Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIOLIFE SOLUTIONS INC | v459802_8k.htm |
Exhibit 99.1
NASDAQ: BLFS Corporate Presentation February 2017 Biopreservation Tools for Cells, Tissues and Organs

Safe Harbor Statement This presentation contains forward - looking statements, including, but not limited to, statements concerning the company’s anticipated business and operations, the potential utility of and market for its products and services, potential revenue growth and market expansion, new products, and third party projections regarding the future market for regenerative medicine and cold chain packaging and instrumentation services . All statements other than statements of historical fact are statements that could be deemed forward - looking statements . These statements are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described in the forward - looking statements, including among other things, uncertainty regarding market adoption of products ; uncertainty regarding third party market projections ; market volatility ; competition ; litigation ; and those other factors described in our risk factors set forth in our filings with the Securities and Exchange Commission from time to time, including our Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q . We undertake no obligation to update the forward - looking statements contained herein or to reflect events or circumstances occurring after the date hereof, other than as may be required by applicable law . 2 © 2017 BIOLIFE SOLUTIONS, INC.

Mission Become the leading provider of biopreservation tools for cells, tissues, and organs; By supplying best in class tools for maintaining the health and function of biologic source material and finished products during manufacturing, storage and distribution. 3 WHAT WHY HOW To facilitate basic and applied research and commercialization of new biologic - based therapies; © 2017 BIOLIFE SOLUTIONS, INC.

4 Executive Team: 200 Years Experience Mike Rice – Chief Executive Officer BS Bus Admin; 10 years as BLFS CEO; chief visionary of BLFS market opportunities, branding, marketing strategies; 18 years medical device sales, sales management, marketing; patient monitoring, defibrillators, implantable CRM, hearing devices, LAN/WAN; 5 issued and 13 pending patents Aby J. Mathew , PhD – CTO, Senior Vice President BS Microbiology, PhD, Cell & Molecular Biology; co - developer of platform HypoThermosol® media; in demand industry thought leader in biopreservation of cells and tissues for clinical applications; catalyst responsible for driving regen med market to adopt BLFS clinical grade biopreservation media; 6 issued and 6 pending patents; numerous journal articles Roderick de Greef – Chief Financial Officer BA Economics, MBA; 25 years CFO experience for 5 public companies; Serves/served on 4 US public company boards including Endologix (NASDAQ: ELGX); Raised >$200mm from US, EU and AP private and institutional investors; Structured, negotiated and closed $400mm of public company mergers and acquisition transactions in the US and Europe Karen Foster – Vice President, Operations BS Biological Sciences, MS Zoology, MBA; 25 year career in quality and manufacturing operations including 13 years VP Manufacturing Operations and Site Leader at ViaCord, 2 positions leading 80 member teams; certified Six Sigma Green Belt. Jim Mathers – Vice President, Global Sales BA , Biology, MBA; 35 years sales and sales and marketing management; repeated achievement in driving early adoption of new medical device technologies for Stryker, MAKO Surgical, BrainLab, AccuRay, Cardiac Science, JNJ Todd Berard – Vice President, Marketing BS , Biochemistry, MBA; 16 years marketing including leadership of marcom, corporate branding, product marketing, and positioning for Verathon, Physio Control (MDT), tech startups © 2017 BIOLIFE SOLUTIONS, INC.

5 Our Biopreservation Tools Media designed to preserve biologics during ex vivo storage and shipment Goal: maximize effectiveness of biologic based medicine Smart, cloud - connected shipping containers engineered to maintain stability of time and temperature sensitive biologic materials from vein to vein © 2017 BIOLIFE SOLUTIONS, INC.

