Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - Accelerated Pharma, Inc. | v451545_ex23-1.htm |
| EX-10.8 - EXHIBIT 10.8 - Accelerated Pharma, Inc. | v451545_ex10-8.htm |
| EX-10.7 - EXHIBIT 10.7 - Accelerated Pharma, Inc. | v451545_ex10-7.htm |
| S-1/A - S-1/A - Accelerated Pharma, Inc. | v451545_s1a.htm |
Exhibit 10.13
MASTER SERVICES AGREEMENT
This Master Services Agreement (the “Agreement”) is entered into as of April 27th, 2015 (the “Effective Date”) by and between Accelerated Pharma Inc. with a place of business at 15W15581st, Burr Ridge 60527 Illinois, USA (“Accelerated”), and Heraeus Precious Metals GmbH & Co. KG, a German limited liability company with a principal place of business located at Heraeusstr. 12- 14, 63450 Hanau, Germany (“Manufacturer” or “Heraeus”), each singly a “Party” and together, the “Parties.”
RECITALS
Accelerated intends to entrust Heraeus with the manufacture of the active pharmaceutical ingredient Picoplatin which is required by Accelerated for a clinical development program.
NOW, THEREFORE, the Parties agree as follows:
AGREEMENT
1 DEFINITIONS. Throughout this Agreement, the following terms will be defined thus:
| 1.1 | “Affiliates” means any company, firm, individual, partnership or other legal entity which controls, or is controlled by, or controlled in common with a Party. “Control” here means holding more than 50% of the nominal value of the issued share capital or of the voting power at the general meeting of shareholders or members or has the power to appoint a majority of the directors or otherwise directs the activities of such company, firm, partnership or other legal entity. |
| 1.2 | “Confidential Information” is defined in Section 6 below. |
| 1.3 | “Day” or “Days” means a calendar day or days. “Business Days” are weekdays, excepting legal holidays and local holidays in Germany and/or Hessen. |
| 1.4 | “Order” means a purchase order for the Products and services, as further described below, pursuant to this Agreement. |
| 1.5 | “Person” means any individual, partnership, company, firm, legal entity, trust, trustee or association. |
| 1.6 | “Personnel” means the employees of a Party, individuals working as subcontractors of a Party, and employees of such Party’s affiliates, contractors and agents. |
| 1.7 | “Product” means the active pharmaceutical ingredient Picoplatin as described in Annex 1. 1.8 “Specifications” means the technical criteria described in Annex 1. |
| 1.9 | Other definitions are found within the Agreement next to the term defined. |
2. SERVICES
Manufacturer will provide Services and Products according to Order(s) made from time to time by both Parties in writing. The Initial Services are described in Annex 1. Each Order, confirmed by the Manufacturer in writing, executed pursuant to this Agreement shall form a separate agreement between the Parties and be governed by the terms of this Agreement as if contained therein in their entirety. In the event of any conflict between the terms of this Agreement and any particular Order, the terms of this Agreement shall take precedence.
2.1 Manufacturing Services. By signing this Agreement, Heraeus is entrusted to manufacture three (3) validation batches under CGMP conditions and to deliver these batches to Accelerated for their clinical development program. A validation batch contains at least 500g of the Product. If Accelerated requires further validation batches, Accelerated may order further validation batches or GMP batches of the Product at a later point in time. Each batch shall consist of a minimum quantity of 500g. By signing this Agreement, Heraeus is also entrusted to create a GMP documentation as well as to conduct a stability study for the Product.
2.2 Shipping, Title and Risk of Loss. The Products will be shipped, Ex Works per Incoterms the Manufacturer’s loading dock, such that title to and risk of loss in all Product will pass to the Accelerated at the point of shipment using the Accelerated’ s carrier account noted on the Order. Orders shall be shipped at a mutually agreed upon date and time. After written notice from Accelerated, Manufacturer will promptly ship the quantities of any missing Product to remedy any shortage.
2.3 Delivery. Manufacturer will ensure Products are packaged sufficiently to maintain their integrity during transportation.
2.4 Acceptance. Products will be deemed accepted upon delivery unless Accelerated makes a rejection of the Products within thirty (30) days of arrival at Accelerated’ s designated destination. Claims for shortages, damage, or obvious defects must be made in writing within that time period. Accelerated shall not unreasonably reject Products that conform to specifications.
2.5 Title. Title and risk of loss to the Products shall pass to Accelerated at the time Products are delivered to Accelerated. However, Manufacturer retains a security interest on Products until payment in accordance with this Agreement.
2.6 Additional Services. Parties may agree to contract for additional services than those described in 2.1. Such agreement will be pursuant to a request for quote (“Request for Quote”) provided by Accelerated to Manufacturer. The Request for Quote will provide sufficient detail to allow the Manufacturer to proceed with each individual manufacturing project, including details on validation protocols, Accelerated’ s production needs and forecasts, if any, and other details as provided.
