Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Avalo Therapeutics, Inc. | cerc_exh9912.htm |
| 8-K - 8-K - Avalo Therapeutics, Inc. | cerc_currentfolio8-k2.htm |
Exhibit 99.2
|
|
Clin301-203 Phase 2 Topline MDD Data Conference Call November 29, 2016 NASDAQ:CERC www.cerecor.com |
|
|
Welcome Mariam E. Morris, CPA CFO Introduction Uli Hacksell, Ph.D. CEO, President and Chairman CERC-301 Phase 2 Trial Design and Results Ronald Marcus, M.D. CMO and Head, Regulatory Affairs Next Steps/Close Uli Hacksell, Ph.D. CEO, President and Chairman Q&A Cerecor Management Agenda 2 |
|
|
Forward-looking Statements This presentation contains forward-looking statements, including statements about our plans to develop and potentially commercialize our product candidates, our ongoing and planned clinical trials and preclinical studies for our product candidates, the timing of the availability of data from our clinical trials, the timing of and our ability to obtain and maintain regulatory approvals for our product candidates, the potential clinical utility and benefits of our product candidates, our intellectual property position and potential acquisitions, in-licenses or collaborations. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. These and other risks concerning our business are described in additional detail in our Quarterly Report on Form 10-Q filed with the SEC on November 8, 2016 and our other Periodic and Current Reports filed with the SEC. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Further, the information contained in this presentation speaks only as the date hereof. While we may elect to update the information in this presentation in the future, we disclaim any obligation to do so except to the extent required by applicable law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. 3 |
|
|
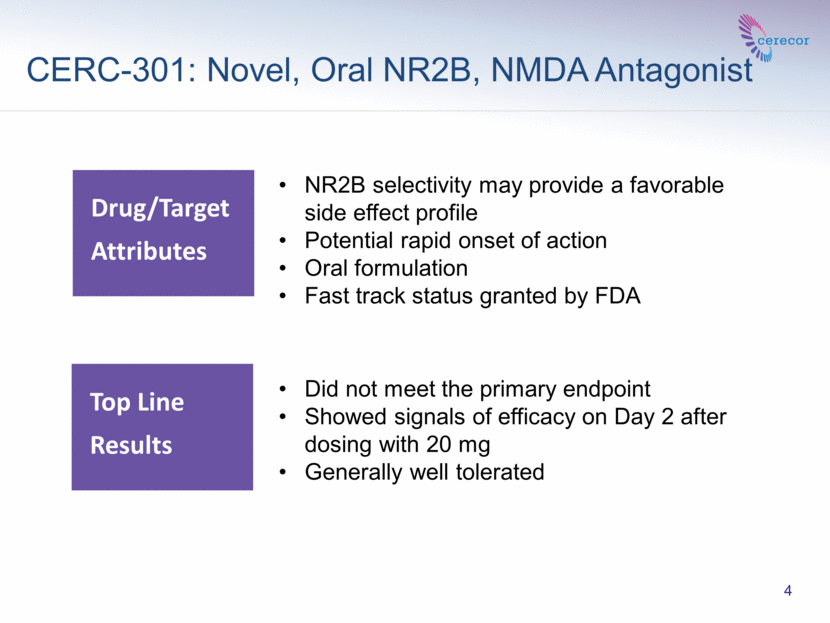
CERC-301: Novel, Oral NR2B, NMDA Antagonist Drug/Target Attributes NR2B selectivity may provide a favorable side effect profile Potential rapid onset of action Oral formulation Fast track status granted by FDA 4 Did not meet the primary endpoint Showed signals of efficacy on Day 2 after dosing with 20 mg Generally well tolerated Top Line Results |
|
|
Phase 2 Study Overview Clin301-203 3-week randomized, double-blind, placebo-controlled trial Sequential parallel comparison design (SPCD) with 2 periods of 7 days followed by a 7 day follow-up Intermittent doses of adjunctive CERC-301 (12 mg or 20 mg) or placebo Subjects with MDD currently experiencing a severe depressive episode despite current stable treatment with an SSRI or SNRI* Subjects were males or females 18-65 years of age with a lifetime history of >2 major depressive episodes and a current episode of <2.5 years 5 * Selective serotonin reuptake inhibitor (SSRI) or serotonin norepinephrine uptake inhibitor (SNRI) |
