Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Revance Therapeutics, Inc. | d307789dex992.htm |
| 8-K - FORM 8-K - Revance Therapeutics, Inc. | d307789d8k.htm |

Revance TherapeutIcs DaxibotulinumtoxinA for Injection (RT002) Investigational Product for the Treatment of Cervical Dystonia RT002 is an investigational product Interim Results for Phase 2 Open-Label Study Exhibit 99.1

Forward-Looking Statements / Safe Harbor This presentation contains forward-looking statements, including statements related to: our financial outlook and other financial performance; the process and timing of anticipated future clinical development of our product candidates; our business strategy, goals, plans and prospects; timing and outcome of our clinical trials; our ability to obtain regulatory approval; the potential therapeutic and economic benefits and value of our product candidates and our technologies; demand for our product candidates and drivers of demand; market size, adoption rate and potential revenue; growth opportunities and product pipeline; our ability to leverage our investment in our development and manufacturing platform; and our intellectual property strategy. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from our expectations. These risks and uncertainties include, but are not limited to: the outcome, cost and timing of our product development activities and clinical trials; the uncertain clinical development process, including the risks that interim results are not indicative of final results and that that clinical trials may not have an effective design; our ability to obtain and maintain regulatory approval of our product candidates; our ability to obtain funding for our operations; our plans to research, develop and commercialize our product candidates; our ability to achieve market acceptance of our product candidates; unanticipated costs or delays in research, development and commercialization efforts; the applicability of clinical study results to actual outcomes; the size and growth potential of the markets for our product candidates; our ability to successfully commercialize our product candidates and the timing of commercialization activities; the rate and degree of market acceptance of our product candidates; our ability to develop sales and marketing capabilities; the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for financing; and our ability to continue obtaining and maintaining intellectual property protection for our product candidates. These and other risks are described in the “Risk Factors” section of our Form 10-Q filed with the Securities and Exchange Commission on November 4, 2016. The “Risk Factors” section of 10-Q speaks only as of the date thereof. These forward-looking