Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LABORATORY CORP OF AMERICA HOLDINGS | form8-k11017jpmppt.htm |

J.P. MORGAN
HEALTHCARE CONFERENCE
JANUARY 10, 2017 | SAN FRANCISCO, CA

1
FORWARD LOOKING STATEMENT
Cautionary Statement Regarding Forward Looking Statements
This presentation contains forward-looking statements including with respect to estimated 2016 results and
guidance and the impact of various factors on operating results. Each of the forward-looking statements is subject
to change based on various important factors, including without limitation, competitive actions in the marketplace,
adverse actions of governmental and other third-party payers and the results from the Company’s acquisition of
Covance. Actual results could differ materially from those suggested by these forward-looking statements. Further
information on potential factors that could affect LabCorp’s operating and financial results is included in the
Company’s Form 10-K for the year ended December 31, 2015, including in each case under the heading risk factors,
and in the Company’s other filings with the SEC, as well as in the risk factors included in Covance’s filings with the
SEC. The information in this presentation should be read in conjunction with a review of the Company’s filings with
the SEC including the information in the Company’s Form 10-K for the year ended December 31, 2015, under the
heading MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The Company assumes no obligation to update any forward-looking information included in this presentation.

2
AGENDA
Company Overview
Update on “Wave One” Initiatives
2016 Highlights
Financial Strength
2017 Priorities

3
WHO WE ARE
Our
Mission
is to
improve
health and
improve lives
LabCorp is
a world-leading
life sciences company
that is deeply integrated
in guiding patient care

4
LABCORP OVERVIEW
1. Based on the midpoint of guidance issued on October 26, 2016
• Provides diagnostic, drug development
and technology-enabled solutions for
>110 million patient encounters per year
• Operates in two segments – LabCorp Diagnostics
and Covance Drug Development
• ~$9.4B revenue expected in 20161
• >50,000 mission-driven employees worldwide
• Leadership in large, growing, fragmented global markets
• Experienced management team
A World-Leading Life Sciences Company

5
LABCORP DIAGNOSTICS OVERVIEW
• ~$6.6B revenue expected in 20161
• National network of 41 primary clinical laboratories
and approximately 1,750 patient service centers
• Offers broad range of 4,800+ clinical, anatomic
pathology, genetic and genomic tests
• Processes ~500,000 patient specimens daily
• >150 million unique patients seen over past 5 years
• Serves hundreds of thousands of customers,
including physicians, government agencies,
managed care organizations, hospitals and
health systems, patients and consumers
1. Based on the midpoint of guidance issued on October 26, 2016
2. Presented on a pro forma basis as if the acquisition of Covance closed on January 1, 2015.
Adjusted operating income and margin exclude unallocated corporate expenses, amortization, restructuring and other special items
Leading National Clinical Laboratory
Pro Forma Segment Financial Summary2
Constant
Nine Months Ended Currency
9/30/2016 9/30/2015 Change Change
Revenue 4,922$ 4,659$ 5.6% 5.9%
Adj. O.I. 1,005$ 942$ 6.7%
Adj. O.I. % 20.4% 20.2% 20 bps

6
Pro Forma Segment Financial Summary2
Constant
Nine Months Ended Currency
9/30/2016 9/30/2015 Change Change
Revenue 2,127$ 1,937$ 9.8% 11.0%
Adj. O.I. 306$ 261$ 17.4%
Adj. O.I. % 14.4% 13.5% 90 bps
COVANCE DRUG DEVELOPMENT OVERVIEW
• ~$2.8B revenue expected in 20161
• Market leader in early development, central
laboratory, and Phase I-IV clinical trial management
services
• Collaborated on 87% of the 45 new drugs approved by
FDA in 2015, including all 14 approved oncology
drugs, and 20 of 21 drugs treating rare and orphan
diseases
• Xcellerate® is the world’s most comprehensive
investigator performance database
1. Based on the midpoint of guidance issued on October 26, 2016
2. Presented on a pro forma basis as if the acquisition of Covance closed on January 1, 2015.
Adjusted operating income and margin exclude unallocated corporate expenses, amortization, restructuring and other special items
Leading CRO / Drug Development Services Provider
7
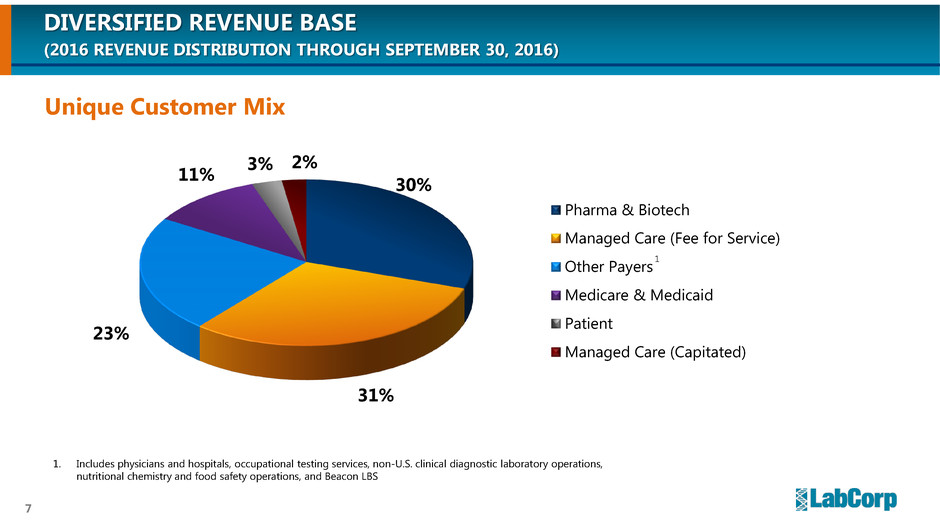
DIVERSIFIED REVENUE BASE
(2016 REVENUE DISTRIBUTION THROUGH SEPTEMBER 30, 2016)
Unique Customer Mix
30%
31%
23%
11%
3% 2%
Pharma & Biotech
Managed Care (Fee for Service)
Other Payers
Medicare & Medicaid
Patient
Managed Care (Capitated)
1. Includes physicians and hospitals, occupational testing services, non-U.S. clinical diagnostic laboratory operations,
nutritional chemistry and food safety operations, and Beacon LBS
1

