Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a17-1645_1ex99d1.htm |
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a17-1645_18k.htm |
Exhibit 99.2
35th Annual J.P. Morgan Healthcare Conference John F. Crowley, Chairman and Chief Executive Officer January 10, 2017

2 Introduction Safe Harbor This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of our product candidates, the timing and reporting of results from preclinical studies and clinical trials, the prospects and timing of the potential regulatory approval of our product candidates, commercialization plans, financing plans, and the projected cash position for the Company. In particular, this presentation relates to the preliminary data from a global Phase 1/2 study (ATB200-02) to investigate ATB200/AT2221. The inclusion of forward-looking statements arising from this preliminary data and study should not be regarded as a representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this presentation may turn out to be wrong and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing, and outcomes of discussions with regulatory authorities, and in particular the potential goals, progress, timing, and results of preclinical studies and clinical trials, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in our business, including, without limitation: the potential that results of clinical or preclinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities, including the FDA, EMA, and PMDA, may not grant or may delay approval for our product candidates; the potential that we may not be successful in commercializing Galafold in Europe or our other product candidates if and when approved; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; and the potential that we will need additional funding to complete all of our studies. Further, the results of earlier preclinical studies and/or clinical trials may not be predictive of future results. The preliminary data and Phase 1/2 study discussed herein is inherently preliminary and early in the study, derived from a limited patient set, and later trial results with this patient set or others may not be consistent with these preliminary results. With respect to statements regarding projections of our cash position, actual results may differ based on market factors and our ability to execute operational and budget plans. In addition, all forward-looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the year ended December 31, 2015 and Quarterly Report on Form 10-Q for the quarter ended September 30, 2016. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update this presentation to reflect events or circumstances after the date hereof.

3 Introduction Building a Top Global Biotech in Devastating Rare Diseases 1 BREAKTHROUGH THERAPY DESIGNATION FIRST ORAL PRECISION MEDICINE FOR FABRY DISEASE TREATING PATIENTS IN 24 COUNTRIES WORLD CLASS SCIENCE & DRUG DEVELOPMENT Two Phase 3 PROGRAMS (FABRY & EB) PROTEIN ENGINEERING & GLYCOBIOLOGY $331M CASH BALANCE $3B+ MARKET OPPORTUNITY FOR CURRENT PIPELINE ATB200/AT2221 NOVEL TREATMENT PARADIGM FOR POMPE IN PHASE 1/2 3 PROGRAMS IN CLINIC IN 3 RARE DISEASES
4 Introduction Key Accomplishments in 2016 2016 • • • EU approval International launch success Regulatory progress Fabry Disease (Galafold™) Pompe Disease (ATB200/AT2221) • Positive preliminary data in Phase 1/2 study in Pompe patients Epidermolysis Bullosa (EB) (SD-101) • Phase 3 enrollment near complete Strong Balance Sheet • $331M in cash (12/31/16)