statements speak only as of the date hereof or the date specified. Revance disclaims any obligation to update these forward-looking statements. “Revance Therapeutics”, TransMTS, “Remarkable Science Changes Everything”, and the Revance logo are registered trademarks of Revance Therapeutics, Inc. All other trademarks or registered trademarks are the property of their respective owners.
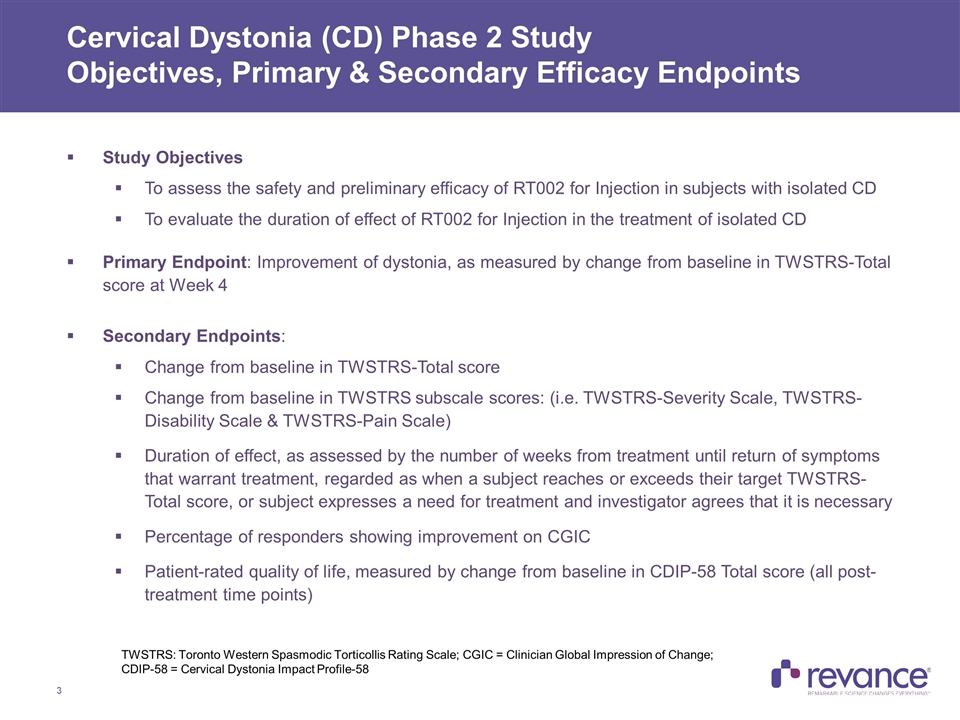
Study Objectives To assess the safety and preliminary efficacy of RT002 for Injection in subjects with isolated CD To evaluate the duration of effect of RT002 for Injection in the treatment of isolated CD Primary Endpoint: Improvement of dystonia, as measured by change from baseline in TWSTRS-Total score at Week 4 Secondary Endpoints: Change from baseline in TWSTRS-Total score Change from baseline in TWSTRS subscale scores: (i.e. TWSTRS-Severity Scale, TWSTRS-Disability Scale & TWSTRS-Pain Scale) Duration of effect, as assessed by the number of weeks from treatment until return of symptoms that warrant treatment, regarded as when a subject reaches or exceeds their target TWSTRS-Total score, or subject expresses a need for treatment and investigator agrees that it is necessary Percentage of responders showing improvement on CGIC Patient-rated quality of life, measured by change from baseline in CDIP-58 Total score (all post-treatment time