Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Medtronic plc | fy17q3earningsrelease.htm |
| EX-99.1 - EXHIBIT 99.1 - Medtronic plc | exhibit991-fy17q3earningsr.htm |

MEDTRONIC PLC
Q3 FY17
EARNINGS PRESENTATION
FEBRUARY 21, 2017
Exhibit 99.2
• CONSOLIDATED RESULTS & GROUP
REVENUE HIGHLIGHTS
• EPS GUIDANCE, REVENUE OUTLOOK, &
OTHER ASSUMPTIONS

Q3 FY17 Earnings Results | February 21, 2017 | 2
FORWARD LOOKING STATEMENTS
This presentation contains forward-looking statements which provide current expectations or forecasts, including those
relating to market and sales growth, growth strategies, changes to the healthcare system, financial results, use of capital,
balance sheet changes, the creation of shareholder value and shareholder returns, product and service development,
introduction, and adoption, partnerships, regulatory matters, restructuring initiatives, mergers/acquisitions/divestitures and
related effects, accounting estimates, working capital adequacy, currency exchange rates, competitive strengths and sales
efforts. They are based on current assumptions and expectations that involve uncertainties or risks. These uncertainties and
risks include, but are not limited to, those described in the filings we make with the U.S. Securities and Exchange Commission
(SEC). Actual results may differ materially from anticipated results. Forward-looking statements are made as of today's date,
and we undertake no duty to update them or any of the information contained in this presentation.
Financial Data
Certain information in this presentation includes calculations or figures that have been prepared internally and have not been
reviewed or audited by our independent registered public accounting firm. Use of different methods for preparing, calculating
or presenting information may lead to differences and such differences may be material. This presentation contains financial
measures and guidance, including free cash flow figures (defined as operating cash flows less property, plant and equipment
additions), revenue, margin and growth rates on a constant currency basis, and adjusted EPS, all of which are considered “non-
GAAP” financial measures under applicable SEC rules and regulations. We believe these non-GAAP measures provide a useful
way to evaluate our underlying performance. Medtronic calculates forward-looking non-GAAP financial measures based on
internal forecasts that omit certain amounts that would be included in GAAP financial measures. For instance, forward-looking
revenue growth and EPS projections exclude the impact of foreign currency exchange fluctuations. Forward-looking non-GAAP
EPS guidance also excludes other potential charges or gains that would be recorded as non-GAAP adjustments to earnings
during the fiscal year, such as amortization of intangible assets and acquisition-related, certain tax and litigation, and
restructuring charges or gains. Medtronic does not attempt to provide reconciliations of forward-looking non-GAAP EPS
guidance to projected GAAP EPS guidance because the combined impact and timing of recognition of these potential charges
or gains is inherently uncertain and difficult to predict, and is unavailable without unreasonable efforts. In addition, we believe
such reconciliations would imply a degree of precision and certainty that could be confusing to investors. Such items could have
a substantial impact on GAAP measures of financial performance. Detail concerning how all non-GAAP measures are
calculated, including all GAAP to non-GAAP reconciliations, are provided on our website and can be accessed using this link.

