Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - PTC THERAPEUTICS, INC. | exhibit991_2017-1x09ptctjp.htm |
| 8-K - 8-K - PTC THERAPEUTICS, INC. | jpmform8k.htm |

PageJan-17
PTC Therapeutics:
A Precision Medicine Platform
35th Annual JP Morgan Healthcare Conference
1

Page
Forward looking statements within the meaning of
The Private Securities Litigation Reform Act of 1995
Jan-17
All statements, other than those of historical fact, contained in this presentation, are forward-looking statements, including the information appearing under the
headings “2016 Preliminary Results” and “2017 Forecast” as well as statements regarding: the future expectations, plans and prospects for PTC; the timing and
outcome of PTC’s regulatory process in the U.S., including as related to a planned file over protest of the new drug application for TranslarnaTM for the treatment of
nmDMD, and in the European Economic Area (EEA), including as related to the European Commission’s determination as to renewal of the marketing authorization
for Translarna for the treatment of nmDMD and PTC’s plan to conduct an additional Phase 3 randomized trial of Translarna in nmDMD; the clinical utility and
potential advantages of Translarna; when top-line results of ACT CF will be available and reported and any statements related to the potential results of such trial;
PTC's estimates regarding the potential market opportunity for Translarna, including the size of eligible patient populations and PTC's ability to identify such
patients; PTC’s ability to maintain the current label under the marketing authorization in the EEA; the timing of, and PTC’s ability to, expand the approved product
label of Translarna for the treatment of nmDMD in the EEA, whether pursuant to its ongoing Phase 2 study of Translarna for nmDMD in pediatric patients, or
otherwise; the timing of, and PTC’s ability to, obtain additional marketing authorizations for Translarna in other territories, including the U.S., or for additional
indications, including nmCF; the timing, results and conduct of PTC’s clinical studies of PTC596 and Translarna for the treatment of other indications; further
advancement of the FIREFISH and SUNFISH studies under the joint SMA collaboration, including transition into the pivotal part of either study; the timing of a
milestone payment, if any, to PTC from Roche; PTC’s ability to increase commercial presence in countries where Translarna is currently available and commercially
expand into new territories; PTC’s strategy, future operations, future financial position, future revenues or projected costs; and the objectives of management. Other
forward-looking statements may be identified by the words “estimate,” “outlook,” “will,” “plan,” “expect,” “anticipate,” “believe,” “estimate,” “intend,” “may,” “potential,”
“possible,” “potential,” “would,” “could,” “should,” “continue,” and similar expressions.
PTC’s actual results, performance or achievements could differ materially from those expressed or implied by forward-looking statements it makes as a result of a
variety of risks and uncertainties, including those related to: the completion of PTC’s year-end audit; PTC’s ability to maintain its marketing authorization of
Translarna for the treatment of nmDMD in the EEA, including whether the European Commission determines to approve the renewal of such authorization and
whether the EMA determines in future annual renewal cycles that the benefit-risk balance of Translarna authorization supports renewal of such authorization; the
final design of the new nmDMD trial that PTC will undertake pursuant to the specific obligation associated with the marketing authorization (if renewed) and PTC’s
ability to enroll, fund and conduct such trial; the timing and outcome of future interactions PTC has with the FDA with respect to Translarna for the treatment of
nmDMD, including PTC's ability to resolve the matters set forth in the Refuse to File letter from the FDA or otherwise advance Translarna for the treatment of
nmDMD in the United States (whether pursuant to the file over protest process or otherwise), including whether PTC is required to perform additional clinical and
non-clinical trials at significant cost; the outcome of ongoing or future clinical trials or studies in Translarna, in particular ACT CF and the Translarna nmDMD
pediatric study; events during, or as a result of, the SUNFISH or FIREFISH studies that could delay or prevent further advancement of the SMA program, including
future actions or activities under the SMA joint development program; the eligible patient base and commercial potential of Translarna and PTC’s other product
candidates; the scope of regulatory approvals or authorizations for Translarna (if any), including labeling and other matters that could affect the availability or
commercial potential of Translarna; PTC’s ability to commercialize and commercially manufacture Translarna in general and specifically as a treatment for nmDMD;
the outcome of pricing and reimbursement negotiations in those territories in which PTC is authorized to sell Translarna; PTC’s scientific approach and general
development progress; the sufficiency of PTC’s cash resources and its ability to obtain adequate financing in the future for its foreseeable and unforeseeable
operating expenses and capital expenditures; and the factors discussed in the “Risk Factors” section of PTC’s most recent Quarterly Report on Form 10-Q as well
as any updates to these risk factors filed from time to time in PTC’s other filings with the SEC. You are urged to carefully consider all such factors.
As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. There
are no guarantees that Translarna will receive full regulatory approval in any territory or maintain its current marketing authorization in the EEA, or prove to be
commercially successful in general, or specifically with respect to the treatment of nmDMD. The forward-looking statements contained herein represent PTC’s views
only as of the date of this presentation and PTC does not undertake to update or revise any such forward-looking statements occurring after the date of this
presentation except as required by law.
2

Page
Our mission
To leverage our knowledge
of RNA biology to bring
novel therapeutics to patients
affected by rare and neglected
disorders
Jan-17 3

Page
Sustainable, growing DMD business enables innovative
research & development engine
Translarna™: Strong commercial growth for DMD ex-US
Development and regulatory milestones for Translarna
CHMP recommended renewal of approval of DMD*
File NDA over protest for DMD with the U.S. FDA in Q1 2017
ACT CF Phase 3 clinical trial results in Q1 2017
Proof-of-concept trials advance: MPS I, Aniridia & Dravet / CDKL5
Expanding pipeline
SMA: advancing into two pivotal studies
BMI1, Huntington’s and FD programs
Strong financial position with healthy balance sheet
Jan-17 3
*Annual renewal subject to approval by the European Commission

