Attached files
| file | filename |
|---|---|
| EX-10.1 - EXHIBIT 10.1 - Salarius Pharmaceuticals, Inc. | amendmenttojmccabeemployme.htm |
| 8-K - 8-K - Salarius Pharmaceuticals, Inc. | a20161213form8-k.htm |

1
Novel Treatments for Neuromuscular Conditions
NASDAQ: FLKS
December 2016

2
Any statements in this presentation and the oral commentary about future expectations, plans and prospects for the company, including
statements about the company’s strategy, future operations, development of its consumer and drug product candidates, plans for potential future
product candidates and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,”
“project,” “suggest,” “target,” “potential,” “will,” “approximately,” “development plans,” “would,” “could,” “should,” “continue,” and similar
expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may
differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the status, timing,
design, costs, results and interpretation of the company’s clinical studies; the uncertainties inherent in conducting clinical studies; results from our
ongoing and planned preclinical development; expectations of our ability to make regulatory filings and obtain and maintain regulatory approvals,
our ability to commercialize our consumer products; positioning and product attributes of our consumer products; results of early clinical studies
as indicative of the results of future trials; availability of funding sufficient for the company’s foreseeable and unforeseeable operating expenses
and capital expenditure requirements; other matters that could affect the availability or commercial potential of the company’s consumer or drug
product candidates; the inherent uncertainties associated with intellectual property; and other factors discussed in the Risk Factors set forth in the
company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC) and in other filings the company makes with
the SEC from time to time. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and
you should not place undue reliance on our forward-looking statements. The forward-looking statements in this presentation represent our views
as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we
may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent
required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date
subsequent to the date of this presentation.
This presentation also contains estimates and other statistical data made by independent parties and by the company relating to market size and
growth and other data about the company’s industry. This data involves a number of assumptions and limitations, and you are cautioned not to
give undue weight to such estimates. In addition, projections, assumptions and estimates of the company’s future performance and the future
performance of the markets in which the company operates are necessarily subject to a high degree of uncertainty and risk.
This presentation contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and
trade names referred to in this presentation, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but
such references are not intended to indicate, in any way, that their respective owners will not assert, to the fullest extent under applicable law,
their rights thereto. We do not intend our use or display of other companies' trade names or trademarks to imply a relationship with, or
endorsement or sponsorship of us by, any other companies.
Forward-Looking Statements

3
3
ORIGIN STORY

4
• Innovative treatments for a broad range of painful and debilitating muscle cramps,
spasms and spasticity based upon novel insights regarding neuromuscular physiology
from our co-founders (ion channels and TRP biology, MacKinnon Nobel Prize 2003)
• FLX-787: MS & ALS exploratory human POC studies initiated in 2016; results in H2
2017. Phase 2 NLC study to initiate H1 2017.
• Data in human settings involving muscle cramps:
1. NLC: efficacy signals in 2 exploratory randomized, blinded, controlled, cross-over
POC studies with FLX-787 (n=37 subanalysis, n=29)
2. NLC: statistically significant positive effect in a randomized, blinded, controlled,
cross-over study with extract formulation (n=50)
3. Electrically-induced cramp (EIC) model: sigmoidal dose response curve (n=5),
p<0.05. Randomized, blinded, controlled efficacy with 100+ subjects & over 200
safety/efficacy data points
Flex Pharma Overview

5
Management Team
• Christoph Westphal, MD PhD, CEO; Cofounder/Lead investor ALNY MNTA XLRN SIRT Alnara CNCE VSTM OVAS
• Rob Hadfield, General Counsel; Cooley LLP, Kiva Systems, SG Cowen
• Kathie Lindemann, COO; DAVIDs TEA, Starbucks
• John McCabe, CFO; Ariad, Charles River Associates, Biogen, Arthur Andersen
• Angelene Simonello, VP Corporate and Program Development; Viacell, Biogen
• Thomas Wessel, MD PhD, CMO; JNJ (Razadyne®), SEPR (Lunesta®), ACOR (Ampyra®)
• Elizabeth Woo, SVP, Investor Relations; Biogen, Ironwood, Cubist
Board of Directors
• Jeff Capello, former CFO Ortho-Clinical Diagnostics; BOD OVAS, former Boston Scientific CFO, PKI, PWC
• Peter Barton Hutt, former Chief Counsel FDA; Sirtris, Momenta, Concert, Covington and Burling
• Marc Kozin, LEK Consulting, former President of North American practice; BOD OVAS, ECYT, DYAX, UFPT
• Rod MacKinnon, MD, Co-founder, Chair, SAB; Nobel Prize 2003, ion channels; Professor, Rockefeller; NAS
• Rob Perez, former CEO Cubist; former Biogen, BOD AMAG, CDTX
• Stuart Randle, Ivenix CEO, former CEO GI Dynamics, former CEO ACT Medical, Baxter
• John Sculley, former CEO Pepsi (current owner of Gatorade), former CEO Apple
• Michelle Stacy, former Keurig President, Gillette/P&G, BOD iRobot
Management Team and Board of Directors