2.7 Additional Terms. Additional terms pertaining to shipment of products, delays, risk of loss, insurance, and other terms not provided in this Agreement will be according to Manufacturer’s terms and conditions of sale (The “Heraeus Terms and Conditions of Sale”), as then in effect. (The current terms are specified in Annex 2) If there is a conflict between the Agreement and the Heraeus Terms and Conditions of Sale, then this Agreement will govern.
3. PRICING AND PAYMENT
3.1 Pricing for Initial Services are described in Annex I. The prices for Products ordered by Accelerated at a later point in time are set forth in Heraeus’ offer which Heraeus will send to Accelerated, if and when required. The prices to be paid to Heraeus are exclusive of any statutory value-added tax as well as exclusive of any other taxes, Pricing duties and charges.
Page 2 of 10
3.2 If not agreed otherwise agreed in writing Payment terms for products and services will be Net 30 days after the date of invoice or date of delivery, whichever occurs later. Invoicing requirements, if any, will be as specified in a mutually agreed electronic format, or if there is no such format, in the applicable Order.
4. QUALITY
4.1 Product Changes
4.1.1 Accelerated Proposed Product Changes. Accelerated may propose changes to any of the Products by submitting such changes to Manufacturer. Accelerated will identify those changes that it deems mandatory in order to make the Product suitable for Accelerated’s intended use. Unless otherwise agreed to in writing by Accelerated, Manufacturer will respond in writing to Accelerated within ten (10) Days after receipt of such proposed or mandatory changes with the following information, as applicable: (i) lead time required to implement the changes, provided that it is technically feasible; (ii) impact of changes on Product, including, but not limited to, any Parts, tooling, and testing, and additional costs; (iii) impact of changes on scrap material and work in process; (iv) any non-recurring engineering changes required in order to implement the changes; and (v) impact of changes on the lead time of the Product.
4.1.2 Manufacturer Proposed Product Changes. Any changes proposed by Manufacturer, including material or process changes, which affect form, fit, function, reliability, serviceability, performance, functional interchange-ability, regulatory compliance, safety, must be submitted along with a written change notice, for Accelerated approval. This may include, but is not limited to, changes of sources of material and parts, changes in accepted manufacturing range, manufacturing processes, test procedures, or replacement of equipment. No such changes may be implemented by Manufacturer without receiving Accelerated’s prior written approval.
4.2 Quality Management System.
4.2.1 Manufacturer shall document, implement, and maintain an acceptable Quality System. Manufacturer will test and inspect all Products in accordance with its existing procedures before shipping any Products to Accelerated. Manufacturer will supply an Accelerated -approved certificate of compliance for all Products shipped, which includes the results of the standard testing and procedures. Manufacturer will document all of its standard operating procedures, including quality assurance, manufacturing, testing and delivery and make copies of those procedures available to Accelerated for its review and audit.
4.2.3 In the event that any Product fails to meet the specifications, whether discovered prior to delivery or after, Manufacturer will investigate such failure and document the nature and root cause of the failure. Manufacturer will devise proposed changes to its processes and procedures as needed in order to avoid repetition of known failures.
Page 3 of 10
5. CONFIDENTIALITY
5.1 Disclosure and Use of Confidential Information. In the course of performing or receiving Services in connection with this Agreement, the Manufacturer or Accelerated (each, when receiving information, the “Receiving Party”) may be given or have access to, confidential and proprietary information as defined as Confidential Information of the other Party below (the “Disclosing Party”). The Disclosing Party’s affiliates, subsidiaries, independent contractors, business partners, and licensors, may disclose information relating to any or all of the Disclosing Party’s products and/or services (whether marketed or in development), business proposals, manufacturing and distribution processes, customer lists, computer software and related documentation, financial information, and employee data, whether tangible or intangible, and including all copies, analyses and derivatives thereof, that is marked or otherwise identified as proprietary or confidential at the time of disclosure, or which if disclosed orally is confirmed in writing by the Disclosing Party to the Receiving Party within thirty (30) days of initial disclosure (collectively, “Confidential Information”). The Receiving Party shall not, without the Disclosing Party’s prior written consent, disclose to any third-party any Confidential Information or use any Confidential Information for any purpose other than performance of the Services. The Receiving Party shall employ the same standard of care in protecting disclosed Confidential Information as it would employ to protect its own confidential information, but shall in no event use less than reasonable care. The Receiving Party shall disseminate Confidential Information to its personnel and Suppliers only on a “need-to-know” basis. The Receiving Party shall cause each of its personnel and Suppliers who have access to Confidential Information to comply with the terms of this Section 6 in the same manner as it is bound by this Section 6, with the Receiving Party remaining responsible for the actions and disclosures of any such personnel.
5.2 Exceptions to Confidential Information. For purposes hereof, “Confidential Information” does not include information that (i) was rightfully in the Receiving Party’s possession without restriction before disclosure hereunder, (ii) was or becomes public knowledge through no fault of the Receiving Party, (iii) was rightfully disclosed to the Receiving Party without restriction by a third-party not bound by a confidentiality restriction, or (iv) was independently developed by the Receiving Party or its personnel. The restrictions in this Section 6 shall not prevent disclosures required by law, court order or other governmental order or demand; provided that, to the extent practicable, the Receiving Party provides prompt written notice and reasonable assistance to the Disclosing Party prior to such disclosure, so that the Disclosing Party may seek a protective order or other appropriate remedy to protect against or limit such disclosure. The confidentiality obligations set forth in this Agreement shall expire five (5) years from the date of the disclosure.
