Attached files
| file | filename |
|---|---|
| 8-K/A - FORM 8-K/A - XBiotech Inc. | f8ka_120516.htm |
Exhibit 99.1

Corporate Presentation 2016 NASDAQ: XBIT Creating Breakthrough Therapies from Natural Human Immunity

1 This presentation contains forward - looking statements, including declarations regarding management's beliefs and expectations,that involve substantial risks and uncertainties . In some cases, you can identify forward - looking statements by terminology such as "may," "will," "should," "would," "could," "expects," "plans," "contemplate," "anticipates," "believes," "estimates," "predicts," "projects," "intend" or "continue" or the negative of such terms or other comparable terminology, although not all forward - looking statements contain these identifying words . Forward - looking statements are subject to inherent risks and uncertainties in predicting future results and conditions that could cause the actual results to differ materially from those projected in these forward - looking statements . These risks and uncertainties are subject to the disclosures set forth in the "Risk Factors" section of certain of our SEC filings . Forward - looking statements are not guarantees of future performance, and our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate, may differ materially from the forward - looking statements contained in this presentation . Any forward - looking statements that we make in this presentation speak only as of the date of this presentation . We assume no obligation to update our forward - looking statements whether as a result of new information, future events or otherwise, after the date of this presentation . Presentation Contains Forward Looking Statements

2 30,000 ft 2 R&D Laboratories, Manufacturing, Administrative 16 ,000 ft 2 Clinical Operations, Manufacturing Filling Suite, Vivarium 40,000 ft 2 Manufacturing, Quality Control Laboratory, Administrative XBiotech Facilities (New 48 acre Campus Location)

3 • Incorporated in 2005, IPO April 2015 (NASDAQ XBIT) • 107 Employees • 86,000 ft 2 Operations in Austin, Texas • Operations include R&D, Manufacturing, Sales & Marketing (planned) • Incorporated in United States, Switzerland, Germany, Japan, Canada • Over 100 Clinical Sites in Operation in Various Indications in 20 Countries Around the World • 180 Patents & Patents Pending Snapshot December 2016

4 Corporate Board Dr. Fabrizio Bonanni , Former Executive VP Operations Amgen, Developed World’s Largest Biologics Manufacturing Operation Thorpe McKenzie Founding Partner of Tiger Fund, One of the Most Iconic Investment Funds in the History of Wall Street. John Simard Founder CTL ImmunoTherapies , AlleCure , MannKind , XBiotech. Dr. Daniel Vasella Former Chairman & CEO Novartis. Founded Novartis in merger between Ciba - Geigy and Sandoz Laboratories, creating what became the largest revenue generating Pharma in the World.

5 Pending Marketing Authorization for First - In - Class Therapy for Colorectal Cancer Rich Pipeline. Three Fast - Tracked Development Paths: Oncology , Cardiovascular, Infectious Disease 100% Ownership of Assets , $ 0 Debt World’s Leading Disposable Manufacturing Program (GMP Certified Oct. 2016) XBiotech Today

6 Healthy Human Volunteers with Natural Immunity to Disease The Human Antibody Repertoire: A Remarkable Platform for Breakthrough Medicines

7 Program 1 Pre - Clinical Phase I Phase II Phase III Pending Marketing Authorization 2 Symptomatic Colorectal Cancer Advanced Colorectal Cancer Type II Diabetes Peripheral Vascular Disease Psoriasis Acne Pyoderma Gangrenosum Hidradentitis Suppurativa S. aureus Bacteremia C. difficile Influenza Herpes Varicella Zoster (Chickenpox) Ebola 1 All Grey Shaded Areas Involve MABp1 Targeting Interleukin - 1 a . Green - shaded programs have individual antibodies specific for each infectious agent. Fast Tracked MAA Decision Pending x x x x x x x x Fast Tracked Fast Tracked x x MAA Submission 2017 = pilot study x x x x

8 Anti - Inflammatory Programs • Type II Diabetes • Peripheral Vascular Disease • Pyoderma Gangrenosum • Psoriasis • Hidradenitis Supperativa • Acne