5 Introduction 2017 Key Strategic Priorities Advance International Galafold Launch Submit Japanese New Drug Application (J-NDA) for Migalastat Establish Definitive Proof of Concept for ATB200/AT2221 with Clear Path to Registration for Pompe Disease Successfully Complete Phase 3 EB Study Maintain Financial Strength We Remain Sharply Focused on FIVE Key Strategic Priorities as We Continue to Build a Top Global Biotechnology Company Focused on Rare Devastating Diseases
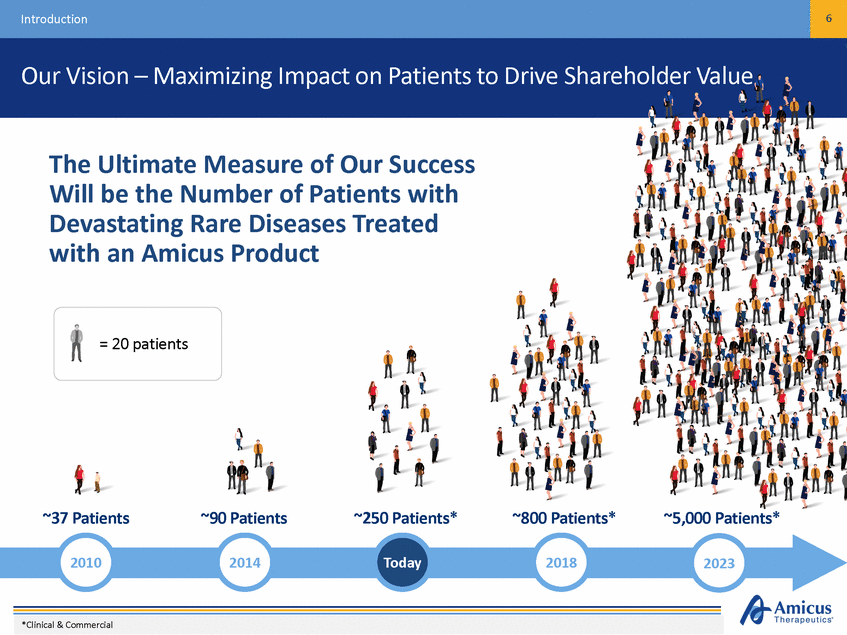
6 Introduction Our Vision – Maximizing Impact on Patients to Drive Shareholder Value The Ultimate Measure of Our Success Will be the Number of Patients with Devastating Rare Diseases Treated with an Amicus Product = 20 patients ~37 Patients ~90 Patients ~250 Patients* ~800 Patients* ~5,000 Patients* 2010 2014 Today 2018 2023 *Clinical & Commercial
Galafold™ (Migalastat) Precision Medicine for Fabry Disease Continue Launch Execution and Geographic Expansion

Galafold: Precision Medicine for Fabry Disease 8 Fabry Disease Overview Leading Causes of Death TRANSIENT ISCHEMIC Life-Limiting Symptoms GASTROINTESTINAL3 and diarrhea • ATTACK (TIA) & STROKE1 Nausea, vomiting, cramping, • Pain/bloating after eating, feeling full Constipation Difficulty managing weight HEART DISEASE2 • • • • • Irregular heartbeat (fast or slow) Heart attack or heart failure Enlarged heart Key Facts Deficiency of α-Gal A enzyme leading to GL-3 accumulation >900 known mutations 5-10K diagnosed WW (51% female/49% male4) Newborn screening studies suggest prevalence of ~1:1000 to ~1:4000 KIDNEY DISEASE3 • • • • Protein in the urine Decreased kidney function Kidney failure • • • 1. Desnick R, et al. Ann Intern Med. 2003 2. Yousef Z, et al. Eur Heart J. 2013 3. Germain D. Orphanet J Rare Dis. 2010 4. Fabry Registry 2011 Fabry Disease is a Fatal Genetic Disorder that Affects Multiple Organ Systems
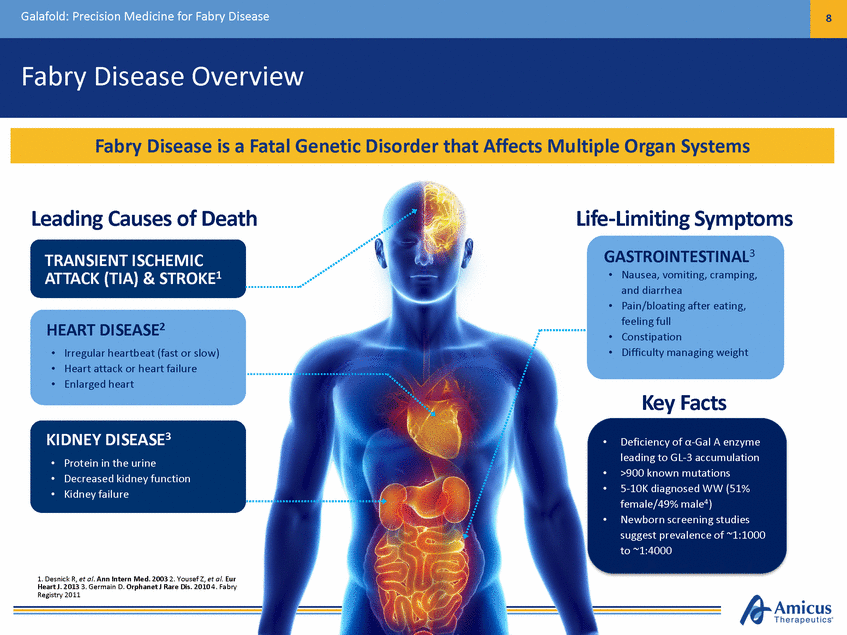
9 Galafold: Precision Medicine for Fabry Disease Precision Medicine Driven by a Patient’s Genotype Amicus Therapeutics is Committed to Innovative R&D to Develop the Highest Future Vision Novel ERT co-formulated with migalastat Growing to ~$2B Global Fabry market Quality Therapies Fabry Patients for ALL ~$1.2B Global Fabry market today Non-Amenable Today Migalastat Oral precision medicine Non-Amenable Amenable Amenable (35-50% of patients) *Artist rendering, not actual product image
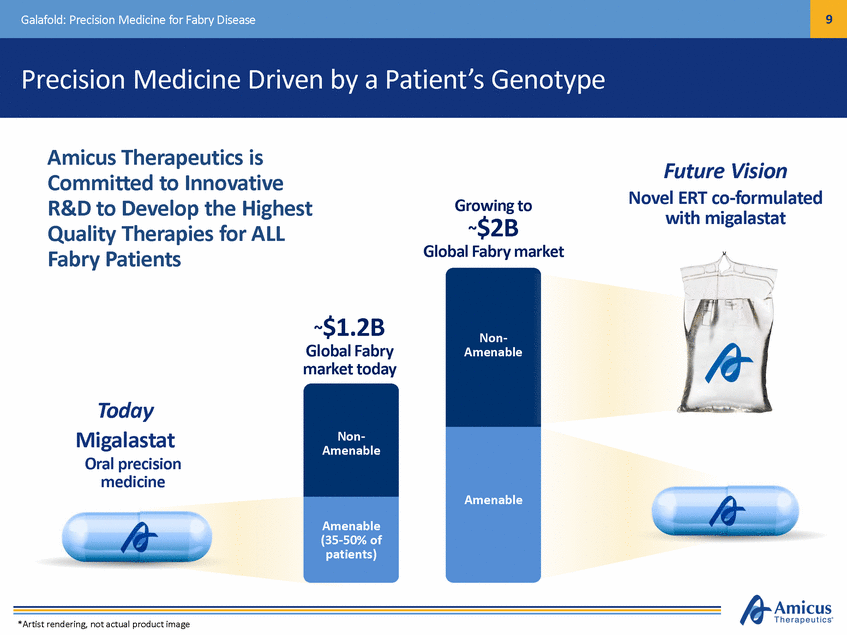
10 Galafold: Precision Medicine for Fabry Disease Full EU Approval as First Oral Precision Medicine for Fabry Disease FIRST new treatment option for Fabry in more than a FIRST oral precision medicine for Fabry disease Galafold Indicated for Long-Term Treatment of Adults and Adolescents Aged > 16 years with a Confirmed Diagnosis of Fabry Disease and Who have an Amenable Mutation3 Strong safety profile, most common side effect reported in clinical trials was headache FIRST searchable, electronic pharmacogenetic label • Approved May 30, 2016 • Launch exceeding expectations Label expanded from 269 to include 313 amenable mutations Established efficacy from 2 pivotal studies (ERT-switch & naïve patients)1,2,3 1.Germain, DP et al., New England Journal of Medicine. 2. Hughes, et al., Journal of Medical Genetics. 3. For important safety information for Galafold visit www.ema.europa.eu.
