Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Karyopharm Therapeutics Inc. | d285942d8k.htm |
Exhibit 99.1

Corporate Presentation January 2017 KARYOPHARM THERAPEUTICS INC. Targetting Disease at the Nuclear Pore

Forward-looking Statements • This presentation contains forward-looking statements within the meaning of the “safe harbor” provisions of The Private Securities Litigation Reform Act of 1995. • Such forward-looking statements include those regarding the therapeutic potential of and potential clinical development plans and commercialization for Karyopharm’s drug candidates, including the timing of initiation of certain trials and of the reporting of data from such trials, and assumptions of management regarding strategic and financial expectations and projections. • Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the company’s current expectations. For example, there can be no guarantee that any of Karyopharm’s SINE™ compounds, including selinexor (KPT-330) and KPT-8602, Karyopharm’s second generation SINE™ compound, or KPT-9274, Karyopharm’s first-in-class oral dual inhibitor of PAK4 and NAMPT, or any other drug candidate Karyopharm is developing, will successfully complete necessary preclinical and clinical development phases or that development of any of Karyopharm’s drug candidates will continue. Further, there can be no guarantee that any positive developments in Karyopharm’s drug candidate portfolio will result in stock price appreciation. In addition, even if Karyopharm receives marketing approval for selinexor or another drug candidate, there can be no assurance that Karyopharm will be able to successfully commercialize that drug candidate. Management’s expectations and, therefore, any forward-looking statements in this presentation could also be affected by risks and uncertainties relating to a number of other factors, many of which are beyond Karyopharm’s control, including the following: Karyopharm’s results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the U.S. Food and Drug Administration and other regulatory authorities, investigational review boards at clinical trial sites and publication review bodies; Karyopharm’s ability to obtain and maintain requisite regulatory approvals and to enroll patients in its clinical trials; unplanned cash requirements and expenditures; development of drug candidates by Karyopharm’s competitors for diseases for which Karyopharm is currently developing its drug candidates; and Karyopharm’s ability to obtain, maintain and