Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIO-PATH HOLDINGS INC | v455157_8k.htm |
Exhibit 99.1

“ A new path in DNA - powered medicine ” Company Presentation December 2016 1

Forward Looking Statements This Presentation contains forward - looking statements with respect to business conducted by Bio - Path Holdings, Inc . By their nature, forward - looking statements and forecasts involve risks and uncertainties because they relate to events and depend on circumstances that will occur in the future . The Company does not undertake to update any forward - looking statements . There are a number of factors that could cause actual results and developments to differ materially and investors should use their own judgment to evaluate risks . 2
DNAbilize™ Technology x Enables the development and delivery of systemic antisense DNA treatments for a broad spectrum of cancers including hematological malignancies, solid tumors, as well as diseases outside of cancer » has the ability to address hard to treat diseases and unmet clinical needs in fragile populations » has been the only technology in therapeutic applications that has shown no evidence of toxicity while producing therapeutic effect. x DNAbilize™ is NOT siRNA and is NOT like other antisense technology that have associated toxicity and no delivery 3
Investment Highlights • Lead candidate, Prexigebersen, (BP1001), a Liposomal Grb2 Antisense in Phase II for acute myeloid leukemia and chronic myeloid leukemia • Second drug candidate being readied for IND to start Phase I • Demonstrated ability to deliver antisense DNA into target cells and down - regulate the target protein in systemic disease • Lack of toxicity allows for development of drugs for hard to treat diseases and unmet needs among fragile populations • UT Southwestern developing clinical and preclinical pipeline for systemic lupus erythematosus (SLE) • MD Anderson developing clinical and preclinical pipeline in pancreatic, triple negative and inflammatory breast and advanced ovarian cancers • Thomas Jefferson University establishing DNAbilize™ Technology for glioblastoma immunotherapy • Applications and development in other cancers including solid