6
Sports Team investors
Wyc Grousbeck, Managing Partner, Governor, CEO
Boston Celtics
KPC Venture Capital (Kraft family), Owner New
England Patriots, New England Revolution
PagsGroup (Steve Pagliuca), Managing Partner, Bain
Capital; Managing Partner, Boston Celtics
Mark Wan, Causeway Partners; Minority Owner: Boston
Celtics, SF 49ers
Christoph Westphal, Minority Owner Boston Celtics
Scientific Advisory Board
Rod MacKinnon, MD, Cofounder, Chair, SAB; Nobel Prize
2003, ion channels; Professor, Rockefeller; National
Academy of Science (NAS)
Bruce Bean, PhD, Cofounder, Chair, SAB; Winthrop
Professor, Harvard Med; Neurophysiology; NAS
W. Larry Kenney, PhD; Penn State Univ Professor
Physiology
Alfred Sandrock, MD, PhD; Neurologist, Chief Medical
Officer of Biogen
Roger Tung, PhD; Medicinal chemist, Vertex, Merck
(inventor multiple drugs); CEO, Concert
Chris Walsh, PhD; Professor Emeritus, Harvard Med;
Genzyme, Verastem, Sirtris; NAS
John Winkelman, MD, PhD; Chief Sleep Disorders, MGH;
BWH, RLS clinical development
Scientific Advisory Board & Select Investors

7
Large and Diverse Market Opportunities
Nocturnal Leg Cramps
Sudden painful contraction
reducing sleep quality
No drug approved in the U.S.
U.S. Patient Population
• 37% prevalence for 50+ yo1
• ~4M over 65 yo suffer daily2
1 Naylor & Young, A General Population Survey of Rest Cramps, Age and Ageing 1994.23 418-420
2 Management estimates based on third party survey results
3 National Institute of Neurological Disorders and Stroke
4 Morbidity and Mortality Weekly Report July 2014
Muscle Cramps & Spasticity
in Neuromuscular Conditions
Multiple Sclerosis, ALS/Motor
Neuron Disease
U.S. Patient Population
• MS: 250K – 350K patients3
• ALS: 12K patients4
8
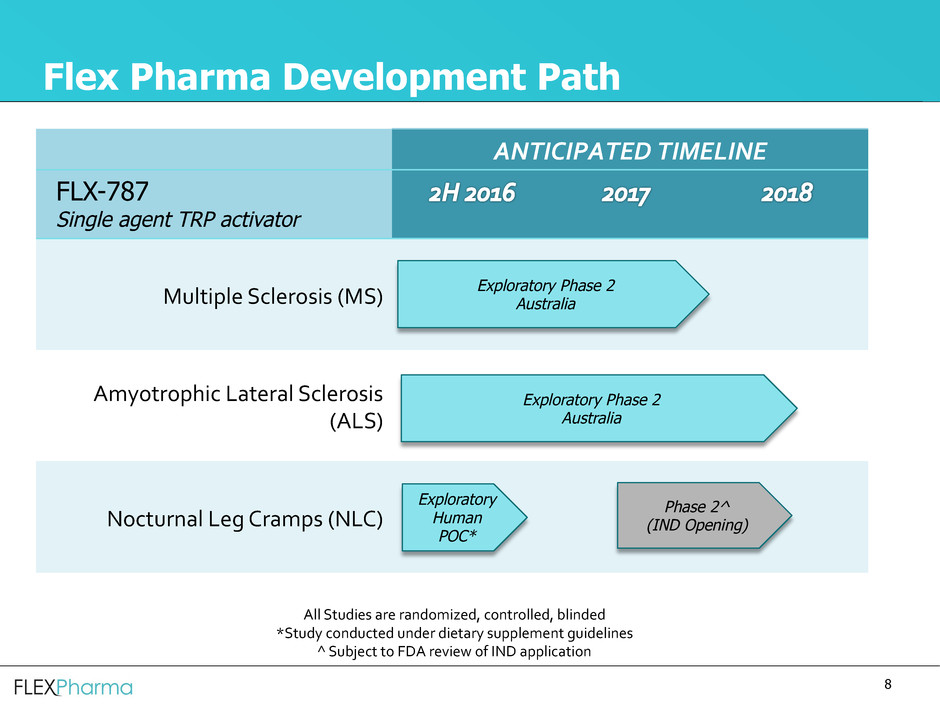
Flex Pharma Development Path
ANTICIPATED TIMELINE
FLX-787
Single agent TRP activator
Multiple Sclerosis (MS)
Amyotrophic Lateral Sclerosis
(ALS)
Nocturnal Leg Cramps (NLC)
Phase 2^
(IND Opening)
Exploratory
Human
POC*
Exploratory Phase 2
Australia
Exploratory Phase 2
Australia
All Studies are randomized, controlled, blinded
*Study conducted under dietary supplement guidelines
^ Subject to FDA review of IND application

