Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REPROS THERAPEUTICS INC. | v453186_8k.htm |
Exhibit 99.1

Repros Therapeutics Development of small molecule drugs for major unmet medical needs that treat male and female reproductive disorders.

Repros Disclaimer Any statements made by Repros Therapeutics Inc . (“Repros” or the “Company”) that are not historical facts contained in these slides (or in any oral accompanying discussion) are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to various risks, uncertainties and other factors that could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by such forward - looking statements . These statements often include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “project,” “potential,” “intend,” “believe,” “plan,” “seek,” “could,” “can,” “should” or similar expressions . These statements are based on assumptions that the Company has made in light of the Company’s experience in the industry, as well as the Company’s perceptions of historical trends, current conditions, expected future developments and other factors the Company believes are appropriate in these circumstances . Forward - looking statements include, but are not limited to, those relating to development of and anticipated milestones for Enclomiphene and Proellex®, the conduct of planned clinical studies and the timing and nature of the results thereof, the markets for the Company’s products and the potential success of the Company in penetrating those markets and that the Company’s need for and use of financial resources . Such statements are based on current expectations that involve a number of known and unknown risks, uncertainties and other factors that may cause actual events to be materially different from those expressed or implied by such forward - looking statements, including the ability to raise additional needed capital on a timely basis in order for the Company to continue to fund development of its Enclomiphene and Proellex® programs, the ability to have success in the clinical development of the Company’s technologies, the reliability of interim results to predict final study outcomes, and such other risks as are identified in the Company's most recent Annual Report on Form 10 - K and the subsequent quarterly reports on Form 10 - Q . These documents are available on request from Repros or at www . sec . gov . Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise . In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry . We obtained this information and these statistics from third - party sources, which we have supplemented where necessary with information from publicly available sources and our own internal estimates . Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information . While we believe that each of these studies and publications is reliable, we have not independently verified such data, and we make no any representation as to the accuracy of such information . Similarly, we believe our internal research is reliable, but it has not been verified by any independent sources .

Proellex® Addressing significant unmet female reproductive disorders

Uterine Fibroids & Endometriosis Poor therapeutic options for debilitating female disorders experienced by women in the prime of their life • Benign, monoclonal, hormone sensitive, smooth muscle tumors of the uterus • Most common tumor of the female reproductive tract – Heavy bleeding / anemia – Abdominal pressure / pain / urinary frequency • Affect 20 - 77% of women age 35 – 55 • 600,000 hysterectomies conducted annually • Definition: the presence of epithelial and stromal endometrial cells outside of the uterine cavity • Complaints of infertility or pregnancy loss • Pelvic pain/back pain • Dyspareunia (pain during sex) • Dysmenorrhea (menstrual cycle cramps) • 5% of women of reproductive age • Estimated that 25 - 40% (2 – 4 million) of infertility cases may be due to endometriosis • 71 - 87% in women with chronic pelvic pain • 53% of teenagers with dysmenorrhea • Many women have it without the diagnosis • Unmet medical need – Oc’s , Lupron, Danazol – Laparascopic procedures • High recurrence rate after treatment Uterus Exhibiting Multiple Fibroids Courtesy of Jay Goldberg, MD, MSCP Director, Jefferson Fibroid Center Director, Division of General OB/GYN Jefferson Medical College, Philadelphia, PA Endometrial lesions in peritoneum of woman suffering with endometriosis Courtesy of Bruce A. Lessey , MD, PhD

