Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - ALPINE IMMUNE SCIENCES, INC. | a16-22234_1ex99d1.htm |
| 8-K - 8-K - ALPINE IMMUNE SCIENCES, INC. | a16-22234_18k.htm |
Exhibit 99.2
Cavosonstat Phase 2 Trial Results Call November 28th, 2016

Introduction Mike Carruthers, Chief Financial Officer Initial Remarks Jon Congleton, President and CEO Data Highlights David Rodman, M.D., Chief Medical Officer Joining for Q&A Jan Troha, Chief Operating Officer Steve Shoemaker, M.D., VP Medical/Clinical Sherif Gabriel, PhD, VP Scientific Search Agenda

This presentation contains forward-looking statements that are based on our management's belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as "may," "might," "could," "would," "will," "should," "expect," "intend," "plan," "anticipate," "believe," "estimate," "predict," "project," "target," "potential," "continue" or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. These risks, uncertainties and other factors include, among others, uncertainty in the potential for cavosonstat or any other GSNOR inhibitors to address other therapeutic areas, the risk that we may be unable to fund future development of cavosonstat or any other GSNOR inhibitors, the risk that unanticipated costs or expenditures arise in connection with our clinical trials that affect our need for capital and the uncertainties inherent in the clinical drug development process. Any forward-looking statements made in this presentation are also subject to the risks and uncertainties that are described more fully in our filings with the