Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a16-20656_2ex99d1.htm |
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a16-20656_28k.htm |
Exhibit 99.2
3Q16 Financial Results and Program Updates November 7, 2016

Safe Harbor This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 relating to preclinical and clinical development of our product candidates, the timing and reporting of results from preclinical studies and clinical trials, the prospects and timing of the potential regulatory approval of our product candidates, commercialization plans, financing plans, and the projected cash position for the Company. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Any or all of the forward-looking statements in this press release may turn out to be wrong and can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. For example, with respect to statements regarding the goals, progress, timing, and outcomes of discussions with regulatory authorities, and in particular the potential goals, progress, timing, and results of preclinical studies and clinical trials, actual results may differ materially from those set forth in this release due to the risks and uncertainties inherent in our business, including, without limitation: the potential that results of clinical or preclinical studies indicate that the product candidates are unsafe or ineffective; the potential that it may be difficult to enroll patients in our clinical trials; the potential that regulatory authorities, including the FDA, EMA, and PMDA may not grant or may delay approval for our product candidates; the potential that we may not be successful in commercializing Galafold in Europe or our other product candidates if and when approved; the potential that preclinical and clinical studies could be delayed because we identify serious side effects or other safety issues; and the potential that we will need additional funding to complete all of our studies. Further, the results of earlier preclinical studies and/or clinical trials may not be predictive of future results. With respect to statements regarding projections of the Company's cash position, actual results may differ based on market factors and the Company's ability to execute its operational and budget plans. In addition, all forward-looking statements are subject to other risks detailed in our Annual Report on Form 10-K for the year ended December 31, 2015 and Quarterly Report on Form 10-Q for the quarter ended September 30, 2016. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update this news release to reflect events or circumstances after the date hereof. Introduction

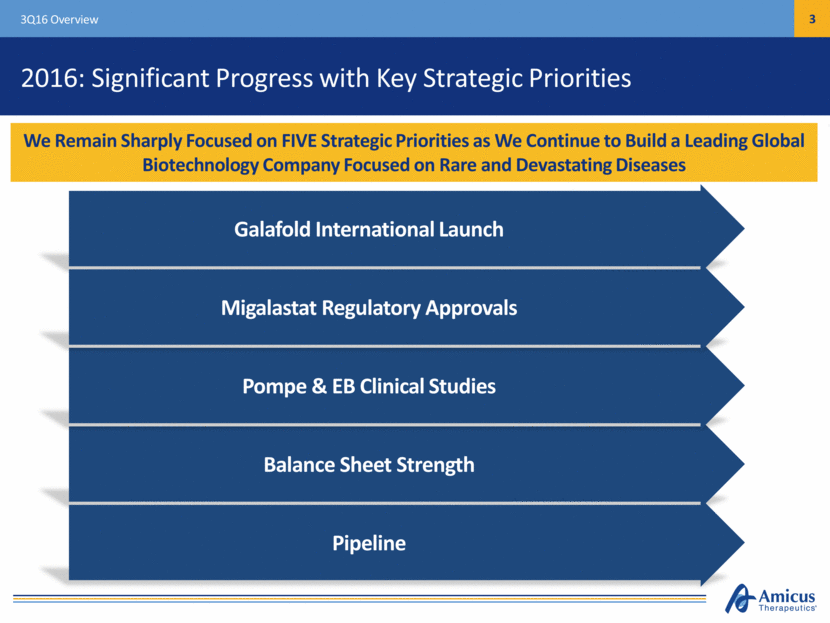
2016: Significant Progress with Key Strategic Priorities 3Q16 Overview We Remain Sharply Focused on FIVE Strategic Priorities as We Continue to Build a Leading Global Biotechnology Company Focused on Rare and Devastating Diseases Galafold International Launch Balance Sheet Strength Pompe & EB Clinical Studies Migalastat Regulatory Approvals Pipeline
Galafold™ (Migalastat) Precision Medicine for Fabry Disease International Launch Underway