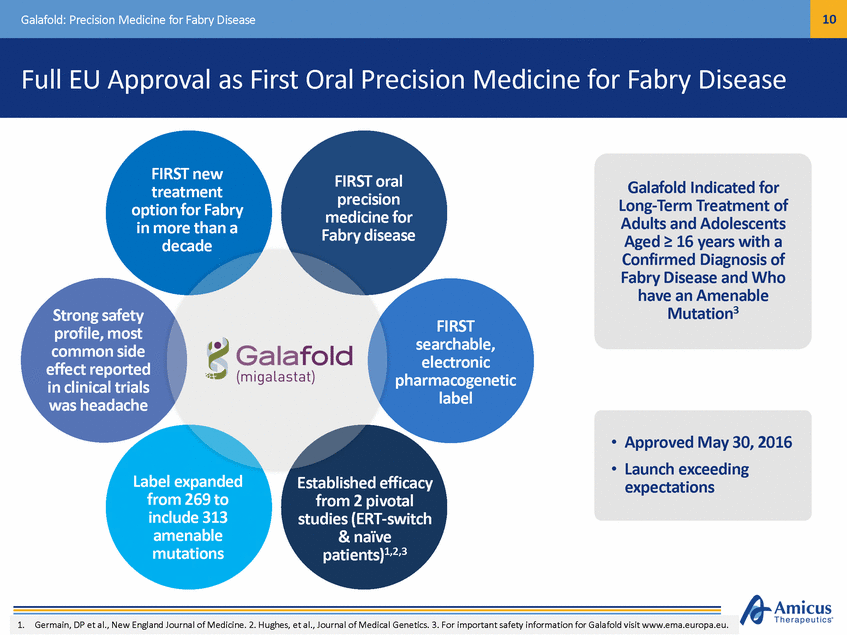
11 Galafold: Precision Medicine for Fabry Disease Galafold Commercial Opportunity Geographic Segments Patient Segments Undiagnosed Diagnosed Untreated U.S. 27% ROW 26% ERT-Treated Japan 13% EU 34% amenable mutations = • • • 5k-10k Patients Diagnosed WW 40%-50% of Diagnosed Patients not on ERT Newborn Screening Studies Suggest Prevalence of ~1:1000 to ~1:40002 1. Company filings and Amicus estimates 2. Burton, LDN WORLD Symposium, 2012 Feb. Mechtler et al., The Lancet, 2011 Dec. Hwu et al., Hum Mutation, 2009 Jun. Spada et al., Am J Human Genet., 2006 Jul Prioritizing EU, Japan, and Other Large Fabry Markets to Address Patients with Amenable Mutations (35%-50% of Fabry Population)
12 Galafold: Precision Medicine for Fabry Disease Early Success with International Launch (as of 12/31/16) Patients (Switch & Naïve) on reimbursed Galafold (12/31/16) 61 300 Countries with available reimbursement* 6 Countries with pricing discussions ongoing 18 Target Number of Patients on Reimbursed Galafold by YE17 22 Countries with Amicus footprint *Commercial and Expanded Access Programs (EAPs) Initial Launch Success Driven by Germany with ERT-Switch & Naïve Patients, Reimbursement Now Available in 6 Countries*
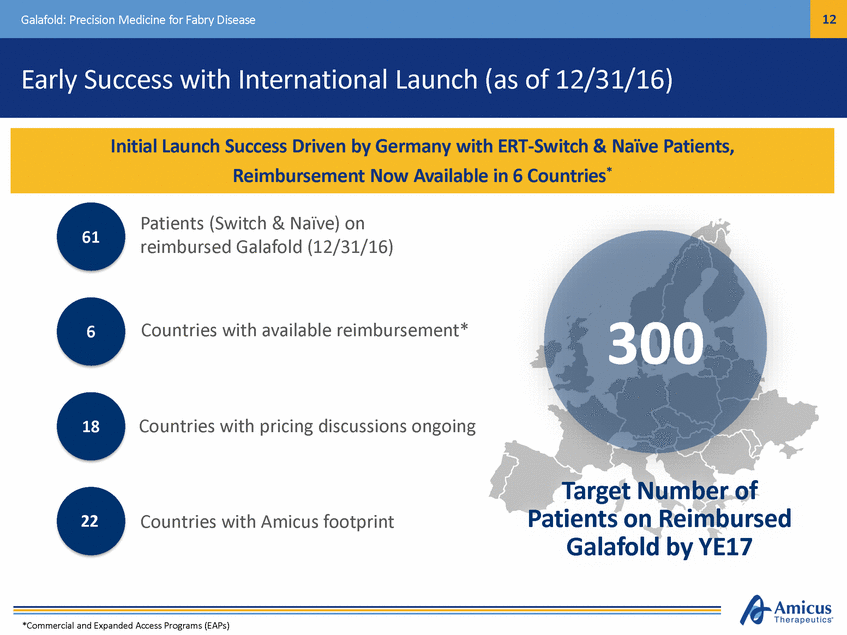
13 Galafold: Precision Medicine for Fabry Disease German Launch Update (as of 12/31/16) Current Approximate Market Share* IMPORTANT EARLY INDICATORS IN GERMANY • • Vast majority switch patients ~25% of eligible switch patients now on Galafold* All newly experienced patients & physicians Majority of switches from Replagal™ Male / female mix 13 unique prescribers Galafold ~25% • ERT ~75% • • • *Market share assumptions based on estimated number of ERT-treated patients with amenable mutations in Germany as of May 2016 Germany is an Important Indicator for EU Launch Success
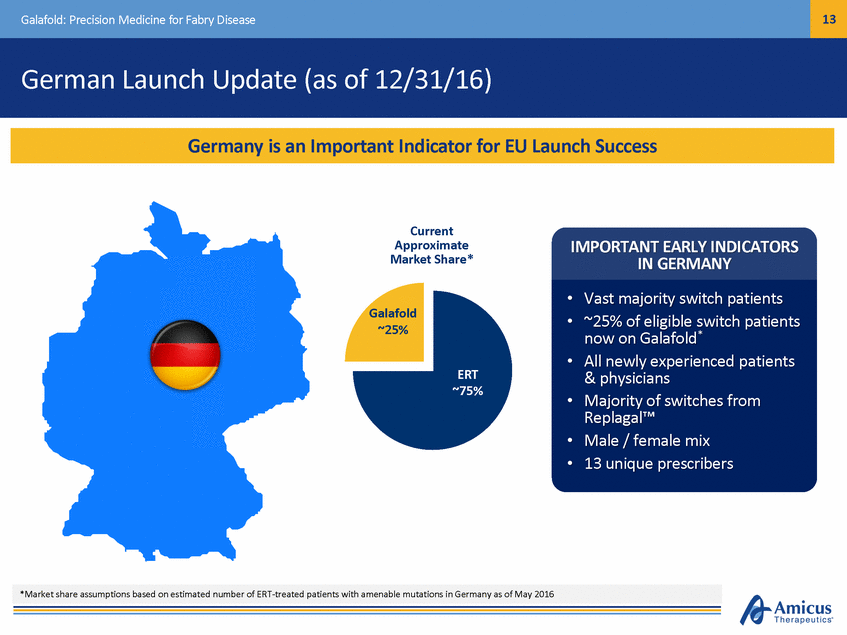