SEC, including our most recent annual report on Form 10K and quarterly report on Form 10-Q. If one or more of these risks, uncertainties or other factors occurs, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change but we undertake no obligation to update these forward-looking statements in the future, except to the extent required by applicable law. All trademarks and registered trademarks are the property of their respective owners. Such use should not be construed as an endorsement of our business. Certain data in this presentation was obtained from various external sources, and neither we nor our affiliates, advisers or representatives has verified such data with independent sources. Accordingly, neither we nor any of our affiliates, advisers or representatives makes any representations as to the accuracy or completeness of that data or to update such data after the date of this presentation. Such data is subject to change based on various factors. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities in any jurisdiction in which such offer or solicitation is not permitted under applicable law. Safe Harbor Statement

Cavosonstat at doses up to 400mg bid was well tolerated with no unanticipated safety findings. Treatment did not result in improvement in lung function over the twelve week period of treatment in this patient population. Modest reduction in sweat chloride, consistent with CFTR modulation, was confirmed in the 200mg cohort but failed to produce durable or dose dependent effects. Cavosonstat Added to Orkambi in F508del Homozygous Patients – SNO-6 Results
SNO-6 Trial Top Line Results David M. Rodman, MD Chief Medical Officer

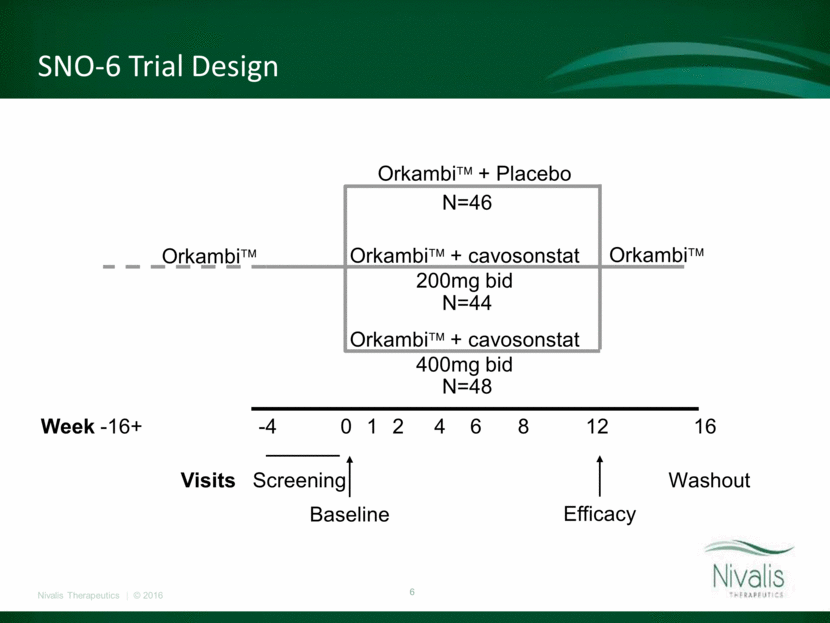
SNO-6 Trial Design Orkambi+ Placebo Orkambi+ cavosonstat 200mg bid Orkambi+ cavosonstat 400mg bid OrkambiN=46 N=44 N=48 Week -16+ -4 0 1 2 4 6 8 12 16 Visits Screening Washout Orkambi Baseline Efficacy
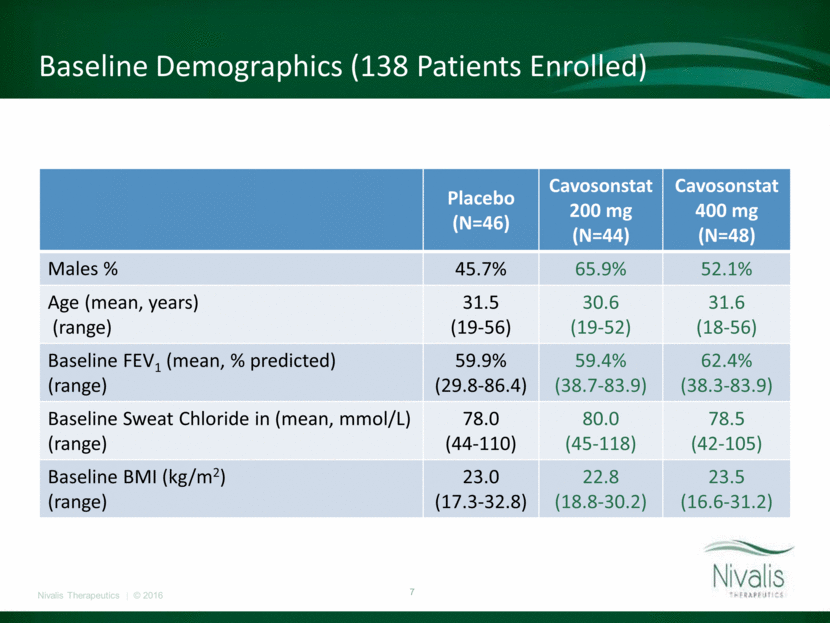
Baseline Demographics (138 Patients Enrolled) Placebo (N=46) Cavosonstat 200 mg (N=44) Cavosonstat 400 mg (N=48) Males % 45.7% 65.9% 52.1% Age (mean, years) (range) 31.5 (19-56) 30.6 (19-52) 31.6 (18-56) Baseline FEV1 (mean, % predicted) (range) 59.9% (29.8-86.4) 59.4% (38.7-83.9) 62.4% (38.3-83.9) Baseline Sweat Chloride in (mean, mmol/L) (range) 78.0 (44-110) 80.0 (45-118) 78.5 (42-105) Baseline BMI (kg/m2) (range) 23.0 (17.3-32.8) 22.8 (18.8-30.2) 23.5 (16.6-31.2)
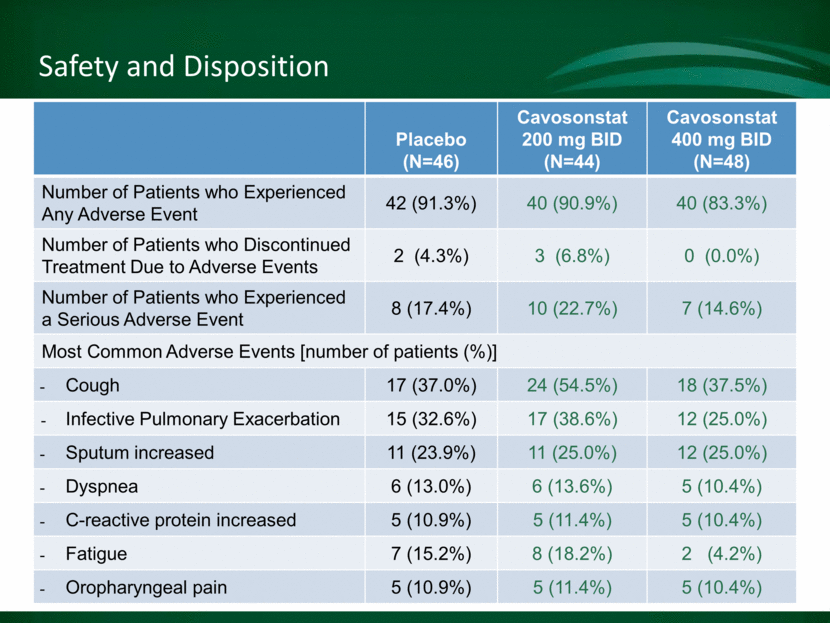
Safety and Disposition Steve to insert safety summary slide here Placebo (N=46) Cavosonstat 200 mg BID (N=44) Cavosonstat 400 mg BID (N=48) Number of Patients who Experienced Any Adverse Event 42 (91.3%) 40 (90.9%) 40 (83.3%) Number of Patients who Discontinued Treatment Due to Adverse Events 2 (4.3%) 3 (6.8%) 0 (0.0%) Number of Patients who Experienced a Serious Adverse Event 8 (17.4%) 10 (22.7%) 7 (14.6%) Most Common Adverse Events [number of patients (%)] Cough 17 (37.0%) 24 (54.5%) 18 (37.5%) Infective Pulmonary Exacerbation 15 (32.6%) 17 (38.6%) 12 (25.0%) Sputum increased 11 (23.9%) 11 (25.0%) 12 (25.0%) Dyspnea 6 (13.0%) 6 (13.6%) 5 (10.4%) C-reactive protein increased 5 (10.9%) 5 (11.4%) 5 (10.4%) Fatigue 7 (15.2%) 8 (18.2%) 2 (4.2%) Oropharyngeal pain 5 (10.9%) 5 (11.4%) 5 (10.4%)