CONSOLIDATED
RESULTS & GROUP
REVENUE HIGHLIGHTS

Q3 FY17 Earnings Results | February 21, 2017 | 4
Balanced growth across groups and geographies
• CVG, MITG, and RTG all MSD growth1; Diabetes HSD growth1
• New products driving growth including Evolut® R 34mm, LigaSure™ instruments,
MiniMed ® 6 series
• Continued improvement in Spine: Best growth in over 7 years
• US MSD growth1; Non-US Developed HSD growth1; Emerging Markets DD growth1
• Western Europe and Japan HSD growth1
• China, Latin America, and Eastern Europe all grew mid-teens or higher1
• Growth Vector Performance:
• New Therapies: above our 200 to 350 bps goal, contributing ~390 bps
• Emerging Markets: in line with our 150 to 200 bps goal, contributing ~150 bps
• Services & Solutions: below our 40 to 60 bps goal, contributing ~20 bps
• MSD Organic Growth2: 4.1%
• Acquisitions & divestitures contributed a net 150 bps to Q3 revenue growth
Meaningful improvement in operating margin; Double-digit EPS1,2 growth
• EPS: 10%1,2 growth; EPS leverage ~480 bps1
• Operating Margin: ~130 bps improvement Y/Y1; ~170bps improvement Y/Y1 on
organic basis2; Operating leverage ~470 bps1
• Covidien synergies: on track for a minimum of $850M in cost savings by FY18
• Delivered $355M in FY16; on track to deliver $225-250M in FY17
Outlook: Continue to expect MSD revenue2 and double-digit EPS2 growth
for the full fiscal year
• Q4 Revenue2: Lower half of MSD range, following strong growth in prior year
• Reiterate FY17 Free Cash Flow3 outlook of $5B - $6B
Capital allocation: Strategically deploying capital against priorities
• Q3: 74% Payout Ratio4; $590M in dividends and $566M in net share repurchases
MDT
Q3 FY17 HIGHLIGHTS
1 Figures represent comparison to Q3 FY16 on a constant currency basis (non-GAAP).
2 Non-GAAP measure
3 Operating cash flows less property, plant and equipment additions (non-GAAP)
4 Dividends plus net share repurchases divided by adjusted net income (non-GAAP)
SOLID QUARTER: IMPROVED RESULTS
ACROSS ALL GROUPS AND GEOGRAPHIES
Revenue:
Other Financial Highlights:
U.S.
56%
Non-
U.S.
Dev
30%
EM
14%
1
Diluted
EPS
Y/Y
CC1
Y/Y%
GAAP $0.59 (23%) NC
Non-GAAP $1.12 6% 10%
Cash Flow
from Ops $2.1B
Free Cash
Flow4 $1.8B
CVG
35%
MITG
33%
RTG
25%
DIAB
7%
Revenue
$M
As Rep
Y/Y %
CC1
Y/Y %
CVG 2,548 5 6
MITG 2,417 5 6
RTG 1,817 4 4
Diabetes 501 6 7
Total $7,283 5% 6%
U.S. 4,106 4 4
Non-U.S. Dev 2,193 6 7
EM 984 9 11
Total $7,283 5% 6%

Q3 FY17 Earnings Results | February 21, 2017 | 5
MDT
Q3 FY17 GAAP SELECT FINANCIAL INFORMATION
Q3
FY17
Q3
FY16
Y/Y Growth /
Y/Y Change
Net Sales ($M) 7,283 6,934 5%
Cost of Products Sold 2,268 2,141 6%
Gross Margin 68.9% 69.1% (20) bps
SG&A ($M) 2,388 2,317 3%
% of Sales 32.8% 33.4% 60 bps
R&D ($M) 530 546 (3%)
% of Sales 7.3% 7.9% 60 bps
Other Expense, Net ($M) 46 9 411%
Operating Profit 1,147 1,355 (15%)
Operating Margin 15.7% 19.5% (380) bps
Diluted EPS ($) 0.59 0.77 (23%)
Q3 FY17 Earnings Results | February 21, 2017 | 6
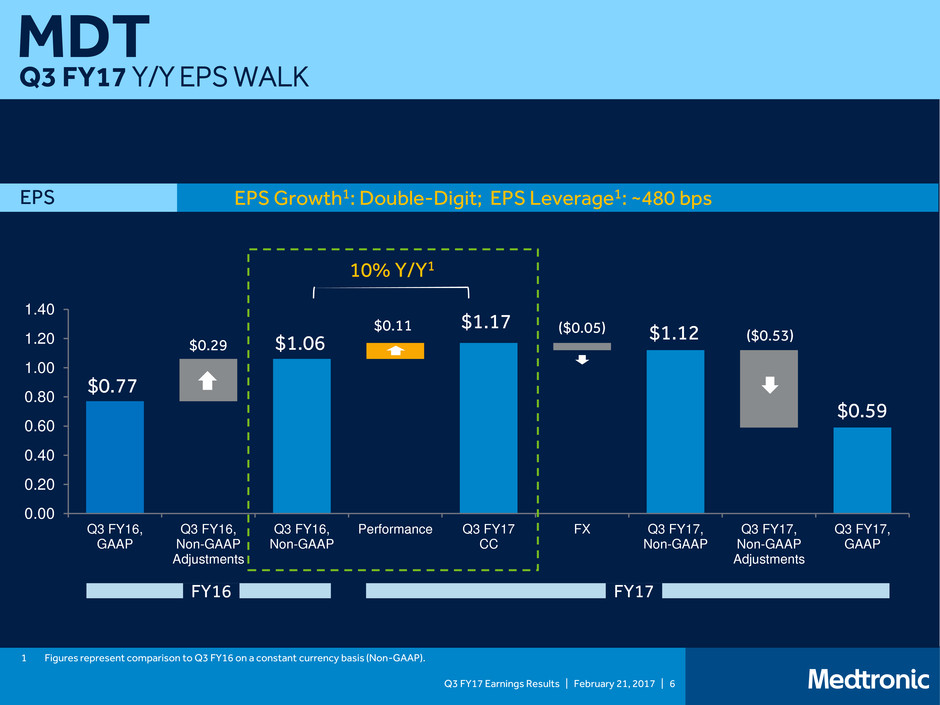
MDT
Q3 FY17 Y/Y EPS WALK
0.00
0.20
0.40
0.60
0.80
1.00
1.20
1.40
Q3 FY16,
GAAP
Q3 FY16,
Non-GAAP
Adjustments
Q3 FY16,
Non-GAAP
Performance Q3 FY17
CC
FX Q3 FY17,
Non-GAAP
Q3 FY17,
Non-GAAP
Adjustments
Q3 FY17,
GAAP
EPS Growth1: Double-Digit; EPS Leverage1: ~480 bps EPS
$0.77
$0.29 $1.06
1 Figures represent comparison to Q3 FY16 on a constant currency basis (Non-GAAP).
$1.17 ($0.05) $1.12 ($0.53)
$0.59
10% Y/Y1
$0.11
FY16 FY17