6. REPRESENTATIONS
6.1 Compliance with Laws. Manufacturer represents and warrants that Manufacturer shall comply with all applicable laws and regulations and customer policies in which the Services will be provided, including the maintenance of necessary government permits, licenses and other means by which to lawfully perform Services. Manufacturer further represents and warrants that no action (or failure to take action) by it or any of its employees will cause Accelerated to violate or incur any penalty under any applicable law or regulations. Manufacturer does not assume any liability for the non-infringement of any third-party rights by the manufacture, the placing on the market or the use of the Product. It shall be the sole responsibility of Accelerated to perform a Freedom to Operate Analysis to ensure that the Product in the application intended by Accelerated does not infringe any third-party rights.
6.2 No Other Restrictive Arrangement. Manufacturer is not subject to, or a party to, any employment agreement, non-competition covenant, non-disclosure agreement, or other agreement, covenant, understanding, or restriction that would prohibit Manufacturer from executing this Agreement and performing fully the duties and responsibilities hereunder.
6.3 Disclosure. A Party shall within three (3) calendar days notify the other Party in the event any representation or warranty by the Party set forth in this Agreement shall no longer be true, correct, or complete.
Page 4 of 10
6.4 Conflict of Interest. Manufacturer covenants that, during the term of this Agreement, Manufacturer will not engage, directly or indirectly, in any activity that materially conflicts with Manufacturer’s faithful performance of the Services.
6.5 Representations and Indemnifications of Accelerated. Accelerated represents and warrants that Accelerated has all IP rights required for the manufacture, the placement on the market and the use of the Product in the application intended by Accelerated. In addition, Accelerated represents and warrants that Accelerated rightfully acquired any and all rights to the manufacturing process of the Product from Poniard Inc. and that Accelerated is entitled to exercise these IP rights. Accelerated will, upon first written request, indemnify and hold Manufacturer harmless from and against any and all claims which are asserted or entered against Manufacturer by any third party on account of the alleged or actual infringement of third party IP rights by the manufacture and/or delivery and/or use of the Product in pharmaceutical products of Accelerated or third parties. Accelerated shall reimburse to Heraeus any and all damages accruing to Heraeus on account of such alleged or actual infringement of third-party property rights by the manufacture and/or delivery to Accelerated.
7. WARRANTY AND LIABILITY
7.1 Warranty
7.1.1 Limited Warranty. Manufacturer warrants that the Products will be free from defects in workmanship and materials and will conform to the specifications set forth in Annex 1 for a period of ninety (90) days from the date of receipt by Accelerated (the “warranty period”).
7.1.2 Subject to Section 5.1.4, the Manufacturer’s responsibility for defective or non-conforming Products is limited, at Manufacturer’s option, to either replacement of the defective Products or return to Accelerated of monies actually received by Manufacturer from Accelerated for those defective Products, less any unrecoverable costs for components or sub-assemblies. Manufacturer may require inspection of the defective or non-conforming Products. However, no Product may be returned to Manufacturer unless such returned Product shall first have been determined to be defective or non-conforming.
7.1.3 Accelerated will pay Manufacturer the cost of all charges, including but not limited to a reasonable charge for examination and rework if the returned Products prove not to be defective or for work requested by Accelerated that exceeds Manufacturer obligations under this Warranty.
7.1.4 This Warranty extends only to the original customer of the Products (that is, Accelerated). This Warranty does not apply to, and Manufacturer assumes no responsibility for, damage or defects due to any cause other than those specified above, including, but not limited to, damage or defects arising as a result of misuse, improper installation by Accelerated’s personnel or subcontractors, accident, neglect, modification, repair by Accelerated, adverse conditions, and demands exceeding performance levels required by applicable specifications by Manufacturer. Provision of Products to any third-party terminates this warranty, even though the warranty period has not expired, unless tests Products against specifications using tests or methods acceptable to Manufacturer prior to that incorporation. In no case, however, shall the warranty extend beyond the 90-day warranty period.
Page 5 of 10
7.1.5 THIS WARRANTY IS EXCLUSIVE AND MADE IN LIEU OF ALL OTHER WARRANTIES, EXPRESSED OR IMPLIED, INCLUDING THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. NO MODIFICATION OR ALTERATION OF THE FOREGOING WARRANTY AND LIMITATION OR REMEDIES PROVISIONS SHALL BE VALD OR ENFORCEABLE UNLESS SET FORTH IN A WRITTEN AGREEMENT SIGNED BY HERAEUS AND THE CUSTOMER.
