Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - HEAT BIOLOGICS, INC. | htbx_8k.htm |
EXHIBIT 99.1

NASDAQ:HTBX January 2017

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2015 and our quarterly report on Form 10-Q for the subsequent quarters (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. 2
Investment Opportunity 3 Platform technologies designed to activate and expand “killer” T cells against multiple tumor antigens Focused on immuno-oncology (I-O) combinations that have the potential to dramatically improve patient outcomes Conducting vaccine + PD-1 checkpoint inhibitor combo trial in non-small cell lung cancer (NSCLC) Clinical proof of concept for platform mechanismIncreased CD8+ T cells in tumors associated with clinical response Favorable safety profile to-date Over 1,000 doses administered in ~200 patients
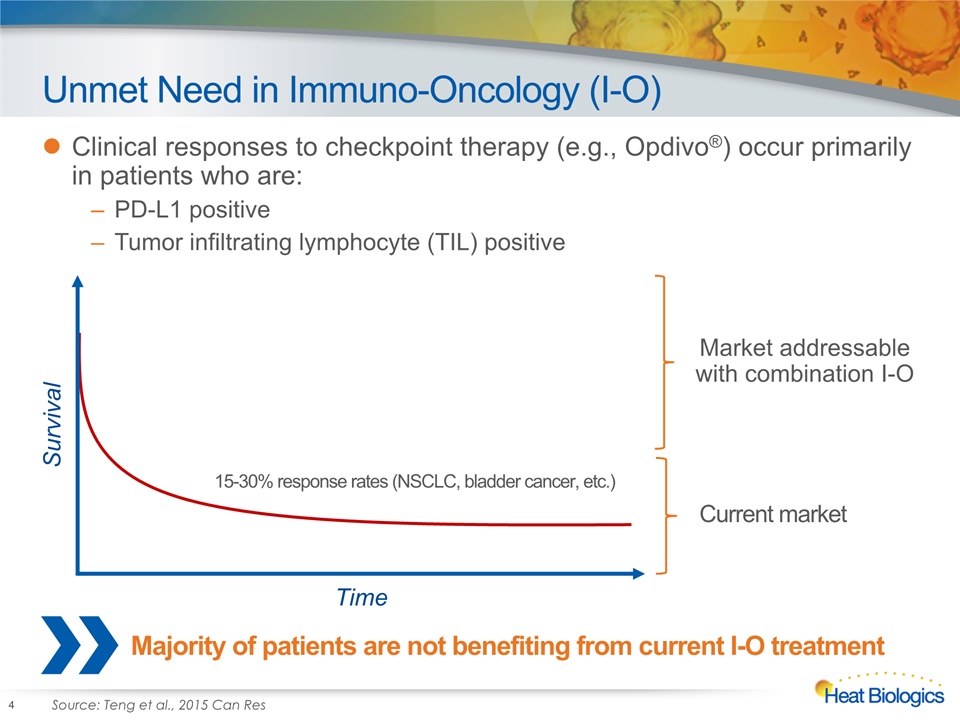
Unmet Need in Immuno-Oncology (I-O) Clinical responses to checkpoint therapy (e.g., Opdivo®) occur primarily in patients who are:PD-L1 positiveTumor infiltrating lymphocyte (TIL) positive 4 Survival Time 15-30% response rates (NSCLC, bladder cancer, etc.) Market addressable with combination I-O Current market Majority of patients are not benefiting from current I-O treatment Source: Teng et al., 2015 Can Res

Majority of Patients do not Respond to Checkpoints 5 Heat is focusing on converting ‘cold’ tumors to ‘hot’ tumors by activating highly specific “killer” T cells Source: Teng et al., 2015 Can Res Heat’s focus is on the majority of patients who do not respond to checkpoint therapy (low TIL) Only ~25% of patients respond to checkpoints

Vision and Strategy Focused on I-O combinations that have the potential to dramatically improve patient outcomes 6 Activation of CD8+ “killer” T cells Co-stimulation to enhance immune response Overcome tumor defenses through combination therapies

Heat’s Product Development 7 Cancer Cell Lines & Patient Samples Heat’s AntigenScreening & Selection Algorithm Transition to CMOto Mass Produce Select Cell Line with Most Antigen Overlap Transfect with Heat’s Technology Platform Technology Highlights Applies to multiple cancers and infectious diseasesDesigned to activate “killer” T cellsTargets multiple tumor antigensEnables scalable, low cost manufacturing relative to autologous cell therapies