enforce patent and other intellectual property protection for any drug candidates it is developing. • These and other risks, including those which may impact management’s expectations, are described in greater detail under the heading “Risk Factors” in Karyopharm’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2016, which is on file with the Securities and Exchange Commission, and in subsequent filings filed by Karyopharm with the Securities and Exchange Commission. • Any forward-looking statements contained in this presentation are for informational purposes only and speak only as of the date hereof. Other than as is required by law, Karyopharm expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. • Karyopharm’s website is http://www.karyopharm.com. Karyopharm regularly uses its website to post information regarding its business, drug development programs and governance. Karyopharm encourages investors to use www.karyopharm.com, particularly the information in the section entitled “Investors,” as a source of information about Karyopharm. References to www.karyopharm.com in this presentation are not intended to, nor shall they be deemed to, incorporate information on www.karyopharm.com into this presentation by reference. • Unless otherwise noted, this presentation contains data that are interim and unaudited based on site reports. ©2017 Karyopharm Therapeutics Inc. 2
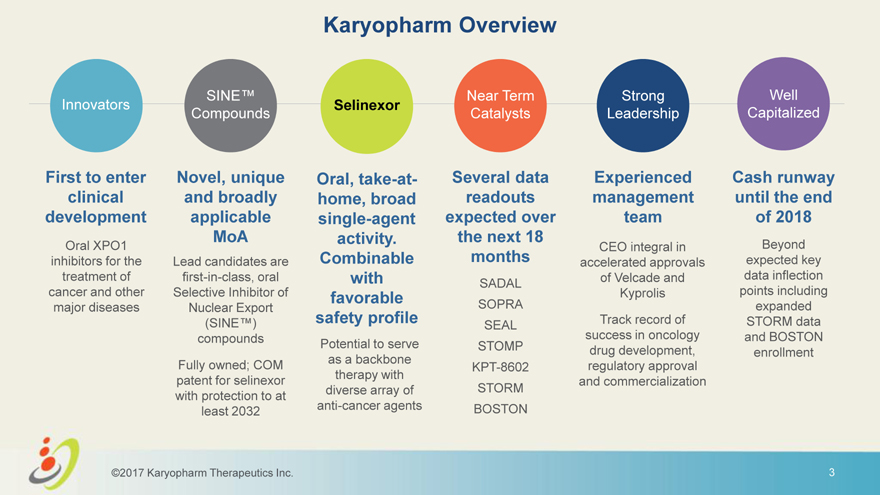
Karyopharm Overview SINE™ Near Term Strong Well Innovators Selinexor Compounds Catalysts Leadership Capitalized First to enter Novel, unique Oral, take-at- Several data Experienced Cash runway clinical and broadly home, broad readouts management until the end development applicable single-agent expected over team of 2018 MoA activity. the next 18 Oral XPO1 CEO integral in Beyond inhibitors for the Lead candidates are Combinable months accelerated approvals expected key treatment of first-in-class, oral with of Velcade and data inflection SADAL cancer and other Selective Inhibitor of favorable Kyprolis points including major diseases Nuclear Export safety profile SOPRA expanded (SINE™) Track record of STORM data SEAL compounds success in oncology and BOSTON Potential to serve STOMP drug development, enrollment as a backbone Fully owned; COM KPT-8602 regulatory approval therapy with patent for selinexor and commercialization diverse array of STORM with protection to at anti-cancer agents BOSTON least 2032 ©2017 Karyopharm Therapeutics Inc. 3
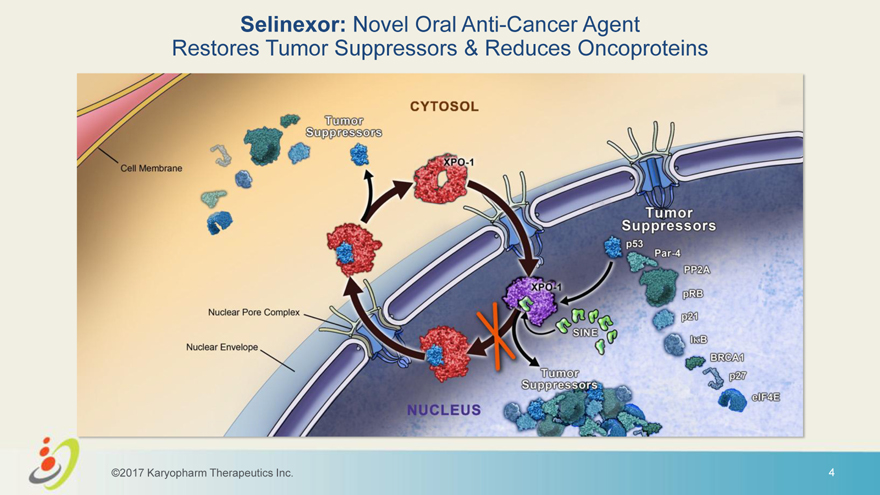