Worldwide Customer Base 392 • 33 Countries • 530 Direct Customers • Excludes Distributor End Customers 21 61 15 8 24 6 3 6 © 2017 BIOLIFE SOLUTIONS, INC.
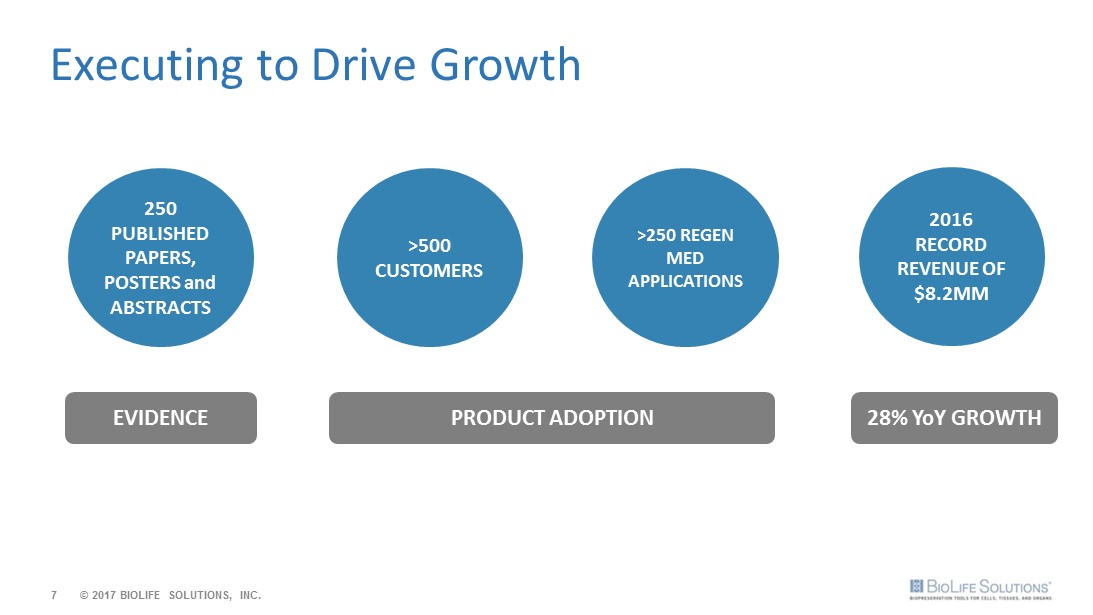
7 Executing to Drive Growth >500 CUSTOMERS >250 REGEN MED APPLICATIONS PRODUCT ADOPTION 2016 RECORD REVENUE OF $8.2MM 28% YoY GROWTH 250 PUBLISHED PAPERS, POSTERS and ABSTRACTS EVIDENCE © 2017 BIOLIFE SOLUTIONS, INC.

8 Preservation of Biologic Material Solid Organs Tumor Tissue Biopsies Apheresis Bone Marrow Allogeneic Transplant Biobanking Drug Discovery Regenerative Medicine Autologous Cell Therapy © 2017 BIOLIFE SOLUTIONS, INC.

9 Biopreservation Challenges Survival – How Long Viability – How Much Function – How Well Ex Vivo Time Viability Ex Vivo Time Survival Ex Vivo Time Function Cold storage is used to preserve biologic integrity and function by lowering metabolism . When removed from the body – cells, tissues, and organs degrade and die – a race against time! Traditional methods and tools are not optimized and offer limited protection from preservation - induced stress, injury, and death. © 2017 BIOLIFE SOLUTIONS, INC.
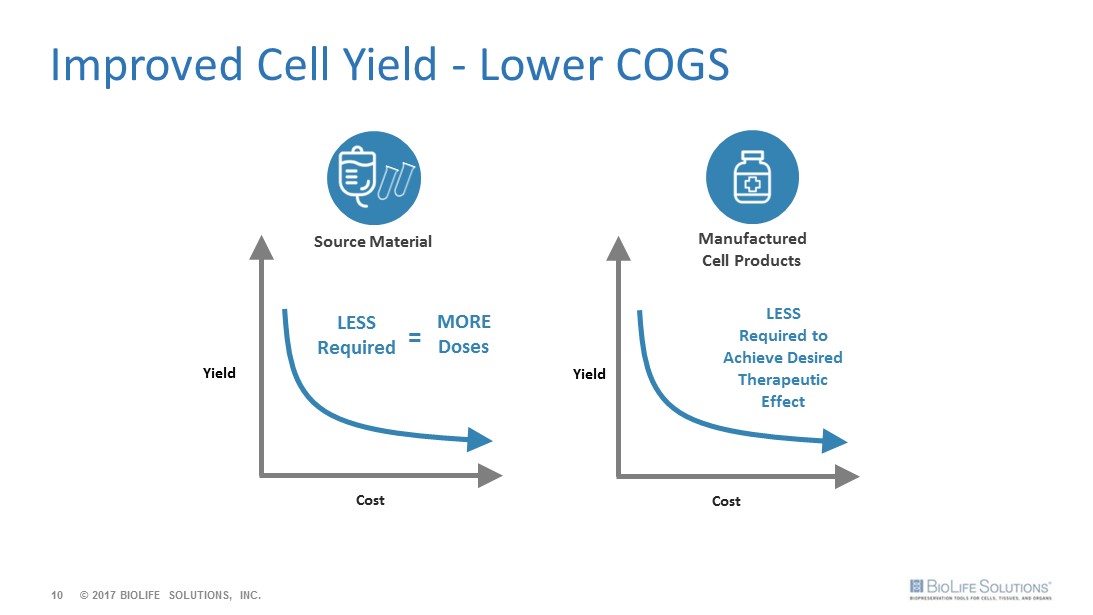
10 Improved Cell Yield - Lower COGS Yield Yield Cost Cost LESS Required to Achieve Desired Therapeutic Effect Source Material Manufactured Cell Products LESS Required MORE Doses = © 2017 BIOLIFE SOLUTIONS, INC.