ImPACT Platform Technology 8 Intradermal injectedImPACT cells ImPACT cells secrete Gp96-Ig chaperone + tumor antigens (TAA) ImPACT Cells Gp96-Ig+TAA Selectively activate CD8+ T Cells CD8+ T-Cells Dendritic Cells CD8+ T cells circulate & eliminate tumor cells Tumor Cells CD8+ CD8+ CD8+ CD8+ 2 Dual antigen carrier & adjuvant activates Dendritic Cells Gp96-Ig+TAA Dendritic Cells 3 4 5 1

Clinical Proof of Concept 9 Secrete Gp96-lg Pan-Antigen T Cell Activation Tumor Infiltration Future I-O combinations will focus on checkpoint blockade and durable T cell responses
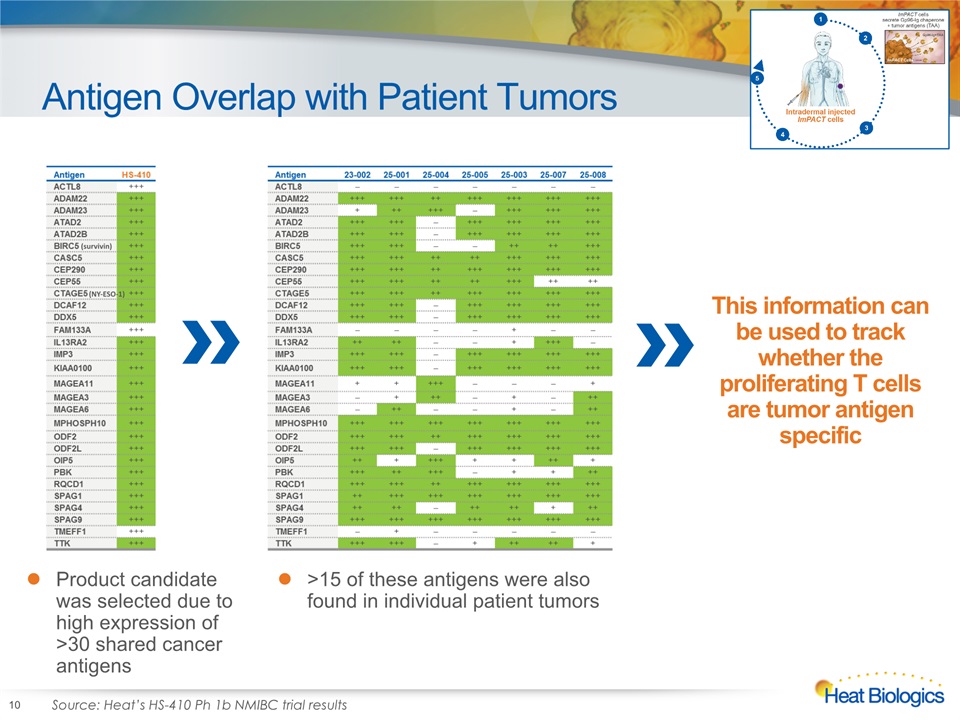
Antigen Overlap with Patient Tumors 10 Product candidate was selected due to high expression of >30 shared cancer antigens >15 of these antigens were also found in individual patient tumors Source: Heat’s HS-410 Ph 1b NMIBC trial results This information can be used to track whether the proliferating T cells are tumor antigen specific (survivin) (NY-ESO-1)
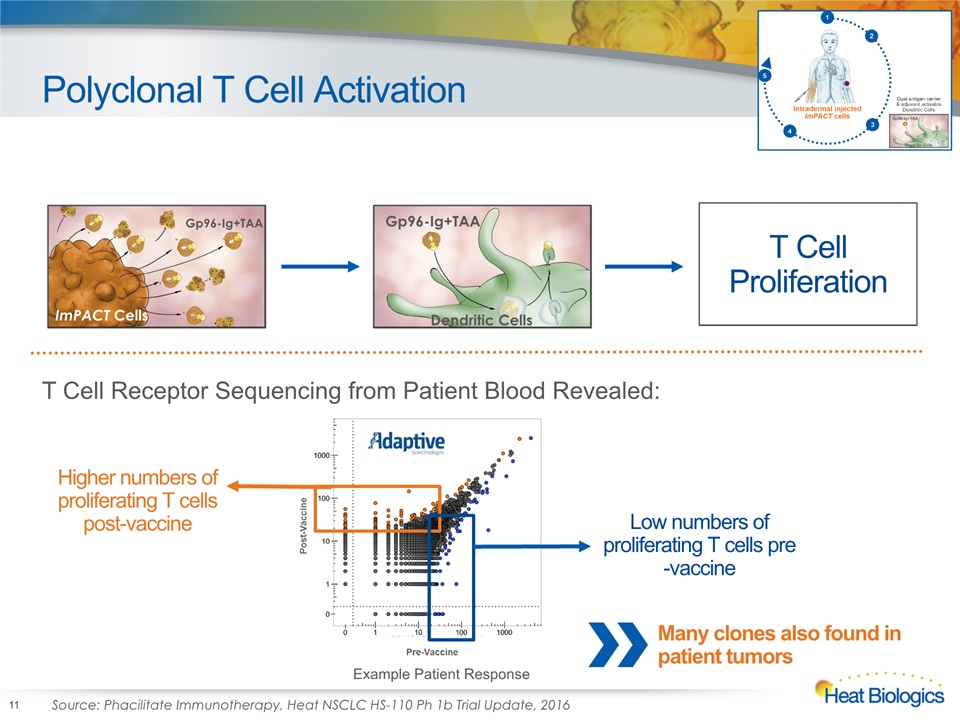
Polyclonal T Cell Activation 11 ImPACT Cells Gp96-Ig+TAA Gp96-Ig+TAA Dendritic Cells Gp96-Ig+TAA Dendritic Cells T Cell Proliferation Low numbers of proliferating T cells pre-vaccine Higher numbers of proliferating T cells post-vaccine T Cell Receptor Sequencing from Patient Blood Revealed: Source: Phacilitate Immunotherapy, Heat NSCLC HS-110 Ph 1b Trial Update, 2016 Example Patient Response Many clones also found in patient tumors

