Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Blueprint Medicines Corp | bpmc_ex991.htm |
| 8-K - 8-K - Blueprint Medicines Corp | bpmc_8ksmpressreleaseandmee.htm |
Exhibit 99.2
|
|
Preliminary Safety and Activity in a Phase 1 study of BLU-285, a Potent, Highly-Selective Inhibitor of KIT D816V in Advanced Systemic Mastocytosis (SM) Mark Drummond1, Daniel DeAngelo2, Michael Deininger3, Deepti Radia4, Albert Quiery5, Elizabeth Hexner6, Hongliang Shi7, Terri Alvarez-Diez7, Erica Evans7, Mary Ellen Healy7, Beni Wolf7, Srdan Verstovsek8 1Beatson West of Scotland Cancer Centre, NHS Greater Glasgow and Clyde, Glasgow, United Kingdom; 2Dana-Farber Cancer Institute, Boston, MA; 3Division of Hematology and Hematologic Malignancies, Huntsman Cancer Institute, The University of Utah, Salt Lake City, UT; 4Guy's & St Thomas NHS Trust, London, United Kingdom; 5University of Michigan, Ann Arbor, MI; 6Abramson Cancer Center of the University of Pennsylvania, Philadelphia, PA; 7Blueprint Medicines, Cambridge, MA; 8Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX American Society of Hematology Annual Meeting San Diego, California, USA, 04 Dec 2016 |
|
|
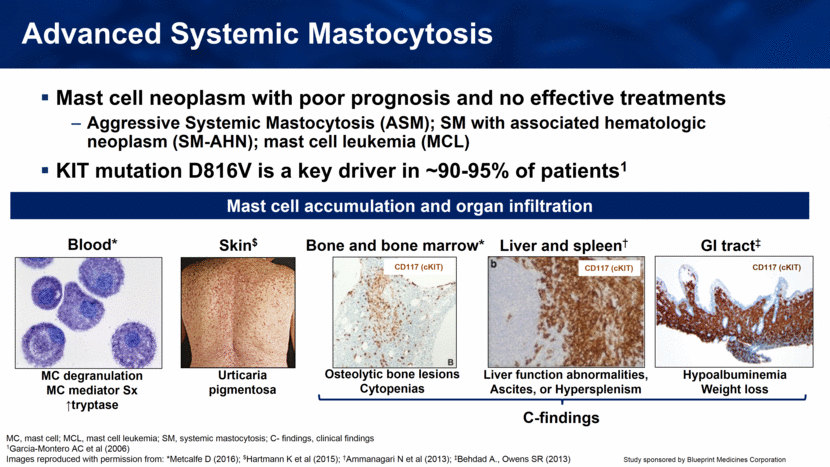
Skin$ Urticaria pigmentosa Mast cell neoplasm with poor prognosis and no effective treatments Aggressive Systemic Mastocytosis (ASM); SM with associated hematologic neoplasm (SM-AHN); mast cell leukemia (MCL) KIT mutation D816V is a key driver in ~90-95% of patients1 Advanced Systemic Mastocytosis Mast cell accumulation and organ infiltration Liver and spleen† Liver function abnormalities, Ascites, or Hypersplenism CD117 (cKIT) Bone and bone marrow* Osteolytic bone lesions Cytopenias CD117 (cKIT) Blood* MC degranulation MC mediator Sx ↑tryptase GI tract‡ Hypoalbuminemia Weight loss CD117 (cKIT) C-findings MC, mast cell; MCL, mast cell leukemia; SM, systemic mastocytosis; C- findings, clinical findings 1Garcia-Montero AC et al (2006) Images reproduced with permission from: *Metcalfe D (2016); $Hartmann K et al (2015); †Ammanagari N et al (2013); ‡Behdad A., Owens SR (2013) |
|
|
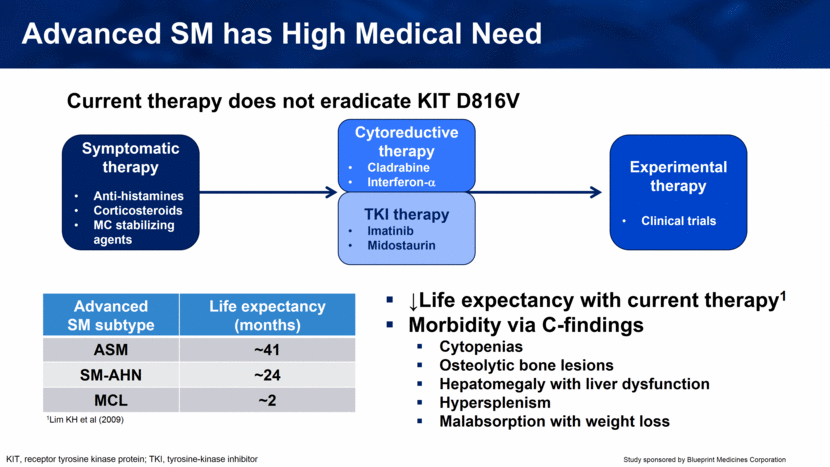
KIT, receptor tyrosine kinase protein; TKI, tyrosine-kinase inhibitor Advanced SM has High Medical Need Symptomatic therapy Anti-histamines Corticosteroids MC stabilizing agents Cytoreductive therapy Cladrabine Interferon- Experimental therapy Clinical trials Current therapy does not eradicate KIT D816V ↓Life expectancy with current therapy1 Morbidity via C-findings Cytopenias Osteolytic bone lesions Hepatomegaly with liver dysfunction Hypersplenism Malabsorption with weight loss Advanced SM subtype Life expectancy (months) ASM ~41 SM-AHN ~24 MCL ~2 TKI therapy Imatinib Midostaurin 1Lim KH et al (2009) |
|
|
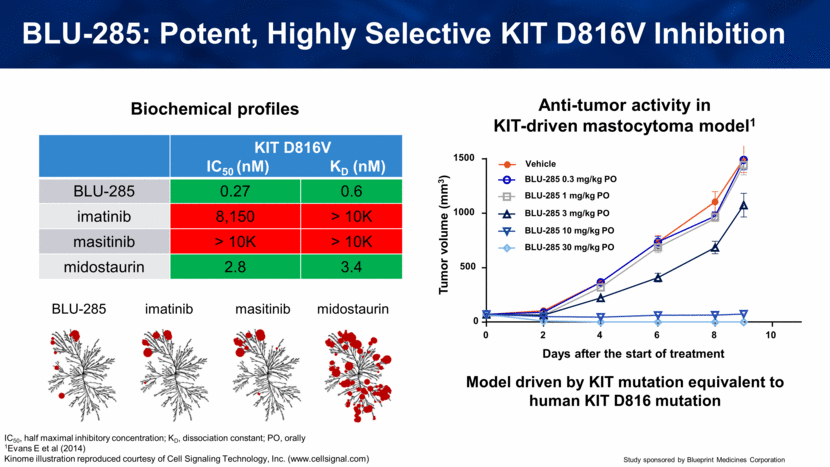
Biochemical profiles Model driven by KIT mutation equivalent to human KIT D816 mutation IC50, half maximal inhibitory concentration; KD, dissociation constant; PO, orally 1Evans E et al (2014) Kinome illustration reproduced courtesy of Cell Signaling Technology, Inc. (www.cellsignal.com) Anti-tumor activity in KIT-driven mastocytoma model1 BLU-285: Potent, Highly Selective KIT D816V Inhibition KIT D816V IC50 (nM) KD (nM) BLU-285 0.27 0.6 imatinib 8,150 > 10K masitinib > 10K > 10K midostaurin 2.8 3.4 0 2 4 6 8 10 0 500 1000 1500 Days after the start of treatment Vehicle BLU-285 0.3 mg/kg PO BLU-285 1 mg/kg PO BLU-285 3 mg/kg PO BLU-285 10 mg/kg PO BLU-285 30 mg/kg PO BLU-285 imatinib masitinib midostaurin Tumor volume (mm3) |
|
|
Any of the following diagnoses: Aggressive Systemic Mastocytosis (ASM)1 SM with associated hematologic disorder (SM-AHN)1 with ≥ 1 C-finding Mast Cell Leukemia (MCL)1 Relapsed or refractory myeloid malignancy (dose escalation only)2 Age ≥ 18 ECOG performance status 0–3 Platelet count ≥ 25 x 109 /L ANC ≥ 0.5 x 109 /L Adequate hepatic and renal function Key Entry Criteria ANC, absolute neutrophil count; ECOG, Eastern Cooperative Oncology Group 1ASM, SM-AHNMD, or MCL per WHO criteria via local diagnosis and retrospective central pathology to confirm mastocytosis subtype. 2Per IWG-MRT or WHO diagnostic criteria |
|
|
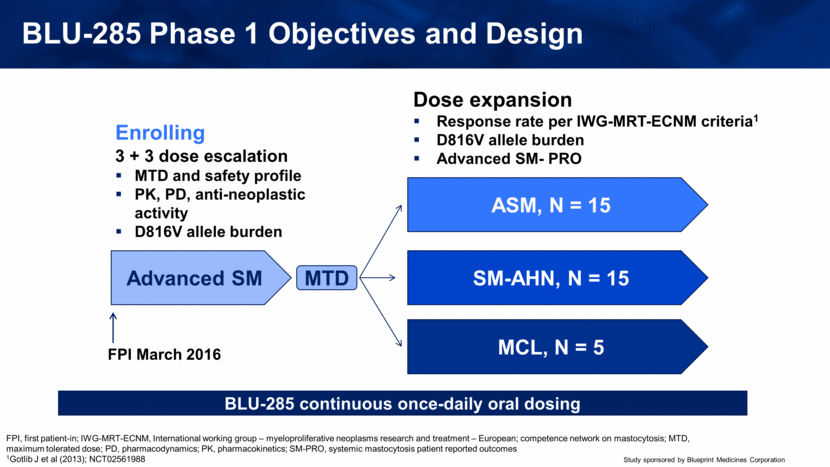
BLU-285 Phase 1 Objectives and Design Advanced SM MCL, N = 5 ASM, N = 15 FPI March 2016 SM-AHN, N = 15 MTD Dose expansion Response rate per IWG-MRT-ECNM criteria1 D816V allele burden Advanced SM- PRO BLU-285 continuous once-daily oral dosing 3 + 3 dose escalation MTD and safety profile PK, PD, anti-neoplastic activity D816V allele burden Enrolling FPI, first patient-in; IWG-MRT-ECNM, International working group – myeloproliferative neoplasms research and treatment – European; competence network on mastocytosis; MTD, maximum tolerated dose; PD, pharmacodynamics; PK, pharmacokinetics; SM-PRO, systemic mastocytosis patient reported outcomes 1Gotlib J et al (2013); NCT02561988 |
|
|
