Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Juno Therapeutics, Inc. | a8-kforjpmpresentationxbody.htm |

January 2017
Corporate Presentation
Juno Therapeutics Proprietary Materials
Exhibit 99.1

Juno Therapeutics 2 Proprietary Materials
Forward-looking Statements, Investigational Status, and Interim Data
This presentation and the accompanying oral commentary contain forward-looking statements that involve risks, uncertainties, and assumptions. If
the risks or uncertainties ever materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by
such forward-looking statements. All statements other than statements of historical fact could be deemed forward-looking, including, but not limited
to, any statements of the plans, strategies, and objectives of management for future operations, including our clinical development and
commercialization plans; any projections of financial information; any statements about historical results that may suggest trends for our business;
any statements of expectation or belief regarding future events, potential markets or market size, technology developments, our product pipeline,
clinical data or the implications thereof, enforceability of our intellectual property rights, competitive strengths or our position within the industry; any
statements regarding the anticipated benefits of our Celgene collaboration or other strategic transactions; and any statements of assumptions
underlying any of the items mentioned.
These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future
performance. Actual results could differ materially from our current expectations as a result of many risks and uncertainties, including but not limited
to, risks associated with: the success, cost, and timing of our product development activities and clinical trials; our ability to obtain regulatory
approval for and to commercialize our product candidates; our ability to establish a commercially-viable manufacturing process and manufacturing
infrastructure; regulatory requirements and regulatory developments; the effects of competition and technological advances; our dependence on
third-party collaborators and other contractors in our research and development activities, including for the conduct of clinical trials and the
manufacture of our product candidates; our dependence on Celgene for the development and commercialization outside of North America and
China of our CD19 product candidates and any other product candidates for which Celgene exercises an option; Juno’s dependence on JW
Therapeutics (Shanghai) Co., Ltd, over which Juno does not exercise complete control, for the development and commercialization of product
candidates in China; our ability to obtain, maintain, or protect intellectual property rights related to our product candidates; among others. For a
further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements,
as well as risks relating to our business in general, see our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on
November 9, 2016 and our other periodic reports filed from time to time with the Securities and Exchange Commission. Except as required by law,