8
EXPANDED GROWTH OPPORTUNITIES WITH INCREASED GLOBAL PRESENCE
1. 2014 revenue excludes Covance. 2016 revenue from January 1st through September 30th
2. Based on industry publications and company estimates
3. Over 30 currencies in 2016 and no single currency (other than US dollar) accounts for more than 10% of 2016 revenue
2014 Revenue Distribution1
>$70 billion
addressable
market2
2016 Revenue Distribution1
0%
25%
50%
75%
100%
USA Rest of World
92.7%
80.9%
7.3%
19.1%
0%
25%
50%
75%
100%
USA Rest of World
92.7%
Markets Served
North American
Clinical Reference Laboratory
Central Laboratory
Market Opportunities
Global Clinical Reference Laboratory Drug Development
Central Laboratory Market Access
Food Safety and Chemistry
>$200 billion
addressable
market2
3

9
OUR MISSION: IMPROVE HEALTH AND IMPROVE LIVES
Build / Acquire
Complementary Capabilities
Organic Growth Through
New Tests, Customers and
Markets
Integrate Diagnostic
Information and Content
Build / Acquire
Complementary Capabilities
Use Tools and Technology to
Improve Success, and Reduce
Time and Cost, of Trials
Commercialize
Technology-Enabled
Solutions
Develop Scalable Platforms
and Applications for
Customers
Delivering
World Class Diagnostics
Bringing Innovative
Medicines to Patients Faster
Using Technology
to Provide Better Care

10
AGENDA
Company Overview
Update on “Wave One” Initiatives
2016 Highlights
Financial Strength
2017 Priorities

11
2015 JP MORGAN CONFERENCE:
COMBINATION PROVIDES SIGNIFICANT NEW GROWTH AVENUES
Prioritized top 3 opportunities based on
materiality, feasibility, and strategic fit
Deliver faster clinical
trial enrollment
1
Partner of choice to
develop and commercialize
companion diagnostics
2
Enhance Phase IV trial experience
and post-market surveillance
3
Wave One
12
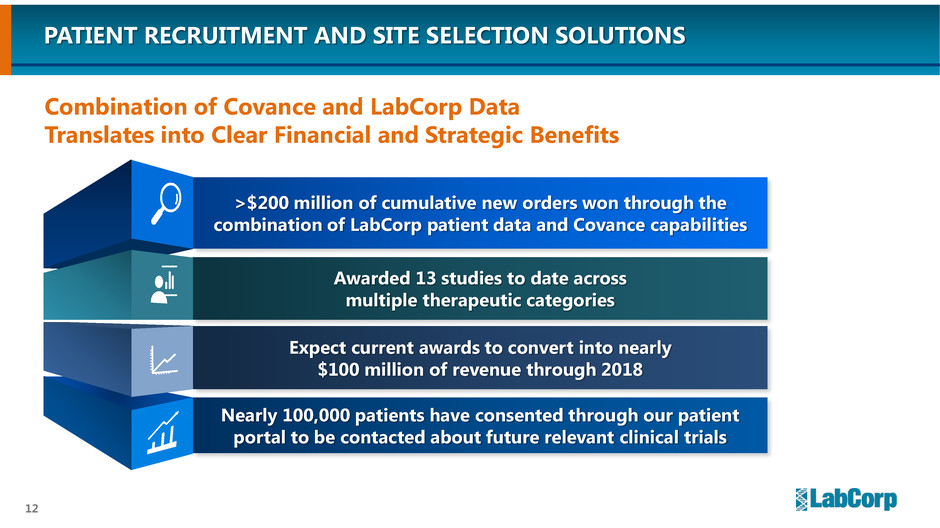
>$200 million of cumulative new orders won through the
combination of LabCorp patient data and Covance capabilities
Awarded 13 studies to date across
multiple therapeutic categories
Expect current awards to convert into nearly
$100 million of revenue through 2018
Nearly 100,000 patients have consented through our patient
portal to be contacted about future relevant clinical trials
PATIENT RECRUITMENT AND SITE SELECTION SOLUTIONS
Combination of Covance and LabCorp Data
Translates into Clear Financial and Strategic Benefits