8. LIMITATION OF LIABILITY. EXCEPT IN THE CASE OF BREACH OF CONFIDENTIALITY, IN NO EVENT SHALL EITHER PARTY OR ITS EMPLOYEES, AGENTS, AFFILIATES, OWNERS OR OFFICERS BE LIABLE TO THE OTHER PARTY FOR SPECIAL, CONSEQUENTIAL, INCIDENTAL, INDIRECT, PUNITIVE OR EXEMPLARY DAMAGES, HOWEVER CAUSED, WHETHER FOR BREACH OF WARRANTY, CONTRACT, TORT (INCLUDING NEGLIGENCE), STRICT LIABILITY OR OTHERWISE.
9. INDEMNIFICATION
9.1 Indemnification by Accelerated. Accelerated shall indemnify, defend and hold harmless Manufacturer, its Affiliates, and their respective officers, directors, agents, employees, and shareholders from and against all claims whether such claim is stated as a product liability claim, a strict liability claim or other similar claims, to the extent such claims on any legal theory relates to or arises from: (a) personal injury or death of any person from any pharmaceutical product manufactured or sold by Accelerated that incorporates a Product, provided that this indemnification obligation shall not extend to a claim to the extent such claim is found to have been directly caused by Manufacturer’s negligence, gross negligence or willful misconduct, or violation of any material applicable local, state, federal, foreign or international law or regulation, in each case in connection with the manufacture, labeling, packaging, sale, storage or shipment of such Product, as proven by a final non-appealable judgment, (b) the infringement or alleged infringement of any third-party’s patent, copyright or trade secret right to the extent such infringement is based upon Accelerated’ s specifications or the design of the products provided by Accelerated to Manufacturer or the end use or application of a product, or (c) Accelerated’ s negligence, gross negligence or willful misconduct, or violation of any material applicable local, state, federal, foreign or international law or regulation, in each case in connection with the manufacture, assembly, design, handling, labeling, packaging, sale, storage or shipment of devices incorporating any Product, as proven by a final non-appealable judgment. Manufacturer shall: (i) give Accelerated prompt written notice of any claims for which Manufacturer may seek indemnification from Accelerated; (ii) permit Accelerated to participate in the defense of the same through its counsel, subject to any applicable privileges; (iii) give relevant information in its possession relating to such claims; (iv) assist in such defense; and (v) not compromise or settle any such claims without Accelerated’s written consent.
9.2 Insurance provided by Accelerated. For the clinical development project, Accelerated is obligated to take out an insurance for the test persons which provides sufficient coverage in the event any test person suffers injuries to life, body or health. Accelerated will include Heraeus as additional insured under this insurance policy.
10. TERM
This Agreement shall commence on the Effective Date and will continue in full force and effect until completion of the Services, unless terminated as provided in Section 13.
11. TERMINATION AND POST-TERMINATION OBLIGATIONS
11.1 Termination by Mutual Consent. This Agreement may be terminated at any time by mutual written agreement of the Parties.
Page 6 of 10
11.2 Optional Termination. This Agreement may be terminated by either of the Parties without cause by giving the other Party fifteen (15) calendar days’ prior written notice.
11.3 Termination by Accelerated. Accelerated may terminate this Agreement, effective immediately upon written notice to Manufacturer, in the event of a material breach of this Agreement (including the breach of any of the representations and warranties contained herein), bankruptcy, loss of commercial registration or cessation of business, except that in the event of a breach caused by Product quality issues, Accelerated will provide Manufacturer with notice and a reasonable opportunity to cure by repair or replacement of non-conforming Products with conforming Products.
11.4 Effect of Termination. On termination, Accelerated’s sole obligation to Manufacturer will be to pay any then-outstanding unpaid fee for Services actually performed hereunder and to reimburse Manufacturer for then-outstanding reimbursable expense. Manufacturer shall waive any and have no claims upon Accelerated in respect of any severance or similar compensation in any form. Manufacturer hereby expressly agrees that termination of this Agreement will not void, invalidate, or otherwise affect Manufacturer’s obligations under Section 6 (Confidentiality). Manufacturer expressly agrees that the terms of Section 6 will survive expiration or termination of this Agreement.
12. NOTICE TO ACCELERATED
12.1 If Manufacturer receives a request or demand to disclose any books, documents, or records relevant to this Agreement for the purpose of an audit or investigation, Manufacturer shall notify Accelerated immediately (within two (2) business days after receipt of such request or demand) in writing of the nature and scope of such request or demand. Manufacturer shall make available to Accelerated, upon written request of Accelerated, all such books, documents, or records.
13. MANUFACTURER PERSONNEL
13.1 Manufacturer shall be solely responsible for the compensation of its Personnel, including the payment of salary, benefits and employment-related taxes and withholding. Manufacturer shall be permitted to use subcontractors to provide Services under this Agreement.
