.20 | |||||||||||||
|
|
|
|
|
|||||||||||||
| Balances at December 31, 2016 |
804,401 | $ | 34.20 | 9.34 | $ | 14,938 | ||||||||||
|
|
|
|
|
|||||||||||||
127
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
The weighted average grant-date fair value of performance-based options granted with an exercise price equal to market was $18.54 for the year ended December 31, 2016. No share based compensation expense has been recorded to date with respect to the First LTIP performance-based options as the conditions for recognizing expense have not yet been met.
RSU activity
The fair value of RSUs is determined based on the closing price of the Company’s common stock on the date of grant.
A summary of RSU activity under the 2007 Plan is as follows:
| Non-vested RSUs |
Share equivalent |
Weighted- average grant date fair value |
||||||
| Non-vested at December 31, 2015 |
1,832,704 | $ | 40.55 | |||||
| Changes during the period: |
||||||||
| Granted |
873,496 | 44.72 | ||||||
| Vested |
(537,545 | ) | 39.46 | |||||
| Forfeited |
(89,716 | ) | 40.53 | |||||
|
|
|
|
|
|||||
| Non-vested at December 31, 2016 |
2,078,939 | $ | 42.58 | |||||
|
|
|
|
|
|||||
The total value of RSUs that vested during 2016 (measured on the date of vesting) was $23.3 million. As of December 31, 2016, there was approximately $45.3 million of total unrecognized compensation cost related to non-vested RSU awards that will be recognized as expense over a weighted-average period of 1.74 years. The Company recognizes compensation cost for RSUs on a straight-line basis over the requisite service period for the entire award, as adjusted for expected forfeitures. The Company will utilize newly issued shares for RSUs that vest.
Employee Stock Purchase Plan
The Company has an Amended and Restated 2000 Employee Stock Purchase Plan, or the Stock Purchase Plan, with a total of 760,809 shares of common stock available for issuance as of December 31, 2016. Activity under the Stock Purchase Plan for the years ended December 31, was as follows:
| Shares Purchased |
Weighted- average purchase price per share |
|||||||
| 2016 |
203,225 | $ | 27.98 | |||||
| 2015 |
201,103 | $ | 26.44 | |||||
| 2014 |
149,576 | $ | 33.02 | |||||
Under the current terms of the Stock Purchase Plan, shares are purchased at the lower of 85 percent of the fair market value of the Company’s common stock on either the first day or the last day of each six month offering period.
14. Employee benefit plan
The Company has a 401(k) Plan for all of its employees. The 401(k) Plan allows eligible employees to defer, at the employee’s discretion, up to 75% of their pretax compensation up to the IRS annual limit. The
128
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Company has a 401(k) matching program whereby the Company may, at its discretion, match a portion of an employee’s contributions, not to exceed a prescribed annual limit. The Company’s matching contribution vests over four years from the start of employment. Under this matching program, the Company contributed a total of approximately $4.7 million in 2016, $2.6 million in 2015, and $2.2 million in 2014.
15. Quarterly Financial Data (unaudited)
The following table contains selected unaudited financial data for each quarter of 2016 and 2015. The unaudited information should be read in conjunction with the Company’s financial statements and related notes included elsewhere in this report. The Company believes that the following unaudited information reflects all normal recurring adjustments necessary for a fair presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
Quarterly Financial Data (in thousands, except per share data):
| Three months ended | ||||||||||||||||
| March 31, | June 30, | September 30, | December 31, | |||||||||||||
| 2016 |
||||||||||||||||
| Total revenues |
$ | 111,155 | $ | 95,402 | $ | 106,315 | $ | 105,275 | ||||||||
| Net loss |
$ | (20,478 | ) | $ | (32,743 | ) | $ | (31,752 | ) | $ | (55,138 | ) | ||||
| Net loss per share—basic and diluted |
$ | (0.15 | ) | $ | (0.23 | ) | $ | (0.23 | ) | $ | (0.39 | ) | ||||
| 2015 |
||||||||||||||||
| Total revenues |
$ | 82,157 | $ | 77,096 | $ | 84,072 | $ | 93,477 | ||||||||
| Net loss |
$ | (21,690 | ) | $ | (47,502 | ) | $ | (26,438 | ) | $ | (24,856 | ) | ||||
| Net loss per share—basic and diluted |
$ | (0.17 | ) | $ | (0.38 | ) | $ | (0.21 | ) | $ | (0.18 | ) | ||||
16. Subsequent events
On January 10, 2017, the Company became a named defendant in a securities class action complaint seeking compensatory damages of an undisclosed amount. The Company does not believe it is feasible to predict or determine the outcome or resolution of this litigation, or to estimate the amount of, or potential range of, loss with respect to this proceeding. In addition, the timing of the final resolution of this proceeding is uncertain. As a result of the lawsuit, the Company will incur litigation expenses and may incur indemnification expenses, and potential resolutions of the lawsuit could include a settlement requiring payments. Those expenses could have a material impact on the Company’s financial position, results of operations, and cash flows.