13
Keytruda is a registered trademark of Merck Sharp & Dohme Corp.,
a subsidiary of Merck & Co., Inc.
OPDIVO is a registered trademark of Bristol-Myers Squibb Company.
cobas is a registered trademark of Roche.
TAGRISSO is a trademark of the AstraZeneca group of companies.
Tarceva is a registered trademark of OSI Pharmaceuticals.
TECENTRIQ is a registered trademark of Genentech, Inc. 1. 2016 full year revenue is estimated based on run-rate through September 30th, 2016
COMPANION AND COMPLEMENTARY DIAGNOSTICS (CDX)
Unmatched CDx Franchise Providing End-to-End Clinical Development
and Commercial Lab Testing Solutions
• Dedicated global CDx team and laboratories
• Worked on 60+ CDx programs supporting
145+ clinical protocols in 2016
• 33% increase in revenue across drug
development and commercial clinical
laboratory testing since 20141
• CDx collaborations with 13 of top 20
pharmaceutical companies
• Only CRO awarded a podium presentation at
World Companion Diagnostics Conference
• PD-L1 IHC 22C3 pharmDx
(Merck’s Keytruda®)
• PD-L1 IHC 28-8 pharmDx
(Bristol-Myers Squibb’s OPDIVO®)
• cobas® EGFR Mutation Test v2
(AstraZeneca’s TAGRISSO™ and
Roche’s Tarceva®)
• Ventana PD-L1 (SP142)
(Genentech’s TECENTRIQ®)
Notable CDx Tests from LabCorp

14
UNIQUELY POSITIONED FOR PARTNERSHIPS
IN REAL-WORLD EVIDENCE AND POST-MARKET SURVEILLANCE
Delivering Integrated Solutions for Commercially-Approved Products
in “Real-World” Setting
• Lab Assist Program with Top 20 pharmaceutical
partner to facilitate required monthly liver testing
• Patient and provider support through
program enrollment, monthly test scheduling,
and follow up on missed appointments
• Convenient access to LabCorp’s Patient Service
Center network for specimen collections or
drop-offs
• Customized informatics enable electronic
delivery of results to providers and patients
• Coordination between Covance Market Access
and LabCorp Diagnostics
• Program Coordinator calls
the patient to schedule
monthly testing
• Sample is collected and
submitted to LabCorp
• Results are delivered to the
provider’s office

15
AGENDA
Company Overview
Update on “Wave One” Initiatives
2016 Highlights
Financial Strength
2017 Priorities

16
ENTERPRISE HIGHLIGHT
Trial Data / Capability Outcome
Prevention of upper
respiratory tract infections
with seasonal incidence
LabCorp-generated data enables Covance to flexibly open
and close sites based on timely insights into viruses of
interest circulating in a particular community
All Studies
Awarded to
Non-alcoholic
steatohepatitis (NASH)
Leveraged the LabCorp database of physicians ordering Fibrosure,
a non-invasive biomarker of fibrosis, in client proposals
Rare genetic disorder Director in Biochemical & Molecular Genetics at LabCorp will serve
as “Geneticist Expert,” and LabCorp team will conduct review,
validation and classification of mutation types
Cardiopulmonary bypass
surgery involving use of
frozen platelets
LabCorp’s Chief Medical Officer served as in-house consultant
for transfusion medicine for RFP
Innovative Use Cases for LabCorp Data and Technical Expertise
Contribute to New Study Awards

17
ENTERPRISE HIGHLIGHT
Integrated “Research Hub” Model for Hospitals and Health Systems,
Adding Value for All Key Stakeholders
• Expand patient
recruitment
• Enhance site
identification
• Greater access to clinical trials
• Improve patient care and
outcomes
• Grow reference testing
• Cultivate long-term,
comprehensive
partnerships
• Collaboration and
medical institution
growth
• Access to new revenue stream
• Differentiate from competitors
• Enhance academic reputation
• Reduce costs under value-based
reimbursement framework
Integrated
“Research Hub”
Model
LabCorp
Diagnostics
Covance Drug
Development
Hospitals, Health
Systems & Large
Provider Networks
Patients

