Attached files
| file | filename |
|---|---|
| 8-K - 8-K TCT DATA RELEASE - SPECTRANETICS CORP | a20168ktctdatarelease.htm |
| EX-99.3 - EXHIBIT 99.3 TCT DATA RELEASE - SPECTRANETICS CORP | tctirpresentation.htm |
| EX-99.1 - EXHIBIT 99.1 TCT DATA RELEASE - SPECTRANETICS CORP | ex9912016tctdatarelease.htm |

ILLUMENATE Pivotal
Stellarex DCB IDE Study
12-Month Results
Sean Lyden, MD
On behalf of Co-PI Prakash Krishnan, MD
and the ILLUMENATE Pivotal Investigators
Cleveland Clinic
Cleveland, Ohio

Disclosure Statement of Financial Interest
• Grant/Research Support
• Consulting Fees/Honoraria
• Other Financial Benefit
• Cook, Cordis, Gore, Endologix,
Bolton, Silkroad, Trivascular,
Medtronic, Spectranetics, Bard
• Spectranetics, Biomet, Endologix,
TVA Medical
• VIVA Physicians Board Member
Within the past 12 months, I or my spouse/partner have had a
financial interest/arrangement or affiliation with the
organization(s) listed below.
Affiliation/Financial Relationship Company