PageJan-17
Translarna™
Precision medicine platform: Realizing Translarna’s full value
through multiple indications
5
Page
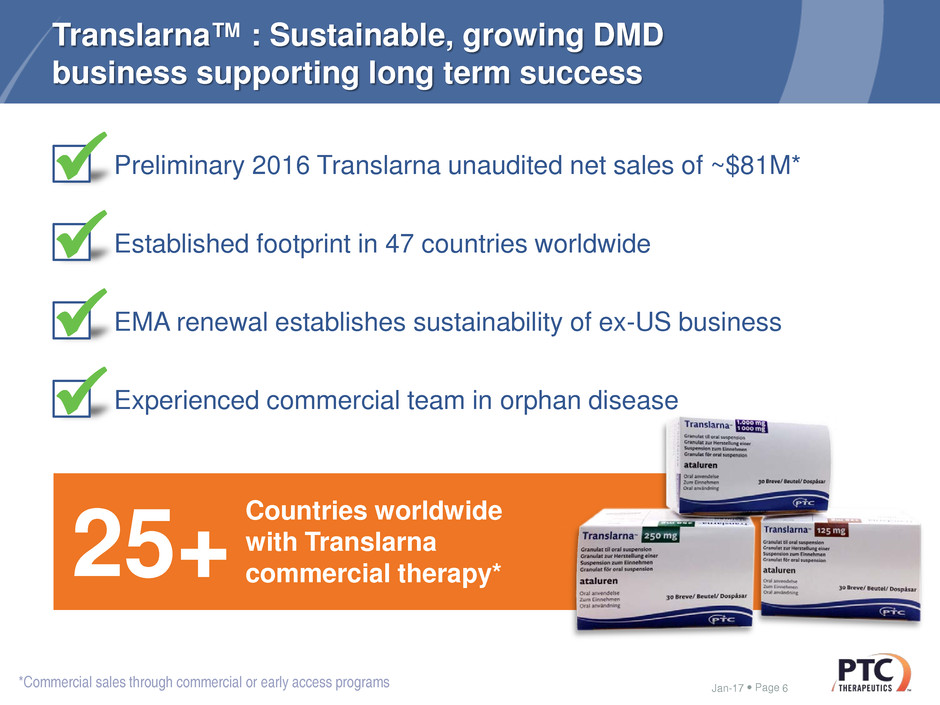
Translarna™ : Sustainable, growing DMD
business supporting long term success
Jan-17 6
25+ Countries worldwide with Translarnacommercial therapy*
Preliminary 2016 Translarna unaudited net sales of ~$81M*
Established footprint in 47 countries worldwide
EMA renewal establishes sustainability of ex-US business
Experienced commercial team in orphan disease
*Commercial sales through commercial or early access programs
Page
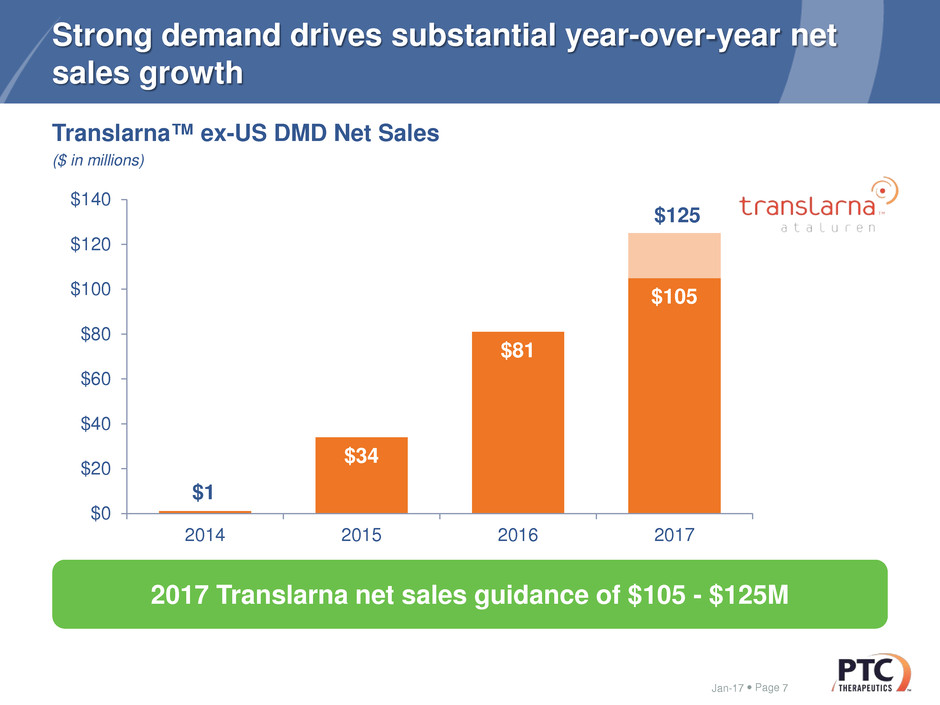
$1
$34
$81
$105
$0
$20
$40
$60
$80
$100
$120
$140
2014 2015 2016 2017
Strong demand drives substantial year-over-year net
sales growth
Jan-17 7
2017 Translarna net sales guidance of $105 - $125M
Translarna™ ex-US DMD Net Sales
($ in millions)
$125
Page
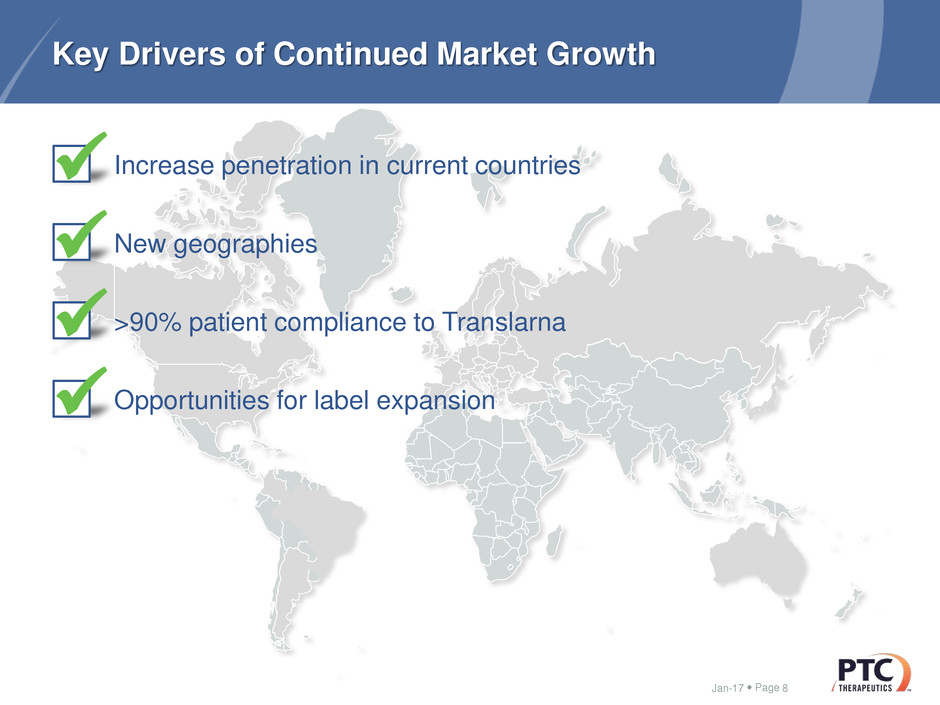
Key Drivers of Continued Market Growth
Jan-17 8
Increase penetration in current countries
New geographies
>90% patient compliance to Translarna
Opportunities for label expansion

