Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Bioverativ Inc. | bioverativjanuary52017.htm |

Confidential and proprietary
Bioverativ Investor Day
January 6th, 2017

2 Confidential and proprietary
Forward-Looking Statements
• This presentation contains forward-looking statements, including statements relating to: the planned separation of Bioverativ from Biogen;
business and strategic objectives; growth prospects and potential opportunities for commercial products and pipeline programs; planned
geographic expansion; manufacturing, supply and distribution arrangements; relationships with collaborators and other third parties; research
and development activities and priorities; anticipated clinical trials and data readouts; business development plans and opportunities; and
expected capitalization, revenues, operating margin, cash flows and other financial guidance. These forward-looking statements may be
accompanied by such words as “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “potential,” “project,”
“target,” “will” and other words and terms of similar meaning. You should not place undue reliance on these statements.
• These forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such
statements, including: Bioverativ’s dependence on revenues from sales of ELOCTATE and ALPROLIX; failure to compete effectively due to
significant product competition in the markets in which Bioverativ operates; product quality or safety concerns, including the occurrence of
adverse safety events; product development risks; risks associated with clinical trials; risks relating to actions of regulatory authorities; risks
related to reliance on third parties for manufacturing, supply and distribution of Bioverativ’s products and product candidates; difficulties in
obtaining and maintaining adequate coverage, pricing and reimbursement for Bioverativ’s products; failure to obtain and maintain adequate
protection for intellectual property and other proprietary rights; risks of doing business in international markets; risks associated with current
and potential future healthcare reforms; failure to identify and execute on business development and research and development opportunities;
Bioverativ’s dependence on relationships with collaborators and other third parties for revenue and other aspects of its business; loss of key
employees or inability to attract and retain key personnel; disruptions to, or other adverse impact on Bioverativ’s relationships with its
customers and other business partners; failure to comply with legal and regulatory requirements affecting Bioverativ’s business; the impact of
global economic conditions; fluctuations in foreign exchange and interest rates; changes in the law concerning the taxation of income; risks
relating to technology failures or breaches; the outcome of any significant legal proceedings; the adequacy of the Bioverativ’s cash flows from
operations; Bioverativ’s lack of operating history as a standalone business; risks relating to the separation from Biogen, including, among
others, risks that the separation will be completed in a timely manner or at all, failure to achieve the anticipated benefits from the separation,
reliance on Biogen and other third parties to provide certain services post-separation, restrictions to preserve the tax-free treatment of the
separation that may impact Bioverativ’s strategic and operating flexibility, and Bioverativ’s ability to satisfy liabilities and potential
indemnification obligations in connection with the separation; and other risks and uncertainties described in the Risk Factors section of
Bioverativ’s Registration Statement on Form 10 and other filings with the Securities and Exchange Commission.
• These statements are based on Bioverativ’s current beliefs and expectations and speak only as of the date of this presentation. Bioverativ
does not undertake any obligation to publicly update any forward-looking statements.
• Note Regarding Trademarks. Bioverativ owns or has rights to use the trademarks, service marks and trade names that it uses in conjunction
with the operation of its business. Some of the trademarks that appear in this presentation include: ALPROLIX® and ELOCTATE®, which
may be registered or trademarked in the U.S. and other jurisdictions. Each trademark, trade name or service mark of any other company
appearing in this presentation is, to Bioverativ’s knowledge, owned by such other company.

3 Confidential and proprietary
Today’s Agenda
Time Topic Speaker
10:05-10:10am Introduction
Paul Clancy
Biogen CFO
10:10-10:50am
Investment
opportunity and
strategic vision
John Cox
Chief Executive Officer
10:50-11:10am
Global
Therapeutic
Operations
Rogerio Vivaldi, MD, MBA
Chief Global Therapeutic
Operations Officer
11:10-11:20am
Financial
overview
John Greene
Chief Financial Officer
11:20-11:25am Closing
John Cox
Chief Executive Officer
11:25-11:50am Break
11:50-12:40pm Q&A Bioverativ Leadership Team

Welcome
Paul Clancy, Biogen CFO

Investment opportunity and strategic vision
John Cox, CEO

6 Confidential and proprietary
leading hematology rare disease company
committed to creating significant progress
for patients
Our Vision is to become the…

7 Confidential and proprietary
A Unique and Compelling
Investment Opportunity
Strong hemophilia
franchise
Integrated
capabilities
Talented team
Capitalized to
create value

8 Confidential and proprietary
Bioverativ: Why Compelling?
Expected revenues of >$800 million in 2016 targeting a $10B+ market in hemophilia
Generated $203 million of net cash flows from operations from Q1-Q3 2016
Geographic presence in U.S., Japan, and Canada with opportunities to expand
R&D, clinical development, regulatory prowess in hematology
Proven commercial execution
Expertise in process development, manufacturing, and supply of complex biologics
Strategic Business Development and on-going R&D are levers for growth
Appropriately sized organization
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

9 Confidential and proprietary
Maximize
Potential of
ELOCTATE &
ALPROLIX
Rapidly
Advance our
Discovery
Molecules
Pursue
Strategic
Opportunities
Positioned for Growth as a Stand Alone Company
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

10 Confidential and proprietary
Accomplished and Driven Executive Leadership Team
John Cox
Chief Executive Officer
John Greene
Chief Financial Officer
Rogerio Vivaldi, MD, MBA
Chief Global Therapeutic
Operations Officer
Andrea DiFabio
Chief Legal Officer
Lucia Celona
Chief HR & Corporate
Communications Officer
Richard Brudnick
EVP of BD & Alliance
Management
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

11 Confidential and proprietary
Strong Scientific, Medical Leaders with Hematology
Expertise
Bill Hobbs II, M.D., Ph.D.
Executive Director,
Clinical Development
10+ years of clinical and
research experience. A leading
treater of SCD and administered
only adult SCD program in
Pacific NW
Michael Poirier, M.S.
SVP, Regulatory & Safety
16+ years in Regulatory Affairs at Biogen;
Global Regulatory lead on key programs
including Avonex, Tysabri, Tecfidera, and
most recently Spinraza
Maha Radhakrishnan, M.D.
SVP, Medical
12+ years in Medical Affairs leadership
roles at BMS, Cephalon and Biogen.
Global experience, most recently Europe
Medical Head
Rob Peters, Ph.D.
SVP, Research
16+ years experience.
Renowned hemophilia scientist.
Inventor of Fc fusion technology
at Syntonix
Nisha Jain, M.D.
Executive Director, Medical
16+ years experience in hematology and
rare diseases including experience in NIH
and FDA
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