Pan-Antigen “Killer” T Cell Activation 12 Antigen-specific immune responses were measured against individual antigens (e.g., survivin and NY-ESO-1) Antigens included in initial analysis Source: Heat’s HS-110 Ph 1b NSCLC trial results reported at World Lung Conf in Dec. 2016; HS-410 Ph 2 NMIBC trial results reported at SUO in Nov. 2016 HS-410 Treated Placebo Patient ELISPOT Responses (IFNγ) (survivin) (NY-ESO-1) Most patients on vaccine responded BCG did not cause T cell activation Some patients had memory response Clinical data indicate antigen specific T cell response

Increasing TILs at the Tumor Site Images of the bladder (above) showed changes that resemble lymphoid (T cell rich) structures, which we believe indicates that HS-410 leads to a localized immune response within the bladder Blue-light cystoscopy from patient treated with HS-410 Tumor biopsy from patient treated with HS-410 Source: Heat’s HS-410 Phase 2 NMIBC interim monotherapy trial results announced January 26, 2016 13
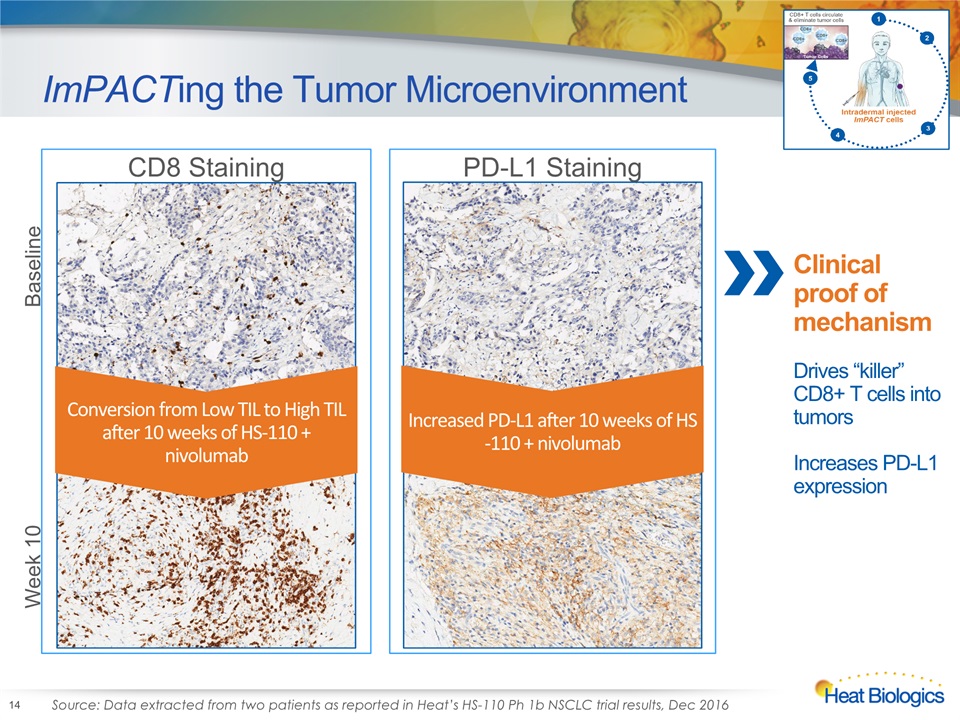
ImPACTing the Tumor Microenvironment 14 PD-L1 Staining CD8 Staining Increased PD-L1 after 10 weeks of HS-110 + nivolumab Conversion from Low TIL to High TIL after 10 weeks of HS-110 + nivolumab Baseline Week 10 Source: Data extracted from two patients as reported in Heat’s HS-110 Ph 1b NSCLC trial results, Dec 2016 Clinical proof of mechanismDrives “killer” CD8+ T cells into tumorsIncreases PD-L1 expression