points) Cervical Dystonia (CD) Phase 2 Study Objectives, Primary & Secondary Efficacy Endpoints TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale; CGIC = Clinician Global Impression of Change; CDIP-58 = Cervical Dystonia Impact Profile-58
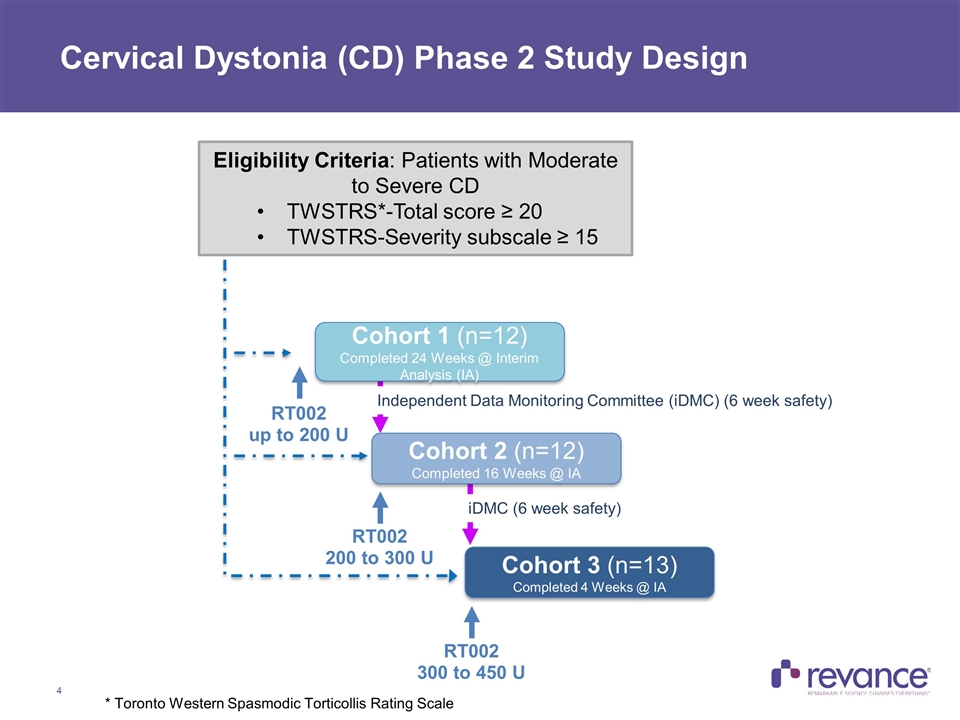
Cervical Dystonia (CD) Phase 2 Study Design RT002 up to 200 U Independent Data Monitoring Committee (iDMC) (6 week safety) iDMC (6 week safety) RT002 200 to 300 U RT002 300 to 450 U Eligibility Criteria: Patients with Moderate to Severe CD TWSTRS*-Total score ≥ 20 TWSTRS-Severity subscale ≥ 15 Cohort 1 (n=12) Completed 24 Weeks @ Interim Analysis (IA) Cohort 2 (n=12) Completed 16 Weeks @ IA Cohort 3 (n=13) Completed 4 Weeks @ IA * Toronto Western Spasmodic Torticollis Rating Scale
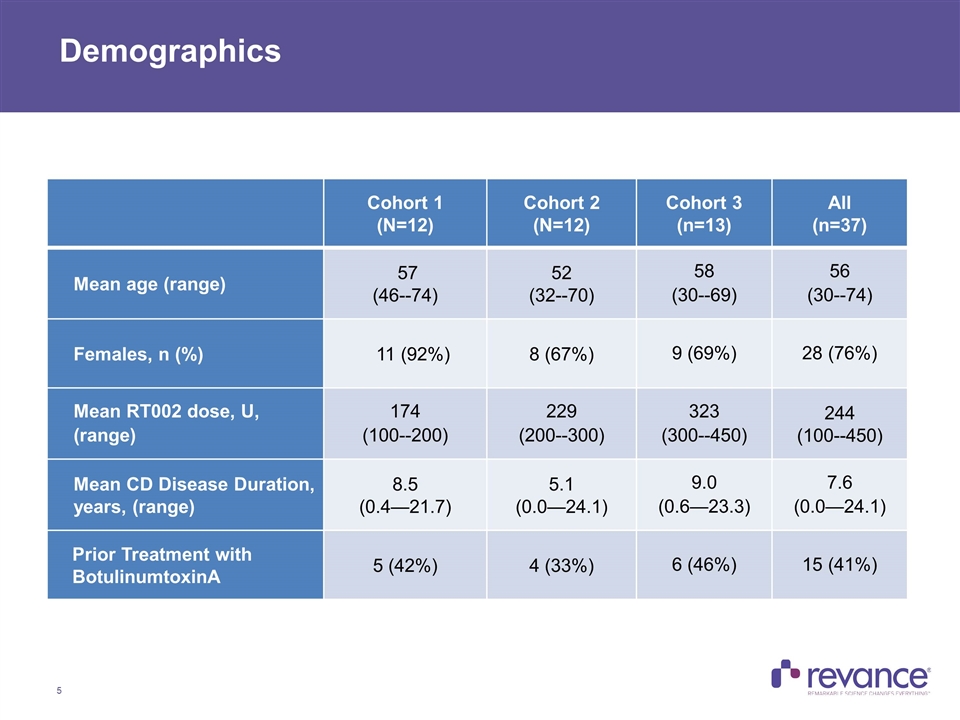
Demographics Cohort 1 (N=12) Cohort 2 (N=12) Cohort 3 (n=13) All (n=37) Mean age (range) 57 (46--74) 52 (32--70) 58 (30--69) 56 (30--74) Females, n (%) 11 (92%) 8 (67%) 9 (69%) 28 (76%) Mean RT002 dose, U, (range) 174 (100--200) 229 (200--300) 323 (300--450) 244 (100--450) Mean CD Disease Duration, years, (range) 8.5 (0.4—21.7) 5.1 (0.0—24.1) 9.0 (0.6—23.3) 7.6 (0.0—24.1) Prior Treatment with BotulinumtoxinA 5 (42%) 4 (33%) 6 (46%) 15 (41%)
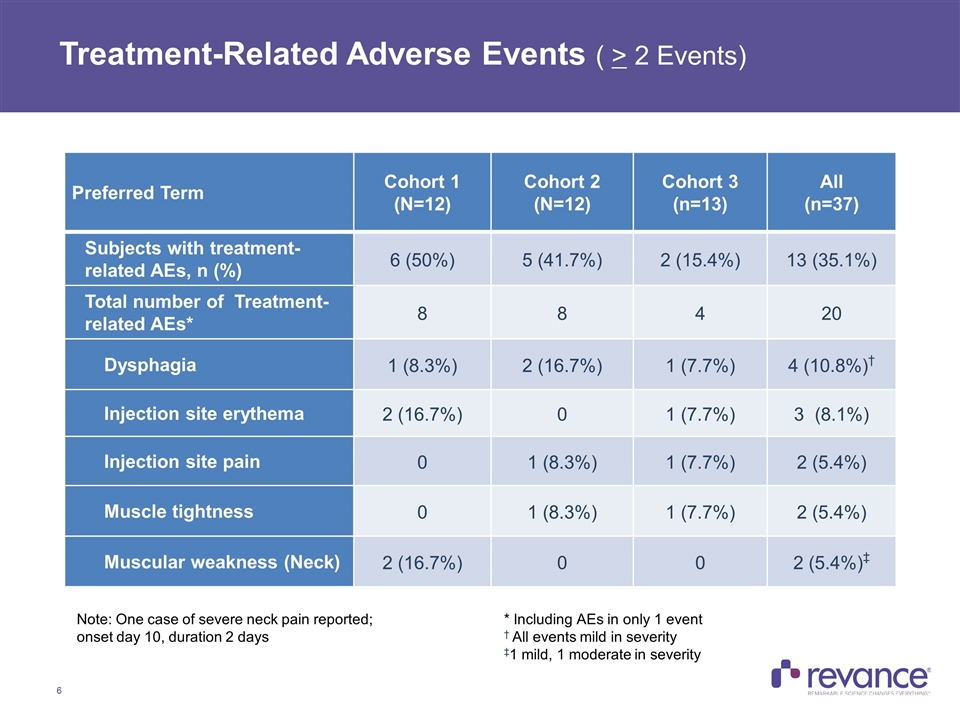