we assume no obligation and do not intend to update these forward-looking statements or to conform these statements to actual results or to
changes in our expectations.
All of Juno’s product candidates are investigational product candidates and their safety and efficacy have not been established. Juno has not
obtained marketing approval for any product, and there is no certainty that any marketing approvals will be obtained or as to the timelines on which
they will be obtained.
Any data presented pertaining to Juno product candidates is interim data, and may include investigator-reported interim data for which Juno has not
yet independently reviewed the source data. The interim data may not be representative of the final results that may be obtained in the
corresponding trial, and results from earlier trials may not be representative of results obtained in later trials or pivotal trials.
Juno Therapeutics 3 Proprietary Materials
Building the Leading T Cell Company
Progress with potential best-in-class CD19 product candidate and platform
– JCAR017 on the market as early as 2018
– Significant advances in defining attributes that correlate with complete response in NHL
– Year-end progress: Human CD19 trial open, JCAR014 + durvalumab combination trial open, and
CD19 / 4-1BBL “armored” CAR trial to begin soon
Moving beyond CD19
– Myeloma program expected to begin dosing patients in 1H17
– Solid organ tumor targets – five in human testing; additional targets in pipeline
– Year-end progress: Lewis Y trial open
Building platform and self-sustaining research capability to support leadership in the
engineered T cell space
With Celgene, global manufacturing, development, and commercial capabilities to bring
engineered T cells to market globally
Strong balance sheet with >$1 billion in cash and equivalents as of 3Q16
Juno Therapeutics 4 Proprietary Materials
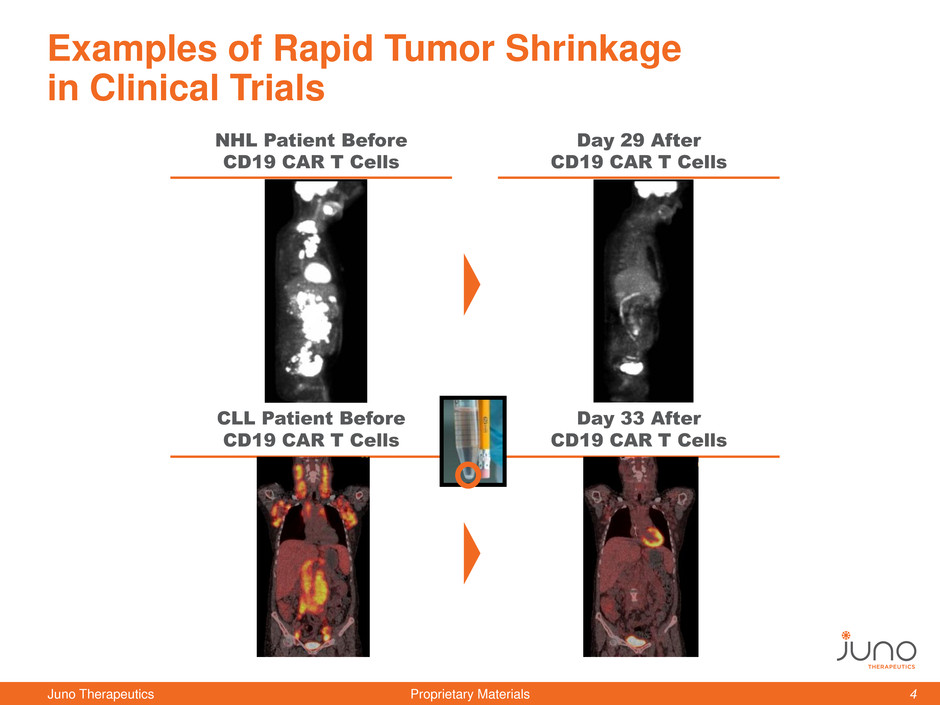
Examples of Rapid Tumor Shrinkage
in Clinical Trials
Day 29 After
CD19 CAR T Cells
NHL Patient Before
CD19 CAR T Cells
Day 33 After
CD19 CAR T Cells
CLL Patient Before
CD19 CAR T Cells
Juno Therapeutics 5 Proprietary Materials
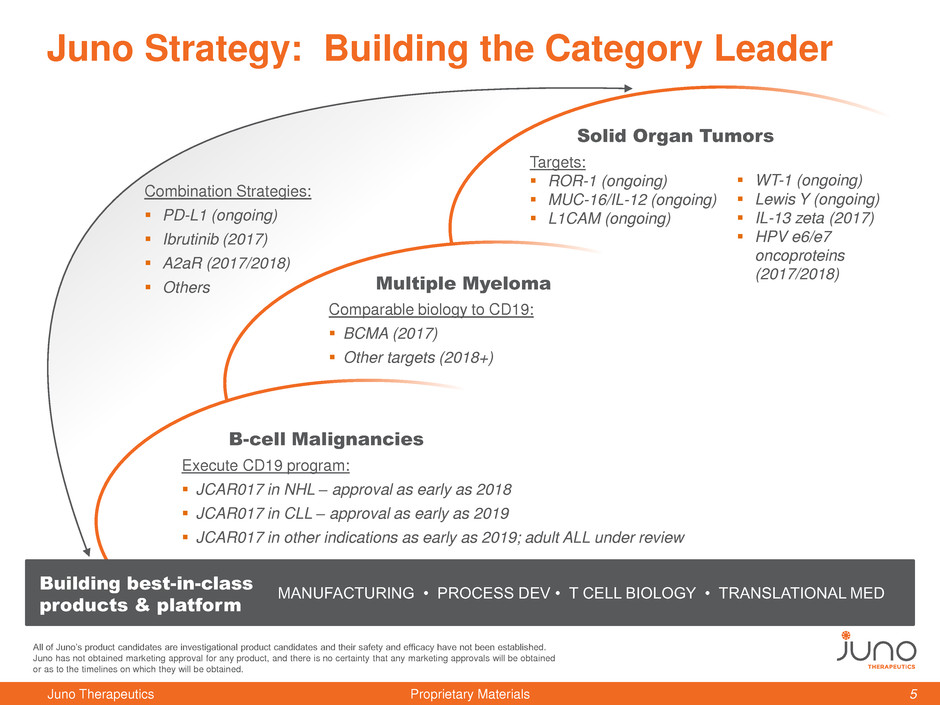
Combination Strategies:
PD-L1 (ongoing)
Ibrutinib (2017)
A2aR (2017/2018)
Others
Juno Strategy: Building the Category Leader
B-cell Malignancies
Execute CD19 program:
JCAR017 in NHL – approval as early as 2018
JCAR017 in CLL – approval as early as 2019
JCAR017 in other indications as early as 2019; adult ALL under review
Multiple Myeloma
Comparable biology to CD19:
BCMA (2017)
Other targets (2018+)
Solid Organ Tumors
Targets:
ROR-1 (ongoing)
MUC-16/IL-12 (ongoing)
L1CAM (ongoing)
MANUFACTURING • PROCESS DEV • T CELL BIOLOGY • TRANSLATIONAL MED
Building best-in-class
products & platform
WT-1 (ongoing)
Lewis Y (ongoing)
IL-13 zeta (2017)
HPV e6/e7
oncoproteins
(2017/2018)
All of Juno’s product candidates are investigational product candidates and their safety and efficacy have not been established.
Juno has not obtained marketing approval for any product, and there is no certainty that any marketing approvals will be obtained
or as to the timelines on which they will be obtained.