Q3 FY17 Earnings Results | February 21, 2017 | 7
MDT
Q3 FY17 Y/Y OPERATING MARGIN CHANGES
0.0%
5.0%
10.0%
15.0%
20.0%
25.0%
30.0%
35.0%
Q3 FY16,
GAAP
Q3 FY16,
Non-GAAP
Adjustments
Q3 FY16,
Non-GAAP
Performance Q3 FY17
CC
FX Q3 FY17,
Non-GAAP
Q3 FY17,
Non-GAAP
Adjustments
Q3 FY17,
GAAP
~130 bps Operational Improvement1 Operating Margin
19.5%
8.3% 27.8%
1.3% 29.1% (0.9%) 28.2% (12.5%)
15.7%
FY16 FY17
1 Figures represent comparison to Q3 FY16 on a constant currency basis (Non-GAAP).

Q3 FY17 Earnings Results | February 21, 2017 | 8
MDT
Q3 FY17 NON-GAAP SELECT FINANCIAL INFORMATION
Q3
FY17
Q3
FY16
FX
Impact
$M / Change
Q3 FY17
Constant
Currency1
Q3 FY17
CC Growth /
Change2
Net Sales ($M) 7,283 6,934 (40) -- 6%
Cost of Products Sold1 2,268 2,132 10 -- 6%
Gross Margin1 68.9% 69.3% (30) bps 69.2% (10) bps
SG&A ($M) 2,388 2,317 (9) -- 3%
% of Sales 32.8% 33.4% 10 bps 32.7% 70 bps
R&D ($M) 530 546 (1) -- (3%)
% of Sales 7.3% 7.9% Flat 7.3% 60 bps
Other Expense, Net ($M) 46 9 38 -- (11%)
Operating Profit1 2,051 1,930 (78) -- 10%
Operating Margin1 28.2% 27.8% (90) bps 29.1% 130 bps
Diluted EPS1 ($) 1.12 1.06 (0.05) 1.17 10%
1 Non-GAAP
2 Figures represent comparison to Q3 FY16 on a constant currency basis (Non-GAAP).
Operating
Leverage2
+470bps
EPS
Leverage2
+480bps