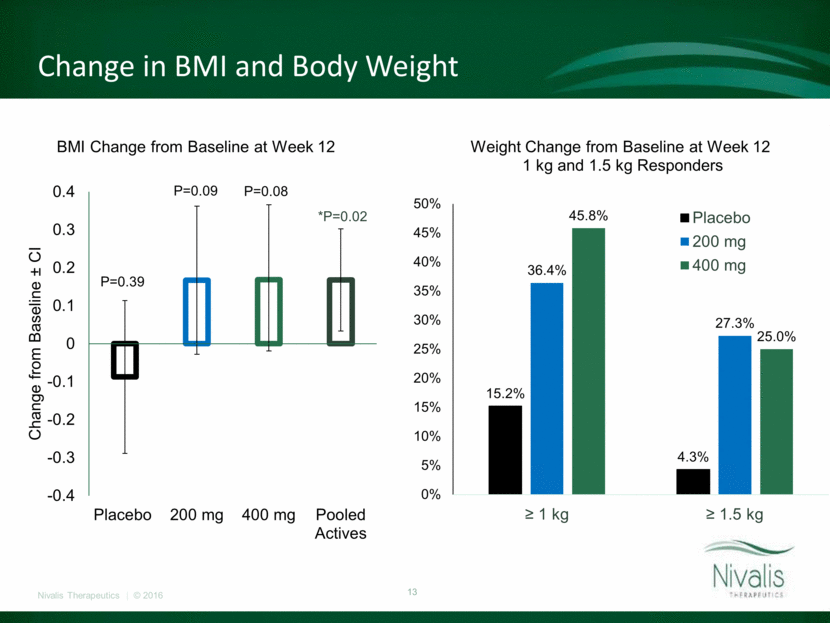
Primary and Key Secondary Outcomes at Week 12* Placebo (N=46) 200 mg BID (N=44) 400 mg BID (N=48) Pooled Active (N=92) Absolute Change in FEV1 (% predicted) (Within group p-value) 0.97 (0.36) 0.39 (0.72) 0.35 (0.73) 0.37 (0.62) Relative Change in FEV1 (% predicted) (Within group p-value) 1.87 (0.31) 0.66 (0.72) 1.11 (0.53) 0.91 (0.48) Absolute Change in Sweat Chloride (mmol/L) (Within group p-value) -2.3 (0.16) -1.2 (0.46) -0.6 (0.69) -0.8 (0.44) Absolute Change in CFQ-R respiratory domain (Within group p-value) -3.03 (0.24) -3.15 (0.23) 3.16 (0.21) 0.16 (0.93) Absolute change in BMI (kg/m2) (Within group p-value) -0.09 (0.39) 0.17 (0.09) 0.17 (0.08) 0.17 (0.02) * MMRM LS Means
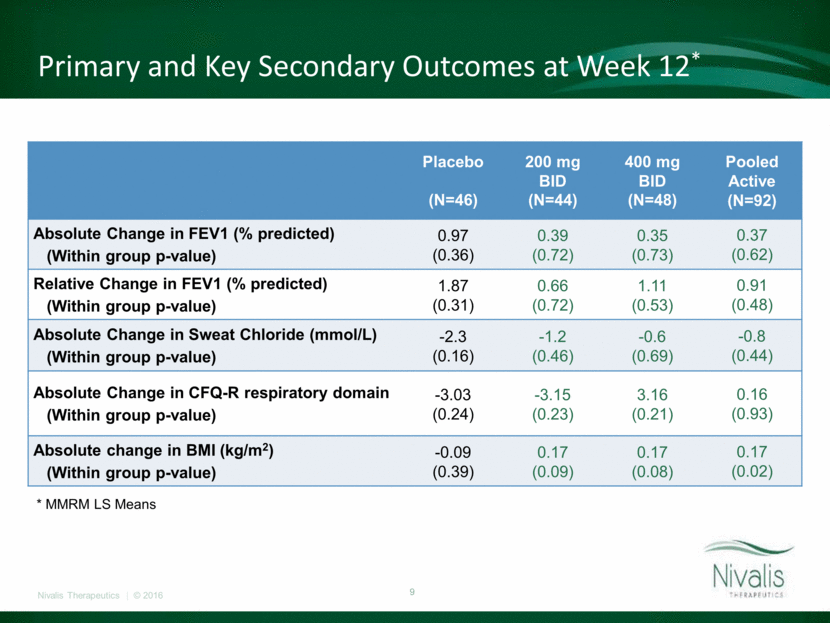
FEV1 (Absolute % Predicted) Change from Baseline -4 -3 -2 -1 0 1 2 3 4 0 4 8 12 16 Change from Baseline ± SEM (%) Study Visit (week) Placebo 200mg bid 400mg bid
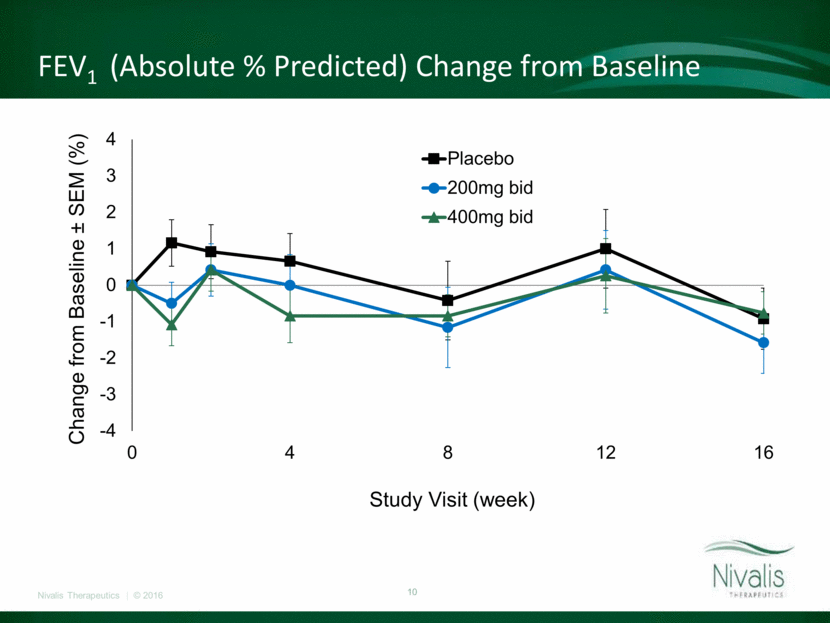
Sweat Chloride Change from Baseline -6 -5 -4 -3 -2 -1 0 1 2 3 0 4 8 12 Change from Baseline ± SEM ( mmol /L) Study Visit (week) P<0.02 P<0.01 Cavosonstat 200mg bid Cavosonstat 400mg bid Placebo
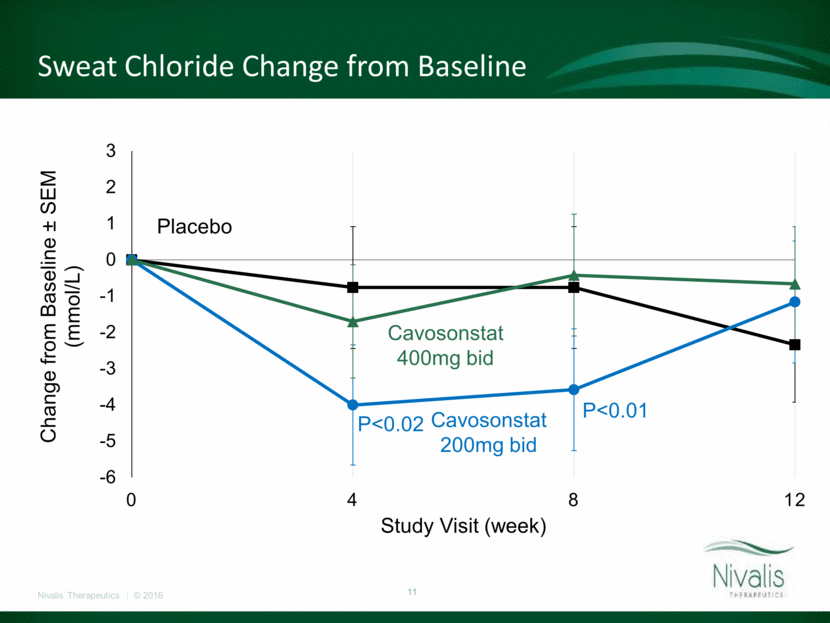
CFQ-R Respiratory Domain Change from Baseline* Placebo Cavosonstat 200mg bid Cavosonstat 400mg bid * Arithmetic Means -10 -8 -6 -4 -2 0 2 4 6 8 0 4 8 12 16 Change from baseline ± CI Study Visit (week)
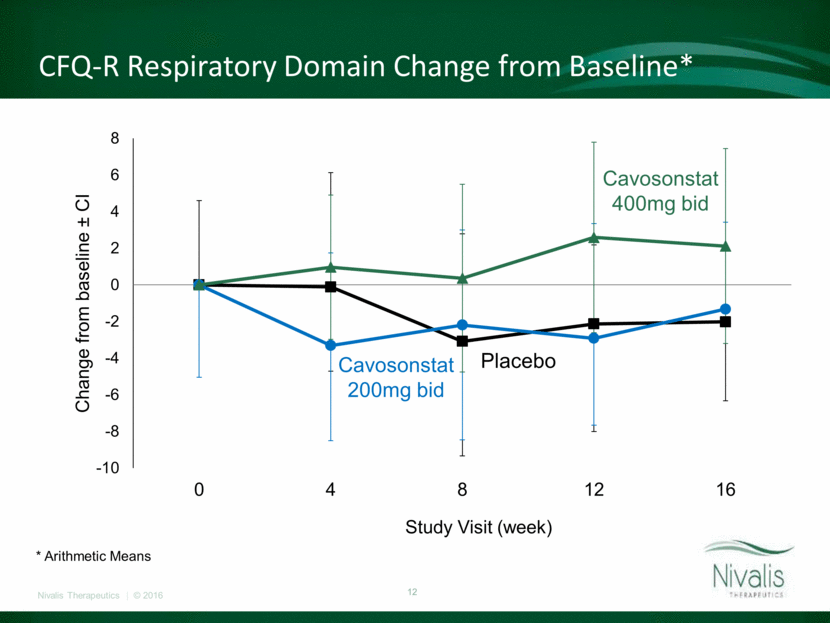
