Attached files
| file | filename |
|---|---|
| EX-21.1 - EXHIBIT 21.1 - LIGAND PHARMACEUTICALS INC | a10-kaforlgnd_12311510kxex.htm |
| EX-32.1 - EXHIBIT 32.1 - LIGAND PHARMACEUTICALS INC | a10-kaexhibit321.htm |
| EX-31.2 - EXHIBIT 31.2 - LIGAND PHARMACEUTICALS INC | a10-kaexhibit312.htm |
| EX-31.1 - EXHIBIT 31.1 - LIGAND PHARMACEUTICALS INC | a10-kafexhibit311.htm |
| EX-23.1 - EXHIBIT 23.1 - LIGAND PHARMACEUTICALS INC | a10-kaexhibit231.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________________________________________________________________________________
FORM 10-K/A
Amendment No. 1
_____________________________________________________________________________________________
Mark One
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2015
OR
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission File No. 001-33093
LIGAND PHARMACEUTICALS INCORPORATED
(Exact name of registrant as specified in its charter)
Delaware | 77-0160744 | |
(State or other jurisdiction of incorporation or organization) | (IRS Employer Identification No.) | |
3911 Sorrento Valley Boulevard San Diego, CA | 92121 | |
(Address of Principal Executive Offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (858) 550-7500
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class | Name of Each Exchange on Which Registered |
Common Stock, par value $.001 per share | The NASDAQ Global Market of The NASDAQ Stock Market LLC |
Preferred Share Purchase Rights | The NASDAQ Global Market of The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934. Yes o No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large Accelerated Filer x | Accelerated Filer o | Non-accelerated Filer o | Smaller reporting company o | |||
(Do not check if a smaller reporting company) | ||||||
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of the Registrant’s voting and non-voting stock held by non-affiliates was approximately $1.9 billion based on the last sales price of the Registrant’s Common Stock on the NASDAQ Global Market of the NASDAQ Stock Market LLC on June 30, 2015. For purposes of this calculation, shares of Common Stock held by directors, officers and 10% stockholders known to the Registrant have been deemed to be owned by affiliates which should not be construed to indicate that any such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the Registrant or that such person is controlled by or under common control with the Registrant.
As of November 1, 2016, the Registrant had 20,900,189 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Proxy Statement for the Registrant’s 2015 Annual Meeting of Stockholders to be filed with the Commission on or before April 29, 2016 are incorporated by reference in Part III of this Annual Report on Form 10-K. With the exception of those portions that are specifically incorporated by reference in this Annual Report on Form 10-K, such Proxy Statement shall not be deemed filed as part of this Report or incorporated by reference herein.
EXPLANATORY NOTE
Ligand Pharmaceuticals Incorporated (“the Company”) is filing this Amendment on Form 10-K/A (“Amended Form 10-K”) to its Annual Report on Form 10-K for the fiscal year ended December 31, 2015 (the “Original Form 10-K”), which was originally filed with the Securities and Exchange Commission (“SEC”) on February 26, 2016 (the “Original Filing Date”), to reflect restatement of financial statements ("Restatement") described below.
For the convenience of the reader, this Annual Report on Form 10-K/A sets forth the Original Form 10-K, in its entirety, as amended by and to reflect the Restatement.
The following Items have been amended as a result of this Restatement:
•Part I, Item 1A, Risk Factors
•Part II, Item 6, Selected Financial Data
•Part II, Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations
•Part II, Item 9A. Controls and Procedures
•Part IV, Item 15, Exhibits and Financial Statement Schedules
This Amended Form 10-K to amends Part II, Item 6 "Selected Consolidated Financial Data", Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, Item 8. “Consolidated Financial Statements and Supplementary Data”, and Item 9A "Controls and Procedures" to correct errors relating to the Company's net operating loss (NOL) carryforward benefits in the United States which resulted in an overstatement of deferred tax assets (DTA) at December 31, 2015. In connection with three acquisitions that were completed prior to February 2010, the Company recognized DTA for a portion of the NOLs, which included capitalized research and development expenses, obtained from the acquired businesses. From the time of the acquisitions until September 2015, there was a full valuation allowance against all of the Company’s NOLs, including those obtained from the entities acquired. In September 2015, the Company concluded that the valuation allowance against substantially all of its DTA was no longer required based on its then recent income and projections of sustained profitability. As a result, the Company released its DTA valuation allowance in full, including $27.5 million related to NOLs recognized as part of the businesses acquired prior to February of 2010.
During the quarter ended September 30, 2016, the Company concluded that for accounting purposes the approximately $27.5 million of DTA that were obtained upon acquiring the businesses prior to February of 2010 did not meet the more likely-than-not criterion for recognition in 2015 and that the related valuation allowance should not have been reversed. In reviewing its prior-year accounting as part of the 2016 third quarter close process, the Company re-evaluated its accounting for income taxes with the assistance of additional third-party tax professionals and determined that the Company's income tax benefit and net income for the year ended December 31, 2015 were overstated by $27.5 million each.
The Company also corrected errors as part of this Restatement relating to the classification of our 2019 Convertible Senior Notes. As of December 31, 2015, the Company's last reported sale price exceeded the 130% threshold described in Note 5 - "Financing Arrangements" and accordingly the 2019 Convertible Senior Notes have been reclassified as a current liability as of December 31, 2015. As a result, the related unamortized discount of $39.6 million was classified as temporary equity component of currently redeemable convertible notes on our Consolidated Balance Sheet.
The following amounts have been restated for the year ended December 31 2015:
• | Decrease in deferred tax asset and income tax benefit by $27.5 million |
• | Income from continuing operations for the year ended December 31, 2015 has been decreased by $27.5 million to $229.8 million, or $10.83 per diluted share, from $257.3 million, or $12.12 per diluted share |
• | Notes payable of $205.3 million has been reclassified from long term debt to short term debt and the related unamortized discount of $39.6 million has been classified as temporary equity. |
In addition, the consolidated financial statements also give effect to the retrospective adoption of ASU 2015-03, Interest Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs on quarter ended March 31, 2016
This Amended Form 10-K also revises the Company’s disclosure under the heading "Evaluation of Disclosure Controls and Procedures” and “Management’s Report on Internal Control over Financial Reporting” in Item 9A for the material weaknesses relating to this Restatement, includes the updated attestations of our independent registered public accounting firm and includes currently-dated certifications from the Company’s Chief Executive Officer and Chief Financial Officer, as required by Sections 302 and 906 of the Sarbanes-Oxley Act of 2002.
In order to preserve the nature and character of the disclosures set forth in the Original Report, except as expressly noted above, this Amended Form 10-K speaks as of the date of the filing of the Original Report, February 26, 2016, and we have not updated the disclosures in the Amended Form 10-K to speak as of a later date. All information contained in this Amended Report is subject to updating and supplementing as provided in our reports filed with the Securities and Exchange Commission subsequent to the date of the Original Report.
Information not affected by the Restatement is unchanged and reflects the disclosures made as of the Original Filing Date. In particular, forward-looking statements included in this Amended Form 10-K that have not been affected by the Restatement represent management’s views as of the Original Filing Date. Such forward-looking statements should not be assumed to be accurate as of any future date. Accordingly, this Amended Form 10-K should be read in conjunction with our subsequent filings with the SEC, as information in such filings may update or supersede certain information contained in this Amended Form 10-K.
The financial information previously disclosed in the Company’s consolidated financial statements included in the Original Form 10-K (and other SEC filings in which such financial statements were included) and the Company’s unaudited interim condensed consolidated financial statements previously included in the Company’s Quarterly Reports on Form 10-Q for the quarterly and year to date periods ended September 30, 2015 should not be relied upon. The unaudited condensed consolidated financial statements for the three and nine months ended September 30, 2015 and the related disclosures in Management’s Discussion and analysis on financial condition and results of operations will not be restated on Form 10-Q/A but will be restated on Form 10-Q for the period ended September 30, 2016.
In connection with the Restatement, the unaudited condensed consolidated financial statements for the three months ended March 31, 2016 and the three and six months ended June 30, 2016 and the related disclosures will be restated on Forms 10-Q/A for those respective periods.
Table of Contents
Part I | ||
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
Part II | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Part III | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
Part IV | ||
Item 15. | ||
GLOSSARY OF TERMS AND ABBREVIATIONS | |
Abbreviation | Definition |
2019 Convertible Senior Notes | $245.0 million aggregate principal amount of convertible senior unsecured notes due 2019 |
ABSSSI | Acute bacterial skin and skin structure infections |
ADHF | Acute decompensated heart failure |
Amended ESPP | Employee Stock Purchase Plan, as amended and restated |
Amgen | Amgen, Inc. |
AML | Acute myeloid leukemia |
ANDA | Abbreviated New Drug Application |
AOCI | Accumulated Other Comprehensive Income |
API | Active pharmaceutical ingredient |
ASU | Accounting Standards Update |
Azure | Azure Biotech, Inc. |
BACE | Beta-secretase |
Baxter | Baxter International, Inc. |
BMS | Bristol Myers Squibb |
Cardioxyl | Cardioxyl Pharmaceuticals, Inc. |
CIT | Chemotherapy-induced thrombocytopenia |
CMC | Chemistry, Manufacturing and Controls |
Coherus Biosciences | Coherus Biosciences, Inc. |
CoM | Composition of Matter |
Company | Ligand Pharmaceuticals Incorporated, including subsidiaries |
COSO | Committee of Sponsoring Organizations of the Treadway Commission |
CRO | Contract Research Organization |
CURx | CURx Pharmaceuticals, Inc. |
CVR | Contingent value right |
CyDex | CyDex Pharmaceuticals, Inc. |
Deciphera | Deciphera Pharmaceuticals, LLC |
DMF | Drug Master File |
EC | European Commission |
Eli Lilly | Eli Lilly and Company |
EPOR | Erythropoietin receptor |
Ethicor | Ethicor Pharmaceuticals, Ltd |
EU | European Union |
FASB | Financial Accounting Standards Board |
FDA | Food and Drug Administration |
FSGS | Focal segmental glomerulosclerosis |
GCSF | Granulocyte-colony stimulating factor |
Hovione | Hovione FarmCiencia |
IND | Investigational New Drug |
IPR&D | In-Process Research and Development |
IRAK-4 | Interleukin-1 Receptor Associated Kinase-4 |
ITP | Chronic immune (idiopathic) thrombocytopenic purpura |
IV | Intravenous |
Ligand | Ligand Pharmaceuticals Incorporated, including subsidiaries |
LSA | Loan and Security Agreement |
LTP | Liver-targeted prodrug |
Lundbeck | Lundbeck A/S |
MDS | Myelodysplastic syndromes |
Melinta | Melinta Therapeutics, Inc. |
Merck | Merck & Co., Inc. |
Merrimack | Merrimack Pharmaceuticals, Inc. |
Millenium | Millenium Pharmaceuticals, Inc. |
MLA | Master License Agreement |
MRSA | Methicillin-resistant Staphylococcus aureu |
NASH | Non-alcoholic steatohepatitis |
NDA | New Drug Application |
NOLs | Net Operating Losses |
OMT | OMT, Inc. or Open Monoclonal Technology, Inc. |
Omthera | Omthera Pharmaceuticals, Inc. |
Orange Book | Publication identifying drug products approved by the FDA based on safety and effectiveness |
Par | Par Pharmaceutical, Inc. |
Pfizer | Pfizer Inc. |
Retrophin | Retrophin Inc. |
SAA | Severe Aplastic Anemia |
SAGE | Sage Therapeutics, Inc. |
SARM | Selective Androgen Receptor Modulator |
Sedor | Sedor Pharmaceuticals, Inc., or RODES, Inc. |
Selexis | Selexis, SA |
Sermonix | Sermonix Pharmaceuticals, LLC |
Spectrum | Spectrum Pharmaceuticals, Inc. |
SRSE | Super-refractory status epilepticus |
Takeda | Takeda Pharmaceuticals Company Limited |
TG Therapeutics | TG Therapeutics, Inc. |
TPE | Third-party evidence |
TR-β | Thyroid hormone receptor beta |
VentiRx | VentiRx Pharmaceuticals Inc. |
VIE | Variable interest entity |
Viking | Viking Therapeutics |
Viking IPO | Viking's initial public offering |
VSOE | Vendor-specific objective evidence |
X-ALD | X-linked adrenoleukodystrophy |
Zydus Cadila | Zydus Cadila Healthcare Ltd |
PART I
Cautionary Note Regarding Forward-Looking Statements:
You should read the following together with the more detailed information regarding our company, our common stock and our financial statements and notes to those statements appearing elsewhere in this document or incorporated by reference.
This report contains forward-looking statements that involve a number of risks and uncertainties. Although our forward-looking statements reflect the good faith judgment of our management, these statements can only be based on facts and factors currently known by us. Consequently, these forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from results and outcomes discussed in the forward-looking statements.
Forward-looking statements can be identified by the use of forward-looking words such as “believes,” “expects,” “may,” “will,” “plan,” “intends,” “estimates,” “would,” “continue,” “seeks,” “pro forma,” or “anticipates,” or other similar words (including their use in the negative), or by discussions of future matters such as those related to our royalties and milestones under license agreements, Capitsol materials sales, and product development, as well as other statements that are not historical. You should be aware that the occurrence of any of the events discussed under the caption “Risk Factors” could negatively affect our results of operations and financial condition and the trading price of our stock.
The cautionary statements made in this report are intended to be applicable to all related forward-looking statements wherever they may appear in this report. We urge you not to place undue reliance on these forward-looking statements, which speak only as of the date of this report. Except as required by law, we assume no obligation to update our forward-looking statements, even if new information becomes available in the future. This caution is made under the safe harbor provisions of Section 21E of the Securities Exchange Act of 1934, as amended.
References to “Ligand Pharmaceuticals Incorporated,” “Ligand,” the “Company,” “we,” “our” and “us” include Ligand Pharmaceuticals Incorporated and our wholly-owned subsidiaries.
Trademarks
Our trademarks, trade names and service marks referenced herein include Ligand®, Captisol®, Captisol-enabled™, LTP technology™, OmniAb®, OmniMouse®, OmniRat® and OmniFlic®. All other trademarks, trade names and service marks including Conbriza®, Duavee®, Kyprolis®, Premarin®, Promacta®, Revolade®, SUREtechnology Platform™, and Viviant® are the property of their respective owners. Use or display by us of other parties’ trademarks, trade dress or products is not intended to and does not imply a relationship with, or endorsement or sponsorship of, us by the trademark or trade dress owners.
1
Item 1. | Business |
Overview
We are a biopharmaceutical company focused on developing and acquiring technologies that help pharmaceutical companies discover and develop medicines. Over our more than 25 year history, we have employed research technologies such as nuclear receptor assays, high throughput computer screening, formulation science, liver targeted pro-drug technologies and antibody discovery technologies to assist companies in their work toward securing prescription drug approvals. We currently have partnerships and license agreements with over 85 pharmaceutical and biotechnology companies, and over 140 different programs under license with us are currently in various stages of commercialization and development. We have contributed novel research and technologies for approved medicines that treat cancer, osteoporosis, fungal infections and low blood platelets, among others. Our partners have programs currently in clinical development targeting seizure, coma, cancer, diabetes, cardiovascular disease, muscle wasting, liver disease, and kidney disease, among others. We have over 500 issued patents worldwide, and over 300 currently pending patent applications.
We have assembled our large portfolio of fully-funded programs either by licensing our own proprietary drug development programs, licensing our platform technologies such as Captisol or OmniAb to partners for use with their proprietary programs, or acquiring existing partnered programs from other companies. Fully-funded programs are those for which our partners pay all of the development and commercialization costs. For our internal programs, we generally plan to advance drug candidates through early-stage drug development or clinical proof-of-concept.
Our business model creates value for stockholders by providing a diversified portfolio of biotech and pharmaceutical product revenue streams that are supported by an efficient and low corporate cost structure. Our goal is to offer investors an opportunity to participate in the promise of the biotech industry in a profitable, diversified and lower-risk business than a typical biotech company. Our business model is based on doing what we do best: drug discovery, early-stage drug development, product reformulation and partnering. We partner with other pharmaceutical companies to leverage what they do best (late-stage development, regulatory management and commercialization) to ultimately generate our revenue. We believe that focusing on discovery and early-stage drug development while benefiting from our partners’ development and commercialization expertise will reduce our internal expenses and allow us to have a larger number of drug candidates progress to later stages of drug development.
Our revenue consists of three primary elements: royalties from commercialized products, license and milestone payments and sale of Captisol material. In addition to discovering and developing our own proprietary drugs, we selectively pursue acquisitions to bring in new assets, pipelines, and technologies to aid in generating additional potential new revenue streams.
2015 Major Business Highlights for Ligand
Late-Stage Clinical Data
• | On December 5, 2015, Amgen announced The Lancet Oncology published results from the Phase 3 ENDEAVOR clinical trial evaluating Kyprolis plus dexamethasone versus Velcade (bortezomib) plus dexamethasone showing that patients with relapsed multiple myeloma treated with Kyprolis lived twice as long without their disease worsening. |
• | Melinta announced positive results from a Phase 3 study to evaluate delafloxacin against vancomycin + aztreonam for the treatment of patients with ABSSSI. |
• | SAGE announced initiation of a Phase 3 study designed to evaluate the safety of SAGE-547 in patients with SRSE. SAGE also announced SAGE-547 demonstrated a 77% response rate in evaluable patients with SRSE in a Phase 1/2 clinical trial. |
• | Spectrum published results from the pivotal clinical study for EVOMELA in the journal Biology of Blood and Marrow Transplantation. |
NDA Submissions, Approvals or Label Expansion for Products Ligand is Entitled to Royalties
• | FDA approved Promacta for the treatment of children six years and older with chronic immune thrombocytopenia who have had an insufficient response to corticosteroids, immunoglobulins or splenectomy. |
2
• | The European Commission approved Revolade (Promacta) for the treatment of adults with acquired SAA who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for hematopoietic stem cell transplantation. |
• | On January 21, 2016, Amgen announced that the FDA approved Kyprolis in combination with dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma who have received one to three lines of therapy. The FDA also approved Kyprolis as a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy, converting to full approval the initial accelerated approval Kyprolis received in July 2012 as a single agent. |
• | On November 19, 2015, Amgen announced the EC approval of Kyprolis in combination with lenalidomide and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. |
• | Zydus Cadila announced the approval and launch of Exemptia, a biosimilar of adalimumab, in India. Ligand gained rights to royalties on sales of Exemptia in the April 2013 Selexis royalty acquisition. |
Licensing Deals Ligand Entered into or Expanded in 2015
• | Worldwide agreement with Sanofi for SAR-125844, a Captisol-enabled program. |
• | Clinical-stage agreement with AiCuris GmbH & Co for an undisclosed anti-infective Captisol-enabled program. |
• | Expanded global license and supply agreements with SAGE to cover the use of Captisol in the development and commercialization of SAGE-689. |
• | License and supply agreement with Vireo Health for use of Captisol in the development and commercialization of cannabinoid-based medications. |
• | Global license and supply agreements with RODES, Inc. (now known as Sedor) for intramuscular (IM)/IV meloxicam, IM/IV fosphenytoin, and intranasal budesonide. |
• | Commercial supply agreement with Gilead Sciences to supply Captisol for use in developing a Captisol-enabled program directed against Ebola virus disease. |
• | Clinical use agreement with XTL Biopharmaceuticals to supply Captisol for use in in the formulation of its lead drug, hCDR1, for the treatment of systemic lupus erythematosus. |
• | License agreement with Sermonix Pharmaceuticals for the development and commercialization of oral lasofoxifene in the U.S. and additional territories. |
Acquisitions
• | Ligand acquired OMT in January 2016, conferring ownership of a large portfolio of licenses and the OmniAb platform, for $178 million in cash and stock. |
• | Ligand acquired financial rights to more than 15 additional development stage programs from Selexis for $4 million in cash. |
Other Highlights
• | Ligand announced results from a Phase 1b trial of LGD-6972 that demonstrated favorable safety, tolerability and pharmacokinetics in normal healthy volunteers and in subjects with type 2 diabetes mellitus. The trial results also demonstrated a robust, dose-dependent reduction of fasting plasma glucose. |
• | In connection with the Viking IPO, Ligand received an equity milestone of 3.4 million shares and invested an additional $9.0 million in the offering. Key programs licensed to Viking include VK5211 (SARM), VK2809/VK0214 (TRβ), VK0612 (FBPase), EPOR and DGAT-1. |
Technologies
A variety of technology platforms that enable elements of drug discovery or development form the basis of our portfolio of fully-funded shots on goal. Platform technologies or individual drugs discovered by Ligand are related to a broad estate of intellectual property that includes over 500 issued patents and over 300 pending patent applications.
3
Captisol Technology
Captisol is Ligand’s patented, uniquely-modified cyclodextrin that is specifically designed to maximize safety, while improving the solubility, stability and bioavailability of APIs. Captisol can enable faster and more efficient development paths for our partners, given its known regulatory acceptance. Ligand maintains both Type IV and Type V DMFs with the FDA. These DMFs contain manufacturing and safety information relating to Captisol that our licensees can reference when developing Captisol-enabled drugs. Ligand also filed a DMF in Japan in 2015. Captisol-enabled drugs are marketed in more than 60 countries, and over 45 partners have Captisol-enabled drugs in development.
OmniAb Technologies (OMT)
In January of 2016, Ligand acquired OMT and the OmniAb Technologies. OmniAb includes three complementary and globally-branded platforms named OmniRat, OmniMouse and OmniFlic. The OmniAb platforms consist of genetically-engineered transgenic rodents that produce a broadly diversified repertoire of antibodies and enable novel fully-human antibody drug discovery and development by our OmniAb partners. Fully-human OmniAb antibodies provide advantages to our partners in that fully-human antibodies have reduced immunogenicity, streamline development timelines and costs, and accelerate novel antibody discovery. Currently, more than 18 partners are utilizing OmniAb animals in their drug discovery and development efforts.
LTP Technology Platform
The LTP Technology platform is a novel prodrug technology designed to selectively deliver a broad range of pharmaceutical agents to the liver. A prodrug is a biologically inactive compound that can be metabolized in the body to produce an active drug. The LTP Technology works by chemically modifying biologically active molecules into an inactive prodrug, which will be administered to a patient and later activated by specific enzymes in the liver. The technology can be used to improve the safety and/or activity of existing drugs, develop new agents to treat certain liver-relayed diseases, and treat diseases caused by imbalances of circulating molecules that are controlled by the liver. The technology is especially applicable to metabolic and cardiovascular indications, among others. Currently 3 partners are utilizing the LTP Technology or related platform(s).
SUREtechnology Platform (owned by Selexis)
Ligand acquired economic rights to over 30 SUREtechnology Platform programs from Selexis in two separate transactions in 2013 and 2015, granting Ligand rights to downstream economics on novel biologics and biosimilars programs. The SUREtechnology Platform, developed and owned by Selexis, is a novel technology that improves the way that cells are utilized in the development and manufacturing of recombinant proteins and drugs. The technology is based on novel DNA-based elements that control the dynamic organization of chromatin within mammalian cells and allow for higher and more stable expression of recombinant proteins. The technology creates advantages over traditional approaches including accelerated development and manufacturing times, high yields and increased compound stability.
4
Partners and Licensees
The following table lists our disclosed partners and licensees. In addition to these 70 Companies, we have over 15 additional undisclosed partners and licensees, mostly biotech companies.
Big Pharma | Ticker | Generics | Ticker | Biotech, continued | Ticker | ||
AstraZeneca | AZN | Alvogen | Private | Genmab | Private | ||
Baxter | BAX | Avion | Private | Gilead Sciences | GILD | ||
BMS | BMY | BioCad | Private | Hanall | Private | ||
Daiichi Sankyo | DSKY | Coherus | Private | Harpoon | Private | ||
Eli Lilly | LLY | Gedeon Richter | Private | Lubris | Private | ||
GSK | GSK | IBC Generium | Private | Marinus | MRNS | ||
Janssen | JNJ | Oncobiologics | Private | MEI | MEIP | ||
Merck | MRK | Zydus Cadila | CADILAHC | Melinta | Private | ||
Merck KGaA | MRK | Meridian Labs | Private | ||||
Novartis | NVS | Biotech | Ticker | Millennium | Private | ||
Otsuka | 4768 | AiCuris | Private | Merrimack | MACK | ||
Pfizer | PFZ | Aldeyra | ALDX | Novogen | NVGN | ||
Sanofi | SNY | Amgen | AMGN | Opthea | Private | ||
Takeda | 4502 | ARMO | Private | Precision Biologics | Private | ||
Azure | Private | Retrophin | RTRX | ||||
bluebird bio | BLUE | ROAR | Private | ||||
Cantex | Private | SAGE | SAGE | ||||
Specialty Pharmaceutical | Ticker | Celgene | CELG | Seattle Genetics | SGEN | ||
Cuda | Private | Chiva | Private | Stemcentrx | Private | ||
Ethicor | Private | CURx | Private | Symphogen | Private | ||
Lundbeck | LUN | Deciphera | Private | TG Therapeutics | TGTX | ||
Sedor | Private | Emergent Biosolutions | EBS | Tizona | Private | ||
Sermonix | Private | Exelixis | EXC | VentiRx | Private | ||
Spectrum | SPPI | Five Prime | FRPX | Viking | VKTX | ||
Vireo Health | Private | ForSight Vision | Private | XTL Bio | XTLB | ||
Upsher-Smith | Private | F-Star | Private | WuXi | Private | ||
5
Portfolio
We have a large portfolio of current and future potential revenue-generating programs, over 140 of which are fully-funded by our partners. In addition to the table below, we also have more than 40 undisclosed programs.
Commercialized | Phase 2 | Pre-Clinical | |||||
Novartis | Promacta | Retrophin | Sparsentan | Viking | EPOR Agonist | ||
Amgen | Kyprolis | Eli Lilly | LY2606368 | Viking | DGAT-1 Inhibitor | ||
Pfizer | Viviant/Conbriza | VentiRx | VTX-2337 | Sedor | CE-Meloxicam | ||
Pfizer | Duavee | CURx | IV Topiramate | Meridian Labs | ML-061 | ||
Baxter | Nexterone | Millennium/Takeda | MLN-4924 | Upsher Smith | CXCR4 | ||
Merck | Noxafil-IV | Viking | VK0612 | Azure | Lasofoxifene | ||
Zydus Cadila | Exemptia | Cantex | ODSH | SAGE | SAGE-689 | ||
Zydus Cadila | Vivitra | Merrimack | MM-121 | TG Therapeutics | IRAK4 | ||
Pfizer | Vfend | Merrimack | MM-141 | Marinus | Ganaxalone IV | ||
Lubris | Lubricin | Cuda | CE-Propofol | ||||
Regulatory Submission Stage | Cardioxyl / BMS | CXL-1427 | CURx | IV Lamotrigine | |||
Lundbeck | Carbella | Exelixis/Daiichi | CS-3150 | Exelixis (BMS) | XL652 | ||
Alvogen | Voriconazole | Precision Biologics | NPC-1C | Omthera/AZ | LTP-O3FA | ||
Spectrum | Evomela | Viking | VK5211 | Novogen | Cantrixil | ||
Sermonix | Lasofoxifene | Viking | TR Beta | Oncobiologics | Rituximab | ||
Ethicor | Fablyn | Aldeyra | NS-2 | Oncobiologics | ONS4010 | ||
Sedor | CE-Fosphenytoin | Novartis | 5921 | AiCuris GmBH | Undisclosed | ||
Baxter | BAX-69 | Vireo Health | CE-Cannabinoids | ||||
Phase 3 | Biocad | BCD-066 | XTL Bio | hCDR1 | |||
Melinta | Baxdela | Sanofi | SAR125844 | Amgen | OmniAb | ||
Merck | Verubecestat | ARMO | OmniAb | ||||
Coherus | CHS-0214 | Phase 1 | Celgene | OmniAb | |||
Oncobiologics | ONS-3010 | Sedor | CE-Budesonide | Emergent Bio | OmniAb | ||
Oncobiologics | ONS-1045 | MEI | ME-344 | Five Prime | OmniAb | ||
SAGE | SAGE-547 | MEI | ME-143 | Genmab | OmniAb | ||
Merrimack | MM-302 | Merrimack | MM-151 | Hanall | OmniAb | ||
Gedeon Richter | RGB-03 | Janssen | OmniAb | ||||
Gedeon Richter | Bevacizumab | Merck KGaA | OmniAb | ||||
Gedeon Richter | Trastuzumab | Pfizer | OmniAb | ||||
Biocad | Interferon beta-1a | Seattle Genetics | OmniAb | ||||
Biocad | EPOR Agonist | Stemcentrx | OmniAb | ||||
Chiva | Pradefovir | Symphogen | OmniAb | ||||
Chiva | MB07133 | Tizona | OmniAb | ||||
Deciphera | Altiratinib | WuXi | OmniAb | ||||
Color Legend | VentiRx | VTX-1463 | |||||
Blood Disorders | Takeda | TAK-020 | |||||
Cardiovascular | Otsuka | OPC-269 | |||||
Central Nervous System | ROAR | UC-961 | |||||
Infectious Disease | Opthea | OPT-302 | |||||
Inflammation/Metabolic | F-Star | F-102 | |||||
Severe and Rare | IBC Generium | GNR-008 | |||||
Cancer | IBC Generium | Deplera | |||||
Other / Undisclosed | Gilead | GS-5734 | |||||
6
Commercial Programs
We have multiple programs under license with other companies that have products that are already being commercialized. The following programs represent components of our current portfolio of revenue-generating assets and potential for near-term growth in royalty and other revenue. For information about the royalties owed to Ligand for these programs, see “Royalties” later in this business section.
Promacta (Novartis)
We are party to a license agreement with Novartis related to Promacta, which is an oral medicine that increases the number of platelets in the blood. Platelets are one of the three components of blood and facilitate clotting in the blood. Individuals with low platelets can be at significant risk of bleeding or death. Because of the importance of having a sufficient number of platelets, Promacta has broad potential applicability to a number of medical situations where low platelets exist.
Promacta is currently approved for three indications: (1) the treatment of thrombocytopenia in patients with ITP who have had an insufficient response to corticosteroids, immunoglobulins or splenectomy, (2) Hepatitis-C associated thrombocytopenia and (3) SAA. Promacta was initially approved in 2008, and the product has been generating royalty revenue for Ligand since 2009. Promacta is known as Revolade in the EU and other non-US markets.
Novartis has been and continues to pursue globalization of the brand and currently markets Promacta in multiple countries for the three approved indications. Specifically, ITP is currently approved in more than 100 countries, the Hepatitis C-related indication is currently approved in more than 50 countries, and the SAA indication is approved in more than 30 counties.
Beyond the currently-approved indications, Novartis is also performing development activities to expand the brand into new indications, including a number of oncology-related indications including MDS, AML and CIT. As of February 2016, there are 42 open clinical trials related to Promacta (listed as recruiting or open, and not yet recruiting) on the clinicaltrials.gov website.
We are entitled to receive royalties related to Promacta during the life of the relevant patents or at a reduced rate for ten years from the first commercial sale, whichever is longer, on a country-by-country basis. Novartis has listed a patent in the FDA’s, Orange Book for Promacta with an expiration date in 2027, and absent early termination for bankruptcy or material breach, the term of the agreement expires upon expiration of the obligation to pay royalties. There are no remaining milestones to be paid under the agreement.
Kyprolis (Amgen)
Ligand supplies Captisol to Amgen for use with carfilzomib, and granted an exclusive product-specific license under our patent rights with respect to Captisol. Kyprolis is formulated with Ligand’s Captisol technology and is approved in the U.S. for the following:
• | In combination with dexamethasone or with lenalidomide plus dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma who have received one to three lines of therapy. |
• | As a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy. |
Kyprolis is also approved in Argentina, Israel, Kuwait, Mexico, Thailand, Columbia, Korea, Canada and the European Union. Kyprolis was initially approved in the U.S. in 2012, and Amgen continues to invest significantly in Kyprolis to further expand its label and geography.
Amgen’s obligation to pay royalties does not expire until four years after the expiration of the last-to-expire patent covering Captisol. Our patents and applications relating to the Captisol component of Kyprolis are not expected to expire until 2033. Our agreement with Amgen may be terminated by either party in the event of material breach or bankruptcy, or unilaterally by Amgen with prior written notice, subject to certain surviving obligations. Absent early termination, the agreement will terminate upon expiration of the obligation to pay royalties. Under this agreement, we are entitled to receive remaining milestones of up to $2.3 million, revenue from clinical and commercial Captisol material sales and royalties on annual net sales of Kyprolis.
7
Duavee or Duavive (bazedoxifene/conjugated estrogens) and Viviant/Conbriza (Pfizer)
Pfizer is marketing bazedoxifene under the brand names Viviant and Conbriza in various territories for the treatment of postmenopausal osteoporosis. Pfizer is responsible for the registration and worldwide marketing of bazedoxifene, a synthetic drug specifically designed to reduce the risk of osteoporotic fractures while also protecting uterine tissue. Pfizer has combined bazedoxifene with the active ingredient in Premarin to create Duavee, a combination therapy for the treatment of post-menopausal symptoms in women. Duavee is approved in the United States and it is anticipated that it will be marketed under the brand name Duavive in the EU. Net royalties on annual net sales of Viviant/Conbriza and Duavee/Duavive are each payable to us through the life of the relevant patents or ten years from the first commercial sale, whichever is longer, on a country by country basis.
Nexterone (Baxter)
We have a license agreement with Baxter, related to Baxter's Nexterone, a Captisol-enabled formulation of amiodarone, which is marketed in the United States and Canada. We supply Captisol to Baxter for use in accordance with the terms of the license agreement under a separate supply agreement. Under the terms of the license agreement we will continue to earn milestone payments, royalties, and revenue from Captisol material sales. We are entitled to earn royalties on sales of Nexterone through early 2033.
Noxafil-IV (Merck)
We have a supply agreement with Merck related to Merck’s NOXAFIL-IV, a Captisol-enabled formulation of posaconazole for IV use. NOXAFIL-IV is marketed in the United States, EU and Canada. We receive our commercial compensation for this program through the sale of Captisol, and we do not receive a royalty on this program.