Juno Therapeutics 6 Proprietary Materials
JCAR015 Phase II ROCKET Trial Update
Clinical hold on November 22
38 total treated patients to date
– 8 with flu/cy conditioning regimen – 3 treatment-related deaths
– 30 with cy alone conditioning regimen – 2 treatment-related deaths
– Early efficacy encouraging and consistent with Phase I experience
Evidence continues to correlate risk to the early kinetics of cellular expansion
Key variables to consider:
– Patient
– Product
– Interventions
JCAR015 potential outcomes:
– Modify ROCKET
– Start another trial that explores opportunities to modify benefit/risk ratio
– Focus on JCAR017 or alternative product candidate
Juno Therapeutics 7 Proprietary Materials
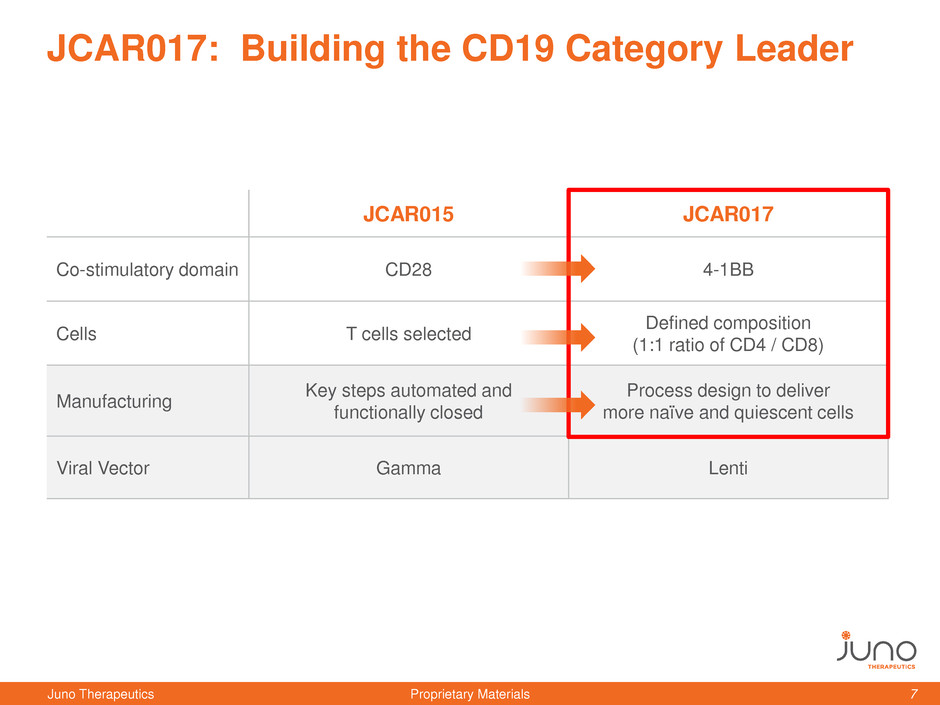
JCAR015 JCAR017
Co-stimulatory domain CD28 4-1BB
Cells T cells selected
Defined composition
(1:1 ratio of CD4 / CD8)
Manufacturing
Key steps automated and
functionally closed
Process design to deliver
more naïve and quiescent cells
Viral Vector Gamma Lenti
JCAR017: Building the CD19 Category Leader
Juno Therapeutics 8 Proprietary Materials
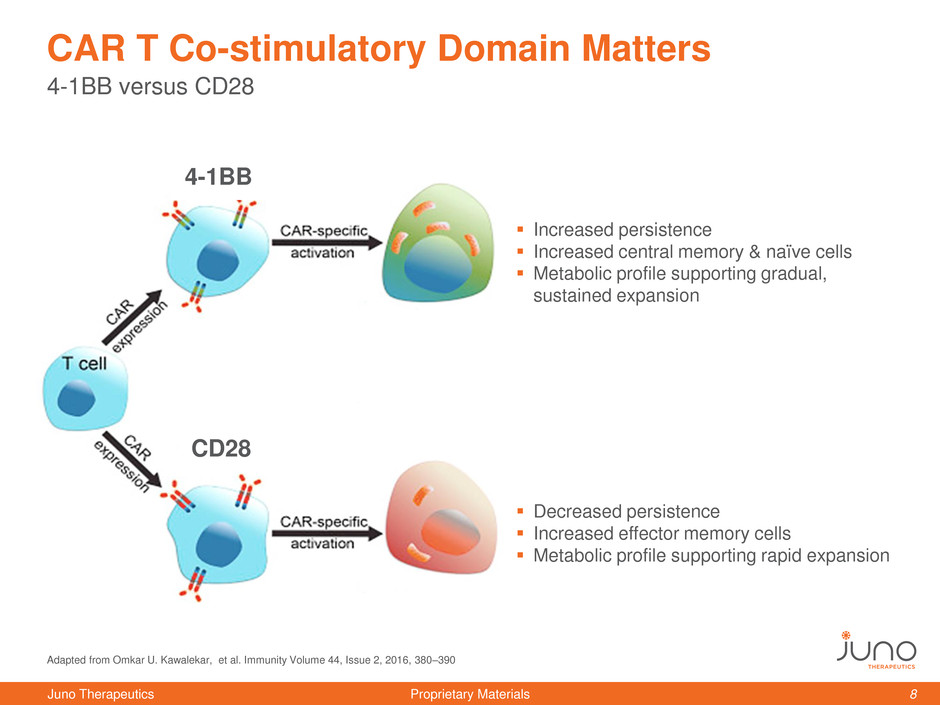
4-1BB versus CD28
CAR T Co-stimulatory Domain Matters
Increased persistence
Increased central memory & naïve cells
Metabolic profile supporting gradual,
sustained expansion
Decreased persistence
Increased effector memory cells
Metabolic profile supporting rapid expansion
4-1BB
CD28
Adapted from Omkar U. Kawalekar, et al. Immunity Volume 44, Issue 2, 2016, 380–390
Juno Therapeutics 9 Proprietary Materials
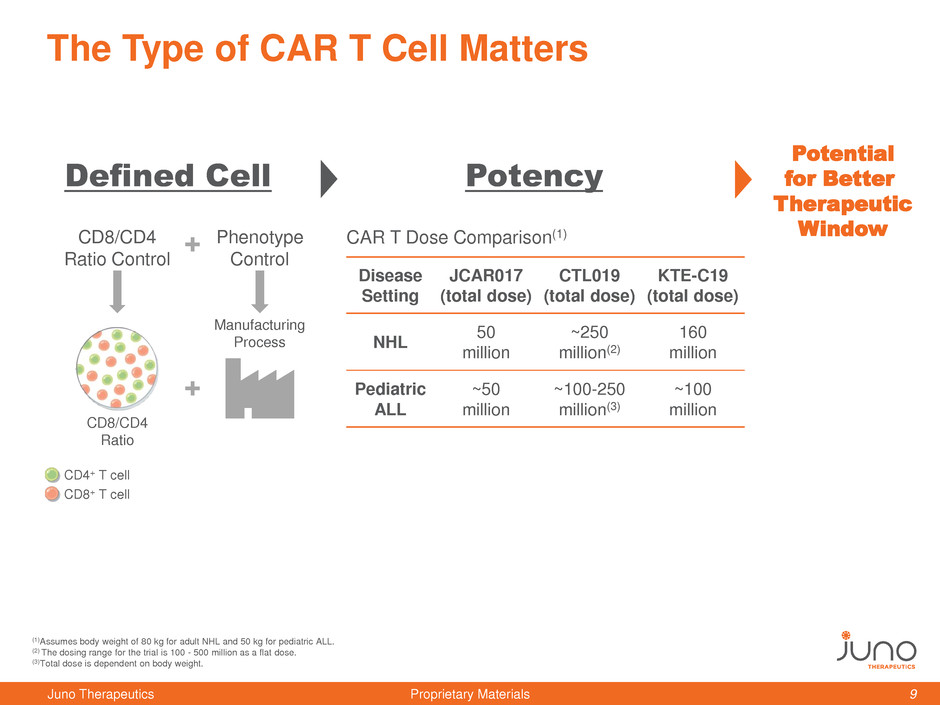
The Type of CAR T Cell Matters
(1)Assumes body weight of 80 kg for adult NHL and 50 kg for pediatric ALL.
(2) The dosing range for the trial is 100 - 500 million as a flat dose.