On February 10, 2017, the Company entered into a development and license agreement with Immunomedics pursuant to which Immunomedics granted, subject to the terms and conditions of the agreement, the Company exclusive worldwide rights to develop, manufacture, and commercialize sacituzumab govitecan, or IMMU-132, for all human therapeutic uses in any and all indications. Sacituzumab govitecan is an antibody-drug conjugate targeted to TROP-2, which is expressed in several solid tumors including cancers of the breast, lung and bladder. Under the agreement, the Company would have primary responsibility for development, regulatory approval and commercialization of IMMU-132. Immunomedics will have the right to exercise a co-promotion option to provide up to 50% of the sales efforts for the commercialization of IMMU-132 in the United States, subject to certain parameters set forth in the agreement.
Under and subject to the agreement, the Company agreed to pay Immunomedics an upfront payment of $250 million following the closing of the agreement. In addition, the Company agreed to pay development, regulatory and sales-dependent milestone payments to Immunomedics across multiple indications and
129
Table of Contents
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
geographic regions of up to a total maximum of approximately $1.7 billion, as well as royalties which are based on a percentage of worldwide annual net sales of licensed products, if any, beginning in the teens and rising to twenty percent based on sales volume. The Company will bear the future costs of worldwide development and commercialization of licensed products. The closing of the agreement is subject to customary closing conditions, including the expiration of the applicable waiting period of the Hart-Scott-Rodino Antitrust Improvements Act of 1976, there being no pending court or administrative challenges to the Immunomedics License and there being no court or administrative orders blocking the closing. On February 20, 2017, the Company and Immunomedics entered into a letter agreement pursuant to which Immunomedics irrevocably waived to the extent applicable to Immunomedics the condition precedent to the closing and effectiveness of the Immunomedics License that there be no pending court or administrative challenges to the transaction. Additionally, under the terms of the Immunomedics License, Immunomedics had the right to continue discussions with a small number of parties that previously expressed interest in licensing IMMU-132 until 11:59 p.m. New York City time on February 19, 2017. If a third party had provided Immunomedics with a financially superior licensing offer, the Company would have had the right to match any such offer, and if the Company had decided not to match, Immunomedics would have had the right to accept the superior offer and terminate the Immunomedics License upon payment of a termination fee to the Company. The Company has not received notice from Immunomedics of any such third party offers during this limited time period, and on February 21, 2017, Immunomedics announced that it is subject to customary “no-shop” restrictions on its and its representatives’ ability to solicit, discuss or negotiate alternative licensing agreement proposals from third parties with regard to IMMU-132.
On February 10, 2017, the Company also entered into an agreement to purchase 3,000,000 shares of common stock of Immunomedics at an aggregate purchase price of $14.7 million, and on February 16, 2017, Immunomedics issued the Company a warrant to purchase up to 8,655,804 additional shares of common stock of Immunomedics at an exercise price of $4.90 until February 10, 2020. The issuances of the purchased common shares and the shares underlying the warrants have not been registered under the Securities Act. The Company entered into a Registration Rights Agreement with Immunomedics under which Immunomedics agreed to file a registration statement to register the common shares and the shares underlying the warrant.