Q3 FY17 Earnings Results | February 21, 2017 | 9
CVG
Q3 FY17 HIGHLIGHTS
CRHF
54%
CSH
29%
APV
17%
U.S.
52%
Non-
U.S.
Dev
32%
EM
16%
Cardiac Rhythm & Heart Failure (CRHF)
KEY PERFORMANCE DRIVERS1
Heart Failure:+Upper-Teens
• Driven by recent HeartWare
acquisition; integration on track
• CRT-D: MSD growth driven by US
• Japan benefitted from continued
share gains following strong launch
of Compia MRI™ and Amplia MRI ™
• CRT-P: share loss from lack of quad
Arrhythmia Mgmt: +MSD
• WW Tachy: +LSD; strong implants in US
• WW Brady: LSD decline
• US: Modest share decline
• Reveal LINQ® pull-through
• Diagnostics: Mid-teens – Reveal LINQ®
• AF Solutions: Mid-twenties – Continued
share gain in EU/US; Japan >100% growth
Coronary & Structural Heart (CSH)
Aortic & Peripheral Vascular (APV)
Services & Solutions: +LDD
Heart Valve Therapies:
+Upper-Teens
• WW TAVR market growing ~30%
• TAVR : In-line with WW market
•US: seq. share gain on large size Evolut®
R 34mm launch; over 200 accounts
currently
•Share stability in EU
•Japan: Evolut® R launch continues in Q4;
modest share gains seen in early centers
Coronary: -MSD
• DES: LDD decline
• US: mid-20s decline - competitive
product launches
• OUS: LSD decline – Resolute
Onyx™ maintaining share
Aortic: +MSD
• US: Flat growth; Heli-FX® EndoAnchor®:
driving strong growth and AAA pull-
through, offset by competitive headwinds
in TAA
• OUS: HSD growth
• AAA: MSD growth; Endurant® with
ChEVAR indication CE Mark received in Q3
Peripheral & endoVenous:
+HSD
• DCB: US & WW market share leader
• IN.PACT® Admiral® DCB high-30s
• Pricing uplift from 150mm length
• Maintained market leadership in EU
despite pressure on price and
competitive registry enrollment
HSD Growth in CRHF and APV;
LSD growth in CSH
Extracorp. Therapies: -LSD
• Cannulae and Revasc growth offset
by Surgical Ablation decline
Compia
MRI™
SureScan®
CRT-D
CoreValve®
Evolut® R
34mm
Resolute
Onyx™
IN.PACT®
Admiral®
Heli-FX®
EndoAnchor®
Revenue
$M
As Rep
Y/Y %
CC1
Y/Y %
CRHF 1,371 7 8
CSH 751 2 3
APV 426 6 6
Total $2,548 5% 6%
U.S. 1,320 5 5
Non-U.S. Dev 815 5 7
EM 413 7 10
Total $2,548 5% 6%
Arctic Front
Advance®
1 Figures represent comparison to Q3 FY16 on a constant currency basis (Non-GAAP).
Q4 Growth Outlook: MSD

Q3 FY17 Earnings Results | February 21, 2017 | 10
MITG
Q3 FY17 HIGHLIGHTS
Surgical Solutions
KEY PERFORMANCE DRIVERS1
MSD Growth in Surgical Solutions
and PMR
Patient Monitoring & Recovery (PMR)
Early Technologies: +HSD
• Strong growth in GI Solutions
driven by new products including
Barrx™ 360 Express, which helps in
the treatment of Barrett's
Esophagus.
General Surgical: Flat
• Solid growth in OR Safety driven by
our Situate™ technology, a
detection system for retained
surgical sponges, offset due to
softness in Electrosurgery.
Patient Care/ DVT/
Nutritional Insufficiency:
-LSD
• Growth in Nutritional Insufficiency
• DVT: affected by reprocessing in US
Endo GIA™
Bellco
Renal Care Solutions
• Benefitted from Bellco acquisition
• Continued strength from dialyzers
and other consumables revenue
Revenue
$M
As Rep
Y/Y %
CC1
Y/Y %
Surg. Sol. 1,343 6 7
PMR 1,074 5 5
Total $2,417 5% 6%
U.S. 1,234 2 2
Non-U.S. Dev 842 8 8
EM 341 12 14
Total $2,417 5% 6%
ValleyLab™
FT10
PMR
44% Surg.
Sol.
56%
U.S.
51%
Non-
U.S.
Dev
35%
EM
14%
Puritan
Bennett™
980
1 Figures represent comparison to Q3 FY16 on a constant currency basis (Non-GAAP).
LigaSure™
Vessel
Sealing
TRUCLEAR™
Advanced Surgical: +HSD
• Solid growth in Advanced Stapling
driven by innovative new products in
endo stapling specialty reloads.
• Strong growth in Advanced Energy
driven by new LigaSure™ Vessel
Sealing Instruments and ValleyLab™
FT10.
• The business also benefitted from
the Smith & Nephew gynecology
acquisition (TRUCLEAR™).
• US surgical volumes appear stable in
1-2% range.
Respiratory and Monitoring
Solutions: +HSD
• Strong growth in Airways and Ventilation
due to the continued adoption of the
Puritan Bennett™ 980 ventilator.
• Solid growth in our Patient Monitoring
business as a result of strength in
Nellcor™ Pulse Oximetry.
Q4 Growth Outlook: MSD