14 Galafold: Precision Medicine for Fabry Disease EU Launch Strategy INITIAL FOCUS ON TOP 5 COUNTRIES INVEST IN KEY MID-SIZED EU COUNTRIES AND SELECT EAP OPPORTUNITIES • • • • • Germany France, Italy, Spain, UK ~2,000 Fabry patients treated ~70-75% of EU market value ~25% of global Fabry market • • • Austria, Nordics (4), Netherlands, Belgium, etc. ~10% of EU market value Selectively invest in key EAP markets Focus on EU Top 5 Plus Key Mid-Sized EU Markets in 2017
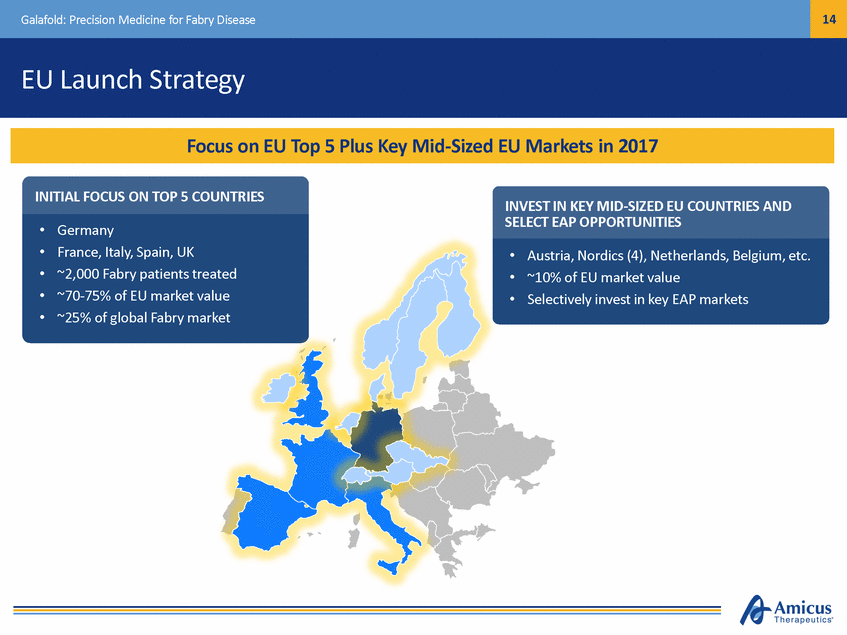
15 Galafold: Precision Medicine for Fabry Disease Global Regulatory Strategy to Reach More Patients EU Approval and Launch (May 30, 2016) U.S. Full Approval Pathway Defined by Amicus Japanese NDA Targeted 1H17 Active Expanded Access Programs (EAP) in 2 Territories with More Initiated 1 Additional Approval (Switzerland) 6 Additional Regulatory Submissions Complete, Process Initiated in Other Key Geographies EU Approval is Gateway to ~75% of Global ERT Market
16 Novel Proprietary Fabry ERT Amicus Proprietary Fabry ERT Building on Biologics Capabilities and CHART™ Platform to Develop Differentiated Novel ERT Development status: •Cell line transferred to manufacturer •Preclinical data update in 2017 Fabry ERT Target Product Profile: • • • • Improved drug targeting to key tissues Significantly more potent dose delivery Co-formulation with chaperone to enhance stability Dosing flexibility
ATB200 Novel ERT for Pompe Disease Establishing Human Proof of Concept and Validating Biologics Platform in 2017

18 Pompe Disease Pompe Disease Overview Respiratory and cardiac failure are leading causes of morbidity and mortality Deficiency of GAA leading to glycogen accumulation Age of onset ranges from infancy to adulthood Symptoms include muscle weakness, respiratory failure, and cardiomyopathy 5,000 – 10,000 patients diagnosed WW1 ~$800M+ Global Pompe ERT sales in FY152 1. National Institute of Neurological Disorders and Stroke (NIH). 2. Sanofi Press Release & 10-K Devastating Disease Symptoms Persist Across a Broad Spectrum of Patients Despite Available Therapy

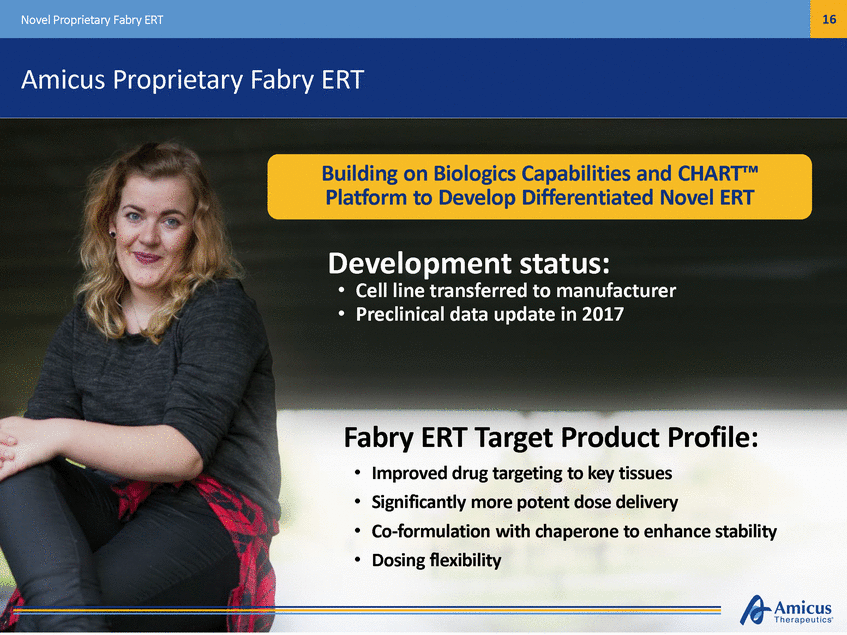