Change in BMI and Body Weight P=0.39 P=0.09 P=0.08 *P=0.02 -0.4 -0.3 -0.2 -0.1 0 0.1 0.2 0.3 0.4 Placebo 200 mg 400 mg Pooled Actives Change from Baseline ± CI BMI Change from Baseline at Week 12 15.2% 4.3% 36.4% 27.3% 45.8% 25.0% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% ? 1 kg ? 1.5 kg Weight Change from Baseline at Week 12 1 kg and 1.5 kg Responders Placebo 200 mg 400 mg
Cavosonstat at doses up to 400mg bid was well tolerated with no unanticipated safety findings. Treatment did not result in improvement in lung function over the twelve week period of treatment in this patient population. Modest reduction in sweat chloride, consistent with CFTR modulation, was confirmed in the 200mg cohort but failed to produce durable or dose dependent effects. Increase in weight and body mass index was observed in both treatment groups, which may indicate potential therapeutic benefit to CF-related gastrointestinal and/or metabolic disease. Summary of SNO-6 Trial
We do not envision moving forward with cavosonstat for treatment of CF-related lung disease in F508del homozygous patients. We await results of the SNO-7 trial in Kalydeco-treated patients heterozygous for F508del and a CFTR gating mutation. The observation of improved weight and BMI in an adult CF population is interesting and we plan further evaluation of the data and consultation with CF GI/Nutrition experts in the near future. We plan to investigate the therapeutic potential of cavosonstat and our S-nitrosoglutathione reductase (GSNOR) inhibitor portfolio and determine next steps. Forward Plans

Q & A