14. INTELLECTUAL PROPERTY
14.1 Accelerated Intellectual Property. Manufacturer acknowledges (a) Accelerated’s exclusive right, title and interest in and to Accelerated’s Intellectual Property; (b) the validity of any and all registrations thereof; (c) that Accelerated is the sole owner of same and all goodwill relating thereto; and (d) that Manufacturer shall not, by reason of this Agreement or otherwise, acquire any right title or other ownership therein other than the limited privilege of use contemplated by this Agreement. For these purposes, -Intellectual Property” means that all trademarks and foreign language equivalents, copyrights and translations of Accelerated copyrights property, domain names, trademarks and trade names, patents, trade dress, emblems, designs (whether registered or not), insignia, models and methods of Accelerate, Accelerated’ s Confidential Information and any other intellectual property relating to Accelerated’ s products or packaging, labeling, advertising and promotional materials used for and/or relating to such products. Manufacturer agrees that he shall take no steps to challenge or impair Accelerated’s Intellectual Property. Subject to these restrictions and upon prior written approval by Accelerated of content and format, Manufacturer may indicate on its stationery, advertising and promotional materials that it is Accelerated’s supplier.
Page 7 of 10
14.2 Manufacturer Intellectual Property.
Accelerated acknowledges that, except as specifically set forth herein, that all right, title and interest in and to Manufacturer’s Intellectual Property shall be solely and exclusively vested in Manufacturer and no ownership, interest, right or license is granted to the other Party.
15. GOVERNING LAW AND ARBITRATION
15.1 Governing Law. This Agreement will be governed by and construed in accordance with the substantive laws of Germany without regard to its conflict of law rules.
15.2 Emergency Remedies. Notwithstanding Section 17.2, the Parties acknowledge and agree that that they will have injunctive and other equitable remedies as described in Section 18.9 (Remedies) below.
16. MISCELLANEOUS
16.1 Force Majeure. If the performance of any obligation under this Agreement by Manufacturer is prevented, restricted, or interfered with by reason of war, revolution, civil commotion, acts of public enemies, blockade, embargo, strikes, interruption of supply, any law, order, proclamation, regulation, ordinance, demand, or requirement having a legal effect of any government or any judicial authority or representative of any such government, which is beyond the reasonable control of the Party affected, then the Party so affected shall, upon giving prior written notice to the other Party, be excused from such performance (other than the obligation to pay amounts due hereunder) to the extent of such prevention, restriction, or interference, provided that the Party so affected shall use reasonable commercial efforts to avoid or remove such causes of nonperformance and shall continue performance hereunder with reasonable dispatch whenever such causes are removed. Neither Party shall be in default if any delay or failure to perform any obligation hereunder (other than the obligation to pay amounts due hereunder) that is caused by events beyond such Party’s reasonable control.
16.2 Waiver of Compliance. Neither party shall by mere lapse of time, without giving notice or taking other action hereunder, be deemed to have waived any breach by the other party of any of the provisions of this Agreement. Further, the waiver by either party of a particular breach of this Agreement by the other shall not be construed as or constitute a continuing waiver of such breach or of other breaches of the same or other provisions of this Agreement.
16.3 Severability. It is intended that this Agreement shall not violate any applicable law and the unenforceability or invalidity of any provision, or part thereof, (other than the provisions obligating Accelerated to make payments to Manufacturer) shall not affect the force and validity of any other provision and such invalid provisions shall be deemed severed from this Agreement, and, if permissible, be replaced with terms which as closely as possible approximate the intent of such invalid provisions.
16.4 Counterparts. This Agreement may be executed in any number of counterparts, including electronic or facsimile produced copy, each of which shall be deemed an original and all of which shall constitute one and the same agreement.
16.5 Headings. The headings contained in this Agreement are for convenience of reference only and do not qualify or affect in any way the meaning or interpretation of this Agreement.
16.6 Construction of Agreement. Each Party represents that it has carefully read and fully understands the scope and effect of all the provisions of this Agreement, and that it was offered such period as it deemed necessary to consider it. Consequently, any language deemed to be ambiguous contained herein shall not be construed in favor of one Party over the other.
Page 8 of 10
16.7 Relationship. Notwithstanding any provision hereof, for all purposes of this Agreement each Party shall be and act as an independent contractor and not as partner, joint venturer, employer, employee or agent of the other and shall not bind nor attempt to bind the other to any contract. Manufacturer is an independent contractor and is solely responsible for all taxes, withholdings, and other statutory or contractual obligations of any sort, including, but not limited to, Workers’ Compensation Insurance.
16.8 Non-assignment. This Agreement is personal to Manufacturer and cannot be assigned by Manufacturer or otherwise transferred to any other person or party without Accelerated’s prior written consent, unless as a part of a sale or transfer of assets to any Manufacturer affiliate. Any assignment without such consent will be cause for immediate termination of this Agreement by Accelerated. Any other attempts to transfer will be void. Manufacturer further agrees not to subcontract services under this Agreement, in whole or in part, without Accelerated’s prior written consent. Manufacturer’s obligations under this Agreement are binding on Manufacturer’s heirs, legal representatives, administrators, and executors.
16.9 Remedies. Notwithstanding and as an exception to Section17.2 above, the Parties acknowledge and agree that in the event of any breach or threatened breach of Section 6 or the breach of its obligations relating to ownership or use of intellectual property, the other Party will suffer irreparable damage for which it will have no adequate remedy at law. Accordingly, each Party shall be entitled to seek injunctive and other equitable remedies to prevent or restrain, temporarily or permanently, such breach or threatened breach, without the necessity of posting any bond or surety, in addition to any other remedy that such Party may have at law or in equity.