9
Neurogenic Origin of Muscle Cramping/Spasms
Cramps and spasms are generally NOT caused by dehydration, lactic acid build-up
or electrolyte imbalances affecting the muscle
• Muscle cramping is caused by excessive firing
of alpha-motor neurons in the spinal cord,
which trigger a painful contraction of the
muscle
• Repetitive muscle use induces
hyperexcitability of alpha-motor neurons,
causing them to fire excessively and trigger
cramping Alpha-motor neuron
Hyperexcitability of alpha-motor neurons is also a likely basis for spasticity and spasms

10
TRP Ion Channel Co-crystal Structures –
Flex Drug Targets in Two Recent Nature Papers
C.E. Paulsen, J. Armache, Y. Gao, Y. Cheng and D. Julius,
Nature, 8 April 2015
E. Cao, M. Liao, Y. Cheng and D. Julius, Nature, 5 Dec 2013
TRPA1 TRPV1

11
Chemical Neuro Stimulation of Vagus Nerve

12
FLX-787 Orally Disintegrating Tablet (ODT)
§ Single agent molecule selected on the
basis of potency and efficacy in human
electrically-induced cramp model
§ Optimized ODT formulation to cover
TRPV1 and TRPA1 receptors in mucous
membranes
§ Reduced risk of aspiration for patients
across all potential indications
FLX-787 Tablets
13
1 1 0 1 0 0
0 . 0
0 . 5
1 . 0
0
l o g D o s e ( m g )
P
e
r
c
e
n
t
R
e
d
u
c
ti
o
n
i
n
r
A
U
C
(
%
)
F l e x - 7 8 7 I n h i b i t i o n o f e l e c t r i c a l l y - i n d u c e d
c r a m p s i n n o r m a l h e a l t h y s u b j e c t s
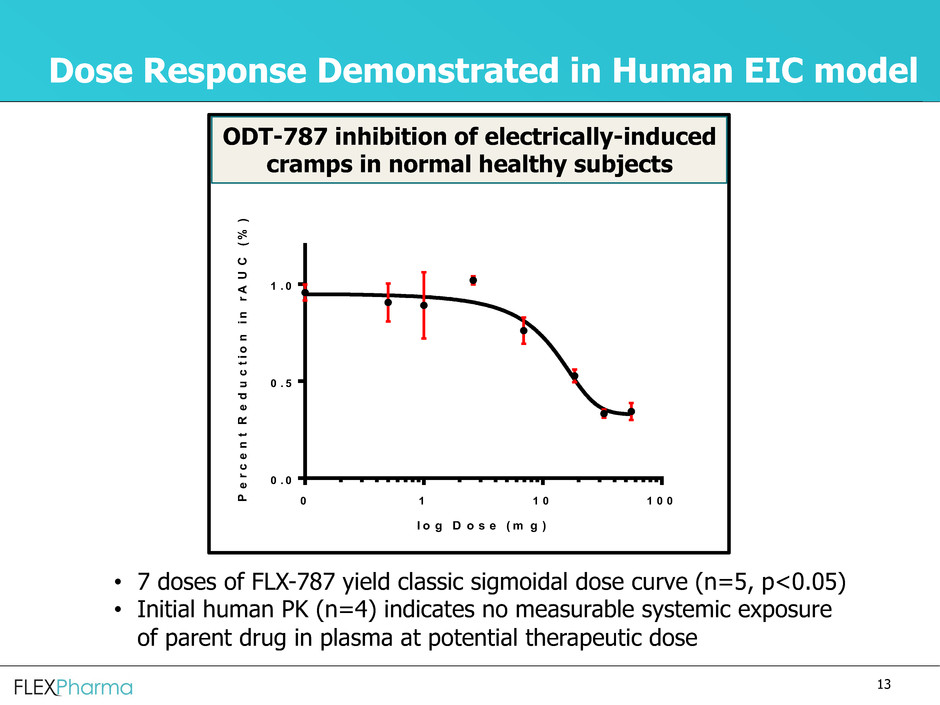
Dose Response Demonstrated in Human EIC model
ODT-787 inhibition of electrically-induced
cramps in normal healthy subjects
• 7 doses of FLX-787 yield classic sigmoidal dose curve (n=5, p<0.05)
• Initial human PK (n=4) indicates no measurable systemic exposure
of parent drug in plasma at potential therapeutic dose