On February 13, 2017, the Company was named as co-defendant in a lawsuit filed by venBio in the Delaware Chancery Court against the members of the board of directors of Immunomedics. The lawsuit alleges that the members of the Immunomedics board have breached their fiduciary duties toward their stockholders by hastily licensing IMMU-132 to the Company. The Company is alleged to have aided and abetted the breach of fiduciary duties. Among other things, venBio seeks to enjoin the closing of the transactions contemplated by the Immunomedics License. As a result of the pending litigation challenging the transactions contemplated by the Immunomedics License, the Company and Immunomedics have committed to the court not to close the transactions contemplated by the Immunomedics License prior to March 10, 2017. The Company does not believe it is feasible to predict or determine the outcome or resolution of this lawsuit, or to estimate the amount of, or potential range of, loss with respect to this lawsuit. In addition, the timing of the final resolution of this lawsuit is uncertain. As a result of the lawsuit, the Company will incur litigation expenses, and potential resolution of the lawsuit could include a settlement requiring payments. Those expenses could have a material impact on the Company’s financial position, results of operations, and cash flows.
130
Table of Contents
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
(a) Evaluation of disclosure controls and procedures. Our Chief Executive Officer and our Chief Financial Officer have evaluated our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended) prior to the filing of this annual report. Based on that evaluation, they have concluded that, as of the end of the period covered by this annual report, our disclosure controls and procedures were, in design and operation, effective.
(b) Changes in internal control over financial reporting. There have not been any changes in our internal control over financial reporting during the quarter ended December 31, 2016 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
(c) Management’s Annual Report on Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Securities Exchange Act of 1934, as amended. Our management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the 2013 framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on its evaluation under the framework in Internal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2016.
The effectiveness of our internal control over financial reporting as of December 31, 2016 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which is included in Item 8 in this Annual Report on Form 10-K.
None.
131
Table of Contents
PART III
The information required by Part III is omitted from this report because we will file a definitive proxy statement within 120 days after the end of our 2016 fiscal year pursuant to Regulation 14A for our 2017 Annual Meeting of Stockholders, or the 2017 Proxy Statement, and the information to be included in the 2017 Proxy Statement is incorporated herein by reference.
Item 10. Directors, Executive Officers and Corporate Governance
(1) The information required by this Item concerning our executive officers and our directors and nominees for director, including information with respect to our audit committee and audit committee financial expert, may be found under the section entitled “Proposal No. 1—Election of Directors” appearing in the 2017 Proxy Statement. Such information is incorporated herein by reference.
(2) The information required by this Item concerning our code of ethics may be found under the section entitled “Proposal No. 1—Election of Directors—Certain Other Corporate Governance Matters—Code of Ethics” appearing in the 2017 Proxy Statement. Such information is incorporated herein by reference.
(3) The information required by this Item concerning compliance with Section 16(a) of the Securities Exchange Act of 1934 may be found in the section entitled “Section 16(a) Beneficial Ownership Reporting Compliance” appearing in the 2017 Proxy Statement. Such information is incorporated herein by reference.
Item 11. Executive Compensation
The information required by this Item may be found under the sections entitled “Proposal No. 1—Election of Directors—Director Compensation” and “Compensation of Executive Officers” appearing in the 2017 Proxy Statement. Such information is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
(1) The information required by this Item with respect to security ownership of certain beneficial owners and management may be found under the section entitled “Security Ownership of Certain Beneficial Owners and Management” appearing in the 2017 Proxy Statement. Such information is incorporated herein by reference.
(2) The information required by this Item with respect to securities authorized for issuance under our equity compensation plans may be found under the sections entitled “Equity Compensation Plan Information” appearing in the 2017 Proxy Statement. Such information is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
(1) The information required by this Item concerning related party transactions may be found under the section entitled “Certain Relationships and Related Party Transactions” appearing in the 2017 Proxy Statement. Such information is incorporated herein by reference.
(2) The information required by this Item concerning director independence may be found under the section entitled “Proposal No. 1—Election of Directors” appearing in the 2017 Proxy Statement. Such information is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services
The information required by this Item may be found under the section entitled “Proposal No. 2—Ratification of Appointment of Independent Registered Public Accounting Firm” appearing in the 2017 Proxy Statement. Such information is incorporated herein by reference.