Serum - free, protein - free, animal origin free; highest quality ingredients; US FDA Master File 11 CryoStor® Freeze Media Improved cell viability and functional recovery compared to commercial and home - brew alternatives in numerous cell types Formulated to mitigate molecular cell stress during freeze/thaw process in cord blood stem cells, T cells, others © 2017 BIOLIFE SOLUTIONS, INC.

12 HypoThermosol® Storage Media Optimized for hypothermic (2 - 8 ° C) storage and shipping of cells and tissues Enables multiple days of cell and tissue storage for transport of source material and manufactured cell products throughout the world Serum - free, protein - free, animal origin free; USP ingredients; US FDA Master File © 2017 BIOLIFE SOLUTIONS, INC.

Cells Preserved in traditional “home brew” Human mesenchymal stem cells isolated from bone marrow; condition of cells after 5 days of cold storage, then returned to culture conditions and assayed 24 hours later 13 Product Performance Cells Preserved in HypoThermosol ® Dead cells Healthy cells © 2017 BIOLIFE SOLUTIONS, INC.

14 Over 250 Articles, Abstracts & Posters © 2017 BIOLIFE SOLUTIONS, INC.

15 Biopreservation Market Source : Biopreservation Market Size By Product (Equipment, Media, Laboratory Information Management System), By Application (Regenerative Medicine, Biobanking, Drug Discovery), Industry Analysis Report, Regional Outlook (U . S . , Canada, UK, Germany, Japan, China, Brazil, Mexico, South Africa), Growth Potential, Price Trends, Competitive Market Share & Forecast, 2016 – 2024 . Global Market Research ; Published September 2016 Addressable media segment 14% CAGR; driven by Regenerative Medicine segment demand Hardware Media LIMS 2024 – $9.7B Total Spend Hardware Media LIMS $448M Addressable in Media $1.3B Addressable in Media 2016 - $3.5B Total Spend Driving change from using home brew © 2017 BIOLIFE SOLUTIONS, INC.

16 3 Strategic Revenue Markets REGENERATIVE MEDICINE C ell therapy companies Hospital - based stem cell transplant centers University - based clinical research labs Cell therapy CDMO, CRO DRUG DISCOVERY Pharmaceutical companies Cell suppliers Toxicity testing labs Personalized medicine labs BIOBANKING Umbilical cord blood banks Adult stem cell banks Tissue banks Biorepositories Hair transplant physicians 15% OF 2016 4 5% OF 2016 40% OF 2016

Regenerative Medicine Opportunity 17 © 2017 BIOLIFE SOLUTIONS, INC.