14
Baseline assessed
over 2-week Run-in
MS & ALS Exploratory Studies
Recruitment and
Screening
Baseline
Run-In:
(14 days)
MS: Placebo
ALS: No Tx
Cross-Over
Period 2
FLX-787
(14 days)
Cross-Over
Period 3
FLX-787
(14 days)
Cross-Over
Period 3
Placebo
(14 days)
? ?
? ? ?
?
?
7-
14
D
W
A
S
H
O
U
T
Cross-Over
Period 2
Placebo
(14 days)
? Study Visit
Unmet Need:
current agents have safety concerns (quinine) or sedating effects (benzodiazepenes)
MS
• n~50; 19mg liquid, 2x/day
• Initiated June 2016; Results H2 2017
• Spasticity (Tardieu, Ashworth), Cramping in patients who cramp, sleep, safety
ALS
• n<50; 30mg ODT, 3x/day
• Initiated Sept 2016; Results 2017/2018
• Cramping, spasticity, sleep
Exclude: Placebo Responders
Parallel Crossover
15
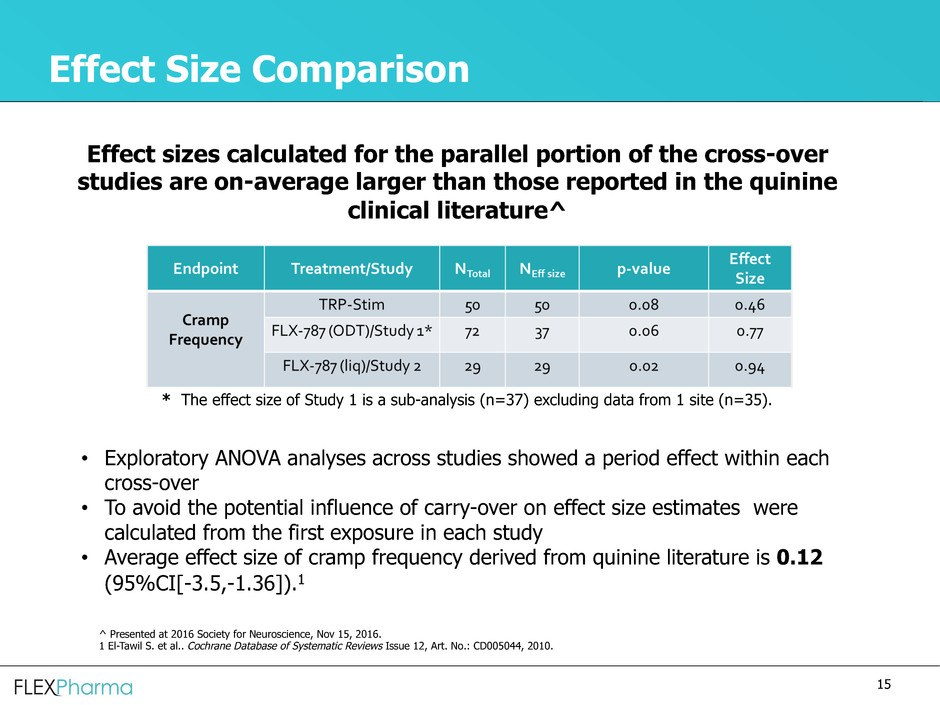
Effect Size Comparison
Effect sizes calculated for the parallel portion of the cross-over
studies are on-average larger than those reported in the quinine
clinical literature^
• Exploratory ANOVA analyses across studies showed a period effect within each
cross-over