Selinexor: Novel Oral Anti-Cancer Agent Restores Tumor Suppressors & Reduces Oncoproteins ©2017 Karyopharm Therapeutics Inc. 4 Nucleus Cytosol
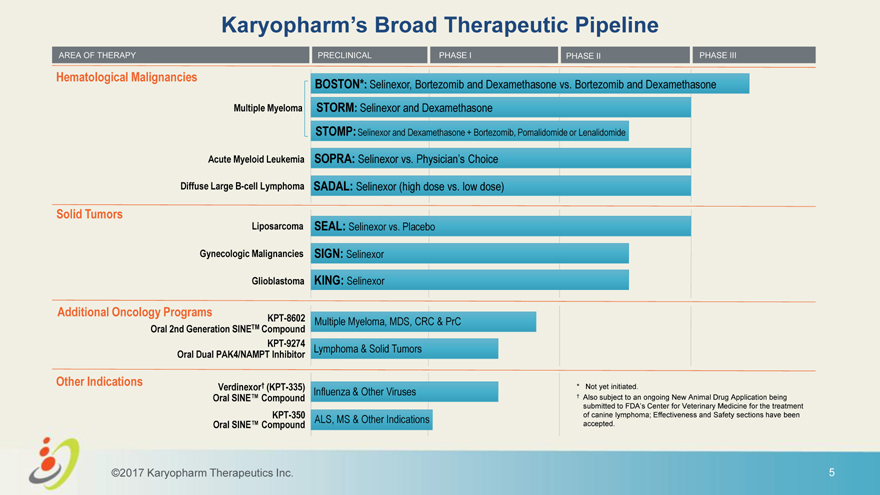
Karyopharm’s Broad Therapeutic Pipeline AREA OF THERAPY PRECLINICAL PHASE I PHASE II PHASE III Hematological Malignancies BOSTON*: Selinexor, Bortezomib and Dexamethasone vs. Bortezomib and Dexamethasone Multiple Myeloma STORM: Selinexor and Dexamethasone STOMP: Selinexor and Dexamethasone + Bortezomib, Pomalidomide or Lenalidomide Acute Myeloid Leukemia SOPRA: Selinexor vs. Physician’s Choice Diffuse Large B-cell Lymphoma SADAL: Selinexor (high dose vs. low dose) Solid Tumors Liposarcoma SEAL: Selinexor vs. Placebo Gynecologic Malignancies SIGN: Selinexor Glioblastoma KING: Selinexor Additional Oncology Programs KPT-8602 Multiple Myeloma, MDS, CRC & PrC Oral 2nd Generation SINETM Compound KPT-9274 Lymphoma & Solid Tumors Oral Dual PAK4/NAMPT Inhibitor Other Indications Verdinexor† (KPT-335) * Not yet initiated. Influenza & Other Viruses Oral SINE™ Compound † Also subject to an ongoing New Animal Drug Application being submitted to FDA’s Center for Veterinary Medicine for the treatment KPT-350 ALS, MS & Other Indications of canine lymphoma; Effectiveness and Safety sections have been Oral SINE™ Compound accepted. ©2017 Karyopharm Therapeutics Inc. 5

Selinexor in Myeloma: First Oral Agent with Novel Mechanism and Single Agent Activity Since the IMiDs
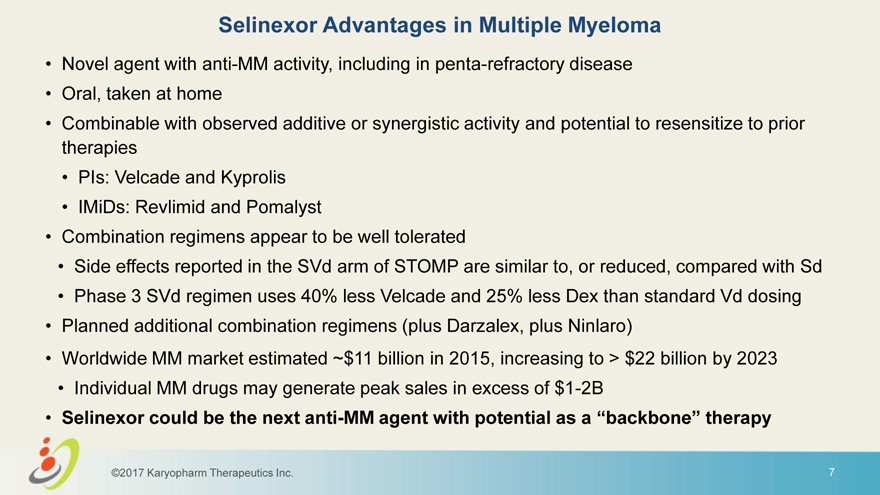
Selinexor Advantages in Multiple Myeloma • Novel agent with anti-MM activity, including in penta-refractory disease • Oral, taken at home • Combinable with observed additive or synergistic activity and potential to resensitize to prior therapies • PIs: Velcade and Kyprolis • IMiDs: Revlimid and Pomalyst • Combination regimens appear to be well tolerated • Side effects reported in the SVd arm of STOMP are similar to, or reduced, compared with Sd • Phase 3 SVd regimen uses 40% less Velcade and 25% less Dex than standard Vd dosing • Planned additional combination regimens (plus Darzalex, plus Ninlaro) • Worldwide MM market estimated ~$11 billion in 2015, increasing to > $22 billion by 2023 • Individual MM drugs may generate peak sales in excess of $1-2B • Selinexor could be the next anti-MM agent with potential as a “backbone” therapy ©2017 Karyopharm Therapeutics Inc. 7
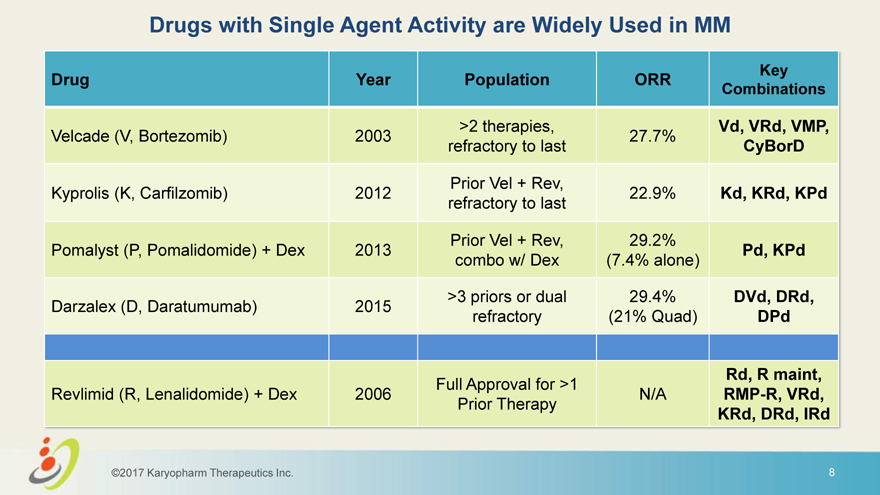
