Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - TherapeuticsMD, Inc. | txmd-8k_120816.htm |
Exhibit 99.1

1 TX - 001HR Supplemental Replenish Trial Design And Topline Phase 3 Results December 2016

2 Forward - Looking Statements This presentation by TherapeuticsMD, Inc . (referred to as “we” and “our”) may contain forward - looking statements . Forward - looking statements may include, but are not limited to, statements relating to our objectives, plans and strategies, as well as statements, other than historical facts, that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future . These statements are often characterized by terminology such as “believe,” “hope,” “may,” “anticipate,” “should,” “intend,” “plan,” “will,” “expect,” “estimate,” “project,” “positioned,” “strategy” and similar expressions and are based on assumptions and assessments made in light of our managerial experience and perception of historical trends, current conditions, expected future developments and other factors we believe to be appropriate . Forward - looking statements in this presentation are made as of the date of this presentation, and we undertake no duty to update or revise any such statements, whether as a result of new information, future events or otherwise . Forward - looking statements are not guarantees of future performance and are subject to risks and uncertainties, many of which may be outside of our control . Important factors that could cause actual results, developments and business decisions to differ materially from forward - looking statements are described in the sections titled “Risk Factors” in our filings with the Securities and Exchange Commission, including our most recent Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q, as well as our current reports on Form 8 - K, and include the following : our ability to maintain or increase sales of our products ; our ability to develop, protect and defend our intellectual property ; our ability to develop and commercialize our hormone therapy drug candidates and obtain additional financing necessary therefore ; whether the company will be able to prepare a new drug application for its TX - 001 HR product candidate and, if prepared, whether the FDA will accept and approve the application ; whether the FDA will approve the company’s new drug application for its TX - 004 HR product candidate and whether any such approval will occur by the PDUFA date ; the length, cost and uncertain results of our clinical trials ; potential adverse side effects or other safety risks that could preclude the approval of our hormone therapy drug candidates ; our reliance on third parties to conduct our clinical trials, research and development and manufacturing ; the availability of reimbursement from government authorities and