|
|
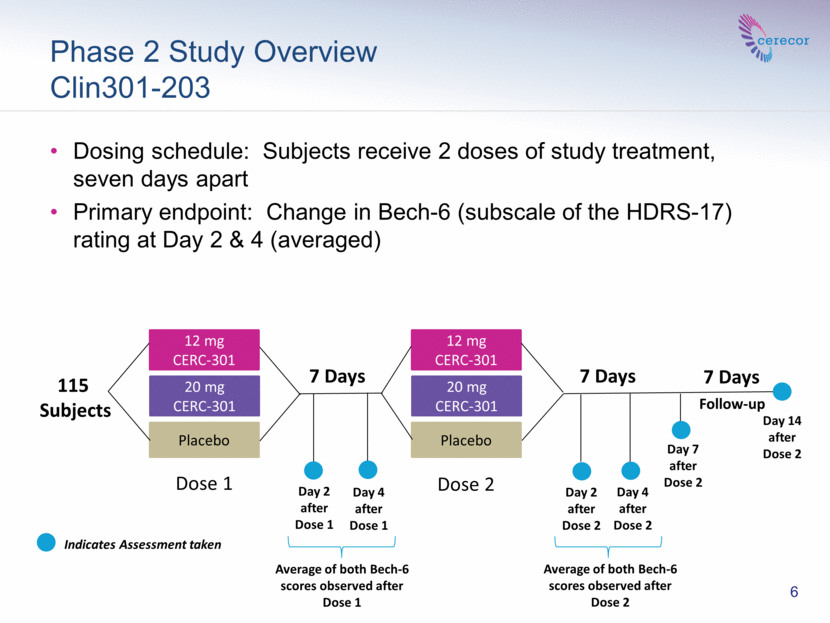
Phase 2 Study Overview Clin301-203 Dosing schedule: Subjects receive 2 doses of study treatment, seven days apart Primary endpoint: Change in Bech-6 (subscale of the HDRS-17) rating at Day 2 & 4 (averaged) 7 Days 7 Days Day 2 after Dose 1 Day 4 after Dose 1 Follow-up 12 mg CERC-301 20 mg CERC-301 Placebo 115 Subjects 12 mg CERC-301 20 mg CERC-301 Placebo Day 2 after Dose 2 Day 4 after Dose 2 Day 7 after Dose 2 7 Days Dose 1 Dose 2 6 Indicates Assessment taken Average of both Bech-6 scores observed after Dose 1 Average of both Bech-6 scores observed after Dose 2 Day 14 after Dose 2 |
|
|
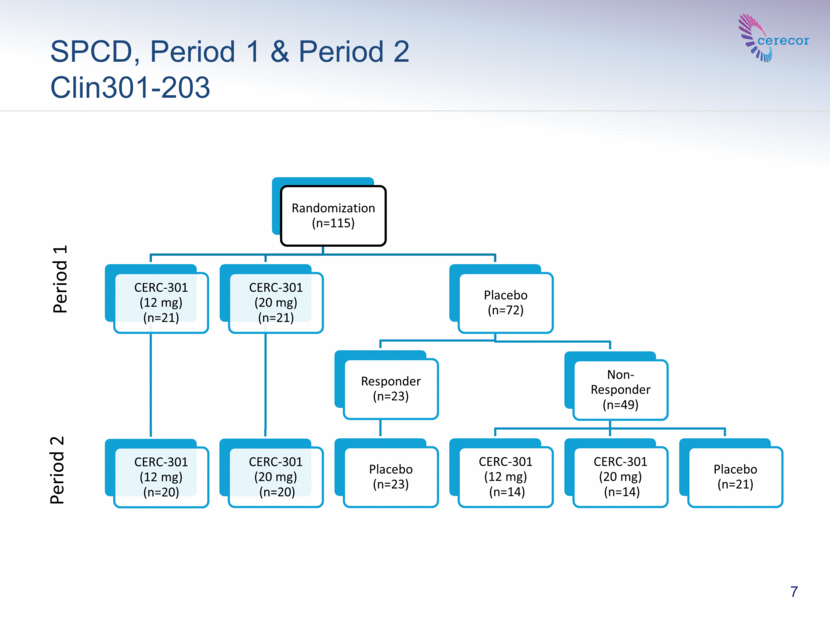
SPCD, Period 1 & Period 2 Clin301-203 7 Period 1 Period 2 Randomization (n=115) CERC-301 (12 mg) (n=21) CERC-301 (12 mg) (n=20) CERC-301 (20 mg) (n=21) CERC-301 (20 mg) (n=20) Placebo (n=72) Responder (n=23) Placebo (n=23) Non-Responder (n=49) CERC-301 (12 mg) (n=14) CERC-301 (20 mg) (n=14) Placebo (n=21) |
|
|
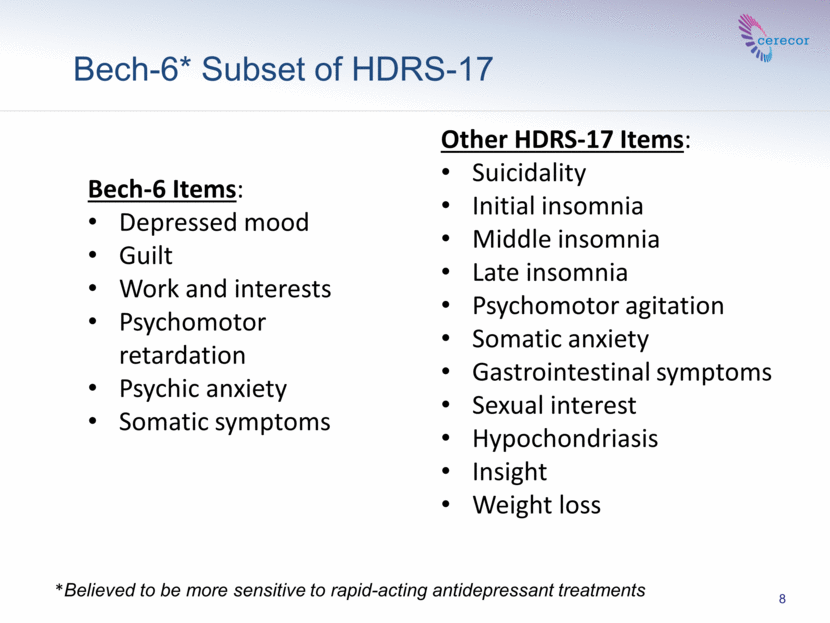