12 Confidential and proprietary
A Unique and Compelling
Investment Opportunity
Strong Hemophilia
Franchise
Integrated
capabilities
Talented team
Capitalized to
create value
A Unique and Compelling
Investment Opportunity
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

13 Confidential and proprietary
Our Therapies; First Innovation in 20 Years
BIV
V
E
v
olu
ti
o
n
In
d
u
s
tr
y
E
v
olu
ti
o
n
Plasma-Derived
Clotting Factors
FDA approval
/
Spin-off from Biogen
Maximize EHL and Lead Next
Generation Products
Recombinant
Extended
Half-Life Factors
(2014–present)
(1969–present)
2014
2017
Next Generation
Therapies
Gene
Therapy
Novel
EHLs
Bispecific
Abs
(Future)
Recombinant
Clotting Factors
Conventional
or “short-acting”
(1990s–present)
Note: Biogen acquired
Syntonix in 2007
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
14 Confidential and proprietary
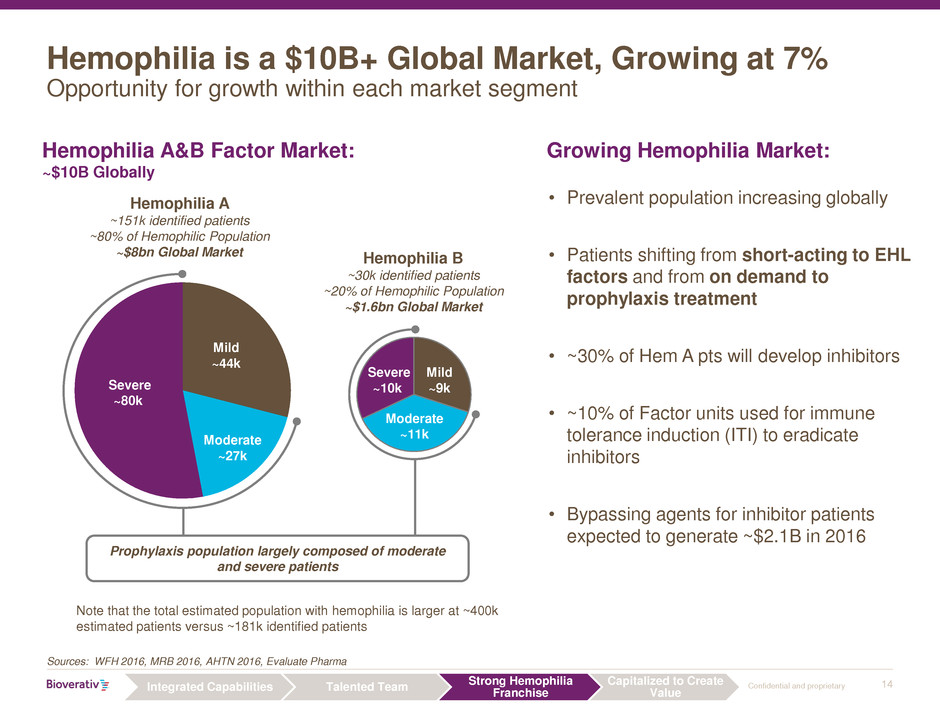
Hemophilia is a $10B+ Global Market, Growing at 7%
Opportunity for growth within each market segment
• Prevalent population increasing globally
• Patients shifting from short-acting to EHL
factors and from on demand to
prophylaxis treatment
• ~30% of Hem A pts will develop inhibitors
• ~10% of Factor units used for immune
tolerance induction (ITI) to eradicate
inhibitors
• Bypassing agents for inhibitor patients
expected to generate ~$2.1B in 2016
Growing Hemophilia Market: Hemophilia A&B Factor Market:
~$10B Globally
Sources: WFH 2016, MRB 2016, AHTN 2016, Evaluate Pharma
Mild
~44k
Moderate
~27k
Severe
~80k
Mild
~9k
Moderate
~11k
Severe
~10k
Hemophilia A
~151k identified patients
~80% of Hemophilic Population
~$8bn Global Market Hemophilia B
~30k identified patients
~20% of Hemophilic Population
~$1.6bn Global Market
Prophylaxis population largely composed of moderate
and severe patients
Note that the total estimated population with hemophilia is larger at ~400k
estimated patients versus ~181k identified patients
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

15 Confidential and proprietary
10
47
77 97
129
156 172
183
205 217
Q2-14 Q3-14 Q4-14 Q1-15 Q2-15 Q3-15 Q4-15 Q1-16 Q2-16 Q3-16
ELOCTATE & ALPROLIX Have Delivered Strong Uptake
Since Launch and are Approaching $800M+ in 2016
Hemophilia Product Revenue ($mm)
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
Q1-Q3 2016: $605M

16 Confidential and proprietary
ELOCTATE and ALPROLIX are the Only Factors Utilizing
Proprietary Fc Fusion Technology
The Fc portion of the
product temporarily binds
to receptors in your body
The product is released back into the bloodstream
where it can temporarily recirculate in the body
before degrading naturally
Binding redirects the
product back toward the
bloodstream, avoiding
degradation pathways
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
17 Confidential and proprietary
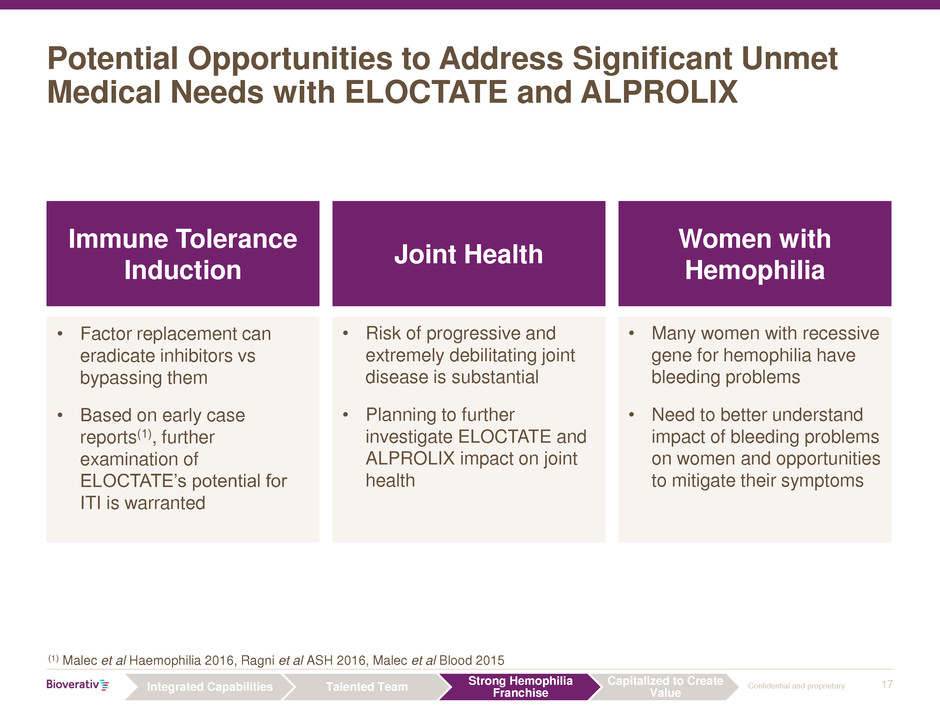
Potential Opportunities to Address Significant Unmet
Medical Needs with ELOCTATE and ALPROLIX
Women with
Hemophilia
Joint Health
Immune Tolerance
Induction
• Risk of progressive and
extremely debilitating joint
disease is substantial
• Planning to further
investigate ELOCTATE and
ALPROLIX impact on joint
health
• Many women with recessive
gene for hemophilia have
bleeding problems
• Need to better understand
impact of bleeding problems
on women and opportunities
to mitigate their symptoms
• Factor replacement can
eradicate inhibitors vs
bypassing them
• Based on early case
reports(1), further
examination of
ELOCTATE’s potential for
ITI is warranted
(1) Malec et al Haemophilia 2016, Ragni et al ASH 2016, Malec et al Blood 2015
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
18 Confidential and proprietary
A Unique and Compelling
Investment Opportunity
Strong Hemophilia
Franchise
Integrated
capabilities
Talented team
Capitalized to
create value
A Unique and Compelling
Investment Opportunity
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

