Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SOLENO THERAPEUTICS INC | d286139d8k.htm |

NASDAQ: CAPN www.capnia.com Corporate Presentation January 2017 Exhibit 99.1

Certain Notices and Disclaimers Forward-Looking Statements This presentation contains forward-looking statements that are subject to many risks and uncertainties. Forward looking statements appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned product development and clinical trials; the timing of, and our ability to make, regulatory filings and obtain and maintain regulatory approvals for our product candidates; our intellectual property position; the degree of clinical utility of our products, particularly in specific patient populations; our ability to develop commercial functions; expectations regarding product launch and revenue; our results of operations, cash needs, and spending of the proceeds from this offering; financial condition, liquidity, prospects, growth and strategies; the industry in which we operate; and the trends that may affect the industry or us. We may, in some cases, use terms such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation. You should also read carefully the factors described in the “Risk Factors” section and other parts of our Annual Report on Form 10-K, available at www.sec.gov, in order to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this presentation will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation or to reflect the occurrence of unanticipated events.
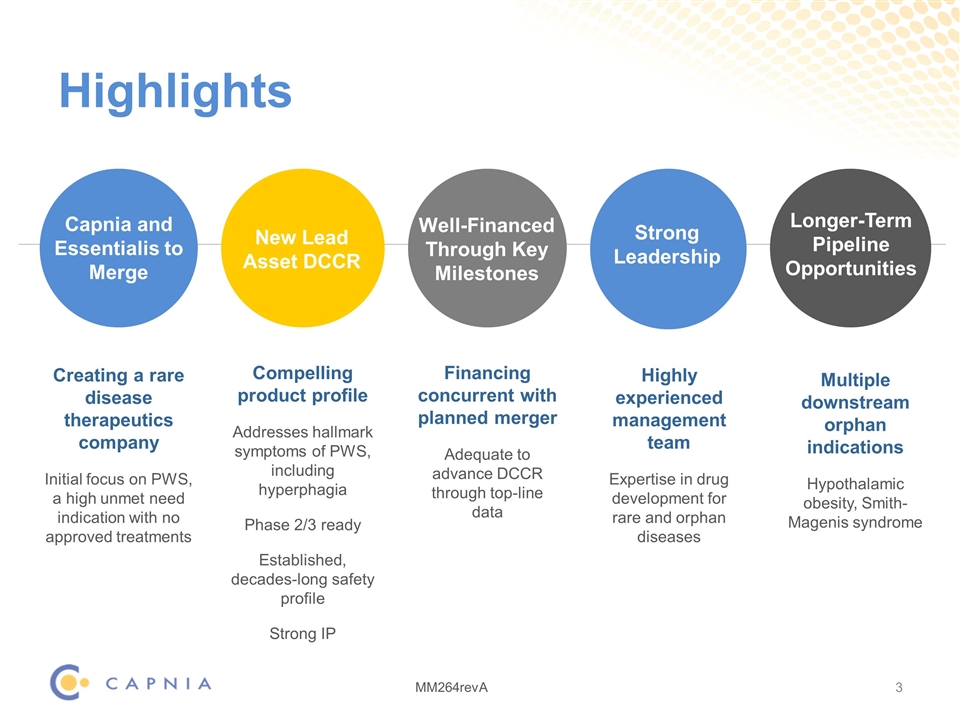
Highlights Capnia and Essentialis to Merge Creating a rare disease therapeutics company Initial focus on PWS, a high unmet need indication with no approved treatments Compelling product profile Addresses hallmark symptoms of PWS, including hyperphagia Phase 2/3 ready Established, decades-long safety profile Strong IP Financing concurrent with planned merger Adequate to advance DCCR through top-line data Multiple downstream orphan indications Hypothalamic obesity, Smith-Magenis syndrome New Lead Asset DCCR Well-Financed Through Key Milestones Longer-Term Pipeline Opportunities Strong Leadership Highly experienced management team Expertise in drug development for rare and orphan diseases

Post Merger Leadership Team Anish Bhatnagar, M.D. Chief Executive Officer David O’Toole Chief Financial Officer Neil Cowen, Ph.D.1 Senior VP of Drug Development Kristen Yen, M.S. VP of Clinical Operations Patricia Hirano, M.P.H. Regulatory Affairs 1 Currently serves as Chief Scientific Officer of Essentialis
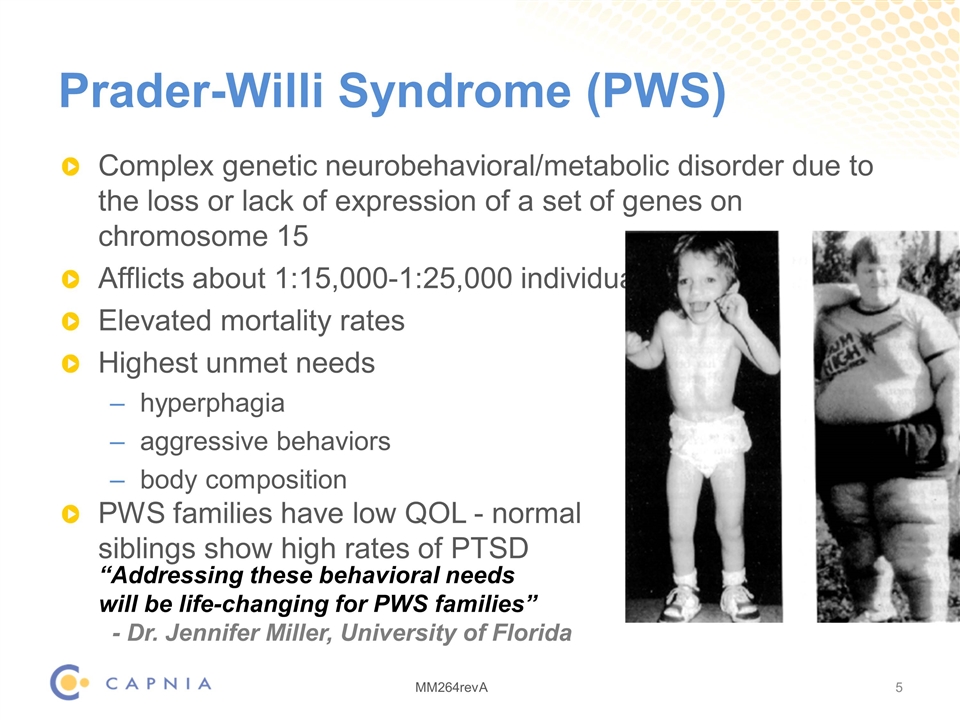