9 Peer - Reviewed Publications on Clinical Findings • MABp1, a first - in - class true human antibody targeting interleukin - 1α in refractory cancers: an open - label, phase 1 dose - escalation and expansion study • Xilonix , a novel true human antibody targeting the inflammatory cytokine interleukin - 1 alpha, in non - small cell lung cancer • Safety, pharmacokinetics, and preliminary efficacy of a specific anti - IL - 1alpha therapeutic antibody (MABp1) in patients with type 2 diabetes mellitus

10 • An open label, phase 2 study of MABp1 monotherapy for the treatment of acne vulgaris and psychiatric comorbidity • A randomized phase II study of Xilonix , a targeted therapy against interleukin 1α, for the prevention of superficial femoral artery restenosis after percutaneous revascularization • Open - label trial of MABp1, a true human monoclonal antibody targeting interleukin 1α, for the treatment of psoriasis Clinical Publications (Continued)

11 Oncology • Xilonix Monotherapy in Colorectal Cancer: Two Phase III Programs • Development of Novel Endpoint Radiological/Quality of Life Measure : A Direct Measure of Clinical Benefit and a S urrogate Measure of Anti - Cancer Activity • Xilonix Suitable for Combination with Multiple Therapies • Pluripotent Anti - Tumor Mechanism Interrupts Tumor Growth and Augments Recovery
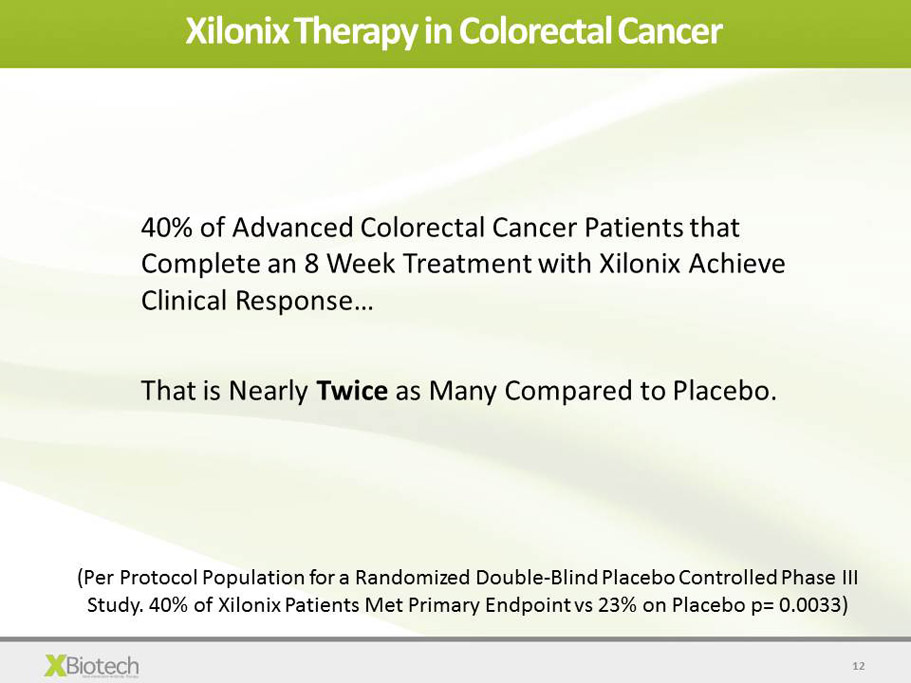
12 Xilonix Therapy in Colorectal Cancer 40% of Advanced Colorectal Cancer Patients that Complete an 8 Week Treatment with Xilonix Achieve Clinical Response … That is Nearly Twice as Many Compared to Placebo. ( Per Protocol Population for a Randomized Double - Blind Placebo Controlled Phase III Study. 40% of Xilonix Patients Met Primary Endpoint vs 23% on Placebo p= 0.0033)