Treatment-Related Adverse Events ( > 2 Events) Preferred Term Cohort 1 (N=12) Cohort 2 (N=12) Cohort 3 (n=13) All (n=37) Subjects with treatment-related AEs, n (%) 6 (50%) 5 (41.7%) 2 (15.4%) 13 (35.1%) Total number of Treatment-related AEs* 8 8 4 20 Dysphagia 1 (8.3%) 2 (16.7%) 1 (7.7%) 4 (10.8%)† Injection site erythema 2 (16.7%) 0 1 (7.7%) 3 (8.1%) Injection site pain 0 1 (8.3%) 1 (7.7%) 2 (5.4%) Muscle tightness 0 1 (8.3%) 1 (7.7%) 2 (5.4%) Muscular weakness (Neck) 2 (16.7%) 0 0 2 (5.4%)‡ * Including AEs in only 1 event † All events mild in severity ‡1 mild, 1 moderate in severity Note: One case of severe neck pain reported; onset day 10, duration 2 days
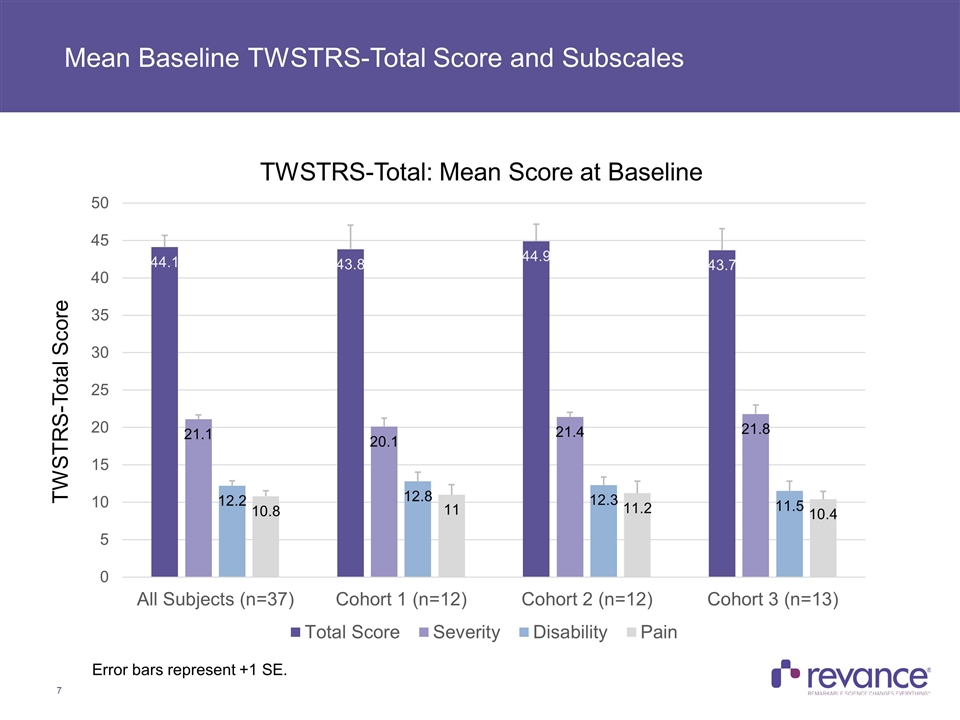
Mean Baseline TWSTRS-Total Score and Subscales Error bars represent +1 SE. TWSTRS-Total Score
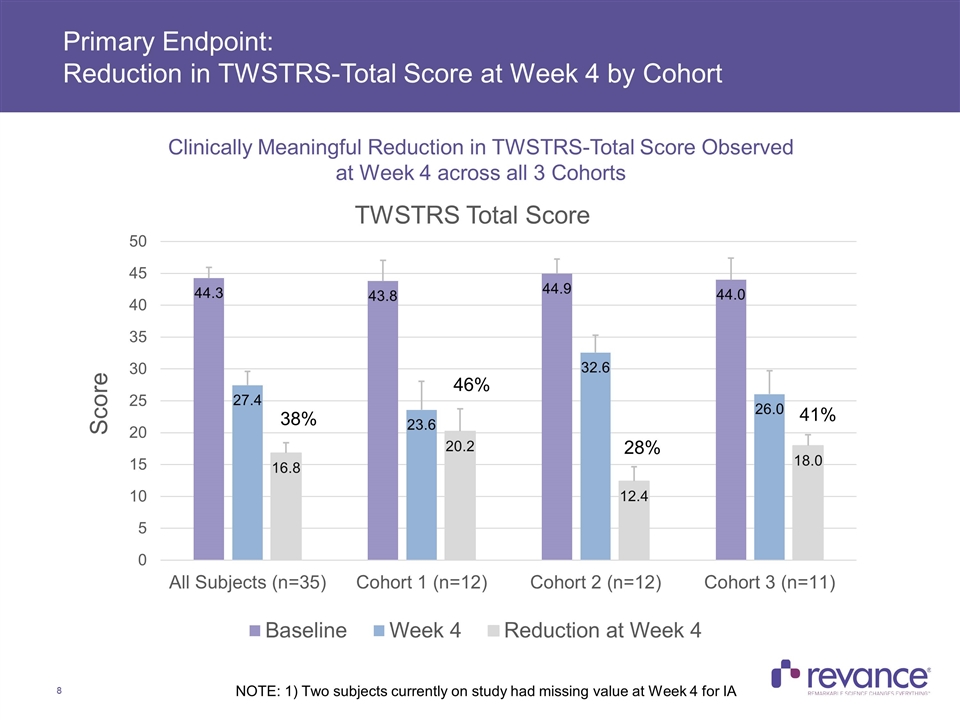
Primary Endpoint: Reduction in TWSTRS-Total Score at Week 4 by Cohort NOTE: 1) Two subjects currently on study had missing value at Week 4 for IA 38% 46% 28% 41% Clinically Meaningful Reduction in TWSTRS-Total Score Observed at Week 4 across all 3 Cohorts
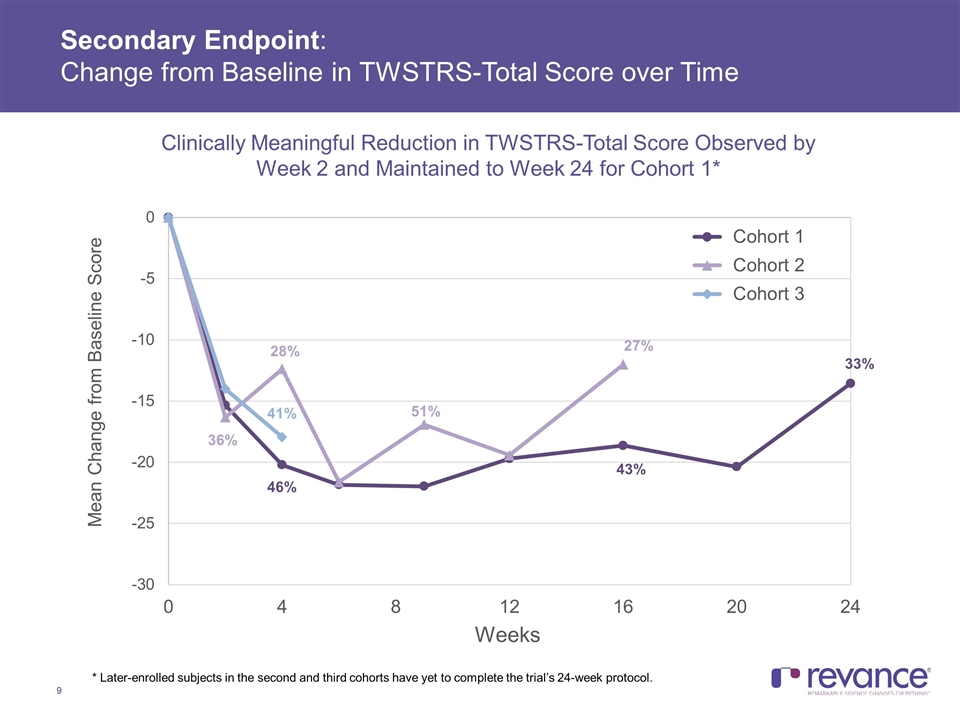
Secondary Endpoint: Change from Baseline in TWSTRS-Total Score over Time 46% 43% 33% 28% 51% 27% 41% Clinically Meaningful Reduction in TWSTRS-Total Score Observed by Week 2 and Maintained to Week 24 for Cohort 1* 36% * Later-enrolled subjects in the second and third cohorts have yet to complete the trial’s 24-week protocol.