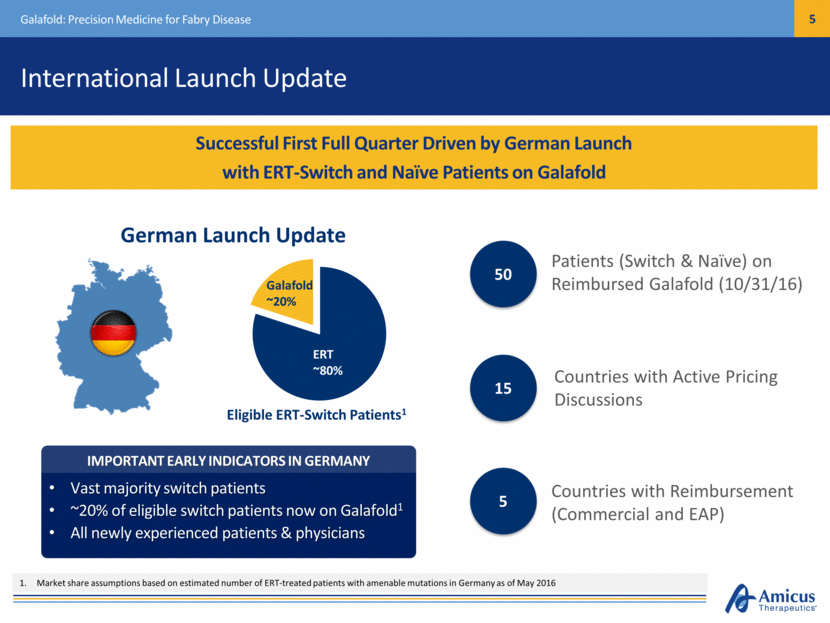
International Launch Update Galafold: Precision Medicine for Fabry Disease Successful First Full Quarter Driven by German Launch with ERT-Switch and Naïve Patients on Galafold Patients (Switch & Naïve) on Reimbursed Galafold (10/31/16)) 50 Countries with Reimbursement (Commercial and EAP) 5 Countries with Active Pricing Discussions 15 Market share assumptions based on estimated number of ERT-treated patients with amenable mutations in Germany as of May 2016 IMPORTANT EARLY INDICATORS IN GERMANY Vast majority switch patients ~20% of eligible switch patients now on Galafold1 All newly experienced patients & physicians Galafold ~20% ERT ~80% German Launch Update Eligible ERT-Switch Patients1
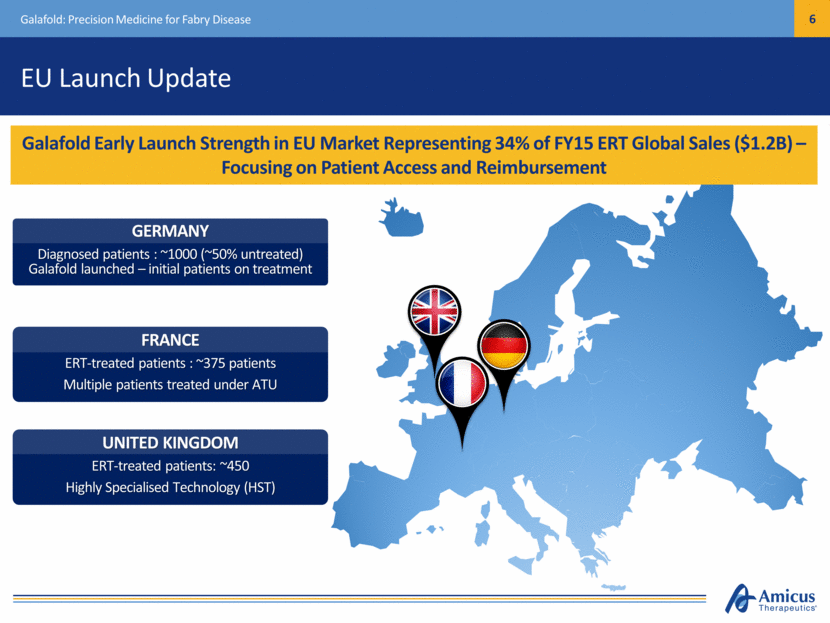
EU Launch Update Galafold: Precision Medicine for Fabry Disease Galafold Early Launch Strength in EU Market Representing 34% of FY15 ERT Global Sales ($1.2B) – Focusing on Patient Access and Reimbursement UNITED KINGDOM ERT-treated patients: ~450 Highly Specialised Technology (HST) FRANCE ERT-treated patients : ~375 patients Multiple patients treated under ATU GERMANY Diagnosed patients : ~1000 (~50% untreated) Galafold launched – initial patients on treatment
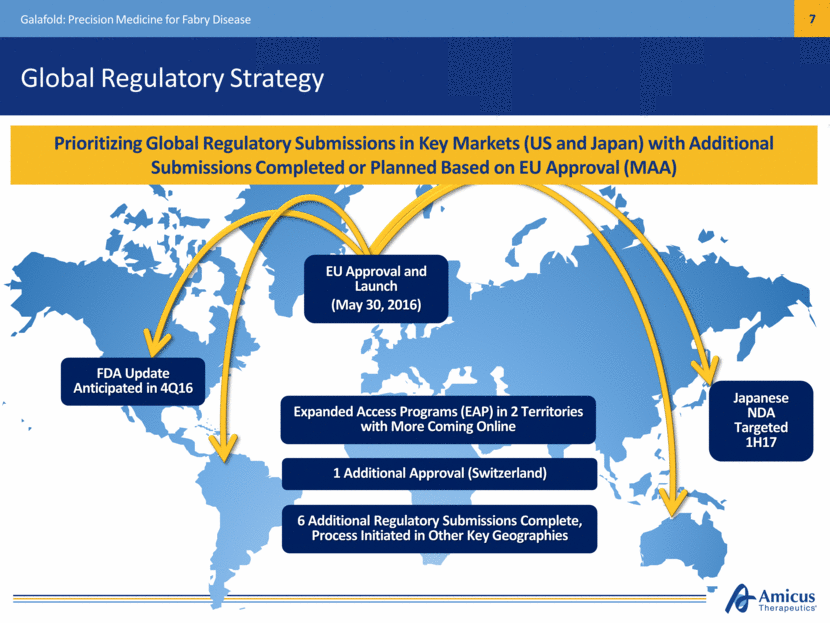
Global Regulatory Strategy Galafold: Precision Medicine for Fabry Disease EU Approval and Launch (May 30, 2016) Expanded Access Programs (EAP) in 2 Territories with More Coming Online Prioritizing Global Regulatory Submissions in Key Markets (US and Japan) with Additional Submissions Completed or Planned Based on EU Approval (MAA) 6 Additional Regulatory Submissions Complete, Process Initiated in Other Key Geographies FDA Update Anticipated in 4Q16 Japanese NDA Targeted 1H17 1 Additional Approval (Switzerland)
ATB200 Novel ERT for Pompe Disease A Proprietary, Clinical-Stage Biologics Program