18 Regen Med Market Investments © 2017 BIOLIFE SOLUTIONS, INC.

19 Vein to Vein Customer Engagement Source Material Preservation Media Cell Factory Manufactured Products Temperature Controlled Container Preservation Media 4 Engagement Opportunities : Media and Cold Chain for Source Material and Final Dose Patient Temperature Controlled Container Temperature Controlled Container © 2017 BIOLIFE SOLUTIONS, INC.
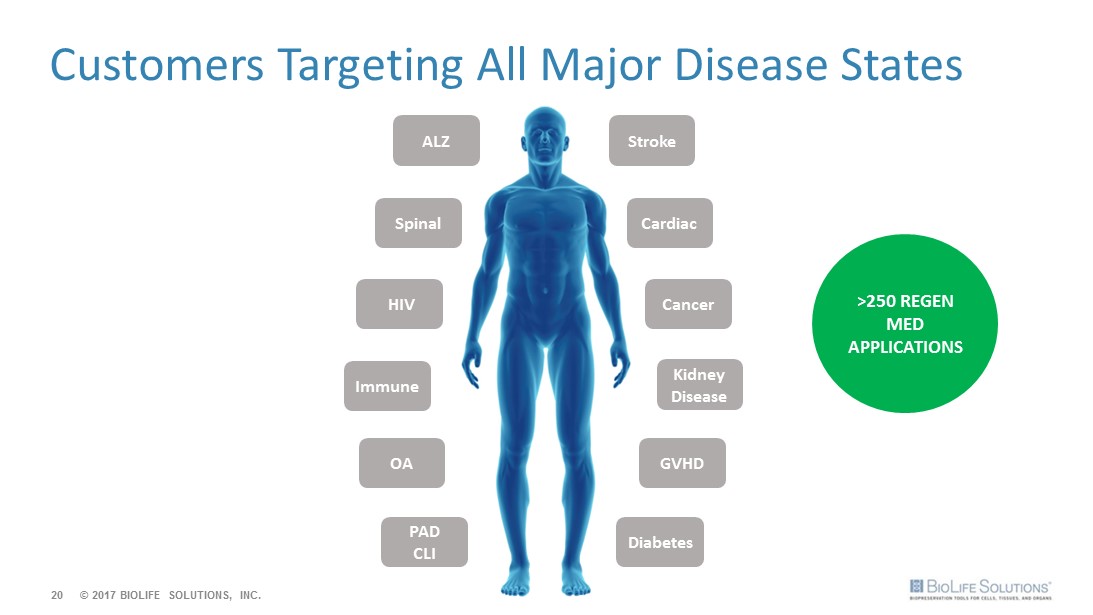
Customers Targeting All Major Disease States 20 © 2017 BIOLIFE SOLUTIONS, INC. Immune Stroke Cardiac Diabetes HIV OA PAD CLI GVHD Cancer Kidney Disease ALZ Spinal >250 REGEN MED APPLICATIONS

21 Marquee Customers DISTRIBUTORS CMO & CDMO DIRECT – 100 REGEN MED COMPANIES © 2017 BIOLIFE SOLUTIONS, INC.

Engagement Strength “Over the last several years we have worked to qualify and adopt CryoStor in our clinical manufacturing process. We are now securing long term supply continuity for this reagent as our cell therapy product candidate continues to progress through multiple clinical trials." Marc Better, PhD Vice President, Product Sciences CUSTOMER: KITE PHARMA CELL THERAPIES IN DEVELOPMENTS: CAR T CELL PLATFORM FOR VARIOUS CANCERS BIOLIFE PRODUCT USED: CRYOSTOR® APPLICATION OF BIOLIFE PRODUCT: FREEZING EVERY DOSE AFTER MANUFACTURING TO ENABLE STORAGE AND DISTRIBUTION TO CLINIC RELATIONSHIP ENGAGEMENT: 10 - YEAR SUPPLY AGREEMENT 22 © 2017 BIOLIFE SOLUTIONS, INC.

23 Significant Growth Potential Annual BioLife Revenue Per Customer Approval: $500,000 - $2,000,000 X = X X X X X = = Annual Doses Produced BioLife Product Used Per Dose (mL) Price per mL Annual Revenue Rare Cancer (2.5K) Common Cancer (10K) Osteoarthritis (200K) X X = 3.5ml $3.00 $2.1M 60ml $3.00 $1.8M 60ml $3.00 $450K © 2017 BIOLIFE SOLUTIONS, INC.

Intelligent, Informed, Precise Biologic Materials Management e vo ® Cold Chain 2.0™ Cloud - Based Cold Chain Management 24 © 2017 BIOLIFE SOLUTIONS, INC.

Patents Pending 25 Vertical SaaS for Cold Chain Management © 2017 BIOLIFE SOLUTIONS, INC.