18
ENTERPRISE HIGHLIGHT
Combined Expertise in Oncology Drives Growth
• Utilized LabCorp data and Covance informatics to secure
Phase III study in Acute Myeloid Leukemia
• Heat map highlighted U.S. physicians with high volume of AML
patients; 50,000+ patients represented in this dataset from LabCorp
• Physicians in LabCorp database evaluated for clinical trial experience
and categorized by expertise and practice type
• Integrated, end-to-end development and commercialization
capabilities in immuno-oncology
• Doubled the number of immuno-oncology study awards
and related backlog year on year
• Performed thousands of PD-L1 tests through Diagnostic and
Drug Development segments
• Published real world utilization data at ASCO
Acute myeloid leukemia cells
19
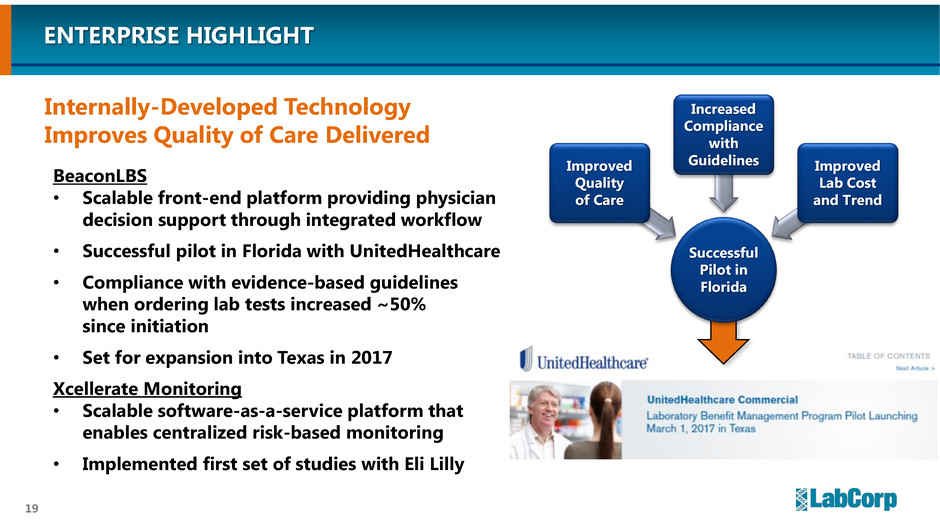
BeaconLBS
• Scalable front-end platform providing physician
decision support through integrated workflow
• Successful pilot in Florida with UnitedHealthcare
• Compliance with evidence-based guidelines
when ordering lab tests increased ~50%
since initiation
• Set for expansion into Texas in 2017
Xcellerate Monitoring
• Scalable software-as-a-service platform that
enables centralized risk-based monitoring
• Implemented first set of studies with Eli Lilly
Internally-Developed Technology
Improves Quality of Care Delivered
ENTERPRISE HIGHLIGHT
Successful
Pilot in
Florida
Improved
Quality
of Care
Increased
Compliance
with
Guidelines Improved
Lab Cost
and Trend

20
LABCORP DIAGNOSTICS HIGHLIGHT
Broad and Flexible Health System and Large Provider Collaborations
Have Been a Successful Model for Over Three Decades
Reference
Testing
Lab Optimization
(incl. Outreach Business)
Research
Hub Model
• 1,800+ hospital clients, and 200+ partnerships
• Average partnership length of ~6 years
• Significant progress on multiple strategic
health system initiatives in 2016
• Enhanced executive leadership focused on
comprehensive partnerships

21
Acquiring Assets of Mount Sinai Health System Clinical Outreach Laboratories
LABCORP DIAGNOSTICS HIGHLIGHT
• LabCorp will provide comprehensive
laboratory services
• Exploring opportunities to collaborate on
companion diagnostics, clinical trials and
medical education
• LabCorp’s differentiators include:
• Access to clinical trials and research
through Covance Drug Development
• Enhanced IT and data analytics
• Standardized testing platforms
• Meets stated financial criteria
“[LabCorp’s] unparalleled reputation and
success ensure our patients will continue to
have access to high-quality, high-value and
convenient testing services.” 1
“LabCorp’s proven track record of service
excellence, breadth of diagnostic capabilities,
and cost-efficiency will benefit our community
now and in years to come.” 2
“We are confident this transaction will provide
great benefits for our patients and physicians
and allow Mount Sinai to continue to invest in
our core strategic programs.” 2
1. Quote attributed to Carlos Cordon-Cardo, MD, PhD, Irene Heinz Given and John LaPorte
Given Professor and Chairman, Department of Pathology, Mount Sinai Health System
2. Quote attributed to Donald Scanlon, Chief Financial Officer and Chief of Corporate Services,
Mount Sinai Health System