Exemptia (Zydus Cadila)
Our partner, Zydus Cadila’s Exemptia (adalimumab biosimilar) is marketed in India for autoimmune diseases. Zydus Cadila uses the Selexis technology platform for Exemptia. We are entitled to earn royalties on sales by Zydus Cadila through at least 2026.
Vivitra (Zydus Cadila)
Our partner, Zydus Cadila’s Vivitra (trastuzumab biosimilar) is marketed in India for breast cancer. Zydus Cadila uses the Selexis technology platform for Vivitra. We are entitled to earn royalties on sales by Zydus Cadila through at least 2026.
Summary of Selected Development-stage Programs
We have multiple fully-funded partnered programs that are either in or nearing the regulatory approval process, or given the area of research or value of the license terms are considered particularly noteworthy. We are eligible to receive milestone payments and royalties off of these programs. For information about the royalties owed to Ligand for these programs, see “Royalties” later in this Business Overview section. In the case of Captisol-related programs, we are also eligible to receive revenue for the sale of Captisol material supply.
Evomela (Spectrum)
We have a license agreement with Spectrum related to Evomela, which is a Captisol-enabled melphalan IV formulation. In December 2014, Spectrum submitted a NDA to the FDA. In October 2015, Spectrum announced that it had received a complete response letter from the FDA requiring additional information regarding its contract manufacturers. Spectrum has indicated that next FDA action date is May 2016. Evomela is intended for use in the multiple myeloma stem cell transplant setting, and has been granted Orphan Designation by the FDA. The Evomela formulation avoids the use of propylene glycol, which has been reported to cause renal and cardiac side-effects that limit the ability to deliver higher quantities of therapeutic compounds. The use of the Captisol technology to reformulate melphalan is anticipated to allow for longer administration durations and slower infusion rates, potentially enabling clinicians to safely achieve a higher dose intensity of pre-transplant chemotherapy.
Under the terms of the license agreement, we granted an exclusive license to Spectrum under our patent rights to Captisol relating to the product. We are eligible to receive over $50 million in potential milestone payments under this
8
agreement and royalties on future net sales of the Captisol-enabled melphalan product. Spectrum’s obligation to pay royalties will expire at the end of the life of the relevant patents or when a competing product is launched, whichever is earlier, but in no event within ten years of the commercial launch. Our patents and applications relating to the Captisol component of melphalan are not expected to expire until 2033. Absent early termination, the agreement will terminate upon expiration of the obligation to pay royalties. The agreement may be terminated by either party for an uncured material breach or unilaterally by Spectrum by prior written notice.
Verubecestat (Merck)
Our partner, Merck is conducting two Phase 3 trials for Verubecestat (MK-8931), a BACE inhibitor for the treatment of Alzheimer’s disease. Alzheimer’s disease is characterized by plaques of amyloid-beta protein within the brain. BACE is believed to be a key enzyme in the production of amyloid-beta protein. Amyloid-beta is formed when the larger amyloid precursor protein is cleaved by two enzymes, BACE and gamma-secretase, which releases the amyloid-beta fragment. A BACE inhibitor is expected to reduce amyloid-beta generation in Alzheimer’s disease patients. Merck expects initial data from Phase 3 trials in mid-2017. We are entitled to a royalty on potential future sales by Merck. Merck is responsible for all development costs related to the program.
SAGE-547 (SAGE)
Our partner, SAGE, is conducting a Phase 3 clinical trial for the development of Captisol-enabled therapeutics for a broad range of debilitating central nervous system conditions. SAGE’s lead clinical program, Captisol-enabled SAGE-547 is an allosteric modulator of both synaptic and extra-synaptic GABAA receptors that is in clinical development as an adjunctive therapy, a therapy combined with current therapeutic approaches, for the treatment of SRSE. SAGE-547 was granted Fast Track designation, which is intended to facilitate the development and expedite the review of drug candidates that are intended to treat serious or life-threatening conditions and demonstrate the potential to address unmet medical needs, and orphan drug designation, which is intended to facilitate drug development for rare diseases, by the FDA for SRSE. Ligand has the potential to receive milestone payments, royalties and revenue from Captisol material sales for Captisol-enabled programs. SAGE is responsible for all development costs related to the program.
Sparsentan (Retrophin)
Our partner Retrophin is currently conducting a Phase 2 clinical trial for the development of Sparsentan for orphan indications of severe kidney diseases including FSGS. Certain patient groups with severely compromised renal function exhibit extreme proteinuria resulting in progression to dialysis and a high mortality rate. Sparsentan, with its unique dual blockade of angiotensin and endothelin receptors, is expected to provide meaningful clinical benefits in mitigating proteinuria in indications where there are no approved therapies. In January 2015, the FDA granted Sparsentan orphan drug designation.
Under our license agreement with Retrophin we are entitled to receive potential net milestones of over $75 million in the future and net royalties on future worldwide sales by Retrophin through the life of the relevant patents, which we currently expect to be through at least 2019 and may be extended until 2024. Retrophin is responsible for all development costs related to the program.
Baxdela (Melinta)
Our partner Melinta is currently completing Phase 3 clinical trials for the development of Baxdela, a Captisol-enabled delafloxacin-IV. Delafloxacin is a novel hospital-focused fluoroquinolone antibiotic candidate with potency against a variety of quinolone-resistant Gram-positive and Gram-negative bacteria, including quinolone-resistant MRSA. In 2015, Melinta reported positive top-line results on the first of two planned Phase 3 clinical trials of delafoxacin for the treatment of ABSSSI, including infections caused by MRSA. Under the terms of the agreement, we may be entitled to up to $3.6 million of development and regulatory milestones, a royalty on potential future sales by Melinta, and revenue from Captisol material sales. Melinta is responsible for all development costs related to the program.
Carbamazepine-IV (Lundbeck)
Lundbeck's Carbella is a Captisol-enabled carbamazepine-IV currently under review by the FDA. Carbella is for the management of acute seizure disorder for hospital or emergency settings. Lundbeck is in the process of responding
9
to a request of CMC data from the FDA’s Complete Response Letter received in late 2014. Under the terms of our agreement with Lundbeck, we may be entitled to development and regulatory milestones, royalties on potential future sales by Lundbeck and revenue from Captisol material sales. Lundbeck is responsible for all development costs related to the program.
SARM - VK5211 (Viking)
Our partner Viking is developing VK5211, a novel, potentially best-in-class SARM for patients recovering from hip-fracture. SARMs retain the beneficial properties of androgens without undesired side-effects of steroids or other less selective androgens. Viking initiated a Phase 2 trial in hip fracture in 2015. Under the terms of the agreement with Viking, we may be entitled to up to $270 million of development, regulatory and commercial milestones and tiered royalties on potential future sales.
TR-β - VK2809 (Viking)
Viking is developing VK2809, a novel selective TR-β agonist with potential in multiple indications, including hypercholesterolemia, dyslipidemia, NASH, and X-ALD. Viking intends to initiate a Phase 2 trial for VK2809 in hypercholesterolemia and fatty liver disease in 2016. Under the terms of the agreement with Viking, we may be entitled to up to $375 million of development, regulatory and commercial milestones and tiered royalties on potential future sales.
IRAK4 Inhibitor Program (TG Therapeutics)
Our partner, TG Therapeutics is developing our IRAK-4 inhibitors. The IRAK-4 program is in preclinical development for potential use in certain cancers and autoimmune diseases. Under the terms of the agreement we are eligible to receive $207 million in potential milestone payments. We are also eligible to receive royalties on future net sales of licensed products containing patented IRAK-4 inhibitors. TG Therapeutics will be responsible for all development costs related to the program.
Topiramate IV (CURx)
The FDA granted our partner, CURx, orphan-drug designation for a proprietary Captisol-enabled Topiramate Injection formulation for the treatment of partial onset or primary generalized tonic-clonic seizures in hospitalized epilepsy patients who are unable to take oral topiramate. Under the terms of our agreement, CURx may be required to pay us an aggregate of $19.6 million, net of amounts owed to third parties upon the achievement of specified milestones. Additionally, we are owed net royalties on future sales. CURx will be responsible for all development costs related to the program.
Lasofoxifene (Azure Biotech, Ethicor, and Sermonix)
Our partner Azure is developing a novel formulation of lasofoxifene. Under the terms of our agreement with Azure, we are entitled to receive up to $2.6 million in potential development and regulatory milestones as well as royalties on future net sales through the later of the life of the relevant patents (currently expected to be at least until 2027) or 10 years after regulatory approval. Azure may terminate the license agreement at any time upon six months’ prior notice.
Lasofoxifene is an estrogen partial agonist for osteoporosis treatment and other diseases, discovered through the research collaboration between us and Pfizer. Under the terms of the license agreement with Azure, we retained the rights to the oral formulation of lasofoxifene originally developed by Pfizer.
Our partner, Ethicor has an agreement with us for the manufacture and distribution of the oral formulation of lasofoxifene in the European Economic Area, Switzerland and the Indian Subcontinent. Under the terms of the agreement, we are entitled to receive potential sales milestones of up to $16 million and royalties on future net sales. Ethicor plans to supply oral lasofoxifene as an unlicensed medicinal product, which may be requested by healthcare professionals to meet the clinical needs of patients when authorized medicines are unsuitable or contraindicated.
Our partner, Sermonix has a license for the development of oral lasofoxifene for the United States and additional territories. Under the terms of the agreement, we are entitled to receive up to $45 million in potential regulatory and commercial milestone payments and royalties on future net sales.
10
SAR-125844 (Sanofi)
Our partner, Sanofi licensed Captisol for use in the development of Captisol-enabled SAR-125844, a potent MET kinase inhibitor. Under the terms of the agreement, we are eligible to receive potential milestone payments, royalties on future net sales and revenue from Captisol material sales. Sanofi will be responsible for all development costs related to the program. SAR-125844 is a potent, selective and reversible ATP-competitive MET tyrosine kinase inhibitor for IV administration. SAR-125844 recently completed a first-in-human, open-label, non-randomized, single agent, Phase 1 study in advanced/refractory solid tumor patients.
CHS-0214 (Coherus Biosciences)
Our partner, Coherus Biosciences is conducting Phase 3 / BLA-enabling clinical trials for CHS-0214 (etanercept biosimilar) for rheumatoid arthritis. Coherus uses the Selexis’ technology platform for CHS-0214. We are entitled to earn regulatory and sales milestones, and royalties on potential future sales through at least 2026.
CXL-1427 (Cardioxyl /BMS)
Our partner, Cardioxyl (acquired by BMS in 2015) is conducting Phase 2 clinical trials for Captisol-enabled CXL-1427 (nitroxyl donor prodrug) for ADHF. Under the terms of the agreement, we may be entitled to development and regulatory milestones, and royalties on potential future sales by BMS and revenue from Captisol material sales.
LY2606368 (Eli Lilly)
Our partner, Eli Lilly is conducting Phase 2 clinical trials for Captisol-enabled LY2606368 (Chk 1/2 inhibitor) for solid tumors. Under the terms of the agreement, we may be entitled to regulatory milestones, royalties on potential future sales by Eli Lilly and revenue from Captisol material sales.
Altiratinib (Deciphera Pharmaceuticals)
Our partner, Deciphera Pharmaceuticals is currently conducting Phase 1 trials for the development of Altiratinib for the treatment of solid tumors. Altiratinib is a Captisol-enabled MET/TIE2/VEGF2/TRK (A,B,C) kinase inhibitor. Under the terms of the clinical-stage agreement, we may be entitled to development milestones from Deciphera and revenue from Captisol material sales.
MM-302 (Merrimack Pharmaceuticals)
Our partner, Merrimack Pharmaceuticals is currently conducting a Phase 2/3 trial for the treatment of advanced metastatic HER2-positive breast cancer. MM-302 is an antibody-drug conjugated liposomal doxorubicin that was developed using the Selexis SUREtechnology Platform. Under the terms of the agreement, we may be entitled to development and commercial milestones, royalties on potential future sales.
Motolimod - VTX-2337 (VentiRx Pharmaceuticals/Celgene)
Our partner, VentiRx is currently conducting Phase 2 trials for the development of Motolimod for the treatment of ovarian cancer and head and neck cancer. Motolimod is a Captisol-enabled Toll-like Receptor 8 agonist. Motolimod was granted Fast Track and Orphan Designations by the FDA for the treatment of recurrent or persistent ovarian cancer. VentiRx has an exclusive worldwide collaboration with Celgene to develop VTX-2337. Under the terms of the clinical-stage agreement, we have earned development milestones from VentiRx and revenue from Captisol material sales.
Pevonedistat - MLN-4924 (Millennium/Takeda)
Our partner, Millennium/Takeda is currently conducting Phase 2 trials for the development of Pevonedistat for the treatment of hematological malignancies and solid tumors. Pevonedistat is a Captisol-enabled Nedd8-Activating Enzyme Inhibitor. Under the terms of the clinical-stage agreement, we may be entitled to development milestones from Millennium/Takeda and revenue from Captisol material sales.
11
Royalty Table
Ligand Licenses With Tiered Royalties, Tiers Disclosed* | |||||||||||||
Promacta (Novartis) | Kyprolis (Amgen) | Duavee (Pfizer) | Viviant/Conbriza (Pfizer) | ||||||||||
< $100 million | 4.7% | < $250 million | 1.5% | <$400 million | 0.5% | <$400 million | 0.5% | ||||||
$100 to $200 million | 6.6% | $250 to $500 million | 2.0% | $400 million to $1.0 billion | 1.5% | $400 million to $1.0 billion | 1.5% | ||||||
$200 to $400 million | 7.5% | $500 to $750 million | 2.5% | >$1.0 billion | 2.5% | >$1.0 billion | 2.5% | ||||||
$400 million to $1.5 billion | 9.4% | >$750 million | 3.0% | ||||||||||
>$1.5 billion | 9.3% | ||||||||||||
CE-Topiramate (CURx) | CE-Budesonide (Sedor) | CE-Meloxicam (Sedor) | |||||||
<$50 million | 6% | < $25 million | 8% | < $25 million | 8% | ||||
$50 to $100 million | 6.75% | >$25 million | 10% | >$25 million | 10% | ||||
>$100 million | 7.5% | ||||||||
Ligand Licenses With Tiered Royalties, Tiers Undisclosed* | ||
Program | Licensee | Royalty Rate |
IRAK4 | TG Therapeutics | 6.0% - 9.5% |
CE-Lamotrigine | CURx | 4.0% - 7.0% |
Lasofoxifene | Sermonix | 6.0% - 10.0% |
FBPase Inhibitor | Viking | 7.5% - 9.5% |
SARM | Viking | 7.25% - 9.25% |
TR Beta | Viking | 3.5% - 7.5% |
Oral EPO | Viking | 4.5% - 8.5% |
DGAT-1 | Viking | 3.0% - 7.0% |
LTP-O3FA | Omthera/AstraZeneca | Tiered mid-to-high single digit royalties |
Ligand Licenses With Fixed Royalties* | ||
Program | Licensee | Royalty Rate |
EVOMELA | Spectrum Pharma | 20.0% |
Baxdela | Melinta | 2.5% |
SAGE-547 | SAGE | 3.0% |
Sparsentan (RE-021) | Retrophin | 9.0% |
CE-Fosphenytoin | Sedor | 11.0% |
Pradefovir | Chiva Pharma | 9.0% |
MB07133 | Chiva Pharma | 6.0% |
Fablyn | Ethicor | 25.0% |
'5921 | Novartis | 14.5% (6.5% in year one) |
Topical lasofoxifene | Azure Biotech | 5.0% |
MM-121 | Merrimack Pharma | <1.0% |
MM-302 | Merrimack Pharma | <1.0% |
MM-151 | Merrimack Pharma | <1.0% |
MM-141 | Merrimack Pharma | <1.0% |
ME-143 | MEI Pharma | Low single digit royalty |
ME-344 | MEI Pharma | Low single digit royalty |
NS-2 | Aldeyra Therapeutics | Low single digit royalty |
*Royalty rates are shown net of sublicense payments. Royalty tier references for specific rates notated in the table are for up to and including the dollar amount referenced. Higher tiers are only applicable for the dollar ranges specified in the table.
12
Primary Internal Development Program - Glucagon Receptor Antagonist Program
We are currently developing a small molecule glucagon receptor antagonist for the treatment of Type 2 diabetes mellitus. Compounds that block the action of glucagon may reduce the hyperglycemia that is characteristic of the disease. Glucagon stimulates the production of glucose by the liver and its release into the blood stream. In diabetic patients, glucagon secretion is abnormally elevated and contributes to hyperglycemia in these patients. We conducted a Phase 1b trial showing robust effects throughout multiple ascending dosing, and plan to initiate a Phase 2 clinical trial in 2016.
The following table represents other internal programs eligible for further development funding, either through Ligand or a partner:
Program | Development Stage | Indication | ||
GCSF Receptor Agonist | Preclinical | Blood disorders | ||
Captisol-enabled Clopidogrel | Phase 3 | Anti-coagulant | ||
Captisol-enabled Busulfan | Preclinical | Oncology | ||
Captisol-enabled Acetaminophen Injection | Preclinical | Pain | ||
Captisol-enabled Sertraline, Oral Concentrate | Phase 1 | Depression | ||
Captisol-enabled Cetirizine Injection | Preclinical | Allergy | ||
Captisol-enabled Silymarin for Topical formulation | Preclinical | Sun damage | ||
Aplindore | Phase 2 | Restless Leg/Parkinson's | ||
Histamine H3 Receptor Antagonist | Preclinical | Cognitive Disorders | ||
Liver Specific Glucokinase Activator | Preclinical | Diabetes | ||
CCR1 Antagonist | Preclinical | Oncology | ||
CRTH2 Antagonist | Preclinical | Inflammation | ||
FLT3 Kinase Inhibitors | Preclinical | Oncology | ||
Manufacturing
We currently have no manufacturing facilities and rely on a third party, Hovione, for Captisol production. Hovione is a global supplier with over 50 years of experience in the development and manufacture of APIs and Drug Product Intermediates. Hovione operates FDA-inspected sites in the United States, Macau, Ireland and Portugal. Manufacturing operations for Captisol are currently performed in both of Hovione's Portugal and Ireland sites with distribution operations also performed from Hovione's Portugal and Ireland sites.
We have ongoing minimum purchase commitments under the agreement and are required to pay Hovione an aggregate minimum amount during the agreement term.
In the event of a Captisol supply interruption, we are permitted to designate and, with Hovione’s assistance, qualify one or more alternate suppliers. If the supply interruption continues beyond a designated period, we may terminate the agreement. In addition, if Hovione cannot supply our requirements of Captisol due to an uncured force majeure event or if the unit price of Captisol exceeds a set figure, we may obtain Captisol from a third party.
The current term of the agreement with Hovione is through December 2019. The agreement will automatically renew for successive two year renewal terms unless either party gives written notice of its intention to terminate the agreement no less than two years prior to the expiration of the initial term or renewal term. In addition, either party may terminate the agreement for the uncured material breach or bankruptcy of the other party or an extended force majeure event. We may terminate the agreement for extended supply interruption, regulatory action related to Captisol or other specified events. For further discussion of these items, see below under “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
13
Competition
Some of the drugs we and our licensees are developing may compete with existing therapies or other drugs in development by other companies. Furthermore, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competing products or technologies and may establish collaborative arrangements with our competitors.
Existing or potential competitors to our licensee’s products, particularly large pharmaceutical companies, may have greater financial, technical and human resources than our licensees. Accordingly, these competitors may be better equipped to develop, manufacture and market products. Many of these companies also have extensive experience in preclinical testing and human clinical trials, obtaining FDA and other regulatory approvals and manufacturing and marketing pharmaceutical products.
Our Captisol business may face competition from other suppliers of similar cyclodextrin excipients or other technologies that are aimed to increase solubility or stability of APIs. Our OmniAb antibody technology faces competition from suppliers of other transgenic animal systems that are also available for antibody drug discovery.
Our competitive position also depends upon our ability to obtain patent protection or otherwise develop proprietary products or processes. For a discussion of the risks associated with competition, see below under “Item 1A. Risk Factors.”
Government Regulation
The research and development, manufacturing and marketing of pharmaceutical products are subject to regulation by numerous governmental authorities in the United States and other countries. We and our partners, depending on specific activities performed, are subject to these regulations. In the United States, pharmaceuticals are subject to regulation by both federal and various state authorities, including the FDA. The Federal Food, Drug and Cosmetic Act and the Public Health Service Act govern the testing, manufacture, safety, efficacy, labeling, storage, record keeping, approval, advertising and promotion of pharmaceutical products and there are often comparable regulations that apply at the state level. There are similar regulations in other countries as well. For both currently marketed and products in development, failure to comply with applicable regulatory requirements can, among other things, result in delays, the suspension of regulatory approvals, as well as possible civil and criminal sanctions. In addition, changes in existing regulations could have a material adverse effect on us or our partners. For a discussion of the risks associated with government regulations, see below under “Item 1A. Risk Factors.”
Patents and Proprietary Rights
We believe that patents and other proprietary rights are important to our business. Our policy is to file patent applications to protect technology, inventions and improvements to our inventions that are considered important to the development of our business. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position.
Patents are issued or pending for the following key products or product families. The scope and type of patent protection provided by each patent family is defined by the claims in the various patents. The nominal patent expiration dates have been provided. The actual patent term may vary by jurisdiction and depend on a number of factors including potential patent term adjustments, patent term extensions, and terminal disclaimers. For each product or product family, the patents and/or applications referred to are in force in at least the United States, and for most products and product families, the patents and/or applications are also in force in European jurisdictions, Japan and other jurisdictions.
Promacta
Patents covering Promacta are owned by Novartis. The United States patent listed in the FDA’s Orange Book relating to Promacta with the latest expiration date is not expected to expire until 2027. Six months of additional exclusivity has been granted due to pediatric studies conducted by GSK. The type of patent protection (e.g., composition of matter or use) for each patent listed in the Orange Book and the expiration date for each patent listed in the Orange Book are provided in the following table. In addition, certain related patents in the commercially important jurisdictions of Europe and Japan are identified in the following table.
14
Promacta | |||||
United States | Corresponding Foreign | ||||
Type of Protection | U.S. Patent No. | U.S. Expiration Date | Jurisdiction | Patent Number | Expiration Date‡ |
CoM / Use | 6,280,959 | 10/30/2018 | N/A | ||
CoM / Use | 7,160,870 | 11/20/2022 | EU | 1,864,981 | 5/24/21 |
EU | 1,294,378 | 5/24/21 | |||
Japan | 3,813,875 | 5/24/21 | |||
Use | 7,332,481 | 5/24/2021 | EU | 1,889,838 | 5/24/21 |
Japan | 4,546,919 | 5/24/21 | |||
CoM / Use | 7,452,874 | 5/24/2021 | EU | 1,889,838 | 5/24/21 |
Japan | 4,546,919 | 5/24/21 | |||
CoM / Use | 7,473,686 | 5/24/2021 | EU | 1,864,981 | 5/24/21 |
EU | 1,294,378 | 5/24/21 | |||
Japan | 3,813,875 | 5/24/21 | |||
CoM / Use | 7,547,719 | 7/13/2025 | EU | 1,534,390 | 5/21/23 |
Japan | 4,612,414 | 5/21/23 | |||
Use | 7,790,704 | 5/24/2021 | N/A | ||
Use | 7,795,293 | 5/21/2023 | N/A | ||
CoM / Use | 8,052,993 | 8/1/2027 | EU | 2,152,237 | 8/1/27 |
Japan | 5,419,866 | 8/1/27 | |||
Japan | 5,735,078 | 8/1/27 | |||
CoM / Use | 8,052,994 | 8/1/2027 | EU | 2,152,237 | 8/1/27 |
Japan | 5,419,866 | 8/1/27 | |||
Japan | 5,735,078 | 8/1/27 | |||
CoM / Use | 8,052,995 | 8/1/2027 | EU | 2,152,237 | 8/1/27 |
Japan | 5,419,866 | 8/1/27 | |||
Japan | 5,735,078 | 8/1/27 | |||
CoM / Use | 8,062,665 | 8/1/2027 | EU | 2,152,237 | 8/1/27 |
Japan | 5,419,866 | 8/1/27 | |||
Japan | 5,735,078 | 8/1/27 | |||
CoM / Use | 8,071,129 | 8/1/2027 | EU | 2,152,237 | 8/1/27 |
Japan | 5,419,866 | 8/1/27 | |||
Japan | 5,735,078 | 8/1/27 | |||
CoM / Use | 8,828,430 | 8/1/2027 | EU | 2,152,237 | 8/1/27 |
Japan | 5,419,866 | 8/1/27 | |||
Japan | 5,735,078 | 8/1/27 | |||
‡Expiration dates of European and Japanese patents are calculated as 20 years from the earliest nonprovisional filing date to which priority is claimed, and do not take into account extensions that are or may be available in these jurisdictions.
Kyprolis
Patents protecting Kyprolis include those owned by Amgen and those owned by Ligand. The United States patent listed in the Orange Book relating to Kyprolis with the latest expiration date is not expected to expire until 2027. Patents and applications owned by Ligand relating to the Captisol component of Kyprolis are not expected to expire until 2033. The type of patent protection (e.g., composition of matter or use) for each patent listed in the Orange Book and the
15
expiration dates for each patent listed in the Orange Book are provided in the following table. In addition, certain related patents in the commercially important jurisdictions of Europe and Japan are identified in the following table.
Kyprolis | |||||
United States | Corresponding Foreign | ||||
Type of Protection | U.S. Patent No. | U.S. Expiration Date | Jurisdiction | Patent Number | Expiration Date‡ |
CoM | 7,232,818 | 4/14/2025 | EU | 1,745,064 | 4/14/25 |
Japan | 5,394,423 | 4/14/25 | |||
CoM | 7,417,042 | 6/7/2026 | EU | 1,781,688 | 8/8/25 |
Japan | 4,743,720 | 8/8/25 | |||
Use | 7,491,704 | 4/14/2025 | EU | 1,745,064 | 4/14/25 |
Japan | 5,394,423 | 4/14/25 | |||
CoM | 7,737,112 | 12/7/2027 | EU | 1,819,353 | 12/7/25 |
EU | 2,260,835 | 12/7/25 | |||
EU | 2,261,236 | 12/7/25 | |||
Japan | 4,990,155 | 12/7/25 | |||
Japan | 5,108,509 | 5/9/25 | |||
Use | 8,129,346 | 12/25/2026 | EU | 1,745,064 | 4/14/25 |
Japan | 5,394,423 | 4/14/25 | |||
CoM | 8,207,125 | 4/14/2025 | EU | 1,781,688 | 8/8/25 |
Japan | 4,743,720 | 8/8/25 | |||
CoM / Use | 8,207,126 | 4/14/2025 | N/A | ||
Use | 8,207,127 | 4/14/2025 | N/A | ||
CoM / Use | 8,207,297 | 4/14/2025 | N/A | ||
‡Expiration dates of European and Japanese patents are calculated as 20 years from the earliest nonprovisional filing date to which priority is claimed, and do not take into account extensions that are or may be available in these jurisdictions.
Captisol
Patents and pending patent applications covering Captisol are owned by Ligand. Other patents and pending patent applications covering methods of making Captisol are owned by Ligand or by Pfizer. The patents covering the Captisol product, if issued, with the latest expiration date would not be set to expire until 2033 (see, e.g., WO 2013/130666 (contains composition of matter and use claims; filed Feb. 27, 2013)). Ligand also owns several patents and pending patent applications covering drug products containing Captisol as a component. The type of patent protection (e.g., composition of matter or use) and the expiration dates for several issued patents covering Captisol are provided in the following table. In addition, certain related patents and applications in the commercially important jurisdictions of Europe and Japan are listed in the following table.
16
Captisol | |||||
United States | Corresponding Foreign | ||||
Type of Protection | U.S. Patent No. | U.S. Expiration Date | Jurisdiction | Patent Number | Expiration Date‡ |
CoM | 8,114,438 | 3/19/28 | EU | 2,708,225 | pending |
Japan | 2,015,163,634 | pending | |||
CoM | 7,629,331 | 10/26/25 | EU | 1,945,228 | 10/26/25 |
EU | 2,335,707 | 10/26/25 | |||
EU | 2,581,078 | 10/26/25 | |||
Use | 8,049,003 | 12/19/26 | EU | 2,583,668 | 10/26/25 |
CoM | 8,846,901 | 10/26/25 | EU | 1,945,228 | 10/26/25 |
EU | 2,335,707 | 10/26/25 | |||
EU | 2,581,078 | 10/26/25 | |||
CoM | 8,829,182 | 10/26/25 | EU | 1,945,228 | 10/26/25 |
EU | 2,335,707 | 10/26/25 | |||
EU | 2,581,078 | 10/26/25 | |||
CoM / Use | 7,635,773 | 3/13/29 | EU | 2,268,269 | pending |
Japan | 4,923,144 | 4/28/29 | |||
Japan | 2,015,110,671 | pending | |||
CoM | 8,410,077 | 3/13/29 | EU | 2,268,269 | pending |
Japan | 4,923,144 | 4/28/29 | |||
Japan | 2,015,110,671 | pending | |||
CoM | 9,200,088 | 3/13/29 | EU | 2,268,269 | pending |
Japan | 4,923,144 | 4/28/29 | |||
Japan | 2,015,110,671 | pending | |||
‡ Expiration date of European and Japanese patents are calculated as 20 years from the earliest nonprovisional filing date to which priority is claimed, and do not take into account extensions that are or may be available in these jurisdictions.
Subject to compliance with the terms of the respective agreements, our rights to receive royalty payments under our licenses with our exclusive licensors typically extend for the life of the patents covering such developments. For a discussion of the risks associated with patent and proprietary rights, see below under “Item 1A. Risk Factors.”
OmniAb
OMT has received patent protection in 27 countries, including the United States, multiple countries throughout Europe, Japan and China (see selected cases listed in the table below) and has 19 patent applications pending worldwide. The patents and applications owned by OMT are expected to expire between 2028 and 2033 and partners are able to use the OMT patented technology to generate novel antibodies, which may be entitled to additional patent protection.
OmniAb | |||||
United States | Corresponding Foreign | ||||
Type of Protection | U.S. Patent No. | U.S. Expiration Date | Jurisdiction | Patent Number | Expiration Date‡ |
CoM | 8,703,485 | 10/10/31 | EU | 2,152,880 | 5/30/28 |
EU | 2,336,329 | 5/30/28 | |||
Japan | 5,823,690 | 5/30/28 | |||
Use | 8,907,157 | 5/30/28 | N/A | ||
17
‡ Expiration date of European and Japanese patents are calculated as 20 years from the earliest nonprovisional filing date to which priority is claimed, and do not take into account extensions that are or may be available in these jurisdictions.
LTP Technology
Patent applications related to our LTP Technology include three families owned by Ligand and one owned by Omthera. Each of these patent families include claims directed to composition of matter and use. Patents resulting from these applications, if granted, would have a latest expiration date in 2036.
LGD-6972 (Glucagon Receptor Antagonist)
Patents and pending patent applications covering LGD-6972 are owned by Ligand. Patents covering LGD-6972, if issued, with the latest expiration date would not be set to expire until 2035 (see, e.g., WO 2015/191900 (contains composition of matter and use claims; filed June 11, 2015)). The type of patent protection (e.g., composition of matter or use) and the expiration dates for several issued patents covering LGD-6972 are provided in the following table. In addition, certain related patents and applications in the commercially important jurisdictions of Europe and Japan are listed in the following table.
LGD-6972 | |||||
United States | Corresponding Foreign | ||||
Type of Protection | U.S. Patent No. | U.S. Expiration Date | Jurisdiction | Patent Number | Expiration Date‡ |
CoM | 8,710,236 | 2/11/28 | EU | 2,129,654 | 2/11/28 |
EU | 2,786,985 | pending | |||
Japan | 5,322,951 | 2/11/28 | |||
Japan | 2015-196171 | pending | |||
CoM | 9,169,201 | 2/11/28 | EU | 2,129,654 | 2/11/28 |
EU | 2,786,985 | pending | |||
Japan | 5,322,951 | 2/11/28 | |||
Japan | 2015-196171 | pending | |||
CoM / Use | 8,907,103 | 1/2/31 | EU | 2,326,618 | 8/13/29 |
EU | 2,799,428 | pending | |||
Japan | 5,684,126 | 8/13/29 | |||
Japan | 2015-129133 | pending | |||
‡ Expiration date of European and Japanese patents are calculated as 20 years from the earliest nonprovisional filing date to which priority is claimed, and do not take into account extensions that are or may be available in these jurisdictions.
Human Resources
As of February 1, 2016, we had 21 full-time employees, of whom seven are involved directly in scientific research and development activities.
Investor Information
Financial and other information about us is available on our website at www.ligand.com. We make available on our website copies of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the U.S. Securities and Exchange Commission (SEC). In addition, we have previously filed registration statements and other documents with the SEC. Any document we file may be inspected, at the SEC’s public reference room at 100 F Street NE, Washington, DC 20549, or at the SEC’s internet address at www.sec.gov. These website addresses are not intended to function as hyperlinks, and
18
the information contained in our website and in the SEC’s website is not intended to be a part of this filing. Information related to the operation of the SEC’s public reference room may be obtained by calling the SEC at 800-SEC-0330.
ITEM 1A. | RISK FACTORS |
The following is a summary description of some of the many risks we face in our business. You should carefully review these risks in evaluating our business, including the businesses of our subsidiaries. You should also consider the other information described in this report.
Future revenue based on Promacta and Kyprolis, as well as sales of our other products, may be lower than expected.
Novartis is obligated to pay us royalties on its sales of Promacta, and we receive revenue from Amgen based on both sales of Kyprolis and purchases of Captisol material for clinical and commercial uses. These payments are expected to be a substantial portion of our ongoing revenues for some time. In addition, we receive revenues based on sales of Duavee, Conbriza, Noxafil IV and Nexterone. Any setback that may occur with respect to any of our products, and in particular Promacta or Kyprolis, could significantly impair our operating results and/or reduce the market price of our stock. Setbacks for the products could include problems with shipping, distribution, manufacturing, product safety, marketing, government regulation or reimbursement, licenses and approvals, intellectual property rights, competition with existing or new products and physician or patient acceptance of the products, as well as higher than expected total rebates, returns, discounts, or unfavorable exchange rates. These products also are or may become subject to generic competition. Any such setback could reduce our revenue.