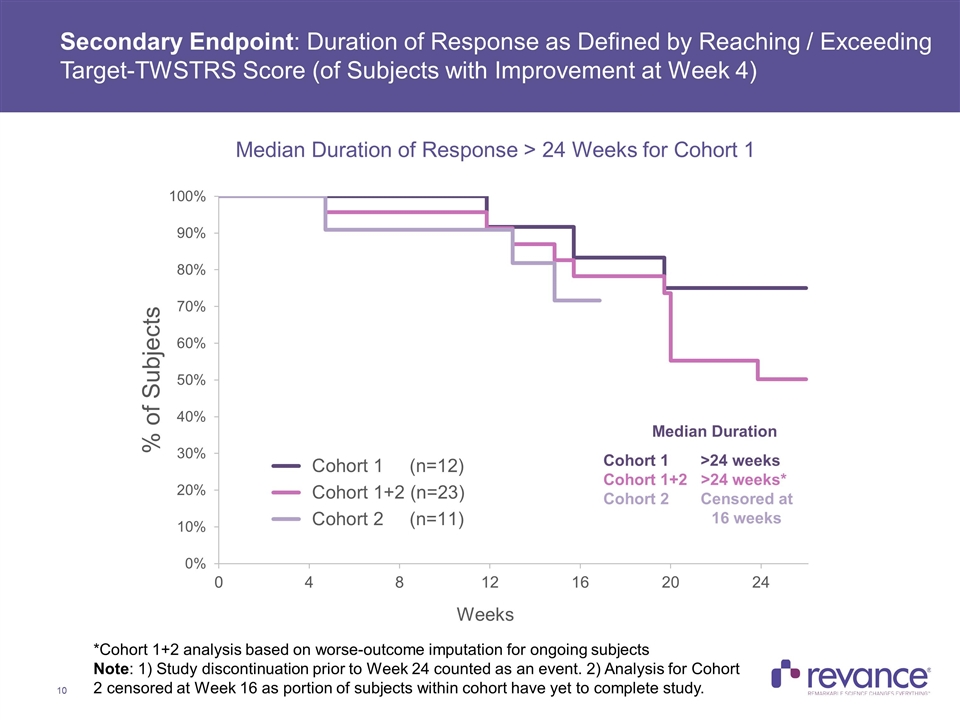
Median Duration Cohort 1 >24 weeks Cohort 1+2 >24 weeks* Cohort 2 Censored at 16 weeks *Cohort 1+2 analysis based on worse-outcome imputation for ongoing subjects Note: 1) Study discontinuation prior to Week 24 counted as an event. 2) Analysis for Cohort 2 censored at Week 16 as portion of subjects within cohort have yet to complete study. Secondary Endpoint: Duration of Response as Defined by Reaching / Exceeding Target-TWSTRS Score (of Subjects with Improvement at Week 4) Median Duration of Response > 24 Weeks for Cohort 1
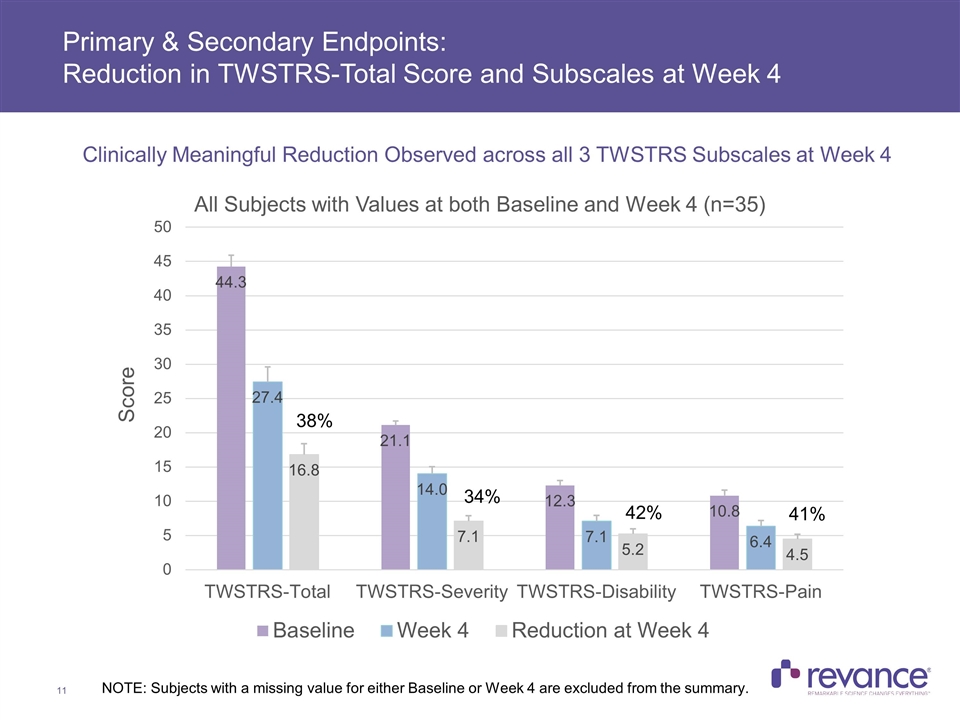