13 Achieving the Clinical Response Means: • Twice as Likely to Have Stable Disease 1 • Five T imes Less Likely to Have Serious Adverse Events 2 1 24.1% of Patients Achieving Primary Endpoint had stable disease at 8 weeks vs 11.7% for those that did not (p=0.0062) 2 5.7% of Patients Achieving Primary Endpoint had SAEs vs 29.3% that Failed To Achieve (p < 0.001)
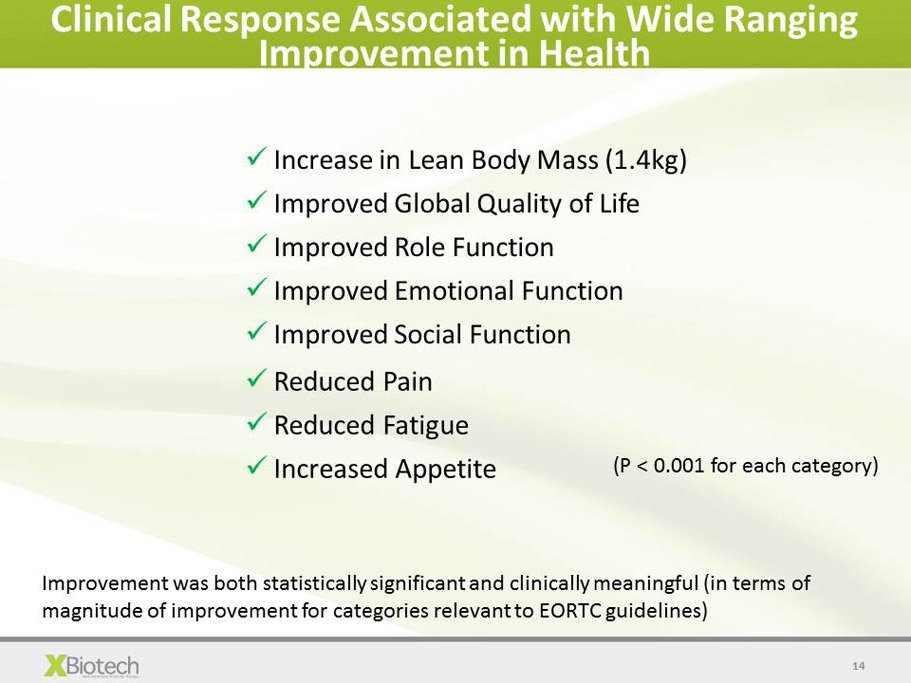
14 Clinical Response Associated with Wide Ranging Improvement in Health x Increase in Lean Body Mass (1.4kg) x Improved Global Quality of Life x Improved Role Function x Improved Emotional Function x Improved Social Function x Reduced Pain x Reduced Fatigue x Increased Appetite ( P < 0.001 for each category) Improvement was both statistically significant and clinically meaningful (in terms of magnitude of improvement for categories relevant to EORTC guidelines)

15 Patients Achieving Clinical Response After 8 Weeks Were Nearly Twice as Likely to Live Longer 1 After 8 weeks on Study 40% of Xilonix Patients Met Primary Endpoint vs 23% on Placebo (p< 0.0033) n =139 P=<0.0001 Patients Achieving Primary Endpoint (Overall Survival 11.7 months) All Other Patients (Overall Survival 5.7 Months)

16 Summary of Patient Benefit During an 8 Week Treatment Regimen with Xilonix • Nearly Twice as Likely to Have Increased Survival • Twice as Likely to Have Stable Disease • Improvement in All Life Quality Measures, Including Physical, Functional and Emotional Health • Five Times Less Likely to have a Serious Adverse Event • Reduced Incidence of Hospitalization or Prolongation of Existing Hospitalization • Reduced Incidence of Persistent or Significant Disability/Incapacity from Disease

17 EU Marketing Authorization • Europe Represents by Far the Largest Unmet Need for Colorectal Cancer Therapy • Discussion with European Medicines Agency for Marketing Approval is Ongoing and Decision Not Expected Until 2017 • XBiotech Plans to Sell and Market Xilonix in Europe Subject to Regulatory Approval • European Market Authorization will Be Used to Seek Registration in Multiple Regions and Countries — Through Partnership — Around the World.