Favorable Safety Profile To-Date Over 1,000 doses administered in ~200 patientsFavorable safety profile and well-toleratedNo additive toxicities or serious adverse reactions No systemic use of steroids was required 15 Injection Reactions Week 1 Week 2 Immune Reactions*(≤ Grade 3 toxicity) *Represents the only patient of ~200 patients dosed who discontinued treatment for a vaccine-related adverse event; Source: Heat’s HS-410 and HS-110 data results, Dec 2016
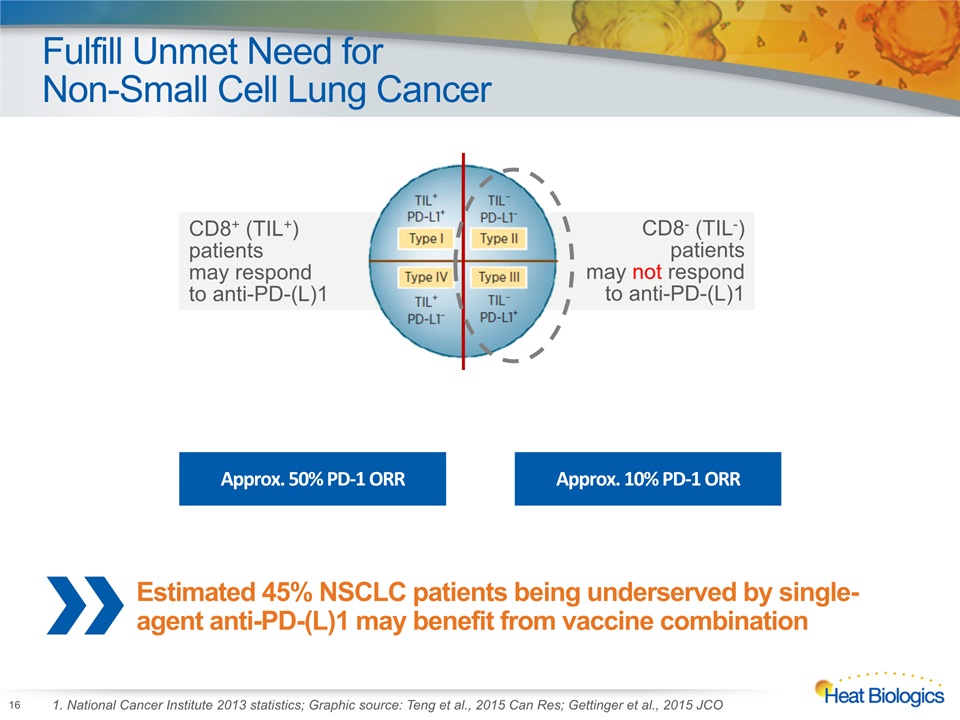
Fulfill Unmet Need for Non-Small Cell Lung Cancer 16 1. National Cancer Institute 2013 statistics; Graphic source: Teng et al., 2015 Can Res; Gettinger et al., 2015 JCO CD8+ (TIL+) patients may respond to anti-PD-(L)1 CD8- (TIL-) patients may not respond to anti-PD-(L)1 Approx. 50% PD-1 ORR Approx. 10% PD-1 ORR Estimated 45% NSCLC patients being underserved by single-agent anti-PD-(L)1 may benefit from vaccine combination
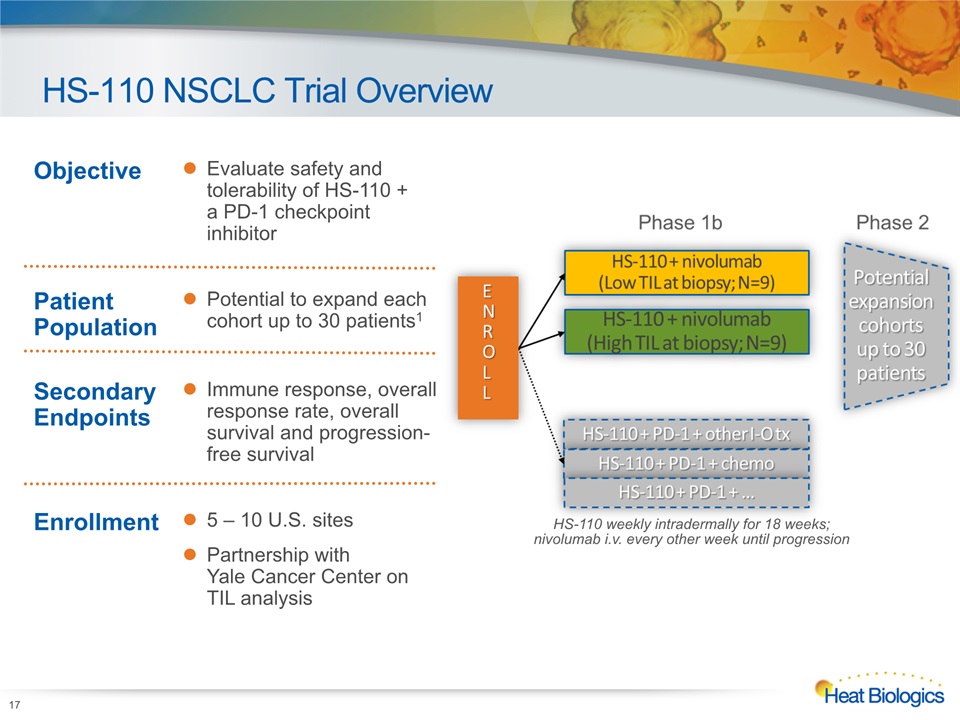
HS-110 NSCLC Trial Overview HS-110 weekly intradermally for 18 weeks; nivolumab i.v. every other week until progression Objective Evaluate safety and tolerability of HS-110 + a PD-1 checkpoint inhibitor Patient Population Potential to expand each cohort up to 30 patients1 Secondary Endpoints Immune response, overall response rate, overall survival and progression-free survival Enrollment 5 – 10 U.S. sitesPartnership with Yale Cancer Center on TIL analysis 17