19 Novel ERT for Pompe Disease – ATB200 + Chaperone Pompe Disease: A Complex Disease with Significant Unmet Needs Enhanced levels of M6P Improved in vitro cellular uptake Enhanced ERT activity & stability preclinical clinical Desired PK profile & half-life key questions Excellent tolerability Early biomarkers of efficacy Safety in ERT-switch Optimized glycosylation Greater ERT uptake into muscle in Pompe KO mice Greater glycogen reduction in Pompe KO mice We’ve Made Great Strides and Expect to Address Key Remaining Questions in 2017
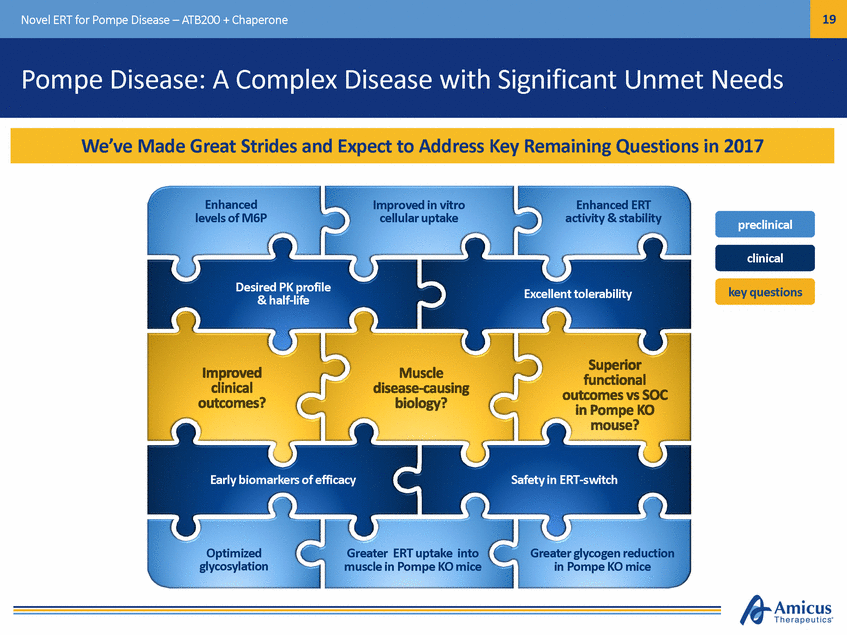
20 Novel ERT for Pompe Disease – ATB200 + Chaperone ATB200 + Chaperone: A Highly Differentiated Approach Optimized mixture of glycans ATB200 (Novel ERT) High levels of M6P and bis M6P Chaperone addition *Artist rendering, not actual product image Novel Pompe Treatment Paradigm with Three Key Differentiators
21 Novel ERT for Pompe Disease – ATB200 + Chaperone Biologics Manufacturing Capabilities Proprietary Process 1000L (Registration Trial & Commercial Scale) 2016-2017+ 5L (Bench Scale) 2013 250L (Clinical Scale) 2014-2015+ Research Scale / MCB Highly Successful Biologics Manufacturing Scale-up in Three Years

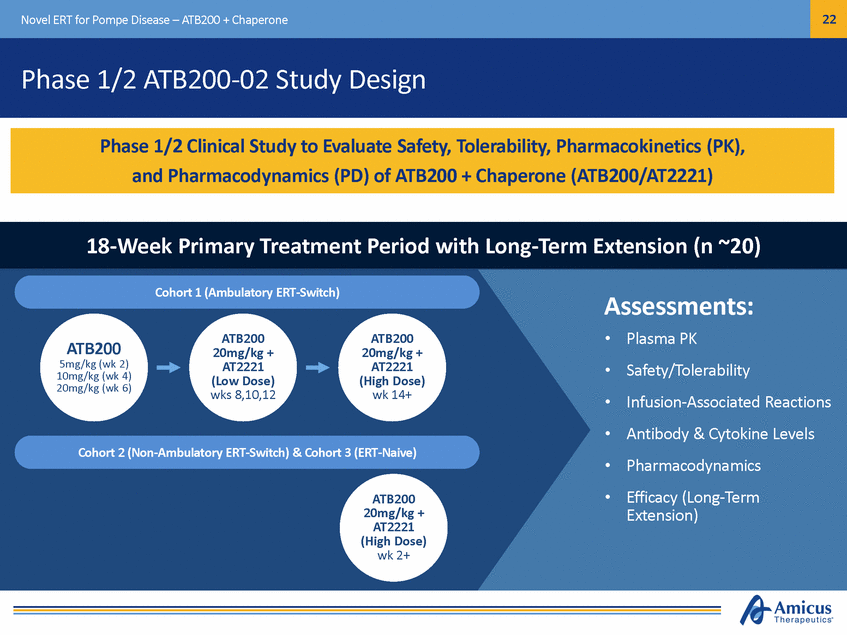
22 Novel ERT for Pompe Disease – ATB200 + Chaperone Phase 1/2 ATB200-02 Study Design 18-Week Primary Treatment Period with Long-Term Extension (n ~20) Cohort 1 (Ambulatory ERT-Switch) Assessments: • • • • • • Plasma PK Safety/Tolerability Infusion-Associated Reactions Antibody & Cytokine Levels Pharmacodynamics Efficacy (Long-Term Extension) ATB200 20mg/kg + AT2221 (Low Dose) wks 8,10,12 ATB200 20mg/kg + AT2221 (High Dose) wk 14+ ATB200 5mg/kg (wk 2) 10mg/kg (wk 4) 20mg/kg (wk 6) Cohort 2 (Non-Ambulatory ERT-Switch) & Cohort 3 (ERT-Naive) ATB200 20mg/kg + AT2221 (High Dose) wk 2+ Phase 1/2 Clinical Study to Evaluate Safety, Tolerability, Pharmacokinetics (PK), and Pharmacodynamics (PD) of ATB200 + Chaperone (ATB200/AT2221)