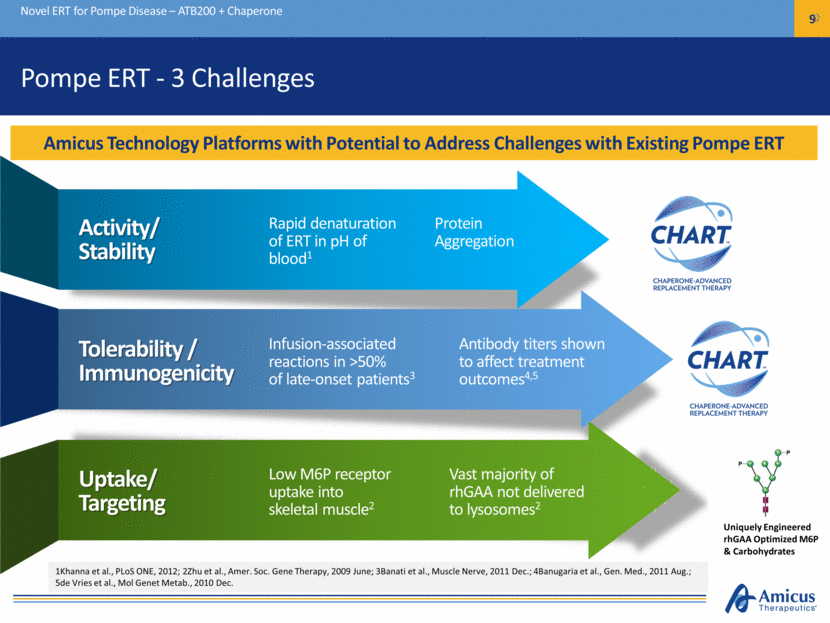
Pompe ERT - 3 Challenges Novel ERT for Pompe Disease – ATB200 + Chaperone 9 Activity/ Stability Rapid denaturation of ERT in pH of blood1 Tolerability / Immunogenicity Infusion-associated reactions in >50% of late-onset patients3 Uptake/ Targeting Low M6P receptor uptake into skeletal muscle2 Antibody titers shown to affect treatment outcomes4,5 Vast majority of rhGAA not delivered to lysosomes2 Protein Aggregation 1Khanna et al., PLoS ONE, 2012; 2Zhu et al., Amer. Soc. Gene Therapy, 2009 June; 3Banati et al., Muscle Nerve, 2011 Dec.; 4Banugaria et al., Gen. Med., 2011 Aug.; 5de Vries et al., Mol Genet Metab., 2010 Dec. Amicus Technology Platforms with Potential to Address Challenges with Existing Pompe ERT Uniquely Engineered rhGAA Optimized M6P & Carbohydrates
Pompe Clinical Study ATB200-02 Data Cascade Novel ERT for Pompe Disease – ATB200 + Chaperone Data in initial ambulatory ERT-switch patients (Cohort 1, n=4) Data in ERT-naïve patients (Cohort 3) Additional extension study data (all Cohorts) A Cascade of Data Points from 4Q16 through 2017 Offer Clear Parameters to Define Success and Differentiate ATB200/AT2221 Pompe Data Cascade 4Q16 Through 2017 Additional data & initial extension data in Cohort 1 Data in non-ambulatory ERT-switch patients (Cohort 2) 18-WEEK DATA Safety / tolerability Pharmacokinetics (PK) Biomarkers Immunogenicity EXTENSION DATA Motor/pulmonary function