132
Table of Contents
PART IV
Item 15. Exhibits, Financial Statement Schedules
| (a) | The following documents are filed as part of this report: |
| (1) | Financial Statements and Report of Independent Registered Public Accounting Firm |
| (2) | Financial Statement Schedules |
Financial Statement Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
| (3) | Exhibits are incorporated herein by reference or are filed with this report as indicated below (numbered in accordance with Item 601 of Regulation S-K). |
| (b) | Exhibits |
|
Exhibit |
Incorporation By Reference | |||||||||||
| Exhibit Description |
Form | SEC File No. | Exhibit | Filing Date | ||||||||
| 3.1 | Fourth Amended and Restated Certificate of Incorporation of Seattle Genetics, Inc. |
10-Q | 000-32405 | 3.1 | 11/7/2008 | |||||||
| 3.2 | Certificate of Amendment of Fourth Amended and Restated Certificate of Incorporation of Seattle Genetics, Inc. |
8-K | 000-32405 | 3.3 | 5/26/2011 | |||||||
| 3.3 | Amended and Restated Bylaws of Seattle Genetics, Inc. |
8-K | 000-32405 | 3.1 | 11/25/2015 | |||||||
| 4.1 | Specimen Stock Certificate. |
S-1/A | 333-50266 | 4.1 | 2/8/2001 | |||||||
| 4.2 | Investor Rights Agreement dated July 8, 2003 among Seattle Genetics, Inc. and certain of its stockholders. |
10-Q | 000-32405 | 4.3 | 11/7/2008 | |||||||
| 4.3 | Registration Rights Agreement, dated September 10, 2015, by and between Seattle Genetics, Inc. and the persons listed on Schedule A attached thereto. |
8-K | 000-32405 | 10.1 | 9/11/2015 | |||||||
| 10.1 | License Agreement dated March 30, 1998 between Seattle Genetics, Inc. and Bristol-Myers Squibb Company. |
10-K/A | 000-32405 | 10.1 | 11/26/2010 | |||||||
| 10.2 | Amendment Letter to the Bristol-Myers Squibb Company License Agreement dated July 29, 1999 between Seattle Genetics, Inc. and Bristol-Myers Squibb Company. |
10-K/A | 000-32405 | 10.2 | 11/26/2010 | |||||||
| 10.3 | Amendment Agreement to the Bristol-Myers Squibb Company License Agreement dated July 26, 2000 between Seattle Genetics, Inc. and Bristol-Myers Squibb Company. |
S-1/A | 333-50266 | 10.7 | 12/5/2000 | |||||||
| 10.4† | Amendment to License Agreement to the Bristol-Myers Squibb Company License Agreement dated December 18, 2015 between Seattle Genetics, Inc. and Bristol-Myers Squibb Company. |
10-K | 000-32405 | 10.4 | 2/19/2016 | |||||||
| 10.5 | License Agreement dated September 20, 1999 between Seattle Genetics, Inc. and the University of Miami. |
10-K/A | 000-32405 | 10.6 | 11/26/2010 | |||||||
133
Table of Contents
| 10.6 | Amendment No. 1 to the University of Miami License Agreement dated August 4, 2000 between Seattle Genetics, Inc. and the University of Miami. |
10-K/A | 000-32405 | 10.7 | 11/26/2010 | |||||||
| 10.7† | Letter Agreement Regarding Royalty between the University of Miami and Seattle Genetics, Inc. dated April 11, 2016 |
10-Q | 000-32405 | 10.1 | 7/26/2016 | |||||||
| 10.8 | Lease Agreement dated December 1, 2000 between Seattle Genetics, Inc. and WCM132-302, LLC. |
S-1/A | 333-50266 | 10.21 | 1/4/2001 | |||||||
| 10.9 | First Amendment to Lease dated May 28, 2003 between Seattle Genetics, Inc. and B&N 141-302, LLC. |
10-Q | 333-50266 | 10.1 | 8/12/2003 | |||||||
| 10.10† | Second Amendment to Lease dated July 1, 2008 between Seattle Genetics, Inc. and B&N 141-302, LLC. |
10-Q | 000-32405 | 10.1 | 11/7/2008 | |||||||
| 10.11† | Third Amendment to Lease dated May 9, 2011 between Seattle Genetics, Inc. and B&N 141-302, LLC. |