Immune ResponseCorrelates with Overall Survival 18 Source: HS-110 Ph 1b NSCLC data (left); HS-110 Ph 1b NSCLC data reported at World Lung Dec. 2016 (right)

ComPACT Platform Technology 19 gp96-Ig OX40L-Fc The first potential dual-acting immunotherapy designed to deliver T cell activation and costimulation in a single product – combination therapy without additive costs

ComPACT Outperforms OX40 Monoclonal Antibodies in Preclinical Models 20 ComPACT alone leads to ~50% complete tumor rejection as compared to ~16% with OX40 agonist antibody combinations1 Source: 1) Demonstrated in preclinical models for CT26 – Colon Cancer; Fromm et al. Society for Immunotherapy of Cancer Annual Meeting, 2016 Memory formation? B16.F10 - Melanoma

ComPACT Synergizes with PD-1 Blockade 21 % of CD8+ T Cells(CD127+KLRG1+) Frequency of Memory CD8+ Cells Source: Fromm et al. Society for Immunotherapy of Cancer Annual Meeting, 2016 ComPACT synergizes with PD-1 blockade to drive memory “killer” T cell differentiation

Leveraging gp96 Platform to Target Zika Virus & Other Infectious Diseases Zolovax, a wholly-owned subsidiary, formed to leverage our platform into infectious diseasesCollaborating with the University of Miami (UM) to explore whether the gp96 vaccine might stimulate a similar virus-specific response in the placenta of Zika-infected women that could clear the virus Focusing on the unmet need of protecting the fetus of Zika-infected womenCompelling data generated In NIH-funded studies, the gp96-based vaccine induced a potent and localized immune response and mucosal immunityU.S. Department of Defense funded research grant awarded to UM in malaria 22

Highlights Clinical proof of conceptFavorable safety profile and low toxicities to-datePan-antigen “killer” T cell activation and expansionApplicable to multiple cancers and infectious diseasesReady-to-use; scalable, low cost manufacturingUnencumbered commercialization rights 23 Focused on I-O combinations that have the potential to dramatically improve patient outcomes

Financial Snapshot 24 1. As of January 4, 2017; 2. As reported for the three months ended September 30, 2016 Nasdaq HTBX Shares Outstanding 23.9M Share Price $0.901 Market Cap $21.5M1 Cash & Equiv. $8.5M2 Founded in 2008, completed IPO 2013

THANK YOU