Background
SFA disease remains a challenge to manage
Current PTA results from DCB trials are associated with
improved outcomes over OPC goals 1,2,3
Drug-coated balloons have improved patency over PTA
in randomized trials 1,2
Severe calcium, diabetes, CKD, women and small
vessels remain a problem for current technologies4,5,6,7
1 N Engl J Med. 2015 Jul 9;373(2):145-53
2 J Am Coll Cardiol. 2015 Dec 1;66(21):2329-38
3 Catheter Cardiovasc Interv. 2007 May 1;69(6):910-9
4 J Endovasc Ther. 2016 Oct;23(5):731-7
5JACC Cardiovasc Interv. 2014 Aug;7(8):923-33
6 J Vasc Interv Radiol. 2016 Aug;27(8):1204-14
7 Cardiovasc Intervent Radiol. 2014 Aug;37(4):898-907
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Study Device: StellarexTM DCB
(Spectranetics)
CAUTION: Investigational device. Not for sale or distribution in the United States.
EnduraCoatTM technology:
• Low dose paclitaxel, 2 µg/mm2
• Excipient: Polyethylene Glycol (PEG)
• Proprietary open-folded coating technology
Balloon catheter features:
• Catheter shaft designed for pushability
• Low 0.039” tip entry profile
• Flexible balloon and tip for tracking through tortuous
anatomy
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Stellarex Program: Sites & Investigators
ILLUMENATE FIH
80 subjects enrolled
3 EU sites
ILLUMENATE
Pivotal IDE
300 subjects enrolled
43 US & EU sites
ILLUMENATE Global
371 subjects enrolled
37 EU & AUS/NZ sites
ILLUMENATE EU RCT
328 subjects enrolled
18 EU sites
ILLUMENATE PK
25 subjects enrolled
2 NZ sites
P. Faries, NY, USA C. Bosarge, FL, USA
K. Niazi, GA, USA O. Rosales, TX, USA
A. Jain, CA, USA M. Shishehbor, OH, USA
R. Sachar, NC, USA G. Al-Khoury, PA, USA
W. Bachinsky, PA, USA M. Goodwin, IL, USA
J. Cardenas, AZ, USA J. Angle, VA, USA
M. Werner, Vienna, Austria J. Park, TX, USA
M. Brodmann, Graz, Austria M. Mewissen, WI, USA
C. Mena-Hurtado, CT, USA E. Korngold, OR, USA
J. Mustapha, MI, USA P. Desai, NC, USA
J. Ricci, MI, USA M. Ghani, OK, USA
M. Khuddus, FL, USA W. Miller, CO, USA
W. Crowder, MS, USA C. Pollock, TN, USA
M. Laiq Raja, TX, USA D. Paolini, OH, USA
G. Ansel, OH, USA D. Fry, IA, USA
C. Joels, TN, USA T. Gensler, VA, USA
J. Sandhu, PA, USA R. Kovach, NJ, USA
L. Lopez, IN, USA G. Schultz, SD, USA
N. Farhat, OH, USA G. Mayeda, CA, USA
E. Kang, GA, USA B. Katzen, FL, USA
C. Metzger, TN, USA A. Nanjundappa, WV, USA
J. Henretta, NC, USA
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16
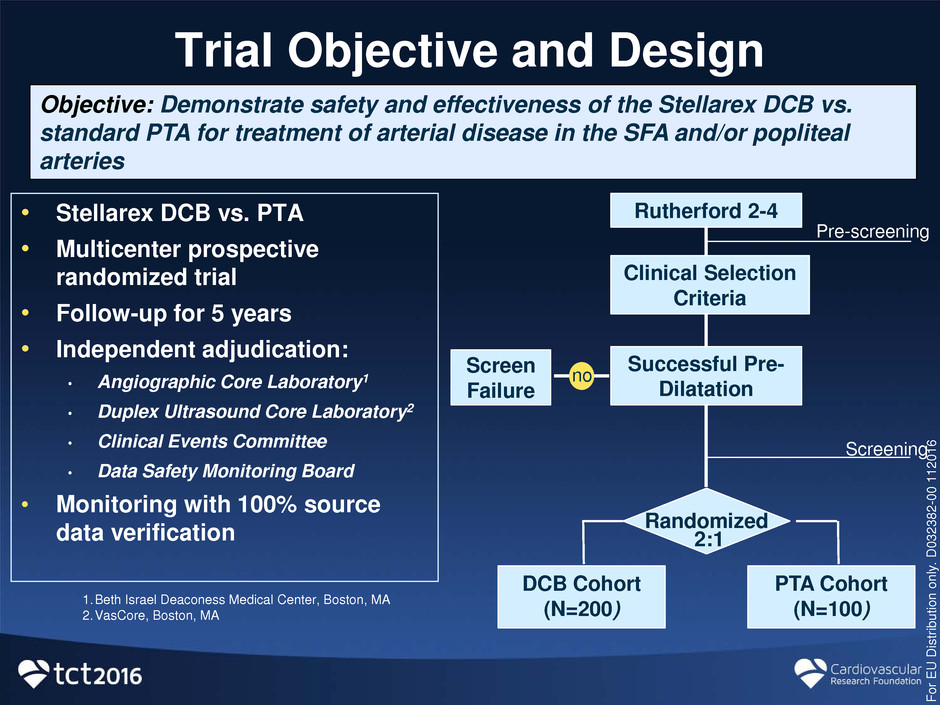
Trial Objective and Design
• Stellarex DCB vs. PTA
• Multicenter prospective
randomized trial
• Follow-up for 5 years
• Independent adjudication:
• Angiographic Core Laboratory1
• Duplex Ultrasound Core Laboratory2
• Clinical Events Committee
• Data Safety Monitoring Board
• Monitoring with 100% source
data verification
Rutherford 2-4
Clinical Selection
Criteria
Successful Pre-
Dilatation
Screen
Failure
Pre-screening
Screening
no
Randomized
2:1
DCB Cohort
(N=200)
PTA Cohort
(N=100) 1.Beth Israel Deaconess Medical Center, Boston, MA 2.VasCore, Boston, MA
Objective: Demonstrate safety and effectiveness of the Stellarex DCB vs.
standard PTA for treatment of arterial disease in the SFA and/or popliteal
arteries
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Primary Endpoints
Primary Safety Endpoint: Freedom from device- and
procedure-related death through 30 days and freedom from
target limb major amputation and clinically-driven TLR
through 12 months
Primary Effectiveness Endpoint: Primary patency at 12
months, defined as freedom from target lesion restenosis
(determined by duplex ultrasound PSVR ≤ 2.5) and freedom
from clinically-driven TLR at 12 months
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Key Eligibility Criteria
Key Inclusion Criteria
• Rutherford class 2, 3 or 4
• Lesion located in the SFA
and/or popliteal
• Has at least one patent run-off
vessel below-the-knee
• Lesion length 3-18 cm
Key Exclusion Criteria
• Acute or sub-acute thrombus
in target vessel
• Significant inflow disease
• In-stent restenosis