tumors, hematological cancers, and indications outside of cancer Clinical Stage Pipeline Novel Mechanism of Action Validating Academic Collaborations Robust Preclinical Pipeline Broad Vision, Focused Strategy • Next generation oligonucleotide based therapeutics combined with high efficiency delivery • Original technology licensed from the MD Anderson Cancer Center with significant improvements from Bio - Path 4
» Antisense - molecules that interfere with the process of producing proteins inside cells (RNAi) » Does not use a toxic agent to kill cells, but blocks production of proteins » Advantage of specificity because it targets the disease - causing protein » No toxicity - In numerous animal studies and human patients in BP1001 clinical trial » DNAbilize ™ liposome structure is similar to the cellular membrane » P - ethoxy DNA does not induce hepatotoxicity or thrombocytopenia » Systemic treatment - I.V. delivery to the main organs via blood flow » High cellular uptake - liposome structure is similar to the cellular membrane » Microscopic - sized liposomes - enable penetration into tumors for delivery of drug » Proven target inhibition - demonstrated that DNAbilize ™ method inhibits target protein, proving delivery technology works DNAbilize ™ Antisense DNA: A Targeted Method for Treating Disease 5
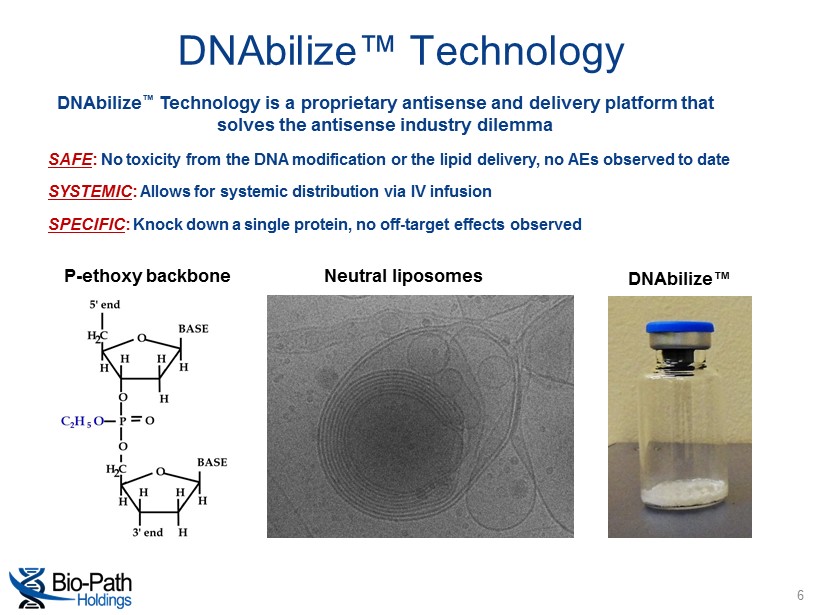
DNAbilize ™ Technology is a proprietary antisense and delivery platform that solves the antisense industry dilemma P - ethoxy backbone Neutral liposomes DNAbilize™ SAFE : No toxicity from the DNA modification or the lipid delivery, no AEs observed to date SYSTEMIC : Allows for systemic distribution via IV infusion SPECIFIC : Knock down a single protein, no off - target effects observed DNAbilize™ Technology 6
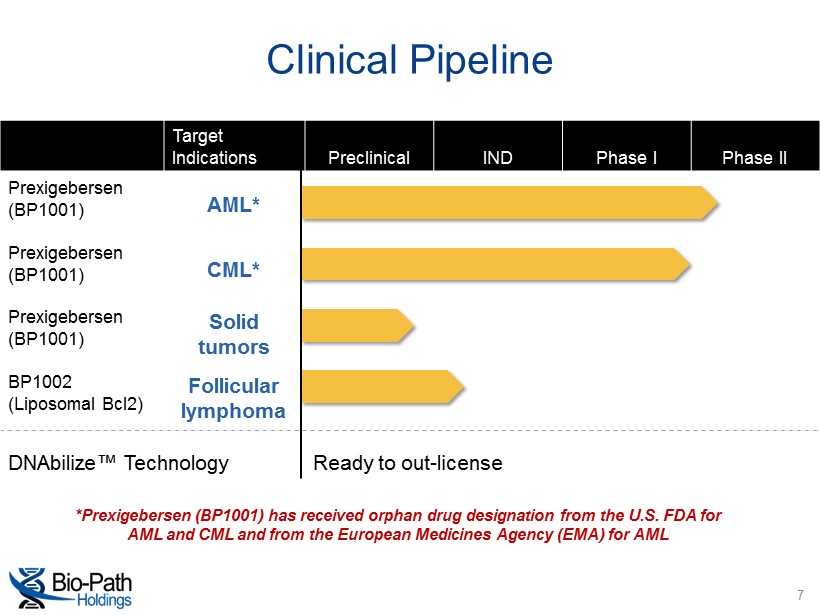
*Prexigebersen (BP1001) has received orphan drug designation from the U.S. FDA for AML and CML and from the European Medicines Agency (EMA) for AML Clinical Pipeline Target Indications Preclinical IND Phase I Phase II Prexigebersen (BP1001) AML* Prexigebersen (BP1001) CML* Prexigebersen (BP1001) Solid tumors BP1002 (Liposomal Bcl2) Follicular lymphoma DNAbilize ™ Technology Ready to out - license 7