Pompe Clinical Study ATB200-02 Parameters for Success Key Questions to Determine Potential for ATB200/AT2221 to Address 3 Major ERT Challenges in Initial 18-Week Treatment Period Novel ERT for Pompe Disease – ATB200 + Chaperone Do patients safely switch from standard of care to ATB200/AT2221? Do patients tolerate ATB200/AT2221 with limited infusion-associated reactions? Is PK profile of ATB200/AT2221 differentiated and in optimal range consistent with preclinical studies? Safety Exposure, Targeting & Uptake Tolerability & Immunogenicity Do antibodies in switch patients remain the same on ATB200/AT2221? CHALLENGES: KEY QUESTIONS: ? ? ? ?

SD-101 for Epidermolysis Bullosa

PHASE 3 ESSENCE STUDY STATUS 28 sites activated as of October 31, 2016 100% conversion to extension study (SD-006) SAP submitted to FDA for finalization Top-line Phase 3 data anticipated 1H17 EB Program Update - Phase 3 ESSENCE Study (SD-005) SD-101 for EB Significant Momentum for Ongoing Study with Data on Track for 1H17
Financial Summary

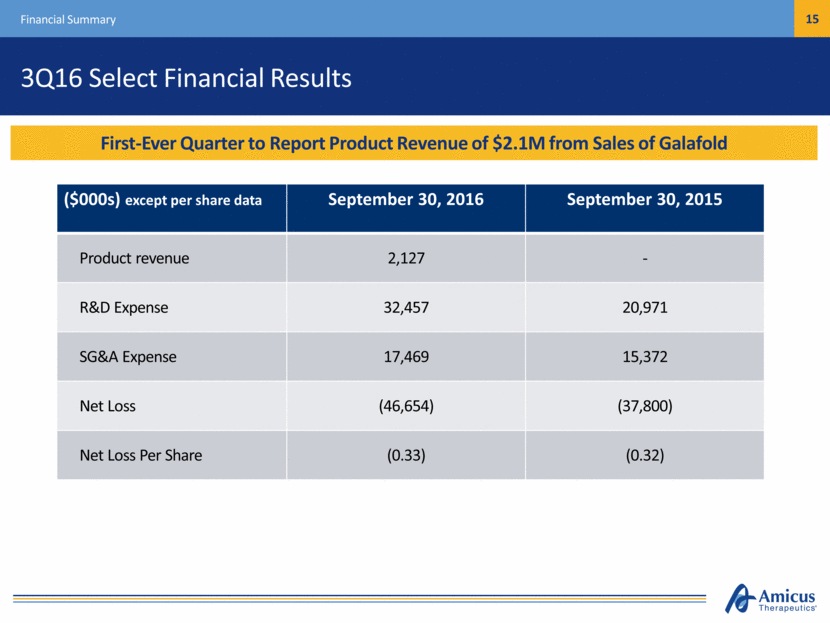
3Q16 Select Financial Results Financial Summary ($000s) except per share data September 30, 2016 September 30, 2015 Product revenue 2,127 - R&D Expense 32,457 20,971 SG&A Expense 17,469 15,372 Net Loss (46,654) (37,800) Net Loss Per Share (0.33) (0.32) First-Ever Quarter to Report Product Revenue of $2.1M from Sales of Galafold
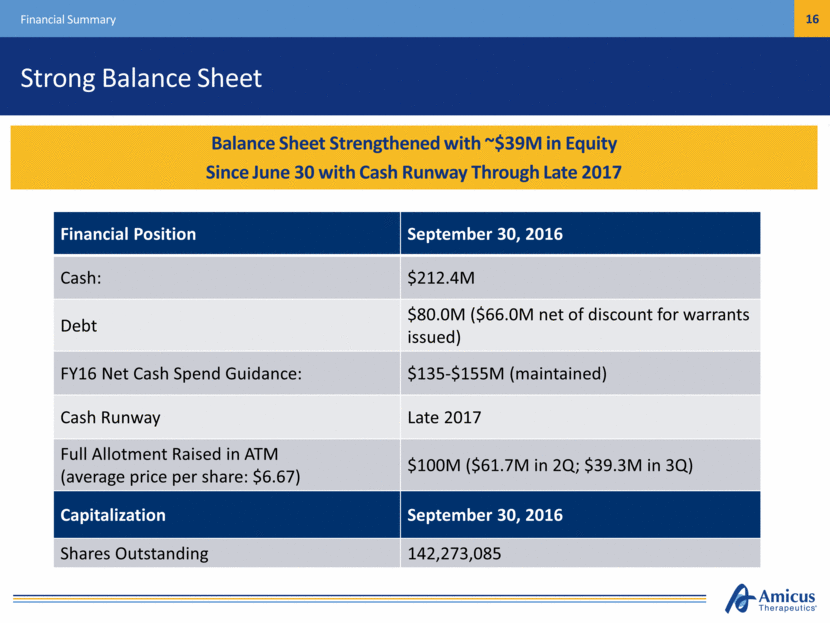
Strong Balance Sheet Financial Summary Financial Position September 30, 2016 Cash: $212.4M Debt $80.0M ($66.0M net of discount for warrants issued) FY16 Net Cash Spend Guidance: $135-$155M (maintained) Cash Runway Late 2017 Full Allotment Raised in ATM (average price per share: $6.67) $100M ($61.7M in 2Q; $39.3M in 3Q) Capitalization September 30, 2016 Shares Outstanding 142,273,085 Cash Position Provides Runway Under Current Operating Plan into mid-2017 Balance Sheet Strengthened with ~$39M in Equity Since June 30 with Cash Runway Through Late 2017
Closing Remarks