Q3 FY17 Earnings Results | February 21, 2017 | 11
RTG
Q3 FY17 HIGHLIGHTS
Spine
36%
Brain
29%
Specialty
20%
Pain
15%
US
68%
Non-US
Dev
21%
EM
11%
KEY PERFORMANCE DRIVERS1
Continued Improvement in Spine;
Solid Brain Therapies & Specialty
Therapies Growth Offsets
Declines in Pain Therapies
Neurosurgery: +HSD
• Growth driven by strong performance in
navigation capital (+20%) and disposables
• WW O-arm® O2 driven by robust OUS
demand
Core Spine: +LSD
• US growth driven by new product and
procedural innovation introductions
• Interbody and Discs launches
(Elevate™, OLIF, and Rialto™ for
sacroiliac joint fusion) strong uptake
• Speed-to-scale and surgical synergy
driving implant growth
BMP: +LSD
• US Pricing remains favorable
• InductOs™ return to market expected
mid-FY18
Brain Modulation: +LSD
• US: LSD share loss; competitive
pressure partially mitigated by MRI-
conditional labelling
• EU: revenue growth; competitive
pressure remains
ENT: +LSD
• Continued strong growth in NuVent® driven by
Fusion® Compact penetration
• US growth driven by Power, Balloon & Service
Advanced Energy: +LDD
• Broad geographic expansion led by
China, APAC, and EMEA
• Strong PEAK PlasmaBlade®
disposable growth in Breast,
Generator Replacement markets
InterStim® II
O-arm® O2
Infuse®
Bone Graft
Spine
Brain Therapies
Specialty Therapies
Pain Therapies
Pelvic Health: +MSD
• US growth driven by both new implant and
replacement demand
Neurovascular: +LDD
• Strong sequential and Y/Y growth despite
headwinds from voluntary Q2 recall
Kanghui: +HSD
Revenue
$M
As Rep
Y/Y %
CC1
Y/Y %
Spine 657 3 3
Brain 518 7 8
Specialty 370 4 5
Pain 272 (3) (2)
Total $1,817 4% 4%
U.S. 1,242 3 3
Non-U.S. Dev 384 5 5
EM 191 8 11
Total $1,817 4% 4%
• Growth driven by LatAm, EMEA, APAC
• China growth driven by Spine product
launches (Peek Cage)
SCS/Pumps: -MSD
• US growth in replacement implants, offset
by share loss and new implant declines
• Ongoing SCS competitive pressure
leading to share loss
Interventional: +HSD
• Growth driven by new product
launches including OsteoCool® in EU
• Japan up 25%+ despite competitive
headwinds
OsteoCool®
1 Figures represent comparison to Q3 FY16 on a constant currency basis (Non-GAAP).
Strongest growth in over 7 years; Continue to gain share
Q4 Growth Outlook: Low End of MSD Range

Q3 FY17 Earnings Results | February 21, 2017 | 12
DIABETES
Q3 FY17 HIGHLIGHTS
US
62%
Non-US
Dev
30%
EM
8%
KEY PERFORMANCE DRIVERS1
Intensive Insulin Management (IIM)
Significant Improvement Over
Last Quarter; Strong Interest in
MiniMed® 6 Series Pumps
MiniMed®
630G
Guardian®
Connect
12
Total Group
Revenue
$501M
Revenue
$M
As Rep
Y/Y %
CC1
Y/Y %
IIM ND HSD LDD
NDT ND >15 >15
DSS ND LSD LSD
Total $501 6% 7%
U.S. 310 6 6
Non-U.S. Dev 152 6 9
EM 39 5 5
Total $501 6% 7%
Q4 Growth Outlook: MSD to HSD
MiniMed®
670G
Non-Intensive Diabetes Therapies (NDT)
iPro®2 CGM
w/ Pattern
Snapshot
Diabetes Service & Solutions (DSS)
Improved Patient Retention:
• Substantial sequential improvement in
CGM retention rates
• Patient and physician excitement
driving both installed base growth and
competitive share gains
MiniMed® 640G System:
• Continued strong sales throughout
EMEA and Australia
• Continuing to launch throughout
APAC and Latin America
• Anticipating Japan launch in Q3FY18
MiniMed® 670G System:
• Strong patient participation in the
Priority Access Program; enrollees will
be first in line for 670G
• Early coverage confirmed with many
commercial payers
CGM Adoption:
• iPro® 2 OUS growth from China launch
• Continue to receive positive feedback
for iPro® Pattern Snapshot
i-Port Advance Technology:
• Launches in India, Argentina and Korea
Consumables:
• Solid constant currency growth in
international markets
• LSD declines in U.S. driven by pricing
and challenging prior year comps
Guardian® Connect:
• Positive response to pilot launches in
major European markets
IBM Watson Partnership:
• Preparing for limited preview of
Sugar.IQ™ app; Medtronic Turning
Point platform with IBM now live
Customer Care Programs:
• OUS growth supported by new pro-
active programs to improve adherence
and retention
MiniMed® 630G System:
• Solid US sales, excellent feedback from
patients and providers
• Ongoing physician training
Fitbit Partnership:
• Strategic partnership reached in Q3 to
integrate health and activity tracking for
patients with diabetes
1 Figures represent comparison to Q3 FY16 on a constant currency basis (Non-GAAP).
Diabeter Clinics:
• Strong patient growth
• Global expansion plans moving forward
UNH Partnership:
• Positive coverage decision on 670G
Henry Schein :
• Sales ramped through the quarter;
expect to continue run-rate