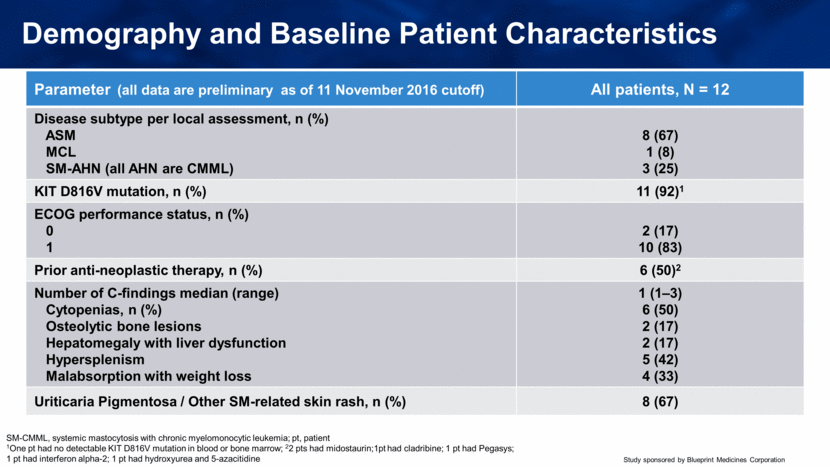
Demography and Baseline Patient Characteristics Parameter (all data are preliminary as of 11 November 2016 cutoff) All patients, N = 12 Disease subtype per local assessment, n (%) ASM MCL SM-AHN (all AHN are CMML) 8 (67) 1 (8) 3 (25) KIT D816V mutation, n (%) 11 (92)1 ECOG performance status, n (%) 0 1 2 (17) 10 (83) Prior anti-neoplastic therapy, n (%) 6 (50)2 Number of C-findings median (range) Cytopenias, n (%) Osteolytic bone lesions Hepatomegaly with liver dysfunction Hypersplenism Malabsorption with weight loss 1 (1–3) 6 (50) 2 (17) 2 (17) 5 (42) 4 (33) Uriticaria Pigmentosa / Other SM-related skin rash, n (%) 8 (67) SM-CMML, systemic mastocytosis with chronic myelomonocytic leukemia; pt, patient 1One pt had no detectable KIT D816V mutation in blood or bone marrow; 22 pts had midostaurin;1pt had cladribine; 1 pt had Pegasys; 1 pt had interferon alpha-2; 1 pt had hydroxyurea and 5-azacitidine |
|
|
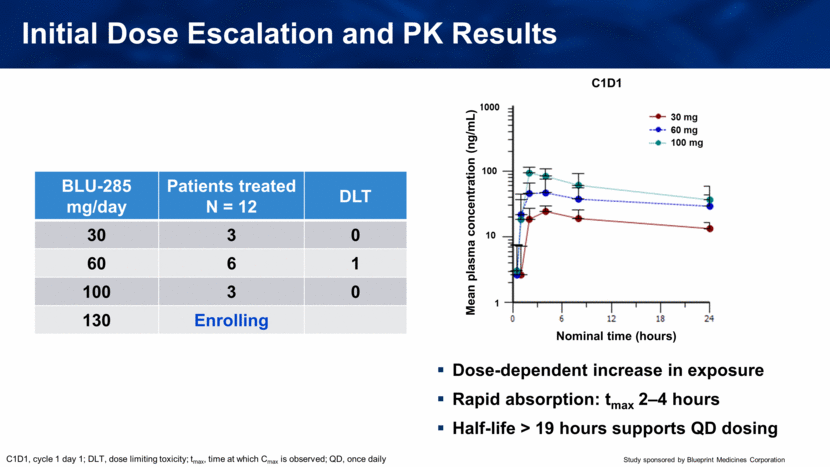
Initial Dose Escalation and PK Results Dose-dependent increase in exposure Rapid absorption: tmax 2–4 hours Half-life > 19 hours supports QD dosing BLU-285 mg/day Patients treated N = 12 DLT 30 3 0 60 6 1 100 3 0 130 Enrolling C1D1, cycle 1 day 1; DLT, dose limiting toxicity; tmax, time at which Cmax is observed; QD, once daily Mean plasma concentration (ng/mL) Nominal time (hours) |
|
|
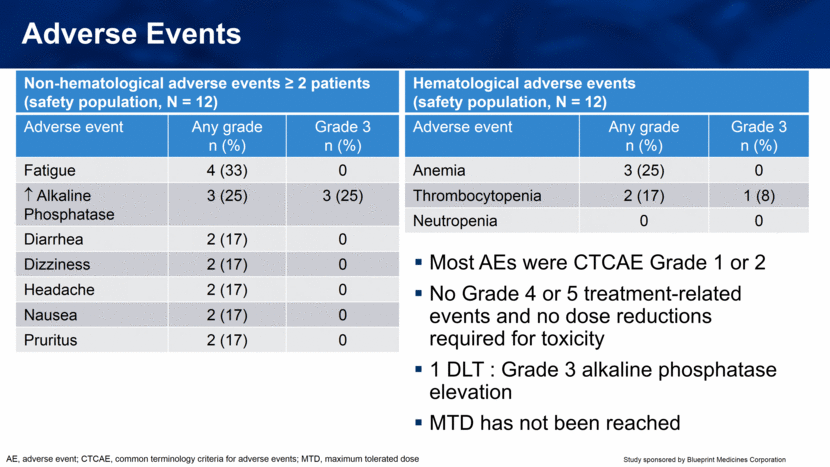