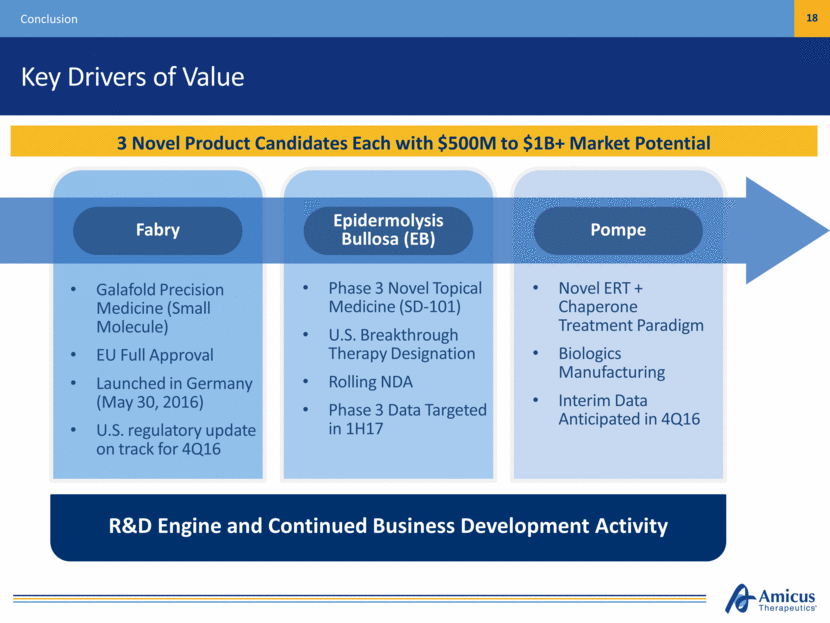
Key Drivers of Value Conclusion 18 3 Novel Product Candidates Each with $500M to $1B+ Market Potential Galafold Precision Medicine (Small Molecule) EU Full Approval Launched in Germany (May 30, 2016) U.S. regulatory update on track for 4Q16 Phase 3 Novel Topical Medicine (SD-101) U.S. Breakthrough Therapy Designation Rolling NDA Phase 3 Data Targeted in 1H17 Novel ERT + Chaperone Treatment Paradigm Biologics Manufacturing Interim Data Anticipated in 4Q16 R&D Engine and Continued Business Development Activity Pompe Epidermolysis Bullosa (EB) Fabry