19 Confidential and proprietary
Pipeline of Novel, Next Generation Hemophilia, Beta-
Thalassemia, and Sickle Cell Disease Candidates
XTEN licensed from Amunix
Drug Indication Description Modality Discovery Preclinical Clinical Marketed
BIVV 001
rFVIIIFc-VWF-XTEN
Hem A
EHL factor
1x/weekly dosing
or less frequent
Biologic
BIVV 002
rFIXFc-XTEN
Hem B
EHL factor
Subcutaneous
Biologic
Sangamo
collaboration
Beta Thal-
assemia
Zinc finger
nuclease (ZFN)
Genome
Editing
Sickle Cell
Zinc finger
nuclease (ZFN)
Genome
Editing
San Raffaele
collaboration
Hem A Lentiviral vector
Gene
Therapy
Hem B Lentiviral vector
Gene
Therapy
FVIIIa mimetic
bispecific ab
Hem A;
Inhibitors
MOA not
disclosed
Biologic
Multiple early stage
programs
Sickle Cell Multiple MOAs
Sm
Molecules
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
20 Confidential and proprietary
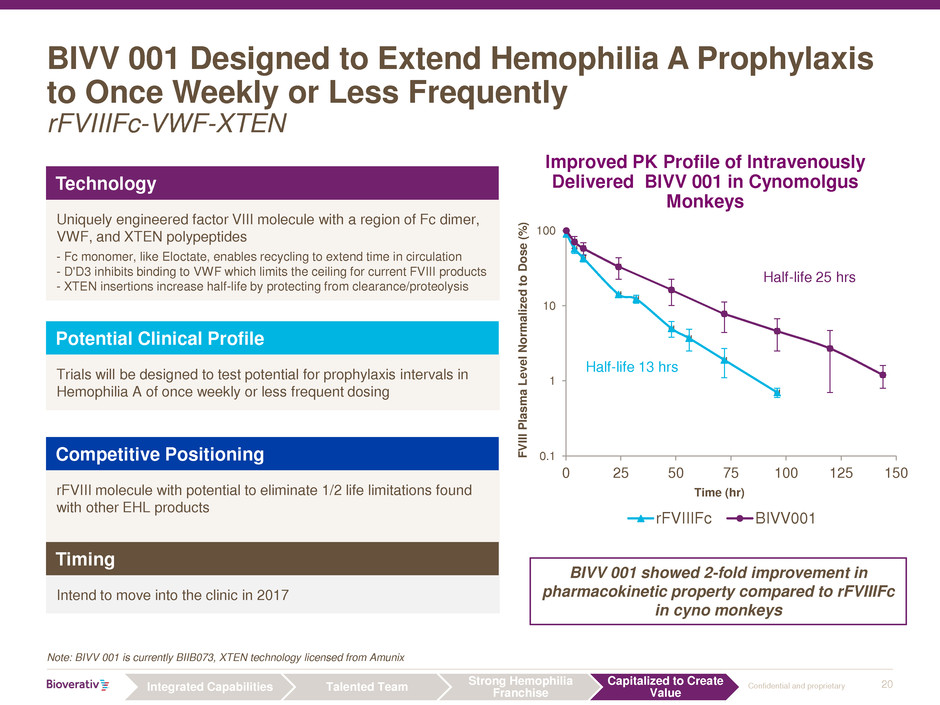
BIVV 001 Designed to Extend Hemophilia A Prophylaxis
to Once Weekly or Less Frequently
rFVIIIFc-VWF-XTEN
Note: BIVV 001 is currently BIIB073, XTEN technology licensed from Amunix
BIVV 001 showed 2-fold improvement in
pharmacokinetic property compared to rFVIIIFc
in cyno monkeys
Improved PK Profile of Intravenously
Delivered BIVV 001 in Cynomolgus
Monkeys
0.1
1
10
100
0 25 50 75 100 125 150
rFVIIIFc BIVV001
Half-life 13 hrs
Half-life 25 hrs
FV
II
I
Pl
a
s
m
a
L
e
v
e
l
Norm
a
li
z
e
d
to
Do
s
e
(
%
)
Time (hr)
Technology
Uniquely engineered factor VIII molecule with a region of Fc dimer,
VWF, and XTEN polypeptides
- Fc monomer, like Eloctate, enables recycling to extend time in circulation
- D'D3 inhibits binding to VWF which limits the ceiling for current FVIII products
- XTEN insertions increase half-life by protecting from clearance/proteolysis
Potential Clinical Profile
Trials will be designed to test potential for prophylaxis intervals in
Hemophilia A of once weekly or less frequent dosing
Competitive Positioning
rFVIII molecule with potential to eliminate 1/2 life limitations found
with other EHL products
Timing
Intend to move into the clinic in 2017
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

