Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zyla Life Sciences | a16-21618_18k.htm |
Exhibit 99.1
NASDAQ: EGLT Egalet Advancing Next-Generation Abuse-Deterrent Opioids November 2016 © Copyright 2016. Egalet Corporation

Founded around Guardian™ Technology Proprietary technology Established commercial infrastructure Product & pipeline target large markets & designed to address top FDA priority © Copyright 2016. Egalet Corporation. 2 2
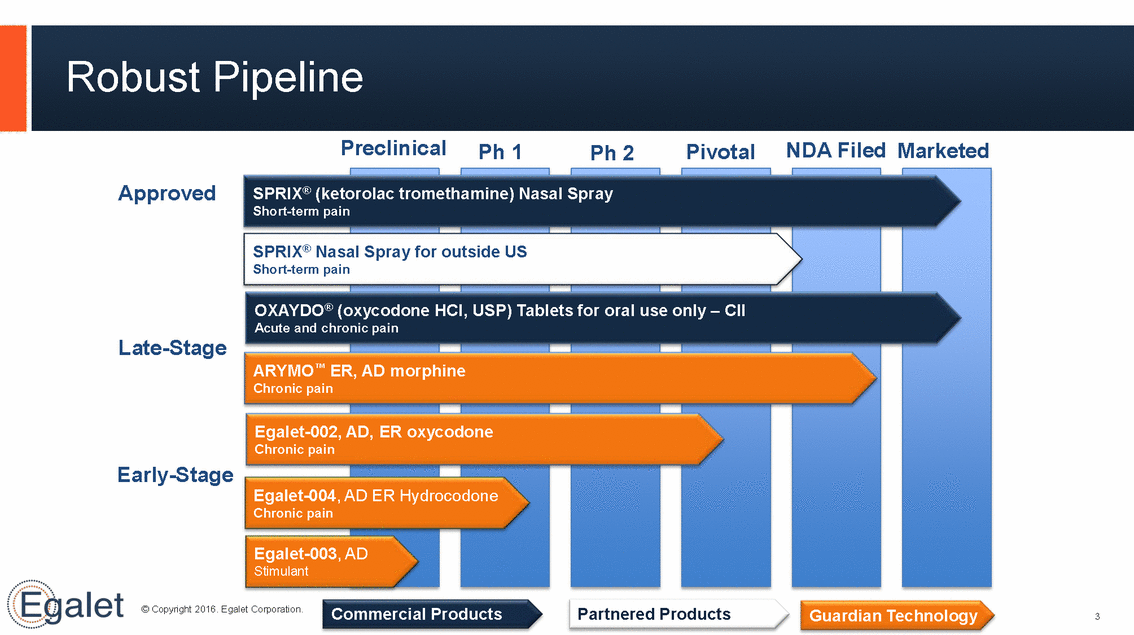
Robust Pipeline Preclinical NDA Filed Marketed Ph 1 Pivotal Ph 2 Approved SPRIX® (ketorolac trometh al Spray Short-term pain SPRIX® Nasal Spray for outside US Short-term pain OXAYDO® (oxycodone HCl, USP) Tablets for oral use only – CII Acute and chronic pain Late-Stage ARYMO™ ER, AD morphine Chronic pain Egalet-002, AD, ER oxycodone Chronic pain Early-Stage Egalet-004, AD ER Hydrocodone Chronic pain Egalet-003, AD Stimulant © Copyright 2016. Egalet Corporation. Commercial Products Partnered Products Guardian Technology 3 amine) Nas
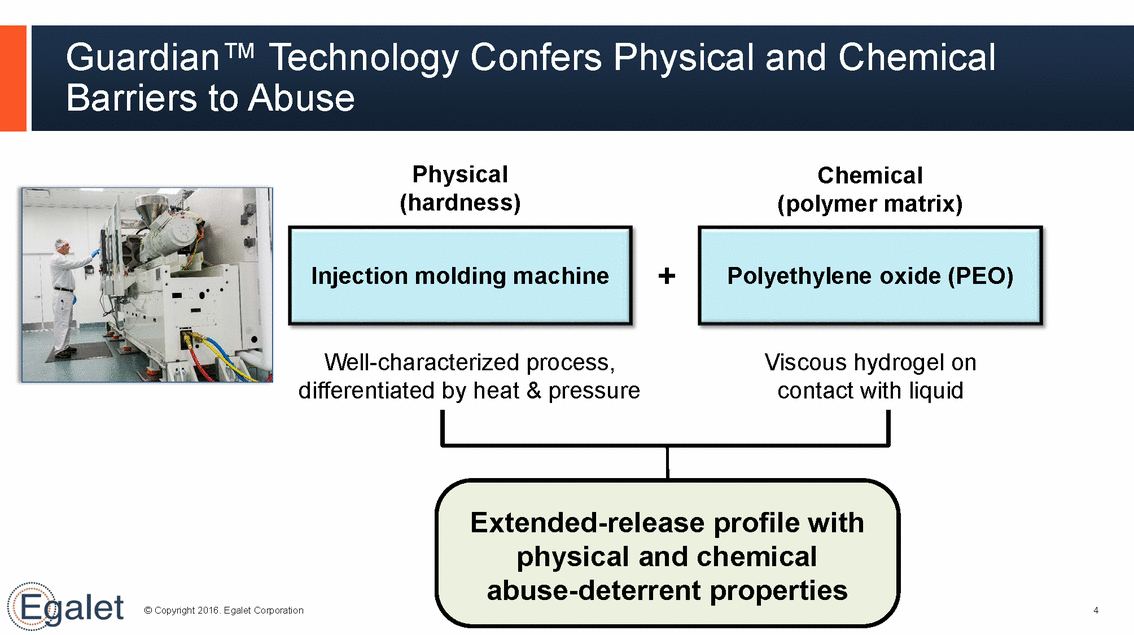
Guardian™ Technology Confers Physical and Chemical Barriers to Abuse Physical (hardness) Chemical (polymer matrix) + Injection molding machine Polyethylene oxide (PEO) Well-characterized process, Viscous hydrogel on differentiated by heat & pressure contact with liquid Extended-release profile with physical and chemical abuse-deterrent properties © Copyright 2016. Egalet Corporation 4