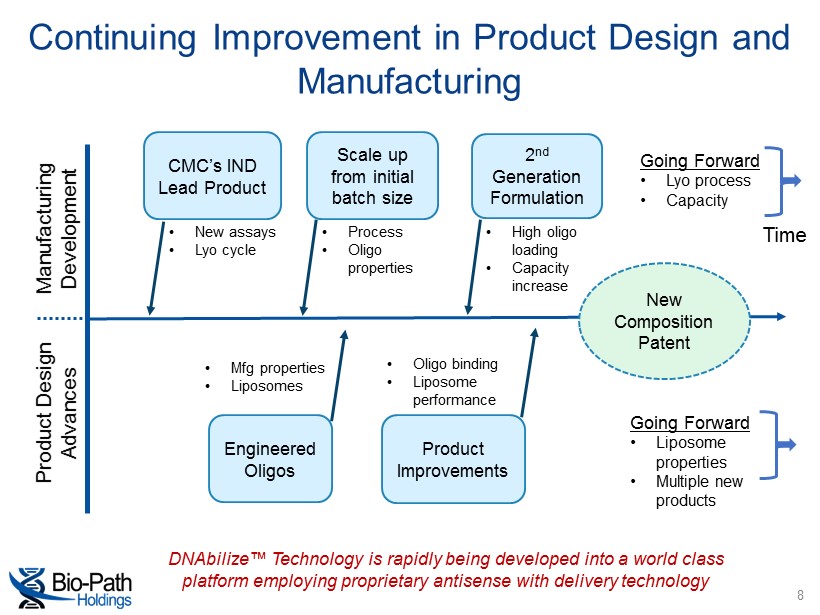
Continuing Improvement in Product Design and Manufacturing 8 Manufacturing Development Product Design Advances CMC’s IND Lead Product Scale up from initial batch size 2 nd Generation Formulation Engineered Oligos Product Improvements Going Forward • Lyo process • Capacity Going Forward • Liposome properties • Multiple new products Time DNAbilize™ Technology is rapidly being developed into a world class platform employing proprietary antisense with delivery technology • New assays • Lyo cycle • Process • Oligo properties • High oligo loading • Capacity increase New Composition Patent • Oligo binding • Liposome performance • Mfg properties • Liposomes
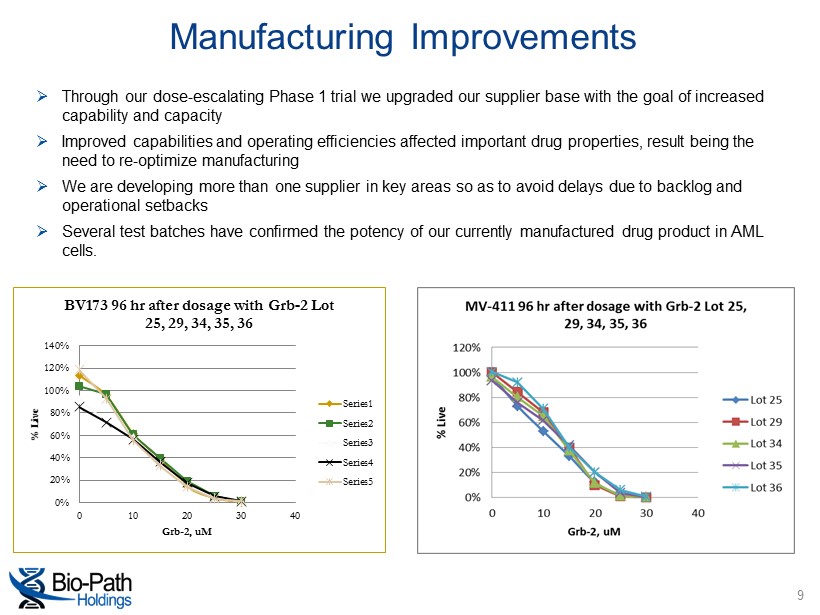
Manufacturing Improvements 9 0% 20% 40% 60% 80% 100% 120% 140% 0 10 20 30 40 % Live Grb - 2, uM BV173 96 hr after dosage with Grb - 2 Lot 25, 29, 34, 35, 36 Series1 Series2 Series3 Series4 Series5 » Through our dose - escalating Phase 1 trial we upgraded our supplier base with the goal of increased capability and capacity » Improved capabilities and operating efficiencies affected important drug properties, result being the need to re - optimize manufacturing » We are developing more than one supplier in key areas so as to avoid delays due to backlog and operational setbacks » Several test batches have confirmed the potency of our currently manufactured drug product in AML cells.
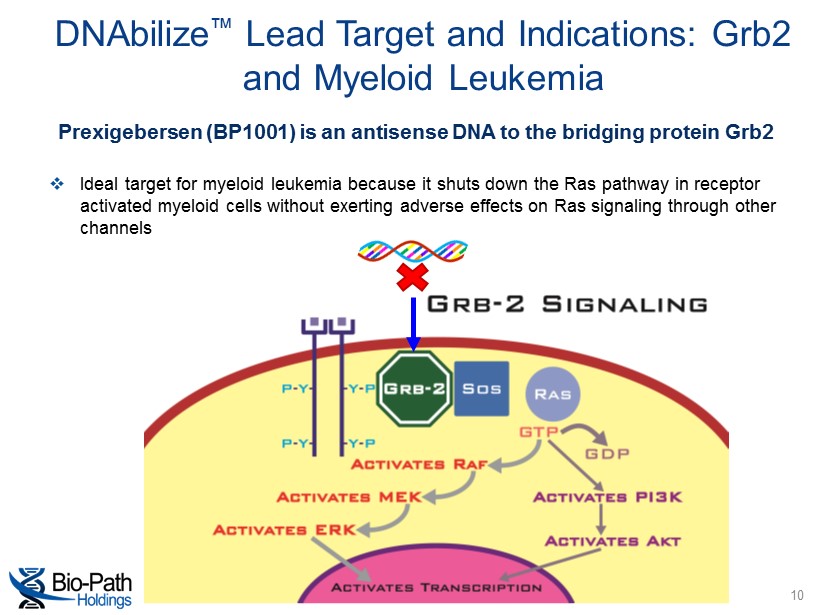
DNAbilize ™ Lead Target and Indications: Grb2 and Myeloid Leukemia 10 Prexigebersen (BP1001) is an antisense DNA to the bridging protein Grb2 □ Ideal target for myeloid leukemia because it shuts down the Ras pathway in receptor activated myeloid cells without exerting adverse effects on Ras signaling through other channels
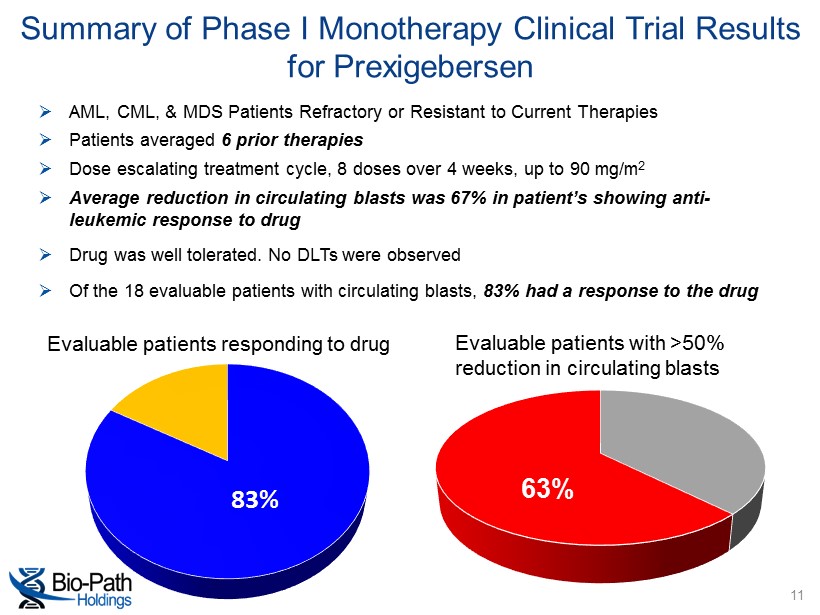
» AML, CML, & MDS Patients Refractory or Resistant to Current Therapies » Patients averaged 6 prior therapies » Dose escalating treatment cycle, 8 doses over 4 weeks, up to 90 mg/m 2 » Average reduction in circulating blasts was 67% in patient’s showing anti - leukemic response to drug » Drug was well tolerated. No DLTs were observed » Of the 18 evaluable patients with circulating blasts, 83% had a response to the drug Summary of Phase I Monotherapy Clinical Trial Results for Prexigebersen 83% Evaluable patients responding to drug Evaluable patients with >50% reduction in circulating blasts 63% 11