21 Confidential and proprietary
Scientific Rationale for BIVV 001 Molecule Design
Novel fusion protein, consisting of:
D’D3 domains of VWF provide protection & stability of VWF while evading half-life limitation of
endogenous VWF
XTEN polypeptides, which improve the pharmacokinetic profile and degrade naturally
rFVIII fused to dimeric Fc which maintains thrombin-mediated release of FVIII from VWF like natural
FVIII. Once released FVIII will then bind phospholipids and participate in the clotting cascade
Native
FVIII-VWF
complex
BIVV 001
A1
A2
A3
C1 C2
D’D3
Fc
Fc
Thrombin
activation
A1
A2
A3
C1 C2
D’D3
VWF
Activated
rFVIIIFc
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
A1
A2
A3
C1 C2
Fc
Fc

22 Confidential and proprietary
BIVV 002 Designed to Enable Subcutaneous
Administration
rFIXFc-XTEN
Note: BIVV 002 is currently BIIB085, XTEN technology licensed from Amunix
Source: Preliminary modeling Arjan van der Flier & Qin Weng, DMPK
rFIXFc (IV)
50 IU/kg
100 IU/kg
200 IU/kg
rFIXFc-XTEN (SC)
100 IU/kg
200 IU/kg
48 96 144 192 240 288 336 384 432 480
140
60
120
50
40
30
20
10
0
30% peak value
5% trough
Hours
IU
/d
L
(
a.
k
.a
.
%
n
o
rmal
)
Technology
Combines Fc dimer and XTEN technology along with
R338L Padua Factor IX variant in the treatment of
Hemophilia B
Potential Clinical Profile
Trials will be designed to explore potential for
subcutaneous dosing. Leverages Fc fusion technology
Competitive Positioning
Potential for subcutaneous dosing could lessen burden
of care and to compete with gene therapy
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

23 Confidential and proprietary
Molecular Design of BIVV 002
WT
GLA
EGF
EGF
catalytic
.
WT
GLA
EGF
EGF
catalytic
XTEN 72
R338L
GLA
EGF
EGF
catalytic
rFIX
(BeneFIX)
rFIXFc
(ALPROLIX)
Fc domain for
extended half-life
XTEN peptide for
improved bioavailability
and extended half-life
rFIXFc-XTEN
R338L (Padua) to
enhance FIX activity
Activation of FIX cleaves and releases the AP domain and attached XTEN
so that the resulting active FIXFc molecules are identical (except for R388L)
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

24 Confidential and proprietary
Collaboration on Sangamo’s Non-viral, Ex-vivo
ZFN-mediated Genome Editing Programs for β-thal, SCD
ZFN-mediated Enhancer Knockout
MOA hypothesis in β-thal: increase HbF to compensate
for no or low β-globin levels allowing for more normal
RBC production and RBC lifespan
MOA hypothesis in SCD: increase HbF levels to dilute the
HbS, block polymerization, allow for more normal RBC
function, and decrease RBC destruction (hemolysis)
Technology
Zinc finger nuclease (ZFN)-mediated genome editing
program for beta-thalassemia and sickle cell disease is
based on the use of genome editing technology to modify a
patient’s own (autologous) hematopoietic stem progenitor
cells (HSPCs)
Potential Clinical Profile
Trials will explore potential in both beta-thalassemia and
sickle cell disease, diseases with significant unmet medical
need
Competitive Positioning
Potential gene therapy treatment for rare diseases with
significant unmet need
β-thal and SCD are rare diseases with significant unmet medical needs
and are priority areas of focus for our Hematology franchise
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

25 Confidential and proprietary
Large Opportunity Exists for Expansion in Rare Hematology
Source: EvaluatePharma, Decision Resources, Company management
Global Non-Malignant Hematology Market (2016)
Additional Rare
Hematology Opportunities:
Bioverativ
(ELOCTATE,
ALPROLIX)
>$800m
Hemophilia +
Sickle Cell Disease +
β-thalassemia
~$20-25B Market Potential
Hemophilia
$10B+ Market
HUS
Aplastic Anemia
PKD
Hemochromatosis
ITP
Wiskott-
Aldrich
Illustrative
Essential
Thrombocytemia
AL Amyloidosis
Hemolytic
Anemias
AGA
HLH
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

26 Confidential and proprietary
Business Development Vision: Sector Expert and
Partner of Choice
• Numerous rare hematologic diseases with
high unmet need and interesting accessible
clinical stage assets
• Committed to exploring such opportunities
to bolster our pipeline
• With our expertise we believe we can drive
programs rapidly through the clinic and we
aim to be the partner of choice
• Financial capacity provides potential
to grow inorganically
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

Global Therapeutic Operations
Rogerio Vivaldi, MD, MBA

28 Confidential and proprietary
Strategic Imperatives for Therapeutic Operations
Maximize
Potential of ELOCTATE
and ALPROLIX
Enhance
Value with Patient-Centric
Approach
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