Adverse Events Most AEs were CTCAE Grade 1 or 2 No Grade 4 or 5 treatment-related events and no dose reductions required for toxicity 1 DLT : Grade 3 alkaline phosphatase elevation MTD has not been reached Non-hematological adverse events ≥ 2 patients (safety population, N = 12) Adverse event Any grade n (%) Grade 3 n (%) Fatigue 4 (33) 0 Alkaline Phosphatase 3 (25) 3 (25) Diarrhea 2 (17) 0 Dizziness 2 (17) 0 Headache 2 (17) 0 Nausea 2 (17) 0 Pruritus 2 (17) 0 Hematological adverse events (safety population, N = 12) Adverse event Any grade n (%) Grade 3 n (%) Anemia 3 (25) 0 Thrombocytopenia 2 (17) 1 (8) Neutropenia 0 0 AE, adverse event; CTCAE, common terminology criteria for adverse events; MTD, maximum tolerated dose |
|
|
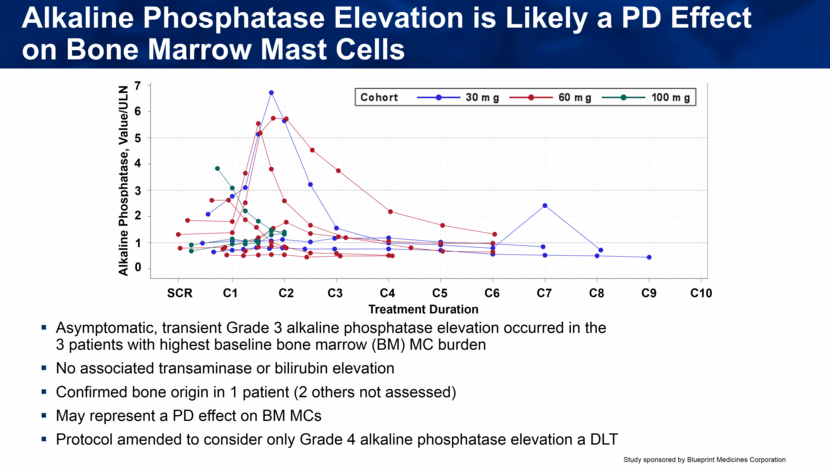
Asymptomatic, transient Grade 3 alkaline phosphatase elevation occurred in the 3 patients with highest baseline bone marrow (BM) MC burden No associated transaminase or bilirubin elevation Confirmed bone origin in 1 patient (2 others not assessed) May represent a PD effect on BM MCs Protocol amended to consider only Grade 4 alkaline phosphatase elevation a DLT Alkaline Phosphatase Elevation is Likely a PD Effect on Bone Marrow Mast Cells Treatment Duration SCR C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 Alkaline Phosphatase, Value/ULN 0 1 2 3 4 5 6 7 SCR C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 Treatment Duration 0 1 2 3 4 5 6 7 A l k a l i n e P h o s p h a t a s e , V a l u e / U L N 100 mg 60 mg 30 mg Cohort R:\Clinical\Clinical Data\BLU285\Study2101\Stat\LFTProfilebyCohort.sas 04NOV2016 12:13 |
|
|
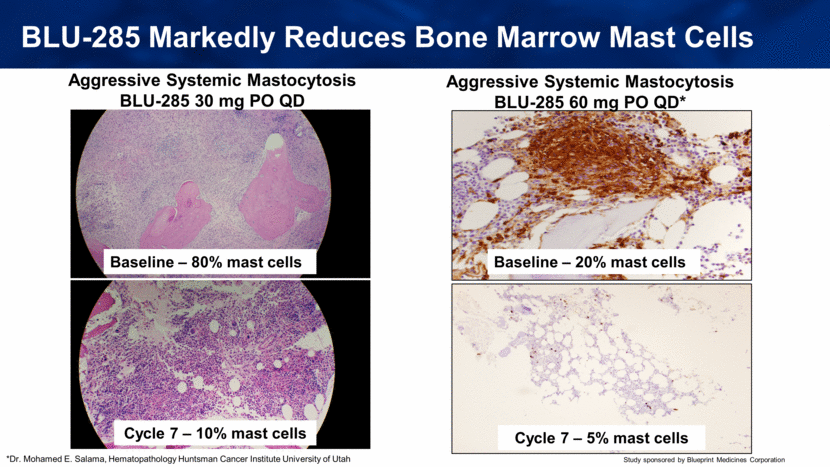
BLU-285 Markedly Reduces Bone Marrow Mast Cells Baseline – 80% mast cells Cycle 7 – 10% mast cells Aggressive Systemic Mastocytosis BLU-285 30 mg PO QD Baseline – 20% mast cells Cycle 7 – 5% mast cells Aggressive Systemic Mastocytosis BLU-285 60 mg PO QD* *Dr. Mohamed E. Salama, Hematopathology Huntsman Cancer Institute University of Utah |