16.10 Notices. Notices or communications to be given under this Agreement shall be provided to the appropriate Party in writing either by personal delivery, overnight delivery service, confirmed telefacsimile, electronic mail or registered or certified mail, postage prepaid, to the addresses first set forth above or to such other addresses and to such other persons as either Party may from time to time designate by notice given as herein provided. Such notices or communications shall be deemed to have been given upon receipt if by personal delivery, five (5) business days after deposit in the United States mail if sent by first class, registered, or certified mail, postage prepaid, one (1) business day after delivery if by an overnight delivery service, or upon transmission confirmation if by telefacsimile or electronic mail.
16.11 Entire Agreement. The Parties hereto acknowledge that this Agreement, together with any Statements of Work attached hereto or executed after the date hereof, is the complete and exclusive statement of agreement respecting the subject matter hereof and supersedes and renders null and void any and all agreements and proposals (oral or written), understandings, representations, conditions, and other communications between the Parties relating hereto and shall constitute the only valid binding and enforceable agreement between them. This Agreement may be amended only by a subsequent writing that specifically refers to this Agreement. An exchange of email correspondence in which both Parties agree to a change or addition to the Agreement shall be treated as an agreement in writing for these purposes.
Page 9 of 10
IN WITNESSETH WHEREOF, the Parties have executed this Agreement as of the Effective Date.
| ACCELERATED PHARMA, INC. | HERAEUS PRECIOUS METALS GMBH & Co. KG | |||
| By: | /s/ Michael Fonstein | By: | /s/ Dr. Marcus Hannakam | |
| Name: | Michael Fonstein | Name: | Dr. Marcus Hannakam | |
| Title: | CEO 5/5/15 | Title: | Global Head of Marketing and Sales | |
| ACCELERATED PHARMA, INC. | HERAEUS PRECIOUS METALS GMBH & Co. KG | |||
| By: | /s/ Michael Fonstein | By: | /s/ Eberhard Rämisch | |
| Name: | Michael Fonstein | Name: | Eberhard Rämisch | |
| Title: | CEO 5/5/15 | Title: | Head of New Sales & Business Development Pharmaceutial Ingredients Business Unit Heraeus Precious Metals GmbH & Co. KG 27.04.2015 | |
| Page 10 of 10 |
Apr. 27th, 15
MASTER SERVICES AGREEMENT
Production of Picoplatin
GMP Grade
Annex I
Production of 3 GMP-Validation Batches of Picoplatin
Including
GMP Documentation and Stability Program
for Regulatory Filing
between Accelerated Pharma Inc. with a place of business at 15W15581 st, Burr Ridge 60527 Illinois, USA ("Accelerated"). and Heraeus Precious Metals GmbH & Co. KG, a German limited liability company with a principal place of business located at Heraeusstr. 12-14. 63450 Hanau, Germany ("Manufacturer" or "Heraeus"), each singly a "Party" and together, the "Parties."
1. Background, scope and objective
Accelerated Pharma Inc. ("Accelerated") is seeking a supplier for the oncology API Picoplatin. Requirement is expected to be approx. 0.5 kg/year to 1.0 kg/year.
Picoplatin is a drug used in cancer chemotherapy and has been manufactured at Heraeus in the past years in several development campaigns and was validated in 2009 on 2.25 kg scale.
The chemical structure and name of Picoplatin is:
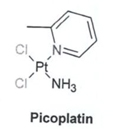
| · | Cis-Rammine)dichloro)(2-methylpyridine)platinum(11)], C61-110C12N2Pt |
| · | CAS number: 181630-15-9 |
| · | CAS name: Platinum. amminedichloro(2-methylpyridine)-, (SP-4-3)- |
2. Proposal Requirements as discussed with Accelerate Pharma Inc.
Heraeus has several years of experience in the manufacturing of Picoplatin. However as the last manufacturing was some years ago, the production process needs to be adapted to the newest standards as well as Accelerate Pharma Ltd. Inc. specific requirements.
This Annex covers the production of Picoplatin drug substance under cGMP conditions. The target quantity is min. 500g of Picoplatin drug substance per batch. The manufactured drug substance is required for a clinical development program by Accelerated Pharma
The basis of our calculations is the synthetic route followed for the production of the last Picoplatin cGMP batches 102090070 to 104090070 at Heraeus in 2009. as well as a site change of the manufacturing plant of the step 3 process. This leads to significant scale and process modifications in step 3 of the process compared to the batches manufactured in 2009.