Future revenue from sales of Captisol material to our collaborative partners may be lower than expected.
Revenues from sales of Captisol material to our collaborative partners represent a significant portion of our current revenues. Any setback that may occur with respect to Captisol could significantly impair our operating results and/or reduce the market price of our stock. Setbacks for Captisol could include problems with shipping, distribution, manufacturing, product safety, marketing, government regulation or reimbursement, licenses and approvals, intellectual property rights, competition with existing or new products and physician or patient acceptance of the products using Captisol, as well as higher than expected total rebates, returns or discounts for such products.
If products or product candidates incorporating Captisol technology were to cause any unexpected adverse events, the perception of Captisol safety could be seriously harmed. If this were to occur, we may not be able to market Captisol products unless and until we are able to demonstrate that the adverse event was unrelated to Captisol, which we may not be able to do. Further, whether or not the adverse event was a result of Captisol, we could be required by the FDA to submit to additional regulatory reviews or approvals, including extensive safety testing or clinical testing of products using Captisol, which would be expensive and, even if we were to demonstrate that the adverse event was unrelated to Captisol, would delay the marketing of Captisol-enabled products and receipt of revenue related to those products, which could significantly impair our operating results and/or reduce the market price of our stock.
We obtain Captisol from a sole source supplier, and if this supplier were to cease to be able, for any reason, to supply Captisol to us in the amounts we require, or decline to supply Captisol to us, we would be required to seek an alternative source, which could potentially take a considerable length of time and impact our revenue and customer relationships. We maintain inventory of Captisol, which has a five year shelf life, at three geographically dispersed storage locations in the United States and Europe. If we were to encounter problems maintaining our inventory, such as natural disasters, at one or more of these locations, it could lead to supply interruptions.
We currently depend on our arrangements with our outlicensees to sell products using our Captisol technology. These agreements generally provide that outlicensees may terminate the agreements at will. If our outlicensees discontinue sales of products using our Captisol technology, fail to obtain regulatory approval for products using our Captisol technology, fail to satisfy their obligations under their agreements with us, or choose to utilize a generic form of Captisol should it become available, or if we are unable to establish new licensing and marketing relationships, our financial results and growth prospects would be materially affected. Furthermore, we maintain significant accounts receivable balances with certain customers purchasing Captisol materials, which may result in the concentration of credit risk. We generally do not require any collateral from our customers to secure payment of these accounts receivable. If any of our major customers were to default in the payment of their obligations to us, our business, financial condition, operating results and cash flows could be adversely affected.
19
Further, under most of our Captisol outlicenses, the amount of royalties we receive will be reduced or will cease when the relevant patent expires. Our high purity patents and foreign equivalents, are not expected to expire until 2029 and our morphology patents and foreign equivalents, are not expected to expire until 2025, but the initially filed patents relating to Captisol expired starting in 2010 in the United States and will expire by 2016 in most countries outside the United States. If our other intellectual property rights are not sufficient to prevent a generic form of Captisol from coming to market and if in such case our outlicensees choose to terminate their agreements with us, our Captisol revenue may decrease significantly.
Third party intellectual property may prevent us or our partners from developing our potential products; our and our partners’ intellectual property may not prevent competition; and any intellectual property issues may be expensive and time consuming to resolve.
The manufacture, use or sale of our potential products or our collaborative partners' products or potential products may infringe the patent rights of others. If others obtain patents with conflicting claims, we may be required to obtain licenses to those patents or to develop or obtain alternative technology. We may not be able to obtain any such licenses on acceptable terms, or at all. Any failure to obtain such licenses could delay or prevent us from pursuing the development or commercialization of our potential products.
Generally, our success will depend on our ability and the ability of us and our partners to obtain and maintain patents and other intellectual property rights for our and their potential products both in the United States and in foreign countries. Our patent position, like that of many biotechnology and pharmaceutical companies, is uncertain and involves complex legal and technical questions for which important legal principles are unresolved. Even if we or our partners do obtain patents, such patents may not adequately protect the technology we own or have licensed. For example, in January 2016, we received a paragraph IV certification from a subsidiary of Par advising us that it had filed an ANDA with the FDA seeking approval to market a generic version of Merck’s NOXAFIL-IV product. The paragraph IV certification alleges that Merck’s U.S. Patent No. 9,023,790 related to NOXAFIL-IV and our U.S. Patent No. 8,410,077 related to Captisol, which we refer to as the ‘077 Patent, are invalid and/or will not be infringed by Par’s manufacture, use or sale of the product for which the ANDA was submitted. If Par succeeds in receiving the ANDA, we could lose the revenues related to NOXAFIL-IV or the ability to enter into new licenses using our ‘077 Patent. For additional information, see “Item 3. Legal Proceedings.”
Any conflicts with the patent rights of others could significantly reduce the coverage of our patents or limit our ability to obtain meaningful patent protection. For example, our European patent related to Agglomerated forms of Captisol was limited during an opposition proceeding, and the rejection of our European patent application related to High Purity Captisol is currently being appealed. In addition, any determination that our patent rights are invalid may result in early termination of our agreements with our collaborative partners and could adversely affect our ability to enter into new collaborations. We also rely on unpatented trade secrets and know-how to protect and maintain our competitive position. We require our employees, consultants, collaborative partners and others to sign confidentiality agreements when they begin their relationship with us. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our competitors may independently discover our trade secrets.
We may also need to initiate litigation, which could be time-consuming and expensive, to enforce our proprietary rights or to determine the scope and validity of others' rights. If this occurs, a court may find our patents or those of our licensors invalid or may find that we have infringed on a competitor's rights. In addition, if any of our competitors have filed patent applications in the United States which claim technology we also have invented, the United States Patent and Trademark Office may require us to participate in expensive interference proceedings to determine who has the right to a patent for the technology.
The occurrence of any of the foregoing problems could be time-consuming and expensive and could adversely affect our financial position, liquidity and results of operations.
We rely heavily on collaborative relationships, and any disputes or litigation with our collaborative partners or termination or breach of any of the related agreements could reduce the financial resources available to us, including milestone payments and future royalty revenues.
Our existing collaborations may not continue or be successful, and we may be unable to enter into future collaborative arrangements to develop and commercialize our unpartnered assets. Generally, our current collaborative partners also have the right to terminate their collaborations at will or under specified circumstances. If any of our collaborative partners breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities successfully (for example, by not making required payments when due, or at all), our product development under these agreements will be delayed or terminated. Disputes or litigation may also arise with our collaborators (with us and/or with one or more third parties), including those over
20
ownership rights to intellectual property, know-how or technologies developed with our collaborators. Such disputes or litigation could adversely affect our rights to one or more of our product candidates and could delay, interrupt or terminate the collaborative research, development and commercialization of certain potential products, create uncertainty as to ownership rights of intellectual property, or could result in litigation or arbitration. The occurrence of any of these problems could be time-consuming and expensive and could adversely affect our business.
Our product candidates, and the product candidates of our partners, face significant development and regulatory hurdles prior to partnering and/or marketing which could delay or prevent licensing, sales-based royalties and/or milestone revenue.
Before we or our partners obtain the approvals necessary to sell any of our unpartnered assets or partnered programs, we must show through preclinical studies and human testing that each potential product is safe and effective. We and/or our partners have a number of partnered programs and unpartnered assets moving toward or currently awaiting regulatory action. Failure to show any product's safety and effectiveness could delay or prevent regulatory approval of a product and could adversely affect our business. The drug development and clinical trials process is complex and uncertain. For example, the results of preclinical studies and initial clinical trials may not necessarily predict the results from later large-scale clinical trials. In addition, clinical trials may not demonstrate a product's safety and effectiveness to the satisfaction of the regulatory authorities. A number of companies have suffered significant setbacks in advanced clinical trials or in seeking regulatory approvals, despite promising results in earlier trials. The FDA may also require additional clinical trials after regulatory approvals are received. Such additional trials may be expensive and time-consuming, and failure to successfully conduct those trials could jeopardize continued commercialization of a product.
The speed at which we and our partners complete our scientific studies and clinical trials depends on many factors, including, but not limited to, our ability to obtain adequate supplies of the products to be tested and patient enrollment. Patient enrollment is a function of many factors, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial and other potential drug candidates being studied. Delays in patient enrollment for our or our partners’ trials may result in increased costs and longer development times. In addition, our collaborative partners have rights to control product development and clinical programs for products developed under our collaborations. As a result, these collaborative partners may conduct these programs more slowly or in a different manner than expected. Moreover, even if clinical trials are completed, we or our collaborative partners still may not apply for FDA approval in a timely manner or the FDA still may not grant approval.
Our drug development programs may require substantial additional capital to complete successfully, arising from costs to: conduct research, preclinical testing and human studies; establish pilot scale and commercial scale manufacturing processes and facilities; and establish and develop quality control, regulatory, marketing, sales and administrative capabilities to support these programs. While we expect to fund our research and development activities from cash generated from royalties and milestones from our partners in various past and future collaborations to the extent possible, if we are unable to do so, we may need to complete additional equity or debt financings or seek other external means of financing. These financings could depress our stock price. If additional funds are required to support our operations and we are unable to obtain them on terms favorable to us, we may be required to cease or reduce further development or commercialization of our products, to sell some or all of our technology or assets or to merge with another entity.
Our OmniAb antibody platform faces specific risks, including the fact that no drug using antibodies from the platform has been tested in clinical trials.
None of our collaboration partners using our OmniAb antibody platform have tested drugs based on the platform in clinical trials and, therefore, none of our OmniAb collaboration partners’ drugs have received FDA approval. If one of our OmniAb collaboration partners’ drug candidates fails during preclinical studies or clinical trials, our other OmniAb collaboration partners may decide to abandon drugs using antibodies generated from the OmniAb platform, whether or not attributable to the platform. All of our OmniAb collaboration partners may terminate their programs at any time without penalty. In addition, our OmniRat and OmniFlic platforms, which we consider the most promising, are covered by two patents within the U.S. and two patents in the European Union and are subject to the same risks as our patent portfolio discussed above, including the risk that our patents may infringe on third party patent rights or that our patents may be invalidated. Further, we face significant competition from other companies selling human antibody-generating rodents, especially mice which compete with our OmniMouse platform, including the VelocImmune mouse, the AlivaMab mouse and the Trianni mouse. Many of our competitors have greater financial, technical and human resources than we do and may be better equipped to develop, manufacture and market competing antibody platforms.
21
If plaintiffs bring product liability lawsuits against us or our partners, we or our partners may incur substantial liabilities and may be required to limit commercialization of our approved products and product candidates.
As is common in our industry, our partners and we face an inherent risk of product liability as a result of the clinical testing of our product candidates in clinical trials and face an even greater risk for commercialized products. Although we are not currently a party to product liability litigation, if we are sued, we may be held liable if any product or product candidate we develop causes injury or is found otherwise unsuitable during product testing, manufacturing, marketing or sale. Regardless of merit or eventual outcome, liability claims may result in decreased demand for any product candidates or products that we may develop, injury to our reputation, discontinuation of clinical trials, costs to defend litigation, substantial monetary awards to clinical trial participants or patients, loss of revenue and the inability to commercialize any products that we develop. We have product liability insurance that covers our clinical trials up to a $10.0 million annual limit. If we are sued for any injury caused by our product candidates or any future products, our liability could exceed our total assets.
Any difficulties from strategic acquisitions could adversely affect our stock price, operating results and results of operations.
We may acquire companies, businesses and products that complement or augment our existing business. We may not be able to integrate any acquired business successfully or operate any acquired business profitably. Integrating any newly acquired business could be expensive and time-consuming. Integration efforts often take a significant amount of time, place a significant strain on managerial, operational and financial resources and could prove to be more difficult or expensive than we predict. The diversion of our management's attention and any delay or difficulties encountered in connection with any future acquisitions we may consummate could result in the disruption of our on-going business or inconsistencies in standards and controls that could negatively affect our ability to maintain third-party relationships. Moreover, we may need to raise additional funds through public or private debt or equity financing, or issue additional shares, to acquire any businesses or products, which may result in dilution for stockholders or the incurrence of indebtedness.
As part of our efforts to acquire companies, business or product candidates or to enter into other significant transactions, we conduct business, legal and financial due diligence with the goal of identifying and evaluating material risks involved in the transaction. Despite our efforts, we ultimately may be unsuccessful in ascertaining or evaluating all such risks and, as a result, might not realize the intended advantages of the transaction. If we fail to realize the expected benefits from acquisitions we may consummate in the future or have consummated in the past, whether as a result of unidentified risks, integration difficulties, regulatory setbacks, litigation with current or former employees and other events, our business, results of operations and financial condition could be adversely affected. If we acquire product candidates, we will also need to make certain assumptions about, among other things, development costs, the likelihood of receiving regulatory approval and the market for such product candidates. Our assumptions may prove to be incorrect, which could cause us to fail to realize the anticipated benefits of these transactions.
In addition, we will likely experience significant charges to earnings in connection with our efforts, if any, to consummate acquisitions. For transactions that are ultimately not consummated, these charges may include fees and expenses for investment bankers, attorneys, accountants and other advisors in connection with our efforts. Even if our efforts are successful, we may incur, as part of a transaction, substantial charges for closure costs associated with elimination of duplicate operations and facilities and acquired IPR&D charges. In either case, the incurrence of these charges could adversely affect our results of operations for particular quarterly or annual periods.
We may be subject to prosecution for violation of federal law due to our agreement with Vireo Health, which is developing drugs using cannabis.
In November 2015, we entered into a license agreement and supply agreement with Vireo Health granting Vireo Health an exclusive right in certain states within the United States and certain global territories to use Captisol in Vireo’s development and commercialization of pharmaceutical-grade cannabinoid-based products. However, state laws legalizing medical cannabis use are in conflict with the Federal Controlled Substances Act, which classifies cannabis as a schedule-I controlled substance and makes cannabis use and possession illegal on a national level. The United States Supreme Court has ruled that it is the Federal government that has the right to regulate and criminalize cannabis, even for medical purposes, and thus Federal law criminalizing the use of cannabis preempts state laws that legalize its use. The Obama administration has effectively stated that it is not an efficient use of resources to direct Federal law enforcement agencies to prosecute those lawfully abiding by state-designated laws allowing the use and distribution of medical and recreational cannabis. Yet, there is no guarantee that the current policy and practice will not change regarding the low-priority enforcement of Federal laws in states where cannabis has been legalized. Any such change in the Federal government’s enforcement of Federal laws could result in Ligand, as the supplier of Captisol, to be charged with violations of Federal laws which may result in significant legal expenses and substantial penalties and fines.
22
We have restated prior consolidated financial statements, which may lead to possible additional risks and uncertainties, including possible loss of investor confidence.
We have restated our consolidated financial statements as of and for the year ended December 31, 2015 (including the third quarter within that year) and for the first and second quarters of fiscal year 2016 in order to correct certain accounting errors as described in Restatement in "Note 1 Summary of significant accounting policies" to the consolidated financial statements (the Restatement). For a description of the material weaknesses in our internal control over financial reporting identified by management in connection with the Restatement and management’s plan to remediate those material weaknesses, see “Part II, Item 9A - Controls and Procedures.”
As a result of the Restatement, we have become subject to possible additional costs and risks, including (a) accounting and legal fees incurred in connection with the Restatement and (b) a possible loss of investor confidence.
We have identified material weaknesses in our internal control over financial reporting that, if not remediated, could result in additional material misstatements in our financial statements.
As described in “Part II, Item 9A - Controls and Procedures,” management identified control deficiencies that represent material weaknesses. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. As a result of the identified material weaknesses, management has concluded that we did not maintain effective internal control over financial reporting as of December 31, 2015. See “Part II, Item 9A - Controls and Procedures.”
We are developing and implementing a remediation plan to address the material weaknesses. If our remediation efforts are insufficient or if additional material weaknesses in our internal control over financial reporting are discovered or occur in the future, our consolidated financial statements may contain material misstatements and we could be required to restate our financial results, which could materially and adversely affect our business, results of operations and financial condition, restrict our ability to access the capital markets, require us to expend significant resources to correct the material weakness, subject us to fines, penalties or judgments, harm our reputation or otherwise cause a decline in investor confidence.
Our shareholder rights plan, concentration of ownership and charter documents may hinder or prevent change of control transactions.
Our shareholder rights plan and provisions contained in our certificate of incorporation and bylaws may discourage transactions involving an actual or potential change in our ownership. In addition, our Board of Directors may issue shares of common or preferred stock without any further action by the stockholders. Our directors and certain of our institutional investors, collectively beneficially own a significant portion of our outstanding common stock. We have in the past granted waivers to investors allowing them to increase their ownership level above the limit set forth in our shareholder rights agreement. Such restrictions, circumstances and issuances may have the effect of delaying or preventing a change in our ownership. If changes in our ownership are discouraged, delayed or prevented, it would be more difficult for our current Board of Directors to be removed and replaced, even if you or our other stockholders believe that such actions are in the best interests of us and our stockholders.
We rely on information technology and any failure, inadequacy, interruption or security lapse of that technology, including any cyber security incidents, could harm our ability to operate our business effectively.
Our business is increasingly dependent on critical, complex and interdependent information technology systems, including internet-based systems, to support business processes as well as internal and external communications. Despite the implementation of security measures, our internal computer systems and those of our collaborative partners are vulnerable to damage from cyber-attacks, computer viruses, security breaches, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. System failures, accidents or security breaches could cause interruptions in our operations, could lead to the loss of trade secrets or other intellectual property, could lead to the public exposure of personal information of our employees and others, and could result in a material disruption of our clinical and commercialization activities and business operations, in addition to possibly requiring substantial expenditures to remedy. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our business and financial condition could be harmed.
23
The occurrence of a catastrophic disaster could damage our facilities beyond insurance limits or we could lose key data which could cause us to curtail or cease operations.
We are vulnerable to damage and/or loss of vital data from natural disasters, such as earthquakes, tornadoes, power loss, fire, floods and similar events, as well as from accidental loss or destruction. If any disaster were to occur, our ability to operate our business could be seriously impaired. We have property, liability, and business interruption insurance which may not be adequate to cover our losses resulting from disasters or other similar significant business interruptions, and we do not plan to purchase additional insurance to cover such losses due to the cost of obtaining such coverage. Any significant losses that are not recoverable under our insurance policies could seriously impair our business, financial condition and prospects.
We sold the 2019 Convertible Senior Notes, which may impact our financial results, result in the dilution of existing stockholders, and restrict our ability to take advantage of future opportunities.
In August of 2014, we sold $245.0 million aggregate principal amount of 0.75% Convertible Senior Notes due 2019, or the 2019 Convertible Senior Notes. We will be required to pay interest on the 2019 Convertible Senior Notes until they come due or are converted, and the payment of that interest will reduce our net income. The sale of the 2019 Convertible Senior Notes may also affect our earnings per share figures, as accounting procedures require that we include in our calculation of earnings per share the number of shares of our common stock into which the 2019 Convertible Senior Notes are convertible. The 2019 Convertible Senior Notes may be converted, under the conditions and at the premium specified in the 2019 Convertible Senior Notes, into cash and shares of our common stock, if any (subject to our right to pay cash in lieu of all or a portion of such shares). If shares of our common stock are issued to the holders of the 2019 Convertible Senior Notes upon conversion, there will be dilution to our shareholders equity. Upon the occurrence of certain circumstances, holders of the 2019 Convertible Senior Notes may require us to purchase all or a portion of their notes for cash, which may require the use of a substantial amount of cash. If such cash is not available, we may be required to sell other assets or enter into alternate financing arrangements at terms that may or may not be desirable. The existence of the 2019 Convertible Senior Notes and the obligations that we incurred by issuing them may restrict our ability to take advantage of certain future opportunities, such as engaging in future debt or equity financing activities.
Impairment charges pertaining to goodwill, identifiable intangible assets or other long-lived assets from our mergers and acquisitions could have an adverse impact on our results of operations and the market value of our common stock.
The total purchase price pertaining to our acquisitions in recent years of CyDex, Metabasis, Pharmacopeia, and Neurogen have been allocated to net tangible assets, identifiable intangible assets, in-process research and development and goodwill. To the extent the value of goodwill or identifiable intangible assets or other long-lived assets become impaired, we will be required to incur material charges relating to the impairment. Any impairment charges could have a material adverse impact on our results of operations and the market value of our common stock.
Our stock price has been volatile and could experience a sudden decline in value.
The market prices for securities of biotechnology and pharmaceutical companies have historically been highly volatile, and the market has recently experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. Continued volatility in the overall capital markets could reduce the market price of our common stock in spite of our operating performance. Further, high stock price volatility could result in higher stock-based compensation expense.
Our common stock has experienced significant price and volume fluctuations and may continue to experience volatility in the future. Many factors may have a significant impact on the market price of our common stock, including, but not limited to, the following factors: results of or delays in our preclinical studies and clinical trials; the success of our collaboration agreements; publicity regarding actual or potential medical results relating to products under development by us or others; announcements of technological innovations or new commercial products by us or others; developments in patent or other proprietary rights by us or others; comments or opinions by securities analysts or major stockholders; future sales of our common stock by existing stockholders; regulatory developments or changes in regulatory guidance; litigation or threats of litigation; economic and other external factors or other disaster or crises; the departure of any of our officers, directors or key employees; period-to-period fluctuations in financial results; and price and volume fluctuations in the overall stock market.
24
Our results of operations and liquidity needs could be materially negatively affected by market fluctuations and economic downturn.
Our results of operations could be materially negatively affected by economic conditions generally, both in the United States and elsewhere around the world. Continuing concerns over inflation, energy costs, geopolitical issues, the availability and cost of credit, and the U.S. financial markets have contributed to increased volatility and diminished expectations for the economy and the markets going forward. Domestic and international equity markets periodically experience heightened volatility and turmoil. These events may have an adverse effect on us. In the event of a market downturn, our results of operations could be adversely affected by those factors in many ways, including making it more difficult for us to raise funds if necessary, and our stock price may further decline. We cannot provide assurance that our investments are not subject to adverse changes in market value. If our investments experience adverse changes in market value, we may have less capital to fund our operations.
Item 1B. | Unresolved Staff Comments |
None.
Item 2. | Properties |
We currently lease premises consisting of approximately 16,500 square feet of office and laboratory space in San Diego, leased through June 2019 which serves as our corporate headquarters. Approximately 6,500 square feet of laboratory space is currently subleased. In 2015, we entered into a lease termination agreement to accelerate the expiration date of the lease to April 30, 2016. In February 2016, we received a notice from our current landlord regarding the termination date of our lease and are currently in discussions to resolve any disputes. The Company requires smaller facility space and accordingly entered into a new lease agreement consisting of approximately 4,000 square feet of office space in San Diego. The new lease has an initial term of approximately 7 years and is expected to commence in May 2016.
We lease approximately 1,500 square feet of laboratory space located at the Bioscience and Technology Business Center in Lawrence, Kansas, leased through December 2017.
We lease approximately 99,000 square feet in three facilities in Cranbury, New Jersey under leases that expire in 2016. We also sublease approximately 11,666 square feet of these facilities with subleases expiring in 2016. We fully vacated these facilities in September 2010.
Item 3. | Legal Proceedings |
From time to time we are subject to various lawsuits and claims with respect to matters arising out of the normal course of our business. Due to the uncertainty of the ultimate outcome of these matters, the impact on future financial results is not subject to reasonable estimates.
Securities Litigation
In 2012, a federal securities class action and shareholder derivative lawsuit was filed in Pennsylvania alleging that the Company and its CEO assisted various breaches of fiduciary duties based on our purchase of a licensing interest in a development-stage pharmaceutical program from the Genaera Liquidating Trust in 2010 and our subsequent sale of half of our interest in the transaction to Biotechnology Value Fund, Inc. Plaintiff filed a second amended complaint in February 2015, which we moved to dismiss in March 2015. The district court granted the motion to dismiss on November 11, 2015. The plaintiff has appealed that ruling to the Third Circuit. The Company intends to continue to vigorously defend against the claims against the Company and its CEO. The outcome of the matter is not presently determinable.
Paragraph IV Certification by Par Pharmaceuticals
On January 7, 2016, we received a paragraph IV certification from Par Sterile Products, LLC, a subsidiary of Par Pharmaceuticals, Inc., or Par, advising us that it had filed an ANDA with the FDA seeking approval to market a generic version of Merck’s NOXAFIL-IV product. The paragraph IV certification states it is Par’s position that Merck’s U.S. Patent No. 9,023,790 related to NOXAFIL-IV and our U.S. Patent No. 8,410,077 related to Captisol are invalid and/or will not be
25
infringed by Par’s manufacture, use or sale of the product for which the ANDA was submitted. On February 19, 2016, Merck filed an action against Par in the United States District Court for the District of New Jersey, asserting that Par’s manufacture, use or sale of the product for which the ANDA was submitted would infringe Merck’s U.S. Patent No. 9,023,790. The case against Par is captioned Merck Sharpe & Dohme Corp. v. Par Sterile Products, LLC, Par Pharmaceuticals, Inc., Par Pharmaceutical Companies, Inc., and Par Pharmaceutical Holdings, Inc., No.16-cv-00948.
Item 4. | Mine Safety Disclosures |
Not applicable.
PART II
Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities |
Market Information
Our common stock is traded on the NASDAQ Global Market under the symbol “LGND.”
The following table sets forth the high and low intraday sales prices for our common stock on the NASDAQ Global Market for the periods indicated:
Price Range | |||||||
Low | High | ||||||
Year Ended December 31, 2015: | |||||||
1st Quarter | $ | 51.54 | $ | 77.11 | |||
2nd Quarter | 75.67 | 100.90 | |||||
3rd Quarter | 82.10 | 111.25 | |||||
4th Quarter | 84.46 | 111.85 | |||||
Year Ended December 31, 2014: | |||||||
1st Quarter | $ | 50.73 | $ | 80.42 | |||
2nd Quarter | 55.90 | 71.44 | |||||
3rd Quarter | 46.32 | 65.66 | |||||
4th Quarter | 41.99 | 58.48 | |||||
As of February 17, 2016, the closing price of our common stock on the NASDAQ Global Market was $90.36
Holders
As of February 17, 2016, there were approximately 604 holders of record of the common stock.
Purchases of Equity Securities By the Issuer and Affiliated Purchasers
The following table presents information regarding repurchases by us of our common stock during the year ended December 31, 2015 under the stock repurchase program approved by our board of directors in September 2015, under which we may acquire up to $200.0 million of our common stock in open market and negotiated purchases for a period of one year.
ISSUER PURCHASES OF EQUITY SECURITIES
Total Number of Shares Purchased | Average Price Paid Per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Maximum Dollar Value of Shares that May Yet Be Purchased Under the Program (in thousands) | |||||||||||
September 1-September 30, 2015 | 6,120 | $ | 79.92 | 6,120 | $ | 199,511 | ||||||||
Total | 6,120 | |||||||||||||
26
Performance Graph
The graph below shows the five-year cumulative total stockholder return assuming the investment of $100 and is based on the returns of the component companies weighted monthly according to their market capitalizations. The graph compares total stockholder returns of our common stock, of all companies traded on the NASDAQ Stock market, as represented by the NASDAQ Composite® Index, and of the NASDAQ Biotechnology Stock Index, as prepared by The NASDAQ Stock Market Inc. The NASDAQ Biotechnology Stock Index tracks approximately 151 domestic biotechnology stocks.
The stockholder return shown on the graph below is not necessarily indicative of future performance and we will not make or endorse any predictions as to future stockholder returns.
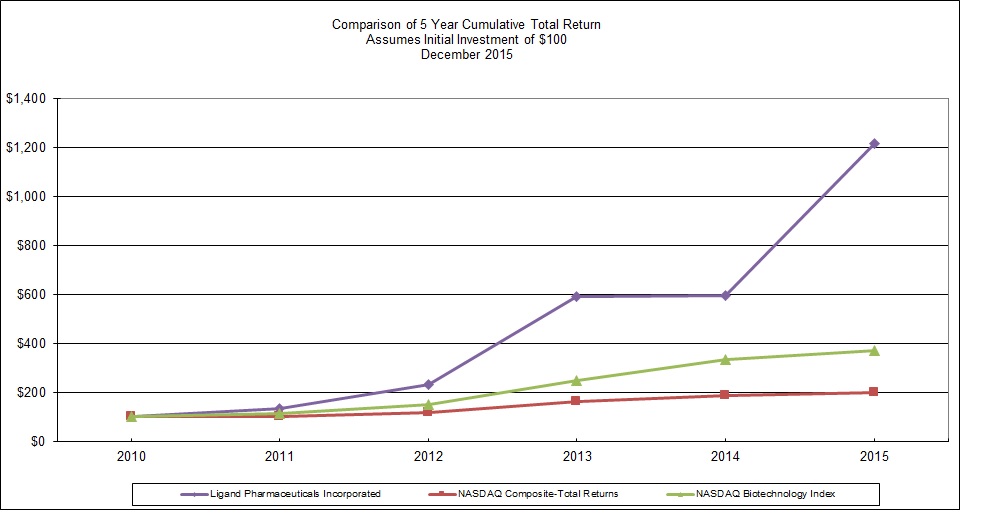
12/31/2010 | 12/31/2011 | 12/31/2012 | 12/31/2013 | 12/31/2014 | 12/31/2015 | ||||||||||||
Ligand | 100 | % | 33 | % | 75 | % | 154 | % | 1 | % | 104 | % | |||||
NASDAQ Market (U.S. Companies) Index | 100 | % | (1 | )% | 17 | % | 40 | % | 15 | % | 7 | % | |||||
NASDAQ Biotechnology Stocks | 100 | % | 12 | % | 33 | % | 66 | % | 34 | % | 12 | % | |||||
27
Item 6. | Selected Consolidated Financial Data |
The following selected historical consolidated financial and other data are qualified by reference to, and should be read in conjunction with, our consolidated financial statements and the related notes thereto appearing elsewhere herein and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our selected statement of operations data set forth below for each of the years ended December 31, 2015, 2014, 2013, 2012, and 2011 and the balance sheet data as of December 31, 2015, 2014, 2013, 2012, and 2011 are derived from our consolidated financial statements.
Year Ended December 31, | |||||||||||||||||||
2015 Restated | 2014 | 2013 | 2012 | 2011 | |||||||||||||||
Consolidated Statements of Operations Data: | (in thousands) | ||||||||||||||||||
Royalties | $ | 38,194 | $ | 29,994 | $ | 23,584 | $ | 14,073 | $ | 9,213 | |||||||||
Material sales | 27,662 | 28,488 | 19,072 | 9,432 | 12,123 | ||||||||||||||
License fees, milestones, and other revenues | 6,058 | 6,056 | 6,317 | 7,883 | 8,701 | ||||||||||||||
Total revenues | 71,914 | 64,538 | 48,973 | 31,388 | 30,037 | ||||||||||||||
Cost of material sales | 5,807 | 9,136 | 5,732 | 3,601 | 4,909 | ||||||||||||||
Research and development expenses | 13,380 | 12,122 | 9,274 | 10,790 | 10,291 | ||||||||||||||
General and administrative expenses | 24,378 | 22,570 | 17,984 | 15,782 | 14,583 | ||||||||||||||
Lease exit and termination costs | 1,020 | 1,084 | 560 | 1,022 | 552 | ||||||||||||||
Write-off of acquired IPR&D | — | — | 480 | — | 2,282 | ||||||||||||||
Total operating costs and expenses | 44,585 | 44,912 | 34,030 | 31,195 | 32,617 | ||||||||||||||
Accretion of deferred gain on sale leaseback | 1,702 | ||||||||||||||||||
Income (loss) from operations | 27,329 | 19,626 | 14,943 | 193 | (878 | ) | |||||||||||||
Income (loss) from continuing operations including noncontrolling interests | 227,444 | 10,892 | 8,832 | (2,674 | ) | 9,712 | |||||||||||||
Loss attributable to noncontrolling interests | (2,380 | ) | (1,132 | ) | — | — | — | ||||||||||||
Income (loss) from continuing operations | 229,824 | 12,024 | 8,832 | (2,674 | ) | 9,712 | |||||||||||||
Discontinued operations (1) | — | — | 2,588 | 2,147 | 3 | ||||||||||||||
Net income (loss) | 229,824 | 12,024 | 11,420 | (527 | ) | 9,715 | |||||||||||||
Basic per share amounts: | |||||||||||||||||||
Income (loss) from continuing operations | $ | 11.61 | $ | 0.59 | $ | 0.43 | $ | (0.14 | ) | $ | 0.49 | ||||||||
Discontinued operations (1) | — | — | 0.13 | 0.11 | — | ||||||||||||||
Net income (loss) | $ | 11.61 | $ | 0.59 | $ | 0.56 | $ | (0.03 | ) | $ | 0.49 | ||||||||
Weighted average number of common shares-basic | 19,790 | 20,419 | 20,312 | 19,853 | 19,656 | ||||||||||||||
Diluted per share amounts: | |||||||||||||||||||
Income (loss) from continuing operations | $ | 10.83 | $ | 0.56 | $ | 0.43 | $ | (0.14 | ) | $ | 0.49 | ||||||||
Discontinued operations (1) | — | — | 0.12 | 0.11 | — | ||||||||||||||
Net income (loss) | $ | 10.83 | $ | 0.56 | $ | 0.55 | $ | (0.03 | ) | $ | 0.49 | ||||||||
Weighted average number of common shares-diluted | 21,228 | 21,433 | 20,745 | 19,853 | 19,713 | ||||||||||||||
28
December 31, | |||||||||||||||||||
2015 Restated | 2014 | 2013 | 2012 | 2011 | |||||||||||||||
(in thousands) | |||||||||||||||||||
Consolidated Balance Sheet Data: | |||||||||||||||||||
Cash, cash equivalents, short-term investments and restricted cash and investments | $ | 229,947 | $ | 168,597 | $ | 17,320 | $ | 15,148 | $ | 18,382 | |||||||||
Working capital | (8,109 | ) | 162,379 | (4,058 | ) | (11,616 | ) | (11,413 | ) | ||||||||||
Total assets | 503,061 | 258,029 | 104,713 | 104,260 | 120,583 | ||||||||||||||
Current portion of deferred revenue, net | 8 | 150 | 116 | 486 | 1,240 | ||||||||||||||
Long-term obligations (excludes long-term portions of deferred revenue, net and deferred gain) | 3,330 | 208,757 | 24,076 | 39,967 | 56,945 | ||||||||||||||
Long-term portion of deferred revenue, net | — | 2,085 | 2,085 | 2,369 | 3,466 | ||||||||||||||
Common stock subject to conditional redemption | — | — | — | 8,344 | |||||||||||||||
Accumulated deficit | (429,491 | ) | (659,315 | ) | (671,339 | ) | (682,759 | ) | (682,232 | ) | |||||||||
Total stockholders’ equity (deficit) | 237,282 | 26,318 | 49,613 | 26,485 | 8,185 | ||||||||||||||
(1) | We sold our Oncology product line (“Oncology”) on October 25, 2006 and we sold our Avinza product line (“Avinza”) on February 26, 2007. The operating results for the Oncology and Avinza product lines have been presented in our consolidated statements of operations as “Discontinued Operations.” |
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Results of Operations
Total revenues for 2015 were $71.9 million compared to $64.5 million in 2014 and $49.0 million in 2013. Our income from continuing operations for 2015 was $229.8 million, or $10.83 per diluted share, compared to income from continuing operations of $12.0 million in 2014, or $0.56 per diluted share, and net income from continuing operations of $8.8 million, or $0.43 per diluted share, in 2013.