Prader-Willi Syndrome (PWS) Complex genetic neurobehavioral/metabolic disorder due to the loss or lack of expression of a set of genes on chromosome 15 Afflicts about 1:15,000-1:25,000 individuals Elevated mortality rates Highest unmet needs hyperphagia aggressive behaviors body composition PWS families have low QOL - normal siblings show high rates of PTSD “Addressing these behavioral needs will be life-changing for PWS families” - Dr. Jennifer Miller, University of Florida

Diazoxide – Long History of Safe Use DCCR – Extensive Pre-Clinical and Clinical Data Diazoxide I.V., Oral Capsule, and Oral Suspension KATP channel agonist approved in 1976 Previously used as IV treatment for malignant hypertension BID/TID oral suspension for the treatment of hypoglycemia due to hyperinsulinism in infants, children and adults - remains global standard of care Diazoxide Choline Controlled-Release (DCCR) Tablet QD tablet formulation of choline salt of diazoxide Characterized in 5 Phase I and 3 Phase II studies in obese, dyslipidemic and PWS subjects More than 210 treated subjects Protected by multiple issued patents, including composition of matter Diazoxide Choline
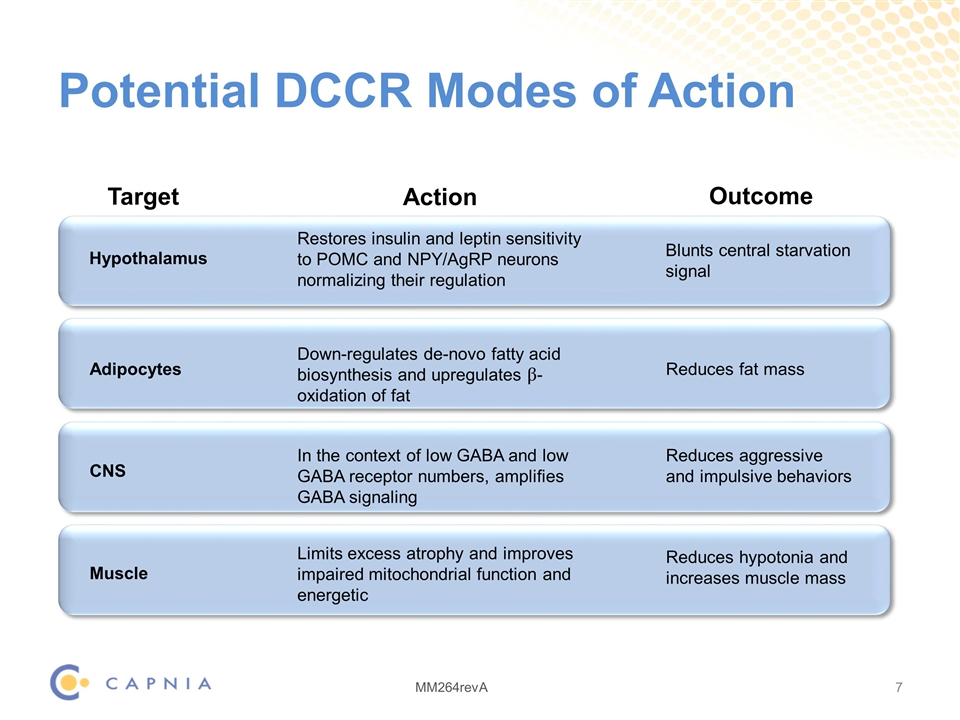