2 - 8 ° C Use with pre - conditioned cold packs 26 Cloud - Connected Smart Shippers CRT 15 ° C - 20 ° C Use with pre - conditioned cold packs CRYO - 80 ° C Use with dry ice Patents Pending © 2017 BIOLIFE SOLUTIONS, INC.

e vo® System Performance "We have been extremely pleased with the performance of the evo® Cold Chain 2 . 0 ™ system for use in our phase 3 study . The unique uni - directional packout prevents errors in packaging and makes it very simple for our clinical sites to package the starting apheresis product for shipment to our manufacturing facilities . Validation of tight temperature stability in every shipment is critical, and we have not experienced any temperature or packaging deviations since switching to evo . It is clear that an enormous amount of thought and testing went in to the design of the evo system . " Marta Schilling Vice President, Cell Therapy Manufacturing CUSTOMER: IMMUNOCELLULAR CELL THERAPIES IN DEVELOPMENTS: DENDRITIC CELL PLATFORM FOR VARIOUS CANCERS PRODUCT USED: evo® SMART SHIPPER PRODUCT APPLICATION: COLD CHAIN 2.0™ SHIPMENT OF APHERESIS FOR EVERY MANUFACTURED DOSE RELATIONSHIP ENGAGEMENT: 120 SITE, 400+ PATIENTS PHASE 3 TRIAL 27 © 2017 BIOLIFE SOLUTIONS, INC.

b iologistex JV Restructuring • All evo® Smart Shipper and biologistex™ Cold Chain SaaS IP and assets now consolidated in one entity to enable additional direct investment in the JV; not via BLFS stock offering • BioLife is the exclusive distributor to the worldwide regen med market • BioLife holds a 45% equity position and will continue to exclusively market, sell and support customers in the regen med space • 20% revenue share for first 3 years • Anticipated $1.6M - $2M annual expense reduction 28 © 2017 BIOLIFE SOLUTIONS, INC.

Financial Information 29 © 2017 BIOLIFE SOLUTIONS, INC.

Biopreservation Media Revenue Growth $0 $500,000 $1,000,000 $1,500,000 $2,000,000 $2,500,000 1Q12 2Q12 3Q12 4Q12 1Q13 2Q13 3Q13 4Q13 1Q14 2Q14 3Q14 4Q14 1Q15 2Q15 3Q15 4Q15 1Q16 2Q16 3Q16 4Q16 29% CAGR 2012 – 2016 30 © 2017 BIOLIFE SOLUTIONS, INC.

2017 Catalysts • Cell therapy customers, including Kite Pharma, are expected to receive regulatory approvals to commence marketing and commercial manufacturing, which, as a result, should drive increased demand for our proprietary biopreservation media products. • Continued adoption of CryoStor® and HypoThermosol® in pre - IND validations and phase 1, 2 and 3 clinical trials of new cell and tissue based products and therapies. • Adoption of the evo Smart Shipper and Cold Chain Management SaaS by leading CAR T cell therapy customers. 31 © 2017 BIOLIFE SOLUTIONS, INC.

2017 Guidance • Biopreservation media revenue growth of 20% - 25% over 2016; revenue in excess of $10 million • Gross margin in a range of 55% to 60% • Operating expenses ranging from $8 - $9 million, reflecting a quarterly decrease of $400,000 - $500,000 resulting from the restructuring of the biologistex joint venture • Restructured debt • Positive quarterly EBITDAS by the end of 2017 32 © 2017 BIOLIFE SOLUTIONS, INC.

Operating Leverage • Two independent cGMP formulation and fill suites • 100,000 liter annual capacity in current facilities • No significant CAPEX required to realize 10X current production level • Potential 10 + percentage point gross margin increase as demand ramps • Minimal SG&A expense increases needed to realize full production and margin potential 3 3 © 2017 BIOLIFE SOLUTIONS, INC.

Capitalization Table 34 ▪ 6.9M priced at $4.75 ▪ 4.0M held by the same two long term shareholders ▪ Management and employee incentives ▪ 1.6M vested ▪ $1.83 wt. avg exercise price ▪ Limited institutional holders ▪ 48% held by two long term shareholders Shares – 13.3M Directors & Officers Affiliates Other Warrants – 7.6M Directors & Officers Affiliates Other Options – 2.3M Directors & Officers Affiliates Other © 2017 BIOLIFE SOLUTIONS, INC.