PageJan-17 9
Europe
~2,000 pts.
Asia Pac:
~600 pts.
U.S. &
Canada:
~1,000 pts.
>7,000 addressable nmDMD patients worldwide
Lat Am/ Mex:
~2,000 pts.
~85% of patients outside the U.S.
MENA
~1,400 pts.
Internal estimates of potential patient population based on data derived from a variety of sources
and are subject to change

Page
Developing insight on patient prevalence
Jan-17 10
1:4,000 male
births are DMD
Birth rate varies
country to country
Global nmDMD prevalence drivers
Current diagnosed population >5yrs =
>7,000* patients
Typically patients not diagnosed <5 years of age
~10-15%
nonsense
mutation
Under current
label 5+yrs and
ambulatory~35%
~65%
Non-ambulatory
*Internal estimates of potential patient population based on data derived from a
variety of sources and are subject to change
Page
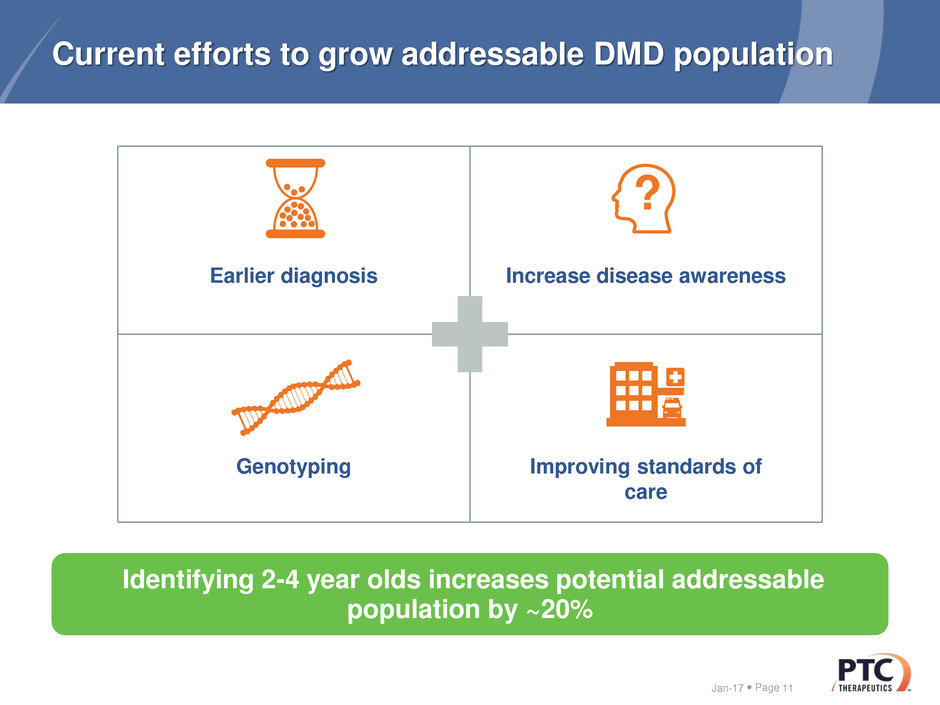
Current efforts to grow addressable DMD population
11
Earlier diagnosis Increase disease awareness
Genotyping Improving standards of
care
Jan-17
Identifying 2-4 year olds increases potential addressable
population by ~20%
?
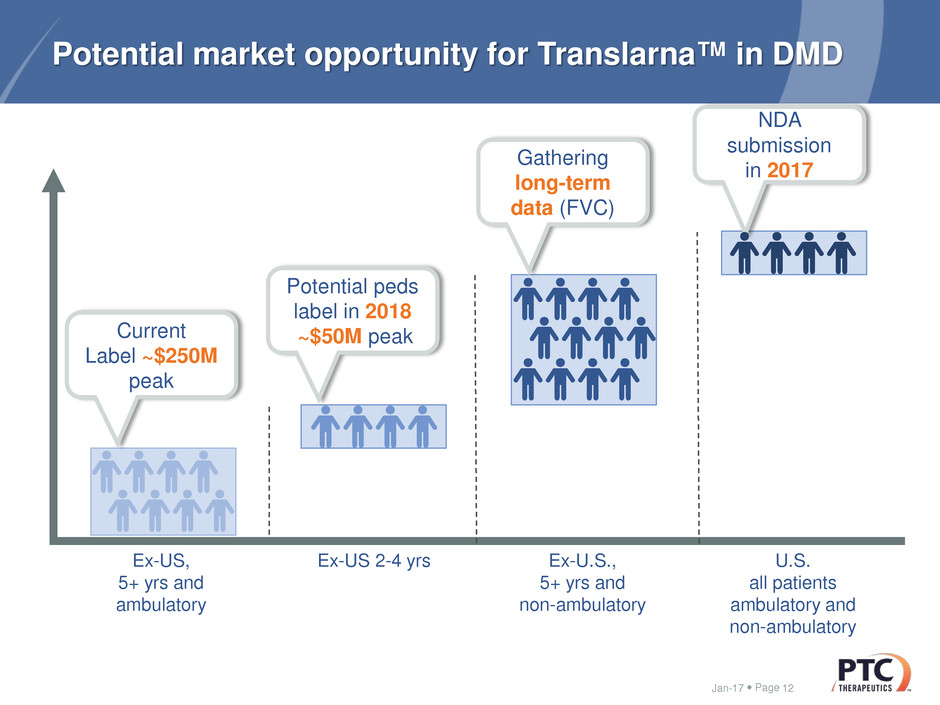
Page
Potential market opportunity for Translarna™ in DMD
Jan-17 12
Ex-US,
5+ yrs and
ambulatory
U.S.
all patients
ambulatory and
non-ambulatory
Ex-US 2-4 yrs
Potential peds
label in 2018
~$50M peak
NDA
submission
in 2017
Ex-U.S.,
5+ yrs and
non-ambulatory
Gathering
long-term
data (FVC)
Current
Label ~$250M
peak