The contract includes:
| · | Update of existing cGMP documents prior to manufacture (cleaning records, update of MBRs. (IQ/OQ packages), reflecting process changes. |
| · | Preparation of new cGMP documents required for the site change of the step 3 process of Picoplatin drug substance (cleaning records, update of MBRs, qualification documents due to changed equipment, process change documentation) |
| · | Required Reference standards for Product and Intermediates |
| · | Installation and testing of equipment in the laboratory isolator (preparations for steps 1, 2 and 3); programming of the process control unit, water runs as well as documented calibration of sensors. |
| · | Production costs as outlined below: step 1 (TCPP). step 2 (Picoplatin Crude) and step 3 (Picoplatin drug substance) |
| · | Pre-batch cleaning of isolator, cleaning between steps and post-production cleaning |
| · | Raw materials for syntheses and cleaning, required single-use equipment (inline filters, hoses etc.) |
| · | Preparation of process documentation after finishing the campaign |
| · | Quality control for raw materials, intermediates, drug substance and cleaning samples |
| · | Waste disposal (syntheses and cleaning) |
| · | Platinum losses and recovery |
| · | Freight, packing and insurance |
| · | QA review of all cGMP documents |
3. Project description
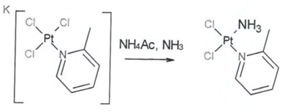
Heraeus has validated the synthesis of Picoplatin on 2.25 kg scale using the chemical route as outlined below
Step1: TCPP
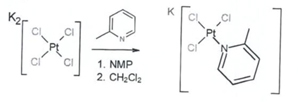
Step 2: Picoplatin Crude
Step 3: Picoplatin
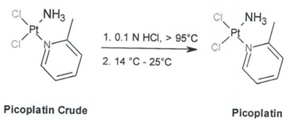
Figure 1: Synthetic route to Picoplatin
The draft specification for Picoplatin drug substance based on the last revision of the specification from 2009 is as follows:
| Parameter | Specification | |
1. Description Appearance |
A yellow powder | |
| 2. Identification by IR | Conforms to reference standard | |
| 3. Identification by HPLC | Retention time of the picoplatin peak in the sample should not differ from that of the reference standard by more than ± 0.2 minutes. | |
4. Color of Solution |
Comparable to or between GY2 to GY3 reference solutions (Ph. Eur.) | |
| 5. Clarity of Solution | Opalescence must be less than opalescence reference solution I (Ph. Eur.) | |
| 6. Picoplatin Assay by HPLC | 98.0- 102.0 `)/0 w/w | |
7. Impurities by HPLC* a) 2-picoline b) TCPP c) TCAP d) Highest single other impurity e) Total impurities |
NMT
0.05 % w/w | |
8. Residual Metals Heavy Metals Process Metals Iron |
NMT 20 ppm NMT 20 ppm NMT 10 ppm | |
| 9 Water content (KF) | NMT 0.3 % w/w | |
| 10. Residual acetone by GC | NMTO 5 °A wiw | |
| 11. Endotoxins, USP | NMT 0.19 EU/mg | |
| 12. Bioburden Total aerobic microbial count (TAMC) | NMT 10 CFU/g | |
| 13. pH of Solution | Informative |
*Impurity profile is not expected to be changed vs. last production campaigns and no new impurities are expected
The final specification has to be agreed and accepted between Accelerated and Heraeus.
Stability testing program of the drug substance to support regulatory filing will be performed
Creation of a Drug Master File and additional other regulatory services are not agreed and included in this quote.
Details are described in fcllowing paragraphs.
| 3.1 | Generation of GMP documentation required for process validation |
Heraeus will prepare, review and approve all GMP relevant documentation required to initiate the following GMP production. such as:
Validation master plan
Risk analyses
Validation protocols
Equipment qualifica:ion documents
Process documents (batch records, other SOPs and form sheets)
Cleaning documents (cleaning validation protocol. cleaning records, other form sheets)
Please note:
Responsibilities regarding documentation and approval of documents and material are described in a separate Quality Agreement.
| 3.2 | Generation of Reference Standards |
Heraeus will prepare and qualify approx. 300 gram of reference standards for Picoplatin and sufficient amount of raw materials and intermediates as requested:
| Picoplatin | |
| TCPP | |
| 2-Picoline | |
| TCAP | (Including CoA) |
Heraeus will send samples of reference standards to Accelerated Pharma on request.
| 3..3 | Manufacture of Picoplatin GMP Grade |
Heraeus will manufacture 3 GMP batches of Picoplatin sequentially on a scale of approx. 500g Picoplatin drug substance in our commercial facility (glove box facility: non-dedicated glass equipment).
- Manufacture of 3 batches of TCPP, 1 batch Picoplatin Crude and 3 batches of Picoplatin drug substance, including documentation of full validation of a different batch scale for step 3 and a site change for step 3.
Deliveries
Target quantity of gross 3 x approx. 500g Picoplatin drug substance
Documentation supplied to Accelerated Pharma Inc.
Certificates of Analysis
Validation protocol / report
Executed batch manufacturing records
Timelines
A new reactor is already on order for the processing in step 3 of the synthesis. The lead time of this reactor represents the critical path in the timeline before manufacturing can start. The generation of the required GMP documentation, sourcing of raw materials and other preparation prior to production will be performed in this time. Manufacturing will be started as soon as the equipment is installed, raw materials are released and the required documentation is in place.
Details see separate Gant Charts
Storage conditions: 25°C based on the existing stability data for Picoplatin.