23 Pompe Phase 1/2 Study ATB200-02 Preliminary Data Preliminary Data Summary • • No serious adverse events (SAEs) AEs generally mild and transient Safety (n=9)* Tolerability • No infusion-associated reactions following 100+ infusions PK (n=4)** • • • Clinical PK profile as predicted consistent with preclinical data ATB200 plasma clearance rate suggests efficient tissue uptake ATB200 alone showed greater than dose-proportional increases in exposure, further enhanced with AT2221 • • Early trend to improvement in 2 patients Stable in 2 patients Muscle damage biomarkers (CK, AST, ALT) (n=4) • • Anti-rhGAA antibodies remained generally stable Cytokines remained low and stable during infusions Immunogenicity (n=4) *N = 8 from Cohort 1 (Ambulatory ERT-Switch) and 1 from Cohort 1 (Non-Ambulatory ERT-Switch); through interim data analysis (maximum 24 weeks) **N = 4 from Cohort 1 ATB200/AT2221 Demonstrates Promising Preliminary Results in First ERT-Switch Patients at the Targeted Therapeutic Dose
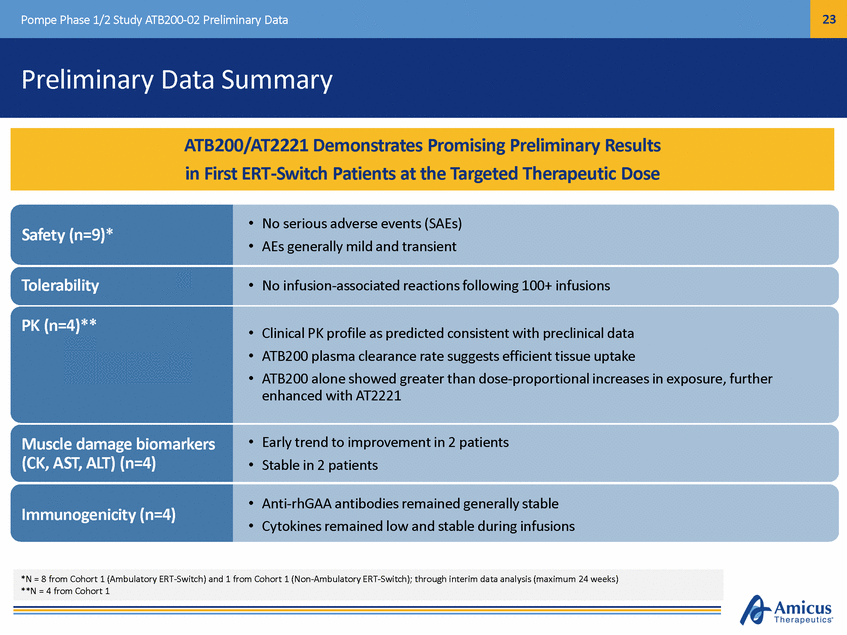
24 Pompe Phase 1/2 Study ATB200-02 Preliminary Data Pharmacokinetics at Week 14: Plasma Exposure (n=4)* Mean GAA Total Protein (n=4) 5, 10, 20 mg/kg ATB200 Alone Mean GAA Total Protein (n=4) 20 mg/kg ATB200 + AT2221 20 mg/KG 20 mg/KG 20 mg/KG + low dose AT2221 10 mg/KG 20 mg/KG + high dose AT2221 5 mg/KG (L/hr) *N = 4 from Cohort 1 (Ambulatory ERT-Switch) Treatment Mean AUC0-∞ (hr*g/ml) Mean Clearance 20 mg/kg 1547 1.11 +low dose AT2221 1676 1.03 +high dose AT2221 1945 0.90 Treatment Mean AUC0-∞ (hr*g/ml) Mean Clearance (L/hr) 5 mg/kg 215 1.97 10 mg/kg 589 1.45 20 mg/kg 1547 1.11 ATB200 Clinical PK Profile as Predicted Based on Preclinical Studies with Greater than Dose Proportional Increases in Exposure that were Enhanced by AT2221
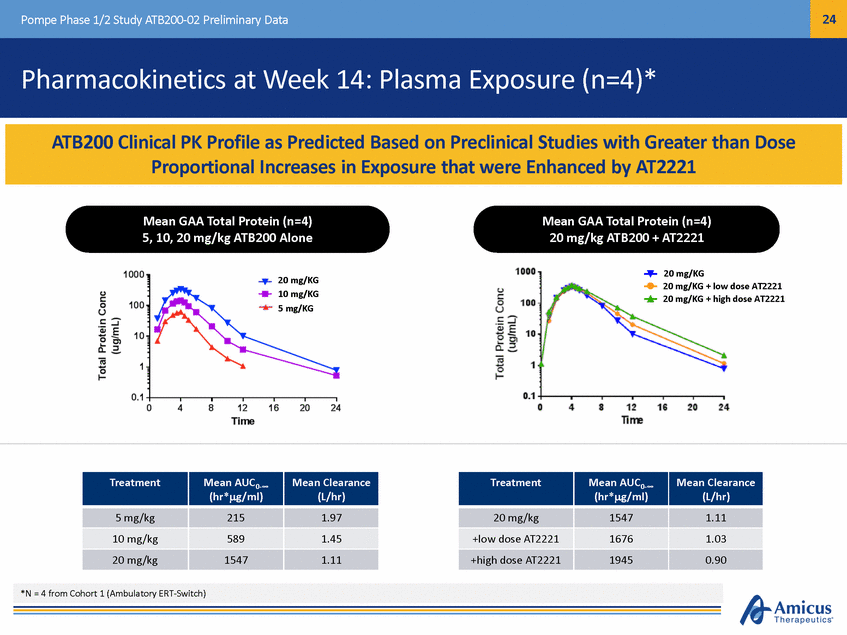
25 Pompe Phase 1/2 Study ATB200-02 Preliminary Data Muscle Damage Biomarkers at Week 14 (n=4)* Two patients showed early trend toward improvement in all three biomarkers: Patient 1 Patient 2 Elevated creatine kinase (CK), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) are indicators of damage to muscle tissue -11% -22% -31% -34% -44% Two patients remained stable *N = 4 from Cohort 1 (Ambulatory ERT-Switch) ALT AST CK -28% ALT AST CK After Switching from Lumizyme™ to ATB200/AT2221, Muscle Damage Biomarkers (CK, AST, ALT) Trended Toward Early Improvement in Two Patients and Were Stable in the Other Two Patients