Page
Translarna™ regulatory update: CHMP recommends
renewal of approval, NDA to be reviewed by FDA
EMA
Renewal of Translarna DMD approval recommended by CHMP
– Commitment to conduct post marketing DMD trial
FDA
Multiple interactions with FDA officials and advisors over the last several
months
Feedback indicated best path forward for full and fair review of the current
Translarna NDA is to file over protest rather than continue appeal of RTF
NDA to be supplemented with additional efficacy data utilized by CHMP in
renewal recommendation
Jan-17 13
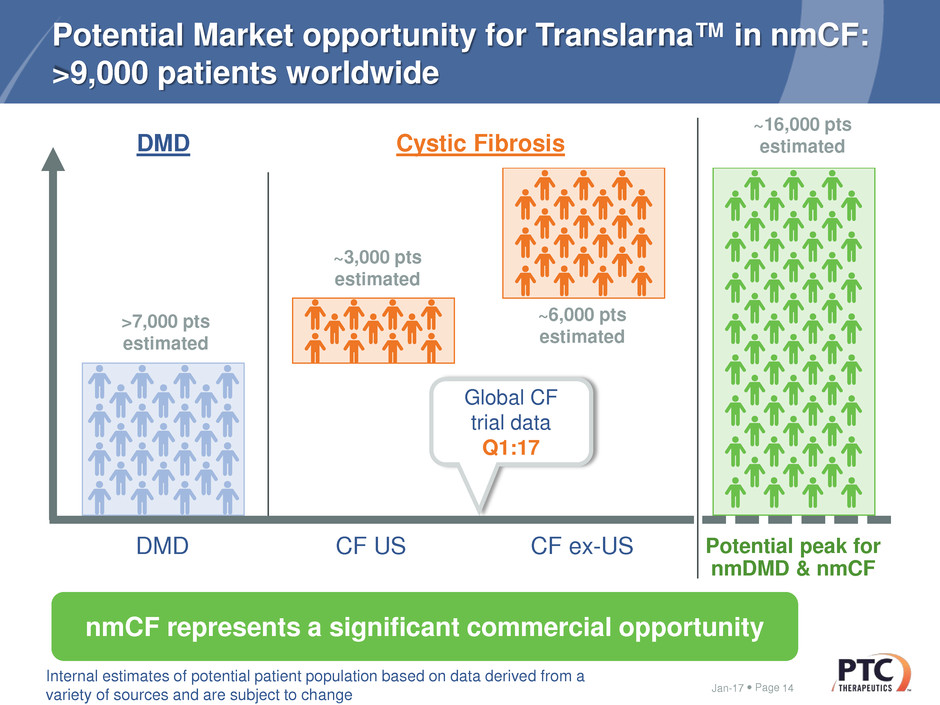
Page
Potential Market opportunity for Translarna™ in nmCF:
>9,000 patients worldwide
Jan-17 14
Cystic Fibrosis
nmCF represents a significant commercial opportunity
DMD CF US CF ex-US Potential peak for
nmDMD & nmCF
Global CF
trial data
Q1:17
DMD
~3,000 pts
estimated
~6,000 pts
estimated
Internal estimates of potential patient population based on data derived from a
variety of sources and are subject to change
>7,000 pts
estimated
~16,000 pts
estimated