• Concentric calcification that
precluded PTA pre-dilatation
• Use of adjunctive therapies (i.e.
atherectomy or cutting/scoring
balloons)
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Baseline Characteristics
ITT Data Set
Stellarex PTA p
Age (years) 68.3 ± 10.3 (200) 69.8 ± 9.8 (100) 0.225
Rutherford Clinical Category 0.735
2 31.5% (63/200) 35.0% (35/100)
3 64.5% (129/200) 60.0% (60/100)
4 4.0% (8/200) 5.0% (5/100)
ABI 0.75±0.21 (193) 0.76± 0.2 (100) 0.508
Hypertension 93.5% (187/200) 94.0% (94/100) 0.867
Hyperlipidemia 88.0% (176/200) 90.0% (90/100) 0.606
Prior Coronary Revasc. 45.0% (90/200) 48.0% (48/100) 0.623
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Baseline Characteristics
ITT Data Set
Stellarex PTA p
Female 44% (88/200) 36% (36/100) 0.185
Diabetes 49.5% (99/200) 52.0% (52/100) 0.683
Renal Insufficiency 18.0% (36/200) 16.0% (16/100) 0.666
BMI ≥ 30 39.5% ( 79 /200) 30.0% (30/100) 0.107
Previous or Current
Smoker
84.0% (168/200) 75.0% (75/100) 0.061
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Baseline Core Lab
Angiographic Data
ITT Data Set
Stellarex PTA p
Lesion Length (cm) 8.0 ± 4.5 (199) 8.9 ± 4.6 (100) 0.105
Restenotic1 9.5% (19/200) 18.0% (18/100) 0.035
Total Occlusion 19.0% (38/200) 18.0% (18/100) 0.834
Severe Calcification 43.9% (87/198) 43.0% (43/100) 0.877
Diameter Stenosis (%) 73.9 ± 16.9 (200) 74.8 ± 17.0 (100) 0.673
Reference Vessel Diameter
(mm)
4.86 ± 0.92 (200) 5.15 ± 1.05 (100) 0.017
0-1 Patent Run-off Vessels 32.5% (54/166) 30.5% (25/82) 0.745
1. Site reported data
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Procedural Characteristics
Stellarex PTA p
Pre-dilatation Performed1 100% (200/200) 100% (100/100) N/A
Study Device Inflation Time1
(min/lesion)
3.9 ± 2.0 (200) 3.7 ± 2.3 (100) 0.557
Post-DCB/PTA Dissection2
Grade D
Grade E/F (Flow-limiting)
20.0% (40/200)
0.0% (0/193)
12.0% (12/100)
0.0% (0/98)
0.084
N/A
Bail-out Stent Placement1 6.0% (12/200) 6.0% (6/100) 1.000
Post-procedure
Diameter Stenosis (%)2
25.2 ± 11.7 (199)
27.4 ± 10.1
(100)
0.107
1. Site-reported data
2. Per Angiographic Core Lab Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Primary Safety Endpoint
ITT Data Set
Composite of freedom from device & procedure-related death through
30 days post-procedure and freedom from target limb major amputation
and CD-TLR through 12 months post-procedure (410 days)
• Stellarex1: 92.1% (174/189)
• PTA1: 83.2% (79/95)
• Difference: [95%CI]2 8.3% [0.03%, 16.57%]
• P=0.0013
Superiority Endpoint Achieved
1 Data are based on complete data without multiple imputation and presented as % (n/N).
2 Estimate of the difference (DCB-PTA) and 95% CI are based on the model based estimates resulting from
multiple-imputation of missing data. p-value is 1-sided for a non-inferiority margin of 5% (for DCB-PTA) and
based on the model based estimates resulting from multiple-imputation of missing data.
3 Since non-inferiority of safety was met and additionally the lower bound of the 95% CI of the difference
was greater than 0%, testing for superiority was conducted. The p-value for the superiority comparison was
0.0246, demonstrating superiority of the DCB group against the PTA group Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Key Safety Outcomes
ITT Data Set
Stellarex PTA
Difference
[95% CI]2
12-Month MAEs1 9.4% (18/191) [18] 17.7% (17/96) [18] -8.3% [-17.0%, 0.4%]
CV Death 1.6% (3/191) [3] 2.1% (2/96) [2] -0.5% [-3.9%, 2.8%]
Target Limb
Amputation
0.0% (0/189) [0] 0.0% (0/95) [0] N/A
Clinically-
Driven TLR
7.9% (15/189) [15] 16.8% (16/95) [16]
-8.9%
[-17.4%, -0.5%]
12-Month All-
Cause Mortality
2.6% (5/192) 2.1% (2/96)
0.5%
[-3.1%, 4.2%]
1. Numbers are % (n/N) [Events]- Denominator includes subjects with an event or those without
an event having follow-up on or past the opening of the visit window.
2. Confidence interval of the difference is exact when the smallest expected cell count was less
than 5. Otherwise the confidence interval of the difference is asymptotic. Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