8 *Believed to be more sensitive to rapid-acting antidepressant treatments Bech-6* Subset of HDRS-17 Bech-6 Items: Depressed mood Guilt Work and interests Psychomotor retardation Psychic anxiety Somatic symptoms Other HDRS-17 Items: Suicidality Initial insomnia Middle insomnia Late insomnia Psychomotor agitation Somatic anxiety Gastrointestinal symptoms Sexual interest Hypochondriasis Insight Weight loss |
|
|
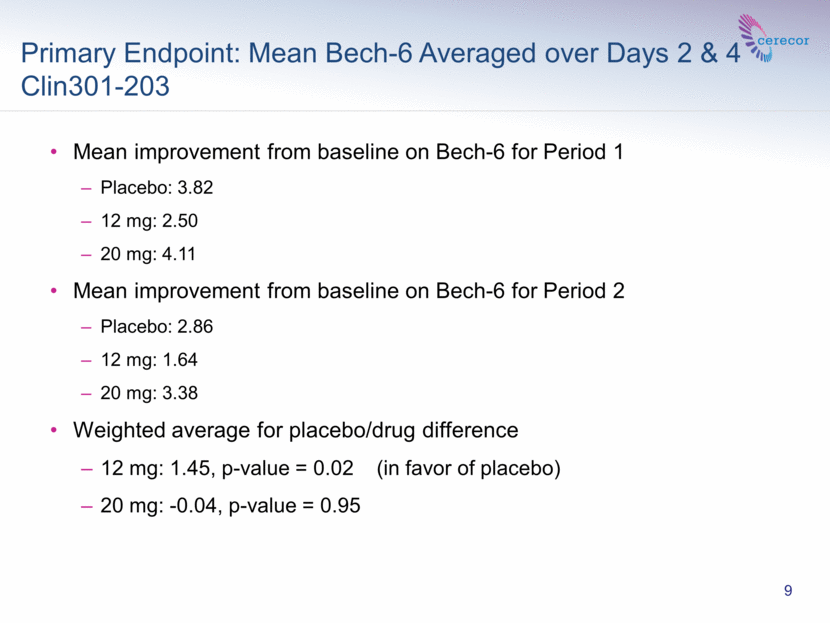
Primary Endpoint: Mean Bech-6 Averaged over Days 2 & 4 Clin301-203 9 Mean improvement from baseline on Bech-6 for Period 1 Placebo: 3.82 12 mg: 2.50 20 mg: 4.11 Mean improvement from baseline on Bech-6 for Period 2 Placebo: 2.86 12 mg: 1.64 20 mg: 3.38 Weighted average for placebo/drug difference 12 mg: 1.45, p-value = 0.02 (in favor of placebo) 20 mg: -0.04, p-value = 0.95 |
|
|
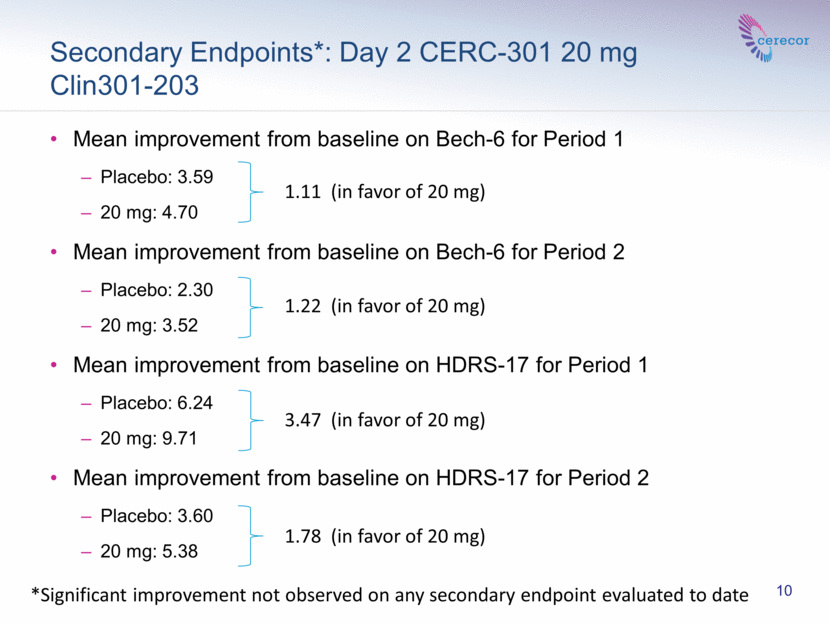
Secondary Endpoints*: Day 2 CERC-301 20 mg Clin301-203 Mean improvement from baseline on Bech-6 for Period 1 Placebo: 3.59 20 mg: 4.70 Mean improvement from baseline on Bech-6 for Period 2 Placebo: 2.30 20 mg: 3.52 Mean improvement from baseline on HDRS-17 for Period 1 Placebo: 6.24 20 mg: 9.71 Mean improvement from baseline on HDRS-17 for Period 2 Placebo: 3.60 20 mg: 5.38 3.47 (in favor of 20 mg) 1.78 (in favor of 20 mg) 1.11 (in favor of 20 mg) 1.22 (in favor of 20 mg) 10 *Significant improvement not observed on any secondary endpoint evaluated to date |
|
|
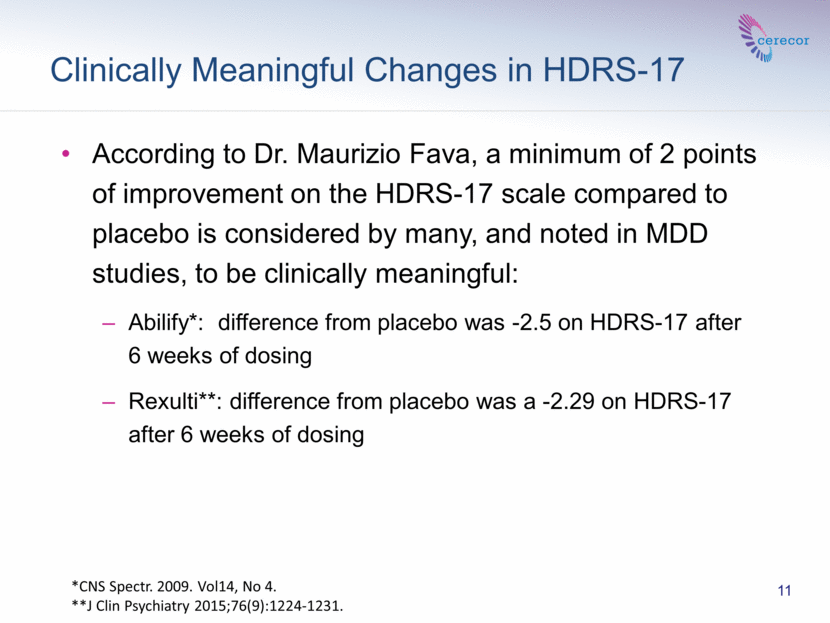