29 Confidential and proprietary
Patient-Centric Model in Rare Hematology
Bioverativ’s commercial structure well-positioned to continue to serve the needs
of all our hematology customers in an efficient way
Work directly with the
community to provide
educational information,
resources, and info about our
programs and services
Provides caregivers, patients
and healthcare providers with
dedicated and individualized
support
Provide HCPs and HTCs with
fair and balanced
information
Dedicated to patient
access working closely with
payers
Patient Services
Account Executives
National Account
Managers
CoRe Managers
Advocacy
Groups
Payers &
Pharmacies
Healthcare
Providers
Patients
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
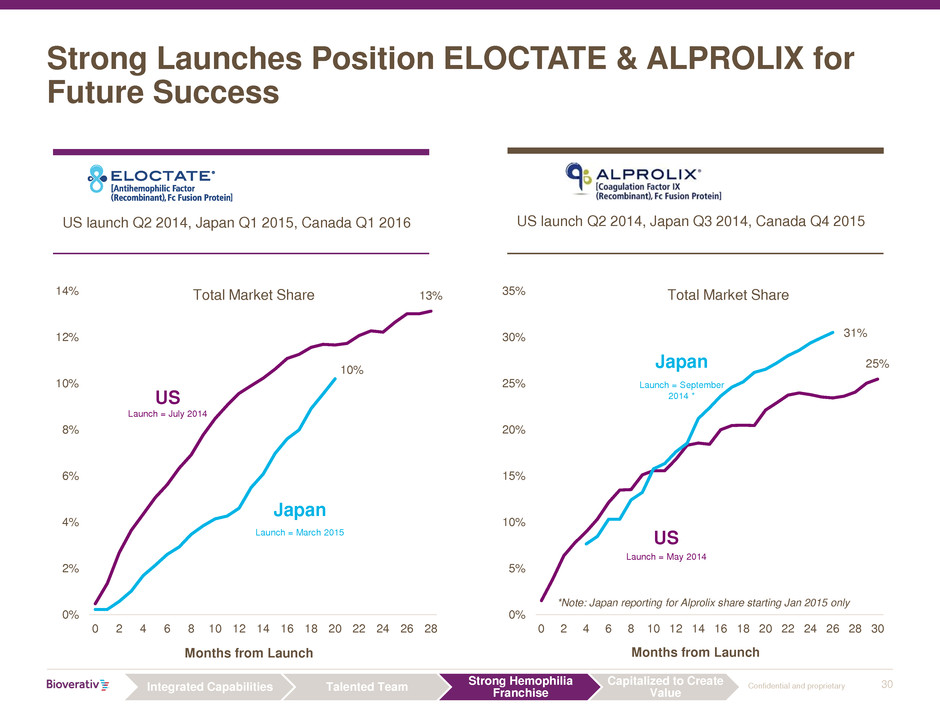
30 Confidential and proprietary
13%
10%
0%
2%
4%
6%
8%
10%
12%
14%
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28
Strong Launches Position ELOCTATE & ALPROLIX for
Future Success
US launch Q2 2014, Japan Q1 2015, Canada Q1 2016 US launch Q2 2014, Japan Q3 2014, Canada Q4 2015
Months from Launch
Total Market Share
US
Japan
25%
31%
0%
5%
10%
15%
20%
25%
30%
35%
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30
Months from Launch
US
Japan
*Note: Japan reporting for Alprolix share starting Jan 2015 only
Launch = March 2015
Launch = July 2014
Launch = September
2014 *
Launch = May 2014
Total Market Share
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
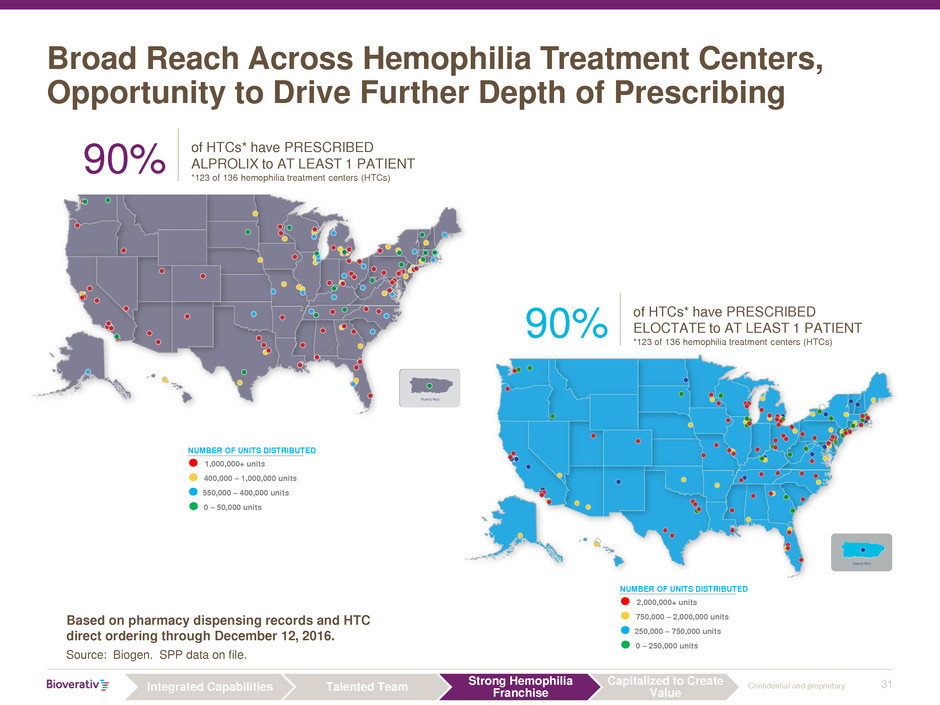
31 Confidential and proprietary
Broad Reach Across Hemophilia Treatment Centers,
Opportunity to Drive Further Depth of Prescribing
Based on pharmacy dispensing records and HTC
direct ordering through December 12, 2016.
Source: Biogen. SPP data on file.
90%
of HTCs* have PRESCRIBED
ALPROLIX to AT LEAST 1 PATIENT
*123 of 136 hemophilia treatment centers (HTCs)
NUMBER OF UNITS DISTRIBUTED
2,000,000+ units
750,000 – 2,000,000 units
250,000 – 750,000 units
0 – 250,000 units
NUMBER OF UNITS DISTRIBUTED
1,000,000+ units
400,000 – 1,000,000 units
550,000 – 400,000 units
0 – 50,000 units
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
90%
of HTCs* have PRESCRIBED
ELOCTATE to AT LEAST 1 PATIENT
*123 of 136 hemophilia treatment centers (HTCs)

32 Confidential and proprietary
Opportunity Remains for Further Growth from Shift to
Prophylaxis and EHL Therapies
H
emo
p
hi
li
a
A
H
emo
p
hi
li
a
B
Market Share of Patients on Prophylaxis (U.S.)
Source: Q2 2016 US HTC Market Tracking FVIII and FIX Report
100%
83% 70%
18%
23%
7%
Q2-14 Q2-15 Q2-16
Other EHL (Pegylated)
Conventional
(Short-Acting)
85%
57% 51%
15%
44% 47%
2%
Q2-14 Q2-15 Q2-16
Other EHL
(Albumin Fusion)
Conventional (Short-
Acting)
On Demand vs Prophylaxis
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
50% 50%
On Demand Prophylaxis
40%
60%
On Demand Prophylaxis

33 Confidential and proprietary
Plans to Expand to New Markets Where Already
Approved or Filed
ELOCTATE and ALPROLIX Approved
Not-yet-commercialized Territory
Elocta and ALPROLIX Approved
Not-yet-commercialized Territory
Launched in the
U.S. and Canada Fully integrated
operation in Japan
with ~80 FTEs
BIVV approved /
filings accepted
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

34 Confidential and proprietary
ELOCTATE has Delivered Strong Performance and
Growth Opportunities Remain
Approved for Hem A to reduce frequency
of bleeding episodes with prophylactic
infusions every 3 to 5 days
Advantage of more than two years of real-
world experience and consistent long-
term safety data
Potential Growth Opportunities
• Continued shift to EHLs, and shift to
prophylaxis
• Expansion into new geographies
• Increased patient access
• Treating women who have the recessive
gene for hemophilia
Source: Investor Presentation, Company Website, Form 10
Note: Approved in EU in November 2015 under trade name Elocta®.
ELOCTATE Revenue ($mm)
58
320
500+
2014 2015 2016
US Japan Canada
Q1-Q3
ELOCTATE Revenue ($M)
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