(3)Total dose is dependent on body weight.
Potency
Disease
Setting
JCAR017
(total dose)
CTL019
(total dose)
KTE-C19
(total dose)
NHL
50
million
~250
million(2)
160
million
Pediatric
ALL
~50
million
~100-250
million(3)
~100
million
CAR T Dose Comparison(1)
Potential
for Better
Therapeutic
Window
Manufacturing
Process
CD4+ T cell
CD8+ T cell
CD8/CD4
Ratio
Phenotype
Control
CD8/CD4
Ratio Control
Defined Cell

Juno Therapeutics 10 Proprietary Materials
Potential to differentiate on efficacy and safety
NHL: Potential Best-in-class Profile
Patient Characteristics
Goal » JCAR017 NHL
registration trial to initiate in 2017
with approval as early as 2018
Dose Level 1
5*107
N=19 – 22
Dose Level 2
1*108
N=2
ORR 16/20
(80%)
2/2
(100%)
CR 12/20
(60%)
2/2
(100%)
CR at 3 months 8/19
(42%)
n/a
Severe Cytokine
Release Syndrome
0/22
(0%)
0/2
(0%)
Severe
Neurotoxicity
3/22
(14%)
0/2
(0%)
JCAR017 in DLBCL(1)
(NCT02631044)
(1)Investigator-reported data as-of November 23, 2016 cut-off date. Includes fludarabine and cyclophosphamide conditioning regimen.
CR = complete response; PR = partial response; ORR = CR + PR
ECOG 0-2 status
Relapsed and chemorefractory
Median of 4 lines of prior therapy
Safety
No prophylactic use of safety
medications in protocol
In 22 safety-evaluable patients (19 r/r DLBCL, 1 follicular lymphoma grade 3B, and 2 MCL patients) treated at dose level 1, single-dose schedule, no severe cytokine release syndrome
(sCRS) was observed. Grade 3-4 neurotoxicity was observed in 3/22 (14%) patients, all of whom received the steroid dexamethasone for neurotoxicity. A single patient received
tocilizumab for early onset grade 2 CRS. The most frequently reported treatment-emergent adverse events were neutropenia (100%), decreased appetite (36%) and fatigue (32%).