10-Q | 000-32405 | 10.2 | 8/5/2011 | |||||||
| 10.12† | Office Lease dated May 9, 2011 between Seattle Genetics, Inc. and WCM Highlands II, LLC. |
10-Q | 000-32405 | 10.1 | 8/5/2011 | |||||||
| 10.13† | Collaboration and License Agreement dated January 7, 2007 between Seattle Genetics, Inc. and Agensys, Inc. |
10-Q | 000-32405 | 10.1 | 5/8/2007 | |||||||
| 10.14† | Amendment to the Collaboration and License Agreement between Seattle Genetics, Inc. and Agensys, Inc. dated effective November 20, 2009. |
10-K | 000-32405 | 10.49 | 3/12/2010 | |||||||
| 10.15† | Collaboration Agreement between Seattle Genetics, Inc. and Millennium Pharmaceuticals, Inc. (a wholly-owned subsidiary of Takeda Pharmaceutical Company Limited) dated December 14, 2009. |
10-K | 000-32405 | 10.50 | 3/12/2010 | |||||||
| 10.16† | Commercial Supply Agreement dated December 1, 2010 between Seattle Genetics, Inc. and SAFC, an operating division of Sigma-Aldrich, Inc. |
10-Q | 000-32405 | 10.1 | 11/4/2011 | |||||||
| 10.17+† | First Amendment to Commercial Supply Agreement effective as of January 20, 2014 between Seattle Genetics, Inc. and SAFC, an operating division of Sigma-Aldrich, Inc. |
— | — | — | — | |||||||
| 10.18+† | Second Amendment to Commercial Supply Agreement effective as of December 2, 2016 between Seattle Genetics, Inc. and SAFC, an operating division of Sigma-Aldrich, Inc. |
— | — | — | — | |||||||
| 10.19 | Development and Supply Agreement dated February 23, 2004 between Seattle Genetics, Inc. and Abbott Laboratories. |
10-K | 000-32405 | 10.15 | 2/27/2015 | |||||||
| 10.20† | First Amendment to Development and Supply Agreement dated April 17, 2008 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. |
10-Q | 000-32405 | 10.1 | 8/8/2008 | |||||||
134
Table of Contents
| 10.21† | Second Amendment to Development and Supply Agreement dated June 15, 2009 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. |
10-Q | 000-32405 | 10.4 | 11/4/2011 | |||||||
| 10.22† | Third Amendment to Development and Supply Agreement dated November 5, 2009 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. |
10-Q | 000-32405 | 10.5 | 11/4/2011 | |||||||
| 10.23† | Fourth Amendment to Development and Supply Agreement dated April 18, 2010 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. |
10-Q | 000-32405 | 10.6 | 11/4/2011 | |||||||
| 10.24† | Fifth Amendment to Development and Supply Agreement dated August 24, 2010 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. |
10-Q | 000-32405 | 10.7 | 11/4/2011 | |||||||
| 10.25† | Sixth Amendment to Development and Supply Agreement dated November 18, 2010 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. |
10-Q | 000-32405 | 10.8 | 11/4/2011 | |||||||
| 10.26† | Seventh Amendment to Development and Supply Agreement dated January 2, 2013 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. |
10-K | 000-32405 | 10.42 | 2/27/2013 | |||||||
| 10.27† | Eighth Amendment to Development and Supply Agreement dated July 7, 2015 between Seattle Genetics, Inc. and AbbVie Inc. (formerly part of Abbott Laboratories, Inc.). |
10-Q | 000-32405 | 10.2 | 7/30/2015 | |||||||
| 10.28† | Ninth Amendment to Development and Supply Agreement, effective as of August 23, 2016 between Seattle Genetics, Inc. and AbbVie Inc. (formerly part of Abbott Laboratories, Inc.). |
10-Q | 000-32405 | 10.1 | 10/27/2016 | |||||||