Drugs with Single Agent Activity are Widely Used in MM Key Drug Year Population ORR Combinations >2 therapies, Vd, VRd, VMP, Velcade (V, Bortezomib) 2003 27.7% refractory to last CyBorD Prior Vel + Rev, Kyprolis (K, Carfilzomib) 2012 22.9% Kd, KRd, KPd refractory to last Prior Vel + Rev, 29.2% Pomalyst (P, Pomalidomide) + Dex 2013 Pd, KPd combo w/ Dex (7.4% alone) >3 priors or dual 29.4% DVd, DRd, Darzalex (D, Daratumumab) 2015 refractory (21% Quad) DPd Rd, R maint, Full Approval for >1 Revlimid (R, Lenalidomide) + Dex 2006 N/A RMP-R, VRd, Prior Therapy KRd, DRd, IRd ©2017 Karyopharm Therapeutics Inc. 8
STORM: Selinexor in Refractory Myeloma: Quad and Penta Populations
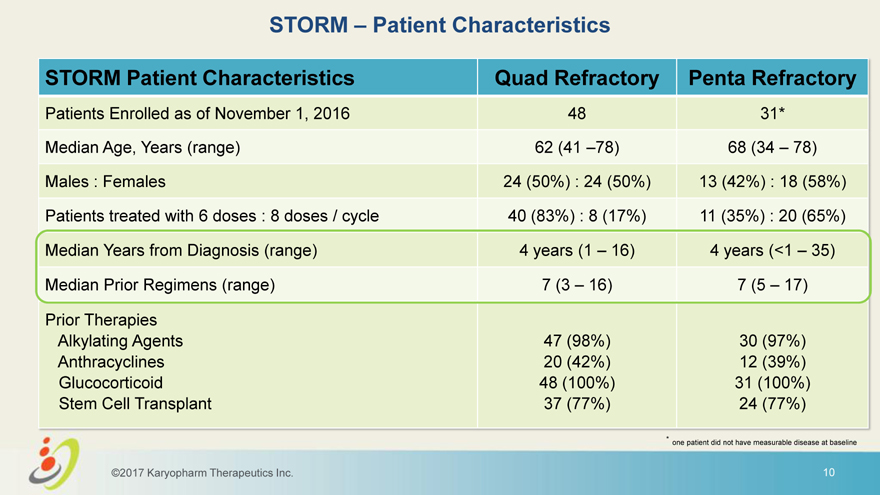
STORM – Patient Characteristics STORM Patient Characteristics Quad Refractory Penta Refractory Patients Enrolled as of November 1, 2016 48 31* Median Age, Years (range) 62 (41 –78) 68 (34 – 78) Males : Females 24 (50%) : 24 (50%) 13 (42%) : 18 (58%) Patients treated with 6 doses : 8 doses / cycle 40 (83%) : 8 (17%) 11 (35%) : 20 (65%) Median Years from Diagnosis (range) 4 years (1 – 16) 4 years (<1 – 35) Median Prior Regimens (range) 7 (3 – 16) 7 (5 – 17) Prior Therapies Alkylating Agents 47 (98%) 30 (97%) Anthracyclines 20 (42%) 12 (39%) Glucocorticoid 48 (100%) 31 (100%) Stem Cell Transplant 37 (77%) 24 (77%) * one patient did not have measurable disease at baseline ©2017 Karyopharm Therapeutics Inc. 10
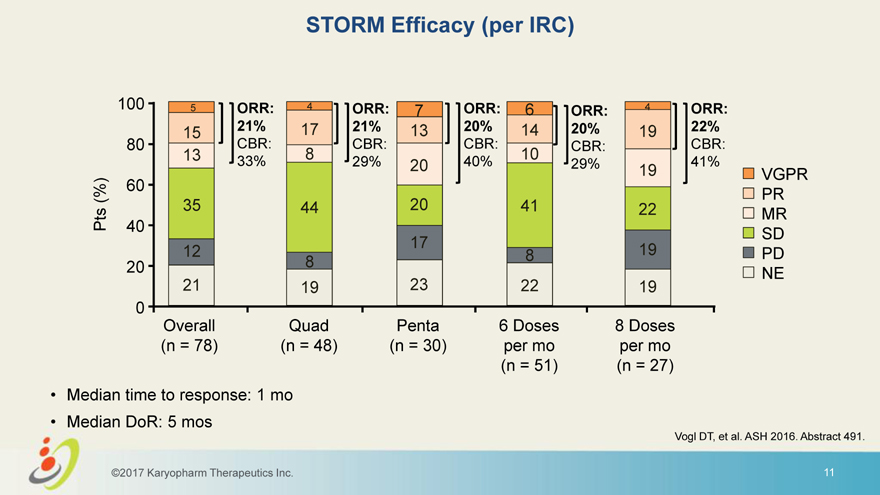
STORM Efficacy (per IRC) 100 5 ORR: 4 ORR: 7 ORR: 6 ORR: 4 ORR: 21% 17 21% 13 20% 14 20% 19 22% 15 80 CBR: CBR: CBR: CBR: CBR: 13 8 10 33% 29% 20 40% 29% 41% 19 VGPR 60 (%) PR Pts 35 44 20 41 22 MR 40 SD 17 19 12 8 PD 20 8 NE 21 19 23 22 19 0 Overall Quad Penta 6 Doses 8 Doses (n = 78) (n = 48) (n = 30) per mo per mo (n = 51) (n = 27) • Median time to response: 1 mo • Median DoR: 5 mos Vogl DT, et al. ASH 2016. Abstract 491. ©2017 Karyopharm Therapeutics Inc. 11
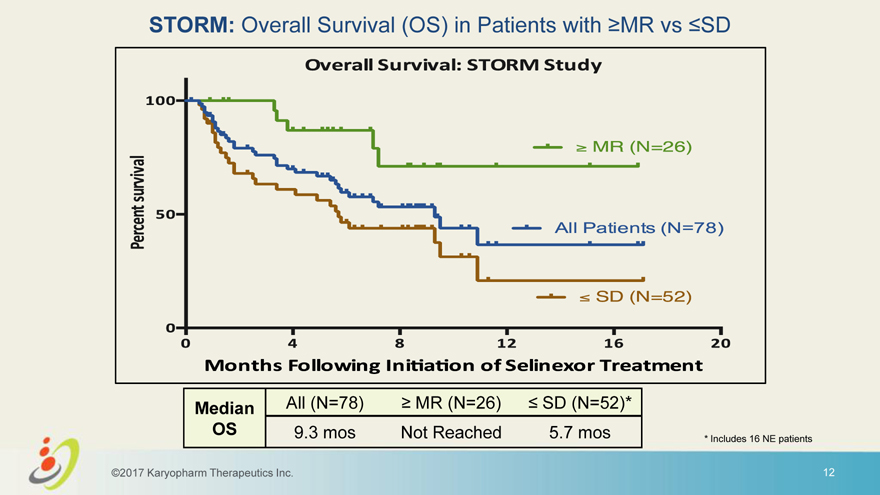
STORM: Overall Survival (OS) in Patients with MR vs SD Median All (N=78) MR (N=26) SD (N=52)* OS 9.3 mos Not Reached 5.7 mos * Includes 16 NE patients ©2017 Karyopharm Therapeutics Inc. 12 Storm study
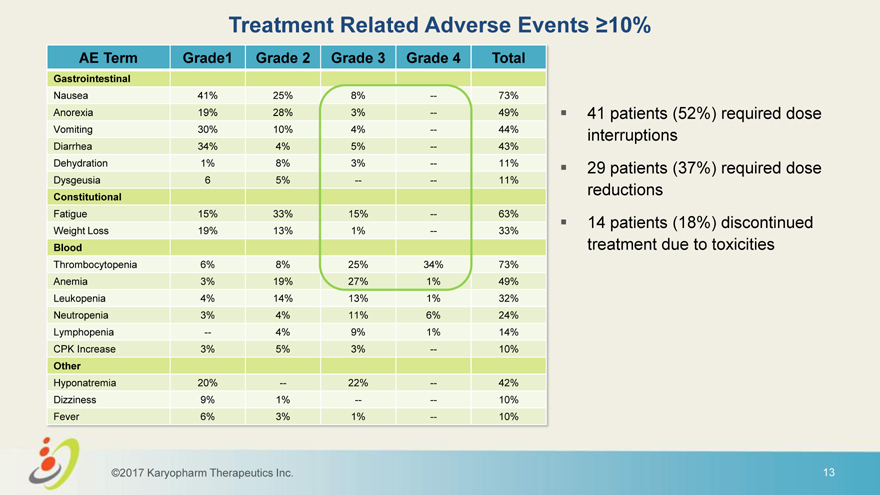
Treatment Related Adverse Events 10% AE Term Grade1 Grade 2 Grade 3 Grade 4 Total Gastrointestinal Nausea 41% 25% 8% — 73% Anorexia 19% 28% 3% — 49% 41 patients (52%) required dose Vomiting 30% 10% 4% — 44% interruptions Diarrhea 34% 4% 5% — 43% Dehydration 1% 8% 3% — 11% 29 patients (37%) required dose Dysgeusia 6 5% — — 11% reductions Constitutional Fatigue 15% 33% 15% — 63% 14 patients (18%) discontinued Weight Loss 19% 13% 1% — 33% Blood treatment due to toxicities Thrombocytopenia 6% 8% 25% 34% 73% Anemia 3% 19% 27% 1% 49% Leukopenia 4% 14% 13% 1% 32% Neutropenia 3% 4% 11% 6% 24% Lymphopenia — 4% 9% 1% 14% CPK Increase 3% 5% 3% — 10% Other Hyponatremia 20% — 22% — 42% Dizziness 9% 1% — — 10% Fever 6% 3% 1% — 10% ©2017 Karyopharm Therapeutics Inc. 13