26 Novel ERT for Pompe Disease – ATB200 + Chaperone Pompe Clinical Study ATB200-02 Data Cascade Pompe Milestones in 2017 Data in non-ambulatory ERT-switch patients (Cohort 2) Data in ERT-naïve patients (Cohort 3) Additional extension study data (all Cohorts) Meeting with U.S. and EU regulators Additional data & initial extension data in Cohort 1 18-WEEK DATA EXTENSION DATA • • •Biomarkers •Immunogenicity Safety / tolerability Pharmacokinetics (PK) •Motor/pulmonary function A Cascade of Additional Data Points During 2017 to Demonstrate Proof of Concept

SD-101 for Epidermolysis Bullosa Potential First-in-Class Treatment with Phase 3 Data Anticipated Mid-2017
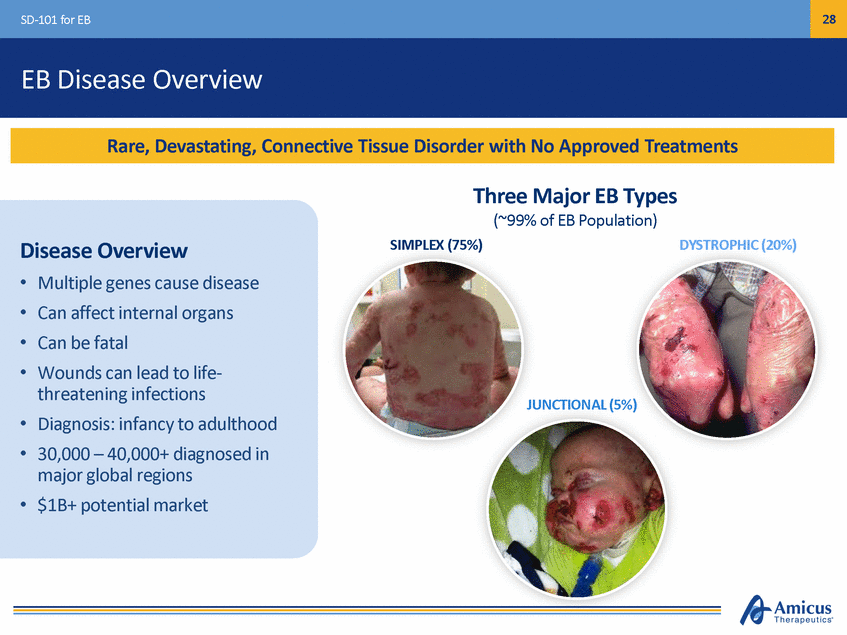
28 SD-101 for EB EB Disease Overview Three Major EB Types (~99% of EB Population) SIMPLEX (75%) DYSTROPHIC (20%) Disease Overview • • • • Multiple genes cause disease Can affect internal organs Can be fatal Wounds can lead to life-threatening infections Diagnosis: infancy to adulthood 30,000 – 40,000+ diagnosed in major global regions $1B+ potential market JUNCTIONAL (5%) • • • Rare, Devastating, Connective Tissue Disorder with No Approved Treatments
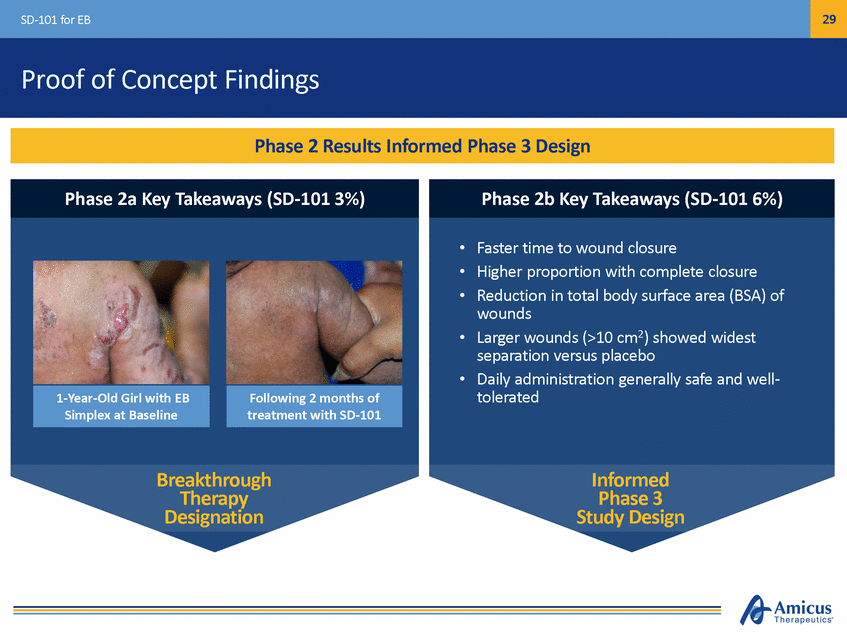
29 SD-101 for EB Proof of Concept Findings • • • Faster time to wound closure Higher proportion with complete closure Reduction in total body surface area (BSA) of wounds Larger wounds (>10 cm2) showed widest separation versus placebo Daily administration generally safe and well-tolerated • • Breakthrough Therapy Designation Informed Phase 3 Study Design Following 2 months of treatment with SD-101 1-Year-Old Girl with EB Simplex at Baseline Phase 2b Key Takeaways (SD-101 6%) Phase 2a Key Takeaways (SD-101 3%) Phase 2 Results Informed Phase 3 Design