Primary & Secondary Endpoints: Reduction in TWSTRS-Total Score and Subscales at Week 4 NOTE: Subjects with a missing value for either Baseline or Week 4 are excluded from the summary. 38% 34% 42% 41% Clinically Meaningful Reduction Observed across all 3 TWSTRS Subscales at Week 4
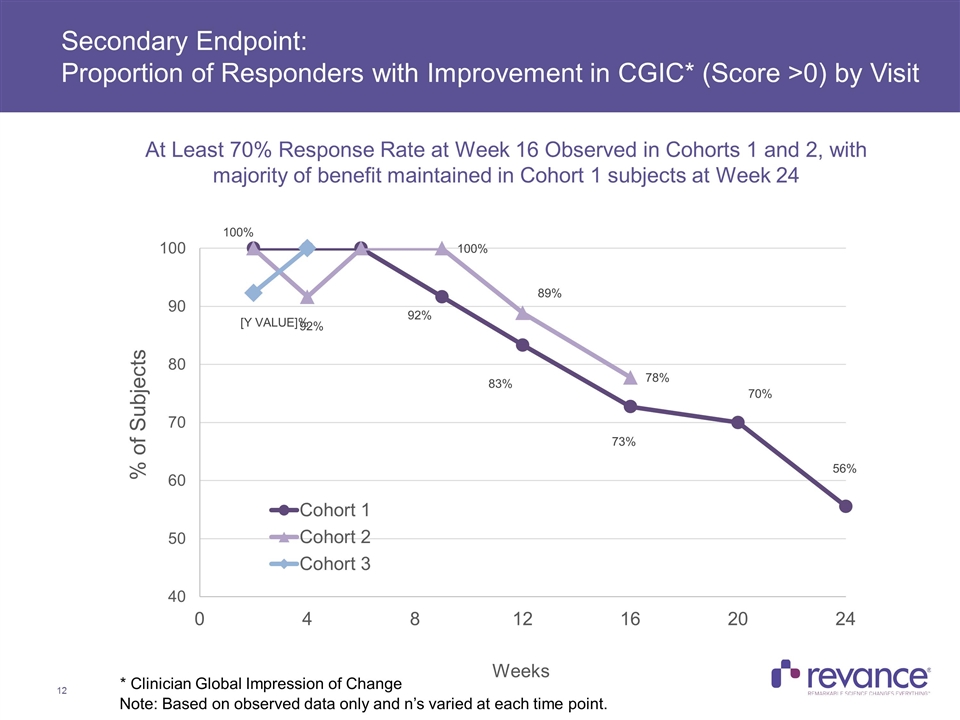
Secondary Endpoint: Proportion of Responders with Improvement in CGIC* (Score >0) by Visit * Clinician Global Impression of Change At Least 70% Response Rate at Week 16 Observed in Cohorts 1 and 2, with majority of benefit maintained in Cohort 1 subjects at Week 24 Note: Based on observed data only and n’s varied at each time point.