Clinically Meaningful Changes in HDRS-17 11 According to Dr. Maurizio Fava, a minimum of 2 points of improvement on the HDRS-17 scale compared to placebo is considered by many, and noted in MDD studies, to be clinically meaningful: Abilify*: difference from placebo was -2.5 on HDRS-17 after 6 weeks of dosing Rexulti**: difference from placebo was a -2.29 on HDRS-17 after 6 weeks of dosing *CNS Spectr. 2009. Vol14, No 4. **J Clin Psychiatry 2015;76(9):1224-1231. |
|
|
Safety and Tolerability Clin301-203 CLIN 301-203 Phase 2 12 Generally well-tolerated No SAEs reported No discontinuations due to AEs Most commonly reported: Increased blood pressure Dizziness Somnolence Paresthesia |
|
|
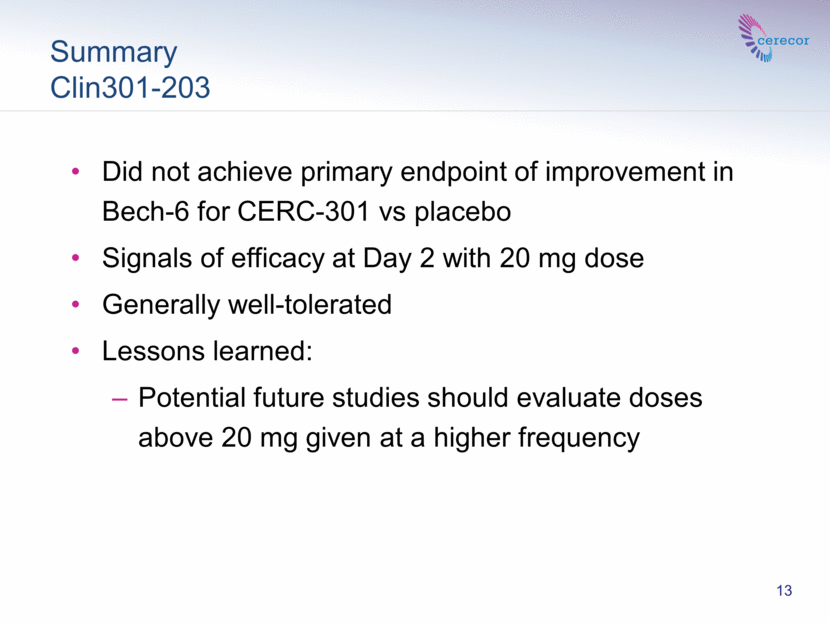
Summary Clin301-203 CLIN 301-203 Phase 2 13 Did not achieve primary endpoint of improvement in Bech-6 for CERC-301 vs placebo Signals of efficacy at Day 2 with 20 mg dose Generally well-tolerated Lessons learned: Potential future studies should evaluate doses above 20 mg given at a higher frequency |
|
|
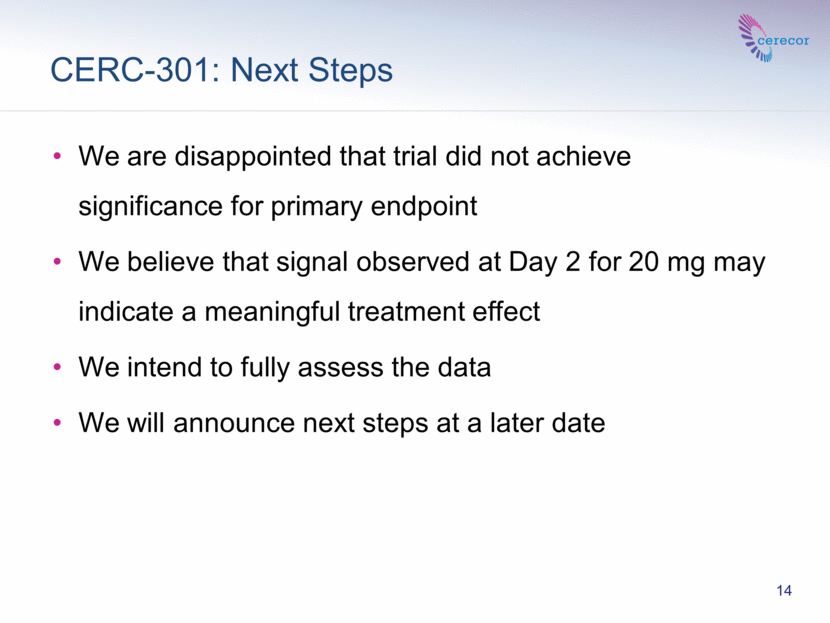
CERC-301: Next Steps 14 We are disappointed that trial did not achieve significance for primary endpoint We believe that signal observed at Day 2 for 20 mg may indicate a meaningful treatment effect We intend to fully assess the data We will announce next steps at a later date |
|
|
Q&A |