Royalty revenue
Royalty revenues were $38.2 million in 2015, compared to $30.0 million in 2014 and $23.6 million in 2013. The increases in royalty revenue of $8.2 million and $6.4 million for the years ended December 31, 2015 and 2014, respectively are primarily due to increases in Promacta and Kyprolis royalties.
The following table represents royalty revenue by program (in thousands):
Year ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Partner A | $ | 29,295 | $ | 23,300 | $ | 16,024 | |||||
Partner B | 7,317 | 4,558 | 3,495 | ||||||||
Partner C | 390 | 1,244 | 3,309 | ||||||||
Other | 1,192 | 892 | 756 | ||||||||
Total | $ | 38,194 | $ | 29,994 | $ | 23,584 | |||||
Material sales
We recorded material sales of Captisol of $27.7 million in 2015 compared to $28.5 million in 2014 and $19.1 million in 2013. The decrease in material sales of $0.8 million for the year ended December 31, 2015 compared to 2014 is due to timing of customer purchases for Captisol for both clinical and commercial uses. The increase in material sales of $9.4 million for the year ended December 31, 2014 compared to 2013 is due to timing of customer purchases of Captisol as well as an increase in customer purchases for commercial use.
The following table represents material sales by clinical and commercial use (in thousands):
Year ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Clinical material sales | $ | 10,049 | $ | 13,798 | $ | 9,685 | |||||
Commercial material sales | 17,613 | 14,690 | 9,387 | ||||||||
Total | $ | 27,662 | $ | 28,488 | $ | 19,072 | |||||
License fees, milestones and other revenues
We recorded license fees, milestones and other revenues of $6.1 million in 2015 compared to $6.1 million in 2014 and $6.3 million in 2013. The decrease in license fees, milestones and other revenues of $0.2 million for the year ended December 31, 2014, compared to 2013 is primarily due to achievement and timing of milestones as well as licensing payments.
Cost of material sales
Cost of material sales were $5.8 million in 2015 compared to $9.1 million in 2014 and $5.7 million in 2013. The decrease of $3.3 million for the year ended December 31, 2015, compared to the same period in 2014 is due to the mix of Captisol sales and lower cost of goods sold overall. The increase of $3.4 million for the year ended December 31, 2014, compared to 2013 is primarily due to an increase in material sales of Captisol.
Research and development expenses
Research and development expenses for 2015 were $13.4 million compared to $12.1 million in 2014 and $9.3 million in 2013. The increase of $1.3 million is primarily due to the timing of costs associated with internal programs and an increase in non-cash stock based compensation expense. The increase in research and development expenses of $2.8 million for the year ended December 31, 2014 compared to 2013 is primarily due to timing of costs associated with internal programs and an increase in non-cash stock based compensation expense.
We are developing several proprietary products. Our programs represent a range of future licensing opportunities to expand our partnered asset portfolio. Our development focus for the year ended December 31, 2015, 2014, and 2013 has been LGD-6972, our novel glucagon receptor antagonist program. We completed a Phase 1b trial in 2015 that demonstrated favorable safety, tolerability and pharmacokinetics and plan to initiate a Phase 2 trial in 2016.
General and administrative expenses
General and administrative expenses were $24.4 million for the year ended December 31, 2015 compared to $22.6 million for 2014 and $18.0 million for 2013. The increase of $1.8 million in general and administrative expenses for the year ended December 31, 2015 compared with 2014 is primarily due to an increase in non-cash stock-based compensation and costs incurred for business development activities in 2015. The increase in expenses for the year ended December 31, 2014 compared with 2013 of $4.6 million is primarily due to costs associated with business development activities and an increase in non-cash stock based compensation expense.
Lease exit and termination costs
For the years ended December 31, 2015 and 2014, we had lease exit obligations of $0.9 million and $3.3 million, respectively. The lease exit obligations are related to a facility in Cranbury, New Jersey. The remaining lease obligations run through August 2016. Portions of the facility are subleased with such subleases expiring August 2016. We recorded lease exit and termination costs of $1.0 million for the year ended December 31, 2015, compared to $1.1 million for 2014, and $0.6 million in 2013. Lease exit and termination costs for the years ended December 31, 2015, 2014, and 2013 consisted of accretion costs and adjustments to the liability for lease exit costs due to changes in leasing assumptions.
Write-off of acquired IPR&D
For the years ended December 31, 2015 and December 31, 2014, there was no write-off of IPR&D recorded. For the year ended December 31, 2013, we recorded a non-cash impairment charge of $0.5 million for the write-off of IPR&D for Clopidogrel. Clopidogrel is an IV formulation of the anti-platelet medication designed for situations where the administration of oral platelet inhibitors is not feasible or desirable.
29
Interest expense, net
Interest expense was $11.8 million for the year ended December 31, 2015 compared to $4.9 million in 2014 and $2.1 million in 2013. The increase in interest expense of $6.9 million for the year ended December 31, 2015 compared with 2014 is due to interest expense and non-cash debt related costs related to the 2019 Convertible Senior Notes, partially offset by a decrease in interest expense related to the term loan facility that we paid off in July 2014. The increase in interest expense of $2.8 million for the year ended December 31, 2014 compared to 2013 was primarily due due to interest expense and non-cash debt related costs related to the 2019 Convertible Senior Notes.
Change in contingent liabilities
We recorded an expense associated with the increase in contingent liabilities of $5.0 million for the year ended December 31, 2015 compared to $5.1 million in 2014 and $3.6 million in 2013. The increase in contingent liabilities for the year ended December 31, 2015 is due to an increase in the fair value of CyDex related contingent liabilities of $3.8 million and an increase in the Metabasis CVRs of $1.2 million. The increase in contingent liabilities for the year ended December 31, 2014 is due to an increase in CyDex related contingent liabilities of $5.7 million, partially offset by a decrease in the fair value of the Metabasis CVR liability of $0.5 million. The increase in contingent liabilities for the year ended December 31, 2013 is due primarily to the increase in the fair value of the Metabasis CVR liability of $4.2 million. This was partially offset by a decrease in the fair value of $0.6 million in CyDex contingent liabilities.
Gain on deconsolidation of Viking
We recorded a $28.2 million gain on deconsolidation of Viking for the year ended December 31, 2015, primarily related to the equity milestone received from Viking upon the close of the Viking IPO in addition to the value received upon the underwriters’ exercise of their overallotment option.
Equity in net losses of Viking
We recorded a $5.1 million equity in net loss of Viking for the year ended December 31, 2015, for our proportionate share of Viking’s losses based on our ownership of Viking common stock.
Other, net
We recorded other income of $1.8 million for the year ended December 31, 2015 compared to other expense of $1.7 million in 2014 and other income of $0.1 million in 2013. Other income for the year ended December 31, 2015 and 2014 is primarily due to the gain on the sale of short-term investments, partially offset by a decrease in amounts owed to sublicensees. Other expense for 2013 is primarily due to an increase in amounts owed to sublicensees, partially offset by changes in certain liabilities.
Income taxes
We recorded an income tax benefit of $192.1 million for the year ended December 31, 2015 compared to an income tax expense from continuing operations of $0.4 million for the year ended December 31, 2014 and an income tax expense of $0.4 million for the year ended December 31, 2013. The income tax benefit for the year ended December 31, 2015 is primarily the result of releasing a valuation allowance against a significant portion of our deferred tax assets. The tax benefit is primarily comprised of U.S. federal and state net operating loss carryforwards, tax credits, and other temporary differences.
The income tax expense recognized in 2014 and 2013 is primarily attributable to deferred taxes associated with the amortization of acquired IPR&D assets for tax purposes.
30
Discontinued operations, net
Avinza Product Line
On September 6, 2006, we and King Pharmaceuticals, now a subsidiary of Pfizer, entered into a purchase agreement, or the Avinza Purchase Agreement, pursuant to which Pfizer acquired all of our rights in and to Avinza in the United States, its territories and Canada, and to assume certain liabilities as set forth in the Avinza Purchase Agreement.
Pursuant to the terms of the Avinza Purchase Agreement, we retained the liability for returns of product from wholesalers that had been sold by us prior to the close of this transaction. Accordingly, as part of the accounting for the gain on the sale of Avinza, we recorded a reserve for Avinza product returns. For the years ended December 31, 2015, 2014 and 2013, we recognized pre-tax gains of $0, $0, and $2.6 million, respectively, due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date.
Net loss attributable to noncontrolling interests
We recorded $2.4 million as a net loss attributable to noncontrolling interests for the year ended December 31, 2015 compared with $1.1 million for the year ended December 31, 2014. The net loss attributable to noncontrolling interests was recorded as a result of our determination that prior to Viking's IPO we held a variable interest in Viking. We recorded 100% of the losses incurred from May 21, 2014 through deconsolidation of Viking, as net loss attributable to noncontrolling interest due to the fact that we are considered a primary beneficiary with no equity interest in the variable interest entity. Viking was deconsolidated upon IPO and we no longer hold a variable interest in Viking.
Liquidity and Capital Resources
We have financed our operations through offerings of our equity securities, borrowings from long-term debt, issuance of convertible notes, product sales and the subsequent sales of our commercial assets, royalties, license fees, milestones and other revenues, capital and operating lease transactions.
We had net income of $229.8 million for the year ended December 31, 2015. At December 31, 2015, our accumulated deficit was $429.5 million and we had a working capital deficit of $8.1 million. We believe that our currently available funds, cash generated from operations as well as existing sources of and access to financing will be sufficient to fund our anticipated operating, capital requirements and debt service requirement. We expect to build cash in the future as we continue to generate significant cash flow from royalty, license and milestone revenue and Captisol material sales primarily driven by continued increases in Promacta and Kyprolis sales, recent product approvals and regulatory developments, as well as revenue from anticipated new licenses and milestones. In addition, we anticipate that our liquidity needs can be met through other sources, including sales of marketable securities, borrowings through commercial paper and/or syndicated credit facilities and access to other domestic and foreign debt markets and equity markets.
Investments
We invest our excess cash principally in U.S. government debt securities, investment-grade corporate debt securities and certificates of deposit. We have established guidelines relative to diversification and maturities of our investments in order to provide both safety and liquidity. These guidelines are periodically reviewed and modified to take advantage of trends in yields and interest rates. Additionally, we own certain securities which are classified as short-term investments that we received in December 2012 and June 2014 as a result of an event-based payment and an upfront license payment, respectively, under licenses.
Borrowings and Other Liabilities
2019 Convertible Senior Notes
We have convertible debt outstanding as of December 31, 2015 related to our 2019 Convertible Senior Notes. In August 2014, we issued $245.0 million aggregate principal amount of convertible senior unsecured notes. The Notes are convertible into common stock upon satisfaction of certain conditions. Interest of 0.75% per year is payable semi-annually on August 15th and February 15th through the maturity of the notes in August 2019.
31
Repurchases of Common Stock
During the year ended December 31, 2015, we repurchased 6,120 common shares at a weighted average price of $79.92 per share pursuant to the repurchase plan, or approximately $0.5 million of common shares.
During the year ended December 31, 2014, we repurchased 1,253,425 common shares at a weighted average price of $54.20 per share pursuant to the repurchase plan, or approximately $68.0 million of common shares.
Contingent Liabilities
CyDex
In connection with the acquisition of CyDex in January 2011, we issued a series of CVRs and also assumed certain contingent liabilities. We may be required to make additional payments upon achievement of certain clinical and regulatory milestones to the CyDex shareholders and former license holders. In addition, through 2016 we will pay CyDex shareholders 20% of all CyDex-related annual revenue exceeding $15.0 million; plus an additional 10% of all CyDex-related annual revenue exceeding $35.0 million.
Metabasis
In connection with the acquisition of Metabasis in January 2010, we entered into four CVR agreements with Metabasis shareholders. The CVRs entitle the holders to cash payments upon the sale or licensing of certain assets and upon the achievement of specified milestones.
Leases and Off-Balance Sheet Arrangements
We lease our office facilities under operating lease arrangements with varying terms through April 2023. The agreements provide for increases in annual rents based on changes in the Consumer Price Index or fixed percentage increases ranging from 3.0% to 3.5%. We also sublease a portion of our facilities through leases which expire in 2016. The sublease agreements provide for a 3% increase in annual rents. We had no off-balance sheet arrangements at December 31, 2015, 2014 and 2013.
Contractual Obligations
As of December 31, 2015, future minimum payments due under our contractual obligations are as follows (in thousands):
Payments Due by Period | |||||||||||||||||||
Total | Less than 1 year | 1-2 years | 3-4 years | Thereafter | |||||||||||||||
Purchase obligations (1) | $ | 12,328 | $ | 10,196 | $ | 2,132 | $ | — | $ | — | |||||||||
Contingent liabilities (2) | $ | 5,390 | $ | 5,390 | $ | — | $ | — | $ | — | |||||||||
Note and interest payment obligations | $ | 252,351 | $ | 1,838 | $ | 3,675 | $ | 246,838 | $ | — | |||||||||
Operating lease obligations (3) | $ | 2,691 | $ | 1,762 | $ | 313 | $ | 275 | $ | 341 | |||||||||
(1) | Purchase obligations represent our commitments under our supply agreement with Hovione for Captisol purchases. |
(2) | Contingent liabilities to former shareholders and licenseholders are subjective and affected by changes in inputs to the valuation model including management’s assumptions regarding revenue volatility, probability of commercialization of products, estimates of timing and probability of achievement of certain revenue thresholds and developmental and regulatory milestones and affect amounts owed to former license holders and CVR holders. As of December 31, 2015, only those liabilities for revenue sharing payments and milestones achieved as a result of 2015 activities are included in the table above. |
(3) | Represents minimum future lease payments under our non-cancellable operating leases. These amounts assume that the lease for our current corporate headquarters terminates on April 30, 2016, pursuant to a termination agreement with our landlord, even though we received a letter from our landlord disputing the date of such termination. If we are obligated to pay rents under the lease after April 30, 2016, we will be required to make aggregate future minimum lease payments totalling $2.3 million (nondiscounted) over the duration of the lease as follows which are not included in the table above: $0.5 million within less than one year, $1.5 million within one to two years, and $0.4 million within three years. Additionally, we sublease portions of office and research facilities located in our current corporate headquarters and would receive additional |
32
sublease income of $1.4 million through the end of such lease which are not in the table above: $0.3 million within less than one year, $0.9 million within one to two years, and $0.2 million within three years.
We are also required under our CyDex CVR Agreement to invest at least $1.5 million per year, inclusive of employee expenses, in the acquired business through 2015. As of December 31, 2015, we exceeded that amount.
Operating Activities
Operating activities provided cash of $41.7 million, $20.6 million and $20.7 million in 2015, 2014 and 2013, respectively.
The cash provided in 2015 reflects net income of $227.4 million and $186.5 million of non-cash items to reconcile the net income to net cash used in operations. These reconciling items primarily reflect a net deferred tax asset of $192.1 million from the release of our valuation allowance, a $28.2 million gain on deconsolidation of Viking, and a $2.6 million gain on the sale of investments. Partially offsetting non-cash change in estimated value of contingent liabilities of $5.0 million, $5.1 million loss on equity investment of Viking, depreciation and amortization of $2.6 million, stock-based compensation of $12.5 million, amortization of debt discount and issuance fees of $10.3 million, and a decrease in the fair value of the Viking convertible note of $0.8 million. The cash provided by operations in 2015 is further impacted by changes in operating assets and liabilities due primarily to a decrease in accounts receivable of $6.5 million and a decrease in restricted cash of $1.3 million. Partially offsetting, other assets increased $0.3 million, accounts payable and accrued liabilities decreased $4.0 million, deferred revenue decreased $2.2 million and inventory increased $0.4 million.
The cash provided in 2014 reflects net income of $10.9 million and $20.6 million of non-cash items to reconcile the net income to net cash used in operations. These reconciling items primarily reflect a non-cash change in estimated value of contingent liabilities of $5.1 million, depreciation and amortization of $2.7 million, stock-based compensation of $11.3 million, amortization of debt discount and issuance fees of $3.7 million, accretion of notes payable of $0.2 million, a non-cash milestone payment received of $1.2 million, realized gain on investments of $1.5 million and net deferred tax assets and liabilities of $0.4 million. The cash provided by operations in 2014 is further impacted by changes in operating assets and liabilities due primarily to an increase in accounts receivable of $10.4 million, an increase in other assets of $1.9 million and a decrease in accounts payable and accrued liabilities of $3.2 million. Partially offsetting this, inventory decreased $4.4 million and restricted cash decreased $0.1 million.
The cash provided in 2013 reflects net income of $11.4 million, adjusted by $2.6 million of gain from discontinued operations and $13.2 million of non-cash items to reconcile the net income to net cash used in operations. These reconciling items primarily reflect a non-cash change in estimated value of contingent liabilities of $3.6 million, depreciation and amortization of $2.7 million, stock-based compensation of $5.7 million, write-off of in-process research and development $0.5 million, accretion of notes payable of $0.4 million, and net deferred tax assets and liabilities of $0.4 million. The cash provided by operations in 2013 is further impacted by changes in operating assets and liabilities due primarily to a decrease in accounts receivable of $2.4 million, a decrease in inventory of $0.6 million, and a decrease in other assets of $0.1 million. Partially offsetting this, accounts payable and accrued liabilities decreased $2.8 million, other liabilities decreased $0.4 million and deferred revenue decreased $0.7 million. Net cash used in operating activities of discontinued operations was $0.6 million in 2013.
Investing Activities
Investing activities used cash of $112.9 million, $2.0 million, and $5.0 million in 2015, 2014, and 2013, respectively.
Cash used by investing activities in 2015 primarily reflects the purchase of short-term investments of $166.0 million, purchase of Viking common stock of $9.0 million, purchase of commercial license rights of $4.0 million, payments to CyDex CVR holders and other contingency payments of $6.7 million, $0.2 million for a reduction in cash due to deconsolidation of Viking and purchases of property and equipment of $0.1 million. Partially offsetting, investing activities generated proceeds from the maturity of short-term investments of $57.2 million and $16.0 million from the sale of short-term investments.
Cash used by investing activities in 2014 primarily reflects the purchase of commercial license rights of $1.0 million and payments to CyDex CVR holders and other contingency payments of $3.5 million, partially offset by proceeds from the sale of short-term investments of $2.3 million and proceeds from the sale of property, building and equipment of $0.1 million.
Cash used by investing activities in 2013 primarily reflects the purchase of commercial license rights of $3.6 million, payments to CyDex CVR holders of $1.0 million, and purchases of property, building and equipment of $0.4 million.
33
Financing Activities
Financing activities provided cash of $8.4 million and $130.0 million in 2015 and 2014, respectively and used cash of $16.5 million in 2013.
Cash provided by financing activities in 2015 primarily reflects the $8.8 million of proceeds received from stock option exercises and our employee stock purchase plan, partially offset by payment for share repurchases of $0.5 million.
Cash provided by financing activities in 2014 primarily reflects the gross proceeds received from the issuance of an aggregate $245.0 million of the 2019 Convertible Senior Notes, proceeds from issuance of warrants of $11.6 million, and $4.6 million of proceeds received from stock option exercises and our employee stock purchase plan, partially offset by repayment of debt of $9.4 million, purchase of convertible bond hedge of $48.1 million, payment for share repurchases of $68.0 million and payment of debt issuance costs of $5.7 million.
Cash used in financing activities in 2013 primarily reflects the repayment of debt of $19.6 million, partially offset by proceeds of $3.1 million received from stock option exercises and purchases under our employee stock purchase plan.
Critical Accounting Policies
Certain of our policies require the application of management judgment in making estimates and assumptions that affect the amounts reported in the consolidated financial statements and disclosures made in the accompanying notes. Those estimates and assumptions are based on historical experience and various other factors deemed to be applicable and reasonable under the circumstances. The use of judgment in determining such estimates and assumptions is by nature, subject to a degree of uncertainty. Accordingly, actual results could differ materially from the estimates made. Our critical accounting policies are as follows:
Revenue Recognition
Royalties on sales of products commercialized by our partners are recognized in the quarter reported by the respective partner. Generally, we receive royalty reports from our licensees approximately one quarter in arrears due to the fact that our agreements require partners to report product sales between 30-60 days after the end of the quarter. The Company recognizes royalty revenues when it can reliably estimate such amounts and collectability is reasonably assured. Under this accounting policy, the royalty revenues reported are not based upon estimates and such royalty revenues are typically reported to the Company by its partners in the same period in which payment is received.
Revenue from material sales of Captisol is recognized upon transfer of title, which normally passes upon shipment to the customer, provided all other revenue recognition criteria have been met. All product returns are subject to the Company's credit and exchange policy, approval by the Company and a 20% restocking fee. To date, product returns by customers have not been material to net material sales in any related period. The Company records revenue net of product returns, if any, and sales tax collected and remitted to government authorities during the period.
Many of the Company's revenue arrangements for Captisol involve a license agreement with the supply of manufactured Captisol product. Licenses may be granted to pharmaceutical companies for the use of Captisol product in the development of pharmaceutical compounds. The supply of the Captisol product may be for all phases of clinical trials and through commercial availability of the host drug or may be limited to certain phases of the clinical trial process. The Company evaluates the deliverables in these agreements to determine whether they have stand-alone value to our customers and therefore meet the criteria to be accounted for as separate units of accounting or they should be combined with other deliverables and accounted for as a single unit of accounting. Management believes that the Company's licenses have stand-alone value at the outset of an arrangement because the customer obtains the right to use Captisol in its formulations without any additional input by the Company.
Other nonrefundable, upfront license fees are recognized as revenue upon delivery of the license, if the license is determined to have standalone value that is not dependent on any future performance by the Company under the applicable collaboration agreement. Nonrefundable contingent event-based payments are recognized as revenue when the contingent event is met, which is usually the earlier of when payments are received or collections are assured, provided that it does not require future performance by the Company. Sales-based contingent payments from partners are accounted for similarly to royalties, with revenue recognized upon achievement of the sales targets assuming all other revenue recognition criteria are met. The Company occasionally has sub-license obligations related to arrangements for which it receives license fees, milestones and royalties. The Company evaluates the determination of gross versus net reporting based on each individual agreement.
Revenue from development and regulatory milestones is recognized when earned, as evidenced by written acknowledgement from the collaborator, provided that (1) the milestone event is substantive, its achievability was not
34
reasonably assured at the inception of the agreement, and the Company has no further performance obligations relating to that event, and (2) collectability is reasonably assured. If these criteria are not met, the milestone payment is recognized over the remaining period of the Company’s performance obligations under the arrangement.
Revenue from research funding under our collaboration agreements is earned and recognized on a percentage-of completion basis as research hours are incurred in accordance with the provisions of each agreement.
Valuation of intangible assets and goodwill
We review the carrying value of our finite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If such circumstances exist, an estimate of undiscounted future cash flows to be generated by the long-lived asset is compared to the carrying value to determine whether an impairment exists. If an asset is determined to be impaired, the loss is measured based on the difference between the asset's fair value and its carrying value. As of December 31, 2015, 2014, and 2013 there has been no impairment of finite-lived assets.
Indefinite-lived intangible assets, composed of IPR&D assets acquired in a business combination and we have not obtained the regulatory approval for marketing or abandoned the associated research and development effors, are reviewed annually for impairment and whenever events or changes in circumstances that would indicate a reduction in the fair value of the IPR&D projects below their respective carrying amounts. If the asset's carrying value exceeds its fair value, an impairment charge is recorded for the difference and its carrying value is reduced accordingly. Estimating future net cash flows of an IPR&D assets for purposes of an impairment analysis requires us to make significant estimates and assumptions regarding the the amount, timing and probability of achieving revenues from various regulatory milestone events and the completed product for the projects we licensed to partners, as well as amount and timing of costs to complete for projects we currently develop independently. Consequently, the eventual realized value of an acquired IPR&D asset may vary from its estimated fair value at the date of acquisition, and IPR&D impairment charges may occur in future periods which could have a material adverse effect on our results of operations. As of December 31, 2015 and 2014, there has been no impairment of IPR&D assets. We recorded $0.5 million impairment to one of the IPR&D assets in 2013.
Similar to IPR&D assets, we perform an impairment analysis for goodwill on at least an annual basis, usually as of December 31 of each year, absent any indicators of earlier impairment. We use the income approach and the market approach, each weighted at 50%, for goodwill impairment analysis. For the income approach, we consider the present value of future cash flows and the carrying value of its assets and liabilities, including goodwill. The market approach is based on an analysis of revenue multiples of guideline public companies. If the carrying value of the assets and liabilities, including goodwill, were to exceed our estimation of the fair value, we would record an impairment charge in an amount equal to the excess of the carrying value of goodwill over the implied fair value of the goodwill. As of December 31, 2015, 2014, and 2013 there has been no impairment of goodwill.
Contingent Liabilities
In connection with our acquisition of CyDex in January 2011, we recorded contingent liabilities for amounts potentially due to holders of the CyDex CVR's and certain other contingency payments. The fair value of the liability is assessed at each reporting date using the income approach incorporating the estimated future cash flows from potential milestones and revenue sharing. The change in fair value is recorded in our consolidated statements of operations. The carrying amount of the liability may fluctuate significantly and actual amounts paid may be materially different than the carrying amount of the liability.
In connection with our acquisition of Metabasis in January 2010, we issued Metabasis stockholders four tradable CVRs, one CVR from each of four respective series of CVR, for each Metabasis share. The CVRs entitle Metabasis stockholders to cash payments as proceed is received by us from the sale or partnering of any of the Metabasis drug development programs. The fair values of the CVRs are remeasured at each reporting date through the term of the related agreement. Changes in the fair values are reported in the statement of operations as income (decreases) or expense (increases). The carrying amount of the liability may fluctuate significantly based upon quoted market prices and actual amounts paid under the agreements may be materially different than the carrying amount of the liability.
Income Taxes
Income taxes are accounted for under the liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of differences between the tax basis of assets or liabilities and their carrying amounts in the consolidated financial statements. The Company provides a valuation allowance for deferred tax assets if it is more likely than not that these items will expire before we are able to realize their benefit. The Company calculates the
35
valuation allowance in accordance with the authoritative guidance relating to income taxes under ASC 740, Income Taxes, which requires an assessment of both positive and negative evidence that is available regarding the reliability of these deferred tax assets, when measuring the need for a valuation allowance. Developing the provision for income taxes requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and, if necessary, any valuation allowances that may be required for deferred tax assets. The Company's judgments and tax strategies are subject to audit by various taxing authorities. While management believes the Company has provided adequately for its income tax liabilities in its consolidated financial statements, adverse determinations by these taxing authorities could have a material adverse effect on the Company's consolidated financial condition and results of operations.
Stock-Based Compensation
Stock-based compensation cost for awards to employees and non-employee directors is recognized on a straight-line basis over the vesting period until the last tranche vests.
The fair-value for options that were awarded to employees and directors was estimated at the date of grant using the Black-Scholes option valuation model with the following weighted average assumptions:
Year Ended December 31, | ||||||
2015 | 2014 | 2013 | ||||
Risk-free interest rate | 1.7%-2.0% | 1.9% | 1.13%-1.82% | |||
Expected volatility | 50%-58% | 62%-69% | 69% | |||
Expected term | 6.5 years | 6 years | 6 years | |||
Forfeiture rate | 8.52% | 8.6%-9.7% | 8.4%-9.8% | |||
Variable Interest Entities
We identify an entity as a variable interest entity, or VIE, if either: (1) the entity does not have sufficient equity investment at risk to permit the entity to finance its activities without additional subordinated financial support, or (2) the entity's equity investors lack the essential characteristics of a controlling financial interest. If the Company is no longer the primary of a VIE or the entity is no longer considered as a VIE as facts and circumstances changed, it deconsolidates the entity under the applicable accounting guidance. When perform the analysis for certain transaction such as our investment in Viking (Refer to Note 2 to the consolidated financials for details), the Company considered certain criteria, including risk and reward sharing, experience and financial condition of its partner, voting rights, involvement in day-to-day operating decisions, the Company’s representation on the entity's executive committee, and level of economics between the Company and the entity.
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
We are exposed to market risk from interest rates and equity prices which could affect our results of operations, financial condition and cash flows. We manage our exposure to these market risks through our regular operating and financing activities.
Investment Portfolio Risk
At December 31, 2015, our investment portfolio included investments in available-for-sale equity securities of $102.8 million. These securities are subject to market risk and may decline in value based on market conditions.
Equity Price Risk
Our 2019 Convertible Senior Notes include conversion and settlement provisions that are based on the price of our common stock at conversion or maturity of the notes, as applicable. The minimum amount of cash we may be required to pay is $245.0 million, but will ultimately be determined by the price of our common stock. The fair values of our 2019 Convertible Senior Notes are dependent on the price and volatility of our common stock and will generally increase or decrease as the market price of our common stock changes. In order to minimize the impact of potential dilution to our common stock upon the conversion of the 2019 Convertible Senior Notes, we entered into convertible bond hedges covering 3,264,643 shares of our common stock. Concurrently with entering into the convertible bond hedge transactions, we entered into warrant transactions whereby we sold warrants with an exercise price of approximately $125.08 per share, subject to adjustment. Throughout the
36
term of the 2019 Convertible Senior Notes, the notes may have a dilutive effect on our earnings per share to the extent the stock price exceeds the conversion price of the notes. Additionally, the warrants may have a dilutive effect on our earnings per share to the extent the stock price exceeds the strike price of the warrants.
Foreign Currency Risk
Through our licensing and business operations, we are exposed to foreign currency risk. Foreign currency exposures arise from transactions denominated in a currency other than the functional currency and from foreign denominated revenues and profit translated into U.S. dollars. Our collaborative partners sell our products worldwide in currencies other than the U.S. dollar. Because of this, our revenues from royalty payments are subject to risk from changes in exchange rates.
We purchase Captisol from Hovione, located in Lisbon, Portugal. Payments to Hovione are denominated and paid in U.S. dollars; however the unit price of Captisol contains an adjustment factor which is based on the sharing of foreign currency risk between the two parties. The effect of an immediate 10% change in foreign exchange rates would not have a material impact on our financial condition, results of operations or cash flows. We do not currently hedge our exposures to foreign currency fluctuations.
Interest Rate Risk
We are exposed to market risk involving rising interest rates. To the extent interest rates rise, our interest costs could increase. An increase in interest costs of 10% would not have a material impact on our financial condition, results of operations or cash flows.
Item 8. | Consolidated Financial Statements and Supplementary Data |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Page | |
37
Report of Independent Registered Public Accounting Firm
Board of Directors and Shareholders of Ligand Pharmaceuticals Incorporated
We have audited the accompanying consolidated balance sheets of Ligand Pharmaceuticals Incorporated (the “Company”) as of December 31, 2015 and 2014, and the related consolidated statements of operations, comprehensive income (loss), changes in shareholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2015. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Ligand Pharmaceuticals Incorporated as of December 31, 2015 and 2014, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2015 in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 1, the 2015 consolidated financial statements have been restated to correct errors.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2015, based on criteria established in the 2013 Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated February 26, 2016 (except for the material weaknesses discussed in the Management’s Report on Internal Control over Financial Reporting, as to which the date is November 14, 2016) expressed an adverse opinion.