• To avoid the potential influence of carry-over on effect size estimates were
calculated from the first exposure in each study
• Average effect size of cramp frequency derived from quinine literature is 0.12
(95%CI[-3.5,-1.36]).1
* The effect size of Study 1 is a sub-analysis (n=37) excluding data from 1 site (n=35).
^ Presented at 2016 Society for Neuroscience, Nov 15, 2016.
1 El-Tawil S. et al.. Cochrane Database of Systematic Reviews Issue 12, Art. No.: CD005044, 2010.
16
MS ALS
Traumatic brain injury
Spinal cord injury
Post-stroke
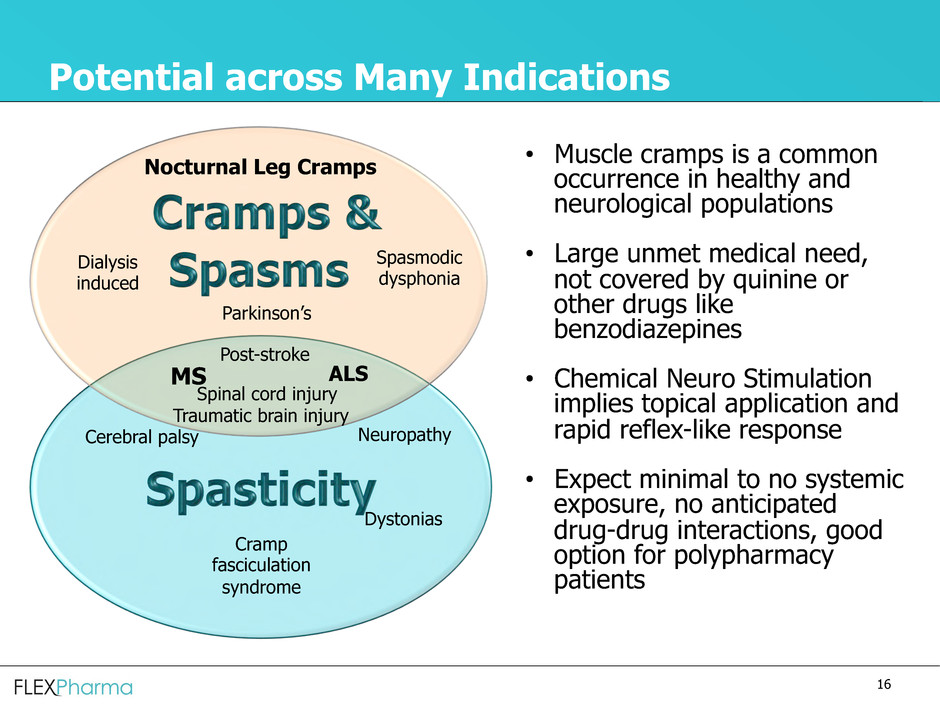
Potential across Many Indications
• Muscle cramps is a common
occurrence in healthy and
neurological populations
• Large unmet medical need,
not covered by quinine or
other drugs like
benzodiazepines
• Chemical Neuro Stimulation
implies topical application and
rapid reflex-like response
• Expect minimal to no systemic
exposure, no anticipated
drug-drug interactions, good
option for polypharmacy
patients
Nocturnal Leg Cramps
Dystonias
Cramp
fasciculation
syndrome
Dialysis
induced
Spasmodic
dysphonia
Neuropathy
Parkinson’s
Cerebral palsy

