Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - PHASERX, INC. | v452454_ex99-2.htm |
| 8-K - FORM 8-K - PHASERX, INC. | v452454_8k.htm |
Exhibit 99.1

Unlocking the value of mRNA ® Non - Human Primate Safety Study Data November 8, 2016 NASDAQ: PZRX

FORWARD - LOOKING STATEMENTS This presentation contains forward - looking statements which are based on current expectations, estimates and projections. Statements that are not historical facts are forward - looking statements and typically are identified by words like “may”, “believe”, “anticipate”, “could”, “should”, “estimate”, “expect”, “intend”, “plan”, “project”, “will”, “forecast”, “budget”, “pro forma”, and similar terms. These statements are not guarantees of future performance, events or results and involve potential risks and uncertainties. Although we believe that such statements are based on reasonable assumptions, these forward - looking statements are subject to numerous factors, risks and uncertainties that could cause actual outcomes and results to be materially different from those projected or assumed in our forward - looking statements. We caution you that any forward - looking statement reflects only our belief at the time the statement is made. Accordingly, the Company’s actual results may differ from our current expectations, estimates and projections. We undertake no obligation to update any forward - looking statements, whether as a result of new information, future events or otherwise. 2
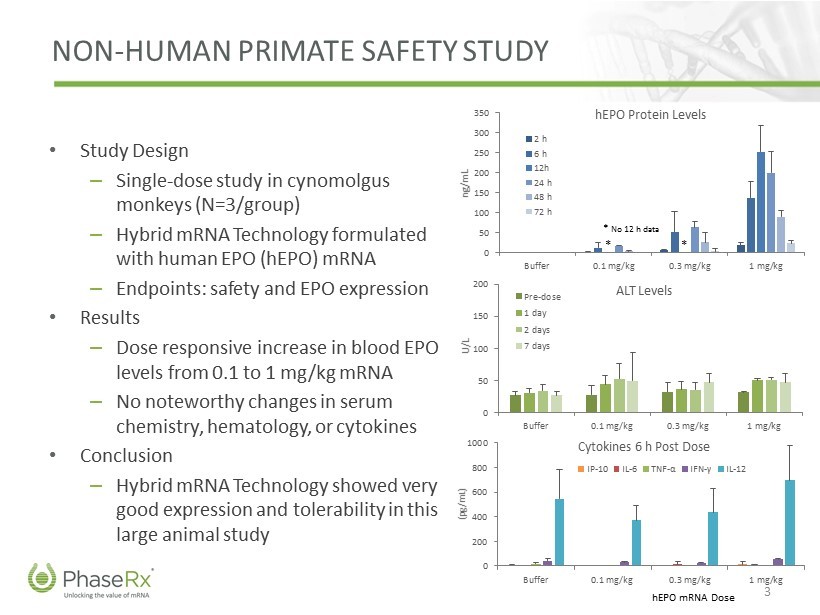
NON - HUMAN PRIMATE SAFETY STUDY • Study Design – Single - dose study in cynomolgus monkeys (N=3/group) – Hybrid mRNA Technology formulated with human EPO ( hEPO ) mRNA – Endpoints: safety and EPO expression • Results – Dose responsive increase in blood EPO levels from 0.1 to 1 mg/kg mRNA – No noteworthy changes in serum chemistry, hematology, or cytokines • Conclusion – Hybrid mRNA Technology showed very good expression and tolerability in this large animal study 3 0 50 100 150 200 250 300 350 Buffer 0.1 mg/kg 0.3 mg/kg 1 mg/kg ng/mL hEPO Protein Levels 2 h 6 h 12h 24 h 48 h 72 h * * * No 12 h data 0 50 100 150 200 Buffer 0.1 mg/kg 0.3 mg/kg 1 mg/kg U/L ALT Levels Pre-dose 1 day 2 days 7 days hEPO mRNA Dose 0 200 400 600 800 1000 Buffer 0.1 mg/kg 0.3 mg/kg 1 mg/kg (pg/mL) Cytokines 6 h Post Dose IP-10 IL-6 TNF - α IFN - γ IL-12