/s/ GRANT THORNTON LLP
San Diego, California
February 26, 2016 (except for 2015 Restatement described in Note 1 and the effects thereof, as to which the date is November 14, 2016)
38
LIGAND PHARMACEUTICALS INCORPORATED
CONSOLIDATED BALANCE SHEETS
(in thousands, except share data)
December 31, | |||||||
2015 | 2014 | ||||||
Restated | |||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 97,428 | $ | 160,203 | |||
Short-term investments | 102,791 | 7,133 | |||||
Accounts receivable, net | 6,170 | 12,634 | |||||
Note receivable from Viking | 4,782 | — | |||||
Inventory | 1,633 | 269 | |||||
Capitalized IPO expenses, VIE | — | 2,268 | |||||
Current debt issuance costs | — | 809 | |||||
Other current assets | 1,908 | 1,842 | |||||
Total current assets | 214,712 | 185,158 | |||||
Deferred income taxes | 189,083 | — | |||||
Investment in Viking | 29,728 | — | |||||
Intangible assets, net | 48,347 | 50,723 | |||||
Goodwill | 12,238 | 12,238 | |||||
Commercial license rights | 8,554 | 4,568 | |||||
Restricted cash | — | 1,261 | |||||
Property and equipment, net | 372 | 486 | |||||
Long-term debt issuance costs | — | 3,388 | |||||
Other assets | 27 | 207 | |||||
Total assets | $ | 503,061 | $ | 258,029 | |||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 4,083 | $ | 7,698 | |||
Accrued liabilities | 5,397 | 4,866 | |||||
Current contingent liabilities | 10,414 | 6,796 | |||||
Current lease exit obligations | 934 | 2,356 | |||||
2019 convertible senior notes, net | 201,985 | ||||||
Other current liabilities | 8 | 1,063 | |||||
Total current liabilities | 222,821 | 22,779 | |||||
Long-term notes payable | — | 195,908 | |||||
Long-term contingent liabilities | 3,033 | 8,353 | |||||
Long-term deferred revenue, net | — | 2,085 | |||||
Long-term lease exit obligations | — | 934 | |||||
Long-term deferred income taxes | — | 2,792 | |||||
Other long-term liabilities | 297 | 770 | |||||
Total liabilities | 226,151 | 233,621 | |||||
Commitments and contingencies | |||||||
Equity component of currently redeemable convertible notes (Note 5) | 39,628 | — | |||||
Stockholders’ equity: | |||||||
Common stock, $0.001 par value; 33,333,333 shares authorized; 19,949,012 and 19,575,150 shares issued and outstanding at December 31, 2015 and 2014, respectively | 20 | 20 | |||||
Additional paid-in capital | 661,850 | 680,660 | |||||
Accumulated other comprehensive income | 4,903 | 4,953 | |||||
Accumulated deficit | (429,491 | ) | (659,315 | ) | |||
Total stockholders’ equity attributable to parent | 237,282 | 26,318 | |||||
Noncontrolling interests | — | (1,910 | ) | ||||
Total stockholder's equity | 237,282 | 24,408 | |||||
Total liabilities and stockholders’ equity | $ | 503,061 | $ | 258,029 | |||
39
LIGAND PHARMACEUTICALS INCORPORATED
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share amounts)
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Restated | |||||||||||
Revenues: | |||||||||||
Royalties | $ | 38,194 | $ | 29,994 | $ | 23,584 | |||||
Material sales | 27,662 | 28,488 | 19,072 | ||||||||
License fees, milestones and other revenues | 6,058 | 6,056 | 6,317 | ||||||||
Total revenues | 71,914 | 64,538 | 48,973 | ||||||||
Operating costs and expenses: | |||||||||||
Cost of material sales | 5,807 | 9,136 | 5,732 | ||||||||
Research and development | 13,380 | 12,122 | 9,274 | ||||||||
General and administrative | 24,378 | 22,570 | 17,984 | ||||||||
Lease exit and termination costs | 1,020 | 1,084 | 560 | ||||||||
Write-off of acquired IPR&D | — | — | 480 | ||||||||
Total operating costs and expenses | 44,585 | 44,912 | 34,030 | ||||||||
Income from operations | 27,329 | 19,626 | 14,943 | ||||||||
Other (expense) income: | |||||||||||
Interest expense, net | (11,802 | ) | (4,860 | ) | (2,077 | ) | |||||
Increase in contingent liabilities | (5,013 | ) | (5,135 | ) | (3,597 | ) | |||||
Gain on deconsolidation of Viking | 28,190 | — | — | ||||||||
Equity in net losses from Viking | (5,143 | ) | — | — | |||||||
Other, net | 1,768 | 1,671 | (63 | ) | |||||||
Total other income (expense), net | 8,000 | (8,324 | ) | (5,737 | ) | ||||||
Income from continuing operations before income tax benefit | 35,329 | 11,302 | 9,206 | ||||||||
Income tax benefit (expense) from continuing operations | 192,115 | (410 | ) | (374 | ) | ||||||
Income from continuing operations including noncontrolling interests | 227,444 | 10,892 | 8,832 | ||||||||
Less: Net loss attributable to noncontrolling interests | (2,380 | ) | (1,132 | ) | — | ||||||
Net income from continuing operations | 229,824 | 12,024 | 8,832 | ||||||||
Discontinued operations: | |||||||||||
Gain on sale of Avinza Product Line, net | — | — | 2,588 | ||||||||
Net income | $ | 229,824 | $ | 12,024 | $ | 11,420 | |||||
Basic per share amounts: | |||||||||||
Income from continuing operations | $ | 11.61 | $ | 0.59 | $ | 0.43 | |||||
Income from discontinued operations | — | — | 0.13 | ||||||||
Net income | $ | 11.61 | $ | 0.59 | $ | 0.56 | |||||
Weighted average number of common shares-basic | 19,790 | 20,419 | 20,312 | ||||||||
Diluted per share amounts: | |||||||||||
Income from continuing operations | $ | 10.83 | $ | 0.56 | $ | 0.43 | |||||
Income from discontinued operations | — | — | 0.12 | ||||||||
Net income | $ | 10.83 | $ | 0.56 | $ | 0.55 | |||||
Weighted average number of common shares-diluted | 21,228 | 21,433 | 20,745 | ||||||||
See accompanying notes to these consolidated financial statements.
40
LIGAND PHARMACEUTICALS INCORPORATED
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(in thousands)
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Restated | |||||||||||
Net income | $ | 229,824 | $ | 12,024 | $ | 11,420 | |||||
Unrealized net gain on available-for-sale securities, net of tax | 1,933 | 3,872 | 2,914 | ||||||||
Less:Reclassification of net realized gains included in net income, net of tax | $ | (1,965 | ) | $ | (1,833 | ) | $ | — | |||
Comprehensive income | $ | 229,792 | $ | 14,063 | $ | 14,334 | |||||
See accompanying notes to these consolidated financial statements.
41
LIGAND PHARMACEUTICALS INCORPORATED
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands, except share data)
Common Stock | Additional paid-in capital | Accumulated other comprehensive income (loss) | Accumulated deficit | Noncontrolling interest | Treasury stock | Total stockholders’ equity (deficit) | |||||||||||||||||||||||||||
Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||||
Balance at December 31, 2012 | 21,278,606 | $ | 21 | $ | 751,503 | $ | — | $ | (682,759 | ) | $ | — | (1,118,222 | ) | $ | (42,280 | ) | $ | 26,485 | ||||||||||||||
Issuance of common stock under employee stock compensation plans, net | 308,137 | 1 | 3,127 | — | — | — | — | — | 3,128 | ||||||||||||||||||||||||
Stock-based compensation | — | — | 5,666 | — | — | — | — | — | 5,666 | ||||||||||||||||||||||||
Retirement of treasury shares | (1,118,222 | ) | (1 | ) | (42,279 | ) | — | — | — | 1,118,222 | 42,280 | — | |||||||||||||||||||||
Unrealized net gain on available-for-sale securities | — | — | — | 2,914 | — | — | — | — | 2,914 | ||||||||||||||||||||||||
Net income | — | — | — | — | 11,420 | — | — | — | 11,420 | ||||||||||||||||||||||||
Balance at December 31, 2013 | 20,468,521 | $ | 21 | $ | 718,017 | $ | 2,914 | $ | (671,339 | ) | $ | — | — | $ | — | $ | 49,613 | ||||||||||||||||
Consolidation of Viking | — | — | — | — | — | (778 | ) | — | — | (778 | ) | ||||||||||||||||||||||
Issuance of common stock under employee stock compensation plans, net | 360,054 | — | 4,561 | — | — | — | — | — | 4,561 | ||||||||||||||||||||||||
Stock-based compensation | — | — | 11,270 | — | — | — | — | — | 11,270 | ||||||||||||||||||||||||
Repurchase of common stock | (1,253,425 | ) | (1 | ) | (67,954 | ) | — | — | — | — | — | (67,955 | ) | ||||||||||||||||||||
Sale of warrants | — | — | 11,638 | — | — | — | — | — | 11,638 | ||||||||||||||||||||||||
Purchase of convertible bond hedge | — | — | (48,143 | ) | — | — | — | — | — | (48,143 | ) | ||||||||||||||||||||||
Equity component of convertible debt issuance, net of issuance costs | — | — | 51,271 | — | — | — | — | — | 51,271 | ||||||||||||||||||||||||
Other comprehensive income | — | — | — | 2,039 | — | — | — | — | 2,039 | ||||||||||||||||||||||||
Net income | — | — | — | — | 12,024 | — | — | — | 12,024 | ||||||||||||||||||||||||
Net loss in noncontrolling interests | — | — | — | — | — | (1,132 | ) | — | — | (1,132 | ) | ||||||||||||||||||||||
Balance at December 31, 2014 | 19,575,150 | $ | 20 | $ | 680,660 | $ | 4,953 | $ | (659,315 | ) | $ | (1,910 | ) | — | $ | — | $ | 24,408 | |||||||||||||||
Issuance of common stock under employee stock compensation plans, net | 379,982 | — | 8,849 | — | — | — | — | — | 8,849 | ||||||||||||||||||||||||
Reclassification of equity component of currently redeemable convertible notes (Restated) | — | — | (39,628 | ) | — | — | — | — | — | (39,628 | ) | ||||||||||||||||||||||
Stock-based compensation | — | — | 12,458 | — | — | — | — | — | 12,458 | ||||||||||||||||||||||||
Repurchase of common stock | (6,120 | ) | — | (489 | ) | — | — | — | — | — | (489 | ) | |||||||||||||||||||||
Other comprehensive income | — | — | — | (50 | ) | — | — | — | — | (50 | ) | ||||||||||||||||||||||
Net income (Restated) | — | — | — | — | 229,824 | — | — | — | 229,824 | ||||||||||||||||||||||||
Net loss in noncontrolling interests | — | — | — | — | — | (2,380 | ) | — | — | (2,380 | ) | ||||||||||||||||||||||
Deconsolidation of Viking | — | — | — | — | — | 4,290 | — | — | 4,290 | ||||||||||||||||||||||||
Balance at December 31, 2015 | 19,949,012 | $ | 20 | $ | 661,850 | $ | 4,903 | $ | (429,491 | ) | $ | — | — | $ | — | $ | 237,282 | ||||||||||||||||
See accompanying notes to these consolidated financial statements.
42
LIGAND PHARMACEUTICALS INCORPORATED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
Year Ended December 31, | |||||||||||
2015 Restated | 2014 | 2013 | |||||||||
Operating activities | |||||||||||
Net income | $ | 227,444 | $ | 10,892 | $ | 11,420 | |||||
Less: gain from discontinued operations | — | — | 2,588 | ||||||||
Income from continuing operations | 227,444 | 10,892 | 8,832 | ||||||||
Adjustments to reconcile net income to net cash used in operating activities: | |||||||||||
Write-off of acquired in-process research and development | — | — | 480 | ||||||||
Change in estimated fair value of contingent liabilities | 5,013 | 5,135 | 3,597 | ||||||||
Realized gain on sale of short-term investment | (2,603 | ) | (1,538 | ) | — | ||||||
Depreciation and amortization | 2,627 | 2,657 | 2,663 | ||||||||
Gain on deconsolidation of Viking | (28,190 | ) | — | — | |||||||
Loss on equity investment in Viking | 5,143 | — | — | ||||||||
Change in fair value of the convertible debt receivable from Viking | 765 | — | — | ||||||||
Amortization of debt discount and issuance fees | 10,274 | 3,694 | — | ||||||||
Non-cash milestone revenue | — | (1,211 | ) | — | |||||||
Stock-based compensation | 12,458 | 11,270 | 5,666 | ||||||||
Deferred income taxes | (192,132 | ) | 410 | 374 | |||||||
Other | 107 | 206 | 422 | ||||||||
Changes in operating assets and liabilities, net of acquisition: | |||||||||||
Accounts receivable, net | 6,489 | (10,412 | ) | 2,367 | |||||||
Inventory | (401 | ) | 4,369 | 646 | |||||||
Restricted cash | 1,261 | — | — | ||||||||
Other current assets | 51 | (426 | ) | (130 | ) | ||||||
Other long term assets | (325 | ) | (1,439 | ) | 218 | ||||||
Accounts payable and accrued liabilities | (4,027 | ) | (3,121 | ) | (3,149 | ) | |||||
Deferred revenue | (2,227 | ) | 80 | (654 | ) | ||||||
Net cash provided by operating activities of continuing operations | 41,727 | 20,566 | 21,332 | ||||||||
Net cash used in operating activities of discontinued operations | — | — | (642 | ) | |||||||
Net cash provided by operating activities | 41,727 | 20,566 | 20,690 | ||||||||
Investing activities | |||||||||||
Purchase of commercial license rights | (4,030 | ) | (1,000 | ) | (3,571 | ) | |||||
Purchase of Viking common stock | (9,000 | ) | — | — | |||||||
Reduction of cash due to deconsolidation of Viking | (247 | ) | — | — | |||||||
Payments to CVR holders and other contingency payments | (6,740 | ) | (3,493 | ) | (989 | ) | |||||
Purchases of property and equipment | (93 | ) | (6 | ) | (377 | ) | |||||
Purchases of short-term investments | (166,025 | ) | — | — | |||||||
Proceeds from sale of short-term investments | 16,039 | 2,342 | — | ||||||||
Proceeds from maturity of short-term investments | 57,234 | — | — | ||||||||
Other, net | — | 130 | (37 | ) | |||||||
Net cash used in investing activities | (112,862 | ) | (2,027 | ) | (4,974 | ) | |||||
Financing activities | |||||||||||
Repayment of debt | — | (9,366 | ) | (19,586 | ) | ||||||
Gross proceeds from issuance of 2019 Convertible Senior Notes | — | 245,000 | — | ||||||||
Payment of debt issuance costs | — | (5,711 | ) | — | |||||||
Proceeds from issuance of warrants | — | 11,638 | — | ||||||||
Purchase of convertible bond hedge | — | (48,143 | ) | — | |||||||
Net proceeds from stock option exercises | 8,849 | 4,561 | 3,128 | ||||||||
43
Share repurchases | (489 | ) | (67,954 | ) | — | ||||||
Net cash provided by (used in) financing activities | 8,360 | 130,025 | (16,458 | ) | |||||||
Net (decrease) increase in cash and cash equivalents | (62,775 | ) | 148,564 | (742 | ) | ||||||
Cash and cash equivalents at beginning of year | 160,203 | 11,639 | 12,381 | ||||||||
Cash and cash equivalents at end of year | $ | 97,428 | $ | 160,203 | $ | 11,639 | |||||
Supplemental disclosure of cash flow information | |||||||||||
Cash paid during the year: | |||||||||||
Interest paid | $ | 1,822 | $ | 494 | $ | 1,816 | |||||
Taxes paid | $ | 28 | $ | 18 | $ | 26 | |||||
Supplemental schedule of non-cash investing and financing activities | |||||||||||
Accrued inventory purchases | $ | 1,333 | $ | 3,246 | $ | 341 | |||||
Unrealized gain on AFS investments | $ | 3,005 | $ | 3,872 | $ | 2,914 | |||||
See accompanying notes to these consolidated financial statements.
44
LIGAND PHARMACEUTICALS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Summary of significant accounting policies
Business
Ligand is a biopharmaceutical company with a business model that is based upon the concept of developing or acquiring royalty revenue generating assets and coupling them with a lean corporate cost structure.
Principles of Consolidation
The accompanying consolidated financial statements include Ligand and its wholly owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires the use of estimates and assumptions that affect the amounts reported in the condensed consolidated financial statements and the accompanying notes. Actual results may differ from those estimates
Correction of Previously Reported Financials
In connection with the preparation of the financial statements for the year ended December 31, 2015, the Company determined that the deferred tax assets and the tax benefit previously reported in our condensed and consolidated financial statements as of and for the three- and nine-month periods ended September 30, 2015 reflected an error in the calculation of certain capital loss carry-forwards at September 30, 2015 related to the sale of the Avinza product line. The error resulted in an understatement of long-term deferred income tax assets of $2.1 million, which represents approximately 1% of the previously reported deferred tax assets as of September 30, 2015, and an understatement of tax benefit as well as net income of approximately $2.1 million for the three- and nine-month periods ended September 30, 2015. The impact on basic and diluted EPS for the same periods of $0.11 per share and $0.10 per share, respectively, represents less than 1% of the previously reported EPS. While concluded the error was not material to any prior periods, individually or in the aggregate, based on our qualitative and quantitative analysis, management opted to correct the error by restating the respective amounts that were previously reported as of and for the three- and nine-month periods ended September 30, 2015 in this 10-K filing. Please refer to Note 11. Summary of Unaudited Quarterly Financial Information for details.
2015 Restatement
The Company is restating its previously issued consolidated financial statements as of and for the year ended December 31, 2015 and the condensed consolidated financial statements as of and for the three and nine months ended September 30, 2015 to correct errors relating to the Company's net operating loss (NOL) carryforward benefits in the United States which resulted in an overstatement of deferred tax assets (DTA). In connection with three acquisitions that were completed prior to February 2010, the Company recognized DTA for a portion of the NOLs, which included capitalized research and development expenses, obtained from the acquired businesses. From the time of the acquisitions until September 2015, there was a full valuation allowance against all of the Company’s NOLs, including those obtained from the entities acquired. In September 2015, the Company concluded that the valuation allowance against substantially all of its DTA was no longer required based on its then recent income and projections of sustained profitability. As a result, the Company released its DTA valuation allowance in full, including $27.5 million related to NOLs recognized as part of the businesses acquired prior to February of 2010.
During the quarter ended September 30, 2016, the Company concluded that for accounting purposes the approximately $27.5 million of DTA that were obtained upon acquiring the businesses prior to February of 2010 did not meet the more likely-than-not criterion for recognition in 2015 and that the related valuation allowance should not have been reversed. As a result, the Company's income tax benefit and net income for the year ended December 31, 2015 were overstated by $27.5 million each.
The Company also recorded adjustments to the consolidated financial statements as part of this restatement relating to the classification of our 2019 Convertible Senior Notes. As of December 31, 2015, the Company's last reported sale price exceeded the 130% threshold described in Note 5 - "Financing Arrangements" and accordingly the 2019 Convertible Senior Notes have been reclassified as a current liability as of December 31, 2015. As a result, the related unamortized discount of $39.6 million was classified as temporary equity component of currently redeemable convertible notes on our Consolidated Balance Sheet.
The account balances labeled As Reported in the following tables as of and for the year ended December 31, 2015 represent the previously reported amounts as presented in the Company's Annual Report on Form 10-K for the year ended December 31, 2015. For the effects of correcting the errors related to the DTA on the condensed and consolidated financial statements for the interim period ended September 30, 2015, please refer to Note 11 Summary of Unaudited Quarterly Financial Information.
The effects of these prior period corrections on the statement of operations and comprehensive income are as follows (in thousands except for per share data):
Year ended December 31, 2015 | |||||||||||
As Reported | Adjustments | As Restated | |||||||||
Income tax benefit | $ | 219,596 | $ | (27,481 | ) | $ | 192,115 | ||||
Net income | 257,305 | (27,481 | ) | 229,824 | |||||||
Comprehensive income | 257,273 | (27,481 | ) | 229,792 | |||||||
Basic earnings per share | 13.00 | (1.39 | ) | 11.61 | |||||||
Diluted earnings per share data | 12.12 | (1.29 | ) | 10.83 | |||||||
The effects of these prior period corrections on the consolidated balance sheet is as follows:
As of December 31, 2015 | |||||||||||||||
As Reported | Adoption of ASU 2015-03(1) | Adjustments | As Restated | ||||||||||||
Current debt issuance costs | $ | 860 | $ | (860 | ) | $ | — | $ | — | ||||||
Long-term debt issuance costs | $ | 2,527 | $ | (2,527 | ) | $ | — | $ | — | ||||||
Deferred income taxes | $ | 216,564 | $ | — | $ | (27,481 | ) | $ | 189,083 | ||||||
Total assets | 533,929 | (3,387 | ) | (27,481 | ) | 503,061 | |||||||||
2019 convertible senior notes, net - current | — | (3,387 | ) | 205,372 | 201,985 | ||||||||||
Total current liabilities | 20,836 | (3,387 | ) | 205,372 | 222,821 | ||||||||||
2019 convertible senior notes, net - long term(1) | 205,372 | — | (205,372 | ) | — | ||||||||||
Total liabilities | 229,538 | (3,387 | ) | — | 226,151 | ||||||||||
Equity component of currently redeemable convertible notes (Note 5) | — | — | 39,628 | 39,628 | |||||||||||
Additional paid-in capital | 701,478 | — | (39,628 | ) | 661,850 | ||||||||||
Accumulated deficit | (402,010 | ) | — | (27,481 | ) | (429,491 | ) | ||||||||
Total stockholders' equity | 304,391 | — | (67,109 | ) | 237,282 | ||||||||||
Total liabilities and stockholders' equity | 533,929 | (3,387 | ) | (27,481 | ) | 503,061 | |||||||||
(1) Unamortized issuance cost was reclassified to debt discount in this 10-K/A form due to that it is filed after the Company's retrospective adoption of ASU 2015-03, Interest-Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs in Q1 2016. | |||||||||||||||
Upon the occurrence of certain circumstances, holders of the 2019 Convertible Senior Notes may require us to purchase all or a portion of their notes for cash, which may require the use of a substantial amount of cash. If such cash is not available, we may be required to sell other assets or enter into alternate financing arrangements at terms that may or may not be desirable. The existence of the 2019 Convertible Senior Notes and the obligations that we incurred by issuing them may restrict our ability to take advantage of certain future opportunities, such as engaging in future debt or equity financing activities.
45
The corrections did not have any impact on the company's cash flow statements for any period.
Correction of Immaterial Errors
During the three and nine months ended September 30, 2015, a clerical error was identified in the calculation of the projections used in the June 30, 2015 and September 30, 2015 valuation of contingent liabilities related to CyDex CVR holders. The error in the June 30, 2015 projection resulted in an understatement of short-term contingent liabilities of $0.6 million as of June 30, 2015, and an overstatement of net income of $0.6 million, or $0.03 per share for the three and six months ended June 30, 2015, respectively. No other error was identified in the other interim period(s) in 2015 or 2014 based on the Company's review in those periods. The impact of correcting the error resulted in an understatement of net income of $0.6 million, or $0.03 per share for the three months ended September 30, 2015. Based on a qualitative and quantitative analysis of the error, the Company concluded that it is immaterial to the interim condensed consolidated financial statements for the three and six months ended June 30, 2015 and had no effect on the trend of financial results. As such, the Company has corrected the error in the condensed consolidated financial statements for the period ended September 30, 2015.
Reclassifications
Certain reclassifications have been made to the previously issued statement of operations for comparability purposes. These reclassifications had no effect on the reported net income, stockholders' equity and operating cash flows as previously reported.
Income Per Share
Basic income per share is calculated by dividing net income by the weighted-average number of common shares outstanding during the period. Diluted income per share is computed by dividing net income by the weighted-average number of common shares and common stock equivalents of all dilutive securities calculated using the treasury stock method and the if-converted method.
The total number of potentially dilutive securities including stock options and warrants excluded from the computation of diluted income per share because their inclusion would have been anti-dilutive, were 3.3 million, 5.1 million and 0.8 million for the years ended December 31, 2015, 2014 and 2013 respectively. In addition, the Company issued 793,594 shares of its common stock in January 2016 as part of the consideration for the acquisition of Open Monoclonal Technology, Inc. (Refer to Note 12 for details), which was not included in basic and diluted income per share for the year ended December 31, 2015.
46
The following table presents the computation of basic and diluted net income per share for the periods indicated (in thousands, except per share amounts):
Year Ended December 31, | |||||||||||
Restated | |||||||||||
EPS Attributable to Common Shareholders | 2015 | 2014 | 2013 | ||||||||
Net income from continuing operations | $ | 229,824 | $ | 12,024 | $ | 8,832 | |||||
Discontinued operations | — | — | 2,588 | ||||||||
Net income | $ | 229,824 | $ | 12,024 | $ | 11,420 | |||||
Shares used to compute basic income per share | 19,790 | 20,419 | 20,312 | ||||||||
Dilutive potential common shares: | |||||||||||
Restricted stock | 56 | 36 | 80 | ||||||||
Stock options | 882 | 978 | 353 | ||||||||
2019 Convertible Senior Notes | 499 | — | — | ||||||||
Shares used to compute diluted income per share | 21,228 | 21,433 | 20,745 | ||||||||
Basic per share amounts: | |||||||||||
Income from continuing operations | $ | 11.61 | $ | 0.59 | $ | 0.43 | |||||
Discontinued operations | — | — | 0.13 | ||||||||
Net income | $ | 11.61 | $ | 0.59 | $ | 0.56 | |||||
Diluted per share amounts: | |||||||||||
Income from continuing operations | $ | 10.83 | $ | 0.56 | $ | 0.43 | |||||
Discontinued operations | — | — | 0.12 | ||||||||
Net income | $ | 10.83 | $ | 0.56 | $ | 0.55 | |||||
Cash Equivalents
Cash equivalents consist of all investments with maturities of three months or less from the date of acquisition.
Short-term Investments
Short-term investments primarily consist of investments in debt securities that have effective maturities greater than three months and less than twelve months from the date of acquisition. The Company classifies its short-term investments as "available-for-sale". Such investments are carried at fair value, with unrealized gains and losses included in the statement of comprehensive income (loss). The Company determines the cost of investments based on the specific identification method.
Restricted Investments
Restricted investments consist of certificates of deposit held with a financial institution as collateral under a facility lease and third-party service provider arrangements.
47
The following table summarizes the various investment categories at December 31, 2015 and 2014 (in thousands):
Cost | Gross unrealized gains | Gross unrealized losses | Estimated fair value | ||||||||||||
December 31, 2015 | |||||||||||||||
Short-term investments | |||||||||||||||
Bank deposits | 43,043 | — | (4 | ) | 43,039 | ||||||||||
Corporate bonds | 41,238 | — | (35 | ) | 41,203 | ||||||||||
Commercial paper | 1,747 | — | — | 1,747 | |||||||||||
Asset backed securities | 10,020 | — | (5 | ) | 10,015 | ||||||||||
Corporate equity securities | 1,843 | 4,944 | — | 6,787 | |||||||||||
$ | 97,891 | $ | 4,944 | $ | (44 | ) | $ | 102,791 | |||||||
December 31, 2014 | |||||||||||||||
Short-term investments (Corporate equity securities) | 2,179 | 4,954 | $ | — | $ | 7,133 | |||||||||
Certificates of deposit-restricted | 1,261 | — | — | 1,261 | |||||||||||
$ | 3,440 | $ | 4,954 | $ | — | $ | 8,394 | ||||||||
Concentrations of Business Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash equivalents and investments.
The Company invests its excess cash principally in United States government debt securities, investment grade corporate debt securities and certificates of deposit. The Company has established guidelines relative to diversification and maturities that maintain safety and liquidity. These guidelines are periodically reviewed and modified to take advantage of trends in yields and interest rates. During 2015, the Company did not experience any significant losses on its cash equivalents, short-term investments or restricted investments.
A relatively small number of partners accounts for a significant percentage of our revenue. Revenue from significant partners, which is defined as 10% or more of our total revenue, was as follows:
December 31, | ||||||||
2015 | 2014 | 2013 | ||||||
Partner A | 27 | % | 37 | % | 33 | % | ||
Partner B | 23 | % | 31 | % | 28 | % | ||
Partner C | 18 | % | 10 | % | 14 | % | ||
The Company obtains Captisol from a single supplier, Hovione. If this supplier were not able to supply the requested amounts of Captisol, the Company would be unable to continue to derive revenues from the sale of Captisol until it obtained an alternative source, which could take a considerable length of time.
Inventory
Inventory, which consists of finished goods, is stated at the lower of cost or market value. The Company determines cost using the first-in, first-out method. The Company analyzes its inventory levels periodically and writes down inventory to its net realizable value if it has become obsolete, has a cost basis in excess of its expected net realizable value or is in excess of expected requirements. There were no write downs related to obsolete inventory recorded for the years ended December 31, 2015 and 2014. As of December 31, 2015, the commitment under our supply agreement with Hovione for Captisol purchases was $12.3 million.
48
Allowance for Doubtful Accounts
The Company maintains an allowance for doubtful accounts based on the best estimate of the amount of probable losses in the Company’s existing accounts receivable. Accounts receivable that are outstanding longer than their contractual payment terms, ranging from 30 to 90 days, are considered past due. When determining the allowance for doubtful accounts, several factors are taken into consideration, including historical write-off experience and review of specific customer accounts for collectability. Account balances are charged off against the allowance after collection efforts have been exhausted and the potential for recovery is considered remote. There was no allowance for doubtful accounts recorded as of December 31, 2015 and 2014.
Goodwill and Other Identifiable Intangible Assets
Goodwill and other identifiable intangible assets consist of the following (in thousands):
December 31, | |||||||
2015 | 2014 | ||||||
Indefinite lived intangible assets | |||||||
IPR&D | $ | 12,556 | $ | 12,556 | |||
Goodwill | 12,238 | 12,238 | |||||
Definite lived intangible assets | |||||||
Complete technology | 15,267 | 15,267 | |||||
Less: Accumulated amortization | (3,762 | ) | (2,999 | ) | |||
Trade name | 2,642 | 2,642 | |||||
Less: Accumulated amortization | (652 | ) | (519 | ) | |||
Customer relationships | 29,600 | 29,600 | |||||
Less: Accumulated amortization | (7,304 | ) | (5,824 | ) | |||
Total goodwill and other identifiable intangible assets, net | $ | 60,585 | $ | 62,961 | |||
Amortization of finite lived intangible assets is computed using the straight-line method over the estimated useful life of the asset of 20 years. Amortization expense of $2.4 million was recognized in each of the three years ending December 31, 2015, 2014, and 2013. Estimated amortization expense for the years ending December 31, 2016 through 2021 is $2.4 million per year. For each of the years ended December 31, 2015, 2014, and 2013, there was no impairment of intangible assets with finite lives.
The Company accounts for goodwill in accordance with Accounting Standards Codification ("ASC"), 350, Goodwill and Other Intangibles. The Company performs its impairment analysis for goodwill and certain non-amortizing intangibles on at least an annual basis. The Company uses the income approach and the market approach, each weighted at 50%, for goodwill impairment analysis. For the income approach, the Company considers the present value of future cash flows and the carrying value of its assets and liabilities, including goodwill. The market approach is based on an analysis of revenue multiples of guideline public companies. If the carrying value of the assets and liabilities, including goodwill, were to exceed the Company’s estimation of the fair value, the Company would record an impairment charge in an amount equal to the excess of the carrying value of goodwill over the implied fair value of the goodwill. The Company performs an evaluation of goodwill as of December 31 of each year, absent any indicators of earlier impairment, to ensure that impairment charges, if applicable, are reflected in the Company's financial results before December 31 of each year. When it is determined that impairment has occurred, a charge to operations is recorded. Goodwill and other intangible asset balances are included in the identifiable assets of the business segment to which they have been assigned. As of December 31, 2015, 2014 and 2013 there has been no impairment of goodwill for continuing operations.
49
Intangible assets related to acquired IPR&D are considered to be indefinite-lived until the completion or abandonment of the associated research and development efforts. During the period the assets are considered to be indefinite-lived, they are not amortized but are tested for impairment on an annual basis and between annual tests if the Company becomes aware of any events occurring or changes in circumstances that would indicate a reduction in the fair value of the IPR&D projects below their respective carrying amounts. If and when development is complete, which generally occurs if and when regulatory approval to market a product is obtained, the associated assets would be deemed finite-lived and would then be amortized based on their respective estimated useful lives at that point in time. For the year ended December 31, 2013, the Company recorded a non-cash impairment charge of $0.5 million for the write-off of IPR&D for Captisol-enabled IV Clopidogrel. The impairment analysis was performed based on the income method using a Monte Carlo analysis. The asset was impaired upon notification from MedCo that they intended to terminate the license agreement and return the rights of the compound to the Company. Captisol-enabled IV Clopidogrel is an intravenous formulation of the anti-platelet medication designed for situations where the administration of oral platelet inhibitors is not feasible or desirable. For the years ended December 31, 2015 and December 31, 2014, there was no impairment of IPR&D assets.
Commercial license rights
Commercial license rights represent a portfolio of future milestone and royalty payment rights acquired from Selexis in April 2013 and April 2015. Individual commercial license rights acquired under the agreement are carried at allocated cost and approximate fair value. The carrying value of the license rights will be reduced on a pro-rata basis as revenue is realized over the term of the agreement. Declines in the fair value of license rights below their carrying value that are deemed to be other than temporary are reflected in earnings in the period such determination is made. As of December 31, 2015, management does not believe there have been any events or circumstances indicating that the carrying amount of its commercial license rights may not be recoverable.
Property and Equipment, net
Property and equipment is stated at cost and consists of the following (in thousands):
December 31, | |||||||
2015 | 2014 | ||||||
Lab and office equipment | $ | 2,248 | $ | 2,232 | |||
Leasehold improvements | 273 | 273 | |||||
Computer equipment and software | 632 | 624 | |||||
3,153 | 3,129 | ||||||
Less accumulated depreciation and amortization | (2,781 | ) | (2,643 | ) | |||
$ | 372 | $ | 486 | ||||
Depreciation of equipment is computed using the straight-line method over the estimated useful lives of the assets which range from three to ten years. Leasehold improvements are amortized using the straight-line method over their estimated useful lives or their related lease term, whichever is shorter. Depreciation expense of $0.2 million, $0.3 million, and $0.3 million was recognized for the years ending December 31, 2015, 2014, and 2013, respectively and is included in operating expenses.
Contingent Liabilities
CyDex contingent liabilities
In connection with the Company’s acquisition of CyDex in January 2011, the Company recorded a contingent liability for amounts potentially due to holders of the CyDex CVRs and former license holders. The liability is periodically assessed based on events and circumstances related to the underlying milestones, royalties and material sales. Any change in fair value is recorded in the Company’s consolidated statements of operations. The carrying amount of the liability may fluctuate significantly and actual amounts paid under the CVR agreements may be materially different than the carrying amount of the liability. The fair value of the liability at December 31, 2015 and 2014 was $9.5 million and $11.5 million, respectively. The Company recorded a fair value adjustment to increase the liability for CyDex related contingent liabilities of $3.8 million for the year ended December 31, 2015, $5.7 million increase in the liability for the year ended December 31, 2014 and a decrease in the liability of $0.6 million for the year ended December 31, 2013. Contingent liabilities decreased for cash payments to CVR holders and other contingency payments by $5.8 million during the year ended December 31, 2015, $3.5 million during the year ended December 31, 2014 and $1.0 million during the year ended December 31, 2013.