|
|
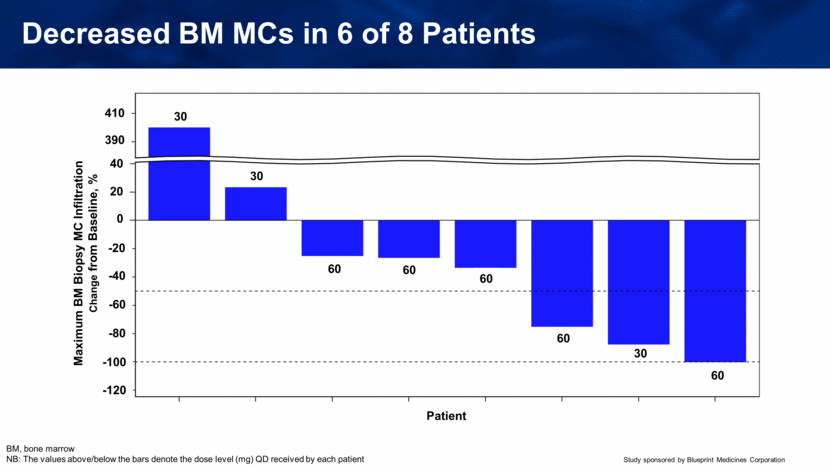
Decreased BM MCs in 6 of 8 Patients BM, bone marrow NB: The values above/below the bars denote the dose level (mg) QD received by each patient Patient Maximum BM Biopsy MC Infiltration Change from Baseline, % 410 390 40 20 0 -20 -40 -60 -80 -100 -120 30 30 60 60 60 60 60 30 |
|
|
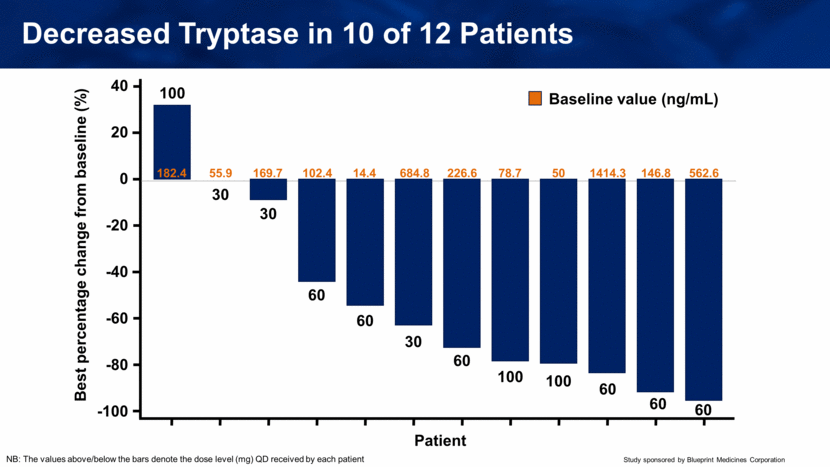
Decreased Tryptase in 10 of 12 Patients NB: The values above/below the bars denote the dose level (mg) QD received by each patient Best percentage change from baseline (%) Patient 40 20 -40 -20 -60 -80 -100 0 100 30 30 60 60 30 60 100 100 60 60 60 Baseline value (ng/mL) 182.4 55.9 169.7 102.4 14.4 684.8 226.6 78.7 50 1414.3 146.8 562.6 |
|
|
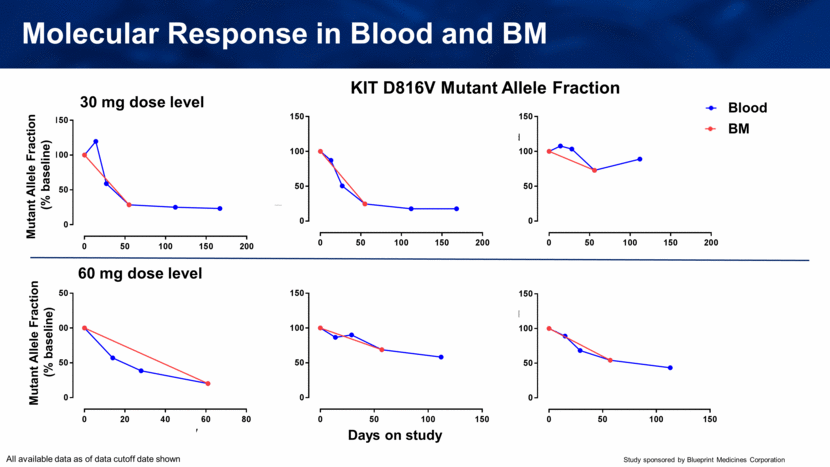
Molecular Response in Blood and BM KIT D816V Mutant Allele Fraction 30 mg dose level 60 mg dose level Blood BM Mutant Allele Fraction (% baseline) Days on study Mutant Allele Fraction (% baseline) All available data as of data cutoff date shown 0 50 100 150 200 0 50 100 150 1-1102-001 SM-AHNMD (CMML) Days on study M A F [ % b a s e l i n e ] Blood BMA 0 50 100 150 200 0 50 100 150 1-2002-001 SM-AHNMD (MDS) Days on study M A F [ % b a s e l i n e ] Blood BMA 0 50 100 150 200 0 50 100 150 1-2004-001 SM-AHNMD (CMML) Days on study M A F [ % b a s e l i n e ] Blood BMA 0 20 40 60 80 0 50 100 150 2-1101-001 ASM Days on study M A F [ % b a s e l i n e ] Blood BMA 0 50 100 150 0 50 100 150 2-2003-001 ASM Days on study M A F [ % b a s e l i n e ] Blood BMA 0 50 100 150 0 50 100 150 2-2004-002 ASM Days on study M A F [ % b a s e l i n e ] Blood BMA |