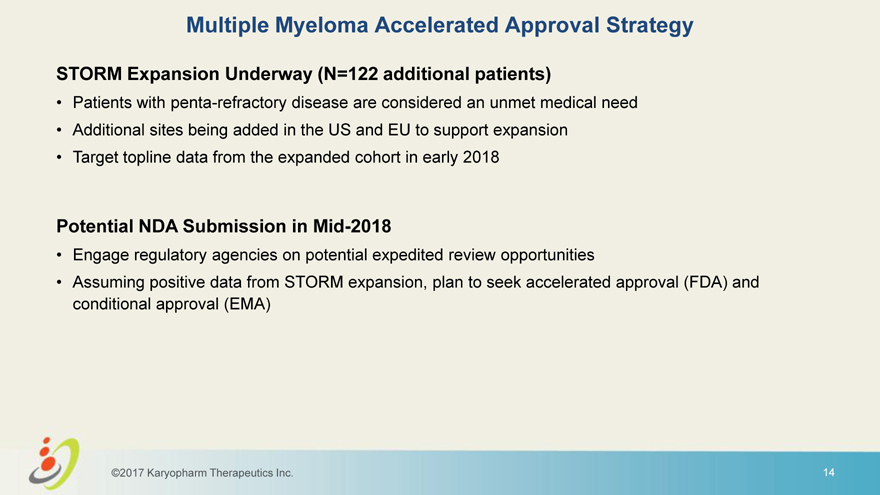
Multiple Myeloma Accelerated Approval Strategy STORM Expansion Underway (N=122 additional patients) • Patients with penta-refractory disease are considered an unmet medical need • Additional sites being added in the US and EU to support expansion • Target topline data from the expanded cohort in early 2018 Potential NDA Submission in Mid-2018 • Engage regulatory agencies on potential expedited review opportunities • Assuming positive data from STORM expansion, plan to seek accelerated approval (FDA) and conditional approval (EMA) ©2017 Karyopharm Therapeutics Inc. 14
Selinexor + velcade bortezomib + dexamethasone svd efficacy phase 1
STOMP: Selinexor and Backbone Treatments for Relapsed/Refractory Multiple Myeloma
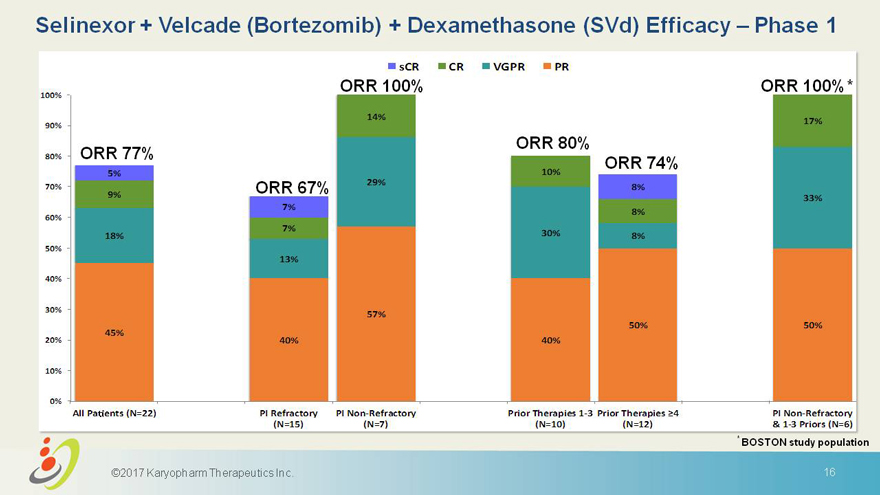
sCR CR VGPR PR 100% ORR 100% ORR 100%* 14% 17% 90% ORR 80% 80% ORR 77% ORR 74% 5% 10% 29% 70% ORR 67% 8% 9% 33% 7% 8% 60% 7% 18% 30% 8% 50% 13% 40% 30% 57% 50% 50% 45% 20% 40% 40% 10% 0% AllPa ents(N=22) PIRefractory PINon-Refractory PriorTherapies1-3 PriorTherapies 4 PINon-Refractory (N=15) (N=7) (N=10) (N=12) &1-3Priors(N=6) * BOSTON study population 2017 Karyopharm Therapeutics Inc. 16
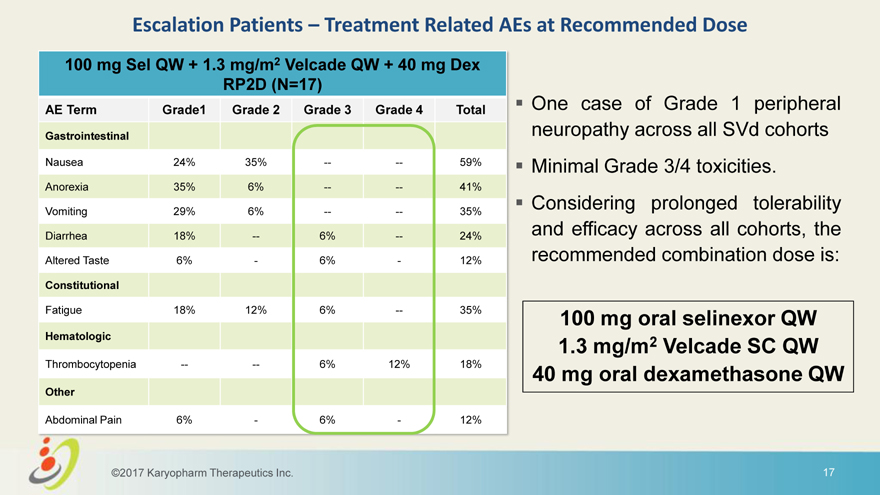
Escalation Patients – Treatment Related AEs at Recommended Dose 100 mg Sel QW + 1.3 mg/m2 Velcade QW + 40 mg Dex RP2D (N=17) AE Term Grade1 Grade 2 Grade 3 Grade 4 Total One case of Grade 1 peripheral Gastrointestinal neuropathy across all SVd cohorts Nausea 24% 35% — — 59% Minimal Grade 3/4 toxicities. Anorexia 35% 6% — — 41% Considering prolonged tolerability Vomiting 29% 6% — — 35% Diarrhea 18% — 6% — 24% and efficacy across all cohorts, the Altered Taste 6%—6%—12% recommended combination dose is: Constitutional Fatigue 18% 12% 6% — 35% 100 mg oral selinexor QW Hematologic 1.3 mg/m2 Velcade SC QW Thrombocytopenia — — 6% 12% 18% 40 mg oral dexamethasone QW Other Abdominal Pain 6%—6%—12% ©2017 Karyopharm Therapeutics Inc. 17
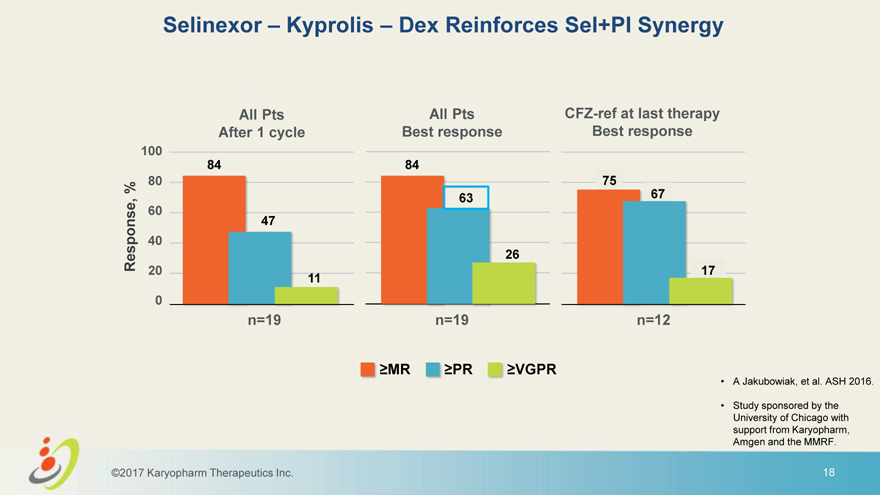