22
• Increased Patient Engagement
• Mobile-Friendly Patient Portal
• Clinical Trial Patient Consents
• Self Service Registration in 2017
(opportunity for clinical trial opt-ins)
• Integrated Clinical Decision Support Capabilities
• LabCorp Link
• LithoLink CDS Platform and Reports
• UpToDate® Advisor
• Enhanced Revenue Cycle Management Tools
• Nationwide Real-Time Eligibility Verification
• Introduced Patient Responsibility Estimate
(Price Transparency)
Continued Commitment to Technology Innovation
to Deliver Improved Patient Care
LABCORP DIAGNOSTICS HIGHLIGHT

23
COVANCE DRUG DEVELOPMENT HIGHLIGHT
Novel Drug Development Solutions Drive Growth and Loyalty
Lab Optimization
(incl. Outreach Business)
Research
Hub Model • Integrated LabCorp Diagnostics’ specialty
test menu into global central laboratory services
• Leveraged the Xcellerate informatics platform to
optimize and execute an enrollment strategy for
12,000-patient Cardiovascular Outcomes trial
• Developed a “One Stop” laboratory solution that
manages all internal and external lab vendors
• Early Phase Development Solutions (EPDS) available
from pre-Clinical Lead Optimization through Clinical
Proof of Concept with consistent and focused
project team
• Nearly 10x increase in number of complex
tests referred from Covance to LabCorp
• Enrollment for this 600 site, 37 country study was
completed 5 months ahead of original
stretch goal
• Executed two multi-year sole source
agreements with top 20 pharmaceutical companies
• Through EPDS, worked with over 50 companies
worldwide in pre-clinical, early clinical or both stages of
development
Solution Result

24
AGENDA
Company Overview
Update on “Wave One” Initiatives
2016 Highlights
Financial Strength
2017 Priorities

25
LONG-TERM REVENUE GROWTH1
$3.3
$3.6
$4.1
$4.5 $4.7
$5.0
$5.5 $5.7
$5.8
$8.5
$0.0
$2.0
$4.0
$6.0
$8.0
$10.0
2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016E
$6.0
~$9.4
CAGR 9%
41%
1. 2005-2014 revenue excludes Covance results. 2008 revenue includes a $7.5 million adjustment relating to certain historic
overpayments made by Medicare for claims submitted by a subsidiary of the Company
2. Guidance issued on October 26, 2016
($ Billions)
Covance
Drug
Development
LabCorp
Diagnostics
Midpoint of Guidance2
~10.5%

26
LONG-TERM ADJUSTED EPS GROWTH1,2
$3.01
$3.53
$4.45
$4.91
$5.24
$5.98
$6.37
$6.82 $6.95 $6.80
$7.91
$8.80
$0.00
$2.00
$4.00
$6.00
$8.00
$10.00
2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016E
CAGR 2%
1. EPS, as presented, represents adjusted, non-GAAP financial measures (excludes amortization, restructuring and other special
charges). Diluted EPS, as reported in the Company’s Annual Report were: $2.71 in 2005; $3.24 in 2006; $3.93 in 2007; $4.16 in
2008; $4.98 in 2009; $5.29 in 2010; $5.11 in 2011; $5.99 in 2012; $6.25 in 2013; $5.91 in 2014; and $4.34 in 2015
2. 2005-2014 figures exclude Covance results, and other items discussed in the Appendix
3. Guidance issued on October 26, 2016
Midpoint of
Guidance3
CAGR 13%
16%