O O OMe N OAc CH 3 H 3 C How Antiprogestins Like Proellex Work Antiprogestins • Selectively block progesterone activity • Allow tonic hormone secretions – Alleviate negative side effects of GnRHa • Potential for chronic use Different than GnRH agonists and antagonists. These agents: • Block hypothalamus / pituitary axis • Shut down hormonal secretions • Long - term side effects include bone loss Proellex Hypothalamus Pituitary Endometrial & Uterine Tissue
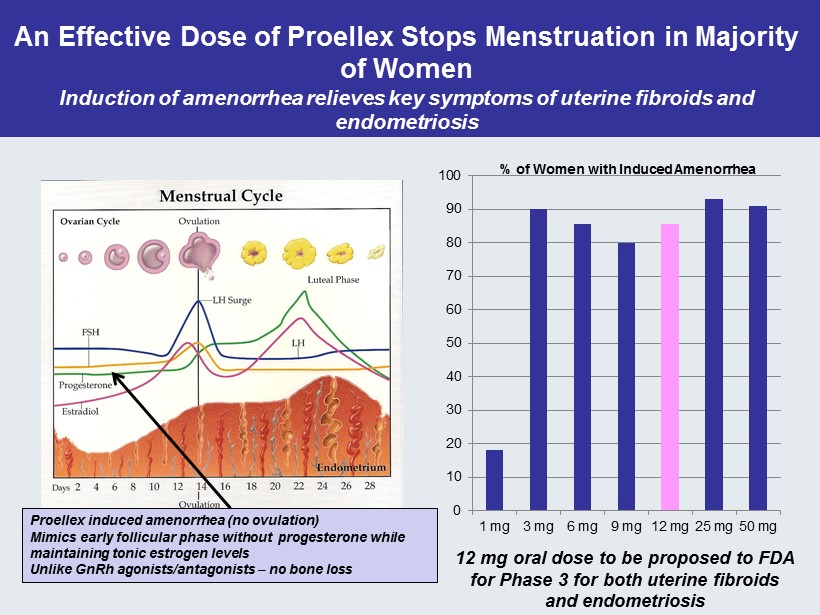
An Effective Dose of Proellex Stops Menstruation in Majority of Women Induction of amenorrhea relieves key symptoms of uterine fibroids and endometriosis 0 10 20 30 40 50 60 70 80 90 100 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg % of Women with Induced Amenorrhea Proellex induced amenorrhea (no ovulation) Mimics early follicular phase without progesterone while maintaining tonic estrogen levels Unlike GnRh agonists/antagonists – no bone loss 12 mg oral dose to be proposed to FDA for Phase 3 for both uterine fibroids and endometriosis

Impact of Proellex Treatment on Uterine Fibroids -80 -60 -40 -20 0 20 40 60 80 100 120 % Amenorrhea % Reduction in Fibroid Volume % Effects of 12 mg Proellex after 36 weeks of treatment of severe uterine fibroids (menstrual blood loss > 80mL/ cycle) LOCF Analysis 12 mg Proellex Placebo p=0.0001 p=0.0004 N = 17 12 mg N = 26 Placebo

Change in UFSQOL Over Time LOCF 0 10 20 30 40 50 60 70 80 90 Baseline End of Cycle 1 End of Cycle 2 Comparison of Quality of Life Outcomes Between Different Doses of Telapristone and Different Routes of Administration in Treatment of Uterine Fibroids Oral Pbo Oral 6mg Oral 12mg Vaginal Pbo Vaginal 6mg Vaginal 12mg At baseline, no difference between groups At end of first cycle, oral 6 & 12 mg stat. sig. compared to pbo At end of cycle 2, 12 mg superior to placebo UFSQOL Score >40 Indicative of Symptomatic Uterine Fibroids

Impact of Proellex Treatment on Endometriosis 0 2 4 6 8 10 12 14 Baseline End of 18 Wk Off Drug Interval Median Sum of BBSS Scored Menstrual Pain Over 28 Day Cycle Proellex Placebo p=0.0013 N=43 Proellex , 17 Placebo 47 in US, 13 in Argentina BBSS Classification Moderate to Severe

Impact of Proellex Treatment on Endometriosis -70 -60 -50 -40 -30 -20 -10 0 4 Wk 8 Wk 12 Wk 18 Wk LOCF Off Drug Interval Median % Change in Non - Prescription Analgesics Proellex Placebo p=0.0093 -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 4 Wk 8 Wk 12 Wk 18 Wk LOCF Off Drug Interval Median % Change in Prescription Analgesics Proellex Placebo p=0.0314 p=0.055 p=0.0273 p=0.0848 Baseline Analgesic Use Per 28 Day Menstrual Cycle Prescription Non - Prescription Proellex 23.14 (40.81) 30.28 (74.96) Placebo 14.38 (17.78) 18.94 (29.05)