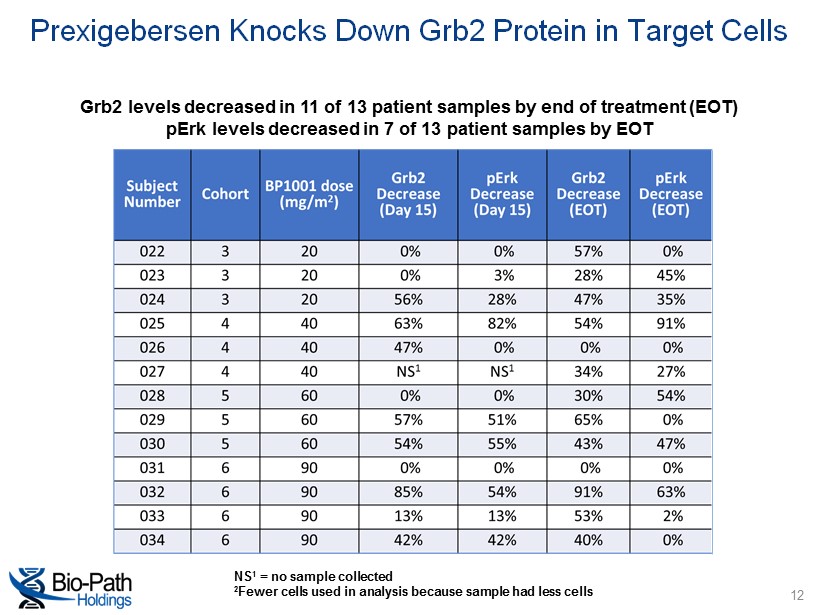
NS 1 = no sample collected 2 Fewer cells used in analysis because sample had less cells Grb2 levels decreased in 11 of 13 patient samples by end of treatment (EOT) pErk levels decreased in 7 of 13 patient samples by EOT Prexigebersen Knocks Down Grb2 Protein in Target Cells 12
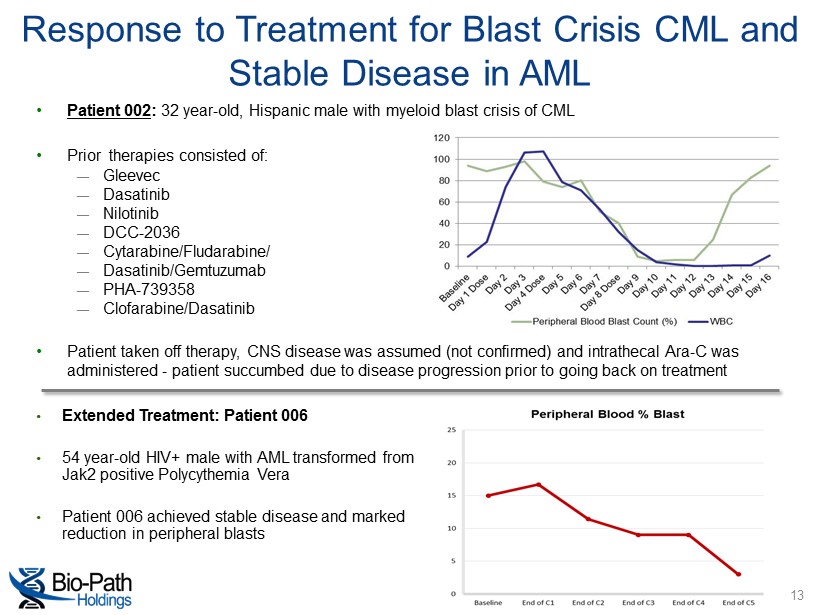
• Patient 002 : 32 year - old, Hispanic male with myeloid blast crisis of CML • Prior therapies consisted of: ― Gleevec ― Dasatinib ― Nilotinib ― DCC - 2036 ― Cytarabine/Fludarabine/ ― Dasatinib/Gemtuzumab ― PHA - 739358 ― Clofarabine/Dasatinib • Patient taken off therapy, CNS disease was assumed (not confirmed) and intrathecal Ara - C was administered - patient succumbed due to disease progression prior to going back on treatment Response to Treatment for Blast Crisis CML and Stable Disease in AML • Extended Treatment: Patient 006 • 54 year - old HIV+ male with AML transformed from Jak2 positive Polycythemia Vera • Patient 006 achieved stable disease and marked reduction in peripheral blasts 13
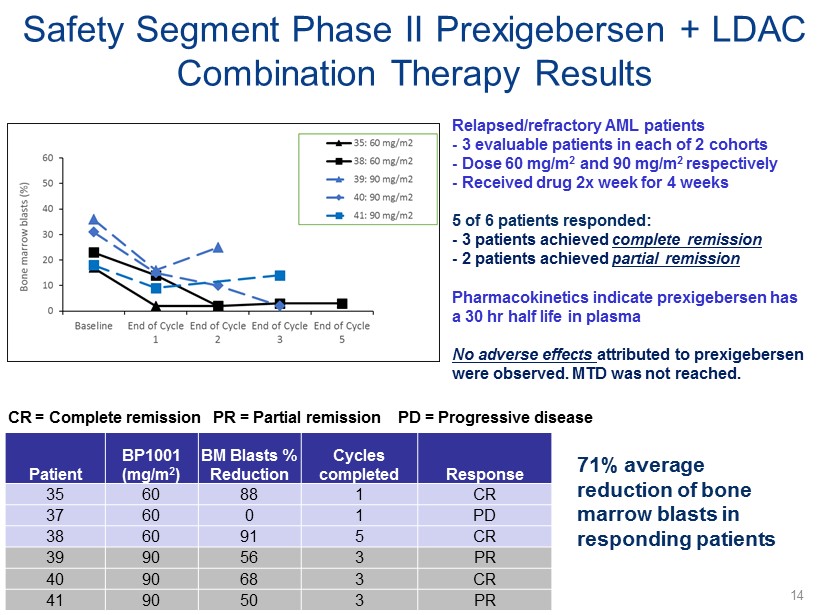
CR = Complete remission Safety Segment Phase II Prexigebersen + LDAC Combination Therapy Results Relapsed/refractory AML patients - 3 evaluable patients in each of 2 cohorts - Dose 60 mg/m 2 and 90 mg/m 2 respectively - Received drug 2x week for 4 weeks 5 of 6 patients responded: - 3 patients achieved complete remission - 2 patients achieved partial remission Pharmacokinetics indicate prexigebersen has a 30 hr half life in plasma No adverse effects attributed to prexigebersen were observed. MTD was not reached. PR = Partial remission PD = Progressive disease Patient BP1001 (mg/m 2 ) BM Blasts % Reduction Cycles completed Response 35 60 88 1 CR 37 60 0 1 PD 38 60 91 5 CR 39 90 56 3 PR 40 90 68 3 CR 41 90 50 3 PR 71% average reduction of bone marrow blasts in responding patients 14
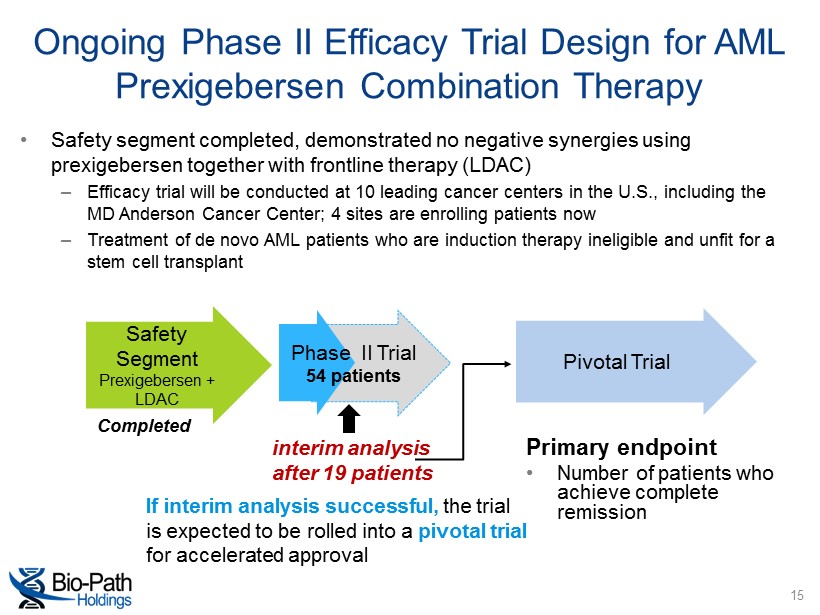