27
FREE CASH FLOW1,2
$516
$567
$624
$748
$758 $759
$668
$617
$536
$727
$860
$0
$200
$400
$600
$800
$1,000
2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016E
1. 2006-2014 figures exclude Covance results
2. Operating Cash Flow and Free Cash Flow in 2011 excludes the $49.5 million Hunter Labs settlement
3. Guidance issued on October 26, 2016
($ Millions) 2006 – 2015 Average Free Cash Flow: $652 million
Midpoint of
Guidance3
28
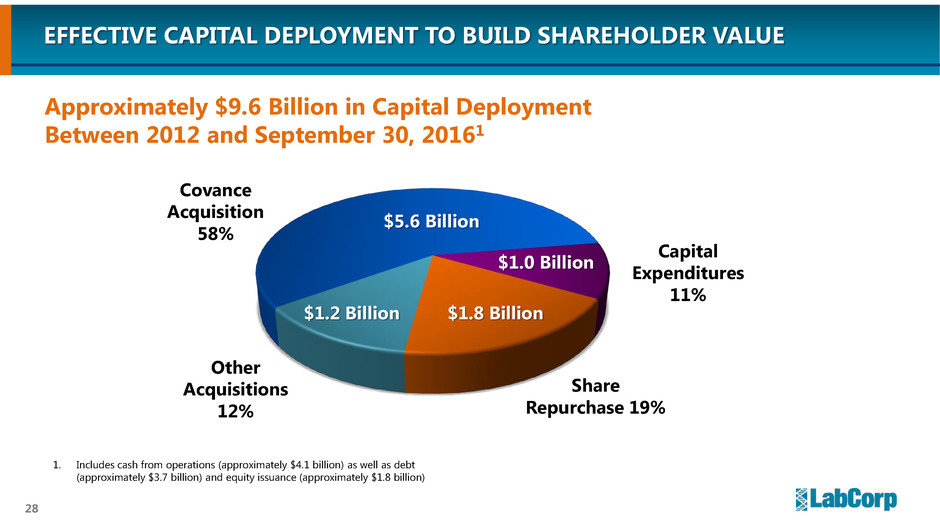
EFFECTIVE CAPITAL DEPLOYMENT TO BUILD SHAREHOLDER VALUE
Capital
Expenditures
11%
$1.8 Billion
Share
Repurchase 19%
Other
Acquisitions
12%
$5.6 Billion
$1.0 Billion
1. Includes cash from operations (approximately $4.1 billion) as well as debt
(approximately $3.7 billion) and equity issuance (approximately $1.8 billion)
Covance
Acquisition
58%
$1.2 Billion
Approximately $9.6 Billion in Capital Deployment
Between 2012 and September 30, 20161

29
AGENDA
Company Overview
Update on “Wave One” Initiatives
2016 Highlights
Financial Strength
2017 Priorities

30
2017 STRATEGIC PRIORITIES
Solution
Focus Fully-
Integrated
Organization
Return of Capital
to Shareholders -
Reinitiated Share
Repurchases

31
The Combination of Covance and LabCorp will:
OUR PURPOSE FOR CREATING
A WORLD LEADING LIFE SCIENCES COMPANY
• Accelerate long-term profitable growth through expanded
market opportunities
• Commercialize new business models in clinical care and
research settings
• Increase shareholder value, including return of capital
• Continue to enhance capabilities that guide patient care,
fulfilling our mission of improving health and improving lives

J.P. MORGAN
HEALTHCARE CONFERENCE
JANUARY 10, 2017 | SAN FRANCISCO, CA

33
APPENDIX

34
YEAR-TO-DATE PRO FORMA SEGMENT RESULTS1
(DOLLARS IN MILLIONS)
Pro forma results assume that the acquisition of Covance closed on January 1, 2015
Nine Months Nine Months
Ended 9/30/16 Ended 9/30/15 % Change
Net Revenue
LabCorp Diagnostics $4,922.1 $4,659.2 5.6%
Covance Drug Development $2,126.6 $1,937.3 9.8%
Total Net Revenue $7,048.2 $6,596.5 6.8%
Adjusted Operating Income2, 3
LabCorp Diagnostics $1,005.1 $942.0 6.7%
Adjusted Operating Margin 20.4% 20.2% 20 bps
Covance Drug Development $306.2 $260.9 17.4%
Adjusted Operating Margin 14.4% 13.5% 90 bps
Unallocated Corporate Expense ($108.9) ($98.5) (10.6%)
Total Adjusted Operating Income $1,202.4 $1,104.4 8.9%
Total Adjusted Operating Margin 17.1% 16.7% 40 bps
(1) The consolidated net revenue and adjusted operating income are presented net of inter-segment transaction eliminations
(2) Adjusted Operating Income excludes amortization, restructuring and special items
(3) See Reconciliation of non-GAAP Financial Measures in Appendix

35
RECONCILIATION OF NON-GAAP FINANCIAL MEASURES
The following consolidated results include Covance as of February 19, 2015;
prior to February 19, 2015, all consolidated results exclude Covance
LABORATORY CORPORATION OF AMERICA HOLDINGS
Reconciliation of Non-GAAP Financial Measures
(in millions, except per share data)
Adjusted Operating Income 2016 2015
Operating Income 989.0$ 760.3$
Acquisition-related costs 15.1 118.0
Restructuring and other special charges 48.6 59.9
Consulting fees and executive transition expenses 7.9 15.2
Wind-down of minimum volume contract operations 4.0 -
LaunchPad system implementation costs 7.1 -
Amortization of intangibles and other assets 130.7 120.6
Adjusted operating income 1,202.4$ 1,074.0$
A justed EPS
Diluted earnings per common share 5.25$ 3.29$
Restructuring and special items 0.56 1.83
Amortization expense 0.86 0.82
Adjusted EPS 6.67$ 5.94$
Nine Months Ended
September 30,