17
Anticipated Upcoming Milestones
• H1 2017: IND filing
• H1 2017: Initiate Phase 2 NLC study
• H2 2017: Exploratory Phase 2 MS study readout
• 2017/2018: Exploratory Phase 2 ALS study readout

18
HOTSHOT WORKS

12/12/16 19
PREVENT
Drink a 1.7 oz. HOTSHOT 15-30 minutes before exercise to boost
your Neuromuscular Performance and prevent muscle cramps. Feel
it work from the first kick to the warm afterglow.
RECOVER
Drink a 1.7 oz. HOTSHOT after activity to
prevent post-exercise cramping.
A proprietary formulation of organic ingredients that stop
muscle cramps where they start. At the nerve.
TREAT
Drink a 1.7 oz. HOTSHOT at the first sign
of cramping. It starts working in minutes.
FIRST & ONLY PRODUCT
SCIENTIFICALLY PROVEN TO
PREVENT & TREAT MUSCLE CRAMPS

20

21
HOW HOTSHOT WORKS:
TRP ACTIVATION

12/12/16 22
THE OPPORTUNITY
Cramp Incidence
• 68% of triathletes (lifetime) 1
• 60% of cyclists (lifetime) 1
• 30-50% of marathon runners (lifetime) 1, 2
1 – Schwellnus MP et al. Muscle cramping in athletes – risk factors, clinical assessment, and management. Clin Sports Med 27(1):183-194, 2008
2 – Minetto MA et al. Origin and development of muscle cramps. Exerc Sports Sci Rev 41(1):3-10, 2013

ENDORSEMENTS
23 23 © 2016 Flex Innovation Group LLC. All Rights Reserved
SHALANE FLANAGAN
USA Marathon Runner
“Thank you
#ITSTHENERVE for a
great workout today.”
EVAN JAGER
USA Steeplechase
“Thanks to
#ITSTHENERVE for
cramp-free steeplechase
training and races!”
AMY CRAGG
USA Marathon Runner
“Happy to have Shalane
Flanagan and
#ITSTHENERVE by my
side for the long run!”
COLLEEN QUIGLEY
USA Steeplechase
“Thanks to #ITSTHENERVE for
keeping me cramp-free during
this very important time in my
training!”

BRAND AMBASSADOR
“It’s been an education.
What I’ve learned from Rod
MacKinnon is quite
opposite from what the
commonly held beliefs are
about cramping. HOTSHOT
can definitely be a game
changer.”
Craig “Crowie” Alexander,
5x Triathlon World Champion/
HOTSHOT Brand Ambassador
24 © 216 Flex Innovation Group LLC. All Rights Reserved

BRAND AMBASSADOR
25 © 216 Flex Innovation Group LLC. All Rights Reserved

26
A MUSCLE CRAMP IS A FAILURE IN
NEURO MUSCULAR PERFORMANCE
BRAIN
SPINAL
CORD
MUSCLE
RECEPTORS
Signals from the brain to the spinal cord
balance the activity of motor neurons
Alpha motor neurons, residing in the spinal
cord, are what control the contraction of
muscles
If alpha motor neurons become hyper-
excitable and start to fire repetitively,
a muscle cramp can occur

27
NMP
Neuro Muscular Performance refers to consistent, and
closely integrated function of nerves and muscles. It’s how
an athlete’s nerves and muscles work together in an optimal
way.
HOTSHOT boosts your NMP to stop muscle cramps by treating
the nerve. When you’re cramp free you can push harder, train
longer, finish stronger.
We continue to study the poten,al benefits of improved Neuro
Muscular Performance.

28
PREMIUM PACKAGING
12-PACK
12-PACK
$65
6-PACK
$35
1.7 OZ.
$7
6-PACK SINGLE BOTTLE

LAUNCHED!
PRODUCT
• Natural, Organic, NSF Certified for Sport®
PLACEMENT:
• TeamHOTSHOT.com
• Specialty retailers in Boston, Boulder & LA
PROMOTION
• Face-to-face events to cultivate influencers
• Authentic brand ambassadors (Crowie, Flanagan)
• Word of mouth amplified through social media
• Targeted media (LAVA, Competitor, VELO, etc)
2016 Net Revenues
• Q2: $113K
• Q3: $586K
• ~20K customers
29
1.7 OZ. of Neuro
Muscular Performance

30
Financial Profile
• NASDAQ: FLKS
• $67.3 M Cash balance as of 9/30/16
• Cash into Q1 2019 based on current operating plan
• ~17.9 million shares outstanding
• No debt