30 SD-101 for EB Phase 3 Study - Delivering on Our EB Vision • • • Sample size of up to 150 patients Larger baseline target wound size Time to wound closure endpoint elevated • • • 95%+ participation in extension study Enrollment near complete Top-line data anticipated mid-2017 Status SD-005 Study Design Optimized Phase 3 Study Optimized for Success with Top-Line Data Anticipated Mid-2017
31 CDKL5 Deficiency Program Cyclin-Dependent Kinase-Like 5 (CDKL5) Deficiency Disease Overview • Genetic mutations in CDKL5 gene result in deficient protein essential for normal brain development Persistent, spontaneous seizures starting in infancy Severe impairment in neurological development Most affected children cannot walk, talk or care for themselves May include scoliosis, visual impairment, sensory issues, and gastrointestinal complications >1,200 documented cases worldwide1 Patient identification rising significantly • • • • • • 1. LouLouFoundation.org Preclinical Development Underway for a Rare, Devastating, Genetic Neurological Disease with No Approved Treatments
Financial Summary & Key Milestones

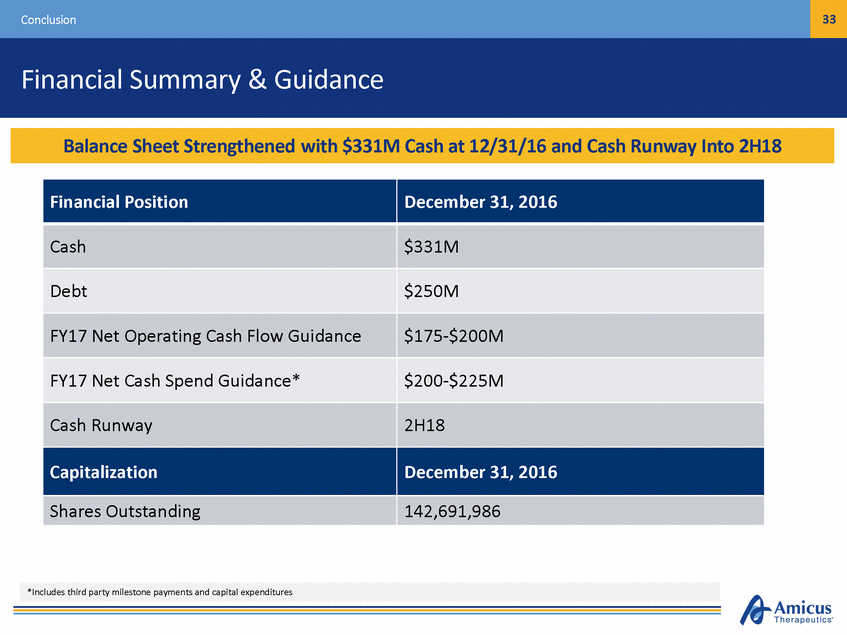
33 Conclusion Financial Summary & Guidance *Includes third party milestone payments and capital expenditures Financial Position December 31, 2016 Cash $331M Debt $250M FY17 Net Operating Cash Flow Guidance $175-$200M FY17 Net Cash Spend Guidance* $200-$225M Cash Runway 2H18 Capitalization December 31, 2016 Shares Outstanding 142,691,986 BalancCeasShhePeotsiSttiorennPgrtohveindeds Rwuitnhw$a3y3U1MndeCrasChurarte1n2t/O3p1e/1ra6tianngdPClanshinRtuonmwiady-2I0n1to7 2H18
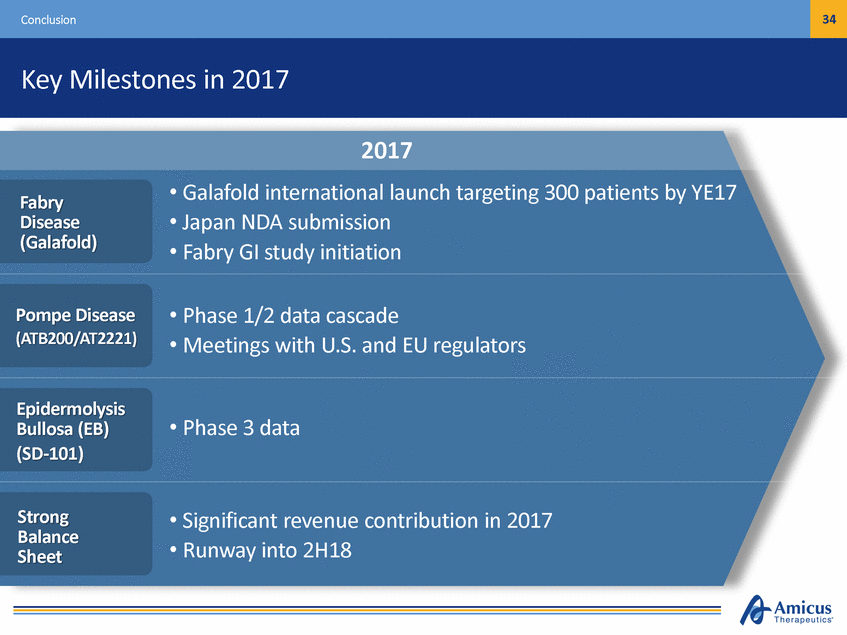
34 Conclusion Key Milestones in 2017 2017 • • • Galafold international launch targeting Japan NDA submission Fabry GI study initiation 300 patients by YE17 Fabry Disease (Galafold) • • Phase 1/2 data cascade Meetings with U.S. and EU regulators Pompe Disease (ATB200/AT2221) Epidermolysis Bullosa (EB) (SD-101) • Phase 3 data Strong Balance Sheet • • Significant revenue contribution in 2017 Runway into 2H18
Thank You