Page
Cystic fibrosis is caused by a malfunction of the
CFTR protein due to different mutations
Jan-17
CFTR channel
through
cell membrane
CFTR protein
cftr gene in
cell nucleus
V
Reduced
SynthesisClass Normal
IV
Conductance
I
No Synthesis
III
Gating
II
Processing
Block
% CF Patients 2%10% ~5%70%
Compounds Translarna Kalydeco®Orkambi®
CFTR
CFTR
CFTR
CFTR
CFTR
CFTR
CFTR
CFTR
CFTR
CFTR
CFTR
CFTR
CFTR
15
Translarna™ is only compound in development targeting Class I CF

Page
Slowing disease progression provides clinically meaningful benefits
to CF patients over the long-term
Translarna™ CF data is comparable to other
approved CF correctors***
Jan-17
0%
2%
4%
6%
8%
10%
12%
14%
A
bs
ol
ut
e
Δ
pp
FE
V
1
Class III
Class I
Class II
* Post hoc analysis; relative Δ 5.7% ** Post hoc analysis; relative Δ 8.4% *** Tested in separate trials
abs. Δ
3.5% *
abs. Δ
5.4% **
Translarna
non TOBI®
subgroup
Translarna
non TOBI® <18
subgroup
abs. Δ
12.5%
ENVISION
(Kalydeco®)
abs. Δ
10.7%
KONNECTION
(Kalydeco®)
abs. Δ
2.1%
KONDUCT
(Kalydeco®)
abs. Δ
3.0%
TRAFFIC and
TRANSPORT
(Orkambi®)
CFTR
CFTR
CFTR
CFTR
CFTR
CFTR
16
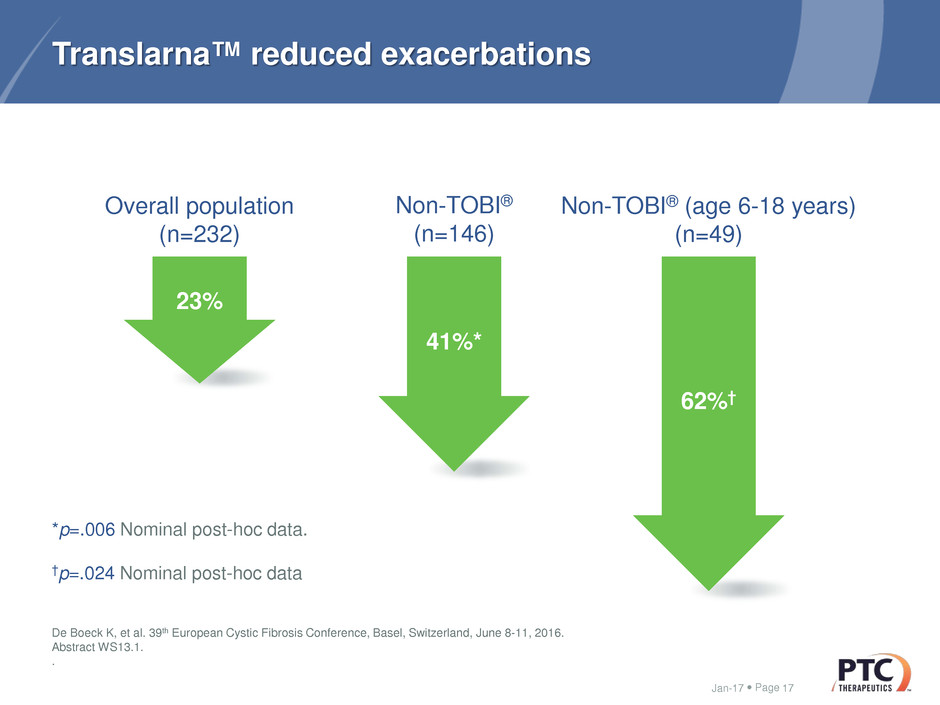
Page
Translarna™ reduced exacerbations
Jan-17 17
62%†
Overall population
(n=232)
Non-TOBI®
(n=146)
Non-TOBI® (age 6-18 years)
(n=49)
*p=.006 Nominal post-hoc data.
†p=.024 Nominal post-hoc data
De Boeck K, et al. 39th European Cystic Fibrosis Conference, Basel, Switzerland, June 8-11, 2016.
Abstract WS13.1.
.
41%*
23%

Page
Length of Trial TranslarnaTM (n) Placebo (n)
48 weeks 140 140
Primary outcome measure:
% predicted FEV1
Secondary outcome measure:
Pulmonary exacerbation rate
CFQ-R
BMI
ACT CF (Ataluren Confirmatory Trial) study:
Topline data expected late Q1 2017
Eligibility Criteria Stratification
Nonsense mutation CF
≥6 years
FEV1 ≥40% and ≤90% predicted
No chronic inhaled TOBI
Age: ˂18 vs ≥18 years
Inhaled antibiotic use (yes vs no)
Baseline FEV1: ≥65% vs <65% of
predicted
Trial powered with 90% confidence for 3% absolute Δ FEV1
Jan-17 18