Selinexor – Kyprolis – Dex Reinforces Sel+PI Synergy * All Pts All Pts CFZ-ref at last therapy After 1 cycle Best response Best response 100 84 84 % 80 75 63 67 60 47 40 Response, 26 20 17 11 0 n=19 n=19 n=12 MR PR VGPR • A Jakubowiak, et al. ASH 2016. • Study sponsored by the University of Chicago with support from Karyopharm, Amgen and the MMRF. ©2017 Karyopharm Therapeutics Inc. 18
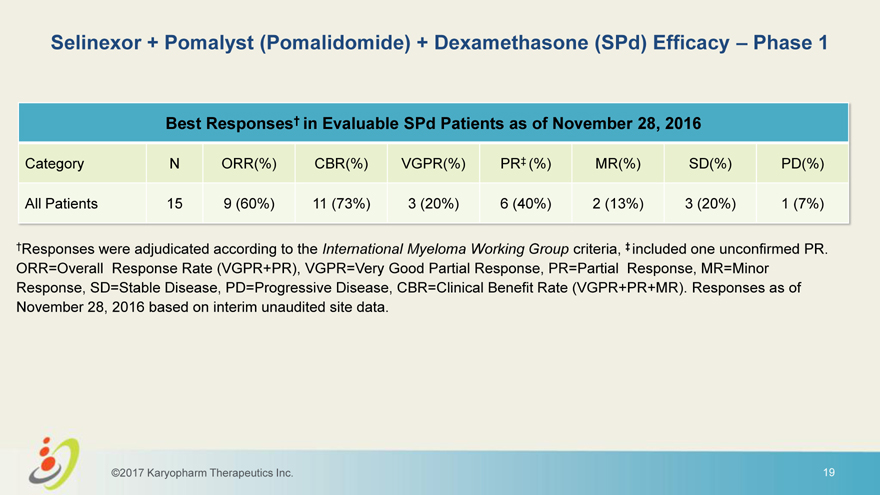
Selinexor + Pomalyst (Pomalidomide) + Dexamethasone (SPd) Efficacy – Phase 1 Best Responses† in Evaluable SPd Patients as of November 28, 2016 Category N ORR(%) CBR(%) VGPR(%) PR‡ (%) MR(%) SD(%) PD(%) All Patients 15 9 (60%) 11 (73%) 3 (20%) 6 (40%) 2 (13%) 3 (20%) 1 (7%) †Responses were adjudicated according to the International Myeloma Working Group criteria, ‡ included one unconfirmed PR. ORR=Overall Response Rate (VGPR+PR), VGPR=Very Good Partial Response, PR=Partial Response, MR=Minor Response, SD=Stable Disease, PD=Progressive Disease, CBR=Clinical Benefit Rate (VGPR+PR+MR). Responses as of November 28, 2016 based on interim unaudited site data. ©2017 Karyopharm Therapeutics Inc. 19
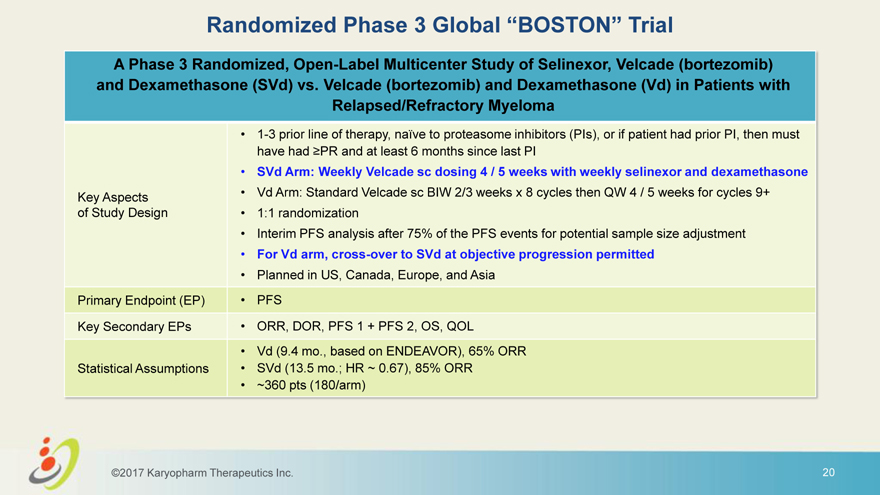
Randomized Phase 3 Global “BOSTON” Trial A Phase 3 Randomized, Open-Label Multicenter Study of Selinexor, Velcade (bortezomib) and Dexamethasone (SVd) vs. Velcade (bortezomib) and Dexamethasone (Vd) in Patients with Relapsed/Refractory Myeloma • 1-3 prior line of therapy, naïve to proteasome inhibitors (PIs), or if patient had prior PI, then must have had PR and at least 6 months since last PI • SVd Arm: Weekly Velcade sc dosing 4 / 5 weeks with weekly selinexor and dexamethasone Key Aspects • Vd Arm: Standard Velcade sc BIW 2/3 weeks x 8 cycles then QW 4 / 5 weeks for cycles 9+ of Study Design • 1:1 randomization • Interim PFS analysis after 75% of the PFS events for potential sample size adjustment • For Vd arm, cross-over to SVd at objective progression permitted • Planned in US, Canada, Europe, and Asia Primary Endpoint (EP) • PFS Key Secondary EPs • ORR, DOR, PFS 1 + PFS 2, OS, QOL • Vd (9.4 mo., based on ENDEAVOR), 65% ORR Statistical Assumptions • SVd (13.5 mo.; HR ~ 0.67), 85% ORR • ~360 pts (180/arm) ©2017 Karyopharm Therapeutics Inc. 20

SADAL (DLBCL) & SOPRA (AML)
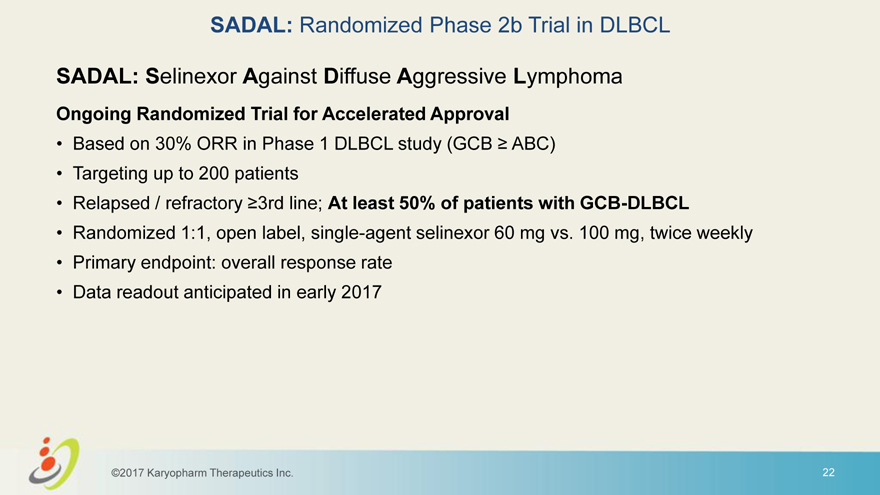
SADAL: Randomized Phase 2b Trial in DLBCL SADAL: Selinexor Against Diffuse Aggressive Lymphoma Ongoing Randomized Trial for Accelerated Approval • Based on 30% ORR in Phase 1 DLBCL study (GCB ABC) • Targeting up to 200 patients • Relapsed / refractory 3rd line; At least 50% of patients with GCB-DLBCL • Randomized 1:1, open label, single-agent selinexor 60 mg vs. 100 mg, twice weekly • Primary endpoint: overall response rate • Data readout anticipated in early 2017 ©2017 Karyopharm Therapeutics Inc. 22

