UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 22, 2016
PRESBIA PLC
(Exact Name of Registrant as Specified in Charter)
|
|
||
|
|
|
|
|
Ireland (State or Other Jurisdiction of Incorporation) |
001-36824 (Commission File Number) |
98-1162329 (IRS Employer Identification No.) |
120/121 Baggot Street Lower
Dublin 2 Ireland
(Address of Principal Executive Offices)(Zip Code)
+353 (1) 659 9446
Registrant's Telephone Number
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 8.01 Update on U.S. Staged Pivotal Clinical Trial
Presbia PLC (the “Company”) hereby reports certain interim data from its U.S. staged pivotal clinical trial. Through November 30, 2016, 421 subjects have undergone insertion of the Company’s microlens during the staged pivotal clinic trial that the Company is performing to obtain the clinical data required to enable the Company to obtain pre-market approval from the FDA. Currently, the Company is 24 months into its 3-year pivotal study and anticipates submitting data to the FDA in September 2017. To date, 100% of the subjects have passed through the 12 month post-operative visit. Data (representing 100% of the study cohort and excluding subjects who explanted the Microlens or did not return for scheduled trial visits and considered lost to follow-up) made available to the Company indicates that:
|
|
• |
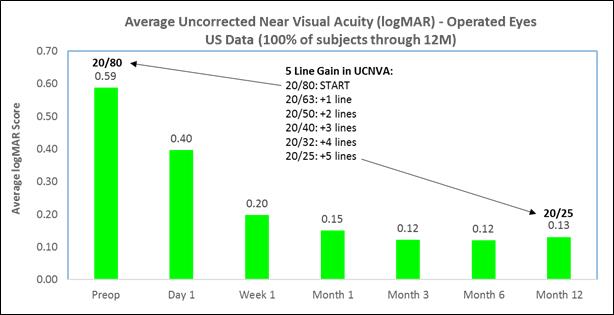
Subjects gained an average of 5 lines of uncorrected near visual acuity (the ability to see close objects without prescription enhancement) in treated eyes (Figure A), |
|
|
• |
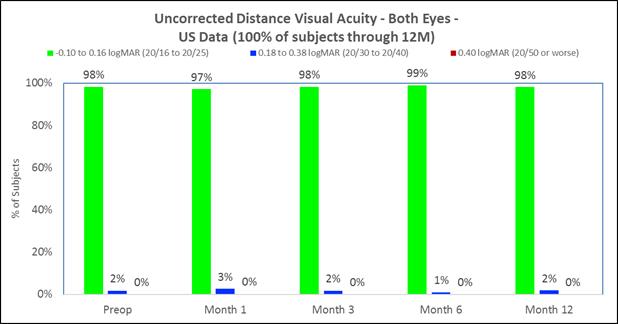
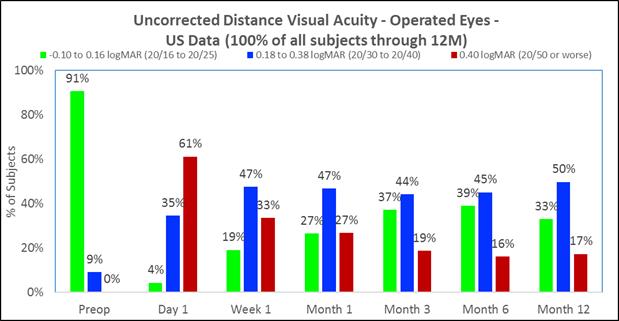
Approximately 83% of subjects achieved 20/40 or better uncorrected distance vision in treated eyes (Figure B) and there was little to no change in binocular uncorrected distance vision (Figure C), and |
|
|
• |
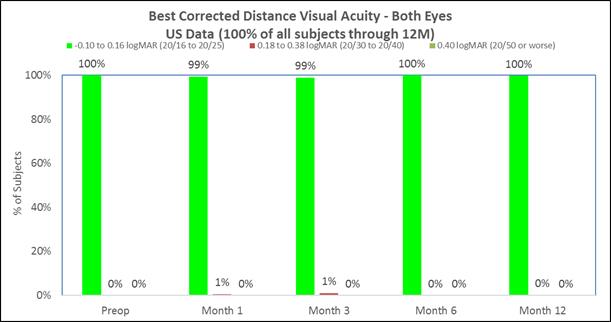
Approximately 98% of subjects achieved 20/40 or better best corrected distance vision in the treated eyes (Figure D) and there was little to no change in binocular best corrected distance vision (Figure E). (Presbia Flexivue Microlens is designed to take advantage of binocular vision as most patients fuse both images in the brain. The brain filters bad images, thus, resulting in accepting the best images. This process is known as “neuroadaptation.”). |
The following chart summarizes the uncorrected near visual acuity in the treated eyes:
Figure A
The following chart summarizes the uncorrected distance vision in the treated eyes:
Figure B
The following chart summarizes the binocular uncorrected distance visual acuity:
Figure C
The following chart summarizes the best corrected distance vision in the treated eyes:
Figure D
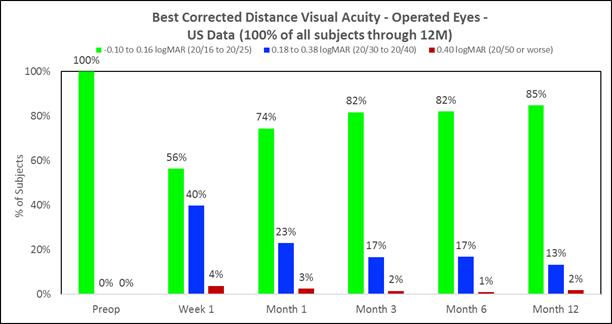
The following chart summarizes the binocular best corrected distance vision:
Figure E
Notwithstanding these results, we cannot assure you when or whether the Company will obtain pre-market approval, or what expenditures the Company will incur whether or not we obtain such approval, given the many significant risks associated with seeking such an approval from the FDA. Furthermore, certain adverse events have been reported as part of the on-going staged pivotal clinical trial. For a discussion of previously reported adverse events please see the risk factors, including the risk factor titled “If concerns regarding side effects from presbyopia correction surgery generally, or our products specifically, develop, including as a result of third-party studies and publications, our business, results of operations and financial condition will be materially and adversely affected.”, in the Company’s annual report on Form 10-K for the year ended December 31, 2015.
The Company requires PMA approval in order to market its products in the United States.
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
PRESBIA PLC |
|
|
|
|
|
|
|
|
|
|
|
By: /s/ Jarett Fenton |
|
|
|
|
|
Name: Jarett Fenton |
|
|
|
|
|
Title:Chief Financial Officer |
|
Dated: December 22, 2016