Lead Proprietary Abuse-Deterrent Candidate: ARYMO™ ER © Copyright 2016. Egalet Corporation

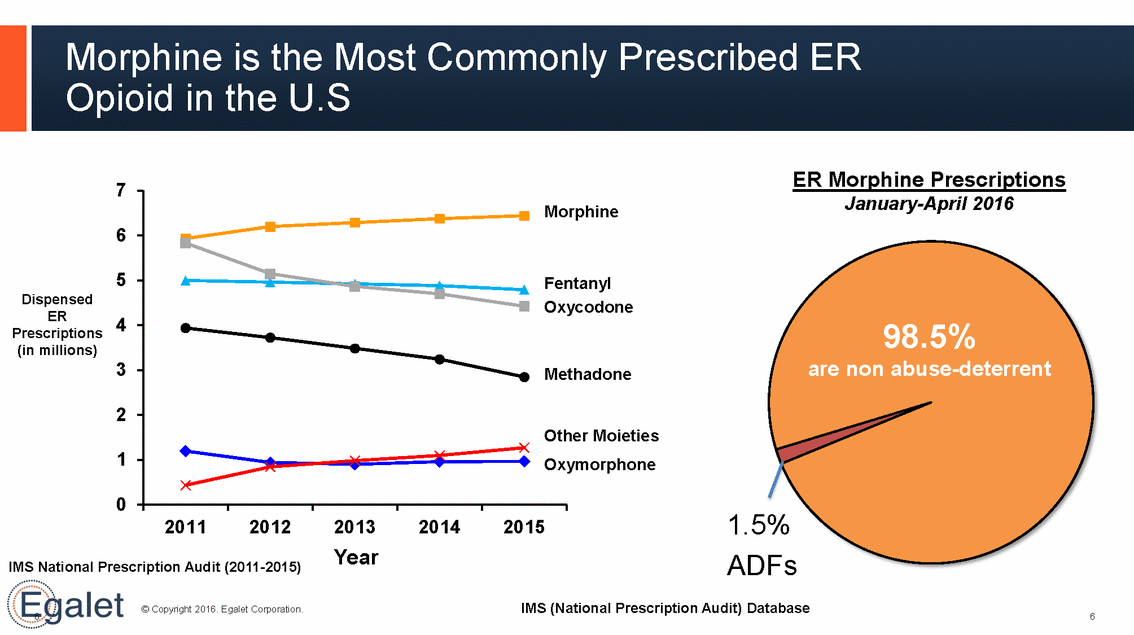
Morphine is the Most Commonly Prescribed ER Opioid in the U.S ER Morphine Prescriptions 7 January-April 2016 rphine 6 5 ntanyl ycodone Dispensed ER Prescriptions (in millions) 4 98.5% non abuse-deterrent are 3 thadone 2 her Moieties ymorphone 1 0 1.5% ADFs IMS (National Prescription Audit) Database 2011 2012 2013 Year 2014 2015 IMS National Prescription Audit (2011-2015) © Copyright 2016. Egalet Corporation. 6 6 Mo Fe Ox Me Ot Ox
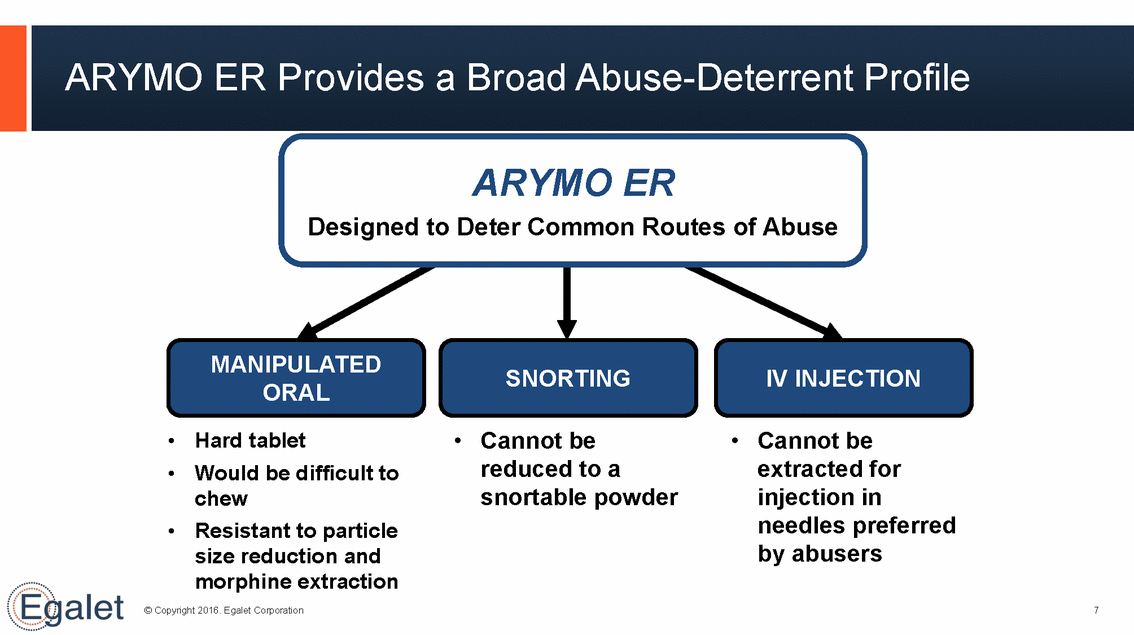
ARYMO ER Provides a Broad Abuse-Deterrent Profile ARYMO ER Designed to Deter Common Routes of Abuse MANIPULATED ORAL SNORTING IV INJECTION • Cannot be reduced to a • Cannot be extracted for injection in needles preferred by abusers • • Hard tablet Would be difficult to chew Resistant to particle size reduction and morphine extraction snortable powder • © Copyright 2016. Egalet Corporation 7
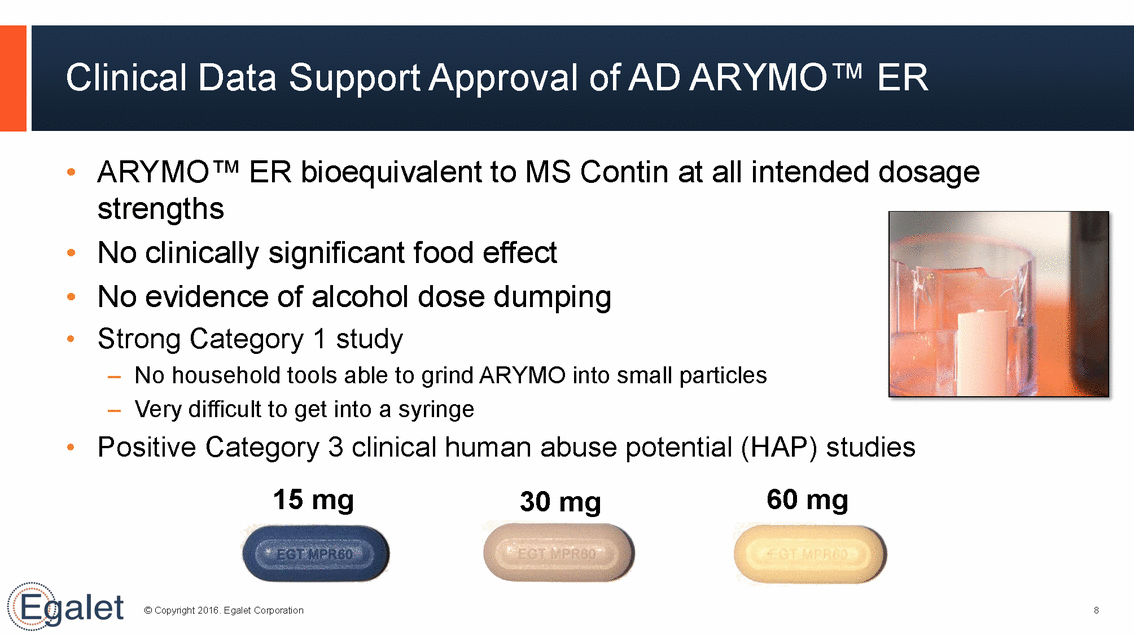
Clinical Data Support Approval of AD ARYMO™ ER • ARYMO™ ER bioequivalent to MS Contin strengths No clinically significant food effect No evidence of alcohol dose dumping Strong Category 1 study –No household tools able to grind ARYMO into small –Very difficult to get into a syringe at all intended dosage • • • particles • Positive Category 3 clinical 15 mg human abuse 30 mg potential (HAP) studies 60 mg © Copyright 2016. Egalet Corporation 8