Currently Heraeus assumes that no amendment in the Heraeus manufacturing license is required at this stage and an eventual approval by German authorities is not included in the timeline.
| 3.4 | Stability study |
Heraeus will perform a stability study on each batch of drug substance manufactured. Conditions for labeled storage set to ambient (25 °C +/- 3 °C; 60% r.H.) conditions, conditions for accelerated storage set to 40 °C +1- 3 °C; 75% r.H.
Testing intervals for each validation batch could be
Labeled conditions: 3, 6, 9. 12, 18. 24, 36, 48, 60 months
Accelerated conditions: 1, 2, 3, 6 months
4. Regulatory services
At any time before successful completion of the work described in sections 3.1 — 3.3. Accelerated and Heraeus should discuss details of the preparation of required regulatory documentation for Picoplatin.
Heraeus offers a professional lifecycle management in all regions of the world, depending on our clients' needs.
Details on required regulatory documentation should be agreed upon between Accelerated and Heraeus.
5. Limitations and options
Heraeus does not provide any warranty with regards to Intellectual Property and assumes that Accelerated is fully responsible for FTO and/or holds IP with regards to active substance and the agreed upon manufacturing processes.
Accelerated Ltd. Inc. has to indemnify Heraeus against third party claims if the manufacturing process and/or the active substance actually or allegedly infringe third party IF rights.
6. Project management
Heraeus will provide an experienced project manager as technical responsible person throughout the project with either a chemical (Ph.D.) or chemical engineering background.
We will work closely with Accelerated Inc. to assure prompt reporting of the current project status and progress made. Regular project up-dates as well as option for regular tele or video conferences are standard offers to Heraeus clients.
Costs for project management are included in this quotation.
7. Prices
| 3.1 | Generation of GMP documentation required for process validation |
| Price: | USD 70 000 lump sum |
| 3.2 | Generation of Reference standards |
| Price: | USD 32 500 lump sum |
Price for external delivery of Picoplatin reference standard:
| USD 100 per gram |
| + price of Platinum s. 3.2 |
| 3..3 | Manufacture of GMP Material |
Our main target is to offer competitive and transparent prices, therefore we usually mention the Platinum prices and manufacturing prices separately for our APIs
While the manufacturing price stays the same for comparable deliveries, the Platinum price may change over time due to its high volatility.
Internal policies ensure that a booking of Platinum is only possible with a valid PO, therefore we ask for your understanding that we can fix the final prices only after receipt of the same.
Manufacture of three validation batches of Picoplatin
| Price: | USD 350 000 lump sum |
| + Platinum ca. 250 gr approx. USD 30. 000 |
| (40, 50 USD/gram as of April 1st, 2015) |
| 3.4 | Stability study |
| Price: | USD 2450 per test point and temperature |
| 4. | Regulatory services |
Additional regulatory services are not agreed / included Current yields and cost estimations are based on the experience Heraeus gathered during the last Picoplatin manufacturing campaign in 2009.
In case the place of delivery requested by Accelerated should be within the European Union, 19% Value Added Tax may apply to invoices regarding delivery within EU. While VAT can be reclaimed by Accelerated, we suggest to jointly discuss this topic asap prior to order confirmation by Heraeus.
9. Terms and conditions
Delivery conditions: CIP place of delivery (lncoterms 2010). incl. packing and insurance Payment terms:
9.1 100 000 USD, 10 days net from submission of PO
9.2 125 000 USD, net immediately at start of first batch production
+ Price for Platinum approx. 40,50 USD per gram
9.3 127 500 USD, 10 days net after finalization of third batch
9.4 100 000 USD, 10 days net after delivery of CoA and Documentation
9.5 Stability program:
at/for each time point 10 days net after submission of CoA/Report
Price for Platinum non binding without obligation until receipt of final PO
10. Outlook on the cost of commercial production
Commercial pricing is driven by the final needs of Accelerated Inc. (e.g. batch size, choice of manufacturing site).
For commercial product supply a license by the German authorities is mandatory. Current lead time around 6-9 months.
This shall be discussed at an appropriate point in time during the project.
11. Validity of Pricing
The prices offered for items 3.1 - 3.4 and price indications provided for items 4, 5 and 10 are based on the current EUR/USD exchange rate of 1.10
Should the EUR/USD rate at submission of Accelerated's PO for each of the offered items be outside a range of 1.05-1.15 we will adjust our prices accordingly.
12. Remarks
Availability of plant capacity is subject to a written agreement.
Heraeus will perform all above mentioned work according to the existing information and will make best efforts to synthesize the required quantities of Picoplatin and meeting the target specification.
Should the procedures fail to give the anticipated products or target quantities, the project scope and costs need to be revised.
IN WITNESSETH WHEREOF, the Parties have executed this Agreement as of the Effective Date.
| ACCELERATED PHARMA, INC. | HERAEUS PRECIOUS METALS GMBH & CO. KG | |||
| By: | Michael Fonstein | By: | Eberhard Ramisch | |
| Title: | CEO | Title: | Head of New Sales and Business Development | |