35 Confidential and proprietary
Near Term
Evolution of the Hemophilia A Landscape
Longer Term
• Competition from other EHL entrants • Advancements in half-life extension
technology including BIVV 001
• New MOAs including bispecific
antibodies, RNAi therapeutics, gene
therapies, etc.
• Bypassing agents, ITI, potential
bispecific antibody
• With few next generation therapies on
the horizon, there is significant unmet
medical need for therapies to treat
inhibitor market
H
e
mo
p
hi
lia
A
Inh
ibitor
s
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

36 Confidential and proprietary
ALPROLIX Has Also Delivered Strong Performance and
Growth Opportunities Remain
• Approved for Hem B to reduce frequency of
bleeding episodes with prophylactic
infusions every 7 to 10 days, with potential to
extend dosing based on individual response
• Advantage of more than two years of real-
world experience and consistent long-term
safety data
Potential Growth Opportunities
• Continued shift to prophylaxis due to
differentiated efficacy and dosing schedule, and
shift to EHLs
• Expansion into new geographies
• Increased patient access
• Treating women who have the recessive gene
for hemophilia
Source: Investor Presentation, Company Website, Form 10
76
237
300+
2014 2015 2016
US Japan Canada
Q1-Q3
ALPROLIX Revenue ($M)
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value
37 Confidential and proprietary
Evolution of the Hemophilia B Landscape
• Competition from other EHL entrants • Potential subcutaneous EHLs, including
BIVV 002
• New MOAs including gene therapies,
RNAi
Near Term Longer Term
Integrated Capabilities Talented Team
Strong Hemophilia
Franchise
Capitalized to Create
Value

Financial Overview
John Greene, CFO

39 Confidential and proprietary
Financial Snapshot
$32
$238
2015
Q1-Q3
2016
Q1-Q3
Operating Income ($M)
Operating Margin 8% 38%
$386
$631
2015
Q1-Q3
2016
Q1-Q3
+63%
Revenues ($M)
Gross Margin 87% 79%
$36
$148
2015
Q1-Q3
2016
Q1-Q3
Net Income ($M)
Tax Rate 37.5% 37.5%
-$12
$203
2015
Q1-Q3
2016
Q1-Q3
Free Cash Flows ($M)
Cash as % of
Net Income
(33%) 137%
Note: 2016 reported with Pro Forma adjustments
POSITIVE
POSITIVE
POSITIVE
Free Cash Flows = Net CFs provided by(used in) operating activities + Net CFs used in investing activities
40 Confidential and proprietary
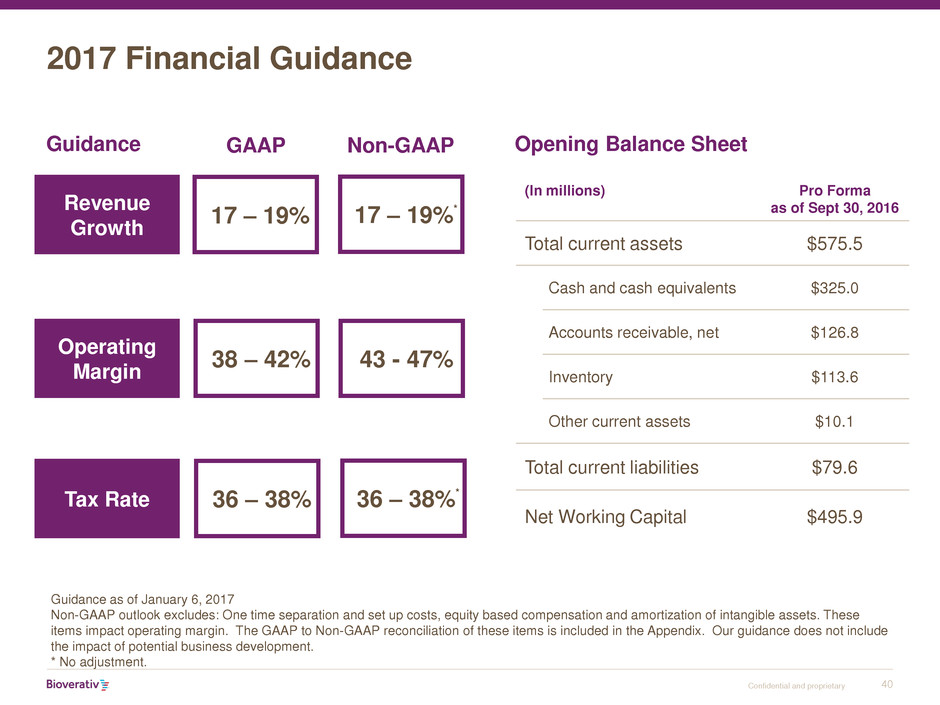
2017 Financial Guidance
Guidance as of January 6, 2017
Non-GAAP outlook excludes: One time separation and set up costs, equity based compensation and amortization of intangible assets. These
items impact operating margin. The GAAP to Non-GAAP reconciliation of these items is included in the Appendix. Our guidance does not include
the impact of potential business development.
* No adjustment.
Revenue
Growth
17 – 19%
Operating
Margin
Tax Rate
Guidance Opening Balance Sheet
(In millions) Pro Forma
as of Sept 30, 2016
Total current assets $575.5
Cash and cash equivalents $325.0
Accounts receivable, net $126.8
Inventory $113.6
Other current assets $10.1
Total current liabilities $79.6
Net Working Capital $495.9
GAAP Non-GAAP
38 – 42% 43 - 47%
36 – 38%
17 – 19%*
36 – 38%*