ARYMO™ ERCould be on Market in 1Q17* NDA submitted Dec 2015 NDA accepted Feb 2016 FDA Advisory Committee supported approval and abuse-deterrent labeling Aug 4, 2016 FDA regulatory action Commercial launch Q1:17 *for the management of pain severe enough to require daily, around-the-clock, opioid treatment and for which alternative treatments are inadequate © Copyright 2016. Egalet Corporation 9
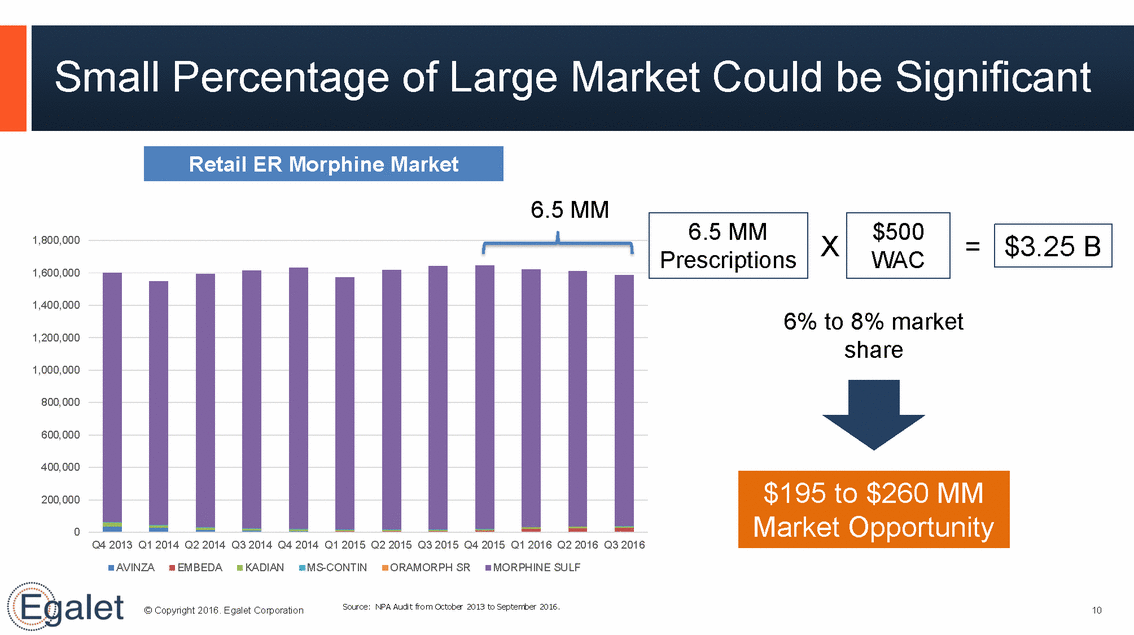
Small Percentage of Large Market Could be Significant 6.5 MM X = 1,800,000 1,600,000 1,400,000 6% to 8% market 1,200,000 share 1,000,000 800,000 600,000 400,000 200,000 0 Q4 2013 Q1 2014 Q2 2014 Q3 2014 Q4 2014 Q1 2015 Q2 2015 Q3 2015 Q4 2015 Q1 2016 Q2 2016 Q3 2016 AVINZA EMBEDA KADIAN MS-CONTIN ORAMORPH SR MORPHINE SULF Source: NPA Audit from October 2013 to September 2016. © Copyright 2016. Egalet Corporation 10 $195 to $260 MM Market Opportunity $3.25 B $500 WAC 6.5 MM Prescriptions Retail ER Morphine Market
Egalet-002, an abuse-deterrent, extended-release oxycodone © Copyright 2016. Egalet Corporation

Egalet-002, AD/ER Oxycodone, in Late-Stage Development* Hard matrix surrounded by hard shell Resistant to particle size reduction & chemical extraction Forms viscous hydrogel on contact with liquid can’t draw up into a