Juno Therapeutics 11 Proprietary Materials
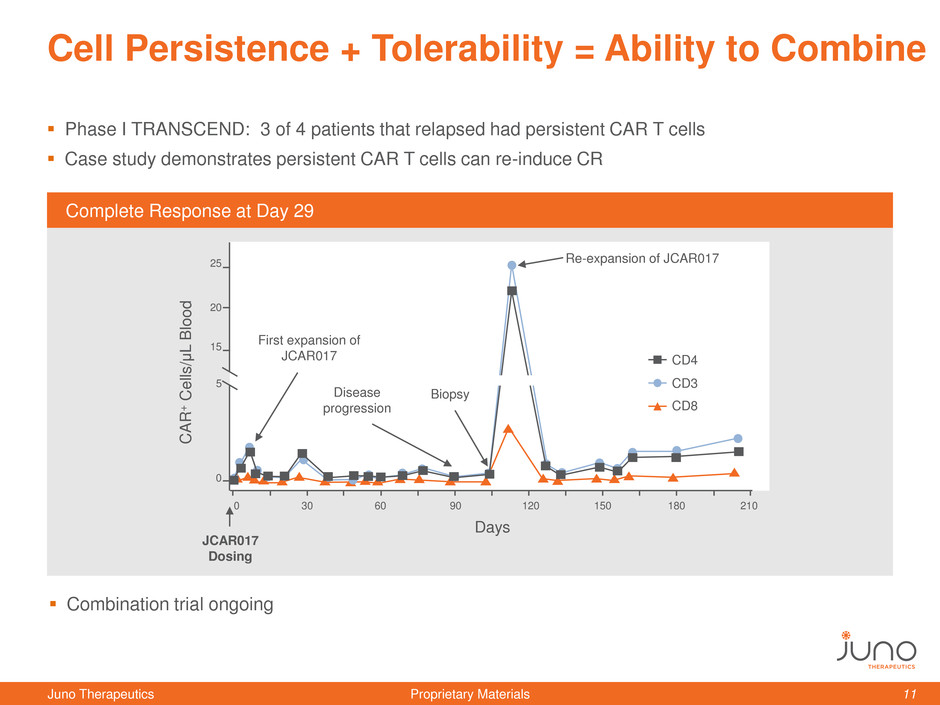
Cell Persistence + Tolerability = Ability to Combine
Phase I TRANSCEND: 3 of 4 patients that relapsed had persistent CAR T cells
Case study demonstrates persistent CAR T cells can re-induce CR
Complete Response at Day 29
25
20
15
5
0
0 30 60 90 120 150 180 210
Days
First expansion of
JCAR017
Disease
progression
Re-expansion of JCAR017
Biopsy
JCAR017
Dosing
CA
R
+
Cells
/μ
L
B
lo
o
d
CD4
CD3
CD8
Combination trial ongoing
Juno Therapeutics 12 Proprietary Materials
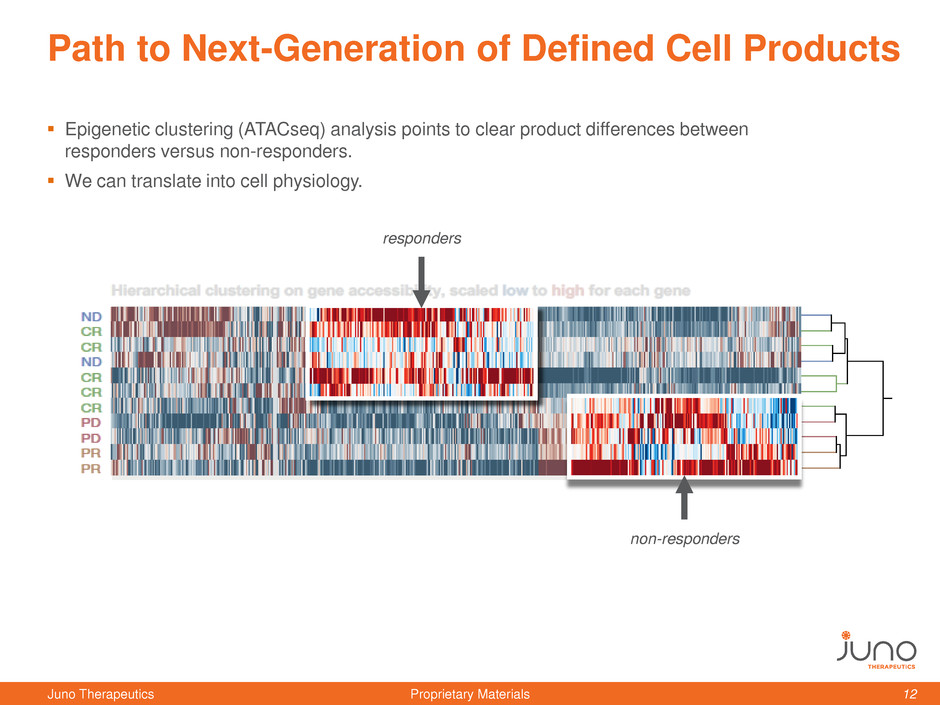
Path to Next-Generation of Defined Cell Products
Epigenetic clustering (ATACseq) analysis points to clear product differences between
responders versus non-responders.