50
Metabasis contingent liabilities
In connection with the Company’s acquisition of Metabasis in January 2010, the Company issued Metabasis stockholders four tradable CVRs, one CVR from each of four respective series of CVR, for each Metabasis share. The CVRs will entitle Metabasis stockholders to cash payments as frequently as every six months as cash is received by the Company from proceeds from Metabasis’ partnership with Roche (which has been terminated) or the sale or partnering of any of the Metabasis drug development programs, among other triggering events. The acquisition-date fair value of the CVRs of $9.1 million was determined using quoted market prices of Metabasis common stock in active markets. The fair values of the CVRs are remeasured at each reporting date through the term of the related agreement. Changes in the fair values are reported in the statement of operations as income (decreases) or expense (increases). The carrying amount of the liability may fluctuate significantly based upon quoted market prices and actual amounts paid under the agreements may be materially different than the carrying amount of the liability. The fair value of the liability was $4.0 million and $3.7 million as of December 31, 2015 and 2014, respectively. The Company recorded an increase in the liability for CVRs of $1.2 million during the year ended December 31, 2015, a decrease of $0.5 million during the year ended December 31, 2014 and an increase of $4.2 million during the year ended December 31, 2013. Contingent liabilities decreased for cash payments to CVR holders by $0.9 million for the year ended December 31, 2015. No cash payments were made to Metabasis CVR holders for the years ended December 31, 2014 and 2013.
Revenue Recognition
Royalties on sales of products commercialized by the Company’s partners are recognized in the quarter reported by the respective partner. Generally, the Company receives royalty reports from its licensees approximately one quarter in arrears due to the fact that its agreements require partners to report product sales between 30 and 60 days after the end of the quarter. The Company recognizes royalty revenues when it can reliably estimate such amounts and collectability is reasonably assured. Under this accounting policy, the royalty revenues reported are not based upon estimates and such royalty revenues are typically reported to the Company by its partners in the same period in which payment is received.
Revenue from material sales of Captisol is recognized upon transfer of title, which normally passes upon shipment to the customer, provided all other revenue recognition criteria have been met. All product returns are subject to the Company's credit and exchange policy, approval by the Company and a 20% restocking fee. To date, product returns have not been material to net material sales in any related period. The Company records revenue net of product returns, if any, and sales tax collected and remitted to government authorities during the period.
The Company analyzes its revenue arrangements and other agreements to determine whether there are multiple elements that should be separated and accounted for individually or as a single unit of accounting. For multiple element contracts, arrangement consideration is allocated at the inception of the arrangement to all deliverables on the basis of relative selling price, using a hierarchy to determine selling price. Management first considers VSOE, then TPE and if neither VSOE nor TPE exist, the Company uses its best estimate of selling price.
Many of the Company's revenue arrangements for Captisol involve a license agreement with the supply of manufactured Captisol product. Licenses may be granted to pharmaceutical companies for the use of Captisol product in the development of pharmaceutical compounds. The supply of the Captisol product may be for all phases of clinical trials and through commercial availability of the host drug or may be limited to certain phases of the clinical trial process. Management believes that the Company's licenses have stand-alone value at the outset of an arrangement because the customer obtains the right to use Captisol in its formulations without any additional input by the Company.
Other nonrefundable, up-front license fees are recognized as revenue upon delivery of the license, if the license is determined to have standalone value that is not dependent on any future performance by the Company under the applicable collaboration agreement. Nonrefundable contingent event-based payments are recognized as revenue when the contingent event is met, which is usually the earlier of when payments are received or collections are assured, provided that it does not require future performance by the Company. The Company occasionally has sub-license obligations related to arrangements for which it receives license fees, milestones and royalties. Management evaluates the determination of gross versus net reporting based on each individual agreement.
Sales-based contingent payments from partners are accounted for similarly to royalties, with revenue recognized upon achievement of the sales targets assuming all other revenue recognition criteria for milestones are met. Revenue from development and regulatory milestones is recognized when earned, as evidenced by written acknowledgement from the collaborator, provided that (1) the milestone event is substantive, its achievability was not reasonably assured at the inception of the agreement, and the Company has no further performance obligations relating to that event, and (2) collectability is
51
reasonably assured. If these criteria are not met, the milestone payment is recognized over the remaining period of the Company’s performance obligations under the arrangement.
Revenue from research funding under our collaboration agreements is earned and recognized on a percentage-of completion basis as research hours are incurred in accordance with the provisions of each agreement.
In May 2014, the Company entered into a licensing agreement and research collaboration with Omthera. The research collaboration will target the development of novel products that utilize the proprietary Ligand developed LTP TECHNOLOGY to improve lipid-lowering activity of certain omega-3 fatty acids. The Company is eligible to receive compensation and reimbursement from Omthera for internal research effort and external costs incurred, as well as development and regulatory event-based payments. The completion of a proof of concept under the development program would trigger a $1.0 million payment which is determined to be a milestone under the milestone method of accounting as (1) it is an event that can only be achieved in part on the Company's past performance, (2) there was substantive uncertainty at the date the arrangement was entered into that the event would be achieved and (3) it results in additional payment being due to the Company. None of the other event-based payments represents a milestone under the milestone method of accounting. No event based payment or milestone was achieved during the periods presented. The Company received $0.5 million from Omthera in 2014 under the agreement and recognized $0.1 million and $0.4 million, respectively, for the years ended December 31, 2015 and 2014 as collaborative revenue based on the percentage of completion of the research program. No milestone payment or contingent payment was received in 2015.
Cost of Material Sales
The Company determines cost using the first-in, first-out method. Cost of material sales include all costs of purchase and other costs incurred in bringing the Captisol inventories to their present location and condition, costs to store, and distribute.
Preclinical Study and Clinical Trial Accruals
Substantial portions of the Company’s preclinical studies and all of the Company’s clinical trials have been performed by third-party laboratories, CROs. The Company accounts for a significant portion of its clinical study costs according to the terms of its contracts with CROs. The terms of its CRO contracts may result in payment flows that do not match the periods over which services are provided to us under such contracts. The Company's objective is to reflect the appropriate preclinical and clinical trial expenses in its financial statements in the same period as the services occur. As part of the process of preparing its financial statements, the Company relies on cost information provided by its CROs. The Company is also required to estimate certain of its expenses resulting from its obligations under its CRO contracts. Accordingly, the Company's preclinical study and clinical trial accrual is dependent upon the timely and accurate reporting of CROs and other third-party vendors. The Company periodically evaluates its estimates to determine if adjustments are necessary or appropriate as more information becomes available concerning changing circumstances, and conditions or events that may affect such estimates. No material adjustments to preclinical study and clinical trial accrued expenses have been recognized to date.
Income Taxes
Income taxes are accounted for under the asset and liability method. This approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of differences between the tax basis of assets or liabilities and their carrying amounts in the consolidated financial statements. The Company provides a valuation allowance for deferred tax assets if it is more likely than not that these items will expire before we are able to realize their benefit. The Company calculates the valuation allowance in accordance with the authoritative guidance relating to income taxes under ASC 740, Income Taxes, which requires an assessment of both positive and negative evidence that is available regarding the reliability of these deferred tax assets, when measuring the need for a valuation allowance. Developing the provision for income taxes requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and, if necessary, any valuation allowances that may be required for deferred tax assets. The Company's judgments and tax strategies are subject to audit by various taxing authorities. While management believes the Company has provided adequately for its income tax liabilities in its consolidated financial statements, adverse determinations by these taxing authorities could have a material adverse effect on the Company's consolidated financial condition and results of operations.
52
Research and Development Expenses
Research and development expense consists of labor, material, equipment, and allocated facilities costs of the Company’s scientific staff who are working pursuant to the Company’s collaborative agreements and other research and development projects. Also included in research and development expenses are third-party costs incurred for the Company’s research programs including in-licensing costs, CRO costs and costs incurred by other research and development service vendors. We expense these costs as they are incurred. When we make payments for research and development services prior to the services being rendered, we record those amounts as prepaid assets on our consolidated balance sheet and we expense them as the services are provided
Stock-Based Compensation
The Company grants options and awards to employees, non-employee consultants, and non-employee directors. Only new shares of common stock are issued upon the exercise of stock options. Non-employee directors are accounted for as employees. Options and restricted stock granted to certain directors vest in equal monthly installments over one year from the date of grant. Options granted to employees vest 1/8 on the six month anniversary of the date of grant, and 1/48 each month thereafter for forty-two months. All option awards generally expire ten years from the date of grant.
Stock-based compensation expense for awards to employees and non-employee directors is recognized on a straight-line basis over the vesting period until the last tranche vests. The fair-value for options that were awarded to employees and directors was estimated at the date of grant using the Black-Scholes option valuation model with the following weighted average assumptions:
Year Ended December 31, | ||||||
2015 | 2014 | 2013 | ||||
Risk-free interest rate | 1.7%-2.0% | 1.9% | 1.13%-1.82% | |||
Expected volatility | 50%-58% | 62%-69% | 69% | |||
Expected term | 6.5 years | 6 years | 6 years | |||
Forfeiture rate | 8.52% | 8.6%-9.7% | 8.4%-9.8% | |||
The risk-free interest rate is based on the U.S. Treasury yield curve at the time of the grant. The expected term of the employee and non-employee director options is the estimated weighted-average period until exercise or cancellation of vested options (forfeited unvested options are not considered) based on historical experience. Volatility is a measure of the expected amount of variability in the stock price over the expected life of an option expressed as a standard deviation. In making this assumption, the Company used the historical volatility of the Company’s stock price over a period equal to the expected term. The forfeiture rate is based on historical data at the time of the grant.
The following table summarizes stock-based compensation expense recorded as components of research and development expenses and general and administrative expenses for the periods indicated (in thousands):
December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Stock-based compensation expense as a component of: | |||||||||||
Research and development expenses | $ | 4,080 | $ | 3,595 | $ | 1,705 | |||||
General and administrative expenses | 8,378 | 7,675 | 3,961 | ||||||||
$ | 12,458 | $ | 11,270 | $ | 5,666 | ||||||
Segment Reporting
Under Accounting Standards Codification No. 280, “Segment Reporting” (ASC 280), operating segments are defined as components of an enterprise about which separate financial information is available that is regularly evaluated by the entity’s chief operating decision maker, in deciding how to allocate resources and in assessing performance. The Company has evaluated its operating segment in accordance with ASC 280, and has determined that the previously identified two reportable segments should be consolidated to one reporting segment at December 31, 2015. In earlier periods, the Company had identified two reporting segments: developing, licensing and manufacturing materials using Captisol reformulation technology by CyDex and development and licensing biopharmaceutical assets by Ligand. Due to the full integration of the these two
53
segments and the organizational changes during the year ended December 31, 2015, especially in the fourth quarter of 2015 as management evaluated, planned for, and executed the acquisition of a new business from Open Monoclonal Technology, Inc. (Refer to Note 12 for details), our chief operating decision maker now evaluates the performance of and manages the Company as one comprehensive business, which is development and licensing biopharmaceutical assets and coupling them with a lean corporate cost structure. As a result, management has concluded that the Company operates under one segment and there is one reporting segment at December 31, 2015, and all the respective disclosure under two reporting segments for 2014 and 2013 have been removed from this 10-K.
Comprehensive Income (Loss)
Comprehensive income (loss) represents net income (loss) adjusted for the change during the periods presented in unrealized gains and losses on available-for-sale securities less reclassification adjustments for realized gains or losses included in net income (loss). The unrealized gains or losses are reported on the Consolidated Statements of Comprehensive Income (Loss).
Variable Interest Entities ("VIE")
The Company identifies an entity as a VIE if either: (1) the entity does not have sufficient equity investment at risk to permit the entity to finance its activities without additional subordinated financial support, or (2) the entity's equity investors lack the essential characteristics of a controlling financial interest. The Company performs ongoing qualitative assessments of its VIEs to determine whether the Company has a controlling financial interest in any VIE and therefore is the primary beneficiary. If the Company is the primary beneficiary of a VIE, it consolidates the VIE under applicable accounting guidance. If the Company is no longer the primary of a VIE or the entity is no longer considered as a VIE as facts and circumstances changed, it deconsolidates the entity under the applicable accounting guidance. Beginning May 2015, the Company deconsolidated Viking, a previously reported VIE, and elected to record its investment in Viking under the equity method of accounting as Viking is no longer considered a VIE, and the Company does not have voting control or other elements of control that would require consolidation. The investment is subsequently adjusted for the Company’s share of Viking's operating results, and if applicable, cash contributions and distributions, which is reported on a separate line in our condensed consolidated statement of operations called “Equity in net losses of Viking”. On the condensed consolidated balance sheet, the Company reports its investment in Viking on a separate line in the non-current assets section called “Investment in Viking”. See Note 2, Investment in Viking, for additional details.
Convertible Debt
In August 2014, the Company completed a $245.0 million offering of convertible senior notes, which mature in 2019 and bear interest at 0.75%. The Company accounts for notes by separating the liability and equity components of the instrument in a manner that reflects the Company's nonconvertible debt borrowing rate. As a result, the Company assigned a value to the debt component of the notes equal to the estimated fair value of similar debt instruments without the conversion feature, which resulted in the Company recording the debt instrument at a discount. The Company is amortizing the debt discount over the life of the notes as additional non-cash interest expense utilizing the effective interest method.
Recent Accounting Pronouncements
In May 2014, FASB issued ASU 2014-09, Revenue from Contracts with Customers. ASU 2014-09 is effective for annual periods beginning after December 15, 2016 and interim periods within those annual periods. The revenue standard’s core principle is built on the contract between a vendor and a customer for the provision of goods and services. It attempts to depict the exchange of rights and obligations between the parties in the pattern of revenue recognition based on the consideration to which the vendor is entitled. To accomplish this objective, the standard requires five basic steps: (1) identify the contract with the customer, (2) identify the performance obligations in the contract, (3) determine the transaction price, (4) allocate the transaction price to the performance obligations in the contract, (5) recognize revenue when (or as) the entity satisfies a performance obligation. Management is currently evaluating the effect the adoption of this standard will have on the Company's financial statements.
54
In February 2015, FASB issued ASU 2015-02 Consolidation (Topic 810): Amendments to the Consolidation Analysis. ASU 2015-02 changes the analysis that a reporting entity must perform to determine whether it should consolidate certain types of legal entities. It is effective for annual reporting periods, and interim periods within those years, beginning after December 15, 2015. Early adoption is permitted, including adoption in an interim period. Management is currently evaluating the impact of the adoption of ASU 2015-02 on our consolidated financial statements.
In April 2015, FASB issued ASU 2015-03, Interest-Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs. This update was issued to simplify the presentation for debt issuance costs. Upon adoption, such costs shall be presented on our consolidated balance sheets as a direct deduction from the carrying amount of the related debt liability and not as a deferred charge presented in Other assets on our consolidated balance sheets. This amendment will be effective for interim and annual periods beginning on January 1, 2016, and is required to be retrospectively adopted. Management adopted the change in the presentation on our consolidated balance sheets in Q1 2016 retrospectively, and also changed the presentation on our consolidated balance sheet for the year ended December 31, 2015 accordingly in this Form 10-K/A, which was filed subsequent to the adoption (see Note 5 for details).
In November 2015, the FASB issued ASU 2015-17 Income Taxes (Topic 740), Balance Sheet Classification of Deferred Taxes that amends the presentation of deferred income taxes on our Consolidated Balance Sheet such that they are presented entirely as noncurrent assets and liabilities. As permitted by the standard, we adopted the new presentation prospectively, beginning January 1, 2015. Consistent with our prospective adoption, presentation of deferred income tax assets and liabilities as of December 31, 2014, was not restated. If they had been restated, Other current liabilities would have be reduced by $0.3 million and Long-term deferred tax liabilities would have been would have increased by $0.3 million.
In January 2016, the FASB issued ASU2016-01 Recognition and Measurement of Financial Assets and Financial Liabilities that amends the accounting and disclosures of financial instruments, including a provision that requires equity investments (except for investments accounted for under the equity method of accounting) to be measured at fair value with changes in fair value recognized in current earnings. The new standard is effective for interim and annual periods beginning on January 1, 2018. We are currently evaluating the impact that this new standard will have on our consolidated financial statements.
2. Investment in Viking
Transaction History
In May 2014, the Company entered into a MLA to license rights to five programs to Viking. Upon the consummation of the Viking IPO, Viking agreed to issue to the Company shares of Viking common stock having an aggregate value of approximately $29.2 million. In addition, Viking agreed to pay the Company royalties and milestone payments on products developed under the MLA. As part of this transaction, the Company extended a $2.5 million loan to Viking under a LSA. The loan accrues interest at a fixed rate equal to 5%.
In April 2015, the Company entered into an amendment to the MLA with Viking ("the MLA Amendment") which among other things, capped the Company’s aggregate ownership of Viking common stock to 49.9% of the Viking capital stock outstanding following the closing of the Viking IPO. Additionally, the Company and Viking entered into an amendment to the LSA Amendment, pursuant to which, the loans were no longer due and payable upon completion of the Viking IPO, but were extended to become due upon the earlier of: (i) a certain private qualified financing transaction or (ii) a public offering subsequent to the Viking IPO or (iii) one year after the closing of the Viking IPO. The Company may elect to receive equity of Viking common stock or cash equal to 200% of the principal amount plus accrued and unpaid interest. As of December 31, 2015, the aggregate fair market value of the note receivable was $4.8 million.
In May 2015, Viking completed the Viking IPO selling 3.5 million shares of its common stock at an initial offering price of $8.00 per share for an aggregate offering price of $27.6 million. In connection with the Viking IPO, the Company purchased 1.1 million shares for $9.0 million. In addition, pursuant to the amended MLA Amendment, the Company received approximately 3.7 million shares of Viking common stock having a value of $29.2 million based on the initial public offering price of $8.00 per share. As a result, the Company including its related parties owned an aggregate of 49.4% of the outstanding common stock of Viking, based on the shares of outstanding Viking common stock at December 31, 2015. As of December 31, 2015, the carrying value of the Company's investment in Viking was $29.7 million.
55
Accounting Consideration
In May 2014, the Company determined it held a variable interest in Viking. The Company's variable interests in Viking included the convertible note issued pursuant to the LSA and the Company’s potential upfront payment of equity pursuant to the MLA. The Company considered certain criteria, including risk and reward sharing, experience and financial condition of its partner, voting rights, involvement in day-to-day operating decisions, the Company’s representation on Viking's executive committee, and level of economics between the Company and Viking. Based on these criteria, and using its judgment, the Company determined that it was the primary beneficiary of Viking and, as a result, the Company consolidated Viking on its financial statements. From May 21, 2014 through May 4, 2015, the date of Viking’s IPO, recorded 100% of the losses incurred as net loss attributable to noncontrolling interest because it was a primary beneficiary with no equity interest in the VIE. The loans issued pursuant to the LSA were included as notes payable by Viking and were eliminated as long as the Company consolidated Viking on its financial statements.
Upon completion of the Viking IPO in May 2015, the Company determined that Viking was no longer a VIE. The Company also determined that it does not have voting control or other elements of control that would require consolidation of Viking. As a result of this assessment, the Company deconsolidated Viking on May 4, 2015 by derecognizing its assets, liabilities, and noncontrolling interest from the Company's consolidated financial statements. Applying deconsolidation accounting guidance, the Company determined, based on an independent valuation, the fair value of its equity investment in Viking upon deconsolidation was approximately $34.9 million after applying a discount on the Viking IPO price due to applicable transfer restrictions applicable to the Company as an affiliate of Viking pursuant to Rule 144 under the Securities Act of 1933. Based on a separate independent valuation, the Company determined that the fair value of the convertible notes receivable was approximately $5.5 million upon deconsolidation. The Company recorded a $28.2 million gain on deconsolidation of Viking in its consolidated statement of operations as of December 31, 2015.
Following the deconsolidation, the Company accounts for its equity investment in Viking under the equity method. For the year ended December 31, 2015, the Company reported approximately $5.1 million, as equity in net losses from Viking. The Company has opted to account for the Viking convertible notes receivable at fair value. For the year ended December 31, 2015, the Company recorded a change in the fair value of the Viking convertible notes of $0.8 million. See Note 3, Fair Value Measurements for additional details.
The following table represents the assets and liabilities, which are owned by and are obligations of Viking and are with no recourse to the Company, as of December 31, 2014 (in thousands):
December 31, 2014 | |||
Cash and cash equivalents | $ | 756 | |
Other current assets | 18 | ||
Capitalized IPO expenses | 2,268 | ||
Total current assets | 3,042 | ||
Other assets | 1 | ||
Total assets | $ | 3,043 | |
Accounts payable | 2,211 | ||
Accrued liabilities | 77 | ||
Current portion of notes payable | 334 | ||
Total current liabilities | 2,622 | ||
Long-term portion of notes payable (eliminates in consolidation) | 2,331 | ||
Total liabilities | $ | 4,953 | |
Metabasis CVR Payouts
In connection with the shares of Viking common stock received pursuant to the MLA, the Company will make a cash payment to the holders of certain Metabasis CVRs. The Company made a cash payment to certain holders of Metabasis CVRs
56
of $0.8 million during the year ended December 31, 2015. The remaining cash payment, made in January 2016, was $2.6 million. See Note 1. Summary of Significant Accounting Policies-Contingent Liabilities for additional information on the Metabasis CVRs.
3. Fair Value Measurement
The Company measures certain financial assets and liabilities at fair value on a recurring basis. Fair value is a market-based measurement that should be determined using assumptions that market participants would use in pricing an asset or liability. The Company establishes a three-level hierarchy to prioritize the inputs used in measuring fair value. The levels are described in the below with level 1 having the highest priority and level 3 having the lowest:
Level 1 - Observable inputs such as quoted prices in active markets
Level 2 - Inputs other than the quoted prices in active markets that are observable either directly or indirectly
Level 3 - Unobservable inputs in which there is little or no market data, which require the Company to develop its own assumptions
The following table provide a summary of the assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2015 and 2014 (in thousands):
Fair Value Measurements at Reporting Date Using | |||||||||||||||
December 31, 2015 | Quoted Prices in Active Markets for Identical Assets | Significant Other Observable Inputs | Significant Unobservable Inputs | ||||||||||||
Total | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Assets: | |||||||||||||||
Cash equivalents (1) | $ | 3,015 | $ | — | $ | 3,015 | $ | — | |||||||
Short-term investments (2) | 92,775 | 6,786 | 85,989 | — | |||||||||||
Note receivable Viking (3) | 4,782 | — | — | 4,782 | |||||||||||
Total assets | $ | 100,572 | $ | 6,786 | $ | 89,004 | $ | 4,782 | |||||||
Liabilities: | |||||||||||||||
Current contingent liabilities - CyDex (4) | $ | 7,812 | $ | — | $ | — | $ | 7,812 | |||||||
Current contingent liabilities-Metabasis (5) | $ | 2,602 | — | 2,602 | — | ||||||||||
Long-term contingent liabilities - Metabasis (5) | 1,355 | — | 1,355 | — | |||||||||||
Long-term contingent liabilities - CyDex (4) | 1,678 | — | — | 1,678 | |||||||||||
Liability for amounts owed to former licensees (6) | 794 | 794 | — | — | |||||||||||
Total liabilities | $ | 14,241 | $ | 794 | $ | 3,957 | $ | 9,490 | |||||||
Fair Value Measurements at Reporting Date Using | |||||||||||||||
December 31, 2014 | Quoted Prices in Active Markets for Identical Assets | Significant Other Observable Inputs * | Significant Unobservable Inputs | ||||||||||||
Total | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Assets: | |||||||||||||||
Cash equivalents (1) | $ | — | |||||||||||||
Current co-promote termination payments receivable (7) | $ | 322 | $ | — | $ | — | $ | 322 | |||||||
Short-term investments (2) | 7,133 | 7,133 | — | — | |||||||||||
Total assets | $ | 7,455 | $ | 7,133 | $ | — | $ | 322 | |||||||
Liabilities: | |||||||||||||||
Current contingent liabilities - CyDex (4) | $ | 6,796 | $ | — | $ | — | $ | 6,796 | |||||||
Current co-promote termination liability (7) | 322 | — | — | 322 | |||||||||||
Long-term contingent liabilities - Metabasis (5) | 3,652 | — | 3,652 | — | |||||||||||
Long-term contingent liabilities - CyDex (4) | 4,701 | — | — | 4,701 | |||||||||||
Liability for amounts owed to former licensees (6) | 773 | 773 | — | — | |||||||||||
Total liabilities | $ | 16,244 | $ | 773 | $ | 3,652 | $ | 11,819 | |||||||
*Adjusted to correct an error in disclosure that was deemed immaterial to the financial statements taken as a whole. Contingent liabilities related to Metabasis were reclassified from Level 1 to Level 2 as market is deemed inactive. Additionally, certain certificates of deposit with maturities less than 90 days were not previously disclosed in the table above.
(1) Highly liquid investments with maturities less than 90 days from the purchase date are recorded as cash equivalents that are classified as Level 2 of the fair value hierarchy, as these investment securities are valued based upon quoted prices for identical or similar instruments in markets that are not active, and model-based valuation techniques for which all significant assumptions are observable in the market.
(2) Investments in equity securities, are classified as level 1 as the fair value is determined using quoted market prices in active markets for the same securities. Short-term investments in marketable securities with maturities greater than 90 days are classified as level 2 of the fair value hierarchy, as these investment securities are valued based upon quoted prices for identical or similar instruments in markets that are not active, and model-based valuation techniques for which all significant assumptions are observable in the market.
(3) The fair value of the convertible note receivable from Viking was determined using a probability weighted option pricing model using a lattice methodology. The fair value is subjective and is affected by certain significant input to the valuation model such as the estimated volatility of the common stock, which was estimated to be 65% at December 31, 2015. Changes in these assumptions may materially affect the fair value estimate. For the year ended December 31, 2015, the Company reported a decrease in the fair value of the Viking convertible notes of $0.8 million in "Other, net" of the consolidated statement of operations.
(4) The fair value of the liabilities for CyDex contingent liabilities were determined based on the income approach using a Monte Carlo analysis. The fair value is subjective and is affected by changes in inputs to the valuation model including management’s assumptions regarding revenue volatility, probability of commercialization of products, estimates of timing and probability of achievement of certain revenue thresholds and developmental and regulatory milestones which may be achieved and affect amounts owed to former license holders and CVR holders. Changes in these assumptions can materially affect the fair value estimate.
(5) The liability for CVRs for Metabasis are determined using quoted market prices in an inactive market for the
underlying CVR.
(6) The liability for amounts owed to former licensees are determined using quoted market prices in active markets for the underlying investment received from a partner, a portion of which is owed to former licensees.
57
(7) The co-promote termination payments receivable represents a receivable for future payments to be made by Pfizer related to product sales and is recorded at its fair value. The receivable and liability will remain equal. The fair value is determined based on a valuation model using an income approach.
The following table represents significant unobservable inputs used in determining the fair value of contingent liabilities assumed in the acquisition of CyDex:
December 31, | ||||
2015 | 2014 | |||
Range of annual revenue subject to revenue sharing (1) | $22.5 million | $17.2 million-$17.3 million | ||
Revenue volatility | 25% | 25% | ||
Average of probability of commercialization | 73% | 81% | ||
Sales beta | 0.40 | 0.60 | ||
Credit rating | BB | B | ||
Equity risk premium | 6% | 6% | ||
(1) | Revenue subject to revenue sharing represent management’s estimate of the range of total annual revenue subject to revenue sharing (i.e. annual revenues in excess of $15 million) through December 31, 2016, which is the term of the CVR agreement. |
A reconciliation of the level 3 financial instruments as of December 31, 2015 is as follows (in thousands):
Assets: | |||
Fair value of level 3 financial instruments as of December 31, 2014 | $ | 322 | |
Assumed payments made by Pfizer or assignee | (390 | ) | |
Fair value adjustments to co-promote termination liability | 68 | ||
Note receivable Viking | 4,782 | ||
Fair value of level 3 financial instrument assets as of December 31, 2015 | $ | 4,782 | |
Liabilities | |||
Fair value of level 3 financial instruments as of December 31, 2014 | $ | 11,819 | |
Assumed payments made by Pfizer or assignee | (390 | ) | |
Payments to CVR holders and other contingency payments | (5,848 | ) | |
Fair value adjustments to contingent liabilities | 3,841 | ||
Fair value adjustments to co-promote termination liability | 68 | ||
Fair value of level 3 financial instruments as of December 31, 2015 | $ | 9,490 | |
Other Fair Value Measurements-2019 Convertible Senior Notes
In August 2014, the Company issued the 2019 Convertible Senior Notes. The Company uses a quoted market rate in an inactive market, which is classified as a Level 2 input, to estimate the current fair value of its 2019 Convertible Senior Notes. The estimated fair value of the 2019 Senior Convertible Notes was $377.9 million as of December 31, 2015. The carrying value of the notes does not reflect the market rate. See Note 7 Financing Arrangements for additional information.
Viking
The Company records its investment in Viking under the equity method of accounting. The investment is subsequently adjusted for the Company’s share of Viking's operating results, and if applicable, cash contributions and distributions. See Note 2 Investment in Viking for additional information. The market value of the Company's investment in Viking was $16.3 million as of December 31, 2015. The carrying value of the investment in Viking does not reflect the market value.
58
4. Lease Obligations
The Company leases office and laboratory facilities in California, Kansas and New Jersey. These leases expire between 2016 and 2019 and are subject to annual increases which range from 3.0% to 3.5%. The Company currently subleases office and laboratory space in California and New Jersey. The following table provides a summary of operating lease obligations and payments expected to be received from sublease agreements as of December 31, 2015 (in thousands):
Operating lease obligations: | Lease Termination Date | Less than 1 year | 1-2 years | 3-4 years | Thereafter | Total | ||||||||||||||||
Corporate headquarters-La Jolla, CA | April 2016 | $ | 230 | — | — | — | $ | 230 | ||||||||||||||
Corporate headquarters-San Diego, CA | April 2023 | 21 | 259 | 275 | 341 | $ | 896 | |||||||||||||||
Bioscience and Technology Business Center-Lawrence, KS | December 2017 | 54 | 54 | — | — | 108 | ||||||||||||||||
Vacated office and research facility-Cranbury, NJ | August 2016 | 1,743 | — | — | — | 1,743 | ||||||||||||||||
Total operating lease obligations | 2,048 | 313 | 275 | 341 | 2,977 | |||||||||||||||||
Sublease payments expected to be received: | ||||||||||||||||||||||
Office and research facility-La Jolla, CA | April 2016 | 145 | — | — | — | 145 | ||||||||||||||||
Office and research facility-Cranbury, NJ | August 2016 | 141 | — | — | — | 141 | ||||||||||||||||
Net operating lease obligations | $ | 1,762 | $ | 313 | $ | 275 | $ | 341 | $ | 2,691 | ||||||||||||
Lease termination
In November 2015, the Company entered into a lease termination agreement with its current lessor for the corporate headquarters facility located in La Jolla, California. The termination agreement accelerated the expiration date of the lease to April 2016, through which date, the Company is obligated to pay all base rent, operating expenses and other obligations due under the current lease. In addition, contingent upon the Company's surrender of the leased space in compliance with the termination agreement on or before April 2016, the Company is entitled to receive from the lessor a one-time lease buy-out payment equal to the base rent and the operating expenses paid for last six months of the revised lease term. In February 2016, the Company received a notice from its current landlord regarding the termination date of the lease and are currently in discussions to resolve any disputes.
In conjunction with the execution of the termination agreement, the Company entered into a new lease agreement with a different lessor for its corporate headquarters located in San Diego, California. The new lease has an initial term of approximately 7 years and is expected to commence in May 2016. The base rent under the new facility lease agreement is approximately $0.1 million per year for the first year, escalating 3.0% annually thereafter over the initial term. The Company has an option to extend the term of the lease for an additional five years. The lease is subject to additional charges for property management, common area maintenance and other costs.
Lease exit obligations
For the years ended December 31, 2015 and 2014, the Company had lease exit obligations of $0.9 million and $3.3 million, respectively. For the years ended December 31, 2015 and 2014, the Company made cash payments, net of sublease payments received of $3.3 million and $3.5 million, respectively. The Company recognized adjustments for accretion and changes in leasing assumptions of $0.9 million, $1.1 million and $0.6 million for the years ended December 31, 2015, 2014 and 2013, respectively.
Rent expense and deferred rent
Total rent expense under all office leases for 2015, 2014 and 2013 was $0.4 million, $0.7 million and $0.7 million, respectively. The Company recognizes rent expense on a straight-line basis. Deferred rent at December 31, 2015 and 2014 was $0.2 million and $0.3 million, respectively.
59
5. Financing Arrangements
2019 Convertible Senior Notes
In August 2014, the Company issued $245.0 million aggregate principal amount of its 2019 Convertible Senior Notes, resulting in net proceeds of $239.3 million. The 2019 Convertible Senior Notes are convertible into common stock at an initial conversion rate of 13.3251 shares per $1,000 principal amount of convertible notes, subject to adjustment upon certain events, which is equivalent to an initial conversion price of approximately $75.05 per share of common stock. The notes bear cash interest at a rate of 0.75% per year, payable semi-annually.
Holders of the 2019 Convertible Senior Notes may convert the notes at any time prior to the close of business on the business day immediately preceding May 15, 2019, under any of the following circumstances:
(1) during any fiscal quarter (and only during such fiscal quarter) commencing after December 31, 2014, if,
for at least 20 trading days (whether or not consecutive) during the 30 consecutive trading day period ending on the last trading day of the immediately preceding fiscal quarter, the last reported sale price of the Company's common stock on such trading day is greater than 130% of the conversion price on such trading day;
(2) during the five business day period immediately following any 10 consecutive trading day period, in which the trading price per $1,000 principal amount of notes was less than 98% of the product of the last reported sale price of the Company's common stock on such trading day and the conversion rate on each such trading day; or
(3) upon the occurrence of certain specified corporate events as specified in the indenture governing the notes.