Page
Ion channel
disorders
Publications: 1
Genetically defined
epilepsy
Metabolic
disorders
Publications: 10
Muscle
disorders
Publications: 6
Eye
disorders
Publications: 3
Skin
disorders
Publications: 2
Neurological
disorders
Publications: 8
Pulmonary
disorders
Publications: 10
Translarna™: Realizing a new paradigm for the treatment
of rare diseases, progressing proof of concept studies
Jan-17 19
~ 40 publications in many disease models
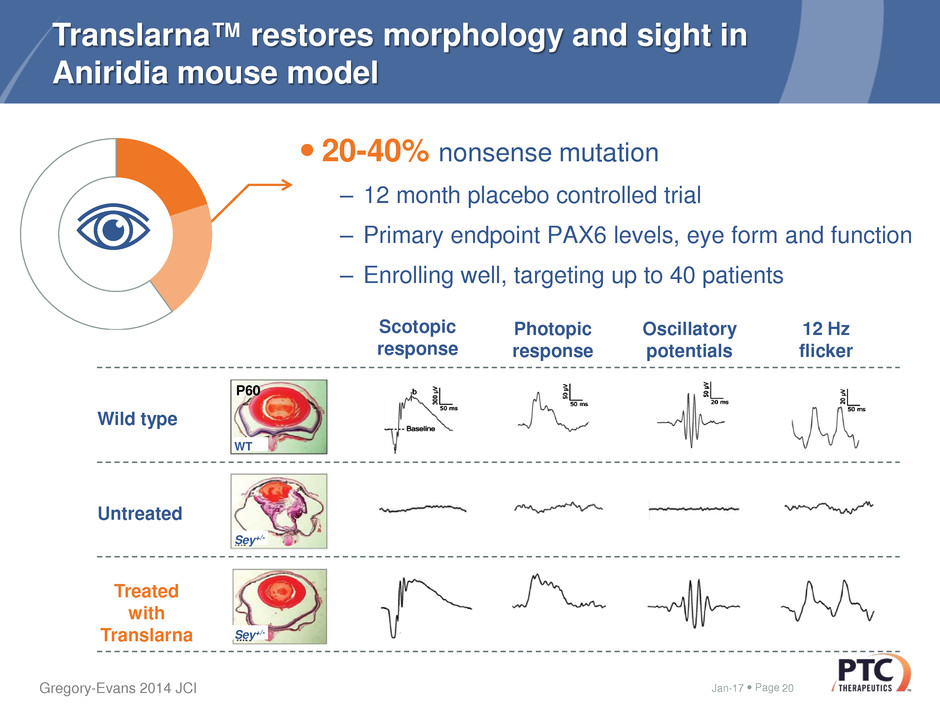
Page
Translarna™ restores morphology and sight in
Aniridia mouse model
20-40% nonsense mutation
– 12 month placebo controlled trial
– Primary endpoint PAX6 levels, eye form and function
– Enrolling well, targeting up to 40 patients
Jan-17 20
Untreated
Wild type
Treated
with
Translarna
Sey+/-
Sey+/-
Scotopic
response
Photopic
response
Oscillatory
potentials
12 Hz
flicker
WT
P60
Gregory-Evans 2014 JCI
Page
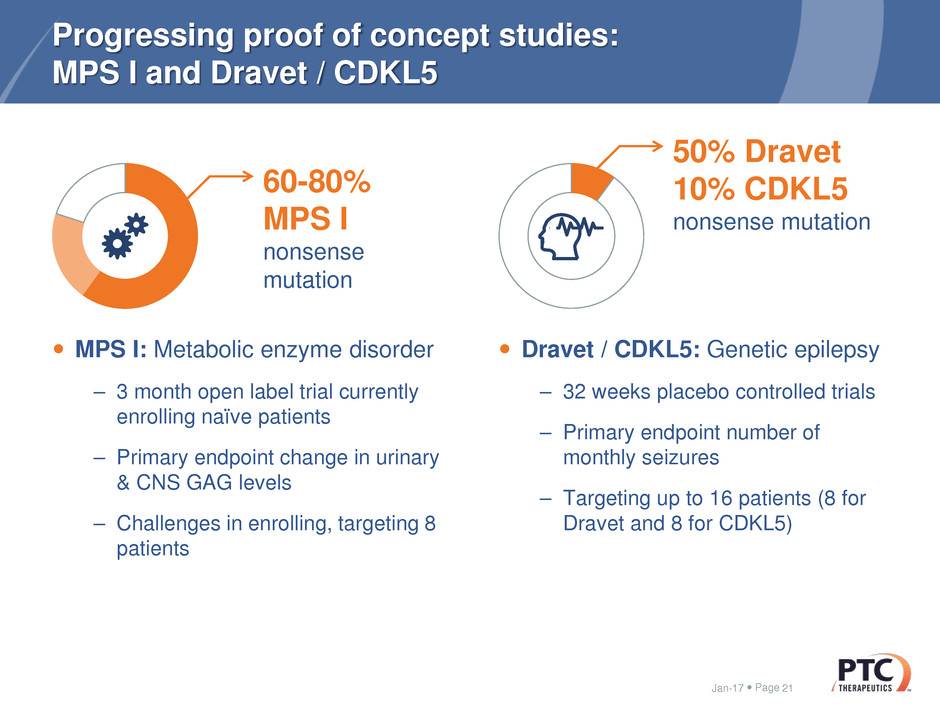
Progressing proof of concept studies:
MPS I and Dravet / CDKL5
MPS I: Metabolic enzyme disorder
– 3 month open label trial currently
enrolling naïve patients
– Primary endpoint change in urinary
& CNS GAG levels
– Challenges in enrolling, targeting 8
patients
Dravet / CDKL5: Genetic epilepsy
– 32 weeks placebo controlled trials
– Primary endpoint number of
monthly seizures
– Targeting up to 16 patients (8 for
Dravet and 8 for CDKL5)
Jan-17 21
60-80%
MPS I
nonsense
mutation
50% Dravet
10% CDKL5
nonsense mutation