All other antiprogestins in development require off - drug - interval after 12 weeks of treatment Key Advantage of Proellex Over Other Antiprogestins in Development Proliferative Disordered Proliferative Benign Endometrial Hyperplasia Mutter et al, 2007 12.5mg Proellex No hyperplasia Estrogen over Time • Required off Drug Interval to allow for: • Menses • Refresh the endometrium • Potential unexpected uterine hemorrhage – Menses returns in 25 - 35 days • Return of symptoms after cessation of treatment Proellex provides 18 weeks of significant symptom relief before Off - Drug - Interval

PROELLEX EVOLUTION OF LEAD COMPOUND O CH 3 O OMe N OAc CH 3 H 3 C 44 Analogues Synthesized to Date O CH 3 OH 3 C N OAc CH 3 H 3 C CDB - 2914 ( Ulipristal Acetate) • NIH precursor to Repros class • Allergan marketing molecule ( Esmya ) in Europe for treatment of uterine fibroids CDB - 4124 Proellex ( telapristone acetate) • Repros worldwide exclusive license from NIH • Composition of matter patent Both molecules metabolized Via CYP3A4 Mono demethylation of amine Active metabolites

Differences Between Proellex and Esmya • Proellex – Duration of drug cycle • 18 weeks – Formulation • Immediate release capsule: 12 mg – Tmax : 0.75 hr – Cmax : 256 ± 113 ng/ml – AUC: 935 ± 492 ng* hr /ml – NIH rabbit model • 95% suppression of progesterone action @ 8 mg oral – Induction of amenorrhea after two 18 weeks cycles • 100% – Fibroid Reduction: 58.2% • Esmya (Label and Fertil Steril , 2015) – Duration of drug cycle • 12 weeks – Formulation • Rapid disintegrating tablet: 10 mg – Tmax : 1 hr – Cmax : 50 ± 34.4 ng/ml – AUC: 134 ± 83.8 ng* hr /ml – NIH rabbit model • 63% suppression of progesterone action @ 8 mg oral – Induction of amenorrhea after two 12 weeks cycles @10 mg • 73% – Fibroid Reduction: 58%

Differences Between Proellex and Esmya Comparison of Proelle x and Esmya Treatment Age BMI Ethnicity % Amenorrhea % Fibroid Reduction UFSQOL Baseline UFSQOL End of Study Proellex (All US @ 36 wks , LOCF) 6 mg 40.55 33.67 100% Black 88.9 32.9% 77.1 33.5 12 mg 41.35 33.49 88.2% Black 94.1 58.2% 69.9 19.3 Esmya (5 mg @ 12 wks in Canada, 5 & 10 mg @ 24 wks in Europe) N.America Black 40.3 27.3 41% ND 51.7 21.6 N.America White 44.5 26.5 66% ND 55.9 17.6 Europe 5 mg 41.6 25.2 92.5% White 61.9% 54.1% 50.0 15.6 10 mg 41.1 25.3 96.0% White 72.7% 58.0% 50.0 12.5

Proellex Clinical Goals • Confirm Phase 3 requirements with FDA • Uterine Fibroids: Request Mtg. Q4 - ’16 • Endometriosis : Request Mtg. Q4 - ’16 • Anticipate meetings Q1 - Q2 ‘17 • Completed Proellex NDA components • Pre - clinical complete (including 2 carcinogenicity studies) • Manufacturing complete (API sourced, stable final drug product) • Phase 1 complete except TQtc – No signal in pilot study

Proellex Summary • Potential Proellex market opportunity > $1 Billion for Fibroids and Endometriosis • Rigorous clinical program working to move into Phase 3 • Significant clinical advantages identified against GnRH agonists/antagonists • Longer symptom relief established against other approved or in development anti - progestins