|
|
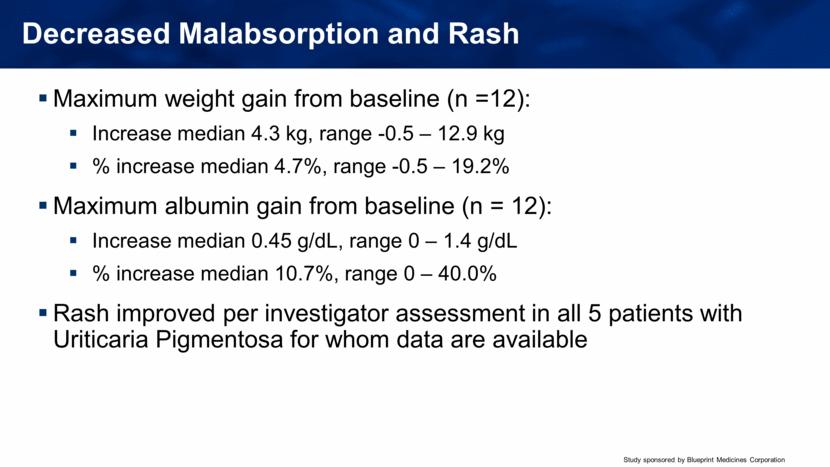
Decreased Malabsorption and Rash Maximum weight gain from baseline (n =12): Increase median 4.3 kg, range -0.5 – 12.9 kg % increase median 4.7%, range -0.5 – 19.2% Maximum albumin gain from baseline (n = 12): Increase median 0.45 g/dL, range 0 – 1.4 g/dL % increase median 10.7%, range 0 – 40.0% Rash improved per investigator assessment in all 5 patients with Uriticaria Pigmentosa for whom data are available |
|
|
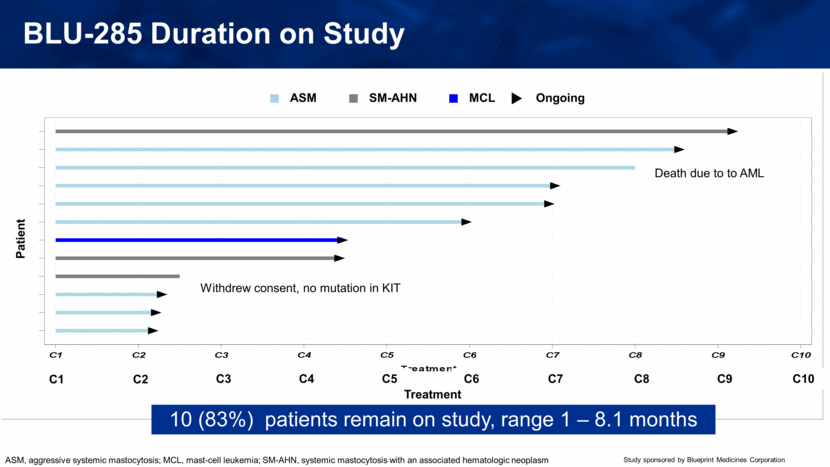
BLU-285 Duration on Study 10 (83%) patients remain on study, range 1 – 8.1 months C1 Patient Treatment C2 C3 C4 C5 C6 C7 C8 C9 C10 ASM SM-AHN MCL Ongoing Withdrew consent, no mutation in KIT Death due to to AML ASM, aggressive systemic mastocytosis; MCL, mast-cell leukemia; SM-AHN, systemic mastocytosis with an associated hematologic neoplasm C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 Treatment P a t i e n t Ongoing MCL SM-CMML ASM |
|
|
BLU-285 has demonstrated encouraging clinical activity in advanced SM with marked decreases in mast cell burden and improved patient symptoms Data support the hypothesis that KIT D816V is a key disease driver in SM Half-life > 19 hours supports QD dosing BLU-285 has been well tolerated over a dose range of 30 to 100 mg - dose escalation (currently at 130 mg QD) BLU-285 deserves continued investigation in advanced SM, and further investigation in other KIT-driven diseases; Phase 1 study of BLU-285 in GIST is ongoing Summary GIST, gastrointestinal stromal tumor |