Share Price © 2017 BIOLIFE SOLUTIONS, INC. 35 0 50,000 100,000 150,000 200,000 250,000 300,000 350,000 400,000 450,000 500,000 $0.00 $0.50 $1.00 $1.50 $2.00 $2.50 1/1/16 2/1/16 3/1/16 4/1/16 5/1/16 6/1/16 7/1/16 8/1/16 9/1/16 10/1/16 11/1/16 12/1/16 1/1/17 2/1/17 Volume Price

NASDAQ: BLFS For additional questions or comments, please contact: Mike Rice | President and CEO mrice@BioLifeSolutions.com | (425) 686 - 6003 Roderick de Greef | CFO rdegreef@BioLifeSolutions.com | (425) 686 - 6002 www.biolifesolutions.com BioLife Solutions, Inc. 3303 Monte Villa Parkway, Suite 310 Bothell, WA 98021 36 © 2017 BIOLIFE SOLUTIONS, INC.

Supplemental Slides 37 © 2017 BIOLIFE SOLUTIONS, INC.

38 evo™ Smart Shipper Integrated cell phone with real time location and payload monitoring via SaaS Durable and reusable – designed for harsh small parcel shipping environment Intuitive, universal pack - out; ALL SEASON™ Cold Packs; eliminates packout errors True payload temp monitored via integrated thermocouple (temperature, humidity, tilt) Cold pack Cold pack Payload Carrier Integrated thermocouple Cell phone Super insulation © 2017 BIOLIFE SOLUTIONS, INC.

The Problem: Cell Therapy Dynamics • Traditional pharma – bulk manufacturing: • 80 million to 130 million annual temperature sensitive shipments requiring cold chain logistics 1 • $12 billion spent annually on cold chain logistics, with $9 billion for transportation and $3 billion for specialized tertiary packaging and instrumentation such as insulated boxes, blankets, phase change materials, temperature sensors and recorders 2 • $15 billion to $35 billion spent annually replacing products lost due to temperature excursions 1 • 6% of revenue spent on logistics 2 yet the average per dose expense for cold chain is de minimis • Cellular therapies – more temp sensitive, personalized medicine: • Fully burdened manufacturing cost per dose could range from $30,000 - $50,000, with reimbursement at $100K - $500K • Critical risk of individual dose delivery failure or temperature excursions • Significant per dose cold chain expense is rational yet unappreciated 39 1 ChainLink Research 2 Pharmaceutical Commerce 2016 Cold Chain Sourcebook © 2017 BIOLIFE SOLUTIONS, INC.

40 Competitive Shippers: Inadequate Foam Coolers • Old technology • Marginal performance • Single - use Data Loggers • Records container, not payload temperature • Data reviewed after clinical product is used Vacuum Panel Shippers • Overly complex • Heavy, expensive to ship • Prone to packout errors © 2017 BIOLIFE SOLUTIONS, INC.

41 Risks – Broken Cold Chain Economic Cost of scrapping biologic source material or manufactured cell products Clinical trial impact from poor biologistics management; lost doses, poor shipping container performance, temperature excursions, trial failure, funding exposure, reimbursement exposure $ Clinical Rx Unknowingly administering a thermally sensitive biologic dose that was exposed to undetected temperature excursions, packout errors or has exceeded it stability period © 2017 BIOLIFE SOLUTIONS, INC.

42 Cloud Based – Integrated Logistics Single app enables simple logistics: Cloud Hosted, Secure SaaS 21 CFR Part 11 Auditable EVO # 31099282738 Alert: Package is within 5 miles of destination as of 5/5/2015 at 21:19PST • biologistex • Shipment 494839 Picked up by carrier • biologistex • Shipment 494839 opened at 1:15PM • biologistex • Shipment 494839 delivered at 10:48AM • biologistex • Shipment 494839 remaining shelf life 00:18 - URGENT Critical Alerts Patents pending • Packout • Select and pay for outbound and return shipping • Tracking during transit • Actionable notifications for sender and recipient • Return logistics for reusable evo Smart Shipper © 2017 BIOLIFE SOLUTIONS, INC.

Source: Pharmaceutical Commerce 2015, Management Estimates 43 Biologistics Addressable Market $500 Million Estimated Addressable Market for Cold Chain Shipping Containers and Data Loggers for Small Payloads and Individualized Cell Therapies $2.6B $500M Small Payload Containers & Data Loggers Medium/Large Format Containers © 2017 BIOLIFE SOLUTIONS, INC.