SOPRA: Randomized Phase 2 Trial in 2nd Line AML SOPRA: Selinexor in Older Patients With Relapsed AML • AML patients >60 years old – after first relapse (n = ~ 170 patients) • Hypomethylating agents or low dose cytarabine or supportive care only • Randomized 2:1, open label, single-agent selinexor (60 mg fixed dose, twice weekly) vs. “Physician’s Choice”• Primary endpoint: overall survival • Interim analysis in early 2017; topline data readout anticipated mid-2017 Phase 1 Clinical Results • Based on 95 total patients, 78 evaluable for response (median 3+ prior therapies) • Overall complete response rate: 10% [8/78 with CR/CR(i/p)] • 45 patients with stable disease (58%) • 68% (53 of 78) disease control rate (CR/CR(i/p) & stable disease) ©2017 Karyopharm Therapeutics Inc. 23

Solid Tumors

SEAL: Phase 2/3 Trial in Liposarcoma SEAL: Selinexor in Advanced Liposarcoma • Phase 2 portion target = ~50 patients • Evaluating patients with advanced unresectable dedifferentiated liposarcoma • Randomized 1:1, double blind, single-agent selinexor (60mg fixed dose, twice weekly) vs. placebo • Primary endpoint: progression-free survival • Trial design and endpoints agreed with FDA & EMA • Top-line data readout from Phase 2 portion anticipated in mid-2017; study may be expanded to Phase 3 following an interim analysis ©2017 Karyopharm Therapeutics Inc. 25
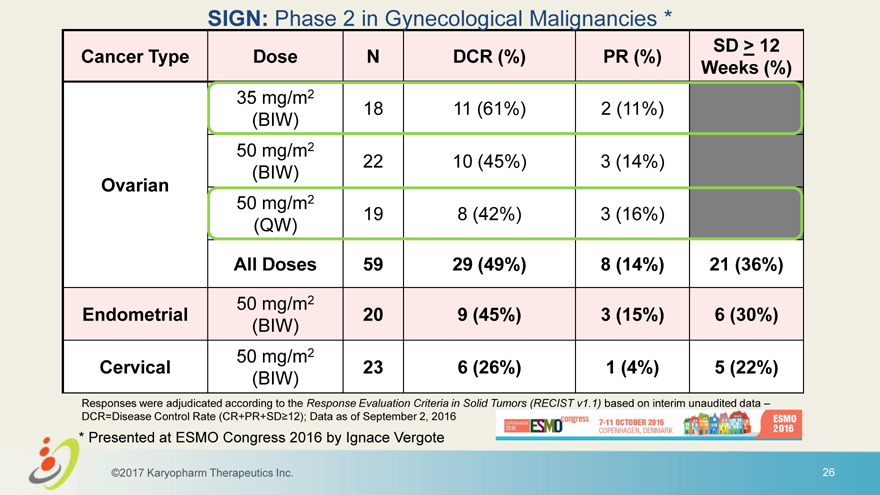
SIGN: Phase 2 in Gynecological Malignancies * SD > 12 Cancer Type Dose N DCR (%) PR (%) Weeks (%) 35 mg/m2 18 11 (61%) 2 (11%) (BIW) 50 mg/m2 22 10 (45%) 3 (14%) (BIW) Ovarian 50 mg/m2 19 8 (42%) 3 (16%) (QW) All Doses 59 29 (49%) 8 (14%) 21 (36%) 50 mg/m2 Endometrial 20 9 (45%) 3 (15%) 6 (30%) (BIW) 50 mg/m2 Cervical 23 6 (26%) 1 (4%) 5 (22%) (BIW) Responses were adjudicated according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) based on interim unaudited data – DCR=Disease Control Rate (CR+PR+SD 12); Data as of September 2, 2016 * Presented at ESMO Congress 2016 by Ignace Vergote ©2017 Karyopharm Therapeutics Inc. 26
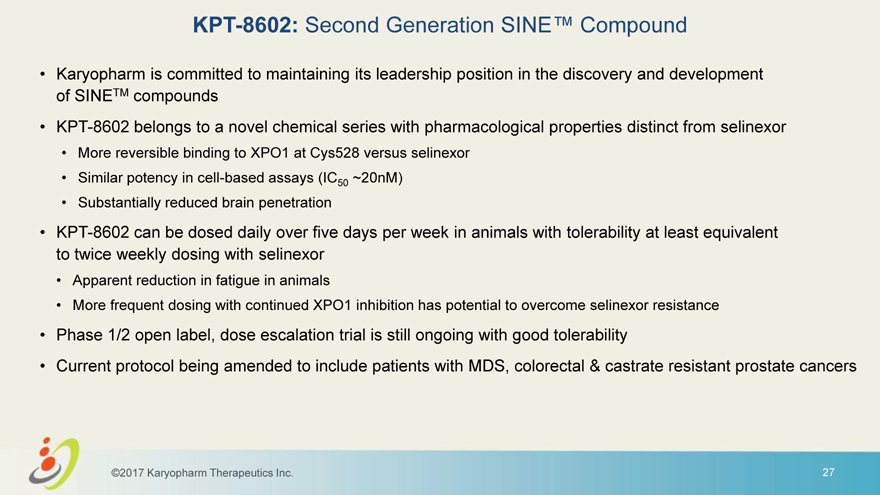