We can translate into cell physiology.
responders
non-responders
Juno Therapeutics 13 Proprietary Materials
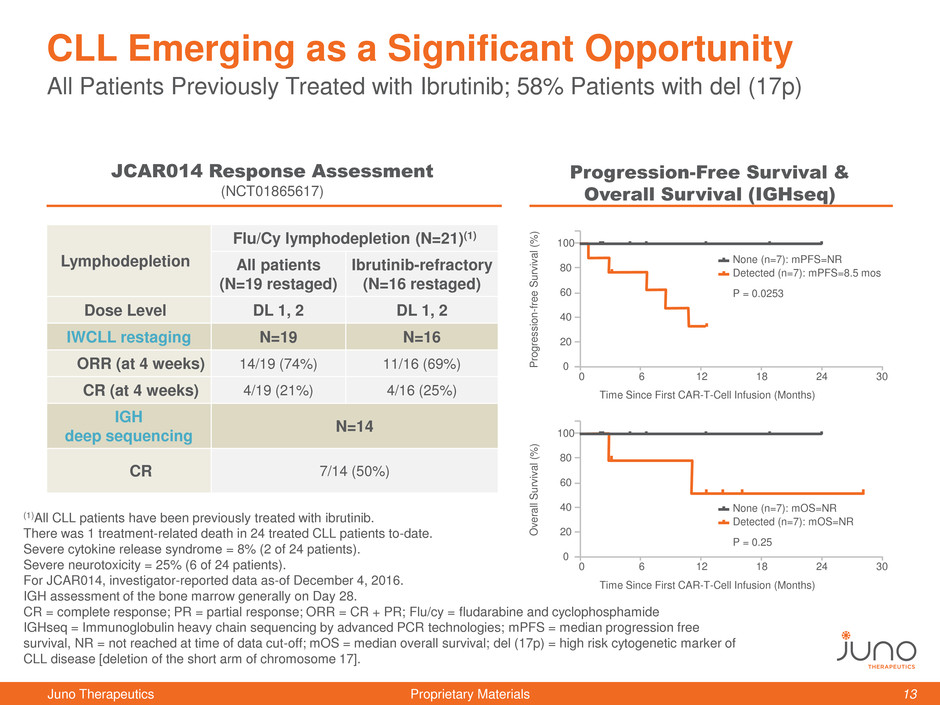
(1)All CLL patients have been previously treated with ibrutinib.
There was 1 treatment-related death in 24 treated CLL patients to-date.
Severe cytokine release syndrome = 8% (2 of 24 patients).
Severe neurotoxicity = 25% (6 of 24 patients).
For JCAR014, investigator-reported data as-of December 4, 2016.
IGH assessment of the bone marrow generally on Day 28.
CR = complete response; PR = partial response; ORR = CR + PR; Flu/cy = fludarabine and cyclophosphamide
IGHseq = Immunoglobulin heavy chain sequencing by advanced PCR technologies; mPFS = median progression free
survival, NR = not reached at time of data cut-off; mOS = median overall survival; del (17p) = high risk cytogenetic marker of
CLL disease [deletion of the short arm of chromosome 17].
All Patients Previously Treated with Ibrutinib; 58% Patients with del (17p)
CLL Emerging as a Significant Opportunity
Lymphodepletion
Flu/Cy lymphodepletion (N=21)(1)
All patients
(N=19 restaged)
Ibrutinib-refractory
(N=16 restaged)
Dose Level DL 1, 2 DL 1, 2
IWCLL restaging N=19 N=16
ORR (at 4 weeks) 14/19 (74%) 11/16 (69%)
CR (at 4 weeks) 4/19 (21%) 4/16 (25%)
IGH
deep sequencing
N=14
CR 7/14 (50%)
JCAR014 Response Assessment
(NCT01865617)
Progression-Free Survival &
Overall Survival (IGHseq)
Detected (n=7): mPFS=8.5 mos
None (n=7): mPFS=NR
P = 0.0253
0
40
100
0 6 12 18 24 30
P
ro
g
re
s
s
io
n
-f
re
e
S
u
rv
iv
a
l
(%
)
20
80
60
Time Since First CAR-T-Cell Infusion (Months)
0
40
100
0 6 12 18 24 30
O
v
e
ra
ll
S
u
rv
iv
a
l
(%
)
20
80
60
Time Since First CAR-T-Cell Infusion (Months)