Closing
John Cox, CEO
42 Confidential and proprietary
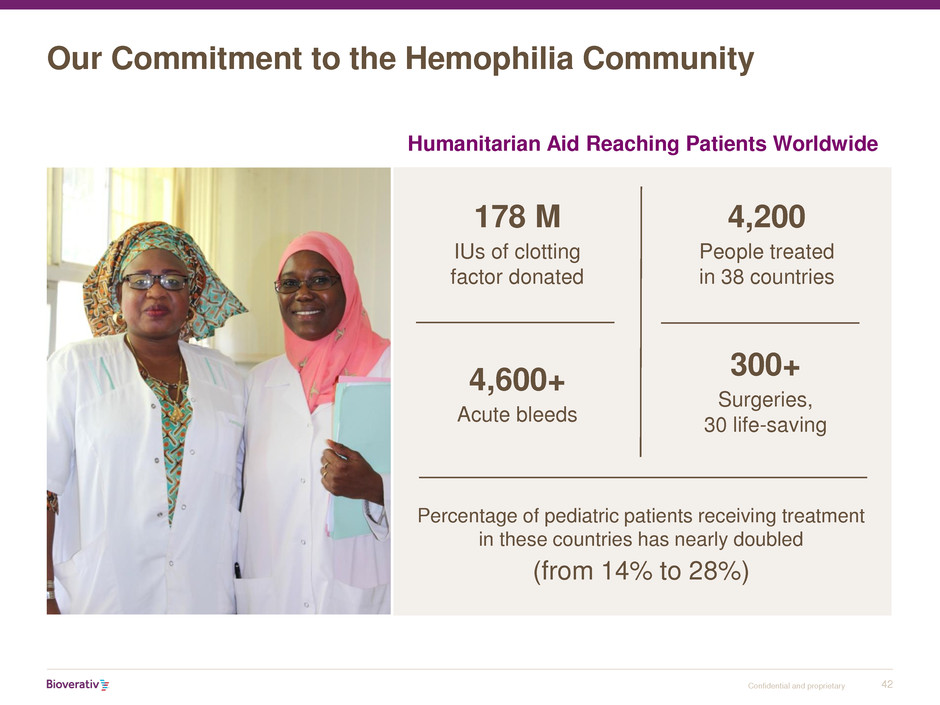
Our Commitment to the Hemophilia Community
178 M
IUs of clotting
factor donated
4,200
People treated
in 38 countries
4,600+
Acute bleeds
300+
Surgeries,
30 life-saving
Percentage of pediatric patients receiving treatment
in these countries has nearly doubled
(from 14% to 28%)
Humanitarian Aid Reaching Patients Worldwide

43 Confidential and proprietary
A Unique and Compelling
Investment Opportunity
Strong Hemophilia
Franchise
Integrated
capabilities
Talented team
Capitalized to
create value

Appendix

45 Confidential and proprietary
Income Statement
2015 2015 2016
2016
Pro Forma
Full year
Nine months
end Sept 30
Nine months
end Sept 30
Adjust
Nine months
end Sept 30
Revenues:
Product, net 554.1 381.7 604.8 604.8
Collaboration revenue 6.2 4.4 26.4 26.4
Total revenues 560.3 386.1 631.2 631.2
Cost and expenses:
Cost of sales 52.9 50.8 162.2 (30.0) A 132.2
Gross Margin % 91% 87% 79%
Research and development 186.1 135.4 122.6 122.6
% revenues 33% 35% 19%
Selling, general and administrative 223.3 167.7 138.4 138.4
% revenues 40% 43% 22%
Total operating expenses 409.4 303.1 261.0 261.0
Income from operations 98.0 32.2 208.0 30.0 238.0
Operating Margin % 17% 8% 38%
Other income (expense), net 0.6 0.6 (1.0) (1.0)
Income before income tax
expense
98.6 32.8 207.0 30.0 237.0
Income tax (benefit) expense (3.3) (3.7) 3.7 B 0.0
37.0 88.9 C 88.9
Net income 61.6 36.1 210.7 (62.6) 148.1
A - Elimination of accelerated depreciation associated with Bio 2
B - Reflects elimination of historical Bioverativ tax benefit
C - Reflects expected tax expense using an effective income tax rate of 37.5%

46 Confidential and proprietary
GAAP/Non-GAAP reconciliation
Operating margin reconciliation:
GAAP operating margin 38 - 42%
One time separation and set up costs 2%
Equity based compensation 2%
Amortization of intangibles 1%
Non-GAAP operating margin 43% - 47%
47 Confidential and proprietary
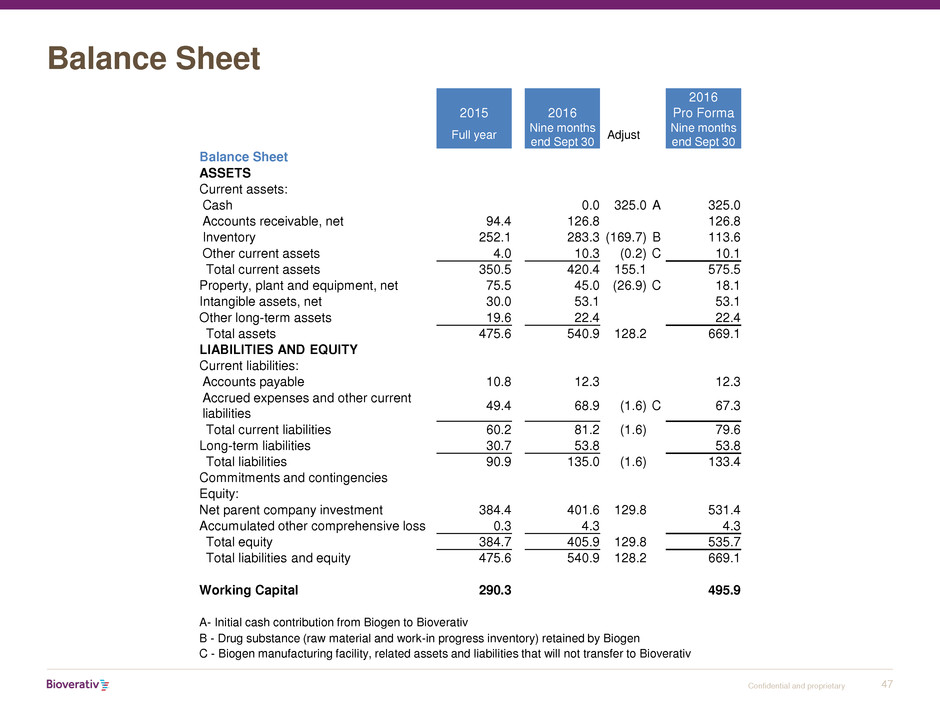
Balance Sheet
2015 2016
2016
Pro Forma
Full year
Nine months
end Sept 30
Adjust
Nine months
end Sept 30
Balance Sheet
ASSETS
Current assets:
Cash 0.0 325.0 A 325.0
Accounts receivable, net 94.4 126.8 126.8
Inventory 252.1 283.3 (169.7) B 113.6
Other current assets 4.0 10.3 (0.2) C 10.1
Total current assets 350.5 420.4 155.1 575.5
Property, plant and equipment, net 75.5 45.0 (26.9) C 18.1
Intangible assets, net 30.0 53.1 53.1
Other long-term assets 19.6 22.4 22.4
Total assets 475.6 540.9 128.2 669.1
LIABILITIES AND EQUITY
Current liabilities:
Accounts payable 10.8 12.3 12.3
Accrued expenses and other current
liabilities
49.4 68.9 (1.6) C 67.3
Total current liabilities 60.2 81.2 (1.6) 79.6
Long-term liabilities 30.7 53.8 53.8
Total liabilities 90.9 135.0 (1.6) 133.4
Commitments and contingencies
Equity:
Net parent company investment 384.4 401.6 129.8 531.4
Accumulated other comprehensive loss 0.3 4.3 4.3
Total equity 384.7 405.9 129.8 535.7
Total liabilities and equity 475.6 540.9 128.2 669.1
Working Capital 290.3 495.9
A- Initial cash contribution from Biogen to Bioverativ
B - Drug substance (raw material and work-in progress inventory) retained by Biogen
C - Biogen manufacturing facility, related assets and liabilities that will not transfer to Bioverativ