interim analysis after 19 patients Ongoing Phase II Efficacy Trial Design for AML Prexigebersen Combination Therapy • Safety segment completed, demonstrated no negative synergies using prexigebersen together with frontline therapy (LDAC) – Efficacy trial will be conducted at 10 leading cancer centers in the U.S., including the MD Anderson Cancer Center; 4 sites are enrolling patients now – Treatment of de novo AML patients who are induction therapy ineligible and unfit for a stem cell transplant Safety Segment Prexigebersen + LDAC Pivotal Trial Phase II Trial 54 patients If interim analysis successful, the trial is expected to be rolled into a pivotal trial for accelerated approval Completed Primary endpoint • Number of patients who achieve complete remission 15
Advances in the CML Program • Presentation at the 58th Annual American Society of Hematology Annual Meeting: – BP1001, a Novel Therapeutic for Chronic Myelogenous Leukemia • Prexigebersen decreased the proliferation of Gleevec ® ( imatinib ) - resistant CML cells in a dose - dependent manner • Prexigebersen pretreatment enhanced the inhibitory effects of Sprycel ® ( dasatinib ) in CML cells, leading to cell death • Five CML blast phase patients were enrolled in the first cohort (5 mg/m 2 dose) of the Phase 1 clinical study • Two CML patients with drug - resistant mutations showed significant reductions in circulating blasts during treatment – One patient’s blasts were reduced from 89% to 12%, while another patient’s blasts were reduced from 24% to 7% Prexigebersen has the potential to treat the 33% of CML patients who are resistant to Gleevec , the current standard of care 16
Prexigebersen Treatment Paradigm • Frontline therapy for de novo AML patients who are induction therapy ineligible and unfit for a stem cell transplant – Prexigebersen combination with LDAC – Current response rate frontline therapy alone less than 20% – Prexigebersen to improve response rates significantly – Reduce treatment related mortality • Salvage therapy for relapsed and refractory AML patients who have failed two prior regimens – Safety segment trial of prexigebersen combination with LDAC demonstrated complete or partial response in 5 of 6 elderly patients – Average patient age 75 years old • Blast crisis and accelerated phase CML patients – Prexigebersen combination with frontline therapy – Current response rate frontline therapy alone is <30% – Average survival 9 months 17