PageJan-17
Small molecule splicing technology platform
Multiple on-going programs
22

Page
The spinal muscular atrophy program validates PTC’s
small molecule splicing platform
Jan-17 23
Target splicing event to restore or
reduce proteinSplicing
Tumor Resistance – MCL1
SMA - SMN2
FD – IKBKAP
HD – HTT

Page
Pivotal portion of both Sunfish & Firefish trials
expected to begin in 2017
SUNFISH
Clinical study in SMA type 2/3 patients initiated in November
– Enrolling 36 patients for dose escalation phase, placebo controlled 2:1
– Pivotal phase will be 150 patients, placebo controlled 1:1, endpoint of total motor
function measure (MFM-32) at 12 months
FIREFISH
Clinical study in SMA type 1 patients <7 months old
– Enrolling 8 patients for dose escalation phase
– Pivotal phase will enroll 40 babies, open label
endpoint of sitting as measured by Bayley infant scale
Jan-17 24
Two pivotal studies expected to begin in 2017
Page
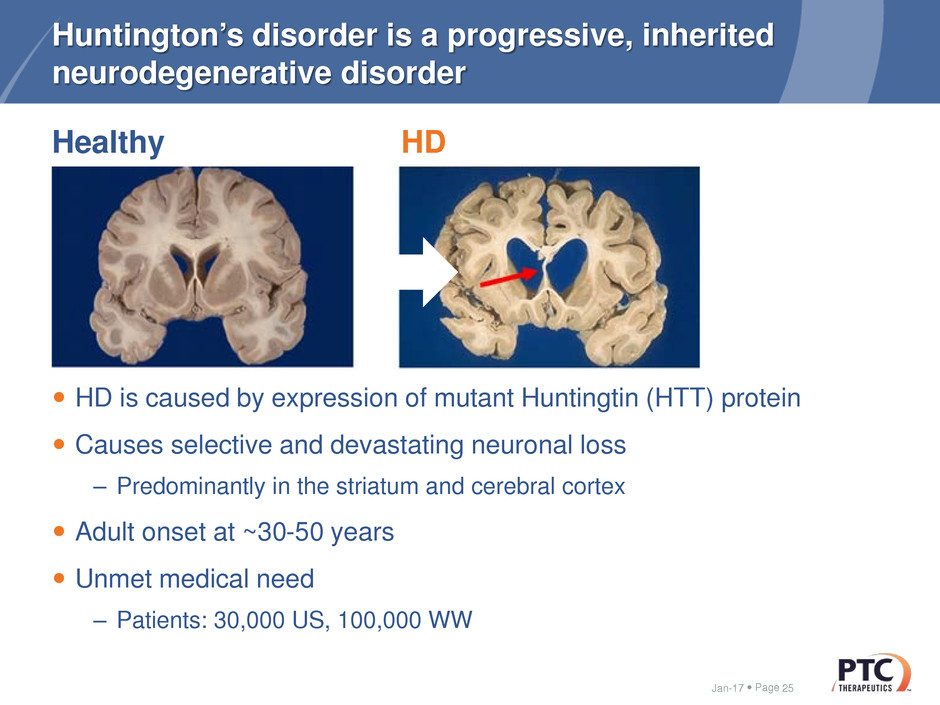
HD
Huntington’s disorder is a progressive, inherited
neurodegenerative disorder
HD is caused by expression of mutant Huntingtin (HTT) protein
Causes selective and devastating neuronal loss
– Predominantly in the striatum and cerebral cortex
Adult onset at ~30-50 years
Unmet medical need
– Patients: 30,000 US, 100,000 WW
25
Healthy
Jan-17
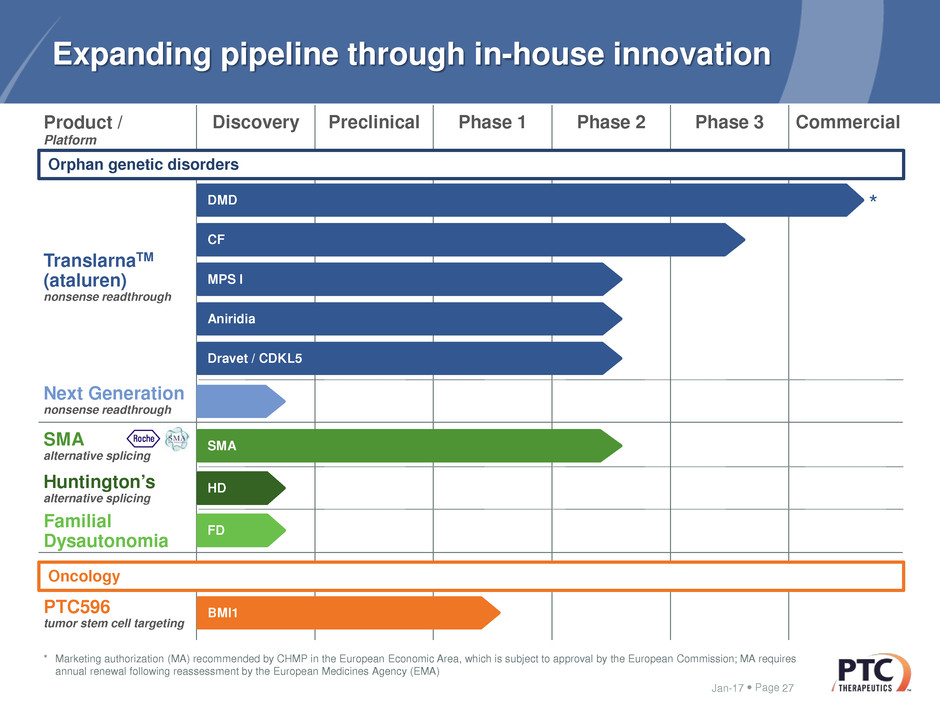
Page
Multiple orally bioavailable, brain penetrant compounds being
optimized
– Lowers HTT by altering splicing
Opportunity to provide first-in-class disease modifying, HTT lowering
small molecule therapy
– Potential to circumvent delivery and distribution challenges seen with the
current modalities
HD program in Lead Optimization – showcases
PTC’s alternative splicing platform
Jan-17 26Jan-17 3
V e h ic le 1 0
0
2 0
4 0
6 0
8 0
1 0 0