48 Confidential and proprietary
Sobi Collaboration
• For the years ended December 31, 2015, 2014 and 2013, the royalty payable to Sobi based upon sales in the company’s
territory was 2%. Upon Sobi’s first commercial sale in 2016, and during the Reimbursement period, the royalty rate the
company will pay Sobi on sales of ELOCTATE and ALPROLIX in our territory is 7%. After the Reimbursement period
concludes, the royalty rate we pay to Sobi increases to 12%. We are recording cost of sales at the effective royalty rate
expected over the term of the agreement of approximately 11%.
• The royalty rate received by the company, during the Reimbursement period on sales of ELOCTATE and ALPROLIX in
Sobi’s territory is 17%. After the Reimbursement period concludes, the royalty we receive decreases to 12%. We are
recording revenue at the effective royalty rate expected over the term of the agreement of approximately 14%.
Rates post Sobi Opt-In
Royalty and Net Revenue Share Rates: Method
Base Rate following
1st commercial sale in
the Sobi Territory:
Rate during the
Reimbursement
Period:
Sobi rate to Biogen on net sales in the Sobi Territory Royalty 12% Base Rate
plus 5%
Biogen rate to Sobi on net sales in the Biogen North
America Territory
Royalty 12% Base Rate
less 5%
Biogen rate to Sobi on net sales in the Biogen Direct
Territory
Royalty 17% Base Rate
less 5%
Biogen rate to Sobi on net revenue
from the Biogen Distributor Territory
Net Revenue Share 50% Base Rate
less 15%

49 Confidential and proprietary
Collaboration on San Raffaele’s Lentiviral Platform
for Hemophilia A and B
AAV Lentiviral
Integration
Safety, persistence
Low Yes
Cargo capacity & regulation
FVIII delivery, immunogenicity
4.7 kb 10 kb
Pre-existing anti-vector immunity
Eligibility, clearance, immunogenicity
Frequent Rare
Manufacturing & clinical experience
Feasibility, dose escalation, cost
More
Advanced
Recent
Technology
Leverages San Raffaele’s expertise in lentiviral vector
development and next-generation lentiviral platform
Potential Clinical Profile
Potential to provide single-dose, lasting treatment for
Hemophilia A and B patients
Competitive Positioning
Persistent gene transfer in most tissues throughout
development, addresses FVIII size challenge and may
circumvent immunity limitations of AAV vectors utilized in
majority of current gene therapy approaches. Lentivirus (self
inactivated) Utilizes same 3rd generation Self-Inactivating
Lentiviral technology used by the Naldini group to cure kids
with WAS and MLD by ex vivo treatment of hematopoietic
stem cells, without any signs of insertional oncogenesis
50 Confidential and proprietary
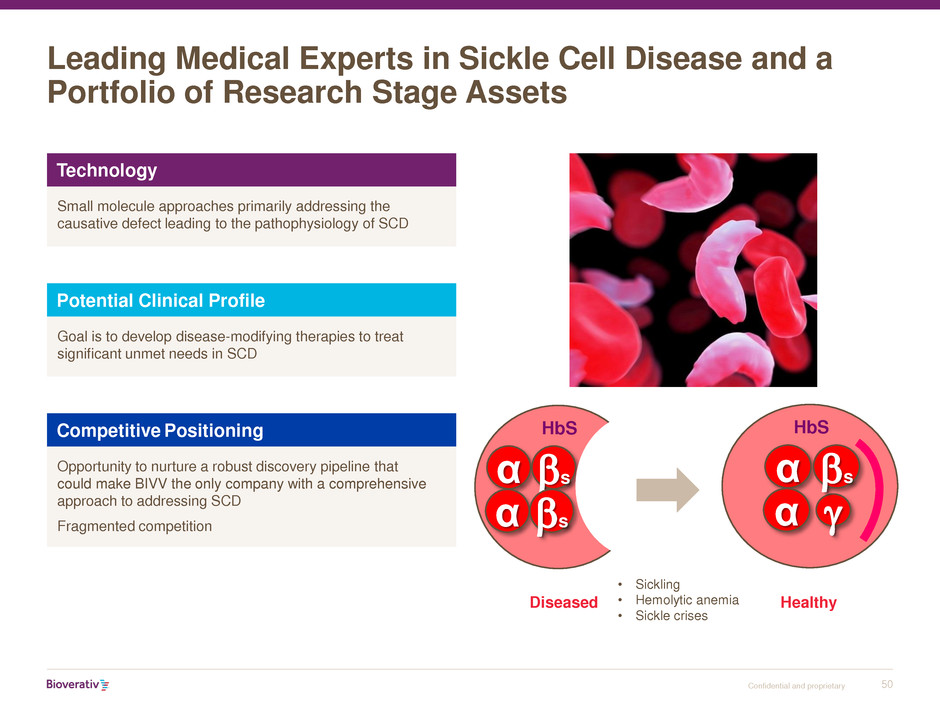
Leading Medical Experts in Sickle Cell Disease and a
Portfolio of Research Stage Assets
α
α g
HbS
bs
Healthy Diseased
α bs
α bs
HbS
• Sickling
• Hemolytic anemia
• Sickle crises
Technology
Small molecule approaches primarily addressing the
causative defect leading to the pathophysiology of SCD
Potential Clinical Profile
Goal is to develop disease-modifying therapies to treat
significant unmet needs in SCD
Competitive Positioning
Opportunity to nurture a robust discovery pipeline that
could make BIVV the only company with a comprehensive
approach to addressing SCD
Fragmented competition