syringe Reduces Reduces injection Category risk risk of of accidental misuse via chewing intential abuse via snorting or IV Oxycodone is highest selling ER opioid; $2.5 B in sales in 2013 2/3 studies to include direct comparison to Oxycontin NDA submission mid-17 Pivotal Phase 3 program ongoing *for the management of pain severe enough to require daily, around-the-clock, opioid treatment and for which alternative treatments are inadequate © Copyright 2016. Egalet Corporation 12
Focused Commercial Strategy © Copyright 2016. Egalet Corporation

Products Brought in Advance of ARYMO ER Launch Accelerated transformation into a commercial company w/ revenues Established commercial groundwork for ARYMO Created pain franchise presence laying ER launch © Copyright 2016. Egalet Corporation 14
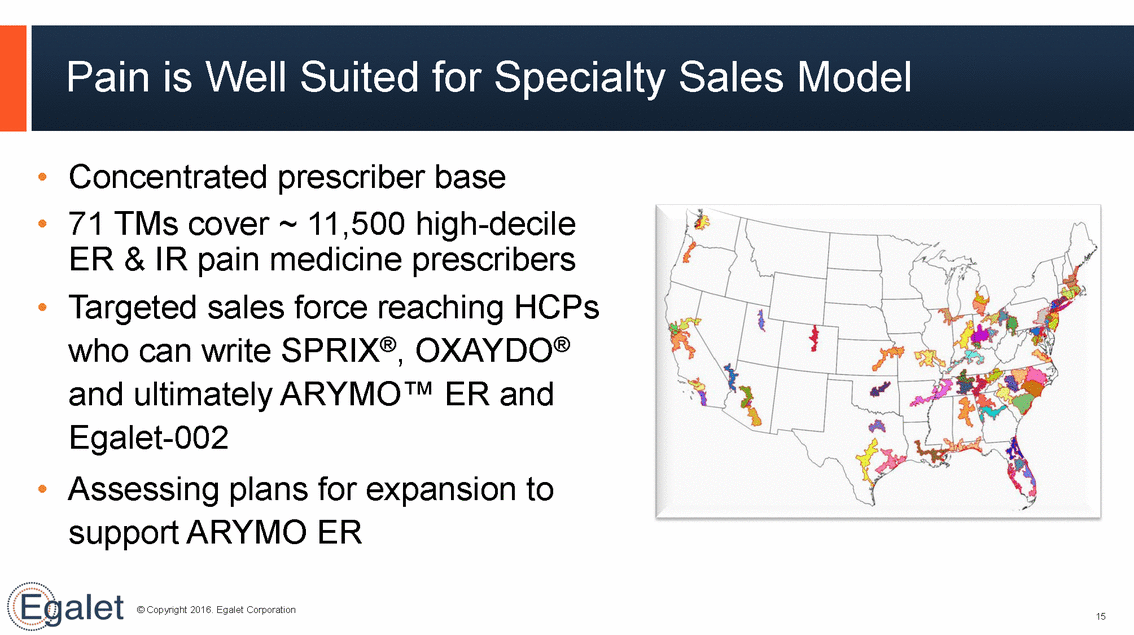
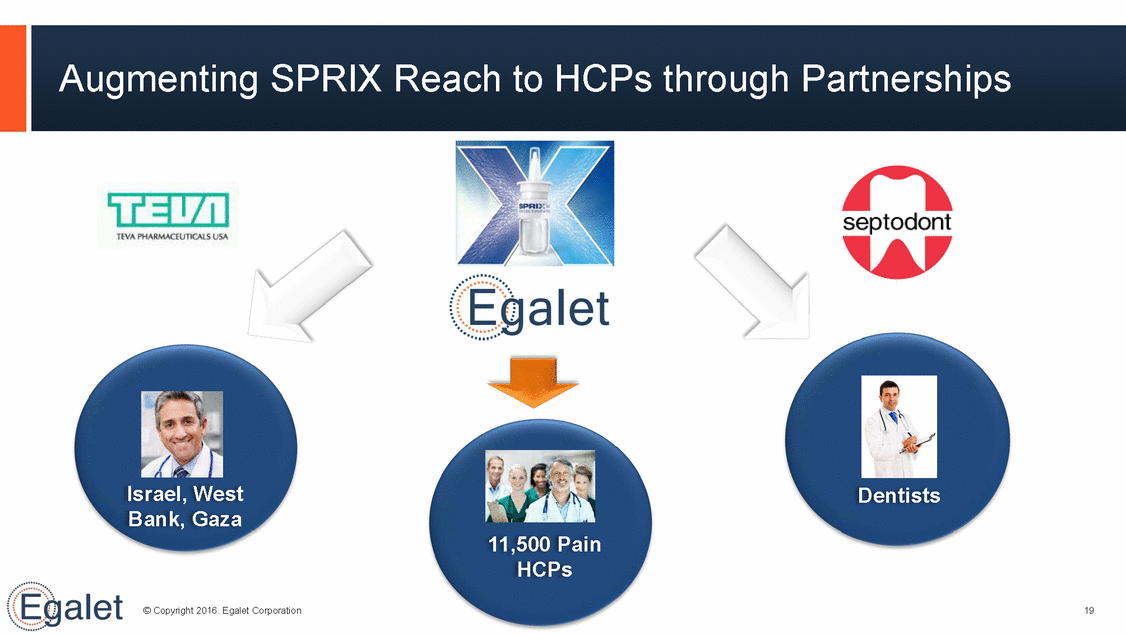
Pain is WellSuited for Specialty Sales Model • • Concentrated prescriber base 71 TMs cover ~ 11,500 high-decile ER & IR pain medicine prescribers Targeted sales force reaching HCPs who can write SPRIX®, OXAYDO® and ultimately ARYMO™ ER and Egalet-002 • • Assessing plans support ARYMO for expansion to ER © Copyright 2016. Egalet Corporation 15