Potential DCCR Modes of Action Hypothalamus Restores insulin and leptin sensitivity to POMC and NPY/AgRP neurons normalizing their regulation Blunts central starvation signal Adipocytes Reduces fat mass Down-regulates de-novo fatty acid biosynthesis and upregulates β-oxidation of fat CNS Reduces aggressive and impulsive behaviors In the context of low GABA and low GABA receptor numbers, amplifies GABA signaling Muscle Reduces hypotonia and increases muscle mass Limits excess atrophy and improves impaired mitochondrial function and energetic Target Action Outcome
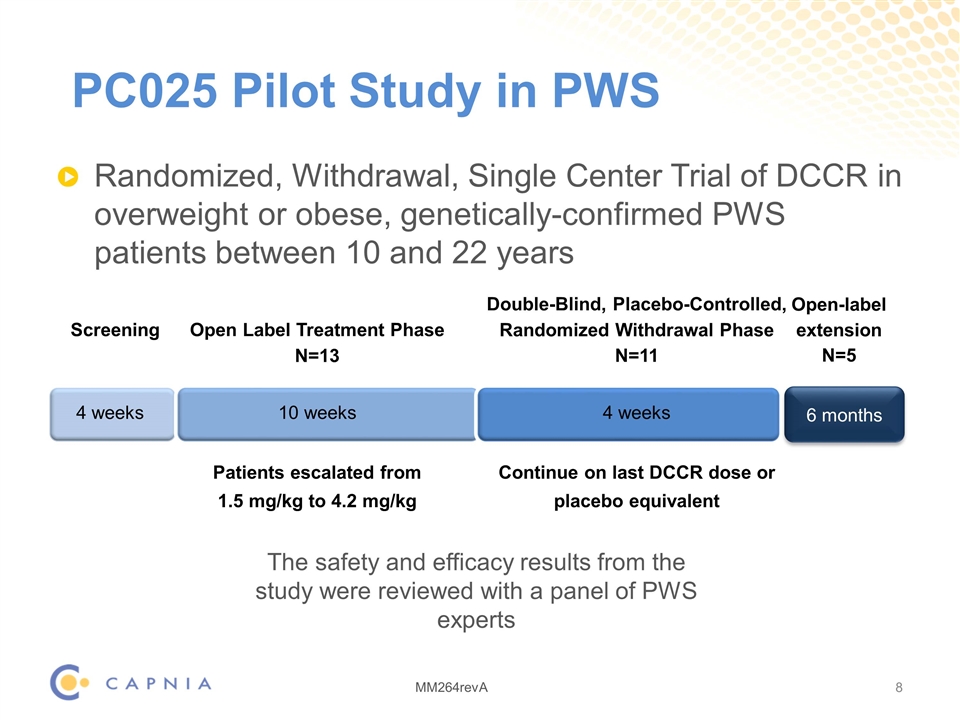
PC025 Pilot Study in PWS Randomized, Withdrawal, Single Center Trial of DCCR in overweight or obese, genetically-confirmed PWS patients between 10 and 22 years 6 months Open-label extension N=5 Screening Open Label Treatment Phase N=13 Double-Blind, Placebo-Controlled, Randomized Withdrawal Phase N=11 4 weeks 10 weeks 4 weeks Patients escalated from 1.5 mg/kg to 4.2 mg/kg Continue on last DCCR dose or placebo equivalent The safety and efficacy results from the study were reviewed with a panel of PWS experts
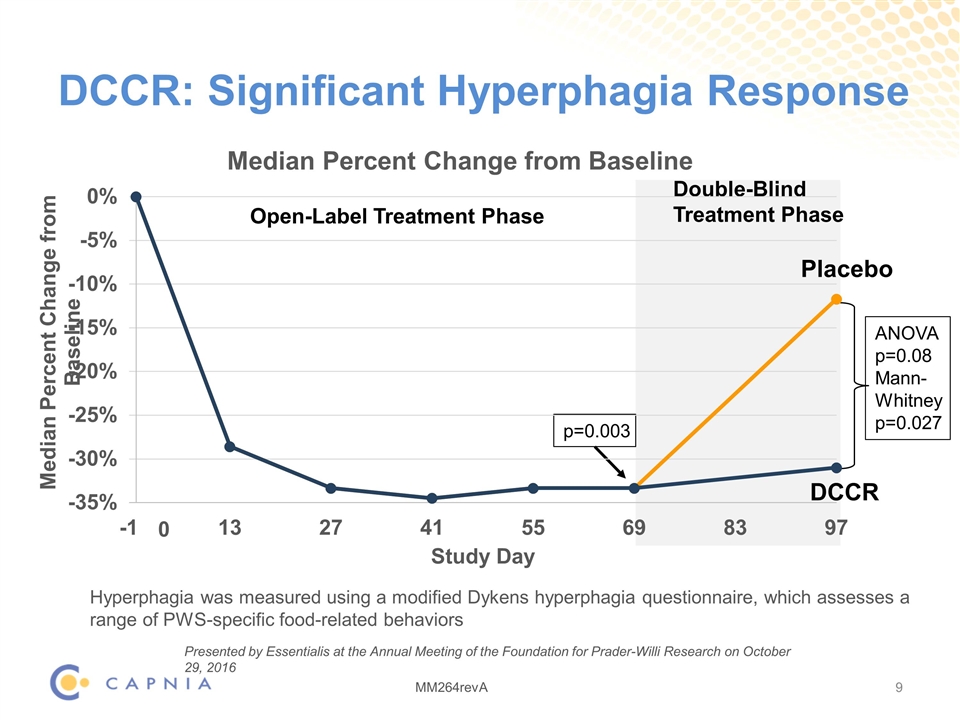
DCCR: Significant Hyperphagia Response DCCR Open-Label Treatment Phase 0 p=0.003 ANOVA p=0.08 Mann- Whitney p=0.027 Hyperphagia was measured using a modified Dykens hyperphagia questionnaire, which assesses a range of PWS-specific food-related behaviors Placebo Double-Blind Treatment Phase Presented by Essentialis at the Annual Meeting of the Foundation for Prader-Willi Research on October 29, 2016