As of December 31 2015, the Company's last reported sale price exceeded the 130% threshold described above and accordingly the Convertible Notes have been classified as a current liability as of December 31, 2015. As a result, the related unamortized discount of $39.6 million was classified as temporary equity component of currently redeemable convertible notes on our Consolidated Balance Sheet. The determination of whether or not the Convertible Notes are convertible as described above is made each quarter until maturity, conversion or repurchase. It is possible that the Convertible Notes may not be convertible in future periods, in which case the Convertible Notes would be classified as long-term debt, and the unamortized discount would be classified as permanent equity unless one of the other conversion events described above were to occur.
On or after May 15, 2019 until the close of business on the second scheduled trading day immediately preceding August 15, 2019, holders of the notes may convert all or a portion of their notes at any time. Upon conversion, Ligand must deliver cash to settle the principal and may deliver cash or shares of common stock, at the option of the Company, to settle any premium due upon conversion.
The Company separately accounted for the debt and equity components of the 2019 Convertible Senior Notes by allocating the $245.0 million total proceeds between the debt component and the embedded conversion option, or equity component, due to Ligand's ability to settle the 2019 Convertible Senior Notes in cash for the principal portion and to settle any premium in cash or common stock, at the Company's election. The debt allocation was performed in a manner that reflected the Company's non-convertible borrowing rate for similar debt of 5.83% derived from independent valuation analysis. The initial debt value of $192.5 million accretes at 5.83% to reach $245.0 million at the maturity date. The equity component of the 2019 Convertible Senior Notes was recognized as a debt discount and represents the difference between the $245.0 million proceeds at issuance of the 2019 Convertible Senior Notes and the fair value of the debt allocation on their respective issuance dates. The debt discount is amortized to interest expense using the effective interest method over the expected life of a similar liability without an equity component. As of December 31, 2015, the “if-converted value” exceeded the principal amount of the 2019 Convertible Senior Notes by $108.9 million.
In connection with the issuance of the 2019 Convertible Senior Notes, the Company incurred $5.7 million of issuance costs, which primarily consisted of underwriting, legal and other professional fees. The portions of these costs allocated to the equity components totaling $1.2 million were recorded as a reduction to additional paid-in capital. The portions of these costs allocated to the liability components totaling $4.5 million were recorded as assets on the balance sheet at the time the debt was issued. Beginning in 2016, the unamortized issuance costs allocated to the liability components are recorded as part of debt discount on the consolidated balance sheet upon the Company's respective adoption of ASU 2015-03, Interest-Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs. As such, we changed the presentation on the consolidated balance sheet for the year ended December 31, 2015 in this 10-K/A accordingly, which is filed subsequent to the adoption of the accounting guidance. As of December 31, 2015, $3.4 million issuance cost was included in the unamortized debt discount.
60
The portions allocated to the liability components are amortized to interest expense using the effective interest method over the expected life of the 2019 Convertible Senior Notes.
The Company determined the expected life of the debt discount for the 2019 Convertible Senior Notes to be equal to the original five-year term of the notes. The carrying value of the equity component related to the 2019 Convertible Senior Notes as of December 31, 2015, net of issuance costs, was $51.3 million.
Convertible Bond Hedge and Warrant Transactions
To minimize the impact of potential dilution to the Company's common stock upon conversion of the 2019 Convertible Senior Notes, the Company entered into convertible bond hedges and sold warrants covering 3,264,643 shares of its common stock. The convertible bond hedges have an exercise price of $75.05 per share and are exercisable when and if the 2019 Convertible Senior Notes are converted. If upon conversion of the 2019 Convertible Senior Notes, the price of the Company's common stock is above the exercise price of the convertible bond hedges, the counterparties will deliver shares of common stock and/or cash with an aggregate value approximately equal to the difference between the price of common stock at the conversion date and the exercise price, multiplied by the number of shares of common stock related to the convertible bond hedge transaction being exercised. The convertible bond hedges and warrants described below are separate transactions entered into by the Company and are not part of the terms of the 2019 Convertible Senior Notes. Holders of the 2019 Convertible Senior Notes and warrants will not have any rights with respect to the convertible bond hedges. The Company paid $48.1 million for these convertible bond hedges and recorded the amount as a reduction to additional paid-in capital.
Concurrently with the convertible bond hedge transactions, the Company entered into warrant transactions whereby it sold warrants to acquire approximately 3,264,643 shares of common stock with an exercise price of approximately $125.08 per share, subject to certain adjustments. The warrants have various expiration dates ranging from November 13, 2019 to April 22, 2020. The warrants will have a dilutive effect to the extent the market price per share of common stock exceeds the applicable exercise price of the warrants, as measured under the terms of the warrant transactions. The Company received $11.6 million for these warrants and recorded this amount to additional paid-in capital. The common stock issuable upon exercise of the warrants will be in unregistered shares, and the Company does not have the obligation and does not intend to file any registration statement with the Securities and Exchange Commission registering the issuance of the shares under the warrants.
The carrying values and the fixed contractual coupon rates of the Company's financing arrangements are as follows (dollars in thousands):
December 31, 2015 Restated | December 31, 2014 | ||||||
Convertible notes payable, 2.16% to 3.84%, due 2015, VIE | $ | — | $ | 334 | |||
2019 Convertible Senior Notes | |||||||
Principal amount outstanding | 245,000 | ||||||
Unamortized discount | (43,015 | ) | |||||
Net carrying amount | 201,985 | ||||||
Total current portion of notes payable | $ | 201,985 | $ | 334 | |||
2019 Convertible Senior Notes | |||||||
Principal amount outstanding | $ | — | $ | 245,000 | |||
Unamortized discount | — | (49,092 | ) | ||||
Net carrying amount | — | 195,908 | |||||
Total long-term portion of notes payable | $ | — | $ | 195,908 | |||
The fair value of the Company’s debt instruments approximates their carrying values as the interest is tied to or approximates market rates. As of December 31, 2015, there were no events of default or violation of any covenants under the Company's financing obligations.
61
6. Discontinued Operations
Avinza Product Line
In 2006, the Company and King, now a subsidiary of Pfizer, entered into a purchase agreement, or the Avinza Purchase Agreement, pursuant to which Pfizer acquired all of the Company's rights in and to Avinza in the United States, its territories and Canada, including, among other things, all Avinza inventory, records and related intellectual property, and assume certain liabilities as set forth in the Avinza Purchase Agreement. Pursuant to the terms of the Avinza Purchase Agreement, the Company retained the liability for returns of product from wholesalers that had been sold by the Company prior to the close of this transaction. Accordingly, as part of the accounting for the gain on the sale of Avinza, the Company recorded a reserve for Avinza product returns. For the years ended December 31, 2015, 2014 and 2013, the Company recognized pre-tax gains of $0, $0 and $2.6 million, respectively, due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date.
7. Other Balance Sheet Details
Other current assets consist of the following (in thousands):
December 31, | |||||||
2015 | 2014 | ||||||
Co-promote termination receivable | — | 322 | |||||
Prepaid expenses | $ | 1,177 | $ | 835 | |||
Other receivables | 731 | 685 | |||||
$ | 1,908 | $ | 1,842 | ||||
Accrued liabilities consist of the following (in thousands):
December 31, | |||||||
2015 | 2014 | ||||||
Compensation | $ | 1,711 | $ | 1,708 | |||
Legal | 726 | 459 | |||||
Amounts owed to former licensees | 915 | 925 | |||||
Royalties owed to third parties | 823 | 705 | |||||
Other | 1,222 | 1,069 | |||||
$ | 5,397 | $ | 4,866 | ||||
Other Long-Term Liabilities
Other long-term liabilities consist of the following (in thousands):
December 31, | |||||||
2015 | 2014 | ||||||
Deferred rent | — | 327 | |||||
Deposits | 268 | 411 | |||||
Other | 29 | 32 | |||||
$ | 297 | $ | 770 | ||||
8. Stockholders’ Equity
Stock Plans
In May 2009, the Company’s stockholders approved the amendment and restatement of the Company’s 2002 Stock Incentive Plan (the “Amended 2002 Plan”). The Company’s 2002 Stock Incentive Plan was amended to (i) increase the number
62
of shares available for issuance under the Amended 2002 Plan by 1.3 million shares, (ii) revise the list of performance criteria that may be used by the compensation committee for purposes of granting awards under the Amended 2002 Plan that are intended to qualify as performance-based compensation under Section 162(m) of the Internal Revenue Code, as amended, and (iii) eliminate the automatic option grant program for non-employee directors, the director fee stock issuance program and the director fee option grant program, which programs have been superseded by the Company’s amended and restated Director Compensation Policy. Additionally, in May 2012, the Company’s stockholders approved an amendment and restatement of the Company’s 2002 Stock Incentive Plan to increase the number of shares available for issuance by 1.8 million shares. As of December 31, 2015, there were 0.7 million shares available for future option grants or direct issuance under the Amended 2002 Plan.
Following is a summary of the Company’s stock option plan activity and related information:
Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term in Years | Aggregate Intrinsic Value (In thousands) | |||||||||
Balance at December 31, 2014 | 1,800,697 | $ | 28.78 | 7.25 | $ | 51,558 | ||||||
Granted | 287,747 | 62.82 | ||||||||||
Exercised | (326,418 | ) | 26.55 | |||||||||
Forfeited | (78,685 | ) | 45.75 | |||||||||
Balance at December 31, 2015 | 1,683,341 | 34.23 | 6.66 | 124,880 | ||||||||
Exercisable at December 31, 2015 | 1,193,288 | 25.41 | 5.98 | 99,061 | ||||||||
Options vested and expected to vest as of December 31, 2015 | 1,683,341 | $ | 34.23 | 6.66 | $ | 124,880 | ||||||
The weighted-average grant-date fair value of all stock options granted during 2015, 2014 and 2013 was $35.39, $46.20 and $14.28 per share, respectively. The total intrinsic value of all options exercised during 2015, 2014 and 2013 was approximately $20.7 million, $15.3 million and $5.9 million, respectively. As of December 31, 2015, there was $13.1 million of total unrecognized compensation cost related to non-vested stock options. That cost is expected to be recognized over a weighted average period of 2.4 years.
Cash received from options exercised, net of fees paid, in 2015, 2014 and 2013 was $8.7 million, $4.4 million and $3.0 million, respectively.
63
Following is a further breakdown of the options outstanding as of December 31, 2015:
Range of exercise prices | Options outstanding | Weighted average remaining life in years | Weighted average exercise price | Options exercisable | Weighted average exercise price | ||||||||||
$8.58 – $12.53 | 353,949 | 5.17 | $ | 10.26 | 353,824 | $ | 10.26 | ||||||||
$12.81-16.14 | 371,551 | 5.58 | 14.75 | 342,754 | 14.78 | ||||||||||
$17.10-32.30 | 361,019 | 6.55 | 23.34 | 270,634 | 23.81 | ||||||||||
$32.76-74.42 | 552,544 | 8.18 | 64.69 | 226,076 | 67.13 | ||||||||||
$89.75-104.59 | 44,278 | 9.47 | 98.13 | — | — | ||||||||||
$8.58 – $104.59 | 1,683,341 | 6.66 | $ | 34.23 | 1,193,288 | $ | 25.41 | ||||||||
Restricted Stock Activity
The following is a summary of the Company’s restricted stock activity and related information:
Shares | Weighted-Average Grant Date Fair Value | |||||
Outstanding at December 31, 2014 | 82,673 | $ | 45.76 | |||
Granted | 112,954 | 63.50 | ||||
Vested | (49,366 | ) | 44.8 | |||
Forfeited | (15,512 | ) | 54.91 | |||
Outstanding at December 31, 2015 | 130,749 | $ | 60.36 | |||
Restricted stock awards generally vest over three years. As of December 31, 2015, unrecognized compensation cost related to non-vested stock awards amounted to $4.7 million. That cost is expected to be recognized over a weighted average period of 1.6 years.
Employee Stock Purchase Plan
The Company’s Amended ESPP allows participants to purchase up to 1,250 shares of Ligand common stock during each offering period, but in no event may a participant purchase more than 1,250 shares of common stock during any calendar year. The length of each offering period is six months, and employees are eligible to participate in the first offering period beginning after their hire date.
The Amended ESPP allows employees to purchase a limited amount of common stock at the end of each six month period at a price equal to 85% of the lesser of fair market value on either the start date of the period or the last trading day of the period (the “Lookback Provision”). The 15% discount and the Lookback Provision make the Amended ESPP compensatory. There were 3,374, 3,774 and 5,016 shares of common stock issued under the Amended ESPP in 2015, 2014 and 2013, respectively, resulting in an expense of $56,000, $54,000 and $45,000, respectively. For shares purchased under the Company’s Amended ESPP, a weighted-average expected volatility of 49%, 40% and 36% was used for 2015, 2014 and 2013, respectively. The expected term for shares issued under the ESPP is 6 months. As of December 31, 2015, 215,821 shares of common stock had been issued under the Amended ESPP to employees and 72,367 shares are available for future issuance.
64
Preferred Stock
The Company has authorized 5,000,000 shares of preferred stock, of which 1,600,000 are designated Series A Participating Preferred Stock (the “Preferred Stock”). The Board of Directors of Ligand has the authority to issue the Preferred Stock in one or more series and to fix the designation, powers, preferences, rights, qualifications, limitations and restrictions of the shares of each such series, including the dividend rights, dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions), liquidation preferences and the number of shares constituting any such series, without any further vote or action by the stockholders. The rights and preferences of Preferred Stock may in all respects be superior and prior to the rights of the common stock. The issuance of the Preferred Stock could decrease the amount of earnings and assets available for distribution to holders of common stock or adversely affect the rights and powers, including voting rights, of the holders of the common stock and could have the effect of delaying, deferring or preventing a change in control of Ligand. As of December 31, 2015 and 2014, there are no preferred shares issued or outstanding.
Shareholder Rights Plan
In October 2006, the Company’s Board of Directors renewed the Company’s stockholder rights plan, which was originally adopted and has been in place since September 2002, and which expired on September 13, 2006, through the adoption of a new 2006 Stockholder Rights Plan (the “2006 Rights Plan”). The 2006 Rights Plan provides for a dividend distribution of one preferred share purchase right (a “Right”) on each outstanding share of the Company’s common stock. Each Right entitles stockholders to buy 1/1000th of a share of Ligand Series A Participating Preferred Stock at an exercise price of $100. The Rights will become exercisable if a person or group announces an acquisition of 20% or more of the Company’s common stock, or announces commencement of a tender offer for 20% or more of the common stock. In that event, the Rights permit stockholders, other than the acquiring person, to purchase the Company’s common stock having a market value of twice the exercise price of the Rights, in lieu of the Preferred stock. In addition, in the event of certain business combinations, the Rights permit the purchase of the common stock of an acquiring person at a 50% discount. Rights held by the acquiring person become null and void in each case. The 2006 Rights Plan expires in 2016.
Corporate Share Repurchase
In September 2015, the Company's Board of Directors authorized the Company to repurchase up to $200 million of its own stock in privately negotiated and open market transactions for a period of up to three years, subject to the Company's evaluation of market conditions, applicable legal requirements and other factors. During the year ended December 31, 2015, the Company repurchased 6,120 common shares at a weighted average price of $79.92 per share pursuant to the repurchase plan, or $0.5 million of common shares.
In August 2014, the Company's Board of Directors authorized the Company to repurchase up to $200 million of its own stock in privately negotiated and open market transactions for a period of up to one year, subject to the Company's evaluation of market conditions, applicable legal requirements and other factors. The plan expired in August 2015. During the year ended December 31, 2014, the Company repurchased 1,253,425 common shares at a weighted average price of $54.20 per share pursuant to the repurchase plan, or $68.0 million of common shares.
9. Litigation
The Company records an estimate of a loss when the loss is considered probable and estimable. Where a liability is probable and there is a range of estimated loss and no amount in the range is more likely than any other number in the range, The Company records the minimum estimated liability related to the claim in accordance with FASB ASC Topic 450 Contingencies. As additional information becomes available, the Company assesses the potential liability related to its pending litigation and revises its estimates. Revisions in the Company's estimates of potential liability could materially impact its results of operations.
Securities Litigation
In 2012, a federal securities class action and shareholder derivative lawsuit was filed in Pennsylvania alleging that the Company and its CEO assisted various breaches of fiduciary duties based on the Company’s purchase of a licensing interest in a development-stage pharmaceutical program from the Genaera Liquidating Trust in 2010 and the Company’s subsequent sale of half of its interest in the transaction to Biotechnology Value Fund, Inc. Plaintiff filed a second amended complaint in February 2015, which the Company moved to dismiss in March 2015. The district court granted the motion to dismiss on
65
November 11, 2015. The plaintiff has appealed that ruling to the Third Circuit. The Company intends to continue to vigorously defend against the claims against the Company and its CEO. The outcome of the matter is not presently determinable.
Paragraph IV Certification by Par Pharmaceuticals
On January 7, 2016, the Company received a paragraph IV certification from Par Sterile Products, LLC, a subsidiary of Par Pharmaceuticals, Inc., or Par, advising us that it had filed an ANDA with the FDA seeking approval to market a generic version of Merck’s NOXAFIL-IV product. The paragraph IV certification states it is Par’s position that Merck’s U.S. Patent No. 9,023,790 related to NOXAFIL-IV and our U.S. Patent No. 8,410,077 related to Captisol are invalid and/or will not be infringed by Par’s manufacture, use or sale of the product for which the ANDA was submitted. On February 19, 2016, Merck filed an action against Par in the United States District Court for the District of New Jersey, asserting that Par’s manufacture, use or sale of the product for which the ANDA was submitted would infringe Merck’s U.S. Patent No. 9,023,790. The case against Par is captioned Merck Sharpe & Dohme Corp. v. Par Sterile Products, LLC, Par Pharmaceuticals, Inc., Par Pharmaceutical Companies, Inc., and Par Pharmaceutical Holdings, Inc., No.16-cv-00948.
10. Income Taxes
At December 31, 2015, the Company had federal net operating loss carryforwards set to expire through 2036 of $537.1 million and $138.4 million of state net operating loss carryforwards. The Company also has $10.6 million of federal research and development credit carryforwards, which expire through 2036. The Company has $14.1 million of California research and development credit carryforwards that have no expiration date.
Sections 382 and 383 of the U.S. tax code impose limitations (“382 and 383 limitations”) on the annual utilization of operating loss and credit carryforwards whenever a greater than fifty percent change in the ownership of a company occurs within a three year period. In addition to the annual limitations on operating loss and credit carryforwards, Section 382 can also restrict the utilization of certain post change losses if the tax basis in assets exceeds the fair value of assets (“net unrealized built in loss”) at the date of an ownership change. Companies with operating loss and credit carryforwards are required to test the cumulative three year change whenever there is an equity transaction that impacts the ownership of holders of more than five percent of the Company’s stock. During 2015, the Company completed a rollforward analysis through December 31, 2014. As a result of the rollforward analysis, it was determined that no additional ownership changes occurred at the Company within the meaning of section 382 since June 20, 2007. Future changes in the ownership of the Company could place additional restrictions on the Company’s ability to utilize operating loss and credit carryforwards arising through December 31, 2015. The components of the income tax expense (benefit) for continuing operations are as follows (in thousands):
Year Ended December 31, | ||||||||||||
2015 Restated | 2014 | 2013 | ||||||||||
Current expense (benefit): | ||||||||||||
Federal | $ | 11 | $ | 15 | $ | — | ||||||
State | 7 | 19 | 33 | |||||||||
18 | 34 | 33 | ||||||||||
Deferred expense (benefit): | ||||||||||||
Federal | (167,413 | ) | 406 | 404 | ||||||||
State | (24,720 | ) | (30 | ) | (63 | ) | ||||||
$ | (192,115 | ) | $ | 410 | $ | 374 | ||||||
In periods prior to the year ended December 31, 2015, the Company concluded that a full valuation allowance was necessary to offset its deferred tax assets, due to a history of operating losses and other key operating factors. As of September 30, 2015, the Company concluded that it was more likely than not that a substantial portion of its deferred tax assets would be realized through future taxable income. The Company's income tax provision for the year ended December 31, 2015, included a discrete income tax benefit related to the release of a majority of the Company’s valuation allowance and various adjustments to its deferred tax assets, including studies validating the Company’s tax attributes and adjustments resulting from the tax return filings during the quarter.
Significant components of the Company’s deferred tax assets and liabilities as of December 31, 2015 and 2014 are shown below. The Company assesses the positive and negative evidence to determine if sufficient future taxable income will be
66
generated to use the existing deferred tax assets. During the third quarter of the year, the Company's evaluation of evidence resulted in management concluding that the majority of the Company's deferred tax assets will be realized. However, the Company maintains a valuation allowance to offset certain net deferred tax assets as management believes realization of such assets are uncertain as of December 31, 2015, 2014 and 2013. The valuation allowance decreased $230.7 million in 2015, decreased $9.8 million in 2014 and decreased $5.4 million in 2013.
December 31, | |||||||
2015 Restated | 2014 | ||||||
(in thousands) | |||||||
Deferred assets: | |||||||
Net operating loss carryforwards | $ | 160,595 | $ | 193,747 | |||
Research and AMT credit carryforwards | 25,613 | 28,288 | |||||
Fixed assets and intangibles | 8,839 | 13,237 | |||||
Accrued expenses | 1,523 | 1,579 | |||||
Contingent liabilities | 707 | 579 | |||||
Deferred revenue | 3 | 771 | |||||
Present value of royalties | 3,007 | 11,686 | |||||
Organon termination asset | — | (111 | ) | ||||
Organon termination liability | — | 111 | |||||
Deferred rent | 68 | 730 | |||||
Other | 17,281 | 5,780 | |||||
217,636 | 256,397 | ||||||
Valuation allowance for deferred tax assets | (9,066 | ) | (240,420 | ) | |||
Net deferred tax assets | $ | 208,570 | $ | 15,977 | |||
Deferred tax liabilities: | |||||||
Retrophin fair value adjustment | $ | (1,256 | ) | $ | (1,396 | ) | |
Convertible debt | (1,844 | ) | (1,436 | ) | |||
Identified intangibles | (12,770 | ) | (13,146 | ) | |||
Identified indefinite lived intangibles | (3,617 | ) | (3,048 | ) | |||
Total | $ | 189,083 | $ | (3,049 | ) | ||
As of December 31, 2015 and 2014, the Company had not recognized as a deferred tax asset $11.5 million and $8.1 million, respectively of unrealized excess tax benefits from stock-based compensation. When realized and the valuation allowance is reversed, such benefits will be credited directly to additional paid-in capital. Changes to the valuation allowance allocated directly to other comprehensive income were $0 million, $0.7 million and $1.0 million for 2015, 2014 and 2013, respectively.
67
A reconciliation of income tax expense (benefit) from continuing operations to the amount computed by applying the statutory federal income tax rate to the net income (loss) from continuing operations is summarized as follows:
Year Ended December 31, | |||||||||||
2015 Restated | 2014 | 2013 | |||||||||
Amounts computed at statutory federal rate | $ | (13,198 | ) | $ | (3,843 | ) | $ | (3,131 | ) | ||
State taxes net of federal benefit | (386 | ) | (697 | ) | (293 | ) | |||||
Meals & entertainment | (16 | ) | (9 | ) | (10 | ) | |||||
Imputed interest | 161 | (53 | ) | (285 | ) | ||||||
Section 162(m) limitation | (197 | ) | (490 | ) | — | ||||||
Contingent liabilities | (1,684 | ) | (1,748 | ) | (2,027 | ) | |||||
Stock-based compensation | (140 | ) | (89 | ) | 556 | ||||||
Expired NOLs | (232 | ) | (88 | ) | — | ||||||
Research and development credits | (304 | ) | 113 | 4,581 | |||||||
Change in uncertain tax positions | (27,188 | ) | (7 | ) | (364 | ) | |||||
Rate change for changes in state law | 5,756 | (119 | ) | (901 | ) | ||||||
Increase in deferred tax assets from completion of 382 analysis | (3,329 | ) | (43 | ) | (786 | ) | |||||
Avinza true up | 2,107 | — | — | ||||||||
Change in valuation allowance | 231,370 | 7,243 | 3,509 | ||||||||
Other | (605 | ) | (580 | ) | (1,223 | ) | |||||
$ | 192,115 | $ | (410 | ) | $ | (374 | ) | ||||
The Company accounts for income taxes by evaluating a probability threshold that a tax position must meet before a financial statement benefit is recognized. The minimum threshold is a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The Company’s remaining liabilities for uncertain tax positions are presented net of the deferred tax asset balances on the accompanying consolidated balance sheet.
A reconciliation of the amount of unrecognized tax benefits at December 31, 2015 and 2014 is as follows (in thousands):
Balance at December 31, 2013 | $ | 8,504 | |
Additions based on tax positions related to the current year | 40 | ||
Additions for tax positions of prior years | (20 | ) | |
Balance at December 31, 2014 | $ | 8,524 | |
Additions based on tax positions related to the current year | 154 | ||
Additions based on tax positions related to prior years | 28,224 | ||
Reductions for tax positions of prior years | (450 | ) | |
Balance at December 31, 2015 | $ | 36,452 | |
Included in the balance of unrecognized tax benefits at December 31, 2015 is $33.5 million of tax benefits that, if recognized would impact the effective rate. There are no positions for which it is reasonably possible that the uncertain tax benefit will significantly increase or decrease within twelve months.
The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2015 and December 31, 2014, there was an accrual related to uncertain tax positions of $29,000 and $32,000, respectively. The Company files income tax returns in the United States and in various state jurisdictions with varying statutes of limitations. The federal statute of limitation remains open for the 2012 tax year to present. The state income tax returns generally remain open for the 2011 tax years through present. Changes to the valuation allowance allocated directly to equity were $0 million, $1.1 million, and $0 million for the years ended December 31, 2015, 2014, and 2013, respectively.
68
As of December 31, 2015, approximately $4.2 million of the valuation allowance for deferred tax assets related to benefits of stock option deductions which, when recognized, will be allocated directly to paid-in-capital.
For the year ended December 31, 2015, the Company has adopted ASU 2015-17, Income Taxes (Topic 740) Balance Sheet Classification of Deferred Taxes which requires noncurrent classification of all deferred tax liabilities and assets.
Under current GAAP, in a classified statement of financial position, deferred tax assets and liabilities are separated into a current amount and a non-current amount on the basis of the classification of the related asset or liability for financial reporting. Deferred tax assets and liabilities that are not related to an asset or liability for financial reporting are classified according to the expected reversal date of the temporary difference. On November 20, 2015, the FASB issued ASU 2015-17, Income Taxes (Topic 740) Balance Sheet Classification of Deferred Taxes which requires non-current classification of all deferred tax assets and liabilities for all public entities for annual periods beginning after December 15, 2016. ASU 2015-17 also provides for early adoption for all entities as of the beginning of an annual period. For the year ended December 31, 2015, the Company has elected to early adopt ASU 2015-17 and will present all its deferred tax assets and liabilities as non-current for the period ended December 31, 2015. The Company has applied the Standard on a prospective basis. Therefore, the classification of deferred tax assets and liabilities in periods prior to the period ended December 31, 2015 have not been changed from the original presentation.
11. Summary of Unaudited Quarterly Financial Information
As discussed in Correction of Previously Reported Financials in Note 1 of the previously filed Annual Report on Form 10-K on February 26, 2016, management opted to restate certain amounts in the previously reported financial statements as of and for the three and nine months ended September 30, 2015 to correct an immaterial error in the calculation of certain capital loss carry-forwards at September 30, 2015 related to the sale of the Avinza product line. The restated amounts are set forth in the following tables (in thousands, except share data):
Three months ended, | Nine months ended, | ||||||||||||||
September 30, 2015 | September 30, 2015 | ||||||||||||||
Previously Reported in 10-Q | Restated | Previously Reported in 10-Q | Restated | ||||||||||||
Income tax benefit | $ | 217,255 | $ | 219,362 | $ | 216,976 | $ | 219,083 | |||||||
Net income | 224,539 | 226,646 | 248,857 | 250,964 | |||||||||||
Basic net income per share | $ | 11.29 | $ | 11.40 | $ | 12.61 | $ | 12.71 | |||||||
Diluted net income per share | $ | 10.46 | $ | 10.56 | $ | 11.78 | $ | 11.88 | |||||||
As of September 30, 2015 | |||||||
Previously Reported in 10-Q | Restated | ||||||
Long-term deferred tax assets | $ | 206,423 | $ | 208,530 | |||
Accumulated deficit | (410,458 | ) | (408,351 | ) | |||
In addition, As discussed in 2015 Restatement in Note 1 of this Annual Report on Form 10K/A, the Company corrected accounting errors related to its DTA at September 30, 2015 by restating the previously reported financial statements as of and for the three months and nine months ended September 30, 2015. The restated amounts are set forth in the following tables:
69
The effects of these prior period corrections on the condensed consolidated statement of operations and comprehensive income are as follows
Nine months ended September 30, 2015 | |||||||||||
As Previously Reported in 10-K(1) | Adjustments | As Restated | |||||||||
Income tax benefit | $ | 219,083 | $ | (27,481 | ) | $ | 191,602 | ||||
Net income | 250,964 | (27,481 | ) | 223,483 | |||||||
Comprehensive income | 251,351 | (27,481 | ) | 223,870 | |||||||
Basic earnings per share | 12.71 | (1.38 | ) | 11.33 | |||||||
Diluted earnings per share data | 11.88 | (1.30 | ) | 10.58 | |||||||
Basic | 19,887 | — | 19,887 | ||||||||
Diluted | 21,122 | — | 21,122 | ||||||||
Three months ended September 30, 2015 | |||||||||||
As Previously Reported in 10-K(1) | Adjustments | As Restated | |||||||||
Income tax benefit (expense) | $ | 219,362 | $ | (27,481 | ) | $ | 191,881 | ||||
Net income | 226,646 | (27,481 | ) | 199,165 | |||||||
Comprehensive income | 222,981 | (27,481 | ) | 195,500 | |||||||
Basic earnings per share | 11.40 | (1.39 | ) | 10.01 | |||||||
Diluted earnings per share data | 10.56 | (1.28 | ) | 9.28 | |||||||
The effects of these prior period corrections on the condensed consolidated balance sheet is as follows
As of September 30, 2015 | |||||||||||
As Previously Reported in 10-K(1) | Adjustments | As Restated | |||||||||
Deferred income taxes | $ | 208,530 | $ | (27,481 | ) | $ | 181,049 | ||||
Total assets | 523,807 | (27,481 | ) | 496,326 | |||||||
Accumulated deficit | (408,351 | ) | (27,481 | ) | (435,832 | ) | |||||
Total stockholders' equity | 294,288 | (27,481 | ) | 266,807 | |||||||
Total liabilities and stockholders' equity | 523,807 | (27,481 | ) | 496,326 | |||||||
(1) The Company opted to restate certain amounts in the previously reported financial statements as of and for the three and nine months ended September 30, 2015 to correct an immaterial error in the calculation of certain capital loss carry-forwards at September 30, 2015 related to the sale of the Avinza product line, which amounted to $2.1 million.
The corrections did not have any impact on the company's cash flow statements for any period.
70
The following is a summary of the unaudited quarterly results of operations for the years ended December 31, 2015 and 2014 (in thousands).
Quarter ended | |||||||||||||||
March 31 | June 30 | September 30 Restated | December 31 | ||||||||||||
2015 | |||||||||||||||
Total revenues | $ | 14,602 | $ | 18,418 | $ | 17,701 | $ | 21,193 | |||||||
Total operating costs and expenses | 11,253 | 14,053 | 9,104 | 10,175 | |||||||||||
Income tax (expense) benefit | (15 | ) | (265 | ) | 191,881 | 514 | |||||||||
Income from continuing operations | (89 | ) | 22,027 | 199,165 | 6,341 | ||||||||||
Net loss attributable to noncontrolling interests | (843 | ) | (1,537 | ) | — | — | |||||||||
Net income | 754 | 23,564 | 199,165 | 6,341 | |||||||||||
Basic per share amounts: | |||||||||||||||
Net income | $ | 0.04 | $ | 1.19 | $ | 10.01 | $ | 0.32 | |||||||
Diluted per share amounts: | |||||||||||||||
Net income | $ | 0.04 | $ | 1.11 | $ | 9.28 | $ | 0.29 | |||||||
Weighted average shares—basic | 19,612 | 19,725 | 19,887 | 19,933 | |||||||||||
Weighted average shares—diluted | 20,631 | 21,276 | 21,460 | 21,542 | |||||||||||
2014 | |||||||||||||||
Total revenues | $ | 15,958 | $ | 10,608 | $ | 14,973 | $ | 22,999 | |||||||
Total operating costs and expenses | 10,858 | 9,250 | 11,441 | 13,363 | |||||||||||
Income tax expense | (53 | ) | 47 | (124 | ) | (280 | ) | ||||||||
Income from continuing operations | 2,097 | 1,288 | 777 | 6,730 | |||||||||||
Net loss attributable to noncontrolling interests | — | (304 | ) | (503 | ) | (325 | ) | ||||||||
Net income | 2,097 | 1,592 | 1,280 | 7,055 | |||||||||||
Basic per share amounts: | |||||||||||||||
Net income | $ | 0.10 | $ | 0.08 | $ | 0.06 | $ | 0.35 | |||||||
Diluted per share amounts: | |||||||||||||||
Net income | 0.10 | 0.07 | 0.06 | 0.34 | |||||||||||
Weighted average shares—basic | 20,601 | 20,738 | 20,417 | 19,878 | |||||||||||
Weighted average shares—diluted | 21,208 | 21,780 | 21,345 | 20,792 | |||||||||||
12. Subsequent Event
Acquisition of OMT
In January 2016, the Company acquired OMT, a leader in genetic engineering of animals for the generation of human therapeutic antibodies through its OmniAb platform. OMT offers three transgenic animal platforms for license, including OmniRat®, OmniMouse® and OmniFlic®. The transaction added 16 partnered programs to the Company's portfolio. As a result of the acquisition, the Company paid $96.4 million in cash and issued $85.4 million in shares of Ligand common stock. The aggregate merger consideration is subject to certain adjustments, including amounts based on changes in OMT's net working capital and cash at closing. The Company issued 793,594 shares of its common stock based on a 20-day volume-weighted average price of $107.66 of its common stock calculated three days prior to closing.