Detected (n=7): mOS=NR
None (n=7): mOS=NR
P = 0.25
Juno Therapeutics 14 Proprietary Materials
Expect BCMA CAR to enter the clinic in 1H2017
Expanding the Franchise into Multiple Myeloma
Variable BCMA surface expression 3
Inadequate CAR T cell persistence 1
Complex tumor micro-environment 2
Multiple targets
Binder selection
Combinations
Fully-human binders
4-1BB and next-generation co-stimulatory domains
Manufacturing technologies
Gene editing
Combinations
Armored CARs
Cell Signaling
Challenges Juno’s potential solutions
B-cell
malignancies
Multiple
myeloma
1. Predictable cell surface protein on
every cancer cell
2. Expression limited to B cell lineage
3. Multiple targets
Juno Therapeutics 15 Proprietary Materials
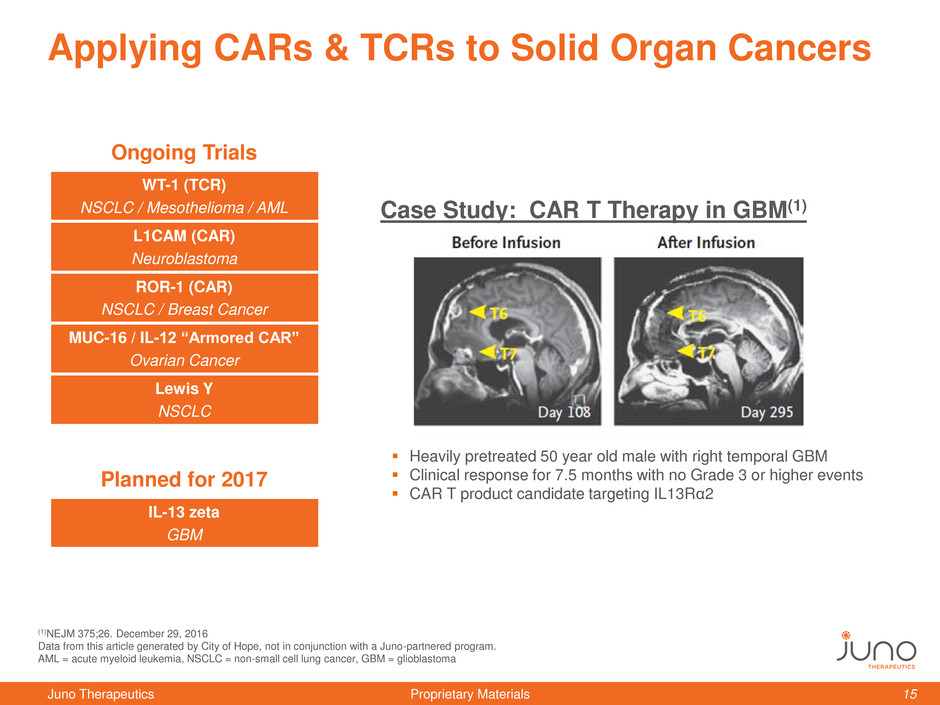
Applying CARs & TCRs to Solid Organ Cancers
Heavily pretreated 50 year old male with right temporal GBM
Clinical response for 7.5 months with no Grade 3 or higher events
CAR T product candidate targeting IL13Rα2
Case Study: CAR T Therapy in GBM(1)
(1)NEJM 375;26. December 29, 2016
Data from this article generated by City of Hope, not in conjunction with a Juno-partnered program.
AML = acute myeloid leukemia, NSCLC = non-small cell lung cancer, GBM = glioblastoma
Ongoing Trials
WT-1 (TCR)
NSCLC / Mesothelioma / AML
L1CAM (CAR)
Neuroblastoma
ROR-1 (CAR)
NSCLC / Breast Cancer
MUC-16 / IL-12 “Armored CAR”
Ovarian Cancer
Lewis Y
NSCLC
Planned for 2017
IL-13 zeta
GBM
Juno Therapeutics 16 Proprietary Materials
Manual process
High operating expenses
ISO 5
staff:
cost: $$$$
Closed and automated
process
Defined cell process
ISO 7 / 8
staff:
cost: $$$
Closed, automated, and
integrated process
1-2 machines