| 10.29+† | Tenth Amendment to Development and Supply Agreement, effective as of December 26, 2016 between Seattle Genetics, Inc. and AbbVie, Inc. (formerly part of Abbott Laboratories, Inc.). |
— | — | — | — | |||||||
| 10.30* | Form of Indemnification Agreement between Seattle Genetics, Inc. and each of its officers and directors. |
S-1/A | 333-50266 | 10.29 | 1/4/2001 | |||||||
| 10.31* | Amended and Restated 1998 Stock Option Plan, effective as of August 5, 2009. |
10-Q | 000-32405 | 10.1 | 8/10/2009 | |||||||
| 10.32* | Form Notice of Grant and Stock Option Agreement under Seattle Genetics, Inc. Amended and Restated 1998 Stock Option Plan. |
10-K | 000-32405 | 10.11 | 3/15/2005 | |||||||
| 10.33* | 2000 Directors’ Stock Option Plan, as amended February 5, 2010. |
10-K | 000-32405 | 10.13 | 3/12/2010 | |||||||
| 10.34* | Form Notice of Grant and Stock Option Agreement under Seattle Genetics, Inc. 2000 Directors’ Stock Option Plan. |
10-K | 000-32405 | 10.12 | 3/15/2005 | |||||||
| 10.35+* | Amended and Restated 2000 Employee Stock Purchase Plan, effective May 20, 2011. |
— | — | — | — | |||||||
135
Table of Contents
| 10.36* | Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan, effective as of May 18, 2012. |
10-Q | 000-32405 | 10.1 | 8/8/2012 | |||||||
| 10.37* | Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan, effective as of May 16, 2014. |
10-Q | 000-32405 | 10.1 | 8/8/2014 | |||||||
| 10.38* | Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan, effective as of May 20, 2016. |
10-Q | 000-32405 | 10.4 | 7/26/2016 | |||||||
| 10.39* | Form Stock Option Agreement for employees under Seattle Genetics, Inc. 2007 Equity Incentive Plan. |
10-K | 000-32405 | 10.44 | 3/13/2009 | |||||||
| 10.40* | Form of Stock Unit Grant Notice and Stock Unit Agreement for employees under Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan. |
8-K | 000-32405 | 10.1 | 8/30/2011 | |||||||
| 10.41* | Form of Notice of Stock Option Grant and Stock Option Agreement for non-employee directors under the Amended and Restated 2007 Equity Incentive Plan. |
10-Q | 000-32405 | 10.4 | 8/5/2011 | |||||||
| 10.42* | Form of Stock Unit Grant Notice and Stock Unit Agreement for non-employee directors under the Amended and Restated 2007 Equity Incentive Plan. |
10-K | 000-32405 | 10.33 | 2/28/2014 | |||||||
| 10.43* | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Clay B. Siegall. |
10-Q | 000-32405 | 10.4 | 10/27/2016 | |||||||
| 10.44* | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Todd E. Simpson. |
10-Q | 000-32405 | 10.5 | 10/27/2016 | |||||||
| 10.45* | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Eric L. Dobmeier. |
10-Q | 000-32405 | 10.6 | 10/27/2016 | |||||||
| 10.46* | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Jonathan Drachman. |
10-Q | 000-32405 | 10.7 | 10/27/2016 | |||||||
| 10.47* | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Vaughn Himes. |
10-Q | 000-32405 | 10.8 | 10/27/2016 | |||||||
| 10.48* | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Jean Liu. |
10-Q | 000-32405 | 10.9 | 10/27/2016 | |||||||
| 10.49* | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Darren Cline. |
10-Q | 000-32405 | 10.10 | 10/27/2016 | |||||||
| 10.50* | Seattle Genetics, Inc. 2016 Senior Executive Annual Bonus Plan. |
8-K | 000-32405 | 10.1 | 1/29/2016 | |||||||
136
Table of Contents
| 10.51* | Seattle Genetics, Inc. 2017 Senior Executive Annual Bonus Plan |