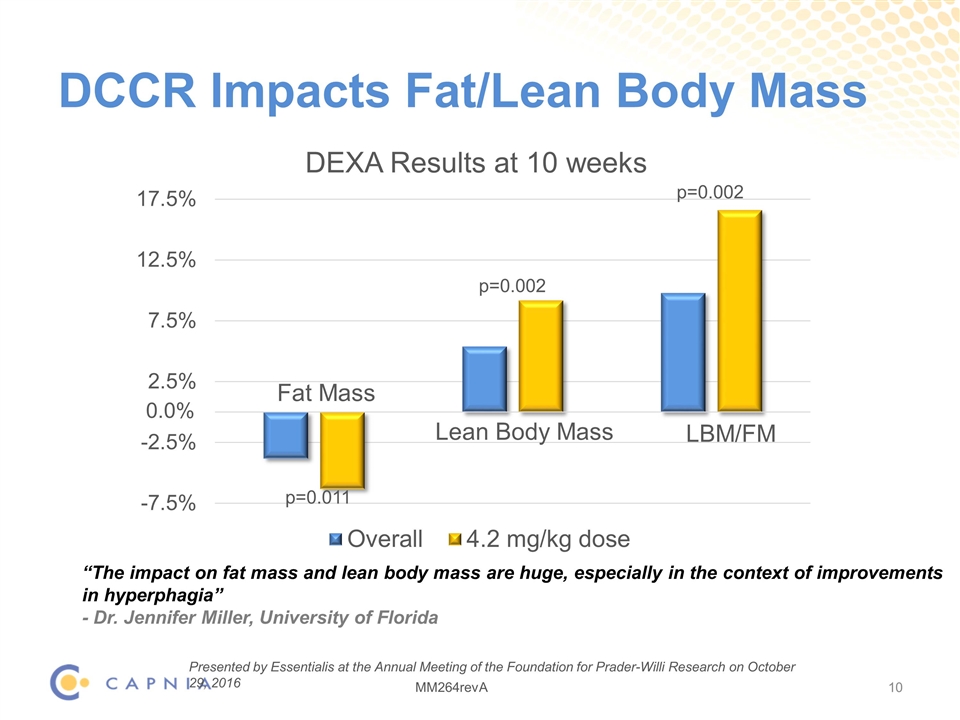
DCCR Impacts Fat/Lean Body Mass Fat Mass Lean Body Mass LBM/FM p=0.011 p=0.002 p=0.002 “The impact on fat mass and lean body mass are huge, especially in the context of improvements in hyperphagia” - Dr. Jennifer Miller, University of Florida Presented by Essentialis at the Annual Meeting of the Foundation for Prader-Willi Research on October 29, 2016

DCCR Reduces Aggressive Behaviors “From a family standpoint, the behavioral changes are huge. Aggression takes kids out of the home.” - Dr. Theresa Strong, FPWR “These behavioral changes can be life-changing for the family” - Dr. Jennifer Miller, University of Florida Based on the Behavioral Assessment Questionnaire from the Prader-Willi Syndrome Natural History Study DCCR improved a range of behaviors that are characteristic of PWS Throwing objects Foul language Destructive behavior
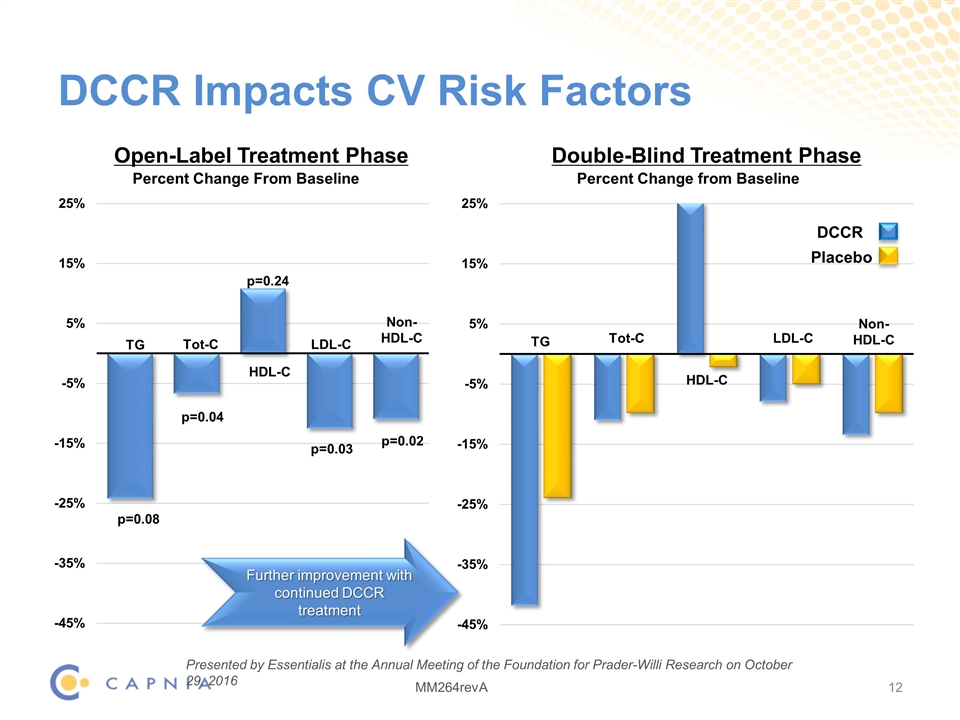
DCCR Impacts CV Risk Factors Open-Label Treatment Phase TG Tot-C HDL-C LDL-C Non- HDL-C p=0.08 p=0.04 p=0.24 p=0.03 p=0.02 TG Tot-C HDL-C LDL-C Non- HDL-C Double-Blind Treatment Phase DCCR Placebo Further improvement with continued DCCR treatment Presented by Essentialis at the Annual Meeting of the Foundation for Prader-Willi Research on October 29, 2016