36
RECONCILIATION OF NON-GAAP FINANCIAL MEASURES
LABORATORY CORPORATION OF AMERICA HOLDINGS
Reconciliation of Non-GAAP Financial Measures
(in millions, except per share data)
Free Cash Flow: 2016 2015
Net cash provided by operating activities 727.0$ 597.8$
Less: Capital expenditures (204.6) (170.7)
Free cash flow 522.4$ 427.1$
Free Cash Flow, Excluding Acquisition Related Charges:
Net cash provided by operating activities 727.0$ 597.8$
Add back: Acquisition related charges - 153.5
Net cash provided by operating activities, excluding
acquisition related charges 727.0$ 751.3$
Less: Capital expenditures (204.6) (170.7)
Free cash flow, excluding acquisition related charges 522.4$ 580.6$
Nine Months Ended
September 30,
The following consolidated results include Covance as of February 19, 2015;
prior to February 19, 2015, all consolidated results exclude Covance

37
RECONCILIATION OF NON-GAAP FINANCIAL MEASURES – FOOTNOTES
1) During the third quarter of 2016, the Company recorded net restructuring and special items of $22.8 million. The charges included $14.1 million in severance
and other personnel costs along with $9.1 million in facility-related costs associated with facility closures and general integration initiatives. The Company
reversed previously established reserves of $0.2 million in unused facility-related costs and $0.2 million in unused personnel costs. The Company incurred
$5.9 million in fees and expenses associated with acquisitions completed during the quarter and incurred additional legal and other costs of $1.3 million
relating to the wind-down of its minimum volume contract operations. The Company also recorded $1.4 million in consulting expenses relating to fees
incurred as part of its Covance integration costs and compensation analysis, along with $0.5 million in short-term equity retention arrangements relating to
the acquisition of Covance and $3.4 million of accelerated equity and other final compensation relating to executive transition announced during the third
quarter and incurred $3.7 million of non-capitalized costs associated with the implementation of a major system as part of its LaunchPad business process
improvement initiative (all recorded in selling, general and administrative expenses). The Company also incurred $5.6 million of interest expense relating to
the early retirement of subsidiary indebtedness acquired as part of its recent acquisition of Sequenom. The after tax impact of these charges decreased net
earnings for the quarter ended September 30, 2016, by $28.5 million and diluted earnings per share by $0.27 ($28.5 million divided by 104.9 million shares).
During the first two quarters of 2016, the Company recorded net restructuring and other special charges of $25.8 million. The charges included $9.0 million
in severance and other personnel costs along with $21.6 million in facility-related costs associated with facility closures and general integration initiatives.
The Company reversed previously established reserves of $2.2 million in unused facility-related costs and $2.6 million in unused severance reserves. The
Company incurred $1.5 million in fees and expenses associated with completed acquisitions and incurred additional legal and other costs of $2.7 million
relating to the wind-down of its minimum volume contract operations. The Company also recorded $3.0 million in consulting expenses relating to fees
incurred as part of its Covance integration costs and compensation analysis, along with $1.8 million in short-term equity retention arrangements relating to
the acquisition of Covance and $4.1 million of accelerated equity compensation relating to the announced retirement of a Company executive and incurred
$4.8 million of non-capitalized costs associated with the implementation of a major system as part of its LaunchPad business process improvement initiative
(all recorded in selling, general and administrative expenses). In conjunction with certain international legal entity tax structuring, the Company recorded a
one-time tax liability of $1.1 million.
The after tax impact of these charges decreased net earnings for the nine months ended September 30, 2016, by $58.1 million and diluted earnings per share
by $0.56 ($58.1 million divided by 104.2 million shares).