CD-TLR1 Free at 12 Months: 93.6%
ITT Data Set
1. Clinically-driven TLR defined as reintervention due to PSVR≥2.5 (or >50% stenosis via angio) with an increase in the RCC >1
category or deterioration in the ABI by >0.15 compared to maximum early post-procedural level. Per subject analysis.
80.0%
@ day 410
91.0%
@ day 410
DCB 93.6% @ day 365
PTA 87.3% @ day 365
*
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

12 Month Primary
Effectiveness Endpoint
ITT Data Set
Absence of restenosis (Duplex PSVR ≤ 2.5) & freedom
from CD-TLR through 12 months (410 days)
• Stellarex1: 76.3% (135/177)
• PTA1: 57.6% (53/92)
• Difference: [95% CI]2 16.9% [5.1%, 28.7%]
• P=0.003
Superiority Endpoint Achieved
1 Data are based on complete data without multiple imputation and presented as % (n/N)
2 Estimate of the difference (DCB-PTA) and 95% CI are based on the model based estimates resulting from multiple-
imputation of missing data. p-value is 1-sided and based on the model based estimates resulting from multiple-
imputation of missing data Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16
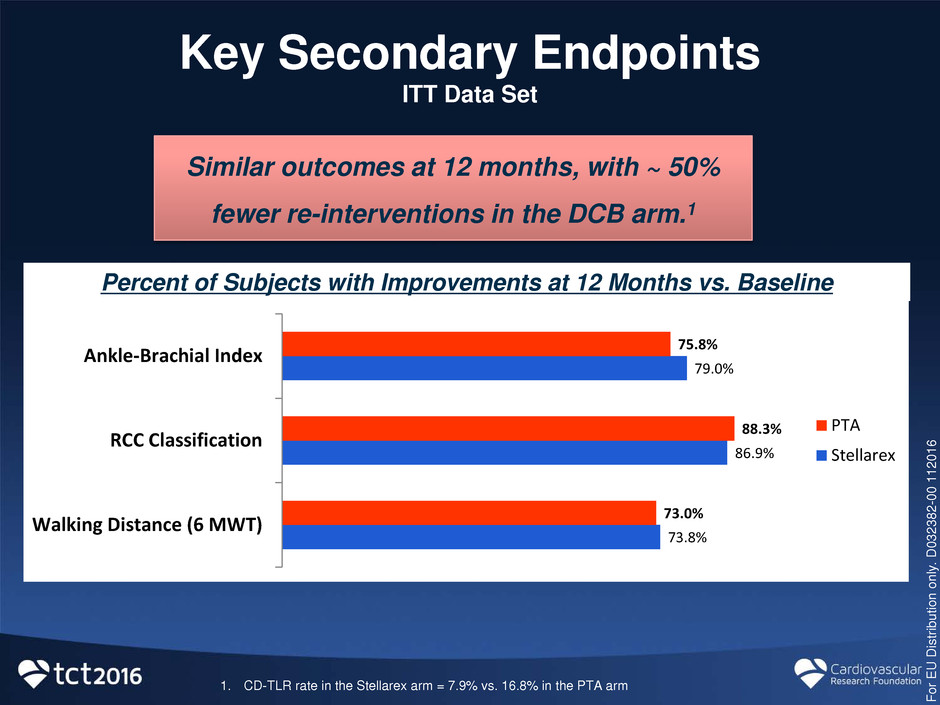
Key Secondary Endpoints
ITT Data Set
Percent of Subjects with Improvements at 12 Months vs. Baseline
1. CD-TLR rate in the Stellarex arm = 7.9% vs. 16.8% in the PTA arm
73.8%
86.9%
79.0%
73.0%
88.3%
75.8%
Walking Distance (6 MWT)
RCC Classification
Ankle-Brachial Index
PTA
Stellarex
Similar outcomes at 12 months, with ~ 50%
fewer re-interventions in the DCB arm.1
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Primary Patency at 12 Months
ITT Data Set
Primary patency is defined as freedom from restenosis (determined by duplex ultrasound PSVR threshold of 2.5) and
freedom from clinically-driven TLR at 12 months. Assessed per lesion. KM estimates reported at day 410 to capture all
patients and events within the full 320-410 follow-up window. Rates from the middle of the protocol visit window (365
days) reported for consistency and comparative purposes with other trials.
50.4%
@ day 410
73.7%
@ day 410
Δ23.3%
DCB 82.3% @ day 365
PTA 70.9% @ day 365
*
For EU Distribution only. D032382-00 112016

Data in Context with Core Lab*
Adjudicated 12-Month Patency Rates
1. Brodmann M. Oral presentation. AMP Symposium, Chicago, IL, Aug 10, 2016
2. Laird JR et al. J Am Coll Cardiol 2015;66:2329-38, P.Krishnan Oral Presentation. VIVA 2016
3. Rosenfield K, Jaff MR, White CJ, et al. The New England Journal of Medicine. 2015;373(2):145-153.
1 2
*VasCore (Boston, MA); PSVR: 2.5, KM estimates at day 365 (360 for IN.PACT SFA)
3
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16

Conclusions
• Stellarex is a low-dose (2 µg/mm2) DCB
• One of the most complex patient groups studied in DCB IDE trials
• Severe calcium 43.9%, diabetes 49.5%, 0-1 runoff 32.5%
• 12-Month DCB Primary Patency: 82.3%
• 12-Month DCB Freedom from CD-TLR: 93.6%
• Both primary safety and effectiveness endpoints
demonstrated superiority of Stellarex over PTA
• Results reaffirm prior data
ILLUMENATE FIH and EU Randomized Trial
Fo
r E
U
D
is
tri
bu
tio
n
on
ly
. D
03
23
82
-0
0
11
20
16