2-4 day manufacturing
time
Cell selection processes
improved
staff:
cost: $$
Automation Lowers Cost and Improves Efficiency
Standard Process Juno Process Today
Goal for Juno Process
in the Medium-term
Juno Therapeutics 17 Proprietary Materials
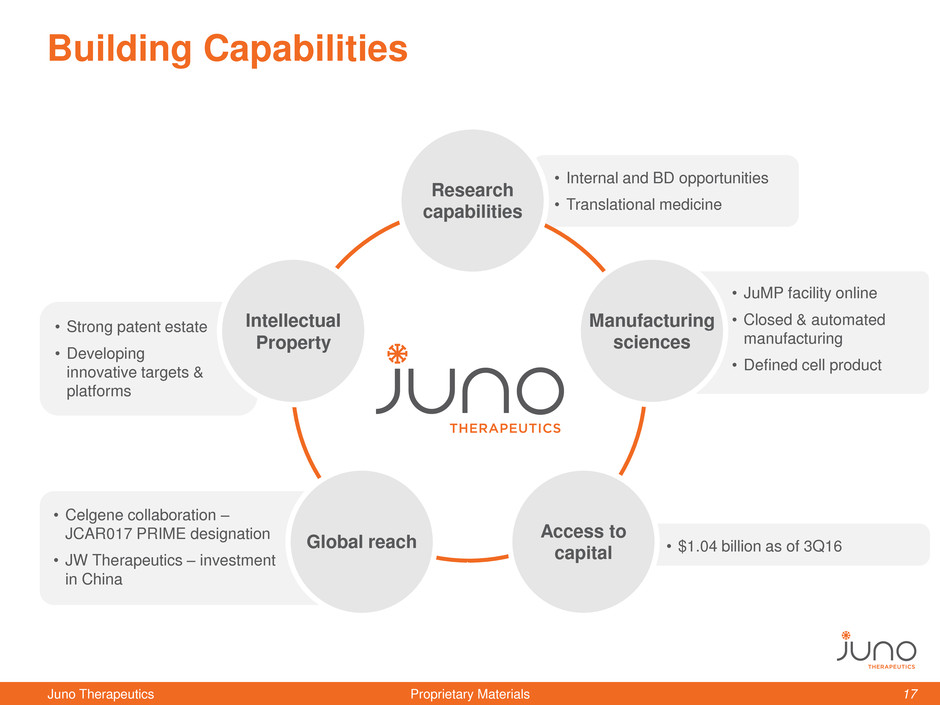
• JuMP facility online
• Closed & automated
manufacturing
• Defined cell product
• Celgene collaboration –
JCAR017 PRIME designation
• JW Therapeutics – investment
in China
• Internal and BD opportunities
• Translational medicine
• $1.04 billion as of 3Q16
• Strong patent estate
• Developing
innovative targets &
platforms
Building Capabilities
Manufacturing
sciences
Global reach
Research
capabilities
Access to
capital
Intellectual
Property
Juno Therapeutics 18 Proprietary Materials
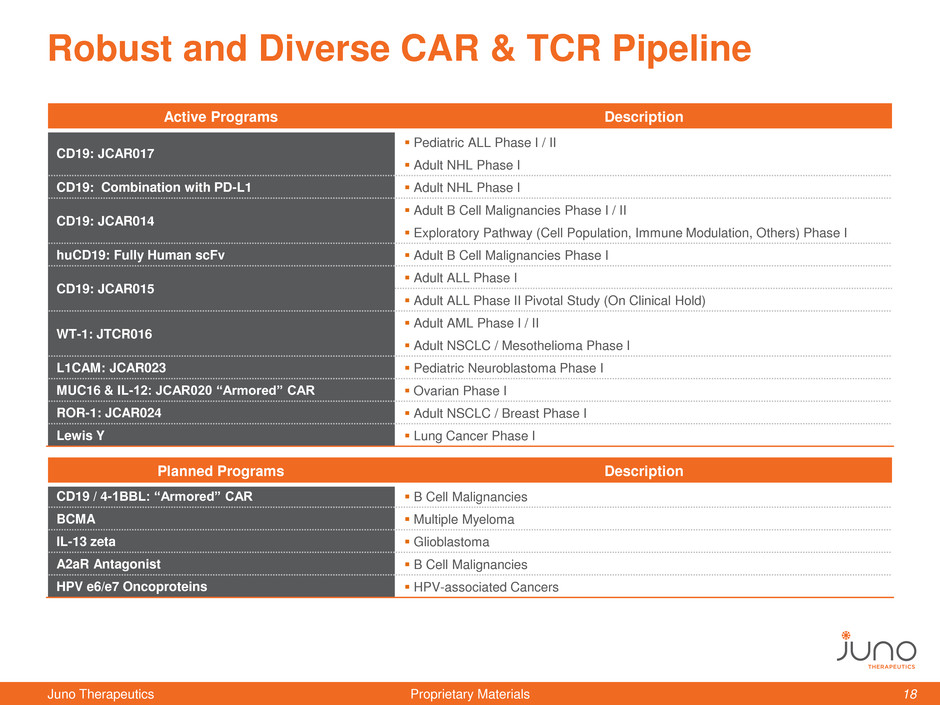
Robust and Diverse CAR & TCR Pipeline
Active Programs Description
CD19: JCAR017
Pediatric ALL Phase I / II
Adult NHL Phase I
CD19: Combination with PD-L1 Adult NHL Phase I
CD19: JCAR014
Adult B Cell Malignancies Phase I / II
Exploratory Pathway (Cell Population, Immune Modulation, Others) Phase I
huCD19: Fully Human scFv Adult B Cell Malignancies Phase I
CD19: JCAR015
Adult ALL Phase I
Adult ALL Phase II Pivotal Study (On Clinical Hold)
WT-1: JTCR016
Adult AML Phase I / II
Adult NSCLC / Mesothelioma Phase I
L1CAM: JCAR023 Pediatric Neuroblastoma Phase I
MUC16 & IL-12: JCAR020 “Armored” CAR Ovarian Phase I
ROR-1: JCAR024 Adult NSCLC / Breast Phase I
Lewis Y Lung Cancer Phase I
Planned Programs Description
CD19 / 4-1BBL: “Armored” CAR B Cell Malignancies
BCMA Multiple Myeloma
IL-13 zeta Glioblastoma
A2aR Antagonist B Cell Malignancies
HPV e6/e7 Oncoproteins HPV-associated Cancers