S tr ia tu m
%
H
T
T
l
o
w
e
ri
n
g
P 3 0 1 9 0 5 (m g /k g )
Ve h ic le 1 0
0
2 0
4 0
6 0
8 0
1 0 0
C o r te x
%
H
T
T
l
o
w
e
ri
n
g
P 3 0 1 9 0 5 (m g /k g )
V e h ic le 1 0
0
2 0
4 0
6 0
8 0
1 0 0
S k in
%
H
T
T
l
o
w
e
ri
n
g
P 3 0 1 9 0 5 (m g /k g )
Page
Expanding pipeline through in-house innovation
Jan-17 27
Product /
Platform
Discovery Preclinical Phase 1 Phase 2 Phase 3
DMD
CF
BMI1
SMA
HD
TranslarnaTM
(ataluren)
nonsense readthrough
SMA
alternative splicing
PTC596
tumor stem cell targeting
Huntington’s
alternative splicing
Commercial
Aniridia
Oncology
Orphan genetic disorders
MPS I
Next Generation
nonsense readthrough
Dravet / CDKL5
* Marketing authorization (MA) recommended by CHMP in the European Economic Area, which is subject to approval by the European Commission; MA requires
annual renewal following reassessment by the European Medicines Agency (EMA)
*
FDFamilial
Dysautonomia
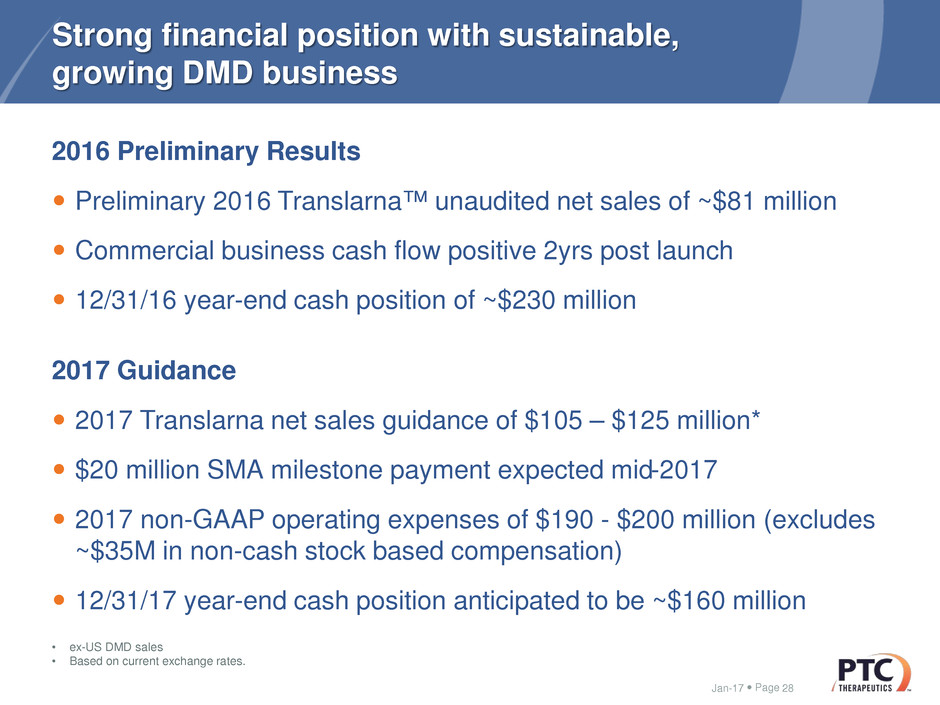
Page
Strong financial position with sustainable,
growing DMD business
2016 Preliminary Results
Preliminary 2016 Translarna™ unaudited net sales of ~$81 million
Commercial business cash flow positive 2yrs post launch
12/31/16 year-end cash position of ~$230 million
2017 Guidance
2017 Translarna net sales guidance of $105 – $125 million*
$20 million SMA milestone payment expected mid-2017
2017 non-GAAP operating expenses of $190 - $200 million (excludes
~$35M in non-cash stock based compensation)
12/31/17 year-end cash position anticipated to be ~$160 million
Jan-17 28
• ex-US DMD sales
• Based on current exchange rates.

Page
PTC Near-term milestones
Jan-17 29
Phase 3
ACT CF data
in late Q1:17
SMA – 2
pivotal
studies
Continued
Translarna™
net sales
growth
ex-US
Additional
pipeline
progress
US FDA
regulatory
outcome for
Translarna™
in DMD