Encyzix ( enclomiphene ) Rational treatment for secondary hypogonadism

Enclomiphene Development Status • Central EU filing ongoing for treatment of secondary hypogonadism – Anticipated marketing authorization Q4 - ’17 • Repros to present as sponsor at 12/6/16 FDA Adcom – “Agenda : The committee will discuss appropriate clinical trial design features, including acceptable endpoints for demonstrating clinical benefit, for drugs intended to treat secondary hypogonadism while preserving or improving testicular function, including spermatogenesis .” – No T replacement to be discussed due to negative effects on testicular function • US Phase 2 “Proof of Concept” to evaluate clinical benefit

CM - 19 Prescription Claims for Testosterone Products Nguyen et al N Eng J Med 2015 73% 9% 3%

CM - 20 Prevalence of Overweight and Obesity Global Burden of Disease Study 2013 (Ng et al Lancet 2014) Prevalence (%) 70 60 50 40 3 0 20 10 0 2 - 9 10 - 19 20 - 29 30 - 39 40 - 49 50 - 59 60 - 69 70 - 79 ≥80 Age (years) 29.0 39.4 32.0 0 20 40 60 20-39 Years 40-59 Years ≥60 Years Percent CDC (Ogden, 2013 ) Men in Developed Countries Overweight and Obese Obese Only

CM - 21 0 20 40 60 80 LH (U/L) 0 10 20 30 40 50 Total Testosterone (nmol/L) Primary Hypogonadism Low T, high LH n=52 (1.7%) n = 3219 40 – 80 yr Categorizing Gonadal Status by Testosterone and LH Eugonadal Normal T and LH Compensated ‘Hypogonadism ’ Normal T, high LH Secondary Hypogonadism Low T, normal/low LH n=318 (10.4%) <300 ng/ dL >9.4 U/L EMAS Tajar, JCEM 2010 Exclusions (n = 150) Known pituitary/testicular diseases and current medications affecting pituitary, testicular function or metabolism of T

CM - 22 EMAS Wu, JCEM 2008 BMI and Age: Different Effects on Hormones Testosterone ( nmol /L) LH (U/L) • With obesity, LH does not respond to fall in testosterone – functional hypothalamic / pituitary suppression • With aging , increasing LH compensates for failing testicular function so that any age - related decline of testosterone is minimized

Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Secondary Hypogonadism at Baseline Age (years) BMI ≥ 25 - <30 kg/m2 BMI ≥ 30 kg/m2 Smoking (current) Alcohol (frequent) Morbidity ( ≥ 1) 0 5 10 15 *** *** * Poor morning erection Sexual thoughts Erectile dysfunction Low vigorous activity Unable to bend Slow walking speed 0 2 4 6 * * Adjusted Relative Risk Ratio *p <0.05 **p<0.01 ***p<0.001 Adjusted Odds Ratio Risk factors Symptoms Tajar et al. JCEM 2010

Obesity Related Hypogonadism is the Leading Cause of Secondary Hypogonadism • Obesity has been shown to attenuate LH pulse amplitude but maintain pulse frequency ( Vermeulen , JCEM,1993) • EMAS ( Tajar , JCEM, 2010) notes a decrease in the T:E ratio in secondary hypogonadal men compared to eugonadal • Anti - estrogens have been shown to increase both LH and T in secondary hypogonadal men T ( nmol /L) E 2 ( pmol /L) T:E molar ratio Eugonadal 17.8 74.1 240.2 Secondary 8.7 57.2 152.1

Weight Related Secondary Hypogonadism is Reversible with Weight Loss CM - 18 n = 2395, *p<0.05, **p<0.01 % Weight loss Weight gain % 0 * ** * * * Camacho et al. European Journal of Endocrinolology 2013 Mean (SEM) Secondary Hypogonadism is REVERSIBLE with WEIGHT LOSS Secondary Hypogonadism DEVELOPS with WEIGHT GAIN Testosterone Changes Related Weight Change Camacho, 2013