The safety profile of Proglycem in chronic use is well-known In the development of DCCR, there have been no new safety findings The doses of DCCR that will continue in development are at the low end or below the labeled range for Proglycem More than 120,000 patient years of chronic use “The medication’s safety profile is well known. It’s a benign medication” - Dr. Jennifer Miller, University of Florida Diazoxide – Long History of Safe Use DCCR – Extensive Pre-Clinical and Clinical Data
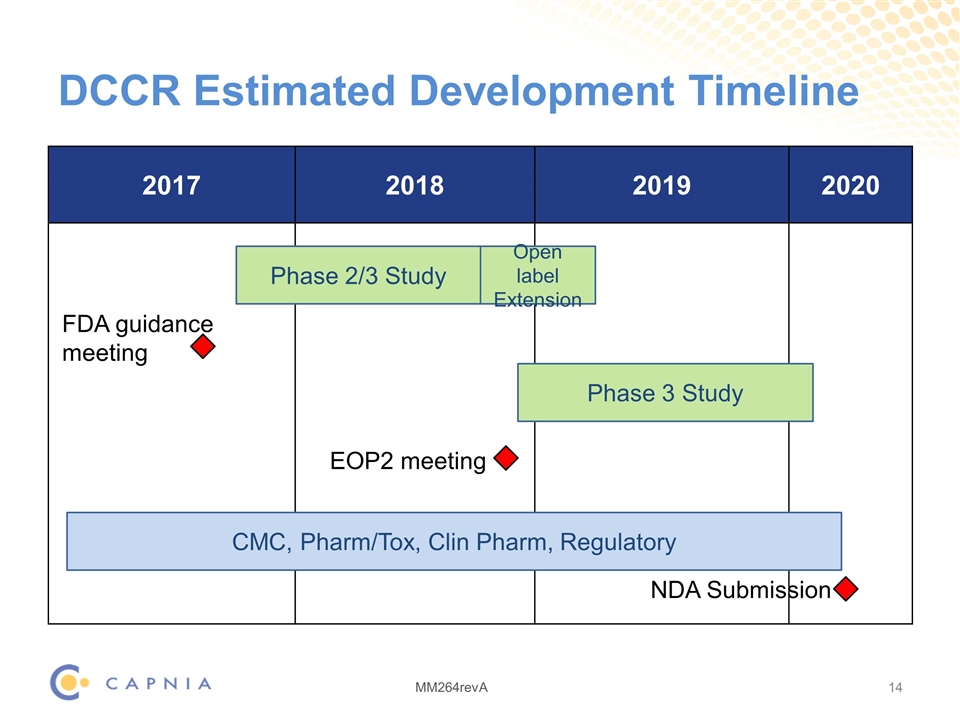
DCCR Estimated Development Timeline 2017 2018 2019 2020 Phase 2/3 Study Phase 3 Study CMC, Pharm/Tox, Clin Pharm, Regulatory NDA Submission Open label Extension EOP2 meeting FDA guidance meeting
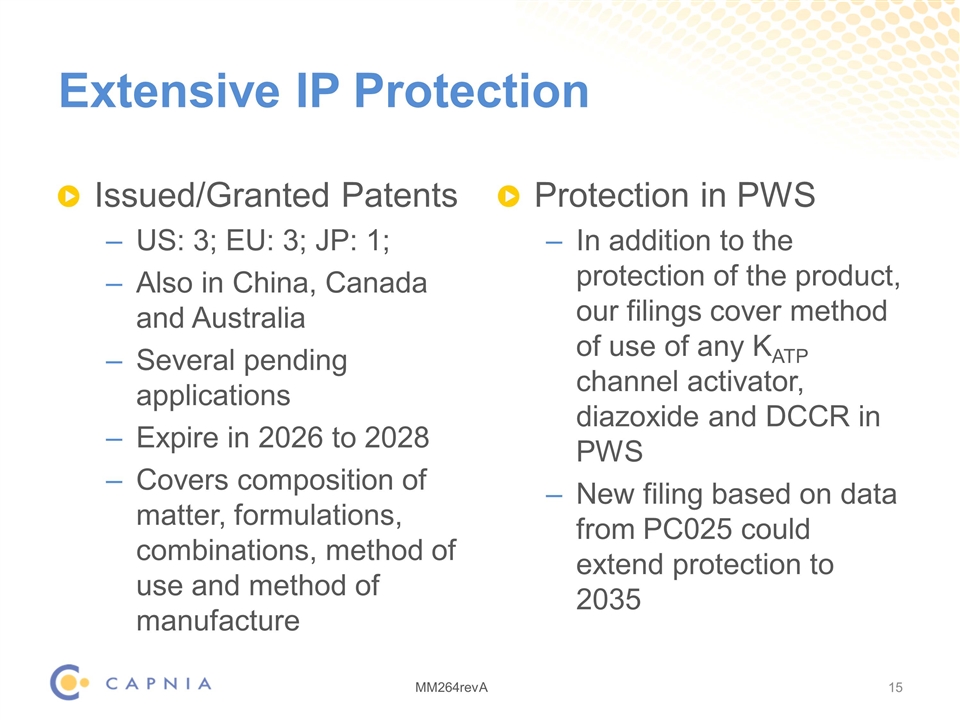
Extensive IP Protection Issued/Granted Patents US: 3; EU: 3; JP: 1; Also in China, Canada and Australia Several pending applications Expire in 2026 to 2028 Covers composition of matter, formulations, combinations, method of use and method of manufacture Protection in PWS In addition to the protection of the product, our filings cover method of use of any KATP channel activator, diazoxide and DCCR in PWS New filing based on data from PC025 could extend protection to 2035