Suppon ror patientl> • _ ,CII5PR!IC ponTor ...,._ao!l._, U'RIX ca"lllG.....,: + Ell<ty II tD IIIICI!In lleaiDlcare proV"tlJ& + 4>10 -......., ..,flllll'UOdcr!o>IUIM • OC>-ply""""'J',.,....i;fboo ,.n-.dhcomnwdJI tor2oWJ:&.r,.,..,....,. 1011bo proo<llood--..ppocl--<lily'"'1'1<111llirJ'IIGitl<ly --..g..,......_..,.,. Sffltx ll1aJCI nat 1>o"""' .a-otw"llct cr.r , l!ljlllll. aa1110r HSMliL A-.. Sra Ill,--.. -.n>C Jq>U; AI!f C:<nIIIIN.l.I.i.n:-m:av"<l.."::'·-:·:-:.=-.:..:1..r:.:::=_.... ...:pcr"<nllei'\-CII1 DO OOXI.IIt1!io1I•IIZOIII--Ita!W!ln pUDQDo""',_o:to:anbt...,._ :=::;-..:,;:. i1la'mlttln, ttiUdrg (kebtll.ctromaharrie) ,,.lilt '"I............. BDUdWll'1'*g. ,....i ii.Aii SPoOfJ Dalt a....l...e.... t ©Copyright 2016. Egalet Corporation 16 Dosingand presaibing smtx af""'tiodtn•s..td'l<l ""*·'Ell:h lxll!locxrDr6 g not...., -. I not•-!il'"ldlhddl!W trmln a:li'IBII-L' "ForpAIQIID---CII-eo.,-<110III,or .-....ylhiii"<IQO[ ii110I"IIIld daloll t 'I"Wf mal'lliiUII--,u :ur. os -· --fCliiiEll<lcD<Risllaf -.g,--,..K<torclx:c:r .=...·lhDrtMtJl)-*tcanlba.-1»\a.dl« SPRI·"..".'·.,' Now available through tiP; f!f Direct -- OroeringSPRL--------------------c.I1-3M.f11-.51?RIXII-..rn-77'111)..a1-...:1pMr:ITII<¥ toc,._,d_I'D"""""'""f&>"l<rl l nlcrtn.abl. .SCRISE bytnyas o.Sa'biii)"'IImm nllllnCl •SffiiX pmcby1,00...ET('Mihm1Dir.b1o1Har-a11on Slq;Jport Line ------------Rlrtlg aSAUllllGciJon,QJ 1-..J'-Iii'ID n-71411). -F.=y!UilJWO>ItOOI'II ET •-lal -pn.....:y......,n -tl1oOlmocsranr rnoanl n---:..:o2"Jpilliir'b-..,.,SI"UX-=--=s::t.,":::;'-tnl""'*-' l'lmaarl9 ®aIet.O,.>,O..IO., ,.,I"Z"I......