Preclinical Pipeline Validating DNAbilize™ with Key Opinion Leaders: • Establishing prexigebersen (BP1001) in triple negative and inflammatory breast cancer and advanced ovarian cancer with the MD Anderson Cancer Center • Developing clinical and preclinical targets for treatment of systemic lupus in collaboration with UT Southwestern Medical Center • Developing clinical and preclinical targets in pancreatic cancer using a patient derived ex vivo tumor model developed by The MD Anderson Cancer Center • Establishing DNAbilize™ technology for systemic immunotherapy for glioblastoma in collaboration with Thomas Jefferson University 18

Achievements and Upcoming Milestones Completed safety segment of the Phase II for AML Enrolled first patient in prexigebersen Phase II efficacy trial for AML Received orphan drug designation in the EU from the European Medicines Agency Expanded pre - clinical development with new target drug candidates (lymphoma, pancreatic, brain) Announced new indication outside of cancer, with UT Southwestern, autoimmune collaboration in systemic lupus Value propositions being advanced: • Enrollment of first 19 patients in Phase II with an interim analysis to be completed with potential for switch to registration trial for accelerated approval • Safety segment of the prexigebersen Phase II clinical trial for blast and accelerated crisis CML will provide insight into toxicity and potentially efficacy • Demonstrating effectiveness of delivery technology (broad drug development, licensing implications) • Pursuing new manufacturing and target IP 19

Core Organization Peter Nielsen Co - Founder, President, Chief Executive Officer and Chief Financial Officer • Officer and Director since founding Company in 2007 • Manufacturing development and evolution of engineered product design Ulrich Mueller, PhD Chief Operating Officer • P reviously Vice President at the Fred Hutchinson Cancer Research Center • Former Managing Director Office of