38
RECONCILIATION OF NON-GAAP FINANCIAL MEASURES – FOOTNOTES
2) During the third quarter of 2015, the Company recorded net restructuring and special items of $26.3 million. The charges included $24.4 million in severance
and other personnel costs along with $2.2 million in facility-related costs associated with facility closures and general integration initiatives. The Company
reversed previously established reserves of $0.3 million in unused facility-related costs. The Company also recorded $3.5 million in consulting expenses
relating to fees incurred as part of its Covance integration costs, along with $1.4 million in short-term equity retention arrangements relating to the
acquisition of Covance (all recorded in selling, general and administrative expenses). In addition, the Company recorded a non-cash loss of $2.3 million, upon
the dissolution of one of its equity investments (recorded in other, net in the accompanying Consolidated Statements of Operations). The after tax impact of
these charges decreased net earnings for the quarter ended September 30, 2015, by $27.7 million and diluted earnings per share by $0.27 ($27.7 million
divided by 102.9 million shares).
During the first two quarters of 2015, the Company recorded net restructuring and other special charges of $33.5 million. The charges included $9.5 million
in severance and other personnel costs along with $9.8 million in costs associated with facility closures and general integration initiatives. The Company
reversed previously established reserves of $0.6 million in unused facility-related costs. In addition, the Company recorded asset impairments of $14.8 million
relating to lab and customer service applications that will no longer be used. The Company also recorded $11.6 million of consulting expenses relating to fees
incurred as part of its LaunchPad business process improvement initiative as well as Covance integration costs. In addition, the Company also expensed $2.9
million in short-term equity retention arrangements relating to the acquisition of Covance.
During the first quarter of 2015, the Company recorded $166.0 million of one-time costs associated with its acquisition of Covance. The costs included $79.5
million of Covance employee equity awards, change in control payments and short-term retention arrangements that were accelerated or triggered by the
acquisition transaction (recorded in selling, general and administrative expenses in the accompanying Consolidated Statements of Operations). The
acquisition costs also included advisor and legal fees of $33.9 million (recorded in selling, general and administrative expenses in the accompanying
Consolidated Statements of Operations), $15.2 million of deferred financing fees associated with the Company’s bridge loan facility as well as a make-whole
payment of $37.4 million paid to call Covance’s private placement debt outstanding at the purchase date (both amounts recorded in interest expense in the
accompanying Consolidated Statements of Operations).
The after tax impact of these charges decreased net earnings for the nine months ended September 30, 2015, by $182.5 million and diluted earnings per
share by $1.83 ($182.5 million divided by 99.7 million shares).

39
RECONCILIATION OF NON-GAAP FINANCIAL MEASURES – FOOTNOTES
3) The Company continues to grow the business through acquisitions and uses Adjusted EPS excluding amortization as a measure of operational
performance, growth and shareholder returns. The Company believes adjusting EPS for amortization provides investors with better insight
into the operating performance of the business. For the quarters ended September 30, 2016 and 2015, intangible amortization was $41.1
million and $44.9 million, respectively ($28.4 million and $31.3 million net of tax, respectively) and decreased EPS by $0.27 ($28.4 million
divided by 104.9 million shares) and $0.30 ($30.8 million divided by 102.9 million shares), respectively. For the nine months ended September
30, 2016 and 2015, intangible amortization was $130.7 million and $120.6 million, respectively ($89.4 million and $81.9 million net of tax,
respectively) and decreased EPS by $0.86 ($89.4 million divided by 104.2 million shares) and $0.82 ($81.9 million divided by 99.7 million
shares), respectively.
4) During the first quarter of 2015, the Company's operating cash flows were reduced due to payment of $153.5 million in acquisition-related
charges. These payments were comprised of $75.5 million in legal and advisor fees, $40.6 million in accelerated Covance employee equity
awards, and $37.4 million in make-whole payments triggered by calling Covance private placement notes outstanding at the time of the
transaction.

40
FOOTNOTES TO “LONG-TERM ADJUSTED EPS GROWTH” SLIDE
(1) EPS, as presented, represents adjusted, non-GAAP financial measures (excludes amortization, restructuring and other special charges).
Diluted EPS, as reported in the Company’s Annual Report were: $2.71 in 2005; $3.24 in 2006; $3.93 in 2007; $4.16 in 2008; $4.98 in 2009;
$5.29 in 2010; $5.11 in 2011; $5.99 in 2012; $6.25 in 2013; $5.91 in 2014; and $4.34 in 2015.
(2) 2005-2014 figures exclude Covance results. Excluding the $0.09 per diluted share impact of restructuring and other special charges and the
$0.21 per diluted share impact from amortization in 2005; excluding the $0.06 per diluted share impact of restructuring and other special
charges and the $0.23 per diluted share impact from amortization in 2006; excluding the $0.25 per diluted share impact of restructuring and
other special charges and the $0.27 per diluted share impact from amortization in 2007; excluding the $0.44 per diluted share impact of
restructuring and other special charges and the $0.31 per diluted share impact from amortization in 2008; excluding the ($0.09) per diluted
share impact of restructuring and other special charges and the $0.35 per diluted share impact from amortization in 2009; excluding the
$0.26 per diluted share impact of restructuring and other special charges and the $0.43 per diluted share impact from amortization in 2010;
excluding the $0.72 per diluted share impact of restructuring and other special charges, the $0.03 per diluted share impact from a loss on the
divestiture of assets and the $0.51 per diluted share impact from amortization in 2011; excluding the $0.29 per diluted share impact of
restructuring and other special charges and the $0.54 per diluted share impact from amortization in 2012; excluding the $0.15 per diluted
share impact of restructuring and other special charges and the $0.55 per diluted share impact from amortization in 2013; excluding the
$0.34 per diluted share impact of restructuring and other special charges and the $0.55 per diluted share impact from amortization in 2014;
and excluding the $2.44 per diluted share impact of restructuring and other special charges and the $1.13 per diluted share impact from
amortization in 2015.
(3) Guidance issued on October 26, 2016.