SPRIX® Nasal Spray Product Opportunity SPRIX NSAIDS Ibuprofen Diclofenac Celecoxib Meloxicam Opioids Hydrocodone Oxycodone Oxymorphone Morphine PATIENTS Broad Indication for Short-term Pain Relief (up to 5 days) © Copyright 2016. Egalet Corporation 17
Quarterly SPRIX Rx Continue to Increase as of 9/30 5,000 4,500 4,000 3,500 3,000 2,500 2,000 1,500 1,000 500 - Q1-15 Q2-15 Q3-15 Q4-15 Q1-16 Q2-16 Q3-16 © Copyright 2016. Egalet Corporation 18 Net revenue: Q3: $3.8 MM; YTD: $8.6 MM Prescription growth: Q3: 21% New prescribers: Q3: + >450
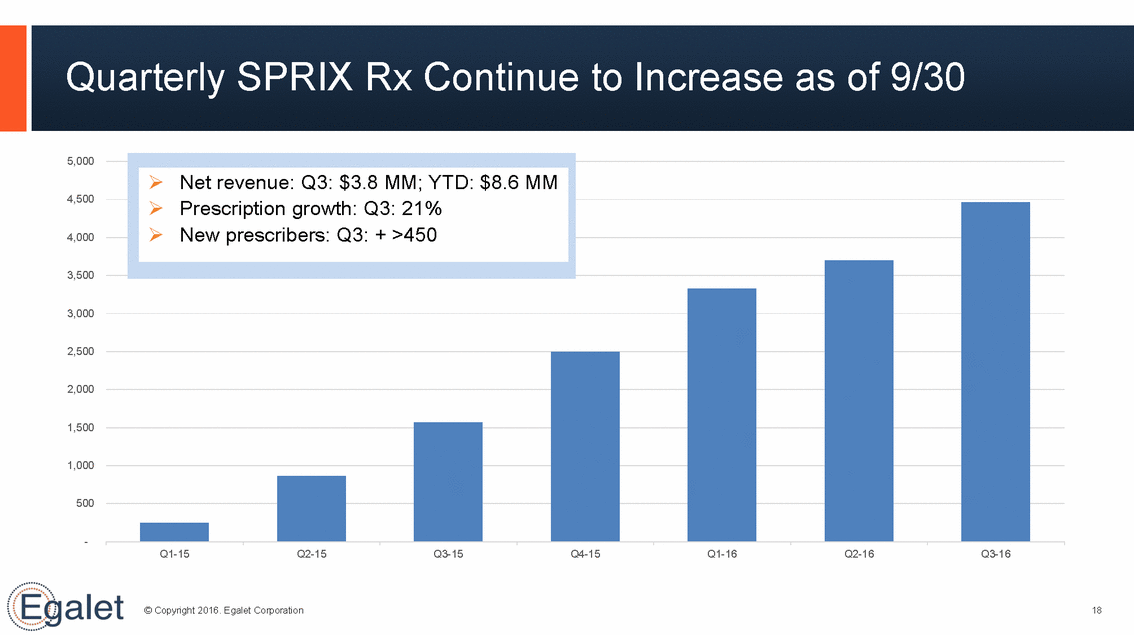
Augmenting SPRIX Reach to HCPs through Partnerships Israel, West Bank, Gaza Dentists 11,500 Pain HCPs © Copyright 2016. Egalet Corporation 19
OXAYDO®, Only IR Oxy Designed to Discourage Abuse For the management of acute and chronic moderate to severe pain where the use of an opioid analgesic is appropriate. • • Immediate-release oxycodone with aversion technology Designed to discourage abuse via most common route of abuse—snorting • • • Approved in 5 mg and 7.5 mg doses Demonstrated BE at 15 mg Abuse-deterrent Category 1 data showed resists syringeability Goal: New dosage second half of 2017 © Copyright 2016. Egalet Corporation 20

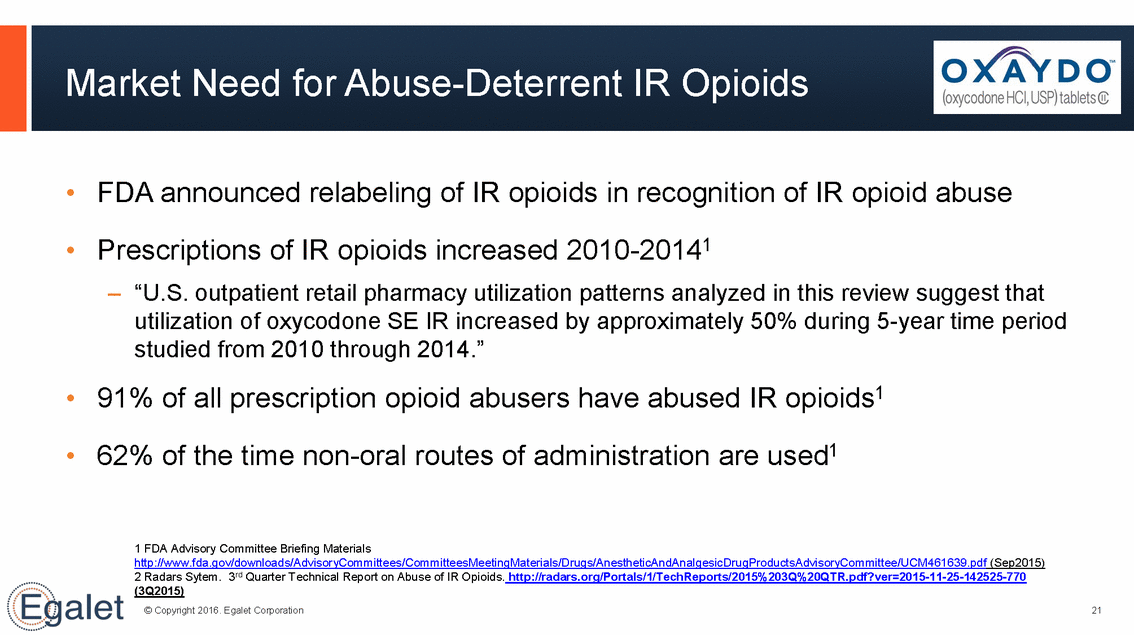