31
Novel Treatments for
Neuromuscular Conditions
NASDAQ: FLKS
32
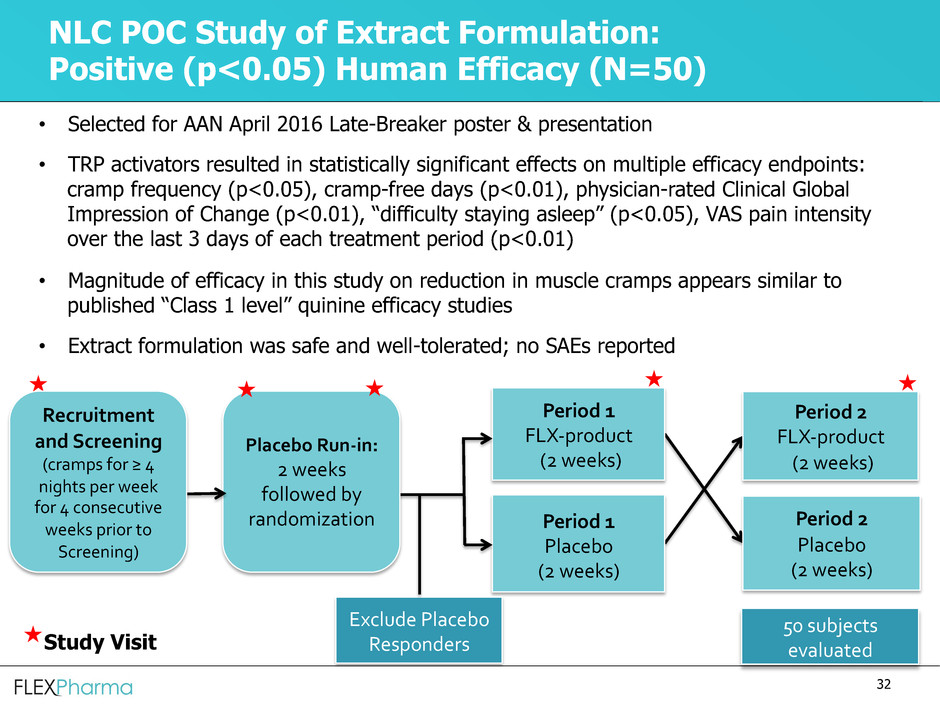
NLC POC Study of Extract Formulation:
Positive (p<0.05) Human Efficacy (N=50)
• Selected for AAN April 2016 Late-Breaker poster & presentation
• TRP activators resulted in statistically significant effects on multiple efficacy endpoints:
cramp frequency (p<0.05), cramp-free days (p<0.01), physician-rated Clinical Global
Impression of Change (p<0.01), “difficulty staying asleep” (p<0.05), VAS pain intensity
over the last 3 days of each treatment period (p<0.01)
• Magnitude of efficacy in this study on reduction in muscle cramps appears similar to
published “Class 1 level” quinine efficacy studies
• Extract formulation was safe and well-tolerated; no SAEs reported
Placebo Run-in:
2 weeks
followed by
randomization
Period 1
FLX-product
(2 weeks)
Period 2
FLX-product
(2 weeks)
Period 1
Placebo
(2 weeks)
Period 2
Placebo
(2 weeks)
Exclude Placebo
Responders
★ ★ ★ ★
★ Study Visit
50 subjects
evaluated
★
Recruitment
and Screening
(cramps for ≥ 4
nights per week
for 4 consecutive
weeks prior to
Screening)
33
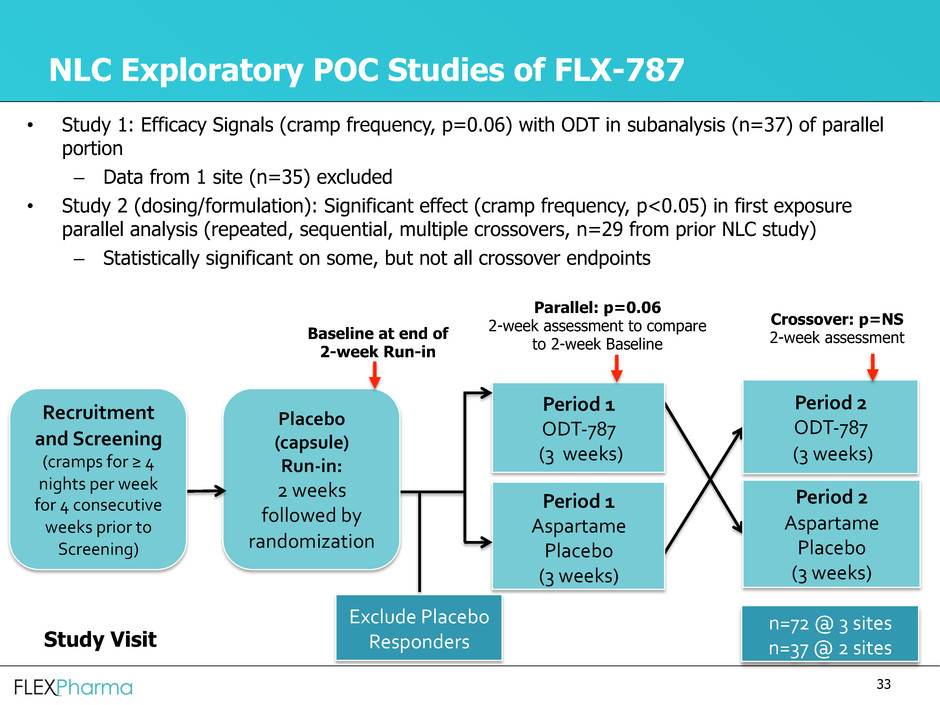
NLC Exploratory POC Studies of FLX-787
Placebo
(capsule)
Run-in:
2 weeks
followed by
randomization
Period 1
ODT-787
(3 weeks)
Period 2
ODT-787
(3 weeks)
Period 1
Aspartame
Placebo
(3 weeks)
Period 2
Aspartame
Placebo
(3 weeks)
Exclude Placebo
Responders Study Visit
n=72 @ 3 sites
n=37 @ 2 sites
Recruitment
and Screening
(cramps for ≥ 4
nights per week
for 4 consecutive
weeks prior to
Screening)
• Study 1: Efficacy Signals (cramp frequency, p=0.06) with ODT in subanalysis (n=37) of parallel
portion
– Data from 1 site (n=35) excluded
• Study 2 (dosing/formulation): Significant effect (cramp frequency, p<0.05) in first exposure
parallel analysis (repeated, sequential, multiple crossovers, n=29 from prior NLC study)
– Statistically significant on some, but not all crossover endpoints
Baseline at end of
2-week Run-in
Parallel: p=0.06
2-week assessment to compare
to 2-week Baseline
Crossover: p=NS
2-week assessment