health insurance companies for our products ; the impact of product liability lawsuits ; the influence of extensive and costly government regulation ; the volatility of the trading price of our common stock ; and the concentration of power in our stock ownership . Yuvvexy TM (TX - 004 HR), TX - 001 HR, TX - 005 HR, and TX - 006 HR are investigational drugs and are not approved by the FDA . This non - promotional presentation is intended for investor audiences only . PDF copies of press releases and financial tables can be viewed and downloaded at our website : www.therapeuticsmd.com / pressreleases.aspx .
3 Replenish Trial Overview A Phase 3, Double - Blind, Placebo - Controlled, Randomized, Multicenter Study to Evaluate the Safety and Efficacy of Estradiol in Combination with Progesterone in Postmenopausal Women with an Intact Uterus
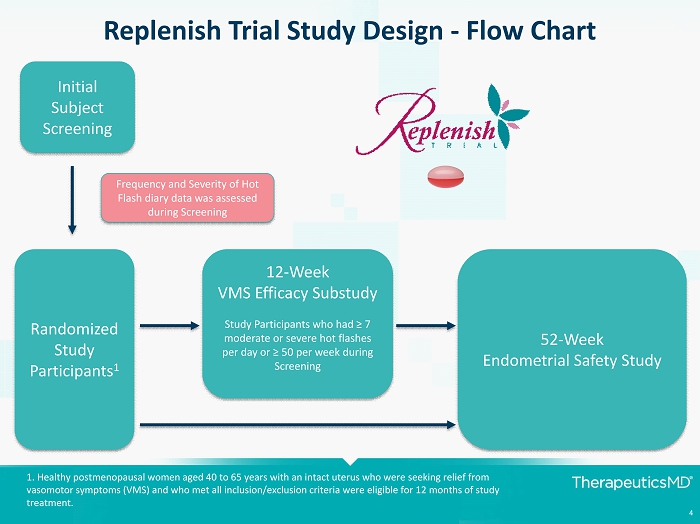
4 Replenish Trial Study Design - Flow Chart 1. Healthy postmenopausal women aged 40 to 65 years with an intact uterus who were seeking relief from vasomotor symptoms (VMS) and who met all inclusion/exclusion criteria were eligible for 12 months of study treatment. Initial Subject Screening Randomized Study Participants 1 52 - Week Endometrial Safety Study 12 - Week VMS Efficacy Substudy Study Participants who had ≥ 7 moderate or severe hot flashes per day or ≥ 50 per week during Screening Frequency and Severity of Hot Flash diary data was assessed during Screening
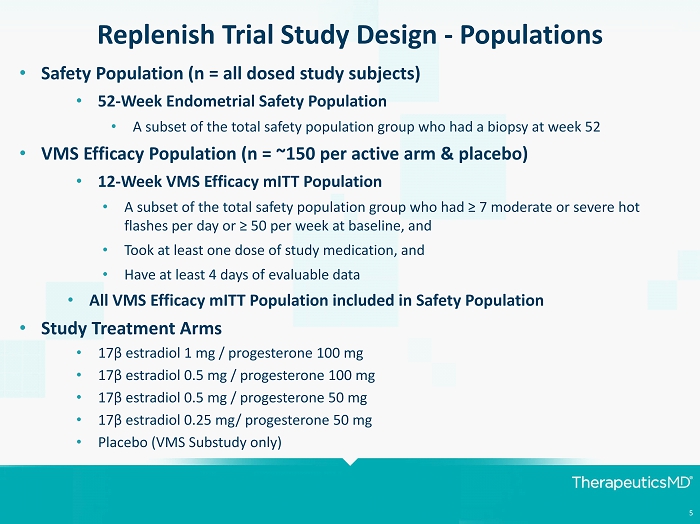
5 Replenish Trial Study Design - Populations • Safety Population (n = all dosed study subjects) • 52 - Week Endometrial Safety Population • A subset of the total safety population group who had a biopsy at week 52 • VMS Efficacy Population (n = ~150 per active arm & placebo) • 12 - Week VMS Efficacy mITT Population • A subset of the total safety population group who had ≥ 7 moderate or severe hot flashes per day or ≥ 50 per week at baseline, and • Took at least one dose of study medication, and • Have at least 4 days of evaluable data • All VMS Efficacy mITT Population included in Safety Population • Study Treatment Arms • 17 β estradiol 1 mg / progesterone 100 mg • 17 β estradiol 0.5 mg / progesterone 100 mg • 17 β estradiol 0.5 mg / progesterone 50 mg • 17 β estradiol 0.25 mg/ progesterone 50 mg • Placebo (VMS Substudy only)
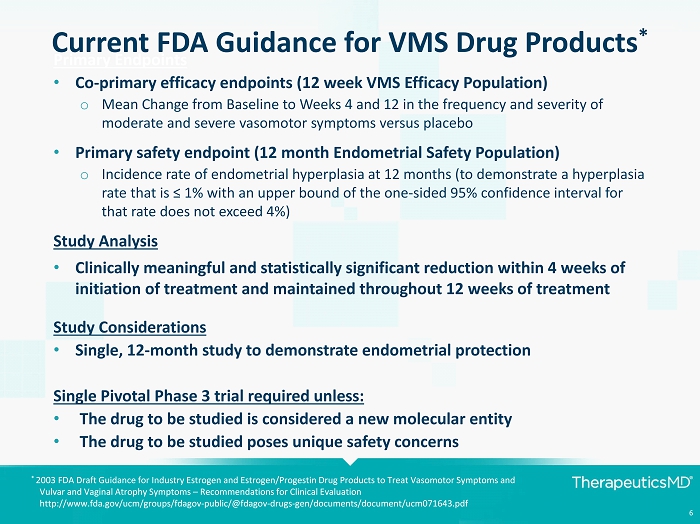
6 Current FDA Guidance for VMS Drug Products * Primary Endpoints • Co - primary efficacy endpoints (12 week VMS Efficacy Population) o Mean Change from Baseline to Weeks 4 and 12 in the frequency and severity of moderate and severe vasomotor symptoms versus placebo • Primary safety endpoint (12 month Endometrial Safety Population) o Incidence rate of endometrial hyperplasia at 12 months (to demonstrate a hyperplasia rate that is ≤ 1% with an upper bound of the one - sided 95% confidence interval for that rate does not exceed 4%) Study Analysis • Clinically meaningful and statistically significant reduction within 4 weeks of initiation of treatment and maintained throughout 12 weeks of treatment Study Considerations • Single, 12 - month study to demonstrate endometrial protection Single Pivotal Phase 3 trial required unless: • The drug to be studied is considered a new molecular entity • The drug to be studied poses unique safety concerns * 2003 FDA Draft Guidance for Industry Estrogen and Estrogen/Progestin Drug Products to Treat Vasomotor Symptoms and Vulvar and Vaginal Atrophy Symptoms – Recommendations for Clinical Evaluation http://www.fda.gov/ucm/groups/fdagov - public/@fdagov - drugs - gen/documents/document/ucm071643.pdf
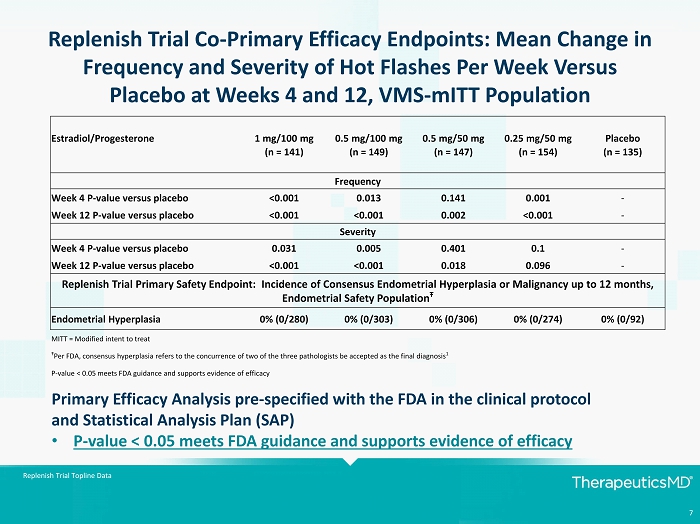
7 Replenish Trial Co - Primary Efficacy Endpoints: Mean Change in Frequency and Severity of Hot Flashes Per Week Versus Placebo at Weeks 4 and 12, VMS - mITT Population Estradiol/Progesterone 1 mg/100 mg 0.5 mg/100 mg 0.5 mg/50 mg 0.25 mg/50 mg Placebo (n = 141) (n = 149) (n = 147) (n = 154) (n = 135) Frequency Week 4 P - value versus placebo <0.001 0.013 0.141 0.001 - Week 12 P - value versus placebo <0.001 <0.001 0.002 <0.001 - Severity Week 4 P - value versus placebo 0.031 0.005 0.401 0.1 - Week 12 P - value versus placebo <0.001 <0.001 0.018 0.096 - Replenish Trial Primary Safety Endpoint: Incidence of Consensus Endometrial Hyperplasia or Malignancy up to 12 months, Endometrial Safety Population Ŧ Endometrial Hyperplasia 0% (0/280) 0% (0/303) 0% (0/306) 0% (0/274) 0% (0/92) MITT = Modified intent to treat Ŧ Per FDA, consensus hyperplasia refers to the concurrence of two of the three pathologists be accepted as the final diagnosis 1 P - value < 0.05 meets FDA guidance and supports evidence of efficacy Primary Efficacy Analysis pre - specified with the FDA in the clinical protocol and Statistical Analysis Plan (SAP) • P - value < 0.05 meets FDA guidance and supports evidence of efficacy Replenish Trial Topline Data
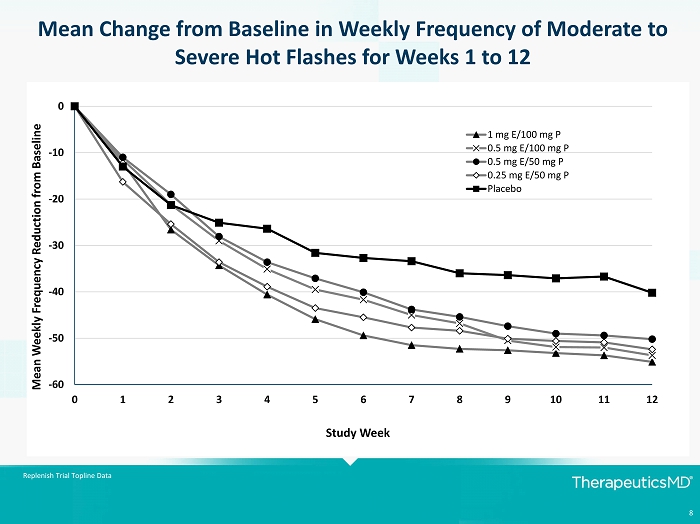
8 Replenish Trial Topline Data Mean Change from Baseline in Weekly Frequency of Moderate to Severe Hot Flashes for Weeks 1 to 12 -60 -50 -40 -30 -20 -10 0 0 1 2 3 4 5 6 7 8 9 10 11 12 Mean Weekly Frequency Reduction from Baseline Study Week 1 mg E/100 mg P 0.5 mg E/100 mg P 0.5 mg E/50 mg P 0.25 mg E/50 mg P Placebo
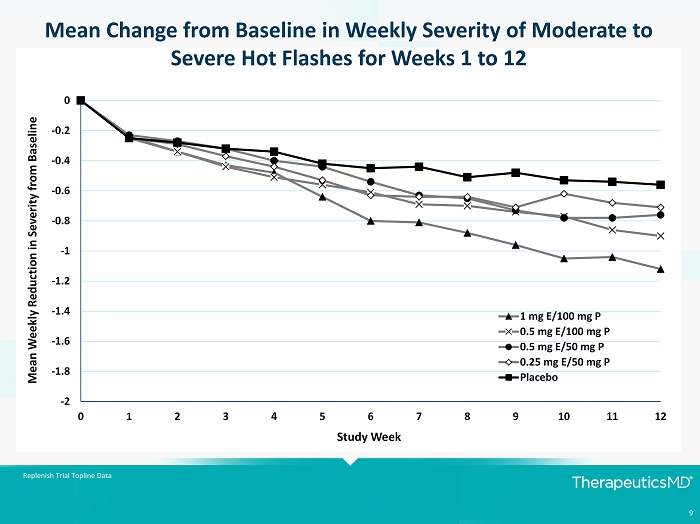
9 Replenish Trial Topline Data -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 Mean Weekly Reduction in Severity from Baseline Study Week 1 mg E/100 mg P 0.5 mg E/100 mg P 0.5 mg E/50 mg P 0.25 mg E/50 mg P Placebo Mean Change from Baseline in Weekly Severity of Moderate to Severe Hot Flashes for Weeks 1 to 12