Relevant Experience Gleaned during the Development of Enclomiphene for the Treatment of Secondary Hypogonadism in Overweight or Obese Men All studies enrolled secondary hypogonadal men – Morning T <300 ng/ dL – LH < 9.4 mIU /mL – BMI > 25 – Age < 60 years Screening failures: Lessons learned from studies assessing testosterone and spermatogenesis effects of enclomiphene – Sperm concentration (studies 301, 302, 304 & 305) • 10.6% (186/1,761 screened) did not meet sperm concentration of > 15 Million/mL – Morning Testosterone (Pivotal studies 304 & 305) • 24% (156/642 screened) did not meet morning T < 300 ng/mL • Average age of T screen failures: 46.4 (9.2) years • Average T of T screen failures: 372 (79.7) ng/ dL

0 100 200 300 400 500 600 700 800 900 1000 0 5 10 15 20 Total Testosterone ( ng /dl) Hours T @ Baseline T @ 6 Weeks 0 100 200 300 400 500 600 700 800 900 1000 0 5 10 15 20 Total Testosterone (ng/ dL ) Hours T @ Baseline T @ 6 Weeks The Anti - estrogen Enclomiphene and Topical Testosterone Exhibit Different Effects on LH in the Overweight/Obese Hypogoandal Male Enclomiphene Androgel • Enclomiphene blocks estradiol, raising LH and maintains pulsatile behavior • T further suppresses the axis Wiehle 2013 0 2 4 6 8 10 12 14 0 5 10 15 20 LH mIU /mL Hours LH@ Baseline LH@ 6Weeks 0 2 4 6 8 10 12 14 0 5 10 15 20 LH mIU /mL Hours LH@ Baseline LH@ 6Weeks
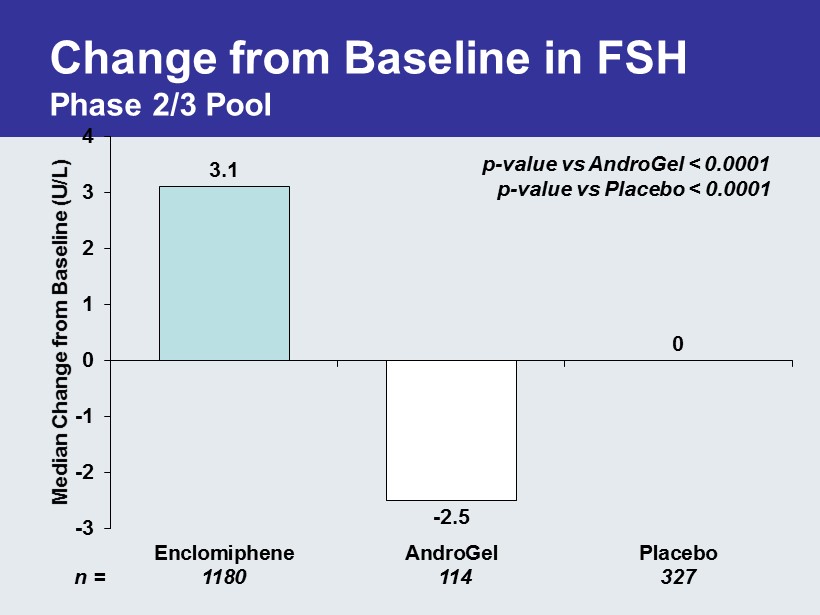
Change from Baseline in FSH Phase 2/3 Pool 3.1 - 2.5 0 -3 -2 -1 0 1 2 3 4 Enclomiphene AndroGel Placebo Median Change from Baseline (U/L) QEF - 38 n = 1180 114 327 p - value vs AndroGel < 0.0001 p - value vs Placebo < 0.0001

Change from Baseline in LH Phase 2/3 Pool 3.0 - 1.8 0.2 -3 -2 -1 0 1 2 3 4 Enclomiphene AndroGel Placebo Median Change from Baseline ( mIU /mL) p - value vs AndroGel < 0.0001 p - value vs Placebo < 0.0001 QEF - 38 n = 1179 109 328