34
NLC Key Learnings: Topical Neurostimulation of TRPA1 &
TRPV1 Channels Reduces Muscle Cramping in Humans
Efficacy Signals Warrant Further
NLC Development
• FLX-787 has shown positive
signals on muscle cramping in the
parallel design portion of two
exploratory human proof-of-
concept NLC studies.
• FLX-787 has shown a sigmoidal
dose-response curve in a human
electrically-induced cramp model.
• FLX-787 thus far is well tolerated
and safe, and no SAEs have been
reported.
Key Learnings
• Patient selection and data capture
& monitoring are critical issues of
focus in the upcoming clinical
studies.
• Given potential carry-over effects
in cross-over studies, and
consistent with FDA guidance,
future FLX-787 studies in NLC will
be parallel design.
Next Steps
• Initiate IND-opening Phase 2
parallel design study in H1 2017,
after the IND has been accepted.

35
Unmet Need
• Sudden painful contractions negatively impacting sleep and quality of life
• No drug approved in the U.S. In 1994, FDA banned use of quinine for
treatment of leg cramps due to association with serious and life-threatening
adverse events (primarily thrombocytopenia)
Affected U.S. Population
• 37% prevalence for 50+ yo1
• Approximately 4M people over 65 yo suffer daily2
• NLC population increasing dramatically given aging US demographics
Significant Demand
• 4.5 Million Quinine sulfate prescriptions in 2013 in UK (1/5 of the US population)
NLC Market Opportunity
1 Naylor & Young, A General Population Survey of Rest Cramps, Age and Ageing 1994.23 418-420
2 Management estimates based on third party survey results
*Two published studies of quinine treatment in muscle cramps that were categorized as Class I level of evidence by Katzberg et al for the American Academy of Neurology in 2010:
• Randomised controlled trial of hydroquinine in muscle cramps. Jansen et al, Lancet 1997; 349: 528-32.
• Effectiveness of quinine in treating muscle cramps: a double-blind, placebo-controlled, parallel-group, multicentre trial. Diener et al. Int J Clin Pract. 2002 May;56(4):243-6.
Comprehensive meta-analysis of quinine studies by the Cochrane Collaboration in 2010.

36
Broad Intellectual Property
Broad initial applications covering HOTSHOT and FLX-787 that should
provide the basis for an expanded patent portfolio
• Applications Covering FLX-787
• Composition and method of use application covering one or more
TRP activators for the treatment of muscle cramps and spasms
associated with disease
• Composition and method of use application covering FLX-787 and
other single molecules for the treatment of muscle cramps and
spasms associated with disease
• Applications Covering HOTSHOT
• Methods of use application covering two or more TRP activators for
treating EAMC and NLC
• Compositions of TRP activators based on HOTSHOT formulation