Market Need for Abuse-Deterrent IR Opioids • FDA announced relabeling of IR opioids in recognition of IR opioid abuse Prescriptions of IR opioids increased 2010-20141 –“U.S. outpatient retail pharmacy utilization patterns analyzed in this review suggest that • utilization of oxycodone SE IR increased by approximately 50% during 5-year studied from 2010 through 2014.” 91% of all prescription opioid abusers have abused IR opioids1 62% of the time non-oral routes of administration are used1 time period • • 1 FDA Advisory Committee Briefing Materials http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndAnalgesicDrugProductsAdvisoryCommittee/UCM461639.pdf (Sep2015) 2 Radars Sytem. 3rd Quarter Technical Report on Abuse of IR Opioids. http://radars.org/Portals/1/TechReports/2015%203Q%20QTR.pdf?ver=2015-11-25-142525-770 (3Q2015) © Copyright 2016. Egalet Corporation 21
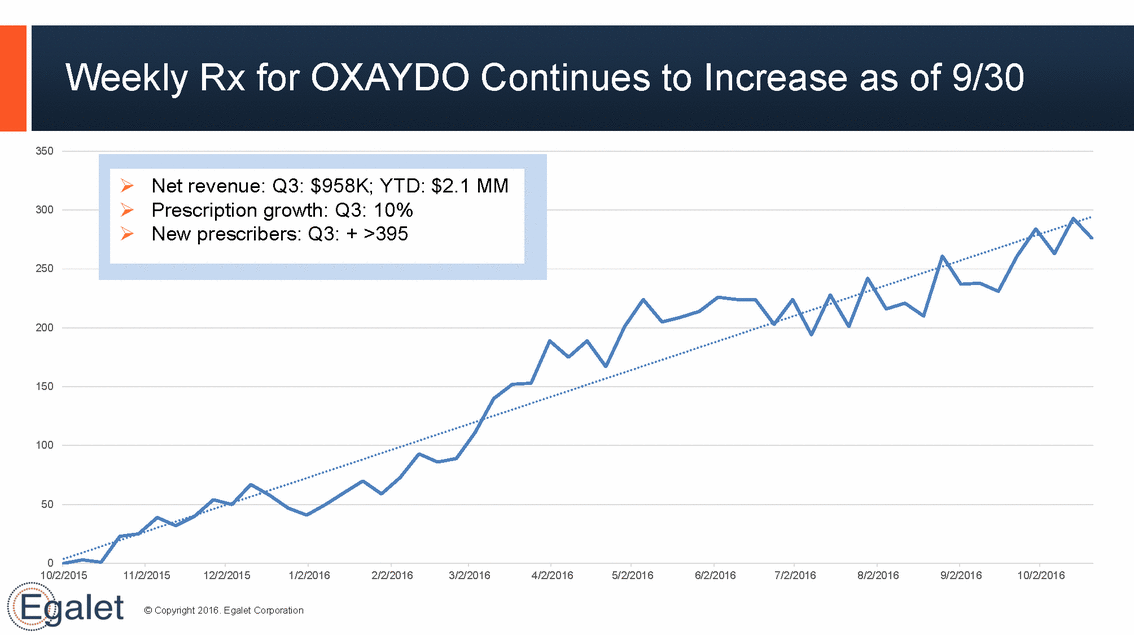
Weekly Rx for OXAYDO Continues to Increase as of 9/30 350 300 250 200 150 100 50 0 10/2/2015 11/2/2015 12/2/2015 1/2/2016 2/2/2016 3/2/2016 4/2/2016 5/2/2016 6/2/2016 7/2/2016 8/2/2016 9/2/2016 10/2/2016 © Copyright 2016. Egalet Corporation Net revenue: Q3: $958K; YTD: $2.1 MM Prescription growth: Q3: 10% New prescribers: Q3: + >395
Financials and Conclusion © Copyright 2016. Egalet Corporation

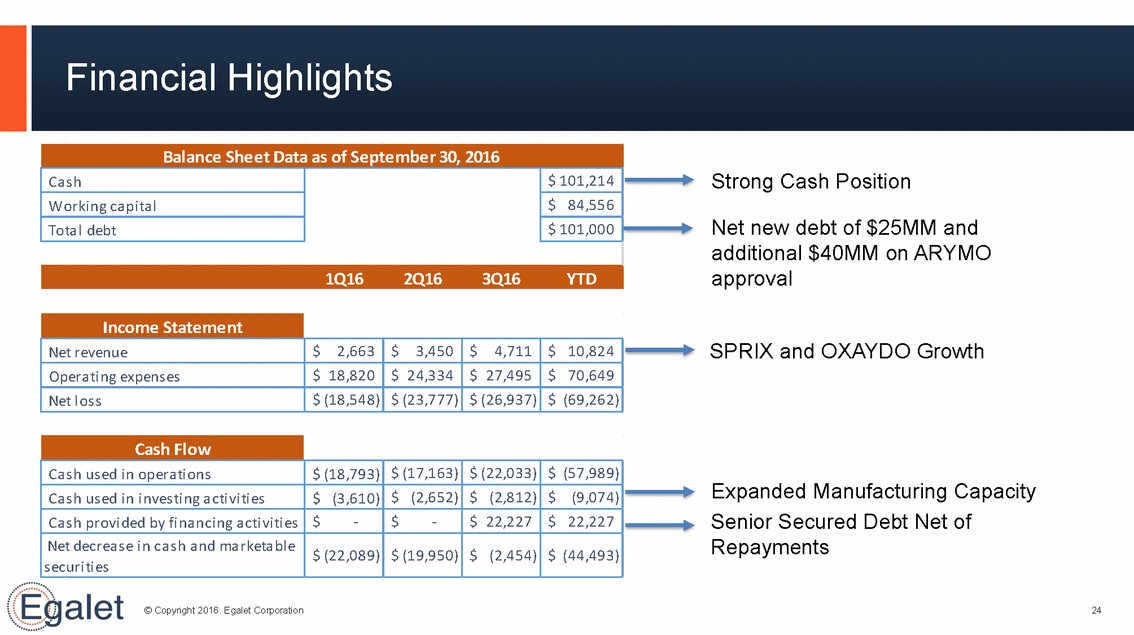