The Effects of Topical T Treatment Enclomiphene restores normal testosterone levels and maintains sperm concentrations Kim 2015, BJU International

5.1 6.8 - 6.8 - 2.2 10.7 5.4 -8 -6 -4 -2 0 2 4 6 8 10 12 ZA-304 ZA-305 Mean Percent Change from Baseline Enclomiphene AndroGel Placebo Percent Change in Testicular Volume by Orchidometry vs. AndroGel p = 0.0074 vs. AndroGel p = 0.0224 n = 41 43 45 44 42 41

Phase 2 Proof of Concept “Diet & Exercise” Study in Obese Hypogonadal Men - Baseline Findings Screen 98 to enroll 50 15 Month Study • Enrollment (n=50 in 5 weeks @ 5 sites) • Demographics ( stdev ) – Age: 43.3 (9.2) – BMI: 36.8 (3.2) – Waist: 46.9” (4.1) – % Body Fat: 38.1 (5.2) • Hormonal Status – Testosterone: 221.9 (52.7) ng/ dL – Estradiol: 48.1 (14.8) pg /mL – T:E Ratio: 4.95 (1.7) normal 20 - 25 • Top 4 Reported Baseline Symptoms (% of Enrollees) – Fatigue/Lack of Energy: 96% – Depression, Irritability Lack of Focus: 74% – Poor Libido: 60% – Muscle Weakness: 48% Seeks to show: • The disorder is reversible with weight loss • Raising endogenous T provides benefit while attempting to diet and exercise

How Enclomiphene Works Enclomiphene blocks estrogen at the level of the H - P axis increasing LH levels which in turn increases endogenous production of T 0 1 2 3 4 5 6 7 Enclomiphene LH Androgel LH Median LH ( mIU /mL) Impact on LH in 16 Wk Study ZA - 304 Baseline End of Study 0 50 100 150 200 250 300 350 400 450 500 Enclomiphene T Androgel T Mean Morning T (ng/ dL ) Impact on T in 16 Wk Study ZA - 304 Baseline End of Study p<0.0001 p=0.0007 Induction of infertility and shrinking testicles in Androgel arm

Interim 9 Month Data ZA - 205 30 31 32 33 34 35 36 37 38 39 Bl 3 mo 6 mo 9 mo 12 mo 15mo BMI BMI Over Time 12.5 mg 25 mg pbo 0 50 100 150 200 250 300 350 400 0 100 200 300 400 500 600 700 Scr BL 3 mo 6 mo 9 mo 12 mo 15 mo Composite PRO CLEIA Morning T (ng/ dL ) Morning T Over Time 12.5 mg 25 mg pbo pro12.5 pro25 propbo Commercial diet ends at 6 months. Personal trainer ends at 12 months. Drug vs Placebo P<0.0001

Interim 6 Month Data Lean Mass, LCMS T & Free T 63.5 64 64.5 65 65.5 66 66.5 67 67.5 68 68.5 Baseline 6 Month 12 Month 15 Month Lean Mass by DXA (kg) 12.5 mg 25 mg Placebo 0 20 40 60 80 100 120 140 160 180 0 100 200 300 400 500 600 700 800 900 1000 Baseline 6 Month 12 Month 15 Month Free T ( pg /mL) Testosterone (ng/ dL ) LCMS T & Equilibrium Free T 12.5 T 25 T Pbo T 12.5 Free T 25 Free T Pbo Free T Drug vs Placebo p=0.1078 Drug vs Placebo T p=0.0017 Free T p=0.002 Enclomiphene arms gaining lean mass Placebo arm losing lean mass

Repros Late Stage Assets Repros seeking regional or global development/commercialization partners

Financial Summary • Cash and equivalents: (unaudited Sept. 30, 2016) $10.5 M • Cash used in 2016 through Sept. 30, 2016: $12.5 M • Cash runway: Q2 2017 • Current shares outstanding: 25.4 M shares