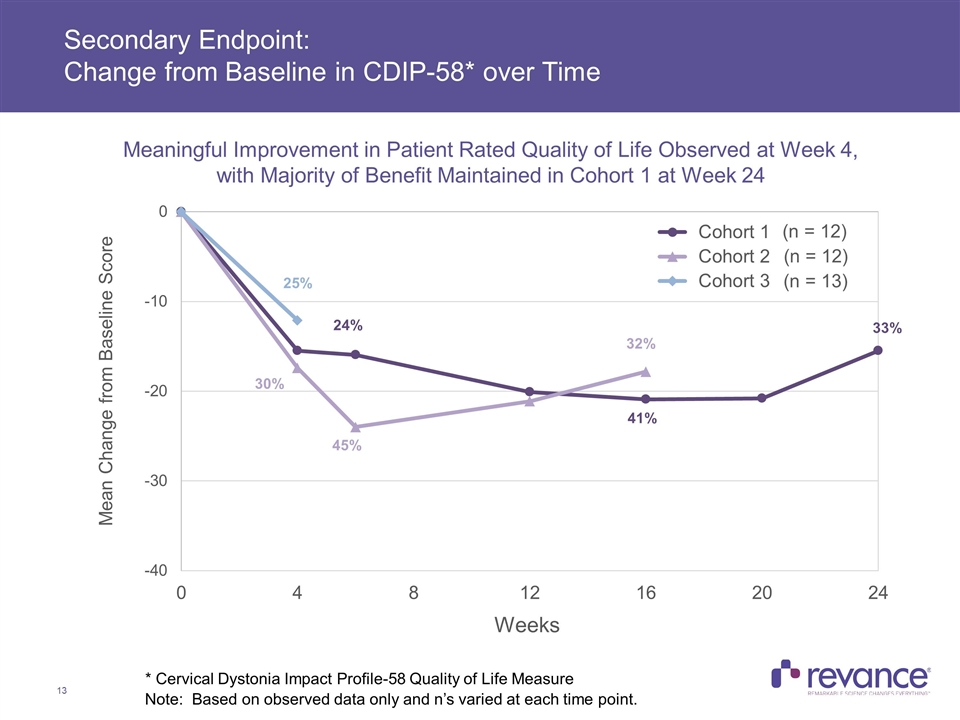
Secondary Endpoint: Change from Baseline in CDIP-58* over Time 25% 24% 41% 33% 30% 45% 32% * Cervical Dystonia Impact Profile-58 Quality of Life Measure Meaningful Improvement in Patient Rated Quality of Life Observed at Week 4, with Majority of Benefit Maintained in Cohort 1 at Week 24 Note: Based on observed data only and n’s varied at each time point. (n = 12) (n = 12) (n = 13)

Summary of Safety Results RT002 appeared to be generally safe and well tolerated across all 3 cohorts, with no increase in treatment-related AE’s upon dose escalation Total of 20 Treatment Related AE’s reported in 13 of 37 subjects Most frequently reported: dysphagia (10.8%), injection site erythema (8.1%) injection site pain (5.4%), muscle tightness (5.4%) and muscular weakness (5.4%) All Treatment Related AE’s were mild or moderate in severity except for one case of neck pain reported as severe (Day 10 onset, duration of 2 days) Trials of other BoNTA products approved to treat CD have rates of dysphagia ranging from 13-39%* No Serious AE’s were reported The following Treatment-Related AE’s associated with distant spread of toxin were not observed: dry mouth, generalized weakness, dysphonia, dysarthria or difficulty breathing * Includes BOTOX, Dysport DBL, Dysport and Xeomin. Data as reported in product prescribing information

Summary of Efficacy Results Clinically meaningful treatment benefit addressing the disabling features of CD was observed in the TWSTRS-Total Score, with a mean reduction of 38% across all subjects at Week 4 In Cohort 1, with a mean dose of 174U, the majority of the treatment benefit observed at Week 4 (46% improvement) was maintained at Week 24 (33%) Clinically meaningful mean reductions in the TWSTRS Severity, Disability and Pain subscales were consistent and also observed at all time points in Cohort 1 Duration of Effect: The median duration of effect, as measured by the time for subjects to reach their Target TWSTRS Score was > 24 weeks for Cohort 1 In Cohort 1, no subjects had returned to their baseline TWSTRS-Total Score at Week 24, while only one subject in Cohort 2 to date returned to baseline, which occurred at the Week 24 visit Complete assessment of RT002 duration was limited by study duration of 24 weeks Current treatment of cervical dystonia calls for injection of botulinum toxin approximately every 3 months, or 4 times per year Clinician Global Impression of Change: > 70% Response Rate at Week 16 Observed in Cohorts 1 and 2, with majority of benefit maintained in Cohort 1 subjects at Week 24 Quality of Life: A meaningful improvement in patient-rated QoL was observed on CDIP-58 at week 4, with the majority of this benefit maintained in Cohort 1 at Week 24