In connection with the closing of the merger, OMT's former chief executive officer, Roland Buelow, joined the Company as an employee and was granted stock options with an aggregate value of $2.0 million on the date of grant and performance stock units with an aggregate value of $18.0 million on the date of grant. Dr. Buelow's performance stock units will vest based on achievement of certain milestones related to the OMT business, including annual revenues, research and development milestones
71
and entering into new business releationships. Dr. Buelow also entered into a noncompetition agreement with the Company pursuant to which he agreed not to engage in any competitive activities for a period of two years following the merger.
Due to the close proximity of the acquisition date and the Company’s filing of its annual report on Form 10-K for the year ended December 31, 2015, the initial accounting for the business combination is incomplete, and therefore the Company is unable to disclose the information required by ASC 805, Business Combinations. Such information will be included in the Company’s subsequent Form 10-Q.
Viking Note Amendment
In January 2016, the Company entered into a second amendment to the LSA with Viking. The Company provided Viking with loans in an aggregate amount of $2.5 million, evidenced by a Secured Convertible Promissory Note. The loan amendment extends the maturity date of the loans under the Viking Note from May 21, 2016 to May 21, 2017, reduces the annual interest rate from 5.0% to 2.5%, and extends the Company’s lock-up period by one year such that the Company may not, sell or otherwise transfer or dispose of any Viking securities prior to January 23, 2017. Additionally, upon the consummation of Viking’s first capital financing transaction, Viking will be required to repay $1.5 million of the Viking Note obligation to the Company, with at least $0.3 million to be paid in cash and the remaining amount to be paid in the form of shares of Viking’s equity securities.
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
72
Item 9A. | Controls and Procedures |
(a) Evaluation of Disclosure Controls and Procedures
We are responsible for maintaining disclosure controls and procedures designed to provide reasonable assurance that information required to be disclosed in reports we file under the Exchange Act is recorded, processed, summarized and reported within the specified time periods and accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. As of the end of the period covered by this Annual Report on Form 10-K, we had carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, and had concluded our disclosure controls and procedures were effective as of December 31, 2015.
On November 14, 2016, the Audit Committee (the “Committee”), after consultation with management, concluded that our unaudited condensed consolidated financial statements for the interim periods ended September 30, 2015 and the audited consolidated financial statements for the year ended December 31, 2015, did not properly account for certain acquisition-related tax benefits that were more likely than not subject to Section 382 limitation at the time we released our valuation allowance at September 30, 2015. In addition, the classification and presentation of our 2019 Convertible Senior Notes was not proper as of December 31, 2015 due to the Company's last reported sale price exceeded the 130% threshold described in Note 5 - "Financing Arrangements" and accordingly the 2019 Convertible Senior Notes should have been reclassified as a current liability as of December 31, 2015. As a result, certain related unamortized discount should have been classified as temporary equity on our Consolidated Balance Sheet. As such, these interim financial statements and annual financial statements should not be relied upon. The Company, under the authorization of the Committee, restated its audited financial statements for the year ended December 31, 2015 and the unaudited interim financial statements for the three and nine months ended September 30, 2015.
Following the identification of the accounting errors as described above, our management, with the participation of our Chief Executive Officer and Chief Financial Officer, re-evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2015. Based on this re-evaluation, our Chief Executive Officer and Chief Financial Officer have now concluded that our disclosure controls and procedures as of such date were not effective as of December 31, 2015 due to the material weaknesses in internal control over financial reporting as discussed in the section (b) below.
(b) Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting for the Company. Internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of our financial reporting for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements in accordance with generally accepted accounting principles; providing reasonable assurance that receipts and expenditures are made in accordance with our management and directors; and providing reasonable assurance that
unauthorized acquisition, use or disposition of company assets that could have a material effect on our financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected.
Management had concluded in the Annual Report on Form 10-K for the year ended December 31, 2015, that our internal controls over financial reporting were effective as of December 31, 2015. Following the identification of the errors as described above, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we re-evaluated the effectiveness of our internal control over financial reporting based on the framework established by the COSO as set forth in the 2013 Internal Control-Integrated Framework. Based on our re-evaluation under the 2013 framework in Internal Control - Integrated Framework, the Audit Committee, after consultation with our management
73
concluded that our internal controls over financial reporting were not effective as of December 31, 2015. Management has identified the following deficiencies that constituted material weaknesses in our internal control over financial reporting as of December 31, 2015:
(1) A material weakness in the design of our internal control over the tax accounting for complex transactions that have a significant tax impact, specifically, management did not have adequate supervision and review of certain technical tax accounting performed by third party tax specialists.
(2) A material weakness in the design of our internal control over the presentation and disclosure of our 2019 convertible senior notes, specifically, management review control is not precise and adequate to capture the appropriate presentation and disclosure of our 2019 convertible senior notes that is triggered by certain specific contractual conditions.
Grant Thornton LLP, an independent registered public accounting firm, has audited the effectiveness of the Company’s internal control over financial reporting as of December 31, 2015; their report is included in Item 9A.
Remediation Plan.
To remediate the material weakness described in bullet point (1) above and to prevent similar deficiencies in the future, we are currently evaluating additional controls and procedures, which may include:
• | engagement of additional independent third party tax experts to assist or review in the tax accounting for non-routine, complex transactions or give a “second opinion” on the same transaction |
• | additional training for staff involved in the tax accounting for non-routine, complex transactions |
With regards to the material weakness described in bullet point (2) above, subsequent to December 31, 2015, management has implemented additional controls and procedures related to management review of the presentation and disclosures of our 2019 convertible senior notes, which include:
• | developed and refined certain spreadsheet tool to review various inputs including our stock price, which would trigger the early conversion conditions under the debt indenture agreement |
• | additional management review of the presentation including the classification of our convertible debt |
Management will continue to strive to improve the respective process and controls over the presentation and disclosure of our convertible debt. However, the material weakness will not be considered remediated until the applicable remedial controls operate for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively.
As disclosed in Form 10-Q for the three- and nine-month periods ended September 30, 2015, we have identified a material weakness in our internal controls related to management’s review of the manual calculation of certain projections used in the valuation of certain contingent liabilities, specifically, the review lacks sufficient precision to confirm the related calculation. We undertook the following steps in the course of our fourth fiscal quarter in order to remediate the identified material weakness:
• | We performed a review of all the manual calculation schedules used in our financial accounting and reporting and identified the key manual schedules based on the complexity of the calculation in the schedule and the associated risk of material misstatement in the financial statements. |
• | For all the identified key manual schedules including the one for the projections used in the valuation of certain contingent liabilities, we enhanced our procedures and controls by implementing additional management review as well as the frequency of the review of the clerical accuracy of all the formulas and calculation within the schedules. |
• | Enhanced the precision of management analytical review of the projections and calculation used in certain contingent liabilities by implementing certain reconciliation with management expectation as well as defining the materiality for acceptable variance from management expectation. |
74
Changes in Internal Control over Financial Reporting
Except for the changes mentioned in the section above, there have been no changes in our internal control over financial reporting that occurred in our fourth fiscal quarter that materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
Board of Directors and Shareholders
Ligand Pharmaceuticals Incorporated
We have audited the internal control over financial reporting of Ligand Pharmaceuticals Incorporated (the “Company”) as of December 31, 2015, based on criteria established in the 2013 Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting (“Management’s Report”). Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or combination of control deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and included in Management’s Report. Management concluded that material weaknesses in internal control over financial reporting existed relating to the accuracy and presentation of the accounting for income taxes related to complex transactions, including the income tax provision and related tax assets and liabilities and controls over the financial reporting classification of convertible debt and temporary equity.
75
In our report dated February 26, 2016, we expressed an unqualified opinion on the Company’s internal control over financial reporting. The material weaknesses discussed above was subsequently identified in connection with the restatement of the Company’s previously issued financial statements. Accordingly, management has revised its assessment about the effectiveness of the Company’s internal control over financial reporting, and our present opinion on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2015, as expressed herein, is different from that expressed in our previous report. The material weaknesses were considered in connection with the aforementioned restatements, and this report does not affect our opinion on the Company’s 2015 financial statements.
In our opinion, because of the effect of the material weaknesses described above on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of December 31, 2015, based on criteria established in the 2013 Internal Control-Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements of the Company as of and for the year ended December 31, 2015. The material weaknesses identified above were considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2015 consolidated financial statements, and this report does not affect our report dated February 26, 2016 (except for the 2015 Restatement described in Note 1 and the effects thereof, as to which the date is November 14, 2016), which expressed an unqualified opinion on those financial statements.
/s/ GRANT THORNTON LLP
San Diego, California
February 26, 2016 (except for the material weaknesses discussed in Management’s Report on Internal Control over Financial Reporting, as to which the date is November 14, 2016)
76
Part III
Item 10. | Directors, Executive Officers and Corporate Governance |
Code of Conduct
The Board of Directors has adopted a Code of Conduct and Ethics Policy (“Code of Conduct”) that applies to all officers, directors and employees. The Company will promptly disclose any material amendment or waiver to the Code of Conduct which affects any corporate officer. The Code of Conduct can be accessed via our website (http://www.ligand.com), Corporate Overview page. You may also request a free copy by writing to: Investor Relations, Ligand Pharmaceuticals Incorporated, 11119 North Torrey Pines Road, Suite 200, La Jolla, CA 92037.
The other information under Item 10 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC on or prior to April 29, 2016.
Item 11. | Executive Compensation |
Item 11 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC on or prior to April 29, 2016.
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Item 12 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC on or prior to April 29, 2016.
Item 13. | Certain Relationships and Related Transactions, and Director Independence |
Item 13 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC on or prior to April 29, 2016.
Item 14. | Principal Accountant Fees and Services |
Item 14 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC on or prior to April 29, 2016.
77
PART IV
Item 15. | Exhibits and Financial Statement Schedule |
(a) The following documents are included as part of this Annual Report on Form 10-K.
(1) Financial statements
(2) Schedules not included herein have been omitted because they are not applicable or the required information is in the consolidated financial statements or notes thereto.
(3) The following exhibits are filed as part of this Form 10-K and this list includes the Exhibit Index.
Exhibit Number | Description | |
2.1 | Agreement and Plan of Merger, dated January 14, 2011 by and among the Company, CyDex Pharmaceuticals, Inc., and Caymus Acquisition, Inc., (incorporated by reference to the Company's Current Report on Form 8-K filed on January 26, 2011). | |
2.2 | Agreement and Plan of Merger, dated as of December 17, 2015, by and among Ligand Pharmaceuticals Incorporated, Open Monoclonal Technology, Inc., OMT, LLC, Schrader 1 Acquisition, Inc., Schrader 2 Acquisition, Inc. and Fortis Advisors LLC (incorporated by reference to the Company’s Current Report on Form 8-K filed on December 18, 2015). | |
3.1 | Amended and Restated Certificate of Incorporation of the Company. (incorporated by reference to the Company's Registration Statement on Form S-4 (No. 333-58823) filed on July 9, 1998). | |
3.2 | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Company, dated June 14, 2000 (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2000). | |
3.3 | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Company, dated June 30, 2004 (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2004). | |
3.4 | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Company, dated November 17, 2010 (incorporated by reference to the Company’s Current Report on Form 8-K filed on November 19, 2010). | |
3.5 | Amended Certificate of Designation of Rights, Preferences and Privileges of Series A Participating Preferred Stock of the Company (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 1999). | |
3.6 | Third Amended and Restated Bylaws of the Company (incorporated by reference to the Company’s Current Report on Form 8-K filed on September 10, 2015). | |
4.1 | Specimen stock certificate for shares of the common stock of the Company (incorporated by reference to the Company’s Registration Statement on Form S-1 (No. 33-47257) filed on April 16, 1992 as amended). | |
4.2 | 2006 Preferred Shares Rights Agreement, by and between the Company and Mellon Investor Services LLC, dated October 13, 2006 (incorporated by reference to the Company’s Current Report Form 8-K filed on October 17, 2006). | |
78
Exhibit Number | Description | |
4.3 | First Amendment to 2006 Preferred Shares Rights Agreement, by and between the Company and Computershare Shareowner Services LLC (f/k/a Mellon Investor Services LLC), dated June 19, 2013 (incorporated by reference to the Company’s Current Report on Form 8-K filed on June 20, 2013). | |
4.4 | Indenture dated August 18, 2014 between the Company and Wilmington Trust, National Association (incorporated by reference to the Company's Current Report on Form 8-K filed August 18, 2014). | |
10.1# | Form of Indemnification Agreement between the Company and each of its directors (incorporated by reference to the Company’s Registration Statement on Form S-1 (No. 33-47257) filed on April 16, 1992 as amended). | |
10.2# | Form of Indemnification Agreement between the Company and each of its officers (incorporated by reference to the Company’s Registration Statement on Form S-1 (No. 33-47257) filed on April 16, 1992 as amended). | |
10.3# | 2002 Stock Incentive Plan (as amended and restated through May 31, 2012) (incorporated by reference to the Company’s Registration Statement on Form S-8 filed on July 5, 2012 as amended). | |
10.4# | 2002 Employee Stock Purchase Plan (as amended effective July 1, 2009) (incorporated by reference to the Company’s Registration Statement on Form S-8 filed on June 22, 2009). | |
10.5# | Form of Stock Option Grant Notice and Stock Option Agreement under the Company’s 2002 Stock Incentive Plan | |
10.6# | Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under the Company’s 2002 Stock Incentive Plan (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2003). | |
10.7# | Form of Stock Issuance Agreement for non-employee directors under the Company’s 2002 Stock Incentive Plan (incorporated by reference to the Company’s Registration Statement on Form S-1 (no. 333-131029) filed on January 13, 2006 as amended). | |
10.8# | Form of Letter Agreement regarding Change of Control Severance Benefits between the Company and its officers (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006). | |
10.9# | Form of Executive Officer Change in Control Severance Agreement (incorporated by reference to the Company’s Current Report on Form 8-K filed on August 22, 2007). | |
10.10# | Amended and Restated Severance Plan, dated December 20, 2008 (incorporated by reference to the Company’s Current Report on Form 8-K filed on December 24, 2012). | |
10.11# | Amended and Restated Director Compensation and Stock Ownership Policy, effective as of June 1, 2011 (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2011). | |
10.12# | Letter Agreement by and between the Company and John L. Higgins, dated January 10, 2007 (incorporated by reference to the Company’s Current Report on Form 8-K filed on January 16, 2007). | |
10.13 | Stock Purchase Agreement, dated September 9, 1992, between the Company and Glaxo, Inc. (incorporated by reference to the Company’s Registration Statement on Form S-1 (No. 33-47257) filed on April 16, 1992 as amended). | |
10.14† | Research and Development Agreement, dated September 9, 1992, between the Company and Glaxo, Inc. (incorporated by reference to the Company’s Registration Statement on Form S-1 (No. 33-47257) filed on April 16, 1992 as amended). | |
10.15† | Option Agreement, dated September 2, 1994, between the Company and American Home Products Corporation, as represented by its Wyeth-Ayerst Research Division (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 1994). | |
10.16† | Research, Development and License Agreement, dated December 29, 1994, between SmithKline Beecham Corporation and the Company (incorporated by reference to the Registration Statement on Form S-1/S-3 (No. 33-87598 and 33-87600) filed on December 20, 1994, as amended). | |
10.17† | Letter of Agreement, dated September 28, 1998, among the Company, Elan Corporation, plc and Elan International Services, Ltd. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 1998). | |
79
Exhibit Number | Description | |
10.18† | Amended and Restated License and Supply Agreement, dated December 6, 2002, between the Company, Elan Corporation, plc and Elan Management Limited (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2002). | |
10.19† | Stock Purchase Agreement by and between the Company and Warner-Lambert Company dated September 1, 1999 (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 1999). | |
10.20† | License Agreement, effective June 30, 1999, by and between the Company and X-Ceptor Therapeutics, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 1999). | |
10.21 | Purchase Agreement, dated March 6, 2002, between the Company and Pharmaceutical Royalties International (Cayman) Ltd. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2002). | |
10.22 | Amendment Number 1 to Purchase Agreement, dated July 29, 2002, between the Company and Pharmaceutical Royalties International (Cayman) Ltd. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2002). | |
10.23 | Amendment Number 2 to Purchase Agreement, dated December 19, 2002, between the Company and Pharmaceuticals Royalties International (Cayman) Ltd. (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2002). | |
10.24† | Amendment Number 3 to Purchase Agreement, dated December 30, 2002, between the Company and Pharmaceuticals Royalties International (Cayman) Ltd. (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2002). | |
10.25† | Purchase Agreement, dated December 30, 2002, between the Company and Pharmaceuticals Royalties International (Cayman) Ltd. (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2002). | |
10.26† | Option Agreement Between Investors Trust & Custodial Services (Ireland) Ltd., as Trustee for Royalty Pharma, Royalty Pharma Finance Trust and the Company, dated October 1, 2003 (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2003). | |
10.27† | Amendment to Purchase Agreement Between Royalty Pharma Finance Trust and the Company, dated October 1, 2003 (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2003). | |
10.28 | Amendment Number 1 to the Option Agreement between Investors Trust & Custodial Services (Ireland) Ltd., solely in its capacity as Trustee for Royalty Pharma, Royalty Pharma Finance Trust and the Company, dated November 5, 2004 (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2004). | |
10.29 | Amendment to Purchase Agreement between Royalty Pharma Finance Trust, the Company and Investors Trust and Custodial Services (Ireland) Ltd., solely in its capacity as Trustee of Royalty Pharma, dated November 5, 2004 (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2004). | |
10.30† | Amended and Restated Research, Development and License Agreement, dated December 1, 2005, between the Company and Wyeth (formerly American Home Products Corporation) (incorporated by reference to the Company’s Registration Statement on Form S-1 (no. 333-131029) filed on January 13, 2006 as amended). | |
10.31 | Purchase Agreement, by and between the Company, King Pharmaceuticals, Inc. and King Pharmaceuticals Research and Development, Inc., dated September 6, 2006 (incorporated by reference to the Company’s Current Report Form 8-K filed on September 11, 2006). | |
10.32 | Loan Agreement by and between the Company and King Pharmaceuticals, 303 Inc., dated October 12, 2006 (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006). | |
10.33 | Letter Agreement by and between the Company and King Pharmaceuticals, Inc. effective as of December 29, 2006 (incorporated by reference to the Company’s Current Report on Form 8-K filed on January 5, 2007). | |
80
Exhibit Number | Description | |
10.34 | Amendment Number 2 to Purchase Agreement, by and between the Company and King Pharmaceuticals, Inc., effective February 26, 2007 (incorporated by reference to the Company’s Current Report on Form 8-K filed on February 28, 2007). | |
10.35 | Purchase Agreement and Escrow Instructions by and between Nexus Equity VI, LLC, the Company and Slough Estates USA Inc., dated October 25, 2006 (incorporated by reference to the Company’s Current Report on Form 8-K filed on October 31, 2006). | |
10.36 | Lease, dated July 6, 1994, between the Company and Chevron/Nexus partnership, First Amendment to Lease dated July 6, 1994 (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 1995). | |
10.37 | Sublease Agreement between the Company and eBIOSCIENCE, INC., dated as of December 16, 2007 (incorporated by reference to the Company’s Current Report on Form 8-K filed on December 19, 2007). | |
10.38 | Lease, dated August 20, 2003, between Pharmacopeia, Inc. and Eastpark at 8A (Building 1000) (incorporated by reference to the Company’s Annual Report on Form 10-K for the period ended December 31, 2008). | |
10.39 | Amendment to Lease, dated September 10, 2007, between Pharmacopeia, Inc. and Eastpark at 8A (Building 1000) (incorporated by reference to Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended September 30, 2007, File No. 000-50523). | |
10.40 | Lease, dated August 20, 2003, between Pharmacopeia, Inc. and Eastpark at 8A (Building 3000) (incorporated by reference to the Company’s Annual Report on Form 10-K for the period ended December 31, 2008). | |
10.41 | Amendment to Lease, dated April 18, 2007, between Pharmacopeia, Inc. and Eastpark at 8A (Building 3000) (incorporated by reference to Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended September 30, 2007, File No. 000-50523). | |
10.42 | Lease Agreement, dated September 5, 2011, between the Company and ARE-SD Region No. 24, LLC (incorporated by reference to the Company’s Current Report on Form 8-K filed on September 9, 2011). | |
10.43† | Collaboration and License Agreement, dated July 9, 2003 and effective August 8, 2003, between Pharmacopeia, Inc. and Schering-Plough Ltd. (incorporated by reference to the Company’s Annual Report on Form 10-K for the period ended December 31, 2008). | |
10.44† | Collaboration and License Agreement, dated July 9, 2003 and effective August 8, 2003, between Pharmacopeia, Inc. and Schering Corporation (incorporated by reference to the Company’s Annual Report on Form 10-K for the period ended December 31, 2008). | |
10.45 | Amendment No. 1, dated July 27, 2006, to the Collaboration and License Agreements, effective as of July 9, 2003, between (i) Pharmacopeia, Inc. and Schering Corporation and (ii) Pharmacopeia, Inc. and Schering-Plough Ltd. (incorporated by reference to Pharmacopeia, Inc.’s Current Report on Form 8-K filed on August 2, 2006, File No. 000-50523). | |
10.46 | License Agreement, dated March 27, 2006, between Pharmacopeia, Inc. and Bristol-Myers Squibb Company (incorporated by reference to Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended March 31, 2006, File No. 000-50523). | |
10.47 | License Agreement, dated October 11, 2007, between Bristol-Myers Squibb Company and Pharmacopeia, Inc. (Filed as Exhibit 10.45) (File No. 000-50523) (incorporated by reference to Pharmacopeia, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2007, File No. 000-50523). | |
10.48† | License Agreement, dated December 17, 2008, between the Company and SmithKline Beecham Corporation, doing business as GlaxoSmithKline (incorporated by reference to the Company’s Annual Report on Form 10-K for the period ended December 31, 2008). | |
10.49 | Settlement Agreement and Mutual Release, by and between the Company and The Rockefeller University, dated February 11, 2009 (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2009). | |
10.50 | TR Beta Contingent Value Rights Agreement, dated January 27, 2010, among the Company, Metabasis Therapeutics, Inc., David F. Hale and Mellon Investor Services LLC (incorporated by reference to the Company’s Current Report on Form 8-K filed on January 28, 2010). | |
81
Exhibit Number | Description | |
10.51 | Glucagon Contingent Value Rights Agreement, dated January 27, 2010, among the Company, Metabasis Therapeutics, Inc., David F. Hale and Mellon Investor Services LLC (incorporated by reference to the Company’s Current Report on Form 8-K filed on January 28, 2010). | |
10.52 | General Contingent Value Rights Agreement, dated January 27, 2010, among the Company, Metabasis Therapeutics, Inc., David F. Hale and Mellon Investor Services LLC (incorporated by reference to the Company’s Current Report on Form 8-K filed on January 28, 2010). | |
10.53 | Amendment of General Contingent Value Rights Agreement, dated January 26, 2011, among the Company, Metabasis Therapeutics, Inc., David F. Hale and Mellon Investor Services LLC (incorporated by reference to the Company’s Current Report on Form 8-K filed on January 31, 2011. | |
10.54 | Purchase and Sale Agreement, dated May 18, 2010, between the Company and The Genaera Liquidating Trust (incorporated by reference to the Company’s Current Report on Form 8-K filed on May 24, 2010). | |
10.55 | Purchase Agreement, dated May 20, 2010, between the Company and Biotechnology Value Fund, L.P., on its own behalf and on behalf of Biotechnology Value Fund II, L.P. and Investment 10, L.L.C. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2010). | |
10.56 | Asset Purchase Agreement, dated July 30, 2010, between Wyeth LLC, Pharmacopeia, Inc. and the Company (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2010). | |
10.57 | Contingent Value Rights Agreement, by and among the Company, CyDex Pharmaceuticals, Inc., and Allen K. Roberson and David Poltack, acting jointly as Shareholders’ Representative, dated January 14, 2011 (incorporated by reference to the Company’s Current Report on Form 8-K filed on January 26, 2011). | |
10.58† | Supply Agreement, dated December 20, 2002, among CyDex Pharmaceuticals, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited, and Hovione International Limited (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.59† | First Amendment to Supply Agreement, dated July 29, 2005, among CyDex Pharmaceuticals, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited, and Hovione International Limited (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.60 | 2nd Amendment to Supply Agreement, dated March 1, 2007, among CyDex Pharmaceuticals, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited, and Hovione International Limited (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.61† | 3rd Amendment to Supply Agreement, dated January 25, 2008, among CyDex Pharmaceuticals, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited, and Hovione International Limited (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.62† | 4th Amendment to Supply Agreement, dated September 28, 2009, among CyDex Pharmaceuticals, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited, and Hovione International Limited (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.63† | License Agreement, dated September 3, 1993, between CyDex Pharmaceuticals, Inc. and The University of Kansas (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.64† | Second Amendment to the License Agreement, dated August 4, 2004, between CyDex Pharmaceuticals, Inc. and The University of Kansas (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.65† | Acknowledgement Agreement, dated March 3, 2008, between CyDex Pharmaceuticals, Inc. and The University of Kansas (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.66† | Exclusive License Agreement, dated June 4, 1996, between Pfizer, Inc. and CyDex Pharmaceuticals, Inc. (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
82
Exhibit Number | Description | |
10.67† | Nonexclusive License Agreement, dated June 4, 1996, between Pfizer, Inc. and CyDex Pharmaceuticals, Inc. (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.68† | Addendum to Nonexclusive License Agreement, dated December 11, 2001, between CyDex Pharmaceuticals, Inc. and Pfizer, Inc. (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.69† | License Agreement, dated January 4, 2006, between CyDex Pharmaceuticals, Inc. and Prism Pharmaceuticals (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.70† | Amendment to License Agreement, dated May 12, 2006, between CyDex Pharmaceuticals, Inc. and Prism Pharmaceuticals (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.71† | Supply Agreement, dated March 5, 2007, between CyDex Pharmaceuticals, Inc. and Prism Pharmaceuticals (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010). | |
10.72† | License and Supply Agreement, dated October 12, 2005, between CyDex Pharmaceuticals, Inc. and Proteolix, Inc. (Filed as Exhibit 10.22)(File No. 000-28298) (incorporated by reference to Onyx Pharmaceuticals, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2009, File No. 000-28298). | |
10.73† | Amended and Restated License Agreement, dated October 31, 2012, between the Company and Chiva Pharmaceuticals, Inc. (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012). | |
10.74† | Settlement Agreement and Mutual Release, dated October 31, 2012, between the Company and Chiva Pharmaceuticals, Inc. (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012). | |
10.75† | Supply Agreement, dated June 13, 2011 by and between CyDex Pharmaceuticals, Inc. and Merck (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2011). | |
10.76 | License Agreement, dated September 5, 2011, between the Company and ARE-3535/3565 General Atomics Court, LLC (incorporated by reference to the Company’s Current Report on Form 8-K filed on September 9, 2011). | |
10.77 | Letter Agreement, dated September 29, 2011, between the Company and Biotechnology Value Fund, L.P. (incorporated by reference to the Company’s Current Report on Form 8-K filed on September 30, 2011). | |
10.78 | Amended Letter Agreement, dated June 19, 2013, between the Company and Biotechnology Value Fund, L.P. (incorporated by reference to the Company’s Current Report on Form 8-K filed on June 20, 2013). | |
10.79† | License Agreement, by and between CyDex and Spectrum Pharmaceuticals, Inc., dated as of March 8, 2013 (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended March 31, 2013). | |
10.80† | Supply Agreement, by and between CyDex and Spectrum Pharmaceuticals, Inc., dated as of March 8, 2013 (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended March 31, 2013). | |
10.81† | Royalty Stream and Milestone Payments Purchase Agreement, dated April 29, 2013, between the Company and Selexis S.A. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended June 30, 2013). | |
10.82† | License Agreement dated July 17, 2013 between the Company and Azure Biotech, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended September 30, 2013). | |
10.83† | Exclusive License and Distribution Agreement dated July 23, 2013 between the Company and Ethicor Pharmaceuticals, Ltd. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended September 30, 2013). | |
10.84† | License Agreement dated August 12, 2013 between CyDex Pharmaceuticals, Inc. and CURx Pharmaceuticals, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended September 30, 2013). | |
83
Exhibit Number | Description | |
10.85† | Supply Agreement dated August 12, 2013 between CyDex Pharmaceuticals, Inc. and CURx Pharmaceuticals, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended September 30, 2013). | |
10.86 | Amendment of “General” Contingent Value Rights Agreement dated May 20, 2014 among the Company, Metabasis Therapeutics, Inc., David F. Hale and Computershare Inc. (incorporated by reference to the Company’s Current Report on Form 8-K filed May 22, 2014). | |
10.87 | Amendment of “TR Beta” Contingent Value Rights Agreement dated May 20, 2014 among the Company, Metabasis Therapeutics, Inc., David F. Hale and Computershare, Inc. (incorporated by reference to the Company’s Current Report on Form 8-K filed May 22, 2014). | |
10.88† | Loan and Security Agreement dated May 21, 2014 between the Company and Viking Therapeutics, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended June 30, 2014). | |
10.89† | Master License Agreement dated May 21, 2014 among the Company, Metabasis Therapeutics, Inc. and Viking Therapeutics, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q | |
10.90† | Research and License Agreement dated May 9, 2014 between the Company and Omthera Pharmaceuticals, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended June 30, 2014). | |
10.91† | License Agreement between dated June 23, 2014 between the Company and TG Therapeutics, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended June 30, 2014). | |
10.92 | Letter Agreement, dated as of August 12, 2014, between Bank of America, N.A. and the Company regarding the Base Convertible Note Hedge Transactions (incorporated by reference to the Company’s Current Report on Form 8-K filed on August 18, 2014). | |
10.93 | Letter Agreement, dated as of August 12, 2014, between Bank of America, N.A. and the Company regarding the Base Warrant Transactions (incorporated by reference to the Company’s Current Report on Form 8-K filed on August 18, 2014). | |
10.94 | Letter Agreement, dated as of August 12, 2014, between Deutsche Bank AG, London Branch and the Company regarding the Base Convertible Note Hedge Transactions (incorporated by reference to the Company’s Current Report on Form 8-K filed on August 18, 2014). | |
10.95 | Letter Agreement, dated as of August 12, 2014, between Deutsche Bank AG, London Branch and the Company regarding the Base Warrant Transactions (incorporated by reference to the Company’s Current Report on Form 8-K filed on August 18, 2014). | |
10.96 | Letter Agreement, dated as of August 14, 2014, between Bank of America, N.A. and the Company regarding the Additional Convertible Note Hedge Transactions (incorporated by reference to the Company’s Current Report on Form 8-K filed on August 18, 2014). | |
10.97 | Letter Agreement, dated as of August 14, 2014, between Bank of America, N.A. and the Company regarding the Additional Warrant Transactions (incorporated by reference to the Company’s Current Report on Form 8-K filed on August 18, 2014). | |
10.98 | Letter Agreement, dated as of August 14, 2014, between Deutsche Bank AG, London Branch and the Company regarding the Additional Convertible Note Hedge Transactions (incorporated by reference to the Company’s Current Report on Form 8-K filed on August 18, 2014). | |
10.99 | Letter Agreement, dated as of August 14, 2014, between Deutsche Bank AG, London Branch and the Company regarding the Additional Warrant Transactions (incorporated by reference to the Company’s Current Report on Form 8-K filed on August 18, 2014). | |
10.100† | First Amendment to Master License Agreement dated September 6, 2014 among the Company, Metabasis Therapeutics, Inc. and Viking Therapeutics, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the Period ended September 30, 2014). | |
10.101† | Second Amendment to Master License Agreement, dated April 8, 2015, among the Company, Metabasis Therapeutics, Inc. and Viking Therapeutics, Inc. (incorporated by reference the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2015). | |
84
Exhibit Number | Description | |
10.102† | First Amendment to Loan and Security Agreement, dated April 8, 2015, among the Company, Metabasis Therapeutics, Inc. and Viking Therapeutics, Inc. (incorporated by reference the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2015). | |
10.103† | Amendment No. 4 to Sublicense Agreement, dated September 17, 2015, among the Company, Pharmacopeia, LLC and Retrophin, Inc. (incorporated by reference the Company’s amended Quarterly Report on Form 10-Q for the period ended September 30, 2015, as filed on December 23, 2015). | |
10.104† | Lease, dated November 3, 2015, between the Company and 3911/3931 SVB, LLC (incorporated by reference to the Company’s Current Report on Form 8-K filed on November 10, 2015). | |
21.1 | Subsidiaries of the Company (incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2011). | |
23.1 | Consent of independent registered public accounting firm-Grant Thornton LLP | |
31.1 | Certification by Principal Executive Officer, Pursuant to Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
31.2 | Certification by Principal Financial Officer, Pursuant to Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
32.1 | Certifications by Principal Executive Officer and Principal Financial Officer, Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
101.INS | XBRL Instance Document. | |
101.SCH | XBRL Taxonomy Extension Schema Document. | |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document. | |
† | Confidential treatment has been requested for portions of this exhibit. These portions have been omitted and submitted separately to the Securities and Exchange Commission. |
# | Indicates management contract or compensatory plan. |
85
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
LIGAND PHARMACEUTICALS INCORPORATED | |
By: | /S/ JOHN L. HIGGINS |
John L. Higgins, | |
Chief Executive Officer | |
Date: November 14, 2016
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ JOHN L. HIGGINS | Chief Executive Officer and Director (Principal Executive Officer) | November 14, 2016 | ||
John L. Higgins | ||||
/s/ MATTHEW KORENBERG | Vice President, Finance and Chief Financial Officer (Principal Financial and Accounting Officer) | November 14, 2016 | ||
Matthew Korenberg | ||||
/s/ TODD C. DAVIS | Director | November 14, 2016 | ||
Todd C. Davis | ||||
/s/ SUNIL PATEL | Director | November 14, 2016 | ||
Sunil Patel | ||||
/s/ STEPHEN L. SABBA | Director | November 14, 2016 | ||
Stephen L. Sabba | ||||
86