|
|
This study was sponsored by Blueprint Medicines We thank the participating patients, their families, all study co-investigators, and research coordinators at the following institutions: Guy's & St Thomas NHS Trust Gartnavel General Hospital, Beatson West of Scotland Cancer Center Abramson Cancer Center at the University of Pennsylvania University of Michigan Comprehensive Cancer Center Dana-Farber Cancer Institute University of Utah, Huntsman Cancer Institute MD Anderson Cancer Center University of Colorado Stanford University Acknowledgments |
|
|
Slide 3 images: Blood, Bone and Bone Marrow Republished with permission of American Society of Hematology, from Mast Cells and mastocytosis, Dean D Metcalfe, volume 112, number 4, 2008 Skin Reprinted from Journal of Allergy and Clinical Immunology, Volume 137, Issue 1, Hartmann et al., Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma, and Immunology; and the European Academy of Allergology and Clinical Immunology, pages 35–45, 2016, with permission from Elsevier Liver and spleen Annals of Hematology, Isolated splenomegaly as the only presentation of systemic mastocytosis, 92, 2013, pg. 1574 Figure 1, Nischala Ammannagari, Sara Grethlein, James J. Longhi, and John M. Fisk, Copyright Springer-Verlag Berlin Heidelberg 2013, With permission from Springer GI tract Reprinted from Behdad A, Owens SR. Systemic Mastocytosis Involving the Gastrointestinal Tract: Case Report and Review. Arch Pathol Lab Med. 2013; 137(9):1220-1223 with permission from Archives of Pathology & Laboratory Medicine. Copyright 2013. College of American Pathologists Permissions |