8-K | 000-32405 | 10.1 | 2/1/2017 | |||||||
| 10.52* | Seattle Genetics, Inc. Long Term Incentive Plan for ECHELON-1. |
10-Q | 000-32405 | 10.2 | 7/26/2016 | |||||||
| 10.53* | Form of Stock Option Agreement for Long Term Incentive Plan for ECHELON-1 under the Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan. |
10-Q | 000-32405 | 10.3 | 7/26/2016 | |||||||
| 10.54* | Seattle Genetics, Inc. Long Term Incentive Plan for SGN-CD33A. |
10-Q | 000-32405 | 10.2 | 10/27/2016 | |||||||
| 10.55* | Form of Stock Option Agreement for Long Term Incentive Plan for SGN-CD33A under the Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan. |
10-Q | 000-32405 | 10.3 | 10/27/2016 | |||||||
| 10.56+* | Compensation Information for Executive Officers and Directors. |
— | — | — | ||||||||
| 21.1+ | Subsidiaries of Seattle Genetics, Inc. |
— | — | — | ||||||||
| 23.1+ | Consent of Independent Registered Public Accounting Firm. |
— | — | — | ||||||||
| 31.1+ | Certification of Chief Executive Officer pursuant to Rule 13a-14(a). |
— | — | — | ||||||||
| 31.2+ | Certification of Chief Financial Officer pursuant to Rule 13a-14(a). |
— | — | — | ||||||||
| 32.1+ | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350. |
— | — | — | ||||||||
| 32.2+ | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350. |
— | — | — | ||||||||
| 101.INS+ | XBRL Instance Document |
— | — | — | ||||||||
| 101.SCH+ | XBRL Taxonomy Extension Schema Document. |
— | — | — | ||||||||
| 101.CAL+ | XBRL Taxonomy Extension Calculation Linkbase Document |
— | — | — | ||||||||
| 101.DEF+ | XBRL Taxonomy Extension Definition Linkbase Document |
— | — | — | ||||||||
| 101.LAB+ | XBRL Taxonomy Extension Labels Linkbase Document |
— | — | — | ||||||||
| 101.PRE+ | XBRL Taxonomy Extension Presentation Linkbase Document |
— | — | — | ||||||||
| + | Filed herewith. |
| † | Pursuant to a request for confidential treatment, portions of this Exhibit have been redacted from the publicly filed document and have been furnished separately to the Securities and Exchange Commission as required by Rule 24b-2 under the Securities Exchange Act of 1934. |
| * | Indicates a management contract or compensatory plan or arrangement. |
Not applicable.
137
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: February 21, 2017 | SEATTLE GENETICS, INC. | |||
| By: | /S/ CLAY B. SIEGALL | |||
| Clay B. Siegall President & Chief Executive Officer (Principal Executive Officer) | ||||
| Date: February 21, 2017 | By: | /S/ TODD E. SIMPSON | ||
| Todd E. Simpson Chief Financial Officer (Principal Financial and Accounting Officer) | ||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
|
/S/ CLAY B. SIEGALL Clay B. Siegall |
Director, President & CEO (Principal Executive Officer) | February 21, 2017 | ||
|
/S/ TODD E. SIMPSON Todd E. Simpson |
Chief Financial Officer (Principal Financial and Accounting Officer) | February 21, 2017 | ||
|
/S/ SRINIVAS AKKARAJU Srinivas Akkaraju |
Director | February 21, 2017 | ||
|
/S/ FELIX BAKER Felix Baker |
Director | February 21, 2017 | ||
|
/S/ DAVID W. GRYSKA David W. Gryska |
Director | February 21, 2017 | ||
|
/S/ MARC E. LIPPMAN Marc E. Lippman |
Director | February 21, 2017 | ||
|
/S/ JOHN A. ORWIN John A. Orwin |
Director | February 21, 2017 | ||
|
/S/ NANCY A. SIMONIAN Nancy A. Simonian |
Director | February 21, 2017 | ||
|
/S/ DANIEL G. WELCH Daniel G. Welch |
Director | February 21, 2017 | ||
138