Financial Highlights Strong Cash Position Net new debt of $25MM and additional $40MM on ARYMO approval SPRIX and OXAYDO Growth Expanded Manufacturing Capacity Senior Secured Debt Net of Repayments © Copyright 2016. Egalet Corporation 24 Cash Flow Ca s h us ed i n opera ti ons $ (18,793) $ (17,163) $ (22,033) $ (57,989) Ca s h us ed i n i nves ti ng a cti vi ti es $ (3,610) $ (2,652) $ (2,812) $(9,074) Ca s h provi ded by fi na nci ng a cti vi ti es $-$- $ 22,227 $ 22,227 Net decrea s e i n ca s h a nd ma rketa bl e s ecuri ti es $ (22,089) $ (19,950) $ (2,454) $ (44,493) Income Statement Net revenue $2,663 $3,450 $4,711 $ 10,824 Opera ti ng expens es $ 18,820 $ 24,334 $ 27,495 $ 70,649 Net l os s $ (18,548) $ (23,777) $ (26,937) $ (69,262) Balance Sheet Data as of September 30, 2016 Ca s h $ 101,214 Worki ng ca pi ta l $ 84,556 Tota l debt $ 101,000 1Q162Q163Q16YTD
Sustainable Growth with Numerous Milestones in Next 24 Months Q4 ARYMO ER Regulatory Action H2:16 H1:17 H1:17 Egalet-002 Cat 3 IN HAP Submit IND for Egalet-003 Complete Egalet-002 Phase 3 program Mid-17 Egalet-002 oral HAP data Mid-17 submit Egalet-002 NDA H2:17 New dose of OXAYDO available 2018 Launch Egalet-002 © Copyright 2016. Egalet Corporation.

Thank You @EgaletCorp Egalet.com © Copyright 2016. Egalet Corporation