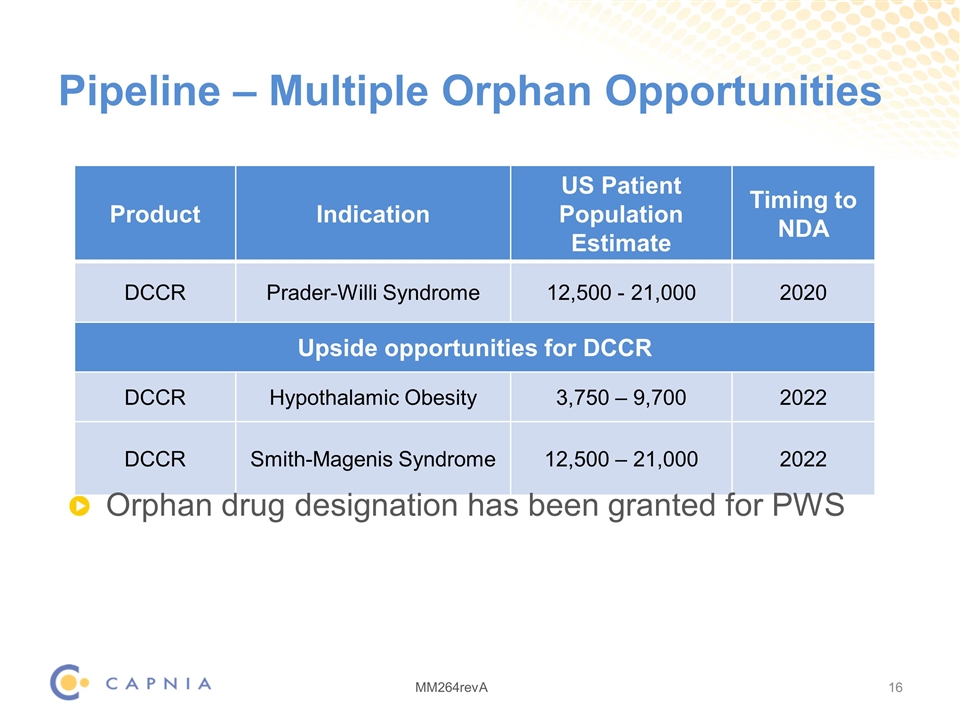
Pipeline – Multiple Orphan Opportunities Product Indication US Patient Population Estimate Timing to NDA DCCR Prader-Willi Syndrome 12,500 - 21,000 2020 Upside opportunities for DCCR DCCR Hypothalamic obesity Hypothalamic Obesity 3,750 – 9,700 2022 DCCR Smith-Magenis syndrome Smith-Magenis Syndrome 12,500 – 21,000 2022 Orphan drug designation has been granted for PWS

Capnia Product Portfolio

Diversified, Revenue Generating Product Portfolio
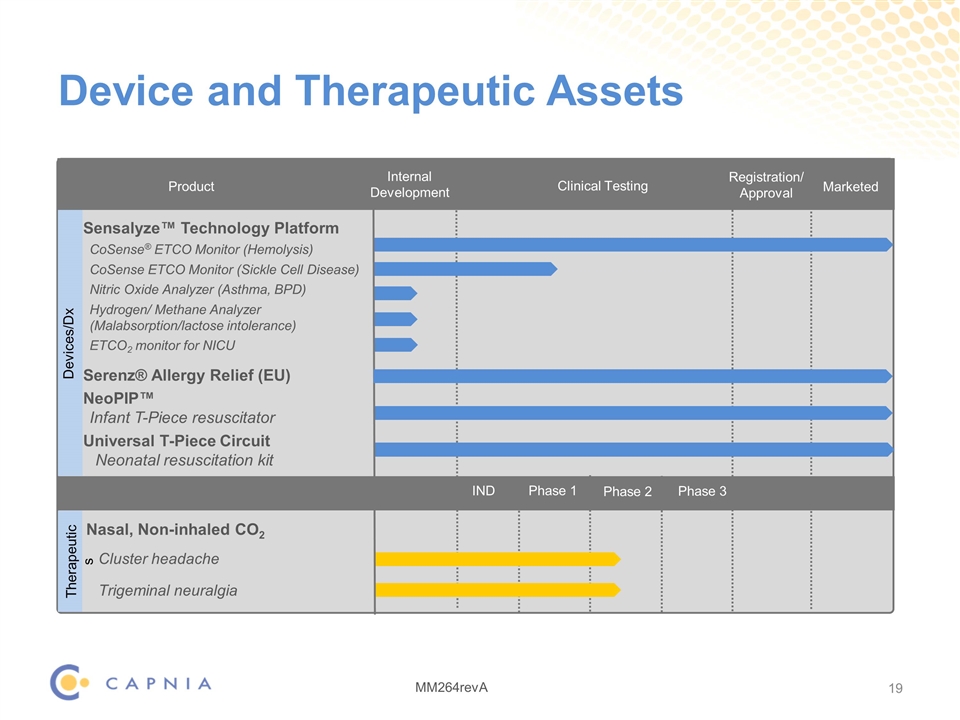
Device and Therapeutic Assets Product Internal Development Marketed Sensalyze™ Technology Platform CoSense® ETCO Monitor (Hemolysis) CoSense ETCO Monitor (Sickle Cell Disease) Nitric Oxide Analyzer (Asthma, BPD) Hydrogen/ Methane Analyzer (Malabsorption/lactose intolerance) ETCO2 monitor for NICU Serenz® Allergy Relief (EU) NeoPIP™ Infant T-Piece resuscitator Universal T-Piece Circuit Neonatal resuscitation kit Nasal, Non-inhaled CO2 Cluster headache Trigeminal neuralgia Registration/ Approval Therapeutics Devices/Dx Clinical Testing Phase 1 Phase 2 Phase 3 IND

2017 Priorities and Milestones 1Q17 – Close merger transaction with Essentialis; complete concurrent $8M financing Mid-2017 – Complete FDA guidance meeting for DCCR 2H17 – Initiate Phase 2/3 clinical study evaluating DCCR for the treatment of PWS 2017 – Explore strategic alternatives for legacy marketed products and product candidates 2017 – Secure orphan drug designation for DCCR in additional indications beyond PWS