18 Ongoing Global Phase III Study Under US FDA Fast - Track Program • Double - Blind Placebo Controlled Randomized Study Involving 600+ Patients • Enrollment Completed On Schedule in November 2016 and Results of First Interim Analysis Expected in 1 st Quarter 2017 • First Interim Analysis Enables Independent Data Monitoring Committee to Unblind Study to Assess Both Safety and Efficacy • Interim Analysis Incorporates Futility Boundary • Overall Survival is Primary Endpoint. Subjects In Study Progressed (or intolerant) After: Oxaliplatin , Irinotecan , Flouropyrimidine , and Cetuximab or Panitumumab if KRAS wildtype

19 Anti - Infective Programs • Staphylococcus aureus • Clostridium difficile • Influenza Virus • Ebola • Herpes Varicella Zoster Virus (Chickenpox)

20 CDC: Staph classified as “Serious Threat” • Staph Kills an Estimated 20,000 Patients in the US Every Year 1 • Antibiotic Resistance is Not Being Solved with Small Molecules • No Effective Vaccines and Growing Consensus that Such an Approach is Difficult, if Not Impossible • Repeated Failures Due to an Immune Evasion Mechanism 1 http :// mrsa - research - center.bsd.uchicago.edu /

21 FDA Fast Track 514G3 in Phase II Study • A Single Dose of Antibody to Mediate Therapy • Neutralize Immune Evasion Mechanism and Facilitate Immune Clearance • Targets All Forms of S. aureus — Including MRSA, MSSA • Double - Blind, Placebo Controlled Randomized Study in 52 Patients • Enrollment Completed in December 2016 • Topline Data Readout 1 st Quarter 2017

Staphylococcus aureus Fc Binding Protein (Protein A) Protein A Binds Antibodies by Fc Serum Antibody Fc Portion of Antibody Cartoon Depiction of S. aureus with Antibody Bound by Fc Region

Serum Antibodies Bind ProA Forming Protective Coat on S. aureus • Evade Detection • Neutralize Antibodies • Prevent Immune Control Protect Coat Helps S. aureus

True Human Antibody (514G3) Binds ProA 514G3 • Fc portion of 514G3 does NOT Bind ProA • 514G3 Binds with Correct Geometry to Block ProA and Mediate Clearance

• 500,000 People in USA Contract Disease Annually • 1/9 Persons Over 65 Years of Age Die Within 30 days of Contracting Disease • 29,000 Deaths Annually in the US Clostridium difficile • Currently No Means of Preventing Disease http:// www.cdc.gov /media/releases/2015/p0225 - clostridium - difficile.html

26 Best Way to Treat is to Prevent Pathological Specimen Showing Pseudomembranous Colitis Image: https :// en.wikipedia.org /wiki/ Clostridium_difficile_infection

27 Developing a Novel Therapy to Prevent Deadly C . difficile infection • FIRST Oral Delivered Monoclonal Antibody Therapy • Targeting Clinical Launch for Year End - 2017 • Prophylaxis Therapy — Only Approach Designed to Eradicate Bacteria Rather than Target only C. difficile toxins • Safety and Tolerability Ideal for Patient Population

28 Strategic Manufacturing — Industry Leading Disposable Platform Artist’s Conception Unprecedented Reduction in Cost and Flexibility

29 Near Term Corporate Goals □ Seek Registration for Xilonix in Europe and other markets for CRC □ Establish Sales and Marketing Capability in Key Markets of Europe □ Continue Clinical Development in Colorectal and Other Oncology Indications, Including Combination Therapies □ Develop Pivotal Study for Fast - tracked Anti - Infective Therapy for S. aureus □ Advance Other MABp1 Clinical Programs in Strategic Therapeutic Areas

30 Thank You