FY17 EPS GUIDANCE,
REVENUE OUTLOOK, &
OTHER ASSUMPTIONS

Q3 FY17 Earnings Results | February 21, 2017 | 14
MDT
FY17 EPS GUIDANCE, REVENUE OUTLOOK & OTHER ASSUMPTIONS
Q4 FY17 FY17
Revenue Growth Outlook – CCCW Lower Half of MSD MSD
CVG Growth – CCCW MSD --
MITG Growth – CCCW MSD --
RTG Growth – CCCW Low End of MSD --
Diabetes Growth – CCCW MSD to HSD --
COV Synergies -- ~$225-250M
EPS Growth Guidance– CCCW -- DD
Free Cash Flow1 -- $5B - $6B
Other than noted, revenue and EPS growth guidance do not include any charges or gains that would be recorded as non-GAAP adjustments to earnings during the fiscal year
1 Operating cash flows less property, plant and equipment additions (non-GAAP)
Note: Medtronic will adopt FASB ASU 2016-09 regarding the change in tax treatment of
stock-based compensation in the first quarter of fiscal year 2018.
Outlook & Guidance
FX Assumptions
Q4 FY17 FY17 FY18
Revenue ($20M) – ($40M) ($20M) – ($40M) ($100M) – ($300M)
EPS ~($0.05) ~($0.20) ($0.05) – ($0.15)
Note: While FX rates are fluid, assumptions above are based on current rates.
Q3 FY17 Earnings Results | February 21, 2017 | 15
APPENDIX
ACRONYMS / ABBREVIATIONS
1
Growth
DD Double Digits
HSD High-Single Digit
LDD Low-Double Digits
LSD Low-Single Digit
MSD Mid-Single Digit
Other
APAC Asia Pacific FY Fiscal Year
Bps Basis Points NC Not Comparable
CC Constant Currency Ops Operations
CCCW
Constant Currency
Constant Weeks
OM Operating Margins
Dev Developed OUS
Outside the United
States
EM Emerging Markets R&D
Research &
Development
EMEA
Europe, Middle East &
Africa
Rep Reported
EPS Earnings per Share SG&A
Selling, General &
Administrative
FCF Free Cash Flow WW Worldwide
FX Foreign Exchange Y/Y Year-over-Year
Business Specific
AAA Abdominal Aortic Aneurysm ENT Ear, Nose, & Throat
AF Atrial Fibrillation Extracorp Extracorporeal
APV Aortic & Peripheral Vascular HF Heart Failure
BMP Bone Morphogenetic Protein IIM Intensive Insulin Management
Brady Bradycardia MDT Medtronic
CGM Continuous Glucose Monitoring MITG Minimally Invasive Therapies Group
CRHF Cardiac Rhythm & Heart Failure MRI Magnetic Resonance Imaging
CRT-D
Cardiac Resynchronization Therapy –
Defibrillator
NDT Non-Intensive Diabetes Therapies
CRT-P
Cardiac Resynchronization Therapy –
Pacemakers
NV Neurovascular
CSH Coronary & Structural Heart PMR Patient Monitoring & Recovery
CVG Cardiac & Vascular Group RTG Restorative Therapies Group
DVT Deep Vein Thrombosis SCS Spinal Cord Stimulation
DCB Drug Coated Balloon Sol Solutions
DES Drug Eluting Stent TAVR Transcatheter Aortic Valve Replacement
DSS Diabetes Services & Solutions