KPT-8602: Second Generation SINE™ Compound • Karyopharm is committed to maintaining its leadership position in the discovery and development of SINETM compounds • KPT-8602 belongs to a novel chemical series with pharmacological properties distinct from selinexor • More reversible binding to XPO1 at Cys528 versus selinexor • Similar potency in cell-based assays (IC50 ~20nM) • Substantially reduced brain penetration • KPT-8602 can be dosed daily over five days per week in animals with tolerability at least equivalent to twice weekly dosing with selinexor • Apparent reduction in fatigue in animals • More frequent dosing with continued XPO1 inhibition has potential to overcome selinexor resistance • Phase 1/2 open label, dose escalation trial is still ongoing with good tolerability • Current protocol being amended to include patients with MDS, colorectal & castrate resistant prostate cancers ©2017 Karyopharm Therapeutics Inc. 27
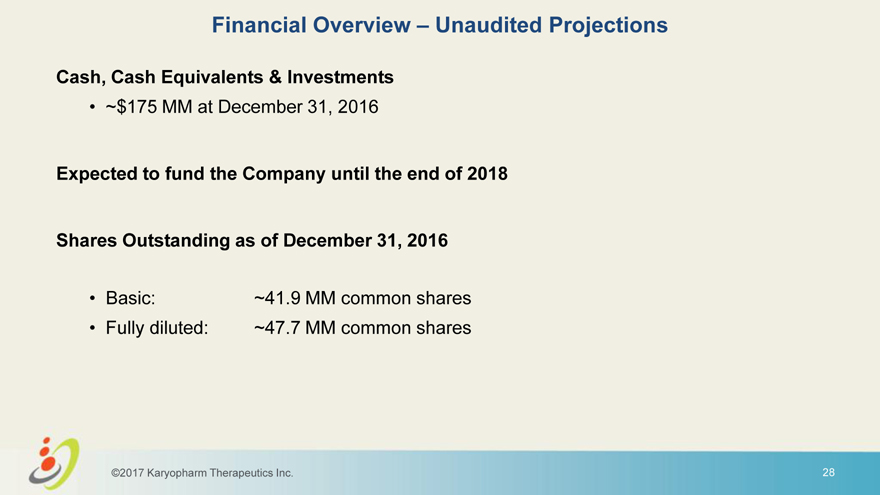
Financial Overview – Unaudited Projections Cash, Cash Equivalents & Investments • ~$175 MM at December 31, 2016 Expected to fund the Company until the end of 2018 Shares Outstanding as of December 31, 2016 • Basic: ~41.9 MM common shares • Fully diluted: ~47.7 MM common shares ©2017 Karyopharm Therapeutics Inc. 28

Anticipated Corporate Milestones STORM: Phase 2b expansion top-line data Early 2018 Multiple Myeloma BOSTON: Phase 3 top-line data 2019 DLBCL SADAL: Phase 2b top-line data (ORR) Early 2017 SOPRA: Phase 2 interim analysis Early 2017 AML Phase 2 top-line data (OS) Mid 2017 Solid Tumors SEAL: Phase 2 top-line data (PFS) Mid 2017 KPT-8602: Phase 1/2 top-line data Late 2017 Pipeline Expansion KPT-9274: Phase 1/2 top-line data (safety and tolerability) Mid 2017 ©2017 Karyopharm Therapeutics Inc. 29
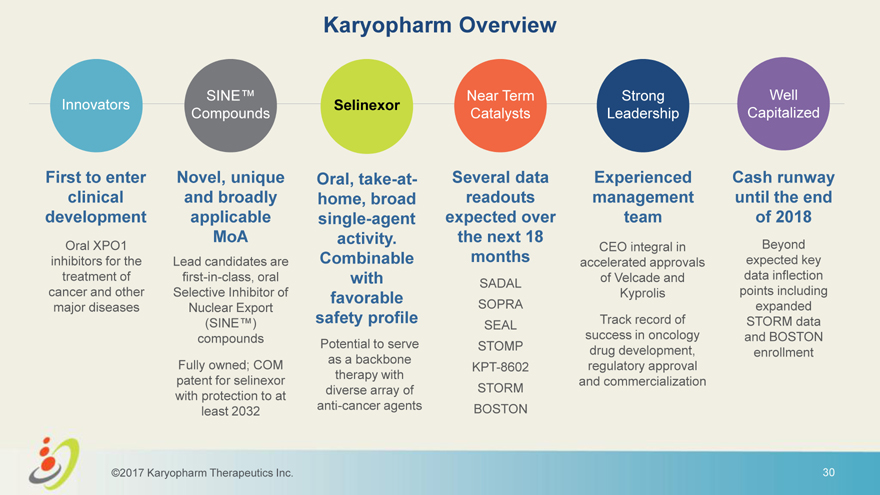
Karyopharm Overview SINE™ Near Term Strong Well Innovators Selinexor Compounds Catalysts Leadership Capitalized First to enter Novel, unique Oral, take-at- Several data Experienced Cash runway clinical and broadly home, broad readouts management until the end development applicable single-agent expected over team of 2018 MoA activity. the next 18 Oral XPO1 CEO integral in Beyond inhibitors for the Lead candidates are Combinable months accelerated approvals expected key treatment of first-in-class, oral with of Velcade and data inflection SADAL cancer and other Selective Inhibitor of favorable Kyprolis points including major diseases Nuclear Export safety profile SOPRA expanded (SINE™) Track record of STORM data SEAL compounds success in oncology and BOSTON Potential to serve STOMP drug development, enrollment as a backbone Fully owned; COM KPT-8602 regulatory approval therapy with patent for selinexor and commercialization diverse array of STORM with protection to at anti-cancer agents BOSTON least 2032 ©2017 Karyopharm Therapeutics Inc. 30

Thank you