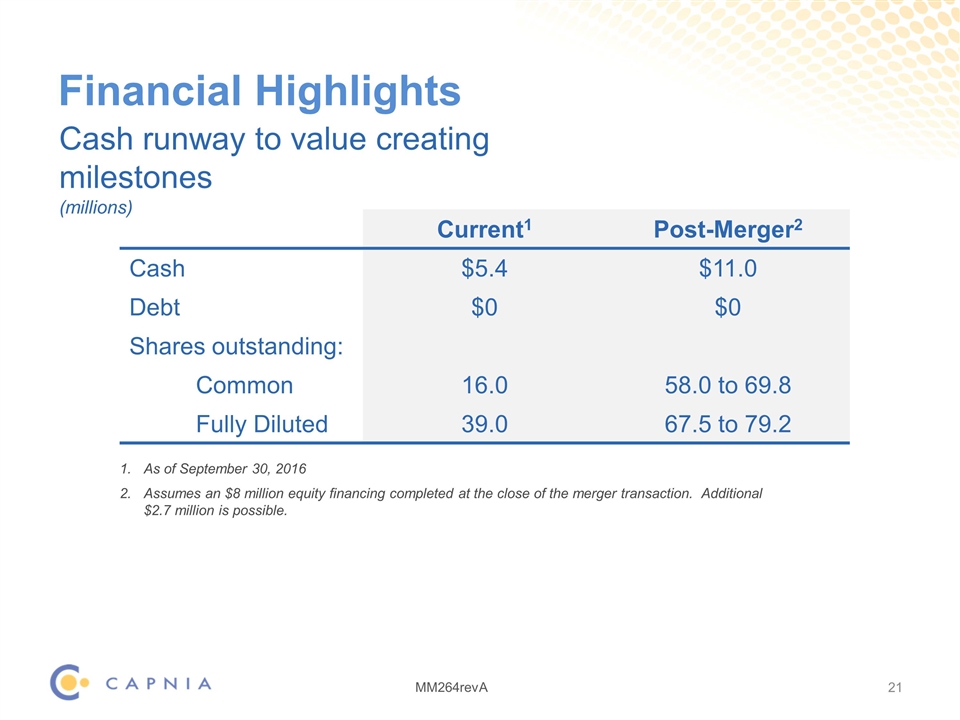
Financial Highlights As of September 30, 2016 Assumes an $8 million equity financing completed at the close of the merger transaction. Additional $2.7 million is possible. Cash runway to value creating milestones (millions) Current1 Post-Merger2 Cash $5.4 $11.0 Debt $0 $0 Shares outstanding: Common 16.0 58.0 to 69.8 Fully Diluted 39.0 67.5 to 79.2
Highlights Capnia and Essentialis to Merge Creating a rare disease therapeutics company Initial focus on PWS, a high unmet need indication with no approved treatments Compelling product profile Addresses hallmark symptoms of PWS, including hyperphagia Phase 2/3 ready Established, decades-long safety profile Strong IP Financing concurrent with planned merger Adequate to advance DCCR through top-line data Multiple downstream orphan indications Hypothalamic obesity, Smith-Magenis syndrome New Lead Asset DCCR Well-Financed Through Key Milestones Longer-Term Pipeline Opportunities Strong Leadership Highly experienced management team Expertise in drug development for rare and orphan diseases

NASDAQ: CAPN www.capnia.com Thank you.