Technology Commercialization at MD Anderson Ana M. Tari, PhD, MBA Director, Preclinical Operations & Research • Key member of the research team that developed our liposomal delivery technology Tara Sadeghi, MPH Director, Clinical Operations • More than 24 years of drug development and clinical operations experience across all phases of clinical development (Phases I through III) Suzanne Kennedy, PhD Director, Corporate Development • More than 15 years of marketing, business development, and research & development experience in the biotech industry Focus • Clinical team added to manage clinical trials and place new candidates into an IND, clinical trial • Expanding preclinical research and manufacturing capabilities 20

Jorge Cortes, M.D . Chairman • M.D. from la Facultad de Medicina , Universidad Nacional Autónoma de México • Jane and John Justin Distinguished Chair in Leukemia Research, Chief of the AML and CML sections, and Deputy Chair of the Department of Leukemia at The University of Texas MDACC • Has consulted leading pharmaceutical companies such as AstraZeneca on development of prenyltransferase inhibitors, GlaxoSmithKline on the use of topotecan in MDS and CMML, and Rhône - Poulenc Rorer on the use of PEG - Asparaginase in adult ALL Amy P. Sing, M.D. Member, Bio - Path’s Board of Directors • M.D. from the Stanford University School of Medicine • Previously Senior Director of Medical Affairs at Genomic Health, Inc. • Former Senior Medical Director at Genentech, Inc., had integral role in the Avastin ™ program • Former Senior Director of Medical and Regulatory Affairs at Seattle Genetics Recruiting additional members Scientific Advisory Board 21
Intellectual Property • Original patents licensed from MD Anderson • New composition and methods of use patents filed to cover DNAbilize technology, solely owned by Bio - Path Financial Snapshot • Ticker: NASDAQ: BPTH • Cash: $11.3 million as of September 30, 2016 • July 2016, $10 million registered direct offering extends cash runway through 2017 • Market Cap: Approximately $120 million • Burn rate: • $1 million per quarter core overhead • External programs: $1.0 - 1.5 million per quarter IP and Financial Snapshot 22
